Abstract
Background
Cardiovascular disease (CVD) is the leading cause of death worldwide. Lifestyle changes are at the forefront of preventing the disease. This includes advice such as increasing physical activity and having a healthy balanced diet to reduce risk factors. Intermittent fasting (IF) is a popular dietary plan involving restricting caloric intake to certain days in the week such as alternate day fasting and periodic fasting, and restricting intake to a number of hours in a given day, otherwise known as time‐restricted feeding. IF is being researched for its benefits and many randomised controlled trials have looked at its benefits in preventing CVD.
Objectives
To determine the role of IF in preventing and reducing the risk of CVD in people with or without prior documented CVD.
Search methods
We conducted our search on 12 December 2019; we searched CENTRAL, MEDLINE and Embase. We also searched three trials registers and searched the reference lists of included papers. Systematic reviews were also viewed for additional studies. There was no language restriction applied.
Selection criteria
We included randomised controlled trials comparing IF to ad libitum feeding (eating at any time with no specific caloric restriction) or continuous energy restriction (CER). Participants had to be over the age of 18 and included those with and without cardiometabolic risk factors. Intermittent fasting was categorised into alternate‐day fasting, modified alternate‐day fasting, periodic fasting and time‐restricted feeding.
Data collection and analysis
Five review authors independently selected studies for inclusion and extraction. Primary outcomes included all‐cause mortality, cardiovascular mortality, stroke, myocardial infarction, and heart failure. Secondary outcomes include the absolute change in body weight, and glucose. Furthermore, side effects such as headaches and changes to the quality of life were also noted. For continuous data, pooled mean differences (MD) (with 95% confidence intervals (CIs)) were calculated. We contacted trial authors to obtain missing data. We used GRADE to assess the certainty of the evidence.
Main results
Our search yielded 39,165 records after the removal of duplicates. From this, 26 studies met our criteria, and 18 were included in the pooled analysis. The 18 studies included 1125 participants and observed outcomes ranging from four weeks to six months. Of quantitatively analysed data, seven studies compared IF with ab libitum feeding, eight studies compared IF with CER, and three studies compared IF with both ad libitum feeding and CER. Outcomes were reported at short term (≤ 3 months) and medium term (> 3 months to 12 months) follow‐up.
None of the included studies reported on all‐cause mortality, cardiovascular mortality, stroke, myocardial infarction or heart failure.
Body weight was reduced with IF compared to ad libitum feeding in the short term (MD ‐2.88 kg, 95% CI ‐3.96 to ‐1.80; 224 participants; 7 studies; low‐certainty evidence). We are uncertain of the effect of IF when compared to CER in the short term (MD ‐0.88 kg, 95% CI ‐1.76 to 0.00; 719 participants; 10 studies; very low‐certainty evidence) and there may be no effect in the medium term (MD ‐0.56 kg, 95% CI ‐1.68 to 0.56; 279 participants; 4 studies; low‐certainty evidence).
We are uncertain about the effect of IF on glucose when compared to ad libitum feeding in the short term (MD ‐0.03 mmol/L, 95% CI ‐0.26 to 0.19; 95 participants; 3 studies; very‐low‐certainty of evidence) and when compared to CER in the short term: MD ‐0.02 mmol/L, 95% CI ‐0.16 to 0.12; 582 participants; 9 studies; very low‐certainty; medium term: MD 0.01, 95% CI ‐0.10 to 0.11; 279 participants; 4 studies; low‐certainty evidence).
The changes in body weight and glucose were not deemed to be clinically significant.
Four studies reported data on side effects, with some participants complaining of mild headaches. One study reported on the quality of life using the RAND SF‐36 score. There was a modest increase in the physical component summary score.
Authors' conclusions
We are uncertain about the effects of intermittent fasting on clinical events such as mortality, myocardial infarction and heart failure due to lack of data for these outcomes. The individual meta‐analyses show that intermittent fasting may be effective in reducing weight when compared to ad libitum feeding and may be as effective as continuous energy restriction. Despite this, these changes appear to be clinically insignificant at short‐term follow‐up. The quality of the available evidence is low to very low which means that many areas of uncertainty remain. Further research is needed to understand which patient groups would and would not benefit from intermittent fasting (e.g. patients with diabetes or eating disorders) as well as the effect on longer‐term outcomes such as all‐cause mortality and myocardial infarction.
Plain language summary
Does limiting the times you eat (intermittent fasting) prevent cardiovascular disease?
What is cardiovascular disease?
Cardiovascular disease (CVD) is the leading cause of death worldwide. Smoking, diabetes and being overweight are risk factors for CVD, which means that they increase your chances of developing CVD. CVD can often be prevented by a healthy lifestyle, such as keeping to a healthy weight or losing weight if you need to.
Following a diet
Some people choose to lose weight by following a diet; for example, by eating less fat, or by reducing the number of calories they eat. Intermittent fasting is a type of diet involving patterns of eating and fasting (not eating foods); it does not limit what foods you eat, but limits when you can eat them. Eating patterns in intermittent fasting include: fasting for one or two days each week; fasting every other day; or eating only during certain hours and fasting for at least 12 hours every day.
Why we did this Cochrane Review
Diets that involve intermittent fasting are becoming popular. We wanted to find out if intermittent fasting could reduce or prevent CVD.
What did we do?
We searched for studies that tested intermittent fasting against 'usual eating' (someone eats whatever foods they want whenever they like), or against 'energy restriction' diets (someone limits the number of calories they eat).
We wanted to find out whether intermittent fasting affected mortality, cardiovascular mortality, risk of stroke, heart attack or heart failure. We also looked at whether intermittent fasting affected body weight and blood sugar levels.
Search date: we included evidence published up to 12 December 2019.
What we found
We found 26 relevant studies; we then used the results from 18 of the studies to compare the different diets. The 18 studies included 1125 adults (aged over 18 years). Some people in the studies had risk factors for CVD and some people had no risk factors. Most studies were funded by universities and research centres; two studies were funded by companies that make diet foods.
The studies compared intermittent fasting against usual eating (in seven studies); energy restriction diets (eight studies); and usual eating and energy restriction diets (three studies). The studies lasted from four weeks to six months. Results were reported after three months (short‐term), and between three and 12 months (medium‐term).
We didn't find any data on mortality, cardiovascular mortality or risk of stroke, heart attack or heart failure.
We found that people may lose more weight by intermittent fasting than by usual eating over three months (evidence from 7 studies in 224 people); but not when compared against energy restriction diets for three months (10 studies; 719 people) or longer (3 to 12 months; 4 studies; 279 people).
We also found that intermittent fasting did not appear to affect blood sugar levels when compared against usual eating over three months (3 studies; 95 people); energy restriction diets over three months (9 studies; 582 people); or energy restriction diets over 3 to12 months (4 studies; 279 people).
The weight losses and changes in blood sugars reported in the studies were small. These changes were not deemed to be clinically significant.
Only four studies reported unwanted effects of intermittent fasting: some people taking part reported mild headaches. Only one study reported on people's well‐being, showing a small increase in scores for physical well‐being.
Our confidence in our results
We are not confident in our results. We found limitations in the ways that the studies were designed, conducted and reported; and in some studies, the results varied widely, or were not consistent. Our results are likely to change if more evidence becomes available.
Key messages
We did not find enough good certainty evidence to know whether intermittent fasting could prevent CVD. We found that intermittent fasting may help people to lose more weight than 'eating as usual' (not dieting) but was similar to energy restriction diets. We need further research to test the benefits and potential harms of intermittent fasting, and to test if it might affect how many people die or develop CVD.
Summary of findings
Summary of findings 1. IF compared to ad libitum (short term) for the prevention of cardiovascular disease.
| IF compared to ad libitum (short term) for the prevention of cardiovascular disease | ||||||
| Patient or population: the prevention of cardiovascular disease Setting: outpatient Intervention: IF Comparison: ad libitum (short term) (≤ 3 months) | ||||||
|
Outcomes (4 to 12 weeks follow‐up) |
Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (RCTs) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ad libitum | Risk with IF | |||||
| All‐cause mortality | No trials reported data on these outcomes | |||||
| CV Mortality | ||||||
| Stroke | ||||||
| MI | ||||||
| Heart failure | ||||||
| Absolute change in body weight (kg) (4 to 12 weeks follow‐up) |
The mean change from baseline ranged from ‐1.4 to 1 kg. | MD 2.88 lower (3.96 lower to 1.80 lower) | ‐ | 224 (7 studies) | ⊕⊕⊝⊝ lowa,b |
The difference between groups is not clinically meaningful, as it represents less than a 5% reduction in baseline body weight. |
| Absolute change in Glucose (mmol/L) (8 to 12 weeks follow‐up) |
The mean change from baseline ranged from ‐0.33 to 0.01 mmol/L. | MD 0.03 lower (0.26 lower to 0.19 higher) | ‐ | 95 (3 studies) | ⊕⊝⊝⊝ very lowc,d |
The difference between groups is not clinically meaningful, as it represents less than a 5% reduction in baseline glucose. |
| *The risk in the intervention group (and its 95% confidence interval) is
based on the assumed risk in the comparison group and the relative effect
of the intervention (and its 95% CI). CI: confidence interval; CV: cardiovascular IF: Intermittent fasting; MD: mean difference; MI: myocardial infarction; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a We downgraded by one level for inconsistency, due to substantial heterogeneity (I2 = 85%).
b We downgraded by one level for study limitations, due to high risk of performance bias in all studies, an unclear or high risk of selection bias (inadequate allocation concealment), and an unclear risk of detection bias in 6 of the 7 studies.
c We downgraded by one level for study limitations, due to high risk of performance bias in all studies, an unclear or high risk of selection bias (inadequate allocation concealment), and a high or unclear risk of attrition bias in 2 of the 3 studies.
d We downgraded by two levels for imprecision, due to very low sample size and a wide confidence interval that includes both a possible benefit and a possible harm.
Summary of findings 2. IF compared to CER (short term) for the prevention of cardiovascular disease.
| IF compared to CER (short term) for the prevention of cardiovascular disease | ||||||
| Patient or population: the prevention of cardiovascular disease Setting: outpatient Intervention: IF Comparison: CER (Short term) (≤ 3 months) | ||||||
|
Outcomes (4 to 12 weeks follow‐up) |
Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (RCTs) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CER | Risk with IF | |||||
| All‐cause mortality | No trials reported data on these outcomes | |||||
| CV Mortality | ||||||
| Stroke | ||||||
| MI | ||||||
| Heart failure | ||||||
| Absolute change in body weight (kg) (4 to 12 weeks follow‐up) |
The mean change from baseline ranged from ‐7.4kg to ‐1.7kg | MD 0.88 lower (1.76 lower to 0.0 higher) | ‐ | 719 (10 studies) | ⊕⊝⊝⊝ very lowa,b,c |
The difference between groups is not clinically meaningful, as it represents less than a 5% reduction in baseline body weight. |
| Absolute change in Glucose (mmol/L) (4 to 12 weeks follow‐up) |
The mean change from baseline ranged from ‐0.4 to 1.1 mmol/L. | MD 0.02 lower (0.16 lower to 0.12 higher) | ‐ | 582 (9 studies) | ⊕⊝⊝⊝ very lowc,d,e |
The difference between groups is not clinically meaningful, as it represents less than a 5% reduction in baseline glucose. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CER: continuous energy restriction; CI: confidence interval; CV: cardiovascular IF: Intermittent fasting; MD: mean difference; MI: myocardial infarction; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a We downgraded by one level for inconsistency, due to substantial heterogeneity (I2 = 66%).
b We downgraded by one level for study limitations, due to high risk of performance bias in all studies, an unclear or high risk of selection bias (inadequate allocation concealment), and an unclear risk of detection bias in 5 of the 10 studies.
c We downgraded by one level for imprecision, due to a wide confidence interval that includes both a possible benefit and a possible harm.
d We downgraded by one level for inconsistency, due to substantial heterogeneity (I2 = 73%).
e We downgraded by one level for study limitations, due to an unclear or high risk of selection bias (inadequate allocation concealment), and an unclear risk of detection bias in 5 of the 10 studies.
Summary of findings 3. IF compared to CER (medium term) for the prevention of cardiovascular disease.
| IF compared to CER (medium term) for the prevention of cardiovascular disease | ||||||
| Patient or population: the prevention of cardiovascular disease Setting: outpatient Intervention: IF Comparison: CER (medium term) (> 3 months to 12 months) | ||||||
|
Outcomes (4 months ‐ 6 months follow‐up) |
Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (RCTs) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CER | Risk with IF | |||||
| All‐cause mortality | No trials reported data on these outcomes | |||||
| CV Mortality | ||||||
| Stroke | ||||||
| MI | ||||||
| Heart failure | ||||||
| Absolute change in body weight (kg) (4 months ‐ 6 months follow‐up) |
The mean change from baseline ranged from ‐9.4kg to ‐5kg | MD 0.56 lower (1.68 lower to 0.56 higher) | ‐ | 279 (4 studies) | ⊕⊕⊝⊝ lowa,b |
The difference between groups is not clinically meaningful, as it represents less than a 5% reduction in baseline body weight. |
| Absolute change in glucose (mmol/L) (4 months ‐ 6 months follow‐up) |
The mean change from baseline ranged from ‐0.2 to 0.09 mmol/L. | MD 0.01 higher (0.10 lower to 0.11 higher) | ‐ | 279 (4 studies) | ⊕⊕⊝⊝ lowa,b |
The difference between groups is not clinically meaningful, as it represents less than a 5% reduction in baseline glucose. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CER: continuous energy restriction; CI: confidence interval; CV: cardiovascular IF: Intermittent fasting; MD: mean difference; MI: myocardial infarction; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a We downgraded by one level for study limitations, due to high risk of performance bias in all studies, an unclear or high risk of selection bias (inadequate allocation concealment), and an unclear risk of detection bias in 2 of the 4 studies.
b We downgraded by one level for imprecision, due to a wide confidence interval that includes both a possible benefit and a possible harm.
Background
Description of the condition
Cardiovascular disease (CVD) is the leading cause of death worldwide and is recognised by the United Nations as a major global health burden (Westerman 2017). CVD encompasses a variety of diseases. These include heart failure, hypertension, ischaemic heart disease, such as stable angina and acute coronary syndromes, cerebrovascular disease such as stroke, valvular abnormalities such as aortic stenosis and arrhythmias such as atrial fibrillation (Lopez 2020; Stewart 2017).
CVD is largely due to the pathological process of atherosclerosis. Atherosclerosis is a chronic inflammatory process involving build up of lipids within the inner wall of arteries, stimulating infiltration by immunocytes and the subsequent formation of a fibrous cap by vascular smooth muscle cells (Bergheanu 2017). As the atherosclerotic plaque develops, a central necrotic core is formed containing necrotic cells, cell debris and cholesterol crystals (Hansson 2011). The plaque can lead to blood vessel stenosis and progress to plaque rupture causing a myocardial infarction (MI) (Sakakura 2013).
Multiple factors increase the risk of developing CVD including leading a sedentary lifestyle, smoking, consuming a diet high in salt, fatty acids and sugar, being overweight/obese, having poor lipid control (elevated plasma low‐density lipoprotein (LDL) and total cholesterol levels), having a raised blood pressure, as well as suffering from diabetes (ESC 2016).
Obesity is strongly associated with CVD, increasing the risk of developing heart failure, coronary heart disease, hypertension and atrial fibrillation (Carbone 2019). Specifically, recent data suggest that obesity increases the risk of heart failure with preserved ejection fraction (HFpEF) (Pandey 2017). Additionally, obesity causes a chronic state of low‐grade inflammation in the body leading to increased macrophage activation and plaque instability, further driving coronary heart disease (De Rosa 2017; Lovren 2015). The prevalence of obesity has also increased drastically over the last few decades and may be a key factor in increasing the prevalence of CVD (Capewell 2008).
Diabetes mellitus (DM) is also strongly associated with CVD (Schmidt 2019). DM leads to 5.2 million deaths globally, increasing the likelihood of developing peripheral arterial disease and heart failure (Glovaci 2019). One of the biggest causes of Type 2 DM is obesity. Obesity leads to end‐organ adipose tissue becoming insulin‐resistant (Hardy 2012). Multiple trials have been published looking at weight loss for the remission of Type 2 DM, including the DiRECT trial which recorded 46% Type 2 DM remission in the weight loss group as opposed to 4% remission in the control group (Lean 2017).
The World Health Organization (WHO) estimates that 80% of premature heart disease and stroke is preventable (WHO EU 2016). Despite CVD mortality declining in the UK by 68% between 1980 and 2013, hospital admissions have increased (Bhatnagar 2016). CVD is also the leading cause of disability‐adjusted life years around the world (Perk 2012), and lead to approximately 31% of global deaths in 2016 (WHO 2016).
Prevention of CVD is a top priority of many public health institutions, promoted by advising patients to maintain a healthy lifestyle. The National Institute for Health and Care Excellence (NICE) guidelines for the prevention of CVD focus on reducing salt intake, saturated fats and increasing physical exercise (NICE 2010). The American Heart Association (AHA) along with the American College of Cardiology (ACC) have published an extensive report on the prevention of CVD (ACC/AHA 2019). The European Society of Cardiology (ESC) has also published 2016 guidelines on CVD prevention in clinical practice (ESC 2016). The World Heart Federation (WHF) is also committed to preventing CVD and has published guidelines on rheumatic heart disease, atherosclerotic disease, metabolic syndrome, as well as general heart disease prevention (WHF 2017). Finally, the WHO also has guidance on the assessment and management of cardiovascular risk (WHO 2007).
Description of the intervention
Intermittent fasting (IF) is a dietary regimen involving energy restriction for specific periods of time (Anton 2018). This may mean consuming all the daily caloric intake in a certain time frame e.g. eight hours, or it may mean eating one day and completely fasting the next day. Intermittent fasting is also referred to as intermittent energy restriction (IER) in the literature. There are a variety of different types of intermittent fasting including alternate‐day fasting (ADF), periodic fasting (PF), time‐restricted feeding (TRF) and religious fasts (Anton 2018; de Cabo 2019) described below.
Periodic fasting: this is defined as a cyclical feeding pattern that entails fasting (consumption of 25% or less of required calories). This includes, but is not exclusive to fasting for one to two days per week with ad libitum feeding for the remaining days, in a once‐weekly or a twice‐weekly regimen (Anton 2018;Cioffi 2018).
Alternate‐day fasting (ADF): this is defined as a cyclical feeding pattern that entails complete fasting (consumption of no calories) for a period of 24 hours, followed by ad libitum feeding for 24 hours (Harris 2018).
Modified alternate‐day fasting: this is a subtype of ADF which involves the consumption of 25% or less of maintenance calories for a period of 24 hours, followed by ad libitum feeding for the next 24 hours (Harris 2018).
Time‐restricted feeding (TRF): this is defined as complete fasting (consumption of no calories) for at least 12 hours per day with ad libitum feeding for the rest of the day; repeated every day (Cioffi 2018).
Common religious fasts:
-
the Islamic Ramadhan fast: Muslims across the world fast for one month every year from sunrise to sunset. This fast refraining from eating and drinking (Trepanowski 2010). The fast varies in the number of hours depending on where in the world the observers are fasting as it depends on the time of sunrise and sunset. The month of Ramadhan shifts every year depending on the Islamic lunar calendar (Adler‐Lazarovits 2019). During the period between sunset and sunrise, Muslims are free to consume food and drink with no caloric restriction (ad libitum feeding) (Trepanowski 2010).
Greek Orthodox fasts: observers abstain from dairy products, eggs, and meat for 40 days during the nativity fast, for 48 days during the Lent fast, and for 15 days during the assumption fast (Trepanowski 2010).
There has been a growing interest in the potential role of intermittent fasting in preventing CVD (Johnstone 2015; Malinowski 2019). In the literature, intermittent fasting is often compared to the following.
ontinuous energy restriction (CER): a reduced daily caloric intake to achieve weight loss with no time restriction (Cioffi 2018). For example, this may involve a deficit of 500 calories daily.
Ad libitum feeding: food intake based on the participants' usual eating habits with no time or calorie restriction (Rynders 2019). For example, a person may consume whatever they wish to eat whenever they want on a daily basis.
A look at the current literature
An AHA investigation into the effect of ADF and PF on preventing CVD found a weight loss reduction of 3% to 8% over three to 24 weeks (AHA 2017). Additionally, they found a reduction in serum cardiovascular markers including a reduction of 6% to 21% in total cholesterol, a reduction of 7% to 32% in LDL cholesterol, and a reduction of 14% to 42% in triglycerides (TG) (AHA 2017). Improvement in blood glucose concentration was only seen in those with elevated blood glucose initially (AHA 2017), indicating that intermittent fasting may benefit patients with diabetes, however, it is difficult to tell as patients with diabetes tend to be excluded from these studies.
Additionally, Trepanowski 2017 published a year‐long randomised controlled trial (RCT) comparing ADF to CER and ad libitum feeding. They found an increase in high‐density lipoprotein (HDL) levels (6.2 mg/dL, 95% confidence interval (CI) 0.1 mg/dL to 12.4 mg/dL) relative to the CER group at six months as well as an increase in LDL levels (11.5 mg/dL, 95% CI, 1.9 mg/dL to 21.1 mg/dL) relative to CER group at 12 months, indicating that ADF did not outperform CER (Trepanowski 2017). Interestingly, no significant difference was seen between intervention groups for weight loss, fat mass, lean mass, visceral fat mass, and a higher dropout rate was seen in the intermittent fasting group, questioning the sustainability of the diet in the long term (Trepanowski 2017 ).
A number of recent systematic reviews have investigated how intermittent fasting/IER compares to CER and ad libitum feeding in reducing weight and preventing CVD (Cioffi 2018; Ganesan 2018; Harris 2018; Meng 2020; Welton 2020). Cioffi 2018 focused on IER versus CER. The authors reported no significant benefit of IER over CER on weight loss, glucose, glycated haemoglobin (HbA1c), and lipid profile (Cioffi 2018). Similarly, Harris 2018 found six RCTs that looked at IER compared to CER, but also compared it to ad libitum feeding. Meta‐analyses showed that IER was superior to ad libitum feeding for weight loss (‐4.14 kg, 95% confidence interval (CI) ‐6.30 kg to ‐1.99 kg; P ≤ 0.001), but also showed no difference between IER and CER for weight loss (‐1.03 kg, 95% CI ‐2.46 kg to 0.40 kg; P = 0.156) (Harris 2018).
How the intervention might work
It has been hypothesised that these intermittent fasting regimens influence cardiometabolic outcomes via effects on circadian biology (Patterson 2017). Regimens that exclude or dramatically restrict evening energy intake lead to reduced postprandial insulin and glucose exposure than during the day (Frape 1997; Gibbs 2014). Specifically, it has been hypothesised that time‐restricted feeding regimens lead to improved oscillations in circadian clock gene expression and improved body weight regulation by imposing a diurnal rhythm of food intake aligned with the 24‐hour light‐dark cycle (Hatori 2012). Furthermore, research in shift‐workers, who eat most of their calories at night and are at an increased risk for obesity, has demonstrated alterations in appetite‐regulating hormones (leptin and ghrelin, for instance) that may increase energy intake (Crispim 2011; Schiavo‐Cardozo 2013; Wirth 2014). Therefore, changes in meal timing with respect to the 24‐hour light‐dark cycle may have an important influence on energy intake, weight control, and glucose metabolism.
It is speculated that intermittent fasting might change the human microbiome. The human microbiome is the collective genomes of micro‐organisms in the human gastrointestinal tract and is known to be dynamic and undergo daily cyclical fluctuations in its composition (Zarrinpar 2014). Obesogenic diets affect the composition of the microbiome and diminish the cyclical fluctuations. Intermittent fasting (especially time‐restricted feeding) is reported to restore key microbiota, reset the composition of the microbiome and restore the cyclical fluctuations (Zarrinpar 2014).
Non‐circadian mechanisms also play a role. Intermittent fasting has been shown to reduce blood pressure in animals as well as humans (Malinowski 2019). A year‐long intermittent fasting study of 1422 participants conducted at the Buchinger Wilhelmi clinic in Germany revealed a reduction in both systolic and diastolic blood pressure, which may be explained by an increase in parasympathetic drive due to an increase in brain‐derived neurotrophic factor (BDNF), an increase in noradrenaline excretion and increased sensitivity of natriuretic peptides and insulin (Wilhelmi 2019). Intermittent fasting without calorie restriction alleviates inflammation (Hatori 2012), which itself is a pathogenic factor in both obesity (Bolus 2018) and diabetes (Donath 2011).
Ramadan fasting has been shown to improve the lipid profile in the healthy, obese, and dyslipidaemic (Santos 2018). Ramadan fasting may increase serum HDL levels and decrease serum very‐low‐density lipoproteins (VLDL), LDL, and small and dense LDL (sdLDL) levels by increasing fatty acid oxidation in the liver, increasing production of the HDL precursor apolipoprotein A (apoA), and decreasing the production of the LDL precursor apolipoprotein B (apoB)(Adlouni 1998; Hammouda 2013).
Studies have shown that the benefit of intermittent fasting may also be due to reduced caloric consumption. Fasting for the whole day or consuming ≤ 25% of the normal caloric intake per day was shown to reduce caloric intake by 30% for the next three days (Antoni 2016). Most fasting regimens force observers to eat in a restricted time period. This may be a 16‐hour fast, whereby the observer would fast from 8 PM the previous day to mid‐day the next day and then ad libitum feed for eight hours. Due to the short nature of the feeding window, less food may be consumed thus reducing caloric intake (Patterson 2017).
Why it is important to do this review
Intermittent fasting is becoming more popular for health and fitness and in October 2016, the search term "diet fasting intermittent alternate day" received 210,000 searches (Patterson 2017). Intermittent fasting was also in the top 10 diet searches on diet on Google trends in the USA in 2018 (Google trends 2018). The popularity of intermittent fasting makes us question whether there is a potential benefit of it as a lifestyle intervention in preventing or reducing the burden of CVD. The majority of existing human studies are cross‐sectional and observational studies which focus on the benefits of religious fasting such as Ramadan fasting. The existing trials report inconsistent results on the benefit of intermittent fasting. Moreover, the comparisons between intermittent fasting and calorie restriction diets are not conclusive. Finally, each trial has addressed a limited number of cardiovascular risk factors and therefore a comprehensive review is needed.
This review aims to bring together all the relevant RCTs in a single systematic review, reporting the effects of intermittent fasting in humans and providing a comprehensive report on the impact of intermittent fasting on CVD.
Objectives
To determine the role of intermittent fasting in preventing and reducing the risk of cardiovascular disease (CVD) in people with or without prior documented CVD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in which the participants undergo intermittent fasting compared to ad libitum feeding (normal diet) or caloric restriction for the primary or secondary prevention of cardiovascular disease (CVD).
Since blinding of participants may not be possible in these types of studies, we included open‐label RCTs. We planned to include cluster‐RCTs. We included trials reported as full text, those published as abstract only, and unpublished data to minimise publication bias. Furthermore, we included relevant arms of multi‐arm trials.
We excluded non‐randomised and observational studies, single‐arm studies, case reports, letters, study protocols, narrative reviews, and meta‐analyses. Furthermore, we excluded cross‐over trials as they are unsuitable to investigate the irreversible primary outcomes of this study.
Types of participants
We included adults (aged 18 years or older) with or without CVD or documented cardiometabolic risk factors. If available, we planned to extract relevant data from trials that included only a subset of eligible patients, if the data were reported separately. If the data were not reported separately, we only included studies if ≥ 80% of the study population were eligible for our review (Naude 2019). We planned to assess this decision with a sensitivity analysis.
Where the data were unavailable, we tried to contact the trial authors to obtain the relevant data.
We excluded trials where the participants were children (aged under 18 years). No other exclusion criteria were applied to the study population.
Types of interventions
The following types of intermittent fasting were focused on in this review.
Alternate day fasting (ADF): this was defined as a cyclical feeding pattern that entails complete fasting (consumption of no calories) for a period of 24 hours, followed by ad libitum feeding for the next 24 hours (Harris 2018).
Modified alternate day fasting (Modified ADF): this was defined as a cyclical feeding pattern that entails fasting (consumption of 25% or less of maintenance calories) for a period of 24 hours, followed by ad libitum feeding for 24 hours (Harris 2018). If the % of caloric intake was not stated, then a cut off of ≤ 600 calories was used (roughly 25% of a recommended daily caloric intake for a male/mixed population).
Periodic fasting (PF): this was defined as a cyclical feeding pattern that entails fasting (consumption of 25% or less of required calories). This includes fasting for one to two days per week with ad libitum feeding for the remaining days, in a once‐weekly or twice‐weekly regimen (Anton 2018; Cioffi 2018). If the % of caloric intake was not stated, then a cut off of ≤ 600 calories was used (roughly 25% of a recommended daily caloric intake for a male/mixed population).
Time‐restricted feeding (TRF): this is defined as complete fasting (consumption of no calories) for at least 12 hours per day with ad libitum feeding for the rest of the day; repeated every day (Cioffi 2018).
We included the Islamic fast, provided that the fasting period exceeded 12 hours with ad libitum feeding between sunset and sunrise (Adler‐Lazarovits 2019; Trepanowski 2010). Notably, religious fasts were only included if the study was an randomised controlled trial. Ramadan fasting studies tend to be observational due to the obligatory nature of the fast and therefore would be excluded the majority of the time.
We excluded RCTs in which the participant fast does not meet the criterion for a minimum of 12 hours of caloric restriction to 25% or less of the maintenance caloric requirement. In addition, we excluded religious fasts that did not meet this criterion; this includes but is not limited to the following.
Christian Lent fasts
Daniel Fasts
Buddhist fasts
Jewish fasts
With regards to the comparator, we included RCTs in which intermittent fasting was compared to either ad libitum feeding (normal diet or no intervention) or continuous energy restriction (CER), defined as a minimum of 25% reduction in caloric intake, which does not meet our aforementioned criterion of intermittent fasting. We excluded studies in which intermittent fasting was compared to exercise therapies, surgical techniques, or pharmacological medications.
Types of outcome measures
Primary outcomes
All‐cause mortality
Cardiovascular (CV) mortality
Stroke
Myocardial infarction (MI)
Heart failure
We assessed all the above‐mentioned outcomes at short‐term follow‐up (≤ 3 months), medium‐term follow‐up (> 3 months to 12 months) and long‐term follow‐up (> 12 months).
Stroke, MI, and heart failure were measured by the number of participants with at least one event during follow‐up.
Secondary outcomes
Absolute change in body weight
Absolute change in body mass index (BMI)
Absolute change in waist circumference
Absolute change in total cholesterol levels (TC)
Absolute change in low‐density lipoprotein cholesterol levels (LDL)
Absolute change in high‐density lipoprotein cholesterol levels (HDL)
Absolute change in total triglyceride levels (TG)
Absolute change in systolic blood pressure (SBP)
Absolute change in diastolic blood pressure (DBP)
Absolute change in C‐reactive protein (CRP)
Absolute change in fasting plasma glucose
Absolute change in glycated haemoglobin (HbA1C)
Incidence of headaches (side effect)
Incidence of dizziness (side effect)
Incidence of weakness (side effect)
Quality of life
We assessed outcomes 1‐12 at short‐term follow‐up (≤ 3 months), medium‐term follow‐up (> 3 months to 12 months) and long‐term follow‐up (>12 months). The absolute change was the mean change from the baseline provided for each group. Where data for the absolute change from baseline were not available, we contacted the trial authors to obtain the absolute changes or the raw values to calculate the standard deviations (SDs). Where we could not contact the trial authors, we were able to impute the SD values using another study in the systematic review given it had a similar intervention and follow‐up. If that was also not the case, then we narratively discussed the study instead.
We assessed outcomes 13‐15 (side effects) at any point during follow‐up and reported them narratively.
We assessed outcome 16 (quality of life) narratively at any point during follow‐up. We intended to use validated quality of life scales such as the World Health Organization Quality Of Life Assessment Instrument (WHOQOL), Medical Outcomes Study 36‐item Short‐Form Health Survey (SF‐36), Nottingham Health Profile (NHP), Euro‐Quality of Life Questionnaire (EuroQoL, EQ‐5D), as well as cardiovascular specific scales such as the Seattle Angina Questionnaire (SAQ), the Minnesota Living with Heart Failure (MLHF) questionnaire and the Atrial Fibrillation Severity Scale (AFSS). The papers included quality of life as an outcome reported on it generally and did not specify specific time points.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases on 12 December 2019.
Cochrane Central Register of Controlled Trials (CENTRAL) Issue 12 of 12, December 2019 (Cochrane Library)
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to December 10, 2019)
Embase (Ovid, 1980 to 2019, week 49)
Search strategies for each database are available in Appendix 1. The Cochrane sensitivity‐precision maximising RCT filter Lefebvre 2011, was applied to MEDLINE (Ovid) and an adaptation of it to Embase. We searched all databases from their inception to the present, and we imposed no restriction on publication language or status.
Searching other resources
In addition, we carried out the following searches.
We searched reference lists of all relevant reviews retrieved by electronic searching to identify other potentially eligible trials or ancillary publications.
We searched the following conference proceedings on 11 January 2020 via their websites, from their inception to present: ESC Congress 365 (congress365.escardio.org)(2013 onwards), ACC Annual Scientific Sessions (http://www.onlinejacc.org/content/ meeting‐abstract‐supplements)(1983 onwards), AHA Annual Scientific Sessions (circ.ahajournals.org)(1983 onwards), American Society for Nutrition meeting (https://meeting.nutrition.org)(2006 onwards), British Nutrition Foundation conference (https://www.nutrition.org.uk/bnfevents.html)(2006 onwards).
We contacted corresponding authors of included studies for any additional published or unpublished data.
We contacted the authors of trials when information in the study report was lacking or unclear.
We also examined any relevant retraction statements and errata for included studies.
We searched the following clinical trial registers for ongoing or unpublished trials on 11 January 2020. The search terms used are in Appendix 1.
ClinicalTrials.gov (clinicaltrials.gov).
European (EU) Clinical Trials Register (clinicaltrialsregister.eu).
WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch).
Data collection and analysis
Selection of studies
Five review authors (MA, HE, OM, SZ, and MFKF) independently screened titles and abstracts of studies retrieved using the aforementioned search strategies and then coded these as ‘retrieve’ (if eligible or potentially eligible/unclear) or ‘do not retrieve’. The studies were screened by a minimum of two review authors. Any discrepancies were resolved through consensus amongst all review authors. Full texts of potentially eligible studies were retrieved. Two review authors (MA and SZ) independently screened these full texts and identified studies that met inclusion criteria.
MA and SZ recorded any articles excluded after full‐text assessment and their reasons for exclusion in the ‘Characteristics of excluded studies' table. We also identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report is the unit of interest in the review.
We also included a PRISMA flowchart to depict the study selection process (see Figure 1).
1.
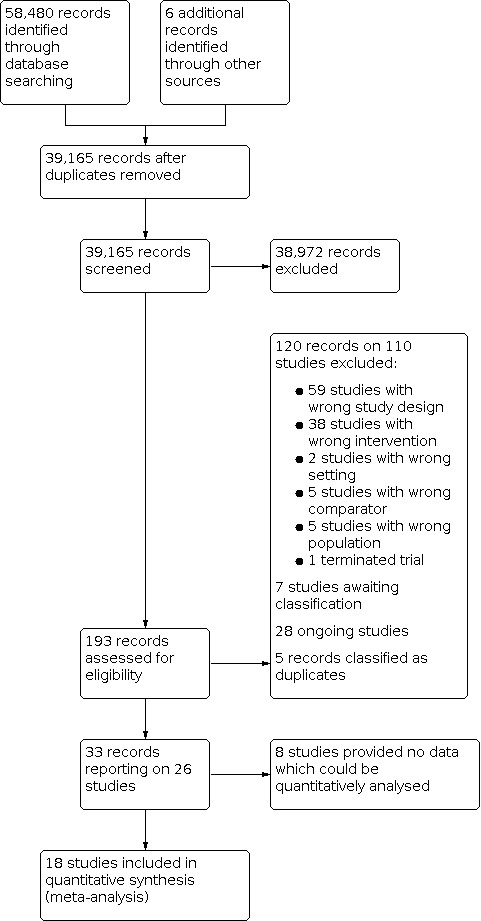
Study flow diagram.
Data extraction and management
We used a data collection form for study characteristics and outcome data that we piloted on at least one study included in the review. Two review authors (HE and OM) extracted study characteristics from included studies. We extracted the following study characteristics.
Reference and design: author, publication year, country of publication, study design, number of centres, and sources of funding.
Interventions: intervention groups in the study.
Participants: the indication for enrolment in the study, the total number of randomised participants and number of participants in each group, the attrition rate, and the inclusion and exclusion criteria for enrolment into the study.
Outcomes: primary and secondary outcomes specified and collected, and time points reported and the length of follow‐up.
Baseline characteristics of participants including: age, number of males, smoking status, hypertension, dyslipidaemia, diabetes mellitus, history of cardiovascular disease (CVD), body mass index (BMI), C‐reactive protein (CRP), systolic blood pressure (SBP), diastolic blood pressure ( DBP), total cholesterol (TC), : triglycerides (TG), low‐density lipoprotein (LDL) and high‐density lipoprotein (HDL) cholesterol, fasting plasma glucose, fasting insulin, leptin, glycated haemoglobin (HbA1C), and homeostatic model assessment of insulin resistance.
Comments: general comments regarding the trial design and conduct or specific methodological comments that we believe may influence the data or outcomes.
Two review authors (HE and OM) independently extracted outcome data from included studies. They spot‐checked study characteristics for accuracy against the trial report. They resolved disagreements by consensus. One review author (MFKF) transferred data into the Review Manager 5 file (RevMan 2014). Two review authors (HE and OM) double‐checked if the data were entered correctly by comparing the data presented in the systematic review with the study reports.
Certain studies reported outcomes in units different to the ones used in this paper. In that case, the following conversions were used.
mg/dL to mmol/L (total cholesterol, HDL, LDL). The value was divided by 38.67 (Rugge B 2011).
mg/dL to mmol/L (triglycerides). The value was divided by 88.57 (Rugge B 2011).
mg/dL to mmol/L (glucose). The value was divided by 18 (Riemsma R 2016).
Standard error (SE) to standard deviation (SD). The value was multiplied by the square root of the sample size (Altman 2005).
Assessment of risk of bias in included studies
Two review authors (HE and MA) independently assessed the risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by consulting a third review author (AD). We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear, using Cochrane ‘Risk of bias' criteria, and we provided a quote from the study report together with a justification for our judgment in the ‘Risk of bias' table. We summarised the ‘Risk of bias' judgements across different studies for each of the domains listed. Where information on the risk of bias related to unpublished data or correspondence with a trial author, we noted this in the ‘Risk of bias' table. When considering treatment effects, we took the risk of bias for the studies that contribute to that outcome into account.
For cluster‐RCTs, two review authors (HE and MA) assessed the risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by consulting a third review author (AD). We assessed the risk of bias according to the following domains.
Recruitment bias.
Baseline imbalance.
Loss of clusters.
Incorrect analysis.
Comparability with individually‐randomised trials.
Assessment of bias in conducting the systematic review
We conducted the review according to this published protocol and we if required, we planned to report any deviations from it in the ‘Differences between protocol and review' section of the review.
Measures of treatment effect
We planned to analyse dichotomous data as risk ratios (RR) with 95% confidence intervals (CIs). We analysed continuous data as mean difference (MD) with 95% CIs as outcomes were measured using the same method. We entered data presented as a scale with a consistent direction of effect.
Defining clinically significant changes in continuous outcomes can be difficult as there is a mixture of opinions in the literature and each change may be specific to a particular patient group with particular baseline characteristics. In this review, we defined clinically meaningful differences as the following.
Body weight: a minimum of 5% reduction in body weight from baseline level (Pi‐Sunyer 2015; Topol 2010; Swift 2016; Williamson 2015).
BMI: a minimum of 5% reduction in BMI from the baseline level. For example, if the average mean baseline BMI was 30 kg/m2, a difference between treatment group and comparator of 1.5 kg/m2 would be considered clinically significant.
Waist circumference: a minimum of 5% reduction in waist circumference from the baseline level. For example, if the average mean baseline waist circumference was 100 cm, a difference between treatment group and comparator of 5 cm would be considered clinically significant.
Lipid profile: a minimum 10% change from baseline (Bradley 2009).
Blood pressure: 5 mm Hg reduction in either systolic or diastolic blood pressure (Bradley 2009).
CRP: 5% reduction
Glucose: 5% reduction
HbA1c: a minimum reduction of 0.5%. (Bradley 2009).
Due to high levels of attrition in the included studies, we used per‐protocol analysis. Intention‐to‐treat analysis was not possible due to missing data. The majority of our included studies reported their outcomes using per‐protocol analysis. We imputed data for Pinto 2019.
Unit of analysis issues
We included individual‐RCTs. No cluster‐RCTs were found.
For any studies with more than two interventions of interest and a single comparator arm, we divided the comparator between the intervention arms to avoid double counting the participants. This only applied to one study (Hutchison 2019). The control group (ad libitum) had a total of 11 participants so could not be divided equally. Therefore six participants were allocated to the Intermittent fasting (IF)70 comparison and five to the IF100 comparison throughout the analysis tables. Alternating the allocated values (in other words five to the IF70 comparator and six to the IF100 comparator) did not change our results. The comparator group for CER had a total of 24 participants which were divided equally (12 and 12) between IF100 and IF70 analyses.
Trials with multiple follow‐up times were used where available. Data given at ≤ 3 months were analysed as short‐term follow‐up and > 3 months to 12 months as medium‐term follow‐up. Where there were multiple follow‐up times within the same time period (e.g. four months and six months in medium‐term follow‐up), the latter value were included. This is with exception of Sundfor 2018 ,which provided data at six and 12 months. The values at six months were used as more of the outcomes in that study were reported at six months compared to 12 months and is coincidently more consistent with the other studies at medium term.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis. Nevertheless, we did not believe any of the data introduced bias to our results; a sensitivity analysis was therefore not performed. We dealt with data that we considered were not missing at random by imputing missing data based on predicted values, using regression analysis.
The following study authors were contacted to gather more data to calculate or have access to absolute changes for our given outcomes: Harvie 2011; Harvie 2013; Pinto 2019; Schubel 2018; Tinsley 2017; Tinsley 2019; Trepanowski 2017.
Standard deviation (SD) values were imputed for Pinto 2019 using correlation coefficients as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Correlation coefficients were generated by looking at Catenacci 2016, which reported the baseline, follow‐up and change from baseline values separately. These coefficients were then used to impute the missing SD values for Pinto 2019. Furthermore, we made sure that Catenacci 2016 had a similar intervention, time of follow‐up and outcomes to Pinto 2019.
Assessment of heterogeneity
We used the I2 statistic, which quantifies inconsistency across studies, to assess the impact of heterogeneity on the meta‐analysis and we used an I2 statistic value of 50% or higher as a measure of substantial heterogeneity. Where we identified substantial heterogeneity, we reported it and explored possible causes by prespecified subgroup analysis if this was possible. We were unable to do this for Analysis 1.1 due to insufficient data. We also inspected forest plots visually for signs of heterogeneity.
1.1. Analysis.

