Abstract
Objective:
The purpose of this study was to quantify the growth of the various craniofacial and velopharyngeal structures and examine sex and race effects.
Methods:
Eight-five healthy children (53 White and 32 Black) with normal velopharyngeal anatomy between 4 and 9 years of age who met the inclusion criteria and successfully completed the MRI scans were included in the study.
Results:
Developmental normative mean values for selected craniometric and velopharyngeal variables by race and sex are reported. Cranial variables (face height, nasion to sella, sella to basion, palate height, palate width) and velopharyngeal variables (levator muscle length, angle of origin, sagittal angle, velar length, velar thickness, velar knee to posterior pharyngeal wall, and posterior nasal spine to levator muscle) demonstrated a trend toward a decrease in angle measures and increase in linear measures as age increased (with the exception of PNS to levator muscle). Only hard palate width and levator muscle length showed a significant sex effect. However, two cranial and six velopharyngeal variables showed a significant race effect. The interactions between sex, race, and age were not statistically significant across all variables, with the exception of posterior nasal spine to posterior pharyngeal wall.
Conclusion:
Findings established a large age and race-specific normative reference for craniometiric and velopharyngeal variables. Data reveal minimal sexual dimorphism variables used in the present study; however, significant racial effects were observed.
Keywords: MRI, velopharyngeal, craniometrics, levator veli palatine, sex differences
Introduction
Investigations of cranial and velopharyngeal structures suggest significant differences based on sex and race, particularly among adults. Adult males demonstrate significantly larger anterior and posterior vertical facial profiles and greater linear values for cephalometric variables compared to adult females (Behrents, 1985; Genecov et al., 1990; Kharbanda et al., 1991; Bishara et al., 1994; Formby et al., 1994; Bishara et al., 1998; Thilander et al., 2005; Pecora et al., 2008). Significant sexual dimorphism of the vocal tract has also been established among adults (Fitch and Giedd, 1999; Simpson, 2001; Xue and Hao, 2006; Xue et al., 2006). Cranial dimensions vary between adults of different race and ethnicity (Yuen et al., 1989; Johannsdottir et al., 2004; Yeong and Huggare, 2004). Adult males use greater velar height, increased velar closure force, and increased velar surface contact against the posterior pharyngeal wall during speech compared to females (McKerns and Bzoch, 1970; Kuehn, 1976; Kuehn and Moon, 1998). Studies have further observed variations between male and female adults for velar movement durations (Kuehn, 1976; Zajac and Mayo, 1996). Using magnetic resonance imaging (MRI), Ettema et al. (2002) observed five adult females to have an overall shorter levator veli palatini (levator) muscle, greater angle of muscle origins (angle created between muscle and base of skull as it converges towards the velum), and a smaller distance between levator muscle origins compared to five adult males. Perry et al. (2016) used similar methods with a larger sample size (N = 89) to demonstrate a significant sex effect for levator muscle measures with adult males demonstrating significantly longer levator muscle length, greater distance between origins, and greater velar insertion distance compared to adult females. Perry et al. (2016) noted that Black adults displayed velopharyngeal measures that placed the velopharyngeal mechanism at a mechanical advantage including a significantly longer and thicker velum compared to White adults.
Investigations of sex and race effects on cranial and velopharyngeal muscles and structures among the child population have been slower to evolve. However, numerous studies related to the vocal tract suggest there are likely differences based on age, sex, and race. Vorperian et al. (2011) demonstrated significant prepubertal sexual dimorphism in the oropharyngeal and nasopharyngeal regions. Other studies have demonstrated no significant difference in the vocal tract between pre-pubertal boys and girls (Roche and Barkla, 1965; Fitch and Giedd, 1999; Vorperian et al., 2005; Vorperian et al., 2009). Lieberman et al. (2001) found sexual dimorphism of the pharyngeal shape and size to be negligible prior to 14 years of age with the exception of oropharyngeal width. The nasopharynx and velum display similar pubertal effects in which sex differences for nasopharyngeal area become significant after 13 years of age (Jeans et al., 1981). Subtelny (1957) also demonstrated no significant difference for velar length and thickness between boys and girls birth to 18 years of age. These studies, however, did not examine the velopharyngeal musculature.
The development of faster MRI sequences (Scott et al., 2012; Fu et al., 2015, 2016; Sagar and Nimkin, 2015) and robust child-friendly protocols (Kollara and Perry, 2014) has enabled investigations of the velopharyngeal variations among child participants. Kollara et al. (2016) used MRI to examine 32 children (16 White and 16 Black, evenly divided for male and female groups) to investigate differences in velopharyngeal structures between Black and White children. Results demonstrated a non-significant race-specific sex effect for velar and levator muscle measures with the exception of velar insertion distance. Significant race effects were observed for velar length, velar thickness, velopharyngeal ratio, and several craniofacial measures (sella to basion, pharyngeal depth, and face height). Specifically, Black children displayed a significantly thicker and longer velum compared to White children. However, these findings are limited by the relatively small sample size and age effects were not reported. It is likely there were significant differences among the children at each age given the relatively large age range of 4 to 8 years of age. Additionally, combining children among this wide age range may have confounded the observations of the sex and race effects.
The purpose of this study was to quantify the growth of the various craniofacial and velopharyngeal structures. By doing so, this study also established a developmental normative database that expands upon the normative values for velar length and thickness provided by Subtelny (1957) to include mean values for additional velopharyngeal and craniofacial structures in the child population from 4 to 9 years of age. Farkas and colleagues (2007) proposed the need for normative child craniometric values that are race-specific as a means to tailor patient-specific craniofacial surgery. Due to significant racial and sex variations in the velopharyngeal anatomy found among adults, Perry et al. (2016) further suggested a patient’s sex and race to be likely variables that should inform velopharyngeal surgical treatment (e.g., cleft palate surgery) and prognosis. Finite element modeling using studies of the velopharyngeal mechanism support these hypotheses of race and sex variations and further highlight the need to examine individual anatomic variations for cleft palate surgical planning (Inouye et al., 2015, 2016). To date, these hypotheses are driven primarily by adult data, however, implications for patient-specific surgery is intended for the child population (e.g., cleft palate craniofacial surgeries).
The second aim of this study was to determine if sex and racial differences in cranial structures, velar measures, and levator muscle measures are present in the child population between 4 to 9 years of age. We hypothesized racial differences among children will exist similarly to the adult study (Perry et al., 2016) for measures of velar length, velar thickness, and cranial measures. We further hypothesized a lack of sexual dimorphism for velar, levator muscle, and cranial measures. Specific study questions are as follows:
Are the expected developmental changes similar in the cranial, facial, and velopharyngeal structures as a function of age?
Are there sex differences in cranial, facial, and velopharyngeal structures, and if so, is there an age effect at which differences are observed?
Are there racial differences in cranial, facial, and velopharyngeal structures, and if so, is there an age effect at which differences are observed?
Method
Participants
In accordance with the Institutional Review Board, we recruited children between the ages of 4 and 9 years of age using flyers placed in the community. To meet the inclusion criteria, children were to display normal velopharyngeal anatomy as determined by a perceptual evaluation and an oral mechanism exam. A speech-language pathologist with over 15 years of experience in resonance assessments conducted perceptual evaluations using a 5-point resonance rating scale at the conversational level and examined structural oral abnormalities through an oral mechanism exam. We excluded individuals with an abnormal perceptual rating (rated as a two through 5 on the 5-point rating scale) and/or oral examination (showing oral abnormalities). Participants with a body mass index of less than 30 were included due to the involvement of fat deposits known to increase velar thickness among obese individuals (Horner et al., 1989), which would confound the normative data. As an inclusionary requirement, parents or caregivers self-reported their child’s racial ancestry and indicated three generations of the same racial origin. Epidemiology studies consider self-report to be the gold standard for racial classification in research studies (Kaufman and Cooper, 2001). Additional inclusion criteria included negative history of reported swallowing, neurological, hearing, craniofacial, or musculoskeletal disorders.
Eighty-five children met the inclusion criteria and enrolled in the study. Child participants were between 4 and 9 years of age (mean = 6. 7 years, SD = 1.7 years) and distributed across two racial groups including 32 Black (15 males and 17 females) and 53 White (28 males and 25 females). An a priori power analysis (assuming equal variance, α= 0.05, with at least 80% power) using variances reported in the literature, indicated that 14 participants per race and sex group were needed to reach statistically significant comparisons (Perry et al., 2016b). We targeted the age range of 4 to 9 years of age because this the typical age at which secondary surgery in cleft palate is often considered and is the time a child is often assessed for resonance and to provide a comparison to current child data in MRI of the velopharynx (Kollara et al., 2016; Tian et al., 2010a, 2010b). Vorperian et al. (2011) used a 5-year age span to examine sexual dimorphism of vocal tract structures and further cautioned sex comparisons across a decade given the known variations in growth rate and growth trends between males and females. Using a moving comparison window, the authors demonstrated horizontal plane vocal tract measures to show pre-pubertal sexual dimorphism. These findings may have implications on particular horizontal measures used in the present study, which we will describe in more detail. We expanded the sample size from the prior comparative study on sexual dimorphism among 32 children (Kollara et al., 2016) to include 85 children, which enabled sufficient power for analyses.
Racial groups, as defined by NIH guidelines (NIH, 2001), were selected to be consistent with those reported in a comparable child MRI study (Kollara et al., 2016). Child participants were recruited as part of three separate MRI studies. As a result, participants were not evenly recruited across racial groups as this was not the focus of the separate studies. No participants indicated Hispanic or Latino ethnicity.
