Summary
We describe a protocol for identifying bacteria-derived lipid metabolites produced in the guts using antibiotic-treated mice, liquid chromatography tandem mass spectrometry-based lipidomics, and feature-based molecular spectrum networking (FBMN). Untargeted lipidomics using the MS-DIAL 4 program provides information on known and unknown complex lipid molecules. The FBMN technique clusters similar MS2 spectra, facilitating the identification of bacterial lipids. Targeted analysis was used as a complementary method to cover oxylipins. Here, we provide details for targeted and untargeted analyses.
For complete details on the use and execution of this protocol, please refer to Yasuda et al. (2020).
Subject areas: Immunology, Metabolomics, Microbiology, Mass Spectrometry
Graphical abstract

Highlights
-
•
LC-MS/MS-based lipidomics on fecal samples collected from antibiotic-treated mice
-
•
Feces homogenization and lipid extraction for targeted and untargeted analyses
-
•
Molecular spectrum networking for identifying bacteria-derived lipid metabolites
We describe a protocol for identifying bacteria-derived lipid metabolites produced in the guts using antibiotic-treated mice, liquid chromatography tandem mass spectrometry-based lipidomics, and feature-based molecular spectrum networking (FBMN). Untargeted lipidomics using the MS-DIAL 4 program provides information on known and unknown complex lipid molecules. The FBMN technique clusters similar MS2 spectra, facilitating the identification of bacterial lipids. Targeted analysis was used as a complementary method to cover oxylipins. Here, we provide details for targeted and untargeted analyses.
Before you begin
Administration of antibiotics
Timing: 6 weeks
The fecal metabolome contains complex components of food, host, and bacterial origins. By comparing the metabolic profiles obtained from fecal samples of antibiotic-treated and control mice, bacteria-derived lipid candidates can be characterized, with marked decreases observed in the antibiotics group compared with the control group. Total bacteria were eliminated by the administeration of an antibiotic cocktail containing ampicillin, vancomycin, neomycin, and metronidazole.
-
1.
Maintain wild type C57BL/6J mice for at least two weeks in a specific pathogen-free facility to equilibrate the gut microbiota to the breeding environment.
-
2.
Prepare the antibiotic cocktail as described in the “materials and equipment” section.
-
3.
Provide antibiotic-treated drinking water to the treatment group of mice for four weeks. Replace with fresh antibiotic-treated drinking water every two weeks.
-
4.
After administration of antibiotics in the drinking water, collect feces into sterile microtubes.
CRITICAL: Be careful not to contaminate fecal samples with urine or bedding. Place the tubes with the collected feces on ice immediately to decrease unexpected enzymatic reactions and lipid oxidation.
-
5.
Store the fecal samples at −80°C immediately.
Pause point: Fecal samples can be stored at −80°C for a year (Jonasdottir et al 2018).
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Acetonitrile for QTofMS | FUJIFILM Wako Pure Chemical | 018-26225 |
| Methanol for QTofMS | FUJIFILM Wako Pure Chemical | 130-18545 |
| 2-Propanol for QTofMS | FUJIFILM Wako Pure Chemical | 164-27515 |
| 1 mol/L Ammonium acetate solution | FUJIFILM Wako Pure Chemical | 018-21041 |
| Ethylenediamine-N,N,N’,N’-tetraacetic acid, diammonium salt (EDTA 2NH4) | Dojindo Laboratories | 346-01971 |
| APCI Positive Calibration Solution | SCIEX | 4460131 |
| APCI Negative Calibration Solution | SCIEX | 4460134 |
| Chloroform | MilliporeSigma | 05-3450-7-1L-J |
| EquiSPLASH LIPIDOMIX Quantitative Mass Spec Internal Standard | Avanti Polar Lipids | 330731-1EA |
| Acetonitrile for LCMS | FUJIFILM Wako Pure Chemical | 018-19853 |
| Methanol for LCMS | FUJIFILM Wako Pure Chemical | 134-14523 |
| Milli-Q water (18 MΩ) | Merck | n/a |
| Acetic acid LC-MS CHROMASOLV | FLUKA | 49199-50ML-F |
| Prostaglandin E2-d4 | Cayman | 314010 |
| Leukotriene B4-d4 | Cayman | 320110 |
| Leukotriene D4-d5 | Cayman | 10006199 |
| 15-Hydroxyeicosatetraenoic acid-d8 | Cayman | 334720 |
| Arachidonic acid-d8 | Cayman | 390010 |
| 14, 15-Epoxy-5, 8, 11-eicosatrienoic acid-d11 | Cayman | 10006410 |
| Prostaglandin B2-d4 | Cayman | 311210 |
| 8-iso Prostaglandin F2α-d4 | Cayman | 316350 |
| Software and Algorithms | ||
| Analyst | SCIEX | Ver. 1.8.1 |
| Binary Solvent Manager | Waters | Ver. 1.72 |
| MassLynx | Waters | Ver. 4.2 |
| MultiQuant | SCIEX | Ver. 3.0.3 |
