Abstract
Aim:
The aim of this study was to compare the osteogenic potential of conventional glass-ionomer cement (GIC) with chitosan-modified GIC (CH-GIC) and bioactive glass-modified GIC (BAG-GIC) as a function of time in varying proportions.
Materials and Methods:
CH-GIC was prepared by adding 10 v/v% (Group II) and 50 v/v% (Group III) CH to the commercial liquid of GIC. BAG-GIC was prepared by the addition of 10 wt% (Group IV) and 30 wt% (Group V) of BAG to the GIC powder. Conventional GIC was kept as Group I. Nine round-shaped samples measuring 2 mm thick and 5 mm in diameter were prepared for every experimental material. Human osteosarcoma cells were cultured and cell proliferation was assessed at 24, 48, and 72 h using 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) assay, and cell differentiation was assessed at 7,14, and 21 days using alkaline phosphatase (ALP) assay. All experiments were done in triplicate. The data obtained were analyzed using one-way analysis of variance and Tukey honestly significant difference post hoc multiple comparisons at 0.05 level significance.
Results:
Cell culture studies showed a significant increase in proliferative activity and ALP activity in Group II, III, IV, and V than Group I at all-time intervals (P < 0.05). There was no statistically significant difference in osteogenic potential between CH-GIC and BAG-GIC groups.
Conclusion:
The osteogenic potential was significantly higher in CH-GIC and BAG-GIC compared to conventional GIC.
Keywords: Bioactiveglass, chitosan, glass-ionomer cement, osteogenic potential
Introduction
Glass-ionomer cements (GIC) possess an edge over other dental materials by its unique property of chemical bonding to the tooth structure, biocompatibility, and fluoride release.[1] However, it lacks strength, toughness, and it is susceptible to leaching of ions on premature exposure to moisture.[2] The potential to release incorporated therapeutic agents, absence of toxic monomers, and absence of heat release during setting have pushed the scope of GIC usage beyond dentistry to the biomedical application as bone cement in orthopedic surgery.[3]
Chitosan (CH) and bioactive glass (BAG) are widely used in bone tissue engineering in recent years. CH being an inexpensive natural biopolymer, with antifungal and antibacterial properties,[4,5] has been used for wound healing[6] and periodontal therapy.[7] BAG has antimicrobial and anti-inflammatory effects and it displays osteoconductive properties which may assist the repair of bony defects.[8]
Yli-Urpo et al. reported that the addition of BAG to GIC compromised the compressive strength but increased the surface hardness, and more calcium was detected in the BAG-containing materials than in the conventional GIC.[9,10] A new polyacid to improve the mechanical properties of GI and BAG was formulated by Xie et al.[11] Petri et al. reported that the addition of 0.0044wt% of CH improved the flexural resistance, but CH contents higher than 0.022wt% led to poor performance.[12] Karthick et al. suggested that the addition of CH improved the microshear bond strength of the conventional GIC.[13] The addition of nanochitosan to GIC was found to improve fluoride release as well as the mechanical properties.[14] However, the synergistic bioactive potential of these materials can be studied for its application in those areas that do not demand good mechanical performance such as a root-end filling material or as bone cement.
The aim of this study was to compare the osteogenic potential of conventional GIC with CH-GIC and bioactive glass-modified GIC (BAG-GIC) as a function of time in varying proportions.
Materials and Methods
Preparation of chitosan-modified glass-ionomer cements
1.8 ml of glacial acetic acid is made up to 100 ml with distilled water in a 100 ml standard flask. Twenty milligram of CH (Sigma-Aldrich, USA) was weighed and dissolved in 0.3N acetic acid and made up to 100 ml with the same acetic acid in a 100 ml standard flask to get 0.2 mg/ml CH solution. 0.1 ml of 0.2 mg/ml of CH solution is added to 0.9 ml of conventional GIC (GC Fuji II, GC Corporation, Tokyo, Japan) liquid to get 10 v/v% CH-GIC. 100 mg of CH was weighed and dissolved in 0.3 N acetic acid and made up to 100 ml with the same acetic acid in a 100 ml standard flask to get 1 mg/ml CH solution. 0.5 ml of 1 mg/ml of CH solution is added to 0.5 ml of conventional GIC liquid to get 50 v/v% CH-GIC.
