Abstract
Recent development regarding mixture of H2 (concentration of ~66%) with O2 (concentration of ~34%) for medical purpose, such as treatment of coronavirus disease-19 (COVID-19) patients, is introduced. Furthermore, the design principles of a hydrogen inhaler which generates mixture of hydrogen (~66%) with oxygen (~34%) for medical purpose are proposed. With the installation of the liquid blocking module and flame arresters, the air pathway of the hydrogen inhaler is divided by multiple isolation zones to prevent any unexpected explosion propagating from one zone to the other. An integrated filtering/cycling module is utilized to purify the impurity, and cool down the temperature of the electrolytic module to reduce the risk of the explosion. Moreover, a nebulizer is provided to selectively atomize the water into vapor which is then mixed with the filtered hydrogen-oxygen mix gas, such that the static electricity of a substance hardly occurs to reduce the risk of the explosion. Furthermore, hydrogen concentration detector is installed to reduce the risk of hydrogen leakage. Result shows that the hydrogen inhaler implementing the aforesaid design rules could effectively inhibit the explosion, even ignition at the outset of the hydrogen inhaler which outputs hydrogen-oxygen gas (approximately 66% hydrogen: 34% oxygen).
Keywords: anti-explosion, cancer, COVID-19, hydrogen, hydrogen concentration, hydrogen inhaler, medical purpose, nebulizer, respiratory disease, static electricity
INTRODUCTION OF HYDROGEN BIOMEDICINE
Hydrogen (H2) has been utilized in diving medicine for many years, and it is proved that hydrogen, unlike other medical gases such as nitric oxide, carbon monoxide, or hydrogen sulfide, is non-toxic for human body even at high pressure or concentration.1,2 However, hydrogen’s therapeutic effects against diseases were unknown or ignored in the past. Until 1975, hydrogen’s inhibitory effect against squamous cell carcinoma of mouse skin was reported by Dole et al.3 Roberts et al.4 also disclosed hydrogen’s prohibition against the growth of mouse tumor cells and leukemia cells in 1978. Especially, in 2007 Ohsawa et al.5 discovered that hydrogen is capable of selectively detoxifying hydroxyl radicals (• OH) and peroxynitrite (ONOO–) among active oxygens (such selectively detoxifying ability was further discussed by Liu et al.6 in 2010). Thereafter, the study and research of hydrogen biomedicine has been rapidly grown, including not limited to alleviation of inflammation,7,8,9 alleviation of cell apoptosis,10,11 alleviation of side effects caused by anti-cancer therapy,12,13 and cancer inhibition.14
Due to lack of safe and durable hydrogen inhalers which can generate inhaled gas with higher H2 concentration, conventionally hydrogen related animal studies or clinical trials were performed based on either inhaled gas with H2 concentration of less than 4%, or hydrogen-rich water with hydrogen concentration far less than 4%,15,16,17,18 nevertheless, the H2 concentration of less than 4% is not necessary for the optimum value in the human body. Especially, Graham’s Law of diffusion states that the rate of diffusion of one gas is inversely proportional to the square root of the density of the gas19 and the Bernoulli principle also states that an increase in the speed of a fluid occurs simultaneously with a decrease in pressure.20 The higher the H2 concentration in the inhaled gas, the lower the density of the inhaled gas. Therefore, the inhaled gas with higher H2 concentration then has the greater diffusion speed and lower pressure. Thus, it will reduce the driving pressure for human body to breathe the inhaled gas with higher H2 concentration.
With the support of hydrogen inhaler AMS-H-01/AMS-H-03 provided by Asclepius Meditec Co., Ltd., tens of animal studies or pilot clinical trials based on inhaled gas which combines H2 (concentration of ~66%) with O2 (concentration of ~34%) were accomplished and published.14,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54 Additionally, recent formal clinic trials based on the same inhaler AMS-H-01/AMS-H-03 also revealed positive results in respiratory diseases, such as allergic rhinitis, asthma, chronic obstructive pulmonary disease, etc. Especially, it is recommended to use the mixed gas of 66.6% H2/33.3% O2 for treating coronavirus disease-19 (COVID-19) patients as described in the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia, 7 Edition released by National Health Commission of China,55 and such guideline is also republished in English by the World Health Organization China Office.56 Moreover, a survey report regarding 82 cancer patients with the help of the same inhaler AMS-H-01 also demonstrated that in patients with advanced cancer, inhaling gas with H2 concentration of 64–67% can improve patients’ quality of life and control cancer progression.53 Therefore, how to provide safe and durable hydrogen-oxygen inhalers with higher H2 concentration is key factor to expedite and realize the hydrogen biomedicine. Search strategy is shown in Additional file 1 (104.7KB, pdf) .
BASIS OF HYDROGEN INHALER
The currently available hydrogen inhalers can be classified as ion exchange membrane type or electrolytic solution type. Figure 1 shows an ion exchange membrane-based hydrogen inhaler which conventionally uses a platinum-coated ion exchange membrane to separate the electrolysis tank into an anode chamber and a cathode chamber. When the anode and cathode electrodes of the electrolysis tank are supplied with electrical power, oxygen is generated in the anode chamber, and the extra protons (H+) are transported from the anode chamber to the cathode chamber through the ion exchange membrane and combine with electrons on the cathode to form hydrogen therein. Since the ion exchange membrane is structured to separate the electrolytic tank, theoretically almost 100% hydrogen gas could be obtained from the cathode chamber.
Figure 1.
