Abstract
Objective:
This study examined whether the frequency of experiences of ethnic microaggressions and the sensitivity to such experiences were associated with cortisol responses to an acute social stressor (Trier Social Stress Test; TSST) among an ethnically diverse sample of young adults (N = 109, Mage = 18.82 years, SD = 1.40 years, 74% female, 44% Latinx).
Method:
Self-reported experiences of and sensitivity to microaggressions were assessed using the Everyday Microaggressions Scale. Participants’ salivary cortisol was collected before, immediately after, and at three 15-min intervals after the TSST (for a total of 5 salivary samples) to assess their cortisol responses to an acute social stressor.
Results:
Mixed model analyses revealed that experiencing a higher frequency of microaggressions (p = .005) and being more sensitive to those experiences (p = .001) were associated with a more blunted cortisol response (i.e., lower cortisol reactivity and recovery) to the TSST, relative to experiencing a lower frequency of microaggressions and being less sensitive to them. Furthermore, this blunted cortisol response to the TSST was more prominent among young adults of Latinx and other ethnic backgrounds (i.e., biracial, African American, and Native American) compared to their Asian American and non-Hispanic White peers (p = .034).
Conclusion:
Findings provide insight into the different ways in which experiences of ethnic microaggressions can be associated with biological markers of stress.
Keywords: cortisol reactivity, TSST, racial/ethnic differences, ethnic microaggressions, young adults
Microaggressions refer to indirect, subtle, or unintentional discrimination against members of a marginalized group (Sue et al., 2007). For example, comments directed at racial and ethnic minorities such as being told that they speak English well or that they are “articulate,” may not always be a compliment, particularly when stated to someone whose first language is English (Byrd, 2018; Sue, Capodilupo, Nadal, & Torino, 2008). These types of comments can be harmful as they can convey demeaning connotations about a person’s abilities and intelligence based on that person’s racial or cultural background (Byrd, 2018; Sue et al., 2008). Although these messages can often be uttered in an unconscious, unintentional, or joking manner (Sue et al., 2007), these subtle and commonplace verbal, behavioral, and environmental indignities have been associated with a host of deleterious physical and mental health outcomes.
Microaggressions have been associated with increased pain and fatigue (Nadal, Griffin, Wong, Davidoff, & Davis, 2017), depression (Torres, Driscoll, & Burrow, 2010; Torres & Taknint, 2015), anxiety and alcohol consumption (Blume, Lovato, Thyken, & Denny, 2012), and marijuana use (Pro, Sahker, & Marzell, 2018). For college students, in particular, experiencing microaggressions on campus has been documented as a significant challenge which has caused students to disengage, and feel alienated and undermined (Casanova, McGuire, & Martin, 2018; Forrest-Bank & Jenson, 2015; McCabe, 2009; Suárez-Orozco et al., 2015; Torres et al., 2010; Torres & Taknint, 2015). Therefore, in order to mitigate these adverse outcomes, it is important to identify factors associated with the harmful effects that microaggressions may have on one’s health.
Although microaggressions have been shown to have repercussions for physical and mental health, the mechanisms through which this occurs are not well understood. One factor that may play a role in how microaggressions affect health is the cognitive or emotional appraisal of such experiences, which has been theorized to be stressful above and beyond the experience of a microaggression (Harrell, 2000). Due to the ambiguous nature of microaggressions, they can yield a wide range of interpretations and reactions (Major, Mendes, & Dovidio, 2013) including anger, resentment, anxiety, confusion, and curiosity (Wang, Leu, & Shoda, 2011). Individuals’ subjective responses to stressful situations, such as microaggressions, can be shaped by many factors including their outlook for the future (Kaiser, Major, & McCoy, 2004), their perception of the perpetrator’s intent, and the attributions they make for these experiences (Crocker, Voelkl, Testa, & Major, 1991). Although research examining individuals’ reactions to microaggressions is scant (Lilienfeld, 2017), we purport that by examining sensitivity to microaggressions (i.e., the extent to which individuals are bothered or upset by such experiences) we can better understand how microaggressions relate to stress responses.
Literature suggests that responses to microaggressions vary across individuals of different ethnic backgrounds. Studies have found experiences of ethnic microaggressions to be related to higher levels of perceived (i.e., self-reported) stress among African American graduate students (Torres et al., 2010), and mild to moderate levels of traumatic stress in Latinx adults (Torres & Taknint, 2015), both of which have, in turn, been related to higher levels of depressive symptoms. Relatedly, a study by Huynh (2012) found perceived stress to mediate the association between ethnic microaggressions and depressive and somatic symptoms among Asian American and Latinx adolescents. Finally, a study by Wang and colleagues (2011) showed that ethnic microaggressions were significantly associated with increased levels of externalizing emotions (e.g., anger, resentment) and internalizing emotions (e.g., anxiety, shame, embarrassment) among Asian American and non-Hispanic White college students. Notably, experiences of microaggressions were also associated with greater levels of positive emotions (i.e., excitement and happiness) among non-Hispanic White students only (Wang et al., 2011).
Although extant research provides valuable evidence regarding the effects of ethnic microaggressions on health, their influence on specific biological markers of stress, such as cortisol, is less understood. Cortisol is regulated by the hypothalamic-pituitary-adrenal (HPA) axis, is released by the adrenal glands, and is considered to be one of the main markers associated with the biological stress response (Hansen, Garde, & Persson, 2008). Short-term activation of the HPA axis, and subsequent release of cortisol in response to stressors is adaptive and necessary for everyday functioning. The release of cortisol consists of a “reactivity” phase, where cortisol levels rise in response to an acute stressor, and a “recovery” phase, where cortisol levels subsequently drop and return to baseline levels after the stressor has ended (McEwen, 2018). HPA axis activation in response to stress is often studied with the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993), which has been found to reliably induce stress and produce changes in salivary cortisol through standardized stress-generating tasks (Allen et al., 2017). Although the release of cortisol is an adaptive biological response to stressors we experience, both excessive and blunted cortisol reactivity and recovery have been associated with negative mental and physical health outcomes including depressive symptomatology, suppressed immune system function, increased abdominal fat, and decreased cognitive abilities (Andrews, Ali, & Pruessner, 2013; Eppel, 2009; Juster, McEwen, & Lupien, 2010; McEwen, 2008).
Recent studies have begun to examine differences in cortisol reactivity by ethnicity with mixed results. Specifically, some studies have reported finding no ethnic differences on cortisol reactivity (Yim, Quas, Cahill, & Hayakawa, 2010), while others have reported that African Americans show blunted cortisol responses compared to other ethnic groups (e.g., non-Hispanic Whites and Latinxs; Chong, Uhart, McCaul, Johnson, & Wand, 2008; Urizar et al., 2019). Furthermore, some studies have reported that Latinxs show greater cortisol reactivity than non-Latinxs (Busse, Yim, & Campos, 2017). The experience of chronic stressors, such as ethnic microaggressions, could potentially account for these ethnic differences in cortisol responses, yet no studies, to our knowledge, have examined whether ethnic microaggressions are associated with cortisol reactivity and recovery.