Comparison 1: IF vs Ad libitum (Short term), Outcome 1: Absolute change in body weight (kg)
Assessment of reporting biases
We planned to create a funnel plot to explore possible reporting bias for the primary outcomes where 10 or more trials met the inclusion criteria (Sterne 2011). Unfortunately, this was not possible as no data was available on the primary outcomes.
Data synthesis
We undertook meta‐analyses only where this was considered meaningful. For example, this includes situations where the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
We performed the following analyses.
Intermittent fasting versus ad libitum feeding
Intermittent fasting versus continuous energy restriction (CER)
Where there was such evidence for homogenous effects across studies, we planned to analyse the data using RR and summarise all data using the fixed‐effect model (Riley 2011; Wood 2008). We used the random‐effects model where we found high levels of heterogeneity, for example as indicated by a high I2 statistic value (50% or higher). We used both models in that case but only reported the most conservative. We performed statistical analyses according to the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Where the studies include a mixture of change‐from‐baseline and final value scores in some of the outcomes, we pooled the analysis of mean differences as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Due to high levels of attrition in the included studies, we used per‐protocol analysis. Intention‐to‐treat analysis was not possible due to missing data. The majority of our included studies reported their outcomes using per‐protocol analysis. We only imputed data for one study (Pinto 2019). We considered imputing missing data for more studies, however, we were uncertain of whether missing data would be indeed imputable. This is because we cannot ascertain if patients lost to follow‐up would show similar outcomes as those who adhered to the dietary intervention.
Subgroup analysis and investigation of heterogeneity
We analysed the following subgroups.
Male and female patients
Overweight and obese (BMI ≥ 25) and non‐overweight patients (BMI < 25).
Patients with and without diabetes.
Intermittent fasting type: alternate‐day fasting, modified alternate‐day fasting, periodic fasting and time‐restricted feeding.
We used the formal test for subgroup interactions in Review Manager 5 (RevMan 2014).
Sensitivity analysis
We undertook the following sensitivity analysis.
To include only published trials where data were available from full‐text publications and excluded trials only available as abstracts, or from trialists.
We had planned to undertake the following but this was not possible.
To include only those trials at low risk of bias, as specified in the Assessment of risk of bias in included studies section. The blinding of participants was not possible for the interventions comparing (Intermittent fasting versus ad libitum eating or calorie restriction), leaving us with six total domains for potential biases. We did not include 'other bias' in the definition of overall low risk of bias, so defined low risk of bias as those determined to have a low risk of bias in at least four of the domain that must include low risk of selection and reporting biases, which are the most important domains of bias in this review.
To only include studies if ≥ 80% of the study population were eligible for our review (Naude 2019), and planned to assess this decision in a sensitivity analysis.
We had planned to conduct a sensitivity analysis to assess the impact of missing data in cases where we thought it introduced serious bias. However, we did not believe any of the missing data introduced bias to our results, so did not perform a sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
Two review authors (SZ and MFKF) used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the pre‐specified outcomes. We constructed the ‘Summary of findings' tables using GRADEpro software (GRADEpro 2015). We justified all decisions to downgrade the certainty of the evidence using footnotes and we made comments to aid the reader's understanding of the review where necessary.
We included the following outcomes in the 'Summary of findings' tables.
All‐cause mortality
Cardiovascular (CV) mortality
Stroke
Myocardial infarction (MI)
Heart failure
Absolute change in body weight
Absolute change in fasting plasma glucose
There are three 'Summary of findings' tables.
IF versus ad libitum feeding at short‐term follow‐up (≤ 3 months)
IF vs CER at short‐term follow‐up (≤ 3 months)
IF vs CER at medium‐term follow‐up (> 3 months to 12 months)
Two review authors (SZ and MFKF) independently assessed the quality of the evidence and resolved any disagreements through consensus. We justified, documented, and incorporated our judgements into the reporting of results for each outcome.
Results
Description of studies
Results of the search
For the review, 39,165 records were identified after removal of duplicates. From reading titles and abstracts 38,972 records were eliminated as not being relevant to the review. Papers were obtained for 193 records. From these 193 records, 120 records on 110 studies were excluded (see Characteristics of excluded studies). Reasons for exclusion include wrong study design, wrong intervention, wrong setting and wrong population. Seven studies were placed in the awaiting classification section and 28 articles were categorised as ongoing trials. Nine9 further articles were found to be duplicates and added to the relevant study as an additional reference. A total of 26 studies were included (see Characteristics of included studies) and 18 were included in the quantitative synthesis.
Included studies
The trials dated from 2011 to 2019 and were conducted worldwide (Australia, the USA, South Korea, the UK, Iran, Germany and Norway). The studies included in the quantitative analysis included: Bhutani 2013; Carter 2018; Catenacci 2016; Cho 2019; Chow 2019; Griffiths 2016; Harvie 2011; Harvie 2013; Hutchison 2019; Parvaresh 2019; Pinto 2019; Schubel 2018; Stekovic 2019; Sundfor 2018; Tinsley 2017; Tinsley 2019; Varady 2011; Varady 2013.
Eight other studies were included Amodio 2016; Conley 2018; Corley 2019; Ferraris 2019; Kroeger 2015; Moro 2016; Trepanowski 2017; Varady 2016a. These studies met the inclusion criteria but were not included in the quantitative analysis. This was due to several reasons which included no available data, no relevant outcomes, data presented in a form other than absolute change. An attempt was made at contacting all authors with some having no contact details, some not replying to emails, and others declining to share data with us.
In total, the quantitatively analysed studies recruited 1125 participants and observed outcomes ranging from four weeks to six months. No studies included data on all‐cause mortality, cardiovascular mortality, stroke, myocardial infarction and heart failure at any point during follow‐up. The majority of the studies actively excluded participants who have a prior history of cardiovascular disease (CVD) and none of them purely focused on patients with current CVD. The most common inclusion criterion was the inclusion of overweight and obese participants.
Out of the studies included in the quantitative analysis; seven studies compared intermittent fasting (IF) to ab libitum feeding, eight studies compared IF with continuous energy restriction (CER) and three studies compared IF with both ad libitum feeding and CER.
Twelve studies recruited participants who were overweight or obese. Three studies only recruited participants who had diabetes mellitus. Two studies focused on alternate day fasting (ADF), six studies focused on modified ADF, seven studies focused on periodic fasting, and three studies focused on time‐restricted feeding (TRF). Seventeen studies reported body weight as an outcome, 14 reported on body mass index (BMI), nine reported on waist circumference, 13 reported on lipid profile, 11 on blood pressure, four on C‐reactive protein (CRP), 11 on glucose and four on glycated haemoglobin (HbA1c).
The attrition rate for recorded, analysed data was 15.2%. Based on quantitatively analysed studies with post‐attrition recorded data for age and sex, the mean age of the participants was 37.3 years. Of these 45.4% of participants were male.
Funding of the included studies was provided by a variety of institutions including: University of Illnois, University of South Australia, Yonsei University College of Medicine, MTI Biotech Inc. and Texas Tech University, University of Minnesota Healthy Foods Healthy Lives, LighterLife (UK) Ltd and Elmholtz Association of German Research Center.
The details of each included study are shown in Characteristics of included studies.
Excluded studies
Of studies excluded, 59 were due to study design, 38 due to wrong intervention, five due to patient population, two due to the wrong setting, one was terminated and five due to the wrong comparator.
Risk of bias in included studies
We display 'Risk of bias' assessments in Figure 2; Figure 3.
2.

'Risk of bia's summary: review authors' judgements about each risk of bias item for each included study.
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
All the included trials were randomised controlled trials, however, detail of the randomisation process was not provided in 17 studies (Amodio 2016; Bhutani 2013; Catenacci 2016; Chow 2019; Conley 2018; Corley 2019; Ferraris 2019; Griffiths 2016; Harvie 2011; Harvie 2013; Hutchison 2019; Kroeger 2015; Tinsley 2017; Trepanowski 2017; Varady 2011; Varady 2013; Varady 2016a). The nine remaining studies which provided some detail of the randomisation process (Carter 2018; Cho 2019; Moro 2016; Parvaresh 2019; Pinto 2019; Schubel 2018; Stekovic 2019; Sundfor 2018; Tinsley 2019) were considered at low risk of bias.
Allocation concealment
With regards to allocation concealment, 15 studies were rated as unclear risk (Amodio 2016; Catenacci 2016; Cho 2019; Chow 2019; Conley 2018; Corley 2019; Ferraris 2019; Griffiths 2016; Harvie 2011; Kroeger 2015; Moro 2016; Tinsley 2017; Varady 2011; Varady 2013; Varady 2016a). Eight studies were rated as low risk (Bhutani 2013; Harvie 2013; Hutchison 2019; Parvaresh 2019; Pinto 2019; Sundfor 2018; Tinsley 2019; Trepanowski 2017) and three studies as high risk (Carter 2018; Schubel 2018; Stekovic 2019).
Blinding
Performance bias
Blinding of participants is not easy in dietary studies, as the participants usually have to follow instructions to attain the specific dietary goals. This is especially the case in intermittent fasting studies, in which specific meal timings are imposed on participants. Where participants are not blinded, it is difficult to ensure that study staff, healthcare providers and outcome assessors are blinded. We therefore judged blinding of participants and personnel to be inadequate in all studies.
Detection bias
Blinding of outcome assessment was deemed unclear in 20 studies (Amodio 2016; Bhutani 2013; Carter 2018; Catenacci 2016; Chow 2019; Conley 2018; Corley 2019; Ferraris 2019; Griffiths 2016; Hutchison 2019; Kroeger 2015; Moro 2016; Pinto 2019; Sundfor 2018; Tinsley 2017; Tinsley 2019; Trepanowski 2017; Varady 2011; Varady 2013; Varady 2016a). Four studies were deemed low risk (Cho 2019; Harvie 2011; Schubel 2018; Stekovic 2019), and two studies were deemed high risk (Harvie 2013; Parvaresh 2019).
Incomplete outcome data
As the primary outcomes for this review (all‐cause mortality and cardiovascular events) were not reported in any of the original studies, owing to their short length of follow‐up, assessment of whether incomplete outcome data had been addressed was based on the extent of dropout and whether dropouts had been considered in the analysis. In five studies, the risk of attrition bias was deemed to be low due to relatively low dropout rates of <7.5% (Parvaresh 2019; Pinto 2019; Stekovic 2019; Sundfor 2018; Varady 2013). In three studies, the risk of attrition bias was deemed to be low because all participants were included in the intention‐to‐treat analysis (Harvie 2013; Schubel 2018; Tinsley 2019). For six studies, as only the abstracts were available, the risk of attrition bias was rated as unclear (Amodio 2016; Chow 2019; Conley 2018; Corley 2019; Kroeger 2015; Varady 2016a). Three further studies were rated as unclear due to lack of information (Moro 2016; Tinsley 2017; Varady 2011). Other studies had high dropout rates which were unaccounted for in the analysis and these studies were therefore deemed to be at high risk of attrition bias.
Selective reporting
Most of the included studies have either reported that the participants did not experience any of our primary outcomes, have published their outcome data, or have provided the data they did possess. For this reason, we have graded almost all the included full‐text studies as at low risk of selective reporting (Cho 2019; Harvie 2011; Pinto 2019; Schubel 2018; Sundfor 2018; Tinsley 2017; Tinsley 2019; Trepanowski 2017). For eight studies that were only available as abstracts, the risk of reporting bias was deemed to be unclear (Amodio 2016; Chow 2019; Conley 2018; Corley 2019; Ferraris 2019; Griffiths 2016; Kroeger 2015; Varady 2016a). With regards to Varady 2011 the results were reported as percentage changes. We tried to contact these authors to provide absolute changes, but it is possible that they did not reply as they felt that their data did not reflect the expected or hoped‐for pattern of events. This study was rated as high risk of reporting bias. Two other studies were rated at high risk (Hutchison 2019; Varady 2013). The remaining seven studies were assessed as unclear risk of bias (Bhutani 2013; Carter 2018; Catenacci 2016; Harvie 2013; Moro 2016; Parvaresh 2019; Stekovic 2019).
Other potential sources of bias
No other potential sources of bias could be identified.
Effects of interventions
See: Table 1; Table 2; Table 3
Primary outcomes
No studies identified in our review included any data on all‐cause mortality, cardiovascular mortality, stroke, myocardial infarction and heart failure at any point during follow‐up.
Physical body parameters
Body weight (kg)
Seven trials of 224 patients compared intermittent fasting (IF) versus ad libitum. Body weight was reduced in the intermittent fasting (IF) group (mean difference (MD) ‐2.88, 95% confidence interval (CI) ‐3.96 to ‐1.80; I2 = 85%) (Analysis 1.1).
Ten trials of 719 patients compared IF to continuous energy restriction (CER) at short‐term follow‐up. Body weight was reduced in the intermittent fasting group (MD ‐0.88, 95% CI ‐1.76 to 0.00; I2 = 66%) (Analysis 2.1).
2.1. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 1: Absolute change in Body Weight (Total) (kg)
Additionally, four trials with a total of 279 patients compared IF with CER at medium‐term follow‐up. Intermittent fasting had no effect on body weight (MD ‐0.56, 95% CI ‐1.68 to 0.56; I2 = 0%) (Analysis 3.1).
3.1. Analysis.

Comparison 3: IF vs CER (Medium term), Outcome 1: Absolute change in Body weight (kg)
Although changes to body weight were seen in Analysis 1.1 and Analysis 2.1, they did not meet our criteria for clinical significance. We attribute the high heterogeneity in Analysis 1.1; Analysis 2.1 primarily to the heterogeneous designs of the included trials. Notably, the investigation of different subtypes of IF in the trials may have added significantly to the heterogeneity. Furthermore, the different inclusion criteria of the included trials may have compounded this; notable variables include gender, baseline weight and diabetic status are notable inclusion and exclusion criteria employed by trialists that may explain the heterogeneity.We explored this using subgroup analysis, as further outlined below.
The GRADE ratings for this outcome when IF was compared with ad libitum feeding (short term) and CER (medium term) (Table 1; Table 3) were low. This is because of high risk of bias in allocation concealment, detection bias, attrition bias and high heterogeneity. When IF was compared to CER at short‐term follow‐up (Table 2), a GRADE rating of very low was given due to substantial heterogeneity, high risk of performance bias, unclear or high risk of selection and detection bias and a wide confidence interval that includes a possible benefit and possible harm.
Subgroup analyses of body weight (kg)
Only Analysis 2.1 (IF versus CER at short‐term follow‐up) met our criteria of a minimum of 10 studies in order to perform subgroup analysis.
Subtypes of intermittent fasting (IF):Analysis 2.2 focused on the different subgroups of IF (alternate day fasting (ADF), PF and modified ADF). No studies focused on time‐restricted feeding. The test for subgroup difference did not identify any difference in effect by type of IF (P = 0.1).
Females only versus non‐females only:Analysis 2.3 focused on studies conducted on only females versus male studies. Female‐only studies showed effect (MD ‐0.56, 95% CI ‐1.96 to 0.84; participants = 226; studies = 3; I2 = 73%). There was no male‐only studies.
Overweight and obese only versus non‐overweight only:Analysis 2.4 focused on overweight versus non‐overweight participants. The test for subgroup differences did not indicate that the effect of IF was different depending on body weight at baseline (P = 0.18).
Diabetes only versus non‐diabetes only:Analysis 2.5 focused on participants with and without diabetes. The test for subgroup differences did not indicate that the effect of IF was different depending on whether or not participants had diabetes (P = 0.16).
2.2. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 2: Absolute change in Body Weight (Fasting subgroups) (kg)
2.3. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 3: Absolute change in Body Weight (Female subgroup) (Kg)
2.4. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 4: Absolute change in Body Weight (Overweight subgroups) (kg)
2.5. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 5: Absolute change in Body Weight (Diabetes subgroups) (kg)
Body mass index (BMI) (kg/m2)
Four trials of 115 patients compared IF to ad libitum with an MD of ‐0.92 kg/m2 (95% CI ‐1.36 to ‐0.48) favouring IF but without clinical significance. There was marked heterogeneity of effects (I² = 61%) (Analysis 1.2).
1.2. Analysis.

Comparison 1: IF vs Ad libitum (Short term), Outcome 2: Absolute change in BMI (kg/m2).
Nine trials of 651 patients compared IF to CER at short‐term follow‐up (MD ‐0.43 kg/m2, 95% CI ‐0.76, to ‐0.10) favouring IF but without clinical significance. There was some heterogeneity of effect (I² = 34%)(Analysis 2.6).
2.6. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 6: Absolute change in BMI (kg/m2).
Additionally, four trials with a total of 279 patients compared IF to CER at medium‐term follow‐up. There was no effect on BMI (MD ‐0.15 kg/m2, 95% CI ‐0.58 to 0.29). There was no heterogeneity (I² = 0%) (Analysis 3.2).
3.2. Analysis.

Comparison 3: IF vs CER (Medium term), Outcome 2: Absolute change in BMI (kg/m2)
We attribute the high heterogeneity in Analysis 1.2 primarily to the heterogeneous designs of the included trials. As mentioned previously, there were insufficient trials to formally assess the effects of baseline variability on the outcomes.
Waist circumference (cm)
Two trials of 87 patients compared IF versus ad libitum feeding. Intermittent fasting was shown to be superior to ad libitum feeding in reducing waist circumference (MD ‐4.19 cm, 95% CI ‐6.38 to ‐2.01; I2 = 0%)(Analysis 1.3). However, this is not clinically significant.
1.3. Analysis.

Comparison 1: IF vs Ad libitum (Short term), Outcome 3: Absolute change in waist circumference (cm)
Eight trials of 557 patients compared IF to CER at short‐term follow‐up. Intermittent fasting showed no effect compared to CER (MD ‐0.83 cm, 95% CI ‐2.11 to 0.44; I2 = 60%) (Analysis 2.7). Additionally, 3 trials of 258 patients compared IF to CER at medium‐term follow‐up. We found no effect on waist circumference (MD ‐0.66 cm, 95% CI ‐2.55 to 1.23) and there was marked heterogeneity of effects (I² = 58%) (Analysis 3.3).
2.7. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 7: Absolute change in waist circumference (cm)
3.3. Analysis.

Comparison 3: IF vs CER (Medium term), Outcome 3: Absolute change in waist circumference (cm)
We attribute the high heterogeneity in Analysis 2.7; Analysis 3.3 primarily to the heterogeneous designs of the included trials. Notably, the investigation of different subtypes of IF in the trials may have added significantly to the heterogeneity. Furthermore, the different inclusion criteria of the included trials may have compounded this; notable variables include gender, baseline weight and diabetic status are notable inclusion and exclusion criteria employed by trialists that may explain the heterogeneity. Unfortunately, there were insufficient trials to formally assess this using subgroup analysis.
Lipid profile
Absolute change in total cholesterol levels (TC) (mmol/L)
Four trials of 125 patients compared IF versus ad libitum. A reduction in total cholesterol was observed favouringIF (MD ‐0.31 mmol/L, 95% CI ‐0.51 to ‐0.12; I2 = 0%) (Analysis 1.4).
1.4. Analysis.

Comparison 1: IF vs Ad libitum (Short term), Outcome 4: Absolute change in total cholesterol levels (TC) (mmol/L)
Eight trials of 539 patients compared IF versus CER at short‐term follow‐up. There was difference in total cholesterol between both groups (MD ‐0.07 mmol/L, 95% CI ‐0.18 to 0.03; I2 = 0%) (Analysis 2.8).
2.8. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 8: Absolute change in total cholesterol (mmol/l)
Additionally, three trials of 258 patients compared IF versus CER at medium‐term follow‐up. There was no difference in total cholesterol between both groups (‐0.04 mmol/L, 95% CI ‐0.17 to 0.10) and there was no heterogeneity (Analysis 3.4).
3.4. Analysis.

Comparison 3: IF vs CER (Medium term), Outcome 4: Absolute change in total cholesterol (mmol/L)
Absolute change in low‐density lipoprotein cholesterol levels (LDL) (mmol/L)
Four trials of 125 patients compared IF with ad libitum. No change was observed in LDL levels (MD ‐0.22 mmol/L, 95% CI ‐0.40 to 0.05; I2 = 0%) (Analysis 1.5).
1.5. Analysis.

Comparison 1: IF vs Ad libitum (Short term), Outcome 5: Absolute change in LDL (mmol/L
Nine trials of 569 patients compared IF with CER at short‐term follow‐up. No change was seen (MD ‐0.07 mmol/L, 95% CI ‐0.16 to 0.01; I2 = 0%) (Analysis 2.9).
2.9. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 9: Absolute change in LDL (mmol/L)
Additionally, three trials of 258 patients compared IF with CER at medium‐term follow‐up. There was no difference in LDL between both groups (MD ‐0.06 mmol/L. 95% CI ‐0.18 to 0.05) and there was no heterogeneity (Analysis 3.5).
3.5. Analysis.

Comparison 3: IF vs CER (Medium term), Outcome 5: Absolute change in LDL (mmol/L)
Absolute change in high‐density lipoprotein cholesterol levels (HDL) (mmol/L)
Four trials of 125 patients compared IF with ad libitum. No change was seen (MD ‐0.10 mmol/L, 95% CI ‐0.25 to 0.05; I2 = 65%) (Analysis 1.6).
1.6. Analysis.

Comparison 1: IF vs Ad libitum (Short term), Outcome 6: Absolute change in HDL (mmol/L
Nine trials of 569 patients compared IF with CER at short‐term follow‐up. No change was seen (MD ‐0.01 mmol/L, 95% CI ‐0.06 to 0.04; I2 = 59%) (Analysis 2.10).
2.10. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 10: Absolute change in HDL (mmol/L)
Additionally, three trials of 258 patients compared IF with CER at medium‐term follow‐up. There was no difference in HDL between both groups (‐0.00 mmol/L, 95% CI ‐0.07 to 0.07) and there was marked heterogeneity (I² = 52%) (Analysis 3.6).
3.6. Analysis.

Comparison 3: IF vs CER (Medium term), Outcome 6: Absolute change in HDL (mmol/L)
Absolute change in total triglyceride levels (TG) (mmol/L)
Four trials of 125 patients compared IF to ad libitum. No change was seen (MD ‐0.06 mmol/L, 95% CI ‐0.25 to 0.14; I2 = 50%) (Analysis 1.7).
1.7. Analysis.

Comparison 1: IF vs Ad libitum (Short term), Outcome 7: Absolute change in TG (mmol/L)
Eight trials of 539 patients compared IF to CER at short‐term follow‐up. There was no difference in change in TG between both groups (MD ‐0.07 mmol/L, 95% CI ‐0.19 to 0.06; I2 = 43%) (Analysis 2.11).
2.11. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 11: Absolute change in TG (mmol/L)
Additionally, four trials of 279 patients compared IF to CER at medium‐term follow‐up. There was no difference in TG between both groups (MD ‐0.02 mmol/L, 95% CI ‐0.16 to 0.12) and there was no heterogeneity (Analysis 3.7).
3.7. Analysis.

Comparison 3: IF vs CER (Medium term), Outcome 7: Absolute change in TG (mmol/L)
We attribute the high heterogeneity in Analysis 1.7; Analysis 3.6 primarily to the heterogeneous designs of the included trials. Notably, the investigation of different subtypes of IF in the trials may have added significantly to the heterogeneity. Furthermore, the different inclusion criteria of the included trials may have compounded this; notable variables include gender, baseline weight and diabetic status are notable inclusion and exclusion criteria employed by trialists that may explain the heterogeneity. Unfortunately, there were insufficient trials to formally assess this using subgroup analysis.
Blood pressure
Absolute change in systolic blood pressure (SBP) (mmHg)
Five trials of 201 patients compared IF versus ad libitum. A reduction in blood pressure was seen favouring IF (MD ‐4.47 mmHg, 95% CI ‐6.94 to ‐2.01; I2 = 0%) (Analysis 1.8).
1.8. Analysis.

Comparison 1: IF vs Ad libitum (Short term), Outcome 8: Absolute change in SBP (mmHg)
Seven trials of 548 patients compared IF with CER at short‐term follow‐up. There was no difference in change in SBP between both groups (MD ‐1.75 mmHg, 95% CI ‐4.61 to 1.11; I2 = 24%) (Analysis 2.12).
2.12. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 12: Absolute change in SBP (mmHg)
Additionally, three trials of 258 patients compared IF with CER at medium‐term follow‐up. There was no change in SBP between both groups (MD 1.37 mmHg, 95% CI ‐4.98 to 7.72) and there was marked heterogeneity (I² = 52%) (Analysis 3.8).
3.8. Analysis.

Comparison 3: IF vs CER (Medium term), Outcome 8: Absolute change in SBP (mmHg)
Absolute change in diastolic blood pressure (DBP) (mmHg)
Five trials of 201 patients compared IF to ad libitum with no difference in change in DBP between both groups (MD ‐1.07 mmHg, 95% CI ‐3.33 to 1.18; I2 = 0%) (Analysis 1.9).
1.9. Analysis.

Comparison 1: IF vs Ad libitum (Short term), Outcome 9: Absolute change in DBP (mmHg)
Seven trials of 548 patients compared IF with CER at short‐term follow‐up. There was no difference in change in DBP between both groups (MD ‐0.97 mmHg, 95% CI ‐2.35 to 0.42; I2 = 0%) (Analysis 2.13).
2.13. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 13: Absolute change in DBP (mmHg)
Additionally, three trials of 258 patients compared IF with CER at medium‐term follow‐up. There was no change in DBP between both groups (MD ‐1.00 mmHg, 95% CI ‐4.67 to 2.67 and there was some heterogeneity (I² = 37%) (Analysis 3.9).
3.9. Analysis.

Comparison 3: IF vs CER (Medium term), Outcome 9: Absolute change in DBP (mmHg)
C‐reactive protein (CRP) (mg/L)
Two trials of 43 patients compared IF with ad libitum with no difference in change in CRP between both groups (MD ‐1.19 mg/L, 95% CI ‐2.54 to 0.16) and there was no heterogeneity (Analysis 1.10).
1.10. Analysis.

Comparison 1: IF vs Ad libitum (Short term), Outcome 10: Absolute change in CRP (mg/L)
Two trials of 190 patients compared IF with CER at short‐term follow‐up. There was no difference in change in CRP between both groups (MD 0.31 mg/L, 95% CI ‐0.56 to 1.17) and there was no heterogeneity (Analysis 2.14).
2.14. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 14: Absolute change in CRP (mg/L)
Additionally, one trial of 89 patients compared IF with CER at medium‐term follow‐up. There was no change in CRP between both groups (MD 0.46 mg/L, 95% CI ‐0.87 to 1.79) (Analysis 3.10).
3.10. Analysis.

Comparison 3: IF vs CER (Medium term), Outcome 10: Absolute change in CRP (mg/L)
Glucose and glycated haemoglobin (HbA1c)
Absolute change in fasting plasma glucose (mmol/L)
Three trials of 95 patients compared IF with ad libitum with no difference in change in glucose between both groups (MD ‐0.03 mmol/L, 95% CI ‐0.26 to 0.19; I2 = 15%) (Analysis 1.11).
1.11. Analysis.

Comparison 1: IF vs Ad libitum (Short term), Outcome 11: Absolute change in Glucose (mmol/L)
Nine trials of 582 patients compared IF with CER at short‐term follow‐up. There was no difference in change in glucose between both groups (MD ‐0.02 mmol/L, 95% CI ‐0.16 to 0.12; I2 = 73%) (Analysis 2.15).
2.15. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 15: Absolute change in Glucose (mmol/L
Additionally, four trials of 279 patients compared IF with CER at medium‐term follow‐up. There was no difference in change in glucose between both groups (MD 0.01 mmol/L, 95% CI ‐0.10 to 0.11) and there was no heterogeneity (Analysis 3.11).
3.11. Analysis.

Comparison 3: IF vs CER (Medium term), Outcome 11: Absolute change in glucose (mmol/L)
We attribute the high heterogeneity in Analysis 2.15 primarily to the heterogeneous designs of the included trials. Notably, the investigation of different subtypes of IF in the trials may have added significantly to the heterogeneity. Furthermore, the different inclusion criteria of the included trials may have compounded this; notable variables include gender, baseline weight and diabetic status are notable inclusion and exclusion criteria employed by trialists that may explain the heterogeneity. Unfortunately, there were insufficient trials to formally assess this using subgroup analysis.
The GRADE rating for this outcome when IF is compared to ad libitum feeding was very low (we are uncertain about our findings). This was because of high risk of performance bias, unclear or high risk of selection and attrition bias, a very low sample size and a wide confidence interval that includes a possible benefit and harm. The same rating was given when IF was compared to CER at short‐term follow‐up. Again, this is due to substantial heterogeneity, unclear or high risk of selection and detection bias and a wide confidence interval that includes a possible benefit and harm. When IF is compared to CER at medium‐term follow‐up, a low rating was given due to risk of bias and a wide confidence interval as above.
Absolute change in glycated haemoglobin (HbA1C) (mmol/L)
Four trials of 310 patients compared IF to CER at short‐term follow‐up. There was no difference in change in HbA1c between both groups (MD 0.01 mmol/L, 95% CI ‐0.07 to 0.08) and there was no heterogeneity (Analysis 2.16).
2.16. Analysis.

Comparison 2: IF vs CER (Short term), Outcome 16: Absolute change in HbA1c (mmol/L)
Side effects and quality of life
A total of four trials (Carter 2018; Harvie 2013; Schubel 2018; Varady 2013) reported on headaches and no studies reported any data on dizziness and weakness. Pooling all the data, 13 out of 187 participants in the intermittent fasting groups had at least a mild headache (7.0%), where two participants withdrew from the study due to the intensity of it (Carter 2018). Only one trial (Harvie 2011) reported on the quality of life using the RAND SF‐36 score. There was a modest increase in the physical component summary score.
Sensitivity analyses
Summary
No major differences were noted between this sensitivity analysis of published trials and the original analyses.
Body weight (kg)
Six trials of 203 patients compared IF to ad libitum (MD ‐3.04 kg, 95% CI ‐4.45 to ‐1.62; I2 = 86%) (Analysis 4.1). Sensitivity analysis did not change our original findings.
4.1. Analysis.

Comparison 4: Sensitivity analysis; published data: IF vs Ad libitum (Short term), Outcome 1: Absolute change in body weight (kg)
Nine trials of 710 patients compared IF to CER at short‐term follow‐up (MD ‐0.77 kg, 95% CI ‐1.66 to 0.12; I2 = 67%) (Analysis 5.1). Sensitivity analysis did not change our original findings.
5.1. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 1: kbsolute change in Body Weight (Total) (kg)
Additionally, four trials with a total of 279 patients compared IF to CER at medium‐term follow‐up (MD ‐0.56 kg, 95% CI ‐1.68 to 0.56; I2 = 0%) (Analysis 6.1). Sensitivity analysis did not change our original findings.
6.1. Analysis.

Comparison 6: Sensitivity analysis: published data: IF vs CER (Medium term), Outcome 1: Absolute change in Body weight (kg)
BMI (kg/m2)
Four trials of 115 patients compared IF to ad libitum (MD ‐0.92, 95% CI ‐1.36 to ‐0.48; I2 = 61%) (Analysis 4.2). Sensitivity analysis did not change our original findings.
4.2. Analysis.

Comparison 4: Sensitivity analysis; published data: IF vs Ad libitum (Short term), Outcome 2: Absolute change in BMI (kg/m2)
Eight trials of 642 patients compared IF with CER at short‐term follow‐up (MD ‐0.40, 95% CI ‐0.74 to ‐0.06; I2 = 38%) (Analysis 5.6). Sensitivity analysis did not change our original findings.
5.6. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 6: Absolute change in BMI (kg/m2)
Additionally, four trials with a total of 279 patients compared IF with CER at medium‐term follow‐up (MD ‐0.15, 95% CI ‐0.58 to 0.29; I2 = 0%) (Analysis 6.2). Sensitivity analysis did not change our original findings.
6.2. Analysis.

Comparison 6: Sensitivity analysis: published data: IF vs CER (Medium term), Outcome 2: Absolute change in BMI (kg/m2)
Waist circumference (cm)
Two trials of 87 patients compared IF with ad libitum (MD ‐4.19 cm, 95% CI ‐6.38 to ‐2.01; I2 = 0%) (Analysis 4.3). Sensitivity analysis did not change our original findings.
4.3. Analysis.

Comparison 4: Sensitivity analysis; published data: IF vs Ad libitum (Short term), Outcome 3: Absolute change in waist circumference (cm)
Seven trials of 548 patients compared IF with CER at short‐term follow‐up (MD ‐0.74 cm, 95% CI ‐2.08 to 0.59; I2 = 64%) (Analysis 5.7). Sensitivity analysis did not change our original findings.
5.7. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 7: Absolute change in waist circumference (cm)
Absolute change in total cholesterol levels (TC) (mmol/L)
Four trials of 125 patients compared IF with ad libitum (MD ‐0.31 mmol/L, 95% CI ‐0.51 to ‐0.12; I2 = 0%) (Analysis 4.4). Sensitivity analysis did not change our original findings.
4.4. Analysis.

Comparison 4: Sensitivity analysis; published data: IF vs Ad libitum (Short term), Outcome 4: Absolute change in total cholesterol levels (TC) (mmol/L)
Eight trials of 573 patients compared IF with CER at short‐term follow‐up (MD ‐0.09 mmol/L, 95% CI ‐0.20 to 0.02; I2 = 0%) (Analysis 5.8). Sensitivity analysis did not change our original findings.
5.8. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 8: Absolute change in total cholesterol (mmol/L)
Absolute change in low‐density lipoprotein cholesterol levels (LDL) (mmol/L)
Four trials of 125 patients compared IF with ad libitum (MD ‐0.22 mmol/L, 95% CI ‐0.40 to ‐0.05; I2 = 0%) (Analysis 4.5). Sensitivity analysis did not change our original findings.
4.5. Analysis.

Comparison 4: Sensitivity analysis; published data: IF vs Ad libitum (Short term), Outcome 5: Absolute change in LDL (mmol/L)
Eight trials of 560 patients compared IF with CER at short‐term follow‐up (MD ‐0.08 mmol/L, 95% CI ‐0.17 to 0.02; participants = 560; studies = 9; I2 = 0%) (Analysis 5.9). Sensitivity analysis did not change our original findings.
5.9. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 9: Absolute change in LDL (mmol/L)
Absolute change in high‐density lipoprotein cholesterol levels (HDL) (mmol/L)
Four trials of 125 patients compared IF with ad libitum (MD ‐0.10 mmol/L, 95% CI ‐0.25 to 0.05; I2 = 65%) (Analysis 4.6). Sensitivity analysis did not change our original findings.
4.6. Analysis.