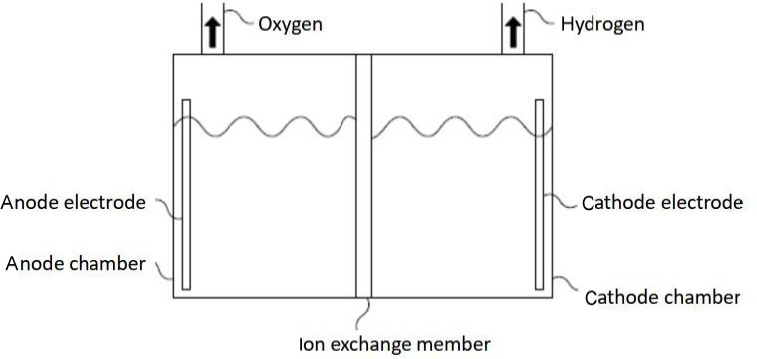
Ion exchange membrane-based hydrogen inhaler.
However, 100% hydrogen gas is not suitable for direct inhalation of human body and shall be mixed with air.57 It is considered as oxygen-deficient gas when the O2 concentration of the inhaled gas is slower than 19.5%, and it is dangerous for human begins when the O2 concentration of the inhaled gas is slower than 16%.58 Mixing 100% hydrogen gas from the ion exchange membrane-based hydrogen inhaler with air for inhaling will inevitably dilute the original O2 concentration (~21%) in the air and likely poses negative impact to subject users. For example, a human body consumes approximately air of 6 L/min. Supposedly the ion exchange membrane-based hydrogen inhaler outputs 100% hydrogen gas of 1 L/min and only half of them (500 mL) is inhaled and mixed with the 5.5 L of air for human body, the oxygen concentration of the mixed gas (air of 5.5 L + 100% hydrogen gas of 500 mL) is approximately reduced to 19.25%. Therefore, it is not recommended to inhale 100% hydrogen gas for a long period of time.
Moreover, deionized water is required for ion exchange membrane-based hydrogen inhaler to maintain the normal operation; otherwise impurities in regular water would block ion channels of the ion exchange membrane and then the function thereof fails. Additionally, the lifetime of such hydrogen inhaler is shorter due to the inevitable decay of the platinum catalyst coated on the ion exchange membrane. Furthermore, ion exchange membrane is a polymer membrane (such as perfluorosulfonic acid proton exchange membrane) mainly used in fuel cells and may not be suitable for medical purpose.59 To be worse, fluoride could be likely released from the perfluorosulfonic acid proton exchange membrane during operation.60
The traditional electrolytic solution-based hydrogen inhaler is illustrated as follows. As shown in Figure 2, two electrodes of the electrolytic tank undergo an electrochemical reaction against water to produce oxygen and hydrogen which are then mixed within the electrolytic tank. The H2 concentration in the hydrogen-oxygen mixed gas is approximately equal to 66%. Unlike the ion exchange membrane-based hydrogen inhaler, deionized water is not necessary for the operation of electrolytic solution-based hydrogen inhaler, but it is recommended to use regular pure water or distilled water.
Figure 2.
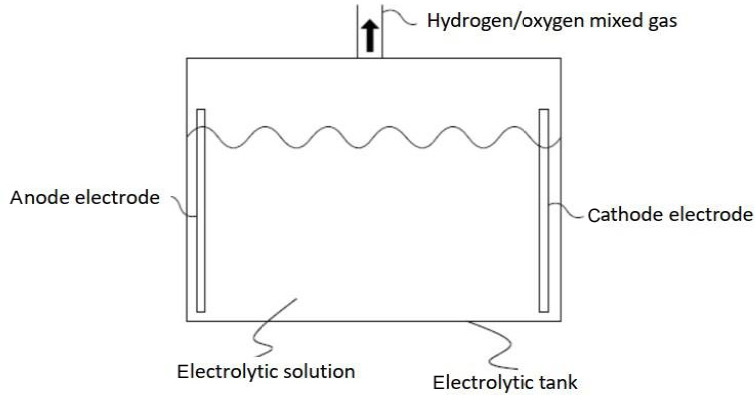
Electrolytic solution-based hydrogen inhaler.
Although the electrolytic solution-based hydrogen inhaler is a simple structure, electrolyte is required in the electrolytic solution to enhance electrolysis efficiency, and alkali mist generated during high temperature may irritate the human body if such alkali mist is not properly filtered and isolated. Moreover, heat released during the electrolysis process unavoidably raises the temperature of the electrolytic tank, and it is necessary to intermittently shut down the traditional electrolytic solution-based hydrogen inhaler for safety purpose. Furthermore, there exists risk of detonation due to the hydrogen (~66%)-oxygen (~34%) mixed gas in the event no suitable anti-explosion devices are designed in the solution-based hydrogen inhaler.
DESIGN OF THE HYDROGEN INHALER FOR MEDICAL PURPOSE
Medical-grade hydrogen inhaler is the prerequisite for the development of hydrogen biomedicine. It should be safe, durable, and can stably generate higher flow rate (e.g., over 2 L/min) and higher hydrogen concentration (e.g., over 50%) over a long period (e.g., at least over 8 hours without any interrupt of operation). The lifetime of the hydrogen inhaler shall be more than 5000 operating hours, and no additional electrolyte shall be supplemented during the above lifetime of the solution-based hydrogen inhaler.
Furthermore, all materials of the hydrogen inhaler used in contact with gas/liquid should be medical-grade and the hydrogen inhaler itself should be made in compliance with all international standards of medical device. Additionally, all impurity generated during the electrolysis process shall be filtered and isolated, ensuring that the outputted hydrogen-oxygen mixture or hydrogen meets the specifications of medical respiratory gas. The examples of available standards are shown in Table 1.
Table 1.