Preparation of bioactive glass-modified glass-ionomer cements
10 wt% BAG (Vivoxid Ltd, Finland) was measured and added to 90 wt% GIC powder (GC Fuji II, GC Corporation, Tokyo, Japan). 30 wt% of BAG was added to 70 wt% GIC powder. The mixtures were then dispensed in 20 ml plastic test tubes, sealed, and placed in a tube roller mixer for 10 min to get uniform filler particle distribution.
The experimental groups considered were as follows:
Group I – Conventional GIC
Group II – GIC+10 v/v% CH
Group III-GIC+50 v/v% CH
Group IV-GIC+10 wt% BAG
Group V-GIC+30 wt% BAG
Control.
Culture of human osteosarcoma cells
Human osteosarcoma (SaOS-2) cell line was procured from the National Center for cell sciences, Pune, India. The cells were grown in culture flasks containing Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Upon reaching confluence, as observed in the inverted phase contrast microscope, the cells were detached using trypsin-ethylenediaminetetraacetic acid (EDTA) solution and used for subculture.
Passaging the cells
The medium from the culture flask was aspirated. The flask was rinsed with 2 ml of phosphate-buffered saline (PBS) and aspirated quickly. One milliliter of trypsin-EDTA solution was added to the flask and swirled gently to cover the entire area for 10 s and aspirated quickly. Then, the flask was incubated at 37°C for 10 min. The detached cells were then resuspended in 10 ml of 10% FBS–DMEM, gently mixed well by pipetting up and down. From the cell suspension, a drop was placed to the edge of the coverslip of Neubauer hemocytometer and the drop was let to run under the coverslip by capillary action. Then, the cells from the E1, E2, E3, E4, and E5 squares were counted under the microscope. The number of cells was calculated using the formula:
Number of cells = Number of cells counted × 50,000
= X cells/ml.
Preparation of test samples
Round-shaped samples measuring 2 mm thick and 5 mm in diameter were prepared using a Teflon mold. The Teflon mold was placed on a glass plate and the experimental materials were mixed and packed into the Teflon mold. Another glass plate was placed on the top and the mold was gently compressed between glass plates until the experimental materials were set. The samples were then carefully removed from the mold.
As all the experiments were done in triplicates for three different time intervals to guarantee the reproducibility, the following number of samples was prepared.
Nine samples for every experimental material were prepared for 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and
Nine samples for every experimental material were prepared for alkaline phosphatase (ALP) assay.
Cell proliferation assessment using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The SaOS-2 cells were plated in 24-well plates at a concentration of 3 × 104 cells/well. Twenty-four hour after plating, cells were washed twice with 100 μl of PBS and starved by incubating the cells with 0.1% bovine serum albumin for 12 h at 37°C in CO2 incubator. The test samples were rinsed three times with PBS and Alpha-MEM (Minimum Essential Medium) and were placed into the wells of 24-well microtiter plates for 24 and 48 and 72 h and incubated at 37°C. All experiments were done in triplicate. At the end of the treatment, the medium from control and test material treated cells were discarded and 100 μl of MTT containing DMEM medium was added to each well. The cells were then incubated for 3 h. The MTT containing medium was then discarded and the cells were washed with PBS (200 μl). The crystals were then dissolved by adding 1 ml of dimethyl sulfoxide. The color developed is directly proportional to the number of live cells. The intensity of purple color was immediately measured in an enzyme-linked immunosorbent assay reader at 545 nm.
