Abstract
During the last two decades, the development in drug discovery is slackening due to drug withdrawal from the market or reported to have postmarket safety events. The vital organ toxicities, especially cardiotoxicity, hepatotoxicity, pulmonary toxicity, and neurotoxicity are the major concerns for high drug attrition rates. The pharmaceutical industry is looking for high throughput, high content analysis based novel assays that would be fast, efficient, reproducible, and cost-effective; would address toxicity, the safety of lead molecules, and complement currently used cell-based assays in preclinical testing. The use of zebrafish, a vertebrate screening model, for preclinical testing is increasing owing to the number of advantages and striking similarities with the mammal. The zebrafish embryo development is fast and all vital organs such as the heart, liver, brain, pancreas, and kidneys in zebrafish are functional within 96–120hpf. The maintenance cost of zebrafish is reasonably low as compared to mammalian systems. Due to these features, zebrafish has arisen as a potential experimental screening model in lead identification and validation in the drug efficacy, toxicity, and safety evaluation. Numbers of drugs and chemicals are screened using zebrafish embryos, and results were found to show 100% concordance with mammalian screening data. The application of zebrafish, being a whole-organism screening model, would show a significant reduction in the cost and time required in the drug development process. The present challenge includes complete automation of the zebrafish screening model, i.e., from sorting, imaging of embryos to data analysis to accelerate the therapeutic target identification, and validation process.
Keywords: Drug discovery, high content analysis, high-throughput screening, organ-specific toxicity, safety pharmacology, zebrafish
Introduction
The research in drug discovery and development is facing many challenges over the last two decades. Very few new drugs (chemical entities and biological) were approved by the US FDA per year (on an average of 30 new drugs per year) in spite of vigorous drug screening by pharmaceutical companies [Figure 1a]. For example, the US FDA approved a total of 496 new drugs in the last two decades. Among 496 approved drugs, 35 drugs were withdrawn from the market and 107 were reported to be associated with postmarket adverse drug events.[1,2,3,4]
Figure 1.
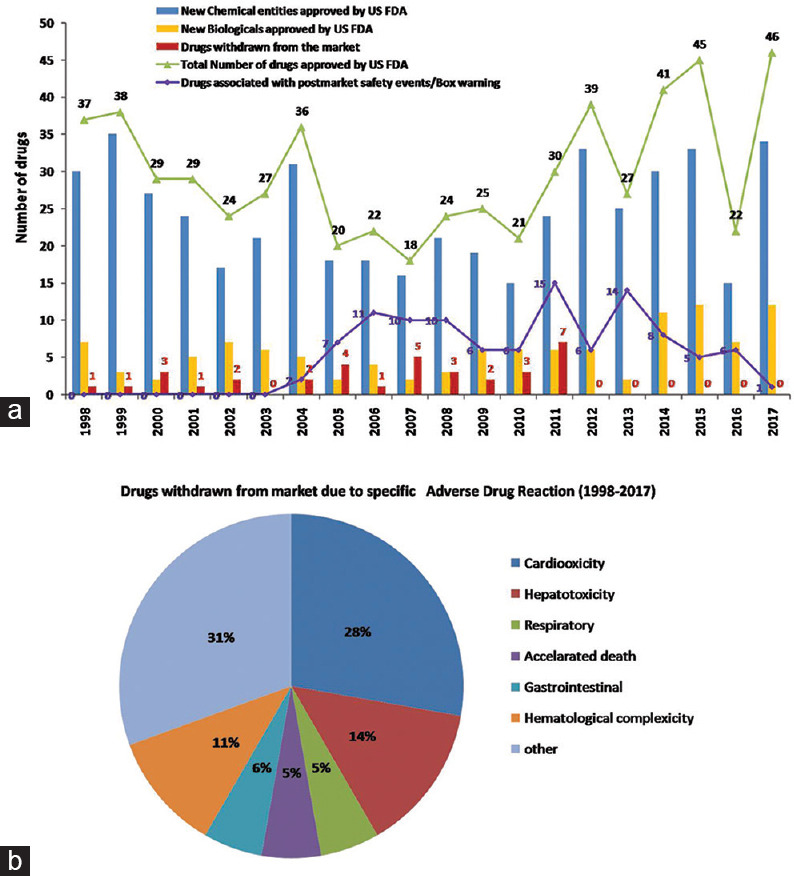
Data of new drug approvals by the US FDA between 1998 and 2017[1,2,3,4] (a) Bar graph depicts new drugs (new molecular entities- chemicals and biological) approved by the US FDA for a period of two decades (1998–2017). The number of drugs approved during this period and later withdrawn or associated with postmarket safety events is depicted by bar graph and line graph, respectively. (b) The different types of toxicities associated with approved drugs in 1998–2017 are denoted
The vital organ toxicity being the major reason for the high drug attrition rate observed the postmarketing launch of the drugs [Figure 1b]. High-throughput screening (HTS) and high content analysis are current technology adopted by the pharmaceutical industry for new drug screening in the view of expired patents of drugs in various therapeutic areas. The pharmaceutical industry requires an innovative, simple, cost-effective, HTS model to overcome the challenges in drug discovery and development.
Although the International Council for Harmonization (ICH) guidelines (ICH S7A) give the design strategy for safety pharmacology studies, a well-defined whole-organism model system for evaluating adverse effects of lead molecules on the central nervous system (CNS), respiratory, cardiovascular, or renal system has not been defined.
