Abstract
Background
Caesarean section increases the risk of postpartum infection for women and prophylactic antibiotics have been shown to reduce the incidence; however, there are adverse effects. It is important to identify the most effective class of antibiotics to use and those with the least adverse effects.
Objectives
To determine, from the best available evidence, the balance of benefits and harms between different classes of antibiotic given prophylactically to women undergoing caesarean section, considering their effectiveness in reducing infectious complications for women and adverse effects on both mother and infant.
Search methods
For this 2020 update, we searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (2 December 2019), and reference lists of retrieved studies.
Selection criteria
We included randomised controlled trials (RCTs) comparing different classes of prophylactic antibiotics given to women undergoing caesarean section. RCTs published in abstract form were also included. We excluded trials that compared drugs with placebo or drugs within a specific class; these are assessed in other Cochrane Reviews. We excluded quasi‐RCTs and cross‐over trials. Cluster‐RCTs were eligible for inclusion but none were identified.
Data collection and analysis
Two review authors independently assessed the studies for inclusion, assessed risk of bias and carried out data extraction. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included 39 studies, with 33 providing data (8073 women). Thirty‐two studies (7690 women) contributing data administered antibiotics systemically, while one study (383 women) used lavage and was analysed separately.
We identified three main comparisons that addressed clinically important questions on antibiotics at caesarean section (all systemic administration), but we only found studies for one comparison, 'antistaphylococcal cephalosporins (1st and 2nd generation) versus broad spectrum penicillins plus betalactamase inhibitors'. We found no studies for the following comparisons: 'antistaphylococcal cephalosporins (1st and 2nd generation) versus lincosamides' and 'antistaphylococcal cephalosporins (1st and 2nd generation) versus lincosamides plus aminoglycosides'.
Twenty‐seven studies (22 provided data) included comparisons of cephalosporins (only) versus penicillins (only). However for this update, we only pooled data relating to different sub‐classes of penicillins and cephalosporins where they are known to have similar spectra of action against agents likely to cause infection at caesarean section.
Eight trials, providing data on 1540 women, reported on our main comparison, 'antistaphylococcal cephalosporins (1st and 2nd generation) versus broad spectrum penicillins plus betalactamase inhibitors'. We found data on four other comparisons of cephalosporins (only) versus penicillins (only) using systemic administration: antistaphylococcal cephalosporins (1st and 2nd generation) versus non‐antistaphylococcal penicillins (natural and broad spectrum) (9 studies, 3093 women); minimally antistaphylococcal cephalosporins (3rd generation) versus non‐antistaphylococcal penicillins (natural and broad spectrum) (4 studies, 854 women); minimally antistaphylococcal cephalosporins (3rd generation) versus broad spectrum penicillins plus betalactamase inhibitors (2 studies, 865 women); and minimally antistaphylococcal cephalosporins (3rd generation) versus broad spectrum and antistaphylococcal penicillins (1 study, 200 women). For other comparisons of different classes of antibiotics, only a small number of trials provided data for each comparison, and in all but one case data were not pooled.
For all comparisons, there was a lack of good quality data and important outcomes often included few women. Three of the studies that contributed data were undertaken with drug company funding, one was funded by the hospital, and for all other studies the funding source was not reported.
Most of the studies were at unclear risk of selection bias, reporting bias and other biases, partly due to the inclusion of many older trials where trial reports did not provide sufficient methodological information. We undertook GRADE assessment on the only main comparison reported by the included studies, antistaphylococcal cephalosporins (1st and 2nd generation) versus broad spectrum penicillins plus betalactamase inhibitors, and the certainty ranged from low to very low, mostly due to concerns about risk of bias, wide confidence intervals (CI), and few events.
In terms of the primary outcomes for our main comparison of 'antistaphylococcal cephalosporins (1st and 2nd generation) versus broad spectrum penicillins plus betalactamase inhibitors': only one small study reported sepsis, and there were too few events to identify clear differences between the drugs (risk ratio (RR) 2.37, 95% CI 0.10 to 56.41, 1 study, 75 women, very low‐certainty evidence). There may be little or no difference between these antibiotics in preventing endometritis (RR 1.10; 95% CI 0.76 to 1.60, 7 studies, 1161 women; low‐certainty evidence). None of the included studies reported on infant sepsis or infant oral thrush. For our secondary outcomes, we found there may be little or no difference between interventions for maternal fever (RR 1.07, 95% CI 0.65 to 1.75, 3 studies, 678 women; low‐certainty evidence). We are uncertain of the effects on maternal: wound infection (RR 0.78, 95% CI 0.32 to 1.90, 4 studies, 543 women), urinary tract infection (average RR 0.64, 95% CI 0.11 to 3.73, 4 studies, 496 women), composite adverse effects (RR 0.96, 95% CI 0.09 to 10.50, 2 studies, 468 women), and skin rash (RR 1.08, 95% CI 0.28 to 4.1, 3 studies, 591 women) (all very low certainty evidence). Although maternal allergic reactions were reported by two studies, there were no events. There were no infant outcomes reported in the included studies.
For the other comparisons, the results for most outcomes had wide CIs, few studies and few women included. None of the included trials reported on longer‐term maternal outcomes, or on any infant outcomes.
Authors' conclusions
Based on the best currently available evidence, 'antistaphylococcal cephalosporins' and 'broad spectrum penicillins plus betalactamase inhibitors' may have similar efficacy at caesarean section when considering immediate postoperative infection, although we did not have clear evidence for several important outcomes. Most trials administered antibiotics at or after cord clamping, or post‐operatively, so results may have limited applicability to current practice which generally favours administration prior to skin incision. We have no data on any infant outcomes, nor on late infections (up to 30 days) in the mother; these are important gaps in the evidence that warrant further research. Antimicrobial resistance is very important but more appropriately investigated by other trial designs.
Plain language summary
Comparing different types of antibiotics given routinely to women at caesarean section to reduce infections
What is the issue?
We wanted to find out if giving specific types of antibiotics routinely at caesarean sections reduced the number of women and babies with infections, when compared with other types of antibiotics. We also looked to see if there were differences in adverse effects. The main types of antibiotics we considered were ones which target infections most commonly seen after giving birth, so we looked mainly at cephalosporins versus penicillins. We collected and analysed all relevant studies (randomised controlled trials) to answer this question (date of latest search 2 December 2019).
Why is this important?
