Abstract
Background
Human milk is the best enteral nutrition for preterm infants. However, human milk, given at standard recommended volumes, is not adequate to meet the protein, energy, and other nutrient requirements of preterm or low birth weight infants. One strategy that may be used to address the potential nutrient deficits is to give a higher volume of enteral feeds. High volume feeds may improve nutrient accretion and growth, and in turn may improve neurodevelopmental outcomes. However, there are concerns that high volume feeds may cause feed intolerance, necrotising enterocolitis, or complications related to fluid overload such as patent ductus arteriosus and chronic lung disease.
This is an update of a review published in 2017.
Objectives
To assess the effect on growth and safety of high versus standard volume enteral feeds in preterm or low birth weight infants. In infants who were fed fortified human milk or preterm formula, high and standard volume feeds were defined as > 180 mL/kg/day and ≤ 180 mL/kg/day, respectively. In infants who were fed unfortified human milk or term formula, high and standard volume feeds were defined as > 200 mL/kg/day and ≤ 200 mL/kg/day, respectively.
Search methods
We used the standard search strategy of Cochrane Neonatal to search Cochrane Central Register of Controlled Trials (CENTRAL; 2020 Issue 6) in the Cochrane Library; Ovid MEDLINE (1946 to June 2020); Embase (1974 to June 2020); and CINAHL (inception to June 2020); Maternity & Infant Care Database (MIDIRS) (1971 to April 2020); as well as previous reviews, and trial registries.
Selection criteria
We included randomised controlled trials (RCTs) that compared high versus standard volume enteral feeds for preterm or low birth weight infants.
Data collection and analysis
Two review authors assessed trial eligibility and risk of bias and independently extracted data. We analysed treatment effects in individual trials and reported risk ratio (RR) and risk difference for dichotomous data, and mean difference (MD) for continuous data, with respective 95% confidence intervals (CIs). We used the GRADE approach to assess the certainty of evidence. The primary outcomes were weight gain, linear and head growth during hospital stay, and extrauterine growth restriction at discharge.
Main results
We included two new RCTs (283 infants) in this update. In total, we included three trials (347 infants) in this updated review.
High versus standard volume feeds with fortified human milk or preterm formula
Two trials (283 infants) met the inclusion criteria for this comparison. Both were of good methodological quality, except for lack of masking. Both trials were performed in infants born at < 32 weeks' gestation. Meta‐analysis of data from both trials showed high volume feeds probably improves weight gain during hospital stay (MD 2.58 g/kg/day, 95% CI 1.41 to 3.76; participants = 271; moderate‐certainty evidence). High volume feeds may have little or no effect on linear growth (MD 0.05 cm/week, 95% CI ‐0.02 to 0.13; participants = 271; low‐certainty evidence), head growth (MD 0.02 cm/week, 95% CI ‐0.04 to 0.09; participants = 271; low‐certainty evidence), and extrauterine growth restriction at discharge (RR 0.71, 95% CI 0.50 to 1.02; participants = 271; low‐certainty evidence). We are uncertain of the effect of high volume feeds with fortified human milk or preterm formula on the risk of necrotising enterocolitis (RR 0.74, 95% CI 0.12 to 4.51; participants = 283; very‐low certainty evidence).
High versus standard volume feeds with unfortified human milk or term formula
One trial with 64 very low birth weight infants met the inclusion criteria for this comparison. This trial was unmasked but otherwise of good methodological quality. High volume feeds probably improves weight gain during hospital stay (MD 6.2 g/kg/day, 95% CI 2.71 to 9.69; participants = 61; moderate‐certainty evidence). The trial did not provide data on linear and head growth, and extrauterine growth restriction at discharge. We are uncertain as to the effect of high volume feeds with unfortified human milk or term formula on the risk of necrotising enterocolitis (RR 1.03, 95% CI 0.07 to 15.78; participants = 61; very low‐certainty evidence).
Authors' conclusions
High volume feeds (≥ 180 mL/kg/day of fortified human milk or preterm formula, or ≥ 200 mL/kg/day of unfortified human milk or term formula) probably improves weight gain during hospital stay. The available data is inadequate to draw conclusions on the effect of high volume feeds on other growth and clinical outcomes. A large RCT is needed to provide data of sufficient quality and precision to inform policy and practice.
Plain language summary
High versus standard volume feeds to promote growth in preterm or low birth weight infants
Review question Does giving preterm (born at < 37 weeks) or low birth weight (< 2500 grams) infants a greater volume of feeds than is usually given, promote growth without causing feeding problems or other side effects?
Background Infants born early (preterm) need extra nutrients for growth. One way to deliver extra nutrition is to give infants a greater volume of feeds than usual (high volume feeds, equal to or greater than 180 to 200 mL/kg/day of milk). Although giving high volumes of milk to preterm or low birth weight infants might increase growth rates, there are concerns that infants may not tolerate high volume feeds and may experience side effects including severe bowel problems. We have looked for evidence from clinical trials that assessed whether high volume feeds are beneficial or harmful for preterm or low birth weight infants.
Study characteristics Search is up‐to‐date as of June 2020. We found three studies that addressed this question.
Key results Evidence from two studies showed that high volume feeds (≥ 180 mL/kg/day) with fortified human milk (human milk with added human milk fortifier) or preterm formula probably improves weight gain during hospital stay, when compared to standard volume of the same. Similarly, evidence from one small study showed that high volume feeds (≥ 200 mL/kg/day) with unfortified human milk or preterm formula probably improves weight gain during hospital stay. The evidence is insufficient to comment on the effect of high volume feeds on increase in length or head size during hospital stay, long‐term growth and development, and the effect on gut problems or other side effects.
Conclusions
High volume feeds probably improve weight gain during hospital stay. The available data is inadequate to draw conclusions on the effect of high volume feeds on other growth and clinical outcomes.
Summary of findings
Background
Description of the condition
The optimal growth rate of infants born preterm or with low birth weight (LBW) is not known (Higgins 2012). Consensus guidelines suggest that caregivers should aim to achieve a postnatal growth rate similar to the gestation‐equivalent foetal growth rate (Agostoni 2010). Many preterm or LBW infants, especially those who are very low birth weight (VLBW) or extremely low birth weight (ELBW), do not achieve these rates of growth and are growth restricted at the time of hospital discharge (Clark 2003; Cooke 2004; Ehrenkranz 1999; Horbar 2015; Lima 2014; Sakurai 2008; Shan 2009; Stevens 2016; Steward 2002;). Growth deficits can persist through childhood and adolescence and into adulthood (Brandt 2005; Dusick 2003; Euser 2008; Hack 2003; Stein 2013). Slow postnatal growth is associated with neurodevelopmental impairment and with poorer cognitive and scholastic outcomes (Brandt 2003; Franz 2009; Leppanen 2014; Neubauer 2013). Furthermore, there are concerns that nutritional deficiency and growth restriction during infancy may have adverse effects on long‐term metabolic and cardiovascular health (Embleton 2013; Higgins 2012; Lapillonne 2013).
Preterm infants have higher nutritional requirements than term infants. An energy intake of 110 to 135 Kcal/kg/day and protein intake of 3.5 to 4 g/kg/day in infants with 1000 to 1499 grams birth weight and 4 to 4.5 g/kg/day in infants with < 1000 grams birth weight are recommended to meet the higher nutrients requirement of preterm infants.
Description of the intervention
Human milk is the best enteral nutrition for newborn infants (Johnston 2012). However, human milk alone, given at volumes that meet the nutritional needs of term infants, may not meet the higher nutritional requirements of preterm or LBW infants (AAP 2004; Agostoni 2010; CPS 1995; Embleton 2007) (Table 3). The strategy most commonly employed to address these potential nutrient deficits is to supplement human milk with a multi‐component human milk fortifier (Cormack 2013; Dutta 2015; Klingenberg 2012; Uhing 2009). A Cochrane Review of randomised controlled trials provides evidence that feeding preterm infants with multi‐nutrient fortified human milk rather than unfortified human milk increases growth rates during the initial hospitalisation period (Brown 2020). However, despite fortification, the rates of extrauterine growth restriction in VLBW or ELBW infants is still high (Lee 2020; Stevens 2016). This signifies the need for further improvement in nutritional intake in these infants.
1. Typical energy and protein content of human milk or formula.
| Per 100 mL |
Expressed breast milk (EBM) |
EBM + fortifier |
Term formula | Preterm formula |
| Energy (kCal) | 67 | 80 | 67 | 80 |
| Protein (g) | 1.5 | 2.0 to 2.3 | 1.5 | 2.0 |
The maximum volume of enteral feeds used in many centres across the world is 150 to 180 mL/kg/day (Klingenberg 2012). Fortified human milk or preterm formula (which usually gives 80 Kcal and 2 grams protein per 100 mL) given at this standard volume meets only the calorie requirement and not the protein requirement of preterm, especially ELBW infants. Increasing the volume of feeds could meet these nutrient deficits. Further, multi‐component fortifiers and nutrient‐enriched preterm formula are expensive and unaffordable in resource‐limited settings (Chawla 2008; Kler 2015). Unfortified human milk or standard term formula is still used as the sole source of enteral nutrition in such settings, where increasing the volume of feeds could be the only way to improve nutrient intake in preterm infants.
Feeding preterm or LBW infants with daily volumes of milk in excess of 180 mL/kg (high volume feeds) has been proposed as a safe and effective growth‐enhancement strategy (Klingenberg 2019; Kuschel 2000; Lewis 1984; Thomas 2012; Valman 1974; Zecca 2014). In order to provide 4 to 4.5 g/kg protein, fortified human milk or preterm formula has to be fed at a volume of 200 to 225 mL/kg/day and unfortified human milk or term formula has to be fed at a volume of 270 to 300 mL/kg/day. However, due to concerns about the risk of necrotising enterocolitis (NEC), feed intolerance and complications of fluid overload patent ductus arteriosus (PDA) and chronic lung disease (CLD), high volume enteral feeding has not become an established practice (Bertino 2009; Chawla 2008; Klingenberg 2012; Raban 2013; Sankar 2008).
How the intervention might work
Feeding preterm or LBW infants higher volumes of milk (more than 180 mL/kg/d) may promote nutrient accretion and improve growth. Higher levels of nutrient intake during this critical period may be important for optimising long‐term growth and neurodevelopment (Embleton 2013). The potential disadvantages of high volume feeds also are known. High volumes of milk may add to the physiological and metabolic stress of the immature gastrointestinal tract and its blood supply, thus increasing the risk of NEC. High volume feeds may result in or worsen gastro‐oesophageal reflux, increasing risk of apnoea or aspiration. High volume feeds may lead to fluid overload and associated complications such as peripheral or pulmonary oedema, PDA and CLD. Furthermore, enteral feeding that is ceased owing to intolerance may reduce total nutrient intake over time, thus adversely affecting growth.
Why it is important to do this review
Given the potential of high volume feeds to increase nutrient accretion and improve growth and developmental outcomes in preterm or LBW infants, as well the potential risks of this feeding strategy, we undertook a systematic review to identify and appraise data from randomised controlled trials and to provide a synthesis of evidence that could inform practice and research.
Objectives
To assess the effect on growth and safety of high versus standard volume enteral feeds in preterm or LBW infants. In infants who were fed fortified human milk or preterm formula, high and standard volume feeds were defined as > 180 mL/kg/day and ≤ 180 mL/kg/day, respectively. In infants who were fed unfortified human milk or term formula, high and standard volume feeds were defined as > 200 mL/kg/day and ≤ 200 mL/kg/day, respectively.
