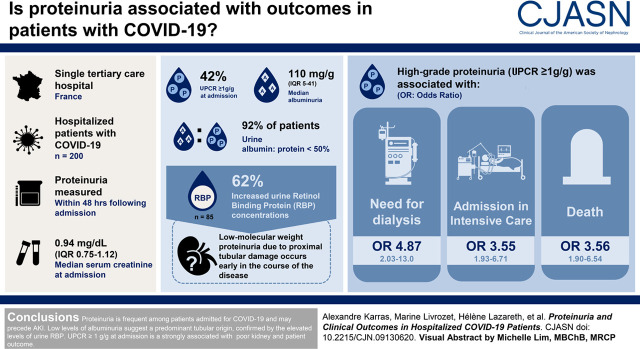
Visual Abstract
Keywords: acute renal failure, COVID-19, proteinuria, proximal tubule, tubular epithelium, prognosis
Abstract
Background and objectives
Kidney involvement is frequent among patients with coronavirus disease 2019 (COVID-19), and occurrence of AKI is associated with higher mortality in this population. The objective of this study was to describe occurrence and significance of proteinuria in this setting.
Design, setting, participants & measurements
We conducted a single-center retrospective study to describe the characteristic features of proteinuria measured within 48 hours following admission among patients with COVID-19 admitted in a tertiary care hospital in France, and to evaluate its association with initiation of dialysis, intensive care unit admission, and death.
Results
Among 200 patients with available data, urine protein-creatinine ratio at admission was ≥1 g/g for 84 (42%), although kidney function was normal in most patients, with a median serum creatinine of 0.94 mg/dl (interquartile range, 0.75–1.21). Median urine albumin-creatinine ratio was 110 mg/g (interquartile range, 50–410), with a urine albumin-protein ratio <50% in 92% of patients. Urine retinol binding protein concentrations, available for 85 patients, were ≥0.03 mg/mmol in 62% of patients. Urine protein-creatinine ratio ≥1 g/g was associated with initiation of dialysis (odds ratio, 4.87; 95% confidence interval, 2.03 to 13.0; P<0.001), admission to the intensive care unit (odds ratio, 3.55; 95% confidence interval, 1.93 to 6.71; P<0.001), and death (odds ratio, 3.56; 95% confidence interval, 1.90 to 6.54; P<0.001).
Conclusions
Proteinuria is very frequent among patients admitted for COVID-19 and may precede AKI. Low levels of albuminuria suggest a predominant tubular origin, confirmed by the elevated levels of urine retinol binding protein. Urine protein-creatinine ratio ≥1 g/g at admission is strongly associated with poor kidney and patient outcome.
Introduction
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus is responsible for coronavirus disease 2019 (COVID-19). Despite predominant respiratory manifestations, COVID-19 has a broad clinical spectrum encompassing asymptomatic infection, mild upper airways illness with fever, and severe pneumonia with respiratory failure, as well as thromboembolic complications, coagulopathy, acute cardiac injury, and neurologic symptoms (1,2). Patients with critical presentations require admission to intensive care units (ICUs) either for acute respiratory distress syndrome or for cytokine storm syndrome leading to multiple organ dysfunction (3). Mortality is particularly important among older patients as well as populations with comorbidities such as obesity, diabetes, hypertension, chronic respiratory disease, or previous immunosuppression (4).
Several teams have reported kidney involvement in SARS-CoV-2–infected patients. Although initial reports suggested that acute kidney failure was rare (<5%) in the entire infected population (1,5), the incidence of this complication is much higher, up to 65%, among patients with severe disease who were admitted to the ICU (6) or those with a fatal outcome (2,7–9). More recently, a retrospective study of 3993 patients with COVID-19 hospitalized in New York revealed that AKI occurred in 46% of patients and that 19% of patients with AKI required dialysis (10). An early report from a large (n=701) cohort in China revealed that 43.9% of patients had proteinuria and 26.7% had hematuria on admission (11). The same authors reported that among 333 patients hospitalized in Wuhan with COVID-19 pneumonia, 75.4% had transient abnormal urine dipstick tests (12). Among the 435 patients with AKI and available urine tests reported by Chan et al. (10), 84% had proteinuria, 81% had hematuria, and 60% had leucocyturia.
In the first published autopsy series (13), postmortem examination of kidney pathology was reported in 26 cases, showing that the most common finding was diffuse proximal acute tubular necrosis (ATN), due to either kidney ischemia or to direct infection of kidney epithelial cells by SARS-CoV-2, as suggested by the presence of viral-like particles on electron microscopy studies (13,14) and also by the presence of viral RNA in kidney cells (15). This tropism of the virus for the kidney was confirmed by other authors detecting SARS-CoV-2 RNA in the kidney of 73% of patients with COVID-19 and AKI and also showing that patient-derived virus can replicate in kidney tubular epithelial cells (16).
Notwithstanding, a comprehensive characterization of kidney injury associated with SARS-CoV-2 is lacking. We undertook this study to describe the clinical and biochemical features of AKI associated with SARS-CoV-2 in a large cohort of patients. The aim of this study was to study the prevalence of proteinuria among hospitalized patients with COVID-19 to describe its nature and to test the prognostic value of proteinuria in this setting.