Women undergoing caesarean section have an increased likelihood of infection compared with women giving birth vaginally. These infections can come from the urine, the surgical incision, or occur in the lining of the womb (endometritis). Infections can be serious, causing, for example, an abscess in the pelvis or infection in the blood. Very occasionally they can lead to a mother's death, particularly in low‐resource settings. Good surgical techniques are important to reduce infection, along with the use of skin antiseptics and giving antibiotics before the initiation of the caesarean section. Antibiotics can, however, cause adverse effects in the mother, such as nausea, vomiting, skin rash and in some rare cases allergic reactions. The mother and the baby can develop thrush (candida). Antibiotics given to women around the time of giving birth can also change the baby's gut flora and may interfere with the baby's developing immune system.
What evidence did we find?
We included 39 studies, of which 33 studies involving 8073 women and their babies provided data. The quality of the individual studies was generally unclear, which led to overall low or very low certainty of the evidence. Three of the 33 studies were undertaken with drug company funding. Most of the studies administered antibiotics at or after cord clamping, although practice now often gives antibiotics before skin incision.
Eight studies with data on 1540 women reported on antistaphylococcal cephalosporins (first and second generation) versus broad spectrum penicillins plus betalactamase inhibitors. We found that these antibiotics may be as effective as each other in reducing endometritis and maternal fever. We were uncertain which antibiotic performed better for wound infection, urinary tract infection, and maternal adverse effects such as nausea, vomiting, diarrhoea and skin rash. We did not find any evidence on longer‐term outcomes for mothers once they left hospital, or on any outcomes for babies. Only one small study (75 women) reported on blood infection (sepsis) in mothers, with too few events to identify any clear differences between the antibiotics.
We identified no studies with evidence on antistaphylococcal cephalosporins versus lincosamides, nor antistaphylococcal cephalosporins versus lincosamides plus aminoglycosides. The other studies looked at a very large number of different comparisons with insufficient data to come to any firm conclusions about specific comparisons.
What does this mean?
At caesarean sections, antistaphylococcal cephalosporins and penicillins plus betalactamase inhibitors may be similarly effective at preventing infections for the mother, although we did not find clear evidence for many important outcomes. In particular, we found no evidence describing the effects of these antibiotics on babies, nor any longer‐term effects on women and children. This is particularly concerning for the studies giving the antibiotics prior to the surgical incision, as these antibiotics may reach the baby. For the other comparisons included in this review, data were sparse. Many studies were old and lacked information on study design and important outcomes, often including small numbers of women and few events. Research on drug‐resistant antibiotics needs to be considered as well.
Summary of findings
Summary of findings 1. Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) compared to broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes for preventing infection at caesarean section.
| Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) compared to broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes for preventing infection at caesarean section | ||||||
| Patient or population: all women undergoing caesarean section Setting: Hospital (Greece, India, Thailand, USA) Intervention: antistaphylococcal cephalosporins 1st and 2nd generation (C1 and C2) Comparison: broad spectrum penicillins plus betalactamase inhibitors (P2+) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with broad spectrum penicillins plus betalactamase inhibitors (P2+) | Risk with Antistaphylococcal cephalosporins (1st and 2nd generation (C1 and C2) | |||||
| Maternal sepsis | Study population | RR 2.37 (0.10 to 56.41) | 75 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Maternal endometritis | Study population | RR 1.10 (0.76 to 1.60) | 1161 (7 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | ||
| 78 per 1000 | 86 per 1000 (60 to 125) | |||||
| Infant sepsis | Study population | ‐ | (0 studies) | ‐ | No included studies reported on this outcome | |
| see comment | see comment | |||||
| Infant oral thrush | Study population | ‐ | (0 studies) | ‐ | No included studies reported on this outcome | |
| see comment | see comment | |||||
| Maternal wound infection | Study population | RR 0.78 (0.32 to 1.90) | 543 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 5 | ||
| 38 per 1000 | 29 per 1000 (12 to 71) | |||||
| Maternal urinary tract infection | Study population | RR 0.64 (0.11 to 3.73) | 496 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 6 7 8 | ||
| 51 per 1000 | 33 per 1000 (6 to 190) | |||||
| Maternal composite adverse effects | Study population | RR 0.96 (0.09 to 10.50) | 468 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 5 | ||
| 5 per 1000 | 5 per 1000 (0 to 56) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 All of pooled effect provided by study or studies at moderate risk of selection bias. Downgrade ‐1.
2 Single study with small sample size and few events. Wide confidence interval including both appreciable reduction and appreciable increase in risk with antistaphylococcal (1st and 2nd generation) cephalosporins. The reported data are for bacteriaemia, not sepsis. Although bacteriaemia is usually accompanied by sepsis, there is the possibility of indirectness for this outcome. Downgrade ‐2.
3 Wide confidence interval including appreciable increase in risk with antistaphylococcal (1st and 2nd generation) cephalosporins, whilst also including no difference in effect. Downgrade ‐1.
4 Majority of pooled effect provided by studies at moderate risk of selection bias or detection bias. Downgrade ‐1.
5 Few events. Wide confidence interval including both appreciable reduction and appreciable increase in risk with antistaphylococcal (1st and 2nd generation) cephalosporins. Downgrade ‐2.
6 Majority of pooled effect provided by studies at moderate risk of bias due to lack of information about random sequence generation and concealment of allocation. Downgrade ‐1.
7 Severe unexplained statistical heterogeneity (I2 = 66%, P value for Chi2 test = 0.05). Downgrade ‐1.
8 Few events. Downgrade ‐1.
Background
The incidence of caesarean sections is increasing annually, with a global estimate of 29.7 million births by caesarean section (20.1% of live births) in 2015, up from 12% in 2000 (Boerma 2018). Rates of caesarean section differ widely by region, from 4.1% in parts of Africa to 44.3% in some areas of Latin America (Boerma 2018). Women undergoing caesarean section have an increased risk of postoperative infection and infectious morbidity compared with women giving birth vaginally (Declercq 2007), therefore the large and potentially increasing number of infections worldwide is a major concern.
Description of the condition
Caesarean sections have been shown to have nearly five times the risk of postpartum infection as vaginal births (and this is with a policy of antibiotic prophylaxis at caesarean section), and just over 75% occur after hospital discharge (Leth 2009). The infectious complications that can occur after caesarean birth include infections of the wound/incision, endometritis (infection of the lining of the uterus) and urinary tract infection (UTI), although fever can occur after any operation and is not necessarily an indicator of infection (Mascarello 2017; van Dillen 2010). However, there can occasionally be more serious infectious complications such as pelvic abscess (collection of pus in the pelvis), bacteraemia (bacterial infection in the blood), sepsis (organ dysfunction resulting from infection), and its most severe form septic shock, necrotising fasciitis (tissue destruction in the abdominal wall), and septic pelvic vein thrombophlebitis (inflammation and infection of the veins in the pelvis). These more serious infectious complications can lead to maternal mortality.
