Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory condition characterised by persistent respiratory symptoms and airflow limitation. Acute exacerbations punctuate the natural history of COPD and are associated with increased morbidity and mortality and disease progression. Chronic airflow limitation is caused by a combination of small airways (bronchitis) and parenchymal destruction (emphysema), which can impact day‐to‐day activities and overall quality of life. In carefully selected patients with COPD, long‐term, prophylactic use of antibiotics may reduce bacterial load, inflammation of the airways, and the frequency of exacerbations.
Objectives
To assess effects of different prophylactic antibiotics on exacerbations, quality of life, and serious adverse events in people with COPD in three separate network meta‐analyses (NMAs), and to provide rankings of identified antibiotics.
Search methods
To identify eligible randomised controlled trials (RCTs), we searched the Cochrane Airways Group Specialised Register of trials and clinical trials registries. We conducted the most recent search on 22 January 2020.
Selection criteria
We included RCTs with a parallel design of at least 12 weeks' duration evaluating long‐term administration of antibiotics prophylactically compared with other antibiotics, or placebo, for patients with COPD.
Data collection and analysis
This Cochrane Review collected and updated pair‐wise data from two previous Cochrane Reviews. Searches were updated and additional studies included. We conducted three separate network meta‐analyses (NMAs) within a Bayesian framework to assess three outcomes: exacerbations, quality of life, and serious adverse events. For quality of life, we collected data from St George's Respiratory Questionnaire (SGRQ). Using previously validated methods, we selected the simplest model that could adequately fit the data for every analysis. We used threshold analysis to indicate which results were robust to potential biases, taking into account each study’s contributions to the overall results and network structure. Probability ranking was performed for each antibiotic class for exacerbations, quality of life, and serious adverse events.
Main results
Characteristics of studies and participants
Eight trials were conducted at multiple sites that included hospital clinics or academic health centres. Seven were single‐centre trials conducted in hospital clinics. Two trials did not report settings. Trials durations ranged from 12 to 52 weeks. Most participants had moderate to severe disease. Mean age ranged from 64 years to 73 years, and more males were recruited (51% to 100%). Forced expiratory volume in one second (FEV₁) ranged from 0.935 to 1.36 L. Most participants had previous exacerbations. Data from 12 studies were included in the NMAs (3405 participants; 16 treatment arms including placebo). Prophylactic antibiotics evaluated were macrolides (azithromycin and erythromycin), tetracyclines (doxycyclines), quinolones (moxifloxacin) and macrolides plus tetracyclines (roxithromycin plus doxycycline).
Risk of bias and threshold analysis
Most studies were at low risk across domains, except detection bias, for which only seven studies were judged at low risk. In the threshold analysis for exacerbations, all comparisons in which one antibiotic was compared with another were robust to sampling variation, especially macrolide comparisons. Comparisons of classes with placebo were sensitive to potential bias, especially macrolide versus placebo, therefore, any bias in the comparison was likely to favour the active class, so any adjustment would bring the estimated relative effect closer to the null value, thus quinolone may become the best class to prevent exacerbations.
Exacerbations
Nine studies were included (2732 participants) in this NMA (exacerbations analysed as time to first exacerbation or people with one or more exacerbations). Macrolides and quinolones reduced exacerbations. Macrolides had a greater effect in reducing exacerbations compared with placebo (macrolides: hazard ratio (HR) 0.67, 95% credible interval (CrI) 0.60 to 0.75; quinolones: HR 0.89, 95% CrI 0.75 to 1.04), resulting in 127 fewer people per 1000 experiencing exacerbations on macrolides. The difference in exacerbations between tetracyclines and placebo was uncertain (HR 1.29, 95% CrI 0.66 to 2.41). Macrolides ranked first (95% CrI first to second), with quinolones ranked second (95% CrI second to third). Tetracyclines ranked fourth, which was lower than placebo (ranked third). Contributing studies were considered as low risk of bias in a threshold analysis.
Quality of life (SGRQ)
Seven studies were included (2237 participants) in this NMA. SGRQ scores improved with macrolide treatment compared with placebo (fixed effect‐fixed class effect: mean difference (MD) ‐2.30, 95% CrI ‐3.61 to ‐0.99), but the mean difference did not reach the minimally clinical important difference (MCID) of 4 points. Tetracyclines and quinolones did not improve quality of life any more than placebo, and we did not detect a difference between antibiotic classes.
Serious adverse events
Nine studies were included (3180 participants) in the NMA. Macrolides reduced the odds of a serious adverse event compared with placebo (fixed effect‐fixed class effect: odds ratio (OR) 0.76, 95% CrI 0.62 to 0.93). There was probably little to no difference in the effect of quinolone compared with placebo or tetracycline plus macrolide compared with placebo. There was probably little to no difference in serious adverse events between quinolones or tetracycline plus macrolide. With macrolide treatment 49 fewer people per 1000 experienced a serious adverse event compared with those given placebo. Macrolides ranked first, followed by quinolones. Tetracycline did not rank better than placebo.
Drug resistance
Ten studies reported drug resistance. Results were not combined due to variation in outcome measures. All studies concluded that prophylactic antibiotic administration was associated with the development of antimicrobial resistance.
Authors' conclusions
This NMA evaluated the safety and efficacy of different antibiotics used prophylactically for COPD patients. Compared to placebo, prolonged administration of macrolides (ranked first) appeared beneficial in prolonging the time to next exacerbation, improving quality of life, and reducing serious adverse events. No clear benefits were associated with use of quinolones or tetracyclines. In addition, antibiotic resistance was a concern and could not be thoroughly assessed in this review. Given the trade‐off between effectiveness, safety, and risk of antibiotic resistance, prophylactic administration of antibiotics may be best reserved for selected patients, such as those experiencing frequent exacerbations. However, none of the eligible studies excluded patients with previously isolated non‐tuberculous mycobacteria, which would contraindicate prophylactic administration of antibiotics, due to the risk of developing resistant non‐tuberculous mycobacteria.
Plain language summary
Prophylactic antibiotics for people with COPD
Review question
Which preventative antibiotic is effective and safe for reducing exacerbations, improving quality of life, and reducing serious side effects in people with COPD?
What is COPD?
COPD is a lung condition that can cause long‐term breathing problems. Symptoms include shortness of breath, cough, and sputum production. Flare‐ups (so‐called exacerbations) can be triggered by infection or inflammation, causing worsening symptoms and lung damage. Frequent exacerbations can lead to reduced quality of life and can increase the risk of death.
Why did we do this review?
We wanted to find out if one type of preventative antibiotic was better than another in reducing exacerbations, improving quality of life, and reducing side effects. We did this by using information from two previous reviews and comparing different antibiotics with each other, and with a control treatment (called placebo), by creating networks. As information was limited, the networks allowed us to combine information and determine the best preventative antibiotics by ranking them in order of ability to reduce exacerbations, improve quality of life, and reduce serious side effects.
What evidence did we find?
We tested three types of antibiotics: macrolides, quinolones, and tetracyclines. Macrolides were better in reducing exacerbations compared to control treatment. There was no clear difference in exacerbations when quinolone or tetracycline was compared with a control treatment. Tetracyclines were ranked lower than placebo in reducing exacerbations. We used the data for each antibiotic group to rank antibiotic groups in order of their ability to reduce exacerbations. We found that macrolides ranked first, followed by quinolones (second). Tetracyclines were ranked fourth and were not better than control treatment (ranked third).
Macrolides improved quality of life compared with control treatment. Quinolones did not appear to impact quality of life, and tetracyclines may have been associated with worsening quality of life compared to control treatment.
Macrolides were more effective in reducing serious unwanted events. There was no clear benefit for serious unwanted events with quinolone, tetracycline, or combined macrolide plus tetracycline compared with control treatment.
We could not clearly show benefit or harm of preventative antibiotic use for microbial resistance.
Quality of the evidence
We did not find any concerns about the ways in which studies were carried out, except that for some studies, people collecting the information knew (1) which patient was included in which treatment group, and (2) patient results when treatments were completed. Overall, the numerical information was robust and was unlikely to be influenced by differences noted between individual studies.
Conclusion
We found that exacerbations were reduced, quality of life was improved, and unwanted events were fewer with macrolides compared with control treatment. We could not determine whether quinolones or tetracyclines were of benefit compared with control treatment. Macrolides were ranked highest, followed by quinolones, which ranked second. Tetracyclines were no better than control treatment (ranked fourth and third, respectively). Although these NMAs show some benefit of using macrolides, they are based on a limited number of studies, and concerns remain about antibiotic resistance with long‐term use of antibiotics.
Summary of findings
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a common and preventable disease that is characterised by persistent respiratory symptoms and airflow obstruction, with or without alveolar abnormalities, usually caused by significant exposure to noxious particles or gases (GOLD 2020). Tobacco smoking is considered the main risk factor for COPD, but other factors, such as biomass fuel and air pollution, can also contribute to development of the disease. In addition, individuals with genetic abnormalities, abnormal lung development, and accelerated ageing are likely to be susceptible to COPD (GOLD 2020). Common respiratory symptoms include dyspnoea, cough with or without sputum production, and recurrent lower respiratory tract infection. People with COPD may experience intermittent worsening of symptoms, known as exacerbations. Exacerbations are associated with increased mortality (Soler‐Cataluña 2005), higher healthcare costs (Pasquale 2012), and a more rapid decline in lung function (Donaldson 2002), as well as negative impact on quality of life (Mathioudakis 2020; Seemungal 1998).
Description of the intervention
The ECLIPSE study has shown that frequent flare‐ups are associated with a moderate to severe COPD phenotype; as disease severity increases, the frequency of exacerbations also increases (Hurst 2010). One approach to reduce the frequency of exacerbations of COPD and reverse this potential ‘vicious cycle' of inflammation is the long‐term use of antibiotics to prevent exacerbations. Such antibiotics are usually given by mouth but can also be inhaled. Depending on the type of antibiotic, it can be taken daily or three times a week, or by ‘pulsed' administration (e.g. 'pulsed' antibiotic may be given daily for several days followed by a break) (Herath 2018).
Authors of a Cochrane Review investigated effects of macrolides and a quinolone compared with control treatment (Herath 2018). Long‐term use of antibiotics was associated with significantly fewer patients experiencing an exacerbation of COPD compared with those receiving control treatment. Patients on prophylactic antibiotics were more likely to experience adverse effects, such as hearing loss with azithromycin and gastrointestinal symptoms with moxifloxacin.
How the intervention might work
Effects of long‐term antibiotics are not completely understood. Antibiotics may offer both antibacterial and anti‐inflammatory effects (Martinez 2008), and therefore may reduce both bacterial load and inflammation as a result of exacerbations from bacteria, viruses, and environmental pollution. Studies have suggested that the lungs of people with COPD may be colonised with more pathogenic bacteria than are found in healthy lungs (Mathioudakis 2020; Sethi 2004). Bacteria are identified in the sputum of approximately 40% to 60% of people experiencing an acute exacerbation (Sethi 2004), and their overgrowth may be a precipitant of exacerbations (Sze 2014). Antibiotics may also reduce neutrophilic airway inflammation by reducing bacterial load, potentially providing clinical benefit (Siva 2014). Choice of prophylactic antibiotic may be guided by factors including clinician and patient preferences and prior experience, previously isolated bacteria, and side effect profiles. Organisms isolated from exacerbating patients include Haemophilus influenzae (11% of all patients), Streptococcus pneumoniae (10%), Moraxella catarrhalis (10%), Haemophilus parainfluenzae (10%), and Pseudomonas aeruginosa (4%) (Sapey 2006).
Prophylactic antibiotics may be of greatest benefit in a subset of patients (Mathioudakis 2017; Miravittles 2015). Compared to placebo, azithromycin (a macrolide antibiotic) reduces exacerbations most markedly in older patients, non‐smokers, and those not using oral or inhaled steroids at baseline (Albert 2011).
Why it is important to do this review
This Cochrane Review included a network meta‐analysis that will accompany the head‐to‐head pair‐wise meta‐analysis review of prophylactic antibiotics (Threapleton 2018); this was supplemented with the addition of antibiotic versus placebo data (Herath 2018). As comparisons of antibiotics for reducing exacerbations and improving quality of life for patients with COPD were limited, a network meta‐analysis (NMA) was important to identify which antibiotic was better for improving these outcomes.
Objectives
To assess effects of different prophylactic antibiotics on exacerbations, quality of life, and serious adverse events in people with COPD in three separate network meta‐analyses, and to provide rankings of identified antibiotics.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), regardless of language or publication status. We included trials of minimum 12 weeks' intervention duration. This duration was considered an appropriate minimum cut‐off to allow for evaluation of the impact of interventions on COPD exacerbations. We excluded cross‐over trials due to carry‐over effects. We did not identify any cluster‐randomised trials.
Types of participants
We included adults 18 years of age and older who had been diagnosed with COPD according to validated criteria (e.g. European Respiratory Society, American Thoracic Society, Global Initiative for Obstructive Lung Disease (GOLD) criteria). We included studies enrolling patients with COPD during a stable disease state, or during exacerbations, provided that antibiotics were administered long‐term, prophylactically. We included study populations with mild, moderate, severe, or very severe COPD according to the GOLD criteria for airflow limitation (GOLD 1: ≥ 80% predicted forced expiratory volume in one second (FEV₁); GOLD 2: 50% to 79%; GOLD 3: 30% to 49%; GOLD 4 < 30%). We anticipated that trials would likely recruit patients with moderate to severe or very severe COPD (GOLD stages 2 to 4). Most patients included in the studies had moderate to very severe COPD. Only one study included patients with mild COPD (5%), but this number was very small and was unlikely to affect our analyses. We included trials that recruited participants with or without a recent history of exacerbations and explored this as a potential source of heterogeneity. We excluded patients with the following co‐morbidities or characteristics: a primary diagnosis of bronchiectasis, asthma, or genetic disease, such as cystic fibrosis or primary ciliary dyskinesia.
Types of interventions
We included any prophylactic oral antibiotic classes given for at least 12 weeks continuously, intermittently (e.g. three times per week), or pulsed, in keeping with the linked pair‐wise meta‐analyses (Herath 2018; Threapleton 2018). Pulsed antibiotics must have been given for a minimum of five consecutive days every eight weeks.
We included trials in which participants had access to the following background treatments provided they were not part of the randomised study treatments.
Short‐acting and long‐acting bronchodilators.
Inhaled corticosteroids.
Oral corticosteroids.
Oxygen.
Pulmonary rehabilitation.
Smoking cessation interventions.
Any other standard treatment for COPD.
Types of outcome measures
Primary outcomes
COPD exacerbation* (we extracted data on time until first exacerbation, estimated using hazard ratios (HRs) as a preference, followed by rate ratio data and numbers of participants with one or more exacerbations)
Quality of life (St George's Respiratory Questionnaire (SGRQ))
All‐cause serious adverse events (number of participants with one or more adverse event)
Drug resistance/Microbial sensitivity (we did not perform NMA on this outcome, but we reported results for this outcome narratively)
Mortality (we anticipated that events would be rare, so we did not perform NMA on this outcome, but we reported results for this outcome narratively)
We reported endpoint data for dichotomous outcomes. Continuous outcomes were extracted, and we reported then at the closest time points to 6 months and 12 months.
*Moderate and severe exacerbations were defined as worsening of respiratory status requiring treatment with systemic corticosteroids and/or antibiotics; severe exacerbations were defined as requiring hospitalisation (see Table 4).
1. Characteristics of studies including prior exacerbation details.
| Author | Class comparison | Concomitant treatments (%, antibiotic/ placebo) | Dose/regimen | COPD severity | Included in NMA? | Exacberations in the previous 12 months before participation in study | Exacerbation definition | Risk of bias |
|
Albert 2011 (N = 1142); USA (12 academic health centres) 52 weeks |
Macrolide vs placebo | ICS only (4%/6%) LAMAs only (6%/8%) LABAs only (3%/1%) ICS + LAMA (19%/22%) ICS + LABA (4%/5%) LABA + LAMA (5%/4%) ICS + LABA + LAMA (49%/46%) None (10%/8%) |
AZM 250 mg daily continuous |
Moderate to severe FEV₁ 1.11 L |
Yes | Approximately 50% of participants in each treatment arm had required hospitalisation or an ED visit in the previous 12 months | Acute exacerbation of COPD: “a complex of respiratory symptoms (increased or new onset) of more than one of the following: cough, sputum, wheezing, dyspnoea, or chest tightness with a duration of at least 3 days requiring treatment with antibiotics or systemic steroids" | Low risk of bias across all domains except attrition (unclear reasoning of missing data for HRQoL) |
|
Banerjee 2005
(N = 67); UK (clinics and lung function units from 2 hospitals) 13 weeks |
Macrolide vs placebo | All participants: ICS (100%), LABAs (18%), inhaled anticholinergics (63%) |
CLR 500 mg daily continuous |
Moderate to severe | No | NR | Not included in exacerbation analysis | Low risk of bias across all domains except detection bias, which was unclear |
|
Berkhof 2013 (N = 84); Netherlands (1 teaching hospital) 12 weeks |
Macrolide vs placebo | LABAs (81%/80%) Long‐acting anticholinergics (64%/57%) ICS (98%/83%) |
AZM 250 mg 3 times a week Intermittent |
Moderate FEV₁ 1.36 L |
Yes | Participants had a median for 1 exacerbation (range 0 to 13) in the previous 12 months | Time to first exacerbation of COPD: sustained worsening of COPD, from stable state and beyond normal day‐to‐day variations, requiring treatment with prednisolone, antibiotics, or a combination of both | Unclear selection bias (allocation) but assumed done, low risk across all other domains |
|
Blasi 2010 (N = 22); Italy (multi‐centre) 26 weeks |
Macrolide vs placebo | Inhaled medication NR LTOT (46% in both groups) | AZM 500 mg 3 times a week Intermittent |
Severe FEV₁ (not reported) |
Yes | Participants in each treatment arm had a mean of 3 exacerbations in the previous 12 months | Worsening of symptoms requiring both a change in regular respiratory medication or medical assistance, or resulting in hospitalisation or ED treatment | Judged as high risk of bias for allocation concealment, performance, detection, attrition, and selective reporting; open‐label |
|
Brill 2015 (N = 99); UK (1 outpatient hospital department) 13 weeks |
Quinolone Tetracycline Macrolide vs placebo |
ICS (84%/76%/72%) ICS in placebo group 57% |
MOX 400 mg daily for 5 days every 4 weeks (pulsed) DOX 100 mg daily (continuous) AZM 250 mg 3 times a week (intermittent) |
Moderate to severe FEV₁ 1.4 L |
Yes | Participants had a mean of 2.5 (SD 2.1) exacerbations with moxifloxacin, 2.1 (SD 1.7) exacerbations with doxycycline, 2.8 (SD 4.0) exacerbations with azithromycin, and 1.5 (SD 1.4) exacerbations with placebo in previous 12 months |
Exacerbations during the study: using diary card criteria, patient reporting to clinical health professionals or study team. Exacerbation was not the primary outcome of the study |
Unclear performance bias; detection bias judged as high |
|
He 2010 (N = 36); China (1 university hospital) 26 weeks |
Macrolide vs placebo | ICS (44%/38%) Theophylline (61%/55%) Inhaled anticholinergic (50%/55%) Inhaled beta‐adrenergic (72%/77%) |
ERY 125 mg 3 times daily (continuous) | Severe FEV₁ 1.07 L at baseline |
Yes | NR |
Moderate exacerbation: sustained worsening of baseline respiratory symptoms for at least 2 days requiring increased treatment or additional therapy (e.g. OCS, antibiotics) Severe exacerbation: all of the above plus requiring hospital admission |
Randomisation and allocation unclear. Double‐blind study, but outcome assessment unclear. Funding not stated |
|
Mygind 2010 (N = 575); Denmark 156 weeks |
Macrolide vs placebo | NR | AZM 500 mg for 3 days every month (pulsed) | NR | No | NR | Not included in exacerbation analysis | Unclear randomisation, allocation concealment, attrition domains. Blinding of participants, personnel, and outcome assessors were judged as low risk of bias |
|
Seemungal 2008 (N = 109); UK (2 outpatient clinics in 2 hospitals) 52 weeks |
Macrolide vs placebo | ICS (77% in both groups) LABAs (66%/61%) LAMAs (28%/38%) Theophylline (7.5%/14%) |
ERY 250 mg twice daily (continuous) | Moderate to severe FEV₁ 1.31 L at baseline |
Yes | 35% of participants had 3 or more exacerbations in the previous 12 months |
Moderate exacerbation: sustained worsening of baseline respiratory symptoms for at least 2 days requiring treatment with OCS (prednisolone) and/or antibiotics Severe exacerbation: requiring admission to hospital |
Low risk of bias across all domains. Funded by British Lung Foundation |
|
Sethi 2010 (N = 1157); (international multi‐centre) 48 weeks |
Quinolone vs placebo |
SABAs (71%/72%) LABAs (44%/45%) ICS (41%/43%) Theophylline (29%/26%) Systemic steroids (0.4%/0.2%) Others (4.7%/5.7%) ICS + long‐acting bronchodilators (25%/26%) |
MOX 400 mg daily for 5 days every 8 weeks (pulsed) | Mild to severe FEV₁ 1.2 L at baseline |
Yes | NR |
Any confirmed AECB: requiring intervention (start of systemic antibiotic and/or start of systemic steroid and/or hospitalisation within 7 days of the start date of exacerbation) and with a minimum of 2 weeks between the start of 2 consecutive exacerbations |
Unclear risk for selection bias (random sequence generation and allocation concealment). Low risk for performance bias and selective reporting |
|
Shafuddin 2015 (N = 292); Australia and New Zealand (multi‐ centre) 12 weeks |
Macrolide Macrolide plus tetracycline vs placebo |
NR | ROX 300 mg daily (continuous) DOX + ROX 100 mg daily plus 300 mg daily (continuous) |
Moderate to severe FEV₁ 0.935 L at baseline |
Yes | Mean 5.11 (SD 2.4) exacerbations within 2 years | Not included in exacerbation analysis | Low risk of bias across all domain except attrition, which was unclear |
|
Simpson 2014 (N = 30); Australia (1 tertiary care respiratory and sleep ambulatory care service, hospital) 12 weeks |
Macrolide vs placebo | ICS (% NR) | AZM 250 mg daily (continuous) | Moderate | Yes | NR | Severe exacerbations of COPD: requiring unscheduled medical attention with treatment of OCS and/or antibiotics | Low risk of bias across all domains |
|
Singh 2019 (N = 60); India (1 outpatient department) 13 weeks |
Tetracycline vs placebo |
NR | DOX 100 mg daily (continuous) | Moderate to severe | No (sensitivity analysis) | NR | Not included in exacerbation analysis | Low risk of bias for allocation concealment, high risk of bias for blinding of participants, personnel, and outcome assessors. Randomisation and selective reporting domains were unclear |
|
Suzuki 2001 (N = 109); Japan (setting NR) 13 weeks |
Macrolide vs placebo |
NR | ERY 200 to 400 mg daily (continuous) | FEV₁ 1.47 L at baseline | No (sensitivity analysis) | NR | Not included in exacerbation analysis | Low risk of bias across most domains except for blinding of participants, personnel, and outcome assessors, which were judged as high risk of bias |
|
Tan 2016 (N = 49); China (1 regional hospital) 52 weeks |
Macrolide vs placebo |
ICS (44%/38%/44%) Theophylline (55%/55%/61%) Inhaled anticholinergic (55%/50%/50%) Inhaled beta2‐adrenergic agonist (66%/66%/72%) |
ERY 125 mg 3 times daily (continuous) ERY 125 mg 3 times daily with 6 months' follow‐up (continuous) |
Moderate to severe FEV₁ 1.04 to 1.08 L |
Yes | NR | Not included in exacerbation analysis | Unclear risk of bias across most domains, high risk of bias for blinding of participants, personnel, and outcome assessors |
|
Uzun 2014 (N = 92); Netherlands (1 regional hospital) 52 weeks |
Macrolide vs placebo |
LABA (96%/91%) LAMA (89%/71%) ICS (89%/96%) SABA (68%/73%) Prednisolone (28%/20%) |
AZM 500 mg 3 times a week (intermittent) | Mild to severe FEV₁ 1.1 L at baseline |
Yes | Participants had a mean of 4 (SD 1.1) acute exacerbations in the previous 12 months | All exacerbations: defined according to Anthonisen criteria, and whether the patient needed treatment with steroids or antibiotics, or both. Severe exacerbation: requiring hospital admission. Mild exacerbation: requiring treatment at the outpatient department |
Low risk of bias across all domains |
|
Vermeersch 2019 (N = 301); Italy (5 centres across Italy) 13 weeks |
Macrolide vs placebo |
LABA (93%/94%) LAMA (80%/80%) ICS (80%/80%) SABA (73%/71%) |
AZM 500 mg once daily (loading dose) for 3 days, followed by 250 mg every 2 days for 13 weeks (intermittent) | FEV₁ 0.925 L | Yes | NR | Not included in exacerbation analysis | Low risk of bias across all domains |
|
Wang 2017 (N = 86); China (1 regional hospital) 26 weeks |
Macrolide vs placebo |
NR | AZM 250 mg once daily plus 20 mg once daily simvastatin (continuous) | FEV₁ 0.67 L | No | NR | Not included in exacerbation analysis | Low risk of bias for randomisation, high risk of bias for blinding of participants, personnel, and outcome assessors |
Abbreviations
AECB: acute exacerbation of chronic bronchitis; AZM: azithromycin; CLR: clarithromycin; COPD: chronic obstructive pulmonary disease; DOX: doxycycline; ED: emergency department; ERY: erythromycin; FEV₁: forced expiratory volume in one second; HRQoL: health‐related quality of life; ICS: inhaled corticosteroid; LABA: long‐acting beta agonist; LAMA: long‐acting muscarinic antagonist; LTOT: long‐term oxygen therapy; MOX: moxifloxacin; NMA: network meta‐analysis; NR: not reported; OCS: oral corticosteroids;ROX: roxithromycin; SABA: short‐acting beta agonist; SD: standard deviation.
Search methods for identification of studies
Electronic searches
We updated the searches for both Herath 2018 and Threapleton 2018.
For this NMA, we identified studies from the Cochrane Airways Trials Register, which is maintained by the Information Specialist of the Cochrane Airways Group. We carried out the first search in October 2018 and updated it on 22 January 2020. At the time of this review, the Cochrane Airways Trials Register contained studies identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, through the Cochrane Register of Studies (inception to Issue 12; 2019);
weekly searches of MEDLINE Ovid SP (1946 to January 2020);
weekly searches of Embase Ovid SP (1974 to January 2020);
monthly searches of PsycINFO Ovid SP (1967 to January 2020);
monthly searches of the Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCO; 1937 to January 2020);
monthly searches of the Allied and Complementary Medicine Database (AMED EBSCO; inception to January 2020); and
handsearches of major respiratory conference proceedings.
Studies contained in the Cochrane Airways Trials Register were identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings, are presented in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We searched the following trials registries.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 22 January 2020).
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 22 January 2020).
We searched the Cochrane Airways Trials Register and additional sources with no restriction on language or type of publication.
Searching other resources
For this NMA, we checked the reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for study information.
On 21 August 2020, we searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
This review was built on two existing Cochrane Reviews (Herath 2018; Threapleton 2018), in which data from included studies in each of the reviews had already been extracted by two pairs of independent review authors. For studies already identified from the two existing Cochrane Reviews that reported exacerbations outcome data, we checked and extracted hazard ratio data if these data were available. New studies that were not included in Herath 2018 and Threapleton 2018 were selected, and data were extracted as outlined below.
Selection of studies
Two review authors (SJ and CT) independently screened the titles and abstracts of search results and coded them as either ‘retrieve' (eligible or potentially eligible/unclear) or ‘do not retrieve'. We retrieved the full‐text study reports of all potentially eligible studies. Two review authors (SJ and CT) independently assessed these for inclusion and recorded the reasons for exclusion of ineligible studies. We selected studies that evaluated clinical efficacy and safety of any prophylactic antibiotic treatments in patients with COPD (e.g. macrolides/quinones, macrolides/tetracyclines, quinones/tetracyclines, combined macrolide plus tetracycline/macrolide). We resolved any disagreement through discussion or, if required, we consulted a third review author (RF). We identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies tables (Moher 2009).
Data extraction and management
We used Microsoft Excel to manage outcome data for the NMA, which we had piloted on at least one trial included in the review. One review author (SJ) extracted the following study characteristics from included trials that had not already been included in the two existing Cochrane Reviews (Herath 2018; Threapleton 2018).
Methods: study design, total duration of study, details of any ‘run‐in' period, number of study centres and locations, study settings, withdrawals, dates of study.
Participants: N, mean age, age range, gender, severity of COPD, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, exclusion criteria, previous history of exacerbations.
Interventions: intervention, comparison, concomitant medications, excluded medications.
Outcomes: primary and secondary outcomes specified and collected, time points reported.
Notes: funding for studies, notable conflicts of interest of trial authors.
