Patients on in-center maintenance hemodialysis (HD) are at potentially high risk of acquiring severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (coronavirus disease 2019 [COVID-19]) inside the HD unit, due to social contacts during the frequent HD sessions. Data concerning the dynamics of anti–SARS-CoV-2 antibodies in patients on HD are scarce.
We prospectively studied the onset and evolution over 3 months of anti–SARS-CoV-2 antibodies in a cohort of adult patients on in-center maintenance HD.
We included all patients on in-center maintenance HD at Cliniques Universitaires Saint-Luc, Brussels, Belgium. On March 19th (day 0), a 77-year-old man presented with cough and fever (patient 1) and had a positive real-time reverse transcription polymerase chain reaction (RT-qPCR) for SARS-CoV-2 (1) on a nasopharyngeal swab. Since then, the use of surgical face masks by all staff members and all patients on HD has become mandatory, as long as they are in the facility. Between March 19th and April 6th, seven additional symptomatic patients (patients 2–8) were diagnosed with COVID-19 by RT-qPCR. On April 6th and 7th, all asymptomatic patients (n=90) were screened by RT-qPCR, and two (patients 9 and 10) were found to be positive.
Serum samples from all patients were tested first by a total anti–SARS-CoV-2 antibodies qualitative test (sensitivity 100% [≥14 days post-PCR confirmation], specificity 99.8%, no crossreaction with other human endemic coronaviruses) using an electrochemiluminescent immunoassay (Roche Diagnostics). Positive samples were tested by a quantitative chemiluminescent test for both specific IgM (sensitivity 78.7%, specificity 97.5%) and IgG (sensitivity 91.2%, specificity 97.3%) binding SARS-CoV-2 spike and nucleocapsid proteins (cutoff value 1.0 AU/ml; Snibe Diagnostic). Samples were drawn approximately monthly (i.e., on days 19–20, 47–48, 75–76, and 110–111 [T1, T2, T3, and T4, respectively]) after RT-qPCR diagnosis of patient 1.
IgG titers were compared across time points using mixed effect models. Depending on the number of days after symptom onset, each blood sample was attributed to a time point (T1, T2, T3, and T4). The date of RT-qPCR screening was considered as illness onset in both asymptomatic patients.
Ninety-eight patients (aged 68.8 [±14] years, 58% men, 47% patients with diabetes) were included. Often patients who were RT-qPCR positive (none with comorbidities suggestive of severe immunocompromised status such as HIV, malignancy, chemotherapy, or immunosuppressive treatment), four required hospitalization, and two died. Eight patients were alive 164–183 days after a SARS-CoV-2–positive RT-qPCR.
Patient 1 did not have detectable antibodies at T1 (i.e., 20 days after symptom onset), and he died before T2. Patient 7 died within 3 days of positive RT-qPCR. The other eight patients who were RT-qPCR positive seroconverted. IgM levels were slightly positive and became negative 1 month after seroconversion. IgG titers varied widely across subjects (1.7–200 AU/ml). Seroconversion was documented 12–34 days after symptom onset (mean 20±9 days).
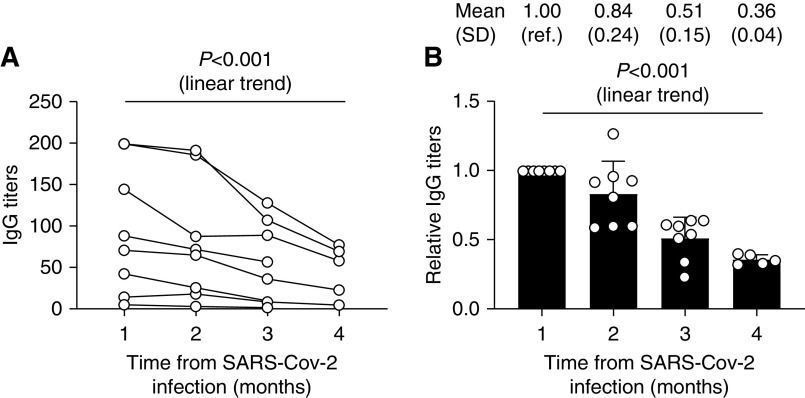
No patient became seronegative during follow-up (mean 96±20 days). Overall, anti–SARS-CoV-2 IgG was still detected 90–128 days after symptom onset. However, IgG levels declined in all patients (mixed effect analysis: P=0.007), with a linear trend (slope −24.8; 95% confidence interval, −15.9 to −33.7; P<0.001) (Figure 1). IgG levels decreased during the study by 62.4%±0.12% (median 65.6% [35.8%–76.8%]), with a linear trend (slope −0.23; 95% confidence interval, −0.18 to −0.28; P<0.001). Among the remaining 88 patients negative on screening RT-qPCR, none seroconverted at any of the four tested time points.
Figure 1.
Progressive decrease of anti-SARS-CoV-2 IgG levels over 3 months. Dynamic absolute (A) and relative (B) changes of antisevere acute respiratory syndrome coronavirus 2 (anti–SARS-CoV-2) IgG levels. IgG levels decreased significantly during follow-up. ref., reference.
