Abstract
Background
Infertility is a prevalent problem that has significant consequences for individuals, families, and the community. Modifiable lifestyle factors may affect the chance of people with infertility having a baby. However, no guideline is available about what preconception advice should be offered. It is important to determine what preconception advice should be given to people with infertility and to evaluate whether this advice helps them make positive behavioural changes to improve their lifestyle and their chances of conceiving.
Objectives
To assess the safety and effectiveness of preconception lifestyle advice on fertility outcomes and lifestyle behavioural changes for people with infertility.
Search methods
We searched the Cochrane Gynaecology and Fertility Group Specialised Register of controlled trials, CENTRAL, MEDLINE, Embase, PsycINFO, AMED, CINAHL, trial registers, Google Scholar, and Epistemonikos in January 2021; we checked references and contacted field experts to identify additional studies.
Selection criteria
We included randomised controlled trials (RCTs), randomised cross‐over studies, and cluster‐randomised studies that compared at least one form of preconception lifestyle advice with routine care or attention control for people with infertility.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. Primary effectiveness outcomes were live birth and ongoing pregnancy. Primary safety outcomes were adverse events and miscarriage. Secondary outcomes included reported behavioural changes in lifestyle, birth weight, gestational age, clinical pregnancy, time to pregnancy, quality of life, and male factor infertility outcomes. We assessed the overall quality of evidence using GRADE criteria.
Main results
We included in the review seven RCTs involving 2130 participants. Only one RCT included male partners. Three studies compared preconception lifestyle advice on a combination of topics with routine care or attention control. Four studies compared preconception lifestyle advice on one topic (weight, alcohol intake, or smoking) with routine care for women with infertility and specific lifestyle characteristics. The evidence was of low to very low‐quality. The main limitations of the included studies were serious risk of bias due to lack of blinding, serious imprecision, and poor reporting of outcome measures.
Preconception lifestyle advice on a combination of topics versus routine care or attention control Preconception lifestyle advice on a combination of topics may result in little to no difference in the number of live births (risk ratio (RR) 0.93, 95% confidence interval (CI) 0.79 to 1.10; 1 RCT, 626 participants), but the quality of evidence was low. No studies reported on adverse events or miscarriage. Due to very low‐quality evidence, we are uncertain whether preconception lifestyle advice on a combination of topics affects lifestyle behavioural changes: body mass index (BMI) (mean difference (MD) ‐1.06 kg/m², 95% CI ‐2.33 to 0.21; 1 RCT, 180 participants), vegetable intake (MD 12.50 grams/d, 95% CI ‐8.43 to 33.43; 1 RCT, 264 participants), alcohol abstinence in men (RR 1.08, 95% CI 0.74 to 1.58; 1 RCT, 210 participants), or smoking cessation in men (RR 1.01, 95% CI 0.91 to 1.12; 1 RCT, 212 participants). Preconception lifestyle advice on a combination of topics may result in little to no difference in the number of women with adequate folic acid supplement use (RR 0.98, 95% CI 0.95 to 1.01; 2 RCTs, 850 participants; I² = 4%), alcohol abstinence (RR 1.07, 95% CI 0.99 to 1.17; 1 RCT, 607 participants), and smoking cessation (RR 1.01, 95% CI 0.98 to 1.04; 1 RCT, 606 participants), on low quality evidence. No studies reported on other behavioural changes.
Preconception lifestyle advice on weight versus routine care Studies on preconception lifestyle advice on weight were identified only in women with infertility and obesity. Compared to routine care, we are uncertain whether preconception lifestyle advice on weight affects the number of live births (RR 0.94, 95% CI 0.62 to 1.43; 2 RCTs, 707 participants; I² = 68%; very low‐quality evidence), adverse events including gestational diabetes (RR 0.78, 95% CI 0.48 to 1.26; 1 RCT, 317 participants; very low‐quality evidence), hypertension (RR 1.07, 95% CI 0.66 to 1.75; 1 RCT, 317 participants; very low‐quality evidence), or miscarriage (RR 1.50, 95% CI 0.95 to 2.37; 1 RCT, 577 participants; very low‐quality evidence). Regarding lifestyle behavioural changes for women with infertility and obesity, preconception lifestyle advice on weight may slightly reduce BMI (MD ‐1.30 kg/m², 95% CI ‐1.58 to ‐1.02; 1 RCT, 574 participants; low‐quality evidence). Due to very low‐quality evidence, we are uncertain whether preconception lifestyle advice affects the percentage of weight loss, vegetable and fruit intake, alcohol abstinence, or physical activity. No studies reported on other behavioural changes.
Preconception lifestyle advice on alcohol intake versus routine care Studies on preconception lifestyle advice on alcohol intake were identified only in at‐risk drinking women with infertility. We are uncertain whether preconception lifestyle advice on alcohol intake affects the number of live births (RR 1.15, 95% CI 0.53 to 2.50; 1 RCT, 37 participants; very low‐quality evidence) or miscarriages (RR 1.31, 95% CI 0.21 to 8.34; 1 RCT, 37 participants; very low‐quality evidence). One study reported on behavioural changes for alcohol consumption but not as defined in the review methods. No studies reported on adverse events or other behavioural changes.
Preconception lifestyle advice on smoking versus routine care Studies on preconception lifestyle advice on smoking were identified only in smoking women with infertility. No studies reported on live birth, ongoing pregnancy, adverse events, or miscarriage. One study reported on behavioural changes for smoking but not as defined in the review methods.
Authors' conclusions
Low‐quality evidence suggests that preconception lifestyle advice on a combination of topics may result in little to no difference in the number of live births. Evidence was insufficient to allow conclusions on the effects of preconception lifestyle advice on adverse events and miscarriage and on safety, as no studies were found that looked at these outcomes, or the studies were of very low quality. This review does not provide clear guidance for clinical practice in this area. However, it does highlight the need for high‐quality RCTs to investigate preconception lifestyle advice on a combination of topics and to assess relevant effectiveness and safety outcomes in men and women with infertility.
Keywords: Female; Humans; Male; Alcohol Drinking; Bias; Caffeine; Caffeine/adverse effects; Central Nervous System Stimulants; Central Nervous System Stimulants/adverse effects; Counseling; Counseling/methods; Diet, Healthy; Exercise; Folic Acid; Folic Acid/administration & dosage; Infertility; Infertility/therapy; Infertility, Female; Infertility, Female/therapy; Life Style; Live Birth; Live Birth/epidemiology; Preconception Care; Preconception Care/methods; Randomized Controlled Trials as Topic; Sex Factors; Smoking Cessation; Vitamin B Complex; Vitamin B Complex/administration & dosage; Weight Loss
Plain language summary
Does preconception lifestyle advice help people with infertility to have a baby?
Background Infertility places a significant burden on individuals, families, and the wider community and impacts more than 45 million couples worldwide. Treatment for infertility includes simple interventions such as fertility awareness and lifestyle advice (counselling about weight, diet, physical activity, and/or smoking) to more complex assisted reproductive technologies such as in vitro fertilisation (IVF). Lifestyle factors such as weight, diet, physical activity, and smoking may affect fertility and the chance of people with infertility having a baby. However, guidelines about what preconception lifestyle advice should be offered are lacking.
Why we did this Cochrane Review We wanted to find out the effects of preconception lifestyle advice compared to routine care or attention control (e.g. treatment advice without lifestyle advice) for people with infertility.
What we did We searched for randomised controlled studies that compared preconception lifestyle advice for people with infertility with routine care or attention control.
We were interested in finding out what preconception lifestyle advice should be given to people with infertility; how well it works for improving lifestyle to increase their chance of having a baby; and whether it had any unwanted effects.
Search date We included evidence published up to 14 January 2021.
What we found We found seven studies in 2130 people with infertility. Only one study also included male partners. The studies were conducted in Canada, Iran, The Netherlands, UK, and USA. Three studies compared preconception lifestyle advice on a combination of topics with routine care or attention control. Four studies compared preconception lifestyle advice on one topic (weight, alcohol intake, or smoking) with routine care in women with infertility and specific lifestyle characteristics.
Key results
Preconception lifestyle advice on a combination of topics versus routine care or attention control Preconception lifestyle advice on a combination of topics may not affect live birth. The evidence suggests that if live birth is assumed to be 48% for those receiving routine care or attention control, then live birth when preconception lifestyle advice is received would be between 38% and 53%. We are uncertain whether preconception lifestyle advice on a combination of topics affects lifestyle behaviour changes such as body mass index (BMI) in women, vegetable intake in men and women, or alcohol abstinence and smoking cessation in men. Preconception lifestyle advice on a combination of topics may not affect adequate use of folic acid supplement, alcohol abstinence, or smoking cessation in women. The evidence suggests that if adequate folic acid supplement use in women is assumed to be 93% for those receiving routine care or attention control, then adequate folic acid supplement use when preconception lifestyle advice is received would be between 89% and 94%. Evidence also suggests that if it is assumed that 75% of women abstain from alcohol with routine care or attention control, then between 74% and 88% of women would abstain from alcohol when receiving preconception lifestyle advice. If it is assumed that smoking cessation is seen in 95% of women receiving routine care or attention control, then smoking cessation would be seen in 93% to 99% of women when they receive preconception lifestyle advice. No study reported on other behavioural changes.
Preconception lifestyle advice on weight versus routine care We are uncertain whether preconception lifestyle advice on weight for women with infertility and obesity affects live birth or adverse events (including gestational diabetes and hypertension) and miscarriage. Regarding behavioural changes, preconception lifestyle advice on weight may slightly reduce BMI, but we are uncertain whether it affects other behavioural changes: percentage of weight loss, vegetable and fruit intake, alcohol intake, and total moderate to vigorous physical activity. No study reported on other behavioural changes.
Preconception lifestyle advice on alcohol intake versus routine care In at‐risk drinking women with infertility, we are uncertain whether preconception lifestyle advice on alcohol intake affects live birth or miscarriage. One study reported behavioural changes in alcohol intake but not as defined in the Review methods. No study reported on any other outcome.
Preconception lifestyle advice on smoking versus routine care One study reported on preconception lifestyle advice with a focus on behavioural changes for smoking cessation in women with infertility who smoke, but not as defined in the Review methods. No study reported on any other outcome.
Quality of the evidence The evidence was of low to very low quality. The main limitations of the evidence were poor study methods in included studies (lack of blinding) and lack of (precision in) findings for live birth, safety outcomes, and reported behavioural changes.
Summary of findings
Summary of findings 1. Preconception lifestyle advice on a combination of topics compared to routine care or attention control for people with infertility.
| Preconception lifestyle advice on a combination of topics compared to routine care or attention control for people with infertility | |||||||
| Patient or population: people with infertility Setting: university/hospital Intervention: preconception lifestyle advice on a combination of topics Comparison: routine care or attention control | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with routine care or attention control | Risk with preconception lifestyle advice on a combination of topics | ||||||
| Live birth | 481 per 1000 | 447 per 1000 (380 to 529) | RR 0.93 (0.79 to 1.10) | 626 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | Preconception lifestyle advice on a combination of topics may result in little to no difference in live birth | |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported on this outcome | |
| Miscarriage | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported on this outcome | |
| Reported behavioural changes in weight: BMI (measured "at study end" ‐ 3 months) |
Mean reported behavioural changes in weight: BMI was 25.52 kg/m² | MD 1.06 kg/m² lower (2.33 lower to 0.21 higher) | ‐ | 180 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c,d | Evidence is very uncertain about the effects on BMI of preconception lifestyle advice on a combination of topics | |
| Reported behavioural changes in diet: vegetable intake assessed with lifestyle questionnaire (measured at 3 months) | Mean reported behavioural changes in diet: vegetable intake was 135.2 grams/d | MD 12.5 grams/d higher (8.43 lower to 33.43 higher) | ‐ | 264 (1 RCT) | ⊕⊝⊝⊝ VERY LOWd,e | Evidence is very uncertain about the effects on vegetable intake of preconception lifestyle advice on a combination of topics | |
| Reported behavioural changes on vitamin or mineral supplement intake: number of women with adequate use of folic acid supplement assessed with lifestyle questionnaire (measured at 3 and 6 months) | 933 per 1000 | 915 per 1000 (887 to 943) | RR 0.98 (0.95 to 1.01) | 850 (2 RCTs) | ⊕⊕⊝⊝ LOWe | Preconception lifestyle advice on a combination of topics may result in little to no difference in the number of women with adequate use of folic acid supplement, but the quality of the evidence was low | |
| Reported behavioural changes in alcohol intake (measured at 6 months) | Number of women abstaining from alcohol assessed with lifestyle questionnaire | 750 per 1000 | 803 per 1000 (742 to 878) | RR 1.07 (0.99 to 1.17) | 607 (1 RCT) | ⊕⊕⊝⊝ LOWe | Preconception lifestyle advice on a combination of topics may result in little to no difference in the number of women abstaining from alcohol, but the quality of the evidence was low |
| Number of men abstaining from alcohol assessed with lifestyle questionnaire | 321 per 1000 | 347 per 1000 (238 to 507) | RR 1.08 (0.74 to 1.58) | 210 (1 RCT) | ⊕⊝⊝⊝ VERY LOWe,f | Evidence is very uncertain about the effect on the number of men abstaining from alcohol of preconception lifestyle advice on a combination of topics | |
| Reported behavioural changes in smoking (measured at 6 months): | Number of women not smoking assessed with lifestyle questionnaire | 951 per 1000 | 961 per 1000 (932 to 989) | RR 1.01 (0.98 to 1.04) | 606 (1 RCT) | ⊕⊕⊝⊝ LOWe | Preconception lifestyle advice on a combination of topics may result in little to no difference in the number of women not smoking, but the quality of the evidence was low |
| Number of men not smoking assessed with lifestyle questionnaire | 873 per 1000 | 881 per 1000 (794 to 977) | RR 1.01 (0.91 to 1.12) | 212 (1 RCT) | ⊕⊝⊝⊝ VERY LOWd,e | Evidence is very uncertain about the effect on the number of men not smoking of preconception lifestyle advice on a combination of topics | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||||
|
GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||||
aDowngraded by one level for serious risk of bias: high risk for performance bias and at multiple domains, unclear risk of bias.
bDowngraded by one level for serious imprecision: optimal information size (OIS) not met.
cDowngraded by one level for serious indirectness: differences in intervention (one study has traditional medicine‐oriented diet regimen).
dDowngraded by one level for serious imprecision: one study, few patients (< 400).
eDowngraded by two levels for very serious risk of bias: high risk for performance, detection, and reporting bias and at multiple domains, unclear risk of bias.
fDowngraded by two levels for very serious imprecision: one study, few events (< 400) and 95% CI includes important benefit and harm.
Summary of findings 2. Preconception lifestyle advice on weight compared to routine care or attention control for people with infertility and obesity.
| Preconception lifestyle advice on weight compared to routine care or attention control for people with infertility and obesity | ||||||
| Patient or population: women with infertility and obesity Setting: university/hospital Intervention: preconception lifestyle advice on weight Comparison: routine care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with routine care or attention control | Risk with preconception lifestyle advice on weight | |||||
| Live birth | 494 per 1000 | 465 per 1000 (306 to 707) | RR 0.94 (0.62 to 1.43) | 707 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c,d | Evidence about the effect on live birth of preconception lifestyle advice on weight is very uncertain |
| Adverse events ‐ Hypertension | 162 per 1000 | 173 per 1000 (107 to 283) | RR 1.07 (0.66 to 1.75) | 317 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c,e | Evidence about the effect on hypertension of preconception lifestyle advice on weight is very uncertain |
| Adverse events ‐ Gestational diabetes | 198 per 1000 | 154 per 1000 (95 to 249) | RR 0.78 (0.48 to 1.26) | 317 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c,e | Evidence about the effect on gestational diabetes of preconception lifestyle advice on weight is very uncertain |
| Miscarriage | 94 per 1000 | 141 per 1000 (89 to 223) | RR 1.50 (0.95 to 2.37) | 577 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c,d | Evidence about the effect on miscarriage of preconception lifestyle advice on weight is very uncertain |
| Reported behavioural changes in weight: BMI (measured at 6 months) | Mean reported behavioural changes in weight: BMI was 35.6 kg/m² | MD 1.3 kg/m² lower (1.58 lower to 1.02 lower) | ‐ | 574 (1 RCT) | ⊕⊕⊝⊝ LOWa,c | Preconception lifestyle advice on weight may result in a slight reduction in BMI |
| Reported behavioural changes in weight: percentage of weight loss (measured at 6 months) | Mean reported behavioural changes in weight: percentage of weight loss was ‐0.97% | MD 3.29 % lower (4.34 lower to 2.24 lower) | ‐ | 380 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,c,d | Evidence about the effect on percentage of weight loss of preconception lifestyle advice on weight is very uncertain |
| Reported behavioural changes in diet: vegetable intake assessed with FFQ (measured at 6 months) | Mean reported behavioural changes in diet: vegetable intake was 128.75 grams/d | MD 0 grams/d (4.18 lower to 4.18 higher) | ‐ | 250 (1 RCT) | ⊕⊝⊝⊝ VERY LOWc,d,f | Evidence about the effect on vegetable intake of preconception lifestyle advice on weight is very uncertain |
| Reported behavioural changes in diet: fruit intake assessed with FFQ (measured at 6 months) | Mean reported behavioural changes in diet: fruit intake was 135.75 grams/d | MD 7.25 g/day lower (7.86 lower to 6.64 lower) | ‐ | 258 (1 RCT) | ⊕⊝⊝⊝ VERY LOWc,d,f | Evidence about the effect on fruit intake of preconception lifestyle advice on weight is very uncertain |
| Reported behavioural changes in alcohol intake assessed with FFQ (measured at 6 months) | Mean reported behavioural change in alcohol consumption was 0 glasses/d | MD 0 glasses/d (0 to 0 ) | ‐ | 239 (1 RCT) | ⊕⊝⊝⊝ VERY LOWc,d,f | Not estimable |
| Reported behavioural changes in physical activity assessed with SQUASH (measured at 6 months) | Mean reported behavioural change in physical activity was 361.24 minutes/week | MD 50.76 minutes/week higher (16.77 higher to 84.75 higher) | ‐ | 254 (1 RCT) | ⊕⊝⊝⊝ VERY LOWc,d,f | Evidence about the effect on physical activity of preconception lifestyle advice on weight is very uncertain |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FFQ: Food Frequency Questionnaire; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by one level for serious risk of bias: high risk for performance bias.
bDowngraded by one level for serious inconsistency: substantial heterogeneity (I² = 68%) and wide variance of point estimates across studies with opposite directions of effect.
cDowngraded by one level for serious indirectness: differences in comparison (access to fertility treatment). Specific population: women with infertility and obesity.
dDowngraded by one level for serious imprecision: < 400 events and 95% CIs overlap.
eDowngraded by two levels for very serious imprecision: one study, few events (< 400) and 95% CIs include important benefit and harm.
fDowngraded by one level for serious risk of bias: high risk for performance and detection bias.
Summary of findings 3. Preconception lifestyle advice on alcohol intake compared to routine care or attention control for at‐risk drinking women with infertility.
| Preconception lifestyle advice on alcohol intake compared to routine care or attention control for at‐risk drinking women with infertility | ||||||
| Patient or population: women with infertility and at‐risk drinking Setting: university/hospital Intervention: preconception lifestyle advice on alcohol intake Comparison: routine care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with routine care or attention control | Risk with preconception lifestyle advice on alcohol intake | |||||
| Live birth | 381 per 1000 | 438 per 1000 (202 to 952) | RR 1.15 (0.53 to 2.50) | 37 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b | Evidence about the effect on live birth of preconception lifestyle advice on alcohol intake is very uncertain |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported on this outcome |
| Miscarriage | 95 per 1000 | 125 per 1000 (20 to 794) | RR 1.31 (0.21 to 8.34) | 37 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b | Evidence about the effect on miscarriage of preconception lifestyle advice on alcohol intake is very uncertain |
| Reported behavioural changes | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported on this outcome in a way defined by this review |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels for very serious risk of bias: high risk for performance bias, attrition bias, and reporting bias.
bDowngraded by two levels for very serious imprecision: one study, few patients (n = 37) and few events; 95% CI includes important benefit and harm.
Summary of findings 4. Preconception lifestyle advice on smoking compared to routine care or attention control for people with infertility.
| Preconception lifestyle advice on smoking compared to routine care or attention control for people with infertility | ||||||
| Patient or population: smoking people with infertility Setting: university/hospital Intervention: preconception lifestyle advice on smoking Comparison: routine care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with routine care or attention control | Risk with preconception lifestyle advice on smoking | |||||
| Live birth or ongoing pregnancy | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported on this outcome |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported on this outcome |
| Miscarriage | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported on this outcome |
| Reported behavioural changes: smoking | ‐ | ‐ | ‐ | ‐ | ‐ | In the single study in this comparison, study authors concluded, "There were no significant differences in the mean delta stage‐of‐change or 12‐month rate of maintained cessation". The rate of maintained cessation was not reported separately for intervention and control groups |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval. | ||||||
|
GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
Background
Description of the condition
Infertility is defined as the failure to establish a clinical pregnancy after 12 months of regular unprotected sexual intercourse (Zegers‐Hochschild 2017). Worldwide, an estimated 48.5 million couples suffer from fertility problems (Mascarenhas 2012). Infertility may be due to male factors, female factors, or a combination of both, and in 20% of cases, the cause of infertility is unknown (Fritz 2011The Fertility Society of Australia 2019). Treatment for people with infertility is referred to as medically assisted reproduction (MAR) and includes assisted reproductive technologies (ART) such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). These treatments have large financial and biopsychosocial costs for individuals and for the community (Gameiro 2012Myers 2008). Therefore, improving treatment success rates and reducing this burden are important research priorities in reproductive medicine (Gameiro 2013).
Description of the intervention
Modifiable lifestyle factors such as weight, diet, alcohol intake, caffeine intake, physical activity, smoking, and other substance abuse may affect the chance of people with infertility having a live birth (Homan 2007Rooney 2014). Research suggests that these factors may have important effects both during the preconception period and on the developing foetus (Atrash 2006Bille 2009World Health Organization 2012). More specifically, Weeks 3 to 8 of pregnancy are the most sensitive in development of the embryo. Therefore, it is important to provide advice about modifying maternal and paternal factors before this period, to reduce the risk of adverse pregnancy outcomes (Atrash 2006Gardiner 2008World Health Organization 2012). Lifestyle factors that may affect fertility and the chance of a live birth include weight, diet, alcohol intake, caffeine intake, physical activity, smoking, and other substance abuse (Government of South Australia 2015Homan 2007Practice Committee 2017Rooney 2014World Health Organization 2017). Providing advice about which modifiable lifestyle factors affect fertility is a crucial first step in helping people with infertility to make modifications that may increase their chances of timely conception and delivery of a healthy baby (Grainger 2006Homan 2007Moran 2016a; Shawe 2015; World Health Organization 2012).
How the intervention might work
Counselling about the aforementioned lifestyle factors may positively influence a couple's behaviour before conceiving, and thus may improve their chance of achieving a live birth.
Weight
For both women and men, fertility may decrease when they are overweight (body mass index (BMI) ≥ 25 kg/m²) or underweight (BMI < 18.5 kg/m²) (Campbell 2015McKinnon 2016Practice Committee 2015). In addition, obesity (BMI ≥ 30 kg/m²) in both women and men has substantial adverse effects on general health but also on reproductive functions and the health of offspring (Campbell 2015Godfrey 2017Lane 2015McKinnon 2016Practice Committee 2015). More specifically, obesity in women is associated with ovulatory dysfunction, reduced ovarian responsiveness to agents that induce ovulation, and lower birth rates. Indeed, fertility treatment is less successful in men and women who are overweight or obese as compared with normal weight couples (Campbell 2015Rittenberg 2011). Moreover obese women are at increased risk of developing maternal and foetal complications during pregnancy (Practice Committee 2015). Therefore, lifestyle modification is first‐line treatment for women and men with obesity (Best 2017Practice Committee 2015). It is recommended that people aiming to conceive should maintain a healthy weight, that is, should aim for BMI between 18.5 and 25 kg/m², by exercising regularly and following a healthy diet (Gardiner 2008Government of South Australia 2015Practice Committee 2017World Health Organization 2016World Health Organization 2017).
Diet
Evidence suggests that adherence to a diet rich in vegetables, fruits, whole grains, fish, and poultry is related to better fertility and higher live birth rates among women and improved semen quality in men, and unhealthy diets may have the opposite effect (Chiu 2018Gaskins 2018a; Giahi 2016Grieger 2018Salas‐Huetos 2017Vujkovic 2010). Given this evidence and the significant benefits of a healthy diet for general health, women and men aiming to conceive should be encouraged to follow a healthy diet (World Health Organization 2016World Health Organization 2017World Health Organization 2020a).
In addition to adherence to a healthy diet, some specific dietary recommendations have been provided for women during the preconception period. Maternal methylmercury exposure through eating predatory fish can affect foetal development (McDiarmid 2008). Thus, it has been recommended that women avoid eating shark, swordfish, king mackerel, and tile fish, and limit their intake of tuna during both the preconception period and pregnancy (Gardiner 2008McDiarmid 2008). Furthermore, it is recommended that pregnant women ensure that any fruits and vegetables are washed before eating; all perishable food is refrigerated correctly and consumed as soon as possible; and they do not eat soft cheeses, unpasteurised milk, raw eggs, or undercooked meat (Government of South Australia 2015Ross 2006). These food items may contain salmonella, toxoplasmosis, campylobacter, or listeria, which may cause infection and consequently adverse pregnancy outcomes.
Regarding mineral intake, it is known that a mother’s iodine level affects foetal neurological development. In many countries, iodine intake is inadequate. For women trying to conceive, this may lead to adverse effects on a pregnancy and on the foetus (Harding 2017). However, until now, no clear effects of iodine supplementation on maternal and foetal outcomes have been noted (Harding 2017). The general recommendation is that the general population, and pregnant women in particular, should enjoy a varied diet with adequate amounts of iodine (usually consumed through iodised salt and bread) (Government of South Australia 2015). Iron supplementation can prevent maternal anaemia and improve birth outcomes including birth weight (Haider 2017World Health Organization 2012). Therefore, daily oral iron supplementation with 30 to 60 mg of elemental iron is recommended for pregnant women (World Health Organization 2017).
Regarding vitamins, excessive vitamin A consumption through supplements or through crustacean or liver products during pregnancy can cause congenital defects, so it is recommended that women avoid these both before and during pregnancy (Government of South Australia 2015). Taking a daily folic acid (vitamin B9) supplement of 400 µg before conception and during the first three months of pregnancy has been shown to decrease the risk of neural tube defects (such as spina bifida) in the foetus (De‐Regil 2017). Evidence from observational studies suggests that higher intake of folic acid may increase a woman’s chances of becoming pregnant (Gaskins 2018a). Given this evidence, it is recommended that women be advised to take 400 µg of folic acid daily to help prevent foetal neural tube defects, starting as early as two months before they try to conceive (Government of South Australia 2015Haider 2017Practice Committee 2017World Health Organization 2017).
At this point, evidence‐based recommendations or high‐quality evidence on the intake of other vitamin or mineral supplements for improving fertility and chances of having a healthy live birth in men and women is lacking (Chiu 2018Gaskins 2018a). Antioxidants are biological and chemical compounds, including vitamins, minerals, and polyunsaturated fatty acids, that reduce oxidative damage. Low‐ to very low‐quality evidence suggests that antioxidant supplementation in women with infertility may increase the chance of pregnancy or live birth rate (Showell 2020). Low‐quality evidence suggests that antioxidant supplementation for men with infertility may improve live birth rates (Smits 2019). No recommendations for antioxidant supplementation are currently available for men and women aiming to conceive.
Alcohol
Consuming high levels of alcohol (> 2 drinks per day, with 1 drink > 10 g of ethanol) can affect both female and male fertility and the success of fertility treatment (Hakim 1998Klonoff‐Cohen 2003Rossi 2011). Additionally, alcohol has well‐documented detrimental effects on a foetus during pregnancy (Mukherjee 2005). Debate continues about the amount of alcohol that must be consumed before conception and pregnancy are affected, but, given the severity of the consequences associated with overindulgence of alcohol, it has been recommended that people avoid drinking alcohol before conception, and that women should avoid alcohol throughout their pregnancy (Government of South Australia 2015Homan 2007Practice Committee 2017World Health Organization 2017).
Caffeine
Evidence on the association between consumption of caffeine and male and female fertility is inconclusive (Lyngso 2017Ricci 2017). Few studies have investigated the effects of caffeine intake on people with infertility as a subpopulation, but no clear associations between caffeine intake and outcomes of fertility treatment have been found (Lyngso 2017). However, consumption of high levels of caffeine (> 300 mg caffeine/d) during pregnancy has been associated with increased risk of spontaneous abortion (Lyngso 2017). Thus, the recommendation has been made that people trying to conceive should limit their caffeine intake to the equivalent of less than two cups of coffee per day (Government of South Australia 2015Homan 2007Practice Committee 2017).
Physical activity
Physical activity has a positive impact on one’s physical, emotional, and general health, and contributes to prevention of non‐communicable disease (Homan 2007Penedo 2005World Health Organization 2020b). Besides these substantial health benefits, evidence suggests a positive association between moderate physical activity and male and female fertility (Homan 2007Ibanez‐Perez 2019McKinnon 2016). For women undergoing fertility treatment, moderate physical activity is associated with increased pregnancy and live birth rates (Rao 2018). Therefore, 150 minutes of moderate‐intensity physical activity such as walking, cycling, and doing sports throughout the week is recommended for couples trying to conceive before conception and during pregnancy (World Health Organization 2017World Health Organization 2020b).
Smoking
Analysis of the literature indicates that active and passive (second‐hand) smoking of tobacco is associated with decreased fertility and reduced chance of a healthy, live birth both in the general population and among couples with infertility (Augood 1998Hyland 2016Practice Committee 2018Radin 2014Sharma 2016Waylen 2008). Moreover, evidence shows that smoking reduces the success of fertility treatments such as IVF and ICSI (Klonoff‐Cohen 2005Mínguez‐Alarcón 2018Practice Committee 2018Waylen 2008). Thus, women and men should be counselled to stop smoking before they try to conceive (NICE 2013Practice Committee 2017World Health Organization 2013).
Other substance use
The use of non‐prescription and recreational drugs before conception has been associated with reduced fertility in both men and women (Frey 2008Fronczak 2012Mueller 1990). Due to the prevalence of poly‐substance use, limited evidence is available on the independent effects of these drugs on pregnancy outcomes. However, current data suggest that non‐prescription and recreational drug use during pregnancy is associated with increased risk of foetal death, low birth weight, and preterm birth (Gouin 2011Gunn 2016Ladhani 2011Metz 2017). Given these data, it is recommended that people should be counselled to stop using non‐prescription and recreational drugs before conception (Fronczak 2012NICE 2013Practice Committee 2017; World Health Organization 2014).
Why it is important to do this review
This is an update of a Cochrane Review that was first published in 2010. For the first version of this review, the search identified no RCTs that assessed effects of preconception advice on the chance of live birth or other fertility outcomes in people with a diagnosis of infertility, and the need for further research into this topic was highlighted (Anderson 2010). Over past years, fertility clinics have tended to see an increasing number of patients with an unhealthy lifestyle, and they have acknowledged more and more the importance of providing preconception lifestyle advice (Gormack 2015Homan 2018). The importance of providing preconception lifestyle advice is also reflected in the guidelines on routine psychosocial care in infertility and medically assisted reproduction of the European Society of Human Reproduction and Embryology (ESHRE) (ESHRE guideline 2015). However, clear and specific information is needed about what preconception advice related to these factors should be given to people presenting for fertility treatment, to help them make positive changes in the hope of improving their chances of conception and delivery of a healthy, live baby (Grainger 2006Moran 2016a). With increased attention to optimising preconception lifestyle advice for people with a diagnosis of infertility and continuous updates of the Methodological Expectations of Cochrane Intervention Reviews (MECIR), an update of this review is indispensable.
Objectives
To assess the safety and effectiveness of preconception lifestyle advice on fertility outcomes and lifestyle behavioural changes for people with infertility.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), randomised cross‐over studies, and cluster‐randomised studies. For cross‐over trials, we had planned to include only data from the first phase (pre‐cross‐over data), as the cross‐over is not a valid design in this context, but we identified no cross‐over trials. We excluded non‐randomised studies, as they are associated with high risk of bias.
Types of participants
Inclusion criteria
Trials that included men or women with infertility in the following phases of treatment were eligible for inclusion.
Pretreatment: from diagnosis of infertility until initiation of fertility treatment.
During treatment: from initiation of fertility treatment until the end of fertility treatment.
Exclusion criteria
Trials that included solely women with polycystic ovary syndrome (PCOS) were excluded.
Types of interventions
RCTs considering at least one form of preconception lifestyle advice were eligible for inclusion. The preconception lifestyle advice had to be the main component of the intervention but could be combined with other care aspects that are not specified in the list below. Preconception lifestyle advice was defined as a combination of counselling about weight, diet, vitamin or mineral supplement intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse.
Setting: preconception lifestyle advice could be provided individually, per couple, or in a group setting.
Mode of delivery: preconception lifestyle advice could be provided face‐to‐face (F2F), through mobile applications, through the Internet, through telephone contact, or through written information in leaflets, booklets, or decision aids, and on websites, or by a combination of delivery modes.
Duration: the duration of preconception lifestyle advice could vary widely, from one session to multiple sessions.
Eligible comparisons consisted of routine care (either no preconception lifestyle advice or unstructured minimal preconception lifestyle advice) or attention control provided to groups.
Types of outcome measures
Primary outcomes
Effectiveness outcomes
-
Live birth or ongoing pregnancy
Live birth defined as delivery of a live foetus after 20 completed weeks of gestation
Ongoing pregnancy defined as evidence of a gestational sac with foetal heart motion at 12 weeks, confirmed by ultrasound
When studies reported both live birth and ongoing pregnancy, data on live birth were utilised
Safety outcomes
Any adverse event in men or women with infertility related to the intervention reported either as a composite measure or separately (including gestational diabetes and hypertension)
Miscarriage, defined as spontaneous loss of an intrauterine pregnancy before 22 completed weeks of gestation
Secondary outcomes
Reported lifestyle behavioural changes (in women and/or men unless otherwise indicated), including maintaining a healthy weight (measured as BMI in kg/m² or % weight loss or number of people with BMI between 18.5 and 25 kg/m² (Mackenzie 2019); improving in efforts to follow a healthy diet (according to World Health Organization (WHO) standards; measured as vegetable intake in grams/d and fruit intake in grams/d or number of people reaching the WHO recommendation of 400 g of vegetables and fruit per day, preferably by a Food Frequency Questionnaire (FFQ) or other validated scales); taking vitamin or mineral supplements when necessary (women) (measured as the number of women taking a daily folic acid (vitamin B9) supplement of 400 µg); stopping/reducing alcohol intake (measured as alcoholic drinks/d, with 1 drink > 10 g of ethanol) or number of people abstaining from alcohol, preferably by an FFQ or other validated scales); reducing caffeine intake (measured as caffeine intake in mg/d, preferably by an FFQ or other validated scales); increasing physical activity (according to WHO standards; measured as minutes of moderate to vigorous physical activity (MVPA) per week or number of people reaching the WHO recommendation of doing 150 minutes of moderate‐intensity physical activity per week, preferably by the Global Physical Activity Questionnaire or other validated scales); and stopping smoking and other substance abuse (measured as number of people not smoking or number of people not abusing other substances)
Birth weight, including small‐for‐gestational‐age and large‐for‐gestational‐age outcomes (measured in grams)
Gestational age, including preterm birth outcome (measured in weeks)
Clinical pregnancy, defined as evidence of a gestational sac, confirmed by ultrasound
Time to pregnancy leading to live birth (measured in months)
Quality of life of women and/or men (measured preferably by the Fertility Quality of Life tool (FERTIQOL) or other validated scales)
Male factor infertility outcomes including sperm motility and sperm concentration (measured according to the WHO laboratory manual for examination and processing of human semen; World Health Organization 2010)
If studies reported outcomes at different time points, the time point at the end of intervention was selected.
Search methods for identification of studies
We searched for all published and unpublished studies addressing preconception advice to influence lifestyle factors in people with the diagnosis of infertility. We applied no language restrictions, and we searched in consultation with the Cochrane Gynaecology and Fertility Group (CGFG) Information Specialist.