To conduct subgroup analyses based on gestational age (< 28 weeks, 28 weeks to 31 weeks, ≥ 32 weeks), birth weight (< 1000 grams, 1000 grams to 1499 grams, ≥ 1500 grams), type of milk (human milk vs formula) and presence of intrauterine growth restriction (using birth weight relative to the reference population as a surrogate). (See Subgroup analysis and investigation of heterogeneity.)
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), quasi‐ and cluster‐RCTs.
Types of participants
We included preterm (< 37 weeks' gestational age) or low birth weight (< 2500 grams birth weight) infants.
Types of interventions
Comparison 1: In infants who were fed fortified human milk or preterm formula
Intervention
High volume feeds, defined as > 180 mL/kg/day
Control
Standard volume feeds, defined as ≤ 180 mL/kg/day
Comparison 2: In infants who were fed unfortified human milk or term formula
Intervention
High volume feeds defined as > 200 mL/kg/day
Control
Standard volume feeds, defined as ≤ 200 mL/kg/day
See Differences between protocol and review.
Infants might have been randomised to the allocated intervention at any stage up to the time of achieving full enteral feeding volumes. The prescribed feeding regimen should have been followed until the infant was able to self‐regulate intake.
Types of outcome measures
Primary outcomes
Rates of weight gain (g/kg/d), linear growth (cm/week), or head growth (cm/week) during hospital stay
Proportion of infants with extrauterine growth restriction at discharge (weight less than 10th percentile for the index population)
Secondary outcomes
Number of infants with NEC (modified Bell stage 2 or 3; Walsh 1986)
Proportion of infants with feed interruption episodes (lasting ≥ 12 hours)
Time to regain birth weight (days)
Growth measures, namely weight, length and head circumference, measured at a specified postmenstrual age prior to discharge
Number of infants with PDA treated pharmacologically or surgically
Number of infants with aspiration pneumonia or pneumonitis (clinical or radiological evidence of lower respiratory tract compromise that has been attributed to covert or evident aspiration of gastric contents)
Number of infants with gastro‐oesophageal reflux diagnosed by clinical features; post‐feed (if bolus‐fed) apnoea, desaturation, irritability, or vomiting; or oesophageal pH monitoring, multiple intraluminal impedance, or endoscopy
Frequency of apnoea (no respiratory effort > 20 seconds) or bradycardia (< 100 beats per minute), or apnoea/bradycardia necessitating stimulation, oxygen administration increase, or positive‐pressure ventilation (number of episodes per day)
Frequency of episodes of spontaneous fall in oxygen saturation (SpO₂) to 85% or less (number of episodes per day)
Number of infants with CLD (requirement of oxygen supplementation at 36 weeks' postmenstrual age)
All‐cause mortality before discharge or up to 44 weeks' postmenstrual age
Duration of hospital stay (days)
Growth measures following discharge from hospital to latest follow‐up
Neurodevelopmental outcomes assessed after 12 months post‐term: neurological evaluations; developmental scores; and classifications of disability, including auditory and visual disability. We defined neurodevelopmental impairment as the presence of one or more of the following: non‐ambulant cerebral palsy; developmental quotient greater than two standard deviations below the population mean; and blindness (visual acuity < 6/60) or deafness (any hearing impairment requiring, or unimproved by, amplification)
Search methods for identification of studies
We used the standard search strategy of Cochrane Neonatal (neonatal.cochrane.org/resources-review-authors).
Electronic searches
We conducted a comprehensive search including Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 6) in the Cochrane Library; Ovid MEDLINE(R) (1946 to 5 June 2020); Embase (1974 to 5 June 2020); CINAHL (inception to 8 June 2020); Maternity & Infant Care Database (MIDIRS), Ovid (1971 to April 2020). We have included the search strategies for each database in Appendix 1. We did not apply language restrictions.
We searched ClinicalTrials.gov for completed or ongoing trials.
This search updates the searches conducted for previous versions of the review (Abiramalatha 2017; Appendix 2).
Searching other resources
We also searched the reference lists of any articles selected for inclusion in this review in order to identify additional relevant articles.
Data collection and analysis
We used standard methods of Cochrane and Cochrane Neonatal (Higgins 2020).
Selection of studies
We screened the title and abstract of all studies identified by the above search strategy, and two review authors (TA and ST) independently assessed the full articles for all potentially relevant trials. We excluded studies that did not meet all of the inclusion criteria, and we stated the reason for exclusion. We discussed disagreements until we achieved consensus or by consulting a third author (NT).
We recorded the selection process in sufficient detail to complete a Characteristics of excluded studies table and a PRISMA flow diagram (Moher 2009).
Data extraction and management
Two review authors (TA and ST) extracted data independently using a data collection form to aid extraction of information on design, methods, participants, interventions, outcomes and treatment effects from each included study. We discussed disagreements until we achieved consensus or by consulting a third author (NT). If data from trial reports were insufficient, we contacted trialists to request further information.
Assessment of risk of bias in included studies
Two review authors (TA and ST) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011), for the following domains.
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
We resolved any disagreements by discussion or by consulting a third author (NT). See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We analysed treatment effects in individual trials using Review Manager 5 and reported risk ratios (RRs) and risk differences (RDs) for dichotomous data, and mean differences (MDs) for continuous data, with respective 95% confidence intervals (CIs) (Review Manager 2020).
Unit of analysis issues
The unit of analysis was the participating infant in RCTs. We combined study results where there was little heterogeneity between study designs, and we considered interactions between effects of the intervention and the choice of randomisation unit to be unlikely.
Dealing with missing data
We requested and obtained additional data from trialists if information on important outcomes was missing or was reported unclearly.
Assessment of heterogeneity
We examined treatment effects of individual trials and heterogeneity between trial results by inspecting forest plots. We calculated the I² statistic for each RR analysis to quantify inconsistency across studies and to describe the percentage of variability in effect estimates that may be due to heterogeneity rather than to sampling error. If we detected moderate or high heterogeneity (I² ≥ 50%), we planned to explore possible causes (e.g. differences in study design, participants, interventions, or completeness of outcome assessments).
Assessment of reporting biases
As we included only two trials in the meta‐analysis, we could not examine a funnel plot for possible publication bias.
Data synthesis
We analysed all infants randomised on an intention‐to‐treat basis and treatment effects in individual trials using a fixed‐effect model to combine the data.
Certainty of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence of the following (clinically relevant) outcomes: weight gain, linear and head growth during hospital stay, extrauterine growth restriction at discharge, and necrotising enterocolitis.
Two review authors (TA and ST) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create Table 1 and Table 2 to report the certainty of the evidence.
Summary of findings 1. High compared to standard volume of fortified human milk or preterm formula in preterm or low birth weight infants.
| High compared to standard volume of fortified human milk or preterm formula in preterm or low birth weight infants | ||||||
| Patient or population: preterm or low birth weight infants Setting: neonatal intensive care unit (Australia and United States) Intervention: high volume feeds with fortified human milk or preterm formula Comparison: standard volume feeds with fortified human milk or preterm formula | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard volumes of fortified human milk or preterm formula | Risk with High volumes of fortified human milk or preterm formula | |||||
| Weight gain during hospital stay (g/kg/day) Follow‐up: Until discharge or 35‐36 weeks PMA |
The mean weight gain during hospital stay varied from 16.5 to 17.9 g/kg/day | MD 2.58 higher (1.41 higher to 3.76 higher) | ‐ | 271 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Probably improves weight gain during hospital stay |
| Linear growth during hospital stay (cm/week) Follow‐up: Until discharge or 35‐36 weeks PMA |
The mean linear growth during hospital stay varied from 0.64 to 0.89 cm/week | MD 0.05 higher (0.02 lower to 0.13 higher) | ‐ | 271 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | May or may not improve linear growth during hospital stay |
| Head growth during hospital stay (cm/week) Follow‐up: Until discharge or 35‐36 weeks PMA |
The mean head growth during hospital stay varied from 0.59 to 0.83 cm/week | MD 0.02 higher (0.04 lower to 0.09 higher) | ‐ | 271 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | May or may not improve linear growth during hospital stay |
| Extrauterine growth restriction at discharge Follow‐up: Until discharge |
Study population | RR 0.71 (0.50 to 1.02) | 271 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | May or may not reduce extrauterine growth restriction at discharge | |
| 312 per 1,000 | 222 per 1,000 (156 to 318) | |||||
| Necrotising enterocolitis Follow‐up: Until discharge |
Study population | RR 0.74 (0.12 to 4.51) | 283 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 | Uncertainty regarding the effect on the risk of NEC | |
| 14 per 1,000 | 10 per 1,000 (2 to 62) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; PMA: Postmenstrual age; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level for serious imprecision due to small sample size
2 Downgraded one level for serious risk of bias due to lack of masking
3Downgraded by two levels for very serious imprecision due to small sample size and wide confidence interval
Summary of findings 2. High compared to standard volume of unfortified human milk or term formula in preterm or low birth weight infants.
| High compared to standard volume of unfortified human milk or term formula in preterm or low birth weight infants | ||||||
| Patient or population: preterm or low birth weight infants Setting: neonatal intensive care units (India) Intervention: high volume feeds with unfortified human milk or term formula Comparison: standard volume feeds with unfortified human milk or term formula | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard volume of unfortified human milk or term formula | Risk with High volumes of unfortified human milk or term formula | |||||
| Weight gain during hospital stay (g/kg/day) Follow‐up: Until babies reach 1700 g weight |
The mean weight gain during hospital stay was 18.7 g/kg/day | MD 6.2 higher (2.71 higher to 9.69 higher) | ‐ | 61 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | Probably improves weight gain during hospital stay |
| Linear growth (cm/week) | ‐ | see comment | ‐ | (0 studies) | ‐ | We found no data on this outcome. |
| Head growth (cm/week) | ‐ | see comment | ‐ | (0 studies) | ‐ | We found no data on this outcome. |
| Extrauterine growth restriction at discharge | Study population | ‐ | (0 studies) | ‐ | We found no data on this outcome. | |
| see comment | see comment | |||||
| Necrotising enterocolitis Follow‐up: Until discharge |
Study population | RR 1.03 (0.07 to 15.78) | 61 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 3 | Uncertainty regarding the effect on the risk of NEC | |
| 32 per 1,000 | 33 per 1,000 (2 to 509) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level for serious imprecision due to small sample size
2 Downgraded one level for serious risk of bias due to lack of masking
3 Downgraded two levels for very serious imprecision due to small sample size and wide confidence intervals
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Subgroup analysis and investigation of heterogeneity
We planned to undertake these subgroup analyses, when possible.
Based on gestational age: < 28 weeks, 28 to 31 weeks, ≥ 32 weeks
Based on birth weight: < 1000 grams, 1000 to 1499 grams, ≥ 1500 grams
Type of milk: human milk versus formula
Infants with birth weight below the 10th percentile for the reference population ('small for gestational age') versus infants with birth weight 'appropriate for gestational age
Sensitivity analysis
We planned to undertake sensitivity analyses to determine whether findings were affected when only studies using adequate methods were included (low risk of bias); adequate methods were defined as adequate randomisation and allocation concealment, blinding of intervention and measurement, and less than 10% loss to follow‐up. However, we did not conduct any sensitivity analysis, as it was not required.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The results of the updated search are shown in Figure 1. We screened 5858 titles and abstracts that were identified via the search strategy. We carried out full‐text review of four articles. We excluded two new studies, and we reported details of the excluded studies. We identified two new eligible studies. Thus, we included a total of three studies in this review.
