Abstract
Metastatic breast cancer cells carry adult and neonatal variants of NaV1.5 voltage-gated activated Na+ channels involved in cell invasion. We hypothesize that instilling lignocaine near the surgical field to anesthetize the pectoral nerves for analgesia will decrease angiogenesis by blocking voltage-gated activated Na+ channels. Twenty patients undergoing unilateral modified radical mastectomy were randomized in a single-blinded, parallel-arm group feasibility pilot study in two groups. In Group I a catheter was placed between the pectoralis major and minor muscle under direct vision before skin closure. Ten milliliters of 2% lignocaine was given as an initial bolus followed by 10 mL of 2% lignocaine every 8 hours up to 24 hours. Group II did not receive any regional block. Primary measure outcomes were pre and postoperative changes in levels of vascular endothelial growth factor. Secondary outcomes were postoperative pain scores and total rescue analgesia used. Nine patients in each group were analyzed. Baseline demographic data of all females were similar with respect to age, body mass, height and duration of anesthesia. Postoperative mean serum levels of vascular endothelial growth factor were decreased by 46.60% from baseline in Group I, while were increased by 84.27% as compared to preoperative values in Group II. Postoperative average pain scores were less in Group I. Postoperative rescue analgesia in 24 hours in Group I was lower than that in Group II. There was no postoperative adverse event related to catheter or lignocaine administration at given doses. Instilling lignocaine to block pectoral nerves provides better postoperative analgesia and decreases a marker of angiogenesis. The study protocol was approved by the Institutional Ethical Committee of the Tertiary Centre (All India Institute of Medical Sciences Rishikesh India) (No. AIIMS/IEC/19/1002) on August 9, 2019, and the larger expansion trial was prospectively registered on Clinical Trial Registry India (No. CTRI/2020/01/022784) on January 15, 2020.
Keywords: analgesia, angiogenesis, lignocaine, modified radical mastectomy, nerve block, pectoral nerve, vascular endothelial growth factor, voltage-gated sodium channel
INTRODUCTION
Even recent advances in cancer management are not able to decrease the morbidity and mortality of cancer recurrence.1 Surgery remains the primary management in resectable tumors though its effects on cancer recurrence and metastasis after removal of the primary is a matter of concern. Surgical handling of tumor led to release of tumor cells into the systematic circulation. Another mechanism of distant spread is the inflammation after surgery. Inflammation after surgery leads to localized shedding of the glycocalyx, impaired vascular integrity and escape of metastatic cells.2
Perioperatively, anesthetic agents cause suppression of innate immunity. Natural killer cells kill circulating tumor cells and decrease metastasis. Hypoxia, as well as certain anesthetic agents, induces the formation of hypoxia-inducible factor-1 alpha (HIF-1α) and vascular endothelial growth factor (VEGF). These factors help in tumor cell proliferation, new blood vessel formation and increased invasiveness or metastasis of tumor cells.3,4
Some studies are completed and some are going on to find those perioperative factors that might change patient’s outcome in terms of cancer-specific survival and time to tumor progression.5,6 Regional anesthesia techniques like paravertebral blocks have shown mixed results.7 Intravenous or direct application of local anesthetics has shown promising results in vivo models due to its both direct and indirect effects on tumor progression.8
We conceptualize that instilling lignocaine directly on the surgical field to anaesthetize the medial and lateral pectoral nerves will indirectly and directly decrease marker of angiogenesis and tumor migration i.e., VEGF. Indirectly, regional anesthesia decreases surgical stress, inflammation and opioid consumption by providing postoperative analgesia. Secondly, lignocaine has direct effects on tumor cells migration by inhibiting proangiogenic factors. Thus our objective is to explore the role of lignocaine instillation as nerve block between pectoralis muscles in decreasing the process of angiogenesis by measuring VEGF levels pre- and postoperatively.
SUBJECTS AND METHODS
Study design
This is a randomized, single-blinded, parallel-arm group feasibility pilot study with 1:1 allocation ratio in a single institute. Permission for conduction of the larger expansion trial (of which this feasibility study is a part) was taken from the Institutional Ethical Committee of the Tertiary Center (All India Institute of Medical sciences Rishikesh India) where the study was conducted (No. AIIMS/IEC/19/1002) on August 9, 2019 (Additional file 1 (317KB, pdf) ). The larger expansion trial was prospectively registered on Clinical Trial Registry India (No. CTRI/2020/01/022784; Registered on January 15, 2020). The study was conducted as per the Helsinki Declaration on human experimentation. Written informed consent (Additional file 2 (493.2KB, pdf) ) was taken from each enrolled patient in the study.