However, a few studies have examined cortisol responses in relation to discrimination. Results of these studies have shown that overt discrimination (i.e., differential treatment based on race) and vicarious experiences of discrimination (i.e., exposure to the negative treatment of others based on race) have been associated with blunted cortisol reactivity (Huynh, Huynh, & Stein, 2017; Lucas et al., 2017; Richman & Jonassaint, 2008). These studies included African American community (n = 85; Lucas et al., 2017) and college student samples (n = 33; Richman & Jonassaint, 2008), as well as Latinx college student samples (n = 36; Huynh et al., 2017), and utilized the TSST and other social-evaluative stress tasks to investigate how discrimination was associated with cortisol reactivity. Although these studies suggest that ethnic discrimination (e.g., overt, vicarious) and ethnic-related stressors more broadly are associated with altered cortisol reactivity, studies examining the role of ethnic microaggressions on cortisol responses are needed. Microaggressions have distinct characteristics such as being more ambiguous, unpredictable, more difficult to interpret, and potentially more stressful than instances of overt discrimination (Sue et al., 2007, 2008). Additional gaps in the literature can be addressed by studies that examine whether one’s sensitivity, or emotional response, to experiences of ethnic microaggressions might influence their cortisol responses to stress, and whether this association differs across ethnic groups.
Therefore, the current study examined whether the frequency of experiences of ethnic microaggressions and the sensitivity to such experiences were associated with cortisol responses to an acute stressor (TSST), while adjusting for participant gender. We also investigated whether these associations differed by ethnicity. We hypothesized that overall, young adults who experienced microaggressions more frequently and who were more sensitive to microaggressions (i.e., were more bothered by microaggressions) would demonstrate a higher cortisol reactivity compared to young adults who experienced microaggressions less frequently and who were less sensitive to them. Furthermore, based on previous research (Busse et al., 2017; Chong et al., 2008), we hypothesized that young adults of Latinx and other ethnic backgrounds would show blunted cortisol reactivity (i.e., lower cortisol reactivity and recovery) compared to young adults of non-Hispanic White and Asian American backgrounds.
Method
Participants
Participants were eligible for the study if they were 18 years of age or older and were willing to participate in a 2-hr study examining the association between stress and health. Participants were excluded from this study if they had type 1 or type 2 diabetes or had smoked any tobacco or nicotine products (e.g., cigarettes, tobacco, or vaporizers), and/or used any medication containing corticosteroids (e.g., asthma inhalers, cortisone, and prednisone) during the past 6 months. Participants were recruited using the Department of Psychology subject pool and flyers posted around campus of a public university in southern California. Participants received course credit and/or a gift card (up to $10) for compensation.
A summary of demographic characteristics is presented in Table 1. The current study included 109 young adult undergraduates at a minority serving institution from diverse ethnic backgrounds (44% Latinx, 24% Asian American, 19% non-Hispanic White, 9% biracial, 3% African American, and 1% Native American). Almost a third (31%) of our sample were second generation Latinx young adults (i.e., at least 1 parent was foreign born).
Table 1.
Sociodemographic and Ethnic Microaggression Characteristics by Ethnicity Among a Sample of Young Adults
| Variable | Total (n = 109) | Latinx (n = 48) | Asian American (n = 26) | Non-Hispanic White (n = 21) | Other ethnicity (n = 14) |
|---|---|---|---|---|---|
| Sociodemographic | |||||
| Age, M (SD) | 18.82 (1.40) | 18.67 (1.16) | 18.92 (1.32) | 18.48 (0.98) | 19.14 (1.70) |
| Gender (% female) | 74 | 77 | 77 | 66 | 71 |
| Generation status (% first generation) | 13 | 11*** | 32 | 0 | 0 |
| Parental education (% high school or less) | 25 | 69*** | 24 | 14 | 14 |
| Ethnic microaggressions | |||||
| Frequency of microaggressions, M (SD) | 1.03 (0.69) | 0.97 (0.72) | 1.00 (0.74) | 0.82 (0.41) | 1.13 (0.93) |
| Sensitivity to microaggressions, M (SD) | 0.75 (0.64) | 0.68 (0.73) | 0.70 (0.44) | 0.94 (0.63) | 0.78 (0.74) |
Note. Pearson’s χ2 and analyses of variance were conducted to test for between group differences among categorical and continuous variables, respectively.
p < .001.
Procedure
All study procedures were approved by the university’s institutional review board. Participants who were interested in the study were asked to refrain from eating, exercising, brushing their teeth and taking any medications at least 1 hr prior to their scheduled visit to the laboratory to avoid any behaviors or substances known to interfere with salivary cortisol (Kirschbaum et al., 1993). Upon arrival, participants responded to a brief eligibility screener and completed written informed consent. Eligible participants were asked to provide the first of five saliva samples to serve as a baseline prior to participating in the laboratory-based TSST (described below).
Following the collection of their first salivary cortisol sample, participants were led to a different room and completed the TSST (Kirschbaum et al., 1993), a protocol that reliably elicits increases in salivary cortisol concentrations, is highly standardized, and has good ecological validity (Allen et al., 2017). Exposure to the TSST protocol has been shown to successfully stimulate HPA axis activity in healthy young adults (Campisi, Bravo, Cole, & Gobeil, 2012) and has been administered with ethnically diverse samples (Busse et al., 2017; Campos et al., 2014; Urizar et al., 2019; Yim et al., 2010) although some ethnic differences in reactivity to the TSST have been reported (Chong et al., 2008; Hostinar, McQuillan, Mirous, Grant, & Adam, 2014; Urizar et al., 2019). Additionally, the TSST was selected for this study because exposure to a nondiscriminatory laboratory stressor allows us to examine the association between HPA axis activity (i.e., cortisol reactivity) and microaggressions, a chronic stressor (Busse, Yim, Campos, & Marshburn, 2017). As part of the TSST, participants performed a video-recorded speech task and a mental arithmetic task in front of a panel of two research confederates who wore lab coats and sat behind a desk to evaluate the participant’s performance. First, participants were given a 5-min speech task (i.e., participant was asked to explain why there were qualified for a job position of their choice), and then a 5-min mental arithmetic task (i.e., participant was asked to quickly subtract the number 13 from a larger number [e.g., 789] serially until he or she reached zero).
Once the TSST tasks ended, participants were escorted back into the first room and were asked to provide a second saliva sample. After providing the second saliva sample, they were debriefed on the purpose of the TSST. Thereafter, participants rested while completing a questionnaire packet, which included demographic questions and a measure assessing their experiences of ethnic microaggressions. During this period, participants also provided saliva samples every 15 min (at 15, 30, and 45 min following the TSST).
Measures
Demographics.
Participants answered several questions to obtain demographic information that included their age (in years), gender (male/female), ethnicity (Latinx, Asian American, non-Hispanic White, biracial, African American, and Native American), annual personal income, and parental education (averaged for both parents and used as a proxy for socioeconomic status). Generational status was also assessed and was defined as first generation if the young adult was not born in the United States, second generation if at least one parent was not born in the United States, and third generation if both parents were born in the United States.
