Abstract
Background
The Milan metropolitan area in Northern Italy was among the most severely hit by the SARS-CoV-2 outbreak. The aim of this study was to examine the seroprevalence trends of SARS-CoV-2 in healthy asymptomatic adults, and the risk factors and laboratory correlates of positive tests.
Materials and methods
We conducted a cross-sectional study in a random sample of blood donors, who were asymptomatic at the time of evaluation, at the beginning of the first phase (February 24th to April 8th 2020; n=789). Presence of IgM/IgG antibodies against the SARS-CoV-2-Nucleocapsid protein was assessed by a lateral flow immunoassay.
Results
The test had a 100/98.3 sensitivity/specificity (n=32/120 positive/negative controls, respectively), and the IgG test was validated in a subset by an independent ELISA against the Spike protein (n=34, p<0.001). At the start of the outbreak, the overall adjusted seroprevalence of SARS-CoV-2 was 2.7% (95% CI: 0.3–6%; p<0.0001 vs 120 historical controls). During the study period, characterised by a gradual implementation of social distancing measures, there was a progressive increase in the adjusted seroprevalence to 5.2% (95% CI: 2.4–9.0; 4.5%, 95% CI: 0.9–9.2% according to a Bayesian estimate) due to a rise in IgG reactivity to 5% (95% CI: 2.8–8.2; p=0.004 for trend), but there was no increase in IgM+ (p=not significant). At multivariate logistic regression analysis, IgG reactivity was more frequent in younger individuals (p=0.043), while IgM reactivity was more frequent in individuals aged >45 years (p=0.002).
Discussion
SARS-CoV-2 infection was already circulating in Milan at the start of the outbreak. The pattern of IgM/IgG reactivity was influenced by age: IgM was more frequently detected in participants aged >45 years. By the end of April, 2.4–9.0% of healthy adults had evidence of seroconversion.
Keywords: blood donors, coronavirus, epidemiology, prevalence
INTRODUCTION
The Milan metropolitan area in Northern Italy was the first in Western countries to be severely hit by the spread of SARS-CoV-2, the virus that causes COVID-19 disease. COVID-19 is frequently complicated by the development of respiratory failure, which in this geographical area was characterised by a 26% mortality rate in critically ill patients1. About 45% of individuals infected by SARS-CoV-2 are asymptomatic or mildly symptomatic, and they represent a major source of viral spread2–4. It has been estimated that in the first half of 2020, 9.8% of the Italian population had already been infected by SARS-CoV-25. However, epidemiological trends in individuals with mild COVID-19 remain unknown. SARS-CoV-2 replication is followed by IgM/IgG seroconversion, which can improve COVID-19 diagnosis and the evaluation of circulation of SARS-CoV-26,7. On the other hand, it is not known whether routine laboratory tests can help to identify asymptomatic carriers.
The transfusion centre at the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico (Policlinico Hospital) is the main blood centre in Milan, collecting almost 40,000 blood donations per year. Over the last 25 years, we have provided evidence that blood donor cohorts represent a special vantage point from which to study subclinical conditions, and to describe the prevalence, incidence and natural course of infectious diseases8,9. Studies in blood donors might help to assess the dynamics of viral circulation, and to model the evolution of the COVID-19 pandemic10.
The main aim of this study was, therefore, to examine the trend in the prevalence of SARS-CoV-2 antibody reactivity among healthy individuals who were asymptomatic at the time of evaluation during the initial outbreak (the first phase of the pandemic) in the Milan area. We also assessed the risk factors and laboratory features associated with positive tests.
MATERIALS AND METHODS
Study cohorts
We considered 789 individuals donating blood between February 24th (the first week of the Italian outbreak) and April 8th 2020 whose plasma samples had been stored for haemovigilance studies.
The main study cohort was composed of blood donors who were apparently healthy subjects, aged 18–70 years. Exclusion criteria were any active infection or other active medical conditions, recent surgical procedures, trips to areas with endemic infective diseases, reported risk factors for parenterally acquired infections, chronic degenerative conditions (except for those with stable arterial hypertension), type 2 diabetes or dyslipidaemia under control with changes in lifestyle or pharmacological therapy, diagnosis of cancer or high risk of cardiovascular events. All donors underwent clinical and medical history evaluation and biochemical testing. To qualify for blood donation, candidates had to be free of recent symptoms possibly related to COVID-19 and not to have had any close contact with confirmed cases. After March 2nd, they had to be symptom free during the preceding 14 days, and not to have had unprotected contact with suspected cases11.
