Abstract
Background
Many people with chronic disease have more than one chronic condition, which is referred to as multimorbidity. The term comorbidity is also used but this is now taken to mean that there is a defined index condition with other linked conditions, for example diabetes and cardiovascular disease. It is also used when there are combinations of defined conditions that commonly co‐exist, for example diabetes and depression. While this is not a new phenomenon, there is greater recognition of its impact and the importance of improving outcomes for individuals affected. Research in the area to date has focused mainly on descriptive epidemiology and impact assessment. There has been limited exploration of the effectiveness of interventions to improve outcomes for people with multimorbidity.
Objectives
To determine the effectiveness of health‐service or patient‐oriented interventions designed to improve outcomes in people with multimorbidity in primary care and community settings. Multimorbidity was defined as two or more chronic conditions in the same individual.
Search methods
We searched MEDLINE, EMBASE, CINAHL and seven other databases to 28 September 2015. We also searched grey literature and consulted experts in the field for completed or ongoing studies.
Selection criteria
Two review authors independently screened and selected studies for inclusion. We considered randomised controlled trials (RCTs), non‐randomised clinical trials (NRCTs), controlled before‐after studies (CBAs), and interrupted time series analyses (ITS) evaluating interventions to improve outcomes for people with multimorbidity in primary care and community settings. Multimorbidity was defined as two or more chronic conditions in the same individual. This includes studies where participants can have combinations of any condition or have combinations of pre‐specified common conditions (comorbidity), for example, hypertension and cardiovascular disease. The comparison was usual care as delivered in that setting.
Data collection and analysis
Two review authors independently extracted data from the included studies, evaluated study quality, and judged the certainty of the evidence using the GRADE approach. We conducted a meta‐analysis of the results where possible and carried out a narrative synthesis for the remainder of the results. We present the results in a 'Summary of findings' table and tabular format to show effect sizes across all outcome types.
Main results
We identified 17 RCTs examining a range of complex interventions for people with multimorbidity. Nine studies focused on defined comorbid conditions with an emphasis on depression, diabetes and cardiovascular disease. The remaining studies focused on multimorbidity, generally in older people. In 11 studies, the predominant intervention element was a change to the organisation of care delivery, usually through case management or enhanced multidisciplinary team work. In six studies, the interventions were predominantly patient‐oriented, for example, educational or self‐management support‐type interventions delivered directly to participants. Overall our confidence in the results regarding the effectiveness of interventions ranged from low to high certainty. There was little or no difference in clinical outcomes (based on moderate certainty evidence). Mental health outcomes improved (based on high certainty evidence) and there were modest reductions in mean depression scores for the comorbidity studies that targeted participants with depression (standardized mean difference (SMD) −0.41, 95% confidence interval (CI) −0.63 to −0.2). There was probably a small improvement in patient‐reported outcomes (moderate certainty evidence). The intervention may make little or no difference to health service use (low certainty evidence), may slightly improve medication adherence (low certainty evidence), probably slightly improves patient‐related health behaviours (moderate certainty evidence), and probably improves provider behaviour in terms of prescribing behaviour and quality of care (moderate certainty evidence). Cost data were limited.
Authors' conclusions
This review identifies the emerging evidence to support policy for the management of people with multimorbidity and common comorbidities in primary care and community settings. There are remaining uncertainties about the effectiveness of interventions for people with multimorbidity in general due to the relatively small number of RCTs conducted in this area to date, with mixed findings overall. It is possible that the findings may change with the inclusion of large ongoing well‐organised trials in future updates. The results suggest an improvement in health outcomes if interventions can be targeted at risk factors such as depression in people with co‐morbidity.
Plain language summary
Improving outcomes for people with multiple chronic conditions
Background
The World Health Organization defines chronic conditions as "health problems that require ongoing management over a period of years or decades". Many people with a chronic health problem or condition, have more than one chronic health condition, which is referred to as multimorbidity. This generally means that people could have any possible combination of health conditions but in some studies the combinations of conditions are pre‐specified to target common combinations such as diabetes and heart disease. We refer to these types of studies as comorbidity studies. Little is known about the effectiveness of interventions to improve outcomes for people with multimorbidity. This is an update of a previously published review.
Review question
This review aimed to identify and summarise the existing evidence on the effectiveness of interventions to improve clinical and mental health outcomes and patient‐reported outcomes including health‐related quality of life for people with multimorbidity in primary care and community settings.
Description of study characteristics
We searched the literature up to September 2015 and identified 17 generally well‐designed randomised controlled trials meeting the eligibility criteria. Nine of these studies focused on specific combinations of health conditions (comorbidity studies), for example diabetes and heart disease. The other eight studies included people with a broad range of conditions (multimorbidity studies) although they tended to focus on elderly people. The majority of studies examined interventions that involved changes to the organisation of care delivery although some studies had more patient‐focused interventions. All studies had governmental or charitable sources of funding.
Key results
Overall the results regarding the effectiveness of interventions were mixed. There were no clear positive improvements in clinical outcomes, health service use, medication adherence, patient‐related health behaviours, health professional behaviours or costs. There were modest improvements in mental health outcomes from seven studies that targeted people with depression. Results indicated that interventions may possibly improve functional outcomes in the studies that reported these outcomes. . Overall the results indicate that it is difficult to improve outcomes for people with multiple conditions. The review suggests that interventions that are designed to target specific risk factors (for example treatment for depression) may be more effective. There is a need for further studies on this topic, particularly involving people with multimorbidity in general across the age ranges.
Quality/certainty of the evidence
All of the included studies were randomised controlled trials. The overall quality of these studies was good though many studies did not fully report on all potential sources of bias. As definitions of multimorbidity vary among studies, the potential to reasonably combine study results and draw overall conclusions is limited. Overall, we judged that the certainty or confidence we can have in the results from this review is moderate but due to small numbers of studies and mixed results we acknowledge the uncertainty remaining and the potential that future studies could change our conclusions.
Summary of findings
Summary of findings 1. Summary of findings.
| Interventions aimed at improving outcomes for people with multimorbidity compared with usual care | |||
|
Participant or population: Adults with multimorbidity (two or more chronic conditions) Settings: Primary care and community settings Intervention: Any intervention designed to improve outcomes for people with multimorbidity including professional‐, organisational‐ and patient‐oriented interventions Comparison: Usual care | |||
| Outcomes | Impacts | Number of studies | Quality of the evidence (GRADE) |
| Clinical outcomes | There is no clear effect on clinical outcomes with a range of standardised effect sizes from 0.01 to 0.78 with a minority having effect sizes > 0.5; interventions aimed at improving management of risk factors in comorbid conditions were more likely to have higher effect sizes. | 10 | ⊕⊕⊕⊖ Moderate |
| Mental health outcomes | There are improved depression‐related outcomes in studies targeting comorbid conditions that include depression with a range of standardised effect sizes from 0.09 to 1.18 with 3 of 7 studies having moderate to large effect sizes (> 0.5) . Standardised mean difference of −0.41 (95% CI, −0.63 to −0.20) was calculated from combining data from 6 studies. | 9 | ⊕⊕⊕⊕ High |
| Patient‐reported outcome measures (PROMs) | There are mixed effects on PROMs with only half of studies that reported these outcomes showing any benefit with a range of standardised effect sizes from 0.03 to 0.84. Only 1 of 5 studies with data available data on self‐efficacy had a moderate effect size (>0.5), 1 of 6 had a moderate effect size for HRQoL, and effect sizes for other psychosocial outcomes were generally low. | 13 | ⊕⊕⊕⊖ Moderate |
| Health Service Utilisation | There were no effects on health service utilisation and changes in visits were difficult to interpret as some interventions could lead to higher numbers of visits if previous unmet need was being addressed. There was no difference in admission‐related outcomes, though numbers of admissions in most of these studies were very small. | 5 | ⊕⊕⊖⊖ Low |
| Medication use and adherence | There are mixed effects on medication use and adherence with half the studies reporting this outcome showing benefit. Proportions adherent to medication were higher in intervention participants with ranges in absolute difference of 10% to 40% but all studies with available data had small effect sizes. | 4 | ⊕⊕⊖⊖ Low |
| Health‐related patient behaviours | Studies measuring this outcome reported a range of effects varying from an additional 18 minutes spent walking per week to an absolute difference in kcals expenditure per week of 2516 (no studies presented data that could be used to calculate effect sizes). | 7 | ⊕⊕⊕⊖ Moderate |
| Provider behaviour | The majority of studies reporting provider behaviour indicated improved provider behaviour relating to care delivery; three studies reported a range of 15% to 40% in proportions of intervention providers improving behaviours such as appropriate referral. | 5 | ⊕⊕⊕⊖ Moderate |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
We downgraded the evidence for effects on clinical and psychosocial outcomes to moderate due to lack of consistency of effect across studies and small effect sizes. We downgraded the evidence for effects on provider behaviour to moderate due to limited available data for calculation of standardised effect sizes (SES) and lack of clarity regarding the clinical importance of the results. We downgraded the evidence for effects on health service utilisation and medication use and adherence to low due to variation across studies and small effect sizes.
Background
Many people with chronic disease have more than one chronic condition, which is referred to as multimorbidity. While this is not a new phenomenon, there is greater recognition of its impact and the importance of improving outcomes for individuals affected. Research in the area to date has focused mainly on descriptive epidemiology and impact assessment (Fortin 2007). There has been limited exploration of the effectiveness of interventions to improve outcomes for people with multimorbidity.
Description of the condition
There has been increasing focus on the enormous personal and societal burden of ill‐health caused by chronic disease. The World Health Organization (WHO) has emphasised the importance of organising healthcare delivery systems to improve health outcomes and has stressed the importance of building integrated healthcare systems that can address chronic disease management (WHO 2002). This can be done by focusing on generic chronic care models, as has happened mainly in the United States of America (USA), or by developing national systems focusing on single chronic conditions as has happened with the National Service Frameworks in the UK (Lewis 2004; Satariano 2013). However, many people with chronic disease have more than one chronic condition, which is referred to as multimorbidity and formally defined as the co‐existence of two or more chronic conditions (Fortin 2005). While this is not a new phenomenon, there is greater recognition of its impact and the importance of improving outcomes for individuals affected (Fortin 2007; Smith 2007).
While the accepted term for people with multiple chronic conditions is now multimorbidity, the term comorbidity has been used interchangeably in the past. It is now accepted that comorbidity should be used when there is a specified index condition or where there are defined combinations of conditions (for example hypertension and cardiovascular disease) as opposed to multimorbidity where any condition could be included (Valderas 2011). Multimorbidity is the more general term and individuals with comorbidity also have multimorbidity but the reverse does not necessarily apply. For the purposes of this review when analysing the included studies, we looked at studies based on the intervention elements but we also considered differences between studies that specifically target comorbid conditions as opposed to those targeting general multimorbidity. This is because interventions in the comorbidity studies are designed to target the specific included conditions. These distinctions are important in the context of developing and evaluating effective interventions and considering their generalisability (Fortin 2013; Smith 2013).
Individuals with multimorbidity are more likely to die prematurely (Deeg 2002; Menotti 2001; Rochon 1996), be admitted to hospital (Bähler 2015; Condelius 2008; Payne 2013), and have longer hospital stays (Bähler 2015; Librero 1999). They have poorer quality of life (Brettschneider 2013), loss of physical functioning (Bayliss 2004; Fortin 2004; Fortin 2006b), and are more likely to suffer from psychological stress (Fortin 2006a; Gunn 2012). Medicines management is often complex, resulting in polypharmacy with its attendant risks of drug interactions and adverse drug events (Duerden 2013; Gandhi 2003; Guthrie 2011). For patients, in addition to understanding and managing their conditions and drug regimes, they must also attend multiple appointments with different healthcare providers and adhere to lifestyle recommendations (Gallacher 2011; Townsend 2006).
Prevalence studies of multimorbidity have been carried out in different countries indicating that, particularly in those over 60 years, the majority of people attending family primary care services had more than one chronic condition (Fortin 2005; Fortin 2006c; van den Akker 1998; Wolff 2002). A subgroup of these service users have a debilitating combination of conditions that have a high impact on their own lives but also on their utilisation of health services and related costs (Hoffman 1996; Marengoni 2011; Parmelee 1995; Smith 2008). This emerging concept may be referred to as 'complex multimorbidity' and has been defined as people with three or more chronic conditions involving three or more body systems (Harrison 2014). These individuals can pose management difficulties, resulting in frequent health care visits, frequent emergency hospital admissions, and repeated investigations with enormous cost both for the individuals and the healthcare system involved. A UK report has examined the costs associated with this group of people who are described as 'high impact users' on the basis of their frequent emergency admissions (Rowell 2006). Fragmentation of care is a significant problem for this group, resulting from the involvement of both primary care and multiple specialists who may not be communicating with each other effectively (Wallace 2015). Starfield found that people with a greater morbidity burden have a higher use of specialists even for conditions that are normally managed in primary care, and concludes that there is a need for a better understanding of the roles of generalists and specialists in managing these individuals (Starfield 2005)
Description of the intervention
Given the complexity of managing people with multiple chronic conditions, potential interventions are likely to be complex and multifaceted if they are to address the varied needs of these individuals. We anticipated that a variety of intervention types could work to improve outcomes for people with multimorbidity and could be included within the scope of this review. Cochrane Effective Practice and Organisation of Care (EPOC) has developed a taxonomy that defines intervention types (EPOC 2002). We have used this taxonomy to define health service and patient‐oriented interventions that have been designed to improve outcomes of people or populations with more than one chronic condition.
1. Professional interventions: for example, education designed to change the behaviour of clinicians. Such interventions may work by altering professionals' awareness of multimorbidity or providing training or education designed to equip clinicians with skills in managing these individuals, thus improving their healthcare delivery.
2. Financial interventions: for example, financial incentives to providers to reach treatment targets. These interventions might work by incentivising health service delivery and providing resources to extend consultation length for people with multimorbidity.
3. Organisational interventions: these can be further divided into organisational changes delivered through practitioners or directly to patients. For example, any changes to care delivery such as case management or the addition of different healthcare workers such as a pharmacist to the healthcare team. These interventions may work by changing care delivery to match the needs of people with multimorbidity across a range of areas such as coordination of care, medicines management, or use of other health professionals such as physiotherapists and occupational therapists to address needs relating to physical and social functioning.
4. Patient‐oriented interventions: this would include any intervention directed primarily at individuals, for example, education or support for self management. These interventions might work by improving self management, thus enabling people to manage their conditions more effectively and to seek appropriate health care.
5. Regulatory interventions: for example, changes to local or national regulations designed to alter care delivery in order to improve outcomes. Such interventions might work by introducing regulatory changes that facilitate and enable the funding of care that is directed towards those with complex health needs. An example could be the introduction of free primary care for people with multimorbidity on the basis that preventive care might prevent subsequent more costly hospital admissions. While we did not find these types of interventions, we believe they could exist and would fall within the scope of this review for future updates.
How the intervention might work
We anticipated that organisational‐type interventions might predominate. We were aware that there has been a focus on case management, based mainly in health maintenance organisations in the USA (Zwarenstein 2000). Case management is defined as the explicit allocation of co‐ordination of tasks to an appointed individual or group and it is postulated that the function of co‐ordination is so important and specialised that responsibility for carrying it out needs to be explicitly allocated (Zwarenstein 2000). Our review included studies where case management was employed but only if it was specifically directed towards individuals identified as having multimorbidity.
The implementation of the Family Medicine Groups in the province of Québec, Canada, is another example of an organisational intervention as it involved new forms of shared responsibilities between physicians and nurses (MSSS 2001). Another example in the United Kingdom (UK) is the community matrons programme, which is being delivered through primary care trusts and is based on nurse‐provided case management for people with complex care needs including those with multimorbidity (London DOH 2005). It is similar to previous programmes delivered through social services in the 1990s and there have been concerns expressed as to the feasibility of achieving the programme targets without real integration of primary and specialist services (Murphy 2004).
The differences outlined earlier between multimorbidity in general and comorbidity where there are defined combinations of conditions also influences how interventions are designed. Interventions targeting specified comorbid conditions can be designed to address the specific challenges for people with those conditions. For example, an intervention that targets people with diabetes and depression will combine elements of diabetes‐focused care with psychotherapy or escalation of antidepressant medication, or both interventions, so as to address both conditions. Interventions for people with multimorbidity in general cannot have a disease focus as there are no pre‐specified conditions so the interventions might address improved coordination of care, improved medicines management or specific functional difficulties experienced by patients.
Since this review was originally planned in 2007, there has been widespread recognition of the need to address the challenge of multimorbidity across health systems with a series of articles in international medical journals highlighting the challenges involved. Two very useful resources highlighting the challenges of multimorbidity and collating research in the area are: i) the BMJ multimorbidity special collections (BMJ Multimorbidity collection) and ii) the International Research Community on Multimorbidity archive IRCMO at the University of Sherbrooke, Quèbec, Canada (IRCMO). The BMJ series includes a series of editorials, original research studies and a clinical review with a multimorbidity focus. IRCMO provides a platform for any researcher interested in multimorbidity to contribute to a regularly updated blog and also compiles a list of multimorbidity related publications.
Why it is important to do this review
This review was originally undertaken based on the clear recognition of the need for integrated care for people with multiple conditions who have complex care needs (Stange 2005). The evidence base for managing chronic conditions is based largely on trials of interventions for single conditions and individuals with multimorbidity are often excluded from such studies (Fortin 2006c; Starfield 2001; Wyatt 2014Zulman 2011). The inadequacy of existing clinical guidelines to support clinicians in managing people with multimorbidity has been highlighted as a significant issue in delivering care (Dumbreck 2015; Wyatt 2014). Clinical guideline developers have attempted to address this issue with the consideration of certain combinations of commonly co‐occurring conditions, for example, diabetes and depression (NICE 2009). However good quality evidence is essential to inform this clinical area and in recent years focus has shifted to intervention development and the need to reorientate clinical practice and healthcare systems for the people who use them most (Satariano 2013).
Objectives
To determine the effectiveness of health‐service or patient‐oriented interventions designed to improve outcomes in people with multimorbidity in primary care and community settings. Multimorbidity was defined as two or more chronic conditions in the same individual.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs), non‐randomised clinical trials (NRCTs), controlled before‐after studies (CBAs), and interrupted time series analyses (ITS), meeting EPOC quality criteria (EPOC 2013). We included NRCTs in the original protocol (Smith 2007b) as we anticipated that, given the challenges in undertaking multimorbidity research (Fortin 2007) and the likelihood that complex interventions would be tested, there would be relatively few RCTs and that non‐randomised designs might be used instead.
Types of participants
We included any people or populations with multimorbidity receiving care in a primary or community care setting. We adopted the most widely used definition of multimorbidity, that is, the co‐existence of multiple chronic diseases and medical conditions in the same individual, usually defined as two or more conditions (Fortin 2004; van den Akker 1998). We used the WHO definition of chronic disease, which is "health problems that require ongoing management over a period of years or decades" (WHO 2002). We included all studies that identified participants or sub‐groups of participants on the basis of multimorbidity, as defined by the study authors. In some studies, additional eligibility criteria were applied (for example, history of high service utilisation) in an effort to identify more vulnerable people who might benefit more from the intervention being studied.
We excluded studies where multimorbidity was assumed to be the norm on the basis of individuals' age as the interventions were not being targeted specifically at multimorbidity and its recognised challenges. This included studies where interventions were directed at communities of people based on location or age of participants in which participants could be presumed to have multimorbidity on the basis of their age or residence in a nursing home but interventions were not designed to specifically target multimorbidity.
Types of interventions
We included any type of intervention that was specifically directed towards a group of people defined as having multimorbidity. Only interventions based in primary care and community settings were included. Interventions included care delivered by family doctors, nurses, or other primary care professionals. Primary health care was defined as providing "integrated, easy to access, health care services by clinicians who are accountable for addressing a large majority of personal health care needs, developing a sustained and continuous relationship with patients, and practicing in the context of family and community" (Vaneslow 1995). However, not all countries have clearly‐defined primary care systems (Starfield 1998), so we included care delivered in community settings by individuals fulfilling the basic criteria for primary care, i.e. if they are available to treat all common conditions in all age groups and have an ongoing relationship with their patients. While some specialists may deliver components of primary care to their patients, practitioners were not included unless they fulfilled the definition of being available to treat all conditions and have an ongoing relationship with their patients.
Interventions were classified as 'simple' if they used one identifiable component or 'multifaceted' if they incorporated more than one feature.
We categorised interventions using the EPOC taxonomy presented in the Background section. Where interventions had multiple elements, we defined each element within the taxonomy and highlighted the predominant element of the intervention (see Table 2).
1. Multimorbidity intervention components.
| Author Year | Professional | Participant | Organisational | Effect of intervention on primary outcome | ||
| Case management or coordination of care | Reorganisation of care/team working | New team member | ||||
| Predominantly organisational | ||||||
| Barley 2014 | Nurse training | Participant information Prioritisation to create goals and health plan |
Case manager provided personalised care | Regular planned participant visits Weekly team meetings |
Nurse case manager | Pilot study and primary outcome was feasibility and deemed successful |
| Bogner 2008 | Individualised programme | Case manager | Regular planned participant visits | Improved blood pressure control and depression scores | ||
| Boult 2011 | Nurse training | Individual management plans Support for self‐management |
Guided care nurses coordinated care |
Guided care 'pods' consisting of nurse and PCP Monthly monitoring of participants |
No impact on healthcare utilisation | |
| Coventry 2015 | Practice team training | Personalised goals and participant workbooks |
Collaborative care using stepped care protocols Joint consultation between participant, psychologist and practice nurse |
Psychologist Supervision and input from team psychiatrist |
Modest reduction in depression scores | |
| Hogg 2009 | Individualised care plans |
Multidisciplinary team‐based management with home based assessment Medication review |
Pharmacist | Modest improvements in quality of chronic care delivery | ||
| Katon 2010 | Individualised management plans and targets Support for self‐management |
Team‐based care Stepped care treatment protocols Weekly team meeting |
Psychologist and psychiatrist supported depression care | Improvements in composite outcome of glycaemic control, blood pressure, lipids and depression scores | ||
| Kennedy 2013 | Practice training | Support for self‐management Participant guidebooks |
Systems‐based approach to self‐management support with practice supports and links made with related local services | No intervention effect noted | ||
| Krska 2001 | Individualised pharmaceutical care plans | Practice team‐implemented care plans | Pharmacist undertook medication review and devised pharmaceutical care plans | Reduction in pharmaceutical care issues | ||
| Martin 2013 | Training for community psychologists | Cognitive behavioural therapy sessions | Psychological care programme designed for headache and depression | Community psychologists | Reduced headaches and improved depression scores | |
| Morgan 2013 | Practice nurse training | Support for self‐management Goal setting Individualised care plans |
Nurse case manager | Quarterly reviews with practice nurse with GP stepping up care as needed | Improved depression scores | |
| Sommers 2000 | Risk reduction plan | Team based care with home assessment followed by team discussion, treatment plan and targets | Social worker | Reduced hospitalisation | ||
| Wakefield 2012 | Participation in home telehealth monitoring | Nurse case manager using telehealth monitoring and treatment algorithms | Improved blood pressure, no effect on glycaemic control | |||
| Predominantly Patient‐oriented interventions | ||||||
| Eakin 2007 | Support for self‐management with focus on diet and physical activity | Regular visits and follow‐up telephone calls | Health educator | Improvements in diet but not in physical activity | ||
| Garvey 2015 | Occupational therapist (OT) training |
OT‐led, group‐based support for self‐management programme (6 weeks) Goal setting and peer support |
GP and primary care team referral | OT with input from physiotherapist and pharmacist | Improvements in activity participation | |
| Hochhalter 2010 | Training for coaches running intervention | Patient Engagement workshop (x1) | Two follow‐up phone calls | Coach who delivered workshop | No effect on outcomes | |
| Lorig 1999 | Training for volunteer lay group leaders |
Chronic Disease Self Management Support Programme (six sessions) Peer support |
Volunteer lay group leaders supported by study team | No primary outcome specified. Multiple outcomes reported with mixed effects | ||
| Lynch 2014 |
Diabetes self management support groups (18 sessions) Peer support Goal setting and behaviour skills training |
Dietician led groups | No effect on primary outcome of weight reduction | |||
The predominant intervention component is highlighted in bold text for each study
No study contained a financial‐type intervention element
We excluded the following interventions:
Professional educational interventions or research initiatives where there was no specified structured clinical care delivered to an identified group of people with multimorbidity.
Interventions including people with comorbid conditions where the intervention was targeted solely at one condition and did not address the full extent of the multimorbidity. This commonly arises in relation to chronic disease and comorbid depression, so called 'depression plus one studies'. These are increasingly common as the link between depression and most chronic conditions has now been well established (Simon 2001). They include interventions designed to address depression in participants rather than targeting all conditions identified. We therefore excluded such studies if the intervention was only targeted at the depression and did not address the full extent of the multimorbidity.
The comparison was usual care.
Types of outcome measures
We included studies if they reported any objective, validated measure of:
Patient clinical or mental health outcomes (e.g. blood pressure, symptom scores, depression scores).
Patient‐reported outcome measures (e.g. quality of life, well‐being, measures of disability or functional status).
Utilisation of health services (e.g. hospital admissions).
Patient behaviour (e.g. measures of medication use and adherence, and other objective measures such as goal attainment (Cox 2002; Gordon 1999; Kiresuk 1968), if measured with a validated scale.
Provider behaviour (e.g. chronic disease management scores).
Acceptability of the service to recipients and providers, and treatment satisfaction were included if it was reported in a study that reported objective outcome measures behaviour.
Economic outcomes (e.g. full economic analyses incorporating measures of efficiency or effectiveness in relation to costs or direct costs depending on what was reported in included studies). Where direct costs were reported alone, we indicated whether these costs related to society, the health service, or the recipients. We also reported, where possible, costs in relation to the specific year and currency presented; whether costs related to total costs or simple fees charged; what was included in the cost calculations; and over what time period costs were calculated.
We excluded attitude and knowledge outcomes.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases without language restrictions up to 28 September 2015:
Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library, 2015, Issue 10, Wiley
Database of Abstracts of Reviews of Effects (DARE), The Cochrane Library, 2015, Issue 3, Wiley
MEDLINE, 1990 to September 2015, In‐Process and other non‐indexed citations, OvidSP
EMBASE, 1980 to September 2015, OvidSP
Cochrane Effective Practice and Organisation of Care (EPOC) Group Specialised Register, Reference Manager
Cumulative Index to Nursing and Allied Health Literature (CINAHL), 1980 to September 2015, EBSCOHost
Allied and Complementary Medicine Database (AMED), 1985 to September 2015, OvidSP
CAB Abstracts, 1973 to September 2015, EBSCOHost
HealthSTAR, 1999 to September 2015, OvidSP
We also searched the following trials registries:
We searched the IRCMO repository for unpublished/grey literature (IRCMO), and invited experts to inform us of other completed or ongoing studies
The search strategy was particularly challenging given the lack of a MeSH terms for multimorbidity. In addition, we were aware from existing epidemiological literature that the recognition of multimorbidity as a concept is relatively recent. Multimorbidity is sometimes used synonymously with the term comorbidity, though this tends to be used in relation to diseases that coexist with an index disease under study (de Groot 2004). However, comorbidity is a MeSH term, whereas multimorbidity is not, so we included both terms in our search. For pragmatic reasons we limited the MEDLINE search to articles indexed from 1990 onwards.
The search strategy published in the protocol (Smith 2007b) was not used; and the search strategy recorded for the 2007 search of MEDLINE was revised in 2009 to better capture the concept of multimorbidity. Results of the search were limited by filters for study design and an extensive list of intervention terms. Search strategies are provided in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5. The MEDLINE search strategy was used in HealthSTAR and AMED; the Cochrane search strategy was used in DARE.
Searching other resources
We also:
(a) Searched the reference lists of included papers (b) Contacted authors of relevant papers regarding any further published or unpublished work where indicated
Data collection and analysis
Selection of studies
All citations identified by the electronic searches were downloaded to reference manager software (EndNote 2013) and duplicates were removed. Potentially relevant studies were identified by review of the titles and abstracts of search results by the lead author (SS). We retrieved full text copies of all articles identified as potentially relevant. Two review authors (SS, HS, or EW) ) independently screened all citations found by the electronic searches and assessed each retrieved article for inclusion. We resolved any disagreement by discussion and consensus.
Data extraction and management
Two review authors (SS, HS or EW ) undertook data abstraction and cross checked data abstraction forms using a modified version of the EPOC data collection checklist (EPOC 2013a). Disagreements about data abstraction and quality were resolved by consensus between the review authors or through adjudication by the Cochrane contact editor.
We extracted the following information from the included studies: (1) Details of the intervention: a full description of the intervention was extracted as were details regarding aims; clinical protocols; use of case workers; remuneration/payment systems; providers involved; and theoretical framework on which the intervention was based; (2) Participants: patients, the nature of multimorbidity and how it was determined; providers, i.e. specialist and primary care providers, family members; (3) Clinical setting; (4) Study design; (5) Outcomes; (6) Results. Results were organised into: (i) Clinical outcomes; (ii) Mental health outcomes; (iii) Patient‐reported outcomes; (iv) Health service use (v) Recipient and provider behaviours; and (vi) Recipient and provider acceptability/satisfaction.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in all included studies using standard EPOC criteria (EPOC 2015) and included the following domains: allocation (sequence generation and concealment); baseline characteristics; incomplete outcome data; contamination; blinding; selective outcome reporting; and other potential sources of bias.
Measures of treatment effect
We reported data in natural units for each study. For RCTs, we reported results as (1) Absolute difference (mean or proportion of outcome in intervention group minus control at study completion); (2) Relative percentage difference (absolute difference divided by post‐intervention score in the control group). We undertook meta‐analysis where appropriate in terms of participants, interventions and outcomes using random‐effects models. Analyses were undertaken for clinical outcomes (glycaemic control and blood pressure) and depression scores in the comorbidity studies. We also undertook meta‐analyses for HRQoL and self‐efficacy in all studies in which these were reported. All meta‐analyses apart from self efficacy had significant statistical heterogeneity so we present the figures for these analyses without the pooled estimates of effect.
Standardised effect sizes (SES) are presented in tables where possible, i.e. where studies reported relevant data for their calculation. We have reported the range of effects using SES in the text of the results and used the generally accepted convention that an SES of more than 0.2 indicates a small intervention effect, an SES of more than 0.5 indicates a moderate intervention effect and an SES of more than 0.8 is a large effect size (Cohen 1988).
For ITS we had planned to report two effect sizes: (1) The change in the outcome immediately after the introduction of the intervention. (2) The change in the slope of the regression lines.
However, no ITS studies were identified.
Unit of analysis issues
None of the included studies had unit of analysis errors.