Zebrafish-A Whole-organism Screening Model
Zebrafish (Danio rerio) belonging to the family Cyprinidae, is a freshwater fish native to northern India, arose in the Ganges region and can be commonly found in countries including India, Bangladesh, Nepal, Myanmar, and Pakistan.[5,6] The interest in zebrafish as an in vivo model system has increased exponentially due to several characteristics of zebrafish. It is found that zebrafish resembles mammalian models. They are small in size (adult zebrafish 3 cm long; larvae 1–4 mm long), having a low cost of maintenance, high fecundity (200–300 embryos by single female at one mating), excessive transparency for several days postfertilization, rapid embryonic development, and simple drug administration.[7,8]
The genome sequencing of zebrafish was completed in 2013[9] and it shows genetic and physiological similarity (~70%) with mammals. The embryogenesis with fully functional organs such as the liver, brain, heart, pancreas, kidney, intestines, nervous, and sensory organs is attained by 120 hpf. These organs of zebrafish show similarity to the mammalian organs anatomically, and physiologically.[8,10]
Although in vitro cellular assays have the scale and volume capacity, a number of leads obtained by these approaches failed in humans. As delineated in Figure 2, the zebrafish model is a whole-organism in vivo screening model close to the mammalian model and overcomes the limitations posed by in vitro cell-based or other in vivo screening tools.
Figure 2.
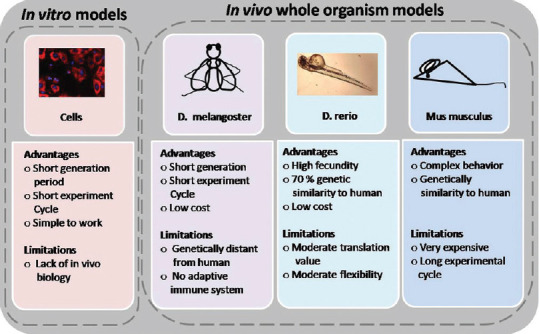
Comparison of in vitro and in vivo whole-organism models
Zebrafish A Cost-effective Experimental Model of the Laboratory
The maintenance of zebrafish is inexpensive in comparison to rodents'. Similar to rodents, the consideration in their maintenance are light conditions, feeding, breeding, and cleaning.[9] For a healthy environment, water quality should be maintained by monitoring various parameters, as enlisted in Table 1. The pH of the water should be between 6.8 and 7.5 and the temperature between 26°C and 28°C, 14:10 h (light: dark) light condition should be maintained.[6,9] The ideal range of water conductivity for a zebrafish system is between 300 and 1500 μS.
Table 1.
Water parameters for maintaining zebrafish
| Parameter | Optimum range |
|---|---|
| Alkalinity (mg/L, CaCO3) | 50-150 |
| pH | 6.8-7.5 (6.0-8.5 tolerated) |
| Temperature (°C) | 26-28.5 |
| Hardness (mg/L, CaCO3) | 50-100 |
| Un-ionized ammonia (mg/L) | <0.02 |
| Nitrate (NO3−) (mg/L) | <50 |
| Nitrite (NO2−) (mg/L) | <0.1 |
| Dissolved oxygen (mg/L) | >6.0 |
| Salinity (g/L) | 0.5-1 |
| Conductivity (µS) | 300-1500 |
A balanced diet is important for the health of zebrafish and reproduction. Two types of feed are given to them and they are fed with 4% of their body weight per day [Table 2]. The larvae of 4–15 days old can be fed with paramecia or rotifers, followed by regular food, especially live brine shrimps. Brine shrimp eggs can be hatched in the laboratory itself that is economical. Overfeeding to zebrafish should be avoided which may affect their breeding or may be fatal due to increase in nitrate level.[6,11] On attainment of sexual maturity and under favorable conditions, the zebrafish start laying the eggs.[6] Within 72–120 hpf, all vital organs are developed and due to transparency; it is easy to visualize the internal organ systems of zebrafish larvae [Figure 3]. Zebrafish embryos are not fed till 5 dpf. The dry food having (~100 microns in size) or live food such as paramecium and rotifers are given to zebrafish embryos after 5 dpf.[11] Laboratory-bred zebrafish have a maximal recorded life-span of 5.5 years, though an average of 3.5 years has been reported.[12]
Table 2.
Zebrafish feed
| Time | Stage | Body weight (mg) | 4% of body weight food/day (mg) | Dry feed (µm) | Live feed |
|---|---|---|---|---|---|
| Day 0 | Embryos | - | - | - | - |
| Day 4 | Larvae | <200 | ~8 | <100 | Rotifers/paramecia |
| Day 7 | Larvae | ~200 | ~8 | 300 | Rotifers/paramecia |
| Day 14 | Larvae | ~250 | ~10 | 300 | Brine shrimps |
| Day 21 | Larvae | ~310 | ~12 | 300-400 | Brine shrimps |
| Day 42 | Larvae | ~400 | ~14 | 300-400 | Brine shrimps |
| Day 66 | Larvae | ~600 | ~24 | 300-400 | Brine shrimps |
| Day 90 | Adult | ~900 | ~48 | 300-400 | Brine shrimps |
Figure 3.
