Abstract
Background
Even though it rarely influences venous thromboembolism (VTE) treatment and the fact that it is generally discouraged, thrombophilia testing is still largely prescribed. We assessed: 1) whether/how frequently Italian thrombosis centres requested thrombophilia testing; 2) what results were obtained; and 3) if the results affected treatment and clinical results.
Materials and methods
We examined data from 4,826 VTE patients enrolled by 19 clinical centres participating in the START 2-Register.
Results
57.2% of patients were tested. Numbers varied widely among centres (2.9–99.7%). Thrombophilic alterations were recorded in 18.2% of patients and the percentage of positive results was inversely correlated with that of patients tested. Significantly less patients with deep vein thrombosis (DVT) were tested, whereas more were tested when the event was idiopathic, presenting as isolated pulmonary embolism (PE), or in unusual sites. Patients with thrombophilic alterations were younger, more frequently treated with direct oral anticoagulants (DOACs), with lower mortality and less frequently discontinued anticoagulation. DOACs were more frequently prescribed in patients with heterozygous Factor V (FV) Leiden or prothrombin mutations, whereas vitamin K antagonists were preferred in patients with inhibitor deficiencies, combined alterations or antiphospholipid syndrome (APLS). There was no difference in duration of treatment among those with or without alterations, though more APLS patients received an extended treatment course. Bleeding and thrombotic complications occurred with a similar and fairly low incidence in patients with or without thrombophilic alterations.
Discussion
Although general testing for thrombophilia in VTE patients is currently discouraged, more than half of the VTE patients included in the START2-Register were tested. However, there were marked differences in practice between Italian thrombosis centres. About 60% of all patients with alterations were treated with DOACs, confirming that DOACs can be a useful option for treatment of thrombophilic VTE patients, with the exclusion of those with APLS.
Keywords: thrombophilia, recurrent events, bleeding, anticoagulant therapy, venous thromboembolism
INTRODUCTION
Inherited thrombophilia is associated with susceptibility to venous thromboembolism (VTE). Thrombophilic alterations are frequent in the general population, involving up to 5–10% of subjects, and can be detected in about 40% of patients with VTE1. The strength of thrombotic predisposition is heterogeneous between the different alterations. Deficiency of antithrombin, protein C, or protein S (rare alterations, each occurring in <0.5% of the general population) is associated with a high risk of developing VTE (>10 times that of the general population). The presence of R506Q Factor V Leiden (FVL) or Prothrombin G20210A (PT) gene mutations is much more frequent in some populations (such as Caucasians) but is associated with modest VTE risk2. In principle, not only does thrombophilia affect the risk of a first VTE but may potentially also influence the initial and long-term therapy of a VTE episode as well as the type and duration of treatment to avoid VTE recurrence. This probably explains why testing for inherited thrombophilia has been so common in clinical practice in recent years3,4. Currently, however, many experts consider testing for thrombophilia to be of little use in the clinical management of most patients with VTE5. Although no randomised trials comparing testing for thrombophilia with no testing are available, observational studies indicate that anticoagulants are equally effective in patients with or without thrombophilia6. Data are available confirming that patients with hereditary protein S, protein C, or antithrombin deficiency have a high absolute risk of recurrence, especially if the first event is spontaneous7. However, these alterations are somewhat rare and so it is not clear if general testing for thrombophilia can reduce the risk of VTE recurrence8.
While thrombophilia rarely influences the treatment of VTE, and general testing is discouraged by experts and guidelines5,9, thrombophilia testing of VTE patients is still largely prescribed in clinical practice. With the present study, we aimed to assess: 1) whether and how frequently Italian thrombosis centres, participating in the prospective, multicentre, Italian START 2-Register and almost all affiliated to the Italian Federation of Anticoagulation Clinics (FCSA), request thrombophilia testing; 2) what results were obtained; and 3) if these results might have affected the treatment and clinical results.
MATERIALS AND METHODS
The START2-Register
The START2-Register is a prospective, observational, multicentre, study that includes adults (≥18 years) who start anticoagulation therapy, whatever the drug, the dosage used, and the indication for treatment. Details on rules and procedures of the registry are provided elsewhere10. The registry was approved in October 2011 (n=142/2010/0/0ss) by the Ethical Committee of the Institution of the Coordinating Member (University Hospital “S. Orsola-Malpighi”, Bologna, Italy). The study is registered at clinicaltrials.gov identifier: NCT02219984 and is still ongoing and recruitment is still open. Patients aged ≥18 years at the time of inclusion who give their written informed consent and who have been receiving anticoagulation therapy for no more than 30 days are eligible for inclusion in the registry. The participating centres are required to enroll patients consecutively, without any a priori exclusion criteria other than life expectancy or geographical inaccessibility. Definition of the time-frame for enrolment (e.g., one week every month or the first month of the year) is left to the discretion of each participating centre as long as it ensures random enrolment of patients. Here we present the results of the cohort of patients included for a VTE event. For the present analysis, data were collected from January 2012 to April 2018.
