B. anthracis, the etiological agent of anthrax, is a highly pathogenic, spore-forming bacterium that causes acute, life-threatening disease in both humans and livestock. A greater understanding of the metabolic determinants governing the fitness of B. anthracis is essential for the development of successful therapeutic and vaccination strategies aimed at lessening the potential impact of this important biodefense pathogen.
KEYWORDS: overflow metabolism, acetate production, metabolic status, fitness, Bacillus anthracis
ABSTRACT
Under conditions of glucose excess, aerobically growing bacteria predominantly direct carbon flux toward acetate fermentation, a phenomenon known as overflow metabolism or the bacterial “Crabtree effect.” Numerous studies of the major acetate-generating pathway, the phosphotransacetylase (Pta)-acetate kinase (AckA) pathway, have revealed its important role in bacterial fitness through the control of central metabolism to sustain balanced growth and cellular homeostasis. In this work, we highlight the contribution of the Pta-AckA pathway to the fitness of the spore-forming bacterium Bacillus anthracis. We demonstrate that disruption of the Pta-AckA pathway causes drastic growth reduction in the mutants and alters the metabolic and energy status of the cells. Our results revealed that inactivation of the Pta-AckA pathway increases the glucose consumption rate, affects intracellular ATP, NAD+, and NADH levels, and leads to a metabolic block at the pyruvate and acetyl coenzyme A (acetyl-CoA) nodes. Consequently, accumulation of intracellular acetyl-CoA and pyruvate forces bacteria to direct carbon into the tricarboxylic acid and/or glyoxylate cycles, as well as fatty acid and poly(3‐hydroxybutyrate) biosynthesis pathways. Notably, the presence of phosphotransbutyrylase (Ptb) in B. anthracis partially compensates for the loss of Pta activity. Furthermore, overexpression of the ptb gene not only eliminates the negative impact of the pta mutation on B. anthracis fitness but also restores normal growth in the pta mutant of the non-butyrate-producing bacterium Staphylococcus aureus. Taken together, the results of this study demonstrate the importance of the Pta-AckA pathway for B. anthracis fitness by revealing its critical contribution to the maintenance of metabolic homeostasis during aerobic growth under conditions of carbon overflow.
IMPORTANCE B. anthracis, the etiological agent of anthrax, is a highly pathogenic, spore-forming bacterium that causes acute, life-threatening disease in both humans and livestock. A greater understanding of the metabolic determinants governing the fitness of B. anthracis is essential for the development of successful therapeutic and vaccination strategies aimed at lessening the potential impact of this important biodefense pathogen. This study is the first to demonstrate the vital role of the Pta-AckA pathway in preserving energy and metabolic homeostasis in B. anthracis under conditions of carbon overflow, thus highlighting this pathway as a potential therapeutic target for drug discovery. Overall, the results of this study provide important insights into the metabolic processes and requirements driving rapid B. anthracis proliferation during vegetative growth.
INTRODUCTION
During growth on glucose and other easily metabolizable carbohydrates, various bacteria, including Bacillus spp., generate acetic acid as one of the most abundant by-products of carbon metabolism (1–8). The major pathway of acetate production in prokaryotes, the phosphotransacetylase (Pta)-acetate kinase (AckA) pathway, is directly linked to central metabolism and is composed of two enzymes, Pta and AckA. In Escherichia coli and the majority of Gram-negative bacteria, the pta and ackA genes, encoding Pta and AckA, respectively, form a single operon; in Bacillus subtilis and other Gram-positive bacteria, however, these genes are located at distant loci on the chromosome (2, 5, 6, 9–12). During acetate fermentation, Pta catalyzes a reaction with acetyl coenzyme A (acetyl-CoA) to generate the high-energy, acid/base-labile intermediate acetyl phosphate (AcP), which is then converted to acetate by AckA in the subsequent reaction of substrate-level phosphorylation to generate ATP (5). In anaerobically growing bacteria, the end product of glycolysis, pyruvate, undergoes mixed-acid fermentation, leading to excretion of lactate, acetate, formate, and ethanol (13–15). In the presence of oxygen, however, pyruvate is decarboxylated to acetyl-CoA by the pyruvate dehydrogenase complex (PDHC). The metabolic fate of aerobically generated acetyl-CoA depends on the growth conditions. In environments with limited glucose, acetyl-CoA is completely oxidized in the tricarboxylic acid (TCA) cycle to generate energy primarily through aerobic respiration (oxidative phosphorylation). Under conditions of glucose excess, however, the TCA cycle activity is restricted by carbon catabolite repression (16–20) and acetyl-CoA is directed into acetate fermentation, where energy is generated by the less efficient process of substrate-level phosphorylation, a phenomenon known as overflow metabolism or the bacterial “Crabtree effect” (5, 21). Overflow metabolism has been widely studied in E. coli and other bacteria over the decades, and numerous works have revealed the critical importance of aerobic acetate fermentation for bacterial fitness, central metabolism, cellular homeostasis, and physiology through the preservation of the intricate balance between glycolytic flux and pathways involved in energy production and biosynthesis (5, 6, 18, 22–29). To date, however, the contribution of acetate fermentation to Bacillus anthracis fitness and physiology during overflow metabolism is still poorly understood, and the Pta-AckA pathway in this medically important pathogen has not been characterized.
In this study, we analyzed the contribution of the Pta-AckA pathway to the fitness and physiology of B. anthracis. We demonstrated that disruption of either pta or ackA significantly impairs growth and affects cellular homeostasis of B. anthracis under conditions of carbon overflow. Our results showed that the fitness defects in the mutants were associated with a metabolic block at the pyruvate and acetyl-CoA nodes, leading to redirection of carbon away from growth into pathways that normally have only limited expression under conditions of carbon overflow.
RESULTS AND DISCUSSION
Inactivation of the Pta-AckA pathway impairs B. anthracis growth.
During growth under conditions of glucose and oxygen excess, Bacillus spp. generate acetate as the major by-product of carbon overflow metabolism (1, 2, 18, 30, 31). This implies that the Pta-AckA pathway, driving carbon flux toward acetate production, can play an essential role in B. anthracis fitness, as has been reported for other bacteria (5–7, 10, 32–35). To determine the contribution of the Pta-AckA pathway to B. anthracis growth and to examine its impact on bacterial metabolic status, we inactivated the ackA and pta genes in the B. anthracis strain V770-NP1-R (see Materials and Methods). Disruption of the Pta-AckA pathway by inactivation of the ackA and pta genes had a negative impact on overall bacterial growth (Fig. 1A) and drastically decreased growth rates during the exponential phase in both mutants, compared to the wild-type strain (Fig. 1B). The impairment of growth was accompanied by a significant decline in the concentrations and rate of acetic acid excretion in both the ackA and pta mutants (Fig. 1C and D). Furthermore, the decreased growth rates in both mutants were reflected in the reduced temporal depletion of glucose from the culture medium (Fig. 1E) and an increase in the glucose consumption rate during the exponential growth phase (Fig. 1F). This suggests that carbon flux was directed into other metabolic pathways, similar to what has been reported for Staphylococcus aureus (6). Importantly, a complementation study using plasmids containing the wild-type alleles of the ackA and pta genes showed restoration of the growth characteristics in the mutants to the wild-type levels (see Fig. S1A and B in the supplemental material), verifying the absence of second-site mutations and confirming that the growth impairment was due to the inactivation of either ackA or pta. Taken together, these results demonstrate an important contribution of the Pta-AckA pathway to B. anthracis fitness during overflow metabolism, as the loss of this pathway impairs bacterial proliferation and increases the glucose consumption rate, suggesting that carbon is directed away from growth into other cellular processes.