Two review authors (SJ and CT) independently extracted outcome data from included trials that had not already been identified by the two existing Cochrane Reviews (Herath 2018; Threapleton 2018), which they managed in Microsoft Excel. We noted in the Characteristics of included studies table if outcome data were not reported in a useable way. We resolved disagreements by reaching consensus or by involving a third review author (RF). We double‐checked that data were entered correctly by comparing data presented in the systematic review against study reports. A second review author (CT) spot‐checked study characteristics for accuracy against the study report. We recorded on the data extraction sheet data extracted from previous Cochrane Reviews that were relevant for this NMA.
Assessment of risk of bias in included studies
Studies that had been identified from Herath 2018 and Threapleton 2018 had previously been assessed for risk of bias by two pairs of independent review authors. Trials that were not already included in Herath 2018 and Threapleton 2018, were assessed for risk of bias as outlined below.
Two review authors (SJ and CT) independently assessed risk of bias for each included trial using the criteria outlined in the recently updated Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2018). We resolved disagreements by discussion or by consultation with a third review author (RF). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We judged each potential source of bias as ‘high', ‘low', or ‘unclear' and provided a quote from the study report together with a justification for our judgement in the ‘Risk of bias' table. We summarised ‘Risk of bias' judgements across different trials for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a trial author, we noted this in the ‘Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for trials that contributed to those outcomes.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and justified any deviations from it in the Differences between protocol and review section of this systematic review.
Measures of treatment effect
Direct pair‐wise meta‐analysis methods
Briefly, two published Cochrane Reviews outlined the pair‐wise meta‐analyses from prophylactic versus placebo‐controlled trials and head‐to‐head antibiotic trials (Herath 2018; Threapleton 2018). These reviews were conducted by standard Cochrane methods. Dichotomous data were analysed as odds ratios and continuous data as mean differences (MDs) or standardised mean differences (SMDs). Data in Threapleton 2018 were insufficient for review authors to conduct meta‐analyses; however, pooled results in Herath 2018 with dichotomous variables were expressed as a random effects model odds ratio (OR) with 95% CI. Rate data were combined (e.g. number of exacerbations per participant per year) via generic inverse variance (GIV) and were expressed as a rate ratio (Herath 2018).
NMA methods
We conducted an NMA of clinical trials to compare all prophylactic antibiotics with each other and with placebo. Bayesian Markov chain Monte Carlo method was implemented in OpenBUGS 3.2.3 (Lunn 2009). We used a hierarchical model with classes of antibiotics composed of individual treatments, which allowed each treatment effect and the overall class mean to be estimated (Dias 2018; Kew 2014).
We combined dichotomous data that took into account exposure time with rate and hazard ratio data for the exacerbations outcome; dichotomous data were combined with hazard ratio or rate ratio data with the assumption that all exacerbations occurred at the same rate (i.e. a patient is not more likely to have a second exacerbation if he or she has had a previous exacerbation). This was done by using a shared parameter model in OpenBUGS, whereby data on the log hazard ratio of exacerbations were modelled with normal likelihood and an identity link. Dichotomous data on the number of patients with at least one exacerbation were modelled using a binomial likelihood with a cloglog link (Dias 2018). Depending on availability, we extracted hazard ratios as a preference because they accounted for time at risk and censoring. We pooled other dichotomous outcomes as odds ratios. We used mean differences for continuous outcomes.
Prior distributions
For all models, vague prior distributions were used for all trial baselines and for relative treatment or class effects (normal(0,100²)). For random treatment effects models, a minimally informative uniform prior distribution was used for the between‐study heterogeneity parameter, with lower limit of zero and upper limit of 5 for exacerbations and serious adverse events (SAEs), and upper limit of 15 for SGRQ. For exchangeable‐class models, a Uniform(0, 5) prior distribution was used for the within‐class standard deviation.
Where the number of studies per comparison is small (usually less than 5), empirically informative prior distributions for the heterogeneity parameter are recommended (Rhodes 2015; Turner 2015). In order to assess sensitivity of results of random‐treatment effects model to the the prior distribution for the heterogeneity, results using empirically based prior distributions were also presented for the change for baseline in SGRQ and SAE outcomes. No empirically based prior distributions were available for outcomes on the log‐hazard ratio scale, so these were not considered for the exacerbations outcome. Therefore, when few studies were available in all comparisons for the exacerbations outcome, a half‐normal prior distribution that expressed the prior belief that 95% of trials would give hazard ratios within a factor of 2 from the estimated median hazard ratio was considered: half‐N(0, 0.32²) (Dias 2018). For exchangeable or fixed class models, the minimally informative uniform prior distribution was used (Turner 2015).
In the random treatment effects model for the change from baseline in SGRQ, we used the empirically based t‐distribution for the log of the between‐study variance for comparisons of pharmacological therapies to placebo for quality of life outcomes in respiratory diseases (t(‐5.07, 2.512, 5) as reported by Rhodes 2015. Because this prior distribution was presented in Rhodes 2015 on a standardised mean difference scale, it was converted to the mean difference scale by multiplying by 14, which is approximately the standard deviation of the SGRQ scale in patients with COPD (Puhan 2006).
For the random treatment effects model for SAE, we used the empirically based log‐normal distribution for the between‐trial variance (LN(‐1.87, 1.522)), as reported by Turner 2015.
Fixed‐class models were chosen for these outcomes (for which there were comparisons with more than 5 studies), and minimally informative uniform prior distributions were used.
Model fit and choice
We chose a model and considered it as the primary analysis for NMAs using the following strategy.
Start with fixed class models (with random and fixed treatment effects). If both fit well (i.e. posterior mean of residual deviance is close to the number of data points), choose the model with the lowest deviance information criterion (DIC) (if the difference is less than 3, choose the fixed effect model) and stop.
If the fixed treatment effect‐fixed class model does not fit well, try the fixed treatment effect‐random class model – assess fit, compare to models in the first step here, and choose the model with the lowest DIC.
If neither of the models in the first or second step fit well, try also random treatment effects with random class model. Choose a final model based on DIC, but interpret with caution if model fit is poor.
Compare results of random class models to the equivalent treatment level model (i.e. no class), if networks are connected.
Threshold analysis
A contrast level threshold analysis was performed to examine the impact of bias on each treatment contrast (Phillippo 2018; Phillippo 2019). Thresholds are provided that quantify how much the evidence could change (due, for example, to potential biases, or simply sampling variation) before the best treatment changes, and what the revised ‘best’ treatment would be. If it is judged that the evidence could not plausibly change by more than this amount, then the ‘best’ treatment choice is considered robust; otherwise, this choice is sensitive to plausible changes in the evidence.
Unit of analysis issues
Pair‐wise analysis
Herath 2018 and Threapleton 2018 reported pair‐wise data. Neither Herath 2018 nor Threapleton 2018 identified any cross‐over or cluster‐randomised trials. Threapleton 2018 used participants rather than events as the unit of analysis (i.e. the number of people admitted to hospital, rather than the number of admissions per person).
NMA
For dichotomous outcomes, participants were used as the unit of analysis to eliminate risk of multiple counting of participants (i.e. number of COPD patients with one exacerbation). If exacerbation data were provided as rate ratios or HRs, these data were extracted and analysed accordingly. Data from cluster‐randomised trials were planned to be included provided the data had been, or could be, adjusted to take clustering into account.
Dealing with missing data
For both pair‐wise and NMA missing data, investigators or trial sponsors were contacted to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a trial is identified as an abstract only). When this was not possible and the missing data were thought to introduce serious bias, we planned to perform a sensitivity analysis to determine whether the missing data could introduce serious bias to the overall results of the NMA (Guyatt 2017). When possible, we used intention‐to‐treat (ITT) data from randomly assigned participants.
Assessment of heterogeneity
Pair‐wise meta‐analysis
Herath 2018 tested for heterogeneity when CIs did not overlap with each other. The I² statistic was used to measure heterogeneity among the studies in each analysis. When we identified heterogeneity (I² ≥ 40%), we explored this using a pre‐specified subgroup analysis. We used the following overlapping cut‐off to define heterogeneity (Higgins 2011).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
As Threapleton 2018 identified insufficient studies for meta‐analysis, the I² statistic was not used to measure heterogeneity nor to perform pre‐specified subgroup analyses.
NMA
Assessment of similarity of participants, interventions, and trial methods
We assessed clinical similarity of studies and statistical consistency (when possible). Note that incoherence, transitivity, and the presence of effect modifiers all relate to the same issue of consistency, which was addressed clinically and statistically (when possible).
Assessment of heterogeneity and statistical consistency in the network meta‐analysis
For the NMA, we assessed consistency by comparing the model fit and between‐trial heterogeneity from NMA models versus those from an unrelated effects (inconsistency) model (Dias 2013a; Dias 2013b). We would use this to determine the presence and area of inconsistency. In networks for exacerbations and SGRQ, all loops were formed by a single multi‐arm study (Brill 2015). For the SAE network, all loops were formed by one study (Shafuddin 2015). Therefore, there was no potential to detect inconsistency in these networks, and inconsistency checks were not carried out.
We planned to qualitatively compare results from direct pair‐wise meta‐analysis versus NMA estimates to check for broad agreement.
Assessment of reporting biases
Pair‐wise meta‐analysis
Both Herath 2018 and Threapleton 2018 aimed to pool data if they identified more than 10 studies, and to examine a funnel plot to explore possible small‐study and publication biases. Threapleton 2018 did not explore reporting bias, as review authors identified insufficient studies. Herath 2018 attempted to contact study authors to ask for missing data when reporting bias was suspected.
NMA
We aimed to minimise reporting bias from unpublished trials or selective outcome reporting by using a broad search strategy, and by checking references of included trials and relevant systematic reviews. For each outcome, we estimated and presented the proportion of trials that contributed to the NMA. When possible, we aimed to combine data reported as HR, rate ratio, or number of participants with, for example, at least one exacerbation to minimise reporting bias.
Data synthesis
Pair‐wise meta‐analysis
Herath 2018 subgrouped all meta‐analyses by regimen (continuous (daily), intermittent (two or three times per week), or pulsed (daily for five days every four weeks)). Meta‐analysis was performed only when study populations were sufficiently similar for pooling to make sense (Herath 2018). As Threapleton 2018 identified insufficient studies, it was not possible to perform meta‐analyses.
NMA
We considered all treatment dosages as individual treatments. We used a class model approach for the NMA (Dias 2018; Kew 2014). We pre‐specified five classes of interventions in the network: macrolides (e.g. azithromycin, erythromycin, roxithromycin), quinolones (ciprofloxacin, moxifloxacin), tetracyclines (doxycycline), combined tetracyclines/macrolides (e.g. roxithromycin/doxycyline), and placebo. We compared models that assumed all interventions within a class had the same effect to models for which effects within a class were exchangeable (i.e. similar) using the deviance information criterion (DIC) and taking into account any changes in estimated heterogeneity. We presented estimates for within‐class variability in treatment effects, as well as between‐class variability in treatment effects, when applicable. We also presented the ranking of each class in one of the five positions (from best to worst).
Subgroup analysis and investigation of heterogeneity
Pair‐wise meta‐analyses
Herath 2018 planned to carry out subgroup analysis for the primary outcome (number of exacerbations) by exploring severity of COPD according to FEV₁ and GOLD criteria, type of antibiotic, duration of antibiotic use, year of conduct of study, whether the antibiotic was used primarily as an antimicrobial or anti‐inflammatory agent, treatment regimen (dose, frequency, route of administration), and history of exacerbations. Threapleton 2018 explored exacerbation history and COPD severity in studies with 70% or more on long‐acting beta‐adrenoceptor agonist/long‐acting muscarinic antagonist/inhaled corticosteroid (LABA/LAMA/ICS) at baseline versus those with less than 70% on LABA/LAMA/ICS at baseline.
NMA
We planned to undertake a flexible and exploratory approach to investigate heterogeneity, depending on the data found. In the event of significant heterogeneity in the NMA, we considered exploring heterogeneity using pre‐specified factors, if extractable.
Exacerbation history: trials that recruit participants with a group mean < 1 versus 2 to 3 or 4 or more exacerbations in the preceding year.
COPD severity: participants predominantly classed as GOLD 1 or 2 versus those predominantly classed as GOLD 3 or 4.
Trials with ≥ 70% of participants on long‐acting beta‐agonists (LABAs) or long‐acting muscarinic receptor agonists (LAMAs) or inhaled corticosteroids (ICSs) at baseline.
Pseudomonas colonisation: trials that recruited participants colonised with Pseudomonas at baseline versus those not colonised with Pseudomonas at baseline.
Methodological issues with randomisation, allocation concealment, participant/personnel blinding, outcome assessor blinding, and attrition.
If data were insufficient for assessment of the pre‐specified factors, we planned to investigate differences (if any) by extracting key severity criteria for each trial, and to summarise data across pair‐wise comparisons.
Sensitivity analysis
Pair‐wise analyses
Herath 2018 and Threapleton 2018 planned to conduct a sensitivity analysis on the primary outcome (people with one or more exacerbations) by removing studies at high risk or unclear risk of sequence generation, allocation concealment, or blinding. Threapleton 2018 also planned to remove cross‐over studies. Herath 2018 used a random effects model for outcome measures.
NMA
We performed sensitivity analyses by primarily excluding from the main analysis studies that were at high risk of bias, then including these studies in a sensitivity analysis.
Reporting biases
We did not investigate reporting bias, as studies were too few for a contour‐adjusted funnel plot to be prepared.
Summary of findings and assessment of the certainty of the evidence
‘Summary of findings' tables were created for the following outcomes: exacerbations, quality of life (SGRQ) and serious adverse events. Judgement of the quality of the evidence was based on the ‘Risk of bias' assessment of included trials, estimates of heterogeneity, and assessment of model fit inconsistency.
Results
Description of studies
Results of the search
Pair‐wise meta‐analysis results
Herath 2018 included nine new studies from a search of 265 additional references for the 2018 update. The previous version of the review included seven studies, resulting in a total of 16 studies included in the review (Albert 2011; Banerjee 2005; Berkhof 2013; Brill 2015; He 2010; Mygind 2010; NCT00524095; NCT02628769; Seemungal 2008; Sethi 2010; Shafuddin 2015; Simpson 2014; Suzuki 2001; Tan 2016; Uzun 2014; Wang 2017). Fourteen studies (N = 3932 participants) were included in the pair‐wise meta‐analyses (Herath 2018).
Threapleton 2018 included for analysis two eligible studies (N = 391) from a search of 1415 references (Brill 2015; Shafuddin 2015).
Further details about the study characteristics of both reviews can be found in Characteristics of included studies, and a summary of the results can be found in Appendix 3. From this point onwards, we will describe the results of the NMA only.
NMA results
We identified 1120 records through database searching, which included the original search in 2018 and updated searches in 2019 and January 2020. We screened all 1120 records in the absence of any duplicate records. We excluded 1052 records on the basis of titles and abstracts, which resulted in 68 full texts to be assessed for eligibility. From the full‐text assessment, we identified 38 manuscripts, reporting on 17 studies that were eligible to be included in the review. The PRISMA flow diagram shows how the final selection of studies was made (Figure 1).
1.
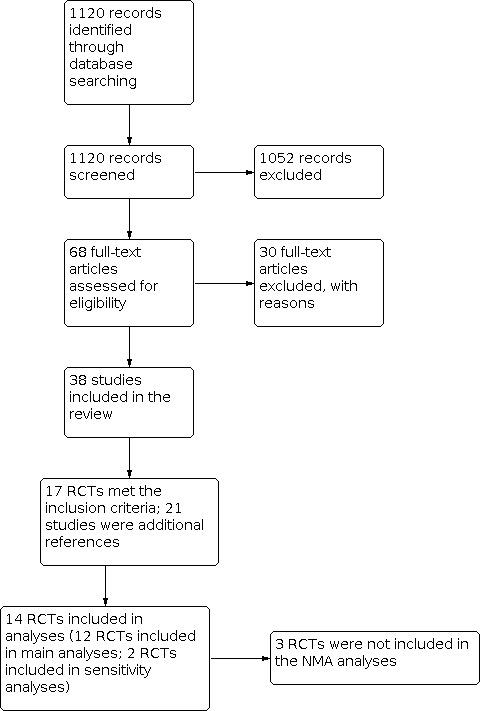
Study flow diagram.
Included studies
NMA included studies
We identified 17 trials that were eligible for inclusion in this review (Albert 2011; Banerjee 2005; Berkhof 2013; Blasi 2010; Brill 2015; He 2010; Mygind 2010; Seemungal 2008; Sethi 2010; Shafuddin 2015; Simpson 2014; Singh 2019; Suzuki 2001; Tan 2016; Uzun 2014; Vermeersch 2019; Wang 2017). Details of each study can be found in Characteristics of included studies. Of these, two were multi‐arm studies from which we could make direct comparisons among all included antibiotic classes (Brill 2015; Shafuddin 2015). We also included 12 studies that compared a single antibiotic with placebo (Albert 2011; Berkhof 2013; Blasi 2010; He 2010; Seemungal 2008; Sethi 2010; Simpson 2014; Singh 2019; Suzuki 2001; Tan 2016; Uzun 2014; Vermeersch 2019).
Of the 17 trials, three studies were not included in the NMA (Banerjee 2005; Mygind 2010; Wang 2017). Banerjee 2005 reported SGRQ symptom score in a format that could not be included in the network; therefore, this was reported as a pair‐wise analysis. Mygind 2010 was a conference abstract for which we could not obtain any further data when we contacted study authors. Wang 2017 did not include data relevant to our outcome criteria.
Two of the studies were not eligible for inclusion in the main NMA analyses but were included in sensitivity analyses (Simpson 2014; Singh 2019). Therefore, of the 12 studies included in the main NMA analysis, a total of 3405 participants with a diagnosis of COPD were randomly assigned to 16 treatment arms of interest (including placebo) (Table 5).
2. Treatments and corresponding abbreviations and classes.
| Treatment | Abbreviation | Class |
| Placebo | Pbo | NA |
| Azithromycin 250 mg once daily | AZM250 once daily | Macrolide |
| Azithromycin 250 mg once daily 3 times per week | AZM250 once daily (3x weekly) | Macrolide |
| Azithromycin 500 mg once daily 3 times per week | AZM500 once daily (3x weekly) | Macrolide |
| Azithromycin 500 mg once daily 3 times per month | AZM500 once daily (3x monthly) | Macrolide |
| Azithromycin 500 mg once daily (for first 3 days), azithromycin 250 mg every 2 days (intermittent) for rest of treatment duration |
AZM500 once daily (3 days) then AZM250 (alternating day days) |
Macrolide |
| Clarithromycin 500 mg once daily | CLR500 once daily | Macrolide |
| Erythromycin 250 mg three times daily | ERY250 three times daily | Macrolide |
| Erythromycin 250 mg twice daily | ERY250 twice daily | Macrolide |
| Erythromycin 125 mg 3 times daily | ERY125 three times daily | Macrolide |
| Erythromycin 200 to 400 mg once daily | ERY200/400 once daily | Macrolide |
| Roxithromycin 300 mg once daily | ROX300 once daily | Macrolide |
| Doxycycline 100 mg once daily | DOX100 once daily | Tetracycline |
| Roxithromycin 300 mg once daily + Doxycycline 100 mg once daily |
ROX300 once daily + DOX100 once daily |
Macrolide + tetracycline |
| Moxifloxacin 400 mg once daily (5 days every 4 weeks) |
MOX400 once daily (5 days every 4 weeks) |
Quinolone |
| Moxifloxacin 400 mg once daily (5 days every 8 weeks) |
MOX400 once daily (5 days every 8 weeks) |
Quinolone |
Abbreviations
AZM: azithromycin; CLR: clarithromycin; ERY: erythromycin; DOX: doxycycline; MOX: moxifloxacin; NA: not applicable; Pbo: placebo; ROX: roxithromycin.
Baseline characteristics of participants in trials included in the NMA
We found that baseline characteristics of trial populations were fairly similar across all studies, and most trial participants had moderate to severe disease. Mean age was similar, ranging from 64 years to 73 years, and considerably more males than females were recruited. Lung function, specifically mean FEV₁, ranged from 0.935 to 1.36 L. Mean pack‐years ranged from 36 to 59 across all studies, and overall there were no serious concerns that there was any imbalance in characteristics expected to modify relative treatment effects. The mean number of exacerbations among trial participants in the 12 months before trial start ranged from two to five (Blasi 2010; Brill 2015; Seemungal 2008; Shafuddin 2015; Uzun 2014; Table 4). Berkhof 2013 reported a median of one exacerbation in the previous 12 months among study participants. In Albert 2011, 50% of study participants were hospitalised or visited the emergency department 12 months before the trial start.
Characteristics of interventions in the NMA
Studies included in the NMA
Across the 12 studies included in the NMA, 15 antibiotics were evaluated and categorised into four classes: macrolides, tetracycline, quinolone, and macrolide plus tetracycline. A description of all studies is found in Characteristics of included studies and in Table 5.
Excluded studies
Fourteen excluded studies are listed in the Characteristics of excluded studies table, along with reasons for exclusion.
Risk of bias in included studies
Judgements for risk of bias and reasons can be found in the Characteristics of included studies table, and an overview of judgements for risk of bias can be found in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Of the included studies, 12 were judged as having low risk of bias for randomisation sequence generation. We judged five studies as having unclear risk, as they did not report methods for the randomisation process (He 2010; Mygind 2010; Sethi 2010; Singh 2019; Tan 2016).
Allocation concealment
We assessed 10 studies as having low risk of bias for allocation concealment. Seven studies were judged as having unclear risk (Berkhof 2013; Blasi 2010; He 2010; Mygind 2010; Sethi 2010; Tan 2016; Wang 2017), as no further information about the treatment allocation process was provided.
Blinding
Blinding of participants and personnel
Eleven studies were judged as having low risk of performance bias (Albert 2011; Banerjee 2005; Berkhof 2013; He 2010; Mygind 2010; Seemungal 2008; Sethi 2010; Shafuddin 2015; Simpson 2014; Uzun 2014; Vermeersch 2019). Blasi 2010, Singh 2019, Suzuki 2001, and Wang 2017 were open‐label trials and therefore were judged to be at high risk of bias. Tan 2016 did not provide any information about blinding of participants or personnel; therefore, we assumed that this was an open‐label study. Brill 2015 was judged as having unclear risk of bias for this domain because it is not clear whether study personnel were blinded.
Blinding of outcome assessors
Seven studies were judged as having low risk of bias for outcome assessment (Albert 2011; Berkhof 2013; Seemungal 2008; Shafuddin 2015; Simpson 2014; Uzun 2014; Vermeersch 2019). Three studies were rated as having high risk of bias (Blasi 2010; Singh 2019; Suzuki 2001), as they were open‐label trials with no documentation regarding blinding of outcome assessors. Brill 2015, Tan 2016, and Wang 2017 were also judged as having high risk of bias, as blinding of outcome assessors was not described in either study. No further information about blinding in this domain was described; therefore, Banerjee 2005, He 2010, Mygind 2010, and Sethi 2010 were judged as having unclear risk of bias.
Incomplete outcome data
Thirteen studies provided adequate descriptions of outcomes of study participants (Albert 2011; Banerjee 2005; Berkhof 2013; Brill 2015; He 2010; Seemungal 2008; Shafuddin 2015; Simpson 2014; Singh 2019; Suzuki 2001; Tan 2016; Uzun 2014; Vermeersch 2019), as they had described the flow of participants throughout the trial using a CONSORT diagram or by including this information in a specific paragraph or table. Blasi 2010 was judged as having high risk of bias because the analysis was not intention‐to‐treat, and participants who died were not included in the analysis. This may have led to an overestimation of beneficial outcomes.
Withdrawal rates in treatment and control arms of most studies were similar, with the exception of four studies that were judged as having unclear risk of bias (Albert 2011Sethi 2010; Shafuddin 2015; Tan 2016). Albert 2011 and Sethi 2010 did not report reasons for missing health‐related quality of life data. Shafuddin 2015 was judged as having unclear risk of bias for this domain, as more participants dropped out of the combined antibiotic treatment arm compared to the single antibiotic and placebo arms, although all participants were included in the intention‐to‐treat analysis. Tan 2016 was judged as having unclear risk of bias, as details of the number of people analysed at each time point were not reported.
Mygind 2010 provided limited information, as it was a conference abstract of unpublished data; therefore we judged this source as having unclear risk of bias. Wang 2017 did not provide any further information about missing data; therefore we judged this study as having unclear risk of bias for this domain.
Selective reporting
We judged 13 studies as having low risk of bias for this domain (Albert 2011; Banerjee 2005; Berkhof 2013; Brill 2015; He 2010; Mygind 2010; Seemungal 2008; Sethi 2010; Shafuddin 2015; Simpson 2014; Suzuki 2001; Uzun 2014; Vermeersch 2019). We judged Blasi 2010 as having high risk as outcomes in the publication were reported differently from those in the protocol on the trial registry website. SAEs were reported only in the antibiotic arm and not in the control arm; therefore it is not clear whether participants in the control group had any SAEs. We also judged Wang 2017 as having high risk of bias, as no prospective trial registration or protocol was identified, and dyspnoea grade was reported as measured in the abstract but there was no description in the methods or results of the publication. We judged two studies as having unclear risk of bias (Singh 2019; Tan 2016), as it was not clear if the outcomes were reported as planned.
No prospective trial registration or protocol was identified.
Other potential sources of bias
We did not consider industry sponsorship as necessarily increasing the risk of bias when studies were well designed. We did not identify any other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Summary of findings: exacerbations.
| Prophylactic antibiotics compared with placebo for COPD | ||||
|
Patients or population: adults with COPD Settings: hospital clinics, multi‐centre Intervention: macrolide, tetracycline, or quinolone Comparison: placebo or standard care | ||||
| Treatment | Anticipated absolute effects (95% CrI)* | Relative effect HR (95% CrI) | No. of participants (studies) | |
| Absolute rate of exacerbations: median (95% CrI) |
Risk difference with treatment (number of people experiencing exacerbations) |
|||
|
Macrolide (weighted mean 50 weeks' duration) |
1.34 (1.19 to 1.50) | 127 fewer per 1000 (168 fewer to 87 fewer) | 0.67 (0.60 to 0.75) | 688 (6) |
|
Tetracycline (13 weeks' duration) |
2.58 (1.33 to 4.81) | 60 more per 1000 (129 fewer to 127 more) | 1.29 (0.66 to 2.41) | 25 (1) |
|
Quinolone (weighted mean 46.5 weeks' duration) |
1.77 (1.50 to 2.08) | 35 fewer per 1000 (87 fewer to 11 more) | 0.89 (0.75 to 1.04) | 594 (2) |
| *The basis for the anticipatedrisk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CrI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). COPD: chronic obstructive pulmonary disease; CrI: credible interval; HR: hazard ratio. | ||||
*Absolute rate of exacerbations per year in the placebo arm = 2; 864 people per 1000 experienced exacerbations over a year.
Summary of findings 2. Summary of findings: change from baseline in SGRQ.
| Prophylactic antibiotics compared with placebo for COPD | |||
|
Patients or population: adults with COPD Settings: hospital clinics, multi‐centre Intervention: macrolide, tetracycline, or quinolone Comparison: placebo | |||
| Treatment | Anticipated absolute effects (95% CrI)* | No. of participants (studies) | |
| Absolute change from baseline in SGRQ (95% CrI) | Mean difference in change from baseline in SGRQ score with treatment** | ||
|
Macrolide (weighted mean 48 weeks' duration) |
‐4.00 (‐5.51 to ‐2.68) | 2.298 point improvement (3.605 to 0.985 point improvement) | 578 (6) |
|
Tetracycline (13 weeks' duration) |
‐0.52 (‐3.21 to 2.16) | 1.179 point worsening (1.509 point improvement to 3.859 point worsening) | 25 (1) |
|
Quinolone (weighted mean 46.5 weeks' duration) |
‐3.03 (‐4.69 to ‐1.37) | 1.33 point improvement (2.986 point improvement to 0.328 point improvement) | 528 (2) |
| *The basis for the anticipatedrisk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CrI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). COPD: chronic obstructive pulmonary disease; CrI: credible interval; SGRQ: St George's Respiratory Questionnaire. | |||
*Absolute change from baseline in the placebo arm was ‐1.7 (1.7 point improvement).
**The minimally clinically important difference for SGRQ is 4 points.
Summary of findings 3. Summary of findings: serious adverse events.
| Prophylactic antibiotics compared with placebo for COPD | ||||
|
Patients or population: adults with COPD Settings: hospital clinics, multi‐centre Intervention: macrolide, tetracycline, or quinolone Comparison: placebo | ||||
| Treatment | Anticipated absolute effects (95% CrI)* | Relative effect OR (95% CrI) | No. of participants (studies) | |
| Absolute probability of an SAE: median (95% CrI) | Risk difference with treatment* | |||
| Macrolide (weighted mean 49 weeks' duration) |
0.21 (0.18 to 0.25) | 49.07 fewer per 1000 (81.18 fewer to 14.23 fewer) | 0.76 (0.62 to 0.93) | 971 (8) |
| Quinolone (48 weeks' duration) |
0.26 (0.20 to 0.32) | 1.873 fewer per 1000 (57.88 fewer to 60.89 more) | 1.00 (0.72 to 1.34) | 569 (1) |
| Macrolide + tetracycline (12 weeks' duration) |
0.25 (0.15 to 0.37) | 9.461 fewer per 1000 (1.07 fewer to 108.5 more) | 0.97 (0.52 to 1.66) | 101 (1) |
| *The basis for the anticipatedrisk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CrI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CrI). COPD: chronic obstructive pulmonary disease; CrI: credible interval; OR: odds ratio. | ||||
*Absolute probability of events in the placebo arm was 0.26; risk of an SAE with placebo was 260 per 1000.