Patient’s clinical features are recorded by centres on web-based case report forms (CRF). Baseline data include patients’ demographic and clinical characteristics including co-morbidities and co-medications, type and site of VTE event, presence of provoking factors or associated risk factors, laboratory routine data, and type (and dosages) of anticoagulant drug used. Information was collected on the nature, site, and seriousness of VTE events. The nature of the index event, first or recurrent, was considered: a) idiopathic when not temporally associated with any potential triggering condition or risk factor; b) associated with weak risk factors (RF) when the event was associated with conditions considered weak risk factors such as minor, arthroscopic or laparoscopic general surgery, pregnancy or puerperium, contraceptive or replacement hormonal therapy, long trip, minor trauma, hospitalisation, reduced mobility (not complete immobilisation); c) provoked by transient major RF in cases where one or more of the following conditions were present within 3 months of VTE diagnosis: major surgery with general or spinal anaesthesia, lower limb fracture, casting or no weight bearing for ≥3 days, bed-bound for >3 days due to acute illness, etc.; d) provoked by permanent major RF when associated with paraplegia, active cancer, chronic active inflammatory disease (e.g., intestinal inflammatory disease) or other chronic serious disease, serious inherited thrombophilic alterations, antiphospholipid syndrome (APLS), severe post-thrombotic syndrome, presence of cava filter.
Participating centres are solicited to regularly follow-up enroled patients at least quarterly, by a phone call or visit to the surgery. A hospital check-up is recommended at least annually. Participants are required to provide detailed clinical reports of any relevant clinical outcome occurring in enroled patients. Though all changes in medical history are recorded, specific outcomes for patients treated for VTE are new or recurrent deep vein thrombosis (DVT), pulmonary embolism (PE), venous thrombosis in different sites or arterial thrombotic complications, major bleeding (MB) events and non-major but clinically relevant bleeding (NMCRB) episodes. MB events were defined as recommended by the International Society on Thrombosis and Haemostasis11. Clinically relevant non-major bleeding was defined as overt bleeding that did not meet the criteria for MB but was associated with the need for medical intervention, contact with a physician, changes in dosing of VKA assumption, or with discomfort or impairment of daily activities12.
Thrombophilia screening
Inherited and acquired thrombophilia screening is neither recommended nor excluded by the registry procedure but left to the judgment of participant clinicians. In patients tested, the following risk factors are recorded: FVL and PT gene mutations; antithrombin, protein C and protein S deficiencies; lupus anticoagulant (LA) phenomenon, anticardiolipin and β2-glycoprotein 1 antibodies. All the analyses are performed according to local protocol. In general, the FCSA and Italian scientific societies recommend performing thrombophilic tests (except genetic analysis) only after 3 months of full anticoagulation and after an adequate withdrawal period of the oral anticoagulant drug.
Statistical analysis
Continuous variables are presented as median and interquartile range (IQR). The Mann-Whitney U-test and the χ2 test (Yates corrected) were used for group comparison, as appropriate; p<0.05 was considered statistically significant. For statistical analysis the SPSS software package (SPSS, Chicago, III, USA) and the GraphPad Software (San Diego, CA, USA) were used.
RESULTS
Participating centres and their practice of testing venous thromboembolism patients for thrombophilia
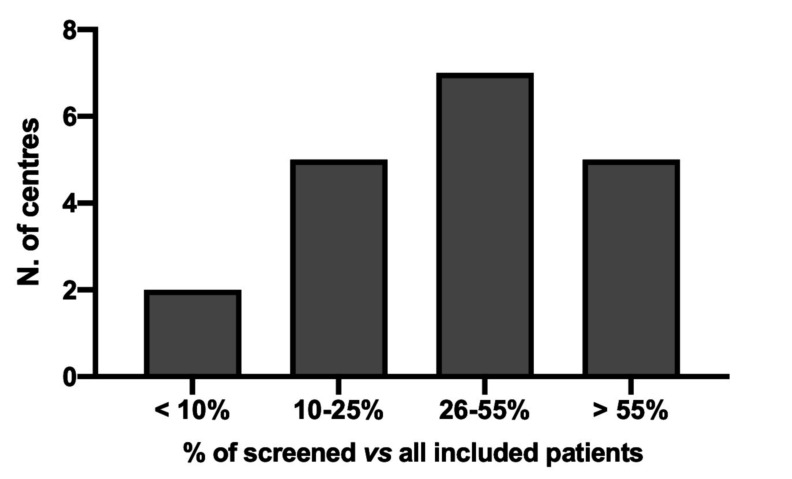
For the scope of the present report, the central database of the START2-Register for VTE was examined in October 2019. Only centres that had contributed to the START2-Register by including at least 40 VTE patients were included in the analysis; 17 of 19 of them were affiliated with the FCSA. Table I shows the participating centres (in decreasing order of the number of patients included in the registry), their geographic position in the country, the number (%) of patients not screened or screened for thrombophilia, and those with positive results. Eleven out of the 19 centres included in the study were located in Northern Italy and the remainder in Central-Southern. Altogether, tests for thrombophilia were performed in more than half (n=2,759; 57.2%) of all included patients (n=4,826), with positive results recorded in 18.2% (n=501). The percentage of tested patients, however, varied widely among centres (range 2.9–99.7%); Figure 1 shows the distribution of centres in classes of tested patient percentages. The rates of positive results in the centres were inversely correlated with the percentages of patients tested (equation: Y= −0.5172*X+58.87; R2=0.2662, p=0.0237).
