In this review, Choi et al. compare the spontaneous activity of the developing visual system between mammals and Drosophila and suggest that Drosophila is an emerging model for mechanistic and functional studies of correlated spontaneous activity.
Keywords: Drosophila, gap junctions, neural development, spontaneous activity, vertebrates versus invertebrates, visual system, wave of neural activity
Abstract
During the development of the vertebrate nervous systems, genetic programs assemble an immature circuit that is subsequently refined by neuronal activity evoked by external stimuli. However, prior to sensory experience, the intrinsic property of the developing nervous system also triggers correlated network-level neuronal activity, with retinal waves in the developing vertebrate retina being the best documented example. Spontaneous activity has also been found in the visual system of Drosophila. Here, we compare the spontaneous activity of the developing visual system between mammalian and Drosophila and suggest that Drosophila is an emerging model for mechanistic and functional studies of correlated spontaneous activity.
How to build a functional nervous system is one of the most fundamental questions in developmental neuroscience. Establishing neuronal circuits that robustly respond to the external environment involves numerous challenging tasks, including the production of a diversity of neurons in the correct number, their assembly into circuits, and their maturation into functional neurons (Sporns et al. 2004; Sanes and Zipursky 2010; Perry et al. 2017; Price et al. 2018). Vertebrate circuits form through the interplay of genetic programs and environmental experience: Genetic programs assemble a hard-wired and immature neuronal network at early developmental stages, starting with neurogenesis, neuronal specification, axonal guidance, and synaptogenesis. During synaptogenesis, neuronal activity across neuronal networks shapes and refines the circuit to a more mature form that is optimized for various external sensory stimuli (Penn 2001; Ganguly and Poo 2013).
Importantly, this network-level neuronal activity can be triggered not only by sensory experience but also by the intrinsic property of the developing nervous system. Prior to sensory stimuli, this early experience-independent, “spontaneous” neuronal activity initially occurs sporadically in individual cells but later becomes correlated in larger neuronal populations. Multiple studies in vertebrates have found this type of spontaneously emerging correlated activity in many developing neural tissues such as the retina, visual cortex, spinal cord, auditory cortex, cerebellum, and hippocampus, suggesting a role in proper circuit maturation and refinement (Katz and Shatz 1996; Friauf and Lohmann 1999; Zhang and Poo 2001; Moody and Bosma 2005; Huberman et al. 2008; Blankenship and Feller 2010; Kirkby et al. 2013; Wang and Bergles 2015; Arroyo and Feller 2016). This indicates that sensory stimulus-independent neuronal activity that is built in the genetically determined neuronal circuitry mediates an important developmental process. After the onset of sensory stimuli, the circuits are further optimized by experience-dependent neuronal activity (Hubel and Wiesel 1970; Ganguly and Poo 2013).
While correlated spontaneous activity has been found in most vertebrates, it was also recently discovered in invertebrates in the developing Drosophila visual system (Akin et al. 2019). It is widely believed that the invertebrate brain mostly forms in a highly stereotyped manner, leaving little space for experience-dependent development (Jefferis et al. 2001; Hiesinger et al. 2006). Naturally, it was also assumed that neuronal activity had a minimum role in building the simple brain of invertebrates. However, the new findings suggest that spontaneously emerging correlated neuronal activity could have a developmental function in the invertebrate nervous system.
We describe below the most extensively studied form of correlated spontaneous activity, the retinal waves in the developing mammalian visual system. We provide a general overview of the known cellular and molecular mechanisms of these retinal waves at different stages and present recent findings of how correlated spontaneous activity regulates circuit connectivity. Then, we focus on the recently discovered correlated spontaneous activity in the developing Drosophila visual system. We propose that these phenomena depend on neuronal features that are conceptually similar between vertebrates and invertebrates, opening up exciting avenues for a mechanistic understanding of correlated network activity. Finally, we illustrate the use of Drosophila to investigate how this spontaneously emerging neuronal activity contributes to the pattern refinement of a “hard-wired” brain.
Correlated spontaneous activity in the developing mammalian visual system
Correlated network activity is generally tightly coupled with sensory-input and/or motor output. It is an important feature of a mature nervous system (Draguhn et al. 2014; Yuste 2015). In contrast, the significance of the correlated network activity spontaneously occurring early in developing neuronal circuits has remained unclear (Hamburger and Balaban 1963; O'Donovan 1999; Kirkby et al. 2013). The best-documented example of such a developmental phenomenon comes from the vertebrate visual system (Huberman et al. 2008; Blankenship and Feller 2010; Kirkby et al. 2013). In ex vivo preparation of developing rabbit retina, spontaneous bursts of action potential in individual retinal ganglion cells (RGCs) were observed early, before the retina can respond to visual stimuli (Masland 1977). In the early 1990s, Shatz's group (Meister et al. 1991) discovered the correlated nature of the spontaneous activity in ex vivo preparation of developing ferret retina, taking advantage of the development of multiple electrode arrays that can measure the neural activity of ∼100 neurons simultaneously. This correlated spontaneous activity, known as “retinal waves,” propagates as a wave-like form where neighboring RGCs exhibit correlated bursts of action potentials periodically across the ganglion cell layers of the retina (Wong 1999). Subsequently, using calcium influxes as a proxy for neuronal activity, both ex vivo and in vivo calcium imaging allowed the characterization of these waves at single-cell resolution (Wong et al. 1995). Subsequent pharmacological and genetic manipulations elucidated some of the underlying molecular and cellular mechanisms of retinal waves as well as their developmental role in the refinement of the visual system circuitry (Blankenship and Feller 2010; Kirkby et al. 2013; Arroyo and Feller 2016).
The stage II and III retinal waves also drive network activity over large domains of higher brain regions along the visual pathway, such as the lateral geniculate nucleus (dLGN) of the thalamus, the superior colliculus (SC), and the primary visual cortex (V1) areas (Hanganu et al. 2006; Ackman et al. 2012; Gribizis et al. 2019). However, correlated activity along the visual pathway can also have a central origin at later stages (Weliky and Katz 1999; Chiu and Weliky 2002; Gribizis et al. 2019). The existence of early neuronal activity correlated between different parts of developing neuronal circuits suggests that the retinal waves have a role not just for refining local retinal circuitry but also for organizing the visual pathway at the global level prior to the onset of visual stimulation (Weliky and Katz 1999; Ackman and Crair 2014; Golding et al. 2014; Arroyo and Feller 2016; Gribizis et al. 2019).
Cellular and molecular mechanisms underlying the generation of retinal waves
Retinal waves appear to mainly propagate between RGCs, but there are distinct circuits and cellular players underlying retinal waves at different developmental stages (Fig. 1). Based on the molecular and cellular mechanisms of depolarization of RGCs, three different stages of retinal waves have been defined in mammals: Embryonic gap junction-mediated waves occur before birth (stage I), waves mediated by cholinergic synaptic transmission occur around the first postnatal week (stage II), and waves mediated by glutamatergic synaptic transmission occur around the second postnatal week, before eye opening (stage III) (Blankenship and Feller 2010; Kirkby et al. 2013; Kerschensteiner 2014; Arroyo and Feller 2016).
Figure 1.
Transitions between mammalian retinal waves during development. Schematic diagram of the inner plexiform layer (IPL) in the mammalian retina. The developmental stages are based on mice. (A) During stage I, gap junctions mediate wave propagation between RGCs, where waves are thought to be initiated cell-autonomously. (B) During stage II, SAC circuits initiate the waves cell-autonomously and mediate their propagation when SACs activate both neighboring SACs and RGCs via cholinergic synaptic transmission. (C) Stage III waves are initiated either from photoreceptors stimulated by light through the closed eyelids, or cell-autonomously from cBCs. The waves propagate between cBCs via extrasynaptic spillover of glutamate that comes from the synaptic transmission between cBCs and RGCs. Gap junction coupling between cBCs further mediates lateral propagation of waves. It also mediates the inhibitory input from On cBC to Off cBC via inhibitory AC. The On sublayer is colored in yellow and the Off sublayer is in gray. (RGC) Retinal ganglion cell, (SAC) starburst amacrine cell, (AC) amacrine cell, (cBC) cone bipolar cell.
Stage I embryonic waves
The first stage of retinal waves occurs at the embryonic stage (approximately E17–P0 in mice) and lasts until birth, when the next stage of waves initiates. The onset of stage I waves coincides with the early development of RGC populations, including their neurogenesis, axonal guidance, and axonal arborization, but occurs prior to synaptic connection forming in the inner plexiform layer (IPL), where the major dendritic processes of RGC are located (Fig. 1A; Sernagor et al. 2001; D'Souza and Lang 2020). At this early stage of the mammalian retina, RGCs are coupled by gap junction channels like neurons in other brain areas (Penn et al. 1994; Singer et al. 2001; Montoro and Yuste 2004; Bloomfield and Völgyi 2009). Pharmacological experiments in rabbits and chicks have supported the model that stage I waves are mainly mediated by gap junction-dependent intercellular interactions (Catsicas et al. 1998; Wong et al. 1998; Syed et al. 2004). However, because monitoring this initial stage of waves in prenatal animals is more difficult, the underlying mechanisms of initiation and propagation of stage I waves between developing RGCs are significantly understudied compared with later stages (Huberman et al. 2008).
In the rabbit retina, where stage I embryonic waves have been most studied, the first waves are observed as soon as RGCs acquire the ability to generate action potentials and their axons reach their target area in the dLGN (Crabtree 1990; Syed et al. 2004). The pharmacological blockage of most neurotransmitter receptors, including acetylcholine, ionotropic glutamate, GABAA/C, and glycine receptors, does not affect the propagation of stage I embryonic waves (Syed et al. 2004). Instead, these early retinal waves are inhibited by gap junction blockers (Fig. 1A; Syed et al. 2004). Interestingly, agonists for the GABAB receptor as well as antagonists of the adenosine receptor also disrupt stage I retinal waves (Syed et al. 2004), suggesting that multiple pathways can modulate this activity prior to synaptogenesis. However, a detailed underlying mechanism awaits to be defined. Embryonic waves in mice have been less studied, and thus, a systematic pharmacological and genetic characterization of this activity is still lacking. For example, although rabbit and chick studies suggest that mice embryonic waves are mediated by gap junction coupling between RGCs (Catsicas et al. 1998; Syed et al. 2004), direct measurement of these waves in prenatal mice retina upon the inhibition of gap junction channel has never been reported. However, treatment of embryonic mice retina by antagonists of nicotinic acetylcholine receptor (nAChR) partially blocks the embryonic waves (Bansal et al. 2000). Because of the lack of canonical synaptic connections in the IPL at this stage, the nAChR might have a modulatory role similar to the GABAB and adenosine receptors in the rabbit retina, but further studies will be required to confirm this model. Still, both rabbit and mice stage I embryonic waves have similar spatiotemporal properties, which are quite distinct from the waves at later stages as they are more than twice as fast and frequent and propagate to the entire retinal field without clear boundaries or refractory periods (Bansal et al. 2000; Syed et al. 2004).