Comparison 4: Sensitivity analysis; published data: IF vs Ad libitum (Short term), Outcome 6: Absolute change in HDL (mmol/L)
Eight trials of 560 patients compared IF with CER at short‐term follow‐up (MD ‐0.00 mmol/L, 95% CI ‐0.05 to 0.04; I2 = 37%) (Analysis 5.10). Sensitivity analysis did not change our original findings.
5.10. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 10: Absolute change in HDL (mmol/L)
Absolute change in total triglyceride levels (TG) (mmol/L)
Four trials of 125 patients compared IF with ad libitum (MD ‐0.06 mmol/L, 95% CI ‐0.25 to 0.14; I2 = 50%) (Analysis 4.7). Sensitivity analysis did not change our original findings.
4.7. Analysis.

Comparison 4: Sensitivity analysis; published data: IF vs Ad libitum (Short term), Outcome 7: Absolute change in TG (mmol/L)
Seven trials of 530 patients compared IF with CER at short‐term follow‐up (MD ‐0.09 mmol/L, 95% CI ‐0.18 to 0.00; I2 = 1%) (Analysis 5.11). Sensitivity analysis did not change our original findings.
5.11. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 11: Absolute change in TG (mmol/L)
Additionally, four trials of 279 patients compared IF with CER at medium‐term follow‐up (MD ‐0.02 mmol/L, 95% CI ‐0.16 to 0.12; I2 = 0%) (Analysis 6.7). Sensitivity analysis did not change our original findings.
6.7. Analysis.

Comparison 6: Sensitivity analysis: published data: IF vs CER (Medium term), Outcome 7: Absolute change in TG (mmol/L)
Absolute change in systolic blood pressure (SBP) (mmHg)
Five trials of 201 patients compared IF with ad libitum (MD ‐4.47 mmHg, 95% CI ‐6.94 to ‐2.01; I2 = 0%) (Analysis 4.8). Sensitivity analysis did not change our original findings.
4.8. Analysis.

Comparison 4: Sensitivity analysis; published data: IF vs Ad libitum (Short term), Outcome 8: Absolute change in SBP (mmHg)
Seven trials of 548 patients compared IF with CER at short‐term follow‐up (MD ‐1.75 mmHg, 95% CI ‐4.61 to 1.11; I2 = 24%) (Analysis 5.12). Sensitivity analysis did not change our original findings.
5.12. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 12: Absolute change in SBP (mmHg)
Absolute change in diastolic blood pressure (DBP) (mmHg)
Five trials of 201 patients compared IF with ad libitum (MD ‐1.07 mmHg, 95% CI ‐3.33 to 1.18; I2 = 0%) (Analysis 4.9). Sensitivity analysis did not change our original findings.
4.9. Analysis.

Comparison 4: Sensitivity analysis; published data: IF vs Ad libitum (Short term), Outcome 9: Absolute change in DBP (mmHg)
Seven trials of 548 patients compared IF to CER at short‐term follow‐up (MD ‐0.97 mmHg, 95% CI ‐2.35 to 0.42; participants = 548; studies = 8; I2 = 0%) (Analysis 5.13). Sensitivity analysis did not change our original findings.
5.13. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 13: Absolute change in DBP (mmHg)
CRP (mg/L)
Two trials of 43 patients compared IF to ad libitum with no difference in change in CRP between both groups (‐1.19 mg/L, 95% CI ‐2.54 to 0.16) and there was no heterogeneity (Analysis 4.10). Sensitivity analysis did not change our original findings.
4.10. Analysis.

Comparison 4: Sensitivity analysis; published data: IF vs Ad libitum (Short term), Outcome 10: Absolute change in CRP (mg/L)
Absolute change in fasting plasma glucose (mmol/L)
Three trials of 95 patients compared IF to ad libitum (MD ‐0.03 mmol/L, 95% CI ‐0.26 to 0.19; I2 = 15%) (Analysis 4.11). Sensitivity analysis did not change our original findings.
4.11. Analysis.

Comparison 4: Sensitivity analysis; published data: IF vs Ad libitum (Short term), Outcome 11: Absolute change in Glucose (mmol/L)
Eight trials of 573 patients compared IF to CER at short‐term follow‐up (MD ‐0.01 mmol/L, 95% CI ‐0.15 to 0.12; I2 = 73%) (Analysis 5.15). Sensitivity analysis did not change our original findings.
5.15. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 15: Absolute change in Glucose (mmol/L)
Additionally, three trials of 279 patients compared IF to CER at medium‐term follow‐up (MD 0.01 mmol/L, 95% CI ‐0.10 to 0.11; I2 = 0%) (Analysis 6.10). Sensitivity analysis did not change our original findings.
6.10. Analysis.

Comparison 6: Sensitivity analysis: published data: IF vs CER (Medium term), Outcome 10: Absolute change in glucose (mmol/L)
Absolute change in glycated haemoglobin (HbA1C) (mmol/L)
Three trials of 301 patients compared IF to CER at short‐term follow‐up. There was no difference in change in HbA1c between both groups (MD 0.01, 95% CI ‐0.07 to 0.09) and there was no heterogeneity (Analysis 5.16). Sensitivity analysis did not change our original findings.
5.16. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 16: Absolute change in HbA1c (mmol/L)
Discussion
Summary of main results
In total, 26 randomised controlled trials (RCTs) were included in this review, 18 of which (1125 participants) provided data for the quantitative synthesis. Of these studies, seven studies recruited participants who were overweight or obese; three recruited patients who were obese; and two studies excluded obese participants. Two studies only recruited participants with type 2 diabetes mellitus. Notably, none of the included RCTs were at low summary risk of bias (randomisation, allocation concealment, selection and detection bias all at low risk for supplementation trials; randomisation, allocation concealment and detection bias all at low risk for dietary advice trials).
Randomised controlled trials of the effects of intermittent fasting (IF) compared to ad libitum feeding, showed a reduction in value of some outcomes in favour of IF. However the results reported did not seem to be clinically significant. We attribute this to the primarily short‐term follow‐up periods for these parameters; the cardiometabolic effects of dietary interventions often need sufficient time to present, which would not be expected in studies reporting short‐term outcomes only. None of the trials found in the literature investigated the long‐term effects of IF on cardiometabolic risk factors. There was insufficient evidence to assess the effects of IF compared to ad libitum feeding on glycated haemoglobin (HbA1C) as it was reported in only one study (Schubel 2018). This was probably designed due to the short follow‐up periods of these studies.
Whilst a statistical difference was found to favour IF compared to ad libitum feeding in reducing patient weight, body mass index (BMI), waist circumference, reduction in total cholesterol (TC), and reduction in systolic blood pressure (SBP), the clinical significance of these findings may not be entirely evident. We decided to use a cut‐off of a difference of 5% or larger from baseline value as a cut‐off for clinically significant difference between both diets in accordance with previous literature (Pi‐Sunyer 2015; Topol 2010). None of the differences in parameters met our criteria for clinical significance, although this may be due to a short follow‐up period.
Compared to continuous energy restriction (CER), IF caused a reduction in some of the outcomes, but this was most obvious in body weight and BMI after short‐term follow‐up. However, none of the differences were large enough to meet our criteria for clinical significance. Furthermore, both diets were equally effective in terms of their effects on changes in TC, low‐density lipoprotein (LDL), high density lipoprotein (HDL), triglycerides (TG), systolic blood pressure (SBP), diastolic blood pressure (DBP), plasma glucose, HbA1C,or C‐reactive protein (CRP).
None of the studies identified in our review included any data on our intended primary outcomes of all‐cause mortality, cardiovascular mortality, stroke, myocardial infarction and heart failure at any point during follow‐up. As such, we cannot currently comment on the effect of IF compared to ad libitum feeding or CER on these outcomes.
Overall completeness and applicability of evidence
We conducted a careful search and used a set of comprehensive search strategies in attempt to find the full set of available RCTs in published literature that assess the effects of IF compared with CER and ad libitum feeding in our specified outcomes. This resulted in 18 trials that randomised a total of 1125 participants to IF, CER and ad libitum feeding. To reduce selection bias, we contacted authors of trials that appeared to have randomised appropriate participants to appropriate intervention and comparator but may not have published relevant outcomes at the time of conducting our search. If trial authors had assessed any of our outcomes, we requested data and included the trial. This enabled us to include several additional trials. Whilst we have made every possible effort to include all available data published on this topic, we acknowledge that there is a small possibility that some data may have been inadvertently missed.
None of the studies identified in our review included any data on our intended primary outcomes of all‐cause mortality, cardiovascular mortality, stroke, myocardial infarction and heart failure at any point during follow‐up. As such, we were unable to comment on the effect of IF compared to ad libitum feeding or CER on these outcomes. This reflects the paucity of available data on the effects of IF on these major adverse cardiovascular events. This illustrates a significant limitation in the clinical utility of the results of this review; whilst they demonstrate promising findings regarding the effects of IF on the well‐documented risk factors of cardiovascular disease, we are unable to comment as to whether this is sufficient to translate into reduced incidence of all‐cause mortality, stroke, myocardial infarction or heart failure.
Quality of the evidence
Figure 2; Figure 3 display the risk of bias of included trials. None of the 18 RCTs that provided data was at low summary risk of bias (at low risk of selection bias, performance bias and detection bias, plus the low risk of performance bias in supplemental trials). Predominantly, this was due to the fact that none of the studies were deemed at low risk of performance bias. This is a significant limitation in investigating lifestyle interventions such as dieting and intermittent fasting as blinding of participants is not easy in dietary studies. This is because the participants usually have to follow instructions to attain specific dietary goals. This is especially the case in intermittent fasting studies, in which specific meal timings are imposed on participants. Where participants are not blinded, it is difficult to ensure that study staff, healthcare providers and outcome assessors are blinded. We, therefore, judged blinding to be inadequate in all studies. However, it is noteworthy that aside from this fact, none of the RCTs in this study were deemed to be at low risk of bias in the remaining domains.
We had planned to assess the validity of evidence in meta‐analyses by running sensitivity analyses that removed trials not at low summary risk of bias. As none of the studies were deemed to be at low risk of bias, this was not possible.
Furthermore, a noteworthy limitation of our study is the exclusion of non‐randomised studies. This allowed for the inclusion of high‐quality studies as it eliminates selection bias as an important confounder of the results. Nevertheless, the use of non‐randomised trials would have increased the clinical generalisability of our results as such studies often reflect clinical practice more accurately than randomised trials.
Potential biases in the review process
Potential adverse effects include psychological, neurological and physical problems; whilst we have collated all available data regarding these adverse effects, there was an overall paucity of data regarding this aspect. However, we did not specifically contact the authors of included and excluded trials for additional data on these outcomes. Unfortunately, the data on adverse effects were insufficient to conduct a quantitative synthesis. We had predicted this in the study protocol and thus planned to conduct a qualitative analysis on this outcome.
Furthermore, one problem with cardiovascular disease outcomes is that they are all interconnected. For example, loss of weight is attributed to improvement in dyslipidaemia. Contrarily, there has been evidence to suggest that weight loss is associated with transient hypertriglyceridaemia (Phinney 1991). As such, it is indiscernible whether the results obtained in this study and its constituent RCTs were directly due to intermittent fasting or whether they arise due to these complex interactions. Moreover, most trials included in this meta‐analysis were short‐to‐medium term, with little data from long‐term trials. As such, we are unable to decipher whether these findings are a transient physiological reaction to a stressor or whether they are indeed secondary to the dietary interventions; thus posing an important confounder.
Similarly, a problem with dietary interventions is that they are strong psychosocial interventions aimed at improving patients' cardiovascular risks. This is often (but not always) accompanied by an increase in the patient's resolve to follow a "healthier" lifestyle. Prominently, studies have shown a significant increase the amount of physical exercise performed in patients who commence dietary interventions (Phinney 2004). This is yet another confounding variable that we did not account for in the analysis. The trials included in this study did not record whether a change in baseline physical activity was noted in the enrolled patients, neither did they report physical activity in patients of different groups. In addition, we did not specifically request such data from the trial authors. As such, this remains a confounding variable which was not accounted for in our analysis.
While we tried hard to locate all available trials and collect additional outcome data where possible, there was evidence of some small‐study bias. Some smaller trials showing increased weight with IF may be missing. If these trials were replaced they would tend to increase risk ratios. This suggests that there is some underlying small‐study bias within our review.
Furthermore, we had planned to conduct several sensitivity analyses. For example, we had planned to conduct a sensitivity analysis where we include only published trials where data are available from full‐text publications and exclude trials only available as abstracts. We had also planned to conduct a sensitivity analysis where we only included studies if ≥ 80% of the study population were eligible for our review; in all trials included, all the patients in the study were eligible for our review and thus this analysis was not possible. Finally, as previously discussed, we had planned to assess the validity of evidence in meta‐analyses by running sensitivity analyses that removed trials, not at low summary risk of bias. As none of the studies were deemed to be at low risk of bias, this was not possible.
Due to high levels of attrition in the included studies, we used per‐protocol analysis. Intention‐to‐treat analysis was not possible due to missing data. The majority of our included studies reported their outcomes using per‐protocol analysis. We only imputed data for one study (Pinto 2019). We considered imputing missing data for more studies, however, we were uncertain of whether missing data would be indeed imputable. This is because we cannot ascertain if patients lost to follow‐up would show similar outcomes as those who adhered to the dietary intervention. We recognise the limitations of this approach, namely introducing bias. Notably, intermittent fasting requires strict adherence; this limits the generalisability of our data as results from per‐protocol analysis only apply to the cohort of patients who have indeed adhered to the intervention. Previous studies have suggested that intermittent fasting may not be sustainable in the long‐term due to high attrition amongst participants in clinical trials (Trepanowski 2017). This is reflected in our findings where a high risk of attrition bias was noted amongst our included studies.
Agreements and disagreements with other studies or reviews
This is the first Cochrane Review investigating the effects of intermittent fasting on cardiovascular disease. One recent review (Welton 2020) included randomised and observational trials of intermittent fasting and its effects on cardiovascular risk factors; most notably, weight loss. This study concluded that studies comparing intermittent fasting to calorie restriction found equivalent results. This conflicts with our findings that intermittent fasting seems to cause a reduction in BMI when compared to CER. There are several reasons for this discrepancy. First, numerous types of intermittent fasting exist, and the inclusion of different types of intermittent fasting may explain this. For example, their review does not provide specific definitions for intermittent fasting. In our review, we have defined intermittent fasting by the different subtypes commonly discussed in the literature. The majority of the time, modified alternate‐day fasting (ADF) is defined as a period of caloric restriction for one day which includes 25% or less of maintenance caloric requirement. However, in certain studies, the percentage caloric intake was not given and instead a numerical caloric intake was provided. For this, we included studies which had a consumption of ≤ 600 calories. With regards to time‐restricted feeding (TRF), the majority of studies highlighted a minimum of around 12 hours of fasting. This was used as a cut‐off in this review.
The discordance in the definition may result in a discrepancy in the inclusion or exclusion of trials, thus leading to different results. Another explanation for the discrepancy may be due to their inclusion of non‐randomised studies. Whilst these studies are invaluable for providing epidemiological data, the observational nature of these trials yields a high risk of bias and introduces several confounding variables. For this reason, we chose to exclude these studies from our analysis, as is the protocol for most reviews conducted under the Cochrane Heart group. Despite these differences, the (Welton 2020) review documented improved glycaemic control with IF, findings comparable to our own.
In addition, another systematic review (Harris 2018) showed similar effects of IF compared to ad libitum feeding for weight loss (−4.14 kg; 95% CI −6.30 to −1.99; P ≤ 0.001), but no significant difference between IF and CER for weight loss (−1.03 kg; 95% CI −2.46 to 0.40; P = 0.156). In addition to the aforementioned reasons, we attribute the discrepancy between their findings and ours to the relatively fewer studies in their review compared to this review.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence regarding the role of intermittent fasting in the primary and secondary prevention of cardiovascular disease. The individual meta‐analyses show that intermittent fasting may be effective in reducing weight when compared to ad libitum feeding and may be as effective as continuous energy restriction. Despite this, these changes appear to be clinically insignificant at short‐term follow‐up. The quality of the available evidence is low to very low which mean sthat many areas of uncertainty remain. Further research is needed to understand which patient groups would and would not benefit from intermittent fasting. This includes patients with diabetes and patients with eating disorders. Currently, there is a scarcity of safety data and future randomised controlled trials (RCTs) need to address the safety of intermittent fasting along with the efficacy and provide a valid risk‐benefit analysis for such patient groups (e.g. patients with diabetes or eating disorders).
Implications for research.
As mentioned above, it would be useful to study intermittent fasting in specific patient groups and to determine where it can and cannot be indicated. RCTs in the future should explicitly integrate the safety of intermittent fasting into each study. Furthermore, this review reported no data on primary outcomes such as all‐cause mortality, cardiovascular mortality, stroke, myocardial infarction and heart failure. It would be useful to see whether intermittent fasting may be beneficial in these outcomes but may require longer‐term studies with regular follow‐up. We suggest a prospectively randomised open blinded end‐point (PROBE) study which compares long‐term outcomes between intermittent fasting, calorie restriction and ad libitum feeding. We especially suggest a follow‐up of five years or greater in order to assess major adverse cardiovascular events. Ideally, separate trials should include patients with and without established cardiovascular disease as well as cardiovascular risk factors. We hope this would ascertain the benefit of intermittent fasting in the primary and secondary prevention of cardiovascular disease.
What's new
| Date | Event | Description |
|---|---|---|
| 25 February 2021 | Amended | The conclusion in the abstract section has been reworded to better reflect the results of this review. |
History
Protocol first published: Issue 11, 2019 Review first published: Issue 1, 2021
Acknowledgements
The background and methods section of this review is based on a standard template provided by Cochrane Heart. The review authors would like to acknowledge Nicole Martin and the rest of the Cochrane Heart group staff for their support in developing this review.
The authors would also like to acknowledge the following in helping review the protocol:
Benjamin D Horne (Intermountain Medical Center, Heart Institute and Stanford University, School of Medicine) for his peer review contribution.
Ann E. Fonfa and Negar Jamshidi (RMIT University, Melbourne, Australia) for their consumer review contribution of the protocol.
The authors would like to acknowledge the following in helping review the full systematic review:
Andrea Takeda (Cochrane Heart group) for her contribution as systematic review specialist
Charlene Bridges (Cochrane Heart group) for her contribution as information specialist
Mahmood Ahmad (Cardiology Department at the Royal Free Hospital, Royal Free London NHS Foundation Trust London UK) as contact editor
Dr. Joana M. Correia (Neuromuscular Research Lab and CIPER, Faculty of Human Motricity, University of Lisbon, Estrada da Costa, 1499‐002 Cruz Quebrada, Dafundo, Portugal) as a peer reviewer
Benjamin D. Horne (Intermountain Medical Center Heart Institute and Stanford University School of Medicine) as a peer reviewer
Ann Fonfa (Annie Appleseed Project) as a consumer reviewer
Appendices
Appendix 1. Search strategies
CENTRAL
#1MeSH descriptor: [Fasting] this term only #2(intermittent* near/3 fast*):ti,ab #3(fast* near/3 diet*).ti,ab #4(alternat* near/3 fast*):ti,ab #5(modified near/2 fast*):ti,ab #6(food next (abstinence or fast*)):ti,ab #7((diet* or food) near/2 restricti*):ti,ab #8time restricted feed*:ti,ab #9time restricted fast*:ti,ab #10whole day fast*:ti,ab #11food tim*:ti,ab #12(Ramadan or Ramadhan):ti,ab #13#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
MEDLINE (Ovid)
1 Fasting/ (34093) 2 Fasting.tw. (103717) 3 (intermittent* adj3 fast*).tw. (520) 4 (fast* adj3 diet*).tw. (1888) 5 (alternat* adj3 fast*).tw. (1362) 6 (modified adj2 fast*).tw. (588) 7 (food adj (abstinence or fast*)).tw. (85) 8 ((diet* or food) adj2 restricti*).tw. (12809) 9 time restricted feed*.tw. (118) 10 time restricted fast*.tw. (1) 11 whole day fast*.tw. (1) 12 food tim*.tw. (81) 13 (Ramadan or Ramadhan).tw. (1189) 14 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 (130713) 15 randomized controlled trial.pt. (488336) 16 controlled clinical trial.pt. (93253) 17 randomized.ab. (453148) 18 placebo.ab. (200411) 19 clinical trials as topic.sh. (188167) 20 randomly.ab. (317270) 21 trial.ti. (203972) 22 15 or 16 or 17 or 18 or 19 or 20 or 21 (1235613) 23 exp animals/ not humans.sh. (4613577) 24 22 not 23 (1136377) 25 14 and 24 (19281)
Embase (Ovid)
1 fasting/ 2 Fasting.tw. 3 (intermittent* adj3 fast*).tw. 4 (fast* adj3 diet*).tw. 5 (alternat* adj3 fast*).tw. 6 (modified adj2 fast*).tw. 7 (food adj (abstinence or fast*)).tw. 8 ((diet* or food) adj2 restricti*).tw. 9 time restricted feed*.tw. 10 time restricted fast*.tw. 11 whole day fast*.tw. 12 food tim*.tw. 13 (Ramadan or Ramadhan).tw. 14 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 random$.tw. 16 factorial$.tw. 17 crossover$.tw. 18 cross over$.tw. 19 cross‐over$.tw. 20 placebo$.tw. 21 (doubl$ adj blind$).tw. 22 (singl$ adj blind$).tw. 23 assign$.tw. 24 allocat$.tw. 25 volunteer$.tw. 26 crossover procedure/ 27 double blind procedure/ 28 randomized controlled trial/ 29 single blind procedure/ 30 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 31 (animal/ or nonhuman/) not human/ 32 30 not 31 33 14 and 32 34 limit 33 to embase
Clinical trials registers
"intermittent fasting" OR "time restricted feed" OR "alternate day fast"
Data and analyses
Comparison 1. IF vs Ad libitum (Short term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Absolute change in body weight (kg) | 7 | 224 | Mean Difference (IV, Random, 95% CI) | ‐2.88 [‐3.96, ‐1.80] |
| 1.2 Absolute change in BMI (kg/m2). | 4 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.36, ‐0.48] |
| 1.3 Absolute change in waist circumference (cm) | 2 | 87 | Mean Difference (IV, Random, 95% CI) | ‐4.19 [‐6.38, ‐2.01] |
| 1.4 Absolute change in total cholesterol levels (TC) (mmol/L) | 4 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.51, ‐0.12] |
| 1.5 Absolute change in LDL (mmol/L | 4 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.40, ‐0.05] |
| 1.6 Absolute change in HDL (mmol/L | 4 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.25, 0.05] |
| 1.7 Absolute change in TG (mmol/L) | 4 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.25, 0.14] |
| 1.8 Absolute change in SBP (mmHg) | 5 | 201 | Mean Difference (IV, Random, 95% CI) | ‐4.47 [‐6.94, ‐2.01] |
| 1.9 Absolute change in DBP (mmHg) | 5 | 201 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐3.33, 1.18] |
| 1.10 Absolute change in CRP (mg/L) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.54, 0.16] |
| 1.11 Absolute change in Glucose (mmol/L) | 3 | 95 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.26, 0.19] |
Comparison 2. IF vs CER (Short term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Absolute change in Body Weight (Total) (kg) | 10 | 719 | Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.76, 0.00] |
| 2.2 Absolute change in Body Weight (Fasting subgroups) (kg) | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 ADF | 2 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐2.34, 1.65] |
| 2.2.2 MADF | 1 | 69 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐3.71, ‐1.09] |
| 2.2.3 PF | 7 | 557 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.77, 0.11] |
| 2.3 Absolute change in Body Weight (Female subgroup) (Kg) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.3.1 Female | 3 | 226 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.96, 0.84] |
| 2.3.2 Male | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 2.4 Absolute change in Body Weight (Overweight subgroups) (kg) | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.4.1 Overweight and obese | 9 | 710 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.66, 0.12] |
| 2.4.2 Non‐overweight | 1 | 9 | Mean Difference (IV, Random, 95% CI) | ‐3.50 [‐7.41, 0.41] |
| 2.5 Absolute change in Body Weight (Diabetes subgroups) (kg) | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.5.1 Diabetics | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐4.14, ‐0.29] |
| 2.5.2 Non‐diabetics | 8 | 573 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.63, 0.26] |
| 2.6 Absolute change in BMI (kg/m2). | 9 | 651 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.76, ‐0.10] |
| 2.7 Absolute change in waist circumference (cm) | 8 | 557 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐2.11, 0.44] |
| 2.8 Absolute change in total cholesterol (mmol/l) | 8 | 539 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.18, 0.03] |
| 2.9 Absolute change in LDL (mmol/L) | 9 | 569 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.16, 0.01] |
| 2.10 Absolute change in HDL (mmol/L) | 9 | 569 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 2.11 Absolute change in TG (mmol/L) | 8 | 539 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.19, 0.06] |
| 2.12 Absolute change in SBP (mmHg) | 7 | 548 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐4.61, 1.11] |
| 2.13 Absolute change in DBP (mmHg) | 7 | 548 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐2.35, 0.42] |
| 2.14 Absolute change in CRP (mg/L) | 2 | 190 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.56, 1.17] |
| 2.15 Absolute change in Glucose (mmol/L | 9 | 582 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.16, 0.12] |
| 2.16 Absolute change in HbA1c (mmol/L) | 4 | 310 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.07, 0.08] |
Comparison 3. IF vs CER (Medium term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Absolute change in Body weight (kg) | 4 | 279 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.68, 0.56] |
| 3.2 Absolute change in BMI (kg/m2) | 4 | 279 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.58, 0.29] |
| 3.3 Absolute change in waist circumference (cm) | 3 | 258 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐2.55, 1.23] |
| 3.4 Absolute change in total cholesterol (mmol/L) | 3 | 258 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.17, 0.10] |
| 3.5 Absolute change in LDL (mmol/L) | 3 | 258 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.18, 0.05] |
| 3.6 Absolute change in HDL (mmol/L) | 3 | 258 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.07, 0.07] |
| 3.7 Absolute change in TG (mmol/L) | 4 | 279 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.16, 0.12] |
| 3.8 Absolute change in SBP (mmHg) | 3 | 258 | Mean Difference (IV, Random, 95% CI) | 1.37 [‐4.98, 7.72] |
| 3.9 Absolute change in DBP (mmHg) | 3 | 258 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐4.67, 2.67] |
| 3.10 Absolute change in CRP (mg/L) | 1 | 89 | Mean Difference (IV, Random, 95% CI) | 0.46 [‐0.87, 1.79] |
| 3.11 Absolute change in glucose (mmol/L) | 4 | 279 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.10, 0.11] |
Comparison 4. Sensitivity analysis; published data: IF vs Ad libitum (Short term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Absolute change in body weight (kg) | 6 | 203 | Mean Difference (IV, Random, 95% CI) | ‐3.04 [‐4.45, ‐1.62] |
| 4.2 Absolute change in BMI (kg/m2) | 4 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.36, ‐0.48] |
| 4.3 Absolute change in waist circumference (cm) | 2 | 87 | Mean Difference (IV, Random, 95% CI) | ‐4.19 [‐6.38, ‐2.01] |
| 4.4 Absolute change in total cholesterol levels (TC) (mmol/L) | 4 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.51, ‐0.12] |
| 4.5 Absolute change in LDL (mmol/L) | 4 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.40, ‐0.05] |
| 4.6 Absolute change in HDL (mmol/L) | 4 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.25, 0.05] |
| 4.7 Absolute change in TG (mmol/L) | 4 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.25, 0.14] |
| 4.8 Absolute change in SBP (mmHg) | 5 | 201 | Mean Difference (IV, Random, 95% CI) | ‐4.47 [‐6.94, ‐2.01] |
| 4.9 Absolute change in DBP (mmHg) | 5 | 201 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐3.33, 1.18] |
| 4.10 Absolute change in CRP (mg/L) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.54, 0.16] |
| 4.11 Absolute change in Glucose (mmol/L) | 3 | 95 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.26, 0.19] |
Comparison 5. Sensitivity analysis; published data: IF vs CER (Short term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 kbsolute change in Body Weight (Total) (kg) | 9 | 710 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.66, 0.12] |
| 5.2 Absolute change in Body Weight (Fasting subgroups) (kg) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.2.1 ADF | 2 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐2.34, 1.65] |
| 5.2.2 MADF | 1 | 69 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐3.71, ‐1.09] |
| 5.2.3 PF | 6 | 548 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.61, 0.24] |
| 5.3 Absolute change in Body Weight (Female subgroups) (kg) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.3.1 Female only | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐2.75, 2.54] |
| 5.3.2 Male only | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 5.4 Absolute change in Body Weight (Overweight subgroups) (kg) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.4.1 Overweight and obese | 9 | 710 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.66, 0.12] |
| 5.4.2 Non‐overweight only | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 5.5 Absolute change in Body Weight (Diabetes subgroups) (Kg) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.5.1 Diabetics | 1 | 137 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐4.01, 0.41] |
| 5.5.2 Non‐diabetics | 8 | 573 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.63, 0.26] |
| 5.6 Absolute change in BMI (kg/m2) | 8 | 642 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.74, ‐0.06] |
| 5.7 Absolute change in waist circumference (cm) | 7 | 548 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐2.08, 0.59] |
| 5.8 Absolute change in total cholesterol (mmol/L) | 8 | 573 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.20, 0.02] |
| 5.9 Absolute change in LDL (mmol/L) | 8 | 560 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.17, 0.02] |
| 5.10 Absolute change in HDL (mmol/L) | 8 | 560 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 5.11 Absolute change in TG (mmol/L) | 7 | 530 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.18, 0.00] |
| 5.12 Absolute change in SBP (mmHg) | 7 | 548 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐4.61, 1.11] |
| 5.13 Absolute change in DBP (mmHg) | 7 | 548 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐2.35, 0.42] |
| 5.14 Absolute change in CRP (mg/L) | 2 | 190 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.56, 1.17] |
| 5.15 Absolute change in Glucose (mmol/L) | 8 | 573 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.15, 0.12] |
| 5.16 Absolute change in HbA1c (mmol/L) | 3 | 301 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.07, 0.09] |
5.2. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 2: Absolute change in Body Weight (Fasting subgroups) (kg)
5.3. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 3: Absolute change in Body Weight (Female subgroups) (kg)
5.4. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 4: Absolute change in Body Weight (Overweight subgroups) (kg)
5.5. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 5: Absolute change in Body Weight (Diabetes subgroups) (Kg)
5.14. Analysis.

Comparison 5: Sensitivity analysis; published data: IF vs CER (Short term), Outcome 14: Absolute change in CRP (mg/L)
Comparison 6. Sensitivity analysis: published data: IF vs CER (Medium term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Absolute change in Body weight (kg) | 4 | 279 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.68, 0.56] |
| 6.2 Absolute change in BMI (kg/m2) | 4 | 279 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.58, 0.29] |
| 6.3 Absolute change in waist circumference (cm) | 3 | 258 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐2.55, 1.23] |
| 6.4 Absolute change in total cholesterol (mmol/L) | 3 | 258 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.17, 0.10] |
| 6.5 Absolute change in LDL (mmol/L) | 3 | 258 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.18, 0.05] |
| 6.6 Absolute change in HDL (mmol/L) | 3 | 258 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.07, 0.07] |
| 6.7 Absolute change in TG (mmol/L) | 4 | 279 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.16, 0.12] |
| 6.8 Absolute change in SBP (mmHg) | 3 | 258 | Mean Difference (IV, Random, 95% CI) | 1.37 [‐4.98, 7.72] |
| 6.9 Absolute change in DBP (mmHg) | 3 | 258 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐4.67, 2.67] |
| 6.10 Absolute change in glucose (mmol/L) | 4 | 279 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.10, 0.11] |
6.3. Analysis.

Comparison 6: Sensitivity analysis: published data: IF vs CER (Medium term), Outcome 3: Absolute change in waist circumference (cm)
6.4. Analysis.

Comparison 6: Sensitivity analysis: published data: IF vs CER (Medium term), Outcome 4: Absolute change in total cholesterol (mmol/L)
6.5. Analysis.

Comparison 6: Sensitivity analysis: published data: IF vs CER (Medium term), Outcome 5: Absolute change in LDL (mmol/L)
6.6. Analysis.

Comparison 6: Sensitivity analysis: published data: IF vs CER (Medium term), Outcome 6: Absolute change in HDL (mmol/L)
6.8. Analysis.

Comparison 6: Sensitivity analysis: published data: IF vs CER (Medium term), Outcome 8: Absolute change in SBP (mmHg)
6.9. Analysis.

