The latent human immunodeficiency virus type 1 (HIV-1) reservoir in persons on antiretroviral therapy (ART) represents a major barrier to a cure. Although most studies have focused on the HIV-1 reservoir in the memory T cell subset, replication-competent HIV-1 has been isolated from TN, and CCR5-tropic HIV-1 has been recovered from CCR5neg TN from ART-suppressed HIV-1-infected individuals.
KEYWORDS: B lymphocytes, HIV-1, dendritic cells, naive CD4+ T cells, trans infection
ABSTRACT
Insight into the establishment and maintenance of HIV-1 infection in resting CD4+ T cell subsets is critical for the development of therapeutics targeting the HIV-1 reservoir. Although the frequency of HIV-1 infection, as quantified by the frequency of HIV-1 DNA, is lower in CD4+ naive T cells (TN) than in the memory T cell subsets, recent studies have shown that TN harbor a large pool of replication-competent virus. Interestingly, however, TN are highly resistant to direct (cis) HIV-1 infection in vitro, in particular to R5-tropic HIV-1, as TN do not express CCR5. In this study, we investigated whether TN could be efficiently HIV-1 trans infected by professional antigen-presenting B lymphocytes and myeloid dendritic cells (DC) in the absence of global T cell activation. We found that B cells, but not DC, have a unique ability to efficiently trans infect TN in vitro. In contrast, both B cells and DC mediated HIV-1 trans infection of memory and activated CD4+ T cells. Moreover, we found that TN isolated from HIV-1-infected nonprogressors (NP) harbor significantly disproportionately lower levels of HIV-1 DNA than TN isolated from progressors. This is consistent with our previous finding that antigen-presenting cells (APC) derived from NP do not efficiently trans infect CD4+ T cells due to alterations in APC cholesterol metabolism and cell membrane lipid raft organization. These findings support that B cell-mediated trans infection of TN with HIV-1 has a more profound role than previously considered in establishing the viral reservoir and control of HIV-1 disease progression.
INTRODUCTION
Latently infected resting CD4+ T cells constitute a major reservoir of persistent HIV-1 infection. Strategies that lead to a significant reduction or elimination of this reservoir could help in the development of either a functional or sterilizing cure (1–4). The CD4+ T cell population is heterogeneous, broadly comprised of naive (TN) and memory cells that differ in life span, proliferative capacity, localization, and HIV-1 coreceptor expression. Memory cells are further categorized by various stages of differentiation, namely, central memory (TCM), transitional memory, and effector memory. The latent HIV-1 reservoir in memory T cell subsets has been extensively studied, whereas TN have been largely overlooked (5, 6). Although resting TN are highly resistant to direct, cis infection with HIV-1 in vitro, we and others have shown that HIV-1 DNA is detectable in TN of viremic and virus-suppressed individuals (7, 8). While the frequency of HIV-1 infection in TN is lower than that in TCM, as much or more virus is produced by TN after reactivation with latency-reversing agents (LRAs) (9). Moreover, paradoxically, CCR5-tropic HIV-1 has been recovered from TN despite the fact that they do not express the CCR5 coreceptor (9–12).
HIV-1 can infect target cells via direct, cis infection or through a cell-to-cell transfer which can result in trans infection (13–15). The latter mechanism has been extensively described as mediated by professional antigen-presenting cells (APC), i.e., monocytes/macrophages, myeloid dendritic cells (DC), and B lymphocytes. Indeed, HIV-1 trans infection mediated by APC is 10- to 1,000-fold more efficient than passive, cis dissemination of virions through the extracellular milieu (16, 17). We have previously shown that APC derived from HIV-1-infected nonprogressors (NP) do not efficiently transfer HIV-1 to CD4+ T cells due to alterations in APC cholesterol metabolism and cell membrane lipid raft organization (18, 19). In the present study, we show that B lymphocytes, but not DC, have the exclusive ability to efficiently trans infect TN with CCR5-tropic HIV-1. Furthermore, TN isolated from NP harbor significantly lower levels of HIV-1 DNA than do TN isolated from HIV-1 progressors (PR). These findings support that B cell-mediated trans infection of TN with HIV-1 has a more profound role than previously considered in establishing the viral reservoir and control of HIV-1 disease progression.
RESULTS
B cells trans infect CD4+ T cells with high efficiency.
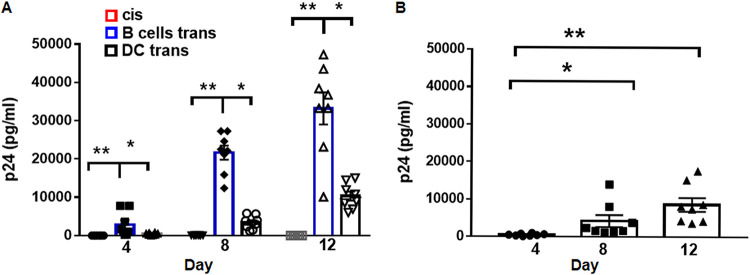
B cells activated with CD40L and interleukin-4 (IL-4), which mimics signals received from activated CD4+ T cells, express the C-type lectin DC-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) and can capture HIV-1, leading to trans infection of CD4+ T cells (15, 20). In this study, we first extended this finding by demonstrating that B cells or DC loaded with a low, 10−3 multiplicity of infection (MOI) of R5-tropic HIV-1BaL could trans infect phytohemagglutinin (PHA)/IL-2-activated CD4+ T cells, with the efficiency of trans infection being significantly greater for B cells than DC (one-way analysis of variance [ANOVA], P < 0.0001, Fig. 1A). This is of importance because, as we showed previously, only about 10 to 15% of activated B cells express DC-SIGN, compared to 100% of DC, therefore potentially implicating B cells as more efficient than DC in mediating HIV-1 trans infection. In agreement with our previous findings (15), CD4+ T cells were refractory to cis HIV-1 infection at the same low, 10−3 MOI but were productively infected with a 100-fold-greater dose of 10−1 MOI (Fig. 1B). We conclude from these data that B lymphocytes, activated by two surrogates for CD4+ T helper cells, i.e., IL-4 and CD40L, are more efficient than myeloid DC in mediating HIV-1 trans infection of activated CD4+ T lymphocytes.