Magnetic Resonance Imaging
Participants were imaged across three MRI sites using MRI sequences with comparable imaging parameters. Three MRI sites were used to increase participant enrollment to obtain a large sample size. MRI site 1 used a Siemens 3 Tesla Trio system (Siemens, Erlangen, Germany) and a 3D turbo-spin-echo (TSE) sequence called Sampling Perfection with Application optimized Contrasts using different flip angle Evolution (SPACE) with repetition time of 2,500 ms, echo time of 268 ms, echo train length of 171, 0 mm spacing, and .8 mm slice thickness. MRI site two used a Philips 1.5 Tesla Intera scanner (Philips, Eindhoven, Netherlands) and a high resolution, T1- weighted turbo-spin-echo (TSE) 3D anatomical SENSitivity Encoding (SENSE) with repetition time of 864 ms, echo time of 11 ms, echo train length of 9, 0 mm spacing, and 1.5 mm slice thickness. MRI site three used a General Electric 3 Tesla Signa Excite scanner (GE Healthcare, Milwaukee, WI) and a T2-fluid attenuation inversion recovery (FLAIR; used for midsagittal images) with repetition time of 2270 ms, echo time of 8.568 ms, 5 mm spacing, and 4 mm slice thickness and a proton density weighted sequence (used for oblique and oblique coronal images) sequence with repetition time of 866.68 ms, echo time of 15.632 ms, echo train length of 10, 2 mm spacing, and 2 mm slice thickness. Three-dimensional MRI scanning sequences were of adequate image resolution (0.8 in-plane isotropic resolution) with reasonable image time (less than 5 minutes) to limit head motion.
Participants were enrolled across the age span at all three MRI sites. The mean age of MRI site 1 was 8 years (SD = 1.3), MRI site 2 was 5.7 years (SD = 1.3), and MRI site 3 was 5.7 years (SD = 1.4). Male and female enrollments were similar across MRI site 1 (21 males and 18 females), site 2 (6 males and 7 females), and site 3 (16 males and 17 females). A larger proportion of White children were enrolled at MRI site 1 (32 out of 39 enrolled being White) compared to sites 2 (3 out of 13 enrolled being White) and 3 (18 out of 33 enrolled being White). Participant enrollment across the three MRI sites demonstrate little to no selection bias based on gender or age, however, MRI site 1 showed greater enrollment of White children compared to Black children. These two sites, however, were demographically in the same region.
All participants were scanned without the use of sedation using previously described behavioral and environmental adaptations (Kollara and Perry, 2014). Environmental and behavioral modifications were consistent across imaging sites. Prior to the MRI study, participants were mailed a coloring book explaining the process of the MRI study. Participants were able to explore the MRI room prior to the initiation of the study. Additionally, the loud scanner noises were played through headphones outside of the MRI scanning room to acclimate the child to the loud MR noises. During the 5-minute scan, participants were instructed to breathe through their nose with their mouth closed. The velum was in a relaxed and lowered position. An elastic strap attached to the sides of the head coil and crossing the participant’s head just above the glabella was used to reduce head motion. Cushions were used around the head to secure the head and prevent motion. A blanket was wrapped around the participant’s body to limit movement of extremities, which result in motion artifacts seen in the head. All participants completed the MRI study demonstrating a 100% success rate using the described environmental, behavioral, and imaging protocol for children between the ages of 4 to 9 years of age.
Image Analyses
Digital Imaging and Communication in Medicine (DICOM) files were analyzed using Amira 4 Visualization Volume Modeling software (Visage Imaging GmbH, Berlin, Germany). The DICOM support system enables the data to preserve original geometry. Midsagittal image plane was determined as the image most clearly depicting the velar midline as noted by the presence of the anterior nasal spine, posterior nasal spine, maximum velar length, fourth ventricle, and genu of the corpus collosum (Ettema et al., 2002). The oblique coronal image plane used for measurements was determined by rotating the oblique slice placing the slice through the bulk of the velar eminence and in the plane, which shows the levator muscle from origin to insertion (Ettema et al., 2002). Measures are described in Table 1 and displayed in Figures 1 and 2 and include craniometric and velopharyngeal variables. Craniometric measures obtained in the present study included cranial breadth (used as a covariate), face width, face height (nasion to menton), nasion to sella, sella to basion, nasion-sella-basion angle, palate height, and palate width. Velopharyngeal measures included posterior nasal spine to levator muscle, posterior nasal spine to posterior pharyngeal wall, velopharyngeal gap (velar knee to posterior pharyngeal wall), length of the levator muscle, distance between levator muscle origins, angles of origin, sagittal angle, velar length, velar thickness, and midline adenoid size (anterior-to-posterior depth). The variables were selected because previous studies showed differences in these variables among adult male and females and are thus of particular interest for the present study. Additionally, these variables are related to the velopharyngeal portal system.
Table 1.
Description of craniometric and velopharyngeal variables
| Craniofacial Variables | Reference Image | |
|---|---|---|
| Cranial breadth | Linear distance (mm) between the two euryon points of the skull as seen in axial image | |
| Face width | Linear distance (mm) between the zygomatic arches as seen on a coronal MR image | Figure 1c |
| Face height | Linear distance (mm) from nasion to menton as seen on sagittal image | Figure 1a |
| Nasion to sella | Linear distance (mm) from nasion to sella (NS) as seen on sagittal image | Figure 1a |
| Sella to basion | Linear distance (mm) from sella to basion (SB) as seen on sagittal image | Figure 1a |
| NSB angle | Inner angle (degrees) created between the intersection of the NS and SB reference lines as seen on sagittal image | Figure 1a |
| Palate height | Perpendicular distance (mm) extending from the palate width line to the roof of the hard palate as seen on a coronal MR image | Figure 1c |
| Palate width | Linear distance (mm) to the free lingual gingival margin of the first molar bilaterally as seen on a coronal MR image | Figure 1c |
| Velopharyngeal Variables | ||
| Levator length | Distance (mm) of the levator veli palatini muscle from the base of the skull (origin) through the midline of the muscle bundle to the midsagittal insertion point in the velum. This measure was taken in the oblique coronal image plane and the right and left muscle bundles and calculated as a combined mean value | Figure 1b |
| Origin to Origin | Linear distance (mm) between the two points of origin for the right and left levator muscle bundles as seen on the oblique coronal image plane | Figure 1b |
| Angle of origin | Angle (degrees) created by the line connecting the two temporal origins of the levator muscle and the line coursing through the levator muscle bundles. Right and left measurements combined for a mean value as seen on the oblique coronal image plane | Figure 1b |
| Sagittal angle | Angle (degrees) created by drawing a vertical line along the anterior boundaries of vertebrae three and four and a line coursing along the sagittal plane of the levator muscle. This angle represents the steepness of the levator muscle as it converges toward the velum from the muscle origin and is seen on the sagittal image plane | Figure 3 |
| Velar length | Curvilinear line (mm) drawn in the velar midline extending from the posterior nasal spine to the tip of the uvula as seen on the sagittal image plane | Figure 1a |
| Velar thickness | Perpendicular distance (mm) between the lines drawn tangent to the velar knee on the nasal side and the velar dimple on the oral side from a sagittal image plane | Figure 1a |
| Adenoid size | Measured as the distance (mm) between the nasopharyngeal margin of the adenoid tissue to the intersection of two reference lines-vertical line along posterior pharyngeal wall and horizontal line through palatal plane as seen on the sagittal image plane | Figure 2 |
| PNS to PPW | Distance (mm) between the posterior nasal spine (PNS) and posterior pharyngeal wall (PPW) as seen on the sagittal image plane | Figure 1a |
| Knee to PPW | Linear distance (mm) from velar knee to the PPW as see on the sagittal image plane | Figure 1a |
| PNS to levator | Linear distance (mm) from the PNS to the middle of the levator muscle sling where it inserts into the body of the velum | Figure 1a |
Figure 1.
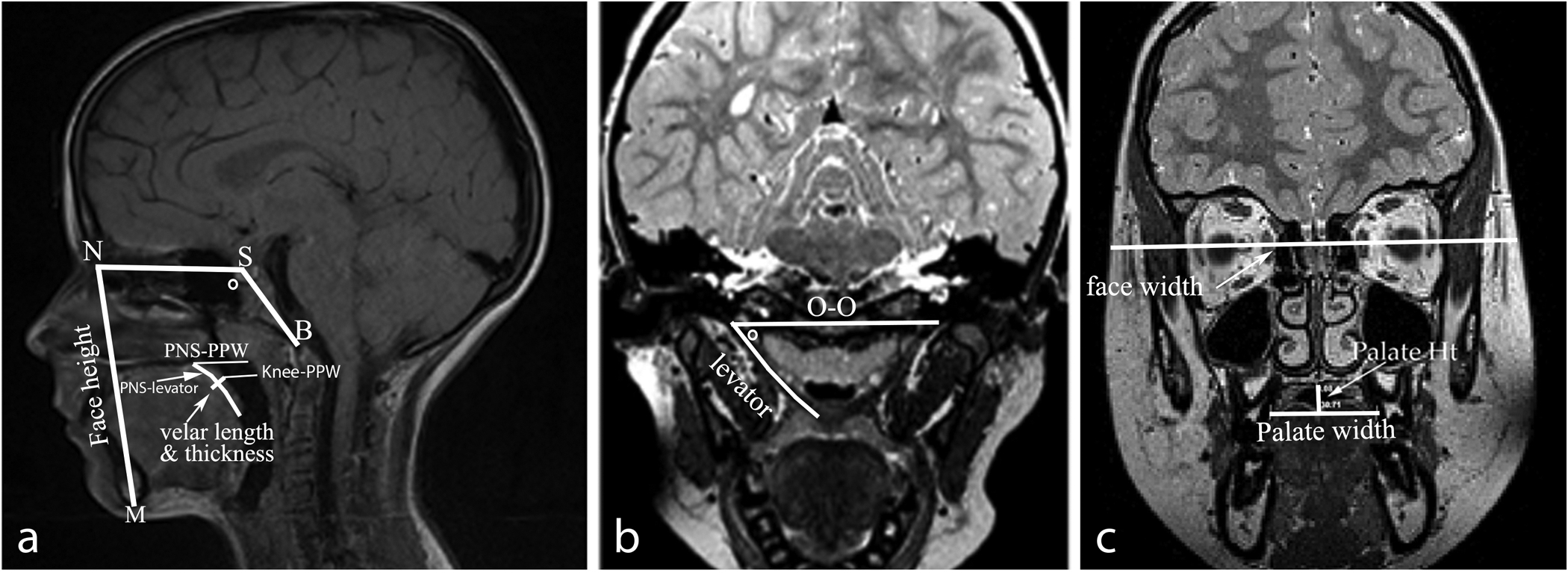
Visual display of the variables measured from midsagittal (a), oblique coronal (b), and coronal (c) planes. Abbreviations: nasion (N), menton (M), sella (S), basion (B), origin to origin (O-O), and NSB angle (left image) and oblique coronal angle of origin (middle image) noted by a degree symbol.
