Abstract
Background
Coenzyme Q10, or ubiquinone, is a non‐prescription nutritional supplement. It is a fat‐soluble molecule that acts as an electron carrier in mitochondria, and as a coenzyme for mitochondrial enzymes. Coenzyme Q10 deficiency may be associated with a multitude of diseases, including heart failure. The severity of heart failure correlates with the severity of coenzyme Q10 deficiency. Emerging data suggest that the harmful effects of reactive oxygen species are increased in people with heart failure, and coenzyme Q10 may help to reduce these toxic effects because of its antioxidant activity. Coenzyme Q10 may also have a role in stabilising myocardial calcium‐dependent ion channels, and in preventing the consumption of metabolites essential for adenosine‐5'‐triphosphate (ATP) synthesis. Coenzyme Q10, although not a primary recommended treatment, could be beneficial to people with heart failure. Several randomised controlled trials have compared coenzyme Q10 to other therapeutic modalities, but no systematic review of existing randomised trials was conducted prior to the original version of this Cochrane Review, in 2014.
Objectives
To review the safety and efficacy of coenzyme Q10 in heart failure.
Search methods
We searched CENTRAL, MEDLINE, Embase, Web of Science, CINAHL Plus, and AMED on 16 October 2020; ClinicalTrials.gov on 16 July 2020, and the ISRCTN Registry on 11 November 2019. We applied no language restrictions.
Selection criteria
We included randomised controlled trials of either parallel or cross‐over design that assessed the beneficial and harmful effects of coenzyme Q10 in people with heart failure. When we identified cross‐over studies, we considered data only from the first phase.
Data collection and analysis
We used standard Cochrane methods, assessed study risk of bias using the Cochrane 'Risk of bias' tool, and GRADE methods to assess the quality of the evidence. For dichotomous data, we calculated the risk ratio (RR); for continuous data, the mean difference (MD), both with 95% confidence intervals (CI). Where appropriate data were available, we conducted meta‐analysis. When meta‐analysis was not possible, we wrote a narrative synthesis. We provided a PRISMA flow chart to show the flow of study selection.
Main results
We included eleven studies, with 1573 participants, comparing coenzyme Q10 to placebo or conventional therapy (control). In the majority of the studies, sample size was relatively small. There were important differences among studies in daily coenzyme Q10 dose, follow‐up period, and the measures of treatment effect. All studies had unclear, or high risk of bias, or both, in one or more bias domains. We were only able to conduct meta‐analysis for some of the outcomes. None of the included trials considered quality of life, measured on a validated scale, exercise variables (exercise haemodynamics), or cost‐effectiveness.
Coenzyme Q10 probably reduces the risk of all‐cause mortality more than control (RR 0.58, 95% CI 0.35 to 0.95; 1 study, 420 participants; number needed to treat for an additional beneficial outcome (NNTB) 13.3; moderate‐quality evidence).
There was low‐quality evidence of inconclusive results between the coenzyme Q10 and control groups for the risk of myocardial infarction (RR 1.62, 95% CI 0.27 to 9.59; 1 study, 420 participants), and stroke (RR 0.18, 95% CI 0.02 to 1.48; 1 study, 420 participants).
Coenzyme Q10 probably reduces hospitalisation related to heart failure (RR 0.62, 95% CI 0.49 to 0.78; 2 studies, 1061 participants; NNTB 9.7; moderate‐quality evidence).
Very low‐quality evidence suggests that coenzyme Q10 may improve the left ventricular ejection fraction (MD 1.77, 95% CI 0.09 to 3.44; 7 studies, 650 participants), but the results are inconclusive for exercise capacity (MD 48.23, 95% CI ‐24.75 to 121.20; 3 studies, 91 participants); and the risk of developing adverse events (RR 0.70, 95% CI 0.45 to 1.10; 2 studies, 568 participants).
We downgraded the quality of the evidence mainly due to high risk of bias and imprecision.
Authors' conclusions
The included studies provide moderate‐quality evidence that coenzyme Q10 probably reduces all‐cause mortality and hospitalisation for heart failure. There is low‐quality evidence of inconclusive results as to whether coenzyme Q10 has an effect on the risk of myocardial infarction, or stroke. Because of very low‐quality evidence, it is very uncertain whether coenzyme Q10 has an effect on either left ventricular ejection fraction or exercise capacity. There is low‐quality evidence that coenzyme Q10 may increase the risk of adverse effects, or have little to no difference.
There is currently no convincing evidence to support or refute the use of coenzyme Q10 for heart failure. Future trials are needed to confirm our findings.
Plain language summary
Coenzyme Q10 for heart failure
Heart failure is a term used to describe the state that develops when the heart cannot maintain adequate cardiac output, or can do so only at the expense of overfilling the heart chambers. People with heart failure commonly experience a relapsing and remitting disease course, with periods of stability and episodes of decompensation (failure to cope with heart damage), leading to worsening symptoms that necessitate hospitalisation.
Treatment options for heart failure range from drugs to heart transplantation, with each having its own limitations. Coenzyme Q10 (or ubiquinone) has been suggested as a treatment option in some trials. Coenzyme Q10 is a non‐prescription nutritional supplement. It is a fat‐soluble molecule that has a role in energy production within the cells of the body. It may also have antioxidant properties.
Low levels of coenzyme Q10 may be related to the severity of heart failure. Coenzyme Q10 has been found in all tissues and organs in the body, with the highest concentrations in the heart. Emerging data have suggested that the harmful effects of reactive oxygen species (unstable molecules that contains oxygen and easily reacts with other molecules) are increased in people with heart failure. Because of its antioxidant activity, coenzyme Q10 may help to reduce these toxic effects, which damage the components of the cardiac cells, and disrupt cellular signalling. Coenzyme Q10 plays an important role in conducting signals within the heart muscle and in generating energy.The concentration of coenzyme Q10 has been inversely related to the severity of heart failure. Supplementation with coenzyme Q10 may improve heart failure. Coenzyme Q10 is sometimes used because it is thought to have an acceptable safety profile, with no significant side effects.
We conducted this review to assess the available evidence on the effects of coenzyme Q10 in people with heart failure. We included 11 randomised controlled trials, involving 1573 participants. They were relatively small, and followed up participants for a relatively short period of time. The analyses show that coenzyme Q10 probably reduces the risk of mortality from all causes, and hospitalisations due to heart failure. It may result in increased, or little or no difference in the risk of myocardial infarction, stroke, or adverse events. The effect of coenzyme Q10 on cardiac function and symptom improvement is uncertain.
The evidence, current to October 2020, is of a moderate quality at best, because of the high risk of bias in some of the included studies and the absence of precise and consistent results. There is currently no convincing evidence to support or refute the use of coenzyme Q10 for heart failure.
Summary of findings
Summary of findings 1. Coenzyme Q10 compared to placebo or conventional therapy for heart failure.
| Coenzyme Q10 compared to placebo or conventional therapy for heart failure | ||||||
| Patient or population: people with heart failure Setting: outpatient departments Intervention: coenzyme Q10 Comparison: placebo or conventional therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or conventional therapy | Risk with coenzyme Q10 | |||||
| All‐cause mortality follow‐up: 26 months | Study population | RR 0.58 (0.35 to 0.95) | 420 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Coenzyme Q10 probably reduces all‐cause mortality | |
| 179 per 1000 | 104 per 1000 (63 to 170) | |||||
| Myocardial infarction follow‐up: 26 months | Study population | RR 1.62 (0.27 to 9.59) | 420 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | The results for the effect of coenzyme Q10 on risk of myocardial infarction are inconclusive. | |
| 9 per 1000 | 15 per 1000 (2 to 88) | |||||
| Stroke follow‐up: 26 months | Study population | RR 0.18 (0.02 to 1.48) | 420 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | The results for the effect of coenzyme Q10 on risk of stroke are inconclusive. | |
| 28 per 1000 | 5 per 1000 (1 to 41) | |||||
| Hospitalisation for heart failure follow‐up: mean 19 months | Study population | RR 0.62 (0.49 to 0.78) | 1061 (2 RCTs) | ⊕⊕⊕⊝ Moderatec | Coenzyme Q10 probably reduces hospitalisation for heart failure. | |
| 276 per 1000 | 171 per 1000 (135 to 215) | |||||
| Left ventricular ejection fraction (%) follow‐up: mean 8 months | MD 1.77 higher (0.09 higher to 3.44 higher) | ‐ | 650 (7 RCTs) | ⊕⊝⊝⊝ Very lowd,e | The evidence is very uncertain about the effect of coenzyme Q10 on left ventricular ejection fraction (%). | |
| Exercise capacity (assessed with treadmill exercise test (duration in seconds)) follow‐up: mean 4 months | MD 48.23 higher (24.75 lower to 121.2 higher) | ‐ | 91 (3 RCTs) | ⊕⊝⊝⊝ Very lowf,g | The evidence is very uncertain about the effect of coenzyme Q10 on exercise capacity. | |
| Adverse events follow‐up: mean 16 months | Study population | RR 0.70 (0.45 to 1.10) | 568 (2 RCTs) | ⊕⊕⊝⊝ Lowb,h | The results for adverse events associated with coenzyme Q10 are inconclusive. | |
| 158 per 1000 | 111 per 1000 (71 to 174) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty. We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty. We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty. We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by one level due to indirectness. The findings are applicable only to the characteristics of participants and dosing regimen as included in this one study. bDowngraded by one level due to imprecision. The effect size has a very wide confidence interval that includes the possibilities of substantial harm, no difference, and a lower risk with coenzyme Q10. cDowngraded by one level due to risk of bias. The included study that contributed most weight to the analysis had unclear risk for selection, detection, and reporting biases, and high risk for attrition bias. dDowngraded by one level due to imprecision. The effect size has a very wide confidence interval that includes the possibility of only a minimal benefit with coenzyme Q10. eDowngraded by two levels due to substantial risk of bias. Within the 7 included studies, selection bias was unclear in 6, performance bias was high in 1, detection bias was unclear in 3 and high in 1, attrition bias was high in 4, reporting bias was unclear in 3 and high in 1, and other bias was high in 1. fDowngraded by two levels for imprecision. The effect size has a very wide confidence interval that includes the possibility of substantial harm, no difference, and a lower risk with coenzyme Q10. Also, the sample size is small. gDowngraded by one level due to risk of bias. The 3 included studies had unclear risk of selection and detection biases, and high risk of attrition bias. Two of them also had unclear risk of reporting bias. hDowngraded by one level due to indirectness. The findings are applicable only to the characteristics of participants and dosing regimen as included in the study that contributed the most weight to this analysis.
Background
Description of the condition
Heart failure is a term used to describe the state that develops when the heart cannot maintain adequate cardiac output, or can do so only at the expense of an elevated filling pressure (Boon 2006; Savarese 2017). Heart failure may be acute or chronic, and can be caused by a variety of conditions, including ischaemic heart disease (coronary artery disease), hypertension, elevated blood pressure, diseases of the heart valves, cardiomyopathy, and congenital heart diseases (Drexler 2004; Ho 1993; Richardson 1996; Teerlink 1991). The most commonly used classification system to quantify the degree of heart failure‐associated functional limitation was first developed by the New York Heart Association (NYHA; (NYHA 1964)). This system assigns people to one of four functional classes, depending on the degree of effort needed to elicit symptoms:
class I – symptoms of heart failure only at activity levels that would limit normal individuals;
class II – symptoms of heart failure with ordinary exertion;
class III – symptoms of heart failure with less than ordinary exertion;
class IV – symptoms of heart failure at rest.
It is now appreciated that heart failure often occurs with preserved left ventricular systolic function (Redfield 2016). Various studies estimate that as many as 40% to 60% of people with heart failure have diastolic dysfunction, as defined by a preserved left ventricular ejection fraction (Elesber 2001; Gottdiener 2002). Thus, heart failure can be classified as ejection fraction reduced, ejection fraction mid‐range, and ejection fraction preserved (Ponikowski 2016).
There are at least 26 million people who have heart failure worldwide (Savarese 2017). It is estimated that 6.2 million Americans older than 20 years of age had heart failure between 2013 and 2016, compared to 5.7 million between 2009 and 2012 (Virani 2020). The prevalence of heart failure in the USA is expected to increase from 2.42% in 2012 to 2.97% in 2030 (Virani 2020).
Chronic heart failure is a common condition, and is one of the most frequent causes of disability and admission to hospital in older individuals (Lippi 2020; Savarese 2017). The prevalence of heart failure increases with age, and is associated with high morbidity and mortality worldwide (Rodriguez 2004; Savarese 2017). Based on European data, the 12‐month all‐cause mortality rate was 17% of hospitalised people with stable heart disease, and 7% in ambulatory people with heart disease, despite major advances in drug treatments (Ponikowski 2016). American data showed even higher mortality rates (Virani 2020).
People with chronic heart failure commonly experience a relapsing and remitting disease course, with periods of stability and episodes of decompensation that lead to worsening symptoms that necessitate hospitalisation. The clinical picture depends on the nature of the underlying heart disease, the type of heart failure that it has evoked, and the neural and endocrine changes that have developed (Boon 2006).
Description of the intervention
Coenzyme Q10, or ubiquinone, is a non‐prescription nutritional supplement. It is a fat‐soluble molecule that acts as an electron carrier in mitochondria, and as a coenzyme for mitochondrial enzymes (Gutierrez‐Mariscal 2019; Raizner 2019). Coenzyme Q10 is obtained through both tissue synthesis and diet (Raizner 2019). Supplementary oral administration of coenzyme Q10 has been found to increase coenzyme Q10 levels in plasma, platelets, and white blood cells (Niklowitz 2007). Absorption of dietary coenzyme Q10 is slow and limited because of its hydrophobicity and large molecular weight. Solubilised coenzyme Q10 formulations show enhanced bioavailability, with a Tmax (time to reach maximum concentration) of approximately six hours, and an elimination half‐life of approximately 33 hours. Oral preparations of coenzyme Q10 are used in human therapeutics (Bhagavan 2007).
Coenzyme Q10 is considered a cell membrane stabiliser, and thought to be useful in preventing atherosclerosis, abnormal protein synthesis, and age‐related degenerative diseases (Migliore 2004). Coenzyme Q10 deficiency may be associated with a multitude of diseases, as diverse as coronary artery disease and congestive heart failure, Parkinson's disease, diabetes, breast cancer, and hypertension (Niklowitz 2007).
Coenzyme Q10 appears to be generally safe, with no significant side effects. Potential adverse effects include abdominal discomfort, headache, nausea, vomiting, and allergic maculopapular rash (Baggio 1994; Singh 1999). Coenzyme Q10 may reduce the effectiveness of warfarin, and may limit or prevent effective anticoagulation (Heck 2000).
Some drugs can cause depletion of coenzyme Q10, such as statins (Berthold 2006; Folkers 1990; Mortensen 1997), and beta‐blockers (Kishi 1977).
How the intervention might work
Emerging data suggest that oxidative stress is increased in people with heart failure, and may predict outcome. Markers of oxidative stress have been shown to be elevated in people with both ischaemic and non‐ischaemic cardiomyopathy (McMurray 1990; McMurray 1993), to be inversely correlated with the left ventricular ejection fraction (Belch 1991), and directly correlated with the chronicity and severity of heart failure (Diaz‐Velez 1996; Nishiyama 1998), and to predict mortality in people with heart failure (Tsutsui 2002). Coenzyme Q10 may reduce oxidative stress because of its antioxidant activity (Rauchova 1995), which has been shown to be similar to that of vitamin E (Tappel 1972). In addition, Coenzyme Q10 supplementation in animal models ameliorated left ventricular dysfunction, decreased left ventricular fibrosis, and improved endothelial function by enhancing nitric oxide bioavailability (De Blasio 2015; Tsai 2012).
Besides preventing oxidative stress in heart failure, coenzyme Q10 may also have a role in stabilising myocardial calcium‐dependent ion channels, and in preventing the consumption of metabolites essential for adenosine‐5'‐triphosphate (ATP) synthesis (Greenberg 1990). Plasma concentration of coenzyme Q10 was found to be an independent predictor of mortality in a cohort of participants with congestive heart failure (Molyneux 2008). Moreover, coenzyme Q10 myocardial tissue levels in people with chronic heart failure, are on average 33% lower than in a control population (Mortensen 1990; Mortensen 1993). The severity of heart failure correlates with the severity of coenzyme Q10 deficiency (Mortensen 1984). Therefore, supplementing this deficiency may play a role in the treatment of heart failure.