Materials and Methods
Study Design, Setting, and Population
This retrospective, single-center, observational study was conducted in the Hôpital Européen Georges Pompidou, an academic hospital belonging to the Assistance Publique-Hôpitaux de Paris (APHP) hospital network and providing health care to the population of Paris area. The local institutional review board (registration no. 00011928, Comité d’Ethique de la Recherche Assistance Publique Hôpitaux de Paris 5) approved this study as noninterventional research using data collected for routine clinical practice. Patients included in this study were all informed that their medical data could be used for research purpose, in accordance with General Data Protection Regulation 2016/679. Our analysis follows recommendations provided by the Reporting of Studies Conducted Using Observational Routinely-Collected Health Data statement.
After the publication of the first data concerning kidney injury during SARS-CoV-2 infection, local recommendations were made in our institution for clinicians in charge of patients with COVID-19 in the different units, such as emergency rooms, internal medicine, pulmonology, vascular medicine, nephrology, and ICUs. In accordance with these recommendations, patients had early routine urine testing for proteinuria and, whenever possible, urine albumin and retinol binding protein assessment. All patients admitted in the hospital between March 17 and April 15, 2020, with proven SARS-CoV-2 infection and available laboratory proteinuria testing within 48 hours following admission were included in this study. The SARS-CoV-2 infection was proven by a positive result on PCR testing of a nasopharyngeal or oropharyngeal swab specimen, bronchoalveolar lavage, or bronchial aspirates. The only exclusion criterion was kidney failure requiring long-term dialysis therapy as history. Clinical outcomes (AKI, ICU admission, and death) were monitored until the end of hospitalization through August 1, 2020, the end date of follow-up. Complete data were available for all patients during hospitalization, retrieved from the institutional electronic medical records. Follow-up data, including kidney function assessment, could be collected for most patients during an outpatient clinic visit in the 2-month period following hospital discharge.
Laboratory Measures
Urine biochemical parameters were obtained prospectively on spot urine collection within 48 hours after admission. Proteinuria was expressed as urine protein-creatinine ratio (UPCR), and albuminuria was expressed as urine albumin-creatinine ratio (UACR). Tubular dysfunction was evaluated in a subset of patients by assessment of urine retinol binding protein concentration, expressed as urine retinol binding protein-creatinine ratio.
The total urinary protein concentration was quantified by measuring pyrogallol red at 600-/800-nm absorbance (urinary protein assay; Beckman Coulter), and the albumin concentration was measured by immunoturbidimetry analysis (DIAgAM assay; Beckman Coulter) using a Beckman Coulter AU680 analyzer. The urinary levels of retinol binding protein were measured using a Siemens BN II Nephelometer Analyzer II. Urine protein concentrations were corrected for creatinine levels, which were measured using a colorimetric assay (modified kinetic Jaffe method) on a Beckman Coulter DXC analyzer (serum) or a Beckman Coulter AU680 analyzer (urine).
Definitions
AKI was defined by the Kidney Disease Improving Global Outcomes (KDIGO) AKI criteria (17). The KDIGO criteria for AKI were applied using only serum creatinine variations, as urine output data were not available for patients developing AKI before entering ICU. Baseline serum creatinine value was defined by the most recent value found in the centralized electronic medical records of the Assistance Publique-Hôpitaux de Paris network preceding the current admission. Whenever data were not available, we used as baseline value the initial serum creatinine level measured at the time of admission. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula (18). CKD was defined as a previously known eGFR below 60 ml/min per 1.73 m2 preceding actual hospitalization or a former history of kidney transplantation.
Indication for ICU admission was on the basis of the presence of acute respiratory distress syndrome requiring high-flow nasal oxygen therapy, noninvasive mechanical ventilation, or invasive mechanical ventilation. Hospitalization in non-ICU departments was decided when patients required only nasal low-flow oxygen administration and standard medical monitoring.
Comorbidities, including diabetes mellitus, hypertension, or CKD, were retrospectively collected in individual medical files of the included patients.
Statistical Analyses
Categorical variables were summarized as percentages, and continuous variables were expressed as the mean ± SD or median with interquartile range (IQR). Characteristics of subgroups were compared with the use of a t test or a Wilcoxon rank-sum test for continuous measures and a chi-squared test or a Fisher exact test for categorical variables. The effect of initial proteinuria on the occurrence of death was examined by Kaplan–Meier analysis with the use of log-rank test. Multivariable logistic regression was performed to evaluate the relationship between UPCR, retinol binding protein-creatinine ratio, and outcomes adjusted for age, sex, body mass index (BMI), hypertension, diabetes, and serum creatinine at admission. Adjusted analysis excluded patients with missing data for one or more covariates. Results are reported as odds ratios (ORs) with 95% confidence intervals (95% CIs). Statistical significance was set at two-sided P=0.05. Analyses were performed with either version 8.0.2 of JMP software (SAS Institute) or version 1.2.5033 of the R Studio software (R Project for Statistical Computing; R Foundation).
