Abstract
The Kidney Precision Medicine Project (KPMP) is a multisite study designed to improve understanding of CKD attributed to diabetes or hypertension and AKI by performing protocol-driven kidney biopsies. Study participants and their kidney tissue samples undergo state-of-the-art deep phenotyping using advanced molecular, imaging, and data analytical methods. Few patients participate in research design or concepts for discovery science. A major goal of the KPMP is to include patients as equal partners to inform the research for clinically relevant benefit. The purpose of this report is to describe patient and community engagement and the value they bring to the KPMP. Patients with CKD and AKI and clinicians from the study sites are members of the Community Engagement Committee, with representation on other KPMP committees. They participate in KPMP deliberations to address scientific, clinical, logistic, analytic, ethical, and community engagement issues. The Community Engagement Committee guides KPMP research priorities from perspectives of patients and clinicians. Patients led development of essential study components, including the informed consent process, no-fault harm insurance coverage, the ethics statement, return of results plan, a “Patient Primer” for scientists and the public, and Community Advisory Boards. As members across other KPMP committees, the Community Engagement Committee assures that the science is developed and conducted in a manner relevant to study participants and the clinical community. Patients have guided the KPMP to produce research aligned with their priorities. The Community Engagement Committee partnership has set new benchmarks for patient leadership in precision medicine research.
Keywords: chronic kidney disease, kidney, kidney biopsy, kidney disease, renal biopsy, acute kidney injury, diabetic nephropathy, hypertensive nephropathy, Precision Medicine
Introduction
Research in human health takes many forms, from observations without intervention to experimental treatments. Study participants and investigators rarely work collaboratively for many reasons, including traditional structures of research as well as differences in motivation, culture, and values. Patient priorities and viewpoints are rarely incorporated into discovery science. As a result, challenges in translating discoveries to improve clinical outcomes have been a barrier to advancing patient care, including for those with kidney diseases. However, when patients participate in study design, the research is more likely to engage affected populations, build public trust, improve recruitment and retention, and produce patient-centered results (1,2).
CKD attributed to diabetes or hypertension and AKI are common conditions that are poorly understood and lack effective diagnostics and treatments to reduce serious morbidity and mortality (3,4). The Kidney Precision Medicine Project (KPMP) is a unique initiative that is obtaining research protocol–driven kidney biopsies from patients with diabetic or hypertensive CKD and hospitalized patients with AKI (5). Using the kidney tissue from these biopsies, along with other biosamples and clinical data, KPMP aims to produce novel discoveries by advanced molecular, imaging, and data analytics to deliver (1) a new kidney atlas to identify and define previously unknown cell types; (2) disease phenotypes on the basis of patient characteristics, clinical outcomes, and molecular features; and (3) identification of cells, pathways, and targets for diagnostics and treatments.
Patients have been absent in priority setting, design, conduct, and dissemination of findings from prior studies of the biologic basis for kidney diseases. To overcome this omission and optimize relevance, a major goal of KPMP, from inception, has been inclusion of patients as equal partners to guide scientific discovery toward clinically meaningful benefit. The purpose of this report is to describe these approaches and the value they bring to KPMP.
Partnerships with Patients
When the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health began planning KPMP, patients were included in pivotal roles leading to the creation of the funding opportunity. This objective was fully encouraged and supported from the outset by NIDDK. The first public event held in Bethesda, Maryland, on May 23–25, 2016 that was tied to the launch of KPMP featured a panel of patients with kidney diseases. They included leaders of stakeholder organizations who discussed their own experiences with kidney disease, biopsies, risk tolerance, and acceptance. Patient insights were helpful in identifying the likely concerns patients would have before agreeing to participate in KPMP kidney biopsies for scientific discovery. One (Richard Knight) of the patients served on the NIDDK Advisory Council and reviewed the KPMP grant applications with a focus on patient engagement plans. He also gave a presentation to the NIDDK Advisory Council on fundamentals of patient engagement. Another patient was selected to serve on the External Expert Evaluation Panel. This action ensured that a 360° patient viewpoint informs the ongoing oversight and operations at every level and throughout the life cycle of KPMP. Patient leaders who participated in the public unveiling of KPMP continue to play leadership roles in the Community Engagement Committee (CEC) and the Expert Evaluation Panel to inform the priorities and scope of KPMP.
After the research proposals were selected, patient partners were added to the CEC from recruitment sites, tissue interrogation sites, and the Central Hub (administrative unit). Their demographic composition has been intentionally diverse to reflect diabetic or hypertensive CKD and AKI populations, 40% (six of 15) Black, and 47% (seven of 15) women, with ages from young adults to seniors. They have a wide range of kidney disease experiences, including CKD, AKI, and progression to kidney failure. Most have undergone a kidney biopsy, dialysis, and/or kidney transplant. Patient input has been facilitated by open dialogue in all KPMP committee and consortium-wide meetings. Encouragement of patients to share their kidney disease stories has been motivating to the researchers and helped to set research goals. They have evaluated scientific objectives, including kidney tissue handling, interpretation of molecular data, and research effect.
