Significance
Psychedelic compounds, such as psilocybin, have beneficial actions in several psychiatric diseases. They also produce strong alterations of consciousness, which may be a barrier to their widespread use. We found that psilocybin has fast-acting antidepressant-like properties in mice. Using multiple assays of hedonic behavior and an antagonist of prohallucinatory 5-HT2A receptors, we further suggest that altered perception may not be necessary for its therapeutic actions. We also showed that psilocybin strengthens connections between brain cells in regions important for processing rewards and emotions. These experiments suggest that it may be possible to retain the beneficial actions of psilocybin while minimizing the alterations in consciousness, thereby accelerating its use in the clinic.
Keywords: depression, hallucination, hallucinogen, psychedelic, serotonin
Abstract
Depression is a widespread and devastating mental illness and the search for rapid-acting antidepressants remains critical. There is now exciting evidence that the psychedelic compound psilocybin produces not only powerful alterations of consciousness, but also rapid and persistent antidepressant effects. How psilocybin exerts its therapeutic actions is not known, but it is widely presumed that these actions require altered consciousness, which is known to be dependent on serotonin 2A receptor (5-HT2AR) activation. This hypothesis has never been tested, however. We therefore asked whether psilocybin would exert antidepressant-like responses in mice and, if so, whether these responses required 5-HT2AR activation. Using chronically stressed male mice, we observed that a single injection of psilocybin reversed anhedonic responses assessed with the sucrose preference and female urine preference tests. The antianhedonic response to psilocybin was accompanied by a strengthening of excitatory synapses in the hippocampus—a characteristic of traditional and fast-acting antidepressants. Neither behavioral nor electrophysiological responses to psilocybin were prevented by pretreatment with the 5-HT2A/2C antagonist ketanserin, despite positive evidence of ketanserin’s efficacy. We conclude that psilocybin’s mechanism of antidepressant action can be studied in animal models and suggest that altered perception may not be required for its antidepressant effects. We further suggest that a 5-HT2AR–independent restoration of synaptic strength in cortico-mesolimbic reward circuits may contribute to its antidepressant action. The possibility of combining psychedelic compounds and a 5-HT2AR antagonist offers a potential means to increase their acceptance and clinical utility and should be studied in human depression.
Psychedelic substances have been used by humans for millennia for spiritual and medicinal purposes (1). Clinical research has recently begun to provide evidence supporting their use as therapeutics for numerous neuropsychiatric disorders, including obsessive-compulsive disorder, posttraumatic stress disorder, and treatment-resistant depression (TRD) (2). A single administration of psilocybin, for example, was recently shown to significantly improve patient-reported depression scores after 1 wk, with improvements persisting for up to 6 mo (3, 4). The FDA has since given psilocybin a fast-track designation for depression, and additional clinical trials are underway. While otherwise safe (5), psilocybin-induced alterations in sensory perception and consciousness are a significant barrier to its widespread utilization, necessitating a costly 6 to 8 h of full-time psychological support in an inpatient setting during administration. These barriers would be greatly reduced if the psychedelic response could be diminished without impairing the antidepressant response.
Psilocin, the active metabolite of psilocybin, is a potent agonist at almost all 5-HTRs, with affinities ranging from 3 to 500 nM (6), comparable to serotonin. In humans, the intensity of psilocybin-induced perceptual changes is correlated with serotonin 2A receptor (5-HT2AR) activation (7). Blocking 5-HT2A/2CRs with ketanserin eliminates self-reported psychedelic perceptual distortions (8). There is a widespread expectation that these psilocybin-induced alterations in consciousness are essential to the antidepressant response (2, 9, 10); however, this hypothesis has never been tested clinically or preclinically. While acute psilocybin-induced changes in processing of negative emotional stimuli in healthy subjects are prevented by ketanserin (11), it is possible that psilocybin relieves depressive symptoms through rapid activation of some other critical 5-HTRs.
Advancement of psychedelic drugs as treatments for neuropsychiatric disorders, both in terms of drug development and increased societal acceptance, would be facilitated by a better mechanistic understanding of how they exert their therapeutic effects. Although the actions of psychedelics have been well characterized in a number of mouse behaviors, including head twitches (12) and escape behaviors (13, 14), the efficacy of psychedelics has not been sufficiently studied in well-validated animal models of neuropsychiatric disorders with phenotypic symptoms relevant to their therapeutic actions. In particular, evidence about the critical serotonin receptors required for the beneficial actions of psilocybin is lacking, as is mechanistic evidence of biological changes in the brain that accompany relief of depressive symptoms.
Human depression results from a combination of genetic susceptibility and environmental factors, such as stress. Anhedonia, the inability to experience pleasure from previously enjoyable activities, is a core symptom of depression. Like human depression, various forms of chronic stress induce an anhedonic state in rodents, which is characterized by attenuated behavioral responses to previously rewarding stimuli (15). Importantly, responses to rewarding stimuli in stressed animals are restored by compounds that have antidepressant efficacy in humans, including fast- and slow-acting compounds such as ketamine and selective serotonin reuptake inhibitors (SSRIs), when administered acutely and chronically, respectively (16).
We tested the hypothesis that psilocybin exerts an antidepressant-like response in chronically stressed mice and, using the same experimental design as employed in human studies of psilocybin and ketanserin, asked whether 5-HT2R activation is necessary for this response. We observed that psilocybin exerted rapid antianhedonic actions in chronically stressed mice, that this response was accompanied by a strengthening of excitatory synapses in the hippocampus, and that neither of these effects were blocked by ketanserin.