Why it is important to do this review
Despite the suggested potential benefits, the quality of evidence for the use of coenzyme Q10 in the treatment of heart failure has not been determined, and it is not included in American or European management guidelines for heart failure (Ponikowski 2016; Yancy 2017). This review is important because it summarises the best available evidence for the safety and efficacy of coenzyme Q10 in people with heart failure. The information derived from this review could assist clinicians and heart associations to determine whether to recommend coenzyme Q10 for heart failure.
An updated review to add new published trials and GRADE analysis is needed to reassess the safety and effectiveness of coenzyme Q10 in heart failure.
Objectives
To review the safety and efficacy of coenzyme Q10 in heart failure.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCT) that assessed the beneficial and harmful effects of coenzyme Q10 in heart failure. Cluster‐randomised clinical trials (cRCTs) were eligible for inclusion. We placed no restrictions on blinding, publication status, abstracts, conference proceedings, or language. We excluded quasi‐randomised and observational studies. When we identified cross‐over studies, we only considered data from the first phase. When first‐phase data were not available, we contacted the authors to obtain these data, if possible.
Types of participants
We included all participants, regardless of age, with chronic heart failure, defined as a clinical syndrome characterised by breathlessness and fatigue, which was caused by an inability of the heart to support adequate circulation, which may limit exercise tolerance, and may lead to pulmonary congestion and peripheral oedema; also, if possible, defined by more objective evidence of left ventricular systolic or diastolic function (e.g. echocardiography, radionuclide ventriculography, cardiac magnetic resonance, cardiac catheterization, and biomarkers, such as brain natriuretic peptide (BNP), and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP).
We included participants with ejection fraction reduced heart failure, ejection fraction mid‐range heart failure, and ejection fraction preserved heart failure.
We included participants with chronic heart failure of any severity.
Chronic heart failure included left‐sided and right‐sided heart failure.
We excluded participants with acute heart failure, defined as rapid onset or worsening of sign, symptoms, or both, of heart failure (Ponikowski 2016).
We included any studies in which the majority of participants met our inclusion criteria, We asked the trial authors for data for the subgroup of interest, and (if no data forthcoming) used sensitivity analysis to investigate the impact of including studies in which not all participants met the inclusion criteria.
Types of interventions
Coenzyme Q10 versus placebo
Coenzyme Q10 versus another active agent for use in heart failure
High‐dose versus low‐dose coenzyme Q10
Types of outcome measures
We used all outcome data from the longest follow‐up. We did not base our decision to include or exclude the study solely on the reporting of our outcomes of interest. When a published report did not appear to report one of these outcomes, we accessed the trial protocol and contacted the trial authors to ascertain whether the outcomes were measured but not reported. We included relevant trials, which measured these outcomes but did not report the data at all, or not in a usable format, in the review as part of the narrative.
Primary outcomes
All‐cause mortality
Cardiovascular (CV) mortality
Fatal and non‐fatal myocardial infarction
Fatal and non‐fatal stroke
Revascularisation procedures (percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG))
Hospitalisation due to heart failure (number of people with at least one hospitalisation due to heart failure)
All‐cause hospitalisation (number of people with at least one hospitalisation for any cause)
New York Heart Association (NYHA) classification of clinical status (four classes, lower = better)
Secondary outcomes
Left ventricular ejection fraction, determined by echocardiography, contrast, or radionuclide angiography
Symptom improvement, measured by individual trials, by exercise capacity (exercise duration or walking distance, or both), or both
Other exercise variables (peak VO₂, exercise haemodynamics)
Quality of life
BNP and NT‐pro BNP
Measurement of post‐therapeutic serum levels of coenzyme Q10
Adverse events (number of people with at least one adverse event)
Cost‐effectiveness
Search methods for identification of studies
Electronic searches
We updated the searches from 2013 on 16 October 2020. We searched the following databases:
The Cochrane Central Register of Controlled Trials (CENTRAL, 2020 Issue 10), in the Cochrane Library (searched 16 October 2020);
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE Ovid (1946 to 15 October 2020);
Embase Ovid (1980 to 2020 week 41);
Web of Science Clarivate Analytics (1900 to 16 October 2020);
CINAHL Plus EBSCO (1981 to 16 October 2020);
AMED Ovid (Allied and Complementary Medicine; 1985 to October 2020).
We also searched the following clinical trials registers for ongoing or unpublished studies:
International Standard Randomised Controlled Trial Number Registry (ISRCTN; www.isrctn.com/; searched 11 November 2019);
ClinicalTrials.gov (clinicaltrials.gov/; searched 16 July 2020).
The RCT filter for MEDLINE is the Cochrane sensitivity‐maximising RCT filter, and for Embase, we applied the terms recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). For CINAHL, we used an adaptation of the Cochrane RCT filter. We applied no language restrictions. Searching the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) was not possible, as the database was not available. The search strategies are in Appendix 1.
Searching other resources
We checked the bibliographic references of the included randomised controlled trials to find randomised controlled trials not identified by the electronic searches. When possible, we approached the principal authors of the identified randomised controlled trials and enquired if they knew of any other randomised controlled trials.
Data collection and analysis
We conducted the meta‐analyses according to Cochrane recommendations (Higgins 2019), using Review Manager 5.4 (Review Manager 2020).
Selection of studies
Five authors (KT, BS, AM, MK, MF) independently assessed the identified references to determine if they fulfilled the inclusion criteria (two authors per reference). First, we screened the references by title, abstract, or both, and excluded those which were clearly not relevant to the review. Then, we obtained the full texts for the references that passed the first screening phase, and evaluated them for inclusion. In cases when references did not have sufficient information to determine their eligibility, we contacted the trial authors, asking for more information; if we did not receive a response, we excluded these studies. We listed the excluded trials with the reasons for exclusion. We resolved disagreements by discussion. We summarised the flow of papers through the search and selection process using a PRISMA flow chart (Figure 1; Moher 2009).
1.
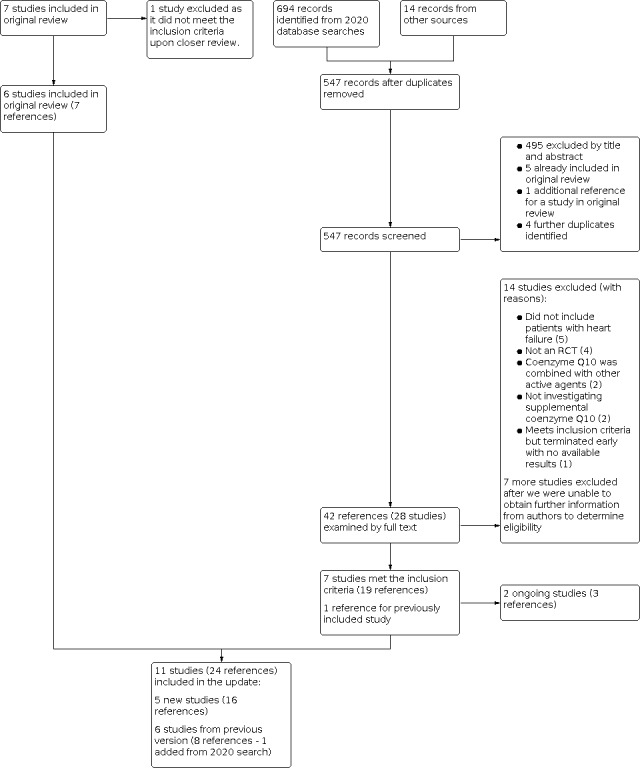
PRISMA flow chart
Data extraction and management
Five authors (KT, BS, AM, MK, MF) independently extracted data (two authors per trial), and resolved disagreements by discussion. We used a standardised data collection form to extract data on methods, participants, interventions, and outcomes. If we identified more than one publication on a single randomised controlled trial, we extracted the most appropriate data.
We extracted data needed to assess risk of bias, conduct meta‐analysis, and investigate any possible heterogeneity. The extracted data included data about study methodology (design, duration, setting, inclusion and exclusion criteria, comparisons groups, randomisation, blinding), participants (total number, demographics, medical conditions), intervention (dose, route, preparation), control (placebo versus active compounds, dosing), outcomes (primary and secondary, outcome measures, time points measured), and funding.
Assessment of risk of bias in included studies
We assessed methodological quality according to our level of confidence that the design and report of a published trial restricted bias in the intervention comparison (Moher 1998). In assessing the risk of bias, we used the Cochrane tool for assessing risk of bias, described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed risk of bias in the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other biases.
Measures of treatment effect
Dichotomous data
We reported absolute effect and risk ratios (RR), with 95% confidence intervals (CI). We calculated the the number needed to treat for an additional beneficial outcome (NNTB), and the number needed to treat for an additional harmful outcome (NNTH), if possible.
Continuous data
We calculated mean differences (MD) with 95% CIs for outcomes reported with the same measure. If the quality of life outcome was reported using different tools in different studies, we had planned to use the standardized mean difference (SMD).
Unit of analysis issues
When we retrieved cross‐over studies from the search, we only took the first arm into consideration.
We used all outcome data from the longest follow‐up, as mentioned above.
We used guidelines from the Cochrane Handbook for Systematic Reviews of Interventions guidelines to inform the inclusion and analysis of cRCTs in our review (Higgins 2011). When analysing results from cRCTs, we had planned to adjust for clustering to avoid artificially narrow confidence intervals for the treatment effect. We also planned to estimate the intracluster correlation coefficient (ICC) to quantify the extent to which data from observations from the same cluster were correlated. However, we did not identify any relevant cluster‐randomised trials.
We handled trials with more than one comparison (e.g. two doses of coenzyme Q10 versus placebo or coenzyme Q10 versus another treatment versus placebo), by splitting the shared group (placebo in the first example and coenzyme Q10 in the second) into two or more groups with smaller sample size, to enable us to include two or more comparisons.
Dealing with missing data
We performed all analyses on an intention‐to‐treat basis, using the last reported observed response (carry forward) and including all participants, regardless of compliance or follow‐up. In addition, we planned to perform a 'worst‐case scenario' analysis, considering all participants with missing data as treatment failures. We used the Review Manager 5 calculator to calculate missing data, like standard deviation (Review Manager 2020).
Assessment of heterogeneity
We assessed statistical heterogeneity using a Chi² test, and we used the I² statistic to quantify inconsistency across included studies (Higgins 2003). We also assessed statistical heterogeneity by visually examining the graphical presentations (forest plots; (Egger 1997)).
Assessment of reporting biases
We tried to locate the protocol for each included randomised controlled trial. If the protocol was available, we compared its outcomes with those in the published RCT report. If it was not available, we compared the outcomes listed in the methods section of the report with the actual reported results. We planned to use a funnel plot of all included trials to check the presence of publication bias, but there were insufficient trials (Egger 1997).
Data synthesis
We undertook meta‐analysis when there were sufficient data of a suitable type, using Review Manager 5 (Review Manager 2020). When there were too few clinically homogeneous trials for us to perform a meta‐analysis, we presented a narrative synthesis.
We used the random‐effects model to avoid the risk that the variability between the studies may be exclusively due to a random sampling variation around a fixed effect. Using the random‐effects model is also recommended by Clinical Evidence (Clinical Evidence).
Subgroup analysis and investigation of heterogeneity
In cases of significant heterogeneity, we aimed to address it by exploring clinical and methodological variations between the studies. If significant differences were identified, we conducted a subgroup analysis to offer new insights and confirm the effect in the different subgroups. Therefore, we conducted a posthoc subgroup analysis to compare studies without a maximum coenzyme Q10 dose against those with a maximum dose of 200 mg daily. Age and gender were the two subgroups planned for analysis in the protocol; however, we were unable to do so, due to insufficient data.
Sensitivity analysis
We did not carry out any sensitivity analysis because of the small number of included studies for each outcome.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the quality of the evidence (Higgins 2019), and GRADEpro GDT (gradepro.org), to import data from Review Manager 5 (Review Manager 2020), to create a ’Summary of findings’ table. This table provides outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to the care of people with the disorder and decision‐making. We selected the following main outcomes for the ’Summary of findings’ table:
All‐cause mortality
Fatal and non‐fatal myocardial infarction
Fatal and non‐fatal stroke
Hospitalisation due to heart failure
Left ventricular ejection fraction
Exercise capacity, measured by treadmill exercise test duration
Adverse events
Although important, we did not include NYHA clinical status as available data were exhaustive and non‐combinable. We intended to include three comparisons (coenzyme Q10 versus placebo or no coenzyme Q10, coenzyme Q10 versus another active agent, and high dose versus low dose coenzyme Q10). However, the identified studies only compared coenzyme Q10 to placebo or standard care, thus, we only created one, instead of three, 'Summary of findings' table.
Results
Description of studies
Results of the search
The search and screening in the original review resulted in seven included studies. After reviewing these seven studies, we found that one study did not meet the inclusion criteria, so we excluded it (Adarsh 2008).
For the update, the initial database search on 16 October 2020 yielded 694 records. We also identified 14 records from other sources (one reference was identified by checking the bibliographic references of the included RCTs; 13 references identified by searching the clinical trials databases). After de‐duplication, there were 547 references for screening. We removed 505 records (we excluded 495 references by their title and abstract; 5 references were already included in the original review; 1 reference was an additional report for a study in the original review; we identified 4 further duplicate references). Of the 42 remaining references, we combined those belonging to the same study, resulting in 28 unique studies. We retrieved and examined the full texts for the 28 studies, and excluded 14 studies. For seven studies, we needed usable data and additional information, so we contacted the study authors. However, we did not get a response from the authors, so we excluded these seven studies. Of the seven remaining studies, two studies were ongoing (confirmed by contacting their authors). As a result, we identified five new studies with the updated search.
Including the six studies from the original review, the updated review includes a total of 11 studies. Please refer to the PRISMA flow chart for details on the updated search (Figure 1).
Included studies
This review included 11 randomised controlled trials (RCT) with 1573 participants. Only four studies had relatively large sample sizes (128 in Zhao 2015; 148 in Mareev 2017; 420 in Mortensen 2014; 641 in Morisco 1993). Sample sizes for the rest of the studies ranged from 20 to 55 participants. Only one study had a cross‐over design (from which only first phase data were used; (Kawashima 2016)). All other studies had a parallel design.
Five studies were conducted in Asia (Berman 2004; Kawashima 2016; Kocharian 2009; Sobirin 2019; Zhao 2015), two studies in Europe (Morisco 1993; Munkholm 1999), one study in Russia (Mareev 2017), one in Australia (Keogh 2003), and one in the USA (Khatta 2000). One study enrolled participants from Europe, Australia, and Asia (Mortensen 2014).
All studies were conducted with adults, except Kocharian 2009, which included participants younger than 18 years. In addition, all studies used placebo plus conventional therapy in the control group except for two studies, which used conventional therapy alone (Sobirin 2019; Zhao 2015).
The daily dose of coenzyme Q10 varied significantly among studies: 30 mg in Zhao 2015; 60 mg in Berman 2004; 150 mg in Keogh 2003; 200 mg in Khatta 2000 and Munkholm 1999; 225 mg in Mareev 2017; 300 mg in Mortensen 2014 and Sobirin 2019; and 400 mg in Kawashima 2016. In Kocharian 2009 and Morisco 1993, participants received 2 mg/kg of coenzyme Q10 daily. In one study, coenzyme Q10 was given in a nasal drop form (Mareev 2017). In all other included studies, it was given orally.
The follow‐up period varied among studies: one month in Sobirin 2019; three months in Berman 2004, Kawashima 2016, Keogh 2003 and Munkholm 1999; and six months in Khatta 2000, Kocharian 2009 and Mareev 2017. Mortensen 2014 reported data at 4 and 26 months, while Zhao 2015 reported data at 6 and 12 months. Morisco 1993 followed participants up to 12 months.
Two studies were partially funded by pharmaceutical companies that manufacture and distribute coenzyme Q10 supplements (Keogh 2003; Mortensen 2014).