1.
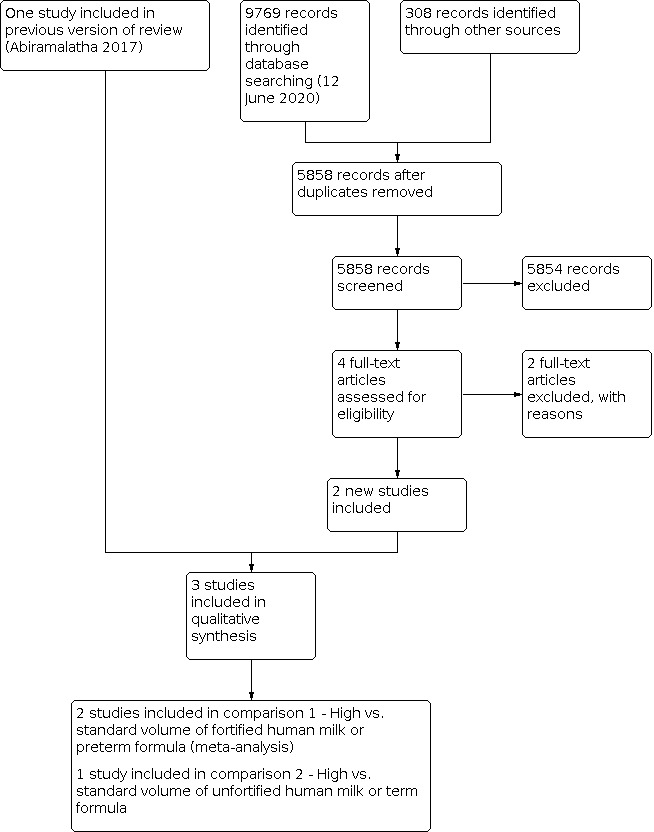
Updated study flow diagram.
Included studies
We included three RCTs with a total of 347 infants (See Characteristics of included studies table).
We included two trials for the comparison of high versus standard volume feeds with fortified human milk or preterm formula (Kuschel 2000; Travers 2020), and one trial for the comparison of high versus standard volume feeds with unfortified human milk or term formula (Thomas 2012).
Kuschel 2000, conducted in Australia, enrolled 59 infants born at less than 30 weeks' gestation. Infants in high and standard volume feeds groups received 200 and 150 mL/kd/day feeds respectively. Either fortified human milk or preterm formula was used for feeding. Fortification was continued until the baby reached 1800 to 2000 grams. The primary outcome was growth measures such as weight, length, head circumference, arm area, arm muscle are, arm fat area at 35 weeks' postmenstrual age (PMA), and mean weight gain in g/kg/day. Secondary outcomes were PMA and weight at which fortification or preterm formula was ceased, PMA when reaching full sucking feeds, PMA and weight at discharge, growth measures such as weight, length and head circumference at 12 months' corrected age, and developmental assessment using Griffiths Mental Development Scales at 12 months' corrected age.
Travers 2020, conducted in the USA, randomised 224 infants born at less than 32 weeks' gestation with a birth weight 1001 to 2500 grams to high (180 to 200 mL/kg/day) and standard volume feeds (140 to 160 mL/kd/day) groups. Either fortified human milk or preterm formula was used for feeding. The primary outcome was weight gain in g/kg/day from birth till 36 weeks' PMA. Secondary outcomes were increase in length, head circumference and mid‐arm circumference, postnatal growth failure (weight less than 10th percentile for PMA), CLD, NEC, haemodynamically significant PDA, duration of respiratory support, culture proven sepsis after study entry and mortality before hospital discharge.
Thomas 2012, conducted in India, enrolled 64 infants with birth weight < 1500 grams. Both appropriate‐ and small‐for‐gestational‐age infants were eligible to participate. Infants in the high volume group received 300 mL/kg/day, and those in the standard volume group received 200 mL/kg/day. Participants were fed with unfortified human milk plus individual micronutrient supplementation for iron, calcium, and vitamins. Multi‐nutrient fortifiers, which supplement calories and protein, were not used. The primary outcome was daily weight gain from enrolment until the infant reached 1700 grams weight. Secondary outcomes were feed intolerance, tachypnoea (respiratory rate > 60 breaths per minute), PDA (diagnosed clinically or by echocardiography), NEC (Bell stage 2A or greater), invasive infection (confirmed by blood culture) and biochemical abnormalities.
Excluded studies
We excluded four studies in total (see Characteristics of excluded studies).
We excluded two new studies (Klingenberg 2019; Zecca 2014), for the following reasons.
Klingenberg 2019 was a retrospective observational study on 99 infants born at < 30 weeks' gestation, who were fed high volume (> 180 mL/kg/day) of fortified human milk.
Zecca 2014 was an RCT. The trial compared 200 mL/kg/day versus 170 mL/kg/day of unfortified human milk, both of which were standard volume feeds. Further, the trial reported different rates of feed volume advancement in the intervention and control groups.
We excluded two studies in the original review (Abiramalatha 2017), because they were not RCTs. One was an observational study of LBW infants fed 250 mL/kg/d of milk (Lewis 1984). The other was a cohort study comparing two enteral feed volumes; 180 and 230 mL/kg/d (Valman 1974).
Risk of bias in included studies
See Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for the included study.
Allocation
All three included trials used computer‐generated random numbers for sequence generation and sealed opaque envelopes for allocation concealment (Kuschel 2000; Thomas 2012; Travers 2020).
Blinding
All the three included studies were open‐label trials.
Incomplete outcome data
Trialists of all the three trials have assessed all participants for primary and secondary outcomes.
Selective reporting
The study protocol was published for Travers 2020 and all proposed outcomes were reported. The study protocol was not published for Kuschel 2000 and Thomas 2012; by personal communication with the trialists, we found that all proposed outcomes were reported.
Other potential sources of bias
We did not identify any other bias in the three included trials.
Effects of interventions
High versus standard volume of fortified human milk or preterm formula (Comparison 1)
Primary outcomes
Rate of weight gain during hospital stay
1.1. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 1: Weight gain during hospital stay (g/kg/day)
3.

Forest plot of comparison: 1 High versus standard volume of fortified human milk or preterm formula, outcome: 1.1 Weight gain during hospital stay (g/kg/day).
Both trials reported this outcome (Kuschel 2000; Travers 2020). The rate of weight gain was greater in infants fed high volumes compared to those fed standard volumes of fortified milk or preterm formula (MD 2.58 g/kg/day, 95% CI 1.41 to 3.76; participants = 271). There was no evidence of heterogeneity (I2 = 0%).
Linear growth during hospital stay
1.2. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 2: Linear growth during hospital stay (cm/week)
Meta‐analysis of data from both trials (Kuschel 2000; Travers 2020), showed no difference in the outcome of linear growth during hospital stay between high and standard volume feeds groups (MD 0.05 cm/week, 95% CI ‐0.02 to 0.13; participants = 271). There was no evidence of heterogeneity (I2 = 0%).
Head growth during hospital stay
1.3. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 3: Head growth during hospital stay (cm/week)
Meta‐analysis of data from both trials (Kuschel 2000; Travers 2020), showed no difference in head growth during hospital stay between the groups (MD 0.02 cm/week, 95% CI ‐0.04 to 0.09; participants = 271). There was no heterogeneity (I2 = 10%).
Extrauterine growth restriction at discharge
1.4. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 4: Extrauterine growth restriction at discharge
Both trials (Kuschel 2000; Travers 2020), reported the outcome. There was no difference in the incidence of extrauterine growth restriction (weight less than 10th percentile) at discharge between the high and standard volume feeds groups (RR 0.71, 95% CI 0.50 to 1.02; participants = 271). There was moderate heterogeneity (I2 = 60%).
Secondary outcomes
NEC stage 2 or 3
1.5. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 5: Necrotising enterocolitis
4.

Forest plot of comparison: 1 High versus standard volume of fortified human milk or preterm formula, outcome: 1.5 Necrotising enterocolitis.
Both trials (Kuschel 2000; Travers 2020) reported the outcome. The meta‐analysis showed no difference in the incidence of NEC between the high and standard volume feeds groups (RR 0.74, 95% CI 0.12 to 4.51; participants = 283). There was mild heterogeneity (I2 = 27%).
Feed interruption episodes
1.6. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 6: Feed interruption episodes
One trial (Travers 2020), reported the outcome. There was no difference in the proportion of infants with feed interruption episodes ≥ 12 hours between the groups (RR 0.72, 95% CI 0.12 to 4.25; participants = 217).
Time to regain birth weight
1.7. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 7: Time to regain birth weight (days)
Both trials reported this outcome (Kuschel 2000; Travers 2020). The meta‐analysis showed a marginal difference, with infants given high volume feeds regaining birth weight earlier than those given standard volume feeds (MD ‐1.23 days, 95% CI ‐2.36 to ‐0.10; participants = 271). There was no evidence of heterogeneity (I2 = 0%).
Weight at a specified PMA
1.8. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 8: Weight at a specified postmenstrual age (g)
5.

Forest plot of comparison: 1 High versus standard volume of fortified human milk or preterm formula, outcome: 1.8 Weight at a specified postmenstrual age (g).
Both trials reported the outcome. Kuschel 2000 reported the growth measures when fortification was stopped, which had a median of 34 to 35 weeks' (range 32 to 40 weeks') PMA. Travers 2020 reported growth measures at discharge or 36 weeks' PMA whichever was earlier. Meta‐analysis showed that babies who were given high volume feeds had reached greater weight at a specified PMA compared to those given standard volume feeds (MD 152.10 g, 95% CI 71.67 to 232.53; participants = 271). There was no evidence of heterogeneity (I2 = 4%).
Length at a specified PMA
1.9. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 9: Length at a specified postmenstrual age (cm)
Meta‐analysis of data from both trials (Kuschel 2000; Travers 2020) showed no difference in the length attained at a specified PMA between the two groups (MD 0.50 cm, 95% CI ‐0.04 to 1.04; participants = 271). There was no heterogeneity (I2 = 0%).
Head circumference at a specified PMA
1.10. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 10: Head circumference at a specified postmenstrual age (cm)
6.

Forest plot of comparison: 1 High versus standard volume of fortified human milk or preterm formula, outcome: 1.10 Head circumference at a specified postmenstrual age (cm).
Meta‐analysis of data from both trials (Kuschel 2000; Travers 2020) showed that infants in the high volume feeds group attained greater head circumference at a specified PMA than those in the standard volume feeds group (MD 0.49 cm, 95% CI 0.15 to 0.83; participants = 271). There was no evidence of heterogeneity (I2 = 0%).
PDA requiring treatment
1.11. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 11: PDA requiring treatment
Both trials (Kuschel 2000; Travers 2020), reported the outcome and there was no difference in the incidence of PDA requiring treatment (RR 0.77, 95% CI 0.28 to 2.12; participants = 271). There was no heterogeneity (I2 = 0%).
Aspiration pneumonia or pneumonitis
This outcome was not reported in the trials.