Electronic searches
We searched the following electronic databases for relevant trials:
Cochrane Gynaecology and Fertility Group (CGFG) Specialised Register of controlled trials; PROCITE platform (searched 12 January 2021) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, via the Cochrane Register of Studies Online; CRSO Web platform (searched 12 January 2021) (Appendix 2);
MEDLINE; OVID platform (searched from 1946 to 12 January 2021) (Appendix 3);
Embase; OVID platform (searched from 1980 to 12 January 2021) (Appendix 4);
PsycINFO; OVID platform (searched from 1806 to 12 January 2021) (Appendix 5);
Allied and Complementary Medicine Database (AMED); OVID platform (searched from 1985 to 12 January 2021) (Appendix 6);
Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCO platform) (searched from 1961 to 20 February 2020). CINAHL content from 20 February 2020 to 12 January 2021 was accessed through the CENTRAL CRSO search (Appendix 7).
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0, Chapter 6, 6.4.11) (Higgins 2020). The Embase search was combined with the trial filter developed by the Scottish Intercollegiate Guidelines Network (SIGN) (https://www.sign.ac.uk/what‐we‐do/methodology/search‐filters/).
Other electronic searches included the following:
Trial registers for ongoing and registered trials: the World Health Organization International Clinical Trials Registry Platform (ICTRP) and clincialtrials.gov (currently included in CENTRAL output);
Google Scholar for recent trials not yet indexed in the major databases;
Epistemonikos database for systematic reviews.
The search output was managed in Covidence (Covidence).
Searching other resources
We handsearched reference lists of relevant trials and systematic reviews retrieved by the search and contacted experts in the field to obtain additional trials. We also handsearched relevant journals and conference abstracts that were not covered in the CGFG Register, in liaison with the Information Specialist.
Data collection and analysis
Selection of studies
Three review authors (TB, ACV, MV) independently performed an initial screen of titles and abstracts retrieved by the search for potentially relevant studies. Each study was screened in duplicate. We retrieved the full texts of all potentially eligible studies, and three review authors independently examined these full‐text articles for compliance with the inclusion criteria and to select eligible studies. We corresponded with study investigators as required, to clarify study eligibility. Disagreements were resolved by discussion, and, if necessary, the independent judgement of a senior review author (SLF) was sought. If any reports required translation, we described the process used for data collection. We documented the selection process with a 'PRISMA' flow chart (Figure 1).
1.
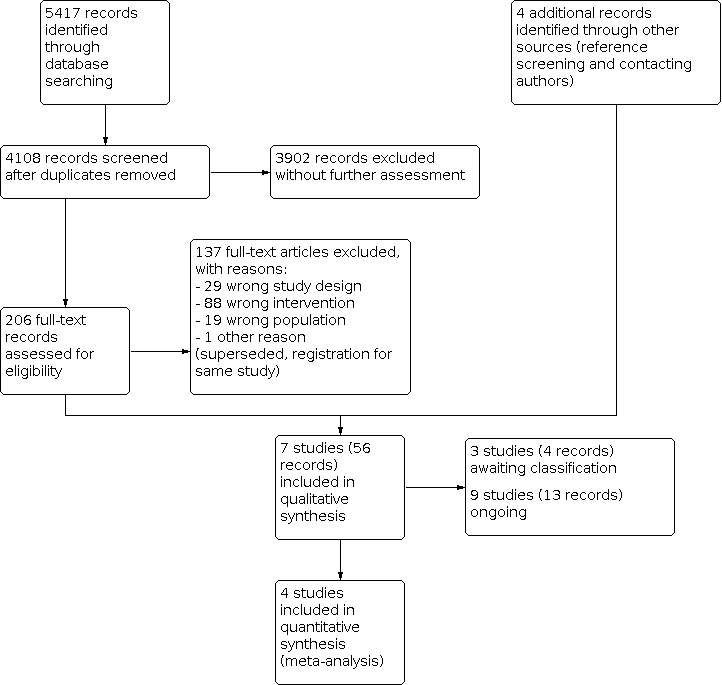
Study flow diagram.
Data extraction and management
Three review authors (TB, ACV, MV) independently extracted data from eligible studies using a data extraction form, which was designed by the authors in Covidence. Any disagreement was resolved by discussion and, if necessary, by consultation with a senior review author (SLF). Data extracted included study characteristics and outcome data (see data extraction table for details; Appendix 8). When studies had multiple publications, the review authors collated multiple reports of the same study under a single study ID with multiple references. We corresponded with study investigators to request further data on methods and/or results, as required.
Assessment of risk of bias in included studies
Three review authors (TB, ACV, MV) independently reviewed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool to assess selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias. Judgements were assigned as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, Section 8.5 (Higgins 2020). We resolved disagreements by discussion. We described all judgements fully and presented conclusions in the 'Risk of bias' table, which is incorporated into the interpretation of review findings by means of sensitivity analyses (see below). With respect to within‐trial selective reporting, when identified studies failed to report the primary outcome of live birth but did report interim outcomes such as pregnancy, we assessed whether the interim values were similar to those reported in studies that also reported live birth.
Measures of treatment effect
For dichotomous data (e.g. live birth rates), we used numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel risk ratios (RRs). For continuous data (e.g. birth weight), if all studies reported exactly the same outcomes, we calculated mean difference (MDs) between treatment groups. If similar outcomes were reported on different scales (e.g. change in quality of life), we planned to calculate the standardised mean difference (SMD), but no such data were identified. We treated ordinal data (e.g. quality of life scores) as continuous data. We planned to reverse the direction of effect of individual studies, if required, to ensure consistency across trials, but we identified no such studies. We presented 95% confidence intervals (CIs) for all outcomes. When data to calculate RRs or MDs were not available, we utilised the most detailed numerical data available that facilitated similar analyses of included studies (e.g. test statistics, P values). We assessed whether estimates calculated in the review for individual studies were compatible in each case with estimates reported in the study publications.
Unit of analysis issues
The primary analysis was per individual randomised; per pregnancy data were also included for some outcomes (e.g. miscarriage). If we encountered data that did not allow valid analysis (e.g. "per cycle" data), we planned to briefly summarise these in an additional table and to not perform meta‐analysis, but we identified no such data. Multiple births were counted as one live birth event. We planned to include only first‐phase data from cross‐over trials, but we identified no cross‐over trials. We planned to include data from cluster‐randomised trials in the meta‐analyses only if the reported outcome measure properly accounted for the cluster design, or if the necessary information was available to account for the clustering. If the intracluster correlation coefficient (ICC) was not reported, we planned to include the trial only if an ICC could be obtained from similar studies (Higgins 2020Rao 1992). However, we found no data from cluster‐randomised trials, and this analysis was not performed.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible (i.e. including all randomised participants in analysis, in the groups to which they were randomised). We attempted to obtain missing data from the original studies. When these were not obtainable, we undertook imputation of individual values for live birth or ongoing pregnancy only: live birth or ongoing pregnancy was assumed not to have occurred in participants without a reported outcome. For other outcomes, we analysed only available data. Any imputation undertaken was subjected to sensitivity analysis (see below). If studies reported sufficient detail to calculate mean differences but no information on associated standard deviation (SD), we assumed the outcome to have a standard deviation equal to the highest SD from other studies within the same analysis, but we identified no such studies.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed heterogeneity by inspecting the forest plot and by using the Chi²‐test and the I² statistic. An I² measurement greater than 50% was taken to indicate substantial heterogeneity (Higgins 2020). If heterogeneity was substantial, we examined the direction of effects before making a decision whether to report the pooled result or to describe the effects narratively.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If ten or more studies were included in an analysis, we planned to use a funnel plot to explore the possibility of small‐study effects (i.e. a tendency for estimates of the intervention effect to be more beneficial in smaller studies), but for each comparison, we identified only one study or a few studies.
Data synthesis
If studies were sufficiently similar, we attempted to combine the data using a random‐effects model for the following comparisons.
Preconception advice versus no preconception advice for a combination of any of the following topics: weight, diet, vitamin or mineral supplement intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse.
Preconception advice versus no preconception advice for one of the aforementioned topics. Each of the topics was analysed as a separate comparison.
A narrative review summary format was chosen as the method for synthesis when it was not possible to conduct meta‐analyses. In this case, MDs were presented for continuous outcomes and RRs for dichotomous outcomes without pooling.
For the secondary outcomes on reported behavioural changes, decision rules based on guidance documents and clinical considerations were followed in selecting outcomes for inclusion in our synthesis, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). In general, if continuous and dichotomous outcomes were reported (e.g. vegetable intake in grams/d versus number of participants with adequate vegetable intake > 200 grams/d), preference was given to continuous outcomes, and study authors were contacted to provide us with these data. If composite scores and individual component scores on behavioural changes were reported, preference was given to inclusion of individual component scores. For example, one study reported on a combination of reported behavioural changes in diet, more specifically, a dietary risk score (DRS) from 0 to 9 (the lower, the better) comprising intake of vegetables, fruits, and folic acid supplement; and on a lifestyle risk score (LRS) from 0 to 9 (the lower, the better) comprising alcohol intake and smoking (Oostingh 2020). Study authors also reported on these individual components, and preference was given to inclusion of these outcomes in our synthesis. We further specified that if outcomes were measured at multiple time points within a time frame, we would select the time point at the end of the intervention.
Subgroup analysis and investigation of heterogeneity
If we detected substantial heterogeneity, and if data were sufficient, we planned to use subgroup analyses to consider differences between studies that might account for heterogeneity (e.g. differences in study populations, therapy settings, timing, design, delivery of the intervention). Due to the limited number of studies identified for each comparison, we were not able to perform subgroup analyses. We took any statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect.
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
eligibility had been restricted to studies at low risk of bias, defined as studies at low risk for sequence generation and allocation concealment, and not at high risk of bias in any domain;
a fixed‐effect model had been adopted;
alternative imputation strategies had been implemented for missing data;
the summary effect measure had been odds ratio rather than risk ratio; or
the primary outcome had been live birth rather than live birth or ongoing pregnancy.
Due to the limited number of studies identified for each comparison, we performed sensitivity analyses only on model and summary effect measures.
Summary of findings and assessment of the certainty of the evidence
We prepared a 'Summary of findings' table using GRADEpro software and Cochrane methods (Higgins 2020). This table shows the overall quality of the body of evidence for the main review outcomes (live birth or ongoing pregnancy, any adverse event, miscarriage, and reported behavioural changes) for the main review comparison (combination of weight, diet, vitamin or mineral supplement intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse preconception advice versus no preconception advice).
We prepared additional 'Summary of findings' tables for the main review outcomes for each of the other comparisons in our data synthesis.
We assessed the quality of evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness, and publication bias. Judgements about evidence quality (high, moderate, low, or very low) were made by two review authors (TB, ACV) working independently, with disagreements resolved by discussion. Judgements were justified, documented, and incorporated into reporting of results for each outcome. We extracted study data, formatted our comparisons in data tables, and prepared a 'Summary of findings' table before writing the results and conclusions of our review.
Results
Description of studies
Results of the search
Due to substantial changes to the criteria for considering studies for this systematic review, a new search was designed for the 2021 update of this systematic review. The search retrieved 4108 records after duplicates were removed. After initial screening, 206 records were found potentially eligible and were retrieved in full text for more detailed evaluation. Reference screening and contact with study authors resulted in four additional records (one additional abstract ‐ Belan 2019; two clinical trial registrations ‐ Ng 2018Oostingh 2020; and an ongoing study ‐ Timmermans 2019). We excluded 137 records for reasons stated in the Characteristics of excluded studies table. Seven studies (56 records), including the study identified in the first version of this systematic review (Hughes 2000), met our inclusion criteria (see Characteristics of included studies table). We categorised three studies (four records) as awaiting classification due to our inability to determine their eligibility or to lack of information (see Characteristics of studies awaiting classification table). Nine studies (13 records) are still ongoing (see Characteristics of ongoing studies table). See Figure 1 for a PRISMA study flow diagram of the search and selection process.
Included studies
Study design and setting
We included seven parallel‐design RCTs with a total of 2130 participants, including Hughes 2000, which was the only included study in the 2010 version of this systematic review (Anderson 2010). Study characteristics are presented in the Characteristics of included studies table. Of the seven included studies, two were published solely as an abstract (Belan 2019Ng 2018). We contacted all corresponding authors of included studies for additional information, five of whom provided (a part of) the requested information (Belan 2019Hughes 2000Mutsaerts 2016Ng 2018Oostingh 2020). Authors of the two studies with a published abstract were unable to report on their full data set at this point (Belan 2019Ng 2018).
The studies were conducted at university medical centres or at general hospitals in Iran (Alibeigi 2020), Canada (Belan 2019Hughes 2000), the UK (Ng 2018), The Netherlands (Mutsaerts 2016;,Oostingh 2020), and the USA (Rossi 2013). Three were single‐centre studies (Alibeigi 2020Belan 2019Rossi 2013), and four were multi‐centre studies (Hughes 2000Mutsaerts 2016Ng 2018Oostingh 2020).
Participants
Studies were heterogeneous with respect to participants.
The studies included 1908 women and 222 men with a diagnosis of infertility. In one study, women were included with their male partner (i.e. as a couple) (Oostingh 2020); no studies focused on men only. The other six studies focused solely on women (Alibeigi 2020Belan 2019Hughes 2000Mutsaerts 2016Ng 2018Rossi 2013). The number of randomised participants per study ranged from 37 in Rossi 2013 to 848 in Oostingh 2020.
We included studies with participants before or during fertility treatment. One study included participants before fertility treatment (Hughes 2000), another included participants during fertility treatment (Alibeigi 2020), and three studies included participants before and during fertility treatment (Belan 2019Mutsaerts 2016Oostingh 2020). In Belan 2019 and Mutsaerts 2016, the control group received immediate fertility treatment (during) and the intervention group received fertility treatment after six months (before). In two studies, this feature was not specified (Ng 2018Rossi 2013). Fertility treatment consisted of IVF in Alibeigi 2020, IVF or ICSI in Oostingh 2020 and all types of fertility treatment according to local protocols in Belan 2019 and Mutsaerts 2016.
The age range of included participants varied across studies from 18 to 45 years (Alibeigi 2020Belan 2019Mutsaerts 2016Ng 2018Oostingh 2020). Two studies did not specify the age of included subjects (Hughes 2000Rossi 2013).
Inclusion and exclusion criteria varied considerably between studies. Three studies included all women with infertility, regardless of the cause of infertility, without specifying a type of infertility factor (Hughes 2000Ng 2018Rossi 2013). One study included only participants with indication for IVF or ICSI (Oostingh 2020). Other inclusion criteria related to infertility were women with ovulatory problems (Alibeigi 2020Belan 2019Mutsaerts 2016), endometriosis or idiopathic infertility (Alibeigi 2020), ovulatory cycle and unsuccessful attempts to conceive for 12 months (Belan 2019Mutsaerts 2016), or six months when older than 35 years (Belan 2019). Ng 2018 also included women suffering from recurrent miscarriages. Exclusion criteria related to infertility were anatomical causes, uterine myoma, or hydrosalpinx (Alibeigi 2020); inability or unlikeliness for natural conception (Belan 2019); and severe endometriosis or premature ovarian failure (Mutsaerts 2016). Three studies excluded severe male factor infertility (Alibeigi 2020Belan 2019Mutsaerts 2016). Other reported exclusion criteria were refusal to go through IVF (Alibeigi 2020), and previously evaluated for infertility, genetic counselling, and occurrence of recurrent miscarriages (Hughes 2000). Four studies included only women with infertility with specific lifestyle characteristics such as being overweight or obese (Belan 2019Mutsaerts 2016); smoking three or more cigarettes per day over the last six months (Hughes 2000); or engaging in at‐risk alcohol drinking (Rossi 2013). Six studies excluded participants with lifestyle‐related diseases, including physical dependence on alcohol or other substances (Rossi 2013), diabetes mellitus (Alibeigi 2020Mutsaerts 2016Ng 2018), planning or undergoing bariatric surgery (Belan 2019); on a diet for medical reasons (Ng 2018Oostingh 2020); and hypertension or other endocrinopathies (Mutsaerts 2016). Additional exclusion criteria related to lifestyle were participants smoking or drinking alcohol (Alibeigi 2020), being in treatment for alcohol and drug abuse (Rossi 2013), undergoing lifestyle interventions (Ng 2018), and following a specific diet (Oostingh 2020).
At baseline, participants in the intervention and control groups were comparable, with the exception of Alibeigi 2020: women in the intervention group had a greater number of previous IVF attempts than women in the control group (P = 0.029); and women in the intervention group were more highly educated than control group women (P = 0.004). Median duration of time trying to conceive was longer in the intervention group than in the control group in Mutsaerts 2016 (P = 0.037); in Rossi 2013 the intervention group had on average 2.1 drinks/drinking per day and the control group 1.8.
Further details on participants are available under Characteristics of included studies.
Interventions
Studies were heterogeneous with respect to interventions and comparisons.
Three studies assessed preconception lifestyle advice on a combination of topics (Alibeigi 2020Ng 2018Oostingh 2020); the remaining four studies assessed preconception lifestyle advice on one topic: weight (Belan 2019Mutsaerts 2016), alcohol intake (Rossi 2013), or smoking (Hughes 2000).
Two studies provided preconception lifestyle advice on a combination of weight, diet, vitamin or mineral supplement intake (folic acid), alcohol intake, physical activity, and smoking (Ng 2018Oostingh 2020). Both studies describe a personalised smart phone lifestyle coaching programme called 'Smarter Pregnancy', with the aim of achieving a healthy preconception lifestyle. Tailored lifestyle advice was generated in a mobile app based on online lifestyle questionnaires. Advice and coaching consisted of text and email messages including tips, recommendations, vouchers, seasonal recipes, and additional questions addressing behaviour, pregnancy status, BMI, and adequacy of the diet. Results and feedback on the questionnaires were shown on a personal online page to reveal participants' progress. The other study consisted of preconception lifestyle advice on diet (advice to follow traditional medicine‐oriented diet) and general healthy lifestyle recommendations according to Iranian Traditional Medicine (ITM) sources, provided through a face‐to‐face (F2F) consult, in combination with a training guidance text and telegram group discussion (Alibeigi 2020). A 24‐hour diet recall questionnaire was used to follow up on the rate and quality of the diet.
Two studies provided preconception lifestyle advice on weight loss for women with infertility and obesity (Belan 2019Mutsaerts 2016). Both studies aimed at modest weight loss by addressing diet and physical activity behaviours. One study combined individual counselling with a dietician and a physiotherapist with group sessions and group physical activity workouts to implement progressive and sustainable lifestyle changes following local guides (Belan 2019). The other study combined individual counselling with a coach on diet and physical activity in combination with motivational counselling to promote awareness of a healthy lifestyle and to formulate individualised goals (Mutsaerts 2016). Both studies followed the principles of motivational interviewing and used a food diary and pedometer to follow‐up diet and physical activity behaviours during the intervention.
One study provided preconception lifestyle advice on alcohol intake (Rossi 2013). Based on interviews on current alcohol intake, at‐risk drinking women with infertility received feedback on alcohol consumption and information on health consequences of drinking alcohol, goal setting, and behavioural modification to reduce alcohol consumption in an F2F consult. Interviews were used to follow up on alcohol use.
One study provided preconception lifestyle advice on smoking cessation (Hughes 2000). Women with infertility smoking three or more cigarettes per day over the last six months received a scripted motivational intervention with advice to quit smoking, "stage‐of‐change" handouts, an offer for counselling at the Smoking Cessation Clinic, and exhaled carbon monoxide measurements to follow up on smoking behaviour.
Interventions varied not only on topics or content of preconception lifestyle advice but also on setting, mode of delivery, and duration.
In four studies, preconception lifestyle advice was provided solely in an individual setting (Hughes 2000Mutsaerts 2016Ng 2018Rossi 2013). In two studies, the partner was also invited to participate in (part of) the intervention (Belan 2019Oostingh 2020). Two studies also used a group setting (Alibeigi 2020Belan 2019). One study used a telegram discussion group (Alibeigi 2020), and one study organised group sessions including group workshops covering various topics related to obesity management, infertility, and physical activity workouts, with partners also invited to these group sessions (Belan 2019).
Regarding mode of delivery, two studies provided preconception lifestyle advice through mobile health (mobile app and email) (Ng 2018Oostingh 2020). In the other studies, preconception lifestyle advice was given by a healthcare provider or coach by F2F contact, combined with written materials (Alibeigi 2020Hughes 2000Rossi 2013), by telephone contact (Alibeigi 2020Belan 2019Mutsaerts 2016), or with tools including a food record and a pedometer (Belan 2019Mutsaerts 2016).
The duration of the intervention and of follow‐up ranged from six months in Ng 2018 and Oostingh 2020 to 24 months in Mutsaerts 2016. In one study, the duration of the intervention was not specified (Alibeigi 2020). All interventions included multiple contacts with participants ranging from three times/week in Ng 2018 and Oostingh 2020 to once every three to six months in Rossi 2013. For one study, the frequency or the number of contacts was not specified (Hughes 2000).
All studies defined preconception lifestyle advice as the intervention, whereas the control group received routine care or attention control. In two studies, routine care consisted of immediate start of fertility treatment and information according to local protocols (Belan 2019Mutsaerts 2016). In both studies, the intervention group first received lifestyle intervention so that fertility treatment was postponed within six months. In the other studies, there was no difference in access to fertility treatment between intervention and control groups. In four studies, participants in the control group received routine care consisting of unstructured minimal preconception lifestyle advice or assessment, including advice on modern dietary recommendations (Alibeigi 2020), standard care information about the impact of smoking on fertility and exhaled carbon monoxide measurements (Hughes 2000), standard preconception advice offered online by national health services (Ng 2018), or assessment of current alcohol consumption and general health in an assessment interview (Rossi 2013). One study compared the intervention with an attention control to adjust for the benefit of attention. For the latter group, lifestyle assessment was conducted with a questionnaire at baseline and at three and six months without feedback, with access to a personal page and one seasonal recipe per week (Oostingh 2020).
All studies described their interventions in their publications; only Mutsaerts 2016 provided a full reproducible protocol on how the intervention was built.
Outcomes
Studies were heterogeneous with respect to outcomes measured.
With respect to primary effectiveness outcomes, three studies reported on live birth in the study publication (Belan 2019Mutsaerts 2016Rossi 2013), and one study reported data on live birth on request (Oostingh 2020). One study reported the number of live births (spontaneous and after IVF) 24 months after randomisation (Mutsaerts 2016). One study reported the percentage of live births 18 months after randomisation (Belan 2019), and from two studies, it was not clear when the numbers of live births were measured (Oostingh 2020Rossi 2013).
One study reported additionally on the numbers of ongoing pregnancies (spontaneous and after IVF) (Mutsaerts 2016). Because both live birth and ongoing pregnancy were reported in this study, data on live birth were used for analysis.
For the primary safety outcomes, two studies reported on adverse events including gestational diabetes and hypertension (Belan 2019Mutsaerts 2016), and two studies reported on miscarriage. One study defined miscarriage as the number of losses of clinical pregnancy at gestational age < 16 weeks (Mutsaerts 2016). The other study defined miscarriage as clinical pregnancy without live birth (Rossi 2013).
For secondary outcome parameters on lifestyle behavioural changes, three studies reported on maintaining a healthy weight (Alibeigi 2020Belan 2019Mutsaerts 2016). Two studies reported on change in BMI (Alibeigi 2020Mutsaerts 2016), and two studies reported on weight. One study reported on weight change (in percentage) at six months (Belan 2019), and the other reported on absolute weight in kilograms at baseline and at three and six months (Mutsaerts 2016).
Four studies reported on following a healthy diet (Belan 2019Mutsaerts 2016Ng 2018Oostingh 2020). One study stated in the methods that investigators measured rate and quality of the diet but reported no data (Alibeigi 2020). All four studies used self‐report questionnaires including a lifestyle questionnaire adapted from the Canadian Health Survey (Belan 2019), a Food Frequency Questionnaire (FFQ) (Mutsaerts 2016), and a self‐validated online lifestyle questionnaire (Ng 2018Oostingh 2020). A variety of outcome measures were reported in these studies. One study reported an overall dietary score more specifically, with change in healthy eating score adapted from the USA's healthy eating index (Belan 2019). Three studies reported on change in vegetable intake (Mutsaerts 2016Ng 2018Oostingh 2020). Two studies reported on vegetable intake in grams/d (Mutsaerts 2016Ng 2018), and one study reported on the number of participants having adequate vegetable intake greater than 200 grams/d (Oostingh 2020). Three studies reported on change in fruit intake based on different scales (grams/d (Mutsaerts 2016); pieces/d (Ng 2018); and number of participants with adequate fruit intake of 2 pieces/d (Oostingh 2020)). One study reported on change in intake of other foods including sugary drinks (glasses/d), savoury snacks (handful/week), and sweet snacks (portion/week) (Mutsaerts 2016). Additionally, one study reported on a combination of reported behavioural changes in diet, more specifically, a dietary risk score (DRS) from 0 to 9 (the lower, the better) comprising intake of vegetables, fruits, and folic acid supplement (Oostingh 2020).
Two studies reported on change in vitamin or mineral supplement intake, more specifically, the number of women with adequate folic acid supplement use (Ng 2018Oostingh 2020).
Three studies reported on stopping/reducing alcohol intake via self‐report questionnaires such as FFQ (Mutsaerts 2016), a self‐validated online lifestyle questionnaire (Oostingh 2020), and the Alcohol Timeline Followback questionnaire (Rossi 2013). A variety of outcome measures were reported in these studies including alcoholic beverages in glasses per day (Mutsaerts 2016), the number of participants not drinking alcohol (Oostingh 2020), decrease in the number of drinks on a drinking day, decrease in percentage of drinking days in the past six months, decrease in the number of weeks of drinking above the daily safety limit in the past six months, and decrease in the number of binges in the past six months (Rossi 2013).
No studies reported on reduction of caffeine intake.
Two studies reported on increase in physical activity, one of which used a triaxial accelerometer to measure changes in total leisure activity energy expenditure in kcal/kg/d (Belan 2019); the other study used the Short Questionnaire to Assess Health‐Enhancing Physical Activity (SQUASH) to measure total minutes per week in moderate to vigorous physical activity (MVPA) including leisure‐time physical activity and commuting activities (Mutsaerts 2016).
Two studies reported on stopping smoking and other substance abuse. One study reported the rate of maintained smoking cessation at 12 months and the delta stage of change towards smoking cessation (difference in stage of motivation to change smoking behaviour) (Hughes 2000). The other study reported on the number of participants not smoking before and after the intervention (Oostingh 2020).
Additionally, one study reported on a combination of lifestyle behavioural changes, more specifically, a lifestyle risk score (LRS) from 0 to 9 (the lower, the better) comprising alcohol intake and smoking (Oostingh 2020).
With respect to the other secondary outcomes, two studies reported on birth weight and gestational age (Belan 2019Mutsaerts 2016). Four studies reported on clinical pregnancy with a variety of definitions, including pregnancy confirmed by ultrasound (Alibeigi 2020), evidence of a foetal heartbeat on ultrasound or pregnancy exceeding 12 weeks (Belan 2019), and evidence of a gestational sac confirmed by ultrasound (Mutsaerts 2016). One study reported clinical pregnancy rates without further definition (Oostingh 2020). Time points for measuring clinical pregnancy varied from 12 months in Oostingh 2020 to 24 months in Mutsaerts 2016 after randomisation. One study did not report the time point of measurement (Alibeigi 2020).
One study reported on time to pregnancy leading to live birth (Mutsaerts 2016).
One study reported on quality of life using the 36‐Item Short Form Health Survey (SF‐36) (Mutsaerts 2016).
No studies reported on male factor infertility outcomes.
Excluded studies
We excluded 137 records from the review after consideration of full text for the following reasons (see also Characteristics of excluded studies table).
29 records on the basis of study design (not RCTs).
88 records based on the intervention (no preconception lifestyle advice), for example, strict weight loss interventions (with supplements/products), strict diet interventions (with supplements), or supervised physical activity interventions) or comparison (no routine care or attention control).
19 records based on study populations (no participants with infertility).
1 record for other reasons (ISRCTN17222161 2017) (this study was superseded by another study (another registration for the same study) ‐ NCT03553927 2018).
Risk of bias in included studies
See Figure 2 and Figure 3 for an overview on risk of bias and the 'Risk of bias' table under Characteristics of included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Six studies were rated at low risk of selection bias related to sequence generation, as they used computer‐generated randomisation or a random numbers table (Belan 2019Hughes 2000Mutsaerts 2016Ng 2018Oostingh 2020Rossi 2013). One study was rated at unclear risk of selection bias of sequence generation (Alibeigi 2020). Study authors describe the use of block randomisation but fail to specify the process of selecting the blocks. We contacted the study authors to clarify the randomisation procedure, but we did not receive a reply.
Allocation concealment
Five studies were rated at low risk of selection bias related to allocation concealment, as they used sequentially numbered, sealed, opaque envelopes or central randomisation by a third party (Belan 2019Hughes 2000Mutsaerts 2016Ng 2018Oostingh 2020). Two studies did not describe the methods of allocation concealment, and no reply was received when we contacted study authors for clarification. These two studies were rated at unclear risk of this bias (Alibeigi 2020Rossi 2013).
Blinding
Blinding of participants and personnel (performance bias)
In all studies, participants were aware of the assigned interventions, as it is difficult to blind participants to behavioural interventions such as preconception lifestyle advice. In two studies, it is not clear if personnel were also blinded during the intervention (Ng 2018Oostingh 2020). In the other five studies, personnel were aware of the assigned interventions (Alibeigi 2020Belan 2019Hughes 2000Mutsaerts 2016Rossi 2013). Deviations from the intended intervention could have arisen in all studies, for example, differential behaviours across groups such as the control group seeking preconception advice outside the study setting, or differential administration of co‐interventions by personnel. We therefore rated all studies at high risk of performance bias.
Blinding of outcome assessors (detection bias)
We considered that not blinding outcome assessors was unlikely to influence the primary review outcomes (live birth, ongoing pregnancy, adverse events, and miscarriage) and certain secondary outcomes, including birth weight, gestational age, clinical pregnancy, and time to pregnancy. These are observer‐reported outcome measures not involving judgement. Five studies were rated at low risk of detection bias for these outcomes (Alibeigi 2020Belan 2019Mutsaerts 2016Oostingh 2020Rossi 2013). Not blinding outcome assessors might influence male factor infertility outcomes, as these are observer‐reported outcomes involving judgement, but no studies reported on this outcome measure. Not blinding outcome assessors might influence patient‐reported outcome measures such as effects of reported behavioural changes on diet, vitamin or mineral supplement intake, alcohol intake, caffeine intake, physical activity, smoking, and quality of life, as participants could report more socially desirable answers for these outcomes. Six studies reported on patient‐reported outcome measures for which the participant was the outcome assessor and was not blinded. Therefore, these studies had high risk of detection bias for these outcomes (Belan 2019Hughes 2000Mutsaerts 2016Ng 2018Oostingh 2020Rossi 2013).
Incomplete outcome data
Two studies were reported as conference abstracts with limited available data, in which missing outcome data were not (yet) described (Belan 2019Ng 2018). We therefore rated these studies at unclear risk of attrition bias at this point. Two studies failed to report the number of participants lost to follow‐up (Hughes 2000Rossi 2013). However, Rossi 2013 provided data on 37 participants, whereas 161 patients with infertility were initially included. This study was rated at high risk of attrition bias. We contacted the study authors for clarification but did not receive a reply. Three studies reported discontinuation of the intervention and/or losses to follow‐up but used intention‐to‐treat (ITT) analysis (Alibeigi 2020Mutsaerts 2016Oostingh 2020). In Alibeigi 2020, missing data and reasons for discontinuation were documented and were balanced between groups (five in the intervention group, six in the control group), and all randomised women were included in the ITT analysis. This study was rated at low risk of attrition bias. In Mutsaerts 2016 and Oostingh 2020, discontinuation was not balanced between groups. In Mutsaerts 2016, discontinuation was 21.8% in the intervention group. Study authors did not impute on ITT for those who withdrew consent (one in the intervention group, two in the control group) and for those lost to follow‐up (nine in the intervention group, one in the control group). In our analysis, we assumed that these dropouts did not have a live birth. Because the reasons for discontinuation of the intervention were well documented and were consistent with the statistical plan of 20% discontinuation of the intervention and 5% loss to follow‐up, we rated this study at low risk of attrition bias (Mutsaerts 2016). In Oostingh 2020, discontinuation in the intervention group was 30.2% versus 18.2% in the control group. Study authors did not impute on ITT for those lost to follow‐up (13 in the intervention group, 14 in the control group), but we assumed in our analysis that these participants did not have a clinical pregnancy. Reasons for discontinuation of the intervention or control were not reported. Therefore, we rated risk of attrition bias as unclear (Oostingh 2020).
Selective reporting
Protocols were available for six studies (Alibeigi 2020Belan 2019Mutsaerts 2016Ng 2018Oostingh 2020Rossi 2013); four were prospectively registered (Alibeigi 2020Belan 2019Mutsaerts 2016Oostingh 2020). Live birth or ongoing pregnancy was reported in three studies (Belan 2019Mutsaerts 2016Rossi 2013), and one study provided data on live birth on request (Oostingh 2020). We considered studies to be at low risk of reporting bias if a protocol was prospectively registered and live birth or ongoing pregnancy was included as an outcome measure. We therefore rated Mutsaerts 2016 at low risk of reporting bias. Six studies were rated at high risk of reporting bias (Alibeigi 2020Belan 2019Hughes 2000Ng 2018Oostingh 2020Rossi 2013). Alibeigi 2020 had a prospectively registered protocol but did not report on live birth or ongoing pregnancy. In addition, data on dietary behaviour were measured but were not reported. The study details of Hughes 2000 could not be verified, as no study protocol was available and the study was not prospectively registered. Additionally, these investigators did not report on live birth or ongoing pregnancy, and some data on smoking (carbon monoxide (CO) measurements) were measured but were not reported. Two studies were reported in conference abstracts and did not report all pre‐specified outcomes at this point. (Belan 2019Ng 2018). Oostingh 2020 had an unreported pre‐specified outcome (Big 3 complications including small‐for‐gestational‐age, premature birth, and congenital malformations) and reported an outcome (lifestyle risk score) not specified in the protocol. Finally, Rossi 2013 was not prospectively registered. Although these study authors did report on live birth, they did not report on quality of life (a pre‐specified outcome), and baseline data on alcohol intake were missing (Rossi 2013).
Other potential sources of bias
One study was rated at high risk of other bias, as it was an interim analysis (Ng 2018). Another study was rated at unclear risk of other bias, as it was not clear which lifestyle questionnaire was used, and if this questionnaire had been validated (Oostingh 2020). We found no potential sources of within‐study bias in the other studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
See Table 1 for the main comparison preconception lifestyle advice on a combination of any of the following topics: weight, diet, vitamin or mineral supplement intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse compared with routine care or attention control for people with infertility.
Comparison 1. Preconception lifestyle advice on a combination of any of the following topics: weight, diet, vitamin or mineral supplement intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse versus routine care or attention control
See Table 1.
Primary effectiveness outcomes
Live birth or ongoing pregnancy
One study reported on the number of live births and compared preconception lifestyle advice on a combination of topics with attention control (Oostingh 2020).
Preconception lifestyle advice on a combination of topics may result in little to no difference in the number of live births (risk ratio (RR) 0.93, 95% confidence interval (CI) 0.79 to 1.10; 1 RCT, 626 participants; low‐quality evidence). This suggests that if the proportion of live births is assumed to be 48% in the control group, then the proportion of live births when preconception lifestyle advice is received would be between 38% and 53% (Analysis 1.1).
1.1. Analysis.

Comparison 1: Preconception lifestyle advice on a combination of any of the following topics: weight, diet, vitamin and mineral intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse vs routine care or attention control, Outcome 1: Live birth
Primary safety outcomes
Any adverse event
No studies reported on adverse events.
Miscarriage
No studies reported on miscarriage.
Secondary outcomes
Reported lifestyle behavioural changes
No studies reported on lifestyle behavioural changes in caffeine intake and physical activity.
Reported behavioural changes in weight
One study reported on BMI and compared preconception lifestyle advice on a combination of topics with routine care (Alibeigi 2020).
We are uncertain whether preconception lifestyle advice on a combination of topics affects BMI in women compared to routine care (mean difference (MD) ‐1.06 kg/m², 95% CI ‐2.33 to 0.21; 1 RCT, 180 participants; very low‐quality evidence) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Preconception lifestyle advice on a combination of any of the following topics: weight, diet, vitamin and mineral intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse vs routine care or attention control, Outcome 2: Reported behavioural changes in weight: BMI
Reported behavioural changes in diet
Two studies reported on behavioural changes in diet (Ng 2018Oostingh 2020). Ng 2018 compared preconception lifestyle advice on a combination of topics with routine care, and Oostingh 2020 compared this with attention control. Only Ng 2018 reported on behavioural changes in diet (vegetable intake in g/day) as defined in the review methods.