FIG 1.
B cells trans infect CD4+ T cells with higher efficiency than DC. (A) B cells or immature DC (iDC) were pulsed with HIV-1BaL (10−3 MOI) and cocultured with PHA/IL-2-activated autologous CD4+ T cells at a 1:10 ratio for up to 12 days as described in Materials and Methods (trans infection). PHA/IL-2-activated CD4+ T cells were also pulsed with HIV-1BaL (10−3 MOI) and cultured alone (cis infection). Coculture supernatants were tested at the indicated time points for HIV-1 Gag p24 levels by ELISA. (B) PHA/IL-2-activated CD4+ T cells were pulsed with HIV-1BaL (10−1 MOI) and cultured up to 12 days. Culture supernatants were tested at the indicated time points for HIV-1 Gag p24 levels by ELISA. Data are mean ± SE; n = 8; *, P < 0.05; **, P < 0.005.
B cells, but not DC, trans infect CD4+ naive T cells in vitro.
TN do not express CCR5; however, in vivo, TN harbor R5-tropic HIV-1 (10, 11, 21, 22). We therefore hypothesized that TN are infected through an APC-mediated trans infection mechanism that does not require CCR5 expression by the T cells. To test this hypothesis, we used purified TN and TCM as targets for trans infection mediated by autologous B lymphocytes or DC that were loaded with 10−3 MOI of R5-tropic HIV-1BaL. Consistent with the approach described in Fig. 1, we initially used PHA/IL-2-activated CD4+ TN and TCM as targets. B cells were able to productively trans infect both TN and TCM with R5-tropic HIV-1, whereas DC were able only to trans infect the TCM subset (Fig. 2A). As previously shown by trans infection of total CD4+ T cells, B cell-mediated trans infection of both TN and TCM was significantly more productive than DC-mediated trans infection of either subset (one-way ANOVA, P < 0.0001).
FIG 2.
Only B cells trans infect TN. (A) B cells or iDC were pulsed with HIV-1BaL (10−3 MOI) and cocultured with PHA/IL-2-activated purified naive (TN; n = 5) or central memory (TCM; n = 4) CD4+ T cells. Coculture supernatants were tested at the indicated time points for HIV-1 Gag p24 levels by ELISA. Data are mean ± SE. (B) TN were treated with CCL-19, washed, and cocultured with HIV-1BaL (10−3 MOI)-pulsed B cells, iDC, or mature DC (mDC). Coculture supernatants were tested at the indicated time points for HIV-1 Gag p24 by ELISA. Data are mean ± SE, n = 6. (C) Total CD4+ T cells or TN were treated with PHA/IL-2 or CCL-19, pulsed with HIV-1BaL (10−1 MOI), and cultured alone. Culture supernatants were tested at the indicated time points for HIV-1 Gag p24 by ELISA. Data are mean ± SE, n = 4. (D) B cells pulsed with HIV-1 92FR_BX08 (10−3 MOI) were cocultured with CCL-19-treated total CD4+ T cells or TN for up to 12 days. Coculture supernatants were tested at the indicated time points for HIV-1 Gag p24 by ELISA. Data are mean ± SE, n = 3 replicates from 2 donors. (E) iDC pulsed with HIV-1 92FR_BX08 (10−3 MOI) were cocultured with CCL-19-treated total CD4+ T cells or TN for up to 12 days. Coculture supernatants were tested at the indicated time points for HIV-1 Gag p24 by ELISA. Data are mean ± SE, n = 3 replicates from 2 donors. Data were analyzed by one-way ANOVA followed by ad hoc Student’s t test. *, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.0001.
PHA/IL-2 treatment of TN and TCM induces T cell activation, thus rendering them more susceptible to HIV-1 infection. Therefore, we next assessed B cell- and DC-mediated HIV-1BaL trans infection of TN treated with the chemokine CCL-19. As described previously (12), CCL-19 neither elicits T cell activation nor induces CCR5 or CXCR4 expression, but enhances cis HIV-1 infection of resting CD4+ T cells. We found that only B cells were able to trans infect CCL-19-treated TN, resulting in detectable HIV-1 Gag p24 in the coculture supernatants (Fig. 2B). In contrast, neither mature DC (mDC) nor immature DC (iDC) mediated trans infection, indicating that the ability of DC to trans infect TN did not depend on their maturation status (one-way ANOVA, P < 0.0001). As expected, TN were refractory to direct cis infection of HIV-1BaL using either PHA/IL-2- or CCL-19-conditioned medium, while total CD4+ T cells were susceptible to productive cis infection (Fig. 2C). Our findings were further confirmed using an R5-tropic patient isolate, HIV-1 BX08(92FR_BX08), obtained from the NIAID AIDS Reagent Repository (23). We showed that B cells could efficiently trans infect both total CD4+ T cells and TN, while iDC could trans infect only total CD4+ T cells (Fig. 2D and E). Cis infection of TN was undetectable (not shown). Taken together, these results support that B lymphocytes have a unique ability to mediate highly productive trans infection of naive CD4+ T cells with R5-tropic HIV-1.
Coculture with B cells or DC does not affect the TN phenotype.
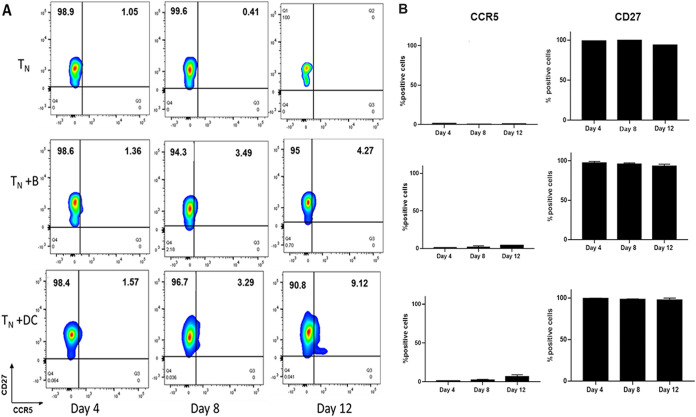
To address whether the CD4+ TN phenotype was altered through coculture with the APC, potentially affecting their efficiency of being infected with HIV-1, we analyzed TN for CCR5 and CD27 expression. TN cultured alone served as a control. CD27 expression was chosen instead of CCR7 expression because CCL-19 can induce downregulation of CCR7 (24). The flow cytometry gating strategy is shown in Fig. S1 of the supplemental material. Neither B cells nor DC induced a significantly higher expression of CCR5 up to 12 days in culture, although we detected a slight increase of CCR5 between day 8 and 12 in the DC-TN cocultures (Fig. 3A). Expression of the CD27 marker also remained unchanged throughout the coculture period (Fig. 3B).
