The process by which bacteria sense environmental cues to regulate their virulence is complex. Several studies have focused on regulating the expression of the locus of enterocyte effacement pathogenicity island in the typical gut pathogenic bacterium, O157.
KEYWORDS: O157:H7, virulence, small RNA, flagella, NtrC
ABSTRACT
Enterohemorrhagic Escherichia coli serotype O157:H7 (O157) is a critical, foodborne, human intestinal pathogen that causes severe acute hemorrhagic diarrhea, abdominal cramping, and even death. Small RNAs (sRNAs) are noncoding regulatory molecules that sense environmental changes and trigger various virulence-related signaling pathways; however, few such sRNAs have been identified in O157. Here, we report a novel sRNA, EsrF that senses high ammonium concentrations in the colon and enhances O157 pathogenicity by promoting bacterial motility and adhesion to host cells. Specifically, EsrF was found to directly interact with the 5′ untranslated regions of the flagellar biosynthetic gene, flhB, mRNA and increase its abundance, thereby upregulating expression of essential flagellar genes, including flhD, flhC, fliA, and fliC, leading to elevated O157 motility and virulence. Meanwhile, an infant rabbit model of O157 infection showed that deletion of esrF and flhB significantly attenuates O157 pathogenicity. Furthermore, NtrC—the response regulator of the NtrC/B two-component system—was found to exert direct, negative regulation of esrF expression. Meanwhile, high ammonium concentrations in the colon release the inhibitory effect of NtrC on esrF, thereby enhancing its expression and subsequently promoting bacterial colonization in the host colon. Our work reveals a novel, sRNA-centered, virulence-related signaling pathway in O157 that senses high ammonium concentrations. These findings provide novel insights for future research on O157 pathogenesis and targeted treatment strategies.
INTRODUCTION
Enterohemorrhagic Escherichia coli (EHEC) serotype O157:H7 (O157) is a foodborne pathogen that causes bloody diarrhea, hemorrhagic colitis, and fatal hemolytic-uremic syndrome in humans (1). O157 usually infects the human colon through contaminated food and water at a low infectious dose (50 CFU) (2). Since no vaccine is available against O157 infections, effective treatments are urgently required to combat these infections (3). O157 typically colonizes the human large intestine (4), and successful O157 colonization is characterized by the formation of attaching and effacing (AE) lesions in the host epithelium (5). These lesions lead to the rearrangement of the actin cytoskeleton and effacement of microvilli, which results in the form of pedestal-like structures and intimate attachment of O157 to the enterocytes (6). The locus of enterocyte effacement (LEE) pathogenicity island mainly encodes genes whose products cause AE lesions (7). The LEE contains five polycistronic operons (LEE1 to LEE5) that express the master LEE regulator, Ler, type III secretion system (T3SS), adhesins (such as intimin and its receptor [Tir]), and other effector proteins (8). In addition to T3SS and effectors encoded by LEE, other adhesins also participate in the initial attachment of bacteria to eukaryotic cells (9).
Flagella are the locomotive organelles important for bacterial pathogenesis and contribute to the initial breakdown and penetration through the mucus layer by bacterial pathogens (10). Moreover, flagella may directly regulate bacterial adhesion and colonization at the sites of infection (11). EHEC flagella directly interact with mucin in the mucus layer (12). O157 flagella also act as adhesins, directly contributing to adhesion to bovine intestinal epithelium in the early stages of colonization (13). Flagella also contribute to the pathogenesis of EHEC O113:H21 infections by promoting the invasion of the intestinal epithelium (14). More than 60 flagellum-related genes have been arranged in hierarchical order in three classes (14). Class 1 contains the master operon, flhDC, the expression of which is needed for transcribing class 2 and class 3 operons (15). Class 2 contains eight operons that encode components for constructing the hook-basal body complex, including the sigma factor fliA, to directly regulate flagellar genes (16). Class 3 encodes components for filament assembly and motor function, including the flagellin gene, fliC. FlhB is a class 2 constituent membrane protein of the basal body required for flagellar appendant synthesis and hook-length control involved in substrate specificity switch (17, 18). Recently, FlhB was found to regulate flagellar gene expression in Listeria monocytogenes (17).
Small RNAs (sRNAs) are regulatory, noncoding, rapid-acting molecules whose synthesis does not require much energy consumption and are involved in many physiological processes of bacteria (19, 20). sRNAs positively or negatively regulate target genes by base pairing with specific mRNAs and often need the sRNA chaperone, Sm-like homohexameric ring protein, Hfq, to function (21, 22). For bacterial pathogens, sRNAs play a vital role in their rapid adaptation to the host environment and regulating virulence gene expression (22). Several virulence-related sRNAs have been identified in O157 (23–26). For example, sRNA, DicF, senses low oxygen concentrations in the environment to induce expression of LEE genes to promote the formation of AE lesions and virulence through direct binding with the transcriptional activator, pchA mRNA (23). In addition, several sRNAs (DsrA, Arl, Esr41, Spot42, sRNA56, sRNA103, sRNA350, GlmY, and GlmZ) regulate LEE operons in O157 (26–29). However, the exact mechanisms by which most sRNAs in O157 regulate downstream genes and their roles in bacterial virulence remain unclear.
Bacterial two-component systems (TCSs) sense various microenvironmental cues, which are transferred from the cytoplasmic membrane to the cytoplasm to activate several processes (30). TCSs contain a sensor histidine kinase and its cognate DNA-binding response regulator (RR) (31). The conformational change of the membrane histidine kinase delivers the phosphate to the aspartic residue on the RR to activate a domain that modulates gene expression (32). Previous studies have identified several TCSs that are essential for the virulence of O157 (33). For example, CpxA/CpxR TCS senses the neurotransmitter, serotonin, and downregulates O157 LEE gene expression (33). FusK/R TCS senses fucose produced by the commensal Bacteroides thetaiotaomicron to promote O157 colonization (34). QseE/QseF TCS senses epinephrine and sulfate to regulate the effector protein synthesis, EspFu (35). It is likely that more TCSs are involved in the virulence regulatory network of O157; further investigation is required to confirm and clarify their roles. Here, we aimed to investigate the role of an sRNA, EsrF, and the signaling pathway and the mechanism involved in the motility and virulence of O157.
RESULTS
Identification and characterization of EsrF in O157.
By analyzing the transcriptomic data of the O157 strain EDL933 during infection of HeLa cells (36), we predicted one potential sRNA in the intergenic region between ntrB (glnL) and glnA and called it EsrF. The transcriptomic data showed that EsrF expression is significantly upregulated by 4.43-fold when infecting HeLa cells compared to its expression in free cultures in Dulbecco modified Eagle medium (DMEM). We inferred that EsrF is associated with O157 virulence.
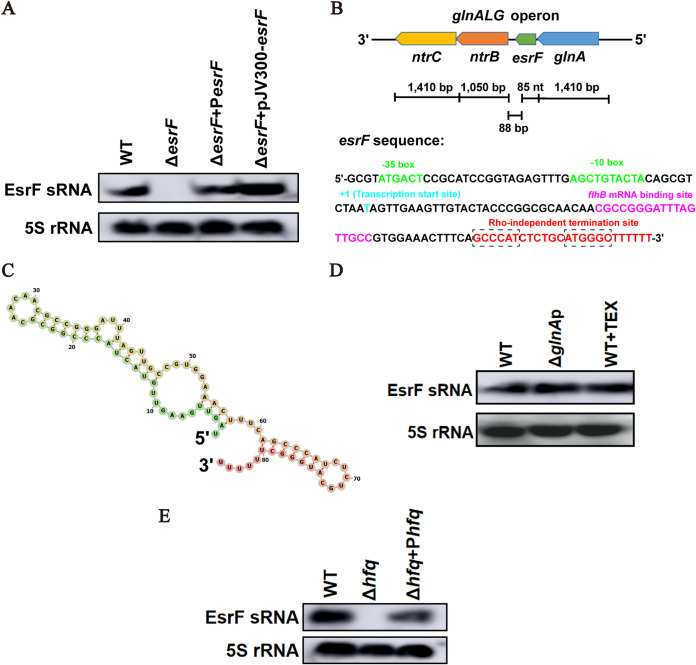
Northern blotting was performed to confirm whether EsrF is an sRNA transcribed in O157 and to determine its size. Briefly, we used an EsrF-specific probe to detect O157 WT (wild type), ΔesrF (esrF mutant), and ΔesrF+PesrF (esrF complement strain) strains, with 5S rRNA as the internal control. The results revealed an RNA band that was approximately 80 to 90 nucleotides (nt) in length in WT and ΔesrF+PesrF strains, but not in the ΔesrF mutant, indicating that EsrF was transcribed from the reverse strand of the EDL933 genome (Fig. 1A).
FIG 1.
Identification and characterization of EsrF sRNA in O157. (A) Northern blotting performed with a specific probe directed against EsrF sRNA in O157 WT, ΔesrF, and ΔesrF+PesrF strains; 5S rRNA was used as a loading control. esrF with its putative promoter was cloned into a high-copy-number plasmid pJV300, and the abundance of EsrF in ΔesrF+pJV300-esrF was determined by Northern blotting. (B) The position of the esrF sequence in the O157 EDL933 genome is shown. The −35 and −10 boxes of the putative promoter region, transcription start site, flhB mRNA binding site, and Rho-independent termination site are depicted in green, blue, purple, and red, respectively. The palindromic sequence before poly(T) in Rho-independent termination site are demarcated with a dashed box. (C) Predicted secondary structure of EsrF sRNA determined using RNAfold (Institute for Theoretical Chemistry, University of Vienna [http://rna.tbi.univie.ac.at/]) and depicted as a color gradient from green to red, representing 5′ to 3′ ends of EsrF sRNA, respectively. (D) EsrF abundance in WT, glnA promoter mutant (ΔglnAp), and WT incubated with 5′ monophosphate-dependent terminator exonuclease (WT+TEX) as determined by Northern blotting. (E) Northern blotting was performed with a specific probe directed against EsrF sRNA in O157 WT, Δhfq, and Δhfq+Phfq strains; 5S rRNA was used as a loading control. The data are presented as means ± the SD (n = 3; ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test.
We then performed the 5′ and 3′ rapid amplification of cDNA ends (RACE) assay to identify the transcription start and termination sites of EsrF. The results show that EsrF was exactly 85 nt in length, which is consistent with the Northern blotting results. Further, the transcription start site (+1) of EsrF coding sequence (CDS) was mapped to T4939965 in the EDL933 genome, while a Rho-independent terminator was mapped to T4940049 (Fig. 1B; see also Fig. S1B in the supplemental material). Using RNAfold (37), we also predicted the secondary structure of EsrF to have one hairpin, two bulges, and three internal loops (Fig. 1C).