Gastro‐oesophageal reflux
This outcome was not reported in the trials.
Frequency of apnoea
This outcome was not reported in the trials.
Frequency of desaturation episodes
This outcome was not reported in the trials.
Chronic lung disease
1.12. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 12: Chronic lung disease
Both trials (Kuschel 2000; Travers 2020), reported this outcome. There was no difference in the incidence of chronic lung disease between the groups (RR 1.01, 95% CI 0.57 to 1.81; participants = 271). There was no evidence of heterogeneity (I2 = 0%).
All‐cause mortality before discharge
1.13. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 13: All‐cause mortality before discharge
Data was available from both the trials (Kuschel 2000; Travers 2020). Meta‐analysis showed no difference in the outcome between the high and standard volume feeds groups (RR 0.24, 95% CI 0.01 to 4.71; participants = 283). There was no evidence of heterogeneity (I2 = 0%).
Duration of hospital stay
Meta‐analysis of data from both the trials showed no difference in the duration of hospital stay between the groups (MD 1.00 day, 95% CI ‐3.54 to 5.54 days; participants = 271). There was no evidence of heterogeneity (I2 = 0%).
Growth measures at 12 months' corrected age
(Analysis 1.15; Analysis 1.16; Analysis 1.17)
1.15. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 15: Weight < 10th percentile at 12 months' corrected age
1.16. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 16: Length < 10th percentile at 12 months' corrected age
1.17. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 17: Head circumference < 10th percentile at 12 months' corrected age
One trial (Kuschel 2000), reported growth measures at 12 months' corrected age. The trial showed no difference in the proportion of infants with weight for age less than 10th percentile (RR 0.70, 95% CI 0.23 to 2.15; participants = 47), length less than 10th percentile (RR 0.35, 95% CI 0.08 to 1.55; participants = 47) or head circumference less than 10th percentile (RR 0.35, 95% CI 0.01 to 8.11; participants = 47) at 12 months' corrected age between the high and standard volume feeds groups.
Neurodevelopmental outcomes assessed at 12 months' corrected age
1.18. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 18: Neurodevelopmental impairment at 12 months' corrected age
One trial (Kuschel 2000), reported neurodevelopmental outcomes at 12 months' corrected age. The trial showed no difference in the proportion of infants with neurodevelopmental impairment (which included cerebral palsy, severe developmental delay and/or deafness) at 12 months' corrected age between the high and standard volume feeds groups (RR 0.52, 95% CI 0.15 to 1.84; participants = 47).
High versus standard volume of unfortified milk or term formula (Comparison 2)
Primary outcomes
Rate of weight gain during hospital stay
2.1. Analysis.

Comparison 2: High versus standard volume of unfortified human milk or term formula, Outcome 1: Weight gain during hospital stay (g/kg/day)
7.

Forest plot of comparison: 2 High versus standard volume of unfortified human milk or term formula, outcome: 2.1 Weight gain during hospital stay (g/kg/day).
Thomas 2012 showed that the rate of weight gain was greater in infants fed higher volume compared to those fed standard volumes of unfortified milk or term formula (MD 6.20 g/kg/day, 95% CI 2.71 to 9.69; participants = 61).
Linear growth during hospital stay
This outcome was not reported on in the included trial.
Head growth during hospital stay
This outcome was not reported on in the included trial.
Extrauterine growth restriction at discharge
This outcome was not reported on in the included trial.
Secondary outcomes
NEC stage 2 or 3
2.2. Analysis.

Comparison 2: High versus standard volume of unfortified human milk or term formula, Outcome 2: Necrotising enterocolitis
Thomas 2012 showed no difference in the incidence of NEC between the high and standard volume feeds groups (RR 1.03, 95% CI 0.07 to 15.78; participants = 61).
Feed interruption episodes
This outcome was not reported on in the included trial.
Time to regain birth weight
2.3. Analysis.

Comparison 2: High versus standard volume of unfortified human milk or term formula, Outcome 3: Time to regain birth weight (days)
Thomas 2012 showed no difference in the time to regain birth weight between the groups (MD ‐0.50 days, 95% CI ‐2.61 to 1.61; participants = 61).
Weight at a specified PMA
This outcome was not reported on in the included trial.
Length at a specified PMA
This outcome was not reported on in the included trial.
Head circumference at a specified PMA
This outcome was not reported on in the included trial.
PDA requiring treatment
This outcome was not reported on in the included trial.
Aspiration pneumonia or pneumonitis
This outcome was not reported on in the included trial.
Gastro‐oesophageal reflux
This outcome was not reported on in the included trial.
Frequency of apnoea
This outcome was not reported on in the included trial.
Frequency of desaturation episodes
This outcome was not reported on in the included trial.
Chronic lung disease
This outcome was not reported on in the included trial.
All‐cause mortality before discharge
This outcome was not reported on in the included trial.
Duration of hospital stay
This outcome was not reported on in the included trial.
Growth measures at 12 months of age
This outcome was not reported on in the included trial.
Neurodevelopmental outcomes assessed at 12 months' corrected age
This outcome was not reported on in the included trial.
Subgroup analyses
Based on gestational age or birth weight
This subgroup analysis was not possible. Both the trials on fortified human milk (Kuschel 2000; Travers 2020), were performed in infants < 32 weeks' gestation. The trial on unfortified human milk (Thomas 2012), was performed in infants with < 1500 grams birth weight.
Human milk‐fed versus formula‐fed infants
This was not possible. Either fortified human milk or preterm formula was used in Kuschel 2000 and Travers 2020, and subgroup data were not reported. Thomas 2012 used only unfortified human milk.
Small‐for‐gestational‐age infants
Kuschel 2000 did not report data on infants who were small‐for‐gestational age. Travers 2020 recruited small‐for‐gestational age infants, but did not report outcomes separately. Thomas 2012 reported rate of weight gain in small‐for‐gestational age infants and showed no difference in the rate of weight gain in the subgroup: high volume (n = 10) 22.5 g/kg/d versus standard volume (n = 14) 17.6 g/kg/d. Trialists did not report standard deviations (SDs) and did not report data for appropriate‐for‐gestational‐age infants, so a subgroup comparison was not possible (Thomas 2012).
Trials conducted in low‐ or middle‐income versus high‐income countries
This subgroup analysis was not possible. Both trials on fortified human milk (Kuschel 2000; Travers 2020), were conducted in high‐income countries (Australia and USA respectively), whereas the trial on unfortified human milk (Thomas 2012), was conducted in a middle‐income country (India).
Discussion
Summary of main results
High versus standard volume feeds with fortified human milk or formula feeds (Comparison 1)
Two trials (Kuschel 2000; Travers 2020), with a total of 283 infants, met the inclusion criteria for this comparison. Both trials were of good methodological quality, except for lack of masking. Meta‐analysis of data from both the trials showed that high volume feeds (≥ 180 mL/kg/day) probably improves weight gain (2.6 g/kg/day more, which amounts to 18.2 g/kg/week and 78 g/kg/month) and weight at a specified postmenstrual age (152 grams more, 72 to 233 grams more). The certainty of evidence was moderate. High volume feeds may have little or no effect on linear growth or length attained at a specified PMA (low‐certainty evidence). Though high volume feeds may slightly increase the head circumference attained (~ 0.5 cm more) at a specified PMA, the analysis did not show a difference in the rate of head growth between the groups (low‐certainty evidence).
High volume feeds may marginally reduce the time taken to regain birth weight. Infants given high volume feeds regained birth weight 1.23 days earlier (2.36 to 0.10 days earlier) (low‐certainty of evidence). High volume feeds may have little or no effect on time to duration of hospital stay and proportion of infants with extrauterine growth restriction at discharge (low‐certainty evidence). We are uncertain whether high volume feeds improve growth or neurodevelopmental outcomes at 12 months' corrected age (very low‐certainty evidence).
We are uncertain as to the effect of high volumes of fortified human milk or preterm formula on adverse effects such as necrotising enterocolitis stage 2 or 3, feed interruption episodes, patent ductus arteriosus requiring treatment, chronic lung disease and all‐cause mortality before discharge (low‐ to very low‐certainty evidence). The included trials did not report outcomes such as aspiration pneumonia, gastro‐oesophageal reflux, apnoea and desaturation episodes.
High versus standard volume feeds with unfortified human milk or term formula (Comparison 2)
One trial (Thomas 2012), met the inclusion criteria for this comparison. This trial was unmasked, but otherwise of good methodological quality. High volume feeds probably improves weight gain during hospital stay (moderate‐certainty evidence). The mean difference of 6.2 g/kg/day amounts to 43 g/kg/week and 186 g/kg/month.
We are uncertain as to the effect of high volume feeds with unfortified human milk on the risk of necrotising enterocolitis (very low‐certainty evidence). The trial did not provide data on other growth and clinical outcomes.
Overall completeness and applicability of evidence
Both trials on fortified milk or preterm formula (Kuschel 2000; Travers 2020), have been performed on infants < 32 weeks' gestation and the trial on unfortified milk (Thomas 2012), has been performed on infants < 1500 grams. Thus, all three trials included very preterm or VLBW infants, the population that is at high risk of extrauterine growth restriction. The results are applicable to small‐for‐gestational‐age infants, since all the trials have included this population. However, there were no clear data on intrauterine growth restriction and antenatal Doppler abnormalities in the included trials. Hence, we are unable to decide whether the findings are applicable to infants with compromised fetal blood flow, who are at high risk for developing NEC. Both the trials in comparison one have used either fortified human milk or preterm formula for feeding (Kuschel 2000; Travers 2020). The trial in comparison two has used only unfortified human milk (Thomas 2012). Hence, the results are not applicable to cow's milk or term formula, which are frequently used in low‐ and middle‐income countries.
The meta‐analysis gave moderate certainty of evidence that high volume feeds improve in‐hospital weight gain. The mean difference between the high and standard volume feed groups was 2.6 g/kg/day in fortified human milk or preterm formula and 6.2 g/kg/day in unfortified human milk or term formula. The difference was greater in the latter, since even 200 mL/kg/day of unfortified milk is nutritionally inferior (providing ~ 3 g/kg of protein only). Though the head circumference attained at a specified PMA was greater and the time to regain birth weight was less in infants given high volume feeds with fortified human milk or preterm formula, the available data is limited by a low‐certainty evidence for both the outcomes.
The major concern while giving high volume feeds is the risk of NEC. Though the meta‐analysis did not find a difference in the incidence of NEC between the groups, we are unable to draw any conclusion since the certainty of evidence was very low for this outcome. Similarly, the available data were insufficient to comment on the effect of high volume feeds on all the other important clinical outcomes.
Quality of the evidence
For the comparison of high versus standard volumes of fortified human milk or preterm formula, the certainty of evidence was moderate for rate of weight gain and weight at a specified postmenstrual age. The evidence was downgraded for serious imprecision due to small sample size. The evidence was not downgraded for lack of masking, as weight is an objective outcome. The certainty of evidence was low for linear growth, head growth, length and head circumference at a specified postmenstrual age. The evidence was downgraded for serious imprecision due to small sample size and serious risk of bias due to lack of masking. Though length and head circumference are objective outcomes, the outcome was downgraded for lack of masking, as the measurement is observer‐dependent and prone to bias.
