Abstract
Background
Major depression is one of the world’s leading causes of disability in adults with long‐term physical conditions compared to those without physical illness. This co‐morbidity is associated with a negative prognosis in terms of increased morbidity and mortality rates, increased healthcare costs, decreased adherence to treatment regimens, and a substantial decline in quality of life. Therefore, preventing the onset of depressive episodes in adults with long‐term physical conditions should be a global healthcare aim.
In this review, primary or tertiary (in cases of preventing recurrences in those with a history of depression) prevention are the focus. While primary prevention aims at preventing the onset of depression, tertiary prevention comprises both preventing recurrences and prohibiting relapses. Tertiary prevention aims to address a depressive episode that might still be present, is about to subside, or has recently resolved. We included tertiary prevention in the case where the focus was preventing the onset of depression in those with a history of depression (preventing recurrences) but excluded it if it specifically focused on maintaining an condition or implementing rehabilitation services (relapse prevention). Secondary prevention of depression seeks to prevent the progression of depressive symptoms by early detection and treatment and may therefore be considered a 'treatment,' rather than prevention. We therefore exclude the whole spectrum of secondary prevention.
Objectives
To assess the effectiveness, acceptability and tolerability of psychological or pharmacological interventions, in comparison to control conditions, in preventing depression in adults with long‐term physical conditions; either before first ever onset of depressive symptoms (i.e. primary prevention) or before first onset of depressive symptoms in patients with a history of depression (i.e. tertiary prevention).
Search methods
We searched the Cochrane Common Mental Disorders Controlled Trials Register, CENTRAL, MEDLINE, Embase, PsycINFO and two trials registries, up to 6 February 2020.
Selection criteria
We included randomised controlled trials (RCTs) of preventive psychological or pharmacological interventions, specifically targeting incidence of depression in comparison to treatment as usual (TAU), waiting list, attention/psychological placebo, or placebo. Participants had to be age 18 years or older, with at least one long‐term physical condition, and no diagnosis of major depression at baseline (primary prevention). In addition, we included studies comprising mixed samples of patients with and without a history of depression, which explored tertiary prevention of recurrent depression. We excluded other tertiary prevention studies. We also excluded secondary preventive interventions. Primary outcomes included incidence of depression, tolerability, and acceptability. Secondary outcomes included severity of depression, cost‐effectiveness and cost‐utility.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 11 RCTs, with one trial on psychological interventions, and 10 trials on pharmacological interventions. Data analyses on the psychological intervention (problem‐solving therapy compared to TAU) included 194 participants with age‐related macular degeneration.
Data analyses on pharmacological interventions included 837 participants comparing citalopram (one trial), escitalopram (three trials), a mixed sample of fluoxetine/nortriptyline (one trial), melatonin (one trial), milnacipran (one trial), and sertraline (three trials), each to placebo. Included types of long‐term physical conditions were acute coronary syndrome (one trial), breast cancer (one trial), head and neck cancer (two trials), stroke (five trials), and traumatic brain injury (one trial).
Psychological interventions
Very low‐certainty evidence of one study suggests that problem solving therapy may be slightly more effective than TAU in preventing the incidence of depression, immediately post‐intervention (odds ratio (OR) 0.43, 95% confidence interval (CI) 0.20 to 0.95; 194 participants). However, there may be little to no difference between groups at six months follow‐up (OR 0.71, 95% CI 0.36 to 1.38; 190 participants; one study; very low‐certainty evidence). No data were available regarding incidence of depression after six months. Regarding acceptability (drop‐outs due to any cause), slightly fewer drop‐outs occurred in the TAU group immediately post‐intervention (OR 5.21, 95% CI 1.11 to 24.40; 206 participants; low‐certainty evidence). After six months, however, the groups did not differ (OR 1.67, 95% CI 0.58 to 4.77; 206 participants; low‐certainty evidence). This study did not measure tolerability.
Pharmacological interventions
Post‐intervention, compared to placebo, antidepressants may be beneficial in preventing depression in adults with different types of long‐term physical conditions, but the evidence is very uncertain (OR 0.31, 95% CI 0.20 to 0.49; 814 participants; nine studies; I2 =0%; very low‐certainty evidence). There may be little to no difference between groups both immediately and at six months follow‐up (OR 0.44, 95% CI 0.08 to 2.46; 23 participants; one study; very low‐certainty evidence) as well as at six to 12 months follow‐up (OR 0.81, 95% CI 0.23 to 2.82; 233 participants; three studies; I2 = 49%; very low‐certainty evidence). There was very low‐certainty evidence from five studies regarding the tolerability of the pharmacological intervention. A total of 669 adverse events were observed in 316 participants from the pharmacological intervention group, and 610 adverse events from 311 participants in the placebo group. There was very low‐certainty evidence that drop‐outs due to adverse events may be less frequent in the placebo group (OR 2.05, 95% CI 1.07 to 3.89; 561 participants; five studies; I2 = 0%). There was also very low‐certainty evidence that drop‐outs due to any cause may not differ between groups either post‐intervention (OR 1.13, 95% CI 0.73 to 1.73; 962 participants; nine studies; I2 = 28%), or at six to 12 months (OR 1.13, 95% CI 0.69 to 1.86; 327 participants; three studies; I2 = 0%).
Authors' conclusions
Based on evidence of very low certainty, our results may indicate the benefit of pharmacological interventions, during or directly after preventive treatment. Few trials examined short‐term outcomes up to six months, nor the follow‐up effects at six to 12 months, with studies suffering from great numbers of drop‐outs and inconclusive results. Generalisation of results is limited as study populations and treatment regimes were very heterogeneous.
Based on the results of this review, we conclude that for adults with long‐term physical conditions, there is only very uncertain evidence regarding the implementation of any primary preventive interventions (psychological/pharmacological) for depression.
Plain language summary
Drug or psychological interventions for preventing depression in people with long‐term physical conditions
Why is this review important?
People with long‐term illness or other physical health conditions have a higher risk than other people of developing depression. This can reduce their quality of life. Depression is characterised by symptoms such as low mood, feelings of hopelessness, loss of interest in things that once gave pleasure, and other symptoms, as well as sleep disturbances. People with long‐term physical conditions who develop depression are more likely to worsen in their illnesses and are more likely to die. Therefore, preventing depression in people with long‐term physical conditions should be an important goal in healthcare.
What questions does this review aim to answer?
We wanted to know whether standard interventions for treating depression (i.e. psychological treatments and antidepressant drugs) can also safely be used to prevent the onset of an depressive episode in those adults at high risk for depression due to their long‐term physical condition, but who do not yet show depressive symptoms. We also wanted to know whether these interventions worked in preventing recurrent depression, in those patients with long‐term physical conditions who had a history of depression.
How did we identify and evaluate the evidence?
First, we searched the medical literature for randomised controlled studies (clinical studies where people are randomly put into one of two or more treatment groups). This type of study provides the most robust evidence about the effects of a treatment. We then compared the results, and summarised the evidence from all the studies. Finally, we assessed how certain the evidence was. To do this, we considered factors such as the way studies were conducted, study sizes, and consistency of findings across studies. Based on our assessments, we categorised the evidence as being of very low, low, moderate or high certainty.
Who will be interested in this review?
Medical and mental health care providers (including physicians and psychologists) and pharmacists, as well as adults with long‐term physical conditions, their relatives and care‐givers.
Which studies were included in the review?
This review includes 11 trials comparing a psychological intervention (problem‐solving therapy) to treatment as usual; or comparing pharmacological antidepressant interventions (citalopram, escitalopram, sertraline, fluoxetine/nortriptyline, milnacipran, or melatonin) to placebo. For the psychological intervention, we found only one trial, including 194 people with age‐related macular degeneration (an eye disease). For pharmacological interventions, we included 10 trials comprising 1009 people. Due to some participants not completing the studies, we could only analyse data for 837 participants.
What does the evidence from the review tell us?
Our analyses show that people with long‐term physical conditions may be less likely to develop depression during treatment with problem solving therapy, or with different types of antidepressants. However, these interventions appear to be beneficial only during treatment. Three to 12 months after treatment, there was no significant difference in onset of depression between the groups that had the interventions and those that did not. Therefore, preventive interventions might be effective in preventing depression onset only for the duration of the intervention. Our conclusions are based on evidence of very low certainty. In addition, there is not enough adequate information on the tolerability (unpleasant but generally medically less important adverse events due to the intervention, e.g. dry mouth) and acceptability (willingness to go through with the intervention even in the presence of adverse events) of these treatments. The interventions may be unsafe, irrespective of their potential to prevent depression.
How‐up‐to date is this review?
The evidence in this Cochrane Review is current to 6 February 2020.
Summary of findings
Background
Description of the condition
Major depressive disorder is characterised by an array of symptoms including depressed mood, loss of interest or pleasure, diminished energy, fatigue, difficulties with concentration, changes in appetite and sleep disturbances (APA 2013). Depression is a common condition with a lifetime prevalence of 16% (Kessler 2003), and a 12‐month prevalence rate between 6% and 10% (Baumeister 2007).The World Health Organisation (WHO) rated depression as the third leading contributor to the burden of disease worldwide (and predicted it to become the first in 2030), accounting for more than 4% of total disability‐adjusted life‐years (DALYs) (WHO 2008).
There is strong evidence for even higher rates of depression in adults with long‐term physical conditions (also termed medical illness or chronic illness) compared to those without it (Gunn 2012; Härter 2007a; Holahan 2010; Patten 2001). Research has not yet established a causal link between depression and long‐term physical conditions, but shows a distinctive decline in health when depression is present (WHO 2007). The presence of depression in adults with long‐term physical conditions is associated with a negative prognosis in terms of increased morbidity and mortality rates (Katon 2003; Katon 2007), increased health care costs (Baumeister 2012a; Haschke 2012; Hutter 2010; Hutter 2011; Katon 2002), decreased adherence to treatment regimens (Evans 2005), and a substantial loss in quality of life (Baumeister 2011a).
While negative effects of depression are apparent, its origins remain yet inconclusive. When long‐term physical conditions precede a depressive episode, as it is the case in prevention trials, depression can be categorised by its assumed main cause or trigger.
It might be caused by the treatment of the illness (i.e. substance‐induced depression).
It might directly result from the illness (i.e. organic depression).
It could be a psychological response of coping with the illness (DGPPN 2012; Freedland 2010; Härter 2007b).
It might possess a common disposition with the illness (i.e. genetic pleiotropy, common socio‐economic and demographic characteristics) (de Geus 2006; McCaffery 2006).
It might co‐occur with the illness due to chance.
Therefore, depression subtypes need to be considered within the context of the interplay between diatheses and stress, as the principal underlying determinants of depression (Baumeister 2012b; Durand 2012). Among adults with long‐term physical conditions, a variety of factors such as worsening condition, unrelieved pain, functional impairment, or social isolation are associated with the possible onset of depression (Clarke 2009). Therefore, these adults are a selected population with an increased risk to (newly) develop depression (Gunn 2012; Härter 2007a; Holahan 2010; Patten 2001). This risk for depression implies the potential benefit of primary prevention trials.
Description of the intervention
To gain a comprehensive understanding of the term prevention as we applied it throughout this review, some considerations have to be taken into account. First of all, prevention needs to be defined. The current body of literature concerning prevention of depression in adults with long‐term physical conditions is considerable, but often lacks clear definition and conceptualisation. Prevention is usually subdivided into primary, secondary and tertiary prevention. While these terms are commonly used, reports and studies often lack explicit operationalisation (Muñoz 1996).
To prevent something, an intervention must take place before the event occurs; in this case, to prevent depression, the preventive intervention must take place before the onset of first depressive symptoms. This is the case only in primary prevention trials. In contrast, secondary prevention of depression seeks to prevent the progression of a disease or disorder by early detection and treatment of the condition, and is therefore considered to be a 'treatment' rather than an actual type of prevention. Tertiary prevention of depression includes interventions aiming to prevent relapse and recurrences of depressive disorders and was reinstated to be considered as 'maintenance' of the condition (Mrazek 1994; O'Connell 2009).
The Institute of Medicine's report on Reducing Risks for Mental Disorders considers only primary prevention strategies as 'prevention' stricto senso (Muñoz 1996). The type of prevention, the population to which it is applied, and the balance of benefits against risk (adverse events) and cost (cost‐effectiveness, cost‐utility) determine the specificity of a primary preventive intervention (Gordon 1983). Therefore, it can be specified as either universal, selective or indicated. While universal prevention aims at the population as a whole, selective and indicated prevention are applied to subgroups of the population whose risk is above average (selective prevention) or who show subthreshold symptoms and/or exhibit risk factors such as biological markers (indicated prevention) (Gordon 1983). While the latter might easily be confused with secondary prevention or treatment, the National Institutes of Health (NIH) argue that a clinical abnormality is distinct from a clinical symptom, and that the treatment of such an abnormality serves to prevent the development of some later symptoms. Hence, indicated prevention is different from treatment (Gordon 1983).
All the same, up to 80% of adults with depression experience at least one recurring episode (Wittchen 2010), implying a great likelihood of a history of depression sometime in their lives. Therefore, adults included in depression prevention trials might match the requirements of tertiary prevention rather than those of primary prevention. The main intent of tertiary prevention, however, differs from primary prevention: while primary prevention aims at preventing the onset of depression, tertiary prevention deals with the aftermath of a depressive episode that might still be present, is about to subside, or has recently dissolved.
With regard to types of preventive interventions, two approaches might be considered that have been shown to be effective for the treatment of depression in adults with long‐term physical conditions. These are psychological interventions (Baumeister 2011b; Baumeister 2012c; Beltman 2010; Stockton 2004) and pharmacological interventions (Baumeister 2011b; Baumeister 2012c; Gill 2000; Krishnan 2005; Rayner 2010; Simon 2005). There is evidence that the onset of depression in general can be prevented or at least be delayed by psychological interventions (Cuijpers 2008). As for pharmacological interventions, there are hints that prophylactic treatment with antidepressants can decrease the incidence of depression in adults with head and neck cancer (Lydiatt 2013).
There is a multitude of psychological interventions such as cognitive behavioural therapy, psychodynamic therapies, behaviour therapy or behaviour modification, systemic therapies, third wave cognitive behavioural therapies, humanistic therapies, or integrative therapies. All interventions are carried out by a healthcare professional in a setting of individual, group, family or couples therapy. Psychological interventions aim to recognise, improve or prevent distress by direct or interactive communication.
Complementary, pharmacological interventions are commonly used in the treatment of depression. Selection of antidepressant drugs depends on the type of depressive disorder, adverse reactions and present co‐morbidities (both psychological and physical). Most prevalent classes of antidepressants are tricyclic antidepressants, heterocyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs) or monoamine oxidase inhibitors (MAOIs).
How the intervention might work
The majority of psychological therapies are based on social‐learning, biosocial, and psychodynamic theories, hypothesising that impairments might be learned, or by assuming an interaction of given dispositions (also called vulnerabilities) and circumstantial influences. They comprise various approaches targeting and altering unhelpful emotions, behaviours and/or cognitions to alleviate depressive symptoms. A number of Cochrane Reviews address the effectiveness of psychological interventions. Even though the certainty of evidence is mainly low to moderate, evidence suggests an effective treatment of depression by means of psychological interventions in adults as well as in the physically ill (Akechi 2008; Baumeister 2012c; Shinohara 2013; Thomas 2006; Whalley 2011).
The majority of pharmacological interventions target monoamine neurotransmitter function, and were assumed to convey their antidepressant effects by enhancement of functional activity of serotonin, noradrenaline and/or dopamine. However, more frequent research approaches challenge a "purely neurotransmitter‐based explanation for antidepressant drug action" (Harmer 2017). At present, it is not yet known exactly how antidepressants exert their effects. Various theories have been put forward, including the neuroplasticity and the neuropsychological theory. Instead of a simple neurochemical understanding, novel theories include a broader understanding of antidepressant drug action by considering the effects of antidepressants on emotional and cognitive function as well as neuroplasticity (Harmer 2017). Several Cochrane Reviews investigate the potential effects of antidepressants, suggesting a beneficial effect of pharmacological treatment for depression (Arroll 2009; Cipriani 2009; Cipriani 2012; van Marvijk 2012). This also appears to be the case in adults with long‐term physical conditions (Gill 2000; Rayner 2010).
While the importance of managing adverse events is emphasised for all antidepressant treatments, including both psychological and pharmacological interventions, special attention has to be given to adults with long‐term physical conditions. Especially for pharmacological agents, the potential benefits of antidepressants need to be balanced against their potential side effects and the challenges of managing the complex drug regimens of adults with multiple morbidities. Despite this, clinical guidelines recommend psychological interventions for the treatment of subthreshold to moderate depression, and pharmacological interventions for the treatment of moderate to severe depression in adults with long‐term physical conditions (NICE 2009).
The effective treatment of depression by means of psychological as well as pharmacological interventions implies that they may also be beneficial in the use of these treatments as preventive interventions.
Why it is important to do this review
Co‐morbid depression in adults with long‐term physical conditions leads to increased symptom burden and medical complications (Evans 2005; Katon 2003; Katon 2007; WHO 2008). It has a considerable effect on health care utilisation and cost, and is associated with substantial suffering (Baumeister 2011a; Baumeister 2012a; Haschke 2012; Hutter 2010; Hutter 2011; Katon 2002). There is evidence from several Cochrane Reviews that depression in adults with long‐term physical conditions can be treated; however, the effectiveness of interventions for manifest depression seems to be limited (Baumeister 2011b; Baumeister 2012c; Gill 2000; Rayner 2010). Thus, the scientific and healthcare implications of preventing depression as a potentially effective and cost‐effective way of dealing with potential depression in adults with long‐term physical conditions would be substantial.
This review gives an overview of available prevention trials for depression in adults with long‐term physical conditions, and offers conclusions on the effects of prevention. We examined different types of interventions (psychological and pharmacological), allowing us to draw conclusions about specific preventive interventions. We define prevention in this review as being either primary (prevention of first onset of depression) or tertiary (prevention of recurrent depression), and exclude secondary prevention and (tertiary) relapse prevention.
Furthermore, follow‐up data and sources of heterogeneity are explored and may help to provide suggestions for the design of future studies. We discuss methodological limitations, problems and shortcomings, which may also help to guide future research. To allow the integration of new evidence and findings, we will continuously maintain and update this review.
According to diagnostic criteria defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM), a 'substance‐induced mood disorder' is characterised by the same symptoms as major depression with the exception that the source of the symptoms is clearly attributed to pharmacological agents (APA 2000). Certain long‐term physical conditions such as cancers, viral diseases, or multiple sclerosis are commonly treated with agents that are considered to cause or convey depressive symptoms (Raison 2005; Wichers 2005). While adults receiving such agents might also benefit from preventive interventions, substance‐induced depression is clearly distinct from depression in adults with long‐term physical conditions, and will therefore be treated separately in a complementary review.
Objectives
To assess the effectiveness, acceptability and tolerability of psychological or pharmacological interventions in comparison to controls in preventing the incidence of depression in adults with long‐term physical conditions, either before first ever onset of depressive symptoms (i.e. primary prevention) or before first onset of depressive symptoms in patients with a history of depression (i.e. tertiary depression). Specifically we aimed to determine:
the effects of preventive psychological interventions; and
the effects of pharmacological interventions.
Methods
Criteria for considering studies for this review
Types of studies
We restricted eligible study designs to randomised controlled trials (RCTs), cluster‐RCTs, and cross‐over trials. We included trials published in any language and with any publication status. We had no restriction on sample size or duration of follow‐up.
Types of participants
Participant characteristics
Adult participants (age 18 years or older) of either sex were eligible for inclusion.
Diagnosis
We included trials if participants had at least one diagnosed long‐term physical condition meeting International Classification of Diseases (ICD)‐10 criteria (WHO 1992), and no major depressive disorder at baseline. To confirm the absence of depression at baseline, and to determine the onset of depression in the course of the trial, depression had to be diagnosed by means of a standardised clinical interview, using ICD or DSM criteria. However, in favour of providing a more comprehensive review, we also included trials using validated self‐reports, or rating scales with specific cut‐off points for depression, or a medical diagnosis made by a healthcare professional. For a full list of included long‐term physical conditions please see Appendix 1.
Co‐morbidities
Due to the nature of this review, participants with one or more co‐morbid physical or mental disorders, other than depression were eligible for inclusion.
Settings
We included trials regardless of the setting (including inpatients and outpatients). Participants might have been treated in acute care hospitals, emergency facilities, general practice, rehabilitation settings, or extended care facilities such as nursing homes or communities.
Subset data
In case of mixed study samples (e.g. a sample of participants who were initially without depression or anxiety; a sample consisting of participants who were mainly with a long‐term physical condition), we planned to obtain 'clear' data of interest from study authors. If data could not be obtained, or if the use of a subsample would break the RCT, we planned to exclude the study.
Types of interventions
Experimental interventions
The following psychological and pharmacological preventive interventions specifically targeting depression were eligible for inclusion:
-
Psychological interventions:
cognitive behavioural therapy, i.e. problem solving, stress management, restructuring;
psychodynamic psychotherapy, i.e. psychoanalytic therapy, countertransference, insight oriented therapy;
behaviour therapy or behaviour modification, i.e. activity scheduling, exposure therapy, social skills training;
systemic therapy, i.e. conjoint therapy (couple, family), narrative therapy, socio‐environmental therapy;
third wave cognitive behavioural therapies, i.e. mindfulness‐based cognitive therapy, behavioural activation, dialectical behaviour therapy, acceptance and commitment therapy;
humanistic therapies, i.e. Rogerian, expressive therapy, supportive therapy;
integrative therapies, i.e. interpersonal therapy, counselling, cognitive analytical therapy;
other psychological‐oriented interventions, i.e. catharsis, drama therapy, bibliotherapy.
-
Pharmacological interventions:
tricyclic antidepressants, i.e. amitriptyline, desipramine, imipramine, clomipramine;
heterocyclic antidepressants, i.e. mianserin, trazodone, amoxapine, maprotiline;
SSRIs, i.e. citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline;
MAOIs, i.e. irreversible: phenelzine, tranylcypromine; reversible: brofaromine, moclobemide;
other antidepressants, i.e. serotonin and norepinephrine reuptake inhibitors (SNRIs), such as venlafaxine, milnacipran; noradrenergic and specific serotonergic antidepressants such as mirtazapine; or unclassified antidepressants such as agomelatine.
Comparator interventions
In the case of psychological prevention trials, the comparison group had to be 'treatment as usual' (TAU), 'waiting list,' 'attention placebo' (which is regarded as inactive by both researchers and participants), or 'psychological placebo' (which is regarded as active by participants and inactive by researchers).
In case of pharmacological prevention trials the comparison group had to be 'placebo.'
Excluded interventions
Secondary preventive interventions seeking to prevent the progression of a disease or disorder by early detection and treatment of the condition (which marked it a 'treatment' rather than an actual prevention)
Tertiary prevention that aimed to prevent relapse, to maintain an aspired condition, or to implement rehabilitation services (including continuation therapy)
Health promotion interventions designed to improve health and well‐being
Types of outcome measures
Primary outcomes
The primary outcomes were incidence of depression following the preventive intervention, as well as tolerability and acceptability of the preventive intervention.
Incidence of depression: depression was defined by meeting the criteria of major depression set out by DSM (DSM‐III (APA 1980), DSM‐III‐R (APA 1987), DSM‐IV (APA 1994), DSM‐IV‐TR (APA 2000); DSM‐V (APA 2013)), or the criteria of a depressive episode set out by ICD (ICD‐9 (WHO 1978), ICD‐10 (WHO 1992)). Depression had to be measured or assessed by standardised interviews (such as the Structured Clinical Interview for DSM‐IV Axis I Disorders (SCID‐I) (First 1996), Composite International Diagnostic Interview (CIDI) (WHO 1990), Mini‐International Neuropsychiatric Interview (MINI) (Sheehan 1998), or Schedules for Clinical Assessment in Neuropsychiatry (SCAN) (Wing 1990)), or treating physician.
-
Tolerability and acceptability: adverse events (harms, side‐effects) and drop‐outs during preventive intervention were a proxy measure for preventive intervention tolerability and acceptability.
total number of adverse events experienced;
drop‐outs due to adverse events; and
drop‐outs due to any cause.
Secondary outcomes
-
Incidence of depression, measured by
scoring above the cut‐off for depressive disorder as defined by symptom scores on standardised, validated rating scales (such as the Hamilton Rating Scale for Depression (HDRS/HAMD/HAM‐D) (Hamilton 1960), or the Montgomery‐Åsberg Depression Scale (MADS) (Montgomery 1979), or
scoring above the cut‐off for depressive disorder as defined by symptom scores on standardised, validated self‐report questionnaires (such as the Beck Depression Inventory (BDI) (Beck 1961), the Center for Epidemiologic Studies Depression Scale (CES‐D) (Radloff 1977), the Hospital Anxiety and Depression Rating Scale (HADS) (Zigmond 1983), or the Patient Health Questionnaire ‐ depression module (PHQ‐9) (Kroenke 2001).
Severity of depression (measured by self‐report questionnaires or rating scales such as BDI, HDRS, HADS, or MADS)
Cost‐effectiveness (measured by the incremental cost‐effectiveness ratio (ICER)
Cost‐utility (measured by quality‐adjusted life years (QALYs))
Hierarchy of outcome measures
Due to the great likelihood of more than one reported eligible outcome, we included data as follows.
In case of available data from both rating scales and self‐report questionnaires, data from rating scales were prioritised.
In case of several outcome measures of the same hierarchy level used in one study, we selected the outcome measure most frequently used across all studies. Therefore, availability were to determine the selection of the outcome measure (e.g. if four studies report data from the Hamilton Rating Scale for Depression and two studies from the Montgomery‐Åsberg Depression Scale, Hamilton Rating Scale for Depression were to be selected).
In case of several outcome measures of the same hierarchy level and the same availability across studies, the outcome measure was to be randomly selected.
Further, if different studies were to use the same outcome measure but still use different cut‐off scores, we were to conduct sensitivity analysis to account for this (see Sensitivity analysis).
Timing of outcome assessment
Due to the nature of this review, we encountered multiple observations and heterogeneity concerning the follow‐up length of the outcome assessment. We analysed follow‐up durations using different time frames:
short term (less than six months post‐intervention);
medium term (six to 12 months post‐intervention); and
long term (more than 12 months post‐intervention).
In case that studies reported more than one assessment per period, we included the assessment that covered a greater period (e.g. we prioritised the assessment at three months over that at two months).
We conducted corresponding sensitivity analysis to evaluate the sustainability of possible antidepressant effects of preventive interventions (follow‐up; post‐randomisation instead of post‐intervention).
Search methods for identification of studies
The searches relied to a large extent on the use of subject headings and indexing terms (e.g. the US National Library of Medicine's 'Medical Subject Headings' (MeSH), Embase's 'Emtree' and the American Psychological Asssociation's 'APA Thesaurus'), as creating a fully comprehensive and sensitive list of keywords (including synonyms, related terms, variant spellings) for 'all physical illness' was unfeasible. The list of keywords was created in collaboration with the review authors and Cochrane Common Mental Disorders (CCMD) Information Specialist, using the ICD‐10 list (Appendix 1) and relevant guidelines (NICE 2009) and reviews (AHRQ 2012) as a source of reference for co‐morbid health conditions associated with depression.
Electronic searches
Searches were first conducted in November 2014, with updates in November 2017 and February 2020. The information specialist with CCMD searched the following databases:
Cochrane Common Mental Disorders Controlled Trials Register (CCMDCTR) (all available years) (Appendix 2)
The Cochrane Library (all issues, to Issue 2, 2020);
Ovid MEDLINE databases (1 January 2005 to 6 February 2020);
Ovid EMBASE (1 January 2011 to 6 Feb 2020);
Ovid PsycINFO (all years, to 9 Feb 2020).
The additional biomedical database searches (Appendix 3) were performed to identify RCTs beyond the scope of the CCMDCTR, due to the inclusion criteria of this review (i.e. adults diagnosed with at least one long‐term physical condition with no depression at baseline). The CCMDCTR also fell out of date during the course of this review.
A date restriction was applied to the MEDLINE and Embase searches, as records from these databases have already been screened for inclusion in CENTRAL (Lefebvre 2008). For further details, see the CENTRAL help file, available on the Cochrane Library website.
No further date restrictions were applied to the search.
International trial registries
Two international trial registries, ClinicalTrials.gov at the US National Institutes of Health, and the WHO International Clinical Trials Registry Portal (ICTRP), were searched to identify additional ongoing or unpublished studies.
Searching other resources
Reference lists
We searched references of included studies for relevant publications and cited unpublished trials. Furthermore, we checked relevant reviews and those articles that cited the studies we intended to include.
Data collection and analysis
Selection of studies
Based on the information gained from title and abstract, two authors (HK, OM) independently assessed all identified studies for the inclusion criteria, using the systematic review web application Rayyan (https://rayyan.qcri.org/welcome). If there was sufficient information for exclusion, the study was rejected. In the second step, the two authors independently accessed the full text of the potentially eligible results and compiled a list of studies that they believed met inclusion criteria. The two lists were compared and discrepancies discussed. Any further disagreements were resolved by involving a third review author (HB). We recorded the selection process in sufficient detail to complete a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram (Figure 1) and a 'Characteristics of excluded studies' table.
1.
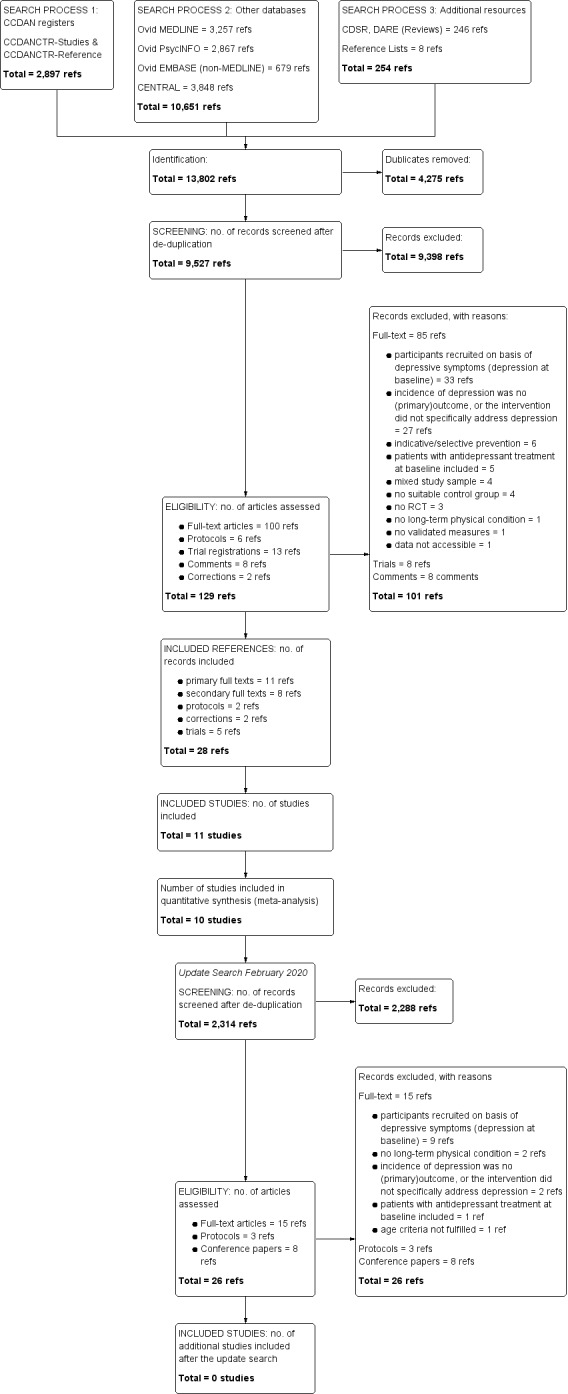
Study flow diagram.
Data extraction and management
Two review authors (HK, OM) independently extracted data. All data was cross‐checked for completeness by also including a research assistant. After discussing and resolving any disagreements by involving a third review author (HB), we entered data in the Cochrane Collaboration statistical software, RevMan 5 (Review Manager 2014). In case of missing data or information, we attempted to contact the trial's primary investigators.
We collected the following data and information from each included study report.
Description of trial: authors, year of publication, funding.
Study population: type of physical illness, sample size (numbers randomised, numbers treated, numbers at follow‐up), social demography, medical records (including history of depression if available).
Diagnostic and assessment of depression: physician's diagnosis (in accordance to DSM or ICD), standardised diagnostic interview, self‐report questionnaire (including cut‐off scores).
-
Types of intervention:
intervention group: psychological, pharmacological, other (type, dose, duration, methods of delivery);
control group: placebo, usual care, waiting list, attention placebo.
Research design: sampling, randomization, follow‐up.
Outcome measures: description of primary and secondary measures, drop outs, adverse events.
Statistics: sample sizes, incidence, means, standard deviation/error, and P values.
Main comparisons
We evaluated the effects of the preventive psychological and pharmacological interventions separately as below:
psychological interventions versus usual care, waiting list, attention placebo, or no intervention;
pharmacological intervention versus placebo.
Furthermore, we planned to conduct subgroup analyses for different types of long‐term physical conditions and classes of antidepressants. This was not possible due to lack of data. We also planned to conduct subgroup analyses for types of psychological interventions but this was not possible as we included only one such trial.
Assessment of risk of bias in included studies
Using the Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011), two authors (HK, OM) independently assessed the risk of bias for all eligible studies. We discussed any disagreements, and sought further advice from a third review author (HB).
We provide 'Risk of bias' tables which present the domain, the review author's judgement and support for such judgement.
We considered the following domains for risk of bias:
Sequence generation: was the allocation sequence adequately generated?
Allocation concealment: was allocation adequately concealed?
Blinding of participants and personnel: was knowledge of the allocated intervention adequately prevented during the study?
Blinding of outcome assessors: was knowledge of the allocated intervention adequately prevented during the study?
Incomplete outcome data: were incomplete outcome data adequately addressed?
Selective outcome reporting: are reports of the study free of suggestion of selective outcome reporting?
Other sources of bias: was the study apparently free of other problems that could put it at a high risk of bias?
The support of judgement ensured transparency by providing a description from which judgements of risk of bias can be made. Furthermore, we present the review author's judgement on the obtained data. Within and across studies risk of bias was judged to be low, unclear or high for each domain.
We provide a description and graphic presentation of all risk of bias data.
Measures of treatment effect
Dichotomous data
For dichotomous data, we calculated odds ratios (ORs) with 95% confidence intervals (CIs) to assess the incidence of depression.
Continuous data
For continuous data, we calculated standardised mean differences (SMDs) with 95% CIs to assess depression severity across different depression scales. We used Hedges' adjusted g statistic to calculate SMDs (it is very similar to Cohen's d statistic, but includes an adjustment for small sample bias). For a descriptive assessment of tolerability (adverse events) and acceptability (drop‐outs), we provide percentages that might offer estimates for these effects.
Unit of analysis issues
Studies with multiple treatment groups
Multiple‐arm studies contain more than two (intervention, comparison) relevant treatment arms (in addition to the control group there might be different types of interventions or different doses of medication). We avoided any possible bias caused by multiple comparisons with one control group by combining the groups to create a single pair‐wise comparison (Higgins 2011).
Cluster‐randomised trials
