Abstract
OBJECTIVES:
The objective was to evaluate the efficacy of antiviral agent valacyclovir compared with famciclovir in the treatment of herpes zoster.
MATERIALS AND METHODS:
A comparative study was conducted over a period of 1 year. Data relevant to the study were collected from 60 patients, with active herpes zoster presenting to the outpatient department within 72 hr of the first occurrence of zoster rash. They were divided in to two groups of 30 patients each. The first group of patients received valacyclovir tablet 1000 mg thrice daily, whereas those in the second group were given famciclovir tablet 500 mg thrice daily. Both the drugs were given for 7 days. Periodic follow-up till 29th day was done for assessment of the effects of given drugs.
RESULTS:
Significant decrease was observed on comparison of pain scores between the two groups using the visual analog scale, with the drug valacyclovir, than in the famciclovir group at day 29. Furthermore, valacyclovir treatment accelerated the resolution of zoster associated pain in more number of patients compared with famciclovir.
CONCLUSION:
Oral valacyclovir administered during acute zoster infection for a period of 7 days offers significant benefit compared to famciclovir by providing a well tolerated and greater resolution of pain while maintaining the favorable safety profile, making valacyclovir more efficacious and a better drug in management of Herpes Zoster in comparison to famciclovir.
Keywords: Famciclovir, herpes zoster, valacyclovir
Introduction
Herpes zoster a localized disease has been known since ancient times and is often referred by different names such as varicella zoster and shingles. It is typically characterized by unilateral radicular pain along with grouped vesicular eruptions.[1] An incidence of 3 − 5/1000 patients/year for herpes zoster in Europe, North America, and Asia-Pacific, was reported by a systematic review in 2014. It also showed an increased incidence at the age of 60 years as 6 − 8/1000 patients/year and also at the age of 80 years as incidence being 8 − 12/1000 patients/year.[2] Immunosuppression and increasing age are well-established risk factors that can lead to latent virus reactivation.[3,4] Pain is a major symptom and usually the reason for patients to seek medical advice.[5] It frequently persists even after the healing of rash, a complication commonly called as the postherpetic neuralgia. This distinctive presentation of signs and symptoms is usually sufficient enough to reach a clinical diagnosis.[6]
The pharmacotherapy for its management comprises antiviral agents, analgesics, and corticosteroids. The ideal time to start the treatment is within 3 days from when the rash first appears. Opioids and tricyclic antidepressants are often used for the treatment of significant persisting pain. Among the antiviral agents, acyclovir is the most commonly used agent, but its prodrug valacyclovir has been observed to be better than acyclovir.[7] Valacyclovir is known to accelerate the resolution of acute pain associated with herpes zoster and also decreases the number of patients complaining of persistent pain.[7] Famciclovir is another antiviral agent, which is a prodrug of penciclovir available with the advantage of a longer intracellular half-live and a better bioavailability. Some studies claim famciclovir to be a better drug when compared to valacyclovir for relief of pain,[8] while others state valacyclovir to be a better drug when compared to famciclovir.[9] However, still, there is a paucity of clinical studies comparing the effects of valacyclovir and famciclovir in the literature available and hence, the present study has been undertaken.
Materials and Methods
The study was conducted on patients with herpes zoster over a period of 1 year who attended the outpatient department (OPD) of Dermatology at BRIMS, Bidar, Karnataka. Data collection was done by recording patient's details in a preformed performa, after taking written informed consent from the patients. Individual parameters including demographic details, history of presenting illness, symptoms, and signs were recorded. Patients included in the study were patients of any gender having age above 18 years, with clinically confirmed diagnosis of herpes zoster; new, untreated cases who presented within 3 days of appearance of zoster associated rash. The exclusion criteria followed were patients with atypical presentations, or with associated complications, pregnant and lactating females; patients with known immunocompromised status; those who have been treated with other antiviral agents and immunomodulatory drugs; and patients with preexisting hepatic or renal impairment. Noncompliant and dropout patients were also excluded from the study.