Examples of available standards for medical device
| International standard number | Title of standard |
|---|---|
| IEC 60601-1:2005 | Medical electrical equipment—Part 1: General requirements for safety |
| IEC 60601-1-11:2015 | Medical electrical equipment – Part 1–11: General requirements for basic safety and essential performance – Collateral Standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment |
| IEC 60601-1-2:2007 | Medical electrical equipment-Part 1–2: General requirements for safety-Collateral standards: Electromagnetic compatibility-Requirements and tests |
| ISO 18562-2:2017 | Biocompatibility evaluation of breathing gas pathways in healthcare applications-Parts 2: Tests for emissions of particulate matter |
| ISO 18562-3:2017 | Biocompatibility evaluation of breathing gas pathways in healthcare applications-Parts 3: Tests for emissions of volatile organic compounds |
| ISO 18562-4:2017 | Biocompatibility evaluation of breathing gas pathways in healthcare applications-Parts 4: Tests for leachable in condensate |
| ISO 10993-5:2016 | Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity |
| ISO 10993-10:2009 | Biological evaluation of medical devices—Part 10: Tests for irritation and skin sensitization |
The following are the proposed design rules of a safe and durable hydrogen inhaler for medical purpose:
Electrolytic module and filtering module
As shown in Figure 3, the electrolytic module is the kernel for the hydrogen inhaler, it will either generates almost 100% hydrogen gas (ion exchange membrane type) or hydrogen-oxygen mixed gas (H2 ~66%: O2 ~34%, electrolytic solution type) which is then combined with air for the breath of human body. It is important to reduce the space of the gas chamber in the electrolytic module, such that only few residuals of hydrogen gas or hydrogen-oxygen mixed gas would be kept in the electrolytic module to reduce the risk or impact of the unexpected explosion.
Figure 3.
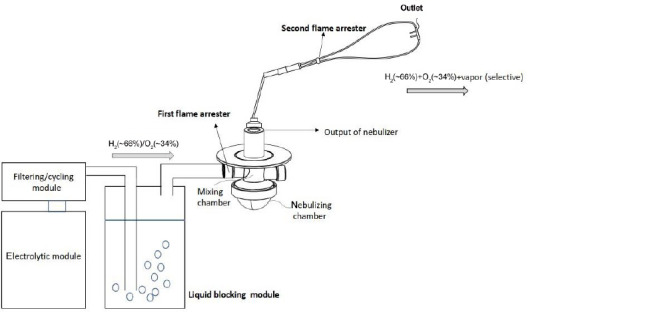
Proposed structure of safe and durable hydrogen inhaler for medical purpose.
Furthermore, filtering module shall be installed to purify the hydrogen gas or hydrogen/oxygen mixed gas in order to comply with available medical gas standards. Additionally, double or triple purification processes are recommended to filter various potential impurities, such as fluoride or electrolyte.
Cycling module
During electrolytic process, heat will be generated and eventually raises the temperature of the electrolytic module. Such continuously raised temperature could shorten the lifetime of the electrolytic module, breaks sealing of the electrolytic module and then causes the leakage of the liquid/gas therein, and likely triggers the explosion. Therefore, a cycling module to circulate and cool down the liquid in the electrolytic module is critical to maintain the lifetime and safety, as shown in Figure 3. A temperature sensor coupled to the electrolytic module can monitor the temperature of the electrolytic module and activates the cycling module whenever necessary.
Moreover, when the cycling module is utilized in the electrolytic solution type hydrogen inhaler, it could also circulate water and flush residual electrolyte in the air pathway of the hydrogen inhaler back to the electrolytic module. Flushing the residual electrolyte back to the electrolytic module could recycle the electrolyte and extend the operation hours of the hydrogen inhaler.
Liquid blocking module
A liquid blocking module in the hydrogen inhaler is an anti-explosion device. It will isolate the electrolytic module from the output of the liquid blocking module; therefore, to block the subject user from any impact of unexpected explosion happened in the electrolytic module. On the other hand, the liquid blocking module prohibits any accidental explosion propagating from the output of the liquid blocking module into the electrolytic module, and therefore to prevent any damage to the electrolytic module caused by any ignition happening outside the liquid blocking module. Thus, a first isolation zone is formed between the electrolytic module and the liquid blocking module, and any unexpected explosion will be restrained therein (Figure 3).
Besides, the liquid blocking module could lower the temperature of the hydrogen gas or hydrogen-oxygen mixed gas generated from the hot zone of the electrolytic module, and further dissolve potential impurity of the generated gas in the liquid as well.
Nebulizer
In the conventional hydrogen inhaler, a carbon dioxide absorbent is common used to partially remove the vapor in the hydrogen gas or hydrogen-oxygen mix gas. As a result, the mucus layer of the respiratory organ could not be kept in a normal state, and the respiratory airway could not be protected by the mucus layer. Therefore, it is necessary to provide appropriate humidity in the hydrogen gas or hydrogen-oxygen mixed gas for the breath of subject users. The nebulizer is provided to selectively atomize the water into vapor at the nebulizing chamber and the vapor will be mixed with the hydrogen gas or hydrogen-oxygen mixed gas in the mixing chamber, as shown in Figure 3. Furthermore, the nebulizer could atomize suitable medicine for breath as well to assist treatment of human body.
Moreover, when the humidity increases, the electrical resistance of a substance (gas or the subject user) decreases and static electricity hardly occurs. The disappearing time during which static electricity is eliminated also decreases exponentially as the absolute humidity increases.61 Therefore, the higher the relative humidity, the less likely static electricity causes explosion. With the help of vapor combined with the hydrogen gas or hydrogen/oxygen mix gas, the static electricity of a substance (gas or the subject user) hardly occurs, and the explosion incurred by the static electricity could be prevented.
