Abstract
The Chronic Renal Insufficiency Cohort (CRIC) Study is an ongoing, multicenter, longitudinal study of nearly 5500 adults with CKD in the United States. Over the past 10 years, the CRIC Study has made significant contributions to the understanding of factors associated with CKD progression. This review summarizes findings from longitudinal studies evaluating risk factors associated with CKD progression in the CRIC Study, grouped into the following six thematic categories: (1) sociodemographic and economic (sex, race/ethnicity, and nephrology care); (2) behavioral (healthy lifestyle, diet, and sleep); (3) genetic (apoL1, genome-wide association study, and renin-angiotensin-aldosterone system pathway genes); (4) cardiovascular (atrial fibrillation, hypertension, and vascular stiffness); (5) metabolic (fibroblast growth factor 23 and urinary oxalate); and (6) novel factors (AKI and biomarkers of kidney injury). Additionally, we highlight areas where future research is needed, and opportunities for interdisciplinary collaboration.
Keywords: risk factors, chronic kidney disease, progression of chronic renal failure
Introduction
CKD is a growing public health problem worldwide (1), with an estimated prevalence of 14% in the United States (2). In response to the rising epidemic of CKD, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) established the Chronic Renal Insufficiency Cohort (CRIC) Study in 2001. Since its inception, the CRIC Study has enrolled nearly 5500 participants with the purpose of identifying factors that contribute to kidney and cardiovascular disease progression, and morbidity and mortality in persons with CKD (3,4).
The CRIC Study uses the tools of clinical epidemiology to evaluate both diabetic and nondiabetic CKD. With its large and diverse population, recruited from seven clinical centers, the study addresses research questions concerning etiology, prognosis, therapy, utilization of health care services, and quality of life pertinent to CKD. CRIC is a unique resource for examining risk factors for CKD progression. In this overview, we present a more comprehensive synthesis of recent findings from the CRIC Study, specifically focusing on factors associated with CKD progression. We also discuss clinical implications and highlight directions for future research.
The CRIC Study is an ongoing, multicenter, prospective cohort study of individuals with CKD in the United States who have been followed through annual in-person visits, as previously described (3,4). The CRIC Research Network designed the study in 2001. During the first phase, which occurred between 2003 and 2008, a cohort of 3939 adults with CKD, aged 21–74 years and with an eGFR of 20–70 ml/min per 1.73 m2, was successfully recruited and characterized (46% women, 42% non-Hispanic White, 42% non-Hispanic Black, and 13% Hispanic). At baseline, 86% of participants had hypertension and 47% had diabetes. During the second phase of the study, which was completed in 2013, annual follow-up of participants continued with data collection and measurements at predetermined intervals (5). During the third phase of the study, >1500 adults with less severe CKD (eGFR of 45–70 ml/min per 1.73 m2) were recruited. In the current, fourth phase, which began in 2018, the CRIC Study is increasing the diversity of the cohort by recruiting 500 American Indian and 126 Hispanic adults, while refocusing data collection by incorporating novel mobile technologies to remotely collect kidney and cardiovascular data from the participants’ homes (Figure 1).
Figure 1.
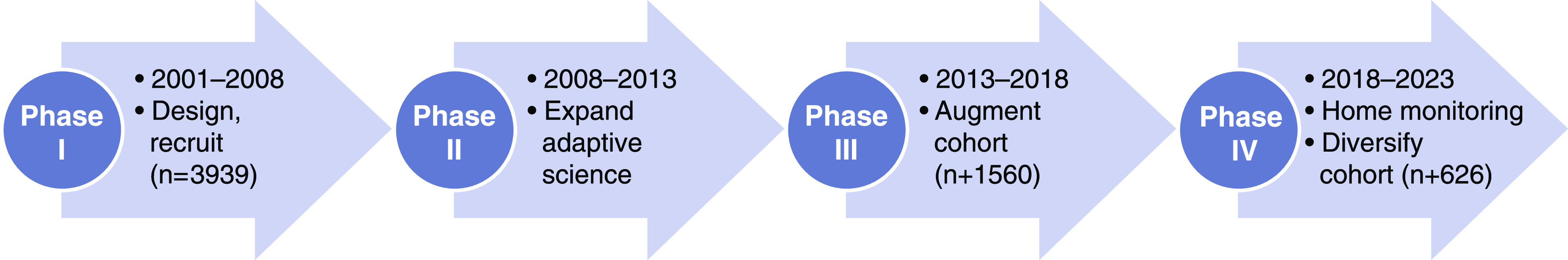
Phases of the Chronic Renal Insufficiency Cohort (CRIC) Study.
We conducted a thorough review of the literature by using the CRIC Study website publications list (www.cristudy.org), and by searching PubMed with relevant Medical Subject Heading terms, to find all publications from the CRIC Study that evaluated factors associated with CKD progression. We identified 44 articles, in which 54 factors were examined. These factors are summarized in Table 1 and are grouped into the following exposure categories: (1) sociodemographic and economic, (2) behavioral, (3) genetic, (4) cardiovascular, (5) metabolic, and (6) novel factors. Below, we present a more in-depth discussion of select publications and their contributions to the field.
Table 1.
Summary of studies evaluating risk factors for kidney failure or CKD progression in the CRIC Study
| First Author | Risk Factor | Follow-Up (yr) | n | Association with Outcomes | |
|---|---|---|---|---|---|
| Sociodemographic and economic factors | |||||
| Ricardo et al. (7) | Sex (women versus men) | 6.9 | 3939 | ↓ Kidney failurea risk in women versus men | |
| Fischer et al. (9) | Hispanic ethnicity | 5.1 | 3785 | Similar kidney failure risk in Hispanic versus non-Hispanic White individuals | |
| Ricardo et al. (10) | Nephrologist care | 6.6 | 3855 | No significant association | |
| Behavioral factors | |||||
| Ricardo et al. (11) | Healthy diet, regular physical activity | 4 | 3006 | No significant association | |
| BMI >25 kg/m2, past/never smoker | ↓ Kidney failure risk in overweight/obese and nonsmokers | ||||
| He et al. (15) | ↑ Urinary Na+, K+ | 15,807 p-y | 3939 | ↑ CKD progressionb risk | |
| Hu et al. (20) | Dietary patterns (DASH, aMED, HEI) | 7 | 2403 | ↓ CKD progression risk in least versus most adherent tertile | |
| Hu et al. (21) | Healthy beverage score | 7 | 2283 | ↓ CKD progression risk with higher scores | |
| Bundy et al. (64) | Tobacco, alcohol, marijuana | 5.5 | 2288 | No significant association | |
| Heroin | ↑ CKD progression risk in users versus nonusers | ||||
| Schrauben et al. (65) | Obese/sedentary pattern in adults <65 yr with diabetes | <3 to >5 | 5499 | ↑ CKD progression risk | |
| Ricardo et al. (22) | ↑ Sleep fragmentation | 4.4 | 431 | ↑ eGFR decline and kidney failure risk | |
| ↓ Sleep duration | ↑ eGFR decline | ||||
| Porter et al. (66) | Health-related quality of life | 6.2 | 3837 | No significant association | |
| Cedillo-Couvert et al. (67) | Medication nonadherence | 6 | 3305 | ↑ CKD progression risk | |
| Schrauben et al. (68) | Self-management behaviors (smoking, poor diet, physical inactivity, and uncontrolled BP) | 3 | 3939 | ↑ CKD progression risk | |
| Genetic factors | |||||
| Parsa et al. (26) | APOL1 gene variants | 4.4 | 2955 | ↑ eGFR decline and CKD progression risk | |
| Parsa et al. (28) | SNPs in LINC00923 (RNA gene expressed in the kidney) | — | 3074 | ↑ eGFR decline and kidney failure risk | |
| Wing et al. (69) | DNA methylation pattern | — | 40 | No significant association | |
| Kelly et al. (29) | Renin-angiotensin-aldosterone system genes | — | 3013 | ↑ eGFR decline and CKD progression risk | |
| Cardiovascular factors | |||||
| Bansal et al. (33) | Atrial fibrillation | 5.9 | 3091 | ↑ Kidney failure risk | |
| Anderson et al. (37) | Time-updated ↑ systolic BP | 5.7 | 3708 | ↑ Kidney failure risk | |
| Thomas et al. (70) | Treatment-resistant HTN | 5 | 3367 | ↑ CKD progression risk | |
