Abstract
Background
Epilepsy is a highly prevalent neurological condition characterised by repeated unprovoked seizures with various aetiologies. Although antiepileptic medications produce clinical improvement in many individuals, nearly a third of individuals have drug‐resistant epilepsy that carries significant morbidity and mortality, and even individuals who have clinical improvement from antiepileptic medications often report iatrogenic symptoms. There remains a need for non‐invasive and more effective therapies for this population. Transcranial magnetic stimulation (TMS) uses electromagnetic coils to excite or inhibit neurons, with repetitive pulses at low‐frequency producing an inhibitory effect that could conceivably reduce cortical excitability associated with epilepsy.
This is an updated version of the original Cochrane Review published in 2016.
Objectives
To assess the evidence for the use of TMS in individuals with drug‐resistant epilepsy compared with other available treatments in reducing seizure frequency, improving quality of life, reducing epileptiform discharges, antiepileptic medication use, and side effects.
Search methods
For the latest update, we searched the Cochrane Register of Studies (CRS Web) and MEDLINE (Ovid 1946 to 2 June 2020). CRS Web includes randomised or quasi‐randomised controlled trials from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the specialised registers of Cochrane Review Groups including Epilepsy.
Selection criteria
We included randomised controlled trials that were double‐blinded, single‐blinded, or unblinded, and placebo controlled, no treatment, or active controlled, which used repetitive transcranial magnetic stimulation (rTMS) without restriction of frequency, coil, duration or intensity on participants with drug‐resistant epilepsy.
Data collection and analysis
We extracted information from each trial including methodological data; participant demographics including baseline seizure frequency, type of epileptic drugs taken; intervention details and intervention groups for comparison; potential biases; and outcomes and time points, primarily change in seizure frequency or responder rates, as well as quality of life and epileptiform discharges, adverse effects, and changes in medication use.
Main results
The original search revealed 274 records from the databases that after selection provided seven full‐text relevant studies for inclusion. The latest search identified 179 new records from the databases that after evaluation against the inclusion and exclusion criteria provided one additional full‐text relevant study. The eight included studies (241 participants) were all randomised trials; seven of the studies were blinded. Methodological and design information in the included studies was unclear, particularly relating to randomisation and allocation concealment methods. We were not able to combine the results of the trials in analysis due to differences in the studies' designs.
For the current update, two of the eight studies analysed showed a statistically significant reduction in seizure rate from baseline (72% and 78.9% reduction of seizures per week from the baseline rate, respectively), whilst the other six studies showed no statistically significant difference in seizure frequency following rTMS treatment compared with controls (low‐certainty evidence). One study assessed quality of life and found that more participants showed improvement in quality of life scores with active treatments compared to the sham treatment, but this only involved seven participants (very low‐certainty evidence).
Four studies evaluated our secondary endpoint of mean number of epileptic discharges, three of which showed a statistically significant reduction in discharges after active rTMS treatment. Adverse effects were uncommon in the studies and typically involved headache, dizziness, and tinnitus; however increased seizure frequency did occur in a small number of individuals. The included trials reported no significant changes in medication use. Overall the risk of bias was either low or unclear, and the certainty of the evidence was low to very low.
Authors' conclusions
Overall, we judged the certainty of evidence for the primary outcomes of this review to be low to very low. We found some evidence to suggest that rTMS is safe but some adverse events were experienced. The variability in technique and outcome reporting prevented meta‐analysis, and the evidence for efficacy of rTMS for seizure reduction is still lacking, despite reasonable evidence that it is effective at reducing epileptiform discharges.
Plain language summary
Transcranial magnetic stimulation for treatment of epilepsy
Background
Epilepsy is a common neurological disorder that appears in various forms. Many individuals with epilepsy have satisfactory seizure control with the use of antiepileptic medications. Yet, nearly a third of people with epilepsy suffer from frequent and uncontrolled seizures despite the use of medication, or are unable to tolerate the side effects of those medications. Surgery is an option for some people with uncontrolled seizures, but it is invasive and not suitable for all individuals. As a result, there remains a substantial unmet need for safe, effective therapies for these harder‐to‐treat epilepsies.
Transcranial magnetic stimulation (TMS) is one of several newer treatments that can potentially offer people with epilepsy a safe and non‐invasive alternative to surgery. Long used as a research tool to study brain function, TMS has also been studied as a possible treatment for a number of nervous system conditions, including epilepsy. This non‐surgical and painless treatment uses induced magnetic currents to regulate brain function in order to reduce the tendency to have seizures.
Objective
We aimed in this review to evaluate the evidence for the use of repetitive transcranial magnetic stimulation (rTMS) in individuals with epilepsy compared with other available treatments in reducing seizure frequency, improving quality of life, reducing epileptiform discharges (abnormalities on brain electrographic testing that suggest underlying brain disturbance or seizure tendency), antiepileptic medication use, and side effects.
Methods
The latest search for trials was 2 June 2020. We assessed the evidence from eight randomised controlled trials (studies in which participants are assigned to one of two or more treatment groups using a random method) involving a total of 241 participants comparing rTMS to control treatments (sham treatment, antiepileptic medication, or low‐frequency rTMS).
Results
Some of the included trials showed that rTMS reduces the number of seizures individuals had compared to before the therapy, but other trials did not show any significant differences in seizure frequency. Four trials showed a reduction in epileptiform discharges following rTMS treatment. One study measured changes in quality of life in seven participants; although not statistically analysed they found that a greater proportion of study participants reported increased quality of life scores with active treatments compared to the sham treatment. One trial reported an increase in antiepileptic medication in a single individual but they had received the control treatment. Side effects were uncommon; the most frequently reported side effect was headache (and the majority of individuals completed the treatment with rTMS). However, one study showed an increase in seizure frequency in two individuals: one during the rTMS treatment (who discontinued the treatment early), and one weeks after the treatment.
Certainty of the evidence
Overall, we judged the certainty of the evidence for the main outcome of reduction in seizure frequency to be low due to unclear information in the published papers about study design and the unclear presentation of results. One included study commented on quality of life, but involved only seven participants.
The evidence is current to June 2020.
Summary of findings
Summary of findings 1. Repetitive transcranial magnetic stimulation (rTMS) compared with control for epilepsy.
| Repetitive transcranial magnetic stimulation (rTMS) compared with control for epilepsy | |||||
|
Patient or population: adults and children with epilepsy Settings: outpatients Intervention: repetitive transcranial magnetic stimulation (rTMS) Comparison: control treatment1 | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI)2 | No. of participants (studies) | Certainty of the evidence (GRADE)2 | |
| Assumed risk | Corresponding risk | ||||
| Control treatment1 | Repetitive transcranial magnetic stimulation (rTMS) | ||||
| Reduction in seizure frequency: the proportion of people with a 50% or greater reduction in seizure frequency following the treatment period Follow‐up period: 8 weeks to 14 weeks |
In Fregni 2006, there was a statistically significant advantage to rTMS compared with sham rTMS, whilst in the other 3 studies no statistically significant difference was found between rTMS and sham rTMS, Cantello 2007; Seynaeve 2016, or between focal rTMS and non‐focal rTMS (Joo 2007). | Not estimable | 109 (4 studies) | ⊕⊕⊝⊝ low certainty3,4 | |
| Reduction in seizure frequency: the difference in pre‐ and post‐treatment seizure rates Follow‐up period: 4 weeks to 14 weeks |
Two studies reported statistically significant reductions in seizure rates post‐treatment in the rTMS group (Fregni 2006; Sun 2012), whilst six studies did not find a statistically significant reduction in seizure frequency in the rTMS group (Cantello 2007; Joo 2007; Seynaeve 2016; Tergau 2003; Theodore 2002; Wang 2008). Between‐group differences were not reported. | Not estimable | 225 (8 studies) | ⊕⊕⊝⊝ low certainty3,5 | |
| Improvement in quality of life: the difference in quality of life scores for participants surveyed before and after treatment Follow‐up period: 10 weeks |
Seynaeve 2016 reported quality of life scores qualitatively. | Not estimable | 7 (1 study) | Very low certainty4 | |
| *The basis for the assumed risk and the corresponding risk are the narrative summaries of the studies contributing to each outcome. A relative effect is not estimable as meta‐analysis was not performed.2 CI: confidence interval | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||
1Control treatments were sham rTMS (placebo), antiepileptic drug treatment, low‐intensity rTMS (compared with high‐intensity rTMS), and non‐focal rTMS (compared with focal rTMS). 2Due to variation in study design, interventions, and outcomes measured in the eight studies, we deemed meta‐analysis to not be appropriate and discussed the eight studies narratively in the review. The GRADE judgement for each outcome is based on the characteristics and narrative results of the studies which contributed to each outcome. 3Downgraded once due to unclear design and methodological information in the included studies (unclear risk of bias). 4Downgraded once due to imprecision: small number of participants contributed to the outcome. 5The presentation of the results did not allow for comparisons between rTMS and control; pre‐ and post‐treatment seizure rates were available only within rTMS group.
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Chen 2016).
Description of the condition
Epilepsy is a highly prevalent neurological disorder affecting an estimated 70 million people worldwide (Ngugi 2010). There are 50 new cases per 100,000 people globally each year, and up to 82 new cases per 100,000 people in low and middle‐income countries (Ngugi 2011). One of the world's oldest recognised conditions, epilepsy affects all age groups and has various presentations and causes. Epilepsy is characterised by repeated unprovoked seizures (episodes of continual discharges of brain activity) and can be considered a consequence of an underlying condition, such as a tumour, or of genetic alterations, brain malformations, infection, intoxication, or another illness (Shorvon 2011). Although antiepileptic medications produce clinical improvement, enabling most individuals to control their seizures, a 2008 study conducted in France estimated that 22.5% of individuals have 'drug‐resistant' epilepsy (Picot 2008), leading to increased risks of premature death, injury, psychosocial dysfunction, and a reduced quality of life (Kwan 2011). Individuals whose epilepsy is resistant to medication may pursue alternative therapies including surgery; high‐fat, low‐carbohydrate diets; and vagus nerve stimulation. Although these other therapies can be quite effective, they have limitations in that the diets have poor adherence, and the procedural treatments are invasive and effective only in selected populations. Consequently, there remains a need for non‐invasive and more effective therapies.