It was an analytical observational cohort study; universal sampling technique was applied. The sample size was calculated as per the average number of active herpes zoster patients, attending the OPD of dermatology, during the period of study. A total of 60 patients; two groups of 30 patients each, were enrolled in the study. Patients presenting on the 1st month were prescribed valacyclovir 1000 mg thrice daily, and those presenting on the 2nd month were prescribed famciclovir 500 mg thrice daily. The treatment was given for 7 days. The same alternating drug method was followed for the rest of the 10 months during the study period. Valacyclovir or famciclovir for 7 days was given to patients of these groups, respectively. To maintain the efficacy of drugs, the same brand drugs were used throughout the study duration. Forty milligrams methyl prednisone once daily in the morning for 1 week from the presentation, followed by tapering over the next 2 weeks was added to all the patients. Acetaminophen 500 mg TDS was also added for 1st week, followed by as and when required. Follow-up was done on days 3, 8, 15, 22, and 29. Patients were requested to visit in the morning without taking prior medication on the day of follow-up. For assessment of the compliance, the pill counting method was applied; patients were asked to bring the drug strip in every scheduled visit, so as to check the number of remaining pills in the pack.
Statistical analysis was conducted using GraphPad Software, 2365 Northside Dr.Suite 560, San Diego, CA 92108. Assessment of the intensity of pain was done using the visual analog scale (VAS). It is a numerical rating scale that shows markings ranging from 0 to 10 that represents a continuum; in the increasing order of severity from no to worst pain.[10] The reduction in pain scores between both the groups was analyzed using unpaired t-test. The rate of pain cessation, i.e., the proportion of patients without pain was compared using the Chi-square test between the two groups after every visit. P < 0.05 was considered to be statistically significant in both the tests. The process from recruitment to treatment for the present study is summarized in Consort (Chart 1).
CHART 1.
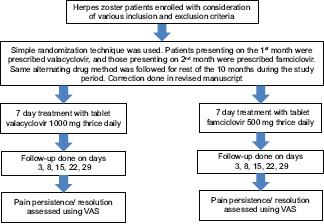
CONSORT CHART
Ethical considerations
The study was done after obtaining clearance from the Institutional Ethical Committee of Bidar Institute of Medical Sciences, Bidar. No. BRIMS/Ethical Committee: BRIMS/IEC/46/2015. IEC No.: IRB 84/2015.
Results
In both of the study groups, maximum participants belonged within 20–59 years of age. In the valacyclovir group, the mean age was 42 ± 14.31 and in the famciclovir group, it was 43 ± 12.56. A comparable gender-wise distribution showing a male predominance was recorded for both the groups. Valacyclovir group had 20 males and 10 females, while the famciclovir group had 22 males and 8 females. The dermatome distribution was similar in both treatment groups as shown in Figures 1 and 2. Table 1 shows the VAS scores comparison at every follow-up visit between both the groups. The mean VAS scores on the day of presentation, i.e., the baseline scores in both the groups were almost similar, and the difference was not statistically significant. The mean VAS scores in the valacyclovir group were significantly reduced at day 29 (P < 0.05) in comparison to the famciclovir group. Results showed a greater number of totally pain-free patients at day 29, i. e., 24 (80%) patients in the valacyclovir group, while in the famciclovir group, 18 (60%) patients were reported to be totally pain free, although it was statistically insignificant (P > 0.05), as depicted in Table 2.
Figure 1.
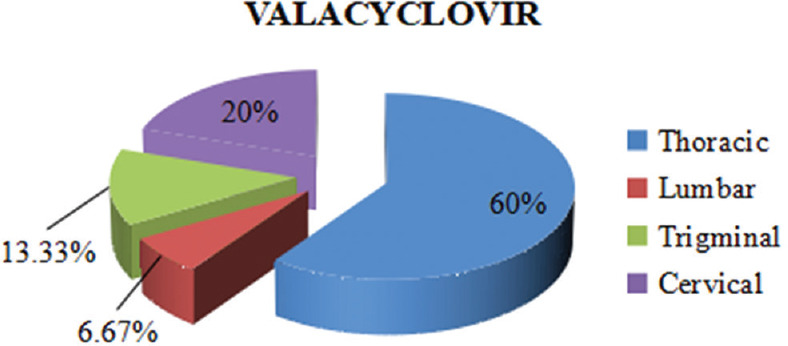
Dermatome distribution
Figure 2.