Comparison 6: Sensitivity analysis: published data: IF vs CER (Medium term), Outcome 9: Absolute change in DBP (mmHg)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amodio 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Menopausal women with metabolic syndrome Inclusion criteria: none provided Exclusion criteria: none provided |
|
| Interventions |
2‐arm trial Intervention (n = 12): moderately hypocaloric diet (1600 Kcal/day) during 8 hours (7 AM‐3 PM; Comparator (n = 11): moderately hypocaloric diet (1600 Kcal/day) for 45 days ad libitum |
|
| Outcomes | Weight loss, fasting plasma glucose, triglycerides | |
| Notes |
Type of paper: abstract only. Funding: no data on sources of funding were provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only and method of randomisation not stated in abstract. |
| Allocation concealment (selection bias) | Unclear risk | Abstract only and allocation concealment not indicated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Abstract only and blinding of outcome assessment not indicated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only and loss to follow‐up not reported in abstract. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. |
| Other bias | Unclear risk | Abstract only. |
Bhutani 2013.
| Study characteristics | ||
| Methods | Randomised, controlled, parallel‐arm feeding trial | |
| Participants | 83 participants based in the USA aged between 25‐65 years. BMI between 30
and 39.9 kg/m2. Inclusion criteria: age 25‐65 years; BMI between 30 and 39.9 kg/m2; weight stable for 3 months prior to the beginning of the study (i.e. less than 5 kg weight loss or weight gain); nondiabetic; no history of cardiovascular disease; lightly active (i.e. <3 hours/week of light intensity exercise at 2.5‐4.0 metabolic equivalents [METs] for 3 months prior to the study); nonsmoker; no history of bariatric surgery; and not taking weight loss, lipid, or glucose lowering medications. Exclusion criteria: peri‐menopausal women were excluded from the study, and post‐menopausal women (absence of menses for more than 2 years) were required to maintain their current hormone replacement therapy regimen for the duration of the study. |
|
| Interventions |
4‐arm trial Intervention (n = 16): modified Alternate Day fasting (ADF) (25% of normal) Comparator (n = 16):a d libitum feeding Other arms not included: combination (ADF+ exercise) and exercise‐alone arm |
|
| Outcomes | Body weight, waist circumference, blood pressure | |
| Notes |
Type of paper: full‐text publication Funding:
ADF‐ alternate day fasting |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed for each stratum by selecting an intervention at random from an opaque envelope." |
| Allocation concealment (selection bias) | Low risk | Quote:"Randomization was performed for each stratum by selecting an intervention at random from an opaque envelope." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Additional participants were randomised to groups that had high dropout rates (i.e. the ADF and exercise group) to ensure that the total number of participants would be the same in each group at the end of the study. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Low risk | Nothing to note. |
Carter 2018.
| Study characteristics | ||
| Methods | Parallel‐arm randomised clinical trial | |
| Participants | 137 participants based in Australia aged ≥ 18 years with type 2 diabetes
who were overweight or obese (BMI ≥ 27). Inclusion criteria: adults (≥18 years of age) with type 2 diabetes who were overweight or obese (BMI ≥ 27 [calculated as weight in kilograms divided by height in meters squared]) Exclusion criteria: pregnant or breastfeeding. |
|
| Interventions |
2‐arm trial Intervention (n = 70): intermittent energy restriction group followed a diet of 500 to 600 kcal/day for 2 days of the week and followed their usual diet for the other 5 days. Comparator (n = 67): continuous energy restriction |
|
| Outcomes | Body weight, BMI, HbA1c | |
| Notes |
Type of paper: abstract Funding: Ms Carter was supported by a University of South Australia postgraduate award. Dr Clifton was supported by a National Health and Medical Research Council principal research fellowship. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Randomization was completed using an online generated random number allocation sequence and was not blinded; participants were allocated to groups by the study dietitian according to the randomization schedule." |
| Allocation concealment (selection bias) | High risk | There was lack of blinding when assigning the interventions to the participants; quote:"participants were allocated to groups by the study dietitian." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rates of dropout; quote: "97 participants (70.8%) completed the study, and the dropout rates were similar in both groups (21 participants [31.3%] in the continuous energy restriction group and 19 participants [27.1%] in the intermittent energy restriction group; P = .71)." |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Low risk | Nothing to note. |
Catenacci 2016.
| Study characteristics | ||
| Methods | Parallel randomised clinical trial | |
| Participants | 29 participants based in the USA aged between 18 and 55 years with a BMI
> 30. Inclusion criteria: (18‐55 years, BMI ≥30 kg/m2, non‐smoker, ≤4.5 kg weight change over past 6 months) were invited to a screening visit. Exclusion criteria: volunteers were excluded if they had diabetes, CVD, uncontrolled hypertension, severe dyslipidaemia (or were on lipid‐lowering therapy), cancer, thyroid disease, seizures, migraines, significant renal, hepatic or gastrointestinal disorders, binge eating disorder, current depression, history of bariatric surgery, or were taking medications known to affect appetite or energy metabolism. Women who were currently pregnant, planning pregnancy, or lactating were also excluded. |
|
| Interventions |
2‐arm trial Intervention (n = 13): zero‐calorie alternate day fasting Comparator (n = 12): continuous calorie restriction |
|
| Outcomes | Body weight, BMI, lipid profile, glucose | |
| Notes |
Type of paper: full‐text publication Funding: NIH R21 AT002617‐02, NIH UL1 TR001082, NIH DK 048520, the Colorado Obesity Research Institute (CORI), and the Intramural Research Program of the National Institute on Aging. Dr. Melanson is supported with resources and the use of facilities at the Denver VA Medical Center. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | There was no mention of a method for allocation sequence concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rates of dropout; Of the 45 people who consented at screening, 16 participants (35.6%) either dropped out or were withdrawn prior to randomisation. Then, 1 of the 15 participants (6.67%) in the ADF group dropped out from the 8‐week intervention, and 2 out of 14 participants (14.3%) withdrawn from the CR group in this period. By the 24‐week follow‐up, the dropout rate was 21.4% (3 out of 14 participants) in the ADF group and 16.7% (2 out of 12 participants) in the CR group. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Low risk | Nothing to note. |
Cho 2019.
| Study characteristics | ||
| Methods | Multiple‐arm randomised controlled clinical trial | |
| Participants | 112 participants based in South Korea aged between 20 and 65 with a BMI
> 23. Inclusion criteria and exclusion criteria: Eligibility requirements were: age 20–65 years; BMI >23.0 kg/m2 (overweight or obese for Asian populations, according to the World Health Organization [17]); stable weight for 3 months prior to the study (i.e. weight loss or weight gain <5 kg); no history of bariatric surgery; no secondary obesity, such as hypothyroidism; non‐diabetic; aspartate amino‐transferase (ASP) or alanine amino‐transferase (ALT) levels < 200 mg/dL; serum creatinine level < 2.0 mg/dL, no pancreatitis or related disorders; no acute infectious diseases (i.e. pneumonia, acute enteritis, or urinary infection); no chronic inflammatory diseases (i.e. rheumatoid arthritis, or lupus); no history of cardiovascular diseases; no history of cancer; not taking anti‐obesity, anti‐diabetic, diuretic, central‐nervous system, antidepressant, antipsychotic, or steroid medications; no pregnant or lactating women; no overeating behaviour; no >30 g of daily alcohol intake; not a night‐time or shift‐work worker; no chronic malabsorption syndrome or cholestasis; no other medical conditions that would preclude subjects from participating in exercise and physical test. |
|
| Interventions |
2‐arm trial Intervention (n = 8): modified alternate day fasting (25% of normal) Comparator (n = 5): ad libitum feeding |
|
| Outcomes | Body weight, BMI, lipid profile, CRP, glucose | |
| Notes |
Type of paper: full‐text publication Funding: This study was supported by a 2013 Faculty Research Grant from the Yonsei University College of Medicine (6-2013-0021) and the Bio & Medical Technology Development Program, through the National Research Foundation of Korea funded by the Ministry of Science and ICT (NRF-2013M3A9B6046413, NRF-2018R1D1A1B07049223). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Block randomization was performed with a computer‐generated random number sequence. An independent statistician generated the allocation sequence." |
| Allocation concealment (selection bias) | Unclear risk | Quote:"An independent statistician generated the allocation sequence, and the study coordinator assigned the participants to interventions in chronological order as the participants enrolled. Only outcome assessors were blinded to group allocation" It is unclear what was meant by assignment in chronological order, and this statement suggests that the allocation sequence was not concealed from the study coordinators, in contradiction to the later statement that "only outcome assessors were blinded to group allocation". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rates of dropout; Dropout of 7 out of 26 participants (26.9%) in ADF group. Dropout of 6 out of 22 participants (27.3%) in control group |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the Methods section were reported in the Results section. An assessment based on ClinicalTrials.gov (NCT03652532) cannot be made because the trial was registered post‐hoc. |
| Other bias | Low risk | Nothing to note. |
Chow 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 21 overweight participants: 18F/3M, mean (SE), age: 44.7 years (2.7),
BMI:34.6 kg/m2(1.7) Inclusion and exclusion criteria: nothing noted |
|
| Interventions |
2 arm trial Intervention (n=11): time restricted feeding Comparator (n=10): ad libitum feeding |
|
| Outcomes | Weight, lean mass, triglycerides, visceral fat, glucose | |
| Notes |
Type of paper: abstract only Funded by: University of Minnesota Healthy Foods Healthy Lives (17SFR‐2YR50LC) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only and method of randomisation not stated in abstract. |
| Allocation concealment (selection bias) | Unclear risk | Abstract only and allocation concealment not indicated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Abstract only and blinding of outcome assessment not indicated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only and loss to follow‐up not reported in abstract. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. |
| Other bias | Unclear risk | Abstract only. |
Conley 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 24 Participants Inclusion and exclusion criteria: nothing noted |
|
| Interventions |
2‐arm trial Intervention: twice‐weekly diet Comparator: standard energy‐restricted diet (SERD) (2050 KJ (500 calorie) reduction per day) for 6 months. |
|
| Outcomes | Weight, waist circumference (WC), fasting blood glucose, blood lipids, blood pressure and dietary intake were measured at baseline | |
| Notes |
Type of paper: abstract only Funding: no data provided Abstract did not provide detail on how many people were in each arm of the trial and no contact details were provided, therefore the few data provided in the abstract cannot be included in the meta‐analysis at this stage. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only and method of randomisation not stated in abstract. |
| Allocation concealment (selection bias) | Unclear risk | Abstract only and allocation concealment not indicated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Abstract only and blinding of outcome assessment is only indicated by quote: "... [outcomes were] measured at baseline, 3 and 6 months by a blinded investigator" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only and loss to follow‐up not reported in abstract. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. |
| Other bias | Unclear risk | Abstract only. |
Corley 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Obese and overweight men Inclusion and exclusion criteria: no data provided |
|
| Interventions |
2‐arm trial Intervention: intermittent fast consisting of two days of 25% restriction and 5 days of isocaloric intake per week over six weeks Comparator: 79% daily restriction |
|
| Outcomes | Primary outcomes: body composition, resting energy expenditure. Secondary outcomes were change in HbA1c, blood pressure, fasting lipids, leptin,ghrelin, adiponectin and thyroid function tests | |
| Notes |
Type of paper: abstract only Funding: no data There were no contact details provided to contact the authors to include any data, therefore this study has not been quantitatively analysed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only and method of randomisation not stated in abstract. |
| Allocation concealment (selection bias) | Unclear risk | Abstract only and allocation concealment not indicated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Abstract only and blinding of outcome assessment not indicated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only and loss to follow‐up not reported in abstract. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. |
| Other bias | Unclear risk | Abstract only. |
Ferraris 2019.
| Study characteristics | ||
| Methods | Randomised controlled clinical trial | |
| Participants | 18 professional cyclists Inclusion and exclusion criteria: none provided |
|
| Interventions |
2‐arm trial Intervention: 30 days of isocaloric time‐restricted feeding performed with the 16/8 method (16 hours fasting and 8 hour window for feeding) Comparator: ad libitum feeding |
|
| Outcomes | Body fat percentage, leucocyte number, IGF1 | |
| Notes |
Type of paper: abstract only Funding: no data provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only and method of randomisation not stated in abstract. |
| Allocation concealment (selection bias) | Unclear risk | Abstract only and allocation concealment not indicated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Abstract only and blinding of outcome assessment not indicated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reported that 1 participant out of 9 (11.1%) left the TRF group and 1 participant out of 9 (11.1%) left the control group. These represent high attrition rates due to the small sample size. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. |
| Other bias | Unclear risk | Abstract only. |
Griffiths 2016.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | 9 participants in Australia Inclusion and exclusion criteria: no data provided |
|
| Interventions |
2‐arm trial Intervention (n = 4): periodic fasting. 3 very low energy diet (VLED) shakes a day (~2,500 kJ/day) on any 2 days a week and to eat to appetite on other days. Comparator (n = 5): participants randomised to continuous energy restriction were instructed to reduce their energy intake by 30% everyday in accordance with the Australian Guide to Healthy Eating |
|
| Outcomes | Body weight, BMI, waist circumference, lipid profile, glucose, HbA1c | |
| Notes |
Type of paper: abstract only Funding: no data provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only and method of randomisation not stated in abstract. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 out of the 5 participants (20%) dropped out in IF arm. |
| Selective reporting (reporting bias) | Unclear risk | Unclear from abstract. |
| Other bias | Unclear risk | Not stated. |
Harvie 2011.
| Study characteristics | ||
| Methods | Parallel‐arm randomised clinical trial | |
| Participants | 107 premenopausal women based in the UK aged between 30 and 45 with a BMI
between 24 and 40 kg/m2 Inclusion criteria and exclusion criteria: participants were non‐smokers, not currently dieting or losing weight, with regular menstrual cycles and no evidence of hyperandrogenism or polycystic ovary syndrome, and no oral contraceptive use during the previous 6 months. They did not have high intakes of alcohol (>28 units/week) or phytoestrogens, and were not suffering from diagnosed diabetes, CVD, major psychiatric morbidity or cancer. |
|
| Interventions |
2‐arm trial Intermittent fasting (n = 45): periodic fasting 2 consecutive days per week consuming 25% of normal caloric intake. Comparator (n = 47): continuous energy restriction |
|
| Outcomes | Weight change, insulin sensitivity, total cholesterol and serum LDL levels, serum HDL levels, triglycerides, andSBP and D BP. | |
| Notes |
Type of paper: full‐text publication Funding: Breast Cancer Campaign, World Cancer Research Fund, Genesis Appeal Manchester UK, Intramural Research Program of the National Institute on Aging of the NIH, The Danish Research Council for Health and Disease, Tanita Europe BV Middlesex UK for provision of Tanita TBF‐300. Author was contacted for raw data to calculate absolute changes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | There was no mention of a method for allocation sequence concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory personnel were blinded to the sample identity. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Eighteen women withdrew from the study before 6 months (IER=11, CER=7) representing 21% IER and 13% CER subjects (χ2 =1.16, P=0.28). The main reasons for drop out were comparable between the groups: stress (IER=3, CER=2), pregnancy (IER=2, CER=1), change in employment (IER=2, CER=1), problems adhering to the diet (IER=3, CER=3) and personal illness (infected pacemaker, IER=1)." |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the Methods section were reported in the Results section. |
| Other bias | Low risk | Nothing to note. |
Harvie 2013.
| Study characteristics | ||
| Methods | Multiple‐arm randomised clinical trial | |
| Participants | 115 overweight women based in the UK aged between 20 and 69 with a BMI
between 24–45 kg/m2 Inclusion criteria: women were eligible for the study if their BMI was 24–45 kg/m2 and/or body fat was >30% of total weight, and their reported adult weight gain since the age of 20 years exceeded 7 kg. There was no age restriction but participants entered were between 20 and 69 years of age. Exclusion criteria: women were excluded if they were currently dieting or losing weight, or suffering from diabetes, major CVD, respiratory, psychiatric or musculoskeletal morbidity. |
|
| Interventions |
3 arms Intervention (n = 33): periodic fasting 2 consecutive days per week consuming 25% of normal caloric intake. Comparator (n = 33): continuous energy restriction. This group was prescribed a daily energy‐restricted Mediterranean‐type diet that was relatively high in protein (25% energy) with moderate carbohydrate (45% energy from low‐glycaemic‐index carbohydrates) and moderate fat (30% fat; 15% MUFA, 8% PUFA and 7% SFA) intakes. A third trial arm included periodic fasting with ad libitum protein and fat and thus was not included in this analysis. |
|
| Outcomes | Lipid profile, HbA1c, glucose, body weight, waist circumference | |
| Notes | Author was contacted for raw data to calculate absolute changes Type of paper: full‐text publication Funding:tThe present study was supported by the Genesis Breast Cancer Prevention (Registered Charity no. 1109839) and, in part, by the Intramural Research Program of the National Institute on Aging, Baltimore, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Low risk | Group allocation was established by opaque, sealed envelopes that contained the assignment for each participant. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Anthropometric measures were performed by research dietitians who were not blinded to the treatment groups. Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Personnel performing laboratory measurements, and inputting and analysing trial data were blinded to group allocations. Anthropometric measures were performed by research dietitians who were not blinded to the treatment groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although data on all randomised participants were analysed (Intention‐to‐treat analysis), the dropout rate was high; 10 out of 38 participants (26.3%) in the intermittent energy and carbohydrate restriction (ad libitum protein and fat) group, 4 our of 37 participants (10.8%) in the intermittent energy and carbohydrate restriction group, and 13 out of 40 participants (32.5%) in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes unspecified. |
| Other bias | Low risk | Nothing to note. |
Hutchison 2019.
| Study characteristics | ||
| Methods | Multiple‐arm randomised controlled clinical trial | |
| Participants | 88 female participants based in Australia aged between 35 and 70 years with
a BMI between 25‐42 kg/m2 Inclusion and exclusion criteria: patients were aged 35 to 70 years; BMI 25 to 42 kg/m2; weight stable (±5% of their screening weight) for > 6 months prior to study entry; nondiabetic; nonsmoker; sedentary or lightly active (i.e., < 2 moderate‐ to high‐intensity exercise sessions/week); consumed < 140 g alcohol per week; no history of CVD, eating disorders, or psychiatric disorders (including those taking antidepressants); not pregnant or breastfeeding; and not taking medication that may affect study outcomes (e.g. phentermine, orlistat, metformin, excluding antihypertensive/lipid‐lowering medication). |
|
| Interventions |
4‐arm trial: IF100, IF70, DR70, Control (ad libitum feeding) Interventions (n = 22, n = 22): IF100, IF70 (Both were used in this analysis) Comparator (n = 24, n = 11): DR70, Control |
|
| Outcomes | Body weight, waist circumference, lipid profile, blood pressure, glucose | |
| Notes |
Type of paper: full‐text publication Funding: the research was funded by a National Health and Medical Research Council (NHMRC) Project Grant APP1023401. LKH was supported by an Australian Research Council Future Fellowship FT120100027. BL was supported by an Australian Government Research Training Program Scholarship. Control (C): continuous energy intake at 100 % of baseline energy requirements; IF100: intermittent fasting diet at 100 % of baseline energy requirements; IF70: intermittent fasting diet at 70 % of baseline energy requirements; DR70: continuous energy restriction at 70 % of baseline energy requirements. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Low risk | Although block randomisation was performed, the block sizes varied between 4 and 8 participants, which reduces the risk of determining allocations. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout rates varied substantially between two of the groups that had different interventions (12% for the IF70 and IF100 groups but 4% for the DF70 group), and different reasons were provided for dropout although it was not stated what reasons corresponded to which group. |
| Selective reporting (reporting bias) | High risk | Outcomes of interest that were pre‐specified in the clinical trial registry (ClinicalTrials.gov: NCT01769976) were not reported in the published study, including energy expenditure, hunger, mood and cognitive function. |
| Other bias | Low risk | Nothing to note. |
Kroeger 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 48 obese participants Inclusion and exclusion criteria: none provided |
|
| Interventions |
2‐arm trial Intervention: ADF (75% restriction fast day alternated with ad libitum feed day), Comparator: CR (25% restriction everyday), for a 6‐month weight loss period followed by a 6‐month weight maintenance period. |
|
| Outcomes | Body weight, fat mass, visceral fat mass, fat free mass, plasma glucose, insulin levels and insulin resistance. | |
| Notes |
Type of paper: abstract only Funding: none provided Other: absolute changes were provided in the abstract but the number of people in each of the arms was not stated. No contact details were provided to contact the authors to provide extra data. ADF‐ Alternate day fasting CR‐ calorie restriction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only and method of randomisation not stated in abstract. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Abstract only and blinding of outcome assessment not indicated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only and loss to follow‐up not reported in abstract. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. |
| Other bias | Unclear risk | Abstract only. |
Moro 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 34 participants based in the USA who have performed resistance training
continuously for the last 5 years. Inclusion and exclusion criteria: the criteria for entering the study were that participants must have performed resistance training continuously for at least 5 years (training 3–5 days/week with at least 3 years experience in split training routines), be presently engaged in regular resistance training at the time of recruitment, be life‐long steroid free, and have no clinical problems that could be aggravated by the study procedures. |
|
| Interventions |
2‐arm trial Intervention (n = 17): time restricted feeding (16 hours fasting, 8 hours feeding) Comparator (n = 17): ad libitum feeding/normal diet |
|
| Outcomes | Lipid profile | |
| Notes |
Other: data presented as percentage changes. Authors contacted for
absolute changes but no response was given. Therefore, data unfortunately
not included in quantitative analysis. Funding: this research was conducted with authors’ institutional founds Type of paper: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to a time‐restricted feeding group (TRF; n = 17) or standard diet group (ND; n = 17) through computer‐generated software. |
| Allocation concealment (selection bias) | Unclear risk | Although it was stated quote: "the research staff conducting outcome assessments was unaware of the assignment of the subjects (i.e. a single blind design)", it was not indicated how the assignment sequence was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The research staff conducting outcome assessments was unaware of the assignment of the subjects (i.e. a single blind design)." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no information provided regarding dropout or the number of completers. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Low risk | Nothing to note. |
Parvaresh 2019.
| Study characteristics | ||
| Methods | Parallel‐arm randomised clinical trial | |
| Participants | 70 participants based in Iran aged between 25 and 60 with a 25 ≤ BMI ≤
40 kg/m2 Inclusion and exclusion criteria: the patients were eligible to enter the study if they were aged 25–60 and overweight (25 ≤ BMI ≤ 40 kg/m2). Individuals with weight changes ≥5% for 3 months preceding the study, history of liver cardiovascular, renal, and metabolic disease, smoking or taking any medication or following a special diet in the last 6 months, which is known to impact on body weight, serum lipids, or glucose metabolism, breast feeding, post‐menopausal and pregnant women were excluded. |
|
| Interventions |
2‐arm trial Intervention (n = 35): ADF (alternate day fasting) Comparator (n = 34): continuous energy restriction |
|
| Outcomes | Body weight, BMI, Waist circumference, lipid profile, blood pressure, glucose | |
| Notes |
Funding: this research did not receive any specific grant from
funding agencies in the public, commercial, or not‐for‐profit sectors. Type of paper: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by using by computer‐generated random numbers and was concealed from the researchers as well as participants." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by using by computer‐generated random numbers and was concealed from the researchers as well as participants." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The compliance to the prescribed diet was recorded using self‐reporting, which might have resulted in misstatements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 dropout out of 35 participants (2.9%) in CER group and no dropout in ADF group. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes pre‐specified in the Methods section were reported in the Results section. |
| Other bias | Low risk | Nothing to note. |
Pinto 2019.
| Study characteristics | ||
| Methods | Parallel‐arm randomised clinical trial | |
| Participants | 45 participants based in the UK aged between 35 and 37 Inclusion and exclusion criteria: the main inclusion criteria were non‐smoking men and women aged 35–75 years with a waist circumference exceeding the cut‐off determined by the World Health Organization to confer a high risk of cardiometabolic disease [32]: >102 cm and >88 cm for men and women, respectively (> 90 cm and > 80 cm, for men and women respectively, with South Asian or East Asian ethnic background [33]). There were no inclusion/exclusion criteria based on BMI since this index does not provide information on body fat distribution. The exclusion criteria included kidney or cardiovascular disease, cancer, diabetes, chronic liver disease; previous bariatric surgery or other major surgery (e.g. organ transplantation); significant psychiatric disorder or uncontrolled depression; eating disorders; participation in a weight management drug trial in the previous 3 months; uncontrolled epilepsy; taking medication likely to affect metabolic rate and/or weight (e.g. beta blockers, corticosteroids, diuretics); lactose intolerant; alcohol or substance abuse. Women who were currently pregnant, lactating or planning pregnancy were also excluded. |
|
| Interventions |
2‐arm trial Intervention (n = 21): periodic fasting on two consecutive days Comparison (n=22): continuous energy restriction |
|
| Outcomes | Body weight, BMI, lipid profile, glucose | |
| Notes |
Other: missing change from baseline standard deviation values were
imputed by generating correlation co‐efficients for each outcome from a
similar paper (Catenacci 2016) and
then imputing the missing standard deviation values. Funding: this work was supported by LighterLife (UK) Ltd, who funded the running costs of the trial and provided the food packs used for the IER fasting days. Type of paper: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment was randomly allocated by the lead researcher using a computer online MinimPy 0.3 (Copyright (c) 2011 Mahmoud Saghaei, http://minimpy.sourceforge.net) by minimization for sex, BMI, ethnicity and waist circumference." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by using by computer‐generated random numbers and was concealed from the researchers as well as participants" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rates; no dropout in the control group and a dropout rate of 2 out of 23 participants (8.7%) in the intermittent fasting group. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes (clinicaltrials.gov NCT02679989) were reported. |
| Other bias | Low risk | Nothing to note. |
Schubel 2018.
| Study characteristics | ||
| Methods | Multiple‐arm randomised controlled trial | |
| Participants | 150 participants based in Germany aged between 35 and 65 with a BMI ≥25 and
<40 Inclusion criteria:
Exclusion criteria
|
|
| Interventions |
3‐arm trial Intervention (n = 49): ICR group were advised to restrict their energy intake on 2 self‐selected nonconsecutive days per week to 25% of the individual energy requirement. The remaining 5 days of the week were based on a eucaloric balanced diet Comparators ( n=49, n = 52): continuous energy restriction arm and control/ad libitum feeding arm |
|
| Outcomes | Lipid profile, glucose, CRP | |
| Notes | All arms were included in analysis. Funding: the HELENA Trial was funded by the Helmholtz Association of German Research Centers (Cross Program Topic: Metabolic Dysfunction). MRI examinations were performed in and financed by the Department of Diagnostic and Interventional Radiology, University Hospital Heidelberg. The financing of the MRI was also supported by the Stiftung zur Förderung der Erforschung der Zivilisationserkrankungen, Baden‐Baden, Germany. CMU was funded by the Huntsman Cancer Foundation, Salt Lake City, UT and by NIH grant U01 CA 206110. Type of paper: full‐text publication ICR‐ Intermittent calorie restriction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The web‐based software RANDI2 was used to generate the random allocation sequence." |
| Allocation concealment (selection bias) | High risk | Participants were assigned by stratified block randomisation with a fixed block size. This means that the executors can predict the next assignment, and this is clearly incompatible with allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because the HELENA Trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The technical staff was blinded for downstream laboratory work and data management. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in intention‐to‐treat analysis; dropout rates were also low at 4 out of 49 participants (8.2%) in the intermittent calorie restriction group and 2 out of 52 participants (3.8%) in the control group. |
| Selective reporting (reporting bias) | Low risk | The vast majority of pre‐specified outcomes (ClinicalTrials.gov: NCT02449148) were reported in the Results section, with the exception of some secondary outcomes such as effect on quality of life. |
| Other bias | Low risk | Nothing to note. |
Stekovic 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 60 participants based in Australia aged between 35 and 65 with a BMI
between 22.0 and 30.0 kg/m2 Inclusion criteria: age between 35 and 65 years, both inclusive
Exclusion criteria:
|
|
| Interventions |
2‐arm trial Intervention (n = 29): ADF 25% of caloric intake Comparator (n = 28): control/ad libitum feeding |
|
| Outcomes | Body weight change, BMI, blood pressure | |
| Notes |
Funding and acknowledgements: F.M. is grateful to the Austrian Science Fund FWF (SFB LIPOTOX F3007& F3012, W1226, P29203, P29262, P27893, P 31727), the Austrian Federal Ministry of Education, Science and Research, and the University of Graz for grants “Unkonventionelle Forschung‐InterFast” and “ flysleep” (BMWFW-80.109/0001-WF/V/3b/2015), as well as the field of excellence program BioHealth. We acknowledge support from NAWI Graz and the BioTechMed‐Graz flagship project “EPIAge.” G.K. is supported by the Ligue Contre le Cancer Comité de Charente‐Maritime (équipe labelisée); Agence National de la Recherche (ANR)–Projets blancs; ANR under the frame of E‐Rare‐2, the ERA‐Net for Research on Rare Diseases; Association pour la Recherche sur le Cancer (ARC); Cancéropôle Île‐de‐France;Institut National du Cancer (INCa); Institut Universitaire de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the Leducq Foundation; the Labex Immuno‐Oncology; the Recherche Hospitalo‐Universitaire Torino Lumière, the Site de Recherche Intégrée sur le Cancer (SIRIC) Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI). T.E. is supported by the FFG COIN‐project 855987 . J.D. is supported by the Canton of Fribourg and the Swiss National Science Foundation. M.A.A. and R.d.C. are funded by the IRP of the NIA, NIH. H.S. is grateful to support from the K1 Comet Center CBmed (Center for Biomarker Research in Medicine) funded by the Austrian Research Promotion Agency (FFG) and the Styrian Research Agency (SFG). Type of paper: full‐text publication ADF‐ alternate day fasting |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was conducted using an online tool "Randomizer for Clinical Trials’’ (https://www.randomizer.at/) and stratifying participants by sex." |
| Allocation concealment (selection bias) | High risk | Quote: "While we were not able to blind participants regarding their intervention, allocation, clinical, and scientific staff were blinded during the process of collection, archiving, and analyses of collected samples and measurements." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"clinical, and scientific staff were blinded during the process of collection, archiving, and analyses of collected samples and measurements." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rates were relatively low for both groups; during these 4 weeks of intervention, 1 participant out of 30 (3.3%) dropped out of the ADF group and 2 participants out of 30 (6.7%) dropped out of the control group 3 participants. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not pre‐specified. |
| Other bias | Unclear risk | Not clear. |
Sundfor 2018.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | 112 participants based in Norway aged between 21 and 70 with a BMI of 30 to
45.0 kg/m2 Inclusion criteria: waist circumference ≥94/80 cm (men/women) and ≥1 additional metabolic syndrome component: circulating levels of TG ≥ 1.7 mmol/L, HDL cholesterol ≤1.0/1.3 (men/women), blood pressure ≥130/85 mmHg or use of antihypertensive drugs or fasting glucose ≥5.6 mmol/L, and weight stability within ±3 kg during the last three months. Exclusion criteria: diabetes if treated with insulin or incretin analogues, bariatric surgery, use of anti‐obesity drugs or other drugs affecting body weight, eating disorder, or psychiatric illness, or alcohol or drug abuse that could contribute to difficulties with study procedures. |
|
| Interventions |
2‐arm trial Intervention (n = 54): twice‐weekly diet. Participants in the intermittent energy restriction group were advised to consume 400/600 (female/male) on each of two nonconsecutive days a week and to consume food as usual the remaining five days a week. Comparator (n = 58): continuous energy restriction |
|
| Outcomes | Body weight, BMI, Waist circumference, lipid profile, blood pressure, glucose, CRP | |
| Notes |
Funding: not stated Type of paper: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A statistician prepared a computer‐generated random number list" |
| Allocation concealment (selection bias) | Low risk | The project leader (TS) opened numbered and sealed envelopes consecutively with no exception. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote:"Measurements were not blinded, but data entry was done by assistants who were blinded to study group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout rates in both groups and intention‐to‐treat analysis was performed; at 12 months, 4 out of 54 (7.4%) dropout rate in the IER group dropouts and 3 out of 58 (5.2%) dropout rate in the CER group. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes (www.clinicaltrials.gov NCT02480504) were reported. |
| Other bias | Unclear risk | Study visits were not scheduled according to fasting days among participants in the intermittent energy restriction group, to limit lack of compliance, as participants were allowed to vary the day of the week on which they fasted. |
Tinsley 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 18 participants based in the USA. Inclusion and exclusion criteria: generally healthy, recreationally active men who had not followed a consistent resistance training programme over the previous three months were eligible for participation in the study. |
|
| Interventions |
3‐arm trial Intervention (n = 10): time restricted feeding Comparator (n = 8): control/ad libitum feeding |
|
| Outcomes | Body weight | |
| Notes |
Funding: not stated Type of paper: full‐text publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The dropout rates per group were not clarified. It was stated: quote: "6 participants dropped out of the study prior to the 4‐week visit (1 from RT‐TRF and 5 from RT‐ND), 3 participants from the RT‐TRF group were excluded from the analysis due to low compliance to the fasting programme (compliance <80%), and one participant from the RT‐ND group was excluded from the analysis due to self‐report of a major lifestyle change that led to substantial unexpected weight loss. The most common reasons for dropout were illness, injury unrelated to the study, and reported lack of time to complete the programme." |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the Methods section were reported in the Results section. |
| Other bias | Low risk | Nothing to note. |
Tinsley 2019.
| Study characteristics | ||
| Methods | Randomised control trial | |
| Participants | 27 participants aged between 18 and 30 Inclusion criteria: participants were required to have prior RT experience, defined as reporting ≥1 year of RT at a frequency of 2 to 4 sessions per week and with weekly training of major upper‐ and lower‐body muscle groups. Additionally, participants were screened for BF% using multi frequency bioelectrical impedance analysis (MFBIA; mBCA 514/515, Seca). The original target BF% range for participants was 15% to 29%; however, due to data from our lab indicating overestimations of body fat via MFBIA compared with a 4‐component (4C) model in resistance‐trained females (19), individuals with ≤33% body fat at screening were considered eligible. Exclusion criteria: individuals were excluded if they did not meet the aforementioned criteria or were pregnant, trying to become pregnant, currently breastfeeding, cigarette smokers, allergic to dairy protein, or had a pacemaker or other electrical implant. |
|
| Interventions |
3‐arm trial Intervention (n = 13): TRF participants were instructed to consume all calories between 12:00 hours and 20:00 hours each day Comparator (n = 14): control diet/ad libitum feeding Third arm was TRF+ β‐hydroxy β‐methylbutyrate (HMB) supplementation and was not included in this analysis |
|
| Outcomes | Body weight | |
| Notes |
Funding: supported by MTI Biotech Inc. and Texas Tech University.
In‐kind donations were received from MTI Biotech Inc. (HMB and placebo
capsule supplements) and Dymatize Enterprises (whey protein
supplements). Type of paper: full‐text publication TRF‐ time restricted feeding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed quote: "using sequences produced from a random sequence generator (http://www.random.org)" |
