Abstract
It is now widely recognized that children exposed to adverse life events in the first years of life are at increased risk for a variety of neural, behavioral and psychological sequelae. As we discuss in this paper, adverse events represent a violation of the expectable environment. If such violations occur during a critical period of brain development, the detrimental effects of early adversity are likely to be long-lasting. Here we discuss the various ways adversity becomes neurobiologically embedded, and how the timing of such adversity plays an important role in determining outcomes. We conclude our paper by offering recommendations for how to elucidate the neural mechanisms responsible for the behavioral sequelae and how best to model the effects of early adversity.
Keywords: Adverse childhood experiences, brain development, neurobiological embedding, developmental programming, early adversity, critical periods
A conceptual framework for considering the effects of early adversity on brain development
There is growing evidence that children exposed to adversity (see Glossary) early in life are at increased risk for atypical variations in brain development that in turn are associated with a variety of psychological, behavioral, and physical health sequelae [1–3]. Adversity generally involves exposure to biological hazards (e.g., malnutrition, environmental toxins, chronic infection), psychological hazards (e.g., maltreatment, neighborhood or domestic violence) or both. And, although one can be exposed to adversity at any point in the lifespan, here we argue that exposure to adversity during critical periods of brain development – many of which occur within the first years of life – can be particularly hazardous to development.
We begin our article by arguing that adversity is best considered through the lens of violations in the expectable environment. These violations include experiences that are atypical (e.g., patterned light is diffused through a cataract; a caregiver is physically or emotionally abusive) or experiences that are entirely absent (e.g., a child born deaf or a child deprived of adequate caregiving). Critical periods of brain development exist to encode the expectable environment with enduring effects on brain and behavior. Thus, it is vital to consider critical periods in the context of how adversity exerts such deleterious effects. Indeed, adversity disrupts both critical period substrates and critical period mechanisms themselves. Thus, adversity that occurs during a critical period of brain development is far more likely to have enduring rather than transient effects on development. We summarize these enduring effects of early adversity on human development, and we offer suggestions for advancing progress on this complex topic. We make the point that it is rare for children to be exposed to a single form of adversity at a single time point; rather, a majority of children are exposed to multiple forms of adversity simultaneously, and in many cases, such adversity extends over time rather than occurring at a single time point. This makes it difficult to disentangle the differential effects of any specific adverse experience on brain development; indeed, one might ask if it makes more sense to develop models that reflect the aggregate and interactive effects of adversity exposures. Finally, because of limitations in the spatial and temporal resolution of human neuroimaging tools, and because of ethical constraints on the kinds of studies that can be performed with human children, understanding how adversity becomes neurobiologically embedded will require the continued development of animal models that closely parallel the human condition.
What do we mean by “adversity?”
Adversity has been used in a variety of ways. Some investigators have drawn analogies between early life stress and adversity [4–6]. However, this can be misleading, as not all forms of adversity will be interpreted and/or encoded as stressful, depending on brain maturity and developmental history (e.g., an impoverished language environment, where a child is exposed to fewer words and less complex language, is likely a form of adversity but it is in and of itself not stressful and doesn’t activate the stress response system). And conversely, not all stressful experiences are adverse (e.g., a child may experience preparation for an exam as stressful, but this would not be considered a form of adversity).
There are also conflicting views about the dimensions of adversity that are most impactful on development. For example, the long-running Adverse Childhood Experiences study (ACEs; [2,7–10]) has argued that it is the number of adverse life events that most influence development, not the nature of these events. However, not all adhere to this view of adversity; for example, McLaughlin and Sheridan [11–13] have offered persuasive evidence that threatening events (e.g., physical abuse) impact the brain differently than neglect does (e.g., absence of caregiving). Moreover, the type of threat (e.g., physical abuse vs. verbal abuse) or the type of deprivation (e.g., lack of caregiving vs. lack of visual or auditory input) likely impacts development differently. Thus, simply considering the number of adverse events without also considering the nature of the adversity and the timing of the adversity (as we discuss in some detail below) likely captures only part of the story.
Adversity as a violation of the expectable environment
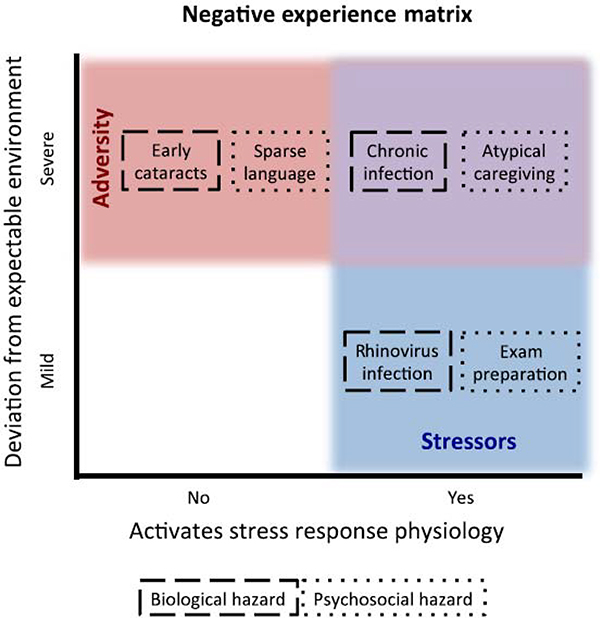
For the purposes of this paper, we argue that adversity should be taken to reflect deviations in or disruptions of the expectable environment [22–23]; that is, experiences that are expected to occur (in order to confer survival and adaptation to the environment) either do not occur (e.g., lack of caregiving; lack of nutrition) or are atypical in some way (e.g., physical abuse). The reason an absence of an expected experience or the presence of an atypical experience matter can be attributed to the experience-driven nature of brain development [20–21]. When cortical specialization is driven by experience, atypical experiences or the lack of experiences during those windows should lead to atypical patterns of brain development [24–25]. This, of course, is well established in sensory systems [26], and increasingly well established in higher cognitive and emotional systems [27–28]. Thus, any deviation in or disruption of an expectable experience should be considered to have potentially adverse consequences (see Figure 1 for conceptual representation of adverse experiences compared with stress experiences in development).
