Visual Abstract
Keywords: frailty, dialysis, chronic kidney disease, decision making
Abstract
Background and objectives
Frailty is common in patients with CKD. Little is known about the prevalence of frailty and its effect on prognosis and decisions surrounding dialysis modalities in patients with advanced CKD (eGFR<30 ml/min per 1.73 m2). Our objective was to determine the agreement between different frailty measures and physical function and their association with dialysis modality choice (home based versus in-center) and all-cause mortality in patients with advanced CKD.
Design, setting, participants, & measurements
Our study was a prospective, multicenter, cohort study. In 603 patients with advanced CKD, we collected demographics, comorbidities, and laboratory results in addition to objective (Fried frailty criteria) and subjective measures of frailty (physician and nurse impressions) and physical function (Short Physical Performance Battery). Logistic regression and Cox proportional hazards models were used to evaluate the association of frailty with dialysis modality choice and all-cause mortality, respectively.
Results
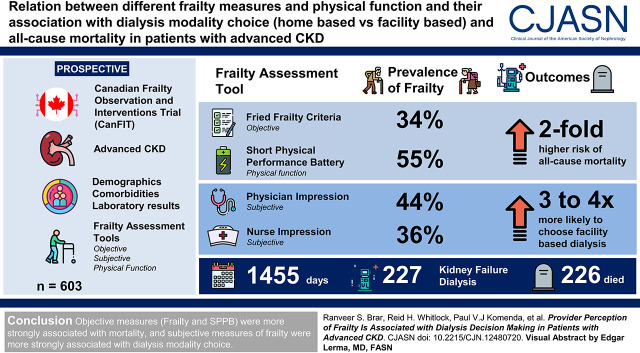
The prevalence of frailty varied with assessment tool used (Fried frailty criteria, 34%; Short Physical Performance Battery, 55%; physician impression, 44%; nurse impression, 36%). The agreement between all frailty and physical function measures was poor. We had 227 patients reach kidney failure and decide on a dialysis therapy, and 226 patients died during a mean follow-up of 1455 days. After adjusting for age, sex, and comorbid conditions, the Fried criteria and Short Physical Performance Battery were associated with a two-fold higher risk of all-cause mortality (hazard ratio, 1.96; 95% confidence interval, 1.47 to 2.61 and hazard ratio, 1.96; 95% confidence interval,1.42 to 2.76, respectively). Patients deemed as frail by physician and nurse frailty impressions were three to four times more likely to choose in-center dialysis (odds ratio, 3.41; 95% confidence interval, 1.56 to 7.44; odds ratio, 3.87; 95% confidence interval, 1.76 to 8.51, respectively).
Conclusions
We found that the agreement between objective and subjective measures of frailty and physical function was poor. Objective measures of frailty and physical function were associated with mortality, and subjective measures of frailty were associated with dialysis modality choice.
Introduction
CKD disproportionately affects nearly one in three individuals over the age of 70 and may be a surrogate of unsuccessful aging (1,2). Older adults with CKD often carry a high burden of comorbid conditions and suffer from worsening frailty and functional decline (3,4). In addition to influencing patient longevity, these changes in frailty may also affect health-related quality of life and influence treatment choices for dialysis modalities.
Frailty is a multidimensional construct, conceptualized as the sum of alterations in physiologic systems causing increased vulnerability for adverse health outcomes, including disability, dependency, falls, and mortality (5). Methods of measuring frailty range from objective measures of physical performance tests and questionnaires used to describe a “frailty phenotype” to a sum of objective deficits in clinical conditions and laboratory tests used to determine a continuous frailty index (6,7). Simpler subjective measures, including a gestalt or “eyeball” assessment of frailty, have also been recommended (8).
In the general population, most measures of frailty are associated with poor clinical outcomes (6). Although there have been several studies in patients with early stages of CKD (9) and those on dialysis (10), very few studies have examined the effect of frailty on the clinical trajectory of patients with advanced CKD (stages 4 and 5; eGFR<30 ml/min per 1.73 m2). In particular, no study has yet examined the effect that frailty may have on decisions surrounding choices for dialysis modalities, which are currently on the basis of subjective patient, caregiver, and care provider factors.
To address this knowledge gap, we measured frailty, its associated risk factors, physical function, and clinical outcomes in a prospective cohort study of patients with advanced CKD. We sought to determine the agreement between objective and subjective frailty measures and physical function. In addition, we wanted to determine the association of frailty and physical function with future dialysis modality decisions and mortality.