Results
Study Population
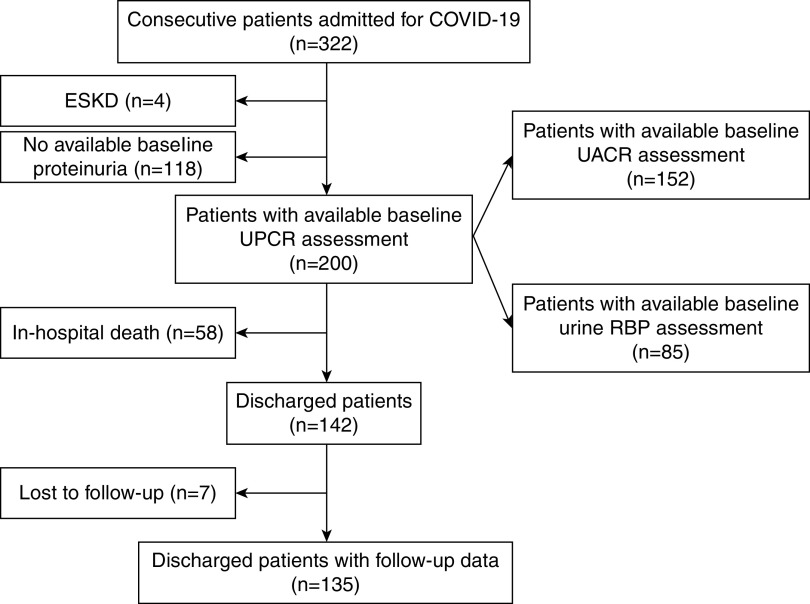
Among the 322 patients admitted by April 15, 2020, 122 patients were excluded from analysis due to lack of data on proteinuria or to presence of preexisting kidney failure requiring long-term dialysis therapy. Figure 1 shows the flow diagram of the study. The characteristics of patients without available proteinuria are detailed in Supplemental Table 1, revealing a population with fewer comorbidities and lower risk of admission to ICU or death.
Figure 1.
Flow diagram. Depicts included and excluded patients, as well as numbers of patients available for each analysis. COVID-19, coronavirus disease 2019; RBP, retinol binding protein; UACR, urine albumin-creatinine ratio; UPCR, urine protein-creatinine ratio.
Overall, 200 patients—143 men and 57 women—were included in this study (Table 1). Median age was 63 years (IQR, 54–73), with 32 of 200 (16%) patients aged above 80 years. BMI was available for 171 patients, with a median value of 26.7 kg/m2 (IQR, 24.3–30.1). Of note, 66 (39%) patients had a BMI between 25 and 30 kg/m2, and 44 (26%) patients had a value above 30 kg/m2. Former history of hypertension was reported in 103 patients (51%), and diabetes mellitus was reported in 51 (25%) patients. Previously known CKD was reported for 18 patients, of whom seven were kidney transplant recipients. Previous use of antihypertensive therapy with angiotensin-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) was reported for 60 (30%) patients, whereas 42 (21%) patients were receiving diuretics. The median delay between self-reported first signs of viral infection and admission was 7 days (IQR, 5–9).
Table 1.
Baseline characteristics for patients admitted for coronavirus disease 2019 according to proteinuria level at admission
| Characteristics | All Patients, n=200 | Urine Protein-Creatinine Ratio, g/g | Urine Retinol Binding Protein-Creatinine Ratio, mg/mmol | ||
|---|---|---|---|---|---|
| <1, n=116 | ≥1, n=84 | <0.03, n=32 | ≥0.03, n=53 | ||
| Men | 143 (71) | 78 (67) | 65 (77) | 21 (65) | 39 (76) |
| Age, yr | 63 (54–73) | 60 (53–69) | 67 (55–79) | 59 (48–64) | 63 (54–75) |
| BMI, kg/m2a | 26.7 (24.3–30.1) | 26.5 (23.9–30.3) | 27.4 (24.6–30.1) | 26.1 (24.5–30) | 27.6 (24.5–31.3) |
| Hypertension | 103 (51) | 53 (46) | 50 (59) | 12 (37) | 29 (55) |
| Diabetes | 51 (25) | 25 (21) | 26 (31) | 7 (22) | 16 (30) |
| CKD | 18 (9) | 10 (9) | 8 (9) | 2 (6) | 5 (9) |
| Use of ACEi or ARB | 60 (30) | 25 (21) | 35 (41) | 7 (22) | 20 (38) |
| Use of diuretics | 42 (21) | 19 (16) | 23 (27) | 5 (16) | 10 (19) |
| First symptoms to admission, d | 7 (5–9) | 7 (6–9.7) | 7 (4–8) | 7 (4–9) | 7 (5–10) |
| UPCR, g/g | 0.86 (0.38–1.50) | 0.41 (0.25–0.72) | 1.6 (1.3–2.1) | 0.24 (0.15–0.51) | 1.22 (0.75–1.8) |
| UACR, mg/gb | 110 (50–410) | 60 (20–100) | 480 (260–830) | 98 (42–382) | 250 (103–533) |
| UACR-UPCR,b % | 18 (9.6–33.4) | 13 (6.7–20.5) | 32 (20.9–43.3) | 16 (8.5–27.6) | 23 (13.2–36.6) |
| URBPCR,c mg/mmol | 0.31 (0–2.76) | 0 (0–0.14) | 2.8 (1–5.8) | 0 (0–0) | 1.35 (0.63–4.8) |
| Serum creatinine, mg/dl | 0.94 (0.75–1.21) | 0.85 (0.69–1.12) | 1.03 (0.85–1.36) | 0.83 (0.63–1.11) | 0.98 (0.77–1.30) |
Quantitative values are given as medians (interquartile ranges), and qualitative values are given as number of patients/percentage. BMI, body mass index; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; UPCR, urine protein-creatinine ratio; UACR, urine albumin-creatinine ratio; URBPCR, urine retinol binding protein-creatinine ratio.