This is the first study assessing the onset and evolution over a 3-month period of anti–SARS-CoV-2 antibodies in adult patients on maintenance HD. Such patients appear to be able to mount a humoral immune response within a delay similar to that observed in the general population (2). Our results expand those by De Vriese and Reynders (3), who detected seroconversion in all six tested patients on HD in the second week after symptom onset and a plateau in specific IgG titers during the third to fifth weeks after symptom onset. In some (4) but not all (5) general population reports, a significant decay of specific IgG levels was documented during the convalescent phase. In our cohort, IgG response persisted for at least 90 days after symptom onset. However, a significant decrease of titers occurred 1 month after seroconversion (i.e., 28–65 days after symptom onset) and continued during follow-up. If confirmed in larger cohorts, this suggests that cross-sectional studies of seroprevalence in HD units may underestimate the actual rates of prior infections, except in recent months. Additionally, the decline of titers suggests that the efficacy of a vaccine in this specific population may be temporary if the level of antibodies predicts protection. Serologic monitoring may thus be needed to determine the timing of boosters after a vaccine is available.
The strength of this study is the prospective longitudinal design and the fact that RT-qPCR screening was performed in all patients on HD, which allowed for identifying two asymptomatic patients who were RT-qPCR positive. Limitations include a monocentric design and a small sample size.
In conclusion, almost all patients on maintenance HD with COVID-19 developed specific antibodies within the first month after symptom onset. However, a regular titer decrease is observed from 30 days after seroconversion through the following 3 months.
Disclosures
J. De Greef reports consultancy agreements with Menarini. M. Jadoul reports consultancy agreements with Amgen, Astellas, AstraZeneca, Boehringer-Ingelheim, Fresenius, Merck (MSD), Mundipharma, and Vifor Med Care Renal Pharma; receiving research funding from Amgen, Janssen-Cilag, MSD, Otsuka, and Roche; receiving honoraria from Amgen, Astellas, Boehringer-Ingelheim, Fresenius, Menarini, Merck (MSD), and Vifor Med Care Renal Pharma; receiving personal fees from Abbvie and Bayer; receiving nonfinancial support from Sanofi; and speakers bureau for Amgen, AstraZeneca, Fresenius, Menarini, MSD, Mundipharma, and Vifor Med Care Renal Pharma, outside the submitted work (all grants and fees are paid to the institution). M. Jadoul also reports serving as theme editor for Nephrology Dialysis Transplantation and serving as cochair of Kidney Disease Improving Global Outcomes since 2019. L. Labriola reports receiving lecture fees and travel support from Amgen, honoraria from Fresenius for conferences, honoraria from Amgen and Otsuka for ongoing trials, and travel support from Vifor. J. Morelle reports receiving research funding from Alexion Pharmaceuticals and Baxter Healthcare, outside the submitted work; serving as a scientific advisor or member of AstraZeneca and Versantis; lecture fees from Baxter HealthCare and Fresenius Medical Care; receiving funding from the Association pour l’Information et la Recherche sur les Maladies Rénales Génétiques (Brussels, Belgium), the National Fund for Scientific Research (Brussels, Belgium), and the Saint-Luc Foundation (Brussels, Belgium); and receiving a travel grant from Sanofi-Genzyme. All remaining authors have nothing to disclose.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Werion A, Belkhir L, Perrot M, Schmit G, Aydin S, Chen Z, Penaloza A, De Greef J, Yildiz H, Pothen L, Yombi JC, Dewulf J, Scohy A, Gérard L, Wittebole X, Laterre PF, Miller SE, Devuyst O, Jadoul M, Morelle J; Cliniques Universitaires Saint-Luc (CUSL) COVID-19 Research Group: SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int 98: 1296–1307, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long QX, Liu B-Z, Deng H-J, Wu G-C, Deng K, Chen Y-K, Liao P, Qiu JF, Lin Y, Cai XF, Wang DQ, Hu Y, Ren JH, Tang N, Xu YY, Yu LH, Mo Z, Gong F, Zhang XL, Tian WG, Hu L, Zhang XX, Xiang JL, Du HX, Liu HW, Lang CH, Luo XH, Wu SB, Cui XP, Zhou Z, Zhu MM, Wang J, Xue CJ, Li XF, Wang L, Li ZJ, Wang K, Niu CC, Yang QJ, Tang XJ, Zhang Y, Liu XM, Li JJ, Zhang DC, Zhang F, Liu P, Yuan J, Li Q, Hu JL, Chen J, Huang AL: Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26: 845–848, 2020. [DOI] [PubMed] [Google Scholar]
- 3.De Vriese AS, Reynders M: IgG antibody response to SARS-CoV-2 infection and viral RNA persistence in patients on maintenance hemodialysis. Am J Kidney Dis 76: 440–441, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, Hu JL, Xu W, Zhang Y, Lv FJ, Su K, Zhang F, Gong J, Wu B, Liu XM, Li JJ, Qiu JF, Chen J, Huang AL: Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 26: 1200–1204, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, Saemundsdottir J, Sigurdsson A, Sulem P, Agustsdottir AB, Eiriksdottir B, Fridriksdottir R, Gardarsdottir EE, Georgsson G, Gretarsdottir OS, Gudmundsson KR, Gunnarsdottir TR, Gylfason A, Holm H, Jensson BO, Jonasdottir A, Jonsson F, Josefsdottir KS, Kristjansson T, Magnusdottir DN, le Roux L, Sigmundsdottir G, Sveinbjornsson G, Sveinsdottir KE, Sveinsdottir M, Thorarensen EA, Thorbjornsson B, Löve A, Masson G, Jonsdottir I, Möller AD, Gudnason T, Kristinsson KG, Thorsteinsdottir U, Stefansson K: Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med 382: 2302–2315, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]