Stage II cholinergic waves
The stage II cholinergic waves emerge around birth and last ∼10 postnatal days (P0–P10 in mice) until stage III waves take over during the second postnatal week. Stage II waves appear as the synaptic connections between RGCs and amacrine cells in the IPL become mature (Fig. 1B; Fan et al. 2016; Nguyen-Ba-Charvet and Rebsam 2020). The pharmacological properties of stage II waves are almost identical between mice and rabbits (Syed et al. 2004; Ford et al. 2012): They are mediated primarily through cholinergic (ACh) synaptic transmission as the application of antagonists of the nAChR and knockout mice of either the nAChR β2 subunit or ChAT (choline acetyltransferase) have significantly disrupted stage II retinal waves (Feller et al. 1996; Bansal et al. 2000; Stacy et al. 2005; Sun et al. 2008). Stage II cholinergic waves initiate in random places across the retina and propagate to a larger area, at a slower speed and lower frequency than stage I waves. Importantly, they propagate homogeneously to the entire retina area in a nonoverlapping manner with clear boundaries that are determined by a refractory period of ∼50 sec (Feller et al. 1997; Maccione et al. 2014). This nonoverlapping wave kinetic results in an even activation of the whole retina by the propagation of the waves (Feller et al. 1997).
The underlying circuit mechanism of stage II waves is well characterized. Starburst amacrine cells (SACs) in the inner nuclear layer and in the ganglion cell layer (Fig. 1B) are the main pacemakers that drive the initiation and propagation of the wave (Fig. 1B; Huberman et al. 2008; Balasubramanian and Gan 2014). SACs form homotypic synaptic connections with nearby SACs and heterotypic synaptic connections with RGCs. The SACs initiate cell-autonomous spontaneous firing that subsequently drives the activation of the neighboring SACs via cholinergic synaptic transmission, thereby laterally propagating waves across SAC circuits (Fig. 1B; Zheng et al. 2006; Huberman et al. 2008; Blankenship and Feller 2010). Along with this propagation across the SAC network, the SACs also activate their downstream RGCs via cholinergic transmission, allowing the synchronized firing of the neighboring RGC clusters (Fig. 1B). This SAC network governs the overall spatiotemporal structure of the waves with their refractory period (Zheng et al. 2006). Individual SACs cell-autonomously generate semiperiodic calcium spikes followed by after-hyperpolarizations (AHPs) and thus act as spontaneous oscillators. During waves, reciprocally excitatory interactions between these spontaneous oscillators generate SAC network activity, and subsequently, the wave pattern (Zheng et al. 2006; Blankenship and Feller 2010; Ford et al. 2013).
Stage III glutamatergic waves
Stage III glutamatergic waves emerge in the second postnatal week (around P10 in mice) and last for the following three to four postnatal days. The waves then disappear around the time of eye opening (approximately P15), regardless of visual experience (Demas et al. 2003; Kerschensteiner 2016). The onset of stage III waves coincides with the emergence of the bipolar cell (BC) circuit, as BCs are generated during stage II waves and begin to form synapses with upstream photoreceptors and with downstream RGCs (Fig. 1C; Fan et al. 2016; Nguyen-Ba-Charvet and Rebsam 2020). The mechanism by which the waves terminate around eye opening is still unknown (Demas et al. 2003; Kerschensteiner 2016). Like stage II, the pharmacological features of stage III waves are comparable between mice and rabbits (Syed et al. 2004; Kerschensteiner 2016). The hallmark of stage III waves is its underlying glutamatergic drive. In mice, glutamate receptor antagonists or mutants of vglut1 (the vesicular glutamate transporter) disrupt stage III waves after approximately P10, when the waves become insensitive to nAChR antagonists (Blankenship et al. 2009). During the transition between stage II and III waves, the cholinergic drive disappears as the SACs stop expressing nAChR, preventing cholinergic drive within the network (Zheng et al. 2004), while the glutamatergic drive increases as the synaptic connections of glutamatergic BCs gradually form and mature (Huberman et al. 2008; Kerschensteiner 2016).
In addition to the distinct neurotransmitters they use, stage III waves have dramatically different initiation and propagating features compared with previous stages (Fig. 1C). While stage II waves are triggered by the spontaneous firing of SACs, stage III waves can be triggered not only by the cell-autonomous firing of BCs (Zhang et al. 2016) but also by ambient light through the closed eyelids (Tiriac et al. 2018) via newly emerging photoreceptor connections at this stage (Fig. 1C). However, despite their distinct initiation mechanisms, the spatiotemporal properties of both light-triggered and spontaneously emerging waves are indistinguishable from each other, suggesting that they use a common underlying circuit mechanism (Tiriac et al. 2018). Thus, this visual stimulus through close eyelids increases the occurrence of glutamatergic waves (Tiriac et al. 2018). The spatiotemporal propagation of glutamatergic waves is faster, nonrandom, and more repetitive within a smaller area than stage II waves, although both waves have clear boundaries with a refractory period (Maccione et al. 2014).
In contrast to stage II waves, which indiscriminately propagate to the SACs and RGCs in the proximity of the initiation point via the SAC circuit, stage III waves occur preferentially between specific neuronal subtypes via the BC circuit (Fig. 1C). In the adult visual system, cone BCs relay signals from cone photoreceptors to RGCs, while rod BCs do not directly connect to RGCs. Cone BCs and their downstream RGCs are further categorized as On if they depolarize in response to light increments or Off if they hyperpolarize to light (Euler et al. 2014; Kerschensteiner 2016). Among these subtypes, action potential firing patterns during stage III waves are more synchronized within the population of On or of Off RGCs, but are anticorrelated between On and Off subtypes (Kerschensteiner and Wong 2008; Kerschensteiner 2016). This alternative firing pattern between On and Off RGCs originates from the BC network: Once BCs fire, either cell-autonomously or by light stimulus via photoreceptors, they subsequently activate RGCs using glutamatergic synaptic transmission (Fig. 1C). BC activation also propagates laterally across neighboring BCs via two nonsynaptic mechanisms (Fig. 1C): (1) Glutamate that is spilled over during synaptic transmission from On BC to On RGC diffuses extrasynaptically and activates the neighboring On BC subpopulation that expresses the ionotropic glutamate receptor (iGluR) (Blankenship et al. 2009; Akrouh and Kerschensteiner 2013; Firl et al. 2013; Rosa et al. 2015). The diffusion of glutamate is limited to the On sublayer of IPL by Müller glial cells (MGCs) that express the glutamate transporter (EAAT1) and prevent the cross-activation of Off BC (Akrouh and Kerschensteiner 2013). (2) Gap junction coupling also mediates lateral propagation of the waves from iGluR-positive On BCs to iGluR-negative On BCs as well as to adjacent inhibitory amacrine cells (Fig. 1C; Akrouh and Kerschensteiner 2013; Kerschensteiner 2016). While this lateral propagation of activity across the On-type BC circuit generates synchronized activation of On RGCs, gap junction-coupled GABAergic and glycinergic amacrine cells with the On BC circuit generate inhibitory inputs to the Off BC circuit (Fig. 1C). This hyperpolarizes Off BCs when On BCs and RGCs are depolarized. Once this inhibition of Off BCs is relieved after the On wave has passed, they can activate Off RGCs and generate a wave in the Off circuit (Matthews and Fuchs 2010; Kerschensteiner 2016). Thus, inhibitory amacrine cells are responsible for anticorrelated activation between On/Off pathways and shape the spatiotemporal properties of stage III waves.
Role of correlated spontaneous activity in the refinement of retinotopic maps
The developmental role of retinal waves is best characterized in the connectivity of RGCs, the main output neurons in the retina (Kirkby et al. 2013; Arroyo and Feller 2016). To appropriately process visual input, RGCs send projections to several main target regions in the brain, including the dLGN and SC (Dhande and Huberman 2014; Dhande et al. 2015; Guido 2018). Processing spatial information of visual input requires retinotopic axonal projections of RGCs, which refers to the topographic distribution of RGC axons receiving input from neighboring neurons in the retina projecting to neighboring locations of the dLGN and SC (Fig. 2). Another feature of the spatial arrangement of RGC axons in binocular animals is the eye-specific map: RGC axons show either ipsilateral or contralateral projections to dLGN and SC (Fig. 2; Dhande and Huberman 2014; Dhande et al. 2015; Guido 2018). Ipsilateral axons project to the center of the dLGN, while contralateral axons project to the surrounding regions. In mice, at P0, ipsilateral and contralateral axons are initially intermingled with each other in the dLGN. From P4 to P8, prior to the onset of visual input, they start to segregate in an eye-specific manner (Fig. 2). While these circuitries are eventually refined and matured by sensory input-dependent activity during the critical period (Hubel and Wiesel 1970; Katz 1999), the refinement of both retinotopic and eye-specific maps requires the retinal waves prior to visual input, making the developing retina a unique model to study the functional role of correlated spontaneous activity in shaping visual circuit development (Huberman et al. 2008; Blankenship and Feller 2010; Kirkby et al. 2013; Kerschensteiner 2014; Arroyo and Feller 2016).
Figure 2.
Development of retinotopic connection and eye-specific segregation of RGCs during stage II cholinergic waves. Schematic diagram of the mouse visual circuit. Yellow and blue represent each eye and the axonal processes of RGCs. The letters in the circle represent retinotopically separated RGCs and their axon arborization in the target areas. Both retinotopic map and eye-specific segregation are established between P4 and P8 during stage II cholinergic waves. (A) At the P4 stage, the precise retinotopic maps of RGCs in the lateral geniculate nucleus (dLGN) and superior colliculus (SC) areas are not established yet, and the axonal arborizations of RGCs from ipsilateral (thinner lines) and contralateral (thicker lines) projections are intermingled with each other in the dLGN and SC. (B) At the P8 stage, the retinotopic maps of RGCs are established, and the axonal arborizations from both eyes have become segregated depending on which eye they came from. (SC) Superior colliculus, (dLGN) lateral geniculate nucleus.