Among the 3,586 individuals who donated blood during the study period, we randomly selected 20 each day to assess SARS-CoV-2 seroprevalence; their clinical features were representative of the overall population (data not shown). Clinical features of the 789 individuals included in the study are shown in Table I.
Table I.
Clinical features of 789 individuals who donated blood between February 24th and April 8th 2020 stratified by the presence of anti-SARS-CoV-2 antibodies
| Characteristics | SARS-CoV-2 antibodies | p* | |
|---|---|---|---|
| Present | Absent | ||
| N | 40 (5.1%) | 749 (94.9%) | |
| Age, years | 42.6±13.4 | 40.7±13.2 | 0.37 |
| Sex, F | 10 (25.0%) | 266 (35.6%) | 0.16 |
| Resident in Milan | 24 (60%) | 497 (66.4%) | 0.91 |
| Health-care profession, yes | 2 (5%) | 35 (5.1%) | 0.75 |
| BMI, kg/m2 | 25.5±3.3 | 24.3±3.7 | 0.040 |
| Arterial hypertension, yes | 2 (3.2%) | 60 (8.1%) | 0.76 |
| Smoking, active | 7 (17.5%) | 197 (27.3%) | 0.27 |
| ABO blood group, N (0/A/A1/AB/B), % | 16/16/0/2/6 (40/40/0/5/15) | 341/287/3/27/90 (45.6/38.4/0.4/3.6/12.0) | 0.92 |
| Week of outbreak, N | 4.5 [2–6] | 4.0 [2–5] | 0.10 |
| Lymphocytes, 103/mm3 | 1.94±0.54 | 1.91±0.52 | 0.70 |
| ALT, IU/L | 25.3±11.9 | 25.6±16.0 | 0.81 |
| GGT, IU/L | 24.3±20.0 | 18.2±13.1 | 0.090 |
Data are shown as mean±standard deviation, median [interquartile range].
At logistic regression analysis.
N: number (%) values; F: female; BMI: body mass index; ALT: alanine aminotransferases, GGT: gamma-glutamyl transferases.
To gain further insight into the epidemiological trends before the outbreak, we also examined anonymised samples of 184 individuals who presented themselves for blood donation between December 2019 and March 2020, and who had been included in a screening programme for metabolic disorders (Bible study, mean age 54.7±6.4, 89.6% of male sex, body mass index [BMI] 28.8±3.4 kg/m2). The study protocol complies with Good Clinical Practice (GCP) rules, the Declaration of Helsinki, European clinical practice, international guidelines and Italian national legal regulations, and was approved by the Ethical Committee of the Policlinico Hospital (“COVID-19 Donors Study” [CoDS] n. 334–2020 of 3rd April 2020). Each blood donor signed written informed consent to allow testing for communicable diseases, storage of anonymised data and biological materials for diagnostic and research purposes, and use of their de-identified data for clinical research. The blood donors’ organisation supporting our centre was involved in planning and designing the study (https://www.donatoriamici.it). Implementation of widespread testing of donors during the next study phase will be carried forward in consideration of the concerns expressed by the blood donors’ organisation.