EsrF expression, verification, and dependency on Hfq. (A) Transcription of the esrF-glnA region as observed in transcriptome data. EsrF appears to be transcribed from its promoter. (B) 5′- and 3′-RACE gel photographs of EsrF cloned into the T vector amplified before sequencing. For 5′-RACE, the size of the product was 130-bp (85-bp esrF CDS + 45-bp adapter). For 3′-RACE, the size of product was 250-bp (85-bp esrF CDS + 30-bp adapter + 135-bp T vector). (C) Sequencing reads recovered from Hfq UV-induced RNA-protein crosslinking and analysis of cDNA by high-throughput sequencing (CRAC) that map to esrF (top) and deletions recovered within sequencing reads (below). The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S1, TIF file, 2.4 MB (2.4MB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Moreover, the transcription start site, “T,” of esrF is the nucleotide adjacent to the glnA CDS stop codon (Fig. 1B). Hence, EsrF is a 3′ untranslated region (UTR) type sRNA that may contain the full glnA 3′ UTR. To investigate whether EsrF is independently transcribed or cotranscribed and cleaved from the full-length glnA transcript, we performed several analyses. First, the transcriptome data suggest that the putative unique promoter region of esrF is located −35 to −10 bp upstream of the esrF transcription start site (Fig. 1B; see also Fig. S1A). Second, after deletion of the glnA promoter in O157 WT and subsequent Northern blotting, no difference was observed in EsrF abundance between ΔglnAp and WT strains (Fig. 1D). Third, considering that cotranscribed sRNAs processed from primary transcripts lead to the conversion of 5′ triphosphorylated RNAs to 5′ monophosphorylated RNAs (38), we treated total RNA samples of O157 WT with the 5′ monophosphate-dependent terminator exonuclease (TEX), which degrades processed transcripts while sparing primary transcripts (39). Northern blotting was subsequently performed, which revealed that the EsrF transcript is resistant to TEX treatment (Fig. 1D). Fourth, we introduced a promoter-less plasmid (pJV300) containing esrF, with its predicted promoter region, into the ΔesrF mutant, which, according to Northern blotting results, lead to the overexpression of EsrF (Fig. 1A). Taken together, these results indicate that EsrF is an independently transcribed 3′ UTR sRNA, the expression of which is regulated by its promoter and does not require glnA expression.
Hfq is a well-known sRNA chaperone that binds to both sRNAs and their target mRNAs to promote their base pairing, while protecting sRNAs from degradation by cellular nucleosidases. The expression of Hfq-binding sRNAs is generally reduced in Gram-negative bacteria hfq deletion mutants. We therefore examined the effect of hfq deletion on EsrF abundance by performing Northern blotting. Results for which demonstrate the presence of EsrF RNA in WT and Δhfq+Phfq strains, which was absent from the Δhfq strain (Fig. 1E), indicating that hfq promotes EsrF abundance.
Ultraviolet (UV) cross-linking has been commonly employed to determine whether a specific protein interacts directly with a specific RNA in living cells (40). Specifically, UV-induced RNA-protein cross-linking and high-throughput sequencing has been applied to identify transcriptome-wide targets of Hfq binding in O157 (41). By analyzing this published raw data, we found that Hfq-cross-linked reads cover the entire sequence of esrF (see Fig. S1C), suggesting that Hfq may directly bind to the “body” of EsrF sRNA and contribute to its stability.
EsrF promotes O157 virulence in vitro and in vivo.
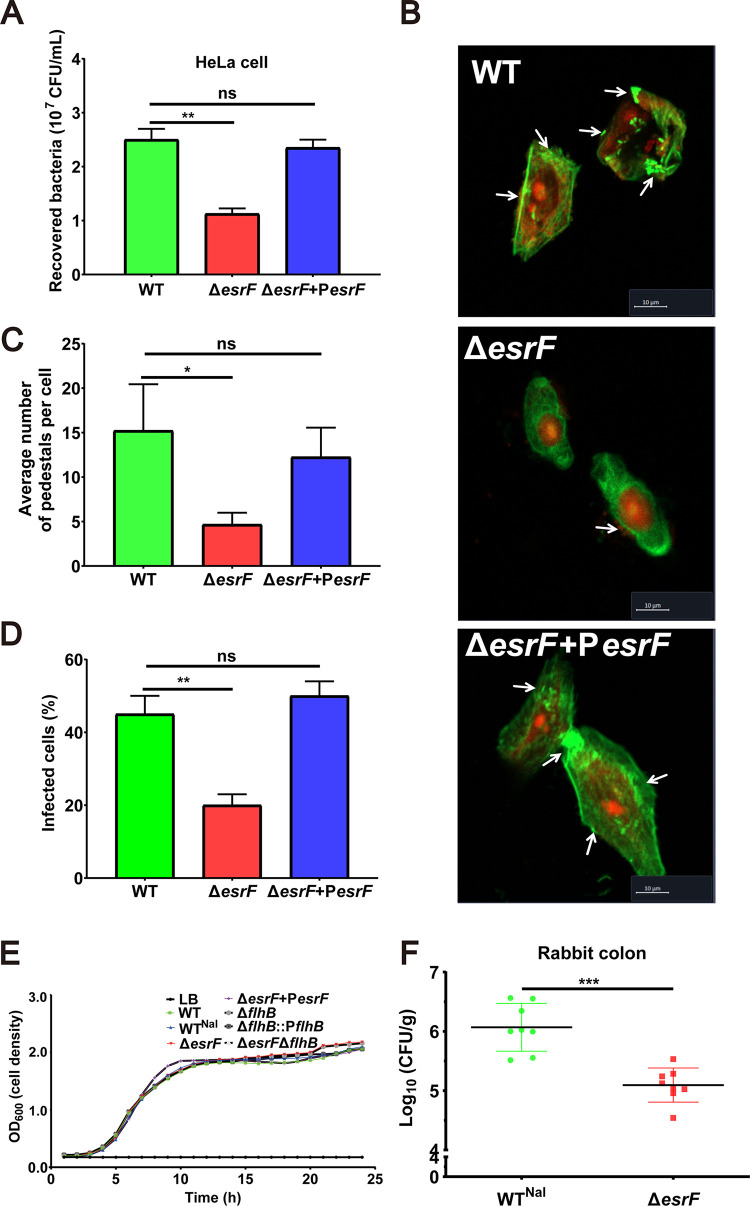
To investigate the impact of EsrF sRNA on the adhesion of O157 to host cells, we performed a HeLa cell adhesion assay. To avoid the influence of motility on bacterial adhesion, the bacteria were first centrifuged onto the HeLa cells before infection. The results show that the ΔesrF mutant adhered to cells at much lower levels (2.3- and 2.1-fold) than did the WT and ΔesrF+PesrF strains (Fig. 2A).
FIG 2.
EsrF promoted O157 virulence in vitro and in vivo. (A) Adhesion of O157 WT, ΔesrF, and ΔesrF+PesrF strains to HeLa cells in DMEM. (B) Quantification of the number of pedestals per infected HeLa cell. (C) Quantification of the proportion of infected HeLa cells. (D) FAS of HeLa cells infected with WT, ΔesrF, and ΔesrF+PesrF strains. DNA was stained with propidium iodide (PI, red), and the HeLa cell actin cytoskeleton was stained with FITC-labeled phalloidin (green). Pedestals were observed as punctate green structures typically associated with bacterial cells. Scale bar, 10 μm. (E) Growth curves for the seven strains (WT, WTNal, ΔesrF, ΔesrF+ΔesrF, ΔflhB, ΔflhB+ΔflhB, and ΔesrF ΔflhB) used in this study. (F) Colonization capacities of WT and ΔesrF strains in rabbit colon. The data are presented as means ± the SD (n = 3; ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test.
Considering that the most important features associated with O157 adhesion to host cells are the AE lesion and pedestal formation (23), fluorescein actin staining (FAS) was performed to observe them in HeLa cells infected by WT, ΔesrF, or ΔesrF+PesrF strains. The results revealed significantly reduced AE lesion formation in HeLa cells infected by the ΔesrF mutant (20%) compared to cells infected by WT (45%) or ΔesrF+PesrF strains (50%), with averages of 15.0, 4.8, and 13.3 pedestals per cell observed in cells infected by WT, ΔesrF, and ΔesrF+PesrF strains, respectively (Fig. 2B, C, and D). Notably, WT, ΔesrF, and ΔesrF+PesrF strains grew at similar rates in vitro (Fig. 2E), indicating that the decreased cell adhesion of the ΔesrF mutant was not due to slower bacterial growth. Cumulatively, these results suggest that EsrF positively regulates O157 adhesion to host HeLa cells.
To further investigate the effect of EsrF sRNA on O157 pathogenicity in vivo, we performed a rabbit colon colonization experiment. Three-day-old New Zealand White infant rabbits were intragastrically inoculated with WT and ΔesrF strains, respectively, and the numbers of bacteria recovered from the colon homogenates were determined. The results showed that rabbit colons infected with ΔesrF had relatively fewer (10.8-fold) bacteria than the colons infected with the WT strain (Fig. 2F). This result indicated that EsrF sRNA had a positive influence on O157 virulence in vivo.
flhB expression is positively regulated by EsrF.
sRNAs influence the expression of their target genes by directly interacting with mRNA molecules (42). We predicted 53 putative target genes of EsrF sRNA using TargetRNA2 (43) (see Table S1A in the supplemental material). Comparative transcriptome sequencing was then performed to detect differences in global gene expression profiles between WT and ΔesrF strains, which identified a total of 244 genes (187 downregulated genes and 57 upregulated genes) as differentially transcribed (>2-fold) between the two strains (see Table S1B). Among these differentially transcribed genes, only flhB was also predicted by TargetRNA2 as a putative target of EsrF sRNA. FlhB is a flagellar export apparatus membrane protein known to induce effector transport (18); however, more recently, FlhB was also found to regulate other flagellar genes in Listeria monocytogenes (44, 45).
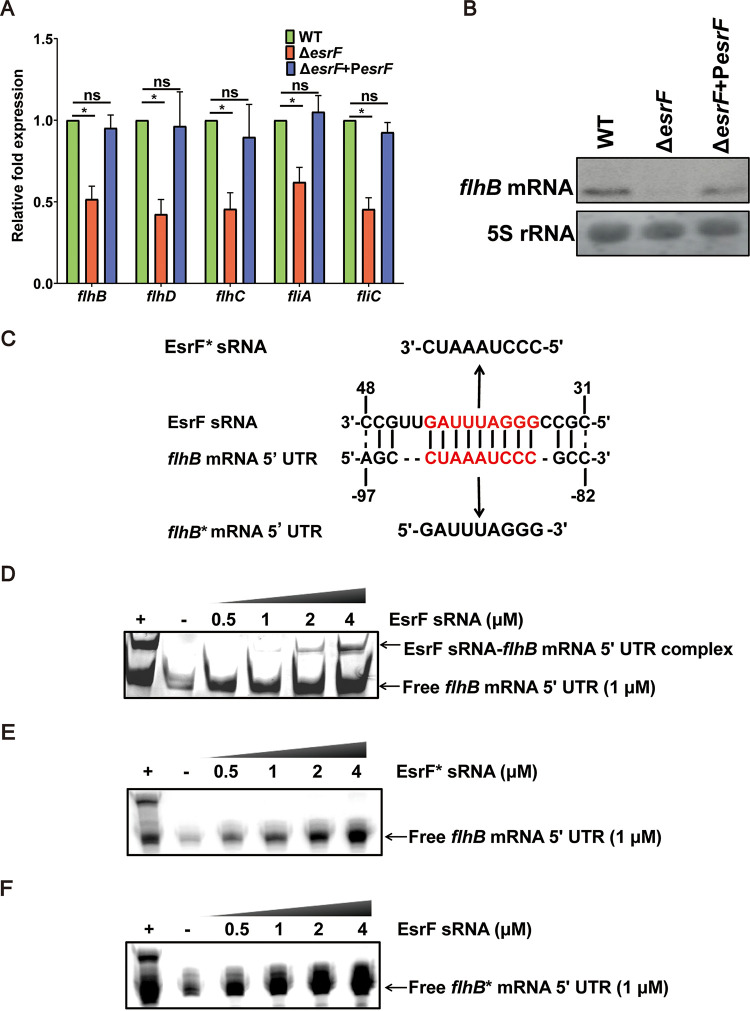
Quantitative reverse transcription-PCR (qRT-PCR) assay results confirmed that flhB expression was downregulated by 2.5- and 2.3-fold in the ΔesrF mutant, respectively, compared to WT and ΔesrF+PesrF strains (Fig. 3A). Moreover, flhB expression was found to be downregulated by 2.0- and 2.1-fold in Δhfq and ΔesrF strains, respectively, compared to the WT (see Fig. S2A). To further confirm the activation of flhB by EsrF, we performed Northern blotting to detect flhB mRNA levels in WT, ΔesrF, and ΔesrF+PesrF strains. Significantly decreased flhB mRNA levels were detected in ΔesrF compared to WT, which were restored in the complemented strain (Fig. 3B; see also Fig. S2B). The 18-nt interaction region between EsrF sRNA (CCGUUGAUUUAGGGCCGC) and flhB mRNA (AGCCUAAAUCCCGCC) 5′ UTRs was predicted by TargetRNA2 (Fig. 3C).