FIG 1.
Inactivation of the Pta-AckA pathway affects growth characteristics of B. anthracis. (A) Growth curves of the wild-type (wt) strain V770-NP1-R and mutant strains V770-ackA and V770-pta grown aerobically in TSB containing 0.25% glucose. The OD600 and the pH of the culture medium were determined at the indicated times. (B) Growth rate of the wild-type strain V770-NP1-R and mutant strains V770-ackA and V770-pta grown aerobically in TSB containing 0.25% glucose, determined between 0 and 3 h of growth. (C) Temporal accumulation and depletion of acetic acid in the culture medium of strains V770-NP1-R, V770-ackA, and V770-pta. (D) Acetate excretion rate determined for strains V770-NP1-R, V770-ackA, and V770-pta between 0 and 3 h of growth. (E) Temporal depletion of glucose from the culture medium of strains V770-NP1-R, V770-ackA, and V770-pta. (F) Glucose consumption rate determined for strains V770-NP1-R, V770-ackA, and V770-pta between 0 and 3 h of growth. For panels A, C, and E, the results are representative of at least three independent experiments. For panels B, D, and F, the results are presented as the means plus standard errors of the means of duplicate determinations for at least three independent experiments. Statistical significance between the wild-type strain and the pta and ackA mutants was determined by using Student's t test. *, P <0.005.
Disruption of the Pta-AckA pathway causes a metabolic block at the pyruvate and acetyl-CoA nodes and alters the cellular energy status.
In previous studies, it was reported that inactivation of the Pta-AckA pathway affects the metabolic and energy status of S. aureus and E. coli by altering carbon flux through the pyruvate and acetyl-CoA nodes (6, 7, 32, 36). To determine whether inactivation of the Pta-AckA pathway in B. anthracis causes a similar effect on carbon flux, we measured the intracellular concentrations of acetyl-CoA and pyruvate in the wild-type and mutant strains during the exponential phase of growth. As anticipated, inactivation of the Pta-AckA pathway in B. anthracis during vegetative growth led to the accumulation of intracellular acetyl-CoA and pyruvate in the mutants (Fig. 2A and B). Furthermore, similar to the results reported earlier for E. coli and S. aureus (6, 10, 32, 37), measurements of the extracellular pyruvate concentrations revealed its significant increase in the culture media for the ackA and pta mutants (Fig. 2C). Because pyruvate is an unusual by-product that is not normally excreted by the wild-type strain of B. anthracis under conditions of carbon overflow (Fig. 2C), its accumulation in the media might indicate leakage and/or transport of excess of pyruvate out of the cells. This suggests that the surge in the levels of intracellular pyruvate and/or acetyl-CoA caused by inactivation of the Pta-AckA pathway might have a negative impact on bacterial fitness, as was earlier proposed for S. aureus and E. coli (6, 32), thus forcing cells to excrete excess pyruvate and direct carbon into other metabolic pathways at the cost of growth.
FIG 2.
Inactivation of the Pta-AckA pathway alters carbon flux at the pyruvate and acetyl-CoA nodes and affects the energy status of B. anthracis. (A) Intracellular acetyl-CoA (Ac-CoA) concentrations determined for strains V770-NP1-R, V770-ackA, and V770-pta after 3 h of aerobic growth in TSB containing 0.25% glucose. wt, wild-type. (B) Intracellular pyruvate concentrations determined for strains V770-NP1-R, V770-ackA, and V770-pta after 3 h of aerobic growth in TSB containing 0.25% glucose. (C) Concentrations of pyruvate in the culture medium determined for strains V770-NP1-R, V770-ackA, and V770-pta after 3 h of aerobic growth in TSB containing 0.25% glucose. (D) Intracellular ATP concentrations determined for strains V770-NP1-R, V770-ackA, and V770-pta after 3 h of aerobic growth in TSB containing 0.25% glucose. (E) Intracellular NAD+ and NADH concentrations determined for strains V770-NP1-R, V770-ackA, and V770-pta after 3 h of aerobic growth in TSB containing 0.25% glucose. The results are presented as the means plus standard errors of the means of duplicate determinations for at least three independent experiments. Statistical significance between the wild-type strain and the pta and ackA mutants was determined by using Student's t test. *, P <0.01.
During overflow metabolism, i.e., aerobic growth in the presence of excess glucose, the activity of the TCA cycle in bacilli is restricted by carbon catabolite repression (18, 38–40). Consequently, to support rapid cell proliferation under these conditions, a substantial part of energy in the form of ATP is generated through substrate-level phosphorylation via glycolysis and the Pta-AckA pathway (5, 41). Hence, the detrimental impact on bacterial fitness in the pta and ackA mutants could be a result of the decreased energy status of bacteria caused by the loss of the generation of two ATP molecules per glucose consumed through the Pta-AckA pathway. To determine whether disruption of the Pta-AckA pathway alters the cellular energy status, we measured the intracellular ATP, NAD+, and NADH concentrations in the ackA and pta mutants during the exponential phase of growth. As seen in Fig. 2D, our results demonstrate that the intracellular ATP levels were indeed lower in the pta and ackA mutants, compared to the wild-type strain. In contrast, determination of the intracellular NAD+ and NADH concentrations revealed significant increases in the pools of these metabolites (Fig. 2E), suggesting enhanced respiration in the mutants to compensate for the loss of ATP. Previously, we demonstrated that inactivation of the Pta-AckA pathway in S. aureus caused increases in the intracellular concentrations of ATP, NAD+, and NADH, which resulted from redirection of carbon into the TCA cycle and enhanced oxidative phosphorylation in the mutants (6). In B. anthracis, however, our experiments demonstrate that disruption of the Pta-AckA pathway reduces the intracellular ATP concentrations in the mutants despite increasing the NAD+ and NADH pools. Therefore, these results suggest that in B. anthracis carbon might be directed toward the less efficient energy-generating glyoxylate bypass of the TCA cycle, which is absent in S. aureus, and/or directed into energy-consuming cellular processes.
Inactivation of the Pta-AckA pathway directs carbon into the TCA and glyoxylate cycles, fatty acid biosynthesis, and PHB production.