Study participants
After screening for enrolment in the study, patients undergoing unilateral modified radicle mastectomy without breast reconstruction under general anesthesia were recruited after verifying inclusion and exclusion criteria. The study was conducted and completed in a tertiary care government-aided teaching institute (All India Institute of Medical Sciences Rishikesh India). Inclusion criteria were age between 35 to 65 years,9 female, American Society of Anesthesiologists class I – III10 and no known presence of tumor extension beyond breasts and axillary lymph nodes.11 Exclusion criteria were patients refusal for enrollment, infection at the surgical site or systemic infection, inflammatory breast cancer, prior breast cancer surgery on the same side, presence of other concomitant cancer, known allergy to any of the anesthetic agents used in the study, presence of coagulation disorder and patient with cognitive impairment or inability to understand the study protocol.
Perioperative anesthesia protocol
Simple 1:1 randomization was done for 20 patients on the day of surgery with a computer-generated table of random numbers that were concealed in a sealed opaque envelope. Two groups were formed. Group I received regional anesthesia with a catheter placed between the pectoralis major and minor muscle under direct vision before skin closure. Ten milliliters of 2% lignocaine (preservative-free; Xylocard; AstraZeneca Pharma India Limited, Bangalore India) was given through catheter as an initial bolus (immediately in post anesthesia care unit) and 10 mL of 2% lignocaine as intermittent boluses every 8 hours up to 24 hours. Group II did not receive regional block (no catheter was inserted) at the end of surgery. Patients were aware of the intervention received and were operated by the same surgeons not blinded to the group allotment. Physician in the recovery period collected all the data and was blinded to group allotment.
Preoperative induction, hemodynamic and bispectral index monitors (Bispectral Index® Aspect Medical Systems, Inc. Norwood, MA, USA) were applied. Induction was done with propofol (2 mg/kg; Neon Laboratories Ltd., Mumbai, India), fentanyl (2 μg/kg; Troikaa Pharmaceuticals Ltd., Ahmedabad India) and endotracheal intubation was done with the aid of vecuronium (0.1 mg/kg; Neon Laboratories Ltd.). Maintenance was done with oxygen/nitrous oxide (1:1 ratio) and propofol infusion at 9–12 μg/kg/min to keep the bispectral index between 40 to 60. Normothermia, normocapnia and vitals (within 20% of baseline) were maintained throughout surgery. Special care was taken to avoid hypoxia (blood oxygen saturation levels < 94%) as it will induce the formation of HIF-1α and VEGF. Fentanyl 1 μg/kg was used in both groups for intraoperative analgesia as per need. Postoperative rescue analgesia up to 24 hours was provided with fentanyl 0.5 μg/kg if Numerical Rating scale (NRS) was more than 4.12 Patients were monitored in the postanesthetic care unit up to 24 hours for any untoward events. After 24 hours, ambulation of the patient was started and oral acetaminophen 325 mg and tramadol 37.5 mg drug combination were given twice a day till the day of discharge.
Measure outcomes
Our objective was to evaluate the role of administering lignocaine as pectoral nerve block to decrease the process of angiogenesis by measuring VEGF levels preoperatively and postoperatively.
Primary outcomes measured for feasibility were changes in levels of VEGF (baseline: 24 hours before the surgery and postoperatively: 24 hours after surgery). Serum concentrations of VEGF were measured 24 hours before and 24 hours after surgery as a marker of neoangiogenesis and distant metastasis.3,4 The venous blood sample was collected in vacutainer (BD India Pvt. Ltd., Gurgaon, India) and then centrifuged at 3000 × g for 20 minutes. The supernatant serum was stored at –80°C for analysis using Enzyme-linked immunosorbent assay (Sandwich detection method; Eon BioTek, Winooski, VT, USA) as per the VEGF kits manufacturer’s instructions (Sinogeneclon Co., Ltd., Hangzhou, China). Levels of VEGF were determined by finding the concentration value corresponding to the sample optical density on the standard curve. The detection range of the kit is 37.5 – 1200 pg/mL and sensitivity is 3.9 pg/mL.