Figure 2.
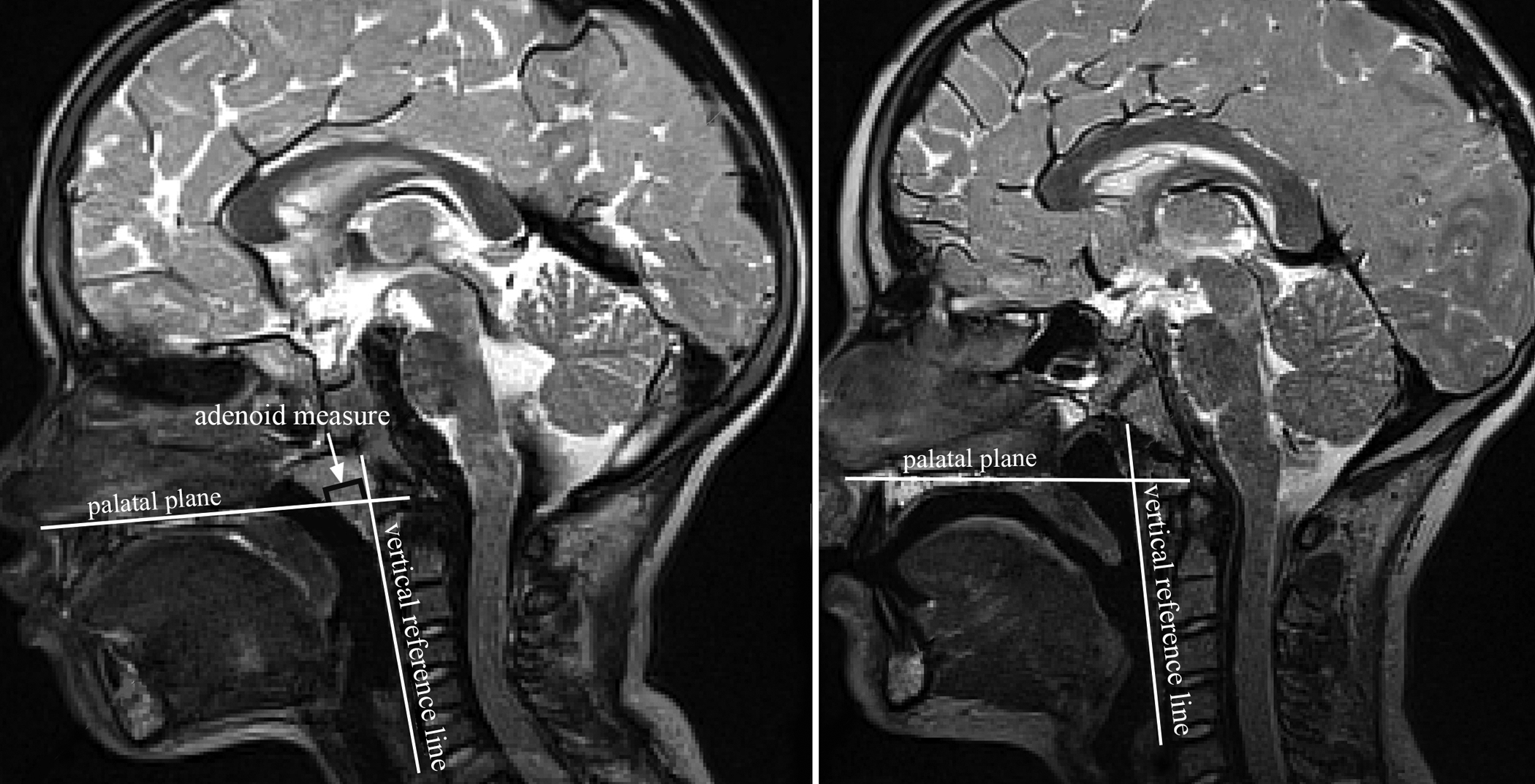
Visual display of the measure of adenoid size measured as the distance (mm) between the nasopharyngeal margin of the adenoid tissue to the intersection of two reference lines-vertical line along posterior pharyngeal wall and horizontal line through palatal plane as seen on the sagittal image plane. The left image represents a 5-year-old with noticeable adenoids compared to the 9-year-old with absent adenoid mass.
A primary and secondary rater with experience in MRI data analyses randomly selected and re-measured all variables from 26 participants (30% of the participants) five months after the first measures were obtained. Paired t tests were conducted to determine intra- and interrater differences across measures. There were no statistically significant differences (p > 0.05) between the first and second time-points of measures nor between the two raters. Intraclass correlation was between .78 and .98 (p < .001) for cranial and velar measures, demonstrating excellent correlation between repeated measures. Measures of highest agreement (between .85 and .98) were craniometric measures, adenoid size, velar measures (length and thickness), and levator muscle origin to origin. Measures of lowest agreement (between .78 and .85) were for levator muscle length and levator muscle angle measures (sagittal angle and oblique coronal angle).
Statistical Analyses
A two-way analysis of covariance (ANCOVA) was used to investigate the effects of race, gender and age (IBM SPSS Version 21.0; IBM Corporation, Armonk, NY, 2012). Age was treated as a continuous variable in order to estimate the trend in age directly and allow for more degrees of freedom for estimating the error variance. This model allows testing for sex, race, and age effects on muscle and cranial measures simultaneously. Cranial breadth was treated as a covariate to control for the effect of head size on the measures (Perry et al., 2016). Levene’s test was used to verify if the equal variance assumption is true. Anderson-Darling and Shapiro-Wilk tests (calculated using residual errors) were used to check on the normality assumption. The test assumption is that the variances can be assumed to be equal or the distribution can be assumed to be normal. Results demonstrated all p-values greater than .05, therefore, we failed to reject all the null hypotheses, i.e., both equal variance and normality assumptions are justified. When significant interactions between race and sex were observed, Tukey-Kramer tests were used to compare all possible pairs of mean values. Tukey-Kramer test (extension of Tukey’s honest significance test to handle unequal sample sizes) allows for multiple comparisons while preserving the family wise type I error rate at a level of .05 and does not require equal sample sizes.
Results
Male and female child participant groups were comparable showing no significant difference for cranial breadth (male mean = 179.34 mm (SD = 9.93 mm), female mean = 176.42 (SD = 8.64 mm); t(83) = 1.452, p = .150). Perry et al. (2016b) showed cranial size to be highly correlated and predictive of muscle measures. In the present study, cranial size correlated with 11 out of 17 variables. Kollara et al. (2016) also demonstrated cranial measures were correlated to height and weight of the child. Qualitative observations of the MRI data across participants displayed a uniform cohesive levator muscle sling with no midline septum separation of levator muscle bundles as they enter the velum. Participants displayed minimal qualitative differences in the velopharyngeal anatomy and levator muscle morphology.
Age Comparisons
This study examined if there are differences in cranial structures and velopharyngeal variables as a function of age. Using an ANCOVA with cranial breadth as the covariate and age as a continuous variable (Table 2), several variables demonstrated a significant age affect after controlling for gender and race. This indicates cranial (5 out of 7 cranial variables) and velopharyngeal variables (7 out of 10 velopharyngeal variables) are significantly impacted by age, particularly between the age ranges of 4 to 9 years of age. Specifically, cranial variables including face height (p = .000), nasion to sella (p =.033), sella to basion (p = .000), palate height (p = .000), and palate width (p = .000) demonstrated a significant age effect (Table 2). Table 3 displays mean values (standard deviation in parentheses) for variables separated for each age. A gradual increase in values across the age span of 4 to 9 years of age is apparent for the large majority of measures indicating a steady growth for cranial variables of face height, nasion to sella, sella to basion, and palate height and width measures. The cranial base angle shows minimal change from 4 years of age (White children mean = 127.5 degrees, SD = 5.8 degrees; Black children mean = 130.7 degrees, SD = 7.6 degrees) to 9 years of age (White children mean = 127.7 degrees, SD = 6.4 degrees; Black children mean = 129.2 degrees, SD = 5.2 degrees).
Table 2.
Results of ANCOVA demonstrating sex, race, and age effects
| β | Std. Error | t | Significance | ||
|---|---|---|---|---|---|
| Craniometric Variables | |||||
| Face Width | Sex | 2.039 | 1.638 | 1.245 | .218 |
| Race | 1.157 | 1.706 | .678 | .500 | |
| Age | −.013 | .586 | −.022 | .982 | |
| Face Height | Sex | .239 | 1.198 | .200 | .842 |
| Race | −2.768 | 1.248 | −2.218 | .030* (W<B) | |
| Age | 2.031 | .429 | 4.736 | .000* | |
| Nasion to Sella | Sex | 1.114 | .820 | 1.358 | .179 |
| Race | 3.454 | .855 | 4.042 | .000* (W>B) | |
| Age | .638 | .294 | 2.174 | .033* | |
| Sella to Basion | Sex | .960 | .715 | 1.343 | .184 |
| Race | .466 | .745 | .625 | .534 | |
| Age | 1.150 | .256 | 4.491 | .000* | |
| NSB Angle | Sex | −.593 | 1.667 | −.355 | .723 |
| Race | −3.095 | 1.737 | −1.781 | .080 | |
| Age | .063 | .597 | .106 | .916 | |
| Palate Height | Sex | .179 | .418 | .427 | .670 |
| Race | −.270 | .436 | −.619 | .538 | |
| Age | .607 | .150 | 4.049 | .000* | |
| Palate Width | Sex | 2.359 | .904 | 2.610 | .011* (M>F) |
| Race | .736 | .942 | .781 | .437 | |
| Age | 1.438 | .324 | 4.444 | .000* | |
| Velopharyngeal Variables | |||||
| Levator length | Sex | 1.991 | .774 | .2571 | .012* (M>F) |
| Race | 1.637 | .807 | 2.029 | .047* (W>B) | |
| Age | 1.466 | .277 | 5.286 | .000* | |
| Origin to Origin | Sex | .740 | 1.042 | .711 | .480 |
| Race | −.235 | 1.085 | −.216 | .829 | |
| Age | .093 | .373 | .249 | .804 | |
| Angle of Origin | Sex | −1.544 | .901 | −1.713 | .091 |
| Race | .611 | .939 | .651 | .518 | |
| Age | −.710 | .323 | −2.202 | .031* | |
| Sagittal Angle | Sex | −1.719 | 1.484 | −1.158 | .251 |
| Race | −2.596 | 1.546 | −1.679 | .098 | |
| Age | −1.222 | .531 | −2.30 | .025* | |
| Velar Length | Sex | 1.227 | 1.071 | 1.145 | .256 |
| Race | −4.275 | 1.116 | −3.830 | .000* (W<B) | |
| Age | .921 | .384 | 2.401 | .019* | |
| Velar Thickness | Sex | .393 | .367 | 1.073 | .287 |
| Race | −8.43 | .382 | −2.207 | .031* (W<B) | |
| Age | .286 | .131 | 2.183 | .033* | |
| Adenoid Size | Sex | .310 | 1.024 | .302 | .763 |
| Race | −3.370 | 1.067 | −3.158 | .002* (W<B) | |
| Age | −.499 | .367 | −1.361 | .178 | |
| PNS to PPW | Sex | 1.524 | .938 | 1.625 | .109 |
| Race | 1.680 | .977 | 1.719 | .090 | |
| Age | −.159 | .336 | −.474 | .637 | |
| Knee to PPW | Sex | −.360 | .693 | −.520 | .605 |
| Race | 2.767 | .722 | 3.831 | .000* (W>B) | |
| Age | .777 | .248 | 3.131 | .003* | |
| PNS to Levator | Sex | .311 | .455 | .684 | .497 |
| Race | −1.030 | .474 | −2.173 | .033* (W<B) | |
| Age | −.371 | .163 | −2.277 | .026* | |
Abbreviations—posterior nasal spine (PNS), posterior pharyngeal wall (PPW).