Composition of the Community Engagement Committee
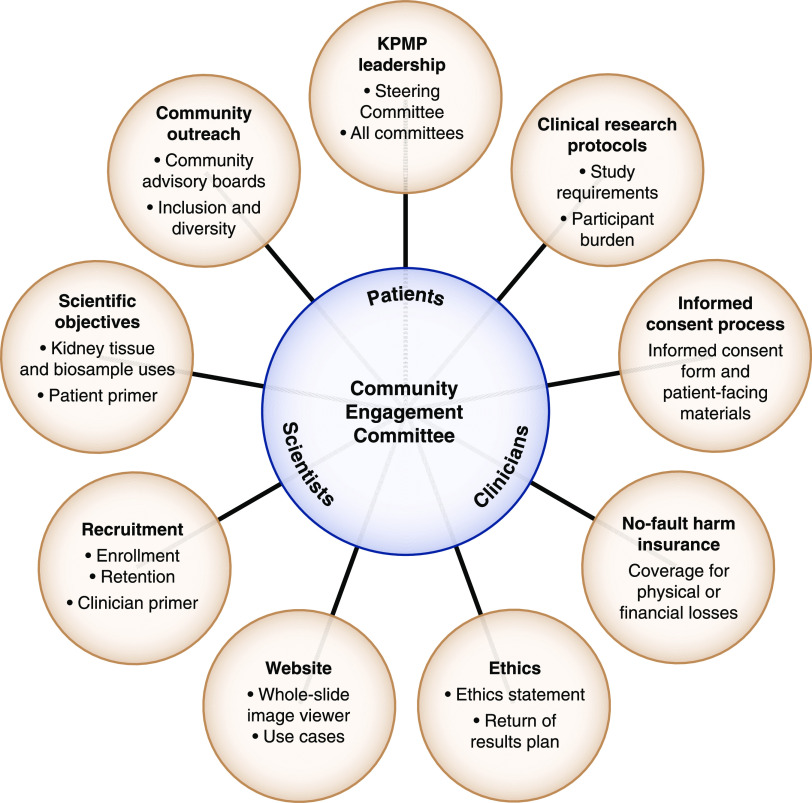
The CEC is composed of patient partners and clinicians who treat CKD and AKI. CEC members have been active across the KPMP committees and work groups (Figure 1). A patient (R.K.) cochairs the CEC along with an investigator (K.R.T.). In addition to patients, clinician members were referred from the KPMP recruitment sites and the Central Hub. They are from different disciplines (e.g., nursing and medicine) and specialties (primary care, endocrinology, intensivists, ethics, and nephrology). These clinicians have contributed to strategies for recruitment and retention as well as to educational approaches for KPMP participants and clinicians.
Figure 1.
Patient representation on Kidney Precision Medicine Project (KPMP) committees. CEC, Community Engagement Committee; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; PI, principal investigators.
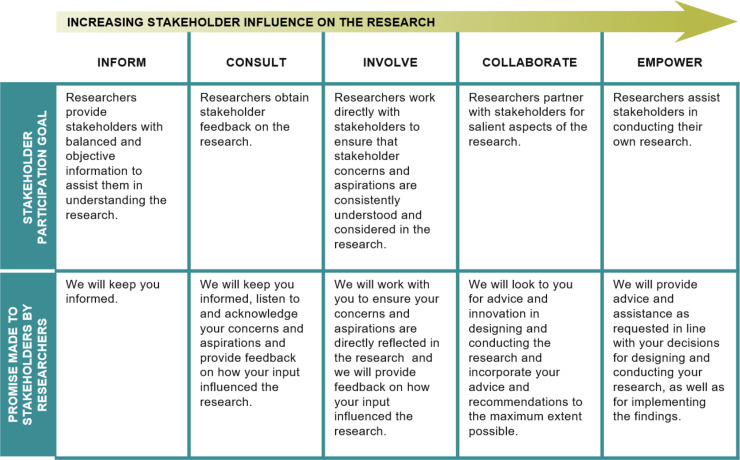
Prior to convening the first in-person KPMP meeting, two teleconferences were held to introduce members, provide educational and meeting materials, and discuss goals and expectations. Foundational principles of community engagement were emphasized (Figure 2): (1) primacy of the patient and patient circle (family, support system), (2) equal partnerships, (3) patient safety, and (4) ethical conduct of research.
Figure 2.
Stakeholder influence by degree of involvement in research. Reprinted from ref. 6, with permission.