Secondary outcomes compared for the feasibility study were pain scores (NRS 0 being no pain and 10 as worst imaginable pain) at 0 (immediate postoperative), 1, 2, 4, 6, 8, 12 and 24 hours at rest. Also, total fentanyl used in post anesthesia care unit for rescue analgesia and any complication postoperatively due to the catheter or local anesthetics up to 24 hours were recorded.
Statistical analysis
Statistical tests were conducted on IBM SPSS Statistics Version 23 (IBM, Armonk, NY, USA) and GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, CA, USA). The normalcy of the data was checked with the Shapiro-Wilk test. Continuous data were presented as the mean ± SD and discrete numbers were taken as percentages and proportions. Unpaired t-test was used to compare mean and chi-square test to compare percentages based on the assumption that population at source were equally distributed. Graphs were plotted in Microsoft Word 2016 (Microsoft, Redmond, WA, USA) sheets. A value of P < 0.05 was considered statistically significant. As it was a pilot study, a priori sample size was not calculated.
RESULTS
In this study, 10 patients in each group were enrolled and received the intervention. One patient in Group I was excluded due to displacement of the catheter in the post anesthesia care unit. One patient in Group II was excluded from analysis due to sending of an unsuitable postoperative blood sample for VEGF estimation. In the final analysis, nine patients in each group were analyzed (Figure 1).
Figure 1.
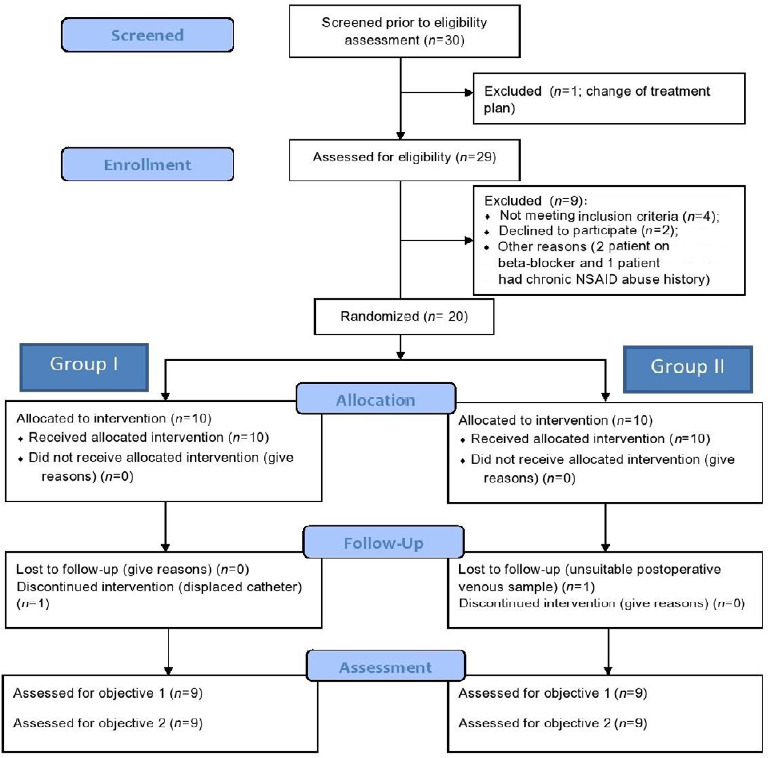
Flow diagram of the progress through phases of a parallel randomized pilot trial of two groups for each pilot trial objective.
Note: Group I: A catheter was placed between the pectoralis major and minor muscle under direct vision before skin closure. Ten milliliters of 2% lignocaine was given as an initial bolus followed by 10 mL of 2% lignocaine every 8 hours up to 24 hours. Group II: No regional block was given. NSAID: Nonsteroidal anti-inflammatory drug.
Baseline demographic data of patients and duration of anesthesia were comparable with no statistically significant difference between the two groups (P > 0.05; Table 1).
Table 1.