p < 0.05
Table 3.
Mean (standard deviation) for cranial and velopharyngeal variables represented in mm with the exception of angle measures (NSB angle, muscle angle origin, and sagittal angle), abbreviations are as follows: nasion (N), sella (S), basion (B), posterior nasal spine (PNS), posterior pharyngeal wall (PPW), Black participants (B), White participants (W)
| Measures | 4 year olds n = 11 (8W, 3B) |
5 year olds n = 11 (6W, 5B) |
6 year olds n = 14 (8W, 6B) |
7 year olds n = 9 (5W, 4B) |
8 year olds n = 21 (11W, 10B) |
9 year olds n = 19 (15W, 4B) |
Combined Mean N = 85 (53W, 32B) |
|---|---|---|---|---|---|---|---|
| Craniometric Variables | |||||||
| Cranial breadth | W: 164.1 (6.5) | W: 175.0 (7.8) | W: 180.6 (8.6) | W: 180.1 (10.1) | W: 179.4 (3.9) | W: 182.7 (9.4) | W: 177.8 (9.8) |
| B: 169.2 (5.1) | B: 175.3 (6.0) | B: 172.3 (3.2) | B: 177.1 (3.2) | B: 182.7 (9.8) | B: 189.1 (8.7) | B: 178.4 (9.1) | |
| Face width | W: 109.9 (6.5) | W: 112.3 (6.4) | W: 111.7 (6.7) | W: 110.1 (8.7) | W: 113.7 (3.2) | W: 112.2 (4.2) | W: 111.9 (5.7) |
| B: 109.4 (3.6) | B: 106.5 (9.1) | B: 106.2 (1.6) | B: 119.1 (10.9) | B: 113.8 (9.4) | B: 110.7 (8.8) | B: 111.4 (8.7) | |
| Face height | W: 83.9 (4.1) | W: 92.9 (4.2) | W: 90.9 (6.3) | W: 92.5 (4.7) | W: 94.4 (4.2) | W: 99.9 (2.7) | W: 93.6 (6.6) |
| B: 88.2 (3.4) | B: 94.5 (6.7) | B: 91.9 (2.9) | B: 94.9 (8.1) | B: 97.9 (5.0) | B: 102.5 (3.0) | B: 95.6 (6.1) | |
| NS | W: 54.3 (1.9) | W: 57.2 (3.6) | W: 57.3 (2.5) | W: 56.1 (3.7) | W: 58.9 (2.6) | W: 61.9 (3.4) | W: 58.3 (3.4) |
| B: 54.5 (4.5) | B: 55.4 (5.2) | B: 51.9 (4.9) | B: 55.5 (2.6) | B: 57.3 (5.6) | B: 60.8 (3.8) | B: 55.9 (5.1) | |
| SB | W: 32.6 (2.1) | W: 34.9 (2.3) | W: 57.3 (2.5) | W: 34.9 (2.8) | W: 38.6 (3.0) | W: 39.1 (3.1) | W: 36.2 (3.7) |
| B: 34.8 (2.9) | B:(35.1 (3.9) | B: 51.9 (4.9) | B: 33.8 (2.8) | B: 37.6 (3.6) | B: 40.5 (3.0) | B: 36.5 (4.0) | |
| NSB angle | W: 127.5 (5.3) | W: 132.3 (6.6) | W: 127.7 (6.0) | W: 130.9 (1.5) | W: 126.7 (5.1) | W: 127.4 (6.4) | W: 128.2 (5.7) |
| B: 130.7 (7.6) | B: 131.8 (14.0) | B: 135.4 (6.1) | B: 135.5 (4.6) | B: 127.7 (8.0) | B:129.2 (5.2) | B: 131.1 (8.3) | |
| Palate height | W: 5.8 (1.0) | W: 8.6 (1.7) | W: 7.6 (1.4) | W: 7.4 (2.8) | W: 9.7 (.9) | W: 10.9 (1.3) | W: 9.9 (2.2) |
| B: 4.9 (2.9) | B: 8.8 (2.3) | B: 5.7 (1.4) | B: 6.3 (3.1) | B: 9.2 (3.2) | B: 11.5 (1.8) | B: 8.0 (3.2) | |
| Palate width | W: 26.7 (2.7) | W: 30.7 (3.6) | W: 29.4 (3.0) | W: 28.0 (2.5) | W: 35.2 (2.1) | W: 35.2 (1.7) | W: 32.1 (4.2) |
| B: 28.8 (1.2) | B: 30.9 (2.8) | B: 31.4 (.45) | B: 30.5 (.6) | B:33.1 (2.8) | B:36.1 (1.9) | B: 32.7 (3.0) | |
| Velopharyngeal Variables | |||||||
| Levator length | W: 32.7 (2.1) | W: 35.6 (2.4) | W: 37.4 (3.7) | W: 35.6 (3.6) | W: 41.5 (3.1) | W: 52.8 (4.3) | W: 54.8 (4.1) |
| B: 31.6 (3.7) | B: 33.9 (.85) | B: 31.9 (2.9) | B: 31.4 (4.3) | B: 37.9 (3.7) | B: 41.5 (2.9) | B: 35.2 (4.9) | |
| Origin to origin | W: 55.3 (3.2) | W: 55.4 (2.3) | W: 55.2 (5.9) | W: 53.8 (.9) | W: 56.7 (2.5) | W: 52.8 (4.3) | W: 54.8 (4.1) |
| B: 51.9 (1.1) | B:54.1 (1.6) | B: 57.1 (3.0) | B: 53.4 (2.5) | B: 55.2 (5.7) | B: 54.2 (2.6) | B: 54.7 (3.9) | |
| Angle of origin | W: 56.3 (3.2) | W: 54.9 (3.7) | W: 55.4 (5.4) | W: 54.9 (1.8) | W: 54.7 (3.5) | W: 52.8 (4.3) | W: 54.4 (3.9) |
| B: 53.8 (1.6) | B: 52.6 (1.9) | B: 56.4 (2.2) | B: 54.0 (1.3) | B: 54.4 (4.3) | B: 53.5 (3.3) | B:54.2 (3.1) | |
| Sagittal angle | W: 56.2 (10.9) | W: 50.4 (5.1) | W: 55.8 (7.7) | W: 52.3 (10.4) | W: 47.4 (6.7) | W: 48.3 (4.6) | W: 51.0 (7.9) |
| B: 67.5 (10.2) | B: 56.3 (4.5) | B: 65.7 (5.1) | B: 56.6 (4.8) | B: 51.1 (6.7) | B:44.1 (6.9) | B: 56.0 (9.5) | |
| Velar length | W: 23.9 (1.6) | W: 25.1 (3.2) | W: 26.0 (3.7) | W: 24.1 (3.7) | W: 27.5 (3.2) | W: 28.0 (3.7) | W: 26.3 (3.6) |
| B: 25.9 (.9) | B: 28.7 (4.2) | B: 28.4 (2.2) | B: 29.8 (5.0) | B: 32.9 (7.0) | B: 28.2 (5.9) | B: 29.8 (5.3) | |
| Velar thickness | W: 6.9 (1.2) | W: 8.7 (1.9) | W: 7.4 (1.6) | W: 7.8 (1.6) | W: 9.1 (1.6) | W: 8.8 (1.0) | W: 8.3 (1.6) |
| B: 9.5 (.3) | B: 8.7 (1.0) | B: 7.5 (1.3) | B:8.4 (.5) | B: 9.0 (1.4) | B: 10.4 (.3) | B: 8.8 (1.5) | |
| Adenoid Size | W: 7.9 (3.4) | W:10.7 (3.6) | W: 10.4 (3.1) | W: 8.6 (3.1) | W: 10.2 (2.3) | W: 10.9 (1.3) | W: 8.1 (4.2) |
| B: 12.5 (2.9) | B:11.2 (4.4) | B: 9.9 (1.8) | B: 11.1 (1.3) | B: 12.7 (3.5) | B: 7.7 (1.6) | B: 11.1 (3.2) | |
| PNS to PPW | W: 18.4 (3.9) | W: 16.9 (2.7) | W: 20.3 (5.8) | W: 22.2 (5.6) | W:18.3 (2.3) | W: 19.5 (3.8) | W: 19.2 (4.1) |
| B: 15.9 (1.8) | B: 20.9 (1.9) | B: 17.5 (3.0) | B: 19.4 (3.4) | B:15.6 (3.2) | B: 13.2 (2.6) | B: 16.9 (3.6) | |
| Knee to PPW | W: 5.8 (2.3) | W: 6.3 (2.9) | W: 6.3 (2.7) | W: 9.8 (3.5) | W: 9.7 (1.6) | W: 11.0 (2.5) | W: 8.6 (3.2) |
| B: 5.9 (4.1) | B: 5.0 (3.7) | B: 6.8 (2.6) | B: 6.3 (4.6) | B: 5.1 (2.9) | B: 10.3 (1.7) | B: 6.3 (3.4) | |
| PNS to levator | W: 11.8 (1.6) | W: 11.9 (1.6) | W: 12.4 (1.5) | W: 11.3 (1.6) | W: 10.1 (1.6) | W: 10.1 (1.8) | W: 11.0 (1.8) |
| B: 10.4 (.5) | B: 12.5 (.5) | B: 12.2 (1.1) | B: 12.8 (1.4) | B: 11.9 (2.9) | B: 9.5 (2.1) | B: 11.7 (2.1) |
Results also indicate that age is a significant factor for velopharyngeal variables including levator muscle length (p = .000), angle of origin (p = .031), sagittal angle (p = .025), velar length (p = .019), velar thickness (p = .033), velar knee to posterior pharyngeal wall (p = .003), and posterior nasal spine to levator muscle (p = .026). Levator muscle length showed (Table 3) the greatest increase in growth between 7 to 9 years of age, increasing in length by 17.2 mm from 7 years of age among White children and 10.1 mm among Black children. This vertical growth spurt is also consistent with that of face height in which the face showed the greatest vertical growth increase from 7 years of age to 9 years of age for both White (increase of 7.4 mm) and Black (increase of 7.6 mm) children. The vertical measure of palate height also reflected a similar growth between 7 years of age to 9 years of age, increasing 3.5 mm for White children and 5.2 mm for Black children. From these observations, the vertical growth of the levator muscle and palate height appears to show similar growth trends as that of the vertical growth in the face height. The sagittal angle of origin demonstrated a decrease in the angle across the age span. This is evident in Figure 3, which demonstrates the difference in the sagittal angle between a 4- and 9-year-old child. It is expected that as the child increased in age, the levator muscle would become steeper due to changes in the angulation of the vocal tract.