| Townsend et al. (45) | ↑ Aortic pulse wave velocity | 4.1 | 2795 | ↑ Kidney failure risk | |
| Grunwald et al. (71) | Baseline retinopathy | 2.3 | 1852 | No significant association | |
| Grunwald et al. (72) | Progression of retinopathy | 3.5 | 1936 | No significant association | |
| Kurella Tamura et al. (73) | Cognitive impairment | 6.1 | 3883 | No significant association | |
| Rahman et al. (74) | Self-reported cardiovascular disease | 6.63 | 3939 | No significant association | |
| Self-reported congestive heart failure | 6.63 | 3939 | ↑ CKD progression risk | ||
| Rahman et al. (75) | Lipids | 4.1 | 3939 | No significant association | |
| Metabolic factors | |||||
| Isakova et al. (48) | ↑ FGF23 | 3.5 | 3879 | ↑ Kidney failure risk (depending on baseline stage of CKD) | |
| Dobre et al. (76) | ↓ Serum bicarbonate | 3.9 | 3939 | ↑ CKD progression risk | |
| Scialla et al. (77) | ↓ Net acid excretion | 6 | 980 | ↑ CKD progression risk | |
| Waikar et al. (50) | ↑ Urinary oxalate | 22,318 p-y | 3123 | ↑ CKD progression risk | |
| Koye et al. (78) | Absence of albuminuria in people with diabetes | 6.3 | 1908 | ↓ CKD progression risk versus those with albuminuria and diabetes | |
| Bansal et al. (79) | Body composition by BIA | 7 | 3751 | No significant association | |
| Srivastava et al. (80) | ↑ Uric acid | 7.9 | 3885 | ↑ Kidney failure risk | |
| Novel factors | |||||
| Afshinnia et al. (81) | AA metabolites: 20-HETE, LOX, CYP450 metabolic pathways | 10 | 300 | ↑ Kidney failure risk (20-HETE) | |
| Rhee et al. (82) | Amino acid metabolites, acylcarnitines, dipeptides, nucleotides, and other cationic polar metabolites | — | 400 | Nominally associated with rapid CKD progression | |
| Foster et al. (83) | Serum BTP and B2M | 6 | 3613 | ↑ Kidney failure risk | |
| Inker et al. (84) | BTP and B2M | 13 | 3938 | No significant association | |
| Amdur et al. (85) | ↑ Fibrinogen, ↑ TNF-α, ↓ albumin | 6.3 | 899 | ↑ CKD progression risk | |
| Liu et al. (55) | ↑ Urinary NGAL | 3.2 | 3386 | ↑ CKD progression risk | |
| Hsu et al. (56) | Urine biomarkers (KIM-1, NGAL, NAG, and FABP) | 9433 p-y | 2466 | No independent association | |
| Hsu et al. (54) | AKI | 9433 p-y | 456 | ↑ Urine protein-creatinine ratio | |
| Orlandi et al. (86) | Hematuria | 2 | 3272 | ↑ CKD progression risk | |
| Zhan et al. (87) | Opioid, NSAID use | 6.8 | 3939 | ↑ CKD progression risk, stronger with opioid use than NSAID use | |
| Anderson et al. (88) | CXCL12, NTproBNP, urine NGAL | 7 | 3379 | ↑ CKD progression risk in those with diabetes | |
| ↓ Serum bicarbonate, ↑ hsTnT, NTproBNP, urine NGAL | ↑ CKD progression risk in those without diabetes | ||||
CRIC, Chronic Renal Insufficiency Cohort; BMI, body mass index; Na+, sodium ion; K+, potassium ion; p-y, person years; DASH, Dietary Approaches to Stop Hypertension; aMED, Alternate Mediterranean Diet; HEI, Healthy Eating Index; SNP, single-nucleotide polymorphism; HTN, hypertension; FGF23, fibroblast growth factor 23; BIA, bioelectrical impedance analysis; AA, amino acid; 20-HETE, 20-hydroxyeicosatetraenoic acid; CYP450, cytochrome P450; BTP, β-trace protein; B2M, β-2 microglobulin; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule 1; NAG, N-acetyl-β-d-glucosaminidase; FABP, liver fatty acid binding protein; NSAID, nonsteroidal anti-inflammatory drug; CXCL12, inflammatory chemokine; NTproBNP, N-terminal pro-B-type natriuretic peptide; hsTnT, high-sensitivity troponin T.
Kidney failure defined as receipt of RRT (dialysis or kidney transplant).
CKD progression defined as kidney failure or 50% reduction of eGFR from baseline.
Sociodemographic and Economic Factors
Sex Disparities
Sex-related disparities in CKD are well established. According to the United States Renal Data System, the prevalence of kidney failure is higher among men, despite a higher prevalence of CKD among women (6), suggesting that women may have slower kidney function decline compared with men, or that women are more likely to die before progressing to kidney failure. The extensive data collection in the CRIC Study afforded the opportunity to examine potential explanations for these disparities. At baseline, compared with men, women were more likely to have adverse risk factors, including lower socioeconomic status; higher body mass index and waist circumference; lower physical activity; higher serum phosphate, fibroblast growth factor 23 (FGF23), and LDL cholesterol levels; and lower HDL cholesterol and eGFR. Furthermore, women were less likely to report the use of cardioprotective medications. However, women experience lower rates of kidney failure (defined as receipt of dialysis or kidney transplant) than men (3.1 versus 3.8 per 100 person-years). In regression analysis, women had a 28% lower risk of kidney failure compared with men, despite extensive adjustment for sociodemographic, clinical, and laboratory characteristics (7). The protective effect of endogenous estrogens has been proposed as a potential explanation for these disparities (8).
Racial/Ethnic Disparities
Racial/ethnic minority populations in the United States are more likely to experience CKD progression compared with White individuals (6). CRIC provides an opportunity to evaluate health disparities in CKD outcomes in greater depth. During a median follow-up of 6.6 years, Hispanic and non-Hispanic Black individuals experienced an almost two-fold higher rate of kidney failure compared with non-Hispanic White individuals (9). In multivariable analyses using death as a competing risk, the risk of kidney failure was similar in Hispanic compared with non-Hispanic White individuals (hazard ratio [HR], 1.32; 95% confidence interval [95% CI], 0.96 to 1.81), and in Hispanic compared with non-Hispanic Black individuals (HR, 0.94; 95% CI, 0.71 to 1.25) after adjustment for sociodemographic (e.g., age, sex, education, income) and clinical characteristics (e.g., BP, diabetes, eGFR, and proteinuria). These findings have important clinical implications because the disparity in CKD progression among these racial/ethnic minority populations is explained, in part, by potentially modifiable risk factors. As detailed below, the APOL1 gene plays an important role in CKD progression among individuals with African ancestry.
Nephrology Care
CRIC Study participants undergo extensive data collection regarding health insurance and access to health care, and there is ongoing work evaluating the role of these factors in CKD progression (defined as 50% decline in eGFR from baseline or kidney failure). Not surprisingly, the two thirds of individuals who reported nephrology care were more likely to be on renin-angiotensin-aldosterone system (RAAS) blockers. However, prior nephrology care was not associated with lower CKD progression (10). Potential reasons for this finding include the overall high achievement of guideline-recommended treatment goals among CRIC participants (making significant differences between patients with and without nephrology care difficult to appreciate), or other factors such as medication copayment or transportation, which were not measured.