Baseline demographic variables of patients undergoing unilateral modified radical mastectomy
| Parameter | Group I | Group II | Mean difference (95% confidence interval) | P-value |
|---|---|---|---|---|
| Age (yr) | 56.22±7.19 | 52.0±9.21 | 4.22 (–4.04 to 12.48) | 0.29 |
| Weight (kg) | 69.33±7.35 | 68.33±5.15 | 1.00 (–5.36 to 7.32) | 0.74 |
| Height (cm) | 165.78±3.19 | 164.56±2.60 | 1.22 (–1.69 to 4.13) | 0.39 |
| Duration of anesthesia (h) | 2.77±0.27 | 2.68±0.31 | 0.09 (–0.2 to 0.38) | 0.52 |
| Intraoperative fentanyl use (μg) | 194.44±11.39 | 197.78±6.67 | –3.34 (–12.61 to 5.93) | 0.46 |
Note: Group I: A catheter was placed between the pectoralis major and minor muscle under direct vision before skin closure. Ten milliliters of 2% lignocaine was given as an initial bolus followed by 10 mL of 2% lignocaine every 8 hours up to 24 hours. Group II: No regional block was given. Data are presented as the mean ± SD, and were analyzed by unpaired t-test.
Shapiro-Wilk test showed preoperative and postoperative serum VEGF levels to be normally distributed (P > 0.05). Preoperative (baseline) serum VEGF levels in Group I vs. Group II were not comparable (228.50 ± 80.94 pg/mL vs. 112.33 ± 69.61 pg/mL; P = 0.004; mean difference = 116.17, 95% confidence interval: 40.73–191.61). Results showed that postoperative serum levels of VEGF (122.00 ± 72.35 pg/mL) were decreased by 46.60% from baseline mean serum VEGF levels in Group I . While postoperative mean serum levels of VEGF (207.00 ± 92.38 pg/mL) were increased by 84.27% as compared to preoperative mean serum VEGF levels in Group II (Figure 2).
Figure 2.
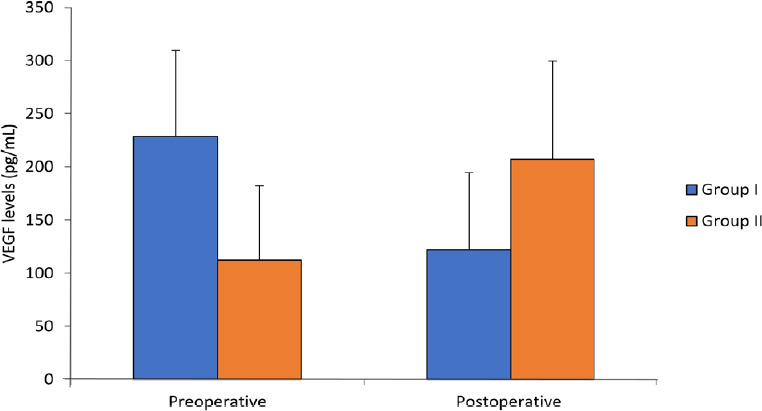
Serum VEGF levels of patients undergoing unilateral modified radical mastectomy preoperatively and postoperatively.
Note: Group I: A catheter was placed between the pectoralis major and minor muscle under direct vision before skin closure. Ten milliliters of 2% lignocaine was given as an initial bolus followed by 10 mL of 2% lignocaine every 8 hours up to 24 hours. Group II: No regional block was given. Data are shown in mean ± SD, and were analyzed by unpaired t-test. VEGF: Vascular endothelial growth factor.
Results of secondary outcomes, i.e., comparison of postoperative analgesia showed that mean NRS was significantly less in Group I as compared to Group II at the 1st, 2nd, 12th and 24th hour (Figure 3).
Figure 3.
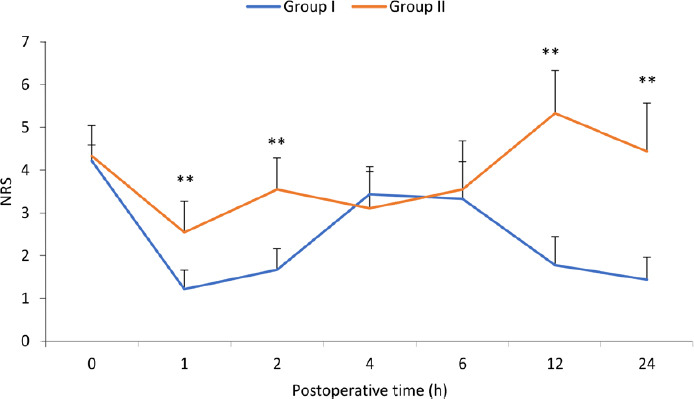
Comparison of postoperative analgesia with NRS over 24 hours.