FIG 3.
Positive regulation of the downstream gene, flhB, by EsrF binding. (A) qRT-PCR of the expression of flhB, flhD, flhC, fliA, and fliC in WT, ΔesrF, and ΔesrF+PesrF strains. (B) Northern blotting was performed with a specific probe directed against the flhB mRNA in O157 WT, ΔesrF, and ΔesrF+PesrF strains; 5S rRNA was used as the loading control. (C) Predicted EsrF sRNA-flhB mRNA 5′ UTR base pairing. The lines and dashed lines indicate the predicted base pairs and incomplete base pairs between EsrF and flhB RNA 5′ UTR, respectively. The red letters represent the predicted, binding motif for EsrF action. EsrF* sRNA represents EsrF with point mutation with the GGGAUUUAG motif mutated to CCCUAAAUC, while flhB* mRNA 5′ UTR represents flhB with point mutations for which the motif CUAAAUCCC was mutated to GAUUUAGGG. (D) RNA-RNA EMSA of EsrF sRNA and flhB mRNA 5′ UTR. The EsrF complement strand (+) and yeast RNA (−) were used as positive and negative controls, respectively. (E) RNA-RNA EMSA of EsrF* sRNA and flhB mRNA 5′ UTR. (F) RNA-RNA EMSA of EsrF sRNA and flhB* mRNA 5′ UTRs. The data are presented as means ± the SD (n = 3; ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test.
EsrF positively regulates flhB transcription. (A) qRT-PCR quantification of flhB expression in WT, ΔesrF, ΔesrF+PesrF, Δhfq, and Δhfq+Phfq strains. (B) Northern blotting quantification of flhB mRNA in WT, ΔesrF, and ΔesrF+PesrF strains. (C) Quantification of shifted flhB mRNA in RNA-RNA EMSA reactions. The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S2, TIF file, 1.4 MB (1.4MB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) TargetRNA2 used to predict 53 putative target genes of EsrF. (B) Differently expressed genes of the O157 ΔesrF transcriptome. Download Table S1, DOCX file, 0.05 MB (47.1KB, docx) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
RNA-RNA electrophoretic mobility shift assay (REMSA) was performed to verify the direct interaction between EsrF sRNA and flhB mRNA in vitro. EsrF, the EsrF complementary strand, and flhB mRNA were transcribed in vitro and purified. The REMSA reaction was performed with different concentrations of EsrF sRNA (0.5, 1, 2, and 4 μM) and a fixed amount of flhB mRNA (1 μM). EsrF sRNA-flhB mRNA complex bands were formed by both EsrF sRNA and flhB mRNA in 8% native polyacrylamide gel electrophoresis (PAGE), and the band intensity increased as the EsrF concentration increased from 0.5 to 4 μM (Fig. 3D; see also Fig. S2C). The REMSA verified the direct interaction between EsrF sRNA and flhB mRNA in vitro and suggested that EsrF sRNA directly upregulated flhB expression.
The 9-nucleotide crucial motif (GGGAUUUAG) for EsrF sRNA action was predicted by TargetRNA2 (Fig. 3C). To determine whether this motif represents the key sequence bound to flhB mRNA, we performed REMSA under the same reaction conditions, using a mutated EsrF (EsrF*) sRNA (the motif GGGAUUUAG was mutated to CUCCCTAAA) generated by in vitro transcription with flhB mRNA. We also performed REMSA using a mutated flhB (flhB*) mRNA (the motif CUAAAUCCC was mutated to GAUUUAGGG) with EsrF sRNA. The results showed no interaction between the EsrF* sRNA and flhB mRNA, or between EsrF sRNA and flhB* mRNA (Fig. 3E and F), indicating that this motif is vital to the ability of EsrF sRNA to bind to flhB mRNA.
EsrF and flhB promote O157 motility.
Considering that flagella are directly related to bacterial motility (11), a swimming motility assay was performed to determine whether esrF or flhB influence O157 motility. The growth radius was found to decrease significantly in the ΔesrF and ΔflhB mutants compared to the WT and the corresponding complement strains (Fig. 4A; see also Fig. S3A). Growth curve analysis further demonstrated that WT, ΔesrF, and ΔflhB strains and the corresponding complement strains grew at similar rates in Luria-Bertani (LB) medium (Fig. 2E), indicating that the decreased motility in ΔesrF and ΔflhB strains was not due to slower growth. Together, these results indicate that both esrF and flhB enhance O157 motility.
FIG 4.
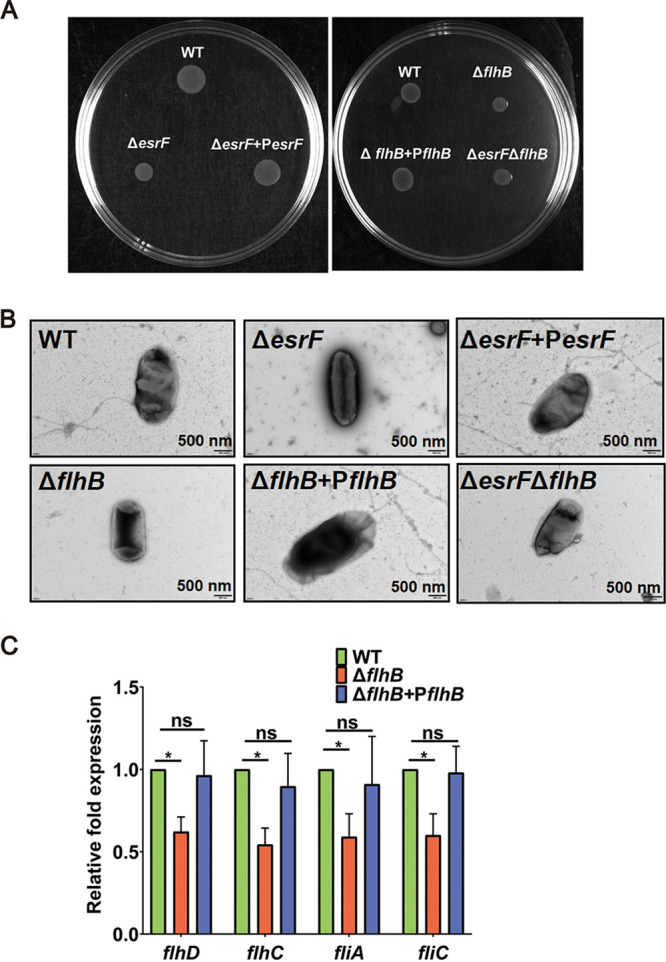
EsrF and flhB promotes O157 motility. (A) Representative images of swimming motility of O157 WT, ΔesrF, ΔesrF+PesrF, ΔflhB, ΔflhB+PflhB, and ΔesrF ΔflhB strains. All strains were observed after 12 h of growth in semisolid LB medium at 25°C. (B) Representative TEM images of O157 WT, ΔesrF, ΔesrF+PesrF, ΔflhB, ΔflhB+PflhB, and ΔesrF ΔflhB strains. Scale bar, 500 nm. (C) qRT-PCR of the expression of flhD, flhC, fliA, and fliC in WT, ΔflhB, and ΔflhB+PflhB strains. The data are presented as means ± the SD (n = 3; ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test.
EsrF and flhB regulate O157 motility and flagella synthesis. (A) Growth radii of WT, ΔesrF, ΔesrF+PesrF, ΔflhB, ΔflhB+PflhB, and ΔesrF ΔflhB strains in motility assays. (B) Percentages of flagella numbers/20 cells of WT, ΔesrF, ΔesrF+PesrF, ΔflhB, ΔflhB+PflhB, and ΔesrF ΔflhB strains. The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S3, TIF file, 0.9 MB (932.4KB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We further investigated whether flagellar synthesis was inhibited in ΔesrF and ΔflhB strains by transmission electron microscopy (TEM). The results showed that approximately 75% of the WT and complemented cells possessed flagella, whereas approximately 85 and 90% of ΔesrF and ΔflhB cells were aflagellate (Fig. 4B; see also Fig. S3B). These results indicated that esrF and flhB promoted flagellar synthesis, which resulted in enhanced bacterial motility.
Although FlhB is generally regarded as a class 2 flagellar biosynthetic protein (46), recently flhB was reported to regulate the expression of several flagellar genes, including flaA (O157 fliC homolog), fliY, fliS, motA, lmo0695, and lmo0698, in Listeria monocytogenes (17). Hence, we performed qRT-PCR to determine whether flhB regulates the expression of flagellar genes in O157. The expression of flhD, flhC, fliA, and fliC was significantly downregulated in the ΔflhB strain compared to WT and ΔflhB+PflhB strains (Fig. 4C). Furthermore, the expression of flhB, flhD, flhC, fliA, and fliC was also significantly downregulated in the ΔesrF mutant compared to WT and ΔesrF+PesrF strains (Fig. 3A). These results indicate that EsrF sRNA promotes flhB-mediated expression of flagellar genes in O157.
To determine whether EsrF influences the motility of O157 directly by regulating flhB expression, we constructed an ΔesrF ΔflhB double mutant. Swimming motility and TEM results showed that the growth radius and flagellar synthesis decreased to similar levels in the ΔflhB and ΔesrF ΔflhB mutants compared to those of the WT strain (Fig. 4A and B). These data indicate that esrF contributed to the motility of O157 by directly inducing the expression of flhB (Fig. 3 and 4).
flhB promotes O157 virulence in vitro and in vivo.