FIG 3.
Coculture with B cells or DC does not affect TN phenotype. (A) CCL-19-treated TN were cultured alone (top row) or cocultured with HIV-1BaL (10−3 MOI)-pulsed B cells or iDC (middle and bottom rows, respectively), sampled at the indicated time points, stained with anti-CCR5 and CD27 MAb, and analyzed by fluorescence-activated cell sorting (FACS) as described in Materials and Methods. Representative data from 3 independent experiments. (B) CCR5 and CD27 percent positive cells in control TN cultures or cocultures. Data are mean± SE, n = 3.
Gating strategy to determine TN phenotype. Lymphocytes were gated first based on forward and side scatter, followed by doublet event exclusion, then by exclusion of dead cells (Aqua dye positive). CD4+ positive cells were then gated into CD45RA-negative and CD45RA-positive populations, with the latter population being 100% CCR7 positive as well as CCR5 negative. Download FIG S1, TIF file, 1.1 MB (1.1MB, tif) .
Copyright © 2021 Gerberick et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
These data show that there is no significant alteration of the TN phenotype during coculture with APC and confirm that B cells can establish HIV-1BaL infection in TN in the absence of significant CCR5 coreceptor expression.
Detection of intracellular HIV-1 p24 in APC-TN cocultures.
We next questioned if the detection of HIV-1 Gag p24 that we measured in the trans infection coculture supernatants reflected p24 intracellular localization. We therefore stained cells collected from the trans infection wells and examined them for HIV-1 p24 expression by flow cytometry. We were able to detect intracellular HIV-1 p24 in the cocultures of B cells with either total CD4+ T cells or TN (Fig. 4, left). In the DC-mediated trans infection cocultures, we could detect HIV-1 p24 only in the DC-total CD4+ T cell wells, with very low levels in the DC-TN cocultures. As shown in Fig. 4 (right), only total CD4+ T cells supported direct, cis infection with HIV-1. Taken together, these data further support the conclusion that only B lymphocytes can efficiently trans infect TN.
FIG 4.
Detection of HIV-1 p24 antigen in trans infection coculture with B cells or DC. (Left) Trans infection. B cells or iDC were pulsed with HIV-1BaL (10−3 MOI) and cocultured with CCL-19-treated total CD4+ T cells or TN. Cocultures were sampled after 8 days, stained with anti-Kc57, -CD4, -CD3, or -CD19, and analyzed by FACS as described in Materials and Methods. (Right) Cis infection. CCL-19-treated total CD4+ T cells or TN were pulsed with HIV-1BaL (10−1 MOI) and cultured alone. Cultures were sampled after 8 days, stained, and analyzed by flow cytometry in parallel to the trans infection cocultures. Controls represent uninfected cultures. Representative data from 2 independent experiments.
B cell trans infection of TN is mediated by DC-SIGN.
We have previously shown that B cell-mediated trans infection of CD4+ T cells is inhibited by blocking of DC-SIGN (15). Therefore, we tested if DC-SIGN was also necessary to mediate trans infection of TN. Treatment of B cells with anti-DC-SIGN monoclonal antibody (MAb) significantly inhibited trans infection of TN (Fig. 5A). We also treated TN with maraviroc, a CCR5 antagonist, to determine if CCR5 expressed by TN in the trans infection cocultures could be responsible for the infection (Fig. 5B). The concentration of maraviroc used was previously determined to significantly inhibit direct, cis infection of total CD4+ T cells, where expression of CCR5 is significantly higher than the very low or negative expression of CCR5 in TN alone (25). Production of HIV-1 Gag p24 detected in the maraviroc-treated coculture supernatants was not significantly different from the untreated cocultures at the same time point. HIV-1 infection is known to induce downregulation of CD4 surface expression. Therefore, we analyzed the effect of maraviroc treatment on the CD3+ CD4neg cell population in the cocultures by flow cytometry (Fig. S3). As shown in Fig. S3, the levels of HIV-1 p24 detected in this subpopulation were not significantly affected by maraviroc treatment. Blocking of HIV-1 trans infection by anti-DC-SIGN and minimal inhibition by maraviroc were confirmed by HIV-1 p24 intracellular staining of the trans infection cocultures (Fig. 5C).
FIG 5.
B cell-mediated trans infection of TN is inhibited by anti-DC-SIGN. (A) B cells were incubated with 20 μg/ml anti-DC-SIGN MAb for 30 min at 4°C prior to pulsing with HIV-1BaL (10−3 MOI) and then cocultured with CCL-19-treated TN for trans infection as described in Materials and Methods. Supernatants were collected after 12 days and tested for HIV-1 Gag p24 by ELISA. B cells treated with mouse IgG (20 μg/ml) were used as the control. Data are mean ± SE, n = 3 replicates from 2 donors. (B) In parallel cultures, CCL-19-treated TN were subsequently treated with maraviroc (1 μM) as described in Materials and Methods and cocultured with HIV-1BaL (10−3 MOI)-pulsed B cells for trans infection. Supernatants were collected after 12 days and tested for HIV-1 Gag p24 by ELISA. Untreated TN were used in the control cocultures. Data are mean ± SE, n = 3 replicates from 2 donors. Data were analyzed by one-way ANOVA followed by ad hoc Student’s t test. **, P < 0.005; ns, not significant. (C) Anti-DC-SIGN- or maraviroc-treated cells and the corresponding controls from the cocultures in panels A and B, as well as uninfected cocultures, were sampled after 12 days, stained with anti-Kc57, -CD4, -CD3, or -CD19, and analyzed by FACS as described in Materials and Methods.