Figure 3.
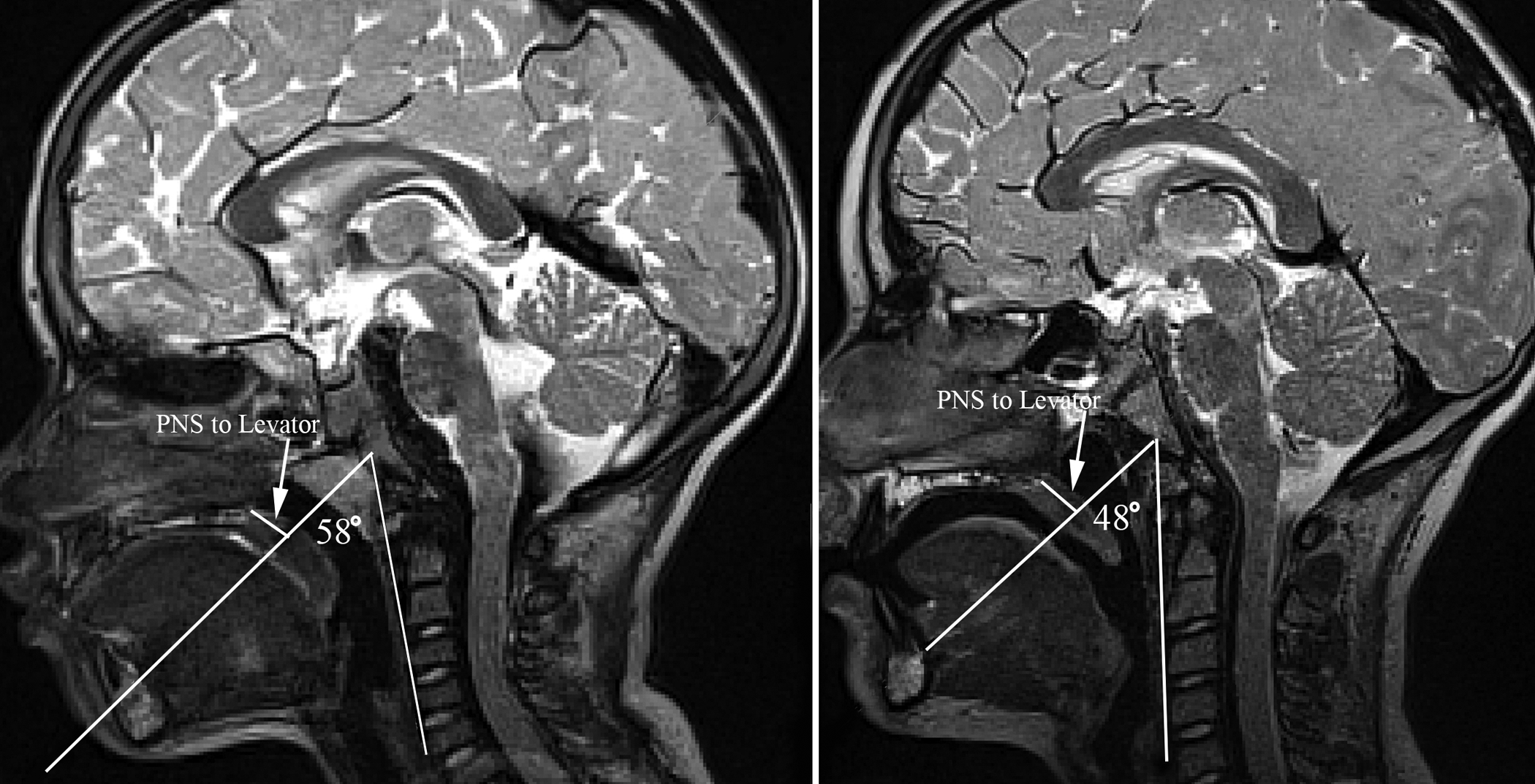
Visual display of the variables measured demonstrating the lack of change in the PNS to levator muscle insertion distance (noted by arrow) between a 5-year-old (left image) and 9-year-old (right image) despite the change in the sagittal angle and velar lengthening with growth.
In contrast, face width demonstrated a minimal increase from 4 to 9 years of age. Palate width (horizontal linear measure) showed the greatest growth spurt between 7 years of age (White children mean = 28.0 mm, Black children mean = 30.5 mm) to 9 years of age (White children mean = 35.2 mm, Black children mean = 36.1 mm) with minimal change in palatal width from 4 to 7 years of age for both races. The horizontal measure of origin to origin showed minimal changes across the age span for both races. Velar thickness displayed steady growth in thickness from 4 years of age (White children mean = 6.9 mm, Black children mean = 9.5 mm) to 9 years of age (White children mean = 8.8 mm, Black children mean = 10.4 mm). Velar length increased by 4.1 mm and 2.3 mm from 4 to 9 years of age for White and Black children, respectively.
There was a minimal change in the distance from the posterior nasal spine to the posterior pharyngeal wall across the age span ranging from 15.6 mm to 22.2 mm with no clear direction of increase or decrease in this measure across the age span of 4 to 9 years of age. The velopharyngeal depth, as measured by velar knee to posterior pharyngeal wall, appeared similar with minimal and inconsistent change across the age span. However, by 9 years of age, the velopharyngeal depth at rest became the greatest at 11.0 mm and 10.3 mm for White and Black children, respectively. This is likely due to the change in adenoid size seen from 4 to 9 years of age. The adenoid size (measured as midsagittal depth, Figure 2) remained consistently between 7.9 mm to 12.7 mm between 4 and 8 years of age with a marked decrease in size at 9 years of age to 4.1 mm in midline thickness for White children and 7.7 mm among Black children. This can be seen in Figure 2 comparing a 5-year-old to a 9-year-old participant. This drastic decrease in adenoid thickness at 9 years of age likely explains the increase in velopharyngeal depth at rest (velar knee to posterior pharyngeal wall measure). This increase in velopharyngeal depth can be visualized in Figure 2 in which the 5-year-old shows a relatively smaller velopharyngeal gap compared to the 9-year-old with no prominent adenoid tissue. The measure of posterior nasal spine to the insertion of the levator muscle was assessed to examine how changes in the pharyngeal depth affect the distance of the muscle from the bony hard palate. As seen in Figure 3, despite the changes in the angulation (sagittal angle) of the levator muscle, the distance from the posterior nasal spine to the insertion of the muscle remained similar showing little change between 4 to 9 years of age.
Sex Comparisons
The second question examined in this study was to determine if there are sex differences in cranial structures and velopharyngeal measures in the child population between 4 to 9 years of age, and to examine if there is an age effect and at which age differences are observed. Sex effects were evident (Table 2) for one of the 7 craniometric variables (i.e., palate width) and one of the 10 velopharyngeal measures (i.e., levator muscle length). Female child participants had a significantly narrower hard palate compared to male child participants (Female mean = 29.2 mm (SD = 5.3 mm) < Male mean = 32.2 mm (SD = 3.7 mm); p = .011). Male child participants had a significantly longer levator muscle compared to females child participants (Male mean=36.7 mm (SD=4.5) > Female mean=34.9 mm (SD=4.5); p = .012). Combined mean values found in Table 4 demonstrate a relatively small trend for larger values in males for variables of cranial breadth, nasion to sella, angle of origin, and distance from posterior nasal spine to posterior pharyngeal wall, although these trends are not significant. The interaction between sex and age was not statistically significant across all variables, with the exception of posterior nasal spine to posterior pharyngeal wall. This means the sex differences observed do not change significantly with age for almost all measures. For posterior nasal spine to posterior pharyngeal wall, males tend to decrease more slowly with age than females (p = 0.022).
Table 4.