Description of the intervention
The potential for prophylactic antibiotics to reduce the incidence of maternal infectious morbidity following caesarean section has now been systematically investigated (Hofmeyr 2010; Smaill 2014). Although evidence has existed for some time to support this practice (Smaill 2014; Wilson 2018), it is not clear whether any one particular agent, dose or route of administration is superior. Many different drug regimens have been reported to be effective in decreasing immediate postoperative infectious morbidity. To date, various penicillins (ampicillin, ticarcillin, mezlocillin, piperacillin), cephalosporins (cefazolin, cephalothin, ceforanide, cefonicid, cefuroxime, ceftazidime, cefoxitin, cefamandole, cephradine, cefotetan, cefotaxime), fluoroquinolones, etc. have been used for caesarean section prophylaxis and overall they have demonstrated some efficacy either alone or in combination with another drug (Smaill 2008). Some of these drugs have activity against a narrow range of potential pathogens (e.g. metronidazole, gentamicin), others have additional specific anaerobic activity (e.g. cefoxitin and cefotetan), and yet others have very broad‐spectrum coverage (imipenem). Their pharmacokinetic properties (e.g. serum half‐life) also differ. Some drugs used in the past are now associated with bacterial resistance (Martinez de Tejada 2014). Despite variability in local policies, the American College of Obstetricians and Gynecologists (ACOG 2018), Infectious Diseases Society of America (IDSA 2013), and the Canadian Society of Obstetrics and Gynaecology (SOGC 2017) have recommended the use of cefazolin or other first‐generation cephalosporins as first choice for prophylaxis at caesarean section. In the UK, the Royal College of Obstetricians and Gynaecologists recommend prophylactic antibiotics before skin incision that are effective against endometritis, UTI and wound infections, however they advise against co‐amoxyclav (amoxicillin plus clavulanic acid) due to an increase in risk of necrotising enterocolitis for babies exposed to this antibiotic (RCOG 2011).
In addition to the choice of drug, there are differences in the route and the timing of administration of prophylactic antibiotics. As well as systemic administration (intravenous, intramuscular or oral), use of intra‐operative irrigation of the uterus and peritoneal cavity with an antibiotic solution has been reported. While some guidelines recommend multiple doses of antibiotics, a single dose at the time of the procedure may be adequate. These considerations will be covered in other Cochrane Reviews ‐ see Differences between protocol and review for details.
How the intervention might work
Since penicillin was introduced during the 1940s, scientists have developed numerous other antibiotics. Today, over 100 different antibiotics are available. For the prevention of surgical infections, it is generally considered that sound surgical technique is important along with skin antiseptics and the use of antibiotics (Martin 2018; Walsh 2010). Antibiotics act by either killing bacteria (bactericidal) or inhibiting bacterial replication (bacteriostatic), but the large variety of different types of bacteria mean a large variety of possible antibiotics may be used (Kapoor 2017).
Classification of antibiotics
Antibiotics can be classified in a number of ways, but classifying by chemical structure is useful because antibiotics within a structural class will generally have similar patterns of effectiveness, toxicity and allergic potential (Bayarski 2006; eMedExpert 2009; Goodman 2008). The most commonly used types of antibiotics for surgical prophylaxis are penicillins with or without betalactamase inhibitors, cephalosporins, aminoglycosides, lincosamide, fluoroquinolones, carbapenems, and macrolides. Each class includes many drugs (Table 2). Penicillins have a common structure which they share with cephalosporins and carbapenems, the betalactam ring. Both penicillins and cephalosporins are bactericidal, acting through inhibiting cell wall synthesis (Letourneau 2020a). Penicillins are grouped into three types and cephalosporins are grouped into five generations with each newer generation having a broader spectrum of activity (Letourneau 2020b; Letourneau 2020d). Fluoroquinolones are synthetic rather than derived from bacteria, and interfere with the ability of bacteria to make DNA. These newer fluoroquinolones are broad‐spectrum bacteriocidal drugs chemically unrelated to penicillins or cephalosporins. Macrolides are derived from streptomyces bacteria and are also bacteriostatic in action, binding to bacterial ribosomes. Aminoglycosides are relatively broad spectrum antibiotics usually used in combination with other antibiotics such as beta‐lactams (Drew 2020).
1. Classification of antibiotics.
| Penicillins (P) | ||
| Penicillins consist of a thiazolidine ring connected to a B‐lactam ring to which is attached to a side chain. The penicillin nucleus itself is the chief structural requirement for biological activity. Penicillins are the oldest class of antibiotics and function by inhibiting cell wall synthesis (bactericidal). | ||
| Class or sub‐class name and detail | Examples | Spectrum |
| Natural penicillins (P1) are based on the original penicillin‐G structure (also known as first‐generation penicillins) | Penicillin G (benzyl penicillin, crystalline penicillin); Procaine; Penicillin V; Benzathine. | Gram‐positive: non‐betalactamase producing gram‐positive cocci (including viridans streptococci, group A streptococci, Streptococcus pneumoniae, anaerobic Streptococcus), Enterococcus spp., non‐penicillinase producing strains of Staphylococcus aureus, coagulase negative Staphylococcus aureus, Clostridium spp. (excluding C. difficile), Actinomyces spp Gram‐negative:Neisseria meningitides, non‐penicillinase producing Neisseria gonorrhoea, Pasteurella multocida |
| Broad spectrum penicillins(P2) which are effective against a wider range of bacteria |
Second‐generation penicillins: Aminopenicillins; Ampicillin; Amoxicillin. |
Gram‐positive:Streptococcus spp, Enterococcus faecalis, Listeria monocytogenes. Gram negative:Escherichia coli, Proteus mirabilis, Salmonella, Shigella, e Haemophilus influenzae Anaerobes: Clostridium spp |
| Third‐generation penicillins: Carbenicillin; Ticarcillin. |
Gram‐positive: Streptococcus spp, Enterococcus faecalis, Listeria monocytogenes. Gram‐negative: Escherichia coli, Proteus mirabilis, Salmonella, Shigella, Haemophilus influenzae, Pseudomonas aeruginosa, Acinetobacter spp Anaerobes: Clostridium spp |
|