Figure 1. Relation between Adversity and Stress Experiences in Development.
This diagram illustrates the conceptual differences and overlap between experiences of adversity and stress in development. We define adverse experience to be a significant deviation from the expectable environment, independent of whether that experience triggers a response in the stress system (adverse exposures highlighted in red). Similarly, early stressors all impact the stress response system, independent of whether the experience reflects a severe deviation from the expectable environment (stress exposures highlighted in blue). Some experiences may be considered both stressful and adverse experiences (highlighted in purple). We provide examples of biological hazards (broken outline) and psychosocial hazards (dotted outline) in each category of adverse and stress experiences.
The role of critical periods
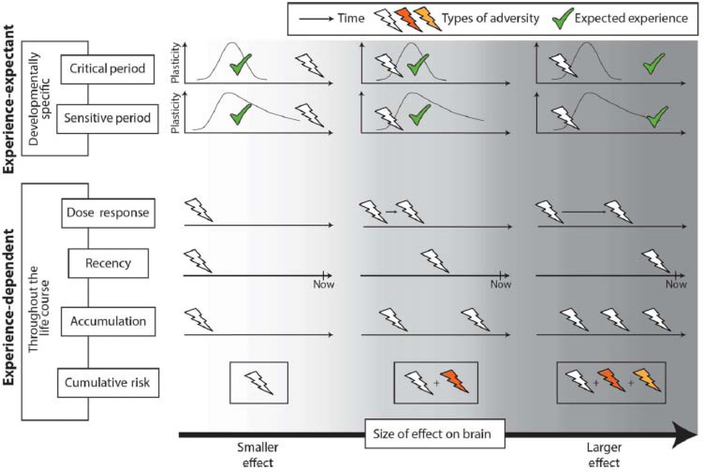
Recently, Gabard-Durnam and McLaughlin [14] have summarized several conceptual models that attempt to explain how adversity impacts neurodevelopment (Figure 2). These models emphasize different dimensions of adversity (e.g. timing, duration, type, number) or focus on how individual-level traits moderate the impact of an adverse experience [12, 15–19]. The conceptual models make assumptions not only about the most relevant features of environmental experience, but also the underlying neurobiological mechanisms involved. The majority of the models assume experience-dependent neural mechanisms; essentially processes that facilitate learning across the lifespan without ontogenetic constraints (e.g. synaptogenesis/pruning [20–21]). However, sensitive and critical period models (see below) rely on experience-expectant mechanisms that facilitate biological encoding of expectable environmental stimuli (e.g. patterned light, speech) during constrained developmental windows of heightened plasticity [20–21]. These neurobiological mechanisms have distinct implications for the impact of adversity. That is, adversity occurring during experience-expectant development, such as during sensitive/critical periods, is more likely to have significant, persistent effects on neural function into adulthood.
Figure 2. Conceptual Models of Environmental Influence on Neurodevelopment.
These models differ in the dimensions of adversity they account for (e.g., duration, timing, number) and the underlying neurobiological mechanisms (i.e., experience-expectant or experience-dependent mechanisms). Adversity may have the most significant, long-lasting effects on the brain when it disrupts or abolishes expected experiences during critical or sensitive periods of development for encoding those experiences (cases at top right of figure). Adapted from [14].
Thus, the timing of exposure is essential in considering the effects of early adversity on brain development, which brings us to the role of sensitive or critical periods. Although these terms are often used interchangeably, they differ in fundamental ways. Knudsen [65], for example, has argued that sensitive period is a more general term used to describe the effects experience has on the brain during narrow windows of time. If experiences essential to cortical specialization fail to occur during this time (e.g., access to patterned light or linguistic input), it may be difficult to redirect development along a typical trajectory; even then, function in the affected domain (e.g., vision, language) may not fully recover. As Nelson et al. [28] argue, the formation of a secure attachment to a caregiver may reflect a sensitive period. Importantly, Knudsen [65] has argued that whatever plasticity exists beyond a sensitive period is constrained by what transpired during a sensitive period; that is, one can reshape existing circuits only to a limited degree. If there is no residual plasticity after the experience-expectant window, however, then this period is a deemed a critical period. Therefore, critical periods result in irreversible changes in brain function. If a key experience fails to occur during a critical period, behavior will be permanently impacted, with little recovery possible. Filial imprinting in animals likely represents a critical period (see, for example, [66]).