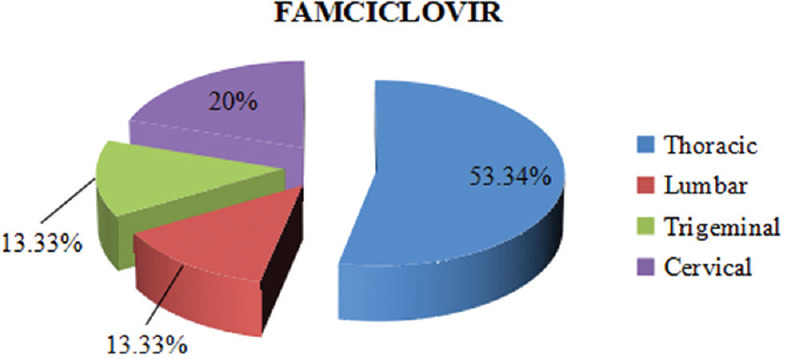
Dermatome distribution
Table 1.
Mean visual analog scale scores comparison at every follow-up visit between both the study groups
| Days | Mean±SD | P* | |
|---|---|---|---|
| Valacyclovir | Famciclovir | ||
| 0 | 6.53±1.51 | 6.27±1.67 | 0.5295 |
| 3 | 4.13±0.99 | 4.33±1.29 | 0.5032 |
| 8 | 2.0±1.36 | 2.33±1.59 | 0.3912 |
| 15 | 1.07±1.28 | 1.33±1.50 | 0.4731 |
| 22 | 0.60±0.83 | 1.2±1.42 | 0.0504 |
| 29 | 0.2±0.41 | 0.87±1.19 | 0.0050 |
*P<0.05 considered significant. SD - Standard deviation
Table 2.
Comparing the number of patients free of zoster pain in both the study groups at each follow-up
| Days | Valacyclovir, n (%) | Famciclovir, n (%) | P* |
|---|---|---|---|
| 3 | 0 | 0 | - |
| 8 | 6 (20) | 6 (20) | 1.000 |
| 15 | 16 (53.34) | 14 (47) | 0.7963 |
| 22 | 18 (60) | 16 (53.34) | 0.7945 |
| 29 | 24 (80) | 18 (60) | 0.1590 |
*P<0.05 significant
Discussion
It is well known that the principal reason for majority of the patients to seek medical attention is the pain of herpes zoster.[11] In our study, 60 patients were chosen and divided randomly in two groups receiving valacyclovir and famciclovir, respectively, in the doses mentioned above. Valacyclovir is a prodrug of acyclovir.[1] Famciclovir is also a prodrug with an active metabolite penciclovir. They both inhibit viral DNA polymerase enzyme, thus preventing the viral replication.[12] When given within 3 days of the appearance of the first lesion, both the drugs provide a reduction in duration not only loss of acute pain but also of full crusting and healing of the zoster associated lesions.[13]
Both valacyclovir and famciclovir were well tolerated. No serious adverse effects were observed in either of the study groups to warrant withdrawal of any patient. A male preponderance was observed as in other studies conducted previously.[14] On observing the dermatome distribution, both the groups showed thoracic dermatomes to be the most commonly involved dermatome segments.
In the present study, greater resolution of zoster associated pain was done by valacyclovir compared to famciclovir. Better reduction of pain with valacyclovir treatment by comparing mean ± standard deviation VAS scores was observed, which was found to be statistically significant only on day 29 (P = 0.005). Although valacyclovir provided a faster resolution of pain, and more number of pain free patients by day 29, the difference in number of pain free patients was not found to be statistically significant when compared with famciclovir (P = 0.1590). For the development of postherpetic neuralgia, an important risk factor considered is the severity of pain experienced by the patients. Valacyclovir, by its virtue of reducing the severity of pain significantly, can be said to have a favorable effect on the development of postherpetic neuralgia. Similar findings were reported by Indradevi et al.[14] at a study conducted in a tertiary care hospital in Puducherry and also by Tyring et al.[15] in their study in the United States.