Dealing with missing data
If data on multimorbidity sub‐groups were missing from potentially eligible studies, we contacted authors to obtain the information. Two studies provided additional data on sub‐groups with multimorbidity (Coventry 2015; Eakin 2007). We did not include any studies with more than 20% missing data in meta‐analyses and did not make any assumptions regarding missing data.
Assessment of heterogeneity
We assessed included studies in terms of clinical and statistical heterogeneity. Statistical heterogeneity was assessed by examining forest plots and considering the I² statistic (Cochrane Handbook). We planned to prepare tables and funnel plots comparing effect sizes of studies grouped according to potential effect modifiers (for example, simple versus multifaceted interventions) if sufficient studies had been identified but this was not possible.
If there had been enough studies, we had planned to use meta‐regression to see whether the effect sizes could be predicted by study characteristics. These could, for example, include duration of the intervention, age groups, and simple versus multifaceted interventions (Cooper 1994). We also considered formal tests of homogeneity (Petitti 1994). None of these quantitative methods were possible for this version of the review but will be considered for future review updates.
Assessment of reporting biases
We assessed incomplete reporting of outcomes, where possible, within the 'Risk of bias' tables. This was only possible for studies that had published protocols or specifically reported different results than the outcomes mentioned in the methods sections of included papers.
Data synthesis
We expected that included studies would measure similar outcomes using different methods. These included either continuous variables (such as different depression scales) or dichotomous process measures (such as proportion of people with recovery from depression). For continuous outcomes, we reported means and standard deviations at study completion with the absolute difference and relative percentage difference. We calculated standardised effect sizes for continuous measures by dividing the difference in mean scores between the intervention and comparison group in each study, by an estimate of the pooled standard deviation. For categorical outcomes, we reported the proportions in the intervention and control groups with the absolute difference and relative percentage difference.
We undertook meta‐analysis of studies that were similar in terms of settings, participants, interventions, outcome assessment and study methods. If there was a high I² indicating statistical heterogeneity, we used graphs to illustrate the results but did not present the combined effects as the heterogeneity indicates that combining the studies in a meta‐analysis is inappropriate. Where meta‐analysis was not possible we carried out a narrative synthesis of the results and presented the results based on outcome groupings. See Additional tables.
We assessed the certainty of the evidence for the main outcomes using the following GRADE (Grading of Recommendations Assessment, Development and Evaluation) criteria (Guyatt 2008); and present the main findings with our judgments in a 'Summary of findings' table
1. Study limitations (i.e. risk of bias). 2. Consistency of effect. 3. Imprecision. 4. Indirectness. 5. Publication bias.
Subgroup analysis and investigation of heterogeneity
We had planned to consider subgroup analyses based on the degree of multimorbidity of participants estimated by the number of conditions per person. These analyses were not possible due to the variation in definitions of multimorbidity and characteristics of participants across studies.
Sensitivity analysis
We planned to undertake sensitivity analyses based on intervention type or clear distinctions in studies with different risk of bias but this was not possible due to the limited number of meta‐analyses undertaken with each containing relatively few studies.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence for the main outcomes using the following GRADE (Grading of Recommendations Assessment, Development and Evaluation) criteria (Guyatt 2008) including risk of bias, consistency of effect, imprecision, indirectness and other potential criteria such as publication bias. We presented the main findings with our judgments in a 'Summary of findings' table. We used the EPOC guidance for preparing a Summary of Findings (SoF) table using GRADE (EPOC 2017a). We included the following outcomes in the Summary of Findings Table: HRQoL, mental health outcomes, clinical outcomes, other patient reported outcomes, health behaviours, healthcare utilisation, medicines outcomes and provider behaviour.
We have included worksheet that outline the decision making process when applying GRADE for the three comparisons (Appendix 2; Appendix 3; Appendix 4).
Results
Description of studies
Results of the search
The electronic searches yielded 30,206 original citations after duplicates were removed (Figure 1). Of these, 30,075 citations were irrelevant and directly excluded. Full texts were retrieved for 131 studies. Of these, 60 studies from 93 papers were excluded with reasons Characteristics of excluded studies. Fifteen studies are on‐going (Characteristics of ongoing studies) ,. Seventeen studies from 19 papers were eligible for inclusion in this review and four other studies are awaiting classification.(Characteristics of studies awaiting classification).
1.
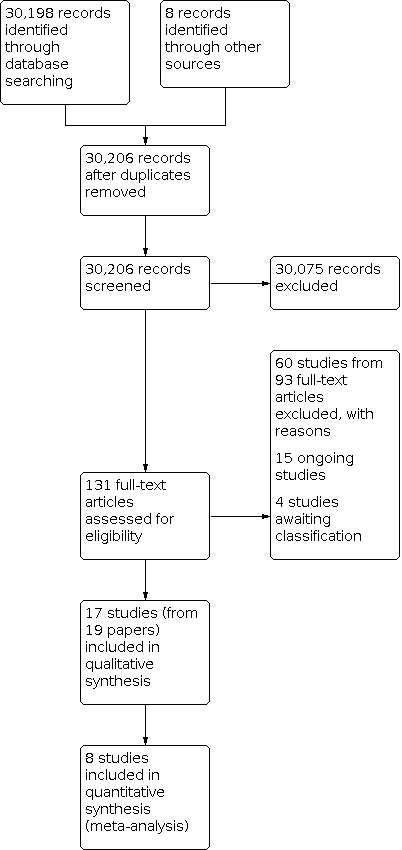
Flow Diagram
Included studies
See Characteristics of included studies table
Study design
We identified 17 RCTs eligible for inclusion in the review, 9 from the original review (Bogner 2008; Boult 2011; Eakin 2007; Hochhalter 2010; Hogg 2009; Katon 2010; Krska 2001; Lorig 1999; Sommers 2000;) and 8 identified in this update (Barley 2014; Coventry 2015; Garvey 2015; Kennedy 2013; Lynch 2014; Martin 2013; Morgan 2013; Wakefield 2012). No other study designs with eligible interventions were identified.
Population/participants
There were a total of 8408 participants across all studies. The interventions varied in duration from eight weeks to two years, with the majority lasting 6 to 12 months. There was also variation in post intervention follow‐up, varying from immediate follow‐up to follow‐up 12 months post intervention cessation.
Eight of the 17 studies recruited participants with a broad range of conditions (Boult 2011; Eakin 2007; Garvey 2015; Hochhalter 2010; Hogg 2009; Krska 2001; Lorig 1999; Sommers 2000), whereas the remaining nine focused on the following comorbidities: depression and hypertension (Bogner 2008); depression and diabetes and/or heart disease (Barley 2014; Coventry 2015; Morgan 2013; Katon 2010); depression and headache (Martin 2013); diabetes and hypertension (Lynch 2014; Wakefield 2012); and a sub‐group of people with at least two of diabetes, chronic obstructive pulmonary disease and irritable bowel syndrome (Kennedy 2013).
Settings
All studies were set in primary care or community settings in the USA, apart from Krska 2001 which was set in the UK National Health Service and Hogg 2009 which was set in Canada. Seven were funded by a government or university grant (Coventry 2015; Garvey 2015; Hogg 2009; Katon 2010; Kennedy 2013; Krska 2001; Lorig 1999); and the remaining studies were funded by charitable foundations. None were funded directly by the pharmaceutical industry.
Comparison intervention
In the majority of included studies, the comparator was usual medical care which in some studies was supplemented by a newsletter or leaflet (Eakin 2007;), or involved a baseline assessment but no follow‐on intervention (Bogner 2008; Garvey 2015; Katon 2010; Krska 2001). These minimal additions to usual care could be considered as being within the variation of usual care provided in different settings. One study invited those allocated to a control group to attend a group session based on an unrelated topic (Hochhalter 2010). This was an attempt to ensure that the intervention effect did not relate to the group setting but related to the intervention content.
Description of interventions
The interventions were all multifaceted and brief descriptions for each study are provided in the Characteristics of included studies. No study specifically reported consumer involvement in the intervention design.
As outlined in the methods, we used the EPOC taxonomy of interventions to describe and categorise the interventions tested in these studies (EPOC 2002). While the interventions identified all involved multiple components they could be divided broadly into two main groups. In 12 of 17 studies, the interventions were primarily organisational, for example case management or addition of a pharmacist to the clinical care team (Barley 2014; Bogner 2008; Boult 2011; Coventry 2015; Hogg 2009; Katon 2010; Kennedy 2013; Krska 2001; Martin 2013; Morgan 2013; Sommers 2000; Wakefield 2012). In the remaining five studies, the interventions were primarily patient‐oriented, for example self‐management support groups (Eakin 2007; Garvey 2015; Hochhalter 2010; Lorig 1999; Lynch 2014). However, there were overlapping elements with some organisational‐type studies including patient‐oriented elements such as education provided by a case manager and vice versa. No study involving financial or regulatory type interventions were identified. We have included an additional table which outlines intervention elements and indicates which elements featured in each of the included studies (Table 2)
Excluded studies
We excluded 75 studies in total, see Characteristics of excluded studies. Thirty‐five studies were excluded on the basis of ineligible participants. In some of these studies there was a potential multimorbidity sub‐group but these data were not reported or not available from authors when requested. Twenty‐six studies were excluded on the basis of an ineligible intervention. This was usually because it was conducted in a specialist setting or had a single‐condition focus despite participants having multiple conditions. The remaining studies were excluded on the basis of study design, largely due to absence of control groups.
Risk of bias in included studies
See Characteristics of included studies table, Figure 2 and Figure 3 for a summary assessment of the risk of bias of the included studies. Overall three of the 17 studies reported all elements for the risk of bias domains. Two studies reported domains with a high risk of bias and in 13 studies there were domains classified as unclear due to lack of reporting.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation concealment was assessed as adequate in eight of the 17 studies (Barley 2014; Boult 2011; Coventry 2015; Garvey 2015; ; Hogg 2009; Katon 2010; Krska 2001; Sommers 2000), but was assessed as unclear in the remainder. Baseline measurement of outcomes was carried out in all studies. All reported adequate follow‐up of participants except Lorig 1999 and Wakefield 2012 where the risk of bias was assessed as unclear. Lorig 1999 did not provide specific details pertaining to follow‐up for the multimorbidity subgroup, although follow‐up for the overall study was assessed as adequate. There was high risk of bias in Martin 2013 with poorer follow‐up in the intervention group (57%) compared to the control group (80%) at study completion. Objective outcomes were used in all but two studies, Krska 2001 and Hogg 2009, where this dimension was assessed as unclear. Krska 2001 used a measure detailing pharmaceutical care issues (PCIs) which was a previously developed classification system modified for the study. Hogg 2009 collected data on chronic and preventive care delivery from individuals' records but the accuracy of this process was not described. Blinding of outcome assessment was assessed as done in six studies (Boult 2011; Coventry 2015;; Hochhalter 2010; Katon 2010; Lorig 1999; Sommers 2000). It was assessed as unclear in nine studies (Barley 2014; Bogner 2008; Eakin 2007; Hogg 2009; Lynch 2014; Martin 2013; Morgan 2013; Wakefield 2012); and assessed as not done in Garvey 2015 and Krska 2001.
Five of the 17 studies had a cluster design that ensured no contamination of control participants (Boult 2011; Coventry 2015; Kennedy 2013; Morgan 2013; Sommers 2000). Contamination of participants allocated to the control group was unlikely in a further seven studies where the intervention was directed at recipients rather than providers (Barley 2014; Bogner 2008; Garvey 2015; Eakin 2007; Lorig 1999; Lynch 2014; Hochhalter 2010), but was possible in the remaining studies four studies (Hogg 2009; Katon 2010; Martin 2013; Wakefield 2012). However, Katon 2010 provided an appendix outlining potential contamination and indicated that it was minimal and, if it had occurred, it would have diluted rather than increased the significant effect size of their intervention. Krska 2001 stated that contamination of control participants who attended the same general practitioners (GPs) as the intervention participants could have occurred but that a cluster design would have been more problematic due to differential prescribing patterns between practices. All studies had low risk of selective outcome reporting and had no apparent other biases.
The five cluster randomised controlled trials accounted for clustering effects in their analysis so there were no unit of analysis errors (Boult 2011; Coventry 2015; Kennedy 2013; Morgan 2013; Sommers 2000).
Certainty of the evidence
See Table 1. In general, while all the included studies were RCTs the main limitation related to a lack of consistency of effect for most outcomes. Only the mental health outcomes, largely relating to depression in the comorbidity studies, were regarded as having a high GRADE ranking. We downgraded the evidence for effects on clinical and patient‐reported outcomes to moderate due to lack of consistency of effect across studies and small effect sizes. We downgraded the evidence for effects on provider behaviour to moderate, due to limited available data for calculation of standardised effect sizes (SES) and lack of clarity regarding the clinical importance of the results. We downgraded the evidence for effects on health service utilisation and medication use and adherence to low, due to variation across studies and small effect sizes. We did not include economic outcomes in the Table 1 due to the lack of robust economic analyses, rather we summarised this outcome in Table 3.
2. Costs.
| Study | Study type | Outcome | Result | Notes |
| Barley | RCT | Cost‐effectiveness | The intervention demonstrated marginal cost effectiveness up to a QALY threshold of GBP 3035 | |
| Boult | RCT | Total healthcare cost | Saving of USD 75,000 per GCN and USD 1364 per participant | USD in 2007 Initial result only ns |
| Katon | RCT | Cost‐effectiveness | Mean reduction of 114 days in depression free days and an estimated difference of 0.335 QALYs (95% CI −0.18 to 0.85). The intervention was associated with lower OPD costs with a reduction of USD 594 per participant (95% CI USD −3241 to USD 2053). | Non‐significant but 99.7% probability that the intervention met the threshold of < USD 20,000 per QALY |
| Krska | RCT | Mean cost of medicines | Int: 38.83 Con: 42.61 Absol diff 3.78 Rel %diff 9% |
GBP in 2000 ns SES = 0.13 |
| Lorig | RCT | Intervention cost per completed participant | USD 70 | USD in 1998 See text for assumptions made |
| Lorig | RCT | Cost savings per individual | USD 750 | USD in 1998 See text for assumptions made |
| Sommers | RCT | Savings per individual | USD 90 | USD in 1994 See text for assumptions made |
* refers to whether original study reported statistically significant improvement in this outcome
Effects of interventions
See: Table 1
Effects by type of interventions
We have presented an overview of intervention components for each study, highlighting the main intervention component in bold text and have included a brief summary of the intervention effect on the study primary outcomes in Table 2. The description of intervention components is based on reporting of intervention components in each paper and this is not consistent across studies. For example, most studies were likely to have included training of practitioners involved in interventions but not all studies reported this as an intervention component. We have also presented an overview of results based on whether the studies addressed general multimorbidity or comorbidity in Table 4.
3. Overview of outcomes.
| Outcome category | Outcome | No. studies with this outcome | No. studies with p< 0.05 for this outcome | |
| Physical Health | Hb1Ac | 5 | 2 | |
| BP | 6 | 2 | ||
| Cholesterol | 2 | 1 | ||
| Other symptom score | 4 | 1 | ||
| Mental Health | Depression scores | 8 | 6 | |
| % improved depression | 1 | 1 | ||
| Anxiety scores | 4 | 3 | ||
| Cognitive symptom management | 1 | 0 | ||
| Psychosocial | QoL/general health | 10 | 4 | |
| Functional impairment & disability | 5 | 2 | ||
| Social (activity/support) | 4 | 1 | ||
| Self efficacy | 7 | 3 | ||
| Home hazards | 1 | 0 | ||
| Health service use | Visits/use service | 5 | 0 | |
| Hospital admission related | 6 | 2 | ||
| Patient health related behaviours | Exercise/diet | 6 | 2 | |
| Medication adherence | 5 | 2 | ||
| Provider behaviour | Prescribing | 3 | 2 | |
| Disease management | 3 | 3 | ||
| Costs | Direct costs or cost effectiveness | 6 | Not applicable |
* Multimorbidity is defined as two or more independent conditions within the same individual whereas comorbidity refers to linked conditions. In this review comorbidity studies included depression and diabetes or depression and hypertension
** The scales or measurements used in each study for the outcomes are described in the Table of included studies
Organisational interventions
Twelve of the 17 included studies had organisational‐type interventions (Barley 2014; Bogner 2008; Boult 2011; Coventry 2015; Hogg 2009; Katon 2010; Kennedy 2013; Krska 2001; Martin 2013; Morgan 2013; Sommers 2000; Wakefield 2012). These predominantly involved case management and coordination of care or the enhancement of skill mix in multidisciplinary teams in addition to delivery of patient care.
1. Clinical outcomes
Eight of the 12 organisational type studies reported clinical outcomes. These studies had a range of standardised effect sizes (SES) varying from 0.01 to 1.6. Interventions aimed at improving management of risk factors in comorbid conditions were more likely to have larger effect sizes (e.g. Bogner 2008; Katon 2010; Morgan 2013).
Five studies reported six measures of glycaemic control (five mean HbA1c and one study reported percentage achieving at least 0.5% reduction in HbA1c). Katon 2010 and Morgan 2013 reported improvements in mean HBA1c; however, Morgan 2013 had a substantial proportion of missing HbA1c data at study completion so these data were not included in the meta‐analysis of HbA1c. Hogg 2009, Lynch 2014 and Wakefield 2012 found little or no difference in HbA1c.Lynch 2014 The SES ranged from 0.05 to 0.38 and none of these studies had an SES greater than 0.5. The mean difference (MD) was ‐0.193 (95% CI −0.47 to 0.10) as outlined in Figure 4.
4.

Forest plot of comparison: 1 Glycaemic control (HbA1c) Diabetes outcome: 1.1 HbA1c at immediate to 6 months post‐intervention follow up.
Five studies reported on systolic blood pressure (SBP). Bogner 2008 and Katon 2010 reported improvements in blood pressure, although this was of minimal clinical significance in Katon 2010. Morgan 2013 and Wakefield 2012 reported little difference. The standardised effect sizes (SES) ranged from 0.01 to 0.78 but only one of these four studies had an SES greater than 0.5. The MD was −3.10 (95% CI −7.26 to 1.06) as illustrated in Figure 5.
5.

Forest plot of comparison: 2 Systolic Blood Pressure: outcome: 2.1 Systolic blood pressure at immediate to 6 months post‐intervention follow up.
Two studies reported on cholesterol. Katon 2010 found a reduction in LDL cholesterol, whereas Morgan 2013 found no meaningful difference (SES ranges 0.22 to 0.26). Katon 2010 reported a composite primary outcome that combined three risk factors, which showed an improvement in intervention participants compared to control (see Table 5).
4. Clinical Outcomes.
| Study | Study type | Outcomes | Results | Notes |
| Barley | RCT | % with angina (Rose Angina score) | Int 22/31 Con 30/37 Absol diff 8, Rel % diff 27% |
ns |
| Bognor | RCT | Systolic BP | Int 127.3 (SD 17.7) Con 141.3 (SD 18.8) Absol diff 14, Rel % diff 10% |
* SES = 0.78 |
| Bognor | RCT | Diastolic BP | Int 83 (SD 10.7) Con 81.4 (SD 11.1) Absol diff 9.2, Rel % diff 11% |
* SES = 0.15 |
| Hogg | RCT | Systolic BP | Int 124.3 Con 124.2 Absol diff 0.1, Rel % diff < 0.1% |
ns (No SDs reported) |
| Hogg | RCT | HbA1c | Int 7.01 Con 6.78 Absol diff 0.23, Rel % diff 3% |
ns |
| Katon | RCT | Systolic BP | Int 131 (SD 18.4) Con 132.3 (SD 17.2) Absol diff 1.3, Rel % diff 1% |
* SES = 0.07 |
| Katon | RCT | HbA1c | Int 7.33 (SD 1.21) Con 7.81 (SD 1.9) Absol diff 0.48, Rel % diff 6% |
* SES = 0.32 |
| Katon | RCT | Cholesterol | Int 91.9 (SD 36.7) Con 101.4 (SD 36.6) Absol diff 9.5, Rel % diff 9% |
* SES = 0.26 |
| Katon | RCT | Composite: all three risk factors (BP, HbA1c and cholesterol) below guidelines |
Int 36/97 (0.37) Con: 19/87 (0.22) Absol diff 15, Rel % diff 68% |
* |
| Lorig | RCT | Pain/ physical discomfort | Int 59.8 (SD 20.1) Con 60.6 (SD 17.1) Absol diff 0.8, Rel % diff 1% |
SES = 0.04 ns |
| Lorig | RCT | Energy/fatigue | Int 2.18 (SD 0.73) Con 2.02 (SD 0.75) Absol diff 0.16, Rel % diff 8% |
ns |
| Lorig | RCT | Shortness of breath | Int 1.34 (SD 0.91) Con 1.58 (SD 0.83) Absol diff 0.24, Rel % diff 15% |
ns |
| Lynch | RCT | HbA1C | Int 7.4 (SD 1.6) Con 7.5 (SD 1.6) Absol diff 0.1, Rel % diff 6.7% |
ns SES = 0.06 |
| Lynch | RCT | % with at least 0.5 absolute reduction in HbA1c | Int 15/30 (0.05) 7/31 Con (0.21) Absol diff 29, Rel % diff 138% |
* |
| Lynch | RCT | Mean SBP | Int 135.8 (SD 21.4) Con 136.7 (SD 23) Absol diff 0.9, Rel % diff 0.6% |
ns SES = 0.04 |
| Morgan | RCT | HbA1C | Int 6.9 (SD 1.21) Con 7.4 (SD 1.42) Absol diff 0.5, Rel % diff 6.7% |
* SES = 0.38 |
| Morgan | RCT | Systolic BP | Int 132.4 (SD 19) Con 131.2(SD 19.6) Absol diff 1.2, Rel % diff 0.9% |
ns SES = 0.02 |
| Morgan | RCT | Cholesterol | Int 4.22 (SD 0.94) Con 4.44 (SD 1.06) Absol diff 0.22, Rel % diff 5% |
ns SES = 0.22 |
| Morgan | RCT | Mean BMI | Int 31.2 (SD 6) Con 31.0 (SD 6) Absol diff 0.2, Rel % diff 0.6% |
ns SES = 0.03 |
| Sommers | RCT | Symptom scores | Int 17.2 Con 18.9 Absol diff 1.7, Rel % diff 9% |
ns |
| Wakefield | RCT | HbA1c | Int 6.9 (1.1) Con 6.95 (1.1) Absol diff 0.05, Rel % diff 0.7% |
ns SES = 0.05 |
| Wakefield | RCT | Systolic BP | Int 133 (16.6) Con 137 (17.3) Absol diff 4, Rel % diff 3% |
ns SES = 0.24 |
| Martin | RCT | Mean headache rating | Int 0.63 (SD 0.5) Con 1.01 (SD 0.83) Absol diff 0.38, Rel % diff 38% |
* SES = 0.58 |
* refers to whether original study reported statistically significant improvement in this outcome
** Total number with final data collected was 384. No final numbers of intervention and control participants presented.
Four studies reported symptom scores relating to clinical outcomes. Barley 2014, Lorig 1999 and Sommers 2000 found little or no difference whereas Martin 2013 reported improvements in mean headache rating (see Table 5).
2. Mental health outcomes
Seven studies presented data on mental health outcomes (Barley 2014; Bogner 2008; Coventry 2015; Katon 2010; Martin 2013; Morgan 2013; Sommers 2000). Five of the seven studies reported improvements in a range of depression measures whereas two showed no improvements in depression outcomes (Barley 2014; Sommers 2000). We undertook two meta‐analyses: a meta‐analysis of Patient Health Questionnaire, version 9 (HQ9) depression scores; and a meta‐analysis of standardised mean difference (SMD) in depression scores for the studies with available data where depression was a targeted condition. This suggests a modest intervention effect. The meta‐analysis for PHQ9 scores had high heterogeneity so we do not report the pooled effect (Figure 6). The SMD for other depression scores was −0.41 (95% CI −0.63 to −0.20) (Figure 7). The range in SESs for depression outcomes across these studies was from 0.09 to 1.18 with four of the nine outcomes indicating moderate to large effect sizes (i.e. SES > 0.5). These higher effect sizes were all reported in the studies in which depression was a focus of the intervention.
6.

Forest plot of comparison: 3 Depression scores: 3.1 PHQ9 Depression scores at immediate to 6 months post‐intervention follow up.
7.

Forest plot of comparison: 4 Depression scores: 4.1 Depression scores at immediate to 6 months post‐intervention follow up.
Three studies reported on anxiety measures, two showed improvements (Coventry 2015 and Martin 2013) whereas Barley 2014 reported little difference (see Table 6). There were small effect sizes in all studies (SES range 0.08 to 0.26).
5. Mental Health Outcomes.
| Study | Study type | Outcome | Result | Notes |
| Barley | RCT | PHQ9 depression score | Int 12.6 (SD 7.1) Con 12 (SD 6.9) Absol diff 0.6, Rel % diff 8% |
ns SES = 0.09 |
| Barley | RCT | HADS depression score | Int 9.5 (SD 4.6) Con 8.8 (SD 4.8) Absol diff 0.7, Rel % diff 8% |
ns SES = 0.15 |
| Barley | RCT | HADS anxiety score | Int 9.9 (SD 7.1) Con 9.5 (SD 5.4) Absol diff 0.4, Rel % diff 4% |
ns SES = 0.08 |
| Bognor | RCT | CES depression score | Int 9.9 (SD 10.7) Con 19.3 (SD 15.2) Absol diff 9.4, Rel % diff 49% |
* SES = 0.75 |
| Coventry | RCT | SCL‐D13 depression score | Int 1.76 (SD 0.9) Con 2.02 (SD 0.9) Absol diff 2.6, Rel % diff 13% |
* SES = 0.28 |
| Coventry | RCT | PHQ9 depression score | Int 11.3 (SD 6.5) Con 13.1 (SD 6.5) Absol diff 1.8, Rel % diff 14% |
* SES = 0.28 |
| Coventry | RCT | GAD‐7 anxiety score | Int 8.2 (SD 5.8) Con 9.7 (SD 5.9) Absol diff 1.5, Rel % diff 15% |
* SES = 0.26 |
| Garvey | RCT | HADS total score | Int 15.6 (SD 8.3) Con 16.7 (SD 8.2) Absol diff 1.1, Rel % diff 6.5% |
ns SES = 0.13 |
| Katon | RCT | SCL 20 depression score | Int 0.83 (SD 0.66) Con 1.14 (SD 0.68) Absol diff 0.31, Rel % diff 27% |
* SES = 0.46 |
| Katon | RCT | Patient global improvement in depression | Int 41/92 Con 16/91 Absol diff 27, Rel % diff 150% |
* |
| Lorig | RCT | Cognitive symptom management score | Int 1.75 Con 0.98 Absol diff 0.77, Rel % diff 79% |
ns |
| Martin | RCT | PHQ9 depression score | Int 6.7 (SD 4.6) Con 12.6 (SD 5.3) Absol diff 5.9, Rel % diff 47% |
* SES = 1.18 |
| Martin | RCT | BDI ‐Depression score | Int 13.1 (SD 8.6) Con 28.7 (SD 9.5) Absol diff 15.6, Rel % diff 54% |
* SES = 1.73 |
| Martin | RCT | BAI Anxiety score | Int 10.5 (SD 10.8) Con 16.4 (SD 9.3) Absol diff 5.9, Rel % diff 36% |
* SES = 0.1 |
| Morgan | RCT | PHQ9 depression score | Int 7.1 (SD 4.7) Con 9.0 (SD 5.5) Absol diff 1.9, Rel % diff 21% |
* SES = 0.37 |
| Sommers | RCT | GDS score (depression) | Int 4.1 Con 4.1 Absol diff 0, Rel % diff 0% |
ns |
* refers to whether original study reported statistically significant improvement in this outcome
3. Patient‐reported outcome measures
Nine of the organisational‐type studies presented patient‐reported outcome measures (PROMs).
Nine of these reported a variety of HRQoL measures with a range of effects from SES of 0.03 to 0.84. Coventry 2015 Three studies reported small effect sizes (Coventry 2015;Katon 2010; Martin 2013). The remaining six studies reported little or no effect (Barley 2014; Hogg 2009; Kennedy 2013; Krska 2001; Morgan 2013; Sommers 2000). Krska 2001 and Morgan 2013 reported that SF36 scores had been analysed across eight domains at study completion and reported little or no difference between groups, but did not present actual data. The mixed evidence regarding HRQoL is illustrated in Figure 8 which includes studies with available data but the pooled effect is not reported due to high statistical heterogeneity (I² = 73%).
8.

Forest plot of comparison: 5 Health related quality of life, outcome: 5.1 HRQoL at immediate to 6 months post‐intervention follow up.
Five organisational studies reported on self‐efficacy with a range in SES of 0.03 to 0.11, suggesting minimal effect. (Barley 2014; Hochhalter 2010; Kennedy 2013; Wakefield 2012; Coventry 2015). We undertook a meta‐analysis of standardised mean self‐efficacy scores in comorbidity studies and found no effect, SMD −0.05 (95% CI −0.12 to 0.22) (Figure 9).
9.

Forest plot of comparison: 6 Self‐Efficacy, outcome: 6.1 Self‐efficacy score at immediate to 6 months post‐intervention follow up.
Two of the organisational studies reported measures relating to disability or impaired activities of daily living (IADL). Hogg 2009 reported no effect of interventions on IADL, whereas Coventry 2015 reported an improvement in the Sheehan Disability score in intervention participants.
Two of the organisational studies reported measures relating to Illness perceptions and both reported no effect (Barley 2014; Coventry 2015).
A range of other PROMs were also reported with mixed effects and none had an SES greater than 0.3. These are presented in Table 7.