Note: Group I: A catheter was placed between the pectoralis major and minor muscle under direct vision before skin closure. Ten milliliters of 2% lignocaine was given as an initial bolus followed by 10 mL of 2% lignocaine every 8 hours up to 24 hours. Group II: No regional block was given. A significant difference (**P < 0.01) was observed at the 1st, 2nd, 12th and 24th hour. Data are shown as the mean ± SD (n = 9), and were analyzed by unpaired t-test. NRS; Numerical Rating Scale.
Postoperative total fentanyl consumed was statistically lower in Group I than that in Group II (70.00 ± 6.61 µg vs. 142.22 ± 40.48 µg; P = 0.00; mean difference = –72.22, 95% confidence interval: –100.59 to –42.62). There was no incidence of any postoperative adverse events related to catheter or lignocaine administration at given doses.
DISCUSSION
In this pilot feasibility study, we evaluated the effects of the regional block where lignocaine is instilled between the pectoralis major and minor muscle close to the surgical dissection plane. Our outcomes were the changes in the markers of angiogenesis and pain scores in patients undergoing modified radicle mastectomy. Our results showed that patients who received regional block at the end of surgery consumed less rescue analgesia in the postoperative period and had lower pain scores. Also, their postoperative VEGF levels were decreased from baseline in contrast to the other group. The amount of decrease of VEGF levels vary between patients but the trend or direction of decrease is very consistent within the group. The causal relationship in the decrease of VEGF levels and regional block cannot be established by this feasibility study.
Angiogenic factors play a very vital role in new vessel formation in the microenvironment of tumor cells. Their raised level serves as a marker of neovascularization, survival, proliferation and metastasis of tumor cells. During the development stage in the embryo as well as in the adult, several angiogenic factors are released to induce angiogenesis. These proteins are VEGF, fibroblast growth factor, platelet-derived growth factors and some others.13
Interaction between VEGF and its receptor, VEGFR is the most crucial step in physiological and pathological vasculogenesis. In cancer cells, there is high metabolism and cells are deficient of oxygen. Hypoxia induces the formation of HIF α which in turn switch on the expression of VEGF and VEGFR genes. VEGF and VEGFR binding play an important role in pathological angiogenesis by causing activation of endothelial cells and progenitor cells. These new vessels formed are fragile and have increased permeability to circulating tumor cells. There are four types of VEGF and three types of VEGFRs. VEGF A binds to VEGFR-1 and VEGFR-2 and is involved in angiogenesis in embryo and adults. While VEGF-C binds to VEGFR-3 and is involved in the formation and proliferation of vascular lymphatic systems in embryo and adults.14
There are indirect and direct effects of lignocaine on tumor invasion, migration and survival. Evidence is accumulating on the beneficial role of amide lignocaine given intravenously or through regional blocks in cancer surgeries.15 Regional blocks decrease postoperative pain and opioid consumption. Opioids have been shown to decrease natural killer cell cytotoxicity. Natural killer cell is the first line of defense against circulating tumor cells and decreases metastasis from the primary site.16 Overexpression of the μ receptor is observed in many cancers. µ Receptor activation by opioids decrease migration of natural killer cell in breast cancer tissue.17 Opioids can activate VEGFR by Src activation and facilitate circulating tumor cell invasion and migration.18
Surgical neuroendocrine activation is mediated by the hypothalamic-pituitary axis and sympathetic nervous system activation. Adequate pain relief by lignocaine is associated with decreased release of catecholamine and prostaglandin E2. These, in turn, decrease VEGF, transforming growth factor-beta and inflammatory cytokines (interleukins 6 and 8) involved in tumor angiogenesis and metastasis.19 Prostaglandin E2 is again associated with VEGF-independent angiogenesis and its inhibition in breast and colon cancer models inhibit angiogenesis.20 All these mechanisms (decrease surgical stress and decrease opioid consumption) indirectly lead to decreased levels of markers of angiogenesis, i.e., VEGF.21,22
Direct effects of lignocaine on tumor cells are reduced cell division (via blocking NaV1.5 channels and inhibiting epidermal growth factor receptor), increased apoptosis and reduced cell migration. In the context of this study and our observation, lignocaine affects the level of proangiogenic factors like VEGF through modulation of inflammatory cytokines levels.8 Tumor necrosis factor-α transactivates Src protein kinase (a regulator of endothelial cell permeability) and increase expression of intracellular adhesion molecule-1. Both Src and intracellular adhesion molecule-1 are found to be involved in the migration of cancerous cells. Piegeler et al.23 demonstrated that amide lignocaine administered with tumor necrosis factor-α cause decreased Src activation and intracellular adhesion molecule-1 phosphorylation in lung cancer cells.