Results
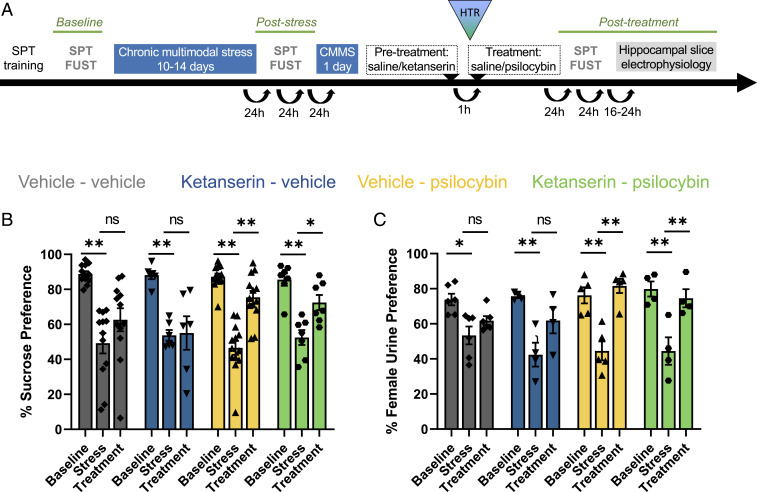
We exposed 8-wk-old male C57BL/6J mice to a chronic multimodal stress paradigm (CMMS) and assayed hedonic state with two well characterized appetitive choice tasks involving different senses: a two-bottle sucrose preference test (SPT), comparing consumption of a 1% sucrose solution and water, and a female urine sniffing test (FUST), comparing interactions with swabs dipped in urine from male mice and female mice in estrous (Fig. 1A). Mice displayed strong preferences for the sucrose solution and for female urine at baseline and significant decreases in both sucrose and female urine preferences after 10 to 14 consecutive days of CMMS (Fig. 1 B and C). Mice were then given a single intraperitoneal (i.p.) injection of psilocybin (1 mg/kg). Mice displayed a significant restoration of their preference for sucrose solution and female urine 24 to 48 h after psilocybin injection, whereas mice given a saline vehicle injection retained low sucrose and female urine preferences. Stress-resilient mice that did not display loss of sucrose preference after CMMS did not display any significant change in their responses after psilocybin injection (SI Appendix, Fig. S1). These data represent evidence of a rapid antianhedonic response to psilocybin in a stress-induced preclinical model of depression-relevant behaviors.
Fig. 1.
Restoration of hedonic behavior after chronic stress by psilocybin is unaffected by ketanserin. (A) Experimental timeline illustrating when hedonic behaviors were measured in relation to CMMS and drug treatment. (B) CMMS significantly decreased sucrose preference (SP) compared to baseline across all treatment groups: vehicle–vehicle (gray; P = 0.0012; n = 12), ketanserin–vehicle (blue; P = 0.0012; n = 6), vehicle–psilocybin (yellow; P = 0.0012; n = 13), and ketanserin–psilocybin (green; P = 0.0012; n = 7). Treatment with psilocybin (1 mg/kg, i.p.) significantly increased SP compared to values after CMMS, whether animals were pretreated with ketanserin (2 mg/kg; P = 0.042) or a vehicle control (P = 0.0012). Neither injection with vehicle (P = 0.078) nor ketanserin alone (P = 0.87; n = 6) had a significant effect on SP following CMMS. Three-way repeated-measures ANOVA revealed a significant effect of stress (F2,68 = 60.26, P < 0.0001) and interaction of Stress × Psilocybin (F2,68 = 4.50, P = 0.015) but not for Stress × Psilocybin × Ketanserin (F2,68 = 0.05917, P = 0.9426). (C) CMMS significantly decreased preference for female urine compared to baseline: vehicle–vehicle (P = 0.013; n = 6), ketanserin–vehicle (P = 0.0012; n = 4), vehicle–psilocybin (P = 0.0012; n = 5), and ketanserin–psilocybin (P = 0.0012; n = 4). Treatment with psilocybin significantly increased preference for the scent of female urine compared to values after CMMS whether animals were pretreated with ketanserin (P = 0.0024) or vehicle (P = 0.0012). Following CMMS, neither injection with vehicle (P = 0.43) nor ketanserin alone (P = 0.072) had a significant effect on female urine preference. Stress significantly reduced female urine preference in all groups (F2,30 = 43.41, P < 0.0001), and a three-way repeated-measures ANOVA showed a significant interaction between Stress × Psilocybin (F2,30 = 4.26, P < 0.024) but not between Stress × Psilocybin × Ketanserin (F2,30 = 0.8677, P = 0.4302). The figure bars represent the group means ± SEM. The reported post hoc comparisons were corrected with the Holm-Sidak method. *P < 0.05; **P < 0.005; ns, not significant.
We also asked whether activation of prohallucinatory 5-HT2Rs was necessary for the antidepressant-like response to psilocybin. Pretreatment with the 5-HT2A/5-HT2C antagonist ketanserin attenuates psilocybin-induced perceptual alterations in humans (11). In mice, 5-HT2ARs are required for psilocybin-induced head twitching (12, 17). We therefore gave stress-susceptible mice an injection of ketanserin (2 mg/kg, i.p.), as shown to be effective in previous rat behavioral studies (18), followed 1 h later by psilocybin (1 mg/kg, i.p.) or vehicle (0.9% saline). Psilocybin significantly increased sucrose and female urine preferences following stress in ketanserin-pretreated mice (Fig. 1 B and C), whereas ketanserin pretreatment alone had no significant effect on either behavior.