| MS-DIAL 4 | Tsugawa et al., (2020) | http://prime.psc.riken.jp/compms/index.html |
| Analysis Base File Converter | Reifycs | https://www.reifycs.com/AbfConverter/ |
| Cytoscape | Cytoscape Consortium | https://cytoscape.org/ |
| Other | ||
| ACQUITY UPLC system | Waters | I class |
| Acquity UPLC Peptide BEH C18 column (50 × 2.1 mm; 1.7 μm) | Waters | 186003554 |
| TripleTOF 6600 mass spectrometer | SCIEX | n/a |
| Multiposition microelectric valve actuators | VICI Valco Instruments | EPC10W |
| Triple Quad 5500 QTrap mass spectrometer | SCIEX | n/a |
| Acquity UPLC BEH C18 column (150 × 1.0 mm; 1.7 μm) | Waters | 186002347 |
| Multi-beads shocker MB1200 | Yasui Kikai | n/a |
| 3 mL Metal corn beads | Yasui Kikai | MC-0316(S) |
| 3 mL Reinforced homogenization tubes | Yasui Kikai | ST-0320PCF |
| 200 μL Large orifice pipette tips | Scientific Specialties | 4297-S0 |
| 2.0 mL Glass jacket tubes | FCR&Bio | JRD-1GS200 |
| 2.0 mL Glass jacket tube caps | FCR&Bio | GC2-1S(HI) |
| MonoSpin C18-AX | GL Sciences | 5010-21736 |
| Glass tips for 0.2 mL | SIBATA SCIENTIFIC TECHNOLOGY | 080130-10021A |
| Dispensing Burette Glass DIGIFIT 0.2 mL | SIBATA SCIENTIFIC TECHNOLOGY | 080130-1002 |
| Disposable Pasteur Pipettes 5 inch | IWAKI | IK-PAS-5P |
| Acura manual 835 Macropipette with Pasteur pipette adapter 0.2 – 2 mL | Socorex | 835.02PP |
| 2 mL Screw Agilent Hplc Vials | Agilent | 5182-0716 |
| 250 μL Glass insert, deactivated | Agilent | 5181-8872 |
| Blue screw cap, pre-slit PTFE/sil septa | Agilent | 5185-5865 |
Materials and equipment
Antibiotic cocktail
| Reagent | Final concentration | Amount |
|---|---|---|
| Ampicillin | 1 g/L | 500 mg |
| Vancomycin | 0.5 g/L | 250 mg |
| Neomycin | 1 g/L | 500 mg |
| Metronidazole | 1 g/L | 500 mg |
| Milli-Q water | n/a | 500 mL |
| Total | n/a | To 500 mL |
Note: Scale-up using a 500-mL volumetric cylinder. Metronidazole is difficult to dissolve in deionized water and should be stirred using magnetic stir plate with magnetic stirrer for about 10 min. The antibiotic cocktail is stable for two weeks at room temperature. In the case of single antibiotic administration, one type of antibiotic should be dissolved in Milli-Q water.
Diluted EquiSPLASH (internal standards for untargeted analysis)
| Reagent | Final concentration | Amount |
|---|---|---|
| EquiSPLASH | n/a | 1 mL |
| Methanol for QTofMS | n/a | 29 mL |
Note: Store at 4°C in a glass vial pre-washed with methanol and chloroform until use.
CRITICAL: Methanol is highly flammable, a suspected fetal toxin, an eye irratant, and is considered an acutely and chronically toxic solvent. A laboratory coat, goggles, and gloves should be worn when working with this solvent. A chemical fume hood should be used when working with large volumes of this solvent.
CRITICAL: Headspace gas must be replaced by nitrogen after each usage.
Solvent A for untargeted analysis
| Reagent | Final concentration | Amount |
|---|---|---|
| Acetonitrile for QTofMS | n/a | 100 mL |
| Methanol for QTofMS | n/a | 100 mL |
| Milli-Q water | n/a | 300 mL |
| 1 M ammonium acetate solution | 5 mM | 2.5 mL |
| 50 μM EDTA 2NH4 solution | 10 nM | 100 μL |
Note: Gently mix the solvent in a circular motion. Degas the solvents by placing the bottles in an ultrasonic bath for 5 min. To prevent the retention time shift, the same set of samples should be analyzed within 2–3 days while mobile phase solvents are fresh.
CRITICAL: Acetonitrile is highly flammable, and is considered acutely toxic solvent on skin exposure. A laboratory coat, goggles, and gloves should be worn when working with this solvent. A chemical fume hood should be used when working with large volumes of this solvent.
CRITICAL: Ammonium acetate may be harmful if absorbed through the skin or injested, and is considered a skin, eye, and respiratory irratant. A laboratory coat, goggles, and gloves should be worn when working with this material.
CRITICAL: Do not use detergents and laboratory dishwasher to wash the solvent bottles. QTOF/MS grade acetonitrile and methanol should be used.
Solvent B for untargeted analysis
| Reagent | Final concentration | Amount |
|---|---|---|
| 2-Propanol for QTofMS | n/a | 400 mL |
| 1 M ammonium acetate solution | 5 mM | 2 mL |
| 50 μM EDTA 2NH4 solution | 10 nM | 80 μL |
Note: Gently mix the solvent in a circular motion. Degas the solvents by placing the bottles in an ultrasonic bath for 5 min. To prevent the retention time shift, the same set of samples should be analyzed within 2–3 days while mobile phase solvents are fresh.