Flame arresters
It is known that flame will be extinguished if the dimension of the air pathway is 0.20 mm or less at any hydrogen gas concentration.62 Based on the 0.20 mm air pathway criteria, a flame arrester made of stainless steel meshes which are randomly compressed and overlapped together can set up pathway the dimension of which is less than 0.2 mm. Therefore, the flame arrester is also an anti-explosion device to extinguish flame and to prevent explosion from propagating. The flame arrester could be installed at the input of the nebulizer such that a second isolation zone is formed between the nebulizer and the liquid blocking module, and any unexpected explosion in the second isolation zone will be confined therein.
For the convenience of breath of the subject user, a comfortable nasal tube is necessary. An additional flame arrester could be installed in the nasal tube such that a third isolation zone is formed between the nasal tube and the nebulizer, and again any unexpected explosion in the third isolation zone will be confined therein. Moreover, such flame arrester is preferred to be installed closed to the output of the nasal tube to reduce the space between such flame arrester and the outlet of the nasal tube (e.g., within the range of 1–2 cm3). In the event any static electricity generated from the subject user or intentional ignition happens at the outlet of the nasal tube, the small space of 1–2 cm3 hardly causes explosion, and therefore prevent damage to the subject user.
Hydrogen concentration detection
It is well discussed that the H2 concentration of the mixed gas higher than 4% may cause explosion. Nevertheless, even the hydrogen inhaler outputs the hydrogen-oxygen mixed gas and the H2 concentration of which is around 66%, H2 concentration around the subject user breathing the hydrogen-oxygen mixed gas is less than 1% (as discussed later). Thus, during normal operation it is almost unlikely to incur explosion even there is unexpected flame or ignition close to the subject user. However, in the event there is any abnormal situation happened during the operation of the hydrogen inhaler, such as leakage in the hydrogen inhaler, H2 concentration around the subject user may be higher than 4% and there still exits risk of detonation. Therefore, a detector could be installed to monitor the H2 concentration and the hydrogen inhaler should be immediately shut down if the detected H2 concentration is approaching 4%.
RESULTS AND DISCUSSION
Pursuant to the aforesaid design principles, AMS-H-03 hydrogen inhaler is developed and built. It provides inhaled gas with H2 concentration of ~66% and O2 concentration of ~34%, at output rate of 2–3 L/min. Since O2 concentration of the inhaled gas is higher than 21%, any combination of the inhaled gas and air for the breath of human body will not be considered as oxygen-deficient gas.
During the operation, the electrolytic module of the AMS-H-03 hydrogen inhaler generates hydrogen-oxygen mixed gas (H2 ~66%: O2 ~34%, electrolytic solution type) which then passes through the filtering module and the liquid blocking module for purification. One additional filter is installed after the liquid blocking module to further percolate the hydrogen-oxygen mixed gas. Such triple purification (including the filtering module, the liquid blocking module, and the other filter) will assure that the outputted hydrogen-oxygen mixed gas is complied with available medical gas standards.
When the temperature of the electrolytic module raises due to the heat generated by the electrolytic process, one cycling module of AMS-H-03 then activated to circulate and cool down the liquid in the electrolytic module. Furthermore, in the event the water in the electrolytic module is consumed or exhausted due to the electrolytic process, another cycling module of AMS-H-03 will supply water into the electrolytic module through the filtering module, and the residual electrolyte originally blocked by the filter module will then be flushed back to the electrolytic module simultaneously. Flushing the residual electrolyte back to the electrolytic module could recycle the electrolyte and extend the operation hours of the hydrogen inhaler.
The gas pathway of AMS-H-03, beginning from the water surface of the electrolytic module to the outlet of the nasal tube, is designed as short as possible. Moreover, such gas pathway is further divided into three isolation zones, one is between the electrolytic module and the liquid blocking module, another is between the liquid blocking module and a flame arrester coupled to the nebulizer, and the other is between the flame arrester coupled to the nebulizer and anther flame arrester close to the outlet of the nasal tube worn by a user. With the installation of the liquid blocking module and flame arresters, any unexpected explosion generated in one isolation zone will be prevented from propagating to the other isolation zone. Moreover, the nebulizer is provided to selectively atomize the water into vapor which is then mixed with the filtered hydrogen/oxygen mix gas, not only to maintain appropriate humidity in the hydrogen gas or hydrogen/oxygen mix gas for the respiratory system of subject users, but also to reduce the risk of explosion incurred by the static electricity.
A multicenter randomized clinical trial that verifies the efficacy and safety regarding inhalation of H2 (~66%) and O2 (~34%) in patients with COVID-19 was reported based on utility of hundreds of AMS-H-03 hydrogen inhalers. It is found that inhalation of H2 (~66%) and O2 (~34%) significantly improves disease severity of patients with COVID-19, such as improvement of chest distress, chest pain, resting oxygen saturation, and dyspnea scale, etc.63 As previously mentioned, the inhaled gas with higher H2 concentration has the greater diffusion speed and lower pressure. Thus, mixture of H2 (~66%) and O2 (~34%) could decrease the inspiratory efforts due to the significantly lower resistance when passing through the respiratory tract,63 and could be an option for relieving respiratory symptoms in patients with COVID-19.
Acid/alkaline measurement and hydrogen concentration measurement
The inhaled gas of the AMS-H-01/AMS-H-03 is subject to the tests set forth in GB8982-2009 (CN) regarding standards of medical gas and aviation gas. With the help of filtering module and cycling module, the inhaled gas of the AMS-H-01/AMS-H-03 passes the section 5.4 of GB8982-2009 (CN) regarding the residual amount of acid material and alkaline material. Therefore, the alkali mist is totally removed from the inhaled gas of the AMS-H-01/AMS-H-03.