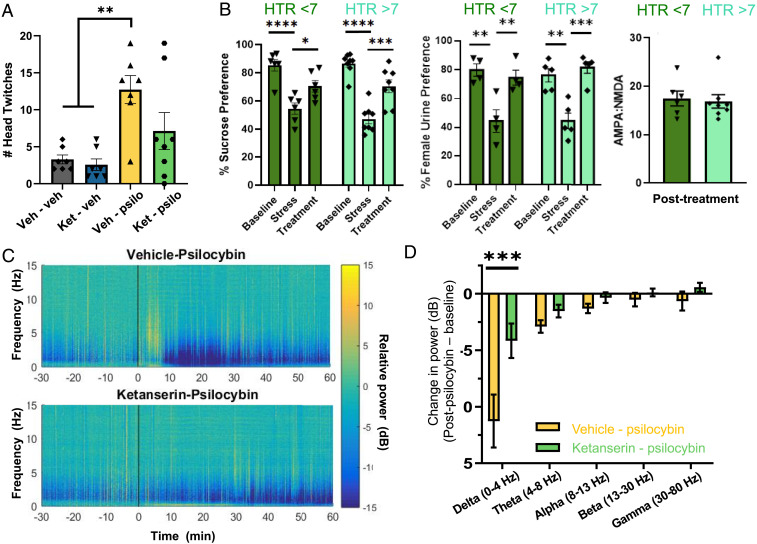
Vehicle-pretreated mice receiving psilocybin demonstrated significantly more head twitching compared to mice receiving vehicle or ketanserin alone, serving as a behavioral indication of 5-HT2AR activation by psychedelic compounds (17). Increases in head-twitching counts in mice given psilocybin alone were comparable to previous reports in mice (from ca. 1 twitch/3 to 4 min to 1 twitch/min) (19). Head-twitching counts were not significantly different in ketanserin-pretreated mice receiving psilocybin compared to mice receiving vehicle alone (Fig. 2A), indicating that ketanserin had sufficiently blocked 5-HT2ARs during psilocybin administration.
Fig. 2.
Pretreatment with ketanserin effectively blocks 5-HT2A activation. (A) Pretreatment with ketanserin (2 mg/kg) 60 min prior to psilocybin (1 mg/kg) administration prevents head twitching during the 15 min immediately following drug injection in mice (ket-psil versus ket-veh, P = 0.26). The difference between groups (F3,25 = 6.96, P = 0.0015) was driven by the increase in head twitching seen in vehicle–psilocybin animals (veh-veh versus veh-psil, P = 0.0045). (B) In animals treated with psilocybin (n = 14), both with and without ketanserin, there was no effect of head twitching (F1,12 = 0.53, P = 0.48) but a significant effect of time (F2,24 = 38.90, P < 0.0001), revealing significant differences between stress and posttreatment SPT in both high head twitching (P = 0.0005) and low head twitching (P = 0.038) mice. Of these also used in the FUST (n = 9), there was no effect of head twitching (F1,7 = 0.1040, P = 0 0.7565) but a significant effect of time (F2,14 = 23.05, P < 0.0001), showing a significant difference in female urine preferences between high (P = 0.0007) and low (P = 0.0086) head twitching mice between poststress and posttreatment timepoints. A two-tailed, unpaired t test comparing AMPA/NMDA ratios between high (n = 8) and low (n = 6) head twitching mice found no difference between groups (P = 0.7717). (C) In a separate cohort of animals, psilocybin (10 mg/kg, n = 4) acutely reduced hippocampal local field potential activity in the low-frequency range, with a peak effect around 15 to 25 min following psilocybin injection. This effect was inhibited by pretreatment with ketanserin (2 mg/kg, n = 4). (D) Pretreatment with ketanserin significantly attenuated psilocybin-induced reductions in the delta frequency band 15 to 25 min following psilocybin injection. Two-way ANOVA revealed significant interaction of Frequency × Ketanserin (F4,30 = 3.66, P = 0.0152), and a post hoc analysis revealed a significant difference between Vehicle–Psilocybin and Ketanserin–Psilocybin in the delta band (t = 4.99, df = 30, P = 0.00012). The figure bars represent the group means ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001.
Psilocybin-induced increases in the number of head twitches require 5-HT2AR activation (12, 17), as shown above. To determine whether psilocybin’s antianhedonic actions resulted from a possible incomplete block of 5-HT2ARs, we did a post hoc separation of mice given psilocybin with or without ketanserin into a high head twitch group (>7/15 min, which is greater than any of the control mice, demonstrating 5-HT2AR activation), and a low head twitching group with little or no increase in head-twitches (<7/15 min, comparable to control mice) and thus displaying little or no evidence of 5-HT2AR activation. Significant improvements in both sucrose and female urine sniffing preferences were observed in mice having both few and many head twitches (Fig. 2B), consistent with our conclusion that the restoration of reward behavior by psilocybin was independent of 5-HT2AR activation. As in previously published reports (6, 20), pretreatment with ketanserin induced hypolocomotion, directly and/or by enhancing the effects of psilocybin (SI Appendix, Fig. S2), confirming its activity at the given dose.
In humans, psilocybin decreases low-frequency electroencephalographic oscillations in cortical and limbic areas via activation of ketanserin-sensitive 5-HT2ARs (21). Similarly, another 5-HT2AR selective psychedelic compound, 2,5-dimethoxy-4-iodoamphetamine, reduces low-frequency oscillatory local field potentials in the prefrontal cortex of anesthetized rats (22). We recorded local field potentials (LFPs) in hippocampal area CA1 in anesthetized mice in vivo and observed that a high concentration of psilocybin (10 mg/kg, i.p.) decreased the power of oscillations in the delta frequency band (0 to 4 Hz) within 15 min, an effect that lasted for >60 min (Fig. 2 B and C). We therefore used this decrease in LFP delta activity to ask whether our ketanserin preinjection protocol was effective in blocking 5-HT2ARs while psilocybin was active. We observed that ketanserin pretreatment greatly attenuated the decrease in LFP delta power in response to this high concentration of psilocybin, providing another strong positive control of its efficacy as a 5-HT2AR antagonist in the hippocampus under our experimental conditions. These results thus strengthen our conclusion that the antianhedonic actions of psilocybin in chronically stressed mice do not require 5-HT2AR activation.