| Allocation concealment (selection bias) | Low risk | Given that the allocation sequence within each stratum was randomly generated, it is likely to have been adequately concealed; quote: "Eligible participants were... randomly assigned to 1 of the 3 study groups [CD plus placebo (CD), TRF plus placebo (TRF), or TRF plus HMB (TRFHMB)] using sequences produced from a random sequence generator (http://www.random.org). Each participant within a given stratum was allocated in a sequential manner to the first available group assignment at the time of baseline [i.e., week 0 (W0)] testing using the random integer sequence for that stratum." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trainers supervising the RT program were asked not to discuss group assignment with participants in order to maintain blinding with respect to the assigned dietary program. However, since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clarified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in intention‐to‐treat analysis. There were high dropout rates in all groups; dropout rate of 5 out of 14 participants (35.7%) in the control group, and 5 out of 13 participants (38.5%) in the TRF group. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes (ClinicalTrials.gov: NCT03404271) were reported in the Results section. |
| Other bias | Low risk | Nothing to note. |
Trepanowski 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Men and women (n = 100) aged 18–65 years with a BMI of 25 to 39.9 kg/m2
were recruited from the University of Illinois at Chicago campus. Inclusion criteria: as above Exclusion criteria: if they had a history of cardiovascular disease, diabetes mellitus, were taking weight loss medications, were not weight stable for 3 months prior to study initiation, were peri‐menopausal, pregnant, or smokers. |
|
| Interventions | Participants were randomised by a stratified random sample (based on age,
sex, and BMI) to 1 of 3 groups for 6‐months Intervention (n = 34): ADF Comparator 1 (n = 35): CR Comparator 2 (n = 31): control The 6‐month trial was divided into a 3‐month quote: “controlled feeding period”, followed by a 3‐month “self‐selected feeding period” |
|
| Outcomes | Body weight, body composition. | |
| Notes | There were no relevant data available to analyse. Funding: National Institutes of Health: NHLBI (R01HL106228, T32HL007034), NIDDK (F32DK107157). Type of paper: full‐text publication ADF‐ alternate day fasting CR‐ Calorie restriction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Low risk | Although block randomisation was performed, the block sizes varied between 1 and 11 participants, which reduces the risk of determining allocations. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of dropout;quote: "A total of n = 100 subjects were randomized to the three intervention groups (ADF n = 34, CR n = 35, control n = 31). After 6 months, n = 9 (26.5%) dropped out of the ADF group, n = 6 (17.1%) dropped out of the CR group, and n = 6 (19.4%) dropped out of the control group. Reasons for subject withdrawals included: dissatisfaction with study diets, scheduling conflicts, and personal reasons." |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes (ClinicalTrials.gov: NCT00960505) were reported. |
| Other bias | Low risk | Nothing of note. |
Varady 2011.
| Study characteristics | ||
| Methods | Multiple‐arm randomised clinical trial | |
| Participants | 60 participants based in the USA aged between 35 and 65 with a BMI between
25 and 39.9 kg/m2 Key inclusion criteria: BMI between 25 and 39.9 kg/m2; age 35 to 65 years; non‐diabetic; no history of cardiovascular disease; non‐smoker; weight stable (< 6 kg weight loss or gain for 3 months prior to the study); sedentary or lightly active (<3 hours/week of light intensity exercise for 3 months prior to the study); and not taking weight loss, lipid‐, or glucose‐lowering medications. |
|
| Interventions |
4‐arm trial Intervention (n = 15): ADF (75% energy restriction for 24 hours alternated with ad libitum feeding for 24 hours) Comparator 1 ( n =15): CR (25% energy restriction every day), 3) exercise (moderate intensity training 3 x/week) Comparator 2 (n = 15): control A fourth arm which focused on exercise (moderate intensity training 3 x/week) was not included in the analysis |
|
| Outcomes | Body weight, lipid profile | |
| Notes | Majority of outcomes presented as percentage change. Author was contacted
to calculate absolute changes but no response. Funding: Departmental grant, Kinesiology and Nutrition, University of Illinois, Chicago Type of paper: full‐text publication ADF‐ alternate day fasting CR‐ calorie restriction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rates per group not provided. It was stated: quote: "60 subjects commenced the study, with 49 completing the 12‐week trial" |
| Selective reporting (reporting bias) | High risk | Majority of outcomes presented as percentage change. Author was contacted to calculate absolute changes but no response. |
| Other bias | Low risk | Nothing to note. |
Varady 2013.
| Study characteristics | ||
| Methods | Randomised clinical trial | |
| Participants | 32 participants based in the USA aged between 35 and 65 with a BMI between
20 and 29.9 kg/m2 Inclusion criteria: BMI between 20 and 29.9 kg/m2; age between 35 and 65 years; pre‐menopausal or post‐menopausal (absence of menses for more than 2 years); lightly active (< 3 hours/week of light intensity exercise at 2.5 to 4.0 metabolic equivalents (METs) for 3 months prior to the study); weight stable for 3 months prior to the beginning of the study (< 4 kg weight loss or weight gain); non‐diabetic; no history of cardiovascular disease; non‐smoker; and not taking weigh‐ loss, lipid‐ or glucose‐lowering medications. |
|
| Interventions |
2‐arm trial Intervention (n = 15): ADF 25% of baseline energy needs on fast day and then ad libitum on each alternating feed day. Comparator (n = 15): control |
|
| Outcomes | Body weight, lipid profile, blood pressure, CRP | |
| Notes |
Funding: Departmental grant from Kinesiology and Nutrition at the
University of Illinois, Chicago. Type of paper: full‐text publication ADF‐ alternate day fasting |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rates of dropout; rate of 1 out of 16 participants (6.3%) in the ADF group and 1 out of 16 participants (6.3%) in the control group. |
| Selective reporting (reporting bias) | High risk | Quote: "This pilot study was originally designed to compare the effects of ADF in normal weight versus overweight individuals on body weight and CHD risk." Due to a low recruitment rate, we were only able to recruit n = 8 subjects into the normal weight group and n = 8 subjects into the overweight group. In view of this, we decided to combine the normal weight and overweight groups into one group to increase sample size." |
| Other bias | Low risk | Nothing to note. |
Varady 2016a.
| Study characteristics | ||
| Methods | This study examined the impact of ADF on markers of bone turnover in a 6‐month randomised controlled trial. | |
| Participants | Obese participants Inclusion and exclusion criteria: none provided |
|
| Interventions |
3‐arm trial Intervention: alternate day fasting (25% energy intake fast day, alternated with 125% intake feast day) Comparator 1: calorie restriction (75% intake every day) Comparator 2: control (ad libitum intake every day) |
|
| Outcomes | Body weight, fat mass, and lean mass | |
| Notes |
Type of paper: abstract only There were no available data from the abstract to analyse and it was no possible to reach the author. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since this trial was a dietary intervention study, it was not feasible for participants or all study personnel to be blinded to the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Abstract only and blinding of outcome assessment not indicated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract‐only and loss to follow‐up not indicated. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. |
| Other bias | Unclear risk | Abstract only. |
ADF: alternate day fasting; BMI: body mass index; BP: blood pressure; CER: Continuous energy restriction; CRP: C‐reactive protein; CVD: cardiovascular disease; DBP: diastolic blood pressure; HbA1c: glycated haemoglobin; HDL: high density lipoprotein; ICR: Intermittent calorie restriction; IER: intermittent energy restriction; IGF‐1: insulin‐like growth factor‐1; LDL: low‐density lipoprotein; SBP: systolic blood pressure; SD: standard deviation; SE: standard error; TC: total cholesterol; TG: triglycerides; TRF: time‐restricted feeding.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Actrn 2013 | Wrong study design |
| Aghasadeghi 2008 | Wrong intervention |
| Aksungar 2005 | Wrong study design |
| Aksungar 2007 | Wrong study design |
| Al‐Barha 2019 | Wrong study design |
| Alhamdan 2016 | Wrong study design |
| Almeneessier 2017 | Wrong study design |
| Almeneessier 2018 | Wrong study design |
| Andrews 1984 | Wrong setting |
| Antoni 2018 | Wrong study design |
| Arguin 2012 | wrong intervention |
| Arnason 2017 | Wrong study design |
| Bachman 2016 | Wrong study design |
| Bahammam 2016 | Wrong study design |
| Bahmani 2013 | Wrong study design |
| Basolo 2019 | Wrong study design |
| Bergendahl 2000 | Wrong study design |
| Bergman 2007 | Wrong comparator |
| Bhutani 2010 | Wrong study design |
| Boden 1996 | Wrong study design |
| Bowen 2018 | Wrong intervention |
| Chan 2007 | Wrong study design |
| Cherif 2017 | wrong study design |
| Chirinos 2016 | Wrong intervention |
| Chowdhury 2016 | Wrong study design |
| Cignarella 2017 | Wrong patient population |
| Cignarella 2018 | Wrong patient population |
| Clayton 2016 | Wrong intervention |
| Clayton 2018 | Wrong intervention |
| Clegg 2014 | Wrong intervention |
| Contaldo 1980 | Wrong setting |
| Corley 2018 | Wrong comparator |
| Corvilain 1995 | Wrong study design |
| Coutinho 2018 | Wrong intervention ‐ 550 kcal /day to 660 kcal /day |
| Ctri 2018 | Wrong intervention |
| Dallongeville 1998 | Wrong intervention |
| Dong 2004 | Paediatric population |
| Edinburgh 2019 | Wrong intervention |
| Fitzgerald 2017 | Wrong intervention |
| Gjedsted 2007 | Wrong intervention |
| Grajower 2019 | Wrong study design |
| Harder‐Lauridsen 2017 | Wrong intervention |
| He 2016 | Wrong study design |
| Headland 2018 | Wrong intervention |
| Hirsh 2019 | Wrong intervention |
| Hoddy 2014 | Wrong comparator |
| Hussin 2013 | Wrong intervention |
| Irct201702269856N 2017 | Wrong study design |
| Irct2017070434897N 2017 | Wrong study design |
| ISRCTN16400313 | Wrong study design |
| ISRCTN65605485 | Wrong intervention |
| ISRCTN89657927 | Wrong study design |
| Jafari 2003 | Wrong intervention |
| Jakubowicz 2015 | Wrong study design |
| Jamshed 2019 | Wrong study design |
| Jensen 1988 | Wrong study design |
| Jimenez 2019 | Wrong intervention |
| Johari 2019 | Wrong intervention |
| Johnson 2010 | Wrong study design |
| Kaikkonen 2019 | Wrong intervention |
| Kalam 2019 | Wrong study design |
| Kalam 2019a | Wrong study design |
| Kamble 2018 | Wrong study design |
| Karimi 2016 | Wrong intervention |
| Kearney 2011 | Wrong intervention |
| Keogh 2014 | Wrong intervention |
| Kessler 2018 | Wrong study design |
| Klempel 2012 | Wrong comparator |
| Kobayashi 2014 | Wrong study design |
| Kohn 2016 | Wrong study design |
| Kolb 1983 | Wrong intervention |
| Kroeger 2012 | Wrong comparator |
| Larijani 2003 | Wrong study design |
| Larson‐Meyer 2008 | Wrong intervention |
| Maly 1996 | wrong study design |
| NCT00183027 | Wrong intervention |
| NCT01754350 | Wrong intervention |
| NCT01895179 | Wrong study design |
| NCT01964118 | Cross‐over trial ‐ wrong study design |
| NCT02287103 | Cross‐over trial ‐ wrong study design |
| NCT02525419 | Wrong study design |
| NCT02633722 | Cross‐over trial ‐ wrong study design |
| NCT02970188 | Cross‐over trial ‐ wrong study design |
| NCT03459703 | cross‐over trial ‐ wrong study design |
| NCT03569852 | Cross‐over trial‐ wrong study design |
| NCT03574103 | wrong intervention |
| NCT04009239 | Cross‐over trial ‐ wrong study design |
| NCT 2017 | Terminated trial |
| Ng 2019 | Wrong patient population |
| Ongsara 2017 | Wrong study design |
| Overland 2017 | Wrong study design |
| Parr 2019 | Wrong study design |
| Pavic 2019 | Wrong intervention |
| Pramanik 2012 | Wrong intervention |
| Ravussin 2019 | Wrong study design |
| Ruiz 2013 | Wrong study design |
| Rynders 2019 | Wrong study design |
| Salas‐Salvado 2019 | Wrong intervention |
| Santos 2018 | Wrong study design |
| Soeters 2009 | Wrong study design |
| Solianik 2018 | Wrong intervention |
| Sutton 2018 | Wrong study design |
| Takahashi 2011 | Wrong patient population |
| Tang 1995 | Wrong intervention |
| Teng 2013 | Wrong intervention |
| Varady 2016 | Review paper ‐ wrong study design |
| Verboeket‐van 1993 | Wrong intervention |
| Washburn 2019 | Wrong study design |
| Webber 1994 | Wrong study design |
| Williams 1998 | Wrong intervention |
Characteristics of studies awaiting classification [ordered by study ID]
Liu 2017.
| Methods | Randomised controlled trial |
| Participants | 75 overweight and obese women |
| Interventions | Participants were randomised to 1 of 3 groups for 8 weeks, and provided with foods at 70% (IF70 and CR70), or 100% (IF100) of energy requirements. |
| Outcomes | Insulin sensitivity |
| Notes | Does not provide a trial registration number, and may be an early abstract of the published paper Hutchison 2019. |
NCT00467220.
| Methods | Randomised controlled trial |
| Participants | 24 Participants Ages eligible for study: 35 years to 65 years (adult, older adult) Sexes eligible for study: both Accepts healthy volunteers: yes Criteria Inclusion criteria: Male and female; Body mass index (BMI) between 20‐30 kg/m2; Age between 35‐65 years; sedentary (light exercise less than 1 hour per week) or moderately active (1 to 2 hours per week); Weight stable for >3 months prior to the beginning of the study; Able to give written informed consent; Female participants must be post‐menopausal for at least 2 years and can not be on hormone replacement therapy (HRT). |
| Interventions | Arm Intervention/treatment No Intervention: control Participants will follow all study tasks but will not be required to follow a calorie‐restricted meal plan. Alternate Day Fasting Arm Participants in this arm will be asked to alternate between one day of eating as they wish versus one day on a calorie‐restricted meal plan. Participants will follow this alternating meal plan for 3 months. Behavioural: calorie restriction Participants in the "calorie restriction" arm and the "alternate day fasting" arm will be asked to follow a menu plan, for three months, that includes some level of calorie restriction. Calorie restriction Participants in this arm will be asked to follow a calorie‐restricted meal plan, daily, for three months. Behavioural: calorie restriction Participants in the "calorie restriction" arm and the "alternate day fasting" arm will be asked to follow a menu plan, for three months, that includes some level of calorie restriction. |
| Outcomes | Primary outcome measures: Adipose tissue dynamics [Time Frame: 12 weeks] Parameters measured will include adipose tissue dynamics (triglyceride turnover, lipolysis, de novo lipogenesis, adipose cell proliferation), adipose tissue morphology (cell size and number), adipose tissue hormone levels (adiponectin, leptin), skin turnover (keratin dynamics), T‐lymphocyte proliferation, as well as plasma lipid and lipoprotein, homocysteine, and C‐reactive protein levels. |
| Notes | NCT00467220. It is unclear whether the information above meets our defined intervention. More information is needed. |
NCT02148458.
| Methods | Randomised controlled trial |
| Participants | 53 participants Ages eligible for study: 30 years to 65 years (adult, older adult) Sexes eligible for study: all Accepts healthy volunteers: yes Criteria Inclusion criteria: ‐ The participants in this study will be 50 men and women in the 30 to 65 year age range, who have a BMI in the high‐normal to moderately overweight range (i.e. 22 to 28 kg/m2), ‐ Participants who are eating usual US diets and are sedentary to moderately active (i.e. not exercise trained). ‐ Participants have at least one of these metabolic abnormalities: pre‐hypertension or hypertension (i.e. systolic blood pressure > 120 mmHg or diastolic blood pressure > 80 mmHg or specific treatment of previously diagnosed hypertension)10, sub‐optimal lipid levels (i.e. LDL‐cholesterol > 100 mg/dL or HDL‐cholesterol < 59 mg/dL or specific treatment for this lipid abnormality)11, impaired fasting glucose or glucose intolerance (i.e. fasting glucose > 100 mg/dL or 2hour‐glucose during OGTT with a glucose load of 75 g > 140 mg/dL or specific treatment for previously diagnosed type 2 diabetes)12, or high‐risk waist circumference (≥ 94 cm in men and ≥ 80 cm in women)13. |
| Interventions | Arm Intervention/treatment Control group Western diet for 8 weeks, followed by 8 weeks of Western diet with intermittent fasting. Other: control group control group eating their usual Western diet for 8 weeks, followed by 8 weeks of usual diet with intermittent fasting (i.e. 2 non‐consecutive days of fasting per week). Experimental: Mediterranean diet Mediterranean diet for 8 weeks, followed by 8 weeks of Mediterranean diet with intermittent fasting. Other: Mediterranean diet Mediterranean diet for 8 weeks, followed by 8 weeks of Mediterranean diet with intermittent fasting (i.e. 2 non‐consecutive days of fasting per week) |
| Outcomes | Primary outcome measures: Decrease in high sensitivity C‐reactive protein (hsCRP) [ Time Frame: 16 weeks‐‐ Baseline, 8 weeks, 16 weeks ] hsCRP is in mg/L |
| Notes | It is unclear from the information above that the intervention meets our predefined criteria. |
NCT02606669.
| Methods | Randomised controlled trial |
| Participants | Ages eligible for study: 21 years to 70 years (adult, older adult) Sexes Eligible for Study: All Accepts healthy volunteers: yes Criteria Inclusion criteria: Ability to provide informed consent. Chinese. (both parents must be Chinese) Age above 21 years old. This study will focus only on adult participants, and 21 years old is chosen as the age cut off, as it is the recognised legal age of independent consent. BMI ≥ 25 kg/m2 |
| Interventions | Experimental: intermittent fasting The intervention of interest here is the Intermittent Energy Restriction (IER) or the Intermittent Fasting approach, specifically, the "twice‐weekly" diet, where adherence to this dietary intervention consists of fasting for two consecutive days and consuming enough to meet energy requirements for the remaining five days. In this study, fasting will be achieved by using a meal replacement product (Optifast®) supplemented by two scoops of protein powder (Propass®) and a multivitamin, making a total of 540kcal (54g protein, 60g carbohydrates) for each fasting day. Dietary supplement: meal replacement Using a meal‐replacement product, supplemented by protein powder and multivitamin No Intervention: control Diet and physical activity advice only. no treatment plan. |
| Outcomes | Primary outcome measures: Change in total body weight (in kg) at 3 months compared to baseline [ Time Frame: Baseline to 3 months ] Secondary outcome measures: Change in quality of life (as determined by RAND Short Form‐36 health survey) at 3 months compared to baseline [ Time Frame: Baseline to 3 months ] Change in insulin sensitivity % (as determined by the Homeostasis Model Assessment (HOMA)) at 3 months compared to baseline [ Time Frame: Baseline to 3 months ] Change in total cholesterol levels (in mmol/L) at 3 months compared to baseline [ Time Frame: Baseline to 3 months ] Change in low‐density lipoprotein (LDL)‐ cholesterol levels (in mmol/L) at 3 months compared to baseline [ Time Frame: Baseline to 3 months ] Change in high‐density lipoprotein (HDL)‐cholesterol levels (in mmol/L) at 3 months compared to baseline [ Time Frame: Baseline to 3 months ] Change in triglyceride levels (in mmol/L) at 3 months compared to baseline [ Time Frame: Baseline to 3 months ] Change in waist circumference (in cm) at 3 months compared to baseline [ Time Frame: Baseline to 3 months ] Change in body fat % (as determined by bio‐electrical impedance analysis) at 3 months compared to baseline [ Time Frame: Baseline to 3 months ] Change in hip circumference (in cm) at 3 months compared to baseline [ Time Frame: Baseline to 3 months ] Change in alanine transaminase (ALT) levels (in U/L) at 3 months compared to baseline [ Time Frame: Baseline to 3 months ] Change in aspartate aminotransferase (AST) levels (in U/L) at 3 months compared to baseline [ Time Frame: Baseline to 3 months ] Change in gamma‐glutamyl transpeptidase (GGT) levels (in U/L) at 3 months compared to baseline [ Time Frame: Baseline to 3 months ] |
| Notes | NCT02606669 ‐ It is unclear from the information above that the intervention meets our predefined criteria. |
NCT02948517.
| Methods | Randomised controlled trial |
| Participants | 31 participants Ages eligible for study: 25 years to 65 years (adult, older adult) Sexes eligible for study: all Accepts healthy volunteers: yes Criteria Inclusion Criteria: Male or female Body mass index (BMI) between 30.0 and 40 kg/m2 Age between 25 and 65 years Sedentary (light exercise less than 1 hour per week) or moderately active (moderate exercise 1 to 2 hours per week) Weight stable for >3 months prior to the beginning of the study (gain or loss <4 kg) Able to give written informed consent |
| Interventions | Arm Intervention/treatment Experimental: Time restricted feeding Time restricted feeding Other: Time restricted feeding Time restricted feeding No Intervention: Control No intervention |
| Outcomes | Primary outcome measures: Body weight [ Time Frame: 12 weeks ] Secondary outcome measures: Plasma lipids [ Time Frame: 12 weeks ] Blood pressure [ Time Frame: 12 weeks ] Insulin resistance measured by Homeostatic model assessment (HOMA) [ Time Frame: 12 weeks ] Inflammatory markers: tumour necrosis factor‐alpha (TNF) and Interleukin‐6 (IL‐6) [ Time Frame: 12 weeks ] |
| Notes | NCT02948517‐ It is unclear from the information above that the intervention meets our predefined criteria. |
Ntr 2009.
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria: 1. Lean healthy male volunteers; 2. Age 18 ‐ 35 years; 3. BMI 20‐25 kg/m2; 4. Stable weight three months prior to study inclusion; 5. Normal oral glucose tolerance test (OGTT) using the ADA‐criteria. |
| Interventions | 1. Two weeks standard diet; 2. Two weeks of intermittent fasting. After these two weeks, a study day as previously described will follow where after volunteers change diet (standard vs IF, or IF vs standard). |
| Outcomes | Primary outcome: insulin sensitivity of glucose metabolism. Secondaryoutcome: insulin sensitivity of lipid and protein metabolism. |
| Notes | It is unclear from the information above that the intervention meets our predefined criteria. |
Spelta 2017.
| Methods | Randomised controlled trial |
| Participants | 40 overweight/slightly obese HF outpatients (NYHA I‐II) |
| Interventions | Participants assigned to IF or control group for six months. IF scheme is a 'twice‐weekly‐diet' (ad libitum food for 5 days during the week and fasting ‐i.e. a maximum of 500 Kcal intake‐ for two nonconsecutive days). |
| Outcomes | |
| Notes | More information needed to determine whether paper meets our eligibility criteria. Only abstract was found. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12619000246189.
| Study name | Mediterranean diet with or without intermittent fasting in type 2 diabetic patients: a randomized clinical trial |
| Methods | Randomised controlled trial |
| Participants | Diagnostic criteria: type 2 diabetes (previously diagnosed with HbA1c
>7.0% [53 mmol/mol] and/or taking anti‐glycaemic medication) Age minimum: 20 years Age maximum: 75 years Gender: both males and females |
| Interventions | Arm 1 Intensive personalised dietary counselling based on a Mediterranean diet (low in saturated fat [<7% of total energy intake (TEI)] and high in fibre (> 40 g/day), consumed ad libitum and delivered by a nutritionist with minimum 5 years' experience. Arm 2 Intensive personalised dietary counselling based on a Mediterranean diet (low in saturated fat ([<7% of TEI and high in fibre (> 40 g/day]), consumed ad libitum accompanied by time restricted feeding (12 hours fasting every day). |
| Outcomes | Changes in HbA1c assessed by using serum essay analysis performed in a
qualified laboratory.[Timepoint: Baseline, 3 months (primary time point) and
6 months after intervention commencement] Changes in CRP assessed by using serum essay analysis performed in a qualified laboratory.[Timepoint: Baseline, and at 3 and 6 months after intervention commencement] Changes in fasting plasma glucose assessed by using serum essay analysis performed in a qualified laboratory.[Timepoint: Baseline, and at 3 and 6 months after intervention commencement] Changes in HDL cholesterol assessed by using serum essay analysis performed in a qualified laboratory.[Timepoint: Baseline, and at 3 and 6 months after intervention commencement] Changes in LDL cholesterol assessed by using serum essay analysis performed in a qualified laboratory.[Timepoint: Baseline, and at 3 and 6 months after intervention commencement] Changes in plasma insulin concentrations assessed by using serum essay analysis performed in a qualified laboratory.[Timepoint: Baseline, and at 3 and 6 months after intervention commencement] Changes in systolic and diastolic blood pressure assessed using automated sphygmomanometry. [Timepoint: Baseline, and at 3 and 6 months after intervention commencement] Changes in total cholesterol assessed by using serum essay analysis performed in a qualified laboratory. [Timepoint: Baseline, and at 3 and 6 months after intervention commencement ] Changes in triglycerides assessed by using serum essay analysis performed in a qualified laboratory. [Timepoint: Baseline, and at 3 and 6 months after intervention commencement] Medication changes assessed using a medication record at each visit, asking specifically if there were any changes to the medications or the dose taken. [Timepoint: Baseline, and at 3 and 6 months after intervention commencement] |
| Starting date | 01/04/2019 |
| Contact information | C.Itsiopoulos@latrobe.edu.au |
| Notes |
ACTRN12619000757112.
| Study name | The effect of intermittent fasting on muscle mass in overweight, middle‐aged men |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1.Male 2.Age: 35‐55 years 3.BMI: 25‐35 kg/m2 Exclusion criteria 1. Current diagnosis of type 2 diabetes (HbA1C > 6.5% or 48 mmol/mol) 2. Unable to attend the laboratory for visits and adhere to the 3‐day lead‐in diet and 10‐day intervention diet 3. Currently meeting physical activity guidelines (i.e. doing more than 150 minutes of physical activity per week or >10,000 steps per day) 4. Major or chronic illness that impairs mobility or eating/digestion 5. Previous bariatric surgery 6. Shift workers; 7. Smoker (cigarette, e‐cigarette or marijuana) 8. Individuals with strict dietary intake regimens (i.e. vegan, avoidance of principal study foods) 9. Individuals who are currently restricting their dietary intake (i.e. actively trying to diet and lose weight) or participating in regular fasting (defined as fasting >16 hours/day or having completed 12 24‐hour fasts within the past year) 10. Participating in shift work (i.e. >3 hours between 22:00 hours and 05:00 hours for 1 day per week (> 50 days per year)) 11. Not weight stable (>5 kg body weight change over last 3 months) 12. Individuals who do not consume breakfast on at least 5 of 7 days per week (i.e. not eating regular meals) 13. On prescribed medications required to be taken with food in the early morning or late evening or taking other prescribed medications for <3 months 14. On prescribed anti coagulation medications (interfering with muscle biopsy procedures) |
| Interventions | The study design consists of a randomised, parallel‐group intervention study in 40 overweight, middle‐aged males. The study will involve a 3‐day lead‐in control diet for all intervention groups, after which subjects will be randomised to 10 consecutive days of either the following. 1. Alternate day fasting (ADF: 25 Energy percentage (En%) food ingestion on day 1, 3, 5, 7 and 100 En% food ingestion on day 2, 4, 6, 8 and 10) 2. Continuous energy restriction (CER: daily intake of 62.5 En% of total energy intake) 3. Time restricted eating (TRE: daily intake of 100 En% of total energy intake in an 8‐hour time window) 4. An energy‐balanced control diet (CON: daily intake of 100 En% of total energy intake in a 12‐hour time window, according to current Australian Healthy Eating guidelines comprising a macronutrient intake of 50% En, CHO, 30% En fat and 20% En protein.). |
| Outcomes | The primary outcome measurement will be muscle protein synthesis rates
(%/day) measured using the rate of labelled water (D2O) uptake from the
muscle biopsy samples and alanine enrichments in saliva. [Day 0 and Day
11] Body composition assessed by a DEXA scan: fat‐free mass (in % and kg) [Days 0, 4, 8 and 11] Body composition assessed by a DEXA scan: fat mass (in % and kg) [Day s0, 4, 8 and 11] Body composition assessed by a DEXA scan: total body mass (in % and kg) [Dasy 0, 4, 8 and 11] Body water 2H2O enrichment by GC‐MS analyses [muscle biopsies at Day 0 and Day 11; plasma samples at days 0,4,8 and 11; and daily saliva samples from day 0‐11] Free L‐[2,3,3,3‐2H4]‐alanine muscle enrichments by GC‐MS analyses [muscle biopsies at Day 0 and Day 11] Free L‐[2,3,3,3‐2H4]‐alanine plasma enrichments by GC‐MS analyses [plasma samples at day 0,4,8 and 11] Glyacemic control (Freestlye Libre continuous glucose monitoring)[Day 0 to Day 11 (15 minutes time intervals throughout the 10‐day intervention period)] Plasma amino acid concentrations (total amino acid profile measured by LC‐MS)[Day 0, 4, 8 and 11] Plasma gastrointestinal hormone concentrations (PYY, CCK, GLP, GIP and ghrelin)[Days 0, 4, 8 and 11] Plasma glucose concentrations in blood samples analysed by YSI 2900[Days 0, 4, 8 and 11] Plasma insulin concentrations (ELISA) [Days 0, 4, 8 and 11] |
| Starting date | 01/06/2019 |
| Contact information | imre.kouw@acu.edu.au |
| Notes |
ChiCTR1800016271.
| Study name | The effect of intermittent fasting in patients with overweight |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria 1. aged 25 to 65 years,male and female 2. BMI = 24 kg/m2; 3. light physical activity (i.e. < 3 hours/week of light intensity exercise at 2.5 to 4.0 metabolic equivalents (METs) for 3 months prior to the study), weight stable for 3 months prior to the start of this study (i.e. less than 4 kg weight loss or weight gain in recent three months) 4. participants are required to sign informed consent Exclusion criteria 1. women who are either in pre‐menopause or 2 years post menopause 2. patients with diabetes (FPG = 7.0mmol/L and / or OGTT 2‐hour PG = 11.1mmol/L) 3. attended in another study or take weight‐loss drugs at the same time or 3 months prior to the beginning of the study 4. obvious renal insufficiency (creatinine = 1.5 mg/dL or > 133umol/L) 5. obvious abnormal liver function (ALT more than 2 times of the normal upper limit) 6. ever suffered from history of stroke or myocardial infarction; be done or are preparing to do coronary angioplasty or coronary artery bypass grafting 7. other serious diseases such as malignant tumour 8. be preparing for pregnancy; other situations that researchers believe that is not suitable for the selected object |
| Interventions | 1:Intermittent fasting 2:Continuous Energy restriction |
| Outcomes | BMI; waist circumference;total cholesterol; |
| Starting date | 2018‐06‐01 |
| Contact information | fan_yibing@163.com |
| Notes |
ChiCTR1800017557.
| Study name | Effect of time‐restricted feeding and continuous feeding in gut microbiome of critically ill patients |
| Methods | Randomised controlled trial |
| Participants | Key inclusion & exclusion criteria
Inclusion criteria ICU patients needing for enteral nutrition by gastric tube Exclusion criteria gastrectomy, enterectomy, gastrointestinal haemorrhage, diabetes, intestinal obstruction |
| Interventions | Group 1:time‐restricted feeding;Group 2:continuous feeding; |
| Outcomes | bacterial diversity; Albumin |
| Starting date | 2018‐08‐28 |
| Contact information | icuyaobo@126.com |
| Notes |
ChiCTR1900020871.
| Study name | Randomized controlled trial for intermittent fasting therapy for nonalcoholic fatty liver disease |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria (1) patients with nonalcoholic fatty liver disease (2) age 18‐70 years old (3)Light physical labourers (4) participants are required to sign informed consent Exclusion criteria (1) other liver diseases and drug‐induced liver damage (2) diabetic patients (FPG = 7.0mmol/L and/or OGTT 2‐hour PG=11.1mmol/L) (3) hypertensive patients (systolic blood pressure =140 mmHg and / or diastolic blood pressure = 90 mmHg or taking antihypertensive drugs) (4) those who participated in the study three months before, or participated in other research trials at the same time (5) those who were enrolled in the study three months ago, or at the same time (6) obvious renal insufficiency (creatinine = 1.5 mg/dL or > 133umol/L) (7) those who have had a history of stroke or myocardial infarction (8) have done or are preparing for coronary balloon dilatation or coronary artery bypass surgery (9) suffering from other serious diseases such as malignant tumours (10) pregnant or lactating women (11)participant considered by the investigator not suitable for inclusion |
| Interventions | 1:Health education, sports guidance,Intermittent fasting for 1 day per week;2:Health education, sports guidance,Intermittent fasting for 2 days per week;3:Health education, sports guidance; |
| Outcomes | BMI; waist circumference; hip circumference; waist‐to‐hip ratio; liver function; |
| Starting date | 2019‐07‐01 |
| Contact information | fan_yibing@163.com |
| Notes |
Irct20150909023957N8.
| Study name | Effect of low‐calorie diets on anthropometric indices, glycemic markers and cardiovascular risk factors in metabolic syndrome |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria
Patients with Metabolic Syndrome
Age
25‐60 years 25 =BMI = 40 kg/m2 Body weight more than 5 kg has not changed during the last 3 months No fasting for 3 months prior to the beginning of the study Ppeople who are willing to cooperate and answer questions and conduct their tests after explaining the work Exclusion criteria Smoker History of cardiovascular, pulmonary, renal, thyroid disorders, digestive and liver problems such as hepatitis and ...follow a special diet Severe physical activity People who have been using drugs that have an effect on weight loss, lipid or glucose metabolism over the past 6 months |
| Interventions | Intervention 1: During 8‐week ADF period, participants consumed very low
calorie diet (75% energy restriction) during the 3 fast days (Saturday,
Monday, Wednesday) and then ate diet that providing 100% of their energy
needs on each feed day (3 days a week). In Friday participants consume ad
libitum without limitation. ADF participants were provided with meals on
each fast day (ranging from 400‐ 600 kcal), and consumed ad libitum at home
on feed day. The feed and fast days began at midnight each day, and all fast
day meals were consumed between 12.00 pm and 2.00 pm to ensure that each
participant was undergoing the same duration of fasting. all food prepared
in the home. Participants were permitted to consume calorie‐free foods (such
as water, green tea, coffee without sugar (< 400 mg caffeine per day),
non‐starchy vegetable (such as lettuce, cucumber, green leaf, tomato) and
sugar‐free gums on the fast day and were encouraged to drink plenty of
water. Intervention 2: Control group: in Calorie‐restriction group, participants consumed 75% energy needs in each day for 8 weeks and includes 3 main meals and 2 snacks. All participants in two groups were required to prepare all of their meals at home. The baseline energy requirements for the subjects were assessed by Mifflin equation. Daily dietary carbohydrate, fat and protein accounted for 52, 30 and 18% of ingested energy, respectively. |
| Outcomes | Primary Outcomes BMI. Timepoint: two times, before and after dietary intervention. Method of measurement: BMI was calculated as the weight in kg divided by the square of the height in meters (kg/m2). Body weight and body composition analysis. Timepoint: two times, before and after dietary intervention. Method of measurement: In light clothing, standing without shoes and hose on metal foot‐ plates while holding the handles of the bio‐impedance analyser (BIA; BC‐418, Tanita Europe, Amsterdam, NL). Fasting blood sugar. Timepoint: two times, before and after dietary intervention. Method of measurement: fasting plasma glucose concentrations were measured using auto‐analyser (glucose oxidase/peroxidase). HDL cholesterol. Timepoint: two times, before and after dietary intervention. Method of measurement: Plasma HDL‐C was measured using detergent oxidase/peroxidase methods. HOMA‐IR. Timepoint: Two times, before and after dietary intervention. Method of measurement: fasting glucose (mmol/L) * fasting insulin (µU/L)/ 22.5. LDL cholesterol. Timepoint: two times, before and after dietary intervention. Method of measurement: LDL‐C concentration was calculated using the Friedwald equation (LDL= total cholesterol – TAG/ 2.18 – HDL). Plasma insulin. Timepoint: two times, before and after dietary intervention. Method of measurement: plasma insulin levels were measured by Elisa method. Total cholesterol. Timepoint: two times, before and after dietary intervention. Method of measurement: plasma total cholesterol was measured in duplicate using cholesterol oxidase/peroxidase. Triglyceride (TG). Timepoint: two times, before and after dietary intervention. Method of measurement: plasma TG concentration was measured glycerol phosphate oxidase/peroxidase method. Waist circumference. Timepoint: two times, before and after dietary intervention. Method of measurement: waist circumference was measured by a flexible tape to the nearest 0.1 cm, in standing participant at the midway between the lower costal margin of the last palpable rib and the top of the iliac crest during a period of expiration. |
| Starting date | 2019‐01‐23 |
| Contact information | |
| Notes |
ISRCTN13374800.
| Study name | Alternate day fasting as an intervention to improve body composition and blood biochemical markers in overweight patients |
| Methods | Randomised controlled trial |
| Participants | Key inclusion & exclusion criteria
Inclusion criteria 1. 20‐55 years of age 2. BMI > 25 kg/m2 3. Previous sedentary lifestyle 4. Absence of diabetes, hypertension and cardiovascular diseases 5. No smoking Exclusion criteria 1. Previously undergone bariatric surgery |
| Interventions | Alternate day fasting (ADF) group: participants in this group have their calorie intake limited to 25% of the baseline energy needs on the fasting days and this is to be ingested within a 12‐hour period and divided into six small meals. On the feeding days, ad libitum food intake was permitted over a 12‐hour period. On both days, ingestion of food is not permitted in the remaining hours during which the participants are awake. On the feeding days, there is no restriction on calorie intake, but the participants are required to record the specific consumed foods in a food diary. Carloric restriction (CR) group: participants in this group have a hypocaloric diet that is restricted to 75% of the estimated calorie expenditure per day throughout the intervention period. |
| Outcomes | 1. Mean body weight loss is measured using the BodPod and InBody770 at
baseline and 4 weeks
2. Fat loss is measured using the BodPod and
InBody770 at baseline and 4 weeks
3. Lean mass loss is measured using
the BodPod and InBody770 at baseline and 4 weeks
4. Carbohydrate
burning is measured using indirect calorimetry at baseline and 4
weeks
5. BMR (Basal Metabolic Rate) is measured using Indirect
calorimetry (Spirostik‐REE) at baseline and 4 weeks 1. Body water is measured using InBody770 at baseline and 4 weeks 2. Food patterns is measured using a Food Reminder Form that was delivered for all subjects at baseline and 4 weeks |
| Starting date | 08/08/2017 |
| Contact information | |
| Notes |
ISRCTN32122407.
| Study name | The effects of early versus late time‐restricted feeding on metabolic disease risk factors in adults at increased risk of developing type 2 diabetes: Is there an optimal time to eat? |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria Participants must: 1. Be aged 18 – 65 years old 2. Have a BMI = 25 kg/m2 3. Have maintained a stable body weight for the 6 months preceding the study (± 2 kg) 4. Be at increased/moderate/high risk of developing type 2 diabetes, as per the Diabetes UK Diabetes Risk Score (scoring = 7) 5. be able and willing to give informed oral and informed written consent 6. Complete and meet the defined criteria of pre‐study questionnaires 7.bBe able and willing to complete daily sleep and food diaries during the study 8. Have an eating period of = 12 hours a day 9. Agree to eat their meals within certain time periods during the day whilst participating in the study 10. Be willing and able to undertake laboratory tests on agreed dates during the study |
| Interventions | After an initial 1‐week baseline period, participants will be randomly assigned to one of three experimental groups for an intervention period of approximately 10 weeks. The first (control) group will be asked to maintain their habitual sleep‐wake and feed‐fast routines throughout the intervention period. The second, ‘early‐TRF’, group will be asked to maintain their habitual sleep‐wake routine, but restrict the duration of their eating times during the day to between 7am and 3pm (± 1 hour). The third, ‘late‐TRF’, group will also be asked to maintain their habitual sleep‐wake routine, but restrict the duration of their eating times during the day to between 12pm and 8pm (± 1 hour). |
| Outcomes | 1. Low‐density lipoprotein (LDL) cholesterol measured using fasted blood tests at baseline, weeks 3, 5, 8 and 10 2. Insulin resistance measured using the homeostatic model assessment (HOMA) which utilises fasted glucose and insulin levels, at baseline, week 5 and week 10 |
| Starting date | 1. Weight measured using scales at baseline, weeks 3, 5, 8 and 10 2. Adiposity measured using the gold standard dual‐energy x‐ray absorptiometry (DEXA) at baseline and week 10 3. Dietary intake assessed through the use of food diaries at baseline, weeks 2, 4, 7 and 9, as well as 24‐hour dietary recalls at baseline, weeks 3, 5, 8 and 10 4. Food preferences assessed using questionnaires at baseline, weeks 3, 5 8 and 10, as well as eye tracking tests at baseline, week 5 and week 10 |
| Contact information | s.lynch@surrey.ac.uk |
| Notes |
NCT03342742.