Table I.
Number of patients tested or not tested, and with thrombophilic alterations enrolled from the participating centres
| Centres (see list in Appendix 1) | Geographic location | Enrolled patients n. | Not tested patients n. | Tested patients n. (%) | Patients with thrombophilic alterations n. (% vs tested) |
|---|---|---|---|---|---|
| 1 | Northern | 1,237 | 4 | 1,233 (99.7) | 117 (9.5) |
| 2 | Central-Southern | 592 | 291 | 301 (50.8) | 98 (32.6) |
| 3 | Northern | 576 | 336 | 240 (41.7) | 73 (30.4) |
| 4 | Northern | 451 | 49 | 402 (89.1) | 38 (9.4) |
| 5 | Central-Southern | 401 | 385 | 16 (4.0) | 7 (43.7) |
| 6 | Northern | 246 | 213 | 33 (13.4) | 7 (21.2) |
| 7 | Central-Southern | 196 | 93 | 103 (52.6) | 13 (12.6) |
| 8 | Central-Southern | 182 | 78 | 104 (57.1) | 37 (35.6) |
| 9 | Northern | 178 | 112 | 66 (37.1) | 10 (15.1) |
| 10 | Central-Southern | 132 | 103 | 29 (22.0) | 26 (89.7) |
| 11 | Northern | 99 | 56 | 43 (43.4) | 17 (39.5) |
| 12 | Northern | 81 | 33 | 48 (59.2) | 7 (14.6) |
| 13 | Central-Southern | 79 | 53 | 26 (32.9) | 18 (69.2) |
| 14 | Central-Southern | 77 | 13 | 64 (83.1) | 12 (18.7) |
| 15 | Northern | 75 | 59 | 16 (21.3) | 8 (50.0) |
| 16 | Northern | 69 | 67 | 2 (2.9) | 2 (100) |
| 17 | Central-Southern | 66 | 51 | 15 (22.7) | 9 (60.0) |
| 18 | Northern | 47 | 35 | 12 (25.5) | 2 (16.7) |
| 19 | Northern | 42 | 36 | 6 (14.3) | 0 (0) |
| Total | Northern=11 Central-Southern=8 | 4,826 | 2,067 | 2,759 (57.2) | 501 (18.2) |
Figure 1.
Number of centres in relation of the percent of screened vs all included patients
Tested population and results of thrombophilia testing
When the personal and clinical characteristics of patients (tested or not tested), were compared (Table II), tested patients were more often young (in particular <50 years of age), presenting with isolated PE or venous thrombosis at unusual sites with events that were idiopathic or associated with weak RF. Conversely, fewer elderly patients (>75 years) and subjects with renal problems, DVT and VTE associated with major or persisting RF were tested.
Table II.
Baseline characteristics of patients
| Tested 2,759 (57.2%) |
Not tested 2,067 (42.8%) |
p# | |
|---|---|---|---|
|
| |||
| Male, n (%) | 1,386 (50.2) | 985 (47.6) | |
|
| |||
| Age, median (IQR), years | 65 (49–76) | 68 (53–78) | <0.0001 |
|
| |||
| Age classes, years | |||
| <50 | 713 (25.8) | 438 (21.2) | 0.0002 |
| 50–65 | 697 (25.3) | 486 (23.5) | |
| 66–75 | 637 (23.1) | 492 (23.8) | |
| >75 | 712 (25.8) | 651 (31.5) | <0.0001 |
|
| |||
| Creatinine clearance | |||
| <60 mL/min | 626 (22.7) | 552 (26.7) | 0.0014 |
| >=60 mL/min | 2,133 (77.3) | 1,515 (73.3) | |
|
| |||
| Type of VTE event | |||
| Proximal leg DVT ± PE | 1,180 (42.8) | 1,098 (53.1) | <0.0001 |
| Distal leg DVT ± PE | 564 (20.4) | 389 (18.8) | |
| Isolated PE | 555 (20.2) | 265 (12.8) | <0.0001 |
| Superficial thrombosis | 185 (6.7) | 143 (6.9) | |
| Arm DVT ± PE | 114 (4.1) | 92 (4.5) | |
| Unusual site VT | 161 (5.8) | 80 (3.9) | 0.0027 |
|
| |||
| Nature of VTE events | |||
| Idiopathic | 2,009 (73.0) | 1,287 (63.8) | <0.0001 |
| Associated with weak RF | 171 (6.2) | 97 (4.8) | 0.0379 |
| Provoked by transient major RF | 297 (10.8) | 352 (17.4) | <0.0001 |
| Provoked by permanent major RF | 275 (10.0) | 282 (14.0) | <0.0001 |
| Missing | 7 | 49 | |
Not statistically significant if not reported.