Several additional points about critical/sensitive periods are worth noting. First, there is not just one critical/sensitive period but rather, cascading critical and sensitive periods for different neural circuits and for different complex phenomena such as caregiving and language (see figure 3). Second, even within a domain there will be different critical and sensitive periods. For example, there are multiple critical/sensitive periods for language [26].
Figure 3.
Multiple Critical and Sensitive Periods Occur in the First Years of Life. Sensitive and critical periods in early brain development exist across sensory, cognitive, and affective domains. There are multiple critical and sensitive periods both across and within domains, as illustrated here for language development. Adversity in early life may have particularly significant, lasting consequences if it disrupts these early critical and sensitive periods of brain development. Reproduced, with permission, from [26].
Considering critical period plasticity in the context of adversity
Here we wish to make several points to clarify the association between adversity and critical period plasticity. First, adversity is not itself an expectable experience that the brain prepares for. For example, the brain does not expect exposure to domestic violence. Adversity reflecting absent or impoverished specific expectable experiences clearly influences critical period inputs, but the adversity is not the expected substrate. Moreover, critical periods are inherently specific to particular types of experience and particular neural circuits [26, 67]. However, many types of adversity reflect complex exposures (e.g. poverty, malnutrition) that can impact multiple critical periods across multiple domains (e.g. co-occurring language and attachment critical periods) and across development (hierarchical language critical periods). Thus, there is unlikely to be one narrow window when these adverse experiences affect a single neural target. Indeed, the ability to exert widespread effects on the brain (and multiple critical periods) is part of what makes adversity like poverty or malnutrition so deleterious to development.
Second, adversity acts on critical period processes. The extent to which adverse experiences activate biological mediators like glucocorticoids or oxidative stress reduces plasticity during critical periods or prolongs plasticity afterwards, respectively (e.g. [68–70]). Other changes are adversity-specific. For example, threat experiences appear to accelerate critical period timing, whereas deprivation in certain domains (e.g. vision, such as cataracts in newborns) causes timing delays [17, 71–72]. Thus, adversity exerts powerful effects on development in part because it impacts both the expectable experience substrates of critical periods, and the critical period mechanisms themselves.
Third, adversity that occurs in the context of critical periods is also potent because it is more likely to have lasting effects on brain function and behavior than adversity following critical periods. Indeed, critical periods “close” via molecular brakes (e.g. perineuronal nets, myelin) that actively dampen plasticity to stabilize and protect experience-driven learning from future insults like adversity [67]. That is, in the context of healthy development, these protective brakes reduce future vulnerability to adversity, as experiences of any kind may only minimally impact brain circuitry. However, in the context of adversity that occurs during critical periods, deleterious effects are similarly preserved and “locked in” brain circuit function. Plasticity brakes then prevent future experience from rescuing function effectively. This shift in developmental priorities from plasticity to stability is thus a double-edged sword with regard to adversity experiences.
A high-level summary of the effects of adversity
We now consider the empirical evidence that early adversity can have enduring effects on human development. Countless studies have demonstrated an association between exposure to early, adverse life events and later maladaptive outcomes, with sequelae spanning a broad number of developmental domains. Below we provide a cursory summary of some of the main findings.
Biological hazards:
There is a host of biological hazards that can disrupt healthy development. Examples of two hazards that are particularly problematic among children growing up in many parts of the world include malnutrition (from an insufficient nutritional environment) and inflammation (e.g. from unsanitary environments, for review, see 29). The literature regarding the effects of malnutrition is ample, and the following are intended just as illustrative examples. First, there is evidence from human postmortem studies that 3- to 4-month-old infants who are malnourished show reduced dendritic growth in the primary motor cortex [30]. In addition, adults who experienced famine during gestation exhibit white matter (WM) hyperintensities over the entire cerebrum. One hypothesis regarding the underpinnings of such changes is that famine may lead to an inadequate supply of the nutrients required to sustain and replace catabolized myelin and gliosis after myelin loss [31]. There is histological evidence from malnourished juvenile rodents of an association between undernutrition and reduced cortical synaptic density and neuronal loss and alterations in callosal connections, likely caused by reduced neuron proliferation and changes in myelination and synaptic pruning [32–34]. The neuronal and volumetric changes in the brain associated with undernutrition may lead to poor cognitive outcomes [35].
Turning specifically to macronutrient deficiencies in children (for review, see [36]), even viewed through the coarse lens of body mass index (BMI), deleterious effects on brain development have been observed. For example, using fMRI, van Meer et al. [37] have reported that as BMI increases, activation in the dorsolateral prefrontal cortex decreases. In terms of micronutrients, deficiencies in a large number of vitamins and minerals have been found to lead to perturbations across multiple levels of brain development (for review, see [38]). An extensively studied single nutrient deficiency is iron, which is known to influence myelination early in life (e.g., [39]) and impact the functioning of the default mode brain network in adults [40].
Inflammatory processes brought about by hazards such as poor sanitation and unclean water have also been linked to poor developmental outcomes. For example, higher levels of inflammation in the first few years of life are associated with reduced scores on the Bayley Scales of Infant Development (e.g., [41]). At the level of the brain, both increased inflammation and adversity generally are associated with decreases in the amplitude of the visual evoked potential [42] and changes in functional networks derived from the EEG that underpin social information processing [43].