We intended to include cluster‐randomised trials if the study had been adjusted for the effect of the clustering, or an estimate of the intraclass correlation coefficient (ICC) could be obtained to adjust for the effect of the clustering (missing data was to be requested from authors). If the ICC were not to be acquired from study authors, external estimates were to be obtained from similar studies (Higgins 2011). If no estimate at all would have been available, we were to exclude the trial from analysis. However, no cluster‐randomised trials were included in this review.
Cross‐over trials
Results of cross‐over trials might be influenced by a carry‐over effect. Here, a treatment in the first randomised treatment period has an effect that carries over to the second randomised treatment period. We also considered cross‐over designs eligible for inclusion but no cross‐over trials were identified. If we identify any cross‐over trials in future updates of this review, we will include only data from the first randomised treatment period.
Dealing with missing data
In keeping with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we dealt with missing data as follows.
We collected and documented information on missing data. Whenever possible, we contacted the original study authors to obtain relevant missing data. We documented all correspondence with authors, and reported which authors responded and what methods they (might) have used for imputing data (such as multiple imputations).
When data can be assumed to be 'missing at random' (due to available information), we were to analyse only the available data.
Study drop‐outs were considered to be 'not missing at random'. We assumed that adverse events might arise due to the treatment or the development of depressive symptoms, and therefore, might pose the major reasons for discontinuation of study participation. To account for this, we conducted per protocol (PP) analyses instead of intention‐to‐treat (ITT) analyses. Therefore, incidence of depression is reported as the ratio of 'n incidence' to ('n randomised' minus 'n drop‐outs').
We performed sensitivity analyses to assess the impact of missing data.
Assessment of heterogeneity
To identify statistical heterogeneity, we visually evaluated the forest plots and assessed Chi2 test of heterogeneity. Given the low power of the Chi2 test when trials are small, we used a significance level of α = 0.10 (Higgins 2011).
In addition, we used the I2 heterogeneity statistic to quantify inconsistency across studies to assess the impact of heterogeneity (Higgins 2003). We interpreted I2 values in accordance with the Cochrane Handbook (Higgins 2011). A rough guide to interpretation is as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
In order to minimise publication bias, we tried to identify unpublished data and included studies published in any language. With more than ten studies included, we graphically examined the risk of existing publication bias by the use of a funnel plot (Higgins 2011). The limitations of funnel plots (asymmetry does not necessarily imply publication bias or publication bias might exist even though there is no asymmetry) were taken into consideration (Guyatt 2011).
Furthermore, we identified outcome reporting bias by comparing planned and reported outcomes. We identified missing outcome data, tried to obtain it by contacting authors, and in case of its unavailability reported its absence.
Data synthesis
Our decision on whether or not we should perform meta‐analyses was based on the assessment of clinical, methodological and statistical heterogeneity:
use of a fixed‐effect model, where we would expect the included trials to estimate the same true value;
use of a random‐effects model, for which we expect the included trials to estimate different but related estimates of effect; or
no meta‐analysis, in which case we expect the included trials to estimate different and unrelated estimates of effect.
We used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses are observational by nature and often involve multiple analyses which increase the likelihood of falsely positive results. They should be interpreted with caution.
We planned to conduct the following subgroup analyses:
Type of long‐term physical condition: we expected differences in the efficiency, acceptability and tolerability of the preventive intervention depending on the type of long‐term physical condition (subgroup analyses were to be conducted for type of long‐term physical conditions if three or more studies deal with one specific disease as listed in Appendix 1);
Type of psychological intervention: we expected differences in the efficiency, acceptability and tolerability of the psychological intervention depending on the type of psychological interventions (subgroup analyses were to be conducted for the type of psychological intervention if three or more studies deal with on specific psychological intervention);
Class of antidepressant: we expected differences in the efficiency, acceptability and tolerability of the pharmacological intervention depending on the class of antidepressant (subgroup analyses were to be conducted for the type of pharmacological intervention if three or more studies deal with one specific pharmacological intervention).
Sensitivity analysis
In order to examine the robustness of the results of the primary outcome, we intended to perform the following sensitivity analyses:
Assessment method: different studies might use the same outcome measure but still use different cut‐off scores to separate depressed from non‐depressed participants; we were to exclude studies using low cut‐off scores and describe the procedure in detail.
-
Quality of studies: we excluded those studies which posed a high risk of bias and might therefore affect the quality of study results; sensitivity analyses were to be conducted for studies who:
fail to double blind (participants and treating health care professional) in case of pharmacological and other interventions (psychological interventions are not expected to blind participants);
lack proper allocation concealment;
-
might show an impact of missing data: in accordance with the Cochrane Handbook (Higgins 2011), we were to conduct 'best case' and 'worst case' analyses to evaluate the impact of participants who were lost to follow‐up, specifically:
best case scenario: all missing data in the control group were to be considered as occurrence of depressive symptoms;
worst case scenario: all missing data in the treatment group were to be considered as occurrence of depressive symptoms;
were cluster‐randomised trials.
-
Selection of participants: we were to exclude those studies which only include selected samples of participants at higher risk for depression, e.g. studies which might:
include all participants in a diagnostic group;
apply eligibility criteria (such as history of depression) which might single out participants at even higher risk for depression; or
enrol only participants who score above a threshold on a self‐report questionnaire (i.e. subthreshold depression), but do not yet meet criteria for mild depression (which might also mark them at being at even higher risk for depression).
-
Length of treatment: we conducted analyses where follow‐up was post‐randomisation instead of post‐intervention. We analysed follow‐up durations using different time frames:
short‐term (less than six months post‐randomisation);
medium‐term (six to 12 months post‐randomisation); and
long‐term (more than 12 months post‐randomisation).
Type of funding: we excluded studies based on commercial funding, as they might have posed a bias towards the sponsors' interests.
Summary of findings and assessment of the certainty of the evidence
We summarised key findings of our review in 'Summary of findings' tables (Higgins 2011). The tables include the primary outcomes the incidence of depression, acceptability and tolerability for both psychological (Table 1) and pharmacological (Table 2) preventive interventions. We assessed the certainty of the body of evidence for each outcome with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Schünemann 2013). The 'Summary of findings' tables include numbers of studies and participants, ORs or SMDs respectively, as well as an estimation of the certainty of the evidence based on the standards of GRADE. Certainty of evidence was assessed considering the risk of bias of included studies, unexplained heterogeneity (inconsistency), the directness of the evidence (indirectness), preciseness of results (imprecision), as well as the risk of publication bias (other considerations).
Summary of findings 1. Psychological interventions compared to treatment as usual in the prevention of the incidence of depression in adults with long‐term physical conditions.
| Psychological interventions compared to treatment as usual in the prevention of the incidence of depression in adults with long‐term physical conditions | ||||||
| Patient or population: adults with long‐term physical conditions (18 years or older) Setting: hospital Intervention: preventive psychological interventions Comparison: treatment as usual | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with treatment as usual | Risk with preventive psychological interventions | |||||
| Incidence of depression ‐ post‐intervention: 0 months follow‐up assessed with: diagnosis by DSM‐IV | Study population | OR 0.43 (0.20 to 0.95) | 194 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | ||
| 23 per 100 | 12 per 100 (6 to 22) | |||||
| Incidence of depression ‐ short term: < 6 months follow‐up assessed with: diagnosis by DSM‐IV | Study population | OR 0.71 (0.36 to 1.38) | 190 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | ||
| 27 per 100 | 21 per 100 (12 to 34) | |||||
| Incidence of depression ‐ medium term: 6‐12 months follow‐up | no data available | ‐ | (0 RCTs) | ‐ | ||
| Incidence of depression ‐ long term: > 12 months follow‐up | no data available | ‐ | (0 RCTs) | ‐ | ||
| Tolerability ‐ total number of adverse events | no data available | ‐ | (0 RCTs) | ‐ | ||
| acceptability ‐ drop‐outs due to adverse events | no data available | ‐ | (0 RCTs) | ‐ | ||
| acceptability ‐ drop‐outs due to any cause: post‐intervention | Study population | OR 5.21 (1.11 to 24.40) | 206 (1 RCT) | ⊕⊕⊝⊝ LOW 2 4 | ||
| 20 per 1.000 | 95 per 1.000 (22 to 330) | |||||
| acceptability ‐ drop‐outs due to any cause: < 6 months | Study population | OR 1.67 (0.58 to 4.77) | 206 (1 RCT) | ⊕⊕⊝⊝ LOW 2 4 | ||
| 59 per 1.000 | 95 per 1.000 (35 to 232) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 The low and high risk values are the two extreme numbers of incidences in the control groups from studies included in the review.
2 Downgraded once due to indirectness. Due to the nature of this review, we included all types of chronic physical illnesses. With only one study addressing psychological interventions, there is some uncertainty with the applicability of the effect to all types of diseases.
3 Downgraded once due to imprecision. The evidence is based on only one study with 194 participants.
4 Publication bias strongly suspected.
Summary of findings 2. Pharmacological interventions compared to placebo in the prevention of the incidence of depression in adults with long‐term physical conditions.
| Pharmacological interventions compared to placebo in the prevention of the incidence of depression in adults with long‐term physical conditions | ||||||
| Patient or population: adults with long‐term physical conditions (18 years or older) Setting: hospitals (especially university medical center) and specialised rehabilitation clinics Intervention: preventive pharmacological interventions Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with preventive pharmacological interventions | |||||
| Incidence of depression ‐ post‐intervention: 0 months follow‐up assessed with: diagnosis by DSM‐IV, ICD‐10 or clinician, & cut‐off (HADS‐D, MDI, QIDS‐RS, HAM‐D‐17) | Study population | OR 0.31 (0.20 to 0.49) | 814 (9 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 4 5 | ||
| 22 per 100 | 8 per 100 (5 to 12) | |||||
| Low | ||||||
| 6 per 100 3 | 2 per 100 (1 to 3) | |||||
| High | ||||||
| 28 per 100 3 | 11 per 100 (7 to 16) | |||||
| Incidence of depression ‐ short‐term: < 6 months follow‐up assessed with: diagnosis by clinician | Study population | OR 0.44 (0.08 to 2.46) | 23 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 4 5 6 | ||
| 50 per 100 | 22 per 100 (4 to 100) | |||||
| Incidence of depression ‐ medium‐term: 6‐12 months follow‐up assessed with: diagnosis by DSM‐IV | Study population | OR 0.81 (0.23 to 2.82) | 233 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 4 5 7 8 | ||
| 18 per 100 | 16 per 100 (5 to 39) | |||||
| Low | ||||||
| 0 per 100 3 | 0 per 100 (0 to 0) | |||||
| High | ||||||
| 30 per 100 3 | 26 per 100 (9 to 55) | |||||
| Incidence of depression ‐ long‐term: >12 months follow‐up | no data available | ‐ | (0 RCTs) | ‐ | ||
| tolerability ‐ total number of adverse events | escitalopram (n = 459) vs. placebo (n = 389): post‐intervention; melatonin (n = 29) vs. placebo (n = 15): post‐intervention; sertraline (n = 181) vs. placebo (n = 206): post‐intervention | ‐ | (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 4 5 9 10 | ||
| acceptability ‐ drop‐outs due to adverse events: post‐intervention | Study population | OR 2.05 (1.07 to 3.89) | 561 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 4 10 11 | ||
| 58 per 1.000 | 111 per 1.000 (61 to 192) | |||||
| acceptability ‐ drop‐outs due to any cause: post‐intervention | Study population | OR 1.13 (0.73 to 1.73) | 962 (9 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 4 5 10 12 | ||
| 226 per 1.000 | 248 per 1.000 (176 to 336) | |||||
| acceptability ‐ drop‐outs due to any cause: 6‐12 months | Study population | OR 1.13 (0.69 to 1.86) | 327 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 4 5 10 12 | ||
| 274 per 1.000 | 299 per 1.000 (207 to 413) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded once due to indirectness. Due to the nature of this review, we included all types of chronic physical illnesses. With five out of ten studies including patients after stroke, they weighted heavily in the meta‐analyses. Therefore, there is some uncertainty with the applicability of the effect to all types of diseases.
2 Publication bias strongly suspected.
3 The low and high risk values are the two extreme numbers of incidences in the control groups from studies included in the review.
4 Downgraded twice due to imprecision. For one or more studies there were only small sample sizes, only few events, and/or wide confidence intervals.
5 Downgraded twice due to risk of bias. Across all included trials, outcome data was incomplete, and bias for selective reporting was either high or unclear. One study was insufficiently blinded, and one study had a high risk for other bias.
6 Downgraded once due to indirectness. Due to the nature of this review, we included all types of chronic physical illnesses. With only one study on the short‐term effect, there is some uncertainty with the applicability of the effect to all types of diseases.
7 Downgraded once due to indirectness. Due to the nature of this review, we included all types of chronic physical illnesses. With two out of three studies including patients after stroke, they weighted heavily in the meta‐analyses. Therefore, there is some uncertainty with the applicability of the effect to all types of diseases.
8 Downgraded once due to inconsistency. One study favoured controls.
9 Downgraded twice due to inconsistency across studies. For SSRIs, two studies report more adverse events in the escitalopram group than the placebo group, while another two studies report fewer adverse events in the sertraline group than the placebo group.
10 Downgraded once due to indirectness. Due to the nature of this review, we included all types of pharmacological interventions. With seven studies including SSRIs, there is some uncertainty with the applicability on other classes of antidepressants.
11 Downgraded twice due to risk of bias. Outcome data was incomplete and reporting bias was either high or unclear.
12 Downgraded twice due to inconsistency across studies with one study favouring the pharmacological intervention.
Results
Description of studies
Results of the search
After de‐duplication, the systematic search yielded 9,527 references. Two review authors (HK, OM) screened the results, excluding 9,398 references. Among these, two studies are still ongoing and recruiting (ACTRN12616000886482; Madsen 2017; see also Characteristics of ongoing studies). A total of 13 studies are still awaiting classification (see Characteristics of studies awaiting classification). Among these, two studies have completed data collection but results are not published yet (ChiCTR‐TRC‐12003489; Sander 2017). For three references, no corresponding study or further data could be identified (EudraCT‐2005‐005266‐37; Guneri 2006; van Zyl 2006). Another eight studies were reported in Chinese and could not be accessed (He 2004; Li 2004; Lu 2010; Wen 2006; Xu 2006; Zhong 2013; Zhou 2008; Zhu 2014). Of the 129 full‐text articles assessed for eligibility, 101 references were excluded. These comprised 85 full‐texts, eight trial registrations, and eight comments. Among the excluded studies, 20 studies (Almeida 2010; Beglinger 2014; Brody 2005; Browne 2013; Burns 2007; Couch 1976; Dean 1969; de Jonge 2009; Hackett 2010; Hackett 2013; Karp 2016; Kong 2007; Mossey 1996; Niedermaier 2004; Pitceathly 2009; Pols 2017; Rovner 2014; van der Aa 2015; Yeung 2014; Zhang 2013) comprising 26 references were excluded for less obvious reasons than not fulfilling inclusion criteria such as 'Types of studies,' ‘Types of participants,' or ‘Types of interventions.' Reasons for excluding these studies are in the Characteristics of excluded studies. Any discrepancies in assessment were resolved by involving another review author (HB).
Finally, we included 28 references addressing 11 different trials (Almeida 2006; Hansen 2012; Hansen 2014; Lydiatt 2008; Lydiatt 2013; Narushima 2002; Novack 2009; Rasmussen 2003; Robinson 2008; Rovner 2007; Tsai 2011; see also Characteristics of included studies). Figure 1 offers a detailed overview of the study selection process in form of a PRISMA flow diagram.
In February 2020, we did an update of the systematic search, yielding a further 2,326 references. Handsearches yielded another six references. After de‐duplication, 2,314 references were once again screened by two of the review authors (HK, OM). 2,288 references were excluded after screening (among these one study (comprising two conference papers) was already included in prior search batches (Hansen 2012) and was not included again). Of the 26 full‐texts assessed for eligibility, 26 references were excluded (comprising 12 trials reported in 15 references, three protocols, and eight conference papers). Among the excluded studies, 4 studies (Borrelli 2019; Hood 2018; Kredentser 2018; Read 2020) comprising 4 references were excluded for less obvious reasons than not fulfilling inclusion criteria such as 'Types of studies,' ‘Types of participants,' or ‘Types of interventions. Therefore, no additional studies were included after the February 2020 update.
Included studies
Design
All 11 included studies were RCTs. Of the 11 included trials, ten compared pharmacological interventions to placebo, and were included in the final meta‐analyses (Almeida 2006; Hansen 2012; Hansen 2014; Lydiatt 2008; Lydiatt 2013; Narushima 2002; Novack 2009; Rasmussen 2003; Robinson 2008; Tsai 2011). Given that only one trial compared psychological interventions to a fitting control condition, pooling of results was not possible (Rovner 2007). Due to the nature of the study design, the psychological intervention was single‐blinded. Two trials included a three‐arm parallel randomised controlled clinical trial design with Robinson 2008 comparing a pharmacological and a psychological intervention (problem‐solving therapy) to placebo, and Narushima 2002 comparing two different types of a pharmacological intervention (fluoxetine, nortriptyline) to placebo. For Robinson 2008 we only included the pharmacological intervention because their comparison of a psychological intervention to placebo was not eligible for inclusion. As for Narushima 2002, we combined the two groups of pharmacological interventions (i.e. fluoxetine/nortriptyline) to create a single pair‐wise comparison. For details of the included trials, see Characteristics of included studies.
We found no eligible cluster‐randomised trials or cross‐over trials.
Sample sizes
Sample sizes of randomised participants varied from 36 participants (Lydiatt 2008) to 239 participants (Hansen 2012). In total, 1279 participants were randomised. Among these, 653 participants were included in the intervention groups, and 626 participants in the control groups. Due to drop‐outs, we analysed the data of 1031 participants. For the comparisons of pharmacological interventions to placebo, we analysed data of 837 participants, among these 423 participants in the intervention groups, and 414 participants in the control groups. For the comparison of a psychological intervention to TAU we analysed data of 194 participants, 95 participants in the intervention group and 99 participants in the control group.
Setting
Trials were conducted in five different countries. Six trials were conducted in the United States of America (Lydiatt 2008; Lydiatt 2013; Narushima 2002; Novack 2009; Robinson 2008; Rovner 2007), three trials in Denmark (Hansen 2012; Hansen 2014; Rasmussen 2003), and one trial each in Australia (Almeida 2006) and Taiwan (Tsai 2011) respectively. Narushima 2002 included participants from Argentina in addition to those from the US.
Subset data
None of the included studies used mixed study samples (e.g. a sample of participants who were initially without depression or anxiety; a sample consisting of adults who were mainly with a long‐term physical condition). Two studies were of potential interest to this review (de Jonge 2009; Pols 2017) but due to mixed study samples and the unavailability of 'clear' data, we excluded them.
Participants
Psychological intervention versus control
For a comparison of psychological interventions and TAU, we analysed the data of one trial including 194 participants with age‐related macular degeneration (Rovner 2007). Thirty‐four percent of participants in the intervention group, and 26% in the control group were male. The average age in both groups was 81 years.
Pharmacological intervention versus placebo
For a comparison of pharmacological interventions and placebo, we analysed the data of five trials including 505 participants after stroke (Almeida 2006; Narushima 2002; Rasmussen 2003; Robinson 2008; Tsai 2011), two trials including 176 participants with head and neck cancer (Lydiatt 2008; Lydiatt 2013), one trial including 54 participants with breast cancer (Hansen 2014), one trial including 239 participants with acute coronary syndrome (Hansen 2012), and one trial including 99 participants with traumatic brain injury (Novack 2009). In the intervention groups, the average age was 60 years (range: 35 to 72 years), and about 61.8% of participants were male (range: 0% to 80%). For the control groups, the average age was 61 years (range: 35 to 68 years), and about 53% of participants were male (range: 0% to 80%). Narushima 2002 offered no information on age, and Hansen 2014 only included female participants, as they were addressing participants with breast cancer.
Interventions
Psychological intervention versus control
Rovner 2007 examined how problem‐solving therapy compared to TAU might prevent the incidence of depression in participants with age‐related macular degeneration. Problem‐solving therapy addresses negative perceptions possibly interfering with participants practical solution skills by teaching problem‐solving skills. This manual‐driven interventions was administered by trained therapists (two nurses and one master's level counsellor), and delivered in six in‐home problem‐solving sessions (45 to 60 minutes) during a period of eight weeks. In order to maintain treatment fidelity, problem‐solving sessions were audiotaped, and partially randomly assessed by one of the study authors. Ranging from 0 ('very poor') to 5 ('very good'), the study author rated 'implementing decision‐making guidelines' with a mean score of 3.70 (SD = 1.30), and 'interpersonal effectiveness' with a means score of 4.97 (SD = 0.24), indicating satisfactory to very good performance. TAU consisted of usual treatments by ophthalmologists as well as other health care providers. Participants who received problem‐solving therapy also received usual care.
Pharmacological intervention versus placebo
Of the ten included trials addressing pharmacological interventions to prevent depressive symptoms in adults with different long‐term physical conditions, eight trials applied SSRIs (Almeida 2006; Hansen 2012; Lydiatt 2008; Lydiatt 2013; Narushima 2002; Novack 2009; Rasmussen 2003; Robinson 2008), one trial tricyclic antidepressants (Narushima 2002), one trial an SNRI (Tsai 2011), and one trial an unclassified antidepressant (Hansen 2014).
Included SSRIs were sertraline in three trials (Almeida 2006; Novack 2009; Rasmussen 2003) with 347 participants; escitalopram in another three trials (Hansen 2012; Lydiatt 2013; Robinson 2008) with 504 participants; and citalopram (Lydiatt 2008) with 28 participants in one trial. One additional trial also administered an SSRI (fluoxetine), but given that Narushima 2002 used a three‐arm parallel randomised controlled clinical design with 48 participants, we avoided the potential for bias caused by multiple comparisons with one control group by combining the two study arms, fluoxetine and nortriptyline, into one group, to create a single pair‐wise comparison for meta‐analysis. The duration and dosage of SSRI treatment varied greatly across studies. While all three sertraline trials administered a constant dosage of 50 mg/daily, Rasmussen 2003 allowed for a dosage increase of sertraline up to 150 mg in case of clinical need. Follow‐up duration was 12 months in all three studies, but duration of treatment varied from three months (Novack 2009) to 24 weeks (Almeida 2006) to 12 months (Rasmussen 2003). As for escitalopram, dosages varied from 5 mg to 20 mg. While Hansen 2012 increased the dosage from 5 mg to 10 mg after one week until study conclusion after 12 months, Lydiatt 2013 started treatment with 10 mg, increased the dosage to 20 mg from weeks two to 16, and decreased it once more to 10 mg until the end of week 16. Robinson 2008, on the other hand, offered a constant dosage of 5 mg to those aged ≥ 65 years, and a dosage of 10 mg to those aged < 65 years during the 12 months of preventive treatment. While Hansen 2012 conducted no further follow‐up after the 12 months of treatment, Robinson 2008 offered follow‐up data after 18 months, and Lydiatt 2013 after 28 weeks. Considering the other two SSRIs, citalopram and fluoxetine, both administered the preventive treatment for a duration of 12 weeks. Lydiatt 2008 started citalopram treatment with a dosage of 20 mg in the first week, continued it with 40 mg from weeks two to 11, and discontinued it after a dosage of 20 mg in week 12. Narushima 2002 steadily increased the fluoxetine dosage from 10 mg in the first three weeks, to 20 mg in weeks four to six, to 30 mg in weeks seven to nine, and finally to 40 mg in weeks 10 to 12. Follow‐ups were available for citalopram after 16 weeks, and for fluoxetine after 6, 9, 12, and 24 months.
The one tricyclic antidepressant studied was nortriptyline (Narushima 2002). Corresponding to the other study arm conducted by Narushima 2002 (i.e. fluoxetine), the treatment duration with nortriptyline was three months, and follow‐ups were available after six, nine, 12, and 24 months. During the treatment period, nortriptyline dosage was steadily increased from 25 mg in the first week to 50 mg in weeks two to three, to 75 mg in weeks three to six, and finally, to 100 mg in weeks 10 to 12.
The SNRI milnacipran was administered by one trial (Tsai 2011) with 92 participants. Starting the preventive treatment with a dosage of 50 mg, the dosage was increased to 100 mg after one week which participants received until the end of treatment after 12 months. No further follow‐ups were available.
One trial (Hansen 2014) with 54 participants used the unclassified antidepressant melatonin in order to prevent depressive symptoms. For the duration of 13 weeks, participants scheduled for lumpectomy or mastectomy for breast cancer received a constant dosage of 6 mg melatonin, with no further follow‐up data available. The first week of antidepressant treatment was administered preoperatively, the remaining 12 weeks postoperatively.
Outcomes
Seven of the 11 included trials determined incidence of depression by clinical diagnosis. Diagnoses were made by a psychiatrist according to DSM‐IV (Tsai 2011), by the SCAN interview according to ICD‐10 (Hansen 2012), by MINI according to DSM‐IV (Lydiatt 2008), by Present State Examination (PSE) (Wing 1974) according to DSM‐IV (Narushima 2002), by SCID‐I according to DSM‐IV (Novack 2009; Robinson 2008), or by the Schedule for Affective Disorders and Schizophrenia (SADS) (Endicott 1978; Rovner 2007). One trial assessed incidence of depression by either receiving a diagnosis of depression by a physician, or by scoring above the cut‐off of a validated self‐report questionnaire (Almeida 2006). The remaining three trials used cut‐off scores of standardised, validated rating scales (Rasmussen 2003), or standardised, validated self‐report questionnaires (Hansen 2014; Lydiatt 2013) to determine incidence of depression. The applied rating scale was the 17‐item version of the HAMD, with Rasmussen 2003 using the cut‐off > 18. Rasmussen 2003 also included information on the shorter 6‐item version of the HAMD. However, we extracted the information of the 17‐item version, as we pre‐specified to select the outcome measure most frequently used across all studies, in cases where several outcome measures of the same hierarchy level were used in one study. Self‐report questionnaires were HADS‐D (cut‐off score ≥ 8) used by Almeida 2006, the Quick Inventory of Depressive Symptomatology ‐ Self‐Rated version (QIDS‐SR) (Rush 2003) (cut‐off: ≥ 11) used by Lydiatt 2013, as well as the Major Depression Inventory (MDI) (Bech 2001) (cut‐off: ≥ 21) used by Hansen 2014.
Adverse events were measured either by counting the total number of adverse events (Almeida 2006; Hansen 2012; Hansen 2014; Rasmussen 2003; Robinson 2008), or by counting drop‐outs due to adverse events (Hansen 2012; Hansen 2014; Lydiatt 2008; Lydiatt 2013; Tsai 2011). Three trials, however, offered no information on adverse events (Narushima 2002; Novack 2009; Rovner 2007).
Two trials offered information on severity of depression (Novack 2009; Robinson 2008). Novack 2009 used the Neurobehavioral Functioning Inventory (NFI) Depression Subscale to determine severity of depression, and Robinson 2008 the HDRS‐17.
Excluded studies
After accessing full texts, we excluded 94 trials comprising 100 full‐text references from this review. We excluded 41 trials due to baseline depression. We excluded another 28 trials either because they did not include an intervention intended to target depressive symptoms, or depression was not an outcome of special interest. For example, (Beglinger 2014) used citalopram to enhance cognitive function; here, changes in depression were only secondary. Another three trials were excluded as they were not RCTs. Other reasons for exclusion were more specific. These 23 trials comprising 26 references are presented in detail in Characteristics of excluded studies.
Studies awaiting classification
Overall, we identified 13 studies still awaiting classification (see also Characteristics of studies awaiting classification). Among these, two are likely to be eligible for inclusion (ChiCTR‐TRC‐12003489; Sander 2017). After contacting study authors, both revealed that data collection was completed and results are in preparation for publication. Another three studies sounded promising (EudraCT‐2005‐005266‐37; Guneri 2006; van Zyl 2006) but no corresponding published study or further data could be found. For the remaining eight studies (He 2004; Li 2004; Lu 2010; Wen 2006; Xu 2006; Zhong 2013; Zhou 2008; Zhu 2014), full texts were not accessible and no further information were available.
Ongoing studies
Two trials, both RCTs, are currently ongoing. One study examines the effects of melatonin on depression in participants with coronary syndrome (NCT02451293; Madsen 2017). The other study looks at the effects of a novel Cognitive Bias Modification intervention in the prevention of depression in participants with Alzheimer’s disease (ACTRN12616000886482). See Characteristics of ongoing studies for further details.
Risk of bias in included studies
For detailed information on the risk of bias for each included study, please see the 'Risk of bias' tables in the Characteristics of included studies. A graphical representation of the overall risk of bias in included studies is presented in Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Out of the 11 included studies, seven had low risk of bias (Almeida 2006; Hansen 2012; Hansen 2014; Lydiatt 2008; Lydiatt 2013; Robinson 2008; Rovner 2007), and four studies an unclear risk of bias (Narushima 2002; Novack 2009; Rasmussen 2003; Tsai 2011) regarding random sequence generation.
Almeida 2006 allocated participants using a computer‐generated random list of numbers. Hansen 2012 used centrally prepared randomization blocs. Hansen 2014 randomly (with a computer‐generated list) assigned subjects in blocks of six. Lydiatt 2008 allocated participants by coin toss in a 1:1 fashion. Lydiatt 2013 had a pharmacist with no involvement in the evaluation assigning participants in a 1:1 ratio to either one of the conditions according to a randomization table prepared by the study statistician. Robinson 2008 used a permuted blocks randomization scheme. Therefore, the sample size was divided randomly (200 participants) into block sizes of three, six and nine and within each block participants were randomly assigned one of the three treatments using computer‐generated random numbers of 1, 2, or 3. Rovner 2007 used a random‐numbers table, sealed envelopes containing treatment assignments, and a fixed randomization scheme with a 1:1 allocation ratio to assign participants to one of the two study groups. To ensure balance between treatment groups Rovner 2007 applied a random block design, with block sizes (4 or 6) randomly chosen to mask the blocking process.
Narushima 2002, Novack 2009 and Rasmussen 2003 did not specify any information about randomization, and Tsai 2011 only states that they randomly assigned participants to the conditions, without more precise information.
Allocation
The risk of bias for allocation concealment was low for eight studies (Almeida 2006; Hansen 2012; Hansen 2014; Lydiatt 2008; Lydiatt 2013; Robinson 2008; Rovner 2007; Tsai 2011). Randomisation was conducted in a decentralised manner in all of the aforementioned studies. The remaining three included studies did not specify any information about the allocation concealment (Narushima 2002; Novack 2009; Rasmussen 2003), and hence, potential bias remained unclear.
Blinding
We assessed blinding separately for performance and detection bias.
Blinding of participants and personnel (performance bias)
Seven studies (Almeida 2006; Hansen 2012; Hansen 2014; Lydiatt 2008; Novack 2009; Robinson 2008; Tsai 2011) showed low risk of bias, three studies (Lydiatt 2013; Rasmussen 2003; Rovner 2007) unclear risk of bias, and one study (Narushima 2002) a high risk of bias. Studies with low risk of bias ensured unawareness of treatment allocation for both participants and study personnel (Almeida 2006; Lydiatt 2008; Hansen 2012; Hansen 2014; Novack 2009; Robinson 2008; Tsai 2011).
Even though sufficient blinding of participants and study personnel by both Lydiatt 2013 and Rasmussen 2003 can be assumed with the heading saying 'double blind', both studies received an unclear risk of bias rating as information on blinding procedures were insufficient. Rovner 2007 also received a unclear risk of bias rating even though great efforts were taken to insure study quality. Due to the nature of the intervention (problem solving therapy), participants and therapists were aware of the treatment condition. Still, research nurses instructed participants on the importance to remain silence about their study participation to avoid further bias. Despite these efforts, unmasking took place in 18% of cases.
Narushima 2002 was rated with a high risk of bias for performance bias due to the insufficient blinding of treating personnel. While efforts were made to ensure participants blinding, treating physicians appear to be aware of study conditions. In reaction to drug induced side effects in the intervention group, treatment doses were decreased for both affected participants in the intervention group and also corresponding numbers of participants in the control group. To do so, treating physicians had to be aware on both study conditions.
Blinding of outcome assessment (detection bias)
Concerning the blinding of the outcome assessment, seven studies were rated at a low risk of bias (Almeida 2006; Hansen 2012; Hansen 2014; Lydiatt 2008; Narushima 2002; Novack 2009; Rovner 2007), and four studies (Lydiatt 2013; Rasmussen 2003; Robinson 2008; Tsai 2011) at an unclear risk of bias.
Almeida 2006 stated that the whole research time was blinded regarding study conditions. Hansen 2012, Hansen 2014, Lydiatt 2008, Novack 2009 and Rovner 2007 specifically mention the outcome assessment to be blinded. While Narushima 2002 offers no information on the blinding of the outcome assessors, we still rated the risk of bias being low because authors made efforts to determine the effectiveness of the blinding by asking both participants and raters if they believed they had received treatment or placebo.
All four studies (Lydiatt 2013; Rasmussen 2003; Robinson 2008; Tsai 2011) receiving an unclear bias rating and offered no information for assessing detection bias.
Incomplete outcome data
Only one of the 11 included studies (the only study offering a psychological intervention) had less than 10% of incomplete outcome data (Rovner 2007). Still, we rated the risk of attrition bias as unclear because drop‐outs for the intervention group were reported incorrectly. The ten studies administering pharmacological interventions (Almeida 2006; Hansen 2012; Hansen 2014; Lydiatt 2008; Lydiatt 2013; Narushima 2002; Novack 2009; Rasmussen 2003; Robinson 2008; Tsai 2011) were rated with a high risk of bias due to more than 10% of missing data.
Selective reporting
For six studies, there were no protocols available, so that the risk of reporting bias remained unclear (Almeida 2006; Lydiatt 2008; Lydiatt 2013; Narushima 2002; Novack 2009; Rovner 2007). For five studies, a high risk of reporting bias was found due to incomplete reporting of outcomes (Hansen 2012; Hansen 2014; Rasmussen 2003; Robinson 2008; Tsai 2011). For only two of these trials were protocols available (Hansen 2012; Hansen 2014). The other three trials did not report completely on outcomes named in the trial registration (Robinson 2008), or in the method section (Rasmussen 2003; Tsai 2011).
Other potential sources of bias
Regarding other possible bias, eight studies showed a low risk of bias (Almeida 2006; Hansen 2012; Hansen 2014; Novack 2009; Rasmussen 2003; Robinson 2008; Rovner 2007; Tsai 2011). For two studies the risk of other bias remained unclear (Lydiatt 2008; Lydiatt 2013). One study was rated to have a high risk of other bias (Narushima 2002).
Lydiatt 2008 only offered socio‐demographic data on those participants who completed visit one after four weeks, instead of those randomised and allocated, and therefore, the risk for other potential sources of bias was rated unclear. Lydiatt 2013 stratified participants by site, sex, stage, and primary modality of treatment which might also imply the potential for other sources of bias.
Narushima 2002 received a high risk of bias rating as they did not provide any information on participants' baseline characteristics (e.g. age) which hampers the evaluation of external validity. In addition, Narushima 2002 did not mention any declaration of interest, so that conflicts of interest cannot be ruled out.
Effects of interventions
Comparison 1: Psychological interventions versus usual care
One study including 194 participants contributed data to this comparison (Rovner 2007). See also: Table 1.
Primary outcomes
1.1 Incidence of depression assessed by diagnosis: post‐intervention and short‐term
There was evidence of very low certainty showing that problem‐solving therapy was more effective in preventing the incidence of depression than usual care immediately post‐intervention (OR 0.43, 95% CI 0.20 to 0.95; participants = 194; studies = 1; Analysis 1.1). In the short‐term, however, this effect was no longer meaningful (OR 0.71, 95% CI 0.36 to 1.38; participants = 190; studies = 1; Analysis 1.2).
1.1. Analysis.