Materials and Methods
Study Design and Population
The Canadian Frailty Observation and Interventions Trial (CanFIT) is a Canadian multicenter, prospective, observational, cohort study that began enrollment in September 2012. Details of the protocol have been previously described (4). Briefly, the study examines the burden and longitudinal trajectory of frailty and its association with morbidity, mortality, and other patient-related outcomes in individuals with advanced CKD (defined by an eGFR<30 ml/min per 1.73 m2) followed in multidisciplinary nephrology clinics. The study aims to recruit 600 patients and follow them annually (9–18 months) until a study end point of death, opt out, loss to follow-up, or study completion (have been assessed 6–12 months after the initiation of KRT) has been reached. Patients are then followed for outcomes of mortality, morbidity, and hospitalization. Patients are excluded if they are unable to provide informed consent, are unable to speak English, have blindness, have known dementia, and had previous dialysis treatment. Data at baseline on the first 603 patients enrolled in the CanFIT study are presented here.
Data Collection
Comorbid Conditions.
Demographic information (date of birth, sex, and ethnicity) and laboratory results were collected through chart review at baseline. This information was used to calculate kidney failure risk (11). Comorbidities were collected at baseline through self-report and supplemented by medical records review. We constructed a comorbidity count on the basis of the comorbidities collected and commonly used comorbidity indices (12,13). These included prior myocardial infarction, cerebrovascular disease, diabetes, peripheral vascular disease, cirrhosis, gastrointestinal disease, chronic obstructive pulmonary disease, congestive heart failure, hypertension, pulmonary hypertension, arthritis, weight loss, depression, anxiety/panic attacks, asthma, visual and hearing impairments, malignancy, and severe psychologic stress or acute medical issue.
Frailty Assessment Tools.
Frailty measures were assessed at baseline directly by a research coordinator through various questionnaires and standardized physical function tests. The objective frailty measurement tool used was the Fried criteria (6), and subjective physician and nurse frailty impression ratings of each participant were also collected.
Fried Criteria.
The Fried criteria were scored on a scale from one to five. The methods used to calculate this score are described in Supplemental Table 1. Briefly, five domains were measured: weight loss, exhaustion, low physical activity, slowness, and weakness. Each domain was given a score of zero (not frail) or one (frail). Patients were considered frail if greater than or equal to three criteria were abnormal (6).
Physician and Nurse Impressions of Frailty.
The clinic nephrologist and nurse were asked to independently rate each participant’s level of frailty on a five-point Likert scale (very fit: one; very frail: five) at the baseline frailty assessment with the question: “On a scale from one to five, one being very fit and five being very frail, where would you rate the participant?” No other forms of visual or verbal aid were provided. Patients were considered frail if they were rated three or higher on the scale.
Physical Function Assessment: Short Physical Performance Battery
Short Physical Performance Battery (SPPB) scores range from zero to 12. The methods used to calculate the SPPB score are described in Supplemental Table 2. Briefly, SPPB incorporates three tests of lower extremity function (each scored from zero to four): chair stand, 4-m gait speed, and balance (side by side, semitandem, and tandem). Subjects who scored less than ten were categorized as having poor physical function. This cutoff is on the basis of previous study protocol and has been associated with all-cause mortality (4,14,15).
Outcomes of Interest
Our primary outcome was to examine the agreement between objective and subjective frailty measures and physical function. Second, we observed the association of these measures with choice of dialysis modality: in-center hemodialysis (HD) or home dialysis (home HD or peritoneal dialysis) at the time of dialysis initiation. In these clinics, the dialysis care plan is discussed and re-evaluated with a multidisciplinary nephrology care team (nephrologist, pharmacist, nurse, and dietician) at every visit (3–6 months). The choice of dialysis modality and initiation are agreed upon by the nephrology care team, patient, and caregiver, and, ultimately, the modality that the patient initiates is defined as the modality of choice. In addition to choice of dialysis modality, all-cause mortality was also an outcome of interest. This was identified by linking provincial electronic kidney health records to our cohort through personal health information numbers. The electronic kidney health records track all patients with treated kidney failure in the province, along with their dialysis start date and treatment modality. All outcomes were assessed as of January 31, 2020. The study protocol was approved by the University of Manitoba Health Research Ethics Board.