IQR: interequartile range; DVT: deep vein thrombosis; PE: pulmonary embolism; RF: risk factors; VTE: venous thromboembolism.
The presence of at least one thrombophilic alteration was detected in 501 patients (18.2% of those tested). Table III shows the prevalence of the different thrombophilic alterations. As expected FVL and PT mutations in heterozygote form were the most prevalent alterations (10.4% of the tested population, 57.7% of all patients with detected alterations), followed by combined alterations. Natural anticoagulant deficiency was identified in 81 patients (3.0% of the tested population, 16.2% of patients with alterations). Altogether, single or associate alterations of acquired antiphospholipid antibodies, with or without LA phenomenon, were detected in 72 subjects (2.5% of the population, 14.4% of patients with alterations).
Table III.
Diagnosed thrombophilic alterations
| Alteration | N. (% vs all screened n=2,759) [% vs all positive; n=501] |
|---|---|
|
| |
| Antithrombin deficiency | 21 (0.8) [4.2] |
|
| |
| Protein C deficiency | 21 (0.8) [4.2] |
|
| |
| Protein S deficiency | 39 (1.4) [7.8] |
|
| |
| Prothrombin G20210A mutation (heterozygous) | 92 (3.3) [18.4] |
|
| |
| R506Q Factor V Leiden mutation (heterozygous) | 197 (7.1) [39.3] |
|
| |
| Antiphospholipid syndrome# | |
| Triple positive | 12 (0.4) [2.4] |
| Double positive | 9 (0.3) [1.8] |
| Single positive | 51(1.8) [10.2] |
|
| |
| Combined alterations§ | 59 (2.1) [11.8] |
Triple positive: LA positive and high anticardiolipin and antibeta2-glycoprotein1 levels. Double positive: two tests positive among LA, anticardiolipin and antibeta2-glycoprotein1; Single positive: one test positive among LA, anticardiolipin and antibeta2-glycoprotein1 (37/51 patients with isolated positive LA).
Included homozygous or double heterozygous FVL and PT mutations, or combination of a mutation (FVL and/or PT) with an inhibitor deficiency.
LA: lupus anticoagulant; FVL: Factor V Leiden; PT: prothrombin G20210A.
Table IV shows the prevalence of thrombophilic alterations in relation to the sites and types of index VTE events. Our results show a lower rate of R506Q FV Leiden in patients with isolated PE than in those with leg DVT with/without PE (3.4% vs 9.3%, p<0.0001). Furthermore, the prevalence of thrombophilic alterations was comparably higher in patients with proximal leg DVT, superficial vein thrombosis and thrombosis in unusual sites vs patients with other index events.
Table IV.
Type of venous thromboembolism event and diagnosed thrombophilic alterations
| Proximal leg DVT ± PE n=1,180 |
Distal leg DVT ± PE n=564 |
Isolated PE n=555 |
Superficial thrombosis n=185 |
Arm DVT ± PE n=114 |
Unusual site VT n=161 |
|
|---|---|---|---|---|---|---|
|
| ||||||
| Antithrombin deficiency, n (%) | 11 (0.93) | 3 (0.53) | 4 (0.72) | 2 (1.1) | 0 | 1 (0.62) |
|
| ||||||
| Protein C deficiency, n (%) | 12 (1.0) | 5 (0.89) | 2 (0.36) | 0 | 0 | 2 (1.2) |
|
| ||||||
| Protein S deficiency, n (%) | 22 (1.9) | 5 (0.89) | 5 (0.90) | 2 (1.1) | 0 | 5 (3.1) |
|
| ||||||
| Prothrombin G20210A mutation (heterozygous), n (%) | 45 (3.8) | 11 (2.0) | 16 (2.9) | 9 (4.9) | 4 (3.5) | 7 (4.3) |
|
| ||||||
| R506Q Factor V Leiden mutation (heterozygous), n (%) | 110 (9.3) | 34 (6.0) | 19 (3.4) | 20 (10.8) | 5 (4.4) | 9 (5.6) |
|
| ||||||
| Antiphospholipid syndrome# | ||||||
| Triple positive, n (%) | 4 (0.34) | 2 (0.35) | 1 (0.18) | 1 (0.54) | 1 (0.88) | 3 (1.9) |
| Double positive, n (%) | 4 (0.34) | 2 (0.35) | 1 (0.18) | 0 | 1 (0.88) | 1 (0.62) |
| Single positive, n (%) | 24 (2.0) | 7 (1.2) | 12 (2.2) | 4 (2.2) | 1 (0.88) | 3 (1.9) |
|
| ||||||
| Combined alterations§, n (%) | 25 (2.1) | 8 (1.4) | 9 (1.6) | 7 (3.8) | 2 (1.8) | 8 (5.0) |
|
| ||||||
| All alterations, n (%) | 257 (21.8) | 77 (13.7) | 69 (12.4) | 45 (24.3) | 14 (12.3) | 39 (24.2 ) |
Triple positive: LA positive and high anticardiolipin and antibeta2-glycoprotein1 levels; double positive: two tests positive among LA, anticardiolipin and antibeta2-glycoprotein1; single positive: one test positive among LA, anticardiolipin and antibeta2-glycoprotein1.