Expression of HIV-1 p24 in CD3+ CD4neg maraviroc-treated cells. Cells from maraviroc-treated cocultures or controls analyzed in Fig. 5C were further analyzed by gating on the CD3+ CD4neg subpopulation and evaluated for HIV-1 p24 expression as described in Materials and Methods. Download FIG S3, TIF file, 0.5 MB (542KB, tif) .
Copyright © 2021 Gerberick et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Reactivation of HIV-1 from TN.
To assess whether HIV-1 trans infection of TN mediated by B cells or DC resulted in HIV-1 latency, TN were cultured with either 10−3 MOI HIV-1BaL-loaded B cells or DC for 8 days and then treated with phorbol myristate acetate (PMA)/PHA. Coculture supernatants were then harvested every 3 days for HIV-1 Gag p24 analysis (Fig. 6A). HIV-1 was recovered only from the B-TN cocultures (Fig. 6B). This indicates that the lack of detectable virus replication in the DC-TN cocultures was not due to the establishment of latency without detectable viral replication in the TN.
FIG 6.

Detection of virus after LRA reactivation. (A) Schematic representation of the experimental approach to measure reversal of HIV-1 latency in TN trans infected by B cells or iDC. (B) Cocultures were treated with LRAs at day 8 and then sampled at the indicated time points after reactivation. Supernatants were tested for HIV-1 Gag p24 by ELISA.
CD4+ TN from HIV-1-infected NP harbor less total HIV-1 DNA.
We have previously shown that APC from NP cannot trans infect autologous and heterologous CD4+ T cells and that this phenotype is under the control of cellular cholesterol homeostasis regulation (18, 19). Furthermore, this characteristic is present prior to infection with HIV-1, indicating that it is an innate, genetically controlled phenotype. If B cell-mediated trans infection of TN is an important mechanism by which these cells become infected with HIV-1, then it is plausible that NP have a reduced or absent level of HIV-1 DNA in this CD4+ T cell subset. We therefore quantified the viral DNA reservoir in total CD4+ T cells and TN from 7 NP not under antiretroviral therapy (ART) and 7 PR on ART (Fig. 7). The results show that we could not detect HIV-1 DNA in TN from NP classified as elite controllers (EC), while a relatively low number of HIV-1 DNA copies were detected in long-term nonprogressors (LTNP) and viremic controllers (VC) (19). Overall, the average copy number of HIV-1 DNA in TN from NP was lower than the number of copies detected in the 7 ART-suppressed PR (P = 0.007). NP and PR had similar levels of HIV-1 DNA copies when total CD4+ T cells were tested. Given that CD4+ T cells from NP as well as CD4+ T cells from PR are susceptible to direct, cis infection (19), the evidence supports the concept that the small amount of HIV-1 DNA detected is the result of direct infection. Taken together, these data support that individuals naturally able to control HIV-1 disease progression have a reduced or absent HIV-1 reservoir in their TN population.
FIG 7.
Quantification of total HIV-1 DNA in CD4+ total T cells and TN. DNA was quantified by qPCR as described in Materials and Methods. Each dot represents a unique donor. Statistical comparison was analyzed using a Wilcoxon matched-pairs signed-rank test. P < 0.05 was considered significant.
DISCUSSION
Here, we show that B lymphocytes have the unique ability to trans infect CD4+ TN in vitro with an R5-tropic HIV-1 laboratory strain (HIV-1BaL) and an R5 clinical isolate [HIV-1 BX08(92FR_BX08)], compared to myeloid DC. Prior studies have shown that TN can be infected in vitro with CXCR4-tropic HIV-1 when pretreated with the chemokine CCL-19, the ligand for the CCR7 receptor, expression of which significantly increases during the acute phase of infection when the latent reservoir is established (9, 24). This treatment does not alter the activation or proliferation state of TN and does not induce significant expression of the CCR5 coreceptor. Therefore, this model was used in our study to preserve the phenotype of the TN population, which remained resistant to cis infection with an R5-tropic HIV-1 strain. Furthermore, exposure of TN to B cells loaded with R5-tropic HIV-1BaL during the coculture period did not induce significantly higher expression of the CCR5 receptor, thus excluding the possibility that the efficient trans infection we observed was the result of in vitro conditions. However, treatment of TN with maraviroc under our culture conditions resulted in some inhibition of trans infection after 12 days of culture. This could be due to a waning effect of the drug in vitro or by de novo infection of cells expressing the CCR5 coreceptor later in the culture induced by the in vitro conditions, and being directly infected in cis by newly produced virus. Since this could also be occurring in the complex in vivo environment, it is possible that enhanced B cell-mediated trans infection of TN is aided by expression of CCR5 on these cells once they start interacting with their cognate counterparts. Efficient transfer of HIV-1 to TN mediated by B cells was also confirmed by detection of intracellular HIV-1 p24 by flow cytometry.
We have previously shown that activated B cells are able to bind and internalize HIV-1 into cytoplasmic vesicles through DC-SIGN and can trans infect CD4+ T cells for up to 2 days with high efficiency (15). Furthermore, we demonstrated that trans infection of total CD4+ T cells can be inhibited by treatment with anti-DC-SIGN MAb. Here, we confirmed that inhibition of DC-SIGN expression on B cells also impairs trans infection of TN. Notably, B cells do not support HIV-1 replication (15). Therefore, the second, cis infection phase in DC-mediated HIV-1 trans infection (26) is not applicable to B lymphocytes. Since cell-to-cell-mediated spread of HIV-1 is several orders of magnitude more efficient than direct cis infection of target cells (17), this mode of virus dissemination could have a significant role in HIV-1 pathogenesis, particularly in T cell-APC-dense anatomical compartments (16, 27). We propose that this trans infection process is likely intertwined with basic immunologic interactions of B lymphocytes and TN. Indeed, B cells were recently described as having a broad role in the development of TN (28). The interaction between B cells and CD4+ T cells thus goes beyond the classical initiation of antigen-specific B cell differentiation into antibody-producing plasma cells. In fact, evidence suggests that B cells are necessary and sufficient to prime and activate TN in response to virus-like particles (28). Thus, unique features of interactions between B cells and TN could drive the transfer and replication of HIV-1. In T-dependent B cell immune responses, antigen-engaged B cells must find their cognate helper T cells to initiate the progression of B cell immune responses. However, within the lymph node follicle, B cells move continuously to survey the subcapsular (SCS) macrophages for surface-displayed antigens (29, 30) and are also receiving survival signals from fibroblastic reticular cells (FRC) (31) such as the B cell activator BAFF, which has been shown to activate B cells to express DC-SIGN (32). These B cells are positioned to capture HIV-1 either as free virus entering through the afferent lymph vessel or through sampling of SCS macrophages (33), which have poor endocytic capacity and limited degradative ability (30). This ultimately prevents them from efficiently degrading HIV-1. In this scenario, subcapsular B cells activated through a T-independent mechanism are perfectly positioned to capture HIV-1 particles while surveying the environment for their specific antigen.
