Abstract
Background
Periorbital and orbital cellulitis are infections of the tissue anterior and posterior to the orbital septum, respectively, and can be difficult to differentiate clinically. Periorbital cellulitis can also progress to become orbital cellulitis.
Orbital cellulitis has a relatively high incidence in children and adults, and potentially serious consequences including vision loss, meningitis, and death. Complications occur in part due to inflammatory swelling from the infection creating a compartment syndrome within the bony orbit, leading to elevated ocular pressure and compression of vasculature and the optic nerve. Corticosteroids are used in other infections to reduce this inflammation and edema, but they can lead to immune suppression and worsening infection.
Objectives
To assess the effectiveness and safety of adjunctive corticosteroids for periorbital and orbital cellulitis, and to assess their effectiveness and safety in children and in adults separately.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2020, Issue 3); Ovid MEDLINE; Embase.com; PubMed; Latin American and Caribbean Health Sciences Literature Database (LILACS); ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We did not use any date or language restrictions in the electronic search for trials. We last searched the electronic databases on 2 March 2020.
Selection criteria
We included studies of participants diagnosed with periorbital or orbital cellulitis. We excluded studies that focused exclusively on participants who were undergoing elective endoscopic surgery, including management of infections postsurgery as well as studies conducted solely on trauma patients. Randomized and quasi‐randomized controlled trials were eligible for inclusion. Any study that administered corticosteroids was eligible regardless of type of steroid, route of administration, length of therapy, or timing of treatment. Comparators could include placebo, another corticosteroid, no treatment control, or another intervention.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane.
Main results
The search yielded 7998 records, of which 13 were selected for full‐text screening. We identified one trial for inclusion. No other eligible ongoing or completed trials were identified. The included study compared the use of corticosteroids in addition to antibiotics to the use of antibiotics alone for the treatment of orbital cellulitis. The study included a total of 21 participants aged 10 years and older, of which 14 participants were randomized to corticosteroids and antibiotics and 7 participants to antibiotics alone. Participants randomized to corticosteroids and antibiotics received adjunctive corticosteroids after initial antibiotic response (mean 5.13 days), at an initial dose of 1.5 mg/kg for three days followed by 1 mg/kg for another three days before being tapered over a one‐ to two‐week period.
We assessed the included study as having an unclear risk of bias for allocation concealment, masking (blinding), selective outcome reporting, and other sources of bias. Risk of bias from sequence generation and incomplete outcome data were low.
The certainty of evidence for all outcomes was very low, downgraded for risk of bias (‐1) and imprecision (‐2). Length of hospital stay was compared between the group receiving antibiotics alone compared to the group receiving antibiotics and corticosteroids (mean difference (MD) 4.30, 95% confidence interval (CI) −0.48 to 9.08; 21 participants). There was no observed difference in duration of antibiotics between treatment groups (MD 3.00, 95% CI −0.48 to 6.48; 21 participants). Likewise, preservation of visual acuity at 12 weeks of follow‐up between group was also assessed (RR 1.00, 95% CI 0.82 to 1.22; 21 participants). Pain scores were compared between groups on day 3 (MD −0.20, 95% CI −1.02 to 0.62; 22 eyes) along with the need for surgical intervention (RR 1.00, 95% CI 0.11 to 9.23; 21 participants). Exposure keratopathy was reported in five participants who received corticosteroids and antibiotics and three participants who received antibiotic alone (RR 1.20, 95% CI 0.40 to 3.63; 21 participants). No major complications of orbital cellulitis were seen in either the intervention or the control group. No side effects of corticosteroids were reported, although it is unclear which side effects were assessed.
Authors' conclusions
There is insufficient evidence to draw conclusions about the use of corticosteroids in the treatment of periorbital and orbital cellulitis. Since there is significant variation in how corticosteroids are used in clinical practice, additional high‐quality evidence from randomized controlled trials is needed to inform decision making. Future studies should explore the effects of corticosteroids in children and adults separately, and evaluate different dosing and timing of corticosteroid therapy.
Plain language summary
Corticosteroids for periorbital and orbital cellulitis
What is the aim of this review? We aimed to find out if steroids are useful in treating serious infections of the area around the eye known as periorbital and orbital cellulitis. These infections can lead to complications like blindness, brain infection, or death. We also wanted to see if steroids worked the same or differently in children and adults.
Key messages We do not know if steroids are useful in treating periorbital and orbital cellulitis. We identified only one small study that looked at this topic, which showed that there may be a benefit to using steroids and antibiotics together. However, larger and higher‐quality studies are needed to better understand this topic.
What was studied in this review? Periorbital and orbital cellulitis are potentially serious infections of the area around the eye. These infections are usually treated in hospital, as they can cause serious complications. One contributing factor to the development of these complications is that there is limited space in the bony structures that surround the eye. When these spaces get swollen as a result of infection, the pressure inside the space increases, which can damage the eye. Steroids are medications that can reduce swelling, but they can also affect the body’s ability to fight infection. There are no clear guidelines on whether or not to use steroids to treat periorbital and orbital cellulitis, and there is a lot of variation in how these conditions are currently treated among doctors. Our review used standard methods to identify studies that assigned patients to receive either steroids or another treatment using a random method, and then compared the results.
What are the main results of the review? We found one study that compared using only antibiotics to using both antibiotics and steroids to treat periorbital and orbital cellulitis. The study included 21 people, 7 who only received antibiotics and 14 who received both antibiotics and steroids. The study included people 10 years or older, but did not see if there were any differences between how children and adults respond to steroids. The study found that people receiving steroids and antibiotics together needed antibiotics for less time and had improved symptoms earlier than the group that received antibiotics alone. There was no difference in length of hospital stay between people receiving steroids and antibiotics and those receiving only antibiotics. However, because there is only one small study on this topic, it is hard to tell if all patients with periorbital and orbital cellulitis should be given steroids with antibiotics; more research is needed before any formal recommendations can be made.
How up‐to‐date is this review? We searched for studies published up to 2 March 2020.
Summary of findings
Summary of findings 1. Standard treatment plus systemic corticosteroids compared to standard treatment alone for periorbital and orbital cellulitis.
| Standard treatment plus systemic corticosteroids compared to standard treatment alone for periorbital and orbital cellulitis | ||||||
| Patient or population: patients age 10 years and older with periorbital or orbital cellulitis Setting: hospital Intervention: systemic corticosteroids plus standard treatment Comparison: standard treatment alone | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard treatment | Risk with systemic corticosteroids | |||||
| Length of hospital stay | The mean length of hospital stay was 18.4 days. | MD 4.3 days fewer (8.8 fewer to 0.07 more) | MD 4.30 (−0.48 to 9.08) | 21 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Evidence regarding the effect of systemic corticosteroids on length of hospital stay is very uncertain. |
| Preservation of visual acuity assessed with: logMAR follow up: 12 weeks | 100 per 100 | 100 per 100 (82 to 100) | RR 1.00 (0.82 to 1.22) | 21 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | All participants in both the corticosteroid and standard of care groups had fully recovered visual acuity by end of study period. |
| Severity of pain on day 3 assessed with: grouped visual analog scale Scale from: 1 to 5 | The mean severity of pain on day 3 was 3.1. | MD 0.2 higher (1.02 lower to 0.78 higher) | MD −0.20 (−1.02 to 0.62) | 22 eyes (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Visual analog scale was grouped to a 5‐point scale from original 10‐point scale. |
| Duration of time requiring intravenous antibiotics | The mean duration of time requiring intravenous antibiotics was 11.6 days. | MD 3 days lower (0.287 lower to 5.713 lower) | MD 3.00 (−0.48 to 6.48) | 21 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Only total duration of intravenous antibiotic use was available; no information on subsequent use of oral antibiotics. |
| Need for surgical intervention | 14 per 100 | 14 per 100 (2 to 100) | RR 1.00 (0.11 to 9.23) | 21 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Evidence regarding the effect of systemic corticosteroids on the proportion of participants who required surgical intervention is very uncertain. |
| Adverse effects: exposure keratopathy follow‐up: 12 weeks | 43 per 100 | 36 per 100 | RR 1.20 (0.40 to 3.63) | 21 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | No other adverse effects were documented. |
| Return visit to health care (not reported) | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; logMAR: logarithm of the minimum angle of resolution; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Evidence was downgraded for risk of bias (−1). Unclear risk of bias from allocation concealment, blinding, and selective reporting. Other potential sources of bias included selection bias from requiring an initial response to antibiotics; 2:1 allocation to corticosteroid group; and using the eye as the unit of analysis rather than the participant. 2Evidence was downgraded (−2) for imprecision as there were only 21 participants. 3Evidence was downgraded (−1) for indirectness. Total duration of intravenous antibiotics was reported; however, oral antibiotics were used following intravenous antibiotics, and total duration of any antibiotic use was not reported.
Background
Description of the condition
Although periorbital (preseptal) and orbital (postseptal) cellulitis are anatomically distinct bacterial infections, clinical differentiation is often difficult, and these two conditions are usually considered together (Wong 2018). Periorbital cellulitis is an infection of the tissues anterior to the orbital septum, caused by spread of a local infection such as conjunctivitis, or following injury (Rimon 2008). Orbital cellulitis is an infection of the tissues posterior to the orbital septum, usually as a complication of sinusitis (Howe 2004). Periorbital and orbital cellulitis are usually due to direct inoculation such as a skin abrasion (e.g. insect bite), sinusitis, or due to hematogenous spread. In both conditions, people present with periorbital swelling and redness. Swelling may be severe, making examination of the eye challenging. Orbital cellulitis is characterized by decreased ocular movement, pain with eye movements, decreased visual acuity, and proptosis, whereas these clinical features are absent in periorbital cellulitis. In 1970, Chandler and colleagues described criteria for categorizing severity (Chandler 1970). Periorbital cellulitis (Chandler criteria I) is defined as an infection of the eyelid, or tissue around the eye; orbital cellulitis (Chandler criteria II) is defined as an infection of the structures posterior to orbital septum; orbital cellulitis is further categorized according to presence of subperiosteal abscess (Chandler criteria III), orbital abscess (Chandler criteria IV), and cavernous sinus thrombosis (Chandler criteria V). Severe complications of orbital cellulitis include loss of vision, meningitis, and death (Crosbie 2016; Lee 2011; Murphy 2014).
The incidence of orbital cellulitis is estimated to range from 1.6 per 100,000 to 6 per 100,000 in children under the age of 18 years, and 0.6 per 100,000 to 2.4 per 100,000 in adults, although few published studies have evaluated incidence (Capra 2015; Murphy 2014; Soroudi 2003). Children under the age of 18 years represent a large proportion of people hospitalized with orbital cellulitis (Marchiano 2016). Based on the US Healthcare Cost and Utilization Project (HCUP) National Inpatient Sample, which uses weighted national estimates, in 2013 there were approximately 6400 hospitalizations across all ages, and approximately 2500 hospitalizations in children (HCUPnet). Across all ages, the estimated cost of these hospitalizations was over USD 41 million per year; the mean age was 33 years; there was a slight male predominance at 52%; and the mean length of stay was four days (HCUPnet). One US study compared the outcomes of children hospitalized with orbital cellulitis in 2000 (prior to the introduction of the heptavalent pneumococcal vaccine) to 2009 and reported that surgical rates nearly doubled and hospital charges more than doubled (Capra 2015).
Microbiologic isolates have changed over the decades. Haemophilus influenzae type B was the most common pathogen prior to the introduction of the vaccine (1985), after which Streptococcus pneumoniae predominated (Donahue 1998; Harris 1994). Peña and colleagues examined sinus and blood cultures of children with orbital cellulitis at one US children's hospital in two eras, pre‐ and postintroduction of the 7‐valent pneumococcal conjugate vaccine (2003) (Peña 2013). Prior to the introduction of the vaccine, S pneumoniae was the predominate pathogen, but was not found in any children postvaccine (from 22% to 0%). There was a similar reduction in Streptococcus viridans (from 12% to 0%). There was an increase in Staphylococcus aureus (from 20% to 42%), and of those, there was methicillin‐resistance only in the postvaccine era (from 0% to 56%). However, in one study from Toronto, Canada (2000 to 2011), of those children with a positive culture, 13.3% grew H influenzae from their abscess drainage, leading the authors to conclude that this pathogen remains a cause of orbital cellulitis, especially in older children with larger abscesses (Sharma 2015).
The indications for computed tomography (CT) imaging have been examined, and are especially important considering the risks to young children from exposure to ionizing radiation. Rudloe and colleagues examined the charts of children attending a US pediatric emergency department (1995 to 2008) presenting with acute periorbital edema or erythema, or both (Rudloe 2010). Of these, 32% underwent CT imaging; 12% of all children and 37% of those with CT scans had significant CT findings (defined as Chandler III or higher), of which 91% were Chandler III (subperiosteal abscess). Children reporting pain on eye movement or having proptosis or ophthalmoplegia at clinical exam had increased risk of having an orbital abscess at CT. The authors of one systematic review concluded that periorbital and orbital cellulitis can be managed medically; subperiosteal abscess can often be managed medically; and orbital abscess and cavernous sinus thrombosis are managed surgically (Wong 2018). Surgical management is also indicated for people who fail to respond to antimicrobial therapy, or if complications develop (e.g. optic nerve compromise). Studies have shown similar efficacy of oral and parenteral antibiotics for uncomplicated periorbital cellulitis (Al‐Nammari 2007), while intravenous (IV) antibiotics are warranted for orbital cellulitis, subperiosteal abscess, and orbital abscess (Wong 2018).
Description of the intervention
A small number of studies have suggested that adding corticosteroids to antimicrobial therapy may improve outcomes in people with periorbital and orbital cellulitis (Chen 2018; Davies 2015; Pushker 2013; Yen 2005). Two prospective, comparative observational studies that evaluated the effectiveness of corticosteroids in children hospitalized with orbital cellulitis noted a three‐day reduction in length of stay (Chen 2018; Davies 2015). One randomized controlled trial of 21 people aged over 10 years with orbital cellulitis admitted to a tertiary care eye hospital in India reported that people treated with corticosteroids had a three‐day reduction in duration of IV antibiotics and a four‐day reduction in mean length of stay, with no difference in observed need for surgical intervention (Pushker 2013). Furthermore, people treated with corticosteroids had less pain, periorbital edema, fever, and chemosis. At 12 weeks' follow‐up, people treated with corticosteroids had less residual proptosis and restrictions in extraocular movement, and no adverse effects. However, there are no clinical practice guidelines to guide practitioners on the use of corticosteroids in people with periorbital and orbital cellulitis.
How the intervention might work
Orbital cellulitis causes inflammation and swelling leading to a compartment syndrome within the enclosed bony orbit, resulting in elevated ocular pressure and compression of the vasculature, musculature, and optic nerve, resulting in ischemia, ophthalmoplegia, and visual impairment respectively. Corticosteroids have several beneficial effects when used for acute illness, including reducing inflammation and edema, and are used for several other infections to reduce inflammation and swelling. For example, corticosteroids are standard of care for children with mild, moderate, and severe croup, a viral infection of the larynx and trachea, leading to a reduced frequency of hospitalization and intensive care unit admission (Gates 2018). There is clinical precedence for the use of corticosteroids in several other acute infections, including bacterial meningitis (Brouwer 2015), tuberculosis meningitis (Prasad 2016), bacterial meningitis in neonates (Ogunlesi 2015), and acute bacterial sinusitis (Venekamp 2014). Some authors have reported clinical reticence to use corticosteroids due to concerns with immune suppression and fear of worsening infection (Chen 2018; Davies 2015), although studies have not supported these assertions.
Why it is important to do this review
The results of studies to date have been inconclusive on the use of corticosteroids in people with periorbital and orbital cellulitis. One large, US‐based, multicenter observational study identified significant variation in the use of adjunctive corticosteroids for children hospitalized with periorbital and orbital cellulitis, highlighting an important evidence gap (Markham 2018). Questions remain on the type of corticosteroids (e.g. dexamethasone, prednisolone), dose, route (IV versus oral versus intranasal), duration, timing (before, during, or after antibiotics or when responding to therapy), and indication (periorbital cellulitis versus orbital cellulitis versus subperiosteal/orbital abscess). No prior systematic review has evaluated whether adjunctive corticosteroids improve outcomes. A synthesized body of evidence is necessary to optimize treatment and outcomes for people with periorbital and orbital cellulitis.
Objectives
To assess the effectiveness and safety of adjunctive corticosteroids for periorbital and orbital cellulitis, and to assess their effectiveness and safety in children and in adults separately.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs), with and without treatment masking, and quasi‐RCTs (i.e. controlled clinical trials). We included studies independent of the language of publication, publication status (unpublished, published), sample size, or risk of bias. We imposed no minimum follow‐up period for trial eligibility.
Types of participants
We included studies that enrolled participants of any age with clinically diagnosed periorbital and orbital cellulitis, including all ranges of severity as defined by Chandler (Chandler 1970). We included studies that enrolled participants with a clinical diagnosis of periorbital or orbital cellulitis, irrespective of whether radiographic imaging was completed. We included studies conducted in both inpatient and outpatient settings (e.g. emergency room, ambulatory care, outpatient clinic).
We excluded studies that focused exclusively on participants who were undergoing elective endoscopic surgery, including management of infections postsurgery. We excluded studies conducted solely on trauma patients.
Types of interventions
We included studies that focused on the short‐term use of adjunctive systemic corticosteroids (e.g. dexamethasone, prednisone, prednisolone, methylprednisolone) or intranasal corticosteroids (e.g. fluticasone, betamethasone) administered for the acute care of people with periorbital and orbital cellulitis. We considered all types of corticosteroids, dose, mode of delivery (i.e. oral, IV, or intranasal), length of therapy, or timing of treatment (i.e. prior to or at the time of antibiotic administration, or delayed). We included comparators that were placebo, another corticosteroid, no treatment control, or another intervention.
Types of outcome measures
There is no current core outcome set for periorbital or orbital cellulitis on which to base outcome selection.
The critical outcomes of this review included the following.
Time to recovery. When this was not reported, we considered mean length of hospital stay (hours or days) for studies focused on hospitalized participants as a surrogate to time to recovery.
Proportion of participants recovered at the end of follow‐up, as defined by each individual study.
Proportion of participants with preservation of visual acuity at the end of the study intervention period, defined as not worsening from baseline measure of best‐corrected visual acuity (BCVA) on a standardized age‐appropriate assessment tool (e.g. logarithm of the minimum angle of resolution (logMAR) values, Snellen chart).
Severity of pain as reported by participant or caregiver as defined using mean difference on a standard age‐appropriate assessment tool (e.g. visual analog score) from baseline and throughout the study period (duration of hospitalization for inpatients, or until resolution of symptoms for outpatients) at days 1, 2, 3, 7, 10, and 14. We chose multiple time points for this outcome because hospitalized patients with orbital cellulitis are frequently assessed daily, which results in management decisions (e.g. surgical intervention). Consequently, these time points are clinically meaningful for treatment decisions, and meaningful to families (i.e. pain improving or worsening). Prior studies on corticosteroids in hospitalized participants identified clinical differences at these time points.
Mean duration (hours or days) requiring IV antibiotics during the study period.
Proportion of participants who required surgical intervention during the study period.
Adverse effects associated with corticosteroids, including behavioral problems, hyperactivity, gastric irritation, hyperglycemia, adrenal suppression, weight gain, cataracts, and immune suppression. We documented other adverse events reported from included studies.
Other important outcomes of this review included the following.
Proportion of participants with normal extraocular muscle movement at the end of the study intervention period, defined using an objective tool (e.g. Kestenbaum limbus test of motility) or at clinical exam and in the absence of any reported diplopia.
Proportion of participants who had a return visit to healthcare provider during the first 7 days and between 7 and 30 days after the end of the study.
Proportion of participants who died at the end of follow‐up.
We did not conduct a formal cost‐effectiveness analysis, as no data were available.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs and controlled clinical trials. There were no restrictions to language or year of publication. The electronic databases were last searched on 2 March 2020.
Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (Issue 3, 2020) (Appendix 1).
MEDLINE Ovid (1946 to 2 March 2020) (Appendix 2).
Embase.com (1947 to 2 March 2020) (Appendix 3).
PubMed (1946 to 2 March 2020) (Appendix 4).
LILACS (Latin American and Caribbean Health Science Information Database (1982 to 2 March 2020) (Appendix 5).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov: searched 2 March 2020) (Appendix 6).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp: searched 2 March 2020) (Appendix 7).
Searching other resources
We searched conference proceedings for abstracts that described potentially relevant trials. We identified additional published, unpublished, or ongoing studies by handsearching reference lists of relevant articles, included and excluded articles (backward searching), using the Science Citation Index for papers cited (forward searching), and by contacting topic experts in the field (hospital‐based pediatricians, pediatric emergency physicians, ophthalmologists, and otolaryngologists).
Data collection and analysis
Selection of studies
Two review authors independently screened all titles, abstracts, and keywords (when available) generated by the electronic searches for inclusion, classifying each record as 'definitely relevant,' 'possibly relevant,' or 'definitely not relevant.' We obtained the full texts for all studies reported in articles classified as 'definitely relevant' or 'possibly relevant,' and two review authors independently assessed each full‐text report as 'include,' 'exclude,' or 'unsure.' Any disagreements were resolved by discussion and consensus or by consulting a third review author if necessary. For articles excluded after full‐text review, we documented reasons for exclusion in the 'Characteristics of excluded studies' table. The search and selection process is described in a PRISMA flow diagram (Moher 2009).
Where studies had multiple publications, we collated the reports of the same study so that each study, rather than each report, was the unit of interest for the review; such studies have a single identifier with multiple references.
Data extraction and management
We adapted a data collection form developed and piloted by Cochrane Eyes and Vision. Two review authors independently extracted study information; any disagreements were resolved by discussion and consensus, or by consulting a third review author if needed to make a final decision. The data extraction form included details related to the trial methodology, characteristics of the participants, interventions, outcomes, and additional information (e.g. funding, conflicts of interest, trial registration). We completed a 'Characteristics of included studies' table; one review author transferred data into Review Manager 5 (Review Manager 2014), and a second review author verified that the data had been entered correctly.
Assessment of risk of bias in included studies
We assessed risk of bias in the included studies using Cochrane's domain‐based evaluation tool as recommended in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We used the Cochrane 'Risk of bias' tool to assess the following domains of bias: selection bias (random sequence generation, allocation concealment before randomization), performance bias (masking of participants and personnel), detection bias (masking of outcome assessment), attrition bias (incomplete outcome data, amount, and handling of missing data), reporting bias (selective outcome reporting), and other sources of bias (e.g. funding source). Two review authors independently assessed risk of bias for each domain (low, high, or unclear risk) and resolved any discrepancies by discussion or by consulting a third review author if needed to make a final judgement. We contacted trial authors and waited two weeks if there was uncertainty in any domain due to lack of information.
Measures of treatment effect
We analyzed dichotomous data using risk ratio with 95% confidence intervals (CIs). Dichotomous outcomes included participant recovery at the end of follow‐up period, preservation of visual acuity, normal extraocular movements, surgical intervention, adverse events, return visit to a healthcare provider, and death.
We analyzed continuous data as mean difference with 95% CIs when measurements were made using the same scale, or standardized mean differences with 95% CIs when measurements were made using different scales. Continuous outcomes included time to recovery, length of hospital stay, severity of pain, and duration of antibiotics.
Unit of analysis issues
The unit of analysis was the participant. The treatments that we evaluated were systemic, therefore potentially affecting both eyes. When trials with more than two arms were eligible for inclusion (e.g. two or more doses), we would evaluate each relevant comparison separately without double counting them in the analysis.
Dealing with missing data
We contacted investigators to obtain missing information, either for a study that had been reported in an abstract only, or when insufficient detail was provided in the full‐text reports. If the study investigators did not respond within two weeks, we made a further attempt at contact before proceeding with the available information and evaluating the impact of the missing data on the overall assessment of results. We managed potentially missing data according to the guidelines in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We would have assessed clinical heterogeneity by comparing the characteristics of individual studies (i.e. study participants, inclusion/exclusion criteria, and outcomes) if multiple studies has been identified. We would have assessed methodologic heterogeneity by comparing design characteristics, including 'Risk of bias' assessment of individual studies. We would have used the I2 statistic to assess statistical heterogeneity in the outcome estimates from included studies (Higgins 2017). Had we detected significant, clinical, methodologic, or statistical heterogeneity (defined as I2 statistic above 60%), we would have explored reasons for the heterogeneity and possibly decided not to combine data in a meta‐analysis.
Assessment of reporting biases
We attempted to mitigate reporting biases by searching the grey literature for unpublished studies. Had 10 or more studies been included, we would have assessed small‐study effect, which could be due to publication bias, using funnel plots.
Data synthesis
We synthesized the data qualitatively, focusing on the comparability of studies and characteristics of studies that may affect the cumulative evidence. We had planned to combine results in a meta‐analysis but did not do so because fewer than two studies were included in the review.
Subgroup analysis and investigation of heterogeneity
Had sufficient data been available, we would have conducted several subgroup analyses of outcomes, according to:
age: children (aged 18 years and under) versus adults (aged over 18 years);
route of corticosteroids: systemic (oral, IV) versus intranasal;
timing of corticosteroids: immediately at diagnosis versus delayed to when responding to therapy;
setting: inpatient versus outpatient.
However, as only one study was identified, subgroup analyses were not conducted.
Sensitivity analysis
Had a sufficient number of studies been available, we would have conducted the following sensitivity analyses by evaluating the effect of:
excluding studies with a high risk of bias for the primary outcome on any domain;
excluding unpublished data (if relevant).
Summary of findings and assessment of the certainty of the evidence
We presented a 'Summary of findings' table for each comparison (Table 1). The planned comparisons were adjunctive systemic or intranasal corticosteroids versus control or another comparator. We included the following seven outcomes at the end of the study period.
Time to recovery, or mean length of hospital stay.
Proportion of participants with preservation of visual acuity at the end of the study period.
Severity of pain as reported by participant or caregiver on day 3.
Mean duration of time requiring antibiotics.
Proportion of participants who required surgical intervention.
Adverse effects associated with corticosteroids.
Proportion of participants who had a return visit to health care at the end of the study period.
We assessed the quality of a body of evidence as high, moderate, low, or very low with respect to each prespecified outcome using the core GRADE components: study limitations, consistency of effect, imprecision of results, indirectness of evidence, and high probability of publication bias. We used GRADEpro GDT software to assist in the preparation of the 'Summary of findings' tables (GRADEpro GDT). We detailed the reasons for upgrading or downgrading of the evidence.
Results
Description of studies
Results of the search
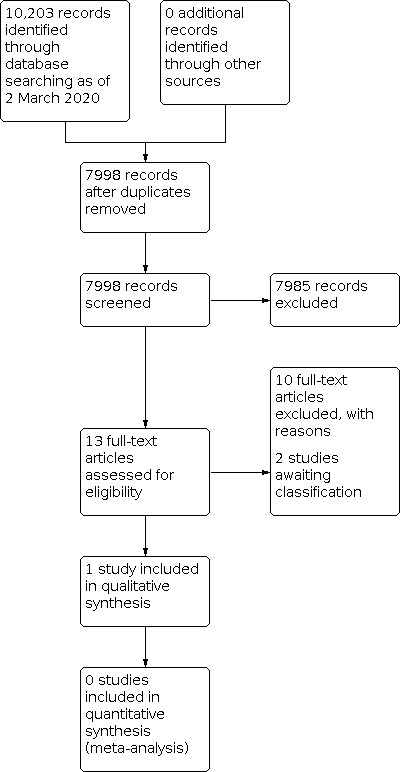
The initial search yielded 10,203 records; after the removal of 2205 duplicates, 7998 unique records remained (Figure 1). We excluded 7985 records based on title and abstract screening and selected 13 records for full‐text screening. We included one study, excluded 10 records, and classified two records as awaiting classification. No additional records were identified after contacting topic experts or after forward and backward citation searching.
1.
Study flow diagram.
Included studies
For details of the included study, see Characteristics of included studies.
Types of studies
We identified one RCT for inclusion in the review (Pushker 2013). The study was conducted at the All India Institute of Medical Sciences in India from 2007 to 2009, and included hospitalized patients with acute onset (defined as within 14 days) orbital cellulitis with or without abscess.
Type of participants
A total of 21 participants aged 10 years and older were enrolled in the trial, of which 7 participants were randomized to receive standard care and 14 to receive corticosteroids in addition to standard care. One participant in the intervention group had two affected eyes, while all others had only a single affected eye.
Type of interventions
Standard care included treatment with IV antibiotics, treatment of concomitant sinusitis, surgical drainage when indicated, and supportive treatment with topical antibiotics and lubricants. In addition to standard care, the intervention group was started on adjuvant oral prednisolone following an initial response to treatment after a minimum of four days in hospital. Participants in the intervention group initially received 1.5 mg/kg/day of prednisolone for three days, then the dose was lowered to 1 mg/kg/day for the next three days before tapering the treatment over a one‐ to two‐week period. No additional information was available on the tapering regimen.
Type of outcomes
The study investigators assessed the effects of corticosteroids on length of hospital stay, length of time requiring IV antibiotics, pain (using a visual analog scale), fever, periorbital edema, conjunctival chemosis, vision (logMAR values), proptosis (measured by Hertel exophthalmometer), and extraocular movements (Kestenbaum limbus test of motility). Outcomes were assessed at baseline and after 3, 7, 10, and 14 days and 12 weeks. The investigators did not describe any potential conflicts of interest or external funding sources. We contacted the study authors to obtain additional data, as well as for separate results by adult and pediatric age group, but the authors indicated they no longer had access to the raw data, and were not able to provide additional details.
Adverse effects
The only reported complication was exposure keratopathy, which occurred in 2 out of 14 (14%) participants in the corticosteroid group and 1 out of 7 (14%) participants in the control group. No participants in either group experienced permanent vision loss, meningitis, or sinus venous thrombosis. No other adverse effects from corticosteroids were reported.
Unit of analysis
We had planned that the unit of analysis was the participant. However, Pushker 2013 used the eye as the unit of analysis rather than the participant for some outcome measures. Corticosteroids are a systemic treatment that would affect both eyes, therefore reporting data without accounting for the correlation between two eyes would result in wrong estimate of standard deviation. However, only one participant had involvement of both eyes, and this participant was in the treatment group.
Excluded studies
Of the 10 records excluded after full‐text screening, eight were not the study design of interest and two were not the population of interest. One study was a registration for a trial on the effects of corticosteroids (NCT01671423); we contacted the lead investigator on the study, who confirmed that no study participants had a diagnosis of periorbital or orbital cellulitis. The other study, a clinical trial protocol that was later withdrawn, assessed the effect of combined antibiotics and steroids for the treatment of bacterial blepharitis and/or keratitis and/or conjunctivitis. Details on the reasons for the exclusion of studies are provided in the Characteristics of excluded studies table.
Awaiting classification
We assessed two studies as awaiting classification. One study was a clinical trial registration for the effects of corticosteroids on cellulitis (NCT02087527). This trial was terminated because an adequate sample size was not achieved. We made several attempts to contact the authors to confirm whether any participants with periorbital or orbital cellulitis were included prior to termination, but received no response. Based on the available study description, it is unlikely that any included participants had periorbital or orbital cellulitis. For example, one outcome measure was change in erythema size, which would typically be used in cellulitis, not periorbital or orbital cellulitis. A second study remains as awaiting classification, as the only copy of the study identified by our research librarian is in a library which is currently closed (Carvalho 1984).
Risk of bias in included studies
We assessed the one study eligible for inclusion for risk of bias, which is summarized in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was adequate, therefore we judged the study to be at low risk of bias for sequence generation. The method of treatment allocation was not reported, therefore we judged this domain as unclear risk of bias for treatment allocation concealment.
Blinding
While the study states that it was single masked, no information was provided as to who was masked (i.e. whether participants or clinicians). There was also no information on whether the outcome assessor was masked, therefore we judged the study to be at unclear risk for performance and detection bias.
Incomplete outcome data
The study had complete follow‐up data for all participants with no attrition, resulting in a judgement of low risk of bias for incomplete outcome data.
Selective reporting
The study was not registered with ClinicalTrials.gov, the WHO ICTRP, or Clinical Trials Registry – India, nor was there a registration number provided in the publication. No protocol with predetermined outcome measures was available or provided by the study authors. Given that all clinically relevant outcomes were described and reported, we rated the risk of bias from selective reporting as unclear.
Effects of interventions
See: Table 1
Healthcare utilization
The mean length of hospital stay in the control group was 18.4 days (standard deviation (SD) 5.9 days) compared to 14.1 days (SD 3.7 days) in the group receiving corticosteroids. Pushker 2013 found this difference in length of hospital stay to be statistically significant, however based on analysis of the available data using Review Manager 5, there was no evidence of a difference between the two groups (mean difference (MD) 4.30, 95% confidence interval (CI) −0.48 to 9.08; 21 participants) (Figure 3; Analysis 1.1). This discrepancy may be the result of different statistical techniques being applied. Pushker 2013 indicated using Mann‐Whitney test, Student t‐test, and Fisher exact test, but it is unclear which statistical tests were used for which analysis. We contacted the authors for clarification but received no response. We rated the certainty of evidence for this outcome as very low, downgrading for risk of bias (−1) and imprecision (−2).
3.