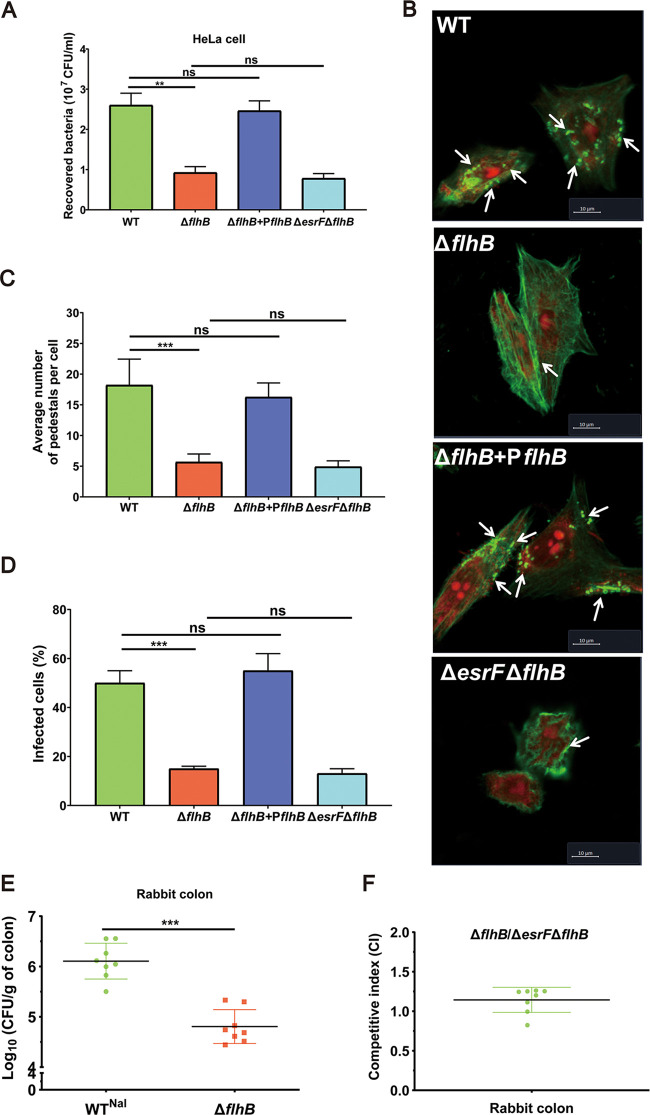
The effect of flhB on O157 virulence was investigated using the HeLa cell adhesion assay and rabbit colon colonization experiments. The results showed that ΔflhB cell adhesion decreased significantly compared with that of WT (2.9-fold) and ΔflhB+PflhB (2.7-fold) strains (Fig. 5A). Moreover, the FAS results revealed significantly reduced AE lesion formation in HeLa cells infected with the ΔflhB mutant (15%) compared to WT (50%) and ΔflhB+PflhB (55%) strains, with averages of 18.3, 5.2, and 15.3 pedestals on each cell infected by WT, ΔflhB, and ΔflhB+PflhB strains, respectively (Fig. 5B to D). Notably, WT, ΔflhB, and ΔflhB+PflhB strains grew at similar rates in vitro (Fig. 2E), indicating that the decreased cell adhesion of ΔflhB was not due to slower bacterial growth. In addition, an infant rabbit model of O157 infection revealed that significantly fewer bacteria were recovered from rabbit colons infected with the ΔflhB mutant (16.7-fold) than with the WT strain (Fig. 5E), thereby validating the inference exerting by flhB on O157 virulence in vitro and in vivo.
FIG 5.
flhB promotes O157 virulence in vitro and in vivo. (A) HeLa cell adhesion of WT, ΔflhB, ΔflhB+PflhB, and ΔesrF ΔflhB strains at an MOI of 10. (B) FAS of HeLa cells infected with WT, ΔflhB, ΔflhB+PflhB, and ΔesrF ΔflhB strains. DNA was stained with propidium iodide (PI, red), and the HeLa cell actin cytoskeleton was stained with FITC-labeled phalloidin (green). Pedestals were observed as punctate green structures typically associated with bacterial cells. Scale bar, 10 μm. (C) Quantification of pedestals on HeLa cells infected with WT, ΔflhB, ΔflhB+PflhB, and ΔesrF ΔflhB strains. (D) Quantification of the proportion of infected HeLa cells. (E) Colonization of rabbit colon by WT and ΔflhB strains. (F) The competitive colonization index of ΔflhB versus ΔesrF ΔflhB strains in rabbit colon. The data are presented as means ± the SD (n = 3; ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test.
We next sought to determine whether EsrF directly influences O157 virulence by regulating flhB expression. HeLa cell adhesion and FAS results showed no further significant decrease in cell adhesion or pedestal formation for the ΔesrF ΔflhB strain compared to the ΔflhB mutant (Fig. 5A to D). Moreover, competitive infection assays in rabbits using ΔflhB and ΔesrF ΔflhB mutants revealed that the competitive index (CI) for ΔesrF ΔflhB versus ΔflhB strains in the colonization of rabbit colon was 1.09, indicating a similar colonization ability (Fig. 5F). Together, these data indicate that esrF contributes to O157 virulence by directly inducing the expression of flhB (Fig. 2 and 5).
NtrC negatively regulates esrF expression.
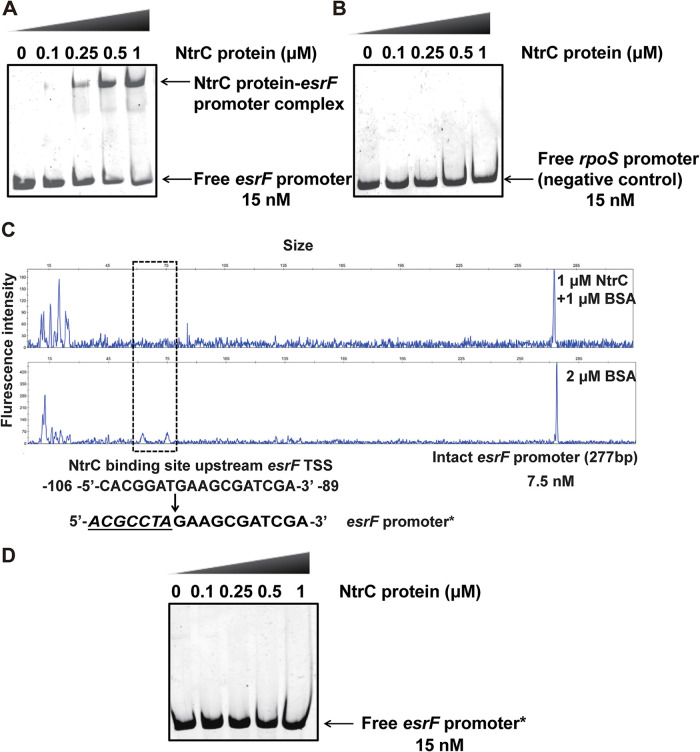
The esrF gene sequence is located within the glnALG operon of the O157 genome; the expressions of all genes within this operon are regulated by NtrC (47). NtrC is the RR of the NtrC/B TCS, which regulates ∼100 nitrogen (N) starvation genes to balance bacterial N metabolism (47). According to the reported NtrC binding sites in the glnA 5′ UTR (NR1 to NR5) (48), we searched the esrF upstream region and found one 13-bp potential NtrC binding site (5′-TCACGGATGAAGC-3′; positions −107 to −95 from the esrF transcriptional start site [TSS]; see Fig. S4A). The NtrC-His6 protein was subsequently purified and used in a protein-DNA promoter EMSA, along with an amplified DNA fragment of the esrF promoter region to verify whether the phosphorylated form of NtrC protein (NtrC-P) directly binds to the esrF promoter. Slowly migrating bands were observed for the esrF promoter with increasing NtrC protein concentrations (Fig. 6A), and the intensities of these bands gradually strengthened as the concentration of NtrC protein increased from 0.25 to 1 μM. Meanwhile, no migrating bands were observed for the negative-control rpoS promoter with increasing NtrC protein concentrations (Fig. 6B), thus verifying the direct binding between the NtrC protein to the esrF promoter in vitro.
FIG 6.
NtrC regulates EsrF by binding to its promoter. (A) EMSAs were performed with purified NtrC protein and esrF promoter. (B) EMSAs were performed with purified NtrC protein and rpoS promoter, used as the negative control. (C) NtrC protein bound to the motif CACGGATGAAGCGATCGA in the esrF promoter region. Electropherograms showed the protection pattern of the esrF promoter region after digestion with DNase I after incubation in the absence or presence of 1 μM NtrC protein. (D) EMSAs of NtrC protein with the modified esrF promoter region (the motif CACGGATGAAGCGATCGA mutated to ACGCCTAGAAGCGATCGA). The data are presented as means ± the SD (n = 3; ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test.
The upstream gene ntrC negatively regulates EsrF expression in high ammonium environment. (A) The five previously known NtrC binding sites and potential NtrC binding sites located upstream of esrF were aligned using the MUltiple Sequence Comparison by Log-Expectation (MUCLE) program in Unipro Ugene software, with the “gCaC-at+aTggtGc” consensus sequence. (B) Quantification of EsrF abundance in WT and ΔntrC after treatment with 3 mM (low ammonium) and 24 mM (high ammonium) NH4Cl. (C) qRT-PCR performed to detect gfp expression to represent the esrFp and flhBp expression of the ΔntrC mutant in the colon in vivo and LB medium-cultured samples. rfp was used as the internal control. The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S4, TIF file, 2.0 MB (2MB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Using the dye-based DNase I footprinting assay, we identified a specific NtrC-bound sequence containing an 18-bp motif (5′-CACGGATGAAGCGATCGA-3′; positions −106 to −89 from the esrF TSS; Fig. 6C). This motif contains the 13-bp predicted NtrC binding site described above. To determine whether the motif was necessary for binding to NtrC, we performed EMSAs using a mutated esrF promoter (the motif CACGGATGAAGCGATCGA was mutated to ACGCCTAGAAGCGATCGA) under the same reaction conditions. The results showed no interaction between NtrC and the mutated esrF promoter (Fig. 6D), indicating that the motif is vital for the binding ability of NtrC to the esrF promoter.
Negative regulation of esrF by NtrC is released in response to the high ammonium environment in rabbit colon.
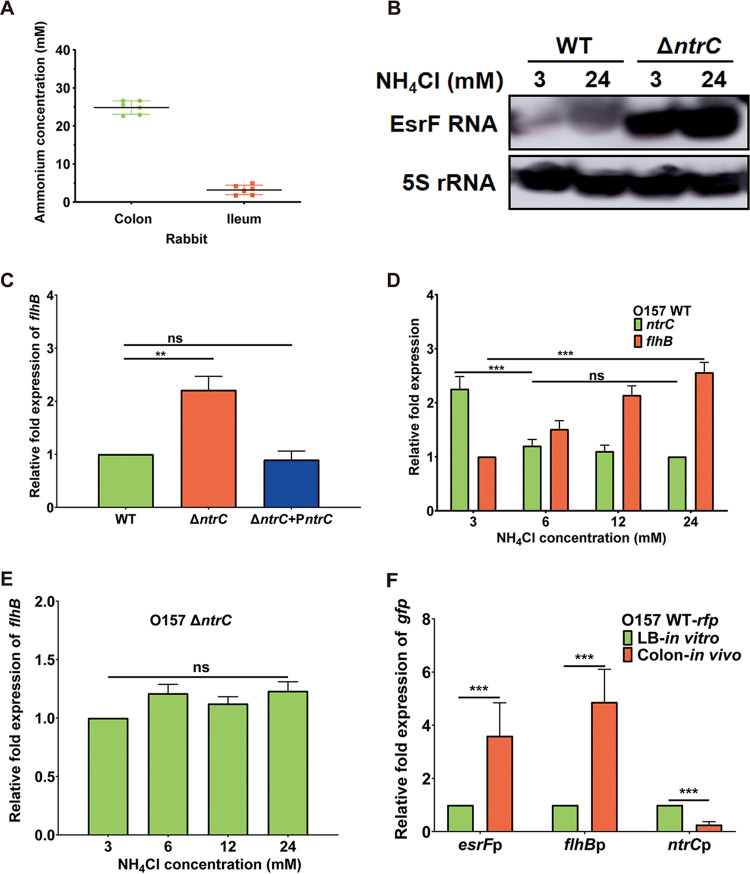
Microenvironmental cues are sensed by various TCSs to regulate bacterial gene expression (30). For instance, the NtrC/B TCS can respond to a low ammonium ion concentration (3 mM) and regulate gene expression; however, it is inhibited under high ammonium ion concentrations (47). The average ammonium concentration in rabbit colon was found to be 24.1 mM (Fig. 7A), representing a high ammonium environment, which is in agreement with the previously reported concentration of 20.8 ± 8.0 mM in rabbit cecum (49).
FIG 7.
NtrC negatively regulates EsrF in different ammonium environments. (A) Ammonium concentrations of the rabbit colon and ileum were measured. (B) Northern blotting of EsrF in WT and ΔntrC strains after treatment with 3 mM (low ammonium) and 24 mM (high ammonium) NH4Cl. (C) Expression of flhB in WT, ΔntrC, and ΔntrC+PntrC strains analyzed using qRT-PCR. (D) ntrC and flhB expression in the WT strain with different concentrations of NH4Cl analyzed using qRT-PCR. (E) flhB in the ΔntrC mutant expression with different concentrations of NH4Cl analyzed using qRT-PCR. (F) qRT-PCR detection of gfp expression to represent the esrFp, flhBp and ntrCp expressions of WT in the colon in vivo and LB medium-cultured samples. rfp was used as the internal control. The data are presented as means ± the SD (n = 3; ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test.