The topographic organization of RGC axons is determined both by cell surface repulsive guidance molecules (Ephrins and their Eph receptors) and by spontaneous activity. Members of the Eph and Ephrin families are expressed in complementary gradients in the retina (Eph) and the target brain regions (Ephrins) to regulate positional information in different axes: EphA and Ephrin-As are important for the nasal–temporal axis, and EphB and Ephrin-Bs for the dorso–ventral axis of the retina (McLaughlin et al. 2003a; McLaughlin and O'Leary 2005; O'Leary and McLaughlin 2005; Triplett and Feldheim 2012). These guidance molecules establish an early coarse map of RGC axonal projection.
The eye-specific segregation of the RGC projections is further refined by the retinal waves that appear to play an instructive (i.e., correlated patterns are important) role in driving map formation (Crair 1999; Chalupa 2009; Feller 2009), as supported by various pharmacological and genetic alterations of retinal activity or manipulations of specific spatiotemporal features of retinal waves (Chandrasekaran et al. 2005; Xu et al. 2011; Zhang et al. 2012; Burbridge et al. 2014; Arroyo and Feller 2016). In this regard, stage II cholinergic waves are the most studied, as they are temporally correlated with the stage of axonal refinement of RGCs (Fig. 2). Two features of retinal waves are thought to be important instructive signals. During stage II waves, the activity of neighboring RGCs is more synchronized than that of distant RGCs within the same retina, suggesting these patterns of local intraretinal waves convey retinotopic information (Eglen et al. 2003). On the contrary, the firing patterns of RGCs from different eyes are not synchronized, allowing eye-specific segregation. In nAChR β2 knocked-out mice, which inhibit stage II waves, both intraretinal and interretinal patterns of waves are severely disrupted, leading to disrupted retinotopic connection and eye-specific segregation of RGCs (Rossi et al. 2001; Muir-Robinson et al. 2002; McLaughlin et al. 2003b; Sun et al. 2008). To disrupt a specific pattern of waves, several genetic and optogenetic manipulations are used (Xu et al. 2011, 2015; Zhang et al. 2012). For example, in mice in which nAChR β2 is exclusively knocked out in the retina from early development, the amplitude of retinal waves is lowered with variable interwave intervals. The reduced activity limits the propagating distance of the waves, which are unable to trigger large-scale asynchronous activity between the two eyes. In contrast, the short-range intraretinal waves still promote locally synchronized activation of RGCs (Xu et al. 2015). As a consequence, the eye-specific segregation of RGC axons of these mutants is specifically defective, while retinotopic connectivity is normal (Xu et al. 2015). This model is further supported by optogenetics, where artificially synchronized activity between eyes induces defective eye-specific segregation, while induced anticorrelated activity between eyes not only preserves eye-specific segregation but also partially rescues the segregation defects of nAChR β2 knocked-out mice (Zhang et al. 2012). Altogether, the correlation of retinal waves instructs different features of visual maps, although the effects may differ between the target brain regions and species examined. However, the molecular and cellular signaling underlying retinal activity that drives the rearrangements of axons for map refinement remains incomplete (Kirkby et al. 2013; Arroyo and Feller 2016).
Correlated spontaneous activity during development in invertebrates
The invertebrate central nervous system (CNS) is believed to be built in a genetically deterministic and hard-wired manner (Jefferis et al. 2001; Scott et al. 2003; Berdnik et al. 2006; Hiesinger et al. 2006), although activity-dependent developmental plasticity of the Drosophila peripheral nervous system (PNS) has been extensively studied in the larval neuromuscular junction (Choi et al. 2014; Vonhoff and Keshishian 2017; Bai and Suzuki 2020). This notion is based on the observation that brain development in invertebrates is largely normal under several genetic manipulations that disrupt canonical mechanisms required for sensory input, neuronal activity, or synaptic transmission (Jefferis et al. 2001; Scott et al. 2003; Berdnik et al. 2006; Hiesinger et al. 2006). These earlier studies have focused on gross morphological phenotypes, without measuring neuronal activity during development, partially owing to the technical challenge of recording in the tiny invertebrate brain.
However, recent studies with more advanced tools suggest that neuronal activity is still required for building a larval motor and sensory CNS circuit in flies, prior to sensory input (Suster and Bate 2002; Giachello and Baines 2015; Akin and Zipursky 2020; Valdes-Aleman et al. 2021). For example, genetic tools with a higher temporal and spatial resolution have been developed for stage-specific activation or inhibition of neuronal activity with single cell type resolution and advanced in vivo imaging technology (Kleinlogel et al. 2011; Chen et al. 2013, 2014; Özel et al. 2015; Akin and Zipursky 2016; Simpson and Looger 2018). These observations raise the question of how neuronal activity actually occurs during development. More recently, the improvement of genetically encoded optical sensors and in vivo imaging platforms have allowed versatile functional imaging of neuronal activity at a population level in the developing invertebrate CNS (Chen et al. 2013; Özel et al. 2015; Akin and Zipursky 2016; Simpson and Looger 2018; Dana et al. 2019). This improved imaging platform has revealed the occurrence of correlated spontaneous activity in the developing invertebrate nervous system (Akin et al. 2019), reminiscent of vertebrates. We describe below correlated spontaneous activity in the developing Drosophila visual system and compare it with its vertebrate counterpart, the retinal waves.
Correlated spontaneous activity in the developing Drosophila visual system
Akin et al. (2019) recently discovered correlated spontaneous activity—called patterned, stimulus-independent neuronal activity (PSINA) in the developing Drosophila CNS—using cutting-edge intravital two-photon imaging of intact pupae and the genetically encoded calcium indicator GCaMP6 (Chen et al. 2013). They found robust and correlated neuronal activity throughout the developing pupal brain, starting ∼50 h after puparium formation (APF), the onset of synaptogenesis (Sanes and Zipursky 2010). Almost all neuronal populations participate in this highly structured and stereotypic network activity throughout the developing brain. In the visual system, they showed that the patterned activity has three distinct stages based on the temporal pattern: a periodic stage (55–65 h APF), a turbulent stage (from 70 h APF to eclosion), and a young adult stage (until they are 5 d old), when visual input-independent activity occurs alongside visual stimulus-evoked activity. This neuronal activity is sensitive to tetrodotoxin (a blocker of voltage-gated sodium channels) and is correlated with neurotransmitter (glutamate) release as shown with a genetically encoded optical sensor for glutamate (Dürst et al. 2019; Leopold et al. 2019). This suggests that this spontaneously emerging neuronal activity is mediated by action potential and neurotransmitter release like the adult visual stimulus-evoked activity.
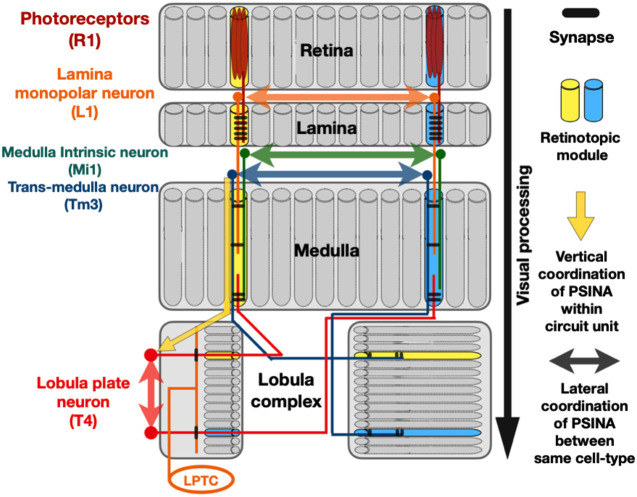
The investigators then focused on 15 different neuronal types from all distinct neuropiles (retina, lamina, medulla, and lobula complex) of the visual system and discovered that each cell type shows its own unique pattern of spontaneous activity (Fig. 3). The Drosophila retina, lamina, and medulla areas are equivalent structures to the vertebrate retinal circuit, while the lobula complex is comparable with the dLGN and SC areas (Sanes and Zipursky 2010). Importantly, two orthogonal axes of correlations have been found during PSINA in these areas (Fig. 3). The fly visual system is composed of ∼800 repeated topographically matched modules, where all 15 neuronal types tested are evenly distributed (Fig. 3). Although there is no direct synaptic connection between neurons of the same type in the adult, their spontaneous activity during PSINA is correlated. Interestingly, while these cell type-specific correlations are stereotypic and quantifiable, only some of them are qualitatively similar to the wave-like pattern seen in the vertebrate retina, suggesting diverse underlying propagating mechanisms.
Figure 3.
Retinotopically organized visual input channels in the Drosophila visual system. Schematic diagram of the fly optic lobe. Yellow and blue represent two retinotopically organized parallel and independent input channels that process visual information. Representative unicolumnar neuronal types from each neuropile and their connectivity are shown: photoreceptor neurons from the retina, a L1 lamina monopolar neuron from the lamina, a Tm3 trans-medulla neurons, an Mi1 medulla intrinsic neuron from the medulla, and a T4 lobula plate neuron from the lobula complex. During visual processing in the adult, visual information flows via synaptic connection down the vertical axis while preserving retinotopic information before the LPTCs. In contrast, during development, PSINA occurs between the same neuronal type, suggesting transient excitatory coupling (shown in double-headed arrows) along the lateral axis of the visual system. (LPTC) Lobula plate tangential cell, (PSINA) patterned, stimulus-independent neuronal activity.
Meanwhile, the pattern of activity between different cell types that are synaptically connected along the visual processing pathway in the adult is also more correlated (see below) (Fig. 3). Preventing synaptic transmission with genetically encoded tetanus toxin in the presynaptic cell population weakens this correlation, suggesting that this vertical correlation depends on functional synaptic coupling that must therefore already exist during synaptogenesis. Still, a potential specific developmental role has not yet been demonstrated.
Comparison of correlated spontaneous activity between vertebrates and invertebrates
Using simpler model systems allows a deeper understanding of complex biological phenomena (Venken et al. 2011; Ugur et al. 2016; Holguera and Desplan 2018; Zheng et al. 2018; Baenas and Wagner 2019; Mirzoyan et al. 2019). However, correlated spontaneous activity was until recently thought to be a unique feature of vertebrates. With the recently described PSINA in the Drosophila visual system, where circuit analogy can be made to the vertebrate system (Sanes and Zipursky 2010), and our deep knowledge about its development (Sanes and Zipursky 2010; Nériec and Desplan 2016; Perry et al. 2017; Holguera and Desplan 2018; Chen and Desplan 2020), it is now possible to compare this developmental process between vertebrate and invertebrate systems. There are intriguing conceptual similarities between the spontaneous activity in the mammalian and fly visual systems, suggesting that this phenomenon may be evolutionarily conserved. We discuss these similarities below and posit how a simpler genetic model system can answer the remaining outstanding questions in the field. Notably, while our comparison uses mammalian retinal waves and Drosophila PSINA in the visual system as specific examples, their properties are often generalizable to those seen with spontaneous emerging patterned activity in other nervous systems (Blankenship and Feller 2010; Kirkby et al. 2013).
