Abstract
Health risks are described from elevated indoor air carbon dioxide (CO2), which often ranges from 1,000 to 4,000 ppm, but the mechanisms are unknown. Here, we demonstrate that mice exposed for 2 h to 2,000 or 4,000 ppm CO2 exhibit, respectively, 3.4 ± 0.9-fold (SE, n = 6) and 4.1 ± 0.7-fold (n = 10) elevations in circulating microparticles (MPs); neutrophil and platelet activation, and vascular leak in brain, muscle, and distal colon. Interleukin (IL)-1β content of MPs also increases after 2,000 ppm by 3.8 ± 0.6-fold (n = 6) and after 4,000 ppm CO2 by 9.3 ± 1.1-fold (n = 10) greater than control. CO2–induced vascular damage is abrogated by treating mice with an antibody to IL-1β or an IL-1β receptor inhibitor. Injecting naïve mice with CO2-induced MPs expressing a protein found on mature neutrophils recapitulates vascular damage as seen with elevated CO2, and destruction of MPs in CO2-exposed mice abrogates vascular injuries without altering neutrophil or platelet activation. We conclude that environmentally relevant elevations of CO2 trigger neutrophils to generate MPs containing high concentrations of IL-1β that cause diffuse inflammatory vascular injury.
NEW & NOTEWORTHY Elevated levels of CO2 are often found in indoor air and cause adverse health effects, but the mechanisms have not been identified. In a murine model, environmentally relevant levels of CO2 were found to cause diffuse vascular damage because neutrophils are stimulated to produce microparticles that contain high concentrations of interleukin-1β.
Keywords: indoor air quality, microparticles, interleukin-1β, neutrophil activation, vasculopathy
health risks from elevations of indoor air carbon dioxide (CO2) are poorly understood. The general population spends 80–90% of their time indoors (12). The CO2 concentration may be as low as that found outdoors (~380 ppm, 0.038%), but due to occupant density and building ventilation rates it is often 5- to 10-fold higher and sometimes reaches over 4,500 ppm (23). Studies performed on four continents have found many buildings have mean levels of ~2,800 ppm (5, 16, 23, 27, 42).
Breathing CO2 at ~1,000 ppm for 8 h, or higher concentrations for 2–3 h, causes cognitive dysfunction (2, 17, 33) and may cause subtle cardiovascular effects (17). No mechanisms are identified to explain these problems. Before these studies, CO2 was considered as a reference parameter for indoor air quality with ill effects arising from a variety of pollutants but not CO2 itself. CO2, more so than other air contaminants, is now considered to most closely correlate with symptoms and thus pose the major factor for a wide range of gastrointestinal, respiratory, and cognitive disorders simply described as “sick building syndrome” (23, 41). In 2016, concern was raised over the air quality in schools worldwide (32). As an example, a time-averaged CO2 concentration of over 1,000 ppm was reported for 88% of 120 classrooms in Texas and peak levels exceeded 3,000 ppm in 21% (6). Elevations in excess of 1,000 ppm over outdoor values were found in 45% of 434 classrooms in Washington and Idaho, where elevations correlated with student absences (34).
We hypothesized that elevations of CO2 cause adverse effects in multiple organs due to circulating proinflammatory microparticles (MPs). MPs are 0.1 to 1 μm in diameter membrane vesicles shed from all vascular cells in response to various stimuli. MPs serve as intercellular messengers because they may contain cytokines or other signaling proteins, microRNA, and messenger RNA and they generate free radicals (24). A growing body of evidence indicates MPs are biological vectors involved with endothelial dysfunction, inflammation, thrombosis, and contribute to the progression of macro- and microvascular complications in numerous disorders.
We recently reported that due to enhanced carboxylation reactions, 1,000 to 4,000 ppm CO2 rapidly triggers human and murine neutrophils to generate MPs containing high concentrations of interleukin (IL)-1β (36). The purpose of this investigation was to evaluate whether these levels of CO2 cause similar effects in vivo. That is, using a mouse model, we assessed whether MPs containing high levels of IL-1β are generated, whether neutrophil and/or platelet activation occur, and whether the MPs can be shown to cause a vascular insult. There are no studies that have examined this question, but there are data to suggest CO2-mediated MPs alterations occur in humans. Those suffering from obstructive sleep apnea (OSA) and chronic obstructive pulmonary disease (COPD) commonly have 2- to 40-fold elevations of blood-borne MPs (3, 29, 35, 38, 40, 46). Mild elevations of arterial CO2 are a common feature among these patients. With quiescent COPD the mean arterial CO2 increases by ~3 mmHg when advancing from mild to severe grades (8, 9, 18). Elevations of ~3–4 mmHg also occur in patients with OSA for minutes-long intervals that may total over 25% of total sleep time (30). These CO2 values are comparable to those resulting from breathing ~4,000 ppm CO2, which is a level below the threshold that triggers hyperventilation (8, 13, 30). These patients exhibit heightened risks for cognitive problems similar to those seen with elevated environmental CO2, as well as cardiovascular disease, stroke, and a puzzlingly high risk for inflammatory bowel diseases (4, 10, 19). The etiology for these effects is speculated upon but unknown (14, 22, 26).
METHODS AND MATERIALS
Materials.
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Compressed gases were purchased from Air Products and Chemicals (Allentown, PA), and anakinra was from Amgen (Thousand Oaks, CA). Information on antibodies is summarized in Table 1.
