Abstract
Background
Several factors contribute to the manifestation of red blood cell (RBC) storage lesions, with one of the most interesting being the “donor variation effect”. Since many haematological characteristics of blood donors are sex-dependent, sex hormones and their age-dependent variation may affect the storage profile of RBCs.
Materials and methods
Fresh blood from 200 healthy male and female donors underwent haematological, biochemical and physiological analysis. Three selected groups of donors (men, n=8; pre-menopausal women, n=8; and post-menopausal women, n=4) exhibiting as similar as possible baseline values were recruited for blood donation in leukoreduced CPD/SAGM units. RBC indices, haemolysis and propensity for haemolysis, reactive oxygen species (ROS) and plasma antioxidant capacity were measured bi-weekly.
Results
Female blood was characterised by lower plasma antioxidant capacity and free haemoglobin (Hb) levels in vivo, in spite of the higher RBC osmotic fragility, compared to male blood. Comparatively low Hb concentration was also measured in stored RBCs from female donors, as in vivo. Mean corpuscular Hb (MCH), mean corpuscular Hb concentration (MCHC), and plasma antioxidant capacity were also lower in female donors throughout storage, even though baseline levels were equal to those of the male group. There was no difference in propensity of stored RBCs for haemolysis between male and female units but intracellular ROS levels were significantly lower in female RBCs. Increased end-of-storage extracellular potassium and recruitment of protein stress markers (clusterin, Hb) to the RBC membrane were observed in the units of post- vs pre-menopausal female donors at mid-storage onwards.
Discussion
Donor’s sex has an impact on Hb concentration and redox parameters of stored RBCs. In addition, menopause seems to promote RBC membrane remodelling, at least during prolonged storage. Our pilot study provides new insights on the different effects on RBC storage lesion according to sex.
Keywords: red cell storage lesion, donor variation, female donors, menopause
INTRODUCTION
Transfusion of red blood cells (RBCs) is the most common medical procedure practiced around the world, thus the optimal preservation of RBC units is a matter of utmost importance. Even under the best available storage conditions, RBCs undergo a series of alterations in their membrane, energy metabolism and mechanical properties1. These deteriorations have been linked to reduced RBC viability and adverse reactions in transfusion recipients2–5. Several factors contribute to the manifestation of storage lesions, with one of the most interesting being the so-called “donor variation effect”. Every blood donor possesses a set of unique genetic and non-genetic features that might affect the RBC capacity to endure storage stresses, and consequently these have an impact on transfusion outcome6.
Many haematological characteristics of blood donors are age- and sex-dependent. After the onset of menstruation, women present lower haemoglobin (Hb) levels and RBC count7, as well as lower ferritin, folate and other iron homeostasis indices8. Moreover, major antioxidant enzymes are differentially regulated by menstrual hormone fluctuations9. Uric acid, the main plasma antioxidant that has been linked to storage quality10, and RBC membrane microvesiculation have both been found lower in premenopausal women than in men11 and postmenopausal women12, respectively. Consequently, the presence or absence of Y chromosome, sex hormones and their age-dependent variation may affect RBC storage lesion and transfusion efficacy and effects.
The probable diversity in response to cold storage conditions between RBCs from male and female donors has so far been little explored, though sex-related variation in blood Hb levels may in the first place differentiate the corresponding RBC units13. Furthermore, stored female RBCs are less susceptible to storage, osmotic, oxidative14,15 and mechanical haemolysis16 when compared to male RBCs. While this feature seems to be an early indication of better recovery of female RBCs in recipients, an adverse association of units from female donors with increased mortality17, especially in sex miss-matched transfusions, was observed18. The aim of this study was to search for biological differences in RBCs of female (both pre- and post-menopausal women) and male donors both at baseline and during storage, in order to unravel some of the aspects of the complex donor variation effect.
MATERIALS AND METHODS
Biological samples and blood unit preparation
Fresh blood from 204 healthy donors (101 men and 99 women aged 18–28 years, and 4 post-menopausal women aged 40–48 years) was collected into EDTA, citrate and serum vacutainer tubes. Twenty leukoreduced RBC units (8 from male, 8 from pre-menopausal female and 4 from post-menopausal female donors) containing citrate-phosphate-dextrose (CPD)/saline-adenine-glucose-mannitol (SAGM) were prepared and stored for 42 days at 4 °C. Sampling was performed aseptically after 2, 14, 28 and 42 days of storage. The smallest as possible baseline differences in the measured parameters were used as criterion for the construction of male/female groups. Classification of post-menopausal female donors was based on age and personal statements that were subsequently verified by serum estradiol measurement.
Haematological and biochemical parameters
BC-3000 PLUS, MINDRAY Celltac E, MEK-7222 K, NIHON KOHDEN automatic blood cell counters were used for complete blood count through double measurements in order to achieve maximum reliability, while the automatic analysers Hitachi 902, AVL Series Electrolyte Analyzer 9180 and Elecsys Systems Analyzer (Roche Diagnostics, Risch-Rotkreuz, Switzerland) were used for the biochemical analysis of triglycerides, lipoproteins (e.g. LDL, HDL), iron (Fe), electrolytes (K, Na), ferritin and estradiol.
Haemolysis parameters
To measure free haemoglobin levels before and during storage, a spectrophotometric method, first reported by Harboe19, was used. Hb absorbance was measured vs blank at 380,415 and 450 nm, and the final Hb concentration was calculated using a 3-point Allen correction. Fresh and/or stored RBCs were also examined for osmotic and mechanical fragility. In the first case, the cells were exposed to increasingly hypo-osmotic saline (NaCl) solutions, and mean corpuscular fragility (MCF), i.e. the concentration of NaCl that causes 50% haemolysis, was determined by measurement of free Hb levels in the supernatant at 540 nm. The mechanical fragility index (MFI) was determined by a similar approach, following mixing and rocking of RBCs with stainless steel beads for 1 hour (h). Free Hb was measured against an un-rocked control, to exclude haemolysis unrelated to mechanical stress16.
Redox parameters
Total antioxidant capacity (TAC) of plasma (and supernatant) was determined by using the ferric reducing antioxidant power (FRAP)20 assay. Treatment of plasma with uricase was used to estimate the UA-dependent (UAdAC) and UA-independent (UAiAC) proportion of the antioxidant capacity. Intracellular reactive oxygen species (ROS) levels were measured by fluorometry (VersaFluor Fluorometer System, BioRad, Hercules, CA, USA) after incubation of RBCs with the membrane permeable and redox-sensitive probe 5-(and-6)-chloromethyl-2′, 7′-dichloro-dihydro-fluoresceindiacetate, acetyl ester (CM-H2DCFDA). Tert-Butyl hydroperoxide- (tBHP) and diamide-induced ROS were also determined, following treatment of RBCs with 100μM tBHP for 20 min at 25 °C or with 2 mM diamide for 45 min at 37 °C. Quantification of ROS levels was achieved after normalisation to protein levels21.