As shown in Table 2, during the normal operation, even H2 concentration at the output of the nebulizer and nasal tube is around 64–67%, the H2 concentration around the subject user is less than 1% and the H2 concentration of exhaled breath of the subject user is less than 4%, both of which are less than the critical explosive 10% H2 concentration in the mixture of hydrogen and air (or oxygen).57,64,65,66 Therefore, during normal operation, in the event there is unexpected flame or ignition close to the subject user, it is almost unlikely to incur explosion or detonation due to the fact that the H2 concentration surrounding the subject user is less than 4%.
Table 2.
H2 concentration measured at different locations
| Subject code | VA (%) | OA (%) | ONT (%) | Test time (min) | A (%) | B (%) | C (%) | D (%) | Exhaled breath (%) |
|---|---|---|---|---|---|---|---|---|---|
| DUYI | 0 | 65 | 65 | 5 | 0 | 0.4 | 0 | 0 | 2.5 |
| 60 | 0.4 | 0 | 0 | 0.3 | |||||
| 120 | 0 | 0 | 0 | 0 | |||||
| LLDI | 0 | 66 | 66 | 5 | 0.3 | 0.3 | 0.3 | 0.3 | 1.9 |
| 60 | 0.3 | 0.3 | 0.4 | 0.3 | |||||
| 120 | 0.3 | 0.5 | 0.5 | 0.3 | |||||
| YSFA | 0 | 64 | 64 | 5 | 0.4 | 0.3 | 0.5 | 0.3 | 0.9 |
| 60 | 0.4 | 0.3 | 0 | 0 | |||||
| 120 | 0 | 0.7 | 0 | 0 | |||||
| MLRO | 0 | 64 | 64 | 5 | 0.3 | 0.3 | 0.3 | 0.4 | 2.8 |
| 60 | 0 | 0 | 0 | 0 | |||||
| 120 | 0 | 0 | 0 | 0 | |||||
| ZCSH | 0 | 64 | 64 | 5 | 0 | 0 | 0 | 0 | 2 |
| 60 | 0.3 | 0.3 | 0.7 | 0.6 | |||||
| 120 | 0.7 | 0.3 | 0.6 | 0 | |||||
| DUYI | 0 | 64 | 64 | 5 | 0.5 | 0 | 0 | 0 | 2.3 |
| 60 | 0 | 0 | 0 | 0 | |||||
| 120 | 0 | 0 | 0 | 0 | |||||
| LLDI | 0 | 66 | 66 | 5 | 0.4 | 0.5 | 0.6 | 0.4 | 1.3 |
| 60 | 0.4 | 0.4 | 0.4 | 0.4 | |||||
| 120 | 0.8 | 0.7 | 0.5 | 0.4 | |||||
| YSFA | 0 | 67 | 67 | 5 | 0.6 | 0.5 | 0.5 | 0.4 | 2 |
| 60 | 0.4 | 0.5 | 0.3 | 0.4 | |||||
| 120 | 0.7 | 0.5 | 0.4 | 0.4 | |||||
| SXYU | 0 | 64 | 64 | 5 | 0.4 | 0.3 | 0.3 | 0.3 | 2.2 |
| 60 | 0.5 | 0.4 | 0.4 | 0.4 | |||||
| 120 | 0.4 | 0.4 | 0.4 | 0.4 | |||||
| HDFE | 0 | 64 | 64 | 5 | 0 | 0 | 0 | 0 | 3.3 |
| 60 | 0 | 0 | 0 | 0 | |||||
| 120 | 0.3 | 0.3 | 0 | 0 | |||||
| CDXI | 0 | 66 | 66 | 5 | 0.3 | 0.3 | 0.3 | 0.3 | 3.8 |
| 60 | 0.4 | 0.4 | 0.4 | 0.4 | |||||
| 120 | 0.4 | 0.4 | 0.4 | 0.4 | |||||
| ZDHU | 0 | 64 | 64 | 5 | 0.7 | 0.4 | 0.7 | 0.4 | 1.4 |
| 60 | 0.4 | 0.5 | 0.7 | 0.5 | |||||
| 120 | 0.5 | 0.4 | 0.6 | 0.5 | |||||
| MLRO | 0 | 64 | 64 | 5 | 0.5 | 0.5 | 0.5 | 0 | 2.5 |
| 60 | 0.5 | 0 | 0 | 0 | |||||
| 120 | 0.5 | 0.5 | 0.5 | 0.5 | |||||
| ZCSH | 0 | 64 | 64 | 5 | 0.7 | 0.5 | 0.3 | 0 | 1.9 |
| 60 | 0.5 | 0.3 | 0.3 | 0.3 | |||||
| 120 | 0 | 0.7 | 0.5 | 0 |
Note: Furthermore, H2 concentration is measured by New Cosmos Electric: XP-3140 (Osaka, Japan). First, when the AMS-H-01 is tuned on, the hydrogen concentration at: (1) ambient environment which is one meter away from the AMS-H-01 (“VA”); (2) output of the nebulizer (“OA”); and (3) output of special nasal tube or nasal mask connected to the nebulizer (“ONT”) are measured separately. Secondly, when each subject user is equipped with the nasal tube, H2 concentration around the subject user is detected at the location of 1 cm away from: (1) right ear (“A”); (2) left ear (“B”); (3) top of head (“C”); and (4) back of head (“D”) of the subject user after 5-minute, 60-minute, and 120-minute operation of the AMS-H-01 respectively. Last, the exhaled breath of the subject was collected using a gas sample collection bag immediately after the nasal tube was removed from the subject user, and then the hydrogen concentration of the exhaled breath was measured.
Ignition experiments
The ignition experiments at flow rate of 3 L/min, 2.5 L/min, and 2 L/min of hydrogen-oxygen gas (approximately 66% H2: 34% O2) generated from the AMS-H-03 are performed. The AMS-H-03 is ignited at output of nasal tube, as shown in Figure 4.
Figure 4.

Ignited at output of nasal tube of AMS-H-03.