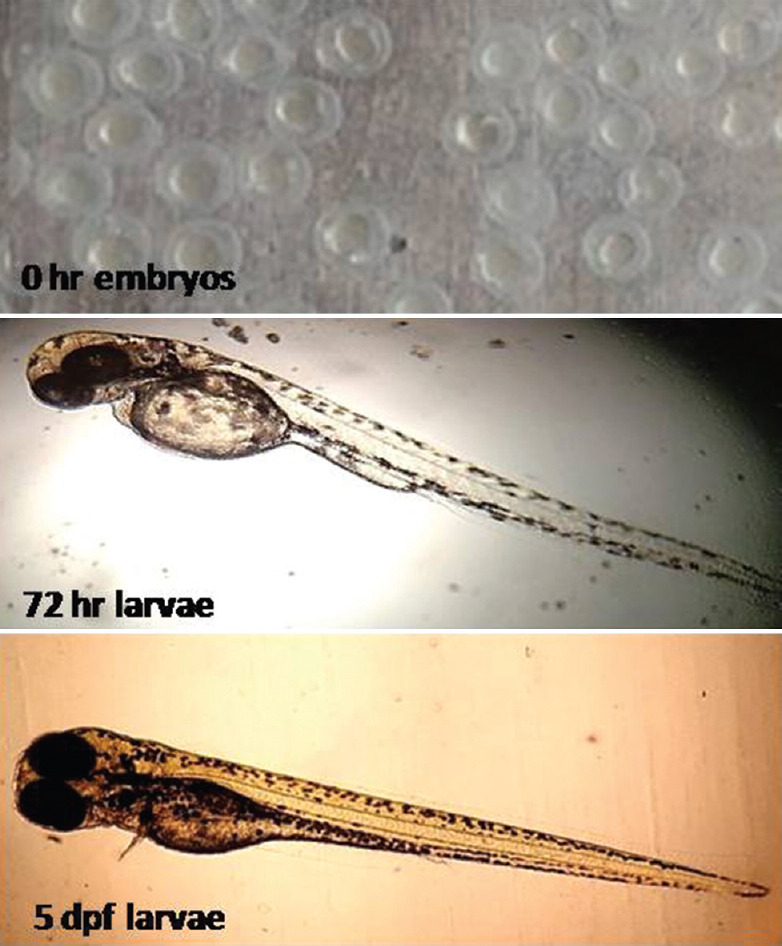
The developmental stages of zebrafish from 0 h embryos, 72 hpf and 5 dpf
Toxicity Studies in Zebrafish
Organ-specific toxicity is of major concern for high drug attrition in recent times. During the last decades, organ-specific toxicity was the main concern for the drug withdrawal from the market [Table 3]. Cardiotoxicity and hepatotoxicity being the major causes, followed by immune-related reaction, neurotoxicity, hematological toxicity reported for the postmarketing withdrawal of the drug.[2] Pharmacological studies, especially pharmacodynamics (i.e., what drug does to the body) are broadly classified as primary pharmacodynamic, secondary pharmacodynamic, and safety pharmacology. Safety pharmacology is mainly concerned with adverse effects on the vital organs such as cardiovascular, respiratory, and nervous systems. The adverse effect on these systems varies depending on various factors such as class of compound and mechanism of action. For example, antiarrhythmic agents are known to cause pro-arrhythmia.[13,14] The cell line-based assays to detect organ-specific toxicity are used; however, in vivo biology cannot be mimicked and toxicity cannot be estimated at the optimal level. Initially, zebrafish have been used for toxicity studies of environmental pollutants, agrochemical compounds; however, in the last 20 years, the pharmaceutical industry witnessed increasing use of zebrafish whole-organism screening model for toxicity studies.[11,14,15]
Table 3.
List of drugs withdrawn from the market
| Name of drug | Year of withdrawal | Cause for withdrawal |
|---|---|---|
| Troglitazone | 2000 | Hepatotoxicity |
| bromfenac | 1998 | Hepatotoxicity |
| Trovafloxacin | 1999 | Hepatotoxicity |
| Ebrotidine | 1998 | Hepatotoxicity |
| Nimesulide | 1999 | Hepatotoxicity |
| Nefazodone | 2003 | Hepatotoxicity |
| Ximelagatran | 2006 | Hepatotoxicity |
| Pemoline | 2010 | Hepatotoxicity |
| Tegaserod maleate | 2007 | Cardiotoxicity |
| Rofecoxib | 2004 | Cardiotoxicity |
| Grepafloxacin | 1999 | Cardiac repolarization; QT interval prolongation; ventricular arrhythmia (torsade de pointes) |
| Efalizumab | 2009 | Progressive multifocal leukoencephalopathy |
| Cisapride | 2000 | Serious cardiac arrhythmias |
| Pergolide | 2007 | Valve regurgitation |
| Gemtuzumab ozogamicin | 2010 | Increased risk of death and veno-occlusive disease |
| Sibutramine | 2010 | Increased cardiovascular and stroke risk |
| Astemizole | 1999 | Torsade de Pointes (LQTS; prolonged QT intervals) |
| Propoxyphene | 2010 | Serious cardiotoxicity |
| Valdecoxib | 2005 | Serious cardiovascular adverse events gastrointestinal bleeding |
| Cerivastatin | 2001 | Rhabdomyolysis which led to kidney failure; |
| Isotretinoin | 2009 | Increased risk of birth defects, miscarriages, and premature births; suicidal tendencies |
| Alatrofloxacin | 2000 | Hepatotoxicity |
LQTS - Long QT syndrome
Cardiotoxicity
Cardiotoxicity is the major cause of drug attrition rate and major concern for investigation. The majority of compounds, withdrawn from the market, account for cardiotoxicity. The drug-induced blockade of the human ether-a-go-go-related gene and prolongation of QT interval was found to cause pro-arrhythmic episodes in the patient having cardiac disease. This is not only a big loss for the pharmaceutical industry but also to the society. The high drug attrition rate is accounted for such rare toxic events resulted from drug–gene interactions.
The mammalian heart made up of four chambers having two atrium and two ventricles, and each is separated by septa and valves. During embryonic development, 1% of cardiac myocytes differentiate into auto rhythmic cells that function as the pacemaker of the heart, responsible for contraction and relaxation of the heart. The impulse generated at the sinus node, a dominant pacemaker located in the wall of the right atrium at the upper part, goes rapidly through the intranodal pathway, ensuring contraction of atria chambers and reaching to the atrioventricular node (AVN) and here, the impulse slows down providing enough time for ventricular filling, atrium emptying from AVN, the impulse travels to the left and right Purkinje fibers through the Bundle of His before dividing and this cycle repeats giving ~ 70–90 beats/min.[16]
Unlike mammalian heart, zebrafish heart consists of two chambers, the atrium and ventricle. The atrioventricular valve separates these two chambers. In zebrafish heart, the deoxygenated blood enters in sinus venous and then flows through single two-chamber heart [Figure 4a]. The heart contraction in zebrafish starts at 24 hpf. The three types of progenitor cells such as atrial ventricular cardiomyocytes and endocardial cells are present at 24 hpf.[17] The atrial and ventricle progenitor cells migrate laterally on both sides to the anterior lateral mesoderm plate by 15 hpf. At 22 hpf, cardiac progenitor cells along with endocardium cells fused to form cardiac disk and show contraction between 22 and 24 hpf. The cardiac disk starts elongating into linear tubes and two-chamber heart is formed by leftward migration of cardiac disk and looping with the addition of second cardiac field cells to the atrium and venous pole at the end of 48 hpf.[18] The deoxygenated blood then pumps to the ventral aorta through the bulbus arteriosus to gills for oxygenation.[17,18]
Figure 4.