We are uncertain whether preconception lifestyle advice on a combination of topics affects vegetable intake (MD 12.50 grams/d, 95% CI ‐8.43 to 33.43; 1 RCT, 264 participants; very low‐quality evidence) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Preconception lifestyle advice on a combination of any of the following topics: weight, diet, vitamin and mineral intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse vs routine care or attention control, Outcome 3: Reported behavioural changes in diet: vegetable intake
Additionally, Ng 2018 reported on fruit intake in pieces/d: MD 0.80, 95% CI 0.36 to 1.24 in 164 women with infertility. Oostingh 2020 reported on the number of participants with adequate intake of vegetables (> 200 grams/d) and fruit (> 2 pieces/d). For the number of participants with adequate intake of vegetables, the RR was 1.46, 95% CI 1.17 to 1.82 in 612 women with infertility, and was 1.42, 95% CI 0.96 to 2.08 in 216 male partners. For the number of participants with adequate fruit intake, study authors reported an RR of 1.18, 95% CI 1.05 to 1.33 in 612 women with infertility, and an RR of 1.57, 95% CI 1.21 to 2.04 in 216 male partners. Table 5 presents an overview of additional behavioural changes not reported according to the definition in the review methods.
1. Additional data on lifestyle behavioural changes not reported according to definitions in the review methods.
| Comparison 1. Preconception lifestyle advice on a combination of any of the following topics: weight, diet, vitamin and mineral intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse compared to routine care or attention control | |
| Reported behavioural changes in diet | |
| Oostingh 2020 |
Number of participants with adequate vegetable intake (> 200 grams/d) at 6 months
Women:
124/299 in experimental group
89/313 in control group
RR 1.46, 95% CI 1.17 to 1.82
Men:
40/103 in experimental group
31/113 in control group
RR 1.42, 95% CI 0.96 to 2.08
Number of participants with adequate fruit intake (> 2 pieces/d) at 6 months
Women:
207/299 in experimental group
182/311 in control group
RR 1.18, 95% CI 1.05 to 1.33 Men: 67/103 in experimental group 46/111 in control group RR 1.57, 95% CI 1.21 to 2.04 |
| Ng 2018 | Fruit intake in pieces/d at 3 months (mean ± SD) 2.82 ± 1.9 in experimental group (n = 131) 2.02 ± 1.7 in control group (n = 133) MD 0.80, 95% CI 0.36 to 1.24 |
| Comparison 2. Preconception lifestyle advice on weight compared to routine care | |
| Reported behavioural changes in diet | |
| Belan 2019 | Composite score: healthy eating index in points at 6 months (mean ± SD) 18.2 ± 13.7 in experimental group (n = 46) 5.3 ± 12.4 in control group (n = 51) MD 12.90, 95% CI 7.68 to 18.12 |
| Mutsaerts 2016 | Sugary drinks in glasses/d at 6 months (mean ± SD) 0.59 ± 0.2 glasses/d in experimental group (n = 88) 0.99 ± 0.3 glasses/d in control group (n = 128) MD ‐0.41, 95% CI ‐0.47 to ‐0.34 Savoury snacks in handfuls/week at 6 months (mean ± SD) 2.17 ± 0.73 handfuls/week in experimental group (n = 100) 2.32 ± 0.81 handfuls/week in control group (n = 139) MD ‐0.15, 95% CI ‐0.35 to 0.05 Sweet snacks in portions/week at 6 months (mean ± SD) 1.725 ± 0.32 portion/week in experimental group (n = 99) 2.72 ± 0.79 portion/week in control group (n = 136) MD ‐1, 95% CI ‐1.14 to ‐0.85 |
| Reported behavioural changes in physical activity | |
| Belan 2019 | Total leisure activity energy expenditure in kcal/kg/d at 6 months (mean ± SD) 0.77 ± 1.63 in experimental group (n = 46) 0.17 ± 1.26 in control group (n = 51) MD 0.60, 95% CI 0.02 to 1.18 |
| Comparison 3. Preconception lifestyle advice on alcohol intake compared to routine care | |
| Reported behavioural changes in alcohol intake | |
| Rossi 2013 | Decrease in number of drinks on a drinking day (mean ± SD) 1 ± 1 in experimental group (n = 16) 0.4 ± 1 in control group (n = 21) MD 0.60, 95% CI ‐0.05 to 1.25 Decrease in % of drinking days in past 6 months (mean ± SD) 0.2 ± 0.3 in experimental group (n = 16) 0.1 ± 0.2 in control group (n = 21) MD 0.10, 95% CI ‐0.07 to 0.27 Decrease in number of weeks drinking above the safety daily limit in the past 6 months(mean ± SD) 5.6 ± 9 in experimental group (n = 16) 1.7 ± 3.5 in control group (n = 21) MD 3.90, 95% CI ‐0.76 to 8.56 Decrease in number of binges in the past 6 months (mean ± SD) 14.5 ± 44.4 in experimental group (n = 16) 1.2 ± 4.5 in control group (n = 21) MD 13.30, 95% CI ‐8.54 to 35.14 |
| Comparison 4. Preconception lifestyle advice on alcohol intake compared to routine care | |
| Reported behavioural changes in smoking | |
| Hughes 2000 | Delta stage‐of‐change = difference in stage of motivation to change smoking behaviour (mean) Experimental group (mean): 0.31 (no SD reported) (n = 47) Control group (mean): 0.26 (no SD reported) (n = 47) Rate of maintained cessation "Rate of maintained cessation rose from 4% to 24% (P < 0.001)" (experimental and control groups reported together) No further data provided |
CI: confidence interval.
MD: mean difference.
RR: risk ratio.
SD: standard deviation.
Reported behavioural changes in vitamin or mineral supplement intake (women only)
Two studies reported on the number of women with adequate use of folic acid supplement (Ng 2018Oostingh 2020). Ng 2018 compared preconception lifestyle advice on a combination of topics with routine care, and Oostingh 2020 compared this with attention control.
Preconception lifestyle advice on a combination of topics may result in little to no difference in the number of women with adequate use of folic acid supplement (RR 0.98, 95% CI 0.95 to 1.01; 2 RCTs, 850 participants; I² = 4%; low‐quality evidence). This suggests that if the proportion of women with adequate use of folic acid supplement is assumed to be 93% in the control group, then the proportion of women with adequate use of folic acid supplement when preconception lifestyle advice is received would be between 89% and 94% (Analysis 1.4).
1.4. Analysis.

Comparison 1: Preconception lifestyle advice on a combination of any of the following topics: weight, diet, vitamin and mineral intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse vs routine care or attention control, Outcome 4: Reported behavioural changes in vitamin and mineral supplement intake: use of folic acid supplement
Reported behavioural changes in alcohol intake
One study reported on the number of men and women abstaining from alcohol and compared preconception lifestyle advice on a combination of topics with attention control (Oostingh 2020).
Preconception lifestyle advice on a combination of topics may result in little to no difference in the proportion of women abstaining from alcohol (RR 1.07, 95% CI 0.99 to 1.17; 1 RCT, 607 participants; low‐quality evidence). This suggests that if the number of women abstaining from alcohol is assumed to be 75% in the control group, then the number of women abstaining from alcohol when receiving preconception lifestyle advice would be between 74% and 88% (Analysis 1.5).
1.5. Analysis.

Comparison 1: Preconception lifestyle advice on a combination of any of the following topics: weight, diet, vitamin and mineral intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse vs routine care or attention control, Outcome 5: Reported behavioural changes in alcohol intake: numbers of women and men abstaining from alcohol
We are uncertain whether preconception lifestyle advice on a combination of topics affects the number of men abstaining from alcohol (RR 1.08, 95% CI 0.74 to 1.58; 1 RCT, 210 participants; very low‐quality evidence).
Reported behavioural changes in smoking and other substance abuse
The same study reported on the numbers of men and women not smoking after preconception lifestyle advice on a combination of topics compared with attention control (Oostingh 2020).
Preconception lifestyle advice on a combination of topics may result in little to no difference in the proportion of women not smoking (RR 1.01, 95% CI 0.98 to 1.04; 1 RCT, 606 participants; low‐quality evidence). This suggests that if the proportion of women not smoking is assumed to be 95.1% in the control group, then the number of women not smoking when preconception lifestyle advice is received would be between 93% and 99% (Analysis 1.6).
1.6. Analysis.

Comparison 1: Preconception lifestyle advice on a combination of any of the following topics: weight, diet, vitamin and mineral intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse vs routine care or attention control, Outcome 6: Reported behavioural changes in smoking: numbers of women and men not smoking
We are uncertain whether preconception lifestyle advice on a combination of topics affects the number of men not smoking (RR 1.01, 95% CI 0.91 to 1.12; 1 RCT, 212 participants; very low‐quality evidence).
Birth weight
No studies reported on birth weight.
Gestational age
No studies reported on gestational age.
Clinical pregnancy
Two studies reported on clinical pregnancy and compared preconception lifestyle advice on a combination of topics to routine care (Alibeigi 2020), or to attention control (Oostingh 2020). Oostingh 2020 reported the percentage of clinical pregnancy at 12 months without specifying a definition for clinical pregnancy. On request, the study authors provided the number of clinical pregnancies that was included in the analysis. Considerable statistical heterogeneity (P = 0.0003 and I² = 92%) and clinical heterogeneity between intervention and comparison were evident. Therefore, the data from these two studies were not pooled for this outcome, as this would not result in a clinically meaningful estimate of the treatment effect (Analysis 1.7).
1.7. Analysis.

Comparison 1: Preconception lifestyle advice on a combination of any of the following topics: weight, diet, vitamin and mineral intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse vs routine care or attention control, Outcome 7: Clinical pregnancy
Due to very low‐quality evidence, we are uncertain whether preconception lifestyle advice on a combination of topics affects the number of clinical pregnancies. Alibeigi 2020 reported an RR of 2.85 (95% CI 1.53 to 5.29) in 180 participants. Oostingh 2020 reported an RR of 0.92 (95% CI 0.81 to 1.05) in 626 participants.
Time to pregnancy
No studies reported on time to pregnancy.
Quality of life
No studies reported on quality of life.
Male factor infertility outcomes
No studies reported on male factor infertility outcomes.
Comparison 2. Preconception lifestyle advice on one topic: weight versus routine care or attention control
See Table 2.
Studies identified for this comparison included only women with infertility and a specific lifestyle characteristic (e.g. being overweight or obese) (Belan 2019Mutsaerts 2016).
Primary effectiveness outcomes
Live birth or ongoing pregnancy
Two studies reported on live birth among women with infertility and obesity and compared preconception lifestyle advice on weight to routine care (Belan 2019Mutsaerts 2016). Belan 2019 reported percentages of live birth, which we were able to recalculate to numbers of live births.
We are uncertain whether preconception lifestyle advice on weight affects the numbers of live births in women with infertility and obesity (RR 0.94, 95% CI 0.62 to 1.43; 2 RCTs, 707 participants; I² = 68%; very low‐quality evidence). The evidence suggests that if the chance of live birth is assumed to be 49% in the control group, then the chance of live birth when preconception lifestyle advice is received would be between 30% and 70% (Analysis 2.1).
2.1. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 1: Live birth
Sensitivity analyses were performed including changing the random‐effects model to a fixed‐effect model and changing RR to OR; these changes did not alter the overall effect.
Primary safety outcomes
Adverse events
Two studies reported on adverse events including gestational diabetes and hypertension in women with infertility and obesity and compared preconception lifestyle advice on weight to routine care (Belan 2019Mutsaerts 2016).
No data on adverse events could be extracted from Belan 2019. These study authors stated that "groups did not differ for the rate of gestational diabetes or preeclampsia." We contacted study authors for more information, but we did not receive any reply as of the time of submission (February 2021).
We are uncertain whether preconception lifestyle advice on weight affects the numbers of adverse events including gestational diabetes (RR 0.78, 95% CI 0.48 to 1.26; 1 RCT, 317 participants; very low‐quality evidence) and hypertension (RR 1.07, 95% CI 0.66 to 1.75; 1 RCT, 317 participants; very low‐quality evidence) in women with infertility and obesity. Evidence suggests that if the chances of gestational diabetes and hypertension are assumed to be 20% and 16% in the control group, then the chances when preconception lifestyle advice is received would be between 10% and 25% for gestational diabetes, and between 11% and 28% for hypertension (Analysis 2.2).
2.2. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 2: Adverse events
Miscarriage
One study reported on miscarriage as the number of losses of clinical pregnancy at gestational age < 16 weeks in women with infertility and obesity and compared preconception lifestyle advice on weight to routine care (Mutsaerts 2016).
We are uncertain whether preconception lifestyle advice on weight affects the numbers of miscarriages in women with infertility and obesity (RR 1.50, 95% CI 0.95 to 2.37; 1 RCT, 577 participants; very low‐quality evidence). Evidence suggests that if the chance of miscarriage is assumed to be 9% in the control group, then the chance of live birth when preconception lifestyle advice is received would be between 9% and 22% (Analysis 2.3).
2.3. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 3: Miscarriage
Secondary outcomes
Reported lifestyle behavioural changes
No studies reported on lifestyle behavioural changes in vitamin or mineral supplement intake, caffeine intake, smoking, or other substance abuse after preconception lifestyle advice on weight.
Reported behavioural changes in weight
In the comparison of preconception lifestyle advice on weight to routine care, two studies reported on behavioural changes in weight in women with infertility and obesity (Belan 2019Mutsaerts 2016). These studies used different parameters to report this outcome: change in BMI and weight (Mutsaerts 2016); and percentage of weight loss (Belan 2019). A minimal data set was provided by the authors of the first study (Mutsaerts 2016), so we were able to recalculate weight into the percentage of weight loss (mean ± SD).
Preconception lifestyle advice on weight may slightly reduce the BMI in women with infertility and obesity (MD ‐1.30 kg/m², 95% CI ‐1.58 to ‐1.02; 1 RCT, 574 participants; low‐quality evidence) (Analysis 2.5).
2.5. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 5: Reported behavioural changes in weight: percentage of weight loss
We are uncertain whether preconception lifestyle advice on weight affects the percentage of weight loss in women with infertility and obesity (MD ‐3.29%, 95% CI ‐4.34 to ‐2.24; 2 RCTs, 380 participants; I² = 24%, very low‐quality evidence) (Analysis 2.4).
2.4. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 4: Reported behavioural changes in weight: BMI
Reported behavioural changes in diet
The same two studies in women with infertility and obesity reported on behavioural changes in diet (Belan 2019Mutsaerts 2016). Only Mutsaerts 2016 reported on behavioural changes in diet (vegetable and fruit intake in grams/d) as defined in the review methods.
We are uncertain whether preconception lifestyle advice on weight affects vegetable intake (MD 0.00 grams/d, 95% CI ‐4.18 to 4.18; 1 RCT, 250 participants; very low‐quality evidence) and fruit intake (MD ‐7.25 grams/d, 95% CI ‐7.86 to ‐6.64; 1 RCT, 258 participants; very low‐quality evidence) in women with infertility and obesity (Analysis 2.6Analysis 2.7).
2.6. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 6: Reported behavioural changes in diet: vegetable intake
2.7. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 7: Reported behavioural changes in diet: fruit intake
Belan 2019 reported on a healthy eating score adapted from the USA's healthy eating index, whereas Mutsaerts 2016 reported on the median intake of vegetables, fruits, sugary drinks, savoury snacks, and sweet snacks. Table 5 presents an overview of additional behavioural changes not reported according to the definition in the review methods.
Reported behavioural changes in alcohol intake
One study reported on the median intake of alcoholic beverages expressed as glasses/d in women with infertility and obesity and compared preconception lifestyle advice on weight to routine care (Mutsaerts 2016). A minimal data set was provided by the study authors and values were recalculated to mean ± SD. The mean intake of alcoholic beverages was 0 glasses/d in the control group and 0.02 ± 0.016 glasses/d in the intervention group, hence the mean difference was not estimable.
We are uncertain whether preconception lifestyle advice on weight decreases alcohol intake in women with infertility and obesity (MD not estimable; 1 RCT, 239 participants; very low‐quality evidence) (Analysis 2.8).
2.8. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 8: Reported behavioural changes in alcohol intake
Reported behavioural changes in physical activity
Two studies in women with infertility and obesity reported on behavioural changes in physical activity and compared preconception lifestyle advice on weight to routine care (Belan 2019Mutsaerts 2016). Only Mutsaerts 2016 reported on behavioural changes in physical activity as defined in the review methods. In this study, physical activity was defined as the median minutes spent in moderate to vigorous physical activity per week, which we were able to recalculate to mean values.
We are uncertain whether preconception lifestyle advice on weight affects the total moderate to vigorous physical activity (MD 50.76 minutes/week, 95% CI 16.77 to 84.75; 1 RCT, 254 participants; very low‐quality evidence) (Analysis 2.9).
2.9. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 9: Reported behavioural changes in physical activity
Belan 2019 reported on total leisure activity energy expenditure in kcal/kg/d: MD 0.60 kcal/kg/d, 95% CI 0.02 to 1.18, in 97 women with infertility and obesity (Table 5).
Birth weight
Two studies in women with infertility and obesity reported on birth weight and compared preconception lifestyle advice on weight to routine care (Belan 2019Mutsaerts 2016).
No data on birth weight could be extracted from Belan 2019. These study authors stated, "there was no significant difference between groups for weight at birth." We contacted the study authors for more information, but we did not receive any reply as of the time of submission (February 2021).
Median birth weight was reported by Mutsaerts 2016. A minimal data set was provided by the study authors; therefore we were able to recalculate mean values and a mean difference.
We are uncertain whether preconception lifestyle advice on weight affects birth weight in women with infertility and obesity (MD ‐29.00 g, 95% CI ‐39.12 to ‐18.88; 1 RCT, 276 participants; very low‐quality evidence) (Analysis 2.10).
2.10. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 10: Birth weight
Gestational age
Two studies in women with infertility and obesity reported on gestational age and compared preconception lifestyle advice on weight to routine care (Belan 2019Mutsaerts 2016).
No data on gestational age could be extracted from Belan 2019. These study authors concluded that "there was no significant difference between groups for gestational age." We contacted the authors for more information, but we did not receive any reply as of the time of submission (February 2021).
Median gestational was reported by Mutsaerts 2016. A minimal data set was provided by the study authors; therefore we were able to recalculate this to mean ± SD to calculate a mean difference.
We are uncertain whether preconception lifestyle advice on weight affects gestational age in women with infertility and obesity (MD 0.45 weeks, 95% CI 0.33 to 0.57; 1 RCT, 276 participants; very low‐quality evidence) (Analysis 2.11).
2.11. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 11: Gestational age
Clinical pregnancy
Two studies reported on clinical pregnancy in women and compared preconception lifestyle advice on weight to routine care (Belan 2019Mutsaerts 2016). Belan 2019 reported the percentage of clinical pregnancy, which we recalculated to the number of clinical pregnancies. Due to statistical (P = 0.008 and I² = 86%) and clinical heterogeneity, pooling the data from both studies would not result in a clinically meaningful estimate of the treatment effect.
From evidence of very low quality, we are uncertain whether preconception lifestyle advice on weight affects the number of clinical pregnancies in women with infertility and obesity. Belan 2019 reported an RR of 1.41 (95% CI 1.06 to 1.87) in 130 participants. Mutsaerts 2016 reported an RR of 0.93 (95% CI 0.82 to 1.06) in 577 participants (Analysis 2.12).
2.12. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 12: Clinical pregnancy
Time to pregnancy
One study reported on time to pregnancy leading to live birth, comparing preconception lifestyle advice on weight to routine care (Mutsaerts 2016). A minimal data set was provided by the study authors, so we were able to calculate the mean (± SD) time to pregnancy and the hazard ratio (HR) from the Kaplan‐Meier curve. Mean time to pregnancy in the intervention group was 19.31 ± 0.67 months and in the control group 16.81 ± 0.73 months.
Delaying the start of fertility treatment to give preconception lifestyle advice on weight might slightly increase the time to pregnancy in women with infertility and obesity (HR 0.79, 95% CI 0.63 to 0.99; 1 RCT, 561 participants; low‐quality evidence) (Analysis 2.14).
2.14. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 14: Quality of life
Quality of life in women and/or men
One study reported on the mental and physical quality of life in women and compared preconception lifestyle advice on weight to routine care (Mutsaerts 2016). A minimal data set was provided by the study authors; therefore we were able to calculate the mean (± SD) quality of life.
We are uncertain whether preconception lifestyle advice on weight affects the mental quality of life in women with infertility and obesity (MD ‐0.34, 95% CI ‐2.91 to 2.24; 1 RCT, 235 participants; very low‐quality evidence) or the physical quality of life in women with infertility and obesity (MD 1.63, 95% CI ‐0.58 to 3.85; 1 RCT, 235 participants; very low‐quality evidence) (Analysis 2.14).
Male factor infertility outcomes
No studies reported on male factor infertility outcomes.
Comparison 3. Preconception lifestyle advice on one topic: alcohol intake versus routine care or attention control
See Table 3.
The identified study for this comparison included only women with infertility and a specific lifestyle characteristic (e.g. at‐risk alcohol drinking) (Rossi 2013).
Primary effectiveness outcomes
Live birth or ongoing pregnancy
One study reported on live birth after preconception lifestyle advice on alcohol intake as compared with routine care (Rossi 2013).
We are uncertain whether preconception lifestyle advice on alcohol intake affects the number of live births in at‐risk drinking women with infertility (RR 1.15, 95% CI 0.53 to 2.50; 1 RCT, 37 participants; very low‐quality evidence). Evidence suggests that if the chance of live birth is assumed to be 38% in the control group, then the chance of live birth when preconception lifestyle advice is received would be between 20% and 95% (Analysis 3.1).
3.1. Analysis.

Comparison 3: Preconception lifestyle advice on alcohol intake vs routine care or attention control, Outcome 1: Live birth
Primary safety outcomes
Any adverse event
No studies reported on adverse events.
Miscarriage
That same study in at‐risk drinking women with infertility reported on miscarriage and compared preconception lifestyle advice on alcohol intake to routine care (Rossi 2013).
We are uncertain whether preconception lifestyle advice on alcohol intake affects miscarriage in at‐risk drinking women with infertility (RR 1.31, 95% CI 0.21 to 8.34; 1 RCT, 37 participants; very low‐quality evidence). Evidence suggests that if the chance of miscarriage is assumed to be 10% in the control group, then the chance of miscarriage when preconception lifestyle advice is received would be between 2% and 79% (Analysis 3.2).
3.2. Analysis.

Comparison 3: Preconception lifestyle advice on alcohol intake vs routine care or attention control, Outcome 2: Miscarriage
Secondary outcomes
Reported lifestyle behavioural changes
No studies reported on lifestyle behavioural changes in weight, diet, vitamin or mineral supplement intake, caffeine intake, physical activity, smoking, or other substance abuse.
Reported behavioural changes in alcohol intake
Behavioural changes in alcohol intake were reported in the same study in at‐risk drinking women with infertility (Rossi 2013). Behavioural changes in alcohol intake were not reported as defined in the review methods; however surrogate outcomes on alcohol consumption were provided. Table 5 presents an overview of the results. Study authors reported the number of drinks on a drinking day (MD 0.60, 95% CI ‐0.05 to 1.25), the percentage of drinking days in the past six months (MD 0.10, 95% CI ‐0.07 to 0.27), the number of weeks drinking above the safety daily limit in the past six months (MD 3.90, 95% CI ‐0.76 to 8.56), and the number of binges in the past six months (MD 13.30, 95% CI ‐8.54 to 35.14) in 37 at‐risk drinking women with infertility.
Birth weight
No studies reported on birth weight.
Gestational age
No studies reported on gestational age.
Clinical pregnancy
No studies reported on clinical pregnancy.
Time to pregnancy
No studies reported on time to pregnancy.
Quality of life
No studies reported on quality of life.
Male factor infertility outcomes
No studies reported on male factor infertility outcomes.
Comparison 4. Preconception lifestyle advice on one topic: smoking versus routine care or attention control
See Table 4.
The study identified for this comparison included only women with infertility and a specific lifestyle characteristic (e.g. smoking three or more cigarettes per day over the last six months) (Hughes 2000).
Primary effectiveness outcomes
Live birth or ongoing pregnancy
No studies reported on live birth or ongoing pregnancy.
Primary safety outcomes
Any adverse event
No studies reported on adverse events.
Miscarriage
No studies reported on miscarriage
Secondary outcomes
Reported lifestyle behavioural changes
No studies reported on lifestyle behavioural changes in weight, diet, vitamin or mineral supplement intake, alcohol intake, caffeine intake, or physical activity.
Reported behavioural changes in smoking or other substance abuse
One study reported on behavioural changes in smoking habits and compared preconception lifestyle advice on smoking to routine care (Hughes 2000). Study authors did not use outcome measures as defined in the review methods but reported delta stage of change (difference in stage of motivation to change smoking behaviour) and rate of maintained smoking cessation at 12 months in women with infertility who smoked three or more cigarettes over the past six months (Table 5). The mean delta stage of change was 0.31 for the experimental group and 0.26 for the control group. Mean differences could not be calculated, as study authors did not report standard deviations, CIs, or P values. In their study, the study authors concluded, "there were no significant differences in the mean delta stage of change or 12‐month rate of maintained cessation." The rate of maintained cessation was not reported separately for intervention and control groups.
Birth weight
No studies reported on birth weight.
Gestational age
No studies reported on gestational age.
Clinical pregnancy
No studies reported on clinical pregnancy.
Time to pregnancy
No studies reported on time to pregnancy.
Quality of life
No studies reported on quality of life.
Male factor infertility outcomes
No studies reported on male factor infertility outcomes.
Discussion
Summary of main results
We found seven randomised controlled trials (RCTs) comparing preconception lifestyle advice with routine care or attention control in a total of 2130 women and men with infertility. Only one of these RCTs also included male partners. Three studies compared preconception lifestyle advice on a combination of topics with routine care or attention control. Four studies compared preconception lifestyle advice on one topic (weight, alcohol intake, or smoking) with routine care in women with infertility and specific lifestyle characteristics. The summary of finding tables present the main outcomes.
Preconception lifestyle advice on a combination of topics versus routine care or attention control
Low‐quality evidence suggests that preconception lifestyle advice on a combination of topics may result in little to no difference in the number of live births.
No studies reported on adverse events or miscarriage.
Regarding lifestyle behavioural changes, we are uncertain whether preconception lifestyle advice on a combination of topics affects body mass index (BMI) and vegetable intake due to very low‐quality evidence. Low‐quality evidence suggests that preconception lifestyle advice on a combination of topics may result in little to no difference in the number of women with adequate use of folic acid supplement, abstaining from alcohol, and not smoking. One study reported on the number of men abstaining from alcohol and not smoking, but the evidence was of very low quality. No studies reported behavioural changes in caffeine intake and physical activity.
Due to very low‐quality evidence, we are uncertain whether preconception lifestyle advice on a combination of topics affects the number of clinical pregnancies. No studies reported on birth weight, gestational age, time to pregnancy, quality of life, or male factor infertility outcomes.
Preconception lifestyle advice on weight versus routine care
Due to very low‐quality evidence, we are uncertain whether preconception lifestyle advice on weight affects the number of live births, the number of adverse events including gestational diabetes and hypertension, and the number of miscarriages in women with infertility and obesity.
Regarding lifestyle behavioural changes, low‐quality evidence suggests that preconception lifestyle advice on weight may slightly reduce the BMI of women with infertility and obesity.
We are uncertain whether preconception lifestyle advice on weight affects the percentage of weight loss, vegetable and fruit intake, alcohol intake, and total moderate to vigorous physical activity in women with infertility and obesity due to very low‐quality evidence. No studies reported behavioural changes in vitamin or mineral supplement intake, caffeine intake, smoking, or other substance abuse. Due to very low‐quality evidence, we are uncertain whether preconception lifestyle advice on weight affects birth weight, gestational age, clinical pregnancy, and quality of life in women with infertility and obesity.
Low‐quality evidence suggests that delaying the start of fertility treatment to give preconception lifestyle advice on weight may slightly increase the time to pregnancy in women with infertility and obesity. The shorter time to pregnancy in the control group can be explained by the fact that these participants received in vitro fertilisation (IVF)/intracytoplasmic sperm injection (ICSI) treatment six months earlier than the intervention group. No studies reported on male factor infertility outcomes.
Preconception lifestyle advice on alcohol intake versus routine care
Due to very low‐quality evidence, we are uncertain whether preconception lifestyle advice on alcohol intake affects the number of live births and decreases the number of miscarriages in at‐risk drinking women with infertility. No studies reported on adverse events. No studies reported lifestyle behavioural changes in weight, diet, vitamin or mineral supplement intake, caffeine intake, physical activity, smoking, or other substance abuse. One study reported on behavioural changes in alcohol intake but not as defined in the review methods. No studies reported on birth weight, gestational age, clinical pregnancy, time to pregnancy, quality of life, or male factor infertility outcomes.
Preconception lifestyle advice on smoking versus routine care
One study reported on lifestyle behavioural changes in smoking but not as defined in the review methods. No studies reported on all other outcomes.
Overall completeness and applicability of evidence
Evidence from identified studies was not sufficient to justify firm conclusions on the effect of preconception lifestyle advice. Seven studies met the inclusion criteria for this review, only three of which reported on live birth or ongoing pregnancy (Belan 2019Mutsaerts 2016Rossi 2013), and one provided data on live birth on request (Oostingh 2020). The predefined safety outcomes (adverse events and miscarriage) were reported in only one study (Mutsaerts 2016). Evidence is also insufficient to address most of the secondary outcomes of the review. For example, birth weight, gestational age, quality of life, and time to pregnancy were adequately reported in only one study, and male factor infertility was not a measured outcome in any of the included studies. Although lifestyle behavioural changes after the intervention were reported in all studies, a variety of measures and definitions were used to report these changes. Likewise, four studies reported on clinical pregnancy using a variety of definitions. Although all definitions used were in accordance with the ICMART (International Committee for Monitoring Assisted Reproduction Technology) Glossary, we were unable to synthesise these data in our meta‐analysis (Zegers‐Hochschild 2017). This inconsistency in reporting outcomes and the small number of studies covering all predefined outcomes contributed to our inability to synthesise results and compare and combine individual studies into a meta‐analysis. Consequently, this limits the usefulness of this evidence in informing clinical practice.
We aimed to assess a wide range of participants (men and women, before and during fertility treatment) and interventions (preconception lifestyle on a combination of topics or on one topic, in different settings, varying mode of delivery or duration) to consider clinical utility of the preconception lifestyle advice, and to identify optimal components of preconception lifestyle advice in a broad population. It may be expected that most evidence may be available for a population with specific risk factors of preconception health such as smoking, obesity, or at‐risk drinking. It may also be expected that interventions started before any fertility treatment is begun may have a different effect than interventions provided during fertility treatment. Given the limited number of identified studies, subgroup analyses on participant or intervention characteristics were not feasible, and inclusion of such a wide range of participants and interventions resulted in clinical heterogeneity.
The paucity of RCTs performed on this topic may be explained by the possible necessity to delay fertility treatment; after receiving preconception lifestyle advice, the couple has to undertake lifestyle changes, which may take up to several months before any effect will be noticeable (e.g. weight loss). Once a cause of infertility has been diagnosed and there is indication for fertility treatment, advice on lifestyle behaviour changes seems an unfavourable option for both patients and clinicians. Nevertheless, our search identified nine ongoing studies, reflecting increasing interest in preconception lifestyle interventions.
Infertility is a couple's condition, and couples may have a correlated lifestyle, for example, in weight and diet (Best 2017). Addressing couples on lifestyle interventions may provide extra support and promote compliance and behavioural change. It may thus be expected that involving both partners in preconception lifestyle advice may have greater impact as compared to advising only the woman, to positively change preconception health. In our review, we included only one study that had invited male participants (Oostingh 2020). However, not all male partners of female participants took part in the study. This may reflect the reluctance of male partners to be involved in lifestyle behavioural changes in preparation for pregnancy. Hence, our review stresses not only the need to further explore the effect of preconception lifestyle advice in couples, but also the need to create awareness in our patients on the importance of preconception health for both partners if a healthy pregnancy is to be achieved.
Quality of the evidence
Based on the GRADE criteria, the quality of the evidence was low to very low.
The main reasons to downgrade the evidence were serious risk of bias and serious imprecision due to the limited number of studies and hence, the limited number of patients or events. Despite the fact that all included studies investigated the effects of preconception lifestyle advice in a population with infertility, live birth was defined as an outcome in only a minority of studies. Failure to report live birth in infertility trials is a major source of bias, as it should be the default primary outcome in such studies (Harbin Consensus Conference Workshop Group 2014). Remarkably, most studies were rated at high risk or at unclear risk of bias due to poor reporting of study methods. In some cases, study authors were not able to provide us with additional data to clarify their study methods. Obviously, all studies were at high risk of detection bias for patient‐reported outcome measures because of the type of intervention and the impossibility of blinding participants to such types of interventions.
We found few studies for each comparison; therefore quality of the evidence was low to very low; it is very likely that further research will have an important impact on our confidence in the effect estimates and may change these estimates.
Potential biases in the review process
This review was conducted according to Cochrane methods, with a pre‐defined protocol to minimise potential bias. Changes were made to the review protocol before the update was undertaken, to improve the structure of the review. Changes are noted in the Differences between protocol and review section. No changes were made as a result of the findings of included studies. We aimed to minimise reporting bias by conducting a comprehensive search for eligible studies and by being alert for duplication of data. Due to the small number of studies included in the review, we were not able to construct a funnel plot, and consequently, we were not able to examine the presence of publication bias. Reporting bias was assessed through careful assessment of included studies for failure to report obvious outcomes or for reporting them in insufficient detail. Risk of bias assessment for reporting bias was independently conducted by two review authors. We have included this level of detail in the 'Risk of bias' table under Characteristics of included studies.
We defined our intervention as follows: "preconception lifestyle advice defined as counselling about weight, diet, vitamin or mineral supplement intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse." Many of the initially identified studies evaluated a well‐defined intervention (e.g. supervised physical activity, a strict diet). Similarly, multiple studies investigated the effects of supplements or study products. Because these interventions were not initiated as preconception lifestyle advice alone, do not reflect daily clinical practice, and do not allow for assessment of lifestyle behavioural changes, we did not include such studies in our review. Consequently, we excluded a large number of studies that may provide useful information on the topic of lifestyle interventions in people with infertility.
Agreements and disagreements with other studies or reviews
We identified one other systematic review on the effects of preconception lifestyle interventions on people with infertility (Lan 2017). This paper reviewed the impact of preconception lifestyle interventions on fertility, obstetrical, foetal, anthropometric, and metabolic outcomes in a population with intent to conceive, including people with infertility. Thus, both women and men were included, but inclusion was not limited to a population with infertility. Additionally, we excluded studies solely focusing on alcohol or smoking cessation and micronutrient supplementation. Finally, interventions were not restricted to preconception lifestyle advice but included any modifications aiming to optimise nutritional and/or physical activity status, such as weight management, dietary changes, exercise regimens, and psychological support.
We searched the literature published up to January 2017 and identified eight studies, which were primarily performed among women with infertility who were overweight or obese. Only one of these studies was included in our review, as the other studies provided strict weight loss interventions or were not conducted in people with infertility (Mutsaerts 2016). Review authors concluded that lifestyle interventions showed benefits for weight loss and BMI reduction in overweight and obese women. Study authors did not use the GRADE system to determine the quality of the evidence, and so we are unable to confirm their findings. Similar to our review, these review authors highlighted the need for further research exploring optimal components of preconception lifestyle interventions in the broader population.
In the first edition of this Cochrane systematic review, the need for further research into this subject was highlighted, as no available RCTs had assessed the effects of preconception advice on the chance of live birth or other fertility outcomes in people with diagnosed infertility (Anderson 2010). Although the importance of providing preconception lifestyle advice is more widely acknowledged at this time (ESHRE guideline 2015Homan 2018), we identified only six additional studies and nine ongoing studies. Consequently, we were able to add only evidence of low to very low quality to the conclusions of the first edition of this review.
Authors' conclusions
Implications for practice.