Evaluation of anti-SARS-CoV-2 antibodies
The presence of IgM/IgG against SARS-CoV-2 were determined on plasma samples (20 μL) by a lateral flow immunoassay against the Nucleocapsid protein (COVID-19 IgG/IgM Rapid Test, Prima Lab, Balerna, Switzerland). The study test was chosen because, at the time of protocol approval, it had already been certified by the European Commission and provided reasonable accuracy in pre-clinical evaluation when compared to real-time-polymerase chain reaction (RT-PCR) results. The study was performed at the Laboratory of Infectious Diseases at “Luigi Sacco” Hospital, Milan. The antibody is directed against the nucleocapsid antigen of SARS-CoV-2. The reported accuracy for the lateral flow immunoassay for IgG was: specificity 98.0%, sensitivity 100%, accuracy 98.6%; for IgM: specificity 96.0%, sensitivity 85.0%, accuracy 92.9%. In addition, it was easy to perform and potentially applicable to rapid pre-donation screening. The test was read by two independent expert biologists: both had to agree on the test results. Results were considered positive when strong or weak immunoreactivity for IgG or IgM was detected, while very weak or dubious positive results were considered not specific. To increase the robustness of the test, the serological assay was locally validated by the same operators who read the donors samples; sample sets were larger than those reported by the manufacturer. The validation of the test performance was carried out by testing 32 consecutive patients admitted to the participating institutions (between February and March 2020; 22 at “Luigi Sacco” Hospital and ten at Policlinico Hospital) with typical symptoms of COVID-19. Diagnosis was confirmed by at least one quantitative (q)RT-PCR test on plasma samples collected at admission and 10 days post admission for the “Luigi Sacco” Hospital and 28 days post admission for the Policlinico Hospital (positive controls). As negative controls, we examined plasma samples of 120 patients who had been assessed for blood transfusion during the year 2009 at the Policlinico Hospital in Milan. In a subset of cases (n=34 blood donors selected according to the reactivity pattern: 5 IgG positive [IgG+], 5 IgG/IgM+, 4 IgM+, 21 negative), the lateral flow immunoassay IgG test was compared with a home-made ELISA evaluating antigens in the SARS-CoV-2 Spike protein (soluble ectodomain)12. The diagnostic threshold for positive results of ELISA IgG (OD=0.386) was selected on the basis of a receiver operating characteristic curve analysis performed on historical negative controls and in an independent set of swab-confirmed COVID-19 cases (95% sensitivity and 97% specificity)12.
Statistical analysis
We assumed that the outbreak of SARS-CoV-2 infection started in the Milan area at the beginning of February 2020, so that we could expect a low rate (95% confidence interval [CI] 0–2%) of IgM+ at the beginning of the study (end of February 2020). We report here the results of an analysis of the first CoDS study period. This study had already been planned to gain a timely insight into the dynamic of viral spread in order to inform healthcare decisions.
For descriptive statistics, continuous traits were summarised as mean±standard deviation (SD). Highly skewed variables were summarised as medians and interquartile range. Categorical variables were shown as percentages. The seroprevalence was reported as rate and 95% CI; these data were used to estimate the population prevalence of SARS-CoV-2 antibodies. The proportion of positive tests (either IgM or IgG) was then adjusted for the diagnostic accuracy (adjusted prevalence = [observed prevalence + Specificity-1]/[Sensitivity+Specificity-1]). We also provided a further estimate of the true population prevalence adjusted for the accuracy of the test (considering the 95% confidence intervals of the test accuracy) by a Bayesian approach, as previously described13. For the Bayesian estimate of SARS-CoV-2 seroprevalence, the 95% CI estimates of sensitivity and specificity of the test were derived from local cases (n=22) and controls (n=120). Analyses were performed by fitting data to logistic regression models in such a way as to examine binary traits (presence of IgM and/or IgG antibodies). Analyses were adjusted for main known confounders, as specified in the Results section. p<0.05 (two-tailed) was considered statistically significant.
Results were reported according to the STROBE (Strengthening the Reporting of Molecular Epidemiology for Infectious Diseases) guidelines. Statistical analysis was carried out using the JMP Pro 14.0 Statistical Analysis Software (SAS Institute, Cary, NC, USA) and R statistical analysis software version 3.5.2 (http://www.R-project.org/).
RESULTS
Validation of the diagnostic test
The lateral flow immunoassay showed a 100% sensitivity for IgG (95% CI: 84–100%) and 68.2% sensitivity for IgM (95% CI: 45–86%) for detecting SARS-CoV-2 infection in the 22 COVID-19 patients either at admission or 10 days after they were hospitalised (Online Supplementary Content Figure S1). The ten patients tested at 28 days post admission were all positive for IgG and negative for IgM. In addition, the test detected a clear IgG reactivity in one mildly symptomatic individual, who was tested two weeks after viral RNA on nasal swab became negative. In 120 patients who were assessed for blood transfusion during the year 2009, the specificity for either IgG or IgM reactivity was 98.3% (95% CI: 94.1–99.5%; 99.2% for IgM reactivity and 99.2% for IgG reactivity). The two false positive individuals were in their 60s and had had a recent history of cancer: active urological cancer in a male with IgG reactivity, while the IgM reactive woman had a diagnosis of rheumatoid arthritis with positive rheumatoid factor.