Forest plot of comparison: 1 Corticosteroids versus standard care, outcome: 1.1 Length of hospital stay.
1.1. Analysis.

Comparison 1: Corticosteroids versus standard care, Outcome 1: Length of hospital stay
The mean duration of IV antibiotics in the treatment group was 8.6 days (SD 1.3 days) compared to 11.6 days (SD 4.6 days) in the control group, with an overall mean difference of 3.0 hours (95% CI −0.48 to 6.48; 21 participants) between groups (Figure 4; Analysis 1.2). No information was available on the duration of oral antibiotics. The number of participants requiring surgical intervention during the study period was similar in both groups: 1 (14%) in the control group compared with 2 (14%) in intervention group (risk ratio (RR) 1.00, 95% CI 0.11 to 9.23; 21 participants) (Figure 5; Analysis 1.3). No participant in either group required intensive care unit admission or additional CT imaging. The trial did not evaluate for changes in readmission to hospital or number of follow‐up visits, although no additional visits or admissions to hospital were described. We rated the certainty of evidence for the mean duration of IV antibiotics and number of participants requiring surgical intervention or intensive care unit admission as very low, downgrading for risk of bias (−1), indirectness (−1), and imprecision (−2).
4.

Forest plot of comparison: 1 Corticosteroids versus standard care, outcome: 1.2 Duration of time requiring antibiotics.
1.2. Analysis.

Comparison 1: Corticosteroids versus standard care, Outcome 2: Duration of time requiring antibiotics
5.

Forest plot of comparison: 1 Corticosteroids versus standard care, outcome: 1.3 Proportion of participants who required surgical intervention.
1.3. Analysis.

Comparison 1: Corticosteroids versus standard care, Outcome 3: Proportion of participants who required surgical intervention
Disease course and symptoms
Although there was no evidence of a difference for pain score on day 0 (MD 0.00, 95% CI −0.38 to 0.38; 22 eyes) and day 3 (MD −0.20, 95% CI −1.02 to 0.62; 22 eyes)(Figure 6; Analysis 1.4), the trial investigators observed a general trend towards earlier symptom resolution after day 3 in the group receiving corticosteroids based on various symptom scores evaluated. Participants receiving corticosteroids had an improvement in pain scores relative to the control group on day 7 (mean re‐grouped VAS score 1.6; SD 0.7 versus 2.0; SD 0), yet at day 10, both groups had comparable pain scores (mean re‐grouped VAS score 1.0; SD 0 versus 1.4; SD 1.1). We graded the certainty of evidence for this outcome for days 0, 3, 7, and 10 as very low, downgrading for risk of bias (−1) and imprecision (−2).
6.

Forest plot of comparison: 1 Corticosteroids versus standard care, outcome: 1.4 Pain.
1.4. Analysis.

Comparison 1: Corticosteroids versus standard care, Outcome 4: Pain
While the mean grade of severity of periorbital edema was observed to be lower in the corticosteroid group relative to the control group on day 7 (mean grade 0.7; SD 0.5 versus 2.1; SD 0.7) and day 10 (mean grade 0.1; SD 0.4 versus 0.9; SD 0.4), no evidence of a difference was observed between the groups on day 7 (MD 1.20, 95% CI 0.62 to 1.78; 22 eyes) and day 10 (MD 0.80, 95% CI 0.44 to 1.16; 22 eyes) (Figure 7; Analysis 1.5). No periorbital edema was observed in both groups by day 14. We graded the certainty of evidence for this outcome as very low, downgrading for risk of bias (−1) and imprecision (−2).
7.

Forest plot of comparison: 1 Corticosteroids versus standard care, outcome: 1.5 Periorbital edema.
1.5. Analysis.

Comparison 1: Corticosteroids versus standard care, Outcome 5: Periorbital edema
Participants in the corticosteroid group showed improvements in extraocular motility relative to the control group using the Kestenbaum limbus test of motility at day 14 and at 12 weeks (data not shown). We graded the certainty of evidence for this outcome as very low, downgrading for risk of bias (−1) and imprecision (−2).
The corticosteroid group also appeared to show faster resolution of proptosis relative to the control group on day 10 (mean 0.7 mm; SD 0.9 versus 1.7 mm; SD 0.8) and day 14 (mean 0.1 mm; SD 0.4 versus 1.1 mm; SD 0.7); there was no evidence of a difference between the two groups for resolution of proptosis on day 7 (MD 1.10, 95% CI −0.05 to 2.25; 22 eyes), day 10 (MD 1.0, 95% CI 0.25 to 1.75; 22 eyes), and day 14 (MD 1.00, 95% CI 0.44 to 1.56; 22 eyes) (Figure 8; Analysis 1.6). At 12 weeks, all participants in the group receiving corticosteroids had fully resolved proptosis, while participants in the control group had mild residual proptosis (data not shown). We graded the certainty of evidence this outcome at all the time points assessed as very low, downgrading for risk of bias (−1) and imprecision (−2).
8.

Forest plot of comparison: 1 Corticosteroids versus standard care, outcome: 1.7 Amount of proptosis (mm).
1.6. Analysis.

Comparison 1: Corticosteroids versus standard care, Outcome 6: Amount of proptosis (mm)
Although there was no evidence of a difference between the corticosteroids group relative to control for day 0 (MD −0.30, 95% CI −0.93 to 0.33; 22 eyes) and day 3 (MD 0.30, 95% CI −0.09 to 0.69; 22 eyes) (Figure 9; Analysis 1.7), the trial investigators observed that in the group receiving corticosteroids, conjunctival chemosis fully resolved in all participants by day 10 in hospital, while in the control group, complete resolution in all participants was not seen until day 14 (data not shown). We graded the certainty of evidence for this outcome as very low, downgrading for risk of bias (−1) and imprecision (−2).
9.

Forest plot of comparison: 1 Corticosteroids versus standard care, outcome: 1.7 Conjunctival chemosis.
1.7. Analysis.

Comparison 1: Corticosteroids versus standard care, Outcome 7: Conjunctival chemosis
All trial participants fully recovered vision (Figure 10; Analysis 1.8). We graded the certainty of evidence for this outcome as very low, downgrading for risk of bias (−1) and imprecision (−2).
10.

Forest plot of comparison: 1 Corticosteroids versus standard care, outcome: 1.8 Preservation of visual acuity at the end of the study period.
1.8. Analysis.

Comparison 1: Corticosteroids versus standard care, Outcome 8: Preservation of visual acuity at the end of the study period
Adverse effects
The only ocular complication reported was exposure keratopathy, which occurred in 5 (36%) participants in the corticosteroid group and 3 (43%) participant in the control group (RR 1.20, 95% CI 0.40 to 3.63; 21 participants) (Figure 11; Analysis 1.9). No participants in either group experienced permanent vision loss, meningitis, or sinus venous thrombosis. Pushker 2013 reported no systemic complications from corticosteroids, and did not describe what specific adverse effects were assessed. The study did not report whether there were behavioral problems, hyperactivity, gastric irritation, hyperglycemia, adrenal suppression, weight gain, cataracts, or immune suppression.
11.

Forest plot of comparison: 1 Corticosteroids versus standard care, outcome: 1.9 Exposure keratopathy.
1.9. Analysis.