Network meta‐analysis outcomes
In general, the NMA supported a common class effect for exacerbations and quality of life. Individual prophylactic antibiotics included in each NMA are provided in tables that have been referenced in the text below.
NMA 1. Primary outcome: exacerbations
Definitions of exacerbations reported in the studies included for this outcome are captured in Table 4. Overall, moderate exacerbations were described as sustained worsening of baseline respiratory symptoms for at least two days requiring treatment with systemic corticosteroids and/or antibiotics, with severe exacerbations requiring additional hospital admission.
Exacerbation data were reported either as time to first exacerbation (Albert 2011; Blasi 2010; He 2010; Seemungal 2008; Simpson 2014; Uzun 2014), or as the number of people with one or more exacerbations during the study period (Berkhof 2013; Brill 2015; Sethi 2010; Suzuki 2001) (Table 6; Table 7).
3. Exacerbations: studies included with time to first exacerbation data.
| Study | Treatments compared | Log hazard ratio | Standard error | |
| Albert 2011 | Pbo | AZM 250 mg once daily | ‐0.31 | 0.07 |
| He 2010 | Pbo | ERY125 mg 3 times daily | ‐0.59 | 0.29 |
| Seemungal 2008 | Pbo | ERY 250 mg twice daily | ‐0.45 | 0.14 |
| Simpson 2014 | Pbo | AZM 250 mg once daily | ‐0.99 | 0.62 |
| Uzun 2014 | Pbo | AZM 500 mg once daily 3 times per week | ‐0.54 | 0.16 |
| Blasi 2010 | Pbo | AZM 500 mg once daily 3 times per week | ‐1.69 | 0.60 |
Abbreviations
AZM: azithromycin; ERY: erythromycin; Pbo: placebo.
4. Exacerbations: studies included with the number of people with one or more exacerbations.
| Study | Treatment 1 (N) | No. of events | Treatment 2 (N) | No. of events | Treatment 3 (N) | No. of events | Treatment 4 (N) | No. of events |
| Berkhof 2013 | Pbo (42) |
17 | AZM 250 mg once daily 3 times a week (42) |
10 | ‐ | ‐ | ‐ | ‐ |
| Brill 2015 | Pbo (24) |
13 | DOX100 mg once daily (25) |
15 | AZM 250 mg once daily 3 times per week (25) |
10 | MOX 400 mg once daily (5 days every 4 weeks) (25) |
10 |
| Sethi 2010 | Pbo (580) |
295 | MOX 400 mg once daily (5 days every 8 weeks) (569) |
269 | ‐ | ‐ | ‐ | ‐ |
| Suzuki 2001* | Pbo (54) |
30 | ERY 200 to 400 mg once daily (55) |
6 | ‐ | ‐ | ‐ | ‐ |
Abbreviations
AZM: azithromycin; DOX: doxycycline; ERY: erythromycin; MOX: moxifloxacin;Pbo: placebo.
*This study was included only as a sensitivity analysis ‐ reported in Appendix 4.
Model selection
Both fixed class models with fixed and random treatment effects fit well. They had similar DIC values; therefore the simpler fixed effect model was chosen, although results for the random effects model were also displayed for comparison (DIC 52.17, SD 0.16, 95% CrI 0.006 to 0.519) (Table 8).
5. Exacerbations: model fit statistics.
| DIC | SD (95% CrI) | Total residual deviance* | |
| Fixed class effect models | |||
| Fixed effect model | 51.31 | ‐ | 15.17 |
| Random effects model | 52.17 | 0.16 (0.006 to 0.519) | 13.61 |
Abbreviations
CrI: credible interval; DIC: deviance information criterion; SD: standard deviation.
*Compared to 14 data points.
Results
The NMA included nine studies and nine interventions from three antibiotic classes (macrolides, quinolones, tetracyclines) and from control treatment (placebo or standard therapy) (2732 participants; Table 6; Table 7; Table 9). Figure 3 represents studies contributing to the NMA (a) at the individual intervention level and (b) at the antibiotic class level. Table 1 shows the hazard ratio (HR) for each class compared to every other. Each class except tetracycline (HR 1.29, 95% CrI 0.66 to 2.41) reduced exacerbations compared to control (placebo or standard therapy). Evidence suggests that macrolides considerably reduced exacerbations compared to placebo or standard therapy (HR 0.67, 95% CrI 0.60 to 0.75), whereas quinolones showed smaller benefit and the 95% CrI included no effect (HR 0.89, 95% CrI 0.75 to 1.04). Furthermore, our analysis suggests that macrolides were superior to quinolones in reducing exacerbations (quinolone versus macrolide; HR 1.32, 95% CrI 1.08 to 1.61) (Table 10). Figure 4 presents the hazard ratios for both fixed effect and random effects models.
6. Exacerbations: interventions and treatment classes.
| Intervention | Treatment class | N | |
| 1 | Pbo | Placebo | 1345 |
| 2 | AZM 250 mg once daily | Macrolide | 573 |
| 3 | AZM 250 mg once daily 3 times per week | Macrolide | 67 |
| 4 | AZM 500 mg once daily 3 times per week | Macrolide | 57 |
| 5 | ERY 250 mg 3 times daily | Macrolide | 53 |
| 6 | ERY 125 mg 3 times daily | Macrolide | 18 |
| 7 | DOX 100 mg once daily | Tetracycline | 25 |
| 8 | MOX 400 mg once daily (5 days every 8 weeks) | Quinolone | 569 |
| 9 | MOX 400 mg once daily (5 days every 4 weeks) | Quinolone | 25 |
Abbreviations
AZM: azithromycin; DOX: doxycycline; ERY: erythromycin; MOX: moxifloxacin; Pbo: placebo.
3.
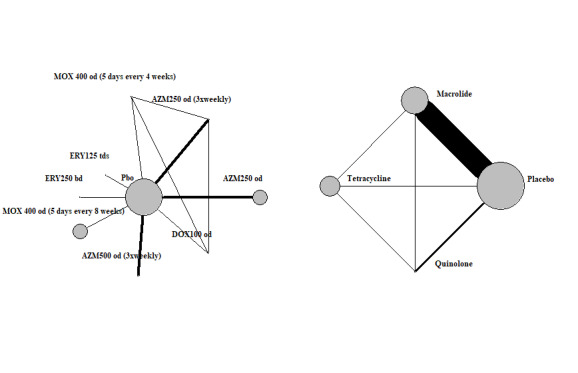
Exacerbations: network diagram of interventions and classes. Treatment abbreviations are defined in Table 4. The size of the nodes is proportionate to the number of participants assigned to the intervention. The thickness of the lines is proportionate to the number of randomised trials that studied the respective comparison.
7. Exacerbations: number of trials, number of participants, and relative effect estimates for all class comparisons.
| Comparison | Hazard ratios | Number of trials | N | ||
| Intervention | Comparator | Median | 95% CrI | ||
| Macrolide | Placebo | 0.67 | 0.60 to 0.75 | 9 | 1509 |
| Tetracycline | Placebo | 1.29 | 0.66 to 2.41 | 1 | 49 |
| Quinolone | Placebo | 0.89 | 0.75 to 1.04 | 2 | 1198 |
| Tetracycline | Macrolide | 1.93 | 0.99 to 3.62 | 1 | 50 |
| Quinolone | Macrolide | 1.32 | 1.08 to 1.61 | 1 | 50 |
| Quinolone | Tetracycline | 0.69 | 0.37 to 1.34 | 1 | 50 |
Abbreviations
CrI: credible interval.
4.
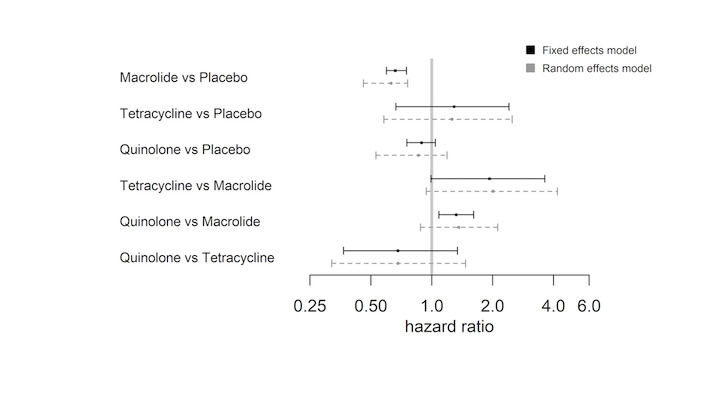
Exacerbations: forest plot of relative effects for each class comparison. Values less than 1 favour the first names class.
Table 11 shows rank statistics for the three antibiotic classes and for control (placebo or standard therapy). The highest ranked class was macrolide, with a median rank of 1 (95% CrI first to second), followed by quinolone (95% CrI second to third). Tetracycline was the worst ranked treatment for this outcome (95% CrI first to fourth). Although control (placebo or standard therapy) was ranked third, it had a 95% CrI of second to fourth, and a similar mean rank to tetracycline (3.1 versus 3.6), which reflected that the HR showed no clear evidence of a difference between tetracycline and control (placebo or standard therapy) (HR 1.29, 95% CrI 0.66 to 2.41) (Figure 4; Table 11).
8. Exacerbations: number of participants and rank statistics for each class (sorted by mean rank).
| Class | N | Mean | Median | 95% CrI |
| Macrolide | 768 | 1.0 | 1 | 1 to 2 |
| Quinolone | 594 | 2.2 | 2 | 2 to 3 |
| Placebo | 1345 | 3.1 | 3 | 2 to 4 |
| Tetracycline | 25 | 3.6 | 4 | 1 to 4 |
Abbreviations
CrI: credible interval.
Figure 5 represents the rank probabilities of each antibiotic class and control (placebo or standard therapy). The vertical axis shows the probability of being ranked best (first) to worst (fourth). The probability of macrolides being ranked first was 0.97 (Figure 5).
5.
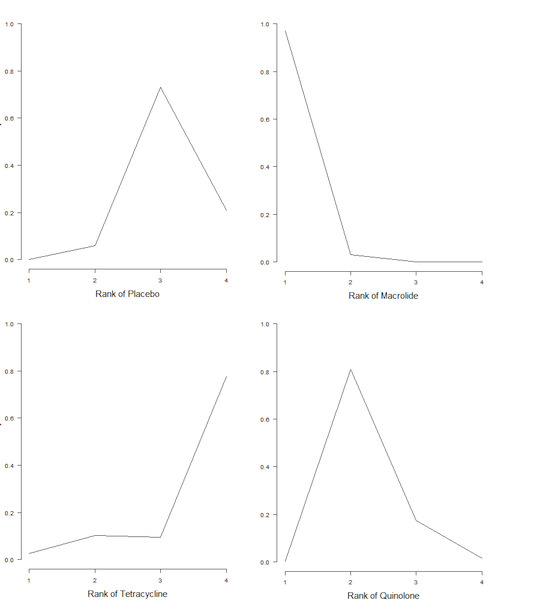
Exacerbations: plot of rank probabilities for each class.
The absolute rate of exacerbations per person per year for each treatment class is reported in Table 1, with the assumption that the absolute rate of exacerbations in the control (placebo or standard therapy) arm was two per person per year. Macrolides had a median rate of exacerbations of 1.34 (CrI 1.19 to 1.50) per person per year compared to control (placebo or standard therapy). Tetracycline was the only class that had a higher median rate of exacerbations per person per year than control (placebo or standard therapy) (2.58, 95% CrI 1.33 to 4.81), suggesting a probable lack of clinical effectiveness.
Threshold analysis and robustness of the evidence
We judged studies contributing to this analysis to be at low risk of bias in most domains. Figure 6 shows the forest plot for the threshold analysis. None of the comparisons had the upper or lower portion of the invariant interval within the 95% CrI of the effect estimate (Figure 6); thus the decision was not sensitive to the level of imprecision in this estimate, that is, the decision that the optimal treatment class to prevent exacerbations is macrolide was robust to sampling variation. However, this decision appeared sensitive to potential bias in comparisons of all classes to placebo (macrolide versus placebo, tetracycline versus placebo, quinolone versus placebo; Figure 6), as some of the thresholds were small. Upon inspection of these thresholds, we noted that only the comparison of macrolides to control (placebo or standard therapy) (2 versus 1; Figure 6) could change due to plausible bias adjustment. This is so because if there was any bias in the comparison, it was likely to favour the active class; therefore any adjustment would bring the estimated relative effect closer to the null value, meaning that quinolone may become the best class to prevent exacerbations. All other comparisons would require a bias that favoured placebo to be present, or an implausibly large bias before the optimal treatment changed. All other invariant intervals were very wide, so comparisons were robust to any changes in the evidence informing those comparisons.
6.
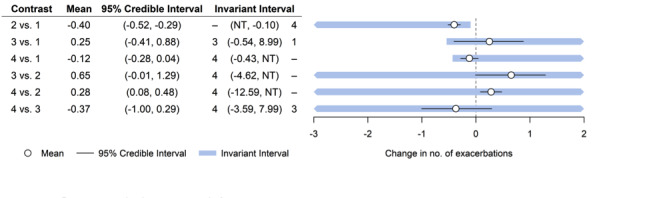
Exacerbations: forest plot with threshold analysis for the log‐HR of exacerbations for each class. Base case optimal treatment set is 2. Class codes: 1 = placebo; 2 = macrolide; 3 = tetracycline; 4 = quinolone. Comparisons are macrolide versus placebo (2 versus 1); tetracycline versus placebo (3 versus 1); quinolone versus placebo (4 versus 1); tetracycline versus macrolide (3 versus 2); quinolone versus macrolide (4 versus 2); quinolone versus tetracycline (4 versus 3).
Exacerbations: sensitivity analysis including Suzuki 2001
In an initial analysis, treatment‐specific comparisons in a fixed effect‐no class model show significantly lower risk of exacerbations, which resulted in macrolides as the highest ranking class‐specific treatment (Appendix 4; Table 9; online supplement Janjua 2021) and a probability of 0.96 for being ranked first. Due to suspicion of increased heterogeneity in the effects of macrolides compared to control treatment and other antibiotic treatments, we investigated further the relative estimates for all treatment comparisons in both fixed effect‐no class and fixed effect‐exchangeable class models. The risk of exacerbations was considerably lower with erythromycin 200 to 400 mg compared with placebo or other antibiotic treatments (Table 7; Appendix 4). We identified Suzuki 2001 as the study contributing to observed effects and investigated characteristics of the study that may have possibly contributed to the analysis result (Table 4). Suzuki 2001 was an open‐label study that was rated at high risk of bias due to lack of blinding. Study authors reported erythromycin dosage as ranging from 200 mg to 400 mg. Unfortunately, they provided no further information in their publication regarding the actual dose of erythromycin that participants received, and there was no possibility of contacting these study authors because the study was published in 2001. For these reasons, we decided to include Suzuki 2001 in a sensitivity analysis rather than in the main analysis.
NMA 2. Primary outcome: quality of life: change from baseline in SGRQ score
Model selection
Fixed class models with both fixed and random treatment effects fit well. Sensitivity to the prior distribution for heterogeneity was assessed by fitting a random effects model with an empirically based prior distribution converted to the mean difference scale (Rhodes 2015). We chose the fixed treatment effect model with fixed class effect (Table 12). We also reported results for the random treatment effects model with fixed class effect using the uniform prior distribution.
9. Change from baseline in SGRQ: number of trials, number of participants, and relative effects for all class comparisons.
| Comparison | Number of trials | N | Fixed effects‐fixed class effect | Random effects‐fixed class effect (uniform prior) | Random effects‐fixed class effect (empirical prior) | |||
| MD | 95% CrI | MD | 95% CrI | MD | 95% CrI | |||
| Macrolide vs placebo | 6 | 1158 | ‐2.30 | ‐3.61 to ‐0.99 | ‐2.34 | ‐4.28 to ‐0.39 | ‐2.28 | ‐5.19 to 1.01 |
| Tetracycline vs placebo | 1 | 49 | 1.18 | ‐1.49 to 3.84 | 1.14 | ‐2.47 to 4.62 | 1.20 | ‐4.62 to 7.19 |
| Quinolone vs placebo | 2 | 1078 | ‐1.33 | ‐2.97 to 0.32 | ‐1.42 | ‐4.04 to 1.05 | ‐1.44 | ‐5.99 to 3.07 |
| Tetracycline vs macrolide | 1 | 50 | 3.47 | 0.92 to 6.03 | 3.47 | 0.01 to 6.83 | 3.47 | ‐2.38 to 9.22 |
| Quinolone vs macrolide | 1 | 50 | 0.97 | ‐0.10 to 2.95 | 0.91 | ‐2.01 to 3.71 | 0.84 | ‐4.24 to 5.51 |
| Quinolone vs tetracycline | 1 | 50 | ‐2.50 | ‐5.32 to 0.30 | ‐2.56 | ‐6.33 to 1.16 | ‐2.63 | ‐8.96 to 3.37 |
Abbreviations
CrI: credible interval;MD: mean difference; SGRQ: St George's Respiratory Questionnaire.
Results
All of the included studies were two‐arm studies except for one study, which had four treatment arms (Brill 2015). Mean differences across the four study arms are correlated; thus a co‐variance between mean differences ‐ V ‐ was required (Dias 2018; Franchini 2012). As this was not reported, we intended to use sampling error (SE²) for mean SGRQ at baseline for participants randomised to placebo (V = 15.04), and to use SE² for the change from baseline (assuming equal baseline and follow‐up variances and correlation of 0.7; V = 9.025) in a sensitivity analysis. However, only the latter (V = 9.025) produced a valid co‐variance matrix for observed mean differences; therefore, only this value was used.
The NMA included seven studies of eight interventions from three antibiotic classes (macrolide, quinolone, tetracycline) and placebo for this outcome (2237 participants; Table 12; Table 13). Figure 7 represents studies contributing to the NMA (a) at the individual intervention level and (b) at the antibiotic class level. Figure 8 shows the mean difference in change from baseline in SGRQ score for each class compared to every other for the preferred model (fixed treatment‐fixed class model), as well as the random treatment‐fixed class model for comparison. Evidence suggests that the macrolide class improved SGRQ score compared to placebo in both models, although only the main analysis yielded significant results (Figure 8; Table 12).
10. Change from baseline in SGRQ: included studies.
| Study | Endpoint (weeks) | Treatments compared | Mean difference vs Placebo | SE of Mean difference | |||||
| Albert 2011 | 52 | Placebo | AZM 250 mg once daily | ‐2.2 | 0.7853 | ||||
| Berkhof 2013 | 12 | Placebo | AZM250 mg once daily 3 times per week | ‐7.5 | 2.5456 | ||||
| He 2010 | 26 | Placebo | ERY 125 mg 3 times daily | ‐3 | 5.6801 | ||||
| Sethi 2010 | 48 | Placebo | MOX 400 mg once daily (5 days every 8 weeks) | ‐1.2 | 0.9231 | ||||
| Simpson 2014 | 12 | Placebo | AZM 250 mg once daily | 6.1 | 5.31927 | ||||
| Uzun 2014 | 52 | Placebo | AZM500 mg once daily 3 times per week | ‐0.61 | 2.622449 | ||||
| Brill 2015* | 13 | Placebo | a. DOX 100 mg once daily b. AZM 250 mg once daily 3 times per week c. MOX 400 mg once daily (5 days every 4 weeks) |
a. 1.02 | b. ‐2.29 | c. ‐1.88 | a. 3.135 | b. 3.212 | c. 3.426 |
| a. 0.88 | b. ‐2.35 | c. ‐2.25 | a. 3.132 | b. 3.085 | c. 3.233 | ||||
Abbreviations
AZM: azithromycin; DOX: doxycycline; ERY: erythromycin; MOX: moxifloxacin; SE: standard error; SGRQ: St George's Respiratory Questionnaire.
*Data in bold used in sensitivity analysis.
7.
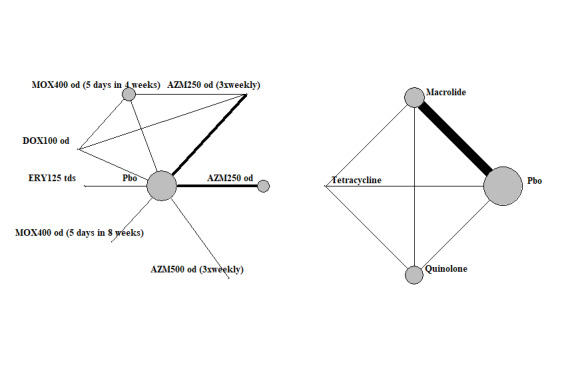
Quality of life: SGRQ network map.
8.
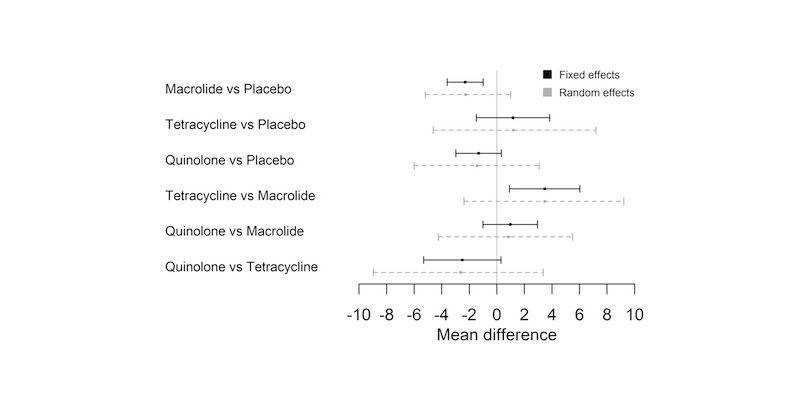
Change from baseline in SGRQ: forest plot of relative effects for each class comparison. Values less than 0 favour the first named class.
Bayesian probabilities of the mean difference exceeding the minimal clinically important difference (MCID) of 4 for SGRQ, when a macrolide is compared to placebo, tetracycline, or quinolone, were calculated as 0.005, 0.345, and 0.001, respectively, under the fixed treatment effect model with fixed class effect.
Table 14 shows rank statistics for the four classes. The highest ranked class was macrolide, with a median rank of 1 (95% CrI first to second). Figure 9 shows plots of rank probabilities for each class. The vertical axis shows the probability of being ranked best (first) to worst (fourth). The probability of macrolides being ranked first was 0.82 (Figure 9).
11. Change from baseline in SGRQ: number of participants and rank statistics for each class (sorted by mean rank).
| Treatment class | Number of participants | Mean | Median | 95% CrI |
| Macrolide | 578 | 1.17 | 1 | 1 to 2 |
| Quinolone | 528 | 1.93 | 2 | 1 to 3 |
| Placebo | 1106 | 3.14 | 3 | 2 to 4 |
| Tetracycline | 25 | 3.76 | 4 | 2 to 4 |
Abbreviations
CrI: credible interval; SGRQ: St. George's Respiratory Questionnaire.
9.
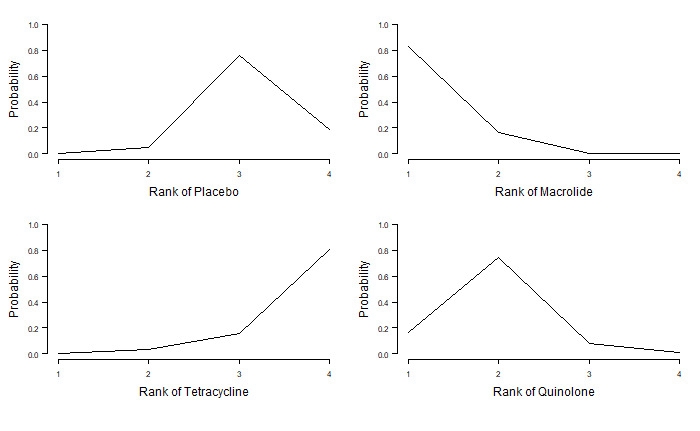
Change from baseline in SGRQ: plot of rank probabilities for each class.
We presented the change in SGRQ from baseline for macrolides, tetracyclines, and quinolones compared with placebo in Table 2. We assumed that the absolute change from baseline for SGRQ in the placebo arm was ‐1.7, for a 1.7 point improvement. The absolute change in SGRQ from baseline with macrolide treatment was ‐4.00 (95% CrI ‐5.51 to ‐2.68), which translated to a 2.30 point improvement compared to placebo (3.61 to 0.99 point improvement). However this did not reach clinical significance (MCID of 4 point improvement). With quinolone treatment, the absolute change from baseline was ‐3.03 (95% CrI ‐4.69 to ‐1.37). This resulted in a 1.33 point improvement compared to placebo (2.99 to 0.33 point improvement). With tetracyclines, there was an absolute change in SGRQ of ‐0.52 (95% CrI ‐3.21 to 2.16), which resulted in a 1.18 point worsening in quality of life compared to placebo (1.51 improvement to 3.86 worsening). Thus, tetracycline was worse than placebo, but there was still improvement compared to baseline.
Quality of life: change from baseline in SGRQ score: sensitivity analysis including Brill 2015
Results of the sensitivity analysis are detailed in Appendix 5. The main NMA included fully adjusted effect estimates from Brill 2015; however, the study also reported relative effects, which were adjusted only for baseline values. With a fixed treatment‐fixed class model, the relative effect for SGRQ score in the sensitivity analysis was similar to the main NMA result and ranking; however, the 95% CrI in the sensitivity analysis no longer included zero when quinolone was compared to tetracycline.
Quality of life: change from baseline in SGRQ: sensitivity analysis including Singh 2019
Results of the sensitivity analysis can be found in Appendix 5. We included Singh 2019 in a sensitivity analysis rather than in the main analysis. Inclusion of Singh 2019 in the sensitivity analysis using a fixed effect‐fixed class model, with effect estimates of each class comparison, including tetracycline, led to a shift when compared to the main analysis (Figure 6; online supplement Janjua 2021, Figure 4.3. For example, the effect estimate for tetracycline versus placebo shifted to the right in the sensitivity analysis, as did the effect estimate for the comparison of tetracycline compared to macrolide (online supplement Janjua 2021; Figure 4.3). Ranking also changed, resulting in tetracycline ranking third and placebo now ranked fourth, in contrast to the main NMA (Appendix 5).
NMA 3. Primary outcome: serious adverse events
Model selection
Fixed class models with both fixed and random treatment effects fit well; thus we chose a fixed treatment effect model with fixed class effect. Results for the random effects model were also displayed for comparison (DIC 113.2, SD 0.44, 95% CrI 0.02 to 1.28) (Table 15). We also reported results for the random treatment effects model with fixed class effect for comparison using the uniform prior distribution (Figure 10; Table 16) and the empirical prior distribution (Table 16) for comparison.
12. Serious adverse events: model fit statistics.
| DIC | Between‐study SD (95% CrI) | Total residual deviance* | |
| Fixed class models | |||
| Fixed treatment effect | 113.8 | ‐ | 21.58 |
| Random treatment effects | 113.2 | 0.44 (0.02 to 1.28) | 18.59 |
Abbreviations
* Compare to 19 data points
CrI: credible interval; DIC: deviance information criterion; SD: standard deviation.
10.
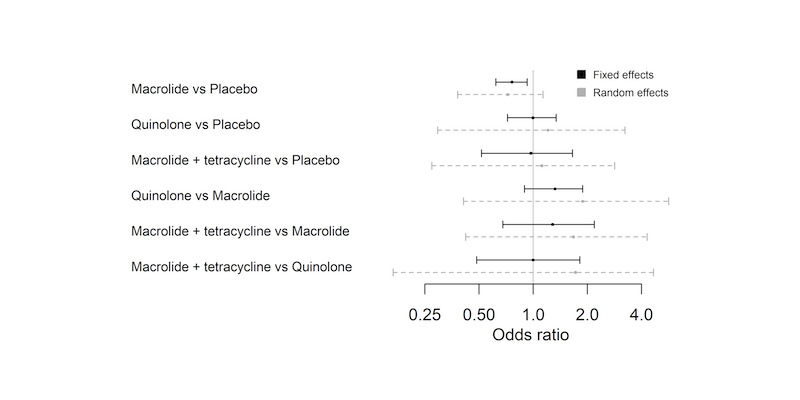
Serious adverse events: forest plot of relative effects for each class comparison. Values less than 1 favour the first named class.