Statistical Analyses
Descriptive characteristics of the cohort at baseline were stratified and analyzed by the Fried criteria. Because of non-normal distribution, continuous variables are presented as medians and interquartile ranges and were analyzed using the Mann–Whitney U test. Categorical variables are presented as frequencies and percentages and were compared using the chi-squared test. Analysis for albuminuria and kidney failure risk was performed with complete cases only (missing urine albumin-to-creatinine ratio=27%). Agreement between the different frailty assessment tools and physical function was calculated using Cohen κ. Values of κ of 0.01–0.20, 0.21–0.39, 0.40–0.59, 0.60–0.79, 0.80–0.90, and above 0.90 indicate levels of agreement of none, minimal, weak, moderate, strong, and almost perfect, respectively (16). The associations between frailty, physical function, and secondary outcomes were evaluated using logistic regression models for modality and Cox proportional hazards models for mortality. We followed patients in our mortality model until January 31, 2020 or until they reached the outcome. Patients in the study who were either lost to follow-up or opted out of future assessments provided consent for passive follow-up. Therefore, patients who did not experience death were not censored at any point other than the end of our follow-up period. Each component of the Fried frailty criteria and physical function was evaluated separately as a predictor of the outcomes and was featured in two models: an unadjusted model and a model adjusted for age, sex, and a comorbidity count as described above. Data on weight, BP, ethnicity, comorbidities, and laboratory values were missing at <5%. Missing data were handled by multiple imputations using the proc MI procedure in SAS 9.3 (17). All analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, NC).
Results
Study Population
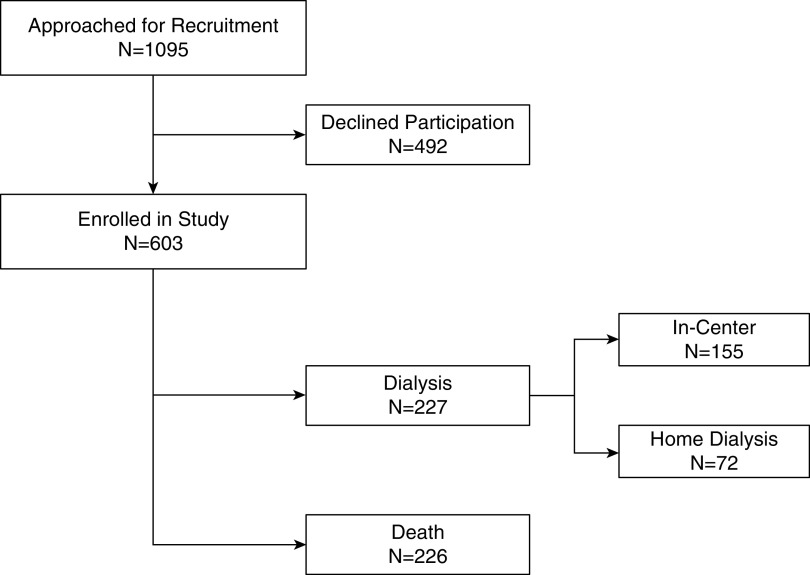
Over the study period, 1095 patients were approached for enrollment, of which 603 consented and were included in the analysis (Figure 1). Of the 603 patients included in the study, 226 patients died, and 227 started dialysis for kidney failure with a mean follow-up period of 1455 days (Figure 1). Of those who started dialysis, 155 started in-center HD, four started home HD, and 68 started peritoneal dialysis (Figure 1). Clinical characteristics of the 603 patients included in this analysis are summarized in Table 1. Patients classified as frail were 8 years older on average and more likely to be women. They weighed an average of 6 kg less, with lower hemoglobin. Comorbid conditions, such as diabetes, peripheral vascular disease, chronic obstructive pulmonary disease, arthritis, congestive heart failure, visual/hearing impairment, and neurologic disease, were more common in frail patients. Albuminuria and kidney failure risk as estimated by the kidney failure risk equation were similar in both groups.
Figure 1.
Study flow diagram.
Table 1.