CRITICAL: 2-propanol is highly flammable, is an eye and respiratory tract irratant, and is considered a chronically toxic solvent. A laboratory coat, goggles, and gloves should be worn when working with this solvent. A chemical fume hood should be used when working with large volumes of this solvent.
CRITICAL: Do not use detergents and laboratory dishwasher to wash the solvent bottles. QTOF/MS grade 2-propanol should be used.
Internal standard for targeted analysis
| Reagent | Final concentration | Amount |
|---|---|---|
| 10 mg/L Arachidonic acid-d8 methanol solution | 320 μM (100 μg/L) | 10 μL |
| 10 mg/L Prostaglandin E2-d4 methanol solution | 281 μM (100 μg/L) | 10 μL |
| 10 mg/L Leukotriene B4-d5 methanol solution | 294 μM (100 μg/L) | 10 μL |
| 10 mg/L Leukotriene D4-d5 methanol solution | 199 μM (100 μg/L) | 10 μL |
| 10 mg/L 15-hydroxyeicosatetraenoic acid-d8 methanol solution | 304 μM (100 μg/L) | 10 μL |
| 10 mg/L 14, 15-epoxy-5, 8, 11-eicosatrienoic acid-d11 methanol solution | 302 μM (100 μg/L) | 10 μL |
| Methanol for LCMS | n/a | 940 μL |
| Total | n/a | To 1 mL |
Note: Use a 1 mL-volmetric flusk for mixing.
External standard for targeted analysis
| Reagent | Final concentration | Amount |
|---|---|---|
| 10 mg/L Prostaglandin B2-d4 methanol solution | 295 μM (100 μg/L) | 10 μL |
| 10 mg/L 8-iso Prostaglandin F2α-d4 methanol solution | 279 μM (100 μg/L) | 10 μL |
| Methanol for LCMS | n/a | 980 μL |
| Total | n/a | To 1 mL |
Note: Use a 1 mL-volmetric flusk for mixing.
Solvent A for targeted analysis
| Reagent | Final concentration | Amount |
|---|---|---|
| Milli-Q water | n/a | 500 mL |
| Acetic acid LC-MS CHROMASOLV | 0.1% (v/v) | 500 μL |
Note: Gently mix the solvent in a circular motion. Degas the solvents by placing the bottles in an ultrasonic bath for 5 min.
CRITICAL: Concentrated acetic acid solutions are considered acutely toxic, highly flammable, and very corrosive to the eyes, skin, and respiratory tract. A laboratory coat, goggles, and gloves should be worn when working with these solutions. A chemical fume hood should be used when working with large volumes of this solvent.
CRITICAL: Do not use detergents and laboratory dishwasher to wash the solvent bottles.
Solvent B for targeted analysis
| Reagent | Final concentration | Amount |
|---|---|---|
| Acetonitrile for LCMS | n/a | 400 mL |
| Methanol for LCMS | n/a | 100 mL |
Note: Gently mix the solvent in a circular motion. Degas the solvents by placing the bottles in an ultrasonic bath for 5 min.
CRITICAL: Do not use detergents and laboratory dishwasher to wash the solvent bottles.
LC-QTOF/MS setup for untargeted analysis
-
•
Untargeted analysis is performed using an ACQUITY UPLC system coupled with a QTOF/MS (TripleTOF 6600 mass spectrometer). The MS parameters are listed in Table 1.
-
•
Lipid metabolites are separated on an Acquity UPLC Peptide BEH C18 column (50 × 2.1 mm; 1.7 μm) maintained at 45°C.
-
•
The mobile phases consist of (A) 1:1:3 (v/v/v) acetonitrile:methanol:Milli-Q water with ammonium acetate (5 mM) and 10 nM EDTA, and (B) 100% 2-propanol with ammonium acetate (5 mM) and 10 nM EDTA are used for gradient elution (Table 2).
-
•
Samples are analyzed in independent runs in positive and negative modes using the parameters shown in Table 1.
Note: overall cycle time per sample was 25 min.
Table 1.
Q-TOF/MS parameter settings
| Parameters | Values |
|---|---|
| Ionization | Electrospray ionization |
| MS1 and MS2 mass ranges | m/z 70 – m/z 1250 |
| MS1 accumulation time | 250 ms |
| MS2 accumulation time | 100 ms |
| Cycle time | 1300 ms |
| Collision gas | Nitrogen |
| Collision energy (positive mode / negative mode) | +40/−42 eV |
| Collision energy spread | 15 eV |
| Ion source gas 1 (air; positive mode / negative mode) | 40/50 psi |
| Ion source gas 2 (air; positive mode / negative mode) | 80/50 psi |
| Curtain gas (nitrogen) | 30 psi |
| Ion source temperature (positive mode / negative mode) | 250°C/300°C |
| Ion spray voltage floating (positive mode / negative mode) | 5500/−4500 V |
| Declustering potential (positive mode / negative mode) | 80/−80 eV |
Table 2.