Description of the intervention
Transcranial magnetic stimulation (TMS) was developed in 1985 in the UK to study and map different areas of brain activity (Kimiskidis 2010). In the study of epilepsy, TMS has been used to probe cortical excitability in various epilepsy syndromes; to assess the effects of antiepileptic drugs on the brain; and to help identify areas of the brain more prone to seizure for surgical removal (Kimiskidis 2010). Once it became known that repeated pulses of TMS could either excite or suppress neural activity for a prolonged period of time, TMS was studied as a potential therapy for a number of neurological and psychiatric conditions, ranging from stroke to depression to amyotrophic lateral sclerosis (Ridding 2007). Recently, studies have looked at TMS as a potential treatment for epilepsy (Kimiskidis 2010). TMS is a procedure that uses magnetic fields to stimulate nerve cells in the brain to improve the symptoms of epilepsy. With TMS, a large electromagnetic coil is placed against the scalp. The electromagnet creates electrical currents that stimulate nerve cells in the region of the brain involved in epilepsy.
How the intervention might work
The prolonged inhibitory effects of TMS are thought to reduce cortical hyperexcitability associated with various epilepsies. Although the exact mechanisms of the treatment remain a topic of active investigation, emerging evidence suggests that TMS can generate either excitatory or inhibitory responses in cortical tissue. A TMS device employs either one or two copper coils, positioned superficial to a site of interest in the brain, to non‐invasively produce a brief (100 to 400 µs) magnetic pulse (generating a 1.5 to 2 T magnetic field) to an estimated depth of ~2 cm. This magnetic pulse induces an electrical current in a patch of cortical tissue of a few square centimetres, causing a depolarisation of nearby axons (Reithler 2011). Such local stimulation can even affect distant areas in ways that are poorly understood (Reithler 2011). It has been observed that repetitive pulses of TMS can cause long‐lasting effects, persisting for more than one hour after a treatment (Huang 2005). Aside from physical positioning of the device, there is no known way to target particular cell types, and various interactions between excitatory and inhibitory processes may occur. However, repetition at higher frequencies generally has an overall excitatory effect, whilst conversely, low‐frequency repetitive pulses have an inhibitory effect on neurons and may suppress the activity related to seizures. Earlier animal studies showed that a single TMS pulse follows a particular time course, producing an initial facilitation or excitation followed by delayed and prolonged suppression (Moliadze 2003). It has thus been hypothesised that low‐frequency repetitive stimulation results in prolonged synaptic depression when each incoming pulse arrives during the late inhibitory phase produced by the previous pulse; however, this has not been not proven (Reithler 2011). Other important parameters in the use of TMS include intensity and duration.
Why it is important to do this review
Repetitive transcranial magnetic stimulation (rTMS) is an emerging therapy for epilepsy, a highly prevalent neurological condition for which a significant proportion of individuals do not achieve an adequate response to medications. Drug‐resistant epilepsy is associated with reduced quality of life, and such individuals often face surgery or other invasive therapies, which carry significant risks. Even individuals responsive to pharmacological therapy may struggle with the possible adverse effects of their medications. In contrast, rTMS is a painless, non‐invasive approach that, if effective, could have significant advantages over both antiepileptic drugs and surgical management. This systematic review will clarify the available scientific evidence to help clinicians and individuals assess the tolerability and effectiveness of this approach for the treatment of epilepsy.
Objectives
To assess the evidence for the use of rTMS in individuals with drug‐resistant epilepsy compared with other available treatments in reducing seizure frequency, improving quality of life, reducing epileptiform discharges, antiepileptic medication use, and side effects.
Methods
Criteria for considering studies for this review
Types of studies
Eligible studies were:
randomised controlled trials (RCTs);
double‐, single‐, or unblinded;
placebo/sham‐controlled, no treatment, or active controlled (e.g. antiepileptic drug treatment).
Types of participants
Any participant of any age, with any type of drug‐resistant epilepsy syndrome, which includes unclassified types of epilepsy and postsurgical epilepsy patients.
Types of interventions
Repetitive transcranial magnetic stimulation of any frequency, either single‐ or double‐coiled, for any duration and at any intensity added to current therapy or used as single therapy.
Types of outcome measures
Primary outcomes
Reduction in seizure frequency
Proportion of people with a 50% or greater reduction in seizure frequency following the treatment period.
Difference in pre‐ and post‐treatment seizure rates.
Improvement in quality of life
Difference in quality of life scores for participants surveyed before and after treatment.
Secondary outcomes
Reduction in epileptiform discharges
Mean number of epileptiform discharges seen on electroencephalography (EEG) during the period between seizures.
Adverse effects
Proportion of people experiencing any of the following adverse effects, which are considered to be common and important potential adverse effects of transcranial magnetic stimulation.
Behavioural changes
Cognitive disturbances
Headache
Tinnitus
Pain/discomfort
Sedation
Seizures
Proportion of people experiencing the six most common adverse effects, if different from the list above.
Changes in medication requirements
Proportion of people who required fewer seizure medications after treatment.
Proportion of people who required more seizure medications after treatment.
Proportion of people who had no changes to their medication after treatment.
Treatment withdrawal
Proportion of people withdrawn from the study for any reason.
Proportion of people withdrawn from the study due to lack of efficacy or adverse effects.
Search methods for identification of studies
Electronic searches
We ran searches for the original review in April 2013 and subsequent searches in June 2014 and March 2016. For the latest update, we searched the following databases on 2 June 2020.
Cochrane Register of Studies (CRS Web), using the search strategy shown in Appendix 1.
MEDLINE (Ovid 1946 to 2 June 2020), using the search strategy shown in Appendix 2.
CRS Web includes randomised or quasi‐randomised controlled trials from PubMed, Embase, US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the specialised registers of Cochrane Review Groups including Epilepsy. We previously searched Scopus (1823 to 1 June 2014) as a substitute for Embase, using the search strategy shown in Appendix 3; however, this was no longer necessary, as RCTs and quasi‐RCTs in Embase are now included in CRS Web.
We did not impose any date or language restrictions.
Searching other resources
We checked the reference lists of retrieved reports for additional reports of relevant studies. We contacted the authors of conference proceedings to identify any unpublished data and experts in the field to identify any further ongoing trials.
Data collection and analysis
Selection of studies
In the original review, two review authors (RC and DS) independently assessed articles for inclusion in the review. For the latest update, two review authors (DW and BDM) undertook this assessment separately. Any disagreements were resolved through mutual discussion; failing this, advice from a third party (JW or SN in the original review, SN in the updated review) was sought and a consensus determination was made.
Data extraction and management
We extracted the following information from each trial using a data extraction sheet.
Methodological/trial design
Method of randomisation and allocation concealment.
Method of blinding.
Number of people excluded from reported analyses.
Duration of baseline period.
Duration of treatment period.
Individual participant/demographic information
Total number of participants allocated to each treatment group.
Age/gender.
Number of participants within each epilepsy type.
Seizure frequency during baseline period.
Type of background antiepileptic drugs taken.
Intervention
Total number of intervention groups and comparisons.
Intervention details.
Potential biases.
Outcomes
Outcomes and time points reported.
Definition of outcome.
Unit of measurement.
Assessment of risk of bias in included studies
In the original review, two review authors (RC and DS) independently assessed the risk of bias for each trial using the Cochrane 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We discussed any disagreements and reached a consensus. For the latest update, two review authors (DW and BDM) performed this assessment. If an agreement could not be reached, a third‐party opinion (SN) was sought. We rated the included studies as at low, high, or unclear risk of bias on six domains of bias applicable to RCTs: method of randomisation, method of concealing allocation, method of blinding, incomplete outcome data, selective outcome reporting, and other sources of bias.
Measures of treatment effect
We intended to present an overall effect estimate for seizure reduction as a risk ratio. We intended to present an overall effect estimate for the difference in pre‐ and post‐treatment seizure rates and the reduction in the number of epileptiform discharges seen on EEG during the period between seizures as a mean difference. We intended to present overall effect estimates for adverse effects, changes in medication requirements, and withdrawals as risk ratios. We intended to present an overall estimate for the difference in quality of life scores before and after treatment as mean difference (or standardised mean difference if varying quality of life scales were used across studies).
Unit of analysis issues
Seizure reduction may be reported using different measures in trials. In the event that this was the case, we sought data from study authors in order to obtain data suitable to be combined in meta‐analysis. The unit of analysis in all included studies was the individual.
If future updates of this review include studies with units of analysis other than the individual (e.g. cluster‐randomised studies), we will implement the methods described in Section 16.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We included three cross‐over studies in the review. We would have preferred to analyse the results of these studies via paired analyses, taking account of the correlated structure of the treatment groups (Elbourne 2002); however, insufficient data were presented in the publications for such an analysis, so we have narratively presented the results of the studies.
Dealing with missing data
We sought missing data from study authors. We intended to carry out intention‐to‐treat, best‐case, and worst‐case analyses in order to account for any missing data (see Data synthesis for details). We intended to present all analyses in the main report.
Assessment of heterogeneity
We intended to assess clinical heterogeneity by comparing important participant and intervention factors amongst trials, including: age, seizure type, duration of epilepsy, number and type of antiepileptic drugs taken at time of randomisation, study methods, loss to follow‐up, and missing data. We intended to examine statistical heterogeneity using a Chi2 test for heterogeneity and the I2 statistic. Providing no significant heterogeneity was present (P > 0.1), we would employ a fixed‐effect meta‐analysis. In the event that heterogeneity was identified, we would carry out a random‐effects analysis and present both results in the main report.
Assessment of reporting biases
We assessed the included studies for reporting biases using the Cochrane 'Risk of bias' tool. In the event that outcome reporting bias was suspected, we investigated this using the Outcome Reporting Bias In Trials (ORBIT) classification system (Kirkham 2010). We requested all protocols from study authors to enable a comparison between a list of a priori‐listed outcomes and what was reported in the matching papers. We intended to examine publication bias via asymmetry of funnel plots if 10 or more trials were combined. However, as insufficient studies were included in the review, we made an informal assessment by identifying certain aspects of each study, including sponsors of the research and research teams involved.