Ethnic microaggressions.
The Everyday Microaggressions Scale (Huynh, 2012) was used to assess the occurrences of ethnic microaggressions in the daily lives of young adults. The instrument consists of 12 items scored on 6-point scale (0 = 0 times [never], 1 = once a year [rarely], 2 = 3–4 times a year [sometimes], 3 = once a month [somewhat frequently], 4 = once a week [frequently], 5 = almost every day [all the time]) and was used to determine the frequency of experiencing ethnic microaggressions across 12 scenarios (e.g., “You are mistaken as a service worker,” “Someone tells you that no one discriminates against your ethnic/racial group”). The frequency of ethnic microaggressions was calculated by taking the average of the scores across all 12 items (range of possible scores = 0–5), with higher scores indicating higher frequency of ethnic microaggressions.
Sensitivity to microaggressions was measured by asking participants to indicate their emotional reaction to the microaggressions they reported to have experienced. Sensitivity was measured on a 5-point scale (−1 = this event made me feel good, 0 = it did not bother me, 1 = it bothered me slightly, 2 = it upset me, 3 = it upset me extremely; Huynh, 2012). Sensitivity to microaggressions was calculated by taking the mean of all items that were endorsed (range of possible scores = −1 to 3), with higher numbers indicating that the microaggression bothered the participant (e.g., higher distress).
Salivary cortisol.
Five total salivary cortisol samples were collected throughout the study, including one sample before (baseline) and immediately after the TSST and 15, 30, and 45 min after the TSST. Participants provided these samples using a passive drool method by allowing saliva to pool in their mouths, and then being asked to hold a saliva collection aid (similar to a straw) inside a collection tube using both hands (Granger et al., 2007). They were instructed to tilt their head forward and carefully spit into the collection tube. They were asked to repeat this procedure until they provided a minimum of 1.8 mL of saliva. Salivary cortisol samples were analyzed via ELISA immunoassay (ELISA, IBL-America, Minneapolis, MN). Intra- and interassay variability were both under 10%. Cortisol values were then logarithmically transformed (base 10, converted from μg/dl to nmol/L), given that salivary cortisol values are typically skewed, with higher values reflecting greater cortisol levels).
Analyses
A power analysis was conducted using G*Power to ensure an adequate sample size was obtained (Faul, Erdfelder, Buchner, & Lang, 2009). Based on the medium effect size found in past research that examined the relationship between ethnic microaggressions and cortisol (Zeiders, Landor, Flores, & Brown, 2018), to obtain 80% power, a sample size of about 84 participants was needed. To account for missing data, a sample size of 109 participants were recruited for the current study to ensure adequate power. Pearson’s chi-square and analyses of variance were conducted for categorical and continuous dependent variables, respectively, to assess whether participant characteristics differed by ethnicity (see Table 1). Spearman and Pearson’s correlations were used to help identify possible covariates. Age, generational status and parental education (a proxy for socioeconomic status) were examined as potential covariates but were not significantly associated with frequency of or sensitivity to microaggressions or cortisol. Therefore, these variables were excluded from final analyses for parsimony. Only participant gender was included as a covariate in subsequent analyses due to well-documented gender differences in cortisol reactivity, with women generally demonstrating lower cortisol reactivity to the TSST than men (see Liu et al., 2017 for review). For analyses testing for group differences by ethnicity, data for biracial, African American, and Native American young adults were grouped together (named “Other ethnicity”) given their small sample sizes in this study (ns = 10, 3, and 1, respectively).
A mixed effect linear model was used to test for possible time and group effects of ethnic microaggressions (frequency and sensitivity) and ethnicity (Latinx, Asian American, non-Hispanic White, Other ethnicity) on salivary cortisol patterns over the five study time points (baseline and 1, 15, 30, and 45 min post-TSST). This approach accounts for intraindividual variation of baseline cortisol, missing data, unequally spaced data points, nonindependence of repeated measures data, and is a more appropriate test for handling data that may violate normality assumptions (Hruschka, Kohrt, & Worthman, 2005; Urizar et al., 2019; Yim et al., 2010). This analytic approach also allows for the examination of within-and between-person variation to the TSST and accounts for auto-correlation of observations across time. Finally, it tests for linear, quadratic, and cubic cortisol patterns, which are particularly important to examine when there are individual differences in participants’ cortisol peak reactivity and recovery following the TSST.
The mixed model was estimated by maximum likelihood using SAS PROC MIXED. Simple effect analyses testing average differences across groups and covariate-adjusted analyses that additionally controlled for gender were conducted. The effect sizes for these time and group effects are presented as partial eta squared , as is recommended for mixed models (Baguley, 2009). A second, exploratory mixed effect linear model was performed to test for significant two-way interactions (i.e., ethnic microaggressions by ethnicity) that could influence ethnic microaggression effects on salivary cortisol. Main effects and interaction terms of p < .05 were considered to be statistically significant. The simple effect slopes of continuous variables (e.g., frequency and sensitivity of microaggressions) were then used (by median split) to illustrate significant moderation effects by ethnicity. More specifically, a dichotomized version of the frequency and sensitivity to microaggressions variables was used via median split to better illustrate group differences (i.e., low vs. high frequency/sensitivity to microaggressions) on cortisol responses. The least-squares means method was used to compare group means for all significant effects. Casewise deletion of missing data was used in all analyses.
Results
Descriptive Characteristics
On average, our sample was about 19 years of age (SD = 1.40 years, range 18–24), the majority of whom were women (74%) and Latinx (44%). Participants of Latinx and Asian American ethnicities were more likely to be first generation (i.e., not United States born), compared to participants of non-Hispanic White and other ethnic backgrounds, χ2(6) = 62.75, p < .001. Participants of Latinx background were also more likely to have parents who had an educational background of high school or less compared to young adults of Asian American, non-Hispanic White, and other ethnic backgrounds, χ2(36) = 59.53, p = .008. In terms of ethnic microaggressions, our sample reported rarely experiencing microaggressions on average (M = 1.01, SD = 0.69) and being slightly bothered by this experience when it did occur (M = 0.74, SD = 0.63). Neither frequency of nor sensitivity to ethnic microaggressions differed by ethnicity (see Table 1).
Cortisol Response to the TSST
Mixed effect linear model analyses of change revealed that the TSST elicited a significant biological stress response among participants (quadratic and cubic patterns), adjusting for gender. The greatest cortisol reactivity (increase in cortisol) occurred from Time Point 1 (baseline, M = 5.61 nmol/L) to Time Point 2 (1 min post-TSST, M = 7.69 nmol/L). The greatest cortisol recovery (decrease in cortisol) was observed from Time Point 2 (1 min post-TSST, M = 7.69 nmol/L) to Time Point 5 [45 min post-TSST, M = 5.43 nmol/L; F(4, 105) = 20.99, p < .001, ].