LC gradient condition for untargeted analysis
| Time (min) | Gradient (% B) | Flow rate (mL/min) |
|---|---|---|
| 0.0 | 0 | 0.3 |
| 1.0 | 0 | 0.3 |
| 5.0 | 40 | 0.3 |
| 7.5 | 64 | 0.3 |
| 12.0 | 64 | 0.3 |
| 12.5 | 82.5 | 0.3 |
| 19.0 | 85 | 0.3 |
| 20.0 | 95 | 0.3 |
| 20.1 | 0 | 0.3 |
| 25.0 | 0 | 0.3 |
LC-tripleQ/MS setup for targeted analysis
-
•
Targeted analysis is performed using an ACQUITY UPLC coupled with a triple quadrupole mass spectrometry (tripleQ/MS; Qtrap 5500). The MS parameters are listed in Table 3.
-
•
Lipid metabolites are separated on an Acquity UPLC BEH C18 column (150 mm × 1.0 mm × 1.7 μm) maintained at 35°C.
-
•
The mobile phases consist of (A) 100:0.1 (v/v) water:acetic acid and (B) 4:1 (v/v) acetonitrile:methanol are used for gradient elution (Table 4).
Note: overall cycle time per sample was 47 min.
Table 3.
TripleQ/MS parameter settings
| Parameters | Values |
|---|---|
| Ionization | Electrospray ionization |
| Collision gas (nitrogen) | Medium |
| Curtain gas (nitrogen) | 30 psi |
| Ion source gas 1 (air) | 15 psi |
| Ion source gas 2 (air) | 50 psi |
| Ion source temperature | 450°C |
| Ion spray voltage floating | −4500 V |
| MRM channels | Described in Data S1 |
Table 4.
LC gradient condition for targeted analysis
| Time (min) | Gradient (% B) | Flow rate (mL/min) |
|---|---|---|
| 0 | 27 | 0.05 |
| 5 | 27 | 0.05 |
| 15 | 70 | 0.05 |
| 25 | 80 | 0.05 |
| 30 | 80 | 0.08 |
| 33 | 80 | 0.10 |
| 35 | 95 | 0.10 |
| 39 | 100 | 0.10 |
| 40 | 27 | 0.05 |
| 47 | 27 | 0.05 |
Step-by-step method details
An overview of the procedures is summarized in Figure 1. The collected frozen fecal samples are homogenized by metal corn beating (steps 1–8). The total lipids are extracted by single phase extraction (steps 9–17) for untargeted lipidomics using LC-QTOF/MS (steps 18–23) (see the left track of Figure 1). The obtained data are analyzed using the MS-DIAL 4 software program (steps 24–25). The FBMN technique clustering similar MS2 spectra is employed to facilitate the identification of bacterial lipid metabolites (steps 26–27). The oxylipins contained in the fecal sample homogenate are enriched by solid phase extraction (steps 28–41) and analyzed by targeted lipidomics using LC-TripleQ/MS (steps 42–48) (see the right track of Figure 1).
Figure 1.
An overview of the procedures
Homogenization of feces samples
Timing: 1–2 h
Feces contain various components, including bacteria, fiber, host cells, and mucus. To extract whole metabolites, it is important to homogenize feces into a complete powder form (Figure 2).
-
1.
Weigh the frozen fecal sample (10–100 mg) and place in a 3-mL reinforced homogenization tube.
-
2.
Place a metal corn in the tube.
-
3.
Close the tube and place it in liquid nitrogen (LN2) for 10 min.
CRITICAL: To get the sample cold enough, the tube should be placed in LN2 until the temperature of the homogenization tube is in thermal equilibrium with LN2, i.e., there are no more bubbles produced from gaseous nitrogen boil-off.
CRITICAL: LN2 is a cryogenic fluid (boiling point temperature of −196°C) that can act as an oxygen displacer in confined spaces causing asphyxiation, and can cause severe tissue burns even in instances of brief exposure. LN2 should only be handled by experienced and trained personnel, using appropriate personal protective equipment, and in well ventilated areas according to appropriate institutional use policies, Guidance from Environmental Health and Safety personnel prior to using LN2 is highly recommended.
-
4.
Install precooled tube-holders into multi-beads shocker.
-
5.
Insert the sample tubes prepared in step 3 to the holders.
-
6.
Homogenize the fecal samples (2,500 rpm, 15 s ×2, pause time, 5s)
-
7.
Add ice-cold methanol (50 μL/10 mg feces) and vortex the samples.
-
8.
Remove the metal cone using a magnet.
Pause point: Feces homogenate can be stored at ‒80°C for one or two weeks.
Figure 2.
A workflow of fecal sample homogenization
Single phase extraction for untargeted lipidomics
Timing: 2–3 h
Single-phase extraction is used for untargeted lipidomics to extract whole lipids (Figure 3).
-
9.
Transfer a 50-μL volume of fecal homogenate into a jacket tube.
Note: Fecal homogenate contains fiberous debris. Use large orifice pipette tips to avoid clogging.
CRITICAL: Chloroform is considered acutely and chronically toxic, and is considered to be a corrosive substance, a reproductive toxin, a carcinogenic substance, and a skin, eye, and respiratory irratant. A laboratory coat, goggles, appropriate gloves, and a chemical fume hood should be used when working with chloroform.
-
10.
Add a 150-μL volume of diluted EquiSPLASH (see. materials and equipment).