As shown in the following Table 3, it is found that only clean ignition noises could be detected for three different flow rates. The results are reasonable since, as previously discussed, the volume between the flame arrester in the nasal tube and the outlet of the nasal tube is quite small, almost no explosive sound is detected when ignition is made at the output of the nasal tube. Therefore, during the operation of AMS-H-03, it is likely that the user who wears the nasal tube for hydrogen inhaling would not be subject to damage even there is ignition caused by static electricity of human body.
Table 3.
Ignition results for three different flow rates
| Flow rate (L/min) | Results |
|---|---|
| 3 | Ignition noise detected, no explosion |
| 2.5 | Ignition noise detected, no explosion |
| 2 | Ignition noise detected, no explosion |
Conclusion
Hydrogen-oxygen mixture (approximately 66% H2: 34% O2) for medical purpose is an oncoming and developing research topic. A safe and durable hydrogen inhaler for generating such hydrogen-oxygen mixture is proposed based on reliable design principles. Experiment and measurement result shows that the hydrogen inhaler implementing the proposed design rules could effectively reduce the explosion, and that the hydrogen-oxygen mixture generated from the hydrogen inhaler is suitable for medical purpose.
Additional file
Additional file 1 (104.7KB, pdf) : Additional file 1.
Acknowledgements
The authors are grateful to Mr. Wang-Fu Chang and Ms. Yun Wang (both from Asclepius Meditec Co., Ltd.) for experiment and measurement.
Footnotes
Conflicts of interest
The corresponding author of the article holds an independent consultant position in Asclepius Meditec Co., Ltd., which does not influence the work completed in this study.
Financial support
None.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
REFERENCES
- 1.Sun XJ. Hydrogen Molecular Medicine. Shanghai, China: Shanghai Second Military Medical University Press; 2013. [Google Scholar]
- 2.Hayashida K, Sano M, Ohsawa I, et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373:30–35. doi: 10.1016/j.bbrc.2008.05.165. [DOI] [PubMed] [Google Scholar]
- 3.Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science. 1975;190:152–154. doi: 10.1126/science.1166304. [DOI] [PubMed] [Google Scholar]
- 4.Roberts BJ, Fife WP, Corbett TH, Schabel FM., Jr Response of five established solid transplantable mouse tumors and one mouse leukemia to hyperbaric hydrogen. Cancer Treat Rep. 1978;62:1077–1079. [PubMed] [Google Scholar]
- 5.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Cui J, Sun Q, Cai J. Hydrogen therapy may be an effective and specific novel treatment for acute radiation syndrome. Med Hypotheses. 2010;74:145–146. doi: 10.1016/j.mehy.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Kajiya M, Silva MJ, Sato K, Ouhara K, Kawai T. Hydrogen mediates suppression of colon inflammation induced by dextran sodium sulfate. Biochem Biophys Res Commun. 2009;386:11–15. doi: 10.1016/j.bbrc.2009.05.117. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Sun Q, He B, Xiao J, Wang Z, Sun X. Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. Int J Cardiol. 2011;148:91–95. doi: 10.1016/j.ijcard.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 9.Buchholz BM, Kaczorowski DJ, Sugimoto R, et al. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am J Transplant. 2008;8:2015–2024. doi: 10.1111/j.1600-6143.2008.02359.x. [DOI] [PubMed] [Google Scholar]
- 10.Cai J, Kang Z, Liu WW, et al. Hydrogen therapy reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci Lett. 2008;441:167–172. doi: 10.1016/j.neulet.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 11.Ge L, Yang M, Yang NN, Yin XX, Song WG. Molecular hydrogen: a preventive and therapeutic medical gas for various diseases. Oncotarget. 2017;8:102653–102673. doi: 10.18632/oncotarget.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakashima-Kamimura N, Mori T, Ohsawa I, Asoh S, Ohta S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother Pharmacol. 2009;64:753–761. doi: 10.1007/s00280-008-0924-2. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Yang H, Fan Y, Li L, Fang J, Yang W. Hydrogen-rich saline attenuates cardiac and hepatic injury in doxorubicin rat model by inhibiting inflammation and apoptosis. Mediators Inflamm. 2016;2016:1320365. doi: 10.1155/2016/1320365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Wang L, Zhang Y, Zhao Y, Chen G. Hydrogen gas inhibits lung cancer progression through targeting SMC3. Biomed Pharmacother. 2018;104:788–797. doi: 10.1016/j.biopha.2018.05.055. [DOI] [PubMed] [Google Scholar]
- 15.Ohsawa I, Nishimaki K, Yamagata K, Ishikawa M, Ohta S. Consumption of hydrogen water prevents atherosclerosis in apolipoprotein E knockout mice. Biochem Biophys Res Commun. 2008;377:1195–1198. doi: 10.1016/j.bbrc.2008.10.156. [DOI] [PubMed] [Google Scholar]
- 16.Sato Y, Kajiyama S, Amano A, et al. Hydrogen-rich pure water prevents superoxide formation in brain slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem Biophys Res Commun. 2008;375:346–350. doi: 10.1016/j.bbrc.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Cardinal JS, Zhan J, Wang Y, et al. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010;77:101–109. doi: 10.1038/ki.2009.421. [DOI] [PubMed] [Google Scholar]