Main Effects of Ethnic Microaggressions and Ethnicity on Cortisol Responses
A mixed effect linear model analysis of change determined that there was a significant main effect of frequency of microaggressions on cortisol responses to the TSST, controlling for gender, F(5, 104) = 8.30, p = .005, . Specifically, young adults who reported experiencing a higher frequency of microaggressions showed a more blunted cortisol response to the TSST (i.e., lower cortisol reactivity and recovery) compared to those who reported experiencing a lower frequency of microaggressions (Figure 1a). Results also showed that there was a significant main effect of sensitivity to microaggressions on cortisol patterns controlling for gender, F(5, 103) = 11.03, p = .001, . Specifically, those who were more sensitive to microaggressions showed a more blunted cortisol response to the TSST compared to those who had lower sensitivity to microaggressions (Figure 1b). Finally, there was a significant main effect of ethnicity on cortisol patterns (adjusting for gender). Latinx and participants in the other ethnic backgrounds (i.e., biracial, African American, and Native American) group showed more blunted cortisol responses to the TSST (i.e., lower cortisol reactivity and recovery) and higher cortisol levels overall than Asian American and non-Hispanic White young adults, F(4, 105) = 16.16, p < .001, .
Figure 1.
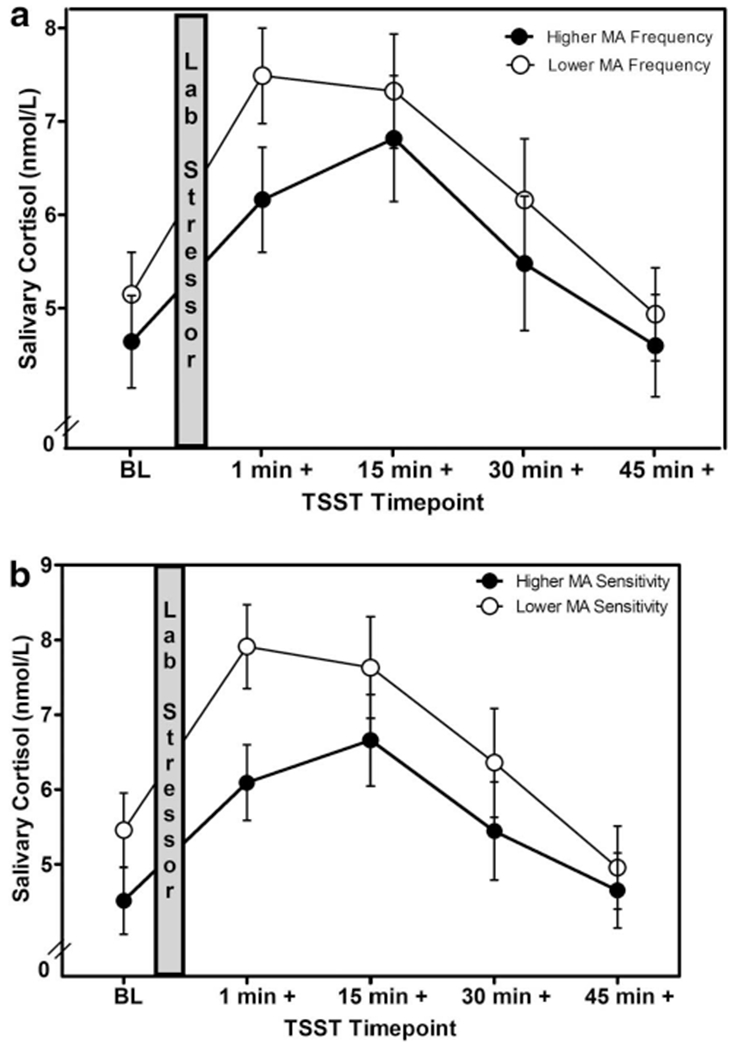
(a and b) Changes in salivary cortisol following a laboratory stressor by microaggression frequency and sensitivity. TSST = Trier Social Stress Test; MA = Microaggressions.
Interaction Effects of Ethnic Microaggressions by Ethnicity on Cortisol Responses
A mixed effect linear model analysis of change showed that there was a significant interaction between frequency of microaggressions and ethnicity on cortisol responses to the TSST controlling for gender, F(5, 104) = 4.64, p = .034, . The dichotomized frequency of microaggressions variable was used to plot these differences (Figure 2a–2d). Latinx participants who experienced a higher frequency of microaggressions showed more blunted cortisol responses to the TSST (i.e., lower cortisol reactivity and recovery) compared to Latinx participants who experienced a lower frequency of microaggressions (see Figure 2a). Similarly, participants in the other ethnic backgrounds (i.e., biracial, African American, and Native American) group who experienced a higher frequency of microaggressions showed more blunted cortisol responses compared to other ethnic background participants who experienced a lower frequency of microaggressions (see Figure 2d). In contrast, Asian American and non-Hispanic White young adults did not show any significant differences in cortisol responses to the TSST regardless of whether they experienced a higher or lower frequency of microaggressions (see Figures 2b and 2c). No significant interaction was found between sensitivity to microaggressions and ethnicity, F(5, 103) = 1.29, p = .259, .
Figure 2.
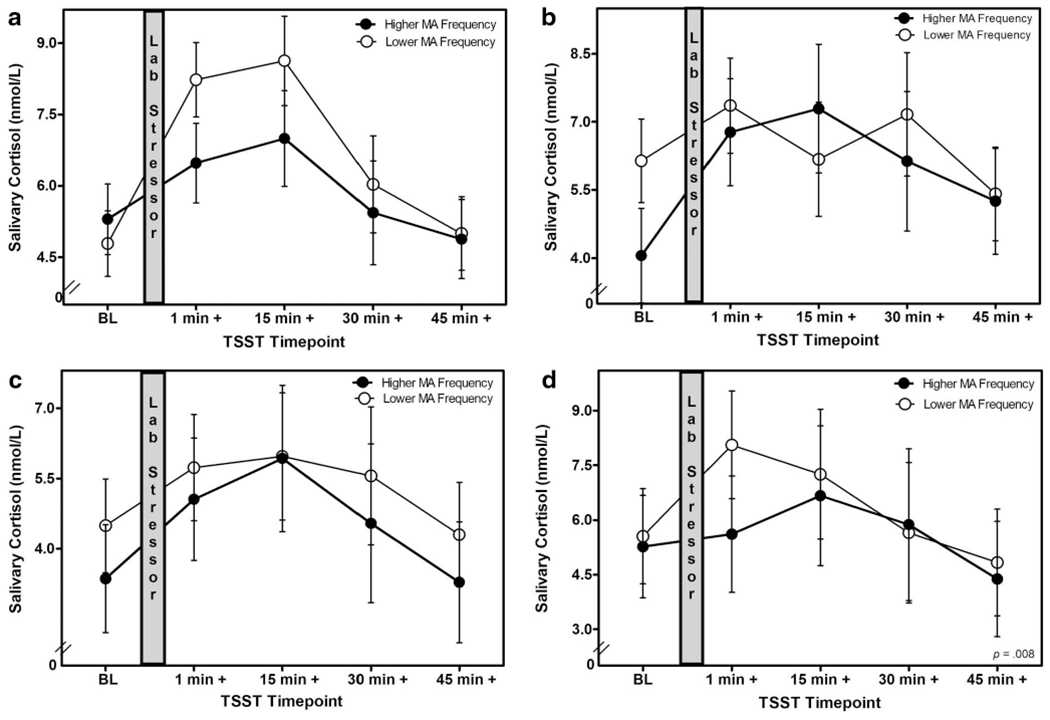
(a–d) Changes in salivary cortisol following a laboratory stressor by microaggression frequency and ethnicity. TSST = Trier Social Stress Test; MA = Microaggressions.