Low‐quality evidence suggests that preconception lifestyle advice on a combination of topics may result in little to no difference in the number of live births nor in the number of women with adequate use of folic acid supplement, abstaining from alcohol, or not smoking. Evidence was insufficient to permit a conclusion on the effects and safety of preconception lifestyle advice on adverse events, miscarriage, birth weight, gestational age, clinical pregnancy, quality of life, or male factor infertility outcomes, as no identified studies looked at these outcomes or the studies were of very low quality. Delaying the start of fertility treatment to give preconception lifestyle advice on weight may slightly reduce BMI and increase time to pregnancy among women with infertility and obesity, but the evidence is of low quality. We are uncertain whether preconception lifestyle advice affects other lifestyle behavioural changes, as no identified studies looked at these outcomes or the studies were of very low quality.
This review does not provide clear guidance for clinical practice on preconception lifestyle advice. However it highlights the lack of evidence on this topic, despite recommendations by international guidelines to provide counselling to all couples with infertility. Our findings also reveal the absence of male participation in the vast majority of studies addressing this topic.
In conclusion, our review highlights the need for high‐quality RCTs investigating effects of preconception lifestyle advice on a combination of topics and assessing relevant effectiveness and safety outcomes for men and women with infertility.
Implications for research.
Based on the findings of the current review, we propose that future research should include well‐designed, adequately powered RCTs with meticulous description of study methods. Future research in this area should explore the effects of preconception lifestyle not only of women with infertility but also of their male partners. Such studies will allow further examination of the interaction within couples. In addition, studies should focus on specific subgroups such as women with infertility and obesity and should also address a broader population with infertility. In most modern countries, unhealthy lifestyle is on the rise; therefore it may be recommended to investigate the beneficial effects of preconception lifestyle counselling among all couples planning to conceive. The effects of various intervention characteristics including different topics on lifestyle advice, duration, setting, behaviour change techniques used, and format of interventions should be explored in future research. Studies should report in detail on these intervention characteristics. Besides the content of advice provided, the mode of delivery and the timing of advice should be considered. This will enable researchers to identify optimal components of preconception lifestyle interventions. In the comparison arm, minimal treatment or attention control that mimics the amount of attention received by the intervention group but is thought not to have a specific effect should be considered.
Of key importance is future assessment of relevant effectiveness and safety outcomes. Future research should record core outcome measures for infertility trials and report accordingly (Duffy 2020aDuffy 2020b). Ideally, core outcome measures on reported lifestyle behavioural changes should be specified and should be reported consistently. Data can thus be combined and synthesised to generate solid evidence to inform clinical practice.
What's new
| Date | Event | Description |
|---|---|---|
| 26 February 2021 | New citation required and conclusions have changed | Searches and results updated. We identified 6 studies in addition to the first version of this review (Alibeigi 2020Belan 2019Mutsaerts 2016Ng 2018Oostingh 2020Rossi 2013) |
| 18 February 2019 | New search has been performed | Background and Methods updated to meet current Cochrane standards |
History
Protocol first published: Issue 1, 2010 Review first published: Issue 4, 2010
Notes
Acknowledgements
We would like to thank the Cochrane Gynaecology and Fertility Group, and in particular Marian Showell, for support provided on the update of the protocol and the search methods.
We would like to thank the authors of the following studies ‐ Belan 2019, Hughes 2000, Mutsaerts 2016, Ng 2018, and Oostingh 2020 ‐ for providing us with additional information on the data as contributed to this review.
We would like to thank the following peer reviewers for their time and commitment: Dr Vanessa Jordan, Dr Elizabeth Glanville, and Professor Andy Vail.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group (CGFG) specialised register search strategy
PROCITE platform
Searched 12 January 2021
Keywords CONTAINS "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or "intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "ART" or "assisted conception" or "assisted reproduction" or "artificial insemination" or "IUI" or "IVF‐ET" or "subfertility" or "Infertility" or "Intrauterine Insemination" or Title CONTAINS "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or "intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "ART" or "assisted conception" or "assisted reproduction" or "artificial insemination" or "IUI" or "IVF‐ET" or "subfertility" or "Infertility" or "Intrauterine Insemination"
AND
Keywords CONTAINS "behavioral coping strategies" or "behavioral therapy" or "therapy group" or "counseling" or "counselling" or "psycho‐educational intervention" or "exercise" or "exercise therapy" or "physical exercise" or "physical well being" or "behavioral treatment" or "Lifestyle" or "lifestyle change" or "lifestyle modification" or "lifestyle program" or "lifestyle programme" or "alcohol" or "smoking" or "Smoking cessation "or "caffeine" or "Diet" or "Diet Supplementation" or "diet therapy" or "dietary" or "dietary intervention" or "dietary self‐monitoring" or "intercourse" or "Nutrition" or "nutritional counseling" or "nutritional supplement "or "nutritional supplements" or "yoga" or "caloric restriction diet" or "Weight Loss" or "Ethanol" or "iodine" or "folate" or "folic acid" or "education" or "educational intervention" or "information focused therapy" or "health behaviour" or "health education" or "preconception" or "Patient Education" or "Patient knowledge" or "decision aid" or "decision making" or "Decision‐making aid"
The total number of records found: 668
Appendix 2. Cochrane CENTRAL Register of Studies Online (CRSO) search strategy
Web platform
Searched 12 January 2021
#1 (icsi or ivf):TI,AB,KY 6745
#2 (intracytoplasmic sperm injection*):TI,AB,KY 1995
#3 (vitro fertili?ation):TI,AB,KY 3531
#4 MESH DESCRIPTOR Reproductive Techniques, Assisted EXPLODE ALL TREES 3175
#5 (IUI or intrauterine insemination*):TI,AB,KY 1262
#6 (artificial insemination*):TI,AB,KY 243
#7 (assisted reproduct*):TI,AB,KY 1454
#8 (ovarian hyperstimulation):TI,AB,KY 1427
#9 (infertil* or subfertil*):TI,AB,KY 9233
#10 fertility:TI,AB,KY 3615
#11 ((want or wanting or hoping or hope or plan or planning or intend* or intention or contemplat*) adj5 pregnan*):TI,AB,KY 589
#12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 16536
#13 MESH DESCRIPTOR Life Style EXPLODE ALL TREES 5449
#14 (lifestyle or life style):TI,AB,KY 20797
#15 MESH DESCRIPTOR Counseling EXPLODE ALL TREES 5398
#16 MESH DESCRIPTOR Health Knowledge, Attitudes, Practice EXPLODE ALL TREES 5962
#17 MESH DESCRIPTOR Preconception Care EXPLODE ALL TREES 114
#18 MESH DESCRIPTOR Behavior Therapy EXPLODE ALL TREES 16571
#19 MESH DESCRIPTOR Patient Education as Topic EXPLODE ALL TREES 8834
#20 (counsel* or care or advice or educat* or intervention* or information or coach* or programme* or program* or advisory):TI,AB,KY 614909
#21 (prepregnan* or periconcept* or antenal or prenatal or preconcept*):TI,AB,KY 7043
#22 (before conception or before conceiving):TI,AB,KY 126
#23 (prior adj3 (conception or conceiving)):TI,AB,KY 84
#24 #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 624850
#25 MESH DESCRIPTOR Life Style EXPLODE ALL TREES 5449
#26 (life style or lifestyle):TI,AB,KY 20797
#27 MESH DESCRIPTOR Adaptation, Psychological EXPLODE ALL TREES 5273
#28 MESH DESCRIPTOR Drinking Behavior EXPLODE ALL TREES 3998
#29 MESH DESCRIPTOR health behavior EXPLODE ALL TREES 35496
#30 MESH DESCRIPTOR Marijuana Use EXPLODE ALL TREES 317
#31 MESH DESCRIPTOR Tobacco Use EXPLODE ALL TREES 233
#32 MESH DESCRIPTOR Street Drugs EXPLODE ALL TREES 0
#33 (recreation* adj2 drug*):TI,AB,KY 172
#34 MESH DESCRIPTOR Cannabis EXPLODE ALL TREES 301
#35 (marijuana or cannabis or cocaine):TI,AB,KY 6176
#36 (smoking adj2 (stop* or reduc*)):TI,AB,KY 851
#37 (smoking adj2 cessation):TI,AB,KY 10114
#38 MESH DESCRIPTOR Alcohol Drinking EXPLODE ALL TREES 3863
#39 alcohol:TI,AB,KY 23853
#40 MESH DESCRIPTOR Caffeine EXPLODE ALL TREES 2083
#41 (coffee or caffeine or caffeinated):TI,AB,KY 5169
#42 MESH DESCRIPTOR Diet EXPLODE ALL TREES 18402
#43 MESH DESCRIPTOR Vitamins EXPLODE ALL TREES 19122
#44 MESH DESCRIPTOR Folic Acid EXPLODE ALL TREES 3462
#45 MESH DESCRIPTOR Iodine EXPLODE ALL TREES 1327
#46 (intercourse adj3 (timing or frequency or compliance)):TI,AB,KY 56
#47 (nutrition* or vitamin* or multivitamin* or nutraceutical*):TI,AB,KY 68491
#48 diet*:TI,AB,KY 89989
#49 (weight or BMI or Body mass index or obes* or overweight):TI,AB,KY 154665
#50 MESH DESCRIPTOR Exercise EXPLODE ALL TREES 24617
#51 MESH DESCRIPTOR Physical Fitness EXPLODE ALL TREES 3307
#52 (exceris* or physical activit*):TI,AB,KY 30718
#53 (iodine or ethanol):TI,AB,KY 10027
#54 (folate or folic acid):TI,AB,KY 5383
#55 MESH DESCRIPTOR Healthy People Programs EXPLODE ALL TREES 11
#56 #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 348833
#57 #12 AND #24 AND #56 1695
Appendix 3. MEDLINE search strategy
OVID platform
Searched from 1946 to 12 January 2021
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (42204) 2 icsi.tw. (8753) 3 intracytoplasmic sperm injection$.tw. (7470) 4 exp reproductive techniques, assisted/ or exp insemination, artificial/ or exp oocyte donation/ (70717) 5 ART.tw. (110634) 6 artificial insemination$.tw. (7004) 7 IUI.tw. (1838) 8 vitro fertili?ation.tw. (23943) 9 ivf.tw. (24492) 10 assisted reproduct$.tw. (15738) 11 intrauterine insemination$.tw. (2578) 12 ovulation induc$.tw. (4209) 13 (ovari$ adj2 stimulat$).tw. (7387) 14 superovulat$.tw. (3492) 15 ovarian hyperstimulation.tw. (5186) 16 COH.tw. (1837) 17 infertil$.tw. (62938) 18 subfertil$.tw. (5258) 19 (ovari$ adj2 induction).tw. (291) 20 (asthenozoospermia or oligospermia or azoospermia).tw. (7839) 21 Asthenospermia.tw. (393) 22 Teratospermia.tw. (185) 23 exp Spermatozoa/ (67655) 24 Sperm$.tw. (139609) 25 semen.tw. (31198) 26 oligoasthenoteratozoospermi$.tw. (429) 27 fertility.tw. (81015) 28 exp ovulation induction/ (13311) 29 exp Infertility/ (66677) 30 exp Infertility, Female/ (28773) 31 ((want or wanting or hoping or hope or plan or planning or intend* or intention or contemplat*) adj5 pregnan$).tw. (4867) 32 or/1‐31 (432235) 33 exp counseling/ or exp directive counseling/ (44750) 34 exp Life Style/ or lifestyle.tw. or life style.tw. (169963) 35 exp Health Knowledge, Attitudes, Practice/ (114433) 36 exp Preconception Care/ (2377) 37 exp Behavior Therapy/ (77368) 38 exp Decision Support Techniques/ or decision$ support.tw. (91449) 39 exp Patient Education as Topic/ (86411) 40 (counsel$ or care or advice or educat$ or intervention$ or information or coach$ or programme or program or advisory).tw. (4039235) 41 (prepregnan$ or periconcept$ or antenal or prenatal or preconcept$).tw. (106522) 42 (before conception or before conceiving).tw. (2045) 43 (prior adj3 (conception or conceiving)).tw. (1109) 44 or/33‐43 (4368296) 45 exp Life Style/ (96337) 46 (life style or lifestyle).tw. (105342) 47 exp adaptation, psychological/ or exp drinking behavior/ or exp health behavior/ or exp "marijuana use"/ or reproductive behavior/ or exp smoking/ or exp "tobacco use"/ (636155) 48 exp Street Drugs/ (12733) 49 (recreation$ adj2 drug$).tw. (2553) 50 Cannabis/ (9439) 51 (marijuana or cannabis or cocaine).tw. (64392) 52 (smoking adj2 (stop$ or reduc$)).tw. (10023) 53 (smoking adj2 cessation).tw. (26369) 54 (tobacco adj2 reduc$).tw. (2305) 55 (tobacco adj2 cessation).tw. (2847) 56 exp Alcohol Drinking/ (70276) 57 alcohol.tw. (261119) 58 exp Caffeine/ (23518) 59 (coffee or caffeine or caffeinated).tw. (41759) 60 diet/ or exp diet, carbohydrate‐restricted/ or diet, reducing/ or healthy diet/ or portion size/ or serving size/ or nutritional status/ (217052) 61 exp Vitamins/ or exp Folic Acid/ or Iodine/ (350897) 62 (intercourse adj3 (timing or frequency or compliance)).tw. (1155) 63 (nutrition$ or vitamin$ or multivitamin$ or nutraceutical$).tw. (484180) 64 diet.tw. (332259) 65 (weight or BMI or Body mass index or obes$ or overweight).tw. (1189791) 66 exercise/ or exp physical conditioning, human/ or exp running/ or jogging/ or swimming/ or walking/ or exp physical fitness/ (207232) 67 (exceris$ or physical activit$).tw. (116985) 68 (yoga or jogging or walk$).tw. (127666) 69 mind‐body therapies/ or biofeedback, psychology/ or breathing exercises/ or exp hypnosis/ or meditation/ or psychophysiology/ or relaxation therapy/ or yoga/ (41552) 70 (hypnosis or mediatation).tw. (7326) 71 (calor$ adj3 restrict$).tw. (8166) 72 (iodine or ethanol).tw. (171269) 73 (folate or folic acid).tw. (41567) 74 exp Weight Loss/ (42762) 75 or/45‐74 (3352043) 76 32 and 44 and 75 (16140) 77 randomized controlled trial.pt. (520465) 78 controlled clinical trial.pt. (94008) 79 randomized.ab. (506731) 80 randomised.ab. (101151) 81 placebo.tw. (220343) 82 clinical trials as topic.sh. (194196) 83 randomly.ab. (349338) 84 trial.ti. (233182) 85 (crossover or cross‐over or cross over).tw. (87670) 86 or/77‐85 (1409876) 87 exp animals/ not humans.sh. (4775224) 88 86 not 87 (1298838) 89 76 and 88 (1745)
Appendix 4. Embase search strategy
OVID platform
Searched from 1980 to 12 January 2021
1 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (72873) 2 embryo$ transfer$.tw. (22306) 3 in vitro fertili?ation.tw. (31672) 4 icsi.tw. (16765) 5 intracytoplasmic sperm injection$.tw. (10056) 6 (blastocyst adj2 transfer$).tw. (2573) 7 ivf.tw. (42188) 8 exp infertility therapy/ or exp artificial insemination/ or exp intrauterine insemination/ or exp ovulation induction/ (104792) 9 assisted reproduct$.tw. (23973) 10 artificial insemination.tw. (6467) 11 iui.tw. (3404) 12 intrauterine insemination$.tw. (3913) 13 ovulation induc$.tw. (5743) 14 (ovari$ adj2 stimulat$).tw. (11588) 15 superovulat$.tw. (3973) 16 ovarian hyperstimulation.tw. (7646) 17 COH.tw. (2593) 18 infertil$.tw. (87986) 19 subfertil$.tw. (7219) 20 exp sperm/ (69097) 21 Sperm$.tw. (151297) 22 semen.tw. (37410) 23 fertility.tw. (96649) 24 ((want or wanting or hoping or hope or plan or planning or intend* or intention or contemplat*) adj4 pregnan$).tw. (6346) 25 or/1‐24 (371200) 26 exp counseling/ or exp e‐counseling/ or exp directive counseling/ (168240) 27 exp lifestyle/ (134633) 28 (lifestyle or life style).tw. (148776) 29 exp attitude to health/ (113919) 30 exp prepregnancy care/ (1927) 31 exp behavior therapy/ (43408) 32 exp decision support system/ (26770) 33 decision$ support.tw. (20407) 34 exp patient education/ (113861) 35 (counsel$ or care or advice or intervention$ or information or coach$ or programme or program or advisory or education$).tw. (5247617) 36 (prepregnan$ or periconcept$ or antenal or prenatal or preconcept$).tw. (132184) 37 (before conception or before conceiving).tw. (2728) 38 (prior adj2 (conception or conceiv$)).tw. (1684) 39 or/26‐38 (5591932) 40 exp lifestyle/ (134633) 41 (life style or lifestyle).tw. (148776) 42 exp drinking behavior/ (48804) 43 exp health behavior/ (422940) 44 exp cannabis/ (33712) 45 exp reproductive behavior/ (1488) 46 exp smoking/ or exp smoking prevention/ or exp paternal smoking/ or exp smoking reduction/ or exp smoking cessation/ or exp smoking cessation program/ (422873) 47 exp "tobacco use"/ (401592) 48 exp street drug/ (3675) 49 exp drug abuse/ or exp recreational drug/ or exp alcohol/ or exp cocaine/ (387436) 50 (recreation$ adj2 drug$).tw. (3948) 51 (marijuana or cannabis or cocaine).tw. (79990) 52 (smoking adj2 reduc$).tw. (6432) 53 (smoking adj2 cessation).tw. (35034) 54 (stop$ adj2 smoking).tw. (6457) 55 (tobacco adj2 reduc$).tw. (2678) 56 (tobacco adj2 cessation).tw. (3724) 57 alcohol.tw. (345384) 58 exp caffeine/ (43615) 59 (coffee or caffeine or caffeinated).tw. (49326) 60 exp low calory diet/ or exp diet restriction/ or exp low carbohydrate diet/ or exp diet/ or exp diet supplementation/ or exp healthy diet/ or exp diet therapy/ or exp low fat diet/ (610166) 61 diet.tw. (423436) 62 (nutrition$ or multivtamin$ or vitamin$ or nutraceutical$).tw. (598738) 63 exp vitamin/ or exp folic acid/ or iodine/ (639695) 64 (intercourse adj3 (timing or frequency or compliance)).tw. (1454) 65 (weight or BMI or Body mass index or obes$ or overweight).tw. (1646258) 66 exp exercise/ (346011) 67 (exceris$ or physical activit$).tw. (158959) 68 (yoga or jogging or walk$).tw. (176596) 69 exp hypnosis/ (12341) 70 exp yoga/ (8133) 71 (hypnosis or mediatation).tw. (7272) 72 (iodine or ethanol).tw. (207477) 73 (folate or folic acid).tw. (50425) 74 exp body weight loss/ (44713) 75 or/40‐74 (4549574) 76 25 and 39 and 75 (16107) 77 Clinical Trial/ (989532) 78 Randomized Controlled Trial/ (636916) 79 exp randomization/ (89856) 80 Single Blind Procedure/ (41459) 81 Double Blind Procedure/ (177538) 82 Crossover Procedure/ (65742) 83 Placebo/ (348188) 84 Randomi?ed controlled trial$.tw. (247480) 85 Rct.tw. (40231) 86 random allocation.tw. (2127) 87 randomly.tw. (461831) 88 randomly allocated.tw. (37212) 89 allocated randomly.tw. (2622) 90 (allocated adj2 random).tw. (833) 91 Single blind$.tw. (25997) 92 Double blind$.tw. (210024) 93 ((treble or triple) adj blind$).tw. (1257) 94 placebo$.tw. (314719) 95 prospective study/ (653806) 96 or/77‐95 (2548752) 97 case study/ (75043) 98 case report.tw. (425262) 99 abstract report/ or letter/ (1138778) 100 or/97‐99 (1627724) 101 96 not 100 (2492394) 102 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (6151881) 103 101 not 102 (2321183) 104 76 and 103 (2429)
Appendix 5. PsycINFO Search Strategy
OVID platform
Searched from 1806 to 12 January 2021
1 exp INFERTILITY/ (2238) 2 exp FERTILITY ENHANCEMENT/ (142) 3 (infertil$ or subfertil$).tw. (3723) 4 or/1‐3 (3964) 5 exp Health Behavior/ or exp Intervention/ or exp Lifestyle/ or exp Lifestyle Changes/ (153728) 6 (life style or lifestyle).tw. (29124) 7 5 or 6 (170685) 8 random.tw. (60343) 9 control.tw. (456434) 10 double‐blind.tw. (23352) 11 clinical trials/ (11837) 12 placebo/ (5845) 13 exp Treatment/ (1075389) 14 or/8‐13 (1482075) 15 4 and 7 and 14 (116)
Appendix 6. Allied and Complementary Medicine (AMED) search strategy
OVID platform
Searched from 1985 to 12 January 2021
1 exp Infertility male/ or exp Infertility female/ (326) 2 (infertil$ or subfertil$).tw. (452) 3 1 or 2 (459) 4 exp Health promotion/ or exp Life style/ (4024) 5 (life style or lifestyle).tw. (2878) 6 4 or 5 (5154) 7 3 and 6 (9)
Appendix 7. Cumulative Index to Nursing and Allied Health Literature (CINAHL) search strategy
EBSCO platform
Searched from 1961 to 20 February 2020 (later CINAHL content, until 12 January 2021, was accessed through the CENTRAL CRSO search)
| # | Query | Results |
| S20 | S7 AND S19 | 95 |
| S19 | S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 | 1,381,761 |
| S18 | TX allocat* random* | 11,476 |
| S17 | (MH "Quantitative Studies") | 24,459 |
| S16 | (MH "Placebos") | 11,631 |
| S15 | TX placebo* | 61,169 |
| S14 | TX random* allocat* | 11,476 |
| S13 | (MH "Random Assignment") | 57,464 |
| S12 | TX randomi* control* trial* | 183,230 |
| S11 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 1,050,648 |
| S10 | TX clinic* n1 trial* | 258,365 |
| S9 | PT Clinical trial | 86,325 |
| S8 | (MH "Clinical Trials+") | 274,131 |
| S7 | S3 AND S6 | 456 |
| S6 | S4 OR S5 | 75,185 |
| S5 | TX(life style or lifestyle) | 75,185 |
| S4 | (MM "Life Style Changes") OR (MM "Life Style") OR (MM "Substance Use Prevention (Iowa NIC)") | 11,599 |
| S3 | S1 OR S2 | 17,356 |
| S2 | TX subfertil* or TX infertil* | 17,356 |
| S1 | (MM "Infertility") | 7,627 |
Appendix 8. Data extraction form ‐ Covidence
| Source and identification | Study ID | ||||||
| Review author | |||||||
| Citations and related records | |||||||
| Contact details of study author | |||||||
| Correspondence required | No | Yes | Questions | ||||
| Sponsorship source | |||||||
| Country | |||||||
| Year study published | |||||||
| Data lacking? | |||||||
| Study author's key conclusions | |||||||
| Other comments of reviewer | |||||||
| Methods | Study design and group | ||||||
| Study duration | |||||||
| Sample size | |||||||
| Setting | |||||||
|
"Risk of bias" table |
Domain | Description | Judgement | ||||
| Adequate sequence generation? | High | Low | Unclear | ||||
| Adequate allocation concealment? | High | Low | Unclear | ||||
| Blinding of participants and personnel? | High | Low | Unclear | ||||
| Blinding of outcome assessors (per outcome)? | High | Low | Unclear | ||||
| Incomplete outcome data assessed? | High | Low | Unclear | ||||
| Free of selective reporting? | High | Low | Unclear | ||||
| Other concerns re bias? | High | Low | Unclear | ||||
| Risk of bias | High | Low | Unclear | ||||
| Participants | Total number of participants randomised | ||||||
| Baseline characteristics (per group) ‐ Age ‐ Sex ‐ Nationality ‐ Number per group ‐ Lifestyle characteristics (BMI...) ‐ Fertility characteristics (type of infertility and treatment, phase of treatment) |
Control | Intervention | |||||
| Baseline differences | |||||||
| Inclusion criteria | |||||||
| Exclusion criteria | |||||||
| Other comments ‐ participants | |||||||
| Interventions | Number of intervention groups | ||||||
| Details of interventions (per group) ‐ Category ‐ Description ‐ Duration ‐ Frequency ‐ Setting ‐ Mode of delivery ‐ Integrity/compliance ‐ Other comments on interventions |
Control | Intervention | |||||
| Outcomes | Outcome name | ||||||
| Outcome type (continuous/dichotomous) | |||||||
| Outcome reported as... (choose from RR, OR, mean, and SD, or custom‐made in Covidence) | |||||||
| Definition and unit of measurement Scale, range, and direction (if relevant) |
|||||||
| Data value (change from baseline/endpoint) | |||||||
| Time point of outcome assessed | |||||||
| Other comments on outcomes | |||||||
| Results | Summary data (in Covidence) | ||||||
| Event: e.g. live birth | No event: e.g. live birth | Total (by group) | |||||
| Intervention: preconception lifestyle advice | |||||||
| Control: no preconception lifestyle advice | |||||||
| Total (by event) | |||||||
| Estimate of effect with confidence interval; P value | |||||||
Data and analyses
Comparison 1. Preconception lifestyle advice on a combination of any of the following topics: weight, diet, vitamin and mineral intake, alcohol intake, caffeine intake, physical activity, smoking, and/or other substance abuse vs routine care or attention control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Live birth | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Reported behavioural changes in weight: BMI | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Reported behavioural changes in diet: vegetable intake | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4 Reported behavioural changes in vitamin and mineral supplement intake: use of folic acid supplement | 2 | 850 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 1.5 Reported behavioural changes in alcohol intake: numbers of women and men abstaining from alcohol | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.1 Number of women abstaining from alcohol | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.2 Number of men abstaining from alcohol | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6 Reported behavioural changes in smoking: numbers of women and men not smoking | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6.1 Number of women not smoking | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6.2 Number of men not smoking | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7 Clinical pregnancy | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Preconception lifestyle advice on weight vs routine care or attention control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Live birth | 2 | 707 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.62, 1.43] |
| 2.2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2.1 Hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2.2 Gestational diabetes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3 Miscarriage | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4 Reported behavioural changes in weight: BMI | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5 Reported behavioural changes in weight: percentage of weight loss | 2 | 380 | Mean Difference (IV, Random, 95% CI) | ‐3.29 [‐4.34, ‐2.24] |
| 2.6 Reported behavioural changes in diet: vegetable intake | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.7 Reported behavioural changes in diet: fruit intake | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.8 Reported behavioural changes in alcohol intake | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.9 Reported behavioural changes in physical activity | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.10 Birth weight | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.11 Gestational age | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.12 Clinical pregnancy | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.13 Time to pregnancy | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.14 Quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.14.1 Mental quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.14.2 Physical quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