The certainty of evidence was low for extrauterine growth restriction at discharge, downgraded for small sample size and lack of masking. Though I2 was 60% for extrauterine growth restriction, the outcome was not downgraded for inconsistency, since the heterogeneity was due to difference between small and large beneficial effects. The certainty of evidence was very low for NEC, downgraded for serious risk of bias due to lack of masking and very serious imprecision due to small sample size and a wide confidence interval.
For the comparison of high versus standard volumes of unfortified human milk or term formula, the certainty of evidence was moderate for weight gain during hospital stay, downgraded for serious imprecision due to small sample size. The certainty of evidence was very low for necrotising enterocolitis, downgraded for very serious imprecision due to small sample size and a wide confidence interval and serious risk of bias due to lack of masking.
Potential biases in the review process
We found only three trials for inclusion in this review. Although we conducted a comprehensive search, including a search of conference proceedings, we could not exclude fully the possibility of publication bias because we do not know whether other published (but not indexed) or unpublished trials have been conducted.
NT was the principal investigator in one included study in the review (Thomas 2012). However, TA performed the 'Risk of bias' assessment and data extraction for the trial.
Agreements and disagreements with other studies or reviews
We are not aware of other systematic reviews on the use of high volume feeds to promote growth in preterm or low birth weight infants.
Zecca 2014 showed that proactive feeding and higher volume feeds (200 versus 170 mL/kg/day) improve weight and length z‐scores at discharge in small‐for‐gestational‐age infants. Klingenberg 2019 showed that infants who received high volume feeds with ≥ 180 mL/kg/day had a lower rate of extrauterine growth restriction at discharge (14%) and lower risk of cerebral palsy (9%); the incidence of both was less compared to incidence data reported in other studies. Lewis 1984 showed that high volume feeds with ~ 250 mL/kg/day of unfortified human milk or term formula achieves a weight gain comparable to in utero growth in preterm infants. Valman 1974 compared 230 versus 180 mL/kg/day feeds and showed that high volume feeds improve weight gain due to calorie, nitrogen and fat retention and not due to fluid accumulation.
Thus, the results of other studies were consistent with those of our meta‐analysis. High volume feeds improves in‐hospital weight gain. Data were insufficient to draw conclusions on other growth and clinical outcomes.
Authors' conclusions
Implications for practice.
High volume feeds (≥ 180 mL/kg/day of fortified human milk or preterm formula, or ≥ 200 mL/kg/day of unfortified human milk or term formula) probably improves weight gain during hospital stay. The available data were inadequate to draw conclusions on the effect of high volume feeds on other growth outcomes such as linear and head growth during hospital stay and post‐discharge growth, or clinical outcomes such as NEC, feed interruption episodes, PDA requiring treatment, chronic lung disease, duration of hospital stay, all‐cause mortality before discharge and long‐term neurodevelopmental outcomes.
Implications for research.
A large RCT is needed to assess whether high volume versus standard volume enteral feeds improve important clinical outcomes for preterm or LBW infants. Such a trial should assess weight and linear and head growth, post‐discharge growth, neurodevelopmental outcomes, and risk of potential complications of high‐volume enteral feeds. A trial of this intervention may be regarded as a research priority, since the incidence of extrauterine growth restriction in VLBW or ELBW infants has not improved much despite multi‐nutrient fortification of feeds in high‐income countries. Further, multi‐nutrient fortifiers are less frequently used in low‐ and middle‐income countries due to cost constraints, and high volume feeds may be a suitable alternative to ensure adequate nutrition in preterm or low birth weight infants in such countries.
What's new
| Date | Event | Description |
|---|---|---|
| 20 November 2020 | New search has been performed | We searched the literature in June 2020. We included two new studies (Kuschel 2000; Travers 2020). |
| 20 November 2020 | New citation required and conclusions have changed | High volume feeds probably improves weight gain during hospital stay (moderate certainty of evidence). There is insufficient data on other growth and clinical outcomes. |
History
Protocol first published: Issue 10, 2016 Review first published: Issue 9, 2017
Acknowledgements
We would like to thank Cochrane Neonatal: Colleen Ovelman, Managing Editor, Jane Cracknell, Assistant Managing Editor, Roger Soll, Co‐coordinating editor, and William McGuire, Co‐coordinating Editor, who provided editorial and administrative support.
Melissa Harden, Information Specialist, Centre for Reviews and Dissemination, York, UK, designed and ran the literature searches.
Sarah Hodgkinson and William McGuire peer reviewed and offered feedback for this update.
We are grateful to Drs Carl Kuschel and Colm Travers for providing further data and details of their trials.
Appendices
Appendix 1. Current search methods
The RCT filters have been created using Cochrane's highly sensitive search strategies for identifying randomised trials (Higgins 2020). Melissa Harden, Information Specialist, Centre for Reviews and Dissemination, York, UK, designed and ran the literature searches.
CENTRAL
Issue 6 of 12, June 2020
#1 MeSH descriptor: [Infant, Newborn] explode all trees 15600
#2 MeSH descriptor: [Premature Birth] this term only 1421
#3 (neonat* or neo next nat*):ti,ab,kw 21423
#4 (newborn* or new next born* or newly next born*):ti,ab,kw 26658
#5 (preterm or preterms or pre next term or pre next terms):ti,ab,kw 13136
#6 (preemie* or premie or premies):ti,ab,kw 50
#7 (prematur* near/3 (birth* or born or deliver*)):ti,ab,kw 2771
#8 (low near/3 (birthweight* or birth next weight*)):ti,ab,kw 5336
#9 (lbw or vlbw or elbw):ti,ab,kw 1630
#10 infan*:ti,ab,kw 60772
#11 (baby or babies):ti,ab,kw 8137
#12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 76894
#13 MeSH descriptor: [Enteral Nutrition] this term only 1802
#14 ((enteral* or enteric*) near/2 (nutrition or feed* or fed*)):ti,ab,kw 5593
#15 ((enteral* or enteric*) near/2 (milk or breastmilk or formula*)):ti,ab,kw 579
#16 ((enteral* or enteric*) near/2 stimulat*):ti,ab,kw 18
#17 ((enteral* or enteric*) near/2 fast*):ti,ab,kw 26
#18 ((oral or sip or tube) near/2 (feed* or fed)):ti,ab,kw 2335
#19 ((nasogastric or gastrostomy or jejunostomy) near/2 tube*):ti,ab,kw 1775
#20 ((high* or increas* or increment* or excess* or full* or standard* or routine* or conventional* or conservative* or moderate* or low* or minimal* or decreas* or reduc* or less* or small*) near/2 (volume* or quantit* or amount*) near/2 (feed* or fed or milk or breastmilk or formula*)):ti,ab,kw 179
#21 ((advanc* or aggressive* or fast or faster or rapid* or slow*) near/3 feed*):ti,ab,kw 394
#22 ((advanc* or aggressive* or fast or faster or rapid* or slow*) near/3 volume*):ti,ab,kw 292
#23 (speed* near/4 (feed* or volume*)):ti,ab,kw 133
#24 (trophic near/2 (feed* or fed or nutrition)):ti,ab,kw 44
#25 ((hypocaloric or (hypo next caloric)) near/2 (feed* or fed or nutrition)):ti,ab,kw 95
#26 ((gut or gastrointestinal or GI) near/2 (priming or prime*)):ti,ab,kw 15
#27 #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 9238
#28 #12 and #27 in Trials 1987
Ovid MEDLINE(R)
ALL <1946 to June 05, 2020>
1 exp Infant, Newborn/ (604669)
2 Premature Birth/ (13651)
3 (neonat$ or neo nat$).ti,ab. (262665)
4 (newborn$ or new born$ or newly born$).ti,ab. (165343)
5 (preterm or preterms or pre term or pre terms).ti,ab. (74485)
6 (preemie$ or premie or premies).ti,ab. (169)
7 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (15620)
8 (low adj3 (birthweight$ or birth weight$)).ti,ab. (34515)
9 (lbw or vlbw or elbw).ti,ab. (8389)
10 infan$.ti,ab. (434517)
11 (baby or babies).ti,ab. (69811)
12 or/1‐11 (1052354)
13 Enteral Nutrition/ (19588)
14 ((enteral$ or enteric$) adj2 (nutrition or feed$ or fed$)).ti,ab. (14396)
15 ((enteral$ or enteric$) adj2 (milk or breastmilk or formula$)).ti,ab. (1278)
16 ((enteral$ or enteric$) adj2 stimulat$).ti,ab. (188)
17 ((enteral$ or enteric$) adj2 fast$).ti,ab. (84)
18 ((oral or sip or tube) adj2 (feed$ or fed)).ti,ab. (11231)
19 ((nasogastric or gastrostomy or jejunostomy) adj2 tube$).ti,ab. (8613)
20 ((high$ or increas$ or increment$ or excess$ or full$ or standard$ or routine$ or conventional$ or conservative$ or moderate$ or low$ or minimal$ or decreas$ or reduc$ or less$ or small$) adj2 (volume$ or quantit$ or amount$) adj2 (feed$ or fed or milk or breastmilk or formula$)).ti,ab. (1264)
21 ((advanc$ or aggressive$ or fast or faster or rapid$ or slow$) adj3 feed$).ti,ab. (3154)
22 ((advanc$ or aggressive$ or fast or faster or rapid$ or slow$) adj3 volume$).ti,ab. (3835)
23 (speed$ adj4 (feed$ or volume$)).ti,ab. (1758)
24 (trophic adj2 (feed$ or fed or nutrition)).ti,ab. (195)
25 ((hypocaloric or hypo caloric) adj2 (feed$ or fed or nutrition)).ti,ab. (230)
26 ((gut or gastrointestinal or GI) adj2 (priming or prime$)).ti,ab. (81)
27 or/13‐26 (47698)
28 12 and 27 (7611)
29 randomized controlled trial.pt. (507002)
30 controlled clinical trial.pt. (93705)
31 randomized.ab. (481626)
32 placebo.ab. (208275)
33 drug therapy.fs. (2208868)
34 randomly.ab. (334529)
35 trial.ab. (507758)
36 groups.ab. (2053908)
37 or/29‐36 (4718557)
38 exp animals/ not humans.sh. (4705042)
39 37 not 38 (4092803)
40 28 and 39 (2165)
CINAHL via EBSCOhost
Inception to 08 June 2020
| # | Query | Results |
| S1 | MH "Infant, Newborn+" | 145,766 |
| S2 | MH "Infant, Low Birth Weight+" | 15,009 |
| S3 | MH "Infant, Premature" | 24,076 |
| S4 | TI ( neonat* or neo‐nat* ) OR AB ( neonat* or neo‐nat* ) | 69,698 |
| S5 | TI ( newborn* or new‐born* or newly N1 born* ) OR AB ( newborn* or new‐born* or newly N1 born* ) | 32,800 |