Proteasomal activity
Proteasomal activities in RBC membranes were determined by fluorescence assays as previously described22. Briefly, 100–200 μg of protein samples in 20 mmol/L Tris-HCl (pH 7.5 or 8.0) were incubated with the fluorogenic peptide substrates Suc-Leu-Leu-Val-Tyr-aminomethylcoumarin (AMC) (for CH-like activity), z-Leu-Leu-Glu-AMC (for CASP-like activity) and Boc-Leu-Arg-Arg-AMC (for TR-like activity) for 1–3 h at 37 °C in the dark in the presence and absence of inhibitors (10 μmol/L bortezomib, 400 μmol/L MG-132, and 500 μmol/L N-Acetyl-Leu-Leu-Methional). All substrates and inhibitors were obtained from Enzo Life Sciences (Osaka, Japan). Fluorescence was measured by using the VersaFluor reader (BIO-RAD Hercules, CA, USA).
Electron microscopy
Morphological evaluation of fresh and stored RBCs was based on scanning electron microscopy. Purified RBCs were fixed with 2% glutaraldehyde and post-fixed with 1% osmium tetroxide in 0.1 mol/L sodium cacodylate buffer, pH 7.4. Ascending ethanol concentration solutions were used for dehydration before coating with gold-palladium. Under observation, cells with spherocytic modifications (spherocytes, spheroechinocytes and spherostomatocytes) as well as those with degenerative shapes were classified as “irreversible”. For each sample, at least 2,000 cells were evaluated by blind examination.
RBC fractionation and immunoblotting analysis
Isolation of membranes from fresh RBCs was performed after leukocyte and platelet reduction through columns consisting of α-cellulose and microcrystalline cellulose mixtures23. RBC membranes and cytosols were isolated by hypotonic lysis in 5 mmol/L sodium phosphate buffer (pH 8.0) supplemented by protease inhibitors. Equal quantity of RBC membrane proteins was loaded in Laemmli gels, blotted to nitrocellulose membranes and probed with primary and horseradish peroxidase (HRP)-conjugated secondary antibodies. Purified membrane proteins were also processed for the detection of carbonyl groups by using the Oxyblot detection kit, according to the manufacturer’s specifications. In both cases, the protein bands were detected by chemiluminescence and quantified by scanning densitometry. Primary antibodies against band 3, calpain, Hsp70, Hb, soluble clusterin (sCLU), spectrin and glucose transporter-1 (Glut1) were used.
Statistical analysis
All measurements were performed in triplicate. For statistical analysis, SPSS (Version 22.0, IBM Corp, Armonk, NY, USA) computer software was used. All variables were tested for normal distribution profile and presence of outliers, by using the Shapiro-Wilk test and detrended normal Q-Q plots. Intergroup differences were evaluated by t-test and Mann-Whitney tests after applying a Bonferroni-like adjustment for multiple comparisons, where needed. Pearson’s and Spearman’s tests were performed to assess correlations between parameters. Regarding correlation analysis, we checked both donor groups for statistically significant and repeatable correlations between each storage day and fresh blood measurements. Only those pairs of variables that exhibited constant correlations between fresh blood and each and every storage time point (i.e. days 2, 14, 28 and 42) were considered reliable in terms of linking the in vivo to the ex vivo state. p<0.05 was considered statistically significant.
RESULTS
Comparative profiling of fresh blood from female versus male eligible donors
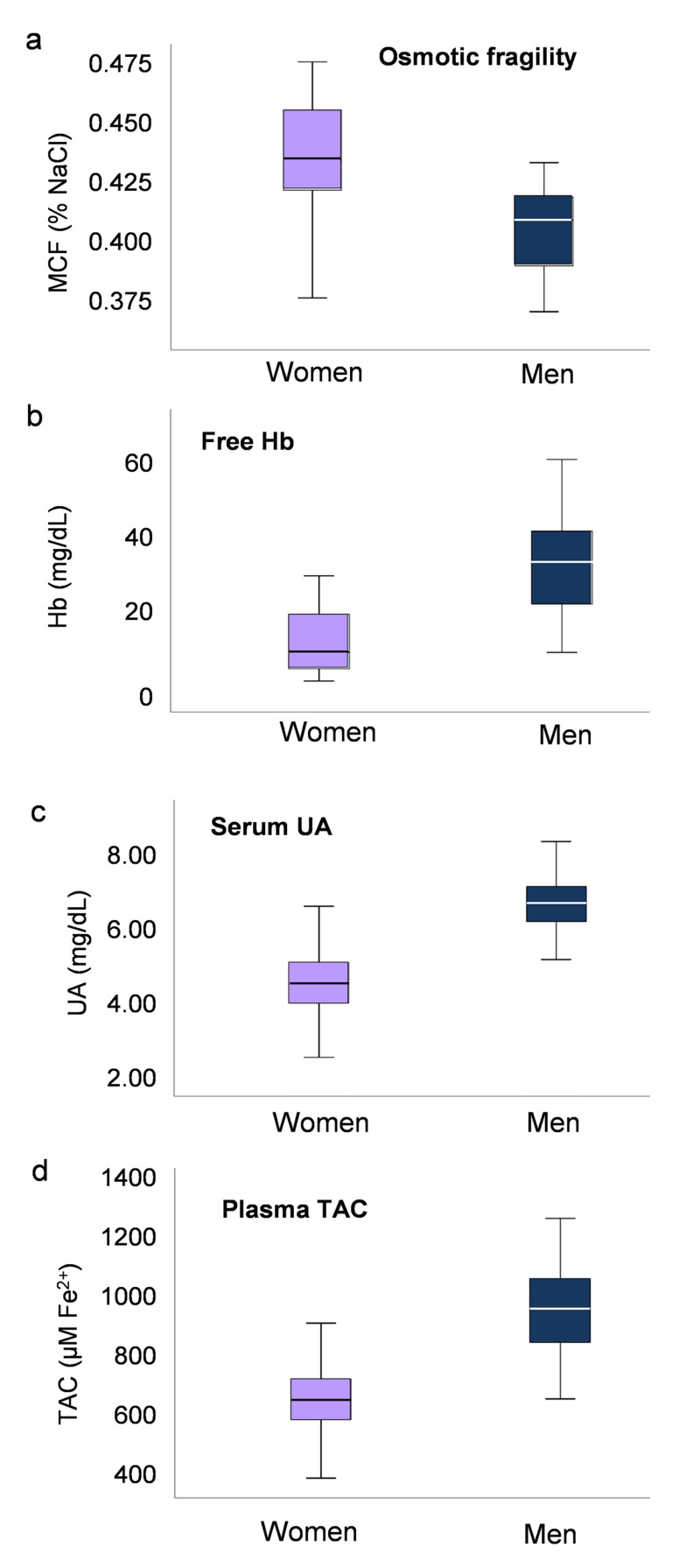
The baseline haematological and RBC biological profiles were determined in two large cohorts of young male and female candidate blood donors. Though within normal range, as expected, significant differences in several variables were detected between the two groups (Table I). The most prominent difference was that women demonstrated lower (p=1.7×10−17) Hb levels, RBC count and serum iron but higher levels of platelet count (p=1.7×10−10). Furthermore, there was a substantial difference in lipid profile between female and male blood, with lower total cholesterol (p=3.4×10−5), low density lipoprotein (LDL) (p=1.2×10−4) and very low density lipoprotein (VLDL) (p=3×10−4) levels, but higher concentration of high density lipoprotein (HDL) (p=1.37×10−16) (Table I). Apart from the classic haematological profiling, we further examined an array of biological/physiological parameters not commonly tested in blood donors. While women demonstrated lower free Hb levels when compared to men (13.48±6.49 vs 34.68±11.99 mg/dL, respectively, p=1.48×10−10), notably, they exhibited higher MCF (0.439±0.023 vs 0 .403±0.029 % NaCl, women vs men, p=2×10−8) (Figure 1). Concerning redox parameters, plasma TAC was lower in female donors (689±123 vs 984±143 μM Fe2+, women vs men, p=7.2×10−12), consistent with the lower serum uric acid (UA) levels (4.59±0.85 vs 6.52±0.74 mg/dL, women vs men, p=1.12×10−15) (Figure 1). Finally, in women donors the serum estradiol levels exhibited a positive correlation with the RBC and PLT counts (r=0.262, p=0.008 and r=0.211, p=0.036, respectively).