Psychosocial hazards:
Psychosocial hazards include a plethora of events such as lack of adequate caregiving, poverty, and maltreatment. A great deal of work has reported associations between psychosocial hazards and a variety of developmental outcomes. At the behavioral level, for example, higher levels of adversity are associated with problems in learning and memory, which in turn may be related to higher rates of academic failure; see [8, 22, 44–49]. Similarly, higher levels of adversity (particularly neglect) are associated with atypical patterns of social-emotional development and higher rates of psychopathology [45, 50], and in stress reactivity [4]. Many of these behavioral sequelae appear to be mediated by a variety of changes in the brain, such as increased/decreased cortical volume [51–52], increased/decreased cortical thinning [12, 53], perturbations in white matter integrity [54–56], and increased/decreased brain activity [57–58].
The intersection of biological and psychosocial hazards:
Although there is a long history of attempting to examine the differential effects of different types of adversity, the reality is the vast majority of children are exposed to multiple and concurrent forms of adversity [23, 59–60]. A case in point is work our lab is conducting in an urban slum in Dhaka, Bangladesh (see [61]). These children are often exposed to very high levels of both biological and psychosocial hazards, including chronic inflammation, diarrheal disease, malnutrition, maltreatment, poverty and exposure to domestic violence. Across a series of studies we have demonstrated that this constellation of factors is associated with reduced brain volume [62], reduced functional activation of various resting state networks [62], reductions in the amplitude of the visual evoked potential [42], an increase in the functional brain networks associated with social information processing [43], and reductions in brain metabolism in response to social and non-social events [90].
Strategies to parse the effects of adversity on development
Given this robust literature linking early adversity with lasting impacts on development, a question that is currently receiving considerable attention empirically is how early adversity becomes neurobiologically embedded. Figure 4 complements the mechanisms highlighted in Figure 2 by illustrating a potential general model of biological embedding and sequelae across the lifespan. How may these conceptual models of biological embedding be translated into productive empirical strategies? At the level of the brain, it is well established that certain regions and circuits are targets for different types of adversity and have different maturation trajectories; so empirical studies must carefully consider adversity type and timing in the context of specific brain targets’ development. For example, receptors for circulating glucocorticoids in the hippocampus make this particular structure vulnerable to chronic early stress [73–74]; as a result, there are now multiple studies that indicate that children who are maltreated [75–76] or adults with a history of maltreatment in childhood [77–79] show reductions in hippocampal volume and perform more poorly on tests of declarative and working memory [80–81]. Below we suggest two additional empirical approaches to parse the complex ways that adversity becomes biologically embedded in brain development.
Figure 4. Sequelae of Adverse Experiences.
Illustration of the consequences of early adverse experience across development (here for the psychosocial hazard type of adversity). Adverse psychosocial hazards in early life co-occurring with critical and sensitive periods to encode psychosocial experiences (e.g., language and caregiver attachment) interact with genetic profiles to produce biological and behavioral changes across development that together lead to a variety of detrimental outcomes persisting into adulthood. Adapted from [1].
New statistical frameworks
First, given the prevalence of co-occurring types of adversity in development, it may be advantageous in future research to take an approach that accounts for these multiple exposures as environmental mixtures. Mixture models facilitate identifying how different types of adversity interact and generate synergistic effects on development. Such an approach has been successfully implemented already in the context of environmental toxicology to model complex effects of toxin mixtures [63–64]. A broadening of the mixture framework for combinations of adverse experiences across biological and psychosocial hazards may thus provide additional insight into the effects of adversity under the conditions most frequently experienced in development.
Coordinated cross-species approaches
Second, given the significant, enduring effects of adversity that occurs during critical periods, we advocate that future research addressing the question of neurobiological embedding of adversity would benefit from a central focus on critical period processes via coordinated cross-species studies (such as has been done in the context of autism, where the visual evoked potential has been used in human children with Rett Syndrome and in MECP2 mice [91]). Importantly, critical periods are carefully orchestrated processes at the molecular level. For example, critical period initiation is regulated by both molecular pacers, which inhibit critical period initiation to prevent precocious plasticity (e.g. PSA-NCAM), and triggers that promote critical period plasticity (e.g. BDNF, GABA-ergic development) [67]. Moreover, manipulations (including adversity-related changes) of these molecular regulators are so powerful that they can shift critical period timing, prevent critical periods from opening, and even reopen critical periods in the adult brain [67,82–84]. However, these molecular-level processes are difficult to observe in human neurodevelopment. Thus, to fully understand how adversity becomes biologically embedded, it is necessary to coordinate human behavioral and neuroimaging studies with those in animal models.
Parallel studies across species to examine the biological embedding of adversity offer several advantages. Insights from molecular-level studies in animal models may identify new neural targets (e.g. a prefrontal cortex critical period [85]) or critical period modulators (e.g. SSRIs, opioids, general anesthetic drugs) that can be studied in human development [83–84, 86]. Animal model experiments may also offer therapeutic solutions for adverse experiences in humans, as with the treatment for amblyopia in the visual domain [87]. Lastly, the timing and nature of adversity in animal models can be manipulated in a controlled manner to complement the natural experiments of adversity exposure in humans [88–89].