Comparison 1: Corticosteroids versus standard care, Outcome 9: Exposure keratopathy
Economic data
Pushker 2013 did not report any cost‐effectiveness data.
Discussion
Summary of main results
Our review evaluated the effectiveness and safety of corticosteroids for periorbital and orbital cellulitis and identified one randomized controlled trial (Pushker 2013). The included study compared the use of systemic corticosteroids following an initial response to antibiotics (mean 5.1 days) to the use of antibiotics alone for the treatment of orbital cellulitis in a population 10 years and older. The study assessed the effects of systemic corticosteroids on length of hospital stay, length of time requiring IV antibiotics, pain, fever, periorbital edema, conjunctival chemosis, vision, proptosis, and extraocular movements. The certainty of evidence for all outcomes is very low. The review evaluated differences between those that received corticosteroids relative to controls in duration of IV antibiotics (MD 3.00, 95% CI −0.48 to 6.48; 21 participants) and length of hospital stay (MD 4.30, 95% CI −0.48 to 9.08; 21 participants).There were limited differences between groups in resolution of symptoms such as fever, pain, periorbital edema, conjunctival chemosis, and visual acuity at day 0 and 3, but reduced periorbital edema (Day 7, 10) and proptosis (day 10, 14) in those treated with corticosteroids. At 12 weeks' follow‐up, participants who received corticosteroids may achieve improvement in extraocular motility and full recovery from proptosis compared to controls. Exposure keratopathy occurred at a similar frequency in both groups, but no other adverse effects were reported although it is unclear which adverse effects were evaluated. No data on cost‐effectiveness were reported.
Overall completeness and applicability of evidence
We performed a robust literature search of seven electronic medical databases and identified only one RCT on the effects of corticosteroids for the treatment of periorbital and orbital cellulitis. We found no evidence of a difference in the effectiveness of corticosteroids, or evidence on different types of corticosteroids, doses, duration, and indications in the treatment of periorbital or orbital cellulitis. We also found no evidence on the effectiveness of corticosteroids specific to children. The lack of age‐specific data is an important knowledge gap given the differences in pathogens and disease course between children and adults and the need for age group‐specific guidelines (Wong 2018). Two prior prospective observational studies that reported a three‐day reduction in length of hospital stay with adjuvant corticosteroids treatment differed in timing of treatment, with corticosteroids administered at the time of admission, Chen 2018, or after a decrease in C‐reactive protein (CRP) (Davies 2015). Evidence regarding the timing of corticosteroids administration remains unknown. One factor that may limit the applicability of the evidence is the long length of hospital stay. Investigators in Pushker 2013 stated that they took a cautious approach to discharging participants, given the experimental nature of corticosteroids, and participants were kept until near complete resolution of symptoms. The mean length of stay of 14 days and over is longer than that of most published observational studies, which is generally less than 10 days (Çağlar 2018; Chen 2018; Davies 2015; Friedel 2019; Gavriel 2018; Goldman 2008; Marchiano 2016; Markham 2018; Santos 2019; Yang 2009; Yen 2005).
Quality of the evidence
We graded the certainty of the evidence for all outcomes as very low given the imprecision in effect estimates and unclear risk of bias. Despite corticosteroids being a systemic treatment, trial investigators used the eye as the unit of analysis for some outcomes rather than the participant without accounting for the correlation between the two eyes in their analyses, which would result in wrong estimate of standard deviation. The trial was grossly underpowered, with no information on trial registration or prior published protocol, thus further warranting downgrading the certainty of the evidence.
Potential biases in the review process
We classified one potentially relevant clinical trial registration that had been terminated due to inadequate sample size as awaiting classification (NCT02087527). Despite our multiple attempts at contact, the authors of this trial did not respond to our request for clarification on whether any participants with periorbital or orbital cellulitis had been enrolled prior to study withdrawal, although this appeared very unlikely. If this study did include participants with periorbital or orbital cellulitis, and had the data been available, not including it in our analysis could potentially have introduced some bias. We attempted to mitigate any biases by conducting a detailed, comprehensive, and sensitive search strategy along with following standard Cochrane methodology. Any differences from the study protocol are outlined in the Differences between protocol and review section.
Agreements and disagreements with other studies or reviews
We identified only one RCT on the use of corticosteroids in the treatment of periorbital or orbital cellulitis. Our findings are generally in agreement with previous studies, although the evidence from these studies is mostly observational. Four retrospective studies have described the use of corticosteroids in orbital and periorbital cellulitis, three of which found negligible differences in outcomes, and one that found a positive effect on length of hospital stay. A retrospective study of 1072 children treated across 41 US hospitals found that corticosteroids did not significantly affect length of hospital stay (adjusted rate ratio (ARR) 1.1, 95% CI 1.0 to 1.2), but did increase cost of care (ARR 1.2, 95% CI 1.1 to 1.4), and led to a small increase in readmission to hospital in patients receiving corticosteroids (Markham 2018). In a study of 122 hospitalized children, Santos 2019 found that corticosteroids had no effect on periorbital cellulitis but increased length of stay for orbital cellulitis. Likewise, a retrospective study by Yen 2005 of 23 children did not find any difference in length of hospital stay between patients treated with and without corticosteroids. In contrast, in a retrospective study of 35 children, investigators found evidence of a difference between patients treated with and without corticosteroids in days until clinically significant improvement occurred, although it was unclear what criterion was used to define clinically significant improvement (Brameli 2018). Limited conclusions can be drawn from these retrospective studies due to the significant practice variation within the study populations, both with regards to how corticosteroids were used and other aspects of treatment (Markham 2018; Yen 2005).
Two non‐randomized prospective studies, both in pediatric populations, suggested a benefit of corticosteroids, particularly in leading to a decrease in length of hospital stay. Davies 2015 included 31 children, of which 21 received oral corticosteroids; the remaining 10 children, whose parents did not consent to treatment with corticosteroids, served as the control group. Children who were started on oral prednisone following a decline in CRP to below 40 mg/L had a three‐day shorter hospital stay compared to those receiving only antibiotics (4.0 days versus 7.2 days). Two patients in the corticosteroids group reported hyperactivity, one of which led to the cessation of treatment. Interestingly, Chen 2018 found similar results when administering corticosteroids immediately following admission to hospital. This study included 43 children, 28 assigned to corticosteroids and 15 to antibiotics alone based on whether the parents consented to the use of corticosteroids. Children receiving corticosteroids in addition to antibiotics had a shorter duration of hospital stay compared to children receiving antibiotics alone (3.8 days versus 6.7 days). Two patients experienced minor hyperactivity, and one experienced minor insomnia. Both observational studies had a shorter mean length of stay than Pushker 2013, although they found a clinically meaningful three‐day reduction in length of hospital stay with corticosteroids. While our review did not find any decrease in hospital stay as was observed in these two non‐randomized prospective studies, some improvements in disease course were seen. These studies further suggest a state of equipoise regarding the use of corticosteroids and call for more high‐quality RCTs on this topic (Markham 2018; Yen 2005).
Authors' conclusions
Implications for practice.
There is currently variation in the use of corticosteroids in individuals with periorbital and orbital cellulitis, which may be due in part to the paucity of evidence on corticosteroids. While it is plausible that corticosteroids could improve the disease course by reducing inflammation and subsequent damage to the eye and surrounding structures, there are also concerns about immune suppression. Our review suggests that at present, there is insufficient high‐quality evidence to inform decisions around the use of corticosteroids for periorbital and orbital cellulitis.
Implications for research.
The one included study provided inadequate evidence to achieve the aims of this review, and highlights the urgent need to conduct comparative effectiveness research on this topic. More high‐quality randomized controlled trials are thus needed before any definitive conclusions can be made. In particular, these studies should explore potential differences in the effects of corticosteroids in pediatric and adult populations. Past research has suggested there may be differences in disease characteristics between children and adults, and at present, there are variations in physicians’ use of corticosteroids based on patient age. Future studies should also explore the timing of administration of corticosteroids, evaluate different dosing and timing regimes of therapy, and the effects of corticosteroids in various practice settings. If corticosteroids are found to have beneficial effects, future trials should explore the optimal timing of administration to maximize benefit from this treatment. Lastly, the role of intranasal corticosteroids in periorbital and orbital cellulitis needs to be explored.
History
Protocol first published: Issue 2, 2020 Review first published: Issue 4, 2021
Acknowledgements
We would like to thank Lily Ren for her assistance with the search. The Methods section of this review is based on a standard template developed by Cochrane Eyes and Vision (CEV). We acknowledge Lori Rosman, Information Specialist for CEV, for developing the search strategies for the electronic databases and executing the searches. We thank the CEV US Project for providing methodological support. We are grateful to the following peer reviewers for their time and comments: Christopher Gappy (University of Michigan) and Richard Mangan (University of Colorado).
This review was managed by CEV@US and was signed off for publication by Tianjing Li and Gianni Virgili.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Cellulitis] explode all trees #2 MeSH descriptor: [Orbital Cellulitis] explode all trees #3 ((periorbit* OR orbit* OR preseptal OR "pre septal" OR postseptal OR "post septal") NEAR/3 celluliti*) #4 ((subperiosteal OR "sub periosteal" OR orbital OR intraconal) NEXT/1 abscess*) #5 MeSH descriptor: [Eye Infections, Bacterial] explode all trees #6 (Bacterial NEAR/3 eye infect*) #7 MeSH descriptor: [Orbital Diseases] this term only #8 orbital NEXT/1 disease* #9 MeSH descriptor: [Sinusitis] explode all trees #10 sinusitis OR (sinus NEXT/2 infect*) OR rhinosinusitis #11 "Sinus venous thrombosis" #12 {OR #1‐#11} #13 MeSH descriptor: [Orbital Cellulitis] explode all trees and with qualifier(s): [drug therapy ‐ DT] #14 MeSH descriptor: [Steroids] explode all trees #15 Steroid* #16 MeSH descriptor: [Adrenal Cortex Hormones] explode all trees #17 (("Adrenal Cortex" NEXT/1 Hormone*) OR Corticosteroid* OR Corticoid* OR Glucocorticoid* OR (Glucorticoid NEXT/1 Effect*) OR Hydroxycorticosteroid* OR ("Adrenal cortical" NEXT/1 hormone*) OR (Adrenocortical NEXT/1 hormone*) OR Adrenocorticosteroid* OR Dermocorticosteroid*) #18 Glucocorticosteroid* OR Glucocorticoidsteroid* OR Glucocortoid* OR Glycocorticoid* OR Glycocorticosteroid* #19 Dexamethasone OR adrecort OR adrenocot OR "aeroseb dex" OR aflucoson OR aflucosone OR alfalyl OR anaflogistico OR arcodexan OR arcodexane OR artrosone OR azium OR bidexol OR calonat OR cebedex OR cetadexon OR colofoam OR corsona OR cortastat OR cortidex OR cortidexason OR cortidrona OR cortidrone OR cortisumman OR "dacortina fuerte" OR "dacortine fuerte" OR dalalone OR danasone OR "de‐sone la" OR decacortin OR decadeltosona OR decadeltosone OR decaderm OR decadion OR decadran OR decadron OR decadronal OR decadrone OR decaesadril OR decaject OR decameth OR decamethasone OR decasone OR decaspray OR decasterolone OR decdan OR decilone OR decofluor OR dectancyl OR dekacort OR delladec OR deltafluoren OR deltafluorene OR dergramin OR deronil OR desacort OR desacortone OR desadrene OR desalark OR desameton OR desametone OR desigdron OR "dexa cortisyl" OR "dexa dabrosan" OR "dexa korti" OR "dexa scherosan" OR "dexa scherozon" OR "dexa scherozone" OR "dexa‐p" OR "dexacen 4" OR dexachel OR dexacort OR dexacortal OR dexacorten OR dexacortin OR dexacortisyl OR dexadabroson OR dexadecadrol OR dexadrol OR dexagel OR dexagen OR dexahelvacort OR dexakorti OR dexalien OR dexalocal OR dexame OR dexamecortin OR dexameson OR dexamesone OR dexametason OR dexametasone OR dexameth OR dexamethason OR dexamethazon OR dexamethonium OR dexamonozon OR dexan OR dexane OR dexano OR dexapot OR dexascheroson OR dexascherozon OR dexascherozone OR dexason OR dexasone OR dexinoral OR dexionil OR dexmethsone OR dexona OR dexone OR dexpak OR dextelan OR dextrasone OR dexycu OR dezone OR dibasona OR doxamethasone OR esacortene OR "ex s1" OR exadion OR exadione OR firmalone OR "fluormethyl prednisolone" OR fluormethylprednisolon OR fluormethylprednisolone OR fluormone OR fluorocort OR fluorodelta OR fluoromethylprednisolone OR fortecortin OR gammacorten OR gammacortene OR grosodexon OR grosodexone OR hexadecadiol OR hexadecadrol OR hexadiol OR hexadrol OR isnacort OR "isopto dex" OR "isopto maxidex" OR "isopto‐maxidex" OR isoptodex OR isoptomaxidex OR "lokalison f" OR loverine OR luxazone OR marvidione OR maxidex OR mediamethasone OR megacortin OR mephameson OR mephamesone OR metasolon OR metasolone OR "methazon ion" OR "methazone ion" OR methazonion OR methazonione OR methylfluorprednisolone OR "metisone lafi" OR mexasone OR millicorten OR millicortenol OR "mk 125" OR mk125 OR mymethasone OR neoforderx OR neofordex OR nisomethasona OR novocort OR "nsc 34521" OR nsc34521 OR "oftan‐dexa" OR opticorten OR opticortinol OR oradexan OR oradexon OR oradexone OR orgadrone OR ozurdex OR pidexon OR policort OR posurdex OR "predni f tablinen" OR "predni‐f" OR "prednisolone f" OR prodexona OR prodexone OR sanamethasone OR santenson OR santeson OR sawasone OR solurex OR spoloven OR sterasone OR thilodexine OR triamcimetil OR vexamet OR visumetazone OR visumethazone OR "50‐02‐2" #20 Prednisone OR ancortone OR biocortone OR colisone OR cortan OR cortancyl OR cortidelt OR cortiprex OR cutason OR dacorten OR "de cortisyl" OR decortancyl OR dacortin OR decortin OR decortine OR decortisyl OR dehydrocortisone OR dekortin OR delitisone OR "dellacort a" OR "delta cortelan" OR "delta cortisone" OR "delta dome" OR "delta e" OR "delta prenovis" OR deltacorten OR deltacortene OR deltacortisone OR deltacortone OR deltasone OR deltison OR deltisona OR deltra OR "di adreson" OR diadreson OR drazone OR encorton OR encortone OR enkorton OR enkortolon OR fernisone OR hostacortin OR insone OR "liquid pred" OR lodotra OR "me‐korti" OR meprison OR metacortandracin OR meticorten OR meticortine OR nisona OR "nsc 10023" OR nsc10023 OR orasone OR orisane OR panafcort OR panasol OR paracort OR pehacort OR precort OR precortal OR "predni tablinen" OR "prednicen‐m" OR prednicorm OR prednicot OR prednidib OR predniment OR prednison OR prednitone OR "pregna 1, 4 diene 3, 11, 20 trione 17, 21 diol" OR pronison OR pronisone OR pronizone OR pulmison OR rayos OR rectodelt OR servisone OR sone OR steerometz OR sterapred OR ultracorten OR urtilone OR winpred OR "53‐03‐2" #21 Prednisolone OR adelcort OR antisolon OR antisolone OR aprednislon OR aprednislone OR benisolon OR benisolone OR berisolon OR berisolone OR caberdelta OR capsoid OR "co hydeltra" OR codelcortone OR compresolon OR cortadeltona OR cortadeltone OR cortalone OR cortelinter OR cortisolone OR cotolone OR dacortin OR "dacortin h" OR dacrotin OR decaprednil OR "decortin h" OR decortril OR "dehydro cortex" OR "dehydro hydrocortisone" OR "dehydro hydrocortisone" OR dehydrocortex OR dehydrocortisol OR dehydrocortisole OR dehydrohydrocortison OR dehydrohydrocortisone OR delcortol OR "delta 1 17 hydroxycorticosterone 21 acetate OR delta 1 hydrocortisone" OR "delta cortef" OR "delta cortril" OR "delta ef cortelan" OR "delta f OR delta hycortol" OR "delta hydrocortisone" OR "delta hydrocortisone" OR "delta ophticor" OR "delta stab" OR "delta‐cortef" OR "delta1 dehydrocortisol" OR "delta1 dehydrohydrocortisone" OR "delta1 hydrocortisone" OR deltacortef OR deltacortenolo OR deltacortil OR deltacortoil OR deltacortril OR deltaderm OR deltaglycortril OR deltahycortol OR deltahydrocortison OR deltahydrocortisone OR deltaophticor OR deltasolone OR deltastab OR deltidrosol OR deltisilone OR deltisolon OR deltisolone OR deltolasson OR deltolassone OR deltosona OR deltosone OR dermosolon OR dhasolone OR DiAdresonF OR "di adreson f" OR "di adresone f" OR "diadreson f" OR "diadresone f" OR dicortol OR domucortone OR encortelon OR encortelone OR encortolon OR equisolon OR "fernisolone‐p" OR glistelone OR hefasolon OR "hostacortin h" OR hydeltra OR hydeltrone OR hydrelta OR hydrocortancyl OR hydrocortidelt OR hydrodeltalone OR hydrodeltisone OR hydroretrocortin OR hydroretrocortine OR inflanefran OR insolone OR "keteocort h" OR "key‐pred" OR lenisolone OR leocortol OR liquipred OR "lygal kopftinktur n" OR mediasolone OR meprisolon OR meprisolone OR metacortalon OR metacortalone OR metacortandralon OR metacortandralone OR metacortelone OR "meti derm" OR meticortelone OR metiderm OR morlone OR mydrapred OR "neo delta" OR nisolon OR nisolone OR "nsc 9120" OR nsc9120 OR opredsone OR panafcortelone OR panafcortolone OR panafort OR paracortol OR phlogex OR "pre cortisyl" OR preconin OR precortalon OR precortancyl OR precortisyl OR "pred‐ject‐50" OR "predacort 50" OR "predaject‐50" OR "predalone 50" OR predartrina OR predartrine OR predate OR predeltilone OR predisole OR predisyr OR "predne dome" OR prednecort OR prednedome OR prednelan OR "predni coelin" OR "predni h tablinen" OR "predni‐helvacort" OR prednicoelin OR prednicort OR prednicortelone OR "prednifor drops" OR predniment OR predniretard OR prednis OR prednisil OR prednisolon OR prednisolona OR prednivet OR prednorsolon OR prednorsolone OR predonine OR predorgasolona OR predorgasolone OR "pregna 1, 4 diene 11beta, 17alpha, 21 triol 3, 20 dione" OR prelon OR prelone OR prenilone OR prenin OR prenolone OR preventan OR prezolon OR rubycort OR scherisolon OR scherisolona OR serilone OR solondo OR solone OR solupren OR soluprene OR spiricort OR spolotane OR sterane OR sterolone OR supercortisol OR supercortizol OR taracortelone OR walesolone OR wysolone OR "50‐24‐8" #22 Methylprednisolone OR "adlone‐40" OR "adlone‐80" OR "dep medalone 80" OR depmedalone OR "depoject‐80" OR depopred OR esametone OR firmacort OR "med‐jec‐40" OR medixon OR mednin OR "medralone 80" OR medrate OR medrol OR medrone OR meprednisolone OR meprelon OR mesopren OR "methacort 40" OR "methacort 80" OR "methyl prednisolone" OR methylcotol OR methylcotolone OR "methylpred dp" OR methylsterolone OR metidrol OR metipred OR metrisone OR metycortin OR metypred OR metypresol OR neomedrone OR nsc 19987 OR nsc19987 OR prednol OR solomet OR "solu decortin" OR urbason OR "6923‐42‐8" OR "83‐43‐2" #23 Hydrocortisone OR acticort OR "aeroseb hc" OR "ala‐cort" OR "ala‐scalp" OR alfacort OR algicortis OR alkindi OR "alpha derm" OR alphaderm OR "anucort‐hc" OR "anumed‐hc" OR "anutone‐hc" OR "aquanil hc" OR "balneol‐hc" OR "barseb hc" OR "beta‐hc" OR biacort OR cetacort OR cobadex OR colocort OR "compound f" OR "cordicare lotion" OR coripen OR "cort dome" OR cortef OR cortenema OR cortibel OR corticorenol OR cortifair OR cortifan OR cortiphate OR cortisol OR cortisole OR cortispray OR cortoderm OR cortril OR cotacort OR covocort OR "cremicort‐h" OR cutaderm OR "derm‐aid cream" OR "dermacrin hc lotion" OR dermaid OR dermocare OR dermocortal OR dermolate OR dioderm OR eczacort OR "ef cortelan" OR efcortelan OR egocort OR eksalb OR eldecort OR "emo‐cort" OR epicort OR epicortisol OR ficortril OR filocot OR flexicort OR "gly‐cort" OR glycort OR "h‐cort" OR "hc no. 1" OR "hc no. 4" OR hebcort OR "hemorrhoidal hc" OR "hemril‐30" OR "hemril‐hc uniserts" OR "hi‐cor" OR hidrotisona OR hycor OR hycort OR hydracort OR hydrasson OR "hydro ricortex" OR "hydro‐rx" OR hydrocort OR hydrocorticosteroid OR hydrocortisate OR hydrocortison OR hydrocortisonum OR hydrocortisyl OR hydrocortone OR hydrogalen OR hydrokort OR hydrokortison OR hydrotopic OR hysone OR hytisone OR hytone OR "incortin h" OR "instacort 10" OR kyypakkaus OR "lacticare hc" OR "lemnis fatty cream hc" OR lenirit OR "medihaler cort" OR "medihaler duo" OR medrocil OR mildison OR "mitocortyl demangeaisons" OR munitren OR "nogenic hc" OR novohydrocort OR "nsc 10483" OR "nsc 741" OR nsc10483 OR nutracort OR optef OR "otosone f" OR penecort OR plenadren OR prepcort OR "prevex hc" OR "pro cort" OR procort OR "procto‐kit" OR proctocort OR "proctosert hc" OR "proctosol‐hc" OR proctosone OR "proctozone hc" OR procutan OR "rectasol‐hc" OR rectocort OR rederm OR sanatison OR "scalp‐aid" OR schericur OR "scherosone f" OR skincalm OR "stie‐cort" OR "substance m" OR synacort OR texacort OR "triburon‐hc" OR unicort OR vasocort OR "50‐23‐7" #24 Loteprednol OR alrex OR "cddd 5604" OR cddd5604 OR CEHOAC OR "chloromethyl 17alpha ethoxycarbonyloxy 11beta hydroxy 3 oxoandrosta 1, 4 diene 17 carboxylate" OR "hgp 1" OR hgp1 OR inveltys OR "kpi 121" OR kpi121 OR "le‐mpp" OR lotemax OR loterex OR loterox OR lotesoft OR "p 5604" OR p5604 OR "82034‐46‐6" #25 Rimexolone OR baxol OR "org 6216" OR org6216 OR "pregna 1, 4 dien 11beta ol 3, 20 dione, 16alpha, 17alpha, 21 trimethyl" OR rimexel OR trimexolone OR vexol OR vexolon OR "49697‐38‐3" #26 "Prednisolone acetate" OR "ak‐pred" OR deppredalone OR econopred OR "flo pred" OR "isopto cetapred" OR "key pred" OR "meticortelone acetate" OR "ocu‐pred" OR omnipred OR optilon OR "poly pred" OR "pred forte" OR "pred mild" OR predforte OR predmet OR "prednefrin sf" OR "predni h" OR "predni‐ophtal" OR prednigalen OR "prednisolone 17 acetate" OR "prednisolone 21 acetate" OR "prednisolone acetate suspension" OR savacort OR "Scherisolone‐Kristall suspension" OR ultracortensol OR ultracortinol OR "52‐21‐1" OR "52628‐64‐5" #27 Beclomethasone OR beclometasone OR "Asmabec Clickhaler" OR Ascocortonyl OR Beclamet OR "Beclo Asma" OR "Beclo AZU" OR Beclocort OR Beclomet OR "Bemedrex Easyhaler" OR Beclorhinol OR Sanasthmax OR Becloturmant OR Beclovent OR Beconase OR Becloforte OR Becodisk OR Becodisks OR Propaderm OR Becotide OR Sanasthmyl OR "Beconase AQ" OR Bronchocort OR Junik OR Qvar OR Ecobec OR Beclazone OR Ventolair OR Prolair OR Filair OR "AeroBec Forte" OR Aerobec OR "Nasobec Aqueous" OR Respocort OR Vancenase OR Vanceril OR Aldecin OR Viarin OR "4419‐39‐0" #28 Betamethasone OR adbeon OR becasone OR beprogel OR "beta methason" OR "beta methasone" OR "beta‐phos/ac" OR betacortril OR betadexamethasone OR betametasone OR betamethasolone OR betamethason OR betamethasonium OR betamethasonum OR betamethazone OR betason OR betnasol OR betnelan OR "betnesol h" OR "betnesol v" OR "betnovate a" OR betsolan OR betsolon OR betsopart OR celestan OR celestene OR celeston OR celestona OR celestone OR cellestoderm OR cidoten OR dermobet OR diprolen OR flubenisolone OR methasone OR "nsc 39470" OR nsc39470 OR "pregna 1, 4 diene 3, 20 dione 11, 17, 21 triol, 9 alpha fluoro 16beta methyl" OR "rg 833" OR rg833 OR rinderon OR "sch 4831" OR sch4831 OR walacort OR "378‐44‐9" #29 Budesonide OR acorspray OR aerox OR allercort OR aquacort OR "b cort" OR "bebe cream" OR bidien OR budecol OR budecort OR budefat OR budeflam OR budelin OR "budenase aq" OR budeno OR budenofalk OR budenoside OR budes OR budeson OR "budesonide easyhaler" OR budiair OR "budicort respules" OR "budo‐san" OR budon OR budosan OR bunase OR buparid OR butacort OR clebudan OR coramen OR cortiment OR cortimentmmx OR cycortide OR "desona nasal" OR desonix OR dexbudesonide OR duasma OR eltair OR entocir OR entocort OR esonide OR "giona easyhaler" OR horacort OR inflammide OR inflanaze OR intesticort OR intestifalk OR jorveza OR larbex OR "map 0010" OR map0010 OR miflo OR miflonid OR miflonide OR miflonil OR mikicort OR nebbud OR "neo‐rinactive" OR novopulmon OR numark OR olfex OR preferid OR "pregna 1, 4 diene 3, 20 dione 16, 17 butyraldehyde cyclic acetal, 11beta, 16alpha, 17, 21 tetrahydroxy" OR pulmaxan OR "pulmicon susp for nebulizer" OR "pulmicon susp for nebulizer" OR pulmicort OR pulmoliseflam OR pulmotide OR respicort OR rhinocort OR ribujet OR ribuspir OR ribuvent OR "s 1320" OR s1320 OR spirocort OR "tafen nasal" OR uceris OR "51333‐22‐3" OR "51372‐29‐3" #30 Ciclesonide OR alvesco OR "b 9207 015" OR "b 9207015" OR "b9207 015" OR "b9207015" OR "by 9010" OR "by9010" OR omnaris OR zetonna OR "126544‐47‐6" #31 Flunisolide OR aerobid OR aerospan OR bronalide OR bronilide OR cyntaris OR flunitec OR gibiflu OR inhacort OR locasyn OR lokilan OR "lunibron‐a" OR lunis OR nasalide OR nasarel OR rhinalar OR "rs 3999" OR rs3999 OR sanergal OR synaclyn OR syntaris OR "val 679" OR val679 OR "3385‐03‐3" #32 Fluticasone OR Flonase OR Flovent OR Cutivate OR Flixonase OR Flixotide OR "90566‐53‐3" #33 Mometasone OR "allermax aqueous" OR asmanex OR belloseta OR bloctimo OR breso OR brusonex OR cutticom OR dermotasone OR dermovel OR desdek OR ecural OR edelan OR elica OR elocom OR elocon OR elocyn OR elomet OR eloson OR flumeta OR frondava OR ivoxel OR kalmente OR lorome OR mefurosan OR metaspray OR metmin OR mipasu OR mofunder OR momate OR momeallerg OR momecutan OR momederm OR momegalen OR momekort OR momesonex OR momespir OR momester OR mometahexal OR mometason OR mometasone OR mometop OR mommox OR monovel OR monovo OR morecort OR motaderm OR mundoson OR muofuder OR nasehaler OR nasoaldo OR nasomet OR nasometin OR nasonex OR nasotasone OR netonox OR "novasone cream" OR "novasone lotion" OR "novasone ointment" OR ovison OR ovixan OR pronasal OR rinelon OR rinometasone OR rivelon OR "sch 32088" OR sch32088 OR sebanez OR sinuva OR sonalox OR temon OR uniclar OR zhekort OR "105102‐22‐5" OR "83919‐23‐7" #34 Triamcinolone OR acetocot OR adcortyl OR aristocort OR aristodan OR azmacor OR celeste OR "cl 19823" OR cl19823 OR clinacort OR clinalog OR delphicort OR fluoxiprednisolone OR fluoxyprednisolone OR "ken‐jec 40" OR kenacort OR korticoid OR ledercort OR omcilon OR polcortolon OR "rp 8357" OR rp8357 OR simacort OR sterocort OR "tac 3" OR tramcinolone OR triacortyl OR "triam‐a" OR "triam‐forte" OR triamcinolon OR triamcinolona OR triamcort OR triamcot OR "triamonide 40" OR triamsicort OR triancinolon OR "u‐tri‐lone" OR volon OR "124‐94‐7" #35 {OR #13‐#34} #36 #12 AND #35
Appendix 2. MEDLINE (Ovid) search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Cellulitis/ 13. exp Orbital Cellulitis/ 14. ((periorbit* or orbit* or preseptal or "pre septal" or postseptal or "post septal") adj3 celluliti*).tw. 15. ((subperiosteal or "sub periosteal" or orbital or intraconal) adj1 abscess*).tw. 16. exp Eye Infections, Bacterial/ 17. (Bacterial adj3 eye infect*).tw. 18. Orbital Diseases/ 19. (orbital adj1 disease*).tw. 20. exp Sinusitis/ 21. (sinusitis or (sinus adj2 infect*) or rhinosinusitis).tw. 22. "Sinus venous thrombosis".tw. 23. or/12‐22 24. exp Orbital Cellulitis/dt [Drug Therapy] 25. exp Steroids/ 26. Steroid*.tw. 27. exp Adrenal Cortex Hormones/ 28. (("Adrenal Cortex" adj1 Hormone*) or Corticosteroid* or Corticoid* or Glucocorticoid* or (Glucorticoid adj1 Effect*) or Hydroxycorticosteroid* or ("Adrenal cortical" adj1 hormone*) or (Adrenocortical adj1 hormone*) or Adrenocorticosteroid* or Dermocorticosteroid*).tw. 29. (Glucocorticosteroid* or Glucocorticoidsteroid* or Glucocortoid* or Glycocorticoid* or Glycocorticosteroid*).tw. 30. (Dexamethasone or adrecort or adrenocot or aeroseb dex or aflucoson or aflucosone or alfalyl or anaflogistico or arcodexan or arcodexane or artrosone or azium or bidexol or calonat or cebedex or cetadexon or colofoam or corsona or cortastat or cortidex or cortidexason or cortidrona or cortidrone or cortisumman or "dacortina fuerte" or "dacortine fuerte" or dalalone or danasone or "de‐sone la" or decacortin or decadeltosona or decadeltosone or decaderm or decadion or decadran or decadron or decadronal or decadrone or decaesadril or decaject or decameth or decamethasone or decasone or decaspray or decasterolone or decdan or decilone or decofluor or dectancyl or dekacort or delladec or deltafluoren or deltafluorene or dergramin or deronil or desacort or desacortone or desadrene or desalark or desameton or desametone or desigdron or "dexa cortisyl" or "dexa dabrosan" or "dexa korti" or "dexa scherosan" or "dexa scherozon" or "dexa scherozone" or "dexa‐p" or "dexacen 4" or dexachel or dexacort or dexacortal or dexacorten or dexacortin or dexacortisyl or dexadabroson or dexadecadrol or dexadrol or dexagel or dexagen or dexahelvacort or dexakorti or dexalien or dexalocal or dexame or dexamecortin or dexameson or dexamesone or dexametason or dexametasone or dexameth or dexamethason or dexamethazon or dexamethonium or dexamonozon or dexan or dexane or dexano or dexapot or dexascheroson or dexascherozon or dexascherozone or dexason or dexasone or dexinoral or dexionil or dexmethsone or dexona or dexone or dexpak or dextelan or dextrasone or dexycu or dezone or dibasona or doxamethasone or esacortene or "ex s1" or exadion or exadione or firmalone or "fluormethyl prednisolone" or fluormethylprednisolon or fluormethylprednisolone or fluormone or fluorocort or fluorodelta or fluoromethylprednisolone or fortecortin or gammacorten or gammacortene or grosodexon or grosodexone or hexadecadiol or hexadecadrol or hexadiol or hexadrol or isnacort or "isopto dex" or "isopto maxidex" or "isopto‐maxidex" or isoptodex or isoptomaxidex or "lokalison f" or loverine or luxazone or marvidione or maxidex or mediamethasone or megacortin or mephameson or mephamesone or metasolon or metasolone or "methazon ion" or "methazone ion" or methazonion or methazonione or methylfluorprednisolone or "metisone lafi" or mexasone or millicorten or millicortenol or "mk 125" or mk125 or mymethasone or neoforderx or neofordex or nisomethasona or novocort or "nsc 34521" or nsc34521 or "oftan‐dexa" or opticorten or opticortinol or oradexan or oradexon or oradexone or orgadrone or ozurdex or pidexon or policort or posurdex or "predni f tablinen" or "predni‐f" or "prednisolone f" or prodexona or prodexone or sanamethasone or santenson or santeson or sawasone or solurex or spoloven or sterasone or thilodexine or triamcimetil or vexamet or visumetazone or visumethazone or "50‐02‐2").tw. 31. (Prednisone or ancortone or biocortone or colisone or cortan or cortancyl or cortidelt or cortiprex or cutason or dacorten or "de cortisyl" or decortancyl or dacortin or decortin or decortine or decortisyl or dehydrocortisone or dekortin or delitisone or "dellacort a" or "delta cortelan" or "delta cortisone" or "delta dome" or "delta e" or "delta prenovis" or deltacorten or deltacortene or deltacortisone or deltacortone or deltasone or deltison or deltisona or deltra or "di adreson" or diadreson or drazone or encorton or encortone or enkorton or enkortolon or fernisone or hostacortin or insone or "liquid pred" or lodotra or "me‐korti" or meprison or metacortandracin or meticorten or meticortine or nisona or "nsc 10023" or nsc10023 or orasone or orisane or panafcort or panasol or paracort or pehacort or precort or precortal or "predni tablinen" or "prednicen‐m" or prednicorm or prednicot or prednidib or predniment or prednison or prednitone or "pregna 1, 4 diene 3, 11, 20 trione 17, 21 diol" or pronison or pronisone or pronizone or pulmison or rayos or rectodelt or servisone or sone or steerometz or sterapred or ultracorten or urtilone or winpred or "53‐03‐2").tw. 32. (Prednisolone or adelcort or antisolon or antisolone or aprednislon or aprednislone or benisolon or benisolone or berisolon or berisolone or caberdelta or capsoid or "co hydeltra" or codelcortone or compresolon or cortadeltona or cortadeltone or cortalone or cortelinter or cortisolone or cotolone or dacortin or "dacortin h" or dacrotin or decaprednil or "decortin h" or decortril or "dehydro cortex" or "dehydro hydrocortisone" or "dehydro hydrocortisone" or dehydrocortex or dehydrocortisol or dehydrocortisole or dehydrohydrocortison or dehydrohydrocortisone or delcortol or "delta 1 17 hydroxycorticosterone 21 acetate OR delta 1 hydrocortisone" or "delta cortef" or "delta cortril" or "delta ef cortelan" or "delta f OR delta hycortol" or "delta hydrocortisone" or "delta hydrocortisone" or "delta ophticor" or "delta stab" or "delta‐cortef" or "delta1 dehydrocortisol" or "delta1 dehydrohydrocortisone" or "delta1 hydrocortisone" or deltacortef or deltacortenolo or deltacortil or deltacortoil or deltacortril or deltaderm or deltaglycortril or deltahycortol or deltahydrocortison or deltahydrocortisone or deltaophticor or deltasolone or deltastab or deltidrosol or deltisilone or deltisolon or deltisolone or deltolasson or deltolassone or deltosona or deltosone or dermosolon or dhasolone or DiAdresonF or "di adreson f" or "di adresone f" or "diadreson f" or "diadresone f" or dicortol or domucortone or encortelon or encortelone or encortolon or equisolon or "fernisolone‐p" or glistelone or hefasolon or "hostacortin h" or hydeltra or hydeltrone or hydrelta or hydrocortancyl or hydrocortidelt or hydrodeltalone or hydrodeltisone or hydroretrocortin or hydroretrocortine or inflanefran or insolone or "keteocort