- 18.Gu Y, Huang CS, Inoue T, et al. Drinking hydrogen water ameliorated cognitive impairment in senescence-accelerated mice. J Clin Biochem Nutr. 2010;46:269–276. doi: 10.3164/jcbn.10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laidler KJ, Meiser JH, Sanctuary BC. Pacific Grove, CA, USA: Brooks/Cole Publishing Company; 2002. Physical Chemistry. [Google Scholar]
- 20.Batchelor GK. An Introduction to Fluid Dynamics. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 21.Geng WY, Fan M, Guan RJ, Zhao XM, Zhang JX. Protective effects of hydrogen inhalation on rat model of chronic obstructive pulmonary disease. Xiandai Shengwu Yixue Jinzhan. 2015;15:3401–3405. [Google Scholar]
- 22.Gao L, Jiang D, Geng J, Dong R, Dai H. Hydrogen inhalation attenuated bleomycin-induced pulmonary fibrosis by inhibiting transforming growth factor-β1 and relevant oxidative stress and epithelial-to-mesenchymal transition. Exp Physiol. 2019;104:1942–1951. doi: 10.1113/EP088028. [DOI] [PubMed] [Google Scholar]
- 23.Peng Z, Chen W, Wang L, et al. Inhalation of hydrogen gas ameliorates glyoxylate-induced calcium oxalate deposition and renal oxidative stress in mice. Int J Clin Exp Pathol. 2015;8:2680–2689. [PMC free article] [PubMed] [Google Scholar]
- 24.Cui J, Chen X, Zhai X, et al. Inhalation of water electrolysis-derived hydrogen ameliorates cerebral ischemia-reperfusion injury in rats - A possible new hydrogen resource for clinical use. Neuroscience. 2016;335:232–241. doi: 10.1016/j.neuroscience.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Gong ZJ, Guan JT, Ren XZ, et al. Protective effect of hydrogen on the lung of sanitation workers exposed to haze. Zhonghua Jie He He Hu Xi Za Zhi. 2016;39:916–923. doi: 10.3760/cma.j.issn.1001-0939.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Wu J, Chen Z, Xia F, Sun Q, Liu L. Postconditioning with inhaled hydrogen promotes survival of retinal ganglion cells in a rat model of retinal ischemia/reperfusion injury. Brain Res. 2016;1632:82–90. doi: 10.1016/j.brainres.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 27.He Y, Shi JZ, Zhang RJ, et al. Effects of hydrogen gas inhalation on endometriosis in rats. Reprod Sci. 2017;24:324–331. doi: 10.1177/1933719116655622. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Ma C, Wang X, et al. Hydrogen coadministration slows the development of COPD-like lung disease in a cigarette smoke-induced rat model. Int J Chron Obstruct Pulmon Dis. 2017;12:1309–1324. doi: 10.2147/COPD.S124547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen O, Cao Z, Li H, et al. High-concentration hydrogen protects mouse heart against ischemia/reperfusion injury through activation of thePI3K/Akt1 pathway. Sci Rep. 2017;7:14871. doi: 10.1038/s41598-017-14072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Q, Song H, Wang XT, et al. Molecular hydrogen increases resilience to stress in mice. Sci Rep. 2017;7:9625. doi: 10.1038/s41598-017-10362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Chen O, Ye Z, et al. Inhalation of high concentrations of hydrogen ameliorates liver ischemia/reperfusion injury through A(2A) receptor mediated PI3K-Akt pathway. Biochem Pharmacol. 2017;130:83–92. doi: 10.1016/j.bcp.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Wang DC, Zhao YX, Zhao ZF, Li YH, Wang LF, Chen G. Effect and mechanism of hydrogen suppressing non-small cell lung cancer. Guoji Huxi Zazhi. 2018;38:561–565. [Google Scholar]
- 33.Lu W, Li D, Hu J, et al. Hydrogen gas inhalation protects against cigarette smoke-induced COPD development in mice. J Thorac Dis. 2018;10:3232–3243. doi: 10.21037/jtd.2018.05.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Cui J, Zhai X, et al. Inhalation of hydrogen of different concentrations ameliorates spinal cord injury in mice by protecting spinal cord neurons from apoptosis, oxidative injury and mitochondrial structure damages. Cell Physiol Biochem. 2018;47:176–190. doi: 10.1159/000489764. [DOI] [PubMed] [Google Scholar]
- 35.Guan P, Lin XM, Yang SC, et al. Hydrogen gas reduces chronic intermittent hypoxia-induced hypertension by inhibiting sympathetic nerve activity and increasing vasodilator responses via the antioxidation. J Cell Biochem. 2019;120:3998–4008. doi: 10.1002/jcb.27684. [DOI] [PubMed] [Google Scholar]
- 36.Li W, Yang S, Yu FY, et al. Hydrogen ameliorates chronic intermittent hypoxia-induced neurocognitive impairment via inhibiting oxidative stress. Brain Res Bull. 2018;143:225–233. doi: 10.1016/j.brainresbull.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Yang SC, Chen LL, Fu T, Li WY, Ji ES. Improvement of hydrogen on liver oxidative stress injury in chronic intermittent hypoxia rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2018;34:61–64. doi: 10.12047/j.cjap.5484.2018.016. [DOI] [PubMed] [Google Scholar]
- 38.Zhou ZQ, Zhong CH, Su ZQ, et al. breathing hydrogen-oxygen mixture decreases inspiratory effort in patients with tracheal stenosis. Respiration. 2019;97:42–51. doi: 10.1159/000492031. [DOI] [PubMed] [Google Scholar]
- 39.Yan W, Chen T, Long P, et al. Effects of post-treatment hydrogen gas inhalation on uveitis induced by endotoxin in rats. Med Sci Monit. 2018;24:3840–3847. doi: 10.12659/MSM.907269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu G, Wang RY, Yu YH, Xie KL. Effect of hydrogen gas inhalation on postoperative delirium in elderly patients receiving hip fracture sur-gery. Linchuang Mazui Xue Zazhi. 2018;34:643–646. [Google Scholar]