Table 1.
Flow cytometry agent suppliers
| Agent | Supplier (Cat. No.) |
|---|---|
| MPs assay agents | |
| Anti-Ly6G eFluor450 | eBioscience (48–5931–82) |
| Anti-mouse CD31 BV510 | BD (563089) |
| Annexin V-FITC | BD (556419) |
| Anti-mouse TSP-1 (GranzymeA) PE | BioLegend (149704) |
| Anti-CD41 PerCP Cy5.5 | BioLegend (133918) |
| Anti-Ter119 PE Cy7 | eBioscience (25–5921–81) |
| Anti-mouse CD14 PE Cy7 | eBioscience (25–0141–82) |
| Anti-mouse CD47 Alexa Fluor647 | BD PharMingen (563584) |
| Neutrophil activation assay agents | |
| Anti-PR3 FITC | Abcam (ab91182) |
| Anti-mouse MPO PE | Hycult Biotechnology (HM1051PE-100) |
| Anti-mouse ly6G eFluor450 | eBioscience (48–5931–82) |
| Anti-mouse CD41 APC | eBioscience (17–0411–82) |
| Anti-mouse CD184 (CXCR4) Alexa Fluor488 | eBioscience (53–9991–80) |
| Anti-mouse CD284 (TLR4) Alexa Fluor488 | eBioscience (53–9041–82) |
| Platelet activation assay agents | |
| Annexin V-BV421 | BD (563973) |
| Anti-mouse CD62P FITC | BD (553744) |
| Anti-mouse TSP PE | Biolegend (149704) |
| Anti-mouse CD41 APC | eBioscience (17–0411–82) |
| Annexin V-BV421 | BD (563973) |
Animals.
All aspects of this study were reviewed and approved by the Institutional Animal Care and Use Committee, by the Department of the Navy, Bureau of Medicine and Surgery, and with adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. C57BL/6J mice (Mus musculus) were purchased from Jackson Laboratories (Bar Harbor, ME), housed in the university animal facility, and fed Laboratory Rodent Diet 5001 (PMI Nutritional, Brentwood, MO) and water ad libitum.
Mice were placed in plastic cages that had been fitted with a solid cover and sealed with cellophane tape. Pure air or air containing 1,000 to 10,000 ppm CO2 in air was flowed through the cages at a rate of 2 l/min for 2 h. Where indicated before exposures, mice were infused via a tail vein with a solution of sterile polyethylene glycol (PEG) telomere B at 0.7 μl of a 0.3% solution (wt/vol) iv/g of mouse or intraperitoneally with 100 mg/kg anakinra or a polyclonal anti-mouse IL-1β neutralizing antibody (Cat. No. BE0246; BioXcell) or a nonspecific control antibody (Cat. No. BE0089; BioXcell) at 50 µg/mouse. Following gas exposures, mice were anesthetized [intraperitoneal administration of ketamine (100 mg/kg) and xylazine (10 mg/kg)] skin was prepared by swabbing with Betadine and blood was obtained into heparinized syringes by aortic puncture.
Preparations of MPs for injection into naïve mice was carried out following our published protocol (44). Briefly, heparinized blood from mice exposed to 4,000 ppm CO2 for 2 h was centrifuged at 15,000 g for 30 min and the supernatant plasma incubated with biotinylated anti-mouse Ly6G for 1 h. MagVigen-streptavadin magnetic nanoparticles (Nvigen, Sunnyvale, CA) were then added and incubated for 12 h before the Ly6G-positive particles were separated using a magnet followed by washing and magnetic bead separation steps according to the manufacturer’s recommended procedure. Ly6G-positive and -negative fractions were then parceled among centrifuge tubes at a ratio of 250 μl + 4 ml PBS and centrifuged at 100,000 g for 60 min (typically 3–4 tubes/experiment were used). Most of the fluid in the tubes was discarded, and 250 μl remaining at the bottom were used to resuspend the particles in the pellets, which were combined. The MPs in the Ly6G-positive and -negative suspensions were counted as annexin V-positive 0.3- to 1-µm diameter particles as described below, and ~40,000 were injected via the tail vein into naïve mice. One hour after injection the mice were euthanized for blood-borne and vascular leak studies.
Standard procedures for MPs isolation.
All reagents and solutions used for MPs isolation and analysis were filtered with a 0.1-µm filter (EMD Millipore, Billerica, MA). MPs were isolated and prepared for analysis by flow cytometry as previously described (37, 44). Briefly, heparinized blood was immediately fixed with 100 µl/ml Fixation Medium A (Invitrogen, Carlsbad, CA) and centrifuged for 5 min at 1,500 g. EDTA was added to the supernatant to achieve 12.5 mM to prevent MPs aggregation. Plasma was centrifuged at 15,000 g for 30 min, and the supernatant was used for MPs count and subtypes analysis by flow cytometry.
MP analysis.
MPs supernatant (10 µl) was labeled for 30 min at room temperature in the dark with optimized concentrations of agents (Table 1) in 100 µl filtered annexin V binding buffer (BD PharMingen, San Jose, CA).
IL-1β measurements and related studies.
Mouse-specific ELISA kits (eBioscience, San Diego, CA) were used to evaluate concentration of IL-1β in MPs samples following the manufacturer’s instructions.
Flow cytometry.