| Study name | Daily caloric restriction and intermittent fasting in overweight and obese adults with autosomal dominant polycystic kidney disease |
| Methods | Randomised controlled trial |
| Participants | 28 Participants Ages eligible for study: 18 years to 65 years (adult, older adult) Sexes Eligible for Study: all Accepts Healthy volunteers: no Inclusion Criteria aged 18‐65 years ADPKD diagnosis based on the modified Pei‐Ravine criteria BMI 25‐45 kg/m2 Normal to mildly declined renal function with an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 by the CKD‐EPI equation Access to the internet with video chat capabilities no plans for extended travel (>2 weeks) during the 3‐month intensive period Not currently participating in another interventional study or weight loss program Ability to provide informed consent |
| Interventions | Arm Intervention/treatment Experimental: Daily Caloric Restriction The daily caloric restriction group will be instructed to reduce energy intake by a 34% daily energy deficit from baseline individual weight maintenance energy requirements. Behavioural: weight loss Weight loss behavioural intervention via one of two strategies. Experimental: intermittent fasting Participants in the intermittent fasting group will be instructed to reduce energy intake to ~20% of estimated energy requirement (delivered as a single meal) three non‐consecutive days per week, resulting in a weekly energy deficit of ~34% (similar to the daily caloric restriction group). Behavioural: weight loss Weight loss behavioural intervention via one of two strategies. |
| Outcomes | Primary outcome measures: Feasibility to enrol and retain participants [ Time Frame: Through study completion, an expected duration of 18 months ] Numbers of individuals pre‐screened Feasibility to enrol participants [ Time Frame: Through study completion, an expected duration of 18 months ] Numbers of individuals screened Feasibility to retain participants [ Time Frame: Through study completion, an expected duration of 18 months ] Numbers of individuals enrolled Feasibility to retain participants [ Time Frame: Through study completion, an expected duration of 18 months ] Numbers of individuals retained Change in Weight Loss [ Time Frame: Baseline, 12 weeks, and 1 year ] Measurement of body weight pre to post intervention in each group Secondary Outcome Measures: Safety and tolerability, measured as adverse events [ Time Frame: 12 weeks and 1 year ] Number of participants with treatment‐related adverse events in each group as evaluated by the Safety Officer Safety and tolerability, measured as treatment‐related adverse events [ Time Frame: 12 weeks and 1 year ] Number of participants with treatment‐related adverse events in each group as evaluated by the Safety Officer Changes in quality of life [ Time Frame: Baseline, 12 weeks and 1 year ] Quality of life (QOL) will be assessed with the RAND 36 Item Health Survey (RAND‐36) physical and mental health component summary score. Changes in mood [ Time Frame: Baseline, 12 weeks and 1 year ] Mood state will be assessed with the Profile of Mood States 2 (POMS‐2) Change in energy intake [ Time Frame: Baseline, 12 weeks and 1 year ] Self‐reported energy intake Change in macronutrient intake [ Time Frame: Baseline, 12 weeks and 1 year ] Self‐reported macronutrient intake Change in serum insulin‐like growth factor‐1 levels [ Time Frame: Baseline, 12 weeks and 1 year ] Serum insulin‐like growth factor‐1 (IGF‐1) levels will be evaluated in each group Change in insulin‐like growth factor binding protein‐1 levels [ Time Frame: Baseline, 12 weeks and 1 year ] Insulin‐like growth factor binding protein‐1 (IGFBP‐1) levels will be evaluated in each group Change in PBMC AMPK expression [ Time Frame: Baseline, 12 weeks and 1 year ] Peripheral blood mononuclear cell protein expression of AMP‐activated kinase (AMPK) in each group Change in PBMC S6K expression [ Time Frame: Baseline, 12 weeks and 1 year ] Peripheral blood mononuclear cell protein expression of S6 kinase (S6K) in each group Change in β‐hydroxybutyrate levels [ Time Frame: Baseline, 12 weeks and 1 year ] Serum β‐hydroxybutyrate levels in each group Change in total kidney volume by magnetic resonance imaging (MRI) [ Time Frame: Baseline, 12 weeks and 1 year ] Change in total kidney volume by MRI in each group |
| Starting date | June 4, 2018 |
| Contact information | Kristen Nowak, Ph.D., MPHUniversity of Colorado ‐ Anschutz Medical Campus |
| Notes | NCT03342742 |
NCT03411356.
| Study name | Intermittent fasting versus daily caloric restriction for weight loss |
| Methods | Randomised controlled trial |
| Participants | 150 participants Ages Eligible for Study: 18 years to 55 years (adult) Sexes Eligible for Study: all Accepts Healthy volunteers: yes Inclusion Criteria Female or Male Age 18‐55 years Body Mass Index 27‐46 kg/m2 Sedentary: defined as <150 minutes per week of voluntary exercise at moderate intensity or greater and < 60 minutes per day of total habitual physical activity (i.e. work‐related, transportation‐related) at moderate intensity or greater, over the past 3 months. No self‐report of acute or chronic disease (CVD, diabetes, gastrointestinal disorders and orthopedic problems in particular) No plans to relocate within the next 12 months No plans for extended travel (> 2 weeks) within the next 12 months No nicotine use Live or work within 30 minutes of the Anschutz Health and Wellness Center (AHWC) (exceptions may be made at the discretion of the Study PI on a case by case basis for highly‐motivated people). Capable and willing to give informed consent, understand exclusion criteria, and accept the randomised group assignment. Have a primary care physician (or are willing to establish care with a primary care physician prior to study enrolment) to address medical issues which may arise during screening or study procedures/interventions. For Females Not currently pregnant or lactating Not pregnant within the past 6 months Not planning to become pregnant in the next 12 months Sexually active women of childbearing potential may be enrolled if they have had a tubal ligation or use a reliable means of contraception |
| Interventions | Arm Intervention/treatment Active Comparator: Daily Caloric Restriction (DCR) Participants in this group will focus on daily calorie restriction as their dietary weight loss strategy. Behavioural: Daily Caloric Restriction (DCR) Participants in this group will be given a calorie goal designed to produce a 34.3% energy deficit from estimated baseline weight maintenance energy requirements. Participants in this group will also receive a 12‐month comprehensive group‐based behavioural weight loss program and will be instructed in specific strategies to support DCR. Randomized groups will meet separately. Participants in this group will also be asked to increase moderate intensity physical activity to a target of 300 minutes per week. Experimental: Intermittent Fasting (IMF) Participants in this group will focus on modified intermittent fasting as their dietary weight loss strategy. Behavioural: Intermittent Fasting (IMF) Participants in this group will be instructed to limit energy intake to 20% of estimated baseline energy requirements on three non‐consecutive days per week, and to eat ad libitum the other 4 days per week. Participants in this group will also receive a 12‐month comprehensive group based behavioural weight loss program and will be instructed in specific strategies to support IMF. Randomised groups will meet separately. Participants in this group will also be asked to increase moderate intensity physical activity to a target of 300 minutes per week. |
| Outcomes | Primary outcome measures: Change in Body Weight [ Time Frame: Baseline and weeks 4, 13, 26, 39, and 52. ] Body weight will be measured via clinic scale. Secondary Outcome Measures: Changes in Body Composition [ Time Frame: Baseline and weeks 26, and 52. ] Body composition will be assessed with dual‐energy x‐ray absorptiometry (DXA). Changes in Blood Pressure [ Time Frame: Baseline and weeks 13, 26, and 52. ] Blood pressure will be measured with a sphygmomanometer. Changes in Fasting Lipids [ Time Frame: Baseline and weeks 26 and 52 ] 12 hour fasting blood sample for measurement of lipid profile. Changes in Insulin Sensitivity [ Time Frame: Baseline and weeks 26 and 52 ] 12 hour fasting blood sample for measurement of insulin and glucose. Insulin sensitivity (homeostasis model assessment of insulin resistance [HOMA‐IR]) will be calculated as ([insulin] x [fasting glucose x 0.055]/22.5). Changes in Objectively Measured Energy Intake (EI) [ Time Frame: Baseline, weeks 26 and 52 ] EI will be measured using the doubly‐labelled water (DLW) intake‐balance method. Changes in Self‐Reported Energy Intake (EI) [ Time Frame: Baseline and weeks 13, 26, and 52. ] Dietary energy intake (kcals/day) will be measured with diet diaries. Changes in Self‐reported Diet Composition [ Time Frame: Baseline and weeks 13, 26, and 52. ] Dietary macronutrient intake will be measured with diet diaries. Changes in Self‐Reported Dietary Adherence [ Time Frame: Weeks 4, 8, 13, 18, 22, 26, 30, 34, 39, 44, 48, 52 ] Adherence to prescribed diet will be assessed with a monthly questionnaire. Changes in Resting Energy Expenditure (REE) [ Time Frame: Baseline and weeks 26, and 52. ] REE will be measured using indirect calorimetry. Changes in Total Daily Energy Expenditure (TDEE) [ Time Frame: Baseline and weeks 26, and 52. ] TDEE will be measured using the doubly‐labelled water (DLW) method. Changes in Physical Activity [ Time Frame: Baseline and weeks 13, 26, and 52. ] Physical activity will be measured with activity monitors. Changes in Sedentary Behaviour [ Time Frame: Baseline and weeks 13, 26, and 52 ] Sedentary behaviour will be measured with activity monitors. Other Outcome Measures: Changes in Waist Circumference [ Time Frame: Baseline and weeks 13, 26, and 52 ] Waist circumference will be measured with a tape measure just over the iliac crest Changes in Highly Sensitive C Reactive Protein (hs CRP) [ Time Frame: Baseline and weeks 26 and 52 ] 12 hour fasting blood sample fro measurement of hs CRP Changes in Leptin [ Time Frame: Baseline and weeks 26 and 52 ] 12 hour fasting blood sample for measurement of Leptin Changes in Ghrelin [ Time Frame: Baseline and weeks 26, and 52 ] 12 hour fasting blood sample for measurement of Ghrelin Changes in Brain‐Derived Neurotrophic Factor (BDNF) [ Time Frame: Baseline and weeks 26 and 52 ] 12 hour fasting blood sample for measurement of BDNF Predictors of Weight Loss [ Time Frame: Baseline and weeks 13, 26, 52, and 78 ] Predictors of weight loss will be assessed in both groups with questionnaires periodically over the 52 week intervention Difficulty of Test Fast [ Time Frame: Baseline ] Participants will be asked to perform a test fast i.e. to limit intake to 25% of estimated baseline energy requirements for 1 day at baseline (prior to randomisation and beginning the interventions). Difficulty of the test fast will be assessed using a 1‐10 Likert scale with 1 representing no difficulty and 10 representing extreme difficulty. Intervention Preference [ Time Frame: Baseline ] Intervention preference will be assessed by asking participants which intervention group (IMF or DCR) they would prefer at baseline (prior to randomisation) 6 Month Post‐Intervention Follow‐Up Body Weight [ Time Frame: 26 weeks after completion of the 52‐week intervention (i.e. at week 78) ] Body weight will be measured via clinic scale. 6 Month Post‐Intervention Follow‐Up Body Composition [ Time Frame: 26 weeks after completion of the 52‐week intervention (i.e. at week 78) ] Body composition will be assessed with dual‐energy x‐ray absorptiometry (DXA). Evaluation of Genotype as Predictor of Weight Loss [ Time Frame: Baseline ] DNA will be isolated from a whole blood sample. DNA genotyping will be performed using a commercial array which covers > 2 million single nucleotide polymorphisms across the human genome. Changes in DNA Methylation [ Time Frame: Baseline and weeks 13, 26 and 52 ] DNA will be isolated from whole blood samples. DNA genotyping will be performed using a commercial array which covers > 2 million single nucleotide polymorphisms across the human genome. DNA methylation will be assessed using commercial arrays which covers >850,000 methylation sites across the human genome. Changes in Stool Microbiome [ Time Frame: Baseline and weeks 13, 26, and 52 ] Stool samples will be collected to evaluate types and relative quantities of bacteria in fecal specimens Changes in Reproductive Hormones [ Time Frame: Over one menstrual cycle at baseline, over the first 3 menstrual cycles after starting the 52 week intervention and over one menstrual cycle after completing the 52 week intervention. ] Daily urine collection over a menstrual cycle in subset of pre‐menopausal women Changes in Peptide YY [ Time Frame: Baseline and weeks 26 and 52 ] 12 hour fasting blood sample for measurement of Peptide YY Changes in Hemoglobin A1C [ Time Frame: Baseline and weeks 26 and 52 ] 12 hour fasting blood sample for measurement of Hemoglobin A1C |
| Starting date | December 22, 2017 |
| Contact information | Victoria Catenacci, MD |
| Notes | NCT03411356 |
NCT03439618.
| Study name | Comparison of time‐restricted feeding and continuous feeding in critically ill patients |
| Methods | Randomised controlled trial |
| Participants | 380 participants |
| Interventions | Arm Intervention/treatment continuous feeding The total amount of every days' Enteral Nutritional Suspension was fed at constant speed for 24 hours Other: continuous feeding At the beginning, all enrolled patients were fed by continuous feeding. When the amount calorie of feeding enteral nutritional suspension increased to 80% target calorie (target calorie: 25kilocalorie/kg/day), the patients was randomly into continuous feeding and time‐restricted feeding group.In the continuous feeding, the total amount of every days' Enteral Nutritional Suspension was fed at constant speed for 24 hours. Time‐restricted feeding The total amount of every days' Enteral Nutritional Suspension was fed at constant speed for 6h (7:00‐9:00,11:00‐13:00,17:00‐19:00). Other: time‐restricted feeding At the beginning, all enrolled patients were fed by continuous feeding. When the amount calorie of feeding enteral nutritional suspension increased to 80% target calorie (target calorie: 25kilocalorie/kg/day), the patients was randomly into continuous feeding and time‐restricted feeding group. In continuous feeding group, the enteral nutritional suspension was fed at constant speed for 24h.In the time restricted feeding, feeding time should be at 7:00‐9:00, 11:00‐13:00 and 17:00‐19:00 at constant feeding speed. |
| Outcomes | Primary outcome measures: nitrogen balance [ Time Frame: at the time point of 10th feeding day ] it equal to Nitrogen intake minus Nitrogen output.Source of nitrogen intake is the enteral nutritional suspension, and the amount of nitrogen can be calculated according to the proportion of nitrogen in enteral nutritional suspension. Main nitrogen losses include urine and faeces. The amount of nitrogen in urine and faeces can be measured by clinical laboratory. Secondary Outcome Measures: delirium [ Time Frame: up to 10 days ] it is disorders of the mental state and medical condition. It can be evaluated by The Confusion Assessment Method for the Intensive Care Unit (CAM‐ICU). Gastric residual volume [ Time Frame: up to 10 days ] This index was to evaluate the feeding complications. Nurse can evaluate the volume by pumping the stomach tube with syringe to measure the gastric content amount. diarrhoea [ Time Frame: up to 10 days ] This index was to evaluate the feeding complications. It is the condition of having at least three loose or liquid bowel movements each day. Incidence of ventilator‐associated pneumonia [ Time Frame: up to 10 days ] This index was to evaluate the feeding complications. Ventilator‐associated pneumonia (VAP) is a type of lung infection that occurs in people who are on mechanical ventilation breathing machines for at least 48 hours. The diagnosis of VAP varies among hospitals and providers but usually requires a new infiltrate on chest x‐ray plus two or more other factors. These factors include temperature of >38 or <36 °C, a white blood cell count of >12 × 10^9/ml, purulent secretions from the airways in the lung, and/or reduction in gas exchange. glucose fluctuation [ Time Frame: up to 10 days ] This index was to evaluate the feeding complications. The glucose is measured at the 11:00, 15:00, 21:00, 1:00 and 5:00 five time points. The glucose fluctuation is the maximum glucose amount plus minimum glucose amount. Albumin [ Time Frame: up to 10 days ] Serum albumin is the main protein of human blood plasma. It can be measured by clinical laboratory. |
| Starting date | May 9, 2018 |
| Contact information | icuyaobo@126.com |
| Notes | NCT03439618 |
NCT03504683.
| Study name | Effect of time‐restricted feeding on 24‐hour glycemic control, blood pressure, and cardiovascular disease risk factors |
| Methods | Randomised controlled trial |
| Participants | 144 participants Ages Eligible for Study: 30 years to 65 years (adult, older adult) Sexes Eligible for Study: all Accepts Healthy volunteers: no Inclusion Criteria Aged 30‐65 years old Prediabetic as determined by HbA1c between 5.7‐6.4% or fasting glucose between 100‐125 mg/dL BMI between 27‐43 kg/m^2 Wake up at a regular time between 5 am‐ to 8 am |
| Interventions | Arm Intervention/treatment Experimental: Early Time‐Restricted FeedingBehavioural: Early Time‐Restricted Feeding Eat between 8 am ‐ 3 pm (or 7 am ‐ 2 pm, if an early riser) Other names: eTRF Early TRF Experimental: Mid‐day Time‐Restricted FeedingBehavioural: Mid‐day Time‐Restricted Feeding Eat between 1 pm ‐ 8 pm (or 12 pm ‐ 7 pm, if an early riser) Other names: mTRF Mid‐day TRF Placebo Comparator: Control ScheduleBehavioural: Control Schedule Eat between 8 am ‐ 8 pm (or 7 am ‐ 7 pm, if an early riser) |
| Outcomes | Primary outcome measures: Mean 24‐hour glucose levels [ Time Frame: 10 weeks ] Mean 24‐hour glucose levels (mg/dl) Mean 24‐hour insulin levels [ Time Frame: 10 weeks ] Mean 24‐hour insulin levels (mU/L) Mean 24‐hour C‐peptide levels [ Time Frame: 10 weeks ] Mean 24‐hour C‐peptide levels (pmol/L). This is also a proxy for total 24‐hour insulin secretion. Insulin sensitivity [ Time Frame: 10 weeks ] Mean value of insulin sensitivity (dl/kg/min/μU/mL) across the three identical meal tolerance tests, as measured by the Oral Minimal Model Beta‐cell responsivity index (a measure of beta‐cell function) [ Time Frame: 10 weeks ] Mean value of beta‐cell responsivity across the three identical meal tolerance tests, as measured by the Oral Minimal Model Glucose AUCs [ Time Frame: 10 weeks ] Glucose area‐under‐the‐curve (mg/dl x hr) during each of three identical meal tolerance tests within a 24‐hour period Insulin AUC [ Time Frame: 10 weeks ] Insulin area‐under‐the‐curve (mU/L x hour) during each of three identical meal tolerance tests within a 24‐hour period C‐peptide AUC [ Time Frame: 10 weeks ] C‐peptide area‐under‐the‐curve (pmol/L x hour) during each of three identical meal tolerance tests within a 24‐hour period Peak glucose and mean amplitude of glycaemic excursions (MAGE) glucose values [ Time Frame: 10 weeks ] mg/dl Secondary Outcome Measures: Mean 24‐hour systolic and diastolic blood pressure [ Time Frame: 10 weeks ] mmHg Daily maximum value, minimum value, and amplitude of systolic and diastolic blood pressure [ Time Frame: 10 weeks ] mmHg Percentage of individuals with non‐dipping blood pressure phenotypes [ Time Frame: 10 weeks ] Heart Rate [ Time Frame: 10 weeks ] beats per minute Lipids [ Time Frame: 10 weeks ] Total cholesterol (mg/dl), LDL cholesterol (mg/dl), HDL cholesterol (mg/dl), and triglycerides (mg/dl) High Sensitivity C‐Reactive Protein (hs‐CRP) [ Time Frame: 10 weeks ] mg/L Cortisol [ Time Frame: 10 weeks ] μg/dL 8‐isoprostane [ Time Frame: 10 weeks ] pg/ml Inflammatory biomarkers [ Time Frame: 10 weeks ] TNF‐alpha, IL‐1beta, IL‐4, IL‐6, IL‐10 (in pg/ml) Other Outcome Measures: Fat mass and lean mass [ Time Frame: 10 weeks ] Changes in fat mass and lean mass as measured by dual‐energy X‐ray absorptiometry (DXA) Body weight [ Time Frame: 10 weeks ] Change in body weight (kg) as measured by scale weight Bone mineral density [ Time Frame: 10 weeks ] Changes in bone mineral density (g/cm^2) as measured by dual‐energy X‐ray absorptiometry (DXA) Chronotype [ Time Frame: 10 weeks ] Chronotype (i.e., mid‐point of sleep in clock time) as measured by the Munich Chronotype Questionnaire Sleep Quality [ Time Frame: 10 weeks ] Sleep quality as assessed by the Pittsburgh Sleep Quality Index (PQSI) (This study will use the Global PQSI score, which ranges from 0‐21, where higher values correspond to worse sleep quality.) |
| Starting date | April 2020 |
| Contact information | cpeterso@uab.edu |
| Notes | NCT03504683 |
NCT03527368.
| Study name | The Time‐Restricted Intake of Meals study (TRIM) |
| Methods | Randomised controlled trial |
| Participants | 41 participants Ages Eligible for Study: 21 years to 69 years (adult, older adult) Sexes Eligible for Study: all Accepts Healthy volunteers: yes Inclusion Criteria Prediabetes defined by HbA1c 5.7‐6.4%, or type 2 diabetes with HbA1c 6.5‐6.9% Class I‐III obesity (BMI 30‐39.9 kg/m2) If on medications for hypertension, stable regimen for at least past 6 months Willingness to adjust timing of feeding Willingness and ability to eat study diet and nothing else during run‐in and intervention Willingness to complete measurement procedures |
| Interventions | Arm Intervention/treatment Experimental: Time‐restricted feeding Behavioural: Time‐restricted feeding Participants consume food earlier in the day Usual feeding pattern Comparison Behavioural: usual feeding pattern Participants consume food later in the day |
| Outcomes | Primary outcome measures: Weight Change [ Time Frame: 12 weeks ] Weight change will be measured in kg Secondary Outcome Measures: Oral glucose tolerance test (OGTT) [ Time Frame: 12 weeks ] Change in 2‐hour glucose on OGTT Blood pressure [ Time Frame: 12 weeks ] 24‐hour ambulatory systolic blood pressure Blood pressure [ Time Frame: 12 weeks ] 24‐hour ambulatory diastolic blood pressure |
| Starting date | September 24, 2018 |
| Contact information | Nisa Maruthur, MDJohns Hopkins University |
| Notes | NCT03527368 |
NCT03539094.
| Study name | Intermittent Fasting in Multiple Sclerosis (IFMS) |
| Methods | Randomised controlled trial |
| Participants | 60 participants Ages Eligible for Study: 18 years and older (adult, older adult) Sexes Eligible for Study: all Accepts Healthy volunteers: no Inclusion Criteria Diagnosis of RRMS (2010 Mc Donald criteria) EDSS <6.0 and disease duration ≤ 15 years On an injectable therapy for MS, glatiramer acetate (GA) or beta‐interferon (beta‐IFN) for at least 3 months prior to the study and with no anticipated changes of the medication for the 12‐week study duration Age ≥18 years BMI > 22 and < 35 kg/m2 with stable weight in the 3 months prior to screening |
| Interventions | Arm Intervention/treatment Experimental: Intermittent fasting The participants randomised to this group will do IF by restricting their diet and consuming few calories two days per week. During the days of fasting, participants will be allowed to drink water, calorie‐free beverages, and eat fresh, steamed or roasted non‐starchy vegetables. Other: intermittent fasting the participants randomised to this group will do IF by restricting their diet and consuming few calories two days per week. During the days of fasting, subjects will be allowed to drink water, calorie‐free beverages, and eat fresh, steamed or roasted non‐starchy vegetables. No Intervention: Western diet The participants randomised to this group will eat a standard Western style diet. |
| Outcomes | Primary Outcome Measures Leptin [ Time Frame: 12 weeks ] Leptin at week 12 measured in the peripheral blood Secondary Outcome Measures Peripheral metabolic and inflammatory profiling [ Time Frame: 12 weeks ] Adipokine and inflammatory markers at week 12 measured in the peripheral blood Anthropometric measure [ Time Frame: 12 weeks ] Weight and height will be combined to report BMI in kg/mg^2 Anthropometric measure [ Time Frame: 12 weeks ] Waist circumference in cm Gut microbiota [ Time Frame: 12 weeks ] Gut microbiota richness and composition |
| Starting date | January 1, 2018 |
| Contact information | picciol@neuro.wustl.edu |
| Notes | NCT03539094 |
NCT03689608.
| Study name | Daily vs Intermittent Restriction of Energy: Controlled Trial to reduce diabetes risk (DIRECT) |
| Methods | Randomised controlled trial |
| Participants | 252 participants Ages Eligible for Study: 35 years to 75 years (adult, older adult) Sexes Eligible for Study: all Accepts Healthy volunteers: yes Criteria Inclusion Criteria Weight‐stable (< 5 % fluctuation in their body weight for past 6‐months at study entry) Score 12 or greater on the AUSDRISK calculator HbA1c <48 mmol/L (measured at screening) |
| Interventions | Arm Intervention/treatment Experimental: Intermittent Fasting (IF) 3 days fasting per week Other: Intermittent Fasting (IF) Participants will fast 3 days per week. In fasting days, meal replacements at 30% of daily energy requirements will be provided for the first 6 months. Participants will have fortnightly nutrition assessment. Experimental: Daily Restriction (DR) daily energy restriction Other: Daily Restriction (DR) Participants are instructed to restrict energy intake by 30% of daily energy requirements. Meal replacements will be provided for the first 6 months. Participants will have fortnightly nutrition assessment. standard care (SC) usual care Other: standard care (SC) Participants will receive current practice guidelines in a static information format, will not take part in any counselling or receive meal replacements. |
| Outcomes | Primary Outcome Measures : Glycaemia [ Time Frame: 6 months ] Change in postprandial glucose HbA1c [ Time Frame: 6 months ] Change in HbA1c Secondary Outcome Measures: Body weight [ Time Frame: 2 months, 6 months, 18 months ] changes in body weight in kilograms Body composition [ Time Frame: 6 months, 18 months ] changes in body fat mass in kilograms waist and hip circumference [ Time Frame: 2 months, 6 months, 18 months ] changes in waist and hip circumference blood lipids [ Time Frame: 2 months, 6 months, 18 months ] changes in blood lipid profile (total cholesterol, HDL‐, LDL‐cholesterol and triglycerides) adherence to intervention [ Time Frame: 2 months, 6 months, 18 months ] assessed by diet records HbA1c [ Time Frame: 2 months, 18 months ] Change in HbA1c blood pressure [ Time Frame: 2 months, 6 months, 18 months ] Changes in systolic blood pressure and diastolic blood pressure Postprandial glucose [ Time Frame: 18 months ] Change in postprandial glucose |
| Starting date | September 26, 2018 |
| Contact information | |
| Notes | NCT03689608 |
NCT03745612.
| Study name | Time restricted feeding on weight loss and cardio‐protection (TREATY Trial) |
| Methods | Randomised controlled trial |
| Participants | 120 participants Ages Eligible for Study: 18 years to 75 years (adult, older adult) Sexes Eligible for Study: all Accepts Healthy volunteers: yes Inclusion Criteria Male of female aged between 18 and 75 years old; Body mass index (BMI)of 28.0 to 45.0 kg/m2; |
| Interventions | Arm Intervention/treatment Experimental: TRF Behavioural: Time restricted feeding Participants will receive a diet of 1500‐1800kcal/d for men and 1200‐1500 kcal/d for women during a window of 8 hours/day (8 am to 4 pm). Active Comparator: CER continuous energy restriction Behavioural: Continuous Energy Restriction Participants will follow receive a diet of 1500‐1800kcal/d for men and 1200‐1500kcal/d for women, without a restriction of feeding time. |
| Outcomes | Primary Outcome Measures : Change in body weight over 6 months and 12 months [ Time Frame: Baseline to 12 months ] Secondary Outcome Measures: Change in body composition [ Time Frame: Baseline to 12 months ] Change in waist circumference [ Time Frame: Baseline to 12 months ] Change in liver fat [ Time Frame: Baseline to 12 months ] Change in visceral fat [ Time Frame: Baseline to 12 months ] Change in HbA1c [ Time Frame: Baseline to 12 months ] Change in Blood pressure [ Time Frame: Baseline to 12 months ] Change in blood lipids [ Time Frame: Baseline to 12 months ] Change in insulin sensitivity [ Time Frame: Baseline to 12 months ] Change in β cell function [ Time Frame: Baseline to 12 months ] Change in pulse wave velocity (PWV) [ Time Frame: Baseline to 12 months ] Depression measured by the Patient Health Questionnaire‐9 (PHQ‐9) [ Time Frame: Baseline to 12 months ] Quality of sleep measured by the Pittsburgh sleep quality index (PSQI) [ Time Frame: Baseline to 12 months ] Quality of life measured by the 12‐item Short‐Form Health Survey Questionnaire (SF‐12) [ Time Frame: Baseline to 12 months ] |
| Starting date | November 30, 2018 |
| Contact information | Huijiezhang2005@126.com |
| Notes | NCT03745612 |
NCT03789409.
| Study name | Intermittent fasting following acute ischemic stroke |
| Methods | Randomised controlled trial |
| Participants | 68 participants Ages Eligible for Study: 20 years and older (adult, older adult) Sexes Eligible for Study: all Accepts Healthy volunteers: no Inclusion Criteria Patients who was diagnosed first ischaemic stroke within preceding 1 year through brain MRI/CT |
| Interventions | Arm Intervention/treatment Experimental: Intermittent Fasting Over rehabilitation treatment during and admission (at least 1 week), intermittent fasting (IF) for more than 12 hours (water can be allowed). For subgroup assignment, participants can choose IF1 (eat early in the evening and late in the morning) or Post‐IF2 (eat the remaining two meals without breakfast), depending on their own their favour. Dietary Supplement: Intermittent Fasting The aforementioned intermittent fasting in arm/group descriptions. No Intervention: ad libitum Participants will be allowed to have hospital meals and all the desired intake without time limit. |
| Outcomes | Primary Outcome Measures : Change of Surface electromyography [ Time Frame: 1 day before the initiation of intervention, and 6 months after the stroke onset ] root mean square and root peak square of compound motor action potential Secondary Outcome Measures: Change of Korean‐modified Barthel index [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Korean version‐Modified Barthel Index (minimum of 0 and maximum scores of 100); higher values and a better outcome. Other Outcome Measures: Change of Mini mental status exam [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Mini mental status exam(minimum of 0 and maximum scores of 30); higher values and a better outcome. Change of Beck depression inventory [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Beck depression inventory(minimum of 0 and maximum scores of 63); higher values and a worse outcome. Change of Wecsler aphasia battery [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Wecsler aphasia battery(minimum of 0 and maximum scores of 100); higher values and a better outcome. Change of Berg balance scale [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Berg balance scale(minimum of 0 and maximum scores of 56); higher values and a better outcome. Change of Functional Ambulation Category [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Functional Ambulation Category(minimum of 0 and maximum scores of 5); higher values and a better outcome. Change of Motricity Index [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Motricity Index(minimum of 0 and maximum scores of 99); higher values and a better outcome. Change of 10m walking test [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] 10m walking test Change of Grasping force (kg) [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Grasping force (kg) Change of 9‐hole pegboard [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] 9‐hole pegboard Change of Jebsen Taylor test [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Jebsen‐Taylor Hand Function Test Change of Nottingham sensory scale [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Nottingham sensory scale(minimum of 0 and maximum scores of 20); higher values and a better outcome. Change of Arm motor Fugl‐Mayer scale [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Arm motor Fugl‐Mayer scale; wrist & hand/proximal arm(minimum of 0 and maximum scores of 24 and of 34, respectively ); higher values and a better outcome. Change of Stroke impact scale [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Hand motor, Stroke Impact Scale (minimum of 12 and maximum scores of 60); higher values and a better outcome. Change of Ashworth scale [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Ashworth scale; elbow, wrist, knee & ankle(minimum of 0 and maximum scores of 4); higher values and a worse outcome. Change of Knee joint kinaesthaesia [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] The smallest iso‐kinetic angle from which the participants could detect any passive flexion or extension movement of their own knee, using Biodex; (minimum of 0 and maximum scores of 360 degree); higher values and a worse outcome. Change of Behavioural intention test [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Behavioural intention test(minimum of 0 and maximum scores of 146); higher values and a better outcome. Change of Apraxia screen of Tulia [ Time Frame: 1 day before the initiation of intervention and 3rd week after start of intervention, 3 months and 6 months after the stroke onset ] Apraxia screen of (minimum of 0 and maximum scores of 12); higher values and a better outcome. Change of motor evoked potential [ Time Frame: 1 day before the initiation of intervention, and 6 months after the stroke onset ] Amplitude (uV) of motor evoked potential was recorded on abductor pollicis brevis and extentor digitorum brevis following trans‐cranial magnetic stimulation for cortico‐spinal excitability. Change of Weight [ Time Frame: 1 day before the start of intervention and 1 weeks and 2 weeks after the start of intervention ] Weight (Kg) Change of Temperature [ Time Frame: 1 day before the start of intervention and 1 weeks and 2 weeks after the start of intervention ] temperature (Celsius) Change of Serum glucose level [ Time Frame: 1 day before the start of intervention and 1 weeks and 2 weeks after the start of intervention ] Serum glucose level (mg/ml) Change of Hypoglycemia‐related severity [ Time Frame: every day following the start of intervention until 2 weeks of intervention ] Assessment of hypoglycaemic symptoms using Likert scale (minimum of 0 and maximum scores of 10); higher values and a worse outcome. |
| Starting date | March 4, 2019 |
| Contact information | chhwang1220ciba@gmail.com |
| Notes | NCT03789409 |
NCT03791203.
| Study name | Effectiveness and adherence of Modified Alternate‐day Calorie Restriction (MACR) in non‐alcoholic fatty liver disease |
| Methods | Randomised controlled trial |
| Participants | Ages Eligible for Study: 18 years to 70 years (adult, older adult) Sexes Eligible for Study: all Accepts Healthy volunteers: Yes Criteria Inclusion Criteria: Have elevated ALT or AST level (ALT >41 or AST>34 IU/L) No evidence of other forms of liver diseases For those with diabetes mellitus and dyslipidaemia, they must be on a stable therapy for at least 6 months prior to study enrolment |
| Interventions | Experimental: Calorie restriction (MACR)
Participants restricted 70%
of their energy needs over 24 hours on a calorie restriction day alternate
with a feeding day for the next 24 hours, where they were allowed eating (ad
libitum). The calorie restriction and feeding days begun at 9 am each day,
and on the calorie restriction day, meals were consumed between 2 pm and 8
pm to ensure that they underwent the same duration of calorie restriction.
On each calorie restriction day, they were allowed energy‐free beverages and
sugar‐free gum and encouraged to drink plenty of water. Diet plans were
self‐selected using detailed individualised food portion lists, meal plans,
and recipes. Participants received phone calls from the investigator and
four 2‐weekly appointments with a dietitian. Adverse experiences were
assessed every 2 weeks. No Intervention: control group Participants in the control group continued their usual habitual diet for 8 weeks. No specific dietary advice or educations were provided throughout the entire trial. |
| Outcomes | Primary Outcome Measures Change from baseline shear wave elastography (SWE) at 8 weeks [ Time Frame: Change from baseline at 8 weeks ] Through the intercostal approach, SWE measurements were performed in the right liver lobe, at the supine position with the right arm in maximal abduction. The sonographer, assisted by an ultrasonic time‐motion image, located a liver portion of at least 6 cm thick, free of large vascular structures. Once the measurement area had been located, the sonographer pressed the probe button to start an acquisition. Patients were asked to hold their breath for about five seconds, while the stiffness of the region of interest was measured and 10 measurements were made for each patient and the median average value of those measurements was recorded in kilopascals (kPa: metric). Change from baseline liver steatosis at 8 weeks [ Time Frame: Change from baseline at 8 weeks ] Ultra‐sonographic measurements including liver steatosis and shear wave elastography (SWE) were performed with the SuperSonic Imagine's Aixplorer® Ultrasound machine (Super Sonic Image, Aix‐en Provence, France). All measurements were performed by a single sonographer where the inter‐observer agreement level with another experienced sonographer was 85%. Concentration of high‐density lipoprotein (HDL) [ Time Frame: Change from baseline at 8‐weeks ] Blood samples (8‐10 hours of fasting blood samples) were collected from participants at 8 am to 10 am at baseline and 8 weeks post intervention for biochemical analysis. It was measured in mmol/L. Concentration of low‐density lipoprotein (LDL) [ Time Frame: Change from baseline at 8 week ] Blood samples (8‐10 hours of fasting blood samples) were collected at 8 am to 10 am at baseline and 8 weeks post intervention for biochemical analysis. It was measured in mmol/L. Concentration of triglycerides (TG) [ Time Frame: Change from baseline at 8weeks ] Blood samples (8‐10 hours of fasting blood samples) were collected at 8 am to10 am at baseline and 8 weeks post intervention for biochemical analysis. It was measured in mmol/L. Concentration of total cholesterol (TC) [ Time Frame: Change from baseline at 8 weeks ] Blood samples (8‐10 hours of fasting blood samples) were collected at 8 am to 10 am at baseline and 8 weeks post intervention for biochemical analysis. It was measured in mmol/L. Concentration of fasting blood sugar (FBS) [ Time Frame: Change from baseline at 8 weeks ] Blood samples (8‐10 hours of fasting blood samples) were collected at 8 am to10 am at baseline and 8 weeks post intervention for biochemical analysis. It was measured in mmol/L. Concentration of alanine aminotransferase (ALT) [ Time Frame: Change from baseline at 8 weeks ] Blood samples (8‐10 hours of fasting blood samples) were collected at 8 am to 10 am at baseline and 8 weeks post intervention for biochemical analysis. It was measured in U/L. Concentration of aspartate aminotransferase (AST) [ Time Frame: Change from baseline at 8 weeks ] Blood samples (8‐10 hours of fasting blood samples) were collected at 8 am to 10 am at baseline and 8 weeks post intervention for biochemical analysis. It was measured in U/L. Secondary Outcome Measures: Dietary plan adherence [ Time Frame: At 8 weeks ] Adherence data were assessed each week as percentage of adherence |
| Starting date | August 1, 2015 |
| Contact information | Muhammad Izzad Bin Johari, Principal Investigator, University of Science Malaysia |
| Notes | NCT03791203 |
NCT03792282.
| Study name | Time‐Restricted Feeding(TRF) on overweight/obese women with Polycystic Ovarian Syndrome (PCOS) (TRF‐PCOS) |
| Methods | Randomised controlled trial |
| Participants | 18 years to 50 years, fFemales Inclusion Criteria
Exclusion Criteria
|
| Interventions | Experimental: TRF Participants in this group will focus on time‐restricted feeding (TRF) in addition to daily calorie restriction. Active Comparator: RCD Participants in this group will focus on daily reduced calorie diet (RCD). |
| Outcomes | Primary Outcome Measures :Changes in body weight (Kilograms) [ Time Frame: Baseline and 16 weeks ]Changes in body weight (kg) Change in insulin resistance [ Time Frame: Baseline and 16 weeks ]Insulin resistance will be assessed by HOMA‐IR Secondary Outcome Measures:Changes in waist circumference (cm) [ Time Frame: Baseline and 16 weeks ] Changes in abdominal circumference (cm) [ Time Frame: Baseline and 16 weeks ] Changes in systolic pressure(SBP) [ Time Frame: Baseline and 16 weeks ] Changes in diastolic pressure (DBP) [ Time Frame: Baseline and 16 weeks ] Change in β cell function [ Time Frame: Baseline and 16 weeks ]β cell function will be assessed by HOMA‐β Change in LDL‐c level [ Time Frame: Baseline and 16 weeks ] Change in TG level [ Time Frame: Baseline and 16 weeks ] Change in CHO level [ Time Frame: Baseline and 16 weeks ] Change in liver fibre [ Time Frame: Baseline and 16 weeks ]liver fibre will be assessed by Controlled attenuation parameter (CAP) evaluated with transient elastography (FibroScan®) Fibroscan Changes in systemic Inflammatory biomarkers [ Time Frame: Baseline and 16 weeks ]Inflammatory biomarkers (TNFa,CRP and Interleukin‐6) are measured by ELISA (enzyme‐linked immunosorbent assay). Changes in Oxidative stress markers [ Time Frame: Baseline and 16 weeks ]Oxidative stress markers include the circulating levels of Catalase,Glutathione Peroxidase, and Malondialdehyde. Change in depressive symptoms as assessed by Patient Health Questionnaire‐9 [ Time Frame: Baseline and 16 weeks ] Change in quality of life measured by the 12‐item Short‐Form Health Survey Quality of life measured by the 12‐item Short‐Form Health Survey Questionnaire (SF‐12) [ Time Frame: Baseline and 16 weeks ] Changes in time to return to normal menstrual cycle [ Time Frame: Baseline and 16 weeks ]Changes in time to return to normal menstrual cycle |
| Starting date | 03/01/2019 |
| Contact information | liuchangqin@xmu.edu.cn |
| Notes |
NCT03802253.
| Study name | Time Restricted Feeding on Impaired Glucose Regulation(TRIG Trial) |
| Methods | Randomised controlled trial |