6. Patient‐reported outcome measures.
| Study | Study type | Outcome | Result | Notes |
| Health Related Quality of Life | ||||
| Barley | RCT | SF12 PCS | Int 32.4 (SD 10.7) Con 33.3 (SD 9.2) Absol diff 0.7, Rel % diff 2% |
ns SES = 0.07 |
| Barley | RCT | SF12 MCS | Int 34.5 (SD 11.6 ) Con 33.6 (SD 12.5 ) Absol diff 0.9, Rel % diff 3% |
ns SES = 0.08 |
| Barley | RCT | HRQoL (WEMWBS) | Int 40.6 (SD 11.2) Con 39.6(SD 12.3) Absol diff 1, Rel % diff 2.5% |
ns SES = 0.08 |
| Coventry | RCT | HRQoL (WHOQOL) | Int 2.99 (SD 0.6) Con 2.91 (SD 0.6) Absol diff 0.08, Rel % diff 3% |
* SES = 0.13 |
| Garvey | RCT | HRQoL (EQ5D VAS) | Int 65.7 (SD 20.2) Con 50.5 (SD 16.3) Absol diff 15.2, Rel % diff 30% |
* SES = 0.84 |
| Hogg | RCT | SF 36 Mental Health | Int 52.4 Con 52.2 Absol diff 0.2, Rel % diff 0.3% |
ns |
| Hogg | RCT | SF 36 Physical Health | Int 44.3 Con 41.5 Absol diff 2.8, Rel % diff 6.7% |
ns |
| Katon | RCT | QoL score | Int 6.0 (SD 2.2) Con 5.2 (SD 1.9) Absol diff 0.8, Rel % diff 15% |
* SES = 0.44 |
| Kennedy | RCT | HRQoL (EQ5D) | Int 0.56 (SD 0.34) Con 0.57 (SD 0.32) Absol diff 0.01, Rel % diff 1% |
ns SES = 0.03 |
| Lorig | RCT | Psychological well‐being | Int 3.47 Con 3.33 Absol diff 0.04, Rel % diff 4% |
ns SES = 0.21 |
| Martin | RCT | HRQol (AQOL) | Int 26.3 (SD 4.76) Con 28.4 (SD 4.97) Absol diff 2.1, Rel % diff 7 % |
* SES = 0.4 |
| Sommers | RCT | SF36 score | Int 2.2 Con 3.3 Absol diff 1.1, Rel % diff 33% |
ns |
| Self‐efficacy | ||||
| Barley | RCT | Self‐efficacy score | Int 28.6 (SD 6.7) Con 27.9 (SD 8.1) Absol diff 0.11, Rel % diff 2.5% |
ns SES = 0.09 |
| Coventry | RCT | Self‐efficacy score | Int 5.72 (SD 1.9) Con 5.53 (SD 1.9) Absol diff 0.18, Rel % diff 3.2% |
ns * SES = 0.09 |
| Garvey | RCT | Self efficacy score | Int 6.8 (SD 1.5) Con 5.3 (SD 1.9) Absol diff 1.47, Rel % diff 28% |
* SES = 0.86 |
| Hochhalter | RCT | Self‐efficacy | Int 7.4 Con 8.0 Absol diff 0.6, Rel % diff 7.5% |
ns |
| Kennedy | RCT | Self‐efficacy | Int 68 (SD 23.4) Con 68.7 (SD 23.1) Absol diff 0.7, Rel % diff 1% |
ns SES = 0.03 |
| Wakefield | RCT | Self‐efficacy | Int 8.1 (SD 1.9) Con 8.3 (SD 1.9) Absol diff 0.2, Rel % diff 2.4% |
ns SES = 0.11 |
| Daily function and disability | ||||
| Coventry | RCT | Sheehan Disability Score | Int 5.73 (SD 2.8) Con 5.83 (SD 2.8) Absol diff 0.1, Rel % diff 2% |
* SES = 0.04 |
| Garvey | RCT | Frenchay Activities Index | Int 21.3 (SD 7.9) Con 18.9 (SD 7.2) Absol diff 2.4, Rel % diff 13% |
* SES = 0.32 |
| Garvey | RCT | Activities daily living: NEADL (total) | Int 47.2 (SD 11.9) Con 40.7 (SD 10.7) Absol diff 6.5, Rel % diff 16% |
* SES = 0.58 |
| Hogg | RCT | IADL | Int 10.6 Con 10.9 Absol diff 0.3, Rel % diff 2.7% |
ns |
| Lorig | RCT | Disability | Int 0.86 Con 0.96 Absol diff 0.1, Rel % diff 10% |
ns |
| Lorig | RCT | Social role/activity limitation | Int 1.91, Con 1.98 Absol diff 0.07, Rel % diff 4% |
ns |
| Illness perceptions | ||||
| Coventry | RCT | Multimorbidity illness perception scale | Int 2.1 (SD 0.9) Con 2.28 (SD 0.9) Absol diff 0.18, Rel % diff 8% |
ns SES = 0.2 |
| Barley | RCT | Illness perceptions (BIPQ) | Int 40 (SD 14.8) Con 43(SD 31.1) Absol diff 3, Rel % diff 7% |
ns SES = 0.22 |
| Social support | ||||
| Coventry | RCT | Social support (ESSI) | Int 3.29 (SD 1.1) Con 3.4 (SD 1.0) Absol diff 0.11, Rel % diff 3% |
ns SES = 0.11 |
| Eakin | RCT | Multilevel support for healthy lifestyle | Int 2.7 Con 2.59 Absol diff 0.11, Rel % diff 4% |
ns |
| Other PROMs | ||||
| Barley | RCT | Patient‐reported needs (PSYCHLOPS) | Int 13.6 (SD 5.1) Con 13.4 (SD 5.4) Absol diff 0.2, Rel % diff 1.5% |
ns SES = 0.04 |
| Hochhalter | RCT | Total unhealthy days | Int 15.3 Con 14.1 Absol diff 1.2, Rel % diff 9% |
ns |
| Hogg | RCT | Total unhealthy days | Int 7.6 Con 9.9 Absol diff 2.3, Rel % diff 23% |
ns |
| Kennedy | RCT | Shared decision making (HCCQ) | Int 67.7 (SD 28) Con 69.3 (SD 26.1) Absol diff 1.6, Rel % diff 2% |
ns SES = 0.06 |
| Lorig | RCT | Self‐rated health | Int 3.42 Con 3.44 Absol diff 0.02, Rel % diff 0.6% |
ns |
| Lorig | RCT | Health distress | Int 1.97 Con: 2.13 Absol diff 0.16, Rel % diff 7.5% |
ns SES = 0.16 |
| Sommers | RCT | Social activities count | Int 8.7 Con:8.6 Absol diff 0.1, Rel % diff 1% |
* (when adjusted for baseline diff) |
| Sommers | RCT | HAQ score | Int 0.44 Con 0.5 Absol diff 0.06, Rel % diff 12% |
ns |
* refers to whether original study reported statistically significant improvement in this outcome
4. Utilisation of health services
Five organisational studies reported outcomes on health services utilisation (Boult 2011; Hogg 2009; Katon 2010; Krska 2001; Sommers 2000). Sommers 2000 reported improvements for intervention group participants across a variety of measures relating to hospital admissions, whereas Boult 2011, Hogg 2009, Katon 2010 and Krska 2001 found no difference in admission‐related outcomes, although numbers of admissions in most of these studies were very small.
Three studies reported data in relation to health service visits with a range of providers none of which showed clear improvements in appropriate health service use (Boult 2011; Hogg 2009; Sommers 2000) (see Table 8). No studies that included health service utilisation reported data that could be used to calculate SESs.
7. Health service use.
| Study | Study type | Outcome | Result | Notes |
| Boult | RCT | No. hospital admissions | Int 0.7 Con 0.72 Absol diff 0.02, Rel % diff 3% |
ns |
| Boult | RCT | No. days in hospital | Int 4.26 Con 4.49 Absol diff 0.23, Rel % diff 5% |
ns |
| Boult | RCT | No. ED visits | Int 0.44 Con 0.44 Absol diff 0, Rel % diff 0 |
ns |
| Boult | RCT | No. PC visits | Int 9.98 Con 9.88 Absol diff 0.1, Rel % diff 1% |
ns |
| Boult | RCT | No. specialist visits | Int 9.04 Con 8.49 Absol diff 0.55, Rel % diff 6% |
ns |
| Boult | RCT | No. home healthcare episodes | Int 0.99 Con 1.3 Absol diff 0.31, Rel % diff 24% |
* |
| Hogg | RCT | No. hospital admissions | Int 0.4 Con 0.46 Absol diff 0.06, Rel % diff 13% |
ns |
| Hogg | RCT | Proportion hospitalised | Int 0.26, Con 0.26 Absol diff 0, Rel % diff 0% |
ns |
| Hogg | RCT | No. ED visits | Int 0.63 Con 0.73 Absol diff 0.01, Rel % diff 14% |
ns |
| Hogg | RCT | Proportion with ED visit | Int 0.38 Con 0.42 Absol diff 0.04, Rel % diff 9% |
ns |
| Katon | RCT | Proportion hospitalised | Int 0.26 Con 0.22 Absol diff 0.04, Rel % diff 18% |
ns |
| Lorig | RCT | No. doctor and ED visits | Int 6.51 Con 7.08 Absol diff 0.57, Rel % diff 8% |
ns |
| Lorig | RCT | No. hospital stays in past 6 months | Int 0.26 Con 0.31 Absol diff 0.05, Rel % diff 6% |
* |
| Lorig | RCT | No. nights in hospital in last 6 months | Int 1.3 Con 1 Absol diff 0.3 Rel % diff 30% |
* |
| Sommers | RCT | No. hospital admissions per individual per year | Int 0.36 Con 0.52 Absol diff 0.16, Rel % diff 31% |
* |
| Sommers | RCT | ≥1 60 day readmission | Int 3.6 Con 9.4 Absol diff 5.8, Rel % diff 62% |
* |
| Sommers | RCT | ≥ 1 hospital admission | Int 8.8 Con 7.7 Absol diff 1.1, Rel % diff 14% |
* |
| Sommers | RCT | No. PCP visits | Int 6.0 Con 6.1 Absol diff 0.1, Rel % diff 2% |
ns |
| Sommers | RCT | No. office visits | Int 11 Con 12.5 Absol diff 1.5, Rel % diff 12% |
* |
| Sommers | RCT | ≥ 1 home care visit | Int 19.5 Con 18.8 Absol diff 0.7, Rel % diff 4% |
ns |
| Sommers | RCT | No. medical specialist visits | Int 1.4 Con 1.7 Absol diff 0.3, Rel % diff 18% |
ns |
| Sommers | RCT | No. other visits | Int 3.9 Con 4.3 Absol diff 0.4, Rel % diff 9% |
* |
| Sommers | RCT | ≥ 1 ED visit | Int 21.4 Con 16.7 Absol diff 4.7, Rel % diff |
ns |
* refers to whether original study reported statistically significant improvement in this outcome
5. Patient behaviour
5.1 Medication use and adherence
Four organisational studies reported measures relating to medication use and adherence. Two of these studies found an effect whereas two did not; and there was a range in SESs from 0.2 to 0.28 indicating minimal intervention effects. Bogner 2008 reported improvements in proportions of intervention participants adhering to both antidepressant and antihypertensive medication as measured using automated counting systems in the caps of medicine bottles (MEMS caps). Morgan 2013 reported a lower proportion of intervention participants were taking anti‐depressant medication. Martin 2013 reported on mean daily medication use which was not significantly different between intervention and control participants. Wakefield 2012 reported two measures of medication‐taking adherence both of which showed no significant difference; (see data in Table 9).
8. Medication use and adherence and prescribing.
| Study | Study type | Outcome | Results | Notes |
| Bognor | RCT | ≥80% adherence to antidepressant medication (MEMS caps) | Int 23/32 Con 10/32 Absol diff 0.41, Rel % diff 132% |
* |
| Bognor | RCT | ≥80% adherence to antihypertensive medication (MEMS caps) | Int 25/32 Con 10/32 Absol diff 0.47, Rel % diff 152% |
* |
| Martin | RCT | Mean daily medication use | Int 2.4 (SD 3.2) Con 3.0 (SD 2.8) Absol diff 0.6, Rel % diff 20% |
ns SES = 0.2 |
| Morgan | RCT | % taking antidepressant medication | Int 34/62 Con 36/113 Absol diff 0.11, Rel % diff 34% |
* |
| Wakefield | RCT | Adherence (Edward's scale) | Int 3.4 (SD 0.5) Con 3.3 (SD 0.5) Absol diff 0.1, Rel % diff 3% |
ns SES = 0.2 |
| Wakefield | RCT | Medication Taking Adherence Score | Int 100 (SD 1.4) Con 98.9 (SD 6.0) Absol diff 1.1, Rel % diff 1% |
ns SES = 0.28 |
* refers to whether original study reported statistically significant improvement in this outcome
5.2 Health related behaviours
Three organisational studies provided data on a variety of outcomes relating to health behaviours by participants (Katon 2010; Morgan 2013; Sommers 2000). Katon 2010 found no difference in relation to adherence to diet and exercise. Morgan 2013 presented self‐report data on three patient‐behaviour outcomes with improvements in proportions of individuals exercising (Int 60% vs Con 29%) and in the proportions smoking (Int 8% vs Con 12%) and consuming alcohol (49% vs 64%). Sommers 2000 found no changes in a nutrition checklist score. No studies reporting health‐related behaviours reported data that could be used to calculate SESs; (see data in Table 10).
9. Health‐related participant behaviours.
| Study | Study type | Outcome | Results | Notes |
| Hochhalter | RCT | PAM (patient activation measure) | Int 66.8 Con 66.2 Absol diff 0.6, Rel % diff 1% |
ns |
| Eakin | RCT | Diet behaviour scores | Int 2.2 Con 2.41 Absol diff 0.21, Rel % diff 9% |
* |
| Eakin | RCT | Change minutes of walking/week | Int +8 Con −10 Absol diff 18, Rel % diff 180% |
* |
| Katon | RCT | General adherence to diet score | Int 0.86 Con 0.81 Absol diff 0.05, Rel % diff 6% |
ns |
| Katon | RCT | General adherence to exercise score | Int 0.54 Con 0.44 Absol diff 0.1, Rel % diff 23% |
ns |
| Lorig | RCT | Exercise: stretching and strengthening (mins/week) | Int 53.1 Con 40.4 Absol diff 12.7,Rel % diff 31% |
ns |
| Lorig | RCT | Exercise: aerobic (mins/week) | Int 101.8 Con 88 Absol diff 13.8, Rel % diff 157% |
ns |
| Lorig | RCT | Communication with doctor (score 1‐5) |
Int 3.34 Con 3.2 Absol diff 0.14, Rel % diff 4% |
ns |
| Lynch | RCT | Physical activity (kcal expenditure per week, CHAMPS) | Int 1913.6 Con −603 Absol diff 2516, Rel % diff 417% |
* |
| Morgan | RCT | Smoking | Int 13/162 Con 13/110 Absol diff 0.04, Rel % diff 33% |
ns |
| Morgan | RCT | Alcohol | Int 51/104 Con 27/42 Absol diff 0.15, Rel % diff 23% |
ns |
| Morgan | RCT | Exercise (30 minutes/day for 5 days/ week) | Int 97/162 Con 22/75 Absol diff 0.31, Rel % diff 106% |
* |
| Sommers | RCT | Nutrition checklist score | Int 2.0 Con1.9 Absol diff 0.1, Rel % diff 5% |
ns |
* refers to whether original study reported statistically significant improvement in this outcome
6. Provider behaviour
6.1 Prescribing
Two organisational studies reported measures relating to practitioner prescribing or medicines management, both of which indicated significant benefits for intervention participants. Katon 2010 reported a measure examining one or more medication adjustments for five classes of drugs related to the comorbid conditions being studied and reported differences for four of these five groups. Krska 2001 reported a reduction in pharmaceutical care issues in intervention participants; (see data in Table 9)
6.2 Other provider behaviours
Provider behaviours relating to chronic disease management or preventive care were reported in four organisational studies. Boult 2011 and Coventry 2015 both presented a validated measure called the Patient Assessment of Chronic Illness Care (PACIC) score, which is a patient assessment of the quality of care received. This score includes five elements and the aggregate quality score derived was improved in the Boult 2011 Guided Care study, and a small effect reported by Coventry 2015 study (SES 0.39). Hogg 2009 reported measures relating to chronic disease management and preventive care based on chart data and both were improved in the intervention group. Morgan 2013 reported on the proportions of particpants referred to exercise and mental health programmes which was higher in intervention than control group participants; (see data in Table 11).
10. Provider behaviour.
| Study | Study type | Outcome | Result | Notes |
| Boult | RCT | PACIC score (patient measure of quality of care received) |
Int 3.14 Con 2.85 Absol diff 0.29, Rel % diff 10% |
* |
| Coventry | RCT | PACIC score | Int 2.37 (SD 1.1) Con 1.98 (SD 1.0) Absol diff 0.39, Rel % diff 20% |
ns SES = 0.39 |
| Hogg | RCT | Chronic Disease Mangement Score | Int 0.84 Con 0.77 Absol diff 0.07, Rel % diff 9% |
* |
| Hogg | RCT | Preventive Care Score | Int 0.89 Con 0.7 Absol diff 0.19, Rel % diff 27% |
* |
| Krska | RCT | % Pharmaceutical care issues resolved from baseline | Int 950/1206 Con 542/1380 Absol diff 0.4, Rel % diff 102% |
* |
| Morgan | RCT | % Referred to mental health | Int 58/162 Con 10/111 Absol diff 0.27, Rel % diff 300% |
* |
| Morgan | RCT | % Referred to exercise programme | Int 58/162 Con 24/114 Absol diff 0.15, Rel % diff 71% |
* |
* refers to whether original study reported statistically significant improvement in this outcome
7. Acceptability of services
Three organisational studies reported treatment satisfaction. Katon 2010 reported the proportion of participants moderately to very satisfied with treatment for depression and diabetes and heart disease at study completion. More intervention participants were satisfied with their care at study completion compared to those experiencing usual care. Boult 2011 reported on the changes in satisfaction for providers as part of an overall examination of the effect of the intervention on providers. The measure incorporated changes in 11 domains of satisfaction with service provision; five domains relating to time spent with participants; six domains relating to provider knowledge of the participant; and four domains relating to care coordination. The only changes reported in the study were improvements in 5 of the 11 domains relating to satisfaction with service provision. Coventry 2015 reported mean Client Satisfaction Scores and reported no difference between intervention and control group participants.
8. Costs
Five organisational studies provided data on costs (Barley 2014; Boult 2011; Katon 2010; Krska 2001; Sommers 2000).
Barley 2014 undertook a parallel economic analysis of the UPBEAT intervention and found that the intervention demonstrated marginal cost effectiveness up to a quality‐adjusted life‐year (QALY) threshold of GBP 3035.
Leff 2009 et al provided initial cost data from Boult 2011 and indicated a saving related to Guided Care of USD 75,000 per guided care nurse (95% CI USD −244,000 to USD 150,900) or USD 1364 per individual. However, these initial results were based on small changes in outcomes with wide confidence intervals. In addition, the final study results were subsequently published and indicated no intervention effect.
Katon 2010 reported the direct mean medical costs relating to the TeamCare intervention over a 12 month period as USD 1224 per individual. A subsequent economic analysis reported that the intervention led to an additional 114 days in depression‐free days and an estimated difference of 0.335 QALYs (95% CI −0.18 to 0.85) (Katon 2012). The intervention was associated with lower OPD costs with a reduction of USD 594 per person (95% CI USD −3241 to USD 2053). There was a 99.7% probability that the intervention met the threshold of less than USD 20,000 per QALY. The authors interpreted this as a high value intervention but this must be interpreted with caution given the wide confidence intervals in the estimates with lack of statistical significance.
Krska 2001 reported the mean medicine cost for the intervention and control groups at study completion and found a marginal benefit for the intervention.
Sommers 2000 reported a net saving per intervention participant of USD 90 though this did not include additional savings from fewer physician visits. It also excluded the costs of implementing the intervention, stated to be USD 118,950, mainly relating to salary costs; (see Table 3).
Patient‐oriented interventions
Five of the 17 included studies had predominantly patient‐oriented interventions, for example education or group‐based self‐management support courses (Eakin 2007; Garvey 2015; ; Hochhalter 2010; Lorig 1999; Lynch 2014). All five aimed to address participant health‐related behaviour and did not engage or involve individuals' current health‐care providers directly. The results from these five studies were mixed and do not suggest that patient‐oriented interventions are generally effective. However, there was an indication that a focus on functional capacity and activity participation may possibly be effective (Garvey 2015)Gitlin 2009Gitlin 2006.
1. Clinical outcomes
Two of the five patient‐oriented studies reported clinical outcomes with mixed results. Gitlin 2009Gitlin 2009). Lorig 1999 reported three measures relating to clinical outcomes all of which showed little or no difference between intervention and control. Lynch 2014 reported on glycaemic and blood pressure control in people with diabetes and hypertension. Mean HbA1c was no different but there was an increase in the proportion of intervention participants who achieved at least an absolute reduction in HbA1c of 0.5%. There was no or little difference in systolic blood pressure; (see Table 5). SESs for clinical outcomes in these studies ranged from 0.01 to 0.31 indicating minimal intervention effects.
2. Mental health outcomes
Two studies presented data on mental health outcomes (Garvey 2015 and Lorig 1999). Garvey 2015 reported Hospital Anxiety and Depression Scores (HADS) and found no overall difference in total HADS scores but modest improvements in the depression and anxiety scores. Lorig 1999 reported a mean difference of 0.77 points on a scale of 0 to 5, suggesting no difference in cognitive symptom management between groups at study completion; (see Table 6).
3. Patient‐reported outcome measures
Four studies reported PROMs (Eakin 2007; Garvey 2015; Hochhalter 2010; Lorig 1999). Garvey 2015's primary and secondary outcomes reflected the occupational therapy basis of the intervention. The intervention was associated with improvements in all three reported occupational participation/functional ability‐type measures. Garvey 2015 also found improvements in HRQol and self‐efficacy but no improvements in the Health Education Impact questionnaire overall. The results relating to HRQol and self‐efficacy are included in the related meta‐analyses (Figure 8; Figure 9). Results of this study have to be interpreted with caution as it is reported as an exploratory trial with immediate post‐intervention follow‐up. A definitive RCT is planned (Garvey 2015). Gitlin 2009Garvey 2015 (see Table 7).
4. Utilisation of health services
Two studies reported outcomes on health services utilisation (Garvey 2015 and Lorig 1999). Garvey 2015 found no difference in primary care visits and hospital admissions although only examined an eight week time frame in a small sample. Lorig 1999 reported improvements for intervention group participants across a variety of measures relating to hospital admissions. Lorig 1999 also reported on primary care and emergency department visits but found no improvements (no data available to calculate SESs); (see Table 8).
5. Patient behaviour
5.1 Medication use and adherence
No study with a patient‐oriented intervention reported on medication use and adherence.
5.2 Health related behaviours
Three studies provided data on a variety of outcomes relating to health behaviours by participants (Eakin 2007, Lorig 1999 and Lynch 2014). Eakin 2007 reported improvements in diet behaviour scores and in changes in minutes of walking per week. Lorig 1999 reported three measures relating to exercise and communication with doctors and while there was moderate differences in favour of the intervention groups these were unlikely to be of clinical significance; (see Table 10). Lynch 2014 reported increased exercise measured by caloric expenditure in the intervention group. There were no data presented to calculate SESs.
6. Provider behaviour
Prescribing and other provider behaviours
No study with a patient‐oriented intervention reported on provider behaviour.
7. Acceptability of services
No study with a patient‐oriented intervention reported on acceptability of services.
8. Costs
One of these studies provided data on costs (, Lorig 1999).
Lorig 1999 reported the mean direct cost of running the course for participants who completed it, although costs did not include the cost of accommodation as this was donated to the study. The significant reduction in hospital admissions shown by the intervention translated to a saving in healthcare costs per participant of USD 750 which the authors point out is ten times the cost of the intervention. This calculation was based on a presumed cost of USD 1000 per day if admitted to hospital (see Table 3).
Discussion
Summary of main results
We have identified 17 studies eligible for inclusion in the review, 9 from the original review and 8 added in the current update. All 17 were randomised controlled trials with a generally low risk of bias. Even within this small number of studies, there was significant variation in participants and interventions. In nine of the 17 studies, the focus was on comorbid conditions, which were eligible for inclusion as their interventions had a multimorbidity focus in that they were directed at the pre‐specified comorbid conditions. The commonest combinations of conditions included depression, diabetes and cardiovascular disease. In the other studies, which included people with general multimorbidity, the focus tended to be on older individuals.
The results suggest that interventions that are targeted at specific risk factor management (for example management of vascular risk factors and depression in people with comorbid vascular disease and depression) or focused on areas where people have difficulties, such as with functional ability or medicines management, may possibly be more likely to be effective. Given the importance of developing interventions for people with multimorbidity, the review provides interesting insights into the types of intervention components that are being examined. However, the majority of interventions in included studies had multiple components incorporating different elements, making comparison of intervention effects difficult. We categorised the intervention components using the EPOC taxonomy and identified the predominant intervention element for each study and then grouped studies depending on whether they had a predominantly organisational or patient focus. When examining the effectiveness of interventions by intervention type, we concluded that organisational type interventions, for example, the introduction of clinical nurse specialists to support treatment of depression or a focus on specific risk factor management in commonly co‐occurring conditions such as diabetes and hypertension may be more effective. Interventions that target areas where people have particular difficulties, such as functional ability, are also more likely to be effective. The current evidence suggests that organisational interventions that have a broader focus, such as case management or changes in care delivery for all individuals with multimorbidity, seem less effective. Patient‐oriented interventions that are not linked to healthcare delivery also seem less effective. Garvey 2015
We have presented results by outcomes pre‐specified in the protocol. In general these results were mixed and inconclusive, though there was a tendency for improvements in the studies that targeted common comorbid conditions that included depression. There was not a strong focus on clinical outcomes, particularly for the multimorbidity studies and this may reflect the challenge in research in multimorbidity when disease‐specific measures cannot be used.
There was limited effect on patient‐reported health outcomes such as HRQoL and on outcomes relating to health service utilisation and mixed effects on hospital admission rates and outcomes relating to medication use, and adherence. Five studies reported patient health behaviour outcomes with a tendency for these to be improved in the studies targeting comorbid conditions. There has been ongoing interest in the potential for improved patient self efficacy to lead to better self management and improved health outcomes. Self efficacy represents an outcome that is not disease or condition focused and was examined in many of the included studies. However, the majority of studies including this outcome showed no effect.
Costs were presented in six studies but only two studies conducted cost‐effectiveness analyses and it was not possible to compare outcomes across studies. The results relating to improved prescribing and risk factor management, in some of the comorbidity trials, indicate a potential for these interventions to reduce health service costs over longer periods of time.
Overall completeness and applicability of evidence
Most of the studies in this review are relatively recent reflecting the fact that this is a new area conceptually and that research to date has focused on description and impact rather than the evaluation of the effectives of interventions. The majority of newer studies included in this update and those studies identified as ongoing, focus on common comorbidities rather than on multimorbidity in general. In the original review (Smith 2012), only two of the ten included studies had interventions that focused on comorbid conditions whereas in this updated review, this has increased to nine of the 18 included studies. The tendency towards significant improvements in mental health outcomes in the comorbidity studies is likely related to the strong focus in these interventions on targeting the specific conditions involved, particularly depression. It is more challenging to design interventions for people with a broad range of conditions. The studies that seem more effective in the general multimorbidity group are those which had interventions targeted at specific areas of concern for participants, such as improving functional ability, which is not disease specific. One of the larger multimorbidity studies included involved a large well‐designed and executed RCT, the Guided Care study, which tested a broad organisational‐type intervention targeted at high risk individuals with multimorbidity, but which found no overall effect (Boult 2011). However, a pre‐planned sub‐group analysis indicated improvements in the use of some health services in the participants enrolled in one of the participating care plans (Kaiser‐Permanente, n = 365, 43% of full sample). Boult 2011 postulated that this result may have been related to the fact that care was already more organised and structured in this system, so that the Guided Care intervention may simply have extended the existing approaches used in that setting whereas its implementation was more challenging in less organised systems. However the results of sub‐group analysis, even when pre‐planned, need to be interpreted with caution given the relatively small samples sizes involved. Nonetheless, the differences in these sub‐groups highlight the importance of the healthcare delivery setting into which new interventions are added. Indeed, some of the patient‐oriented interventions seemed to run independently of people's healthcare delivery, particularly those that recruited participants from the community rather than through healthcare providers. Most of these studies had limited effectiveness, highlighting the importance of considering the overall recipient experience and integrating interventions into the healthcare system. The results of the patient‐oriented intervention studies are consistent with the Foster 2007 Cochrane review on lay‐led self‐management support programme, which concluded that there is no evidence that these interventions improve psychological health, symptoms or health‐related quality of life, or that they significantly alter healthcare use.
The evidence from this review partially addresses the review question, i.e. what interventions can effectively improve outcomes in people with multimorbidity. It suggests that interventions such as the addition of clinical care protocols need to be targeted at populations with defined combinations of common conditions such as diabetes and depression or heart disease; or need to focus on specific problems experienced by people in multimorbidity populations, for example a multidisciplinary team intervention that addresses functional difficulties. However, even when interventions are targeted they may not be effective for appropriate use of medications. The Haynes 2008 Cochrane review of Interventions for enhancing medication adherence concludes that "current methods of improving adherence for chronic health problems are mostly complex and not very effective" and suggests further research is needed. People with multimorbidity may have more specific problems with medicines use that relate to polypharmacy and managing complex drug treatment regimens, so medicines management interventions targeting these specific difficulties may be more effective.
Most of the multimorbidity studies in this review focused on older people; however, it is important to address the needs of younger individuals as there are issues relating to employability and absenteeism. Research in Scotland has highlighted that individuals in the poorest socioeconomic groups are more likely to develop multimorbidity at a younger age and more likely to die prematurely as a result (Barnett 2012). Acting upstream for younger people with multimorbidity is preventive and has potential to bring about significant quality of life benefits for individuals as well as cost savings for healthcare systems. However, even in ageing populations, multimorbidity worsens outcomes so there is still likely to be room for improvement, at least in ambulatory care patients.
The evidence to guide intervention development for individuals with multimorbidity is increasing and evolving rapidly. A number of ongoing studies have been identified and we anticipate that future updates of the review will improve the available evidence to inform policy makers and those planning services for individuals with multimorbidity.
Quality of the evidence
All of the included studies were randomised controlled trials. Overall they were of reasonable quality with minimal risk of bias, although blinding of participants and clinicians involved in the types of interventions included in this review is often impossible. Multimorbidity is a complex area because the characteristics of participants can vary depending on definitions used. This limits the potential to reasonably combine study results for meta‐analysis which is reflected in the high heterogeneity in the meta‐analyses undertaken for the review update, and potentially limits the internal validity of the results of the review.
Potential biases in the review process
The review was carried out in accordance with EPOC guidelines and using the updated Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook). Potential limitations in the search process relate to the lack of a MeSH term for multimorbidity. This meant that we had to use broad search terms which led to a high yield of citations to be searched. However, the authors are active researchers in the field of multimorbidity and are unaware of any potentially eligible studies that were missed by the search. We were also unable to retrieve some missing data from authors. However, as limited meta‐analyses were undertaken this did not lead to any appreciable measurement bias.
In addition, it must be noted that when we address complex interventions in primary care, there is always a context in which those interventions take place. A systematic review does not address the context that could have influenced the results in individual studies as there was limited reporting of external validity or generalisability in individual trials. The usual limitations relating to publication bias apply but we have searched the grey literature and contacted experts in the field to try and identify published and ongoing trials in this area.