Flow cytometry was performed with an eight-color, triple laser MACSQuant Analyzer (Miltenyi Biotec, Auburn, CA) using MACSQuantify software version 2.5 to analyze data. MACSQuant was calibrated every other day with calibration beads ((Miltenyi Biotec). Forward scatter and side scatter were set at logarithmic gain. Photomultiplier tube voltage and triggers were optimized to detect sub-micron particles. Microbeads of three different diameters of 0.3 µm (Sigma) and of 1.0 and 3.0 µm (Spherotech, Lake Forest, IL) were used for initial settings and before each experiment as an internal control. Annexin V-positive particles with diameters up to 1 µm were defined as MPs. The concentration of MPs in sample tubes was determined by MACSQuant Analyzer according to exact volume of solution from which MPs were analyzed.
Neutrophil and platelet activation analysis.
Whole fixed blood (100 µl) was stained for 30 min at room temperature in the dark with optimized concentrations of antibodies as listed in Table 1. After being stained, 2 ml PBS were added to dilute each sample tube before analysis, with the cytometer acquisition set to use anti-mouse Ly6G as the fluorescence trigger to recognize mouse neutrophils. Platelets were analyzed by anti-mouse CD41 along with selection for particles that were annexin V-negative and 3 to 5 µm in diameter.
Vascular permeability assay.
Mice were injected via the tail vein with lysine-fixable tetramethylrhodamine-conjugated dextran (2 × 106 Da; Invitrogen, Carlsbad, CA) and endothelium-enriched homogenates of brain, quadriceps femoris leg muscle, and distal 2 cm of colon were prepared using colloidal silica following published methods (37). Vascular permeability, quantified as perivascular dextran uptake in the experimental group, was compared against a control mouse included in each experiment.
Statistical analysis.
Results are expressed as the means ± SE for three or more independent experiments. Data were compared by ANOVA using SigmaStat (Jandel Scientific, San Jose, CA) and Newman-Keuls post hoc test. The level of statistical significance was defined as P < 0.05.
RESULTS
Mouse CO2 exposures, MPs, and IL-1β content.
Mice were exposed for 2 h to air containing 1,000 to 10,000 ppm CO2. Figure 1 demonstrates a biphasic effect, with significant elevations in the total number of MPs following 2,000 and 4,000 ppm CO2 exposures, consistent with ex vivo studies (36). When MPs were probed for cell-specific membrane surface proteins, statistically significant differences from control were identified for those exhibiting Ly6G, a protein found on mature neutrophils, and MPs of endothelial origin that exhibit CD31 (platelet-endothelial cell adhesion molecule) but not platelet-specific CD41.
Fig. 1.
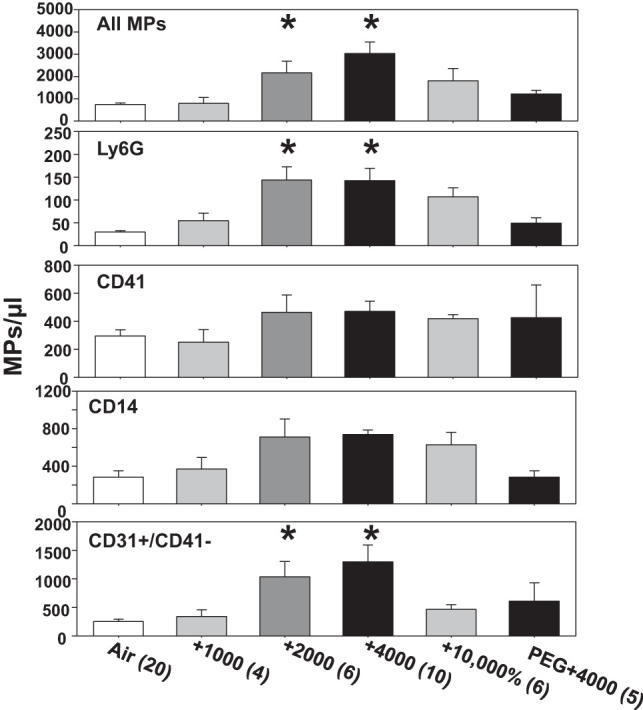
Microparticles (MPs) in mice following 2-h exposures. Blood-borne MPs were quantified in control mice (air only), and mice were killed immediately after exposure for 2 h to air + 1,000 to 10,000 ppm CO2. Where shown, mice were injected before exposure with PEG (see materials and methods). Flow cytometric measurements were made to quantify the number of all 0.3 to 1 µm diameter Annexin V-positive particles (top), as well as those expressing proteins specific to certain cells: Ly6G (mature neutrophils), CD41 (platelets), CD14 (predominantly monocytes), and CD31+/CD41-dim (endothelium). Data are means ± SE; n is shown for each sample. *P < 0.05, significantly different from control by ANOVA.
The concentration of IL-1β in MPs also increased. MPs from control mice had a concentration of 9.5 ± 1.4 (SE, n = 9) pg/million MPs. Those from mice exposed, respectively, to 1,000, 2,000, 4,000, or 10,000 ppm CO2 had the following: 10.0 ± 4.0 (n = 4, not statistically significant), 35.9 ± 5.3 (n = 6, P < 0.05), 88.8 ± 10.9 (n = 10, P < 0.05), and 21.6 ± 1.9 (n = 4, not statistically significant) pg/million MPs.
Mouse CO2 exposures, neutrophil, and platelet activation.