Concluding remarks
Epidemiological studies dating back several decades advanced the idea that early adversity is associated with compromised neural and psychological outcomes. Recent work in neuroscience has begun to shed light on how a violation in experience-expected development during critical periods of brain development accounts for altered developmental outcomes. Not surprisingly, many questions remain unanswered (see Outstanding Questions). Moving forward, we advocate that this critical period approach will be just as important for an interventionist agenda. Specifically, interventions informed by our knowledge of how and when critical periods unfold in the context of adversity may leverage that critical period plasticity to redirect development along a typical trajectory. Moreover, these interventions, which can often be seen by themselves as enriched experiences, can shed additional light on developmental plasticity and critical period mechanisms. Thus, critical periods provide a framework for the synthesis of basic neuroscience and translational interventions in the context of early life adversity, and for development of new interventional strategies that are urgently needed to address the clinical and public health repercussions of adversity.
Outstanding Questions Box.
How do genetic variants interact with environments and developmental timing to influence critical periods and in so doing confer risk/protection from adversity?
Which critical period mechanisms in the context of adversity generalize or differ between animal models and human neurodevelopment, and across different circuits within the human brain (e.g. sensory vs. associative cortex?)
How may simulations of the effects of adversity using computational approaches without the constraints of human and animal studies improve our understanding of how adversity becomes neurobiologically embedded?
In considering the development of new interventions for individuals exposed to early adversity, the question arises as to the most beneficial approach – targeting specific behaviors, or trying and manipulate brain circuitry itself?
Is it possible to lift the molecular brakes that impact critical period closure noninvasively to target specific circuitry and behaviors, or are the tools too coarse, such that the entire brain is impacted? Under what conditions, if any, would it be advisable to lift these molecular brakes?
Acknowledgments:
Writing of this paper was made possible by support to Charles A. Nelson from the Bill and Melinda Gates Foundation (OPP1111625), the National Institute of Mental Health (MH091363), and the Richard David Scott Chair in Pediatric Developmental Medicine Research, Boston Children’s Hospital, and to Laurel Gabard-Durnam from the University of Tokyo International Research Center for Neurointelligence.
Glossary
- Adversity
a violation of the expectable environment that takes the form of biological hazards, psychosocial hazards, of complex exposures of both hazard types, with negative effects on development
- Biological embedding
The mechanisms through which environmental experiences impact neurobiology such that these experiences have enduring consequences on brain structure and function
- Biological hazard
Adverse biological factors in the environment that have negative effects on development, such as insufficient nutrients, environmental toxins, and pathogens that induce chronic infection and inflammation
- Critical period
Window of heightened brain plasticity for encoding specific environmental inputs through experience-expectant mechanisms that results in irreversible changes in brain function with permanent effects on behavior, for example, as in filial imprinting
- Experience-dependent mechanism
neural plasticity mechanism facilitating learning in response to experiences across the lifespan without developmental constraints, for example strengthening or weakening neural synapse connections
- Experience-expectant mechanism
neural plasticity mechanism facilitating the encoding of specific, expectable environmental stimuli, like patterned light, or auditory tones, during constrained developmental windows; underlies critical and sensitive period phenomena
- Mixture model
A conceptual and statistical framework for complex adversity exposures that accounts for how different types of adversity interact and generate synergistic effects on development, for example, implemented with toxin mixtures
- Psychosocial hazard
Adverse cognitive, affective, or social experiences that negatively impact development, such as poverty, inadequate caregiving, and maltreatment
- Sensitive period
constrained window of time when the environment most impacts brain function via experience-expectant mechanisms; similar to a critical period, but with residual plasticity after the period ends such that experiences may continue to affect brain function, for example, as in caregiver attachment formation
References
- 1.Berens A, Jensen S, & Nelson CA (2017). Biological embedding of early adversity: From physiological mechanisms to clinical implications. BMC Medicine, 15(135): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanier P, Maguire-Jack K, Lombardi B, Frey J & Rose R.A. (2018). Adverse Childhood Experiences and Child Health Outcomes: Comparing Cumulative Risk and Latent Class Approaches. Maternal Health Journal, 22(3): 288–297. [DOI] [PubMed] [Google Scholar]
- 3.Shonkoff JP, Garner AS, and the committee on psychosocial aspects of child and family health, committee on early childhood, adoption, and dependent care, and section on developmental and behavioral pediatrics (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129:e232–e246 [DOI] [PubMed] [Google Scholar]