Discussion
Race-related stressors remain a significant challenge for ethnic minorities in the United States. Ethnic discrimination is associated with a host of deleterious health outcomes across different ethnic and age groups including increased risk of chronic conditions (Molina & Simon, 2014), cardiovascular disease (Chae, Lincoln, Adler, & Syme, 2010), substance use (Borrell et al., 2010), poor sleep (Slopen & Williams, 2014), and pain (Burgess et al., 2009). Similarly, ethnic microaggressions, a subtle type of discrimination, have been found to have negative associations with physical and mental health (Huynh, 2012; Slaughter-Acey et al., 2016; Torres et al., 2010; Torres & Taknint, 2015). Pascoe and Smart Richman (2009) suggested that chronic, heightened cardiovascular and cortisol responses to ethnic stressors could contribute to the occurrence of negative health outcomes by increasing allostatic load. In light of this, a growing body of research has linked ethnic discrimination to biological measures of stress (i.e., salivary cortisol; Adam et al., 2015; Busse et al., 2017; Huynh, Guan, Almeida, McCreath, & Fuligni, 2016; Kim, Zhang, Zeiders, Sim, & Gleason, 2018; Lapp, Ahmed, Moore, & Hunter, 2019; Zeiders, Doane, & Roosa, 2012). However, fewer studies have examined the biological toll of ethnic microaggressions and whether there are ethnic differences in these relationships. Thus, the current study sought to examine whether the frequency with which young adults experience ethnic microaggressions and their sensitivity to these microaggressions (i.e., whether they were bothered by those experiences) were related to salivary cortisol responses to an acute stressor (TSST), and to explore whether these associations differed between ethnic groups.
Results indicated that individuals who experienced ethnic microaggressions more frequently and those who were more sensitive to (i.e., bothered by) those experiences showed a blunted cortisol response (i.e., lower cortisol reactivity and recovery) to the TSST. These results are contrary to those of previous studies that found racial stressors to be associated with increased cortisol reactivity following acute laboratory-based stressors. Lucas and colleagues (2017), for instance, found perceived discrimination to be associated with increased cortisol levels throughout the TSST among African American adults. Similarly, Busse et al. (2017) found experiences of discrimination to be related to greater cortisol increases in response to the TSST among a sample of ethnically diverse adults. Lastly, Lapp and colleagues (2019) found lifetime ethnic discrimination to be associated with elevated baseline cortisol levels among a community sample that was exposed to the TSST.
Our findings suggest that overt ethnic discrimination versus ethnic microaggressions may differentially impact activation of the HPA axis. Whereas instances of ethnic discrimination involve major events in which a person was purposefully made to feel inferior, treated unfairly, harassed, or insulted due to their race (Krieger, Smith, Naishadham, Hartman, & Barbeau, 2005; Williams, Yan, Jackson, & Anderson, 1997), experiences of ethnic microaggressions involve daily interactions in which racial prejudice or bias may be communicated unbeknownst to the perpetrator or is more covert and nebulous (Sue et al., 2007), which can make microaggressions more chronic rather than acute stressors. This is a key distinction that could explain the differences in cortisol reactivity to the TSST in our sample compared to those of other studies. As posited by the weathering hypothesis (Geronimus, 2013), chronic stressors result in physiological changes to the systems involved in the stress response (e.g., cardiovascular, HPA axis). Over time and because of these stressors, these systems deteriorate and fail to respond adequately, which can result in blunted cortisol responses. According to Adam, Klimes-Dougan, and Gunnar (2007), the frequency of exposure to stressors and the cumulative history of these stressors are factors that can result in activation and dysregulation of the stress systems. Our study documented that those who encountered microaggressions more frequently than their peers had a blunted cortisol response to the TSST despite the relatively low frequency of microaggressions in our sample, potentially pointing to the cumulative effects of microaggressions encountered over the life span (Sue et al., 2008).
Adam et al. (2007) also purported that the cognitive and emotional appraisal of those stressors can alter cortisol outcomes. A study by Campos and colleagues (2014) that examined the cortisol responses of individuals who tend to experience negative emotions or easily get upset found this group to have blunted cortisol responses to the TSST. In our study, young adults who reported being bothered by their experiences of microaggressions had more blunted cortisol responses to the TSST compared to those who were not bothered by such experiences. These results provide support for Adam and colleagues’ (2007) postulate that the negative appraisal of the events itself and the subsequent subjective distress seem to be related to blunted cortisol responses.
Upon further examining how frequency of microaggressions interacted with ethnicity, we found that young adults’ salivary cortisol patterns differed by ethnicity. There were no significant differences in cortisol patterns among Asian American and non-Hispanic White young adults regardless of how frequently they experienced microaggressions. However, young adults of Latinx and other ethnic backgrounds (i.e., biracial, African American, Native American) who experienced more frequent microaggressions exhibited a blunted cortisol pattern relative to their peers who encountered microaggressions less frequently. To our knowledge, only one other study examined the impact of a racial stressor (i.e., discrimination) on cortisol responses to the TSST by ethnicity (Busse et al., 2017). They found that Latinx individuals who reported experiencing higher levels of discrimination had higher cortisol reactivity to the TSST compared to non-Latinxs.
Although Busse and colleagues (2017) examined overt lifetime discrimination rather than microaggressions, both their findings and ours highlight that Latinxs’ biological responses are differentially affected by racial stressors compared to those of other ethnic groups, which could stem from the multitude of social, health, and economic disadvantages that ethnic minorities encounter (Nazroo & Williams, 2006; Williams & Jackson, 2005). Interestingly, Busse and colleagues’ (2017) study documented relatively low levels of lifetime discrimination much like our study found low frequency of experiences of microaggressions. The frequency reported by individuals in our sample is comparable to that reported in previous studies conducted with ethnic minority and non-Hispanic White individuals in demographically diverse and homogeneous settings (e.g., Huynh, 2012; Torres et al., 2010; Torres & Taknint, 2015; Torres-Harding, Andrade, & Romero Diaz, 2012). This may indicate that these experiences can have a cumulative and/or lasting effect on the biological systems of individuals who experience them even if they are not encountered frequently.