Correlated activity in developmentally transient neuronal circuits
The correlation of neuronal stimulus-induced activity in adult circuits is different from the correlation of spontaneous activity during development (Blankenship and Feller 2010). In the mammalian visual system, the adult circuit is organized as iterative structures in a retinotopic manner (Arcaro et al. 2009; Arcaro and Livingstone 2017; Seabrook et al. 2017). Thus, neuronal activity evoked by sensory input propagates to higher brain areas through multiple parallel channels, while lateral excitatory synaptic transmission between these channels is limited in order to preserve spatial information of visual cues (Wässle 2004; Demb and Singer 2015). In contrast, retinal waves in the developing visual system propagate laterally between the same cell types (RGCs), therefore activating RGC clusters covering very large visual receptive fields (Blankenship and Feller 2010). This excitatory lateral coupling is mediated by developmentally transient circuit mechanisms (Fig. 1). For example, the SACs only express the nAChR subunit during stage II waves, thereby allowing temporary cholinergic transmission between SAC circuits via their lateral synaptic connections (Fig. 1B; Masland 2005; Blankenship and Feller 2010; Ford et al. 2012). In stage III waves, SACs no longer express nAChR while maturing bipolar cells express iGluR, which mediates lateral coupling between the same On or Off types of bipolar cells via extrasynaptic glutamate spillover (Fig. 1C; Kerschensteiner 2016).
Similar phenomena are also observed for PSINA in the developing Drosophila visual system. The adult Drosophila visual circuit is also retinotopically organized as ∼800 repeated topographically matched modules (i.e., ommatidia in the retina, cartridges in the lamina, and columns in the medulla) (Fig. 3; Morante and Desplan 2008; Sanes and Zipursky 2010; Meinertzhagen and Lee 2012; Nériec and Desplan 2016). These individual modules act as parallel input channels to propagate visual information vertically through three distinct neuropils: lamina, medulla, and lobula complex. Since retinotopic information is preserved until it is integrated in the lobula plate tangential cells (LPTCs) (Fig. 3; Borst et al. 2020; Wei et al. 2020), their upstream neuronal pathway presumably has minimum lateral excitatory coupling between parallel input channels. Each individual module is formed by ∼200 distinct neuronal types. Some of these cell types are unicolumnar neurons as they mainly project within a single module (lamina monopolar, medulla intrinsic, and trans-medullar neurons in Fig. 3; Morante and Desplan 2008; Nériec and Desplan 2016; Erclik et al. 2017). However, during PSINA, all unicolumnar neurons tested show cell type-specific correlation of activity across multiple modules (Fig. 3; Akin et al. 2019), indicating a temporary lateral excitatory coupling during development. While the exact cellular and molecular mechanisms of this lateral correlation of PSINA are not known, it is tempting to speculate that the multicolumnar neurons that innervate multiple columns could be one of the substrates involved in this transient excitatory lateral communication (Morante and Desplan 2008; Nériec and Desplan 2016; Erclik et al. 2017) between individual modules, like the SAC circuits for mammalian retinal waves (Blankenship and Feller 2010; Ford et al. 2012).
Developmental timing of correlated spontaneous activity during synaptogenesis
In vertebrates, retinal waves occur just after initial synaptic contact and last through complete synaptogenesis (Blankenship and Feller 2010; Kirkby et al. 2013; Kerschensteiner 2014). This is the developmental window when a rough connectome is established by a genetically hard-wired process, including axon guidance, but further refinement and maturation of synaptic coupling are required for generating a fully functional adult circuit. Multiple studies in vertebrates have suggested that the spontaneously emerging correlated activity triggered by the intrinsic properties of this immature and developing circuit mediates this synaptic maturation process. At each developmental stage, a transient circuit like the SAC network during stage II waves is mediated by still-developing synaptic coupling and drives the specific correlation of network activity, which feeds back into refining the developing circuit to advance the next maturation stage (Blankenship and Feller 2010; Kirkby et al. 2013; Kerschensteiner 2014; Arroyo and Feller 2016).
In Drosophila, the occurrence time window of PSINA is closely reminiscent of this vertebrate model. PSINA initiates at ∼50 h APF when initial circuit assembly is complete in response to secreted signals and contact-dependent signaling (Perry et al. 2017; Akin et al. 2019; Sanes and Zipursky 2020). Then, the pattern of PSINA changes in a stereotypic manner through the entire synaptogenesis process, potentially depending on a transient circuitry via immature synaptic coupling. Whether and how the fly PSNIA has a developmental role for refining circuitry, as it does in vertebrates, still remain to be determined.
Long-distance correlation of spontaneous activity between distinct brain areas
In the mouse visual system, retinal waves are not confined to the retina but also propagate to higher brain areas such as the dLGN, SC, and V1. While neuronal activity can occur and be correlated autonomously in higher brain areas such as SC and V1 of the visual cortex without retinal activity (Weliky and Katz 1999), during stages II and III, retinal waves instruct the pattern of network activity in these higher regions through synaptic transmission (Ackman and Crair 2014; Gribizis et al. 2019). This correlation between distinct brain areas that are synaptically connected suggests a potential developmental role of this activity as long-distance signaling at the global circuit level.
In the developing fly visual system, PSINA is also correlated between multiple cell types from distinct neuropils that are synaptically connected in adults along the visual processing pathway (Akin et al. 2019). For example, along the On motion detection circuit between the medulla and lobula complex, which are comparable with the inner retina and dLGN/SC area, respectively, PSINA in Tm3 transmedullary neurons is closely correlated with the activity patterns of the lobula plate T4 neurons that are synaptically downstream from Tm3 neurons (Sanes and Zipursky 2010; Silies et al. 2014). The expression of tetanus toxin, which disrupts evoked synaptic vesicle release (Venken et al. 2011) in the presynaptic side (Tm3) but not in the postsynaptic side (T4), weakens the correlation of PSINA between Tm3 and T4, suggesting that this coupling is mediated by synaptic transmission. As a negative control, lobula plate T5 neurons that are not connected to Tm3 but are developmentally and spatially closely related to T4 neurons do not exhibit a high correlation of PSINA with Tm3, demonstrating circuit specificity. Whether and how spontaneous activity patterns from potentially presynaptic neurons instruct the pattern of their downstream partners and how they propagate during synaptogenesis remain unknown.
Neuron–glia interaction during correlated activity
In vertebrate, recent studies have found that stage II and III retinal waves propagate not just to neuronal population but also to Müller glial cells (MGCs), the sole astroglia cell type in the inner plexiform layer that contacts most neuronal types in the retina (Rosa et al. 2015; Zhang et al. 2019). In mice, this neuron–glia correlation is mediated by neurotransmitter spillover (Rosa et al. 2015). Interestingly, MGCs express multiple neurotransmitter receptors during this stage, allowing them to respond to both cholinergic and glutamatergic drives. During stage II cholinergic waves, extrasynaptic acetylcholine release from SACs on RGCs also diffuses to activate muscarinic AChRs in MGCs, thus inducing calcium activity in these glia cells. During stage III glutamatergic waves, extrasynaptically released glutamate between BCs on RGCs activates MGCs via AMPA-type iGluRs. This neuronal–glial wave propagation decreases at later stages (from P9 to P11) when MGCs express higher levels of the glutamate transporter (EAAT1), which limits extrasynaptic glutamate (Rosa et al. 2015). While astrocytes like MGCs have been shown to have an essential role in the synaptogenesis process (Clarke and Barres 2013; Chung et al. 2015), the functional significance of these neuronal–MGC interactions remains to be determined.
During fly PSINA, the astrocyte glial population also shows calcium activity correlated with the neuronal population (Akin et al. 2019). This correlation is initially weak but becomes more obvious throughout the periodic stage of PSINA as neuronal and glial activity patterns are phased-shifted with each other. Drosophila astrocytes also play a critical role in synaptogenesis (Muthukumar et al. 2014; Richier et al. 2017), but both the propagating mechanism and developmental function of this glial calcium activity remain unanswered.
Using an invertebrate model to study correlated spontaneous activity
With these intriguing conceptual similarities between mammalian retinal waves and fly PSINA, could the fly model system strengthen decades of previous studies and provide solutions to the remaining questions in the field? Compared with the vertebrate model systems commonly used to study correlated spontaneous activity such as mice, ferrets, rabbits, chickens, and zebrafish, which often depend on electrophysiology, functional imaging, and pharmacology (Chiu and Weliky 2001; Blankenship and Feller 2010; Momose-Sato and Sato 2016; Marachlian et al. 2018), Drosophila has unparalleled genetic and anatomical accessibility with its tiny brain size (Bellen et al. 2010). Through the manipulation of specific cellular functions using quickly-evolving genetic and optogenetic tools, this simple invertebrate model will greatly facilitate future studies of this important developmental phenomenon (Simpson and Looger 2018). Below, we present several examples illustrating the power of the fly model to address the remaining outstanding questions.
The role of gap junction coupling during correlated spontaneous activity.