Excluded studies
In the original review, out of the 721 papers identified in the search, we excluded 567 early in the selection process because the title was irrelevant to the main area of interest. Of the remaining papers, 139 did not meet one or more of the inclusion criteria. We excluded randomised controlled studies with a cross‐over design (which were identified in the original review) later, as their data were unsuitable for analysis.
In the update, we excluded 505 out of 547 references by title, abstract, or both, as they were clearly irrelevant to our review. After combining references belonging to the same study, and assessing the full text, we excluded 14 studies: five studies excluded people with heart failure (Fedacko 2009; IRCT2015070223018N1; NCT03586414; Rivera 2017; Turk 2013), four studies were not RCTs (Chen 2017; Chen 2018; JPRN‐UMIN000020203; Miyazaki 2013), two studies investigated coenzyme Q10 combined with other active agents (Johansson 2013; Pourmoghaddas 2014), two studies did not investigate supplemental coenzyme Q10 (JPRN‐UMIN000007695; McMurray 2009), and one study met the inclusion criteria but was terminated early and had no available results (JPRN‐UMIN000027248). We excluded seven more studies, as they had no usable data and their authors did not respond to our queries (Kukharchik 2016; Kukharchik 2016a; Kukharchik 2017; Kumar 2015; Leonova 2018; Oleg 2016; Saurabh 2014).
When we re‐examined the studies included in the original review, we found that one study did not meet the inclusion criteria (Adarsh 2008). The study did not randomise its participants, and thus, is not an RCT. We deleted the study's findings from the updated review, and added it to the list of excluded studies.
See Characteristics of excluded studies.
Ongoing studies
We identified two ongoing studies (NCT02779634; Pierce 2018). We contacted authors from both studies; they confirmed that the studies are still ongoing, with no published results yet. Both studies are randomised, blinded, placebo‐controlled trials with parallel design. Pierce 2018 also investigates D‐ribose supplements and has four arms (placebo only, coenzyme Q10 only, D‐ribose only, coenzyme Q10 plus D‐ribose). NCT02779634 includes participants with heart failure with preserved ejection fraction, and follows them for 16 weeks. Pierce 2018 also includes participants with heart failure with preserved ejection fraction, and follows them for 12 weeks.
Risk of bias in included studies
Two review authors independently assessed each of the included studies, and found that there was risk of bias in the included studies (see the 'Risk of bias' tables in the 'Characteristics of included studies' tables). We re‐evaluated studies included in the original review for risk of bias.
We used the 'Risk of bias' summary and graph figures to illustrate the proportion of studies with each of the judgements (low risk, high risk, unclear risk of bias) for each domain of the tool (Figure 2; Figure 3).
2.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
3.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
Allocation
All included studies stated they were randomised. However, methods used for random sequence generation were not mentioned in seven studies, and thus, we deemed the risk of selection bias (random sequence generation) in these studies to be unclear (Berman 2004; Kawashima 2016; Keogh 2003; Kocharian 2009; Mareev 2017; Munkholm 1999; Zhao 2015). We assessed the remaining four studies at low risk of bias for random sequence generation.
Nine studies did not mention how they achieved allocation concealment, and therefore, we deemed the risk of selection bias (allocation concealment) to be unclear (Kawashima 2016; Keogh 2003; Khatta 2000; Kocharian 2009; Mareev 2017; Morisco 1993; Munkholm 1999; Sobirin 2019; Zhao 2015). We assessed the remaining two studies at low risk of bias in this domain.
Blinding
Only one study was unblinded, and thus, had a high risk of performance bias (Sobirin 2019). All other included studies were double blinded, and thus, we deemed them at low risk of performance bias.
Sobirin 2019 also had a high risk of detection bias, as it was not blinded. Only three studies mentioned that assessors were blinded, and thus, had a low risk of detection bias (Kocharian 2009; Mortensen 2014; Zhao 2015). The rest of the studies did not mention blinding of the assessors, and thus, we deemed them at an unclear risk of detection bias (Berman 2004; Kawashima 2016; Keogh 2003; Khatta 2000; Mareev 2017; Morisco 1993; Munkholm 1999).
Incomplete outcome data
In eight studies, some of the participants did not finish the study. They were not included in the analysis. We considered these studies to be at high risk of attrition bias (Berman 2004; Kawashima 2016; Keogh 2003; Khatta 2000; Mareev 2017; Morisco 1993; Sobirin 2019; Zhao 2015). In Munkholm 1999 and Kocharian 2009, all participants completed the trial, therefore, we considered these studies to be at low risk of attrition bias. Mortensen 2014 also had participants who did not finish the study, however, their data were included in the intention‐to‐treat analysis for the primary outcomes. Trial authors did not find significant differences between groups in the reasons for withdrawal. Thus, we deemed the risk of attrition bias for this study to be low.
Selective reporting
Comparing outcomes reported in the published study was not possible for seven studies, as no published protocols were available (Berman 2004; Keogh 2003; Khatta 2000; Mareev 2017; Morisco 1993; Munkholm 1999; Zhao 2015). Subsequently, we classified the risk of reporting bias as unclear for these studies. We assessed only one study at a high risk of reporting bias, as some of the outcomes listed in the protocol were not reported in the manuscript (Sobirin 2019). We assessed the remaining three studies at low risk of reporting bias.
Other potential sources of bias
None reported.
Effects of interventions
See: Table 1
We found no studies that compared coenzyme Q10 to other active agent for heart failure, or compared a high dose to a low dose of coenzyme Q10. All eleven included studies compared coenzyme Q10 to either placebo or conventional therapy. We combined the results from all of the studies into one comparison, coenzyme Q10 versus control, where control referred to placebo plus conventional therapy, or conventional therapy alone.
Primary outcomes
Two studies reported on some or all of the following primary outcomes: total mortality, major cardiovascular events, myocardial infarction, stroke, revascularisation procedures, and hospitalisation (Morisco 1993; Mortensen 2014). Seven studies reported on the New York Heart Association (NYHA) functional class (Berman 2004; Kawashima 2016; Keogh 2003; Mareev 2017; Morisco 1993; Mortensen 2014; Munkholm 1999).
In the primary report, Mortensen 2014 reported these outcomes as components of adverse events, and compared them between the two study groups as adverse events rather than as separate outcomes. We extracted these data and directly compared between coenzyme Q10 and control.
All‐cause mortality
One study reported data on mortality (Mortensen 2014). Coenzyme Q10 (CoQ10) was probably reduces all‐cause mortality (10% in the intervention group versus 18% in the control group; risk ratio (RR) 0.58, 95% confidence interval (CI) 0.35 to 0.95; one study, 420 participants; Analysis 1.1; moderate‐quality evidence) at 106 weeks, and was also superior to control for all‐cause mortality survival (hazard ratio (HR) 0.51; 95% CI 0.30 to 0.89; P = 0.018, as reported by Mortensen 2014). The number needed to treat for an additional beneficial outcome (NNTB) was 13.3. We downgraded the evidence by one level due to indirectness (Table 1).
1.1. Analysis.

Comparison 1: Coenzyme Q10 versus control, Outcome 1: All cause mortality
Cardiovascular mortality
One study reported data on cardiovascular mortality (Mortensen 2014). Coenzyme Q10 probably reduces the risk of cardiovascular mortality (9% in CoQ10 group versus 16% in control group; P = 0.039) at 106 weeks, with a NNTB of 15. We downgraded the evidence by one level, to moderate quality, due to indirectness.
Mortensen 2014 also reported on major cardiovascular events (defined as unplanned hospital stay resulting from worsening heart failure, cardiovascular death, mechanical assist implantation, or urgent cardiac transplantation). There were fewer cardiovascular events in the coenzyme Q10 group compared to the control group (15% in CoQ10 group versus 26% in control group; P = 0.005) at 106 weeks. CoQ10 was also superior to control in time‐to‐event analysis for cardiovascular events (HR 0.50; 95% CI 0.32 to 0.80; P = 0.003, as reported by Mortensen 2014).
Fatal and non‐fatal myocardial infarction
One study reported data on myocardial infarction (Mortensen 2014). The results were inconclusive for the risk of myocardial infarction between the CoQ10 and control groups (RR 1.62, 95% CI 0.27 to 9.59; one study, 420 participants; low‐quality evidence; Analysis 1.2). We downgraded the quality of the evidence two levels for indirectness and imprecision (Table 1).
1.2. Analysis.

Comparison 1: Coenzyme Q10 versus control, Outcome 2: Myocardial infarction
Fatal and non‐fatal stroke
One study reported data on strokes (Mortensen 2014). There was little or no difference in the risk of stroke between the CoQ10 and control groups (RR 0.18, 95% CI 0.02 to 1.48; one study, 420 participants; low‐quality evidence; Analysis 1.3). Thus, the results were inconclusive between groups for the risk of stroke. We downgraded the quality of the evidence two levels for indirectness and imprecision (Table 1).
1.3. Analysis.

Comparison 1: Coenzyme Q10 versus control, Outcome 3: Stroke
Revascularisation procedures (percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG))
One study reported data on revascularisation procedures (Mortensen 2014). The results were inconclusive between groups for the risk of revascularization procedures compared to control (RR 0.86, 95% CI 0.24 to 3.17; one study, 420 participants; Analysis 1.4).
1.4. Analysis.

Comparison 1: Coenzyme Q10 versus control, Outcome 4: Revascularisation procedures
Hospitalisation due to heart failure
Two studies reported data on hospitalisation due to heart failure (Morisco 1993; Mortensen 2014). Coenzyme Q10 probably reduces hospitalisations due to heart failure when compared to control (RR 0.62, 95% CI 0.49 to 0.78; two studies, 1061 participants; I² = 0%; moderate‐quality evidence; Analysis 1.5). The NNTB was 9.7.
1.5. Analysis.

Comparison 1: Coenzyme Q10 versus control, Outcome 5: Hospitalisation for heart failure
It is worth mentioning that those two studies were published more than 20 years apart, and reflect significant changes in medical care and hospitalisation criteria for people with heart failure over this time period. In Mortensen 2014, hospital stays within 30 days of randomisation were not counted. We downgraded the quality of the evidence due to high risk of bias (Table 1).
All‐cause hospitalisation
None of the included studies reported on all‐cause hospitalisation.
New York Heart Association (NYHA) classification of clinical status
Seven trials reported on NYHA functional classification (Berman 2004; Kawashima 2016; Keogh 2003; Mareev 2017; Morisco 1993; Mortensen 2014; Munkholm 1999). They used different methods to report the change in NYHA clinical status, and therefore, we were unable to pool the results.
Berman 2004 reported that the median NYHA class in the coenzyme Q10 group decreased from 3.1 to 2.4, whereas no change was reported in the control group (from median 3.68 to 3.6).
Keogh 2003 reported that the NYHA class in the coenzyme Q10 group showed a small (0.5) but significant (P = 0.0001) improvement, whereas the control group showed no significant change. In the coenzyme Q10 group, the NYHA class improved from 2.9 (± 0.06) to 2.4 (± 0.12); P = 0.001. The difference in improvement between the coenzyme Q10 group and the control group in mean NYHA class was 0.5, which was statistically significant for the t‐test (P = 0.012) and for the Wilcoxon test (P = 0.02).
Morisco 1993 reported that there was a progressive reduction in the NYHA class in the coenzyme Q10 group, indicating an improvement in functional status that was statistically significant after three, six, and at 12 months. No significant change in functional class was observed in the control group.
Munkholm 1999 reported that the participants in the coenzyme Q10 group tended to improve with respect to their functional class (from 3A to 2B), whereas no improvement was reported in the control group (2B). However, the improvement in the treatment group was not statistically significant.
Kawashima 2016, a cross‐over trial, reported data on NYHA functional class, however, they did not provide data separately for the first phase.
Mortensen 2014 reported non‐significant improvement in NYHA functional class in both the coenzyme Q10 and control groups at 16 weeks. However, at 106 weeks, a significantly larger proportion of participants in the coenzyme Q10 group showed NYHA functional class improvement compared to the control group (58% versus 45%; P = 0.028).
Mareev 2017 reported a greater change of NYHA functional class in the coenzyme Q10 group (NYHA class change ‐0.16) compared to the control group (‐0.08; P = 0.033).
Secondary outcomes
None of the included trials measured quality of life, measured by a recognised scale, exercise variables (exercise haemodynamics), or cost‐effectiveness.
Left ventricular ejection fraction (LVEF)
Eight trials reported on left ventricular ejection fraction (Kawashima 2016; Khatta 2000; Kocharian 2009; Mareev 2017; Mortensen 2014; Munkholm 1999; Sobirin 2019; Zhao 2015). Data from Khatta 2000 were not useable as they did not report standard deviations. Five studies used echocardiography to assess ejection fraction (Kawashima 2016; Kocharian 2009; Mortensen 2014; Sobirin 2019; Zhao 2015). One study used radionuclide ventriculography (Munkholm 1999), and one study used either echocardiography, contrast, or radionuclide ventriculography (Mareev 2017). Two studies reported LVEF at two separate time points (16 and 106 weeks for Mortensen 2014; 6 and 12 months for Zhao 2015), however, we only used the data from the longest follow‐up.
Coenzyme Q10 was associated with a small change in LVEF (mean difference (MD) 1.77, 95% CI 0.09 to 3.44; seven studies, 650 participants; I² = 38%; very low‐quality evidence; Analysis 1.6). We assessed the quality of evidence for this outcome as very low, because of the substantial risk of bias in most of the studies, and the lack of precision, due to the wide confidence intervals (Table 1). Thus, it is uncertain whether Coenzyme Q10 improves LVEF or not.
1.6. Analysis.

Comparison 1: Coenzyme Q10 versus control, Outcome 6: Left ventricular ejection fraction (%)
Symptom improvement
Overall, six trials reported on symptom improvement (Berman 2004; Kawashima 2016; Keogh 2003; Khatta 2000; Mareev 2017; Mortensen 2014).
Treadmill exercise test duration (seconds)
Three studies reported on exercise capacity, using the duration of treadmill exercise (Kawashima 2016; Keogh 2003; Khatta 2000). Reviewing data from the original version of the review, we found that we had mistakenly switched the numbers for coenzyme Q10 and control groups in the analysis; and input inaccurate numbers of participants in each group for Keogh 2003; and inaccurately converted minutes to seconds for the coenzyme Q10 group in Khatta 2000, although the accurate numbers were not significantly different. We subsequently corrected those numbers for this version.
The results were inconclusive for exercise duration between the coenzyme Q10 and control groups (MD 48.23, 95% CI ‐24.75 to 121.20; three studies, 91 participants; I² = 41%; Analysis 1.7). The sample size for this outcome is very small; it includes studies with high risk of bias; and the effect is imprecise (very wide confidence interval that includes possible harm, no effect, or possible benefit). Thus, the quality of evidence is very low, and it is uncertain whether coenzyme Q10 affects this outcome (Table 1).
1.7. Analysis.

Comparison 1: Coenzyme Q10 versus control, Outcome 7: Exercise capacity: treadmill exercise test duration (seconds)
Treadmill exercise metabolic equivalents (MET)
One study reported data on exercise capacity using treadmill exercise test metabolic equivalents (METs) (Kawashima 2016). Because this cross‐over study did not report analysis for the first phase, we conducted the analysis using data provided by the authors. At three months, the results were inconclusive for METs on the treadmill exercise test between the coenzyme Q10 and control groups (MD ‐2.51, 95% CI ‐6.35 to 1.33; one study, nine participants; Analysis 1.8). This effect is imprecise and potentially biased.
1.8. Analysis.

Comparison 1: Coenzyme Q10 versus control, Outcome 8: Exercise capacity: metabolic equivalent on treadmill exercise test (METs)
6‐minute walk distance (metres)
Four studies reported on exercise capacity using 6‐minute walk test distance (Berman 2004; Keogh 2003; Mortensen 2014, Mareev 2017).
Berman 2004 noted improvement from baseline in the coenzyme Q10 group (269.5 to 382.2 metres; P < 0.0001), and deterioration in the control group (254 to 177 metres).
Keogh 2003 found no difference in response between coenzyme Q10 and placebo (an increase of 21 meters in the coenzyme Q10 group, and a decrease of 16 meters in the control group; P = 0.29).