Cell Differentiation assessment using alkaline phosphatase assay
2 × 104 SaOS-2 cells were seeded on test samples under culture conditions in osteogenic medium and the level of ALP activity was determined at days 7, 14, and 21. The cells were detached from discs using trypsin/EDTA and centrifuged for 5 min at 1000 rpm after being washed twice with PBS. Cell lysate was obtained, and ALP activity was determined using p-nitrophenyl phosphate (pNPP) as the substrate. All experiments were done in triplicate. Upon dephosphorylated by ALP, pNPP turned yellow and its color change was directly proportional to ALP. The reaction was stopped by the addition of 1 N sodium hydroxide to reaction mixture. This colorimetric assay was finished by detecting the absorbance at 405 nm optical density value using Autoanalyzer. The ALP activity was expressed as micromoles of p-nitrophenol formed/min/microgram of protein. The ALP activity was calculated using the formula:
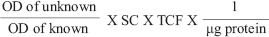
= μ moles of p-nitrophenol formed per min per μg protein
* SC-Standard concentration
* TCF-Time correction factor
In case if alignment changes - to avoid confusion - attached a jpeg image for the same formula mentioned above for your kind reference.
Statistical analysis
The raw data obtained were analyzed using analysis of variance to examine the effect of materials and time points and the interaction of these two factors on cell proliferation and ALP activity. The Tukey honestly significant difference test was used for comparison among groups at 0.05 level significance.
Results
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
To assess immediate and late toxic effects of the test materials on cell viability, the MTT assay was carried out at 24, 48, and 72 h. At the end of 24 h, there was increased proliferative activity than control in all groups except Group I (conventional GIC). After 48 h and 72 h, Group I was not significantly different from control. There was a statistically significant increase in proliferative activity in Groups II, III, IV, and V than Group I (conventional GIC) at 24, 48, and 72 h (P < 0.05) [Table 1]. There was no statistically significant difference between Group II, Group III, and Group V. At 24 and 48 h, Group IV showed significantly less proliferative activity than Groups II, III, and V (P < 0.05) [Figure 1].
Table 1.
One-way ANOVA for MTT assay
| Duration | Groups | Mean | SD | F | Significant |
|---|---|---|---|---|---|
| 24 h | Group I | 0.82700 | 0.032187 | 186.813 | 0.000 |
| Group II | 2.77433 | 0.233204 | |||
| Group III | 2.80700 | 0.033422 | |||
| Group IV | 1.69733 | 0.057204 | |||
| Group V | 2.79867 | 0.039323 | |||
| Control | 1.57700 | 0.077621 | |||
| Total | 2.08022 | 0.790549 | |||
| 48 h | Group I | 0.83000 | 0.022869 | 143.197 | 0.000 |
| Group II | 2.83000 | 0.171852 | |||
| Group III | 2.88533 | 0.119039 | |||
| Group IV | 1.84633 | 0.216632 | |||
| Group V | 2.88133 | 0.165618 | |||
| Control | 0.89867 | 0.057839 | |||
| Total | 2.02861 | 0.932860 | |||
| 72 h | Group I | 0.85367 | 0.111935 | 56.156 | 0.000 |
| Group II | 2.89667 | 0.213294 | |||
| Group III | 2.92667 | 0.113931 | |||
| Group IV | 2.27800 | 0.469920 | |||
| Group V | 2.91467 | 0.170016 | |||
| Control | 0.87367 | 0.064143 | |||
| Total | 2.12389 | 0.965627 |
SD: Standard deviation; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Figure 1.
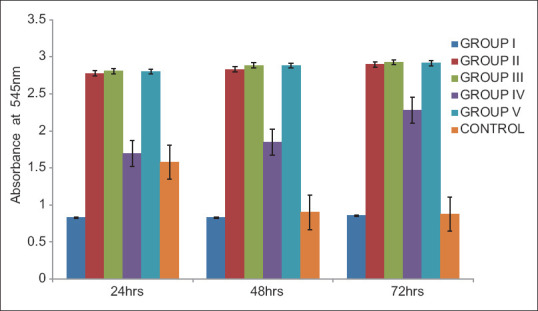
Comparison of optical density values for 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
Alkaline phosphatase assay
ALP activity was tested at 7, 14, and 21 days to assess long-term bioactivity of the test materials.
Throughout the observation period, Group II, Group III, Group IV, and Group V showed higher ALP activity than Group I (conventional GIC) and it was statistically significant (P < 0.05) [Table 2]. There was no statistically significant difference in ALP activity between Group II, Group III, and Group IV and V. At the end of 21 days, Group II, Group III, Group IV, and Group V showed significantly greater ALP activity than control (P < 0.05) [Figure 2].