Contributions from the Community Engagement Committee
The role of the CEC in KPMP follows the principles of the research-modified International Association for Public Participation spectrum (Figure 2) (6). Patients are integrated with clinicians and scientists across key KPMP activities through the CEC (Figure 3). Patients participate in policy decisions and lead meeting sessions. They bring patient insight along with their professional skills in health care, law, education, and business. The CEC has devised strategies for study recruitment, enrollment, and retention. It has been a primary committee for vetting ethical aspects of KPMP, including the protocol kidney biopsy, no-fault harm insurance, use of kidney tissue and biosamples for ‘omics (genomics, proteomics, metabolomics, microbiomics) research, and return of results. The clinical study protocol for KPMP was developed in concert with the CEC and the Clinical Protocol Committee. Patients provided input into study requirements and the appropriate level of research participation in KPMP. They have advised regarding the KPMP website and tools. A patient (G.R.) assisted with developing the whole-slide image viewer and conducted patient interviews for the kidney atlas.
Figure 3.
Integration of patients, clinicians, and scientists across Kidney Precision Medicine Project activities through the Commmunity Engagement Committee.
Patient and Clinician Primers
A central contribution from the CEC was the “Patient Primer” to provide education about patients as research partners (Table 1). The primer describes experiences of living with kidney diseases and guiding principles for incorporating patient priorities into research. It also addresses questions that scientists often have about patient engagement. The Patient Primer is available on the KPMP website (https://drive.google.com/file/d/1xVfkoLD5TqdGteIpXcIsZdZVmF_bDDep/view) to inform scientists and the public about patient partners in research. The CEC also contributed a “Clinician Primer” that outlines the overall structure of the KPMP consortium and clarifies questions that clinicians may have (Table 1). The Clinician Primer is available on the KPMP website (https://drive.google.com/file/d/1ZLB8SJTrY8zdETIW3SfHCrUCaZhCeWVm/) to inform the clinical community about KPMP.
Table 1.
Contributions from the Community Engagement Committee to the Kidney Precision Medicine Project
| Community Engagement Activities | Contribution | Users or Target Audience |
|---|---|---|
| 1 | Patient primer | Scientists |
| 2 | Clinician primer | Clinicians |
| 3 | Informed consent form | CKD recruitment sites |
| AKI recruitment sites | ||
| 4 | Patient-facing materials | Public |
| Videos | CKD recruitment sites | |
| Brochures | AKI recruitment sites | |
| Posters | ||
| 5 | No-fault harm insurance coverage | Study participants |
| CKD recruitment sites | ||
| AKI recruitment sites | ||
| 6 | Ethics statement | Public |
| Scientists | ||
| Clinicians | ||
| Study participants | ||
| CKD recruitment sites | ||
| AKI recruitment sites | ||
| 7 | Return of results | Clinicians |
| Study participants | ||
| CKD recruitment sites | ||
| AKI recruitment sites | ||
| 8 | Community advisory boards | Local communities |
| CKD recruitment sites | ||
| AKI recruitment sites |
Informed Consent Form and Patient-Facing Materials
The CEC led the development of the informed consent process. KPMP has a unique design whereby study participants assume risks of a kidney biopsy that may not provide clinical benefit, with a primary purpose of advancing scientific knowledge. Patient engagement in the CEC was critical in framing this choice transparently and avoiding therapeutic misconception (7,8). The CEC developed the initial patient-centric draft of the informed consent forms (ICFs) in a manner that satisfied research regulations and policies while communicating clearly. To enhance readability, Sage Bionetworks revised the ICFs into a format that increased ease of understanding. Three versions of the ICF were produced to tailor language to participants with CKD, those with AKI, and participants with AKI undergoing a surgical kidney biopsy (Table 1) (https://kpmp.org/researcher-resources/). The ICFs discuss the risks of KPMP primarily in the context of anticipated benefits to future patients. Other benefits, including the possibility of generating new information that may inform a participant’s clinical care or life choices, were shared with suitable caution. The ICFs were finalized in English and Spanish versions, with iterative input from the single institutional review board at Washington University, KPMP site investigators and staff, the Central Hub, and the CEC.
KPMP videos and print materials were developed to align with the ICFs (Table 1). Patient members of the CEC were featured in informational videos to explain their experiences and viewpoints on research. Another video provided an overview of KPMP and the importance of diverse individuals and communities participating. Print materials, including flyers and posters, for recruitment were mapped to the ICFs for a streamlined introduction to the study. Research coordinators from the recruitment sites provided input into the patient-facing materials. They ensured that the materials were helpful for communication with participants and families and that they covered common queries and concerns. Patient members of the CEC assisted with training the research coordinators by participation in mock interviews for informed consent. A “Frequently Asked Questions” document was generated with input from the research coordinators who conduct the KPMP informed consent process (Supplemental Material).