Baseline characteristics by Fried frailty criteria of participants in the Canadian Frailty Observation and Interventions Trial
| Variable | Not Frail | Frail |
|---|---|---|
| N | 399 | 204 |
| Demographics | ||
| Agea | 66 (54–73) | 73 (65–82) |
| Sex (% women)b | 144 (36) | 103 (51) |
| Race (% White)b | 326 (82) | 163 (80) |
| Clinical measurementsa | ||
| Systolic BP, mm Hg | 136 (124–149) | 138 (123–153) |
| Diastolic BP, mm Hg | 75 (67–83) | 70 (63–80) |
| Weight, kg | 86 (72–101) | 81 (70–92) |
| Creatinine, mg/dl | 3.05 (2.25–4.01) | 3.06 (2.26–3.96) |
| eGFR, ml/min per 1.73 m2 | 19 (13–25) | 18 (13–24) |
| Hemoglobin, g/dl | 11.6 (10.7–12.7) | 11.1 (10.1–12.2) |
| Serum albumin, g/dl | 3.6 (3.3–3.9) | 3.5 (3.2–3.8) |
| Serum phosphate, mg/dl | 4.1 (3.5–4.7) | 4.3 (3.7–4.9) |
| Urine ACR, mg/gc | 51 (7–173) | 71 (9–237) |
| HbA1c, %c | 6.2 (5.6–7.5) | 6.3 (5.7–7.6) |
| Comorbiditiesb | ||
| Previous MI (%) | 80 (20) | 43 (22) |
| Diabetes, type 1 or 2 (%) | 209 (52) | 135 (66) |
| Peripheral vascular disease (%) | 31 (8) | 44 (22) |
| Gastrointestinal disease (%) | 71 (18) | 47 (24) |
| Malignancy (%) | 81 (20) | 41 (20) |
| COPD (%) | 22 (6) | 24 (12) |
| Hypertension (%) | 336 (84) | 187 (92) |
| Arthritis (%) | 138 (35) | 108 (53) |
| Congestive heart failure (%) | 32 (8) | 35 (17) |
| Depression (%) | 59 (15) | 42 (21) |
| Visual/hearing impairment (%) | 196 (49) | 138 (68) |
| Neurologic disease (%) | 36 (9) | 53 (26) |
| Comorbidity indexa | 3 (2–4) | 4 (3–5) |
| CKD stage at enrollmentb (% stage 5) | ||
| CKD stage | 115 (28.8) | 63 (30.9) |
| Median kidney failure risk,c % | ||
| 5-yr risk | 21 | 21 |
ACR, albumin-to-creatinine ratio; HbA1c, hemoglobin A1c; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease.
Continuous variables are reported as medians and interquartile ranges. Mann–Whitney U test was used to compare continuous variables.
Categorical variables are reported as percentages and compared using chi-squared tests.
Variables are reported in complete cases (urine ACR missing =27%, HbA1c missing =35%).
Comparison of Frailty Assessment Tools and Physical Function
The prevalence of frailty varied depending on the assessment tool used. The Fried criteria identified 34% of patients as frail, 44% were identified by physician impression ratings, and 36% by nurse impression ratings. The prevalence of poor physical function was 55% on the basis of SPPB. Agreement between objective frailty (Fried) and physical function (SPPB) was weak (Cohen κ; κ=0.43). The agreement was also weak between subjective measures of frailty (physician and nurse impressions; κ=0.45). Distribution of Likert raw scores is presented in Supplemental Figure 1. Every comparison between an objective and a subjective measurement demonstrated minimal agreement at best (κ=0.29–0.33) (Table 2).
Table 2.
Agreement between frailty and physical function assessments
| Assessment Tool | Prevalence, % | Agreement, κ | |||
|---|---|---|---|---|---|
| Fried | Short Physical Performance Battery | Physician Impression | Nurse Impression | ||
| Fried | 34 | 0.43 | 0.33 | 0.31 | |
| Short Physical Performance Battery | 55 | 0.43 | 0.29 | 0.32 | |
| Physician impression | 44 | 0.33 | 0.29 | 0.45 | |
| Nurse impression | 36 | 0.31 | 0.32 | 0.45 | |
Association of Objective and Subjective Frailty and Physical Function with Dialysis Modality Choice
The associations between frailty and physical function measures and dialysis modality decisions are presented in Table 3. Unadjusted physician and nurse impressions were associated with choosing in-center HD over home dialysis (odds ratio [OR], 3.72; 95% confidence interval [95% CI], 1.79 to 7.72 and OR, 4.05; 95% CI, 1.96 to 8.33, respectively). After adjusting for age, sex, and comorbid conditions, patients designated as frail by physicians and nurses were three to four times more likely to choose in-center HD (OR, 3.41; 95% CI, 1.56 to 7.44 and OR, 3.87; 95% CI, 1.76 to 8.51, respectively). In contrast, after adjusting for all covariates, the Fried criteria and SPPB were not associated with choice of dialysis (OR, 1.55; 95% CI, 0.77 to 3.13 and OR, 1.86; 95% CI, 0.99 to 3.53, respectively).
Table 3.