Mean values and standard deviations (in parentheses) for each craniometric and velopharyngeal variable. Values are noted in millimeters, with the exception of the angle measures (in degrees) of NSB (nasion-sella-basion), sagittal angle, and angle of origin.
| White | Black | Combined | ||||||
|---|---|---|---|---|---|---|---|---|
| Measures | Boy | Girl | M | Boy | Girl | M | Boy | Girl |
| Craniometric Variables | ||||||||
| Cranial breadth | 179.0 (9.3) | 176.4 (10.2) | 177.8 (9.7) | 180.4 (11.0) | 176.7 (6.9) | 178.5 (9.1) | 179.5 (9.8) | 176.5 (8.9) |
| Face width | 107.3 (7.9) | 111.2 (7.2) | 109.1 (7.8) | 115.1 (8.6) | 107.2 (7.8) | 111.5 (8.9) | 113.3 (6.7) | 109.9 (7.4) |
| Face height | 91.5 (6.1) | 90.3 (5.9) | 90.9 (5.6) | 95.3 (7.3) | 93.8 (4.1) | 94.5 (5.9) | 93.0 (6.8) | 91.9 (7.4) |
| Nasion-Sella | 57.8 (3.3) | 55.8 (2.6) | 56.9 (3.1) | 57.3 (4.6) | 53.2 (4.5) | 55.2 (5.0) | 57.6 (3.8) | 54.6 (3.8) |
| Sella-Basion | 35.7 (3.1) | 34.3 (3.7) | 35.1 (3.4) | 36.7 (3.9) | 35.2 (3.8) | 35.9 (3.9) | 36.1 (3.4) | 34.7 (3.7) |
| NSB Angle | 128.4 (4.1) | 128.6 (6.9) | 128.5 (5.4) | 129.6 (10.7) | 133.1 (6.0) | 131.4 (8.7) | 128.9 (7.3) | 130.6 (6.8) |
| Palate height | 8.1 (2.2) | 7.8 (1.8) | 7.9 (1.9) | 8.3 (3.0) | 6.8 (2.9) | 7.5 (3.0) | 8.1 (2.5) | 7.3 (2.4) |
| Palate width | 32.3 (4.1) | 29.3 (3.9) | 30.8 (4.2) | 32.2 (3.2) | 29.1 (7.9) | 30.8 (5.7) | 32.2 (3.7) | 29.2 (5.3) |
| Velopharyngeal Variables | ||||||||
| Levator length | 37.5 (4.2) | 36.5 (4.5) | 37.1 (4.3) | 35.4 (4.7) | 33.2 (3.8) | 34.3 (4.4) | 36.7 (4.5) | 34.9 (4.5) |
| Origin-origin | 55.2 (3.2) | 55.8 (4.4) | 55.5 (3.7) | 54.8 (4.0) | 54.8 (4.2) | 54.8 (4.4) | 55.1 (3.5) | 55.3 (4.3) |
| Angle of origin | 54.9 (3.5) | 55.6 (3.8) | 55.2 (3.6) | 54.3 (2.8) | 54.5 (3.6) | 54.4 (4.0) | 54.6 (3.2) | 55.1 (3.6) |
| Sagittal angle | 51.2 (9.4) | 53.1 (7.7) | 52.1 (8.9) | 56.8 (9.9) | 58.5 (7.2) | 57.7 (8.6) | 53.4 (9.9) | 55.6 (3.8) |
| Velar length | 25.7 (3.6) | 25.5 (3.0) | 25.6 (3.3) | 30.4 (6.4) | 29.5 (4.4) | 29.9 (5.3) | 27.6 (5.4) | 27.3 (4.0) |
| Velar thickness | 8.2 (2.0) | 7.9 (1.3) | 8.1 (1.7) | 8.9 (1.7) | 8.2 (.94) | 8.6 (1.4) | 8.5 (1.9) | 8.0 (1.2) |
| Adenoid size | 9.8 (2.7) | 9.5 (3.6) | 9.6 (3.1) | 11.9 (3.3) | 11.3 (2.9) | 11.6 (3.1) | 10.6 (3.1) | 10.3 (3.4) |
| PNS—PPW | 19.5 (4.4) | 18.4 (3.9) | 19.0 (4.2) | 18.5 (2.6) | 16.5 (3.9) | 17.5 (3.4) | 19.1 (3.7) | 17.5 (3.9) |
| Knee—PPW | 8.1 (2.8) | 7.1 (3.2) | 7.6 (3.0) | 5.7 (3.1) | 5.6 (3.3) | 5.7 (3.1) | 7.1 (3.1) | 6.4 (3.3) |
| PNS—levator | 11.5 (1.7) | 11.2 (1.7) | 11.4 (1.7) | 12.0 (1.9) | 12.1 (1.4) | 11.9 (2.4) | 11.7 (1.6) | 11.6 (2.0) |
Race Comparisons
The third research question examined in this study was to determine if there are racial differences in cranial and velopharyngeal variables, and if any observed differences are related to age effects. Two craniometric measures (Table 2) showed a significant race effect, that is, face height (p = .030) and nasion to sella (p = .000). Black children showed a greater mean face height compared to White children (Black children mean = 94.5 mm (SD = 5.9 mm) > White children mean = 90.9 mm (SD = 5.6 mm); p = .030). White children demonstrated a significantly greater nasion to sella distance compared to that of Black children (White children mean = 56.9 mm (SD = 3.1 mm) > Black children mean = 55.2 mm (SD = 5.0 mm), p = .000).
Six out of the 10 velopharyngeal variables also demonstrated a significant race effect. Specifically, race effects were observed for levator muscle length, velar length, velar thickness, adenoid size, velar knee to posterior pharyngeal wall, and posterior nasal spine to levator muscle distance. White children displayed a significantly longer levator muscle compared to Black children (White children mean = 37.1 mm (SD = 4.3 mm) > Black children mean = 34.3 mm (SD 4.4 mm); p = .047). Black children presented with a longer velum compared to White children (Black children mean = 29.9 mm (SD = 5.3 mm) > White children mean = 25.6 (SD = 3.3 mm); p = .000). Black children also displayed a significantly thicker velum compared to that of White children (Black children mean = 8.6 (SD = 1.4 mm) > White children mean = 8.1 mm (SD = 1.7 mm); p = .031). White children had a significantly smaller adenoid compared to Black children (White children mean = 9.6 mm (SD = 3.1 mm) < Black children mean = 11.6 mm (SD = 3.1 mm); p = .002). Black children displayed a significantly smaller distance from the velar knee to the posterior pharyngeal wall compared to that of White children (Black children mean = 5.7 mm (SD = 3.1 mm) < White children mean = 7.6 mm (SD = 3.0 mm); p = .000). This is likely related to the increased velar thickness observed among Black children compared to White children. White children also displayed a shorter distance from the posterior nasal spine to the levator muscle compared to Black children suggesting a more posterior positioned levator sling in Black children compared to White children. The interaction of age and race also showed no interaction effect between variables. This indicates that the differences observed among the racial groups are not significantly affected by age.
Discussion
These findings demonstrate significant impact of age on variables. Of particular interest, the age effects appear to not be related to the observed sex and race trends. However, only hard palate width and levator muscle length showed a significant sex effect. Race effects were significant for two cranial and six velopharyngeal variables
Normative Database
To the best of our knowledge, this study provides the first large-scale (N = 85) normative database of the craniometric and velopharyngeal variables (velopharyngeal and levator muscle measures) among children between 4 to 9 years of age. These data provide an important contribution to the current literature related to velopharyngeal and craniometric measures among children, particularly for comparisons to clinical populations with abnormal velopharyngeal measures such as cleft palate and/or syndromes (e.g., 22q11.2DS). The present study observed a cohesive levator sling through the velar midline among all child participants between 4 to 9 years of age. This is consistent with the histologic findings in adult specimens reported by Kuehn and Moon (2005). A seminal paper by Subtelny (1957) used cephalometric analyses to report mean values for velar length, velar thickness, and posterior nasal spine to posterior pharyngeal wall distance for children from birth to 18 years of age. Comparative normative data from the present study are similar to those of Subtelny (1957) and expand upon this database by including additional measures that cannot be derived from cephalometric analysis (e.g., muscle tissue imaging).
Normative data are particularly important when providing comparisons to studies in the clinical population, such as cleft palate. For example, Yellinedi and Damalacheruvu (2013) used lateral videofluoroscopy to examine 117 children with repaired cleft palate between 8 and 15 years of age to determine if the resting velopharyngeal depth (distance from velar knee to posterior pharyngeal wall) was correlated to outcomes of hypernasality. Findings showed that resting velopharyngeal depth for 15 normal participants ranged from 6.5 to 8.3 mm. Of the 117 patients with repaired cleft palate, 30% had a velopharyngeal depth less than 6 mm, 30% had a depth between 6 to 10 mm, and 40% displayed resting velopharyngeal depth greater than 10 mm. Results further observed individuals with resting velopharyngeal depth greater than 10 mm did not achieve velopharyngeal closure on phonation and those with velopharyngeal depth between 6 to 10 mm displayed borderline closure during phonation. The authors proposed that a resting velopharyngeal depth of 5.1 mm is an optimal depth for ensuring the child will be able to achieve velopharyngeal closure. However, the authors only assessed 15 noncleft participants and velopharyngeal depth values across all patients were not reported relative to the age despite the relatively large age span from 8 to 15 years of age. The distance from the velar knee to posterior pharyngeal wall (equivalent to resting velopharyngeal gap size, as termed in the study) for children with normal anatomy from the present study varied from group (by age and race) means of 5.0 mm to 11.0 mm. As evident, children with normal anatomy and normal resonance in the present study have values that are well into the cautionary range (6 to 10+ mm) proposed by Yellinedi and Damalacheruvu (2013). This highlights the importance of considering restrictive age ranges when drawing clinical conclusions. Thus, the importance of having a large age-specific normative database, such as the values from the present study, to utilize as a reference for appropriate comparisons, are crucial for proper diagnostics. Additional studies are necessary to further understand velopharyngeal growth and its typical variability and to examine the effect of cleft palate on these growth patterns. Consideration of the entire velopharyngeal system and muscle morphology provides a more comprehensive view of the complex nature of velopharyngeal function for speech.