Behavioral Factors
Healthy Lifestyle
Ricardo et al. (11) evaluated how healthy behaviors (i.e., not smoking, healthy diet, regular physical activity, and healthy weight) influence CKD progression. Having previously or never having smoked was associated with reduced risk of CKD progression compared with current smoking; however, there was no significant association with self-reported healthy diet (11). Physical activity was not associated with lower CKD progression, but those individuals who met the American Heart Association (AHA)–recommended physical activity guidelines had lower risk of death. A greater emphasis on lifestyle counseling for individuals with CKD has the potential of improving outcomes in this population. Paradoxically, individuals with a body mass index of ≥25 kg/m2 had lower risk of CKD progression (11). Reasons for this finding are not clear, but similar observations have been reported by others (12).
Diet
Observational studies and clinical trials have documented that high dietary sodium and low potassium intake are associated with elevated BP (13,14). However, data on the effects of these nutrients on the risk of CKD progression are sparse and inconsistent. He et al. (15) evaluated the effect of dietary sodium and potassium on CKD progression using 24-hour urine measurements. Individuals in the highest quartile of urinary sodium excretion (≥195 mmol per 24 hours) were more likely to experience CKD progression compared with those in the lowest quartile (<117 mmol per 24 hours). High urinary potassium excretion (≥67 versus <39.4 mmol per 24 hours) was also associated with higher risk for CKD progression (15). These findings are in contrast with previous epidemiologic studies and clinical trials documenting an inverse association between dietary potassium intake and BP in general populations (14,16), suggesting this association is more complex in the setting of CKD because of abnormal potassium homeostasis and the effect of RAAS blockers.
Healthy dietary patterns have been shown to reduce risk of incident CKD in general populations (17–19), but there is less evidence of an association with CKD progression. Using food frequency questionnaires, Hu et al. (20) calculated the Healthy Eating Index 2015, Alternative Healthy Eating Index 2010, Alternate Mediterranean Diet, and Dietary Approaches to Stop Hypertension diet scores for 2403 CRIC participants. They observed an inverse association between healthy dietary scores and risk of CKD progression, with the strongest association for the Alternate Mediterranean Diet (20). In addition, a healthy beverage score—consistent with higher consumption of low-fat milk, coffee, tea; moderate alcohol; and lower consumption of 100% fruit juice, whole-fat milk, artificially sweetened beverages, and sugar-sweetened beverages—was also associated with lower risk of CKD progression (21). These findings suggest that, in addition to managing single nutrients, nutritional counseling for individuals with CKD should include assessment of, and recommendations for, overall food-based dietary patterns.
Sleep
There is increased recognition of the effect of sleep on health outcomes. Ricardo et al. (22) evaluated habitual sleep using wrist actigraphy in 431 individuals and found higher sleep fragmentation to be associated with higher risk for kidney failure. In addition, higher sleep fragmentation and shorter sleep duration were each associated with steeper decline in eGFR and increase in proteinuria over time (22). These findings suggest impaired sleep is a clinically significant, but unrecognized, risk factor for CKD progression that should be assessed by providers of patients with CKD. The deleterious effects of poor-quality sleep that might be responsible for these associations include acute increases in BP and heart rate, activation of the sympathetic nervous system, increased salt retention, and alterations of glucose metabolism (23). Future work is needed to evaluate interventions to improve sleep habits in patients with CKD and assess whether the observed association with CKD progression is causal.
Genetic Factors
The APOL1 gene has been implicated in the higher risk of CKD progression observed among African American individuals (24,25). Parsa et al. (26) examined the effects of two common sequence variants (G1 and G2) in the gene encoding APOL1 on CKD progression, using data from the CRIC Study and the African American Study of Kidney Disease and Hypertension. Black individuals in the APOL1 high-risk group had more rapid eGFR decline and higher risk of the composite kidney outcome than did White individuals, independent of diabetes status or BP level (26). Findings from this seminal study helped stimulate expansion of basic and translational research in this area. The National Institutes of Health (NIH)–sponsored, ongoing, multicenter APOLLO (APOL1 Long-Term Kidney Transplantation Outcomes Network) study will prospectively assess the effects of APOL1 nephropathy risk variants in kidney donors and recipients (27), and may define the clinical role of APOL1 testing.
A genome-wide association study performed among CRIC participants reported that four single-nucleotide polymorphisms with a minor allele frequency of >0.03 were significantly associated with progression at the genome-wide threshold of P≤5×10−8, and 14 gene regions reached nominal significance (P<10−6). In particular, they identified that LINC00923, an RNA gene expressed in glomeruli and endothelial cells of the kidney, was associated with CKD progression in participants without diabetes (28). In another study, the AGT and RENBP genes involved in the RAAS, and the entire RAAS pathway, were associated with CKD progression (P<1.00×10−6 for each) (29). Future replication and validation studies will continue to move the field toward the goal of personalizing care for individuals with CKD.
Cardiovascular Factors
Atrial Fibrillation
Atrial fibrillation is highly prevalent among adults with CKD and is associated with adverse cardiovascular outcomes (30–32). However, the link between atrial fibrillation and kidney failure has not been fully elucidated. Bansal et al. (33) examined the risk of kidney failure among CRIC participants who developed atrial fibrillation during up to 9 years of follow-up, using marginal structural models with inverse probability weighting to accommodate for the bidirectional relationship between atrial fibrillation and kidney failure. Incident atrial fibrillation was associated with a three-fold higher risk of kidney failure, particularly among non-Hispanic White individuals. Potential explanations for the findings include the altered cardiac hemodynamics caused by atrial fibrillation over time (34,35), which may lead to a decline in kidney function. This study highlights the clinical importance of identifying atrial fibrillation in adults with CKD. Although there is strong evidence supporting the benefit of anticoagulation for atrial fibrillation in non-CKD populations, the clinical benefit among patients with CKD is less clear (36). Therefore, studies designed to address this clinical question are needed.
Hypertension
There is great controversy regarding the optimal BP target to prevent CKD progression. Anderson et al. (37) used marginal structural analysis to evaluate the association between time-updated systolic BP and CKD progression. The findings suggested a systolic BP of >130 mm Hg was associated with higher risk of kidney failure, which is consistent with AHA recommending a BP target of <130/80 mm Hg in individuals with CKD (38). This article furthered our understanding of this important association, which was stronger than that previously reported on the basis of systolic BP measured only at one time point (37).
Vascular Stiffness
Prior studies have shown that arterial stiffness contributes to death and heart failure events in CKD (39,40). It has been hypothesized that increased aortic pulse pressure can lead to microvascular kidney damage, resulting in albuminuria (41). However, the role of aortic stiffness in CKD progression has not been thoroughly investigated, and findings from previous studies have been inconsistent (42,43). Aortic stiffness is estimated from the pulse wave velocity (PWV) traveling along the aorta, in which the carotid and femoral artery sites are used to capture the wave forms (44). Townsend et al. (45) measured aortic stiffness by PWV in >2500 CRIC participants and found that, compared with individuals in the lowest tertile of PWV (<7.9 m/s), those in the highest tertile (>10.3 m/s) were at 37% higher risk for kidney failure (HR, 1.37; 95% CI, 1.05 to 1.80), independent of brachial BP. These findings suggest PWV may play a role in risk stratification, and future interventional trials focused on reducing vascular stiffness are needed.
Metabolic Factors
FGF23
Mineral and bone disorder is a well-recognized complication of CKD, but less is known about its influence on the natural history of CKD. FGF23 induces phosphaturia by decreasing phosphate reabsorption in the proximal tubule (46). In cross-sectional analyses, elevated FGF23 was found to be a common manifestation of CKD that develops earlier than increases in serum phosphate or parathyroid hormone (47). Isakova et al. (48) expanded this area of knowledge with the identification of FGF23 as a risk factor for CKD progression among individuals with an eGFR of ≥30 ml/min per 1.73 m2, but not those with an eGFR of <30 ml/min per 1.73 m2, suggesting FGF23 testing might help in risk stratification. Furthermore, these findings led to the design of the COMBINE (CKD Optimal Management with Binders and Nicotinamide) trial that evaluated the effects of lanthanum carbonate and/or nicotinamide on serum phosphate and FGF23 among adults with stage 3b/4 CKD (49). The study showed that neither treatment significantly lowered serum phosphate or FGF23 over 12 months; therefore, reducing phosphate and FGF23 in patients with CKD will require new approaches.