In a subset of the study cohort, the lateral flow immunoassay had a good agreement with ELISA test to detect IgG (k=0.59±0.16; p<0.001). Interestingly, 3 out of 5 discordant cases between the lateral flow and ELISA tests occurred in IgM+ samples. In IgM negative samples, the agreement of lateral flow immunoassay and ELISA IgG increased to k=0.71±0.19 (p<0.001).
Seroprevalence trends during the outbreak
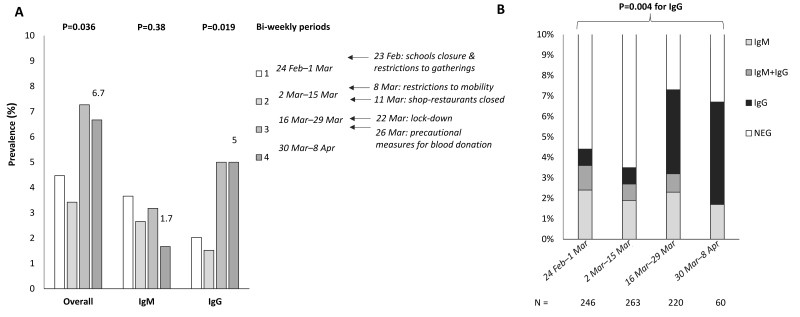
The trend in the overall reactivity to IgM, IgG and the combined profile is reported in Figure 1. During the first week, the baseline prevalence was 4.6% (95% CI: 2.3 to 7.9%; p<0.0001 vs historical controls). We observed a 3.7% prevalence of IgM reactivity and 2.0% of IgG reactivity, respectively. After adjustment for possible inaccuracies in the test, the estimated prevalence was 2.7% (95% CI: 0.3–6%). During the study (Figure 1A), there was a trend for an increase in the overall seroprevalence (p=0.036) due to an increase in IgG reactivity (p=0.019). We observed a tendency for a reduction in the prevalence of IgM reactivity, although this was not significant.
Figure 1.
Seroprevalence trends during the COVID-19 outbreak and lockdown in Milan, Italy
(A) Overall seropositivity (presence of either IgM or IgG reactivity), IgM reactivity and IgG reactivity trends in 789 healthy blood donors enrolled in the COVID-19 Donors Study (CoDS), stratified by time of evaluation (every 2 weeks). p-values were adjusted for age, sex, and body mass index. Main political measures to limit the contagion have been highlighted in the timeline. (B) Frequency and pattern of antibody positivity during the study period (n=789).
The evolution of the combined IgM/IgG reactivity during the study period is presented in Table II and Figure 1B. There was a significant trend for an increase in IgG reactivity over time (p=0.004). At multivariate logistic regression analysis, adjusted for age, sex and BMI, the rate of seroconversion to IgG+ in the study cohort was 1.4±0.7% per week (p=0.005). During the last three weeks of the study (last two periods in Table II and Figure 1), the prevalence of IgG reactivity was 5% (95% CI: 2.8–8.2%) and the overall prevalence of reactivity was 6.7% (95% CI: 4.4–10.8). After adjustment for possible inaccuracies in the test, the estimated prevalence was 5.2% (95% CI: 2.4–9.0).
Table II.