As mentioned above and similar to S. aureus (6), the loss of ATP production caused by inactivation of either pta or ackA increased the glucose consumption rate (Fig. 1F), suggesting enhanced carbon flux through the glycolytic machinery. In support of this, a quantitative real‐time reverse transcriptase PCR (RT‐PCR) analysis using primers specific to pfkA, the gene encoding the key glycolytic enzyme phosphofructokinase, showed >2.5-fold increases in the levels of pfkA mRNA transcripts in both mutants (Fig. 3A). Given that the metabolic block at the pyruvate and acetyl-CoA nodes increased the glucose consumption rate as well as intracellular NAD+ and NADH levels in the mutants, we speculated that, similar to S. aureus and E. coli (6, 32), carbon flux in B. anthracis would be directed into the TCA and/or glyoxylate cycles in order to fulfill the ATP requirements through oxidative phosphorylation. To determine whether inactivation of the Pta-AckA pathway alters transcription of genes involved in the control of the TCA cycle, we performed a quantitative RT-PCR analysis using primers specific to the citZ, citC, and sucA genes, encoding the TCA cycle enzymes citrate synthase, isocitrate dehydrogenase, and 2‐oxoglutarate dehydrogenase (E1), respectively, as well as primers specific to the aceB gene, encoding malate synthase of the glyoxylate bypass. Using this approach, we found that inactivation of ackA led to >2.5‐fold increases in the levels of the citZ, citC, and aceB transcripts and >3.5‐fold increases in the levels of the sucA transcripts (Fig. 3A). Although similar, a less pronounced positive impact on the accumulation of the corresponding transcript levels was observed for the pta mutant (Fig. 3A). To confirm that disruption of the Pta-AckA pathway directs carbon into the TCA cycle and/or the glyoxylate shunt, we measured the intracellular citrate levels in the pta and ackA mutants and the wild-type strain. In agreement with the RT-PCR data, our results showed that inactivation of the Pta-AckA pathway increased the intracellular citrate pools in both mutants, compared to the wild-type strain (Fig. 3B).
FIG 3.
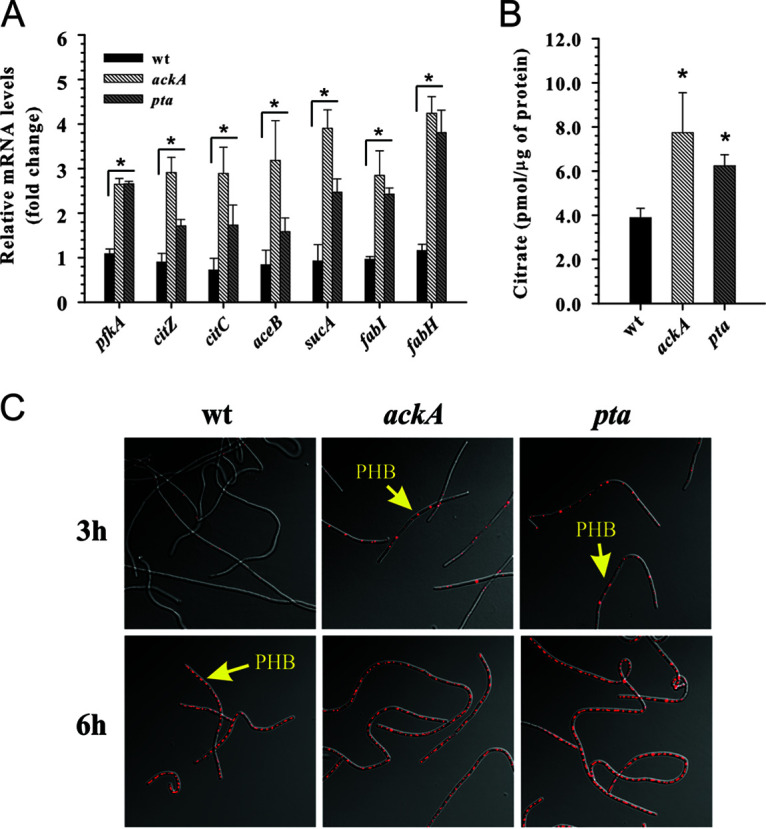
Inactivation of the Pta-AckA pathway in B. anthracis directs carbon into the TCA cycle, fatty acid biosynthesis, and PHB production. (A) Relative transcript levels of the pfkA, citZ, citC, aceB, sucA, fabH, and fabI genes determined by quantitative RT‐PCR after 3 h of aerobic growth in TSB containing 0.25% glucose. Transcript levels in the V770-ackA and V770-pta mutants are presented as a fold difference, compared to those in the wild‐type (wt) strain. (B) Intracellular citrate concentrations determined for strains V770-NP1-R, V770-ackA, and V770-pta after 3 h of aerobic growth in TSB containing 0.25% glucose. (C) Visualization of PHB granules for strains V770-NP1-R, V770-ackA, and V770-pta after 3 and 6 h of aerobic growth in TSB supplemented with 0.25% glucose by confocal laser scanning microscopy using the fluorescent dye Nile red. For panels A and B, the results are presented as the means plus standard errors of the means of duplicate determinations for at least three independent experiments. Statistical significance between the wild-type strain and the pta and ackA mutants was determined by using Student's t test. *, P <0.01.
B. anthracis and other members of the Bacillus cereus group accumulate poly(3‐hydroxybutyrate) (PHB) granules as a carbon and energy storage reservoir during growth (42). In particular, it was shown that aerobically growing B. cereus produced PHB through the catabolism of acetate when glucose was depleted from the medium (42, 43). In agreement with this, we previously demonstrated that in B. anthracis the contribution of the PHB biosynthesis pathway to carbon flux during overflow metabolism is minimal (44). Interestingly, previous studies reported that inactivation of the pta gene in Ralstonia eutropha and cyanobacteria significantly enhanced production of PHB, suggesting that the metabolic block at the acetyl-CoA node directs carbon into the PHB biosynthesis pathway (45, 46). Furthermore, overexpression of the phbCAB genes from Alcaligenes eutrophus was shown to restore the defective growth and survival of the pta mutant in the non-PHB-producing bacterium E. coli (32). Therefore, to determine whether disruption of the Pta-AckA pathway in B. anthracis directs carbon toward production of PHB, we visualized intracellular accumulation of PHB granules in the wild-type strain and pta and ackA mutants during the exponential (3 h) and postexponential (6 h) phases of growth by confocal laser scanning microscopy using the fluorescent dye Nile red. In agreement with the results of previous studies (42–44), the B. anthracis V770-NP1-R wild-type strain produced negligible amounts of PHB during exponential growth but accumulated PHB during acetate catabolism in the postexponential phase of growth (Fig. 1C and 3C). In contrast, the pta and ackA mutants produced substantial amounts of PHB during the exponential growth phase (Fig. 3C) in the presence of glucose (Fig. 1E), suggesting that the metabolic block at the pyruvate and acetyl-CoA nodes caused by inactivation of the Pta-AckA pathway directs carbon flux toward the production of PHB, similar to findings for R. eutropha and cyanobacteria (45, 46).