Urinary Oxalate
Waikar et al. (50) examined urinary oxalate as a potential novel predictor of CKD progression. The main sources of urinary oxalate are hepatic synthesis, breakdown of ascorbic acid, and diet (51). Oxalate nephropathy is a well-known complication of rare genetic disorders, and oxalate overabsorption and calcium oxalate crystal deposition in parenchyma of the kidney can cause tissue injury and inflammation (52). Among 3123 CRIC participants, Waikar et al. (50) found that higher 24-hour urinary oxalate excretion was associated with a 45% higher risk of kidney failure in the highest versus lowest quintile. Whether dietary and pharmacologic interventions to lower urinary oxalate excretion have an effect on CKD progression needs to be evaluated.
Novel Factors
AKI
The relationship between AKI and CKD progression is being increasingly recognized (53). In an analysis of pooled data from CRIC and the Assessment, Serial Evaluation, and Subsequent Sequelae of AKI Study, AKI was independently associated with an increase in urine protein-creatinine ratio in individuals with CKD (54). An ongoing study by Hsu et al. (Available at: https://projectreporter.nih.gov/project_info_description.cfm?aid=10149875&icde=52121974&ddparam=&ddvalue=&ddsub=&cr=3&csb=default&cs=ASC&pball=), designed to systemically evaluate the severity of hospitalized AKI in CRIC, will shed additional light onto this association. In addition, during the current phase of CRIC, eligible participants are performing monthly finger-stick creatinine testing, with additional weekly tests during two of the 12 testing months, to examine longitudinal patterns of CKD progression and intraindividual variability in kidney function, and occurrence of episodes of outpatient AKI.
Biomarkers of Tubular Injury
Several manuscripts evaluated tubular injury biomarkers as predictors of CKD progression. Liu et al. (55) reported that higher levels of 24-hour urine neutrophil gelatinase-associated lipocalin were significantly associated with CKD progression (HR, 1.70, highest to lowest quartile), independent of eGFR and proteinuria, but this biomarker did not substantially improve prediction of outcome events. Similarly, Hsu et al. (56) found that prediction of CKD progression risk with a clinical model that includes eGFR and albuminuria was not improved with the addition of kidney tubular injury biomarkers (i.e., neutrophil gelatinase-associated lipocalin, urinary kidney injury molecule-1, N-acetyl-β-d-glucosaminidase, and liver fatty acid binding protein). These findings underscore the high predictive-performance value of traditional biomarkers, such as eGFR and albuminuria (in addition to readily available clinical parameters), and the fact that measurement of tubular injury biomarkers does not provide additional information for risk prediction of CKD progression. However, CKD biomarkers may provide important pathophysiologic insight that can then lead to novel therapeutic targets or better understanding of drug toxicity (56).
Contributions to Statistical Methodology for Cohort Studies of CKD
In addition to the contributions to the medical field outlined above, CRIC Study investigators published several manuscripts addressing methodologic issues relevant to the cohort, including statistical methods for modeling time-updated exposures in cohort studies of CKD (57), survival analysis in the setting of competing risks (58), prediction modeling (59), recurrent event analysis (60), definition of kidney disease outcomes to identify risk factors for CKD progression (61), and repeated measures of eGFR (62). In these feature articles, mixed-effects, Poisson-regression, and survival models are illustrated by analyzing CRIC data to identify risk factors for different outcomes, and novel statistical approaches are compared with standard analyses.
Clinical Implications and Limitations of CRIC Study Findings
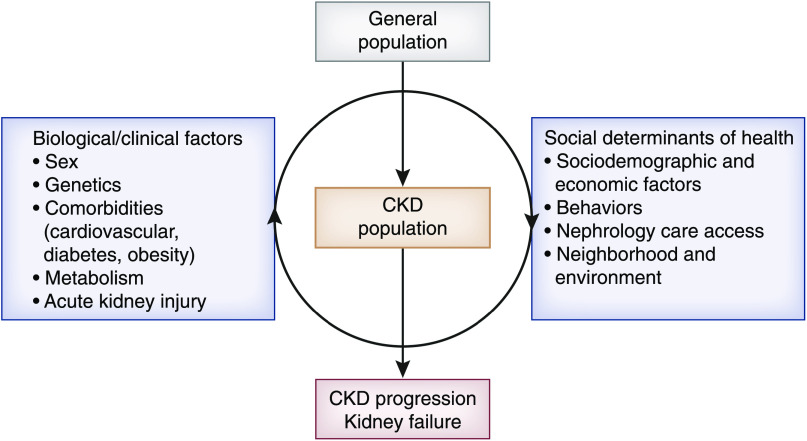
Over the years, the CRIC Study has made substantial contributions to our understanding of risk factors for CKD progression. The study has evaluated a broad array of factors addressed in a theoretic model developed by Norton et al. (63), which illustrates the complex interplay between biologic/clinical factors and social determinants of health in CKD progression (Figure 2). In particular, the CRIC findings regarding modifiable lifestyle factors have important implications for clinical practice (Table 2). As discussed in detail above, study findings can be used by clinicians to make recommendations to patients regarding smoking abstinence, diet, physical activity, and sleep habits. We acknowledge that, given the observational study design of CRIC, statements regarding causal inference should not be made. In addition, findings might not be generalizable to all patients with CKD because study participants were recruited from clinical centers and certain exclusion criteria, such as GN and polycystic kidney disease, were applied. However, the CRIC Study is representative of the ESKD population in which diabetes and hypertension are the primary diagnosis for >70% of patients (3).
Figure 2.
Theoretic model: Interaction of biologic and clinical factors with the social determinants of health affecting CKD progression. Biologic and clinical factors likely interact with the social determinants of health at several levels to increase risk of CKD progression. Adapted from ref. 63, with permission.
Table 2.
Modifiable CKD progression risk factors and potential treatment targets evaluated in the CRIC Study
| Risk Factor | Potential Treatment Target |
|---|---|
| Behavioral | Smoking |
| Healthy dietary patterns | |
| ↑ Urine sodium and potassium intake | |
| Fragmented sleep | |
| Hard illicit drug use (heroin) | |
| Poor self-management behaviors | |
| Medication nonadherence | |
| Genetic | APOL1 gene variants |
| SNPs in LINC00923 (RNA gene expressed in the kidney) | |
| RAAS genes | |
| Cardiovascular | ↑ Systolic BP |
| Treatment-resistant hypertension | |
| Aortic stiffness | |
| Heart failure | |
| Atrial fibrillation | |
| Metabolic | ↑ FGF23 |
| ↓ Serum bicarbonate | |
| ↓ Net acid excretion | |
| ↑ Urinary oxalate | |
| ↑ Serum uric acid | |
| Novel factors | 20-HETE (AA metabolite) |
| Inflammation (fibrinogen, TNF-α, albumin) | |
| Urine NGAL | |
| AKI |
CRIC, Chronic Renal Insufficiency Cohort; SNP, single-nucleotide polymorphism; RAAS, renin-angiotensin-aldosterone system; FGF23, fibroblast growth factor 23; 20-HETE, 20-hydroxyeicosatetraenoic acid; AA, amino acid; NGAL, neutrophil gelatinase-associated lipocalin.
Future Directions
In the summer of 2018, the CRIC Study embarked on its fourth phase to continue to examine key outstanding research questions (Table 3). During this phase, the study protocol has incorporated several innovative, remote data-collection activities that include monthly monitoring of kidney function with finger-stick creatinine testing and urine albumin measurements. Other home-based testing include an assessment of integrated heart rate and activity (Zephyr BioPatch; Medtronic Corporation), arrhythmias (ZIO XT Patch; iRhythm Technologies), and sleep-disordered breathing (ApneaLink Air; ResMed). CRIC is also exploring innovative, integrative statistical techniques to evaluate high-dimensional data.
Table 3.