Upon encounter with antigen, signaling via the B cell receptor (BCR) starts the sequence of events that will bring the antigen-specific B cells to the follicle-T zone boundary where they search for their cognate CD4+ T cell among the CD4+ T helper cells or TN residing there (34). This interaction provides an opportunity for transfer of HIV-1 that has been captured by B cells to the CD4+ T cells. This interaction at the follicle-T cell zone interface of lymph nodes can last from several minutes to an hour (35, 36) and requires the interaction of integrins, such as LFA1 on T helper cells interacting with ICAM-1 or ICAM-2 on B cells, as well as costimulatory molecule CD86 signaling of CD28. Crucially, these interactions are stabilized by the antigenic peptide presented by B cell-expressed major histocompatibility complex (MHC) class II. Notably, it is known that B cells are superior to DC in capturing high doses of cognate antigen through high-affinity antigen-specific receptors, therefore rendering B cell-mediated antigen stimulation more efficient than that by DC (33). B cells capture antigen with high affinity through the BCR, allowing for even a low concentration of antigen to result in high internalization and subsequent presentation to T cells (37) and upregulation of the costimulatory molecule CD86 expression. On the other hand, DC capture antigen through nonspecific binding, requiring higher levels of antigen to induce a CD4+ T cell response. Moreover, the interaction between DC and TN is not prolonged, resulting in a lower chance of virus being transferred, even though activated B cells and DC express costimulatory molecules involved in the formation of the immunological synapse. Thus, the unique features of the interactions between B and TN could drive the transfer of HIV-1 to TN with higher efficiency than that of DC. It has been reported that high expression of Siglec-1 (CD169) on DC matured with lipopolysaccharide (LPS) and type I interferon (IFN) significantly contributes to trans infection (38, 39), and its importance in retrovirus infections has been shown in a mouse model (40). The different conditions under which DC can be matured and the effect on their ability to trans infect human TN are beyond the scope of our study, but it is possible that DC matured under different stimuli could be able to infect TN through trans mechanisms.
Although TN represent the more abundant fraction of CD4+ T cells, most studies of the latent HIV-1 reservoir have focused on memory T cells because they harbor the highest levels of HIV-1 DNA in people under ART. We and others (9, 12, 22) have shown that although the frequency of HIV-1 infection in these cells is lower than other subsets, as much or more virus is produced by these cells after treatment with LRA. This is true also when TN isolated from HIV-1-infected individuals under ART are exposed to LRA. Paradoxically, although TN do not express the HIV-1 coreceptor CCR5, they harbor CCR5-tropic virus in vivo. Therefore, an understanding on how this subset of CD4+ T cells becomes infected could provide important clues in the development of strategies to thwart the early establishment of HIV-1 infection.
As we have previously shown, efficient APC-mediated trans infection is regulated by APC membrane cholesterol content and is related to the control of HIV-1 disease progression (18, 19). In fact, APC derived from HIV-1-infected NP have an innate inability to trans infect CD4+ T cells, and this phenotype can be reversed by replenishing cell membrane cholesterol. On the other hand, APC from HIV-1-infected individuals with progressing disease, i.e., PR, mediate efficient HIV-1 trans infection (18, 19). Here, we quantified the viral reservoirs in total CD4+ T cells and TN from NP and PR in the Pittsburgh clinical site of the MACS/WIHS Combined Cohort Study (MWCCS). Notably, while the PR studied here were under suppressive ART, all the NP tested were therapy naive at the time of the study. We could not detect viral DNA in NP classified as EC, while a significantly smaller number of HIV-1 DNA copies was detected in LTNP and VC than in PR. These data strongly suggest that the altered ability to trans infect CD4+ T cells in NP results in a small or negligible pool of latently infected TN, with HIV-1 DNA levels even lower than those detectable in patients under suppressive ART, thus contributing to the maintenance of the NP phenotype. Although limited in scope, our findings are also consistent with that observed in the French Virological and Immunological Studies in Controllers After Treatment Interruption (VISCONTI) cohort of individuals who received ART within 10 weeks of primary infection (41), where viremia was controlled for 24 months post-treatment interruption. In that cohort, HIV-1 DNA was detected in TN of only 2 out of 11 patients, while the other T cell subsets harbored comparable levels of HIV-1 DNA. Our present study suggests that early, B cell-mediated trans infection could be an important mechanism by which HIV-1, regardless of its basic cell tropism, establishes infection in TN. We propose an additional role for B cell-mediated trans infection, not only as an efficient means to spread HIV-1 to CD4+ T cells but as the driver in establishing the HIV-1 reservoir in TN and potential consequent control of HIV-1 disease progression.
MATERIALS AND METHODS
Ethics statement.
Biological samples were acquired and studied from consenting individuals according to University of Pittsburgh International Review Board-approved protocols. All recruited participants were over the age of 18 and provided written consent prior to sample collection or use.
Cohort.