As noted above, the intracellular ATP levels in the pta and ackA mutants were found to be lower than those in the wild-type strain under conditions of carbon overflow (Fig. 2D), suggesting that carbon is directed toward energy-consuming cellular processes. The generation of malonyl‐CoA, the rate‐limiting step of the de novo fatty acid biosynthesis catalyzed by the ATP-dependent acetyl‐CoA carboxylase, represents one such energy-consuming reaction (47). To determine whether inactivation of the Pta-AckA pathway directs carbon toward lipid biosynthesis, we performed a quantitative RT-PCR analysis using primers specific to the fabH and fabI genes, which encode two key enzymes in fatty acid synthesis, i.e., β‐ketoacyl‐acyl carrier protein synthase III and enoyl‐acyl carrier protein reductase I, respectively. Using this approach, we found that inactivation of the Pta-AckA pathway led to an ∼2.5‐fold increase in the levels of fabI transcripts and a >3.5‐fold increase in the levels of fabH transcripts (Fig. 3A), suggesting enhanced carbon flux toward de novo lipid biosynthesis in the pta and ackA mutants. It has been shown that in Gram‐positive bacteria the expression of genes involved in fatty acid synthesis, including fabH and fabI, is negatively regulated by the malonyl‐CoA-responsive transcriptional repressor FapR, for which malonyl‐CoA serves as a concentration‐dependent DNA binding inhibitor (48–50). Therefore, the observed elevated levels of the fabH and fabI mRNA transcripts not only suggest increased expression of their corresponding genes but also corroborate higher intracellular malonyl‐CoA levels in the pta and ackA mutants.
Overexpression of the ptb-encoded Ptb restores growth of the B. anthracis and S. aureus pta mutants.
Previously, it was proposed that in B. subtilis and E. coli the increase in the intracellular AcP levels might be the cause of the more severe growth inhibition seen in the ackA mutant, compared to the pta mutant (2, 35). Similarly, our current work demonstrated that inactivation of the pta gene in B. anthracis resulted in less pronounced negative impacts on bacterial growth and the metabolic status, compared to disruption of the ackA gene (Fig. 1, 2, and 3A and B). In contrast, our study of the Pta-AckA pathway in S. aureus revealed a more severe detrimental effect of the pta mutation on bacterial fitness, compared to inactivation of the ackA gene (6). Taken together, these results suggested that perturbations in the intracellular levels of AcP might not be a primary cause of the growth defect in the mutants; rather, this difference is likely based on the presence of a species-specific enzyme or pathway that compensates for the loss of the Pta function within the cells. An earlier study of Pta from B. subtilis showed its ability to use the short-chain fatty acid esters propionyl-CoA and butyryl-CoA (which differ from acetyl-CoA by only one and two saturated carbon atoms, respectively) as less efficient substrates for the reaction (51). More recent characterization of Pta from Thermotoga maritima revealed that the N-terminal amino acid sequence of the protein showed high homology to phosphotransbutyrylases (Ptbs) from Clostridium acetobutylicum ATCC 824 and NCIMB 8052 (52). Furthermore, Bock and colleagues (52) showed that, similar to B. subtilis Pta (51), the Pta from T. maritima not only catalyzes a reaction with acetyl-CoA but also, to a lesser extent, can use propionyl-CoA and butyryl-CoA as substrates. Similarly, Sirobhushanam and colleagues (53) recently demonstrated that the Ptb of Listeria monocytogenes not only catalyzes a reversible reaction with butyryl-CoA to form butyryl phosphate but also has broad substrate specificity, with different affinities for different acyl-CoA substrates. Therefore, considering the higher levels of extracellular acetate production in the pta mutant, compared to the ackA mutant (Fig. 1C and D), and the ability of the Pta/Ptb family proteins to catalyze the reactions with various acyl-CoA substrates, we argued that the Ptb activity in B. anthracis would partially compensate for the loss of the Pta function within cells. To test this hypothesis, we constructed the plasmid pMRS183, overexpressing the Ptb of B. anthracis V770-NP1-R (see Materials and Methods). We then introduced this multicopy plasmid into the pta mutant to determine whether expression of the Ptb would complement growth. As anticipated, overexpression of Ptb restored growth of the B. anthracis V770-NP1-R pta mutant to wild-type levels (Fig. S1C). As noted earlier, a pta mutation in S. aureus caused a more severe growth defect, compared to the inactivation of ackA (6). Therefore, we introduced the pMRS183 plasmid into S. aureus strain UAMS-1, which lacks a Ptb orthologue, to determine whether expression of the B. anthracis Ptb would restore growth characteristics of the pta mutant. As shown in the Fig. 4A and B, heterologous expression of the B. anthracis ptb gene in S. aureus completely restored growth and temporal glucose consumption in the UAMS-1 pta mutant. Importantly, in confirmation of the ability of the B. anthracis Ptb to utilize acetyl-CoA as a substrate and thus to compensate for the loss of Pta function, its overexpression restored the production of acetic acid in the pta mutant of S. aureus to wild-type levels (Fig. 4C).
FIG 4.
Overexpression of the ptb gene restores growth of the pta mutant in S. aureus. (A) Growth curves of the wild-type (wt) strain UAMS-1, the mutant strain UAMS-1-pta, and UAMS-1-pta with the plasmid pMRS183, containing the ptb gene from B. anthracis, grown aerobically in TSB containing 0.25% glucose. The OD600 and the pH of the culture medium were determined at the indicated times. (B) Temporal depletion of glucose from the culture medium of strains UAMS-1, UAMS-1-pta, and UAMS-1-pta with the plasmid pMRS183. (C) Temporal accumulation and depletion of acetic acid in the culture medium of strains UAMS-1, UAMS-1-pta, and UAMS-1-pta with the plasmid pMRS183. In the experiments, the wild-type strain and the pta mutant contain pCN51 (empty vector) plasmid. The results are representative of at least three independent experiments.
Concluding remarks.