| S6 | TI ( preterm or preterms or pre‐term or pre‐terms ) OR AB ( preterm or preterms or pre‐term or pre‐terms) | 34,099 |
| S7 | TI ( preemie* or premie or premies ) OR AB ( preemie* or premie or premies ) | 329 |
| S8 | TI ( prematur* N3 (birth* or born or deliver*) ) OR AB ( prematur* N3 (birth* or born or deliver*) ) | 4,895 |
| S9 | TI ( low N3 (birthweight* or birth‐weight*) ) OR AB ( low N3 (birthweight* or birth‐weight*) ) | 13,011 |
| S10 | TI ( lbw or vlbw or elbw ) OR AB ( lbw or vlbw or elbw ) | 3,565 |
| S11 | TI infan* OR AB infan* | 120,057 |
| S12 | TI ( baby or babies ) OR AB ( baby or babies ) | 35,040 |
| S13 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 | 272,581 |
| S14 | (MH "Randomized Controlled Trials") | 120,017 |
| S15 | (MH "Double‐Blind Studies") | 50,142 |
| S16 | (MH "Single‐Blind Studies") | 15,328 |
| S17 | (MH "Random Assignment") | 68,436 |
| S18 | (MH "Pretest‐Posttest Design") | 51,092 |
| S19 | (MH "Cluster Sample") | 5,269 |
| S20 | TI randomised OR randomized | 110,769 |
| S21 | AB random* | 333,960 |
| S22 | TI trial | 112,830 |
| S23 | MH (sample size) AND AB (assigned OR allocated OR control) | 4,850 |
| S24 | MH (placebos) | 13,734 |
| S25 | PT (randomized controlled trial) | 132,192 |
| S26 | AB (control W5 group) | 120,174 |
| S27 | MH (crossover design) OR MH (comparative studies) | 354,078 |
| S28 | AB (cluster W3 RCT) | 372 |
| S29 | S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 | 820,523 |
| S30 | (MH "Control Group") | 14,106 |
| S31 | TI ( group or groups ) OR AB ( group or groups ) | 808,208 |
| S32 | TI assign* OR AB assign* | 82,495 |
| S33 | (MH "Multicenter Studies") | 245,030 |
| S34 | TI ( multicentre* or multi‐centre* or multicenter* or multi‐center* ) OR AB ( multicentre* or multi‐centre* or multicenter* or multi‐center* ) | 53,577 |
| S35 | (MH "Controlled Before‐After Studies") | 167 |
| S36 | TI before N3 after OR AB before N3 after | 83,436 |
| S37 | S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 | 1,078,716 |
| S38 | S29 OR S37 | 1,387,574 |
| S39 | (MH "Enteral Nutrition") | 10,163 |
| S40 | TI ( (enteral* or enteric*) N2 (nutrition or feed* or fed*) ) OR AB ( (enteral* or enteric*) N2 (nutrition or feed* or fed*) ) | 7,253 |
| S41 | TI ( (enteral* or enteric*) N2 (milk or breastmilk or formula*) ) OR AB ( (enteral* or enteric*) N2 (milk or breastmilk or formula*) ) | 616 |
| S42 | TI ( (enteral* or enteric*) N2 stimulat* ) OR AB ( (enteral* or enteric*) N2 stimulat* ) | 37 |
| S43 | TI ( (enteral* or enteric*) N2 fast* ) OR AB ( (enteral* or enteric*) N2 fast* ) | 28 |
| S44 | TI ( (oral or sip or tube) N2 (feed* or fed) ) OR AB ( (oral or sip or tube) N2 (feed* or fed) ) | 5,259 |
| S45 | TI ( (nasogastric or gastrostomy or jejunostomy) N2 tube* ) OR AB ( (nasogastric or gastrostomy or jejunostomy) N2 tube* ) | 3,288 |
| S46 | TI ( (high* or increas* or increment* or excess* or full* or standard* or routine* or conventional* or conservative* or moderate* or low* or minimal* or decreas* or reduc* or less* or small*) N2 (volume* or quantit* or amount*) N2 (feed* or fed or milk or breastmilk or formula*) ) OR AB ( (high* or increas* or increment* or excess* or full* or standard* or routine* or conventional* or conservative* or moderate* or low* or minimal* or decreas* or reduc* or less* or small*) N2 (volume* or quantit* or amount*) N2 (feed* or fed or milk or breastmilk or formula*) ) | 340 |
| S47 | TI ( (advanc* or aggressive* or fast or faster or rapid* or slow*) N3 feed* ) OR AB ( (advanc* or aggressive* or fast or faster or rapid* or slow*) N3 feed* ) | 756 |
| S48 | TI ( (advanc* or aggressive* or fast or faster or rapid* or slow*) N3 volume* ) OR AB ( (advanc* or aggressive* or fast or faster or rapid* or slow*) N3 volume* ) | 924 |
| S49 | TI ( speed* N4 (feed* or volume*) ) OR AB ( speed* N4 (feed* or volume*) ) | 271 |
| S50 | TI ( trophic N2 (feed* or fed or nutrition) ) OR AB ( trophic N2 (feed* or fed or nutrition) ) | 68 |
| S51 | TI ( (hypocaloric or hypo‐caloric) N2 (feed* or fed or nutrition) ) OR AB ( (hypocaloric or hypo‐caloric) N2 (feed* or fed or nutrition) ) | 90 |
| S52 | TI ( (gut or gastrointestinal or GI) N2 (priming or prime*) ) OR AB ( (gut or gastrointestinal or GI) N2 (priming or prime*) ) | 17 |
| S53 | S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 | 19,324 |
| S54 | S13 AND S53 | 3,327 |
| S55 | S38 AND S54 | 1,401 |
| S56 | TI (rat or rats or mouse or mice or swine or porcine or murine or pig or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs) | 96,133 |
| S57 | S55 NOT S56 | 1,367 |
Embase via Ovid
<1974 to 2020 June 05>
1 newborn/ (523847)
2 prematurity/ (101320)
3 exp low birth weight/ (61651)
4 (neonat$ or neo nat$).ti,ab. (340459)
5 (newborn$ or new born$ or newly born$).ti,ab. (192461)
6 (preterm or preterms or pre term or pre terms).ti,ab. (104368)
7 (preemie$ or premie or premies).ti,ab. (261)
8 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (21473)
9 (low adj3 (birthweight$ or birth weight$)).ti,ab. (43508)
10 (lbw or vlbw or elbw).ti,ab. (11455)
11 infan$.ti,ab. (495460)
12 (baby or babies).ti,ab. (96465)
13 or/1‐12 (1135142)
14 enteric feeding/ (31737)
15 ((enteral$ or enteric$) adj2 (nutrition or feed$ or fed$)).ti,ab. (23134)
16 ((enteral$ or enteric$) adj2 (milk or breastmilk or formula$)).ti,ab. (1742)
17 ((enteral$ or enteric$) adj2 stimulat$).ti,ab. (249)
18 ((enteral$ or enteric$) adj2 fast$).ti,ab. (99)
19 ((oral or sip or tube) adj2 (feed$ or fed)).ti,ab. (17060)
20 ((nasogastric or gastrostomy or jejunostomy) adj2 tube$).ti,ab. (13270)
21 ((high$ or increas$ or increment$ or excess$ or full$ or standard$ or routine$ or conventional$ or conservative$ or moderate$ or low$ or minimal$ or decreas$ or reduc$ or less$ or small$) adj2 (volume$ or quantit$ or amount$) adj2 (feed$ or fed or milk or breastmilk or formula$)).ti,ab. (1526)
22 ((advanc$ or aggressive$ or fast or faster or rapid$ or slow$) adj3 feed$).ti,ab. (3756)
23 ((advanc$ or aggressive$ or fast or faster or rapid$ or slow$) adj3 volume$).ti,ab. (5166)
24 (speed$ adj4 (feed$ or volume$)).ti,ab. (2183)
25 (trophic adj2 (feed$ or fed or nutrition)).ti,ab. (255)
26 ((hypocaloric or hypo caloric) adj2 (feed$ or fed or nutrition)).ti,ab. (282)
27 ((gut or gastrointestinal or GI) adj2 (priming or prime$)).ti,ab. (107)
28 or/14‐27 (71577)
29 13 and 28 (10955)
30 randomized controlled trial/ (606861)
31 controlled clinical trial/ (464630)
32 random$.ti,ab. (1541142)
33 randomization/ (87000)
34 intermethod comparison/ (261115)
35 placebo.ti,ab. (307083)
36 (compare or compared or comparison).ti. (509950)
37 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (2117373)
38 (open adj label).ti,ab. (79514)
39 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. (232612)
40 double blind procedure/ (173436)
41 parallel group$1.ti,ab. (25603)
42 (crossover or cross over).ti,ab. (105321)
43 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. (330349)
44 (assigned or allocated).ti,ab. (388709)
45 (controlled adj7 (study or design or trial)).ti,ab. (349049)
46 (volunteer or volunteers).ti,ab. (246875)
47 human experiment/ (498692)
48 trial.ti. (300650)
49 or/30‐48 (5021873)
50 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ (1064124)
51 Animal experiment/ not (human experiment/ or human/) (2246211)
52 50 or 51 (2294010)
53 49 not 52 (4709817)
54 29 and 53 (2628)
Maternity & Infant Care Database (MIDIRS) via Ovid
<1971 to April 2020>
1 ((enteral$ or enteric$) adj2 (nutrition or feed$ or fed$)).mp. (909)
2 ((enteral$ or enteric$) adj2 (milk or breastmilk or formula$)).mp. (18)
3 ((enteral$ or enteric$) adj2 stimulat$).mp. (2)
4 ((enteral$ or enteric$) adj2 fast$).mp. (6)
5 ((oral or sip or tube) adj2 (feed$ or fed)).mp. (530)
6 ((nasogastric or gastrostomy or jejunostomy) adj2 tube$).mp. (150)
7 ((high$ or increas$ or increment$ or excess$ or full$ or standard$ or routine$ or conventional$ or conservative$ or moderate$ or low$ or minimal$ or decreas$ or reduc$ or less$ or small$) adj2 (volume$ or quantit$ or amount$) adj2 (feed$ or fed or milk or breastmilk or formula$)).mp. (111)
8 ((advanc$ or aggressive$ or fast or faster or rapid$ or slow$) adj3 feed$).mp. (158)
9 ((advanc$ or aggressive$ or fast or faster or rapid$ or slow$) adj3 volume$).mp. (58)
10 (speed$ adj4 (feed$ or volume$)).mp. (12)
11 (trophic adj2 (feed$ or fed or nutrition)).mp. (26)
12 ((hypocaloric or hypo caloric) adj2 (feed$ or fed or nutrition)).mp. (3)
13 ((gut or gastrointestinal or GI) adj2 (priming or prime$)).mp. (7)
14 or/1‐13 (1622)
ClinicalTrials.gov
https://clinicaltrials.gov/ct2/
12 June 2020
362 Studies found for: enteral OR enteric | Infant OR Preterm OR pre‐term OR premature OR prematurity OR preemie OR premie OR low birth weight OR low birthweight OR LBW OR VLBW OR ELBW OR neonate OR neo‐nate OR newborn
Appendix 2. Previous search methods
Previous search methods for Abiramalatha 2017
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2) in the Cochrane Library; MEDLINE (1946 to November 2016); Embase (1974 to November 2016); the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to November 2016); and Maternity and Infant Care (1971 to November 2016). We limited search outputs with relevant search filters for clinical trials, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We did not apply any language restrictions.
We searched ClinicalTrials.gov, Current Controlled Trials, and the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/) for completed or ongoing trials.
Searching other resources
We examined reference lists in related reviews, included, and excluded studies. We searched the proceedings of annual meetings of the Pediatric Academic Societies (1993 to 2016), the European Society for Paediatric Research (1995 to 2016), the Royal College of Paediatrics and Child Health (2000 to 2017), and the Perinatal Society of Australia and New Zealand (2000 to 2016). Trials reported only as abstracts were eligible if sufficient information was available from the report, or from contact with trial authors, to fulfil inclusion criteria.