In the present study, the adenoid tissue showed minimal developmental change with possible hypertrophy or increase at 8 years of age followed by a rapid decrease in size at 9 years of age. These findings were similar to that of Handelman and Osborne (1976) in which the adenoid tissue among 12 children using cephalometry showed minimal adenoid tissue at age one, evident hypertrophy by 2 years of age, maximum hypertrophy during the early school ages, and involution during adolescence. However, the effects of adenoid tissue changes on the velopharyngeal portal were not investigated. The present study demonstrated a similar increase in velopharyngeal depth at 9 years of age at the same time point of adenoid involution. Despite changes in the velopharyngeal depth due to adenoid involution, it is well known that most children do not develop velopharyngeal insufficiency. Velopharyngeal insufficiency was found to not develop in 20 children (10 with normal anatomy, 5 with repaired cleft palate, 5 with unrepaired submucous cleft palate) with normal speech post adenoid involution (Siegel-Sadewitz and Shprintzen, 1986). Reduced lateral pharyngeal wall movement from pre- to postpubertal life in all 10 children with normal velopharyngeal anatomy and three children with cleft palate were observed. Findings by Siegel-Sadewitz and Shprintzen (1986) suggest adaptation effect of the velopharyngeal portal during adenoid involution may be related to functional changes as opposed to anatomic changes. Further studies should consider functional differences based on age, race, and sex to better understand the causes of successful or failed velopharyngeal adaptation during development, particularly during the time of adenoid involution.
Craniometric Measures
Craniometric variables examined in the present study were not significantly different between male and female child participants with the exception of palate width. Male child participants displayed a trend toward larger craniometrics values compared to females. However, only palate width was significantly different between male and female child participants. Male child participants also displayed a more acute nasion-sella-basion cranial base angle compared to females. The absence of sexual dimorphism of these selected craniometric variables among this age range is consistent with previous studies (Lewis and Roche, 1977; Ursi et al., 1993; Kollara et al., 2016). Ursi et al. (1993) used lateral cephalograms to identify that sexual dimorphism became prominent for bony cranial structures after 14 years of age. Significant craniofacial variations, however, were observed based on race in the present study. Specifically, Black children showed greater values for face height and a smaller distance between the nasion and sella. Using a smaller sample size (N=32), Kollara et al. (2016) using MRI observed a significant difference by race for face height with Black children displaying a greater mean value compared to females. Porter and Olson (2001) observed significantly greater horizontal facial measures among Black women compared to White women, using photographs. Our study did reflect a significant difference by race for face height, similar to Kollara et al. (2016), but did not observe significant difference for horizontal measures such as face width as seen among adult subjects by Porter and Olson (2001). However, Porter and Olson used photographs as opposed to bony landmarks used in the present study and difference may be due to soft tissue thickness variations.
Velopharyngeal Measures
Velopharyngeal variables also reflected a lack of sexual dimorphism with the exception of levator muscle length. Kollara et al. (2016) observed a lack of sexual dimorphism among all velopharyngeal measures. Lieberman et al. (2001) used lateral radiographs and observed a lack of sexual dimorphism of the pharyngeal shape and size among children under 14 years of age. Jeans et al. (1981) also observed (using lateral radiographs) the nasopharynx to be similar between male and female participants under 13 years of age. Subtelny (1957) demonstrated no significant difference for velar length and thickness between boys and girls from birth to 18 years of age. These studies, however, did not assess race effects.
Using acoustic pharyngometry, Xue et al. (2006) and Xue and Hao (2006) observed race to have a significant effect on vocal tract volume and pharyngeal length among adult participants. Our findings suggest within the age range of 4 to 9 years of age, children display a different mean levator muscle length, velar length, velar thickness, adenoid size, velar knee to posterior pharyngeal wall, and posterior nasal spine to levator muscle distance based on race. Similar to Kollara et al. (2016), we observed Black children to have a significantly longer and thicker velum compared to White children. Our findings suggest a significantly longer levator muscle in White children compared to Black children. Kollara et al. (2016) observed the race effect for levator length to be approaching statistical significance at p = .56. This difference between our findings of significant difference in the levator muscle length is likely due to the larger sample size.
Future Directions and Clinical Implications
Future studies should examine the effects of race and sex on growth rates of velopharyngeal structures to understand the age at which sexual dimorphism of velopharyngeal measures becomes apparent, as seen in the adult population (Perry et al., 2016). Vorperian et al. (1999) examined longitudinal MRI data of two participants from birth to 50 months and observed coordinated growth between almost all areas of vocal tract. For example, the hard palate, oro-naso-pharyngeal length, upper and lower face height, vocal tract length, and tongue length and area display a coordinated growth through early infancy. Vorperian et al. (2009) later examined 605 head or neck imaging studies (MRI or computed tomography) representing an age span from birth to 19 years of age. Results demonstrate a significant post-pubertal sex difference for the oral and pharyngeal segments of the vocal tract. Horizontal variables (vocal tract horizontal measure and anterior oral cavity horizontal length) showed earlier pubertal sex differences which varied during the course of development. Durtschi et al. (2009) using MRI and computed tomography found no significant race effect in the overall growth of selected vocal tract variables, including nasopharyngeal length; however, the study demonstrated variations in growth rates between horizontal and vertical vocal tract measures. Similar investigations among nasopharyngeal variables are important in understanding how growth of the bony and soft tissue structures change across the age span and how surgical intervention (e.g., cleft palate surgery) may affect those growth trajectories. In the present study, the majority of cranial measures (i.e., face height, nasion to sella, sella to basion, and palate height and width measures) showed a steady growth across the age span, however, cranial base angle showed minimal change. Vertical measures (i.e., face height, palate height, levator length) display a vertical growth spurt from 7 to 9 years of age. Palate width similarly displayed a growth spurt between 7 to 9 years of age. However, other horizontal measures (i.e., face width, origin to origin) showed minimal changes across the age span.
Studies have described the effects of a cleft palate to the bony structures of the cranial base and facial skeleton (Shibasaki and Ross, 1969; Krogman et al., 1973; Smahel, 1984; Smahel and Mullerova, 1995). Individuals with cleft palate have been observed to have a more superiorly rotated posterior bony palate compared to those without a cleft palate (Lu et al., 2006). Shibasaki and Ross (1969) observed an overall decrease in maxillary growth in children with cleft palate. Inhibition in maxillary growth may result in asynchronous growth of the nasopharyngeal structures, which may be linked to the occurrence of velopharyngeal dysfunction at an older age (Mason et al., 2016). Numerous studies have examined the effect of cleft palate and/or surgery on the development of the bony skeleton (Cheung et al., 2004; Figueroa et al., 2004; Gursoy et al., 2010). However, there is a dearth of literature to explain the growth and development of velopharyngeal structures and muscles and the effects of cleft palate deformities and surgical techniques on these growth trajectories.
Study Limitations
Limitations of the present study include the unequal sample size and the use of three different MRI scanners. Data in the present study are limited to anatomical interpretations and do not provide evidence of sexual dimorphism in velopharyngeal function. Studies among adults have demonstrated significant differences between males and females in functional aspects of velopharyngeal valving for speech (Kuehn and Moon, 1998; McKerns and Bzoch, 1970; Zajac and Mayo, 1996). We hypothesize that there would not be a velopharyngeal functional difference between boys and girls within the target age group, due to the observed lack of sexual dimorphism in resting velopharyngeal measures. Rather prepubescent children likely have adenoid to velar contact and velar movement is likely controlled by the amount of adenoid tissue present in the velopharynx. Given the non-significant difference in midline adenoid volume between boys and girls in the present study, velopharyngeal functional measures such as velar height, would likely be similar. Future studies should further examine when variations in closure speed, closure force, and velar height become evident in the developing velopharyngeal system and how these relate to levator muscle function. Additionally, future studies should include other racial and ethnic study groups.
Conclusion
The present study provides a large normative database of velopharyngeal and craniometric norms for children ages 4 to 9 years. This is an important contribution as it can be used as a reference for comparison to studies using similar variables in the cleft population, specifically when providing insight into anatomic outcomes using different surgical approaches. A lack of sexual dimorphism was noted across the majority of variables in the present study. Significant race and age effects were noted for several cranial and velopharyngeal measures.