SARS-CoV-2 seroprevalence trends in 789 healthy blood donors according to the study period, in the overall cohort and in participants stratified according to age and sex
| Date | N | IgM+ | IgM+/IgG+ | IgG+ |
|---|---|---|---|---|
| Overall | ||||
| 24/2–1/3 | 246 | 6 (2.4) | 3 (1.2) | 3 (0.8) |
| 2/3–15/3 | 263 | 5 (1.9) | 2 (0.8) | 2 (0.8) |
| 16/3–29/3 | 220 | 5 (2.3) | 2 (0.9) | 9 (4.1) |
| 30/3–8/4 | 60 | 1 (1.7) | 0 | 3 (5.0) |
| Male | ||||
| 24/2–1/3 | 159 | 4 (2.5) | 2 (1.3) | 1 (0.6) |
| 2/3–15/3 | 174 | 4 (2.3) | 1 (0.6) | 2 (1.2) |
| 16/3–29/3 | 142 | 4 (2.8) | 2 (1.4) | 7 (4.9) |
| 30/3–8/4 | 37 | 1 (2.7) | 0 | 2 (5.4) |
| Female | ||||
| 24/2–1/3 | 87 | 2 (2.3) | 1 (1.2) | 1 (1.2) |
| 2/3–15/3 | 89 | 1 (1.1) | 1 (1.1) | 0 |
| 16/3–29/3 | 78 | 1 (1.3) | 0 | 2 (2.6) |
| 30/3–8/4 | 23 | 0 | 0 | 1 (4.4) |
| Age <45 years | ||||
| 24/2–1/3 | 131 | 1 (0.8) | 1 (0.8) | 1 (0.8) |
| 2/3–15/3 | 148 | 0 | 1 (0.7) | 2 (1.4) |
| 16/3–29/3 | 142 | 2 (1.4) | 1 (0.7) | 9 (6.3) |
| 30/3–8/4 | 35 | 0 | 0 | 2 (5.7) |
| Age >45 years | ||||
| 24/2–1/3 | 115 | 5 (4.4) | 2 (1.8) | 1 (0.9) |
| 2/3–15/3 | 115 | 5 (4.4) | 1 (0.9) | 0 |
| 16/3–29/3 | 78 | 3 (3.9) | 1 (1.3) | 0 |
| 30/3–8/4 | 25 | 1 (4.0) | 0 | 1 (4.0) |
As we found some evidence that the SARS-CoV-2 may have been circulating before the end of February 2020, we also tested an independent cohort of 184 donors with dysmetabolism recruited between December 2019 and February 2020. The prevalence of reactive tests, stratified for the combined IgM and IgG reactivity, during the 3 months preceding the study as compared to the study period is reported in the Online Supplementary Content (Supplementary Results and Figure S2).
By using a Bayesian approach considering a wide range of test performance based on the accuracy estimates we had obtained, the estimated mode of the prevalence during the first 2 weeks was 1.0% (95% CI: 0.1–5.6%) compared with 4.5% (95% CI: 0.9–9.2%) during the last 3 weeks. The probability distribution is shown in Online Supplementary Content (Figure S3).
Clinical features of seropositive individuals
The predictors of a serological pattern suggestive of previous SARS-CoV-2 infection (IgG reactivity) and recent infection (IgM reactivity) are shown in Table III. IgG reactivity increased progressively over time (p=0.039) and was more frequently detected in younger individuals (p=0.043). After adjustment for age, sex, and BMI, IgG reactivity was not associated with altered laboratory parameters (data not shown).
Table III.
Independent predictors of the presence of anti-SARS-CoV-2 IgG and IgM antibodies in 789 healthy individuals who donated blood between February 24th and April 8th 2020
| Predictors | IgG+ | IgM+ | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p* | OR | 95% CI | p° | |
| Age, per year | 0.96 | 0.93–1.00 | 0.043 | 1.05 | 1.02–1.09 | 0.002 |
| Sex, F | 0.61 | 0.24–1.61 | 0.31 | 0.78 | 0.30.2.03 | 0.60 |
| BMI, kg/m2 | 1.07 | 0.97–1.17 | 0.21 | 1.07 | 0.96–1.18 | 0.24 |
| Time, weeks | 1.26 | 1.00–1.57 | 0.039 | 0.85 | 0.24–3.02 | 0.80 |
At logistic regression analysis adjusted for the independent predictors shown.
Further adjusted for IgG.
N: number; OR: odds ratio; CI: confidence interval; F: female; BMI: body mass index.
Consistently with data reported in Table II, IgM reactivity was more frequent in older study subjects (p=0.002) but did not change over time. In particular, IgM reactivity was more frequently observed in donors aged >45 years (18 of 331, 5.4% vs 6 of 457, 1.3%; p=0.001). At logistic regression analysis adjusted for age, sex, BMI and IgG+ (Table IV), IgM reactivity was associated with higher triglycerides (p=0.0078), circulating eosinophils (p=0.036), and lymphocytes (p=0.048).
Table IV.
Biochemical and haematologic parameters associated with detection of IgM antibodies (n=24) against SARS-CoV-2 among 789 healthy individuals who donated blood between February 24th and April 8th 2020
| Parameters | N | Estimate±SE | p* |
|---|---|---|---|
| Triglycerides, mg/dL | 409 | +0.017±0.006 | 0.0078 |
| HDL cholesterol, mg/dL | 409 | −0.068±0.035 | 0.058 |
| Eosinophils, 103/mm3 | 789 | +2.59±1.24 | 0.036 |
| Lymphocytes, 103/mm3 | 789 | +0.81±0.42 | 0.048 |
At logistic regression analysis adjusted for age, sex, body mass index, presence of IgG antibodies.