Data synthesis
We were not able to perform meta‐analysis of the included studies due to variation in study design, interventions, and outcomes measured.
However, for future updates of this review, if further studies are included and meta‐analysis is possible, we will employ a fixed‐effect meta‐analysis to synthesise data, or in the case that substantial heterogeneity is present, a random‐effects method (see Assessment of heterogeneity). Comparisons we expect to carry out include intervention group versus control on: seizure reduction; reduction in epileptiform discharges; adverse effects; and treatment withdrawal.
We will stratify each comparison by type of control group (e.g. placebo, other active treatment, no treatment) to enable the appropriate combination of study data.
Our preferred effect estimate is a risk ratio. For all outcomes, except adverse effects, we will use 95% confidence intervals. For individual adverse effects, we will use 99% confidence intervals to make allowance for multiple testing.
All analyses will include all participants in the treatment group to which they had been allocated. For the efficacy outcome of seizure reduction, we intend to employ three analyses, as follows.
Primary (intention‐to‐treat (ITT)) analysis: participants not completing follow‐up or with inadequate seizure data are assumed to be non‐responders. To test the effect of this assumption, we will employ the following sensitivity analyses.
Worst‐case analysis: participants not completing follow‐up or with inadequate seizure data are assumed to be non‐responders in the magnetic stimulation group, and responders in the control group.
Best‐case analysis: participants not completing follow‐up or with inadequate seizure data are assumed to be responders in the magnetic stimulation group and non‐responders in the control group.
Subgroup analysis and investigation of heterogeneity
If further studies are included in an update of this review and meta‐analysis is possible, we will carry out subgroup analysis of any varying trial and participant characteristics (e.g. cross‐over compared to parallel trial design, adults compared to children, epilepsy types, etc.) to explore heterogeneity, if identified.
Sensitivity analysis
If further studies are included in an update of this review and meta‐analysis is possible, we will carry out sensitivity analysis if deemed appropriate, including the sensitivity analyses described in Data synthesis to account for the presence of missing data. If peculiarities are found between studies with regard to quality, characteristics of participants, interventions and/or outcomes, we will conduct sensitivity analysis to explore these differences.
Summary of findings and assessment of the certainty of the evidence
In a post hoc change from the protocol for this review, we have added a 'Summary of findings' table (Table 1), which reports the primary outcomes of the review (reduction in seizure frequency and quality of life).
We determined the certainty of the evidence using the GRADE considerations of study limitations, consistency of effect, imprecision, indirectness, and publication bias (Atkins 2004).
We downgraded the certainty of the evidence by one level if we considered the limitation serious and by two levels if we considered it to be very serious. With the GRADE approach, evidence may also be upgraded if a large treatment effect is demonstrated with no obvious biases, or if a dose‐response effect exists.
Results
Description of studies
Results of the search
In the original review, the search revealed 274 records from the databases outlined in Electronic searches. After removal of 92 duplicates, we screened the remaining 182 records for eligibility. We excluded 173 studies that were irrelevant, and assessed one study as ongoing (NCT01745952). This left eight full‐text articles for assessment, of which seven were included in the review (Cantello 2007; Fregni 2006; Joo 2007; Sun 2012; Tergau 2003; Theodore 2002; Wang 2008), and one was excluded due to study design (NCT00382707); see also Included studies and Excluded studies.
In the current update, the search revealed 179 new records from the databases outlined in Electronic searches. After removal of 24 duplicates, we screened the remaining 155 records for eligibility. We excluded 149 studies that were irrelevant, and assessed five studies as ongoing (CTRI/2017/10/010067; CTRI/2019/02/017440; NCT02757547; NCT03154307; Oberman 2015). This left one full‐text article for assessment, which we included in the latest review (Seynaeve 2016). This was the published article for the ongoing trial NCT01745952 documented in the original review.
Due to variation in study design, interventions, and outcomes measured in the eight studies, we deemed meta‐analysis to be inappropriate and have discussed the eight studies narratively in the review; see Figure 1 for further information.
1.
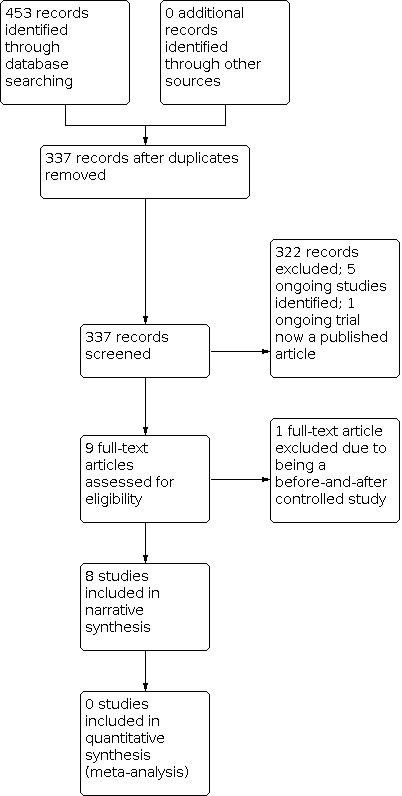
Study flow diagram
Included studies
We included eight RCTs that compared rTMS with active or placebo controls (Cantello 2007; Fregni 2006; Joo 2007; Seynaeve 2016; Sun 2012; Tergau 2003; Theodore 2002; Wang 2008). Three studies were placebo controlled (Cantello 2007; Fregni 2006; Theodore 2002); one study compared rTMS of different intensities (Sun 2012); one study compared rTMS of different intensities versus placebo (Tergau 2003); one study compared focal to non‐focal application of rTMS (Joo 2007); one study compared different rTMS coil types versus placebo (Seynaeve 2016); and one study compared rTMS versus antiepileptic drug treatment (Wang 2008).
All recruited participants had drug‐resistant epilepsy of varying definitions across studies, which was generally defined as at least one complex focal or secondarily generalised seizure per month (but most studies required three or more seizures per week) and an unchanging drug regimen of at least two antiepileptic medications. Except for Seynaeve 2016, which compared figure‐8 coils and round coils to placebo, the included studies used standard figure‐8 coils to deliver rTMS. Sham methods differed between studies.
Cantello 2007 was an Italian multi‐centre, cross‐over, randomised, double‐blind, sham‐controlled trial, with a pre‐treatment period of 12 weeks, five days of active treatment, and a follow‐up evaluation period of six weeks. Forty‐three participants were randomised to either active first or placebo first treatment, with six weeks of follow‐up after each treatment phase (active or sham). Active rTMS was administered twice daily for five days using two circular coils of 500 stimuli at 0.3 Hz, separated by a 30‐second interval at an intensity of 100% of resting motor threshold (RMT).
Fregni 2006 was a randomised, double‐blind, sham‐controlled trial in Brazil with a baseline period of four weeks, five days of active treatment, and a post‐treatment evaluation period of eight weeks. Twenty‐one participants were randomised to active (n = 12) and sham (n = 9) treatment arms. Participants in the treatment group received either focal rTMS, based on the known location of abnormalities on their electroencephalography (EEG), or at midline (CZ site) if there were diffuse EEG abnormalities. rTMS was administered for 20 minutes a day for five days at settings of 1 Hz, 1200 pulses, at 70% of RMT intensity.
Joo 2007 was a Korean randomised, double‐blind, but not placebo‐controlled study using a baseline surveillance period of eight weeks, five days of active treatment, and a post‐treatment evaluation period of eight weeks. Thirty‐five participants with focal, non‐focal, or multifocal epilepsy were randomised into one of four subgroups (F‐1500, F‐3000, NF‐1500, NF‐3000) to either receive focal or non‐focal rTMS for five days, delivering a total of either 1500 pulses (50 minutes) or 3000 pulses (100 minutes) a day, at an intensity of 100% of RMT, 0.5 Hz frequency. Participants were assessed with daily symptom logs before the treatment period and at eight weeks post‐stimulation.
Seynaeve 2016 was a Belgian single‐centre, randomised, double‐blind, sham‐controlled, cross‐over trial with a baseline period of eight weeks, treatment period over two weeks, and observation period of 10 weeks. The study aimed to recruit 20 participants for their main comparison, but only 11 participants with well‐defined focal epilepsy were recruited. These participants were randomly allocated to three treatment arms: placebo stimulation first; 8‐coil stimulation first; or round coil stimulation first. They subsequently received the remaining two coils after the observation period. Participants underwent treatment of 10 sessions over two weeks of 1500 stimuli a day at a frequency of 0.5 Hz at 90% RMT. A focal epileptic zone, decided upon by a multidisciplinary team and investigations (magnetic resonance imaging (MRI), video‐EEG, fluorodeoxyglucose–positron emission tomography (FDGPET), and Subtraction Ictal SPECT co‐registered to MRI (SISCOM) data) was targeted.
Sun 2012 was a Chinese randomised, single‐blind, non‐placebo‐controlled study with a baseline evaluation period of four weeks, two weeks of active treatment, and eight weeks of clinical follow‐up. Sixty participants were randomised to one of two treatment arms: high‐intensity rTMS at 90% of RMT (n = 31) and low‐intensity rTMS at 20% RMT (n = 29). rTMS was delivered three times a day for two weeks to the focal epileptic zone best reflected on EEG with 500 stimuli at 0.5 Hz, separated by a 600‐second interval.
Tergau 2003 was an interim analysis of a randomised, multi‐centre, cross‐over study conducted over three centres in Germany with three treatment arms; placebo stimulation, 0.333 Hz stimulation, and 1 Hz stimulation. The baseline period was three months, and treatment periods were five days followed by a four‐week observation period. All three treatment periods were separated by at least eight weeks. Treatment was delivered unifocally for all three arms, with 1000 pulses each day (500 monopolar pulses with clockwise current direction followed directly by 500 pulses in an anticlockwise direction). Data were available for 17 participants in the interim analysis who had received all three treatments.
Theodore 2002 was a randomised, double‐blind, placebo‐controlled trial conducted at the National Institutes of Health, with a baseline evaluation of eight weeks, one week of active treatment, and a post‐treatment follow‐up period of eight weeks. Twenty‐four participants with localisation‐related drug‐resistant epilepsy were randomised into active treatment (n = 12) and placebo (n = 12) arms. rTMS was administered at an intensity of 120% RMT at 1 Hz frequency, for 15 minutes, twice a day, for one week.