We choose 10 mL of lignocaine 2% for pectoral nerves block based on Blanco et al.’s24 ultrasound description of Pecs block. They used 10 mL of levobupivacaine 0.25% to be injected between the pectoralis major and minor muscles and 20 mL between pectoralis minor and serratus anterior followed by infusion of levobupivacaine 0.125% at 5 mL/h. We used lignocaine because amide lignocaines, particularly lidocaine, have more anti-inflammatory effects on immune cells as compared to levobupivacaine and ropivacaine.25 Also lignocaine is one of the more extensively researched amide lignocaines for regional or systemic administration for its anti-cancer effects.8
We have not measured serum concentration of lignocaine after administration in the tissue plane but we kept doses of lignocaine below the toxic limits (5 mg/kg). It must be desirable to measure serum levels of local anesthetic for this therapeutic indication in vivo, though in vitro studies have been performed to know the desirable inhibitory concentrations of local anesthetics for tumor cells.8
Looney et al.26 compared propofol paravertebral block combination with sevoflurane and morphine-based balanced anesthesia. Preoperative and postoperative values of VEGF-C and other factors were assessed from patients of both the groups. Medan values of serum VEGF-C is significantly increased from 806 pg/mL to 1385 pg/mL (P = 0.01) in sevoflurane group while postoperative levels of serum VEGF-C remain same in patients who received propofol and paravertebral block. Total morphine use and postoperative pain scores were significantly low in patients who receive a paravertebral block.26 In our study the catheter was inserted between the muscle plane of the pectoralis major and pectoralis minor under direct vision which increases the accuracy of instillation of lignocaine and reduced volumes of lignocaine as compared to instillation done in the paravertebral block. The paravertebral block has also many potential complications which can be avoided when the regional block between pectoralis muscles is given under direct vision.
VEGF is expressed by many organs in healthy human beings and it is overexpressed in some cancers. VEGF levels differ among healthy volunteers, patients with benign breast disease and patients with breast cancer. VEGF levels in patients with breast cancer depend upon different stages of cancer, tumor bulk, local invasion, on cycles of neoadjuvant chemo-radiotherapy received, estrogen receptor status and menopausal state.27 Baseline serum VEGF levels varied between two groups in our study, though our small study group was homogenous about hormone receptor-positive status, tumor grade and stage (II and III), surgery performed and neoadjuvant chemotherapy received. One of the common causes of increased levels of VEGF measured in serum samples is its release from platelets at the time of venipuncture. Presence of estrogen receptor is directly associated with increased expression of VEGF levels while no correlation was found with age.28
We did not use an inhalational agent for maintenance of anesthesia in both groups to remove their confounding effect on VEGF levels. Volatile anesthetic agents are now known to impair innate immunity (neutrophils and natural killer cells) of the body to destroy escaped circulating tumor cells in the blood. Volatile agents at higher concentration induce the formation of HIF-1α and VEGF that promote tumor proliferation. Propofol, on the other hand, have anti-inflammatory and antitumor effects by preserving functions of natural killer cells and suppress the angiogenic switch induced by surgical trauma.22 In any of the patient in both groups no blood products transfusion was done perioperatively. The transfusion is associated with tumor recurrence in colorectal cancer surgeries.29 We also prevented hypoxia perioperatively which is a strong stimulant for VEGF formation.
The following are the limitations of our study; first, this is a single-blinded study due to the regional block given to the patient. Second, we use opioid fentanyl intraoperatively in both groups. Opioids (Morphine) are known to modulate angiogenesis in tumor and wound healing and have both pro and anti-effects on VEGF production.30 Also, in our study the separate effects of propofol and lignocaine cannot be assessed; however, it can be useful to formulate a combined balanced anesthesia protocol. We did not explore the long term effects on cancer recurrence or disease-free survival in our study.