13. Serious adverse events: number of trials, participants, and relative effects for all class comparisons.
| Treatment class comparison | Number of trials | N | Fixed effect‐fixed class effect model | Random effects‐fixed class effect (uniform prior) | Random effects‐fixed class effect (empirical prior) | |||
| OR | 95% CrI | OR | 95% CrI | OR | 95% CrI | |||
| Macrolide vs placebo | 8 | 1930 | 0.76 | 0.62 to 0.93 | 0.72 | 0.38 to 1.14 | 0.73 | 0.45 to 1.07 |
| Quinolone vs placebo | 1 | 1149 | 1.00 | 0.72 to 1.34 | 1.21 | 0.29 to 3.24 | 1.08 | 0.42 to 2.27 |
| Macrolide + tetracycline vs placebo | 1 | 195 | 0.97 | 0.52 to 1.66 | 1.12 | 0.27 to 2.84 | 1.00 | 0.36 to 2.19 |
| Quinolone vs macrolide | 0 | 0 | 1.32 | 0.90 to 1.89 | 1.88 | 0.41 to 5.67 | 1.56 | 0.55 to 3.62 |
| Macrolide + tetracycline vs macrolide | 1 | 198 | 1.28 | 0.68 to 2.19 | 1.67 | 0.42 to 4.31 | 1.41 | 0.52 to 3.15 |
| Macrolide + tetracycline vs quinolone | 0 | 0 | 1.00 | 0.49 to 1.82 | 1.72 | 0.17 to 4.67 | 1.13 | 0.26 to 3.11 |
Abbreviations
CrI: credible interval; OR: odds ratio.
Results
The NMA included nine studies, nine interventions from three antibiotic classes (macrolide, macrolide plus tetracycline, quinolone), and placebo for this outcome (3180 participants; Table 17; Table 18). Figure 11 represents studies contributing to the NMA (a) at the individual intervention level and (b) at the antibiotic class level. Brill 2015 was not included in the analysis, as no events were reported in any treatment arm. We presented the odds ratio (OR) of serious adverse events for each class compared to every other (Table 16). Evidence suggests that the macrolide class reduced the odds of having a serious adverse event compared to placebo when the fixed treatment effect model with fixed class effects was used (Table 16; Figure 10). However, use of the random treatment effects model with fixed class effect resulted in the 95% CrI crossing the line of no effect (Table 16; Figure 10).
14. Serious adverse events: table of interventions and treatment classes.
| Intervention | Treatment class | N | |
| 1 | Pbo | Pbo | 1539 |
| 2 | AZM 250 mg od | Macrolide | 573 |
| 3 | ERY 125 mg tds | Macrolide | 54 |
| 4 | ERY 250 mg bd | Macrolide | 53 |
| 5 | MOX 400 mg od (5 days every 8 weeks) | Quinolone | 569 |
| 6 | ROX 300 mg od + DOX 100 mg od | Macrolide + tetracycline | 101 |
| 7 | ROX 300 mg od | Macrolide | 97 |
| 8 | AZM 500 mg od (3x weekly) | Macrolide | 47 |
| 9 | AZM 500 mg od (for first 3 days), AZM 250 mg every 2 days (intermittent) for rest of treatment duration |
Macrolide | 147 |
Abbreviations
AZM: azithromycin; DOX: doxycycline; ERY: erythromycin; MOX: moxifloxacin; Pbo: placebo; ROX: roxithromycin.
15. Serious adverse events: studies included.
| Study name | Treatments compared | Number of participants | Number of events | ||||||
| Treatment 1 | Treatment 2 | Treatment 3 | Treatment 1 | Treatment 2 | Treatment 3 | ||||
| Albert 2011 | Pbo | AZM 250 mg once daily | 559 | 558 | NA | 212 | 184 | NA | |
| He 2010 | Pbo | ERY125 mg 3 times daily | 18 | 18 | NA | 3 | 2 | NA | |
| Seemungal 2008 | Pbo | ERY 250 mg twice daily | 56 | 53 | NA | 12 | 14 | NA | |
| Sethi 2010 | Pbo | MOX 400 mg once daily (5 days every 8 weeks) | 580 | 569 | NA | 97 | 94 | NA | |
| Shafuddin 2015 | Pbo | ROX 300 mg once daily + DOX 100 mg once daily |
ROX 300 mg once daily | 94 | 101 | 97 | 20 | 24 | 23 |
| Simpson 2014 | Pbo | AZM 250 mg once daily | 15 | 15 | NA | 4 | 1 | NA | |
| Tan 2016 | Pbo | ERY 125 mg 3 times daily | 18 | 36 | NA | 3 | 2 | NA | |
| Uzun 2014 | Pbo | AZM 500 mg once daily 3 times per week | 45 | 47 | NA | 5 | 3 | NA | |
| Vermeersch 2019 | Pbo | AZM 500 mg once daily (for first 3 days), AZM 250 mg every 2 days (intermittent) for rest of treatment duration |
15 | 147 | NA | 48 | 25 | NA | |
Abbreviations
AZM: azithromycin; DOX: doxycycline; ERY: erythromycin; MOX: moxifloxacin; NA: not applicable; Pbo: placebo; ROX: roxithromycin.
11.
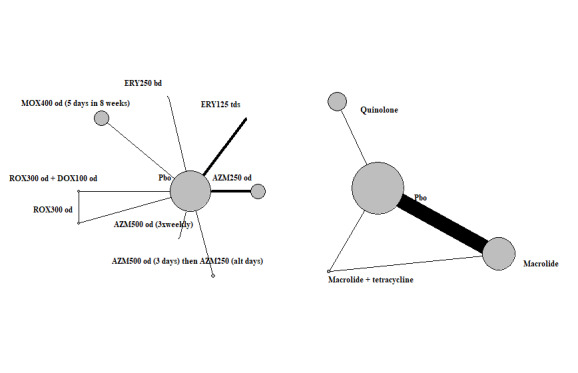
Serious adverse events: network map.
Table 19 shows rank statistics for the four classes. The highest ranked class was macrolide, with a median rank of 1 (95% CrI first to second). Figure 12 represents the plots for ranking probabilities of each class. The vertical axis shows the probability of being ranked best (first) to worst (fourth). The probability of macrolides being ranked first was 0.70 (Figure 12).
16. Serious adverse events: total number of participants and rank statistics for each class (sorted by mean rank).
| Treatment class | N | Mean | Median | 95% CrI |
| Macrolide | 971 | 1.33 | 1 | 1 to 3 |
| Macrolide + tetracycline | 101 | 2.61 | 2 | 1 to 4 |
| Quinolone | 569 | 2.95 | 3 | 1 to 4 |
| Pbo | 1539 | 3.12 | 3 | 2 to 4 |
Abbreviations
CrI: credible interval; Pbo: placebo.
12.
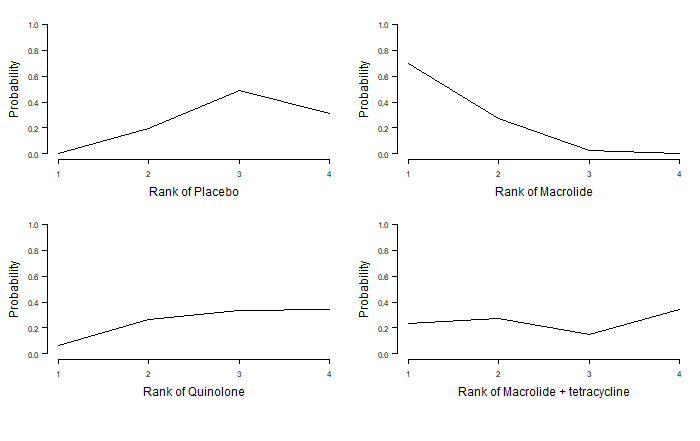
Serious adverse events: plot of rank probabilities for each antibiotic class
The relative effect and the absolute risk difference with macrolide, quinolone, or macrolide plus tetracycline compared with placebo are presented in Table 3, with the assumption that the absolute probability of events in the placebo arm was 0.26, and the risk of serious adverse events with placebo was 260 per 1000. A greater magnitude of effect was evident upon treatment with macrolides compared with placebo. The relative effect was OR 0.76 (95% CrI 0.62 to 0.93), that is, 49 fewer people per 1000 experienced a serious adverse event with macrolides compared to placebo. For quinolones or macrolides plus tetracycline, there was uncertainty in effect between classes and placebo, as the 95% credible interval crossed the line of no effect.
Non‐NMA outcomes
Antimicrobial resistance
Antibiotic resistance was investigated by 10 studies (Albert 2011; Banerjee 2005; Berkhof 2013; Blasi 2010; Brill 2015; He 2010; Seemungal 2008; Sethi 2010; Uzun 2014; Vermeersch 2019) (Table 20).
17. Drug resistance or microbial sensitivity reported in included studies.
| Study, drug, duration (weeks) | Drug resistance/microbial sensitivity methods | Results | Conclusion |
|
Albert 2011 AZM (52) |
Nasopharyngeal swabs and expectorated sputum samples taken at baseline and every 3 months, assessment for resistance to macrolides. Only 15% of participants were able to produce sputum by the third month; therefore, assessments were limited to nasopharyngeal swabs | Prevalence of resistance to macrolides was 52% and 57%, respectively (P = 0.64) During the study, 81% AZM and 41% placebo and bacteria were resistant to macrolides (P < 0.001) |
People receiving AZM were less likely to be colonised with respiratory pathogens compared to placebo but were more likely to become colonised with macrolide‐resistant organisms. No association with exacerbations |
|
Banerjee 2005 CLR (13) |
Sputum sample was tested for potential pathogenic microorganisms: H influenzae, S pneumoniae, M catarrhalis, H parainfluenzae, S aureus, P aeruginosa, K pneumoniae | At the start, 90% of isolates were due to S pneumoniae, H influenzae, M catarrhalis. Some patients had more than one PPM in sputum. After 3 months of CLR, number of people with sputum PPM increased from 12 to 15. Number of bacterial isolates did not increase. In the placebo group, this increased from 10 to 16, and the number of bacterial isolates increased from 15 to 25 CLR did not significantly reduce mean number of H influenzae, S pneumoniae, or M catarrhalis bacterial isolate compared to placebo No multi‐resistant gram‐negative organisms emerged in the CLR group. CLR did not significantly change the mean CFU number per bacterial isolate compared to placebo |
Contradicts other CLR studies that show the opposite due to lack of compliance in the trial. The study did not measure MIC90, which would have been ideal to detect changes in antibiotic susceptibility or resistance with time |
|
Berkhof 2013 AZM (12) |
Sputum samples collected | A reduction in respiratory pathogens was seen in the AZM group compared to the placebo group. One patient in the AZM group at 12 weeks had AZM‐resistant bacteria (S aureus) | Dose given seemed effective compared to other studies |
|
Blasi 2010 AZM (26) |
Minimum inhibitory concentration (MIC) used to determine bacterial counts | P aeruginosa became resistant to ceftazidime after 6 months of treatment in 1 patient in the AZM group. An ERY‐resistant S pneumoniae was found in 1 patient in the AZM group at 6 months | Not associated with significant effects of reduction in bacterial load or bacterial eradication. Patients with long‐term use of AZM had no resistance except for 1 person |
|
Brill 2015 MOX, DOX, AZM (13) |
Resistance to the 3 tested antibiotics, change in sputum bacterial load via quantitative culture (qPCR) | Pulsed MOX demonstrated the largest fall in bacterial numbers on culture but was associated with increased adverse events Resistance was found in all 3 antibiotic arms of at least 3 times pre‐treatment values. With baseline adjustment of MIC, MOX was associated with an increase in MIC for isolates cultured in sputum compared to placebo (e.g. DOX group were more likely to be resistant to DOX vs placebo) |
There was an increase in resistance of airway bacteria to all 3 antibiotics |
| He 2010 | Sputum samples/bacteriology | 9 ERY and 7 placebo patients had bacterial growth at baseline. 4 had > 1 organism. At 6 months, there was significant bacterial growth, and 3 specimens had > 1 organism. There was no detection difference in the rate of identifying the 3 main micro‐organisms between the 2 groups | |
|
Seemungal 2008 ERY (52) |
Sputum samples Sensitivity testing |
H influenzae detection positive in 27% of stable samples and in 40% of exacerbation samples. All H influenzae were resistant to ERY. S pneumoniae was found in 7% and 10%, respectively. No difference in detection rate for any organism between both arms at any follow‐up time points. Sensitivity testing found that 33/69 showed no growth at baseline. Those who tested positive at baseline were resistant to H influenzae (ERY = 10, P = 12), S pneumoniae (ERY = 1, P = 5 all sensitive), M catarrhalis (ERY = 1, P = 2 all sensitive) At 12 months, 26/43 samples had no significant growth. Of those samples that were positive, H influenzae (ERY = 1, P = 3), S pneumoniae (ERY = 1 resistant, P = 2 all sensitive), M catarrhalis (2 = sensitive, P = 2) |
Microbial resistance was not dependent on the use of ERY. Only 1 case of ERY resistance occurred in the macrolide group at 52 weeks. The number of participant in the study was small; therefore interpretation of these results is not definitive |
|
Sethi 2010 MOX (48) |
Sputum samples | Over the 48‐week treatment, there was a reduction in the number of participants with pathogens isolated, with greater reduction with MOX vs placebo. No difference in MIC increases that were sustained Isolates showed that 1 patient in the MOX group was S pneumoniae resistant at week 40, which was not associated with exacerbations and was not persistent at further visits. For S aureus, 1 to 3 isolates were MOX resistant at baseline and at other time points but did not persist and were not related to exacerbations. Median MIC of MOX against P aeruginosa at 24 weeks was 4 mg/L but was reduced to 1 mg/L to levels at randomisation for the MOX group. Median MIC in placebo group increased from 0.5 to 2 mg/L among those who completed treatment |
No further comments |
|
Uzun 2014 AZM (52) |
Macrolide resistance by sputum culture | 32/47 AZM gave samples. 32/45 in placebo gave samples. Most common bacteria were H influenzae, S pneumoniae, and P aeruginosa. At follow‐up fewer in the AZM group had positive cultures compared to the placebo group Macrolide resistance was seen in 3 AZM and in 11 placebo (P = 0.036) |
The number of sputum samples overall was low. Like Albert 2011, AZM group was less likely to be colonised with respiratory pathogens and acquisition of macrolide‐resistant bacteria was significantly reduced |
|
Vermeersch 2019 AZM |
Sputum samples | Bacterial samples obtained contained H influenzae, S pneumoniae, P aeruginosa, M catarrhalis, and S aureus. At follow‐up, there were no significant group differences (AZM or placebo) for positive sputum cultures with acquired pathogens, neither for acquired macrolide‐resistant bacteria | Macrolide resistance was monitored, but induced sputum was not required per protocol; the limited number of spontaneous sputum samples did not allow for thorough evaluation of antibiotic resistance induced by AZM on top of standard treatment |
Abbreviations
AZM: azithromycin; B catarrhalis:Branhamella catarrhalis;CLR: clarithromycin; CFU: colony‐forming unit; DOX: doxycycline; ERY: erythromycin; H influenzae: Haemophius influenzae;MIC: minimum inhibitory concentration; MIC90: MIC required to inhibit growth of 90% or organisms; M catarrhalis: Morexella catarrhalis;MOX: moxifloxacin; NA: not applicable; P aeruginosa: Pseudomonas aeruginosa;PPM: parts per million; qPCR: quantitative polymerase chain reaction; S aureus:Staphylococcus aureus;S pneumoniae: Streptococcus pneumoniae.
Macrolide: azithromycin
Albert 2011 was a 52‐week trial that assessed azithromycin 250 mg once daily compared with placebo (1142 randomised participants). Organisms most commonly identified in the antibiotic group and in the placebo group were Staphylococcus aureus (azithromycin N = 60 (10.7%), placebo N = 71 (12.7%)), Moraxella species (azithromycin N = 13 (2.3%), placebo N = 6 (1%)), and Streptococcus pneumoniae (azithromycin N = 6 (1.1%), placebo N = 6 (1.1%)). S aureus was cultured more frequently, as would be expected from nasopharyngeal sampling. Participants who were not colonised at the start of the study (N = 66 azithromycin, N = 172 placebo) became colonised during the course of the study, and resistance to macrolides was higher in the antibiotic arm than in the placebo arm (81% versus 41%; P < 0.001).
Berkhof 2013 was a 12‐week trial (84 randomised participants) that reported a reduction in respiratory pathogens in sputum samples of those taking azithromycin (250 mg once daily, 3 times a week). At 12 weeks, only one participant in the azithromycin group had antibiotic‐resistant S aureus. Study authors did not report mean inhibitory concentration (MIC)90, which would have allowed detection of changes in antibiotic resistance over time.
Uzun 2014 (92 randomised participants) found that at 52 weeks, fewer people had resistant bacteria when taking azithromycin (500 mg, 3 times a week) compared with placebo (3 versus 11; P = 0.036).
Vermeersch 2019 (301 randomised participants) did not find significant group differences between azithromycin (500 mg once daily for 3 days, followed by 250 mg every 2 days) and placebo for acquired macrolide‐resistant bacteria. At 13 weeks, only one participant in the placebo treatment group had newly acquired macrolide‐resistant bacteria.
Blasi 2010 (22 randomised participants) found that one participant in the azithromycin group (500 mg daily, 3 times a week) had erythromycin‐resistant S pneumoniae at 26 weeks.
Brill 2015 (99 randomised participants) measured sputum bacterial load and antibiotic resistance at 13 weeks post antibiotic treatment (azithromycin 250 mg, 3 times a week) compared with placebo. The most common organisms were S pneumoniae and Streptococcus species. Antibtiotic resistance was increased, with a factor increase of mean inhibitory concentration of 6.23 (95% confidence interval (CI) 1.66 to 23.35; P = 0.01) compared with placebo for sputum‐isolated cultures (Brill 2015).
Macrolide: clarithromycin
Banerjee 2005 (67 randomised participants) found no multi‐resistant gram‐negative pathogens among those taking long‐acting clarithromycin 500 mg daily compared with placebo at 13 weeks.
Macrolide: erythromycin
At 26 weeks of treatment, He 2010 (36 randomised participants) did not report any significant group differences (erythromycin 250 mg, 3 times a day versus placebo) in the emergence of antibiotic‐resistant organisms.
Seemungal 2008 (109 randomised participants) found that at the end of 52 weeks of treatment, only one participant had colonisation of S pneumoniae resistant to erythromycin in the erythromycin (250 mg twice daily) treatment arm. All Haemophilus influenzae isolates (22/109) were found to be resistant to erythromycin.
Quinolone: moxifloxacin
Brill 2015 (99 randomised participants) reported that moxifloxacin (400 mg daily for 5 days, every 4 weeks) was associated with a factor increase in MIC of 4.82 (95% CI 1.44 to 16.19; P = 0.01) compared to placebo from baseline to 13 weeks (Brill 2015). The odds of isolates cultured in sputum being resistant to moxifloxacin was above 2, but this result was not statistically significant.
Sethi 2010 (1149 randomised participants) reported a reduction in the number of participants with pathogens isolated over 48 weeks of treatment, with a more significant reduction with moxifloxacin treatment (400 mg daily for 5 days, repeated every 8 weeks) compared to placebo. One participant taking moxifloxacin had resistant Streptococcus pneumoniae at 40 weeks, which was not associated with exacerbations and did not persist at subsequent visits. This was similarly found in up to three isolates from participants taking moxifloxacin who were positive for S aureus at baseline and also at various time points in the study. The median MIC of moxifloxacin against Pseudomonas aeruginosa at 24 weeks was 4 mg/L, which was reduced further to 1 mg/L during the rest of the treatment period. The opposite was observed in the placebo group, in which median MIC increased from 0.5 mg/L to 2 mg/L among people who completed treatment.
Tetracycline: doxycycline
Brill 2015 (99 randomised participants) reported a change in MIC of 3.74 (95% CI 1.46 to 16.19) with doxycyline (100 mg once daily) compared with placebo at 13 weeks. Moreover, isolates from participants taking doxycycline were more likely to be resistant to doxycycline than from those taking placebo (OR 5.77, 95% CI 1.40 to 23.74; P = 0.02).
Mortality
Overall, eight studies reported mortality data (Albert 2011; Blasi 2010; Mygind 2010; Seemungal 2008; Sethi 2010; Shafuddin 2015; Uzun 2014; Vermeersch 2019) (Table 21). Refer to Appendix 3 for Herath 2018 and Threapleton 2018 results.
18. Mortality: numbers of deaths in treatment and placebo groups in included studies.
| Study ID | Antibiotic class | Antibiotic | Placebo or control or standard treatment |
| Albert 2011 | Macrolide | AZM: 18/570 (3%) | 20/572 (4%) |
| Banerjee 2005 | Macrolide | CLR: 0/31 | 0/36 |
| Berkhof 2013 | Macrolide | AZM: 0/42 | 0/42 |
| Blasi 2010 | Macrolide | AZM: 1/11 (9%) | 5/11 (45%) |
| Brill 2015 | Quinolone Tetracycline Macrolide |
MOX: 0/25 DOX: 0/25 AZM: 0/25 |
0/24 |
| He 2010 | Macrolide | ERY: 0/18 | 0/18 |
| Mygind 2010 | Macrolide | AZM: 74/287 (25%) | 81/288 (28%) |
| Seemungal 2008 | Macrolide | ERY: 0/53 (0%) | 1/56 (2%) |
| Sethi 2010 | Quinolone | MOX: 13/753 (2%) | 13/584 (2%) |
| Shafuddin 2015 | Macrolide+ tetracycline |
ROX: 3/97 (3%) DOX+ROX: 5/101 (5%) |
5/94 (5%) |
| Simpson 2014 | Macrolide | AZM: 0/15 | 0/15 |
| Singh 2019 | Tetracycline | DOX: 0/30 | 0/30 |
| Suzuki 2001 | Macrolide | ERY: 0/55 | 0/54 |
| Tan 2016 | Macrolide | ERY: 0/18 | 0/18 |
| Uzun 2014 | Macrolide | AZM: 0/47 (0%) | 2/45 (4%) |
| Vermeersch 2019 | Macrolide | AZM: 3/147 (2%) | 6/154 (3%) |
| Wang 2017 | Macrolide | AZM: 0/43 | 0/43 |
Abbreviations
AZM: azithromycin; CLR: clarithromycin; DOX: doxycycline; ERY: erythromycin; MOX: moxifloxacin; ROX: roxithromycin.
Overall, the total number of deaths with prophylactic antibiotic was 117/2394 participants compared with 133/2066 participants (Table 21). Death rates reported in the included studies ranged from 2% to 5% with antibiotics (Albert 2011; Sethi 2010; Shafuddin 2015; Vermeersch 2019), and from 2% to 5% with placebo. However, two studies showed higher rates of death compared with other studies (Blasi 2010; Mygind 2010). Mygind 2010, a 156‐week trial, included participants with moderate to severe COPD and reported more than 20% deaths. The number of deaths was similar between treatment groups. Blasi 2010, a 26‐week trial that included participants with severe COPD, showed a significantly higher rate of death in the placebo group compared to the azithromycin group during the treatment period (placebo 45%, azithromycin 9%).
Discussion
Summary of main results
We identified 17 studies that met the inclusion criteria for this review. Twelve studies assessed effects of macrolides (azithromycin and erythromycin) , quinolone (moxifloxacin), tetracycline (doxycycline), or tetracyclines plus macrolides (roxithromycin plus doxycycline) compared with placebo or standard therapy and were included in the network meta‐analysis (NMA) (3405 total participants).
We investigated the safety and clinical effectiveness of prophylactic antibiotics for adults with chronic obstructive pulmonary disease (COPD), specifically focusing on differences among antibiotic classes and individual antibiotics identified. Specifically, we evaluated the impact of different antibiotic classes and individual treatments on the frequency of exacerbations, quality of life evaluated using St George's Respiratory Questionnaire (SGRQ), and serious adverse events (SAEs). Overall, evidence included in the NMA was at low risk of bias and generally supported that macrolides were the highest ranking antibiotic treatment for reducing exacerbations and SAEs, and for improving quality of life, among people with moderate to severe COPD. More specifically, macrolides appear to be superior to placebo in reducing exacerbations and SAEs, with a probability of 0.97 and 0.7, respectively. In addition, macrolides were superior to placebo in improving quality of life, with a probability of 0.82, although the difference from placebo did not exceed the minimum clinically important difference (MCID).
Overall, results of the NMA are consistent with findings of the two previous pair‐wise reviews (Herath 2018; Threapleton 2018).
Exacerbations
Macrolides were more beneficial than placebo in reducing exacerbations (hazard ratio (HR) 0.67, 95% credible interval (CrI) 0.60 to 0.75), with an absolute risk reduction of 127 per 1000 people per year, that is, 127 fewer people had exacerbations per 1000 people treated for a year. The effect between quinolones and placebo was probably smaller (HR 0.89, 95% CrI 0.75 to 1.04). Exacerbations may be increased when tetracyclines are compared to placebo (HR 1.29, 95% CrI 0.66 to 2.41). When the four treatment classes were ranked, macrolides were ranked first, followed byquinolones. Placebo ranked third, although it was only marginally better than tetracyclines. As tetracyclines ranked fourth, this was reflected in the absolute risk, which resulted in an increase in the number of exacerbations by 60 per 1000 people per year compared with placebo. However, there was uncertainty in this result because confidence intervals ranged from 129 fewer to 127 more, and this evidence was based on data from one study. In comparison with each other, macrolides were also superior to quinolones and were borderline superior to tetracyclines in reducing exacerbations.
Quality of life
Treatment with macrolides improved quality of life compared to placebo by 2.29 points on the SGRQ (3.61 to 0.99 point improvement). Smaller improvement was seen with quinolone treatment compared with placebo (1.33 points on the SGRQ (2.99 to 0.33 point improvement)). There may be little to no difference in quality of life with tetracycline treatment compared to placebo (1.18 point worsening in quality of life compared to placebo (1.51 improvement to 3.86 worsening)), although the results were very uncertain. These results are reflected in ranking of these treatments, in which macrolides were ranked first, followed by quinolone. Tetracycline was ranked fourth after placebo.
Serious adverse events
Evidence suggests that macrolides reduced the odds of having an SAE compared to placebo only when the fixed treatment‐fixed class effect model was used. The relative effect of SAEs compared with placebo was odds ratio (OR) 0.76 (95% CrI 0.62 to 0.93). There was probably little to no difference in the effect with quinolones (OR 1.00, 95% CrI 0.72 to 1.34) or with macrolide plus tetracycline (OR 0.97, 95% CrI 0.52 to 1.66). When ranked, macrolides were ranked the highest (i.e. the highest probability of having the largest reduction in SAEs), with a probability of 0.7 of being ranked first. Macrolide plus tetracycline ranked second, quinolone ranked third, and placebo ranked fourth. These results were reflected in the absolute risk of each antibiotic treatment compared with placebo. Absolute risk in the placebo group was 260 per 1000. Absolute risk was 49 fewer per 1000 with macrolide, approximately 10 fewer per 1000 with macrolide plus tetracycline, and approximately 2 fewer per 1000 with quinolone.
Overall completeness and applicability of evidence
To date, this is the first NMA investigating the effectiveness of prophylactic antibiotics for people with COPD. For this reason, we decided to include placebo‐controlled trials to indirectly compare antibiotic classes with each other or with placebo. We identified four antibiotic classes: macrolides, quinolones, tetracyclines, and macrolide plus tetracycline; however most included studies compared macrolides with placebo.
Participants included in the NMA overall were similar, and although there could have been variation in history of exacerbations and maintenance inhaled therapies, there were no concerns for inconsistency across studies. It should be noted, however, that results from the NMA for exacerbations, quality of life, and SAEs can be generalised only to the subgroup of moderate to severe COPD (forced expiratory volume (FEV₁) ranging from 0.935 to 1.36 L) between 64 and 73 years of age.
Analyses show that treatment with macrolides overall reduced exacerbations compared to placebo or standard treatment. We assumed that with placebo or standard treatment, individuals would be expected to experience two exacerbations per year. Based on this assumption, the absolute risk was considerably lower with macrolides compared with placebo (127 per 1000 compared with 864 per 1000, respectively) (Table 1). With quinolones and tetracyclines, there was uncertainty about the difference in exacerbations, as credible intervals crossed the line of no effect. In current clinical practice, long‐term macrolide treatment could be considered for people with COPD who have more than three acute exacerbations per year, and an accurate assessment of baseline exacerbation rate should be determined before long‐term antibiotics are started (Smith 2020). National Institute for Health and Care Excellence (NICE) guidance suggests that the macrolide azithromycin (250 mg 3 times a week) could be used on the condition that individuals who continue to have frequent exacerbations or prolonged exacerbations, or exacerbations requiring hospitalisation, are not smoking, and that such treatment should be continued only as required, and if benefits outweigh risks (NICE 2018).
Similarly, the SGRQ analysis shows that treatment with macrolides resulted in greater improvement in quality of life compared to placebo (mean difference (MD) 2.298), as did treatment with quinolones (MD 1.33). Although these results did not reach the MCID of 4 points, there was modest benefit for quality of life. This result is in line with findings of a previous review showing modest improvement on the SGRQ but not reaching clinical significance with macrolides (i.e. a decrease of 2.12 points) (Ni 2015). Overall, from these findings, it is unclear how much long‐term antibiotics may impact individuals' quality of life beyond 12 months, as the duration of treatment in our analysis ranged from 12 weeks to 52 weeks. Only three studies lasted longer than 48 to 52 weeks (Albert 2011; Sethi 2010; Uzun 2014). Therefore, it may be important to take into consideration the risk and benefit for each individual with regular monitoring at 6 and 12 months using the SGRQ tool, as suggested in clinical guidance (Smith 2020). We did not investigate responder analysis data for SGRQ in trials or other systematic reviews, which could have been informative; however, this could be revisited in the future.