Table I.
Statistically significant differences (p<0.001) in haematological and biochemical parameters between young female and male eligible blood donors. Data are presented as mean ± SD
| Women (n=99) | Men (n=101) | |
|---|---|---|
| RBC (×106/μL ) | 4.47±0.45 | 5.01±0.41 |
| Haemoglobin (g/dL) | 13.45±0.81 | 14.85±1.17 |
| Haematocrit (%) | 39.16±2.36 | 43.77±2.76 |
| MCHC (g/dL) | 34.42±1.11 | 33.90±1.12 |
| Platelets (×106/μL) | 303.34±69.71 | 245.01±50.90 |
| Plateletcrit (%) | 0.24±0.08 | 0.19±0.07 |
| Iron (μg/dL) | 72.24±33.20 | 129.10±27.30 |
| Total cholesterol (mg/dL) | 166.80±38.00 | 187.59±31.26 |
| LDL (mg/dL) | 94.07±40.37 | 113.09±26.62 |
| HDL (mg/dL) | 69.14±15.78 | 52.38±8.64 |
| VLDL (mmol/L) | 16.00±11.92 | 22.13±11.85 |
RBC: red blood cell; MCHC: mean corpuscular haemoglobin concentration.
Figure 1.
Box plots showing variation in osmotic fragility (A), free Hb (B), serum uric acid (UA) (C) and total antioxidant capacity (TAC) of plasma (D) in fresh blood derived from healthy male (n=101) and female (n=99) donors
All differences between groups are statistically significant; p<0.05. MCF: mean corpuscular fragility; UA: uric acid; TAC: total antioxidant capacity.
Storage quality of RBC units categorised by donor sex
Having determined fluctuations and differences in several variables between the two cohorts of donors in vivo, we proceeded with the evaluation of storage lesion metrics in blood samples donated by two sub-groups of young female and male volunteers (n=8 each) throughout standard storage. To ascertain the “net effect” of sex on RBC storage lesion, these subgroups were set up on the basis of minimal (as far as possible) baseline differences in the levels of parameters such as mean corpuscular Hb, UA, cellular fragilities and ROS, all known to be affected by the storage conditions (Figure 2).
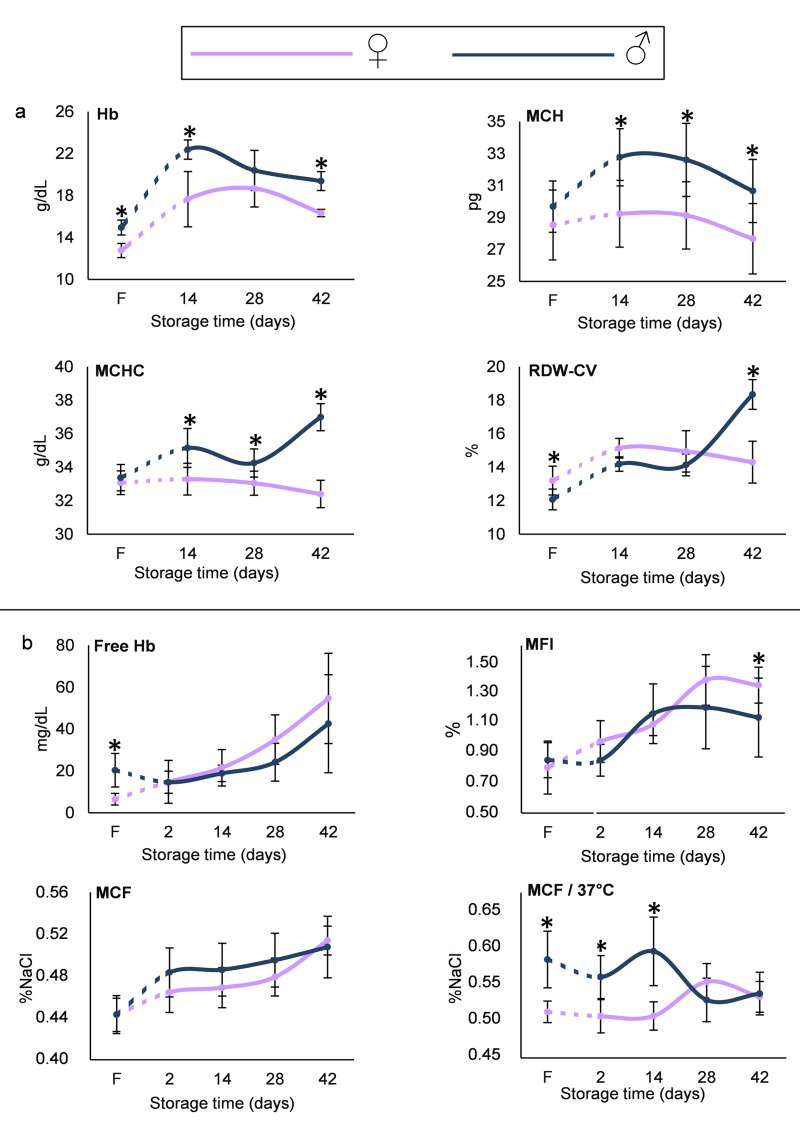
Figure 2.
Haematological (A) and haemolysis parameters (B) in fresh RBCs/plasma in vivo (F) and in RBCs/supernatant isolated from RBC units during storage
*p<0.05 men vs women. Error bars: ±standard deviation (SD).
RBCs: red blood cells Hb: haemoglobin; MCHC: mean corpuscular haemoglobin concentration; MCF: mean corpuscular fragility; RDW-CV: red blood cell distribution width; MFI: mechanical fragility index.