Table 2.
One-way ANOVA for alkaline phosphatase assay
| Duration | Groups | Mean | SD | F | Significanct |
|---|---|---|---|---|---|
| 7 days | Group I | 268.700 | 36.6083 | 9.751 | 0.001 |
| Group II | 443.600 | 52.8071 | |||
| Group III | 448.600 | 50.4107 | |||
| Group IV | 422.533 | 32.2598 | |||
| Group V | 445.000 | 33.4591 | |||
| Control | 392.900 | 5.8592 | |||
| Total | 403.556 | 72.6631 | |||
| 14 days | Group I | 455.37 | 49.14 | 15.378 | 0.000 |
| Group II | 318.77 | 24.94 | |||
| Group III | 542.47 | 48.69 | |||
| Group IV | 543.53 | 53.81 | |||
| Group V | 527.07 | 30.28 | |||
| Control | 543.47 | 15.11 | |||
| Total | 488.44 | 90.73 | |||
| 21 days | Group I | 402.933 | 25.0847 | 26.297 | 0.000 |
| Group II | 574.433 | 23.1641 | |||
| Group III | 582.900 | 15.1700 | |||
| Group IV | 564.367 | 26.0983 | |||
| Group V | 576.833 | 10.6444 | |||
| Control | 477.567 | 38.8805 | |||
| Total | 529.839 | 72.1643 |
SD: Standard deviation
Figure 2.
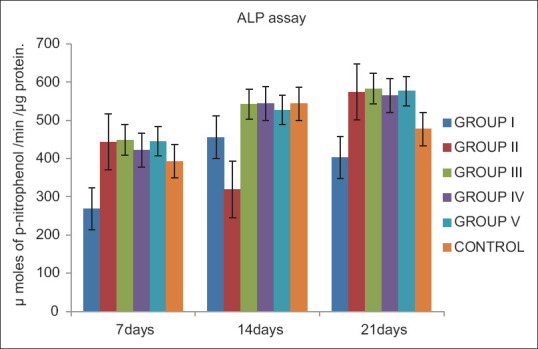
Comparison of alkaline phosphatase activity expressed as μ moles of p-nitrophenol formed/min/μg protein
Discussion
GIC is a versatile material which is being subjected to various modifications to improve its properties. Currently, researches are oriented toward the development of biomaterials having therapeutic functions in addition to its inherent properties.
Yli-Urpo et al. researched on addition of 10 wt% and 30 wt% BAG to conventional GIC and found the combination to be bioactive with compressive strength being compromised on increase in the amount of BAG.[9,10] Petri et al. reported that the addition of CH in lesser concentration to GIC improved the flexural strength, but an increase in CH concentration was found to compromise the material properties.[12] Karthick et al. reported increased microshear bond strength with 10 v/v% and 50 v/v% CH-GIC when compared to conventional GIC.[13] Similar results of increased microshear bond strength were reported by Debnath et al., with 10 v/v% CH-GIC. It was also suggested that 10 v/v% CH-GIC resulted in improved antibacterial property against Streptococcus mutans.[15] Therefore, the study intended to determine the osteogenic potential of GIC by addition of bioactive materials in both lower and higher concentrations.