- 4.Koss KJ, & Gunnar MR (2018). Annual research review: Early adversity, the hypothalamic-pituitary-adrenocortical axis, and child psychopathology. The Journal of Child Psychology and Psychiatry, 59(4):327–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purewal Boparai SK, Au V, Koita K, Oh DL, Briner S, Burke Harris N & Bucci M (2018). Ameliorating the biological impacts of childhood adversity: A review of intervention programs. [DOI] [PubMed]
- 6.Van Tieghem MR & Tottenham N (2018). Neurobiological Programming of Early Life Stress: Functional Development of Amygdala-Prefrontal Circuitry and Vulnerability for Stress-Related Psychopathology. Current Topics in Behavioral Neuroscience, 38:117–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anda RF, Croft JB, Felitti VJ, et al. (1999). Adverse childhood experiences and smoking during adolescence and adulthood. Journal of the American Medical Association, 282 (17):1652–1658. [DOI] [PubMed] [Google Scholar]
- 8.Anda RF, Felitti VJ, Bremner JD, et al. (2006). The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience, 256(3):174 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felitti VJ, Anda RF, Nordenberg D, et al. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14(4):245–258 [DOI] [PubMed] [Google Scholar]
- 10.Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, Jones L, Dunne MP (2017). The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health, 2(8):e356–e366 [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin KA Sheridan MA (2016). Beyond Cumulative Risk: A Dimensional Approach to Childhood Adversity. Current Directions in Psychological Science, 25(4):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlin KA, Sheridan MA, Lambert HK. (2014). Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neuroscience and Biobehavioral Reviews, 47:578–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheridan MA, McLaughlin KA. (2014). Dimensions of early experience and neural development: deprivation and threat. Trends Cognitive Science, 18(11):580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabard-Durnam LJ & McLaughlin KA (2019). Do Sensitive Periods Exist for Exposure to Adversity? Biological Psychiatry, 85(10):789–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans GW et al. (2013) Cumulative risk and child development. Psychol. Bull. 139, 1342 1396. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert ME (1997) Towards the Development of a Biologically based Dose-Response Model of Lead Neurotoxicity 1. Am. Zool. 37, 389–398. [Google Scholar]
- 17.Belsky J and Pluess M (2009) Beyond Diathesis Stress: Differential Susceptibility to Environmental Influences. Psychol. Bull. 135, 885–908. [DOI] [PubMed] [Google Scholar]
- 18.Ellis BJ and Boyce WT (2008) Biological Sensitivity to Context. Curr. Dir. Psychol. Sci. 17, 183–187. [Google Scholar]
- 19.Dunn EC et al. (2019) Sensitive periods for the effect of childhood adversity on DNA methylation: Results from a prospective, longitudinal study. Biol. Psychiatry DOI: 10.1016/J.BIOPSYCH.2018.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black JE, Jones TA, Nelson CA, & Greenough WT (1998). Neuronal plasticity and the developing brain. In Alessi NE, Coyle JT, Harrison SI, & Eth S. (Eds.), Handbook of Child and Adolescent Psychiatry. Vol 6. Basic Psychiatric Science and Treatment (pp. 31–53). New York: John Wiley & Sons. [Google Scholar]
- 21.Greenough WT, Black JE, & Wallace CS (1987). Experience and brain development. Child Development, 58(3):539–59. [PubMed] [Google Scholar]
- 22.Nelson CA (2007). A neurobiological perspective on early human deprivation. Child Development Perspectives, 1, 13–18. [Google Scholar]
- 23.McLaughlin K, Sheridan MA, & Nelson CA (2017). Neglect as a violation of species expectant experience: Neurodevelopmental consequences. Biological Psychiatry, 82(7): 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox SE, Levitt P, & Nelson CA (2010). How the timing and quality of early experiences influence the development of brain architecture. Child Development, 81(1): 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reh R, Dias B, Nelson CA, Kaufer D, Werker J, Kolb B, Levine J, & Hensch TK. (in press). Critical period regulation across multiple timescales. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werker JF, Hensch TK (2015). Critical periods in speech perception: new directions. Annual Review of Psychology, 66:173–96. [DOI] [PubMed] [Google Scholar]
- 27.Kanjila S, Pant R, & Bedny M (2018). Sensitive Period for Cognitive Repurposing of Human Visual Cortex. Cerebral Cortex,; 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson CA, Zeanah CH, & Fox NA (2019; published on line). How Early Experience Shapes Human Development: The Case of Psychosocial Deprivation. Neural Plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suchdev PS, Boivin MJ, Forsyth BW, Georgieff MK, Guerrant RL, & Nelson CA (2017). Assessment of Neurodevelopment, Nutrition, and Inflammation From Fetal Life to Adolescence in Low-Resource Settings, Pediatrics, 139, xxx–xxx [DOI] [PubMed] [Google Scholar]
- 30.Cordero ME, et al. , (1993). Dendritic development in neocortex of infants with early postnatal life undernutrition. Pediatric Neurology, 9(6): 457–64. [DOI] [PubMed] [Google Scholar]
- 31.Hulshoff Pol HE, et al. (2000) Prenatal exposure to famine and brain morphology in schizophrenia. American Journal of Psychiatry, 157(7): p. 1170–2. [DOI] [PubMed] [Google Scholar]
- 32.Coti Bertrand P, O’Kusky JR, and Innis SM, (2006). Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. Journal of Nutrition, 136(6): p. 1570–5. [DOI] [PubMed] [Google Scholar]
- 33.Morgane PJ, et al. , (1993). Prenatal malnutrition and development of the brain. Neuroscience and Biobehavioral Reviews,17(1): 91–128. [DOI] [PubMed] [Google Scholar]
- 34.Soto-Moyano R, et al. , (1998). Prenatal malnutrition-induced functional alterations in callosal connections and in interhemispheric asymmetry in rats are prevented by reduction of noradrenaline synthesis during gestation. Journal of Nutrition, 128(7):1224 1231. [DOI] [PubMed] [Google Scholar]