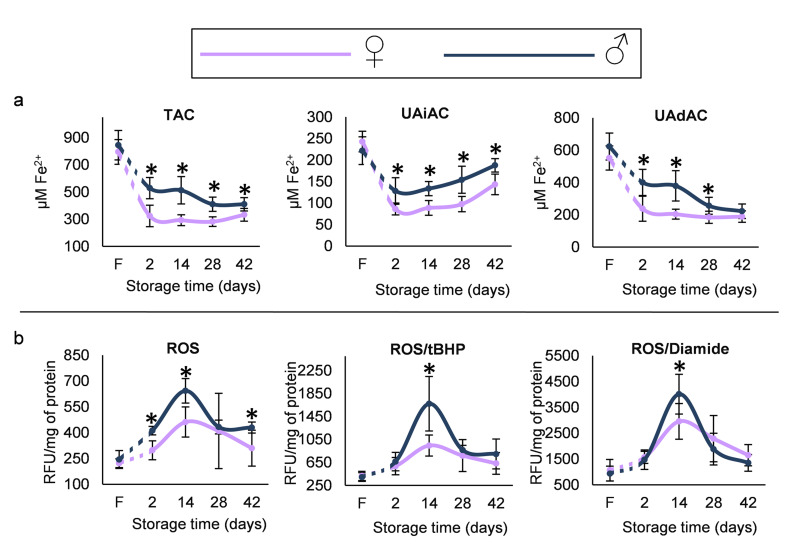
Total Hb concentration was lower in female compared to male donors and remained so throughout storage (e.g. 16.33±0.35 vs 19.39±0.90 g/dL, respectively, p=3×10−4, day 42). Mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) indices, while not different at baseline, were lower in the stored RBCs of female donors. Moreover, even though the RDW index was higher in the fresh blood of female vs male group, it showed a reverse pattern on the last day of storage (Figure 2A). With regard to the propensity of RBCs to haemolysis, less free Hb was detected in female donors in vivo, but no difference was observed throughout the storage period. Cell fragility under osmotic stress was also equal between the two groups, but under mechanical stress a trend for increased levels was observed in female RBCs at late-storage. Following incubation of RBCs for 24 h at 37 °C, female RBCs presented lower MCF values, both in vivo and in early storage (e.g. day 14, 0.508±0.020 vs 0.598±0.048 %NaCl, p=10−4, women vs men) (Figure 2B). As regards to redox parameters, the total, UA-dependent and UA-independent antioxidant capacities were lower in the supernatant of RBC units of female compared to male donors (e.g. TAC for day 2, 324±79 vs 528±78 μM Fe2+, p=5×10−5, respectively), in spite of similar baseline levels in fresh blood and similar time course variation during storage (Figure 3A). However, the inter-group differences were less evident as storage progressed. Starting by equal levels, the two groups further showed similar storage time-course profile in intracellular ROS accumulation (both intrinsic and following stimulation by oxidants). However, RBCs from female donors were characterised by significantly lower intrinsic ROS concentration throughout the storage period (e.g. for day 14, 462±88 vs 643±71 RFU/mg of protein, p=2×10−4, women vs men) and lower levels of tBHP- and diamide-induced ROS on day 14 but not after (Figure 3B). This finding may be associated with the fact that the metabolic shift towards the NADPH-producing pentose phosphate pathway usually occurs after the second week of storage24.
Figure 3.
(A) Antioxidant capacity (total [TAC], uric acid independent [UAiAC] and uric acid dependent [UAdAC]) of fresh plasma in vivo (F) and of red blood cell (RBC) unit supernatant during storage. (B) Reactive oxygen species (ROS) levels, both intrinsic and induced by oxidants (tBHP and diamide), in fresh and stored RBCs
*p<0.01 men vs women. Error bars: ±standard deviation (SD).
RBCs: red blood cells.
Correlations between fresh blood and stored RBC units in male and female donors
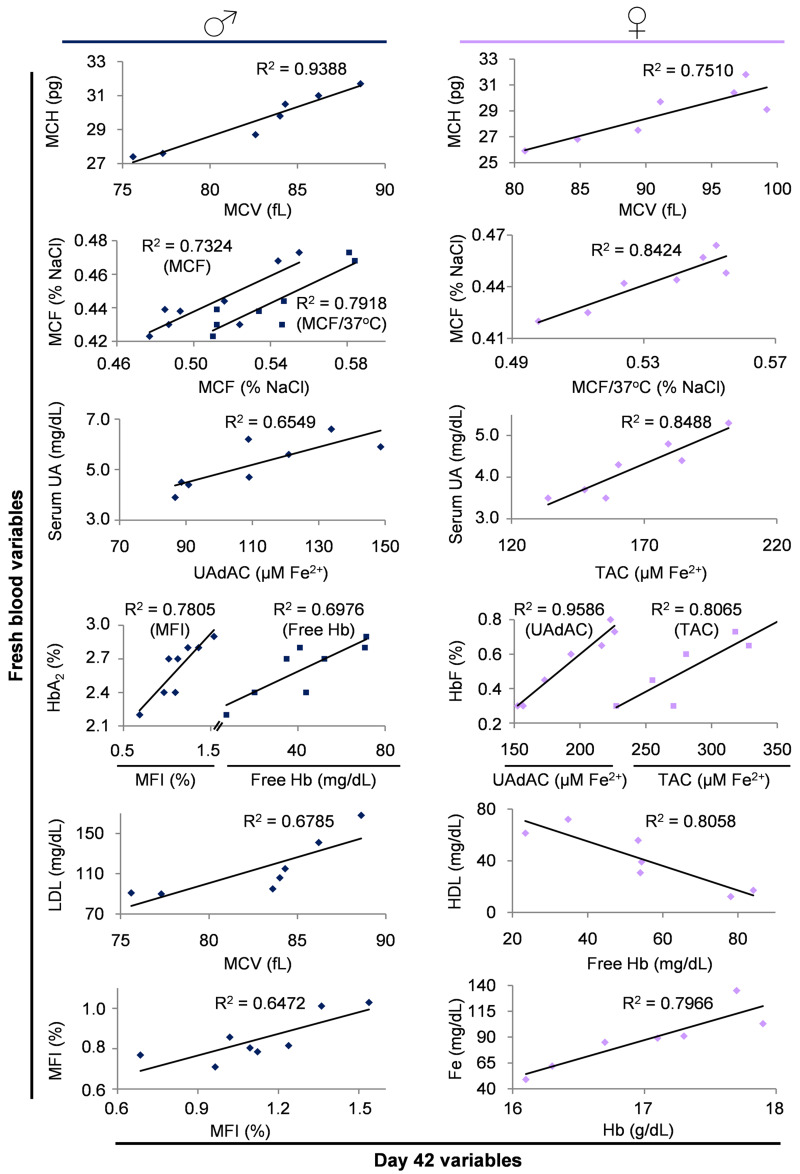
The above-mentioned outcomes of the female vs male comparisons pointed out specific differences in several RBC aspects, both in vivo and during storage. To understand more about whether, how and to what extent the differential storage profile might be related to the in vivo state, we searched for probable correlations between fresh and stored blood variables in both groups, while bearing in mind that correlation does not imply causation. In both donor groups, we found that MCH in vivo positively correlated with MCV of stored RBCs, and that resistance to osmotic stress constitutes an almost “inheritable” RBC feature showing strong, repeatable and significant correlations between fresh blood and stored RBCs (Figure 4). In a similar way, serum UA seemed to be a key player in maintaining the antioxidant potential in the supernatant of blood units regardless of sex.