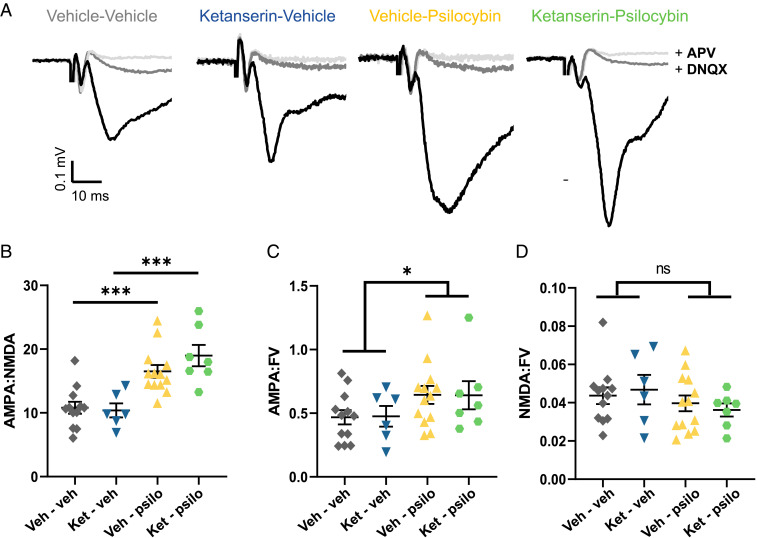
What mechanisms underlie the antidepressant-like behavioral response to psilocybin? A common element linking stress with the therapeutic actions of antidepressants is their shared, but opposing, effects on excitatory synapses. Chronic stress exerts deleterious effects on excitatory synaptic structure and function in multiple brain regions that are associated with cognition, reward, emotion, and motivation to work for reward (16, 23–25). In contrast, antidepressants promote restoration of excitatory synaptic transmission in reward circuits after chronic stress (16, 23–25) and restoration of functional connectivity in humans (26). 5-HT2ARs are expressed at high levels in the cortico-mesolimbic reward circuitry, including the cerebral cortex, ventral striatum, and hippocampus (27, 28). Psychedelic compounds exert acute electrophysiological effects via 5-HT2AR activation in the cortex and hippocampus (29, 30). There is anatomical evidence that psilocybin may promote synaptic connectivity in the prefrontal cortex in stress-naive mice via a 5-HT2R–dependent mechanism (31). We therefore examined whether restoration of hedonic state by psilocybin was accompanied by a potentiation of excitatory synaptic strength, quantified as the ratio of the components of the field excitatory postsynaptic potential mediated by GluA (AMPA) and GluN (NMDA) receptors. After completion of the behavioral assays in Fig. 1, hippocampal brain slices were taken from the animals for extracellular recordings. AMPA/NMDA ratios were measured at the archetypical stress-sensitive excitatory synapse formed by temporoammonic inputs to the distal dendrites of CA1 pyramidal cells (TA-CA1; Fig. 3A) (24). AMPA/NMDA ratios in slices taken from psilocybin-injected CMMS-susceptible mice were significantly greater than those in slices taken from CMMS-susceptible animals injected with vehicle or ketanserin alone (Fig. 3B). Normalization of the individual components of the synaptic responses across slices to their fiber volley amplitudes revealed a greater amplitude of the AMPAR-mediated component of the response (Fig. 3C) and no significant change in the NMDAR-mediated component (Fig. 3D) in mice treated with psilocybin, consistent with the effects of chronically administered fluoxetine described previously (24). These results demonstrate that a single psilocybin administration in rodents promotes persistent synaptic strengthening in a depression-relevant brain region days after its elimination from the body (hours), much like the persistent effects of psilocybin on human brain functional connectivity (32).
Fig. 3.
Psilocybin strengthens hippocampal TA-CA1 synapses following CMMS. (A) Example field EPSPs (fEPSPs) from a single stimulation intensity from one hippocampal slice per group, recorded in Mg2+-free ACSF (black), after wash-in of DNQX (50 µM; dark gray) and then APV (80 µM; light gray) to isolate AMPA- and NMDAR-mediated components. (B) Mice subjected to CMMS and treated with psilocybin had higher AMPA/NMDA ratios compared to stressed mice given only vehicle (gray, n = 12) or ketanserin (blue, n = 7), regardless of whether they were pretreated with ketanserin (green, n = 7; P = 0.0002) or vehicle (yellow, n = 13; P = 0.0003). Two-way ANOVA showed a significant effect of psilocybin (F1,34 = 34.79, P < 0.0001) but not ketanserin × psilocybin (F1,34 = 1.422, P = 0.2414). (C) Psilocybin increased the AMPA/FV ratio of the fEPSP (two-way ANOVA: F1,34 = 4.378, P = 0.044). (D) Treatment with psilocybin did not change the NMDA/FV ratio of the fEPSP (two-way ANOVA: F1,34 = 2.077, P = 0.16). AMPA/NMDA, AMPA/FV and NMDA/FV for each animal is shown along with group means ± SEM; *P < 0.05; ***P < 0.0005; ns, not significant.