Caution is warranted in concluding our results as this is only a pilot feasibility study. The pathophysiology and biological behavior of the tumor is complex and interlinked to many clinical, genetical and environmental factors. Attributing role of only an aesthetic agent in preventing recurrence may be spurious. We have measured serum levels of VEGF from a venous sample that may be significantly different from localized residual breast cancer tissue concentration. Though our results corroborate to our hypothesis that a selected anesthetic protocol in breast cancer surgery can decrease markers of angiogenesis and thus decreases chances of recurrence in the long term follow up. We plan to carry out a larger trial with other markers of tumor recurrence and immune suppression. We also plan to follow up the cases over 5 years with more stringent control on randomization and blinding to build upon the small evidence collected from this study.
We conclude that instilling lignocaine near the surgical field in blocking medial and lateral pectoral nerve provides better postoperative analgesia as well as it decreases markers of angiogenesis and metastasis, i.e., VEGF. Whether this extrapolates to an actual decrease in recurrence of the tumor has to be examined in the larger expansion phase of this feasibility trial.
Additional files
Additional file 1 (317KB, pdf) : Hospital ethic approval.
Additional file 2 (493.2KB, pdf) : Informed consent form.
Acknowledgements
We thanked Mr. Gaurav (Department of Biochemistry, All India Institute of Medical Sciences, Rishikesh, India) for his help in storing the blood samples, Dr. Mohit Saini (Department of Anaesthesiology, All India Institute of Medical Sciences Rishikesh, India) in collecting the samples and Dr. Manisha Naithani (Department of Biochemistry, All India Institute of Medical Sciences, Rishikesh, India) for providing ELISA kit. Thanks to the All India Institute of Medical Sciences Rishikesh India for providing study equipment and support.
Footnotes
Conflicts of interest
None declared.
Financial support
None.
Institutional review board statement
The study protocol was approved by the Institutional Ethical Committee of the Tertiary Centre (All India Institute of Medical Sciences Rishikesh India) (No. AIIMS/IEC/19/1002) on August 9, 2019, and the larger expansion trial was prospectively registered on Clinical Trial Registry India (No. CTRI/2020/01/022784) on January 15, 2020.
Declaration of patient consent
The authors certify that they have obtained patients consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published.
Reporting statement
The writing and editing of the article were performed in accordance with the CONsolidated Standards of Reporting Trials (CONSORT) Statement.
Biostatistics statement
The statistical methods of this study were reviewed by the epidemiologist of Department of Community and Family Medicine, All India Institute of Medical Sciences, Rishikesh, India.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15:205–218. doi: 10.1038/nrclinonc.2017.194. [DOI] [PubMed] [Google Scholar]
- 3.Wall T, Sherwin A, Ma D, Buggy DJ. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth. 2019;123:135–150. doi: 10.1016/j.bja.2019.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossaint J, Zarbock A. Perioperative inflammation and its modulation by anesthetics. Anesth Analg. 2018;126:1058–1067. doi: 10.1213/ANE.0000000000002484. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki M, Zhao H, Jaffer T, et al. Volatile anaesthetics enhance the metastasis related cellular signalling including CXCR2 of ovarian cancer cells. Oncotarget. 2016;7:26042–26056. doi: 10.18632/oncotarget.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley A, McQuaid S, Johnson P, Buggy DJ. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth. 2014;113(Suppl 1):i56–62. doi: 10.1093/bja/aeu200. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Buggy D, Fleischmann E, et al. Thoracic paravertebral regional anesthesia improves analgesia after breast cancer surgery: a randomized controlled multicentre clinical trial. Can J Anaesth. 2015;62:241–251. doi: 10.1007/s12630-014-0285-8. [DOI] [PubMed] [Google Scholar]
- 8.Grandhi RK, Perona B. Mechanisms of action by which local anesthetics reduce cancer recurrence: a systematic review. Pain Med. 2020;21:401–414. doi: 10.1093/pm/pnz139. [DOI] [PubMed] [Google Scholar]
- 9.McGuire A, Brown JA, Malone C, McLaughlin R, Kerin MJ. Effects of age on the detection and management of breast cancer. Cancers (Basel) 2015;7:908–929. doi: 10.3390/cancers7020815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Society of Anesthesiologists. ASA Physical Status Classification System. [Accessed by October 23, 2019]. https://www.asahq.org/standards-and-guidelines/asaphysical-status-classification-system .