Figure 4.
Indicative scatterplots of statistically significant (p<0.05) correlations between variables of fresh blood and stored RBC units
Only the correlations of day 42 with fresh blood measurements are shown; however, similar significant correlations (with slightly different r) were detected throughout the storage period (days 2, 14, 28 and 42). MFI: mechanical fragility index; UAdAC: uric acid dependent antioxidant capacity; TAC: total antioxidant capacity; MCF: mean corpuscular fragility; MCH: mean corpuscular haemoglobin; MCV: mean corpuscular volume; Hb: haemoglobin.
On the contrary, the normal range variation in the secondary Hb species HbA2 and HbF differentially correlated with haemolysis variables (MFI and free Hb) or supernatant antioxidant capacity in men and women, respectively. Serum lipoproteins (HDL and LDL) exhibited distinct correlations with stored RBCs parameters in male vs female donors (MCV or free Hb, respectively) (Figure 4), while serum iron levels were positively correlated with the intracellular Hb concentration of stored RBC units only in women. Finally, and in contrast to osmotic fragility, the resistance of stored RBCs to mechanical stress was strongly linked to the baseline in vivo levels only in male donors (Figure 4).
The effect of menopause on storage lesion profile
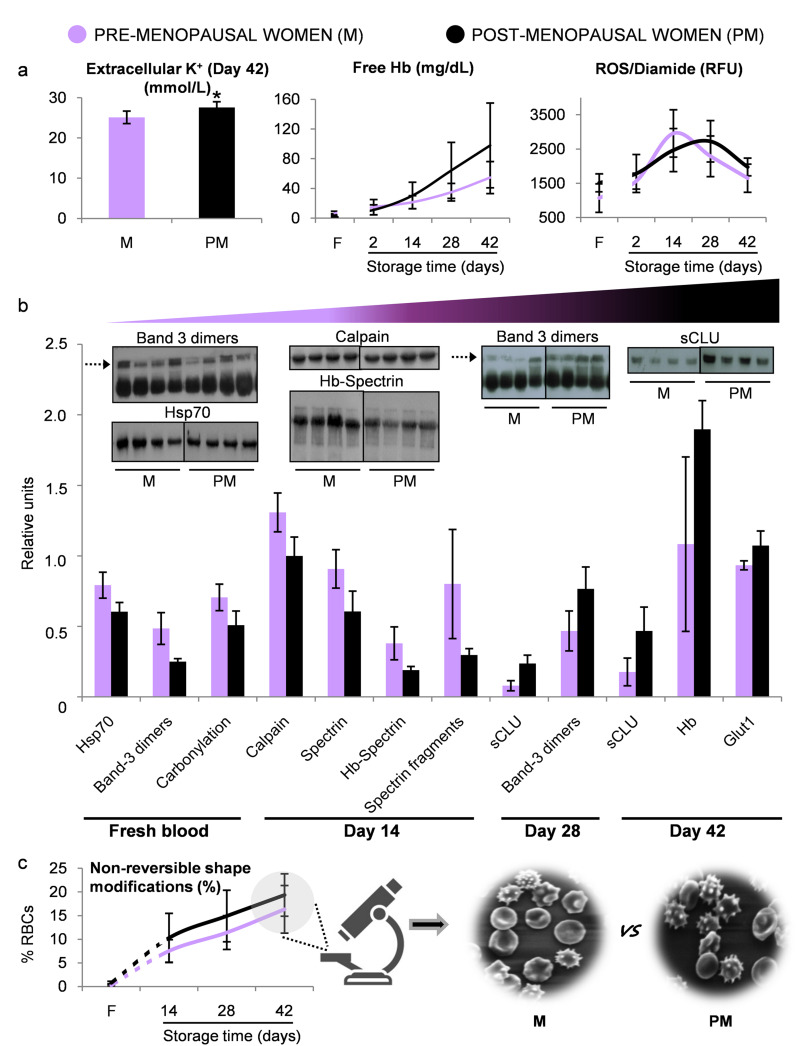
Taking into consideration that sex was not neutral regarding fine parameters of storage lesion, the last step of this study was to investigate the effect of age-related variation in female hormones on RBC storability. For this purpose, we examined an additional small group of postmenopausal female donors characterised by higher age (45.8±2.4 vs 20.2±1.5 years) and lower estradiol levels (23.5±6.8 vs 83.5±24.0 pg/mL) compared to the previously assessed premenopausal female group. This pilot study showed significantly higher concentration of end-of-storage extracellular potassium in post- compared to premenopausal women (27.6±1.4 vs 25.1±1.5 nM, respectively). In addition, and despite the fact that both female groups had equal baseline levels of ROS (diamide stimulated), free Hb (Figure 5A) and irreversible RBC shape modifications (Figure 5C), postmenopausal women were characterised by a trend for higher levels in those parameters after the mid-storage period (Figure 5A and 5C). Finally, although proteasome activities proved to be similar between the two groups (e.g. caspase-like activity of the RBC membrane on day 28, 40,497±10,593 vs 63,195±37,099 RFU/mg of protein, post- vs premenopausal group), comparative immunoblotting analysis of membrane samples from fresh and stored RBCs revealed an interesting pattern of protein composition. Protein stress markers such as carbonylation, band 3 oligomerisation, spectrin fragmentation and complexation with Hb, as well as membrane-bound Hsp70 and calpain were substantially lower in the RBCs of postmenopausal women in both fresh and stored RBCs during the first two weeks of storage (p<0.05) (Figure 5B). However, during the second half of the storage period, the opposite pattern appeared through accumulation of stress protein markers (band 3 oligomerisation, binding of Hb and clusterin) mainly in the RBC membrane of postmenopausal women over their younger counterparts (p<0.05) (Figure 5B).
Figure 5.
Biological evaluation of pre- vs post-menopausal women
(A) Variables showing a trend for different levels between the two groups. (B) Statistically significant differences (p<0.05) in the levels of red blood cell (RBC) membrane proteins (inserts: representative immunoblotting images, dashed arrows: band 3 dimers). (C) Morphological analysis using scanning electron microscopy (magnification ×1,000). *p<0.05 pre- (M) vs post- (PM) menopausal female donors. F: fresh blood. Error bars: ±standard deviation (SD).