Wang 2008 was a randomised, open‐label, antiepileptic drug‐controlled trial at a single centre in China. Fifteen participants were randomised to 1 Hz rTMS at 90% RMT threshold, simulation frequency of 500 times, once a day for seven days, and 15 participants were randomised to 600 mg to 800 mg oral carbamazepine per day for at least 60 days. Outcomes were measured 30 days after treatment with rTMS.
Excluded studies
We only excluded one study from the review after full‐text evaluation, due to the study design, as the trial was a controlled before‐and‐after study, and not an RCT (NCT00382707).
Risk of bias in included studies
In the original review, two review authors (RC and DS) independently assessed the risk of bias for each trial using the Cochrane 'Risk of bias' tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In the current review, this was performed by two review authors (DW and BDM). Any disagreements were discussed and resolved by consensus or by consulting with a third review author (SN). We assessed six domains for each trial: allocation concealment, randomisation method, blinding, completeness of data, selective outcome reporting, and other bias. Notable risks of bias are highlighted as follows. We considered Wang 2008, a randomised but open‐label study, to be at high risk of detection bias, as outcome assessors were not blinded. We deemed Tergau 2003, an interim analysis, to be at high risk of attrition bias due to incomplete outcome data, and at unclear risk of performance bias because blinding was not described. We found Joo 2007 and Seynaeve 2016 to have a high risk of reporting bias, as no primary or secondary outcomes were defined in their methods sections. We assessed the majority of studies as at unclear risk of selection bias when the allocation concealment method was not specified. More detailed findings for each study are summarised in the 'Risk of bias' tables in Characteristics of included studies and in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two studies described adequate methods of generating random sequences (computer‐assisted generation of random sequence) and were judged as at low risk of bias (Cantello 2007; Sun 2012). Three studies described that studies were 'randomised' but provided no information on methods of generating random sequences (Tergau 2003; Theodore 2002; Wang 2008), and one study reported that a "randomization code" was used, but provided no details of how the code was generated (Joo 2007). Another study reported using a computerised random number generator to choose the order of treatments in individuals, with a permutation for each block of three participants (Seynaeve 2016); on further analysis the initial treatment arm appeared to be randomly generated, but the subsequent treatment arms were delivered in a set pattern. We judged these five studies as at unclear risk of bias. One study stated that the "order of entrance" into the trial was used as well as computer‐generated randomisation blocks (Fregni 2006). It is unclear exactly how the "order of entrance" was taken into account in the randomisation and whether this could have led to a predictable randomisation sequence, therefore we judged this study to be at unclear risk of selection bias.
None of the studies reported how allocation was concealed, so we judged all eight studies to be at unclear risk of bias.
Blinding
Six of the eight included studies were described as 'double‐blind' (Cantello 2007; Fregni 2006; Joo 2007; Seynaeve 2016; Sun 2012; Theodore 2002). In five of the six studies, only the investigator(s) responsible for initiating rTMS treatment were not blinded; participants, all other personnel, and outcome assessors were blinded, therefore we judged these five studies to be at low risk of performance and detection bias (Cantello 2007; Fregni 2006; Seynaeve 2016; Sun 2012; Theodore 2002). However, in Seynaeve 2016 two different shaped coils (figure‐8 and round) were used along with a sham coil; it is not stated how the structural difference was kept from participants, and Seynaeve 2016 examined blinding by asking participants to guess the treatments they were receiving. In the other study (Joo 2007), only blinding of the EEG reader was described; it is unclear if participants and other personnel and outcome assessors were blinded, therefore we judged this study to be at unclear risk of performance and detection bias.
In Tergau 2003, blinding of interventions was not mentioned, even though a "placebo stimulation" was used; we judged this study to be at unclear risk of performance and detection bias.
Wang 2008 randomised participants to rTMS or drug treatment, so blinding or participants and personnel would not be possible by design; it is unclear how this design may have influenced outcomes, therefore we judged this study to be at unclear risk of performance bias. Blinding of outcome assessors in a trial of this design would be possible; however, it appears that outcome assessors were not blinded, therefore we judged this study to be at high risk of detection bias.
Incomplete outcome data
In five studies there were no apparent missing data; study attrition was reported (if there were any withdrawals); and an ITT approach to analysis was used, so we judged these five studies to be at low risk of attrition bias (Cantello 2007; Fregni 2006; Sun 2012; Theodore 2002; Wang 2008).
In one study there were no apparent missing data and no withdrawals from treatment reported, but ITT analysis was not specified (Joo 2007). In another study two participants withdrew: one participant was excluded due to incomplete data, and one was excluded due to medication change secondary to toxicity, and ITT analysis was also not specified (Seynaeve 2016). However, the study attrition was reported and data documented. We judged these studies to be at unclear risk of attrition bias.
In Tergau 2003, an interim analysis was presented for 17 participants out of 28 randomised (5 participants were yet to complete the study, and 6 had dropped out). An ITT approach was not used for analysis, and it was unclear when participants dropped out and how many of the cross‐over arms had been completed. We judged this study to be at high risk of attrition bias.
Selective reporting
Study protocols were not available for any of the included studies, therefore we made judgements regarding reporting bias based on study publications alone. In five studies, all primary and secondary outcomes stated in the methods section were reported in the results, and all expected outcomes were reported; we judged these studies to be at low risk of reporting bias (Cantello 2007; Fregni 2006; Sun 2012; Theodore 2002; Wang 2008).
In three studies (Joo 2007; Seynaeve 2016; Tergau 2003), no primary or secondary outcomes were specified in the methods section, so we judged these studies to be at high risk of reporting bias.
Other potential sources of bias
We did not identify any other sources of bias in five studies, which we judged to be at low risk of other bias (Fregni 2006; Joo 2007; Sun 2012; Theodore 2002; Wang 2008).
In the three cross‐over studies (Cantello 2007; Seynaeve 2016; Tergau 2003), the carry‐over effect was not formally assessed, but the observation period of six weeks, 10 weeks, and eight weeks, respectively, between treatments is likely to be a sufficient 'wash‐out' period. However, none of these studies stated how many participants were randomised to each treatment arm first, and whether these randomised groups were balanced for clinical demographics at baseline. Furthermore, for Tergau 2003, it is unclear how a three‐arm cross‐over trial design was to be implemented. We therefore judged these studies to be at unclear risk of other bias.
Effects of interventions
See: Table 1
Due to variation in study design, interventions, and outcomes measured in the eight studies, we deemed meta‐analysis to not be appropriate, and we discussed the eight studies narratively in the review. See Table 1 for a summary of the certainty of evidence for the primary outcomes of the review. We judged the certainty of the evidence for the primary outcomes of this review to be low (reduction in seizure frequency) to very low (quality of life).
Primary outcomes
Reduction in seizure frequency
Proportion of people with a 50% or greater reduction in seizure frequency following the treatment period
Four studies reported on this outcome. Only Fregni 2006 reported a statistically significant and high responder rate of 10 out of 12 participants in their intervention arm experiencing > 50% reduction in seizures (including 3 participants who were seizure‐free), compared with zero responders in the sham procedure group. The proportion of responders was not statistically different between the intervention and sham arms in Cantello 2007; for example, four weeks after treatment, 9/43 and 3/43 participants in the active and sham procedure groups, respectively, exhibited a 50% reduction of seizures. Joo 2007 also reported no statistically significant difference between groups in responder rates, but compared only focal versus non‐focal use of rTMS without a placebo control. Seynaeve 2016 reported no statistically significant difference between condition arms compared to baseline with either figure‐8 or round coils, and no participants achieved a 50% reduction in seizure frequency.
Difference in pre‐ and post‐treatment seizure rates
All eight included studies reported on this outcome; however, generally within‐treatment group results were reported rather than comparisons between groups. Fregni 2006 showed a statistically significant reduction of seizure frequency by 72% of baseline after two weeks of treatment that continued (53% at four weeks, 58% at eight weeks) in the active‐treated group. There was no significant change in seizure frequency in the sham‐treated group. Sun 2012 also reported a statistically significant 78.9% reduction of seizure frequency from baseline (8.9 to 1.8 seizures per week) in the high‐intensity treatment group. There was no significant change in frequency after treatment in the low‐intensity treatment group. Sun 2012 study did not have a placebo control group. In Theodore 2002, there was no significant reduction in seizure frequency in either the active or placebo groups, although a trend was reported. The study by Tergau 2003 showed no significant reduction of seizure frequency in the two intervention groups (0.333 Hz and 1.0 Hz) compared with placebo overall. However, there was a statistically significant, approximately 40% reduction in seizure frequency, two weeks following intervention when compared with baseline. This difference was not significant when compared with placebo. Similarly, Joo 2007, Cantello 2007, Seynaeve 2016, and Wang 2008 reported no significant reduction in seizure frequency in either treatment group.
Improvement in quality of life
Difference in quality of life scores for participants surveyed before and after treatment
Only one study reported on this outcome, using Quality of Life in Epilepsy‐31 (QOLIE‐31) (Seynaeve 2016). Only seven participants filled out the questionnaires, and the results were discussed in a qualitative manner with no formal statistical analysis performed. Overall, a greater proportion of participants had an improvement in quality of life scores in the active treatment arms compared to the sham treatment arm. One participant in all three arms showed an improvement compared to baseline, but the smallest effect size was seen with the sham treatment arm. One participant in the round and sham coil arms had a worse quality of life score.
Secondary outcomes
Reduction in epileptiform discharges
Mean number of epileptiform discharges seen on electroencephalography (EEG) during the period between seizures
Four studies reported on this outcome. Fregni 2006 reported a statistically significant reduction of epileptiform discharges in the active treatment group of 31% immediately after the five‐day treatment and 16% at four weeks prior to washing out. There was no significant change in the number of epileptiform discharges in the sham group. Sun 2012 reported a significant reduction of epileptiform discharges to 65.8% in the first 24 hours in the high‐intensity treatment group, with no change from baseline in the low‐intensity treatment group. The mean reduction in number of epileptiform discharges in Cantello 2007 was not statistically significant. In Joo 2007, epileptiform discharges were significantly reduced by 54.9% after rTMS treatment in all groups combined. The mean number of epileptiform discharges was not studied in Seynaeve 2016, Tergau 2003, Theodore 2002, and Wang 2008. Two studies reported a responder rate based on epileptiform discharges. For example, Cantello 2007 reported a statistically significant decrease in epileptiform discharges by 50% or more from baseline in one‐third of participants receiving rTMS versus less than 5% of those receiving sham treatment. Wang 2008 noted significantly fewer participants with epileptiform discharges after treatment with rTMS (27% of participants) than in the drug‐only group (73% of participants), compared with 100% with such discharges at baseline.