Experiments were performed using peripheral blood mononuclear cells (PBMC) obtained from anonymous donors (HIV-1 negative, n = 6) from the Pittsburgh blood bank (Vitalant Pittsburgh) or archived PBMC obtained from 7 HIV-1-infected NP and 7 HIV-1-infected PR enrolled in the Pittsburgh portion of the MACS/WIHS Combined Cohort Study (MWCCS). The NP cohort consisted of 3 NP (LTNP, CD4+ T cell counts >500 cells/mm3 over >7 years postinfection), 3 elite controllers (EC, undetectable viral load >7 years postinfection), and 1 viremic controller (VC, at least two viral load measures below 2,000 copies of HIV-1 RNA/ml).
Generation of CD4+ T cell subsets.
Naive and central memory CD4+ T lymphocytes were obtained from resting PBMC by magnetic bead negative selection according to the manufacturer’s instructions (Miltenyi Biotech). CD4+ TN were defined as CD45RA+ CCR7+ CCR5−, while CD4+ TCM were defined as CD45RA− CCR7+ CCR5+. The relative purity of the separated fractions was determined by flow cytometry.
Cell isolation and culture.
CD4+ T lymphocytes, B lymphocytes, and CD14+ monocytes were positively selected from PBMC using anti-CD4, -CD19, or -CD14 monoclonal antibody (MAb)-coated magnetic beads (Miltenyi Biotech). Immature DC (iDC) were derived from CD14+ monocytes cultured with 1,000 U/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; Miltenyi Biotech) and 1,000 U/ml recombinant human interleukin-4 (rhIL-4) for 5 days in AIM-V medium, with additional GM-CSF and rhIL-4 added on day 3. Mature DC (mDC) were derived from iDC by the addition of 0.1 μg/ml trimeric CD40L (Enzo) on day 5 and cultured for an additional 2 days. Prior to coculture, CD4+ T cells and B cells were activated for 48 h with 10 U/ml IL-2 (Roche) and 2 μg/ml phytohemagglutinin (PHA; Sigma) or 1,000 U/ml rhIL-4 and 0.1 μg/ml trimeric CD40L (Enzo), respectively. CD4+ TN or TCM were treated with either 100 nM CCL-19 (R&D Systems) or 10 U/ml IL-2 and 2 μg/ml PHA for 48 h as previously described (12, 15).
Cell phenotyping.
Cells were assessed for surface protein expression by flow cytometry. B cell + TN and DC + TN cocultures or TN alone were incubated with LIVE/DEAD fixable Aqua viability cell stain kit (Invitrogen) for 20 min and then incubated with MAb against CD3 (allophycocyanin-H7), CD4 (V450), CCR5 (phycoerythrin [PE]), CD45RA (PE-CF594), CCR7 (allophycocyanin), and CD27 (fluorescein isothiocyanate [FITC]) for 20 min. Cells were fixed with 1% paraformaldehyde (PFA), acquired with a BD LSR Fortessa and analyzed with FlowJo V10. The gating strategy is described in Fig. S1 in the supplemental material.
Virus stock titration and experimental p24 measurements were acquired by enzyme-linked immunosorbent assay (ELISA) using the HIV-1 p24 antigen capture immunoassay (Leidos Biomedical Research, Frederick National Laboratory for Cancer Research) per the manufacturer's instructions. HIV-1 Gag p24 was also evaluated in trans and cis infection cocultures by flow cytometry. Briefly, cocultures were harvested and incubated with LIVE/DEAD fixable Aqua viability stain kit (Invitrogen) for 20 min and then incubated for surface staining with MAb against CD3 (allophycocyanin-H7), CD4 (PE), and CD19 (PE-CF594) for 20 min. Cells were then permeabilized with PermII buffer (BD) for 20 min, washed, incubated with anti-HIV-1 p24 antibody Kc57-FITC (Coulter) for 20 min, washed, and resuspended in 1% PFA prior to analysis with a BD LSR Fortessa. Acquired data were analyzed with FlowJo V10. The gating strategy is described in Fig. S2.
Gating strategy to determine intracellular HIV-1 Gag p24. Lymphocytes were gated first based on forward and side scatter, followed by doublet event exclusion, then by exclusion of dead cells (Aqua dye positive). CD4+/HIV-1 Gag p24+ cells and CD4neg/HIV-1 Gag p24+ cells were then gated within the CD4+/CD3+ population. Download FIG S2, TIF file, 1.3 MB (1.3MB, tif) .
Copyright © 2021 Gerberick et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Trans and cis infection.
R5-tropic HIV-1BaL, grown in and purified from PM1 cells (42) (American Type Culture Collection), was used for cis and trans infection experiments. A patient isolate, R5-tropic HIV-1 BX08(92FR_BX08), used in trans and cis experiments was obtained from the NIH AIDS Reagents Program, Division of AIDS, NIAID, NIH [HIV-1 BX08(92FR_BX08) virus (catalog no. 11420) from Victoria Polonis] (23).
(i) Trans infection. 1 × 106 APC were incubated with a low concentration of HIV-1BaL or HIV-1BX08 (10−3 MOI) for 2 h at 37°C and then washed 3 times with cold medium. Virus-loaded APC were cocultured with autologous CD4+ T cell targets at a 1:10 effector/target ratio in R10 medium.
(ii) Cis infection. Activated CD4+ T cells or TN were incubated with a high concentration of HIV-1BaL or HIV-1BX08 (10−1 MOI) for 2 h at 37°C, washed 3 times with cold medium, and cultured independently. For both trans and cis infection, HIV-1 Gag p24 levels were quantified in cell-free supernatants at days 4, 8, and 12 post coculture. In some experiments, stimulated B cells were incubated with 20 μg/ml anti-DC-SIGN MAb (clone 120507; R&D Systems) or mouse IgG (R&D Systems) for 30 min at 4°C prior to incubation with virus. In some experiments, TN were incubated with maraviroc (1 μM) as previously described (25).
Reactivation of latent HIV-1 from APC-TN cocultures.
Eight days after the start of APC-TN cocultures, cells were treated with 10 nM phorbol myristate acetate (PMA; Sigma-Aldrich) and 10 μg/ml PHA (PMA-PHA). Supernatants were collected at days 11, 14, and 17. Levels of HIV-1 Gag p24 were then tested by ELISA. Parallel untreated cultures were used as control.