- 35.Peter CJ, Fischer LK ,, Kundakovic M, Garg P, Jakovcevski M, Dincer A, Amaral AC, Ginns EI, Galdzicka M, Bryce CP, Ratner C, Waber DP, Mokler D, Medford G, Champagne FA, Rosene DL, McGaughy JA, Sharp AJ, Galler JR, Akbarian S (2016) DNA Methylation Signatures of Early Childhood Malnutrition Associated With Impairments in Attention and Cognition. Biological Psychiatry 80(10):765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prado EL, Dewey KG (2014). Nutrition and brain development in early life. Nutrition Reviews, 72(4):267–84. [DOI] [PubMed] [Google Scholar]
- 37.van Meer F, van der Laan LN, Eiben G, Lissner L, Wolters M , Rach S, Herrmann M, Erhard P, Molnar D, Orsi G, Viergever MA, Adan RAH, Smeets PAM; Family Consortium (2019). [DOI] [PubMed]
- 38.Polavarapu A & Hasbani D (2017). Neurological Complications of Nutritional Disease. Seminars in Pediatric Neurology, 24(1):70–80 [DOI] [PubMed] [Google Scholar]
- 39.Guitart ME, Vence M, Correale J, Pasquini JM, Rosato-Siri MV (2019). Ontogenetic oligodendrocyte maturation through gestational iron deprivation: The road not taken. Glia, 67(9):1760–1774. [DOI] [PubMed] [Google Scholar]
- 40.Algarin C, Karunakaran KD, Reyes S, Morales C, Lozoff B, Peirano P, Biswal B (2017). Differences on Brain Connectivity in Adulthood Are Present in Subjects with Iron Deficiency Anemia in Infancy. Frontiers in Aging Neuroscience, 9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donowitz JR, Cook H, Alam M, Kabir M, Colgate ER, Carmolli MP, Kirkpatrick BD, Nelson CA, Ma JZ, Haque R, & Petri WA (2018). Role of Maternal Health and Inflammation in Infancy in Nutritional and Neurodevelopmental Outcomes of Two-Year Old Bangladeshi Children. PLOS Neglected Tropical Diseases, 12(5): e0006363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen SKG, Kumar S, Xie W, Tofail F, Haque R, Petri WA, & Nelson CA (2019). Neural correlates of early adversity among Bangladeshi infants. Scientific Reports, 9(1): 3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie W, Kumar S, Kakon SH, Haque R, Petri WA, & Nelson CA (in press) Chronic Inflammation Is Associated with Neural Responses to Faces in Bangladeshi Children. Neuroimage [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ.(2013). Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci U S A,110(45):18274–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colvert E, Rutter M, Kreppner J, Castle J, Groothues C, Hawkins A, Stevens S, Sonuga-Barke EJ(2008). Do theory of mind and executive function deficits underlie the adverse outcomes associated with profound early deprivation?; findings from the English and Romanian adoptees study. Journal of Abnormal Child Psychology, 36(7):105–1068. [DOI] [PubMed] [Google Scholar]
- 46.Van den Bos R, Harteveld M, Stoop H (2009). Stress and decision-making in humans: performance is related to cortisol reactivity, albeit differently in men and women. Psychoneuroendrocinology, 34(10):1449–58. [DOI] [PubMed] [Google Scholar]
- 47.Beers Sue R., and De Bellis Michael D.. “Neuropsychological function in children with maltreatment-related posttraumatic stress disorder.” American Journal of Psychiatry 159, no. 3 (2002): 483–486. [DOI] [PubMed] [Google Scholar]
- 48.Loman Michelle M., Johnson Anna E., Westerlund Alissa, Pollak Seth D., Nelson Charles A., and Gunnar Megan R.. “The effect of early deprivation on executive attention in middle childhood.” Journal of Child Psychology and Psychiatry 54, no. 1 (2013): 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pechtel P, and Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berlin), 214(1):55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Humphreys KL, Gleason MM, Drury SS, Miron D, Nelson CA, Fox NA, & Zeanah CH (2015). Effects of early deprivation on psychopathology at age 12 years: Follow-up of a randomized controlled trial. The Lancet Psychiatry, 2 (7), 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luby JL, Barch D, Whalen D, Tillman R, & Belden A, (2017). Association Between Early Life Adversity and Risk for Poor Emotional and Physical Health in Adolescence: A putative Mechanistic Neurodevelopmental Pathway JAMAPediatrics, 171(12):1168–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackey S et al. (2017) Brain Regions Related to Impulsivity Mediate the Effects of Early Adversity on Antisocial Behavior. Biol. Psychiatry 82, 275–282. [DOI] [PubMed] [Google Scholar]
- 53.Whittle S et al. (2016) Observed Measures of Negative Parenting Predict Brain Development during Adolescence. PLoS One 11, e0147774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanson JL et al. (2013) Early Neglect Is Associated With Alterations in White Matter Integrity and Cognitive Functioning. Child Dev. 84, 1566–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang H et al. (2012) White Matter Disruptions in Adolescents Exposed to Childhood Maltreatment and Vulnerability to Psychopathology. Neuropsychopharmacology 37, 2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daniels JK et al. (2013) White matter integrity and its relationship to PTSD and childhood trauma-a systematic review and meta-analysis. Depress. Anxiety 30, 207–216. [DOI] [PubMed] [Google Scholar]
- 57.Marshall PJ, Fox NA, & the Bucharest Early Intervention Project Core Group (2004). A comparison of the electroencephalogram between institutionalized and community children in Romania. Journal of Cognitive Neuroscience, 16, 1327–1338. [DOI] [PubMed] [Google Scholar]
- 58.Vanderwert RE , Marshall PJ, Nelson CA, Zeanah CH, & Fox NA (2010). Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLoSOne, 5(7): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finkelhor D et al. (2011) Polyvictimization: Children’s Exposure to Multiple Types of Violence, Crime, and Abuse. Natl. Surv. Child. Expo. to Violence. [Google Scholar]