Acknowledgments:
This study was made possible by grant number 1R03DC009676-01A1 from the National Institute on Deafness and Other Communicative Disorders. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- Behrents RG. The biological basis for understanding craniofacial growth during adulthood. Prog Clin Biol Res. 1985;187:307–319. [PubMed] [Google Scholar]
- Bishara SE, Jakobsen JR, Hession TJ, Treder JE. Soft tissue profile changes from 5 to 45 years of age. Am J Orthod Dentofac. 1998;114:698–706. [DOI] [PubMed] [Google Scholar]
- Bishara SE, Treder JE, Jakobsen JR. Facial and dental changes in adulthood. Am J Orthod Dentofac. 1994;106:175–186. [DOI] [PubMed] [Google Scholar]
- Cheung LK, Chua HD, Bendeus M. Distraction or osteotomy for the correction of maxillary cleft deformities: which is better? Ann R Australas Coll Dent Surg. 2004;17:57–63. [PubMed] [Google Scholar]
- Durtschi RB, Chung D, Gentry LR, Chung MK, Vorperian HK. Developmental craniofacial anthropometry: Assessment of race effects. Clin Anat. 2009;22:800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettema SL, Kuehn DP, Perlman AL, Alperin N. Magnetic resonance imaging of the levator veli palatini muscle during speech. Cleft Palate Craniofac J. 2002;39:130–144. [DOI] [PubMed] [Google Scholar]
- Farkas LG, Katic MJ, Forrest CR. Comparison of craniofacial measurements of young adult African-American and North American white males and females. Ann Plas Surg. 2007;59:692–698. [DOI] [PubMed] [Google Scholar]
- Figueroa AA, Polley JW, Friede H, Ko EW. Long-term skeletal stability after maxillary advancement with distraction osteogenesis using a rigid external distraction device in cleft maxillary deformities. Plas Reconstr Surg. 2004;114:1382–1392. [DOI] [PubMed] [Google Scholar]
- Fitch WT, Giedd J. Morphology and development of the human vocal tract: a study using magnetic resonance imaging. J Acoust Soc Am. 1999;106:1511–1522. [DOI] [PubMed] [Google Scholar]
- Formby WA, Nanda RS, Currier GF. Longitudinal changes in the adult facial profile. Am J Orthod Dentofacial Orthop. 1994;105:464–476. [DOI] [PubMed] [Google Scholar]
- Fu M, Barlaz MS, Holtrop JL, Perry JL, Kuehn DP, Shosted RK, Liang Z, Sutton BP. High-resolution full-vocal-tract 3D dynamic speech imaging. Magn Reson Med. 2016-in press. [DOI] [PubMed] [Google Scholar]
- Fu M, Bo Z, Shosted RK, Perry JL, Kuehn DP, Liang Z, Sutton BP. High-resolution dynamic speech imaging with joint low-rank and sparsity constraints. Magn Reson Med. 2015;73:1820–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genecov JS, Sinclair PM, Dechow PC. Development of the nose and soft tissue profile. Angle Orthod. 1990;60:191–198. [DOI] [PubMed] [Google Scholar]
- Gursoy S, Hukki J, Hurmerinta K. Five-year follow-up of maxillary distraction osteogenesis on the dentofacial structures of children with cleft lip and palate. J Oral Maxillofac Surg. 2010;68:744–750. [DOI] [PubMed] [Google Scholar]
- Handelman CS, Osborne G. Growth of the nasopharynx and adenoid development from one to eighteeen years. Angle Orthod. 1976;46:243–259. [DOI] [PubMed] [Google Scholar]
- Horner RL, Mohiaddin RH, Lowell DG, Shea SA, Burnman ED, Longmore DB, Guz A. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnea and weight matched controls. Euro Respir J. 1989;2:613–622. [PubMed] [Google Scholar]
- Inouye JM, Pelland CM, Lin KY, Borowitz KC, Blemker SS. A computational model of velopharyngeal closure for simulating cleft palate repair. J Craniofac Surg. 2015;26:658–662. [DOI] [PubMed] [Google Scholar]
- Inouye JM, Perry JL, Pelland CM, Lin KY, Borowitz KC, Blemker SS. A computational model quantifies the effect of anatomical parameters on velopharyngeal function. J Speech, Lang Hear Res. 2016;58:1119–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeans WD, Fernando DC, Maw AR, Leighton BC. A longitudinal study of the growth of the nasopharynx and its contents in normal children. British Journal Radiology, 1981;54:117–121. [DOI] [PubMed] [Google Scholar]
- Johannsdottir B, Thordarson A, Magnusson T. Craniofacial skeletal and soft tissue morphology in Icelandic adults. Euro J Ortho. 2004;26:245–250. [DOI] [PubMed] [Google Scholar]
- Kaufman JS, Cooper RS. Commentary: Considerations for use of racial/ethnic classification in etiologic research. Am J Epidemiol. 2001;154:291–298. [DOI] [PubMed] [Google Scholar]
- Kharbanda OP, Sidhu SS, Sundrum KR. Vertical proportions of face: a cephalometric study. Int J Orthod. 1991;29:6–8. [PubMed] [Google Scholar]
- Kollara L, Perry JL. Effects of gravity on the velopharyngeal structures in children using upright magnetic resonance imaging. Cleft Palate Craniofac J 2014;51:669–676. [DOI] [PubMed] [Google Scholar]
- Kollara L, Perry JL, Hudson S. Racial Variations in Velopharyngeal and craniometric morphology in children: an imaging study. J Speech Lang Hear Res, 2016;59:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn DP. A cineradiographic investigation of velar movement variables in two normal. Cleft Palate Craniofac J. 1976;13:88–103. [PubMed] [Google Scholar]
- Kuehn DP, Moon JB. Velopharyngeal closure force and levator veli palatine activation levels in varying phonetic contexts. J Speech Lang Hear Res. 1998;41:51–62. [DOI] [PubMed] [Google Scholar]
- Kuehn DP, Moon JB. Histologic study of intravelar structures in normal human adult specimens. Cleft Palate Craniofac J. 2005;42:481–489. [DOI] [PubMed] [Google Scholar]
- Krogman WM. Craniofacial growth and development: An appraisal. J Am Dental Assoc. 1973;87:1037–1043. [DOI] [PubMed] [Google Scholar]
- Lewis AB, Roche AF. The saddle angle: constancy or change? Angle Orthod. 1977;47:46–54. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, McCarthy RC, Hiiemae JB, Palmer JB. Ontogeny of postnatal hyoid and larynx descent in humans. Arch Oral Bio. 2001;46:117–128. [DOI] [PubMed] [Google Scholar]
- Lu Y, Shi B, Zheng Q, Xiao W, Li S. Analysis of velopharyngeal morphology in adults with velopharyngeal incompetence after surgery of a cleft palate. Ann Plast Surg. 2006;57:50–54. [DOI] [PubMed] [Google Scholar]
- Mason K, Perry JL, Riski JE, Fang X. Age related changes between the level of velopharyngeal closure and the cervical spine. J Craniofac Surg. 2016-in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerns D, Bzoch KR. Variations in velopharyngeal valving: the factor of sex. Cleft Palate J. 1970;7:652–662. [PubMed] [Google Scholar]
- Pecora NG, Baccetti T, McNamara JA Jr. The aging craniofacial complex: a longitudinal cephalometric study from late adolescence to late adulthood. Am J Orthod Dentofac Orthop. 2008;134(4):496–505. [DOI] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP, Gamage JK, Fang X. Anthropometric Analysis of the Velopharynx and Related Craniometric Dimensions in Three Adult Populations Using MRI. Cleft Palate Craniofac J. 2016;53(1):e1–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JP, Olson KL. Anthropometric facial analysis of the African American woman. Arch Fac Plast Surg. 2001;3:191–197. [DOI] [PubMed] [Google Scholar]
- Roche AF, Barkla DH. The level of the larynx during childhood. Ann Otol Rhino Laryngol. 1965;74:645–654. [DOI] [PubMed] [Google Scholar]
- Sagar P, Nimkin K. Feasibility study to assess clinical applications of 3-T cine MRI coupled with synchronous audio recordings during speech in evaluation of velopharyngeal insufficiency in children. Pediatr Radiol. 2015;45:217–227. [DOI] [PubMed] [Google Scholar]
- Siegel-Sadewitz VL, Shprintzen RJ. Changes in velopharyngeal valving with age. Int J Pediatr Otorhinolaryngol. 1986;11:171–182. [DOI] [PubMed] [Google Scholar]
- Scott AD, Boubertakh R, Birch MJ, Miquel ME. Towards clinical assessment of velopharyngeal closure using MRI: evaluation of real-time MRI sequences at 1.5 and 3T. Br J Radiol. 2012;85:e1083–e1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki Y, Ross R. Facial growth in children with isolated cleft palate. Cleft Palate J. 1969;6:49–62. [PubMed] [Google Scholar]
- Simpson P Dynamic consequences of differences in male and female vocal tract dimensions. J Acoust Soc Am. 2001;109:2153–64. [DOI] [PubMed] [Google Scholar]
- Smahel Z Variations in craniofacial morphology with severity of isolated cleft palate. Cleft Palate J. 1984;21:140–158. [PubMed] [Google Scholar]
- Smahel S, Mullerova Z. Craniofacial growth and development in unilateral cleft lip and palate: clinical implications (a review). Acta Chirugiae Plasticae. 1995;37:29–32. [PubMed] [Google Scholar]
- Subtelny JD. A cephalometric study of the growth of the soft palate. Plast Recon Surg. 1957;19(1):49–62. [DOI] [PubMed] [Google Scholar]
- Thilander B, Persson M, Adolfsson U. Roentgencephalometric standards for a Swedish population. A longitudinal study between the ages of 5 and 31 years. Eur J Orthod. 2005;27:370–89. [DOI] [PubMed] [Google Scholar]
- Tian W, Li Y, Yin H, Zhao SF, Li S, Wang Y, Shi B. Magnetic resonance imaging assessment of velopharyngeal motion in Chinese children after primary palatal repair. J Craniofac Surg. 2010a;21:578–587. [DOI] [PubMed] [Google Scholar]
- Tian W,Yin H, Li Y, Zhao S, Zheng Q, Shi B. Magnetic resonance imaging assessment of velopharyngeal structures in Chinese children after primary palatal repair. J Craniofac Surg. 2010b;21:568–577. [DOI] [PubMed] [Google Scholar]
- Ursi WJ, Trotman CA, McNamara JA Jr, Behrents RG. Sexual dimorphism in normal craniofacial growth. Angle Orthod. 1993;63:47–56. [DOI] [PubMed] [Google Scholar]
- Vorperian HK, Kent RD, Gentry LR, Yandell BS. Magnetic resonance imaging procedures to study the concurrent anatomic development of vocal tract structures: preliminary results. Int J Pediatr Otorhinolaryngol, 1999;49:197–206. [DOI] [PubMed] [Google Scholar]
- Vorperian HK, Kent RD, Lindstrom MJ, Kalina CM, Gentry LR, Yandell BS. Development of vocal tract length during early childhood: A magnetic resonance imaging study. J Acoust Soc Am. 2005;117:338–350. [DOI] [PubMed] [Google Scholar]
- Vorperian HK, Wang S, Chung MK, Schimek EM, Durtschi RB, Kent RD, … Gentry LR. Anatomic development of the oral and pharyngeal portions of the vocal tract: an imaging study. J Acoust Soc Am. 2009;125:1666–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorperian HK, Wang S, Schimek EM, Durtschi RB, Kent RD, Gentry LR, Chung MK. Developmental sexual dimorphism of the oral and pharyngeal portions of the vocal tract: an imaging study. J Speech Lang Hear Res. 2011;54:995–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue SA, Hao JG. Normative standards for vocal tract dimensions by race as measured by acoustic pharyngometry. J Voice. 2006;20:391–400. [DOI] [PubMed] [Google Scholar]
- Xue SA, Hao JG, Mayo R. Volumetric measurements of vocal tracts for male speakers from different races. Clin Linguist Phon. 2006;20:691–702. [DOI] [PubMed] [Google Scholar]
- Yellinedi R, Damalacheruvu MR. Is there an optimal resting velopharyngeal gap in operated cleft palate patients? Indian J Plast Surg. 2013;46:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeong P, Huggare J. Morphology of Singapore Chinese. Eur J Orthod. 2004;26:605–612. [DOI] [PubMed] [Google Scholar]
- Yuen S, Hwang J, Poon P. EMG power spectrum patterns of anterior temporal and masseter muscles in children and adults. J of Dent Res. 1989;68(5):800–4. [DOI] [PubMed] [Google Scholar]
- Zajac DJ, Mayo R. Aerodynamic and temporal aspects of velopharyngeal function in normal speakers. J Speech Lang Hear Res. 1996;39:1199–1207. [DOI] [PubMed] [Google Scholar]