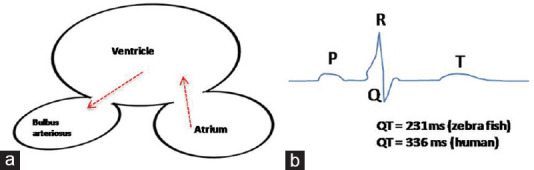
Schematic representation of (a) Two-chamber zebrafish heart and (b) electrocardiogram
Cardiac action potential includes four phases. In the depolarization phase, i.e., Phase 0, there is stimulation of sodium influx and the cell becomes positively charged. In Phase 1, there is an efflux of potassium that is early repolarization. In Phase 2 (plateau phase), there is an equilibrium between potassium efflux and calcium influx. The repolarization phase, i.e., Phase 3 in which the calcium channel closes and potassium efflux exceeds. Phase 3 is followed by Phase 4, the resting phase, in which only potassium channels remain open and due to potassium efflux, there is an establishment of negative resting membrane potential.[13] The QT interval represents the time between ventricular depolarization and repolarization. The ST segment is the start of ventricular repolarization. The QRS interval gives the time of ventricular depolarization. In electrocardiogram (ECG), the P wave represents atrium depolarization and the beginning of the P wave to the QRS complex is the PR interval. As shown in schematic diagram of ECG in [Figure 4b], the QT intervals for zebrafish and humans are 231 and 336 ms, respectively. The ECG of zebrafish heart shows all phases like mammalian ECG, except Phase 1. The early repolarization indicating Phase 1 in ECG of the zebrafish heart is absent. The expression of the ERG gene in zebrafish occurs in the early stage of development and the pore-forming amino acid sequence of the ERG gene shows 90% conservation between humans and zebrafish. The amino acid sequence of the pore-forming unit of ERG gene (ether a go-go related gene) in zebrafish and human is ~ 90% conserved and the ERG gene expression occurs in the early stage of zebrafish development.[19]
Various pathological abnormalities may lead to the changes in these intervals such as cardiac arrhythmias lead to QT prolongation, Torsades des Pointes, ventricular tachycardia, diabetic ketoacidosis, and thyroid disorders. ST-segment elevation may indicate myocardial infarction.[13,14,20,21,22] As enlisted in Table 4, numbers of drugs are screened for cardiotoxicity in zebrafish embryos model.[23,24,25] There is a high concordance in results obtained with cardiotoxic and noncardiotoxic drugs tested in humans and zebrafish.[26,27,28] Since many drugs are withdrawn from the market due to abnormalities caused by the drug, it is important to detect these toxicities in preclinical testing itself.[27,28,29] Zebrafish embryos are well-recognized as an alternative in vivo experimental system that serves as the best prefilter to detect such toxicities.
Table 4.
Summary of cardiotoxicity studies using the zebrafish model
| Drug | Cardiotoxicity in zebrafish |
|---|---|
| Ion channel ligands library | Sampurana BP, et al. Cells 2019;8:566 In silico molecular docking and zebrafish screen - Identification of 5 compounds with cardiotoxicity |
| Evodiamine | Yang et al. Molecules 2017;22:943 Cardiovascular side effects |
| METH; KT; methadone | Fang et al. Int J Pub Health Safe 2016;1:3 METH - Severe manifestations of cardiac and pericardial abnormalities KT - Severe pericardial edema, weak pigmentation, hemorrhage, and morphological abnormalities Methadone - Decreased the heart rate and exhibited an acute and strong cardiotoxicity |
| Human cardiotoxic drugs (aspirin, clomipramine hydrochloride, cyclophosphamide, nimodipine, quinidine, terfenadine, and verapamil hydrochloride) and two noncardiovascular toxicity drugs (gentamicin sulfate and tetracycline hydrochloride) | Zhu et al. J Appl Toxicol 2014;34:139-48 Aspirin - accelerated heart rate (tachycardia) Clomipramine hydrochloride, cyclophosphamide, nimodipine, quinidine, terfenadine, and verapamil hydrochloride - bradycardia Quinidine and terfenadine- AV block. Nimodipine - atrial arrest with much slower but regular ventricular heart beating Gentamicin sulfate and tetracycline hydrochloride - no sign of cardiovascular toxicity |
METH - Methamphetamine; KT - Ketamine; AV - Atrioventricular
Hepatotoxicity
Drug-induced hepatotoxicity or drug-induced liver injury (DILI) is another prime factor affecting the economy of the pharmaceutical sector due to drug safety-related issues aroused in the early and late steps of the drug development process.[29,30,31] For example, a nonsteroidal anti-inflammatory drug, bromfenac, analgesic drug for orthopedic patients was withdrawn from the US market in 1998 due to severe hepatic injury.[31] The FDA added a warning in prescribing propylthiouracil in 2010 because of severe liver injury, failure, and fatal incidents associated with propylthiouracil use in 2009. A total of 320 cases of hepatotoxicity reported including 12 deaths, 5 liver transplants in adult patients, 6 liver transplants in pediatric patients, and one death.[32,33] Troglitazone, an antidiabetic drug, was recalled from the market due to DILI. It is a peroxisomal proliferator activator receptor γ (PPAR) agonist. The patients, taking this medication, showed serum alanine transaminase values greater than three times normal. Approximately 1 person among 1250 and 1 person among 40,000–50,000 developed jaundice and irreversible liver failure, respectively, leading to the replacement of liver or eventually death.[34]
The liver is the vital organ responsible for the metabolism of xenobiotics and other important functions such as emulsification of fats by producing bile, digestion, detoxification, protein synthesis. The drug undergoes three phases of biotransformations. Three phases of biotransformation include drug oxidation, drug conjugation, and drug transport systems.[35]
The liver development in mammals and zebrafish comprises three steps, namely specification, differentiation, and growth. The liver in zebrafish is derived from the foregut endoderm. The committed progenitor cells are derived from the anterior endoderm to form hepatoblasts and can be visualized at 20–22 hpf. The differentiation of hepatoblasts begins at 24–50 hpf. The orthologous genes encoding albumin, and alpha fetoproteins found in humans, are absent in zebrafish. The proteins, transferrin, and liver fatty acid-binding protein are expressed by hepatocytes. Most of the zebrafish CYP genes are direct orthologs of human CYPs and they are involved in similar functions in humans [Table 5].[36] The zebrafish has many genes homologous to mammalian genes. The lipid metabolizing enzymes including 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) synthase that catalyzes the condensation reaction between acetyl-CoA and HMG, HMG-CoA lyase, and the PPAR.[37] Mechanisms that may be responsible for drug-induced liver toxicity include hepatocytes, transport protein and mitochondrial disruption, bile duct injury, cytolytic T-cell activation, intrinsic drug reactions, idiosyncratic drug reactions, and neoplasm.