When SAEs were assessed, it was noted that fewer people experienced adverse events (AEs) with macrolides (49 fewer per 1000 people with macrolides versus placebo treatment (260 per 1000)). Ni 2015 reported a slightly higher rate of AEs in the macrolide group compared to the placebo group (OR 1.55, 95% confidence interval (CI) 1.003 to 2.39; P = 0.049). There was no difference in the effects of taking quinolones or macrolide plus tetracycline compared with placebo. It is important to note that the absolute risk of events in the placebo arm was calculated from the included studies and may not be applicable in general. Furthermore, we did not investigate the association between duration of antibiotic treatment and the impact of SAEs that people may experience. One recent systematic review found that macrolide use for 3 or 12 months resulted in more side effects than control treatment, but at 6 months, there was no difference between antibiotics and control treatment (Cui 2018). We did not investigate the impact of antibiotics on individual side effects; however, a previous Cochrane Review assessed this in detail (Herath 2018). Among the studies that we included in this review, hearing impairment and gastrointestinal problems were more commonly associated with long‐term use of macrolides. Exacerbations could have been reported as AEs or SAEs; however, exacerbations were reported separately from AEs among the studies included in the analysis (Table 22).
19. Adverse events across all studies.
| Study ID, drug, dose, schedule (weeks' duration) | Adverse events | ||
| Antibiotic (n) | Comparator (n) | Reporting of exacerbations as AEs | |
| Albert 2011, AZM, 250 mg once daily (52) | Discontinuation due to: audiogram‐confirmed hearing decrement (142), tinnitus (4), gastrointestinal tract (11), QTc prolongation (6), allergic reaction (5), abnormal laboratory test (4), other (10) | Discontinuation due to: audiogram‐confirmed hearing decrement, tinnitus (4), neoplasm (3), GI tract (6), QTc prolongation (4), allergic reaction (8), abnormal laboratory test (3), other (17) | Exacerbation was not reported as an AE |
| He 2010, ERY, 125 mg 3 times daily (26) | Discontinued due to: abdominal pain (1), complication of left heart failure (1) | Discontinued due to: respiratory insufficiency (2), other (1) | Exacerbation was not reported as an AE |
| Seemungal 2008 ERY, 250 mg twice daily (52) | Upper gastrointestinal (5), lower gastrointestinal (3), rash (3), other (3) | Upper gastrointestinal (5), lower gastrointestinal (3), rash (2), other (2) | Exacerbation was not reported as an AE |
| Sethi 2010, MOX, 400 mg once daily (5 days every 8 weeks) (48) | Cardiac disorders (3), gastrointestinal (diarrhoea, nausea, vomiting) (27), general disorders/administration site conditions (4), asthenia (3), immune system disorders (4), hypersensitivity (3), infections and infestations (5), musculoskeletal and connective tissue disorders (3), nervous system disorders (6), dizziness (3), respiratory, thoracic and mediastinal disorders (8), dyspnoea (4), skin and subcutaneous tissue disorders (5), AEs leading to discontinuation (26) | Cardiac disorders (1), gastrointestinal (diarrhoea, nausea, vomiting) (4), general disorders and administration site conditions (2), asthenia (0), hypersensitivity (0), infections and infestations (3), musculoskeletal and connective tissue disorders (1), nervous system disorders (4), dizziness (1), respiratory, thoracic, and mediastinal disorders (0), dyspnoea (0), skin and subcutaneous tissue disorders (5), AEs leading to discontinuation (16) | Exacerbation was not reported as an AE |
| Shafuddin 2015, ROX 300 mg once daily + DOX 100 mg once daily; or ROX 300 mg once daily (12) | Roxithromycin + doxycycline: nausea (12), diarrhoea (2), headache (4), abdominal pain (3), reflux (2), vomiting (1), abnormal liver function (1), abnormal ECG (1), rash (1), dyspnoea (0), dizziness (0), oral candidiasis (0), gastrointestinal upset (0) Roxithromycin alone: nausea (13), diarrhoea (3), headache (1), abdominal pain (1), reflux (1), vomiting (3), abnormal liver function (2), abnormal ECG (0), rash (1), dyspnoea (1), dizziness (4), oral candidiasis (2), gastrointestinal upset (2) |
Nausea (1), diarrhoea (1), headache (1), abdominal pain (1), reflux (0), vomiting (0), abnormal liver function (0), abnormal ECG (0), dyspnoea (2), dizziness (0), oral candidiasis (3), gastrointestinal upset (2) | Exacerbation was not reported as an AE |
| Simpson 2014, AZM, 250 mg once daily (12) | Diarrhoea (1), abdominal pain (0), nausea (0), vomiting (0), fever (0), headache (0), rash (0), hearing loss (0), other (10) | Diarrhoea (5), abdominal pain (0), nausea (0), vomiting (0), fever (0), headache (0), rash (0), hearing loss (0), other (9) | Exacerbation was not reported as an AE |
| Tan 2016, ERY, 125 mg 3 times daily (52) | Withdrawal due to: abdominal pain (1), left‐sided heart failure (1) | Withdrawal due to: respiratory insufficiency (2), unknown (1) | Exacerbation was not an outcome in the study |
| Uzun 2014, AZM, 500 mg once daily 3 times per week (52) | Diarrhoea (9), nausea/vomiting (3), other (4) | Diarrhoea (1), nausea/vomiting (2), other (7) | Exacerbaiton was not reported as an AE. 2 fatal SAEs occurred due to COPD respiratory failure, which were counted in the mortality outcome |
| Vermeersch 2019, AZM, 500 mg once daily (for first 3 days), AZM 250 mg every 2 days for rest of treatment duration (13) | Diarrhoea (20), nausea (12), anorexia (9), hearing loss (1), syncope (1) | Diarrhoea (15), nausea (11), anorexia (8), hearing loss (6), syncope (2) | Exacerbation was not reported as an AE |
Abbreviations
AE: adverse event;AZM: azithromycin; DOX: doxycyline; ECG: electrocardiogram; ERY: erythromycin; MOX: moxifloxacin; NR: not reported; QTc: corrected QT interval; ROX: roxithromycin; SAE: serious adverse event.
Most data on microbial resistance that we identified assessed macrolides (azithromycin) with varying durations of treatment. Macrolide use led to microbial resistance in Albert 2011 at 52 weeks; however, other studies were of shorter duration, and they found no difference between antibiotic and placebo treatments (Vermeersch 2019), or they reported microbial resistance in only one participant taking macrolide (Blasi 2010). With quinolone treatment, fewer individuals had pathogens compared with placebo (Sethi 2010), and although some quinolone‐resistant bacteria were isolated, they did not persist at the end of treatment. Based on limited evidence, concern is ongoing that the association of antibiotic treatment and microbial resistance may increase over time, prompting careful clinical assessment of risk and benefit before such treatment is started, and regular monitoring once antibiotic treatment is under way.
Limited data are available regarding the persistence of microbial resistance after discontinuation of prophylactic antibiotics for patients with COPD. This question was not rigorously assessed in any of the included studies. Indirect data are available from the AZISAST trial, which evaluated microbial resistance after discontinuation of prophylactic macrolides in exacerbation‐prone patients with severe asthma (Brusselle 2013). In the AZISAST trial, the percentage of macrolide‐resistant streptococci was reduced from 74% to 46% within four weeks from macrolide discontinuation. In the same period, microbiome characteristics returned to the pretreatment condition. As a result of this observation, annual brief periods of discontinuation of prophylactic antibiotics, preferably during summer, when disease is better controlled, are often suggested for patients with COPD (Miravittles 2015; Smith 2020). Such strategies will need to be evaluated by well‐designed randomised controlled trials (RCTs).
Overall, we found that death was a rare event in clinical studies. In most studies, no deaths occurred in either treatment group. When deaths were reported, they were similar in antibiotic and treatment groups. Mygind 2010 recorded a higher death rate (although similar in each treatment group) (azithromycin 25%, placebo 28%), but the study provided no further information. The death rate in Blasi 2010 was higher in the placebo group compared with the azithromycin group (azithromycin 9%, placebo 45%).
In summary, findings of this NMA show that macrolides are superior to other prophylactic antibiotics identified in the review. The superiority of macrolides compared to other antibiotics in preventing exacerbations has long been suspected and may be attributed to the anti‐inflammatory and immunomodulatory effects that this class of antibiotics exerts, in contrast to the other evaluated classes (Loukides 2013). In line with current clinical guidance, azithromycin at the doses identified may be of benefit for reducing exacerbations and improving quality of life in a subgroup of patients who have moderate to severe COPD that is managed by inhaled therapies but who encounter frequent exacerbations that may result in hospitalisation.
Current clinical guidance recommends that prophylactic antibiotic treatment should be considered for a minimum of 6 to 12 months while changes in exacerbations are observed (Smith 2020). The duration of antibiotic use among studies in the NMA ranged from 12 weeks to 52 weeks, which could be considered long enough to detect differences in exacerbations, but also in quality of life and SAEs. Studies in the NMA represent a wide range of populations geographically (China, Europe, United Kingdom, and USA); however, generalisation of prophylactic antibiotics may be impaired by our inability to include two studies from India and Japan in the main analyses (Singh 2019; Suzuki 2001).
We did not investigate the cost‐effectiveness of one antibiotic compared to another; however, these treatments are generally considered to be of low cost, and exacerbations pose a significant health and economic burden. It should be noted that although such treatments may be cost‐effective, potential costs of monitoring and follow‐up of those taking antibiotics over a long time would need to be considered but are likely to be off‐set by potential benefits for health status.
However, significant gaps in the evidence need to be addressed. First, we did not identify any data on the impact of long‐term antibiotic administration in different types of exacerbations (i.e. exacerbations triggered by bacteria or viruses, or those associated with enhanced eosinophilic inflammation) (Mathioudakis 2020). Although it is clear that prophylactic antibiotics are effective only for a subgroup of selected patients with COPD, this finding has not clearly emerged from available evidence in this or previous Cochrane Reviews (Herath 2018, Threapleton 2018). In addition, we did not identify data on the effectiveness of other antibiotics that may be used to treat exacerbations. For example, penicillins are often used to treat acute exacerbations, but we did not identify any trials in which penicillins are used for prophylaxis. Limited information is available about tetracyclines and combined antibiotic treatment; however, available evidence indicates that these antibiotic combinations are not necessarily superior (Shafuddin 2015).
Quality of the evidence
The methodological quality of all included studies was assessed and overall risk of bias was deemed low across the five domains. Some studies lack clarity regarding randomisation, allocation concealment, and attrition. We did not contact study authors for further information for unclear domains, as these studies were also included in Herath 2018 and Threapleton 2018, and study authors would have been contacted already. We were more confident in our findings from the main analyses when two studies at high risk of bias were excluded (Singh 2019; Suzuki 2001). These studies were instead included in sensitivity analyses that did not significantly change our findings. In all networks, all loops were formed by a single multi‐arm study; therefore, there was no potential to detect inconsistency, and inconsistency checks were not carried out. For most networks, the fixed effect model was selected, as there was no evidence of heterogeneity. When random effects models were used, the standard deviation for between‐study heterogeneity was reported along with its credible interval (exacerbations: Table 8; SAEs: Table 15). Imprecision was reflected in the 95% CrI, which was reported and commented on when appropriate.
GRADE headings were not used to assess validity of the evidence, as we did not think this approach would be informative because more than two treatments were being compared in the NMA. Similarly, we did not consider use of Confidence in Network Meta‐Analysis (CINeMA) because this application cannot be used when a Bayesian analysis is conducted. We used threshold analysis to examine the impact of bias on each treatment contrast (relative effect of each treatment comparison) (Phillippo 2019), which quantified how much the evidence could change before the best treatment changed, and what the new 'best' treatment would be. This approach indicates which results were robust to potential biases in the evidence, taking into account the contribution of each study to the overall results and network structure. We interpreted threshold results with respect to the risk of bias identified for each study, and this is reflected in the conclusions. For exacerbations, the comparison of macrolides to control could change due to plausible bias adjustment, suggesting the possibility that quinolones might be the best class of treatment agents for preventing exacerbations. All other antibiotic versus placebo comparisons were robust to any changes in the evidence, as no implausibly large bias was present that would favour placebo. Consequently, direct comparison of macrolides with quinolones in future RCTs could be informative.
Potential biases in the review process
We followed our pre‐published protocol when carrying out this network meta‐analysis. We included reviews that intended to compare placebo‐controlled trials and head‐to‐head antibiotic comparisons. We checked our included studies with two previously published Cochrane Reviews (Herath 2018; Threapleton 2018). As most of the studies that we identified were also included in these reviews, we were confident that we had identified all relevant studies for the NMA. These studies had already been assessed for risk of bias, and data had been extracted by two review authors; however, we arranged the data in the format required for NMAs. Updated searches identified two new studies that were published in 2019, which met the inclusion criteria for the NMAs. We did not use GRADE nor CINeMA to assess certainty of evidence, as we used the threshold analysis as an alternative approach.
Agreements and disagreements with other studies or reviews
To date, no published network meta‐analyses have specifically investigated prophylactic antibiotics for people with COPD. However, several published systematic reviews and meta‐analyses have investigated the effectiveness of prophylactic antibiotics for a subgroup of people with moderate to severe COPD (Cui 2018; Donath 2013; Herath 2018; Huckle 2018; Lee 2013; Ni 2015; Wang 2018; Yao 2013).
Pair‐wise comparisons of prophylactic antibiotics with placebo or with each other were previously published in two Cochrane Reviews (Herath 2018; Threapleton 2018). Herath 2018 compared prophylactic antibiotics with placebo and included 14 trials (3932 participants). These review authors found that prophylactic antibiotics, specifically macrolides (continuous or intermittent), were beneficial in reducing exacerbations among COPD patients. There was probable benefit for patient‐reported quality of life with antibiotics compared with placebo, but this did not reduce the number of deaths due to any cause nor frequency of hospitalisation nor lung function loss during the study period (Herath 2018). Threapleton 2018 compared different classes of prophylactic antibiotics with each other and identified only two trials (391 participants) of short duration in which antibiotics were compared head‐to‐head in COPD patients. There was no clear difference between one antibiotic and another in reducing exacerbations or quality of life. No deaths were reported in one study, but eight people died in the other study. Very similar numbers of people in both studies experienced serious side effects. These numbers were small, and overall it is unclear whether one antibiotic treatment type caused more side effects than another (Threapleton 2018).
Herath 2018 concluded that prophylactic macrolide antibiotics used up to 12 months are likely to reduce the number of people who experience one or more exacerbations (exacerbation frequency) and to increase the median time to first exacerbation, while improving health‐related quality of life. As the head‐to‐head comparison of antibiotics was not clear due to insufficient information, no specific inferences were made in the review (Threapleton 2018). Evidence for exacerbations in Herath 2018 was of moderate certainty and shows overall benefit of antibiotics in reducing exacerbations using GRADE. This review did not conduct subgroup analysis according to treatment class, but when antibiotics were subgrouped according to antibiotic regimen, macrolides seemed to be most effective in reducing the number of people who had exacerbations (Herath 2018).
In our search of relevant evidence, we have identified the same published trials that were included in published reviews, with the exception of some new trials published in 2019. By using these placebo‐controlled trials, we have been able to indirectly compare different antibiotic classes with each other and with placebo treatment. Previous published evidence shows that macrolides can be of benefit for reducing exacerbations in people with moderate to severe COPD. In addition, British Thoracic Society (BTS) and NICE guidance recommends macrolide use for the moderate to severe COPD population subgroup who have frequent exacerbations requiring steroid therapy, who do not currently smoke, and who have had at least one exacerbation requiring hospitalisation per year (NICE 2016; Smith 2020). BTS guidance suggests that long‐term macrolide treatment could be considered for a minimum of 6 months up to 12 months until its impact on exacerbations is assessed (Smith 2020). Our NMA results show that macrolides rank higher than quinolones, tetracyclines, and placebo in reducing exacerbations, even though the duration of treatment across studies varies from 12 to 52 weeks.
Previous published evidence suggests that macrolides can improve quality of life in people with moderate to severe COPD who are taking macrolides (Cui 2018; Herath 2018; Ni 2015; Wang 2018); however, this improvement was not shown to reach the MCID of 4 points. Similarly, results from the NMA show some improvement in quality of life with macrolide treatment, as measured by SGRQ, and in line with published evidence, this improvement does not reach the MCID.
Published reviews have reported increased risk of side effects associated with longer antibiotic treatment duration (Lee 2013; Ni 2015; Wang 2018; Yao 2013). Results from our NMA indicate that with macrolide treatment, the number of people experiencing one or more SAE is reduced compared to placebo. However, we did not investigate all adverse events or side effects, which may be increased overall by antibiotic treatment. Adverse effects most commonly associated with macrolide treatment were hearing impairment, gastrointestinal problems, and nausea, as well as others (Table 22). As suggested by BTS guidance, the risk‐to‐benefit balance needs to be considered by clinicians when they administer macrolides (Smith 2020).
Authors' conclusions
Implications for practice.
The NMA in this review compared macrolides, quinolones, and tetracyclines with each other and with placebo in people with moderate to severe COPD, who were already taking concomitant medications (e.g. long‐acting beta agonist (LABA), long‐acting muscarinic antagonist (LAMA), inhaled corticosteroid (ICS), anticholinergics, short‐acting beta agonist (SABA)), and who may have experienced exacerbations, as not all studies reported previous exacerbations. Overall, this NMA shows that treatment with macrolide reduced exacerbations, improved quality of life scores, and reduced serious adverse events in comparison to placebo. This was reflected in ranking of antibiotics, as macrolide was ranked first in reducing exacerbations. Smaller benefit of taking quinolone compared to placebo was noted (ranked second); however, taking tetracycline was not better than taking placebo. It should be noted that certainty of these effects, as measured by precision, was reflected in the 95% CrI, and certainty with respect to robustness of results was seen in results of the threshold analysis.
Our findings are also in line with other guidance, specifically, that provided in GOLD 2020 and NICE 2018. Although we did not explore the impact of prophylactic antibiotics in different patient subgroups, given the trade‐off between effectiveness, safety, and risk of antibiotic resistance, it is only appropriate to consider prophylactic administration of antibiotics for selected patients, such as those experiencing frequent exacerbations. Decisions on antibiotic use would depend on clinical assessment and discussion with patients about benefits and risks associated with long‐term use of prophylactic antibiotics.
It it interesting to note that none of the eligible studies excluded patients with previously isolated non‐tuberculous mycobacteria, with a prolonged QTc on their electrocardiogram, or with hearing loss. In clinical practice, the former group would not be eligible for prophylactic administration of antibiotics due to the risk of developing resistant non‐tuberculous mycobacteria. Moreover, long‐term use of macrolides would be contraindicated for patients with prolonged QTc, and long‐term use of quinolones should be discouraged. Hearing loss is also a contraindication for the use of long‐term macrolides due to ototoxicity (Rubinstein 2002; Smith 2020).
Implications for research.
Most of the evidence in this NMA comes from studies investigating macrolides and quinolones, with sparse data derived from studies of tetracyclines and combinations of drug classes. Larger studies of head‐to‐head comparisons between macrolides and quinolones may help to further clarify the relative benefit of each drug and to determine subgroups that may respond more favourably to alternative classes. Careful and transparent reporting of baseline characteristics and potential effect modifiers by trialists will assist future evidence synthesis. Evaluation of the impact of prophylactic antibiotics on more selected populations would also be useful, as would evaluation of their impact on different types of COPD exacerbations.
Although studies involving tetracyclines may be informative, given the lack of evidence of benefit and the suggestion of possible harm, it seems unlikely that such trials will be carried out in the future.
Studies investigating alternatives to oral antibiotics may be useful, for example, studies of inhaled antibiotics. This route of administration was not considered in this review but could be considered for future network meta‐analysis if sufficient evidence became available.
Given the anti‐inflammatory effects of macrolides, understanding their impact on infective versus non‐infective exacerbations also warrants further investigation.
Given that this NMA was based on two previous reviews that undertook pair‐wise analyses separately, it will be decided in the future whether we will update the two previous reviews, or update this NMA to incorporate the pair‐wise analyses to keep all information in one review.
History
Protocol first published: Issue 11, 2018 Review first published: Issue 1, 2021
Acknowledgements
Ian Yang was the Editor of this review and commented critically on the review.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
The review authors acknowledge the voluntary translational work performed by RJP Scholten, Hans van der Wouden, and Rachael Kelly.
The review authors and the Cochrane Airways editorial team are grateful to Richard Albert, Terence Ho, and David JW Evans for their peer review comments.
This project was funded by the National Institute for Health Research Systematic Reviews Programme (project number 16/114/21). This project was also supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Health Research Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Register of Trials
Electronic searches: core databases
| Database | Dates searched | Frequency of search |
| CENTRAL (via the Cochrane Register of Studies (CRS)) | From inception | Monthly |
| MEDLINE (Ovid) | 1946 onwards | Weekly |
| Embase (Ovid) | 1974 onwards | Weekly |
| PsycINFO (Ovid) | 1967 onwards | Monthly |
| CINAHL (EBSCO) | 1937 onwards | Monthly |
| AMED (EBSCO) | From inception | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify studies in other electronic databases.
Appendix 2. Search strategies to identify relevant studies
Cochrane Airways Trials Register (via Cochrane Register of Studies)
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD OR AECOPD):TI,AB,KW
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 MESH DESCRIPTOR Anti‐Bacterial Agents EXPLODE ALL
#8 (antibiotic* or antibacterial or anti‐bacterial):ti,ab,kw
#9 (prophylactic or prophylaxis or prevent*):ti,ab,kw
#10 (long‐term OR "long term"):ti,ab,kw
#11 (#7 OR #8) AND (#9 OR #10)
#12 MESH DESCRIPTOR Antibiotic Prophylaxis EXPLODE ALL
#13 Penicillin or phenoxymethylpenicillin or phenethicillin or amoxicillin or amoxicillin or "clavulanic acid" or tetracycline or oxytetracycline or doxycycline or quinolone or ciprofloxacin or moxifloxacin or gemifloxacin or levofloxacin or macrolide or erythromycin or roxithromycin or azithromycin or clarithromycin or telithromycin or sulphonamide or co‐trimoxazole or sulphaphenazole or trimethoprim or sigmamycin or tetracycline or oleandomycin or sulfamethoxazole or sulfaphenazole or sulphonamide or cephalosporin
#14 #11 OR #12 OR #13
#15 #14 AND #6
#16 INREGISTER
#17 #15 AND #16
ClinicalTrials.gov
| Search field | Search terms |
| Study type | Interventional |
| Condition | COPD |
| Intervention | Penicillin OR phenoxymethylpenicillin OR phenethicillin OR amoxicillin OR amoxicillin OR "clavulanic acid" OR tetracycline OR oxytetracycline OR doxycycline OR quinolone OR ciprofloxacin OR moxifloxacin OR gemifloxacin OR levofloxacin OR macrolide OR erythromycin OR roxithromycin OR azithromycin OR clarithromycin OR telithromycin OR sulphonamide OR co‐trimoxazole OR sulphaphenazole OR trimethoprim OR sigmamycin OR tetracycline OR oleandomycin OR sulfamethoxazole OR sulfaphenazole OR sulphonamide OR cephalosporin |
WHO Trials Registry
| Search field | Search terms |
| Condition | COPD |
| Intervention | Penicillin OR phenoxymethylpenicillin OR phenethicillin OR amoxicillin OR amoxicillin OR "clavulanic acid" OR tetracycline OR oxytetracycline OR doxycycline OR quinolone OR ciprofloxacin OR moxifloxacin OR gemifloxacin OR levofloxacin OR macrolide OR erythromycin OR roxithromycin OR azithromycin OR clarithromycin OR telithromycin OR sulphonamide OR co‐trimoxazole OR sulphaphenazole OR trimethoprim OR sigmamycin OR tetracycline OR oleandomycin OR sulfamethoxazole OR sulfaphenazole OR sulphonamide OR cephalosporin |
Appendix 3. Summary of results from Herath 2018 and Threapleton 2018
| Review | Results | Conclusions | Studies included in the review |
|
Herath 2018 Herath 2018 Comparison: prophylactic antibiotic vs placebo |
Outcomes Exacerbations
Health‐related quality of life
Serious adverse events
Antibiotic resistance
|
Conclusions Use of macrolide antibiotics for up to 12 months is likely to reduce the number of patients experiencing exacerbations and to improve quality of life. Selection of patients for prophylactic antibiotic use is critical, but the evidence base is poor, as selection criteria were different across studies. Some serious adverse events may occur, and antibiotic resistance is a concern, especially for those who are colonised with Pseudomonas. There is still uncertainty about long‐term effects of prophylactic antibiotic use |
Albert 2011; Banerjee 2005; Berkhof 2013; Brill 2015; He 2010; Mygind 2010; Seemungal 2008; Sethi 2010; Shafuddin 2015; Simpson 2014; Suzuki 2001; Tan 2016; Uzun 2014; Wang 2017 |
|
Threapleton 2018 Threapleton 2018 Comparison: prophylactic antibiotic vs another |
Macrolide plus tetracycline vs macrolide (roxithromycin plus doxycycline vs roxithromycin) Outcomes reported by Shafuddin 2015 Exacerbations
Quality of life CRQ sub‐scales for dyspnoea, fatigue, emotional function, and mastery: no differences between continuous combined treatment compared to continuous single‐antibiotic treatment, and did not reach clinical significance Drug resistance No evidence Serious adverse events
Lung function No clear differences in FEV₁ or FVC Mortality and adverse events No clear differences in all‐cause mortality nor all‐cause and treatment‐related adverse events (all very low‐certainty evidence) Quinolone vs tetracycline (moxifloxacin vs doxycycline) Outcomes reported by Brill 2015 Exacerbations
Quality of life No evidence Drug resistance No evidence Serious adverse events No reported serious adverse events in either treatment arm Mortality No deaths reported in either treatment arm Lung function, hospitalisations, number of people colonised with P aeruginosa No evidence Quinolone vs macrolide (moxifloxacin vs azithromycin) Outcomes reported by Brill 2015 Exacerbations
Quality of life No evidence Drug resistance No evidence Serious adverse events No reported serious adverse events in either treatment group Mortality No deaths reported Lung function, adverse events, number of people colonised with P aeruginosa No evidence Macrolide vs tetracycline (azithromycin vs doxycycline) Outcomes reported by Brill 2015 Exacerbations
Quality of life No evidence Drug resistance No evidence Serious adverse events No reported serious adverse events in either treatment group Mortality No deaths reported Lung function, adverse events, number of people colonised with P aeruginosa No evidence |
Unclear evidence about efficacy or safety between different classes or regimens of prophylactic antibiotics for 12 to 13 weeks for people with COPD. Small sample sizes in both RCTs and no head‐to‐head comparisons of antibiotic resistance resulted in very low certainty of findings. Evidence was insufficient to inform clinical practice | Brill 2015; Shafuddin 2015 |
Abbreviations
CI: confidence interval; COPD: chronic obstructive pulmonary disease; CRQ: Chronic Respiratory Disease Questionnaire; FEV₁: forced expiratory volume in one second; FVC: forced vital capacity; MD: mean difference; MID: minimally important difference; NNTB: number needed to treat for an additional beneficial outcome; OR: odds ratio; RCT: randomised controlled trial; SAE: serious adverse event; SGRQ: St George’s Respiratory Questionnaire.
Appendix 4. Exacerbations: sensitivity analysis
Methods
Suzuki 2001 was included in the network meta‐analysis (NMA) for exacerbations as a sensitivity analysis, and model fit was re‐evaluated to select the preferred NMA model. Study characteristics are summarised in Table 1. The network plot of interventions is presented in the online supplement (Janjua 2021, Figure A4.1). As no loops of evidence were added, there was no potential to detect inconsistency, so this was not checked.
Table 1. Characteristics of Suzuki 2001
| Author | Class/Antibiotic | Dose/Regimen | COPD severity | Definition of exacerbations | Duration of treatment | Risk of bias |
| Suzuki 2001 (N = 109); Japan (unclear setting) | Macrolide/ Erythromycin |
200 to 400 mg once daily continuous |
Moderate/severe FEV₁ 1.38 L at baseline |
Acute and sustained worsening of COPD symptoms requiring changes to regular treatment, including antimicrobial therapy and/or short courses of systemic steroids Mild/moderate exacerbation: treatment without hospitalisation Severe exacerbation: requiring hospitalisation |
52 weeks | Study was not blinded, high risk of bias for performance and detection domains, funding not stated |
Footnotes:
COPD: chronic obstructive pulmonary disease; FEV₁: forced expiratory volume in one second.