Mortensen 2014 reported improvement in the 6‐minute walk test in both the coenzyme Q10 and control groups at 16 weeks; the difference between groups was not significantly different.
Mareev 2017 reported improvement in both the coenzyme Q10 (288 to 320 metres; P = 0.001) and control groups (311 to 324 metres; P = 0.103); as well as in between the two groups (P = 0.03).
We could not pool data for the 6‐minute walk distance as Berman 2004 and Mareev 2017 did not report standard deviation (SD), and Mortensen 2014 reported only the differences from baseline.
Visual analogue scale (VAS)
One study evaluated symptom improvement with a visual analogue scale (VAS) at 16 weeks (Mortensen 2014). It found improvement in both groups; but no difference between groups was found.
The Minnesota living with heart failure questionnaire
One study reported on symptom improvement by measuring changes in the Minnesota living with heart failure questionnaire at 24 weeks (Mareev 2017). They found a decrease of 9.1 points in the scores for both the coenzyme Q10 and control groups.
The Kansas city cardiomyopathy questionnaire (KCCQ)
One study reported on symptom improvement by measuring changes in the Kansas city cardiomyopathy questionnaire score at 24 weeks (Mareev 2017). There was a larger increase in score for the coenzyme Q10 group (+9.9) compared to the control (+1.4; P = 0.034).
Other exercise variables – peak oxygen consumption
One study reported data for peak oxygen consumption (Khatta 2000). Data showed an increase in the coenzyme Q10 group and a decrease in the control group; but no significant difference between the two groups.
Brain natriuretic peptide (BNP) and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP)
Two studies reported on BNP (Kawashima 2016; Mareev 2017). BNP blood levels were less in the coenzyme Q10 group compared to control (MD ‐91.97, 95% CI ‐103.11 to ‐80.83; two studies, 162 participants; I² = 0%; Analysis 1.9).
1.9. Analysis.

Comparison 1: Coenzyme Q10 versus control, Outcome 9: Brain natriuretic peptide (pg/mL)
One study reported on NT‐proBNP (Mortensen 2014). It found no difference in change from baseline for NT‐proBNP blood levels between the two groups at 16 and 106 weeks.
Serum levels of coenzyme Q10
Seven studies reported data on coenzyme Q10 serum levels (Berman 2004; Kawashima 2016; Keogh 2003; Khatta 2000; Mareev 2017; Mortensen 2014; Munkholm 1999). We were unable to use data from Berman 2004 as they did not report standard deviations. One study reported coenzyme Q10 serum levels at two separate time points (16 and 106 weeks; (Mortensen 2014)).
Coenzyme Q10 serum levels were higher for those taking the supplement compared to control (MD 1.25, 95% CI 1.09 to 1.42; six studies, 489 participants; I² = 91%; Analysis 1.10). The high level of heterogeneity was likely due to the differences in coenzyme Q10 levels, caused by different daily coenzyme Q10 regimens administered among the studies. The daily dose of coenzyme Q10 was higher in three studies (400 mg in Kawashima 2016; 300 mg in Mortensen 2014; 225 mg in Mareev 2017), compared to the other studies (150 mg in Keogh 2003; 200 mg in Khatta 2000; 200 mg in Munkholm 1999). Therefore, we conducted a subgroup analysis to compare studies without a maximum coenzyme Q10 dose against those with a maximum dose of 200 mg daily.
1.10. Analysis.

Comparison 1: Coenzyme Q10 versus control, Outcome 10: Serum levels of coenzyme Q10 (μg/mL)
Subgroup analysis
Analysis for studies with doses ≤ 200 mg daily continued to show a higher coenzyme Q10 level in those who received coenzyme Q10 compared to the control group (MD 1.46, 95% CI 1.19 to 1.72; three studies, 112 participants; I² = 29%; Analysis 1.10).
Test for subgroup differences indicated evidence for a difference between effect based on maximum dosage (P = 0.04). Heterogeneity, however, remained high in the analysis for studies with dose > 200mg daily, which is unexplained (I² = 93%; Analysis 1.10). The effect is inconsistent given the unexplained heterogeneity and potentially biased given the high risk of bias in the included studies.
Adverse events
Two studies reported on adverse events (Mareev 2017; Mortensen 2014). The results were inconclusive between groups (RR 0.70, 95% CI 0.45 to 1.10; two studies, 568 participants; I² = 0%; low‐quality evidence; Analysis 1.11). We downgraded the quality of the evidence two levels, due to indirectness and imprecision, shown by the wide confidence interval (Table 1).
1.11. Analysis.

Comparison 1: Coenzyme Q10 versus control, Outcome 11: Aderse events
Other cardiovascular outcomes
Morisco 1993 reported that the percentage incidence of acute pulmonary oedema was significantly smaller in the coenzyme Q10 group than in the control group (P < 0.001). They also found the incidence of cardiac asthma was lower in participants treated with coenzyme Q10 than those in the control group (P < 0.001). The incidence of arrhythmias was higher in the control group than in the coenzyme Q10 group (P < 0.001).
Discussion
Summary of main results
We included 11 studies comparing coenzyme Q10 to placebo. All had small sample size and all but one study (Kocharian 2009) were performed on adults. Only one of the included studies had a crossover design (Kawashima 2016). Follow up periods varied among the included studies: up to 6 months for 8 studies (Berman 2004; Kawashima 2016; Keogh 2003; Khatta 2000; Kocharian 2009; Mareev 2017; Munkholm 1999; Sobirin 2019), up to 12 months for two studies (Morisco 1993; Zhao 2015), and 26 months for only one study (Mortensen 2014).
Many of the outcomes were reported by only one of the included studies so no meta‐analyses were done. For those outcomes reported by multiple studies, we were not able to combine some of them (NYHA functional class and symptoms improvement in terms of 6‐minutes walk test) due to the variability in reporting.
Primary outcomes
Moderate‐level evidence showed that coenzyme Q10 probably reduces the risk of all cause and cardiovascular mortality at 106 weeks (evidence from one study) and hospitalisations for heart failure (evidence from two studies).
Results from individual studies showed lower incidence of acute pulmonary oedema, cardiac asthma and arrhythmia, and fewer cardiovascular events (defined as unplanned hospital stay resulting from worsening HF, cardiovascular death, mechanical assist implantation, or urgent cardiac transplantation) with coenzyme Q10. However results were inconclusive for risk of myocardial infarction, stroke or revascularisation procedures.
Although seven studies reported on NYHA classification, we were unable to pool the results since they used different methods to report the change. Nevertheless, individual studies showed evidence of improvement in NYHA classification with coenzyme Q10.
Secondary outcomes
Although many studies reported on LVEF, low‐quality evidence leaves us uncertain about the effect of coenzyme Q10 on left ventricular ejection fraction (LVEF). The analysis showed improvement in the LVEF with coenzyme Q10, however, the effect size was small and likely not clinically significant (from 0.09% to 3.44% higher).
Overall, coenzyme Q10 was not associated with better outcomes for peak oxygen consumption, the duration of treadmill exercise, treadmill exercise metabolic equivalents, or heart failure symptoms measured on a visual analogue scale and the Minnesota living with heart failure questionnaire. It, however, improves the distance of the 6‐minutes walk test and heart failure symptoms measured by the Kansas city cardiomyopathy questionnaire.
Coenzyme Q10 supplements results in higher serum levels of coenzyme Q10, even with a maximum daily dose of 200 mg daily. These findings, however, have significant inconsistency due to unexplained heterogeneity. Furthermore, coenzyme Q10 lowers BNP blood levels; but not lower NT‐proBNP. The results for adverse events associated with coenzyme Q10 were inconclusive.
Overall completeness and applicability of evidence
We were unable to pool the data for most of the outcomes, since they were reported in different ways in different studies, reported in only one study, or not reported in any study. For instance, important outcomes in heart failure (e.g. mortality, cardiovascular events, hospitalisation, adverse events) were rarely investigated. For other important outcomes, like symptom improvement and New York Heart Association classification status, assessment methods and numbers reported varied among studies, limiting our ability to conduct meta‐analyses. The dose of coenzyme Q10 also varied among the studies.
Small sample sizes, short follow up periods and lack of usable data inhibited our ability to pool the data and draw robust conclusions and recommendations for clinical practice. We did not investigate the effect of coenzyme Q10 on several biomarkers, such as C‐reactive protein, high sensitivity troponin, myeloperoxidase, and uric acid, as we felt they were less relevant to the objectives of the review. Nevertheless, studying these outcomes in the future might enable a better understanding of the role of coenzyme Q10 in heart failure.
Quality of the evidence
As noted in the Risk of bias in included studies, most of the included studies had unclear or high risks of bias. There were also multiple pooled outcome measures with wide confidence intervals, often crossing the line of no effect, which we assessed as imprecision. These two factors decreased the quality and certainty of the evidence.
This was evident for five primary outcomes: risk of myocardial infarction, risk of stroke, left ventricular ejection fraction, exercise capacity, and adverse events. We found high levels of heterogeneity for one outcome (serum levels of coenzyme Q10); pooled analysis showed unexplained heterogeneity. Evidence derived from this review ranged from very low to moderate quality. Indeed, this confers the need for larger studies that are clear of risk of evidence evaluating coenzyme Q10.
Potential biases in the review process
We conducted our review according to a protocol, and following the recommendations of Cochrane. Per the protocol, we used the random‐effects model in our analyses. One limitation was the unavailability of data from the first phase of cross‐over studies; these data were not published, nor were they provided by the trial authors after we contacted them, except for Kawashima 2016. Therefore, we excluded all cross‐over studies expect for Kawashima 2016. For variables reported in different ways in different studies, we were unable to pool data. This review combined participants with both heart failure with reduced ejection fraction (HFrEF), and heart failure with preserved ejection fraction (HFpEF). Given the differences in the pathophysiology and treatment of these two types of heart failure, separate analyses for each type would be beneficial in subsequent updates of this review. Finally, there is a lag time between the last search and the publication of this review; it is possible that new trials have been published.
Agreements and disagreements with other studies or reviews
One meta‐analysis concluded that the use of coenzyme Q10 in participants with congestive heart failure improved stroke volume, ejection fraction, cardiac output, cardiac index, and end diastolic volume index (Soja 1997).
A systematic review with a meta‐analysis of studies ranging from 3 to 12 months long, reported non‐significant trends towards increased ejection fraction and reduced mortality; they had an insufficient number of participants for meaningful results (Rosenfeldt 2003).
A systematic review with a meta‐analysis concluded that the use of coenzyme Q10 in participants with heart failure improved ejection fraction more in participants who were not receiving angiotensin‐converting enzyme inhibitors, and improved cardiac output. Treatment periods ranged from one to six months (Sander 2006).
A systematic review with a meta‐analysis of studies ranging from 4 to 28 weeks long concluded that the use of coenzyme Q10 in participants with congestive heart failure improved ejection fraction, and showed a trend towards improved NYHA functional class that did not reach statistical significance (Fotino 2013).
Compared to these previous reviews, we are uncertain as to whether the use of coenzyme Q10 in heart failure improves LVEF or not, since the evidence is of very low quality. However, our review shows that coenzyme Q10 in heart failure probably reduces all‐cause and cardiovascular mortality. Results in our review are limited by small studies that were at significant risk of bias. Thus, we are unable to draw robust conclusions.
The original Cochrane review concluded that the use of coenzyme Q10 in participants with heart failure did not have an effect on mortality (Madmani 2014). However, this was derived from the only study that reported on mortality, which we excluded in the current review, due to lack of randomisation (Adarsh 2008). The original review also concluded no effect on LVEF. This does not contradict the current review, since the studies in the original review only reported short‐term follow‐up.
Authors' conclusions
Implications for practice.
The currently available evidence is not sufficient to draw robust conclusions about the safety and efficacy of coenzyme Q10 in heart failure.
Included studies provide moderate‐quality evidence for a probable benefit for coenzyme Q10 in reducing all‐cause mortality, cardiovascular mortality, and hospitalisation for heart failure. The results were inconclusive for risk of myocardial infarction, stroke, or revascularisation procedures.
With very low‐quality evidence, it is very uncertain whether coenzyme Q10 has an effect on left ventricular ejection fraction or exercise capacity. Coenzyme Q10 may raise serum levels of coenzyme Q10, however, there is significant heterogeneity. Coenzyme Q10 appeared to lower BNP but not NT‐proBNP blood levels. For adverse events, there is a low‐quality evidence that coenzyme Q10 may result in possible harm, or little to no difference.
Implications for research.
There is a need for high quality, randomised controlled trials with large sample size, comparing coenzyme Q10 to placebo. Such studies should focus on long‐term high‐yield outcomes, especially mortality, cardiovascular events, hospitalisations, and side effects. It would also be helpful to examine similar doses of coenzyme Q10, and unified measures of treatment effects across the studies to allow for meta‐analyses. Comprehensive reporting of the results of such studies is also key.
What's new
| Date | Event | Description |
|---|---|---|
| 16 October 2020 | New citation required and conclusions have changed | Five new studies included, one of the previously included studies removed. Conclusions changed from ‘no convincing evidence’ to ‘moderate evidence of a probable benefit’. |
| 16 October 2020 | New search has been performed | Review updated – the evidence is current to 16 October 2020 |
History
Protocol first published: Issue 9, 2010 Review first published: Issue 6, 2014
Acknowledgements
We are grateful for peer review by Christopher M Florkowski and Taher Entezari‐Maleki.