Included homozygous or double heterozygous FVL and PT mutations, or combination of a mutation (FVL and/or PT) with an inhibitor deficiency.
LA: lupus anticoagulant; FVL: Factor V Leiden; PT: prothrombin G20210A.
Follow-up of patients with or without thrombophilic alterations
Patients with positive thrombophilia results were significantly younger than those without alterations (p<0.0001) (Table V). Compared to those without thrombophilia, patients with thrombophilic alterations were more frequently treated with a DOAC, had lower mortality, and less frequently discontinued anticoagulant treatment. There was no difference in the incidence of complications during treatment, either haemorrhagic or thrombotic, between the two types of patients. Patients with thrombophilic alterations were categorised in: group A, with serious inherited alterations, including inhibitor deficiencies and combined alterations; group B, with heterozygous FVL or PT mutations; and group C, with APLS. Patients in groups A and C were more frequently treated with VKAs, whereas patients with heterozygous FVL or PT mutations (group B) were more often treated with a DOAC and more frequently stopped anticoagulant treatment before 180 days. There was no difference in the occurrence of haemorrhagic or thrombotic complications across the three groups of different thrombophilic alterations. However, the rate of venous thrombotic recurrences was significantly higher in patients with APLS vs those without thrombophilic alterations (2.73 vs 0.61%, p=0.0307). It is of interest to note that among the 72 patients in group C, two venous thrombotic recurrences occurred in the 12 (16.7%) who were carriers of the triple alteration (LA phenomenon, increased levels of anticardiolipin and β2-glycoprotein1 antibodies), whereas only one event occurred in the remaining patients (1.7%; p=0.018).
Table V.
Oral anticoagulant drugs, duration of treatment, bleeding and thromboembolic complications recorded during follow-up
| Patients | Tested without thrombophilic alterations n=2,258 |
All with thrombophilic alterations n=501 |
p | Group A (inhibitor deficiencies or combined alterations) n=140 |
Group B (heterozygous FVL or PT mutations) n=289 |
Group C (antiphospholipid syndrome) n=72 |
|---|---|---|---|---|---|---|
|
| ||||||
| Age at inclusion, median (IQR), years | 67 (52–77) | 53.5 (42–67) | <0.0001 | 52.5 (42–68) | 54 (44–66) | 52 (40–69) |
|
| ||||||
| Total follow-up, years | 2,441 | 566 | 163 | 293 | 110 | |
|
| ||||||
| Oral anticoagulant drug used, n. (%) | ||||||
| VKA | 930 (41.2) | 179 (35.7) | 0.0231 | 58 (41.4)£ | 85 (29.4)& | 36 (50.0) |
| DOAC | 1,150 (50.9) | 301 (60.1) | 0.0002 | 80 (57.2) | 186 (64.4)# | 35 (48.6) |
| Other | 178 (7.9) | 21 (4.2) | 0.0038 | 2 (1.4)$ | 18 (6.2) | 1 (1.4) |
|
| ||||||
| Duration of treatment, n. (%), days | ||||||
| = <180 | 1,013 (44.9) | 222 (44.3) | 60 (42.8) | 138 (47.8)§ | 24 (33.3) | |
| >180 | 1,245 (55.1) | 279 (55.7) | 80 (57.2) | 151 (52.2) | 48 (66.7) | |
|
| ||||||
| Major and CRNMB, n. (% pt/y) | 60 (2.46) | 21 (3.71) | 7 (4.29) | 12 (4.09) | 2 (1.82) | |
|
| ||||||
| Thrombotic events, n. (% pt/y) | ||||||
| Venous | 15 (0.61) | 6 (1.06) | 1 (0.61) | 2 (0.68) | 3 (2.73)@ | |
| Arterial | 6 (0.25) | 1 (0.18) | 0 (0) | 1 (0.34) | 0 (0) | |
|
| ||||||
| Deaths during follow-up, n. (%) | 128 (5.7) | 7 (1.4) | 0.0001 | 1 (0.7) | 3 (1.0) | 3 (4.2) |
| Cancer | 48 | 1 | 1 | 0 | 0 | |
| Other | 66 | 6 | 0 | 3 | 3 | |
|
| ||||||
| Patients who discontinued treatment, n. (%) | 648 (28.7) | 102 (20.4) | 0.0002 | 30 (21.4) | 58 (20.1) | 14 (19.4) |
| End of expected treatment period | 517 | 66 | 17 | 39 | 10 | |
| Moved to different centres | 80 | 21 | 9 | 10 | 2 | |
| Shift to a different treatment | 16 | 7 | 2 | 4 | 1 | |
| Other | 14 | 6 | 2 | 3 | 1 | |
| Cancer | 7 | 1 | 0 | 1 | 0 | |
| Lost at follow-up | 14 | 1 | 0 | 1 | 0 | |
Group A vs Group B, p=0.0135;
Group A vs Group B, p=0.0268; and Group B vs Group C, p=0.0009;
Group B vs Group C, p=0.0139;
Group B vs Group C, p=0.0271;
Patients with antiphospholipid syndrome vs patients without thrombophilic alterations, p=0.0307.