DISCUSSION
Variability in haemolysis and plasma antioxidant capacity constitute sex-associated aspects of the biological profile of eligible blood donors
Over the years, several studies on blood physiology have focused on the elucidation of factors that contribute to sex-related differences. In this study, women exhibited typically7,8 lower levels of Hb, haematocrit, Fe and RBC count and higher platelet count compared to men. Except for menstruation, those differences are partly attributed to an interplay between those factors that exists regardless of ageing and health status25–27. The positive correlation between estradiol levels and RBC/PLT counts in female donors might reflect a promoting effect of endogenous estradiol on haematopoiesis, including the division potential of haematopoietic stem cells28. In addition, the favourable lipid profile of women is traditionally related to sex differences in dietary habits29 and to the decreased levels of serum UA30. The latter, which is a major compound of the non-enzymatic total antioxidant capacity of plasma31, was accordingly found lower in young women compared to men of the same age in our study. The opposite pattern of lower overall free Hb levels in spite of higher osmotically induced haemolysis in women vs men was intriguing. However, as we have shown in previous studies, haemolysis represents a multivariate “phenotype”, affected by genetic, metabolical and biological parameters that altogether produce a complex network32.
Haematological and redox parameters differ between male and female donors during storage
Transition from the in vivo state to the stressful environment of storage in the cold represents a challenging condition for RBCs regardless of their origin. However, recent research indicates that intrinsic donor characteristics such as serum UA10 or reduced glutathione33: (i) can directly link donor physiology with specific parameters of stored RBCs; and (ii) are not neutral to either the quality of stored RBCs or the transfusion outcome. Indeed, linked- and meta-analyses studying the effect of donor characteristics34 or donor/recipient sex-mismatch18 on transfusion therapy efficacy and mortality, respectively, reported that donor’s sex is a factor that needs to be taken into account. Such observations justify the late increase in studies that focus on sex-related differences in the storability of RBCs14,35. Our study provides new insights into the sex-related aspects of RBC storage lesion by focusing on the storability of RBCs from male and female groups that exhibited minimal variation at baseline in variables that are affected by storage, such as the osmotic haemolysis, extracellular TAC and intracellular ROS. As previously reported13, the lower intracellular Hb levels of female RBCs in vivo were maintained throughout the storage period. Moreover, the RBC indices (MCH, MCV) interconnect in vivo and ex vivo states in both groups36. The correlation between serum iron levels and Hb levels during storage, reported only in female blood, might be shown to be informative in terms of blood unit labelling13, since iron deficiency anaemia is common among female donors. The sex-associated differences in both MCH and MCHC indices, which emerged during storage in spite of equal baseline levels, indicate an unfavourable effect of storage on intracellular Hb profile in stored RBCs from female donors. On the contrary, the RBCs of the same group seemed to be less susceptible to lesions related to anisocytosis compared to those of males at late storage, in spite of the opposite pattern in vivo37.
Overall, no statistically significant intra-group difference was found in storage, osmotic and mechanical haemolysis levels, as opposed to previous studies showing lower haemolysis in women14,16,38,39. This contradiction could be related to the baseline features of our cohorts as well as to the wide inter-donor variability in the storage haemolysis levels32. Time course variation in the RBC osmotic haemolysis, a storage lesion phenotype that varies in proportion to the in vivo levels36, is similar in male and female donors. Since our cohorts were characterised by equal osmotic fragility at baseline, the reported fresh/stored blood correlation profile might partly explain the similar osmotic haemolysis levels during storage. Of note, incubation at body temperature seemed to be a “boost” needed to reveal the higher resistance of female RBCs to osmotic stress, a superiority for which sex hormones could take credit. Indeed, it has been considered that sex hormones may have favourable (progesterone)40 or unfavourable (testosterone)35 effects upon RBCs. The observed inverse correlation between the serum HDL levels and the gold quality standard of storage haemolysis in the female samples was really interesting. It is tempting to speculate that a threshold of HDL that is protective to the RBCs in vivo, either through a direct effect on membrane flexibility or through reducing the oxidative stress and the inflammatory mediators, offers further protection against the storage-induced haemolysis41. Finally, it is not the first time36 that normal range variation in the levels of secondary Hb species (HbA2 and HbF) in fresh blood was found to relate with haemolysis or extracellular antioxidant variables of the blood unit.
While Bardyn et al.42, reported that the extracellular antioxidant power strongly correlates with the UA concentration of the RBC units and that both of them were lower in females throughout storage, we currently show that: (i) units from both sexes present significant correlations of the non-enzymatic antioxidant activity of their supernatants with serum UA (in vivo); and (ii) female donors are characterised by steadily worse absolute levels of all three antioxidant activities measured during the whole storage period. Taken together, while bearing in mind that both groups had similar baseline levels and that the “activity gap” between fresh and day-2 blood (that might be associated with the plasma dilution by the additive solution) is significantly larger in women, it seems plausible to suspect that all plasma non-enzymatic antioxidant molecules are more susceptible to depletion during blood processing procedures, such as leukoreduction, in female donors. It is known that procedures that include filtering such as haemodialysis have been implicated in the loss of certain non-enzymatic antioxidants (e.g. ascorbic acid)43, while serum UA also decreases after haemodialysis sessions44. Whether a similar reduction occurs during leukofiltration or blood processing, especially in the case of female donors, remains to be determined.
Finally, another novelty of our study is the finding that female donor-derived blood units are superior in terms of intracellular ROS accumulation, both intrinsic and chemically induced, as evidenced by the smoother storage time-course profile and the lower “peak” of ROS generation compared to male RBCs, during most of the storage period. This pattern suggests better preservation of intracellular antioxidant capacity in stored female RBCs. While not measuring the same variables, the results of D’Alessandro et al. are in line with our observation, as stored RBCs from women were characterised by decreased levels of oxidative haemolysis compared to those of males15. Indeed, several studies provide evidence that female RBCs can cope better with oxidative stress because of their higher glutathione peroxidase activity9, as well as the antioxidant effects of estradiol45,46 or progesterone, with the latter remaining attached to the RBC membrane even after repeated washes, especially in young stored RBCs40.
Menopause may affect the RBC storability through protein remodelling of the membrane at prolonged storage
Menopausal status is characterised by a rapid decline in the levels of sex hormones, including estradiol and progesterone. These hormones, as well as the regular loss of blood through menstruation that leads to a younger RBC population in premenopausal women, are beneficial for RBCs, making them47 less susceptible to haemolysis48 and lipid peroxidation49. In the light of these observations, our results pointed towards a trend for worse levels of storage-induced haemolysis, potassium leakage and irreversible shape modifications of stored RBCs of postmenopausal women when compared to their premenopausal counterparts. Despite the fact that the small size of the postmenopausal group studied here does not allow broad conclusions to be drawn, these findings are in line with those of Raval et al. who demonstrated higher levels of mechanically-induced haemolysis in postmenopausal female donors’ RBCs during storage50. Furthermore, and apart from menopause, the extremely wide age range of our female donor cohorts (>25 years) should be noted. Shape modifications, that come hand in hand with ion loss, might not only be attributed to dysregulation of RBC mechanical properties, but also to the erythrocyte membrane remodelling, which is a result of the RBC ageing observed in older individuals51,52. Interestingly, our postmenopausal cohort presented signs of accelerated ageing in the membrane after the mid-storage period, highlighting a differential effect of storage duration on protein stress markers with respect to the menopausal status. In terms of storage time, this profile coincided with a parallel shift in ROS generation after oxidation of thiol residues (ROS/diamide) (Figure 5A). In addition, the statistically insignificant but still interesting trend for lower caspase-like proteasome activity observed in the RBC membrane samples of postmenopausal women, especially during mid-storage, is an intriguing observation that needs further investigation in order to clarify which branch of RBC proteostatic mechanisms is modified in this specific group.