| Fourth‐generation penicillins: Piperacillin; Mezlocillin. | Gram‐positive: Streptococcus spp, Enterococcus faecalis, Listeria monocytogenes, Staphylococcus aureus. Gram‐negative:Escherichia coli, Proteus mirabilis, Salmonella, Shigella, e Haemophilus influenzae Anaerobes: Clostridium spp, Bacteroides fragilis | |
| Penicillins plus betalactamase inhibitors (P2+) are active against gram‐positive, gram‐negative and anaerobic bacteria, including S.aureus, Enterococci, Streptococci, many Enterobacterales and Bacteroides spp | Co‐amoxyclav = amoxicillin + clavulanic acid (Trade names include: Augmentin; Clavamox; Tyclav) Ampicillin + sulbactam (Trade names include: Ampictam; Unasyn) Timentin = ticarcillin + clavulanate | Gram‐positive: Streptococcus spp, Enterococcus faecalis, Listeria monocytogenes, Staphylococcus aureus. Gram‐negative:Escherichia coli, Proteus mirabilis, Salmonella, Shigella, e Haemophilus influenzae Anaerobes: Clostridium spp, Bacteroides fragilis |
| Antistaphylococcal penicillins(P3) are active even in the presence of the bacterial enzyme that inactivates most natural penicillins (also known as penicillinase‐resistant penicillins) | Cloxacillin; Dicloxacillin; Methicillin; Nafcillin; Oxacillin. | Staphylococcus aureus |
| Cephalosporins (C) | ||
| Cephalosporins have a similar basic structure to penicillins but with different side chains. They function by inhibiting cell wall synthesis. | ||
| First‐generation cephalosporins (C1) | Cephalothin; cefazolin; cephapirin; cephradine; cephalexin; cefadroxil. |
Gram‐positive: (Streptococcus spp, Staphylococcus aureus) Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae Anaerobes: except Bacteroides |
| Second‐generation cephalosporins (C2) | Cefoxitin; cefaclor; cefuroxime; cefotetan; cefprozil; cefamandole, cefonicid; ceforanide, cefotiam. |
Gram‐positive: (Streptococcus spp, Staphylococcus aureus) Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae. Anaerobes: including Bacteroides (Cephamycins) |
| Third‐generation cephalosporins (C3) | Cefotaxime; ceftizoxime; ceftriaxone; cefpodoxime; cefditoren; ceftibuten; ceftazidime; cefcapene; cefdaloxime; cefetamet; cefixime; cefmenoxime; cefodizime; cefoperazone; cefpimizole. |
Gram‐negative: Enterobacterales, Neisseria spp, Haemophilus spp Gram‐positive: Streptococcus spp Anaerobes:Bacteroides fragilis, Clostridium spp, Peptostreptococcus spp, Prevotella sp |
| Fourth‐generation cephalosporins (C4) | Cefepime; cefpirome; cefclidine; cefluprenam; cefozopran; cefquinome. | Gram‐negatives: Enterobacterales, Neisseria spp, Haemophilus spp, Acinetobacter spp, Pseudomonas aeruginosa Gram‐positive:Staphylococcus aureus, Streptococcus spp |
| Cephalosporin plus betalactamase inhibitors (C+) | Ceftolozane‐tazobactam; ceftazidime‐avibactam. |
Ceftolozane‐tazobactam Gram‐negative:Enterobacterales, P aeruginosa, Gram‐positive: limited activity against streptococci, general low activity against staphilococcal and enterococcal species. Ceftzidime‐avibactam extends the spectrum of ceftazidime against AmpC beta‐lactamase, ESBL and some specific carbapenemases |
| Other classes of antibiotics | ||
| Aminoglycosides (A) are first‐line therapy for a limited number of very specific, often historically prominent infections, such as plague, tularemia and tuberculosis. They are used to treat resistant infections caused by Gram‐negative bacilli | Streptomycin; gentamicin, kanamycin, amikacin. | Gram‐negative: Enterobacterales, Pseudomonas spp, Acinetobacter spp Synergism with beta‐lactams and glycopeptides Enterococcus spp and S. aureus |
| Amphenicols (Am) inhibit bacterial protein synthesis. Very rarely used nowadays. | Chloramphenicol | Chloramphenicol is considered to have similar action to tetracycline (see below). |
| Other beta‐lactams: carbapenems (Ca) Carbapenems are beta‐lactams that have a broader spectrum of activity than most other beta‐lactam antibiotics. | Examples include Imipenem; meropenem; ertapenem; aztreonam. |
Gram‐negative: including Extended‐sectrum betalactamase producing bacteria (ESBL+), H. influenzae e N. gonorrhoeae, Enterobacterales, Acinetobacter spp, P. aeruginosa
Gram‐positive: including Enterococcus faecalis, Listeria S. aureus Anaerobes: including B. fragilis |
| Fluoroquinolones (F) target the bacterial DNA gyrase and topoisomerase. They are potent bacteriocidal agents against a broad variety of micro‐organisms. | Ciprofloxacin; levofloxacin; lomefloxacin; norfloxacin; sparfloxacin; clinafloxacin; gatifloxacin; ofloxacin; trovafloxacin, maxifloxacin. | Gram‐negative: Enterobacterales, Pseudomonas spp, Acinetobacter spp Moxifloxacin and Levofloxacin: as above plus Streptococci |
| Lincosamides (L) are protein synthesis inhibitors which bind to the 50s subunit of bacterial ribosomes and inhibit early elongation of peptide chain by inhibiting transpeptidase reaction. | Lincomycin; clindamycin. | Gram‐positive aerobes and anaerobes, including S. Aureus and Streptococci, not Enterococci |
| Macrolides (M) inhibit bacterial protein synthesis. Resistance can arise. | Erythromycin; clarithromycin; azithromycin. | Streptococcus pneumoniae,S. aureus, Listeria monocytogenes, Neisseria spp, Chlamydia spp, Legionella spp, Haemophilus spp |
| Nitroimidazoles (N) Nitroimidazole is an imidazole derivative that contains a nitro group. It is used for the treatment of infection with anaerobic organisms. | Metronidazole; tinidazol. | Clostridium spp, Eubacterium spp, Peptococcus spp, Peptostreptococcus spp, Fusobacterium spp, Gardnerella, Mobiluncus, Trichomonas, Entamoeba spp |
| Tetracyclines (T) are bacteriostatic antibiotics active against a wide range of aerobes and anaerobic gram‐positive and gram‐negative bacteria. They inhibit bacterial protein synthesis by binding to the 30S bacterial ribosome. Tetracyclines should not be used with children under 8 and specifically during teeth development as they can cause a permanent brown discolouration to the teeth. This antibiotic is, therefore, unlikely to be used at caesarean section. | Tetracycline; doxycycline; minocycline. | Staphylococcus aureus, Streptococcus pneumonia, Streptococcus pyogenes, Streptooccus agalacticae, Campylobacter jejuni, Haemophilus influenzae, Neisseria gonorrhoeae, Neisseria meningitides,Clostridium spp., Peptostreptococcus spp., Peptococcus spp. Bacteroides melaninogenicus, Bacteroides fragilis |
This table was originally adapted from information at https://www.emedexpert.com/classes/antibiotics.shtml, and has been revised for the 2020 update (Drew 2020; Letourneau 2020a; Letourneau 2020b; Letourneau 2020c; Letourneau 2020d; WHO 2020.