Adverse effects
Seven of 43 participants in Cantello 2007 experienced adverse effects, without a significant difference between active and sham treatments; dizziness and headache were the most frequently reported adverse effects. In Sun 2012, two participants experienced mild adverse effects, such as headache and tinnitus, in the high‐intensity group. Theodore 2002 reported rare adverse effects, with one participant experiencing mild discomfort, and another participant withdrawing after having a seizure during treatment (but then had an 80% decrease in seizure frequency two weeks after treatment). In Joo 2007, five of 35 participants complained of a mild and transient headache during and immediately after rTMS. Several participants had a mild headache, and one had insomnia in the Fregni 2006 study, with no significant difference between intervention and sham groups. In the Wang 2008 study, five of 15 participants in the rTMS group experienced headache compared with none in the placebo group. There were increases in seizure frequency in two participants in Seynaeve 2016. One participant had an initial reduction in seizure frequency followed by an abrupt rebound compared to baseline for up to 20 weeks following the two active treatment arms. The other participant reported increased seizure frequency during the rTMS treatment and withdrew from the study. Four participants also experienced headaches: three reported these as minor, but one reported significant pain over an operation scar within minutes of rTMS taking place. Tergau 2003 reported no significant side effects of rTMS.
Changes in medication requirements
Proportion of people who required fewer seizure medications after treatment
None of the studies reported on this outcome.
Proportion of people who required more seizure medications after treatment
Only one study reported on this outcome. One out of nine participants after sham treatment compared with zero of the active treatment participants required an increase in medication in the Fregni 2006 study.
Proportion of people who had no changes to their medication after treatment
None of the studies reported on this outcome.
Treatment withdrawal
Proportion of people withdrawn from the study for any reason
Seven studies reported on this outcome. Cantello 2007, Fregni 2006, and Joo 2007 reported no withdrawals. Sun 2012 had no withdrawals in the high‐intensity treatment group, but two out of 29 participants were lost to follow‐up in the low‐intensity treatment group. In Theodore 2002, one of 12 active treatment participants developed an unrelated medical condition (cancer of the colon) and was not included in the eight‐week analysis. Another active treatment participant did not complete the full week of stimulation due to having had a seizure during stimulation. One control participant did not keep evaluable seizure calendars for the post‐treatment period and had reported a seizure frequency eight times the population mean during baseline; this participant was not included in the analysis. In Tergau 2003, out of 28 participants enrolled, five are yet to complete the study, and six were dropped from analysis with no reason provided. In Seynaeve 2016, two participants withdrew: one due to an exacerbation of seizures with the first treatment arm, and the other after two treatment arms citing lack of efficacy and pain associated with the treatments. Two further participants were excluded, one because of changes in medication due to toxicity, and the other due to an incomplete seizure diary; the latter was not included in the analysis. Wang 2008 did not report treatment withdrawal.
Proportion of people withdrawn from the study due to lack of efficacy or adverse effects
Withdrawals were reported as above and were rare. There were three instances of withdrawal due to an adverse effect: one of 12 active participants in Theodore 2002 after experiencing a seizure during stimulation; one participant in Seynaeve 2016 after experiencing an increase in seizure frequency during treatment; and one in Seynaeve 2016 experiencing the treatments as painful and not effective.
Discussion
Summary of main results
All of the eight studies included in the review were randomised trials. Seven trials were blinded with Wang 2008 being an open‐label study. Two of the studies showed a statistically significant reduction in seizure rate from baseline (72% and 78.9% reduction of seizures per week from the baseline rate, respectively) (Fregni 2006; Sun 2012). The other six studies showed no statistically significant difference in seizure frequency following rTMS treatment compared to baseline (Cantello 2007; Joo 2007; Seynaeve 2016; Tergau 2003; Theodore 2002; Wang 2008). However, in Tergau 2003, there was a trend towards response reported at two weeks after rTMS with the 0.333 Hz stimulation subgroup, with a 40% reduction in seizure frequency compared with baseline that failed to reach significance when compared with the placebo group.
Across studies, seizure frequency and seizure rates were reported by different measures, time points, and techniques, which precluded comparison between groups. Of the four studies that reported on the proportion of responders (Cantello 2007; Fregni 2006; Joo 2007; Seynaeve 2016), only Fregni 2006 showed a significant high responder rate of 10 out of 12 active participants experiencing > 50% reduction in seizure frequency, compared with zero responders in the sham procedure group.
Of the eight included studies, four evaluated our secondary outcome of change in mean number of epileptic discharges. Fregni 2006, Sun 2012, and Joo 2007 demonstrated a statistically significant reduction in epileptiform discharges. In Cantello 2007, there was no difference in the mean reduction in number of epileptiform discharges, but there was a statistically significant decrease in epileptiform discharges by 50% in more active treatment participants compared with sham treatment participants. Similarly, Wang 2008 showed significantly fewer participants with any epileptiform discharges after treatment than in controls.
One study assessed quality of life (Seynaeve 2016), but only involved seven participants and was analysed in a qualitative manner rather than statistically. Adverse effects were uncommon amongst the studies and typically involved headache, dizziness, and tinnitus. Two participants experienced a seizure when receiving treatment while another had a marked increase in the number of seizures following an initial reduction following treatment. Only one study reported changes in medication requirements, finding no significant need to increase medications in participants after active rTMS. Treatment withdrawal was well reported and was uncommon amongst studies. Three participants reportedly withdrew from further treatment with rTMS due to adverse effects (i.e. either seizures or pain).
Overall completeness and applicability of evidence
We reviewed a total of eight studies, including 241 randomised participants. One study, Tergau 2003, was only an interim analysis of a study that was never completed; the rest were completed published studies. In most studies, focal epilepsy with or without secondary generalisation was more common than diffuse or multifocal epilepsies. In all studies, participants had drug‐resistant epilepsy, and were typically either not good surgical candidates or had declined surgery. Although the inclusion criteria required a minimum of one to three seizures per week at baseline (depending on the study) despite medication, the average number of baseline seizures in the participants were generally greater than 10 per week in most studies, suggesting these were truly drug‐resistant participants. The participants studied are thus likely representative of a clinically relevant population, making the results applicable.
Quality of the evidence
The included studies had a substantial amount of methodological variability, and for many studies the details provided regarding design were insufficient to permit an accurate assessment of study quality.
The study of Theodore 2002 leaves open the possibility of a mild treatment effect. A trend towards positive effect was noted, but the study was only powered to detect a large (70%) reduction in seizures, making a type II error possible. Their subgroup analysis showed that focal cortical epilepsies may respond better to rTMS. Joo 2007 also reported a trend in their focal stimulation, long‐duration treatment subgroup, with 30.6% reduction (P = 0.059) from baseline seizure frequency. Their study measured seizure frequency by averaging over eight weeks before and after intervention, and did not report discrete data points, making it difficult to evaluate whether a potential shorter‐lived initial effect was masked by averaging seizure frequency over such a long period.
Three studies were placebo controlled, whilst the other studies compared focal to non‐focal application of rTMS, application of rTMS at different intensities or durations, or application of different coil types. Even between the placebo‐controlled studies, differing parameters of frequency (0.3, 0.5, or 1 Hz), intervals, duration (between 500 to 3000 stimuli, daily for five to seven days in most trials, except Sun 2012, which had a 14‐day treatment), and intensity (low of 20% RMT to high of 120% RMT, and one study that used a fixed high intensity) were found. We noted that the low‐intensity treatment arm in the Sun 2012 study could serve as a de facto placebo control group, given that the level of stimulation at that intensity would be unlikely to be effective, and indeed did not result in a statistically significant treatment effect in their study.
Due to this high degree of variability in study design, we were not able to synthesise results in meta‐analysis. We therefore have presented a discussion of the results narratively. Overall, we judged the certainty of the evidence for the primary outcomes of this review to be low to very low due to the limited and methodologically unclear information available. Of the eight included studies, two studies did show a significant effect, whereas six did not. Given that the variability of study design and techniques and reported parameters precluded meta‐analysis, this narrative review cannot refute a beneficial effect of rTMS on seizure reduction, though strong supportive and comparative evidence for efficacy is still lacking.
Potential biases in the review process
Our searches were comprehensive, and included searches of unpublished literature and ongoing studies; we hope to include the identified ongoing studies in future updates of the review (CTRI/2017/10/010067; CTRI/2019/02/017440; NCT02757547; NCT03154307; Oberman 2015). The possibility remains that our searches may have missed relevant studies; however, we believe this to be unlikely.
Given the extent of variability in the designs of the included studies, we felt performing a meta‐analysis of study results would be inappropriate, and that a narrative review, although less informative and concise than a meta analysis, would provide more reliable and appropriate interpretations of the results and conclusions drawn from this review.
Agreements and disagreements with other studies or reviews
A prior systematic review of 11 studies by Hsu 2011 found a small but significant effect of low‐frequency rTMS on medically intractable epilepsy. A further systematic review of 12 studies (Cooper 2018), which performed an individual participant data meta‐analysis, found a significant reduction in seizure frequency with low‐frequency rTMS, and highlighted parameters that conferred favourability. However, these prior systematic reviews included additional open‐label, non‐randomised, observational cohort studies, where all participants received the intervention. The present review includes only randomised studies comparing intervention and control cohorts.
We found in our review that no appropriate meta‐analysis could be done due to the wide variability of technique as well as time points reported in each study, as the first measurement could be at one day, two weeks, or eight weeks after treatment, with demonstrable differences in effect within individual study parameters. Whilst Cooper 2018 performed an individual participant data meta‐analysis, this involved only 34 participants from five of the 12 studies, four of which were cohort studies. Moreover, the effect seen in Hsu 2011 was based on first measurement after the intervention, despite differences in technique and outcome reporting within each study.