Figure 2 demonstrates that 2,000 or 4,000 ppm CO2 caused neutrophil activation assessed as increased surface expression of Toll-like receptor 4 (TLR4), CXC chemokine receptor 4 (CXCR4), and proteinase 3 (PR3), and after 4,000 ppm CO2, there was also an increase in myeloperoxidase. Neutrophil-platelet interactions were demonstrated as an increase in platelet-specific CD41 on the neutrophil surface after 4,000 ppm CO2. Platelet activation occurred with 2,000 to 10,000 ppm CO2 as shown by an increase in surface thrombospondin-1 (TSP), which is a soluble component within α-granules that on release will noncovalently bind to activated platelet membranes (21). However, there was no statistically significant increase in surface CD62P (P-selectin), an integral membrane protein of α-granules (data not shown) (20). There were no statistically significant differences vs. control in total number of all leukocytes, neutrophils or platelets in mice exposed to any of the elevations in CO2 (data not shown).
Fig. 2.
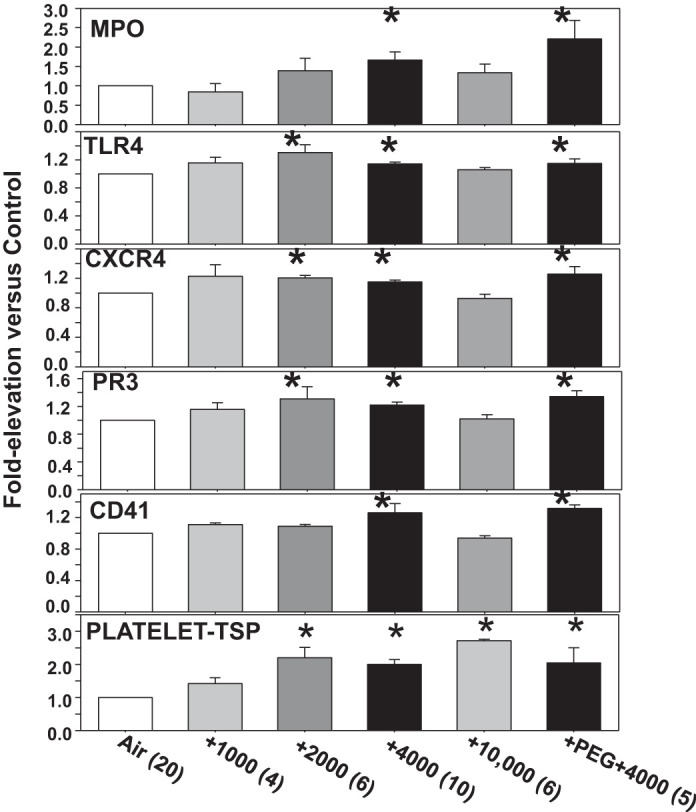
Neutrophil and platelet activation. Surface proteins on neutrophils and platelets were quantified in mice manipulated as described in the caption for Fig. 1. Frames 1–5: analyses of Ly6G-positive cells (neutrophils) where surface expression was evaluated for myeloperoxidase (MPO), Toll-like receptor 4 (TLR4), CXC chemokine receptor 4 (CXCR4), proteinase 3 (PR3), and presence of platelet-specific CD41 as reflecting platelet-neutrophil interactions. Frame 6: elevation in surface expression of thrombospondin-1 (TSP) on platelets, which were identified as CD41-positive, between 3 and 5 µm diameter and annexin V-negative. Data are means ± SE; n is shown for each sample. *P < 0.05, significantly different from control by ANOVA.
Mouse CO2 exposures and dextran extravasation.
Vascular injury in brain, skeletal muscle and distal colon was assessed as uptake of lysine-fixable tetramethylrhodamine-conjugated dextran (2 × 106 Da) injected into mice immediately following CO2 exposures. Statistically significant increases were observed after 2,000 and 4,000 ppm CO2 (Fig. 3), but changes were transient as all had resolved by 13 h postexposure (data not shown). Lung injury was also evaluated in mice exposed to 4,000 ppm CO2 as lung wet-to-dry weight ratio. In control mice, the value was 4.02 ± 0.08, and immediately after the 2 h CO2 exposure the value was 4.5 ± 0.6 (n = 6, P < 0.05).
Fig. 3.
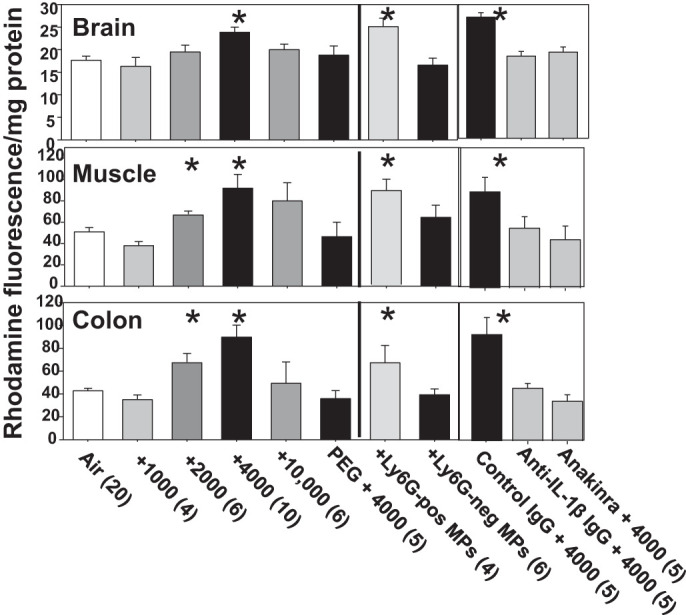
Vascular leakage of 2 × 106 Da rhodamine-labeled dextran. Brain, skeletal muscle and distal colon were prepared and evaluated as described in materials and methods. Values reflect fluorescence from rhodamine as arbitrary units/mg tissue protein in homogenates from mice subjected to 2 h of air or air plus 1,000 to 10,000 ppm CO2. Where shown, mice were injected with PEG, nonspecific control IgG, anti-IL-1β IgG, or anakinra just before exposure to 4,000 ppm CO2. Others were injected with Ly6G-positive or -negative MPs prepared from mice exposed to 4,000 ppm CO2 and killed 1 h later. Data are means ± SE. *P < 0.05, significantly different from control by ANOVA.