Agreements and disagreements with other studies or reviews
We were unable to group sufficient numbers of studies with similar interventions in order to comment on which elements of interventions (e.g. the use of community pharmacists) seemed most effective and compare our review to other reviews of these interventions. The most consistent intervention element across all included studies was the use of case managers, but even these varied in that some were clinical case managers and others were administrative managers. The Cochrane review of the effect of case management on health care outcomes is ongoing but does plan to address differences in effectiveness between different types of case management (Zwarenstein 2000). Systematic reviews of community‐based case management in general have indicated mixed effects with improvements in client and professional satisfaction with care and reductions in caregiver strain but no impact on healthcare utilisation (Challis 2014).
Authors' conclusions
Implications for practice.
Multimorbidity is common in clinical practice and is an important problem in most healthcare systems. While the evidence supporting specific intervention types is limited, it does suggest that clinicians and policy makers should prioritise interventions that target specific problems experienced by people with multimorbidity or should target common comorbid conditions. However, we can only be moderately certain that this is the case and new services and interventions should be evaluated robustly to contribute to the much‐needed evidence to support clinical practice. The epidemiological data on the impact of multimorbidity highlights the specific challenges for people who are socioeconomically disadvantaged (Barnett 2012); and interventions targeting this population have the potential to address health inequalities. One of the ongoing studies specifically targets multimorbidity in areas of deprivation (Mercer ongoing).
The sub‐group analysis from the Guided Care study discussed above suggests that multimorbidity interventions need to be integrated into existing healthcare systems to support implementation and sustainability (Boult 2011). Independent interventions that do not integrate with existing healthcare systems will be difficult to sustain. Many of the included studies focused on integration of care between practitioners, but we also need to consider how interventions can be integrated into healthcare systems. It is likely that local adaptations will need to be made even for interventions that are effective. For example, we are confident in the review findings that interventions targeting comorbid depression are effective but these interventions require training and support for primary care clinicians which may not be available in all settings.
The literature on multimorbidity indicates that it is generally associated with poorer outcomes for patients. However, health planners and policy makers need to consider which outcomes they want to target in an intervention. This should be considered in the early stages of the development of a potential new intervention. People with multimorbidity are not only at higher risk of many adverse outcomes, but they are also more likely to experience 'treatment burden', that is that the effort needed to engage in the multiple treatments offered to them actually make their lives more difficult (May 2009). Having the individual participate in priority setting based on his/her values and preferences becomes both the rational and the ethical thing to do.
Implications for research.
Definitions
There is a need for a clear conceptual definition of multimorbidity and its differentiation from other related concepts such as comorbidity, complexity, frailty, and vulnerability. The variation in definitions in the studies included in this review highlight the need for clear reporting of participant characteristics to allow consideration of external validity and generalisability. This will be particularly important given the need to account for the heterogeneity of multimorbidity; interventions could have differential effects depending on the definition or degree of multimorbidity and the socioeconomic status of participants.
Without these definitions and consideration of related concepts, the generalisability or applicability of studies for people with multimorbidity (with a broader definition than only two or three specific diseases) will be uncertain, as is the case for many of the studies in this review, particularly those with the specific comorbidity focus (Fortin 2013).
We would also advocate for including multimorbidity as a MeSH term as the search strategy for this review and for ongoing work on multimorbidity is particularly complex and time consuming, given the growing concern and interest in the issue.
Study design
While the risk of bias was generally low in this review and all studies were RCTs, we acknowledge the challenge of conducting organisational type interventions using optimal RCT designs, so pragmatic trials or quasi‐experimental studies may also be appropriate while still maintaining rigour. This could include the use of stepped wedge cluster RCTs that would involve regional introduction of organisational or health system delivery change while still allowing for robust evaluation.
Future studies need to carefully consider the comparison or control group, particularly in relation to contamination of control participants. Cluster randomised designs are likely to be optimal if interventions are delivered through care providers. This needs to be taken into account both in terms of power calculations and in the analysis of results.
Interventions
This review indicates that interventions that are targeted at either specific combinations of common conditions such as comorbid depression, or at specific problems for people with multiple conditions, may be more effective. When designing interventions researchers should be clear about the theoretical assumptions underlying the intervention, consider its individual components and the evidence base behind each and then link these to outcomes as outlined below. They should also consider interventions that are likely to be reproducible and applicable within the context of primary care. The Medical Research Council Framework for the Design and Evaluation of complex interventions designed to improve health, provides useful guidance in designing and undertaking these trials (MRC Framework 2008).
A group of researchers active in the area of multimorbidity has developed a specific framework for the development of interventions for multimorbidity which is based on a series of workshops undertaken over a two‐year period combined with the experience of this expert group (Smith 2013). This framework highlights the potential for other study designs such as stepped wedge designs that may be more suited to multimorbidity intervention initiatives and that can be undertaken within service/ research partnerships. The framework also stresses the importance of clearly describing all intervention components to allow replicability and generalisability to other settings.
Within this review, inter professional collaboration was embedded in all interventions. This is worth building on for future intervention development. Most of the included studies focused on changing professional care provision; it may also be worthwhile incorporating the participants' perspective. This could be achieved by adopting a participatory approach to intervention development. People with multimorbidity, their family members, and a range of professionals involved should be consulted during the modelling and exploratory phases of service and intervention planning.
The majority of the evidence for effective chronic disease management has been based on a single disease paradigm. However, it is likely that participants in these trials had some degree of multimorbidity, though sicker individuals may have been excluded. This is also the case for trials examining interventions for frail older people or for interventions seeking to improve care transitions as many participants in these studies also have multimorbidity though this is not usually clearly reported or addressed as a potential confounding variable. We should therefore seek to build on and apply the evidence regarding effective interventions for single conditions or related interventions to people with multimorbidity, rather than designing interventions with no consideration of the existing evidence base for single conditions.
In its broadest sense, multimorbidity encompasses a large variety of individuals which must be considered as it is not pragmatic to design interventions that change systems completely. For this reason, parallel economic analyses that link outcomes to costs and benefits are better than providing simple cost data alone, which make comparison across studies difficult.
Outcomes
The challenge with multimorbidity is to define a set of outcomes that can be used for different combination of diseases, so there is a need for generic outcomes measures that incorporate physical functioning, quality of life and measure of treatment burden that are responsive to change over time. Other outcomes to consider include goal attainment, self care, self efficacy, health related quality of life, distress, adherence to treatment, behavioural changes regarding health habits, individuals' knowledge about care plans, shared decision making, and participation in care. However, unless validated measures are used, many of these outcomes will not be comparable across studies. The recent work of PROMIS (PROMIS 2011) provides validated and useful patient‐reported outcomes that will be particularly relevant for those researching interventions to improve outcomes for people with multimorbidity. Work to develop a core outcome set for multimorbidity using methodology recommended by the COMET initiative (http://www.comet‐initiative.org/) is ongoing.
Most of the interventions in this review used a conceptual model, particularly the Chronic Care Model. In general there needs to be clearer reporting of intervention development and outcomes chosen to reflect the theoretical underpinning as to how and why an intervention might work. It would also be helpful if authors clearly identified intervention elements and matched outcomes to these elements in an effort to clarify which components of multifaceted interventions are more effective than others.
Conclusion
This review highlights the relatively limited but growing evidence underpinning interventions to improve outcomes for people with multimorbidity with the focus to date being on comorbid conditions or multimorbidity in older individuals. The results suggest that interventions to date have had mixed effects but have shown a tendency to improve outcomes if organisational interventions can be targeted at risk factors in common comorbidities such as depression or multidisciplinary team interventions focused on specific functional difficulties in people with multimorbidity. Due to the number of studies and their low risk of bias, we can be confident that there is an effect on depression outcomes in the comorbidity studies that included treatment for depression but there are fewer studies supporting the conclusions for targeting functional difficulties in multimorbidity generally and these findings may change as new evidence becomes available. However, further research is needed and future interventions should be developed in ways that allow rigorous evaluations to be performed that will add to the evidence. There is a need for clear and broader definitions of participants, consideration of appropriate outcomes, and further pragmatic studies based in primary care settings.
What's new
| Date | Event | Description |
|---|---|---|
| 30 April 2021 | Amended | An error in the Summary of Findings table for the PROMs outcome was corrected. |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 4, 2012
| Date | Event | Description |
|---|---|---|
| 20 November 2020 | Amended | A study by Gitlin et al. (2009) has now been excluded from the review. Errors identified in the analyses and PRISMA flow‐chart have been corrected. A summary of findings table has been added. |
| 20 November 2020 | New citation required but conclusions have not changed | A study by Gitlin et al. (2009) has now been excluded from the review. |
| 5 September 2017 | Amended | We corrected an error in the analysis relating to Health Related Quality of Life, presented in Figure 8. The SD in the intervention group for Coventry 2015 was corrected, the labels indicating whether the results favoured intervention or control were corrected and the pooled effect was switched off as indicated in the text of the results, based on the high heterogeneity. The corresponding text on the effects of Organisational interventions (see Effects of interventions) on Patient‐reported outcome measures has been updated to reflect this change. |
| 15 January 2016 | New citation required but conclusions have not changed | Conclusions of the review are similar to those previously reported, but the addition of new studies in this update allows us to be more confident that certain interventions seem more effective than others. This review includes 18 studies |
| 28 September 2015 | New search has been performed | New searches performed to 28 September 2015. Eight new studies identified. |
| 18 March 2015 | Amended | New author added (E Wallace) and two original authors withdrew (H Soubhi and C Hudon) |
| 1 May 2013 | Amended | Minor edits, fixed ref for Katon 2010 |
| 24 May 2011 | Amended | Search updated Feb 2011 |
| 12 June 2008 | Amended | Converted to new review format. |
Notes
May 2021: an error in the text of the Summary of Findings table for the PROMs outcome was identified and corrected. Previously, the sentence read 'only 1 of 5 studies with available data on HRQoL'. ' and this should read 'only 1 of 6 studies with available data on HRQoL'.
November 2020: Errors in the review were identified and they have been corrected. The errors related to: i) a study by Gitlin et al. (2009) being included incorrectly and, ii) errors in analysis (some effect sizes were calculated incorrectly).
Acknowledgements
We would like to acknowledge Julia Worswick, Mary Ann O'Brien, Luciana Ballini, Gerd Flodgren, and Alain Mayhew who provided editorial advice and Sasha Shepperd, Merrick Zwarenstein and Jeremy Grimshaw, the contact editors for this review; Paul Miller, Doug Salzwedel and Michelle Fiander, information specialists and Ailbhe Mealy, Trish Doherty and Linda O'Connor who provided administrative support during the drafting of the review.
We would also like to acknowledge the suggestions and comments received during the review process from Elizabeth Bayliss, John Furler, Craig Ramsey, Sophie Hill, Sarah Dennis, Luciana Ballini, and Martin Eccles. We would also like to particularly acknowledge our previous co‐authors Dr Hassan Soubhi and Dr Catherine Hudon who contributed to the first version of this Cochrane review.
As well, the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Effective Practice and Organisation of Care (EPOC) Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
Medline (OVID)
1 Comorbidity/ (70760)
2 (comorbid$ or co‐morbid$).ti,ab. (88707)
3 (multimorbid$ or multi‐morbid$).ti,ab. (1544)
4 (multidisease? or multi‐disease? or (multiple adj (ill$ or disease? or condition? or syndrom$ or disorder?))).ti,ab. (2649)
5 or/1‐4 (139905)
6 Chronic disease/ (223377)
7 (chronic$ adj3 (disease? or ill$ or care or condition? or disorder$ or health$ or medication$ or syndrom$ or symptom$)).ti,ab. (239744)
8 or/6‐7 (414800)
9 5 or 8 (539044)
10 exp diabetes mellitus/ or diabet$.ti,ab. (481769)
11 exp hypertension/ or (hypertens$ or "high blood pressure?").ti,ab. (383340)
12 exp heart diseases/ or (((heart or cardiac or cardiovascular or coronary) adj (disease? or disorder? or failure)) or arrythmia?).ti,ab. (1058106)
13 exp cerebrovascular disorders/ or ((cerebrovascular or vascular or carotoid$ or arter$) adj (disorder? or disease?)).ti,ab. (391125)
14 exp asthma/ or asthma$.ti,ab. (142524)
15 exp pulmonary disease chronic obstructive/ or (copd or (pulmonary adj2 (disease? or disorder?))).ti,ab. (75165)
16 exp hyperlipidemia/ or (hyperlipidem$ or Hypercholesterolemia$ or hypertriglyceridemia$).ti,ab. (77201)
17 exp Thyroid diseases/ or ((thyroid adj (disease? or disorder)) or hyperthyroid$ or hypothyroid$).ti,ab. (133490)
18 exp arthritis rheumatoid/ or rheumatoid arthritis.ti,ab. (117852)
19 exp mental disorders/ or (((mental or anxiety or mood or psychological or sleep) adj (disease? or disorder?)) or ((substance or drug or marijuana or cocaine or Amphetamine) adj2 abuse) or depression or schizophren$ or psychos$ or "substance abuse" or addiction?).ti,ab. (1197324)
20 exp epilepsy/ or (epileps$ or seizure?).ti,ab. (172872)
21 exp hiv infections/ or (HIV or acquired immune$ deficiency syndrome? or (aids adj (associated or related or arteritis))).ti,ab. (315376)
22 exp neoplasms/ or (neoplasm? or cancer?).ti,ab. (2892349)
23 exp kidney diseases/ or (kidney adj (disease? or disorder?)).ti,ab. (427328)
24 exp liver diseases/ or (liver adj (disease? or disorder?)).ti,ab. (459512)
25 exp osteoporosis/ or osteoporosis.ti,ab. (63622)
26 or/10‐25 (7120398)
27 ((coocur$ or co‐ocur$ or coexist$ or co‐exist$ or multipl$) adj3 (disease? or ill$ or care or condition? or disorder$ or health$ or medication$ or symptom$ or syndrom$)).ti,ab. (49345)
28 chronic$.ti,ab,hw. (1019698)
29 27 or 28 (1061841)
30 26 and 29 (617619)
31 exp *education, continuing/ (30518)
32 (education$ adj2 (program$ or intervention? or meeting? or session? or strateg$ or workshop? or visit?)).tw. (45769)
33 (behavio?r$ adj2 intervention?).tw. (8293)
34 *pamphlets/ (1414)
35 (leaflet? or booklet? or poster or posters).tw. (22084)
36 ((written or printed or oral) adj information).tw. (1553)
37 (information$ adj2 campaign).tw. (374)
38 (education$ adj1 (method? or material?)).tw. (4879)
39 *advance directives/ (3056)
40 outreach.tw. (8261)
41 ((opinion or education$ or influential) adj1 leader?).tw. (1038)
42 facilitator?.tw. (13634)
43 academic detailing.tw. (367)
44 consensus conference?.tw. (4268)
45 *guideline adherence/ (9909)
46 practice guideline?.tw. (15069)
47 (guideline? adj2 (introduc$ or issu$ or impact or effect? or disseminat$ or distribut$)).tw. (3304)
48 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 training program$).tw. (601)
49 *reminder systems/ (1357)
50 reminder?.tw. (7383)
51 (recall adj2 system$).tw. (400)
52 (prompter? or prompting).tw. (5108)
53 algorithm?.tw. (141491)
54 *feedback/ or feedback.tw. (89372)
55 chart review$.tw. (24320)
56 ((effect? or impact or records or chart?) adj2 audit).tw. (789)
57 compliance.tw. (82344)
58 marketing.tw. (17569)
59 or/31‐58 (512958)
60 exp *reimbursement mechanisms/ (17061)
61 fee for service.tw. (3598)
62 *capitation fee/ (2001)
63 *"deductibles and coinsurance"/ (634)
64 cost shar$.tw. (1215)
65 (copayment? or co payment?).tw. (1350)
66 (prepay$ or prepaid or prospective payment?).tw. (4140)
67 *hospital charges/ (957)
68 formular?.tw. (2972)
69 fundhold?.tw. (1)
70 *medicaid/ (10050)
71 *medicare/ (17571)
72 blue cross.tw. (1120)
73 or/60‐72 (51790)
74 *nurse clinicians/ (5524)
75 *nurse midwives/ (4677)
76 *nurse practitioners/ (10903)
77 (nurse adj (rehabilitator? or clinician? or practitioner? or midwi$)).tw. (10788)
78 *pharmacists/ (7289)
79 clinical pharmacist?.tw. (1469)
80 paramedic?.tw. (3418)
81 *patient care team/ (21291)
82 exp *patient care planning/ (23599)
83 (team? adj2 (care or treatment or assessment or consultation)).tw. (10376)
84 (integrat$ adj2 (care or service?)).tw. (7706)
85 (care adj2 (coordinat$ or program$ or continuity)).tw. (19568)
86 (case adj1 management).tw. (7865)
87 exp *ambulatory care facilities/ (25328)
88 *ambulatory care/ (15804)
89 or/74‐88 (153339)
90 *home care services/ (19987)
91 *hospices/ (3299)
92 *nursing homes/ (19931)
93 *office visits/ (2217)
94 *house calls/ (1457)
95 *day care/ (2918)
96 *aftercare/ (2761)
97 *community health nursing/ (14848)
98 (chang$ adj1 location?).tw. (381)
99 domiciliary.tw. (2254)
100 (home adj1 treat$).tw. (1406)
101 day surgery.tw. (2030)
102 *medical records/ (15972)
103 *medical records systems, computerized/ (12787)
104 (information adj2 (management or system?)).tw. (26991)
105 *peer review/ (3134)
106 *utilization review/ (2535)
107 exp *health services misuse/ (3679)
108 or/90‐107 (130606)
109 *physician's practice patterns/ (25519)
110 quality assurance.tw. (18829)
111 *process assessment/ [health care] (1489)
112 *program evaluation/ (7465)
113 *length of stay/ (7564)
114 (early adj1 discharg$).tw. (2209)
115 discharge planning.tw. (2210)
116 offset.tw. (19713)
117 triage.tw. (10142)
118 exp *"Referral and Consultation"/ and "consultation"/ (19518)
119 *drug therapy, computer assisted/ (1138)
120 near patient testing.tw. (188)
121 *medical history taking/ (4446)
122 *telephone/ (4226)
123 (physician patient adj (interaction? or relationship?)).tw. (1987)
124 *health maintenance organizations/ (9387)
125 managed care.tw. (16116)
126 (hospital? adj1 merg$).tw. (370)
127 or/109‐126 (146797)
128 ((standard or usual or routine or regular or traditional or conventional or pattern) adj2 care).tw. (40705)
129 (program$ adj2 (reduc$ or increas$ or decreas$ or chang$ or improv$ or modify$ or monitor$ or care)).tw. (41613)
130 (program$ adj1 (health or care or intervention?)).tw. (30335)
131 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 treatment program$).tw. (299)
132 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 care program$).tw. (138)
133 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 screening program$).tw. (531)
134 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 prevent$ program$).tw. (439)
135 (computer$ adj2 (dosage or dosing or diagnosis or therapy or decision?)).tw. (4108)
136 ((introduc$ or impact or effect? or implement$ or computer$) adj2 protocol?).tw. (2897)
137 ((effect or impact or introduc$) adj2 (legislation or regulations or policy)).tw. (1622)
138 or/128‐137 (110128)
139 or/59,73,89,108,127,138 (995154)
140 randomized controlled trial.pt. (388780)
141 controlled clinical trial.pt. (89842)
142 random$.ti,ab. (743745)
143 (control$ adj2 (trial? or study or studies)).ti,ab. (296267)
144 double‐blind method/ or random allocation/ or single‐blind method/ (220817)
145 ((double or single or triple or treble) adj2 blind$).ti,ab. (131548)
146 (quasi‐experiment$ or quasiexperiment$).ti,ab. (6593)
147 interrupt$ time series.ti,ab. (1126)
148 or/140‐147 (1109791)
149 9 and 139 and 148 (7478)
150 30 and 139 and 148 (5488)
151 149 or 150 [FINAL RESULTS] (9386)
152 (2012$ or 2013$ or 2014$).yr,ed,ep,dp. [Date Limits] (3331408)
153 151 and 152 (2759)
Appendix 2. EMBASE Search Strategy
1 Comorbidity/ (128699)
2 (comorbid$ or co‐morbid$).ti,ab. (139103)
3 (multimorbid$ or multi‐morbid$).ti,ab. (2207)
4 (multidisease? or multi‐disease? or (multiple adj (ill$ or disease? or condition? or syndrom$ or disorder?))).ti,ab. (3322)
5 or/1‐4 (203297)
6 Chronic disease/ (153980)
7 (chronic$ adj3 (disease? or ill$ or care or condition? or disorder$ or health$ or medication$ or syndrom$ or symptom$)).ti,ab. (327098)
8 or/6‐7 (428343)
9 5 or 8 (611739)
10 exp diabetes mellitus/ or diabet$.ti,ab. (753978)
11 exp hypertension/ or (hypertens$ or "high blood pressure?").ti,ab. (678777)
12 exp heart disease/ or exp myocardial disease/ or (((heart or cardiac or cardiovascular or coronary) adj (disease? or disorder? or failure)) or arrythmia?).ti,ab. (1607262)
13 cerebrovascular disease/ or carotid artery disease/ or ((cerebrovascular or vascular or carotoid$ or arter$) adj (disorder? or disease?)).ti,ab. (215400)
14 exp asthma/ or asthma$.ti,ab. (224969)
15 Chronic Obstructive Lung Disease/ or (copd or ((pulmonary or lung?) adj2 (disease? or disorder?))).ti,ab. (154404)
16 exp hyperlipidemia/ or exp hypercholesterolemia/ or (hyperlipidem$ or Hypercholesterolemia$ or hypertriglyceridemia$).ti,ab. (129249)
17 exp Thyroid disease/ or ((thyroid adj (disease? or disorder)) or hyperthyroid$ or hypothyroid$).ti,ab. (205528)
18 exp rheumatoid arthritis/ or rheumatoid arthritis.ti,ab. (178070)
19 exp mental disease/ or (((mental or anxiety or mood or psychological or sleep) adj (disease? or disorder?)) or ((substance or drug or marijuana or cocaine or Amphetamine) adj2 abuse) or depression or schizophren$ or psychos$ or "substance abuse" or addiction?).ti,ab. (1851216)
20 exp epilepsy/ or (epileps$ or seizure?).ti,ab. (255624)
21 Human Immunodeficiency Virus/ or (HIV or acquired immune$ deficiency syndrome? or (aids adj (associated or related or arteritis)) or human immunodeficiency).ti,ab. (306076)
22 exp neoplasm/ or (neoplasm? or cancer?).ti,ab. (3892558)
23 exp kidney disease/ or ((kidney? or renal) adj (disease? or disorder? or failure)).ti,ab. (763592)
24 exp liver disease/ or (liver adj (disease? or disorder?)).ti,ab. (762255)
25 exp osteoporosis/ or osteoporosis.ti,ab. (110294)
26 or/10‐25 (9690063)
27 ((coocur$ or co‐ocur$ or coexist$ or co‐exist$ or multipl$) adj3 (disease? or ill$ or care or condition? or disorder$ or health$ or medication$ or symptom$ or syndrom$)).ti,ab. (69381)
28 chronic$.ti,ab,hw. (1368721)
29 27 or 28 (1427673)
30 26 and 29 (901927)
31 exp primary health care/ or exp primary medical care/ (110049)
32 (primary adj2 (care? or medical$ or health$ or clinic$ or practitioner? or doctor?)).ti,ab. (120119)
33 General practitioner/ (68322)
34 (((family or general or generalist? or communit$) adj2 (physician? or doctor? or practitioner? or practice)) or GP).ti,ab. (150993)
35 General Practice/ (71860)
36 exp Community Care/ (99509)
37 (communit$ adj2 (health or healthcare or service? or clinic$ or setting? or centre? or center?)).ti,ab. (58469)
38 or/31‐37 (457608)
39 (education$ adj2 (program$ or intervention? or meeting? or session? or strateg$ or workshop? or visit?)).tw. (58968)
40 (behavio?r$ adj2 intervention?).tw. (10199)
41 (leaflet? or booklet? or poster or posters).tw. (32641)
42 ((written or printed or oral) adj information).tw. (2345)
43 (information$ adj2 campaign).tw. (490)
44 (education$ adj1 (method? or material?)).tw. (7770)
45 outreach.tw. (10348)
46 ((opinion or education$ or influential) adj1 leader?).tw. (1264)
47 facilitator?.tw. (16200)
48 academic detailing.tw. (457)
49 consensus conference?.tw. (5507)
50 practice guideline?.tw. (19137)
51 (guideline? adj2 (introduc$ or issu$ or impact or effect? or disseminat$ or distribut$)).tw. (4969)
52 ((introduc$ or impact or effect? or implement$ or computer$ or compli$) adj2 protocol?).tw. (5088)
53 ((introduc$ or impact or effect? or implement$ or computer$ or compli$) adj2 algorithm?).tw. (6439)
54 clinical pathway?.tw. (2896)
55 critical pathway?.tw. (1515)
56 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 training program$).tw. (766)
57 reminder?.tw. (10142)
58 (recall adj2 system$).tw. (478)
59 (prompter? or prompting).tw. (6829)
60 advance directive?.tw. (3150)
61 *feedback/ or feedback.tw. (107266)
62 chart review$.tw. (39281)
63 ((effect? or impact or records or chart?) adj2 audit).tw. (1119)
64 compliance.tw. (117170)
65 marketing.tw. (23206)
66 ((cost or clinical or medical) adj information).tw. (25105)
67 *medical education/ (100293)
68 *medical audit/ (11760)
69 continuing education/ (27529)
70 postgraduate education/ (12837)
71 or/39‐70 (622952)
72 fee for service.tw. (4267)
73 cost shar$.tw. (1439)
74 (copayment? or co payment?).tw. (1808)
75 (prepay$ or prepaid or prospective payment?).tw. (4856)
76 formular?.tw. (4857)
77 fundhold?.tw. (1)
78 blue cross.tw. (1407)
79 voucher?.tw. (1195)
80 (free adj2 care).tw. (1345)
81 exp *health insurance/ (86446)
82 *health care costs/ (29401)
83 *health care financing/ (3133)
84 *medical fee/ (4231)
85 *prospective payment/ (3776)
86 or/72‐85 (131153)
87 (nurse adj (rehabilitator? or clinician? or practitioner? or midwi$)).tw. (12811)
88 ((nurse or midwi$ or practitioner) adj managed).tw. (568)
89 clinical pharmacist?.tw. (2991)
90 paramedic?.tw. (4598)
91 exp *paramedical personnel/ (196136)
92 *general practitioner/ (16290)
93 *physician/ (49244)
94 (team? adj2 (care or treatment or assessment or consultation)).tw. (14717)
95 (integrat$ adj2 (care or service?)).tw. (9942)
96 (care adj2 (coordinat$ or program$ or continuity)).tw. (25142)
97 (case adj1 management).tw. (9465)
98 *patient care/ (46965)
99 (chang$ adj1 location?).tw. (459)
100 domiciliary.tw. (3320)
101 (home adj1 (treat$ or visit?)).tw. (8141)
102 day surgery.tw. (2960)
103 exp *primary health care/ (42028)
104 *ambulatory surgery/ (5920)
105 *nursing home/ (22544)
106 *day hospital/ (1428)
107 *outpatient care/ (3552)
108 *terminal care/ (14735)
109 *group practice/ (5739)
110 *general practice/ (38840)
111 *rural health care/ (6703)
112 *community mental health center/ (1910)
113 information system/ (32025)
114 *medical record/ (31657)
115 (information adj2 (management or system?)).tw. (33281)
116 *peer review/ (5654)
117 *professional standards review organization/ (1501)
118 exp *clinical practice/ (26676)
119 quality assurance.tw. (25192)
120 exp *health care delivery/ (497480)
121 *health care quality/ (61935)
122 *professional practice/ (18160)
123 (early adj1 discharg$).tw. (3064)
124 discharge planning.tw. (2722)
125 offset.tw. (22234)
126 triage.tw. (13930)
127 near patient testing.tw. (257)
128 *patient referral/ (12390)
129 (physician patient adj (interaction? or relationship?)).tw. (2250)
130 managed care.tw. (18746)
131 *health care organization/ (46743)
132 *health maintenance organization/ (8566)
133 *health care system/ (13067)
134 *health care access/ (5586)
135 (hospital? adj1 merg$).tw. (418)
136 (computer$ adj2 (dosage or dosing or diagnosis therapy or decision?)).tw. (1690)
137 (computer$ adj2 (diagnosis or therapy)).tw. (3200)
138 gatekeep$.tw. (3814)
139 or/87‐138 (1198920)
140 ((standard or usual or routine or regular or traditional or conventional or pattern) adj2 care).tw. (57422)
141 (program$ adj2 (reduc$ or increas$ or decreas$ or chang$ or improv$ or modify$ or monitor$ or care)).tw. (53287)
142 (program$ adj1 (health or care or intervention?)).tw. (37166)
143 ((effect or impact or introduc$) adj2 (legislation or regulations or policy)).tw. (2053)
144 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 treatment program$).tw. (408)
145 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 care program$).tw. (176)
146 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 screening program$).tw. (688)
147 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 prevent$ program$).tw. (486)
148 or/140‐147 (136072)
149 71 or 86 or 139 or 148 (1892565)
150 randomized controlled trial/ or controlled clinical trial/ or clinical trial/ or controlled study/ (4910245)
151 random$.ti,ab. (926713)
152 (control$ adj2 (trial? or study or studies)).ti,ab. (363648)
153 ((double or single or triple or treble) adj2 blind$).ti,ab. (168110)
154 (quasi‐experiment$ or quasiexperiment$).ti,ab. (7604)
155 interrupt$ time series.ti,ab. (1243)
156 or/150‐155 (5433480)
157 9 and 38 and 149 and 156 (4079)
158 9 and 38 and 149 and (intervent$.ti,ab,pt. or evaluat$.ti,hw. or impact$.ti.) (4402)
159 30 and 38 and 149 and 156 (2945)
160 30 and 38 and 149 and (intervent$.ti,ab,pt. or evaluat$.ti,hw. or impact$.ti.) (3015)
161 157 or 159 (4899)
162 (2011$ or 2012$ or 2013$ or 2014$).em,dp,yr. (5117979)
163 161 and 162 (1895)
Appendix 3. CAB Abstracts. Healthstar
Healthstar (OVID)
1 Comorbidity/ (136781)
2 (comorbid$ or co‐morbid$).ti,ab. (157159)
3 (multimorbid$ or multi‐morbid$).ti,ab. (2688)
4 (multidisease? or multi‐disease? or (multiple adj (ill$ or disease? or condition? or syndrom$ or disorder?))).ti,ab. (4246)
5 or/1‐4 (255167)