Authors' conclusions
Implications for practice.
There is some evidence to suggest that repetitive transcranial magnetic stimulation (rTMS) is safe and in some cases effective at reducing epileptiform discharges on electroencephalography (EEG). A narrative review of the currently available studies included two studies that showed a significant effect on seizure frequency, and six studies that did not show a significant effect. Given the variability in technique and outcome reporting, which prevented meta‐analysis, definitive evidence for the efficacy of rTMS for seizure reduction in focal drug‐resistant epilepsies is still lacking.
Implications for research.
The use of rTMS is still a relatively new therapy for seizures, and future studies should aim to scientifically establish a standard technique for its application. There is some evidence that focal epilepsies with imaging findings may be more amenable to treatment with rTMS, and further randomised trials are needed to assess its efficacy for drug‐resistant epilepsies. It is important that future trials are of sufficient duration, and from the outset must be adequately powered in sample size to inform longer‐term outcomes of efficacy (seizure reduction), quality of life, and any adverse effects related to rTMS treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 2 June 2020 | New search has been performed | Searches updated 2 June 2020; one new study has been included (Seynaeve 2016). |
| 2 June 2020 | New citation required but conclusions have not changed | Conclusions are unchanged. |
History
Protocol first published: Issue 4, 2014 Review first published: Issue 8, 2016
Acknowledgements
We would like to thank and acknowledge two of the authors of the original protocol and review, Ricky Chen and Jennifer Weston.
This review update was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health and Social Care.
Appendices
Appendix 1. CRS Web search strategy
1. MESH DESCRIPTOR Transcranial Magnetic Stimulation EXPLODE ALL AND CENTRAL:TARGET
2. "transcranial magnetic stimulation":AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
3. #1 OR #2
4. MESH DESCRIPTOR Epilepsy EXPLODE ALL AND CENTRAL:TARGET
5. MESH DESCRIPTOR Seizures EXPLODE ALL AND CENTRAL:TARGET
6. (epilep* OR seizure* OR convuls*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
7. #4 OR #5 OR #6 AND CENTRAL:TARGET
8. eclampsia:TI AND CENTRAL:TARGET
9. #7 NOT #8 AND CENTRAL:TARGET
10. #3 AND #9
Appendix 2. MEDLINE search strategy
This strategy includes a modification of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2020).
1. exp Transcranial Magnetic Stimulation/
2. transcranial magnetic stimulation.tw.
3. 1 or 2
4. exp Epilepsy/
5. exp Seizures/
6. (epilep$ or seizure$ or convuls$).tw.
7. 4 or 5 or 6
8. exp *Pre‐Eclampsia/ or exp *Eclampsia/
9. 7 not 8
10. exp controlled clinical trial/ or (randomi?ed or placebo or randomly).ab.
11. clinical trials as topic.sh.
12. trial.ti.
13. 10 or 11 or 12
14. exp animals/ not humans.sh.
15. 13 not 14
16. 3 and 9 and 15
17. remove duplicates from 16
Appendix 3. Scopus search strategy
(TITLE‐ABS‐KEY("Transcranial Magnetic Stimulation")) and ((TITLE‐ABS‐KEY(epilep* OR "infantile spasm" OR seizure OR convuls* OR (syndrome W/2 (aicardi OR angelman OR doose OR dravet OR janz OR jeavons OR "landau kleffner" OR "lennox gastaut" OR ohtahara OR panayiotopoulos OR rasmussen OR rett OR "sturge weber" OR tassinari OR "unverricht lundborg" OR west)) OR "ring chromosome 20" OR "R20" OR "myoclonic encephalopathy" OR "pyridoxine dependency") AND NOT (TITLE(*eclampsia) OR INDEXTERMS(*eclampsia))) OR (TITLE‐ABS‐KEY(lafora* W/4 (disease OR epilep*)) AND NOT (TITLE(dog OR canine) OR INDEXTERMS(dog OR canine)))) and (TITLE((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study)) OR ABS((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study)))
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cantello 2007.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo controlled, cross‐over trial 2 treatment arms: 1 placebo first, 1 rTMS first Pre‐randomisation baseline period: 12 weeks Treatment period: twice daily for 5 days in each arm Follow‐up evaluation period: 6 weeks after each treatment or placebo phase for observation of effect Total study duration: 14 weeks |
|
| Participants | Multicentre study in Italy 43 people randomised. 26 participants were male and 17 were female. Mean age was 36.9 years (SD 13 years) All with drug‐resistant epilepsy (treated with 2 to 4 AEDs and experiencing 3 or more seizures per week), majority were focal epilepsy Not stated how many participants were randomised to each treatment arm (placebo first or rTMS first) |
|
| Interventions | Sham procedure treatment followed by active rTMS, or active rTMS followed by sham treatment. Treatment parameters: 2 circular coils of 500 stimuli at 0.3 Hz, separated by a 30‐second interval at an intensity of 100% of RMT, placed at the vertex regardless of type of epilepsy. |
|
| Outcomes | Proportion of people with a 50% or greater reduction in seizure frequency following the treatment period Change in seizure frequency per week post‐treatment Mean number of ED seen on EEG during the period between seizures (during and after the rTMS cycle) Proportion of people experiencing adverse events and withdrawals from treatment |
|
| Notes | This trial differs from the others because of its cross‐over design (carry‐over effect is not formally assessed, but the observation period of 6 weeks between treatments is likely to be a sufficient 'wash‐out' period) and that rTMS was placed at the vertex regardless of type of epilepsy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study used computer‐assisted randomisation to initial active treatment or sham treatment |
| Allocation concealment (selection bias) | Unclear risk | The allocation concealment method was not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The same apparatus and 'noise' was used to blind participants and personnel. Only the investigator initiating the treatment was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded to intervention group of participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study attrition was reported, and there were no missing data |
| Selective reporting (reporting bias) | Low risk | Study protocols were not available. Primary and secondary outcomes stated in the methods section were reported in the results, all expected outcomes were reported. |
| Other bias | Unclear risk | Observation period of 6 weeks between treatments is likely to be a sufficient 'wash‐out' period in this cross‐over study. Not stated how many participants were randomised to each treatment arm (placebo first or rTMS first) and whether these randomised groups were balanced for clinical demographics at baseline. |
Fregni 2006.
| Study characteristics | ||
| Methods | Randomised, double‐blind, sham‐controlled, parallel‐design trial 2 treatment arms: active rTMS versus sham rTMS Baseline observation period: 4 weeks Follow‐up period: 8 weeks |
|
| Participants | Single‐centre study in Brazil 21 participants were randomised (12 to active rTMS, 9 to sham rTMS). All participants had drug‐resistant epilepsy, with a mean frequency of seizures greater than 10 per month despite 2 or more AEDs. The majority had focal epilepsy compared with generalised epilepsy. Participants either had refused surgery or were poor surgical candidates 12 female participants, 9 male participants. Mean age was 21.9 years (SD 8.1 years) |
|
| Interventions | Treatment period: once a day for 5 consecutive days Treatment parameters: 1 Hz frequency, fixed intensity of 70% of max stimulator output, for a duration of 20 minutes |
|
| Outcomes | Proportion of people with a 50% or greater reduction in seizure frequency following the treatment period Change in seizure frequency per week post‐treatment Mean number of EDs in the EEG Proportion of people experiencing adverse events and withdrawals from treatment Proportion of people who required a change in seizure medication |
|
| Notes | The design of this study was different from the others due to its use of a fixed intensity, rather than adjusted to resting motor threshold. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was performed using the order of entrance in the study and a randomization table previously generated by a computer using randomization blocks of seven (for each seven participants, three were randomized to sham and four to active rTMS) to minimize the risk for unbalanced group sizes." Unclear how the "order of entrance" was taken into account in the randomisation, and whether this could have led to a predictable randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | The allocation concealment method was not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and study personnel were blinded except for those delivering therapy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded to intervention group of participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data, and study attrition reported |
| Selective reporting (reporting bias) | Low risk | Study protocols were not available. Primary and secondary outcomes stated in the methods section were reported in the results, all expected outcomes were reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Joo 2007.
| Study characteristics | ||
| Methods | Randomised, double‐blind, non‐placebo‐controlled, parallel‐design trial 4 treatment arms: focal rTMS for 3000 pulses (n = 8), focal rTMS for 1500 pulses (n = 10); non‐focal rTMS for 3000 pulses (n = 8), and non‐focal rTMS for 1500 pulses (n = 9). Baseline period: 8 weeks Follow‐up period: 8 weeks |
|
| Participants | Single‐centre South Korean study 35 people with focal, non‐focal, or multifocal epilepsy drug‐resistant to medications (range 2 to 7 AEDs). 18 male participants, 17 female participants Mean age was 25 years (range 18 to 46 years). Mean seizure frequency was 9.1 per week. |
|
| Interventions | Focal (over epileptogenic zone) or non‐focal (at vertex) rTMS for 1500 or 3000 pulses Treatment period: once a day for 5 consecutive days Treatment parameters: 0.5 Hz frequency, 100% RMT intensity, 50‐minute duration |
|
| Outcomes | Proportion of people with a 50% or greater reduction in seizure frequency following the treatment period Change in seizure frequency per week post‐treatment Percentage reduction interictal spikes Proportion of people experiencing adverse events |
|
| Notes | The design of this study was different from the others due to no placebo control arm: there were four active treatment arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Use of "randomization code"; no further information reported to assess if the code was likely to be predictable. |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Only blinding of the EEG reader is specified. Blinding of participants is not stated, but they all received active therapy. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only blinding of the EEG reader is specified, unclear if other outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No apparent issues with missing data, and no withdrawals from treatment reported, but ITT analysis is not specified. |
| Selective reporting (reporting bias) | High risk | Outcomes are not adequately specified in the methods section (no primary or secondary outcomes defined) |
| Other bias | Low risk | No other sources of bias identified |
Seynaeve 2016.