Appendices
Appendix 1. Search strategies 2020
CENTRAL #1MeSH descriptor: [Ubiquinone] this term only #2ubiquinon* #3ubidecarenone #4q10 or "q 10" #5coq10 or "coq 10" #6coenzyme next Q* #7co‐enzyme next Q* #8neuquinon* #9"quinone" #10ubiquinol #11Bio‐Quinone next Q10 #12(#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11) #13MeSH descriptor: [Heart Failure] explode all trees #14heart next failure* #15cardiac next failure* #16cardiomyopath* #17#13 or #14 or #15 or #16 #18#12 and #17 MEDLINE Ovid 1. Ubiquinone/ 2. ubiquinon*.tw. 3. ubidecarenone.tw. 4. q10.tw. 5. coq10.tw. 6. coenzyme Q*.tw. 7. co‐enzyme Q*.tw. 8. neuquinon*.tw. 9. quinone.tw. 10. ubiquinol.tw. 11. Bio‐Quinone Q10.tw. 12. q 10.tw. 13. coq 10.tw. 14. or/1‐13 15. exp Heart Failure/ 16. (heart adj2 failure*).tw. 17. (cardiac adj2 failure*).tw. 18. (myocardial adj2 failure*).tw. 19. cardiomyopath*.tw. 20. or/15‐19 21. 14 and 20 22. randomized controlled trial.pt. 23. controlled clinical trial.pt. 24. randomized.ab. 25. placebo.ab. 26. drug therapy.fs. 27. randomly.ab. 28. trial.ab. 29. groups.ab. 30. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 31. exp animals/ not humans.sh. 32. 30 not 31 33. 21 and 32 34. limit 33 to ed=20130124‐20201016 Embase Ovid 1. ubidecarenone/ 2. ubiquinone/ 3. ubiquinon*.tw. 4. ubidecarenone.tw. 5. q10.tw. 6. coq10.tw. 7. coenzyme Q*.tw. 8. co‐enzyme Q*.tw. 9. neuquinon*.tw. 10. quinone.tw. 11. ubiquinol.tw. 12. Bio‐Quinone Q10.tw. 13. q 10.tw. 14. coq 10.tw. 15. or/1‐14 16. exp heart failure/ 17. (heart adj2 failure*).tw. 18. (cardiac adj2 failure*).tw. 19. (myocardial adj2 failure*).tw. 20. cardiomyopath*.tw. 21. or/16‐20 22. 15 and 21 23. random$.tw. 24. factorial$.tw. 25. crossover$.tw. 26. cross over$.tw. 27. cross‐over$.tw. 28. placebo$.tw. 29. (doubl$ adj blind$).tw. 30. (singl$ adj blind$).tw. 31. assign$.tw. 32. allocat$.tw. 33. volunteer$.tw. 34. crossover procedure/ 35. double blind procedure/ 36. randomized controlled trial/ 37. single blind procedure/ 38. 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 39. (animal/ or nonhuman/) not human/ 40. 38 not 39 41. 22 and 40 42. limit 41 to dd=20130124‐20201016 ISI Web of Science #16 #15 AND #10 Timespan 2013‐2020 #15 #14 OR #13 OR #12 OR #11 #14 TS=cardiomyopath* #13 TS=(myocardial SAME failure*) #12 TS=(cardiac SAME failure*) #11 TS=(heart SAME failure*) #10 #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #9 TS="Bio‐Quinone Q10" #8 TS=ubiquinol #7 TS=quinone #6 TS=neuquinon* #5 TS=("coenzyme Q*" or "co‐enzyme Q*") #4 TS=ubidecarenone #3 TS=ubiquinon* #2 TS=("q 10" or "coq 10") #1 TS=(q10 or coq10) CINAHL S40 S21 and S39 Published Date: 20130101‐ 20201031 S39 S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 S38 TX cross‐over* S37 TX crossover* S36 TX volunteer* S35 (MH "Crossover Design") S34 TX allocat* S33 TX control* S32 TX assign* S31 TX placebo* S30 (MH "Placebos") S29 TX random* S28 TX (doubl* N1 mask*) S27 TX (singl* N1 mask*) S26 TX (doubl* N1 blind*) S25 TX (singl* N1 blind*) S24 TX (clinic* N1 trial?) S23 PT clinical trial S22 (MH "Clinical Trials+") S21 S14 and S20 S20 S15 or S16 or S17 or S18 or S19 S19 (TI cardiomyopath*) or (AB cardiomyopath*) S18 (TI (myocardial N2 failure*)) or (AB (myocardial N2 failure*)) S17 (TI (cardiac N2 failure*)) or (AB (cardiac N2 failure*)) S16 (TI (heart N2 failure*)) or (AB (heart N2 failure*)) S15 (MH "Heart Failure +") S14 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 S13 (TI coq 10) or (AB coq 10) S12 (TI q 10) or (AB q 10) S11 (TI Bio‐Quinone Q10) or (AB Bio‐Quinone Q10) S10 (TI ubiquinol) or (AB ubiquinol) S9 (TI quinone) or (AB quinone) S8 (TI neuquinon*) or (AB neuquinon*) S7 (TI co‐enzyme Q*) or (AB co‐enzyme Q*) S6 (TI coenzyme Q*) or (AB coenzyme Q*) S5 (TI coq10) or (AB coq10) S4 (TI ubidecarenone) or (AB ubidecarenone) S3 (TI ubiquinon*) or (AB ubiquinon*) S2 (TI q10) or (AB q10) S1 (MH "Coenzyme Q") AMED 1 coenzymes/ 2 Ubiquinone.tw. 3 ubidecarenone.tw. 4 Q10.tw. 5 CoQ10.tw. 6 coenzym$ Q$.tw. 7 co‐enzym$ Q$.tw. 8 neuquinon$.tw. 9 quinone.tw. 10 ubiquinol.tw. 11 Q‐10.tw. 12 or/1‐11 13 heart failure congestive/ 14 heart failure.tw. 15 cardiac failure.tw. 16 cardiomyopathies/ 17 cardiomyopath$.tw. 18 or/13‐17 19 12 and 18 20 limit 19 to yr="2013 ‐ 2020"
International Standard Randomised Controlled Trial Number Register (ISRCTN)
("coenzyme Q" OR "Ubiquinone" OR "ubidecarenone" OR "Q10" OR "CoQ10" OR "coenzyme Q" OR "co‐enzyme" OR "Q neuquinon" OR "quinone" OR "ubiquinol Q‐10") AND ("heart failure" OR "congestive heart failure" OR "cardiac failure" OR "cardiomyopathies" OR "cardiomyopath")
National Institutes of Health (NIH) ClinicalTrials.gov
Condition: "heart failure" OR "congestive heart failure" OR "cardiac failure" OR "cardiomyopathies" OR "cardiomyopath"
Other terms: "coenzyme Q" OR "Ubiquinone" OR "ubidecarenone" OR "Q10" OR "CoQ10" OR "coenzyme Q" OR "co‐enzyme" OR "Q neuquinon" OR "quinone" OR "ubiquinol Q‐10"
Data and analyses
Comparison 1. Coenzyme Q10 versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Myocardial infarction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3 Stroke | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4 Revascularisation procedures | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5 Hospitalisation for heart failure | 2 | 1061 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.49, 0.78] |
| 1.6 Left ventricular ejection fraction (%) | 7 | 650 | Mean Difference (IV, Random, 95% CI) | 1.77 [0.09, 3.44] |
| 1.7 Exercise capacity: treadmill exercise test duration (seconds) | 3 | 91 | Mean Difference (IV, Random, 95% CI) | 48.23 [‐24.75, 121.20] |
| 1.8 Exercise capacity: metabolic equivalent on treadmill exercise test (METs) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.9 Brain natriuretic peptide (pg/mL) | 2 | 162 | Mean Difference (IV, Random, 95% CI) | ‐91.97 [‐103.11, ‐80.83] |
| 1.10 Serum levels of coenzyme Q10 (μg/mL) | 6 | 489 | Mean Difference (IV, Random, 95% CI) | 1.25 [1.09, 1.42] |
| 1.10.1 Studies with coenzyme Q10 dose >200 mg daily | 3 | 377 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.96, 1.30] |
| 1.10.2 Studies with maximum coenzyme Q10 dose of 200 mg daily | 3 | 112 | Mean Difference (IV, Random, 95% CI) | 1.46 [1.19, 1.72] |
| 1.11 Aderse events | 2 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.45, 1.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berman 2004.
| Study characteristics | ||
| Methods | RCT with parallel design | |
| Participants | 32 participants with end‐stage heart failure awaiting heart transplantation were randomly allocated to receive 60 mg U/day of ultrasome coenzyme Q10 or placebo for 3 months Adults (> 18 years) All participants continued their regular medication regimen | |
| Interventions | Intervention: oral coenzyme Q10 60 mg daily Control: placebo |
|
| Outcomes | Symptoms improvement (NYHA classification) Symptoms improvement measured on the Minnesota Living with Heart Failure Questionnaire Quality of life on 6‐minute walk test Blood tests for atrial natriuretic peptide and tumor necrosis factor Echocardiography |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no details about random sequence generation |
| Allocation concealment (selection bias) | Low risk | Group allocation was done by a third (external) party. Participants were given a personal addressed, sealed envelope containing the words "code A" or "code B" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 participants failed to complete the study because of death, need for heart transplantation, drug‐induced intestinal upset, inconvenient travel connections and lack of compliance (1 participant each). Study also did not report the number of participants in the treatment and placebo groups |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available. |
| Other bias | Low risk | No other bias could be found |
Kawashima 2016.
| Study characteristics | ||
| Methods | Randomised double‐blinded RCT with cross‐over design: participants randomised to coenzyme Q10 or placebo at initiation of study and received therapy for 3 months. They underwent 1‐month wash‐out period and then participants were crossed over to receive the other therapy (coenzyme Q10 or placebo). | |
| Participants | 20 adult participants with chronic heart failure and ejection fraction ≤ 40% as documented with the modified Simpson method via echocardiogram, and who had received standard heart failure treatment for at least 1 month. Excluded: people with malignant disease, severe infectious disease, trauma, people undergoing haemodialysis, and who were receiving other supplements. Some people also excluded based on the decision of the attending doctor. |
|
| Interventions | Intervention with dose: oral ubiquinol 200 mg twice daily (400 mg/day) Control: placebo |
|
| Outcomes | Used in this review (only from the first phase, at 3 months):
Not used in this review (not outcomes of interest): weight, systolic and diastolic blood pressure, heart rate, white blood cell, haemoglobin, creatinine, eGFR, cystatin‐C, total cholesterol, LDL cholesterol, triglycerides, high‐sensitivity troponin I, C‐reactive protein, urinary 8‐OHdG/Cr, CoQ: reduced form, oxidized form, ratio for reduced form to total CoQ10, ratio for reduced form to oxidized form Data about NYHA clinical status from the first phase was not provided by the authors and thus, was not used. |
|
| Notes | 5 participants dropped out of the treatment group and 1 participant dropped out of the control group. Only 14 out of 20 participants completed the study and were included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was mentioned to be randomised, however, random sequence generation method was not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Details about allocation concealment were not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding the assessors given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 14 out of 20 participants completed the study and were included in the analysis. 5 participants dropped out of the treatment group and 1 participant dropped out of the control group. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the protocol were reported in the manuscript. |
| Other bias | Low risk | No other bias could be found. |
Keogh 2003.
| Study characteristics | ||
| Methods | RCT with parallel design | |
| Participants | 39 adult participants with NYHA class II or III heart failure were randomised in a double‐blind, placebo‐controlled study to 150 mg/day of oral coenzyme Q10 or placebo for 3 months 19 participants in the coenzyme Q10 group and 20 in the placebo group Adults (> 18 years) | |
| Interventions | Intervention: oral coenzyme Q10 150 mg/day Control: placebo |
|
| Outcomes | Symptom class by NYHA and specific activities scale Exercise tolerance by a 6‐minute walk test Walk test and treadmill exercise test (modified Naughton stress test) assessment for clinical outcomes of heart failure Plasma levels of coenzyme Q10 Assessment for the clinical outcomes of heart failure including readmission, transplantation or death, serum creatinine, sodium and potassium |
|
| Notes | Study was partially funded by pharmaceutical companies that manufacture and distribute coenzyme Q10 supplements. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no details about random sequence generation mentioned |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the placebo group, 2 participants withdrew: 1 because of a rash and nausea and the other for epigastric burning with a history of peptic ulceration In the active group, 1 participant withdrew after 56 days due to increased lethargy and 1 withdrew in order to start carvedilol (prohibited medication) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available. |
| Other bias | Low risk | No other bias could be found. |
Khatta 2000.
| Study characteristics | ||
| Methods | RCT with parallel design | |
| Participants | 55 participants who had congestive heart failure with NYHA class III and IV symptoms to receive either coenzyme Q10 200 mg/day or placebo for a period of 6 months 28 in the treatment group and 27 in the placebo group Adults (> 18 years) | |
| Interventions | Intervention: oral coenzyme Q10 200 mg/day Control: placebo |
|
| Outcomes | Left ventricular ejection fraction (measured by radionuclide ventriculography) Peak oxygen consumption |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a random number generator |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and study personnel were blinded to study group assignment until all data were final Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 participants did not finish the study: 5 in the coenzyme Q10 group and 4 in the placebo group. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available. |
| Other bias | Low risk | No other bias could be found |
Kocharian 2009.
| Study characteristics | ||
| Methods | RCT with parallel design | |
| Participants | 38 participants younger than 18 years with idiopathic dilated cardiomyopathy were assigned to receive either coenzyme Q10 or placebo for a period of 6 months 17 participants in the coenzyme Q10 group and 21 in the placebo group Children (< 18 years) |
|
| Interventions | Intervention: oral coenzyme Q10 2 mg/kg/day in 2 or 3 divided doses, these being increased to the maximum dose of 10 mg/kg/day according to tolerance or the appearance of side effects Control: placebo |
|
| Outcomes | Left ventricular ejection fraction | |
| Notes | This is the only study that evaluated the use of coenzyme Q10 in children | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no details about random sequence generation provided. |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the protocol were reported in the manuscript. |
| Other bias | Low risk | No other bias could be found |
Mareev 2017.
| Study characteristics | ||
| Methods | double‐blinded, randomised controlled clinical trial with parallel design | |
| Participants | 148 participants with heart failure NYHA functional class I to IV and LVEF < 45% who were on optimal medical therapy. Randomisation ratio was 2:1 101 participants in the coenzyme Q10 group and 47 participants in the placebo group |
|
| Interventions | Intervention with dose: coenzyme Q10 nasal drops (90 mg/day = equivalent 225 mg/day for liposoluble tablets) Control: placebo |
|
| Outcomes | All outcomes measured at 24 weeks dynamics of NYHA functional class, The Minnesota Living with Heart Failure Questionnaire, the Kansas City Cardiomyopathy Questionnaire, distance of 6‐minute walking test, LVEF, brain natriuretic peptide level, coenzyme Q10 levels, adverse events |
|
| Notes | 300 participants were initially included in the study, 152 of them failed to follow up and were excluded from the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method was not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Details about allocation concealment were not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information about blinding the assessors given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 300 enrolled participants, 152 participants were excluded as they failed to follow up; only 148 participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available |
| Other bias | Low risk | No other bias could be found |
Morisco 1993.
| Study characteristics | ||
| Methods | RCT with parallel design | |
| Participants | 641 adult (> 18 years) participants with NYHA III or IV heart failure 319 participants in the coenzyme Q10 group and 322 in the placebo group |
|
| Interventions | Intervention: coenzyme Q10 50 mg twice or 3 times daily Control: placebo |
|
| Outcomes | NYHA clinical status Incidence of severe cardiovascular complications (pulmonary oedema, cardiac asthma, arrhythmia) Length of hospitalisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation |
| Allocation concealment (selection bias) | Unclear risk | Details about allocation concealment were not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 37 participants dropped out in the treatment group and 41 dropped out in the placebo group |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available |
| Other bias | Low risk | No other bias was found |
Mortensen 2014.
| Study characteristics | ||
| Methods | Randomized, double‐blinded RCT with parallel design with short‐term (16 weeks) and long‐term (106 weeks) follow‐up | |
| Participants | 420 adult participants with heart failure were randomised. Inclusion criteria: chronic heart failure in NYHA functional class III or IV with stable HF therapy Exclusion criteria: myocardial infarction, unstable angina pectoris, percutaneous coronary intervention, cardiac resynchronisation device, cardiac surgery, or stroke, all within 6 weeks of enrolment; HF from congenital heart disease; uncorrected valvular heart disease or planned valve surgery; planned other cardiac surgery or resynchronisation therapy; on urgent waiting list for heart transplantation (status 1 person); implanted mechanical assist device; on continuous inotropic support for HF; restrictive or hypertrophic cardiomyopathy; alcoholic heart disease; acute inflammatory myocarditis; severe non‐cardiac disease including malignancy with life expectancy < 1 year; psychosocial instability or anticipated problems with compliance; women of childbearing potential and lactating women; allergy to the constituents of the test medication; supplementary Coenzyme Q10 intake within the last month before run‐in; 6‐minute walk distance > 450 metres at run‐in; participation in another controlled trial 202 participants randomised to coenzyme Q10 group and 218 participants to the placebo group |
|
| Interventions | Intervention with dose: oral coenzyme Q10 100 mg 3 times a day with standard HF therapy Control: placebo with standard HF therapy. |
|
| Outcomes | Used in this review: all‐cause mortality (at 106 weeks); cardiovascular mortality (at 106 weeks); major adverse cardiovascular events (at 106 weeks); myocardial infarction (at 106 weeks); stroke (at 106 weeks); revascularisation procedures percutaneous intervention and coronary artery bypass grafting (at 106 weeks); hospitalisation due to heart failure (at 106 weeks); left ventricular ejection fraction (at 16 and 106 weeks); symptoms improvement: NYHA, visual analogue scale score, 6‐minutes walk test (at 16 and 106 weeks); N‐terminal pro‐brain natriuretic peptide (NT‐proBNP; at 16 and 106 weeks); serum levels of coenzyme Q10 (at 16 and 106 weeks); adverse events (at 106 weeks). Not used in this review (not outcomes of interest): heart rate, systolic and diastolic blood pressure, left ventricular end systolic and end diastolic pressure (at 16 and 106 weeks) |
|
| Notes | 36 participants (22 in the coenzyme Q10 group and 14 in the placebo group) withdrew from the study; however, were included in the intention‐to‐treat analysis. 8 participants (4 in each group) were lost to follow‐up at the end of the study period. Intention‐to‐treat analysis was used for all‐cause mortality, cardiovascular mortality, hospitalisations, and adverse events (including revascularisation procedures, strokes, and MI). Study was partially funded by pharmaceutical companies that manufacture and distribute coenzyme Q10 supplements. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study was randomised. Random sequence generation method was detailed in the methods section. |
| Allocation concealment (selection bias) | Low risk | Random numbers generated for randomisation were kept in sealed envelopes. Randomisation code was unavailable to investigators, participants, or statisticians. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The randomisation code was unavailable to investigators, participants, or statisticians at any time during the study until all data and materials had been collected, all blood samples had been analysed, and statistical analyses had been performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 36 participants (22 in the coenzyme Q10 group and 14 in the placebo group) withdrew from the study; however, were included in the intention‐to‐treat analysis. Analysing withdrawal reasons did not show any significant differences between the groups. 8 participants (4 in each group) were lost to follow‐up at the end of the study period. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the protocol were reported in the manuscript. |
| Other bias | Low risk | No other risk of bias was found. |
Munkholm 1999.