Potential adverse effects of antibiotics
On the mother
The benefits of antibiotics are well‐known, but there are potential adverse effects which also need to be considered. Antibiotic use is associated with some gastrointestinal symptoms (nausea, vomiting or diarrhoea), skin rashes, thrush/candidiasis (infection with candida which can affect both mother and baby), and joint pain (Dancer 2004). Occasionally there can also be blood problems, or kidney or liver damage (Dancer 2004; Martinez de Tejada 2014; Seedat 2017), and very occasionally anaphylaxis (a hypersensitivity reaction leading to pallor, shock and collapse, which is sometimes fatal). Possible interactions with other drugs the mother may be taking also need to be considered.
On the infant
Some antibiotics can reach the baby during labour or through breastfeeding, and these may upset the pattern of friendly bacterial flora being established in the baby's gut as part of the baby's immune system (Bedford Russell 2006; Penders 2006). There is evidence that this impact can continue for up to six months after birth and the consequences of this may occasionally be late‐onset serious bacterial infections (Glasgow 2005). It has been proposed that perinatal exposure to certain agents can cause irreversible changes to health conditions in adulthood through impact on hormonal imprinting (Csaba 2007; Korpela 2018; Mueller 2015). It is also possible that babies born prematurely, with less mature immune systems, may be affected more (Madhok 2015). Tetracyclines are usually not recommended during pregnancy or childbirth (BNF 2020). The current evidence favours the administration of antibiotics 15 to 60 minutes before incision, which was recognised to be better for preventing maternal infections and with no proven harm on the baby when short‐term outcomes were assessed (Mackeen 2014). However, the possibility that antibiotic exposure may adversely effect the newborn's developing immune system and microbiome needs to be assessed by collection of longer‐term data.
Drug‐resistant strains of bacteria
Resistance of bacteria to antibiotics is spreading, and develops when a strain of bacteria evolves ways to escape the effects of the antibiotics. The antibiotic kills the non‐resistant bacteria allowing the resistant ones to colonise and spread or pressures them into evolving resistance mechanisms. Widespread use of antibiotics can contribute to the development of drug‐resistant strains of bacteria, which means that these antibiotics become ineffective because of bacterial resistance (Dancer 2004). At a population level this is a critical problem which may cause an increase in serious morbidity from hospital‐acquired drug‐resistant infections (Dancer 2004). This drug resistance is unlikely to be detected in randomised controlled trials and other types of research are needed to assess the potential problem of drug‐resistant strains (e.g. MRSA (Methicillin‐resistant Staphylococcus aureus), C difficile) in hospitals. The dose and number of antibiotic administrations given are a major consideration in relation to antibiotic resistance. These issues will be addressed in the other research ‐ seeDifferences between protocol and review for details.
Why it is important to do this review
Since there are an overwhelming number of effective antibiotics available, attempts to define an antibiotic regimen of choice have been problematic. Ideally, such a drug regimen should be: (1) proven to be effective in well‐designed prospective, randomised, double‐blind clinical trials, (2) active against the majority of pathogens likely to be involved, (3) able to attain adequate serum and tissue levels throughout the procedure, (4) not associated with the development of antimicrobial resistance, (5) inexpensive, and (6) well‐tolerated. In many respects penicillins and cephalosporins meet these criteria. Many investigators have used these drugs and have recommended that drugs from these classes represent the antibiotics of choice for caesarean section prophylaxis (Lamont 2011; RCOG 2011; Skeith 2017). However, current knowledge of bacterial resistance may challenge these recommendations.
The past several decades have seen an increase in the incidence of caesarean section, associated with an increase in maternal postoperative infection. Studies indicate that wound infection can be as high as 30% and endometritis as high as 60% where prophylactic antibiotics have not been utilised (Hofmeyr 2010). Therefore, infectious complications that occur following caesarean section are an important contributor to maternal morbidity and mortality (Martin 2014; Pierson 2018). Such complications are also an important source of increased hospital stay and consumption of financial resources. Prophylactic antibiotics for caesarean section can be expected to result in a major reduction in postoperative infectious morbidity. The question that remains, therefore, is which regimen to use? This review is an update of the review last published in 2014 (Gyte 2014).
Other Cochrane Reviews have addressed: effectiveness against placebo (Smaill 2014), different routes of administration (Nabhan 2016) and various timings of administration (Mackeen 2014). In addition, two other reviews are proposed on dosage by the various sub‐types of cephalosporins and pencillins (still to be undertaken).
Objectives
To determine, from the best available evidence, the balance of benefits and harms between different classes of antibiotic given prophylactically to women undergoing caesarean section, considering their effectiveness in reducing infectious complications for women and adverse effects on both mother and infant.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) where the intention was to allocate participants randomly to one of at least two alternative classes of regimens of antibiotic prophylaxis for caesarean section. We excluded quasi‐RCTs. Cluster‐RCTs were eligible for inclusion but none were identified. Cross‐over trials were not eligible for inclusion.
Types of participants
Women undergoing caesarean section, both elective and non‐elective.
Types of interventions
Prophylactic antibiotic regimens comparing different classes of antibiotics. We included studies where there was a comparison between two or more antibiotics from the different classes. We looked at antibiotics administered singly or in combination with antibiotics of other classes or in combination with other drugs. The different classes of antibiotics are described and categorised, and also given a shorthand code for ease of reference (e.g. C1 for first‐generation cephalosporins) in Table 2. Where we identified different drugs in the same class of antibiotics being studied, we pooled these data, with some exceptions described below.
The main causative agents of caesarean section infection are skin colonizers, primarily gram‐positive cocci (particularly including Staphylococcus aureus (S. aureus) and Streptococci); and vaginal colonizers, including anaerobes and, to a lesser extent, gram‐negative bacilli.
In the previous update of this review, the main comparison was between cephalosporins and penicillins. We have revised our main comparisons to reflect trends in global practice to include the following.