Notably, when we examined sensitivity to microaggressions across different ethnic groups, we did not find their salivary cortisol patterns to differ. It has been suggested that ethnic minorities may experience more negative outcomes following instances in which they perceive prejudice or hostility (Lilienfeld, 2017). However, our study found that both non-Hispanic White and ethnic minority individuals were similarly sensitive to microaggressions and that their cortisol patterns did not significantly differ based on how sensitive they were to such experiences. These results are consistent with a recent study by West (2019), which found that both ethnic minority and ethnic majority individuals were similarly impacted by microaggressions. West (2019), who studied how microaggressions related to affect and life satisfaction, suggested that it is the frequency with which ethnic minority individuals encounter ethnic microaggressions that explains their negative effects, not the sensitivity to these experiences. Our study partially supports this view, as a higher frequency of microaggressions was associated with more blunted cortisol responses, particularly for young adults of Latinx and other ethnic backgrounds, whereas higher sensitivity to microaggressions was associated with blunted cortisol across all ethnic groups.
Our results should be interpreted with caution given several limitations. First, our sample was mostly comprised of female undergraduate students in their first year of college at a public university. This is a population for whom ethnic and racial identity is salient in a variety of ways including greater exploration and deeper reflection about their identities (Umaña-Taylor et al., 2014), which can shape how they perceive experiences of ethnic microaggressions compared to older or younger populations. Regarding cortisol, some research suggests that cortisol patterns in response to a laboratory stressor do not significantly differ across age groups (Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004); however, future studies might still explore whether the experience of ethnic microaggressions affects cortisol patterns in different age groups. Second, our study relied on retrospective self-report data regarding experiences of ethnic microaggressions, which is subject to recall bias. Additionally, it is possible that our sample underreported their experiences with microaggressions, given that past studies have shown that experiences of racial stressors tend to be reported less due to the psychological consequences of identifying oneself as a victim of discrimination (Leaper & Brown, 2008).
Lastly, due to small subgroup sample sizes, biracial, African American, and Native American participants were grouped together (named ‘Other Ethnicity’ group). Given that the demographic makeup of our sample reflects the diversity within the student population of the university in which the study was conducted and may reflect that of other institutions, we chose to retain these groups to illuminate their experiences and retain statistical power in our analyses. However, we acknowledge that this group is heterogeneous and their experiences of microaggressions may vary, and their cortisol reactivity could be more nuanced. Additional studies examining cortisol patterns across these different ethnic groups are needed to better understand how microaggressions may differentially impact their cortisol responses, particularly given the medium to large effect sizes found in the current study for microaggression frequency and ethnicity on cortisol (Baguley, 2009). These limitations notwithstanding, our study contributes to the growing literature documenting the biological toll of race-related stressors by examining how experiences of ethnic microaggressions and sensitivity to such experiences is related to a biological marker of stress in response to a well-established laboratory protocol (i.e., TSST) among an ethnically diverse sample of young adults.
In sum, the results of this study underscore the role of ethnic microaggressions on cortisol responses, showing that individuals who experience ethnic microaggressions more frequently and who are more sensitive to these microaggressions exhibit blunted cortisol reactivity patterns. Consequently, blunted cortisol responses can result in dysregulation of biobehavioral systems including inflammation, metabolism, cognitive and memory functioning, and affect, which could lead to a number of deleterious health effects (Adam et al., 2007, 2017; Lovallo, 2011). Given the health implications of blunted cortisol responses to stress, additional studies are needed to build upon the results of this study by examining microaggressions, prospectively and longitudinally, to determine how they shape cortisol levels and health outcomes long term. Additionally, future research should examine experiences of ethnic discrimination and ethnic microaggressions concurrently using idiographic approaches to evaluate the extent to which they have an impact on salivary cortisol outcomes. Lastly, studies should strive to further understand the cognitive and emotional appraisal of experiences of ethnic microaggressions and examine whether factors such as rumination moderate the cortisol responses to these events.
Public Significance Statement.
College students of color report experiencing ethnic microaggressions which are associated with increased stress levels. The results of this study suggest that ethnic microaggressions impact cortisol, a biological marker of stress, which could be an important mechanism that explains the negative impact of microaggressions on health.
Acknowledgments
Research support was provided by grants from the National Institute of General Medical Sciences of the National Institutes of Health (Awards UL1GM118979, TL4GM118980, and RL5GM118978).
Contributor Information
Angelina Majeno, Department of Psychological Science, University of California, Irvine.
Guido G. Urizar, Jr., Department of Psychology, California State University, Long Beach
May Ling D. Halim, Department of Psychology, California State University, Long Beach.
Selena T. Nguyen-Rodriguez, Department of Health Science, California State University, Long Beach.
Araceli Gonzalez, Department of Psychology, California State University, Long Beach.
References
- Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, Ehrlich KB, … Eccles JS. (2015). Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: A 20-year prospective study. Psychoneuroendocrinology, 62, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Klimes-Dougan B, & Gunnar MR (2007). Social regulation of the adrenocortical response to stress in infants, children, and adolescents: Implications for psychopathology and education. In Coch D, Dawson G, & Fischer KW (Eds.), Human behavior, learning, and the developing brain: Atypical development (pp. 264–304). New York, NY: Guilford Press. [Google Scholar]
- Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, & Gilbert KE (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 83, 25–41. 10.1016/j.psyneuen.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Dockray S, Cryan JF, Dinan TG, & Clarke G (2017). The Trier Social Stress Test: Principles and practice. Neurobiology of Stress, 6, 113–126. 10.1016/j.ynstr.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J, Ali N, & Pruessner JC (2013). Reflections on the interaction of psychogenic stress systems in humans: The stress coherence/compensation model. Psychoneuroendocrinology, 38, 947–961. 10.1016/j.psyneuen.2013.02.010 [DOI] [PubMed] [Google Scholar]