Fixed class models with fixed‐ and random‐treatment effects were considered, but the fixed‐treatment effect model fitted poorly. A fixed treatment effect model with exchangeable (random) class effects for the macrolide class and fixed class effect for quinolone and tetracycline classes (these classes had only 1 and 2 elements, respectively; therefore, there was not enough information to estimate within‐class variability) was fitted. Within‐class variance for macrolides was given ‐ uniform(0,5) priori distribution.
Between‐study heterogeneity was explained by splitting the macrolide class into its component treatments in the exchangeable class model with fixed treatment effects across studies; therefore this model was selected (Table 2). A fixed effect model with no class effect (i.e. assuming each treatment had a distinct effect) was also fitted for comparison and had equivalent deviance information criterion (DIC) and similar fit to the chosen model (Table 2). As this was a more complex model, it was not selected.
Table 2. Sensitivity analysis: model fit statistics
| DIC | SD (95% CrI) | Total residual deviance* | |
| Fixed class models | |||
| Fixed treatment effect | 76.66 | ‐ | 31.52 |
| Random treatment effects | 68.72 | 0.44 (0.11‐0.98) | 17.4 |
| Exchangeable (random) class models | |||
| Fixed treatment effect | 69.0 | ‐ | 19.19 |
| Separate treatment effects (no class) models | |||
| Fixed treatment effect | 68.81 | ‐ | 17.76 |
Footnotes:
* Compare to 16 data points
CrI: credible interval; DIC: deviance information criterion; SD: standard deviation.
Sensitivity analysis
The network meta‐analysis (NMA) with Suzuki 2001 included a total of 2880 participants (online supplement, Janjua 2021, Figure A4.2; Table 3). Hazard ratios (HRs) for each class were compared to each other. Within‐class variance for macrolides was estimated as 0.57 (95% credible interval (CrI) 0.12 to 4.88) on the log‐HR scale (online supplement,Janjua 2021, Figure A4.2; Table 3).
Table 3. Numbers of trials and participants and relative estimates for all class comparisons
| Comparison | Hazard ratios | No. of trials | No. of patients | ||
| Intervention | Comparator | Median | 95% CrI | ||
| Macrolide | Placebo | 0.53 | 0.27 to 0.90 | 9 | 1618 |
| Tetracycline | Placebo | 1.26 | 0.63 to 2.33 | 1 | 49 |
| Quinolone | Placebo | 0.88 | 0.75 to 1.04 | 2 | 1198 |
| Tetracycline | Macrolide | 2.39 | 1.01 to 5.78 | 1 | 49 |
| Quinolone | Macrolide | 1.66 | 0.97 to 3.32 | 1 | 50 |
| Quinolone | Tetracycline | 0.70 | 0.37 to 1.40 | 1 | 50 |
Footnotes:
CrI: credible interval interval.
We compared each treatment to placebo (online supplement, Janjua 2021, Figure A4.3; Table 4). HR treatment effects from the model with no class showed similar results to the exchangeable‐class model, with all treatments except doxycycline reducing exacerbations against placebo (HR 1.12, 95% CrI 0.54 to 2.25).
Table 4. Sensitivity analysis: numbers of trials and participants and relative estimates for all treatment comparisons
| Treatment comparison | Hazard ratios: fixed effects (no class model) | Hazard ratios: fixed effects (exchangeable class model) | No. of trials (no. of participants) | |||
| Intervention | Comparator | Median | 95% CrI | Median | 95% CrI | |
| AZM 250 mg once daily | Placebo | 0.72 | 0.62 to 0.84 | 0.71 | 0.61 to 1.82 | 2 (1147) |
| AZM 250 mg once daily (three times per week ) | AZM 250 mg once daily | 0.80 | 0.44 to 1.45 | 0.84 | 0.50 to 1.36 | 0 (0) |
| AZM 250 mg once daily (three times per week) | Placebo | 0.58 | 0.32 to 1.03 | 0.60 | 0.36 to 0.96 | 2 (133) |
| AZM 500 mg once daily (three times per week) | AZM 250 mg once daily (3x weekly) |
0.93 | 0.48 to 1.78 | 0.91 | 0.51 to 1.57 | 0 (0) |
| AZM 500 mg once daily (three times per week) | AZM 250 mg once daily | 0.74 | 0.52 to 1.04 | 0.75 | 0.54 to 1.04 | 0 (0) |
| AZM 500 mg once daily (three times per week) | Placebo | 0.53 | 0.39 to 0.73 | 0.54 | 0.40 to 0.72 | 2 (108) |
| ERY 250 mg twice daily | AZM 500 mg once daily (three times per week) |
1.19 | 0.79 to 1.81 | 1.16 | 0.79 to 1.74 | 0 (0) |
| ERY 250 mg twice daily | AZM 250 mg once daily (three times per week) |
1.12 | 0.58 to 2.10 | 1.05 | 0.62 to 1.83 | 0 (0) |
| ERY 250 mg twice daily | AZM 250 mg once daily | 0.88 | 0.64 to 1.21 | 0.88 | 0.65 to 1.18 | 0 (0) |
| ERY 250 mg twice daily | Placebo | 0.64 | 0.48 to 0.85 | 0.63 | 0.48 to 0.82 | 10 (109) |
| ERY 125 mg three times daily | ERY 250 mg twice daily | 0.86 | 0.46 to 1.62 | 0.88 | 0.50 to 1.50 | 0 (0) |
| ERY 125 mg three times daily | AZM 500 mg once daily (three times per week) |
1.03 | 0.54 to 1.98 | 1.02 | 0.58 to 1.80 | 0 (0) |
| ERY 125 mg three times daily | AZM 250 mg once daily (three times per week) |
0.96 | 0.42 to 2.14 | 0.93 | 0.47 to 1.80 | 0 (0) |
| ERY 125 mg three times daily | AZM 250 mg once daily | 0.76 | 0.42 to 1.37 | 0.78 | 0.46 to 1.27 | 0 (0) |
| ERY 125 mg three times daily | Placebo | 0.55 | 0.31 to 0.97 | 0.55 | 0.33 to 0.89 | 1 (36) |
| ERY 200 to 400 mg once daily | ERY 125mg three times daily | 0.24 | 0.08 to 0.67 | 0.47 | 0.15 to 1.06 | 0 (0) |
| ERY 200 to 400 mg once daily | ERY 250 mg twice daily | 0.21 | 0.07 to 0.51 | 0.41 | 0.14 to 0.97 | 0 (0) |
| ERY 200 to 400 mg once daily | AZM 500 mg once daily (three times per week) |
0.25 | 0.09 to 0.62 | 0.48 | 0.17 to 1.04 | 0 (0) |
| ERY 200 to 400 mg once daily | AZM 250 mg once daily (three times per week) |
0.23 | 0.07 to 0.64 | 0.44 | 0.14 to 1.02 | 0 (0) |
| ERY 200 to 400 mg once daily | AZM 250 mg once daily | 0.18 | 0.07 to 0.43 | 0.36 | 0.13 to 0.89 | 0 (0) |
| ERY 200 to 400 mg once daily | Placebo | 0.13 | 0.05 to 0.31 | 0.25 | 0.09 to 0.60 | 1 (109) |
| DOX 100 mg once daily | ERY 200‐400 mg once daily | 8.35 | 2.77 to 27.91 | 4.93 | 1.66 to 15.88 | 0 (0) |
| DOX 100 mg once daily | ERY 125 mg three times daily | 2.02 | 0.81 to 4.97 | 2.28 | 1.00 to 5.02 | 0 (0) |
| DOX 100 mg once daily | ERY 250 mg twice daily | 1.74 | 0.80 to 3.74 | 2.01 | 0.97 to 3.93 | 0 (0) |
| DOX 100 mg once daily | AZM 500 mg once daily (three times per week) |
2.09 | 0.94 to 4.53 | 2.34 | 1.12 to 4.67 | 0 (0) |
| DOX 100 mg once daily | AZM 250 mg once daily (three times per week) |
1.93 | 0.89 to 4.08 | 2.11 | 1.00 to 4.31 | 1 (50) |
| DOX 100 mg once daily | AZM 250 mg once daily | 1.54 | 0.73 to 3.19 | 1.76 | 0.88 to 3.33 | 0 (0) |
| DOX 100 mg once daily | Placebo | 1.12 | 0.54 to 2.25 | 1.26 | 0.63 to 2.33 | 1 (49) |
| MOX 400 mg once daily (5 days every 4 weeks) |
DOX 100 mg once daily | 0.55 | 0.23 to 1.23 | 0.70 | 0.37 to 1.40 | 1 (50) |
| MOX 400 mg once daily (5 days every 4 weeks) |
ERY 200‐400 mg once daily | 4.59 | 1.37 to 15.69 | 3.47 | 1.47 to 9.44 | 0 (0) |
| MOX 400 mg once daily (5 days every 4 weeks) |
ERY 125 mg three times daily | 1.10 | 0.41 to 2.89 | 1.60 | 0.96 to 2.71 | 0 (0) |
| MOX 400 mg once daily (5 days every 4 weeks) |
ERY 250 mg twice daily | 0.96 | 0.40 to 2.17 | 1.41 | 1.03 to 1.92 | 0 (0) |
| MOX 400 mg once daily (5 days every 4 weeks) |
AZM 500 mg once daily (3x weekly) |
1.14 | 0.47 to 2.62 | 1.64 | 1.18 to 2.31 | 0 (0) |
| MOX 400 mg once daily (5 days every 4 weeks) |
AZM 250 mg once daily (3x weekly) |
1.06 | 0.45 to 2.40 | 1.48 | 0.91 to 2.47 | 1 (50) |
| MOX 400 mg once daily (5 days every 4 weeks) |
AZM 250 mg once daily | 0.85 | 0.37 to 1.86 | 1.24 | 1.00 to 1.55 | 0 (0) |
| MOX 400 mg once daily (5 days every 4 weeks) |
Placebo | 0.61 | 0.27 to 1.32 | 0.88 | 0.75 to 1.04 | 1 (49) |
| MOX 400 mg once daily (5 days every 8 weeks) |
MOX 400 mg once daily (5 days every 4 weeks) |
1.47 | 0.67 to 3.40 | 1.00 | 1.00 to 1.00 | 0 (0) |
| MOX 400 mg once daily (5 days every 8 weeks) |
DOX 100 mg once daily | 0.81 | 0.39 to 1.69 | 0.70 | 0.37 to 1.40 | 0 (0) |
| MOX 400 mg once daily (5 days every 8 weeks) |
ERY 200‐400 mg once daily | 6.68 | 2.83 to 18.35 | 3.47 | 1.47 to 9.44 | 0 (0) |
| MOX 400 mg once daily (5 days every 8 weeks) |
ERY 125 mg three times daily | 1.63 | 0.90 to 2.95 | 1.60 | 0.96 to 2.71 | 0 (0) |
| MOX 400 mg once daily (5 days every 8 weeks) |
ERY 250 mg twice daily | 1.41 | 1.01 to 1.96 | 1.41 | 1.03 to 1.92 | 0 (0) |
| MOX 400 mg once daily (5 days every 8 weeks) |
AZM 500 mg once daily (three times per week) |
1.68 | 1.18 to 2.39 | 1.64 | 1.18 to 2.31 | 0 (0) |
| MOX 400 mg once daily (5 days every 8 weeks) |
AZM 250 mg once daily (three times per week) |
1.55 | 0.85 to 2.82 | 1.48 | 0.91 to 2.47 | 0 (0) |
| MOX 400 mg once daily (5 days every 8 weeks) |
AZM 250mg once daily2 (133) | 1.25 | 1.00 to 1.56 | 1.24 | 1.00 to 1.55 | 0 (0) |
| MOX 400 mg once daily (5 days every 8 weeks) |
Placebo | 0.90 | 0.76 to 1.06 | 0.88 | 0.75 to 1.04 | 1 (1149) |
Footnotes:
AZM: azithromycin; CrI: credible interval; DOX: doxycycline; ERY: erythromycin; MOX: moxifloxacin.
Rank statistics for the four classes were investigated (online supplement, Janjua 2021, Figure A4.4; Table 5). Figure 3.4 shows the rank probabilities of each class; the vertical axis represents the probability of being ranked best (first) to worst (fourth). The probability of macrolides being ranked first was 0.96. Treatment‐specific rankings of the exchangeable class model were analysed (Table 6) and showed that erythromycin 200 to 400 mg specifically was the highest ranking treatment.
Table 5. Sensitivity analysis: total number of participants and rank statistics for each class (sorted by mean rank)
| Class | Number of patients | Mean | Median | 95% CrI |
| Macrolide | 823 | 1.1 | 1 | 1 to 2 |
| Quinolone | 594 | 2.2 | 2 | 2 to 3 |
| Placebo | 1399 | 3.2 | 3 | 2 to 4 |
| Tetracycline | 25 | 3.6 | 4 | 2 to 4 |
Footnotes:
CrI: credible interval.
Table 6. Sensitivity analysis: rank statistics for each treatment (fixed treatment‐exchangeable class model)
| Treatment | Class | Mean | Median | 95% CrI |
| ERY 200 to 400 once daily | Macrolide | 1.1 | 1 | 1 to 3 |
| AZM 500 od (three times weekly) | Macrolide | 3.1 | 3 | 2 to 6 |
| ERY 125 three times daily | Macrolide | 3.4 | 3 | 1 to 8 |
| AZM 250 once daily (three times weekly) | Macrolide | 4.0 | 4 | 2 to 8 |
| ERY 250 twice daily | Macrolide | 4.3 | 4 | 2 to 6 |
| AZM 250 once daily | Macrolide | 6.1 | 6 | 4 to 8 |
| MOX 400 once daily (5 days every 8 weeks) | Quinolone | 8.1 | 8 | 7 to 9 |
| MOX 400 once daily (5 days every 4 weeks) | Quinolone | 8.1 | 8 | 7 to 9 |
| Placebo | ‐ | 9.1 | 9 | 7 to 10 |
| DOX 100 once daily | Tetracycline | 9.3 | 10 | 5 to 10 |
Footnotes:
AZM: azithromycin; CrI: credible interval; DOX: doxycycline; ERY: erythromycin; MOX: moxifloxacin.
Inclusion of Suzuki 2001 led to increased heterogeneity in effects of macrolides compared to placebo, which meant that the fixed effect class model could no longer be selected. Figure A4.3 (Supplement 1) illustrates this heterogeneity in the different estimated effects for macrolides compared to placebo. This increased within‐class variability led to wider 95% credible interval (CrI) around the estimated effect of macrolides compared to placebo, although there was no meaningful change in ranking of classes compared to the main analysis.
Appendix 5. Change from baseline in SGRQ: sensitivity analysis with inclusion of Brill 2015 study
Sensitivity analysis with estimates included for Brill 2015 study
In the main analysis, relative effect estimates extracted from the fully adjusted analysis presented in Brill 2015 were used.
Table 1. Characteristics of Brill 2015 study
| Author | Class/antibiotic | Dose | COPD severity | Duration of treatment | Risk of bias |
|
Brill 2015 (N = 99); UK (1 outpatient hospital department) |
|
|
Moderate to severe FEV₁ 1.4 L |
13 weeks | Unclear performance bias; detection bias judged as high |
Abbreviations
COPD: chronic obstructive pulmonary disease; FEV₁: forced expiratory volume in one second.
In a sensitivity analysis, we included relative effects from the additional analysis presented in Brill 2015, which was adjusted only for baseline values (Table 2).
Table 2. Change in baseline of SGRQ: included studies
| Study |
Endpoint (weeks) |
Treatments compared | Mean difference vs placebo | SE of mean difference | |||||||
| Albert 2011 | 52 | Placebo | Azithromycin 250 mg once daily |
‐2.2 | 0.7853 | ||||||
| Berkhof 2013 | 12 | Placebo | Azithromycin 250 mg once daily three times per week |
‐7.5 | 2.5456 | ||||||
| He 2010 | 26 | Placebo | Erythromycin 125 mg three times daily |
‐3 | 5.6801 | ||||||
| Sethi 2010 | 48 | Placebo | Moxifloxacin 400 mg once daily (5 days every 8 weeks) |
‐1.2 | 0.9231 | ||||||
| Simpson 2014 | 12 | Placebo | Azithromycin 250 mg once daily |
6.1 | 5.31927 | ||||||
| Uzun 2014 | 52 | Placebo | Azithromycin 500 mg once daily three times per week |
‐0.61 | 2.622449 | ||||||
| Brill 2015* | 13 | Placebo | Doxycycline 100 mg once daily |
Azithromycin 250 mg once daily three times per week |
Moxifloxacin 400 mg once daily (5 days every 4 weeks) |
1.02 | ‐2.29 | ‐1.88 | 3.135 | 3.212 | 3.426 |
| 0.88 | ‐2.35 | ‐2.25 | 3.132 | 3.085 | 3.233 | ||||||
Footnotes:
mg: milligrams; SE: standard error; SGRQ: St George's Respiratory Questionnaire.
Both models fit well and had a similar DIC. Fixed‐treatment effect, fixed‐class effect model was selected as it was the least complex model (Table 3).
Table 3. Sensitivity analysis: model fit statistics for fixed class models
| DIC | Between‐study SD (95% CrI) | Total residual deviance* | |
| Fixed class models | |||
| Fixed treatment effect | 43.2 | ‐ | 10.5 |
| Random treatment effects | 44.3 | 2.04 (0.08 to 6.91) | 9.4 |
Footnotes:
* Compare to 9 data points
CrI: credible interval; DIC: device information criterion; SD: standard deviation.
Results produced by the main analysis and the sensitivity analysis were broadly similar in terms of both relative effects (online supplement, Janjua 2021,Table 4; Figure A5.1) and rank statistics (Table 5). However, in the sensitivity analysis, the 95% credible interval (CrI) for the mean difference in St George's Respiratory Questionnaire (SGRQ) score for quinolone compared to tetracycline no longer included zero.
Table 4. Sensitivity analysis (Brill 2015): number of trials, number of patients, and relative effects for all class comparisons
| Treatment class comparison | Number of trials | Number of participants | Sensitivity analysis | |||||
| Random effects ‐ fixed class effect (uniform prior) | Random effects ‐ fixed class effect (empirical prior) | Fixed effects ‐ fixed class effect | ||||||
| MD | 95% CrI | MD | 95% CrI | MD | 95% CrI | |||
| Macrolide versus placebo | 6 | 1158 | ‐2.23 | ‐5.16 to 0.87 | ‐2.25 | ‐4.13 to ‐0.39 | ‐2.21 | ‐3.47 to ‐0.96 |
| Tetracycline versus placebo | 1 | 49 | 1.16 | ‐4.40 to 6.85 | 1.13 | ‐2.23 to 4.39 | 1.16 | ‐1.17 to 3.50 |
| Quinolone versus placebo | 2 | 1078 | ‐1.60 | ‐5.91 to 2.72 | ‐1.59 | ‐4.03 to 0.71 | ‐1.49 | ‐3.03 to 0.06 |
| Tetracycline versus macrolide | 1 | 50 | 3.39 | ‐2.24 to 8.86 | 3.39 | 0.28 to 6.47 | 3.38 | 1.25 to 5.51 |
| Quinolone versus macrolide | 1 | 50 | 0.63 | ‐4.13 to 5.12 | 0.66 | ‐1.94 to 3.18 | 0.72 | ‐1.02 to 2.48 |
| Quinolone versus tetracycline | 1 | 50 | ‐2.76 | ‐8.69 to 2.96 | ‐2.73 | ‐6.17 to 0.66 | ‐2.65 | ‐5.11 to ‐0.20 |
Footnotes:
CrI: credible interval; MD: mean difference.
Table 5. Change from baseline in SGRQ‐ sensitivity analysis (Brill 2015): number of participants and rank statistics for each class (sorted by mean rank)
| Treatment class | Number of patients | Mean | Median | 95% CrI |
| Macrolide | 578 | 1.46 | 1 | 1 to 3 |
| Quinolone | 528 | 1.93 | 2 | 1 to 4 |
| Placebo | 1106 | 3.08 | 3 | 2 to 4 |
| Tetracycline | 25 | 3.54 | 4 | 1 to 4 |
Footnotes:
CrI: credible interval;SGRQ: St. George's Respiratory Questionnaire
Sensitivity analysis including data from Singh 2019 study
Updated searches conducted in February 2020 identified one study (Singh 2019), which was eligible for inclusion. The two‐arm study compared doxycycline 100 od to standard therapy. A network diagram of interventions including Singh 2019 is presented in Figure A5.2 (online supplement, Janjua 2021). Data from the study have been included in a sensitivity analysis (Table 6). The fixed treatment effect‐fixed class effect model was selected as it was the least complex model (online supplement, Janjua 2021, Figure A5.3, Table 6, Table 7).
Table 6. Table of interventions and treatment classes including data from Singh 2019
| Intervention | Treatment class | Number of patients randomised to each treatment | |
| 1 | Placebo | Placebo | 1136 |
| 2 | Azithromycin 250 mg once daily | Macrolide | 459 |
| 3 | Azithromycin 250 mg once daily three times per week | Macrolide | 62 |
| 4 | Doxycycline 100 mg once daily | Tetracycline | 55 |
| 5 | Moxifloxacin 400 mg once daily (5 days every 4 weeks) | Quinolone | 25 |
| 6 | Erythromycin 125 mg three times per day | Macrolide | 16 |
| 7 | Moxifloxacin 400 mg once daily (5 days every 8 weeks) | Quinolone | 503 |
| 8 | Azithromycin 500 mg once daily three times per week | Macrolide | 41 |
Footnotes:
mg: milligrams
The network meta‐analysis (NMA) included a total of 2792 participants. Compared to the main analysis, inclusion of data from Singh 2019 shifted the estimates of each class comparison including tetracycline in favour of the comparator. Relative effects for each class favoured macrolides compared with placebo using a fixed effect model. However, this effect was not observed in a random effects model (Supplement 2, Figure A5.4). Median rank scores also differed from those in the main analysis: quinolone median rank 2 (95% CrI second to third), tetracycline median rank 3 (95% CrI third to fourth), and placebo median rank 4 (95% CrI third to fourth) (Table 8).
Table 7. Sensitivity analysis including Singh 2019, model fit statistics
| DIC | Between‐study SD (95% CrI) | Total residual deviance* | |
| Fixed class models | |||
| Fixed treatment effect | 51.1 | ‐ | 13.97 |
| Random treatment effects | 51.31 | 2.37 (0.09 to 6.92) | 10.79 |
| Fixed treatment effect, random class effect | 51.38 | ‐ | 12.82 |
Footnotes:
* Compare to 10 data points
CrI: credible interval; DIC: device information criterion.
Table 8. Sensitivity analysis including Singh 2019, number of participants and rank statistics for each class (sorted by mean rank)
| Treatment class | Number of patients | Mean | Median | 95% CrI |
| Macrolide | 578 | 1.18 | 1 | 1 to 2 |
| Quinolone | 528 | 3.26 | 3 | 2 to 4 |
| Placebo | 1136 | 3.53 | 4 | 3 to 4 |
| Tetracycline | 55 | 2.03 | 2 | 1 to 3 |
Abbreviations
CrI: credible interval.
Data and analyses
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albert 2011.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, placebo‐controlled, parallel‐group design Duration: 52 weeks Location: 17 sites associated with 12 academic health centres across the USA |
|
| Participants |
Population: 1142 adults with moderate to severe COPD were randomly assigned to azithromycin (n = 570) or placebo (n = 572) Baseline characteristics: age (mean years): 65.5 (SD 8.5); % male (mean): 59; % FEV₁ predicted (mean): 39.5 (SD 16); pack‐years (mean): 58.5 (SD 32); exacerbation history. Approximately 50% of participants in each treatment arm had required hospitalisation or an ED visit in the previous 12 months Inclusion criteria: severity of COPD moderate or worse as defined by GOLD criteria; mean FEV₁ (L): 1.10 (SD 0.50) (azithromycin) and 1.12 (SD 0.52) (placebo); presence of either (a) using continuous supplemental oxygen or (b) received systemic glucocorticoids within the previous year/had gone to an emergency room/hospitalisation for an acute exacerbation; no acute exacerbation of COPD for at least 4 weeks Exclusion criteria: asthma; resting heart rate > 100/min; prolonged QT interval > 450 ms; using medications that prolong QTc; hearing impairment documented by audiometry |
|
| Interventions |
Allowed co‐medications: supplemental oxygen or systemic glucocorticoids received in the last year |
|
| Outcomes | Number of people with ≥1 exacerbations, time to first acute exacerbation of COPD, quality of life (SGRQ, SF‐36), nasopharyngeal colonisation with selected respiratory pathogens, adherence to taking study medication as prescribed, serious adverse events. | |
| Notes |
Funding: National Institutes of Health Identifier: NCT00325897 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The stratified random sequence generation was well described in the journal article under "protocol" |
| Allocation concealment (selection bias) | Low risk | Well explained. Central allocation was pharmacy controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active drug and placebo will be identical in appearance. Both patients and treating medical staff were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial staff were unaware of the randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All outcome data were accounted for in a CONSORT diagram for the entire study However data on the secondary outcome: HRQoL ‐ reported loss to follow‐up of 20% in the prophylactic antibiotic arm and 18% in the placebo arm. Reasons for missing data pertaining to HRQoL were not given |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias was identified |
Banerjee 2005.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, placebo‐controlled, parallel‐group design. Duration: 13 weeks Location: 2 UK hospital clinics |
|
| Participants |
Population: 67 adults with COPD were randomly assigned to clarithromycin (n = 31) or placebo (n = 36) Baseline characteristics: mean age 65.1 (clarithromycin) vs 68.1 years (placebo); mean FEV₁ 1.12 L (clarithromycin) vs 1.13 L (placebo); severity of COPD moderate or worse according to BTS guidelines. All patients were taking ICS; exacerbation history was not reported Inclusion criteria: patients enrolled from hospital clinics; no acute exacerbations of COPD over the past 6 weeks Exclusion criteria: previous documented allergies to macrolides; clinical history of lung cancer, asthma, or bronchiectasis |
|
| Interventions |
Allowed co‐medications: participants were allowed to take their medication as prescribed by their primary care doctor. All participants were taking ICS, LABA (18%), inhaled anticholinergics (63%). Equal numbers of participants in each treatment arm were taking both LABA and anticholinergics. |
|
| Outcomes | Health‐related quality of life, infective exacerbation rate, shuttle walk test, serum C‐reactive protein level, sputum bacterial quantities load | |
| Notes |
Funding: Abbott Pharmaceuticals Identifier: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was carried out |
| Allocation concealment (selection bias) | Low risk | Patient randomisation was not known to trial staff. Randomisation was carried out by the Birmingham Hospital Pharmacy Department |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial staff were unaware of randomisation, but blinding of outcome assessors was not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data were described |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias was identified |
Berkhof 2013.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, placebo‐controlled, parallel‐group design Duration: 12 weeks Location: 1 large teaching hospital in Zwolle, Netherlands |
|
| Participants |
Population: 84 adults with COPD (GOLD stage ≥ 2) were randomly assigned to azithromycin (n = 42) or placebo (n = 42) Baseline characteristics: age (mean years): 67.5 (SD 9.5); % male (mean): 75; % FEV₁ predicted (mean): 48.6 (SD 14.6); pack‐years (median): azithromycin 30.5 (range 0 to 46), placebo 30 (range 1 to 69); exacerbation history: participants had a median of 1 exacerbation (range 0 to 13) in the previous 12 months Inclusion criteria: mean % FEV₁ predicted 49.8 (SD 16.4) (azithromycin) and 47.4 (SD 12.9) (placebo); clinical diagnosis of COPD GOLD stage ≥ 2 (defined as post‐bronchodilator FEV₁ < 80% and ratio of FEV₁‐to‐FVC < 70%); chronic productive cough, defined as cough for at least the last 12 weeks, in 2 subsequent years Exclusion criteria: prior history of asthma; use of intravenous or OCS and/or antibiotics for an exacerbation 3 weeks before inclusion; other relevant lung or liver disease at the discretion of the treating physician; pregnancy or lactation; use of macrolides in the last 6 weeks before inclusion; allergy or intolerance to macrolides; use of other investigational medication started 2 months before inclusion |
|
| Interventions |
Allowed co‐medications: long‐term treatment with aerosolised antibiotics, inhaled corticosteroids, and/or bronchodilators was permitted during the trial provided that it was kept constant |
|
| Outcomes | LCQ, quality of life (SGRQ, SF‐36), spirometry (FEV₁, % FEV₁ predicted), blood values, microbiology. Other endpoints included number of people with ≥ 1 exacerbations, time to first exacerbation of COPD, exacerbation rates and hospitalisation rates for COPD, adverse events | |
| Notes |
Funding: Stichting Astma Bestrijding (SAB) Identifier: NCT01071161 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes were generated using a computer allocation programme at a 1:1 ratio and a permutated block size of 4 |
| Allocation concealment (selection bias) | Unclear risk | This was not specifically described but was probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators, research nurses, and participants were masked to treatment allocation until final analyses of data were performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, research nurses, and participants were masked to treatment allocation until final analyses of data were performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout was low and balanced. All participants were accounted for in a flow diagram |
| Selective reporting (reporting bias) | Low risk | Outcomes were measured but were not reported in a way that allowed inclusion in meta‐analysis in the published paper, but study authors supplied additional data on request |
| Other bias | Low risk | No other bias was identified |
Blasi 2010.