Associations of frailty and physical function measures with dialysis choice and all-cause mortality
| Modela | Death, n=226 | Dialysis Choice: In Center = 155; Home = 72)c | ||||
|---|---|---|---|---|---|---|
| N (%) | Unadjusted Hazard Ratio (95% Confidence Interval) | Adjustedb Hazard Ratio (95% Confidence Interval) | N (%) | Unadjusted Odds Ratio (95% Confidence Interval) | Adjustedb Odds Ratio (95% Confidence Interval) | |
| Fried criteria | ||||||
| Present | 117 (52) | 2.79 (2.14 to 3.64) | 1.96 (1.47 to 2.61) | 58 (37) | 1.85 (0.97 to 3.49) | 1.55 (0.77 to 3.13) |
| Not present | 109 (48) | 1 (reference) | 1 (reference) | 97 (63) | 1 (reference) | 1 (reference) |
| Physician impression | ||||||
| Present | 115 (51) | 2.32 (1.78 to 3.04) | 1.48 (1.11 to 1.98) | 61 (39) | 3.72 (1.79 to 7.72) | 3.41 (1.56 to 7.44) |
| Not present | 111 (49) | 1 (reference) | 1 (reference) | 94 (61) | 1 (reference) | 1 (reference) |
| Nurse impression | ||||||
| Present | 104 (46) | 2.27 (1.64 to 3.16) | 1.52 (1.09 to 2.11) | 71 (46) | 4.05 (1.96 to 8.33) | 3.87 (1.76 to 8.51) |
| Not present | 122 (54) | 1 (reference) | 1 (reference) | 84 (54) | 1 (reference) | 1 (reference) |
| Short Physical Performance Battery | ||||||
| Present | 165 (73) | 2.87 (2.14 to 3.86) | 1.96 (1.42 to 2.70) | 85 (55) | 2.12 (1.19 to 3.76) | 1.86 (0.99 to 3.53) |
| Not present | 61 (27) | 1 (reference) | 1 (reference) | 70 (45) | 1 (reference) | 1 (reference) |
All exposures in models were categorical.
Models adjusted for age, sex, and comorbidity count.
Analysis was performed in participants who initiated dialysis for kidney failure. Odds ratio refers to odds of choosing in-center dialysis.
Association of Objective and Subjective Frailty and Physical Function with All-Cause Mortality
The associations between frailty and physical function measures and all-cause mortality are presented in Table 3. Frailty measured objectively using the Fried criteria and SPPB was associated with a two-fold higher risk of mortality in adjusted analysis (hazard ratio [HR], 1.96; 95% CI, 1.47 to 2.61 and HR, 1.96; 95% CI, 1.42 to 2.70, respectively). After adjustment for potential confounders, both physician impression and nurse impression of frailty were less strongly associated with mortality (HR, 1.48; 95% CI, 1.11 to 1.98 and HR, 1.52; 95% CI, 1.09 to 2.11, respectively). There were no differences in the proportions of deaths that occurred pre- or postdialysis initiation when stratified by frailty assessment or physical function (Supplemental Table 3).
Association of Fried Components with Secondary Outcomes
In adjusted analysis, all components of the Fried were not associated with choosing in-center HD, other than low physical activity (OR, 2.15; 95% CI, 1.19 to 3.86) (Supplemental Table 4). Fried components that were strongly associated with all-cause mortality were objective (slowness and weakness; HR, 1.99; 95% CI, 1.49 to 2.65 and HR, 1.61; 95% CI, 1.20 to 2.18, respectively) in adjusted models (Supplemental Table 4).
Association of Short Physical Performance Battery Components with Secondary Outcomes
In adjusted analysis, only the chair stand test was associated with choosing in-center HD (OR, 2.61; 95% CI, 1.35 to 5.09) (Supplemental Table 5). All components of SPPB (chair stand, gait, and balance) were strongly associated with all-cause mortality (HR, 2.52; 95% CI, 1.61 to 3.94; HR, 1.82; 95% CI, 1.36 to 2.43; and HR, 1.85; 95% CI, 1.39 to 2.46, respectively) in adjusted models (Supplemental Table 2).
Discussion
In this prospective, observational, cohort study of patients with advanced CKD, we found that the prevalence of frailty varied widely depending on the frailty assessment tool used. Furthermore, different frailty and physical function assessment tools did not identify the same individuals as being frail or having poor physical function, as demonstrated by their weak agreement. The subjective measures of frailty (physician and nurse impression ratings) were more strongly associated with treatment decisions (in-center HD versus home dialysis), whereas objective frailty and poor physical function (slowness and weakness from Fried and all components of SPPB) were more strongly associated with all-cause mortality. Together, these findings emphasize that although frailty and poor physical function are common in patients with CKD, the choice of measurement tool used to identify frailty and poor physical function is important in determining diagnostic and prognostic values of this concept in the advanced CKD population.