No significant association was found between IgM+ and aspartate aminotransferase (AST), alanine aminotransferases (ALT), gamma-glutamyl transferases (GGT), glucose, total cholesterol, creatinine, haemoglobin (Hb), mean corpuscular volume (MCV), circulating neutrophils, basophils, monocytes or presence of arterial hypertension. HDL: high-density lipoprotein; SE: standard error. Results are shown for p<0.1.
DISCUSSION
In this study, we examined the trend for SARS-CoV-2 seroprevalence in a random sample of healthy blood donors during the Milan COVID-19 outbreak. We exploited a lateral membrane immunoassay directed against the Nucleocapsid protein that, in our hands, showed acceptable specificity (>99% for IgG and IgM) and concordance with an independent ELISA test. The diagnostic accuracy of this assay was comparable to that reported in the literature for the best performing lateral flow assays, though we observed a particularly high specificity and an excellent sensitivity to detect severe COVID-19 after 14 days following hospital admission14. In previous studies, the same assay showed 100% sensitivity and 90.3% specificity as compared to nasopharyngeal swabs for IgG reactivity (91.3% specificity for IgM), and 92% sensitivity and 98% specificity as compared to a reference ELISA test14,15. Notably, antibodies against the Nucleocapsid protein may be more persistent, especially in individuals who did not develop severe COVID-19, making them possibly more suitable for the evaluation of viral circulation in asymptomatic individuals12.
The first main study finding was that, during the last week of February (considered the start of the outbreak in the northern Lombardy region which includes Milan16), approximately 4.6% of healthy adults were already positive for IgM (3.7%) and/or IgG (2.0%) antibodies, leading to an estimated adjusted prevalence of SARS-CoV-2 infection of approximately 2.7%. The true prevalence in the study population was estimated at 1.0% by a conservative Bayesian approach that considered the lower confidence intervals of the assay specificity. These data indicate that the infection was spreading in the general population before the rapid rise in severe COVID-19 cases was observed. During the study period, we observed a progressive increase in reactivity to IgG antibodies. However, seroconversion to IgG reactivity likely reflected infections acquired before major social distancing measures were put in place. Notably, the prevalence of IgG reactivity observed at the end of the observation period was in line with that reported in independent epidemiological studies conducted according to different methodological approaches adopted by health care workers in Northern Italy17,18, suggesting that, despite the inherent limitations of this approach, monitoring blood donors may help track viral circulation in the general population. In addition, the conclusion that the virus was already circulating in the population during February 2020 is consistent with environmental monitoring data suggesting that SARS-CoV-2 infection hit Northern Italy in December 201919.
The divergent impact of age on seroprevalence trends (i.e., IgG reactivity associated with younger and IgM reactivity with older age) is consistent with the possibility that, before the lockdown restrictions, SARS-CoV-2 was more widely prevalent in younger individuals, whereas after the closure of schools and universities, the spread was mainly generated by work contacts among older active individuals. These residual infections may account for the lack of a significant decrease in IgM reactivity, despite a non-significant trend, during the observation period. If confirmed, these data would be consistent with the view that school closures and lockdown restrictions helped control the spread of the disease16. Alternatively, IgM reactivity may also be accounted for by false positive results (in the range of the specificities reported in other studies) or by preferential seroconversion to IgM in participants aged >45 years. In contrast, we did not observe any significant impact of having an A blood group on IgM/G reactivity20.
In the present study, at the end of the observation period, it was estimated that 4.5–5.2% of blood donors had developed antibodies against SARS-CoV-2 according to adjusted estimates of the true prevalence taking into account the limited accuracy of the test. Therefore, among healthy young adults who did not develop severe COVID-19 symptoms and who were asymptomatic at the time of evaluation, about 1 in 22 was not diagnosed by nasal swabs during the first wave of the outbreak, although they were probably infected by SARS-CoV-2. This figure does not rule out the possibility that a larger fraction (up to 1 in 2 individuals) may have developed mild symptoms4.