Injecting purified MPs.
Because Ly6G-expressing MPs are elevated by CO2, studies were undertaken to assess if they were responsible for vascular injuries. Blood was obtained from mice exposed to 4,000 ppm CO2 for 2 h, and MPs samples were prepared (see materials and methods). Naïve mice, that is those breathing only normal air and no elevations of CO2, were injected with Ly6G-positive MPs (36,338 ± 5,652/mouse, n = 4) or Ly6G-negative MPs (41,487 ± 2,351/mouse, n = 4, not statistically significantly different), and 1 h later assessments, were made for neutrophil and platelet activation, and vascular injury. Those injected with Ly6G-positive, but not Ly6G-negative MPs, demonstrated vascular leakage (Fig. 3). Neutrophil activation parameters were insignificantly different from control for all values except CXCR4, where elevations occurred in mice exposed to Ly6G-positive MPs (1.54 ± 0.08-fold over control, n = 4, P < 0.05) and also Ly6G-negative MPs (1.36 ± 0.05-fold over control, n = 6, P < 0.05). Platelets exhibiting TSP increased by 2.0 ± 0.1-fold (n = 7, P < 0.05) for mice injected with Ly6G-positive MPs and 2.0 ± 0.04-fold (n = 7, P < 0.05) for mice injected with Ly6G-negative MPs.
MPs lysis effects.
To address whether blood-borne components other than MPs may be responsible for vascular effects of CO2, a group of mice was infused with PEG telomere B immediately before 4,000 ppm CO2. This is a detergent that is Food and Drug Administration approved for intravenous use that will emulsify MPs in mice (37). Mice so treated did not exhibit statistically significant elevations of MPs (Fig. 1) or vascular injuries (Fig. 3). PEG-treated mice did, however, demonstrate neutrophil and platelet activation (Fig. 2).
IL-1β antagonism.
Injections (intraperitoneal) before CO2 exposure with a neutralizing antibody to IL-1β (50 µg/mouse) or an IL-1β receptor inhibitor (100 mg/kg anakinra) prevented tissue elevations of intravenously injected dextran in mice exposed to 4,000 ppm CO2 (Fig. 3). This did not occur in mice injected with a nonspecific control antibody (50 µg/mouse). CO2-mediated neutrophil activation parameters among all three treatment groups (nonspecific and IL-1β antibodies and anakinra) were comparable to values in mice exposed to only 4,000 ppm CO2, except for surface expression of CD41. Whereas evidence for platelet-neutrophil interactions in those injected with control antibody was similar to those exposed to only 4,000 ppm CO2, 1.27 ± 0.11-fold (n = 5, P < 0.05 vs. control), no significant elevation occurred for mice injected with antibody to IL-1β (1.14 ± 0.05, n = 5) or with anakinra (1.03 ± 0.04, n = 5). These mice exhibited a modest, but statistically insignificant, increase in TSP on platelets, 1.45 ± 0.40 (antibody to IL-1β) and 1.37 ± 0.26-fold (anakinra).
DISCUSSION
We conclude that CO2 at levels found in many buildings and among those with chronic respiratory diseases triggers production of MPs, neutrophil and platelet activation, and vascular damage in multiple tissues. The data indicate that CO2 activates neutrophils that produce MPs containing high concentrations of IL-1β and that these MPs cause vascular damage reflected as elevations of endothelial MPs and dextran extravasation. The biphasic response, with resolution of MPs elevations at 10,000 ppm (1%) CO2, is consistent with ex vivo findings (36). Accelerated carboxylation reactions at very high CO2 levels cause metabolic build-up of intracellular malate, which inhibits the on-going oxidative stress responses and MPs production by neutrophils (36). We did not find changes in intracellular pH with CO2 elevations causing accelerated MPs production, but in the current study an effect from systemic acidosis due to low-level hypercapnia in vivo cannot be ruled out.
We believe this is the first study showing pathophysiological effects from environmentally relevant elevations of CO2. There has been substantial effort focused on higher concentrations of CO2. Production of inflammatory mediators by lung cells ex vivo and in lungs of mice has been reported but only with exposures to more than 50,000 ppm (5%) CO2 (1). Levels of ~120,000 ppm (12%) protect against lung damage due to many insults, prompting therapeutic “permissive hypercapnia” to be administered under a variety of conditions (39).