Table 5.
CYP genes in zebrafish and human
| Zebrafish | Human | Zebrafish | Human | Zebrafish | Human |
|---|---|---|---|---|---|
| CYP1A1 | CYP1A1/1A2 | CYP3A65 | CYP3A-se1,-se2a | CYP19A1, 2 | CYP19A1 |
| CYP1B1 | CYP1B1 | CYP3C1-4 | CYP3A3, 4, 7 | CYP20A1 | CYP20A1 |
| CYP1C1, 2 | - | CYP4F43 | CYP4F | CYP21A1 | CYP21A2 |
| CYP1D1 | CYP1D1P | CYP4V7, 8 | CYP4V2 | CYP24A1 | CYP24A1 |
| CYP2Ks | CYP2W1 | CYP4T8 | - | CYP26A1 | CYP26A1/C1 |
| CYP2N13 | CYP2J2 | CYP5A1 | CYP5A1 | CYP26B1 | CYP26B1 |
| CYP2Ps | CYP2J2 | CYP7A1 | CYP7A1 | CYP26C1 | - |
| CYP2R1 | CYP2R1 | CYP7B1 | CYP7B1 | CYP27A3-7 | CYP27A1 |
| CYP2U1 | CYP2U1 | CYP7C1 | - | CYP27B1 | - |
| CYP2V1 | CYP2J2 | CYP8A1 | CYP8A1 | CYP27C1 | - |
| CYP2X1-10 | - | CYP8B1-3 | CYP8B1 | CYP39A1 | CYP39A1 |
| CYP2Y3, 4 | CYP2A/B/F/S | CYP11A1, 2 | CYP11A1 | CYP46A1 | CYP46A1 |
| CYP2AA1-12 | - | CYP11C1 | - | CYP46A2, 4, 5 | - |
| CYP2AD2, 3, 6 | CYP2J2 | CYP17A1, 2 | CYP17A1 | CYP51A1 | CYP51A1 |
| CYP2AE1, 2 | - |
a-In human, CYP3A-se1 and CYP3A-se2 are single exon pseudogene and share synteny with zebrafish CYP3A65 gene. Zebrafish CYP3A65 is induced by PXR agonist.
The number of features of zebrafish models enables us to help in eliminating hepatotoxicity at an early stage of drug screening. The high volume screening due to high fecundity and short time organogenesis, which could be difficult in rodents as they are time-consuming and cannot be studied at a large scale, makes zebrafish an attractive model. Enzyme reporter assays, liver-specific gene profiling, and liver histopathology methods are applied to assess the DILI.
He et al. quantitatively assessed known hepatotoxic drugs and nonhepatotoxic compounds in zebrafish experimental assay. The findings of the study were 100% in concordance with mammalian results.[30] Another study by Verstraelen et al. screened nonhepatotoxic and hepatotoxic compounds using zebrafish larvae. There was significant liver degeneration with the downregulation of hepatocyte markers with proven human hepatotoxic compounds.[38] The evaluation of DILI using transgenic zebrafish reporter line further corroborated the potential of the zebrafish model for hepatotoxicity studies.[39] The distinct enzymes involved in drug metabolism pathways and upregulated in DILI isolated, and transgenic reporter zebrafish created. Transgenic reporter zebrafish showed dose-dependent, and time-dependent toxicity to reference drugs, providing a platform for DILI evaluation.[39]
Neurotoxicity
The neurotoxicity profile of many approved drugs is not available or incomplete. Drug-induced neurotoxicity is another public concern that needs to be addressed, looking at costly cases of diamthazole, vinyl chloride, and clioquinol neurotoxicity.[40] In general, neurotoxicity assessment includes behavioral changes, biochemical estimation, and morphological (neurohistopathological) analysis using rodents or higher animals in a preclinical setting. Considering the current models used for neurotoxicity assessment, the high throughput, whole-organism predictive model is urgently needed, and the zebrafish model provides this platform.