| Study characteristics | ||
| Methods | RCT with parallel design | |
| Participants | 22 adult participants with NYHA II or III heart failure 11 participants in the treatment group and 11 in the placebo group Adults (> 18 years) Before and after the treatment period, a right heart catheterisation was done |
|
| Interventions | Intervention: oral coenzyme Q10 100 mg twice daily for 12 weeks Control: placebo |
|
| Outcomes | Baseline and post‐therapeutic serum levels of coenzyme Q10 Left ventricular ejection fraction NYHA clinical status |
|
| Notes | This study is an invasive study investigating the treatment of congestive heart failure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no information about random sequence generation provided. |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available |
| Other bias | Low risk | No other risk of bias was found |
Sobirin 2019.
| Study characteristics | ||
| Methods | unblinded, randomised controlled clinical trial with parallel design | |
| Participants | 30 participants with clinical heart failure were randomised. Inclusion criteria: age ≥ 45 years, NYHA functional status II to IV, EF ≥ 50%, evidence of diastolic dysfunction on non‐invasive imaging, and who had received standard heart failure treatment for 4 weeks before the study Exclusion criteria: chronic atrial fibrillation, acute coronary syndrome or coronary revascularisation within 60 days, clinically significant valvular disease, significantly low systolic blood pressure (< 100 mmHg) or high blood pressure, people with a prior LVEF < 40%, known infiltrative cardiomyopathy (e.g. amyloidosis), hypertrophic cardiomyopathy or chronic pericardial disease, dyspnoea or oedema due to non‐cardiac causes, such as pulmonary disease, and anaemia (Hb < 8.0 g/dL), inability or refusal to provide informed consent, poor echocardiographic recordings 15 participants were randomised to coenzyme Q10 group and 15 participants to the control group |
|
| Interventions | Intervention with dose: oral coenzyme Q10 100 mg three times a day + conventional therapy Control: conventional therapy alone |
|
| Outcomes | Outcomes measured at 30 days Used in this review: left ventricular ejection fraction measured by echocardiography Not used in this review (not outcomes of interest): parameters for diastolic function (E/e' ratio, medial and lateral e', E velocity, E/A velocity ratio, deceleration t and LAVI), diastolic and systolic left ventricle internal diameter. left ventricular mass index |
|
| Notes | 1 participant in the treatment group and 1 participant in the control group were lost to follow‐up, and were excluded from the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | study is randomised; randomisation done by permuted blocks with a ratio of 1:1 |
| Allocation concealment (selection bias) | Unclear risk | Details about allocation concealment were not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study was unblinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the randomised participants, 2 participants were not included in the analysis |
| Selective reporting (reporting bias) | High risk | Some outcomes listed in the protocol were not reported in the manuscript (plasma malondialdehyde level, quality of life was measured using the Minnesota Living with Heart Failure Questionnaire (MLHFQ)) |
| Other bias | Low risk | No other risk of bias was found |
Zhao 2015.
| Study characteristics | ||
| Methods | Randomized double‐blinded RCT with parallel design | |
| Participants | 128 consecutive adult participants with non‐ischaemic heart failure were randomised. Inclusion criteria: NYHA functional status II to IV, EF < 40%, and who had received standard heart failure treatment for 3 to 6 months before the study. Exclusion criteria: people with atrial fibrillation before the study, renal or liver dysfunction, acute heart failure, acute coronary syndrome, or heart failure of ischaemic origin 62 participants randomised to coenzyme Q10 group and 66 participants to the control group. |
|
| Interventions | Intervention with dose: oral coenzyme Q10 30 mg/day + conventional therapy Control: conventional therapy alone |
|
| Outcomes | All outcomes measured at 6 and 12 months Used in this review: left ventricular ejection fraction measured by echocardiography Not used in this review (not outcomes of interest): incidence of atrial fibrillation, left ventricular end diastolic diameter (LVED) and left atrial end‐diastolic diameter, TNF‐α and IL‐6, high‐sensitivity C‐reactive protein (hs‐CRP), malondialdehyde (MDA). |
|
| Notes | 14 participants died and 12 participants lost to follow‐up and were excluded. Only 102 participants completed the study and were included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was mentioned to be randomised, however, random sequence generation method was not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Details about allocation concealment were not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physicians, echocardiography staff, laboratory, and the statistician were all blinded to the study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14 participants died and 12 participants lost to follow‐up and were excluded. Only 102 participants (of 128 randomised) completed the study and were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol could not be located |
| Other bias | Low risk | No other risk of bias was found. |
LVEF: left ventricular ejection fraction
NYHA: New York Heart Association RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adarsh 2008 | No randomisation; not an RCT |
| Azuma 1992 | Title is not related to our main area of interest |
| Baggio 1993 | Not a RCT |
| Belardinelli 2005 | RCT study with a cross‐over design; first phase data are unavailable |
| Belardinelli 2008 | Title is not related to our main area of interest |
| Cascone 1985 | Not a RCT |
| Chen 2017 | Not a RCT, review article |
| Chen 2018 | Not a RCT, no control arm |
| Chew 2008 | Title is not related to our main area of interest |
| Davini 1992 | Title is not related to our main area of interest |
| Fedacko 2009 | Participants in the study did not have heart failure |
| Hall 1990 | Not a RCT |
| Hanping 1997 | Title is not related to our main area of interest |
| Hofman‐Bang 1995 | RCT study with a cross‐over design; first phase data are unavailable |
| IRCT2015070223018N1 | Peoeple with heart disease were excluded |
| Ishiyama 1976 | Not a RCT |
| Iwabuchi 1972 | Not a RCT |
| Johansson 2013 | Investigating coenzyme Q10 combined with selenium |
| JPRN‐UMIN000007695 | Not investigating coenzyme Q10 |
| JPRN‐UMIN000020203 | Not a RCT, no control arm |
| JPRN‐UMIN000027248 | Study meets inclusion criteria, but was terminated before it was finished, and no results were generated or reported, according to the first author. |
| Khatta 1999 | Title is not related to our main area of interest |
| Kukharchik 2016 | Abstract has no usable data, authors were contacted but did not respond |
| Kukharchik 2016a | Abstract has no usable data, authors were contacted but did not respond |
| Kukharchik 2017 | Abstract has no usable data, authors were contacted but did not respond |
| Kumar 2007 | Title is not related to our main area of interest |
| Kumar 2015 | Abstract has no usable data, authors were contacted but did not respond |
| Lampertico 1993 | Not a RCT |
| Langsjoen 1985 | Not a RCT |
| Langsjoen 1985a | Title is related to our main area of interest, but none of the selected participants were in frank congestive heart failure |
| Langsjoen 1988 | Not a RCT |
| Langsjoen 1990 | Not a RCT |
| Langsjoen 1994 | Not a RCT |
| Langsjoen 2008 | Title is not related to our main area of interest |
| Leonova 2018 | Abstract has no usable data, authors were contacted but did not respond |
| Manzoli 1990 | Not a RCT |
| Mazzola 1987 | RCT study with a cross‐over design; first phase data are unavailable |
| McMurray 2009 | Not investigating supplemental coenzyme Q10 |
| Miyazaki 2013 | Not a RCT, not investigating supplemental coenzyme Q10 |
| Morisco 1994 | RCT study with a cross‐over design; first phase data are unavailable |
| Mortensen 1985 | Not a RCT |
| NCT03586414 | Includes only healthy individuals, people with cardiovascular disease were excluded |
| Nishimura 1996 | Title is not related to our main area of interest |
| Oleg 2016 | Abstract has no usable data, authors were contacted but did not respond |
| Permanetter 1992 | RCT study with a cross‐over design; first phase data are unavailable |
| Poggesi 1991 | Title is related to our main area of interest, but none of the selected participants were in frank congestive heart failure |
| Pourmoghaddas 2014 | Investigating coenzyme Q10 combined with atorvastatin |
| Rivera 2017 | Did not include people with heart failure |
| Sacher 1997 | Not a RCT |
| Saurabh 2014 | No usable data in the abstract; authors contacted but did not respond |
| Schneeberger 1984 | RCT study with a cross‐over design; first phase data are unavailable |
| Sinatra 2000 | Title is not related to our main area of interest |
| Sinatra 2004 | Title is not related to our main area of interest |
| Turk 2013 | Did not include people with heart failure |
| Watson 1999 | RCT study with a cross‐over design; first phase data are unavailable |
| Witte 2005 | Title is not related to our main area of interest |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT02779634.
| Study name | Ubiquinol treatment in participants with heart failure and preserved ejection fraction |
| Methods | Randomized, triple‐blinded (participant, investigator, outcomes assessor), placebo‐controlled, parallel trial |
| Participants | Adults > 50 years old, meeting the following inclusion criteria:
|
| Interventions | Placebo: sugar pill three times daily
Ubiquinol (coenzyme Q10): pills of 100 mg, three times daily Interventions administered for 16 weeks |
| Outcomes |
Outcomes assessed at 4 months |
| Starting date | July 2016 |
| Contact information | Sara Elias (sarae@hadassah.org.il) David Leibowitz, MD (oleibo@hadassah.org.il) |
| Notes | Recruitment done, finishing follow‐up |
Pierce 2018.
| Study name | Reducing symptom burden in participants with heart failure with preserved ejection fraction using ubiquinol and/or D‐ribose |
| Methods | Randomized, double‐blinded (participant, investigator), placebo‐controlled, parallel trial |
| Participants | Adults, 50 years and older, meeting the following inclusion criteria:
|
| Interventions | Arm 1 (placebo only): placebo pills that are 300 mg capsules, two times daily plus 15 grams placebo powder, mixed with non‐carbonated liquid, one time daily Arm 2 (coenzyme Q10 only): 300 mg capsules of coenzyme Q10, two times daily plus 15 grams placebo powder, mixed with non‐carbonated liquid, one time daily Arm 3 (D‐ribose only): 15 grams D‐Ribose powder, mixed with non‐carbonated liquid, one time daily plus placebo pills that are 300 mg capsules, two times daily Arm 4 (coenzyme Q10 + D‐ribose): 300 mg capsules of coenzyme Q10, two times daily plus 15 grams D‐Ribose powder, mixed with non‐carbonated liquid, one time daily Interventions administered for up to 12 weeks |
| Outcomes | Primary outcomes: change in health status of participants, measured by the Kansas City Cardiomyopathy Questionnaire Secondary outcomes:
|
| Starting date | February 5, 2018 |
| Contact information | Faith Rahman (frahman2@kumc.edu) Janet Pierce, PhD (jpierce@kumc.edu) |
| Notes |
Differences between protocol and review
In this update, we re‐ran searches for all databases listed in the Methods section from 2013. We reassessed studies included in the original review for inclusion, and subsequently excluded one. We also reassessed these studies for risk of bias. We added a ‘Summary of findings’ table to summarise available data on all outcomes we rated as important to care and decision making. Age and gender were the two subgroups planned for analysis in the protocol; however, this was not possible, due to insufficient data. We conducted a posthoc subgroup analysis to compare studies without a maximum coenzyme Q10 dose against those with a maximum dose of 200 mg daily.
Contributions of authors
TA: trial identification, data extraction, 'Risk of bias, assessment, data analysis, 'Summary of findings' table, developed and finalised the protocol and the review.
YA: trial identification, data extraction, 'Risk of bias' assessment, developed and finalised the protocol and the review.
MF: trial identification, data extraction, 'Risk of bias' assessment, drafting the review.
KT: trial identification, data extraction, 'Risk of bias' assessment, 'Summary of findings' table, and drafting the review.
AM: trial identification, data extraction, 'Risk of bias' assessment, drafting the review.
BS: trial identification, data extraction, 'Risk of bias' assessment, drafting the review.
MK: trial identification, data extraction, 'Risk of bias' assessment, drafting the review.
AE: provided methodological support, trial identification, developed and finalised the protocol and the review
MM: provided methodological support, trial identification, developed and finalised the protocol and the review
All authors contributed to, and approved the final version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR Infrastructure Funding, UK
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to the Heart Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
TA has no conflicts of interest to declare
YA has no conflicts of interest to declare
MF has no conflicts of interest to declare
KT has no conflicts of interest to declare
AM has no conflicts of interest to declare
BS has no conflicts of interest to declare
MK has no conflicts of interest to declare
AE has no conflicts of interest to declare
MM has no conflicts of interest to declare
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Berman 2004 {published data only}
- Berman M, Erman A, Ben-Gal T, Dvir D, Georghiou GP, Stamler A, et al. Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: a randomized, placebo-controlled study. Clinical Cardiology 2004;27(10):A26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M, Erman A, Ben-Gal T, Dvir D, Georghiou GP, Stamler A, et al. Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: a randomized, placebo-controlled study. Clinical Cardiology 2004;27(5):295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawashima 2016 {published data only}
- JPRN-UMIN000012604. Effect of the REduced form of COenzyme Q10 Supplementation on endothelial function in chronic heart failure (RECOQS). apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000012604 (date of registration 17 December 2013).
- Kawashima C, Matsuzawa Y, Konishi M, Akiyama E, Suzuki H, Sato R, et al. Ubiquinol improves endothelial function in patients with heart failure with reduced ejection fraction: a single-center, randomized double-blind placebo-controlled crossover pilot study. American Journal of Cardiovascular Drugs 2020;20(4):363-72. [DOI] [PubMed] [Google Scholar]
- Kawashima C, Matsuzawa Y, Akiyama E, Sato R, Konishi M, Suzuki H, et al. Ubiquinol improves endothelial function in patients with heart failure with reduced ejection fraction: a single center, randomized double-blind placebo-controlled cross-over study. Circulation 2016;134(Suppl 1):Abstract 14946. [DOI] [PubMed] [Google Scholar]
Keogh 2003 {published data only}
- Keogh A, Fenton S, Leslie C, Aboyoun C, Macdonald P, Zhao YC, et al. Randomised double-blind, placebo-controlled trial of coenzyme Q10 therapy in class II and III systolic heart failure. Heart, Lung and Circulation 2003;12(3):135-41. [DOI] [PubMed] [Google Scholar]
Khatta 2000 {published data only}
- Khatta M, Alexander BS, Krichten CM, Fisher ML, Freudenberger R, Robinson SW, et al. The effect of coenzyme Q10 in patients with congestive heart failure. Annals of Internal Medicine 2000;132(8):636-40. [DOI] [PubMed] [Google Scholar]
Kocharian 2009 {published data only}
- Kocharian A, Shabanian R, Rafiei-Khorgami M, Kiani A, Heidari-Bateni G, Kocharian Armen, et al. Coenzyme Q10 improves diastolic function in children with idiopathic dilated cardiomyopathy. Cardiology in the Young 2009;19(5):501-6. [DOI] [PubMed] [Google Scholar]
- NCT02115581. Coenzyme Q10 supplementation in children with idiopathic dilated cardiomyopathy. clinicaltrials.gov/show/nct02115581 (first posted 16 April 2014).
Mareev 2017 {published data only}
- Mareev VY, Minina YV, Mareev YV. Coenzyme Q-10 in treatment of patients with heart failure: results Russian multicenter double blind placebo controlled study. European Journal of Heart Failure 2017;19(Suppl 1):56. [Google Scholar]
Morisco 1993 {published data only}
- Morisco C, Trimarco B, Condorelli M. Effect of coenzyme Q10 therapy in patients with congestive heart failure: a long-term multicenter randomized study. Clinical Investigator 1993;71(8 Suppl):S134-6. [DOI] [PubMed] [Google Scholar]
Mortensen 2014 {published data only}
- EUCTR 2005-002960-27-HU. Coenzyme Q10 as adjunctive treatment of chronic heart failure. A randomised double-blind multicenter trial with focus on symptoms, biomarker status (BNP) and long-term outcome (hospitalisation/mortality) (Q-Symbio). www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2005-002960-27-HU (first entered 20 January 2006).