Key outstanding research questions for CRIC phase 4
| Research Questions |
|---|
| Prevalence of gut microbiome dysbiosis in CKD and its contribution to CKD progression |
| Prognostic implications of nonlinear trajectories and short-term variations in kidney function and damage measured frequently using home-based monitoring of serum creatinine and urine albumin |
| Prognostic value of biometric monitoring (e.g., measurement of heart rate) in CKD to identify subgroups at highest cardiovascular risk |
| Significance of undiagnosed atrial fibrillation and other atrial and ventricular dysrhythmias detected using a 14-d continuous ECG monitoring |
| Burden of noncardiovascular morbidity in CKD (e.g., frailty, fractures, pulmonary disease, utilization of health care resources) |
| Usefulness of machine learning methods in electronic phenotyping and characterization of clinical states for the purposes of cohort identification, classification, and prediction |
CRIC, Chronic Renal Insufficiency Cohort; ECG, electrocardiogram.
Developing and testing equations that yield individual risk predictions based on the individual’s values on various risk factors will be a high-priority task during CRIC phase 4. Innovative methods, such as machine learning and electronic phenotyping, and various categories of risk information will be used to derive models that address two objectives: (1) maximizing practicality of use in typical clinical situations, and (2) maximizing predictive power using all available data. Outcomes in these models will be CKD progression, cardiovascular events, and death.
The CRIC Study offers rich opportunities for collaboration with the broad scientific community, as evidenced by the active ancillary study program that includes >22 training grants, ten diversity supplements, and 45 R01 awards. In addition, in the current phase, CRIC continues to have engagement with existing CKD consortia and study groups, such as the CKD Biomarkers Consortium and the CKD Prognosis Consortium, to maximally leverage the resources and data across these groups to affect the lives of patients with CKD. Furthermore, during CRIC phase 4, NIDDK established the CRIC Opportunity Pool Program (http://cristudy.org/Chronic-Kidney-Disease/Chronic-Renal-Insufficiency-Cohort-Study/opportunity-pool), which has set aside funds of >$2.5 million to support innovative projects led by the broader scientific community, including investigators who have not previously worked on CKD epidemiology, and those from outside the field of nephrology. Moreover, the NIDDK Central Repository of CRIC Study data (https://repository.niddk.nih.gov/studies/cric/) provides additional opportunities for investigators to leverage the detailed characterization of CKD progression. Lastly, the CRIC Study has also launched a tool (CRICDataView.org) that permits researchers to query study data.
Disclosures
A. Anderson reports receiving grants from NIH/NIDDK during the conduct of the study; and personal fees from Kyowa Hakko Kirin, outside the submitted work. H. Feldman reports receiving grants from NIH/NIDDK during the conduct of the study. He also reports consulting for Kyowa Hakko Kirin Co., Ltd. since January 2016 and serving as editor-in-chief of American Journal of Kidney Disease since January 2017, outside the submitted work. M. Hannan is a Robert Wood Johnson Foundation Future of Nursing Scholar Postdoctoral Fellow. E. Horwitz is an employee of MetroHealth Medical Center. J. Lash reports receiving grants from NIH during the conduct of the study. N. Meza reports receiving grants from NIDDK during the conduct of the study. A. Ricardo reports receiving grants from NIH during the conduct of the study. M. Saunders reports receiving grants from NIH NIDDK during the conduct of the study. A. Srivastava reports receiving personal fees from AstraZeneca, CVS Caremark, and Horizon Pharma PLC, outside the submitted work. J. Taliercio reports employment at Glickman Urological and Kidney Institute, Cleveland Clinic. S. Waikar reports receiving an investigator-initiated grant from Allena Pharmaceuticals and personal fees for serving on academic steering committee for phase 3 trials of a drug to treat hyperoxaluria; personal fees from Barron and Budd (versus Fresenius) for being an expert witness on litigation against Fresenius for Granuflo; personal fees from Bunch and James for being an expert witness on litigation related to mercury exposure; personal fees from Cerus for being a consultant on a device for AKI prevention; personal fees from CVS for consulting on clinical programs; personal fees from GE Healthcare as expert witness on litigation related to Omniscan and nephrogenic systemic fibrosis; personal fees from GlaxoSmithKline for serving on steering committee for phase 3 trials of dapradustat (hypoxia-inducible factor stabilizer for anemia of CKD); personal fees from Harvard Clinical Research Institute (also known as Baim) for serving on clinical end-points adjudication committees; personal fees from Kantum Pharma for serving on a scientific advisory board; personal fees from JNJ for speaking at a meeting; personal fees from Mallinckrodt for serving on an expert panel meeting; personal fees from Mass Medical International for consulting on global nephrology; personal fees from Pfizer for for being a consultant on litigation related to statins and diabetes mellitus; personal fees from Public Health Advocacy Institute as an expert witness against Philip Morris for cisplatin-induced lung injury from smoking; personal fees from Roth Capital Partners for speaking to a group of investors interested in learning about oxalate-lowering therapies; personal fees from Strataca for advisory about design of trials for a device for AKI; personal fees from Takeda for serving on a steering committee for phase 4 trial on febuxostat; personal fees from Venbio for speaking to a small group of investors about therapies for CKD; and personal fees from Wolters Kluewer for UpToDate editing; outside the submitted work. M. Weir reports receiving grants from NIDDK-CRIC during the conduct of the study. He also reports receiving personal fees from Boehringer-Ingelheim, Janssen, and MSD for serving on scientific advisory boards; personal fees from Boston Scientific for serving on steering committee; and personal fees from Relypsa/Vifor for serving on the steering committee/advisory board; outside the submitted work. K. Wolfrum reports receiving grants from NIH/NIDDK, which funded the study through which this data were collected. The funds from these grants, through employment at the University of Pennsylvania while working on the CRIC study, finance her salary. All remaining authors have nothing to disclose.
Funding
Funding for the CRIC Study was obtained under a cooperative agreement from the NIDDK under grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, U01DK060902, and U24DK060990. In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131, Department of Internal Medicine, University of New Mexico School of Medicine Albuquerque, NM R01DK119199. M. Hannan has received National Heart, Lung, and Blood Institute award T32HL134634 as a T32 Postdoctoral Fellow. J. Lash is funded by NIDDK grants K24DK092290 and R01DK072231-91. A. Ricardo is funded by NIDDK grant R01DK118736. A. Srivastava is funded by NIDDK grant K23DK120811.