- Baguley T (2009). Standardized or simple effect size: What should be reported? British Journal of Psychology, 100(Part 3), 603–617. 10.1348/000712608X377117 [DOI] [PubMed] [Google Scholar]
- Blume AW, Lovato LV, Thyken BN, & Denny N (2012). The relationship of microaggressions with alcohol use and anxiety among ethnic minority college students in a historically White institution. Cultural Diversity and Ethnic Minority Psychology, 18, 45–54. 10.1037/a0025457 [DOI] [PubMed] [Google Scholar]
- Borrell LN, Diez Roux AV, Jacobs DR Jr., Shea S, Jackson SA, Shrager S, & Blumenthal RS (2010). Perceived racial/ethnic discrimination, smoking and alcohol consumption in the Multi-Ethnic Study of Atherosclerosis (MESA). Preventive Medicine: An International Journal Devoted to Practice and Theory, 51, 307–312. 10.1016/j.ypmed.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess DJ, Grill J, Noorbaloochi S, Griffin JM, Ricards J, van Ryn M, & Partin MR (2009). The effect of perceived racial discrimination on bodily pain among older African American men. Pain Medicine, 10, 1341–1352. 10.1111/j.1526-4637.2009.00742.x [DOI] [PubMed] [Google Scholar]
- Busse D, Yim IS, & Campos B (2017). Social context matters: Ethnicity, discrimination and stress reactivity. Psychoneuroendocrinology, 83, 187–193. 10.1016/j.psyneuen.2017.05.025 [DOI] [PubMed] [Google Scholar]
- Busse D, Yim IS, Campos B, & Marshburn CK (2017). Discrimination and the HPA axis: Current evidence and future directions. Journal of Behavioral Medicine, 40, 539–552. 10.1007/s10865-017-9830-6 [DOI] [PubMed] [Google Scholar]
- Byrd MC (2018). Microaggressions self-defense: A role-playing workshop for responding to microaggressions. Social Sciences, 7, 96. 10.3390/socsci7060096 [DOI] [Google Scholar]
- Campisi J, Bravo Y, Cole J, & Gobeil K (2012). Acute psychosocial stress differentially influences salivary endocrine and immune measures in undergraduate students. Physiology & Behavior, 107, 317–321. 10.1016/j.physbeh.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Campos B, Busse D, Yim IS, Dayan A, Chevez L, & Schoebi D (2014). Are the costs of neuroticism inevitable? Evidence of attenuated effects in U.S. Latinas. Cultural Diversity and Ethnic Minority Psychology, 20, 430–440. 10.1037/a0035329 [DOI] [PubMed] [Google Scholar]
- Casanova S, McGuire K, & Martin M (2018). “Why you throwing subs?”: An exploration of community college students’ immediate responses to microaggressions. Teachers College Record, 120, 1–48 [Google Scholar]
- Chae DH, Lincoln KD, Adler NE, & Syme SL (2010). Do experiences of racial discrimination predict cardiovascular disease among African American men? The moderating role of internalized negative racial group attitudes. Social Science & Medicine, 71, 1182–1188. 10.1016/j.socscimed.2010.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong RY, Uhart M, McCaul ME, Johnson E, & Wand GS (2008). Whites have a more robust hypothalamic-pituitary-adrenal axis response to a psychological stressor than blacks. Psychoneuroendocrinology, 33, 246–254. 10.1016/j.psyneuen.2007.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker J, Voelkl K, Testa M, & Major B (1991). Social stigma: The affective consequences of attributional ambiguity. Journal of Personal-ityand Social Psychology, 60, 218–228. 10.1037/0022-3514.60.2.218 [DOI] [PubMed] [Google Scholar]
- Epel ES (2009). Psychological and metabolic stress: A recipe for accelerated cellular aging? Hormones, 8, 7–22. 10.14310/horm.2002.1217 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, & Lang A-G (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41, 1149–1160. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- Forrest-Bank SS, & Jenson JM (2015). The relationship among childhood risk and protective factors, racial microaggression and ethnic identity, and academic self-efficacy and antisocial behavior in young adulthood. Children and Youth Services Review, 50, 64–74. 10.1016/j.childyouth.2015.01.005 [DOI] [Google Scholar]
- Geronimus AT (2013). Deep integration: Letting the epigenome out of the bottle without losing sight of the structural origins of population health. American Journal of Public Health, 103, S56–S63. 10.2105/AJPH.2013.301380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, & Whembolua GL (2007). Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology & Behavior, 92, 583–590. 10.1016/j.physbeh.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Hansen ÅM, Garde AH, & Persson R (2008). Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: A review. Scandinavian Journal of Clinical and Laboratory Investigation, 68, 448–458. 10.1080/00365510701819127 [DOI] [PubMed] [Google Scholar]
- Harrell SP (2000). A multidimensional conceptualization of racism-related stress: Implications for the well-being of people of color. American Journal of Orthopsychiatry, 70, 42–57. 10.1037/h0087722 [DOI] [PubMed] [Google Scholar]
- Hostinar CE, McQuillan MT, Mirous HJ, Grant KE, & Adam EK (2014). Cortisol responses to a group public speaking task for adolescents: Variations by age, gender, and race. Psychoneuroendocrinology, 50, 155–166. 10.1016/j.psyneuen.2014.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, & Worthman CM (2005). Estimating between- and within-individual variation in cortisol levels using multi-level models. Psychoneuroendocrinology, 30, 698–714. 10.1016/j.psyneuen.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Huynh VW (2012). Ethnic microaggressions and the depressive and somatic symptoms of Latino and Asian American adolescents. Journal of Youth and Adolescence, 41, 831–846. 10.1007/s10964-012-9756-9 [DOI] [PubMed] [Google Scholar]
- Huynh VW, Guan SA, Almeida DM, McCreath H, & Fuligni AJ (2016). Everyday discrimination and diurnal cortisol during adolescence. Hormones and Behavior, 80, 76–81. 10.1016/j.yhbeh.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh VW, Huynh QL, & Stein MP (2017). Not just sticks and stones: Indirect ethnic discrimination leads to greater physiological reactivity. Cultural Diversity and Ethnic Minority Psychology, 23, 425–434. 10.1037/cdp0000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews, 35, 2–16. 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Kaiser CR, Major B, & McCoy SK (2004). Expectations about the future and the emotional consequences of perceiving prejudice. Personality and Social Psychology Bulletin, 30, 173–184. 10.1177/0146167203259927 [DOI] [PubMed] [Google Scholar]
- Kim SY, Zhang M, Zeiders KH, Sim L, & Gleason MEJ (2018). Acute salivary cortisol response among Mexican American adolescents in immigrant families. Cultural Diversity and Ethnic Minority Psychology, 24, 510–520. 10.1037/cdp0000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The ‘Trier Social Stress Test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76–81. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Krieger N, Smith K, Naishadham D, Hartman C, & Barbeau EM (2005). Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Social Science & Medicine, 61, 1576–1596. 10.1016/j.socscimed.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, & Kirsch-baum C (2004). HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology, 29, 83–98. 10.1016/S0306-4530(02)00146-4 [DOI] [PubMed] [Google Scholar]
- Lapp HE, Ahmed S, Moore CL, & Hunter RG (2019). Toxic stress history and hypothalamic-pituitary-adrenal axis function in a social stress task: Genetic and epigenetic factors. Neurotoxicology and Teratology, 71, 41–49. 10.1016/j.ntt.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Leaper C, & Brown CS (2008). Perceived experiences with sexism among adolescent girls. Child Development, 79, 685–704. 10.1111/j.1467-8624.2008.01151.x [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO (2017). Microaggressions. Perspectives on Psychological Science, 12, 138–169. 10.1177/1745691616659391 [DOI] [PubMed] [Google Scholar]