Comparison 1: Psychological intervention versus usual care, Outcome 1: Incidence of depression (diagnosis) ‐ post‐intervention
1.2. Analysis.

Comparison 1: Psychological intervention versus usual care, Outcome 2: Incidence of depression (diagnosis) ‐ short‐term
1.2 Tolerability and acceptability of psychological interventions
The total number of adverse events, the number of drop‐outs due to adverse events, and the number of drop‐outs due to any cause were used as a proxy measure for tolerability and acceptability of the preventive intervention.
1.2.1 Adverse events
No data were available for this outcome.
1.2.2 Drop‐outs due to adverse events
No data were available for this outcome.
1.2.3 Drop‐outs due to any cause
Out of 206 participants randomised (intervention: 105; control: 101), Rovner 2007 reported on 10 participants (9.5 %) who dropped‐out from the problem solving therapy study‐arm, and six (2.0 %) participants who dropped out from the TAU study arm, post‐intervention. Short‐term, still a total of 10 participants (9.5 %) had dropped‐out from the problem solving therapy study arm, and six (5.9 %) from the TAU study arm. Based on evidence of low certainty, groups differed post‐intervention, with fewer drop‐outs in the usual care group (OR 5.21, 95% CI 1.11 to 24.40; participants = 206; studies = 1; Analysis 1.3); but not in the short‐term (OR 1.67, 95% CI 0.58 to 4.77; participants = 206; studies = 1; Analysis 1.4). For more details, see Characteristics of included studies.
1.3. Analysis.

Comparison 1: Psychological intervention versus usual care, Outcome 3: Dropouts due to any cause ‐ post‐intervention
1.4. Analysis.

Comparison 1: Psychological intervention versus usual care, Outcome 4: Dropouts due to any cause ‐ short‐term
Secondary outcomes
1.3 Incidence of depression assessed by cut‐off
No data were available for this outcome.
1.4 Severity of depression
No data were available for this outcome.
1.5 Cost‐effectiveness
No data were available for this outcome.
1.6 cost‐utility
No data were available for this outcome.
Comparison 2: Pharmacological interventions versus placebo
Ten studies including 837 participants contributed data to this comparison (Almeida 2006; Hansen 2012; Hansen 2014; Lydiatt 2008; Lydiatt 2013; Narushima 2002; Novack 2009; Rasmussen 2003; Robinson 2008; Tsai 2011). Among these, six studies (Hansen 2012; Lydiatt 2008; Narushima 2002; Novack 2009; Robinson 2008; Tsai 2011) reported on the incidence of depression assessed by diagnosis, our primary outcome. Three studies (Hansen 2014; Lydiatt 2013; Rasmussen 2003) assessed incidence of depression by scoring above a certain cut‐off score (i.e. secondary outcome), and one study (Almeida 2006) assessed incidence of depression either by diagnosis of a treating physician, or by scoring above a certain cut‐off. For the post‐intervention effect, Analysis 2.1 offers an overview on the incidence of depression irrespective of the pre‐defined primary and secondary outcomes. (Only nine studies are presented for the post‐intervention effect because one study (Lydiatt 2008) only offered data on the short‐term effect (see Primary outcomes)). Hence, there was evidence of very low certainty showing that pharmacological interventions (escitalopram, fluoxetine/nortriptyline, melatonin, milnacipran, sertraline) were more effective than placebo in preventing the incidence of depression, assessed either by diagnosis or cut‐off, immediately post‐intervention (OR 0.31, 95% CI 0.20 to 0.49; 814 participants; nine studies; I2 = 0%). In the medium‐term, differences between pharmacological interventions (escitalopram, sertraline) and placebo were no longer meaningful (OR 0.81, 95% CI 0.23 to 2.82; 233 participants; three studies; I2 = 49%; Analysis 2.2). The considerable heterogeneity is most likely attributable to the one study favouring the placebo group instead of the pharmacological group.
2.1. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 1: Incidence of depression (all) ‐ post‐intervention
2.2. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 2: Incidence of depression (all) ‐ medium‐term
The following sections report results in accordance to the prespecified classifications of primary or secondary outcomes.
For overall results, see also: Table 2.
Primary outcomes
2.1 Incidence of depression assessed by diagnosis: post‐intervention, short‐term, and medium‐term
Assessed by diagnosis, there was evidence of very low certainty showing that pharmacological interventions (escitalopram, fluoxetine/nortriptyline, milnacipran, sertraline) were more effective than placebo in preventing the incidence of depression immediately post‐intervention (OR 0.26, 95% CI 0.13 to 0.52; participants = 474; studies = 5; I2 = 0%: Analysis 2.3). Very low‐certainty evidence on the short‐term effect (OR 0.44, 95% CI 0.08 to 2.46; participants = 23; studies = 1; Analysis 2.4), or the medium‐term effect (OR 1.50, 95% CI 0.06 to 39.71; participants = 139; studies = 2; I2 = 75%; Analysis 2.5) yielded no meaningful group differences (short term: only citalopram; medium‐term: escitalopram, sertraline).
2.3. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 3: Incidence of depression (diagnosis) ‐ post‐intervention
2.4. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 4: Incidence of depression (diagnosis) ‐ short‐term
2.5. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 5: Incidence of depression (diagnosis) ‐ medium‐term
2.2 Tolerability and acceptability for pharmacological interventions
2.2.1 Adverse events
Five studies reported adverse events in detail (Almeida 2006; Hansen 2012; Hansen 2014; Rasmussen 2003; Robinson 2008). In total, 669 adverse events were reported from 316 participants in the pharmacological group, and 610 adverse events from 311 participants in the placebo group (escitalopram: n = 459 versus placebo: n = 389; melatonin: n = 29 versus placebo: n = 15; sertraline: n = 181 versus placebo: n = 206).
Almeida 2006 found 46 adverse events in participants after stroke, treated with sertraline, and 25 events in those treated with placebo. According to Fisher's exact test, group differences existed for tremor (P = .005), and agitation (P = .026), with less tremor (23.3% vs. 0.0%) and less agitation (23.3% vs. 3.2%) in the placebo group.
Also treated with sertraline, and measured by the Udvalg for Kliniske Undersøgelser (UKU) Side Effect Rating Scale, Rasmussen 2003 reported no meaningful group differences for the incidence of adverse events in participants after stroke. In total, the intervention group experienced about 170 adverse events, and the control group about 200 adverse events including tiredness: 44% (sertraline) and 50% (placebo) respectively; restlessness/tension: 37% and 49%; depressed mood: 36% and 47%; dizziness: 23% and 24%; poor concentration: 20% and 32%; insomnia: 16% and 25%; constipation: 11% and 16%; diarrhoea: 16% and 11%; nausea: 16% and 11%; increased sleep: 11% and 10%; headache: 9% and 10%; and poor memory: 4% and 13%.
Robinson 2008 assessed a variety of adverse events in participants after stroke treated with escitalopram. In the escitalopram group, 2 to 29% of participants experienced some kind of gastrointestinal adverse events (total number: 73 gastrointestinal adverse events), 7 to 20% some kind of sexual adverse events (total number: 22 sexual adverse events), 8 to 64% some kind of cardiovascular adverse events (total number: 51 cardiovascular adverse events), and 2 to 63% some other kind of adverse event like dizziness (total number: 74 other adverse events). Including all‐cause hospitalizations, nausea, and adverse effects associated with escitalopram were not significantly different between the study groups. In the placebo group, 2 to 47% of participants after stroke experienced some kind of gastrointestinal adverse events (total number: 81 gastrointestinal adverse events), 2 to 16% some kind of sexual adverse events (total number: 14 sexual adverse events), 5 to 62% some kind of cardiovascular adverse events (total number: 46 cardiovascular adverse events), and 2 to 59% some other kind of adverse event like dizziness (total number: 61 other adverse events). While no test statistics were reported, Robinson 2008 state that there were no significant differences between groups. In total, the escitalopram group experienced 220 adverse events, and the placebo group 202 adverse events.
Hansen 2012 assessed the effects of escitalopram on participants with acute coronary syndrome. Also using the UKU side effect scale, authors state there were no differences between groups except for increased dream activity. In total, the escitalopram group experienced about 239 adverse events, and the placebo group about 187, including gastrointestinal adverse effects (23 vs. 16), sexual adverse effects (42 vs. 30), or other adverse effects (174 vs. 141)
For participants with breast cancer, Hansen 2014 report no significant group differences between the melatonin group (15/27 = 56%) and the placebo group (12/24 = 50%) regarding adverse events (P = 0.78). In the melatonin group, most frequent adverse events were dizziness (14%), headache, (10%) and paraesthesia in the mouth region, arms or legs (10%). In the placebo group, the most common adverse events were headache (27%), difficulty falling asleep (13%), and nausea (13%). The total numbers of adverse events were 29 in the melatonin group, and 15 in the placebo group.
2.2.2 Drop‐outs due to adverse events
Five studies reported data on drop‐outs associated with the incidence of adverse events Hansen 2012; Hansen 2014; Lydiatt 2008; Lydiatt 2013; Tsai 2011 (for details see Characteristics of included studies). There was evidence of very low certainty showing that a placebo intervention resulted in fewer drop‐outs due to adverse events than a pharmacological intervention (OR 2.05, 95% CI 1.07 to 3.89; participants = 561; studies = 5; I2 = 0%; Analysis 2.6).
2.6. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 6: Acceptability ‐ dropouts due to adverse events
2.2.3 Drop‐outs due to any cause
Nine studies included data on drop‐outs due to any cause directly post‐intervention (Hansen 2012; Hansen 2014; Lydiatt 2008; Lydiatt 2013; Narushima 2002; Novack 2009; Rasmussen 2003; Robinson 2008; Tsai 2011). There was evidence of very low certainty showing no group difference between pharmacological interventions and placebo regarding drop‐outs due to any cause (OR 1.13, 95% CI 0.73 to 1.73; participants = 962; studies = 9; I2 = 28%; Analysis 2.7). Rasmussen 2003 reported a total drop‐out of 12.9% (9/70) in the sertraline group, and 14.9% (10/67) in the placebo group for participants after stroke. Novack 2009 reported a total drop‐out of 0.5% (1/49) in the sertraline group, and 4.0% (2/50) in the placebo group for participants with traumatic brain injury. Robinson 2008 reported a total drop‐out of 11.9% (7/59) in the escitalopram group, and 8.6% (5/58) in the placebo group for participants after stroke. Lydiatt 2013 reported a total drop‐out of 51.4% (38/74) in the escitalopram group, and 40.5% (30/74) in the placebo group for participants with head and neck cancer. Hansen 2012 reported a total drop‐out of 29.2% (35/120) in the escitalopram group, and 25.2% (30/119) in the placebo group for participants with acute coronary syndrome. One additional participant of the placebo group was excluded due to protocol violation. Hansen 2014 reported a significant difference between groups (P = .002), with a total drop‐out of 3.6% (1/28) in the melatonin group, and 38.5% (10/26) in the placebo group for participants with breast cancer. Lydiatt 2008 reported a total drop‐out of 13.3% (2/15) in the citalopram group, and 23.1% (3/13) in the placebo group for participants with head and neck cancer. Narushima 2002 reported a total drop‐out of 12.5% (4/32) in the fluoxetine/nortriptyline group, and 6.3% (1/16) in the placebo group for participants after stroke. Tsai 2011 reported no significant differences between groups (P = .20), with a total drop‐out of 45.7% (21/46) in the milnacipran group, and 32.6% (15/46) in the placebo group for participants after stroke.
2.7. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 7: Acceptability ‐ dropouts due to any cause ‐ post‐intervention
Three studies included data on drop‐outs due to any cause medium‐term (Almeida 2006; Novack 2009; Robinson 2008). There was evidence of very low certainty showing no group difference between pharmacological interventions and placebo regarding drop‐outs due to any cause (OR 1.13, 95% CI 0.69 to 1.86; participants = 327; studies = 3; I2 = 0%; Analysis 2.8). Almeida 2006 reported a total drop‐out of 20.0% (11/55) in the sertraline group, and 10.7% (6/56) in the placebo group for participants after stroke. Novack 2009 reported a total drop‐out of 26.5% (13/49) in the sertraline group, and 28.0% (14/50) in the placebo group for participants with traumatic brain injury. Robinson 2008 reported a total drop‐out of 42.4% (25/59) in the escitalopram group, and 43.1% (25/58) in the placebo group for participants after stroke.
2.8. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 8: Acceptability ‐ dropouts due to any cause ‐ medium‐term
For more detailed information on drop‐outs due to any cause see Characteristics of included studies.
Secondary outcomes
2.3 Incidence of depression assessed by cut‐off
There was evidence of very low certainty on pharmacological interventions (escitalopram, melatonin, sertraline) being more effective than placebo in preventing the incidence of depression immediately post‐intervention (OR 0.26, 95% CI 0.13 to 0.53; participants = 228; studies = 3; I2 = 0%; Analysis 2.9
2.9. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 9: Incidence of depression (cut‐off) ‐ post‐intervention
2.4 Incidence of depression assessed by either diagnosis or cut‐off
One trial of very low certainty assessed incidence of depression by either diagnosis or cut‐off. The pharmacological intervention sertraline showed no meaningful group differences post‐intervention (OR 0.73, 95% CI 0.26 to 2.00; participants = 99; studies = 1; Analysis 2.10), or medium‐term (OR 0.69, 95% CI 0.27 to 1.74; participants = 94; studies = 1: Analysis 2.11).
2.10. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 10: Incidence of depression (diagnosis or cut‐off) ‐ post‐intervention
2.11. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 11: Incidence of depression (diagnosis or cut‐off) ‐ medium‐term
2.5 Severity of depression
Evidence addressing severity of depression was of very low certainty. Comparing pharmacological interventions (sertraline, escitalopram) to placebo yielded no meaningful differences regarding severity of depression, neither post‐intervention (SMD ‐0.19, 95% CI ‐0.91 to 0.54; participants = 163; studies = 2; I2 = 81%; Analysis 2.12), nor short‐term (SMD ‐0.33, 95% CI ‐0.75 to 0.09; participants = 87; studies = 1; Analysis 2.13), nor in the medium‐term (SMD 0.18, 95% CI ‐0.72 to 1.09; 139 participants; two studies; I2 = 86%; Analysis 2.14). Note that heterogeneity was considerable for the post‐intervention and medium‐term effect, which is most likely attributable to the different types of depression scales used (HDRS‐17 and NFI).
2.12. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 12: Severity of depression ‐ post‐intervention
2.13. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 13: Severity of depression ‐ short‐term
2.14. Analysis.

Comparison 2: Pharmacological intervention versus placebo, Outcome 14: Severity of depression ‐ medium‐term
2.6 Cost‐effectiveness
No data were available for this outcome.
2.7 Cost‐utility
No data were available for this outcome.
Subgroup analyses
Subgroup analyses were planned for the type of long‐term physical condition, type of psychological intervention, as well as the class of antidepressant. To compare at least two types either of long‐term physical conditions, two types of psychological interventions, or two classes of antidepressants, three or more studies had to be available for each particular group.
3.1 Type of long‐term physical condition
Due to a lack of more studies on the same type of disease (except for stroke), no subgroup analyses were conducted for a comparison between acute coronary syndrome, age‐related macular degeneration, breast cancer, head and neck cancer, stroke or traumatic brain injury.
3.2 Type of psychological intervention
Only one study (Rovner 2007) addressed a psychological intervention (problem solving therapy), and hence, no additional subgroup analyses were conducted.
3.3 Class of antidepressant
Across studies, different classes of antidepressant medication were applied to prevent the onset of depressive symptoms. Due to a lack of studies on other classes of antidepressant medications like SSRIs (e.g. escitalopram and sertraline), SNRIs (e.g. milnacipran), tricyclic antidepressant medication (e.g. nortriptyline), and unclassified antidepressant (e.g. melatonin) we were unable to conduct subgroup analyses by class of antidepressant.
Sensitivity analyses
4.1 Assessment method
4.1.1 Comparison 1: Psychological interventions versus usual care
Not applicable. Only one study was included for this outcome.
4.1.2 Comparison 2: Pharmacological interventions versus placebo
Not applicable. Studies using cut‐offs to assess incidence of depression (Hansen 2014; Lydiatt 2013; Rasmussen 2003) used different types of instruments (MDI: Hansen 2014; QIDS‐SR: Lydiatt 2013; HAM‐D 17‐item version: Rasmussen 2003).
4.2 Quality of studies
4.2.1 Comparison 1: Psychological interventions versus usual care
4.2.1.1 Failing to double‐blind
Not applicable.
4.2.1.2 Lacking proper allocation concealment
Not applicable.
4.2.1.3 Impact of missing data
We conducted best‐case analyses where all drop‐outs in the control group were considered to have an occurrence of depression. In the best‐case scenario, problem‐solving therapy was more effective in the prevention of the incidence of depression than usual care, both immediately post‐intervention (OR 0.36, 95% CI 0.16 to 0.77; 206 participants; one study), and in the short‐term (OR 0.51, 95% CI 0.27 to 0.96; 206 participants; one study; Analysis 3.1). Compared to original analyses, in a best‐case scenario the short‐term effect was beneficial as well.
3.1. Analysis.

Comparison 3: Sensitivity analyses on quality of studies ‐ impact of missing data (best & worst case scenarios) ‐ incidence of depression: psychological intervention versus placebo, Outcome 1: best case scenario
We also conducted worst‐case analyses where all drop‐outs in the intervention group were considered to have an occurrence of depression. In the worst‐case scenario, effects were no longer favouring either group, neither immediately post‐intervention (OR 0.85, 95% CI 0.44 to 1.65; 206 participants; one study), nor in the short‐term (OR 1.15, 95% CI 0.62 to 2.13; 206 participants; one study; Analysis 3.2). Assuming a worst‐case scenario, the post‐intervention effect was no longer beneficial with respect to the intervention group.
3.2. Analysis.

Comparison 3: Sensitivity analyses on quality of studies ‐ impact of missing data (best & worst case scenarios) ‐ incidence of depression: psychological intervention versus placebo, Outcome 2: worst case scenario
4.2.1.4 Cluster‐randomised trials
Not applicable.
4.2.2 Comparison 2: Pharmacological interventions vs. placebo
4.2.2.1 Failing to double‐blind
Only one study posed a high risk of bias by lacking proper blinding (Narushima 2002). Therefore, we conducted a sensitivity analysis excluding this study in order to assess the incidence of depression after preventive pharmacological treatment. Hence, even after exclusion of Narushima 2002 there was evidence that pharmacological interventions (escitalopram, milnacipran, sertraline) were more effective than placebo in preventing the incidence of depression assessed by diagnosis immediately post‐intervention (OR 0.24, 95% CI 0.11 to 0.53; 431 participants; four studies; I2 = 0%; Analysis 4.1). Overall, the tendency of the effect did not change after excluding Narushima 2002.
4.1. Analysis.

Comparison 4: Sensitivity analyses on quality of studies ‐ failing to double‐blind ‐ incidence of depression: pharmacological intervention versus placebo, Outcome 1: post‐intervention
4.2.2.2 Lacking proper allocation concealment
Not applicable.
4.2.2.3 Impact of missing data
We conducted best‐case analyses where all drop‐outs in the placebo group were considered to have an occurrence of depression. Overall interpretation of results did not change after conducting best case analyses. Again, we found group differences favouring the intervention group immediately post‐intervention (OR 0.13, 95% CI 0.07 to 0.24; 1045 participants; nine studies; I2 = 50%), and no group differences in the short term (OR 0.31, 95% CI 0.06 to 1.51; 28 participants; one study), and the medium term (OR 0.16, 95% CI 0.06 to 0.43; 327 participants; three studies; I2 = 59%; Analysis 5.1).
5.1. Analysis.

Comparison 5: Sensitivity analyses on quality of studies ‐ impact of missing data (best & worst case scenarios) ‐ incidence of depression: pharmacological intervention versus placebo, Outcome 1: best case scenario
We also conducted worst‐case analyses where all drop‐outs in the intervention group were considered to have an occurrence of depression. Immediately post‐intervention (OR 1.35, 95% CI 0.74 to 2.44; 1045 participants; nine studies; I2 = 69%) as well as in the short‐term (OR 1.07, 95% CI 0.23 to 4.89; 28 participants; one study; I2 = 0%), worst‐case scenarios yielded no meaningful group differences (Analysis 5.2). Compared to the original analyses, the worst‐case scenario no longer found a beneficial post‐intervention effect for the intervention group post‐intervention. In the medium term, worst‐case analyses showed a meaningful effect favouring the placebo group (OR 4.81, 95% CI 0.92 to 25.29; 327 participants; three studies; I2 = 81%). Original results favoured neither group but worst‐case analyses indicated a beneficial effect for the placebo group.
5.2. Analysis.