We, therefore, investigated whether ammonium concentrations influence EsrF and flhB expression in vitro. Northern blotting results found that the abundance of O157 EsrF sRNA significantly increased in the presence of a high ammonium (24 mM) concentration compared to a low ammonium (3 mM) concentration (Fig. 7B; see also Fig. S4B). The qRT-PCR results further demonstrated that flhB expression increased significantly in ΔntrC compared to the WT or ΔntrC+PntrC strains in DMEM (Fig. 7C). Further, the qRT-PCR results found that O157 flhB expression significantly increased with increasing ammonium concentrations (3, 6, 12, and 24 mM), while the reverse trend was observed for ntrC (Fig. 7D). However, the expression of esrF and flhB of the ΔntrC strain exhibited no change between high-ammonium and low-ammonium conditions (Fig. 7B and E). This result indicates that repression of esrF by NtrC becomes released in a high-ammonium environment, resulting in upregulation of flhB expression in vitro.
In addition, in vivo qRT-PCR results showed that the ntrC expression of O157 WT strains in the colon of infected rabbits decreased significantly compared to that in the LB-cultured sample, indicating that ntrC expression was repressed in the high ammonium environment in vivo (Fig. 7F). Furthermore, in the colon of an infected rabbit, the expression of esrF and flhB of the O157 strain WT was enhanced compared to that in the LB medium-cultured sample. However, no difference was observed in the expression of esrF or flhB of the ΔntrC strain in the colon of an infected rabbit and LB medium-cultured samples (see Fig. S4C). Together, these data indicate that O157 sensed the high ammonium concentrations in the rabbit colon and responded by turning off the negative regulation of esrF by NtrC, thus inducing the expression of esrF and flhB to promote its motility and virulence in vivo.
The expression of glnA, which is located upstream esrF in the genome, is also negatively regulated by NtrC (50). The qRT-PCR results showed that the expression of glnA in the ΔntrC mutant increased significantly compared to that in WT and ΔntrC+PntrC strains (see Fig. S5A). Moreover, the expression of glnA in O157 significantly increased with increasing concentrations of ammonium (3, 6, 12, and 24 mM; see Fig. S5B). However, no changes were observed in the expression of glnA in the ΔntrC mutant when exposed to different ammonium conditions (see Fig. S5C). The trend observed for glnA expression was the same as that for esrF under the regulation by NtrC in high-ammonium conditions, indicating that NtrC represses the expression of esrF and glnA through interacting with two unique independent promoters, respectively (Fig. 1D; see also Fig. S5).
ntrC negatively regulates glnA expression in different ammonium environments. (A) Expression of glnA in WT, ΔntrC, and ΔntrC+PntrC strains analyzed using qRT-PCR. (B) Expression of glnA in WT with different concentrations of NH4Cl analyzed using qRT-PCR. (C) Expression of glnA in the ΔntrC mutant with different concentrations of NH4Cl analyzed using qRT-PCR. The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S5, TIF file, 1.3 MB (1.3MB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
O157 senses different host environmental signals, such as butyrate, fucose, and pH, via different TCSs that help to regulate virulence factor expression and promote bacteria colonization in the colon (51). For instance, a low biotin signal activates BirA-mediated expression of LEE genes to promote O157 virulence (52). In fact, most of the signal transduction pathways regulate O157 virulence by influencing the expression of LEE genes. However, the mechanisms by which microenvironmental cues in the colon regulate other non-LEE virulence genes remain unknown. In this study, we revealed a regulatory signal transduction pathway in which O157 utilizes a novel sRNA, EsrF, to sense high ammonium concentrations in the host colon to enhance bacterial motility and virulence. Specifically, the high ammonium concentration in the colon caused the repression of EsrF by NtrC to be lost, resulting in high levels of EsrF expression. EsrF then directly bound the 5′ UTR of flhB mRNA to promote flhB expression, which enhanced the expression of other flagellar genes to facilitate flagellar biosynthesis. This mechanism functions to significantly promote O157 motility and colonization in the colon.
Flagella play diverse roles in bacterial pathogenesis, such as in the migration to an optimal site in the host, colonization, and survival at the site of infection (53). They act as adhesins to promote host cell adhesion and microcolony formation (13). Flagella were identified as adhesins when a fliC mutation was found to reduce EHEC virulence (54). For pathogens, it is necessary to upregulate the expression of flagellar genes during the early stages of infection. Here, we discovered that EsrF promoted O157 motility and host cell adhesion during these early stages. FlhDC and FliA are well-known master flagellar transcriptional activators in E. coli that regulate other flagellar genes (16). In our study, we found that EsrF positively regulated flagellar gene expression via FlhB in O157, which upregulated the expression of flhD, flhC, fliA, and fliC. This observation is consistent with the findings of a previous report that FlhB can regulate flagellar gene expression in Listeria monocytogenes (17). However, the detailed mechanism of how FlhB regulates flagellar genes is unknown.
The potential mechanism underlying the increased flhB mRNA abundance induced by EsrF-flhB mRNA interaction is likely related to the loss of Rho termination in O157. In bacterial cells, the Rho factor mediates termination of transcription at specific sites, which can be relieved by the Rho-specific inhibitor bicyclomycin (BCM) (55). Certain sRNAs in bacteria can anneal within 5′ UTRs to inhibit premature Rho termination and activate the expression of corresponding genes (27). Hence, we analyzed the sequencing reads in the flhB 5′ UTR region from the published raw data generated by Sedlyarova et al. from an RNA-seq analysis on E. coli, with or without BCM treatment (27) (see Fig. S6A). The upregulated expression and released termination within flhB 5′ UTRs in response to BCM treatment was found to occur between sites 0 and 100 (counted from flhB translational start site), indicating that Rho factor targets are located within flhB 5′ UTRs. Moreover, our identified EsrF binding sites were determined to be located at flhB 5′ UTR positions −82 to −97, within the Rho termination region. Besides, we analyzed the transcription of flhB open reading frame (ORF) in O157 WT, with or without BCM treatment, by qRT-PCR assays and found that flhB expression in O157 WT with BCM treatment exhibited a 2.2-fold increase compared to that in O157 WT without BCM treatment (see Fig. S6B). Taken together, these results indicate that the interaction between EsrF with the flhB 5′ UTR may release the Rho-dependent termination within the flhB 5′ UTR, leading to increased flhB expression.
EsrF-flhB mRNA interaction increases flhB mRNA abundance related to relieved Rho-termination. (A) Transcriptomic coverage plots for flhB 5′ UTR before (up) and after (down) treatment with bicyclomycin (BCM). (B) Transcription of flhB ORF in O157 WT without BCM (WT-BCM) or with BCM (WT+BCM) treatment (15 min, 50 μg/ml) analyzed using qRT-PCR. The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S6, TIF file, 1.7 MB (1.7MB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Inorganic N, produced by colonic microflora and digested food, is the secondmost important element after carbon in organisms and is a crucial constituent of proteins, nucleic acids, and cell walls. Various types of inorganic and organic N are assimilated by mammals, including ammonium (NH4+), nitrate (NO3–), nitrite (NO2–), urea, purine, and amino acids. Ammonium, widely used in various food additives, is the most effective N source for bacteria, which is assimilated directly into important biosynthetic reactions (47). Bacterial N metabolism is mainly regulated by the NtrC/B TCS, which regulates approximately 100 genes, mainly for N assimilation under N starvation (56). During N limitation, the NtrB/C TCS positively modulates Pseudomonas aeruginosa virulence by producing rhamnolipids (57) and exopolyphosphatase (Ppx) (58, 59). NtrC-regulated exopolysaccharides are involved in biofilm formation and the pathogenic interaction of Vibrio vulnificus (60). However, NtrB/C has not been reported to be related to the virulence of pathogenic E. coli. In this study, we have demonstrated that NtrC bound directly to the esrF promoter, sensing high ammonium concentrations in the colon to induce O157 motility and host cell adhesion.
Genome sequence analysis for 226 representative E. coli strains revealed that esrF is highly conserved and ubiquitous in different E. coli strain pathotypes. Specifically, the strains containing esrF include EHEC strains O157:H7, O157 Sakai, O157 Xuzhou21, enteropathogenic E. coli (EPEC) strains O55:H7, neonatal meningitis-associated E. coli strain RS218, urinary pathogenic E. coli strain CFT073, avian pathogenic E. coli strain LF82, as well as other clinical isolates of E. coli strains (see Fig. S7A and Table S2 in the supplemental material). However, esrF is absent in nonpathogenic E. coli strains, such as K-12, indicating that EsrF is a horizontal transfer-acquired regulatory sRNA of pathogenic E. coli, while esrF may be associated with the evolution from nonpathogenic E. coli to pathogenic strains.
EsrF is widely found in pathogenic Escherichia coli. (A) Maximum-likelihood tree constructed in PhyML based on 1,112 single-copy core genes shared by 226 E. coli strains. The strains in different clades containing esrF are shown in different colors. (B) flhB expression compared between three representative pathogenic E. coli strains, G1352 (EHEC O26:H11), G4943 (EPEC O55:H7), and G1474 (EPEC O86:H34) with ΔesrF ortholog mutants. The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S7, TIF file, 2.1 MB (2.1MB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Prevalence of esrF among different E. coli strains. Download Table S2, DOCX file, 0.02 MB (20.8KB, docx) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To investigate whether homologous EsrF in other flagellated E. coli strains also regulate flhB expression, we selected three representative strains (EHEC O26:H11, EPEC O55:H7, and EPEC O86:H34) and deleted the esrF orthologous genes to construct corresponding deletion strains. Compared to the corresponding WT strains, the flhB expression level of these mutants was significantly reduced (see Fig. S7B), indicating that the EsrF-mediated mechanism for flhB regulation may be present in various pathogenic E. coli strains.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
The bacterial strains, plasmids, and primers used in this study are listed in Table S3 in the supplemental material. Mutant strains were generated by substitution of the chloramphenicol or kanamycin resistance genes in plasmid pKD3 or pKD4 with sRNA or the relevant genes, respectively, by using the λ Red recombinase system (61). The complementary strains were constructed by cloning the ORF and upstream promoter sequence of corresponding genes into the vector, pBluescript II SK(+) (62). The pET-ntrC strain was generated by cloning the ntrC gene into the downstream region of the His tag element in plasmid pET-28a. All the resulting clones were verified by PCR amplification and DNA sequencing.
Bacterial strains, plasmids, and primers used in this study. Download Table S3, DOCX file, 0.03 MB (30.1KB, docx) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Bacterial growth and cell culture conditions.
O157 and its derivatives were cultured in LB medium or DMEM to simulate in vivo colon conditions. IPTG (isopropyl-β-d-thiogalactopyranoside) and antibiotics were used whenever necessary. After adding overnight subcultures into 96-well plates, the growth curves of all strains were measured over 24 h by using a plate reader. Each experiment was independently performed three times. The HeLa (ATCC CCL-2) cell line was grown in DMEM supplemented with 10% fetal bovine serum (Gibco) and penicillin-streptomycin and incubated at 37°C in a 5% CO2-containing atmosphere.
RNA isolation and qRT-PCR.