| Participants | 140 participants Ages Eligible for Study: 18 years to 70 years (adult, older adult) Sexes Eligible for Study: all Accepts Healthy volunteers: no Inclusion Criteria Aged ≥ 18 years Diagnosis of impaired glucose regulation (i.e. FG between 5.7‐6.9mmol/L) +/‐ impaired glucose tolerance (i.e. 2‐hour postprandial PG between 7.8‐11.1mmol/L) confirmed by latest OGTT results within 3 months prior to recruitment Body mass index (BMI)of 23.0 to 45.0 kg/m2; |
| Interventions | Arm Intervention/treatment Experimental: TRF Participants in this group will focus on time restricted feeding (TRF) in addition to daily calorie restriction. Behavioural: Time Restricted Feeding(TRF) Participants will receive a diet of 1200‐1500 kcal/day and be instructed to eat only during a window of 8 hours (Finishing the last meal before 4pm) in the first 6 months. 6 months later,Participants will receive a diet of 1200‐1500 kcal/day without a restriction of feeding time. Active comparator: RCD Participants in this group will focus on standard care with daily reduced calorie diet (RCD) Behavioural: Reduced Calorie Diet (RCD) Participants will receive a diet of 1200‐1500kcal/d and keep their usual eating pattern. |
| Outcomes | Primary Outcome Measures Changes in fasting blood glucose levels (mmol/L) [ Time Frame: 6 months and 12 months ] Change in insulin sensitivity [ Time Frame: 6 months and 12 months ] Insulin sensitivity will be assessed by HOMA‐IR Incidence of regression to normoglycaemia among the studied population [ Time Frame: 6 months and 12 months ] Secondary Outcome Measures Changes in body weight (Kilograms) [ Time Frame: 6 months and 12 months ] Changes in HbA1c levels (%) [ Time Frame: 6 months and 12 months ] Change in β cell function [ Time Frame: 6 months and 12 months ] β cell function will be assessed by HOMA‐β Changes in serum triglycerides levels (mmol/L) [ Time Frame: 6 months and 12 months ] Changes in serum low‐density lipoprotein levels (mmol/L) [ Time Frame: 6 months and 12 months ] Changes in serum total cholesterol levels(mmol/L) [ Time Frame: 6 months and 12 months ] Changes in waist circumference (cm) [ Time Frame: 6 months and 12 months ] Changes in abdominal circumference (cm) [ Time Frame: 6 months and 12 months ] Changes in diastolic pressure (DBP) [ Time Frame: 6 months and 12 months ] Changes in systolic pressure(SBP) [ Time Frame: 6 months and 12 months ] Incidence of DM [ Time Frame: 6 months and 12 months ] the incidence of DM among Chinese primary care patients with impaired fasting glucose (including isolated IFG or combined IFG / IGT) |
| Starting date | June 18, 2019 |
| Contact information | liuchangqin@xmu.edu.cn |
| Notes | NCT03802253 |
NCT03805776.
| Study name | Intermittent fasting in dyslipidemia |
| Methods | Randomised controlled trial |
| Participants | 60 participants. Ages Eligible for Study: 18 years to 80 years (adult, older adult) Sexes Eligible for Study: all Accepts Healthy volunteers: yes Inclusion Criteria General population with serum HDL less than 40 mg/dL for men and women Adult ages 18‐ 80 years will be included in the study. |
| Interventions | Experimental: interventional Will observe intermittent fasting Other: fasting (diet restriction for specific period) 12‐14 hours fasting No Intervention: control |
| Outcomes | Primary Outcome Measures : Lipid profile [ Time Frame: 6 weeks ] Change in HDL more than 3mg/dL Change in LDL more than 3mg/dl Cholesterol and TG weight loss [ Time Frame: 6 weeks ] Change in body weight (kg), as measured by scale weight Blood pressure [ Time Frame: 6 weeks ] Reduction in systolic and diastolic Secondary Outcome Measures: Fasting Glucose [ Time Frame: 6 weeks ] Fasting glucose mg/dl Fasting Insulin [ Time Frame: 6 weeks ] Fasting insulin (IU/L) Waist circumference Waist circumference (cm) [ Time Frame: 6 weeks ] WC in cm Lipid profile HbA1c (%) Lipids [ Time Frame: 6 weeks ] Total cholesterol (mg/dl), LDL cholesterol (mg/dl), HDL cholesterol (mg/dl), and triglycerides (mg/dl) Waist circumference [ Time Frame: 6 weeks ] Waist circumference (cm) |
| Starting date | February 20, 2019 |
| Contact information | javeria.farooq@aku.edu |
| Notes | NCT03805776 |
NCT04004403.
| Study name | AlternatedDay fasting combined and NAFLD for the treatment of Non‐alcoholic Fatty Liver Disease (NAFLD) |
| Methods | Randomised controlled trial |
| Participants | Inclusion Criteria ‐ Age between 18 to 65 years old ‐ BMI between 30.0 and 49.9 kg/m2 ‐ NAFLD (hepatic steatosis = 5%) ‐ Prediabetic ‐ Sedentary (<20 min, 2x/week of light activity for 3 mo prior to study) Exclusion Criteria ‐ Have chronic liver disease other than NAFLD (hepatitis B or C, primary biliary cirrhosis, sclerosing cholangitis, autoimmune hepatitis, haemochromatosis, Wilson's disease, a1‐antitrypsin deficiency) ‐ Consume excessive amounts of alcohol (Michigan Alcohol Screening Test score > 4) ‐ Have a history of known cardiovascular, pulmonary or renal disease ‐ Diagnosed T1DM or T2DM (fasting glucose: >126 mg/dl, 2‐h glucose OGTT = 200 mg/dL, HbA1c: >6.5%)) ‐ Have contraindications for participation in an exercise program based on ACSM recommendations ‐ Are not weight stable for 3 months prior to the beginning of study (weight gain or loss > 4 kg) ‐ Are claustrophobic or have implanted metallic/electrical devices (e.g. cardiac pacemaker, neuro‐stimulator) ‐ Are not able to keep a food diary or activity log for 7 consecutive days during screening ‐ Are taking drugs that induce steatosis (e.g. corticosteroids, oestrogens, methotrexate, Ca channel blockers) ‐ Are taking drugs that benefit NAFLD (e.g. betaine, pioglitazone, rosiglitazone, metformin, or gemifibrozil) ‐ Are taking drugs that influence study outcomes (weight loss, lipid‐lowering, glucose‐lowering medications) ‐ Are perimenopausal or have an irregular menstrual cycle (menses that does not appear every 27‐32 days) ‐ Are pregnant, or trying to become pregnant ‐ Are smokers |
| Interventions | Other: Alternate day fasting Other: Exercise |
| Outcomes | Change in body weight [Time Frame: Change from week 1 to week
24]
Change in hepatic steatosis [Time Frame: Change from week 1 to
week 24] Change in HbA1c [Time Frame: Change from week 1 to week 24] Change in hepatic insulin sensitivity [Time Frame: Change from week 1 to week 24] Change in triglyceride levels [Time Frame: Change from week 1 to week 24] |
| Starting date | 28/06/2019 |
| Contact information | https://clinicaltrials.gov/show/NCT04004403 |
| Notes | NCT04004403 |
NCT04057339.
| Study name | The Influence of time‐restricted eating in patients with Metabolic Syndrome (TIMET) |
| Methods | Randomised controlled trial |
| Participants | 144 participants |
| Interventions | Arm Intervention/treatment Placebo comparator: SOC (Standard of Care) Everyone in this arm will receive standard of care nutritional behavioural counselling and will be required to log their caloric intake through the use of a smartphone app. Behavioural: SOC Participants in this arm will receive nutritional counselling from the study dietician, but will not be required to adopt a 10‐hOUr eating window. Experimental: TRE + SOC Everyone in this arm will receive SOC nutritional behavioural counselling and will implement a daily 10‐hour window within which they must consume their calories. They will also be required to log their caloric intake through the use of a smartphone app. Behavioural: TRF + SOC Participants in this arm will adhere to a daily, consistent 10‐hour eating window for the course of the study as well as receive nutritional counselling from the study dietitian. Other name: Time Restricted Eating |
| Outcomes | Primary Outcome Measures : Change in fasting glucose [ Time Frame: Baseline and 14 weeks ] Fasting glucose (mg/dl) Change in fasting glucose levels [ Time Frame: Baseline and 14 weeks ] Glucose levels as measured by continuous glucose monitor (mg/dl) for 14 days at baseline and end of intervention Secondary Outcome Measures: Change in LDL particle number [ Time Frame: Baseline and 14 weeks ] LDL particle number (nmol/L) via NMR lipoprofile Change in LDL cholesterol [ Time Frame: Baseline and 14 weeks ] LDL cholesterol (mg/dl) Change in HDL cholesterol [ Time Frame: Baseline and 14 weeks ] HDL cholesterol (mg/dl) Change in Triglycerides [ Time Frame: Baseline and 14 weeks ] Triglycerides (mg/dl) Change in % fat mass [ Time Frame: Baseline and 14 weeks ] Fat mass as measured by dual‐energy X‐ray absorptiometry (DXA) Change in hs‐CRP [ Time Frame: Baseline and 14 weeks ] high sensitivity C‐reactive protein (mg/L) Change in HbA1c [ Time Frame: Baseline and 14 weeks ] HbA1c (%) |
| Starting date | April 8, 2019 |
| Contact information | Ages Eligible for Study: 18 years to 75 years (adult, older adult) Sexes Eligible for Study: all Accepts Healthy volunteers: no Inclusion Criteria Age 18‐75 years BMI > 25 and Metabolic syndrome, as defined as presence of 3 or more of the following criteria. Elevated fasting plasma glucose ≥ 100 mg/dL Elevated waist circumference: In Asians: ≥ 90 cm in men, ≥ 80 cm in women, all other races: ≥ 102 cm in men, ≥ 88 cm in women. Fasting plasma triglycerides ≥ 150 mg/dL, or on drug treatment for elevated triglycerides Reduced High‐density lipoprotein (HDL)‐cholesterol < 40 mg/dL in males or < 50 mg/dL in females, or drug treatment for reduced HDL‐cholesterol, elevated blood pressure, systolic blood pressure ≥ 135 mm Hg and/or diastolic blood pressure ≥ 85 mm Hg or drug treatment for hypertension. Own a smartphone (Apple iOS or Android OS) Baseline eating period > 14 hours/day If patients are on cardiovascular medications (HMG CoA reductase inhibitors (statins), other lipid modifying drugs (including over the counter drugs such as red yeast rice and fish oil), anti‐hypertensive, anti‐diabetes drugs), no dose adjustments will be allowed during the study period. |
| Notes | NCT04057339 |
NCT04062773.
| Study name | Time‐restricted feeding on glucose homeostasis and quality of life |
| Methods | 12 wk Randomised controlled trial |
| Participants | 50 participants. Ages Eligible for Study: 21 years to 65 years (adult, older adult) Sexes Eligible for Study: all Accepts Healthy volunteers: no Inclusion Criteria Must agree to follow time restricted feeding protocol if randomised to the TRF arm. Age 21‐65 years T2DM with any diabetes medication BMI of 25‐45 kg/m2 Wake up at a regular time between 5 am to 8 am Able to provide informed consent. |
| Interventions | Arm Intervention/treatment Experimental: TRF Eating restricted to between midday and 6 pm. Behavioural: TRF Time restricted feeding group Placebo comparator: normal timing of food intake. Eating between 8 am and 11 pm. Behavioural: Normal timing of food intake No time restricted feeding |
| Outcomes | Primary Outcome Measures : HbA1c [ Time Frame: 12 weeks ] Change in HbA1c between the intervention and control arms. Secondary Outcome Measures: Body weight [ Time Frame: 12 weeks ] Change in body weight between the intervention and control arms. Insulin sensitivity [ Time Frame: 12 weeks ] Change insulin sensitivity assessed by OGTT between the intervention and control arms. Diabetes medications [ Time Frame: 12 weeks ] Change in diabetes medications between the intervention and control arms. Inflammatory markers [ Time Frame: 12 weeks ] Change in TNF‐alpha in pg/mL between the intervention and control arms. Inflammatory markers [ Time Frame: 12 weeks ] Change in IL‐10 in pg/mL between the intervention and control arms. Inflammatory markers [ Time Frame: 12 weeks ] Change in IL‐6 in pg/mL between the intervention and control arms. Inflammatory markers [ Time Frame: 12 weeks ] Change in IL‐18 in pg/mL between the intervention and control arms. Inflammatory markers [ Time Frame: 12 weeks ] Change in CRP in mg/L between the intervention and control arms. Inflammatory markers [ Time Frame: 12 weeks ] Change in adiponectin in µg/mL the intervention and control arms. |
| Starting date | July 10, 2019 |
| Contact information | Nicola.Guess@dasmaninstitute.org |
| Notes | NCT04062773 |
NCT04138160.
| Study name | Effects of two weeks of twice‐weekly intermittent energy restriction on basal and postprandial metabolism |
| Methods | Randomised controlled trial |
| Participants | Ages Eligible for Study: 20 years to 35 years (adult) Sexes Eligible for Study: all Accepts Healthy volunteers: yes Inclusion Criteria Ages between 20 ‐ 35 years Healthy with a BMI between 20 and 27 kg·m‐2 Waist circumference < 94 cm for males and < 80 cm for females Ability to give informed consent |
| Interventions | Experimental: twice‐weekly intermittent energy restriction The twice‐weekly intermittent energy restriction (IER) of 70% restriction (~600 kcal) delivered for two non‐consecutive days/week and no restriction (so sufficient energy to meet the requirement of participants) on the other 5 days/week. Other: twice‐weekly Intermittent energy restriction Substantial (70%) energy restriction for 2 non‐consecutive days/week interspersed with normal energy intake (isoenergetic) on the remaining 5 days of the week. Other name: twice‐weekly IER Continuous energy restriction The continuous energy restriction (CER) of 20% restriction below the estimated requirement of participants (~1600 kcal) 7 days/week. Other: Continuous energy restriction 20% energy restriction each day relative to the energy requirement. Other name: CER |
| Outcomes | Primary Outcome Measures: Incremental area under the curve for insulin [ Time Frame: Over three hours from baseline ] Incremental area under the curve for serum insulin will be calculated using samples at 20 minute intervals between baseline and three hours. Secondary Outcome Measures: Incremental area under the curve for arterialised whole blood glucose [ Time Frame: Over four hours post baseline ] Incremental area under the curve for arterialised whole blood glucose will be calculated using samples collected at 10 minute intervals between baseline and four hours. Incremental area under the curve for composite appetite score [ Time Frame: Over four hour from baseline ] Composite appetite score will be calculated using 100mm visual analogue score ratings of satiety, fullness, hunger and prospective food consumption collected very 20 minutes between baseline and four hours. Weight of consumption of a pasta meal three hours after baseline [ Time Frame: Three hours post baseline ] Weight of pasta consumed from a bowl refilled prior to being empty until participants feel comfortably full. Incremental area under the curve for free fatty acid [ Time Frame: Over three hours from baseline ] Incremental area under the curve for FFA will be calculated using samples at 20 minute intervals between baseline and three hours. Incremental area under the curve for TAG [ Time Frame: Over three hours from baseline ] Incremental area under the curve for TAG will be calculated using samples at 20 minute Continuous glucose monitoring [ Time Frame: Over last 6 days of intervention ] Average glucose of 6 days |
| Starting date | October 18, 2018 |
| Contact information | Ian Macdonald, PhD University of Nottingham |
| Notes | NCT04138160 |
NCT04143971.
| Study name | Intermittent fasting in hypertriglyceridaemic overweight or obese subjects |
| Methods | Randomised controlled trial |
| Participants | 90 Participants |
| Interventions | Arm Intervention/treatment Placebo comparator: continuous low calorie diets Low calorie diet with daily calorie restriction Other: low calorie diet continuous low calorie diet Active Comparator: Intermittent Fasting Intermittent fasting every other day, in which daily calorie intake will be up to 30% of required calorie. Other: intermittent fasting diet intermittent fasting diet |
| Outcomes | Primary Outcome Measures : Weight loss [ Time Frame: at 8 weeks ] Mean weight change of participants (kg) after 8 weeks of diets Secondary Outcome Measures: Plasma Triglycerides [ Time Frame: at 8 weeks ] Mean change in plasma triglycerides of participants after 8 weeks of diets |
| Starting date | December 2, 2019 |
| Contact information | mahwoon3424@gmail.com |
| Notes | NCT04143971 |
NCT04155619.
| Study name | Using early time restricted feeding and timed light therapy to improve glycemic control in adults with type 2 diabetes |
| Methods | Randomised controlled trial |
| Participants | Ages Eligible for Study: 30 years to 80 years (adult, older adult) Sexes Eligible for Study: all Accepts Healthy volunteers: no Inclusion Criteria Aged 30‐80 years old HbA1c between 7.0 % to 10.0% On a stable dose of metformin, sulphonylureas, DPP‐IV inhibitors, and/or GLP‐1 receptor agonists for at least 6 months, or taking no diabetes medications Stable values of HbA1c for the past 6 months (within 0.7%) Wake up at a regular time between 5 am to 9 am |
| Interventions | Active Comparator: no change in eating or light exposure habits Behavioural: no change in meal timing Participants will eat within an ≥11‐hour daily period (no change in meal timing habits). Behavioural: no change in light exposure Participants will not change their light exposure habits. Experimental: early Time‐Restricted Feeding Behavioural: no change in light exposure Participants will not change their light exposure habits. Behavioural: early Time‐Restricted Feeding Participants will eat within an 8‐hour daily period early in the day, starting within 2 hours of waking up. Other name: eTRF, early TRF Experimental: Timed Light TherapyBehavioural: No change in meal timing Participants will eat within an ≥11‐hour daily period (no change in meal timing habits). Behavioural: Timed Light Therapy Participants will use bright light therapy for 60 minutes between 6 am to 3 pm, blue light‐blocking glasses for one hour before bedtime, and blackout curtains at night. Other name: Bright Light Therapy Experimental: Early Time‐Restricted Feeding and Timed Light TherapyBehavioural: Early Time‐Restricted Feeding Participants will eat within an 8‐hour daily period early in the day, starting within 2 hours of waking up. Other name: eTRF, early TRF Behavioural: Timed Light Therapy Participants will use bright light therapy for 60 minutes between 6 am to 3 pm, blue light‐blocking glasses for one hour before bedtime, and blackout curtains at night. Other name: Bright Light Therapy |
| Outcomes | Primary Outcome Measures : 24‐hour glucose levels [ Time Frame: 16 weeks ] Time‐weighted mean, fasting, peak, standard deviation, and excursion (maximum ‐ minimum) values (mg/dl) 24‐hour insulin levels [ Time Frame: 16 weeks ] Time‐weighted mean, fasting, peak, standard deviation, and excursion values (mU/L) 24‐hour C‐peptide levels [ Time Frame: 16 weeks ] Time‐weighted mean, fasting, peak, standard deviation, and excursion values (pmol/L). This is also a proxy for total 24‐hour insulin secretion. Hemoglobin A1C [ Time Frame: 16 weeks ] Insulin sensitivity [ Time Frame: 16 weeks ] Insulin sensitivity (dl/kg/min/μU/mL) during three identical meal tolerance tests, as measured by the Oral Minimal Model. The individual, mean, and excursion values, and time of the peak value will also be calculated. Beta‐cell responsivity index (a measure of beta‐cell function) [ Time Frame: 16 weeks ] Beta‐cell responsivity during three identical meal tolerance tests, as measured by the Oral Minimal Model. The individual, mean, and excursion values, and time of the peak value will also be calculated. Insulin secretion [ Time Frame: 16 weeks ] Insulin secretion (mU) across three identical meal tolerance tests, as measured by the Oral Minimal Model. The individual, mean, and excursion values, and time of the peak value will also be calculated. Secondary Outcome Measures: Melatonin Amplitude [ Time Frame: 16 weeks ] Peak value (pg/mL) Cortisol Amplitude [ Time Frame: 16 weeks ] Amplitude (μg/dL) Melatonin Phase [ Time Frame: 16 weeks ] Clock time of dim light melatonin onset (DLMO) Cortisol Phase [ Time Frame: 16 weeks ] Clock time of cortisol phase Glycemic ("Peripheral") Rhythm Amplitude [ Time Frame: 16 weeks ] Amplitude or diurnal variation in glucose levels (mg/dl) during a constant glucose infusion procedure Glycemic ("Peripheral") Rhythm Phase [ Time Frame: 16 weeks ] Time of day that glucose levels experience a nadir during a constant glucose infusion procedure |
| Starting date | March 2020 |
| Contact information | cpeterso@uab.edu |
| Notes | NCT04155619 |
SLCTR/2018/001.
| Study name | Effectiveness of time restricted diet versus a diet taken throughout the day on weight reduction and metabolic parameters in obese individuals. |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
1.Men and women aged 18‐50 years with a BMI
of 25 kg/m2 to 35 kg/m2.
Exclusion criteria 1. Patients with identified secondary cause for obesity 2. Inability to adhere to the suggested meal plan and exercise regimen. 3. Any underlying medical condition which can interfere with the proposed dietary plan and exercise regimen (i.e. diabetes mellitus, Cushing’s disease, rheumatoid arthritis and osteoarthritis of knees.) 4. Patients who are on drugs which can interfere with the proposed diet (i.e. prednisolone) 5. Women who are pregnant or breast feeding 6. Patients with diagnosed psychiatric disorder. |
| Interventions | One group (Group 1) will be given the choice to take the restricted calorie intake at any time during the day. Group 2 will be advised to take the calorie restricted diet during a twelve hour period (e.g. from 6 am to 6 pm). |
| Outcomes | Weight [At baseline and at the end of 3 months] Fasting blood sugar level [At baseline and at the end of 3 months] HbA1C level [At baseline and at the end of 3 months] Lipid Profile [At baseline and at the end of 3 months] Alanine transaminase level [At baseline and at the end of 3 months] |
| Starting date | 2018‐01‐02 |
| Contact information | noelsomasundaram@gmail.com |
| Notes |
ADF: alternate day fasting; BMI: body mass indexCER: continuous energy restriction; CRP: C‐reactive protein; CVD: cardiovascular disease; DBP: diastolic blood pressure; HbA1c: glycated haemoglobin; HDL: high density lipoprotein; IER: intermittent energy restriction; IF: intermittent fasting; LDL: low‐density lipoprotein; OCTT: oral glucose tolerance test; SBP: systolic blood pressure; TC: total cholesterol; TG: triglycerides; TRF: time‐restricted feeding.
Differences between protocol and review
None
Contributions of authors
MA, HE, OM, SZ, MF, AD, AS and KS all contributed to the search, data analysis, writing and drafting of this review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR, UK
This project was supported by the NIHR via Cochrane Infrastructure funding to the Heart Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
MA, HE, OM, SZ, MF, AD, AS and KS all state that there is no conflict of interest.
These authors contributed equally to this work.
These authors contributed equally to this work.
Edited (no change to conclusions)
References
References to studies included in this review
Amodio 2016 {published data only}
- Amodio D, D'Amico M, Meret L, Gaizo A, Laviano A. Time restricted feeding (TRF) enhances weight loss efficiency in dietary restricted women with metabolic syndrome. Clinical Nutrition 2016;35(1):S39. [Google Scholar]
Bhutani 2013 {published data only}
- Bhutani S, Klempel MC, Kroege CM, Trepanowski JF, Phillips SA, Norkeviciute E, et al. Alternate day fasting with or without exercise: effects on endothelial function and adipokines in obese humans. e-SPEN Journal 2013;8(5):e205–e209. [Google Scholar]
- Bhutani S, Klempel MC, Kroeger CM, Aggour E, Calvo Y, Trepanowski JF, et al. Effect of exercising while fasting on eating behaviors and food intake. Journal of the International Society of Sports Nutrition 2013;10(1):50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity 2013;21(7):1370-9. [DOI] [PubMed] [Google Scholar]
- Bhutani S, Klempel MC, Kroeger CM, Varady KA. OP024 combining alternate day fasting with exercise improves plasma lipids and ldl particle size in obese humans. Abstracts of the 34th ESPEN Congress 2012;7(1):10-1. [Google Scholar]
Carter 2018 {published data only}
- Carter S, Clifton PM, Keogh JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Network Open 2018;1(3):e180756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S, Clifton PM, Keogh JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes. JAMA Network Open 2018;1(3):e180756. [PMID: 30646030 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Catenacci 2016 {published data only}
- Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, et al. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016;24(9):1874–83. [https://onlinelibrary.wiley.com/doi/full/10.1002/oby.21581] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2019 {published data only}
- Cho AR, Moon JY, Kim S, An KY, Oh M, Jeon JY, et al. Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: a pilot randomized controlled trial. Metabolism: Clinical and Experimental 2019;93:52–60. [DOI] [PubMed] [Google Scholar]
- Lee JW. Alternate day fasting and exercise in overweight or obese adults. https://clinicaltrials.gov/show/NCT03652532 2018;(): 2018. [DOI] [PubMed]
Chow 2019 {published data only}
- Chow LS, Manoogian E, Alvear AC, Wang Q, Panda S, Mashek DG. Time restricted eating (TRE) promotes weight loss, alters body composition, and improves metabolic parameters in overweight humans. In: American Diabetes Association. Vol. 68. 2019.
Conley 2018 {published data only}
- Conley M, Le Fevre L, Haywood C, Proietto J. Is two days of intermittent energy restriction per week a feasible weight loss approach in obese males? A randomised pilot study. Nutrition & Dietics 2018;75(1):65-72. [DOI] [PubMed] [Google Scholar]
Corley 2019 {published data only}
- Corley B, Khouri C, Theaude L, Hawke P, Hall R, Weatherall M, et al. Changes in resting energy expenditure with intermittent fasting versus continuous daily restriction-a randomised controlled trial. Internal Medicine Journal 2019;49(53):5. [Google Scholar]
Ferraris 2019 {published data only}
- Ferraris C, Marcolin G, Veneto A, Tagliabue A, Paoli A. Time restricted feeding in high-level athletes: a pilot study. Nutrition Metabolism and Cardiovascular Diseases 2019;29(8):877‐8. [Google Scholar]
Griffiths 2016 {published data only}
- Griffiths C, Overland J, Sainsbury A, Little T, Franklin J, Gibson A, et al. Intermittent fasting: appears an effective and safe strategy for weight loss and metabolic control in Type 2 Diabetes. In: ADS-ADEA. 2016. [http://ads-adea-2016.m.asnevents.com.au/schedule/session/9323/abstract/36569]
Harvie 2011 {published data only}
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. International Journal of Obesity 2010;35(5):714–27. [www.ncbi.nlm.nih.gov/pmc/articles/PMC3017674/] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harvie 2013 {published data only}ISRCTN52913838
- Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. British Journal of Nutrition 2013;110(8):1534–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutchison 2019 {published data only}
- Hutchison AT, Liu B, Wood RE, Vincent AD, Thompson CH, O'Callaghan NJ, et al. Effects of intermittent versus continuous energy intakes on insulin sensitivity and metabolic risk in women with overweight. Obesity 2018;27(1):50-8. [DOI] [PubMed] [Google Scholar]
Kroeger 2015 {published data only}
- Kroeger C, Trapanowski J, Barnosky A, Klempel M, Varady K. Effect of 1 year of alternate day fasting versus daily calorie restriction on type 2 diabetes risk. FASEB Journal 2015;29(S1):254.1. [DOI: ] [Google Scholar]
Moro 2016 {published data only}
- Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. Journal of Translational Medicine 2016;14(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parvaresh 2019 {published data only}IRCT201509092395N8
- Parvaresh A, Razavi R, Abbasi B, Yaghoobloo K, Hassanzadeh A, Mohammadifard N, et al. Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: a randomized clinical trial. Complementary Therapies in Medicine 2019;47:102187. [DOI] [PubMed] [Google Scholar]
Pinto 2019 {published data only}
- Pinto AM, Bordoli C, Buckner LP, Kim C, Kaplan PC, Del Arenal IM, et al. Intermittent energy restriction is comparable to continuous energy restriction for cardiometabolic health in adults with central obesity: a randomized controlled trial; the Met-IER study.. Clinical Nutrition 2019;39(6):1753-63. [https://www.ncbi.nlm.nih.gov/pubmed/31409509] [DOI] [PubMed] [Google Scholar]
Schubel 2018 {published data only}
- Schübel R, Nattenmüller J, Sookthai D, Nonnenmacher T, Graf ME, Riedl L, et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. American Journal of Clinical Nutrition 2018;108(5):933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stekovic 2019 {published data only}
- Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metabolism 2019;30(3):462-76.e5. [DOI] [PubMed] [Google Scholar]
- Tripolt NJ, Stekovic S, Aberer F, Url J, Pferschy PN, Schröder S, et al. Intermittent fasting (alternate day fasting) in healthy, non-obese adults: protocol for a cohort trial with an embedded randomized controlled pilot trial. Advances in Therapy 2018;35(8):1265-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sundfor 2018 {published data only}
- Sundfør TM, Svendsen M, Tonstad S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutrition, Metabolism and Cardiovascular Diseases 2018;28(7):698–706. [DOI] [PubMed] [Google Scholar]
Tinsley 2017 {published data only}
- Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, et al. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. European Journal of Sport Science 2016;17(2):200–7. [DOI] [PubMed] [Google Scholar]
Tinsley 2019 {published data only}
- Tinsley GM, Moore ML, Graybeal AJ, Paoli A, Kim Y, Gonzales JU, et al. Time-restricted feeding plus resistance training in active females: a randomized trial. American Journal of Clinical Nutrition 2019;110(3):628–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trepanowski 2017 {published data only}
- Barnosky A, Kroeger CM, Trepanowski JF, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of alternate day fasting on markers of bone metabolism: an exploratory analysis of a 6-month randomized controlled trial. Nutrition and Healthy Aging 2017;4(3):255-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults. JAMA Internal Medicine 2017;177(7):930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Varady 2011 {published data only}
- Varady KA, Bhutani S, Klempe, MC Kroeger CM. Comparison of effects of diet versus exercise weight loss regimens on LDL and HDL particle size in obese adults. Lipids in Health and Disease 2011;10(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Varady 2013 {published data only}
- Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial.. Nutrition Journal 2013;12(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Varady 2016a {published data only}
- Varady K. Alternate-day fasting. Menopause 2016;23(12):1368. [Google Scholar]
References to studies excluded from this review
Actrn 2013 {published data only}
- Hodge A, Mack A, Tuck C, Tchongue J, Darcy H, Sievert W, et al. Non-alcoholic fatty liver disease Intermittent Fasting Time Intervention: fasting without calorie restriction improves hepatic transient elastography, visceral adiposity and insulin resistance compared to standard care. Journal of Gastroenterology and Hepatology 2013;29:68. [Google Scholar]
Aghasadeghi 2008 {published data only}
- Aghasadeghi K, Zare D. Efficacy of alternate day dosing of atorvastatin. Central European Journal of Medicine volume 2008;3(2):163-6. [Google Scholar]
Aksungar 2005 {published data only}
- Aksungar FB, Eren A, Ure S, Teskin O, Ates G. Effects of intermittent fasting on serum lipid levels, coagulation status and plasma homocysteine levels. Annals of Nutrition and Metabolism 2005;49(2):77-82. [DOI] [PubMed] [Google Scholar]
Aksungar 2007 {published data only}
- Aksungar FB, Topkaya AE, Akyildiz M. Interleukin-6, C-reactive protein and biochemical parameters during prolonged intermittent fasting. Annals of Nutrition and Metabolism 2007;51(1):88-95. [DOI] [PubMed] [Google Scholar]
Al‐Barha 2019 {published data only}
- Al-Barha N S, Aljaloud KS. The effect of Ramadan fasting on body composition and metabolic syndrome in apparently healthy men. American Journal of Men's Health 2019;13(1):1557988318816925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alhamdan 2016 {published data only}
- Alhamdan BA, Garcia-Alvarez A, Alzahrnai AH, Karanxha J, Stretchberry DR, Contrera KJ, et al. Alternate-day versus daily energy restriction diets: which is more effective for weight loss? A systematic review and meta-analysis. Obesity Science & Practice 2016;2(3):293-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Almeneessier 2017 {published data only}
- Almeneessier A, Bahammam A, Sharif M, Bahammam S, Nashwan S, Pandi Perumal S, et al. The influence of intermittent fasting on the circadian pattern of melatonin while controlling for caloric intake, energy expenditure, light exposure, and sleep schedules: a preliminary report. Annals of Thoracic Medicine 2017;12(3):183-90. [DOI: 10.4103/atm.ATM_15_17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Almeneessier 2018 {published data only}
- Almeneessier AS, Alzoghaibi M, Bahammam AA, Ibrahim MG, Olaish AH, Nashwan S Z, et al. The effects of diurnal intermittent fasting on the wake-promoting neurotransmitter orexin-A. Annals of Thoracic Medicine 2018;13(1):48-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrews 1984 {published data only}
- Andrews J, Kashiwagi A, Verso M. Effects of four-day fast on triglyceride mobilization in human adipocytes. International Journal of Obesity 1984;8(4):355-63. [PubMed] [Google Scholar]
Antoni 2018 {published data only}
- Antoni R, Johnston KL, Collins AL, Robertson MD. Intermittent v. continuous energy restriction: Differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. British Journal of Nutrition 2018;119(5):507-16. [DOI] [PubMed] [Google Scholar]
Arguin 2012 {published data only}
- Arguin H, Dionne IJ, Sénéchal M, Bouchard DR, Carpentier AC, Ardilouze JL, et al. Short- and long-term effects of continuous versus intermittent restrictive diet approaches on body composition and the metabolic profile in overweight and obese postmenopausal women: a pilot study. Menopause 2012;19(8):870-6. [DOI] [PubMed] [Google Scholar]
Arnason 2017 {published data only}
- Arnason TG, Bowen MW, Mansell KD. Effects of intermittent fasting on health markers in those with type 2 diabetes: A pilot study. World Journal of Diabetes 2017;8(4):154-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bachman 2016 {published data only}
- Bachman J L, Deitrick R W, Hillman A R. Exercising in the fasted state reduced 24-hour energy intake in active male adults. Journal of Nutrition and Metabolism 2016;2016:1984198. [DOI: 10.1155/2016/1984198] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahammam 2016 {published data only}
- Bahammam A, Pandi-Perumal S, Alzoghaibi M. The effect of Ramadan intermittent fasting on lipid peroxidation in healthy young men while controlling for diet and sleep: a pilot study. Annals of Thoracic Medicine 2016;11(1):43-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahmani 2013 {published data only}
- Bahmani A. Islamic fasting and its effect on pre-diabetic population. [Persian]. Scientific Journal of Kurdistan University of Medical Sciences 2013;18(1):40-6. [Google Scholar]
Basolo 2019 {published data only}
- Basolo A, Begaye B, Hollstein T, Vinales K L, Walter M, Santini F, et al. Effects of short-term fasting and different overfeeding diets on thyroid hormones in healthy humans. Thyroid 2019;29(9):1209-12. [DOI: 10.1089/thy.2019.0237] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergendahl 2000 {published data only}
- Bergendahl M, Iranmanesh A, Evans WS, Veldhuis JD. Short-term fasting selectively suppresses leptin pulse mass and 24-hour rhythmic leptin release in healthy midluteal phase women without disturbing leptin pulse frequency or its entropy control (pattern orderliness). Journal of Clinical Endocrinology & Metabolism 2000;85(1):207-13. [DOI: 10.1210/jcem.85.1.6325] [DOI] [PubMed] [Google Scholar]
Bergman 2007 {published data only}
- Bergman BC, Cornier MA, Horton TJ, Bessesen DH. Effects of fasting on insulin action and glucose kinetics in lean and obese men and women. American Journal of Physiology-Endocrinology and Metabolism 2007;293(4):E1103-11. [DOI: 10.1152/ajpendo.00613.2006] [DOI] [PubMed] [Google Scholar]
Bhutani 2010 {published data only}
- Bhutani S, Klempel M C, Berger RA, Varady KA. Improvements in coronary heart disease risk indicators by alternate-day fasting involve adipose tissue modulations. Obesity (Silver Spring) 2010;18(11):2152-9. [DOI] [PubMed] [Google Scholar]
Boden 1996 {published data only}
- Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. Journal of Clinical Endocrinology & Metabolism 1996;81(9):3419-23. [DOI] [PubMed] [Google Scholar]
Bowen 2018 {published data only}ACTRN12616000110482
- Bowen J, Brindal E, James-Martin G, Noakes M. Randomized trial of a high protein, partial meal replacement program with or without alternate day fasting: similar effects on weight loss, retention status, nutritional, metabolic, and behavioral outcomes. Nutrients 2018;10(9):1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2007 {published data only}
- Chan JL, Mietus J E, Raciti M, Goldberger A L, Mantzoros CS. Short-term fasting-induced autonomic activation and changes in catecholamine levels are not mediated by changes in leptin levels in healthy humans. Clinical Endocrinology (Oxford) 2007;66(1):49-57. [DOI] [PubMed] [Google Scholar]
Cherif 2017 {published data only}
- Cherif A, Meeusen R, Farooq A, Ryu J, Fenneni MA, Nikolovski Z, et al. Three days of intermittent fasting: repeated-sprint performance decreased by vertical-stiffness impairment. Intrnational Journal of Sports Physiology and Performance 2017;12(3):287-94. [DOI] [PubMed] [Google Scholar]
Chirinos 2016 {published data only}
- Chirinos DA, Goldberg RB, Llabre MM, Gellman M, Gutt M, McCalla J, et al. Lifestyle modification and weight reduction among low-income patients with the metabolic syndrome: the CHARMS randomized controlled trial. Journal of Behavioal Medicine 2016;39(3):483-92. [DOI] [PubMed] [Google Scholar]
Chowdhury 2016 {published data only}ISRCTN31521726
- Chowdhury EA, Richardson JD, Tsintzas K, Thompson D, Betts JA. Effect of extended morning fasting upon ad libitum lunch intake and associated metabolic and hormonal responses in obese adults. International Journal of Obesity (London) 2016;40(2):305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cignarella 2017 {published data only}
- Cignarella F, Cantoni C, Ghezzi L, Zhou Y, Cross AH, Piccio L. Intermittent fasting in experimental autoimmune encephalomyelitis and multiple sclerosis. In: ACTRIMS Forum 2017. Vol. 23. 2017:69.
Cignarella 2018 {published data only}
- Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metabolism 2018;27(6):1222-1235.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clayton 2016 {published data only}
Clayton 2018 {published data only}
- Clayton D J, Biddle J, Maher T, Funnell MP, Sargeant J A, King JA, et al. 24-h severe energy restriction impairs postprandial glycaemic control in young, lean males. British Journal of Nutrition 2018;120(10):1107-16. [DOI] [PubMed] [Google Scholar]
Clegg 2014 {published data only}
- Clegg ME, Shafat A. The effect of agar jelly on energy expenditure, appetite, gastric emptying and glycaemic response. European Journal of Nutrition 2014;53(2):533‐9. [DOI] [PubMed] [Google Scholar]
Contaldo 1980 {published data only}
- Contaldo F, Di Biase G, Scalfi L, Presta E, Mancini M. Protein-sparing modified fast in the treatment of severe obesity: weight loss and nitrogen balance data. Internaional Journal of Obesity 1980;4(3):189-96. [PubMed] [Google Scholar]
Corley 2018 {published data only}
- Corley BT, Carroll RW, Hall RM, Weatherall M, Parry-Strong A, Krebs JD. Intermittent fasting in Type 2 diabetes mellitus and the risk of hypoglycaemia: a randomized controlled trial. Diabetic Medicine 2018;35(5):588-94. [DOI] [PubMed] [Google Scholar]
Corvilain 1995 {published data only}
- Corvilain B, Abramowicz M, Fery F, Schoutens A, Verlinden M, Balasse E, et al. Effect of short-term starvation on gastric emptying in humans: Relationship to oral glucose tolerance. American Journalof Physiology 1995;269(4 32-4):G512-7. [DOI] [PubMed] [Google Scholar]
Coutinho 2018 {published data only}
- Coutinho SR, Halset EH, Gåsbakk S, Rehfeld JF, Kulseng B, Truby H, et al. Compensatory mechanisms activated with intermittent energy restriction: a randomized control trial. Clinical Nutrition 2018;37(3):815-23. [DOI] [PubMed] [Google Scholar]
Ctri 2018 {published data only}
- Dr Naveen G H. Scientific study to know the effect of fasting and food in excessive weight individuals. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=22916 2018. [CTRI/2018/02/011978]
Dallongeville 1998 {published data only}
- Dallongeville J, Hecquet B, Lebel P, Edme JL, Le Fur C, Fruchart J C, et al. Short term response of circulating leptin to feeding and fasting in man: influence of circadian cycle. International Journal of Obesity 1998;22(8):728-33. [DOI] [PubMed] [Google Scholar]
Dong 2004 {published data only}
- Dong W K, Hoon CK, Jung C P, Heung DK. Benefits of the nonfasting ketogenic diet compared with the initial fasting ketogenic diet. Pediatrics 2004;114(6):1627-30. [DOI] [PubMed] [Google Scholar]
Edinburgh 2019 {published data only}
- Edinburgh RM, Hengist A, Smith HA, Travers R L, Betts JA, Thompson D, et al. Skipping breakfast before exercise creates a more negative 24-hour energy balance: a randomized controlled trial in healthy physically active young men. Journal of Nutrition 2019;149(8):1326-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzgerald 2017 {published data only}
- Fitzgerald S K C, Vizthum D, Barron B, Sullivan P, Baer D, Mowry EM. Calorie restriction diets and changes in the metabolome in people with multiple sclerosis. ECTRIMS Online Library 2017;82:S191. [Google Scholar]
Gjedsted 2007 {published data only}
- Gjedsted J, Gormsen LC, Nielsen S, Schmitz O, Djurhuus CB, Keiding S, et al. Effects of a 3-day fast on regional lipid and glucose metabolism in human skeletal muscle and adipose tissue. Acta Physiologica 2007;191(3):205-16. [DOI] [PubMed] [Google Scholar]
Grajower 2019 {published data only}
- Grajower MM, Horne BD. Clinical management of intermittent fasting in patients with diabetes mellitus. Nutrients 2019;11(4):873. [DOI: 10.3390/nu11040873] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harder‐Lauridsen 2017 {published data only}
- Harder-Lauridsen NM, Nielsen ST, Mann SP, Lyngbæk MP, Benatti FB, Langkilde AR, et al. The effect of alternate-day caloric restriction on the metabolic consequences of 8 days of bed rest in healthy lean men: a randomized trial. Journal of Appiedl Physiology (1985) 2017;122(2):230‐41. [DOI: 10.1152/japplphysiol.00846.2016] [DOI] [PubMed] [Google Scholar]