6 Chronic disease/ (354063)
7 (chronic$ adj3 (disease? or ill$ or care or condition? or disorder$ or health$ or medication$ or syndrom$ or symptom$)).ti,ab. (391706)
8 or/6‐7 (662434)
9 5 or 8 (888824)
10 exp diabetes mellitus/ or diabet$.ti,ab. (734524)
11 exp hypertension/ or (hypertens$ or "high blood pressure?").ti,ab. (593254)
12 exp heart diseases/ or (((heart or cardiac or cardiovascular or coronary) adj (disease? or disorder? or failure)) or arrythmia?).ti,ab. (1783211)
13 exp cerebrovascular disorders/ or ((cerebrovascular or vascular or carotoid$ or arter$) adj (disorder? or disease?)).ti,ab. (654221)
14 exp asthma/ or asthma$.ti,ab. (221525)
15 exp pulmonary disease chronic obstructive/ or (copd or (pulmonary adj2 (disease? or disorder?))).ti,ab. (121690)
16 exp hyperlipidemia/ or (hyperlipidem$ or Hypercholesterolemia$ or hypertriglyceridemia$).ti,ab. (118845)
17 exp Thyroid diseases/ or ((thyroid adj (disease? or disorder)) or hyperthyroid$ or hypothyroid$).ti,ab. (186611)
18 exp arthritis rheumatoid/ or rheumatoid arthritis.ti,ab. (170399)
19 exp mental disorders/ or (((mental or anxiety or mood or psychological or sleep) adj (disease? or disorder?)) or ((substance or drug or marijuana or cocaine or Amphetamine) adj2 abuse) or depression or schizophren$ or psychos$ or "substance abuse" or addiction?).ti,ab. (1987918)
20 exp epilepsy/ or (epileps$ or seizure?).ti,ab. (250120)
21 exp hiv infections/ or (HIV or acquired immune$ deficiency syndrome? or (aids adj (associated or related or arteritis))).ti,ab. (543535)
22 exp neoplasms/ or (neoplasm? or cancer?).ti,ab. (4417173)
23 exp kidney diseases/ or (kidney adj (disease? or disorder?)).ti,ab. (660283)
24 exp liver diseases/ or (liver adj (disease? or disorder?)).ti,ab. (674611)
25 exp osteoporosis/ or osteoporosis.ti,ab. (102526)
26 or/10‐25 (11129474)
27 ((coocur$ or co‐ocur$ or coexist$ or co‐exist$ or multipl$) adj3 (disease? or ill$ or care or condition? or disorder$ or health$ or medication$ or symptom$ or syndrom$)).ti,ab. (79174)
28 chronic$.ti,ab,hw. (1549612)
29 27 or 28 (1616705)
30 26 and 29 (984205)
31 exp Primary Health Care/ or (primary adj2 care).ti,ab. or Physicians, Family/ or (((family or general or generalist? or community) adj2 (physician? or doctor? or practitioner? or practice)) or GP).ti,ab. or Family Practice/ or exp Community Health Services/ or (communit$ adj2 (health or healthcare or service?)).ti,ab. (1375407)
32 (or/9,30) and 31 [Multimorb & PC] (89751)
33 exp *education, continuing/ (57497)
34 (education$ adj2 (program$ or intervention? or meeting? or session? or strateg$ or workshop? or visit?)).tw. (83498)
35 (behavio?r$ adj2 intervention?).tw. (14793)
36 *pamphlets/ (2616)
37 (leaflet? or booklet? or poster or posters).tw. (35076)
38 ((written or printed or oral) adj information).tw. (2893)
39 (information$ adj2 campaign).tw. (670)
40 (education$ adj1 (method? or material?)).tw. (8793)
41 *advance directives/ (5936)
42 outreach.tw. (14955)
43 ((opinion or education$ or influential) adj1 leader?).tw. (1889)
44 facilitator?.tw. (20009)
45 academic detailing.tw. (675)
46 consensus conference?.tw. (7903)
47 *guideline adherence/ (19292)
48 practice guideline?.tw. (27928)
49 (guideline? adj2 (introduc$ or issu$ or impact or effect? or disseminat$ or distribut$)).tw. (6072)
50 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 training program$).tw. (1078)
51 *reminder systems/ (2650)
52 reminder?.tw. (12878)
53 (recall adj2 system$).tw. (702)
54 (prompter? or prompting).tw. (8090)
55 algorithm?.tw. (222548)
56 *feedback/ or feedback.tw. (127818)
57 chart review$.tw. (42420)
58 ((effect? or impact or records or chart?) adj2 audit).tw. (1488)
59 compliance.tw. (141617)
60 marketing.tw. (31868)
61 or/33‐60 (845682)
62 exp *reimbursement mechanisms/ (33927)
63 fee for service.tw. (6823)
64 *capitation fee/ (3985)
65 *"deductibles and coinsurance"/ (1253)
66 cost shar$.tw. (2265)
67 (copayment? or co payment?).tw. (2521)
68 (prepay$ or prepaid or prospective payment?).tw. (8075)
69 *hospital charges/ (1893)
70 formular?.tw. (5328)
71 fundhold?.tw. (2)
72 *medicaid/ (19969)
73 *medicare/ (34885)
74 blue cross.tw. (2138)
75 or/62‐74 (101523)
76 *nurse clinicians/ (10760)
77 *nurse midwives/ (8932)
78 *nurse practitioners/ (21179)
79 (nurse adj (rehabilitator? or clinician? or practitioner? or midwi$)).tw. (20081)
80 *pharmacists/ (13810)
81 clinical pharmacist?.tw. (2662)
82 paramedic?.tw. (6281)
83 *patient care team/ (42259)
84 exp *patient care planning/ (46645)
85 (team? adj2 (care or treatment or assessment or consultation)).tw. (19258)
86 (integrat$ adj2 (care or service?)).tw. (13964)
87 (care adj2 (coordinat$ or program$ or continuity)).tw. (36271)
88 (case adj1 management).tw. (14772)
89 exp *ambulatory care facilities/ (48811)
90 *ambulatory care/ (29666)
91 or/76‐90 (292090)
92 *home care services/ (38726)
93 *hospices/ (6323)
94 *nursing homes/ (38729)
95 *office visits/ (4307)
96 *house calls/ (2784)
97 *day care/ (5461)
98 *aftercare/ (5287)
99 *community health nursing/ (29572)
100 (chang$ adj1 location?).tw. (562)
101 domiciliary.tw. (4012)
102 (home adj1 treat$).tw. (2478)
103 day surgery.tw. (3851)
104 *medical records/ (31654)
105 *medical records systems, computerized/ (25331)
106 (information adj2 (management or system?)).tw. (49115)
107 *peer review/ (6217)
108 *utilization review/ (5041)
109 exp *health services misuse/ (7251)
110 or/92‐109 (250991)
111 *physician's practice patterns/ (50210)
112 quality assurance.tw. (35457)
113 *process assessment/ [health care] (2910)
114 *program evaluation/ (14556)
115 *length of stay/ (14615)
116 (early adj1 discharg$).tw. (4031)
117 discharge planning.tw. (4209)
118 offset.tw. (29706)
119 triage.tw. (18422)
120 exp *"Referral and Consultation"/ and "consultation"/ (37594)
121 *drug therapy, computer assisted/ (2172)
122 near patient testing.tw. (354)
123 *medical history taking/ (8799)
124 *telephone/ (8039)
125 (physician patient adj (interaction? or relationship?)).tw. (3700)
126 *health maintenance organizations/ (18678)
127 managed care.tw. (31562)
128 (hospital? adj1 merg$).tw. (722)
129 or/111‐128 (274522)
130 ((standard or usual or routine or regular or traditional or conventional or pattern) adj2 care).tw. (72645)
131 (program$ adj2 (reduc$ or increas$ or decreas$ or chang$ or improv$ or modify$ or monitor$ or care)).tw. (73494)
132 (program$ adj1 (health or care or intervention?)).tw. (55222)
133 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 treatment program$).tw. (540)
134 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 care program$).tw. (255)
135 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 screening program$).tw. (990)
136 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 prevent$ program$).tw. (810)
137 (computer$ adj2 (dosage or dosing or diagnosis or therapy or decision?)).tw. (7595)
138 ((introduc$ or impact or effect? or implement$ or computer$) adj2 protocol?).tw. (4756)
139 ((effect or impact or introduc$) adj2 (legislation or regulations or policy)).tw. (2959)
140 or/130‐139 (196108)
141 or/61,75,91,110,129,140 (1750807)
142 randomized controlled trial.pt. (751676)
143 controlled clinical trial.pt. (175463)
144 random$.ti,ab. (1251769)
145 (control$ adj2 (trial? or study or studies)).ti,ab. (524250)
146 double‐blind method/ or random allocation/ or single‐blind method/ (386514)
147 ((double or single or triple or treble) adj2 blind$).ti,ab. (246759)
148 (quasi‐experiment$ or quasiexperiment$).ti,ab. (11844)
149 interrupt$ time series.ti,ab. (2028)
150 or/142‐149 (1900410)
151 32 and 150 (12342)
152 9 and 141 and 150 (13829)
153 30 and 141 and 150 (10150)
154 152 or 153 [FINAL RESULTS] (17364)
155 limit 154 to yr="2011 ‐Current" (5406)
156 (2011$ or 2012$ or 2013$ or 2014$).ed,ep,dp. [Date Limits] (6025081)
157 (or/152‐153) and 156 (5893)
158 155 or 157 (5893)
159 exp Primary Health Care/ or (primary adj2 care).ti,ab. or Physicians, Family/ or (((family or general or generalist? or community) adj2 (physician? or doctor? or practitioner? or practice)) or GP).ti,ab. or Family Practice/ or exp Community Health Services/ or (communit$ adj2 (health or healthcare or service?)).ti,ab. (1375407)
160 (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti. (1679885)
161 exp animals/ not humans.sh. (4076816)
162 160 not 161 [Cochrane RCT Filter] (1607573)
163 5 and 159 and 162 [Multimorbidity & PC & Cochrane RCT Filter] (2678)
164 163 not 158 [RCT Multimorbidity all years ML] (2222)
165 remove duplicates from 164 (1103)
166 remove duplicates from 158 (3111)
167 from 165 keep 1‐966 (966)
168 from 166 keep 1‐2435 (2435)
Appendix 4. Cochrane Central Register of Controlled Trials Strategy
#1 MeSH descriptor: [Comorbidity] this term only 2634
#2 (comorbid* or co‐morbid* or multimorbid* or multi‐morbid* or multidisease or multidiseases or multi‐disease or multi‐diseases):ti 992
#3 MeSH descriptor: [Chronic Disease] this term only 11236
#4 #1 or #2 or (#2 and #3) 3340
#5 MeSH descriptor: [Diabetes Mellitus] 1 tree(s) exploded 16930
#6 diabet*:ti,ab 33844
#7 MeSH descriptor: [Hypertension] explode all trees 14236
#8 (hypertens* or "high blood pressure"):ti,ab 30517
#9 MeSH descriptor: [Heart Diseases] explode all trees 38087
#10 MeSH descriptor: [Cerebrovascular Disorders] 1 tree(s) exploded 10092
#11 (cerebrovascular disorder* or cerebrovascular disease* or vascular disorder* or vascular disease* or carotoid* disorder* or carotoid disease* or arter* disorder* or arter* disease*):ti 5257
#12 MeSH descriptor: [Asthma] 1 tree(s) exploded 9189
#13 asthma*:ti 17198
#14 MeSH descriptor: [Pulmonary Disease, Chronic Obstructive] explode all trees 2683
#15 (copd or pulmonary disease* or pulmonary disorder*):ti 7855
#16 MeSH descriptor: [Hyperlipidemias] explode all trees 4608
#17 (hyperlipidem* or Hypercholesterolemia* or hypertriglyceridemia*):ti 2357
#18 MeSH descriptor: [Thyroid Diseases] explode all trees 1689
#19 (thyroid disease* or thyroid disorder*):ti 129
#20 MeSH descriptor: [Mental Disorders] explode all trees 44778
#21 ((mental or anxiety or mood or psychological or sleep) near/2 (disease* or disorder*)):ti 2376
#22 ((substance or drug or marijuana or cocaine or Amphetamine) near/2 abuse):ti 740
#23 (depression or schizophren* or psychos* or "substance abuse" or addiction or addictions):ti 22617
#24 MeSH descriptor: [Epilepsy] explode all trees 2330
#25 (epileps* or seizure or seizures):ti 3195
#26 MeSH descriptor: [HIV Infections] 1 tree(s) exploded 8289
#27 (HIV or acquired immune* deficiency syndrome*):ti 7826
#28 MeSH descriptor: [Neoplasms] explode all trees 54236
#29 (neoplasm or cancer):ti 48221
#30 MeSH descriptor: [Kidney Diseases] 1 tree(s) exploded 10187
#31 (kidney disease* or kidney disorder*):ti 1400
#32 MeSH descriptor: [Liver Diseases] explode all trees 10502
#33 (liver disease* or liver disorder*):ti 1024
#34 MeSH descriptor: [Osteoporosis] explode all trees 3230
#35 osteoporosis:ti 2125
#36 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 302338
#37 ((coocur* or co‐ocur* or coexist* or co‐exist* or multipl*) near/2 (disease or diseases or ill* or care or condition or conditions or disorder* or health* or medication* or symptom* or syndrom*)):ti,ab 1292
#38 #36 and #37 510
#39 #4 or #38 3797
#40 #39 Publication Year from 2011 to 2014 957
Appendix 5. CINHAL search
| Search ID# | Search Terms | Search Options | Actions |
| S70 | S26 or S66 or S67 or S68 or S69 |
Limiters ‐ Published Date: 20110101‐20141031; Exclude MEDLINE records Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (477) |
| S69 | S3 AND S51 AND S64 |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (9,758) |
| S68 | (S24 or S25) AND S51 |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (628) |
| S67 | (S24 or S25) AND S58 |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (167) |
| S66 | S3 and S58 |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (3,313) |
| S65 | S59 or S60 or S61 or S62 or S63 |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (1,253,245) |
| S64 | S59 or S60 or S61 or S62 or S63 |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (1,253,245) |
| S63 | MW care or patient or community |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (1,171,117) |
| S62 | (MH "Community Health Services+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (240,736) |
| S61 | (MH "Primary Health Care") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (30,993) |
| S60 | (MH "Physicians, Family") or TI (family physician? or family doctor?) or AB (family doctor? or family physician?) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (10,844) |
| S59 | (MH "Family Practice") or (family practice) or (general practice) or (family practitioner*) or (general practitioner*) or (family doctor*) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (23,973) |
| S58 | S52 or S53 or S54 or S55 or S56 or S57 |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (139,879) |
| S57 | TI controlled |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (22,469) |
| S56 | TI ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ) or AB ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (1) |
| S55 | TI random* or AB random* |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (118,220) |
| S54 | TI ( “clinical study” or “clinical studies” ) or AB ( “clinical study” or “clinical studies” ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (7,503) |
| S53 | (MM "Clinical Trials+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (8,746) |
| S52 | TI ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) ) or AB ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (9,047) |
| S51 | S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (463,591) |
| S50 | TI ( (time points n3 over) or (time points n3 multiple) or (time points n3 three) or (time points n3 four) or (time points n3 five) or (time points n3 six) or (time points n3 seven) or (time points n3 eight) or (time points n3 nine) or (time points n3 ten) or (time points n3 eleven) or (time points n3 twelve) or (time points n3 month*) or (time points n3 hour*) or (time points n3 day*) or (time points n3 "more than") ) or AB ( (time points n3 over) or (time points n3 multiple) or (time points n3 ... |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (1,824) |
| S49 | TI ( (control w3 area) or (control w3 cohort*) or (control w3 compar*) or (control w3 condition) or (control w3 group*) or (control w3 intervention*) or (control w3 participant*) or (control w3 study) ) or AB ( (control w3 area) or (control w3 cohort*) or (control w3 compar*) or (control w3 condition) or (control w3 group*) or (control w3 intervention*) or (control w3 participant*) or (control w3 study) ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (50,695) |
| S48 | TI ( multicentre or multicenter or multi‐centre or multi‐center ) or AB random* |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (107,535) |
| S47 | TI random* OR controlled |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (37,554) |
| S46 | TI ( trial or (study n3 aim) or "our study" ) or AB ( (study n3 aim) or "our study" ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (94,494) |
| S45 | TI ( pre‐workshop or preworkshop or post‐workshop or postworkshop or (before n3 workshop) or (after n3 workshop) ) or AB ( pre‐workshop or preworkshop or post‐workshop or postworkshop or (before n3 workshop) or (after n3 workshop) ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (354) |
| S44 | TI ( demonstration project OR demonstration projects OR preimplement* or pre‐implement* or post‐implement* or postimplement* ) or AB ( demonstration project OR demonstration projects OR preimplement* or pre‐implement* or post‐implement* or postimplement* ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (1,447) |
| S43 | (intervention n6 clinician*) or (intervention n6 community) or (intervention n6 complex) or (intervention n6 design*) or (intervention n6 doctor*) or (intervention n6 educational) or (intervention n6 family doctor*) or (intervention n6 family physician*) or (intervention n6 family practitioner*) or (intervention n6 financial) or (intervention n6 GP) or (intervention n6 general practice*) Or (intervention n6 hospital*) or (intervention n6 impact*) Or (intervention n6 improv*) or (interven ... |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (46,411) |
| S42 | TI ( collaborativ* or collaboration* or tailored or personalised or personalized ) or AB ( collaborativ* or collaboration* or tailored or personalised or personalized ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (40,174) |
| S41 | TI pilot |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (13,068) |
| S40 | (MH "Pilot Studies") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (32,990) |
| S39 | AB "before‐and‐after" |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (18,791) |
| S38 | AB time series |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (1,950) |
| S37 | TI time series |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (282) |
| S36 | AB ( before* n10 during or before n10 after ) or AU ( before* n10 during or before n10 after ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (35,192) |
| S35 | TI ( (time point*) or (period* n4 interrupted) or (period* n4 multiple) or (period* n4 time) or (period* n4 various) or (period* n4 varying) or (period* n4 week*) or (period* n4 month*) or (period* n4 year*) ) or AB ( (time point*) or (period* n4 interrupted) or (period* n4 multiple) or (period* n4 time) or (period* n4 various) or (period* n4 varying) or (period* n4 week*) or (period* n4 month*) or (period* n4 year*) ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (52,920) |
| S34 | TI ( ( quasi‐experiment* or quasiexperiment* or quasi‐random* or quasirandom* or quasi control* or quasicontrol* or quasi* W3 method* or quasi* W3 study or quasi* W3 studies or quasi* W3 trial or quasi* W3 design* or experimental W3 method* or experimental W3 study or experimental W3 studies or experimental W3 trial or experimental W3 design* ) ) or AB ( ( quasi‐experiment* or quasiexperiment* or quasi‐random* or quasirandom* or quasi control* or quasicontrol* or quasi* W3 method* or quasi* W3 s ... |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (13,277) |
| S33 | TI pre w7 post or AB pre w7 post |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (10,391) |
| S32 | MH "Multiple Time Series" or MH "Time Series" |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (1,476) |
| S31 | TI ( (comparative N2 study) or (comparative N2 studies) or evaluation study or evaluation studies ) or AB ( (comparative N2 study) or (comparative N2 studies) or evaluation study or evaluation studies ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (11,498) |
| S30 | MH Experimental Studies or Community Trials or Community Trials or Pretest‐Posttest Design + or Quasi‐Experimental Studies + Pilot Studies or Policy Studies + Multicenter Studies |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (38,238) |
| S29 | TI ( pre‐test* or pretest* or posttest* or post‐test* ) or AB ( pre‐test* or pretest* or posttest* or "post test* ) OR TI ( preimplement*" or pre‐implement* ) or AB ( pre‐implement* or preimplement* ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (7,482) |
| S28 | TI ( intervention* or multiintervention* or multi‐intervention* or postintervention* or post‐intervention* or preintervention* or pre‐intervention* ) or AB ( intervention* or multiintervention* or multi‐intervention* or postintervention* or post‐intervention* or preintervention* or pre‐intervention* ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (161,816) |
| S27 | (MH "Quasi‐Experimental Studies") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (6,593) |
| S26 | TI ( multimorbid* or multi‐morbid* ) or AB ( multimorbid* or multi‐morbid* ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (314) |
| S25 | s22 and s23 |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (2,157) |
| S24 | S6 and S23 |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (223) |
| S23 | TI ( coocurr* or coexist* or co‐ocurr* or coexist* or co‐exist*) or AB (coocurr* or coexist* or co‐ocurr* or coexist* or co‐exist*) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (3,577) |
| S22 | S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or 21 |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (911,807) |
| S21 | TI diabet* or asthma* or chronic or disease |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (170,650) |
| S20 | MW ( disease OR diseases ) or MW syndrome? or MW chronic |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (355,215) |
| S19 | (MM "Kidney Diseases+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (22,918) |
| S18 | (MM "Osteoporosis+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (6,954) |
| S17 | (MM "Neoplasms+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (157,781) |
| S16 | (MM "Liver Diseases+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (16,641) |
| S15 | (MM "Human Immunodeficiency Virus+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (2,596) |
| S14 | (MH "Mental Disorders, Chronic") OR (MM "Mental Disorders+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (188,092) |
| S13 | (MM "Epilepsy+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (5,260) |
| S12 | (MM "Arthritis+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (22,648) |
| S11 | (MM "Thyroid Diseases+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (4,262) |
| S10 | (MM "Lung Diseases, Obstructive+") OR (MM "Pulmonary Disease, Chronic Obstructive+") OR (MM "Asthma+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (24,299) |
| S9 | (MM "Cardiovascular Diseases+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (205,579) |
| S8 | (MM "Hypertension+") OR (MM "Cerebrovascular Disorders+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (59,999) |
| S7 | (MH "Diabetes Mellitus+") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (72,183) |
| S6 | S4 or S5 |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (46,597) |
| S5 | TI ( chronic* W3 disease? or chronic* W3 ill* or chronic* W3 care or chronic* W3 condition? or chronic* W3 disorder* or chronic* W3 health* or chronic* W3 medication* or chronic* W3 syndrom* or chronic* W3 symptom* ) or AB ( chronic* W3 disease? or chronic* W3 ill* or chronic* W3 care or chronic* W3 condition? or chronic* W3 disorder* or chronic* W3 health* or chronic* W3 medication* or chronic* W3 syndrom* or chronic* W3 symptom* ) |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (23,896) |
| S4 | (MH "Chronic Disease") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (30,061) |
| S3 | S1 or S2 |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (39,568) |
| S2 | TI ( multimorbid* or multi‐morbid* or comorbid* or co‐morbid* or multidisease? or multi‐disease? ) or AB ( multimorbid* or multi‐morbid* or comorbid* or co‐morbid* or multidisease? or multi‐disease? ) or TI (multiple N2 ill* or multiple N2 disease? or multiple N2 condition? or multiple N2 syndrom* or multiple N2 disorder?) or AB (multiple N2 ill* or multiple N2 disease? or multiple N2 condition? or multiple N2 syndrom* or multiple N2 disorder?) or TI ( coocur* N3 disease? or coocur* N3 ill* or ... |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
View Results (22,735) |
| S1 | (MH "Comorbidity") |
Expanders ‐ Apply related words Search modes ‐ Boolean/Phrase |
Appendix 6. EPOC search
EPOC Specialised Register, Reference Manager 12
| Connector | Field | Parameter | Results |
| All Indexed Fields | |||
| OR | All Non‐Indexed Fields |
Appendix 7. AMED (Allied and Complimentary Medicine) (OVID)
1 Comorbidity/ (71202)
2 (comorbid$ or co‐morbid$).ti,ab. (90329)
3 (multimorbid$ or multi‐morbid$).ti,ab. (1577)
4 (multidisease? or multi‐disease? or (multiple adj (ill$ or disease? or condition? or syndrom$ or disorder?))).ti,ab. (2688)
5 or/1‐4 (141817)
6 Chronic disease/ (228383)
7 (chronic$ adj3 (disease? or ill$ or care or condition? or disorder$ or health$ or medication$ or syndrom$ or symptom$)).ti,ab. (246673)
8 or/6‐7 (425175)
9 5 or 8 (550980)
10 exp diabetes mellitus/ or diabet$.ti,ab. (486603)
11 exp hypertension/ or (hypertens$ or "high blood pressure?").ti,ab. (385060)
12 exp heart disease/ or (((heart or cardiac or cardiovascular or coronary) adj (disease? or disorder? or failure)) or arrythmia?).ti,ab. (1063644)
13 exp cerebrovascular disorders/ or ((cerebrovascular or vascular or carotoid$ or arter$) adj (disorder? or disease?)).ti,ab. (399702)
14 exp asthma/ or asthma$.ti,ab. (144387)
15 exp pulmonary disease chronic obstructive/ or (copd or (pulmonary adj2 (disease? or disorder?))).ti,ab. (78374)
16 exp hyperlipidemia/ or (hyperlipidem$ or Hypercholesterolemia$ or hypertriglyceridemia$).ti,ab. (77660)
17 exp Thyroid disease/ or ((thyroid adj (disease? or disorder)) or hyperthyroid$ or hypothyroid$).ti,ab. (133854)
18 exp arthritis rheumatoid/ or rheumatoid arthritis.ti,ab. (120182)
19 exp mental disorders/ or (((mental or anxiety or mood or psychological or sleep) adj (disease? or disorder?)) or ((substance or drug or marijuana or cocaine or Amphetamine) adj2 abuse) or depression or schizophren$ or psychos$ or "substance abuse" or addiction?).ti,ab. (1228206)
20 exp epilepsy/ or (epileps$ or seizure?).ti,ab. (173788)
21 exp hiv infections/ or (HIV or acquired immune$ deficiency syndrome? or (aids adj (associated or related or arteritis))).ti,ab. (317967)
22 exp neoplasms/ or (neoplasm? or cancer?).ti,ab. (2911249)
23 exp kidney disease/ or (kidney adj (disease? or disorder?)).ti,ab. (428395)
24 exp liver disease/ or (liver adj (disease? or disorder?)).ti,ab. (461371)
25 exp osteoporosis/ or osteoporosis.ti,ab. (65004)
26 or/10‐25 (7197068)
27 ((coocur$ or co‐ocur$ or coexist$ or co‐exist$ or multipl$) adj3 (disease? or ill$ or care or condition? or disorder$ or health$ or medication$ or symptom$ or syndrom$)).ti,ab. (50430)
28 chronic$.ti,ab,hw. (1036888)
29 27 or 28 (1079867)
30 26 and 29 (624934)
31 exp Primary Health Care/ or (primary adj2 care).ti,ab. or Physicians, Family/ or (((family or general or generalist? or community) adj2 (physician? or doctor? or practitioner? or practice)) or GP).ti,ab. or Family Practice/ or exp Community Health Services/ or (communit$ adj2 (health or healthcare or service?)).ti,ab. (744825)
32 (or/9,30) and 31 [Multimorb & PC] (48396)
33 exp *education, continuing/ (30556)
34 (education$ adj2 (program$ or intervention? or meeting? or session? or strateg$ or workshop? or visit?)).tw. (47997)
35 (behavio?r$ adj2 intervention?).tw. (8798)
36 *pamphlets/ (1418)
37 (leaflet? or booklet? or poster or posters).tw. (22316)
38 ((written or printed or oral) adj information).tw. (1616)
39 (information$ adj2 campaign).tw. (375)
40 (education$ adj1 (method? or material?)).tw. (5063)
41 *advance directives/ (3060)
42 outreach.tw. (8506)
43 ((opinion or education$ or influential) adj1 leader?).tw. (1064)
44 facilitator?.tw. (14027)
45 academic detailing.tw. (371)
46 consensus conference?.tw. (4351)
47 *guideline adherence/ (9932)
48 practice guideline?.tw. (15621)
49 (guideline? adj2 (introduc$ or issu$ or impact or effect? or disseminat$ or distribut$)).tw. (3402)
50 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 training program$).tw. (797)
51 *reminder systems/ (1364)
52 reminder?.tw. (7519)
53 (recall adj2 system$).tw. (400)
54 (prompter? or prompting).tw. (5216)
55 algorithm?.tw. (142589)
56 *feedback/ or feedback.tw. (91349)
57 chart review$.tw. (24793)
58 ((effect? or impact or records or chart?) adj2 audit).tw. (842)
59 compliance.tw. (84218)
60 marketing.tw. (18043)
61 or/33‐60 (523395)
62 exp *reimbursement mechanisms/ (17092)
63 fee for service.tw. (3651)
64 *capitation fee/ (2001)
65 *"deductibles and coinsurance"/ (635)
66 cost shar$.tw. (1221)
67 (copayment? or co payment?).tw. (1351)
68 (prepay$ or prepaid or prospective payment?).tw. (4227)
69 *hospital charges/ (960)
70 formular?.tw. (2997)
71 fundhold?.tw. (1)
72 *medicaid/ (10071)
73 *medicare/ (17594)
74 blue cross.tw. (1125)
75 or/62‐74 (52023)
76 *nurse clinicians/ (5524)
77 *nurse midwives/ (4679)
78 *nurse practitioners/ (10912)
79 (nurse adj (rehabilitator? or clinician? or practitioner? or midwi$)).tw. (10929)
80 *pharmacists/ (7303)
81 clinical pharmacist?.tw. (1484)
82 paramedic?.tw. (3441)
83 *patient care team/ (21355)
84 exp *patient care planning/ (23648)
85 (team? adj2 (care or treatment or assessment or consultation)).tw. (12673)
86 (integrat$ adj2 (care or service?)).tw. (8332)
87 (care adj2 (coordinat$ or program$ or continuity)).tw. (20929)
88 (case adj1 management).tw. (8243)
89 exp *ambulatory care facilities/ (25365)
90 *ambulatory care/ (15818)
91 or/76‐90 (158006)
92 *home care services/ (20009)
93 *hospices/ (3300)
94 *nursing homes/ (19956)
95 *office visits/ (2222)
96 *house calls/ (1459)
97 *day care/ (2919)
98 *aftercare/ (2761)
99 *community health nursing/ (14860)
100 (chang$ adj1 location?).tw. (398)
101 domiciliary.tw. (2351)
102 (home adj1 treat$).tw. (1496)
103 day surgery.tw. (2080)
104 *medical records/ (15985)
105 *medical records systems, computerized/ (12790)
106 (information adj2 (management or system?)).tw. (27432)
107 *peer review/ (3136)
108 *utilization review/ (2535)
109 health services misuse.hw. (3823)
110 or/92‐109 (131421)
111 *physician's practice patterns/ (25602)
112 quality assurance.tw. (19322)
113 *process assessment/ [health care] (1490)
114 *program evaluation/ (7476)
115 *length of stay/ (7593)
116 (early adj1 discharg$).tw. (2271)
117 discharge planning.tw. (2337)
118 offset.tw. (19965)
119 triage.tw. (10232)
120 exp *"Referral and Consultation"/ and "consultation"/ (19551)
121 *drug therapy, computer assisted/ (1140)
122 near patient testing.tw. (187)
123 *medical history taking/ (4454)
124 *telephone/ (4231)
125 (physician patient adj (interaction? or relationship?)).tw. (2036)
126 *health maintenance organizations/ (9388)
127 managed care.tw. (16605)
128 (hospital? adj1 merg$).tw. (370)
129 or/111‐128 (148510)
130 ((standard or usual or routine or regular or traditional or conventional or pattern) adj2 care).tw. (42136)
131 (program$ adj2 (reduc$ or increas$ or decreas$ or chang$ or improv$ or modify$ or monitor$ or care)).tw. (44374)
132 (program$ adj1 (health or care or intervention?)).tw. (31916)
133 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 treatment program$).tw. (347)
134 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 care program$).tw. (169)
135 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 screening program$).tw. (540)
136 ((effect? or impact or evaluat$ or introduc$ or compar$) adj2 prevent$ program$).tw. (458)
137 (computer$ adj2 (dosage or dosing or diagnosis or therapy or decision?)).tw. (4570)
138 ((introduc$ or impact or effect? or implement$ or computer$) adj2 protocol?).tw. (3051)
139 ((effect or impact or introduc$) adj2 (legislation or regulations or policy)).tw. (1689)
140 or/130‐139 (115558)
141 or/61,75,91,110,129,140 (1015057)
142 randomized controlled trial.pt. (392659)
143 controlled clinical trial.pt. (89968)
144 random$.ti,ab. (758832)
145 double‐blind method/ or random allocation/ or single‐blind method/ (222022)
146 ((double or single or triple or treble) adj2 blind$).ti,ab. (133866)
147 (quasi‐experiment$ or quasiexperiment$).ti,ab. (6980)
148 interrupt$ time series.ti,ab. (1141)
149 (control$ adj2 (trial? or study or studies)).ti,ab. (304877)
150 or/142‐149 (1128989)
151 32 and 150 (6732)
152 9 and 141 and 150 (7802)
153 30 and 141 and 150 (5700)
154 152 or 153 [FINAL RESULTS] (9769)
155 limit 154 to yr="2011 ‐Current" (3258)
156 from 155 keep 1‐81 (81)
Data and analyses
Comparison 1. Glycaemic control (HbA1c) studies targeting diabetes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 HBA1c | 3 | 561 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.47, 0.10] |