Acknowledgments
The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the NIH. The views expressed here do not necessarily reflect the views of the Robert Wood Johnson Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Contributor Information
Collaborators: Chronic Renal Insufficiency Cohort (CRIC) Study Investigators, Lawrence J. Appel, Harold I. Feldman, Alan S. Go, Jiang He, James P. Lash, Robert G. Nelson, Mahboob Rahman, Panduranga S. Rao, Vallabh O. Shah, Raymond R. Townsend, and Mark L. Unruh
References
- 1.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FD: Global prevalence of chronic kidney disease - A systematic review and meta-analysis. PLoS One 11: e0158765, 2016. 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute of Diabetes and Digestive and Kidney Diseases: Kidney disease statistics for the United States. Available at: https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed August 9, 2017
- 3.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group: Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function [published correction appears in Clin J Am Soc Nephrol 6: 2548–2553, 2011]. Clin J Am Soc Nephrol 4: 1302–1311, 2009. 10.2215/CJN.00070109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: The Chronic Renal Insufficiency Cohort (CRIC) study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Denker M, Boyle S, Anderson AH, Appel LJ, Chen J, Fink JC, Flack J, Go AS, Horwitz E, Hsu CY, Kusek JW, Lash JP, Navaneethan S, Ojo AO, Rahman M, Steigerwalt SP, Townsend RR, Feldman HI; Chronic Renal Insufficiency Cohort Study Investigators: Chronic Renal Insufficiency Cohort study (CRIC): Overview and summary of selected findings. Clin J Am Soc Nephrol 10: 2073–2083, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United States Renal Data System: United States Renal Data System 2018. Annual Data Report. Available at: https://www.usrds.org/annual-data-report/previous-adrs/. Accessed October 10, 2020
- 7.Ricardo AC, Yang W, Sha D, Appel LJ, Chen J, Krousel-Wood M, Manoharan A, Steigerwalt S, Wright J, Rahman M, Rosas SE, Saunders M, Sharma K, Daviglus ML, Lash JP; CRIC Investigators: Sex-related disparities in CKD progression. J Am Soc Nephrol 30: 137–146, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubey RK, Jackson EK: Estrogen-induced cardiorenal protection: Potential cellular, biochemical, and molecular mechanisms. Am J Physiol Renal Physiol 280: F365–F388, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Fischer MJ, Hsu JY, Lora CM, Ricardo AC, Anderson AH, Bazzano L, Cuevas MM, Hsu CY, Kusek JW, Renteria A, Ojo AO, Raj DS, Rosas SE, Pan Q, Yaffe K, Go AS, Lash JP; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: CKD progression and mortality among hispanics and non-hispanics. J Am Soc Nephrol 27: 3488–3497, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricardo AC, Roy JA, Tao K, Alper A, Chen J, Drawz PE, Fink JC, Hsu CY, Kusek JW, Ojo A, Schreiber M, Fischer MJ; CRIC Study Investigators: Influence of nephrologist care on management and outcomes in adults with chronic kidney disease. J Gen Intern Med 31: 22–29, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricardo AC, Anderson CA, Yang W, Zhang X, Fischer MJ, Dember LM, Fink JC, Frydrych A, Jensvold NG, Lustigova E, Nessel LC, Porter AC, Rahman M, Wright Nunes JA, Daviglus ML, Lash JP; CRIC Study Investigators: Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 65: 412–424, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu JL, Kalantar-Zadeh K, Ma JZ, Quarles LD, Kovesdy CP: Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol 25: 2088–2096, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He FJ, Li J, Macgregor GA: Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev (4): CD004937, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ: Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 277: 1624–1632, 1997. [DOI] [PubMed] [Google Scholar]
- 15.He J, Mills KT, Appel LJ, Yang W, Chen J, Lee BT, Rosas SE, Porter A, Makos G, Weir MR, Hamm LL, Kusek JW; Chronic Renal Insufficiency Cohort Study Investigators: Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol 27: 1202–1212, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Intersalt Cooperative Research Group: Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 297: 319–328, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu EA, Steffen LM, Grams ME, Crews DC, Coresh J, Appel LJ, Rebholz CM: Dietary patterns and risk of incident chronic kidney disease: The Atherosclerosis Risk in Communities study. Am J Clin Nutr 110: 713–721, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khatri M, Moon YP, Scarmeas N, Gu Y, Gardener H, Cheung K, Wright CB, Sacco RL, Nickolas TL, Elkind MS: The association between a Mediterranean-style diet and kidney function in the Northern Manhattan Study cohort. Clin J Am Soc Nephrol 9: 1868–1875, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rebholz CM, Crews DC, Grams ME, Steffen LM, Levey AS, Miller ER 3rd, Appel LJ, Coresh J: DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am J Kidney Dis 68: 853–861, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu EA, Coresh J, Anderson CAM, Appel LJ, Grams ME, Crews DC, Mills KT, He J, Scialla J, Rahman M, Navaneethan SD, Lash JP, Ricardo AC, Feldman HI, Weir MR, Shou H, Rebholz CM; CRIC Study Investigators: Adherence to healthy dietary patterns and risk of CKD progression and all-cause mortality: Findings from the CRIC (Chronic Renal Insufficiency Cohort) study [published online ahead of print August 5, 2020]. Am J Kidney Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu EA, Anderson CAM, Crews DC, Mills KT, He J, Shou H, Taliercio JJ, Mohanty MJ, Bhat Z, Coresh J, Appel AJ, Rebholz CM; CRIC Study Investigators: A healthy beverage score and risk of chronic kidney disease progression, incident cardiovascular disease, and all-cause mortality in the chronic renal insufficiency cohort. Curr Dev Nutr 4: nzaa088, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricardo AC, Knutson K, Chen J, Appel LJ, Bazzano L, Carmona-Powell E, Cohan J, Kurella Tamura M, Steigerwalt S, Thornton JD, Weir M, Turek NF, Rahman M, Van Cauter E, Lash JP; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: The association of sleep duration and quality with CKD progression. J Am Soc Nephrol 28: 3708–3715, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turek NF, Ricardo AC, Lash JP: Sleep disturbances as nontraditional risk factors for development and progression of CKD: Review of the evidence. Am J Kidney Dis 60: 823–833, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB; SK Investigators: Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 83: 114–120, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsa A, Kao WHL, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr, Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ; AASK Study Investigators; CRIC Study Investigators: APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freedman BI, Moxey-Mims M: The APOL1 long-term kidney transplantation outcomes network-APOLLO. Clin J Am Soc Nephrol 13: 940–942, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsa A, Kanetsky PA, Xiao R, Gupta J, Mitra N, Limou S, Xie D, Xu H, Anderson AH, Ojo A, Kusek JW, Lora CM, Hamm LL, He J, Sandholm N, Jeff J, Raj DE, Böger CA, Bottinger E, Salimi S, Parekh RS, Adler SG, Langefeld CD, Bowden DW, Groop PH, Forsblom C, Freedman BI, Lipkowitz M, Fox CS, Winkler CA, Feldman HI; FIND Consortium; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Genome-wide association of CKD progression: The chronic renal insufficiency cohort study. J Am Soc Nephrol 28: 923–934, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly TN, Raj D, Rahman M, Kretzler M, Kallem RR, Ricardo AC, Rosas SE, Tao K, Xie D, Hamm LL, He J; CRIC Study Investigators: The role of renin-angiotensin-aldosterone system genes in the progression of chronic kidney disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Nephrol Dial Transplant 30: 1711–1718, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, Soliman EZ, Astor BC, Coresh J: Chronic kidney disease is associated with the incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 123: 2946–2953, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bansal N, Fan D, Hsu C-Y, Ordonez JD, Go AS: Incident atrial fibrillation and risk of death in adults with chronic kidney disease. J Am Heart Assoc 3: e001303, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bansal N, Xie D, Sha D, Appel LJ, Deo R, Feldman HI, He J, Jamerson K, Kusek JW, Messe S, Navaneethan SD, Rahman M, Ricardo AC, Soliman EZ, Townsend R, Go AS: Cardiovascular events after new-onset atrial fibrillation in adults with CKD: Results from the Chronic Renal Insufficiency Cohort (CRIC) study. J Am Soc Nephrol 29: 2859–2869, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bansal N, Xie D, Tao K, Chen J, Deo R, Horwitz E, Hsu CY, Kallem RK, Keane MG, Lora CM, Raj D, Soliman EZ, Strauss L, Wolf M, Go AS; CRIC Study: Atrial fibrillation and risk of ESRD in adults with CKD. Clin J Am Soc Nephrol 11: 1189–1196, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grogan M, Smith HC, Gersh BJ, Wood DL: Left ventricular dysfunction due to atrial fibrillation in patients initially believed to have idiopathic dilated cardiomyopathy. Am J Cardiol 69: 1570–1573, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Chen S-C, Su H-M, Hung C-C, Chang JM, Liu WC, Tsai JC, Lin MY, Hwang SJ, Chen HC: Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol 6: 2750–2758, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S, Lim E, Covic A, Verhamme P, Gale CP, Camm AJ, Goldsmith D: Anticoagulation in concomitant chronic kidney disease and atrial fibrillation: JACC review topic of the week. J Am Coll Cardiol 74: 2204–2215, 2019. [DOI] [PubMed] [Google Scholar]