To test the influence of virulence genes, strains were grown in DMEM up to the exponential phase (optical density at 600 nm [OD600] ∼0.6). To test the influence of ammonium concentrations on gene expression, strains were grown in M9 medium under low-ammonium (3 mM) and high-ammonium (24 mM) conditions. To quantify gene expression in vivo, the constitutively expressed red fluorescence protein (rfp) gene, inserted into the O157 chromosome, was used as the reference control for normalization. We also constructed a plasmid containing the esrF, flhB, and ntrC promoter transcriptionally fused to a green fluorescent protein (gfp) gene and introduced it into O157 cells with rfp. The expression of esrF, flhB, and ntrC was then indirectly determined by qRT-PCR analysis for the expression of gfp (to avoid interference of esrF, flhB, and ntrC homologs from intestinal commensal bacteria). The overnight cultures were separated into one sample for RNA extraction and another for oral gavage to a rabbit, the colon of which was dissected for RNA extraction. Total RNA was extracted using the TRIzol LS reagent (catalog no. 10296028; Invitrogen, Carlsbad, CA) and treated with RNase-free DNase I to eliminate genomic DNA contamination. qRT-PCR was performed as follows. Briefly, 2 μg of diluted, extracted RNA was converted to cDNA with Superscript IV VILO Master Mix (Invitrogen). Validated primers (see Table S3) and SYBR green were added to the cDNA, and the mixture was amplified using QuantStudio 5 (Applied Biosystems). Data were collected using QuantStudio Real-Time PCR Software v1.3, normalized to endogenous rpoA levels, and analyzed using the comparative critical threshold (CT) method. Each experiment was performed in triplicate.
RNA-seq and other bioinformatic tools.
Total RNA was purified by using an RNeasy minikit (Qiagen) and a Ribo-Zero rRNA removal kit (Epicentre Biotechnologies, RZNB1056). Libraries of RNA samples were generated by using an NEBNext R Ultra Directional RNA Library Prep kit for Illumina R (NEB). The libraries were sequenced using an Illumina HiSeq platform to generate paired-end reads. To search for potential sRNAs of O157, plot sequence reads were aligned to the reference sequence of O157 using Burrows-Wheeler Aligner software and SAMtools. TargetRNA2 was used to predict target genes of sRNA by searching the 5′ regions (ca. nt −120 to +20 relative to the start codon) of all O157 gene mRNAs for potential RNA duplex formation sequences (43).
Northern blotting.
Northern blotting was performed using the DIG Northern Starter kit (Roche). RNA was separated on a 1.2% agarose gel containing 37% formaldehyde. The size of the RNA was determined by comparing it to the RNA Century-Plus Markers (Invitrogen). Gels were electroblotted onto Brightstar Plus nylon membranes (Applied Biosystems) and immobilized at 120°C for 30 min. Cross-linked membranes were prehybridized for 30 min in digoxigenin (DIG) Easy Hyb buffer. Single-stranded RNA probes were labeled with DIG added to fresh Easy Hyb buffer, and the blots were incubated with hybridization buffer overnight. After high- and low-stringency washes, the blots were further washed using the Wash and Block Buffer, and CDP-Star (Roche) was added as the substrate. Hybridization signals were visualized by using Amersham Imager 680, 5S rRNA being used as an internal control. ImageJ software was used to measure the band intensities. The specific DIG-labeled RNA probes used in the Northern blotting are presented in Table S3.
Exonuclease digestion of RNA.
RNA samples were prepared from O157 WT grown overnight in LB. Treatment of RNA samples with terminator 5-monophosphate-dependent exonuclease (TEX; Epicentre Biotechnologies) was performed as described previously (38). Total RNA (10 μg/sample) samples were incubated with TEX in a final 20-μl reaction volume containing 2 μl of 10× reaction buffer (500 mM Tris-HCl [pH 8.0], 20 mM MgCl2, 1 M NaCl), 1 μl of RNasin (40 U; Promega), and 1 μl of TEX for 1 h at 30°C. The reaction mixture was then purified and analyzed by Northern blotting as described above.
5′ and 3′ RACE assay.
RACE was performed with the 5′/3′-RACE system (Invitrogen). For 5′-RACE, 5 μg of RNA was reverse transcribed using an sRNA-specific antisense primer and SuperScript reverse transcriptase (Invitrogen). cDNA was then purified, dC tailed, and used as a template in a PCR with the Abridged Anchor Primer (AAP) and a nested gene-specific primer. 3′-RACE was performed by ligating a poly(A) tail using a poly(A) polymerase tailing kit (Epicentre Biotechnologies) before reverse transcription. Specific cDNAs were then directly amplified by PCR using an anchor primer (AP) that targets the poly(A) tail region and a gene-specific primer that anneals to a region of known sRNA sequence. PCR products were cloned into the pEASY-T1 simple cloning vector (TransGen) before sequencing.
Cell adhesion assay.
Subcultured bacteria were grown in DMEM until the OD600 was 0.6; the OD600 was then adjusted to 0.3 with DMEM. Next, bacterial cells (2 ml each well) were added to HeLa cell monolayers in 6-well plates at a multiplicity of infection (MOI) of 100. To avoid motility-related effects, all bacteria were centrifuged onto the surface of cells and incubated for 1 h at the early infection stage. The cells were then washed, lysed, and plated on LB agar plates. The adhesion rates were calculated as percentages of the number of bacteria recovered relative to the total bacteria inoculated. All assays were performed in triplicate.
Fluorescein actin staining.
Fluorescence actin staining was performed as previously described (63). Human cervical adenocarcinoma (HeLa) cells were grown on coverslips to 60% confluence and infected by bacteria as described for the cell adhesion assay. After infection, the coverslips were washed and fixed with 4% formaldehyde, permeabilized with 0.2% Triton X-100, and treated with fluorescein isothiocyanate (FITC)-labeled phalloidin to visualize actin accumulation, followed by propidium iodide (PI) to visualize the DNA. The coverslips were mounted on slides and observed under a Leica TCS SP8 microscope. The AE lesions formed in each cell were enumerated for 50 HeLa cells; three slides were observed for each strain in each experiment.
Infant rabbit model of O157 infection.
To prepare the inoculum, bacteria were grown overnight in LB broth, harvested by centrifugation, resuspended in sterile phosphate-buffered saline, and adjusted to a cell density of ∼109 CFU ml−1. Three-day-old New Zealand White rabbits were intragastrically inoculated with ∼5 × 108 CFU/90 g (body weight) of WT or mutant in a 0.5-ml inoculum, which was followed by the administration of 2.5 ml of sterile 0.85% saline solution to ensure delivery of the entire inoculum. Rabbits were euthanized 48 h postinfection. At necropsy, the intestinal tract from the duodenum to the anus was removed to collect samples for microbiologic analyses. Rabbit competition experiments were performed as follows: ΔflhB and ΔesrF ΔflhB mutant strains, both in logarithmic phase, were mixed at a 1:1 ratio for oral inoculation of rabbits, as described above. The CI value of the ΔflhB mutant versus the ΔesrF ΔflhB mutant was calculated. All experiments were performed in triplicate.
Motility assay and transmission electron microscopy.
Overnight cultures were adjusted to an OD600 of 0.2, and then 5 μl was stab inoculated onto 0.35% semi-LB-agar plates. The agar plates were incubated at 25°C for 12 h, and the diameter of the swimming zone around the inoculation site was measured. TEM was performed to observe the flagellum morphology on the surface of O157, as previously described (17, 64). The strains were cultured in LB plates overnight. Single colonies were resuspended in 50 μl of medium; 10 μl was then dropped onto and absorbed for 5 min into carbon-stabilized Formvar supports on 200-mesh copper grids. Cells were then stained by submerging the grids for 5 min in 2% (wt/vol) uranyl acetate and imaged using a JEM-1400 Plus transmission electron microscope (JEOL) operating at 100 kV and fitted with a high-sensitivity real-time charge-coupled device camera. All strains were tested in triplicate, and the number of flagella were counted per 30 bacteria.
Purification of NtrC protein and protein-DNA EMSA.
NtrC-6×His protein was expressed using BL21(DE3)-containing pET-NtrC and purified by using a MagneHis protein purification system (Promega). Protein-DNA EMSAs were performed as described previously (65). EsrF promoter regions were amplified and purified. NtrC protein shift assays were performed by incubating EsrF promoter fragments (15 nM) at 37°C for 30 min with various concentrations of NtrC-6×His protein (0 to 3 μM) in a 20-μl solution containing bandshift buffer (24 mM Tris-HCl [pH 7.5], 80 mM NaCl, 0.1 mM EDTA, 1 mM dithiothreitol [DTT]). In addition, 30 mM acetyl phosphate (AcP) was added as the donor to phosphorylate NtrC to NtrC-P. The samples were loaded on an 8% polyacrylamide gel. The DNA fragments were stained for 10 min with StarGreen (Genstar) and visualized by UV transillumination. ImageJ software was used to measure the band intensities.
Dye primer-based DNase I footprinting assay.
The EsrF promoter (7.5 nM) was amplified with a forward primer (with a 6-FAM modification at the 5′ end) and a reverse primer and then incubated with 1 μM NtrC and 1 μM bovine serum albumin (BSA) in bandshift buffer. The protein-DNA mixtures were then partially digested with 0.05 U of DNase I for 5 min at 25°C, quenched by using 0.25 mol/liter EDTA, and purified. Control samples were prepared with 2 μM BSA. All genotype samples were analyzed using the ABI 3730 DNA analyzer.
In vitro transcription and RNA-RNA EMSA.
The in vitro transcription DNA templates of EsrF (+), EsrF (−; positive control), and flhB mRNA were PCR amplified using O157 genome as the template. Then, DNA templates were transcribed into EsrF (+), EsrF (−), and flhB mRNA using the T7 high-efficiency transcription kit (TransGen) and purified by using the EasyPure RNA purification kit (TransGen). The purified RNA was checked in an 8% Tris-Acr-urea gel. An RNA-RNA EMSA (REMSA) was performed to verify the interaction of RNAs, as previously described (23, 66). REMSAs were performed with EsrF (+, 0.5, 1, 2, and 4 μM), EsrF (−, positive control, 1 μM) and flhB mRNA (1 μM), 10× REMSA binding buffer (100 mM HEPES [pH 7.3], 200 mM KCl, 24 mM MgCl2, and 24 mM DTT), and RNase-free water (Beyotime). The reaction mixtures were incubated for 2 min at 85°C and then at 37°C for 30 min. RNA was separated by 8% Native PAGE using a Native-PAGE preparation kit (Sangon), stained with SYBR Gold nucleic acid gel stain (Invitrogen) for 10 min, and visualized under UV light. ImageJ software was used to measure band intensities.
Ammonium concentration measurements.
The colons from standard 3-day-old New Zealand White rabbits were collected, washed, and homogenized using a Tissueprep Lyser. The samples were pelleted, and the supernatants were collected. The ammonium concentrations of the serially diluted supernatants were measured by using a Quantofix ammonium test kit (Merck Millipore, UK) according to the manufacturer’s instructions.
Phylogenetic analysis.
Orthologous groups were identified using OrthoFinder (67) by which all nucleotide sequences were compared using a BLASTN all-against-all search with an E value cutoff of <10−4. Nucleotide sequences used to construct the phylogenetic tree were aligned in MAFFT (68), and a maximum-likelihood tree was constructed in PhyML and FigTreev1.4.3 based on the GTR model of nucleotide substitution with c-distributed rates among sites.
Ethics statement.
All animal experiments were carried out according to the standards set forth in the Guide for the Care and Use of Laboratory Animals published by the Institute of Laboratory Animal Resources of the National Research Council (United States). The experimental protocols were approved by the Institutional Animal Care Committee at Nankai University. Every effort made was to minimize animal suffering and to reduce the number of animals used.
Statistical analysis.