1.1. Analysis.

Comparison 1: Glycaemic control (HbA1c) studies targeting diabetes, Outcome 1: HBA1c
Comparison 2. Systolic Blood Pressure: studies targeting hypertension.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Systolic blood pressure | 5 | 892 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐7.26, 1.06] |
2.1. Analysis.

Comparison 2: Systolic Blood Pressure: studies targeting hypertension, Outcome 1: Systolic blood pressure
Comparison 3. PHQ9 depression scores: studies targeting depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 PHQ9 Depression scores | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
3.1. Analysis.

Comparison 3: PHQ9 depression scores: studies targeting depression, Outcome 1: PHQ9 Depression scores
Comparison 4. Depression scores: studies targeting depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Depression scores | 6 | 1062 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.63, ‐0.20] |
4.1. Analysis.

Comparison 4: Depression scores: studies targeting depression, Outcome 1: Depression scores
Comparison 5. Health related quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 HRQoL | 6 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
5.1. Analysis.

Comparison 5: Health related quality of life, Outcome 1: HRQoL
Comparison 6. Self‐Efficacy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Self‐efficacy score | 5 | 3639 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.12, 0.22] |
| 6.1.1 Studies targeting self‐efficacy | 5 | 3639 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.12, 0.22] |
6.1. Analysis.

Comparison 6: Self‐Efficacy, Outcome 1: Self‐efficacy score
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barley 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial (Pilot) UK |
|
| Participants | 81 participants with coronary heart disease (with current chest pain) and depression (identified using two stage screening process to confirm diagnosis), mean age 64, | |
| Interventions | UPBEAT intervention: Nurse case manager who undertook biopsychosocial assessment and developed patient‐held personalised care; 3 problems prioritised with behaviour change approach aiming to increase self‐efficacy. Initial face‐to‐face meeting then weekly telephone calls during intervention period. Weekly team meetings for nurse case manager, GP and psychiatrist |
|
| Outcomes |
Primary: Depression (HADS‐D and PHQ scores) Chest pain (Rose Angina questionnaire) Secondary: Self‐efficacy; IIlness Perceptions (BIPQ); HRQol (SF12); HADS‐A; PSYCHLOPS; Well‐being scores (WEMBWBS); Functional status (Specific Activity Schedule); Moriskey Adherence scale; Social Problems Questionnaire Cost effectiveness |
|
| Notes | Intervention lasted 6 months with follow‐up 6 months post intervention completion Comparison: usual care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permuted block design |
| Allocation concealment (selection bias) | Low risk | Allocation by Clinical Trials Unit |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Outcome assessors and statistician blinded; not possible to blind professionals and participants due to nature of intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 85% follow‐up, balanced |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Protection against contamination | Low risk | Controls had no access to intervention |
| Reliable primary outcomes | Low risk | Validated measures |
| Baseline measurement | Low risk | Groups comparable at baseline |
Bogner 2008.
| Study characteristics | ||
| Methods | Randomised controlled trial USA |
|
| Participants | 64 participants aged 50 years and older with hypertension and depression (defined as a diagnosis of depression or prescription of antidepressant within the past year) Integrated care manager and 12 family physicians in primary care clinic |
|
| Interventions | Integration of depression and hypertension treatment coordinated by integrated care manager; individualised program comprising three 30 minute in‐person sessions with participants and two 15 minute follow‐up phone calls | |
| Outcomes |
Primary and secondary (no distinction specified): Depression scores (Centre for Epidemiological Studies depression scale (CES‐D)) Blood pressure Medication (% adherent to antidepressant medication; % adherent to antihypertensive medication (adherence measured using electronic measuring devices (MEMS caps)) |
|
| Notes | Intervention lasted 6 weeks and follow‐up 2 weeks later Comparison: usual care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper states "patients were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated in text |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Automated measurement devices were used but authors don't specifically state that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up reported |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Protection against contamination | Low risk | 25% control consultations monitored to check for contamination |
| Reliable primary outcomes | Low risk | Validated measures and automated tests |
| Baseline measurement | Low risk | Groups comparable at baseline |
Boult 2011.
| Study characteristics | ||
| Methods | Cluster randomised controlled trial USA |
|
| Participants | 904 participants aged 65 years or more with history of high service use and multiple medical conditions, covered by Medicare or other insurance 8 practices with 49 primary care practitioners (PCPs); 7 Guided Care nurses (GCNs) Arranged in 'pods' of 1 GCN, 2 to 5 PCPs and 50 to 60 participants |
|
| Interventions | 'Guided Care' programme comprising eight clinical services including home‐based assessment, individual management plan, coaching for self‐management with monthly monitoring and coordination of care provision Delivered by trained GCNs | |
| Outcomes |
Primary: Health service use Secondary: PACIC (Patient assessment of chronic illness care) score Health care costs (6 months' data only available) |
|
| Notes | Intervention duration 18 months; follow‐up at 6 and 18 months Controls received usual care with PCPs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Carried out independently by study statistician |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Interviewers blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | > 90% follow‐up, balanced |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Protection against contamination | Low risk | Cluster design |
| Reliable primary outcomes | Low risk | Validated measures |
| Baseline measurement | Low risk | Groups comparable at baseline |
Coventry 2015.
| Study characteristics | ||
| Methods | Cluster randomised controlled trial UK |
|
| Participants | 387 participants with depression and diabetes and/or ischaemic heart disease, mean age 59, 62% female, mean of 6.2 chronic conditions 36 general practice teams |
|
| Interventions | COINCIDE collaborative care model Stepped care protocols with: Brief psychotherapy ‐ up to 8 sessions Standardised treatment manual and workbook with problem statement and personalised goals At visit 2 and visit 8 had 10‐minute joint consultation between participant, psychologist and practice nurse to link depression and chronic condition care with targets Drug review with GP if needed Training half‐day workshop for clinicians with video and simulated patients One hour weekly supervision for Practice nurses from psychologist and monthly case meetings Telephone support from trial psychiatrist |
|
| Outcomes |
Primary: Depression (SCL‐D13 scores) Secondary: Depression (PHQ9 scores) Anxiety (GAD scores) Social support (ENRICHID inventory) HRQol (WHO‐QOL BREF, diabetes QOL) Seattle angina questionnaire Sheehan disability index Self‐efficacy Heath education (HEiQ) Ilness beliefs (multimorbidity illness perceptions scale) Treatment satisfaction (CSQ) Process of care (PACIC scores) |
|
| Notes | Intervention duration 3 months, follow‐up at 4 months (1 month post intervention completion) 22% of intervention participants never engaged with programme, mean 4.4 sessions attended Comparison: Usual care with referral to mental health services but no access to COINCIDE psychologists |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation by Clinical Trials Unit (CTU), using minimisation |
| Allocation concealment (selection bias) | Low risk | Independently conducted by CTU |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not possible to blind clinicians and participants but cluster design. Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All practices retained once participants recruited, 90% follow‐up participants, balanced |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Protection against contamination | Low risk | Cluster design |
| Reliable primary outcomes | Low risk | Validated |
| Baseline measurement | Low risk | Comparable at baseline |
Eakin 2007.
| Study characteristics | ||
| Methods | Randomised controlled trial USA |
|
| Participants | Sub‐group of 175 Urban Latinos with multimorbidity (defined as two or more chronic conditions) (data on sub‐group directly from authors) Bilingual health educator |
|
| Interventions | Diet and physical activity intervention with self‐management support delivered by a health educator; involving two face‐to‐face visits (60 to 90 min) 3 months apart; 3 follow‐up phone calls and 3 newsletters | |
| Outcomes |
Primary: Physical activity (Behavioural Risk Factor Surveillance Survey Physical Activity scores) Dietary behaviour (Kristal Fat and Fiber Behaviour (FFB) questionnaire) Secondary: Chronic Illness Resource Survey (CIRS) |
|
| Notes | Intervention 16 weeks, follow‐up 6 months post intervention Comparison: usual care plus a guide to local services and three newsletters | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated scheme |
| Allocation concealment (selection bias) | Unclear risk | Sequentially numbered envelopes used ‐ unclear if were opaque |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated in text |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 80% follow‐up, balanced |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Protection against contamination | Low risk | Not a cluster design but authors state that providers not involved in intervention delivery and intracluster correlation coefficients low previously in this population |
| Reliable primary outcomes | Low risk | Validated measures used |
| Baseline measurement | Low risk | Groups comparable at baseline |
Garvey 2015.
| Study characteristics | ||
| Methods | RCT (exploratory) | |
| Participants | 50 participants with multimorbidity (at least 2 chronic conditions and 4 repeat medications), median age 66, 64% female, median 4.5 conditions | |
| Interventions | OPTIMAL, a 6‐week occupational therapy‐led self‐management support course, weekly meetings in local health centre Focus on goal setting and prioritisation and input from physiotherapy and pharmacist Peer support through group meetings |
|
| Outcomes |
Primary: Activity participation (Frenchay Activities Index) Secondary: Occupational performance (COPM and NEADL) Self Efficacy (SSE) HRQoL (EQ5D) Mental health (HADS) Heathcare utilisation (PC visits and admissions) Health education (HEiQ) |
|
| Notes | Intervention duration: 6 weeks with 2‐week post intervention follow‐up Comparison: Usual care (waiting list for intervention on study completion) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised sequence |
| Allocation concealment (selection bias) | Low risk | Independently done by statistician |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible due to nature of intervention and outcomes collected by research due to limited resources |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 88% follow‐up, balanced |
| Selective reporting (reporting bias) | Low risk | None reported |
| Other bias | Low risk | None reported |
| Protection against contamination | Low risk | Control participants had no access to the intervention |
| Reliable primary outcomes | Low risk | Validated measures |
| Baseline measurement | Low risk | Groups comparable at baseline |
Hochhalter 2010.
| Study characteristics | ||
| Methods | Randomised controlled trial USA |
|
| Participants | 79 participants aged 65 or older with at least 2 of 7 qualifying chronic illnesses who had received treatment in previous 12 months Primary health care providers in “large Internal Medicine Clinic” in Medical School Teaching Hospital |
|
| Interventions | participant engagement intervention: “Making the most of your healthcare” comprising one 2‐hour workshop and 2 follow‐up phone calls before and after a subsequent routine medical appointment, delivered by ‘coaches’ | |
| Outcomes |
Primary: Patient activation measure (PAM) Secondary: Communication with physicians scale HRQoL (HRQOL‐14); Self‐Efficacy for CDM |
|
| Notes | Intervention ran during first 3 months after baseline data collection; follow‐up at 6 months from baseline Comparison was 'attention control' ‐ workshop on safety in the home Study presented as a feasibility study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported as 'randomly assigned' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "interviews carried out by a research assistant blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 81% follow‐up, balanced |
| Selective reporting (reporting bias) | Low risk | None reported |
| Other bias | Low risk | None reported |
| Protection against contamination | Low risk | Control group had no access to patient‐oriented intervention |
| Reliable primary outcomes | Low risk | Valid measures used |
| Baseline measurement | Low risk | Groups comparable at baseline |
Hogg 2009.
| Study characteristics | ||
| Methods | Randomised controlled trial Canada |
|
| Participants | 241 participants aged 50 years or older with at least 2 chronic conditions and at risk of experiencing adverse health outcomes 8 Family Practitioners, 5 nurses and 11 administrative staff in one family‐health network in rural Ontario |
|
| Interventions | APTCare Intervention: Home‐based multidisciplinary team management with an initial assessment by a nurse practitioner and a medication review by pharmacist and individualised patient care plan |
|
| Outcomes |
Primary: Chronic disease management score (CDM score) based on 12 indicators for 1 of 4 chronic diseases Secondary: Clinical outcomes where applicable: BP and HbA1c Quality of preventive care using 6 preventive indicators from the Canadian Task Force on Preventive Health Care (Quality of preventive care score) HRQoL (SF36 scores) Instrumental activities of daily living (IADL score) Health service use (hospitalisation, ED visits) |
|
| Notes | Intervention duration 15 months, follow‐up at intervention completion. Comparison: usual care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Done centrally through automated telephone system |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear for primary outcome, reported as done for secondary outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 95% follow‐up, balanced |
| Selective reporting (reporting bias) | Low risk | None reported |
| Other bias | Low risk | None reported |
| Protection against contamination | Unclear risk | Potential contamination as not cluster randomised Only intervention participants received intervention but FPs and existing nurses could have modified their behaviour with control participants based on their experience with intervention participants |
| Reliable primary outcomes | Unclear risk | Unclear for primary outcome Required chart review which was carried out by a physician; where the data were not clearly recorded in the chart, a nurse double‐checked and they reached agreement No reporting of assessment of process |
| Baseline measurement | Low risk | Groups comparable at baseline |
Katon 2010.
| Study characteristics | ||
| Methods | Randomised controlled trial USA |
|
| Participants | 214 participants with depression and diabetes and/or coronary heart disease Primary Care Practitioners (PCPs) in 14 primary care clinics and 3 trained medically supervised nurses |
|
| Interventions | TEAMcare intervention integrating a treat‐to‐target programme with structured visits with nurses, individualised care plans and treatment targets, support for self‐care combined with pharmacotherapy, provision of self‐care materials for participants, weekly meetings to discuss case progression between nurses, PCPs, psychiatrist and psychologist, electronic registry used to track participant risk factors and depression scores | |
| Outcomes |
Primary: Composite measure of risk factor control incorporating HbA1c; LDL cholesterol, SBP and scores on the SCL‐20 depression scale Secondary: Depression (SCL‐20 scores) participant global rating of improvement score (i.e. > 50% improvement in SCL‐20 score); medication adjustments; medication adherence Adherence with diet and exercise plans HRQoL Satisfaction with care Economic analysis |
|
| Notes | Intervention duration 12 months; follow‐up data collection at 12 months Comparison: “Enhanced primary care” i.e. usual care plus PCPs informed of depression diagnosis and of results at baseline, 6 and 12 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “randomly assigned by a centrally randomised process” |
| Allocation concealment (selection bias) | Low risk | Carried out centrally |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “research assistants who were unaware of the intervention status implemented study procedures” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12‐month follow‐up > 83% all measures, majority > 90% |
| Selective reporting (reporting bias) | Low risk | None reported |
| Other bias | Low risk | None reported |
| Protection against contamination | Unclear risk | Control group did not have access to study nurses but managed by same group of PCPs as intervention group |
| Reliable primary outcomes | Low risk | Automated measures or validated scales |
| Baseline measurement | Low risk | Comparable groups at baseline |
Kennedy 2013.
| Study characteristics | ||
| Methods | Cluster randomised controlled trial UK Primary outcome data from authors for multimorbidity sub‐group. No secondary outcome data available for multimorbidity sub‐group |
|
| Participants | Data on primary outcomes for sub‐group of 4023 participants with multimorbidity, defined as at least two of Diabetes/COPD/Irritable Bowel Syndrome (IBS). 19% of full trial population (n = 5599) had 4 or more conditions, 50% 65 years or older. | |
| Interventions | WISE intervention: System‐based approach to self‐management support. Practice level: training; provision of resources for staff participants: guidebooks on self‐management; web‐based directory of local services; some IBS specific material |
|
| Outcomes |
Primary: Shared decision making Self‐efficacy HRQol (EQ5D) Secondary: No data available for multimorbidity sub‐group |
|
| Notes | Intervention 12 months, immediate follow‐up at 12 months Sub‐group data on primary outcomes from authors Comparison: usual care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "minimisation algorithm by the trial statistician". |
| Allocation concealment (selection bias) | Low risk | "Research staff recruiting practices are unaware of the next allocation in the sequence at the time of recruitment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Postal questionnaires and "analyst blind to practice allocation to trial arms" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72% follow‐up at 12 months, balanced |
| Selective reporting (reporting bias) | Low risk | None reported |
| Other bias | Low risk | None reported |
| Protection against contamination | Low risk | Cluster design |
| Reliable primary outcomes | Low risk | Validated measures |
| Baseline measurement | Low risk | Comparable at baseline |
Krska 2001.
| Study characteristics | ||
| Methods | Randomised controlled trial UK |
|
| Participants | 332 participants aged over 65 years with at least 2 chronic conditions and taking at least 4 prescribed medications regularly; 6 general practices in Grampian, UK | |
| Interventions | Pharmaceutical care plan drawn up by a pharmacist based on medical records and participant interviews, and then implemented by the practice team | |
| Outcomes |
Primary and secondary (no distinction specified): Pharmaceutical care issues (PCI scale) HRQoL (SF36 scores) Health service utilisation including GP and practice nurse contacts, OPD attendance, use of social services and hospital admissions Economic: direct monthly costs of prescribed medications per participant |
|
| Notes | Study duration and follow‐up 3 months Comparison: controls had review of drug therapy by pharmacist but no pharmaceutical care plan implemented |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Text simply states "patients were randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method not specified |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 87% follow‐up, balanced |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Protection against contamination | High risk | Authors state that contamination of control participants could have occurred but stated that a cluster design would have been more problematic due to differential prescribing patterns between practices |
| Reliable primary outcomes | Unclear risk | PCIs previously used but unclear whether validated as outcome measure |
| Baseline measurement | Low risk | Some baseline imbalance between groups for PCIs and admissions; not clinically significant |
Lorig 1999.
| Study characteristics | ||
| Methods | Randomised controlled trial USA |
|
| Participants | Subgroup of 536 participants over 40 years with at least 2 of the following chronic conditions: heart disease, lung disease, stroke or arthritis; recruited through mass media Volunteer lay leaders ran weekly group meetings |
|
| Interventions | Chronic Disease Self Management Programme: weekly meetings for 7 weeks delivered in community settings by trained volunteer lay leaders with professional training of lay leaders and support throughout the programme | |
| Outcomes |
Primary and secondary (no distinction specified): Self‐rated health scale (from the National Health Interview Survey) Health Assessment Questionnaire (HAQ) Disability scale Psychological well‐being (MHI‐5 well‐being scale) HRQoL: pain and physical discomfort scale (adaptation of the Medical Outcomes Survey (MOS) pain scale); energy and fatigue scale (MOS energy and fatigue scale) health distress scale (modified MOS health distress scale) Health behaviours including duration exercise taken, use of cognitive symptom management, communication with physicians, social and role activity limitations Health service utilisation including physician, emergency department and hospital visits and number nights as hospital inpatient Economic: direct programme costs and savings |
|
| Notes | Study duration and follow‐up 6 months Comparison: waiting list controls |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study reports "randomisation was conducted serially" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated specifically for multimorbidity sub‐group; overall follow‐up 85% |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Protection against contamination | Low risk | Controls on waiting list so no exposure to those delivering community‐based intervention |
| Reliable primary outcomes | Low risk | Validated measures used |
| Baseline measurement | Low risk | Groups comparable at baseline |
Lynch 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial (pilot) USA |
|
| Participants | 61 African American participants with diabetes and hypertension, mean age 54 years, 47% taking insulin therapy, 32% with depression | |
| Interventions | LIFE intervention: Diabetes self‐management groups led by dietician, 18 2‐hour classes, incorporating nutrition education, behaviour skills training, self‐monitoring, goal‐setting and problem‐solving skills Social support provided by weekly telephone calls from a peer supporter |
|
| Outcomes |
Primary: % achieving 5% weight loss at 6 months Secondary: HbA1c; % achieving 5% reduction in HbA1c; BP; Diabetes self care activities (SDSCA scores) |
|
| Notes | Intervention duration 6 months with immediate follow‐up Comparison: usual care with two 3‐hour education sessions led by a community health worker |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "we used a block randomisation scheme supervised by the study statistician" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. Outcome data collected by 'study staff' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90% follow‐up, balanced |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Protection against contamination | Low risk | Controls had no access to group‐based sessions or peer support |
| Reliable primary outcomes | Low risk | Clinical outcomes measured using standard clinic protocols |
| Baseline measurement | Low risk | Groups balanced at baseline |
Martin 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial Australia |
|
| Participants | 66 people with depression and headache (migraine (66%); and tension‐type headache (33%)), mean age 41, 74% female Community clinical psychologists |
|
| Interventions | Cognitive behavioural therapy programme for both depression and headache Twelve 50‐minute weekly sessions incorporating pain‐ and lifestyle‐management training Training for community psychologists Treatment manual (44 pages) Client handbook and relaxation CD |
|
| Outcomes |
Primary: Depression (BDI and PHQ9 scores) Medication consumption Secondary: Anxiety (BDA scores) HRQoL (AQOL) |
|
| Notes | Intervention 12 weeks with immediate follow‐up. Additional follow‐up at 4 months for intervention group only so data not used Comparison: usual care and GPs asked not to refer to psychology but could use other mental health services |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Stratified randomisation procedure' |
| Allocation concealment (selection bias) | Low risk | Done by independent researcher |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only analysed those who completed the programme and excluded those who dropped out early |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Protection against contamination | Unclear risk | Unlikely as control participants had no access to programme but were treated by same GPs |
| Reliable primary outcomes | Low risk | Validated scores |
| Baseline measurement | Low risk | Done |
Morgan 2013.
| Study characteristics | ||
| Methods | Cluster randomised controlled trial Australia |
|
| Participants | 400 people with depression and diabetes and/or ischaemic heart disease, mean age 68, 40% IHD, 44% diabetes and 17% both, mean HbA1c at baseline 7.1%, mean SBP at baseline 134 mmHg 11 general practices |
|
| Interventions | TrueBlue collaborative care model Practice nurse case manager Reviews: 3 monthly 45‐minute reviews with practice nurse covering lifestyle risk factors, review of results and support for self‐management and goal setting; followed by 15‐minute review with GP who stepped up treatment if needed Indivudal care plans, copy held by participant Educational resources and fact sheets Practice nurse training, 2‐day workshop |
|
| Outcomes |
Primary: Depression (PHQ9 scores) Secondary: Clinical outcomes: HbA1c, SBP, Cholesterol, BMI, 10‐year CVD risk HRQoL: SF36 scores Medication: on anti‐depressant medication participant behaviours: smoking, alcohol, exercise (30 min/day on 5 days/week, attending exercise programme, attending mental health programme Provider behaviour: referrals to mental health and to exercise programme |
|
| Notes | Intervention duration 6 months with immediate follow‐up and additional follow‐up at 12 months for intervention group only (12 month data not included) Comparison: usual care and offered intervention after 6 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Random number generation' but no report on who undertook it |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not possible to blind participants and clinicians due to nature of intervention. Outcome assessors: data collected from care plans |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 72% follow‐up, balanced |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Protection against contamination | Low risk | Cluster design |
| Reliable primary outcomes | Low risk | Validated measures used |
| Baseline measurement | Low risk | Validated measures, balanced |
Sommers 2000.
| Study characteristics | ||
| Methods | Cluster randomised controlled trial USA |
|
| Participants | 543 participants older than 65 years with at least 2 chronic conditions and living independently, attending 18 private office practices of primary care physicians | |
| Interventions | Senior Care Connections (SCC) intervention delivered by a team including the primary care physician, a nurse with geriatric medicine training and a social worker Home visit assessment followed by team discussion and development of a risk reduction plan and treatment targets | |
| Outcomes |
Primary and secondary (no distinction specified): Physical functioning (Health activities questionnaire (HAQ)) Emotional functioning (short form geriatric depression scale (GDS)) HRQoL (SF36 scores) Social activities count Symptom scale Medication count Nutrition checklist Health service utilisation including office, emergency room and home care visits, hospital admissions, skilled nursing facility admissions, length of hospital stay and nursing home placements Economic: direct costs of the intervention |
|
| Notes | Study duration 2 years, 12‐month follow‐up post completion intervention Comparison: usual care from their primary care physician |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study reports "physicians randomised" |
| Allocation concealment (selection bias) | Unclear risk | Unclear at cluster level but no bias at participant level as recruited through clusters |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Healthcare utilisation measured from automated data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 86% follow‐up for service use measures; 74% follow‐up questionnaire data, balanced |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Protection against contamination | Low risk | Cluster randomisation |
| Reliable primary outcomes | Low risk | Automated data used and validated measures used |
| Baseline measurement | Low risk | Groups comparable at baseline |
Wakefield 2012.
| Study characteristics | ||
| Methods | Randomised controlled trial USA |
|
| Participants | 302 adults with diabetes and hypertension, mean age 68, 96% male (VA system setting), 95% white, baseline mean HbA1c 7.2%, baseline mean SBP 136 mmHg | |
| Interventions | Intervention 1: home telehealth with nurse case manager using high intensity treatment algorithms Intervention 2: home telehealth with nurse case manager using low intensity treatment algorithms Comparison: usual care in primary care clinic with access to PCP, endocrinologist, diabetes education and nurse manager (different to study nurse case manager) |
|
| Outcomes |
Primary: HbA1c and blood pressure Secondary: Medication adherence Knowledge scores Self‐efficacy participant perception of the intervention |
|
| Notes | Intervention duration 6 months, follow‐up 6 months post intervention completion Comparison: usual care in primary care clinic with access to PCP, endocrinologist, diabetes education and nurse manager (different to study nurse case manager) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study nurse used 'sequentially numbered envelopes' |
| Allocation concealment (selection bias) | Unclear risk | Study nurse used opaque envelopes, prepared in advance by project director |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported but primary outcomes automated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 81% follow‐up, balanced |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Protection against contamination | Unclear risk | Unlikely as controls had no access to intervention but treated in same centres |
| Reliable primary outcomes | Low risk | Automated |
| Baseline measurement | Low risk | Balanced at baseline |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Addolorato 2004 | Specialist setting |
| Agarwal 2015 | Participants not defined as having multimorbidity as per review protocol |
| Balaban 2014 | Participants not defined as having multimorbidity as per review protocol |
| Beck 1997 | Participants not defined as having multimorbidity as per review protocol |
| Beretta 2014 | Intervention directed at one condition only (epilepsy) |
| Bove 2015 | Participants not defined as having multimorbidity as per review protocol |
| Brand 2004 | Specialist setting |
| Chow 2014 | Specialist in‐patient setting for first stage of intervention delivery |
| Coburn 2012 | Participants not defined as having multimorbidity as per review protocol |
| Dorr 2008 | No appropriate data for sub‐group with multimorbidity |
| Dougados 2015 | Specialist setting |
| Drake 1998 | Specialist setting |
| Dwinger 2013 | Participants not defined as having multimorbidity as per review protocol |
| Eklund 2013 | Participants not defined as having multimorbidity as per review protocol |
| Essock 2006 | Specialist setting |
| Fischer 2015 | Specialist setting |
| Freund 2011 | Participants not defined as having multimorbidity as per review protocol |
| Ganz 2010 | Participants not defined as having multimorbidity as per review protocol |
| Gitlin 2009 | Inclusion criteria for participants did not specify that they had multimorbidity |
| Harpole 2005 | Intervention not based on multimorbidity: the study presents an analysis of whether co‐morbidity alters response to a depression intervention |
| Hermanns 2015 | Intervention directed at one condition only |
| Hien 2004 | Specialist setting |
| Hinrichs 2013 | Not multimorbidity |
| Hutchings 2013 | Not multimorbidity |
| Katon 2004 | Intervention directed at one condition only (depression) |
| Leveille 1998 | Participants not defined as having multimorbidity as per review protocol |
| Lin 2003 | Intervention directed at one condition only |
| Liss 2013 | Participants not defined as having multimorbidity as per review protocol. |
| Lyles 2003 | Participants had medically unexplained symptoms, not multimorbidity. |
| Martinez 2013 | Participants not defined as having multimorbidity as per review protocol |
| McCall 2011 | Participants not defined as having multimorbidity as per review protocol |
| McCusker 2015 | Participants not defined as having multimorbidity as per review protocol |
| Meeuwissen 2011 | Intervention directed at one condition only |
| Morey 2006 | Participants defined as having a range of chronic conditions (from 0‐15) with no sub‐group eligible for inclusion in this review. |
| Morris 2012 | Intervention directed at one condition only |
| Park 2014 | Study setting ‐ residential care |
| Petersen 2014 | Intervention directed at one condition only |
| Plant 2013 | Study setting: inpatients |
| Reuben 2012 | Participants not defined as having multimorbidity as per review protocol |
| Rodriguez‐Pascual 2013 | Intervention directed at one condition (heart failure). |
| Rosenman 2006 | No multimorbidity sub‐group data |
| Ruikes 2012 | Participants not defined as having multimorbidity as per review protocol |
| Schraeder 2005 | No multimorbidity subgroup |
| Sharpe 2012 | Intervention directed at one condition only (protocol) |
| Shaw 2014 | Participants not defined as having multimorbidity as per review protocol |
| Srinivasan 2014 | Specialist setting |
| Takahashi 2012 | Participants not defined as having multimorbidity as per review protocol. |
| Taveira 2011 | Intervention directed at one condition only |
| van der Weegen 2013 | Participants not defined as having multimorbidity as per review protocol |
| van Mourik 2012 | Intervention around detection and screening |
| Venter 2012 | Participants not defined as having multimorbidity as per review protocol, had either CCF or COPD. |
| Via‐Sosa 2013 | Participants not defined as having multimorbidity as per review protocol |
| Von Korff 2012 | Participants not defined as having multimorbidity as per review protocol. |
| Weber 2012 | Setting: specialist multidisciplinary clinics with no primary care involvement. An alternative model of specialist care. |
| Wilhelmson 2011 | Participants not defined as having multimorbidity as per review protocol |
| Willard‐Grace 2013 | Participants not defined as having multimorbidity as per review protocol |
| Williams 2012 | Participants not defined as having multimorbidity as per review protocol |
| Williams 2013 | Participants not defined as having multimorbidity as per review protocol. |
| Wrede 2013 | Participants not defined as having multimorbidity as per review protocol. |
| Wu 2013 | Specialist setting |
Characteristics of studies awaiting classification [ordered by study ID]
Alexopoulos 2014.