n.: number; IQR: interquartile range; CRNMB: non-major but clinically relevant bleeding; FVL: R506Q Factor V Leiden; PT: prothrombin G20210A.
DISCUSSION
General testing for thrombophilia in VTE patients is discouraged by experts and guidelines5,9,13. However, the present study shows that the approach to this issue varies significantly in clinical practice. We examined a large series of VTE patients whose data were included in a prospective registry (the START2-Register) by Italian thrombosis centres and found that >50% them were tested for thrombophilia. This practice, however, differed markedly among centres: some of them tested almost all, or the major part of patients, whereas others limited the testing to very few or highly selected patients. This difference in practice was not influenced by the geographic distribution of centres (Table I), by the numbers of patients included in the registry, or by patient age, since approximately 50% of tested patients were >65 years old when enroled. As expected, more patients were tested when the index event was idiopathic, presenting as isolated PE or when the thrombosis involved unusual sites. The different approach in clinical practice among the participating centres to testing for thrombophilia is of particular interest since they were almost all affiliated to the same scientific society (FCSA), thus suggesting a lack of discussion on the issue and harmonisation between centres.
At least one thrombophilic alteration was diagnosed in about 20% of tested patients; this was lower than that found in previous studies which included large series of VTE patients (35%14) or of subjects with only DVT (approx. 27%15). The rather low rate of thrombophilic alterations recorded in the present study was almost completely accounted for by the unexpectedly low prevalence of FVL gene mutation (7.1%) and of PT gene mutation (3.3%), the prevalence of which ranged in other studies from 12 to 19% for FVL mutation, and from 7 to 12% for PT mutation14–16. It is reasonable to attribute the lower prevalence of thrombophilic alterations detected in the present study to the more extensive testing generally undertaken by the participating centres and to the older age of investigated patients (median, IQR: 67 years, range: 52–77 years).
In line with previous reports that have described the FV Leiden paradox17, our results confirm a lower rate of R506Q FV Leiden in patients with isolated PE than in those with leg DVT with/without PE (p<0.0001). Furthermore, as also reported by other studies15,18,19, the prevalence of thrombophilic alterations was comparably higher in patients with proximal leg DVT, superficial vein thrombosis and thrombosis in unusual sites than in those with different index events.
Though the present study was not designed to investigate the clinical course of patients after anticoagulation was stopped, we assessed the effects of thrombophilia testing on management and outcomes during anticoagulant treatment. DOACs were more frequently prescribed for long-term treatment in the group of patients with heterozygous FVL or PT mutations, whereas the standard treatment with VKAs was preferred for patients with inhibitor deficiencies, combined alterations or APLS, conditions likely considered at higher risk of complications, and probably because VKAs are expected to assure adequate and stable anticoagulation. Altogether there was no difference in the duration of treatment among patients with or without alterations; it was, however, also influenced by the testing results since a significantly higher proportion of APLS patients received extended treatment. Bleeding and thrombotic complications occurred with a similar and fairly low incidence in patients with or without thrombophilic alterations, and were also similar across the three different groups of alterations. However, the rate of venous thrombotic recurrences was higher in APLS patients than in those without thrombophilic alterations. It should be noted, however, that of the three thrombotic complications recorded in the APLS group of patients (n=72), two occurred in the 12 patients who were triple positive, and only one in the remaining 60 patients, thus confirming the higher thrombotic risk associated with the presence of triple positivity.
Though DOACs are currently used in most VTE cases, their role in the management of thrombophilia-associated VTE is still controversial20,21 and many physicians are reluctant to use them, especially in cases with high-risk alterations22. Furthermore, a recent randomised trial concluded that the use of rivaroxaban in patients with APLS was associated with an increased rate of thrombotic events compared with warfarin23. More than 60% of patients with thrombophilic alterations in the present study were treated with a DOAC, and only two, out of the seven thrombotic events recorded during follow-up in all thrombophilic patients, occurred in association with DOAC treatment. These results seem to agree with the conclusions of a recent systematic review and meta-analysis24 which confirmed that DOACs are an appropriate option for VTE treatment in thrombophilic patients with the exclusion of patients with APLS.