Consistent with the behavioral results, pretreatment with ketanserin did not impair the ability of psilocybin to restore AMPA/NMDA ratios (Fig. 3B). AMPA/NMDA ratios were significantly higher in slices taken from CMMS animals given psilocybin compared to vehicle, regardless of ketanserin pretreatment. As for the behavioral response to psilocybin, AMPA/NMDA ratios in slices from mice given psilocybin with and without ketanserin were elevated to the same extent in mice showing few and many head twitches (Fig. 2B). There was a significant positive correlation between AMPA/NMDA ratios in slices from a given mouse and its performance in the SPT (SI Appendix, Fig. S3). We thus conclude that both the antianhedonic behavioral response and the hippocampal synaptic response to psilocybin in mice are independent of 5-HT2R activation.
Delayed effects of psilocybin in the forced swim test have been reported previously in Wistar-Kyoto rats, but not Flinders Sensitive or Resilient rats (13, 33). Although female mice do not show a robust anhedonic response to chronic stress, they do respond to the fast-acting antidepressant ketamine in the forced swim test (34). We therefore tested the effects of psilocybin in the forced swim test in unstressed male or female C57BL/6J mice. We found no effect of psilocybin (1mg/kg) on immobility time at 1, 3, or 7 d postinjection (SI Appendix, Fig. S4), however. Future studies evaluating antidepressant-like effects of psilocybin in female mice are needed when robust stress-sensitive endpoints are identified.
Discussion
Our results provide evidence that psilocybin exerts a rapid beneficial action in well-studied and well-validated models of chronic stress-induced deficits in depression-relevant hedonic behaviors. Although depression is a uniquely human disease, findings from animal experiments can provide insights into psilocybin’s mechanisms of action that are challenging to obtain in humans, such as receptor pharmacology. Indeed, our results recapitulate the rapid antidepressant actions of psilocybin in humans (3, 4) and alterations in brain functional connectivity that outlast the presence of the drug, as reported previously (32). Although psilocybin has been reported to have delayed antidepressant-like effects in the forced swim test in some, but not all, strains of rats (13, 33), we were unable to replicate this finding in mice.
Psychedelic compounds alter consciousness through activation of 5-HT2ARs. The prevailing view in developing psychedelic compounds for psychiatry is that the mind-altering effects of these compounds contribute to, or are responsible for, the therapeutic benefits (2, 9, 10). This assumption has never been tested, however. Our test of this hypothesis requires that the pretreatment with ketanserin sufficiently attenuated the activation of 5-HT2ARs at the time we administered psilocybin. We present two lines of evidence that indicate this is true. We observed that ketanserin blocked 5-HT2AR–dependent head twitching (Fig. 2A) and 5-HT2AR–dependent decreases in low-frequency oscillatory activity (Fig. 2 C and D). Our preclinical results suggest that 5-HT2ARs, and thus psychedelic responses, may not be required for an antidepressant response to psilocybin. We also found that mice with little or no evidence of psilocybin-induced head twitches, indicative of a lack of 5-HT2AR activation (12, 17), still exhibited a robust psilocybin-induced antianhedonic effect (Fig. 2B). Our preclinical results therefore suggest that 5-HT2ARs, and thus psychedelic responses in humans, may not be required for an antidepressant response to psilocybin, although that can only be definitively established with tests in human TRD. We cannot exclude that 5-HT2AR activation is required for some antidepressant activity at postpsilocybin time points longer than the 24 to 48 h we examined here. If 5-HT2AR activation is not necessary, then the combination of psilocybin and a 5-HT2R antagonist safe for human use, such as ketanserin, offers a potential means to eliminate, attenuate, or shorten the duration of psilocybin-induced alterations of perception while retaining its therapeutic benefits. This would reduce barriers hindering psilocybin’s widespread clinical use and perhaps increase its acceptance and utility. Use of nonhallucinogenic derivatives of psychedelic compounds (14) is another potential approach, although an antianhedonic response to these compounds has not yet been demonstrated.
Resetting synaptic strength within cortico-mesolimbic circuits responsible for integration of reward and emotion, such as those demonstrated here, could provide a neurobiological substrate for lasting improvements in psychological and cognitive processing. In this study, we found a significant correlation between synaptic strength and change in hedonic state after psilocybin. Indeed, human studies with healthy volunteers and TRD patients reveal persistent increases in functional connectivity in these same circuits after psilocybin administration (11, 32, 35). The 5-HTRs underlying this synaptic response to psilocybin remain to be determined, but their definition could reveal a strategy for developing alternatives to psilocybin that are biased toward synaptic strengthening relative to perceptual alteration. Our previous work has suggested that 5-HT1BRs contribute to the synaptic and behavioral antidepressant-like actions of SSRIs (36). It is possible that psilocin and novel ibogaine analogs, which both have a high affinity for 5-HT1BRs (6, 14), exert their beneficial actions through activation of 5-HT1BRs. Although animal models cannot provide insight into the potential synergistic effects of the psychedelic experience and traditional psychotherapy, the effectiveness of these interactions may be improved with a better preclinical understanding of the pharmacological and physiological basis of psilocybin’s actions.
Materials and Methods
All procedures were approved by the University of Maryland Baltimore Animal Use and Care Committee and were conducted in full accordance with the NIH Guide for the Care and Use of Laboratory Animals (37).
Animals.
Two cohorts of male C57BL/6J mice were bred in-house and used in the stress-induced anhedonia experimental protocols of this study. Separate cohorts of C57BL/6J mice from Jacksonville Laboratories were used to test ketanserin’s activity through in vivo electrophysiology and locomotion effects. All mice were 8 wk old at the start of the experiment, kept on a 12 h light/dark cycle (lights on at 7 AM), and provided food and water ad libitum. Animals were group housed prior to the experiment but single housed at the onset of the behavioral and stress protocols until the end of the study. Mice were assigned to balanced experimental and control groups based on hedonic behaviors assessed after stress.