| Methods | RCT |
| Participants | 138 participants with COPD and Depression |
| Interventions | A personalised intervention for depressed participants with COPD (PID‐C) aimed to mobilise participants to participate in the care of both conditions |
| Outcomes | Primary outcome measures were the 17‐item Hamilton Depression Rating Scale and the Pulmonary Functional Status and Dyspnea Questionnaire‐Modified. Other measures were adherence to rehabilitation exercise (> 2 hours per week) and adherence to adequate antidepressant prescriptions. |
| Notes |
Buhrman 2015.
| Methods | RCT |
| Participants | 52 participants with chronic pain and comorbid depression and anxiety |
| Interventions | An individualized cognitive‐behavioural treatment delivered through the Internet |
| Outcomes | Depressive symptoms and pain disability |
| Notes |
Ekdahl 2014.
| Methods | RCT |
| Participants | 383 participants > 75 years, hospitalised at least 3 times during the past 12 months, with at least 3 conditions |
| Interventions | Comprehensive Geriatric Assessment |
| Outcomes | Hospitalisations, mortality, health‐related quality of life (HRQoL) and costs of care |
| Notes |
Katz 2015.
| Methods | RCT |
| Participants | 40 participants with Diabetes and Hypertension |
| Interventions | mHealth group with smartphone and the WellDoc™ Diabetes Manager application providing real time feedback on glucose and blood pressure (BP) entries. Case managers viewed glucose and BP via a web portal. A monthly report was entered into EMR |
| Outcomes | Primary outcome was change in the Patient Activation Measure (PAM) at 6 months. Secondary outcomes included A1c, BP, HEDIS measures, hospitalisations, and ER visits. |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12609000726257.
| Study name | Reed 2011 |
| Methods | RCT |
| Participants | n = 252 aged 60 years or older with two or more chronic conditions |
| Interventions | Flinders programme which is a chronic disease self‐management support programme. Clinician‐led generic self‐management intervention. Usual care group to receive health information only. |
| Outcomes | Primary outcome: self‐rated health, multiple secondary outcomes including health status measures, health behaviours and healthcare utilisation. |
| Starting date | Not recorded, protocol published in 2011 |
| Contact information | Richard.Reed@flinders.edu.au |
| Notes | See usual care to receive health information |
Crowley ongoing.
| Study name | Tailored case management for diabetes and hypertension (TEAM‐DM) |
| Methods | Multicentre RCT involving 9 community practices |
| Participants | n = 377, adults (aged over 21 years), enrolled in a participating clinic for at least 1 year, have type 2 diabetes mellitus requiring medication, have hypertension requiring medication and poor diabetes control (most recent HbA1C in past year over 7.5% |
| Interventions | Telephone‐delivered behavioural intervention. Targets three areas: 1) cultivation of healthy behaviours for diabetes and hypertension; 2) provision of fundamentals to support attainment of healthy behaviours; and 3) identification and correction of patient‐specific barriers to adopting healthy behaviours |
| Outcomes |
Primary: HbA1C and blood pressure measured at 6, 12 and 24 months Secondary: Self‐efficacy, self‐reported medication adherence, exercise and cost‐effectiveness |
| Starting date | Recruitment began on 11 June 2009 and concluded 27 July 2011 |
| Contact information | matthew.crowley@dm.duke.edu |
| Notes |
ISRCTN24874457.
| Study name | Web‐based cognitive behavioural therapy (W‐CBT) for people with diabetes and co‐morbid depression |
| Methods | RCT |
| Participants | N = 286 |
| Interventions | 8‐week, moderated self‐help course tailored to the needs of persons living with diabetes and offered on an individual basis. Participants receive feedback on their homework assignments by email from their coach |
| Outcomes |
Primary: depressive symptoms and diabetes‐specific emotional distress Secondary: satisfaction with the course, perceived health status, self‐care behaviours, glycaemic control, and days in bed/absence from work |
| Starting date | Protocol published in 2008 |
| Contact information | k.vanbastelaar@vumc.nl |
| Notes | Additional publication in 2011; van Bastellar et al Patient Education and Counselling 2011;84(1):49‐55. Further details on intervention development and recruitment |
ISRCTN 83908315.
| Study name | Practice network‐based care management for people with type 2 diabetes and multiple comorbidities (GEDIMAplus) |
| Methods | Multicentre RCT involving 30 study centres |
| Participants | n = 582 adults with type 2 diabetes mellitus and enrolled in the DMP Diabetes programme and at least 2 severe chronic conditions and 1 informal caregiver per participant |
| Interventions | Three main elements: 1) 3 home visits including structured assessment of medical and social needs; 2) 24 structured telephone monitoring contacts; and 3) self‐monitoring of blood glucose levels at 3‐monthly intervals. Delivered by trained healthcare assistants as an add‐on to usual care. |
| Outcomes |
Primary: between‐group differences in changes of diabetes‐related self care behaviours using the revised Summary of Diabetes Self‐Care Activities (SDSCA‐G). Secondary: between‐group differences in the SDSCA‐G subscales, glycosylated haemoglobin A level, health‐related quality of life, self‐efficacy, differences in severe symptomatic hypoglycaemia, cost‐effectiveness, and financial family burden |
| Starting date | participant recruitment started on 1 February 2014 |
| Contact information | kayvan.bozorgmehr@med.uni‐heidelberg.de |
| Notes |
Lassere 2015.
| Study name | Improving quality of care and long‐term health outcomes through continuity of care with the use of an electronic or paper patient‐held portable health file (COMMUNICATE): |
| Methods | RCT |
| Participants | 792 participants aged 60 years or older living independently in the community, but who have 2 or more chronic medical conditions that require prescription medication and regular care by at least 3 medical practitioners (general and specialist care) |
| Interventions | An electronic and paper patient‐held Portable Health File (PHF) |
| Outcomes | The primary outcome is a combined endpoint of deaths, overnight hospitalizations and blindly adjudicated serious out‐of‐hospital events. |
| Starting date | March 2010 with recruitment due to complete in September 2015 |
| Contact information | Marissa N Lassere M.Lassere@unsw.edu.au |
| Notes |
Mercer ongoing.
| Study name | Care Plus Study |
| Methods | Exploratory/Pilot RCT |
| Participants | Multimorbidity and socioeconomic deprivation |
| Interventions | System level organisational type intervention |
| Outcomes | |
| Starting date | 2012 |
| Contact information | Prof Stewart Mercer, University of Glasgow, Scotland |
| Notes |
NCT01328639.
| Study name | Trial Registration number NCT01572389 |
| Methods | Controlled on‐off time series (monthly basis) |
| Participants | Aged 18 years or older, diagnosis of type 2 diabetes and under the care of a primary care network family physician, score ≥ 10 on the Patient Health Questionnaire‐9, speak English and have adequate hearing to complete telephone interviews, aim to recruit n = 168 participants |
| Interventions | Nurse care manager guides patient‐centred care with family physicians and consultant physicians to monitor progress and develop tailored plans. Three phases: 1) Improving depressive symptoms; 2) improving blood glucose, blood pressure and cholesterol; and 3) improving lifestyle behaviours |
| Outcomes |
Primary: change in depressive symptoms and a multivariable, scaled marginal model for the combined outcome of global disease control (i.e. haemoglobin A1C, systolic blood pressure, LDL cholesterol) Secondary; healthcare utilisation, costs |
| Starting date | Not recorded, protocol published August 2012 |
| Contact information | jeff.johnson@ualberta.ca |
| Notes |
NCT01572389.
| Study name | Healthy outcomes through patient empowerment (HOPE) |
| Methods | RCT |
| Participants | n = 242 veterans with Patient Health Questionnaire (PHQ)‐9 score > 10 and haemoglobin A1C > 7.5% |
| Interventions | Blended diabetes/depression behavioural health coaching for 6 months (active intervention), followed by 6 months without coaching (maintenance period) vs enhanced usual care (provision of educational materials) |
| Outcomes |
Primary: PHQ‐9 score and haemoglobin A1C values at 6‐ and 12‐month follow‐up Secondary: 1) Problem area in diabetes questionnaire to assess diabetes‐related distress; 2) Penn State worry questionnaire to assess changes in worry/anxiety; 3) Goal‐setting evaluation tool for diabetes |
| Starting date | Not recorded, protocol published March 2014 |
| Contact information | jcully@bcm.edu |
| Notes |
NCT01719991.
| Study name | Trial Registration number NCT01719991 |
| Methods | Controlled before‐after study; randomised participants, delayed intervention for control group, before and after intervention analysis |
| Participants | n = 400, 25 participants per nurse case manager group, 8 nurse case managers |
| Interventions | First component is nurse‐led case management including 4 elements: 1) evaluation of participant needs and resources; 2) establishment and maintenance of a patient‐centred individualised service plan; 3) co‐ordination of services among partners; and 4) self‐management support for participants and families. Second component includes group meetings (10 to 12 participants) for self‐management support in accordance with the Stanford programme. |
| Outcomes | Personal self‐efficacy, self‐management practices, health habits, patient activation, psychological distress, patient satisfaction, patient empowerment, quality of life, health services utilisation, health professional satisfaction, services integration, long‐term morbidity, and mortality |
| Starting date | Not recorded, protocol published in February 2013 |
| Contact information | mcchouin@uqca.ca |
| Notes |
NTR1847.
| Study name | The effectiveness of case management for people with comorbid diabetes type 2; the CasCo study |
| Methods | RCT |
| Participants | People with type 2 diabetes mellitus who participate in the diabetes care system and have at least one additional chronic condition from a condition list. Aim to recruit 230 participants. |
| Interventions | Case‐management programme in addition to diabetes‐management programme. Based on Guided Care Model with six elements. |
| Outcomes |
Primary: quality of care perceived by participants Secondary: quality of care perceived by GP, health status of the participant, diabetes control, and healthcare utilisation |
| Starting date | Recruitment started February 2011 |
| Contact information | n.versnel@nivel.nl |
| Notes | Usual care is the primary care‐based diabetes management programme |
NTR2626.
| Study name | Trial Registration number NTR2626 |
| Methods | Multicentre RCT |
| Participants | Aged 18 years or older enrolled in a disease‐management programme for asthma and/or COPD and elevated score on depression/anxiety screening instruments. Aim to screen n = 1142 for participation in this trial. |
| Interventions | Stepped care disease management programme for comorbid anxiety and depression. |
| Outcomes |
Primary: Depression score on PHQ‐9 Anxiety scores on Generalized Anxiety disorder‐7 scale Mini international neuropsychiatric interview Quality of life measures (clinical COPD questionnaire, asthma control questionnaire, health survey (SF‐12)) |
| Starting date | Not recorded, protocol published in January 2012 |
| Contact information | F.Pouwer@uvt.nl |
| Notes |
NTR3715.
| Study name | |
| Methods | Cluster RCT |
| Participants | Aged 18 years or older, treated for type 2 diabetes mellitus and/or coronary heart disease in primary care and have subthreshold depressive symptoms (score ≥ 6 on PHQ‐9) without fulfilling the criteria for major depression. Aim to include n = 236 |
| Interventions | Nurse‐led stepped care intervention with four components: 1) watchful waiting; 2) guided self‐help treatment; 3) problem solving treatment; and 4) referral to the GP. |
| Outcomes |
Primary: cumulative incidence of major depressive disorder as measured by the mini international neuropsychiatric interview. Secondary: include severity of depressive symptoms, quality of life, anxiety, and clinical outcomes. |
| Starting date | Not recorded, state first results expected early 2015 |
| Contact information | s.e.m.van.dijk@vu.nl |
| Notes |
Pr1MaC ongoing.
| Study name | Pr1MaC |
| Methods | RCT (sub‐group) |
| Participants | 270 participants with combinations of diabetes, COPD, Astma, CVD and other risk factors such as obesity |
| Interventions | Integration of chronic disease management and prevention |
| Outcomes | SF12, HeiQ, nutrition and physical activity |
| Starting date | 2011 |
| Contact information | Prof Martin Fortin, University of Sherbrooke, Canada |
| Notes |
Salisbury ongoing.
| Study name | 3D Study |
| Methods | Cluster RCT |
| Participants | Multimorbidity |
| Interventions | Organisational‐type general practice‐based intervention |
| Outcomes | HRQoL, treatment burden, self‐efficacy, healthcare utilisation |
| Starting date | 2014 |
| Contact information | Prof Chris Salisbury, University of Bristol, UK |
| Notes |
Schneider ongoing.
| Study name | |
| Methods | pilot RCT |
| Participants | Women with type 2 diabetes mellitus with the following additional criteria: 1) inadequately controlled type 2 diabetes as defined by HbA1C ≥ 7 and ≤ 10; 2) meeting criteria for major depressive disorder as defined by the structured clinical interview for DSM‐IV disorders; 3) not physically active, defined as engaging in moderate intensity exercise less than 3 times per week for 20 min; 4) BMI 18.5 to 45 kg/m²; and 5) aged 21 to 65 years |
| Interventions | Exercise intervention involving 38 behavioural activation‐enhanced group exercise classes over 24 weeks in addition to usual care. Usual care receive depression treatment referrals and print information on diabetes management via diet and physical activity. |
| Outcomes | HbA1C, Depression scores on Beck's depression inventory, fitness measure, BMI, waist circumference, blood pressure, self efficacy for exercise, quality of life and several process measures |
| Starting date | Not recorded, protocol published in June 2011 |
| Contact information | Kirstin.Schneider@umassmed.edu |
| Notes | Pilot RCT, usual care group receive depression referral treatment and written information re diabetes management |
Differences between protocol and review
During the initial review process, the authors decided, following a suggestion from a peer reviewer, that interventions should be excluded if they only targeted one condition as this was contrary to the emphasis on multimorbidity. This led to the exclusion of some studies examining comorbid depression and other conditions where the intervention was only targeted at depression treatment.
Changes were also made to the original search strategy in the protocol, based on initial results from the original searches. The searches used in the review are presented as appendices.
We had planned to contact authors of other reviews in the field of multimorbidity that were retrieved during the search process regarding relevant studies that they may be aware of, but no other reviews of interventions were identified.
We had planned to prepare tables and funnel plots comparing effect sizes of studies grouped according to potential effect modifiers (for example, simple versus multifaceted interventions) if sufficient studies had been identified but this was not possible.
If there had been enough studies, we had planned to use meta‐regression to see whether the effect sizes could be predicted by study characteristics. These could, for example, include duration of the intervention, age groups, and simple versus multifaceted interventions (Cooper 1994). We also considered formal tests of homogeneity (Petitti 1994). None of these quantitative methods were possible for this version of the review but will be considered for future review updates
We had planned, if possible, to consider subgroup analyses based on the degree of multimorbidity of participants. This would have been based on the number of conditions per person. This was not possible.
We initially used the term psychosocial measures to group measures of well‐being, quality of life, function and psychological measures such as illness perceptions. We have replaced this with the more commonly used term 'patient‐reported outcome measures'. We have re‐named 'physical health outcomes' as 'clinical outcomes' for this update of the review.
Contributions of authors
Susan Smith (SS) conceived, co‐ordinated, and designed the review. Emma Wallace (EW) helped co‐ordinate the update of the review, assessed studies for inclusion, and extracted data from included studies. Susan Smith, Martin Fortin (MF) , Emma Wallace, and Tom O'Dowd (TOD) contributed to all stages of the review, and were involved in writing all review drafts and responding to peer review comments.
Sources of support
Internal sources
Health Research Board Primary Care Research Centre, Ireland
External sources
No sources of support supplied
Declarations of interest
SS has no conflict of interest. EW has no conflict of interest. TOD has no conflict of interest. MF has no conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Barley 2014 {published data only}
- Barley EA, Walters P, Haddad M, Phillips R, Achilla E, McCrone P, et al. The UPBEAT nurse-delivered personalized care intervention for people with coronary heart disease who report current chest pain and depression: A randomised controlled pilot study. PLOS One June 2014;9(6):e98704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bogner 2008 {published data only}
- Bogner HR, Vries HF. Integration of depression and hypertension treatment: a pilot, randomized controlled trial. Annals of Family Medicine 2008;6(4):295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boult 2011 {published data only}
- Boult C, Reider L, Frey K, Leff B, Boyd CM, Wolff JL, et al. Early effects of "Guided Care" on the quality of healthcare for multimorbid older persons: A cluster randomized controlled trial. The Journals of Gerontology 2008;63(3):321-7. [DOI] [PubMed] [Google Scholar]
- Leff B, Reider L, Frick KD, Scharfstein DD, Boyd CM, Frey K, et al. Guided Care and the cost of complex healthcare: A preliminary report. American Journal of Managed Care 2009;15(8):555-9. [PubMed] [Google Scholar]
Coventry 2015 {published data only}
- Coventry P, Lovell K, Dickens C, Bower P, Chew-Graham C, McElvenny D, et al. Integrated primary care for patients with mental and physical multimorbidity: cluster randomised controlled trial for patients with depression comorbid with diabetes or cardiovascular disease. BMJ 2015;350:h368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eakin 2007 {published and unpublished data}
- Eakin EGB, Bull SS, Riley KM, Reeves MM, McLaghlin M, Gutierrez S. Resources for Health: A Primary-Care-Based Diet and Physical Activity Intervention Targeting Urban Latinos With Multiple Chronic Conditions. Health Psychology 2007;26(4):392-400. [DOI] [PubMed] [Google Scholar]
Garvey 2015 {published data only}
- Garvey J, Connolly D, Boland F, Smith SM. OPTIMAL, an occupational therapy led self-management support programme for people with multimorbidity in primary care: a randomized controlled trial. BMC Family Practice 2015;16:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hochhalter 2010 {published data only}
- Hochhalter AK, Song J, Rush J, Sklar L, Stevens A. Making the Most of Your Healthcare intervention for older adults with multiple chronic illnesses. Patient Education and Counselling 2010;81(2):207-13. [DOI] [PubMed] [Google Scholar]
Hogg 2009 {published data only}
- Hogg W, Lemelin J, Dahrouge S, Liddy C, Armstrong CD, Legault F, et al. Randomized controlled trial of Anticipatory and Preventive multidisciplinary Team Care. Canadian Family Physician December 2009;55(12):e76-85. [PMC free article] [PubMed] [Google Scholar]
Katon 2010 {published data only}
- Katon W, Lin EHB, Von Korff M, Ciechanowski P, Ludman E, Young B, et al. Collaborative care for patients with depression and chronic illnesses. New England Journal of Medicine 2010;363(27):3611-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon W, Russo J, Lin EH, Schmittdiel J, Ciehanowski P, Ludman E, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Archives of General Psychiatry 2012;69(5):506-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kennedy 2013 {published and unpublished data}
- Kennedy A, Bower P, Reever D, Blakeman T, Bowen R, Chew-Graham C. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013;346:f2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krska 2001 {published data only}
- Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PRS, et al. Pharmacist-led medication review in patients over 65: a randomized controlled trial in primary care. Age and Ageing 2001;30(3):205-11. [DOI] [PubMed] [Google Scholar]
Lorig 1999 {published data only (unpublished sought but not used)}
- Lorig KR, Sobel DS, Stewart AL, Brown Jr BW, Ritter PL, González VM, et al. Evidence suggesting that a chronic disease self-management programme can improve health status and reduce hospitalisation. Medical Care 1999;37(1):5-14. [DOI] [PubMed] [Google Scholar]
Lynch 2014 {published data only}
- Lynch EB, Liebman R, Ventrelle J, Avery EF, Richardson D. A self-management intervention for African Americans with comorbid diabetes and hypertension: a pilot randomized controlled trial. Preventing Chronic Disease 2014;11:E90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2013 {published and unpublished data}
- Martin PR, Aiello R, Gilson K, Meadows G, Milgrom J, Reece J. Co-morbid recurrent headaches and major depressive disorder: An RCT of cognitive behavior therapy. In: Cephalalgia : an international journal of headache. Conference: 2013 International Headache Congress of the International Headache Society and American Headache Society Boston, MA United States. Vol. 2013. 2013:33.
Morgan 2013 {published data only}
- Morgan M, Coates A, Dunbar J Reddy A, Schlicht P, Fuller J. The TrueBlue model of collaborative care using practice nurses as case managers for depression alongside diabetes or heart disease: a randomised trial. BMJ Open 2013;3(1):e002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sommers 2000 {published data only}
- Sommers LS, Marton KI, Barbaccia JC, Randolph J. Physician, nurse and social worker collaboration in primary care for chronically ill seniors. Archives Internal Medicine 2000;160(12):1825-33. [DOI] [PubMed] [Google Scholar]
Wakefield 2012 {published data only}
- Wakefield BJ, Holman JE, Ray A, Scherubel M, Adams MR, Hillis SL, et al. Outcomes of a home telehealth intervention for patients with diabetes and hypertension. Telemedicine Journal and e-Health 2012;18(8):575-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Addolorato 2004 {published data only}
- Addolorato G, De Lorenzi G, AbenavoliL, Leggio L, Capristo E, Gasbarrini G. Psychological support counselling improves gluten-free diet compliance in coeliac patients with affective disorders. Alimentary Pharmacology & Therapeutics 2004;20(7):777-82. [DOI] [PubMed] [Google Scholar]
Agarwal 2015 {published data only}
- Agarwal G, McDonough B, Angeles R, Pirrie M, Marzanek F, McLeod B, et al. Rationale and methods of a multicentre randomised controlled trial of the effectiveness of a Community Health Assessment Programme with Emergency Medical Services (CHAP-EMS) implemented on residents aged 55 years and older in subsidised seniors' housing buildings in Ontario, Canada. BMJ Open 2015;5(6):e008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balaban 2014 {published data only}
- Balaban R, Galbraith B, Burns A, Vialle-Valentin M, Larochelle C, Ross-Degnan MR. A Patient Navigator Intervention to Reduce Hospital Readmissions among High-Risk Safety-Net Patients: A Randomized Controlled Trial. Journal of General Internal Medicine 2015;30(7):907-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1997 {published data only}
- Beck A, Scott J, Williams P, Robertson B, Jackson D, Gade G, et al. A randomized trial of group outpatient visits for chronically ill older HMO members: The Cooperative Health Care Clinic. Journal of the American Geriatrics Society 1997;45(5):543-9. [DOI] [PubMed] [Google Scholar]
Beretta 2014 {published data only}
- Beretta S, Beghi E, Messina P, Gerardi F, Pescini R, La L, et al. Comprehensive educational plan for patients with epilepsy and comorbidity (EDU-COM): a pragmatic randomised trial. Journal of Neurology, Neurosurgery and Psychiatry 2014;85(8):889-94. [DOI] [PubMed] [Google Scholar]
Bove 2015 {published data only}
- Bove DG, Overgaard D, Lomborg K, Lindhardt B, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention versus usual care for managing anxiety and dyspnoea in patients with severe chronic obstructive pulmonary disease: a randomised controlled trial protocol. BMJ Open 2015;5(7):e008031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brand 2004 {published data only}
- Brand CA, Jones CT, Lowe AJ, Nielsen DA, Roberts CA, King BL, et al. A transitional care service for elderly chronic disease patients at risk of readmission. Australian Health Review 2004;28(3):275-84. [DOI] [PubMed] [Google Scholar]
Chow 2014 {published data only}
- Chow SK, Wong FK. A randomized controlled trial of a nurse led case management programme for hospital-discharged older adults with co-morbidities. Journal of Advanced Nursing 2014;70(10):2257-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coburn 2012 {published data only}
- Coburn KD, Marcantonia S, Lazansky R, Keller M, Davis N. Effect of a community-based nursing intervention on mortality in chronically ill older patients: a randomized controlled trial. PLoS Medicine/Public Library of Science 2012;9(7):e1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorr 2008 {published data only}
- Dorr DA, Wilcox A, Brunker CP, Burdon RE, Donnelly S. The effect of technology-supported, multidisease care management on the mortality and hospitalisation of seniors. Journal of the American Geriatrics Society 2008;56(12):2195-202. [DOI] [PubMed] [Google Scholar]
Dougados 2015 {published data only}
- Dougados M, Soubrier M, Perrodeau E, Gossec L, Fayet F, Gilson M, et al. Impact of a nurse-led programme on comorbidity management and impact of a patient self-assessment of disease activity on the management of rheumatoid arthritis: results of a prospective, multicentre, randomised, controlled trial (COMEDRA). Annals of the Rheumatic Diseases 2015;74(9):1725-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drake 1998 {published data only}
- Drake RE, McHugo GJ, Clark RE, Teague GB, Xie H, Miles K, et al. Assertive community treatment for patients with co-occurring severe mental illness and substance use disorder: a clinical trial. American Journal of Orthopsychiatry 1998;68(2):201-15. [DOI] [PubMed] [Google Scholar]
Dwinger 2013 {published data only}
- Dwinger S, Dirmaier S, Herbarth J, Konig L, Eckardt H, Kriston M, et al. Telephone-based health coaching for chronically ill patients: study protocol for a randomized controlled trial. Trials 2013;14:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eklund 2013 {published data only}
- Eklund K, Wilhelmson K, Gustafsson H, Landahl S, Dahlin-Ivanoff S. One-year outcome of frailty indicators and activities of daily living following the randomised controlled trial; "Continuum of care for frail older people". BMC Geriatrics 2013;13:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Essock 2006 {published data only}
- Essock SM, Mueser KT, Drake RE, Covell NH, McHugo GJ, Frisman LK, et al. Comparison of ACT and standard case management for delivering integrated treatment for co-occuring disorders. Psychiatric Services 2006;57(2):185-96. [DOI] [PubMed] [Google Scholar]
Fischer 2015 {published data only}
- Fischer A, Schroder A, Vettorazzi J, Wolf E, Pottgen OT, Lau J, et al. An online programme to reduce depression in patients with multiple sclerosis: a randomised controlled trial. The Lancet Psychiatry 2015;2(3):217-23. [DOI] [PubMed] [Google Scholar]
Freund 2011 {published data only}
- Freund T, Peters-Klimm T, Rochon F, Mahler J, Gensichen C, Erler J, et al. Primary care practice-based care management for chronically ill patients (PraCMan): study protocol for a cluster randomized controlled trial [ISRCTN56104508]. Trials 2011;12:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganz 2010 {published data only}
- Ganz DA, Koretz BK, McCreath HE, Wenger NS, Roth CP, Bail JK, et al. The effect of nurse practitioner co-management on quality of care of patients with chronic conditions in an academic geriatric practice [S]. Journal of the American Geriatrics Society 2010;58(Supplement s1):Pages iii–vi, 1–279. [Google Scholar]
Gitlin 2009 {published and unpublished data}
- Giltin LN, Hauck WW, Dennis MP, Winter L, Hodgson N, Schinfeld S. Long-term effect on mortality of a home intervention that reduces functional difficulties in older adults: Results from a randomized trial. Journal of the American Geriatrics Society 2009;57(3):476-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Dennis MP, Corcoran M, Schinfeld S, Hauck WW. A randomized trial of a multicomponent home intervention to reduce functional difficulties in older adults. Journal of the American Geriatrics Society 2006;54(5):809-16. [DOI] [PubMed] [Google Scholar]
Harpole 2005 {published data only}
- Harpole LH, Williams JW Jr, Olsen MK, Stechuchak KM, Oddone E, Callahan CM, et al. Improving depression outcomes in older adults with comorbid medical illness. General Hospital Psychiatry 2005;27(1):4-12. [DOI] [PubMed] [Google Scholar]
Hermanns 2015 {published data only}
- Hermanns N, Schmitt N, Gahr A, Herder A, Nowotny C, Roden B, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care 2015;38(4):551-60. [DOI] [PubMed] [Google Scholar]
Hien 2004 {published data only}
- Hien DA, Cohen LR, Miele GM, Litt LC, Capstick C. Promising treatments for women with comorbid PTSD and substance use disorders. American Journal of Psychiatry 2004;161(8):1426-32. [DOI] [PubMed] [Google Scholar]
Hinrichs 2013 {published data only}
- Hinrichs T, Moschny T, Brach A, Bücker M, Klaassen-Mielke B, Trampisch R, et al. Recruitment of Chronically Ill and Mobility-Restricted Older Adults for an Exercise Intervention Study Supported by the General Practitioner's Practice. PM&R 2013;5(9, Supplement):S211. [Google Scholar]
Hutchings 2013 {published data only}
- Hutchings H, Evans HA, Fitzsimmons BA, Harrison D, Heaven DJ, Huxley M, et al. Predictive risk stratification model: a progressive cluster-randomised trial in chronic conditions management (PRISMATIC) research protocol. Trials 2013;14:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katon 2004 {published data only}
- Katon W, Von Korff M, Lin EHB, Simon G, Ludman E, Russo J, et al. The Pathways Study: A randomized trial of collaborative care in patients with diabetes and depression. Archives of General Psychiatry 2004;61(10):1042-9. [DOI] [PubMed] [Google Scholar]
Leveille 1998 {published data only}
- Leveille SG, Wagner EH, Davis C, Grothaus L, Wallace J, LoGerfo M, et al. Preventing disability and managing chronic illness in frail older adults: a randomized trial of a community-based partnership with primary care. Journal of the American Geriatrics Society 1998;46(10):1191-8. [DOI] [PubMed] [Google Scholar]
Lin 2003 {published data only}
- Lin EH, Katon W, Von Korff M, Tang L, Williams JW Jr, Kroenke K, IMPACT Investigators. Effect of improving depression care on pain and functional outcomes among older adults with arthritis. Journal of the American Medical Association 2003;290(18):2428-9. [DOI] [PubMed] [Google Scholar]
Liss 2013 {published data only}
- Liss DT, Fishman PA, Rutter CM, Grembowski D, Ross TR, Johnson EA, et al. Outcomes among chronically ill adults in a medical home prototype. The American Journal of Managed Care 2013;19(10):e348-e58. [PMC free article] [PubMed] [Google Scholar]
Lyles 2003 {published data only}
- Lyles JS, Hodges A, Collins C, Lein C, Given CW, Given B, et al. Using nurse practitioners to implement an intervention in primary care for high-utilizing patients with medically unexplained symptoms. General Hospital Psychiatry 2003;25(2):63-73. [DOI] [PubMed] [Google Scholar]
Martinez 2013 {published data only}
- Martinez H, Fort M, Dengo AL, Castro M, Pena L, Alvarado M, et al. A cardiovascular risk reduction intervention in patients with hypertension and/or type 2 diabetes in San José, Costa Rica and Chiapas, Mexico. FASEB Journal 2013;27:1055. [Google Scholar]
McCall 2011 {published data only}
- McCall N, Cromwell J. Results of the Medicare Health Support disease-management pilot program. New England Journal of Medicine 2013;365(18):1704-12. [DOI] [PubMed] [Google Scholar]
McCusker 2015 {published data only}
- McCusker J, Cole J, Yaffe G, Strumpf M, Sewitch E, Sussman M, et al. A randomized trial of a depression self-care toolkit with or without lay telephone coaching for primary care patients with chronic physical conditions. General Hospital Psychiatry 2015;37(3):257-65. [DOI] [PubMed] [Google Scholar]
Meeuwissen 2011 {published data only}
- Meeuwissen J, Holleman AC, Jong GJM, Nuyen J, Feltz-Cornelis CM. Screening and guided self-help intervention for anxiety and depression in patients with type 2 diabetes. European Diabetes Nursing 2011;8(2):47-52a. [Google Scholar]
Morey 2006 {published data only}
- Morey MC, Ekelund C, Pearson M, Crowley G, Peterson M, Sloane R, et al. Project LIFE: a partnership to increase physical activity in elders with multiple chronic illnesses. Journal of Aging & Physical Activity 2006;14(3):324-43. [DOI] [PubMed] [Google Scholar]
Morris 2012 {published data only}
- Morris D, Budhwar W, Husain N, Wisniewski M, Kurian SR, Luther BT, et al. Depression treatment in patients with general medical conditions: results from the CO-MED trial. Annals of Family Medicine 2012;10(1):23-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2014 {published data only}
- Park YH, Chang H. Effect of a health coaching self-management program for older adults with multimorbidity in nursing homes. Patient Preference and Adherence 2014;8:959-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petersen 2014 {published data only}
- Petersen I, Hanass Hancock J, Bhana A, Govender K. A group-based counselling intervention for depression comorbid with HIV/AIDS using a task shifting approach in South Africa: a randomized controlled pilot study. Journal of Affective Disorders 2014;158:78-84. [DOI] [PubMed] [Google Scholar]
Plant 2013 {published data only}
- Plant N, Mallitt N, Kelly KA, Usherwood PJ, Gillespie T, Boyages J, et al. Implementation and effectiveness of 'care navigation', coordinated management for people with complex chronic illness: rationale and methods of a randomised controlled trial. BMC Health Serv Res 2013;13:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reuben 2012 {published data only}
- Reuben DB. Team care to manage chronic illnesses in older persons. In: Journal of the American Geriatrics Society Conference 2012 Annual Scientific Metting of the American Geriatrics Society Seattle, WA United States. 2012.