Statistical analysis was performed using MedCalc 15.6; figures were drawn by GraphPad Prism 8 and integrated by using Adobe Illustrator CC 2020. All data are expressed as means ± the standard deviations (SD). Differences between two groups were evaluated using a two-tailed Student t test or a Mann-Whitney U test. P values of ≤0.05, 0.01, or 0.001 were considered statistically significant (∗), highly significant (∗∗), or extremely significant (∗∗∗), respectively.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China Program (32070130, 81772148, 31820103002, 31770144, and 81871624), Distinguished Young Scholar of Tianjin (20JCJQJC00180), the Natural Science Foundation (Key Project) of Tianjin (20JCZDJC00820), and National Key Programs for Infectious Diseases of China (2017ZX10303405-001 and 2018ZX10714002-001-006).
We thank Yanjie Chao at the Institut Pasteur of Shanghai, Chinese Academy of Sciences, for kindly providing plasmid pJV300.
Footnotes
Citation Jia T, Liu B, Mu H, Qian C, Wang L, Li L, Lu G, Zhu W, Guo X, Yang B, Huang D, Feng L, Liu B. 2021. A novel small RNA promotes motility and virulence of enterohemorrhagic Escherichia coli O157:H7 in response to ammonium. mBio 12:e03605-20. https://doi.org/10.1128/mBio.03605-20.
Contributor Information
Melissa M. Kendall, University of Virginia School of Medicine.
Gerald B. Pier, Harvard Medical School.
REFERENCES
- 1.Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, Davis BR, Hebert RJ, Olcott ES, Johnson LM, Hargrett NT, Blake PA, Cohen ML. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med 308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 2.Scallan E, Griffin PM, Angulo FJ, Tauxe RV, Hoekstra RM. 2011. Foodborne illness acquired in the United States: unspecified agents. Emerg Infect Dis 17:16–22. doi: 10.3201/eid1701.091101p2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews L, Reeve R, Gally DL, Low JC, Woolhouse MEJ, McAteer SP, Locking ME, Chase-Topping ME, Haydon DT, Allison LJ, Hanson MF, Gunn GJ, Reid SWJ. 2013. Predicting the public health benefit of vaccinating cattle against Escherichia coli O157. Proc Natl Acad Sci U S A 110:16265–16270. doi: 10.1073/pnas.1304978110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. 2012. Fucose sensing regulates bacterial intestinal colonization. Nature 492:113–117. doi: 10.1038/nature11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowley CA, Anderson CJ, Kendall MM. 2018. Ethanolamine influences human commensal Escherichia coli growth, gene expression, and competition with enterohemorrhagic Escherichia coli O157:H7. mBio 9:e01429-18. doi: 10.1128/mBio.01429-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clements A, Young JC, Constantinou N, Frankel G. 2012. Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes 3:71–87. doi: 10.4161/gmic.19182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. 2003. Bacterium-host communication: the language of hormones. Proc Natl Acad Sci U S A 100:8951–8956. doi: 10.1073/pnas.1537100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald SF, Beckett AE, Palarea-Albaladejo J, McAteer S, Shaaban S, Morgan J, Ahmad NI, Young R, Mabbott NA, Morrison L, Bono JL, Gally DL, McNeilly TN. 2019. Shiga toxin subtype 2a increases the efficiency of Escherichia coli O157 transmission between animals and restricts epithelial regeneration in bovine enteroids. PLoS Pathog 15:e1008003. doi: 10.1371/journal.ppat.1008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bardiau M, Szalo M, Mainil JG. 2010. Initial adherence of EPEC, EHEC, and VTEC to host cells. Vet Res 41:57. doi: 10.1051/vetres/2010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaban B, Hughes HV, Beeby M. 2015. The flagellum in bacterial pathogens: for motility and a whole lot more. Semin Cell Dev Biol 46:91–103. doi: 10.1016/j.semcdb.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 11.McWilliams BD, Torres AG. 2014. EHEC Adhesins. Microbiol Spectr 2:Ehec00032013. doi: 10.1128/microbiolspec.EHEC-0003-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdem AL, Avelino F, Xicohtencatl-Cortes J, Girón JA. 2007. Host protein binding and adhesive properties of H6 and H7 flagella of attaching and effacing Escherichia coli. J Bacteriol 189:7426–7435. doi: 10.1128/JB.00464-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahajan A, Currie CG, Mackie S, Tree J, McAteer S, McKendrick I, McNeilly TN, Roe A, La Ragione RM, Woodward MJ, Gally DL, Smith DGE. 2009. An investigation of the expression and adhesin function of H7 flagella in the interaction of Escherichia coli O157: H7 with bovine intestinal epithelium. Cell Microbiol 11:121–137. doi: 10.1111/j.1462-5822.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- 14.Rogers TJ, Thorpe CM, Paton AW, Paton JC. 2012. Role of lipid rafts and flagellin in invasion of colonic epithelial cells by Shiga-toxigenic Escherichia coli O113:H21. Infect Immun 80:2858–2867. doi: 10.1128/IAI.00336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg HC. 2003. The rotary motor of bacterial flagella. Annu Rev Biochem 72:19–54. doi: 10.1146/annurev.biochem.72.121801.161737. [DOI] [PubMed] [Google Scholar]
- 16.Chevance FF, Hughes KT. 2008. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol 6:455–465. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng C, Wang H, Ma T, Han X, Yang Y, Sun J, Chen Z, Yu H, Hang Y, Liu F, Fang W, Jiang L, Cai C, Song H. 2018. Flagellar basal body structural proteins FlhB, FliM, and FliY are required for flagellar-associated protein expression in Listeria monocytogenes. Front Microbiol 9:208. doi: 10.3389/fmicb.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhlen L, Johnson S, Zeitler A, Bäurle S, Deme JC, Caesar JJE, Debo R, Fisher J, Wagner S, Lea SM. 2020. The substrate specificity switch FlhB assembles onto the export gate to regulate type three secretion. Nat Commun 11:1296. doi: 10.1038/s41467-020-15071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta T, Srivastava S. 2018. Small RNA-mediated regulation in bacteria: a growing palette of diverse mechanisms. Gene 656:60–72. doi: 10.1016/j.gene.2018.02.068. [DOI] [PubMed] [Google Scholar]
- 20.Hör J, Matera G, Vogel J, Gottesman S, Storz G. 2020. trans-Acting small RNAs and their effects on gene expression in Escherichia coli and Salmonella enterica. EcoSal Plus 9. doi: 10.1128/ecosalplus.ESP-0030-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Xue D, Sun W, Han J, Li J, Gao R, Zhou Z, Zhang W, Chen M, Lin M, Wang J, Zuo K. 2019. sRNA OsiA stabilizes catalase mRNA during oxidative stress response of Deincoccus radiodurans R1. Microorganisms 7:422. doi: 10.3390/microorganisms7100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottesman S, Storz G. 2011. Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb Perspect Biol 3:a003798. doi: 10.1101/cshperspect.a003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melson EM, Kendall MM. 2019. The sRNA DicF integrates oxygen sensing to enhance enterohemorrhagic Escherichia coli virulence via distinctive RNA control mechanisms. Proc Natl Acad Sci U S A 116:14210–14215. doi: 10.1073/pnas.1902725116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han R, Xu L, Wang T, Liu B, Wang L. 2017. A small regulatory RNA contributes to the preferential colonization of Escherichia coli O157:H7 in the large intestine in response to a low DNA concentration. Front Microbiol 8:274–274. doi: 10.3389/fmicb.2017.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hücker SM, Simon S, Scherer S, Neuhaus K. 2017. Transcriptional and translational regulation by RNA thermometers, riboswitches and the sRNA DsrA in Escherichia coli O157:H7 Sakai under combined cold and osmotic stress adaptation. FEMS Microbiol Lett 364:fnw262. doi: 10.1093/femsle/fnw262. [DOI] [PubMed] [Google Scholar]
- 26.Hoe C-H, Raabe CA, Rozhdestvensky TS, Tang T-H. 2013. Bacterial sRNAs: regulation in stress. Int J Med Microbiol 303:217–229. doi: 10.1016/j.ijmm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Sedlyarova N, Shamovsky I, Bharati BK, Epshtein V, Chen J, Gottesman S, Schroeder R, Nudler E. 2016. sRNA-mediated control of transcription termination in Escherichia coli. Cell 167:111–121. doi: 10.1016/j.cell.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D, McAteer SP, Wawszczyk AB, Russell CD, Tahoun A, Elmi A, Cockroft SL, Tollervey D, Granneman S, Tree JJ, Gally DL. 2018. An RNA-dependent mechanism for transient expression of bacterial translocation filaments. Nucleic Acids Res 46:3366–3381. doi: 10.1093/nar/gky096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruber CC, Sperandio V. 2015. Global analysis of posttranscriptional regulation by GlmY and GlmZ in enterohemorrhagic Escherichia coli O157:H7. Infect Immun 83:1286–1295. doi: 10.1128/IAI.02918-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zschiedrich CP, Keidel V, Szurmant H. 2016. Molecular mechanisms of two-component signal transduction. J Mol Biol 428:3752–3775. doi: 10.1016/j.jmb.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao R, Bouillet S, Stock AM. 2019. Structural basis of response regulator function. Annu Rev Microbiol 73:175–197. doi: 10.1146/annurev-micro-020518-115931. [DOI] [PubMed] [Google Scholar]
- 32.Gao R, Mack TR, Stock AM. 2007. Bacterial response regulators: versatile regulatory strategies from common domains. Trends Biochem Sci 32:225–234. doi: 10.1016/j.tibs.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A, Russell RM, Pifer R, Menezes-Garcia Z, Cuesta S, Narayanan S, MacMillan JB, Sperandio V. 2020. The serotonin neurotransmitter modulates virulence of enteric pathogens. Cell Host Microbe 28:41–53. doi: 10.1016/j.chom.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curtis MM, Hu Z, Klimko C, Narayanan S, Deberardinis R, Sperandio V. 2014. The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host Microbe 16:759–769. doi: 10.1016/j.chom.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreira CG, Sperandio V. 2016. The epinephrine/norepinephrine/autoinducer-3 interkingdom signaling system in Escherichia coli O157:H7. Adv Exp Med Biol 874:247–261. doi: 10.1007/978-3-319-20215-0_12. [DOI] [PubMed] [Google Scholar]
- 36.Yang B, Feng L, Wang F, Wang L. 2015. Enterohemorrhagic Escherichia coli senses low biotin status in the large intestine for colonization and infection. Nat Commun 6:6592. doi: 10.1038/ncomms7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denman RB. 1993. Using RNAFOLD to predict the activity of small catalytic RNAs. Biotechniques 15:1090–1095. [PubMed] [Google Scholar]
- 38.Kröger C, Dillon SC, Cameron AD, Papenfort K, Sivasankaran SK, Hokamp K, Chao Y, Sittka A, Hébrard M, Händler K, Colgan A, Leekitcharoenphon P, Langridge GC, Lohan AJ, Loftus B, Lucchini S, Ussery DW, Dorman CJ, Thomson NR, Vogel J, Hinton JC. 2012. The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. Proc Natl Acad Sci U S A 109:E1277–E1286. doi: 10.1073/pnas.1201061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao Y, Papenfort K, Reinhardt R, Sharma CM, Vogel J. 2012. An atlas of Hfq-bound transcripts reveals 3′ UTRs as a genomic reservoir of regulatory small RNAs. EMBO J 31:4005–4019. doi: 10.1038/emboj.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sei E, Conrad NK. 2014. UV cross-linking of interacting RNA and protein in cultured cells. Methods Enzymol 539:53–66. doi: 10.1016/B978-0-12-420120-0.00004-9. [DOI] [PubMed] [Google Scholar]