The intercellular signaling mediated by gap junction is important but often understudied during correlated spontaneous activity. From the early days, the study of correlated spontaneous activity has been considered as a subset of activity-dependent developmental plasticity (Kirkby et al. 2013; Pan and Monje 2020). Naturally, investigations of the developmental role of spontaneous activity have focused on neuronal circuits (Kirkby et al. 2013). This neuron-centric approach often ignored nonneuronal aspects of development in guiding the correlation of spontaneous activity. Specifically, most studies focused on canonical synaptic transmission as the major intercellular signaling mechanism (Blankenship and Feller 2010; Kirkby et al. 2013; Kerschensteiner 2014; Arroyo and Feller 2016). However, throughout species, the very first correlation of activity emerges prior to synaptogenesis, like stage I embryonic retinal waves of mammals, when gap junction coupling is the major intercellular pathway (Cook and Becker 2009; Nagy et al. 2018). Furthermore, robust gap junction networks exist between various retinal cell types throughout development and in the adult retina, where they have important functions such as in motion sensitivity and in injury response (Bloomfield and Völgyi 2009; Völgyi et al. 2013). Indeed, gap junction coupling exists during all three stages of retinal waves (Cook and Becker 2009). In addition to mediating lateral wave propagation along the BC circuit during stage III waves (Fig. 1C), recent studies also have shown that the retinal gap junction network is important for homeostatic mechanism in response to the disruption of retinal waves (Stacy et al. 2005; Blankenship and Feller 2010; Arroyo et al. 2016). In both ChAT and nAChR β2 mutants where stage II cholinergic waves are severely disrupted, the residual waves are sensitive to a gap junction blocker, although stage II waves in wild-type retina are not inhibited by the same gap junction blocker (Stacy et al. 2005; Sun et al. 2008). These gap junction-mediated waves are thought to be the homeostatic compensatory response to the disruption of the cholinergic waves (Stacy et al. 2005; Blankenship and Feller 2010). When cholinergic waves are inhibited, the emergence of these compensatory waves appears to be mediated by strengthening the gap junction network between a subtype of RGCs, the intrinsically photosensitive retinal ganglion cells (Kirkby and Feller 2013; Arroyo et al. 2016). These findings suggest that gap junction coupling not only mediates the embryonic retinal waves but also plays a significant role at all stages of retinal waves.
However, the retinal gap junction network during the waves is not well understood due to several technical challenges in vertebrate systems. For example, a robust genetic model system for gap junction is missing (Bansal et al. 2000; Syed et al. 2004; Sun et al. 2008; Blankenship et al. 2011). In contrast to chemical synaptic transmission where nAChR-β2, chat, or vglut mouse mutants are specific for retinal waves and extensively characterized, mammalian gap junctions have multiple paralogs (20 connexins and three pannexins in mice) (Cook and Becker 1995; Söhl et al. 2005; Burnstock 2018), which are usually essential for early development. Cells express multiple combinations of connexins, hindering the use of versatile loss-of-function strategies. We also lack specific pharmacological antagonists (Swayne and Bennett 2016; Wörsdörfer et al. 2018). Furthermore, despite the growing evidence of the functional significance, complexity, and potential plasticity of gap junction coupling, conventional approaches for investigating chemical synaptic connection might not be suited to study the gap junction network (Bloomfield and Völgyi 2009; Nagy et al. 2018). For example, unlike chemical synapses that can be well characterized by electron micrograph, gap junction coupling is very challenging to visualize (Nagy et al. 2018), and the electrical connectome in vertebrate systems is generally unknown.
The Drosophila model has powerful advantages for addressing these challenges. The fly has eight gap junction genes (eight innexins), six of which are expressed in the developing visual system (Stebbings et al. 2002; Dolezelova et al. 2007). While these genes are dynamically expressed with unique temporal and spatial patterns throughout pupal development, the peak expression of inx8 (also called ShakB), a major gap junction gene in most neuronal populations (Crompton et al. 1995; Stebbings et al. 2002), occurs at ∼50 h APF (Kurmangaliyev et al. 2020), just prior to synaptogenesis, suggesting a potential role in generating initial stages of correlated activity similar to the embryonic retinal waves in mammals. As the developing fly neurons, glia, and epithelial accessory cell types each expresses a cell type-specific combination of the six innexins just prior to PSINA (Stebbings et al. 2002; BJ Choi and C Desplan, unpubl.), this makes the developing fly visual system an ideal model system to study the specific role of these genes during PSINA. The robust genetic accessibility of Drosophila can greatly facilitate the characterization of the gap junction coupling during PSINA and could lead to a systematic mapping of the gap junction network as has been done in C. elegans (Bhattacharya et al. 2019; Jin et al. 2020). In vivo functional assays in specific populations of neurons by genetically encoded optical sensors are also easily performed in flies (Wu et al. 2019).
Cell type-specific role for generating correlated spontaneous activity
A comprehensive determination of cellular diversity in the developing vertebrate nervous system is still technically challenging (Sanes and Masland 2015). For example, the vertebrate retina is composed of six main neuronal classes, rod and cone photoreceptors, amacrine, horizontal, bipolar interneurons, and RGCs (Cepko 2014). However, RGCs alone can be further classified into at least 30 distinct cell types (Sanes and Masland 2015), while there are at least 63 amacrine cell types (Yan et al. 2020). These diverse cell types are critical for the correlation of spontaneous activity. During stage III waves, although they have similar morphologies, On BCs can be subdivided into at least two groups depending on their expression of iGluR. During the lateral propagation of stage III waves in the BC circuit, iGluR-positive On BCs can be activated by extrasynaptic glutamate diffused from neighboring BCs, while iGluR-negative On BCs can participate in wave propagation through gap junction coupling (Fig. 1C; Kerschensteiner 2016). However, despite the recent development of advanced genetic and genomic tools, the determination of retinal cell types is still far from complete (Sanes and Masland 2015), making it difficult to understand whether they have specific contributions to the waves and are affected by them.
Meanwhile, the cellular diversity of the Drosophila visual system has been rigorously determined by taking advantage of the powerful genetic tools as well as the smaller brain size that allows for the completion of a detailed EM-level connectome (Venken et al. 2011; Takemura et al. 2013; Zheng et al. 2018; Scheffer et al. 2020). Through multiple systematic screening studies, tools are available for robust cell type-specific genetic manipulation at multiple developmental stages (Nern et al. 2015; Luan et al. 2020), making the fly an ideal model system to study how individual cell types participate in the correlation of spontaneous activity.
Furthermore, recent advances in genomics, such as single-cell mRNA sequencing (scRNA-seq), enable a robust and systematic determination of cellular diversity and its transcriptome throughout development. A combination of the large-scale use of scRNAseq during development and machine-learning algorithms has described the transcriptome of nearly all major optic lobe neuronal and glial cell types at different developmental stages (Kurmangaliyev et al. 2020; Özel et al. 2021), from the time the neurons are specified to the periods of axonal projection, synaptogenesis, and adult function. Intriguingly, initial analysis of these data shows that well-studied presynaptic genes critical for membrane depolarization (para) and synaptic vesicle release (brp and cac) have peak expression levels in pan-neuronal population ∼60 h APF (Kittel and Heckmann 2016; Kurmangaliyev et al. 2020), which roughly coincides with the onset of PSINA and is consistent with the requirement of synaptic coupling for this process. Similarly, the hr38 (hormone receptor-like in 38), the best-characterized activity-dependent immediate early gene in insects (Fujita et al. 2013), is highly expressed in all neuronal types during the second half of pupal development when PSINA actively occurs and well before the start of sensory-evoked activity (Kurmangaliyev et al. 2020). This suggests not just that both spontaneous and sensory-evoked activity activate similar downstream signaling pathways but also that hr38 can be used as an activity marker for correlated spontaneous activity. By taking advantage of these comprehensive resources, it is now possible to systematically identify how individual cell types engage in the PSINA process through single-cell sequencing of the transcriptional profiles of flies where PSNIA has been specifically disrupted.
Concluding remarks
Since the first discovery of retinal waves ∼30 yr ago, correlated spontaneous activity has been a fascinating and general phenomenon of vertebrate neurodevelopment with its striking visual spectacle. However, its transient nature and delicate phenotype have made it challenging to obtain a comprehensive understanding of this process. Recent advances in Drosophila suggest that spontaneously emerging correlated activity is an evolutionarily conserved developmental process. With an ever-advancing field of genetic and genomic tools, this new avenue of investigation using a simpler and genetically accessible model system will greatly promote our understanding of this fundamental feature of the nervous system.
Acknowledgments
We thank Jennifer Malin, Nikos Konstantinides, Sergio Cordoba, and Neset Özel for their critical reading and comments on the manuscript. This work was supported by the National Institutes of Health to C.D. (R01 EY13012). B.J.C. was supported by a postdoctoral National Research Service Award grant (5F32EY027682-02).
Footnotes
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.348241.121.
Competing interest statement
The authors declare no competing interests.