The limitations faced by our study were that antivirals could not be given exclusively as considering the severity of pain, analgesics, and corticosteroids had to be added. Although to remove bias, they were given in equal doses. Some patients even required an added dose of pregabalin 75 mg BD depending on the pain severity, thus increasing the chances of error. The study sample size was restricted by number of patients presenting to the hospital over the duration of the study. Studies of longer duration and bigger sample size are needed for more appropriate results. Furthermore, despite our request to not take any medications in the morning on the day of follow-up, some patients still confirmed taking pain relief medication, thereby affecting the results.
Conclusion
Oral valacyclovir administered during acute zoster infection for a period of 7 days offers a significant benefit by its virtue of providing a greater resolution of pain along with good tolerance compared to famciclovir while maintaining the favorable safety profile.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to express their thanks to professors and other staff members of the skin and VD department, for permitting us to conduct the study. We would also like to express our gratitude to other staff members of the department of pharmacology for their constant support.
References
- 1.Strauss SE, Oxman MN, Schmader KE. Varicella and herpes zoster. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's Dermatology in General Medicine. 7th ed. New York: Mc Graw Hill Publishing Division; 2008. pp. 1885–98. [Google Scholar]
- 2.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: Towards a global perspective. BMJ Open. 2014;4:e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26–33. doi: 10.1016/s1473-3099(03)00857-0. [DOI] [PubMed] [Google Scholar]
- 4.Wareham DW, Breuer J. Herpes zoster. BMJ. 2007;334:1211–5. doi: 10.1136/bmj.39206.571042.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon YH. Herpes zoster and postherpetic neuralgia: Practical consideration for prevention and treatment. Korean J Pain. 2015;28:177–84. doi: 10.3344/kjp.2015.28.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glaser RB. Clinical aspects of herpes zoster (Topics in primary care medicine) West J Med. 1983;139:718–20. [PMC free article] [PubMed] [Google Scholar]
- 7.Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546–53. doi: 10.1128/aac.39.7.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ono F, Yasumoto S, Furumura M, Hamada T, Ishii N, Gyotoku T, et al. Comparison between famciclovir and valacyclovir for acute pain in adult Japanese immunocompetent patients with herpes zoster. J Dermatol. 2012;39:902–8. doi: 10.1111/j.1346-8138.2012.01584.x. [DOI] [PubMed] [Google Scholar]
- 9.Wald A, Selke S, Warren T, Aoki FY, Sacks S, Diaz-Mitoma F, et al. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex Transm Dis. 2006;33:529–33. doi: 10.1097/01.olq.0000204723.15765.91. [DOI] [PubMed] [Google Scholar]
- 10.Dworkin RH, Gnann JW, Jr, Oaklander AL, Raja SN, Schmader KE, Whitley RJ. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain. 2008;9:S37–44. doi: 10.1016/j.jpain.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Araújo LQ, Macintyre CR, Vujacich C. Epidemiology and burden of herpes zoster and post-herpetic neuralgia in Australia, Asia and South America. Herpes. 2007;14(Suppl 2):40–4. [PubMed] [Google Scholar]
- 12.Heninger U, Seward SF. Varicella. Lancet. 2006;368:1365–76. doi: 10.1016/S0140-6736(06)69561-5. [DOI] [PubMed] [Google Scholar]
- 13.Basickes V, Demarin V. Postherpetic neuralgia. Acta Clin Croat. 2007;46:279–82. [Google Scholar]
- 14.Indradevi R, Manoharan K, Oudeacoumar P, Karthikraja S, Azeem Jaffar N. A comparative study to evaluate the efficacy and safety of valacyclovir and famcyclovir in the management of herpes zoster in a tertiary care hospital in Puducherry. J Evol Med Dent Sci. 2014;3:5804–12. [Google Scholar]
- 15.Tyring SK, Beutner KR, Tucker BA, Anderson WC, Crooks RJ. Antiviral therapy for herpes zoster randomized, controlled clinical trial of valacyclovir and famciclovir therapy in immunocompetent patients 50 years and older. Arch Fam Med. 2000;9:863–9. doi: 10.1001/archfami.9.9.863. [DOI] [PubMed] [Google Scholar]