Rodriguez‐Pascual 2013 {published data only}
- Rodriguez-Pascual C, Paredes-Galan E, Ferrero-Martinez AL, Gonzalez-Guerrero JL, Hornillos M, Abizanda P, et al. A disease management program intervention in elderly patients with high comorbidity: Results of a randomized-controlled trial. In: European Geriatric Medicine 2013 Conference: 9th Congress of the European Union Geriatric Medicine Society, EUGMS Venice Italy. 2013:S15.
Rosenman 2006 {published data only}
- Rosenman MB, Holmes AM, Ackermann RT, Murray MD, Doebbeling CC, Katz B, et al. The Indiana Chronic Disease Management Program. Milbank Quarterly 2006;84(1):135-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruikes 2012 {published data only}
- Ruikes F, Meys FG, de Wetering AR, Akkermans G, Gaal P, Zuidema BG, et al. The CareWell-primary care program: design of a cluster controlled trial and process evaluation of a complex intervention targeting community-dwelling frail elderly. BMC Family Practice 2012;13:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schraeder 2005 {published data only}
- Schraeder C, Dworak D, Stoll JF, Kucera C, Waldschmidt V, Dworak MP. Managing elders with comorbidities. Journal of Ambulatory Care Management 2005;28(3):201-9. [DOI] [PubMed] [Google Scholar]
Sharpe 2012 {published data only}
- Sharpe L, Gittins L, Correia CB, Meade HM, Nicholas T, Raue MK, et al. Problem-solving versus cognitive restructuring of medically ill seniors with depression (PROMISE-D trial): study protocol and design. BMC Psychiatry 2012;12:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 2014 {published data only}
- Shaw W, Besen S, Pransky E, Boot G, Nicholas CR, McLellan MK, et al. Manage at work: a randomized, controlled trial of a self-management group intervention to overcome workplace challenges associated with chronic physical health conditions. BMC Public Health 2014;14:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Srinivasan 2014 {published data only}
- Srinivasan S, Reagan S, Hardin LP, Matthews JW, Leaphart M, Grillo E, et al. Adjunctive tai chi in geriatric depression with comorbid arthritis: A randomized, controlled trial. American Journal of Geriatric Psychiatry 2014;22(3 SUPPL. 1):S135-S136. [Google Scholar]
Takahashi 2012 {published data only}
- Takahashi PY, Pecine JL, Upatising B, Chaudhry R, Shah ND, Van HH, et al. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Archives of Internal Medicine 2012;172(10):773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taveira 2011 {published data only}
- Taveira T, Dooley H, Cohen AG, Khatana LB, Wu SA, et al. Pharmacist-led group medical appointments for the management of type 2 diabetes with comorbid depression in older adults. The Annals of Pharmacotherapy 2011;45(11):1346-55. [DOI] [PubMed] [Google Scholar]
van der Weegen 2013 {published data only}
- Weegen S, Verwey S, Spreeuwenberg R, Tange M, Weijden H, Witte T. The development of a mobile monitoring and feedback tool to stimulate physical activity of people with a chronic disease in primary care: a user-centered design. JMIR Mhealth Uhealth 2013;1(2):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Mourik 2012 {published data only}
- Mourik FH, Moons Y, Bertens KG, Reitsma LC, Hoes JB, Rutten AW. Triage of frail elderly with reduced exercise tolerance in primary care (TREE). A clustered randomized diagnostic study. BMC Public Health 2012;12:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Venter 2012 {published data only}
- Venter A, Burns R, Hefford M, Ehrenberg N. Results of a telehealth-enabled chronic care management service to support people with long-term conditions at home. Journal of Telemedicine & Telecare 2012;18(3):172-5. [DOI] [PubMed] [Google Scholar]
Via‐Sosa 2013 {published data only}
- Via-Sosa M, Lopes MA, March N. Effectiveness of a drug dosing service provided by community pharmacists in polymedicated elderly patients with renal impairment--a comparative study. BMC Family Practice 2013;14:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Von Korff 2012 {published data only}
- Von Korff M, Vitiello MV, McCurry SM, Balderson BH, Moore AL, Baker LD, et al. Group interventions for co-morbid insomnia and osteoarthritis pain in primary care: The lifestyles cluster randomized trial design. Contemporary Clinical Trials 2012;33(4):759-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weber 2012 {published data only}
- Weber C, Beaulieu M, Djurdjev O, Er L, Taylor P, Ignaszewski A, et al. Towards rational approaches of health care utilization in complex patients: an exploratory randomized trial comparing a novel combined clinic to multiple specialty clinics in patients with renal disease-cardiovascular disease-diabetes. Nephrology Dialysis Transplantation Suppl 3;iii:104-10. [DOI] [PubMed] [Google Scholar]
Wilhelmson 2011 {published data only}
- Wilhelmson K, Duner A, Eklund K, Gosman-Hedström G, Blomberg S, Hasson H, et al. Design of a randomized controlled study of a multi-professional and multidimensional intervention targeting frail elderly people. BMC Geriatriatrics 2011;14(11):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Willard‐Grace 2013 {published data only}
- Willard-Grace R, DeVore D, Chen EH, Hessler D, Bodenheimer T, Thom DH. The effectiveness of medical assistant health coaching for low-income patients with uncontrolled diabetes, hypertension, and hyperlipidemia: protocol for a randomized controlled trial and baseline characteristics of the study population. BMC Family Practice 2013;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2012 {published data only}
- Williams A, Manias E, Liew D, Gock H, Gorelik A. Working with CALD groups: testing the feasibility of an intervention to improve medication self-management in people with kidney disease, diabetes, and cardiovascular disease. Renal Society of Australasia Journal 2012;8(2):62-9. [Google Scholar]
Williams 2013 {published data only}
- Williams AM, Bloomfield L, Milthorpe E, Aspinall D, Filocamo K, Wellsmore T, et al. Effectiveness of Moving On: an Australian designed generic self-management program for people with a chronic illness. BMC Health Services Research 2013;13:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wrede 2013 {published data only}
- Wrede J, Voigt I, Bleidorn J, Hummers-Pradier E, Dierks ML, Junius-Walker U. Complex health care decisions with older patients in general practice: patient-centeredness and prioritization in consultations following a geriatric assessment. Patient Education & Counseling 2013;90(1):54-60. [DOI] [PubMed] [Google Scholar]
Wu 2013 {published data only}
- Wu C, Sung J, Chang HC, Atherton AM, Kostner J, Courtney K, et al. Protocol for a randomised blocked design study using telephone and text-messaging to support cardiac patients with diabetes: a cross cultural international collaborative project. BMC Health Services Research 2013;13:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Alexopoulos 2014 {published data only}
- Alexopoulos G, Kiosses S, Sirey D, Kanellopoulos J, Seirup D, Novitch JK, et al. Untangling therapeutic ingredients of a personalized intervention for patients with depression and severe COPD. American Journal of Geriatric Psychiatry 2014;22(11):1316-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buhrman 2015 {published data only}
- Buhrman M, Syk M, Burvall M, Hartig O, Gordh T, Andersson TG. Individualized guided internet-delivered cognitive-behavior therapy for chronic pain patients with comorbid depression and anxiety: A randomized controlled trial. Clinical Journal of Pain 2015;31(6):504-16. [DOI] [PubMed] [Google Scholar]
Ekdahl 2014 {published data only}
- Ekdahl A, Wirehn W, Jaarsma AB, Unosson T, Alwin M, Husberg J, et al. Caring for elderly with multimorbidity: Evaluation of ambulatory geriatric unit (AGU) (the AGe-FIT-study) - A randomized controlled trial. European Geriatric Medicine 2014;5(Supplement 1):S63-64. [Google Scholar]
Katz 2015 {published data only}
- Katz R, Patel J, Young S, Cohen H. Enhancing diabetes and hypertension self-management: A randomized trial of a mhealth strategy in a community clinic setting. Diabetes 2015;64(Supplement 1):A192. [Google Scholar]
References to ongoing studies
ACTRN12609000726257 {published data only}
- Trial Registration number ACTRN12609000726257. Protocol for a randomised controlled trial of chronic disease self-management support for older Australians with multiple chronic diseases. Contemporary Clinical Trials 2011;32(6):946-52. [DOI] [PubMed] [Google Scholar]
Crowley ongoing {published data only}
- Crowley M, Bosworth MJ, Coffman B, Lindquist CJ, Neary JH, Harris AM, et al. Tailored Case Management for Diabetes and Hypertension (TEACH-DM) in a community population: study design and baseline sample characteristics. Contemporary Clinical Trials 2013;36(1):298-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN24874457 {published data only}
- Trial Registration number ISRCTN24874457. Web-based cognitive behavioural therapy (W-CBT) for diabetes patients with co-morbid depression: design of a randomised controlled trial. BMC Psychiatry 2008;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN 83908315 {published data only}
- Current Controlled Trials ISRCTN 83908315. Practice network-based care management for patients with type 2 diabetes and multiple comorbidities (GEDIMAplus): study protocol for a randomized controlled trial. Trials 2014;15:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lassere 2015 {published data only}
- Lassere MN, Baker N, Parle S, Sara A, Johnson KR. Improving quality of care and long-term health outcomes through continuity of care with the use of an electronic or paper patient-held portable health file (COMMUNICATE): study protocol for a randomized controlled trial. Trials 2015;16:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mercer ongoing {published data only}
- Mercer S. Care Plus Study. In: Annual Scientific meeting Society for Academic Primary Care, UK. July 2014.
NCT01328639 {published data only}
- Trial registration number NCT01328639. Controlled trial of a collaborative primary care team model for patients with diabetes and depression: rationale and design for a comprehensive evaluation. BMC Health Services Research 2012;12:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01572389 {published data only}
- Trial registration number NCT01572389. Behavioral health coaching for rural veterans with diabetes and depression: a patient randomized effectiveness implementation trial. BMC Health Services Research 2014;14:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01719991 {published data only}
- Trial Registration number NCT01719991. Case management and self-management support for frequent users with chronic disease in primary care: a pragmatic randomized controlled trial. BMC Health Services Research 2013;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
NTR1847 {published data only}
- Trial Registration number NTR1847. The effectiveness of case management for comorbid diabetes type 2 patients; the CasCo study. Design of a randomized controlled trial. BMC Family Practice 2011;12:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
NTR2626 {published data only}
- NTR2626. Managing co-morbid depression and anxiety in primary care patients with asthma and/or chronic obstructive pulmonary disease: study protocol for a randomized controlled trial. Trials 2012;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
NTR3715 {published data only}
- Trial Registration number NTR3715. Cost-effectiveness of a stepped-care intervention to prevent major depression in patients with type 2 diabetes mellitus and/or coronary heart disease and subthreshold depression: design of a cluster-randomized controlled trial. BMC Psychiatry 2013;13:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pr1MaC ongoing {unpublished data only}
- Pr1MaC. Ongoing study. 2011. Contact author for more information.
Salisbury ongoing {published data only}
- Salisbury C, et al. The 3D Study. In: Annual Scientific meeting of the Society for Academic Primary Care, UK. July 2014.
Schneider ongoing {published data only}
- Schneider K, Pagoto L, Handschin SL, Panza B, Bakke E, Liu S. Design and methods for a pilot randomized clinical trial involving exercise and behavioral activation to treat comorbid type 2 diabetes and major depressive disorder. Mental Health Phys Act 2011;4(1):13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Bähler 2015
- Bähler C, Huber CA, Brüngger B, Reich O. Multimorbidity, health care utilization and costs in an elderly community-dwelling population: a claims data based observational study. BMC Health Services Research 2015;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnett 2012
- Barnett K, Mercer S, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. The Lancet 2012;380:37-43. [DOI] [PubMed] [Google Scholar]
Bayliss 2004
- Bayliss E, Bayliss M, Ware J, Steiner J. Predicting declines in physical function in persons with multiple chronic medical conditions: what we can learn from the medical problem list. Health and Quality of Life Outcomes 2004;2(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
BMJ Multimorbidity collection
- BMJ Multimorbidity Collection. www.bmj.com/specialties/multimorbidity.
Brettschneider 2013
- Brettschneider C, Leicht H, Bickel H, Dahlhaus A, Fuchs A, MultiCare Study Group. Relative impact of multimorbid chronic conditions on health-related quality of life - results from the MultiCare Cohort Study. PLoS One 2013;8(6):e66742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Challis 2014
- Challis D and Hughes J. Intensive care/case management, Expert Briefing Paper 1. Manchester: Personal Social Services Research Unit. http://sites.nursing.manchester.ac.uk/pssru/research/nihrsscr/productsandtoolkits/IntensiveCaseManagement.pdf (accessed 13 December 2014).
Cochrane Handbook
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Cohen 1988
- Cohen J. Statistical power analysis for the behavioural sciences. Lawrence Erlbaum, 1988. [Google Scholar]
Condelius 2008
- Condelius A, Edberg AK, Jakobsson U, Hallberg IR. Hospital admissions among people 65+ related to multimorbidity, municipal and outpatient care. Archives of Gerontology and Geriatrics 2008;46(1):41-55. [DOI] [PubMed] [Google Scholar]
Cooper 1994
- Cooper H, Hedes LV. A handbook of research synthesis. New York: Russell Sage, 1994. [Google Scholar]
Cox 2002
- Cox R, Amsters D. Goal Attainment Scaling: an effective outcome measure for rural and remote health services. Australian Journal of Rural Health 2002;10(5):256-61. [DOI] [PubMed] [Google Scholar]
Deeg 2002
- Deeg DJ, Portrait F, Lindeboom M. Health profiles and profile-specific health expectancies of older women and men. Journal of Women & Aging 2002;14(1-2):27-46. [DOI] [PubMed] [Google Scholar]
de Groot 2004
- Groot V, Beckerman H, Lankhorst G, Bouter L. How to measure comorbidity: a critical review of available methods. Journal of Clinical Epidemiology 2004;57(3):323. [DOI] [PubMed] [Google Scholar]
Duerden 2013
- Duerden M, Avery T, Payne R. Polypharmacy and medicines optimisation. http://www.kingsfund.org.uk/sites/files/kf/field/field_publication_file/polypharmacy-and-medicines-optimisation-kingsfund-nov13.pdf (accessed September 2015).
Dumbreck 2015
- Dumbreck S, Flynn A, Nairn M, Wilson M, Treweek S, Mercer SW. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ 2015;350:h949. [DOI] [PMC free article] [PubMed] [Google Scholar]
EndNote 2013 [Computer program]
- EndNote. Version X7. New York: Thomson Reuters, 2013.
EPOC 2002
- Effective Practice and Organisation of Care (EPOC). EPOC Taxonomy. Oslo: Norwegian Knowledge Centre for the Health Services; 2002. Available at: https://epoc.cochrane.org/epoc-taxonomy.
EPOC 2013
- Effective Practice and Organisation of Care (EPOC). What study designs should be included in an EPOC review? EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services.. http://epoc.cochrane.org/epoc-specific-resources-review-authors.
EPOC 2013a
- Effective Practice and Organisation of Care (EPOC). Data collection form. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2013. Available at http://epoc.cochrane.org/epoc-specific-resources-review-authors.
EPOC 2015
- Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2015.. Available at: http://epoc.cochrane.org/epoc-specific-resources-review-authors.
Fortin 2004
- Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu A, Maltais D. Multimorbidity and quality of life in primary care: a systematic review. Health and Quality of Life Outcomes. 2004;2(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fortin 2005
- Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in Family Practice. Annals of Family Medicine 2005;3(3):223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fortin 2006a
- Fortin M, Bravo G, Hudon C, Lapointe L, Dubois MF, Almirall J. Psychological distress and multimorbidity in primary care. Annals of Family Medicine 2006;4(5):417-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fortin 2006b
- Fortin M, Bravo G, Hudon C, Lapointe L, Almirall J, Dubois MF, et al. Relationship between multimorbidity and health-related quality of life of patients in primary care. Quality of Life Research 2006;15(1):83-91. [DOI] [PubMed] [Google Scholar]
Fortin 2006c
- Fortin M, Dionne J, Pinho G, Gignac J, Almirall J, Lapointe L. Randomized clinical trials: do they have external validity for patients with multiple comorbidities? Annals of Family Medicine 2006;4(2):104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fortin 2007
- Fortin M, Soubhi H, Hudon C, Bayliss E, den Akker M. Multimorbidity's many challenges. BMJ 2007;334(7602):1016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fortin 2013
- Fortin M, Smith SM. External Validity of Clinical Trials in Multiple Chronic Conditions. Journal of Comorbidity 2013;3(2):30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2007
- Foster G, Taylor SJC, Eldridge S, Ramsay J, Griffiths CJ. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD005108. [DOI: 10.1002/14651858.CD005108.pub2] [DOI] [PubMed] [Google Scholar]
Gallacher 2011
- Gallacher K, May CR, Montori VM, Mair FS. Understanding patients’ experiences of treatment burden in chronic heart failure using normalization process theory. Annals of Family Medicine 2011;9(3):235-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gandhi 2003
- Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, et al. Adverse drug events in ambulatory care. New England Journal of Medicine 2003;348(16):1556-64. [DOI] [PubMed] [Google Scholar]
Gitlin 2006
- Gitlin LN, Winter L, Dennis MP, Corcoran M, Schinfeld S, Hauck WW. A randomized trial of a multicomponent home intervention to reduce functional difficulties in older adults. Journal of the American Geriatrics Society 2006;54(5):809-16. [DOI] [PubMed] [Google Scholar]
Gordon 1999
- Gordon JE, Powell C, Rockwood K. Goal attainment scaling as a measure of clinically important change in nursing-home patients. Age & Aging 1999;28(3):275-81. [DOI] [PubMed] [Google Scholar]
Gunn 2012
- Gunn JM, Ayton DR, Densley K, Pallant JF, Chondros P, Herrman HE. The association between chronic illness, multimorbidity and depressive symptoms in an Australian primary care cohort. Social Psychiatry and Psychiatric Epidemiology 2012;47(2):175-84. [DOI] [PubMed] [Google Scholar]
Guthrie 2011
- Guthrie B, McCowan C, Davey P, Simpson CR, Dreischulte T, Barnett K. High risk prescribing in primary care patients particularly vulnerable to adverse drug events: cross sectional population database analysis in Scottish general practice. BMJ 2011;342:d3514. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. GRADE Working Group. Rating quality of evidence and strength of recommendations: What is "quality of evidence" and why is it important to clinicians? BMJ 2008;336(7651):995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrison 2014
- Harrison C, Britt H, Miller G, Henderson J. Examining different measures of multimorbidity, using a large prospective cross-sectional study in Australian general practice. BMJ Open 2014;4(7):e004694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haynes 2008
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD000011. [DOI: 10.1002/14651858.CD000011.pub3] [DOI] [PubMed] [Google Scholar]
Hoffman 1996
- Hoffman C, Rice D, Sung HY. Persons with chronic conditions. Their prevalence and costs. Journal of the American Medical Association 1996;276(18):1473-9. [PubMed] [Google Scholar]
IRCMO
- International Research Community on Multimorbidity. http://crmcspl-blog.recherche.usherbrooke.ca.ca/?page_id=248.
Katon 2012
- Katon W, Russo J, Lin EH, Schmittdiel J, Ciehanowski P, Ludman E, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Archives of General Psychiatry 2012;69(5):506-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiresuk 1968
- Kiresuk TJ, Sherman RE. Goal attainment scaling: a general method for evaluating comprehensive community health programs. Community Mental Health Journal 1968;4(6):443-53. [DOI] [PubMed] [Google Scholar]
Leff 2009
- Leff B, Reider L, Frick KD, Scharfstein DD, Boyd CM, Frey K, et al. Guided Care and the cost of complex healthcare: A preliminary report. American Journal of Managed Care 2009;15(8):555-9. [PubMed] [Google Scholar]
Lewis 2004
- Lewis R, Dixon J. Rethinking management of chronic diseases. BMJ 2004;328(7433):220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Librero 1999
- Librero J, Peiro S, Ordinana R. Chronic comorbidity and outcomes of hospital care: length of stay, mortality, and readmission at 30 and 365 days. Journal of Clinical Epidemiology 1999;52(3):171-9. [DOI] [PubMed] [Google Scholar]
London DOH 2005
- Department of Health London. Supporting people with long term conditions. Department of Health 2005.
Marengoni 2011
- Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Research Reviews 2011;10(4):430-9. [DOI] [PubMed] [Google Scholar]
May 2009
- May C, Montori V, Mair F. We need minimally disruptive medicine. BMJ 2009;339:b2803. [DOI] [PubMed] [Google Scholar]
Menotti 2001
- Menotti A, Mulder I, Nissinen A, Giampaoli S, Feskens EJ, Kromhout D. Prevalence of morbidity and multimorbidity in elderly male populations and their impact on 10-year all-cause mortality: The FINE study (Finland, Italy, Netherlands, Elderly). Journal of Clinical Epidemiology 2001;54(7):680-6. [DOI] [PubMed] [Google Scholar]
MRC Framework 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, the Medical Research Council. Developing and evaluating complex interventions: new guidance. www.mrc.ac.uk/complexinterventionsguidance.
MSSS 2001
- Ministère de la Santé et des Services sociaux. Emerging solutions - Report and Recommendations (of the Commission d'etude sur les services de sante et les services sociaux) [Les solutions émergentes – Rapport et recommandations (Rapport Clair)]. http://tools.hhr-rhs.ca/index.php?option=com_mtree&task=visit&link_id=5093&Itemid=109&lang=en 2001;ISBN 2-550-36960-2.
Murphy 2004
- Murphy E. Case management and community matrons for long term conditions. BMJ 2004;329(7477):1251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health and Care Excellence. Depression in adults with a chronic physical health problem: treatment and management (Clinical guideline 91). www.nice.org.uk/guidance/cg91. 2009. [PubMed]
Parmelee 1995
- Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. Journal of American Geriatrics Society 1995;43(2):130-7. [DOI] [PubMed] [Google Scholar]
Payne 2013
- Payne RA, Abel GA, Guthrie B, Mercer SW. The effect of physical multimorbidity, mental health conditions and socioeconomic deprivation on unplanned admissions to hospital: a retrospective cohort study. Canandian Medical Association Journal 2013;185(5):E221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petitti 1994
- Petitti DB. Metaanalysis, decision analysis, and cost effectiveness analysis. New York: Oxford University Press, 1994. [Google Scholar]
PROMIS 2011
- PROMIS. http://nihpromis.org/ accessed 14/07/2011.
Rochon 1996
- Rochon PA, Katz JN, Morrow LA, McGlinchey-Berroth R, Ahsquist MM, Sarkarati M, et al. Comorbid illness is associated with survival and length of hospital stay in patients with chronic disability. A prospective comparison of three comorbidity indices. Medical Care 1996;34(11):1093-101. [DOI] [PubMed] [Google Scholar]
Rowell 2006
- Hilary Rowell, Dr Foster (Organization). Keeping People Out of Hospital: The Challenge of Reducing Emergency Admissions. London: Dr Foster Intelligence, 2006. [Google Scholar]
Satariano 2013
- Satariano WA and Boyd CM. Improving the evidence base on multimorbidities through better research: a commentary on the US HHS initiative, Multiple Chronic Conditions: A Strategic Framework. Journal of Comorbidity 2013;3:18-21. [PMC free article] [PubMed] [Google Scholar]
Simon 2001
- Simon GE. Treating depression in patients with chronic disease. Western Journal of Medicine 2001;175(5):292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2007
- Smith SM, O'Dowd T. Chronic diseases: what happens when they come in multiples? British Journal of General Practice 2007;57(537):268-70. [PMC free article] [PubMed] [Google Scholar]
Smith 2008
- Smith SM, Ferede A, O'Dowd T. Multimorbidity in younger deprived patients: an exploratory study of research and service implications in general practice. BMC Family Practice 2008;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2013
- Smith SM, Bayliss E, Mercer S, Gunn J, Vestergaard M, Wyke S, et al. How to design and evaluate interventions to improve outcomes for patients with multimorbidity. Journal of Comorbidity 2013;3(1):10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stange 2005
- Stange KC. Radical ideas. Annals of Family Medicine 2005;3(4):369-71. [Google Scholar]
Starfield 1998
- Starfield B. Primary care: balancing health needs, services and technology. New York: Oxford University Press, 1998. [Google Scholar]
Starfield 2001
- Starfield B. New paradigms for quality in primary care. British Journal of General Practice 2001;51(465):303-9. [PMC free article] [PubMed] [Google Scholar]
Starfield 2005
- Starfield B, Lemke KW, Herbert R, Pavlovich WD, Anderson G. Comorbidity and the use of primary care and specialist care in the elderly. Annals of Family Medicine 2005;3(3):215-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Townsend 2006
- Townsend A, Hunt K, Wyke S. Managing multiple morbidity in mid-life: a qualitative study of attitudes to drug use. BMJ 2006;327(7419):837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valderas 2011
- Valderas JM, Mercer S, Fortin M. Research on patients with multiple health conditions: different constructs, different views, one voice. Journal of Comorbidity 2011;1(1):1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Akker 1998
- den Akker M, Buntinx F, Metsemakers JF, Roos S, Knotternerus JA. Multimorbidity in general practice: prevalence, incidence and determinants of co-occurring chronic and recurrent diseases. Journal of Clinical Epidemiology 1998;51(5):367-75. [DOI] [PubMed] [Google Scholar]
Vaneslow 1995
- Vaneslow N, Donaldson M, Yordy K. A new definition of primary care. Journal of Americal Medical Association 1995;272(3):192. [Google Scholar]
Wallace 2015
- Wallace E, Salisbury C, Guthrie B, Lewis C, Fahey T, Smith SM. Managing patients with multimorbidity in primary care. BMJ 2015;350:h176. [DOI] [PubMed] [Google Scholar]
WHO 2002
- World Health Organization. Innovative care for chronic conditions: building blocks for action: global report. Global Report, Geneva 2002.
Wolff 2002
- Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Archives of Internal Medicine 2002;162(20):2269-76. [DOI] [PubMed] [Google Scholar]
Wyatt 2014
- Wyatt KD, Stuart LM, Brito JP, Carranza Leon B, Domecq JP, Prutsky GJ, et al. Out of context: clinical practice guidelines and patients with multiple chronic conditions: a systematic review. Medical Care 2014;52 (Suppl 3):S92-S100. [DOI] [PubMed] [Google Scholar]
Zulman 2011
- Zulman DM, Sussman JB, Chen X, Cigolle CT, Blaum CS, Hayward RA. Examining the evidence: a systematic review of the inclusion and analysis of older adults in randomized controlled trials. Journal of General Internal Medicine 2011;26(7):783-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwarenstein 2000
- Zwarenstein M, Reeves S, Straus SE, Pinfold P, Goldman J. Case management: effects on professional practice and health care outcomes (Protocol). Cochrane Database of Systematic Reviews 2000, Issue 4. Art. No.: CD002797. Art. No: CD002797. [DOI: 10.1002/14651858.CD002797] [DOI] [Google Scholar]
References to other published versions of this review
Smith 2007b
- Smith SM, Soubhi H, Fortin M, Hudon C, O'Dowd T. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD006560. [DOI: 10.1002/14651858.CD006560] [DOI] [PubMed] [Google Scholar]
Smith 2012
- Smith SM, Soubhi H, Fortin M, Hudon C, O'Dowd T. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No: CD006560. [DOI: 10.1002/14651858.CD006560] [DOI] [PubMed] [Google Scholar]