The results showed that conventional GIC exhibited less proliferative activity than other experimental groups in first 24 h. This could be explained by the fact that the in vitro toxicity of GICs was due to a complex mechanism based on both ion release, in particular aluminum and fluoride ions, and pH effects.[16,17,18]
The increased cell proliferative activity in Group II (GIC + 10 v/v% CH) and Group III (GIC + 50 v/v% CH) was in accordance with the previous studies.[19,20] Shi et al. showed CH-coated iron oxide nanoparticles enhanced osteoblast proliferation, decreased cell membrane damage, and promoted cell differentiation, as indicated by an increase in ALP and extracellular calcium deposition.[20] Mathews et al. stated that CH upregulated genes associated with calcium binding and mineralization.[21]
The higher proliferative activity of Group IV (GIC +10wt% BAG) and GroupV (GIC + 30wt % BAG) than Group I (conventional GIC) could be explained by the fact that BAG has the ability to stimulate cell cycling and subsequently enhance osteoblastic turnover of human primary osteoblasts.[22,23] At the end of 24 h, the proliferative activity of Group IV was not significantly different from control. However, at 48 and 72 h, Group IV showed higher proliferative activity. The probable reason could be less amount of BAG (10 wt %) added to GIC. With time, the reactivity of BAG increased resulting in release of calcium and phosphate ions on the surface that lead to increased osteoblastic proliferation. De Caluwé et al. reported that BAG-GIC improved the bioactivity of the GIC by the formation of an apatite layer, but the strength was compromised with increase in the proportion of BAG and concluded that BAG-GIC with 10 mol% Al3+ yielded better physiochemical properties when added in ≤20 wt% to a GIC.[24]
Within the limitations of this study, both CH- and BAG-modified GIC exhibited excellent osteogenic potential with no statistically significant difference between the two materials. Further studies shall be performed employing CH and BAG into GIC to establish its usage as root-end filling material or as a bone cement with excellent healing properties.
Conclusion
From the present study, it can be concluded that the addition of CH and BAG into GIC resulted in a significant increase in osteogenic effect when compared to conventional GIC. Therefore, CH-GIC and BAG-GIC shall be suggested as a promising bioactive material with added therapeutic advantage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would wish to thank Department of Endocrinology, Dr. ALM P.G. Institute of Basic Medical Sciences, University of Madras, Sekkizhar Campus, Taramani, Chennai, Tamil Nadu, India.
References
- 1.Wilson AD, McLean JW. Glass Ionomer Cement. Chicago, IL, USA: Quintessence Publishing Co; 1988. [Google Scholar]
- 2.Prosser HJ, Powis DR, Brant P, Wilson AD. Characterisation of glass-ionomer cements. 7. The physical properties of current cements. J Dent. 1984;12:231–40. doi: 10.1016/0300-5712(84)90067-8. [DOI] [PubMed] [Google Scholar]
- 3.Brook IM, Hatton PV. Glass-ionomers: Bioactive implant materials. Biomaterials. 1998;19:565–71. doi: 10.1016/s0142-9612(98)00138-0. [DOI] [PubMed] [Google Scholar]
- 4.Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133–42. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 5.Pradip KD, Joydeep D, Tripathi VS. Chitin and chitosan: Chemistry, properties and applications. J Sci Ind Res. 2004;63:20–31. [Google Scholar]
- 6.Xiahong W, Yan Y, Renji Z. A comparison of chitosan & collagen sponges as hemostatic dressings. J Bioact Compat Polym. 2006;21:39–54. [Google Scholar]
- 7.Qasim SB, Najeeb S, Delaine-Smith RM, Rawlinson A, Rehman IU. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent Mater. 2017;33:71–83. doi: 10.1016/j.dental.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Mehrvarzfar P, Akhavan H, Rastgarian H, Akhlagi NM, Soleymanpour R, Ahmadi A. An In vitro comparative study on the antimicrobial effects of bioglass 45S5 vs. calcium hydroxide on Enterococcus faecalis. Iran Endod J. 2011;6:29–33. [PMC free article] [PubMed] [Google Scholar]