- Liu JJW, Ein N, Peck K, Huang V, Pruessner JC, & Vickers K (2017). Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): A meta-analysis. Psychoneuroendocrinology, 82, 26–37. 10.1016/j.psyneuen.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Lovallo WR (2011). Do low levels of stress reactivity signal poor states of health? Biological Psychology, 86, 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Wegner R, Pierce J, Lumley MA, Laurent HK, & Granger DA (2017). Perceived discrimination, racial identity, and multisystem stress response to social evaluative threat among African American men and women. Psychosomatic Medicine, 79, 293–305. 10.1097/PSY.0000000000000406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major B, Mendes WB, & Dovidio JF (2013). Intergroup relations and health disparities: A social psychological perspective. Health Psychology, 32, 514–524. 10.1037/a0030358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe J (2009). Racial and gender microaggressions on a predominantly-White campus: Experiences of Black, Latina/o and White undergraduates. Race, Gender, & Class, 16, 133–151. [Google Scholar]
- McEwen BS (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology, 583, 174–185. 10.1016/j.ejphar.2007.11.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2018). Redefining neuroendocrinology: Epigenetics of brain-body communication over the life course. Frontiers in Neuroendocrinology, 49, 8–30. 10.1016/j.yfrne.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Molina KM, & Simon Y (2014). Everyday discrimination and chronic health conditions among Latinos: The moderating role of socioeconomic position. Journal of Behavioral Medicine, 37, 868–880. 10.1007/s10865-013-9547-0 [DOI] [PubMed] [Google Scholar]
- Nadal K, Griffin KE, Wong Y, Davidoff KC, & Davis LS (2017). The injurious relationship between racial microaggressions and physical health: Implications for social work. Journal of Ethnic & Cultural Diversity in Social Work: Innovation in Theory, Research & Practice, 26, 6–17. 10.1080/15313204.2016.1263813 [DOI] [Google Scholar]
- Nazroo JY, & Williams DR (2006). The social determination of ethnic/racial inequalities in health. In Marmot MG & Wilkinson RG (Eds.), Social determinants of health (pp. 238–266). New York, NY: Oxford University Press. [Google Scholar]
- Pascoe EA, & Smart Richman L (2009). Perceived discrimination and health: A meta-analytic review. Psychological Bulletin, 135, 531–554. 10.1037/a0016059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pro G, Sahker E, & Marzell M (2018). Microaggressions and marijuana use among college students. Journal ofEthnicity in Substance Abuse, 17, 375–387. 10.1080/15332640.2017.1288191 [DOI] [PubMed] [Google Scholar]
- Richman LS, & Jonassaint C (2008). The effects of race-related stress on cortisol reactivity in the laboratory: Implications of the Duke lacrosse scandal. Annals of Behavioral Medicine, 35, 105–110. 10.1007/s12160-007-9013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter-Acey JC, Sealy-Jefferson S, Helmkamp L, Caldwell CH, Osypuk TL, Platt RW, … Misra DP. (2016). Racism in the form of micro aggressions and the risk of preterm birth among black women. Annals of Epidemiology, 26, 7–13.e1. 10.1016/j.annepidem.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, & Williams DR (2014). Discrimination, other psychosocial stressors, and self-reported sleep duration and difficulties. Sleep: Journal of Sleep and Sleep Disorders Research, 37, 147–156. 10.5665/sleep.3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Orozco C, Casanova S, Martin M, Katsiaficas D, Cuellar V, Smith NA, & Dias SI (2015). Toxic rain in class: Classroom interpersonal microaggressions. Educational Researcher, 44, 151–160. 10.3102/0013189X15580314 [DOI] [Google Scholar]
- Sue DW, Capodilupo CM, Nadal KL, & Torino GC (2008). Racial microaggressions and the power to define reality. American Psychologist, 63, 277–279. 10.1037/0003-066X.63.4.277 [DOI] [Google Scholar]
- Sue DW, Capodilupo CM, Torino GC, Bucceri JM, Holder AMB, Nadal KL, & Esquilin M (2007). Racial microaggressions in everyday life: Implications for clinical practice. American Psychologist, 62, 271–286. 10.1037/0003-066X.62.4.271 [DOI] [PubMed] [Google Scholar]
- Torres L, Driscoll MW, & Burrow AL (2010). Racial microaggressions and psychological functioning among highly achieving African-Americans: A mixed-methods approach. Journal of Social and Clinical Psychology, 29, 1074–1099. 10.1521/jscp.2010.29.10.1074 [DOI] [Google Scholar]
- Torres L, & Taknint JT (2015). Ethnic microaggressions, traumatic stress symptoms, and Latino depression: A moderated mediational model. Journal of Counseling Psychology, 62, 393–401. 10.1037/cou0000077 [DOI] [PubMed] [Google Scholar]
- Torres-Harding SR, Andrade AL Jr., & Romero Diaz CE (2012). The Racial Microaggressions Scale (RMAS): A new scale to measure experiences of racial microaggressions in people of color. Cultural Diversity and Ethnic Minority Psychology, 18, 153–164. 10.1037/a0027658 [DOI] [PubMed] [Google Scholar]
- Umaña-Taylor AJ, Quintana SM, Lee RM, Cross WE Jr., Rivas-Drake D, Schwartz SJ, … the Ethnic and Racial Identity in the 21st Century Study Group. (2014). Ethnic and racial identity during adolescence and into young adulthood: An integrated conceptualization. Child Development, 85, 21–39. 10.1111/cdev.12196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urizar GG, Yim IS, Kofman YB, Tilka N, Miller K, Freche R, & Johnson A (2019). Ethnic differences in stress-induced cortisol responses: Increased risk for depression during pregnancy. Biological Psychology, 147, 107630. 10.1016/j.biopsycho.2018.12.005 [DOI] [PubMed] [Google Scholar]
- Wang J, Leu J, & Shoda Y (2011). When the seemingly innocuous “stings”: Racial microaggressions and their emotional consequences. Personality and Social Psychology Bulletin, 37, 1666–1678. 10.1177/0146167211416130 [DOI] [PubMed] [Google Scholar]
- West K (2019). Testing hypersensitive responses: Ethnic minorities are not more sensitive to microaggressions, they just experience them more frequently. Personality and Social Psychology Bulletin, 45, 1619–1632. 10.1177/0146167219838790 [DOI] [PubMed] [Google Scholar]
- Williams DR, & Jackson PB (2005). Social sources of racial disparities in health. Health Affairs, 24, 325–334. 10.1377/hlthaff.24.2.325 [DOI] [PubMed] [Google Scholar]
- Williams DR, Yan Y, Jackson JS, & Anderson NB (1997). Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of Health Psychology, 2, 335–351. 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- Yim IS, Quas JA, Cahill L, & Hayakawa CM (2010). Children’s and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrinology, 35, 241–248. 10.1016/j.psyneuen.2009.06.014 [DOI] [PubMed] [Google Scholar]
- Zeiders KH, Doane LD, & Roosa MW (2012). Perceived discrimination and diurnal cortisol: Examining relations among Mexican American adolescents. Hormones and Behavior, 61, 541–548. 10.1016/j.yhbeh.2012.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiders KH, Landor AM, Flores M, & Brown A (2018). Microaggressions and diurnal cortisol: Examining within-person associations among African-American and Latino young adults. Journal of Adolescent Health, 63, 482–488. 10.1016/j.jadohealth.2018.04.018 [DOI] [PubMed] [Google Scholar]