h" or "key‐pred" or lenisolone or leocortol or liquipred or "lygal kopftinktur n" or mediasolone or meprisolon or meprisolone or metacortalon or metacortalone or metacortandralon or metacortandralone or metacortelone or "meti derm" or meticortelone or metiderm or morlone or mydrapred or "neo delta" or nisolon or nisolone or "nsc 9120" or nsc9120 or opredsone or panafcortelone or panafcortolone or panafort or paracortol or phlogex or "pre cortisyl" or preconin or precortalon or precortancyl or precortisyl or "pred‐ject‐50" or "predacort 50" or "predaject‐50" or "predalone 50" or predartrina or predartrine or predate or predeltilone or predisole or predisyr or "predne dome" or prednecort or prednedome or prednelan or "predni coelin" or "predni h tablinen" or "predni‐helvacort" or prednicoelin or prednicort or prednicortelone or "prednifor drops" or predniment or predniretard or prednis or prednisil or prednisolon or prednisolona or prednivet or prednorsolon or prednorsolone or predonine or predorgasolona or predorgasolone or "pregna 1, 4 diene 11beta, 17alpha, 21 triol 3, 20 dione" or prelon or prelone or prenilone or prenin or prenolone or preventan or prezolon or rubycort or scherisolon or scherisolona or serilone or solondo or solone or solupren or soluprene or spiricort or spolotane or sterane or sterolone or supercortisol or supercortizol or taracortelone or walesolone or wysolone or "50‐24‐8").tw. 33. (Methylprednisolone or "adlone‐40" or "adlone‐80" or "dep medalone 80" or depmedalone or "depoject‐80" or depopred or esametone or firmacort or "med‐jec‐40" or medixon or mednin or "medralone 80" or medrate or medrol or medrone or meprednisolone or meprelon or mesopren or "methacort 40" or "methacort 80" or "methyl prednisolone" or methylcotol or methylcotolone or "methylpred dp" or methylsterolone or metidrol or metipred or metrisone or metycortin or metypred or metypresol or neomedrone or nsc 19987 or nsc19987 or prednol or solomet or "solu decortin" or urbason or "6923‐42‐8" or "83‐43‐2").tw. 34. (Hydrocortisone or acticort or "aeroseb hc" or "ala‐cort" or "ala‐scalp" or alfacort or algicortis or alkindi or "alpha derm" or alphaderm or "anucort‐hc" or "anumed‐hc" or "anutone‐hc" or "aquanil hc" or "balneol‐hc" or "barseb hc" or "beta‐hc" or biacort or cetacort or cobadex or colocort or "compound f" or "cordicare lotion" or coripen or "cort dome" or cortef or cortenema or cortibel or corticorenol or cortifair or cortifan or cortiphate or cortisol or cortisole or cortispray or cortoderm or cortril or cotacort or covocort or "cremicort‐h" or cutaderm or "derm‐aid cream" or "dermacrin hc lotion" or dermaid or dermocare or dermocortal or dermolate or dioderm or eczacort or "ef cortelan" or efcortelan or egocort or eksalb or eldecort or "emo‐cort" or epicort or epicortisol or ficortril or filocot or flexicort or "gly‐cort" or glycort or "h‐cort" or "hc no. 1" or "hc no. 4" or hebcort or "hemorrhoidal hc" or "hemril‐30" or "hemril‐hc uniserts" or "hi‐cor" or hidrotisona or hycor or hycort or hydracort or hydrasson or "hydro ricortex" or "hydro‐rx" or hydrocort or hydrocorticosteroid or hydrocortisate or hydrocortison or hydrocortisonum or hydrocortisyl or hydrocortone or hydrogalen or hydrokort or hydrokortison or hydrotopic or hysone or hytisone or hytone or "incortin h" or "instacort 10" or kyypakkaus or "lacticare hc" or "lemnis fatty cream hc" or lenirit or "medihaler cort" or "medihaler duo" or medrocil or mildison or "mitocortyl demangeaisons" or munitren or "nogenic hc" or novohydrocort or "nsc 10483" or "nsc 741" or nsc10483 or nutracort or optef or "otosone f" or penecort or plenadren or prepcort or "prevex hc" or "pro cort" or procort or "procto‐kit" or proctocort or "proctosert hc" or "proctosol‐hc" or proctosone or "proctozone hc" or procutan or "rectasol‐hc" or rectocort or rederm or sanatison or "scalp‐aid" or schericur or "scherosone f" or skincalm or "stie‐cort" or "substance m" or synacort or texacort or "triburon‐hc" or unicort or vasocort or "50‐23‐7").tw. 35. (Loteprednol or alrex or "cddd 5604" or cddd5604 or CEHOAC or "chloromethyl 17alpha ethoxycarbonyloxy 11beta hydroxy 3 oxoandrosta 1, 4 diene 17 carboxylate" or "hgp 1" or hgp1 or inveltys or "kpi 121" or kpi121 or "le‐mpp" or lotemax or loterex or loterox or lotesoft or "p 5604" or p5604 or "82034‐46‐6").tw. 36. (Rimexolone or baxol or "org 6216" or org6216 or "pregna 1, 4 dien 11beta ol 3, 20 dione, 16alpha, 17alpha, 21 trimethyl" or rimexel or trimexolone or vexol or vexolon or "49697‐38‐3 #26 Prednisolone acetate" or "ak‐pred" or deppredalone or econopred or "flo pred" or "isopto cetapred" or "key pred" or "meticortelone acetate" or "ocu‐pred" or omnipred or optilon or "poly pred" or "pred forte" or "pred mild" or predforte or predmet or "prednefrin sf" or "predni h" or "predni‐ophtal" or prednigalen or "prednisolone 17 acetate" or "prednisolone 21 acetate" or "prednisolone acetate suspension" or savacort or "Scherisolone‐Kristall suspension" or ultracortensol or ultracortinol or "52‐21‐1" or "52628‐64‐5").tw. 37. (Beclomethasone or beclometasone or "Asmabec Clickhaler" or Ascocortonyl or Beclamet or "Beclo Asma" or "Beclo AZU" or Beclocort or Beclomet or "Bemedrex Easyhaler" or Beclorhinol or Sanasthmax or Becloturmant or Beclovent or Beconase or Becloforte or Becodisk or Becodisks or Propaderm or Becotide or Sanasthmyl or "Beconase AQ" or Bronchocort or Junik or Qvar or Ecobec or Beclazone or Ventolair or Prolair or Filair or "AeroBec Forte" or Aerobec or "Nasobec Aqueous" or Respocort or Vancenase or Vanceril or Aldecin or Viarin or "4419‐39‐0").tw. 38. (Betamethasone or adbeon or becasone or beprogel or "beta methason" or "beta methasone" or "beta‐phos/ac" or betacortril or betadexamethasone or betametasone or betamethasolone or betamethason or betamethasonium or betamethasonum or betamethazone or betason or betnasol or betnelan or "betnesol h" or "betnesol v" or "betnovate a" or betsolan or betsolon or betsopart or celestan or celestene or celeston or celestona or celestone or cellestoderm or cidoten or dermobet or diprolen or flubenisolone or methasone or "nsc 39470" or nsc39470 or "pregna 1, 4 diene 3, 20 dione 11, 17, 21 triol, 9 alpha fluoro 16beta methyl" or "rg 833" or rg833 or rinderon or "sch 4831" or sch4831 or walacort or "378‐44‐9").tw. 39. (Budesonide or acorspray or aerox or allercort or aquacort or "b cort" or "bebe cream" or bidien or budecol or budecort or budefat or budeflam or budelin or "budenase aq" or budeno or budenofalk or budenoside or budes or budeson or "budesonide easyhaler" or budiair or "budicort respules" or "budo‐san" or budon or budosan or bunase or buparid or butacort or clebudan or coramen or cortiment or cortimentmmx or cycortide or "desona nasal" or desonix or dexbudesonide or duasma or eltair or entocir or entocort or esonide or "giona easyhaler" or horacort or inflammide or inflanaze or intesticort or intestifalk or jorveza or larbex or "map 0010" or map0010 or miflo or miflonid or miflonide or miflonil or mikicort or nebbud or "neo‐rinactive" or novopulmon or numark or olfex or preferid or "pregna 1, 4 diene 3, 20 dione 16, 17 butyraldehyde cyclic acetal, 11beta, 16alpha, 17, 21 tetrahydroxy" or pulmaxan or "pulmicon susp for nebulizer" or "pulmicon susp for nebulizer" or pulmicort or pulmoliseflam or pulmotide or respicort or rhinocort or ribujet or ribuspir or ribuvent or "s 1320" or s1320 or spirocort or "tafen nasal" or uceris or "51333‐22‐3" or "51372‐29‐3").tw. 40. (Ciclesonide or alvesco or "b 9207 015" or "b 9207015" or "b9207 015" or "b9207015" or "by 9010" or "by9010" or omnaris or zetonna or "126544‐47‐6").tw. 41. (Flunisolide or aerobid or aerospan or bronalide or bronilide or cyntaris or flunitec or gibiflu or inhacort or locasyn or lokilan or "lunibron‐a" or lunis or nasalide or nasarel or rhinalar or "rs 3999" or rs3999 or sanergal or synaclyn or syntaris or "val 679" or val679 or "3385‐03‐3").tw. 42. (Fluticasone or Flonase or Flovent or Cutivate or Flixonase or Flixotide or "90566‐53‐3").tw. 43. (Mometasone or "allermax aqueous" or asmanex or belloseta or bloctimo or breso or brusonex or cutticom or dermotasone or dermovel or desdek or ecural or edelan or elica or elocom or elocon or elocyn or elomet or eloson or flumeta or frondava or ivoxel or kalmente or lorome or mefurosan or metaspray or metmin or mipasu or mofunder or momate or momeallerg or momecutan or momederm or momegalen or momekort or momesonex or momespir or momester or mometahexal or mometason or mometasone or mometop or mommox or monovel or monovo or morecort or motaderm or mundoson or muofuder or nasehaler or nasoaldo or nasomet or nasometin or nasonex or nasotasone or netonox or "novasone cream" or "novasone lotion" or "novasone ointment" or ovison or ovixan or pronasal or rinelon or rinometasone or rivelon or "sch 32088" or sch32088 or sebanez or sinuva or sonalox or temon or uniclar or zhekort or "105102‐22‐5" or "83919‐23‐7").tw. 44. (Triamcinolone or acetocot or adcortyl or aristocort or aristodan or azmacor or celeste or "cl 19823" or cl19823 or clinacort or clinalog or delphicort or fluoxiprednisolone or fluoxyprednisolone or "ken‐jec 40" or kenacort or korticoid or ledercort or omcilon or polcortolon or "rp 8357" or rp8357 or simacort or sterocort or "tac 3" or tramcinolone or triacortyl or "triam‐a" or "triam‐forte" or triamcinolon or triamcinolona or triamcort or triamcot or "triamonide 40" or triamsicort or triancinolon or "u‐tri‐lone" or volon or "124‐94‐7").tw. 45. or/24‐44 46. 23 and 45 47. 11 and 46
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'cellulitis'/exp #34 'orbit cellulitis'/exp #35 ((periorbit* OR orbit* OR preseptal OR 'pre septal' OR postseptal OR 'post septal') NEAR/3 celluliti*):ab,ti,kw #36 ((subperiosteal OR 'sub periosteal' OR orbital OR intraconal) NEXT/1 abscess*):ab,ti,kw #37 'bacterial eye infection'/exp #38 ((bacterial NEAR/3 'eye infect*':ab,ti,kw #39 'orbit disease'/de #40 (orbital NEXT/1 disease*):ab,ti,kw #41 'sinusitis'/exp #42 (sinusitis OR (sinus NEXT/2 infect*) OR rhinosinusitis):ab,ti,kw #43 'sinus venous thrombosis':ab,ti,kw #44 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 #45 'orbit cellulitis'/exp AND 'drug therapy'/lnk #46 'steroid'/exp #47 steroid*:ab,ti,kw #48 'corticosteroid'/exp #49 ("Adrenal Cortex Hormone*" OR Corticosteroid* OR Corticoid* OR Glucocorticoid* OR "Glucorticoid Effect*" OR Hydroxycorticosteroid* OR "adrenal cortical hormone*" OR "adrenocortical hormone*" OR adrenocorticosteroid* OR dermocorticosteroid*):ab,ti,kw,tn #50 (glucocorticosteroid* OR glucocorticoidsteroid* OR glucocortoid* OR glycocorticoid* OR glycocorticosteroid*):ab,ti,kw,tn #51 (Dexamethasone OR adrecort OR adrenocot OR aeroseb dex OR aflucoson OR aflucosone OR alfalyl OR anaflogistico OR arcodexan OR arcodexane OR artrosone OR azium OR bidexol OR calonat OR cebedex OR cetadexon OR colofoam OR corsona OR cortastat OR cortidex OR cortidexason OR cortidrona OR cortidrone OR cortisumman OR "dacortina fuerte" OR "dacortine fuerte" OR dalalone OR danasone OR "de‐sone la" OR decacortin OR decadeltosona OR decadeltosone OR decaderm OR decadion OR decadran OR decadron OR decadronal OR decadrone OR decaesadril OR decaject OR decameth OR decamethasone OR decasone OR decaspray OR decasterolone OR decdan OR decilone OR decofluor OR dectancyl OR dekacort OR delladec OR deltafluoren OR deltafluorene OR dergramin OR deronil OR desacort OR desacortone OR desadrene OR desalark OR desameton OR desametone OR desigdron OR "dexa cortisyl" OR "dexa dabrosan" OR "dexa korti" OR "dexa scherosan" OR "dexa scherozon" OR "dexa scherozone" OR "dexa‐p" OR "dexacen 4" OR dexachel OR dexacort OR dexacortal OR dexacorten OR dexacortin OR dexacortisyl OR dexadabroson OR dexadecadrol OR dexadrol OR dexagel OR dexagen OR dexahelvacort OR dexakorti OR dexalien OR dexalocal OR dexame OR dexamecortin OR dexameson OR dexamesone OR dexametason OR dexametasone OR dexameth OR dexamethason OR dexamethazon OR dexamethonium OR dexamonozon OR dexan OR dexane OR dexano OR dexapot OR dexascheroson OR dexascherozon OR dexascherozone OR dexason OR dexasone OR dexinoral OR dexionil OR dexmethsone OR dexona OR dexone OR dexpak OR dextelan OR dextrasone OR dexycu OR dezone OR dibasona OR doxamethasone OR esacortene OR "ex s1" OR exadion OR exadione OR firmalone OR "fluormethyl prednisolone" OR fluormethylprednisolon OR fluormethylprednisolone OR fluormone OR fluorocort OR fluorodelta OR fluoromethylprednisolone OR fortecortin OR gammacorten OR gammacortene OR grosodexon OR grosodexone OR hexadecadiol OR hexadecadrol OR hexadiol OR hexadrol OR isnacort OR "isopto dex" OR "isopto maxidex" OR "isopto‐maxidex" OR isoptodex OR isoptomaxidex OR "lokalison f" OR loverine OR luxazone OR marvidione OR maxidex OR mediamethasone OR megacortin OR mephameson OR mephamesone OR metasolon OR metasolone OR "methazon ion" OR "methazone ion" OR methazonion OR methazonione OR methylfluorprednisolone OR "metisone lafi" OR mexasone OR millicorten OR millicortenol OR "mk 125" OR mk125 OR mymethasone OR neoforderx OR neofordex OR nisomethasona OR novocort OR "nsc 34521" OR nsc34521 OR "oftan‐dexa" OR opticorten OR opticortinol OR oradexan OR oradexon OR oradexone OR orgadrone OR ozurdex OR pidexon OR policort OR posurdex OR "predni f tablinen" OR "predni‐f" OR "prednisolone f" OR prodexona OR prodexone OR sanamethasone OR santenson OR santeson OR sawasone OR solurex OR spoloven OR sterasone OR thilodexine OR triamcimetil OR vexamet OR visumetazone OR visumethazone OR "50‐02‐2"):ab,ti,kw,tn #52 (Prednisone OR ancortone OR biocortone OR colisone OR cortan OR cortancyl OR cortidelt OR cortiprex OR cutason OR dacorten OR "de cortisyl" OR decortancyl OR dacortin OR decortin OR decortine OR decortisyl OR dehydrocortisone OR dekortin OR delitisone OR "dellacort a" OR "delta cortelan" OR "delta cortisone" OR "delta dome" OR "delta e" OR "delta prenovis" OR deltacorten OR deltacortene OR deltacortisone OR deltacortone OR deltasone OR deltison OR deltisona OR deltra OR "di adreson" OR diadreson OR drazone OR encorton OR encortone OR enkorton OR enkortolon OR fernisone OR hostacortin OR insone OR "liquid pred" OR lodotra OR "me‐korti" OR meprison OR metacortandracin OR meticorten OR meticortine OR nisona OR "nsc 10023" OR nsc10023 OR orasone OR orisane OR panafcort OR panasol OR paracort OR pehacort OR precort OR precortal OR "predni tablinen" OR "prednicen‐m" OR prednicorm OR prednicot OR prednidib OR predniment OR prednison OR prednitone OR "pregna 1, 4 diene 3, 11, 20 trione 17, 21 diol" OR pronison OR pronisone OR pronizone OR pulmison OR rayos OR rectodelt OR servisone OR sone OR steerometz OR sterapred OR ultracorten OR urtilone OR winpred OR "53‐03‐2"):ab,ti,kw,tn #53 (Prednisolone OR adelcort OR antisolon OR antisolone OR aprednislon OR aprednislone OR benisolon OR benisolone OR berisolon OR berisolone OR caberdelta OR capsoid OR "co hydeltra" OR codelcortone OR compresolon OR cortadeltona OR cortadeltone OR cortalone OR cortelinter OR cortisolone OR cotolone OR dacortin OR "dacortin h" OR dacrotin OR decaprednil OR "decortin h" OR decortril OR "dehydro cortex" OR "dehydro hydrocortisone" OR "dehydro hydrocortisone" OR dehydrocortex OR dehydrocortisol OR dehydrocortisole OR dehydrohydrocortison OR dehydrohydrocortisone OR delcortol OR "delta 1 17 hydroxycorticosterone 21 acetate OR delta 1 hydrocortisone" OR "delta cortef" OR "delta cortril" OR "delta ef cortelan" OR "delta f OR delta hycortol" OR "delta hydrocortisone" OR "delta hydrocortisone" OR "delta ophticor" OR "delta stab" OR "delta‐cortef" OR "delta1 dehydrocortisol" OR "delta1 dehydrohydrocortisone" OR "delta1 hydrocortisone" OR deltacortef OR deltacortenolo OR deltacortil OR deltacortoil OR deltacortril OR deltaderm OR deltaglycortril OR deltahycortol OR deltahydrocortison OR deltahydrocortisone OR deltaophticor OR deltasolone OR deltastab OR deltidrosol OR deltisilone OR deltisolon OR deltisolone OR deltolasson OR deltolassone OR deltosona OR deltosone OR dermosolon OR dhasolone OR DiAdresonF OR "di adreson f" OR "di adresone f" OR "diadreson f" OR "diadresone f" OR dicortol OR domucortone OR encortelon OR encortelone OR encortolon OR equisolon OR "fernisolone‐p" OR glistelone OR hefasolon OR "hostacortin h" OR hydeltra OR hydeltrone OR hydrelta OR hydrocortancyl OR hydrocortidelt OR hydrodeltalone OR hydrodeltisone OR hydroretrocortin OR hydroretrocortine OR inflanefran OR insolone OR "keteocort h" OR "key‐pred" OR lenisolone OR leocortol OR liquipred OR "lygal kopftinktur n" OR mediasolone OR meprisolon OR meprisolone OR metacortalon OR metacortalone OR metacortandralon OR metacortandralone OR metacortelone OR "meti derm" OR meticortelone OR metiderm OR morlone OR mydrapred OR "neo delta" OR nisolon OR nisolone OR "nsc 9120" OR nsc9120 OR opredsone OR panafcortelone OR panafcortolone OR panafort OR paracortol OR phlogex OR "pre cortisyl" OR preconin OR precortalon OR precortancyl OR precortisyl OR "pred‐ject‐50" OR "predacort 50" OR "predaject‐50" OR "predalone 50" OR predartrina OR predartrine OR predate OR predeltilone OR predisole OR predisyr OR "predne dome" OR prednecort OR prednedome OR prednelan OR "predni coelin" OR "predni h tablinen" OR "predni‐helvacort" OR