MP content of IL-1β increases in conjunction with increased production of MPs. This is consistent with work showing that pathways for MPs production and nucleotide-binding domain-like receptor 3 (NLRP3) inflammasome activation overlap (36). It is important to note that inflammasome activation due to CO2 occurs within 30 min, which is remarkably rapid. NLRP3 inflammasome activation is classically considered an hours-long two-step process requiring both transcriptional priming and posttranslational activation to generate functional cytokines. Recently, a one-step noncanonical activation pathway by lipopolysaccharide was shown in human and porcine monocytes, but this too involved a 14-h incubation (11). The prompt NLRP3 response obviously raises risks associated with elevated CO2. Because antibody to IL-1β and pharmacological inhibition of the IL-1β receptor both abrogate vascular CO2-mediated injuries, we conclude that this cytokine is responsible for tissue damage.
Numerous soluble inflammatory mediators are released by activated neutrophils and platelets (25). Results suggest they play a nominal role in CO2-induced vascular leak. Injecting Ly6G-positive MPs caused dextran extravasation but little evidence of neutrophil activation. Along similar lines, platelet activation occurred irrespective of whether vascular leak was found in mice injected with MPs. PEG injection inhibited vascular leakage but not platelet or neutrophil activation. In prior work we showed that PEG will emulsify newly generated MPs, presumably because of a tenuous cytoskeletal structure, without causing neutrophil activation (37). Platelet activation by CO2 is not correlated with MPs elevations (Figs. 1 and 2). Moreover, we found an increase in surface TSP but not CD62P, which is atypical (20). IL-1β plays a major role with development of platelet-neutrophil aggregates, however, because antibody to IL-1β and anakinra blocked the response.
Vascular leak that was present immediately after 2-h exposures to CO2 resolved within 13 h. Whether changes are more severe or persistent with longer exposures, or whether compensatory/adaptive responses occur, requires investigation. The mice in our study exhibited no overt evidence of compromised physical or gastrointestinal function, but this could change with longer exposures. These questions and direct studies of human exposures obviously have relevance to issues related to indoor air quality.
There are a number of additional situations where our findings may have relevance. Exposures to CO2 elevations equivalent to breathing ~4,000 ppm CO2 occur when health care workers use particulate filtering (N95) respirator face masks for over 15 min (31); CO2 often rises to this level during SCUBA diving because of apparatus dead space, gas density, and slowed respiration related to an elevated O2 partial pressure and MPs could contribute to decompression sickness, and, because of engineering issues with CO2 scrubbers, the levels in submarines and spacecraft are allowed to be 4,000 to 7,000 ppm, or more, depending on a number of factors (15, 28). In this regard, there are extensive data demonstrating immune dysregulation occurs with space flight, including elevations of cytokines indicative of mild inflammation thought possibly related to an altered gut microbiome or other factors, and CO2 could play a role. Plasma levels of IL-1 receptor antagonist, an endogenous inhibitor of the proinflammatory effects of IL-1, are consistently elevated during spaceflight and have been speculated to represent an adaptive physiological response to inflammatory stress (7). The roles of MPs in chronic respiratory disorders also require study as they may contribute to risks for central nervous system, cardiovascular, and inflammatory bowel diseases (4, 10, 19). Further evaluations may also identify prophylactic interventions, as some relatively benign agents can abrogate MPs production in response to some stimuli (43) and dietary changes can inhibit the NLRP3 inflammasome (45).
GRANTS
This project was supported by Office of Naval Research Grant N00014-16-1-2868.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.R.T. conceived and designed research; S.R.T., V.M.B., J.H., and M.Y. performed experiments; S.R.T. analyzed data; S.R.T. interpreted results of experiments; S.R.T. prepared figures; S.R.T. drafted manuscript; S.R.T., V.M.B., J.H., and M.Y. edited and revised manuscript; S.R.T., V.M.B., J.H., and M.Y. approved final version of manuscript.
REFERENCES
- 1.Abolhassani M, Guais A, Chaumet-Riffaud P, Sasco AJ, Schwartz L. Carbon dioxide inhalation causes pulmonary inflammation. Am J Physiol Lung Cell Mol Physiol 296: L657–L665, 2009. doi: 10.1152/ajplung.90460.2008. [DOI] [PubMed] [Google Scholar]
- 2.Allen JG, MacNaughton P, Satish U, Santanam S, Vallarino J, Spengler JD. Associations of cognitive function scores with carbon dioxide, ventilation, and volatile organic compound exposures in office workers: A controlled exposure study of green and conventional office environments. Environ Health Perspect 124: 805–812, 2016. doi: 10.1289/ehp.1510037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayers L, Stoewhas AC, Ferry B, Stradling J, Kohler M. Elevated levels of endothelial cell-derived microparticles following short-term withdrawal of continuous positive airway pressure in patients with obstructive sleep apnea: data from a randomized controlled trial. Respiration 85: 478–485, 2013. doi: 10.1159/000342877. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology 129: 827–836, 2005. doi: 10.1053/j.gastro.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Colton MD, MacNaughton P, Vallarino J, Kane J, Bennett-Fripp M, Spengler JD, Adamkiewicz G. Indoor air quality in green vs conventional multifamily low-income housing. Environ Sci Technol 48: 7833–7841, 2014. doi: 10.1021/es501489u. [DOI] [PubMed] [Google Scholar]