Previous studies have examined the association of frailty and adverse outcomes in patients with CKD and have concluded that frailty leads to worse outcomes, including death and kidney failure (9,18). These larger studies, on the basis of secondary analyses of data from national health surveys, suggested that frailty is common and associated with adverse clinical outcomes in patients with early-stage CKD. Important limitations to these studies were the inclusion of a relatively low-risk CKD population and the need to modify the Fried criteria in order to accommodate the available data, both of which limited the validity and generalizability of these findings to patients with later stages of CKD referred to and cared for in multidisciplinary CKD clinics.
More recently, investigators from the Seattle Kidney study prospectively evaluated frailty using a slightly modified Fried criteria in >300 patients with CKD followed in nephrology clinics (18). They found that patients with an earlier stage of CKD (mean eGFR of 51 ml/min per 1.73 m2) had a nearly two-fold higher prevalence of frailty compared with an older, community-dwelling, non-CKD reference population. These same investigators then studied another cohort of 385 patients with more advanced CKD (mean eGFR of 41 ml/min per 1.73 m2) and found that gait speed (a criterion in the Fried construct of frailty) was independently associated with mortality in the population, whereas handgrip strength and other biomarkers, such as hemoglobin, albumin, or C-reactive protein, were not (19). However, participants in these studies had relatively preserved kidney function, and investigators did not assess agreement between different methods of measuring frailty or examine the effect of frailty on dialysis treatment modality selection.
To our knowledge, this study is the largest to examine the agreement between subjectively and objectively measured frailty in patients with CKD. Salter et al. (20) recently conducted a cross-sectional analysis examining the agreement between physician-, nurse-, and patient-perceived frailty with objective frailty using the Fried criteria in 146 prevalent patients on HD. Similar to our findings, the investigators reported poor agreement (κ=0.24–0.27) between perceived and objective frailty and concluded that perceived frailty is an inadequate proxy for objective measurement.
Our study suggests that patients who were perceived as frail by nurses and physicians (independent of age, sex, and comorbidity) were less likely to choose or perform home dialysis modalities. These findings are novel because the relationship between frailty and decisions around dialysis commencement and modality has not been previously explored. The decision regarding when and what type of dialysis modality to initiate in a patient with progressive CKD is complex, involving both objective and subjective physician, nurse, and patient/caregiver factors. Although we did not ask patients to classify themselves as frail versus nonfrail, our findings suggest that physicians and nurses may be more likely to recommend in-center HD to patients they perceive are frail versus those diagnosed as frail or having poor physical function using the Fried or SPPB-based criteria, respectively. This discordance is concerning, as provider perception had poor agreement with objective measures of frailty or physical function and was not as strongly associated with a definitive clinical outcome (mortality), whereas the Fried and SPPB criteria were. This important discordance between clinician perception and objectively measured frailty and physical function suggests that clinical gestalt alone may be an inappropriate guide for dialysis modality choice and that subjective and objective definitions of frailty should not be considered interchangeable.
In addition, it is possible that nephrologists and nursing staff encouraged in-center dialysis for more frail patients as part of regular clinical encounters on the basis of their subjective assessment. However, objective measures of frailty have stronger associations with clinical outcomes, and perhaps, advice on treatment decisions, if appropriate, should at least include some objective measures as they are easily implemented by the bedside. In particular, measures of lower-extremity physical function (chair stand test) had strong associations with death and modality selection and can be easily performed at the bedside. Furthermore, our previous study examining technique failure in patients on peritoneal dialysis suggested that these objective criteria were more strongly associated with clinical outcomes when compared with physician/nurse impression (21). Future studies should examine this concept and whether incorporation of objective frailty measures or physical function tests into a dialysis decision support tool for use by patients and physicians leads to better modality decision making and technique survival.
Our findings have important clinical and research implications in this population that is at high risk of progression to kidney failure, cardiovascular events, and death. From a clinical perspective, SPPB is simple, takes minimal time to perform in clinic, and therefore, can be integrated into the bedside. Furthermore, individual components of the Fried (gait speed and grip strength) were independently associated with all-cause mortality, which suggests that an objective frailty assessment needs to be integrated into clinical examination. Whether objective frailty measures improve risk prediction beyond traditional models using clinical and demographic variables, however, remains unknown and needs to be examined further in prospective studies. In addition, our findings on nurse and physician impressions of frailty and its association with the choice of in-center HD need to be validated in independent cohorts.