- 37.Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW, Charleston J, He J, Kallem R, Lash JP, Miller ER 3rd, Rahman M, Steigerwalt S, Weir M, Wright JT Jr, Feldman HI; Chronic Renal Insufficiency Cohort Study Investigators: Time-updated systolic blood pressure and the progression of chronic kidney disease: A cohort study. Ann Intern Med 162: 258–265, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones AW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr.: 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71: 1269–1324, 2018. [DOI] [PubMed] [Google Scholar]
- 39.Baumann M, Wassertheurer S, Suttmann Y, Burkhardt K, Heemann U: Aortic pulse wave velocity predicts mortality in chronic kidney disease stages 2–4. J Hypertens 32: 899–903, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Chirinos JA, Khan A, Bansal N, Dries DL, Feldman HI, Ford V, Anderson AH, Kallem R, Lash JP, Ojo A, Schreiber M, Sheridan A, Strelsin J, Teal V, Roy J, Pan Q, Go AS, Townsend RR; CRIC Study Investigators; CRIC Study Investigators: Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study. Circ Heart Fail 7: 709–716, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashimoto J, Ito S: Central pulse pressure and aortic stiffness determine renal hemodynamics: Pathophysiological implication for microalbuminuria in hypertension. Hypertension 58: 839–846, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Weber T, Ammer M, Gündüz D, Bruckenberger P, Eber B, Wallner M: Association of increased arterial wave reflections with decline in renal function in chronic kidney disease stages 3 and 4. Am J Hypertens 24: 762–769, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Chandra P, Sands RL, Gillespie BW, Levin NW, Kotanko P, Kiser M, Finkelstein F, Hinderliter A, Rajagopalan S, Sengstock D, Saran R: Relationship between heart rate variability and pulse wave velocity and their association with patient outcomes in chronic kidney disease. Clin Nephrol 81: 9–19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeLoach SS, Townsend RR: Vascular stiffness: Its measurement and significance for epidemiologic and outcome studies. Clin J Am Soc Nephrol 3: 184–192, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Townsend RR, Anderson AH, Chirinos JA, Feldman HI, Grunwald JE, Nessel L, Roy J, Weir MR, Wright JT Jr, Bansal N, Hsu CY; CRIC Study Investigators: Association of pulse wave velocity with chronic kidney disease progression and mortality: Findings from the CRIC study (Chronic Renal Insufficiency Cohort). Hypertension 71: 1101–1107, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf M: Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol 21: 1427–1435, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Group: Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ix JH, Isakova T, Larive B, Raphael KL, Raj DS, Cheung AK, Sprague SM, Fried LF, Gassman JJ, Middleton JP, Flessner MF, Block GA, Wolf M: Effects of nicotinamide and lanthanum carbonate on serum phosphate and fibroblast growth factor-23 in CKD: The COMBINE trial. J Am Soc Nephrol 30: 1096–1108, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waikar SS, Srivastava A, Palsson R, Shafi T, Hsu CY, Sharma K, Lash JP, Chen J, He J, Lieske J, Xie D, Zhang X, Feldman HI, Curhan GC; Chronic Renal Insufficiency Cohort Study Investigators : Association of urinary oxalate excretion with the risk of chronic kidney disease progression. JAMA Intern Med 179: 542–551, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holmes RP, Goodman HO, Assimos DG: Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int 59: 270–276, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Mulay SR, Anders H-J: Crystallopathies. N Engl J Med 374: 2465–2476, 2016. [DOI] [PubMed] [Google Scholar]
- 53.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu CY, Hsu RK, Liu KD, Yang J, Anderson A, Chen J, Chinchilli VM, Feldman HI, Garg AX, Hamm L, Himmelfarb J, Kaufman JS, Kusek JW, Parikh CR, Ricardo AC, Rosas SE, Saab G, Sha D, Siew ED, Sondheimer J, Taliercio JJ, Yang W, Go AS; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators and the Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury (ASSESS-AKI) Study: Impact of AKI on urinary protein excretion: Analysis of two prospective cohorts. J Am Soc Nephrol 30: 1271–1281, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu KD, Yang W, Anderson AH, Feldman HI, Demirjian S, Hamano T, He J, Lash J, Lustigova E, Rosas SE, Simonson MS, Tao K, Hsu CY; Chronic Renal Insufficiency Cohort (CRIC) study investigators : Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int 83: 909–914, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu CY, Xie D, Waikar SS, Bonventre JV, Zhang X, Sabbisetti V, Mifflin TE, Coresh J, Diamantidis CJ, He J, Lora CM, Miller ER, Nelson RG, Ojo AO, Rahman M, Schelling JR, Wilson FP, Kimmel PL, Feldman HI, Vasan RS, Liu KD; CRIC Study Investigators; CKD Biomarkers Consortium: Urine biomarkers of tubular injury do not improve on the clinical model predicting chronic kidney disease progression. Kidney Int 91: 196–203, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie D, Yang W, Jepson C, Roy J, Hsu JY, Shou H, Anderson AH, Landis JR, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Statistical methods for modeling time-updated exposures in cohort studies of chronic kidney disease. Clin J Am Soc Nephrol 12: 1892–1899, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu JY, Roy JA, Xie D, Yang W, Shou H, Anderson AH, Landis JR, Jepson C, Wolf M, Isakova T, Rahman M, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Statistical methods for cohort studies of CKD: Survival analysis in the setting of competing risks. Clin J Am Soc Nephrol 12: 1181–1189, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roy J, Shou H, Xie D, Hsu JY, Yang W, Anderson AH, Landis JR, Jepson C, He J, Liu KD, Hsu CY, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Statistical methods for cohort studies of CKD: Prediction modeling. Clin J Am Soc Nephrol 12: 1010–1017, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang W, Jepson C, Xie D, Roy JA, Shou H, Hsu JY, Anderson AH, Landis JR, He J, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Statistical methods for recurrent event analysis in cohort studies of CKD. Clin J Am Soc Nephrol 12: 2066–2073, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang W, Xie D, Anderson AH, Joffe MM, Greene T, Teal V, Hsu CY, Fink JC, He J, Lash JP, Ojo A, Rahman M, Nessel L, Kusek JW, Feldman HI; CRIC Study Investigators: Association of kidney disease outcomes with risk factors for CKD: Findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 63: 236–243, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shou H, Hsu JY, Xie D, Yang W, Roy J, Anderson AH, Landis JR, Feldman HI, Parsa A, Jepson C; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Analytic considerations for repeated measures of eGFR in cohort studies of CKD. Clin J Am Soc Nephrol 12: 1357–1365, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norton JM, Moxey-Mims MM, Eggers PW, Narva AS, Star RA, Kimmel PL, Rodgers GP: Social determinants of racial disparities in CKD. J Am Soc Nephrol 27: 2576–2595, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bundy JD, Bazzano LA, Xie D, Cohan J, Dolata J, Fink JC, Hsu CY, Jamerson K, Lash J, Makos G, Steigerwalt S, Wang X, Mills KT, Chen J, He J; CRIC Study Investigators: Self-reported tobacco, alcohol, and illicit drug use and progression of