prednicoelin OR prednicort OR prednicortelone OR "prednifor drops" OR predniment OR predniretard OR prednis OR prednisil OR prednisolon OR prednisolona OR prednivet OR prednorsolon OR prednorsolone OR predonine OR predorgasolona OR predorgasolone OR "pregna 1, 4 diene 11beta, 17alpha, 21 triol 3, 20 dione" OR prelon OR prelone OR prenilone OR prenin OR prenolone OR preventan OR prezolon OR rubycort OR scherisolon OR scherisolona OR serilone OR solondo OR solone OR solupren OR soluprene OR spiricort OR spolotane OR sterane OR sterolone OR supercortisol OR supercortizol OR taracortelone OR walesolone OR wysolone OR "50‐24‐8"):ab,ti,kw,tn #54 (Methylprednisolone OR "adlone‐40" OR "adlone‐80" OR "dep medalone 80" OR depmedalone OR "depoject‐80" OR depopred OR esametone OR firmacort OR "med‐jec‐40" OR medixon OR mednin OR "medralone 80" OR medrate OR medrol OR medrone OR meprednisolone OR meprelon OR mesopren OR "methacort 40" OR "methacort 80" OR "methyl prednisolone" OR methylcotol OR methylcotolone OR "methylpred dp" OR methylsterolone OR metidrol OR metipred OR metrisone OR metycortin OR metypred OR metypresol OR neomedrone OR nsc 19987 OR nsc19987 OR prednol OR solomet OR "solu decortin" OR urbason OR "6923‐42‐8" OR "83‐43‐2"):ab,ti,kw,tn #55 (Hydrocortisone OR acticort OR "aeroseb hc" OR "ala‐cort" OR "ala‐scalp" OR alfacort OR algicortis OR alkindi OR "alpha derm" OR alphaderm OR "anucort‐hc" OR "anumed‐hc" OR "anutone‐hc" OR "aquanil hc" OR "balneol‐hc" OR "barseb hc" OR "beta‐hc" OR biacort OR cetacort OR cobadex OR colocort OR "compound f" OR "cordicare lotion" OR coripen OR "cort dome" OR cortef OR cortenema OR cortibel OR corticorenol OR cortifair OR cortifan OR cortiphate OR cortisol OR cortisole OR cortispray OR cortoderm OR cortril OR cotacort OR covocort OR "cremicort‐h" OR cutaderm OR "derm‐aid cream" OR "dermacrin hc lotion" OR dermaid OR dermocare OR dermocortal OR dermolate OR dioderm OR eczacort OR "ef cortelan" OR efcortelan OR egocort OR eksalb OR eldecort OR "emo‐cort" OR epicort OR epicortisol OR ficortril OR filocot OR flexicort OR "gly‐cort" OR glycort OR "h‐cort" OR "hc no. 1" OR "hc no. 4" OR hebcort OR "hemorrhoidal hc" OR "hemril‐30" OR "hemril‐hc uniserts" OR "hi‐cor" OR hidrotisona OR hycor OR hycort OR hydracort OR hydrasson OR "hydro ricortex" OR "hydro‐rx" OR hydrocort OR hydrocorticosteroid OR hydrocortisate OR hydrocortison OR hydrocortisonum OR hydrocortisyl OR hydrocortone OR hydrogalen OR hydrokort OR hydrokortison OR hydrotopic OR hysone OR hytisone OR hytone OR "incortin h" OR "instacort 10" OR kyypakkaus OR "lacticare hc" OR "lemnis fatty cream hc" OR lenirit OR "medihaler cort" OR "medihaler duo" OR medrocil OR mildison OR "mitocortyl demangeaisons" OR munitren OR "nogenic hc" OR novohydrocort OR "nsc 10483" OR "nsc 741" OR nsc10483 OR nutracort OR optef OR "otosone f" OR penecort OR plenadren OR prepcort OR "prevex hc" OR "pro cort" OR procort OR "procto‐kit" OR proctocort OR "proctosert hc" OR "proctosol‐hc" OR proctosone OR "proctozone hc" OR procutan OR "rectasol‐hc" OR rectocort OR rederm OR sanatison OR "scalp‐aid" OR schericur OR "scherosone f" OR skincalm OR "stie‐cort" OR "substance m" OR synacort OR texacort OR "triburon‐hc" OR unicort OR vasocort OR "50‐23‐7"):ab,ti,kw,tn #56 (Loteprednol OR alrex OR "cddd 5604" OR cddd5604 OR CEHOAC OR "chloromethyl 17alpha ethoxycarbonyloxy 11beta hydroxy 3 oxoandrosta 1, 4 diene 17 carboxylate" OR "hgp 1" OR hgp1 OR inveltys OR "kpi 121" OR kpi121 OR "le‐mpp" OR lotemax OR loterex OR loterox OR lotesoft OR "p 5604" OR p5604 OR "82034‐46‐6"):ab,ti,kw,tn #57 (Rimexolone OR baxol OR "org 6216" OR org6216 OR "pregna 1, 4 dien 11beta ol 3, 20 dione, 16alpha, 17alpha, 21 trimethyl" OR rimexel OR trimexolone OR vexol OR vexolon OR "49697‐38‐3"):ab,ti,kw,tn #58 ("Prednisolone acetate" OR "ak‐pred" OR deppredalone OR econopred OR "flo pred" OR "isopto cetapred" OR "key pred" OR "meticortelone acetate" OR "ocu‐pred" OR omnipred OR optilon OR "poly pred" OR "pred forte" OR "pred mild" OR predforte OR predmet OR "prednefrin sf" OR "predni h" OR "predni‐ophtal" OR prednigalen OR "prednisolone 17 acetate" OR "prednisolone 21 acetate" OR "prednisolone acetate suspension" OR savacort OR "Scherisolone‐Kristall suspension" OR ultracortensol OR ultracortinol OR "52‐21‐1" OR "52628‐64‐5"):ab,ti,kw,tn #59 (Beclomethasone OR beclometasone OR "Asmabec Clickhaler" OR Ascocortonyl OR Beclamet OR "Beclo Asma" OR "Beclo AZU" OR Beclocort OR Beclomet OR "Bemedrex Easyhaler" OR Beclorhinol OR Sanasthmax OR Becloturmant OR Beclovent OR Beconase OR Becloforte OR Becodisk OR Becodisks OR Propaderm OR Becotide OR Sanasthmyl OR "Beconase AQ" OR Bronchocort OR Junik OR Qvar OR Ecobec OR Beclazone OR Ventolair OR Prolair OR Filair OR "AeroBec Forte" OR Aerobec OR "Nasobec Aqueous" OR Respocort OR Vancenase OR Vanceril OR Aldecin OR Viarin OR "4419‐39‐0"):ab,ti,kw,tn #60 (Betamethasone OR adbeon OR becasone OR beprogel OR "beta methason" OR "beta methasone" OR "beta‐phos/ac" OR betacortril OR betadexamethasone OR betametasone OR betamethasolone OR betamethason OR betamethasonium OR betamethasonum OR betamethazone OR betason OR betnasol OR betnelan OR "betnesol h" OR "betnesol v" OR "betnovate a" OR betsolan OR betsolon OR betsopart OR celestan OR celestene OR celeston OR celestona OR celestone OR cellestoderm OR cidoten OR dermobet OR diprolen OR flubenisolone OR methasone OR "nsc 39470" OR nsc39470 OR "pregna 1, 4 diene 3, 20 dione 11, 17, 21 triol, 9 alpha fluoro 16beta methyl" OR "rg 833" OR rg833 OR rinderon OR "sch 4831" OR sch4831 OR walacort OR "378‐44‐9"):ab,ti,kw,tn #61 (Budesonide OR acorspray OR aerox OR allercort OR aquacort OR "b cort" OR "bebe cream" OR bidien OR budecol OR budecort OR budefat OR budeflam OR budelin OR "budenase aq" OR budeno OR budenofalk OR budenoside OR budes OR budeson OR "budesonide easyhaler" OR budiair OR "budicort respules" OR "budo‐san" OR budon OR budosan OR bunase OR buparid OR butacort OR clebudan OR coramen OR cortiment OR cortimentmmx OR cycortide OR "desona nasal" OR desonix OR dexbudesonide OR duasma OR eltair OR entocir OR entocort OR esonide OR "giona easyhaler" OR horacort OR inflammide OR inflanaze OR intesticort OR intestifalk OR jorveza OR larbex OR "map 0010" OR map0010 OR miflo OR miflonid OR miflonide OR miflonil OR mikicort OR nebbud OR "neo‐rinactive" OR novopulmon OR numark OR olfex OR preferid OR "pregna 1, 4 diene 3, 20 dione 16, 17 butyraldehyde cyclic acetal, 11beta, 16alpha, 17, 21 tetrahydroxy" OR pulmaxan OR "pulmicon susp for nebulizer" OR "pulmicon susp for nebulizer" OR pulmicort OR pulmoliseflam OR pulmotide OR respicort OR rhinocort OR ribujet OR ribuspir OR ribuvent OR "s 1320" OR s1320 OR spirocort OR "tafen nasal" OR uceris OR "51333‐22‐3" OR "51372‐29‐3"):ab,ti,kw,tn #62 (Ciclesonide OR alvesco OR "b 9207 015" OR "b 9207015" OR "b9207 015" OR "b9207015" OR "by 9010" OR "by9010" OR omnaris OR zetonna OR "126544‐47‐6"):ab,ti,kw,tn #63 (Flunisolide OR aerobid OR aerospan OR bronalide OR bronilide OR cyntaris OR flunitec OR gibiflu OR inhacort OR locasyn OR lokilan OR "lunibron‐a" OR lunis OR nasalide OR nasarel OR rhinalar OR "rs 3999" OR rs3999 OR sanergal OR synaclyn OR syntaris OR "val 679" OR val679 OR "3385‐03‐3"):ab,ti,kw,tn #64 (Fluticasone OR Flonase OR Flovent OR Cutivate OR Flixonase OR Flixotide OR "90566‐53‐3"):ab,ti,kw,tn #65 (Mometasone OR "allermax aqueous" OR asmanex OR belloseta OR bloctimo OR breso OR brusonex OR cutticom OR dermotasone OR dermovel OR desdek OR ecural OR edelan OR elica OR elocom OR elocon OR elocyn OR elomet OR eloson OR flumeta OR frondava OR ivoxel OR kalmente OR lorome OR mefurosan OR metaspray OR metmin OR mipasu OR mofunder OR momate OR momeallerg OR momecutan OR momederm OR momegalen OR momekort OR momesonex OR momespir OR momester OR mometahexal OR mometason OR mometasone OR mometop OR mommox OR monovel OR monovo OR morecort OR motaderm OR mundoson OR muofuder OR nasehaler OR nasoaldo OR nasomet OR nasometin OR nasonex OR nasotasone OR netonox OR "novasone cream" OR "novasone lotion" OR "novasone ointment" OR ovison OR ovixan OR pronasal OR rinelon OR rinometasone OR rivelon OR "sch 32088" OR sch32088 OR sebanez OR sinuva OR sonalox OR temon OR uniclar OR zhekort OR "105102‐22‐5" OR "83919‐23‐7"):ab,ti,kw,tn #66 (Triamcinolone OR acetocot OR adcortyl OR aristocort OR aristodan OR azmacor OR celeste OR "cl 19823" OR cl19823 OR clinacort OR clinalog OR delphicort OR fluoxiprednisolone OR fluoxyprednisolone OR "ken‐jec 40" OR kenacort OR korticoid OR ledercort OR omcilon OR polcortolon OR "rp 8357" OR rp8357 OR simacort OR sterocort OR "tac 3" OR tramcinolone OR triacortyl OR "triam‐a" OR "triam‐forte" OR triamcinolon OR triamcinolona OR triamcort OR triamcot OR "triamonide 40" OR triamsicort OR triancinolon OR "u‐tri‐lone" OR volon OR "124‐94‐7"):ab,ti,kw,tn #67 #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 #68 #44 AND #67 #69 #32 AND #68
Appendix 4. PubMed search strategy
1. ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) 2. ((periorbit*[tw] OR orbit*[tw] OR preseptal[tw] OR "pre septal"[tw] OR postseptal[tw] OR "post septal"[tw]) AND celluliti*[tw]) 3. ((subperiosteal[tw] OR "sub periosteal"[tw] OR orbital[tw] OR intraconal[tw]) AND abscess*[tw]) 4. bacterial eye infect*[tw] 5. orbital disease*[tw] 6. sinusitis[tw] OR sinus infect*[tw] OR rhinosinusitis[tw] 7. sinus venous thrombosis[tw] 8. #2 OR #3 OR #4 OR #5 OR #6 OR #7 9. steroid*[tw] 10. Adrenal Cortex Hormone*[tw] OR Corticosteroid*[tw] OR Corticoid*[tw] OR Glucocorticoid*[tw] OR Glucorticoid Effect*[tw] OR Hydroxycorticosteroid*[tw] OR Adrenal cortical hormone*[tw] OR Adrenocortical hormone*[tw] OR Adrenocorticosteroid*[tw] OR Dermocorticosteroid*[tw] 11. Glucocorticosteroid*[tw] OR Glucocorticoidsteroid*[tw] OR Glucocortoid*[tw] OR Glycocorticoid*[tw] OR Glycocorticosteroid*[tw] 12. Dexamethasone[tw] OR adrecort[tw] OR adrenocot[tw] OR "aeroseb dex"[tw] OR aflucoson[tw] OR aflucosone[tw] OR alfalyl[tw] OR anaflogistico[tw] OR arcodexan[tw] OR arcodexane[tw] OR artrosone[tw] OR azium[tw] OR bidexol[tw] OR calonat[tw] OR cebedex[tw] OR cetadexon[tw] OR colofoam[tw] OR corsona[tw] OR cortastat[tw] OR cortidex[tw] OR cortidexason[tw] OR cortidrona[tw] OR cortidrone[tw] OR cortisumman[tw] OR "dacortina fuerte"[tw] OR "dacortine fuerte"[tw] OR dalalone[tw] OR danasone[tw] OR "de‐sone la"[tw] OR decacortin[tw] OR decadeltosona[tw] OR decadeltosone[tw] OR decaderm[tw] OR decadion[tw] OR decadran[tw] OR decadron[tw] OR decadronal[tw] OR decadrone[tw] OR decaesadril[tw] OR decaject[tw] OR decameth[tw] OR decamethasone[tw] OR decasone[tw] OR decaspray[tw] OR decasterolone[tw] OR decdan[tw] OR decilone[tw] OR decofluor[tw] OR dectancyl[tw] OR dekacort[tw] OR delladec[tw] OR deltafluoren[tw] OR deltafluorene[tw] OR dergramin[tw] OR deronil[tw] OR desacort[tw] OR desacortone[tw] OR desadrene[tw] OR desalark[tw] OR desameton[tw] OR desametone[tw] OR desigdron[tw] OR "dexa cortisyl"[tw] OR "dexa dabrosan"[tw] OR "dexa korti"[tw] OR "dexa scherosan"[tw] OR "dexa scherozon"[tw] OR "dexa scherozone"[tw] OR "dexa‐p"[tw] OR "dexacen 4"[tw] OR dexachel[tw] OR dexacort[tw] OR dexacortal[tw] OR dexacorten[tw] OR dexacortin[tw] OR dexacortisyl[tw] OR dexadabroson[tw] OR dexadecadrol[tw] OR dexadrol[tw] OR dexagel[tw] OR dexagen[tw] OR dexahelvacort[tw] OR dexakorti[tw] OR dexalien[tw] OR dexalocal[tw] OR dexame[tw] OR dexamecortin[tw] OR dexameson[tw] OR dexamesone[tw] OR dexametason[tw] OR dexametasone[tw] OR dexameth[tw] OR dexamethason[tw] OR dexamethazon[tw] OR dexamethonium[tw] OR dexamonozon[tw] OR dexan[tw] OR dexane[tw] OR dexano[tw] OR dexapot[tw] OR dexascheroson[tw] OR dexascherozon[tw] OR dexascherozone[tw] OR dexason[tw] OR dexasone[tw] OR dexinoral[tw] OR dexionil[tw] OR dexmethsone[tw] OR dexona[tw] OR dexone[tw] OR dexpak[tw] OR dextelan[tw] OR dextrasone[tw] OR dexycu[tw] OR dezone[tw] OR dibasona[tw] OR doxamethasone[tw] OR esacortene[tw] OR "ex s1"[tw] OR exadion[tw] OR exadione[tw] OR firmalone[tw] OR "fluormethyl prednisolone"[tw] OR fluormethylprednisolon[tw] OR fluormethylprednisolone[tw] OR fluormone[tw] OR fluorocort[tw] OR fluorodelta[tw] OR fluoromethylprednisolone[tw] OR fortecortin[tw] OR gammacorten[tw] OR gammacortene[tw] OR grosodexon[tw] OR grosodexone[tw] OR hexadecadiol[tw] OR hexadecadrol[tw] OR hexadiol[tw] OR hexadrol[tw] OR isnacort[tw] OR "isopto dex"[tw] OR "isopto maxidex"[tw] OR "isopto‐maxidex"[tw] OR isoptodex[tw] OR isoptomaxidex[tw] OR "lokalison f"[tw] OR loverine[tw] OR luxazone[tw] OR marvidione[tw] OR maxidex[tw] OR mediamethasone[tw] OR megacortin[tw] OR mephameson[tw] OR mephamesone[tw] OR metasolon[tw] OR metasolone[tw] OR "methazon ion"[tw] OR "methazone ion"[tw] OR methazonion[tw] OR methazonione[tw] OR methylfluorprednisolone[tw] OR "metisone lafi"[tw] OR mexasone[tw] OR millicorten[tw] OR millicortenol[tw] OR "mk 125"[tw] OR mk125[tw] OR mymethasone[tw] OR neoforderx[tw] OR neofordex[tw] OR nisomethasona[tw] OR novocort[tw] OR "nsc 34521"[tw] OR nsc34521[tw] OR "oftan‐dexa"[tw] OR opticorten[tw] OR opticortinol[tw] OR oradexan[tw] OR oradexon[tw] OR oradexone[tw] OR orgadrone[tw] OR ozurdex[tw] OR pidexon[tw] OR policort[tw] OR posurdex[tw] OR "predni f tablinen"[tw] OR "predni‐f"[tw] OR "prednisolone f"[tw] OR prodexona[tw] OR prodexone[tw] OR sanamethasone[tw] OR santenson[tw] OR santeson[tw] OR sawasone[tw] OR solurex[tw] OR spoloven[tw] OR sterasone[tw] OR thilodexine[tw] OR triamcimetil[tw] OR vexamet[tw] OR visumetazone[tw] OR visumethazone[tw] OR "50‐02‐2"[tw] 13. Prednisone[tw] OR ancortone[tw] OR biocortone[tw] OR colisone[tw] OR cortan[tw] OR cortancyl[tw] OR cortidelt[tw] OR cortiprex[tw] OR cutason[tw] OR dacorten[tw] OR "de cortisyl"[tw] OR decortancyl[tw] OR dacortin[tw] OR decortin[tw] OR decortine[tw] OR decortisyl[tw] OR dehydrocortisone[tw] OR dekortin[tw] OR delitisone[tw] OR "dellacort a"[tw] OR "delta cortelan"[tw] OR "delta cortisone"[tw] OR "delta dome"[tw] OR "delta e"[tw] OR "delta prenovis"[tw] OR deltacorten[tw] OR deltacortene[tw] OR deltacortisone[tw] OR deltacortone[tw] OR deltasone[tw] OR deltison[tw] OR deltisona[tw] OR deltra[tw] OR "di adreson"[tw] OR diadreson[tw] OR drazone[tw] OR encorton[tw] OR encortone[tw] OR enkorton[tw] OR enkortolon[tw] OR fernisone[tw] OR hostacortin[tw] OR insone[tw] OR "liquid pred"[tw] OR lodotra[tw] OR "me‐korti"[tw] OR meprison[tw] OR metacortandracin[tw] OR meticorten[tw] OR meticortine[tw] OR nisona[tw] OR "nsc 10023"[tw] OR nsc10023[tw] OR orasone[tw] OR orisane[tw] OR panafcort[tw] OR panasol[tw] OR paracort[tw] OR pehacort[tw] OR precort[tw] OR precortal[tw] OR "predni tablinen"[tw] OR "prednicen‐m"[tw] OR prednicorm[tw] OR prednicot[tw] OR prednidib[tw] OR predniment[tw] OR prednison[tw] OR prednitone[tw] OR "pregna 1, 4 diene 3, 11, 20 trione 17, 21 diol"[tw] OR pronison[tw] OR pronisone[tw] OR pronizone[tw] OR pulmison[tw] OR rayos[tw] OR rectodelt[tw] OR servisone[tw] OR sone[tw] OR steerometz[tw] OR sterapred[tw] OR ultracorten[tw] OR urtilone[tw] OR winpred[tw] OR "53‐03‐2"[tw] 14. Prednisolone[tw] OR adelcort[tw] OR antisolon[tw] OR antisolone[tw] OR aprednislon[tw] OR aprednislone[tw] OR benisolon[tw] OR benisolone[tw] OR berisolon[tw] OR berisolone[tw] OR caberdelta[tw] OR capsoid[tw] OR "co hydeltra"[tw] OR codelcortone[tw] OR compresolon[tw] OR cortadeltona[tw] OR cortadeltone[tw] OR cortalone[tw] OR cortelinter[tw] OR cortisolone[tw] OR cotolone[tw] OR dacortin[tw] OR "dacortin h"[tw] OR dacrotin[tw] OR decaprednil[tw] OR "decortin h"[tw] OR decortril[tw] OR "dehydro cortex"[tw] OR "dehydro hydrocortisone"[tw] OR "dehydro hydrocortisone"[tw] OR dehydrocortex[tw] OR dehydrocortisol[tw] OR dehydrocortisole[tw] OR dehydrohydrocortison[tw] OR dehydrohydrocortisone[tw] OR delcortol[tw] OR "delta 1 17 hydroxycorticosterone 21 acetate[tw] OR delta 1 hydrocortisone"[tw] OR "delta cortef"[tw] OR "delta cortril"[tw] OR "delta ef cortelan"[tw] OR "delta f[tw] OR delta hycortol"[tw] OR "delta hydrocortisone"[tw] OR "delta hydrocortisone"[tw] OR "delta ophticor"[tw] OR "delta stab"[tw] OR "delta‐cortef"[tw] OR "delta1 dehydrocortisol"[tw] OR "delta1 dehydrohydrocortisone"[tw] OR "delta1 hydrocortisone"[tw] OR deltacortef[tw] OR deltacortenolo[tw] OR deltacortil[tw] OR deltacortoil[tw] OR deltacortril[tw] OR deltaderm[tw] OR deltaglycortril[tw] OR deltahycortol[tw] OR deltahydrocortison[tw] OR deltahydrocortisone[tw] OR deltaophticor[tw] OR deltasolone[tw] OR deltastab[tw] OR deltidrosol[tw] OR deltisilone[tw] OR deltisolon[tw] OR deltisolone[tw] OR deltolasson[tw] OR deltolassone[tw] OR deltosona[tw] OR deltosone[tw] OR dermosolon[tw] OR dhasolone[tw] OR DiAdresonF[tw] OR "di adreson f"[tw] OR "di adresone f"[tw] OR "diadreson f"[tw] OR "diadresone f"[tw] OR dicortol[tw] OR domucortone[tw] OR encortelon[tw] OR encortelone[tw] OR encortolon[tw] OR equisolon[tw] OR "fernisolone‐p"[tw] OR glistelone[tw] OR hefasolon[tw] OR "hostacortin h"[tw] OR hydeltra[tw] OR hydeltrone[tw] OR hydrelta[tw] OR hydrocortancyl[tw] OR hydrocortidelt[tw] OR hydrodeltalone[tw] OR hydrodeltisone[tw] OR hydroretrocortin[tw] OR hydroretrocortine[tw] OR inflanefran[tw] OR insolone[tw] OR "keteocort h"[tw] OR "key‐pred"[tw] OR lenisolone[tw] OR leocortol[tw] OR liquipred[tw] OR "lygal kopftinktur n"[tw] OR