| Study characteristics | ||
| Methods | Randomised, double‐blind, sham‐controlled, cross‐over trial 3 treatment arms: placebo stimulation first, figure‐8 coil stimulation first, round coil stimulation first Baseline period: 8 weeks Treatment period: once daily for 2 weeks on weekdays (10 sessions) Follow‐up period: 10 weeks |
|
| Participants | Single‐centre Belgian study 11 participants (7 female, 4 male) between 27 and 61 years of age with drug resistant, focal epilepsy with a single epileptic zone (range 1 to 5 AEDs) Median 24 seizures a month (range 18/day to 2/month) 4 participants had unsuccessful epilepsy surgery |
|
| Interventions | Round coil first, figure‐8 coil first, and sham coil first followed by the other 2 treatments after an observation period Treatment period: 1 session a day over 2 weeks on weekdays (10 sessions) rTMS was delivered to a focal epileptic zone using 1500 stimuli a day at a frequency of 0.5 Hz at 90% RMT |
|
| Outcomes | Proportion of people with a 50% or greater reduction in seizure frequency following the treatment period Changes of seizure activity per week after active rTMS treatment Quality of life scores Proportion of people experiencing adverse events and withdrawals from treatment |
|
| Notes | This trial used a cross‐over design (carry‐over effect is not formally assessed, but the observation period of 10 weeks between treatments is likely to be a sufficient 'wash‐out' period). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised using "a computerized random number generator and a permutation for each block of three patients". Initial therapy appears to be randomly allocated, but there is a fixed order for successive therapies. |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded to order of therapy. Investigator administering therapy blinded to seizure frequency. However, concealment analysis did show that participants were better than average at guessing their therapy with successive treatments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both outcome assessors were blinded to order of treatments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant not analysed due to incomplete seizure diary on multiple days. 3 other participants failed to complete all 3 treatment arms, but study attrition documented. ITT analysis is not specified. |
| Selective reporting (reporting bias) | High risk | Outcomes are not adequately defined in the methods section. Primary and other outcomes referred to in the abstract, results, and discussion sections but not in the methods section. |
| Other bias | Unclear risk | Observation period of 10 weeks between treatments is likely to be a sufficient 'wash‐out' period in this cross‐over study |
Sun 2012.
| Study characteristics | ||
| Methods | Randomised, single‐blind, non‐placebo‐controlled, parallel‐design study Baseline evaluation period: 4 weeks Follow‐up period: 8 weeks 2 treatment arms: high‐intensity rTMS at 90% of RMT (n = 31) and low‐intensity rTMS at 20% RMT (n = 29) |
|
| Participants | 60 participants randomised. Mean age 20.5 years (SD 7 years). 41 male participants, 19 female participants Various types of epilepsies, but the majority were focal epilepsy with or without secondary generalisation. 20 participants had previously undergone surgical resection which failed to control the seizures. |
|
| Interventions | Treatment period: 2 weeks rTMS was delivered 3 times a day for 2 weeks to the focal epileptic zone best reflected on EEG with 500 stimuli at 0.5 Hz, separated by a 600‐second interval |
|
| Outcomes | Change in seizure frequency per week post‐treatment Proportion of people experiencing adverse events and withdrawals from the study Effect of rTMS on interictal ED at 60 minutes |
|
| Notes | The design of this study was different from the others due to no placebo control arm: there were four active treatment arms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence numbers generated by computer‐assisted randomisation program |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and study personnel blinded except for those delivering therapy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded to intervention group of participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of missing data, and study attrition reported. ITT analysis used. |
| Selective reporting (reporting bias) | Low risk | Study protocols were not available. Primary and secondary outcomes stated in the methods section were reported in the results, all expected outcomes were reported. |
| Other bias | Low risk | No other sources of bias identified |
Tergau 2003.
| Study characteristics | ||
| Methods | Randomised, placebo‐ and active‐controlled cross‐over trial 3 treatment arms: placebo stimulation first, 0.333 Hz stimulation first, 1 Hz stimulation first Baseline period: 3 months Follow‐up period: 4‐week observation and treatment periods separated by at least 8 weeks |
|
| Participants | Multicentre study across 3 centres in Germany Results presented for 17 randomised participants. Mean age was 29 years (SD 10 years) Participants had any type of medically intractable epilepsy and at least 2 seizures per week on average during 3‐month baseline period |
|
| Interventions | Treatment period: 5 days Treatment with 1000 pulses each day (500 monopolar pulses with clockwise current direction followed directly by 500 pulses in anticlockwise direction) Treatment delivered "unifocally" |
|
| Outcomes | Change in seizure frequency per week post‐treatment Proportion of people experiencing adverse events and withdrawals from the study |
|
| Notes | At the time of study publication, 28 participants had been enrolled, 5 participants had yet to complete, and 6 had dropped out of the study (reasons stated). Carry‐over effect is not formally assessed, but the observation period of at least 8 weeks between treatments is likely to be a sufficient 'wash‐out' period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study is described as randomised, no further information given |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Method of placebo stimulation described, but it is not stated who was blinded, if anyone |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of placebo stimulation described, but it is not stated who was blinded, if anyone |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data presented for 17 participants out of 28 randomised, ITT approach not used in analysis. Unclear how many of the cross‐over arms were completed by the participants who dropped out |
| Selective reporting (reporting bias) | High risk | Study protocol was not available. Outcomes are not adequately specified in the methods section (no primary or secondary outcomes defined) |
| Other bias | Unclear risk | A period of at least 8 weeks between treatments is likely to have been a sufficient 'wash‐out' period in this cross‐over study. Not stated how many participants were randomised to each treatment arm (placebo first, 0.333 Hz first, or 1 Hz first), and whether these randomised groups were balanced for clinical demographics at baseline. Unclear how a 3‐arm cross‐over trial design was implemented (i.e. a second randomisation after first arm?). |
Theodore 2002.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐design trial 2 treatment arms: active rTMS (n = 12) and sham rTMS (n = 12) Baseline period: 8 weeks Follow‐up period: 8 weeks |
|
| Participants | Single‐centre National Institutes of Health 24 participants were randomised (13 female, 11 male). Mean age was 40 years (SD 14 years) Participants either had localisation‐related or secondary generalised epilepsy that was resistant to medications (at least 1 CPS or secondary GTCS per week on stable AEDs over 8‐week baseline) |
|
| Interventions | Treatment period: 1 week Treatment parameters: focal rTMS administered at 1 Hz for 15 minutes twice a day, at 120% RMT |
|
| Outcomes | Change in seizure frequency per week post‐treatment Proportion of people experiencing adverse events and withdrawals from the study |
|
| Notes | Authors cited in their post hoc analysis that the study was underpowered to detect less than a 70% reduction in seizure frequency at 2 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study is described as randomised, no further information given |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and assessment team blinded; only treatment team unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment team blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No significant issues with missing data. Study attrition reported |
| Selective reporting (reporting bias) | Low risk | Study protocols were not available. Primary outcome measure described in the methods and reported in the results. Secondary measures were less clearly stated in the methods, but the authors make limited claims based on these secondary results. |
| Other bias | Low risk | No additional sources of bias identified |
Wang 2008.
| Study characteristics | ||
| Methods | Randomised, open‐label, AED‐controlled, parallel‐design study 2 treatment arms: rTMS plus carbamazepine (n = 15) and "drug treatment" (n = 15) Baseline period: not stated Follow‐up period: 30 days |
|
| Participants | Single‐centre study in China 30 participants randomised (15 to rTMS group and 15 to AED group) rTMS group: mean age 27.9 years (SD 4.1 years); 10 males, 5 females. AED group: mean age 27.6 years (SD 3.9 years); 9 males, 6 females People with temporal lobe epilepsy and epileptiform discharges were enrolled |
|
| Interventions | Treatment period: 7 days (for rTMS group), 60 days for AED group 1‐hertz rTMS at 90% RMT threshold, simulation frequency of 500 times, once a day for 7 days. Drug treatment group received 600 to 800 mg oral carbamazepine per day for at least 60 days. |
|
| Outcomes | Change in seizure frequency per week post‐treatment Positive rate of epileptiform charges reported Proportion of people experiencing adverse events |
|
| Notes | Imbalance in treatment time across the intervention groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study is described as randomised, no further information given |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label study (cannot be blinded due to design), unclear if outcomes were influenced |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study, outcome assessors not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of missing data, no withdrawals from the study. ITT analysis used. |
| Selective reporting (reporting bias) | Low risk | Study protocols were not available. Primary and secondary outcomes stated in the methods section were reported in the results, all expected outcomes were reported. |
| Other bias | Low risk | No additional sources of bias identified |
AED: antiepileptic drug CPS: complex partial seizure ED: epileptiform discharges EEG: electroencephalogram GTCS: generalised tonic clonic seizure ITT: intention‐to‐treat RMT: resting motor threshold rTMS: repetitive transcranial magnetic stimulation SD: standard deviation TMS: transcranial magnetic stimulation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| NCT00382707 | Before‐and‐after controlled study |
Characteristics of ongoing studies [ordered by study ID]
CTRI/2017/10/010067.
| Study name | Efficacy of adjunctive neuronavigated rTMS in localization related epilepsy in children and adolescent: a sham controlled study |
| Methods | Randomised, parallel‐group, active‐controlled trial |
| Participants | Aged between 8 to 18 years with localised epilepsy, on adequate medication for at least 1 month with 4 or more seizures in the previous month |
| Interventions | rTMS versus sham rTMS |
| Outcomes | Control of paroxysms of seizures up to 3 months after rTMS Reduction in Hamilton anxiety rating scale score and childhood depression inventory scores up to 3 months after rTMS Before and after treatment comparison of EEG abnormalities |
| Starting date | August 2015 |
| Contact information | N Mukherjee, VK Sinha |
| Notes | Authors contacted for more information, no response received. |
CTRI/2019/02/017440.
| Study name | A randomised double blind sham controlled trial of targeted low frequency repetitive transcranial magnetic stimulation (rTMS) therapy in childhood and adolescent with focal onset refractory epilepsy |
| Methods | Randomised, parallel‐group, placebo‐controlled trial |
| Participants | Aged between 5 and 18 years diagnosed with focal onset drug‐refractory epilepsy defined as ≥ 1 seizure/week OR 4 seizures/month despite on ≥ 2 appropriately chosen and optimally prescribed antiepileptic drugs. |
| Interventions | rTMS versus sham rTMS |
| Outcomes | 50% seizure reduction Change in cortical excitability induced by rTMS therapy in terms of pre‐ and post‐therapy change in MT, SICI, and LICI by single‐pulse rTMS Effect of rTMS on behavioural profile and cognition |
| Starting date | February 2019 |
| Contact information | P Jauhari |
| Notes | Authors contacted: enrolment completed, but data analysis still ongoing. |
NCT02757547.