References
- Ackman JB, Crair MC. 2014. Role of emergent neural activity in visual map development. Curr Opin Neurobiol 24: 166–175. 10.1016/j.conb.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackman JB, Burbridge TJ, Crair MC. 2012. Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490: 219–225. 10.1038/nature11529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin O, Zipursky SL. 2016. Frazzled promotes growth cone attachment at the source of a Netrin gradient in the Drosophila visual system. Elife 5: e20762. 10.7554/eLife.20762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin O, Zipursky SL. 2020. Activity regulates brain development in the fly. Curr Opin Genet Dev 65: 8–13. 10.1016/j.gde.2020.04.005 [DOI] [PubMed] [Google Scholar]
- Akin O, Bajar BT, Keles MF, Frye MA, Zipursky SL. 2019. Cell-type-specific patterned stimulus-independent neuronal activity in the Drosophila visual system during synapse formation. Neuron 101: 894–904.e5. 10.1016/j.neuron.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akrouh A, Kerschensteiner D. 2013. Intersecting circuits generate precisely patterned retinal waves. Neuron 79: 322–334. 10.1016/j.neuron.2013.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro MJ, Livingstone MS. 2017. A hierarchical, retinotopic proto-organization of the primate visual system at birth. Elife 6: e26196. 10.7554/eLife.26196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro MJ, McMains SA, Singer BD, Kastner S. 2009. Retinotopic organization of human ventral visual cortex. J Neurosci 29: 10638–10652. 10.1523/JNEUROSCI.2807-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo DA, Feller MB. 2016. Spatiotemporal features of retinal waves instruct the wiring of the visual circuitry. Front Neural Circuits 10: 54. 10.3389/fncir.2016.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo DA, Kirkby LA, Feller MB. 2016. Retinal waves modulate an intraretinal circuit of intrinsically photosensitive retinal ganglion cells. J Neurosci 36: 6892–6905. 10.1523/JNEUROSCI.0572-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenas N, Wagner AE. 2019. Drosophila melanogaster as an alternative model organism in nutrigenomics. Genes Nutr 14: 14. 10.1186/s12263-019-0641-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Suzuki T. 2020. Activity-dependent synaptic plasticity in Drosophila melanogaster. Front Physiol 11: 161. 10.3389/fphys.2020.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian R, Gan L. 2014. Development of retinal amacrine cells and their dendritic stratification. Curr Ophthalmol Rep 2: 100–106. 10.1007/s40135-014-0048-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. 2000. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming On and Off circuits in the inner retina. J Neurosci 20: 7672–7681. 10.1523/JNEUROSCI.20-20-07672.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen HJ, Tong C, Tsuda H. 2010. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat Rev Neurosci 11: 514–522. 10.1038/nrn2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdnik D, Chihara T, Couto A, Luo L. 2006. Wiring stability of the adult Drosophila olfactory circuit after lesion. J Neurosci 26: 3367–3376. 10.1523/JNEUROSCI.4941-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Aghayeva U, Berghoff EG, Hobert O. 2019. Plasticity of the electrical connectome of C. elegans. Cell 176: 1174–1189.e16. 10.1016/j.cell.2018.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. 2010. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci 11: 18–29. 10.1038/nrn2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Ford KJ, Johnson J, Seal RP, Edwards RH, Copenhagen DR, Feller MB. 2009. Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron 62: 230–241. 10.1016/j.neuron.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Hamby AM, Firl A, Vyas S, Maxeiner S, Willecke K, Feller MB. 2011. The role of neuronal connexins 36 and 45 in shaping spontaneous firing patterns in the developing retina. J Neurosci 31: 9998–10008. 10.1523/JNEUROSCI.5640-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Völgyi B. 2009. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci 10: 495–506. 10.1038/nrn2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst A, Haag J, Mauss AS. 2020. How fly neurons compute the direction of visual motion. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 206: 109–124. 10.1007/s00359-019-01375-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbridge TJ, Xu HP, Ackman JB, Ge X, Zhang Y, Ye MJ, Zhou ZJ, Xu J, Contractor A, Crair MC. 2014. Visual circuit development requires patterned activity mediated by retinal acetylcholine receptors. Neuron 84: 1049–1064. 10.1016/j.neuron.2014.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. 2018. Purine and purinergic receptors. Brain Neurosci Adv 2: 239821281881749. 10.1177/2398212818817494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsicas M, Bonness V, Becker D, Mobbs P. 1998. Spontaneous Ca2+ transients and their transmission in the developing chick retina. Curr Biol 8: 283–288. 10.1016/S0960-9822(98)70110-1 [DOI] [PubMed] [Google Scholar]
- Cepko C. 2014. Intrinsically different retinal progenitor cells produce specific types of progeny. Nat Rev Neurosci 15: 615–627. 10.1038/nrn3767 [DOI] [PubMed] [Google Scholar]
- Chalupa LM. 2009. Retinal waves are unlikely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev 4: 25. doi 10.1186/1749-8104-4-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran AR, Plas DT, Gonzalez E, Crair MC. 2005. Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse. J Neurosci 25: 6929–6938. 10.1523/JNEUROSCI.1470-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Desplan C. 2020. Gene regulatory networks during the development of the Drosophila visual system. Curr Top Dev Biol 139: 89–125. 10.1016/bs.ctdb.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499: 295–300. 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Akin O, Nern A, Tsui CY, Pecot MY, Zipursky SL. 2014. Cell-type-specific labeling of synapses in vivo through synaptic tagging with recombination. Neuron 81: 280–293. 10.1016/j.neuron.2013.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Weliky M. 2001. Spontaneous activity in developing ferret visual cortex in vivo. J Neurosci 21: 8906–8914. 10.1523/JNEUROSCI.21-22-08906.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Weliky M. 2002. Relationship of correlated spontaneous activity to functional ocular dominance columns in the developing visual cortex. Neuron 35: 1123–1134. 10.1016/S0896-6273(02)00867-X [DOI] [PubMed] [Google Scholar]
- Choi BJ, Imlach WL, Jiao W, Wolfram V, Wu Y, Grbic M, Cela C, Baines RA, Nitabach MN, McCabe BD. 2014. Miniature neurotransmission regulates Drosophila synaptic structural maturation. Neuron 82: 618–634. 10.1016/j.neuron.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Allen NJ, Eroglu C. 2015. Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol 7: a020370. 10.1101/cshperspect.a020370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. 2013. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci 14: 311–321. 10.1038/nrn3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JE, Becker DL. 1995. Gap junctions in the vertebrate retina. Microsc Res Tech 31: 408–419. 10.1002/jemt.1070310510 [DOI] [PubMed] [Google Scholar]
- Cook JE, Becker DL. 2009. Gap-junction proteins in retinal development: new roles for the ‘nexus.’ Physiology 24: 219–230. 10.1152/physiol.00007.2009 [DOI] [PubMed] [Google Scholar]
- Crabtree JW. 1990. Prenatal development of retinogeniculate projections in the rabbit: an HRP study. J Comp Neurol 299: 75–88. 10.1002/cne.902990106 [DOI] [PubMed] [Google Scholar]
- Crair MC. 1999. Neuronal activity during development: permissive or instructive? Curr Opin Neurobiol 9: 88–93. 10.1016/S0959-4388(99)80011-7 [DOI] [PubMed] [Google Scholar]
- Crompton D, Todman M, Wilkin M, Ji S, Davies J. 1995. Essential and neural transcripts from the Drosophila shaking-B locus are differentially expressed in the embryonic mesoderm and pupal nervous system. Dev Biol 170: 142–158. 10.1006/dbio.1995.1203 [DOI] [PubMed] [Google Scholar]
- Dana H, Sun Y, Mohar B, Hulse BK, Kerlin AM, Hasseman JP, Tsegaye G, Tsang A, Wong A, Patel R, et al. 2019. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat Methods 16: 649–657. 10.1038/s41592-019-0435-6 [DOI] [PubMed] [Google Scholar]
- Demas J, Eglen SJ, Wong RO. 2003. Developmental loss of synchronous spontaneous activity in the mouse retina is independent of visual experience. J Neurosci 23: 2851–2860. 10.1523/JNEUROSCI.23-07-02851.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Singer JH. 2015. Functional circuitry of the retina. Annu Rev Vis Sci 1: 263–289. 10.1146/annurev-vision-082114-035334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Huberman AD. 2014. Retinal ganglion cell maps in the brain: implications for visual processing. Curr Opin Neurobiol 24: 133–142. 10.1016/j.conb.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Stafford BK, Lim JA, Huberman AD. 2015. Contributions of retinal ganglion cells to subcortical visual processing and behaviors. Annu Rev Vis Sci 1: 291–328. 10.1146/annurev-vision-082114-035502 [DOI] [PubMed] [Google Scholar]
- Dolezelova E, Nothacker HP, Civelli O, Bryant PJ, Zurovec M. 2007. A Drosophila adenosine receptor activates cAMP and calcium signaling. Insect Biochem Mol Biol 37: 318–329. 10.1016/j.ibmb.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Draguhn A, Keller M, Reichinnek S. 2014. Coordinated network activity in the hippocampus. Front Neurol Neurosci 34: 26–35. 10.1159/000357026 [DOI] [PubMed] [Google Scholar]
- D'Souza S, Lang RA. 2020. Retinal ganglion cell interactions shape the developing mammalian visual system. Development 147: dev196535. 10.1242/dev.196535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst CD, Wiegert JS, Helassa N, Kerruth S, Coates C, Schulze C, Geeves MA, Török K, Oertner TG. 2019. High-speed imaging of glutamate release with genetically encoded sensors. Nat Protoc 14: 1401–1424. 10.1038/s41596-019-0143-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen SJ, Demas J, Wong RO. 2003. Mapping by waves. Patterned spontaneous activity regulates retinotopic map refinement. Neuron 40: 1053–1055. 10.1016/S0896-6273(03)00808-0 [DOI] [PubMed] [Google Scholar]
- Erclik T, Li X, Courgeon M, Bertet C, Chen Z, Baumert R, Ng J, Koo C, Arain U, Behnia R, et al. 2017. Integration of temporal and spatial patterning generates neural diversity. Nature 541: 365–370. 10.1038/nature20794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Haverkamp S, Schubert T, Baden T. 2014. Retinal bipolar cells: elementary building blocks of vision. Nat Rev Neurosci 15: 507–519. 10.1038/nrn3783 [DOI] [PubMed] [Google Scholar]
- Fan WJ, Li X, Yao HL, Deng JX, Liu HL, Cui ZJ, Wang Q, Wu P, Deng JB. 2016. Neural differentiation and synaptogenesis in retinal development. Neural Regen Res 11: 312–318. 10.4103/1673-5374.177743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller MB. 2009. Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev 4: 24. 10.1186/1749-8104-4-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. 1996. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science 272: 1182–1187. 10.1126/science.272.5265.1182 [DOI] [PubMed] [Google Scholar]
- Feller MB, Butts DA, Aaron HL, Rokhsar DS, Shatz CJ. 1997. Dynamic processes shape spatiotemporal properties of retinal waves. Neuron 19: 293–306. 10.1016/S0896-6273(00)80940-X [DOI] [PubMed] [Google Scholar]
