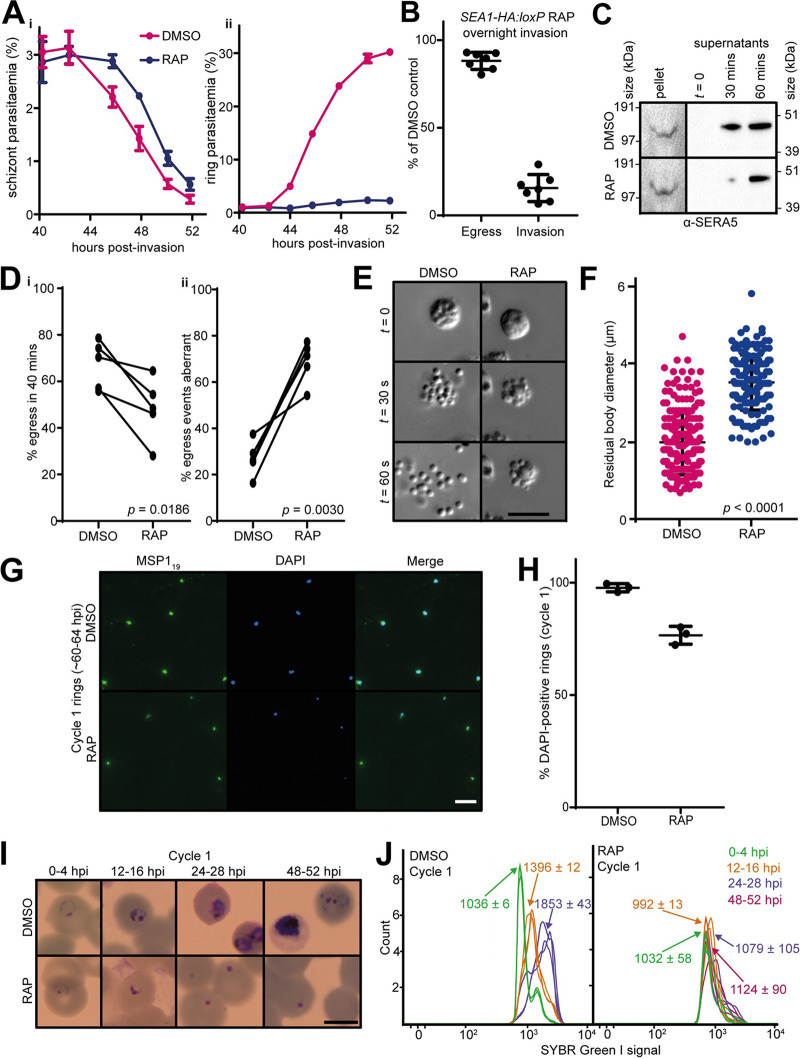
FIG 5.
SEA1-null parasites egress aberrantly and fail to proliferate. (A) Time course showing the results of monitoring schizont (i) and ring (ii) parasitemia around the expected time of egress of DMSO- and RAP-treated SEA1-HA:loxP schizonts. Parasitemia values were measured by flow cytometry. Data points are means from three technical replicates and are representative of four independent experiments. Error bars, SD. (B) Quantification of egress and invasion of RAP-treated SEA1-HA:loxP parasites relative to controls. Schizonts were enriched, added to fresh RBCs, and left to invade under shaking conditions for at least 8 h before egress and invasion rates were measured by flow cytometry. (C) Western blots monitoring release in culture supernatants of the processed PV protein SERA5 (p50) as a proxy for egress of DMSO- and RAP-treated SEA1-HA:loxP schizonts. (D, i) Quantification of egress events observed by microscopic monitoring of preparations of highly synchronous DMSO- and RAP-treated SEA1-HA:loxP schizonts. (ii) Proportion of these egress events in panel i that were aberrant. An egress event was defined as aberrant if more than 3 daughter merozoites remained associated with the residual body (29) and/or if fewer than 3 merozoites were released upon schizont rupture. At least 40 schizonts per condition were observed in each of five matched pairs of videos. P values derive from paired t tests. (E) Stills selected from time-lapse video microscopy (see Movie S1) showing aberrant egress and an enlarged residual body in RAP-treated SEA1-HA:loxP parasites compared to DMSO-treated controls. Scale bar, 10 μm. (F) Quantification of residual body size measured following egress of DMSO- or RAP-treated SEA1-HA:loxP schizonts. More than 150 residual bodies from a total of five videos per condition were measured, and each point represents an individual residual body. Mean values and standard errors of the means are indicated, and P values were derived from Student's t test. (G) IFA showing control and SEA1-null ring-stage parasites from the cycle following DMSO/RAP treatment (cycle 1). Ring-stage parasites were identified by staining with an antibody against the C-terminal fragment of MSP1 (MSP119). Scale bar, 10 μm. (H) Quantification of the proportions of MSP119-positive cycle 1 rings possessing detectable DNA by IFA (DAPI positive). Totals of 549 and 301 merozoites were analyzed for the DMSO- and RAP-treated populations, respectively. Error bars, SD. (I) Images from Giemsa-stained thin films showing the fate of SEA1-HA:loxP parasites that successfully invade following the cycle of treatment with DMSO or RAP. Rings derived from the RAP-treated (SEA1-null) merozoites failed to develop. Scale bar, 5 μm. (J) Flow cytometry plots showing SYBR green I fluorescence (indicating DNA content) associated with parasite-infected RBC over the course of the erythrocytic cycle following treatment of SEA1-HA:loxP parasites with DMSO or RAP (cycle 1). Labels indicate the mean fluorescence intensity of each population ± SD from three replicates. Parasites derived from the RAP-treated (SEA1-null) population failed to develop in cycle 1, consistent with the microscopic images shown in panel H.