The development and functional process of the CNS is well conserved between mammals and zebrafish.[41] Neurons are derived from the neuroectodermal epithelium and neurogenesis is spatially and temporally controlled, precise process involving external and internal clues. Inhibition of bone morphogenic protein signaling pathway and activation of fibroblast growth factor signaling, proteins belonging to the SoxB1 family maintains the pools of neuronal progenitor cells for further differentiation, specification, and growth. The development of the nervous system in zebrafish involves two steps. In the first neurulation, the specified, committed neural ectoderm forms a neural plate that converges to the solid neural keel. Neural keel fuses with dorsal midline to give solid neural rod. In the secondary neurulation process, the neural rod expands and forms a vertebral tube similar to the mammalian system. Inside the neural tube, there is a rapid asymmetric division of daughter cells; apical daughter cells become neurons while the distal daughter cells replenish the pool of progenitor cells. Thus, the apical-basal polarity of neuroepithelial cells is a key step in CNS maturation. The development of the blood–brain barrier is another feature zebrafish possess similar to the mammalian nervous system.[41]
Exposure to chemical, drugs during the development process pose a high risk to human health and there are several reports of neurological impairment leading to range of neuropsychological functional disorder or diseases (attention deficit, autism spectral disorder). A recent study by Prat et al. showed that exposure of 5 days post fertilized larvae to water-soluble acrylamide altered the motor function, specifically at the neuromuscular junction at presynaptic neurons. Moreover, the traces of acrylamide adducts were formed on synaptic proteins.[42] The gene expression profiling study by Fan et al.[43] showed overexpression of astrocytes markers in zebrafish developing brain exposed to different concentrations of ethanol. Furthermore, similar results were observed in mice, rats, and humans.[44,45,46] The effect of ibuprofen, diclofenac, and paracetamol assessment for neurotoxicity in zebrafish larvae showed differential pattern of gene expression of neurog1.[47] Using whole-mount zebrafish embryos, immunostaining for primary motoneurons and secondary motoneurons to determine the neurotoxicity of three compounds such as thiocyclam, cartap, and disulfiram showed the disulfiram to be more toxic among three compounds tested.[48]
Teratogenicity and Developmental Toxicity
Zebrafish is a distinct species with highly conserved developmental process. Zebrafish showed sensitivity toward compounds that were found to be teratogenic in mammals. Zebrafish genome is completely mapped.[9] and thereby, molecular targets for dysmorphology/pathophysiology can be linked to genomic targets to decipher the signaling pathways for compound-induced teratogenicity.[8] In mammals, the placenta is responsible for the transfer of nutrients and drugs to the fetus as well as for gas exchange, hormone secretion, and fetus protection.[49] Drug transport through the placenta depends on the placental membrane and drug. The underlying mechanism for teratogenicity includes number of events. The underlying mechanisms for teratogenicity includes number of events like oxidative stress, vascular or neural crest dysfunction, folate antagonism, endocrine disruption and enzyme-mediated teratogenesis.[50] For example, retinoic acid is teratogenic on overexposure. The study by Lee et al. showed that excess exposure of retinoic acid inhibits kidney formation in the fetus, with downregulated levels of Raldh transcripts encoding retinoic acid-synthesizing enzymes and increased levels of enzymes (Cyp26a1 and Cyp26b1) responsible for the catabolism of retinoic acid.[51]
Various known teratogens and nonteratogens have been evaluated on zebrafish and results are very much similar to mammalian results, even though the developmental process in zebrafish is ex vivo. Teratogens such as alcohol, valproic acid, retinoic acid, methotrexate, aspirin, caffeine, cisplatin, diazepam, mefenamic acid, and indomethacin showed similar results to that of the mammalian in different research findings.
Yamashita et al. tested 59 compounds for teratogenicity, and out of 59 test compounds, 32 compounds were known in vivo mammalian teratogens and 27 were nonteratogenics. The evaluation was based on morphological scoring at each drug concentration. The result showed 90% overall agreement between zebrafish and in vivo mammalian models.[52] Topiramate, an antiepileptic drug used for children and adults, was evaluated for teratogenicity using adult female zebrafish. The adult female and their offspring were screened for altered morphology. The findings of this study showed reduced oogenesis rate, offspring survival rate, and survived offspring with abnormalities like cartilage malformation and bone dysplasia.[53] A number of natural medicinal plant extracts or herbal formulations are evaluated for teratogenicity using zebrafish to provide significant safety guidance for use during pregnancy.[54,55]
Limitation of zebrafish as an experimental model
On a phylogeny tree, zebrafish is far distant from mammals that limit its use. The ex vivo development of zebrafish larvae occurs within chorion that may inhibit the absorption of the compounds. Furthermore, water-insoluble drugs at higher concentrations cannot be screened. Being an aquatic animal, the respiratory system, one of the vital organ systems in the safety pharmacology framework, cannot be evaluated using this model.
Conclusion and Future Path
A major objective of the present review is to highlight the importance of the zebrafish screening system as a potential, predictive, high-throughput whole-organism emerging alternative aid in the drug discovery process. A plethora of research findings has demonstrated that the zebrafish model can be used as a whole-organism HTS model for assessing the safety, efficacy, and toxicity of therapeutics. It can serve as a gap-filler between cellular assays and rodent experiments, thereby reinforcing the 3R concept of “Reduce, Refine and Replace” mammalian usage for pharmacological preclinical screening, and safety profiling of drugs. In spite of various studies and positive results, there is still more to explore about the zebrafish as a model for efficacy, toxicity. The improved protocols are needed to be established for zebrafish and reduce mammalian models.
In addition, new visualization methods, live imaging, advanced microscopy, and computational means for image analysis are continuously being developed across diverse model organisms and adapt to the zebrafish screening model to unearth its potential in drug discovery and development.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zeitoun J, Krumholz HM, Ross JS. Postmarket safety events among novel therapeutics approved by the US food and drug administration between 2001 and 2010. 2017;06520:1854–63. doi: 10.1001/jama.2017.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: A systematic review of the world literature. BMC Med. 2016;14:1–11. doi: 10.1186/s12916-016-0553-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Torre BG, Albericio F. The pharmaceutical industry in 2017. An analysis of FDA drug approvals from the perspective of molecules. Molecules. 2018;23:533. doi: 10.3390/molecules23030533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration web site. [Last accessed on 03 Nov 2018]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf .
- 5.Tavares B, Lopes SS. The importance of Zebrafish in biomedical. Acta Med Port. 2013;26:583–92. [PubMed] [Google Scholar]
- 6.Lawrence C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture. 2007;269:1–20. [Google Scholar]
- 7.McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2016;131:1796–803. doi: 10.1161/CIRCULATIONAHA.114.010270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mcgrath P, Li C. Zebrafish: A predictive model for assessing drug-induced toxicity. Drug Discov Today. 2008;13:394–401. doi: 10.1016/j.drudis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 11.Avdesh A, Chen M, Martin-iverson MT, Mondal A, Ong D, Rainey-Smith S, et al. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. J Vis Exp. 2012;69:e4196. doi: 10.3791/4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasales CJ, Gerhard GS, Kauffman EJ, Wang X, Stewart R, Moore JL, et al. Life spans and senescent phenotypes in two strains of Zebrafish (Danio rerio) Exp Gerontol. 2002;37:1055–68. doi: 10.1016/s0531-5565(02)00088-8. [DOI] [PubMed] [Google Scholar]
- 13.Lahrouchi N, Wilde AA. Ion Channels in Health and Disease. Amsterdam, Netherlands: Elsevier Inc; 2016. Complexity of molecular genetics in the inherited cardiac arrhythmias; pp. 345–68. [Google Scholar]
- 14.Sun W. Cardiotoxicity testing in drug development. SMJ Cardiovasc Dis. 2016;1:1005. [Google Scholar]
- 15.Barros TP, Alderton WK, Reynolds HM, Roach AG, Berghmans S. Zebrafish: An emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br J Pharmacol. 2008;154:1400–13. doi: 10.1038/bjp.2008.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park DS, Fishman GI. Development and function of the cardiac conduction system in health and disease. J Cardiovasc Dev Dis. 2017;4:7. doi: 10.3390/jcdd4020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stainier DY, Lee RK, Fishman MC. Cardiovascular development in the zebrafish I. Myocardial fate map and heart tube formation. Development. 1993;119:31–40. doi: 10.1242/dev.119.1.31. [DOI] [PubMed] [Google Scholar]
- 18.Brown DR, Samsa LA, Qian L, Liu J. Advances in the study of heart development and disease using Zebrafish. J Cardiovasc Dev Dis. 2016;3:13. doi: 10.3390/jcdd3020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langheinrich U, Vacun G, Wagner T. Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. Toxicol Appl Pharmacol. 2003;193:370–82. doi: 10.1016/j.taap.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Hanna EB, Glancy DL. ST-segment elevation: Differential diagnosis, caveats. Cleve Clin J Med. 2015;82:373–84. doi: 10.3949/ccjm.82a.14026. [DOI] [PubMed] [Google Scholar]
- 21.Brana I, Zamora E, Oristrell G, Tabernero J. Cardiotoxicity. Side Effect Med Cancer Ther Prev Treat Second Ed. 2018;21(Suppl 7):367–406. [Google Scholar]
- 22.Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol. 2015;12:547–58. doi: 10.1038/nrcardio.2015.65. [DOI] [PubMed] [Google Scholar]
- 23.Sampurna BP, Santoso F, Lee JH, Yu WH, Wu CC, Audira G, et al. Cardiac rhythm and molecular docking studies of ion channel ligands with cardiotoxicity in zebrafish. Cells. 2019;10:E566. doi: 10.3390/cells8060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W, Ma L, Li S, Cui K, Lei L, Ye Z. Evaluation of the cardiotoxicity of evodiamine in vitro and in vivo. Molecules. 2017;22:943. doi: 10.3390/molecules22060943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang M, Peng J, Zhu D, Luo C, Li C, Lin Y, et al. An in vivo assessment: Cardiotoxicity induced by three kinds of addictive drugs (methamphetamine, ketamine, and methadone) in zebrafish embryos. Int J Public Health Safe. 2016;1:118. [Google Scholar]
- 26.Zhu JJ, Xu YQ, He JH, Yu HP, Huang CJ, Gao JM, et al. Human cardiotoxic drug delivered by soaking and microinjection induce cardiovascular toxicity in zebrafish. J Appl Toxicol. 2014;34:139–48. doi: 10.1002/jat.2843. [DOI] [PubMed] [Google Scholar]
- 27.Zakaria ZZ, Benslimane FM, Nasrallah GK, Shurbaji S, Younes NN, Mraiche F, et al. Using zebrafish for investigating the molecular mechanisms of drug-induced cardiotoxicity. Biomed Res Int. 2018;2018:1642684. doi: 10.1155/2018/1642684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han Y, Zhang JP, Qian JQ, Hu CQ. Cardiotoxicity evaluation of anthracyclines in zebrafish (Danio rerio) J Appl Toxicol. 2015;35:241–52. doi: 10.1002/jat.3007. [DOI] [PubMed] [Google Scholar]
- 29.Siramshetty VB, Nickel J, Omieczynski C, Gohlke BO, Drwal MN, Preissner R. WITHDRAWN a resource for withdrawn and discontinued drugs. Nucleic Acids Res. 2016;44:D1080–6. doi: 10.1093/nar/gkv1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He JH, Guo SY, Zhu F, Zhu JJ, Chen YX, Huang CJ, et al. A zebrafish phenotypic assay for assessing drug-induced hepatotoxicity. J Pharmacol Toxicol Methods. 2013;67:25–32. doi: 10.1016/j.vascn.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Goldkind L, Laine L. A systematic review of NSAIDs withdrawn from the market due to hepatotoxicity: Lessons learned from the bromfenac experience. Pharmacoepidemiology and Drug Safety. 2006;15:213–20. doi: 10.1002/pds.1207. [DOI] [PubMed] [Google Scholar]
- 32.Malozowski S, Chiesa A. Propylthiouracil-induced hepatotoxicity and death hopefully, never more. J Clin Endocrinol Metab. 2010;95:3161–3. doi: 10.1210/jc.2010-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivkees SA, Szarfman A. Dissimilar hepatotoxicity profiles of propylthiouracil and methimazole in children. J Clin Endocrinol Metab. 2010;95:3260–7. doi: 10.1210/jc.2009-2546. [DOI] [PubMed] [Google Scholar]