| Study name | Registered electrical sources for effective TMS treatment of epilepsy |
| Methods | Randomised, cross‐over assignment, quadruple‐blind |
| Participants | 22 years and above with treatment‐resistant epilepsy with 1 localised seizure onset focus |
| Interventions | rTMS versus sham rTMS |
| Outcomes | Reduction in seizure frequency compared to baseline |
| Starting date | April 2016 |
| Contact information | P Olsen |
| Notes | Authors contacted for more information, no response received. |
NCT03154307.
| Study name | Repeated TMS at low frequencies to reduce seizure occurrence |
| Methods | Randomised, parallel‐arm, double‐blind |
| Participants | 18 to 80 years old, who experienced ≥ 3 seizures/month in the month prior to starting study (any seizure) |
| Interventions | Low‐frequency rTMS: rTMS versus sham rTMS and comparing weekly versus monthly rTMS |
| Outcomes | Average weekly seizure frequency change compared to baseline after treatment |
| Starting date | May 2017 |
| Contact information | AK Starosciak |
| Notes | Authors contacted: still enrolling participants. |
Oberman 2015.
| Study name | Safety and tolerability of 1 Hz deep repetitive transcranial magnetic stimulation (RTMS) for treatment of temporal lobe epilepsy |
| Methods | Randomised control |
| Participants | Individuals with temporal lobe epilepsy |
| Interventions | rTMS versus sham rTMS |
| Outcomes | Suppression of seizure activity Adverse events Memory performance Cognitive performance |
| Starting date | Unclear |
| Contact information | A Rotenberg |
| Notes | Authors contacted: currently writing article for publication. |
EEG: electroencephalogram LICI: long‐interval intracortical inhibition MT: motor threshold rTMS: repetitive transcranial magnetic stimulation SICI: short‐interval intracortical inhibition TMS: transcranial magnetic stimulation
Differences between protocol and review
Addition to Types of interventions that interventions will be included if "added to current therapy or used as single therapy".
Measure of treatment effect for 'quality of life' specified as mean difference (or standardised mean difference) depending on outcome scale used.
Additional information added to Unit of analysis issues to specify methods to be used to analyse cluster‐randomised trials and cross‐over trials.
In a post hoc change from protocol, we have added a 'Summary of findings' table (Table 1), reporting the primary outcomes of the review (reduction in seizure frequency and quality of life).
Contributions of authors
Ricky Chen, David C Spencer, and Jennifer Weston wrote the review protocol. All authors were responsible for carrying out the review methods and writing up the review. Ricky Chen was responsible for updating the initial finished review. DW and BDM are responsible for the updated review.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute for Health Research (NIHR), UK
Declarations of interest
DW: none known DS: none known SJN: none known BDM has received funding from UK Research and Innovation, Medical Research Council, National Institute for Health Research, Wellcome Trust, Academy of Medical Sciences, and British Medical Association.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cantello 2007 {published data only}
- Cantello R, Rossi S, Varrasi C, Ulivelli M, Civardi C, Bartalini S, et al. Slow repetitive TMS for drug-resistant epilepsy: clinical and EEG findings of a placebo-controlled trial. Epilepsia 2007;48(2):366-74. [DOI] [PubMed] [Google Scholar]
Fregni 2006 {published data only}
- Fregni F, Otachi PT, Do Valle A, Boggio PS, Thut G, Rigonatti SP, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Annals of Neurology 2006;60(4):447-55. [DOI] [PubMed] [Google Scholar]
Joo 2007 {published data only}
- Joo E, Sun H, Chung S, Cho J, Seo D, Hong S. Antiepileptic effects of low-frequency repetitive transcranial magnetic stimulation by different stimulation durations and locations. Clinical Neurophysiology 2007;118(3):702-8. [DOI] [PubMed] [Google Scholar]
Seynaeve 2016 {published data only}
- Seynaeve L, Devroye A, Dupont P, Van Paesschen W. Randomized crossover sham-controlled clinical trial of targeted low-frequency transcranial magnetic stimulation comparing a figure-8 and a round coil to treat refractory neocortical epilepsy. Epilepsia 2016;57(1):141-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sun 2012 {published data only}
- Sun W, Mao W, Meng X, Wang D, Qiao L, Tao W, et al. Low-frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy: a controlled clinical study. Epilepsia 2012;53(10):1782-9. [DOI] [PubMed] [Google Scholar]
Tergau 2003 {published data only}
- Tergau F, Neumann D, Rosenow F, Nitsche MA, Paulus W, Steinhoff B. Can epilepsies be improved by repetitive transcranial magnetic stimulation? Interim analysis of a controlled study. Supplements to Clinical Neurophysiology 2003;56:400-5. [DOI] [PubMed] [Google Scholar]
Theodore 2002 {published data only}
- Theodore WH, Hunter K, Chen R, Vega-Bermudez F, Boroojerdi B, Reeves-Tyer P, et al. Transcranial magnetic stimulation for the treatment of seizures: a controlled study. Neurology 2002;59(4):560-2. [DOI] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang X, Yang D, Wang S, Zhao X, Zhang L, Chen Z, et al. Effects of low-frequency repetitive transcranial magnetic stimulation on electroencephalogram and seizure frequency in 15 patients with temporal lobe epilepsy following dipole source localization. Neural Regeneration Research 2008;3(11):1257-60. [Google Scholar]
References to studies excluded from this review
NCT00382707 {published data only}
- NCT00382707. Transcranial magnetic stimulation and anti-epileptic effect: optimization and evaluation with electrophysiology. clinicaltrials.gov/show/NCT00382707 (first received 29 September 2006).
References to ongoing studies
CTRI/2017/10/010067 {published data only (unpublished sought but not used)}
- CTRI/2017/10/010067. Experiment of magnetic stimulation of brain for treatment of fits in children. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=15419 (first received 12 October 2017).
CTRI/2019/02/017440 {published data only (unpublished sought but not used)}
- CTRI/2019/02/017440. Transcranial magnetic stimulation device for focal refractory epilepsy in childhood and adolescent. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=30169 (first received 5 February 2019).
NCT02757547 {published data only (unpublished sought but not used)}
- NCT02757547. Transcranial magnetic stimulation for epilepsy. clinicaltrials.gov/show/nct02757547 (first received 2 May 2016).
NCT03154307 {published data only (unpublished sought but not used)}
- NCT03154307. Repeated TMS at low frequencies to reduce seizure occurrence. clinicaltrials.gov/show/nct03154307 (first received 16 May 2017).
Oberman 2015 {published data only (unpublished sought but not used)}
- Oberman L, Gersner R, Zangen A, Rotenberg A. Safety and tolerability of 1 HZ deep repetitive transcranial magnetic stimulation (RTMS) for treatment of temporal lobe epilepsy. Epilepsy Currents 2015;15(1):547. [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2018
- Cooper YA, Pianka ST, Alotaibi NM, Babayan D, Salavati B, Weil AG, et al. Repetitive transcranial magnetic stimulation for the treatment of drug-resistant epilepsy: a systematic review and individual participant data meta-analysis of real-world evidence. Epilepsia Open 2018;3(1):55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hsu 2011
- Hsu WY, Cheng CH, Lin MW, Shih YH, Liao KK, Lin YY. Antiepileptic effects of low frequency repetitive transcranial magnetic stimulation: a meta-analysis. Epilepsy Research 2011;96(3):231-40. [DOI] [PubMed] [Google Scholar]
Huang 2005
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 2005;45(2):201-6. [DOI] [PubMed] [Google Scholar]
Kimiskidis 2010
- Kimiskidis VK. Transcranial magnetic stimulation for drug-resistant epilepsies: rationale and clinical experience. European Neurology 2010;63(4):205-10. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham J, Dwan K, Altman D, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. Research Methods and Reporting 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Kwan 2011
- Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. New England Journal of Medicine 2011;365(10):919-26. [DOI] [PubMed] [Google Scholar]
Lefebvre 2020
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Moliadze 2003
- Moliadze V, Zhao Y, Eysel U, Funke K. Effect of transcranial magnetic stimulation on single-unit activity in the cat primary visual cortex. Journal of Physiology 2003;553(2):665-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01745952
- NCT01745952. Multimodal image‐guided repetitive transcranial magnetic stimulation (rTMS) in the treatment of refractory partial epilepsy. clinicaltrials.gov/show/NCT01745952 (first received 10 December 2012). [CRSREF: 4352906]
Ngugi 2010
- Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia 2010;51(5):883-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ngugi 2011
- Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Incidence of epilepsy: a systematic review and meta-analysis. Neurology 2011;77(10):1005-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Picot 2008
- Picot MC, Baldy-Moulinier M, Daurès JP, Dujols P, Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population-based study in a Western European country. Epilepsia 2008;49(7):1230-8. [DOI] [PubMed] [Google Scholar]
Reithler 2011
- Reithler J, Peters JC, Sack AT. Multimodal transcranial magnetic stimulation: using concurrent neuroimaging to reveal the neural network dynamics of noninvasive brain stimulation. Progress in Neurobiology 2011;94(2):149-65. [DOI] [PubMed] [Google Scholar]
Ridding 2007
- Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nature Reviews Neuroscience 2007;8(7):559-67. [DOI] [PubMed] [Google Scholar]
Shorvon 2011
- Shorvon S. The causes of epilepsy: changing concepts of etiology of epilepsy over the past 150 years. Epilepsia 2011;52(6):1033-44. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chen 2014
- Chen R, Spencer DC, Pulman J. Transcranial magnetic stimulation for the treatment of epilepsy. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No: CD011025. [DOI: 10.1002/14651858.CD011025] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2016
- Chen R, Spencer DC, Weston J, Nolan SJ. Transcranial magnetic stimulation for the treatment of epilepsy. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD011025. [DOI: 10.1002/14651858.CD011025.pub2] [DOI] [PubMed] [Google Scholar]