chronic kidney disease. Clin J Am Soc Nephrol 13: 993–1001, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schrauben SJ, Hsu JY, Wright Nunes J, Fischer MJ, Srivastava A, Chen J, Charleston J, Steigerwalt S, Tan TC, Fink JC, Ricardo AC, Lash JP, Wolf M, Feldman HI, Anderson AH; CRIC Study Investigators: Health behaviors in younger and older adults with CKD: Results from the CRIC study. Kidney Int Rep 4: 80–93, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Porter AC, Lash JP, Xie D, Pan Q, DeLuca J, Kanthety R, Kusek JW, Lora CM, Nessel L, Ricardo AC, Wright Nunes J, Fischer MJ; CRIC Study Investigators: Predictors and outcomes of health-related quality of life in adults with CKD. Clin J Am Soc Nephrol 11: 1154–1162, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cedillo-Couvert EA, Ricardo AC, Chen J, Cohan J, Fischer MJ, Krousel-Wood M, Kusek JW, Lederer S, Lustigova E, Ojo A, Porter AC, Sharp LK, Sondheimer J, Diamantidis C, Wang X, Roy J, Lash JP; CRIC Study Investigators: Self-reported medication adherence and CKD progression. Kidney Int Rep 3: 645–651, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schrauben SJ, Hsu JY, Rosas SE, Jaar BG, Zhang X, Deo R, Saab G, Chen J, Lederer S, Kanthety R, Hamm LL, Ricardo AC, Lash JP, Feldman HI, Anderson AH; CRIC Study Investigators: CKD self-management: Phenotypes and associations with clinical outcomes. Am J Kidney Dis 72: 360–370, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wing MR, Devaney JM, Joffe MM, Xie D, Feldman HI, Dominic EA, Guzman NJ, Ramezani A, Susztak K, Herman JG, Cope L, Harmon B, Kwabi-Addo B, Gordish-Dressman H, Go AS, He J, Lash JP, Kusek JW, Raj DS; Chronic Renal Insufficiency Cohort (CRIC) Study: DNA methylation profile associated with rapid decline in kidney function: Findings from the CRIC study. Nephrol Dial Transplant 29: 864–872, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomas G, Xie D, Chen HY, Anderson AH, Appel LJ, Bodana S, Brecklin CS, Drawz P, Flack JM, Miller ER Jr, Steigerwalt SP, Townsend RR, Weir MR, Wright JT Jr, Rahman M; CRIC Study Investigators: Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: Report from the chronic renal insufficiency cohort study. Hypertension 67: 387–396, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grunwald JE, Pistilli M, Ying G-S, Daniel E, Maguire MG, Xie D, Whittock-Martin R, Parker Ostroff C, Lo JC, Townsend RR, Gadegbeku CA, Lash JP, Fink JC, Rahman M, Feldman HI, Kusek JW; Chronic Renal Insufficiency Cohort Study Investigators: Retinopathy and progression of CKD: The CRIC Study. Clin J Am Soc Nephrol 9: 1217–1224, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grunwald JE, Pistilli M, Ying G-S, Daniel E, Maguire M, Xie D, Roy J, Whittock-Martin R, Parker Ostroff C, Lo JC, Townsend RR, Gadegbeku CA, Lash JP, Fink JC, Rahman M, Feldman HI, Kusek JW; Chronic Renal Insufficiency Cohort Study Investigators: Association between progression of retinopathy and concurrent progression of kidney disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. JAMA Ophthalmol 137: 767–774, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurella Tamura M, Yaffe K, Hsu CY, Yang J, Sozio S, Fischer M, Chen J, Ojo A, DeLuca J, Xie D, Vittinghoff E, Go AS; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Cognitive impairment and progression of CKD. Am J Kidney Dis 68: 77–83, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rahman M, Xie D, Feldman HI, Go AS, He J, Kusek JW, Lash J, Miller ER 3rd, Ojo A, Pan Q, Seliger SL, Steigerwalt S, Townsend RR; CRIC Study Investigators: Association between chronic kidney disease progression and cardiovascular disease: Results from the CRIC Study. Am J Nephrol 40: 399–407, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rahman M, Yang W, Akkina S, Alper A, Anderson AH, Appel LJ, He J, Raj DS, Schelling J, Strauss L, Teal V, Rader DJ; CRIC Study Investigators: Relation of serum lipids and lipoproteins with progression of CKD: The CRIC study. Clin J Am Soc Nephrol 9: 1190–1198, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dobre M, Yang W, Chen J, Drawz P, Hamm LL, Horwitz E, Hostetter T, Jaar B, Lora CM, Nessel L, Ojo A, Scialla J, Steigerwalt S, Teal V, Wolf M, Rahman M; CRIC Investigators: Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: A report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 62: 670–678, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scialla JJ, Asplin J, Dobre M, Chang AR, Lash J, Hsu CY, Kallem RR, Hamm LL, Feldman HI, Chen J, Appel LJ, Anderson CA, Wolf M; Chronic Renal Insufficiency Cohort Study Investigators: Higher net acid excretion is associated with a lower risk of kidney disease progression in patients with diabetes. Kidney Int 91: 204–215, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koye DN, Magliano DJ, Reid CM, Jepson C, Feldman HI, Herman WH, Shaw JE: Risk of progression of nonalbuminuric CKD to end-stage kidney disease in people with diabetes: The CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis 72: 653–661, 2018. [DOI] [PubMed] [Google Scholar]
- 79.Bansal N, Zelnick LR, Himmelfarb J, Chertow GM: Bioelectrical impedance analysis measures and clinical outcomes in CKD. Am J Kidney Dis 72: 662–672, 2018. [DOI] [PubMed] [Google Scholar]
- 80.Srivastava A, Kaze AD, McMullan CJ, Isakova T, Waikar SS: Uric acid and the risks of kidney failure and death in individuals with CKD. Am J Kidney Dis 71: 362–370, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Afshinnia F, Zeng L, Byun J, Wernisch S, Deo R, Chen J, Hamm L, Miller ER, Rhee EP, Fischer MJ, Sharma K, Feldman HI, Michailidis G, Pennathur S; CRIC Study Investigators: Elevated lipoxygenase and cytochrome P450 products predict progression of chronic kidney disease. Nephrol Dial Transplant 35: 303–312, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rhee EP, Clish CB, Wenger J, Roy J, Elmariah S, Pierce KA, Bullock K, Anderson AH, Gerszten RE, Feldman HI: Metabolomics of chronic kidney disease progression: A case-control analysis in the chronic renal insufficiency cohort study. Am J Nephrol 43: 366–374, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Foster MC, Coresh J, Hsu CY, Xie D, Levey AS, Nelson RG, Eckfeldt JH, Vasan RS, Kimmel PL, Schelling J, Simonson M, Sondheimer JH, Anderson AH, Akkina S, Feldman HI, Kusek JW, Ojo AO, Inker LA; CKD Biomarker Consortium and the CRIC Study Investigators: Serum β-trace protein and β2-microglobulin as predictors of ESRD, mortality, and cardiovascular disease in adults with CKD in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 68: 68–76, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Inker LA, Coresh J, Sang Y, Hsu CY, Foster MC, Eckfeldt JH, Karger AB, Nelson RG, Liu X, Sarnak M, Appel LJ, Grams M, Xie D, Kimmel PL, Feldman H, Ramachandran V, Levey AS; CKD Biomarkers Consortium: Filtration markers as predictors of ESRD and mortality: Individual participant data meta-analysis. Clin J Am Soc Nephrol 12: 69–78, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amdur RL, Feldman HI, Gupta J, Yang W, Kanetsky P, Shlipak M, Rahman M, Lash JP, Townsend RR, Ojo A, Roy-Chaudhury A, Go AS, Joffe M, He J, Balakrishnan VS, Kimmel PL, Kusek JW, Raj DS; CRIC Study Investigators: Inflammation and progression of CKD: The CRIC Study. Clin J Am Soc Nephrol 11: 1546–1556, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Orlandi PF, Fujii N, Roy J, Chen HY, Lee Hamm L, Sondheimer JH, He J, Fischer MJ, Rincon-Choles H, Krishnan G, Townsend R, Shafi T, Hsu CY, Kusek JW, Daugirdas JT, Feldman HI; CRIC Study Investigators: Hematuria as a risk factor for progression of chronic kidney disease and death: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. BMC Nephrol 19: 150, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhan M, Doerfler RM, Xie D, Chen J, Chen HY, Diamantidis CJ, Rahman M, Ricardo AC, Sondheimer J, Strauss L, Wagner LA, Weir MR, Fink JC; CRIC Study Investigators: Association of opioids and nonsteroidal anti-inflammatory drugs with outcomes in CKD: Findings from the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis 76: 184–193, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anderson AH, Xie D, Wang X, Baudier RL, Orlandi P, Appel LJ, Dember LM, He J, Kusek JW, Lash JP, Navaneethan SD, Ojo A, Rahman M, Roy J, Scialla JJ, Sondheimer J, Steigerwalt SP, Wilson SP, Wolf M, Feldman HI; CRIC Study Investigators: Novel risk factors for progression of diabetic and non-diabetic CKD: Findings for the Chronic Renal Insufficiency Cohort (CRIC) Study [published online ahead of print August 28, 2020]. Am J Kidney Dis 10.1053/j.ajkd.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]