2.13. Analysis.

Comparison 2: Preconception lifestyle advice on weight vs routine care or attention control, Outcome 13: Time to pregnancy
Comparison 3. Preconception lifestyle advice on alcohol intake vs routine care or attention control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Live birth | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.2 Miscarriage | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alibeigi 2020.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomised controlled trial Setting: single centre/hospital Study duration: enrolment April 2017 to November 2017 + 3 months' follow‐up; no further details Sample size calculation: yes: "the sample size was calculated using a significance level of 0.05 and a power of 80% to detect at least a difference of 25% between groups in 1 outcome of the study for the baseline success rate of 30%. The sample size consisted of 62 patients in each group. Due to the prediction of a high dropout rate during the study, we assumed a sample size of 90 patients per group and 180 patients were randomised" |
|
| Participants |
Number of participants randomised: 180; 89 in control, 91 in intervention Baseline characteristics (mean ± SD) Control: age: 30.0 ± 4.7; lifestyle characteristics: BMI: 25.4 ± 4.4; fertility characteristics: IVF treatment duration (years): 5.1 ± 3.1, previous IVF attempts: 0.19 ± 0.50 Intervention: age: 30.0 ± 4.2; lifestyle characteristics: BMI: 25.0 ± 4.4; fertility characteristics: IVF treatment duration (years): 4.7 ± 2.4, previous IVF attempts: 0.39 ± 0.67 Baseline differences: yes, differences in in previous IVF attempts (P = 0.029) and differences in education level (P = 0.004) between control and intervention (intervention group had more previous IVF attempts and was better educated) Inclusion criteria: women with infertility with ovulatory problems or endometriosis or idiopathic infertility, age 20 to 40 years Exclusion criteria: Infertility due to anatomical causes, with sterility of husbands, with uterine myoma or hydrosalpinx, smoking, drinking alcohol, diabetes mellitus, lack of interest or cooperation during the study, or refusal to go through IVF/ICSI Phase of fertility treatment: during treatment ("study continued until ongoing IVFs lasted at least 3 months") |
|
| Interventions |
Control: routine care + unstructured minimal preconception lifestyle advice Description: routine health care and modern dietary recommendations provided every 2 weeks by telephone and once a month face‐to‐face (F2F) Intervention: combination: diet + general healthy lifestyle recommendations Description: traditional medicine‐oriented diet regimen and general healthy lifestyle recommendations
Duration: "study continued until ongoing IVFs lasted at least 3 months" Frequency: multiple contacts, follow‐up every 2 weeks by telephone, once a month face‐to‐face Setting: individual + group discussion Mode of delivery: F2F + written training guidance text for self‐learning + follow‐up via phone and telegram discussion group Integrity/Compliance: "the individuals in the intervention group were carefully followed, and the rate and quality of their diets were checked based on a 24h recall questionnaire which was completed at 3 different times every 2 weeks." Data were not reported |
|
| Outcomes |
Reported behavioural changes in weight: BMI in kg/m² measured at baseline and "after the study" Clinical pregnancy: defined as pregnancy from IUI, IVF, or spontaneous pregnancy measured with sonography; no further details specified |
|
| Identification |
Sponsorship source: grant from the Research and Technology Deputy of the Iran University of Medical Sciences Protocol available/trial registration: IRCT2017013032245N2 Country: Iran |
|
| Notes | Clinical pregnancy split into IUI, IVF, and spontaneous. Information regarding diet behaviour (adherence to intervention) measured (qualitatively) but not reported Study authors contacted twice for clarification regarding allocation concealment, interventions, and outcomes (diet behaviour, definition and time point of clinical pregnancy, and time point of BMI assessment). No reply as of February 2021 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Unclear risk | Quote: "randomization was stratified according to age groups. According to the order of entrance to the study, based on permuted blocks of size four, the patients in each of the 4 age groups of < 30, 30–34, 35–37, and 38–40 years were randomly assigned to the intervention and control groups" Comment: study authors stated that block randomisation was used, but the process of selecting the blocks was not specified. Study authors were contacted twice for clarification; no reply as of February 2021 |
| Allocation concealment | Unclear risk | Quote: "the random allocation sequence was generated by an investigator who was not involved in eliciting data or conducting the study" Comment: not clear how allocation was concealed. Study authors were contacted twice; no reply as of February 2021 |
| Blinding of participants and personnel All outcomes | High risk | Quote: "another limitation of our study was the lack of blinding patients and physicians in our study" Comment: participants and personnel were aware of assigned intervention. Deviations from the intended intervention could have occurred |
| Blinding of outcome assessors Objective outcomes | Low risk | Quote: "however, all outcomes were measured based on clinical tests. They were all objective and quantitative not qualitative, so they could not be biased" |
| Blinding of outcome assessors Patient reported outcome measures | Unclear risk | Comment: outcome assessors were not blinded. BMI and clinical pregnancy were not likely to be influenced, as they are observer‐reported outcomes not involving judgement |
| Incomplete outcome data | Low risk | Quote: "the study flowchart for IVF is shown in Figure 1. Five women in the intervention group and 6 women in the control group were excluded due to lack of willingness and cooperation. Their data were used to assess the outcome of spontaneous pregnancy, but spontaneous pregnancies did not occur to them" Comment: ITT analysis. Outcome data were available for nearly all participants randomised. Missing data were documented and balanced per group. However, reasons for lack of willingness and cooperation were not reported in detail |
| Selective outcome reporting | High risk | Comment: prospectively registered study protocol was available. Most outcomes were analysed and reported in accordance with the protocol. Change in dietary behaviour and adherence to intervention were measured but not reported. Live birth or ongoing pregnancy was not included in the protocol and was not reported. Study authors were contacted twice for clarification; no reply as of February 2021 |
| Other sources of bias | Low risk | Comment: study appears free of other sources of bias |
Belan 2019.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomised controlled trial Setting: single centre/hospital Study duration: start date: November 2011; estimated study completion date: September 2020 Sample size calculation: yes: "based on the outcome of live birth and the Fertility Fitness Programme, the inclusion of 58 women per group provides 80% power to identify a doubling in live‐birth rates with this programme (25% to 50%, α=5%). Assuming a dropout rate of 10% the final estimate sample size will be 128 women" |
|
| Participants |
Number of participants randomised: 130 randomised (3 screen failures due to severe male factor infertility), 108 analysed at this point (57 in control, 51 in intervention) Baseline characteristics (mean ± SD): not specified (abstract data only) Baseline differences: no, study author replied by email: "there were no statistical or clinical differences between the intervention and control groups for the mPP analysis" Inclusion criteria: obese (BMI ≥ 30 kg/m²) or overweight if PCOS (BMI ≥ 27 kg/m²), women with infertility, age 18 to 40 years; infertility defined as (1) failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse in women who are under 35 years with regular menstrual cycles; (2) women who do not have regular menstrual cycles or are older than 35 years and have not conceived during a 6‐month trial period; or (3) women with a known cause of infertility Exclusion criteria: "bariatric surgery or plan for it and/or if natural conception is impossible or highly unlikely (tubal factor, severe male factor infertility, etc)" Phase of fertility treatment: intervention group pre‐treatment first 6 months, control group during treatment |
|
| Interventions |
Control: routine care Description: standard fertility treatments, initiated as soon as clinically indicated. Standard fertility treatments may include lifestyle counselling by the obstetrician‐gynaecologist certified in gynaecological reproductive endocrinology and infertility (GREI), the reproductive endocrinologist, or the fertility specialist in charge of patient care Intervention: weight Description: individual counselling with a dietician and a kinesiologist (using S.M.A.R.T. goals and motivational interviewing) in combination with group sessions. Aim is to implement progressive and sustainable lifestyle changes to reach modest weight loss. Nutritional counselling with a dietician using 3‐day food diaries to evaluate women’s food intake throughout the programme, and Canada’s Food Guide and the “Healthy Plate” to help improve patients’ diet, The kinesiologist will be responsible for taking anthropometric measures and vital signs and for coaching women to increase their physical activity level. A pedometer will be offered to assist the patient + group sessions: 12 educational group sessions conducted by the dietician, the psychologist, or the kinesiologist (45‐minute interactive small group workshops and 45‐minute physical activity) covering various topics relevant to obesity management and fertility. These group sessions will take place weekly. When a pregnancy is confirmed, the woman will be met to set new objectives specific to her pregnancy, including optimal gestational weight gain based on Institute of Medicine guidelines. Partners will be invited to join individual meetings and will be strongly encouraged to attend all group sessions Duration: 18 months. First 6 months without additional fertility treatment, then 12 months in association with fertility treatment Frequency: multiple contacts, every 3 to 6 weeks, individual meetings with a dietician and a kinesiologist (20 to 30 minutes each). Follow‐up email or call in‐between meetings + 12 weekly educational group sessions (45‐minute interactive small group workshops and 45‐minute physical activity) Setting: individual + couple + group Mode of delivery: F2F + telephone + mail + group discussion + tools (food diary and online pedometer) |
|
| Outcomes |
Live birth: percentage of live birth measured at 18 months Adverse events: gestational diabetes and preeclampsia (not further specified) Reported behavioural changes in weight: percentage of weight loss measured at 6 months Reported behavioural changes in diet: healthy eating score in points for measuring adherence to Canadian's Food Guide recommendations adapted from USA’s Healthy Eating Index (HEI). Measured at 6 months with questionnaire adapted from the Canadian Health Survey Reported behavioural changes in physical activity: total leisure activity energy expenditure in kcal/kg/d measured at 6 months with tri‐axial accelerometer Clinical pregnancy: study authors replied that they first used positive bhCG, following confirmation of a foetal heartbeat at ultrasound, or if the pregnancy exceeded 12 weeks (pregnancy from IVF + spontaneous pregnancy reported). Measured at 18 months Birth weight: not further specified Gestational age: not further specified |
|
| Identification |
Sponsorship source: Canadian Institutes for Health Research (CIHR; FRN‐114125) and Ministère de la santé et des services sociaux of Québec (MSSS, Programme québécois de contrepartie au Programme departenariats pour l’amélioration du système de santé des IRSC) Protocol available/trial registration: NCT01483612 Country: Canada |
|
| Notes | No paper on RCT is available yet (abstract Endocrine Society 2019 + abstract ASRM 2019 with same data presented). Study author replied by email 07 July 2020, the following: "we are currently working on a paper, but it is not completed yet. Other than the information already published in the previous abstracts, we can't provide any new information at this point" Study authors contacted for more clarification on the numbers of participants randomised and analysed, definition and timing of assessing pregnancy, calculation of healthy score, and baseline differences. They replied: "current analyses are mainly according to our modified per protocol (mPP), in which we included the 108 women (57 control and 51 intervention) who have completed at least 6 months into the study or became pregnant during those 6 months" After update of the searches (January 2021), study authors were contacted for more information on adverse events, birth weight, and gestational age; awaiting reply as of February 2021 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "an independent statistician will use computerised random number generation to allocate each participant to the intervention or control group. Randomisation will be stratified according to the PCOS or non‐PCOS status, based on clinical diagnosis in the patient’s record. Sequences will be generated using permuted block randomisation, with block sizes of two, four or six entries" |
| Allocation concealment | Low risk | Quote: "the allocation sequence will be concealed in corresponding sequentially numbered opaque envelopes. After a participant has completed baseline assessments, the research coordinator will open the envelope to reveal the group allocation to the participant. Participants will be informed of their group assignment at that time" |
| Blinding of participants and personnel All outcomes | High risk | Comment: participants and personnel were aware of assigned intervention; deviations from the intended intervention could have occurred |
| Blinding of outcome assessors Objective outcomes | Low risk | Comment: outcome assessors were not blinded. Live birth, weight, and clinical pregnancy were not likely to be influenced, as they are observer‐reported outcomes not involving judgement |
| Blinding of outcome assessors Patient reported outcome measures | High risk | Comment: outcome assessor is participant and is not blinded; reported behavioural changes are likely to be influenced. Eating healthy and moving is socially desirable |
| Incomplete outcome data | Unclear risk | Comment: only abstracts with limited data were available as of February 2021, so risk of attrition bias is unclear at this point 130 participants (65 intervention, 65 control) were randomised; 108 (83%) participants (51 intervention, 57 control) completed already 6 months of the trial and were analysed in modified per‐protocol analysis. For 97 (75%) participants (46 intervention and 51 control), follow‐up data regarding behavioural changes were available. Missing data were documented, but reasons were not (yet) reported |
| Selective outcome reporting | High risk | Comment: prospectively registered study protocol is available. Not all pre‐specified outcomes are reported at this point. Only abstracts with limited data were available as of February 2021, so risk of reporting bias is high at this point |
| Other sources of bias | Low risk | Comment: only abstracts with limited data are available |
Hughes 2000.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomised controlled trial
Setting: multi‐centre/hospitali Study duration: January 1996 to July 1999 Sample size calculation: yes, but not achieved: sample size estimation was based on what was considered to be a clinically significant difference between intervention and control in delta stage‐of‐change of 1.0. Standard assumptions of alpha (2‐tailed) = 0.05 and beta = 0.20 were used. The sample required was 107 patients per study arm. Although this target was reached for combined infertile and pregnant participants (N = 214), it was not attained for each independent sample,,as had been the original intention. The authors of this study state that it was stopped early due to funding issues, and that the study was underpowered |
|
| Participants |
Number of participants randomised: 94; 47 in control, 47 in intervention (and 110 pregnant women) Baseline characteristics (mean ± SD) Control: age: 32.15 ± 4.55; lifestyle characteristics: number of quit smoking attempts: 3.40 ± 3.94; number of cigarettes: 13.80 ± 7.40; fertility characteristics: number of months infertile: 37.83 ± 29.40 Intervention: age: 32.13 ± 5.0; lifestyle characteristics: number of quit smoking attempts: 2.40 ± 2.10; number of cigarettes: 12.19 ± 6.81; fertility characteristics: number of months infertile: 38.08 ± 31.51 Baseline differences: no Inclusion criteria: all newly referred women with infertility who reported that they had smoked ≥ 3 cigarettes over the last 6 months Exclusion criteria: attending clinics for genetic counselling, habitual abortion, previously evaluated in consultation Phase of fertility treatment: pre‐treatment |
|
| Interventions |
Control: routine care + unstructured minimal preconception lifestyle advice including assessment Description: standard care information about the impact of smoking on fertility and pregnancy + exhaled carbon monoxide measurements at enrolment, at 6 and 12 months Intervention: smoking Description: scripted motivational intervention with advice to quit smoking according to "stage‐of‐change" + "stage‐of‐change"–specific information booklet (5 prompt cards) + an offer for more in‐depth counselling at the hospital's smoking cessation clinic + assessment = exhaled carbon monoxide measurements at enrolment, at 6 and 12 months. Follow‐up: updated stage‐of‐change–oriented booklets as they progressed from one stage to the next Duration: 12 months Frequency: multiple contacts, not specified, probably baseline, at 6 and 12 months Setting: individual Mode of delivery: F2F + written |
|
| Outcomes | Reported behavioural changes in smoking: smoking self‐identified "delta stage‐of‐change" (difference in stage of motivation to change smoking behaviour) and rate of maintained smoking cessation at 12 months, measured with questionnaire (no further details) | |
| Identification |
Sponsorship source: Father Sean O’Sullivan Foundation, Hamilton Health Science Foundation, Ron Herkimer and Susan Sakowski, and Department of Obstetrics and Gynecology, McMaster University, Hamilton, Ontario, Canada Protocol available/trial registration: no Country: Canada |
|
| Notes | Study author was contacted for clarification on duration and frequency of intervention, baseline data, and outcomes (for delta stage‐of‐change, no SE, SD, or CI, and P values were reported; for rate of maintained cessation, no information on group with infertility was available and data from CO₂ exhaler as objective measure of smoking cessation were not reported). Study author replied that he was happily retired and does not have access to further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "randomised using a computer‐generated, blocked schedule" |
| Allocation concealment | Low risk | Quote: "numbered, opaque, sealed envelopes" Comment: not specified whether envelopes were sequentially numbered, but likely to be at low risk of bias given that the envelopes were at least numbered, opaque, and sealed, and no baseline differences indicated issues |
| Blinding of participants and personnel All outcomes | High risk | Comment: as blinding was not described, study authors were contacted for the previous version of this systematic review Quote: "the primary care‐giver was unblinded because he had to do the intervention and the patient was unblinded because they were given the intervention". Comment: participants and personnel were aware of assigned intervention; deviations from the intended intervention could have occurred |
| Blinding of outcome assessors Objective outcomes | Unclear risk | Not applicable; no objective outcome measures in this study |
| Blinding of outcome assessors Patient reported outcome measures | High risk | Comment: outcome assessor is participant and is not blinded; reported behavioural changes in smoking are likely to be influenced. Having quit smoking is a more desirable answer |
| Incomplete outcome data | Low risk | Comment: as there was no description of completeness of reported data, study authors were contacted for the previous version of this systematic review. They reported that all patients were accounted for and were included in follow‐up |
| Selective outcome reporting | High risk | Comment: no study protocol was available to further verify selective outcome reporting. Study authors were contacted for the previous version of this systematic review on reporting of live birth. They reported that this information was not collected. this information could have been collected at 12‐month follow‐up. Secondary outcome (rate of maintained cessation): no data on infertile and pregnant women were provided separately. CO exhalation (to verify smoking cessation) was tested in all arms yet was not reported |
| Other sources of bias | Low risk | Comment in the previous version of this systematic review: "women in the control group were aware that their smoking status was to be measured several times throughout the study, the comparison is actually 'tailored advice + measurement versus measurement and therefore may not be an accurate assessment of tailored advice alone" This is now covered under indirectness (differences in comparison). |
Mutsaerts 2016.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomised controlled trial
Setting: multi‐centre/hospital Study duration: recruitment June 2009 to June 2012 + 24 months' follow‐up Sample size calculation: yes: "the power calculations were based on an assumption of an increase in the rate of vaginal birth of a healthy singleton at term from 45% in the control group to 60% in the intervention group, a 20% discontinuation rate during the lifestyle intervention, and a 5% loss to follow‐up. We calculated that a sample of 285 women per group would provide the trial with a power of 80% at a two sided alpha level of 5%" |
|
| Participants |
Number of participants randomised: 577; 287 in control, 290 in intervention (2 in control and 1 in intervention withdrew consent) Baseline characteristics (mean ± SD) Control: age: 29.8 ± 4.6; lifestyle characteristics: median (IQR) BMI at baseline: 36 (33.5 to 38.2); fertility characteristics: median (IQR) duration of time attempting to conceive: 19.0 (13.0 to 32.0) Intervention: age: 29.7 ± 4.5; lifestyle characteristics: median (IQR) BMI at baseline: 36 (33.4 to 38.2); fertility characteristics: median (IQR) duration of time attempting to conceive: 22.0 (14.0 to 36.0) Baseline differences: yes, there were significant differences between groups for the median duration of time attempting to conceive (P = 0.037) Inclusion criteria: women with infertility with BMI ≥ 29 kg/m², age 18 to 39 years. Infertility defined as (1) chronic anovulation (oligomenorrhoea or amenorrhoea and low levels of gonadotropins and low or undetectable levels of oestrogen (World Health Organization (WHO) class I anovulation), or (2) oligomenorrhoea or amenorrhoea and serum follicle‐stimulating hormone and estradiol levels within the normal range (WHO class II anovulation)), or (3) ovulatory cycle and unsuccessfully tried to conceive for at least 12 months Exclusion criteria: "women with severe endometriosis, premature ovarian failure, or endocrinopathy (e.g., women with type 1 diabetes or Cushing’s syndrome) and those who were eligible for donor insemination because of azoospermia, women with untreated preexisting hypertension and those with hypertension‐related complications in a previous pregnancy" Phase of fertility treatment: intervention group pre‐treatment, control group during treatment |
|
| Interventions |
Control: routine care Description: control group received prompt treatment in accordance with Dutch infertility guidelines, irrespective of BMI Intervention: weight Description: 6 months of lifestyle intervention preceding 18 months of infertility treatment. Aim: realistic weight loss of 5% to 10%. The programme was developed according to the recommendations of the National Institutes of Health. Combination of healthy diet (advised to reduce energy intake with 600 kcal/d with assistance of online food diary and minimum caloric intake of 1200 kcal/d) and physical activity (advice by coach to increase moderate‐intensity physical activity (10,000 steps/d measured with step counter and 2 to 3 times, 30 minutes of moderate physical activity a week)) + behavioural modification (motivational counselling to promote awareness of a healthy lifestyle and to formulate individualised goals) + capturing of body weight, menstrual dates, and calorie intake in a computerised system by coaches. After women completed the intervention, infertility treatment was initiated according to the Dutch infertility guidelines, irrespective of BMI Duration: 6 months of lifestyle programme + 18 months of infertility treatment Frequency: multiple contacts; 6 outpatient visits and 4 telephone consults Setting: individual Mode of delivery: F2F + phone + tools (online food diary and pedometer) Integrity/Compliance: participants were guided by intervention coaches who had a degree in nursing or by dieticians who were trained before the trial. Intervention coaches were supervised on site by 1 trained nurse, had yearly group training sessions, and used a standardised computerised system to minimise practice variation. Women who missed ≥ 2 consecutive sessions were considered to have not completed the intervention and received treatment according to local protocols.Participants were informed in advance that they would not automatically receive infertility treatment if they did not complete the intervention To enhance adherence to the intervention, women who lost 5% to 10% of their initial weight or reached BMI < 29 in the first 6 months after randomisation could proceed with their indicated fertility treatment before the intervention was finished |
|
| Outcomes |
Live birth: measured at 24 months
Ongoing pregnancy: defined as viable pregnancy of at least 10 weeks of gestation, measured at 24 months Adverse events: gestational diabetes and hypertension measured at 24 months Miscarriage: defined as loss of a clinical pregnancy at gestational age < 16 weeks, measured at 24 months Reported behavioural changes in weight: BMI in kg/m² and weight in kg measured at baseline, at 3 and 6 months Reported behavioural changes in diet:vegetable intake (raw as well as cooked) in grams/d, fruit intake in grams/d, sugary drinks (fruit juices and soda) in glasses/d, savoury snacks (crisps, pretzels, nuts, and peanuts) in handfuls/week, and sweet snacks in portions/week (1 portion = 2 biscuits or 2 pieces of chocolate or 5 candies or 5 liquorice) measured at baseline, at 3, 6, and 12 months, with FFQ Reported behavioural changes in alcohol intake: alcoholic beverages in glasses/d measured at baseline, at 3, 6, and 12 months with FFQ Reported behavioural changes in physical activity: total moderate to vigorous physical activity (MVPA) including commuting activities and leisure‐time PA in minutes/week measured at baseline, at 3, 6, and 12 months, with SQUASH Birth weight: measured at 24 months Gestational age: measured at 24 months Clinical pregnancy: defined as pregnancy in which the gestational sac was visible on ultrasonography, measured at 24 months Time to pregnancy: time to pregnancy leading to live birth presented in Kaplan‐Meier curve Quality of life (QOL): mental and physical QOL measured at baseline, at 3, 6, and 12 months, with SF‐36 |
|
| Identification |
Sponsorship source: The Netherlands Organization for Health Research and Development Protocol available/trial registration: NTR 1530 Country: The Netherlands |
|
| Notes | Minimal data set is available in the articles We identified 25 publications on this study, including secondary analyses and follow‐up studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "randomization was performed online and was stratified according to trial centre and ovulatory status" |
| Allocation concealment | Low risk | Quote: "central randomisation centre" Comment: central randomisation by third party |
| Blinding of participants and personnel All outcomes | High risk | Quote: "blinding was not possible. However, we specified the type of infertility treatment before randomisation in order to minimize differences in treatment assignments; this led to similar distributions of infertility treatment in both groups" Comment: participants and personnel were aware of assigned intervention; deviations from the intended intervention could have occurred (e.g. control group seeking weight loss advice) |
| Blinding of outcome assessors Objective outcomes | Low risk | Comment: outcome assessor is not blinded. Live birth, adverse events, miscarriage, weight, birth weight, gestational age, clinical pregnancy, and time to pregnancy are not likely to be influenced, as they are observer‐reported outcomes not involving judgement |
| Blinding of outcome assessors Patient reported outcome measures | High risk | Comment: outcome assessor is participant and is not blinded; reported behavioural changes are likely to be influenced. Eating healthy, moving, drinking less, higher QOL are socially desirable |
| Incomplete outcome data | Low risk | Quote: "a total of 63 women (21.8%) discontinued the lifestyle intervention after a median of 2.8 months (interquartile range, 14 days to 3.9 months)" Quote: "a total of 10 women were lost to follow‐up, so data on 280 women in the intervention group and 284 women in the control group were available for the intention‐to‐treat analysis (Fig. 1)" Comment: ITT analysis, but researchers did not impute on ITT for those who withdrew consent (1 intervention, 2 control) and for those lost to follow‐up (9 intervention, 1 control), but we assumed in our analysis that they did not have a live birth. Missing data and reasons for discontinuation were documented but were not balanced between groups, in line with the statistical plan of 20% discontinuation of intervention and 5% lost to follow‐up |
| Selective outcome reporting | Low risk | Comment: prospectively registered study protocol was available; outcomes were analysed and were reported in accordance with the protocol. Many secondary analyses and follow‐up studies were performed |
| Other sources of bias | Low risk | Study appears free of other sources of bias |
Ng 2018.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomised controlled trial
Setting: multi‐centre/hospital Study duration: June 2016 to August 2019 Sample size calculation: yes, but not (yet) achieved: protocol says 440, abstract reported on 264: "in order to show a difference in the proportion of participants achieving a high composite lifestyle score from 30% in the control arm to 50% in the study arm after 24 weeks of the intervention, with 80% power at a P value of < 0.05, 93 patients will be required in each arm. We have assumed a randomisation rate of 50% and assuming a drop‐out rate of 15–20%, 220 patients will be randomized to each arm (440 patients to be recruited in total)" |
|
| Participants |
Number of participants randomised: 264; 133 in control, 131 in intervention Baseline characteristics (mean ± SD): not specified (abstract data only) Baseline differences: no baseline differences in vegetable intake, fruit intake, or folic acid intake; no further details on other baseline characteristics Inclusion criteria: women with infertility or women suffering from recurrent miscarriages, actively trying to conceive, age 18 to 45 years, fluent in the use and understanding of English, having a smartphone capable of running the online application Exclusion criteria: "women who are on a specific diet for medical reasons, women with insulin diabetes, and those undergoing any other means of lifestyle coaching, for example, personal trainer or group lifestyle coaching" Phase of fertility treatment: not specified |
|
| Interventions |
Control: routine care + unstructured minimal preconception lifestyle advice
Description: standard preconception advice offered by the UK National Health Service Intervention: combination of weight, diet, vitamin or mineral supplement intake (folic acid), alcohol intake, physical activity, and smoking Description: a personalised smartphone lifestyle coaching programme "Smarter Pregnancy". Through baseline and follow‐up lifestyle questionnaires (at 6, 12, 18, and 24 weeks) sent out via email, tailored lifestyle advice is generated. Tailored coaching includes a maximum of 3 interventions per week comprising text and email messages containing tips, recommendations, vouchers, seasonal recipes, and additional questions addressing behaviour, pregnancy status, body mass index (BMI), or adequacy of the diet. Coaching is focused on study participants who report inadequate intake of vegetables and fruit, and absence of folic acid supplementation, and those with unfavourable alcohol and smoking habits. Results from the questionnaires are shown on a personal online page to track a participant’s progress Duration: 6 months Frequency: multiple sessions, 2‐ to 3‐week email contact; screening at baseline, at 6, 12,18, and 24 weeks Setting: individual Mode of delivery: mobile app + email |
|
| Outcomes |
Reported behavioural changes in diet: vegetable intake in grams/d and fruit intake in pieces/d, measured at baseline and at 3 months with on online lifestyle questionnaire Reported behavioural changes in vitamin or mineral supplement intake: number of women with adequate use of folic acid supplement (400 mg/d) measured at baseline and at 3 months with an online lifestyle questionnaire |
|
| Identification |
Sponsorship source: NIHR Southampton Biomedical Research Centre Protocol available/trial registration: ISRCTN89523555 (not prospectively registered) Country: United Kingdom |
|
| Notes | No paper on RCT available yet (abstract ESHRE 2019). Study author replied by email: "we are in the process of submitting the iPLAN trial results for publication. We will be in touch when this is published" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "participating women will be randomised in programme by the computer generation of a series of validation codes which is unique for each participant" |
| Allocation concealment | Low risk | Quote: "computer generation of a series of validation codes which is unique for each participant. After completion of the baseline questionnaires, women will be randomised to the intervention or the control group. The randomisation process will be concealed" Comment: central randomisation by third party. |
| Blinding of participants and personnel All outcomes | High risk | Quote: "although clinicians were blinded throughout, due to the nature of the study, it was not possible to blind women who were randomised" Comment: clinicians were blinded but not clear if personnel delivering the intervention (research nurses) were blinded. Participants were aware of assigned intervention. Deviations from the intended intervention could have occurred |
| Blinding of outcome assessors Objective outcomes | Unclear risk | Not applicable; no objective outcome measures in this study |
| Blinding of outcome assessors Patient reported outcome measures | High risk | Quote: "the women’s diet and lifestyle were self reported through questionnaires and it was not possible to eliminate reporting biases" Comment: outcome assessor is participant and is not blinded; reported behavioural changes are likely to be influenced. Eating more vegetables and fruit and adequate use of folic acid supplement are socially desirable |
| Incomplete outcome data | Unclear risk | Comment: only abstract with limited data is available as of February 2021, so risk of attrition bias is unclear at this point ITT analysis is planned. 400 women are recruited, 236 randomised (131 intervention, 133 control); no further information on missing data is available (yet) |
| Selective outcome reporting | High risk | Comment: prospectively registered study protocol is available. Not all pre‐specified outcomes are reported at this point. Only abstracts with limited data are available as of February 2021, so risk of reporting bias is high at this point |
| Other sources of bias | High risk | Comment: only abstracts with interim analyses are available |
Oostingh 2020.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomised controlled trial
Setting: multi‐centre/hospital Study duration: July 2014 to March 2017 Sample size calculation: yes, but not achieved. Aimed for 3000 (general preconception cohort + subfertile cohort) in trial register. Aimed for 1000 general and 1000 subfertile in protocol: "based on our previous studies and the survey using Smarter Pregnancy, we expect a reduction of approximately 0.5 DRS points (based on a standard deviation of 2.7) in the intervention group compared to the control group. Considering alpha = 0.05 and power = 0.80, we will need to include a total of 916 women in our study (2 arms of 458 each). Due to expected dropouts of approximately 10%, we aim to include 1000 fertile (2 arms of 500 each) and 1000 subfertile women (2 arms of 500 each) in our study. For 50% of these women, we expect their male partner (n = 250 in each arm) to participate as well. Due to the lower SD (2.0) in men, with this sample size we are also able to demonstrate a reduction of at least 0.5 DRS points in the male partners" |
|
| Participants |
Number of participants randomised: 848 (626 women and 222 men); 318 women and 116 men in control, 308 women and 106 men in intervention Baseline characteristics (mean ± SD) Control: median (IQR) age women: 33 (30 to 36), median (IQR) age men: 35 (31 to 41); lifestyle characteristics: median BMI (IQR) women: 23.8 (21.6 to 26.3), median (IQR) BMI men: 25.2 (23.3 to 28.3), adequate dietary risk score women: 56/318, adequate dietary risk score men: 12/116, adequate lifestyle risk score women: 175/318, adequate lifestyle risk score men: 26/116; fertility characteristics: not specified Intervention: median (IQR) age women: 33 (29 to 37), median (IQR) age men: 35 (31 to 49); lifestyle characteristics: median BMI (IQR) women: 23.7 (21.6 to 26.7), median (IQR) BMI men: 25.1 (22.7 to 26.9), adequate dietary risk score women: 49/308, adequate dietary risk score men: 17/106, adequate lifestyle risk score women: 175/308, adequate lifestyle risk score men: 27/106; fertility characteristics: not specified Baseline differences: no, study author replied by email that there were no baseline differences between groups Inclusion criteria: couples with indication for IVF/ICSI, age 18 to 45 years for women, no upper age limit for male participants, residing in The Netherlands, and having a smartphone with Internet access Exclusion criteria: "women and their male partners with insufficient knowledge or understanding of the Dutch language, who are treated by a dietician to lose weight in the context of a fertility treatment, and who have a specific diet (e.g. vegans)" Phase of fertility treatment: before and during fertility treatment |
|
| Interventions |
Control: attention control Description: at baseline, at 12 and 24 weeks, participants in the control group receive the monitoring questionnaire about nutrition and lifestyle, but without feedback on the results. They receive access to a personal page and 1 seasonal recipe per week to maintain adherence and prevent dropout. Also, every 6 weeks, controls receive a request to adjust their pregnancy status if needed Intervention: combination of weight, diet, vitamin or mineral supplement intake (folic acid), alcohol intake, physical activity, and smoking Description: a personalised smartphone lifestyle coaching programme "Smarter Pregnancy". Through baseline and follow‐up lifestyle questionnaires (at 6, 12, 18, and 24 weeks) sent out via email, tailored lifestyle advice is generated. Tailored coaching includes a maximum of 3 interventions per week comprising text and email messages containing tips, recommendations, vouchers, seasonal recipes, and additional questions addressing behavior, pregnancy status, body mass index (BMI), or adequacy of the diet. Coaching is focused on study participants who report inadequate intake of vegetables and fruit and absence of folic acid supplementation, and those with unfavourable alcohol and smoking habits. Results from the questionnaires are shown on a personal online page to track a participant’s progress + the personal page provides access to additional modules (i.e. applications) to support physical activity, an agenda to improve compliance with hospital appointments and medicine adherence, and a module to monitor the safety of prescribed medication Duration: 6 months Frequency: multiple sessions, 2‐ to 3‐week email contact; screening at baseline, at 6, 12,18, 24, and 36 weeks Setting: individual + couple Mode of delivery: mobile app + email |
|
| Outcomes |
Live birth: reported after request Reported behavioural changes in diet: numbers of men and women with adequate vegetable intake (> 200 grams/d), numbers of men and women with adequate fruit intake (> 2 pieces/d), measured at baseline, at 6 and 9 months, with online lifestyle questionnaire Reported behavioural changes in vitamin or mineral supplement intake: number of women with adequate use of folic acid supplement (400 mg/d) measured at baseline, at 6 and 9 months, with online lifestyle questionnaire Reported behavioural changes in alcohol intake: numbers of men and women not drinking alcohol measured at baseline, at 6 and 9 months, with online lifestyle questionnaire Reported behavioural changes in smoking: numbers of men and women not smoking measured at baseline, at 6 and 9 months, with online lifestyle questionnaire Reported behavioural changes in combination: a dietary risk score (DRS) comprising intake of vegetables, fruits, and folic acid supplement and a lifestyle risk score (LRS) comprising smoking and alcohol use (score from 0 to 9; the lower, the better) were calculated at baseline, at 6 and 9 months, from an online lifestyle questionnaire Clinical pregnancy: no further details on definition, measured at 12 months through questionnaire |
|
| Identification |
Sponsorship source: Department of Obstetrics and Gynecology, Erasmus MC, University Medical Centre, Rotterdam, The Netherlands, a grant of ZonMW Health Care Efficiency Research and the Erasmus MC Mrace programme "Health Care Efficiency Research" Protocol available/trial registration: NTR4150 Country: The Netherlands |
|
| Notes | Study on general preconception population including subfertile cohort Study authors provided data on live birth as of February 2021. We contacted study authors for clarification of data on big 3 complications and raw data on vegetable and fruit intake in grams/d. We are awaiting reply as of February 2021 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "for each stratum, a permuted block design is used and programmed before‐hand" Comment: computer generated |
| Allocation concealment | Low risk | Quote: "for each stratum, a permuted block design is used and programmed before‐hand. Hereby, allocation concealment is ensured" Comment: study authors contacted for more information; they replied: “allocation concealment was used to ensure that researchers did not know the order of group assignment at recruitment and randomisation. Moreover, researchers were blinded to the allocation of the participants” |
| Blinding of participants and personnel All outcomes | High risk | Comment: no blinding of participants and personnel (according to clinical trial register). Not clear from the paper if personnel delivering the intervention were blinded. Participants were aware of assigned intervention. Deviations from the intended intervention could have occurred |
| Blinding of outcome assessors Objective outcomes | Low risk | Comment: not clear if outcome assessors were blinded. Clinical pregnancy not likely to be influenced, as this is an observer‐reported outcome not involving judgement |
| Blinding of outcome assessors Patient reported outcome measures | High risk | Comment: outcome assessor is participant and is not blinded; reported behavioural changes are likely to be influenced. Eating healthy, moving, adequate use of folic acid supplement, not drinking, and not smoking are socially desirable |
| Incomplete outcome data | Unclear risk | Quote: "intervention: lost to follow‐up (n = 13) due to incomplete answers; ♦Women, n = 10 ♦Men, n = 3. Discontinued intervention (n = 125) ♦Women, n = 97 ♦Men, n = 28. Control: lost to follow‐up (n = 14) due to incomplete answers; ♦Women, n = 9 ♦ Men, n = 5. Discontinued intervention (n = 79) ♦Women, n = 61 ♦Men, n = 1" Comment: ITT analysis, but study authors did not impute on ITT those lost to follow‐up; we assumed in our analysis that they did not have a clinical pregnancy. Missing data documented but not balanced between groups. 30.2% in intervention group vs 18.2% in control group. Reasons for discontinuation not reported |
| Selective outcome reporting | High risk | Comment: prospectively registered study protocol is available. Most outcomes are analysed and reported in accordance with the protocol. However, tertiary outcomes are reported in the protocol and not in Netherlands Trial Register. Additionally, study authors reported an outcome not pre‐specified in the protocol: lifestyle risk score, and did not (yet) report on the pre‐specified outcome: BIG 3 complications |
| Other sources of bias | Unclear risk | Not clear which lifestyle questionnaire was used, and if this questionnaire was validated |
Rossi 2013.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomised controlled trial
Setting: single centre/hospital Study duration: January 2005 to May 2009 Sample size calculation: no, sub‐analyses of larger trial |
|
| Participants |
Number of participants randomised: 37 (subgroup of RCT in different populations); 21 in control, 16 in intervention Baseline characteristics (mean ± SD) Control: age 36.4 ± 3.4; lifestyle characteristics: 5/21 lifetime alcohol use/dependence, 0/21 current alcohol use/dependence, 1.8 drinks/drinking/d; fertility characteristics: not specified Intervention: age 34.9 ± 4.5; lifestyle characteristics: 7/16 lifetime alcohol use/dependence, 2/16 current alcohol use/dependence, 2.1 drinks/drinking/d; fertility characteristics: not specified Baseline differences: yes, control group (AO) had 1.8 drinks/drinking/d on average; intervention group (BI) had 2.1 drinks/drinking/d on average (no P value reported) Inclusion criteria: "women with infertility that practiced at‐risk drinking (at least 7 drinks/week or more than 3 drinks/1 d or T‐ACE‐positive). T‐ACE is a 4‐item screening questionnaire, validated in prenatal alcohol use studies, that asks about tolerance to alcohol, being annoyed by others’ comments about drinking, attempts to decrease use, and having a drink first thing in the morning (“eye‐opener”). As the number of previous IVF cycles influences cycle success, we included only each woman’s first IVF cycle with an embryo transfer" Exclusion criteria: "women with current treatment for alcohol or drug abuse, physical dependence on alcohol, or use of opiates, cocaine, or other illicit substances" Phase of fertility treatment: not specified (asked of study author but no reply received, probably before and during) |
|
| Interventions |
Control: routine care + assessment Description: 1‐hour assessment interview by research assistants. Measures included (a) alcohol and drug abuse modules from the Structured Clinical Interview for DSM‐IV, to obtain current and lifetime alcohol and drug disorder diagnoses; (b) alcohol timeline follow‐back (TLFB), to obtain estimates of daily drinking for the 6 months before study enrolment; and (c) the Medical Outcomes Study 36‐Item Short Form Health Survey (SF‐36), to assess general health status, among others Intervention: alcohol Description: assessment (as described above) + an intervention using "Personal Steps to a Healthy Choice: A Woman’s Guide and Helping Patients Who Drink Too Much" with 3 follow‐up interviews. Assessment and feedback on individuals' drinking pattern and standardised information on health consequences of drinking + Goal setting and contracting (drinking goals and important reasons for modifying drinking behaviour) + Behavioral modification (identify circumstances individual would be at risk for drinking + develop alternative behaviours + written materials of "Personal steps" annotated with personal information Duration: 12 months. Frequency: multiple times, 1‐hour interview and assessment at baseline, at 3, 6, and 12 months Setting: individual Mode of delivery: F2F + written Integrity/Compliance: observation, practice with mock patients + audio tape and feedback on delivery of intervention |
|
| Outcomes |
Live birth: number of live births, not clear when measured, 12 months? Miscarriage: pregnancy loss, defined as clinical pregnancy without live birth. Not clear when measured, 12 months? Reported behavioural changes in alcohol intake: decrease in number of drinks on a drinking day, decrease in % of drinking days in past 6 months, decrease in number of weeks drinking above SDL in past 6 months, decrease in number of binges in past 6 months measured at 12 months with alcohol timeline follow‐back questionnaire |
|
| Identification |
Sponsorship source: National Institute on Alcohol Abuse and Alcoholism and Office of Research on Women's Health Protocol available/trial registration: NCT00846638 (not prospectively registered) Country: USA |
|
| Notes | Specific population (at‐risk drinkers on IVF) Corresponding author and other study authors contacted for clarification on participants, baseline data, outcomes (time point of live birth measured). No reply as of February 2021 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence generation | Low risk | Quote: "computer‐generated random assignment list" |
| Allocation concealment | Unclear risk | Comment: computer‐generated random assignment list. No information on allocation concealment. Study authors were contacted twice, with no reply as of February 2021 |
| Blinding of participants and personnel All outcomes | High risk | Quote: "the participants and the study staff could not be blinded to treatment group assignment, similar to other studies taking place in medical settings (Saitz et al., 2007)" Comment: participants and personnel were aware of assigned intervention. Deviations from the intended intervention could have occurred |
| Blinding of outcome assessors Objective outcomes | Low risk | Comment: outcome assessors were not blinded. Live birth and miscarriage were not likely to be influenced, as they are observer‐reported outcomes not involving judgement |
| Blinding of outcome assessors Patient reported outcome measures | High risk | Comment: outcome assessor is participant and is not blinded; reported behavioural changes regarding drinking are likely to be influenced, as less drinking is known to be socially desirable |
| Incomplete outcome data | High risk | Comment: in Chang 2011 (table 2), 161 out of 511 participants randomised are reported to have infertility. However outcome data are available only for 37 participants. Study authors were contacted twice for clarification, but no reply was received as of February 2021 |
| Selective outcome reporting | High risk | Comment: study protocol not prospectively registered. Subgroup analysis (on population with infertility) not pre‐specified in the study protocol. Baseline for several alcohol use outcomes not reported, although this information should be available. Pre‐specified outcome: SF‐36 data not reported |
| Other sources of bias | Low risk | Comment: study appears free of other sources of bias |
BMI: body mass index.
DRS: dietary risk score.
F2F: face‐to‐face.
FFQ: Food Frequency Questionnaire.
ICSI: intracytoplasmic sperm injection.
IQR: interquartile ratio.
ITM: Iranian Traditional Medicine.
ITT: intention‐to‐treat.
IUI: intrauterine insemination.
IVF: in vitro fertilisation.
LRS: lifestyle risk score.
MVPA: moderate to vigorous physical activity.
PCOS: polycystic ovarian syndrome.
QOL: quality of life.
RCT: randomised controlled trial.
SD: standard deviation.
SDL: sensible drinking limit.
SF‐36: 36‐Item Short Form Health Survey.
SQUASH: Short Questionnaire to Assess Health‐Enhancing Physical Activity.
TLFB: timeline follow‐back.
WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12610000631000 2010 | Wrong intervention: acupuncture; no minimal comparison group (lifestyle advice in both groups) |
| Aflatoonian 2014 | Wrong intervention: no preconception lifestyle advice (vitamin D) |
| Agricola 2014 | Wrong study design: no RCT |
| Asemi 2014 | Wrong patient population: not infertile (gestational diabetes) |
| Ashcroft 1997 | Wrong intervention: no preconception lifestyle advice (patient education) |
| Bai 2019 | Wrong intervention: intervention on psychological distress |
| Bailey 2015 | Wrong patient population: recurrent miscarriages |
| Barbour 2020 | Wrong intervention: no preconception lifestyle advice (counselling on infertility treatment) |
| Becker 2015 | Wrong intervention: main component LGI ‐ diet (no advice) |
| Beerendonk 1996a | Wrong intervention: no preconception lifestyle advice; sodium restriction to reduce bloating |
| Beerendonk 1996b | Wrong intervention: no preconception lifestyle advice; sodium restriction to reduce bloating |
| Beerendonk 1999 | Wrong intervention: no preconception lifestyle advice; sodium restriction to reduce bloating |
| Bell 2000 | Wrong patient population: not infertile (antenatal population) |
| Bernard 2020 | Wrong intervention: no preconception lifestyle advice (counselling on infertility treatment) |
| Bisht 2018 | Wrong intervention: no RCT |
| Bodin 2017 | Wrong patient population: not infertile |
| Bodin 2018 | Wrong patient population: not infertile |
| Carpenter 2016 | Wrong intervention: strict diet intervention; walnut supplementation |
| Chan 2012 | Wrong intervention: preconception lifestyle advice not main component (integrative body/mind/body intervention) |
| Chan 2015 | Wrong study design: no RCT |
| Chavarro 2016 | Wrong study design: no RCT |
| Christensen 2017 | Wrong study design: no RCT |
| Clark 2000 | Wrong intervention: no minimal control (standard weight loss advice vs attendance at a group session) |
| Clifton 2017 | Wrong intervention: preconception lifestyle advice not main component (mind/body intervention) |
| Clifton 2018 | Wrong intervention: preconception lifestyle advice not main component (mind/body intervention) |
| Connolly 1993 | Wrong intervention: no preconception lifestyle advice (psychological counselling) |
| CTRI201711010514 2017 | Wrong intervention: strict exercise intervention (supervised yoga visits) |
| Dancet 2019 | Wrong intervention: preconception lifestyle advice not main component (sexual education intervention) |
| Domar 2009 | Wrong intervention: preconception lifestyle advice not main component (mind/body intervention) |
| Domar 2011 | Wrong intervention: preconception lifestyle advice not main component (mind/body intervention) |
| Domar 2019 | Wrong intervention: preconception lifestyle advice not main component (mind/body intervention) |
| Dortaj 2016 | Wrong intervention: preconception lifestyle advice not main component (mindfulness) |
| DRKS00017554 2019 | Wrong patient population: not infertile |
| Einarsson 2017a | Wrong intervention: strict weight loss intervention with liquid formula diet |
| Einarsson 2017b | Wrong intervention: strict weight loss intervention with liquid formula diet |
| Einarsson 2018 | Wrong intervention: strict weight loss intervention with liquid formula diet |
| Einarsson 2019 | Wrong intervention: strict weight loss intervention with liquid formula diet |
| Elsinga 2008 | Wrong study design: no RCT |
| Emery 2001 | Wrong intervention: no preconception lifestyle advice (psychological counselling) |
| Emery 2002 | Wrong intervention: no preconception lifestyle advice (psychological counselling) |
| Emery 2003 | Wrong intervention: no preconception lifestyle advice (psychological counselling) |
| Emery 2004 | Wrong intervention: no preconception lifestyle advice (psychological counselling) |
| Emery 2006 | Wrong intervention: no preconception lifestyle advice (psychological counselling) |
| Espinos 2017 | Wrong intervention: main component LGI ‐ diet (no advice) |
| Fauque 2007 | Wrong study design: no RCT |
| Galhardo 2013 | Wrong study design: no RCT |
| Garruti 2017 | Wrong study design: no RCT |
| Gaskins 2018 | Wrong study design: no RCT |
| Gaskins 2019 | Wrong study design: no RCT |
| Gautam 2019a | Wrong intervention: strict exercise intervention (supervised yoga visits) |
| Gautam 2019b | Wrong intervention: strict exercise intervention (supervised yoga visits) |
| Goswami 2015 | Wrong study design: no RCT |
| Guzick 1994 | Wrong patient population: not infertile |
| Hamzehgardeshi 2019 | Wrong intervention: preconception lifestyle advice not main component (fertility education and group counselling) |
| Hoirisch Clapauch 2017 | Wrong patient population: recurrent miscarriages |
| HuMl 2016 | Wrong study design: no RCT |
| IRCT201110267915N1 2014 | Wrong intervention: no preconception lifestyle advice (psychological counselling) |
| IRCT201301189463N 2017 | Wrong study design: no RCT |
| IRCT20150119020719N6 2018 | Wrong intervention: preconception lifestyle advice not main component (fertility education and group counselling) |
| IRCT2016012610324N 2016 | Wrong patient population: not infertile (preconception) |
| IRCT20160605028270N2 2020 | Wrong intervention: strict exercise intervention (no exercise allowed outside intervention) |
| ISRCTN03259732 2014 | Wrong patient population: not infertile (women planning pregnancy) |
| ISRCTN11081163 2016 | Wrong patient population: not infertile (preconception) |
| ISRCTN17222161 2017 | Other reason, trial superseded by another trial |
| Jaffar 2018 | Wrong study design: no RCT |
| Jamali 2016 | Wrong intervention: no preconception lifestyle advice; psychological and sexual counselling |
| Jiménez Tuñón 2017 | Wrong intervention: no preconception lifestyle advice; supplements: melatonin, myo‐inositol, folic acid, selenium |
| Kaya 2016 | Wrong study design: no RCT (interventional) |
| Kermack 2014 | Wrong intervention: no preconception lifestyle advice; strict diet intervention |
| Kermack 2017 | Wrong intervention: no preconception lifestyle advice; strict diet intervention |
| Kersten 2015 | Wrong intervention: preconception lifestyle advice not main component; tailored expected management and implementation |
| Kiel 2018 | Wrong intervention: no preconception lifestyle advice; strict exercise intervention (supervised HITT) |
| Kirca 2019 | Wrong intervention: no preconception lifestyle advice; strict exercise intervention (supervised yoga visits) |
| Li 2016 | Wrong study design: no RCT |
| Li 2017 | Wrong intervention: preconception lifestyle advice not main component (fertility education and counselling) |
| Lundgren 2016 | Wrong intervention: strict exercise intervention (supervised) |
| Maleki 2017a | Wrong intervention: strict exercise intervention (no exercise allowed outside intervention) |
| Maleki 2017b | Wrong intervention: no preconception lifestyle advice; strict exercise intervention (no exercise allowed outside intervention) |
| Maleki 2017c | Wrong intervention: no preconception lifestyle advice; strict exercise intervention (no exercise allowed outside intervention) |
| Maleki 2018 | Wrong intervention: strict exercise intervention (no exercise allowed outside intervention) |
| Maleki 2020 | Wrong intervention: strict exercise intervention (no exercise allowed outside intervention) |
| Mirghafourvand 2020 | Wrong patient population: not infertile |
| Moran 2011 | Wrong intervention: no preconception lifestyle advice; strict weight loss intervention and no minimal comparison group |
| Moran 2016b | Wrong intervention: no preconception lifestyle advice; strict weight loss intervention and no minimal comparison group |
| Mori 2009 | Wrong intervention: preconception lifestyle advice not main component (stress management) |
| Mumford 2020 | Wrong study design: no RCT |
| Naab 2019 | Wrong intervention: no preconception lifestyle advice (depression intervention) |
| Nayar 2017 | Wrong intervention: no preconception lifestyle advice; strict exercise intervention (supervised yoga visits) |
| Nayar 2018 | Wrong intervention: no preconception lifestyle advice; strict exercise intervention (supervised yoga visits) |
| NCT01509066 2012 | Wrong patient population: not infertile |
| NCT01566929 2012 | Wrong intervention: no preconception lifestyle advice; strict weight loss intervention with liquid formula diet |
| NCT01716429 2012 | Wrong patient population: solely PCOS |
| NCT01892111 2013 | Wrong patient population: solely PCOS |
| NCT01933633 2013 | Wrong intervention: no preconception lifestyle advice; strict exercise intervention (supervised) |
| NCT01952795 2013 | Wrong intervention: no preconception lifestyle advice; strict weight loss intervention |
| NCT01954498 2013 | Wrong intervention: no preconception lifestyle advice; strict diet intervention (walnut trial) |
| NCT02063256 2014 | Wrong study design: no RCT |
| NCT02432209 2015 | Wrong intervention: no minimal comparison group (lifestyle advice in both groups) |
| NCT02541487 2015 | Wrong study design: no RCT |
| NCT02648555 2016 | Wrong patient population: recurrent miscarriages |
| NCT02746601 2016 | Wrong study design: no RCT |
| NCT02752555 2016 | Wrong intervention: only lifestyle advice in control group (Intervention = Oral Carnitine) |
| NCT03050944 2017 | Wrong study design: no RCT (association) |
| NCT03343405 2017 | Wrong intervention: preconception lifestyle advice not main component (mind/body intervention) |
| NCT03348865 2017 | Wrong intervention: psychological counselling |
| NCT03475199 2018 | Wrong intervention: no preconception lifestyle advice; strict lifestyle intervention |
| NCT03553927 2018 | Wrong intervention: no preconception lifestyle advice; strict weight loss intervention |
| NCT03898037 2019 | Wrong intervention: no minimal comparison group (lifestyle advice in both groups compared to metformin) |
| NCT04002414 2019 | Wrong intervention: no preconception lifestyle advice; strict exercise intervention depending on activity level |
| NCT04273048 2020 | Wrong intervention: no preconception lifestyle advice; strict weight loss intervention |
| NCT04585581 2020 | Wrong patient population: not infertile (< 6 months) |
| Nieschlag 1998 | Wrong intervention: psychological counselling |
| Overdijkink 2018 | Wrong study design: no RCT |
| PACTR201611001683280 2016 | Wrong study design: no RCT |
| Pedro 2019 | Wrong patient population: people desiring to become pregnant |
| Poehl 1999 | Wrong study design: no RCT (and psychological counselling) |
| Psaros 2015 | Wrong study design: no RCT (and mind/body intervention) |
| Rasoulzadeh 2013 | Wrong intervention: preconception lifestyle advice not main component (psychological counselling) |
| Rasoulzadeh 2018 | Wrong intervention: preconception lifestyle advice not main component (psychological counselling) |
| RBR‐7by76r 2016 | Wrong intervention: no minimal comparison group (lifestyle advice in both groups) |
| Rothberg 2016 | Wrong intervention: no preconception lifestyle advice; strict weight loss intervention (Optifast) |
| Sant'Anna 2017 | Wrong intervention: no minimal comparison group |
| Schick 2019 | Wrong intervention: positive adjustment coping intervention |
| Shahrestani 2012 | Wrong intervention: mindfulness‐based cognitive group therapy (MBCT) |
| Sim 2012 | Wrong intervention: strict weight loss intervention (VLED) |
| Sim 2014 | Wrong intervention: strict weight loss intervention (VLED) |
| Skogsdal 2019 | Wrong patient population: preconception lifestyle advice |
| Tolahunase 2017 | Wrong intervention: no preconception lifestyle advice; strict exercise intervention (supervised yoga visits) |
| Tolahunase 2018 | Wrong intervention: no preconception lifestyle advice; strict exercise intervention (supervised yoga visits) |
| Tolahunase 2018a | Wrong intervention: no preconception lifestyle advice; strict exercise intervention (supervised yoga visits) |
| Tolahunase 2018b | Wrong intervention: no preconception lifestyle advice; strict exercise intervention (supervised yoga visits) |
| Tsagareli 2006 | Wrong study design: no RCT and strict weight loss intervention (Optifast) |
| Twigt 2012 | Wrong study design: no RCT (association) |
| UMIN000009034 2012 | Wrong study design: no RCT |
| UMIN000016168 2015 | Wrong intervention (and study population): preconception lifestyle advice not main component (fertility education) |
| UMIN000027424 2017 | Wrong study design: no RCT |
| Vause 2018 | Wrong intervention: preconception lifestyle advice not main component (fertility education intervention) |
HITT: XXX.
LGI: XXX.
MBCT: mindfulness‐based cognitive therapy.
PCOS: polycystic ovarian syndrome.
RCT: randomised controlled trial.
VLED: very low energy diet.
Characteristics of studies awaiting classification [ordered by study ID]
Chang 2006.
| Methods | Randomised controlled trial |
| Participants | 23 women with drinking problem waiting for evaluation appointments at the centre for reproductive medicine |
| Interventions | Brief face‐to‐face intervention to reduce alcohol consumption based on review of current drinking behaviour in combination with guide: "Personal Steps to a Healthy Choice: A Women’s Guide" |
| Outcomes | Reported behavioural changes in alcohol intake (drinks/drinking/d and percentage drinking days) |
| Notes | Not enough information on population to determine eligibility. Not sure if this study presents preliminary data of Rossi 2013. Corresponding author and other study authors contacted for more information. Awaiting reply after 2 reminders |
Jamebozorg 2018.
| Methods | Probably randomised controlled trial |
| Participants | 60 women with infertility (ovarian factor infertility) |
| Interventions | Traditional medicine–oriented diet and lifestyle recommendations |
| Outcomes | Clinical pregnancy (foetal heart rate on ultrasound) |
| Notes | Not enough information on randomisation procedure and intervention to determine eligibility at this point. Only abstract available. Corresponding author contacted for more information. Awaiting reply after 2 reminders |
Liu 2020.
| Methods | Probably randomised controlled trial |
| Participants | 120 men with infertility (women normal fertility) |
| Interventions | Complex lifestyle intervention: "Green Model" to improve ability to self‐manage lifestyle |
| Outcomes | Lifestyle behaviour (not further specified) |
| Notes | Not enough information on intervention and outcomes at this point. Corresponding author contacted for more information. Awaiting reply after 2 reminders |
Characteristics of ongoing studies [ordered by study ID]
Bivia‐Roig 2020.
| Study name | HLRP‐RCT |
| Methods |
Study design: parallel‐group randomised controlled trial Setting: single centre/hospital (Spain) |
| Participants | 94 women with diagnosed primary infertility and BMI between 25 kg/m² and 40 kg/m² |
| Interventions |
Control: routine care and unstructured minimal preconception lifestyle advice Standard treatment, which consists of regular gynaecological visits. The Reproduction Service gynaecologists will recommend healthy lifestyle habits and will give patients a document detailing a specific diet they should follow for weight loss Intervention: weight (weight, diet, and physical activity) "A 3‐month internet‐based program focusing on the promotion of healthy lifestyles. The treatment protocol comprises 9 modules which incorporate psychological strategies to promote healthy lifestyles by gradually changing eating and physical activity habits" |
| Outcomes | Ongoing pregnancy ("pregnancy with ultrasound visualisation of the gestational sac and heartbeat after 20 weeks of gestation" = primary outcome); reported behavioural changes in weight (BMI), diet (Mediterranean diet adherence measured with MEDAS), physical activity (measured with IPAQ). Additional outcomes specified in protocol paper: diet (measured with FFQ) and quality of life (measured with FertiQOL) |
| Starting date | February 2020 |
| Contact information | gemma.bivia@uchceu.es (PhD); juanfran@uchceu.es (PI) |
| Notes |
Status of study: ongoing: recruiting. Estimated study completion date on clinicaltrials.gov is December 2021 Other notes: additional outcomes described in the protocol but not on clinicaltrials.gov, including diet and quality of life |
Boedt 2019.
| Study name | PreLiFe |
| Methods |
Study design: parallel‐group randomised controlled trial Setting: multi‐centre/hospital (Belgium) |
| Participants | 460 heterosexual couples with infertility about to start a first IVF cycle (with or without ICSI) |
| Interventions |
Control: attention control Routine care + attention control programme: a mobile application with treatment information. This implies no advice on lifestyle Intervention: combination Routine care + a mobile preconception lifestyle programme (the PreLiFe‐programme) for 12 months or until an ongoing pregnancy is confirmed by ultrasound. The PreLiFe‐programme includes a mobile application (PreLiFe‐app) with the same treatment information as the attention control group in combination with a lifestyle programme. This includes tailored advice and skills training on diet and physical activity and mindfulness exercises. Additionally, couples will be offered interaction with a healthcare provider through text messages and telephone interaction in keeping with the concept of blended care |
| Outcomes | Ongoing pregnancy (a viable intrauterine pregnancy of at least 12 weeks' duration confirmed on ultrasound = primary outcome); any adverse event; miscarriage; reported behavioural changes in weight (BMI), diet (quality of diet and dietary pattern including vegetable and fruit intake measured with FFQ), and physical activity (moderate to vigorous PA measured with IPAQ); clinical pregnancy; time to pregnancy; quality of life (measured with FertiQOL) |
| Starting date | January 2019 |
| Contact information | tessy.boedt@kuleuven.be |
| Notes |
Status of study: ongoing; estimated completion date on clinicaltrials.gov is August 2021 Other notes: details extracted by ACV and MV because TB, ED, SLF, and CM are involved in this trial |
CTRI201908020997 2019.
| Study name | Effect of lifestyle modification and education on information needs and satisfaction level of infertile couples |
| Methods |
Study design: parallel‐group randomised controlled trial Setting: single centre/hospital (India) |
| Participants | 50 couples taking treatment for infertility |
| Interventions |
Control: routine care "Couples will take treatment as usual and recommended by physician apart from lifestyle modification advice" Intervention: combination Group 1: face‐to‐face counselling on lifestyle One‐to‐one/group training through videos, lectures/demonstrations, display of posters, and PowerPoint presentation on lifestyle Group 2: face‐to‐face counselling on lifestyle + self‐instruction manual (SIM) A customised, patient‐centred self‐instruction manual (SIM) to cater to needs of infertile couples developed based on baseline survey and felt needs of respondents. "It includes general information regarding reproductive system, causes of infertility (related to both male and female), various treatment options available, lifestyle modification approaches, psychological aspects, basic information for child adoption etc. Lifestyle changes are the non‐pharmacological approaches for behavior modifications to encourage positive changes in one’s life like eating right or physical activity. Dietary advice includes eating right potion (both quantity and quality) of food which includes all essential nutrients. Diet high in unsaturated fats, whole grains, vegetables, fruits, nuts and fish is advised. Limit intake caffeine, smoking, alcohol consumption, high glycemic index foods etc. Physical activity should be made part of daily life. Exercise of minimum 30 minutes is advised each day. Apart from this Yoga and meditation is also advised to couples" |
| Outcomes | Quality of life (measured with FertiQOL = primary outcome) and conception rate defined as the number of infertile women who get pregnant spontaneously or from ART during the study time period |
| Starting date | January 2020 |
| Contact information | drdavinderkaur89@gmail.com (PhD); dramarjeet56@gmail.com (PI) |
| Notes |
Status of study: ongoing: study authors replied by email 28 January 2021 that trial was still ongoing, and that no data are available that can be included in this review at this point. Estimated study completion date is after 1 year Other notes: additional information on intervention and definition of pregnancy would be valuable when study will be included |
CTRI202006025577 2020.
| Study name | A study to evaluate the effect of nursing management on emotional distress among infertile women |
| Methods |
Study design: parallel‐group randomised controlled trial Setting: single centre/hospital (India) |
| Participants | 400 women with diagnosed infertility and undergoing fertility treatment at Sri Guru Ram Das Medical College and Hospital |
| Interventions |
Control: routine care "Routine care and medical treatment given by doctors to infertile patients" Intervention: combination Nursing intervention for 6 months with progressive muscle relaxant exercises and lifestyle modification tips |
| Outcomes | Pregnancy outcome (not further specified) |
| Starting date | July 2020? |
| Contact information | getyashraj2006@gmail.com; harjittuppal3@gmail.com |
| Notes |
Status of study: ongoing: not yet recruiting (although date of first enrolment is noted as 1 July 2020). Estimated study completion date is after 2 years Other notes: additional information on intervention, definition of pregnancy, and start of study would be valuable when study will be included. Awaiting reply from study authors |
Dupont 2020.
| Study name | PEPCI |
| Methods |
Study design: parallel‐group randomised controlled trial (on the fly) Setting: multi‐centre/hospital (France) |
| Participants | 750 heterosexual couples with infertility or couples attending a visit at an ART reproductive centre |
| Interventions |
Control: routine care and unstructured minimal preconception lifestyle advice Routine care + a booklet about a French national nutrition and health programme dedicated to pregnancy Intervention: combination Routine care + multi‐disciplinary assessment to establish a baseline periconceptional profile + 2 to 3 tailored objectives on lifestyle negotiated with the couple if necessary: consultation with a psychiatrist, then psychologist follow‐up if required and needed. Addiction specialist physician consultation, then liaison nurse follow‐up if required. Endocrinologist consultation, then dietician follow‐up (3‐month supervised diet programme on web platform) if required. Actiphysician consultation and follow‐up (3‐month personalised PA programme on web platform) if required + personalized follow‐up from the multi‐disciplinary consultation |
| Outcomes | Reported behavioural changes in weight (BMI in kg/m²) and physical activity (measured with IPAQ); clinical pregnancy (evidence of gestational sac on ultrasound exam at 6 weeks (primary outcome) measured at the first ART attempt 3 to 12 months after initial visit) |
| Starting date | January 2018 |
| Contact information | charlotte.dupont@aphp.fr; rachel.levy@aphp.fr |
| Notes |
Status of study: ongoing: study author replied by email 19 January 2020 that PEPCI was still ongoing, and that no data are available that can be included in this review at this point. Estimated study completion data on clinicaltrials.gov is November 2021 Other notes: additional outcomes described in the protocol but not on clinical trials.gov, including reported behavioural changes in diet (dietary intake measured with SUVIMAX Questionnaire); quality of life (measured with Duke Questionnaire), and male factor infertility outcomes |
NCT03395067 2018.
| Study name | PRO‐FIV |
| Methods |
Study design: parallel‐group randomised controlled trial Setting: single centre/hospital (Spain) |
| Participants | 104 women with infertility and obesity (BMI ≥ 30 kg/m²) before IVF |
| Interventions |
Control: routine care Start of IVF immediately after randomisation Intervention: weight (weight, diet, and physical activity) "Multidisciplinary lifestyle counselling coupled with psycho therapeutical intervention. The aim is a weight loss of at least 10% in a 16‐week period of treatment based on a multidisciplinary approach and support groups, which includes diet, physical activity and psychological therapy. IVF will be started immediately after this period" |
| Outcomes | Live birth or ongoing pregnancy (live birth of a healthy baby in a non‐complicated pregnancy measured 10 months after start of IVF treatment = primary outcome); any adverse event (perinatal complications of mother and child); miscarriage (3 months after IVF); reported behavioural changes in weight (weight change in kg and BMI 4 months after intervention); birth weight and clinical pregnancy |
| Starting date | January 2018 |
| Contact information | gcasals@clinic.cat |
| Notes |
Status of study: ongoing; estimated completion date on clinicaltrials.gov is December 2020 Other notes: none |
NCT03908099 2019.
| Study name | Fit‐for‐Fertility |
| Methods |
Study design: parallel‐group randomised controlled trial Setting: multi‐centre/hospital (Canada) |
| Participants | 616 women with infertility (BMI ≥ 30 kg/m² or 27 kg/m² for Asian and Latin American), or BMI ≥ 27 kg/m² for women with PCOS |
| Interventions |
Control: routine care Start of usual fertility care immediately after randomisation. No additional information is provided to control participants on the topics of lifestyle habits, other than recommendations given to them by their gynaecologist and physicians working with participating fertility clinics Intervention: weight (diet, alcohol, physical activity, and smoking) First, Fit for Fertility programme for 6 months followed by usual fertility care for an additional 12 months. Individual sessions with dietician and kinesiologist every 6 to 12 weeks + telephone and email follow‐up via motivational counselling + 8 educational group sessions on addressing healthy lifestyle (healthy eating, sleep, alcohol and tobacco use, behaviour modification and motivation) (45 minutes) in combination with supervised exercise classes (walking, strength training, yoga, circuit training, step workout, and zumba) (45 minutes). Aim is to implement progressive and sustainable lifestyle changes |
| Outcomes | Live birth or ongoing pregnancy (cumulative incidence of live birth after 24 months (primary outcome) and viable pregnancy of ≥ 10 weeks' gestation). Any adverse event such as gestational diabetes, gestational hypertensive disorders, frequency of complications due to MAR procedures; miscarriage (rate of spontaneous miscarriage). Reported behavioural changes in weight (BMI), diet (nutrient intake (measured with FFQ), physical activity (physical activity behaviour measured with IPAQ, daily energy expenditure measured with fit‐bit and physical fitness measured with 6MWT), alcohol, smoking, and other substance abuse; birth weight; clinical pregnancy (pregnancy rate spontaneous and from MAR), and quality of life (measured with anxiety and depression scales) |
| Starting date | April 2019 |
| Contact information | farrah.jean‐denis.ciusse‐chus@ssss.gouv.qc.ca |
| Notes |
Status of study: ongoing: recruitment started in 1 hospital. Estimated study completion date on Clinicaltrials.gov is October 2023. Study author replied by email on 7 July 2020: "unfortunately, it would be too preliminary at this point to share any data. The trial is still ongoing and we plan to be able to publish the results in 2024‐2025" Other notes: none |
NCT04589793 2020.
| Study name | COLIFE |
| Methods |
Study design: parallel‐group randomised controlled trial Setting: multi‐centre?/hospital (Finland) |
| Participants | 780 couples with infertility and BMI between 27 kg/m² and 34.9 kg/m². Not specified if BMI counts for both partners |
| Interventions |
Control: routine care Conventional infertility treatment Intervention: combination "4 sessions of video‐mediated motivational interview, anthropometric measurements, laboratory tests and epigenetic samples. Lifestyle intervention includes physical activity, diet, sleep and stress" |
| Outcomes | Live birth rate (primary outcome); any adverse event (pregnancy and delivery complications including gestational diabetes and hypertension); miscarriage rate; reported behavioural changes in combined score from 1 to 4 (smoking + daily vegetable and fruit use > 500 g + BMI 19 to 27 kg/m² + 150 minutes of moderate exercise/week = primary outcome); reported behavioural changes in weight (weight reduction in kg = primary outcome and BMI), diet (dietary content and pattern measured with FinTerveys dietary questionnaire), alcohol (alcohol consumption measured with FinTerveys dietary questionnaire), and physical activity (minutes per week measured with IPAQ); birth weight; quality of life (measured with FertiQOL) |
| Starting date | Estimated starting date: November 2020 |
| Contact information | maritta.poyhonen‐alho@hus.fi |
| Notes |
Status of study: ongoing: not yet recruiting. Estimated study completion date on clinicaltrials.gov is September 2025 Other notes: additional information on intervention would be valuable when study will be included. Awaiting reply from study authors |
Timmermans 2019.
| Study name | TOP‐mums |
| Methods |
Study design: parallel‐group randomised controlled trial Setting: region (South of Limburg, The Netherlands) |
| Participants | 112 overweight or obese women planning to conceive within 1 year |
| Interventions |
Control: routine care Access to the general practitioner and child wish consultations by a midwife. In addition, ART as part of care as usual for subfertile women according to the Dutch infertility guidelines Intervention: combination Multi‐disciplinary lifestyle intervention from preconception to 1 year postpartum. Lifestyle programme based on lifestyle habits measured with nutritional diary and activity tracker and developed by medical background lifestyle coach trained in motivational interviewing + mHealth Coaching "Smarter Pregnancy" = digital tailored coaching on nutrition and lifestyle, 3 digital posts/week, advice, seasonal recipes, and additional questions addressing lifestyle behaviour during pregnancy (26 weeks) + psychological guidance on eating behaviour if necessary (2 to 3 times a week for 4 months) + personal dietary guidance by dietician every 1 or 2 months + physical activity programme (with physiotherapist) |
| Outcomes | Live birth or ongoing pregnancy; any adverse event (perinatal complications of mother and child); miscarriage; reported behavioural changes in weight (difference in weight in kg from baseline to 6 weeks postpartum = primary outcome), smoking (smoking cessation and biochemical verification of tobacco use), diet (dietary habits), and physical activity (physical activity habits); birth weight; gestational age; time to pregnancy; quality of life |
| Starting date | July 2016 |
| Contact information | a.vreugdenhil@mumc.nl |
| Notes |
Status of study: ongoing: study author replied by email on 26 May 2020 that trial was still ongoing, and that no data are available that can be included in the review at this point. Estimated study completion date on Clinicaltrials.gov is December 2021 Other notes: inclusion of fertile couples and couples with infertility. Intervention from preconception to 1 year postpartum |
6MWT: 6‐Minute Walk test.
ART: assisted reproductive technology.
BMI: body mass index.
FertiQOL: Fertility Quality of Life tool.
FFQ: Food Frequency Questionnaire.
ICSI: intracytoplasmic sperm injection.
IPAQ: International Physical Activity Questionnaire.
IVF: in vitro fertilisation.
MEDAS: XXX.
Differences between protocol and review
Differences between 2010 and 2020 version
For the 2020 update, the review authors agreed to change the title using the now preferred term "infertility" (Zegers‐Hochschild 2017).
For the 2020 update, the background and methods sections were updated to meet current Cochrane standards.
Update of Background section
Addition of new references (WHO guidelines, ESHRE guidelines, Cochrane systematic reviews)
-
Update of lifestyle factors in "How the intervention might work"
Update of diet part based on new studies and WHO recommendations; focus on healthy diet rather than on vitamin or mineral supplement use, removal of vitamin D recommendation, addition of iron recommendation, and addition of evidence regarding antioxidants for fertility
Update of "Why is it important to do this review" based on new guidelines and studies
Update of Methods section
Update of types of studies: addition of randomised cross‐over studies and cluster‐randomised trials based on advice from Cochrane Belgium
Update of types of participants: extension of definition, and exclusion of women with PCOS based on advice from CGFG
Update of types of intervention: extension of definition based on other Cochrane systematic reviews and adapted based on new evidence in updated background on "How the intervention might work"
Update of types of outcome measures: addition of ongoing pregnancy as primary effectiveness outcome, addition of safety outcomes, update of secondary outcomes based on updated background, based on advice from CGFG, and taking into account the core outcome measures for infertility trials
Update of search methods to meet current Cochrane standards
Update of Data collection and analysis to meet current Cochrane standards: regarding measures of treatment effects of binary outcomes, the review authors agreed on RR instead of OR, as one of the three considerations highlighted by the Cochrane Handbook for Systematic Reviews of Interventions is ease of consideration; regarding data synthesis, new strata are defined based on the updated Background, and the review authors agreed to use a random‐effects model instead of a fixed‐effect model because large variation between studies is expected (in populations and/or interventions), subgroup analyses are updated based on current evidence and interests, and the sensitivity analysis is updated to reflect current Cochrane standards
Addition of description of overall body of evidence: "Summary of findings" table
Update of Results section
Six studies were added to the update of this review. Description of studies, risk of bias, and effects of interventions sections therefore were updated. GRADE assessment was performed on the outcomes, and "Summary of findings" tables were added to the review
Differences between 2020 protocol and review
-
As advised by the CGFG, we adapted our safety outcomes to the most clinically relevant
Any adverse event (including bleeding, drug reactions, neonatal mortality, congenital abnormality) reported either as a composite measure or separately was redefined: any adverse event in men or women with infertility related to the intervention reported either as a composite measure or separately (including gestational diabetes and hypertension)
Pregnancy loss (miscarriage, ectopic pregnancy, stillbirth, and other (e.g. pregnancy of unknown location, termination of pregnancy) was redefined: miscarriage, defined as spontaneous loss of an intrauterine pregnancy prior to 22 completed weeks of gestation)
We added details (definitions, scales, time points) of acceptable outcome measures on reported behavioural changes. Decision rules, clinical relevance, and guidance documents were used
If methods were planned but were not implemented, reasons were added
In data synthesis, we added that a narrative review summary format was chosen as the method for synthesis when it was not possible to conduct meta‐analyses; we added information on time points of outcomes and how we identified outcomes for inclusion in our synthesis
Contributions of authors
Main review author
Ms Boedt
Content experts
Ms Boedt, Ms Vercoe, Prof Matthys, Ms Dancet, and Prof Lie Fong
Methodological expert
Ms Vanhove
Drafting the protocol
Ms Boedt with help from Ms Vanhove (for the methods) and Ms Vercoe (for the background), with editing by Prof Matthys, Ms Dancet, and Prof Lie Fong
Developing a search strategy
Ms Boedt and Ms Vanhove, with help from the Cochrane Gynaecology and Fertility Group Information Specialist
Searching for trials
The Cochrane Gynaecology and Fertility Group Information Specialist and Ms Boedt
Obtaining copies of trials
Ms Boedt, Ms Vanhove, and Ms Vercoe
Selecting trials
Ms Boedt, Ms Vanhove, and Ms Vercoe
Extracting data from trials
Ms Boedt, Ms Vanhove, and Ms Vercoe
Assessing risk of bias
Ms Boedt, Ms Vanhove, and Ms Vercoe
Entering data into RevMan
Ms Boedt
Carrying out the analysis
Ms Boedt, with help from Ms Vanhove
Interpreting the analysis
Ms Boedt, with help from Ms Vanhove, Prof Matthys, Ms Dancet, and Prof Lie Fong
Drafting the final review
Ms Boedt, with help from Ms Vanhove, Ms Vercoe, Prof Matthys, Ms Dancet, and Prof Lie Fong
Updating the review
Ms Boedt, with help from Ms Vanhove, Ms Vercoe, Prof Matthys, Ms Dancet, and Prof Lie Fong
Sources of support
Internal sources
KU Leuven, Belgium
External sources
-
Research Foundation Flanders, Belgium
Research grant: Evaluation of a Mobile Preconception Lifestyle Programme in Couples Undergoing In Vitro Fertilisation (PreLiFe‐RCT)
Declarations of interest
TB, ACV, MV, CM, ED, and SLF declare that they do not have any conflicts of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Alibeigi 2020 {published data only (unpublished sought but not used)}
- Alibeigi Z, Jafari-Dehkordi E, Kheiri S, Nemati M, Mohammadi-Farsani G, Tansaz M. The impact of traditional medicine-based lifestyle and diet on infertility treatment in women undergoing assisted reproduction: a randomized controlled trial. Complementary Medicine Research 2020;27:230-41. [DOI: ] [DOI] [PubMed] [Google Scholar]
- IRCT2017013032245N2. The impact of diet based on Iranian Traditional Medicine on IVF/ICSI outcomes of infertile women. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2017013032245N2 (first received 8 March 2017).
Belan 2019 {published data only (unpublished sought but not used)}
- Belan M, Carranza-Mamane B, AinMelk Y, Pesant MH, Duval K, Jean-Denis F, et al. A lifestyle intervention targeting women with obesity and infertility improves their fertility outcomes, especially in women with PCOS: a randomized controlled trial. Fertility and Sterility 2019;112 Suppl(3):e40. [Google Scholar]
- Belan M, Carranza-Mamane B, AinMelk Y, Pesant MH, Duval K, Jean-Denis F, et al. Lifestyle modifications in male partners of subfertile couples in which the spouse is obese improves the chances of the couple to conceive. Fertility and Sterility 2019;112(3):e213. [DOI: 10.1016/j.fertnstert.2019.07.670] [DOI] [PubMed] [Google Scholar]
- Belan M, Carranza-Mamane B, Pesant MH, AinMelk Y, Duval K, Jean-Denis F, et al. Male partners of subfertile couples in which the spouse is obese display adverse weight and lifestyle associated with reduced sperm quality. Obesity Research and Clinical Practice 2019;13(3):226-32. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Belan M, Duval K, Jean-Denis F, Pesant MH, Langlois MF, Ainmelk Y, et al. Impacts of lifestyle and anthropometric changes in male partners of obese infertile women on couples' fertility - preliminary results from a cohort study. In: Endocrine Reviews. Vol. 36. 2015.
- Belan M. A lifestyle program targeting women with obesity and infertility improves their fertility: a randomized controlled trial. In: Journal of the Endocrine Society. Vol. 3. 2019:11.
- Duval K, Belan M, Jean-Denis F, Baillargeon J. An interdisciplinary lifestyle intervention improves clinically relevant fertility outcomes in obese infertile women – preliminary results of a randomized controlled trial. Canadian Journal of Diabetes 2015;39(6):532-3. [DOI: 10.1016/j.jcjd.2015.09.028] [DOI] [Google Scholar]
- Duval K, Belan M, Jean-Denis F, Baillargeon J. An interdisciplinary lifestyle intervention improves clinically relevant fertility outcomes in obese infertile women - preliminary results. Fertility and Sterility 2015;104 Suppl(3):e97. [Google Scholar]
- Duval K, Langlois MF, Carranza-Mamane B, Pesant MH, Hivert MF, Poder TG, et al. The Obesity-Fertility Protocol: a randomized controlled trial assessing clinical outcomes and costs of a transferable interdisciplinary lifestyle intervention, before and during pregnancy, in obese infertile women. BMC Obesity 2015;2(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01483612. Evaluation of clinical outcomes and costs of a lifestyle intervention in obese infertile women. https://clinicaltrials.gov/show/NCT01483612 (first received 1 December 2011).
- Rouissi M, Jean-Denis F, Belan M, Baillargeon JP. A preconception lifestyle intervention improves some gestational outcomes and neonatal markers of adiposity in women with obesity and infertility. Fertility and Sterility 2020;114(3):e466-7. [Google Scholar]
Hughes 2000 {published data only (unpublished sought but not used)}
- Hughes EG, Beecroft ML, Lamont D, Rice S, Wilson D, Freebury M, et al. A randomised controlled trial of a 'State-of-Change' smoking cessation intervention for subfertile and pregnant patients. Fertility and Sterility 1999;72(3 Suppl 1):S61-2. [Google Scholar]
- Hughes EG, Lamont DA, Beecroft ML, Wilson DMC, Brennan BG, Rice SC. Randomized trial of a 'stage-of-change' oriented smoking cessation intervention in infertile and pregnant women. Fertility and Sterility 2000;74(3):498-503. [DOI] [PubMed] [Google Scholar]
Mutsaerts 2016 {published and unpublished data}
- Den Harink T, Gemke RBJB, Hoek A, Groen H, Blom NA, Roseboom TJ, et al. Preconception lifestyle intervention in obese women improves echocardiographic indices of cardiovascular function in their offspring: follow up of a randomised controlled trial. Cardiology in the Young 2019;29(Suppl 1):S11-2. [Google Scholar]
- Karsten MD, Oers AM, Groen H, Mutsaerts MA, Poppel MN, Geelen A, et al. Determinants of successful lifestyle change during a 6-month preconception lifestyle intervention in women with obesity and infertility. European Journal of Nutrition 2019;58(6):2463-75. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol BWJ, Van Oers AM, Mutsaerts MAQ, Land JA, Groen H, Hoek A. Effects of preconceptional weight loss on maternal and fetal outcome in obese subfertile women. Journal of Paediatrics and Child Health 2015;51:36. [Google Scholar]
- Mutsaerts MA, Van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WK, Perquin DA, et al. Randomized trial of a lifestyle program in obese infertile women. New England Journal of Medicine 2016;374(20):1942-53. [DOI] [PubMed] [Google Scholar]
- Mutsaerts MA, Van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WK, Perquin DA, et al. Randomized trial of a lifestyle program in obese infertile women. New England Journal of Medicine 2018;378(26):2546. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Mutsaerts MAQ, Groen H, ter Bogt NCW, Bolster JHT, Land JA, Bemelmans WJE, et al. The LIFESTYLE study: costs and effects of a structured lifestyle program in overweight and obese subfertile women to reduce the need for fertility treatment and improve reproductive outcome. A randomised controlled trial. BMC Women's Health 2010;10(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaerts MAQ, Van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WKH, Perquin DAM, et al. The effectiveness of a structured lifestyle program in overweight and obese subfertile women. Preliminary data from a randomised controlled trial (LIFEstyle study). Human Reproduction 2014;29(Suppl 1):179. [Google Scholar]
- Mutsaerts MAQ. Randomized trial of a lifestyle program in obese infertile women. Nederlands Tijdschrift Voor Geneeskunde 2017;161(6):916. [PubMed] [Google Scholar]
- NTR1530. Lifestyle [Costs and effects of a structured lifestyle program in overweight and obese subfertile couples to prevent unnecessary treatment and improve reproductive outcome]. https://www.trialregister.nl/trial/1461 (first received 16 November 2008).