Quantification of total HIV-1 DNA.
Total HIV-1 DNA in CD4+ T cells and TN was quantified as described previously (43).
Statistics.
Data were tested by the Shapiro-Wilk normality test and analyzed by one-way analysis of variance (one-way ANOVA) followed by Student t tests. GraphPad Prism 7.0 software was used for statistical analysis.
ACKNOWLEDGMENTS
We thank Kathleen Hartle and Patrick Mehta for technical assistance and the Pitt Men’s Study clinical and laboratory staff and study participants for their support.
This work was supported by the National Institutes of Health grants R01AI118403, R56AI139010, U01-AI35041, U01 HL146208, and T32AI065380.
Footnotes
Citation Gerberick A, DeLucia DC, Piazza P, Alaoui-El-Azher M, Rinaldo CR, Sluis-Cremer N, Rappocciolo G. 2021. B lymphocytes, but not dendritic cells, efficiently HIV-1 trans infect naive CD4+ T cells: implications for the viral reservoir. mBio 12:e02998-20. https://doi.org/10.1128/mBio.02998-20.
Contributor Information
Akira Ono, University of Michigan Medical School.
Monica J. Roth, Rutgers-Robert Wood Johnson Medical School.
REFERENCES
- 1.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisele E, Siliciano RF. 2012. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 37:377–388. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 4.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 5.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnittman SM, Lane HC, Greenhouse J, Justement JS, Baseler M, Fauci AS. 1990. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci U S A 87:6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai J, Agosto LM, Baytop C, Yu JJ, Pace MJ, Liszewski MK, O’Doherty U. 2009. Human immunodeficiency virus integrates directly into naive resting CD4+ T cells but enters naive cells less efficiently than memory cells. J Virol 83:4528–4537. doi: 10.1128/JVI.01910-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zerbato JM, Purves HV, Lewin SR, Rasmussen TA. 2019. Between a shock and a hard place: challenges and developments in HIV latency reversal. Curr Opin Virol 38:1–9. doi: 10.1016/j.coviro.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zerbato JM, Serrao E, Lenzi G, Kim B, Ambrose Z, Watkins SC, Engelman AN, Sluis-Cremer N. 2016. Establishment and reversal of HIV-1 latency in naive and central memory CD4+ T cells in vitro. J Virol 90:8059–8073. doi: 10.1128/JVI.00553-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostrowski MA, Chun TW, Justement SJ, Motola I, Spinelli MA, Adelsberger J, Ehler LA, Mizell SB, Hallahan CW, Fauci AS. 1999. Both memory and CD45RA+/CD62L+ naive CD4(+) T cells are infected in human immunodeficiency virus type 1-infected individuals. J Virol 73:6430–6435. doi: 10.1128/JVI.73.8.6430-6435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wightman F, Solomon A, Khoury G, Green JA, Gray L, Gorry PR, Ho YS, Saksena NK, Hoy J, Crowe SM, Cameron PU, Lewin SR. 2010. Both CD31(+) and CD31(-) naive CD4(+) T cells are persistent HIV type 1-infected reservoirs in individuals receiving antiretroviral therapy. J Infect Dis 202:1738–1748. doi: 10.1086/656721. [DOI] [PubMed] [Google Scholar]
- 12.Zerbato JM, McMahon DK, Sobolewski MD, Mellors JW, Sluis-Cremer N. 2019. Naive CD4+ T cells harbor a large inducible reservoir of latent, replication-competent HIV-1. Clin Infect Dis 69:1919–1925. doi: 10.1093/cid/ciz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron PU, Freudenthal PS, Barker JM, Gezelter S, Inaba K, Steinman RM. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 14.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 15.Rappocciolo G, Piazza P, Fuller CL, Reinhart TA, Watkins SC, Rowe DT, Jais M, Gupta P, Rinaldo CR. 2006. DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes. PLoS Pathog 2:e70. doi: 10.1371/journal.ppat.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigal A, Baltimore D. 2012. As good as it gets? The problem of HIV persistence despite antiretroviral drugs. Cell Host Microbe 12:132–138. doi: 10.1016/j.chom.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Zhong P, Agosto LM, Ilinskaya A, Dorjbal B, Truong R, Derse D, Uchil PD, Heidecker G, Mothes W. 2013. Cell-to-cell transmission can overcome multiple donor and target cell barriers imposed on cell-free HIV. PLoS One 8:e53138. doi: 10.1371/journal.pone.0053138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLucia DC, Rinaldo CR, Rappocciolo G. 2018. Inefficient HIV-1 trans infection of CD4(+) T cells by macrophages from HIV-1 nonprogressors is associated with altered membrane cholesterol and DC-SIGN. J Virol 92:e00092-18. doi: 10.1128/JVI.00092-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rappocciolo G, Jais M, Piazza P, Reinhart TA, Berendam SJ, Garcia-Exposito L, Gupta P, Rinaldo CR. 2014. Alterations in cholesterol metabolism restrict HIV-1 trans infection in nonprogressors. mBio 5:e01031-13. doi: 10.1128/mBio.01031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rappocciolo G, Jenkins FJ, Hensler HR, Piazza P, Jais M, Borowski L, Watkins SC, Rinaldo CR, Jr.. 2006. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J Immunol 176:1741–1749. doi: 10.4049/jimmunol.176.3.1741. [DOI] [PubMed] [Google Scholar]
- 21.Heeregrave EJ, Geels MJ, Brenchley JM, Baan E, Ambrozak DR, van der Sluis RM, Bennemeer R, Douek DC, Goudsmit J, Pollakis G, Koup RA, Paxton WA. 2009. Lack of in vivo compartmentalization among HIV-1 infected naive and memory CD4+ T cell subsets. Virology 393:24–32. doi: 10.1016/j.virol.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venanzi Rullo E, Cannon L, Pinzone MR, Ceccarelli M, Nunnari G, O’Doherty U. 2019. Genetic evidence that naive T cells can contribute significantly to the human immunodeficiency virus intact reservoir: time to re-evaluate their role. Clin Infect Dis 69:2236–2237. doi: 10.1093/cid/ciz378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown BK, Darden JM, Tovanabutra S, Oblander T, Frost J, Sanders-Buell E, de Souza MS, Birx DL, McCutchan FE, Polonis VR. 2005. Biologic and genetic characterization of a panel of 60 human immunodeficiency virus type 1 isolates, representing clades A, B, C, D, CRF01_AE, and CRF02_AG, for the development and assessment of candidate vaccines. J Virol 79:6089–6101. doi: 10.1128/JVI.79.10.6089-6101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleh S, Solomon A, Wightman F, Xhilaga M, Cameron PU, Lewin SR. 2007. CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood 110:4161–4164. doi: 10.1182/blood-2007-06-097907. [DOI] [PubMed] [Google Scholar]