I. Antistaphylococcal (i.e. potentially active against S.aureus) cephalosporins C1 and C2 (1st and 2nd generation) versus lincosamides (especially clindamycin)
II. Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) versus lincosamides (especially clindamycin) plus aminoglycosides (especially gentamicin)
III. Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) versus penicillins P2+ (broad spectrum penicillins plus betalactamase inhibitors)
As these comparisons indicate, for this update we have not pooled the data for all penicillins or for all cephalosporins, because of important variations in spectra of action between different sub‐classes (including different generations, sub‐types and co‐formulations) of both of these classes of drugs. Where sub‐classes of these drugs are known to differ in their potential to act against agents that are the principle causes of infection at caesarean section, we have meta‐analysed the results of trials of different sub‐classes separately. Where sub‐classes of drugs are known to have similar potential action against these agents, we have pooled the results. In the case of penicillins, both natural penicillins (P1, also referred to as first‐generation penicillins) and broad spectrum penicillins (P2; encompassing second‐, third‐ and fourth‐generation penicillins), are not active against S. aureus. By contrast, broad spectrum penicillins plus betalactamase inhibitors (P2+; available as co‐formulations, or administered together), and antistaphylococcal penicillins (P3), and are potentially effective against S. aureus. The natural penicillins and broad spectrum penicillins do not differ substantially in their potential action against other relevant agents, therefore, we have pooled results for these drugs (P1 and P2). Although both are potentially active against S. aureus, we analysed each of broad spectrum penicillins plus betalactamase inhibitors (P2+) and antistaphylococcal penicillins (P3) separately, because the former have a much broader spectrum of activity than antistaphylococcal penicillins (including action against gram‐negative bacilli and anaerobes including Bacteroides fragilis). Interventions combining penicillins with other classes of antibiotics have not been pooled with findings for penicillins either alone or in combination with betalactamase inhibitors. For comparisons including penicillins, penicillins are analysed as the control drug. In terms of cephalosporins, we have pooled the findings on first‐ and second‐generation drugs (C1 and C2) because both of these subclasses are potentially active against gram‐positive cocci. However, third‐generation cephalosporins (C3) have only minimal action against S. aureus, so results from trials where women were given any third‐generation drug have been analysed separately. We have analysed fourth‐generation cephalosporins (C4) separately, because they have a much broader spectrum of activity than the other three generations (including action against S. aureus, some gram‐negative bacilli, and potential action against Pseudomonas aeruginosa (P. aeruginosa) and Acinetobacter baumannii (A. baumannii)). Cephalosporins co‐formulated with betalactamase inhibitors result in a broader spectrum especially regarding gram‐negative and anaerobes, and, therefore we have also analysed them separately. Interventions combining cephalosporins with other classes of antibiotics have not been pooled with findings for cephalosporins either alone or in combination with betalactamase inhibitors. For both penicillins and cephalosporins, while we have pooled different subclasses of drugs due to similarities in potential action against agents that cause infection at caesarean section, we acknowledge that there are nevertheless other differences in the spectra of action between the different subclasses of drugs. So, whilst we have structured the meta‐analysis based on the hypothesis that these differences will have little clinical impact when used for prophylaxis at caesarean section, where sufficient data were available, we have analysed the pooled findings for subgroup differences in order to assess whether there were, in fact, differences between natural penicillins and broad spectrum penicillins, and between first‐ and second‐generation cephalosporins, for this problem.
We excluded comparisons of different drugs within the same class of antibiotics, because it is anticipated that these will be assessed in four other Cochrane Reviews. Two of these reviews are as yet unpublished.
Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section
Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section
Two published reviews assess the appropriate timing and route of administration of prophylactic antibiotics at caesarean section.
Timing of prophylactic antibiotics for preventing infectious morbidity in women undergoing caesarean section (Mackeen 2014)
Routes of administration for antibiotic given to women routinely for preventing infection after caesarean section (Nabhan 2016)
Comparisons included
For classification of antibiotics and a key to the letter codes used throughout this review see Table 2.
Main comparisons
I. Antistaphylococcal (i.e. potentially active against S.aureus) cephalosporins C1 and C2 (1st and 2nd generation) versus lincosamides (especially clindamycin)
II. Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) versus lincosamides (especially clindamycin) plus aminoglycosides (especially gentamicin)
III. Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+
Additional comparisons
IV. Cephalosporins versus penicillins (all remaining comparisons within these classes), including comparisons of different types of cephalosporin versus different types of penicillin as described below.
Antistaphylococcal cephalosporins C1 and C2 (1st and/or 2nd generation cephalosporins); or
Minimally antistaphylococcal (i.e. minimally active against S.aureus) cephalosporins C3 (3rd generation cephalosporins); or
Cephalosporins potentially active against S. aureus, P. aeruginosa and A. baumannii C4 (4th generation cephalosporins); or
Cephalospoprins plus betalactamase inhibitors C+
versus
Non‐antistaphylococcal (i.e. inactive against S. aureus) penicillins P1 and P2 (natural and broad spectrum penicillins); or
Penicillins plus betalactamase inhibitors P2+; or
Antistaphylococcal penicillins P3
V. All other comparisons of a single class versus a single class of antibiotic
VI. Comparisons including regimens of mixed classes in one or both groups
Types of outcome measures
Primary outcomes
Maternal
Maternal sepsis (suspected or proven)
Maternal endometritis
Infant
Infant sepsis (suspected or proven)
Infant oral thrush
Secondary outcomes
Maternal
Maternal fever (febrile morbidity)
Maternal wound infection
Maternal urinary tract infection
Maternal thrush
Maternal serious infectious complication (such as bacteraemia, septic shock, septic thrombophlebitis, necrotising fasciitis, or death attributed to infection)
Maternal adverse effects (e.g. allergic reactions, nausea, vomiting, diarrhoea, skin rashes)
Maternal length of hospital stay
Maternal infections ‐ post‐hospital discharge to 30 days postoperatively (not pre‐specified in the protocol)
Maternal readmissions (not pre‐specified in the protocol)
Infant
Immediate adverse effects of antibiotics on the infant (unsettled, diarrhoea, rashes)
Infant length of hospital stay
Infant long‐term adverse effects (e.g. general health, frequency of visits to hospital)
Infant's immune system development (using a validated scoring assessment)
Additional outcomes
Costs
Search methods for identification of studies
The following search methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (2 December 2019).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (2 December 2019) using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists at the end of papers for further studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeGyte 2014.
For this update, the following methods were used for assessing the 17 reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received (> 20% attrition) from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
Had we identified any cluster‐RCTs we would have included them in the analyses along with individually‐randomised trials, following the methods described in Higgins 2011 and the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. In future updates, if we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity subgroup analysis to investigate the effects of the randomisation unit.
Other unit of analysis issues
No special methods were used for trials with more than one treatment group.
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if the Tau² was greater than zero or the I² was greater than 30% and there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Where we identified substantial heterogeneity (above 30%), we explored it by pre‐specified subgroup analysis.
Assessment of reporting biases
Had we found 10 or more studies in the meta‐analysis, we would have investigated reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we explored possible reasons for this.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
For the 2020 update, we planned to undertake subgroup analyses.