In this study, we demonstrated that overflow metabolism in the form of aerobic acetate excretion by B. anthracis represents an important physiological characteristic of this dangerous human pathogen. Our results revealed that the activity of the major acetate-generating pathway, the Pta-AckA pathway, has a direct impact on central metabolism and fitness of B. anthracis, as disruption of this pathway abolished rapid proliferation during exponential growth and globally altered the metabolic status of the cells. We found that the loss of ATP generation by substrate-level phosphorylation increased glucose consumption and glycolytic rates in the mutants and directed carbon into the TCA and/or glyoxylate cycles. Furthermore, our results demonstrated that inactivation of the Pta-AckA pathway resulted in a metabolic block at the pyruvate and acetyl-CoA nodes. Consequently, the surge in the intracellular pyruvate and acetyl-CoA concentrations forced the cells to excrete pyruvate and to direct carbon away from growth toward other metabolic pathways and cellular processes that normally do not operate under these conditions. Interestingly, the fitness and metabolic differences between the pta and ackA mutants in B. anthracis were not associated with perturbations in the AcP pools, as suggested for other bacteria (2, 5, 35), but rather were a result of the presence of a Ptb that was able to partially compensate for the loss of Pta activity within the cells. Overall, the results of this study not only revealed the essential function of the Pta-AckA pathway in regulating cellular homeostasis by maintaining optimal carbon fluxes in central metabolism but also demonstrated the unique features of aerobic acetate production in B. anthracis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Strains and plasmids used in this study are listed in Table S1 in the supplemental material. Escherichia coli strains were grown in LB medium (EMD Millipore) or on LB agar. B. anthracis strains were grown in tryptic soy broth (TSB) (BD Biosciences) supplemented with 0.25% glucose (Sigma-Aldrich). B. anthracis cultures were inoculated to an optical density at 600 nm (OD600) of 0.05 from overnight cultures (grown in TSB without dextrose [BD Biosciences]), incubated at 37°C, and aerated at 250 rpm with a flask/medium ratio of 10:1. Bacterial growth was assessed by measuring the OD600. The OD600 for stationary-phase cultures was measured following dilution of cultures in TSB to remain within the linear range of a spectrophotometer. Antibiotics were purchased from Thermo Fisher Scientific and were used at the following concentrations: ampicillin, 100 μg/ml; chloramphenicol, 10 μg/ml; spectinomycin, 100 μg/ml; erythromycin, 5 μg/ml; and kanamycin, 50 μg/ml.
Construction of the ackA and pta mutants in B. anthracis.
Primers (see Table S2 in the supplemental material) used for construction and confirmation of the ackA and pta mutations were generated based on the sequence of B. anthracis strain Sterne (GenBank accession number NC_005945). The ackA mutant was constructed by replacing a 0.6-kb internal region of the ackA gene with a kanamycin resistance gene (kan), using the gene splicing by overlap extension (SOE) technique (54). The kan antibiotic resistance cassette was amplified from pDG780 (55) using ackA-kan-f and ackA-kan-r primers, which contain sequences homologous to the ackA gene. The primers BamHI-BAS4536-f and kan-ackA-r were used for amplification of a 1.4-kb region upstream of the ackA gene, while a 1.2-kb region downstream of the ackA gene was amplified using kan-ackA-f and SalI-BAS4533-f primers. The three PCR fragments were mixed in equimolar ratio (1:1:1) and amplified using BamHI-BAS4536-f and SalI-BAS4533-f primers. The resulting 4.1-kb PCR product consisted of the 1.5-kb kan cassette flanked by the sequences upstream and downstream of the ackA gene. Following digestion with the restriction endonucleases BamHI and SalI, the 4.1-kb product was cloned into pCL52.2 (56) to generate the pMRS130 plasmid.
The pta mutant was constructed by replacing a 0.7-kb internal region of the pta gene with a spectinomycin resistance gene (spc), using the gene SOE technique (54). The spc antibiotic resistance cassette was amplified from pDG1726 (55) using pta-spc-f and pta-spc-r primers, which contain sequences homologous to the pta gene. The primers BamHI-BAS5239-r and spc-pta-r were used for amplification of a 1.2-kb region upstream of the pta gene, while a 1.1-kb region downstream of the pta gene was amplified using spc-pta-f and SalI-lplA-f primers. The three PCR fragments were mixed in equimolar ratio (1:1:1) and amplified using BamHI-BAS5239-r and SalI-lplA-f primers. The resulting 3.5-kb PCR product consisted of the 1.2-kb spc cassette flanked by the sequences upstream and downstream of the pta gene. Following digestion with the restriction endonucleases BamHI and SalI, the 3.5-kb product was cloned into pJA175 (44) to generate the pJM2 plasmid.
Plasmids pMRS130 and pJM2 were propagated in E. coli strain GM2929 (57), transformed into B. anthracis strain V770-NP1-R (58) by electroporation, and used to construct V770-ackA and V770-pta mutants through standard allelic exchange methodology at 37°C as described (59). The replacement of the ackA gene in the mutant with the kan cassette was confirmed by PCR using primers BAS4536-f and BAS4533-f. The replacement of the pta gene in the mutant with the spc cassette was confirmed by PCR using primers BAS5239-r and BAS5237-f.
Complementation of the ackA and pta mutations.
For complementation of the ackA mutation, a 1.2-kb PCR product containing the wild-type ackA gene was amplified using primers B-SalI-ackA-f and B-SacI-ackA-r. Following digestion with the restriction endonucleases SalI and SacI, the PCR product was cloned into the plasmid pCN51 under the control of a cadmium-inducible promoter (60). The resulting recombinant plasmid was designated pMRS180. For complementation of the pta mutation, a 0.8-kb PCR product containing the wild-type pta gene was amplified using primers Ba-SalI-pta-f and B-SacI-pta-r. Following digestion with the restriction endonucleases SalI and SacI, the PCR product was cloned into the plasmid pCN51 under the control of a cadmium-inducible promoter (60). The resulting recombinant plasmid was designated pMRS200. The plasmids pMRS180 and pMRS200 were propagated in the E. coli strain GM2929 (57) and introduced into the V770-ackA and V770-pta mutants by electroporation correspondingly.
For heterologous complementation of the pta mutations in B. anthracis and S. aureus by the B. anthracis ptb (BAS4071) gene, a 1.0-kb PCR product containing the wild-type ptb gene was amplified using primers SalI-BAS4071-f and SacI-BAS4071-r. Following digestion with the restriction endonucleases SalI and SacI, the PCR product was cloned into the plasmid pCN51 under the control of a cadmium-inducible promoter (60). The resulting recombinant plasmid was designated pMRS183. For complementation of the pta mutation in B. anthracis, the plasmid pMRS183 was propagated in E. coli strain GM2929 (57) and introduced into the V770-pta mutant by electroporation. For complementation of the pta mutation in S. aureus, the plasmid pMRS183 was transformed into strain RN4220 by electroporation and then introduced into the UAMS-1-Δpta strain by phage Φ11-mediated transduction (61).
Measurement of extracellular glucose, acetic acid, and pyruvate concentrations.
Aliquots of bacterial cultures (1 ml) were centrifuged at 18,407 × g for 3 min at 4°C. The supernatants were removed and stored at −20°C until use. Acetate and glucose concentrations were determined using kits purchased from R-Biopharm, according to the manufacturer's protocol and as described previously (6). Pyruvate concentrations were determined using the pyruvate assay kit (MBL), according to the manufacturer’s protocol.
Determination of intracellular pyruvate, citrate, ATP, NAD+, NADH, and acetyl-CoA concentrations.
Intracellular pyruvate concentrations were determined using the pyruvate assay kit (MBL). Aliquots of bacterial cultures (10 ml) were harvested by centrifugation at 3,630 × g at 4°C for 10 min. The bacterial pellets were washed twice with 1 ml of phosphate-buffered saline (PBS) (pH 7.4), resuspended in 0.35 ml of pyruvate assay buffer, incubated for 20 min at 80°C, and lysed using Lysing Matrix B tubes (MP Biomedicals) in a FastPrep instrument (Qbiogene). The lysates were centrifuged at 18,407 × g at 4°C for 5 min. Pyruvate concentrations were determined according to the manufacturer's protocol and normalized to the corresponding total cellular protein concentration at the time of harvest.