- 41.Fang W, Wang G, Tang L, et al. Hydrogen gas inhalation protects against cutaneous ischaemia/reperfusion injury in a mouse model of pressure ulcer. J Cell Mol Med. 2018;22:4243–4252. doi: 10.1111/jcmm.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shang L, Xie F, Li J, et al. Therapeutic potential of molecular hydrogen in ovarian cancer. Transl Cancer Res. 2018;7:988–995. [Google Scholar]
- 43.Qi GW, Liu CQ, Xu ZZ, et al. an animal model and protection of hidden hearing loss. Zhonghua Erke Xue Zazhi. 2018;16:463–468. [Google Scholar]
- 44.Fang S, Li X, Wei X, et al. Beneficial effects of hydrogen gas inhalation on a murine model of allergic rhinitis. Exp Ther Med. 2018;16:5178–5184. doi: 10.3892/etm.2018.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Wang R, Yang D, et al. Hydrogen postconditioning promotes survival of rat retinal ganglion cells against ischemia/reperfusion injury through the PI3K/Akt pathway. Biochem Biophys Res Commun. 2018;495:2462–2468. doi: 10.1016/j.bbrc.2017.12.146. [DOI] [PubMed] [Google Scholar]
- 46.Zhao YS, An JR, Yang S, et al. Hydrogen and oxygen mixture to improve cardiac dysfunction and myocardial pathological changes induced by intermittent hypoxia in rats. Oxid Med Cell Longev. 2019;2019:7415212. doi: 10.1155/2019/7415212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guan P, Sun ZM, Luo LF, et al. Hydrogen gas alleviates chronic intermittent hypoxia-induced renal injury through reducing iron overload. Molecules. 2019;24:1184. doi: 10.3390/molecules24061184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guan P, Sun ZM, Luo LF, et al. Hydrogen protects against chronic intermittent hypoxia induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 2019;225:46–54. doi: 10.1016/j.lfs.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Huang P, Wei S, Huang W, et al. Hydrogen gas inhalation enhances alveolar macrophage phagocytosis in an ovalbumin-induced asthma model. Int Immunopharmacol. 2019;74:105646. doi: 10.1016/j.intimp.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 50.Long P, Yan W, He M, et al. Protective effects of hydrogen gas in a rat model of branch retinal vein occlusion via decreasing VEGF-α expression. BMC Ophthalmol. 2019;19:112. doi: 10.1186/s12886-019-1105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu MY, Xie F, Zhang Y, et al. Molecular hydrogen suppresses glioblastoma growth via inducing the glioma stem-like cell differentiation. Stem Cell Res Ther. 2019;10:145. doi: 10.1186/s13287-019-1241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen JB, Pan ZB, Du DM, et al. Hydrogen gas therapy induced shrinkage of metastatic gallbladder cancer: A case report. World J Clin Cases. 2019;7:2065–2074. doi: 10.12998/wjcc.v7.i15.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen JB, Kong XF, Lv YY, et al. “Real world survey” of hydrogen-controlled cancer: a follow-up report of 82 advanced cancer patients. Med Gas Res. 2019;9:115–121. doi: 10.4103/2045-9912.266985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang N, Deng C, Zhang X, Zhang J, Bai C. Inhalation of hydrogen gas attenuates airway inflammation and oxidative stress in allergic asthmatic mice. Asthma Res Pract. 2018;4:3. doi: 10.1186/s40733-018-0040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Health Commission. Notice of the 7th version of guidelines for the diagnosis and management of Covid-19. [Accessed by March 11, 2020]. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml .
- 56.National Health Commission. Diagnosis and Treatment Protocol for COVID-19 (Trial Version 7) [Accessed by March 29, 2020]. http://en.nhc.gov.cn/2020-03/29/c_78469.htm .
- 57.Kurokawa R, Hirano SI, Ichikawa Y, Matsuo G, Takefuji Y. Preventing explosions of hydrogen gas inhalers. Med Gas Res. 2019;9:160–162. doi: 10.4103/2045-9912.266996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Don Galman. Oxygen Deficiency, Honeywell International. [Accessed by October 2019]. https://www.honeywellanalytics.com/en/solutions/articles/oxygen-deficiency .
- 59.Oxygen Deficiency, Honeywell International. [Accessed by October 2019]. https://www.honeywellanalytics.com/en/solutions/articles/oxygen-deficiency .
- 60.Deborah Jones. Perfluorosulfonic Acid Membranes for Fuel Cell and Electrolyser Applications. [Accessed by December, 2019]. https://www.sigmaaldrich.com/technical-documents/articles/materials-science/perfluorosulfonicacid-membranes.html .
- 61.Manabu Hirai. Static Electricity. What is the Relationship with Humidity? Osaka Prefectural Industrial Technology Research, Technical Sheet, No.10001, April 27, 2010 [Google Scholar]
- 62.Basic Course. TIIS News, Industrial Safety Technology Tree Association No.248 p.4-7 April 10, 2012 [Google Scholar]
- 63.Guan WJ, Wei CH, Chen AL, et al. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J Thorac Dis. 2020;12:3448–3452. doi: 10.21037/jtd-2020-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas GO. Flame acceleration and the development of detonation in fuel-oxygen mixtures at elevated temperatures and pressures. J Hazard Mater. 2009;163:783–794. doi: 10.1016/j.jhazmat.2008.07.105. [DOI] [PubMed] [Google Scholar]
- 65.Yagyu S, Matsui H, Masuda T, Yamamoto H. Study on the explosion risk of hydrogen (1st Report). Effect of pressure on the explosive limit of hydrogen. Research Report of National Institute for Industrial Safety. 1969 [Google Scholar]
- 66.Yagyu S, Masuda T, Yamamoto H. Study on the explosion danger of hydrogen (2nd Report). Explosion pressure of hydrogen air mixture. Research Report of National Institute for Industrial Safety. 1973 [Google Scholar]