CONCLUSIONS
In line with previous studies, we showed that donor’s sex affects the redox homeostasis of stored RBCs. Moreover, the menopausal status of the female donor can be considered a significant modifier of the RBC membrane. Women’s plasma is characterised by lower free Hb levels vs men but women’s RBCs exhibit higher osmotic fragility in circulation, in contrast to recent findings in storage. Though our small sub-groups of RBC units were not representative of random donors, they were able to show the clear effects of storage conditions on sex differences observed in haemolysis variables in the past. Indeed, they showed that such differences might not exist in a context of similar baseline levels. Our study provides novel data on sex-related aspects of storage lesion and their connection to fresh blood. They might prove to be useful in studies on the clinical effects of transfusion as a function of the donor’s biological profile, including the still challenging sex miss-matched transfusion events.
ACKNOWLEDGEMENTS
The Authors would like to thank all blood donors that voluntarily participated in this study and M.S. Jacovides Hellas S.A. for the kind offer of the LTRC blood bags. The Authors also thank graduate students Violetta Anastasopoulou and Ariadne Liakopoulou for their kind contribution in a part of the experimental procedure.
Footnotes
AUTHORSHIP CONTRIBUTIONS
VLT and ATA are equal first Authors. VLT, MHA and AGK conceived the study concept and designed the study. VLT, ATA, PVD and DGK performed the experiments. VLT and ATA performed the statistical analysis. VLT, ATA and MHA analysed the data. VLT, ATA and MHA wrote the paper. KES, SIV, MP and AGK provided the RBC units, performed the biochemical and haematological analyses, and contributed to the final draft of the paper. ISP critically contributed to the final draft of the paper. All Authors read and approved the final manuscript.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Orlov D, Karkouti K. The pathophysiology and consequences of red blood cell storage. Anaesthesia. 2015;70(Suppl 1):29–37. e9–12. doi: 10.1111/anae.12891. [DOI] [PubMed] [Google Scholar]
- 2.Tinmouth A, Fergusson D, Yee IC, et al. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–27. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida T, Prudent M, D’Alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 2019;17:27–52. doi: 10.2450/2019.0217-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaphan E, Laurin D, Lafeuillade B, et al. Impact of transfusion on survival in patients with myelodysplastic syndromes: current knowledge, new insights and transfusion clinical practice. Blood Rev. 2020;41:100649. doi: 10.1016/j.blre.2019.100649. [DOI] [PubMed] [Google Scholar]
- 5.D’Alessandro A, Reisz JA, Zhang Y, et al. Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv. 2019;3:884–96. doi: 10.1182/bloodadvances.2018029629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzounakas VL, Kriebardis AG, Papassideri IS, Antonelou MH. Donor-variation effect on red blood cell storage lesion: A close relationship emerges. Proteomics Clin Appl. 2016;10:791–804. doi: 10.1002/prca.201500128. [DOI] [PubMed] [Google Scholar]
- 7.Rushton DH, Dover R, Sainsbury AW, et al. Why should women have lower reference limits for haemoglobin and ferritin concentrations than men? BMJ. 2001;322:1355–7. doi: 10.1136/bmj.322.7298.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cafolla A, Dragoni F, Girelli G, et al. Folate status in Italian blood donors: relation to gender and smoking. Haematologica. 2000;85:694–8. [PubMed] [Google Scholar]
- 9.Massafra C, Gioia D, De Felice C, et al. Effects of estrogens and androgens on erythrocyte antioxidant superoxide dismutase, catalase and glutathione peroxidase activities during the menstrual cycle. J Endocrinol. 2000;167:447–52. doi: 10.1677/joe.0.1670447. [DOI] [PubMed] [Google Scholar]
- 10.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Uric acid variation among regular blood donors is indicative of red blood cell susceptibility to storage lesion markers: a new hypothesis tested. Transfusion. 2015;55:2659–71. doi: 10.1111/trf.13211. [DOI] [PubMed] [Google Scholar]
- 11.Ishizaka N, Ishizaka Y, Toda E, et al. Association between serum uric acid, metabolic syndrome, and carotid atherosclerosis in Japanese individuals. Arterioscler Thromb Vasc Biol. 2005;25:1038–44. doi: 10.1161/01.ATV.0000161274.87407.26. [DOI] [PubMed] [Google Scholar]
- 12.Gustafson CM, Shepherd AJ, Miller VM, Jayachandran M. Age- and sex-specific differences in blood-borne microvesicles from apparently healthy humans. Biol Sex Differ. 2015;6:10. doi: 10.1186/s13293-015-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agnihotri N, Pal L, Thakur M, Kumar P. The need to label red blood cell units with their haemoglobin content: a single centre study on haemoglobin variations due to donor-related factors. Blood Transfus. 2014;12:520–6. doi: 10.2450/2014.0231-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv. 2017;1:1132–41. doi: 10.1182/bloodadvances.2017004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Alessandro A, Fu X, Kanias T, et al. Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica. 2020 doi: 10.3324/haematol.2020.246603.. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raval JS, Waters JH, Seltsam A, et al. The use of the mechanical fragility test in evaluating sublethal RBC injury during storage. Vox Sang. 2010;99:325–31. doi: 10.1111/j.1423-0410.2010.01365.x. [DOI] [PubMed] [Google Scholar]
- 17.Edgren G, Ullum H, Rostgaard K, et al. Association of donor age and sex with survival of patients receiving transfusions. JAMA Intern Med. 2017;177:854–60. doi: 10.1001/jamainternmed.2017.0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeller MP, Rochwerg B, Jamula E, et al. Sex-mismatched red blood cell transfusions and mortality: a systematic review and meta-analysis. Vox Sang. 2019;114:505–16. doi: 10.1111/vox.12783. [DOI] [PubMed] [Google Scholar]
- 19.Harboe M. A method for determination of hemoglobin in plasma by near-ultraviolet spectrophotometry. Scand J Clin Lab Invest. 1959;11:66–70. doi: 10.3109/00365515909060410. [DOI] [PubMed] [Google Scholar]
- 20.Benzie IF, Strain JJ. The ferric reducing ability of plasma ( FRAP) as a measure of “ antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 21.Antonelou MH, Tzounakas VL, Velentzas AD, et al. Effects of pre-storage leukoreduction on stored red blood cells signaling: a time-course evaluation from shape to proteome. J Proteomics. 2012;76(Spec No):220–38. doi: 10.1016/j.jprot.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Homma T, Kurahashi T, Lee J, et al. SOD1 deficiency decreases proteasomal function, leading to the accumulation of ubiquitinated proteins in erythrocytes. Arch Biochem Biophys. 2015;583:65–72. doi: 10.1016/j.abb.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Beutler E, West C, Blume KG. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976;88:328–33. [PubMed] [Google Scholar]