Chronic Multimodal Stress.
CMMS was used to induce an anhedonic-like phenotype in the animals (15). The CMMS protocol consists of 4 h/d of restraint stress, in which mice were immobilized in appropriately sized plastic restraint tubes and exposed to strobe lighting and white noise to minimize habituation, for 10 to 14 consecutive days. Stress was initiated in the morning hours, between 9 and 10 AM, near the onset of the animals’ light cycle. Following stress, rodents were returned to their home cages and singly housed.
Hedonic Behavior.
Hedonic state was assessed using the SPT and FUST prior to stress (baseline), after 10 to 14 d of CMMS, and 24 h after drug injection (Fig. 1A). For the SPT, mice were trained to the presence of a 2% sucrose solution in their home cages prior to the baseline measurement. The baseline measurements began 1 d later. On each test day, one bottle containing tap water and another bottle containing a 1% sucrose solution were placed in the cages 1 to 2 h prior to the onset of the animal’s dark cycle. Mice were free to consume liquid from either bottle for 14 to 16 h, after which bottles were weighed to measure consumption and replaced. The procedure was repeated for a second night with the position of the bottles reversed. Preference is expressed as a percentage and was calculated for each night as (the weight of 1% sucrose solution consumed/total liquid weight consumed) × 100, and the preferences for the two nights were averaged.
For the FUST, mice were individually transferred to empty, freshly made cages and allowed to habituate for 15 min. A fresh single cotton swab was then affixed to the rim of the cage, such that the tip was within reach of the mouse. One hour later, the swab was removed and replaced with two swabs spaced apart at the same end of the cage, one soaked in freshly collected urine from male mice and the other with urine from female mice in estrous. Video recording was started and animals were given 3 min to interact with the swabs. Videos were later scored by a trained experimenter blinded to the position of male and female urine swabs. Time spent sniffing each swab was recorded and percent preference scored as (time spent sniffing the female urine swab/total time spent sniffing both swabs) × 100. The position of the female urine swab was switched between timepoints to account for potential side preference.
As an a priori criteria for inclusion in the study, mice had to have a preference for sucrose of >65% at baseline (1 out of 54 mice excluded). Mice that displayed a decrease in sucrose preference to <70% following CMMS, representing >3 SDs less than our historical mean baseline sucrose preference for C57 mice (88.7 ± 7.4% [SD], n = 118 animals), were considered stress susceptible. Mice having a sucrose preference >70% after 14 d of CMMS were classified as resilient (15 out of 53 mice resilient). Of those mice, only those displaying a female urine preference of >65% at baseline (8 out of 29 mice excluded) and <70% following CMMS were included in the FUST arm of the study (2 out of 21 mice excluded).
Head Twitch Response.
Head twitch responses was quantified in a subset of mice exposed to CMMS and treated with vehicle–vehicle (n = 7), ketanserin–vehicle (n = 7), vehicle–psilocybin (n = 7), and ketanserin–psilocybin (n = 8). Immediately following the second injection of either vehicle or psilocybin, mice were placed in their home cage and video recording was initiated with a camera positioned directly above the cage. Three trained experimenters scored the first 0 to 15 min of activity for head twitches while blinded to treatment, noting the total number of and time stamp of each head twitch. Time stamps were compared across scorers and individual head twitches were defined as events detected by at least two of the three scorers.
Locomotion.
A separate cohort of 8-wk-old male mice were used to analyze the effects of psilocybin and ketanserin pretreatment on locomotion. As in the stress paradigm, mice were pretreated with ketanserin (2 mg/kg) or saline and returned to their home cage. Mice were injected with a high dose of psilocybin (5 mg/kg) known to induce hypolocomotion (6) 60 min after pretreatment. Following the psilocybin injection, animals were immediately placed in an open field arena, and their behavior was recorded. Locomotion was scored with TopScan Suite software (Clever Sys).
Forced Swim Test.
A separate cohort of 9-wk-old male (n = 20) and female (n = 20) mice were used for the forced swim test (FST). Animals were group housed and injected with vehicle or psilocybin (1 mg/kg). The first FST session occurred 24 h after injection. Briefly, each swim session consisted of a 6 min swim in a Plexiglas cylinder filled with 15 cm of water (23 to 25 °C). Each swim was recorded with a video camera. Immobility time was scored with ANY-maze (Stoelting) for the last 4 min of the 6 min swim, with 2 s of immobility set as the threshold within the program before scoring began.
In Vitro Electrophysiology.
Standard methods were used to prepare 400 µM thick hippocampal slices. Briefly, mice were euthanized via exposure to isoflurane followed by decapitation. Brains were excised, and the hippocampus was quickly dissected from the brain and sectioned on a Leica VT1200 series vibratome in ice-cold artificial cerebrospinal fluid (ACSF) bubbled with 95% O2/5% CO2. The ACSF contained: 124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1.5 mM MgSO4, 2.5 mM CaCl2, 26 mM NaHCO3, and 10 mM glucose. Slices were allowed to recover for a minimum of 60 min at room temperature in ACSF in a humidified interface chamber before recording.