-
11.
Add a 100-μL volume of chloroform using a glass tip-installed dispensing burette.
-
12.
Vortex and incubate for 2 h at room temperature.
-
13.
Add a 20-μL volume of Milli-Q water.
-
14.
Vortex-mix the sample, and incubate for 10 min at room temperature.
-
15.
Centrifuge at 2000 × g for 10 min at room temperature using a swinging rotor centrifuge.
-
16.
Transfer approximately a 200-μL volume of the supernatant into a glass insert assembled in a glass vial, and then close the cap tightly.
-
17.
Prepare a pooled quality control (QC) sample by mixing all the samples (10–20 μL for each depending on the sample numbers).
CRITICAL: Glassware should be used for tips and tubes in the presence of chloroform.
Figure 3.
A workflow of single phase extraction
Untargeted analysis using LC-QTOF/MS
Timing: 25 min/sample
The lipid containing fecal sample extracts were analyzed by LC-QTOF/MS. The data-dependent acquisition mode is employed for quantitation and identification of unknown lipids.
-
18.
Prepare solvents as described in the “materials and equipment” section.
-
19.
Install the HPLC solvent lines into the HPLC solvent reservoirs.
-
20.
Purge the solvent lines for 5 min.
-
21.
Equilibrate the LC-QTOF/MS system as shown in Table 1 and 2.
Note: See troubleshooting 1 if the LC shows an error message of excess pressure.
-
22.
Create a batch table. Insert pooled QC samples once every ten times.
-
23.
Analyze samples in both positive and negative ion modes.
Note: Injection volumes can be changed from 1 to 3 μL, depending on the concentration of the samples. See troubleshooting 2 and 3 if the obtained data are not of good quality.
CRITICAL: To prevent retention time shifting, the same set of samples should be analyzed within 2–3 days while mobile phase solvents are fresh.
Data analysis using MS-DIAL
Timing: 2–3 h
Untargeted lipidomic data are processed by MS-DIAL (Tsugawa et al., 2020). The procedures are detailed in online tutorial of MS-DIAL environment (https://mtbinfo-team.github.io/mtbinfo.github.io/). The MS-DIAL forum is also available to ask questions, feedback, and comments to the developers (http://www.metabolomics-forum.com/index.php). The procedure is explained by the latest version of software programs (December 30th, 2020). The timing for data conversion and processing depends on the number and size of data files, as well as the PC performance. The original file size of a typical LC-MS data file (.wiff and .wiffscan) is approximately 160 MB. Fifty-two sets of LC-MS data obtained in Yasuda et al. (2020), including the alignment procedure, were processed in 40 min using a Windows 10 pro 64 bit-based machine with Intel (R) Xeon (R) Silver 4116 CPU @ 2.10 GHz (2 processors) and 192 GB random access memory.
-
24.Convert data format for MS-DIAL by ABF converter (freely available).
-
a.Open AnalysisBaseFileConverter.exe.
-
b.Drag and drop the wiff file format data into the ABF converter.
-
c.Click the “Convert” button. The abf format data are generated in the same directory.
-
a.
-
25.Analyze data in MS-DIAL.
-
a.Open MSDIAL.exe.
-
b.Click “File” -> “New project” -> “Start up a project”. A new window is shown.
-
c.Select the directory containing abf files as “Project file path”. The following parameters should be selected: soft ionization, chromatography, conventional LC/MS or data-dependent MS/MS, profile data for MS1 and MS2, negative ion mode (for this paper), and lipidomics project. Then, click “Next”.
-
d.Click “browse”. Choose abf filles. Set the file type of procedure blank as “Blank” Add class ID values appropriately for grouping the samples.Note: Data table can be copied using the Ctrl + C shortcut and pasted into Microsoft Excel. After the metadata is organized in Excel, it can be pasted into the data table using the Ctrl + V shortcut.
-
e.Set parameters in the analysis setting window as shown in Table 5. Default values should be used for the other parameters.Note: Unless an identical LC condition is used, set the retention time tolerance to 100 min and untick “Use retention time for scoring and filtering options” in the identification tab.
-
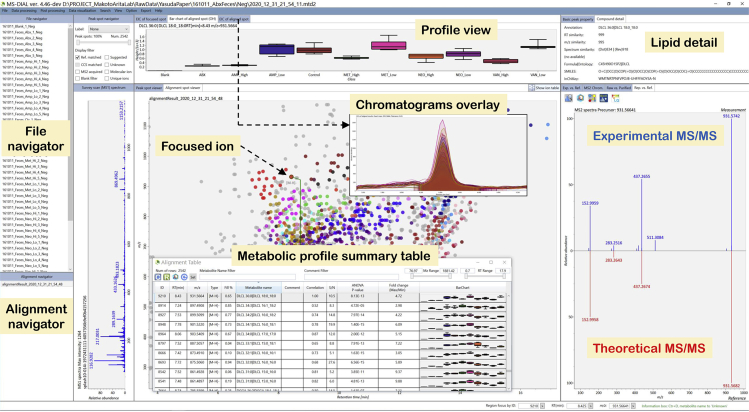
f.Open the alignment result by double-clicking in the alignment navigator (Figure 4).