- Firl A, Sack GS, Newman ZL, Tani H, Feller MB. 2013. Extrasynaptic glutamate and inhibitory neurotransmission modulate ganglion cell participation during glutamatergic retinal waves. J Neurophysiol 109: 1969–1978. 10.1152/jn.00039.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KJ, Felix AL, Feller MB. 2012. Cellular mechanisms underlying spatiotemporal features of cholinergic retinal waves. J Neurosci 32: 850–863. 10.1523/JNEUROSCI.5309-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KJ, Arroyo DA, Kay JN, Lloyd EE, Bryan RM Jr., Sanes JR, Feller MB. 2013. A role for TREK1 in generating the slow afterhyperpolarization in developing starburst amacrine cells. J Neurophysiol 109: 2250–2259. 10.1152/jn.01085.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E, Lohmann C. 1999. Development of auditory brainstem circuitry. Activity-dependent and activity-independent processes. Cell Tissue Res 297: 187–195. 10.1007/s004410051346 [DOI] [PubMed] [Google Scholar]
- Fujita N, Nagata Y, Nishiuchi T, Sato M, Iwami M, Kiya T. 2013. Visualization of neural activity in insect brains using a conserved immediate early gene, Hr38. Curr Biol 23: 2063–2070. 10.1016/j.cub.2013.08.051 [DOI] [PubMed] [Google Scholar]
- Ganguly K, Poo MM. 2013. Activity-dependent neural plasticity from bench to bedside. Neuron 80: 729–741. 10.1016/j.neuron.2013.10.028 [DOI] [PubMed] [Google Scholar]
- Giachello CN, Baines RA. 2015. Inappropriate neural activity during a sensitive period in embryogenesis results in persistent seizure-like behavior. Curr Biol 25: 2964–2968. 10.1016/j.cub.2015.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding B, Pouchelon G, Bellone C, Murthy S, Di Nardo AA, Govindan S, Ogawa M, Shimogori T, Lüscher C, Dayer A, et al. 2014. Retinal input directs the recruitment of inhibitory interneurons into thalamic visual circuits. Neuron 81: 1057–1069. 10.1016/j.neuron.2014.01.032 [DOI] [PubMed] [Google Scholar]
- Gribizis A, Ge X, Daigle TL, Ackman JB, Zeng H, Lee D, Crair MC. 2019. Visual cortex gains independence from peripheral drive before eye opening. Neuron 104: 711–723.e3. 10.1016/j.neuron.2019.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W. 2018. Development, form, and function of the mouse visual thalamus. J Neurophysiol 120: 211–225. 10.1152/jn.00651.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Balaban M. 1963. Observations and experiments on spontaneous rhythmical behavior in the chick embryo. Dev Biol 7: 533–545. 10.1016/0012-1606(63)90140-4 [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Ben-Ari Y, Khazipov R. 2006. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J Neurosci 26: 6728–6736. 10.1523/JNEUROSCI.0752-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesinger PR, Zhai RG, Zhou Y, Koh TW, Mehta SQ, Schulze KL, Cao Y, Verstreken P, Clandinin TR, Fischbach KF, et al. 2006. Activity-independent prespecification of synaptic partners in the visual map of Drosophila. Curr Biol 16: 1835–1843. 10.1016/j.cub.2006.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguera I, Desplan C. 2018. Neuronal specification in space and time. Science 362: 176–180. 10.1126/science.aas9435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1970. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol 206: 419–436. 10.1113/jphysiol.1970.sp009022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. 2008. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci 31: 479–509. 10.1146/annurev.neuro.31.060407.125533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis GS, Marin EC, Stocker RF, Luo L. 2001. Target neuron prespecification in the olfactory map of Drosophila. Nature 414: 204–208. 10.1038/35102574 [DOI] [PubMed] [Google Scholar]
- Jin EJ, Park S, Lyu X, Jin Y. 2020. Gap junctions: historical discoveries and new findings in the Caenorhabditis elegans nervous system. Biol Open 9: bio053983. 10.1242/bio.053983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC. 1999. What's critical for the critical period in visual cortex? Cell 99: 673–676. 10.1016/S0092-8674(00)81665-7 [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. 1996. Synaptic activity and the construction of cortical circuits. Science 274: 1133–1138. 10.1126/science.274.5290.1133 [DOI] [PubMed] [Google Scholar]
- Kerschensteiner D. 2014. Spontaneous network activity and synaptic development. Neuroscientist 20: 272–290. 10.1177/1073858413510044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D. 2016. Glutamatergic retinal waves. Front Neural Circuits 10: 38. 10.3389/fncir.2016.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D, Wong RO. 2008. A precisely timed asynchronous pattern of On and Off retinal ganglion cell activity during propagation of retinal waves. Neuron 58: 851–858. 10.1016/j.neuron.2008.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby LA, Feller MB. 2013. Intrinsically photosensitive ganglion cells contribute to plasticity in retinal wave circuits. Proc Natl Acad Sci 110: 12090–12095. 10.1073/pnas.1222150110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby LA, Sack GS, Firl A, Feller MB. 2013. A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 80: 1129–1144. 10.1016/j.neuron.2013.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel RJ, Heckmann M. 2016. Synaptic vesicle proteins and active zone plasticity. Front Synaptic Neurosci 8: 8. 10.3389/fnsyn.2016.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinlogel S, Feldbauer K, Dempski RE, Fotis H, Wood PG, Bamann C, Bamberg E. 2011. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat Neurosci 14: 513–518. 10.1038/nn.2776 [DOI] [PubMed] [Google Scholar]
- Kurmangaliyev YZ, Yoo J, Valdes-Aleman J, Sanfilippo P, Zipursky SL. 2020. Transcriptional programs of circuit assembly in the Drosophila visual system. Neuron 108: 1045–1057.e6. 10.1016/j.neuron.2020.10.006 [DOI] [PubMed] [Google Scholar]
- Leopold AV, Shcherbakova DM, Verkhusha VV. 2019. Fluorescent biosensors for neurotransmission and neuromodulation: engineering and applications. Front Cell Neurosci 13: 474. 10.3389/fncel.2019.00474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan H, Diao F, Scott RL, White BH. 2020. The Drosophila split Gal4 system for neural circuit mapping. Front Neural Circuits 14. 10.3389/fncir.2020.603397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccione A, Hennig MH, Gandolfo M, Muthmann O, van Coppenhagen J, Eglen SJ, Berdondini L, Sernagor E. 2014. Following the ontogeny of retinal waves: pan-retinal recordings of population dynamics in the neonatal mouse. J Physiol 592: 1545–1563. 10.1113/jphysiol.2013.262840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marachlian E, Avitan L, Goodhill GJ, Sumbre G. 2018. Principles of functional circuit connectivity: insights from spontaneous activity in the zebrafish optic tectum. Front Neural Circuits 12: 46. 10.3389/fncir.2018.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. 1977. Maturation of function in the developing rabbit retina. J Comp Neurol 175: 275–286. 10.1002/cne.901750303 [DOI] [PubMed] [Google Scholar]
- Masland RH. 2005. The many roles of starburst amacrine cells. Trends Neurosci 28: 395–396. 10.1016/j.tins.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Matthews G, Fuchs P. 2010. The diverse roles of ribbon synapses in sensory neurotransmission. Nat Rev Neurosci 11: 812–822. 10.1038/nrn2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, O'Leary DD. 2005. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci 28: 327–355. 10.1146/annurev.neuro.28.061604.135714 [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, O'Leary DD. 2003a. Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr Opin Neurobiol 13: 57–69. 10.1016/S0959-4388(03)00014-X [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O'Leary DD. 2003b. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 40: 1147–1160. 10.1016/S0896-6273(03)00790-6 [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Lee CH. 2012. The genetic analysis of functional connectomics in Drosophila. Adv Genet 80: 99–151. 10.1016/B978-0-12-404742-6.00003-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA, Shatz CJ. 1991. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science 252: 939–943. 10.1126/science.2035024 [DOI] [PubMed] [Google Scholar]
- Mirzoyan Z, Sollazzo M, Allocca M, Valenza AM, Grifoni D, Bellosta P. 2019. Drosophila melanogaster: a model organism to study cancer. Front Genet 10: 51. 10.3389/fgene.2019.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose-Sato Y, Sato K. 2016. Development of spontaneous activity in the avian hindbrain. Front Neural Circuits 10: 63. 10.3389/fncir.2016.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoro RJ, Yuste R. 2004. Gap junctions in developing neocortex: a review. Brain Res Brain Res Rev 47: 216–226. 10.1016/j.brainresrev.2004.06.009 [DOI] [PubMed] [Google Scholar]
- Moody WJ, Bosma MM. 2005. Ion channel development, spontaneous activity, and activity-dependent development in nerve and muscle cells. Physiol Rev 85: 883–941. 10.1152/physrev.00017.2004 [DOI] [PubMed] [Google Scholar]
- Morante J, Desplan C. 2008. The color-vision circuit in the medulla of Drosophila. Curr Biol 18: 553–565. 10.1016/j.cub.2008.02.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir-Robinson G, Hwang BJ, Feller MB. 2002. Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J Neurosci 22: 5259–5264. 10.1523/JNEUROSCI.22-13-05259.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumar AK, Stork T, Freeman MR. 2014. Activity-dependent regulation of astrocyte GAT levels during synaptogenesis. Nat Neurosci 17: 1340–1350. 10.1038/nn.3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Pereda AE, Rash JE. 2018. Electrical synapses in mammalian CNS: past eras, present focus and future directions. Biochim Biophys Acta Biomembr 1860: 102–123. 10.1016/j.bbamem.2017.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nériec N, Desplan C. 2016. From the eye to the brain: development of the Drosophila visual system. Curr Top Dev Biol 116: 247–271. 10.1016/bs.ctdb.2015.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nern A, Pfeiffer BD, Rubin GM. 2015. Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc Natl Acad Sci 112: E2967–E2976. 10.1073/pnas.1506763112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet KT, Rebsam A. 2020. Neurogenesis and specification of retinal ganglion cells. Int J Mol Sci 21: 451. 10.3390/ijms21020451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan MJ. 1999. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol 9: 94–104. 10.1016/S0959-4388(99)80012-9 [DOI] [PubMed] [Google Scholar]
- O'Leary DD, McLaughlin T. 2005. Mechanisms of retinotopic map development: ephs, ephrins, and spontaneous correlated retinal activity. Prog Brain Res 147: 43–65. 10.1016/S0079-6123(04)47005-8 [DOI] [PubMed] [Google Scholar]
- Özel MN, Langen M, Hassan BA, Hiesinger PR. 2015. Filopodial dynamics and growth cone stabilization in Drosophila visual circuit development. Elife 4: e10721. 10.7554/eLife.10721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özel MN, Simon F, Jafari S, Holguera I, Chen YC, Benhra N, El-Danaf RN, Kapuralin K, Malin JA, Konstantinides N, et al. 2021. Neuronal diversity and convergence in a visual system developmental atlas. Nature 589: 88–95. 10.1038/s41586-020-2879-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Monje M. 2020. Activity shapes neural circuit form and function: a historical perspective. J Neurosci 40: 944–954. 10.1523/JNEUROSCI.0740-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AA. 2001. Early brain wiring: activity-dependent processes. Schizophr Bull 27: 337–347. 10.1093/oxfordjournals.schbul.a006880 [DOI] [PubMed] [Google Scholar]