mediasolone[tw] OR meprisolon[tw] OR meprisolone[tw] OR metacortalon[tw] OR metacortalone[tw] OR metacortandralon[tw] OR metacortandralone[tw] OR metacortelone[tw] OR "meti derm"[tw] OR meticortelone[tw] OR metiderm[tw] OR morlone[tw] OR mydrapred[tw] OR "neo delta"[tw] OR nisolon[tw] OR nisolone[tw] OR "nsc 9120"[tw] OR nsc9120[tw] OR opredsone[tw] OR panafcortelone[tw] OR panafcortolone[tw] OR panafort[tw] OR paracortol[tw] OR phlogex[tw] OR "pre cortisyl"[tw] OR preconin[tw] OR precortalon[tw] OR precortancyl[tw] OR precortisyl[tw] OR "pred‐ject‐50"[tw] OR "predacort 50"[tw] OR "predaject‐50"[tw] OR "predalone 50"[tw] OR predartrina[tw] OR predartrine[tw] OR predate[tw] OR predeltilone[tw] OR predisole[tw] OR predisyr[tw] OR "predne dome"[tw] OR prednecort[tw] OR prednedome[tw] OR prednelan[tw] OR "predni coelin"[tw] OR "predni h tablinen"[tw] OR "predni‐helvacort"[tw] OR prednicoelin[tw] OR prednicort[tw] OR prednicortelone[tw] OR "prednifor drops"[tw] OR predniment[tw] OR predniretard[tw] OR prednis[tw] OR prednisil[tw] OR prednisolon[tw] OR prednisolona[tw] OR prednivet[tw] OR prednorsolon[tw] OR prednorsolone[tw] OR predonine[tw] OR predorgasolona[tw] OR predorgasolone[tw] OR "pregna 1, 4 diene 11beta, 17alpha, 21 triol 3, 20 dione"[tw] OR prelon[tw] OR prelone[tw] OR prenilone[tw] OR prenin[tw] OR prenolone[tw] OR preventan[tw] OR prezolon[tw] OR rubycort[tw] OR scherisolon[tw] OR scherisolona[tw] OR serilone[tw] OR solondo[tw] OR solone[tw] OR solupren[tw] OR soluprene[tw] OR spiricort[tw] OR spolotane[tw] OR sterane[tw] OR sterolone[tw] OR supercortisol[tw] OR supercortizol[tw] OR taracortelone[tw] OR walesolone[tw] OR wysolone[tw] OR "50‐24‐8"[tw] 15. Methylprednisolone[tw] OR "adlone‐40"[tw] OR "adlone‐80"[tw] OR "dep medalone 80"[tw] OR depmedalone[tw] OR "depoject‐80"[tw] OR depopred[tw] OR esametone[tw] OR firmacort[tw] OR "med‐jec‐40"[tw] OR medixon[tw] OR mednin[tw] OR "medralone 80"[tw] OR medrate[tw] OR medrol[tw] OR medrone[tw] OR meprednisolone[tw] OR meprelon[tw] OR mesopren[tw] OR "methacort 40"[tw] OR "methacort 80"[tw] OR "methyl prednisolone"[tw] OR methylcotol[tw] OR methylcotolone[tw] OR "methylpred dp"[tw] OR methylsterolone[tw] OR metidrol[tw] OR metipred[tw] OR metrisone[tw] OR metycortin[tw] OR metypred[tw] OR metypresol[tw] OR neomedrone[tw] OR nsc 19987[tw] OR nsc19987[tw] OR prednol[tw] OR solomet[tw] OR "solu decortin"[tw] OR urbason[tw] OR "6923‐42‐8"[tw] OR "83‐43‐2"[tw] 16. Hydrocortisone[tw] OR acticort[tw] OR "aeroseb hc"[tw] OR "ala‐cort"[tw] OR "ala‐scalp"[tw] OR alfacort[tw] OR algicortis[tw] OR alkindi[tw] OR "alpha derm"[tw] OR alphaderm[tw] OR "anucort‐hc"[tw] OR "anumed‐hc"[tw] OR "anutone‐hc"[tw] OR "aquanil hc"[tw] OR "balneol‐hc"[tw] OR "barseb hc"[tw] OR "beta‐hc"[tw] OR biacort[tw] OR cetacort[tw] OR cobadex[tw] OR colocort[tw] OR "compound f"[tw] OR "cordicare lotion"[tw] OR coripen[tw] OR "cort dome"[tw] OR cortef[tw] OR cortenema[tw] OR cortibel[tw] OR corticorenol[tw] OR cortifair[tw] OR cortifan[tw] OR cortiphate[tw] OR cortisol[tw] OR cortisole[tw] OR cortispray[tw] OR cortoderm[tw] OR cortril[tw] OR cotacort[tw] OR covocort[tw] OR "cremicort‐h"[tw] OR cutaderm[tw] OR "derm‐aid cream"[tw] OR "dermacrin hc lotion"[tw] OR dermaid[tw] OR dermocare[tw] OR dermocortal[tw] OR dermolate[tw] OR dioderm[tw] OR eczacort[tw] OR "ef cortelan"[tw] OR efcortelan[tw] OR egocort[tw] OR eksalb[tw] OR eldecort[tw] OR "emo‐cort"[tw] OR epicort[tw] OR epicortisol[tw] OR ficortril[tw] OR filocot[tw] OR flexicort[tw] OR "gly‐cort"[tw] OR glycort[tw] OR "h‐cort"[tw] OR "hc no. 1"[tw] OR "hc no. 4"[tw] OR hebcort[tw] OR "hemorrhoidal hc"[tw] OR "hemril‐30"[tw] OR "hemril‐hc uniserts"[tw] OR "hi‐cor"[tw] OR hidrotisona[tw] OR hycor[tw] OR hycort[tw] OR hydracort[tw] OR hydrasson[tw] OR "hydro ricortex"[tw] OR "hydro‐rx"[tw] OR hydrocort[tw] OR hydrocorticosteroid[tw] OR hydrocortisate[tw] OR hydrocortison[tw] OR hydrocortisonum[tw] OR hydrocortisyl[tw] OR hydrocortone[tw] OR hydrogalen[tw] OR hydrokort[tw] OR hydrokortison[tw] OR hydrotopic[tw] OR hysone[tw] OR hytisone[tw] OR hytone[tw] OR "incortin h"[tw] OR "instacort 10"[tw] OR kyypakkaus[tw] OR "lacticare hc"[tw] OR "lemnis fatty cream hc"[tw] OR lenirit[tw] OR "medihaler cort"[tw] OR "medihaler duo"[tw] OR medrocil[tw] OR mildison[tw] OR "mitocortyl demangeaisons"[tw] OR munitren[tw] OR "nogenic hc"[tw] OR novohydrocort[tw] OR "nsc 10483"[tw] OR "nsc 741"[tw] OR nsc10483[tw] OR nutracort[tw] OR optef[tw] OR "otosone f"[tw] OR penecort[tw] OR plenadren[tw] OR prepcort[tw] OR "prevex hc"[tw] OR "pro cort"[tw] OR procort[tw] OR "procto‐kit"[tw] OR proctocort[tw] OR "proctosert hc"[tw] OR "proctosol‐hc"[tw] OR proctosone[tw] OR "proctozone hc"[tw] OR procutan[tw] OR "rectasol‐hc"[tw] OR rectocort[tw] OR rederm[tw] OR sanatison[tw] OR "scalp‐aid"[tw] OR schericur[tw] OR "scherosone f"[tw] OR skincalm[tw] OR "stie‐cort"[tw] OR "substance m"[tw] OR synacort[tw] OR texacort[tw] OR "triburon‐hc"[tw] OR unicort[tw] OR vasocort[tw] OR "50‐23‐7"[tw] 17. Loteprednol[tw] OR alrex[tw] OR "cddd 5604"[tw] OR cddd5604[tw] OR CEHOAC[tw] OR "chloromethyl 17alpha ethoxycarbonyloxy 11beta hydroxy 3 oxoandrosta 1, 4 diene 17 carboxylate"[tw] OR "hgp 1"[tw] OR hgp1[tw] OR inveltys[tw] OR "kpi 121"[tw] OR kpi121[tw] OR "le‐mpp"[tw] OR lotemax[tw] OR loterex[tw] OR loterox[tw] OR lotesoft[tw] OR "p 5604"[tw] OR p5604[tw] OR "82034‐46‐6"[tw] 18. Rimexolone[tw] OR baxol[tw] OR "org 6216"[tw] OR org6216[tw] OR "pregna 1, 4 dien 11beta ol 3, 20 dione, 16alpha, 17alpha, 21 trimethyl"[tw] OR rimexel[tw] OR trimexolone[tw] OR vexol[tw] OR vexolon[tw] OR "49697‐38‐3"[tw] 19. "Prednisolone acetate"[tw] OR "ak‐pred"[tw] OR deppredalone[tw] OR econopred[tw] OR "flo pred"[tw] OR "isopto cetapred"[tw] OR "key pred"[tw] OR "meticortelone acetate"[tw] OR "ocu‐pred"[tw] OR omnipred[tw] OR optilon[tw] OR "poly pred"[tw] OR "pred forte"[tw] OR "pred mild"[tw] OR predforte[tw] OR predmet[tw] OR "prednefrin sf"[tw] OR "predni h"[tw] OR "predni‐ophtal"[tw] OR prednigalen[tw] OR "prednisolone 17 acetate"[tw] OR "prednisolone 21 acetate"[tw] OR "prednisolone acetate suspension"[tw] OR savacort[tw] OR "Scherisolone‐Kristall suspension"[tw] OR ultracortensol[tw] OR ultracortinol[tw] OR "52‐21‐1"[tw] OR "52628‐64‐5"[tw] 20. Beclomethasone[tw] OR beclometasone[tw] OR "Asmabec Clickhaler"[tw] OR Ascocortonyl[tw] OR Beclamet[tw] OR "Beclo Asma"[tw] OR "Beclo AZU"[tw] OR Beclocort[tw] OR Beclomet[tw] OR "Bemedrex Easyhaler"[tw] OR Beclorhinol[tw] OR Sanasthmax[tw] OR Becloturmant[tw] OR Beclovent[tw] OR Beconase[tw] OR Becloforte[tw] OR Becodisk[tw] OR Becodisks[tw] OR Propaderm[tw] OR Becotide[tw] OR Sanasthmyl[tw] OR "Beconase AQ"[tw] OR Bronchocort[tw] OR Junik[tw] OR Qvar[tw] OR Ecobec[tw] OR Beclazone[tw] OR Ventolair[tw] OR Prolair[tw] OR Filair[tw] OR "AeroBec Forte"[tw] OR Aerobec[tw] OR "Nasobec Aqueous"[tw] OR Respocort[tw] OR Vancenase[tw] OR Vanceril[tw] OR Aldecin[tw] OR Viarin[tw] OR "4419‐39‐0"[tw] 21. Betamethasone[tw] OR adbeon[tw] OR becasone[tw] OR beprogel[tw] OR "beta methason"[tw] OR "beta methasone"[tw] OR "beta‐phos/ac"[tw] OR betacortril[tw] OR betadexamethasone[tw] OR betametasone[tw] OR betamethasolone[tw] OR betamethason[tw] OR betamethasonium[tw] OR betamethasonum[tw] OR betamethazone[tw] OR betason[tw] OR betnasol[tw] OR betnelan[tw] OR "betnesol h"[tw] OR "betnesol v"[tw] OR "betnovate a"[tw] OR betsolan[tw] OR betsolon[tw] OR betsopart[tw] OR celestan[tw] OR celestene[tw] OR celeston[tw] OR celestona[tw] OR celestone[tw] OR cellestoderm[tw] OR cidoten[tw] OR dermobet[tw] OR diprolen[tw] OR flubenisolone[tw] OR methasone[tw] OR "nsc 39470"[tw] OR nsc39470[tw] OR "pregna 1, 4 diene 3, 20 dione 11, 17, 21 triol, 9 alpha fluoro 16beta methyl"[tw] OR "rg 833"[tw] OR rg833[tw] OR rinderon[tw] OR "sch 4831"[tw] OR sch4831[tw] OR walacort[tw] OR "378‐44‐9"[tw] 22. Budesonide[tw] OR acorspray[tw] OR aerox[tw] OR allercort[tw] OR aquacort[tw] OR "b cort"[tw] OR "bebe cream"[tw] OR bidien[tw] OR budecol[tw] OR budecort[tw] OR budefat[tw] OR budeflam[tw] OR budelin[tw] OR "budenase aq"[tw] OR budeno[tw] OR budenofalk[tw] OR budenoside[tw] OR budes[tw] OR budeson[tw] OR "budesonide easyhaler"[tw] OR budiair[tw] OR "budicort respules"[tw] OR "budo‐san"[tw] OR budon[tw] OR budosan[tw] OR bunase[tw] OR buparid[tw] OR butacort[tw] OR clebudan[tw] OR coramen[tw] OR cortiment[tw] OR cortimentmmx[tw] OR cycortide[tw] OR "desona nasal"[tw] OR desonix[tw] OR dexbudesonide[tw] OR duasma[tw] OR eltair[tw] OR entocir[tw] OR entocort[tw] OR esonide[tw] OR "giona easyhaler"[tw] OR horacort[tw] OR inflammide[tw] OR inflanaze[tw] OR intesticort[tw] OR intestifalk[tw] OR jorveza[tw] OR larbex[tw] OR "map 0010"[tw] OR map0010[tw] OR miflo[tw] OR miflonid[tw] OR miflonide[tw] OR miflonil[tw] OR mikicort[tw] OR nebbud[tw] OR "neo‐rinactive"[tw] OR novopulmon[tw] OR numark[tw] OR olfex[tw] OR preferid[tw] OR "pregna 1, 4 diene 3, 20 dione 16, 17 butyraldehyde cyclic acetal, 11beta, 16alpha, 17, 21 tetrahydroxy"[tw] OR pulmaxan[tw] OR "pulmicon susp for nebulizer"[tw] OR "pulmicon susp for nebulizer"[tw] OR pulmicort[tw] OR pulmoliseflam[tw] OR pulmotide[tw] OR respicort[tw] OR rhinocort[tw] OR ribujet[tw] OR ribuspir[tw] OR ribuvent[tw] OR "s 1320"[tw] OR s1320[tw] OR spirocort[tw] OR "tafen nasal"[tw] OR uceris[tw] OR "51333‐22‐3"[tw] OR "51372‐29‐3"[tw] 23. Ciclesonide[tw] OR alvesco[tw] OR "b 9207 015"[tw] OR "b 9207015"[tw] OR "b9207 015"[tw] OR "b9207015"[tw] OR "by 9010"[tw] OR "by9010"[tw] OR omnaris[tw] OR zetonna[tw] OR "126544‐47‐6"[tw] 24. Flunisolide[tw] OR aerobid[tw] OR aerospan[tw] OR bronalide[tw] OR bronilide[tw] OR cyntaris[tw] OR flunitec[tw] OR gibiflu[tw] OR inhacort[tw] OR locasyn[tw] OR lokilan[tw] OR "lunibron‐a"[tw] OR lunis[tw] OR nasalide[tw] OR nasarel[tw] OR rhinalar[tw] OR "rs 3999"[tw] OR rs3999[tw] OR sanergal[tw] OR synaclyn[tw] OR syntaris[tw] OR "val 679"[tw] OR val679[tw] OR "3385‐03‐3"[tw] 25. Fluticasone[tw] OR Flonase[tw] OR Flovent[tw] OR Cutivate[tw] OR Flixonase[tw] OR Flixotide[tw] OR "90566‐53‐3"[tw] 26. Mometasone[tw] OR "allermax aqueous"[tw] OR asmanex[tw] OR belloseta[tw] OR bloctimo[tw] OR breso[tw] OR brusonex[tw] OR cutticom[tw] OR dermotasone[tw] OR dermovel[tw] OR desdek[tw] OR ecural[tw] OR edelan[tw] OR elica[tw] OR elocom[tw] OR elocon[tw] OR elocyn[tw] OR elomet[tw] OR eloson[tw] OR flumeta[tw] OR frondava[tw] OR ivoxel[tw] OR kalmente[tw] OR lorome[tw] OR mefurosan[tw] OR metaspray[tw] OR metmin[tw] OR mipasu[tw] OR mofunder[tw] OR momate[tw] OR momeallerg[tw] OR momecutan[tw] OR momederm[tw] OR momegalen[tw] OR momekort[tw] OR momesonex[tw] OR momespir[tw] OR momester[tw] OR mometahexal[tw] OR mometason[tw] OR mometasone[tw] OR mometop[tw] OR mommox[tw] OR monovel[tw] OR monovo[tw] OR morecort[tw] OR motaderm[tw] OR mundoson[tw] OR muofuder[tw] OR nasehaler[tw] OR nasoaldo[tw] OR nasomet[tw] OR nasometin[tw] OR nasonex[tw] OR nasotasone[tw] OR netonox[tw] OR "novasone cream"[tw] OR "novasone lotion"[tw] OR "novasone ointment"[tw] OR ovison[tw] OR ovixan[tw] OR pronasal[tw] OR rinelon[tw] OR rinometasone[tw] OR rivelon[tw] OR "sch 32088"[tw] OR sch32088[tw] OR sebanez[tw] OR sinuva[tw] OR sonalox[tw] OR temon[tw] OR uniclar[tw] OR zhekort[tw] OR "105102‐22‐5"[tw] OR "83919‐23‐7"[tw] 27. Triamcinolone[tw] OR acetocot[tw] OR adcortyl[tw] OR aristocort[tw] OR aristodan[tw] OR azmacor[tw] OR celeste[tw] OR "cl 19823"[tw] OR cl19823[tw] OR clinacort[tw] OR clinalog[tw] OR delphicort[tw] OR fluoxiprednisolone[tw] OR fluoxyprednisolone[tw] OR "ken‐jec 40"[tw] OR kenacort[tw] OR korticoid[tw] OR ledercort[tw] OR omcilon[tw] OR polcortolon[tw] OR "rp 8357"[tw] OR rp8357[tw] OR simacort[tw] OR sterocort[tw] OR "tac 3"[tw] OR tramcinolone[tw] OR triacortyl[tw] OR "triam‐a"[tw] OR "triam‐forte"[tw] OR triamcinolon[tw] OR triamcinolona[tw] OR triamcort[tw] OR triamcot[tw] OR "triamonide 40"[tw] OR triamsicort[tw] OR triancinolon[tw] OR "u‐tri‐lone"[tw] OR volon[tw] OR "124‐94‐7"[tw] 28. #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 29. #8 AND #28 30. #1 AND #29 31. Medline[sb] 32. #30 NOT #31
Appendix 5. LILACS search strategy
(MH:C01.539.800.130$ OR MH:C01.539.830.200$ OR MH:C17.300.185$ OR MH:C23.550.470.756.200$ OR MH:C11.675.387$ OR MH:C01.252.354$ OR MH:C01.539.375.354$ OR MH:C11.294.354 OR MH:C11.675 OR MH:C08.460.692.752$ OR MH:C08.730.749$ OR MH:C09.603.692.752$ OR (periorbit$ celluliti$) OR (orbit$ celluliti$) OR (preseptal celluliti$) OR ("pre septal" celluliti$) OR (postseptal celluliti$) OR ("post septal" celluliti$) OR (subperiosteal abscess$) OR ("sub periosteal" abscess$) OR (orbital abscess$) OR (intraconal abscess$) OR (Bacterial eye infect$) OR (orbital disease$) OR sinusitis OR (sinus infect$) OR rhinosinusitis OR "Sinus venous thrombosis") AND ((mh:("Orbital Cellulitis/DT")) OR MH:D04.210.500$ OR steroid$ OR MH:D06.472.040$ OR ("Adrenal Cortex" Hormone$) OR Corticosteroid$ OR Corticoid$ OR Glucocorticoid$ OR (Glucorticoid Effect$) OR Hydroxycorticosteroid$ OR ("Adrenal cortical" hormone$) OR (Adrenocortical hormone$) OR Adrenocorticosteroid$ OR Dermocorticosteroid$ OR Glucocorticosteroid$ OR Glucocorticoidsteroid$ OR Glucocortoid$ OR Glycocorticoid$ OR Glycocorticosteroid$ OR Dexamethasone OR Prednisone OR Prednisolone OR Methylprednisolone OR Hydrocortisone OR Loteprednol OR Rimexolone OR Beclomethasone OR Betamethasone OR Budesonide OR Ciclesonide OR Flunisolide OR Fluticasone OR Mometasone OR Triamcinolone)
Appendix 6. ClinicalTrials.gov search strategy
(Cellulitis OR subperiosteal abscess OR sub periosteal abscess OR orbital abscess OR intraconal abscess OR bacterial eye infection OR orbital disease OR sinusitis OR rhinosinusitis OR Sinus venous thrombosis) AND (steroid OR Adrenal Cortex Hormones OR Corticosteroid OR Corticoid OR Glucocorticoid OR Glucorticoid Effect OR Hydroxycorticosteroid OR Adrenal cortical hormone OR Adrenocortical hormone OR Adrenocorticosteroid OR Dermocorticosteroid OR Glucocorticosteroid OR Glucocorticoidsteroid OR Glucocortoid OR Glycocorticoid OR Glycocorticosteroid OR Dexamethasone OR Prednisone OR Prednisolone OR Methylprednisolone OR Hydrocortisone OR Loteprednol OR Rimexolone OR Beclomethasone OR Betamethasone OR Budesonide OR Ciclesonide OR Flunisolide OR Fluticasone OR Mometasone OR Triamcinolone)
Appendix 7. WHO ICTRP search strategy
Cellulitis AND steroid OR Cellulitis AND Adrenal Cortex Hormones OR Cellulitis AND Corticosteroid OR Cellulitis AND Corticoid OR Cellulitis AND Glucocorticoid OR Cellulitis AND Glucorticoid Effect OR Cellulitis AND Hydroxycorticosteroid OR Cellulitis AND Adrenal cortical hormone OR Cellulitis AND Adrenocortical hormone OR Cellulitis AND Adrenocorticosteroid OR Cellulitis AND Dermocorticosteroid OR Cellulitis AND Glucocorticosteroid OR Cellulitis AND Glucocorticoidsteroid OR Cellulitis AND Glucocortoid OR Cellulitis AND Glycocorticoid OR Cellulitis AND Glycocorticosteroid OR Cellulitis AND Dexamethasone OR Cellulitis AND Prednisone OR Cellulitis AND Prednisolone OR Cellulitis AND Methylprednisolone OR Cellulitis AND Hydrocortisone OR Cellulitis AND Loteprednol OR Cellulitis AND Rimexolone OR Cellulitis AND Beclomethasone OR Cellulitis AND Betamethasone OR Cellulitis AND Budesonide OR Cellulitis AND Ciclesonide OR Cellulitis AND Flunisolide OR Cellulitis AND Fluticasone OR Cellulitis AND Mometasone OR Cellulitis AND Triamcinolone
Data and analyses
Comparison 1. Corticosteroids versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2 Duration of time requiring antibiotics | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.3 Proportion of participants who required surgical intervention | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.4 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 Day 0 | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.38, 0.38] |
| 1.4.2 Day 3 | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.02, 0.62] |
| 1.5 Periorbital edema | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Day 0 | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.69, 0.69] |
| 1.5.2 Day 3 | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.41, 0.61] |
| 1.5.3 Day 7 | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.62, 1.78] |
| 1.5.4 Day 10 | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.44, 1.16] |
| 1.6 Amount of proptosis (mm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 Day 0 | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.84, 1.44] |
| 1.6.2 Day 3 | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.38, 1.38] |
| 1.6.3 Day 7 | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐0.05, 2.25] |
| 1.6.4 Day 10 | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [0.25, 1.75] |
| 1.6.5 Day 14 | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [0.44, 1.56] |
| 1.7 Conjunctival chemosis | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Day 0 | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.93, 0.33] |
| 1.7.2 Day 3 | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.09, 0.69] |
| 1.8 Preservation of visual acuity at the end of the study period | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.9 Exposure keratopathy | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.10 Other adverse events | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only |
1.10. Analysis.