Use of extracellular recording, rather than whole-cell recording, was chosen for quantification of AMPA/NMDA ratios because of the complications of stress-induced changes in dendritic structure and their electrotonic influence on recordings of distal TA-CA1 synapses. For quantification of AMPA/NMDA ratios, ACSF was prepared as stated but without MgSO4 to leave NMDA channels unblocked. Picrotoxin (100 µM) and CGP54626 (2 µM) were added to block GABAA and GABAB receptors, respectively. Slices were placed in a recording chamber and perfused with this ACSF (1 mL/min) for the duration of the experiment. Glass recording electrodes with resistance of 3 to 5 MΩ were prepared and filled with recording ACSF. These electrodes were placed in stratum lacunosum moleculare (SLM) of area CA1. Concentric bipolar tungsten electrodes were positioned in SLM at least 500 µM from the recording electrode to stimulate temporoammonic afferents (TA). Field excitatory postsynaptic potentials (fEPSPs) were acquired using Clampex software (pCLAMP 10 Series, Molecular Devices), amplified (×1,000, npi electronic), filtered (3 kHz), and digitized (10 kHz, Digidata 1440a, Molecular Devices). Slices were stimulated (100 µs) at 0.1 Hz at five different intensities ranging from 0.01 to 1.0 mA in order to collect a range of responses around a fiber volley (FV) of 0.1 mV. DNQX (50 µM) was then washed onto the slice for 15 min to block the AMPA component of the fEPSP and reveal the NMDA component. Five fEPSPs were again collected at the same stimulation intensities recorded prior to DNQX. The NMDAR antagonist D-APV (80 µM) was then washed onto the slice for 15 min to confirm that the fEPSP response remaining after DNQX was indeed NMDAR-mediated.
AMPA/NMDA ratios of the TA-CA1 fEPSPs were quantified as described previously (24) in order to provide a measure of synaptic strength across slices from different mice. All traces at each intensity were first averaged, and the amplitude of FVs was quantified. The AMPA component of the fEPSP was quantified as the slope over 1.5 ms at the earliest part of the linear portion of the response for each stimulation intensity, typically 0.1 to 2.0 ms from its initiation. The NMDA component of the fEPSP slope was quantified over 4 ms at the earliest point of the post-DNQX response fully eliminated by APV. Both AMPA and NMDA slopes were normalized to their respective FVs. For quantification, pairs of responses at the same stimulation intensities were chosen in which the response in the presence of DNQX was closest to 0.1 mV in amplitude. AMPA/NMDA ratios from each slice (1 to 6/mouse) were averaged to calculate each individual animal’s mean AMPA/NMDA. As an independent measure of synaptic strength, we also computed AMPA/FV and NMDA/FV ratios, calculated from the same pair of responses used for AMPA/NMDA ratios. Analysis occurred while blinded to the treatment condition, and values were confirmed by a second experimenter.
In Vivo Electrophysiology.
Anesthesia was induced using 3 to 4% isoflurane. Mice were placed in a stereotactic frame and anesthesia was maintained with continuous flow of 2 to 3% isoflurane. A Q4 silicon probe (Neuronexus Technologies, MI) was lowered into the pyramidal layer of the hippocampal CA1 region (AP: −1.8, ML: −1.0, DV: −1.0). Local field potentials (LFPs) were amplified (headstage 20× amplification, Plexon Instruments), digitized (Digidata 1322A, Axon Instruments), and recorded with Clampex 10.3 (Molecular Devices) with a sampling rate of 3 kHz and bandpass filtering between 1 and 300 Hz. Mice were injected with either saline or 2 mg/kg ketanserin (i.p.). LFP recordings were initiated 30 min after ketanserin injections. Baseline LFPs were recorded for 30 min before mice were injected with psilocybin (10 mg/kg, i.p.). LFPs were then recorded for another 90 min. Electrode placement was histologically confirmed at the end of the experiment.
Binary files were loaded into MATLAB 2015a (Mathworks) using abfload script (Forrest Collman: fcollman/abfload, https://github.com/fcollman/abfload). Power spectra were analyzed using Chronux 2.12 spectral analysis script (chronux.org) and normalized to baseline using custom MATLAB script.
Drug Treatment.
Psilocybin was obtained from Cayman Chemical and diluted to 1 mg/mL in sterile 0.9% saline. Psilocybin was prepared within 1 wk of administration and stored at −20 °C. Ketanserin (+)-tartrate salt was purchased from MilliporeSigma and also diluted to 1 mg/mL. Ketanserin was administered 60 min prior to injection with either vehicle control or psilocybin, consistent with previous studies of ketanserin’s ability to block hallucinogenic behavioral responses in humans (8) and rats (18). Psilocybin injections were given at 1 mg/kg and ketanserin at 2 mg/kg, consistent with previous rodent studies (13, 38), or equivalent volumes of saline. Each experimental animal received two injections to control for any effects of injection or handling.
For locomotion experiments or in vivo electrophysiology experiments, psilocybin was prepared at a 5 mg/mL concentration in sterile 0.9% saline and injected at 5 mg/kg or 10 mg/kg, respectively.
Statistics.
Statistical analysis consisted of Student’s t tests; one-, two-, and three-way ANOVAs using GraphPad Prism 8, with Tukey’s correction for multiple comparisons performed in Prism 8; or Holm-Sidak multiple-comparison corrections performed manually in Excel (Microsoft) for our comparisons of interest. The results from the two cohorts of animals were not statistically different and were therefore pooled. Statistical tests used are indicated within the figure legends. Where indicated, n = number of animals.
Supplementary Material
Acknowledgments
We thank Dr. Jonathan Fischell for performing preliminary experiments, Dr. Adam Puche for assistance with psilocybin, Dr. Dongil Keum for assistance with the in vivo electrophysiological recordings, Drs. Mary Kay Lobo and Hyungwoo Nam for assistance with the locomotion experiment and analysis, Drs. Todd Gould and Sarah Clark for assistance with the forced swim test, Dr. Adam Halberstadt (University of California, San Diego) for training in head twitch scoring, and Drs. Tara LeGates, Todd Gould, and Panos Zanos for their comments on the manuscript. This work was supported by NIH Grants R01 MH086828 (S.M.T.) and T32 GM092237.