- Dammen L, Bush NR, Rooij S, Mol BW, Mutsaerts M, van Oers A et al. A lifestyle intervention randomized controlled trial in obese women with infertility improved body composition among those who experienced childhood adversity. Stress and Health 2020;37:93–102. [DOI: 10.1002/smi.2976] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammen L, Wekker V, Rooij SR, Mol BW, Groen H, Hoek A, et al. The effects of a pre-conception lifestyle intervention in women with obesity and infertility on perceived stress, mood symptoms, sleep and quality of life. PLoS One 2019;14(2):e0212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammen L, Wekker V, Oers A, Mutsaerts M, Painter R, Zwinderman A, et al. Effect of a lifestyle intervention in obese infertile women on cardiometabolic health and quality of life. Obesity Facts 2017;10(Suppl 1):3-4. [DOI: 10.1159/000468958] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammen L, Wekker V, Oers AM, Mutsaerts MA, Painter RC, Zwinderman AH, et al. Effect of a lifestyle intervention in obese infertile women on cardiometabolic health and quality of life: a randomized controlled trial. PLoS One 2018;13(1):e0190662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beek C, Hoek A, Painter RC, Gemke RJ, Poppel MN, Geelen A, et al. Women, their Offspring and iMproving lifestyle for Better cardiovascular health of both (WOMB project): a protocol of the follow-up of a multicentre randomised controlled trial. BMJ Open 2018;8(1):e016579. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elten TM, Karsten MD, Geelen A, Gemke RJ, Groen H, Hoek A, Poppel MN, et al. Preconception lifestyle intervention reduces long term energy intake in women with obesity and infertility: a randomised controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2019;16(1):3. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elten TM, Karsten MD, Geelen A, Oers AM, Poppel MN, Groen H, et al. Effects of a preconception lifestyle intervention in obese infertile women on diet and physical activity: a secondary analysis of a randomized controlled trial. PLoS One 2018;13(11):e0206888. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elten TM, de Beek C, Geelen A, Gemke RJ, Groen H, Hoek A, et al. Preconception lifestyle and cardiovascular health in the offspring of overweight and obese women. Nutrients 2019;11(10):2446. [DOI: 10.3390/nu11102446] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oers A, Groen H, Mutsaerts M, Burggraaff J, Kuchenbecker W, Perquin D, et al. Is there a different effect of lifestyle intervention in subgroups of infertile obese women? Prespecified subgroup analyses of the LIFEstyle randomized controlled trial. Human Reproduction 2016;31(Supp1):29. [Google Scholar]
- Oers A, Mutsaerts M, Burggraaff J, Kuchenbecker W, Perquin D, Koks C, et al. Effects of preconceptional weight loss in subfertile obese women on maternal and fetal outcome. American Journal of Obstetrics and Gynecology 2015;212(Suppl 1):S3. [Google Scholar]
- Oers AM, Groen H, Mutsaerts MA, Burggraaff JM, Kuchenbecker WK, Perquin DA, et al. Effectiveness of lifestyle intervention in subgroups of obese infertile women: a subgroup analysis of a RCT. Human Reproduction 2016;31(12):2704-13. [DOI] [PubMed] [Google Scholar]
- Oers AM, Mutsaerts MA, Burggraaff JM, Kuchenbecker WK, Perquin DA, Koks CA, et al. Association between periconceptional weight loss and maternal and neonatal outcomes in obese infertile women. PLoS One 2018;13(3):e0192670. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oers AM, Mutsaerts MA, Burggraaff JM, Kuchenbecker WK, Perquin DA, Koks CA, et al. Cost-effectiveness analysis of lifestyle intervention in obese infertile women. Human Reproduction 2017;32(7):1418-26. [DOI: 10.1093/humrep/dex092] [DOI] [PubMed] [Google Scholar]
- Oers AM, Mutsaerts MA, Burggraaff JM, Kuchenbecker WK, Perquin DA, Koks CA, et al. Cost-effectiveness of a structured lifestyle program in overweight and obese subfertile women. Preliminary data from a randomised controlled trial the LIFEstyle study. Human Reproduction 2014;29(Suppl 1):354-5. [Google Scholar]
- Wekker V, Karsten MD, Painter RC, De Beek C, Groen H, Mol BW, et al. A lifestyle intervention improves sexual function of women with obesity and infertility: a 5 year follow-up of a RCT. PLoS One 2018;13(10):e0205934. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekker V, Karsten MDA, Painter RC, de Beek C, Groen H, Mol BWJ, et al. Sexual function in obese infertile women 5-6 years after a preconception lifestyle intervention. International Journal of Gynecology and Obstetrics 2018;143(Suppl 3):402. [DOI: ] [Google Scholar]
- Wekker V, Dammen L, Oers AM, Mutsaerts MAQ, Painter RC, Zwinderman AH, et al. Effect of a lifestyle intervention in obese infertile women on cardiometabolic health and quality of life: results of a randomised controlled trial. Human Reproduction 2017;32(Suppl 1):i28-9. [Google Scholar]
Ng 2018 {published data only (unpublished sought but not used)}
- ISRCTN89523555. Assessing the effectiveness of a personalised lifestyle coaching phone application in changing behaviours of women planning a pregnancy [iLAN: Impact of a Personalised Lifestyle coaching phone ApplicatioN in modifying peri-conceptional behaviours: a randomised controlled trial]. http://www.isrctn.com/ISRCTN89523555 (received 12 February 2018).
- Ng KY, Wellstead S, Cheong Y, Macklon N. A randomised controlled trial of a personalised lifestyle coaching application in modifying periconceptional behaviours in women suffering from reproductive failures (iPLAN trial). BMC Women's Health 2018;18(1):196. [DOI: 10.1186/s12905-018-0689-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KYB, Steegers-Theunissen R, Willemsen SP, Wellstead S, Cheong Y, Macklon N. RCT of the impact of the online lifestyle coaching platform 'Smarter Pregnancy' on modifying peri-conceptual behaviours in women presenting with subfertility or recurrent miscarriages. Human Reproduction 2019;34(Suppl 1):i336. [Google Scholar]
Oostingh 2020 {published and unpublished data}
- NTR4150. Erasmus MC Care innovation for a healthy pregnancy. Efficacy of "Smarter Pregnant", an interactive food and lifestyle coaching program on the mobile phone. http://www.who.int/trialsearch/Trial2.aspx?TrialID=NTR4150 (first received 19 August 2013).
- Oostingh EC, Koster MP, Dijk MR, Willemsen SP, Steegers EA, Laven JS, et al. Improvement of periconception nutrition and lifestyle behaviors using the mHealth program smarter pregnancy: a randomized controlled trial. Reproductive Sciences 2018;25(1):197A. [Google Scholar]
- Oostingh EC, Koster MPH, Dijk MR, Willemsen SP, Broekmans FJM, Hoek A, et al. First effective mHealth nutrition and lifestyle coaching program for subfertile couples undergoing in vitro fertilization treatment: a single-blinded multicenter randomized controlled trial. Fertility and Sterility 2020;114(5):945-54. [DOI: 10.1016/j.fertnstert.2020.04.051] [DOI] [PubMed] [Google Scholar]
- Oostingh EC, Ophuis RH, Koster MPH, Polinder S, Lingsma HF, Laven JSE, et al. Mobile health coaching on nutrition and lifestyle behaviors for subfertile couples using the smarter pregnancy program: model-based cost-effectiveness analysis. JMIR mHealth uHealth 2019;7(10):e13935. [DOI: 10.2196/13935] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegers-Theunissen RP. Preconceptional personalised mHealth lifestyle coaching: first results of a randomized controlled trial in couples undergoing IVF/ICSI treatment. Human Reproduction 2018;33(Suppl 1):75. [DOI: ] [Google Scholar]
- Dijk MR, Huijgen NA, Willemsen SP, Laven JS, Steegers EA, Steegers-Theunissen RP. Impact of an mHealth platform for pregnancy on nutrition and lifestyle of the reproductive population: a survey. JMIR mHealth and uHealth 2016;4(2):e53. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk MR, Koster MPH, Oostingh EC, Willemsen SP, Steegers EAP, Steegers-Theunissen RP. A mobile app lifestyle intervention to improve healthy nutrition in women before and during early pregnancy: single-center randomized controlled trial. Journal of Medical Internet Research 2020;22(5):e15773. [DOI: 10.2196/15773] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk MR, Oostingh EC, Koster MP, Willemsen SP, Laven JS, Steegers-Theunissen RP. The use of the mHealth program Smarter Pregnancy in preconception care: rationale, study design and data collection of a randomized controlled trial. BMC Pregnancy and Childbirth 2017;17(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossi 2013 {published data only (unpublished sought but not used)}
- Chang G, Fisher ND, Hornstein MD, Jones JA, Hauke SH, Niamkey N, et al. Brief intervention for women with risky drinking and medical diagnoses: a randomized controlled trial. Journal of Substance Abuse Treatment 2011;41(2):105-14. [DOI: 10.1016/j.jsat.2011.02.011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarinis V, Caplan J, Chang G. Brief intervention for risk-drinking women: a mixed methods analysis of content and process. American Journal on Addictions 2013;22(1):67-74. [DOI: 10.1111/j.1521-0391.2013.00331.x] [DOI] [PubMed] [Google Scholar]
- NCT00846638. Screening and brief intervention for problem drinking women. https://clinicaltrials.gov/show/NCT00846638 (first received 19 February 2009).
- Rossi BV, Chang G, Berry K, Mark HD, Missmer SA. In vitro fertilization (IVF) outcomes and alcohol consumption in risk drinkers: effects of a randomized intervention. Fertility and Sterility 2010;94(4):S224. [Google Scholar]
- Rossi BV, Chang G, Berry KF, Hornstein MD, Missmer SA. In vitro fertilization outcomes and alcohol consumption in at-risk drinkers: the effects of a randomized intervention. American Journal on Addictions 2013;22(5):481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12610000631000 2010 {published data only}
- ACTRN12610000631000. Fertility acupuncture clinical trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610000631000 (first received 9 December 2009).
Aflatoonian 2014 {published data only}
- Aflatoonian A, Arabjahvani F, Eftekhar M, Sayadi M. Effect of vitamin D insufficiency treatment on fertility outcomes in frozen-thawed embryo transfer cycles: a randomized clinical trial. Iranian Journal of Reproductive Medicine 2014;12(9):595-600. [PMC free article] [PubMed] [Google Scholar]
Agricola 2014 {published data only}
- Agricola E, Pandolfi E, Gonfiantini MV, Gesualdo F, Romano M, Carloni E, et al. A cohort study of a tailored web intervention for preconception care. BMC Medical Informatics and Decision Making 2014;14(101088682):33. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Asemi 2014 {published data only}
- Asemi Z, Samimi M, Tabassi Z, Esmaillzadeh A. The effect of DASH diet on pregnancy outcomes in gestational diabetes: a randomized controlled clinical trial. European Journal of Clinical Nutrition 2014;68(4):490-5. [DOI: ] [DOI] [PubMed] [Google Scholar]
Ashcroft 1997 {published data only}
- Ashcroft SA, Hinks JA, Jenkins JM. The value of patient education by nurses to improve folate uptake during IVF treatment. Journal of Reproduction and Fertility 1997;19:19-20. [Google Scholar]
Bai 2019 {published data only}
- Bai CF, Cui NX, Xu X, Mi GL, Sun JW, Shao D et al. Effectiveness of two guided self-administered interventions for psychological distress among women with infertility: a three-armed, randomized controlled trial. Human Reproduction 2019;34(7):1235-48. [DOI] [PubMed] [Google Scholar]
Bailey 2015 {published data only}
- Bailey S, Bailey C, Boivin J, Cheong Y, Reading I, Macklon N. A feasibility study for a randomised controlled trial of the Positive Reappraisal Coping Intervention, a novel supportive technique for recurrent miscarriage. BMJ Open 2015;5(4):e007322. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbour 2020 {published data only (unpublished sought but not used)}
- Barbour A, Bernard A, Madeira J, Lindheim S, Goodman L. The impact of an interactive e-learning platform on patient comprehension regarding infertility treatment: a randomized clinical trial. Fertility and Sterility 2020;114(Suppl3):e25-6. [Google Scholar]
Becker 2015 {published data only}
- Becker GF, Passos EP, Moulin CC. Short-term effects of a hypocaloric diet with low glycemic index and low glycemic load on body adiposity, metabolic variables, ghrelin, leptin, and pregnancy rate in overweight and obese infertile women: a randomized controlled trial. American Journal of Clinical Nutrition 2015;102(6):1365-72. [DOI] [PubMed] [Google Scholar]
Beerendonk 1996a {published data only}
- Beerendonk C, Derkx F, Schellekens A, Hop W, Dop P. The influence of dietary sodium restriction on renal and ovarian renin and prorenin production during ovarian stimulation. Human Reproduction 1996;11(5):956-61. [DOI] [PubMed] [Google Scholar]
Beerendonk 1996b {published data only}
- Beerendonk C, Scheepers H, Oostsdam E, Dop P. Anxiety during an IVF procedure the influence of dietary sodium restrictions, several infertility characteristics and early pregnancy. Human Reproduction 1996;11(Suppl 1):162. [Google Scholar]
Beerendonk 1999 {published data only}
- Beerendonk C, Hendriks J, Scheepers H, Braat D, Merkus J, Oostdam B, et al. The influence of dietary sodium restriction on anxiety levels during an in vitro fertilization procedure. Journal of Psychosomatic Obstetrics & Gynecology 1999;20(2):97-103. [DOI] [PubMed] [Google Scholar]
Bell 2000 {published data only}
- Bell R, Palma S. Antenatal exercise and birthweight. Australian & New Zealand Journal of Obstetrics & Gynaecology 2000;40(1):70-3. [DOI] [PubMed] [Google Scholar]
Bernard 2020 {published data only (unpublished sought but not used)}
- Bernard A, Barbour A, Madeira J, Lindheim S, Goodman L. Emotional well-being during fertility treatment: a randomized controlled trial to evaluate the use of an online learning platform as resource. Fertility and Sterility 2020;114(Suppl 3):e449. [Google Scholar]
Bisht 2018 {published data only}
- Bisht S, Chaurasiya P, Khetan RK, Tolahunase M, Dada R. Sperm DNA damage: consequences of the impact of yogic cognitive behavior practices. Andrology 2018;6:75-6. [DOI: ] [Google Scholar]
Bodin 2017 {published data only}
- Bodin M, Tyden T, Kall L, Larsson M. The effect of introducing Reproductive Life Plan-based counselling during men's sexual health visits: a randomized controlled trial. Human Reproduction 2017;32:i395-6. [Google Scholar]
Bodin 2018 {published data only}
- Bodin M, Tyden T, Kall L, Larsson M. Can Reproductive Life Plan-based counselling increase men's fertility awareness? Upsala Journal of Medical Sciences 2018;123(4):255-63. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 2016 {published data only}
- Carpenter Cl, Robbins W, Henning SM. Dietary compensation in randomized nutrition trials: can we identify and adjust? FASEB Journal 2016;30(Suppl 1):1153-61. [Google Scholar]
Chan 2012 {published data only}
- Chan CHY, Chan CLW, Ng EHY, Ho PC, Chan THY, Lee GL et al. Incorporating spirituality in psychosocial group intervention for women undergoing in vitro fertilization: a prospective randomized controlled study. Psychology and Psychotherapy 2012;85(4):356-73. [DOI: ] [DOI] [PubMed] [Google Scholar]
Chan 2015 {published data only}
- Chan C, Wong S, Tam M. Effectiveness of a self-help integrative Body-mind-spirit intervention (I-BMS) in reducing infertile women's anxiety during their in vitro fertilization (IVF) treatment result awaiting period. Fertility and Sterility 2015;104(3):e358. [Google Scholar]
Chavarro 2016 {published data only}
- Chavarro JE, Rich-Edwards JW, Gaskins AJ, Farland LV, Terry KL, Cuilin Z, et al. Contributions of the Nurses' Health Studies to Reproductive Health Research. American Journal of Public Health 2016;106(9):1669-76. [DOI: 10.2105/AJPH.2016.303350] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensen 2017 {published data only}
- Christensen P, Cloherty J, Ryan H, Downer R, Conway U, Lowe E, et al. Intervention to reduce sperm DNA damage prior to fertility treatment. Human Reproduction 2017;32:i170-1. [Google Scholar]
Clark 2000 {published data only}
- Clark AM, Roberts B, Galletly C, Tomlinson L, Norman RJ. Maximizing weight loss in the overweight infertile patient - a prospective randomized controlled trial. Human Reproduction 2000;15:65-6. [Google Scholar]
Clifton 2017 {published data only}
- Clifton J. A randomized pilot trial: an internet-based mind/body intervention to mitigate anxiety in women experiencing infertility. Dissertation Abstracts International: Section B: The Sciences and Engineering 2017;77(10).
Clifton 2018 {published data only}
- Clifton J, Parent J, Worrall G, Seehuus M, Domar A. A randomized pilot trial update: an internet-based mind/body intervention to mitigate distress in women experiencing infertility. Fertility and Sterility 2018;110(4):e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Connolly 1993 {published data only}
- Connolly KJ, Edelmann RJ, Barlett H, Cooke ID, Lenton E, Pike S. An evaluation of counselling for couples going undergoing treatment for in vitro fertilization. Human Reproduction 1993;8:1332-8. [DOI] [PubMed] [Google Scholar]
CTRI201711010514 2017 {published data only}
- CTRI201711010514. To study the impact of counseling with yoga - based stress management in women undergoing assisted reproduction. http://ctri.icmr.org.in/ (first received 2017).
Dancet 2019 {published data only}
- Dancet EAF, D'Hooghe TM, Dreischor F, Wely M, Laan ETM, Lambalk CB, et al. The 'Pleasure & Pregnancy' web-based interactive educational programme versus expectant management in the treatment of unexplained subfertility: protocol for a randomised controlled trial. BMJ Open 2019;9(7):e025845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Domar 2009 {published data only}
- Domar AD, Backman K, Alper MM, Berger BM, Wiegand B, Nikolovski J. The impact of group mind/body participation on pregnancy rates in IVF patients: a randomized controlled trial. Fertility and Sterility 2009;92(3):2. [Google Scholar]
Domar 2011 {published data only}
- Domar AD, Rooney KL, Wiegand B, Orav EJ, Alper MM, Berger BM, et al. Impact of a group mind/body intervention on pregnancy rates in IVF patients. Fertility and Sterility 2011;95(7):2269-73. [DOI] [PubMed] [Google Scholar]
Domar 2019 {published data only}
- Domar AD, Jasulaitis L, Jasulaitis S, Grill EA, Uhler ML. The impact of the fertistrong app on anxiety and depression in men. Fertility and Sterility 2019;112(3):e379. [Google Scholar]
Dortaj 2016 {published data only}
- Dortaj A, Mehdizadeh A, Dortaj H, Bahari H. Effectiveness of mindfulness-based cognitive therapy on improvement of stress and infertility in infertile women undergoing IVF. Journal of Reproduction and Infertility 2016;17(2):264. [Google Scholar]
DRKS00017554 2019 {published data only}
- DRKS00017554. The effect of counselling on preconceptional care knowledge, attitude and lifestyle behaviors in Turkish women: a randomized controlled trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00017554 (first received 2019).
Einarsson 2017a {published data only}
- Einarsson S, Bergh C, Friberg B, Pinborg A, Klajnbard A, Karlstrom PO, et al. Weight reduction intervention for obese infertile women prior to In vitro fertilisation: a randomised controlled trial. Human Reproduction 2017;32:i29. [DOI] [PubMed] [Google Scholar]
Einarsson 2017b {published data only}
- Einarsson S, Bergh C, Friberg B, Pinborg A, Klajnbard A, Karlstrom PO, et al. Weight reduction intervention for obese infertile women prior to IVF: a randomized controlled trial. Human Reproduction 2017;32(8):1621-30. [DOI] [PubMed] [Google Scholar]
Einarsson 2018 {published data only}
- Einarsson S, Christina B, Kluge L, Thurin-Kjellberg A. The effect of weight intervention on obstetric and neonatal outcome in obese women scheduled for IVF treatment. Human Reproduction 2018;33:320. [DOI: ] [Google Scholar]
Einarsson 2019 {published data only}
- Einarsson S, Bergh C, Kluge L, Thurin-Kjellberg A. No effect of weight intervention on perinatal outcomes in obese women scheduled for in vitro fertilization treatment. Acta Obstetricia et Gynecologica Scandinavica 2019;98(6):708-14. [DOI: ] [DOI] [PubMed] [Google Scholar]
Elsinga 2008 {published data only}
- Elsinga J, Jong-Potjer LC, Pal-de Bruin KM, le Cessie S, Assendelft WJJ, Buitendijk SE. The effect of preconception counselling on lifestyle and other behaviour before and during pregnancy. Women's Health Issues 2008;18(6):S117-25. [DOI: ] [DOI] [PubMed] [Google Scholar]
Emery 2001 {published data only}
- Emery M, Beran M, Darwish J, Oppizzi L, Joris V, Capel R, et al. Does counselling prior to IVF affect anxiety and depression scores? Preliminary results of a randomized clinical trial. Human Reproduction 2001;16:201. [Google Scholar]
Emery 2002 {published data only}
- Emery M, Beran M, Darwiche J, Oppizzi L, Joris V, Capel R, et al. What do couples expect and what do the receive through systematic pre-IVF counselling? Results from a randomized controlled study. Human Reproduction 2002;17(1):192. [Google Scholar]
Emery 2003 {published data only}
- Emery M, Beran M, Darwiche J, Oppizzi L, Joris V, Capel R, et al. Results from a prospective, randomized, controlled study evaluating the acceptability and effects of routine pre-IVF counselling. Human Reproduction 2003;18(12):2647-53. [DOI] [PubMed] [Google Scholar]
Emery 2004 {published data only}
- Emery M, Joris V, Beran M, Darwiche J, Capel R, Guex P, et al. Assessing pre-IVF counselling: one-year follow-up. Human Reproduction 2004;19(Suppl 1):i31. [DOI] [PubMed] [Google Scholar]
Emery 2006 {published data only}
- Emery M, Joris V, Beran M, Darwiche J, Capel R, Guex P, et al. Prospective study of pre-IVF counselling; one year study follow-up. Journal of Reproductive Medicine and Endocrinology 2006;1:77-8. [Google Scholar]
Espinos 2017 {published data only}
- Espinos JJ, Polo A, Sanchez-Hernandez J, Bordas R, Pares P, Martinez O, et al. Weight decrease improves live birth rates in obese women undergoing IVF: a pilot study. Reproductive BioMedicine Online 2017;35(4):417-24. [DOI] [PubMed] [Google Scholar]
Fauque 2007 {published data only}
- Fauque P. Medical treatments and hygiene and dietary measures. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2007;36:S78-84. [DOI: ] [DOI] [PubMed] [Google Scholar]
Galhardo 2013 {published data only}
- Galhardo A, Cunha M, Pinto-Gouveia J. Mindfulness-based program for infertility: efficacy study. Fertility and Sterility 2013;100(4):1059-67. [DOI: ] [DOI] [PubMed] [Google Scholar]
Garruti 2017 {published data only}
- Garruti G, De Palo R, De Angelisc M. Weighing the impact of diet and lifestyle on female reproductive function. Current Medicinal Chemistry 2017;26(19):3584-92. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gaskins 2018 {published data only}
- Gaskins AJ. Recent advances in understanding the relationship between long- and short-term weight change and fertility. F1000 Research 2018;7(1):1702. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaskins 2019 {published data only}
- Gaskins AJ, Nassan FL, Chiu YH, Arvizu M, Williams PL, Keller MG, et al. Dietary patterns and outcomes of assisted reproduction. American Journal of Obstetrics and Gynecology 2019;220(6):567. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gautam 2019a {published data only}
- Gautam S, Chaurasia P, Rana D, Kumar U, Dada R. Yoga enhances fertility potential and improves quality of life in infertile men with rheumatoid arthritis on disease-modifying antirheumatic drugs. Andrology 2019;7:78. [DOI: 10.1111/andr.12624] [DOI] [Google Scholar]
Gautam 2019b {published data only}
- Gautam S, Kumar M, Chaurasia P, Rana D, Dada R. Impact of yoga based lifestyle intervention on quality of life, depression and sperm oxidative DNA damage: a randomized controlled trial on infertile men with rheumatoid arthritis. Fertility and Sterility 2019;112(3):e409. [DOI: 10.1016/j.fertnstert.2019.07.1161] [DOI] [Google Scholar]
Goswami 2015 {published data only}
- Goswami SK, Yasmin S, Chakraborty P, Chattopadhyay R, Ghosh S, Goswami M, et al. Role of dietary antioxidant supplementation in treatment of idiopathic male infertility: promising evidence from a sub-continental study. Fertility and Sterility 2015;104:e288. [Google Scholar]
Guzick 1994 {published data only}
- Guzick DS, Wing R, Smith D, Berga SL, Winters SJ. Endocrine consequences of weight loss in obese, hyperandrogenic, anovulatory women. Fertility and Sterility 1994;61(4):598-604. [PubMed] [Google Scholar]
Hamzehgardeshi 2019 {published data only}
- Hamzehgardeshi Z, Yazdani F, Elyasi F, Moosazadeh M, Peyvandi S, Gelehkolaee KS, et al. The efficacy of group counselling on perceived stress among infertile women undergoing in vitro fertilization treatment: an RCT. International Journal of Reproductive Biomedicine 2019;17(1):57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoirisch Clapauch 2017 {published data only}
- Hoirisch-Clapauch S, Sant’Anna MC, Moreira EC, Frankel PP, Neves HC, Valle MP, et al. Lifestyle modification increases the take-home baby rate in women with recurrent early miscarriages: a randomised study. Thrombosis Research 2017;151(Suppl 1):S104. [Google Scholar]
HuMl 2016 {published data only}
- Hu Ml, Chang MY, Chu MC, Wang HS, Huang HY. The effectiveness of brief mindfulness-based stress reduction program on stress, mindful awareness attention, sleep quality in infertile women: a quasi-experimental study. Human Reproduction 2016;31(Suppl 1):i343. [DOI: 10.1093/humrep/31.Supplement_1.1] [DOI] [Google Scholar]
IRCT201110267915N1 2014 {published data only}
- IRCT201110267915N1. The effect of collaborative counseling on psychological subsequent in infertile women. http://en.irct.ir/trial/8359 (first received 25 July 2014).
IRCT201301189463N 2017 {published data only}
- IRCT201301189463N12. The effect of an educational program based on BASNEF model on sexual health promotion in infertile women. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201301189463N12 (first received 3 February 2017).
IRCT20150119020719N6 2018 {published data only}
- IRCT20150119020719N6. The effect of counseling on stress and female behaviors of infertile women. http://en.irct.ir/trial/29348 (first received 14 April 2018).
IRCT2016012610324N 2016 {published data only}
- IRCT2016012610324N28. The effect of preconception counseling on women lifestyle. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016012610324N28 (first received 10 April 2016).
IRCT20160605028270N2 2020 {published data only (unpublished sought but not used)}
- IRCT20160605028270N2. The effects of different exercise training modalities as a treatment and prevention strategies on body composition measures in association with semen parameters in married middle age fertile and at-risk infertile patients. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20160605028270N2 (first received 7 August 2020).
ISRCTN03259732 2014 {published data only}
- ISRCTN03259732. Start at the beginning: a nutrition and lifestyle digital intervention for women planning a pregnancy. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN03259732 (first received 31 July 2014).
ISRCTN11081163 2016 {published data only}
- ISRCTN11081163. A Swedish population-based reproductive life plan intervention. https://doi.org/10.1186/ISRCTN11081163 (first received 1 November 2016).
ISRCTN17222161 2017 {published data only}
- ISRCTN17222161. Changes in male fertility during chronic illness. https://doi.org/10.1186/ISRCTN17222161 (first received 24 February 2017).
Jaffar 2018 {published data only}
- Jaffar M, Andrabi SW, Prakash Babu SML, Subramani SA. Impact of weight loss on reproductive hormones in obese men. International Journal of Infertility and Fetal Medicine 2018;9(3):32-6. [DOI: ] [Google Scholar]
Jamali 2016 {published data only}
- Jamali S, Mosallanekhad Z, Zarei F. The effects of education on anxiety levels and sexual satisfaction in infertile women in Iran. Journal of Reproduction and Infertility 2016;17(2):260-1. [Google Scholar]
Jiménez Tuñón 2017 {published data only}
- Jiménez Tuñón JM, Trilles PP, Molina MG, Duvison MH, Pastor BM, Martín PS, et al. A double-blind, randomized prospective study to evaluate the efficacy of previous therapy with melatonin, myo-inositol, folic acid, and selenium in improving the results of an assisted reproductive treatment. Clinical Medicine Insights: Therapeutics 2017;9:1-6. [DOI: 10.1177/1179559X17742902] [DOI] [Google Scholar]
Kaya 2016 {published data only}
- Kaya Y, Beji NK, Aydin Y, Hassa H. The effect of health-promoting lifestyle education on the treatment of unexplained female infertility. European Journal of Obstetrics & Gynecology and Reproductive Biology 2016;207:109-14. [DOI] [PubMed] [Google Scholar]
Kermack 2014 {published data only}
- Kermack AJ, Calder PC, Houghton FD, Godfrey KM, Macklon NS. A randomised controlled trial of a preconceptional dietary intervention in women undergoing IVF treatment (PREPARE trial). BMC Women's Health. 2014;14:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kermack 2017 {published data only}
- Kermack AJ, Lowen PK, Wellstead SJ, Fisk HL, Montag M, Houghton FD, et al. PREPARE trial: a randomised double blinded controlled trial of a preconception Omega 3 and vitamin D rich dietary supplement in couples undergoing assisted reproduction treatment. Human Reproduction 2017;32(Suppl 1):290. [Google Scholar]
Kersten 2015 {published data only}
- Kersten FAM, Nelen WLDM, den Boogaard NM, Braat DDM, Koks CA, Spinder T, et al. Improving the implementation of tailored expectant management in couples with unexplained infertility: results from a cluster randomized controlled trial. Human Reproduction 2015;30(Suppl 1):57-8. [Google Scholar]
Kiel 2018 {published data only}
- Kiel IA, Lundgren KM, Mørkved S, Kjøtrød SB, Salvesen Ø, Romundstad LB, et al. Women undergoing assisted fertilisation and high-intensity interval training: a pilot randomised controlled trial. BMJ Open Sport & Exercise Medicine 2018;4(1):e000387. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirca 2019 {published data only}
- Kirca N, Pasinlioglu T. The effect of yoga on stress level in infertile women. Perspectives in Psychiatric Care 2019;55(2):319-27. [DOI: ] [DOI] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li J, Long L, Liu Y, He W, Li M. Effects of a mindfulness-based intervention on fertility quality of life and pregnancy rates among women subjected to first in vitro fertilization treatment. Behaviour Research and Therapy 2016;77:96-104. [DOI: ] [DOI] [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li XQ, Sun CF, Guo M. Benefits of nursing care service in the assisted reproduction clinic to self-cycle-management and self-efficiency of infertility patients. National Journal of Andrology 2017;23(6):536-9. [PubMed] [Google Scholar]
Lundgren 2016 {published data only}
- Lundgren KM, Romundstad LB, During V, Morkved S, Kjotrod S, Moholdt T. Exercise prior to assisted fertilization in overweight and obese women (FertilEX): study protocol for a randomized controlled trial. Trials 2016;17(1):268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maleki 2017a {published data only}
- Maleki BH, Tartibian B. Combined aerobic and resistance exercise training for improving reproductive function in infertile men: a randomized controlled trial. Applied Physiology, Nutrition, and Metabolism 2017;42(12):1293-306. [DOI] [PubMed] [Google Scholar]
Maleki 2017b {published data only}
- Maleki BH, Tartibian B. Moderate aerobic exercise training for improving reproductive function in infertile patients: a randomized controlled trial. Cytokine 2017;92:55-67. [DOI] [PubMed] [Google Scholar]
Maleki 2017c {published data only}
- Maleki BH, Tartibian B. High-intensity exercise training for improving reproductive function in infertile patients: a randomized controlled trial. Journal of Obstetrics and Gynaecology Canada 2017;39(7):545-58. [DOI] [PubMed] [Google Scholar]
Maleki 2018 {published data only}
- Maleki BH, Tartibian B. Resistance exercise modulates male factor infertility through anti-inflammatory and antioxidative mechanisms in infertile men: a RCT. Life Sciences 2018;203:150-60. [DOI] [PubMed] [Google Scholar]
Maleki 2020 {published data only}
- Maleki BH, Tartibian B. High-intensity interval training modulates male factor infertility through anti-inflammatory and antioxidative mechanisms in infertile men: a randomized controlled trial. Cytokine 2020;125:154861. [DOI] [PubMed] [Google Scholar]
Mirghafourvand 2020 {published data only (unpublished sought but not used)}
- Mirghafourvand M, Babapour J, Mohammad-Alizadeh-Charandabi S, Ghasemi Yngyknd S. The effect of preconception counselling on health locus of control and stress in Iranian women: a randomized control trial. Women & Health 2020;60(3):314-29. [DOI] [PubMed] [Google Scholar]
Moran 2011 {published data only}
- Moran L, Tsagareli V, Norman R, Noakes M. Diet and IVF pilot study: short-term weight loss improves pregnancy rates in overweight/obese women undertaking IVF. Australian and New Zealand Journal of Obstetrics and Gynaecology 2011;51(5):455-9. [DOI] [PubMed] [Google Scholar]
Moran 2016b {published data only}
- Moran LJ, Tsagareli V, Noakes M, Norman R. Altered preconception fatty acid intake is associated with improved pregnancy rates in overweight and obese women undertaking in vitro fertilisation. Nutrients 2016;8(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mori 2009 {published data only}
- Mori A. Supporting stress management for women undergoing the early stages of fertility treatment: a cluster-randomized controlled trial. Japan Journal of Nursing Science 2009;6(1):37-49. [DOI: ] [DOI] [PubMed] [Google Scholar]
Mumford 2020 {published data only (unpublished sought but not used)}
- Mumford SL, Johnstone E, Kim K, Ahmad M, Salmon S Summers K, et al. A prospective cohort study to evaluate the impact of diet, exercise, and lifestyle on fertility (IDEAL): design and baseline characteristics. American Journal of Epidemiology 2020;189(11):1254-65. [DOI: 10.1093/aje/kwaa073] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naab 2019 {published data only (unpublished sought but not used)}
- Naab F, Brown R, Ward EC. Culturally adapted depression intervention to manage depression among women with infertility in Ghana. Journal of Health Psychology 2019 June 20 [Epub ahead of print]. [DOI: 10.1177/1359105319857175] [DOI] [PubMed] [Google Scholar]
Nayar 2017 {published data only}
- Nayar P, Nayar KD, Ahuja R, Singh M, Kant G, Sharma N, et al. Can yoga affect IVF outcomes? Fertility and Sterility 2017;108 Suppl 1(3):e300. [Google Scholar]
Nayar 2018 {published data only}
- Nayar P, Nayar KD, Singh M, Aggarwal N, Gupta M, Gupta RA, et al. Yoga as an adjuvant to enhance the outcome of IVF treatment. Human Reproduction 2018;33(Suppl 1):393-4. [Google Scholar]
NCT01509066 2012 {published data only}
- NCT01509066. Healthy eating for reproductive health (HERHealth). https://clinicaltrials.gov/show/NCT01509066 (first received 12 January 2012).