Comparison 5: Sensitivity analyses on quality of studies ‐ impact of missing data (best & worst case scenarios) ‐ incidence of depression: pharmacological intervention versus placebo, Outcome 2: worst case scenario
4.2.2.4 Cluster‐randomised trials
Not applicable.
4.3 Selection of patients
4.3.1 Comparison 1: Psychological interventions vs. usual care
No data were available for this outcome.
4.3.2 Comparison 2: Pharmacological interventions vs. placebo
No data were available for this outcome.
4.4 Lengths of treatment
To evaluate the sustainability of possible antidepressant effects we conducted analyses where follow‐up was post‐randomisation instead of post‐intervention.
4.4.1 Comparison 1: Psychological interventions vs. usual care
Only one study addressing problem solving therapy was included (Rovner 2007). Therefore, effects were the same as post‐intervention but time frames for the considered length of treatment changed. If follow‐up was post‐randomisation instead of post‐intervention, the post‐intervention effect would be a short‐term effect (Analysis 1.1), and the short‐term effect a medium‐term effect (Analysis 1.2).
4.4.2 Comparison 2: Pharmacological interventions vs. placebo
For the primary outcome, we derived short‐term effects from six studies (assessed by diagnosis: Lydiatt 2008, Narushima 2002, Novack 2009; assessed by cut‐off: Hansen 2014, Lydiatt 2013; assessed by either diagnosis or cut‐off: Almeida 2006), a medium‐term effect from six studies (assessed by diagnosis: Hansen 2012, Novack 2009, Robinson 2008, Tsai 2011; assessed by cut‐off: Rasmussen 2003; assessed by either diagnosis or cut‐off: Almeida 2006), and a long‐term effect from one study (assessed by diagnosis: Robinson 2008). There was evidence that pharmacological interventions were more effective than placebo in preventing the incidence of depression in the short‐term (OR 0.36, 95% CI 0.21 to 0.64; 384 participants; six studies; I2 = 2%; Analysis 6.1). In the medium term, there was also a meaningful effect favouring pharmacological interventions to placebo in the prevention of the incidence of depression (OR 0.37, 95% CI 0.22 to 0.61; 619 participants; six studies; I2 = 0%; Analysis 6.2). In the long term, groups were not different from one another (OR 9.89, 95% CI 0.51 to 191.27; 67 participants; one study; Analysis 6.3).
6.1. Analysis.

Comparison 6: Sensitivity analyses on length of treatment ‐ post‐randomisation ‐ incidence of depression: pharmacological intervention versus placebo, Outcome 1: short‐term
6.2. Analysis.

Comparison 6: Sensitivity analyses on length of treatment ‐ post‐randomisation ‐ incidence of depression: pharmacological intervention versus placebo, Outcome 2: medium‐term
6.3. Analysis.

Comparison 6: Sensitivity analyses on length of treatment ‐ post‐randomisation ‐ incidence of depression: pharmacological intervention versus placebo, Outcome 3: long‐term
4.5 Type of funding
To evaluate potential bias towards funders' interests, we excluded studies based on commercial funding.
4.5.1 Comparison 1: Psychological interventions versus usual care
Not applicable.
4.5.2 Comparison 2: Pharmacological interventions versus placebo
We excluded Rasmussen 2003 as they received funding by Pfizer A/S. Robinson 2008 received no funding from pharmaceutical companies but authors were involved with e.g. Hamilton Pharmaceutical Company, Avanir Pharmaceutical Company, Forest Laboratories, or Pfizer. Even though authors stated that Hamilton Pharmaceutical Company and Avanir Pharmaceutical Company had no financial interest in their prevention study, we excluded Robinson 2008 as well.
Even after the exclusion of Rasmussen 2003 and Robinson 2008, there was evidence that pharmacological interventions (escitalopram, fluoxetine/nortriptyline, melatonin, milnacipran, sertraline) were still more effective than placebo in preventing the incidence of depression assessed by either diagnosis or cut‐off immediately post‐intervention (OR 0.31, 95% CI 0.18 to 0.54; 591 participants; seven studies; I2 = 2%; Analysis 7.1). As before, in the medium term group differences between pharmacological interventions (escitalopram, sertraline) and placebo were no longer meaningful (OR 0.58, 95% CI 0.26 to 1.26; 166 participants; two studies; I2 = 0%; Analysis 7.2).
7.1. Analysis.

Comparison 7: Sensitivity analyses on type of funding ‐ incidence of depression: pharmacological intervention versus placebo, Outcome 1: post‐intervention
7.2. Analysis.