- 6.Corsi RL, Torres VM, Sanders M, Kinney KA. Carbon dioxide levels and dynamics in elementary schools: results from the TESIAS study. In: Indoor Air 2002, 9th International Conference on Indoor Air Quality and Climate, Monterey, California, edited by Monterey Levin H., CA: Indoor Air, 2002, p. 74–79. [Google Scholar]
- 7.Crucian BE, Zwart SR, Mehta S, Uchakin P, Quiriarte HD, Pierson D, Sams CF, Smith SM. Plasma cytokine concentrations indicate that in vivo hormonal regulation of immunity is altered during long-duration spaceflight. J Interferon Cytokine Res 34: 778–786, 2014. doi: 10.1089/jir.2013.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cukic V. The changes of arterial blood gases in COPD during four-year period. Med Arh 68: 14–18, 2014. doi: 10.5455/medarh.2014.68.14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dal Negro RW, Tognella S, Bonadiman L, Turco P. Changes in blood hemoglobin and blood gases PaO2 and PaCO2 in severe COPD overa three-year telemonitored program of long-term oxygen treatment. Multidiscip Respir Med 7: 15, 2012. doi: 10.1186/2049-6958-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: Relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res 93: 1778–1794, 2015. doi: 10.1002/jnr.23634. [DOI] [PubMed] [Google Scholar]
- 11.Elliott EI, Sutterwala FS. Monocytes take their own path to IL-1β. Immunity 44: 713–715, 2016. doi: 10.1016/j.immuni.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Freijer JI, Bloemen HJ. Modeling relationships between indoor and outdoor air quality. J Air Waste Manag Assoc 50: 292–300, 2000. doi: 10.1080/10473289.2000.10464007. [DOI] [PubMed] [Google Scholar]
- 13.Hirshman CA, McCullough RE, Weil JV. Normal values for hypoxic and hypercapnic ventilaroty drives in man. J Appl Physiol 38: 1095–1098, 1975. [DOI] [PubMed] [Google Scholar]
- 14.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 169: 348–353, 2004. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 15.James JT. Carbon dioxide. In: National Research Council, Spacecraft Maximum Allowable Concentrations for Selected Airborne Contaminants. Washington, DC: National Academy Press: 2008, vol 5, p. 112–124, [Google Scholar]
- 16.Jurado SR, Bankoff ADP, Sanchez A. Indoor air quality in Brazilian universities. Int J Environ Res Public Health 11: 7081–7093, 2014. doi: 10.3390/ijerph110707081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kajtar L, Herczeg L. Influence of carbon-dioxide concentration on human well-being and intensity of mental work. Q J Hung Meteorol Serv 116: 145–169, 2012. [Google Scholar]
- 18.Lacedonia D, Carpagnano GE, Aliani M, Sabato R, Foschino Barbaro MP, Spanevello A, Carone M, Fanfulla F. Daytime PaO2 in OSAS, COPD and the combination of the two (overlap syndrome). Respir Med 107: 310–316, 2013. doi: 10.1016/j.rmed.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Lahousse L, Tiemeier H, Ikram MA, Brusselle GG. Chronic obstructive pulmonary disease and cerebrovascular disease: A comprehensive review. Respir Med 109: 1371–1380, 2015. doi: 10.1016/j.rmed.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Larsen E, Celi A, Gilbert GE, Furie BC, Erban JK, Bonfanti R, Wagner DD, Furie B. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell 59: 305–312, 1989. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- 21.Lawler J. The structural and functional properties of thrombospondin. Blood 67: 1197–1209, 1986. [PubMed] [Google Scholar]
- 22.Liu H, Ding L, Zhang Y, Ni S. Circulating endothelial microparticles involved in lung function decline in a rat exposed in cigarette smoke maybe from apoptotic pulmonary capillary endothelial cells. J Thorac Dis 6: 649–655, 2014. doi: 10.3978/j.issn.2072-1439.2014.06.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu CY, Lin JM, Chen YY, Chen YC. Building-related symptoms among office employees associated with indoor carbon dioxide and total volatile organic compounds. Int J Environ Res Public Health 12: 5833–5845, 2015. doi: 10.3390/ijerph120605833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res 107: 1047–1057, 2010. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 25.Nacher M, Hidalgo A. Fire within the vessels: interactions between blood cells and inflammatory vascular injury. Front Biosci (Schol Ed) 3: 1089–1100, 2011. doi: 10.2741/213. [DOI] [PubMed] [Google Scholar]
- 26.Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med 169: 354–360, 2004. doi: 10.1164/rccm.200306-756OC. [DOI] [PubMed] [Google Scholar]
- 27.Persily AK, Gorfain J. Analysis of Ventilation Data from the U.S. Environmental Protection Agency Building Assessment Survey and Evaluation (BASE) Study. NISTIR-7145-Revised. Gaithersburg, MD: National Institute for Standards and Technology, 2008. doi: 10.6028/NIST.IR.7145r [DOI] [Google Scholar]