The strength of our study lies in the analysis of different thrombophilia testing approaches by several anticoagulation clinics and antithrombotic Italian centres. The analysis included a large contemporary number of VTE patients who altogether are representative of the real-world population of VTE patients in Italy. The study suffers, however, from several limitations. The START2-Register has an observational design; therefore, the results of any direct comparison between patients’ characteristics and drugs used (whose choice was entirely left to the attending physician’s discretion) should be interpreted with caution. The registry only enrolls Italian patients and may not be representative of other geographic areas.
CONCLUSIONS
Although general testing for thrombophilia in VTE patients is currently discouraged, more than half of a large series of VTE patients included in the START2-Register by Italian thrombosis centers were tested, a practice that, however, differed markedly across centres. The prevalence of thrombophilic alterations detected in the tested patients (about 20%) was lower than expected given the results of prior studies, probably because of the rather low number of patients selected for testing and their median older age. Overall, the rates of bleeding or thrombotic events during follow-up were low and there was no difference between patients with or without thrombophilic alterations. About 60% of all patients with alterations were treated with DOACs, which would seem to confirm they can be a useful option for treatment of thrombophilic VTE patients with the exclusion of patients with APLS.
ACKNOWLEDGMENTS
We thank Stephen Jewkes for his correction of English.
APPENDIX 1
List of the START2 Register Investigators
Sophie Testa, Oriana Paoletti, Rossella Morandini - Centro Emostasi e Trombosi - Laboratorio Analisi chimico-cliniche emicrobiologiche - Azienda Socio Sanitaria Territoriale di Cremona, Cremona
Daniela Poli, Rossella Marcucci, Niccolò Maggini - SOD Malattie Aterotrombotiche, Azienda Ospedaliero Universitaria-Careggi, Firenze
Benilde Cosmi, Giuliana Guazzaloca - UO di Angiologia e Malattie Coagulazione, AOU S. Orsola-Malpighi, Bologna, Bologna
Anna Falanga, Teresa Lerede, Luca Barcella - USC SIMT, Centro Emostasi e Trombosi, Ospedale Papa Giovanni XXIII, Bergamo
Daniela Mastroiacovo - UOSD Angiologia e Diagnostica Vascolare, Ospedale SS Filippo e Nicola, Avezzano (AQ)
Eugenio Bucherini - Struttura Semplice Dipartimentale, Medicina Vascolare, Angiologia, Ospedale Civile Faenza, Faenza (RA)
Paolo Chiarugi - Ambulatorio Antitrombosi per la sorveglianza dei pazienti in terapia anticoagulante orale, U.O. Analisi Chimico-Cliniche, Azienda Ospedaliero Universitaria Pisana, Pisa
Elvira Grandone, Donatella Colaizzo - Centro Trombosi Casa del Sollievo e della Sofferenza, S. Giovanni Rotondo (FG)
Vittorio Pengo - Clinica Cardiologica, AOU di Padova, Padova
Pasquale Pignatelli, Daniele Pastori - Centro Trombosi, Clinica Medica Policlinico Umberto I°, Roma
Antonietta Piana, Francesco Cibecchini - Centro Prevenzione e Cura Malattie Tromboemboliche Azienda Ospedaliera Universitaria San Martino-IST, Genova
Giuliana Martini - Centro Emostasi, Spedali Civili Di Brescia, Brescia
Salvatore Bradamante – Centro Emostasi e Trombosi, Centro Trasfusionale, Ospedale SS Annunziata, Taranto
Lucilla Masciocco - Centro Controllo Coagulazione, Medicina Interna, Presidio Ospedaliero Lastaria, Lucera (FG)
Carmelo Paparo - Patologia Clinica, Ospedale Maggiore, Chieri (TO)
Walter Ageno, Giovanna Colombo - Degenza Breve Internistica e Centro Trombosi ed Emostasi, Azienda Socio Sanitaria Territoriale dei Sette Laghi, Varese Ospedale di Circolo, Varese
Claudio Vasselli - Laboratorio Patologia Clinica, Policlinico Casilino, Roma
Andrea Toma - UOC di Patologia Clinica, Ambulatorio Terapia Anticoagulante Orale, O.C. “L. Cazzavillan” Arzignano, (VI)
Adriana Visonà, Diego Tonello - UO Angiologia, Azienda ULSS 2 Marca Trevigiana, Castelfranco Veneto (TV)
Footnotes
AUTHORSHIP CONTRIBUTIONS
CL wrote the first draft of the paper and contributed to data analysis; GP conceived and designed the analysis and reviewed the manuscript; EA monitored the data collection and contributed to data analysis; DP, BC, AF, DM and ST collected the data and reviewed the manuscript; START 2-Register Investigators collected the data and their contributions are acknowledged.
The Authors declare no conflicts of interest.