Data available for 171 patients.
Data available for 152 patients.
Data available for 85 patients.
Proteinuria
Median UPCR at admission was 0.86 g/g (IQR, 0.38–1.50). Proteinuria was <0.2 g/g in 24 (12%) patients, <0.5 g/g in 72 (36%) patients, between 0.5 and 1 g/g in 44 (22%) patients, between 1 and 3 g/g in 68 (34%) patients, and above 3 g/g in only 16 (8%) patients. Despite the fact that urine specimens were collected within 48 hours of admission, 45 patients had these urine tests performed after the AKI diagnosis was made on the basis of serum creatinine elevation.
Baseline UACR was available for only 152 patients (76% of the entire study population). Median UACR was 0.11 g/g (IQR, 0.05–0.41). The median UACR-UPCR ratio was 18% (IQR, 9.6–33.4). UACR-UPCR was above 50% for only 8% of patients.
Baseline urine retinol binding protein was measured in 85 patients. All but two of them had UPCR and UACR measured on the same urine specimen. The urine retinol binding protein-creatinine ratio was below the detection threshold (0.03 mg/mmol) in 32 (37%) patients. For the 53 (63%) patients with detectable urine retinol binding protein, the median value of urine retinol binding protein-creatinine ratio was 1.35 (IQR, 0.63–4.81) mg/mmol. Among the 85 patients with available urine retinol binding protein assessment (Table 1), those with urine retinol binding protein-creatinine ratio above the detection threshold (≥0.03 mg/mmol) had higher levels of baseline proteinuria and slightly higher serum creatinine at admission.
In order to compare baseline characteristics, patients were divided according to their initial UPCR level (<1 versus ≥1 g/g). The main characteristics of the two groups are shown in Table 1. Patients with UPCR≥1 g/g were older, more likely to have hypertension, and more likely to receive ACEi or ARB, but there was no difference regarding the sex ratio, the prevalence of diabetes, the prevalence of CKD, or the level of BMI when compared with patients with UPCR<1 g/g. Interestingly, high levels of proteinuria were observed in patients with or without former history of diabetes or CKD (Supplemental Tables 2–4).
Patients with UPCR≥1 g/g at admission had significantly higher levels of UACR and UACR-UPCR ratio. However, only a minority (n=30 of 84; 36%) of those patients had significant glomerular proteinuria with UACR>0.5 g/g, and only 11 of 86 (13%) had an UACR-UPCR above 50%. Patients with UPCR≥1 g/g also had higher urine retinol binding protein levels (Table 1). Nevertheless, the predominantly nonglomerular origin of proteinuria was confirmed by the negative correlation between UACR and urine retinol binding protein-creatinine ratio found at high levels of albuminuria (Supplemental Figure 1).
Outcomes
Median serum creatinine level at admission was 0.94 mg/dl (IQR, 0.75–1.21). Only 29 (14%) patients had initial serum creatinine >1.5 mg/dl at admission. Median peak serum creatinine was 1.19 mg/dl (IQR, 0.84–2.16), with 79 (39%) patients having at least one value above 1.5 mg/dl during their hospitalization.
AKI was diagnosed in 88 (44%) patients, with 28 (14%), 24 (12%), and 36 (18%) patients presenting stage 1, 2, or 3 AKI, respectively. KRT was required for 27 (13%) patients.
During hospitalization, 118 (59%) patients were admitted to the ICU for severe COVID-19 pneumonia. At last follow-up, 58 (29%) had died after a median delay of 10 days (IQR, 6–20) from admission; 142 (71%) were discharged, with a median length of stay of 11 days (IQR, 7–26) from admission.
Long-term follow-up data after hospitalization for COVID-19 were available for 135 of 142 surviving patients. After a median follow-up period of 36 (IQR, 27–54) days, the median serum creatinine was 0.82 (IQR, 0.67–1) mg/dl. Among the ten surviving patients with last known serum creatinine >1.5 mg/dl, eight had preexisting CKD.