By type of caesarean section. In the previous version of this review, type of surgery was differentiated by elective caesarean section versus non‐elective caesarean section versus mixed or not defined (rupture of membranes for more than six hours or the presence of labour was used to differentiate a non‐elective caesarean section from an elective procedure). For this update, we intended to revise these subgroup distinctions, and differentiate surgery by urgency according the Royal College of Obstetrics and Gynaecology definitions, category 1 versus category 2 and 3 versus category 4 versus mixed or not defined (RCOG 2011), due to the fact that other infection control measures are especially compromised in the most urgent situations. However, the information reported in the available trials was not specific enough to support investigation by urgency in line with these definitions, therefore we retained the previous categorisation as described. Although we have presented results from these subgroup analyses for ease of reference, for most comparisons, there were too few trials to make the results of subgroup analyses meaningful.
By generation of cephalosporin. Where we combined results for 1st and 2nd generation cephalosporins, we included an exploratory subgroup analysis by generation of cephalosporin. While we have presented these subgroup analyses in order to enable readers to easily see the distribution of different generations of cephalosporins in the included trials, there were too few trials and subgroups were too imbalanced in size for findings from these analyses to either support or bring in to question our hypothesis that data from these trials should be pooled.
By type of penicillin. Where we combined results for natural and broad spectrum penicillins, we included an exploratory subgroup analysis by generation of penicillins. As for cephalosporins, while we have presented these subgroup analyses in order to enable readers to easily see the distribution of different generations of penicillins in the included trials, there were too few trials and subgroups were too imbalanced in size for findings from these analyses to either support or bring in to question our hypothesis that data from these trials should be pooled.
Although the regimen of antibiotics varied between trials, we did not plan to undertake subgroup analysis by the number of doses given because this is better assessed in other reviews (Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section and Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section). Other reviews cover timing and routes of administration (Timing of prophylactic antibiotics for preventing infectious morbidity in women undergoing caesarean sectionMackeen 2014 and Routes of administration for antibiotic given to women routinely for preventing infection after caesarean sectionNabhan 2016).
We planned to assess subgroup differences by interaction tests (Deeks 2001) available within RevMan (RevMan 2014) and to report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analysis to explore the effect of risk of bias for important outcomes in the review. Where there was a high risk of bias associated with a particular aspect of a study, for example, inadequate sequence generation and allocation concealment (Schultz 1995), we planned to explore this by sensitivity analysis (Higgins 2011). However, there were too few studies included in any analysis assessed as being at low risk of bias for any meaningful sensitivity analysis in this update.
Summary of findings and assessment of the certainty of the evidence
For this update the certainty of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook. We planned to assess the certainty of the body of evidence relating to the following outcomes for the main comparisons of: 1st and 2nd generation cephalosporins versus lincosamides; 1st and 2nd generation cephalosporins versus lincosamides plus gentamycin; 1st and 2nd generation cephalosporins versus penicillins plus betalactamase inhibitors. However, no trials reported on the first two comparisons, therefore we assessed the certainty of the evidence relating to 1st and 2nd generation cephalosporins versus penicillins plus betalactamase inhibitors; these assessments are reported in Table 1.
Maternal sepsis
Maternal endometritis
Infant sepsis
Infant oral thrush
Maternal wound infection
Maternal urinary tract infection
Maternal composite adverse effects (e.g. allergic reactions; nausea, vomiting, diarrhoea, skin rashes)
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. We used the GRADE approach to provide a summary of the intervention effect alongside an assessment of our confidence in the effect estimate for each of the above outcomes. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence was downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations relating to each of these five considerations.
Results
Description of studies
Results of the search
See Figure 1
1.
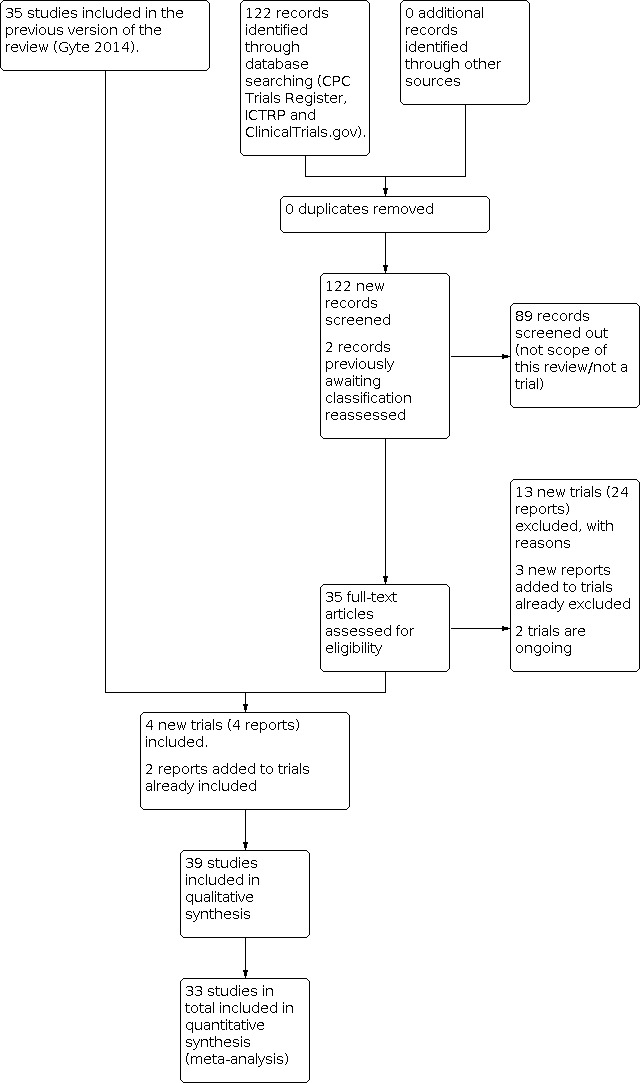
Study flow diagram.
The classification of antibiotics is set out in Table 2.
We assessed 33 new trial reports and we also revisited the two trial reports that were awaiting classification in the previous version of the review. We included four new trials (four reports) (Alekwe 2008; Deng 2007; Rohan 2014; Rudge 2006) and excluded 13 new trials (24 reports) (Azizi 2014; El Aish 2018; Gideon 2016; Jalai 2019; Jayawardena 2019; Mihailovic 1989; Mokhtar 2019; Opoku 2007; Sivasankari 2015; Tita 2016; Vathana 2018; Wajsfeld 2019; Westen 2015). We also added two new reports to trials already included (Mivumbi 2014; Ziogos 2010), and added three new reports to two previously excluded studies (Ijarotimi 2013; Lyimo 2013). We have no studies awaiting further classification, and have two ongoing studies (Abdalmageed 2019; Karamali 2013).
In all, we have identified 170 reports for 150 studies. For a detailed description of studies seeCharacteristics of included studies, Characteristics of excluded studies; Characteristics of studies awaiting classification and Characteristics of ongoing studies.