Footnotes
Competing interest statement: The University of Maryland Baltimore has filed a provisional patent, in which S.M.T. is listed as an inventor on the use of psychedelics combined with 5-HT2R antagonists to treat psychiatric disease.
This article is a PNAS Direct Submission. S.E.A. is a guest editor invited by the Editorial Board.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2022489118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Nichols D. E., Psychedelics. Pharmacol. Rev. 68, 264–355 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nutt D., Erritzoe D., Carhart-Harris R., Psychedelic psychiatry’s brave new world. Cell 181, 24–28 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Carhart-Harris R. L., et al., Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology (Berl.) 235, 399–408 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis A. K., et al., Effects of psilocybin-assisted therapy on major depressive disorder: A randomized clinical trial. JAMA, 10.1001/jamapsychiatry.2020.3285 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson M. W., Griffiths R. R., Hendricks P. S., Henningfield J. E., The abuse potential of medical psilocybin according to the 8 factors of the controlled substances act. Neuropharmacology 142, 143–166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halberstadt A. L., Geyer M. A., Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 61, 364–381 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madsen M. K., et al., Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 44, 1328–1334 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vollenweider F. X., Vollenweider-Scherpenhuyzen M. F., Bäbler A., Vogel H., Hell D., Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9, 3897–3902 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Roseman L., Nutt D. J., Carhart-Harris R. L., Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front. Pharmacol. 8, 974 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaden D. B., Griffith R. R., The subjective effects of psychedelics are necessary for their enduring therapeutic effects. ACS Pharmacol. Transl. Sci., 10.1021/acsptsci.0c00194 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kometer M., et al., Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol. Psychiatry 72, 898–906 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Halberstadt A. L., Chatha M., Klein A. K., Wallach J., Brandt S. D., Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 167, 107933 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hibicke M., Landry A. N., Kramer H. M., Talman Z. K., Nichols C. D., Psychedelics, but not ketamine, produce persistent antidepressant-like effects in a rodent experimental system for the study of depression. ACS Chem. Neurosci. 11, 864–871 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Cameron L. P., et al., A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 589, 474–479 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willner P., The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 6, 78–93 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson S. M., et al., An excitatory synapse hypothesis of depression. Trends Neurosci. 38, 279–294 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halberstadt A. L., Koedood L., Powell S. B., Geyer M. A., Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol. 25, 1548–1561 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winter J. C., Rice K. C., Amorosi D. J., Rabin R. A., Psilocybin-induced stimulus control in the rat. Pharmacol. Biochem. Behav. 87, 472–480 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González-Maeso J., et al., Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53, 439–452 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Galvanho J. P., et al., Profiling of behavioral effects evoked by ketamine and the role of 5HT2 and D2 receptors in ketamine-induced locomotor sensitization in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 97, 109775–109788 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Kometer M., Schmidt A., Jäncke L., Vollenweider F. X., Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J. Neurosci. 33, 10544–10551 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celada P., Puig M. V., Díaz-Mataix L., Artigas F., The hallucinogen DOI reduces low-frequency oscillations in rat prefrontal cortex: Reversal by antipsychotic drugs. Biol. Psychiatry 64, 392–400 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Duman R. S., Sanacora G., Krystal J. H., Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 102, 75–90 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallarackal A. J., et al., Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses. J. Neurosci. 33, 15669–15674 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moda-Sava R. N., et al., Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 364, eaat8078 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans J. W., et al., Default mode connectivity in major depressive disorder measured up to 10 days after ketamine administration. Biol. Psychiatry 84, 582–590 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q. H., et al., Unique expression patterns of 5-HT2A and 5-HT2C receptors in the rat brain during postnatal development: Western blot and immunohistochemical analyses. J. Comp. Neurol. 469, 128–140 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Allen Institute for Brain Science , Allen mouse brain atlas. mouse.brain-map.org. Accessed 27 September 2020.
- 29.Wyskiel D. R., Andrade R., Serotonin excites hippocampal CA1 GABAergic interneurons at the stratum radiatum-stratum lacunosum moleculare border. Hippocampus 26, 1107–1114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambe E. K., Aghajanian G. K., Hallucinogen-induced UP states in the brain slice of rat prefrontal cortex: Role of glutamate spillover and NR2B-NMDA receptors. Neuropsychopharmacology 31, 1682–1689 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Ly C., et al., Psychedelics promote structural and functional neural plasticity. Cell Rep. 23, 3170–3182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrett F. S., Doss M. K., Sepeda N. D., Pekar J. J., Griffiths R. R., Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci. Rep. 10, 2214 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jefsen O., et al., Psilocybin lacks antidepressant-like effect in the Flinders Sensitive Line rat. Acta Neuropsychiatr. 31, 213–219 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Dossat A. M., Wright K. N., Strong C. E., Kabbaj M., Behavioral and biochemical sensitivity to low doses of ketamine: Influence of estrous cycle in C57BL/6 mice. Neuropharmacology 130, 30–41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carhart-Harris R. L., et al., Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci. Rep. 7, 13187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai X., et al., Local potentiation of excitatory synapses by serotonin and its alteration in rodent models of depression. Nat. Neurosci. 16, 464–472 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
- 38.Klein A.K.et al., Investigation of the structure–Activity relationships of psilocybin analogues. ACS Pharmacol. Translational Sci., 10.1021/acsptsci.0c00176 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.