Comparison 7: Sensitivity analyses on type of funding ‐ incidence of depression: pharmacological intervention versus placebo, Outcome 2: medium‐term
Reporting bias
We intended to minimise the impact of reporting bias by conducting an extensive and sensitive systematic search for literature.
5.1 Comparison 1: Psychological interventions vs. usual care
Not applicable, there were insufficient studies to assess reporting bias.
5.2 Comparison 2: Pharmacological interventions vs. placebo
The funnel plot for the primary outcome pharmacological intervention showed evidence of asymmetry (see Figure 4), and therefore, a risk of reporting bias has to be assumed.
Discussion
Our review gives a comprehensive overview of primary preventive interventions to prevent depression in adults with long‐term physical conditions, irrespective of the type of intervention or the type of long‐term physical condition. Therefore, we included both psychological and pharmacological interventions applied in primary prevention trials. Primary prevention trials are characterised by starting a treatment before any relevant symptoms develop; in our case, symptoms of depression. Due to the fact that a majority of antidepressant treatments are associated with a variety of adverse events and increased healthcare costs, the initiation of antidepressant treatment before the onset of any clinical relevant symptoms needs to be highly justified. Hence, the WHO encourages applying certain prevention programs and treatments only to a selected group of people known to be at high risk for a disease; such as, for example, depression (selective prevention) (WHO 2016). People with long‐term physical conditions are considered to be at high risk for developing depressive symptoms, and hence, are considered to be a appropriate group for prevention trials (WHO 2016). However, the types of long‐term physical conditions are not specified, even though some types like coronary heart disease, stroke, cancer, or diabetes are suggested (WHO 2016). Therefore, we included all types of long‐term physical conditions in order to also identify those potentially receptive to prevention approaches. To do so, we developed an extensive search strategy in close collaboration with the Cochrane Common Mental Disorder Group, which intended to incorporate at once all types of long‐term physical conditions according to ICD‐10 criteria. With the magnitude of different types of long‐term physical conditions as well as the specialised expertise required to deal with them, it is very hard for clinicians and researchers alike to have an overview on current developments. By including all types of diseases and applying a sensitive search scheme, we also hoped to identify types of long‐term physical conditions that might show a potential for primary prevention treatments. When conducting this review, we did not expect any concluding realisations in this broad field. However, we wanted to summarise international prevention trials on certain diseases already deemed potentially receptive to primary prevention by experts around the world. We hope that our findings can aid future research to broach certain types of long‐term physical conditions when considering future primary prevention trials.
Summary of main results
Regarding psychological interventions, there was evidence of very low certainty for a beneficial post‐intervention effect of problem‐solving therapy compared to TAU in preventing the incidence of depression in adults with age‐related macular degeneration. However, no group differences could be found at six to 12 months follow‐up. For our other primary outcomes, tolerability and acceptability for psychological interventions, there were no data available on adverse events, or drop‐outs due to adverse events. As for drop‐outs due to any cause, there was evidence of low certainty showing fewer drop‐outs in the usual care group directly post‐intervention. in the short term, however, the two groups no longer differed.
There were no data on our secondary outcomes (severity of depression, cost‐effectiveness, or cost‐utility). See also Table 1.
For psychological interventions, we undertook sensitivity analyses on the 'quality of studies ‐ impact of missing data,' as well as the 'length of treatment.'
To assess the 'impact of missing data', we conducted best‐case analyses where all drop‐outs in the control group were considered to have an occurrence of depression, and worst‐case analyses where all drop‐outs in the intervention group were considered to have an occurrence of depression. In the best‐case scenario, in addition to the beneficial post‐intervention effect, problem‐solving therapy appeared to be also beneficial in the short‐term. In the worst case scenario, post‐intervention effects initially favouring the intervention group were no longer favouring either group. In the short term, there was no difference between original analyses and the worst‐case scenario, and no meaningful group differences in either scenario.
In order to analyse the sustainability of possible antidepressant effects after the preventive intervention has been finished, we conducted the primary outcome analyses where follow‐up was post‐intervention. This approach might mix preventive interventions with very short and very long intervention periods (e.g. combining ultra brief prevention measures of only a few sessions within days compared to an e.g. 12 months prevention measure). In both cases, we would examine the maintenance effects of these interventions, while we would obviously expect a stronger maintenance effect from a prevention intervention lasting 12 months. However, to gain information on the prevention effect of an intervention in a given time period, e.g. 12 months following recruitment/intervention start, we conducted sensitivity analyses where follow‐up was post‐randomisation instead of post‐intervention. In this case, we mixed interventions that already ended (e.g. a short‐term intervention) and already looked at sustainability effects with interventions that are at its post‐treatment effect after 12 months. For psychological interventions, results remained unchanged. If follow‐up was post‐randomisation instead of post‐intervention, the post‐intervention effect would be a less than six months post‐randomisation effect, and the short‐term effect a six to 12 months post‐randomisation effect.
Regarding pharmacological interventions, there was evidence of only very low certainty for a beneficial post‐intervention effect of antidepressants (escitalopram, fluoxetine/nortriptyline, melatonin, milnacipran, or sertraline) compared to placebo in preventing the incidence of depression in adults with acute coronary syndrome, breast cancer, head and neck cancer, stroke or traumatic brain injury. One trial including the antidepressant citalopram did not provide post‐intervention data but data for the 0 to 6 months follow‐up. Here, a comparison of citalopram to placebo for adults with head and neck cancer yielded no meaningful group differences. Assessing the effects for the 6 to 12 months follow‐up revealed no group differences for sertraline in the preventive treatment of adults with stroke or traumatic brain injury either. For our other primary outcome ‐ tolerability and acceptability of pharmacological interventions ‐ only some of the studies reported on these outcomes. As for adverse events, the total number was somewhat comparable between the pharmacological intervention (n = 669) and the placebo group (n = 610). Analysing drop‐outs due to adverse events, data synthesis of five studies revealed less drop‐outs due to adverse events in the placebo group. Concerning drop‐outs due to any cause, no group differences could be found. With respect to our secondary outcomes, only two studies reported on severity of depression, and no data at all was available for cost‐effectiveness, or cost‐utility. Regarding severity of depression, evidence of very low certainty showed no group differences comparing pharmacological interventions (sertraline, escitalopram) to placebo post‐intervention, 0 to 6 months follow‐up, or six to 12 months follow‐up. For an overview of our major findings, see also Table 2.
Due to lack of data, we were unable to conduct subgroup analyses on types of long‐term physical conditions, types of psychological interventions, or classes of antidepressants.
Our sensitivity analyses on the quality of 'studies ‐ failing to double blind' as well as the 'type of funding' revealed no changes in the overall interpretation of results.
When conducting sensitivity analyses on the 'quality of studies ‐ impact of missing data' for pharmacological interventions, our overall interpretation of results did not change after conducting best‐case analyses. Worst‐case analyses annihilated the beneficial post‐intervention effect. At less than six months follow‐up there were no differences. However, at six to 12 months follow‐up, the worst‐case scenario revealed a meaningful effect favouring the placebo group instead of antidepressant treatments.
Regarding the 'length of treatment', conducted sensitivity analyses revealed that less than six months post‐randomisation pharmacological interventions (citalopram, escitalopram, fluoxetine/nortriptyline, melatonin, sertraline) were more effective than placebo. Six to 12 months post‐randomisation, there was also a meaningful effect favouring pharmacological interventions (escitalopram, milnacipran, sertraline) to placebo in the prevention of the incidence of depression. Only In the long‐term (more than 12 months follow‐up), groups were not different from one another.
Overall completeness and applicability of evidence
In total, we included six different types of long‐term physical conditions (acute coronary syndrome, age‐related macular degeneration, breast cancer, head and neck cancer, stroke, and traumatic brain injury) and eight types of treatments. The psychological intervention was problem solving therapy, and the seven types of pharmacological interventions included citalopram, escitalopram, fluoxetine/nortriptyline, melatonin, milnacipran, and sertraline. Stroke was the only condition addressed more extensively with five included trials. Most common pharmacological treatments were SSRIs with three trials including escitalopram and sertraline respectively, as well as one trial including citalopram.
Our analyses were conducted following the 'per protocol' principle. Especially with regard to pharmacological antidepressant agents, we assumed that adverse events might arise due to the treatment itself or the development of depressive symptoms, and therefore, might pose the major reasons for discontinuation of study participation. Of the included trials, six conducted ITT analyses (Hansen 2012; Hansen 2014; Lydiatt 2013; Novack 2009; Robinson 2008; Tsai 2011), and five conduced PP analyses (Almeida 2006; Lydiatt 2008; Narushima 2002; Rasmussen 2003; Rovner 2007). In order to report incidences of depression following the PP principle, we re‐calculated the total N of the ITT trials, i.e. we took the total number of randomised participants (e.g. N = 100) and subtracted the total number of drop‐outs (e.g. N = 5) for each corresponding follow‐up (e.g. 100 minus 15 = 85). An exemplary incidence of 20 would be 20/85 (i.e. PP) instead of 20/100 (ITT). To account for the missing data due to drop‐outs as well, we conducted sensitivity analyses on best case and worst case scenarios.
The interpretation of results derived for psychological interventions aimed at the prevention of depression is limited. Even though relatively well conducted, only one trial (Rovner 2007) dealt with the beneficial effects of psychological preventive interventions. Next to the general reporting of drop‐outs, no information on our second primary outcome 'acceptability and tolerability' were reported. In addition, no information on our secondary outcomes severity of depression, cost‐effectiveness, or cost‐utility were available. One trial (Robinson 2008) included a third study arm on a psychological intervention which was not reported due to the fact that an appropriate control group was missing. Therefore, only the comparison of escitalopram and placebo was included in our review, even though results indicate an even greater beneficial effect of problem solving therapy than escitalopram in adults after stroke. Also due to unsuitable control groups, two studies were not included in our review (Niedermaier 2004; Zhang 2013). Both trials included pharmacological interventions which were compared to treatment as usual. With our focus on psychotherapeutic psychological interventions, we did not explore the wide range of preventative social or lifestyle interventions that might also prove to be beneficial in the prevention of depression (Natale 2019).
The interpretation of results derived for pharmacological interventions aimed at the prevention of depression is limited as well. Only six of the ten included trials offered information on our primary outcome 'incidence of depression' assessed by diagnosis (Hansen 2012; Lydiatt 2008; Narushima 2002; Novack 2009; Robinson 2008; Tsai 2011). To enhance the visibility of results regarding the incidence of depression, we decided to include both the primary (i.e. diagnosis) and the secondary (i.e. cut‐off) outcome in one forest plot. Three trials (Hansen 2014; Lydiatt 2013Rasmussen 2003) assessed the incidence of depression only by scoring above a certain cut‐off (i.e. secondary outcome), and one trial (Almeida 2006) used the mixed method of assessing the incidence of depression by either diagnosis by a physician, or cut‐off. This made it possible to compute an overall effect for the incidence of depression as well as subgroup results for either diagnosis (i.e. high quality of assessment), or cut‐off (i.e. low quality of assessment). Unfortunately, one trial (Lydiatt 2008) offered no information on the post‐intervention effect but only on the short‐term effect. Our major analyses (Analysis 3.1, Figure 8) on the preventive effects of pharmacological interventions therefore only includes the data of nine trials. Interpretation of data was further complicated by imprecise reporting. One trial (Rasmussen 2003) only reported percentages instead of total numbers, so we had to calculate the latter. Two trials (Novack 2009; Rasmussen 2003) presented inconclusive numbers on drop‐outs. After re‐calculation of e.g. percentages, we chose the data adding up best. One study (Narushima 2002) had a mixed study‐arm regarding the intervention. Here, participants received either fluoxetine, a SSRI, or nortriptyline, a tricyclic antidepressant. Given that both antidepressants origin from a different antidepressant class makes interpretation of the data even harder. Even though Narushima 2002 included 3, 6, 9, 12, and 24 months follow‐ups, we could not extract the corresponding data except for the 3 months follow‐up. For the latter, data was presented in the text. For the other follow‐ups, only a graphical presentation of the outcome data was a available. The kind of graphical representation, however, made it impossible for us to do reasonable data extractions. As for our second primary outcome, only five out of ten trials reported information on drop‐outs due to adverse events (Hansen 2012; Hansen 2014; Lydiatt 2008; Lydiatt 2013; Tsai 2011), and five studies report on adverse events in total (Almeida 2006; Hansen 2012; Hansen 2014; Rasmussen 2003; Robinson 2008). While this offers first hints of the acceptability and tolerability of the study medication, more detailed information are necessary to allow for a recommendation of safe administration of preventive antidepressant medication in adults with long‐term physical conditions. As of right now, it appears that the study medication caused more drop‐outs due to adverse events than the placebo medication, and hence, should implicitly be considered when interpreting these results.
Regarding the remaining secondary outcomes, no conclusions can be drawn. Using different instruments, only two of the included studies (Novack 2009; Robinson 2008) addressed severity of depression for adults after stroke or with traumatic brain injury. Cost‐effectiveness and cost‐utility were not assessed at all. The latter is a common shortcoming in these kind of studies.
Studies were excluded mostly due to an inconsistent understanding of prevention. A lot of studies included complete samples or subsamples of adults who were already depressed. In these cases, major study aims mainly were the decline in disease progression, or the treatment of subthreshold depression (i.e. secondary prevention) (see also Characteristics of excluded studies). Future research might benefit from a more specific definition of the kind of prevention in accordance with e.g. classifications made by the WHO (e.g. WHO 2016).
Surprisingly, one trial could not be included as it did not report any incidence of depression, even though it aspired to examine the preventive effects of fluoxetine in adults after stroke (Kong 2007).
In summary, generalisation of results and corresponding conclusions cannot be drawn. Regarding the type of long‐term physical condition, adults after stroke pose the only subgroup that might allow for any conclusions on the preventive effects of pharmacological interventions for the incidence of depression. However, types of treatments varied greatly, with two trials applying sertraline, one trial fluoxetine/nortriptyline, one trial escitalopram, and one trial milnacipran. Among these, three trials assessed incidence by diagnosis, one trial by cut‐off, and one trial by either diagnosis or cut‐off. Heterogeneity regarding sample size was great as well, and hence, no generalisable conclusions can be drawn for adults after stroke.
Quality of the evidence
Using the risk of bias assessments for each study as well as GRADEpro GDT to rate the certainty of the evidence, we find there are serious shortcomings regarding the certainty of the evidence. Overall, the certainty of the evidence was rated very low to low. We were unable to identify any studies free of risk of bias. One of the major concerns of this review was the lack of complete outcome data, and inadequate reporting of adverse events and reasons for drop‐out. We assume that adverse events might arise due to the treatment or the development of depressive symptoms, and therefore, might pose the major reasons for discontinuation of study participation. Incomplete or inadequate reporting of this very central outcome caused us to assume a strong risk of bias. Also, all studies were rated at high or unclear risk of bias regarding selective reporting. For only two of the included trials (Hansen 2012; Hansen 2014) could we identify a corresponding protocol. The trial conducted by Rovner 2007 on the preventive effect of a psychological intervention was at the least risk of bias. Overall, inconsistency was rated mostly 'not serious' with minor exceptions regarding the medium‐term effect of pharmacological interventions. Due to the nature of this review (several types of long‐term physical conditions as well as different types of pharmacological treatments and applied dosages), we had to downgrade the certainty of the evidence once. Regarding the primary outcomes, imprecision was not serious for the psychological prevention trial but very serious for pharmacological interventions (downgraded twice). The latter showed small sample sizes, only few events, and/or wide confidence intervals.
Potential biases in the review process
By predefining the methodological procedures and the selection of outcomes and analyses in the protocol, by involving the Cochrane Mental Disorders Group when defining both the definitions of 'primary prevention' and 'selective prevention' as well as the list of diseases considered to be 'long‐term physical conditions', and by assigning two independent review authors to the task of study selection, we do not consider the review process to be likely to be biased. Still, it is limited be the small number of identified relevant studies. In addition, we might not have identified all grey literature and unpublished studies, and therefore, missing grey literature and unpublished RCTs might have led to an overestimation of prevention effects (McAuley 2000).
Agreements and disagreements with other studies or reviews
A variety of Cochrane Reviews have been undertaken to address depression in long‐term physical conditions. There is evidence for beneficial effects of antidepressant interventions for depressed adults with cancer (Ostuzzi 2018), coronary artery disease (Baumeister 2011b), coronary heart disease (Richards 2017), diabetes mellitus (Baumeister 2012c), and stroke (Hackett 2008b). These data imply the potential of treatment approaches in several long‐term physical conditions, and might guide decision making when choosing the types of interventions that could also serve as preventive interventions for certain types of long‐term physical conditions. For example, there is also evidence for beneficial effects of pharmacological preventive interventions (including secondary prevention approaches) on depression in adults after stroke (Hackett 2008a). This corresponds with our results but it must be noted that some of the included studies are the same as in our review. The potential of preventive psychological interventions for patients after stroke is yet unknown. As for primary prevention, we could not identify any studies including psychological interventions. However, Hackett 2008a found no beneficial effects for psychological interventions in the general prevention of depression in adults after stroke, and therefore, it might be less likely that psychological interventions might be useful as a primary prevention in adults after stroke. For other long‐term physical conditions such as diabetes (Baumeister 2012c), or coronary heart and artery diseases (Baumeister 2011b; Richards 2017), psychological interventions were effective in treating depression and might be considered as primary preventive interventions in these diseases as well. At present, we could identify no RCTs addressing primary preventive psychological interventions in these diseases.
Authors' conclusions
Implications for practice.
With only 11 trials of low‐ to very‐low certainty of evidence included in this review, there is only uncertain evidence regarding the implementation of primary prevention treatments. The available evidence included six different types of long‐term physical conditions, but also different types of treatments (problem‐solving therapy as well as seven types of antidepressant medications). Adults after stroke were the group of people addressed most often (five trials) but results remain heterogeneous, as participants received five different types of antidepressant medication of different dosages. Heterogeneity across trials was substantial and limited the interpretation and certainty of the results. In addition, knowledge on the acceptability and tolerability of the applied treatments is sparse, but this must be given detailed consideration when dealing with these kinds of treatments.
Implications for research.
There is a great need for randomised controlled trials focusing on the short‐, medium‐, and especially long‐term follow‐up of participants, where reasons for drop‐outs, especially if they are treatment‐related, are put into consideration. This includes the importance of examining discontinuation effects in psychopharmacological trials; future prevention trials should collect data on this topic. Also, sample sizes are often small, and need to be larger. More regard should be given to the planning and publication of study protocols. Final publications of study results should report on all study outcomes, especially if they are of interest to the research question. We noted that some studies used generic instruments and cut‐offs to measure incidence and severity of depression, but hardly any information was offered on the severity of depression. Here, researchers should be more thorough when reporting outcomes. Overall, the quality of study conduct and reporting needs to improve.
With regard to our outcomes of interest, results were very heterogeneous. In part, this is due to the nature of this review, focusing on so many aspects at once. Still, we would like to recommend future research to focus on corresponding proceedings with prior research instead of attempting new approaches, using different procedures, most of the time. Heterogeneity could be minimised when considering the type of long‐term physical conditions, the type of treatment, the classes of antidepressant treatment, the applied dosages, the assessment of incidence of depression (e.g. diagnosis or cut‐off), the time‐frames of treatment and follow‐up, the used instruments or versions of instruments (e.g. long versions versus short‐versions), or the applied cut‐offs of the same instrument, for the conduct of future trials. Greater comparability of studies, more care regarding their quality, and larger trials; and specifically in the case of prevention trials, a clear and predefined definition of the type of prevention (primary, secondary or tertiary) that is intended for investigation; can help to improve conclusions drawn from trials and to make well‐founded recommendations for clinical practice.
Moreover, future research might consider other proximal outcomes for depression, like risk factors, instead of focusing on depression as the only primary outcome of an RCT. Addressing proxy measures of depression might also prove to be beneficial regarding other methodological problems like high drop‐out rates and the need for long follow‐up periods. In addition, future research should be much more considerate of the benefit to harms ratio of long‐term antidepressant treatment, not only for pharmacological but also for psychological interventions (Herzog 2019; Klatte 2018).
What's new
| Date | Event | Description |
|---|---|---|
| 6 March 2021 | Amended | Correction made to formatting of Figure 1 following a system error. |
History
Protocol first published: Issue 8, 2014 Review first published: Issue 3, 2021
Acknowledgements
We are grateful for the advice and support received from the editorial team of the Cochrane Common Mental Disorders (CCMD) Group, and the German Cochrane Centre Freiburg for providing a very helpful workshop for review authors. We'd also like to thank Sarah Dawson, the Information Specialist assigned to us by the Cochrane Common Mental Disorders Group. Her expertise and endurance improved the search strategy greatly. Also, a big 'thank you' to our research assistant Hannah Frinken for cross‐checking extracted data with a very keen eye.
Our special regards goes to Prof. Dr. Wilfried Jäckel (WHJ) who was one of the co‐authors of the protocol but was unavailable to be part of the review as well. We'd like to offer him our sincere gratitude regarding his countless contributions and insights.
The review authors and the CCMD Editorial Team, are grateful to the peer reviewers for their time and comments including: Peter Coventry, Nuala Livingstone, Parashar Ramanuj, Lindsay Robertson and Ellen Thomas (Lay Reviewer). They would also like to thank copy editor, Hacsi Horvath.
Cochrane Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer: The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health and Social Care.
Appendices
Appendix 1. List of chronic diseases according to ICD‐10 code
| ICD‐10 Code | Long‐term physical condition |
| A15‐A19 | Tuberculosis |
| A81 | Poliomyelitis |
| B15‐B19 | Viral hepatitis |
| B20‐B24 | Human immunodeficiency virus [HIV] disease |
| C00‐D48 | Malignant Neoplasms |
| E03, E05 | Hypothyroidism |
| E10‐E14 | Diabetes mellitus |
| E24 | Cushing syndrome |
| E66 | Obesity |
| F01‐F03 | Dementia |
| G20 | Parkinson disease |
| G23 | Other degenerative diseases of basal ganglia |
| G24 | Dystonia |
| G25 | Extrapyramidal and movement disorders |
| G30; F00 | Alzheimer disease |
| G31.0 | Circumscribed brain atrophy |
| G31.0, F02.0 | Frontotemporal dementia |
| G31.0, F02.0 | Pick disease |
| G35 | Multiple sclerosis |
| G40 | Epilepsy |
| G43 | Migraine |
| G44.2 | Tension‐type headache |
| G47 | Sleep disorders |
| G62, G63 | Polyneuropathy |
| G70 | Myasthenia gravis |
| G81 | Hemiplegia |
| G82 | Paraplegia |
| G82 | Tetraplegia |
| G93.3 | Fatigue syndrome |
| H35.3 | Macula Degeneration |
| H81 | Disorders of vestibular function |
| H81 | Otosclerosis |
| H90‐H91 | Hearing loss |
| I10‐I15 | Hypertensive diseases (Hypertension) |
| I20‐I25 | Ischaemic heart diseases |
| I20 | Angina pectoris |
| I21‐I22 | Myocardial infarction |
| I42 | Cardiomyopathy |
| I50 | Heart failure |
| I60‐I69 | Cerebrovascular diseases |
| I60 | Subarachnoid haemorrhage |
| I61 | Intracerebral haemorrhage |
| I62 | Intracranial haemorrhage |
| I63 | Cerebral infarction |
| I64 | Stroke |
| I65 | Occlusion of precerebral arteries |
| I65 | Stenosis of precerebral arteries |
| I66 | Occlusion of cerebral arteries |
| I66 | Stenosis of cerebral arteries |
| I73.9 | Peripheral vascular disease |
| I95 | Hypotension |
| J40‐J42 | Chronic bronchitis |
| J43 | Emphysema |
| J44 | Chronic obstructive pulmonary disease |
| J45, J46 | Asthma |
| J47 | Bronchiectasis |
| J60‐J70 | Lung diseases due to external agents |
| J60‐J65 | Pneumoconiosis |
| K25 | Gastric ulcer |
| K26 | Duodenal ulcer |
| K29 | Gastritis and duodenitis |
| K50 | Crohn disease |
| K51 | Ulcerative colitis |
| K58 | Irritable bowel syndrome |
| K70‐K77 | Liver diseases |
| K72.1 | Chronic hepatic failure |
| K74 | Fibrosis and cirrhosis of liver |
| K86.1 | Chronic Pancreatitis |
| L40‐L41 | Papulosquamous disorders |
| L41 | Parapsoriasis |
| L93 | Lupus erythematosus |
| M00‐M99 | Musculoskeletal disorders |
| M00‐M25 | Arthropathies |
| M05, M06 | Rheumatoid arthritis |
| M05‐M14 | Polyarthropathies |
| M08, M09 | Arthritis |
| M10 | Gout |
| M15 | Polyarthrosis |
| M15‐M19 | Arthrosis; Osteoarthrosis; Osteoarthritis |
| M16 | Coxarthrosis |
| M30‐M36 | Systemic disorders of connective tissue |
| M30 | Polyarteritis nodosa |
| M31 | Necrotizing vasculopathies |
| M32 | Systemic lupus erythematosus |
| M33 | Dermatopolymyositis |
| M34 | Systemic sclerosis |
| M40 | Kyphosis and lordosis |
| M40‐M43; M50‐M54 | Dorsopathies |
| M41 | Scoliosis |
| M42 | Spinal osteochondrosis |
| M45‐M49 | Spondylopathies |
| M54.5 | Low back pain |
| M79.7 | Fibromyalgia |
| M80‐M85 | Disorders of bone density and structure |
| M80‐M82 | Osteoporosis |
| N00‐N08 | Glomerular diseases |
| N02 | Haematuria |
| N03 | Chronic nephritic syndrome |
| N04 | Nephrotic syndrome |
| N18 | Chronic kidney disease |
| N30.2 | Chronic cystitis |
| N41.1 | Chronic prostatitis |
| N80 | Endometriosis |
Appendix 2. Cochrane Specialized Register
Cochrane Common Mental Disorders Controlled Trials Register (CCMDCTR)
The Cochrane Common Mental Disorders Group (CCMD) maintains an archived controlled trials register known as the CCMDCTR. This specialized register contains over 40,000 reference records (reports of RCTs) for anxiety disorders, depression, bipolar disorder, eating disorders, self‐harm, and other mental disorders within the scope of this Group. The CCMDCTR is a partially studies‐based register, with more than 50% of reference records tagged to around 12,500 individually PICO‐coded study records. Reports of studies in the register were collated from (weekly) generic searches of key bibliographic databases to June 2016, which included: MEDLINE (1950 onwards), Embase (1974 onwards), PsycINFO (1967 onwards), quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), and review‐specific searches of additional databases. Reports of studies were also sourced from international trials registries, drug companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) are on the Group's website, with an example of the core MEDLINE search displayed below.
[MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/ OR [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).tw,kf. AND [RCT filter]: (controlled clinical trial.pt. or randomised controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomised controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
Records were screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs were tagged to the appropriate study record.
The rationale of maintaining a comprehensive specialised register was reviewed when the editorial group moved from the University of Bristol to the University of York in June 2016. At this time, the Group decided to archive the CCMDCTR and return to searching the medical and psychological literature directly, on a review‐by‐review basis.
*****************************************************************************************************************************
For this review the CCMDCTR was searched (all years to June 2016) using the following terms:
CCMDCTR‐Studies Register: Age Group = (adult or aged or unclear or "not stated") and Condition or Comorbidity = (depress*) and Comorbidity field = (not empty) [depress*:SCO and (adult or aged or unclear or "not stated"):XAGE and not empty:SCM] Studies including participants with physical comorbidities were manually selected.
CCMDCTR‐References Register: The references register was searched using a precision maximizing strategy (all years to 17 June 2016) to identify additional untagged/uncoded references.
#1. ((prevent* or prophyl* or reduc* or relaps* or risk) NEAR depress*):ti,ab,kw,ky,emt,mh,mc #2. (depress* or mental or mood or well being or "well being" or "quality of life"):ti #3. ((“chronic disease” or (chronic* NEAR2 (ill* or condition*1 or disease* or disorder* or health))) not "chronic depression"):ti #4. (“physical* ill*” or “medical* ill*” or (long term NEXT (condition*1 or sick*)) or “medical* morbid*” or (medical* NEXT (comorbid* or co morbid*)) or multimorbid* or (multi* NEXT (morbid* or “co morbid*” or comorbid* or physical))):ti #5.(Alzheimer* or angina or aneurysm or “ankylosing spondylitis” or arthropath* or arthriti* or arthrosis or arthroses or asthma* or “atrial fibrillation” or “autoimmune disease*” or “back pain” or blindness or “brain atroph*” or (bone NEXT (disease* or disorder*)) or ((bronchi* or bowel) NEXT (disease* or disorder*)) or bypass or (cancer or neoplasm* or neoplastic or malignan*) or (cardiac NEXT (arrest or arrhythmia* or surg*)) or cardiomyopath* or ((cardiovascular or coronary) NEAR2 (disease* or disorder* or event*)) or (cerebrovascular NEAR2 (disease* or disorder* or event*)) or “chronic obstructive” or COPD or (chronic NEAR2 pain) or cirrhosis or colitis or “congenital abnormalit*” or coxarthrosis or Crohn* or Cushing* or cystitis):ti #6. (deformit* or disabled or (physical NEXT (deform* or disab* or impair*)) or *degenerative or dement* or dermato* or dorsopath* or diabet* or “digestive system*” or duoden* or dystonia or eczema or (endocrine NEXT (disease* or disorder*)) or emphysema or endometriosis or epilep* or extrapyramidal or “eye disease*” or (“fatigue syndrome” or “chronic fatigue”) or fibromyalgia or fibrosis or (gastr* NEXT (disease* or disorder*)) or gastritis or gout or (glomerul* NEXT (disease* or disorder*)) or headache* or ((h?emic or lymph*) NEXT (disease* or disorder*)) or h?ematuria or h?emophili* or h?emorrhage or ((hearing or visual or vision) NEAR2 (aid* or impair* or loss)) or hemiplegi* or hepatitis or h?emodialysis or ((renal or kidney) NEXT (disease* or disorder* or failure)) or (heart NEXT (attack* or disease* or disorder* or failure or surg*)) or HIV or “human immunodeficiency virus” or hypertensi* or hypotensi*):ti #7. (“inflammatory disease*” or incontinen* or “irritable bowel” or isch?emi* or (joint NEXT (disease* or disorder*)) or kyphosis or leuk?emia or ((liver or hepatic) NEXT (disease* or disorder* or failure)) or lordosis or “lung disease*” or “lupus erythemat*” or lymphoma or “macular degeneration” or migraine* or “movement disorder*” or musculoskeletal or necrotizing or nephrotic* or neuromuscular or “multiple sclerosis” or “myasthenia gravis” or myeloma or “myocardial infarct*”):ti #8. (((nutritional or metabolic) NEXT (disease* or disorder or syndrome*)) or (organ* NEAR2 (transplant* or recipient*)) or (neurological NEXT (disease* or disorder*)) or “occupational disease*” or occlusion* or obesity or orthop?edic* or osteo* or otorhinolaryngology* or otosclerosis or pancrea* or papulosquamous or paraplegi* or Parkinson's* or “peripheral vascular” or “pick disease*” or pneumoconiosis or polio* or polyarthropath* or polyarteritis or polyarthrosis or polyneuropath* or prostat* or psoriasis or parapsoriasis or (pulmonary NEAR2 (disease* or disorder*))):ti #9. ((respiratory NEXT (disease* or disorder*)) or rheumat* or sclerosis or scoliosis or ((skin or “connective tissue”) NEXT (disease* or disorder*)) or (“sleep disorder*” or “sleep apn?ea” or insomnia* or dyssomnia* or hypersomnia*) or spondylo* or stenosis* or stoma* or (stroke or strokes or poststroke or “cerebral infarct*”) or tetraplegi* or ((thyroid NEAR (disease* or disorder* or dysfunction*)) or hyperthyroidism or hypothyroidism) or tuberculosis or (systemic NEAR (disorder* or disease*)) or ulcer* or (urogenital NEXT (disease* or disorder*)) or vasculopath* or (vascular NEAR (disease* or disorder*)) or vestibular or ((virus or viral) NEXT disease)):ti #10. syndrome*:ti #11.((depress* or mood) and ((*agia or *algia or *dynia or *emia or *enia or *itis or *oma or *omata or *omnia or *opath* or *osis or *oses or *penia or *phagy or *philia or *plasia or *plasty or *plegi* or *rrhag* or *trophy) not (diagnos* or doses or hypnos* or neuros* or psychos* or psychopath* or prognos* or schizophreni*))):ti #12. (((#1 or #2) and (#3 or #4 or #5 or #6 or #7 or #8 or #9 or #10)) or #11) #13. ((depress* or mood) and prevent* and (intervention or program* or strategy or study or *therap* or treat* or train* or trial)):ti #14. (Lines #4 to #9):all fields #15. ((#13 and #14) or #12) [Note:The strategy reported in the protocol was too sensitive, retrieving a lot of noise around treatment studies and chronic depression.]
Appendix 3. Biomedical database search strategies
1. COCHRANE LIBRARY (Reviews and Trials (CENTRAL)) The Cochrane Library was searched (all years to Issue 11, November 2014 and again in November 2017 and again in February 2020) using the following terms:
[Depression prevention] #1. ((prevent* or prophyl* or reduc* or relaps* or risk) near depress*):ti,ab #2. MeSH descriptor: [DEPRESSION] this term only and with qualifier(s): [Prevention & control ‐ PC] #3. MeSH descriptor: [DEPRESSIVE DISORDER] this term only and with qualifier(s): [Prevention & control ‐ PC] #4. MeSH descriptor: [DEPRESSIVE DISORDER, MAJOR] this term only and with qualifier(s): [Prevention & control ‐ PC] #5. ((depress* or dysthymi* or affective disorder* or affective symptom* or melanchol* or mood) near/3 (first episode* or onset or prevent* or relaps* or recurr* or risk or at‐risk or symptom*)):ti,ab #6. (depress* near/3 (subclinical* or sub‐clinical* or subthreshold* or sub‐threshold* or subsyndrom* or sub‐syndrom*)):ti,ab #7. ((mild* or minor or nonmajor or non major) next depress*):ti,ab #8. "low mood*" #9. (depress* or mood):ti and (prevent* near/2 (primary or tertiary or universal or selective or indicated)):ti,ab #10. (depressi* or depressed):ti #11. (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9) [Chronic physical illness] #12. MeSH descriptor: [CHRONIC DISEASE] this term only #13. (("chronic disease" or (chronic* near/3 (ill* or condition*1 or disease* or disorder* or health))) not "chronic depression") #14. ("physical* ill*" or "medical* ill*") #15. ("long term" next (condition* or sick*)) #16. ("medical* morbid*" or (medical* next (comorbid* or "co morbid*"))) #17. (multimorbid* or (multi* next (morbid* or "co morbid*" or comorbid* or physical))) #18. (Alzheimer* or angina or aneurysm or "ankylosing spondylitis" or arthropath* or arthriti* or arthrosis or arthroses or asthma* or "atrial fibrillation" or "autoimmune disease*" or "back pain" or blindness or "brain atroph*" or (bone next (disease* or disorder*)) or bronchi* or (bowel next (disease* or disorder*)) or bypass or (cancer or neoplasm* or neoplastic or malignan*) or (cardiac next (arrest or arrhythmia* or surg*)) or cardiomyopath* or ((cardiovascular or coronary) near/3 (disease* or disorder* or event*)) or (cerebrovascular near/3 (disease* or disorder* or event*)) or "chronic obstructive" or COPD or (chronic near/3 pain) or cirrhosis or colitis or "congenital abnormalit*" or coxarthrosis or Crohn* or Cushing* or cystitis or disabled or (physical* near/3 (deform* or disab* or impair*)) or (degenerative or neurodegenerative) or dementia or dermato* or dorsopath* or diabet* or "digestive system*" or duoden* or dystonia or eczema or (endocrine next (disease* or disorder*)) or emphysema or endometriosis or epilepsy or extrapyramidal or "eye disease*" or ("fatigue syndrome" or "chronic fatigue") or fibromyalgia or fibrosis or (gastr* next (disease* or disorder*)) or gastritis or gout or (glomerul* next (disease* or disorder*)) or headache* or ((hemic or haemic or lymph*) next (disease* or disorder*)) or hematuria or haematuria or hemophili* haemophili* or hemorrhag* or haemorrhag* or ((hearing or visual or vision) near/3 (aid* or impair* or loss)) or hemiplegi* or hepatitis or hemodialysis or haemodialysis or ((renal or kidney) next (disease* or disorder* or failure)) or (heart next (attack* or disease* or disorder* or failure or surg*)) or HIV or "human immunodeficiency virus" or hypertensi* or hypotensi* or "inflammatory disease*" or incontinen* or "irritable bowel" or ischemi* or ischaemi* or (joint next (disease* or disorder*)) or kyphosis or leukemia or leukaemia or ((liver or hepatic) next (disease* or disorder* or failure)) or lordosis or "lung disease*" or "Lupus erythemat*" or lymphoma or "macular degeneration" or migraine* or "movement disorder*" or musculoskeletal or necrotizing or nephrotic* or neuromuscular or ((nutritional or metabolic) next (disease* or disorder or syndrome*)) or "multiple sclerosis" or "Myasthenia gravis" or myeloma or "myocardial infarction" or (organ* near/3 (transplant* or recipient*)) or (neurological next (disease* or disorder*)) or "occupational disease*" or occlusion* or obesity or orthopedic* or orthopaedic* or osteo* or otorhinolaryngology or otosclerosis or pancrea* or papulosquamous or paraplegi* or Parkinson* or "peripheral vascular" or "Pick disease*" or pneumoconiosis or polio* or polyarthropath* or polyarteritis or polyarthrosis or polyneuropath* or prostat* or psoriasis or parapsoriasis or (pulmonary near/3 (disease* or disorder*)) or (respiratory next (disease* or disorder*)) or rheumat* or sclerosis or scoliosis or (sleep disorder* or "sleep apn*ea" or "sleep apnaea" insomnia* or dyssomnia* or hypersomnia*) or spondylo* or stenosis* or (stroke or strokes or poststroke or cerebral infarct*) or tetraplegi* or ((thyroid next (disease* or disorder* or dysfunction*)) or hyperthyroidism or hypothyroidism) or tuberculosis or (systemic next (disorder* or disease*)) or ulcer* or (urogenital next (disease* or disorder*)) or vasculopath* or (vascular next (disease* or disorder*)) or vestibular or ((virus or viral) next disease)) #19. MeSH descriptor: [NEOPLASMS] explode all trees #20. MeSH descriptor: [MUSCULOSKELETAL DISEASES] explode all trees #21. MeSH descriptor: [DIGESTIVE SYSTEM DISEASES] explode all trees #22. MeSH descriptor: [RESPIRATORY TRACT DISEASES] explode all trees #23. MeSH descriptor: [STOMATOGNATHIC DISEASES] explode all trees #24. MeSH descriptor: [OTORHINOLARYNGOLOGIC DISEASES] explode all trees #25. MeSH descriptor: [NERVOUS SYSTEM DISEASES] explode all trees #26. MeSH descriptor: [EYE DISEASES] explode all trees #27. MeSH descriptor: [MALE UROGENITAL DISEASES] explode all trees #28. MeSH descriptor: [FEMALE UROGENITAL DISEASES] explode all trees #29. MeSH descriptor: [CARDIOVASCULAR DISEASES] explode all trees #30. MeSH descriptor: [HEMIC AND LYMPHATIC DISEASES] explode all trees #31. MeSH descriptor: [SKIN AND CONNECTIVE TISSUE DISEASES] explode all trees #32. MeSH descriptor: [NUTRITIONAL AND METABOLIC DISEASES] explode all trees #33. MeSH descriptor: [ENDOCRINE SYSTEM DISEASES] explode all trees #34. MeSH descriptor: [IMMUNE SYSTEM DISEASES] explode all trees #35. MeSH descriptor: [DISORDERS OF ENVIRONMENTAL ORIGIN] explode all trees #36. MeSH descriptor: [PATHOLOGICAL CONDITIONS, SIGNS AND SYMPTOMS] explode all trees #37. MeSH descriptor: [OCCUPATIONAL DISEASES] explode all trees #38. MeSH descriptor: [VIRUS DISEASES] explode all trees #39. syndrome*:ti,ab,kw #40. MeSH descriptor: [QUALITY OF LIFE] this term only and with qualifier(s): [Psychology ‐ PX] #41. MeSH descriptor: [REHABILITATION] this term only and with qualifier(s): [Psychology ‐ PX] #42. MeSH descriptor: [ADAPTATION, PSYCHOLOGICAL] explode all trees #43. (#14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39) [Combing search sets ‐ depression prevention + chronic physical illness] #44. #11 and #43 #45. (#1 or #2 or #3 or #4) and (#12 or #13) #46. #10 and (#40 or #41 or #42) #47. (#44 or #45 or #46) [INTERVENTIONS: Psychological interventions] #48. MeSH descriptor: [PSYCHOTHERAPY] explode all trees #49. ((psychologic* or behavior or behaviour or cognitive) near/3 (intervent* or therap* or treat* or manag*)) #50. (abreaction or "acting out" or "acceptance and commitment" or "activity scheduling" or adlerian or "age regression" or "analytical therap*" or "anger control" or "anger management" or "art therap*" or "assertive* training" or "attention bias modification" or "autogenic training" or autosuggestion or "aversion therap*" or "balint group" or "behavio* activation" or "behavio* contracting" or "behavio* modification" or "behavio* therap*" or bibliotherap* or biofeedback or "body therap*" or "brief therapy" or "caregiver support" or catharsis or "client cent* therapy" or "cognitive behavio* therap*" or "cognitive therap*" or CBT or cCBT or iCBT or "cognitive behavio* stress management" or "cognitive rehabilitation" or "cognitive restructur*" or "colo* therap*" or "compassion focus*" or "compassionate therap*" or "conjoint therap*" or "contingency management" or "conversion therap*" or "conversational therap*" or countertransference or "coping skill*" or counsel* or "couples therap*" or "covert sensitization" or "crisis intervention" or "dance therap*" or (dialectic* near/2 therap*) or "diffusion therap*" or "distraction therap*" or "drama therapy" or (dream* near/2 analys*) or "eclectic therap*" or "emotion* focus* therap*" or "emotional freedom technique" or "encounter group therap*" or existential or experiential or "exposure therap*" or "expressive therap*" or "eye movement desensiti*" or "family therap*" or "feminist therap*" or "focus oriented" or "free association" or freudian or "functional analysis" or "geriatric therap*" or gestalt or griefwork or "group therap*" or "guided image*" or "holistic therap*" or humanistic or hypnosis or hypnotherapy or hypnoti* or "implosive therap*" or "insight therap*" or "integrative therap*" or "interpersonal therap*" or Jungian or kleinian or logotherap* or "logo therap*" or "marathon group therap*" or "marital therap*" or meditation or "mental healing" or metacognitive or meta‐cognitive or milieu or "mind train*" or mindfulness or morita or "multimodal therap*" or music or "narrative therap*" or "nondirective therap*" or non‐directive therap* or "nondirective therap*" or "non‐specific therap*" or "nonspecific therap*" or "object relations" or "personal construct therap*" or "person cent* therap*" or "persuasion therap*" or "pet therap*" or "play therap*" or ((pleasant or pleasing) near/2 event*) or "present cent* therap*" or "primal therap*" or "problem focus* therap*" or "problem sol*" or "process experiential" or psychoanaly* or psychodrama or psychodynamic or psychoeducat* or psychotherap* or "rational emotive" or "reality therap*" or "reciprocal inhibition" or "relationship therap*" or relaxation or "reminiscence therap*" or rogerian or "role play*" or schema or "self analys*" or "self esteem building" or "sensitivity training" or "sex therap*" or "sleep phase chronotherap*" or "socioenvironment* therap*" or "social skill*" or sociotherap* or "solution focused" or "stress management" or "support group*" or (support near/2 psycho*) or "supportive therap*" or "systematic desensit*" or "systemic * therap*" or "therapeutic communit*" or "therapeutic technique" or "third wave" or "time limited therap*" or "transference therap*" or "transactional analysis" or transtheoretical or "validation therap*") #51. (#48 or #49 or #50) [INTERVENTIONS: Antidepressants] #52. MeSH descriptor: [ANTIDEPRESSIVE AGENTS] explode all trees #53. MeSH descriptor: [NEUROTRANSMITTER UPTAKE INHIBITORS] explode all trees #54. MeSH descriptor: [MONOAMINE OXIDASE INHIBITORS] explode all trees #55. (antidepress* or "anti depress*" or MAOI* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) and (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic* or psychotropic*) #56. (Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Buproprion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 or Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or St John*) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or (Lu AA21004 or Vortioxetine) or Lu AA24530 or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* or Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone) #57. #52 or #53 or #54 or #55 or #56 [Combing search sets ‐ depression prevention + chronic physical illness + intervention] #58. (#47 and (#51 or #57)) #59. ((depress* or mood) and prevent* and (intervention or program* or *therap* or strategy or study or trial)):ti #60. (depress* or mood):ti and (prevent* near/2 (primary or tertiary or universal or selective or indicated)):ti,ab #61. (#59 or #60) and #43 #62. #58 or #61 [Results, 2014: CENTRAL(Trials)=2944; CDSR=148 ; DARE=40 ;HTA=2; NHS‐EED=21]
In 2017, the following exclusion criteria was added to the search: NOT (schizo* or ((severe or outpatient or inpatient) near/2 psychiatric) or suicid* or bipolar or psychosis or ((resistant or refractory) next depress*) or (treatment near/2 (major depress*)) or PTSD or "posttraumatic stress disorder" or "post traumatic stress disorder" or ((postpartum or post‐partum or postnatal or post‐natal) next depress*) or electroconvulsive or rTMS):ti
2. OVID MEDLINE was search (from 1 January 2004 to 6 February 2020) using the following terms:
[Physical illness] 1. (physical* ill* or medical* ill*).tw. 2. CHRONIC DISEASE/ 3. (chronic* adj2 (ill* or condition*1 or disease* or disorder* or health)).tw. 4. (long term adj (condition*1 or sick*)).tw. 5. (medical* morbid* or (medical* adj (comorbid* or co morbid*))).tw. 6. (multimorbid* or (multi* adj (morbid* or co morbid* or comorbid* or physical))).tw. 7. exp NEOPLASMS/ or exp MUSCULOSKELETAL DISEASES/ or exp DIGESTIVE SYSTEM DISEASES/ or exp STOMATOGNATHIC DISEASES/ or exp RESPIRATORY TRACT DISEASES/ or exp OTORHINOLARYNGOLOGIC DISEASES/ or exp NERVOUS SYSTEM DISEASES/ or exp EYE DISEASES/ or exp MALE UROGENITAL DISEASES/ or exp FEMALE UROGENITAL DISEASES/ or exp CARDIOVASCULAR DISEASES/ or exp "HEMIC AND LYMPHATIC DISEASES"/ or exp "SKIN AND CONNECTIVE TISSUE DISEASES"/ or exp "NUTRITIONAL AND METABOLIC DISEASES"/ or exp ENDOCRINE SYSTEM DISEASES/ or exp IMMUNE SYSTEM DISEASES/ or exp "DISORDERS OF ENVIRONMENTAL ORIGIN"/ or exp "PATHOLOGICAL CONDITIONS, SIGNS AND SYMPTOMS"/ or exp OCCUPATIONAL DISEASES/ or exp VIRUS DISEASES/ 8. (Alzheimer* or angina or aneurysm or ankylosing spondylitis or arthropath* or arthriti* or arthrosis or arthroses or asthma* or atrial fibrillation or autoimmune disease* or back pain or blindness or brain atroph* or (bone adj (disease* or disorder*)) or bronchi* or (bowel adj (disease* or disorder*)) or bypass or (cancer or neoplasm* or neoplastic or malignan*) or (cardiac adj (arrest or arrhythmia* or surg*)) or cardiomyopath* or ((cardiovascular or coronary) adj2 (disease* or disorder* or event*)) or (cerebrovascular adj2 (disease* or disorder* or event*)) or chronic obstructive or COPD or (chronic adj2 pain) or cirrhosis or colitis or congenital abnormalit* or coxarthrosis or Crohn*1 or Cushing*1 or cystitis or disabled or (physical* adj2 (deform* or disab* or impair*)) or (degenerative or neurodegenerative) or dementia or dermato* or dorsopath* or diabet* or digestive system* or duoden* or dystonia or eczema or (endocrine adj (disease* or disorder*)) or emphysema or endometriosis or epilepsy or extrapyramidal or eye disease* or (fatigue syndrome or chronic fatigue) or fibromyalgia or fibrosis or (gastr* adj (disease* or disorder*)) or gastritis or gout or (glomerul* adj (disease* or disorder*)) or headache* or ((h?emic or lymph*) adj (disease* or disorder*)) or h?ematuria or h?emophili* or h?emorrhage or ((hearing or visual or vision) adj2 (aid*1 or impairment* or loss)) or hemiplegi* or hepatitis or h?emodialysis or ((renal or kidney) adj (disease* or disorder* or failure)) or (heart adj (attack* or disease* or disorder* or failure or surg*)) or HIV or human immunodeficiency virus or hypertensi* or hypotensi* or inflammatory disease* or incontinen* or irritable bowel or isch?emi* or (joint adj (disease* or disorder*)) or kyphosis or leuk?emia or ((liver or hepatic) adj (disease* or disorder* or failure)) or lordosis or lung disease* or Lupus erythemat* or lymphoma or macular degeneration or migraine* or movement disorder* or musculoskeletal or necrotizing or nephrotic* or neuromuscular or ((nutritional or metabolic) adj (disease* or disorder or syndrome*)) or multiple sclerosis or Myasthenia gravis or myeloma or myocardial infarction or (organ*1 adj2 (transplant* or recipient*)) or (neurological adj (disease* or disorder*)) or occupational disease* or occlusion* or obesity or orthop?edic* or osteo* or otorhinolaryngology or otosclerosis or pancrea* or papulosquamous or paraplegi* or Parkinson* or peripheral vascular or Pick disease* or pneumoconiosis or polio* or polyarthropath* or polyarteritis or polyarthrosis or polyneuropath* or prostat* or psoriasis or parapsoriasis or (pulmonary adj2 (disease* or disorder*)) or (respiratory adj (disease* or disorder*)) or rheumat* or sclerosis or scoliosis or (sleep disorder* or sleep apn?ea or insomnia* or dyssomnia* or hypersomnia*) or spondylo* or stenosis* or (stroke or strokes or poststroke or cerebral infarct*) or tetraplegi* or ((thyroid adj (disease* or disorder* or dysfunction*)) or hyperthyroidism or hypothyroidism) or tuberculosis or (systemic adj (disorder* or disease*)) or ulcer* or (urogenital adj (disease* or disorder*)) or vasculopath* or (vascular adj (disease* or disorder*)) or vestibular or ((virus or viral) adj disease)).mp. 9. syndrome*1.mp. 10. or/1‐9 [Depression prevention] 11. DEPRESSION/pc [prevention & control] 12. DEPRESSIVE DISORDER/pc [prevention & control] 13. DEPRESSIVE DISORDER, MAJOR/pc [prevention & control] 14. *MENTAL HEALTH/ 15. ((depress* or dysthymi* or affective disorder* or affective symptom* or melanchol* or mood) adj2 (first episode* or onset or prevent* or relaps* or recurr* or risk or at‐risk or symptom*)).tw. 16. (depress* adj2 (subclinical* or sub‐clinical* or subthreshold* or sub‐threshold* or subsyndrom* or sub‐syndrom*)).tw. 17. ((mild* or minor or nonmajor or non major) adj depress*).tw. 18. low mood.tw. 19. (depressi* or depressed).ti. and (*"QUALITY of LIFE"/ or exp *REHABILITATION/ or ADAPTATION, PSYCHOLOGICAL/) 20. (depress* or mood).ti. and (prevent* adj1 (primary or tertiary or universal or selective or indicated)).ti,ab. 21. or/11‐20 [RTC Filter ‐ precision maximizing] 22. randomized controlled trial.pt. 23. (randomi#ed or RCT).ti,ab. 24. (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. 25. placebo*.ab. 26. ((control* or group*1) and (waitlist* or wait* list* or treatment as usual or TAU)).ab. 27. trial.ti. 28. (animals not (humans and animals)).sh. 29. or/21‐26 30. 28 not 27 31. (10 and 21 and 30) 32. (2005* or 2006* or 2007* or 2008* or 2009* or 2010* or 2011* or 2012* or 2013* or 2014*).ed,yr. 33. (31 and 32) In 2017, the MEDLINE search results were limited to records containing the following terms: ((risk or predict* or prevent* or prophyla*).ti,ab,kf,hw. or (prevention & control).fs.) and (2014* or 2015* or 2016* or 2017*).yr,ed,dc,ep.
In 2020, the MEDLINE search results were based on the 2017 restrictions and limited to records containing the following terms: (2017/11 or 2018* or 2019* or 2020*).yr,ed,dc,ep.
3. OVID EMBASE was searched (i) 2010 to 2014 and (ii) 2014 to 9 November 2017 and (iii) November 2017 to 6 February 2020, using the following terms:
[Physical illness] 1. (physical* ill* or medical* ill*).tw. 2. (chronic* adj2 (ill* or condition*1 or disease* or disorder* or health)).tw. 3. (long term adj (condition*1 or sick*)).tw. 4. (medical* morbid* or (medical* adj (comorbid* or co morbid*))).tw. 5. (multimorbid* or (multi* adj (morbid* or co morbid* or comordid* or physical))).tw. 6. CHRONIC DISEASE/ 7. PHYSICAL DISEASE/ or exp PHYSICAL DISEASE BY ANATOMICAL STRUCTURE/ or exp PHYSICAL DISEASE BY BODY FUNCTION/ or exp "PHYSICAL DISEASE BY COMPOSITION OF BODY FLUIDS, EXCRETA AND SECRETIONS"/ or exp PHYSICAL DISEASE BY DEVELOPMENTAL AGE/ or exp "PHYSICAL DISEASE BY ETIOLOGY AND PATHOGENESIS"/ 8. ibid line 8 of MEDLINE search 9. syndrome*1.mp. 10. or/1‐9 [Depression prevention] 11. MOOD DIORDER/pc [prevention & control] 12. *DEPRESSION/pc or AGITATED DEPRESSION/pc or DYSTHYMIA/pc or INVOLUTIONAL DEPRESSION/pc or LATE LIFE DEPRESSION/pc or MAJOR DEPRESSION/pc or MASKED DEPRESSION/pc or "MIXED ANXIETY and DEPRESSION"/pc or "MIXED DEPRESSION AND DEMENTIA"/pc or ORGANIC DEPRESSION/pc or POSTOPERATIVE DEPRESSION/pc or REACTIVE DEPRESSION/pc 13. *MENTAL HEALTH/ 14. ((depress* or dysthymi* or affective disorder* or affective symptom* or melanchol* or mood) adj2 (first episode* or onset or prevent* or relaps* or recurr* or risk or at‐risk or symptom*)).tw. 15. (depress* adj2 (subclinical* or sub‐clinical* or subthreshold* or sub‐threshold* or subsyndrom* or sub‐syndrom*)).tw. 16. ((mild* or minor or nonmajor or non major) adj depress*).tw. 17. low mood.tw. 18. (depressi* or depressed).ti. and (*QUALITY of LIFE/ or exp *REHABILITATION/) 19. (depress* or mood).ti. and (prevent* adj1 (primary or tertiary or universal or selective or indicated)).ti,ab. 20. or/11‐19 [RTC Filter ‐ precision maximizing] 21. randomized controlled trial.de. 22. (randomi#ed or RCT).ti,ab. 23. (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. 24. placebo.de. 25. placebo*.ti,ab. 26. ((control* and group*1) and (waitlist* or wait* list* or treatment as usual or TAU)).ab. 27. controlled trial.ti. 28. ((animal or nonhuman) not (human and (animal or nonhuman))).de. 29. or/21‐27 30. 29 not 28 31. (10 and 20 and 30) In 2017, the EMBASE search results were limited to records containing the following terms: ((risk or predict* or prevent* or prophyla*).ti,ab,kw,hw. or (prevention).fs.) and (2014* or 2015* or 2016* or 2017*).yr,em. The results were also limited to non‐MEDLINE journals.
In 2020, the EMBASE search results were based on the 2017 restrictions and limited to records containing the following terms: (2017/11 or 2018* or 2019* or 2020*).yr,em.
4. OVID PsycINFO was search (all years to 9 February 2020) using the following terms:
[Physical illness] 1. (physical* ill* or medical* ill*).ti,ab,id. 2. (chronic* adj2 (ill* or condition*1 or disease* or disorder* or health)).ti,ab,id. 3. (long term adj (condition*1 or sick*)).ti,ab,id. 4. (medical* morbid* or (medical* adj (comorbid* or co morbid*))).ti,ab,id. 5. (multimorbid* or (multi* adj (morbid* or co morbid* or comorbid* or physical))).tw. 6. CHRONIC ILLNESS/ or "CHRONICITY (Disorders)"/ or CHRONIC PAIN/ or CHRONIC FATIGUE SYNDROME/ 7. exp PHYSICAL DISORDERS/ 8. ibid line 8 of MEDLINE search (.ti,ab,id,sh. PsycINFO fields only) 9. syndrome*1.ti,ab,id,sh. 10. or/1‐9 [Depression prevention] 11. (ATYPICAL DEPRESSION/ OR "DEPRESSION (EMOTION)"/ or MAJOR DEPRESSION/ or DYSTHYMIC DISORDER/ or ENDOGENOUS DEPRESSION/ or REACTIVE DEPRESSION/) and prevention.ti,ab,id,sh. 12. MENTAL HEALTH/ 13. ((depress* or dysthymi* or affective disorder* or affective symptom* or melanchol* or mood) adj2 (first episode* or onset or prevent* or relaps* or recurr* or risk or at‐risk or symptom*)).ti,ab,id. 14. (depress* adj2 (subclinical* or sub‐clinical* or subthreshold* or sub‐threshold* or subsyndrom* or sub‐syndrom*)).ti,ab,id. 15. ((mild* or minor or nonmajor or non major) adj depress*).ti,ab,id. 16. low mood.ti,ab,id. 17. (depress* or mood).ti,id. and (REHABILITATION/ or OCCUPATIONAL THERAPY/ or "QUALITY OF LIFE"/ or LIFE CHANGES/ or LIFESTYLE CHANGES/) 18. (depress* or mood).ti,id. and (prevent* adj1 (primary or tertiary or universal or selective or indicated)).ti,ab,id. 19. or/11‐18 [RTC Filter ‐ precision maximizing] 20. treatment effectiveness evaluation.sh. 21. "2000".md. 22. randomi#ed.ti,ab,id. 23. (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. 24. placebo*.ab,sh. 25. ((control* or group*1) and (waitlist* or wait* list* or treatment as usual or TAU)).ab. 26. trial.ti. 27. or/19‐25 28. (10 and 19 and 27)
Data and analyses
Comparison 1. Psychological intervention versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incidence of depression (diagnosis) ‐ post‐intervention | 1 | 194 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.20, 0.95] |
| 1.2 Incidence of depression (diagnosis) ‐ short‐term | 1 | 190 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.36, 1.38] |
| 1.3 Dropouts due to any cause ‐ post‐intervention | 1 | 206 | Odds Ratio (M‐H, Random, 95% CI) | 5.21 [1.11, 24.40] |
| 1.4 Dropouts due to any cause ‐ short‐term | 1 | 206 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.58, 4.77] |
Comparison 2. Pharmacological intervention versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Incidence of depression (all) ‐ post‐intervention | 9 | 814 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.20, 0.49] |
| 2.2 Incidence of depression (all) ‐ medium‐term | 3 | 233 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.23, 2.82] |
| 2.3 Incidence of depression (diagnosis) ‐ post‐intervention | 5 | 474 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.13, 0.52] |
| 2.4 Incidence of depression (diagnosis) ‐ short‐term | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.08, 2.46] |
| 2.5 Incidence of depression (diagnosis) ‐ medium‐term | 2 | 139 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.06, 39.71] |
| 2.6 Acceptability ‐ dropouts due to adverse events | 5 | 561 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [1.07, 3.89] |
| 2.7 Acceptability ‐ dropouts due to any cause ‐ post‐intervention | 9 | 962 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.73, 1.73] |
| 2.8 Acceptability ‐ dropouts due to any cause ‐ medium‐term | 3 | 327 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.69, 1.86] |
| 2.9 Incidence of depression (cut‐off) ‐ post‐intervention | 3 | 241 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.13, 0.51] |
| 2.10 Incidence of depression (diagnosis or cut‐off) ‐ post‐intervention | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.26, 2.00] |
| 2.11 Incidence of depression (diagnosis or cut‐off) ‐ medium‐term | 1 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.27, 1.74] |
| 2.12 Severity of depression ‐ post‐intervention | 2 | 163 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.91, 0.54] |
| 2.13 Severity of depression ‐ short‐term | 1 | 87 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.75, 0.09] |
| 2.14 Severity of depression ‐ medium‐term | 2 | 139 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.72, 1.09] |
Comparison 3. Sensitivity analyses on quality of studies ‐ impact of missing data (best & worst case scenarios) ‐ incidence of depression: psychological intervention versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 best case scenario | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.27, 0.72] |
| 3.1.1 post‐intervention | 1 | 206 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.16, 0.77] |
| 3.1.2 short‐term | 1 | 206 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.27, 0.96] |
| 3.2 worst case scenario | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.64, 1.57] |
| 3.2.1 post‐intervention | 1 | 206 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.44, 1.65] |
| 3.2.2 short‐term | 1 | 206 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.62, 2.13] |
Comparison 4. Sensitivity analyses on quality of studies ‐ failing to double‐blind ‐ incidence of depression: pharmacological intervention versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 post‐intervention | 4 | 431 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.11, 0.53] |
| 4.1.1 diagnosis | 4 | 431 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.11, 0.53] |
Comparison 5. Sensitivity analyses on quality of studies ‐ impact of missing data (best & worst case scenarios) ‐ incidence of depression: pharmacological intervention versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 best case scenario | 10 | 1400 | Odds Ratio (M‐H, Random, 95% CI) | 0.15 [0.09, 0.24] |
| 5.1.1 post‐intervention | 9 | 1045 | Odds Ratio (M‐H, Random, 95% CI) | 0.13 [0.07, 0.24] |
| 5.1.2 short‐term | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.06, 1.51] |
| 5.1.3 medium‐term | 3 | 327 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.06, 0.43] |
| 5.2 worst case scenario | 10 | 1400 | Odds Ratio (M‐H, Random, 95% CI) | 1.63 [0.97, 2.73] |
| 5.2.1 post‐intervention | 9 | 1045 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.74, 2.44] |
| 5.2.2 short‐term | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.23, 4.89] |
| 5.2.3 medium‐term | 3 | 327 | Odds Ratio (M‐H, Random, 95% CI) | 4.81 [0.92, 25.29] |
Comparison 6. Sensitivity analyses on length of treatment ‐ post‐randomisation ‐ incidence of depression: pharmacological intervention versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 short‐term | 6 | 384 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.21, 0.64] |
| 6.1.1 diagnosis | 3 | 162 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.12, 0.96] |
| 6.1.2 cut‐off | 2 | 123 | Odds Ratio (M‐H, Random, 95% CI) | 0.21 [0.06, 0.72] |
| 6.1.3 diagnosis OR cut‐off | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.26, 2.00] |
| 6.2 medium‐term | 6 | 619 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.22, 0.61] |
| 6.2.1 diagnosis | 4 | 407 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.13, 0.57] |
| 6.2.2 cut‐off | 1 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.10, 0.91] |
| 6.2.3 diagnosis OR cut‐off | 1 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.27, 1.74] |
| 6.3 long‐term | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 9.89 [0.51, 191.27] |
| 6.3.1 diagnosis | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 9.89 [0.51, 191.27] |
Comparison 7. Sensitivity analyses on type of funding ‐ incidence of depression: pharmacological intervention versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 post‐intervention | 7 | 591 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.18, 0.54] |
| 7.1.1 diagnosis | 4 | 369 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.09, 0.54] |
| 7.1.2 cut‐off | 2 | 123 | Odds Ratio (M‐H, Random, 95% CI) | 0.21 [0.06, 0.72] |
| 7.1.3 diagnosis OR cut‐off | 1 | 99 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.26, 2.00] |
| 7.2 medium‐term | 2 | 166 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.26, 1.26] |
| 7.2.1 diagnosis | 1 | 72 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.09, 1.59] |
| 7.2.2 diagnosis OR cut‐off | 1 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.27, 1.74] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Almeida 2006.
| Study characteristics | ||
| Methods |
Study design: randomised (2‐arm parallel randomised controlled clinical trial) Sample size: 981 Method of randomisation: block randomisation Method of concealment: centralised Blinding: double‐blind Analysis: intention‐to‐treat Date the study was conducted: June 2002 ‐ June 2004 |
|
| Participants |
Location: Australia Total N randomised: 111 Intervention n: 55 (67 % male; age M = 68, SD = 13; haemorrhagic stroke n = 7, Ischaemic stroke n = 104) Control n: 56 (63 % male; age M = 67, SD = 13) Inclusion criteria: acute Ischaemic or haemorrhagic stroke and no depression at baseline Diagnostic criteria ‐ long‐term physical condition: acute Ischaemic or haemorrhagic stroke diagnosed according to ICD‐10 Diagnostic criteria ‐ depression: depression is assessed by the HADS ‐ depression sub scale [HADS‐D] (a cut‐off score > 7 implies depression) Exclusion criteria: alcohol dependence; severe communication difficulties (aphasia or limited ability to communicate in English); unstable medical condition as determined by the treating physician; severe cognitive impairment (Mini‐Mental State Examination [MMSE] score ≤ 10); and depression (HADS‐D score > 7); taking· antidepressants (within 4 weeks of stroke); prior history of clinically significant adverse reactions to sertraline History of depression: no information specified |
|
| Interventions |
Intervention: sertraline: fixed daily dose of 50 mg at night Control: matched placebo Other treatment: intervention/placebo: within a period up to 2 weeks after stroke symptoms became apparent Duration of treatment: 24 weeks Length of follow‐up: 52 weeks after randomisation (at baseline, 12, 24, 36 and 52 weeks) Other study arms: No |
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes |
Language: English Funding: Unrestricted grant from the Rotary Health Research Fund of Australia, Parramatta, Australia. No other financial affiliation is relevant to the subject of this article. Declaration of interest: No other financial affiliation is relevant to the subject of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "subjects were allocated ... according to a computer‐generated random Iist of numbers..." (P. 1105) "the lists were produced in random blocks of 8, 10, or 12 subjects to minimize the risk of unbalanced treatment groups and nonblinding" (P. 1105) |
| Allocation concealment (selection bias) | Low risk | "...according to a computer‐generated random Iist of numbers that was maintained centrally and independently by the pharmacist of the Royal Perth Hospital..." (P. 1105) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | both the research team and participants were unaware of treatment allocation until the final endpoint was collected from the last participant enrolled into the study. (P. 1105) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | both the research team and participants were unaware of treatment allocation until the final endpoint was collected from the last participant enrolled into the study. (P. 1105) |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
numbers randomised: sertraline: n = 55; placebo: n = 56; total: n = 111 number of drop‐outs at week 24: n = 12, no further information specified number of drop‐outs at week 52: sertraline: n = 11; placebo: n = 6; total: n = 17 numbers analysed post‐intervention: sertraline: n = 48; placebo: n = 51; total: n = 99 numbers analysed follow‐up: sertraline: n = 44; placebo: n = 50; total: n = 94 reasons for drop‐out: no further information specified |
| Selective reporting (reporting bias) | Unclear risk | no protocol available |
| Other bias | Low risk | "only I breach of protocol was recorded: treatment allocation was disclosed upon request from the treating physician and Ethics Committee after a participant developed seizures. Treatment was discontinued at the end of week 24." (P. 1105) |
Hansen 2012.
| Study characteristics | ||
| Methods |
Study design: randomised (2‐arm parallel randomised controlled clinical trial) Sample size: 11594 Method of randomisation: centrally prepared randomisation blocks Method of concealment: us of the consecutive number of the study medication Blinding: double‐blind Analysis: intention‐to‐treat Date the study was conducted: November 2004 ‐ December 2007 |
|
| Participants |
Location: Denmark Total N randomised: 240 (one patient was excluded due to protocol violation) Intervention n: 120 (63 % male; age M = 65, SD = 12) Control n: 119 (63 % male; age M = 64, SD = 12) Inclusion criteria: acute coronary syndrome (ACS) and no depression at baseline; age > 18 Diagnostic criteria ‐ long‐term physical condition: diagnosis of ACS based on symptoms, electrocardiogram and cardiac enzymes according to contemporary guidelines Diagnostic criteria ‐ depression: depression diagnosis assessed by SCAN interview and confirmation by a psychiatrist Exclusion criteria: current depression as determined by a structured interview and confirmed by a psychiatrist; use of antidepressants or antipsychotics; previous intolerance to SSRI; severe, life‐threatening medical conditions; severe heart failure; current alcohol or substance abuse, psychosis or dementia; participation in other intervention trials; pregnancy and lactation; linguistic difficulties History of mental disorder: Intervention n = 20 (16.7 %); Control n = 18 (15.1 %) |
|
| Interventions |
Intervention: escitalopram
Control: matched placebo Other treatment: aspirin, clopidogrel, beta‐blockers, digoxin, angiotensin‐II antagonists, ACE inhibitors, statins, calcium antagonists, long acting nitrates, diuretics, benzodiazepines, hypnotic drugs, antidiabetics, eltroxin and antacids Duration of treatment: 12 month Length of follow‐up: 12 month (at baseline, 2, 8 and approximately 15, 22, 29, 36, 43, and 50 weeks) Other study arms: no |
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes | Patients were screened for depression by study investigators using the HDS at all assessments. In case of positive screening (cut‐off score ≥ 13),
Language: English Funding: The Danish Hearts Foundation (01‐1‐9‐F13‐22884), Danish Medical Research Council (22‐02‐0221) and H. Lundbeck Ltd. The sponsors of the study had no role in study design, manuscript draft or decision to submit the manuscript. Declaration of interest: The authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization was done in centrally prepared randomization blocs unknown to the investigators." (P. 12) |
| Allocation concealment (selection bias) | Low risk | "the allocation sequence was implemented using the consecutive number of the study medication." (P. 12) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "investigators, nurses, outcome assessors as well as included patients remained blinded to group assignment during the entire intervention and data analysis." (p.12) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "investigators, nurses, outcome assessors as well as included patients remained blinded to group assignment during the entire intervention and data analysis." (p.12) |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
numbers randomised: escitalopram: n = 120; placebo: n = 120; total: N = 240 note: n = 1 participant was excluded due to protocol violation; total: N = 239 number of drop‐outs at 12 months: escitalopram: n = 35; placebo: n = 30; total: N = 65 numbers analysed post‐intervention: escitalopram: n = 85; placebo: n = 89; total: N = 174 reasons for drop‐out:
|
| Selective reporting (reporting bias) | High risk | protocol available
|
| Other bias | Low risk | no |
Hansen 2014.
| Study characteristics | ||
| Methods |
Study design: randomised (2‐arm parallel randomised controlled clinical trial) Sample size: 703 Method of randomisation: random assignment in blocks of six based on a list that was computer generated Method of concealment: centralised Blinding: double‐blind Analysis: intention‐to‐treat Date the study was conducted: July 2011 ‐ December 2012 |
|
| Participants |
Location: Denmark Total N randomised: 54 Intervention n: 28 (0 % male; age M = 51, range = 46 ‐ 66) Control n: 26 (0 % male; age M = 60, range = 49 ‐ 68) Inclusion criteria: women; age 30‐75 years; breast cancer Diagnostic criteria ‐ long‐term physical condition: scheduled for lumpectomy or mastectomy for breast cancer, with American Society of Anesthesiologists (ASA) class 1‐lll Diagnostic criteria ‐ depression: MDI cut‐off score ≥ 21 Exclusion criteria: signs of depression on the Major Depression Inventory (MDI); pregnancy; neoadjuvant chemotherapy; treatment with selective serotonin reuptake inhibitors, antithrombotic drug therapy (except 75 mg acetylsalicylic acid daily), monoaminoxidase (MAO) inhibitors, calcium channel blockers, rotor or Dubine Johnson syndrome; epilepsy; known allergic reaction to melatonin; known and treated sleep apnoea; diabetes mellitus treated with insulin; ongoing or previous medically treated depression or bipolar disorder; known autoimmune diseases (systemic lupus erythematosus, rheumatoid arthritis or only sclerosis); incompensated liver cirrhosis; severe kidney disease (receiving dialysis); previous or other current cancer; known medically treated sleep disorder (insomnia, restless legs, etc.); shift work or night work; daily intake of > 5 units (1 unit = 8 g pure alcohol); preoperative continuous treatment with psychopharmacological drugs of any kind, opioids, anxiolytics or hypnotics; predicted poor compliance; breast feeding; preoperative Mini Mental State Examination score < 24 History of depression: no |
|
| Interventions |
Intervention: daily dose of 6 mg melatonin 1h before bedtime (1 week preoperatively and 12 weeks postoperatively) Control: matched placebo Duration of treatment: 13 weeks Length of follow‐up: 13 weeks (at 1 week preoperatively, baseline, 2, 4, 8, 12 weeks) Other study arms: no |
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes |
Language: English Funding: grants from The University of Copenhagen, The Aase and Ejnar Danielsens Foundation, The A.P. Møller Foundation for the Advancement of Medical Science, The Else and Mogens Wedell Wedellborgs Foundation, The Beckett Foundation, The Hede Nielsen Family Foundation, The Dagmar Marshalls Foundation and Manufacturer Einar Willumsen's Memorial Scholarship Declaration of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "on inclusion patients were randomly assigned, in blocks of six, to melatonin or placebo" (p. 684) "the randomisation Iist was computer generated using dedicated software (http://www.randomization.com)" (p. 684) |
| Allocation concealment (selection bias) | Low risk | "this procedure was completed by the hospital pharmacy, who received the medicine directly from Pharma Nord. The pharmacy packed the melatonin/placebo in identical, sequentially numbered, sealed boxes" (p. 684) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "the participants, the health care providers, the research staff... were all blinded to allocation and the allocation sequence by taking the sequentially numbered sealed boxes" (p. 684) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "... and the investigators assessing the outcomes were all blinded to allocation and the allocation sequence by taking the sequentially numbered sealed boxes" (p. 684) |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
numbers randomised: melatonin: n = 28; placebo: n = 26; total: N = 54 number of drop‐outs at 3 months: melatonin: n = 1; placebo: n = 10; total: N = 11 numbers analyzed post‐intervention: melatonin: n = 27; placebo: n = 16; total: N = 43 reasons for drop‐out (p. 692): melatonin: unable to cope with participation in trial placebo: adverse events, noncompliance, lost to follow‐up, unable to cope with participation in trial |
| Selective reporting (reporting bias) | High risk | protocol available outcomes on e.g. cognitive function have not been reported "were tested with the neuropsychological test battery (data not reported here)" (p. 684) |
| Other bias | Low risk | "...the only change after trial commencement was that the upper Iimit of the age criteria was raised from 70 to 75 years on 19 October 2011, because of slow recruitment" (p. 684) "patients were recruited from July 2011‐ to December 2012 where the trial was terminated prematurely (n = 54) due to restructuring of surgery for breast cancer in the region resulting in an overall Iow inclusion rate. We tried to include other centres in other regions, but this was not possible because of Iack of funding" (p. 692) |
Lydiatt 2008.
| Study characteristics | ||
| Methods |
Study design: randomised (2‐arm parallel randomised controlled clinical trial) Sample size: 75 Method of randomisation: coin toss, randomisation ratio 1:1 Method of concealment: no information specified Blinding: double‐blind (participants and all investigators) Analysis: intention‐to‐treat Date the study was conducted: July 2002 ‐ April 2005 |
|
| Participants |