No-Fault Harm Insurance Coverage
US federal regulations governing research with human participants, the “Common Rule,” require that the benefits of research warrant the risks and that risks are minimized. Kidney biopsies may be complicated by adverse events, particularly bleeding, which require additional interventions. Clinical research studies in the United States usually do not offer insurance protection to individuals who suffer losses due to study participation. Instead, such risks are managed by minimizing the chance of complications. Study budgets do not have funds to compensate participants for these costs. One of the patient CEC members (K.B.) had a key role in making clear the need for insurance protection for KPMP participants. He strongly voiced the belief that such losses should not be the responsibility of a study participant.
The CEC catalyzed a process with the Central Hub, recruitment sites, insurance underwriters and brokers, lawyers, and professional and advocacy organizations to purchase “no-fault” harm insurance to provide compensation for participants who suffer injury or financial loss from KPMP participation in the absence of medical negligence (Table 1). Therefore, the financial risks a participant may experience have been considerably mitigated.
Ethics Statement and Return of Results Plan
To ensure that the highest ethical standards are met in KPMP, the CEC paid careful attention to the balance of risks and benefits for conducting kidney biopsies. The CEC authored an ethics statement to codify the guiding principles for the ethical conduct of KPMP (Table 1, Supplemental Material). The CEC has concentrated on ethics and has provided specific guidance to tissue interrogation sites on use of kidney tissue and other biosamples given by altruistic donors for research. The CEC emphasized the ethical mandate for rigorous processes and procedures that maintain sample integrity to ensure optimal use across technologies and the critical importance of avoiding sample loss or wastage. Additionally, the CEC has driven protections, safety monitoring, and procedures for maintaining participant confidentiality. These efforts satisfy the seven ethical criteria set forth by the Common Rule for institutional review board–approved conduct of research in human participants (9).
The CEC has worked with the Return of Results Committee on the plan for returning results to study participants (Table 1). The CEC has emphasized timely return of research results. Some of the KPMP kidney biopsies will be done exclusively for research, whereas others may be clinically indicated with extra tissue obtained for research. For either, a clinical pathologist at the recruitment site will provide an interpretation of the kidney biopsy to the participant’s clinician to be shared with the participant. A template cover letter in lay language to return the kidney biopsy results has been developed. Results obtained in a Clinical Laboratory Improvement Amendments–certified laboratory will be communicated similarly. An analogous approach is envisioned for other actionable information discovered through research tests in the future.
Community Advisory Boards
For KPMP science to be relevant and translatable, recruitment sites must build trusting relationships with communities extensively affected by diabetic or hypertensive CKD and AKI. These are often communities of color, where participant recruitment for research has been challenging. Although trust is important for all research relationships, precision medicine raises distinct challenges because the implications and future directions of the research are unknown (Table 2) (10). To address these concerns, the CEC led in the creation of Community Advisory Boards (CABs). The Central Hub and the CEC assisted recruitment sites in establishing structure and content and identifying CAB members (Table 1).
Table 2.
Themes affecting community trust in precision medicine research (10)
| Themes |
|---|
| 1. Patient expectations for research benefits vary on the basis of racial/ethnic background |
| 2. Historical discrimination in health care and research creates distrust across minority groups |
| 3. Challenges with navigating the health care system influence trust in research, particularly among recent immigrant groups |
| 4. Patients fear inappropriate data use |
| 5. Consent is necessary but insufficient |
| 6. Institutional trustworthiness requires robust oversight |
CABs enlist patients and leaders with strong connections to the community to build trust in KPMP. They intentionally reflect the local population by their demographic diversity in sex, age, race, and ethnicity. CAB members are a voice for insight into their community’s history, culture, norms, and values. They address ethics and offer advice on outreach. Because of different scenarios for participant involvement by KPMP recruitment sites, they address recruitment and retention at CKD sites, whereas their role at the AKI sites is more focused on retention and sustaining long-term engagement. CAB members share their views about research, as well as review and disseminate information about KPMP to their community. After the CAB members were established and trained, the ongoing management of CABs transitioned to the recruitment sites with ready availability of the CEC for advice and direction.
Discussion
Patients demonstrate leadership in all aspects of KPMP. Investigators in the past had difficulty recognizing how patients could contribute to scientific discovery or clinical translation. Qualitative studies of patient perspectives on research in kidney diseases found a lack of inclusion in all stages and emphasized that as the ultimate end users, patients’ needs and expectations should drive research priorities (11,12). Recognizing the importance of science to solve problems important to patients, the National Health Council and the Patient Centered Outcomes Research Initiative undertook major efforts to include patient voices (13,14).