The strengths of this study include its prospective, multicenter design and the collection of a broad range of clinical, demographic, and laboratory variables in addition to data on frailty and disability. Furthermore, the unique study population of patients with advanced CKD (mean eGFR 20 ml/min) examines a previously unstudied population at exceptionally high risk for kidney failure and all-cause mortality. Finally, the study’s longitudinal design will allow us to continue to prospectively study changes in frailty and physical/cognitive function that occur with dialysis initiation and to assess the effect of these clinical trajectories on clinical outcomes and quality of life.
This study has several limitations. We used a single baseline measurement of frailty and examined the association with downstream adverse events. As frailty status changes, particularly with kidney failure and the initiation of dialysis, the effect of these changes on mortality and dialysis decision making remains unstudied. In addition, most of our mortality outcomes occurred before dialysis, and as such, we are unable to evaluate the effect of dialysis treatment on the frailty trajectory. As we continue follow-up in our study population, we expect that additional outcomes will accrue, and we may be able to examine the association of frailty with postdialysis outcomes. Also, we were unable to collect data on choice of conservative or palliative management of CKD and, therefore, are not able to address the effect this may have had on mortality and dialysis modality choice. We were also not able to attain data on social factors such as caregiver support, education level, and socioeconomic status that may have affected the choice of dialysis modality. We also recognize that a validated SPPB cutoff for patients with CKD has not been established; therefore, a lower cutoff (less than nine) may be more specific for this population. However, we chose the cutoff of ten on the basis of our a priori–defined study protocol. Furthermore, our Canadian study population was mostly of White European descent, so our findings may not be generalizable to other high-risk populations, such as Hispanic and Black populations. Finally, our results may be less applicable to patients with earlier stages of CKD and patients with CKD not followed in nephrology clinics.
In summary, in this prospective cohort study of 603 patients with CKD stages 4 and 5 (eGFR<30 ml/min per 1.73 m2), we have demonstrated that the definition of the frailty construct is important, as there is limited agreement between frailty definitions, and important differences in the relationship of each construct with clinical outcomes. Patients diagnosed as frail using the objective Fried frailty criteria were more likely to die, and patients considered frail by nurses and physicians were more likely to choose in-center HD for KRT. These findings suggest that the methods used to diagnose or define frailty are important in determining its utility for clinical decision making.
Disclosures
C. Bohm reports ownership interest in Precision Advanced Digital Manufacturing and receiving research funding from Hope Pharmaceuticals. R.S. Brar reports employment with the Chronic Disease Innovation Centre. P.V.J. Komenda reports consultancy agreement as Chief Medical Officer of Quanta Dialysis Technologies; ownership interest in Quanta Dialysis Technologies; receiving research funding from AstraZeneca, Baxter, and NxStage; receiving honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Otsuka, and Quanta; and other interests/relationships with the Canadian Society of Nephrology Executive, the Chronic Disease Innovation Centre, and the Seven Oaks Hospital Foundation Council. B. Prasad reports employment with Regina Qu Appelle Health Region; receiving research funding from Baxter and Medtronic; and speakers bureau with AstraZeneca. C. Rigatto reports consultancy agreements with AstraZeneca, Boehringer Ingelheim, Lilly, and Sanofi; receiving research funding from Sanofi; receiving honoraria from Boehringer Ingelheim, Lilly, and Sanofi; and serving as a scientific advisor or member of the Canadian Journal of Kidney Health and Disease. N. Tangri reports consultancy agreements with Mesentech Inc., PulseData Inc., Renibus, and Tricida Inc.; ownership interest in Clinpredict Ltd., Mesentech Inc., PulseData Inc., Renibus, and Tricida Inc.; receiving research funding from AstraZeneca Inc., Janssen, Otsuka, and Tricida Inc.; receiving honoraria from AstraZeneca Inc., Bayer, BI-Janssen, Lilly, Otsuka Pharmaceuticals, and Pfizer; serving as a scientific advisor or member of Mesentech Inc., PulseData Inc., Renibus, and Tricida Inc.; and personal fees from Boehringer Ingelheim/Eli Lilly, Otsuka Inc., and Roche Inc. R.H. Whitlock reports employment with the Chronic Disease Innovation Centre.
Funding
This research was funded by the Canadian Frailty Network (Technology Evaluation in the Elderly Network; grant CAT2018-11), which is supported by the Government of Canada through the Networks of Centres of Excellence program.
Supplementary Material
Acknowledgments
The authors thank the patients who participated in the CanFIT.