Location: USA Total N randomised: 36 (among these n = 5 with baseline depression, and n = 1 who failed Mini Mental State Examination) Randomised and Eligible: 30 (among these n = 2 who failed to show‐up for allocation and baseline visit) Randomised and Allocated: 28 Intervention n: 15 demographics are only available for those participants who completed visit one after 4 weeks n = 13 (62 % male; age M = 61, range 43 ‐ 81) Control n: 13 demographics are only available for those participants who completed visit one after 4 weeks n = 12 (33 % male; age M = 61, range 48 ‐ 76) Inclusion criteria: newly diagnosed or recurrent cancers of the oral cavity, larynx, pharynx, neck, or paranasal sinuses requiring more than limited excision; age ≥ 19 Diagnostic criteria ‐ long‐term physical condition: newly diagnosed or recurrent cancers of the oral cavity, larynx, pharynx, neck, or paranasal sinuses requiring more than limited excision; assessment not specified Diagnostic criteria ‐ depression: depression diagnosis according to DSM‐IV assessed by the MINI Exclusion criteria: meeting diagnostic criteria for major depressive disorder; cognitive impairment (MMSE score ≤ 24); use of antidepressant medication; contraindication to citalopram; meeting diagnostic criteria for psychosis or schizophrenia; suicidal tendencies History of depression: no information specified |
|
| Interventions |
Intervention: citalopram hydrobromide
Control: matched placebo Other treatment: conventional cancer therapy according to the National Comprehensive Cancer Network Guidelines (surgery, radiation therapy with and without chemotherapy) Other possible treatment: supportive care and support group (no psychotherapy and antidepressants) Duration of treatment: 12 weeks Length of follow‐up: 16 weeks (at baseline, 4, 8, 12 and 16 weeks) Other study arms: yes Due to an error, five ineligible participants with depression at baseline were randomised. "Subjects with HDRS scores of at least 15 at baseline were continued in the study because data on the treatment vs the natural history of untreated MDD have also not been studied in HNC. They were excluded from the primary analysis of MDD prevention because, by definition, MDD was already present." (p. 530) |
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes |
Language: English Funding: University of Nebraska Clinical Research Center and an unrestricted grant from Ted Kooser. Forest Laboratories Inc provided matching drug and placebo. Declaration of interest: Dr Burke has served as a consultant and received honoraria and research support from Forest Laboratories Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "subjects were randomised in the University of Nebraska pharmacy by coin toss in a 1:1 fashion to receive placebo or citalopram" (p. 529) |
| Allocation concealment (selection bias) | Low risk | "subjects were randomised in the University of Nebraska pharmacy" (p. 529) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "all assessments were performed in a blinded fashion. Neither the investigators nor the subjects knew whether they were receiving active drug or placebo." (p. 530) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all assessments were performed in a blinded fashion. Neither the investigators nor the subjects knew whether they were receiving active drug or placebo." (p. 530) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | pre‐randomisation:
numbers randomised: citalopram: n = 15; placebo: n = 13; total: N = 28 number of drop‐outs at 16 weeks: citalopram: n = 2; placebo: n = 3; total: N = 5 numbers analysed post‐intervention: citalopram: n = 13; placebo: n = 10; total: N = 23 reasons for drop‐out (fig. 1, p. 530): citalopram: adverse events placebo: decision to withdraw, placement in care facility, or hospitalisation for depression |
| Selective reporting (reporting bias) | Unclear risk | no protocol available |
| Other bias | Unclear risk | data on demographics is only available for those participants who completed visit one after 4 weeks |
Lydiatt 2013.
| Study characteristics | ||
| Methods |
Study design: randomised (2‐arm parallel randomised controlled clinical trial) Sample size: 298 Method of randomisation: 1:1 ratio according to a randomisation table prepared by the study statistician Method of concealment: centralised Blinding: double‐blind Analysis: per protocol Date the study was conducted: January 2008 ‐ December 2011 |
|
| Participants |
Location: USA Total N randomised: 148 Intervention n: 74 (80 % male; age M = 63, SD = 11) Control n: 74 (80 % male; age M = 63, SD = 13) Inclusion criteria: > 18 years; newly diagnosed or recurrent stage II to IV epidermoid cancer of the head and neck Diagnostic criteria ‐ long‐term physical condition: newly diagnosed or recurrent stage II to IV epidermoid cancer of the head and neck (no assessment specified) Diagnostic criteria ‐ depression:
Exclusion criteria: cognitively impaired; advanced cancer or other conditions that limited life expectancy to less than 6 months; met diagnostic criteria for psychosis, schizophrenia, or major depressive disorder; receiving treatment for depression or anxiety; persistent inability to verbally communicate; uncontrolled pain; currently participating in another research study involving a therapeutic intervention; were females of childbearing age who were pregnant, nursing, or not practicing a reliable method of birth control History of depression: no information specified |
|
| Interventions |
Intervention: escitalopram
Control: matched placebo Duration of treatment: 16 weeks Length of follow‐up: 28 weeks (baseline, at 2, 4, 6, 8, 10, 12, 16, 20, 24, and 28 weeks) Other study arms: no |
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes |
Language: English Funding: grant R01 MH079420 from the National Institute of Mental Health additional support was provided by a research support fund grant from the Nebraska Medical Center and the UNMC Declaration of interest: Dr Lydiatt is on the head and neck panel for guideline development for the National Comprehensive Cancer Network but does not receive honorarium for participation in guideline development. Dr Burke has received grant support for his institution from Forest Research Institute and has served as a consultant to Forest and on their speakers’ bureau. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...patients were randomised by a pharmacist with no involvement in the evaluation in a 1:1 ratio to either escitalopram or matching placebo according to a randomisation table prepared by the study statistician" (p. 680) |
| Allocation concealment (selection bias) | Low risk | "patients were randomised by a pharmacist with no involvement in the evaluation ..." (p. 680) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | no information specified except for headline saying "...double‐blind ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | no information specified except for headline saying "...double‐blind ..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
numbers randomised: escitalopram: n = 74; placebo: n = 74; total: N = 148 number of drop‐outs at 28 weeks: escitalopram: n = 38; placebo: n = 30; total: N = 68 numbers analysed post‐intervention: escitalopram: n = 36; placebo: n = 44; total: N = 80 reasons for drop‐out (p. 681, fig. 1) escitalopram:
placebo:
|
| Selective reporting (reporting bias) | Unclear risk | no protocol available |
| Other bias | Unclear risk | "patients were stratified by site (UNMC or NMCC), sex, stage (early [stage II] vs advanced [stage III/IV]), and primary modality of treatment (radiation with or without chemotherapy vs surgery [not biopsy] with or without radiation)" (p. 679) |
Narushima 2002.
| Study characteristics | ||
| Methods |
Study design: randomised (3‐arm parallel randomised controlled clinical trial) Sample size: 347 (USA: 343; Argentina: 4) Method of randomisation: no information specified Method of concealment: no information specified Blinding: double‐blind Analysis: intention‐to‐treat Date the study was conducted: July 1991 ‐ June 1997 |
|
| Participants |
Location: USA and Argentina Total N randomised: 48 Intervention n: 32
Control n: 16 (75 % male; no information on age available) Inclusion criteria: thromboembolic stroke or intracerebral haemorrhage; no depression at baseline; age 18‐85 Diagnostic criteria ‐ long‐term physical condition: thromboembolic stroke or intracerebral haemorrhage (assessed by neurologists or neuroradiologists who evaluated computerized tomography or magnetic resonance scans) Diagnostic criteria ‐ depression: depression diagnosed according to DSM‐IV assessed by the PSE Exclusion criteria: severe comprehension deficit (unable to answer correctly part 1 of the token test); use of antidepressant; contraindication to nortriptyline or fluoxetine; acute stroke (within 6 month of study entry) History of depression: no information specified |
|
| Interventions |
Intervention: fluoxetine
nortriptyline
Control: matched placebo Duration of treatment: 3 months Length of follow‐up: 24 month (at baseline, 3, 6, 9, 12, and 24 months) [no follow‐up for participants from Argentina (duration of participation: 3 months)] Other study arms: no |
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes | Decreasement of medication in case of severe side effects Language: English Funding: National Institute of Mental Health Grants MH‐40355, MHH‐52879, MH‐53592 and Research Scientist Award MH‐00163 (RGR) Declaration of interest: no information specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information specified "Patients were randomly assigned to 12 weeks of treatment with fluoxetine, nortriptyline, or placebo unless one of the active drugs was contraindicated. Nortriptyline was contraindicated in patients with cardiac conduction abnormalities, and fluoxetine was contraindicated in patients with intracerebral hemorrhage. Of the 48 nondepressed patients, 7 had a contraindication to nortriptyline and 5 had a contraindication to fluoxetine. These patients were randomly assigned to receive noncontraindicated medication or placebo. Thus, 75% of the patients were randomly assigned to nortriptyline or fluoxetine, whereas all patients were randomly assigned to active or placebo medication." |
| Allocation concealment (selection bias) | Unclear risk | no information specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The effectiveness of the blind was determined by asking patients and raters to guess whether they were taking active or placebo medication. For active medication, patients were correct 59 % and raters 65 % of the time. For placebo, patients were correct 55 % and raters 46 % of the time. There was no statistically significant difference from a random distribution of guesses for either patients or raters." (p. 298) While blinding for participants and outcome assessment received low risk of bias ratings, further study personnel appeared to be aware of study allocation: "Doses were decreased if side effects were severe, which was the case for six nondepressed patients. Of these six patients, four were treated with nortriptyline and two with fluoxetine. To maintain the double‐blind design, doses were decreased for equal numbers of placebo patients. Nortriptyline‐treated patients were monitored for serum drug concentrations throughout the treatment period to adjust the drug level within the therapeutic window." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The effectiveness of the blind was determined by asking patients and raters to guess whether they were taking active or placebo medication. For active medication, patients were correct 59 % and raters 65 % of the time. For placebo, patients were correct 55 % and raters 46 % of the time. There was no statistically significant difference from a random distribution of guesses for either patients or raters." (p. 298) |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
numbers randomised: fluoxetine: n = 17; nortriptyline: n = 15; placebo: n = 16; total: N = 48 number of drop‐outs at 3 months: fluoxetine: n = 2; nortriptyline: n = 2; placebo: n = 1; total: N = 5 numbers analysed post‐intervention: fluoxetine: n = 15; nortriptyline: n = 13; placebo: n = 15; total: N = 43 reasons for drop‐out (pp. 298‐299): fluoxetine: gastrointestinal symptoms, refused treatment (note: the authors initially name n = 4 drop‐outs for the treatment study, however, among these n = 2 who became depressed, and therefore are not considered drop‐outs for the prevention task but incidence events); nortriptyline: sedative effects, refused treatment placebo: rash |
| Selective reporting (reporting bias) | Unclear risk | no protocol available |
| Other bias | High risk |
|
Novack 2009.
| Study characteristics | ||
| Methods |
Study design: randomised (2‐arm parallel randomised controlled clinical trial) Sample size: 371 Method of randomisation: no information specified Method of concealment: no information specified Blinding: double‐blind Analysis: intention‐to‐treat Date the study was conducted: no information specified |
|
| Participants |
Location: USA Total N randomised: 99 Intervention n: 49 (79 % male; age M = 35, SD = 17) Control n: 50 (66 % male; age M = 35, SD = 16) Inclusion criteria: within 8 weeks of injury ( = traumatic brain injury); injury sufficient to require inpatient rehabilitation; age 19‐75 Diagnostic criteria ‐ long‐term physical condition: traumatic brain injury: Glasgow Coma Scale (GCS) score of ≤ 12, or neuroimaging results consistent with the effects of trauma (e.g., contusion, subdural hematoma) at admission Diagnostic criteria ‐ depression: diagnosis according to DSM‐IV assessed by SCID‐I Exclusion criteria: existing neurological difficulties; use of antidepressant medication at the time of injury; administration of antidepressant medication in the hospital prior to enrolment; ongoing steroid treatment; depression necessitating treatment at the time of enrolment; pregnancy, alcohol or drug abuse in the year prior to the injury; systemic medical illnesses that would independently limit outcome (such as severe renal disease and cardiac difficulties) History of depression: no |
|
| Interventions |
Intervention: daily dose of 50 mg sertraline Control: placebo Duration of treatment: 3 months Length of follow‐up: 12 months (3, 6, and 12 months) Other study arms: no |
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes |
Language: English Funding: National Institute of Disability and Rehabilitation Research Grant H133A980010 Traumatic Brain Injury Model System Project Declaration of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no information specified |
| Allocation concealment (selection bias) | Unclear risk | no information specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "If depression was confirmed, the blind was broken for the treating physician, but not for the psychometrician performing the outcome assessments" (p. 1923) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "If depression was confirmed, the blind was broken for the treating physician, but not for the psychometrician performing the outcome assessments" (p. 1923) |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
numbers randomised: sertraline: n = 49; placebo: n = 50; total: N = 99 number of drop‐outs at 3 months: sertraline: n = 1; placebo: n = 2; total: N = 3 number of drop‐outs at 12 months: sertraline: n = 13; placebo: n = 14; total: N = 27 numbers analysed post‐intervention at 3 months: sertraline: n = 48; placebo: n = 48; total: N = 96 numbers analysed at follow‐up at 12 months: sertraline: n = 36; placebo: n = 36; total: N = 72 reasons for drop‐out (p. 1926): sertraline: stopped taking study meds, lost or withdrew, O‐Log < 25 or nursing home, adverse events, expired placebo: stopped taking study meds, lost or withdrew, O‐Log < 25 or nursing home, expired |
| Selective reporting (reporting bias) | Unclear risk | no protocol available |
| Other bias | Low risk | no |
Rasmussen 2003.
| Study characteristics | ||
| Methods |
Study design: randomised (2‐arm parallel randomised controlled clinical trial) Sample size: 155 Method of randomisation: division in terms of gender and age (over and under 65 years of age) Method of concealment: no information specified Blinding: double‐blind Analysis: no information specified Date the study was conducted: January 1996 ‐ May 1998 |
|
| Participants |
Location: Denmark Total N randomised: 137 Intervention n: 70 (50 % male; age M = 72, SD = 9) Control n: 67 (51 % male; age M = 68, SD = 11) Inclusion criteria: stroke in the preceding 4 weeks Diagnostic criteria ‐ long‐term physical condition: stroke is diagnosed according to clinical criteria (WHO) Diagnostic criteria ‐ depression: diagnosis of depression according to ICD‐10 and assessed by clinical interview Exclusion criteria: current depression (total score > 13 on the HAM‐D‐17); stroke occurrence not within the preceding four weeks; significant aphasia; use of antidepressants (within the preceding 4 weeks); dementia; history of schizophrenia, psychosis, or severe drug abuse; present preexisting neurological illness; cardiovascular illness (within preceding 6 months) History of depression: no information specified |
|
| Interventions |
Intervention: daily dose of 50 mg sertraline (in case of clinical need, dosage is flexibly increased up to a maximum dose of 150 mg/day) Control: matched placebo Duration of treatment: 12 months Length of follow‐up: 12 months (at baseline, and 11 post‐randomisation visits (occurring at approximately 4‐5 week intervals) Other study arms: no |
|
| Outcomes |
Primary outcome:
Secondary outcome:
(Note: due to the predefined hierarchy of outcome measures we used data from the HAM‐D 17‐item version when reporting the incidence of depression.)
|
|
| Notes |
Language: English Funding: Pfizer A/S, Gert Jørgensen legat, and the Brain Cause (Hjernesagen) Declaration of interest: no information specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were fairly evenly divided in terms of gender and those over versus under 65 years of age..." (p. 218) |
| Allocation concealment (selection bias) | Unclear risk | no information specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | no information specified except for headline and p. 217 saying "...double‐blind ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | no information specified except for headline and p. 217 saying "...double‐blind ..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
numbers randomised: sertraline: n = 70; placebo: n = 67; total: N = 137 number of drop‐outs at 12 months: sertraline: n = 9; placebo: n = 10; total: N = 19 numbers analysed post‐intervention: sertraline: n = 61; placebo: n = 57; total: N = 118 reasons for drop‐out: no information specified |
| Selective reporting (reporting bias) | High risk | no protocol available but not all outcomes were reported, e.g. Newastle II scale |
| Other bias | Low risk | no |
Robinson 2008.
| Study characteristics | ||
| Methods |
Study design: 3‐arm parallel randomised controlled clinical trial (note: the third study‐arm problem‐solving therapy was not extracted due to no suitable control group) Sample size: 200 Method of randomisation: permuted blocks randomisation scheme; sample size randomly divided into block sizes of 3, 6, and 9; within each block random assignment to numbers of 1, 2, or 3 (the three treatment arms) Method of concealment: centralised Blinding: double‐blind Analysis: intention‐to‐treat Date the study was conducted: July 2003 ‐ October 2007 |
|
| Participants |
Location: USA Total N randomised: 176 but we excluded the third study‐arm problem‐solving therapy (n = 59) resulting in N = 117 Intervention n: 59 (64 % male; age M = 61, SD = 14) Control n: 58 (64 % male; age M = 64, SD = 13) Inclusion criteria: no depression at baseline; hemispheric, brainstem, or cerebellar (including Ischaemic or haemorrhagic) stroke in the preceding 3 moths; age 50‐90 Diagnostic criteria ‐ long‐term physical condition: stroke is diagnosed by clinical and neurological findings consistent with either hemispheric, brainstem, or cerebellar stroke Diagnostic criteria ‐ depression: depression is diagnosed according to DSM‐IV and assessed by the HAM‐D (17‐item version; a cut‐off score > 11 implies depression) Exclusion criteria: severe comprehension deficits (inability to complete part 1 of the Token Test); impaired decision‐making capacity (neuropsychological testing); alcohol or substance abuse or dependence according to DSM‐IV (within the last past 12 months); acute coronary syndromes; neurodegenerative disorders; life‐threatening heart or respiratory failure; renal or hepatic failure; severely disabling musculoskeletal disorder; cancer, and neurodegenerative disorders such as idiopathic Parkinson disease or Alzheimer disease; occurrence of stroke secondary to complications from an intracranial aneurysm, arterial‐venous malformation, intracranial tumour or neoplastic process; stroke during the course of myocardial infarction, aortic dissection, or revascularization surgery; stroke due to complications of an intracranial aneurysm, arteriovenous malformation, or neoplastic disease History of depression:
|
|
| Interventions |
Intervention: escitalopram:
Control: matched placebo Duration of treatment: 12 months Length of follow‐up: 18 months (at baseline, 3, 6, 9, 12, and 18 months) Other study arms: problem‐solving therapy
|
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes |
Language: English Funding: National Institute of Mental Health (NIMH) grant R01 MH‐65134; NIMH grant funds were use to purchase all of study medications. All of the authors received salary contributions from the grant supporting this study. Over the past five years, Dr Robinson reports serving as a consultant to the former Hamilton Pharmaceutical Company and Avanir Pharmaceutical Company; receiving support or honoraria from Lubeck, Forest Laboratories, and Pfizer; being on the speakers’ bureau for Forest Laboratories and Pfizer; and receiving grant support from the National Institute of Mental Health. Dr Small reports that he conducted a research study funded by Northstar Neuroscience that was unrelated to this prevention study. Dr Arndt reports inheriting Pfizer stock, which he owned from January 6, 2005, to December 23, 2006, and receiving grant support from the National Institute of Mental Health. The former Hamilton Pharmaceutical Company and Avanir Pharmaceutical Company had no financial interest in this prevention study. Declaration of interest: no other authors reported any financial disclosures |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...using a permuted blocks randomisation scheme Specifically, at the beginning of the study, the targeted sample size was divided randomly (200 patients) into block sizes of 3, 6, and 9 and within each block patients were randomly assigned 1 of the 3 treatments using computer‐generated random numbers of 1, 2, or 3..." (p. 4) |
| Allocation concealment (selection bias) | Low risk | "patients were centrally randomised...by a team member who was not involved in any evaluation..." (p. 4) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | low risk for escitalopram: "the examiners were unaware of each patient’s treatment assignment and double‐blinded assessments were done for escitalopram and placebo" (p. 5) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | no information specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
numbers randomised: escitalopram: n = 59; placebo: n = 58; total: N = 117 number of drop‐outs at 12 months: escitalopram: n = 7; placebo: n = 5; total: N = 12 number of drop‐outs at 18 months: escitalopram: n = 25; placebo: n = 25; total: N = 50 (note: no information specified, drop‐outs were calculated by comparing the data of the two corresponding publications conducted by Robinson 2008 and Mikami 2011). numbers analyzed post‐intervention at 12 months: escitalopram: n = 52; placebo: n = 53; total: N = 105 numbers analysed at follow‐up 18 months: escitalopram: n = 34; placebo: n = 33; total: N = 67 reasons for drop‐out: at 12 months (p. 14): escitalopram: death, intercurrent disease, could not be reached, protocol violation; placebo: could not be reached, adverse events, intercurrent disease, protocol violations at 18 months (Mikami et al. 2011): escitalopram: no information specified; placebo: no information specified note: Even though Mikami et al. 2011 published further data (12 months to 18 months) on the study conducted by Robinson et al. 2008, analyses were conducted independently from preceding results. Information on drop‐outs between 12 and 18 months are not available. Information on depression scores of the re‐evaluated sub‐sample are also not available. |
| Selective reporting (reporting bias) | High risk | no protocol available; trial registration included the Stroke Impact Scale which was not reported on |
| Other bias | Low risk | no |
Rovner 2007.
| Study characteristics | ||
| Methods |
Study design: randomised (2‐arm parallel randomised controlled clinical trial) Sample size: 602 Method of randomisation: random‐numbers table and fixed randomisation scheme with a 1:1 allocation ratio; permuted random block design; block sizes of 4 or 6 chosen at random, to mask the blocking process Method of concealment: sealed envelopes containing treatment assignments Blinding: single‐blind Analysis: intention‐to‐treat Date the study was conducted: December 2001 ‐ July 2005 |
|
| Participants |
Location: USA Total N randomised: 206 Intervention n: 105 (34 % male; age M = 81, SD = 5) Control n: 101 (26 % male; age M = 81, SD = 6) Inclusion criteria: preexisting age‐related macular degeneration and neovascular age‐related macular degeneration in the other eye; age > 64 Diagnostic criteria ‐ long‐term physical condition: age‐related macular degeneration is diagnosed by ophthalmologists' dictated reports of fluorescein angiograms and ophthalmologists' confirmation of diagnosis Diagnostic criteria ‐ depression: diagnosis according to DSM‐IV criteria Exclusion criteria: diagnosis of a depressive disorder according to DSM‐IV; current treatment for depression; cognitive impairment; confounding eye conditions History of depression:
|
|
| Interventions |
Intervention: problem solving therapy delivered in 6 in‐home sessions (45‐60 minutes long) Control: TAU Other treatment: ‐ Duration of treatment: 2 months Length of follow‐up: 2 months (at baseline, 2, and 6 months) Other study arms: no |
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes |
Language: English Funding: Grant RO1 MH61331 from the National Institute of Mental Health; grant U01 EY 015839 from the National Eye Institute; Farber Institute for Neurosciences of Thomas Jefferson University Declaration of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "to obtain the sample, we reviewed ophthalmologists’ dictated reports of fluorescein angiograms, confirmed diagnoses with treating ophthalmologists, and contacted potential subjects...this method of ascertainment was objective and verifiable, avoided relying on ophthalmologists to identify eligible cases, and prevented other selection biases" (p. 887) "we used a random‐numbers table, sealed envelopes containing treatment assignments, and a fixed randomisation scheme with a 1:1 allocation ratio to assign subjects to 1 of the 2 study groups. We used a permuted random block design to ensure balance between treatment groups according to their time of patient enrolment. Block sizes (4 or 6) were chosen at random to mask the blocking process." (p. 887) |
| Allocation concealment (selection bias) | Low risk | "...sealed envelopes containing treatment assignments" (p. 887) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "personnel masked to treatment assignment completed central data collection, measurement, and data entry. Only the project director, statistician, and PST therapists were aware of treatment assignment. Following randomisation, the research nurses instructed all subjects on the purpose and importance of masked treatment assignment and requested that subjects not reveal any information about their study participation. We assessed rates of breaches in masking and found that unmasking occurred in 26 PST and 11 control subjects (18.0%). In all instances, subjects inadvertently revealed their treatment assignment. We compared depression rates in unmasked and masked subjects to determine whether unmasking influenced rates of diagnosing depression and found no difference (P=.80)." (p. 888) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "the outcome assessors were unaware of subjects’ treatment assignment in this single masked study." (p. 887) "personnel masked to treatment assignment completed central data collection, measurement, and data entry" (p. 888) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
numbers randomised: problem solving therapy: n = 105; TAU: n = 101; total: N = 206 number of drop‐outs at 2 months: problem solving therapy: n = 10; TAU: n = 2; total: N = 12 number of drop‐outs at 6 months: problem solving therapy: n = 10; TAU: n = 6; total: N = 16 numbers analysed post‐intervention at 2 months: problem solving therapy: n = 95; TAU: n = 99; total: N = 194 numbers analysed follow‐up at 6 months weeks: problem solving therapy: n = 95; TAU: n = 95; total: N = 190 reasons for drop‐out (p. 887): problem solving therapy: loss of interest, too ill, death TAU: loss of interest, too ill, death Note: drop‐outs for the intervention group were reported incorrectly: the authors state that 11 subjects in the problem‐solving therapy group dropped‐out; based on other data and conducted analyses, however, only 10 subjects dropped‐out |
| Selective reporting (reporting bias) | Unclear risk | no protocol available |
| Other bias | Low risk | no |
Tsai 2011.
| Study characteristics | ||
| Methods |
Study design: randomised (2‐arm parallel randomised controlled clinical trial) Sample size: 519 Method of randomisation: no information specified Method of concealment: centralised Blinding: double‐blind (participants, interviewers) Analysis: no information specified Date the study was conducted: July 2007 ‐ June 2010 |
|
| Participants |
Location: Taiwan Total N randomised: 92 Intervention n: 46 (65 % male; age M = 61, SD = 11) Control n: 46 (63 % male; age M = 65, SD = 11) Inclusion criteria: first or recurrent Ischaemic stroke (within last 4 weeks); no depression at baseline Diagnostic criteria ‐ long‐term physical condition: stroke is diagnosed by abnormal neurological symptoms based on the participant's statement and imaging Diagnostic criteria ‐ depression:
Exclusion criteria: possible concurrent depression (HAM‐D ≥ 10); impairment of communication; severe cognitive impairment (MMSE score ≤ 15); use of antidepressants (within 2 weeks of stroke); history of depression, psychosis or severe substance abuse; transit Ischaemic attack History of depression: no |
|
| Interventions |
Intervention: milnacipran
Control: matched placebo Other treatment: ‐ Duration of treatment: 12 months Length of follow‐up: 12 months (at baseline, 1, 3, 6, 9, and 12 months) Other study arms: no |
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
|
| Notes |
Language: English Funding: ChangGung Medical Research Program (CMRPG 660343 and CMRPG 690491) Declaration of interest: None reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...the patients were randomly assigned to two groups" (p.264) |
| Allocation concealment (selection bias) | Low risk | "the randomisation was carried out by our pharmacy department, which has a professional team in charge of the clinical drug trials in our hospital. They prepared the drug with an allocation number for each participant based on their random assignment." (p. 264) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "the placebo was tailor‐made, and the appearance and weight (starch inside) were the same as that of the active drug, milnacipran." (p. 264) "all the interviewers were blinded to the patient's medication." (p. 264) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | no information specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
numbers randomised: milnacipran: n = 46; placebo: n = 46; total: N = 92 number of drop‐outs at 12 months: milnacipran: n = 21; placebo: n = 15; total: N = 36 numbers analysed post‐intervention: milnacipran: n = 25; placebo: n = 31; total: N = 56 reasons for drop‐out (p. 265) milnacipran: n = 21 drop‐outs (among these, 7 due to adverse events) placebo: n = 15 drop‐outs (among these, 4 due to adverse events) no further information specified |
| Selective reporting (reporting bias) | High risk | no protocol available; data on HAM‐D, National Institutes of Health Stroke Scale, and Barthel Index was only reported for baseline assessment even though they were "assessed in each of the follow‐up visits" (p. 264) |
| Other bias | Low risk | no |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Almeida 2010 | participants with antidepressant treatment at baseline included |
| Beglinger 2014 | incidence of depression was no (primary)outcome |
| Borrelli 2019 | no long‐term physical condition |
| Brody 2005 | participants recruited on basis of depressive symptoms |
| Browne 2013 | no long‐term physical condition |
| Burns 2007 | participants with antidepressant treatment at baseline included |
| Couch 1976 | incidence of depression was no outcome |
| Dean 1969 | no validated measures |
| de Jonge 2009 | mixed study sample |
| Hackett 2010 | participants with antidepressant treatment at baseline included |
| Hackett 2013 | participants with antidepressant treatment at baseline included |
| Hood 2018 | age criteria not fulfilled (14‐18 years) |
| Karp 2016 | indicative/selective prevention |
| Kong 2007 | incidence of depression was not reported |
| Kredentser 2018 | no long‐term physical condition |
| Mossey 1996 | participants with antidepressant treatment at baseline included |
| Niedermaier 2004 | no suitable control group |
| Pitceathly 2009 | indicative/selective prevention |
| Pols 2017 | mixed study sample |
| Read 2020 | participants with antidepressant treatment at baseline included |
| Rovner 2014 | no suitable control group |
| van der Aa 2015 | indicative/selective prevention |
| Yeung 2014 | data not accessible |
| Zhang 2013 | no suitable control group |
Characteristics of studies awaiting classification [ordered by study ID]
ChiCTR‐TRC‐12003489.
| Methods | randomised parallel controlled trial |
| Participants | adults after stroke; target size: 244 |
| Interventions | acupuncture therapy |
| Outcomes | poststroke depression measured by the Hamilton Depression Rating Scale (HAMD) |
| Study details | |
| Publication details | |
| Stated aim of study | |
| Notes | The study is led by Sun Jian‐Hua (principle investigator) in China. Contacting Wei Zhang revealed that data collection was completed and results are planned to be published late 2018. Sun Jian‐Hua was contacted again in March 2020 but we were unable to receive any additional information about the status of the study. |
EudraCT‐2005‐005266‐37.
| Methods | double blinded randomised placebo‐controlled trial |
| Participants | adults after acute middle cerebral artery territory infarction; target size: 60 |
| Interventions | escitalopram 10 mg |
| Outcomes | primary: incidence of poststroke depression measured by the Montgomery‐Åsberg Depression Rating Scale (MADRS) after 180 days following acute middle cerebral artery territory infarction, as well as incidence of dementia measured by the Clinical Dementia Rating Scale (CDR, >0,5) after 180 days following acute middle cerebral artery territory infarction. |
| Study details | |
| Publication details | 2006‐01‐04 |
| Stated aim of study | unknown |
| Notes | Sponsor Name: Central Institute for Mental Health, Mannheim, Div. of Gerontopsychiatry |
Guneri 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study details | |
| Publication details | |
| Stated aim of study | |
| Notes | no corresponding published study or further data could be found |
He 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study details | |
| Publication details | |
| Stated aim of study | |
| Notes | full texts were not accessible, no further information were available |
Li 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study details | |
| Publication details | |
| Stated aim of study | |
| Notes | full texts were not accessible, no further information were available |
Lu 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study details | |
| Publication details | |
| Stated aim of study | |
| Notes | full texts were not accessible, no further information were available |
Sander 2017.
| Methods | multicenter randomised controlled clinical trial |
| Participants | adults with chronic back pain; target size: 406 |
| Interventions | internet‐ and mobile‐based intervention (IMI) for the prevention of depression |
| Outcomes | |
| Study details | |
| Publication details | |
| Stated aim of study | |
| Notes | The study is led by Harald Baumeister (principal investigator) in Germany. Contacting Lasse Sander revealed that data collection was completed and results are about to be published. As of now, there were no available data to be included in the review. |
van Zyl 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study details | |
| Publication details | |
| Stated aim of study | |
| Notes | conference paper; no corresponding published study or further data could be found |
Wen 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study details | |
| Publication details | |
| Stated aim of study | |
| Notes | full texts were not accessible, no further information were available |
Xu 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study details | |
| Publication details | |
| Stated aim of study | |
| Notes | full texts were not accessible, no further information were available |
Zhong 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study details | |
| Publication details | |
| Stated aim of study | |
| Notes | full texts were not accessible, no further information were available |
Zhou 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study details | |
| Publication details | |
| Stated aim of study | |
| Notes | full texts were not accessible, no further information were available |
Zhu 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Study details | |
| Publication details | |
| Stated aim of study | |
| Notes | full texts were not accessible, no further information were available |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616000886482.
| Study name | Randomised trial aiming to prevent development of depression and improve quality of life in individuals with dementia (Alzheimer’s disease) through a novel Cognitive Bias Modification intervention |
| Methods | blinded randomised controlled trial |
| Participants | target size: 300 |
| Interventions | cognitive bias modification (CBM) |
| Outcomes | primary: Incidence of clinically significant depressive symptoms in adults with Alzheimer's disease measured by the Cornell Scale for Depression in Dementia (CSDD). |
| Starting date | 2016‐07‐06 |
| Contact information | andrew.ford@uwa.edu.au; varsha,hirani@uwa.edu.au |
| Notes | This study is led by Dr. Andrew Ford (principal investigator) in Australia (ACTRN12616000886482). Dr. Ford was contacted in March 2020: the trial is still ongoing. |
Madsen 2017.
| Study name | The effect of MElatonin on Depressive symptoms, Anxiety, CIrcadian and Sleep disturbances in patients after acute coronary syndrome (MEDACIS): study protocol for a randomised controlled trial |
| Methods | multicenter, double‐blinded, placebo‐controlled, randomised clinical trial |
| Participants | target size: 240 |
| Interventions | 25 mg exogenous melatonin |
| Outcomes | primary: Incidence of clinically significant depressive symptoms measured by the Major Depression Inventory (MDI) |
| Starting date | 2015 |
| Contact information | michael_madsen88@hotmail.com |
| Notes | The study is led by Dr. Michael Tvilling Madsen (principal investigator) in Denmark (NCT02451293). Dr. Madsen was contacted again in March 2020 but we were unable to receive any additional information about the status of the study. |
Differences between protocol and review
Changes in authorship: After contributing to the protocol, WHJ was unavailable for the review. To ensure the quality of the review, we were very grateful to have JB join us as an author to offer his comprehensive expertise on medical and psychological aspects.
Title: In keeping with the terminology used by the CCMD group, we changed the title from 'Prevention of depression in chronically physically ill adults' to 'Prevention of depression in adults with long‐term physical conditions.'