- Nina Majlund Harder-Lauridsen. Bed Rest, Alternate Daily Fasting and Incretin Effect. https://clinicaltrials.gov/show/NCT02134860 2014;(): 2014.
He 2016 {published data only}
- He F, Zuo L, Emery W, Arciero P. High protein intermittent fasting increases serum polychlorinated biphenyls and decreases oxidative stress in obese adults. Journal of Hypertension 2016;34:e258. [Google Scholar]
Headland 2018 {published data only}
- Headland, M, Clifton, P, Keogh, J. Intermittent compared to continuous energy restriction on weight loss and weight maintenance: effects after 12 months. Obesity Research & Clinical Practice 2019;13(3):268‐9. [Google Scholar]
- Headland, ML, Clifton PM, Keogh JB. Effect of intermittent compared to continuous energy restriction on weight loss and weight maintenance after 12 months in healthy overweight or obese adults. International Journal of Obesity (Lond) 2018;43(10):2028-36. [DOI] [PubMed] [Google Scholar]
Hirsh 2019 {published data only}
- Hirsh SP, Pons M, Joyal SV, Swick AG. Avoiding holiday seasonal weight gain with nutrient-supported intermittent energy restriction: a pilot study. Journal of Nutritional Science 2019;8:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoddy 2014 {published data only}
- Hoddy K, Kroeger C, Trepanowski J, Bhutani S, Barnosky A, Varady K. Meal timing during alternate day fasting: effects on body weight and coronary heart disease risk in obese adults. In: ASEB Journal. Vol. 28. 2014. [DOI] [PubMed]
- Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, Varady KA. Meal timing during alternate day fasting: Impact on body weight and cardiovascular disease risk in obese adults. Obesity (Silver Spring) 2014;22(12):2524-31. [DOI: 10.1002/oby.20909] [DOI] [PubMed] [Google Scholar]
Hussin 2013 {published data only}
- Hussin NM, Shahar S, Teng NI, Ngah WZ, Das SK. Efficacy of fasting and calorie restriction (FCR) on mood and depression among ageing men. Journal of Nutrition,Health & Aging 2013;17(8):674-80. [DOI] [PubMed] [Google Scholar]
Irct201702269856N 2017 {published data only}IRCT201702269856N5
- Pasdar Y. The effect of nutrition education on blood glucose and insulin resistance in men fasting in Kermanshah. Iranian Registry of Clinical Trials 2017.
Irct2017070434897N 2017 {published data only}
- Irct2017070434897N. effect of the traditional medicine on health during fasting. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2017070434897N1 2017.
ISRCTN16400313 {published data only}ISRCTN16400313
- Ladmore D. Is Time Restricted Eating (TRE) a practical way to help people lose weight? http://www.isrctn.com/ISRCTN16400313?q=&filters=&sort=&offset=1&totalResults=16396&page=1&pageSize=10&searchType=basic-search 2017.
ISRCTN65605485 {published data only}ISRCTN65605485
- Macdonald I. Effects of short-term energy restriction on liver lipids. http://www.isrctn.com/ISRCTN65605485 2012.
ISRCTN89657927 {published data only}ISRCTN89657927
- Reeves S. Differences between breakfast eaters and breakfast skippers. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN89657927 2014;(): 2014.
Jafari 2003 {published data only}
- Jafari M, Ebrahimi R, Ahmadi-Kashani M, Balian H, Bashir M. Efficacy of alternate-day dosing versus daily dosing of atorvastatin. Journal of Cardiovascular Pharmacological Therapy 2003;8(2):123-6. [DOI] [PubMed] [Google Scholar]
Jakubowicz 2015 {published data only}
- Jakubowicz D, Wainstein J, Ahren B, Landau Z, Bar-Dayan Y, Froy O. Fasting until noon triggers increased postprandial hyperglycemia and impaired insulin response after lunch and dinner in individuals with type 2 diabetes: a randomized clinical trial. Diabetes Care 2015;38(10):1820-6. [DOI] [PubMed] [Google Scholar]
Jamshed 2019 {published data only}
- Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019;11(6):1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 1988 {published data only}
- Jensen MD, Miles J M, Gerich JE, Cryer PE, Haymond MW. Preservation of insulin effects on glucose production and proteolysis during fasting. American Journey of Physiology 1988;254(6 Pt 1):E700-7. [DOI] [PubMed] [Google Scholar]
Jimenez 2019 {published data only}
- Jimenez AM, Oliva SL, Vilar EG, De Cuevillas B, Morais Moreno MD, Gabella De Prado J, et al. The Mediterranean diet pattern with intermittent semi-fasting may facilitate weight loss: Randomised controlled trial. Mediterranean Journal of Nutrition and Metabolism 2019;12(2):153-61. [Google Scholar]
Johari 2019 {published data only}
- Johari MI, Yusoff K, Haron J, Nadarajan C, Ibrahim KN, Wong MS, et al. A randomised controlled trial on the effectiveness and adherence of modified alternate-day calorie restriction in improving activity of non-alcoholic fatty liver disease. Scientific Reports 2019;9(1):11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2010 {published data only}
- Johnson S, Leck K. The effects of dietary fasting on physical balance among healthy young women. Nutrition Journal 2010;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaikkonen 2019 {published data only}
- Kaikkonen KM, Saltevo SS, Korpelainen JT, Vanhala ML, Jokelainen J, Korpelainen I, et al. Effective weight loss and maintenance by intensive start with diet and exercise. Med Sci Sports Exerc 2019;51(5):920-9. [DOI] [PubMed] [Google Scholar]
Kalam 2019 {published data only}
- Kalam F, Kroeger CM, Trepanowski JF, Gabel K, Song JH, Cienfuegos S, et al. Beverage intake during alternate-day fasting: relationship to energy intake and body weight. Nutrition and Health 2019;25(3):167-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalam 2019a {published data only}
- Kalam F, Gabel K, Cienfuegos S, Wiseman E, Ezpeleta M, Steward M, et al. Alternate day fasting combined with a low-carbohydrate diet for weight loss, weight maintenance, and metabolic disease risk reduction. Obesity Science and Practice 2019;5(6):531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamble 2018 {published data only}
- Kamble S, Hiremath S. Insulin resistance and blood lipid levels during fasting. National Journal of Physiology, Pharmacy and Pharmacology 2018;8(8):1158-61. [Google Scholar]
Karimi 2016 {published data only}
- Karimi G, Azadbakht L, Haghighatdoost F, Esmaillzadeh A. Low energy density diet, weight loss maintenance, and risk of cardiovascular disease following a recent weight reduction program: a randomized control trial. Journal of Research andMedical Sciences 2016;21:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kearney 2011 {published data only}
- Kearney PM, O'Regan C, Cronin H, Kenny RA. Effect of fasting on participation in clinical research among older people. European Geriatric Medicine 2011;2(3):187-9. [Google Scholar]
Keogh 2014 {published data only}ACTRN12612000197831.
- Keogh J B, Pedersen E, Petersen KS, Clifton PM. Effects of intermittent compared to continuous energy restriction on short-term weight loss and long-term weight loss maintenance. Clinical Obesity 2014;4(3):150‐6. [DOI] [PubMed] [Google Scholar]
Kessler 2018 {published data only}
- Kessler CS, Stange R, Schlenkermann M, Jeitler M, Michalsen A, Selle A, et al. A nonrandomized controlled clinical pilot trial on 8 wk of intermittent fasting (24 h/wk). Nutrition 2018;46:143-152.e2. [DOI] [PubMed] [Google Scholar]
Klempel 2012 {published data only}
- Klempel MC, Kroeger CM, Bhutani S, Trepanowski JF, Varady KA. Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutrition Journal 2012;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempel MC, Kroeger CM, Norkeviciute E, Goslawski M, Phillips SA, Varady KA. Benefit of a low-fat over high-fat diet on vascular health during alternate day fasting. Nutrition & Diabetes 2013;3:e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempel MC, Kroeger CM, Varady KA. Alternate day fasting (ADF) with a high fat background diet produces similar weight loss and cardio-protection when compared to ADF with a low fat background diet. FASEB Journal 2012;26:lb339-lb339. [DOI] [PubMed] [Google Scholar]
- Klempel MC, Kroeger CM, Varady KA. Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism 2013;62(1):137-43. [DOI] [PubMed] [Google Scholar]
- Klempel M C, Kroeger C M, Varady K A. Alternate day fasting increases LDL particle size independently of dietary fat content in obese humans. European Journal of Clinical Nutrition 2013;67(7):783-5. [DOI] [PubMed] [Google Scholar]
Kobayashi 2014 {published data only}
- Kobayashi F, Ogata H, Omi N, Nagasaka S, Yamaguchi S, Hibi M, et al. Effect of breakfast skipping on diurnal variation of energy metabolism and blood glucose. Obesity Research & Clinical Practice 2014;8(3):e201-98. [DOI] [PubMed] [Google Scholar]
Kohn 2016 {published data only}
- Kohn N, Wassenberg A, Toygar T, Kellermann T, Weidenfeld C, Berthold-Losleben M, et al. Prolonged fasting impairs neural reactivity to visual stimulation. Brain Structure & Function 2016;221(1):147-58. [DOI] [PubMed] [Google Scholar]
Kolb 1983 {published data only}
- Kolb S, Sailer D. Does short-term fasting lower the cardiovascular risk in obesity? Effects of the composition of the reducing diet. Fortschritte Medizin 1983;101(35):1550-3. [PubMed] [Google Scholar]
Kroeger 2012 {published data only}
- Kroeger CM, Klempel MC, Bhutani S, Trepanowski JF, Tangney CC, Varady KA. Improvement in coronary heart disease risk factors during an intermittent fasting/calorie restriction regimen: Relationship to adipokine modulations. Nutrition & Metabolism 2012;9(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger CM, Klempel MC, Bhutani S, Trepanowski JF, Varady KA. Improvement in coronary heart disease risk factors during an intermittent fasting/calorie restriction regimen: relationship to adipokine modulations. FASEB Journal 2013;27:-. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larijani 2003 {published data only}
- Larijani B, Zahedi F, Sanjari M, Amini M R, Jalili RB, Adibi H, et al. The effect of Ramadan fasting on fasting serum glucose in healthy adults. Medical Journal of Malaysia 2003;58(5):678-80. [PubMed] [Google Scholar]
Larson‐Meyer 2008 {published data only}
- Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 2006;29(6):1337-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, et al. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 2008;16(6):1355-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maly 1996 {published data only}
- Maly J, Mala H, Prixova M, Pecka M, Zadak Z, Blaha M. Effect of a modified fasting and reduction diet on some haemostatic and biochemical parameters. British Journal of Haematology 1996;93(Suppl 2):189. [Google Scholar]
NCT00183027 {published data only}
- Roberts SB. Dietary energy restriction and metabolic aging in humans. https://clinicaltrials.gov/show/NCT00183027 2005;(): 2005.
NCT01754350 {published data only}
- Rieger J. Calorie-restricted, ketogenic diet and transient fasting during reirradiation for patients with Recurrent glioblastoma. https://clinicaltrials.gov/show/NCT01754350 2012;(): 2012.
NCT01895179 {published data only}
- Peterson C. Comparison of time-restricted feeding versus grazing. https://clinicaltrials.gov/ct2/show/NCT01895179?term=Comparison+of+Time-Restricted+Feeding+Versus+Grazing&draw=2&rank=1 2013.
NCT01964118 {published data only}
- Fontana L. Can intermittent fasting mimic the metabolic and cardiovascular and anti-aging effects of calorie restriction? NCT01964118 2013.
NCT02287103 {published data only}
- Jakubowicz D. Eating vs skipping breakfast on postprandial hyperglycemia after lunch and dinner in T2D. https://clinicaltrials.gov/ct2/show/NCT02287103?cond=Eating+vs+Skipping+Breakfast+on+Postprandial+Hyperglycemia+After+Lunch+and+Dinner+in+T2D&draw=2&rank=1 2014.
NCT02525419 {published data only}
- Arciero P. Intermittent fasting, caloric restriction and body composition in obese men and women. https://clinicaltrials.gov/show/NCT02525419 2015;(): 2015.
- Zuo L, He F, Tinsley GM, Pannell BK, Ward E, Arciero PJ. Comparison of high-protein, intermittent fasting low-calorie diet and heart healthy diet for vascular health of the obese. Frontiers in Physiology 2016;7(Aug):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02633722 {published data only}
- Heilbronn L. Intermittent fasting for metabolic health, does meal timing matter? https://clinicaltrials.gov/ct2/show/NCT02633722?cond=Intermittent+Fasting+for+Metabolic+Health%2C+Does+Meal+Timing+Matter%3F&draw=2&rank=1 2015.
NCT02970188 {published data only}
- Seals D. The effect of time-restricted feeding on physiological function in middle-aged and older adults. https://clinicaltrials.gov/ct2/show/NCT02970188?cond=The+Effect+of+Time-restricted+Feeding+on+Physiological+Function+in+Middle-aged+and+Older+Adults&draw=2&rank=1 2016.
NCT03459703 {published data only}
- Peterson CM. Effect of time-restricted feeding on fat loss and cardiometabolic risk factors in overweight adults. https://clinicaltrials.gov/ct2/show/NCT03459703 2018.
NCT03569852 {published data only}
- Casazza G. Time restricted feeding in male runners. https://clinicaltrials.gov/ct2/show/NCT03569852?term=Time+Restricted+Feeding+in+Male+Runners&draw=2&rank=1 2018.
NCT03574103 {published data only}
- Ferreira AV. Chronic effect of fasting. https://clinicaltrials.gov/ct2/show/NCT03574103?cond=Chronic+Effect+of+Fasting&draw=2&rank=1 2018.
NCT04009239 {published data only}
- Rynders CA. Time restricted feeding and metabolic rhythms. https://clinicaltrials.gov/ct2/show/NCT04009239?term=Time+Restricted+Feeding+and+Metabolic+Rhythms&draw=2&rank=1 2019.
NCT 2017 {published data only}
- NCT03365531. RCT of caloric restriction vs. alternate-day fasting in non-alcoholic fatty liver disease. https://clinicaltrials.gov/ct2/show/NCT03365531 2017.
Ng 2019 {published data only}
- Ng GY, Kang SW, Kim J, Alli-Shaik A, Baik SH, Jo DG, et al. Genome-wide transcriptome analysis reveals intermittent fasting-induced metabolic rewiring in the liver. Dose Response 2019;17(3):1559325819876780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ongsara 2017 {published data only}
- Ongsara S, Boonpol S, Prompalad N, Jeenduang N. The effect of Ramadan fasting on biochemical parameters in healthy Thai subjects. Journal of Clinical and Diagnostic Research 2017;11(9):BC14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Overland 2017 {published data only}
- Overland J, Griffiths C, Gauld A, Gibson A, Franklin J, Toth K, et al. Intermittent fasting vs. continuous energy restriction: a pilot study in people with type 2 diabetes and overweight and obesity. Diabetes 2017;66:A202. [Google Scholar]
Parr 2019 {published data only}
- Parr EB, Devlin BL, Brennan L, Hawley JA. Effects of time-restricted feeding on mood, hunger and fatigue. Obesity Rsearch & Clinical Practice 2019;13(3):245. [Google Scholar]
Pavic 2019 {published data only}
- Pavic E, Hadziabdic MO, Mucalo I, Martinis I, Romic Z, Bozikov V, et al. Effect of the Mediterranean diet in combination with exercise on metabolic syndrome parameters: 1-year randomized controlled trial. International Journal for Vitamin and Nutrition Research 2019;89(3-4):132-43. [DOI] [PubMed] [Google Scholar]
Pramanik 2012 {published data only}
- Pramanik S, Das AK, Chakrabarty M, Bandyopadhyay SK, Ghosh M, Dalai C K. Efficacy of alternate-day versus everyday dosing of atorvastatin. Indian Journal of Pharmacology 2012;44(3):362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ravussin 2019 {published data only}
- Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity (Silver Spring) 2019;27(8):1244-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruiz 2013 {published data only}
- Ruiz JR, Ortega FB, Labayen I. A weight loss diet intervention has a similar beneficial effect on both metabolically abnormal obese and metabolically healthy but obese premenopausal women. Annals of Nutrition and Metabolism 2013;62(3):223-30. [DOI] [PubMed] [Google Scholar]
Rynders 2019 {published data only}
- Rynders CA, Thomas EA, Zaman A, Pan Z, Catenacci VA, Melanson EL. Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients 2019;11(10):2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salas‐Salvado 2019 {published data only}
- Salas-Salvado J, Diaz-Lopez A, Ruiz-Canela M, Basora J, Fito M, Corella D, et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: One-year results of the PREDIMED-Plus trial. Diabetes Care 2019;42(5):777-88. [DOI] [PubMed] [Google Scholar]
Santos 2018 {published data only}
- Santos HO, Macedo RC. Impact of intermittent fasting on the lipid profile: assessment associated with diet and weight loss. Clinical Nutrition ESPEN 2018;24:14-21. [DOI] [PubMed] [Google Scholar]
Soeters 2009 {published data only}
- Soeters MR, Lammers NM, Dubbelhuis PF, Ackermans MT, Jonkers-Schuitema CF, Fliers E, et al. Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. American Journal of Clinical Nutrition 2009;90(5):1244-51. [DOI] [PubMed] [Google Scholar]
Solianik 2018 {published data only}
- Solianik R, Sujeta A. Two-day fasting evokes stress, but does not affect mood, brain activity, cognitive, psychomotor, and motor performance in overweight women. Behavioural Brain Research 2018;338:166-72. [DOI] [PubMed] [Google Scholar]
Sutton 2018 {published data only}
- Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metabolism 2018;27(6):1212‐1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takahashi 2011 {published data only}
- Takahashi LS, Biller JD, Criscuolo-Urbinati E, Urbinati EC. Feeding strategy with alternate fasting and refeeding: effects on farmed pacu production. Journal of Animal Physiology and Animal Nutrition 2011;95(2):259‐66. [DOI] [PubMed] [Google Scholar]
Tang 1995 {published data only}
- Tang FC, Kung I L, Hsieh S, Sheu W HH, Chang JB. Effects of a very-low-calorie diet and aerobic exercise on the metabolism of skeletal muscle. Journal of the Chinese Nutrition Society 1995;20(3):239-48. [Google Scholar]
Teng 2013 {published data only}
- Teng NI, Shahar S, Manaf ZA, Haron H, Ngah WZ. Fasting calorie restriction improved the quality of dietary intake among aging men in Klang Valley, Malaysia. Pakistan Journal of Nutrition 2013;12(7):607-14. [Google Scholar]
Varady 2016 {published data only}
- Varady KA. Impact of intermittent fasting on glucose homeostasis. Currrent Opinion in Clinical Nutrition andMetabolic Care 2016;19(4):300-2. [DOI] [PubMed] [Google Scholar]
Verboeket‐van 1993 {published data only}
- Verboeket-van de Venne W P, Westerterp KR. Frequency of feeding, weight reduction and energy metabolism. International Journal of Obesity and Relatated Metabolic Disorders 1993;17(1):31-6. [PubMed] [Google Scholar]
Washburn 2019 {published data only}
- Washburn RL, Cox JE, Muhlestein JB, May HT, Carlquist JF, Le VT, et al. Pilot study of novel intermittent fasting effects on metabolomic and trimethylamine N-oxide changes during 24-hour water-only fasting in the FEELGOOD trial. Nutrients 2019;11(2):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Webber 1994 {published data only}
- Webber J, Taylor J, Greathead H, Dawson J, Buttery PJ, Macdonald I A. Effects of fasting on fatty acid kinetics and on the cardiovascular, thermogenic and metabolic responses to the glucose clamp. Clinical Science (Lond) 1994;87(6):697-706. [DOI] [PubMed] [Google Scholar]
Williams 1998 {published data only}
- Williams KV, Mullen ML, Kelley DE, Wing RR. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. Diabetes Care 1998;21(1):2-8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Liu 2017 {published data only}
- Liu B, Hutchison AT, Thompson CH, Wittert GA, Heilbronn L. Adipose tissue remodeling following eight-week calorie restriction or intermittent fasting in females who are overweight and obese. Diabetes 2017;66:A550. [Google Scholar]
NCT00467220 {published data only}
- Hellerstein M. Effect of daily calorie or alternate-day calorie reductions on risk for cardiovascular disease and cancer. https://clinicaltrials.gov/ct2/show/NCT00467220 2007.
NCT02148458 {published data only}
- Fontana L. Short term intermittent fasting and Mediterranean diet. https://clinicaltrials.gov/show/NCT02148458 2014;(): 2014.
NCT02606669 {published data only}
- Lam B. The effect of intermittent energy restriction using meal replacements in overweight Chinese subjects. https://clinicaltrials.gov/ct2/show/NCT02606669 2015.
NCT02948517 {published data only}
- Varady K. Time restricted feeding for weight loss and cardio-protection. https://clinicaltrials.gov/show/NCT02948517 2016;(): 2016.
Ntr 2009 {published data only}
- Ntr. Intermittent fasting and insulin sensitivity In lean healthy male subjects. http://www.who.int/trialsearch/Trial2.aspx?TrialID=NTR1841 2009;(): 2009.
Spelta 2017 {published data only}
- Spelta F, Pasini AM, Cominacini L. Intermittent fasting in heart failure: a pilot study. European Journal of Clinical Investigation 2017;47:62. [Google Scholar]
References to ongoing studies
ACTRN12619000246189 {published data only}
- ACTRN12619000246189. Mediterranean diet with or without intermittent fasting in Type 2 Diabetic patients: a randomized clinical trial. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12619000246189 2019.
ACTRN12619000757112 {published data only}
- ACTRN12619000757112. The effect of intermittent fasting on muscle mass in overweight, middle-aged men. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12619000757112 2019.
ChiCTR1800016271 {published data only}
- ChiCTR1800016271. The effect of intermittent fasting in patients with overweight. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1800016271 2018.
ChiCTR1800017557 {published data only}
- ChiCTR1800017557. Effect of time-restricted feeding and continuous feeding in gut microbiome of critically ill patients. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1800017557 2018.
ChiCTR1900020871 {published data only}
- ChiCTR1900020871. Randomized controlled trial for intermittent fasting therapy for nonalcoholic fatty liver disease. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900020871 2019.
Irct20150909023957N8 {published data only}
- Irct20150909023957N8. Effect of low-calorie diets on anthropometric indices, glycemic markers and cardiovascular risk factors in metabolic syndrome. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20150909023957N8 2019.
ISRCTN13374800 {published data only}
- ISRCTN13374800. Alternate day fasting for obesity treatment. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN13374800 2017.
ISRCTN32122407 {published data only}
- ISRCTN32122407. The effects of early versus late time-restricted feeding on metabolic disease risk factors in adults at increased risk of developing type 2 diabetes: is there an optimal time to eat? https://apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN32122407 2019.
NCT03342742 {published data only}
- NCT03342742. Daily caloric restriction and intermittent fasting in overweight and obese adults with autosomal dominant polycystic kidney disease. https://clinicaltrials.gov/ct2/show/NCT03342742?term=Daily+Caloric+Restriction+and+Intermittent+Fasting+in+Overweight+and+Obese+Adults+With+Autosomal+Dominant+Polycystic+Kidney+Disease&draw=2&rank=1 2017.
NCT03411356 {published data only}
- NCT03411356. Intermittent fasting versus daily caloric restriction for weight loss. https://clinicaltrials.gov/ct2/show/NCT03411356?term=Intermittent+Fasting+Versus+Daily+Caloric+Restriction+for+Weight+Loss&draw=2&rank=1 2018.
NCT03439618 {published data only}
- NCT03439618. Comparison of time-restricted feeding and continuous feeding in critically ill patients. https://clinicaltrials.gov/ct2/show/NCT03439618?cond=Comparison+of+Time-restricted+Feeding+and+Continuous+Feeding+in+Critically+Ill+Patients&draw=2&rank=1 2018.
NCT03504683 {published data only}
- NCT03504683. Effect of time-restricted feeding on 24-hour glycemic control, blood pressure, and cardiovascular disease risk factors. https://clinicaltrials.gov/ct2/show/NCT03504683?term=Effect+of+Time-Restricted+Feeding+on+24-hour+Glycemic+Control%2C+Blood+Pressure%2C+and+Cardiovascular+Disease+Risk+Factors&draw=2&rank=1 2018.
NCT03527368 {published data only}
- NCT03527368. The Time-restricted Intake of Meals Study. https://clinicaltrials.gov/ct2/show/NCT03527368?term=The+Time-Restricted+Intake+of+Meals+Study&draw=1&rank=1 2018.
NCT03539094 {published data only}
- NCT03539094. Intermittent fasting in multiple sclerosis. https://clinicaltrials.gov/ct2/show/NCT03539094?term=Intermittent+Fasting+in+Multiple+Sclerosis&draw=2&rank=1 2018.
NCT03689608 {published data only}
- NCT03689608. Daily vs intermittent restriction of energy: controlled trial to reduce diabetes risk (DIRECT). https://clinicaltrials.gov/ct2/show/NCT03689608?term=Daily+vs+Intermittent+Restriction+of+Energy%3A+controlled+Trial+to+Reduce+Diabetes+Risk+%28DIRECT%29&draw=2&rank=1 2018.
NCT03745612 {published data only}
- NCT03745612. Time restricted feeding on weight loss and cardio-protection (TREATY trial). https://clinicaltrials.gov/ct2/show/NCT03745612?cond=Time+Restricted+Feeding+on+Weight+Loss+and+Cardio-protection+%28TREATY+Trial%29&draw=2&rank=1 2018.
NCT03789409 {published data only}
- NCT03789409. Intermittent fasting following acute ischemic stroke. https://clinicaltrials.gov/ct2/show/NCT03789409?term=Intermittent+Fasting+Following+Acute+Ischemic+Stroke&draw=1&rank=1 2018.
NCT03791203 {unpublished data only}
- NCT03791203. Effectiveness and adherence of Modified Alternate-day Calorie Restriction (MACR) in non-alcoholic fatty liver disease. https://clinicaltrials.gov/ct2/show/NCT03791203?term=Effectiveness+and+Adherence+of+Modified+Alternate-day+Calorie+Restriction+%28MACR%29+in+Non-Alcoholic+Fatty+Liver+Disease&draw=1&rank=1 2019. [DOI] [PMC free article] [PubMed]
NCT03792282 {published data only}
- NCT03792282. Time-Restricted Feeding(TRF) on overweight/obese women with Polycystic Ovarian Syndrome (PCOS) (TRF-PCOS). https://clinicaltrials.gov/ct2/show/NCT03792282.
NCT03802253 {published data only}
- NCT03802253. Time Restricted Feeding on Impaired fasting Glucose(TRIG trial). https://clinicaltrials.gov/ct2/show/NCT03802253?term=Time+Restricted+Feeding+on+Impaired+Fasting+Glucose%28TRIG+Trial%29&draw=1&rank=1 2019.
NCT03805776 {published data only}
- NCT03805776. Intermittent fasting in dyslipidemia. https://clinicaltrials.gov/ct2/show/NCT03805776?term=Intermittent+Fasting+in+Dyslipidemia&draw=2&rank=1 2019.
NCT04004403 {published data only}
- NCT04004403. Alternate day fasting combined and NAFLD for the treatment of Non-alcoholic Fatty Liver Disease (NAFLD). https://clinicaltrials.gov/ct2/show/NCT04004403?term=Alternate+Day+Fasting+Combined+and+NAFLD+for+the+Treatment+of+Non-alcoholic+Fatty+Liver+Disease+%28NAFLD%29&draw=1&rank=1 2019.
NCT04057339 {published data only}
- NCT04057339. The influence of time-restricted eating in patients metabolic syndrome. https://clinicaltrials.gov/ct2/show/NCT04057339?term=The+Influence+of+Time-Restricted+Eating+in+Patients+Metabolic+Syndrome&draw=1&rank=1 2019.
NCT04062773 {published data only}
- NCT04062773. Time-restricted feeding on glucose homeostasis and quality of life. https://clinicaltrials.gov/ct2/show/NCT04062773?cond=Time-Restricted+Feeding+on+Glucose+Homeostasis+and+Quality+of+Life&draw=2&rank=1 2019.
NCT04138160 {published data only}
- NCT04138160. Effects of two weeks of 5: 2 intermittent energy restriction on basal and postprandial metabolism. https://clinicaltrials.gov/ct2/show/NCT04138160?term=Effects+of+Two+Weeks+of+5%3A+2+Intermittent+Energy+Restriction+on+Basal+and+Postprandial+Metabolism&draw=2&rank=1 2019.
NCT04143971 {published data only}
- NCT04143971. Intermittent fasting in hypertriglyceridemic overweight or obese subjects. https://clinicaltrials.gov/ct2/show/NCT04143971?term=Intermittent+Fasting+in+Hypertriglyceridemic+Overweight+or+Obese+Subjects&draw=2&rank=1 2019.
NCT04155619 {published data only}
- NCT04155619. Using early time restricted feeding and timed light therapy to improve glycemic control in adults with Type 2 Diabetes. https://clinicaltrials.gov/ct2/show/NCT04155619?term=Using+Early+Time+Restricted+Feeding+and+Timed+Light+Therapy+to+Improve+Glycemic+Control+in+Adults+With+Type+2+Diabetes&draw=2&rank=1.
SLCTR/2018/001 {published data only}
- SLCTR/2018/001. Effectiveness of time restricted diet versus a diet taken throughout the day on weight reduction and metabolic parameters in obese individuals. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=SLCTR/2018/001 2018.
Additional references
ACC/AHA 2019
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140(11):e596-e646. [https://www.ahajournals.org/doi/10.1161/CIR.0000000000000678] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adler‐Lazarovits 2019
- Adler-Lazarovits C, Weintraub AY. Physicians’ attitudes and views regarding religious fasting during pregnancy and review of the literature. European Journal of Obstetrics & Gynecology and Reproductive Biology 2019;233:76-80. [DOI] [PubMed] [Google Scholar]
Adlouni 1998
- Adlouni A, Ghalim N, Saïle R, Hda N, Parra HJ, Benslimane A. Beneficial effect on serum apo AI, apo B and Lp AI levels of Ramadan fasting. Clinica Chimica Acta 1998;271(2):179-89. [PMID: ] [DOI] [PubMed] [Google Scholar]
AHA 2017
- St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, et al. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation 2017;135(9):e96-e121. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 2005
- Altman DG, Bland JM. Standard deviations and standard errors. BMJ (Clinical research ed.) 2005;331(7521):903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anton 2018
- Anton SD, Moehl K, Donahoo WT, Marosi KR, Lee SA, Mainous AG 3rd, et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity 2018;26(2):254-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Antoni 2016
- Antoni R, Johnston KL, Collins AL, Robertson MD. Investigation into the acute effects of total and partial energy restriction on postprandial metabolism among overweight/obese participants. British Journal of Nutrition 2016;115(6):951-9. [DOI] [PubMed] [Google Scholar]
Bergheanu 2017
- Bergheanu SC, Bodde MC, Jukema JW. Pathophysiology and treatment of atherosclerosis: Current view and future perspective on lipoprotein modification treatment. Netherlands heart journal : monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation 2017;25(4):231-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhatnagar 2016
- Bhatnagar P, Wickramasinghe K, Wilkins E, Townsend N. Trends in the epidemiology of cardiovascular disease in the UK. Heart (British Cardiac Society) 2016;102(24):1945-52. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolus 2018
- Bolus WR, Hasty AH. Contributions of innate type 2 inflammation to adipose function. Journal of Lipid Research 2018;60:10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 2009
- Bradley Rn, Kozura E, Buckle H, Kaltunas J, Tais S, Standish LJ. Description of clinical risk factor changes during naturopathic care for type 2 diabetes. Journal of Alternative and Complementary Medicine (New York, N.Y.) 2009;15(6):633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Capewell 2008
- Capewell S, O’Flaherty M. What explains declining coronary mortality? Lessons and warnings. Heart 2008;94(9):1105. [DOI] [PubMed] [Google Scholar]
Carbone 2019
- Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ. Obesity paradox in cardiovascular disease: where do we stand? Vascular Health and Risk Management 2019;15:89-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cioffi 2018
- Cioffi I, Evangelista A, Ponzo V, Ciccone G, Soldati L, Santarpia L, et al. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. Journal of Translational Medicine 2018;16(1):371. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crispim 2011
- Crispim CA, Waterhouse J, Damaso AR, Zimberg IZ, Padilha HG, Oyama LM, et al. Hormonal appetite control is altered by shift work: a preliminary study. Metabolism: Clinical and Experimental 2011;60(12):1726-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
de Cabo 2019
- Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. New England Journal of Medicine 2019;381(26):2541-51. [DOI] [PubMed] [Google Scholar]
De Rosa 2017
- De Rosa R, Vasa-Nicotera M, Leistner DM, Reis SM, Thome CE, Boeckel JN, et al. Coronary atherosclerotic plaque characteristics and cardiovascular risk factors - insights from an optical coherence tomography study. Circulation Journal : official journal of the Japanese Circulation Society 2017;81(8):1165-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Donath 2011
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nature Reviews. Immunology 2011;11(2):98-107. [PMID: ] [DOI] [PubMed] [Google Scholar]
ESC 2016
- Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons S, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). European Heart Journal 2016;37(29):2315-81. [https://academic.oup.com/eurheartj/article/37/29/2315/1748952] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frape 1997
- Frape DL, Williams NR, Scriven AJ, Palmer CR, O'Sullivan K, Fletcher RJ. Diurnal trends in responses of blood plasma concentrations of glucose, insulin, and C-peptide following high- and low-fat meals and their relation to fat metabolism in healthy middle-aged volunteers. British Journal of Nutrition 1997;77(4):523-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ganesan 2018
- Ganesan K, Habboush Y, Sultan S. Intermittent fasting: the choice for a healthier lifestyle. Cureus 2018;10(7):e2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibbs 2014
- Gibbs M, Harrington D, Starkey S, Williams P, Hampton S. Diurnal postprandial responses to low and high glycaemic index mixed meals. Clinical Nutrition 2014;33(5):889-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Glovaci 2019
- Glovaci D, Fan W, Wong ND. Epidemiology of diabetes mellitus and cardiovascular disease. Current Cardiology Reports 2019;21(4):21. [DOI] [PubMed] [Google Scholar]
Google trends 2018
- Google. Google Trends 2018. Google Trends 2018. [https://trends.google.com/trends/yis/2018/US/]
GRADEpro 2015 [Computer program]
- GRADEpro GDT. McMaster University (developed by Evidence Prime). Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Hammouda 2013
- Hammouda O, Chtourou H, Aloui A, Chahed H, Kallel C, Miled A, et al. Concomitant effects of Ramadan fasting and time-of-day on apolipoprotein AI, B, Lp-a and homocysteine responses during aerobic exercise in Tunisian soccer players. PLOS One 2013;8(11):e79873. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansson 2011
- Hansson GK, Hermansson A. The immune system in atherosclerosis. Nature. Immunology 2011;12(3):204-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hardy 2012
- Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Current Opinion in Endocrinology, Diabetes and Obesity 2012;19(2):81-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2018
- Harris L, Hamilton S, Azevedo LB, Olajide J, De Brun C, Waller G, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database of Systematic Reviews and Implementation Reports 2018;16(2):507-47. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hatori 2012
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabolism 2012;15(6):848-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Johnstone 2015
- Johnstone A. Fasting for weight loss: an effective strategy or latest dieting trend? International Journal of Obesity (2005) 2015;39(5):727-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lean 2017
- Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2017;391(10120):541-51. [https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(17)33102-1/fulltext?cc=y=#articleInformation] [PMID: PMID: 29221645 ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. IAvailable from www.cochrane-handbook.org.
Lopez 2020
- Lopez EO, Ballard BD, Jan A. Cardiovascular Disease. In: https://www.ncbi.nlm.nih.gov/books/NBK535419/. StatPearls, Jan 2020. [Google Scholar]
Lovren 2015
- Lovren F, Teoh H, Verma S. Obesity and atherosclerosis: mechanistic insights. Canadian Journal of Cardiology 2015;31(2):177-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Malinowski 2019
- Malinowski B, Zalewska K, Węsierska A, Sokołowska MM, Socha M, Liczner G, et al. Intermittent Fasting in Cardiovascular Disorders-An Overview. Nutrients 2019;11(3):673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meng 2020
- Meng H, Zhu L, Kord-Varkaneh H, O Santos Hr, Tinsley GM, Fu P. Effects of intermittent fasting and energy-restricted diets on lipid profile: A systematic review and meta-analysis. Nutrition 2020;77:110801. [DOI] [PubMed] [Google Scholar]
Naude 2019
- Naude CE, Schoonees A, Nguyen KA, Senekal M, Young T, Garner P, et al. Low carbohydrate versus balanced carbohydrate diets for reducing weight and cardiovascular risk. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD013334. [DOI: 10.1002/14651858.CD013334] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2010
- NICE. Cardiovascular disease prevention. https://www.nice.org.uk/guidance/ph25/chapter/1-Recommendations June 2010.
Pandey 2017
- Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, et al. Relationship between physical activity, body mass index, and risk of heart failure. Journal of the American College of Cardiology 2017;69(9):1129-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patterson 2017
- Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annual Review of Nutrition 2017;37:371-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Perk 2012
- Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). European Heart Journal 2012;33(13):1635-701. [PMID: 22555213] [DOI] [PubMed] [Google Scholar]
Phinney 1991
- Phinney SD, Tang AB, Waggoner CR, Tezanos-Pinto RG, Davis PA. The transient hypercholesterolemia of major weight loss. American Journal of Clinical Nutrition 1991;53(6):1404-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Phinney 2004
- Phinney SD. Ketogenic diets and physical performance. Nutrition & Metabolism 2004;1(1):2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pi‐Sunyer 2015
- Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New England Journal of Medicine 2015;373(1):11-22. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager 5 (RevMan 5). The Cochrane Collaboration, Nordic Cochrane Centre, Version 5.3. Copenhagen: The Cochrane Collaboration, Nordic Cochrane Centre, 2014.
Riemsma R 2016
- Riemsma R, Corro Ramos I, Birnie R. Integrated Sensor-augmented Pump Therapy Systems [the MiniMed® Paradigm™ Veo system and the Vibe™ and G4® PLATINUM CGM (Continuous Glucose Monitoring) System] for Managing Blood Glucose Levels in Type 1 Diabetes: a Systematic Review and Economic Evaluation. Southampton UK: NIHR Journals Library, 2016 Feb. [https://www.ncbi.nlm.nih.gov/books/NBK348987/] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ (Clinical research ed.) 2011;342:d549. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rugge B 2011
- Rugge B, Balshem H, Sehgal R. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism. Rockville (MD): Agency for Healthcare Research and Quality, Oct 2011. [https://www.ncbi.nlm.nih.gov/books/NBK83505/] [PubMed] [Google Scholar]
Sakakura 2013
- Sakakura K, Nakano M, Otsuka F, Ladich E, Kolodgie FD, Virmani R. Pathophysiology of atherosclerosis plaque progression. Heart, Lung & Circulation 2013;22(6):399-411. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schiavo‐Cardozo 2013
- Schiavo-Cardozo D, Lima MM, Pareja JC, Geloneze B. Appetite-regulating hormones from the upper gut: disrupted control of xenin and ghrelin in night workers. Clinical Endocrinology 2013;79(6):807-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schmidt 2019
- Schmidt A M. Diabetes mellitus and cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology 2019, 2019;39(4):558-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Stewart 2017
- Stewart J, Manmathan G, Wilkinson P. Primary prevention of cardiovascular disease: a review of contemporary guidance and literature. JRSM Cardiovascular Disease 2017;6:2048004016687211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swift 2016
- Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Blair SN, Church TS. Effects of clinically significant weight loss with exercise training on insulin resistance and cardiometabolic adaptations. Obesity (Silver Spring, Md.) 2016;24(4):812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Topol 2010
- Topol EJ, Bousser MG, Fox KA, Creager MA, Despres JP, Easton JD, et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet 2010;376(3974):517-23. [DOI] [PubMed] [Google Scholar]
Trepanowski 2010
- Trepanowski JF, Bloomer RJ. The impact of religious fasting on human health. Nutrition Journal 2010;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welton 2020
- Welton S, Minty R, O'Driscoll T, Willms H, Poirier D, Madden S, et al. Intermittent fasting and weight loss: Systematic review. Canadian Family Physician (Medecin de Famille Canadien) 2020;66(2):117-25. [PMC free article] [PubMed] [Google Scholar]
Westerman 2017
- Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. Journal of the American College of Cardiology 2017;70(1):1-25. [PMID: 28527533 ] [DOI] [PMC free article] [PubMed]
WHF 2017
- World Heart Federation. Scientific statements and guidelines. https://www.world-heart-federation.org/resources/scientific-statements-guidelines/ 2017.
WHO 2007
- WHO. Guidelines for assessment and management of cardiovascular risk. https://www.who.int/cardiovascular_diseases/guidelines/Full%20text.pdf.
WHO 2016
- WHO. Cardiovascular diseases (CVDs). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
WHO EU 2016
- WHO. The challenge of cardiovascular disease – quick statistics. http://www.euro.who.int/en/health-topics/noncommunicable-diseases/cardiovascular-diseases/data-and-statistics 2016.
Wilhelmi 2019
- Wilhelmi de Toledo F, Grundler F, Bergouignan A, Drinda S, Michalsen A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLOS One 2019;14(1):e0209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williamson 2015
- Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity 2015;23(12):2319-20. [DOI] [PubMed] [Google Scholar]
Wirth 2014
- Wirth MD, Burch J, Shivappa N, Steck SE, Hurley TG, Vena JE, et al. Dietary inflammatory index scores differ by shift work status: NHANES 2005 to 2010. Journal of Occupational and Environmental Medicine 2014;56(2):145-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ (Clinical research ed.) 2008;336(7644):601-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zarrinpar 2014
- Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metabolism 2014;20(6):1006-17. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Allaf 2019
- Allaf M, Elghazaly H, Mohamed OG, Fareen MF, Zaman S, Salmasi AM, et al. Intermittent fasting for the prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD013496. [DOI: 10.1002/14651858.CD013496] [DOI] [PMC free article] [PubMed] [Google Scholar]