Intracellular citrate concentrations were determined using the citrate colorimetric/fluorometric assay kit (BioVision). Aliquots of bacterial cultures (24 ml) were harvested by centrifugation at 3,630 × g at 4°C for 10 min. Bacterial pellets were washed twice with 1 ml of PBS and resuspended in 0.5 ml of PBS, followed by the addition of 0.1 ml of ice-cold 3 M perchloric acid. Cells were lysed using Lysing Matrix B tubes (MP Biomedicals) in a FastPrep instrument (Qbiogene). The lysates were then centrifuged at 18,407 × g for 3 min. Subsequently, 300 μl of supernatants was neutralized with 75 μl of a saturated solution of potassium bicarbonate and centrifuged at 18,407 × g at 4°C for 3 min. Citrate concentrations were determined according to the manufacturer’s protocol and normalized to the corresponding total cellular protein concentration at the time of harvest.
Intracellular ATP concentrations were determined using the BacTiter-Glo kit (Promega) according to the manufacturer's protocol and normalized to the total cellular protein concentration at the time of harvest.
Intracellular NAD+ and NADH concentrations were determined using the Fluoro NAD/NADH kit (Cell Technology). Aliquots of bacterial cultures (24 ml) were harvested by centrifugation at 3,630 × g at 4°C for 10 min. The bacterial pellets were washed twice with 1 ml of PBS and then resuspended in 0.2 ml of the NAD/NADH extraction buffer and 0.2 ml of the lysis buffer. Cells were lysed using Lysing Matrix B tubes (MP Biomedicals) in a FastPrep instrument (Qbiogene). The lysates were then incubated at 60°C for 15 min and centrifuged at 18,407 × g at 4°C for 3 min. NAD+ and NADH concentrations in the lysates were determined according to the manufacturer's protocol and normalized to the total cellular protein concentration at the time of harvest.
Intracellular acetyl-CoA concentrations were determined using the PicoProbe acetyl-CoA assay kit (BioVision). Aliquots of bacterial cultures (24 ml) were harvested by centrifugation at 3,630 × g at 4°C for 10 min. The bacterial pellets were washed twice with 1 ml of PBS and resuspended in 0.5 ml of PBS, followed by the addition of 0.1 ml of ice-cold 3 M perchloric acid. The cells were lysed using Lysing Matrix B tubes (MP Biomedicals) in a FastPrep instrument (Qbiogene). The lysates were centrifuged at 18,407 × g at 4°C for 3 min. Subsequently, 300 μl of supernatants was neutralized with 75 μl of a saturated solution of potassium bicarbonate and centrifuged at 18,407 × g at 4°C for 3 min. Acetyl-CoA concentrations were determined according to the manufacturer's protocol and normalized to the total cellular protein concentration at the time of harvest.
All assays were performed in duplicate for at least three independent experiments. Protein concentrations for all assays were determined by the Lowry method (62).
Confocal microscopy.
PHB granules in B. anthracis were visualized with the fluorescent dye Nile red (Sigma-Aldrich) as described (63). Bacterial samples collected after 3 and 6 h of growth in TSB supplemented with 0.25% glucose were stained with Nile red by addition of 0.5 volumes of Nile red solution (1 μg/ml in ethanol). Bacteria were immobilized by addition of 1 volume of 1% agarose (55°C), and then 15 μl of the cell suspension was placed on a microscope slide and covered with a coverslip. Bacteria were imaged with an inverted Zeiss 510 Meta confocal laser scanning microscope fitted with a Plan-Apochromat 63×/1.40 numerical aperture oil differential interference contrast (DIC) M27 objective set to a 1.7 digital zoom. In addition to the acquisition of DIC images, a 561-nm diode-pumped solid-state (DPSS) laser was used to excite Nile red and the emissions were collected with a 575- to 615-nm band-pass filter.
mRNA quantification.
RNA isolation from B. anthracis cultures after 3 h of growth in TSB supplemented with 0.25% glucose was carried out as described previously (64). Quantitative real-time PCR was performed using rpoD-, citZ-, citC-, sucA-, aceB-, fabI-, fabH-, and pfkA-specific primers, as listed in Table S2. Briefly, cDNA was synthesized from 500 ng of total RNA using the QuantiTect reverse transcription kit (Qiagen). The samples were then diluted 1:50, and the cDNA products were amplified using the LightCycler FastStart DNA Master SYBR green I kit (Roche Applied Science) following the manufacturer's protocol. The relative transcript levels were calculated using the comparative threshold cycle (CT) method (65) with normalization to the amount of rpoD transcripts. The results were recorded in duplicate and are representative of three independent experiments.
Supplementary Material
ACKNOWLEDGMENTS
H.I.W. and S.M.W. were supported by National Institute for General Medical Science INBRE grant P20GM103427.
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Speck EL, Freese E. 1973. Control of metabolite secretion in Bacillus subtilis. J Gen Microbiol 78:261–275. 10.1099/00221287-78-2-261. [DOI] [PubMed] [Google Scholar]
- 2.Presecan-Siedel E, Galinier A, Longin R, Deutscher J, Danchin A, Glaser P, Martin-Verstraete I. 1999. Catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis. J Bacteriol 181:6889–6897. 10.1128/JB.181.22.6889-6897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JN, Ahn SJ, Burne RA. 2015. Genetics and physiology of acetate metabolism by the Pta-Ack pathway of Streptococcus mutans. Appl Environ Microbiol 81:5015–5025. 10.1128/AEM.01160-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernal V, Castano-Cerezo S, Canovas M. 2016. Acetate metabolism regulation in Escherichia coli: carbon overflow, pathogenicity, and beyond. Appl Microbiol Biotechnol 100:8985–9001. 10.1007/s00253-016-7832-x. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe AJ. 2005. The acetate switch. Microbiol Mol Biol Rev 69:12–50. 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadykov MR, Thomas VC, Marshall DD, Wenstrom CJ, Moormeier DE, Widhelm TJ, Nuxoll AS, Powers R, Bayles KW. 2013. Inactivation of the Pta-AckA pathway causes cell death in Staphylococcus aureus. J Bacteriol 195:3035–3044. 10.1128/JB.00042-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall DD, Sadykov MR, Thomas VC, Bayles KW, Powers R. 2016. Redox imbalance underlies the fitness defect associated with inactivation of the Pta-AckA pathway in Staphylococcus aureus. J Proteome Res 15:1205–1212. 10.1021/acs.jproteome.5b01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt KA, Flynn JM, Naranjo B, Shikhare ID, Gralnick JA. 2010. Substrate-level phosphorylation is the primary source of energy conservation during anaerobic respiration of Shewanella oneidensis strain MR-1. J Bacteriol 192:3345–3351. 10.1128/JB.00090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latimer MT, Ferry JG. 1993. Cloning, sequence analysis, and hyperexpression of the genes encoding phosphotransacetylase and acetate kinase from Methanosarcina thermophila. J Bacteriol 175:6822–6829. 10.1128/jb.175.21.6822-6829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakuda H, Shiroishi K, Hosono K, Ichihara S. 1994. Construction of Pta-Ack pathway deletion mutants of Escherichia coli and characteristic growth profiles of the mutants in a rich medium. Biosci Biotechnol Biochem 58:2232–2235. 10.1271/bbb.58.2232. [DOI] [PubMed] [Google Scholar]