Interestingly, we detected an association between a serological pattern consistent with recent infection (IgM reactivity) and higher triglycerides, eosinophils and lymphocytes. Hypertriglyceridaemia has been associated with inflammation in patients with COVID-19, but severe infection is usually associated with a decrease in circulating lymphocytes and eosinophils21,22. It could be speculated that an effective immune reaction against SARS-CoV-2 is marked by a distinct pattern of immune and circulating leukocyte response. Of note, eosinophils have recently been implicated in the mucosal response to viral infections in the lung, and the immune homeostasis and IgA production in the intestine23. However, the interpretation of these findings is limited by the fact that, given the diagnostic accuracy of the test and disease prevalence, in more than one-third of cases positive tests are likely not specific. Furthermore, ageing and the associated immunological modifications may have influenced the preferential seroconversion to IgM.
This study has other limitations. Lateral flow immunoassays may have limited accuracy, although they showed adequate performance for epidemiological studies24. Although follow-up data are not yet available, the study test was validated in a subset of cases by ELISA. In addition, an overestimate of the sensitivity of the test may have led to a modest underestimate of the true prevalence of SARS-CoV-2 infection, but the adjusted rate of IgG+ increase during the study was less likely affected. However, due to the abrupt and unexpected sanitary emergency that Northern Italy had to face in March 2020, the study could only be conducted retrospectively.
Therefore, we were unable to systematically validate all positive results against a reference test, and donors who tested positive for IgM could not be evaluated by molecular biology approaches to assess persistence of evidence of viral replication. These data, together with the evaluation of the presence of SARS-CoV-2 viremia in blood products25, are now being collected prospectively within the CoDS study in order to better inform transfusion medicine policies in the next months25. Finally, blood donors are generally healthier than the average general population and do not include either the very young or the very elderly. Therefore, their patterns of social interactions and COVID-19 susceptibility may differ to those in the overall population.
CONCLUSIONS
In conclusion, SARS-CoV-2 infection was already circulating in Milan at the start of the COVID-19 outbreak and, by April 8th 2020, 2.4–9.0% of healthy adults had evidence of seroconversion.
Supplementary Information
ACKNOWLEDGEMENTS
We thank Prima Lab, for providing the COVID-19 IgG/IgM Rapid Test free of charge for the present study. Prima Lab had no role in data collection, analysis, or interpretation; it was not involved in the writing of the report or in the decision to submit it for publication. We also thank the Fondazione Pesenti (Bergamo, Italy), for an unrestricted grant to support the CoDS study.
Appendix 1. The COVID-19 Donors Study (CoDS) network
Daniele Prati, Luca Valenti, Serena Pelusi, Luigi Santoro, Guido Baselli, Elisa Erba, Valeria Ferri - Transfusion Medicine Dept., Fondazione IRCCS Ca’ Granda, Milan
Gianguglielmo Zehender, Massimo Galli - Laboratory of Infectious Diseases, "Luigi Sacco" Hospital, Milan
Silvano Bosari and Luigia Scudeller - Scientific Direction, Fondazione IRCCS Ca' Granda, Milan
Giancarlo Liumbruno - Italian National Blood Centre, Rome, Italy
Giovanna Lunghi - Laboratory Medicine, Fondazione IRCCS Ca’ Granda, Milan
Andrea Gori, Alessandra Bandera - Infectious Diseases, Fondazione IRCCS Ca’ Granda, Milan
Federica Facciotti, Marina Mapelli, Sebastiano Pasqualato - Mucosal Immunology Lab., European Institute of Oncology, Milan
Footnotes
FUNDING
The study was funded by Ricerca Corrente Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan (institutional funding to DP), COVID-19 Network Registry Biobanking, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan (to LV), Finalized Research of the Italian Ministry of Health (Ministero della Salute) RF-2016-02364358 (to LV), Fondazione Sviluppo Ca’ Granda, Milan, for the project Liver-Bible-PR-0361 (to LV), “Finanziamento 5permille” Fondazione IRCCS Ca’ Granda “COVID-19 Biobank” (to LV), the EU Programme Horizon 2020 (under grant agreement no. 777377) for the project LITMUS (to LV).