All authors were responsible for research idea and study design; R.S. Brar was responsible for data acquisition; R.S. Brar, N. Tangri, and R.H. Whitlock were responsible for data analysis/interpretation; R.S. Brar and R. H. Whitlock were responsible for statistical analysis; C. Bohm, P.V.J. Komenda, B. Prasad, C. Rigatto, and N. Tangri provided supervision or mentorship; and all authors participated in editing the manuscript and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12480720/-/DCSupplemental.
Supplemental Table 1. Association of Fried components with dialysis choice and all-cause mortality.
Supplemental Table 2. Association of SPPB components with dialysis choice and all-cause mortality.
Supplemental Table 3. Fried criteria.
Supplemental Table 4. Short physical performance battery scoring.
Supplemental Table 5. Number of deaths before and after dialysis initiation on the basis of objective and subjective measures of frailty and physical function.
Supplemental Figure 1. Distribution of Likert-scale responses from physician and nurse impressions of frailty.
References
- 1.Cook WL: The intersection of geriatrics and chronic kidney disease: Frailty and disability among older adults with kidney disease. Adv Chronic Kidney Dis 16: 420–429, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Pugh J, Aggett J, Goodland A, Prichard A, Thomas N, Donovan K, Roberts G: Frailty and comorbidity are independent predictors of outcome in patients referred for pre-dialysis education. Clin Kidney J 9: 324–329, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker SR, Brar R, Eng F, Komenda P, Rigatto C, Prasad B, Bohm CJ, Storsley LJ, Tangri N: Frailty and physical function in chronic kidney disease: The CanFIT study. Can J Kidney Heal Dis 2: 32, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G: Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 59: 255–263, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group: Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Rockwood K, Mitnitski AB, MacKnight C: Some mathematical models of frailty and their clinical implications. Rev Clin Gerontol 12: 109–117, 2002 [Google Scholar]
- 8.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A: A global clinical measure of fitness and frailty in elderly people. CMAJ 173: 489–495, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilhelm-Leen ER, Hall YN, K Tamura M, Chertow GM: Frailty and chronic kidney disease: The Third National Health and Nutrition Evaluation Survey. Am J Med 122: 664–71.e2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen KL, Chertow GM, Jin C, Kutner NG: Significance of frailty among dialysis patients. J Am Soc Nephrol 18: 2960–2967, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45: 613–619, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Bandinelli S, Lauretani F, Boscherini V, Gandi F, Pozzi M, Corsi AM, Bartali B, Lova RM, Guralnik JM, Ferrucci L: A randomized, controlled trial of disability prevention in frail older patients screened in primary care: The FRASI study. Design and baseline evaluation. Aging Clin Exp Res 18: 359–366, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavasini R, Guralnik J, Brown JC, di Bari M, Cesari M, Landi F, Vaes B, Legrand D, Verghese J, Wang C, Stenholm S, Ferrucci L, Lai JC, Bartes AA, Espaulella J, Ferrer M, Lim JY, Ensrud KE, Cawthon P, Turusheva A, Frolova E, Rolland Y, Lauwers V, Corsonello A, Kirk GD, Ferrari R, Volpato S, Campo G: Short physical performance battery and all-cause mortality: Systematic review and meta-analysis. BMC Med 14: 215, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McHugh ML: Interrater reliability: The kappa statistic. Biochem Med (Zagreb) 22: 276–282, 2012 [PMC free article] [PubMed] [Google Scholar]
- 17.SAS : The MI Procedure. SAS/STAT(R) 14.1 User’s Guide, 2015. Available at: https://support.sas.com/documentation/cdl/en/statug/68162/HTML/default/viewer.htm#statug_mi_gettingstarted.htm. Accessed May 1, 2020.
- 18.Roshanravan B, Khatri M, Robinson-Cohen C, Levin G, Patel KV, de Boer IH, Seliger S, Ruzinski J, Himmelfarb J, Kestenbaum B: A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis 60: 912–921, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roshanravan B, Robinson-Cohen C, Patel KV, Ayers E, Littman AJ, de Boer IH, Ikizler TA, Himmelfarb J, Katzel LI, Kestenbaum B, Seliger S: Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 24: 822–830, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salter ML, Gupta N, Massie AB, McAdams-DeMarco MA, Law AH, Jacob RL, Gimenez LF, Jaar BG, Walston JD, Segev DL: Perceived frailty and measured frailty among adults undergoing hemodialysis: A cross-sectional analysis. BMC Geriatr 15: 52, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brar R, Whitlock R, Komenda P, Lerner B, Prasad B, Bohm C, Thorsteinsdottir B, Rigatto C, Tangri N: The impact of frailty on technique failure and mortality in patients on home dialysis. Perit Dial Int 39: 532–538, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.