Comparison 1: Corticosteroids versus standard care, Outcome 10: Other adverse events
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Pushker 2013.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized (total and by group):
Exclusions after randomization: none reported Number analyzed (total and by group):
Unit of analysis: eye Losses to follow‐up: no loss to follow‐up How were missing data handled?: not applicable Sample size calculation: not reported |
|
| Participants |
Age mean (SD): Group 1: 22.6 (18.6 years) Group 2: 22.9 (11.2 years) Overall: not reported Mean duration of illness at presentation mean (SD): Group 1: 7.4 (2.8 days) Group 2: 7.9 (5.1) days Overall: not reported Gender: n(%) women: Group 1: not reported Group 2: not reported Overall: 6 (29) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Standard of care: “All patients were initially started on standard treatment of intravenous vancomycin (1 g twice a day or 40 mg/kg/day in 2 divided doses) and intravenous ceftriaxone (1 g twice a day or 100 mg/kg/day in 2 divided doses). In cases not responding within 48 hours or where anaerobic foci were suspected, intravenous metronidazole (500 mg 3 times a day or 30 mg/kg/day in 3 divided doses) was added. Concomitant sinusitis, if present, was treated. Surgical drainage was done as and when required and pus was sent for cultures. Supportive treatment in the form of topical antibiotics and lubricants was started.” “Ultrasonography of the orbit was done to look for the resolution of abscess.” Corticosteroids regimen: adjuvant oral prednisolone starting from day 4 to day 7 (after an initial response to antibiotics was seen). The starting dose was 1.5 mg/kg/day for 3 days followed by 1 mg/kg/day for another 3 days, then tapered over 1 to 2 weeks. Planned length of follow‐up: 12 weeks following enrollment |
|
| Outcomes |
Outcomes measured:
Interval at which outcomes were measured: Day of admission; days 3, 7, 10, and 14; and on the 12th week (final) |
|
| Notes | Trial registration: not reported Funding sources: no funding support Disclosures of interest: none reported Study period: August 2007 to January 2009 Reported subgroup analyses: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “After an initial response to antibiotics, the patients were randomized into 2 groups (using a random number generator).” |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Only stated that study was “single‐masked”; it is unclear who was masked |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only stated that study was “single‐masked”; it is unclear who was masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data were available for all participants. |
| Selective reporting (reporting bias) | Unclear risk | Unclear, as no trial registration or predetermined protocol was available |
| Other bias | Unclear risk | Used a 2:1 randomization ratio Used the eye as the unit of analysis, though corticosteroids are a systemic treatment |
IV: intravenous; logMAR: logarithm of the minimum angle of resolution; SD: standard deviation; VAS: visual analog scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 2007 | Not an RCT: treatment guideline: guideline on medications for common eye disorders |
| Baiu 2020 | Not an RCT: JAMA Patient Page: a patient education sheet on periorbital and orbital cellulitis |
| Borello 1965 | Not an RCT: narrative review |
| Brameli 2018 | Not an RCT: retrospective review of children treated for orbital cellulitis at a pediatric hospital in Israel with and without corticosteroids |
| Ekhlassi 2017 | Not an RCT: narrative review of treatment and management of periorbital and orbital cellulitis |
| James 2018 | Not an RCT: retrospective review of how periorbital cellulitis was managed in a pediatric emergency department in Singapore over a 10‐year period |
| Lee 2013 | Not an RCT: commentary on the included trial by Pushker and colleagues |
| NCT01671423 | Not the population of interest: clinical trial registration on the use of a single dose of oral prednisone in the treatment of cellulitis. The trial did not enroll any participants with periorbital or orbital cellulitis. |
| NCT01721694 | Not the population of interest: withdrawn clinical trial protocol of antibiotic steroids combination for the treatment of bacterial blepharitis and/or keratitis and/or conjunctivitis (not orbital or periorbital cellulitis patients) |
| O'Day 1991 | Not an RCT: guest editorial: a review of the use of corticosteroids in infectious keratitis |
RCT: randomized controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Carvalho 1984.
| Methods | Unavailable |
| Participants | Unavailable |
| Interventions | Unavailable |
| Outcomes | Unavailable |
| Notes | Unavailable |
NCT02087527.
| Methods | Randomized controlled trial |
| Participants | Children with cellulitis |
| Interventions | Corticosteroids |
| Outcomes | Length of stay |
| Notes | Unclear if includes periorbital or orbital cellulitis |
Differences between protocol and review
We stated in the protocol that the unit of analysis was the participant. However, in the single study included in the review, the unit of analysis was the eye for some outcome measures. The study did not include sufficient data to conduct a further analysis using the individual participant as the unit of analysis. When we contacted the study authors, they indicated that they no longer had access to the raw data and were therefore unable to provide additional details. Consequently, when no further data were provided, the unit of analysis used was the eye.
Contributions of authors
EK: screened articles for inclusion, conducted data extraction and analysis, drafted the initial version of the manuscript, and reviewed subsequent revisions.
SM: conceived and designed the study, reviewed manuscript, and commented on it critically for intellectual content.
PP: conceived and designed study, reviewed manuscript, and commented on it critically for intellectual content.
YAR: reviewed manuscript in detail and commented on it critically for intellectual content, particularly relating to outcome measurement.
SS: reviewed manuscript in detail and commented on it critically for intellectual content.
PG: conceived and designed study, screened articles for inclusion, conducted data extraction and analysis, reviewed the initial draft manuscript and subsequent revisions.
All authors reviewed and approved the review.
Sources of support
Internal sources
-
Department of Paediatrics, University of Toronto, Toronto, ON, Canada
Pediatric Research and Clinical Summer (PeRCS) Program at Hospital for Sick Children.
-
Paediatric Outcomes Research Team (PORT), Canada
Hospital for Sick Children Foundation
External sources
Cochrane Eyes and Vision Group US Project, supported by grant 1 U01 EY020522‐01, National Eye Institute, National Institutes of Health, USA
-
National Institute for Health Research (NIHR), UK
The NIHR funds the CEV Editorial Base in the UK. The views expressed in this publication are those of the authors and not necessarily those of the NIHR.
Declarations of interest
EK: none known. SM: none known. PP: none known. YAR: none known. SS: none known. PG: Has received grants from the Canadian Institute of Health Research (CIHR) and The Hospital for Sick Children; nonfinancial support from the EBMLive Steering Committee, and the CIHR Institute of Human Development, Child and Youth Health. He is also a member of the CMAJ Open and BMJ Evidence Based Medicine Editorial Board.
New
References
References to studies included in this review
Pushker 2013 {published data only}
- Pushker N, Tejwani LK, Bajaj MS, Khurana S, Velpandian T, Chandra M. Role of oral corticosteroids in orbital cellulitis. American Journal of Ophthalmology 2013;156(1):178-83. [DOI: 10.1016/j.ajo.2013.01.031] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 2007 {published data only}
- Drugs for some common eye disorders. Treatment Guidelines from the Medical Letter 2007;5(53):1-8. [PubMed]
Baiu 2020 {published data only}
- Baiu I, Melendez E. Periorbital and orbital cellulitis. JAMA 2020;323(2):196. [DOI: 10.1001/jama.2019.18211] [DOI] [PubMed] [Google Scholar]
Borello 1965 {published data only}
- Borello ED. The combination of broad-spectrum antibiotics and corticoids in acute diffused cellulitis of the head and neck of dental origin. Divulgacion Cultural Odontologica 1965;103:19-21. [PubMed] [Google Scholar]
Brameli 2018 {published data only}
- Brameli A, Ashkenazi-Hoffnung L, Giloni D, Friling R, Chodick G, Landau D, et al. Systemic corticosteroids may be beneficial for managing severe or refractory orbital cellulitis in children. Acta Paediatrica 2018;107(11):2028-9. [DOI: 10.1111/apa.14467] [DOI] [PubMed] [Google Scholar]
Ekhlassi 2017 {published data only}
- Ekhlassi T, Becker N. Preseptal and orbital cellulitis. Disease-a-Month 2017;63(2):30-2. [DOI: 10.1016/j.disamonth.2016.09.002] [DOI] [PubMed] [Google Scholar]
James 2018 {published data only}
- James V, Mohamad Ikbal MF, Min NC, Chan YH, Ganapathy S. Periorbital cellulitis in paediatric emergency medicine department patients. Annals of the Academy of Medicine, Singapore 2018;47(10):420-3. [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee MS. Literature Commentary: Pushker N, Tejwani LK, Bajaj MS, Khurana S, Velpandian T, Chandra M. Role of oral corticosteroids in orbital cellulitis. Am J. Ophthalmol. 2013. pii: s0002-9394(13) 00104-9. doi: 10.1016/j.ajo2013. epub ahead of print. Journal of Neuro-Ophthalmology 2013;33(3):305-6. [DOI: 10.1097/WNO.0b013e3182a161be] [DOI] [PubMed] [Google Scholar]
NCT01671423 {published data only}
- NCT01671423. Use of a single dose of oral prednisone in the treatment of cellulitis. clinicaltrials.gov/show/nct01671423 (first received 23 August 2012).
NCT01721694 {published data only}
- NCT01721694. Antibiotic steroid combination compared with individual administration in the treatment of ocular inflammation and infection [Association of azithromycin 1,5%/loteprednol 0,5% eye drops versus individual administration of azithromycin 1,5% and loteprednol 0,5% in the treatment of ocular inflammation and infection]. clinicaltrials.gov/ct2/show/NCT01721694 (first received 6 November 2012).
O'Day 1991 {published data only}
- O'Day DM. Corticosteroids: an unresolved debate. Ophthalmology 1991;98(6):845-6. [DOI: 10.1016/s0161-6420(91)32212-7] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Carvalho 1984 {published data only}
- Carvalho ES. Corticosteroids in infantile infectology. II - Specific indications [Corticosteroides em infectologia infantil. II - Indicacoes especificas]. Jornal de Pediatria 1984;56(1/2):33-8. [Google Scholar]
NCT02087527 {published data only}
- NCT02087527. Use of corticosteroids in children with cellulitis. clinicaltrials.gov/ct2/show/NCT02087527 (first received 14 March 2014).
Additional references
Al‐Nammari 2007
- Al-Nammari S, Roberton B, Ferguson C. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Should a child with preseptal periorbital cellulitis be treated with intravenous or oral antibiotics? Emergency Medicine Journal 2007;24(2):128-9. [DOI: 10.1136/emj.2006.045245] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brouwer 2015
- Brouwer MC, McIntyre P, Prasad K, de Beek D. Corticosteroids for acute bacterial meningitis. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD004405. [DOI: 10.1002/14651858.CD004405.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Çağlar 2018
- Çağlar İ, Kafes C, Korcum M, Düzgöl M, Kara A, Bayram SN, et al. Hospital cost analysis of children with preseptal cellulitis. International Journal of Pediatric Otorhinolaryngology 2018;106:96-9. [DOI: 10.1016/j.ijporl.2018.01.007] [DOI] [PubMed] [Google Scholar]
Capra 2015
- Capra G, Liming B, Boseley ME, Brigger MT. Trends in orbital complications of pediatric rhinosinusitis in the United States. JAMA Otolaryngology Head and Neck Surgery 2015;141(1):12-7. [DOI: 10.1001/jamaoto.2014.2626] [DOI] [PubMed] [Google Scholar]
Chandler 1970
- Chandler JR, Langenbrunner DJ, Stevens ER. The pathogenesis of orbital complications in acute sinusitis. Laryngoscope 1970;80(9):1414-28. [DOI: 10.1288/00005537-197009000-00007] [DOI] [PubMed] [Google Scholar]
Chen 2018
- Chen L, Silverman N, Wu A, Shinder R. Intravenous steroids with antibiotics on admission for children with orbital cellulitis. Ophthalmic Plastic and Reconstructive Surgery 2018;34(3):205-8. [DOI: 10.1097/IOP.0000000000000910] [DOI] [PubMed] [Google Scholar]
Crosbie 2016
- Crosbie RA, Nairn J, Kubba H. Management of paediatric periorbital cellulitis: our experience of 243 children managed according to a standardised protocol 2012-2015. International Journal of Pediatric Otorhinolaryngology 2016;87:134-8. [DOI: 10.1016/j.ijporl.2016.06.025] [DOI] [PubMed] [Google Scholar]
Davies 2015
- Davies BW, Smith JM, Hink EM, Durairaj VD. C-reactive protein as a marker for initiating steroid treatment in children with orbital cellulitis. Ophthalmic Plastic and Reconstructive Surgery 2015;31(5):364-8. [DOI: 10.1097/IOP.0000000000000349] [DOI] [PubMed] [Google Scholar]
Donahue 1998
- Donahue SP, Schwartz G. Preseptal and orbital cellulitis in childhood. A changing microbiologic spectrum. Ophthalmology 1998;105(10):1902-5. [DOI: 10.1016/S0161-6420(98)91038-7] [DOI] [PubMed] [Google Scholar]
Friedel 2019
- Friedel N, Scolnik D, Rimon A, Orbach R, Laat S, Glatstein M. Are we over-treating insect bite related periorbital cellulitis in children? The experience of a large, tertiary care pediatric hospital. American Journal of Therapeutics 2019;26(1):e1-4. [DOI: 10.1097/MJT.0000000000000596] [DOI] [PubMed] [Google Scholar]
Gates 2018
- Gates A, Gates M, Vandermeer B, Johnson C, Hartling L, Johnson DW, et al. Glucocorticoids for croup in children. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD001955. [DOI: 10.1002/14651858.CD001955.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gavriel 2018
- Gavriel H, Jabarin B, Israel O, Eviatar E. Conservative management for subperiosteal orbital abscess in adults: a 20-year experience. Annals of Otology, Rhinology and Laryngology 2018;127(3):162-6. [DOI: 10.1177/0003489417751155] [DOI] [PubMed] [Google Scholar]
Goldman 2008
- Goldman R, Dolansky G, Rogovik A. Predictors for admission of children with periorbital cellulitis presenting to the pediatric emergency department. Pediatric Emergency Care 2008;24(5):279-83. [DOI: 10.1097/PEC.0b013e31816ecb43] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 20 July 2020. Available at gradepro.org.
Harris 1994
- Harris GJ. Subperiosteal abscess of the orbit. Age as a factor in the bacteriology and response to treatment. Ophthalmology 1994;101(3):585-95. [DOI: 10.1016/s0161-6420(94)31297-8] [DOI] [PubMed] [Google Scholar]
HCUPnet
- HCUPnet. Healthcare cost and utilization project. hcupnet.ahrq.gov/#setup (accessed 7 January 2020).
Higgins 2011
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Howe 2004
- Howe L, Jones NS. Guidelines for the management of periorbital cellulitis/abscess. Clinical Otolaryngology and Allied Sciences 2004;29(6):725-8. [DOI: 10.1111/j.1365-2273.2004.00889.x] [DOI] [PubMed] [Google Scholar]
Lee 2011
- Lee S, Yen MT. Management of preseptal and orbital cellulitis. Saudi Journal of Ophthalmology 2011;25(1):21-9. [DOI: 10.1016/j.sjopt.2010.10.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchiano 2016
- Marchiano E, Raikundalia MD, Carniol ET, Echanique KA, Kalyoussef E, Baredes S, et al. Characteristics of patients treated for orbital cellulitis: an analysis of inpatient data. Laryngoscope 2016;126(3):554-9. [DOI: 10.1002/lary.25529] [DOI] [PubMed] [Google Scholar]
Markham 2018
- Markham JL, Hall M, Bettenhausen JL, Myrs AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hospital Pediatrics 2018;8(1):28-35. [DOI: 10.1542/hpeds.2017-0040] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2014
- Murphy C, Livingstone I, Foot B, Murgatroyd H, MacEwen CJ. Orbital cellulitis in Scotland: current incidence, aetiology, management and outcomes. British Journal of Ophthalmology 2014;98(11):1575-8. [DOI: 10.1136/bjophthalmol-2014-305222] [DOI] [PubMed] [Google Scholar]
Ogunlesi 2015
- Ogunlesi TA, Odigwe CC, Oladapo OT. Adjuvant corticosteroids for reducing death in neonatal bacterial meningitis. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD010435. [DOI: 10.1002/14651858.CD010435.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peña 2013
- Peña MT, Preciado D, Orestes M, Choi S. Orbital complications of acute sinusitis: changes in the post-pneumococcal vaccine era. JAMA Otolaryngology Head and Neck Surgery 2013;139(3):223-7. [DOI: 10.1001/jamaoto.2013.1703] [DOI] [PubMed] [Google Scholar]
Prasad 2016
- Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD002244. [DOI: 10.1002/14651858.CD002244.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rimon 2008
- Rimon A, Hoffer V, Prais D, Harel L, Amir J. Periorbital cellulitis in the era of Haemophilus influenzae type B vaccine: predisposing factors and etiologic agents in hospitalized children. Journal of Pediatric Ophthalmology and Strabismus 2008;45(5):300-4. [DOI: 10.3928/01913913-20080901-14] [DOI] [PubMed] [Google Scholar]
Rudloe 2010
- Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics 2010;125(4):e719-26. [DOI: 10.1542/peds.2009-1709] [DOI] [PubMed] [Google Scholar]
Santos 2019
- Santos JC, Pinto S, Ferreira S, Maia C, Alves S, da Silva A. Pediatric preseptal and orbital cellulitis: a 10-year experience. International Journal of Pediatric Otorhinolaryngology 2019;120:82-8. [DOI: 10.1016/j.ijporl.2019.02.003] [DOI] [PubMed] [Google Scholar]
Sharma 2015
- Sharma A, Liu ES, Le TD, Adatia FA, Buncic JR, Blaser S, et al. Pediatric orbital cellulitis in the Haemophilus influenzae vaccine era. Journal of AAPOS 2015;19(3):206-10. [DOI: 10.1016/j.jaapos.2015.02.004] [DOI] [PubMed] [Google Scholar]
Soroudi 2003
- Soroudi A, Casey R, Pan D, Baker R. An epidemiologic survey of orbital cellulitis. Investigative Ophthalmology and Visual Science 2003;44(13):786. [Google Scholar]
Venekamp 2014
- Venekamp RP, Thompson MJ, Hayward G, Heneghan CJ, Del Mar CB, Perera R, et al. Systemic corticosteroids for acute sinusitis. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD008115. [DOI: 10.1002/14651858.CD008115.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2018
- Wong SJ, Levi J. Management of pediatric orbital cellulitis: a systematic review. International Journal of Pediatric Otorhinolaryngology 2018;110:123-9. [DOI: 10.1016/j.ijporl.2018.05.006] [DOI] [PubMed] [Google Scholar]
Yang 2009
- Yang M, Quah BL, Seah LL, Looi A. Orbital cellulitis in children—medical treatment versus surgical management. Orbit 2009;28:124-36. [DOI: 10.1080/01676830902765891] [DOI] [PubMed] [Google Scholar]
Yen 2005
- Yen MT, Yen KG. Effect of corticosteroids in the acute management of pediatric orbital cellulitis with subperiosteal abscess. Ophthalmic Plastic and Reconstructive Surgery 2005;21(5):363-6. [DOI: 10.1097/01.iop.0000179973.44003.f7] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gill 2020
- Gill PJ, Parkin P, Reginald YA, Shah SS, Kornelsen E, Mahant S. Corticosteroids for periorbital and orbital cellulitis. Cochrane Database of Systematic Reviews 2020, Issue 2. Art. No: CD013535. [DOI: 10.1002/14651858.CD013535] [DOI] [PMC free article] [PubMed] [Google Scholar]