- 25.Rappocciolo G, Sluis-Cremer N, Rinaldo CR. 2019. Efficient HIV-1 trans infection of CD4(+) T cells occurs in the presence of antiretroviral therapy. Open Forum Infect Dis 6:ofz253. doi: 10.1093/ofid/ofz253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turville SG, Santos JJ, Frank I, Cameron PU, Wilkinson J, Miranda-Saksena M, Dable J, Stossel H, Romani N, Piatak M, Jr, Lifson JD, Pope M, Cunningham AL. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- 27.Sattentau Q. 2008. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol 6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 28.Hong S, Zhang Z, Liu H, Tian M, Zhu X, Zhang Z, Wang W, Zhou X, Zhang F, Ge Q, Zhu B, Tang H, Hua Z, Hou B. 2018. B cells are the dominant antigen-presenting cells that activate naive CD4(+) T cells upon immunization with a virus-derived nanoparticle antigen. Immunity 49:695–708.e4. doi: 10.1016/j.immuni.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Batista FD, Harwood NE. 2009. The who, how and where of antigen presentation to B cells. Nat Rev Immunol 9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 30.Carrasco YR, Batista FD. 2007. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, Ebisuno Y, Katagiri K, Kinashi T, Shimizu A. 2008. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol 181:6189–6200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- 32.He B, Qiao X, Klasse PJ, Chiu A, Chadburn A, Knowles DM, Moore JP, Cerutti A. 2006. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol 176:3931–3941. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 33.Cyster JG. 2010. B cell follicles and antigen encounters of the third kind. Nat Immunol 11:989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 34.Cyster JG, Allen CDC. 2019. B cell responses: cell interaction dynamics and decisions. Cell 177:524–540. doi: 10.1016/j.cell.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen CD, Okada T, Cyster JG. 2007. Germinal-center organization and cellular dynamics. Immunity 27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi H, Kastenmuller W, Germain RN. 2014. Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu Rev Cell Dev Biol 30:141–167. doi: 10.1146/annurev-cellbio-100913-013254. [DOI] [PubMed] [Google Scholar]
- 37.Zaretsky I, Atrakchi O, Mazor RD, Stoler-Barak L, Biram A, Feigelson SW, Gitlin AD, Engelhardt B, Shulman Z. 2017. ICAMs support B cell interactions with T follicular helper cells and promote clonal selection. J Exp Med 214:3435–3448. doi: 10.1084/jem.20171129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E, Pino M, Erkizia I, Glass B, Clotet B, Keppler OT, Telenti A, Krausslich HG, Martinez-Picado J. 2012. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol 10:e1001448. doi: 10.1371/journal.pbio.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puryear WB, Akiyama H, Geer SD, Ramirez NP, Yu X, Reinhard BM, Gummuluru S. 2013. Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog 9:e1003291. doi: 10.1371/journal.ppat.1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sewald X, Ladinsky MS, Uchil PD, Beloor J, Pi R, Herrmann C, Motamedi N, Murooka TT, Brehm MA, Greiner DL, Shultz LD, Mempel TR, Bjorkman PJ, Kumar P, Mothes W. 2015. Retroviruses use CD169-mediated trans-infection of permissive lymphocytes to establish infection. Science 350:563–567. doi: 10.1126/science.aab2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C, ANRS VISCONTI Study Group. 2013. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sankapal S, Gupta P, Ratner D, Ding M, Shen C, Sanyal A, Stolz D, Cu-Uvin S, Ramratnam B, Chen Y. 2016. HIV exposure to the epithelia in ectocervical and colon tissues induces inflammatory cytokines without tight junction disruption. AIDS Res Hum Retroviruses 32:1054–1066. doi: 10.1089/AID.2015.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong F, Aga E, Cillo AR, Yates AL, Besson G, Fyne E, Koontz DL, Jennings C, Zheng L, Mellors JW. 2016. Novel assays for measurement of total cell-associated HIV-1 DNA and RNA. J Clin Microbiol 54:902–911. doi: 10.1128/JCM.02904-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gating strategy to determine TN phenotype. Lymphocytes were gated first based on forward and side scatter, followed by doublet event exclusion, then by exclusion of dead cells (Aqua dye positive). CD4+ positive cells were then gated into CD45RA-negative and CD45RA-positive populations, with the latter population being 100% CCR7 positive as well as CCR5 negative. Download FIG S1, TIF file, 1.1 MB (1.1MB, tif) .
Copyright © 2021 Gerberick et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Expression of HIV-1 p24 in CD3+ CD4neg maraviroc-treated cells. Cells from maraviroc-treated cocultures or controls analyzed in Fig. 5C were further analyzed by gating on the CD3+ CD4neg subpopulation and evaluated for HIV-1 p24 expression as described in Materials and Methods. Download FIG S3, TIF file, 0.5 MB (542KB, tif) .
Copyright © 2021 Gerberick et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Gating strategy to determine intracellular HIV-1 Gag p24. Lymphocytes were gated first based on forward and side scatter, followed by doublet event exclusion, then by exclusion of dead cells (Aqua dye positive). CD4+/HIV-1 Gag p24+ cells and CD4neg/HIV-1 Gag p24+ cells were then gated within the CD4+/CD3+ population. Download FIG S2, TIF file, 1.3 MB (1.3MB, tif) .
Copyright © 2021 Gerberick et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.