Association of Proteinuria with Outcomes
The presence of a UPCR≥1 g/g was associated with higher peak median serum creatinine (1.79 versus 1.06 mg/dl; P<0.001), but it was also associated with significant risk of AKI (OR, 3.61; 95% CI, 2.02 to 6.58; P<0.001) and requirement of KRT (OR, 4.87; 95% CI, 2.03 to 13.00; P<0.001), as well as with risk of admission in the ICU (OR, 3.55; 95% CI, 1.93 to 6.71; P<0.001) and occurrence of death during hospitalization (OR, 3.56; 95% CI, 1.90 to 6.84; P<0.001) in unadjusted analysis (Table 2). When studying only the 138 patients with normal kidney function (eGFR>60 ml/min per 1.73 m2) at admission, the criterion UPCR≥1 g/g was still associated with ICU admission (77% versus 44%; P<0.001), subsequent AKI (45% versus 18%; P=0.004), need for KRT (22% versus 4.5%; P=0.001), and occurrence of death (43% versus 14%; P<0.001). Of note, the delay from first symptoms to admission was not statistically different between the two groups.
Table 2.
Study outcomes (unadjusted analysis)
| Outcomes | Urine Protein-Creatinine Ratio | Urine Retinol Binding Protein-Creatinine Ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| <1 g/g, n=116 | ≥1 g/g, n=84 | Odds Ratio (95% Confidence Interval) | P Value | <0.03 mg/mmol, n=32 | ≥0.03 mg/mmol, n=53 | Odds Ratio (95% Confidence Interval) | P Value | |
| Peak serum creatinine, mg/dl | 1.06 (0.77–1.54) | 1.79 (1.13–3.79) | <0.001 | 0.92 (0.77–1.19) | 1.44 (0.99–1.44) | 0.004 | ||
| AKI, all stages | 36 (31) | 52 (62) | 3.61 (2.02 to 6.58) | <0.001 | 6 (19) | 28 (52) | 5.24 (1.95 to 15.98) | 0.002 |
| Stage 1 | 15 (13) | 13 (15) | 4 (12) | 11 (21) | ||||
| Stage 2 | 10 (9) | 14 (17) | 1 (3) | 8 (15) | ||||
| Stage 3 | 11 (9) | 25 (30) | 1 (3) | 9 (17) | ||||
| KRT | 7 (6) | 20 (24) | 4.87 (2.03 to 13.00) | <0.001 | 0 (0) | 6 (11) | ||
| ICU required | 55 (47) | 64 (76) | 3.55 (1.93 to 6.71) | <0.001 | 8 (25) | 41 (77) | 10.25 (3.82 to 30.23) | <0.001 |
| Death | 21 (18) | 37 (44) | 3.56 (1.90 to 6.54) | <0.001 | 1 (3) | 21 (40) | 22 (4.21 to 406) | <0.001 |
Quantitative values are given as medians (interquartile ranges), and qualitative values are given as number of patients/percentage. Odds ratios and P values are given for unadjusted analysis. ICU, intensive care unit.
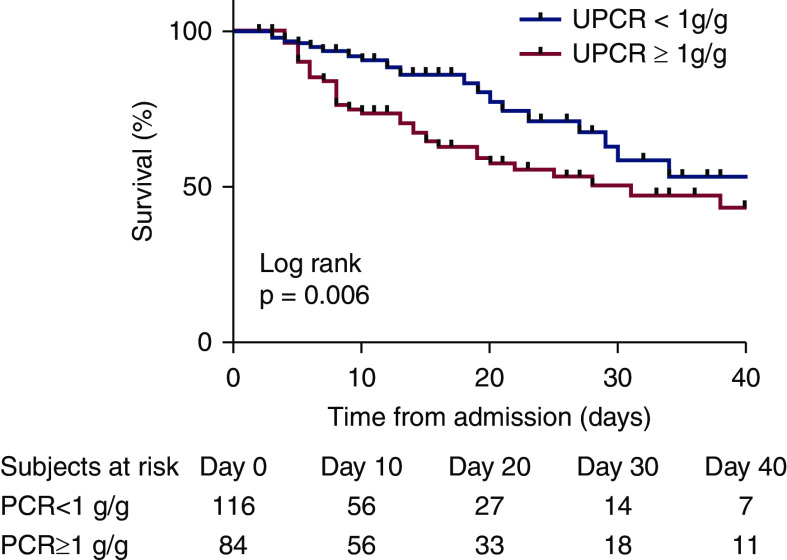
Kaplan–Meier unadjusted survival curves according to baseline proteinuria (<1 or ≥1 g/g) are shown in Figure 2, with a significant difference between groups as concerning occurrence of death (P=0.006).
Figure 2.
Patient survival with Kaplan–Meier analysis. Analysis of event-free survival according to admission proteinuria ≥1 or <1 g/g.
A multivariable analysis was performed, including UPCR≥1 g/g, age, sex, BMI, hypertension, diabetes, and serum creatinine at admission (Supplemental Table 2). The criterion UPCR≥1 g/g remained associated with need for KRT (OR, 4.84; 95% CI, 1.93 to 13.5; P=0.001), admission to the ICU (OR, 3.66; 95% CI, 1.72 to 8.20; P=0.001), occurrence of death (OR, 3.03; 95% CI, 1.46 to 6.39; P=0.003), and the composite outcome of death or need for KRT (OR, 4.24; 95% CI, 2.08 to 8.89; P<0.001).