AUTHORSHIP CONTRIBUTIONS
LV and AB contributed equally to this work as first Author. LV, DP and GZ are responsible for study conception and design. LV, DP, GZ and MG are responsible for study funding. DP, MG and SB supervised the study. LV is responsible for the first draft of the manuscript. LV, DP, AB, GZ and FC are responsible for data analysis and interpretation. SP, AL, AL, AB, LS, GB and CV are responsible for data collection. MT, FF, AL and EE are responsible for processing and testing the samples. All Authors contributed to writing the manuscript and approved the final version for publication.
The Authors declare no conflict of interests.
REFERENCES
- 1.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–81. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382:2302–15. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–93. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavezzo E, Franchin E, Ciavarella C, et al. Suppression of COVID-19 outbreak in the municipality of Vo, Italy. medRxiv. 2020 2020.04.17.20053157. [Google Scholar]
- 5.Flaxman S, Mishra S, Gandy A, et al. Estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries. 2020. [Accessed on 05/04/2020.]. https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-13-europe-npi-impact/
- 6.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. MedRXiv. 2020 2020.03.02.20030189. [Google Scholar]
- 7.Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–8. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 8.Prati D, Capelli C, Zanella A, et al. Influence of different hepatitis C virus genotypes on the course of asymptomatic hepatitis C virus infection. Gastroenterology. 1996;110:178–83. doi: 10.1053/gast.1996.v110.pm8536854. [DOI] [PubMed] [Google Scholar]
- 9.Prati D, Capelli C, Zanella A, et al. Asymptomatic hepatitis G virus infection in blood donors. Transfusion. 1997;37:1200–4. doi: 10.1046/j.1537-2995.1997.37111298088052.x. [DOI] [PubMed] [Google Scholar]
- 10.Chinazzi M, Davis JT, Ajelli M, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368:395–400. doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchini M, Farrugia A, Velati C, et al. The impact of the SARS-CoV-2 outbreak on the safety and availability of blood transfusions in Italy. Vox Sang. 2020;115:603–5. doi: 10.1111/vox.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruni M, Cecatiello V, Diaz-Basabe A, et al. Persistence of anti-SARS-CoV-2 antibodies in non-hospitalized COVID-19 convalescent health care workers. J Clin Med. 2020;9:3188. doi: 10.3390/jcm9103188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diggle PJ. Estimating prevalence using an imperfect test. Epidemiology Research International. 2011;2011 Article ID 608719. [Google Scholar]
- 14.Van Elslande J, Houben E, Depypere M, et al. Diagnostic performance of seven rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin Microbiol Infect. 2020;26:1082–7. doi: 10.1016/j.cmi.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perico L, Tomasoni S, Peracchi T, et al. COVID-19 and lombardy: testing the impact of the first wave of the pandemic. EBioMedicine. 2020;61:103069. doi: 10.1016/j.ebiom.2020.103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giordano G, Blanchini F, Bruno R, et al. Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy. Nat Med. 2020;26:855–60. doi: 10.1038/s41591-020-0883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plebani M, Padoan A, Fedeli U, et al. SARS-CoV-2 serosurvey in health care workers of the Veneto Region. Clin Chem Lab Med. 2020;58:2107–11. doi: 10.1515/cclm-2020-1236. [DOI] [PubMed] [Google Scholar]
- 18.Spreafico M, Raffaele L, Guarnori I, et al. Prevalence and 9-year incidence of hepatitis E virus infection among North Italian blood donors: Estimated transfusion risk. J Viral Hepat. 2020;27:858–61. doi: 10.1111/jvh.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Rosa G, Mancini P, Bonanno Ferraro G, et al. SARS-CoV-2 has been circulating in northern Italy since December 2 019: Evidence from environmental monitoring. Sci Total Environ. 2021;750:141711. doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Yang Y, Huang H, et al. Relationship between the ABO blood group and the COVID-19 susceptibility. medRxiv. 2020 2020.03.11.20031096. [Google Scholar]
- 21.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–9. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah K, Ignacio A, McCoy KD, et al. The emerging roles of eosinophils in mucosal homeostasis. Mucosal Immunol. 2020;13:574–83. doi: 10.1038/s41385-020-0281-y. [DOI] [PubMed] [Google Scholar]
- 24.Adams ER, Ainsworth M, Anand R, et al. Evaluation of antibody testing for SARS-Cov-2 using ELISA and lateral flow immunoassays. medRxiv. 2020 2020.04.15.20066407. [Google Scholar]
- 25.Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–24. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.