- ISRCTN94506234. Coenzyme Q10 as adjunctive treatment of chronic heart failure: a randomised, double-blind, multicentre trial with focus on SYMptoms, BIOmarker status (Brain-Natriuretic Peptide (BNP)), and long-term outcome (hospitalisations/mortality). www.isrctn.com/ISRCTN94506234 (date assigned 23 April 2007).
- Mortensen AL, Rosenfeldt F, Filipiak KJ. Effect of coenzyme Q10 in Europeans with chronic heart failure: a sub-group analysis of the Q-SYMBIO randomized double-blind trial. Cardiology Journal 2019;26(2):147-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SA, Dolliner P, Filipiak KJ, Alehagen U, Pella D, Steurer G, et al. Is the therapeutic efficacy of coenzyme Q10 replicated in a geographical subgroup of the Q-SYMBIO study? European Heart Journal 2015;36:659-60. [Google Scholar]
- Mortensen SA, Kumar A, Dolliner P, Filipiak KJ, Pella D, Alehagen U, et al. Long-term results with coenzyme Q10 as adjunctive therapy in chronic heart failure. Heart 2013;99:A55-6. [Google Scholar]
- Mortensen SA, Kumar A, Dolliner P, Filipiak KJ, Pella D, Alehagen U, et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure. Results from the Q-SYMBIO study. European Journal of Heart Failure 2013;12:S21. [DOI] [PubMed] [Google Scholar]
- Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, et al. Coenzyme Q10 therapy blocks the vicious metabolic cycle in chronic heart failure. Journal of Cardiac Failure 2013;1:S25. [Google Scholar]
- Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC: Heart Failure 2014;2(6):641-9. [DOI] [PubMed] [Google Scholar]
Munkholm 1999 {published data only}
- Munkholm H, Hansen HH, Rasmussen K. Coenzyme Q10 treatment in serious heart failure. Biofactors 1999;9(2-4):285-9. [DOI] [PubMed] [Google Scholar]
Sobirin 2019 {published data only}
- ISRCTN96610559. Coenzyme Q10 supplementation in heart failure with preserved ejection fraction patients. www.isrctn.com/ISRCTN96610559 (date assigned 2 March 2018).
- Sobirin MA, Herry Y, Sofia SN, Uddin I, Rifqi S, Tsutsui H. Effects of coenzyme Q10 supplementation on diastolic function in patients with heart failure with preserved ejection fraction. Drug Discoveries & Therapeutics 2019;13(1):38-46. [DOI] [PubMed] [Google Scholar]
Zhao 2015 {published data only}
- Qingyan Z, Okello E, Yanhong T, Bing W, Congxin H. Effect of coenzyme Q10 administration on the incidence of atrial fibrillation in patients with heart failure. Circulation 2010;122(2):e209. [Google Scholar]
- Zhao Q, Kebbati AH, Zhang Y, Tang Y, Okello E, Huang C. Effect of coenzyme Q10 on the incidence of atrial fibrillation in patients with heart failure. Journal of Investigative Medicine 2015;63(5):735-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adarsh 2008 {published data only}
- Adarsh K, Kaur H, Mohan V. Coenzyme Q10 (CoQ10) in isolated diastolic heart failure in hypertrophic cardiomyopathy (HCM). BioFactors (Oxford, England) 2008;32(1-4):145-9. [DOI] [PubMed] [Google Scholar]
Azuma 1992 {published data only}
- Azuma J, Sawamura A, Awata N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Japanese Circulation Journal 1992;56(1):95-9. [DOI] [PubMed] [Google Scholar]
Baggio 1993 {published data only}
- Baggio E, Gandini R, Plancher AC, Passeri M, Carmosino G, the CoQ10 Drug Surveillance Investigators. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure (interim analysis). Clinical Investigator 1993;71(8 Suppl):S145-9. [DOI] [PubMed] [Google Scholar]
Belardinelli 2005 {published data only}
- Belardinelli R, Mucaj A, Lacalaprice F, Solenghi M, Principi F, Tiano L, et al. Coenzyme Q10 improves contractility of dysfunctional myocardium in chronic heart failure. Biofactors 2005;25(1-4):137-45. [DOI] [PubMed] [Google Scholar]
Belardinelli 2008 {published data only}
- Belardinelli R, Tiano L, Littarru GP. Oxidative stress, endothelial function and coenzyme Q10. Biofactors 2008;32(1-4):129-33. [DOI] [PubMed] [Google Scholar]
Cascone 1985 {published data only}
- Cascone A, Cascone G, Alaimo A. Treatment of patients with congestive heart failure with coenzyme Q10 in an open trial. Bollettino Chimico Farmaceutico 1985;124(5):43S-52S. [PubMed] [Google Scholar]
Chen 2017 {published data only}
- Yuanyuan C, Guozhen S, Xiaoran M. Research progress on correlation and intervention of micronutrients and chronic heart failure. Chinese Nursing Research 2017;31(34):4334-7. [Google Scholar]
Chen 2018 {published data only}
- Chen FL, Chang PS, Lin YC, Lin PT. A pilot clinical study of liquid ubiquinol supplementation on cardiac function in pediatric dilated cardiomyopathy. Nutrients 2018;10(11):07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chew 2008 {published data only}
- Chew GT, Watts GF, Davis TME, Stuckey BGA, Beilin LJ, Thompson PL, et al. Hemodynamic effects of fenofibrate and coenzyme Q10 in type 2 diabetic subjects with left ventricular diastolic dysfunction. Diabetes Care 2008;31(8):1502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davini 1992 {published data only}
- Davini A, Cellerini F, Topi PL. Coenzyme Q10: contractile dysfunction of the myocardial cell and metabolic therapy. Minerva Cardioangiologica 1992;40(11):449-53. [PubMed] [Google Scholar]
Fedacko 2009 {published data only}
- Fedacko J, Pella D, Rybar R. Influence of coenzyme Q10 supplementation in statin treated patients on left ventricular diastolic dysfunction. Results of randomised double-blind clinical study. European Heart Journal 2009;30:369-70. [Google Scholar]
Hall 1990 {published data only}
- Hall JH, Judy WV, Folkers K. Long-term survival in coenzyme Q10 treated congestive-heart-failure patients. Circulation 1990;82(4):675. [Google Scholar]
Hanping 1997 {published data only}
- Hanping Z, Guolong Y, Jing L, Jin H. The changes of PRA, ATII, ALD, ET and ANP in patients with left ventricular and intervention with enalapril. Bulletin of Hunan Medical University 1997;22(4):323-6. [PubMed] [Google Scholar]
Hofman‐Bang 1995 {published data only}
- Hofman-Bang C, Rehnqvist N, Swedberg K, Wiklund I, Astrom H, the Q10 Study Group. Coenzyme Q10 as an adjunctive in the treatment of chronic congestive heart failure. Journal of Cardiac Failure 1995;1:101-7. [DOI] [PubMed] [Google Scholar]
IRCT2015070223018N1 {published data only}
- IRCT2015070223018N1. Effect of Q10 coenzyme in improving cardiac function. en.irct.ir/trial/19746 (date registered 11 August 2015).
Ishiyama 1976 {published data only}
- Ishiyama T, Morita Y, Toyama S, Yamagami T, Tsukamoto N. A clinical study of the effect of coenzyme Q on congestive heart failure. Japanese Heart Journal 1976;17(1):32-42. [DOI] [PubMed] [Google Scholar]
Iwabuchi 1972 {published data only}
- Iwabuchi T. Clinical efficacy of coenzyme Q10 for cardiac failure: a double-blind controlled comparison. Rinsho to Kenkyu (Japanese Journal of Clinical and Experimental Medicine) 1972;49(9):2604-8. [Google Scholar]
Johansson 2013 {published data only}
- Johansson P, Dahlstrom O, Dahlstrom U, Alehagen U. Effect of selenium and Q10 on the cardiac biomarker NT-proBNP. Scandinavian Cardiovascular Journal 2013;47(5):281-8. [DOI] [PubMed] [Google Scholar]
- Johansson P, Dahlstrom O, Dahlstrom U, Alehagen U. Improved health-related quality of life, and more days out of hospital with supplementation with selenium and coenzyme Q10 combined. Results from a double blind, placebo-controlled prospective study. Journal of Nutrition Health & Aging 2015;19(9):870-7. [DOI] [PubMed] [Google Scholar]
JPRN‐UMIN000007695 {published data only}
- JPRN-UMIN000007695. Comparison of effects of pitavastatin and rosuvastatin on patients with chronic heart failure. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000007695 (date of disclosure 10 April 2012).
JPRN‐UMIN000020203 {published data only}
- JPRN-UMIN000020203. Study of a heart failure treatment using a combination drug consisting of reduced coenzyme Q10, astaxanthin, citrulline, and zinc. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000020203 (date registered 15 December 2015).
JPRN‐UMIN000027248 {published data only}
- JPRN-UMIN000027248. Effects of reduced form of coenzyme Q10 (ubiquinol) on heart failure and arrhythmias. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000027248 (date of registration 1 October 2017).
Khatta 1999 {published data only}
- Khatta M, Alexander BS, Krichten CM, Fisher ML, Freudenberger RS, Robinson SW, et al. Long-term efficacy of coenzyme Q10 therapy for congestive heart failure. Circulation 1999;100(18):3411. [Google Scholar]
Kukharchik 2016 {published data only}
- Kukharchik G, Sichinava L, Burbello A, Yubrina I, Gaykovaya L, Sorokin L, et al. Analysis of changes of ECHO-cardiogram indices in patients with chronic heart failure during therapy with coenzyme Q10. European Journal of Heart Failure 2016;18:109. [Google Scholar]
Kukharchik 2016a {published data only}
- Kukharchik G, Sichinava L, Gaykovaya L, Burbello A, Yubrina I, Sorokin L. Some aspects of the effect of coenzyme Q10 on inflammatory processes, remodeling and functional status of endothelium in chronic heart failure. European Journal of Heart Failure 2016;18:399. [Google Scholar]
Kukharchik 2017 {published data only}
- Kukharchik G, Lobanova O, Sichinava L, Gaikovaya L, Ermakov A. The effect of coenzyme Q10 on the content of proinflammatory makers and the realization of the mitochondrial mechanism of apoptosis in chronic heart failure. Journal of the American College of Cardiology 2017;70(16):C145. [Google Scholar]
Kumar 2007 {published data only}
- Kumar A, Singh RB, Saxena M, Niaz MA, Josh SR, Chattopadhyay P, et al. Effect of carni Q-gel (ubiquinol and carnitine) on cytokines in patients with heart failure in the Tishcon study. Acta Cardiologica 2007;62(4):349-54. [DOI] [PubMed] [Google Scholar]
Kumar 2015 {published data only}
- Kumar A, Mohan V, Harharpreet K. Coenzyme Q10 as an adjuvant therapy in heart failure (HF). Cardiology 2015;131:345. [Google Scholar]
Lampertico 1993 {published data only}
- Lampertico M, Comis S. Italian multicenter study on the efficacy and safety of coenzyme Q10 as adjuvant therapy in heart-failure. Clinical Investigator 1993;71(8):S129-33. [DOI] [PubMed] [Google Scholar]
Langsjoen 1985 {published data only}
- Langsjoen PH, Vadhanavikit S, Folkers K. Effective treatment with coenzyme Q10 of patients with chronic myocardial disease. Drugs Under Experimental & Clinical Research 1985;11(8):577-9. [PubMed] [Google Scholar]
Langsjoen 1985a {published data only}
- Langsjoen PH, Vadhanavikit S, Folkers K. Response of patients in classes III and IV of cardiomyopathy to therapy in a blind and crossover trial with coenzyme Q10. Proceedings of the National Academy of Sciences of the United States of America 1985;82(12):4240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langsjoen 1988 {published data only}
- Langsjoen PH, Folkers K, Lyson K, Muratsu K, Lyson T, Langsjoen P. Effective and safe therapy with coenzyme Q10 for cardiomyopathy. Klinische Wochenschrift 1988;66(13):583-90. [DOI] [PubMed] [Google Scholar]
Langsjoen 1990 {published data only}
- Langsjoen PH, Langsjoen PH, Folkers K. Long-term efficacy and safety of coenzyme Q10 therapy for idiopathic dilated cardiomyopathy. American Journal of Cardiology 1990;65(7):521-3. [DOI] [PubMed] [Google Scholar]
Langsjoen 1994 {published data only}
- Langsjoen H, Langsjoen P, Langsjoen P, Willis R, Folkers K. Usefulness of coenzyme Q10 in clinical cardiology: a long-term study. Molecular Aspects of Medicine 1994;15 Suppl:s165-75. [DOI] [PubMed] [Google Scholar]
Langsjoen 2008 {published data only}
- Langsjoen PH, Langsjoen AM. Supplemental ubiquinol in patients with advanced congestive heart failure. Biofactors 2008;32(1-4):119-28. [DOI] [PubMed] [Google Scholar]
Leonova 2018 {published data only}
- Leonova I A, Boldueva S, Kuharchik G, Sichinava L, Gaykovaya L. Changing of some markers of inflammation and remodeling in post-infarction patients with chronic heart failure after ubidecarenone treatment. European Journal of Heart Failure 2018;20(Suppl 1):82-3. [Google Scholar]
Manzoli 1990 {published data only}
- Manzoli U, Rossi E, Littarru GP, Frustaci A, Lippa S, Oradei A, et al. Coenzyme Q10 in dilated cardiomyopathy. International Journal of Tissue Reactions 1990;12(3):173-8. [PubMed] [Google Scholar]
Mazzola 1987 {published data only}
- Mazzola C, Guffanti EE, Vaccarella A. Noninvasive assessment of coenzyme Q10 in patients with chronic stable effort angina and moderate heart failure. Current Therapeutic Research, Clinical and Experimental 1987;41(6):923-32. [Google Scholar]
McMurray 2009 {published data only}
- McMurray JJV, Kjekshus J, Dunselman P, Hjalmarson A, Wedel H, Lindberg M, et al. Prognostic importance of co-enzyme Q10 in heart failure and interaction with statin therapy in the controlled rosuvastatin Mmltinational trial in heart failure trial (CORONA). European Heart Journal 2009;30:1027. [Google Scholar]
Miyazaki 2013 {published data only}
- Miyazaki T, Shimizu M, Takagi A, Kato T, Suda S, Hiki M, et al. Coenzyme Q10 levels are associated with occurrence of cardiogenic shock and in-hospital death in patients admitted to coronary care unit. Circulation 2013;128(22):Abstract 17490. [Google Scholar]
Morisco 1994 {published data only}
- Morisco C, Nappi A, Argenziano L, Sarno D, Fonatana D, Imbriaco M, et al. Noninvasive evaluation of cardiac hemodynamics during exercise in patients with chronic heart failure: effects of short-term coenzyme Q10 treatment. Molecular Aspects of Medicine 1994;15 Suppl:s155-63. [DOI] [PubMed] [Google Scholar]
Mortensen 1985 {published data only}
- Mortensen SA, Vadhanavikit S, Baandrup U, Folkers K. Long-term coenzyme Q10 therapy: a major advance in the management of resistant myocardial failure. Drugs under Experimental and Clinical Research 1985;11(8):581-93. [PubMed] [Google Scholar]
NCT03586414 {published data only}
- NCT03586414. MitoQ supplementation and cardiovascular function in healthy men and women. clinicaltrials.gov/show/nct03586414 (first posted 13 July 2018).