Types of participants ‐ Diagnosis: in order to exclude adults with an initial major depressive order, assessment of major depression could also be made by scoring above a specific cut‐off point on standardized, validated rating scales, or standardized, validated self‐report questionnaires for depression.
Types of interventions ‐ Experimental interventions: we added 'milnacipran' in the list of experimental interventions.
Types of outcome measures ‐ Timing of outcome assessment: to use consistent phrasing throughout the review, we changed the term post‐treatment into post‐intervention.
Data extraction and management: In the protocol we stated that we would extract 'mean and standard deviation/error, effect sizes, confidence intervals (CIs), odds ratios (ORs), hazard ratios (HRs), risk ratios (RRs), P values'. We corrected this. It now says 'sample sizes, incidence, means, standard deviation/error, and P values'.
Measures of treatment effect: In the protocol the statement addressing the descriptive assessment of tolerability (adverse events) and acceptability (drop‐outs) was under the heading 'dichotomous data'. We moved this to 'continuous data'.
Sensitivity analysis: To use consistent phrasing throughout the review, we changed the term post‐treatment into post‐intervention.
Dealing with missing data: In the protocol we stated that we would account for missing data 'not missing at random' by conducting ITT analyses. However, this might result in an too optimistic assessment of incidences. Therefore, conducted analyses in the review were PP. As pre‐described, we conducted sensitivity analyses according to best and worst case scenarios to gain information on the impact of missing data.
Contributions of authors
Drafting of protocol: HK, HB, WHJ, & OM Developing of search strategy: HK, HB, WHJ, & OM Trial search and selection: HK & OM Data extraction: HK & OM Entering data into RevMan: HK Data analysis: HK Drafting the review: HK, HB, JB, & OM
Sources of support
Internal sources
-
Quality Management and Social Medicine, Germany
Funding the review project
External sources
No sources of support supplied
Declarations of interest
HK: None known HB: None known JB: None known OM: None known
Edited (no change to conclusions)
References
References to studies included in this review
Almeida 2006 {published data only}
- Almeida OP, Waterreus A, Hankey GJ. Preventing depression after stroke: results from a randomized placebo-controlled trial. Journal of Clinical Psychiatry 2006;67(7):1104-9. [DOI] [PubMed] [Google Scholar]
Hansen 2012 {published data only}
- *Hansen BH, Hanash JA, Rasmussen A, Hansen JF, Andersen NL, Nielsen OW, Birekt-Smith M. Effects of escitalopram in prevention of depression in patients with acute coronary syndrome (DECARD). Journal of Psychosomatic Research 2012;72:11-6. [DOI] [PubMed] [Google Scholar]
- Hanash JA, Hansen BH, Hansen JF, Nielsen OW, Rasmussen A, Birket-Smith M. Cardiovascular safety of one-year escitalopram therapy in clinically nondepressed patients with acute coronary syndrome: results from the depression in patients with coronary artery disease (DECARD) trial. Journal of Cardiovascular Pharmacology 2012;60(4):397-405. [DOI] [PubMed] [Google Scholar]
- Hanash JA. Impact of prevention of DEpression in patients with acute coronary syndrome on CARDiac risk profile (DECARD): a double-blind, placebo-controlled study of escitalopram. International Journal of Cardiology 2007;119:14-5. [Google Scholar]
- Hansen B, Hanash J, Rasmussen A, Fischer HJ, Traerup AN, Birket-Smith M. Effects of escitalopram in prevention of depression in patients with acute coronary syndrome (DECARD): randomised controlled trial. Abstracts from the 13th Annual Meeting of the European Association for Consultation-Liaison Psychiatry and Psychosomatics, EACLPP and the 28th European Conference on Psychosomatic Research, ECPR. In: Journal of Psychosomatic Research. Innsbruck, Austria, 2010 Jun 30-July 3.
- Hansen B, Hanash J, Rasmussen A, Hansen J, Andersen N, Birket-Smith M. Escitalopram prevents depression in patients with acute coronary syndrome (DECARD): a randomized controlled trial [NCT00140257]. In: European Journal of Cardiovascular Prevention and Rehabilitation [Abstracts from the EuroPRevent 2010 Conference. Prague Czech Republic, May 5-7 2010.
- Hansen BH, Hanash JA, Rasmussen A, Hansen JF, Birket-Smith M. Comparison of participants and non-participants in a randomized study of prevention of depression in patients with acute coronary syndrome. Nordic Journal of Psychiatry 2011;65:22-5. [DOI] [PubMed] [Google Scholar]
- Hansen BH, Hanash JA, Rasmussen A, Hansen JF, Birket-Smith M. Rationale, design and methodology of a double-blind, randomized,placebo-controlled study of escitalopram in prevention of depression in acute coronary syndrome (DECARD) - study protocol. Trials 2009;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorthøj CR, Hansen BH, Hanash JA, Rasmussen A, Birket-Smith M. Prevention of depression in patients with acute coronary syndrome (DECARD) randomized trial:effects on and by self-reported health. Early Intervention in Psychiatry 2015;9:370-7. [DOI] [PubMed] [Google Scholar]
Hansen 2014 {published data only}
- *Hansen MV, Andersen LT, Madsen MT, Hageman I, Rasmussen LS, Bokmand S, Rosenberg J, Gögenur I. Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Breast Cancer Research and Treatment 2014;145:683-95. [DOI] [PubMed] [Google Scholar]
- Hansen MV, Madsen MT, Hageman I, Rasmussen LS, Bokmand S, Rosenberg J, Gögenur I. The effect of MELatOnin on Depression,anxietY, cognitive function and sleep disturbances in patients with breast cancer. The MELODY trial: protocol for a randomised, placebo-controlled, double-blinded trial. BMJ Open 2012;2:e000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lydiatt 2008 {published data only}
- *Lydiatt WM, Denman D, McNeilly DP, Puumula SE, Burke WJ. A randomized, placebo-controlled trial of citalopram for the prevention of major depression during treatment for head and neck cancer. Archives of Otolaryngology - Head & Neck Surgery 2008;134(5):528-35. [DOI] [PubMed] [Google Scholar]
- Lazure KE, Lydiatt WM, Denman D, Burke WJ. Association between depression and survival or disease recurrence in patients with head and neck cancer enrolled in a depression prevention trial. Head & Neck 2009;31(7):888-92. [DOI] [PubMed] [Google Scholar]
Lydiatt 2013 {published data only}
- Lydiatt WM, Bessette D, Schmid KK, Sayles H, Burke WJ. Prevention of depression with escitalopram in patients undergoing treatment for head and neck cancer randomized, double-blind, placebo-controlled clinical trial. JAMA Otolaryngology - Head & Neck Surgery 2013;139(7):678-86. [DOI] [PubMed] [Google Scholar]
Narushima 2002 {published data only}
- Narushima K, Kosier TJ, Robinson RG. Preventing poststroke depression: a 12-week double-blind randomized treatment trial and 21-month follow-up. Journal of Nervous and Mental Disease 2002;190(5):296-303. [DOI] [PubMed] [Google Scholar]
Novack 2009 {published data only}
- Novack TA, Baños JH, Brunner R, Renfroe S, Meythaler JM. Impact of early administration of sertraline on depressive symptoms in the first year after traumatic brain injury. Journal of Neurotrauma 2009;26:1921-8. [DOI] [PubMed] [Google Scholar]
Rasmussen 2003 {published data only}
- *Rasmussen A, Lunde M, Poulsen DL, Sörensen K, Qvitzau S, Bech P. A double-blind, placebo-controlled study of sertraline in the prevention of depression in stroke patients. Psychosomatics 2003;44(3):216-21. [DOI] [PubMed] [Google Scholar]
- Rasmussen A, Lunde M, Poulsen DL, Sörensen K, Qvitzau S, Bech P. A double-blind, placebo-controlled study of sertraline in the prevention of depression in stroke patients - correction. Psychosomatics 2004;45(1):91. [DOI] [PubMed] [Google Scholar]
Robinson 2008 {published data only}
- *Robinson RG, Jorge RE, Moser DJ, Acion L, Solodkin A, Small SL, et al. Escitalopram and problem-solving therapy for prevention of poststroke depression. JAMA 2008;299(20):2391-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge RE, Acion L, Moser D, Adams Jr HP, Robinson RG. Ricardo E. Jorge, MD, Laura Acion, MS, David Moser, PhD, Harold P. Adams Jr, MD, and Robert G. Robinson, MD. Archives of General Psychiatry 2010;67(2):187-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami K, Jorge RE, Moser DJ, Arndt S, Jang M, Solodkin A, Small SL, Fonzetti P, Hegel MT, Robinson RG. Increased frequency of first episode poststroke depression following discontinuation of escitalopram. Stroke 2011;42(11):3281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RG, Arndt S. Escitalopramand problem-solving therapy for prevention of poststroke depression: a randomized controlled trial - correction. JAMA 2009;301(10):1023-4. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Jorge RE, Long J. Prevention of poststroke mortality using problem-solving therapy or escitalopram. American Journal of Geriatric Psychiatry 2017;25:512-9. [DOI] [PubMed] [Google Scholar]
Rovner 2007 {published data only}
- *Rovner Barry W, Casten Robin J, Hegel Mark T, Leiby Benjamin E, Tasman William S. Preventing depression in age-related macular degeneration. Archives of General Psychiatry 2007;64(8):886-92. [DOI] [PubMed] [Google Scholar]
- Rovner BW, Casten RJ. Preventing late-life depression in age-related macular degeneration. American Journal of Geriatric Psychiatry 2008;16(6):454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 2011 {published data only}
- Tsai C-S, Wu C-L, Chou S-Y, Tsang H-Y, Hung T-H, Su J-A. Prevention of poststroke depression with milnacipran in patients with acute ischemic stroke: a double-blind randomized placebo-controlled trial. International Clinical Psychopharmacology 2011;26:263-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Almeida 2010 {published data only}
- Almeida OP, Marsh K, Alfonso H, Flicker L, Davis TME, Hankey GJ. B-vitamins reduce the long-term risk of depression after stroke:the VITATOPS-DEP Trial. Annals of Neurology 2010;68:503-10. [DOI] [PubMed] [Google Scholar]
Beglinger 2014 {published data only}
- Beglinger LJ, Adams WH, Langbehn D, Fiedorowicz JG, Jorge R, Biglan K, Caviness J, Olson B, Robinson RG, Kieburtz K, Paulsen JS. Results of the Citalopram to Enhance Cognition in HuntingtonDisease Trial. Movement Disorder 2014;29(3):401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borrelli 2019 {published data only}
- Borrelli J Jr, Starr A, Downs DL, North CS. Prospective Study of the Effectiveness of Paroxetine on the Onset of Posttraumatic Stress Disorder, Depression, and Health and Functional Outcomes After Trauma. Journal of Orthopaedic Trauma 2019;33(2):e58-e63. [DOI] [PubMed] [Google Scholar]
Brody 2005 {published data only}
- Brody BL, Roch-Levecq A-C, Thomas RG, Kaplan RM, Brown SI. Self-management of age-related macular degeneration at the 6-month follow-up. Archives of ophthalmology 2005;123:46-53. [DOI] [PubMed] [Google Scholar]
Browne 2013 {published data only}
- Browne AL, Appleton S, Fong K, Wood F, Coll F, Munck S, et al. A pilot randomized controlled trial of an early multidisciplinary model to prevent disability following traumatic injury. Disability & Rehabilitation 2013;35(14-15):1149-63. [DOI] [PubMed] [Google Scholar]
Burns 2007 {published data only}
- Burns A, Banerjee S, Morris J, Woodward Y, Baldwin R, Proctor R, et al. Treatment and prevention of depression after surgery for hip fracture in older people: randomized, controlled trials. Journal of the American Geriatrics Society 2007;55(1):75-80. [DOI] [PubMed] [Google Scholar]
Couch 1976 {published data only}
- Couch JR, Hassanein RS. Migraine and depression: effect of amitriptyline prophylaxis. Transactions of the American Neurological Association 1976;101:234-7. [PubMed] [Google Scholar]
Dean 1969 {published data only}
- Dean G. A double-blind trial with an antidepressant drug, imipramine, in multiple sclerosis. South African Medical Journal 1969;43(4):86-7. [PubMed] [Google Scholar]
de Jonge 2009 {published data only (unpublished sought but not used)}
- Jonge P, Hadj FB, Boffa D Zdrojewski C, Dorogi Y, So A, et al. Prevention of major depression in complex medically ill patients: preliminary results from a randomized, controlled trial. Psychosomatics 2009;50(3):227-33. [DOI] [PubMed] [Google Scholar]
- Stiefel F, Zdrojewski C, Bel Hadj F, Boffa D, Dorogi Y, So A, et al. Eligible patients were included and, if identified as complex, randomized to the intervention or care as usual by means of a computer-generated list. Psychotherapy and Psychosomatics 2008;77:247-56. [DOI] [PubMed] [Google Scholar]
Hackett 2010 {published data only}
- Hackett ML, Carter G, Crimmims D, Clarke T, Maddock K, Sturm JW. ImProving Outcomes after STroke clinical pilot trial protocol. International Journal of Stroke 2010;5:52-6. [DOI] [PubMed] [Google Scholar]
Hackett 2013 {published data only}
- Hackett ML, Carter G, Crimmins D, Clarke T, Arblaster L, Billot L, et al. ImPrOving outcomes after STroke (POST): results from the randomized clinical pilot trial. International Journal of Stroke 2013;8:707-10. [DOI] [PubMed] [Google Scholar]
Hood 2018 {published data only}
- Hood KK, Iturralde E, Rausch J, Weissberg-Benchell J. Preventing Diabetes Distress in Adolescents With Type 1 Diabetes: Results 1 Year After Participation in the STePS Program. Diabetes Care 2018;41(8):1623-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karp 2016 {published data only}
- Karp JF, Dew MA, Washed AS, Fitzgerald K, Bolon CA, Weiner DK, et al. Challenges and solutions for depression prevention research: methodology for a depression prevention trial for older adults with knee arthritis and emotional distress. American Journal Geriatric Psychiatry 2016;24(6):433-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kong 2007 {published data only}
- Kong Y, Dong W, Liu C. Fluoxetine for poststroke depression: A randomized placebo controlled clinical trial. Neural Regeneration Research 2007;2(3):162-5. [Google Scholar]
Kredentser 2018 {published data only}
- Kredentser MS, Blouw M, Marten N, Sareen J, Bienvenu OJ, Ryu J, Beatie BE, Logsetty S, Graff LA, Eggertson S, Sweatman S, Debroni B, Cianflone N, Arora RC, Zarychanski R, Olafson K. Preventing Posttraumatic Stress in ICU Survivors: A Single-Center Pilot Randomized Controlled Trial of ICU Diaries and Psychoeducation. Critical Care Medicine 2018;46(12):1914-22. [DOI] [PubMed] [Google Scholar]
Mossey 1996 {published data only}
- Mossey JM, Knott KA, Higgins M, Talerico K. Effectiveness of a psychosocial intervention, interpersonal counseling, for subdysthymic depression in medically ill elderly. Journal of Gerontology 1996;51A(4):172-8. [DOI] [PubMed] [Google Scholar]
Niedermaier 2004 {published data only}
- Niedermaier N, Bohrer E, Schulte K, Schlattmann P, Heuser I. Prevention and treatment of poststroke depression with mirtazapine in patients with acute stroke. Journal of Clinical Psychiatry 2004;65(12):1619-23. [DOI] [PubMed] [Google Scholar]
Pitceathly 2009 {published data only}
- Pitceathly C, Maguire P, Fletcher I, Parle M, Tomenson B, Creed F. Can a brief psychological intervention prevent anxiety or depressive disorders in cancer patients? A randomised controlled trial. Annals of Oncology 2009;20:928-34. [DOI] [PubMed] [Google Scholar]
Pols 2017 {published data only}
- *Pols AD, Dijk SE, Bosmans JE, Hoekstra T, Marwijk HW, Tulder MW, et al. Effectiveness of a stepped-care intervention to prevent major depression in patients with type 2 diabetes mellitus and/or coronary heart disease and subthreshold depression: a pragmatic cluster randomized controlled trial. PLOS One 2017;12(8):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk SE, Pols AD, Adriaanse MC, Bosmans JE, Elders PJ, Marwijk HW, et al. Cost-effectiveness of a stepped-care intervention to prevent major depression in patients with type2 diabetes mellitus and/or coronary heart disease and subthreshold depression: design of a cluster-randomized controlled trial. BMC Psychiatry 2013;13:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Read 2020 {published data only}
- Read J, Sharpe L, Burton AL, Arean PA, Raue PJ, McDonald S, Titov N, Gandy M, Dear BF. A randomized controlled trial of internet-delivered cognitive behaviour therapy to prevent the development of depressive disorders in older adults with multimorbidity. Journal of Affective Disorders 2020;264:464-3. [DOI] [PubMed] [Google Scholar]
Rovner 2014 {published data only}
- Deemer AD, Massof RW, Rovner BW, Casten RJ, Piersol CV. Functional outcomes of the low vision depression prevention trial in age-related macular degeneration. Investigative Ophthalmology & Visual Science 2017;58:1514-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovner BW, Casten RJ, Hegel MT, Massof RW, Leibly BE, Ho AC, et al. Low vision depression prevention trial in age-related macular degeneration: a randomized clinical trial. Ophthalmology 2014;121(11):2204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Aa 2015 {published data only}
- Corrections. Stepped care for depression and anxiety in visually impaired older adults: multicentre randomised controlled trial. BMJ 2016;353:i1995. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Aa HP, Rens GH, Bosmans JE, Comijs HC, Nispen RM. Economic evaluation of stepped-care versus usual care for depression and anxiety in older adults with vision impairment: randomized controlled trial. BMC Psychiatry 2017;17:280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aa HP, Rens GH, Comijs HC, Bosmans JE, Margrain TH, Nispen RM. Stepped-care to prevent depression and anxiety in visually impaired older adults – design of a randomised controlled trial. BMC Psychiatry 2013;13:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aa HP, Rens GH, Comijs HC, Margrain TH, Gallindo-Garre F, Twisk JW, et al. Stepped care for depression and anxiety in visually impaired older adults: multicentre randomised controlled trial. BMJ 2015;351:h6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeung 2014 {published data only}
- Yeung A, Kiat H, Denniss AR, Cheema BS, Bensoussan A, Machliss B, et al. Randomised controlled trial of a 12 week yoga intervention on negative affective states, cardiovascular and cognitive function in post-cardiac rehabilitation patients. BMC Complementary and Alternative Medicine 2014;14:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang L-S, Hu X-Y, Yao L-Y, Geng Y, Wei l-L, Zhang J-H, et al. Prophylactic effects of duloxetine on post-stroke depression symptoms: an open single-blind trial. European Neurology 2013;69:336-43. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ChiCTR‐TRC‐12003489 {unpublished data only}
- ChiCTR-TRC-12003489. Early intervention and prevention with acupuncture therapy on the post stroke depression: A randomized controlled clinical trial [针刺疗法早期预防中风后抑郁的随机对照试验]. chictr.org.cn/hvshowproject.aspx?id=6651 2012.
EudraCT‐2005‐005266‐37 {published data only}
- EudraCT-2005-005266-37. Influence of escitalopram on the incidence of depression and dementia following acute middle cerebral artery territory infarction. A randomized, placebo-controlled, double blind study. clinicaltrialsregister.eu/ctr-search/trial/2005-005266-37/DE (first received 1 April 2006).
Guneri 2006 {published data only}
- Guneri A, Gursoy E, Celebi A, Arslan E, Vardar N, Kaya S. The effects of antidepressant prophylaxis on prognosis of non-depressed acute stroke patients. Journal of Neurology 2006;253:162. [Google Scholar]
He 2004 {published data only}
- He P, Kong Y, Xu L-Z. Randomized controlled observation on the effect of early application of fluoxetine in preventing depression after stroke. Chinese Journal of Clinical Rehabilitation 2004;8(28):6016-7. [Google Scholar]
Li 2004 {published data only}
- Li J, He QY, Han MF. Recent effect of fluoxetine in improving neurologic impairment and preventing post-stroke depression in the early stage. Chinese Journal of Clinical Rehabilitation 2004;8:1208-9. [Google Scholar]
Lu 2010 {published data only}
- Lu R, Miao D, Wang H, Tan L. Prophylactic small dose amitriptyline in prevention of poststroke depression in first stroke patients [早期应用小剂量阿米替林干预首发脑卒中患者脑卒中后抑郁]. Chinese Journal of Neurology 2010;43(5):355-7. [Google Scholar]
Sander 2017 {unpublished data only}
- Sander L, Paganini S, Lin J, Schlicker S, Ebert DD, Buntrock C, et al. Effectiveness and cost-effectiveness of a guided internet- and mobile-based intervention for the indicated prevention of major depression in patients with chronic back pain—study protocol of the PROD-BP multicenter pragmatic RCT. BMC Psychiatry 2017;17(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Zyl 2006 {published data only}
- Zyl LT, Abdollah H, Parker K. Antidepressant prevention of depression and cardiovascular sequelae post-acute coronary syndrome: The 'AVERT' trial. Conference Paper 2006. [Google Scholar]
Wen 2006 {published data only}
- Wen Z, Li H. The influence of post-stroke prophylactic antidepression treatment on nerve functional rehabilitation. Acta Aacademiae Medical Quingdao Universitatis 2006;12:253-4. [Google Scholar]
Xu 2006 {published data only}
- Xu J, Wang JP, Liu J. Preventive effects of antidepressants on post-stroke depression [抗抑郁剂在脑卒中患者中的预防性应用]. Chinese Mental Health Journal 2006;20:186-8. [Google Scholar]
Zhong 2013 {published data only}
- Zhong Y. Preliminary study on effect of venlafaxine sustained-release tablet combined with pinaverium bromide in treating diarrhea-predominant irritable bowel syndrome without mood disorders. Chinese Journal of Gastroenterology 2013;18(11):658-62. [Google Scholar]
Zhou 2008 {published data only}
- Zhou Z, Liang L, Yan Y. Preventive Effects of Fluoxetine on Post-stroke Depression. Chinese Journal of Modern Applied Pharmacy 2008;25:262-4. [Google Scholar]
Zhu 2014 {published data only}
- Zhu J, Hu CM, Guo SS, Wang F, Zhou Y, Zhang SY. Wuling capsule played an assistant role in primary prevention of post-stroke depression: a clinical research [乌灵胶囊辅助治疗对卒中后抑郁一级预防作用的临床观察]. Chinese Journal of Integrated Traditional and Western Medicine 2014;34(6):676-9. [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616000886482 {unpublished data only}
- ACTRN12616000886482. Randomised trial aiming to prevent development of depression and improve quality of life in individuals with dementia (Alzheimer’s disease) [Randomised trial aiming to prevent development of depression and improve quality of life in individuals with dementia (Alzheimer’s disease) through a novel Cognitive Bias Modification intervention]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371029 (first received 4 July 2016).
Madsen 2017 {unpublished data only}
- Madsen MT, Isbrand A, Andersen UO, Andersen LJ, Taskiran M, Simonsen E, Gögenur I. The effect of MElatonin on Depressivesymptoms, Anxiety, CIrcadian and Sleepdisturbances in patients after acute coronary syndrome (MEDACIS): study protocol for a randomized controlled trial. Trials 2017;18(1):81. [DOI: 10.1186/s13063-017-1806-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AHRQ 2012
- Agency for Healthcare Research and Quality (AHRQ), RTI International–University of North Carolina Evidence-based Practice Center. Practice-based interventions addressing concomitant depression and chronic medical conditions in the primary care setting: Comparative effectiveness review no. 75 (prepared by the RTI international–university of North Carolina evidence-based practice center under contract No. 290-2007-10056-I). AHRQ publication no. 12-EHC106-EF. Rockville, MD: agency for healthcare research and quality. August 2012. Available from http://effectivehealthcare.ahrq.gov/ehc/products/297/1219/CER75_Concomitant-Depression_FinalReport_20120808.pdf. [PubMed]
Akechi 2008
- Akechi T, Okuyama T, Onishi J, Morita T, Furukawa TA. Psychotherapy for depression among incurable cancer patients. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD005537. [DOI: 10.1002/14651858.CD005537.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-III). 3rd edition. Washington, DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-III-R). 3rd edition - revised. Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). 4th edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV-TR). 4th edition. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). 5th edition. Arlington, Va: American Psychiatric Association, 2013. [Google Scholar]
Arroll 2009
- Arroll B, Elley CR, Fishman T, Goodyear-Smith FA, Kenealy T, Blashki G, et al. Antidepressants versus placebo for depression in primary care. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007954. [DOI: 10.1002/14651858.CD007954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumeister 2007
- Baumeister H, Härter M. Prevalence of mental disorders based on general population surveys. Social Psychiatry and Psychiatric Epidemiology 2007;42(7):537-46. [DOI] [PubMed] [Google Scholar]
Baumeister 2011a
- Baumeister H, Hutter N, Bengel J, Härter M. Quality of life in medically ill persons with comorbid mental disorders: a systematic review and meta-analysis. Psychotherapy and Psychosomatics 2011;80(12):275-86. [DOI] [PubMed] [Google Scholar]
Baumeister 2011b
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD008012. [DOI: 10.1002/14651858.CD008012.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumeister 2012a
- Baumeister H, Knecht A, Hutter N. Direct and indirect costs in persons with chronic back pain and comorbid mental disorders - A systematic review. Journal of Psychosomatic Research 2012;73(2):79-85. [DOI] [PubMed] [Google Scholar]
Baumeister 2012b
- Baumeister H, Parker G. Meta-review of depressive subtyping models. Journal of Affective Disorders 2012;139:126-40. [DOI] [PubMed] [Google Scholar]
Baumeister 2012c
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD008381. [DOI: 10.1002/14651858.CD008381.pub2] [DOI] [PubMed] [Google Scholar]
Bech 2001
- Bech P, Rasmussen NA, Olsen LR, Noerholm V, Abildgaard W. The sensitivity and specificity of the Major Depression Inventory, using the Present State Examination as the index of diagnostic validity. Journal of Affective Disorders 2001;66(2-3):159-64. [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561-71. [DOI] [PubMed] [Google Scholar]
Beltman 2010
- Beltman MW, Oude Voshaar RC, Speckens AE. Cognitive-behavioural therapy for depression in people with a somatic disease: meta-analysis of randomised controlled trials. British Journal of Psychiatry 2010;197:11-19. [DOI] [PubMed] [Google Scholar]
Cipriani 2009
- Cipriani A, Santilli C, Furukawa TA, Signoretti A, Nakagawa A, McGuire H, et al. Escitalopram versus other antidepressive agents for depression. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD006532. [DOI: 10.1002/14651858.CD006532.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2012
- Cipriani A, Purgato M, Furukawa TA, Trespidi C, Imperadore G, Signoretti A, et al. Citalopram versus other anti-depressive agents for depression. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD006534. [DOI: 10.1002/14651858.CD006534.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2009
- Clarke DM, Currie KC. Depression, anxiety and their relationship with chronic diseases: a review of the epidemiology, risk and treatment evidence. Medical Journal of Australia 2009;190(7):54-60. [DOI] [PubMed] [Google Scholar]
Cuijpers 2008
- Cuijpers P, Straten A, Smit F, Mihalopoulos C, Beekman A. Preventing the onset of depressive disorders: a meta-analytic review of psychological interventions. American Journal of Psychiatry 2008;165(10):1272-80. [DOI] [PubMed] [Google Scholar]
de Geus 2006
- Geus EJ. Genetic pleiotropy in depression and coronary artery disease. Psychosomatic Medicine 2006;68(2):185-6. [DOI] [PubMed] [Google Scholar]
DGPPN 2012
- DGPPN, BÄK, KBV, AWMF, AkdÄ, BPtK, et al. S3-Leitlinie/Nationale VersorgungsLeitlinie Unipolare Depression Langfassung. Version 1.3, Januar 2012. Available from http://www.versorgungsleitlinien.de/themen/depression/pdf/s3_nvl_depression_lang.pdf.
Durand 2012
- Durand VM, Barlow DH. An integrative approach to psychopathology. In: Essentials of Abnormal Psychology. 6th edition. Cengage Learning Emea, 2012:30-68. [Google Scholar]
Endicott 1978
- Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Archives in General Psychiatry 1978;35(7):837-44. [DOI] [PubMed] [Google Scholar]
Evans 2005
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, et al. Mood disorders in the medically ill: scientific review and recommendations. Biological Psychiatry 2005;58(3):175-89. [DOI] [PubMed] [Google Scholar]
First 1996
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders, clinician version (SCID-CV). Washington, DC: American Psychiatric Press, Inc., 1996. [Google Scholar]
Freedland 2010
- Freedland KE, Carney RM. Depression and medical illness. In: Gotlib IA, Hammen CL, editors(s). Handbook of Depression. 2nd edition. New York: The Guilford Press, 2010:113-41. [Google Scholar]
Gill 2000
- Gill D, Hatcher S. Antidepressants for depression in medical illness. Cochrane Database of Systematic Reviews 2000, Issue 4. Art. No: CD001312. [DOI: 10.1002/14651858.CD001312.pub2] [DOI] [PubMed] [Google Scholar]
Gordon 1983
- Gordon RS Jr. An operational classification of disease prevention. Public Health Reports 1983;98(2):107-9. [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime, Inc.) GRADEpro Guideline Development Tool. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2020. Available at gradepro.org.
Gunn 2012
- Gunn JM, Ayton DR, Densley K, Pallant JF, Chondros P, Herrman HE, et al. The association between chronic illness, multimorbidity and depressive symptoms in an Australian primary care cohort. Social Psychiatry and Psychiatric Epidemiology 2012;47(2):175-84. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. rating the quality of evidenced - publication bias. Journal of Clinical Epidemiology 2011;64(12):1277-82. [DOI] [PubMed] [Google Scholar]
Hackett 2008a
- Hackett ML, Anderson CS, House A, Halteh C. Interventions for preventing depression after stroke (Review). Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No: CD003689. [DOI: 10.1002/14651858.CD003689.pub3] [DOI] [PubMed] [Google Scholar]
Hackett 2008b
- Hackett ML, Anderson CS, House A, Xia J. Interventions for treating depression after stroke. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD003437. [DOI: 10.1002/14651858.CD003437.pub3] [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harmer 2017
- Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 2017;4(5):409-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Härter 2007a
- Härter M, Baumeister H, Reuter K, Jacobi F, Höfler M, Bengel J, et al. Increased 12-month prevalence rates of mental disorders in patients with chronic somatic diseases. Psyhotherapy and Psychosomatics 2007;76(6):354-60. [DOI] [PubMed] [Google Scholar]
Härter 2007b
- Härter M, Baumeister H, Bengel J. Etiology of mental disorders in the chronic physically ill [Ätiologie psychischer Störungen bei chronischen körperlichen Erkrankungen]. In: Psychische Störungen bei körperlichen Erkrankungen. Heidelberg: Springer, 2007:2-11. [Google Scholar]
Haschke 2012
- Haschke A, Hutter N, Baumeister H. Indirect costs in patients with coronary artery disease and mental disorders: a systematic review and meta-analysis. International Journal of Occupational Medicine and Enviroment Health 2012;4:319-29. [DOI] [PubMed] [Google Scholar]
Herzog 2019
- Herzog P, Lauff S, Rief W, Brakemeier E-L. Assessing the unwanted: A systematic review of instrumentsused to assess negative effects of psychotherapy. Brain and Behavior 2019;9(12):e01447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Holahan 2010
- Holahan CJ, Pahl SA, Cronkite RC, Holahan CK, North RJ, Moos RH. Depression and vulnerability to incident physical illness across 10 years. Journal of Affective Disorders 2010;123(1-3):222-9. [DOI] [PubMed] [Google Scholar]
Hutter 2010
- Hutter N, Schnurr A, Baumeister H. Healthcare costs in patients with diabetes mellitus and comorbid mental disorders - a systematic review. Diabetologia 2010;53:2470-9. [DOI] [PubMed] [Google Scholar]
Hutter 2011
- Hutter N, Knecht A, Baumeister H. Health care costs in persons with asthma and comorbid mental disorders: A systematic review. General Hospital Psychiatry 2011;33:443-53. [DOI] [PubMed] [Google Scholar]
Katon 2002
- Katon W, Ciechanowski P. Impact of major depression in chronic medical illness. Journal of Psychosomatic Research 2002;53:859-63. [DOI] [PubMed] [Google Scholar]
Katon 2003
- Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biological Psychiatry 2003;54(3):216-26. [DOI] [PubMed] [Google Scholar]
Katon 2007
- Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. General Hospital Psychiatry 2007;29:147-55. [DOI] [PubMed] [Google Scholar]
Kessler 2003
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289(23):3095-105. [DOI] [PubMed] [Google Scholar]
Klatte 2018
- Klatte R, Strauss B, Flückiger C, Rosendahl J. Adverse effects of psychotherapy: protocol for a systematic review and meta-analysis. Systematic Reviews 2018;7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krishnan 2005
- Krishnan KR. Treatment of depression in the medically ill. Journal of Clinical Psychopharmacology 2005;25(1):14-8. [DOI] [PubMed] [Google Scholar]
Kroenke 2001
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine 2001;16(9):606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Eisinga A, McDonald S, Paul N. Enhancing access to reports of randomized trials published world-wide – the contribution of EMBASE records to the CochraneCentral Register of Controlled Trials (CENTRAL) in The CochraneLibrary. Emerging Themes in Epidemiology 2008;5:13. [DOI: 10.1186/1742-7622-5-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
McAuley 2000
- McAuley L, Pham B, Tugwel P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 2000;356(9237):1228-31. [DOI] [PubMed] [Google Scholar]
McCaffery 2006
- McCaffery JM, Frasure-Smith N, Dubé M-P, Théroux P, Rouleau GA, Duan Q, et al. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosomatic Medicine 2006;68:187-200. [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382-9. [DOI] [PubMed] [Google Scholar]
Mrazek 1994
- Mrazek PJ, Haggerty RJ. Reducing risks for mental disorders: Frontiers for preventive intervention research. Washington DC: National Academy Press, 1994. [PubMed] [Google Scholar]
Muñoz 1996
- Muñoz RF, Mrazek PJ, Haggerty RJ. Institute of medicine report on prevention of mental disorders. Summary and commentary. American Psychologist 1996;51(11):1116-22. [DOI] [PubMed] [Google Scholar]
Natale 2019
- Natale P, Palmer SC, Ruospo M, Saglimbene VM, Rabindranath KS, Strippoli GF. Psychosocial interventions for preventing and treating depression in dialysis patients. Cochrane Database of Systematic Reviews 2019, Issue 12. Art. No: CD004542. [DOI: 10.1002/14651858.CD004542.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health and Care Excellence. Depression in adults with a chronic physical health problem: Treatment and management. NICE guidelines [CG91]. October 2009. Available from http://www.nice.org.uk/guidance/CG91. [PubMed]
O'Connell 2009
- O'Connell ME, Boat T, Warner KE. Preventing mental, emotional, and behavioral disorders among young people. Progress and possibilities. Washington DC: The National Academies Press, 2009. [PubMed] [Google Scholar]
Ostuzzi 2018
- Ostuzzi G, Matcham F, Dauchy S, Barbui C, Hotopf M. Antidepressants for the treatment of depression in people with cancer. Cochrane Database of Systematic Reviews 2018, Issue 4. Art. No: CD011006. [DOI: 10.1002/14651858.CD011006.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patten 2001
- Patten SB. Long-term medical conditions and major depression in a Canadian population study at waves 1 and 2. Journal of Affective Disorders 2001;63(1-3):35-41. [DOI] [PubMed] [Google Scholar]
Radloff 1977
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1977;1(3):385-401. [Google Scholar]
Raison 2005
- Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. Journal of Clinical Psychiatry 2005;66(1):41-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rayner 2010
- Rayner L, Price A, Evans A, Valsraj K, Higginson IJ, Hotopf M. Antidepressants for depression in physically ill people. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No: CD007503. [DOI: 10.1002/14651858.CD007503.pub2] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richards 2017
- Richards SH, Anderson L, Jenkinson CE, Whalley B, Rees K, Bennett P, Liu Z, West R, Thompson DR, Taylor RS. Psychological interventions for coronary heart disease. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD002902. [DOI: 10.1002/14651858.CD002902.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rush 2003
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry 2003;54(5):573-83. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. [Google Scholar]
Sheehan 1998
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 1998;59 Suppl 20:22-33. [PubMed] [Google Scholar]
Shinohara 2013
- Shinohara K, Honyashiki M, Imai H, Hunot V, Caldwell DM, Davies P, et al. Behavioural therapies versus other psychological therapies for depression. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD008696. [DOI: 10.1002/14651858.CD008696.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2005
- Simon GE, Korff M, Lin E. Clinical and functional outcomes of depression treatment in patients with and without chronic medical illness. Psychological Medicine 2005;35:271-9. [DOI] [PubMed] [Google Scholar]
Stockton 2004
- Stockton P, Gonzales JJ, Stern NP, Epstein SA. Treatment patterns and outcomes of depressed medically ill and non-medically ill patients in community psychiatric practice. General Hospital Psychiatry 2004;26:2-8. [DOI] [PubMed] [Google Scholar]
Thomas 2006
- Thomas PW, Thomas S, Hillier C, Galvin K, Baker R. Psychological interventions for multiple sclerosis. Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No: CD004431. [DOI: 10.1002/14651858.CD004431.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Marvijk 2012
- Marwijk H, Allick G, Wegman F, Bax A, Riphagen II. Alprazolam for depression. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD007139. [DOI: 10.1002/14651858.CD007139.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whalley 2011
- Whalley B, Rees K, Davies P, Bennett P, Ebrahim S, Liu Z, et al. Psychological interventions for coronary heart disease. Cochrane Database of Systematic Reviews 2011, Issue 8. Art. No: CD002902. [DOI: 10.1002/14651858.CD002902.pub3] [DOI] [PubMed] [Google Scholar]
WHO 1978
- World Health Organization. The ninth revision of the international classification of diseases and related health problems (ICD-9). Geneva: World Health Organization, 1978. [Google Scholar]
WHO 1990
- World Health Organization. Composite international diagnostic interview. Geneva: World Health Organization, 1990. [Google Scholar]
WHO 1992
- World Health Organization. ICD-10 classification of mental and behavioural disorders. Available from http://www.who.int/substance_abuse/terminology/icd_10/en/.
WHO 2007
- World Health Organization. World health statistics. 2007. Available from http://www.who.int/gho/publications/world_health_statistics/en/.
WHO 2008
- World Health Organization. Part 4 - Burden of disease: DALYs. The global burden of disease: 2004 update. 2008. Available from http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/.
WHO 2016
- World Health Organization. Preventing depression in the WHO European Region. 2016. Available from http://www.ncbi.nlm.nih.gov/books/NBK304267/.
Wichers 2005
- Wichers MC, Koek GH, Robaeys G, Praamstra AJ, Maes M. Early increase in vegetative symptoms predicts IFN-α-induced cognitive-depressive changes. Psychological Medicine 2005;35:433-41. [DOI] [PubMed] [Google Scholar]
Wing 1974
- Wing JK, Cooper JE, Sartorius N. The measurement and classification of psychiatric symptoms: An instructional manual for the PSE and CATEGO programs. New York: CambridgeUniversity Press, 1974. [Google Scholar]
Wing 1990
- Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Archives of General Psychiatry 1990;47(6):589-93. [DOI] [PubMed] [Google Scholar]
Wittchen 2010
- Wittchen HU, Jacobi F, Klose M, Ryl L. Gesundheitsberichterstattung des Bundes - Depressive Erkrankungen - Heft 51. Berlin: Robert Koch Institut, 2010. [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI] [PubMed] [Google Scholar]