REFERENCES
- 1.De Stefano V, Rossi E, Paciaroni K, Leone G. Screening for inherited thrombophilia: indications and therapeutic implications. Haematologica. 2002;87:1095–108. [PubMed] [Google Scholar]
- 2.Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301:2472–85. doi: 10.1001/jama.2009.853. [DOI] [PubMed] [Google Scholar]
- 3.Coppens M, Van Mourik JA, Eckmann CM, et al. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5:1979–81. doi: 10.1111/j.1538-7836.2007.02658.x. [DOI] [PubMed] [Google Scholar]
- 4.Laberge AM, Psaty BM, Hindorff LA, Burke W. Use of Factor V Leiden genetic testing in practice and impact on management. Genet Med. 2009;11:750–6. doi: 10.1097/GIM.0b013e3181b3a697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connors JM. Thrombophilia testing and venous thrombosis. N Engl J Med. 2017;377:1177–87. doi: 10.1056/NEJMra1700365. [DOI] [PubMed] [Google Scholar]
- 6.Kearon C. Influence of hereditary or acquired thrombophilias on the treatment of venous thromboembolism. Curr Opin Hematol. 2012;19:363–70. doi: 10.1097/MOH.0b013e328356745b. [DOI] [PubMed] [Google Scholar]
- 7.Brouwer JL, Lijfering WM, Ten Kate MK, et al. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost. 2009;101:93–9. [PubMed] [Google Scholar]
- 8.Coppens M, Reijnders JH, Middeldorp S, et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474–7. doi: 10.1111/j.1538-7836.2008.03055.x. [DOI] [PubMed] [Google Scholar]
- 9.De Stefano V, Rossi E. Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost. 2013;110:697–705. doi: 10.1160/TH13-01-0011. [DOI] [PubMed] [Google Scholar]
- 10.Antonucci E, Poli D, Tosetto A, et al. The Italian START-Register on anticoagulation with focus on atrial fibrillation. PLoS One. 2015;10:e0124719. doi: 10.1371/journal.pone.0124719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaatz S, Ahmad D, Spyropoulos AC, et al. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119–26. doi: 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 13.Chong LY, Fenu E, Stansby G, Hodgkinson S. Management of venous thromboembolic diseases and the role of thrombophilia testing: summary of NICE guidance. BMJ. 2012;344:e3979. doi: 10.1136/bmj.e3979. [DOI] [PubMed] [Google Scholar]
- 14.Makelburg AB, Veeger NJ, Middeldorp S, et al. Different risk of deep vein thrombosis and pulmonary embolism in carriers with factor V Leiden compared with non-carriers, but not in other thrombophilic defects. Results from a large retrospective family cohort study. Haematologica. 2010;95:1030–3. doi: 10.3324/haematol.2009.017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legnani C, Cini M, Cosmi B, et al. Inherited and acquired thrombophilic alterations in patients with superficial vein thrombosis of lower limbs. Thromb Haemost. 2014;111:1194–6. doi: 10.1160/TH13-11-0925. [DOI] [PubMed] [Google Scholar]
- 16.Dentali F, Ageno W, Bozzato S, et al. Role of factor V Leiden or G20210A prothrombin mutation in patients with symptomatic pulmonary embolism and deep vein thrombosis: a meta-analysis of the literature. J Thromb Haemost. 2012;10:732–7. doi: 10.1111/j.1538-7836.2012.04656.x. [DOI] [PubMed] [Google Scholar]
- 17.Corral J, Roldan V, Vicente V. Deep venous thrombosis or pulmonary embolism and factor V Leiden: enigma or paradox. Haematologica. 2010;95:863–6. doi: 10.3324/haematol.2010.023432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinelli I. Unusual forms of venous thrombosis and thrombophilia. Pathophysiol Haemost Thromb. 2002;32:343–5. doi: 10.1159/000073595. [DOI] [PubMed] [Google Scholar]
- 19.Ma K, Wells P, Guzman C, et al. A multicenter prospective study of risk factors and treatment of unusual site thrombosis. Thromb Res. 2016;144:100–5. doi: 10.1016/j.thromres.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Wypasek E, Potaczek DP, Alhenc-Gelas M, Undas A. PROS1 mutations associated with protein S deficiency in Polish patients with residual vein obstruction on rivaroxaban therapy. Thromb Res. 2014;134:199–201. doi: 10.1016/j.thromres.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Boey JP, Jolley A, Nicholls C, et al. Novel protein C gene mutation in a compound heterozygote resulting in catastrophic thrombosis in early adulthood: diagnosis and long-term treatment with subcutaneous protein C concentrate. Br J Haematol. 2016;172:811–3. doi: 10.1111/bjh.13538. [DOI] [PubMed] [Google Scholar]
- 22.Sindet-Pedersen C, Pallisgaard JL, Staerk L, et al. Temporal trends in initiation of VKA, rivaroxaban, apixaban and dabigatran for the treatment of venous thromboembolism - A Danish nationwide cohort study. Sci Rep. 2017;7:3347. doi: 10.1038/s41598-017-03596-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365–71. doi: 10.1182/blood-2018-04-848333. [DOI] [PubMed] [Google Scholar]
- 24.Elsebaie MAT, van Es N, Langston A, et al. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: a systematic review and meta-analysis. J Thromb Haemost. 2019;17:645–56. doi: 10.1111/jth.14398. [DOI] [PubMed] [Google Scholar]