We used the following search terms
De‐duplicated search results from: PubMed, Embase, CINAHL, Cochrane Library (Search date: No limit – November 14, 2016)
Search terms: breast milk OR diet supplementation OR ((fortif* OR supplemented OR supplementation) near ((human OR breast OR expressed) NEAR milk))
Plus the following database‐specific terms:
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Appendix 3. 'Risk of bias' tool
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. High versus standard volume of fortified human milk or preterm formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Weight gain during hospital stay (g/kg/day) | 2 | 271 | Mean Difference (IV, Fixed, 95% CI) | 2.58 [1.41, 3.76] |
| 1.2 Linear growth during hospital stay (cm/week) | 2 | 271 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.02, 0.13] |
| 1.3 Head growth during hospital stay (cm/week) | 2 | 271 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.04, 0.09] |
| 1.4 Extrauterine growth restriction at discharge | 2 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.50, 1.02] |
| 1.5 Necrotising enterocolitis | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.12, 4.51] |
| 1.6 Feed interruption episodes | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.12, 4.25] |
| 1.7 Time to regain birth weight (days) | 2 | 271 | Mean Difference (IV, Fixed, 95% CI) | ‐1.23 [‐2.36, ‐0.10] |
| 1.8 Weight at a specified postmenstrual age (g) | 2 | 271 | Mean Difference (IV, Fixed, 95% CI) | 152.10 [71.67, 232.53] |
| 1.9 Length at a specified postmenstrual age (cm) | 2 | 271 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.04, 1.04] |
| 1.10 Head circumference at a specified postmenstrual age (cm) | 2 | 271 | Mean Difference (IV, Fixed, 95% CI) | 0.49 [0.15, 0.83] |
| 1.11 PDA requiring treatment | 2 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.28, 2.12] |
| 1.12 Chronic lung disease | 2 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.57, 1.81] |
| 1.13 All‐cause mortality before discharge | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.71] |
| 1.14 Duration of hospital stay (days) | 2 | 271 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.54, 5.54] |
| 1.15 Weight < 10th percentile at 12 months' corrected age | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.23, 2.15] |
| 1.16 Length < 10th percentile at 12 months' corrected age | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.08, 1.55] |
| 1.17 Head circumference < 10th percentile at 12 months' corrected age | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.11] |
| 1.18 Neurodevelopmental impairment at 12 months' corrected age | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.15, 1.84] |
1.14. Analysis.

Comparison 1: High versus standard volume of fortified human milk or preterm formula, Outcome 14: Duration of hospital stay (days)
Comparison 2. High versus standard volume of unfortified human milk or term formula.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Weight gain during hospital stay (g/kg/day) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [2.71, 9.69] |
| 2.2 Necrotising enterocolitis | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.78] |
| 2.3 Time to regain birth weight (days) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐2.61, 1.61] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kuschel 2000.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: infants less than 30 weeks' gestation at birth Exclusion criteria: babies who did not survive until reaching full enteral feeds, babies with congenital malformations that are associated with poor postnatal growth |
|
| Interventions | Intervention: 200 mL/kg/day enteral feeds Control: 150 mL/kg/day feeds Randomisation was done when baby was approaching 150 mL/kg per day feed volume. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Setting: Australia Study period: 1995 to 1996 Infants with < 1500 grams birth weight received human milk fortified with human milk fortifier or preterm formula. The fortification was continued until the infant reached 1800 to 2000 grams. If a minimum weight gain of 8 g/kg/day was not achieved, feed volume was increased beyond 150 mL/kg/day in the control group and caloric supplement was added in the intervention group. If infants in the intervention group developed feed intolerance, feed volume was decreased to the maximum tolerated volume. If feed volume was decreased, every effort was made to increase the volume back to 200 mL/kg/day. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal communication: (quote:) "computer‐generated random sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Clinicians and nursing staff were not blinded to the intervention". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Anthropometric measurements were performed by investigators unmasked to the infant’s volume allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All babies enrolled in the study were accounted for. |
| Selective reporting (reporting bias) | Low risk | Personal communication (quote:) "all proposed outcomes reported" |
| Other bias | Low risk | None |
Thomas 2012.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 64 newborn VLBW infants were enrolled when they achieved 200 mL/kg/d enteral feeds. Both appropriate‐for‐gestational‐age and small‐for‐gestational‐age infants were included. Only birth weight (not gestational age) criteria were used for enrolment. | |
| Interventions | Intervention arm (N = 32): *feeds were graded up by 20 mL/kg/d up to 300 mL/kg/d Control arm (N = 32): *feeds were continued at 200 mL/kg/d Babies in both intervention and control arms were given expressed breast milk along with individual micronutrient supplements for calcium, iron, and vitamins. Multi‐nutrient milk fortifiers, which supplement calories and proteins, were not used. Feeds were given by nasogastric tube at 2‐ to 3‐hourly intervals. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Setting: India Study period: 2010 *12 infants in the high‐volume group did not achieve the targeted 300 mL/kg/d (although all achieved feed volumes > 250 mL/kg/d), and 6 infants in the standard‐volume group received higher volumes than targeted (up to 215 mL/kg/d), but analyses were done by (quote:) "intention‐to‐treat". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal communication (quote:) "computer‐generated random sequence" |
| Allocation concealment (selection bias) | Low risk | Personal communication (quote:) "sealed opaque envelopes opened by the principal investigator only at the time of allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 (of 64) randomised infants were removed from the study by parents, did not complete the intervention, and were not included in analyses. |
| Selective reporting (reporting bias) | Low risk | Personal communication (quote:) "all proposed outcomes reported" |
| Other bias | Low risk | None |
Travers 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Inclusion criteria: infants born at < 32 weeks' gestation with birth weight of 1001 to 2500 grams and had achieved a feeding volume of > 120 mL/kg/day Exclusion criteria: haemodynamically significant PDA, NEC ≥ stage 2, known gastrointestinal or neurological malformation, terminal illness, or decision to withdraw or limit support. |
|
| Interventions | Intervention: high volume feeds of 180 to 200 mL/kg/day Control: standard volume feeds of 140 to 160 mL/kg/day |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Setting: USA Study period: 2015‐8 Fortified human milk or preterm formula was used as enteral feeds. Feeds were advanced at the rate of 20 to 30 mL/kg/day. Additional increases in caloric density of feedings was done in infants with inadequate growth in either group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random‐block sequences of 2, 4, 6 and 8" |
| Allocation concealment (selection bias) | Low risk | Quote: "placed in sequentially numbered, opaque, sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "masking could not be performed as staff were aware of volumes ordered and received". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "masking: none (open label) in the study protocol" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All babies enrolled in the study were accounted for. |
| Selective reporting (reporting bias) | Low risk | Quote: "All proposed outcomes reported". Trial protocol available |
| Other bias | Low risk | None |
CLD: chronic lung disease NEC: necrotising enterocolitis PDA: patent ductus arteriosus PMA: postmenstrual age RCT: randomised controlled trial VLBW: very low birth weight
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Klingenberg 2019 | Retrospective observational study |
| Lewis 1984 | Retrospective observational study |
| Valman 1974 | Cohort study |
| Zecca 2014 | Randomised trial on unfortified human milk at 170 versus 200 mL/kg/day, both being standard volume feeds according to our protocol. |
Differences between protocol and review
2020
We have done the following changes to the previous publication of the review (Abiramalatha 2017).
We compared high versus standard volume of 'fortified human milk or preterm formula' and 'unfortified human milk or term formula' in two separate comparisons in this updated review. The volume cut‐offs for high and standard volume feeds for each comparison were chosen based on the prevailing practice and nutritional requirements of preterm infants, as described in the background section.
The objectives of this updated review have been modified. In infants who were fed fortified human milk or preterm formula, high and standard volume feeds were defined as > 180 mL/kg/day and ≤ 180 mL/kg/day, respectively. In infants who were fed unfortified human milk or term formula, high and standard volume feeds were defined as > 200 mL/kg/day and ≤ 200 mL/kg/day, respectively.
-
Some of the outcomes of this review have been modified as follows:
-
Changes to primary outcomes:
"Z‐scores of weight, length and head circumference" have been changed to "growth measures namely weight, length and head circumference, measured at a specified postmenstrual age prior to discharge" and this outcome was moved to secondary outcomes;
Growth measures following discharge from hospital to latest follow‐up: moved to secondary outcomes;
"Number of infants with feed intolerance: vomiting, excessive gastric residual volumes (defined by investigators), or abdominal distension that results in reduction or cessation of enteral feeding)" has been changed to "Proportion of infants with feed interruption episodes (lasting ≥ 12 hours)".
We have added one new secondary outcome: Time to regain birth weight (days).
-
In subgroup analysis, "Very preterm (< 32 weeks' gestation) or VLBW (< 1500 grams) infants versus preterm infants born at between 32 and 36 weeks' gestation or with birth weight 1500 to 2499 grams": changed to "Based on gestational age: < 28 weeks, 28 to 31 weeks, ≥ 32 weeks and 2. Based on birth weight: < 1000 grams, 1000 to 1499 grams, ≥ 1500 grams".
We updated the 'Risk of bias' and the certainty of the evidence.
For the 2020 update, we developed a new search strategy. The previous search methods are available in Appendix 2.
We added new external sources of support.
Contributions of authors
TA (along with NT and ST) revised the previous protocol (Abiramalatha 2016). TA, NT and ST revised the previous published review (Abiramalatha 2017).
For this review update, TA and ST screened search outputs, assessed study eligibility, and extracted and synthesised data. TA and ST assessed risk of bias across key domains and undertook GRADE assessment. All review authors revised the final review update.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK
This report is independent research funded by a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
-
The Gerber Foundation, USA
Editorial support for this review, as part of a suite of preterm nutrition reviews, has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private, 501(c)(3) foundation not related to Gerber Products Company in any way.
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
TA has no interest to declare.
NT was the principal investigator in one study included in this review (Thomas 2012). However, TA performed the 'Risk of bias' assessment and data extraction for the trial. NT received no funding for Thomas 2012.
ST has no interest to declare.