The presence of a urine retinol binding protein-creatinine ratio ≥0.03 mg/mmol at admission was associated with a higher incidence of AKI (OR, 5.24; 95% CI, 1.95 to 15.98; P=0.002), admission to the ICU (OR, 10.25; 95% CI, 3.82 to 30.23; P<0.001), and death (OR, 22; 95% CI, 4.21 to 406; P<0.001) in unadjusted analysis (Table 2). The multivariable analysis performed using the urine retinol binding protein revealed that a urine retinol binding protein-creatinine ratio >0.03 mg/mmol was associated with occurrence of AKI (OR, 5.18; 95% CI, 1.18 to 29.9; P=0.04), admission to the ICU (OR, 15.5; 95% CI, 4.35 to 69.7; P<0.001), and death (OR, 20.9; 95% CI, 3.79 to 394; P=0.005) (Supplemental Table 3).
Discussion
This study confirms that proteinuria is very frequent among patients with symptomatic SARS-CoV-2 infection admitted to the hospital, with a UPCR above 1 g/g at admission for 43% of them. Moreover, our results indicate that this proteinuria contains very small amounts of albuminuria, as the urine albumin-protein ratio is >50% for only 8% of patients, even among patients with UPCR≥1 g/g. In line with this, the urine levels of retinol binding protein are elevated in >50% of tested patients, an observation that strengthens the hypothesis that COVID-19–associated proteinuria reflects low-molecular weight proteins, which cannot be reabsorbed by the proximal kidney tubule in this condition due to acute tubular damage. Critically, in accordance with previous reports (19,20), we demonstrate that low-molecular weight proteinuria reflects early tubular dysfunction sometimes preceding AKI as suggested by the frequent normal-range serum creatinine level at admission.
The kidney histopathologic data published to date have indeed described diffuse injury of proximal tubular epithelial cells with loss of brush border and vacuolar degeneration followed by ATN (13,21). Causes of ATN are multiple in the setting of severe infection (22), as systemic hypoxia, local hypoxia due to microvascular damage, drug toxicity, or sepsis-induced rhabdomyolysis can all promote tissue injury. Nevertheless, electron microscopic examination of kidney biopsy from patients with COVID-19 has revealed virus-like particles within the tubular epithelium, suggesting direct evidence of tubular cells invasion by SARS-CoV-2 (14), and viral RNA has been found in kidney tissue from patients with COVID-19–associated AKI (16). Interestingly, it has been shown that proximal tubule kidney cells express the surface angiotensin-converting enzyme 2 (ACE2), which is instrumental for the coronavirus to bind and penetrate target cells (13,23). More recently, proximal tubule dysfunction has been demonstrated in patients with COVID-19 attested by the presence of low-molecular weight proteinuria (70%–80%), neutral aminoaciduria (46%), and defective handling of both uric acid (46%) and phosphate (19%), corresponding to a partial Fanconi syndrome (24).
Of note, viral-like particles have also been observed inside podocytes and endothelial cells, a finding that may promote glomerular damage. Although two recent cases of collapsing glomerulopathy associated with COVID-19 have been published (25,26), glomerulopathy due to SARS-CoV-2 infection seems to be rare in Asian and European populations, an idea supported by the findings of this study. Nevertheless, this complication may be especially prevalent among patients of African ancestry, in particular owing to a specific genetic background and the presence of APOL1 genetic variants, which predispose to the occurrence of collapsing glomerulopathy (25). This severe form of FSGS could be secondary to a direct effect of the virus on podocytes or could be the consequence of the SARS-CoV-2–induced cytokine storm, a condition that has been associated with glomerular lesions (27).
Our data confirm that overt proteinuria is more frequent among older patients with history of hypertension. The use of renin-angiotensin-aldosterone system inhibitors, such as ACEi and ARB, was more frequent among patients with high-grade proteinuria. Early reports had suggested that these drugs could modify cellular mRNA expression and protein level of ACE2, the SARS-CoV-2 receptor (28). Nevertheless, their use has not been associated with greater severity of disease, and recent studies have not confirmed ACE2 overexpression in the kidney after renin-angiotensin-aldosterone system inhibitor exposure (29).
In addition, we demonstrate that the presence of low-molecular weight proteinuria may precede elevation of serum creatinine and predict both AKI and KRT requirement, as it has been shown in non–COVID-19–associated ATN and AKI associated with sepsis (20,30). Although we have no control non–COVID-19 group, we show that UPCR≥1 g/g and retinol binding protein-creatinine ratio >0.03 mg/mmol are associated with a higher risk of severe COVID-19 that requires admission in the ICU and leads to higher mortality, even among patients with no significant elevation of serum creatinine at admission.