Participation by patients in traditional nephrology research has been unusual. Several groups led the way in clinical research by developing core outcomes (15), harmonized metrics (16), and study designs and priorities informed by patient preferences (17–20). Recent NIDDK-sponsored studies, KPMP and the APOL1 Long-Term Kidney Transplantation Outcomes project, have included patients in development of discovery science, such as genomics and deep phenotyping by ‘omics panels (21). KPMP is distinguished by involving patients with clinicians and scientists in a full arc of stakeholder involvement in research. All are critical to major KPMP initiatives, from the kidney atlas to raising patients’ scientific literacy and understanding of treatment advances. This integrated approach is designed to increase public trust in federally funded research and health care initiatives. KPMP demonstrates that patients can be included at all research stages and that their inclusion increases relevance and opportunities for dissemination and implementation of scientific advances into clinical practice (22,23).
The willingness of research participants to accept risks is balanced against potential benefits. In fields such as cancer prevention and control, participation in research frequently offers hope for patients and families when standard treatments are exhausted. With limited treatment options and a disease viewed as often fatal, patients and families tolerate a high level of risk to volunteer for studies (2,24). In chronic diseases, such as CKD, where immediate benefit is less obvious, study recruitment can be more challenging. In KPMP, participants are asked to undergo a kidney biopsy that may not provide direct benefit to them. Patients, clinicians, and ethicists expressed concerns about risks of participating in KPMP, but they also recognized that kidney biopsies with novel tissue interrogation offer the best possibility for developing better treatments. KPMP found that patients understand and support a study that advances generalizable knowledge from which new discoveries will improve future care, even though immediate benefits may be limited and despite the risks. In a first of kind effort to mitigate risks, in this case from a kidney biopsy, the patient-led CEC and KPMP leadership developed an insurance plan for physical or financial losses resulting from study participation. To our knowledge, KPMP is the first National Institutes of Health–sponsored study to ever insure participants against no-fault harm in the United States. Insurance coverage and the carefully vetted study protocol address risks and benefits, fulfilling the spirit and letter of the Common Rule and the KPMP ethics statement.
Inclusion of clinicians in the CEC also brings perspectives that enhance research relevance for KPMP. For example, clinicians who manage diabetes find that patients are routinely diagnosed with diabetic CKD, despite the fact that one-third to one-half of patients with type 2 diabetes have nondiabetic CKD (25,26). This misconception may result in failure to address other causes of kidney disease. AKI is usually a problem of hospitalized patients and is found in more than half of critically ill patients. AKI frequently complicates emergency surgical procedures (27,28). Nephrologists are often consulted to manage AKI in the hospital (28,29). Clinician-patient engagement is a key aspect of participant recruitment and retention, and peer-to-peer education among clinicians is essential to convey KPMP findings (30). The clinician partnership in the CEC will advance knowledge translation to improve patient care.
The CEC has strengths and limitations. Equity, diversity, and inclusion have permeated KPMP, with a focus on using science to solve problems important to patients. However, effect has not yet been assessed. We will assess barriers and pathways for informed consent, recruitment and retention, utility of no-fault harm insurance, return of results, and CAB activities. KPMP participants are also asked to complete a survey on their experience, with an emphasis on the study information provided, informed consent, and participation requirements. Additional patient perspectives will be obtained through rotating membership on the CEC and CABs. The CEC tracks national policies on return of results and will update procedures accordingly with expertise from ethicists (P.S.A. and D.E.H.) and the Return of Results Committee (S.E.R.). Patient inclusion on research teams could increase cost and time. However, expenses for patient participation (travel, time and effort, patient-facing materials, CAB support) were prescribed by the NIDDK funding mechanism and have not led to study delays or budgetary issues.
In conclusion, patients have guided KPMP to produce research aligned with their priorities. They have led in the informed consent process, no-fault harm insurance coverage, ethics statement, return of results plan, and community-based participation. The CEC partnership has set new benchmarks for patient leadership in precision medicine research.
Disclosures
S. Bansal reports receiving author royalties from UpToDate, other from Home Therapy Institute, and other from Osprey Medical, outside the submitted work. As a KPMP patient partner, K. Brown receives reimbursement for his travel to in-person meetings. There are typically two in-person meetings per year. He also receives a consulting fee of $200 per meeting day for these in-person meetings. As a KPMP patient partner, C. Campbell receives reimbursement for her travel to in-person meetings. There are typically two in-person meetings per year. She also receives a consulting fee of $200 per meeting day for these in-person meetings. I. de Boer reports receiving grants from the American Diabetes Association, Juvenile Diabetes Research Foundation, the National Heart, Lung, and Blood Institute, and NIDDK; consulting fees from Boehringer Ingelheim, Cyclerion Therapeutics, George Clinical, Goldfinch Bio, and Ironwood; and research equipment and supplies from Abbott and Medtronic, outside the submitted work. K. Mehl reports receiving grants from Columbia University during the conduct of the study. R. Murugan reports receiving grants and personal fees from LaJolla, Inc., grants from Bioporto, and grants from NIDDK during the conduct of the study. He also reports receiving personal fees from AM Pharma and Beckman Coulter, outside the submitted work. G. Roberts reports receiving grants from the Center for Dialysis Innovation; travel and living expenses from the American Society of Nephrology, APOL1 CAB, APOL1 Delphi, the International Society of Nephrology, and the National Kidney Foundation; and honoraria from the APOL1 Long-term Kidney Transplantation Outcomes Network (NIDDK) and KPMP, outside the submitted work. S. Rosas attended one scientific advisory board each for Bayer and Reata in 2019, for which she was compensated. K. Tuttle reports receiving personal fees as a consultant for diabetes and CKD from Astra Zeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, and Novo Nordisk and grants and personal fees from Goldfinch Bio, outside the submitted work. All remaining authors have nothing to disclose.