-
g.See metabolic profile summary table (Figure 4) by clicking the “show ion table” button.
-
h.Check retention time shifts and intensity drifts of internal standards in samples and QC.Note: The peak features exceeding 20% coefficient of variation (CV) values in QC samples are excluded according to the previous report (Dunn et al., 2011).
-
i.Curate the MS-DIAL results on the graphical user interface to reduce false positive annotations.
-
a.
Note: MS-DIAL can automatically annotate the metabolite peaks by the similarity calculation of retention time, precursor m/z, isotopic ratios, and MS/MS spectra with the reference databases. In lipidomics, the rule-based annotation system is also executed to describe lipid structures based on the quality of the MS/MS spectrum. Unfortunately, there are also false positive assignments in the resulting peak annotations, as well as true positives. Therefore, we recommend that the original result is manually checked and some of the identified peaks are curated and modified. For example, identification of molecules such as fatty acids, for which diagnostic ions are not sufficiently obtained, requires to check whether the peak spots are regularly aligned depending on the carbon numbers and unsaturation degree (Figure 5), in adtdition to retention time comparison with the standard compounds. For details on the MS-DIAL graphical user interface, see the online tutorial (https://mtbinfo-team.github.io/mtbinfo.github.io/).
Table 5.
Parameter settings for MS-DIAL
| Section | Parameter | Value |
|---|---|---|
| Data collection | RT begin | 0.5 min |
| RT end | 18.0 min | |
| Mass range begin (MS1&2) | 0 Da | |
| Mass range end (MS1&2) | 2,000 Da | |
| MS1 mass tolerance | 0.01 Da | |
| MS2 mass tolerance | 0.025 Da | |
| Number of threads | 2 | |
| Peak detection | Minimum peak height | 500 amplitude |
| Mass slice width | 0.1 Da | |
| Smoothing level | 3 scans | |
| Minimum peak width | 5 scans | |
| Identification | Retention time tolerance | 2 min |
| MS1 mass tolerance | 0.01 Da | |
| MS2 mass tolerance | 0.05 Da | |
| Identification score cutoff | 80% | |
| Retention time for scoring | True | |
| Retention time for filtering | True | |
| Targeted lipid subclasses | Check all | |
| Alignment | Retention time tolerance | 0.05 min |
| MS1 mass tolerance | 0.015 Da | |
| Remove features based on blank information | TRUE |
Figure 4.
A MS-DIAL analysis screen
Figure 5.
A curation example of fatty acid annotations
The molecules of free fatty acids often provide no informative MS/MS spectrum for the structure characterization. Therefore, the confirmation of retention time is essential. Since the elution of free fatty acids in C18 column highly depends on the carbon- and desaturation properties, the mis-annotation can be identified by checking the elution behaviors. This figure shows one mis-annotation of FA 20:0 (marked as False 20:0) having the unexpected retention time value when considered to the other FA peaks' behaviors.
Molecular spectrum networking
Timing: 10 min
Molecular spectrum networking is applied to the untargeted analysis to group unidentified lipids based on the similarity of their MS/MS spectra. The Cytoscape program is used for network visualization. Java Runtime Environment (JRE) or AdoptOpenJDK should be installed before installing Cytoscape. The node and edge files are generated in the MS-DIAL environment.
-
26.Perform molecular spectrum networking
-
a.Click “Export” -> “Molecular spectrum networking” in MS-DIAL environment.
-
b.After the parameter setting window of molecular spectrum networking is shown, select an appropriate directory for the result export. Click “export” then the node and edge files are generated.
-
c.Add the appropriate metadata (compound names, ontologies, and abundance fold changes) to the node file. Example files of the node and edge are available in the Data S2 and S3. The metadata is created using the usual MS-DIAL alignment output file, which can be generated by clicking “Export” -> “Alignment result export”.
-
a.
-
27.Visualize the result in the Cytoscape program.
-
a.Click “File” -> “Import” -> “Network from file...” -> choose the edge file generated by MS-DIAL in step 26-b.Note: Check the automatic determination of Cytoscape for the source and target nodes. In our experimental condition, the columns of “source (ID)” and “target (ID)” are set to the source node and target node, respectively.
-
b.Click “File” -> “Import” -> “Table from file...” -> choose the node file generated by MS-DIAL in step 26-c.Note: The “Key” column should be set appropriately. In our condition, the “title” column is automatically set as the “Key” column by Cytoscape, but here, set “ID” column as the “Key” for mapping the node information into the network.
-
c.Create an appropriate style to visualize the molecular networking result on the Cytoscape (Figure 6). See the online tutorial (https://cytoscape.org/) of Cytoscape for further details.
-
a.
Figure 6.
A Cytoscape screen of molecular spectrum networking
Solid phase extraction for targeted lipidomics
Timing: 2 h
Oxylipins are enriched by a solid-phase extraction step independent from the single phase extraction for untargeted analysis, since their signals are easily masked due to their low abundance in comparison to other groups of lipids.
-
28.
Mix a 5-μL volume of fecal homogenate obtained in step 8 (equivalent to 1.0 mg feces) and a 150-μL volume of Milli-Q water in a microtube.
-
29.
Add a 10-μL volume of internal standard mix (see materials and equipment).
-
30.