- 41.Tree JJ, Granneman S, McAteer SP, Tollervey D, Gally DL. 2014. Identification of bacteriophage-encoded anti-sRNAs in pathogenic Escherichia coli. Mol Cell 55:199–213. doi: 10.1016/j.molcel.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim W, Lee Y. 2020. Mechanism for coordinate regulation of rpoS by sRNA-sRNA interaction in Escherichia coli. RNA Biol 17:176–187. doi: 10.1080/15476286.2019.1672514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kery MB, Feldman M, Livny J, Tjaden B. 2014. TargetRNA2: identifying targets of small regulatory RNAs in bacteria. Nucleic Acids Res 42:W124–W129. doi: 10.1093/nar/gku317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMurry JL, Minamino T, Furukawa Y, Francis JW, Hill SA, Helms KA, Namba K. 2015. Weak interactions between Salmonella enterica FlhB and other flagellar export apparatus proteins govern type III secretion dynamics. PLoS One 10:e0134884. doi: 10.1371/journal.pone.0134884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Login FH, Wolf-Watz H. 2015. YscU/FlhB of Yersinia pseudotuberculosis harbors a C-terminal type III secretion signal. J Biol Chem 290:26282–26291. doi: 10.1074/jbc.M114.633677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meshcheryakov VA, Barker CS, Kostyukova AS, Samatey FA. 2013. Function of FlhB, a membrane protein implicated in the bacterial flagellar type III secretion system. PLoS One 8:e68384. doi: 10.1371/journal.pone.0068384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrero A, Flores E, Imperial J. 2019. Nitrogen assimilation in bacteria, p 280–300. In Schmidt TM (ed), Encyclopedia of microbiology, 4th ed. Academic Press, Oxford, United Kingdom. doi: 10.1016/B978-0-12-809633-8.20680-8. [DOI] [Google Scholar]
- 48.Reitzer LJ, Magasanik B. 1986. Transcription of glnA in Escherichia coli is stimulated by activator bound to sites far from the promoter. Cell 45:785–792. doi: 10.1016/0092-8674(86)90553-2. [DOI] [PubMed] [Google Scholar]
- 49.Marounek M, Miśta D, Volek Z, Savka O, Kalachnyuk L, Kalachnyuk GI. 2013. Comparative study on caecal fermentation pattern in adult domestic rabbits and wild hares. Anim Biol 15:94–101. [Google Scholar]
- 50.Pahel G, Rothstein DM, Magasanik B. 1982. Complex glnA-glnL-glnG operon of Escherichia coli. J Bacteriol 150:202–213. doi: 10.1128/JB.150.1.202-213.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Connolly JPR, Finlay BB, Roe AJ. 2015. From ingestion to colonization: the influence of the host environment on regulation of the LEE encoded type III secretion system in enterohaemorrhagic Escherichia coli. Front Microbiol 6:568. doi: 10.3389/fmicb.2015.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schumacher J, Behrends V, Pan Z, Brown DR, Heydenreich F, Lewis MR, Bennett MH, Razzaghi B, Komorowski M, Barahona M, Stumpf MP, Wigneshweraraj S, Bundy JG, Buck M. 2013. Nitrogen and carbon status are integrated at the transcriptional level by the nitrogen regulator NtrC in vivo. mBio 4:e00881-13. doi: 10.1128/mBio.00881-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osterman IA, Dikhtyar YY, Bogdanov AA, Dontsova OA, Sergiev PV. 2015. Regulation of flagellar gene expression in bacteria. Biochemistry (Mosc) 80:1447–1456. doi: 10.1134/S000629791511005X. [DOI] [PubMed] [Google Scholar]
- 54.Best A, La Ragione RM, Sayers AR, Woodward MJ. 2005. Role for flagella but not intimin in the persistent infection of the gastrointestinal tissues of specific-pathogen-free chicks by Shiga toxin-negative Escherichia coli O157:H7. Infect Immun 73:1836–1846. doi: 10.1128/IAI.73.3.1836-1846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boudvillain M, Figueroa-Bossi N, Bossi L. 2013. Terminator still moving forward: expanding roles for Rho factor. Curr Opin Microbiol 16:118–124. doi: 10.1016/j.mib.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Zimmer DP, Soupene E, Lee HL, Wendisch VF, Khodursky AB, Peter BJ, Bender RA, Kustu S. 2000. Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc Natl Acad Sci U S A 97:14674–14679. doi: 10.1073/pnas.97.26.14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wenner N, Maes A, Cotado-Sampayo M, Lapouge K. 2014. NrsZ: a novel, processed, nitrogen-dependent, small noncoding RNA that regulates Pseudomonas aeruginosa PAO1 virulence. Environ Microbiol 16:1053–1068. doi: 10.1111/1462-2920.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallarato LA, Sánchez DG, Olvera L, Primo ED, Garrido MN, Beassoni PR, Morett E, Lisa AT. 2014. Exopolyphosphatase of Pseudomonas aeruginosa is essential for the production of virulence factors, and its expression is controlled by NtrC and PhoB acting at two interspaced promoters. Microbiology (Reading) 160:406–417. doi: 10.1099/mic.0.074773-0. [DOI] [PubMed] [Google Scholar]
- 59.Alford MA, Baghela A, Yeung ATY, Pletzer D, Hancock REW. 2020. NtrBC regulates invasiveness and virulence of Pseudomonas aeruginosa during high-density infection. Front Microbiol 11:773. doi: 10.3389/fmicb.2020.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim HS, Park SJ, Lee KH. 2009. Role of NtrC-regulated exopolysaccharides in the biofilm formation and pathogenic interaction of Vibrio vulnificus. Mol Microbiol 74:436–453. doi: 10.1111/j.1365-2958.2009.06875.x. [DOI] [PubMed] [Google Scholar]
- 61.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao WD, Liu DX, Wei JY, Miao ZW, Zhang K, Su ZK, Zhang XW, Li Q, Fang WG, Qin XX, Shang DS, Li B, Li QC, Cao L, Kim KS, Chen YH. 2018. Caspr1 is a host receptor for meningitis-causing Escherichia coli. Nat Commun 9:2296. doi: 10.1038/s41467-018-04637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knutton S, Baldwin T, Williams PH, McNeish AS. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun 57:1290–1298. doi: 10.1128/IAI.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beckham KSH, Connolly JPR, Ritchie JM, Wang D, Gawthorne JA, Tahoun A, Gally DL, Burgess K, Burchmore RJ, Smith BO, Beatson SA, Byron O, Wolfe AJ, Douce GR, Roe AJ. 2014. The metabolic enzyme AdhE controls the virulence of Escherichia coli O157:H7. Mol Microbiol 93:199–211. doi: 10.1111/mmi.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang L, Feng L, Yang B, Zhang W, Wang P, Jiang X, Wang L. 2017. Signal transduction pathway mediated by the novel regulator LoiA for low oxygen tension induced Salmonella Typhimurium invasion. PLoS Pathog 13:e1006429. doi: 10.1371/journal.ppat.1006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morita T, Maki K, Aiba H. 2012. Detection of sRNA-mRNA interactions by electrophoretic mobility shift assay. Methods Mol Biol 905:235–244. doi: 10.1007/978-1-61779-949-5_15. [DOI] [PubMed] [Google Scholar]
- 67.Emms DM, Kelly S. 2015. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol 16:157. doi: 10.1186/s13059-015-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EsrF expression, verification, and dependency on Hfq. (A) Transcription of the esrF-glnA region as observed in transcriptome data. EsrF appears to be transcribed from its promoter. (B) 5′- and 3′-RACE gel photographs of EsrF cloned into the T vector amplified before sequencing. For 5′-RACE, the size of the product was 130-bp (85-bp esrF CDS + 45-bp adapter). For 3′-RACE, the size of product was 250-bp (85-bp esrF CDS + 30-bp adapter + 135-bp T vector). (C) Sequencing reads recovered from Hfq UV-induced RNA-protein crosslinking and analysis of cDNA by high-throughput sequencing (CRAC) that map to esrF (top) and deletions recovered within sequencing reads (below). The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S1, TIF file, 2.4 MB (2.4MB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
EsrF positively regulates flhB transcription. (A) qRT-PCR quantification of flhB expression in WT, ΔesrF, ΔesrF+PesrF, Δhfq, and Δhfq+Phfq strains. (B) Northern blotting quantification of flhB mRNA in WT, ΔesrF, and ΔesrF+PesrF strains. (C) Quantification of shifted flhB mRNA in RNA-RNA EMSA reactions. The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S2, TIF file, 1.4 MB (1.4MB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) TargetRNA2 used to predict 53 putative target genes of EsrF. (B) Differently expressed genes of the O157 ΔesrF transcriptome. Download Table S1, DOCX file, 0.05 MB (47.1KB, docx) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
EsrF and flhB regulate O157 motility and flagella synthesis. (A) Growth radii of WT, ΔesrF, ΔesrF+PesrF, ΔflhB, ΔflhB+PflhB, and ΔesrF ΔflhB strains in motility assays. (B) Percentages of flagella numbers/20 cells of WT, ΔesrF, ΔesrF+PesrF, ΔflhB, ΔflhB+PflhB, and ΔesrF ΔflhB strains. The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S3, TIF file, 0.9 MB (932.4KB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The upstream gene ntrC negatively regulates EsrF expression in high ammonium environment. (A) The five previously known NtrC binding sites and potential NtrC binding sites located upstream of esrF were aligned using the MUltiple Sequence Comparison by Log-Expectation (MUCLE) program in Unipro Ugene software, with the “gCaC-at+aTggtGc” consensus sequence. (B) Quantification of EsrF abundance in WT and ΔntrC after treatment with 3 mM (low ammonium) and 24 mM (high ammonium) NH4Cl. (C) qRT-PCR performed to detect gfp expression to represent the esrFp and flhBp expression of the ΔntrC mutant in the colon in vivo and LB medium-cultured samples. rfp was used as the internal control. The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S4, TIF file, 2.0 MB (2MB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ntrC negatively regulates glnA expression in different ammonium environments. (A) Expression of glnA in WT, ΔntrC, and ΔntrC+PntrC strains analyzed using qRT-PCR. (B) Expression of glnA in WT with different concentrations of NH4Cl analyzed using qRT-PCR. (C) Expression of glnA in the ΔntrC mutant with different concentrations of NH4Cl analyzed using qRT-PCR. The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S5, TIF file, 1.3 MB (1.3MB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
EsrF-flhB mRNA interaction increases flhB mRNA abundance related to relieved Rho-termination. (A) Transcriptomic coverage plots for flhB 5′ UTR before (up) and after (down) treatment with bicyclomycin (BCM). (B) Transcription of flhB ORF in O157 WT without BCM (WT-BCM) or with BCM (WT+BCM) treatment (15 min, 50 μg/ml) analyzed using qRT-PCR. The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S6, TIF file, 1.7 MB (1.7MB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
EsrF is widely found in pathogenic Escherichia coli. (A) Maximum-likelihood tree constructed in PhyML based on 1,112 single-copy core genes shared by 226 E. coli strains. The strains in different clades containing esrF are shown in different colors. (B) flhB expression compared between three representative pathogenic E. coli strains, G1352 (EHEC O26:H11), G4943 (EPEC O55:H7), and G1474 (EPEC O86:H34) with ΔesrF ortholog mutants. The data are presented as means ± the SD (ns, no significance; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). All P values were calculated using a Student t test. Download FIG S7, TIF file, 2.1 MB (2.1MB, tif) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Prevalence of esrF among different E. coli strains. Download Table S2, DOCX file, 0.02 MB (20.8KB, docx) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Bacterial strains, plasmids, and primers used in this study. Download Table S3, DOCX file, 0.03 MB (30.1KB, docx) .
Copyright © 2021 Jia et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.