Funding
The KPMP is funded by National Institute of Diabetes and Digestive and Kidney Diseases grants U2C DK114886, UH3DK114861, UH3DK114866, UH3DK114870, UH3DK114907, UH3DK114908, UH3DK114915, UH3DK114920, UH3DK114923, UH3DK114926, UH3DK114933, and UH3DK114937.
Supplementary Material
Acknowledgments
The authors and the entire CEC acknowledge the resolute support and leadership provided by the NIDDK program officials and staff, the Data Safety Monitoring Board, and the External Expert Panel for KPMP. In particular, we recognize pivotal contributions of Mr. Paul Conway, patient member of the External Expert Panel, and Ms. Toya Evans, consultant to KPMP, for CAB development and implementation.
The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the National Institutes of Health. Because Dr. Ian H. de Boer is a Deputy Editor of CJASN, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Contributor Information
Collaborators: Kidney Precision Medicine Project, Richard Knight, Stewart Lecker, Isaac Stillman, Sushrut Waikar, Mia Colona, Zoe Kibblear, Gearoid Mcmahon, Astrid Weins, Nir Hacohen, Paul Hoover, Mark Aulisio, Leslie Cooperman, Leal Herlitz, John O’Toole, Emilio Poggio, John Sedor, Paul Appelbaum, Jonathan Barasch, Olivia Balderes, Andrew Bomback, Vivette D’agati, Krzysztof Kiryluk, Karla Mehl, Ning (Sunny) Shang, Chenhua Weng, Laura Barisoni, Theodore Alexandrov, Tarek Ashkar, Daria Barwinska, Pierre Dagher, Kenneth Dunn, Michael Eadon, Michael Ferkowicz, Katherine Kelly, Timothy Sutton, Seth Winfree, Steven Menez, Chirag Parikh, Avi Rosenberg, Pam Villalobos, Alison Slack, Sylvia Rosas, Mark Williams, Evren Azeloglu, Jens Hansen, Cijang (John) He, Ravi Iyengar, Samir Parikh, Brad Rovin, Chris Anderton, Ljiljana Pasa-Tolic, Dusan Velickovic, George (Holt) Oliver, Joseph Ardayfio, Jack Bebiak, Keith Brown, Taneisha Campbell, Catherine Campbell, Lynda Hayashi, Nichole Jefferson, Robert Koewler, Glenda Roberts, John Saul, Anna Shpigel, Edith Christine Stutzke, Lorenda Wright, Leslie Miegs, Rachel Sealfon, Olga Troyanskaya, Katherine Tuttle, Blue Lake, Kun Zhang, Maria Joanes, Zoltan Laszik, Ulysses Balis, Oliver He, Jeffrey Hodgin, Matthias Kretzler, Laura Mariani, Rajasree Menon, Edgar Otto, Jennifer Schaub, Becky Steck, Michele Elder, Daniel Hall, John Kellum, Raghav Murugan, Paul Palevsky, Parmjeet Randhawa, Matthew Rosengart, Sunny Sims-Lucas, Mary Stefanick, Mitchell Tublin, Charles Alpers, Ian de Boer, Malia Fullerton, Jonathan Himmelfarb, Robyn Mcclelland, Sean Mooney, Stuart Shankland, Kayleen Williams, Kristina Blank, Ashveena Dighe, Jonas Carson, Frederick Dowd, Shweta Bansal, Kumar Sharma, Guanshi Zhang, Asra Kermani, Simon Lee, Tyler Miller, Orson Moe, Harold Park, Francisco Sanchez, Jose Torrealba, Robert Toto, Miguel Vazquez, Nancy Wang, Joe Gaut, Sanjay Jain, Anitha Vijayan, Tanima Arora, Dennis Moledina, Ugwuowo Ugochukwu, and Francis Perry Wilson
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10270620/-/DCSupplemental.
Supplemental Material. Kidney Precision Medicine Project ethics statement and frequently asked questions.