- 28.Persson O, Ostberg C, Pagels J, Sebastian A. Air contaminants in a submarine equipped with air independent propulsion. J Environ Monit 8: 1111–1121, 2006. doi: 10.1039/B605331A. [DOI] [PubMed] [Google Scholar]
- 29.Priou P, Gagnadoux F, Tesse A, Mastronardi ML, Agouni A, Meslier N, Racineux JL, Martinez MC, Trzepizur W, Andriantsitohaina R. Endothelial dysfunction and circulating microparticles from patients with obstructive sleep apnea. Am J Pathol 177: 974–983, 2010. doi: 10.2353/ajpath.2010.091252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rimpilä V, Hosokawa K, Huhtala H, Saaresranta T, Salminen AV, Polo O. Transcutaneous carbon dioxide during sleep-disordered breathing. Respir Physiol Neurobiol 219: 95–102, 2015. doi: 10.1016/j.resp.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Roberge RJ, Coca A, Williams WJ, Powell JB, Palmiero AJ. Physiological impact of the N95 filtering facepiece respirator on healthcare workers. Respir Care 55: 569–577, 2010. [PubMed] [Google Scholar]
- 32.Salthammer T, Uhde E, Schripp T, Schieweck A, Morawska L, Mazaheri M, Clifford S, He C, Buonanno G, Querol X, Viana M, Kumar P. Children’s well-being at schools: Impact of climatic conditions and air pollution. Environ Int 94: 196–210, 2016. doi: 10.1016/j.envint.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Satish U, Mendell MJ, Shekhar K, Hotchi T, Sullivan D, Streufert S, Fisk WJ. Is CO2 an indoor pollutant? Direct effects of low-to-moderate CO2 concentrations on human decision-making performance. Environ Health Perspect 120: 1671–1677, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shendell DG, Prill R, Fisk WJ, Apte MG, Blake D, Faulkner D. Associations between classroom CO2 concentrations and student attendance in Washington and Idaho. Indoor Air 14: 333–341, 2004. doi: 10.1111/j.1600-0668.2004.00251.x. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi T, Kobayashi S, Fujino N, Suzuki T, Ota C, Tando Y, Yamada M, Yanai M, Yamaya M, Kurosawa S, Yamauchi M, Kubo H. Annual FEV1 changes and numbers of circulating endothelial microparticles in patients with COPD: a prospective study. BMJ Open 4: e004571, 2014. doi: 10.1136/bmjopen-2013-004571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thom SR, Bhopale VM, Hu J, Yang M. Increased carbon dioxide levels stimulate neutrophils to produce microparticles and activate the nucleotide-binding domain-like receptor 3 inflammasome. Free Radic Biol Med 106: 406–416, 2017. doi: 10.1016/j.freeradbiomed.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Thom SR, Yang M, Bhopale VM, Huang S, Milovanova TN. Microparticles initiate decompression-induced neutrophil activation and subsequent vascular injuries. J Appl Physiol (1985) 110: 340–351, 2011. doi: 10.1152/japplphysiol.00811.2010. [DOI] [PubMed] [Google Scholar]
- 38.Thomashow MA, Shimbo D, Parikh MA, Hoffman EA, Vogel-Claussen J, Hueper K, Fu J, Liu CY, Bluemke DA, Ventetuolo CE, Doyle MF, Barr RG; The Multi-Ethnic Study of Atherosclerosis Chronic Obstructive Pulmonary Disease Study . Endothelial microparticles in mild chronic obstructive pulmonary disease and emphysema. Am J Respir Crit Care Med 188: 60–68, 2013. doi: 10.1164/rccm.201209-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torbati D. Carbon dioxide: a “waste product” with potential therapeutic utilities in critical care. Crit Care Med 31: 2705–2707, 2003. doi: 10.1097/01.CCM.0000089943.05031.B3. [DOI] [PubMed] [Google Scholar]
- 40.Trzepizur W, Priou P, Paris A, Nardi J, Tual-Chalot S, Meslier N, Urban T, Andriantsitohaina R, Martinez MC, Gagnadoux F. Nocturnal release of leukocyte-derived microparticles in males with obstructive sleep apnoea. Eur Respir J 37: 1293–1295, 2011. doi: 10.1183/09031936.00150010. [DOI] [PubMed] [Google Scholar]
- 41.Tsai DH, Lin JS, Chan CC. Office workers’ sick building syndrome and indoor carbon dioxide concentrations. J Occup Environ Hyg 9: 345–351, 2012. doi: 10.1080/15459624.2012.675291. [DOI] [PubMed] [Google Scholar]
- 42.Wallner P, Munoz U, Tappler P, Wanka A, Kundi M, Shelton JF, Hutter HP. Indoor environmental quality in mechanically ventilated, energy-efficient buildings vs. conventional buildings. Int J Environ Res Public Health 12: 14132–14147, 2015. doi: 10.3390/ijerph121114132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang M, Barak OF, Dujic Z, Madden D, Bhopale VM, Bhullar J, Thom SR. Ascorbic acid supplementation diminishes microparticle elevations and neutrophil activation following SCUBA diving. Am J Physiol Regul Integr Comp Physiol 309: R338–R344, 2015. doi: 10.1152/ajpregu.00155.2015. [DOI] [PubMed] [Google Scholar]
- 44.Yang M, Milovanova TN, Bogush M, Uzun G, Bhopale VM, Thom SR. Microparticle enlargement and altered surface proteins after air decompression are associated with inflammatory vascular injuries. J Appl Physiol (1985) 112: 204–211, 2012. doi: 10.1152/japplphysiol.00953.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Youm Y-H, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21: 263–269, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yun CH, Jung KH, Chu K, Kim SH, Ji KH, Park HK, Kim HC, Lee ST, Lee SK, Roh JK. Increased circulating endothelial microparticles and carotid atherosclerosis in obstructive sleep apnea. J Clin Neurol 6: 89–98, 2010. doi: 10.3988/jcn.2010.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]