Centrifuge the tube at 17000 × g for 5 min at 4°C.
-
31.
Set a C18-AX spin column on a flow through tube.
-
32.Conditioning step:
-
a.Add a 300-μL volume of methanol to the extraction tube.
-
b.Centrifuge the tube at 3500 × g for 30 s at room temperature.
-
a.
-
33.Equilibration step:
-
a.Add a 300-μL volume of Milli-Q water to the extraction tube.
-
b.Centrifuge the tube at 3500 × g for 30 s at room temperature.
-
a.
-
34.Sample loading step:
-
a.Apply the supernatant obtained in step 30.
-
b.Centrifuge the tube at 3500 × g for 1 min at room temperature.
-
a.
-
35.Primary wash step:
-
a.Apply a 300-μL volume of Milli-Q water to the extraction tube.
-
b.Centrifuge the tube at 3500 × g for 1 min at room temperature.
-
c.Discard the flowthrough.
-
a.
-
36.Seconday wash step:
-
a.Apply a 300-μL of 50:50 (v/v) methanol and Milli-Q water solution to the extraction tube.
-
b.Centrifuge the tube at 3500 × g for 2 min at room temperature.
-
c.Discard the flowthrough.
-
d.Change the flow through tube to a new collection tube.
-
a.
-
37.Elution step:
-
a.Apply a 100-μL volume of 90:2:8 (v/v/v) methanol, acetic acid, and Milli-Q water solution to the extraction tube.
-
b.Centrifuge the tube at 3500 × g for 2 min at room temperature.
-
c.Check the tube to confirm that all of the elution solution has passed through the extraction cartridge. If not, centrifuge again.
-
d.Remove the spin column.
-
a.
-
38.
Transfer the supernatant into the glass insert assembled in a glass autosampler vial.
-
39.
Add a 10-μL volume of the external standards mix (see materials and equipment).
-
40.
Close the cap.
-
41.
Vortex briefly.
Targeted analysis using LC-tripleQ/MS
Timing: [50 min/samples]
The extracted oxylipins are analyzed by LC-tripleQ/MS. The separation of oxylipin isomers with slight structural differences (position of hydroxy groups and double bonds) requires relatively slower gradient elution using a longer column than that of untargeted analysis.
-
42.
Prepare the solvents as described in the “materials and equipment” section.
-
43.
Set the solvents.
-
44.
Purge the solvent lines for 5 min.
-
45.
Equilibrate the LC-tripleQ/MS system following Table 3 and 4.
Note: See troubleshooting 1 if the LC shows an error message of excess pressure.
-
46.
Analyze the samples. Sample injection volume is 1 μL at a maximum.
Note: See troubleshooting 2 and 3 if the obtained data are not of good quality.
-
47.
Open the data in MultiQuant software.
-
48.
Integrate the peaks.
Note: To avoid miss annotation, peak picking should be performed by comparing the retention time of each peak with their corresponding authentic standards. We usually prepare a standard mixture containing approximately 300 compounds for each analysis. The experimental deviation of retention time is within 0.05–0.1 min.
-
49.
Check retention time shifts and intensity drifts of internal and external standards.
Note: The peak features exceeding 20% CV values in QC samples are excluded according to the previous report (Dunn et al., 2011).
Expected outcomes
Targeted analysis detected 139 oxylipins, 33 of which were reduced more than 10-fold in the antibiotic cocktail (Abx) group (Figure S1). For example, the levels of microbiome-derived fatty acid metabolites, namely 10 hydroxy-cis-12-octadecenoid acid (HYA) and 10 oxo-cis-12-octadecenoid acid (KetoA) (Kishino et al., 2013), were significantly reduced in in the feces of mice treated with Abx (Figure 7A). These results demonstrated that a significant portion of oxylipins in the feces were dramatically affected by Abx treatment.
Figure 7.
Expected outcomes
(A) The levels of microbiome-derived oxylipins were decreased in Abx-treated mice feces. The results are the mean ± SEM (n=5).
(B) Molecular spectrum networking results. Nodes corresponding to molecular species are linked based on the similarity of MS/MS spectra (similarity cut-off [%] = 85 in step 26-b). The nodes of circle and up- and down-arrows represent lipid ions with less than 10-fold changes and over 10-fold increases and decreases, respectively, in the Abx-treatment group compared to the control group. The node size and thickness of the links denote the magnitude of measured ion intensity and Bonanza score, respectively. Nomenclatures of identified lipids are listed in http://prime.psc.riken.jp/compms/msdial/lipidnomenclature.html. Both figures were generated from Yasuda et al., (2020).
Usually, when 10 mg of feces is analyzed by untargeted lipidomics, approximately 6,000 ion features are obtained after excluding procedure blank features. Among those, 10%–20% of ion features are annotated by MS-DIAL. By applying molecular spectrum networking, these ion features are clustered into each lipid subclass (Figure 7B). Among these, bacteria-dependent lipid clusters are visualized by dramatic reduction (>10-fold) by Abx treatment (shown as a triangle in Figure 7B).
Limitations
The precise determination of acyl chain structures (straight, iso, or anteiso), unsaturation properties (unsaturated bond or cyclopropane), and sugar isomers (glucose or galactose) are not achieved in our untargeted analysis system. The complete chemical assignment of lipid structures should be confirmed using standards prepared from authentic reference materials.