- 24.D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015;55:205–19. doi: 10.1111/trf.12804. [DOI] [PubMed] [Google Scholar]
- 25.Lozano M, Narvaez J, Faundez A, et al. [Platelet count and mean platelet volume in the Spanish population]. Med Clin (Barc) 1998;110:774–7. [In Spanish.] [PubMed] [Google Scholar]
- 26.Park MJ, Park PW, Seo YH, et al. The relationship between iron parameters and platelet parameters in women with iron deficiency anemia and thrombocytosis. Platelets. 2013;24:348–51. doi: 10.3109/09537104.2012.699641. [DOI] [PubMed] [Google Scholar]
- 27.Karolczak K, Soltysik B, Kostka T, et al. Platelet and red blood cell counts, as well as the concentrations of uric acid, but not homocysteinaemia or oxidative stress, contribute mostly to platelet reactivity in older adults. Oxid Med Cell Longev. 2019;2019:9467562. doi: 10.1155/2019/9467562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakada D, Oguro H, Levi BP, et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505:555–8. doi: 10.1038/nature12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leblanc V, Begin C, Corneau L, et al. Gender differences in dietary intakes: what is the contribution of motivational variables? J Hum Nutr Diet. 2015;28:37–46. doi: 10.1111/jhn.12213. [DOI] [PubMed] [Google Scholar]
- 30.Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120:442–7. doi: 10.1016/j.amjmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 31.Duplancic D, Kukoc-Modun L, Modun D, Radic N. Simple and rapid method for the determination of uric acid-independent antioxidant capacity. Molecules. 2011;16:7058–68. doi: 10.3390/molecules16087058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reisz JA, Tzounakas VL, Nemkov T, et al. Metabolic linkage and correlations to storage capacity in erythrocytes from glucose 6-phosphate dehydrogenase-deficient donors. Front Med (Lausanne) 2017;4:248. doi: 10.3389/fmed.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van ‘t Erve TJ, Doskey CM, Wagner BA, et al. Heritability of glutathione and related metabolites in stored red blood cells. Free Radic Biol Med. 2014;76:107–13. doi: 10.1016/j.freeradbiomed.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roubinian NH, Plimier C, Woo JP, et al. Effect of donor, component, and recipient characteristics on hemoglobin increments following red blood cell transfusion. Blood. 2019;134:1003–13. doi: 10.1182/blood.2019000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanias T, Sinchar D, Osei-Hwedieh D, et al. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion. 2016;56:2571–83. doi: 10.1111/trf.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Donor variation effect on red blood cell storage lesion: a multivariable, yet consistent, story. Transfusion. 2016;56:1274–86. doi: 10.1111/trf.13582. [DOI] [PubMed] [Google Scholar]
- 37.Lippi G, Salvagno GL, Guidi GC. Red blood cell distribution width is significantly associated with aging and gender. Clin Chem Lab Med. 2014;52:e197–9. doi: 10.1515/cclm-2014-0353. [DOI] [PubMed] [Google Scholar]
- 38.Lanteri MC, Kanias T, Keating S, et al. Intradonor reproducibility and changes in hemolytic variables during red blood cell storage: results of recall phase of the REDS-III RBC-Omics study. Transfusion. 2019;59:79–88. doi: 10.1111/trf.14987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordan A, Chen D, Yi QL, et al. Assessing the influence of component processing and donor characteristics on quality of red cell concentrates using quality control data. Vox Sang. 2016;111:8–15. doi: 10.1111/vox.12378. [DOI] [PubMed] [Google Scholar]
- 40.DeVenuto F, Wilson SM. Distribution of progesterone and its effect on human blood during storage. Transfusion. 1976;16:107–12. doi: 10.1046/j.1537-2995.1976.16276155103.x. [DOI] [PubMed] [Google Scholar]
- 41.Sparrow RL. Red blood cell components: time to revisit the sources of variability. Blood Transfus. 2017;15:116–25. doi: 10.2450/2017.0326-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bardyn M, Maye S, Lesch A, et al. The antioxidant capacity of erythrocyte concentrates is increased during the first week of storage and correlated with the uric acid level. Vox Sang. 2017;112:638–47. doi: 10.1111/vox.12563. [DOI] [PubMed] [Google Scholar]
- 43.Morena M, Cristol JP, Bosc JY, et al. Convective and diffusive losses of vitamin C during haemodiafiltration session: a contributive factor to oxidative stress in haemodialysis patients. Nephrol Dial Transplant. 2002;17:422–7. doi: 10.1093/ndt/17.3.422. [DOI] [PubMed] [Google Scholar]
- 44.Clermont G, Lecour S, Lahet J, et al. Alteration in plasma antioxidant capacities in chronic renal failure and hemodialysis patients: a possible explanation for the increased cardiovascular risk in these patients. Cardiovasc Res. 2000;47:618–23. doi: 10.1016/s0008-6363(00)00117-6. [DOI] [PubMed] [Google Scholar]
- 45.Djordjevic NZ, Babic GM, Markovic SD, et al. The antioxidative effect of estradiol therapy on erythrocytes in women with preeclampsia. Reprod Toxicol. 2010;29:231–6. doi: 10.1016/j.reprotox.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Sawicka E, Dlugosz A. The role of 17beta-estradiol metabolites in chromium-induced oxidative stress. Adv Clin Exp Med. 2017;26:215, 21, 47. doi: 10.17219/acem/62217. [DOI] [PubMed] [Google Scholar]
- 47.Kameneva MV, Watach MJ, Borovetz HS. Gender difference in rheologic properties of blood and risk of cardiovascular diseases. Clin Hemorheol Microcirc. 1999;21:357–63. [PubMed] [Google Scholar]
- 48.Kanias T, Gladwin MT. Nitric oxide, hemolysis, and the red blood cell storage lesion: interactions between transfusion, donor, and recipient. Transfusion. 2012;52:1388–92. doi: 10.1111/j.1537-2995.2012.03748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rembacz KP, Sawicka E, Dlugosz A. Role of estradiol in chromium-induced oxidative stress. Acta Pol Pharm. 2012;69:1372–9. [PubMed] [Google Scholar]
- 50.Raval JS, Waters JH, Seltsam A, et al. Menopausal status affects the susceptibility of stored RBCs to mechanical stress. Vox Sang. 2011;100:418–21. doi: 10.1111/j.1423-0410.2010.01439.x. [DOI] [PubMed] [Google Scholar]
- 51.Bosman GJ, Werre JM, Willekens FL, Novotny VM. Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med. 2008;18:335–47. doi: 10.1111/j.1365-3148.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 52.Glass GA, Gershon D, Gershon H. Some characteristics of the human erythrocyte as a function of donor and cell age. Exp Hematol. 1985;13:1122–6. [PubMed] [Google Scholar]