- 34.Fukano M, Amano S, Sato JI, Yamamoto K, Adachi H, Okabe H, et al. Subacute hepatic failure associated with a new antidiabetic agent, troglitazone: A case report with autopsy examination. Hum Pathol. 2000;31:250–3. doi: 10.1016/s0046-8177(00)80229-4. [DOI] [PubMed] [Google Scholar]
- 35.Chiang J. Pathobiology of Human Disease. Amsterdam, Netherlands: Elsevier Inc; 2017. Liver physiology: Metabolism and detoxification; pp. 1770–82. [Google Scholar]
- 36.Goldstone JV, McArthur AG, Kubota A, Zanette J, Parente T, Jönsson ME, et al. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics. 2010;11:643. doi: 10.1186/1471-2164-11-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinlivan VH. Lipid uptake, metabolism, and transport in the larval zebrafish. Front Endocrinol. 2017;8:319. doi: 10.3389/fendo.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verstraelen S, Peers B, Maho W, Hollanders K, Remy S, Berckmans P, et al. Phenotypic and biomarker evaluation of zebrafish larvae as an alternative model to predict mammalian hepatotoxicity. J Appl Toxicol. 2016;36:1194–206. doi: 10.1002/jat.3288. [DOI] [PubMed] [Google Scholar]
- 39.Poon KL, Wang X, Lee SG, Ng AS, Goh WH, Zhao Z, et al. Transgenic zebrafish reporter lines as alternative in vivo organ toxicity models. Toxicol Sci. 2017;156:133–48. doi: 10.1093/toxsci/kfw250. [DOI] [PubMed] [Google Scholar]
- 40.Wysowski DK. Adverse drug event surveillance, and drug withdrawals in the United States 1969-2002. Arch Intern Med. 2005;165:1363–9. doi: 10.1001/archinte.165.12.1363. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt R, Strähle U, Scholpp S. Neurogenesis in zebrafish from embryo to adult. Neural Dev. 2013;8:1–13. doi: 10.1186/1749-8104-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prats E, Gómez-Canela C, Ben-Lulu S, Ziv T, Padrós F, Tornero D, et al. Modelling acrylamide acute neurotoxicity in zebrafsh larvae. Sci Rep. 2017;7:13952–63. doi: 10.1038/s41598-017-14460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan CY, Cowden J, Simmons SO, Padilla S, Ramabhadran R. Gene expression changes in developing zebrafish as potential markers for rapid developmental neurotoxicity screening. Neurotoxicol Teratol. 2010;32:91–8. doi: 10.1016/j.ntt.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 44.Alfonso-Loeches S, Pascual M, Guerri C. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology. 2013;311:27–34. doi: 10.1016/j.tox.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez A, Pariente JA, Salido GM. (2007). ETEMPthanol stimulates ROS generation by mitochondria through Ca2+ mobilization and increases GFAP content in rat hippocampal astrocytes. Brain Res. 2007;1178:28–37. doi: 10.1016/j.brainres.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 46.Jung KH, Das ND, Park JH, Lee HT, Choi MR, Chung MK, et al. Effects of acute eTEMPthanol treatment on NCCIT cells and NCCIT cell-derived embryoid bodies (EBs) Toxicol In Vitro. 2010;24:1696–704. doi: 10.1016/j.tiv.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 47.Xia L, Zheng L, Zhou JL. Effects of ibuprofen, diclofenac and paracetamol on hatch and motor behavior in developing zebrafish (Danio rerio) Chemosphere. 2017;182:416–25. doi: 10.1016/j.chemosphere.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 48.Muth-Köhne E, Wichmann A, Delov V, Fenske M. The classification of motor neuron defects in teh zebrafish embryo toxicity test (ZFET) as an animal alternative approach to assess developmental neurotoxicity. Neurotoxicol Teratol. 2012;34:413–24. doi: 10.1016/j.ntt.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Griffiths SK, Hons B, Bs BM, Campbell JP, Hons M, Frca M. Placental structure, function and drug transfer. Contin Educ Anaesth Crit Care Pain. 2015;15:84–9. [Google Scholar]
- 50.Van Gelder MM, Van Rooij IA, Miller RK, Zielhuis GA, Den Berg LT, Roeleveld N. Teratogenic mechanisms of medical drugs. Hum Reprod Update. 2010;16:378–94. doi: 10.1093/humupd/dmp052. [DOI] [PubMed] [Google Scholar]
- 51.Lee LM, Leung C, Tang WW, Choi H, Leung Y, Mccaffery PJ. A paradoxical teratogenic mechanism for retinoic acid. Proc Natl Acad Sci USA. 2012;109:13668–73. doi: 10.1073/pnas.1200872109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamashita A, Inada H, Chihara K, Yamada T, Deguchi J, Funabashi H. Improvement of the evaluation method for teratogenicity using zebrafish embryos. J Toxicol Sci. 2014;39:453–64. doi: 10.2131/jts.39.453. [DOI] [PubMed] [Google Scholar]
- 53.Lai YH, Ding YJ, Moses D, Chen YH. Teratogenic effects of topiramate in a Zebrafish Mode. lnt J Mol Sci. 2017;18:1721. doi: 10.3390/ijms18081721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan MF, Abutaha N, Nasr FA, Alqahtani AS, Noman OM, Wadaan MA. Bitter gourd (Momordica charantia) possess developmental toxicity as revealed by screening the seeds and fruit extracts in zebrafish embryos. BMC Complement Altern Med. 2019;19:184. doi: 10.1186/s12906-019-2599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alafiatayo AA, Lai KS, Syahida A, Mahmood M, Shaharuddin NA. Phytochemical evaluation, embryotoxicity, and teratogenic effects of Curcuma longa extract on zebrafish (Danio rerio) Evid Based Complement Alternat Med. 2019;2019:3807207. doi: 10.1155/2019/3807207. [DOI] [PMC free article] [PubMed] [Google Scholar]