The prognostic value of kidney function impairment during COVID-19 has already been suggested in other series (2,10,11,31), but our study is the first large series to show that the outcome is associated with simple urine tests, such as UPCR and UACR, used in daily practice in contrast with new biomarkers, such as neutrophil gelatinase–associated lipocalin or kidney injury marker-1 (22,32), that have been evaluated in other situations with high risk of AKI but not yet in early phases of COVID-19.
This study has some limitations. First, the study population probably included patients who have the most severe forms of COVID-19, with a high proportion of patients requiring intensive care. In consequence, our results cannot be generalized to nonhospitalized patients with COVID-19. Second, some clinical data are missing, notably regarding level of inflammatory markers, severity of respiratory disease, and incidence of other complications, such as coagulopathy or cardiovascular events. These parameters strongly affect patient survival in this setting, and therefore, we cannot affirm that proteinuria is indeed an independent predictor of patient outcome. Third, the use of the UPCR should be interpreted with caution in AKI, as urinary creatinine excretion is variably decreased in this setting. Nevertheless, the aim of this study was not to estimate the 24-hour proteinuria by this ratio, as it is proposed in CKD, but was only to validate this simple test as a prognostic marker during COVID-19. Fourth, urine retinol binding protein—which was used as a marker for proximal tubular dysfunction in our study—was available for only 42% of the study population, and we do not have data about other tubular biomarkers, such as glycosuria, phosphaturia, or neutrophil gelatinase–associated lipocalin, or kidney pathology data to confirm the hypothesis of a proximal tubule injury. However, both proximal tubular lesions and presence of other features of Fanconi syndrome have recently been reported among patients with COVID-19 (24). To our knowledge, this study is the first large series to report on the composition of proteinuria during COVID-19—in contrast with previous publications based on dipstick analysis (10,11)—and to demonstrate that proteinuria in this setting contains mostly low-molecular weight proteins rather than albumin.
In conclusion, this study reveals that COVID-19 is associated with early and frequent tubular proteinuria, which is associated with poor kidney outcome and higher mortality among patients with symptomatic COVID-19. The comprehension of the precise mechanisms underlying this tubular injury requires further investigations.
Disclosures
J.-L. Diehl reports receiving research funding from Alung, General Electrc Healthcare, and Xenios-Novalung (Fresenius Medical Care); receiving honoraria from Baxter and Fresenius Medical Care; and serving on the editorial board of the Annals of Intensive Care. A. Godier reports receiving honoraria from Aguettant, Bayer-Healthcare, Boehringer-Ingelheim, Bristol-Myers-Squibb/Pfizer, and Sanofi. J.-S. Hulot reports consultancy agreements with Bayer and Bioserenity and receiving speaker, advisory board, or consultancy fees from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Novartis, and WeHealth. A. Karras reports receiving honoraria from Abbvie, Amgen, Gilead, and Roche Pharmaceuticals. T. Mirault reports receiving honoraria from Bayer Healthcare SAS and Incyte Biosciences France and receiving nonfinancial support from Abbott France, Alexion Pharma France, Amgen SAS, Bayer Healthcare SAS, Boehringer Ingelheim France, Bristol-Myers Squibb, ICOMED, Incyte Biosciences France, MSD France, and Pfizer SAS. O. Sanchez reports receiving research funding from Bayer, BMS, Daiichi Sankyo, MSD, and Pfizer and receiving honoraria from Bayer, BMS, Boston Scientifics, Chiesi, MSD, Pfizer, and Sanofi Aventis. E. Thervet reports consultancy agreements with Pfizer and Vifor; receiving research funding from Alexion and GSK; and receiving honoraria from Pfizer and Vifor. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Contributor Information
Collaborators: Jérôme Pinot, Sébastien Clerc, Jean-Benoît Arlet, Anne Godier, and Bernard Cholley
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09130620/-/DCSupplemental.
Supplemental Table 1. Characteristics and outcome of admitted patients with and without proteinuria data available at admission.
Supplemental Table 2. Multivariable logistic regression analysis of outcomes odds according to UPCR≥1 g/g, age, sex, BMI, hypertension, diabetes, and initial serum creatinine.
Supplemental Table 3. Multivariable logistic regression analysis of outcomes odds according to urine retinol binding protein-creatinine ratio ≥0.03 mg/mmol, age, sex, BMI, hypertension, diabetes, and initial serum creatinine.
Supplemental Table 4. Characteristics of proteinuria in patients with and without diabetes and with and without CKD.
Supplemental Figure 1. Correlation between urine retinol binding protein-creatinine ratio and UACR-UPCR, UACR, and UPCR.