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent from the Gerber Products Company. The grantor has no input on the content of the review or the editorial process (see Sources of support).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Kuschel 2000 {published and unpublished data}
- Kuschel CA, Evans N, Askie L, Bredemeyer S, Nash J, Polverino J. A randomized trial of enteral feeding volumes in infants born before 30 weeks' gestation. Journal of Paediatrics and Child Health 2000;36(6):581-6. [DOI: 10.1046/j.1440-1754.2000.00577.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thomas 2012 {published and unpublished data}
- Thomas N, Cherian A, Santhanam S, Jana AK. A randomized control trial comparing two enteral feeding volumes in very low birth weight babies. Journal of Tropical Pediatrics 2012;58(1):55-8. [DOI: 10.1093/tropej/fmr011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Travers 2020 {published and unpublished data}
References to studies excluded from this review
Klingenberg 2019 {published data only}
- Klingenberg C, Kragh FM, Isaksen CE, Elde C, Nilsen T, Torgersen M, et al. Growth and neurodevelopment in very preterm infants receiving a high enteral volume-feeding regimen – a population-based cohort study. Journal of Maternal-Fetal & Neonatal Medicine 2019;32(10):1664-72. [DOI: 10.1080/14767058.2017.1414796] [PMID: ] [DOI] [PubMed]
Lewis 1984 {published data only}
- Lewis MA, Smith BA. High volume milk feeds for preterm infants. Archives of Disease in Childhood 1984;59(8):779-81. [DOI: 10.1136/adc.59.8.779] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Valman 1974 {published data only}
- Valman HB, Aikens R, David-Reed Z, Garrow JS. Retention of nitrogen, fat, and calories in infants of low birth weight on conventional and high-volume feeds. British Medical Journal 1974;3(5926):319-20. [DOI: 10.1136/bmj.3.5926.319] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zecca 2014 {published data only}
- Zecca E, Costa S, Barone G, Giordano L, Zecca C, Maggio L. Proactive enteral nutrition in moderately preterm small for gestational age infants: a randomised clinical trial. Journal of Pediatrics 2014;165(6):1135-9.e1. [DOI: 10.1016/j.jpeds.2014.08.065] [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
AAP 2004
- American Academy of Pediatrics Committee on Nutrition. Nutritional needs of preterm infants. In: Kleinman RE, editors(s). Pediatric Nutrition Handbook. Elk Grove Village, IL: American Academy of Pediatrics, 2004:23–54. [Google Scholar]
Agostoni 2010
- Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al, ESPGHAN Committee on Nutrition. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):85-91. [DOI: 10.1097/MPG.0b013e3181adaee0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bertino 2009
- Bertino E, Giuliani F, Prandi G, Coscia A, Martano C, Fabris C. Necrotizing enterocolitis: risk factor analysis and role of gastric residuals in very low birth weight infants. Journal of Pediatric Gastroenterology and Nutrition 2009;48(4):437-42. [DOI: 10.1097/mpg.0b013e31817b6dbe] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brandt 2003
- Brandt I, Sticker EJ, Lentze MJ. Catch-up growth of head circumference of very low birth weight, small for gestational age preterm infants and mental development to adulthood. Journal of Pediatrics 2003;142(5):463-8. [DOI: 10.1067/mpd.2003.149] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brandt 2005
- Brandt I, Sticker EJ, Gausche R, Lentze MJ. Catch-up growth of supine length/height of very low birth weight, small for gestational age preterm infants to adulthood. Journal of Pediatrics 2005;147(5):662-8. [DOI: 10.1067/mpd.2003.149] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brown 2020
- Brown JV, Lin L, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD000343. [DOI: 10.1002/14651858.CD000343.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chawla 2008
- Chawla D, Agarwal R, Deorari AK, Paul VK. Fluid and electrolyte management in term and preterm neonates. Indian Journal of Pediatrics 2008;75(3):255-9. [DOI: 10.1007/s12098-008-0055-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Clark 2003
- Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003;111(5 Pt 1):986-90. [DOI: 10.1542/peds.111.5.986] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cooke 2004
- Cooke RJ, Ainsworth SB, Fenton AC. Postnatal growth retardation: a universal problem in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89(5):F428-30. [DOI: 10.1136/adc.2001.004044] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cormack 2013
- Cormack B, Sinn J, Lui K, Tudehope D. Australasian neonatal intensive care enteral nutrition survey: implications for practice. Journal of Paediatrics and Child Health 2013;49(4):E340-7. [DOI: 10.1111/jpc.12016] [PMID: ] [DOI] [PubMed] [Google Scholar]
CPS 1995
- Nutrition Committee, Canadian Paediatric Society. Nutrition needs and feeding of premature infants. Canadian Medical Association Journal 1995;152(11):1765-85. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Dusick 2003
- Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Seminars in Perinatology 2003;27(4):302-10. [DOI: 10.1016/s0146-0005(03)00044-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dutta 2015
- Dutta S, Singh B, Chessell L, Wilson J, Janes M, McDonald K, et al. Guidelines for feeding very low birth weight infants. Nutrients 2015;7(1):423-42. [DOI: 10.3390/nu7010423] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehrenkranz 1999
- Ehrenkranz RA, Younes N, Lemons JA, Fanaroff AA, Donovan EF, Wright LL, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics 1999;104(2 Pt 1):280-9. [DOI: 10.1542/peds.104.2.280] [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2007
- Embleton ND. Optimal protein and energy intakes in preterm infants. Early Human Development 2007;83(12):831-7. [DOI: 10.1016/j.earlhumdev.2007.10.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2013
- Embleton ND. Early nutrition and later outcomes in preterm infants. World Review of Nutrition and Dietetics 2013;106:26-32. [DOI: 10.1159/000342553] [PMID: ] [DOI] [PubMed] [Google Scholar]
Euser 2008
- Euser AM, De Wit CC, Finken MJ, Rijken M, Wit JM. Growth of preterm born children. Hormone Research 2008;70(6):319-28. [DOI: 10.1159/000161862] [PMID: ] [DOI] [PubMed] [Google Scholar]
Franz 2009
- Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics 2009;123(1):e101-9. [DOI: 10.1542/peds.2008-1352] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 10 May 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2014. Available at gradepro.org.
Hack 2003
- Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E. Growth of very low birth weight infants to age 20 years. Pediatrics 2003;112(1 Pt 1):e30-8. [DOI: 10.1542/peds.112.1.e30] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2012
- Higgins RD, Devaskar S, Hay WW Jr, Ehrenkranz RA, Greer FR, Kennedy K, et al. Executive summary of the workshop "Nutritional Challenges in the High Risk Infant". Journal of Pediatrics 2012;160(3):511-6. [DOI: 10.1016/j.jpeds.2011.12.024] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Horbar 2015
- Horbar JD, Ehrenkranz RA, Badger GJ, Edwards EM, Morrow KA, Soll RF, et al. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000-2013. Pediatrics 2015;136(1):e84-92. [DOI: 10.1542/peds.2015-0129] [PMID: ] [DOI] [PubMed] [Google Scholar]
Johnston 2012
- Johnston M, Landers S, Noble L, Szucs K, Viehmann L. Breastfeeding and the use of human milk. Pediatrics 2012;129(3):e827-41. [DOI: 10.1542/peds.2011-3552] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kler 2015
- Kler N, Thakur A, Modi M, Kaur A, Garg P, Soni A, et al. Human milk fortification in India. Nestle Nutrition Institute Workshop Series 2015;81:145-51. [DOI: 10.1159/000365904] [PMID: ] [DOI] [PubMed] [Google Scholar]
Klingenberg 2012
- Klingenberg C, Embleton ND, Jacobs SE, O'Connell LA, Kuschel CA. Enteral feeding practices in very preterm infants: an international survey. Archives of Disease in Childhood. Fetal and Neonatal Edition 2012;97(1):F56-61. [DOI: 10.1136/adc.2010.204123] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lapillonne 2013
- Lapillonne A, Griffin IJ. Feeding preterm infants today for later metabolic and cardiovascular outcomes. Journal of Pediatrics 2013;162(3 Suppl):S7-16. [DOI: 10.1016/j.jpeds.2012.11.048] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2020
- Lee LY, Jiun L, Niduvaje K, Seah SS, Atmawidjaja RW, Cheah F. Nutritional therapies in the neonatal intensive care unit and post‐natal growth outcomes of preterm very low birthweight Asian infants. Journal of Paediatrics and Child Health 2020;56(3):400-7. [DOI: 10.1111/jpc.14634] [PMID: ] [DOI] [PubMed]
Leppanen 2014
- Leppanen M, Lapinleimu H, Lind A, Matomaki J, Lehtonen L, Haataja L, et al, PIPARI Study Group. Antenatal and postnatal growth and 5-year cognitive outcome in very preterm infants. Pediatrics 2014;133(1):63-70. [DOI: 10.1542/peds.2013-1187] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lima 2014
- Lima PA, Carvalho MD, Costa AC, Moreira ME. Variables associated with extrauterine growth restriction in very low birth weight infants. Jornal de Pediatria 2014;90(1):22-7. [DOI: 10.1016/j.jped.2013.05.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
Neubauer 2013
- Neubauer V, Griesmaier E, Pehbock-Walser N, Pupp-Peglow U, Kiechl-Kohlendorfer U. Poor postnatal head growth in very preterm infants is associated with impaired neurodevelopment outcome. Acta Paediatrica 2013;102(9):883-8. [DOI: 10.1111/apa.12319] [PMID: ] [DOI] [PubMed] [Google Scholar]
Raban 2013
- Raban MS, Joolay Y, Horn AR, Harrison MC. Enteral feeding practices in preterm infants in South Africa. South African Journal of Child Health 2013;7:8-12. [DOI: 10.7196/SAJCH.503] [Google Scholar]
Review Manager 2020 [Computer program]
- Cochrane Collaboration Review Manager (RevMan). Version 5.4. Copenhagen: Cochrane Collaboration, 2020.
Sakurai 2008
- Sakurai M, Itabashi K, Sato Y, Hibino S, Mizuno K. Extrauterine growth restriction in preterm infants of gestational age ≤ 32 weeks. Pediatrics International 2008;50(1):70-5. [DOI: 10.1111/j.1442-200X.2007.02530.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sankar 2008
- Sankar MJ, Agarwal R, Mishra S, Deorari AK, Paul VK. Feeding of low birth weight infants. Indian Journal of Paediatrics 2008;75(5):459-69. [DOI: 10.1007/s12098-008-0073-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed prior to 18 Feb 2021).
Shan 2009
- Shan HM, Cai W, Cao Y, Fang BH, Feng Y. Extrauterine growth retardation in premature infants in Shanghai: a multicenter retrospective review. European Journal of Pediatrics 2009;168(9):1055-9. [DOI: 10.1007/s00431-008-0885-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stein 2013
- Stein AD, Barros FC, Bhargava SK, Hao W, Horta BL, Lee N, et al. Birth status, child growth, and adult outcomes in low- and middle-income countries. Journal of Pediatrics 2013;163(6):1740-6.e4. [DOI: 10.1016/j.jpeds.2013.08.012] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2016
- Stevens TP, Shields E, Campbell D, Combs A, Horgan M, La Gamma EF, et al. Variation in enteral feeding practices and growth outcomes among very premature Infants: a report from the New York State Perinatal Quality Collaborative. American Journal of Perinatology 2016;33(1):9-19. [DOI: 10.1055/s-0035-1554794] [PMID: ] [DOI] [PubMed] [Google Scholar]
Steward 2002
- Steward DK, Pridham KF. Growth patterns of extremely low-birth-weight hospitalized preterm infants. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2002;31(1):57-65. [DOI: 10.1111/j.1552-6909.2002.tb00023.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Uhing 2009
- Uhing MR, Das UG. Optimizing growth in the preterm infant. Clinics in Perinatology 2009;36(1):165-76. [DOI: 10.1016/j.clp.2008.09.010] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 1986
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America 1986;33(1):179-201. [DOI: 10.1016/s0031-3955(16)34975-6] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Abiramalatha 2016
- Abiramalatha T, Thomas N, Gupta V, Viswanathan A, McGuire W. High versus standard volumes of enteral feeds for preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD012413. [DOI: 10.1002/14651858.CD012413] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abiramalatha 2017
- Abiramalatha T, Thomas N, Gupta V, Viswanathan A, McGuire W. High versus standard volume enteral feeds to promote growth in preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD012413. [DOI: 10.1002/14651858.CD012413.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