NCT01566929 2012 {published data only}
- NCT01566929. Weight management interventions for obese women and the live birth outcome of in vitro fertilization (IVF): a randomized controlled trial. https://clinicaltrials.gov/show/NCT01566929 (first received 30 March 2012).
NCT01716429 2012 {published data only}
- NCT01716429. Healthy eating for reproductive health: Greenville. https://clinicaltrials.gov/show/NCT01716429 (first received 29 October 2012).
NCT01892111 2013 {published data only}
- NCT01892111. Structured exercise training in infertile obese patients treated with assisted reproductive techniques: a randomized controlled trial. https://clinicaltrials.gov/show/NCT01892111 (first received 3 July 2013).
NCT01933633 2013 {published data only}
- NCT01933633. Improved fertility after exercise in overweight/obese women. https://clinicaltrials.gov/show/NCT01933633 (first received 2 September 2013).
NCT01952795 2013 {published data only}
- NCT01952795. Effect of hypocaloric diet and exercise in obese women who are subjected to IVF cycle. https://clinicaltrials.gov/show/NCT01952795 (first received 30 September 2013).
NCT01954498 2013 {published data only}
- NCT01954498. Effect of walnuts on sperm parameters and male fertility. https://clinicaltrials.gov/show/NCT01954498 (first received 1 October 2013).
NCT02063256 2014 {published data only}
- NCT02063256. 7 NUTS Study. A randomized dietary intervention trial on the influence of diet on human sperm quality in subfertile men. https://clinicaltrials.gov/show/NCT02063256 (first received 14 February 2014).
NCT02432209 2015 {published data only}
- NCT02432209. Improving reproductive fitness through pretreatment with lifestyle modification in obese women with unexplained infertility. https://clinicaltrials.gov/show/NCT02432209 (first received 4 May 2015).
NCT02541487 2015 {published data only}
- NCT02541487. Fetal effects of pre-pregnancy lifestyle interventions in unexplained infertility patients. https://clinicaltrials.gov/ct2/show/NCT02541487 (first received 4 September 2015).
NCT02648555 2016 {published data only}
- NCT02648555. A lifestyle intervention to improve in vitro fertilization results. https://clinicaltrials.gov/show/NCT02648555 (first received 7 January 2016).
NCT02746601 2016 {published data only}
- NCT02746601. My Health eSnapshot - a preconception health research study. https://clinicaltrials.gov/ct2/show/NCT02746601 (first received 21 April 2016).
NCT02752555 2016 {published data only}
- NCT02752555. Investigating the effect of modifiable lifestyle factors in severe oligoasthenotertozoospermic men combined with antioxidant treatment prior ICSI. https://clinicaltrials.gov/ct2/show/NCT02752555 (first received 27 April 2016).
NCT03050944 2017 {published data only}
- NCT03050944. Association of the Mediterranean diet with fertility outcomes: a study among couples undergoing in vitro fertilization treatment. https://clinicaltrials.gov/ct2/show/NCT03050944 (first received 13 February 2017).
NCT03343405 2017 {published data only}
- NCT03343405. Fertility & well-being: mind/body protocol. https://clinicaltrials.gov/show/NCT03343405 (first received 17 November 2017).
NCT03348865 2017 {published data only}
- NCT03348865. Fertility Life Counselling Aid (FeLiCiA). https://clinicaltrials.gov/ct2/show/NCT03348865 (first received 21 November 2017).
NCT03475199 2018 {published data only}
- NCT03475199. Evaluation of personalised support program effectiveness in sperm quality improvement. https://clinicaltrials.gov/show/NCT03475199 (first received 23 March 2018).
NCT03553927 2018 {published data only}
- NCT03553927. Investigating the physiological effects of weight loss on male fertility. https://clinicaltrials.gov/show/NCT03553927 (first received 12 June 2018).
NCT03898037 2019 {published data only}
- NCT03898037. Effect of lifestyle and/or metformin intervention on pregnancy outcome: a pilot randomized controlled trial. https://clinicaltrials.gov/show/NCT03898037 (first received 1 April 2019).
NCT04002414 2019 {published data only}
- NCT04002414. Physical activity and fertility care study. https://clinicaltrials.gov/show/NCT04002414 (first received 26 June 2019).
NCT04273048 2020 {published data only (unpublished sought but not used)}
- NCT04273048. The MOM TO BE Study. https://clinicaltrials.gov/show/NCT04273048 (first received 17 February 2020).
NCT04585581 2020 {published data only (unpublished sought but not used)}
- NCT04585581. Preconception lifestyle interventions to improve future metabolic health (before the beginning). https://clinicaltrials.gov/show/NCT04585581 (first received 14 October 2020).
Nieschlag 1998 {published data only}
- Nieschlag E, Hertle L, Fischedick A, Abshagen K, Behre HM. Update on treatment of varicocele: counselling as effective as occlusion of the vena spermatica. Human Reproduction 1998;13(8):2147-50. [DOI] [PubMed] [Google Scholar]
Overdijkink 2018 {published data only}
- Overdijkink SB, Opstal LM, Baart EB, Oostingh EC, Willemsen SP, Laven JS, et al. The impact of inadequate maternal nutritional factors on embryo morphokinetic quality after in vitro fertilization or intracytoplasmic sperm injection. Reproductive Sciences 2018;25(1):250A. [Google Scholar]
PACTR201611001683280 2016 {published data only}
- PACTR201611001683280. Personalized IVF to maximize success rate [Individualized controlled ovarian stimulation for IVF to maximize success rate]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201611001683280 (first received 14 June 2016).
Pedro 2019 {published data only}
- Pedro J, Fernandes J, Barros A, Xavier P, Oliveira C, Schmidt L, et al. Does watching an educational video increase fertility awareness (FA)? Results from a randomised controlled trial with partnered people desiring to become parents. Human Reproduction 2019;34(Suppl 1):i38. [Google Scholar]
Poehl 1999 {published data only}
- Poehl M, Bichler K, Wicke V, Dorner V, Feichtinger W. Psychotherapeutic counseling and pregnancy rates in in vitro fertilization. Journal of Assisted Reproduction and Genetics 1999;16(6):302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Psaros 2015 {published data only}
- Psaros C, Kagan L, Shifren JL, Willett J, Jacquart J, Alert MD, et al. Mind-body group treatment for women coping with infertility: a pilot study. Journal of Psychosomatic Obstetrics & Gynecology 2015;36(2):75-83. [DOI: ] [DOI] [PubMed] [Google Scholar]
Rasoulzadeh 2013 {published data only}
- Bidgoly MR, Roudsari RL. Adopting problem-focused coping strategies following implementation of a collaborative counseling program in infertile women undergoing IVF. International Journal of Fertility & Sterility 2013;7(Suppl 1):46. [Google Scholar]
Rasoulzadeh 2018 {published data only}
- Rasoulzadeh BM, Latifnejad RR. The effect of the collaborative infertility counseling model on coping strategies in infertile women undergoing in vitro fertilization: a randomized controlled trial. International Journal of Women's Health and Reproduction Sciences 2018;6(1):47-54. [Google Scholar]
RBR‐7by76r 2016 {published data only}
- RBR‐7by76r. Effects of "anti-stress therapy" on behavior and stress hormones in infertile women and also on weight loss after diet and exercise guidance in those overweight. http://www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-7by76r (first received 2016).
Rothberg 2016 {published data only}
- Rothberg A, Lanham M, Randolph J, Fowler C, Miller N, Smith Y. Feasibility of a brief, intensive weight loss intervention to improve reproductive outcomes in obese, subfertile women: a pilot study. Fertility and Sterility 2016;106(5):1212-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sant'Anna 2017 {published data only}
- Sant'Anna EM, Paiva SPC, Santos RP, Rodrigues AM, Nery SF, Maia FP, et al. Mindfulness-based intervention for lifestyle modification and weight loss in infertile women: randomized controlled trial. Human Reproduction 2017;32(Suppl 1):i398-9. [Google Scholar]
Schick 2019 {published data only}
- Schick M, Roesner S, Germeyer A, Moessner M, Bauer S, Ditzen B, et al. Smartphone-supported positive adjustment coping intervention (PACI) for couples undergoing fertility treatment: a randomised controlled trial protocol. BMJ Open 2019;9(7):e025288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shahrestani 2012 {published data only}
- Shahrestani M, Qanbari BA, Nemati SH, Rahbardar H. The effectiveness of mindfulness based cognitive group therapy (MBCT) on improving perceived infertility-related stress and irrational parenthood cognitions among infertile women undergoing IVF treatment. Iranian Journal of Obstetrics, Gynecology and Infertility 2012;15(19):28-38. [Google Scholar]
Sim 2012 {published data only}
- Sim K, Denyer G, Caterson I. Weight loss improves reproductive outcomes for obese women undergoing assisted reproductive technology. Obesity Facts 2012;5(Suppl 1):36. [DOI] [PubMed] [Google Scholar]
Sim 2014 {published data only}
- Sim KA, Dezarnaulds GM, Denyer GS, Skilton MR, Caterson ID. Weight loss improves reproductive outcomes in obese women undergoing fertility treatment: a randomized controlled trial.. Clinical Obesity 2014;4(2):61-8. [DOI: 10.1111/cob.12048] [DOI] [PubMed] [Google Scholar]
Skogsdal 2019 {published data only}
- Skogsdal Y, Fadl H, Cao Y, Karlsson J, Tydén T. An intervention in contraceptive counseling increased the knowledge about fertility and awareness of preconception health - a randomized controlled trial. Upsala Journal of Medical Sciences 2019;124(3):203-12. [DOI: 10.1080/03009734.2019.1653407] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tolahunase 2017 {published data only}
- Tolahunase M, Sagar R, Kumar R, Malhotra N, Singh N, Dada R. Yoga and meditation promotes quality of life by decreasing depression severity and cellular aging in male infertility: randomized controlled trial. Andrology 2017;5:116. [Google Scholar]
Tolahunase 2018 {published data only}
- Tolahunase MR, Sagar R, Chaurasia P, Dada R. Impact of yoga- and meditation-based lifestyle intervention on depression, quality of life, and cellular aging in infertile couples. Fertility and Sterility 2018;110(4):e67. [Google Scholar]
Tolahunase 2018a {published data only}
- Tolahunase M, Sagar R, Chaurasia P, Dada R. Yoga optimized neuroplasticity and regulatory feedback improve semenogram and sperm quality in infertile men with major depressive disorder: a randomized controlled trial. Andrology 2018;6(Suppl 1):80. [Google Scholar]
Tolahunase 2018b {published data only}
- Tolahunase M, Kumar R, Sagar R, Dada R. Impact of yoga- and meditation-based lifestyle intervention on depression and quality of life in infertile couples: a randomized controlled trial. Human Reproduction 2018;33(Suppl 1):308-9. [DOI: ] [Google Scholar]
Tsagareli 2006 {published data only}
- Tsagareli V, Noakes M, Norman RJ. Effect of a very-low-calorie diet on in vitro fertilization outcomes. Fertility and Sterility 2006;86(1):227-9. [DOI: 10.1016/j.fertnstert.2005.12.041] [DOI] [PubMed] [Google Scholar]
Twigt 2012 {published data only}
- Twigt J, Bolhuis M, Steegers E, Hammiche F, Inzen W, Laven J, et al. The preconception diet influences the chance of ongoing pregnancy even after IVF/ICSI treatment. Reproductive Sciences 2012;19(3):245A. [DOI: 10.1177/1933719112442493] [DOI] [Google Scholar]
UMIN000009034 2012 {published data only}
- UMIN000009034. The influence of physical constitution, life-style and environmental factors on sex hormones and anti-mullerian hormone. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000010601 (first received 1 November 2012).
UMIN000016168 2015 {published data only}
- UMIN000016168. Effects of fertility information on people's knowledge, life-planning, and psychology. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000016168 (first received 13 January 2015).
UMIN000027424 2017 {published data only}
- UMIN000027424. Impact of guidance and consultation management program on women undergoing non-ART infertility treatment. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000027424 (first received 7 January 2017).
Vause 2018 {published data only}
- Vause TD, Allison DJ, Vause T, Tekok-Kilic A, Ditor DS, Min JK. Comparison of a web-based teaching tool and traditional didactic learning for in vitro fertilization patients: a preliminary randomized controlled trial. Journal of Obstetrics and Gynaecology Canada 2018;40(5):588-94. [DOI: 10.1016/j.jogc.2017.08.029] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Chang 2006 {published data only}
- Chang G, McNamara TK, Haimovici F, Hornstein M. Problem drinking in women evaluated for infertility. American Journal on Addictions 2006;15(2):174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamebozorg 2018 {published data only}
- IRCT2016081629388N1. Evaluation of impact of diet program based on traditional medicine on ovulation and fertility rate in woman with induction of ovulation. http://en.irct.ir/trial/23634 (first received 4 April 2017).
- Jamebozorg N, Samani LN, Naseri M, Beigi RM, Haghani H. The effect of Iranian traditional medicine based dietary intervention on incidence of pregnancy in women undergoing ovulation stimulation. Journal of Reproduction and Infertility 2018;19(2 Suppl 2):160-1. [Google Scholar]
Liu 2020 {published data only (unpublished sought but not used)}
- Liu J, Wang JG, Xu HX, Sun Y, Peng L, Sun J, et al. Application of the Green Model to lifestyle intervention in male sterility patients. Zhonghua nan ke xue = National Journal of Andrology 2020;26(5):441-5. [PubMed] [Google Scholar]
References to ongoing studies
Bivia‐Roig 2020 {published data only (unpublished sought but not used)}
- Bivia-Roig G, Blasco-Sanz R, Boldo-Roda A, Vara MD, Escriva-Martinez T, Herrero R, et al. Efficacy of an internet-based intervention to promote a healthy lifestyle on the reproductive parameters of overweight and obese women: study protocol for a randomised controlled trial. International Journal of Environmental Research and Public Health 2020;17(22):1-15. [DOI: 10.3390/ijerph17228312] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04275869. Internet-based intervention to promote a healthy lifestyle on the reproductive parameters of overweight and obese women. https://clinicaltrials.gov/show/NCT04275869 (first received 19 February 2020). [DOI] [PMC free article] [PubMed]
Boedt 2019 {published data only (unpublished sought but not used)}
- Boedt T, Dancet E, Fong SL, Peeraer K, De Neubourg D, Pelckmans S, et al. Effectiveness of a mobile preconception lifestyle programme in couples undergoing in vitro fertilisation (IVF): the protocol for the PreLiFe randomised controlled trial (PreLiFe-RCT). BMJ Open 2019;9(7):e029665. [DOI: 10.1136/bmjopen-2019-029665] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03790449. Evaluation of a mobile preconception lifestyle programme in couples undergoing in vitro fertilisation: a multicentre randomized controlled trial (PreLiFe-RCT). https://clinicaltrials.gov/ct2/show/NCT03790449 (first received 31 December 2018).
CTRI201908020997 2019 {published data only (unpublished sought but not used)}
- CTRI201908020997. Effect of lifestyle modification and education on information needs and satisfaction level of infertile couples. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/08/020997 (first received 30 August 2019).
CTRI202006025577 2020 {published data only (unpublished sought but not used)}
- CTRI202006025577. A study to evaluate the effect of nursing management on emotional distress among infertile women. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/06/025577 (first received 3 June 2020).
Dupont 2020 {published data only (unpublished sought but not used)}
- Dupont C, Aegerter P, Foucaut AM, Reyre A, Lhuissier FJ, Bourgain M, et al. Effectiveness of a therapeutic multiple-lifestyle intervention taking into account the periconceptional environment in the management of infertile couples: study design of a randomized controlled trial – the PEPCI study. BMC Pregnancy and Childbirth 2020;20(322):1-13. [DOI: 10.1186/s12884-020-2855-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02961907. Pathway taking into account periconceptional environment for infertile couple. https://clinicaltrials.gov/show/NCT02961907 (first received 11 November 2016).
NCT03395067 2018 {published data only (unpublished sought but not used)}
- NCT03395067. Multidisciplinary treatment of obesity prior to in vitro fertilization: impact on global reproductive outcomes (PRO-FIV Study). https://clinicaltrials.gov/show/NCT03395067 (first received 10 January 2018).
NCT03908099 2019 {published data only (unpublished sought but not used)}
- NCT03908099. Fit-for-Fertility multicenter randomized controlled trial: improving reproductive, maternal and neonatal outcomes in obese and infertile. https://clinicaltrials.gov/show/NCT03908099 (first received 9 April 2019).
NCT04589793 2020 {published data only (unpublished sought but not used)}
- NCT04589793. COaching Lifestyle Intervention for FErtility (COLIFE). https://clinicaltrials.gov/show/NCT0458979 (first received 19 October 2020).
Timmermans 2019 {published data only (unpublished sought but not used)}
- NCT02703753. TOwards Prepared Mums (TOP-mums), for a healthy start: a lifestyle intervention to reduce overweight and smoking in women with a pregnancy wish to prevent perinatal morbidity. https://clinicaltrials.gov/ct2/show/NCT02703753 (first received 9 March 2016).
- Timmermans YE, de Kant KD, Reijnders D, Kleijkers LM, Dompeling E, Kramer BW, et al. Towards prepared mums (TOP-mums) for a healthy start, a lifestyle intervention for women with overweight and a child wish: study protocol for a randomised controlled trial in the Netherlands. BMJ Open 2019;9(11):e030236. [DOI: 10.1136/bmjopen-2019-030236] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Atrash 2006
- Atrash HK, Johnson K, Adams M, Cordero JF, Howse J. Preconception care for improving perinatal outcomes: the time to act. Maternal and Child Health Journal 2006;10 Suppl:3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Augood 1998
- Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Human Reproduction 1998;13(6):1532-9. [DOI] [PubMed] [Google Scholar]
Best 2017
- Best D, Avenell A, Bhattacharya S. How effective are weight-loss interventions for improving fertility in women and men who are overweight or obese? A systematic review and meta-analysis of the evidence. Human Reproduction Update 2017;23(6):681–705. [DOI: 10.1093/humrep/dex313.] [DOI] [PubMed] [Google Scholar]
Bille 2009
- Bille C, Nybo Andersen A-M. Preconception care. BMJ 2009;338:b22. [DOI] [PubMed] [Google Scholar]
Campbell 2015
- Campbell JM, Lane M, Owens JA, Bakos HW. Paternal obesity negatively affects male fertility and assisted reproduction outcomes: a systematic review and meta-analysis. Reproductive Biomedicine Online 2015;31(5):593-604. [DOI: 10.1016/j.rbmo.2015.07.012.] [DOI] [PubMed] [Google Scholar]
Chiu 2018
- Chiu YH, Chavarro JE, Souter I. Diet and female fertility: doctor, what should I eat? Fertility and Sterility 2018;110(4):560-9. [DOI: 10.1016/j.fertnstert.2018.05.027.] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 18 February 2019. Melbourne, Australia: Veritas Health Innovation. Available at www.covidence.org.
De‐Regil 2017
- De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD007950. [DOI: 10.1002/14651858.CD007950.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffy 2020a
- Duffy JM, Alhawany H, Bhattacharya S, Collura B, Curtis C, Evers JL et al. Developing a core outcome set for future infertility research: an international consensus development study. Human Reproduction 2020;35(12):2725-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffy 2020b
- Duffy JM, Bhattacharya S, Bofill M, Collura B, Curtis C, Evers JL, et al. Standardizing definition and reporting guidelines for the infertility core outcome set: an international consensus development study. Human Reproduction 2020;35(12):2735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
ESHRE guideline 2015
- Gameiro S, Boivin J, Dancet E, Klerk C, Emery M, Lewis-Jones C, et al, ESHRE. ESHRE guideline: routine psychosocial care in infertility and medically assisted reproduction - a guide for fertility staff. Human Reproduction 2015;30(11):2476-85. [DOI: 10.1093/humrep/dev177.] [DOI] [PubMed] [Google Scholar]
Frey 2008
- Frey KA, Navarro SM, Kotelchuck M, Lu MC. The clinical content of preconception care: preconception care for men. American Journal of Obstetrics and Gynecology 2008;199 Suppl(6):389-95. [DOI] [PubMed] [Google Scholar]
Fritz 2011
- Fritz MA, Speroff L. Clinical Gynecologic Endocrinology and Infertility. 8th edition. Philadelphia: Lippincott Williams and Wilkins, 2011. [Google Scholar]
Fronczak 2012
- Fronczak CM, Kim ED, Barqawi AB. The insults of illicit drug use on male fertility. Journal of Andrology 2012;33(4):515-28. [DOI] [PubMed] [Google Scholar]
Gameiro 2012
- Gameiro S, Boivin J, Peronace L, Verhaak CM. Why do patients discontinue fertility treatment? A systematic review of reasons and predictors of discontinuation in fertility treatment. Human Reproduction Update 2012;18(6):652-69. [DOI: 10.1093/humupd/dms031.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gameiro 2013
- Gameiro S, Boivin J, Domar A. Optimal in vitro fertilization in 2020 should reduce treatment burden and enhance care delivery for patients and staff. Fertility and Sterility 2013;100(2):302-9. [DOI: 10.1016/j.fertnstert.2013.06.015] [DOI] [PubMed] [Google Scholar]
Gardiner 2008
- Gardiner PM, Nelson L, Shellhaas CS, Dunlop AL, Long R, Andrist S, et al. The clinical content of preconception care: nutrition and dietary supplements. American Journal of Obstetrics and Gynecology 2008;199 Suppl(6):345-56. [DOI] [PubMed] [Google Scholar]
Gaskins 2018a
- Gaskins AJ, Chavarro JE. Diet and fertility: a review. American Journal of Obstetrics and Gynecology 2018;218(4):379-89. [DOI: 10.1016/j.ajog.2017.08.010.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giahi 2016
- Giahi L, Mohammadmoradi S, Javidan A, Sadeghi MR. Nutritional modifications in male infertility: a systematic review covering 2 decades. Nutrition Reviews 2016;74(2):118-30. [DOI: 10.1093/nutrit/nuv059.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Godfrey 2017
- Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinology 2017;5(1):53-64. [DOI: 10.1016/S2213-8587(16)30107-3.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gormack 2015
- Gormack AA, Peek JC, Derraik JG, Gluckman PD, Young NL, Cutfield WS. Many women undergoing fertility treatment make poor lifestyle choices that may affect treatment outcome. Human Reproduction 2015;30(7):1617-24. [DOI: 10.1093/humrep/dev094.] [DOI] [PubMed] [Google Scholar]
Gouin 2011
- Gouin K, Murphy K, Shah PS. Effects of cocaine use during pregnancy on low birthweight and preterm birth: systematic review and metaanalyses. American Journal of Obstetrics and Gynecology 2011;204(4):340.e1-12. [DOI] [PubMed] [Google Scholar]
Government of South Australia 2015
- Government of South Australia Department of Health, Maternal and Neonatal Clinical Network. Clinical practice guideline for preconception advice. www.sahealth.sa.gov.au (accessed 18 February 2019).
Grainger 2006
- Grainger DA, Frazier LM, Rowland CA. Preconception care and treatment with assisted reproductive technology. Maternal and Child Health Journal 2006;10 Suppl:161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grieger 2018
- Grieger JA, Grzeskowiak LE, Bianco-Miotto T, Jankovic-Karasoulos T, Moran LJ, Wilson RL, et al. Pre-pregnancy fast food and fruit intake is associated with time to pregnancy. Human Reproduction 2018;33(6):1063-70. [DOI: 10.1093/humrep/dey079.] [DOI] [PubMed] [Google Scholar]
Gunn 2016
- Gunn JKL, Rosales CB, Center KE , Nuñez A, Gibson SJ, Christ C, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open 2016;6(4):e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haider 2017
- Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD004905. [DOI: 10.1002/14651858.CD004905.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hakim 1998
- Hakim RB, Gray RH, Zacur H. Alcohol and caffeine consumption and decreased fertility. Fertility and Sterility 1998;70:632-7. [DOI] [PubMed] [Google Scholar]
Harbin Consensus Conference Workshop Group 2014
- The Harbin Consensus Conference Workshop Group. Improving the reporting of clinical trials of infertility treatments (IMPRINT): modifying the CONSORT statement. Human Reproduction 2014;29(10):2075-82. [DOI: 10.1093/humrep/deu218] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harding 2017
- Harding KB, Peña-Rosas JP, Webster AC, Yap CM, Payne BA, Ota E, et al. Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD011761. [DOI: 10.1002/14651858.CD011761.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Homan 2007
- Homan GF, Davies M, Norman RJ. The impact of lifestyle factors on reproductive performance in the general population and those undergoing fertility treatment: a review. Human Reproduction Update 2007;13:209-23. [DOI] [PubMed] [Google Scholar]
Homan 2018
- Homan GF, Delacey S, Tremellen K. Promoting healthy lifestyle in fertility clinics: an Australian perspective. Human Reproduction Open 2018;1(1):1-7. [DOI: 10.1093/hropen/hox028] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyland 2016
Ibanez‐Perez 2019
- Ibañez-Perez J, Santos-Zorrozua B, Lopez-Lopez E, Matorras R, Garcia-Orad A. An update on the implication of physical activity on semen quality: a systematic review and meta-analysis. Archives of Gynecology and Obstetrics 2019;Epub ahead of print:1-21. [DOI: 10.1007/s00404-019-05045-8.] [DOI] [PubMed] [Google Scholar]
Klonoff‐Cohen 2003
- Klonoff-Cohen H, Lam-Kruglick P, Gonzalez C. Effects of maternal and paternal alcohol consumption on the success rates of in vitro fertilization and gamete intrafallopian transfer. Fertility and Sterility 2003;79(2):330-9. [DOI] [PubMed] [Google Scholar]
Klonoff‐Cohen 2005
- Klonoff-Cohen H. Female and male lifestyle habits and IVF: what is known and unknown. Human Reproduction Update 2005;11(2):179-203. [DOI] [PubMed] [Google Scholar]
Ladhani 2011
- Ladhani NNN, Shah PS, Murphy KE. Prenatal amphetamine exposure and birth outcomes: a systematic review and metaanalysis. American Journal of Obstetrics and Gynecology 2011;205(3):219.e1-7. [DOI] [PubMed] [Google Scholar]
Lan 2017
- Lan L, Harrison CL, Misso M, Hill B, Teede HJ, Mol BW, et al. Systematic review and meta-analysis on the impact of preconception lifestyle interventions on fertility, obstetric, fetal, anthropometric and metabolic outcomes in men and women. Human Reproduction 2017;32(9):1925-40. [DOI: 10.1093/humrep/dex241] [DOI] [PubMed] [Google Scholar]
Lane 2015
- Lane M, Zander-Fox DL, Robker RL, McPherson NO. Peri-conception parental obesity, reproductive health, and transgenerational impacts. Trends In Endocrinology And Metabolism 2015;26(2):84-90. [DOI: 10.1016/j.tem.2014.11.005.] [DOI] [PubMed] [Google Scholar]
Lyngso 2017
- Lyngsø J, Ramlau-Hansen CH, Bay B, Ingerslev HJ, Hulman A, Kesmodel US. Association between coffee or caffeine consumption and fecundity and fertility: a systematic review and dose-response meta-analysis. Clinical Epidemiology 2017;15(9):699-719. [DOI: 10.2147/CLEP.S146496.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mackenzie 2019
- Mackenzie R, Ells LJ, Simpson SA, Logue J. Core outcome set for behavioural weight management interventions for adults with overweight and obesity: standardised reporting of lifestyle weight management interventions to aid evaluation (STAR‐LITE). Obesity Reviews 2019;21:e12961. [DOI: 10.1111/obr.12961] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mascarenhas 2012
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Medicine 2012;9(12):e1001356. [DOI: 10.1371/journal.pmed.1001356.] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDiarmid 2008
- McDiarmid MA, Gardiner PM, Jack BW. The clinical content of preconception care: environmental exposures. American Journal of Obstetrics and Gynecology 2008;199 Suppl(6):357-61. [DOI] [PubMed] [Google Scholar]
McKinnon 2016
- McKinnon CJ, Hatch EE, Rothman KJ, Mikkelsen EM, Wesselink AK, Hahn KA, et al. Body mass index, physical activity and fecundability in a North American preconception cohort study. Fertility and Sterility 2016;106(2):451-9. [DOI: 10.1016/j.fertnstert.2016.04.011.] [DOI] [PubMed] [Google Scholar]
Metz 2017
- Metz TD, Allshouse AA, Hogue CJ, Goldenberg RL, Dudley DJ, Varner MW, et al. Maternal marijuana use, adverse pregnancy outcomes, and neonatal morbidity. American Journal of Obstetrics and Gynecology 2017;217(4):478.e1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mínguez‐Alarcón 2018
- Mínguez-Alarcón L, Chavarro JE, Gaskins AJ. Caffeine, alcohol, smoking, and reproductive outcomes among couples undergoing assisted reproductive technology treatments. Fertility and Sterility 2018;110(4):587-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moran 2016a
- Moran LJ, Spencer L, Russell DL, Hull ML, Robertson SA, Varcoe TJ, Robinson Research Institute Consortium of Fertility and Conception Practitioners. Research priorities for fertility and conception research as identified by multidisciplinary health care practitioners and researchers. Nutrients 2016;13(8):E35. [DOI: 10.3390/nu8010035.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mueller 1990
- Mueller BA, Daling JR, Weiss NS, Moore DE. Recreational drug use and the risk of primary infertility. Epidemiology 1990;1(3):195-200. [DOI] [PubMed] [Google Scholar]
Mukherjee 2005
- Mukherjee RAS, Hollins S, Abou-Saleh MT, Turk J. Low level alcohol consumption and the fetus: abstinence from alcohol is the only safe message in pregnancy. BMJ 2005;330(7488):375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 2008
- Myers ER, McCrory DC, Mills AA, Price TM, Swamy GK, Tantibhedhyangkul J, et al. Effectiveness of assisted reproductive technology. Evidence Report/Technology Assessment No. 167 (Prepared by the Duke University Evidence-based Practice Center under Contract No. 290-02-0025). AHRQ Publication No. 08-E012 2008. [PMC free article] [PubMed]
NICE 2013
- National Institute for Health and Clinical Excellence. Fertility Problems: Assessment and Treatment (CG156). London: National Institute for Health and Clinical Excellence (NICE), 2013. [PubMed] [Google Scholar]
Penedo 2005
- Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry 2005;18(2):189-93. [DOI] [PubMed] [Google Scholar]
Practice Committee 2015
- Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: an educational bulletin. Fertility and Sterility 2015;104(5):1116-26. [DOI] [PubMed] [Google Scholar]
Practice Committee 2017
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility. Fertility and Sterility 2017;107(1):52-8. [Google Scholar]
Practice Committee 2018
- Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility. Fertility and Sterility 2018;110(4):611–8. [DOI] [PubMed] [Google Scholar]
Radin 2014
- Radin R, Hatch E, Rothman K, Mikkelsen E, Sørensen H, Riis A, et al. Active and passive smoking and fecundability in Danish pregnancy planners. Fertility and Sterility 2014;102(1):183-91.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 1992
- Rao JN, Scott AJ. A simple method for the analysis of clustered binary data. Biometrics 1992;48(2):577-85. [PubMed] [Google Scholar]
Rao 2018
- Rao M, Zeng Z, Tang L. Maternal physical activity before IVF/ICSI cycles improves clinical pregnancy rate and live birth rate: a systematic review and meta-analysis. Reproductive Biology and Endocrinology 2018;16(1):11. [DOI: 10.1186/s12958-018-0328-z.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ricci 2017
- Ricci E, Viganò P, Cipriani S, Somigliana E, Chiaffarino F, Bulfoni A, et al. Coffee and caffeine intake and male infertility: a systematic review. Nutrition Journal 2017;16(1):37. [DOI: 10.1186/s12937-017-0257-2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rittenberg 2011
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reproductive Biomedicine Online 2011;23(4):421-39. [DOI: 10.1016/j.rbmo.2011.06.018.] [DOI] [PubMed] [Google Scholar]
Rooney 2014
- Rooney KL, Domar AD. The impact of lifestyle behaviors on infertility treatment outcome. Current Opinion in Obstetrics and Gynecology 2014;26(3):181-5. [DOI: 10.1097/GCO.0000000000000069.] [DOI] [PubMed] [Google Scholar]
Ross 2006
- Ross DS, Jones JL, Lynch MF. Toxoplasmosis, cytomegalovirus, listeriosis and preconception care. Maternal and Child Health Journal 2006;10 Suppl:187-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossi 2011
- Rossi BV, Berry KF, Hornstein MD, Cramer DW, Ehrlich S, Missmer SA. Effect of alcohol consumption on in vitro fertilization. Obstetrics & Gynecology 2011;117(1):136-42. [DOI: 10.1097/AOG.0b013e31820090e1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salas‐Huetos 2017
- Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Human Reproduction Update 2017;23(4):371-89. [DOI: 10.1093/humupd/dmx006.] [DOI] [PubMed] [Google Scholar]
Sharma 2016
- Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization laboratory methods for the examination of human semen. European Urology 2016;70(4):635-45. [DOI] [PubMed] [Google Scholar]
Shawe 2015
- Shawe J, Delbaere I, Ekstrand M, Hegaard HK, Larsson M, Mastroiacovo P, et al. Preconception care policy, guidelines, recommendations and services across six European countries: Belgium (Flanders), Denmark, Italy, the Netherlands, Sweden and the United Kingdom. European Journal of Contraception & Reproductive Health Care 2015;20(2):77-87. [DOI: 10.3109/13625187.2014.990088.] [DOI] [PubMed] [Google Scholar]
Showell 2020
- Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Antioxidants for female subfertility. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD007807. [DOI: 10.1002/14651858.CD007807.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smits 2019
- Smits RM, Mackenzie‐Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell MG. Antioxidants for male subfertility. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD007411. [DOI: 10.1002/14651858.CD007411.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
The Fertility Society of Australia 2019
- The Fertility Society of Australia. Home. www.fertilitysociety.com.au/ (accessed 18 February 2019).
Vujkovic 2010
- Vujkovic M, Vries JH, Lindemans J, Macklon NS, Spek PJ, Steegers EA, et al. The preconception Mediterranean dietary pattern in couples undergoing in vitro fertilization/intracytoplasmic sperm injection treatment increases the chance of pregnancy. Fertility and Sterility 2010;94(6):2096-101. [DOI: 10.1016/j.fertnstert.2009.12.079.] [DOI] [PubMed] [Google Scholar]
Waylen 2008
- Waylen AL, Metwally M, Jones GL, Wilkinson AJ, Ledger WL. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: a meta-analysis. Human Reproduction Update 2008;15(1):31-44. [DOI] [PubMed] [Google Scholar]
World Health Organization 2010
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th edition. Geneva, Switzerland: WHO, 2010. [Google Scholar]
World Health Organization 2012
- World Health Organization. Preconception care to reduce maternal and childhood mortality and morbidity: meeting report and packages of interventions. www.who.int/maternal_child_adolescent/documents/concensus_preconception_care/en/ (2012).
World Health Organization 2013
- World Health Organization. WHO recommendations for the prevention and management of tobacco use and second-hand smoke exposure in pregnancy. http://apps.who.int/iris/bitstream/handle/10665/94555/9789241506076_eng.pdf?sequence=1 (2013). [PubMed]
World Health Organization 2014
- World Health Organization. Guidelines for the identification and management of substance use and substance use disorders in pregnancy. http://apps.who.int/iris/bitstream/handle/10665/107130/9789241548731_eng.pdf?sequence=1 (2014). [PubMed]
World Health Organization 2016
- World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/anc-positive-pregnancy-experience/en/ (2016). [PubMed]
World Health Organization 2017
- World Health Organization. Recommendations on maternal health. www.who.int/maternal_child_adolescent/documents/maternal-health-recommendations/en/ (2017).
World Health Organization 2020a
- World Health Organization. Healthy diet. https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed 29 April 2020).
World Health Organization 2020b
- World Health Organization. Physical activity. https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed 26 November 2020).
Zegers‐Hochschild 2017
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care. Human Reproduction 2017;32(9):1786-801. [DOI: 10.1093/humrep/dex234.] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Anderson 2010
- Anderson K, Norman RJ, Middleton P. Preconception lifestyle advice for people with subfertility. Cochrane Database of Systematic Reviews 2010, Issue 14. Art. No: CD008189. [DOI: 10.1002/14651858.CD008189.pub2] [DOI] [PubMed] [Google Scholar]