| Study characteristics | ||
| Methods |
Design: randomised, uncontrolled, open‐label, parallel‐group design Duration: 26 weeks Location: 5 centres across Italy |
|
| Participants |
Population: 22 adults with severe COPD were randomly assigned to azithromycin (n = 11) or standard care (n = 11) Baseline characteristics: age (mean years): 72.5 (SD 7); % male (mean): 86; pack‐years (mean): 36 (SD 19.5); no current smokers, nearly all participants were former smokers (100% in the azithromycin group; 91% in the standard care group); exacerbation history: participants in each treatment arm had a mean of 3 exacerbations in the previous 12 months Inclusion criteria: 45 years of age with a history of severe COPD diagnosed with pulmonary function test and tracheostomy Exclusion criteria: allergy to macrolides; life expectancy < 1 year |
|
| Interventions |
Allowed co‐medications: not stated |
|
| Outcomes | Reduction in number of exacerbations, time to first exacerbation, reductions in number of hospitalisations, time to first hospitalisation, quality of life, SAEs, AEs | |
| Notes |
Funding: Pfizer, University of Milan Identifier: NCT00323986 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated block sequence |
| Allocation concealment (selection bias) | Unclear risk | No further information was provided about methods to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was open‐label, and participants and personnel would have been unblinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was open‐label, and outcome assessors would not have been blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | This was not an ITT analysis, so the analysis did not include participants who died. This may have led to overestimation of outcomes. More deaths occurred in the SC group than in the AZI group, but whether or not deaths were treatment related was not reported |
| Selective reporting (reporting bias) | High risk | Outcomes reported in the publication are reported differently from those in the protocol at clinicaltrials.gov. Mortality, AEs, and SAEs are additional outcomes that were not reported at the website. Data for SAEs are reported only for the AZI group. It is not clear whether or not people in the SC group had any SAEs |
| Other bias | Low risk | No other bias was identified |
Brill 2015.
| Study characteristics | ||
| Methods |
Design: randomised, single‐blind, placebo‐controlled, parallel‐group design Duration: 13 weeks. Location: single‐centre, UK‐based hospital (London) |
|
| Participants |
Population: 99 adults with moderate to severe COPD were randomly assigned to moxifloxacin (n = 25), doxycycline (n = 25), azithromycin (n = 25), or placebo (n = 24) Baseline characteristics: age (mean years): 69.4 (SD 8.4); % male (mean): 69; % FEV₁ predicted (mean): 50 (SD 14); pack‐years (mean): 53 (SD 38); exacerbation history: participants had a mean of 2.5 (SD 2.1) exacerbations with moxifloxacin, 2.1 (SD 1.7) exacerbations with doxycycline, 2.8 (SD 4.0) exacerbations with azithromycin, and 1.5 (SD 1.4) exacerbations with placebo in the previous 12 months Inclusion criteria: mean % FEV₁ predicted: 52 (SD 13) (moxifloxacin), 53 (SD 14) (doxycyline), 44 (SD 17), (azithromycin), and 53 (SD 13) (placebo). Stable patients with chronic bronchitis (self‐reported sputum expectoration on most days when clinically stable) and spirometrically confirmed COPD (defined by FEV₁ < 80% predicted, FEV₁‐to‐FVC ratio < 0.7, and a history of smoking) Exclusion criteria: treatment for an exacerbation or an episode of symptom worsening in the 4 weeks before screening; unable to enrol for safety reasons (significant hepatic/renal impairment; QT prolongation; pre‐existing long‐term antibiotic use; hypersensitivity to treatments under investigation |
|
| Interventions |
Allowed co‐medications: not reported |
|
| Outcomes | Change in sputum bacterial load, change in resistance to the 3 study antibiotics, change in FEV₁, adherence to therapy, health‐related quality of life (SGRQ),number of people with ≥ 1exacerbations,adverse events | |
| Notes |
Funding: National Institute for Health Research Identifier: NCT01398072 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Internet randomisation into groups of 1:1:1:1 was performed using a computer‐generated permutated block system of variable sizes (Sealed Envelope, UK) |
| Allocation concealment (selection bias) | Low risk | Internet randomisation into groups of 1:1:1:1 was performed using a computer‐generated permutated block system of variable sizes (Sealed Envelope, UK) "Patients remained blinded to treatment allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients remained blinded to treatment allocation. However, it is not clear whether study personnel were blinded. This was described as a single‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No description of outcome assessor blinding was provided, although blinded participants assessed outcomes such as quality of life |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout was low and balanced. All participants were accounted for in a flow diagram |
| Selective reporting (reporting bias) | Low risk | Planned outcomes according to trial registration relevant to this review were reported |
| Other bias | Low risk | No other bias was identified |
He 2010.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, placebo‐controlled, parallel‐group design Duration: 26 weeks Location: Affiliated Hospital of Guangxi Medical University, China |
|
| Participants |
Population: 36 adults with COPD (severity not reported) were randomly assigned to erythromycin (n = 18) or placebo (n = 18) Baseline characteristics: age (mean years): 69 (SD 7.6); % male (mean): 86; % FEV₁ predicted (mean): 43.2 (SD 18.2); pack‐years (mean): 41.7 (SD 18.8); exacerbation history: not reported Inclusion criteria: % FEV₁ predicted between 30 and 70; mean FEV₁ (L): 1.12 (erythromycin) vs 1.02 (placebo); ≥ 10 pack‐year smoking history; no acute exacerbations during the previous 1 month Exclusion criteria: patients with significant other respiratory disorders other than COPD; history of unstable cardiovascular disease; hypersensitivity to macrolides |
|
| Interventions |
Allowed co‐medications: present treatment included: inhaled corticosteroid (41%), theophylline (58%), inhaled anticholinergic (52%), inhaled β‐adrenergic (75%) |
|
| Outcomes | Analysis of sputum samples (total and differential inflammatory cell counts, sputum bacterial culture), quality of life (SGRQ, SF‐36), number of people with exacerbations, time to first exacerbation, spirometry, adverse events | |
| Notes |
Funding: not reported Identifier: ChiCTR‐TRC‐0000036 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was done but was not clearly explained |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not well explained |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This is unknown |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data were described using a CONSORT diagram |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias was identified |
Mygind 2010.
| Study characteristics | ||
| Methods |
Design: randomised, placebo‐controlled, double‐blind study Duration: 156 weeks Location: NR |
|
| Participants |
Population: 575 adults with moderate to severe COPD were randomly assigned to azithromycin (n = 287) or placebo (n = 288) Baseline characteristics: age > 50 years; moderate to severe COPD; % FEV₁ predicted < 60; previous documented allergies to macrolides; clinical history of lung cancer, asthma, or bronchiectasis; exacerbation history: not reported Inclusion criteria: ≥ 1 admission to hospital with an exacerbation of COPD; ex‐smoker or current smoker Exclusion criteria: end‐stage COPD patients (if not expected to survive over 3 years) or bedridden patients; history of asthma, bronchiectasis, or other significant respiratory disease; history of azithromycin allergy; heart, liver, or renal insufficiency; already receiving prophylactic antibiotic |
|
| Interventions |
Allowed co‐medications: not reported |
|
| Outcomes | Rate of decline in lung function (FEV₁), frequency of exacerbation, health‐related quality of life, adverse events, mortality, duration of exacerbations, number of days of hospitalisation, frequency of hospitalisation | |
| Notes |
Funding: not reported Identifier: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but paucity of data was available on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | This was not explained well |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unknown. This study was double‐blind, but it is unclear whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All outcome data were presented. Only 55% completed 3 years |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Unclear risk | This was a conference presentation ‐ not a full publication. Attempts to contact study authors were not successful. Only limited data are available for evaluation of the risk of bias |
Seemungal 2008.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, single‐centre, placebo‐controlled, parallel‐group design Duration: 52 weeks Location: 2 outpatient chest clinics at 2 hospitals in the UK (London) |
|
| Participants |
Population: 109 adults with moderate to severe COPD were randomly assigned to erythromycin (n = 53) or placebo (n = 56) Baseline characteristics: age (mean years, SD): 67.1 (8.5); % male (mean): 63; % FEV₁ predicted (mean, SD): 49.9 (18); pack‐years (mean): 51.6; exacerbation history: 35% of participants had ≥ 3 exacerbations in the previous 12 months Inclusion criteria: severity of COPD was moderate to severe; FEV₁ between 30% and 70% predicted; mean FEV₁ (L): 1.27 (erythromycin) and 1.36 (placebo) Exclusion criteria: history of asthma; bronchiectasis; neoplasia; unstable cardiac status (including prolonged QTc and arrhythmias); macrolide allergy or history of abnormal liver functions |
|
| Interventions |
Allowed co‐medications: inhaled steroids, no changes were made unless there was a clinical need, which excluded the participant from the study. No antibiotics or oral steroids during 1‐month run‐in period of exacerbation‐free symptoms |
|
| Outcomes | Number of people with an exacerbation, exacerbation frequency, time to first exacerbation, spirometry and inflammatory markers, bacteriology, adverse events | |
| Notes |
Funding: British Lung Foundation Identifier: NCT00147667 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated permutated block random sequence generation was carried out |
| Allocation concealment (selection bias) | Low risk | Randomisation numbers were stored in sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and erythromycin were concealed in identical capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unblinding occurred only after data entry |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes/dropouts were explained in a CONSORT diagram |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias was identified |
Sethi 2010.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, placebo‐controlled, parallel‐group design Duration: 48 weeks Location: multi‐centre study across 15 countries in the USA |
|
| Participants |
Population: 1149 adults with COPD (GOLD stage ≥ 2) were randomly assigned to moxifloxacin (n = 569) or placebo (n = 580) Baseline characteristics: age (mean years): 66 (SD 8.9); % male (mean): 74; % FEV₁ predicted (mean): 41 (SD 16); pack‐years (mean): 53 (SD 30); exacerbation history: not reported Inclusion criteria: severity of COPD was GOLD stage ≥ 2; had ≥ 2 exacerbations requiring treatment with antibiotics and/or oral steroids in the 12 months before enrolment Exclusion criteria: known hypersensitivity to treatment antibiotic or other quinolones; history of tendon disease/disorder; known congenital or documented‐acquired QT prolongation; hypokalaemia; clinically relevant bradycardia; clinically relevant heart failure with reduced ventricular ejection fraction; previous history of symptomatic arrhythmias; concomitant use of any antiarrhythmics class IA or class III; neuroleptics; certain antihistamines; post‐menopausal women (< 1 year); not using acceptable birth control; any known disease with life expectancy < 24 months; severe hepatic impairment; used investigational drug in the last 30 days; known bronchial carcinoma; pulmonary tuberculosis; cystic fibrosis; documented chronic bronchial asthma; diffuse bronchiectasis; part of pulmonary rehabilitation programme; history of chronic colonisation of resistant pathogenic organisms (moxifloxacin); systemic/inhaled antibiotic therapy during 6 weeks before screening and any long‐term antibiotic use; home ventilatory support; tracheostomy; inability to attend on specified visit dates |
|
| Interventions |
Allowed co‐medications: nearly all participants had concomitant medications. Long‐acting bronchodilators and inhaled steroids (any adjustment to medication was a reason to exclude the participant from the per‐protocol population) |
|
| Outcomes | Frequency of exacerbations, number of people with exacerbations, hospitalisations, mortality, quality of life (SGRQ), change in lung function, seriousadverse events | |
| Notes |
Funding: Bayer HealthCare AG Identifier: NCT00473460 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was done but sequence generation was not well explained |
| Allocation concealment (selection bias) | Unclear risk | This was not explained |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This was not explained |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All outcome data were described using a CONSORT diagram for the entire study However data on the secondary outcome: HRQoL ‐ reported loss to follow‐up of 12% in the prophylactic antibiotic arm and 10% in the placebo arm. Reasons for missing data pertaining to HRQoL outcome were not given |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were well described |
| Other bias | Low risk | No other bias was identified |
Shafuddin 2015.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, placebo‐controlled, double‐dummy, parallel‐group design Duration: 12 weeks Location: 16 centres across Australia and New Zealand |
|
| Participants |
Population: 292 adults with moderate to severe COPD were randomly assigned to roxithromycin plus doxycycline (n = 101), roxithromycin (n = 97), or matched placebo (n = 94) Baseline characteristics: age (mean) 67 (SD 8.5); % male (mean): 73; % FEV₁ predicted (mean): 34 (SD 10); pack‐years (mean): 55.6 (SD 33); exacerbation history: participants had a mean 5.11 (SD 2.4) exacerbations in the last 24 months Inclusion criteria: mean % FEV₁ predicted, mean: 32.53 (SD 13.55) (roxithromycin/doxycyline), 33.93 (SD 15.3) (doxycyline), 35.8 (SD 15.2) (placebo); meeting spirometric criteria for COPD (FEV₁ ≤ 70% predicted, FEV₁‐to‐FVC ≤ 60%; reversibility of ≤ 10% of predicted FEV₁ or ≤ 200 mL if predicted FEV₁ ≤ 2 L); smoking history ≥ 20 pack‐years; ≥ 3 confirmed moderate or severe COPD exacerbations in the past 2 years (i.e. requiring treatment with antibiotics and/or OCS and/or hospitalisation); positive serology for C pneumoniae (IgG antibody titre ≥ 1:64) Exclusion criteria: pulmonary disease other than COPD; treatment with antibiotics; exacerbation or an investigational drug in the 4 weeks before randomisation; pregnancy (serum pregnancy test) or breast‐feeding; history of hypersensitivity to macrolides, tetracyclines, beta‐lactams, or sulphamethoxazole:trimethoprim; serious cardiovascular, hepatic, renal, or other systemic disease; known long QT syndrome or QTc > 450 ms; sick sinus syndrome; bradycardia (< 50 bpm) or severe hypokalaemia; epilepsy; treatment with medicine known to have important interaction with macrolides or tetracyclines; impaired hepatic function (aspartate aminotransferase or alanine aminotransferase ≥ 2 times the ULN, alkaline phosphatase ≥ 1.25 times the ULN, bilirubin > 2 times the ULN, and albumin < 30 g/L); unlikely to comply |
|
| Interventions |
Allowed co‐medications: not stated |
|
| Outcomes | Exacerbations, quality of life, lung function, adverse events | |
| Notes |
Funding: Sanofi‐Aventis Australia Pty Ltd. Identifier: ANZCTRN 12615000052538 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each eligible participant was assigned to a sequential subject number followed by randomisation number provided by Hoescht Marion Roussel, Australia. Participants were supplied with one of three treatments according to their randomisation number" Clinical trials registry clarifies: computer sequence generation used for randomisation of participants into treatment arms at 1:1:1 ratio |
| Allocation concealment (selection bias) | Low risk | Study medication was packed by Hoechst Marion Roussel in bottles labelled with randomisation and batch numbers. Investigators, pharmacists, and subjects were blinded to study medication in these bottles |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triallists confirm that all participants, personnel, and outcome assessors remained blinded until data had been analysed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Triallists confirm that all participants, personnel, and outcome assessors remained blinded until data had been analysed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | More patients dropped out of combined antibiotics treatment arm (21 vs 13 in single antibiotic arm and 10 in placebo arm), although according to triallists, reasons were not related to study medication. All patients were included in the ITT analysis |
| Selective reporting (reporting bias) | Low risk | Planned outcomes according to trial registration relevant to this review were reported |
| Other bias | Low risk | No other bias was identified |
Simpson 2014.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, placebo‐controlled, parallel‐group design Duration: 12 weeks Location: tertiary care setting (respiratory and sleep medicine ambulatory care service), New South Wales, Australia |
|
| Participants |
Population: 30 adults with moderate COPD were randomly assigned to azithromycin (n = 15) or placebo (n = 15) Baseline characteristics: age (mean years): 70.8 (SD 7.5); % male (mean): 63; % FEV₁ predicted (mean): 53.7 (SD 13.7); pack‐years (mean): 46 (SD 36.6); exacerbation history: not reported Inclusion criteria: COPD diagnosis (by health professional). Post‐bronchodilator FEV₁‐to‐FVC < 70% and < 80%, persistent neutrophil bronchitis (> 61% sputum neutrophil proportion) on 2 occasions, no reported exacerbations or changes to respiratory medication in the last month Exclusion criteria: FEV₁ < 0.5 L, currently stopping or stopped smoking in the last 6 months, hypersensitive to study antibiotic, liver impairment, inability to provide sputum sample |
|
| Interventions |
Allowed co‐medications: not reported |
|
| Outcomes | Reduction in sputum CXCL8, change in sputum neutrophil proportion, total bacterial load in sputum, health care utilisation, QoL (SGRQ), severe exacerbations, pulmonary function tests, chest CT to measure airway thickness, adverse events | |
| Notes |
Funding: National Health and Medical Research Council of Australia Identifier: ACTRN12609000259246 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed random allocation was undertaken by a blinded staff member who took no further part in the study. A random numbers table was computer generated (www.randomization.com) for treatment allocation using permutated blocks of 6, and participants were stratified according to smoking history (never or previous smokers) |
| Allocation concealment (selection bias) | Low risk | Concealed random allocation was undertaken by a blinded staff member who took no further part in the study. Active medication and placebo were prepared and packaged identically by a compounding chemist and were dispensed by the John Hunter Hospital Pharmacy according to the random numbers table |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and study staff were blinded to assignment of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | People assessing outcomes are described as blinded in the trial registration |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout was low and balanced. Reasons for discontinuation were unrelated to study medication |
| Selective reporting (reporting bias) | Low risk | Planned outcomes according to trial registration relevant to this review were reported |
| Other bias | Low risk | No other bias was identified |
Singh 2019.
| Study characteristics | ||
| Methods |
Design: randomised, controlled, parallel‐group design Duration: 13 weeks Location: outpatient department in Kolkata, India |
|
| Participants |
Population: 60 adults with COPD (GOLD stages 2 to 3) were randomly assigned to doxycycline (n = 30) or to standard therapy (n = 30) Baseline characteristics: age (mean): 65 (SD 5.9); % male (mean): 100; % FEV₁ predicted post bronchodilator (mean): 59.3 (SD 3.72); pack‐years: not reported; former smokers (n): 42/60; current smokers (n): 18/60; exacerbation history: not reported Inclusion criteria: COPD diagnosis by GOLD 2 and 3 guidelines Exclusion criteria: moderate or severe exacerbations in the last 6 weeks, significant co‐morbidities, co‐existing respiratory condition that may have interfered with COPD assessment, doxycycline allergy |
|
| Interventions |
Allowed co‐medications: standard therapy (see above) |
|
| Outcomes | FEV₁ % predicted (post bronchodilator); FEV₁‐to‐FVC % (post bronchodilator); CAT; SGRQ; eosinophil count; neutrophil count; platelet count; malondialdehyde concentration; lipid hyper peroxide concentration; uric acid concentration; bilirubin concentration; glutathione‐S‐transferase concentration; total antioxidant status; total oxidant status; peroxy nitrite concentration; interleukins 1, 6, 8, and 10; tumour necrosis factor‐alpha; MMP9, 12, and 2 concentrations | |
| Notes |
Funding: Government of India, Ministry of Human Resource Development (HRD) under Signals and Systems for Life Science (SSLS) Identifier: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was reported as randomised, but process was not described in detail |
| Allocation concealment (selection bias) | Low risk | Participants were randomised according to the 'conventional sealed envelope method' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors reported in the discussion that clinicians and patients were not blinded due to ethical constraints |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study authors reported in the discussion that clinicians and patients were not blinded due to ethical constraints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 participants in the treatment group were excluded overall because of nausea (n = 2) and diarrhoea (n = 1) |
| Selective reporting (reporting bias) | Unclear risk | It is unclear if all outcomes were reported as planned, as no protocol details or trial registry information was available |
| Other bias | Low risk | No other bias was identified |
Suzuki 2001.
| Study characteristics | ||
| Methods |
Design: randomised, controlled, open‐label, parallel‐group design Duration: 52 weeks Location: Japan (no other information) |
|
| Participants |
Population: 109 adults with COPD (severity not reported) were randomly assigned to erythromycin (n = 55) or control (n = 54) Baseline characteristics: age (mean years): 70.4; % male (mean): 83.4; FEV₁ (mean): 2.64 (SE 0.05); pack‐years: not reported; former or current smokers: not reported; exacerbation history: not reported Inclusion criteria: mean FEV₁ (L): 1.47 (erythromycin) and 1.30 (placebo); females 13% in erythromycin group vs 18% in placebo group; all study participants were treated with sustained‐release theophylline and inhaled anticholinergic agents Exclusion criteria: diagnosis of bronchiectasis or diffuse pan bronchiolitis |
|
| Interventions |
Allowed co‐medications: sustained‐release theophylline and inhaled anticholinergic agents. Corticosteroids were not allowed |
|
| Outcomes | Acute exacerbations of COPD, adverse events | |
| Notes |
Funding: not reported Identifier: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a random numbers table |
| Allocation concealment (selection bias) | Low risk | The randomisation list was held independently from the investigators |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This study was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | As the study was not blinded, assessment of outcomes would be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was excluded due to adverse events of erythromycin; all participants were clearly accounted for |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias was identified |
Tan 2016.
| Study characteristics | ||
| Methods |
Design: randomised, placebo‐controlled, parallel‐group design Duration: 52 weeks Location: outpatient department at a medical university hospital |
|
| Participants |
Population: 49 adults with COPD (GOLD stages 2 to 4) were randomly assigned to erythromycin (n = 36) or control (n = 18) Baseline characteristics: age (mean years): 68.4 (SD 6.6); % male: 89; % FEV₁ predicted (mean): 44.4 (SD 13.8); pack‐years (mean): 41.3 (SD 25.8); exacerbation history: not reported Inclusion criteria: stable COPD diagnosis according to GOLD guidelines, FEV₁ < 80% predicted, FEV₁‐to‐FVC < 70%; no acute exacerbation, no change in therapeutic treatment, no antibiotic treatment in the last 4 weeks Exclusion criteria: bronchial asthma; primary bronchiectasis; diffuse pan bronchiolitis; active tuberculosis; lung cancer; pneumoconiosis; other lung disease with restrictive ventilatory impairment; cardiovascular, nervous system, or endocrine disease; blood, hepatic, or kidney disease; malignant tumour, inability to communicate, serious adverse reaction to erythromycin |
|
| Interventions |
Allowed co‐medications: supplemental oxygen, theophylline, inhaled bronchodilators and corticosteroids. Other macrolides, histamine antagonists, non‐steroidal anti‐inflammatory drugs, and oral glucocorticosteroids were not allowed |
|
| Outcomes | Concentrations of IL‐17 and IL‐23 in peripheral blood and induced sputum, 6‐minute walk distance, serious adverse events | |
| Notes |
Funding: National Nature Science Foundation of China (81460009) and Guangxi Natural Science Foundation Identifier: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly divided"; no other details were provided |
| Allocation concealment (selection bias) | Unclear risk | No further information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel was described. Study was assumed to be open‐label (although abstract states double‐blind). Study authors have been contacted; we await clarification response |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors was described. Study was assumed to be open‐label (although abstract states double‐blind). Study authors have been contacted; we await clarification response |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout was low and balanced, but details of how many people were analysed at each time point were not given |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration or protocol was identified, so it is not clear if outcomes of interest for this review may have been collected but not reported (e.g. serious adverse events, exacerbations, quality of life) |
| Other bias | Low risk | No other bias was identified |
Uzun 2014.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, placebo‐controlled, single‐centre design. Duration: 52 weeks Location: a hospital in the Netherlands |
|
| Participants |
Population: 92 adults with mild to very severe COPD were randomly assigned to azithromycin (n = 47) or placebo (n = 45) Baseline characteristics: age (mean); 64.8 (SD 10.2); FEV₁ (L): 1.1 (SD 0.45); % FEV₁ predicted: 44.6 (SD 19,4); FEV₁‐to‐FVC (mean %, SD): 39.2 (SD 12), exacerbation history: participants had a mean of 4 (SD 1.1) acute exacerbations in the previous 12 months Inclusion criteria: mean % FEV₁ predicted: 44.2 (SD 19.3) (azithromycin) and 45.0 (SD 19.5) (placebo). Diagnosis of COPD according to GOLD guidelines; had received treatment for ≥ 3 exacerbations of COPD in the previous year for which they received steroids or antibiotic treatment; clinically stable; could not have had a COPD exacerbation or respiratory tract infection in the month before involvement in the study Exclusion criteria: history of other clinically significant respiratory disease (e.g. asthma, cystic fibrosis); presence of bronchiectasis, as assessed by CT scan; maintenance antibiotic treatment; use of > 10 mg prednisolone a day; allergy to macrolides; pregnancy or lactation in women; liver disease (alanine transaminase or aspartate transaminase concentrations that were ≥ 2 times the upper limit of normal); malignant disease of any kind for which the patient received treatment or was being monitored as part of follow‐up after treatment; heart failure; use of drugs that could adversely interact with macrolides and for which therapeutic monitoring could not be undertaken |
|
| Interventions |
Allowed co‐medications: no more than 10 mg prednisolone per day. No maintenance antibiotic treatment was allowed |
|
| Outcomes | Rate of COPD exacerbations, time to first exacerbation, hospital admission for acute exacerbations, change in proportion of exacerbations needing admission to hospital vs treatment in an outpatient department compared with the previous year, treatment for an acute COPD exacerbation, FEV₁ after bronchodilation, FVC after bronchodilation, 6MWT, quality of life (SF36 and SGRQ), colonisation of macrolide‐resistant micro‐organisms in sputum, adverse events | |
| Notes |
Funding: SoLong (Department of Respiratory Medicine, Amphia Hospital, Breda, The Netherlands) Identifier: NCT00985244 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent pharmacy randomly assigned patients (1:1) via a computer‐generated randomisation sequence with permutated blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Patients were automatically given the next allocated treatment by clinical trials staff at the hospital pharmacy. Participants and investigators were masked to treatment allocation throughout the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were masked to treatment allocation throughout the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | After data collection and data cleaning were completed, and after final database lock, investigators were unmasked and could assess outcomes and complete the data analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Higher dropout was reported in the placebo arm, but results from unadjusted and adjusted per‐protocol analyses were almost identical to those from the intention‐to‐treat analysis, and all participants were included in the safety analysis |
| Selective reporting (reporting bias) | Low risk | Planned outcomes according to trial registration relevant to this review were reported |
| Other bias | Low risk | No other bias was identified |
Vermeersch 2019.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, placebo‐controlled, parallel‐group design Duration: 13 weeks Location: 5 centres across Italy |
|
| Participants |
Population: 301 adults with COPD (GOLD stages B to D) were randomly assigned to azithromycin (n = 147) or placebo (n = 154) Baseline characteristics: age (mean years): 66.5 (SD 9.5); % male (mean): 51; % FEV₁ (mean, pre‐bronchodilator): 0.925 L; pack‐years (mean): 44; exacerbation history: in the previous 12 months; 89 participants = 1 AECOPD exacerbation, 78 = 2 AECOPD exacerbations, 50 = 3 AECOPD exacerbations, 84 = ≥ 3 exacerbations Inclusion criteria: history of severe COPD diagnosed with a pulmonary function test and tracheostomy Exclusion criteria: allergy to macrolides, life expectancy < 1 year |
|
| Interventions |
Allowed co‐medications: not reported |
|
| Outcomes | Reduction in number of exacerbations, time to first exacerbation, reductions in number of hospitalisations, time to first hospitalisation, quality of life, SAEs, AEs | |
| Notes |
Funding: not reported Identifier: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This was not reported in the actual pre‐publication document but in the protocol. Randomisation was performed using an online randomisation schedule; a unique randomisation code was obtained through a secured web‐based programme, as reported on clinicaltrials.gov. We assume that they carried out what they intended |
| Allocation concealment (selection bias) | Low risk | This was not reported in the pre‐publication document but in the protocol. It was reported on clinicaltrials.gov that they intended to use identical packaging, labelling, schedule of administration, and appearance. We assume that they carried out what they intended to do as stated in their protocol |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was reported as double‐blind in the pre‐publication document; at clinicaltrials.gov, this study was reported as triple‐blind, but this was not mentioned anywhere else |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study was reported as triple‐blind at clinicaltrials.gov, but it is unclear whether or not outcome assessors were investigators. This is not mentioned anywhere in the pre‐publication document, but we assume that they carried out what they intended to do as stated in their protocol |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was 13%. Azithromycin group had 11% attrition, placebo group had 16% attrition. In the PRISMA diagram at 90 days, dropout, withdrawal, and mortality were similar, so we considered attrition as low. We requested further information about how attrition was handled in the ITT analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported according to the protocol |
| Other bias | Low risk | This study was stopped early due to slow participant recruitment, but this would not affect the overall analysis |
Wang 2017.
| Study characteristics | ||
| Methods |
Design: randomised, controlled, parallel‐group trial Duration: 26 weeks Location: Zhengzhou Hospital, China |
|
| Participants |
Population: 86 adults with moderate to severe COPD were randomly assigned to azithromycin (n = 43) or placebo (n = 43) Baseline characteristics: age (mean years): 71.4 (SD 8.2), % male (mean): 59, FEV₁ (mean): 0.67 L (SD 0.095); exacerbation history: not reported Inclusion criteria: 45 years of age with history of severe COPD diagnosed with pulmonary function test and tracheostomy Exclusion criteria: allergy to macrolides, life expectancy < 1 year |
|
| Interventions |
Allowed co‐medications: cough relief medication, aminophylline, beta2 receptor agonist |
|
| Outcomes | Blood gas analysis, FEV₁, FVC, 6MWT, pulmonary arterial pressure | |
| Notes |
Funding: "Grant Support & Financial Disclosures: None" Identifier: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly divided into an observation group and a control group using random number table, 43 in each group" |
| Allocation concealment (selection bias) | Unclear risk | No further information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel was described. Study was assumed to be open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors was described. Study was assumed to be open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No further information was provided |
| Selective reporting (reporting bias) | High risk | No prospective trial registration or protocol was identified. Dyspnoea grade was reported as measured in the abstract but was not described in the methods. It is not clear if FEV₁ and FVC variance are SDs or SEs |
| Other bias | Low risk | No other bias was identified |
Abbreviations
6MWT: six‐minute walk test; AE: adverse event; AECOPD: acute exacerbation of chronic obstructive pulmonary disease; bpm: beats per minute; BTS: British Thoracic Society; CAT: COPD assessment test; COPD: chronic obstructive pulmonary disease; ED: emergency department; FEV₁: forced expiratory volume in one second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; HRQoL: health‐related quality of life; ICS: inhaled corticosteroid; ITT: intention‐to‐treat; LABA: long‐acting beta‐agonist; LAMA: long‐acting muscarinic antagonist; LCQ: Leicester Cough Questionnaire; NR: not reported; OCS: oral corticosteroids; QT: uncorrected QT interval (measurement of electrical properties of the heart); QTc: corrected QT interval; SABA: short‐acting beta‐agonist; SAE: serious adverse event; SAMA: short‐acting muscarinic antagonist; SD: standard deviation; SE: standard error; SF36: short form 36; SGRQ: St George's Respiratory Questionnaire; ULN: upper limit of normal.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bussi 1980 | Study compared ciprofloxacin 200 mg, erythromycin 200 mg, or combined ciprofloxacin + erythromycin for 6 months. Study population included bronchiectasis (n = 19), chronic bronchitis (n = 15), pulmonary emphysema (n = 8), diffuse pan bronchiolitis (n = 6), pulmonary fibrosis (n = 1), and old pulmonary tuberculosis (n = 1). Study authors show distribution of characteristics at baseline but not at endpoint ‐ only during the study ‐ the same for adverse events; children and adults were included; no clear diagnosis of bronchiectasis |
| Calder 1968 | No spirometric diagnosis of COPD |
| Cooper 1961 | No spirometric diagnosis of COPD |
| Edwards 1958 | Haemophilus influenzae vaccination was co‐administered |
| EUCTR2011‐004351‐39‐IT | Primary condition is not COPD |
| Francis 1960 | No spirometric diagnosis of COPD |
| ISRCTN72035428 | Wrong population (acute exacerbations of COPD) |
| Milito 2019 | Primary condition is not COPD |
| Murdoch 1959 | No spirometric diagnosis of COPD |
| Nicholson 2016 | Wrong study design (not an RCT) |
| O'Reilly 2013 | Study investigated sputum proline‐glycine‐proline levels during azithromycin/placebo treatment |
| Velzen 2016 | Wrong population (acute exacerbations of COPD) |
| Watanabe 1994 | No spirometric diagnosis of COPD |
| Zykov 2008 | Duration of intervention only 10 days |
Abbreviations
COPD: chronic obstructive pulmonary disease; RCT: randomised controlled trial.