- 11.Cullen SC. Cybernesthesia. Anesthesiology. 1963;24:110–111. doi: 10.1097/00000542-196301000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure. Pain Pract. 2003;3:310–316. doi: 10.1111/j.1530-7085.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoo S, Lee HB, Han W, et al. Total Intravenous anesthesia versus inhalation anesthesia for breast cancer surgery: a retrospective cohort study. Anesthesiology. 2019;130:31–40. doi: 10.1097/ALN.0000000000002491. [DOI] [PubMed] [Google Scholar]
- 14.Park SA, Jeong MS, Ha KT, Jang SB. Structure and function of vascular endothelial growth factor and its receptor system. BMB Rep. 2018;51:73–78. doi: 10.5483/BMBRep.2018.51.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forget P, Aguirre JA, Bencic I, et al. How anesthetic, analgesic and other non-surgical techniques during cancer surgery might affect postoperative oncologic outcomes: a summary of current state of evidence. Cancers (Basel) 2019;11:592. doi: 10.3390/cancers11050592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singleton PA, Mirzapoiazova T, Hasina R, Salgia R, Moss J. Increased μ-opioid receptor expression in metastatic lung cancer. Br J Anaesth. 2014;113(Suppl 1):i103–108. doi: 10.1093/bja/aeu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boland JW, Pockley AG. Influence of opioids on immune function in patients with cancer pain: from bench to bedside. Br J Pharmacol. 2018;175:2726–2736. doi: 10.1111/bph.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connolly C, Buggy DJ. Opioids and tumour metastasis: does the choice of the anesthetic-analgesic technique influence outcome after cancer surgery. Curr Opin Anaesthesiol. 2016;29:468–474. doi: 10.1097/ACO.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 19.Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. 2018;16:8. doi: 10.1186/s12967-018-1389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Stevens J, Hilton MB, et al. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci Transl Med. 2014;6:242ra284. doi: 10.1126/scitranslmed.3008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez MF, Gorur A, Cata JP. The role of opioids in cancer progression. Int Anesthesiol Clin. 2020;58:57–63. doi: 10.1097/AIA.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 22.Sekandarzad MW, van Zundert AAJ, Lirk PB, Doornebal CW, Hollmann MW. Perioperative anesthesia care and tumor progression. Anesth Analg. 2017;124:1697–1708. doi: 10.1213/ANE.0000000000001652. [DOI] [PubMed] [Google Scholar]
- 23.Piegeler T, Votta-Velis EG, Liu G, et al. Antimetastatic potential of amide-linked local anesthetics: inhibition of lung adenocarcinoma cell migration and inflammatory Src signaling independent of sodium channel blockade. Anesthesiology. 2012;117:548–559. doi: 10.1097/ALN.0b013e3182661977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanco R, Fajardo M, Parras Maldonado T. Ultrasound description of Pecs II (modified Pecs I): a novel approach to breast surgery. Rev Esp Anestesiol Reanim. 2012;59:470–475. doi: 10.1016/j.redar.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Van Der Wal S, Vaneker M, Steegers M, et al. Lidocaine increases the anti-inflammatory cytokine IL-10 following mechanical ventilation in healthy mice. Acta Anaesthesiol Scand. 2015;59:47–55. doi: 10.1111/aas.12417. [DOI] [PubMed] [Google Scholar]
- 26.Looney M, Doran P, Buggy DJ. Effect of anesthetic technique on serum vascular endothelial growth factor C and transforming growth factor β in women undergoing anesthesia and surgery for breast cancer. Anesthesiology. 2010;113:1118–1125. doi: 10.1097/ALN.0b013e3181f79a69. [DOI] [PubMed] [Google Scholar]
- 27.Zajkowska M, Lubowicka E, Fiedorowicz W, Szmitkowski M, Jamiołkowski J, Ławicki S. Human plasma Levels of VEGF-A, VEGF-C, VEGF-D, their soluble receptor - VEGFR-2 and applicability of these parameters as tumor markers in the diagnostics of breast cancer. Pathol Oncol Res. 2019;25:1477–1486. doi: 10.1007/s12253-018-0527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams J, Carder PJ, Downey S, et al. Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res. 2000;60:2898–2905. [PubMed] [Google Scholar]
- 29.Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahbuba W, Lambert DG. Opioids and neovascularization; pro or anti. Br J Anaesth. 2015;115:821–824. doi: 10.1093/bja/aev357. [DOI] [PubMed] [Google Scholar]