- 60.Kessler RC et al. (2010) Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry 197, 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Storrs C (2017). How poverty affects the brain. Nature, 547, 150–152. [DOI] [PubMed] [Google Scholar]
- 62.Turesky T, Xie W, Kumar S, Sliva D, Gagoski B, Vaughn J, Zollei L, Haque R, Hafiz Kakon S, Islam N, Petri WA, Nelson CA, & Gaab N. Relating anthropometric indicators to brain structure in 2-month-old Bangladeshi infants growing up in poverty: a pilot study. NeuroImage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu SH et al. (2018) Bayesian varying coefficient kernel machine regression to assess neurodevelopmental trajectories associated with exposure to complex mixtures. Stat. Med. 37, 4680–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu SH et al. (2018) Modeling the health effects of time-varying complex environmental mixtures: Mean field variational Bayes for lagged kernel machine regression. Environmetrics 29, e2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knudsen EI (2004). Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience, 16, 1412–1425. [DOI] [PubMed] [Google Scholar]
- 66.Lorenz Konrad Z. (1958). The evolution of behavior. Scientific American, Vol. 199: 67–82 [DOI] [PubMed] [Google Scholar]
- 67.Takesian AE and Hensch TK (2013) Balancing Plasticity/Stability Across Brain Development. DOI: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed]
- 68.Daw NW et al. (1991) Cortisol reduces plasticity in the kitten visual cortex. J. Neurobiol. 22, 158–168. [DOI] [PubMed] [Google Scholar]
- 69.Morishita H et al. (2015) Prolonged Period of Cortical Plasticity upon Redox Dysregulation in Fast-Spiking Interneurons. Biol. Psychiatry 78, 396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang Z et al. (2013) Social Isolation Exacerbates Schizophrenia-Like Phenotypes via Oxidative Stress in Cortical Interneurons. Biol. Psychiatry 73, 1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moriceau S et al. (2004) Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int. J. Dev. Neurosci. 22, 415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis TL and Maurer D (2005) Multiple sensitive periods in human visual development: Evidence from visually deprived children. Dev. Psychobiol. 46, 163–183. [DOI] [PubMed] [Google Scholar]
- 73.McEwen BS et al. (2015) Mechanisms of stress in the brain. Nat. Neurosci. 18, 1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calem M et al. (2017) Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. NeuroImage Clin. 14, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hanson JL et al. (2015) Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol. Psychiatry 77, 314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lambert HK et al. (2017) Hippocampal Contribution to Context Encoding across Development Is Disrupted following Early-Life Adversity. J. Neurosci. 37, 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frodl T and O’Keane V (2013) How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol. Dis. 52, 24–37 [DOI] [PubMed] [Google Scholar]
- 78.Teicher MH et al. (2012) Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. U. S. A. 109, E563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riem MME et al. (2015) Beating the brain about abuse: Empirical and meta-analytic studies of the association between maltreatment and hippocampal volume across childhood and adolescence. Dev. Psychopathol. 27, 507–520. [DOI] [PubMed] [Google Scholar]
- 80.Gould F et al. (2012) The effects of child abuse and neglect on cognitive functioning in adulthood. J. Psychiatr. Res. 46, 500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheatham CL et al. (2010) Declarative memory in abused and neglected infants. Adv. Child Dev. Behav. 38, 161–182. [DOI] [PubMed] [Google Scholar]
- 82.Lee HHC et al. (2017) Genetic Otx2 mis-localization delays critical period plasticity across brain regions. Mol. Psychiatry 22, 680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gervain J et al. (2013) Valproate reopens critical-period learning of absolute pitch. Front. Syst. Neurosci. 7, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iwai Y et al. (2003) Rapid Critical Period Induction by Tonic Inhibition in Visual Cortex. J. Neurosci. 23, 6695–6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang E-J et al. (2012) Critical period for acoustic preference in mice. Proc. Natl. Acad. Sci.U. S. A. 109 Suppl, 17213–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weikum WM et al. (2012) Prenatal exposure to antidepressants and depressed maternal mood alter trajectory of infant speech perception. Proc. Natl. Acad. Sci. 109, 17221 17227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hensch TK and Quinlan EM (2018) Critical periods in amblyopia. Vis. Neurosci. 35, E014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perry RE et al. (2019) Developing a neurobehavioral animal model of poverty: Drawing cross-species connections between environments of scarcity-adversity, parenting quality, and infant outcome. Dev. Psychopathol. 31, 399–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Callaghan B et al. (2019) Using a Developmental Ecology Framework to Align Fear Neurobiology Across Species. Annu. Rev. Clin. Psychol. 15, 345–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perdue K, Jensen S, Kumar S, Fox S, Elwell C, & Nelson CA (2019, Epub ahead of print). Using functional near-infrared spectroscopy to assess social information processing in poor urban Bangladeshi infants and toddlers. Developmental Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.LeBlanc J, DeGregorio G, Centofante E, Vogel-Farley V, Barnes K, Kaufmann WE, Fagiolini M, & Nelson CA (2015). Visual evoked potentials detect cortical processing deficits in Rett syndrome. Annals of Neurology, 78 (5): 775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]