Differences between protocol and review
NMA methods: change from winBUGS 1.4.3 to openBUGS 3.2.3; addition of description of prior distributions; description of model fit and choice with references; description of threshold analysis with references; explanation of loops for SGRQ and SAE analyses in the 'Assessment of heterogeneity and statistical consistency in the NMA' section; description of sensitivity analysis method.
Authors: two review authors ‐ RW and SS ‐ joined the review author team as they conducted the NMAs.
Contributions of authors
SJ drafted the Background and Methods sections of the review.
SD drafted the NMA methods for the review.
CT drafted the Background and Methods sections of the review.
AGM drafted the Background and Methods sections of the review.
RN provided conceptual and clinical advice and critical review of the review.
RW carried out the NMA under the supervision of SD.
SS carried out the NMA under the supervision of SD.
All review authors read and approved the final review version.
Contributions of editorial team
Chris Cates (Co‐ordinating Editor) checked data entry before the full write‐up of the review; edited the review; advised on methods; and approved the review before publication.
Ian Yang (Contact Editor) edited the review and advised on methods, interpretation, and content.
Emma Dennett (Managing Editor) co‐ordinated the editorial process; advised on interpretation and content; and edited the review.
Emma Jackson (Assistant Managing Editor) conducted peer review; obtained translations; and edited the references and other sections of the review.
Elizabeth Stovold (Information Specialist) designed the search strategy; ran the searches; and edited the search methods section.
Sources of support
Internal sources
-
All authors, Other
The review authors declare that no such funding was received for this systematic review.
External sources
-
National Institute for Health Research (NIHR), UK
Cochrane Programme Grant 16/114/21: NHS priorities in the management of chronic respiratory disease
-
Alexander G. Mathioudakis, UK
Alexander G. Mathioudakis was supported by the NIHR Manchester Biomedical Research Centre (NIHR Manchester BRC
Declarations of interest
SJ is employed full‐time by an NIHR Programme Grant to complete work on this Cochrane Review.
SD was a co‐applicant on a grant whereby Pfizer partially sponsored a researcher but was not sponsored by Pfizer herself.
CT was employed part‐time in 2017‐18 by an NIHR Programme Grant to complete work on this Cochrane Review. He is currently a Specialty Registrar in Clinical Pharmacology and Therapeutics and General Internal Medicine.
AGM has received a research grant for an investigator‐initiated study by Boehringer Ingelheim. He has received honoraria from Boehringer Ingelheim and GlaxoSmithKline, which are not related to the content of this manuscript.
RF is employed part‐time by an NIHR Programme Grant to complete work on this Cochrane Review and is a qualified general practitioner.
RW is a research fellow employed by the University of York.
SS is a research fellow employed by the University of York.
New
References
References to studies included in this review
Albert 2011 {published data only}
- Albert R, Bailey WC, Casaburi R, Connett J, Cooper A, Criner GJ, et al. Chronic azithromycin decreases the frequency of chronic obstructive pulmonary disease exacerbations. American Journal of Respiratory and Critical Care Medicine 2011;183:A6416. [Google Scholar]
- Albert R, Connett J, Bailey WC, Casaburi R, Cooper JAD, Criner GJ, et al. Long-term azithromycin decreases COPD exacerbations. Respirology 2013;18:5. [Google Scholar]
- Albert R, Connett J, Bailey WC, Casaburi R, Cooper JA Jr, Criner GJ, et al. Azithromycin for prevention of exacerbations of COPD. New England Journal of Medicine 2011;365(8):689-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correction: Azithromycin for prevention of exacerbations of COPD. New England Journal of Medicine 2012;366:14.
- Han MJ, Tayob N, Murray S, Dransfield MT, Washko G, Scanlon PD, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. American Journal of Respiratory and Critical Care Medicine 2014;189(12):1503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FJ, Connett J, Voelker H, Criner GJ, Han MJ, Make BJ, et al. Chronic azithromycin therapy decreases the risk of re-hospitalization in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2013;187:A4383. [Google Scholar]
Banerjee 2005 {published data only}
- Banerjee D, Honeybourne D, Khair OA. The effect of oral clarithromycin on bronchial airway inflammation in moderate-to-severe stable COPD: a randomised controlled trial. Treatments in Respiratory Medicine 2004;3:59-65. [DOI] [PubMed] [Google Scholar]
- Banerjee D, Khair OA, Honeybourne D. The effect of oral clarithromycin on health status and sputum bacteriology in stable COPD. Respiratory Medicine 2005;99:208-15. [DOI: 10.1016/j.rmed.2004.06.009] [DOI] [PubMed] [Google Scholar]
Berkhof 2013 {published data only}
- Berkhof FF, Hertog NED, Uil SM, Kerstjens AM, den Berg JWK. Azithromycin and cough-specific health status in patients with chronic obstructive pulmonary disease and chronic cough: a randomised controlled trial. Respiratory Research 2013;14:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhof FF, Ten Hertog NE, Uil SM, Kerstjens HAM, Van Den Berg JK, CACTUS Study Group. Randomized controlled trial of prophylactic azithromycin on cough-specific health status in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2013;187:A2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blasi 2010 {published data only}
- Blasi F, Bonardi D, Aliberti S, Tarsia P, Confalonieri M, Amir O, et al. Long-term azithromycin use in patients with chronic obstructive pulmonary disease and tracheostomy. Pulmonary Pharmacology & Therapeutics 2010;23:200-7. [DOI: 10.1016/jpupt.2009.12.002] [DOI] [PubMed] [Google Scholar]
Brill 2015 {published data only}
- Brill S, James P, Cuthbertson L, Cookson W, Moffatt M, Wedzicha J. Haemophilus dominance of the stable COPD microbiome is associated with greater bacterial load and inflammation and is modulated by prophylactic antibiotic therapy. European Respiratory Journal 2015;46:OA4746. [Google Scholar]
- Brill S, Law M, Allinson J, El-Emir E, McHugh T, Donaldson G, et al. Bacterial resistance induction with prophylactic antibiotics in COPD. In: European Respiratory Journal. Vol. 44. 2014:P4731.
- Brill SE, Law M, El-Emir E, Allinson JP, James P, Maddox V, et al. Effects of different antibiotic classes on airway bacteria in stable COPD using culture and molecular techniques: a randomised controlled trial. Thorax 2015;70:930-8. [DOI: 10.1136/thoraxjnl-2015-207194] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill SE, Law M, El-Emir E, Allinson P, Nazareth I, Donaldson GC, et al. Effect of antibiotics on airway bacteria in patients with chronic obstructive pulmonary disease. In: American Journal of Respiratory and Critical Care Medicine. Vol. 198. 2014:A2874.
He 2010 {published data only}
- He Z-Y, Ou L-M, Zhang J-Q, Bai J, Lui G-N, Li M-H, et al. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respiration 2010;80:445-52. [DOI: 10.1159/000321374] [DOI] [PubMed] [Google Scholar]
Mygind 2010 {published data only}
- Mygind LH, Pedersen C, Vestbo J, Christensen JJ, Frimodt-Moller N, Kristiansen IS, et al. A randomised, placebo-controlled 3 years study of prophylactic azithromycin in 575 patients with chronic obstructive pulmonary disease. In: European Respiratory Society 20th Annual Congress; 2010 Sep 18-22; Barcelona. 2010.
Seemungal 2008 {published data only}
- Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. American Journal of Respiratory and Critical Care Medicine 2008;178:1139-47. [DOI] [PubMed] [Google Scholar]
Sethi 2010 {published data only}
- Sethi S, Jones PW, Theron MS, Miravitlles M, Rubenstein E, Wedzicha JA, et al. Correction: pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomised controlled trial. Respiratory Research 2010;11:88. [DOI] [PMC free article] [PubMed]
- Sethi S, Jones PW, Theron MS, Miravitlles M, Rubinstein E, Wedzicha JA, et al. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomised control trial. Respiratory Research 2010;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shafuddin 2015 {published data only}
- Shafuddin E, Mills GD, Holmes MD, Poole PJ, Mullins PR, Black PN. A double-blind, randomised, placebo-controlled study of roxithromycin and doxycycline combination, roxithromycin alone, or matching placebo for 12 weeks in adults with frequent exacerbations of chronic obstructive pulmonary disease. Journal of Negative Results in Biomedicine 2015;14:15. [DOI: 10.1186/s12952-015-0034-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 2014 {published data only}
- Simpson JL, Powell H, Baines KJ, Milne D, Coxson HO, Hansbro PM, et al. The effect of azithromycin in adults with stable neutrophilic COPD: a double blind randomised, placebo controlled trial. PLOS One 2014;9(8):e105609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2019 {published data only}
- Singh B, Ghosh N, Saha D, Sarkar S, Bhattacharyya P, Chaudhury K. Effect of doxycycline in chronic obstructive pulmonary disease - an exploratory study. Pulmonary Pharmacology and Therapeutics 2019;101831:1-9. [DOI: 10.1016/j.pupt.2019/101831] [DOI] [PubMed] [Google Scholar]
Suzuki 2001 {published data only}
- Suzuki T, Yani M, Yamaya M, Satoh Nakagawa T, Sekizawa K, Ishida S, et al. Erythromycin and common cold in COPD. Chest 2001;120:730-3. [DOI] [PubMed] [Google Scholar]
Tan 2016 {published data only}
- Tan C, Huang H, Zhang J, He Z, Zhong X, Bai J. Effects of low-dose and long-term treatment with erythromycin on IL-17 and IL-23 in peripheral blood and induced sputum in patients with stable chronic obstructive pulmonary disease. In: Chest. Vol. 149. 2016:387A. [DOI] [PMC free article] [PubMed]
- Tan C , Huang H , Zhang J , He Z , Zhong X, Bai J. Effects of low-dose and long-term treatment with erythromycin on interleukin-17 and interleukin-23 in peripheral blood and induced sputum in patients with stable chronic obstructive pulmonary disease. Mediators of Inflammation 2016;4173962:1-11. [DOI: 10.1155/2016/4173962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uzun 2014 {published data only}
- Djamin RS, Uzun S, Ermens AAM, Kerstens R, Hoogsteden HC, Aerts JGJV, et al. Which predictors in COPD patients with the frequent exacerbator phenotype predict the treatment response to maintenance therapy with azithromycin? In: European Respiratory Journal. Vol. 48. 2016:PA3713.
- Uzun S, Djamin RS, Aerts JGJV, Van Der Eerden MM. Patients with COPD Gold C & D: the effect of long-term treatment with azithromycin on exacerbation risk assessed by the Gold Framework. In: American Journal of Respiratory and Critical Care Medicine. Vol. 189. 2014:A5967.
- Uzun S, Djamin RS, Kluytmans JA, Mulder PG, Van't Veer NE, Ermens AA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind,placebo-controlled trial. Lancet Respiratory Medicine 2014;2(5):361-8. [DOI] [PubMed] [Google Scholar]
- Uzun S, Djamin RS, Mulder PGH, Kluytmans JAJW, Pelle AJ, Van't Veer NE, et al. Effect of azithromycin maintenance treatment in patients with frequent exacerbations of COPD (COLUMBUS): a randomised, double-blind, placebo-controlled trial. In: American Journal of Respiratory and Critical Care Medicine. Vol. 189. 2014:A2884. [DOI] [PubMed]
Vermeersch 2019 {published data only}
- Vermeersch K, Gabrovska M, Aumann J, Demedts IK, Corhay J-L, Marchard E, et al. Azithromycin during acute chronic obstructive pulmonary disease exacerbations requiring hospitalisation (BACE). A multi-centre, randomised, double-blind, placebo-controlled trial. American Journal of Respiratory and Critical Care Medicine 2019;200(7):857-68. [10.1164/rccm.201901-0094oc] [DOI] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang P, Yang J, Yang Y, Ding Z. Effect of azithromycin in combination with simvastatin in the treatment of chronic obstructive pulmonary disease complicated by pulmonary arterial hypertension. Pakistan Journal of Medicine Science 2017;33(2):260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bussi 1980 {published data only}
- Bussi S, Murciano D, Botto MJ, Pariente R. Assessment of chemoprophylaxis with intermittent tetracycline in chronic bronchitis. A functional follow-up for 3 years. Revue Francaise des Malades Respiratoires 1980;8(5):351-6. [PubMed] [Google Scholar]
Calder 1968 {published data only}
- Calder MA, Lutz W, Schonell ME. A five year study of bacteriology and prophylactic chemotherapy in patients with chronic bronchitis. British Journal of Diseases of the Chest 1968;62:93. [DOI] [PubMed] [Google Scholar]
Cooper 1961 {published data only}
- Cooper AW, Williamson GM, Zinnemann K. Chronic bronchitis: changes in the bacterial flora of the sputum associated with exacerbations and long-term antibacterial treatment. British Journal of Diseases of the Chest 1961;55:23. [DOI] [PubMed] [Google Scholar]
Edwards 1958 {published data only}
- Edwards G, Fear EC. Adult chronic bronchitis - continuous antibiotic therapy. British Medical Journal 1958;2(5102):1010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
EUCTR2011‐004351‐39‐IT {published data only}
- EUCTR2011-004351-39-IT. Phase II, randomised, double arm, multi-centre study evaluating the efficacy and safety of azithromycin for the long term prophylactic treatment of COPD in primary antibody deficiency patients with clinical spirometrically confirmed COPD suffering from repeated acute exacerbations. www.clinicaltrialsregister.eu/ctr-search/search?query=2011-004351-39 (first received 28 October 2011).
Francis 1960 {published data only}
- Francis RS, Spicer CC. Influence of daily penicillin and tetracycline on exacerbations and their cost. British Medical Journal 1960;30(5169):297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN72035428 {published data only}
- ISRCTN72035428. The effectiveness of antibiotics compared to no antibiotics for exacerbations of chronic obstructive pulmonary disease: a feasibility study. www.isrctn.com/ISRCTN72035428 (first received 12 March 2010).
- Staa TP, Dyson L, McCann G, Padmanabhan S, Belatri R, Goldacre B et al. The opportunity and challenges of pragmatic point-of-care randomised trials using routinely collected electronic records: evaluations of two exemplar trials. Health Technology Assessment 2014;18(43):1-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milito 2019 {published data only}
- Milito C, Pulvirenti F, Tabolli S, Carello R, Quinti I. Antibiotic prophylaxis in primary antibody deficiency patients: study design. Journal of Clinical Immunology 2017;37:197-266. [Google Scholar]
- Milito C, Pulvirenti F, Tabolli S, Carrabba M, Fabio G, Rizzo F, et al. Low dose azithromycin prophylaxis in primary antibody deficiencies. European Respiratory Journal 2019;54(Suppl 63):RCT5100. [DOI: 10.1183/13993003.congress-2019.RCT5100] [DOI] [Google Scholar]
Murdoch 1959 {published data only}
- Murdoch HM, Leckie WJ, Downie J, Swain RH, Gould JC. Evaluation of continuous antibiotic therapy in chronic bronchitis. British Medical Journal 1959;2(5162):1277-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicholson 2016 {published data only}
- Nicholson TT, Franciosi A, Landers S, Butler MW. Assessing potential risks of treatment with long-term azithromycin in COPD patients: long-term oxygen users beware? Irish Journal of Medical Science 2016;185(4):993-7. [DOI] [PubMed] [Google Scholar]
O'Reilly 2013 {published data only}
- O'Reilly PJ, Jackson PL, Wells JM, Dransfield MT Scanlon PD, Blalock JE. Sputum PGP is reduced by azithromycin treatment in patients with COPD and correlates with exacerbations. BMJ Open 2013;3(12):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Velzen 2016 {published data only}
- Van Velzen P, Ter Riet G, Bresser P, Van Den Berg BTJ, Van Den Berg JWK, Daniels JMA, et al. Long term effects of antibiotics in COPD exacerbations: a randomised clinical trial. American Journal of Respiratory and Critical Care Medicine 2016;193:A1021. [Google Scholar]
Watanabe 1994 {published data only}
- Watanabe M, Motomiya T, Nuwika Y, Nakai Y, Honda K, Konno K, et al. A well-controlled comparative clinical study of the combination regimen of ciprofloxacin plus erythromycin for the treatment of repeated acute exacerbations of chronic respiratory tract infections. Chemotherapy 1994;42(10):1194-201. [Google Scholar]
Zykov 2008 {published data only}
- Zykov K, Rvatcheva A, Pustovalov A, Averyanov A. Long term treatment with clarithromycin of stage II COPD patients with frequent exacerbations. In: European Respiratory Society 18th Annual Congress; 2008 Oct 3-7; Berlin. 2008.
Additional references
Brusselle 2013
- Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZIAST): a multi-centre randomised double-blind placebo-controlled trial. Thorax 2013;68(4):322-9. [DOI] [PubMed] [Google Scholar]
Cui 2018
- Cui Y, Luo L, Li C, Chen P, Chen Y. Long-term macrolide treatment for the prevention of acute exacerbations in COPD: a systematic review and meta-analysis. International Journal of COPD 2018;13:3813-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2013a
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. Medical Decision Making 2013;33(5):607-17. [DOI: 10.1177/0272989X12458724] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2013b
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomised controlled trials. Medical Decision Making 2013;33(5):641-56. [DOI: 10.1177/0272989X12455847] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2018
- Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Bias adjustment methods. In: Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ, editor(s). In: Network Meta-Analysis for Decision-Making. New York: John Wiley and Sons Ltd., 2018. [DOI: 10.1002/9781118951651.ch9] [DOI] [Google Scholar]
Donaldson 2002
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57(10):847-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donath 2013
- Donath E, Chaudhry A, Hernandez-Aya LF, Lit L. A meta-analysis on the prophylactic use of macrolide antibiotics for the prevention of disease exacerbations in patients with chronic obstructive pulmonary disease. Respiratory Medicine 2013;107:1385-92. [DOI] [PubMed] [Google Scholar]
Franchini 2012
- Franchini A, Dias S, Ades AE, Jansen J, Welton N. Accounting for correlation in mixed treatment comparisons with multi-arm trials. Research Synthesis Methods 2012;3:142-60. [DOI] [PubMed] [Google Scholar]
GOLD 2020
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2020 Report. www.guidelines.co.uk/respiratory/gold-copd-2020-strategy/455088.article (accessed 1 May 2020).
Guyatt 2017
- Guyatt GH, Ebrahim S, Alonso-Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE Guidelines 17: Assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14-22. [DOI] [PubMed] [Google Scholar]
Herath 2018
- Herath SC, Normansell R, Maisey S, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD009764. [DOI: 10.1002/14651858.CD009764.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2018
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Huckle 2018
- Huckle AW, Fairlough LC, Todd IT. Prophylactic antibiotic use in COPD and the potential anti-inflammatory activities of antibiotics. Respiratory Care 2018;63(5):609-19. [DOI] [PubMed] [Google Scholar]
Hurst 2010
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. New England Journal of Medicine 2010;363(12):1128-38. [DOI: 10.1056/NEJMoa0909883] [DOI] [PubMed] [Google Scholar]
Janjua 2021
- Janjua S , Mathioudakis AG , Fortescue R , Walker RAE , Sharif S, Threapleton CJD et al. Supplementary material: Prophylactic antibiotics for adults with chronic obstructive pulmonary disease: a network meta-analysis. [data set]. Zenodo 2021. [DOI: 10.5281/zenodo.4428848] [DOI] [PMC free article] [PubMed]
Kew 2014
- Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD010844. [DOI: 10.1002/14651858.CD010844.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2013
- Lee JS, Park D-A, Hong Y, Jo KW, Lee SW, Huh JW, et al. Systematic review and meta-analysis of prophylactic antibiotics in COPD and/or chronic bronchitis. International Journal of Tuberculosis and Lung Disease 2013;17(2):153-62. [DOI] [PubMed] [Google Scholar]
Loukides 2013
- Loukides S, Bartziokas K, Vestbo J, Singh D. Novel anti-inflammatory agents in COPD: targeting lung and systemic inflammation. Current Drug Targets 2013;14(2):235-45. [DOI] [PubMed] [Google Scholar]
Lunn 2009
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions (with discussion). Statistics In Medicine 2009;28:3049-82. [DOI] [PubMed] [Google Scholar]
Martinez 2008
- Martinez FJ, Curtis JL, Albert R. Role of macrolide therapy in chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease 2008;3(3):331-50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathioudakis 2017
- Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. European Respiratory Review 2017;26(143):160073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathioudakis 2020
- Mathioudakis AG, Janssens W, Sivapalan P, Singanayagam A, Dransfield MT, Jensen JS, et al. Acute exacerbations of chronic obstructive pulmonary disease: in search of diagnostic biomarkers and treatable traits. Thorax 2020;75(6):520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miravittles 2015
- Miravittles M, Anzueto A. Antibiotic prophylaxis in COPD: why, when, and for whom? Pulmonary Pharmacology and Therapeutics 2015;32:119-23. [DOI: 10.1016/j.pupt.2014.05.002] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00524095
- NCT00524095. Bronchiectasis in Chronic Obstructive Pulmonary Disease (COPD) Patients: Role of Prophylaxis. https://clinicaltrials.gov/ct2/show/NCT00524095 2009.
NCT02628769
- NCT02628769. A study to evaluate the anti-inflammatory effects of solithromycin in chronic obstructive pulmonary disease. https://clinicaltrials.gov/ct2/show/NCT02628769 2017.
Ni 2015
- Ni W, Shao X, Cai X, Wei C, Cui J, Wang R, et al. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. PLoS One 2015;10(3):e0121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2016
- National Institute of Health and Clinical Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management (NG115). www.nice.org.uk/guidance/ng115 (accessed prior to 18 August 2020).
NICE 2018
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in 16s: diagnosis and management NG115. Recommendations. www.nice.org.uk/guidance/ng115/chapter/Recommendations (accessed prior to 18 August 2020).
Pasquale 2012
- Pasquale MK, Sun SX, Song F, Hartnett HJ, Stemkowski SA. Impact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly Medicare population. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:757-64. [DOI: 10.2147/COPD.S36997] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillippo 2018
- Phillippo DM, Dias S, Ades AE, Didelez V, Welton NJ. Sensitivity of treatment recommendations to bias in network meta-analysis. Journal of the Royal Statistical Society 2018;181(3):843-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillippo 2019
- Phillippo DM, Dias S, Welton NJ, Caldwell DM, Taske N, Ades AE. Threshold analysis as an alternative to GRADE for assessing confidence in guideline recommendations based on network meta-analyses threshold analysis in guideline development. Annals of Internal Medicine 2019;170(8):538-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puhan 2006
- Puhan MA, Soesilo I, Guyatt GH, Schünemann HJ. Combining scores from different patient reported outcome measures in meta-analyses: when is it justified? Health and Quality of LIfe Outcomes 2006;7(4):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rhodes 2015
- Rhodes KM, Turner RM, Higgins JPT. Predictive distributions were developed for the extent of heterogeneity in meta-analysis of continuous outcome data. Journal of Clinical Epidemiology 2015;68:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubinstein 2002
- Rubinstein I, Camm J. Cardiotoxicity of fluoroquinolones. Journal of Antimicrobial Chemotherapy 2002;49(4):593-6. [DOI] [PubMed] [Google Scholar]
Sapey 2006
- Sapey E, Stockley RA. COPD exacerbations. 2: Aetiology. Thorax 2006;61(3):250-8. [DOI: 10.1136/thx.2005.041822] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seemungal 1998
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Americal Journal of Respiratory and Critical Care Medicine 1998;157(5 Pt 1):1418-22. [DOI] [PubMed] [Google Scholar]
Sethi 2004
- Sethi S. Bacteria in exacerbations of chronic obstructive pulmonary disease: phenomenon or epiphenomenon? Proceedings of the American Thoracic Society 2004;1(2):109-14. [DOI] [PubMed] [Google Scholar]
Siva 2014
- Siva R, Bafadhel M, Monteiro W, Brightling CE, Pavord ID. Effect of levofloxacin on neutrophilic airway inflammation in stable COPD: a randomized, double-blind, placebo-controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:179-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2020
- Smith D, Du Rand I, Addy CL, Collyns T, Hart SP, Mitchelmore PJ, et al. British Thoracic Society guideline for the use of long-term macrolides in adults with respiratory disease. British Medical Journal 2020;0:1-35. [DOI: 10.1136/ thoraxjnl-2019-213929] [DOI] [PubMed] [Google Scholar]
Soler‐Cataluña 2005
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60(11):925-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sze 2014
- Sze MA, Hogg JC, Sin DD. Bacterial microbiome of lungs in COPD. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:229-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2015
- Turner RM, Jackson D, Wei Y, Thompson SG, Higgins JPT. Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Statistics in Medicine 2015;34(6):984-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2018
- Wang Y, Zijp TR, Bahar MA, Kocks JWH, Wilffert B, Hak E. Effects of prophylactic antibiotics on patients with stable COPD: a systematic review and meta-analysis of randomised controlled trials. Journal of Antimicrobial Chemotherapy 2018;73:3231-43. [DOI] [PubMed] [Google Scholar]
Yao 2013
- Yao G-Y, Ma Y-L, Zhang M-O, Gao Z-C. Macrolide therapy decreases chronic obstructive pulmonary disease exacerbation: a meta-analysis. Respiration 2013;86:254-60. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Threapleton 2018
- Threapleton CJD, Normansell R, Baker EH. Head-to-head oral prophylactic antibiotics therapy for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD013024. [DOI: 10.1002/14651858.CD013024] [DOI] [PMC free article] [PubMed] [Google Scholar]