References
- 1.Ciemins EL, Mollis BL, Brant JM, Hassell LA, Albritton S, Amoroso P, Lloyd A, Smith JM, Pflugeisen BM, Tuttle KR, Baldwin LM: Clinician engagement in research as a path toward the learning health system: A regional survey across the northwestern United States. Health Serv Manage Res 33: 33–42, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramanadhan S, Davis MM, Armstrong R, Baquero B, Ko LK, Leng JC, Salloum RG, Vaughn NA, Brownson RC: Participatory implementation science to increase the impact of evidence-based cancer prevention and control. Cancer Causes Control 29: 363–369, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webster AC, Nagler EV, Morton RL, Masson P: Chronic kidney disease. Lancet 389: 1238–1252, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Moledina DG, Parikh CR: Phenotyping of acute kidney injury: Beyond serum creatinine. Semin Nephrol 38: 3–11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer IH, Alpers CE, El-Achkar TM, Azeloglu E, Balis UGJ, Barasch JM, Barisoni L, Blank K, Bomback AS, Brown K, Dagher PC, Dighe AL, Eadon MT, Gaut JP, Hacohen N, He Y, Hodgin JB, Jain S, Kellum JA, Kiryluk K, Knight R, Laszik ZG, Lienczewski C, Mariani LH, Mcclelland RL, Menez S, Moledina D, Mooney SD, O'Toole J, Palevsky PM, Parikh CR, Poggio E, Rosas S, Rosengart MR, Sarwal M, Schaub JA, Sedor JR, Sharma K, Steck B, Toto R, Troyanskaya O, Tuttle K, Vazquez M, Waikar SS, Williams K, Wilson FP, Zhang K, Iyengar SR, Kretzler M, and Himmelfarb J, for the Kidney Precision Medicine Project : Rationale and design of the Kidney Precision Medicine Project. Kidney Int 99: 498–510, 2021 [DOI] [PMC free article] [PubMed]
- 6.Bammer G: Key Issues in co-creation with stakeholders when research problems are complex. Evid Policy 15: 423–435, 2019 [Google Scholar]
- 7.Goldberg DS: Eschewing definitions of the therapeutic misconception: A family resemblance analysis. J Med Philos 36: 296–320, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Sugarman J, Lavori PW, Boeger M, Cain C, Edsond R, Morrison V, Yeh SS: Evaluating the quality of informed consent. Clin Trials 2: 34–41, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Lidz CW, Appelbaum PS, Arnold R, Candilis P, Gardner W, Myers S, Simon L: How closely do institutional review boards follow the common rule? Acad Med 87: 969–974, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraft SA, Cho MK, Gillespie K, Halley M, Varsava N, Ormond KE, Luft HS, Wilfond BS, Soo-Jin Lee S: Beyond consent: Building trusting relationships with diverse populations in precision medicine research. Am J Bioeth 18: 3–20, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindenmeyer A, Hearnshaw H, Sturt J, Ormerod R, Aitchison G: Assessment of the benefits of user involvement in health research from the Warwick Diabetes Care Research User Group: A qualitative case study. Health Expect 10: 268–277, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nierse CJ, Schipper K, van Zadelhoff E, van de Griendt J, Abma TA: Collaboration and co-ownership in research: Dynamics and dialogues between patient research partners and professional researchers in a research team. Health Expect 15: 242–254, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Health Council : National Health Council rubric to capture the patient voice: A guide to incorporating the patient voice into the heath ecosystem, 2016. Available at: https://nationalhealthcouncil.org/patient-engagement-rubric/. Accessed April 16, 2020
- 14.Fleurence R, Selby JV, Odom-Walker K, Hunt G, Meltzer D, Slutsky JR, Yancy C: How the Patient-Centered Outcomes Research Institute is engaging patients and others in shaping its research agenda. Health Aff (Millwood) 32: 393–400, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Tong A, Craig JC, Nagler EV, Van Biesen W; SONG Executive Committee and the European Renal Best Practice Advisory Board; SONG Executive Committee and the European Renal Best Practice Advisory Board: Composing a new song for trials: The Standardized Outcomes in Nephrology (SONG) initiative. Nephrol Dial Transplant 32: 1963–1966, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Verberne WR, Das-Gupta Z, Allegretti AS, Bart HAJ, van Biesen W, García-García G, Gibbons E, Parra E, Hemmelder MH, Jager KJ, Ketteler M, Roberts C, Al Rohani M, Salt MJ, Stopper A, Terkivatan T, Tuttle KR, Yang CW, Wheeler DC, Bos WJW: Development of an international standard set of value-based outcome measures for patients with chronic kidney disease: A report of the International Consortium for Health