Nishimura 1996 {published data only}
- Nishimura T, Hori M. Therapeutic effects of coenzyme Q10 on dilated cardiomyopathy: assessment by 123I-BMIPP myocardial single photon emission computed tomography (SPECT): a multicenter trial in Osaka University Medical School Group. Kaku Igaku (Japanese Journal of Nuclear Medicine) 1996;33(1):27-32. [PubMed] [Google Scholar]
Oleg 2016 {published data only}
- Medvedev O, Tokareva OG, Gorodetskaya EA, Kalenikova EI, Sizova ZM. CoQ10 treatment of patients with CHF and effects of BNP level. European Journal of Heart Failure 2016;18:107. [Google Scholar]
Permanetter 1992 {published data only}
- Permanetter B, Rossy W, Klein G, Weingartner F, Seidl KF, Blomer H. Ubiquinone (coenzyme Q10) in the long-term treatment of idiopathic dilated cardiomyopathy. European Heart Journal 1992;13(11):1528-33. [DOI] [PubMed] [Google Scholar]
Poggesi 1991 {published data only}
- Poggesi L, Galanti G, Comeglio M, Toncelli L, Vinci M. Effect of coenzyme Q10 on left ventricular function in patients with dilative cardiomyopathy. A medium-term randomized double-blind study versus placebo. Current Therapeutic Research, Clinical and Experimental 1991;49(5):878-86. [Google Scholar]
Pourmoghaddas 2014 {published data only}
- NCT01925937. Atorvastatin/coenzymeQ10 in congestive heart failure. clinicaltrials.gov/show/nct01925937 (first posted 20 August 2013).
- Pourmoghaddas M, Rabbani M, Shahabi J, Garakyaraghi M, Khanjani R, Hedayat P. Combination of atorvastatin/coenzyme Q10 as adjunctive treatment in congestive heart failure: a double-blind randomized placebo-controlled clinical trial. Arya Atherosclerosis 2014;10(1):1-5. [PMC free article] [PubMed] [Google Scholar]
Rivera 2017 {published data only}
- Rivera MB, Yeung CK, Robinson-Cohen C, Phillips BR, Ruzinski J, Rock D, et al. Effect of coenzyme Q10 on biomarkers of oxidative stress and cardiac function in hemodialysis patients: the CoQ10 biomarker trial. American Journal of Kidney Diseases 2017;69(3):389-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sacher 1997 {published data only}
- Sacher HL, Sacher ML, Landau SW, Kersten R, Dooley F, Sacher A, et al. The clinical and hemodynamic effects of coenzyme Q10 in congestive cardiomyopathy. American Journal of Therapeutics 1997;4(2-3):66-72. [DOI] [PubMed] [Google Scholar]
Saurabh 2014 {published data only}
- Saurabh S, Yadav A, Tiwari RK, Sharma A, Goyal YK, Jain A. Comparative study of Ubiquinone (CoQ10) and Krill oil in dilated cardiomyopathy. Indian Journal of Pharmacology 2014;46:S30. [Google Scholar]
Schneeberger 1984 {published data only}
- Schneeberger W, Zilliken F, Moritz J. Clinical studies with coenzyme Q10 in patients with congestive heart failure. Drugs Under Experimental and Clinical Research 1984;10(7):503-12. [Google Scholar]
Sinatra 2000 {published data only}
- Sinatra ST. Coenzyme Q10 and congestive heart failure. Annals of Internal Medicine 2000;133(9):745-6. [DOI] [PubMed] [Google Scholar]
Sinatra 2004 {published data only}
- Sinatra ST, Berman M, Ben-Gal T. Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: a randomized, placebo-controlled study (multiple letters). Clinical Cardiology 2004;27(10):A26, A30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turk 2013 {published data only}
- Turk S, Baki A, Solak Y, Kayrak M, Atalay H, Gaipov A, et al. Coenzyme Q10 supplementation and diastolic heart functions in hemodialysis patients: a randomized double-blind placebo-controlled trial. Hemodialysis International 2013;17(3):374-81. [DOI] [PubMed] [Google Scholar]
Watson 1999 {published data only}
- Watson PS, Scalia GM, Galbraith A, Burstow DJ, Bett N, Aroney CN, et al. Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure. Journal of the American College of Cardiology 1999;33(6):1549-52. [DOI] [PubMed] [Google Scholar]
Witte 2005 {published data only}
- Witte KK, Nikitin NP, Parker AC, Von Haehling S, Volk HD, Anker SD, et al. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. European Heart Journal 2005;26(21):2238-44. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02779634 {published data only}
- NCT02779634. Ubiquinol treatment in patients with heart failure and preserved ejection fraction. clinicaltrials.gov/show/nct02779634 (first posted 20 May 2016).
Pierce 2018 {published data only}
- NCT03133793. CoQ10 and d-ribose in patients with diastolic heart failure. clinicaltrials.gov/show/nct03133793 (first posted 28 April 2017).
- Pierce J D, Mahoney D E, Hiebert J B, Thimmesch A R, Diaz F J, Smith C, et al. Study protocol, randomized controlled trial: reducing symptom burden in patients with heart failure with preserved ejection fraction using ubiquinol and/or D-ribose. BMC Cardiovascular Disorders 2018;18(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Baggio 1994
- Baggio E, Gandini R, Plancher AC, Passeri M, Carmosino G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure. Molecular Aspects of Medicine 1994;15:s287-94. [DOI] [PubMed] [Google Scholar]
Belch 1991
- Belch JJ, Bridges AB, Scott N, Chopra M. Oxygen free radicals and congestive heart failure. British Heart Journal 1991;65(5):245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berthold 2006
- Berthold HK, Naini A, Di Mauro S, Hallikainen M, Gylling H, Krone W, et al. Effect of ezetimibe and/or simvastatin on coenzyme Q10 levels in plasma: a randomised trial. Drug Safety 2006;29(8):703-12. [DOI] [PubMed] [Google Scholar]
Bhagavan 2007
- Bhagavan HN, Chopra RK. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 2007;7S:S78-88. [DOI] [PubMed] [Google Scholar]
Boon 2006
- Boon NA, Colledge NR, Walker BR, Hunter JAA. Davidson's Principles and Practice of Medicine. 20th edition. Churchill Livingstone, 2006. [Google Scholar]
Clinical Evidence
- BMJ Clinical Evidence. EBM Tools: Glossary. clinicalevidence.bmj.com/ceweb/resources/glossary.jsp#Random_effects (accessed 16 January 2010).
De Blasio 2015
- De Blasio MJ, Huynh K, Qin C, Rosli S, Kiriazis H, Ayer A, et al. Therapeutic targeting of oxidative stress with coenzyme Q10 counteracts exaggerated diabetic cardiomyopathy in a mouse model of diabetes with diminished PI3K (p110α) signaling. Free Radical Biology and Medicine 2015;87:137-47. [DOI] [PubMed] [Google Scholar]
Diaz‐Velez 1996
- Diaz-Velez CR, Garcia-Castineiras S, Mendoza-Ramos E, Hernandez-Lopez E. Increased malondialdehyde in peripheral blood of patients with congestive heart failure. American Heart Journal 1996;131(1):146-52. [DOI] [PubMed] [Google Scholar]
Drexler 2004
- Drexler H, Hasenfuss G. Physiology of the normal and failing heart. In: Crawford MH, DiMarco JP, Paulus WJ, editors(s). Cardiology. 2nd edition. Edinburgh: Mosby, 2004:829. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elesber 2001
- Elesber AA, Redfield MM. Approach to patients with heart failure and normal ejection fraction. Mayo Clinic Proceedings 2001;76(10):1047-52. [DOI] [PubMed] [Google Scholar]
Folkers 1990
- Folkers K, Langsjoen P, Willis R, Richardson P, Xia LJ, Ye CQ, et al. Lovastatin decreases coenzyme Q levels in humans. Proceedings of the National Academy of Sciences of the United States of America 1990;87(22):8931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fotino 2013
- Fotino AD, Thompson-Paul AM, Bazzano LA. Effect of coenzyme Q(10) supplementation on heart failure: a meta-analysis. American Journal of Clinical Nutrition 2013;97(2):268-75. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gottdiener 2002
- Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Annals of Internal Medicine 2002;137(8):631-9. [DOI] [PubMed] [Google Scholar]
Greenberg 1990
- Greenberg S, Frishman WH. Co-enzyme Q10: a new drug for cardiovascular disease. Journal of Clinical Pharmacology 1990;30(7):596-608. [DOI] [PubMed] [Google Scholar]
Gutierrez‐Mariscal 2019
- Gutierrez-Mariscal FM, Yubero-Serrano EM, Villalba JM, Lopez-Miranda J. Coenzyme Q10: from bench to clinic in aging diseases, a translational review. Critical Reviews in Food Science and Nutrition 2019;59(14):2240-57. [DOI] [PubMed] [Google Scholar]
Heck 2000
- Heck AM, DeWitt BA, Lukes AL. Potential interactions between alternative therapies and warfarin. American Journal of Health-system Pharmacy 2000;57(13):1221-7. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Ho 1993
- Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham study. Journal of the American College of Cardiology 1993;22:6A-13A. [DOI] [PubMed] [Google Scholar]
Kishi 1977
- Kishi T, Watanabe T, Folkers K. Bioenergetics in clinical medicine XV. Inhibition of coenzyme Q10 enzymes by clinically used adrenergic blockers of beta-receptors. Research Communications in Chemical Pathology and Pharmacology 1977;17(1):157-64. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Lippi 2020
- Lippi G, Sanchis-Gomar F. Global epidemiology and future trends of heart failure. AME Medical Journal 2020;5(15):1-6. [DOI: 10.21037/amj.2020.03.03] [DOI] [Google Scholar]
Madmani 2014
- Madmani ME, Yusuf Solaiman A, Tamr Agha K, Madmani Y, Shahrour Y, Essali A, et al. Coenzyme Q10 for heart failure. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD008684. [DOI: 10.1002/14651858.CD008684.pub2] [DOI] [PubMed] [Google Scholar]
McMurray 1990
- McMurray J, McLay J, Chopra M, Bridges A, Belch JJ. Evidence for enhanced free radical activity in chronic congestive heart failure secondary to coronary artery disease. American Journal of Cardiology 1990;65(18):1261-2. [DOI] [PubMed] [Google Scholar]
McMurray 1993
- McMurray J, Chopra M, Abdullah I, Smith WE, Dargie HJ. Evidence of oxidative stress in chronic heart failure in humans. European Heart Journal 1993;14(11):1493-8. [DOI] [PubMed] [Google Scholar]
Migliore 2004
- Migliore L, Molinu S, Naccarati A, Mancuso M, Rocchi A, Siciliano G. Evaluation of cytogenetic and DNA damage in mitochondrial disease patients: effects of coenzyme Q10 therapy. Mutagenesis 2004;19(1):43-9. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad A, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analysis. Lancet 1998;352:609-13. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:B2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Molyneux 2008
- Molyneux SL, Florkowski CM, George PM, Pilbrow AP, Frampton CM, Lever M, et al. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. Journal of the American College of Cardiology 2008;52(18):1435-41. [DOI] [PubMed] [Google Scholar]
Mortensen 1984
- Mortensen SA, Vadhanavikit S, Folkers K. Deficiency of coenzyme Q10 in myocardial failure. Drugs under Experimental and Clinical Research 1984;X(7):497-502. [PubMed] [Google Scholar]
Mortensen 1990
- Mortensen SA, Vadhanavikit S, Muratsu K, Folkers K. Coenzyme Q10: clinical benefits with biochemical correlates suggesting a scientific breakthrough in the management of chronic heart failure. International Journal of Tissue Reactions 1990;12(3):155-62. [PubMed] [Google Scholar]
Mortensen 1993
- Mortensen SA. Perspectives on therapy of cardiovascular diseases with coenzyme Q10 (ubiquinone). Clinical Investigator 1993;71(8 Suppl):S116-23. [DOI] [PubMed] [Google Scholar]
Mortensen 1997
- Mortensen SA, Leth A, Agner E, Rohde M. Dose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitors. Molecular Aspects of Medicine 1997;18(Suppl):S137-44. [DOI] [PubMed] [Google Scholar]
Niklowitz 2007
- Niklowitz P, Sonnenschein A, Janetzky B, Andler W, Menke T. Enrichment of coenzyme Q10 in plasma and blood cells: defence against oxidative damage. International Journal of Biological Sciences 2007;3(4):257-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nishiyama 1998
- Nishiyama Y, Ikeda H, Haramaki N, Yoshida N, Imaizumi T. Oxidative stress is related to exercise intolerance in patients with heart failure. American Heart Journal 1998;135(1):115-20. [DOI] [PubMed] [Google Scholar]
NYHA 1964
- Criteria Committee, New York Heart Association. Diseases of the Heart and Blood Vessels: Nomenclature and Criteria for Diagnosis. 6th edition. Boston: Little, Brown and Co, 1964. [Google Scholar]
Ponikowski 2016
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal 2016;37(27):2129-200. [DOI] [PubMed] [Google Scholar]
Raizner 2019
- Raizner AE. Coenzyme Q10. Methodist DeBakey Cardiovascular Journal 2019;15(3):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rauchova 1995
- Rauchova H, Drahota Z, Lenaz G. Function of coenzyme Q in the cell: some biochemical and physiological properties. Physiological Research 1995;44(4):209-16. [PubMed] [Google Scholar]
Redfield 2016
- Redfield MM. Heart failure with preserved ejection fraction. New England Journal of Medicine 2016;375(19):1868-77. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. The Cochrane Collaboration, 2020.
Richardson 1996
- Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, et al. Report of the 1995 Word Health Organization/International Society and Federation of Cardiology Task Force on the Definition and classification of cardiomyopathies. Circulation 1996;93:841-2. [DOI] [PubMed] [Google Scholar]
Rodriguez 2004
- Rodriguez-Artalejo F, Banegas Banegas JR, Guallar-Castillon P. Epidemiology of heart failure [Epidemiologia de la insuficiencia cardiaca]. Revista Espanola de Cardiologia 2004;57(2):163-70. [PubMed] [Google Scholar]
Rosenfeldt 2003
- Rosenfeldt F, Hilton D, Pepe S, Krum H. Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. BioFactors (Oxford, England) 2003;18(1-4):91-100. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sander 2006
- Sander S, Coleman CI, Patel AA, Kluger J, White CM. The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. Journal of Cardiac Failure 2006;12(6):464-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Savarese 2017
- Savarese G, Lund LH. Global public health burden of heart failure. Cardiac Failure Review 2017;3(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 1999
- Singh RB, Niaz MA, Rastogi SS, Shukla PK, Thakur AS. Effect of hydrosoluble coenzyme Q10 on blood pressures and insulin resistance in hypertensive patients with coronary artery disease. Journal of Human Hypertension 1999;13(2):203-8. [DOI] [PubMed] [Google Scholar]
Soja 1997
- Soja AM, Mortensen SA. Treatment of congestive heart failure with coenzyme Q10 illuminated by meta-analyses of clinical trials. Molecular Aspects of Medicine 1997;18 Suppl:S159-68. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tappel 1972
- Tappel AL. Vitamin E and free radical peroxidation of lipids. Annals of The New York Academy of Sciences 1972;203:12-21. [DOI] [PubMed] [Google Scholar]
Teerlink 1991
- Teerlink JR, Goldhaber SZ, Preffer MA. An overview of contemporary etiologies of congestive heart failure. American Heart Journal 1991;121:1852-3. [DOI] [PubMed] [Google Scholar]
Tsai 2012
- Tsai KL, Huang YH, Kao CL, Yang DM, Lee HC, Chou HY, et al. A novel mechanism of coenzyme Q10 protects against human endothelial cells from oxidative stress-induced injury by modulating NO-related pathways. Journal of Nutritional Biochemistry 2012;23(5):458-68. [DOI] [PubMed] [Google Scholar]
Tsutsui 2002
- Tsutsui T, Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, et al. Plasma oxidized low-density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. Journal of the American College of Cardiology 2002;39(6):957-62. [DOI] [PubMed] [Google Scholar]
Virani 2020
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics 2020 update: a report from the American Heart Association. Circulation 2020;141(9):e139-596. [DOI: 10.1161/CIR.0000000000000757] [DOI] [PubMed] [Google Scholar]
Yancy 2017
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136(6):e137-61. [DOI: 10.1161/CIR.0000000000000509] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Al Saadi 2014
- Al Saadi T, Assaf Y, Farwati M, Turkmani K, Al-Mouakeh A, Shebli B, et al. Coenzyme Q10 for heart failure. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD008684. [DOI: 10.1002/14651858.CD008684.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