- 9.Yli-Urpo H, Vallittu PK, Närhi TO, Forsback AP, Väkiparta M. Release of silica, calcium, phosphorus, and fluoride from glass ionomer cement containing bioactive glass. J Biomater Appl. 2004;19:5–20. doi: 10.1177/0085328204044538. [DOI] [PubMed] [Google Scholar]
- 10.Yli-Urpo H, Lassila LV, Närhi T, Vallittu PK. Compressive strength and surface characterization of glass ionomer cements modified by particles of bioactive glass. Dent Mater. 2005;21:201–9. doi: 10.1016/j.dental.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Xie D, Zhao J, Weng Y, Park JG, Jiang H, Platt JA. Bioactive glass-ionomer cement with potential therapeutic function to dentin capping mineralization. Eur J Oral Sci. 2008;116:479–87. doi: 10.1111/j.1600-0722.2008.00562.x. [DOI] [PubMed] [Google Scholar]
- 12.Petri DF, Donegá J, Benassi AM, Bocangel JA. Preliminary study on chitosan modified glass ionomer restoratives. Dent Mater. 2007;23:1004–10. doi: 10.1016/j.dental.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 13.Karthick A, Kavitha M, Loganathan SC, Malarvizhi D. Evaluation of microshear bond strength of chitosan modified GIC. World J Med Sci. 2014;10:169–73. [Google Scholar]
- 14.Kumar RS, Ravikumar N, Kavitha S, Mahalaxmi S, Jayasree R, Kumar TS, et al. Nanochitosan modified glass ionomer cement with enhanced mechanical properties and fluoride release. Int J Biol Macromol. 2017;104:1860–65. doi: 10.1016/j.ijbiomac.2017.05.120. [DOI] [PubMed] [Google Scholar]
- 15.Debnath A, Kesavappa SB, Singh GP, Eshwar S, Jain V, Swamy M, et al. Comparative evaluation of antibacterial and adhesive properties of chitosan modified glass ionomer cement and conventional glass ionomer cement: An in vitro study. J Clin Diagn Res. 2017;11:ZC75–8. doi: 10.7860/JCDR/2017/25927.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devlin AJ, Hatton PV, Brook IM. Dependence of in vitro biocompatibility of ionomeric cements onion release. J Mater Sci Mater Med. 1998;9:737–41. doi: 10.1023/a:1008907119794. [DOI] [PubMed] [Google Scholar]
- 17.Sasanaluckit P, Albustany KR, Doherty PJ, Williams DF. Biocompatility of glass ionomer cements. Biomaterials. 1993;14:906–16. doi: 10.1016/0142-9612(93)90132-l. [DOI] [PubMed] [Google Scholar]
- 18.Oliva A, Ragione FD, Salerno A, Riccio V, Tartaro G, Cozzolino A, et al. Biocompatibility studies on glass ionomer cements by primary cultures of human osteoblasts. Biomaterials. 1996;17:1351–6. [PubMed] [Google Scholar]
- 19.Lahiji A, Shorabi A, Hungerford DS, Frondoza CG. Chitosan supports expression of extra cellular matrix proteins in human osteoblasts and chondrocytes. J Biomed Mater Res. 2000;51:586–95. doi: 10.1002/1097-4636(20000915)51:4<586::aid-jbm6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Shi SF, Jia JF, Guo XK, Zhao YP, Chen DS, Guo YY, et al. Biocompatibility of chitosan-coated iron oxide nanoparticles with osteoblast cells. Int J Nanomedicine. 2012;7:5593–602. doi: 10.2147/IJN.S34348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathews S, Gupta PK, Bhonde R, Totey S. Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by up regulating the associated genes. Cell Prolif. 2011;44:537–49. doi: 10.1111/j.1365-2184.2011.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xynos ID, Hukkanen MV, Batten JJ, Buttery LD, Hench LL, Polak JM. Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation in vitro: Implications and applications for bone tissue engineering. Calcif Tissue Int. 2000;67:321–9. doi: 10.1007/s002230001134. [DOI] [PubMed] [Google Scholar]
- 23.Foppiano S, Marshall SJ, Marshall GW, Saiz E, Tomsia AP. The influence of novel bioactive glasses on in vitro osteoblast behavior. J Biomed Mater Res A. 2004;71:242–9. doi: 10.1002/jbm.a.30159. [DOI] [PubMed] [Google Scholar]
- 24.De Caluwé T, Vercruysse CW, Ladik I, Convents R, Declercq H, Martens LC, et al. Addition of bioactive glass to glass ionomer cements: Effect on the physico-chemical properties and biocompatibility. Dent Mater. 2017;33:e186–203. doi: 10.1016/j.dental.2017.01.007. [DOI] [PubMed] [Google Scholar]