- 11.Singh-Wissmann K, Ferry JG. 1995. Transcriptional regulation of the phosphotransacetylase-encoding and acetate kinase-encoding genes (pta and ack) from Methanosarcina thermophila. J Bacteriol 177:1699–1702. 10.1128/jb.177.7.1699-1702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser CM, Gocayne JD, White O, Adams MD, Clayton RA, Fleischmann RD, Bult CJ, Kerlavage AR, Sutton G, Kelley JM, Fritchman RD, Weidman JF, Small KV, Sandusky M, Fuhrmann J, Nguyen D, Utterback TR, Saudek DM, Phillips CA, Merrick JM, Tomb JF, Dougherty BA, Bott KF, Hu PC, Lucier TS, Peterson SN, Smith HO, Hutchison CA, III, Venter JC. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397–403. 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 13.Sawers RG, Blokesch M, Bock A. 2004. Anaerobic formate and hydrogen metabolism. EcoSal Plus 10.1128/ecosalplus.3.5.4. [DOI] [PubMed] [Google Scholar]
- 14.Nakano MM, Dailly YP, Zuber P, Clark DP. 1997. Characterization of anaerobic fermentative growth of Bacillus subtilis: identification of fermentation end products and genes required for growth. J Bacteriol 179:6749–6755. 10.1128/jb.179.21.6749-6755.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strasters KC, Winkler KC. 1963. Carbohydrate metabolism of Staphylococcus aureus. J Gen Microbiol 33:213–229. 10.1099/00221287-33-2-213. [DOI] [PubMed] [Google Scholar]
- 16.Warner JB, Lolkema JS. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol Mol Biol Rev 67:475–490. 10.1128/mmbr.67.4.475-490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorke B, Stulke J. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613–624. 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 18.Sonenshein AL. 2007. Control of key metabolic intersections in Bacillus subtilis. Nat Rev Microbiol 5:917–927. 10.1038/nrmicro1772. [DOI] [PubMed] [Google Scholar]
- 19.Seidl K, Muller S, Francois P, Kriebitzsch C, Schrenzel J, Engelmann S, Bischoff M, Berger-Bachi B. 2009. Effect of a glucose impulse on the CcpA regulon in Staphylococcus aureus. BMC Microbiol 9:95. 10.1186/1471-2180-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadykov MR, Hartmann T, Mattes TA, Hiatt M, Jann NJ, Zhu Y, Ledala N, Landmann R, Herrmann M, Rohde H, Bischoff M, Somerville GA. 2011. CcpA coordinates central metabolism and biofilm formation in Staphylococcus epidermidis. Microbiology (Reading) 157:3458–3468. 10.1099/mic.0.051243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crabtree HG. 1929. Observations on the carbohydrate metabolism of tumours. Biochem J 23:536–545. 10.1042/bj0230536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han K, Lim HC, Hong J. 1992. Acetic acid formation in Escherichia coli fermentation. Biotechnol Bioeng 39:663–671. 10.1002/bit.260390611. [DOI] [PubMed] [Google Scholar]
- 23.Farmer WR, Liao JC. 1997. Reduction of aerobic acetate production by Escherichia coli. Appl Environ Microbiol 63:3205–3210. 10.1128/AEM.63.8.3205-3210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majewski RA, Domach MM. 1990. Simple constrained-optimization view of acetate overflow in E. coli. Biotechnol Bioeng 35:732–738. 10.1002/bit.260350711. [DOI] [PubMed] [Google Scholar]
- 25.Varma A, Palsson BO. 1994. Stoichiometric flux balance models quantitatively predict growth and metabolic by-product secretion in wild-type Escherichia coli W3110. Appl Environ Microbiol 60:3724–3731. 10.1128/AEM.60.10.3724-3731.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vemuri GN, Altman E, Sangurdekar DP, Khodursky AB, Eiteman MA. 2006. Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redox ratio. Appl Environ Microbiol 72:3653–3661. 10.1128/AEM.72.5.3653-3661.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nahku R, Valgepea K, Lahtvee PJ, Erm S, Abner K, Adamberg K, Vilu R. 2010. Specific growth rate dependent transcriptome profiling of Escherichia coli K12 MG1655 in accelerostat cultures. J Biotechnol 145:60–65. 10.1016/j.jbiotec.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Basan M, Hui S, Okano H, Zhang Z, Shen Y, Williamson JR, Hwa T. 2015. Overflow metabolism in Escherichia coli results from efficient proteome allocation. Nature 528:99–104. 10.1038/nature15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szenk M, Dill KA, de Graff AMR. 2017. Why do fast-growing bacteria enter overflow metabolism? Testing the membrane real estate hypothesis. Cell Syst 5:95–104. 10.1016/j.cels.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Hanson RS, Srinivasan VR, Halvorson HO. 1963. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol 85:451–460. 10.1128/JB.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakata HM, Halvorson HO. 1960. Biochemical changes occurring during growth and sporulation of Bacillus cereus. J Bacteriol 80:801–810. 10.1128/JB.80.6.801-810.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang DE, Shin S, Rhee JS, Pan JG. 1999. Acetate metabolism in a pta mutant of Escherichia coli W3110: importance of maintaining acetyl coenzyme A flux for growth and survival. J Bacteriol 181:6656–6663. 10.1128/JB.181.21.6656-6663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rucker N, Billig S, Bucker R, Jahn D, Wittmann C, Bange FC. 2015. Acetate dissimilation and assimilation in Mycobacterium tuberculosis depend on carbon availability. J Bacteriol 197:3182–3190. 10.1128/JB.00259-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grundy FJ, Waters DA, Allen SH, Henkin TM. 1993. Regulation of the Bacillus subtilis acetate kinase gene by CcpA. J Bacteriol 175:7348–7355. 10.1128/jb.175.22.7348-7355.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schutze A, Benndorf D, Puttker S, Kohrs F, Bettenbrock K. 2020. The impact of ackA, pta, and ackA-pta mutations on growth, gene expression and protein acetylation in Escherichia coli K-12. Front Microbiol 11:233. 10.3389/fmicb.2020.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang YT, Bennett GN, San KY. 1999. Effect of inactivation of nuo and ackA-pta on redistribution of metabolic fluxes in Escherichia coli. Biotechnol Bioeng 65:291–297. . [DOI] [PubMed] [Google Scholar]