Outcomes Measurement (ICHOM) CKD working group. Am J Kidney Dis 73: 372–384, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Cukor D, Cohen LM, Cope EL, Ghahramani N, Hedayati SS, Hynes DM, Shah VO, Tentori F, Unruh M, Bobelu J, Cohen S, Dember LM, Faber T, Fischer MJ, Gallardo R, Germain MJ, Ghahate D, Grote N, Hartwell L, Heagerty P, Kimmel PL, Kutner N, Lawson S, Marr L, Nelson RG, Porter AC, Sandy P, Struminger BB, Subramanian L, Weisbord S, Young B, Mehrotra R: Patient and other stakeholder engagement in Patient-Centered Outcomes Research Institute funded studies of patients with kidney diseases. Clin J Am Soc Nephrol 11: 1703–1712, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin A, Adams E, Barrett BJ, Beanlands H, Burns KD, Chiu HH, Chong K, Dart A, Ferera J, Fernandez N, Fowler E, Garg AX, Gilbert R, Harris H, Harvey R, Hemmelgarn B, James M, Johnson J, Kappel J, Komenda P, McCormick M, McIntyre C, Mahmud F, Pei Y, Pollock G, Reich H, Rosenblum ND, Scholey J, Sochett E, Tang M, Tangri N, Tonelli M, Turner C, Walsh M, Woods C, Manns B: Canadians seeking solutions and innovations to overcome chronic kidney disease (Can-SOLVE CKD): Form and function. Can J Kidney Health Dis 5: 2054358117749530, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flythe JE, Hilliard T, Lumby E, Castillo G, Orazi J, Abdel-Rahman EM, Pai AB, Rivara MB, St Peter WL, Weisbord SD, Wilkie CM, Mehrotra R; Kidney Health Initiative Prioritizing Symptoms of ESRD Patients for Developing Therapeutic Interventions Stakeholder Meeting Participants: Fostering innovation in symptom management among hemodialysis patients: Paths forward for insomnia, muscle cramps, and fatigue. Clin J Am Soc Nephrol 14: 150–160, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidney X: Patient Innovator Challenge, 2019. Available at: https://www.kidneyx.org/prizecompetitions/PatientInnovatorChallenge. Accessed April 16, 2020
- 21.Freedman BI, Moxey-Mims MM, Alexander AA, Astor BC, Birdwell KA, Bowden DW, Bowen G, Bromberg J, Craven TE, Dadhania DM, Divers J, Doshi MD, Eidbo E, Fornoni A, Gautreaux MD, Gbadegesin RA, Gee PO, Guerra G, Hsu CY, Iltis AS, Jefferson N, Julian BA, Klassen DK, Koty PP, Langefeld CD, Lentine KL, Ma L, Mannon RB, Menon MC, Mohan S, Moore JB, Murphy B, Newell KA, Odim J, Ortigosa-Goggins M, Palmer ND, Park M, Parsa A, Pastan SO, Poggio ED, Rajapakse N, Reeves-Daniel AM, Rosas SE, Russell LP, Sawinski D, Smith SC, Spainhour M, Stratta RJ, Weir MR, Reboussin DM, Kimmel PL, Brennan DC: APOL1 long-term kidney transplantation outcomes network (APOLLO): Design and rationale. Kidney Int Rep 5: 278–288, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson L: How we get to the engaged patient. Applied Clinical Trials, 2017. Available at: https://www.appliedclinicaltrialsonline.com/view/how-we-get-engaged-patient. Accessed April 16, 2020
- 23.Coleman E: Through the patient perspective: Collaborating to improve research and development. Applied Clinical Trials, 2017. Available at: https://www.appliedclinicaltrialsonline.com/view/through-patient-perspective-collaborating-improve-research-and-development. Accessed April 16, 2020
- 24.Selig S: Hope for a rare disease through TCGA: My perspective as a caregiver, patient advocate, and physician. National Cancer Institute, Center for Cancer Genomics, Updates and Insights Blog, 2017. Available at: www.cancer.gov/about-nci/organization/ccg/blog/2017/cure-om. Accessed May 26, 2020
- 25.Alicic RZ, Rooney MT, Tuttle KR: Diabetic kidney disease: Challenges, progress and possibilities. Clin J Am Soc Nephrol 12: 2032–2045, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anders HJ, Huber TB, Isermann B, Schiffer M: CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol 14: 361–377, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA: Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015 [DOI] [PubMed] [Google Scholar]
- 28.O’Connor ME, Kirwan CJ, Pearse RM, Prowle JR: Incidence and associations of acute kidney injury after major abdominal surgery. Intensive Care Med 42: 521–530, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Balasubramanian G, Al-Aly Z, Moiz A, Rauchman M, Zhang Z, Gopalakrishnan R, Balasubramanian S, El-Achkar TM: Early nephrologist involvement in hospital-acquired acute kidney injury: A pilot study. Am J Kidney Dis 57: 228–234, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Kimmel PL, Jefferson N, Norton JM, Star RA: How community engagement is enhancing NIDDK research. Clin J Am Soc Nephrol 14: 768–770, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.