This method cannot rule out the possibility of including host-derived lipid metabolites that are induced in the presence of commensal bacteria. These should be referred to as bacteria-dependent lipid metabolites.
Troubleshooting
Problem 1
Excess pressure occurs in LC (steps 21 and 45).
Potential solution
The LC lines may be clogged. After each batch analysis, the lines and column should be washed by flowing 2-propanol for 1–2 h.
Problem 2
Peak shape worsens and/or retention time shifts (steps 23 and 46).
Potential solution
The column is considered to have deteriorated. Analyze the system check standards before and after each analysis to check the condition of the column. We use deuterated mix of representative lipid classes.
Problem 3
Sensitivity decreases and/or precision mass shifts (steps 23 and 46).
Potential solution
The ion source may have been contaminated. The pores of the MS inlet should be wiped carefully using clean paper soaked in 50% (v/v) methanol and Milli-Q water solution and wrung out tightly. The electrode needs to be flushed with methanol solution. Soak the electrode in methanol solution in a glass beaker and place in an ultrasonic bath for 10 min. If the problem persists, stop the vacuum pump and clean Q0 according to the instruction manual of the instrument. After starting the vacuum pump and waiting until the MS reaches operational pressure, use the calibration solution to calibrate the instrument and to assess the mass error and sensitivity of specific calibrant ions across the mass range of the instrument.
Problem 4
A single cluster with too many nodes or many clusters with a few nodes appear by molecular spectrum networking (step 27-C).
Potential solution
Optimize the similarity cut-off [%] in step 26 so that known lipid subclasses (e.g., phosphatidylethanolamine, and ceramide) are clustered in a single group.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Makoto Arita (makoto.arita@riken.jp).
Materials availability
This study did not generate new unique materials.
Data and code availability
MS data are available at the DropMet section of RIKEN PRIMe (http://prime.psc.riken.jp/) via the index of DM0032.
Acknowledgments
This work was supported by the AMED LEAP (JP18gm0010003 to M.A.) and JSPS Grant-in-Aid for Scientific Research on Innovative Areas ‘‘LipoQuality’’ (15H05897 and 15H05898 to M.A.) and JSPS KAKENHI (18H02432 and 18K19155 to H.T.). We thank our lab members, especially Kazutaka Ikeda for the development of untargeted lipidomics system, and Mie Honda, Aya Hori, Kanako Igarashi, and Mimi Mitsuji for their skillful technical support.
Author contributions
N.O. organized the protocol design. M.U. wrote the sample preparation protocol. S.Y. wrote the mouse experiment protocol. N.O., M.U., and S.Y. wrote the LC-MS/MS method protocols for targeted and untargeted lipidomics. H.T. wrote the data processing and molecular networking protocols. M.A. initiated and designed this work, and all authors wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2021.100492.
Supplemental information
The changes in fecal oxylipin levels in the antibiotic-treated group relative to the mean of the control group were described. The green-colored oxylipin is a known metabolites of bacteria (Kishino et al., 2013). The blue bars are oxylipins that were reduced more than 10-fold in the antibiotic group.
References
- Dunn W.B., Broadhurst D., Begley P., Zelena E., Francis-mcintyre S., Anderson N., Brown M., Knowles J.D., Halsall A., Haselden J.N. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011;6:1060–1083. doi: 10.1038/nprot.2011.335. [DOI] [PubMed] [Google Scholar]
- Jonasdottir H.S., Brouwers H., Toes R.E.M., Ioan-Facsinay A., Giera M. Effects of anticoagulants and storage conditions on clinical oxylipid levels in human plasma. Biochim. Biophys. Acta. 2018;1863:1511–1522. doi: 10.1016/j.bbalip.2018.10.003. [DOI] [PubMed] [Google Scholar]
- Kishino S., Takeuchi M., Park S.B., Hirata A., Kitamura N., Kunisawa J., Kiyono H., Iwamoto R., Isobe Y., Arita M. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc. Natl. Acad. Sci. U S A. 2013;110:17808–17813. doi: 10.1073/pnas.1312937110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugawa H., Ikeda K., Takahashi M., Satoh A., Mori Y., Uchino H., Okahashi N., Yamada Y., Tada I., Bonini P. A lipidome atlas in MS-DIAL 4. Nat. Biotechnol. 2020;38:1159–1163. doi: 10.1038/s41587-020-0531-2. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Okahashi N., Tsugawa H., Ogata Y., Ikeda K., Suda W., Arai H., Hattori M., Arita M. Elucidation of gut microbiota-associated lipids using LC-MS/MS and 16S rRNA sequence analyses. iScience. 2020;23:101841. doi: 10.1016/j.isci.2020.101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The changes in fecal oxylipin levels in the antibiotic-treated group relative to the mean of the control group were described. The green-colored oxylipin is a known metabolites of bacteria (Kishino et al., 2013). The blue bars are oxylipins that were reduced more than 10-fold in the antibiotic group.
Data Availability Statement
MS data are available at the DropMet section of RIKEN PRIMe (http://prime.psc.riken.jp/) via the index of DM0032.