References
- 1.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSC, Du B, Li L-J, Zeng G, Yuen K-Y, Chen R-C, Tang C-L, Wang T, Chen PY, Xiang J, Li S-Y, Wang J-L, Liang Z-J, Peng Y-X, Wei L, Liu Y, Hu Y-H, Peng P, Wang J-M, Liu J-Y, Chen Z, Li G, Zheng Z-J, Qiu S-Q, Luo J, Ye C-J, Zhu S-Y, Zhong N-S; China Medical Treatment Expert Group for Covid-19: Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B: Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395: 1054–1062, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen SF, Ho Y-C: SARS-CoV-2: A storm is raging. J Clin Invest 130: 2202–2205, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan RE, Adab P, Cheng KK: Covid-19: Risk factors for severe disease and death. BMJ 368: m1198, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y: Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [published correction appears in JAMA Intern Med 180: 1031, 2020 10.1001/jamainternmed.2020.1429]. JAMA Intern Med 180: 934–943, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fanelli V, Fiorentino M, Cantaluppi V, Gesualdo L, Stallone G, Ronco C, Castellano G: Acute kidney injury in SARS-CoV-2 infected patients. Crit Care 24: 155, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q: Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 368: m1091, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, Wang X, Hu C, Ping R, Hu P, Li T, Cao F, Chang C, Hu Q, Jin Y, Xu G: Clinical features of 85 fatal cases of COVID-19 from Wuhan: A retrospective observational study. Am J Respir Crit Care Med 201: 1372–1379, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP; Northwell COVID-19 Research Consortium: Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area [published correction appears in JAMA 323: 2098, 2020 10.1001/jama.2020.7681]. JAMA 323: 2052–2059, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, Paranjpe I, Somani S, Richter F, Miotto R, Lala A, Kia A, Timsina P, Li L, Freeman R, Chen R, Narula J, Just AC, Horowitz C, Fayad Z, Cordon-Cardo C, Schadt E, Levin MA, Reich DL, Fuster V, Murphy B, He JC, Charney AW, Böttinger EP, Glicksberg BS, Coca SG, Nadkarni GN; Mount Sinai COVID Informatics Center (MSCIC): AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 32: 151–160, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, Ma Z, Huang Y, Liu W, Yao Y, Zeng R, Xu G: Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y, Yi F, Yang H-C, Fogo AB, Nie X, Zhang C: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farkash EA, Wilson AM, Jentzen JM: Ultrastructural evidence for direct renal infection with SARS-CoV-2 [published correction appears in J Am Soc Nephrol 31: 2494, 2020 10.1681/ASN.2020081117]. J Am Soc Nephrol 31: 1683–1687, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB: Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383: 590–592, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun F, Lütgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, Nörz D, Heinrich F, Meißner K, Wichmann D, Kluge S, Gross O, Pueschel K, Schröder AS, Edler C, Aepfelbacher M, Puelles VG, Huber TB: SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 396: 597–598, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group: Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit Care 17: 204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norden AG, Scheinman SJ, Deschodt-Lanckman MM, Lapsley M, Nortier JL, Thakker RV, Unwin RJ, Wrong O: Tubular proteinuria defined by a study of Dent’s (CLCN5 mutation) and other tubular diseases. Kidney Int 57: 240–249, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Herget-Rosenthal S, Poppen D, Hüsing J, Marggraf G, Pietruck F, Jakob H-G, Philipp T, Kribben A: Prognostic value of tubular proteinuria and enzymuria in nonoliguric acute tubular necrosis. Clin Chem 50: 552–558, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Santoriello D, Khairallah P, Bomback AS, Xu K, Kudose S, Batal I, Barasch J, Radhakrishnan J, D’Agati V, Markowitz G: Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol 31: 2158–2167, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronco C, Bellomo R, Kellum JA: Acute kidney injury. Lancet 394: 1949–1964, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Pan X-W, Xu D, Zhang H, Zhou W, Wang L-H, Cui X-G: Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single-cell transcriptome analysis. Intensive Care Med 46: 1114–1116, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werion A, Belkhir L, Perrot M, Schmit G, Aydin S, Chen Z, Penaloza A, De Greef J, Yildiz H, Pothen L, Yombi JC, Dewulf J, Scohy A, Gérard L, Wittebole X, Laterre P-F, Miller SE, Devuyst O, Jadoul M, Morelle J; Cliniques universitaires Saint-Luc (CUSL) COVID-19 Research Group: SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int 98: 1296–1307, 2020. 10.1016/j.kint.2020.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA: Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep 5: 935–939, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garandeau C, Figueres L, Renaudin K, Fakhouri F: The Case | A neoplastic cause of acute kidney injury. Kidney Int 97: 621–622, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Thaunat O, Delahousse M, Fakhouri F, Martinez F, Stephan J-L, Noël LH, Karras A: Nephrotic syndrome associated with hemophagocytic syndrome. Kidney Int 69: 1892–1898, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE: Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111: 2605–2610, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Wysocki J, Lores E, Ye M, Soler MJ, Batlle D: Kidney and lung ACE2 expression after an ACE inhibitor or an Ang II receptor blocker: Implications for COVID-19. J Am Soc Nephrol 31: 1941–1943, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagshaw SM, Langenberg C, Bellomo R: Urinary biochemistry and microscopy in septic acute renal failure: A systematic review. Am J Kidney Dis 48: 695–705, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium: Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desanti De Oliveira B, Xu K, Shen TH, Callahan M, Kiryluk K, D’Agati VD, Tatonetti NP, Barasch J, Devarajan P: Molecular nephrology: Types of acute tubular injury. Nat Rev Nephrol 15: 599–612, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.