- Penn AA, Wong RO, Shatz CJ. 1994. Neuronal coupling in the developing mammalian retina. J Neurosci 14: 3805–3815. 10.1523/JNEUROSCI.14-06-03805.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M, Konstantinides N, Pinto-Teixeira F, Desplan C. 2017. Generation and evolution of neural cell types and circuits: insights from the Drosophila visual system. Annu Rev Genet 51: 501–527. 10.1146/annurev-genet-120215-035312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DJ, Jarman A, Mason JO, Kind P. 2018. Building brains: an introduction to neural development. Wiley, Hoboken, NJ. [Google Scholar]
- Richier B, Vijandi CM, Mackensen S, Salecker I. 2017. Lapsyn controls branch extension and positioning of astrocyte-like glia in the Drosophila optic lobe. Nat Commun 8: 317. 10.1038/s41467-017-00384-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa JM, Bos R, Sack GS, Fortuny C, Agarwal A, Bergles DE, Flannery JG, Feller MB. 2015. Neuron-glia signaling in developing retina mediated by neurotransmitter spillover. Elife 4: e09590. 10.7554/eLife.09590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changeux JP. 2001. Requirement of the nicotinic acetylcholine receptor β2 subunit for the anatomical and functional development of the visual system. Proc Natl Acad Sci 98: 6453–6458. 10.1073/pnas.101120998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Masland RH. 2015. The types of retinal ganglion cells: current status and implications for neuronal classification. Annu Rev Neurosci 38: 221–246. 10.1146/annurev-neuro-071714-034120 [DOI] [PubMed] [Google Scholar]
- Sanes JR, Zipursky SL. 2010. Design principles of insect and vertebrate visual systems. Neuron 66: 15–36. 10.1016/j.neuron.2010.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Zipursky SL. 2020. Synaptic specificity, recognition molecules, and assembly of neural circuits. Cell 181: 536–556. 10.1016/j.cell.2020.04.008 [DOI] [PubMed] [Google Scholar]
- Scheffer LK, Xu CS, Januszewski M, Lu Z, Takemura SY, Hayworth KJ, Huang GB, Shinomiya K, Maitlin-Shepard J, Berg S, et al. 2020. A connectome and analysis of the adult Drosophila central brain. Elife 9: e57443. 10.7554/eLife.57443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EK, Reuter JE, Luo L. 2003. Dendritic development of Drosophila high order visual system neurons is independent of sensory experience. BMC Neurosci 4: 14. 10.1186/1471-2202-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook TA, Burbridge TJ, Crair MC, Huberman AD. 2017. Architecture, function, and assembly of the mouse visual system. Annu Rev Neurosci 40: 499–538. 10.1146/annurev-neuro-071714-033842 [DOI] [PubMed] [Google Scholar]
- Sernagor E, Eglen SJ, Wong RO. 2001. Development of retinal ganglion cell structure and function. Prog Retin Eye Res 20: 139–174. 10.1016/S1350-9462(00)00024-0 [DOI] [PubMed] [Google Scholar]
- Silies M, Gohl DM, Clandinin TR. 2014. Motion-detecting circuits in flies: coming into view. Annu Rev Neurosci 37: 307–327. 10.1146/annurev-neuro-071013-013931 [DOI] [PubMed] [Google Scholar]
- Simpson JH, Looger LL. 2018. Functional imaging and optogenetics in Drosophila. Genetics 208: 1291–1309. 10.1534/genetics.117.300228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Mirotznik RR, Feller MB. 2001. Potentiation of L-type calcium channels reveals nonsynaptic mechanisms that correlate spontaneous activity in the developing mammalian retina. J Neurosci 21: 8514–8522. 10.1523/JNEUROSCI.21-21-08514.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söhl G, Maxeiner S, Willecke K. 2005. Expression and functions of neuronal gap junctions. Nat Rev Neurosci 6: 191–200. 10.1038/nrn1627 [DOI] [PubMed] [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. 2004. Organization, development and function of complex brain networks. Trends Cogn Sci 8: 418–425. 10.1016/j.tics.2004.07.008 [DOI] [PubMed] [Google Scholar]
- Stacy RC, Demas J, Burgess RW, Sanes JR, Wong RO. 2005. Disruption and recovery of patterned retinal activity in the absence of acetylcholine. J Neurosci 25: 9347–9357. 10.1523/JNEUROSCI.1800-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings LA, Todman MG, Phillips R, Greer CE, Tam J, Phelan P, Jacobs K, Bacon JP, Davies JA. 2002. Gap junctions in Drosophila: developmental expression of the entire innexin gene family. Mech Dev 113: 197–205. 10.1016/S0925-4773(02)00025-4 [DOI] [PubMed] [Google Scholar]
- Sun C, Warland DK, Ballesteros JM, van der List D, Chalupa LM. 2008. Retinal waves in mice lacking the β2 subunit of the nicotinic acetylcholine receptor. Proc Natl Acad Sci 105: 13638–13643. 10.1073/pnas.0807178105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suster ML, Bate M. 2002. Embryonic assembly of a central pattern generator without sensory input. Nature 416: 174–178. 10.1038/416174a [DOI] [PubMed] [Google Scholar]
- Swayne LA, Bennett SA. 2016. Connexins and pannexins in neuronal development and adult neurogenesis. BMC Cell Biol 17: 10. 10.1186/s12860-016-0089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed MM, Lee S, Zheng J, Zhou ZJ. 2004. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol 560: 533–549. 10.1113/jphysiol.2004.066597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura SY, Bharioke A, Lu Z, Nern A, Vitaladevuni S, Rivlin PK, Katz WT, Olbris DJ, Plaza SM, Winston P, et al. 2013. A visual motion detection circuit suggested by Drosophila connectomics. Nature 500: 175–181. 10.1038/nature12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Smith BE, Feller MB. 2018. Light prior to eye opening promotes retinal waves and eye-specific segregation. Neuron 100: 1059–1065.e4. 10.1016/j.neuron.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett JW, Feldheim DA. 2012. Eph and ephrin signaling in the formation of topographic maps. Semin Cell Dev Biol 23: 7–15. 10.1016/j.semcdb.2011.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugur B, Chen K, Bellen HJ. 2016. Drosophila tools and assays for the study of human diseases. Dis Model Mech 9: 235–244. 10.1242/dmm.023762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Aleman J, Fetter RD, Sales EC, Heckman EL, Venkatasubramanian L, Doe CQ, Landgraf M, Cardona A, Zlatic M. 2021. Comparative connectomics reveals how partner identity, location, and activity specify synaptic connectivity in Drosophila. Neuron 109: 105–122.e7 10.1016/j.neuron.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken KJ, Simpson JH, Bellen HJ. 2011. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron 72: 202–230. 10.1016/j.neuron.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völgyi B, Kovács-Öller T, Atlasz T, Wilhelm M, Gábriel R. 2013. Gap junctional coupling in the vertebrate retina: variations on one theme? Prog Retin Eye Res 34: 1–18. 10.1016/j.preteyeres.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Vonhoff F, Keshishian H. 2017. Activity-dependent synaptic refinement: new insights from Drosophila. Front Syst Neurosci 11: 23. 10.3389/fnsys.2017.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HC, Bergles DE. 2015. Spontaneous activity in the developing auditory system. Cell Tissue Res 361: 65–75. 10.1007/s00441-014-2007-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H. 2004. Parallel processing in the mammalian retina. Nat Rev Neurosci 5: 747–757. 10.1038/nrn1497 [DOI] [PubMed] [Google Scholar]
- Wei H, Kyung HY, Kim PJ, Desplan C. 2020. The diversity of lobula plate tangential cells (LPTCs) in the Drosophila motion vision system. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 206: 139–148. 10.1007/s00359-019-01380-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weliky M, Katz LC. 1999. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science 285: 599–604. 10.1126/science.285.5427.599 [DOI] [PubMed] [Google Scholar]
- Wong RO. 1999. Retinal waves and visual system development. Annu Rev Neurosci 22: 29–47. 10.1146/annurev.neuro.22.1.29 [DOI] [PubMed] [Google Scholar]
- Wong RO, Chernjavsky A, Smith SJ, Shatz CJ. 1995. Early functional neural networks in the developing retina. Nature 374: 716–718. 10.1038/374716a0 [DOI] [PubMed] [Google Scholar]
- Wong WT, Sanes JR, Wong RO. 1998. Developmentally regulated spontaneous activity in the embryonic chick retina. J Neurosci 18: 8839–8852. 10.1523/JNEUROSCI.18-21-08839.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wörsdörfer P, Wagner N, Ergün S. 2018. The role of connexins during early embryonic development: pluripotent stem cells, gene editing, and artificial embryonic tissues as tools to close the knowledge gap. Histochem Cell Biol 150: 327–339. 10.1007/s00418-018-1697-2 [DOI] [PubMed] [Google Scholar]
- Wu L, Dong A, Dong L, Wang SQ, Li Y. 2019. PARIS, an optogenetic method for functionally mapping gap junctions. Elife 8: e43366. 10.7554/eLife.43366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HP, Furman M, Mineur YS, Chen H, King SL, Zenisek D, Zhou ZJ, Butts DA, Tian N, Picciotto MR, et al. 2011. An instructive role for patterned spontaneous retinal activity in mouse visual map development. Neuron 70: 1115–1127. 10.1016/j.neuron.2011.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HP, Burbridge TJ, Chen MG, Ge X, Zhang Y, Zhou ZJ, Crair MC. 2015. Spatial pattern of spontaneous retinal waves instructs retinotopic map refinement more than activity frequency. Dev Neurobiol 75: 621–640. 10.1002/dneu.22288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Laboulaye MA, Tran NM, Whitney IE, Benhar I, Sanes JR. 2020. Mouse retinal cell atlas: molecular identification of over 60 amacrine cell types. J Neurosci 40: 5177–5195. 10.1523/JNEUROSCI.0471-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R. 2015. From the neuron doctrine to neural networks. Nat Rev Neurosci 16: 487–497. 10.1038/nrn3962 [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. 2001. Electrical activity and development of neural circuits. Nat Neurosci 4: 1207–1214. 10.1038/nn753 [DOI] [PubMed] [Google Scholar]
- Zhang J, Ackman JB, Xu HP, Crair MC. 2012. Visual map development depends on the temporal pattern of binocular activity in mice. Nat Neurosci 15: 298–307. 10.1038/nn.3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RW, Li XQ, Kawakami K, Du JL. 2016. Stereotyped initiation of retinal waves by bipolar cells via presynaptic NMDA autoreceptors. Nat Commun 7: 12650. 10.1038/ncomms12650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RW, Du WJ, Prober DA, Du JL. 2019. Müller glial cells participate in retinal waves via glutamate transporters and AMPA receptors. Cell Rep 27: 2871–2880.e2. 10.1016/j.celrep.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JJ, Lee S, Zhou ZJ. 2004. A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron 44: 851–864. 10.1016/j.neuron.2004.11.015 [DOI] [PubMed] [Google Scholar]
- Zheng J, Lee S, Zhou ZJ. 2006. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nat Neurosci 9: 363–371. 10.1038/nn1644 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Lauritzen JS, Perlman E, Robinson CG, Nichols M, Milkie D, Torrens O, Price J, Fisher CB, Sharifi N, et al. 2018. A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell 174: 730–743.e22. 10.1016/j.cell.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]