- 37.Dittrich CR, Vadali RV, Bennett GN, San KY. 2005. Redistribution of metabolic fluxes in the central aerobic metabolic pathway of E. coli mutant strains with deletion of the ackA-pta and poxB pathways for the synthesis of isoamyl acetate. Biotechnol Prog 21:627–631. 10.1021/bp049730r. [DOI] [PubMed] [Google Scholar]
- 38.Tobisch S, Zuhlke D, Bernhardt J, Stulke J, Hecker M. 1999. Role of CcpA in regulation of the central pathways of carbon catabolism in Bacillus subtilis. J Bacteriol 181:6996–7004. 10.1128/JB.181.22.6996-7004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HJ, Roux A, Sonenshein AL. 2002. Direct and indirect roles of CcpA in regulation of Bacillus subtilis Krebs cycle genes. Mol Microbiol 45:179–190. 10.1046/j.1365-2958.2002.03003.x. [DOI] [PubMed] [Google Scholar]
- 40.van der Voort M, Kuipers OP, Buist G, de Vos WM, Abee T. 2008. Assessment of CcpA-mediated catabolite control of gene expression in Bacillus cereus ATCC 14579. BMC Microbiol 8:62. 10.1186/1471-2180-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amarasingham CR, Davis BD. 1965. Regulation of α-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem 240:3664–3668. 10.1016/S0021-9258(18)97196-6. [DOI] [PubMed] [Google Scholar]
- 42.Kominek LA, Halvorson HO. 1965. Metabolism of poly-β-hydroxybutyrate and acetoin in Bacillus cereus. J Bacteriol 90:1251–1259. 10.1128/JB.90.5.1251-1259.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakata HM. 1966. Role of acetate in sporogenesis of Bacillus cereus. J Bacteriol 91:784–788. 10.1128/JB.91.2.784-788.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadykov MR, Ahn JS, Widhelm TJ, Eckrich VM, Endres JL, Driks A, Rutkowski GE, Wingerd KL, Bayles KW. 2017. Poly(3-hydroxybutyrate) fuels the tricarboxylic acid cycle and de novo lipid biosynthesis during Bacillus anthracis sporulation. Mol Microbiol 104:793–803. 10.1111/mmi.13665. [DOI] [PubMed] [Google Scholar]
- 45.Miyake M, Miyamoto C, Schnackenberg J, Kurane R, Asada Y. 2000. Phosphotransacetylase as a key factor in biological production of polyhydroxybutyrate. Appl Biochem Biotechnol 84-86:1039–1044. 10.1385/ABAB:84-86:1-9:1039. [DOI] [PubMed] [Google Scholar]
- 46.Miyake M, Takase K, Narato M, Khatipov E, Schnackenberg J, Shirai M, Kurane R, Asada Y. 2000. Polyhydroxybutyrate production from carbon dioxide by cyanobacteria. Appl Biochem Biotechnol 84-86:991–1002. 10.1385/ABAB:84-86:1-9:991. [DOI] [PubMed] [Google Scholar]
- 47.Tong L. 2005. Acetyl-coenzyme A carboxylase: crucial metabolic enzyme and attractive target for drug discovery. Cell Mol Life Sci 62:1784–1803. 10.1007/s00018-005-5121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schujman GE, Guerin M, Buschiazzo A, Schaeffer F, Llarrull LI, Reh G, Vila AJ, Alzari PM, de Mendoza D. 2006. Structural basis of lipid biosynthesis regulation in Gram-positive bacteria. EMBO J 25:4074–4083. 10.1038/sj.emboj.7601284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujita Y, Matsuoka H, Hirooka K. 2007. Regulation of fatty acid metabolism in bacteria. Mol Microbiol 66:829–839. 10.1111/j.1365-2958.2007.05947.x. [DOI] [PubMed] [Google Scholar]
- 50.Ellis JM, Wolfgang MJ. 2012. A genetically encoded metabolite sensor for malonyl-CoA. Chem Biol 19:1333–1339. 10.1016/j.chembiol.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rado TA, Hoch JA. 1973. Phosphotransacetylase from Bacillus subtilis: purification and physiological studies. Biochim Biophys Acta 321:114–125. 10.1016/0005-2744(73)90065-X. [DOI] [PubMed] [Google Scholar]
- 52.Bock A-K, Glasemacher J, Schmidt R, Schönheit P. 1999. Purification and characterization of two extremely thermostable enzymes, phosphate acetyltransferase and acetate kinase, from the hyperthermophilic eubacterium Thermotoga maritima. J Bacteriol 181:1861–1867. 10.1128/JB.181.6.1861-1867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sirobhushanam S, Galva C, Sen S, Wilkinson BJ, Gatto C. 2016. Broad substrate specificity of phosphotransbutyrylase from Listeria monocytogenes: a potential participant in an alternative pathway for provision of acyl CoA precursors for fatty acid biosynthesis. Biochim Biophys Acta 1861:1102–1110. 10.1016/j.bbalip.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horton RM, Cai ZL, Ho SN, Pease LR. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8:528–535. [PubMed] [Google Scholar]
- 55.Guerout-Fleury AM, Shazand K, Frandsen N, Stragier P. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335–336. 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 56.Sau S, Sun J, Lee CY. 1997. Molecular characterization and transcriptional analysis of type 8 capsule genes in Staphylococcus aureus. J Bacteriol 179:1614–1621. 10.1128/jb.179.5.1614-1621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palmer BR, Marinus MG. 1994. The dam and dcm strains of Escherichia coli: a review. Gene 143:1–12. 10.1016/0378-1119(94)90597-5. [DOI] [PubMed] [Google Scholar]
- 58.Wright GG, Puziss M, Neely WB. 1962. Studies on immunity in anthrax. IX. Effect of variations in cultural conditions on elaboration of protective antigen by strains of Bacillus anthracis. J Bacteriol 83:515–522. 10.1128/JB.83.3.515-522.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahn JS, Chandramohan L, Liou LE, Bayles KW. 2006. Characterization of CidR-mediated regulation in Bacillus anthracis reveals a previously undetected role of S-layer proteins as murein hydrolases. Mol Microbiol 62:1158–1169. 10.1111/j.1365-2958.2006.05433.x. [DOI] [PubMed] [Google Scholar]
- 60.Charpentier E, Anton AI, Barry P, Alfonso B, Fang Y, Novick RP. 2004. Novel cassette-based shuttle vector system for Gram-positive bacteria. Appl Environ Microbiol 70:6076–6085. 10.1128/AEM.70.10.6076-6085.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Novick RP. 1991. Genetic systems in staphylococci. Methods Enzymol 204:587–636. 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 62.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 63.Jendrossek D, Selchow O, Hoppert M. 2007. Poly(3-hydroxybutyrate) granules at the early stages of formation are localized close to the cytoplasmic membrane in Caryophanon latum. Appl Environ Microbiol 73:586–593. 10.1128/AEM.01839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sadykov MR, Olson ME, Halouska S, Zhu Y, Fey PD, Powers R, Somerville GA. 2008. Tricarboxylic acid cycle-dependent regulation of Staphylococcus epidermidis polysaccharide intercellular adhesin synthesis. J Bacteriol 190:7621–7632. 10.1128/JB.00806-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.