Included studies
Overall, our searches identified 39 included studies of which 33 provided data in a format that could be included in this review (Ahmed 2004; Alekwe 2008; Benigno 1986; Bracero 1997; Busowski 2000; Chantharojwong 1993; Deng 2007; Faro 1990; Ford 1986; Gidiri 2014; Jyothi 2010; Kamilya 2012; Kayihura 2003; Koppel 1992; Lehapa 1999; Lewis 1990; Louie 1982; Lumbiganon 1994; Mansueto 1989; Mivumbi 2014; Mothilal 2013; Noyes 1998; Parulekar 2001; Rehu 1980; Rohan 2014; Rosaschino 1988; Rudge 2006; Saltzman 1985; Saltzman 1986; Shah 1998; Spinnato 2000; van der Linden 1993; Ziogos 2010). These studies included data on 8073 women. The six studies which did not provide data for the analyses were: four full‐text papers (Dashow 1986; Graham 1993; Ng 1992; Voto 1986); and two of the conference abstracts (De‐Lalla 1988; Wells 1994).
The studies were published between 1980 and 2014. Five studies were reported as conference abstracts only (De‐Lalla 1988; Lehapa 1999; Lumbiganon 1994; Rohan 2014; Wells 1994). In this update, we have not included the data from two trials from which data were previously included: because there was inconsistency in the denominators between tables in the paper for one report (Dashow 1986); and because there was inconsistency between the tables and the main text in the other (Ng 1992). In both cases, the trials took place too long ago to obtain clarification from the authors.
Two studies reported sponsorship by a drug company (Bracero 1997; van der Linden 1993). One study reported that the drugs were donated by a drug company (Ahmed 2004), and one study reported that the hospital sponsored the research (Ziogos 2010). None of the other studies reported on their source of funding.
Four studies reported they had no conflicts of interest relating to their study (Alekwe 2008; Gidiri 2014; Mivumbi 2014; Ziogos 2010). One study was unclear as the original paper was not written in English and we need to seek help to ascertain this information (Mansueto 1989). The rest of the studies did not report if there was any conflict of interest or not.
Of the 39 studies included in the review, 22 were conducted in high‐income countries, eight in upper‐middle income, seven in lower‐middle income and two in low‐income countries (see Characteristics of included studies).
Participants
Of the trials contributing data to our analyses, six trials included only women who were having elective caesarean sections (Ahmed 2004; Alekwe 2008; Deng 2007; Jyothi 2010; Rohan 2014; Shah 1998); 11 trials included only women having non‐elective caesarean sections (Chantharojwong 1993; Faro 1990; Kayihura 2003; Lehapa 1999; Louie 1982; Lumbiganon 1994; Mansueto 1989; Noyes 1998; Rehu 1980; Saltzman 1986; van der Linden 1993); eight trials included a mixture of elective and non‐elective (Benigno 1986; Gidiri 2014; Kamilya 2012; Lewis 1990; Mivumbi 2014; Mothilal 2013; Spinnato 2000; Ziogos 2010); and in the remaining eight trials the type of caesarean section was not clearly described (Bracero 1997; Busowski 2000; Ford 1986; Koppel 1992; Parulekar 2001; Rosaschino 1988; Rudge 2006; Saltzman 1985).
Interventions and comparators
The studies contributing data to our analyses included women who received the following specific drugs within each class, either singly or in combination with other classes (see Table 2 for more information on each category of drug):
Cephalosporins (C):
1st generation cephalosporins (C1): cefalothin; cefazolin; cephradine
2nd generation cephalosporins (C2): cefotetan; cefoxitin; cefuroxime
3rd generation cephalosporins (C3): cefotaxime; cefotoxime; ceftriaxone; ceftizoxime
Penicillins (P):
Natural penicillins (P1): benzathine penicillin; benzyl penicillin; crystalline penicillin; procaine penicillin
Broad spectrum penicillins (P2): ampicillin; mezlocillin; piperacillin; ticarcillin
Penicillins plus betalactamase inhibitors (P2+): ampicillin plus sulbactam; co‐amoxyclav = amoxicillin plus clavulanic acid; ticarcillin plus clavulanic acid
Antistaphylococcal penicillins (P3): cloxacillin
Other beta‐lactams, carbapenems (Ca): imipenem
Aminoglycasides (A): gentamicin
Amphenicols (Am): chloramphenicol
Fluoroquinolones: ciproflaxin
Lincosamides (L): clindamycin
Macrolides (M): azithromycin; erythromycin
Nitroimadazoles (N): metronidazole
The specific drugs that women received are described alongside the results for each comparison in Effects of interventions.
Most trials administered prophylactic antibiotics after skin incision. Of the trials contributing data to the analyses, only eight trials gave all women in both groups antibiotics prior to skin incision (administration continued postoperatively for groups given multiple doses), with timing described as: preoperative (Gidiri 2014; Rosaschino 1988); at induction of anaesthesia (Ahmed 2004; Rohan 2014; van der Linden 1993); 30 minutes prior to surgery (Mothilal 2013; Rehu 1980); and < 60 minutes before incision (Mivumbi 2014). One further trial (Kayihura 2003), gave women in the intervention group antibiotics pre‐operatively, with all women in the control group receiving them postoperatively. Nineteen trials gave all women in both groups antibiotics at or just after cord clamping (Benigno 1986; Bracero 1997; Busowski 2000; Chantharojwong 1993; Deng 2007; Faro 1990; Ford 1986; Jyothi 2010; Kamilya 2012; Koppel 1992; Louie 1982; Lumbiganon 1994; Mansueto 1989; Noyes 1998; Saltzman 1985; Saltzman 1986; Shah 1998; Spinnato 2000; Ziogos 2010); three trials gave antibiotics just after cord clamping in the intervention group and postoperatively in the control (Alekwe 2008; Parulekar 2001; Rudge 2006). In one trial, timing of administration was not reported (Lehapa 1999). One further trial used intraoperative irrigation (Lewis 1990).
Fifteen studies administered a single dose of antibiotic systemically in all groups, and in most trials administration was specified as intravenous (Bracero 1997; Busowski 2000; Faro 1990; Jyothi 2010; Kamilya 2012; Koppel 1992; Mivumbi 2014; Noyes 1998; Rehu 1980; Rosaschino 1988; Spinnato 2000; Ziogos 2010), although in others this was not explicitly described (Lumbiganon 1994; Mothilal 2013; Rohan 2014). Seven studies administered multiple doses systemically in all groups, intravenously (Benigno 1986; Chantharojwong 1993; Deng 2007; Lehapa 1999; Louie 1982), or route not explicitly described Ford 1986; Saltzman 1985). Ten two‐arm studies administered a single dose to one group and multiple doses to the other, all systemically but with the route of administration varying somewhat between trials. In four of these studies, all women received all antibiotics intravenously (Ahmed 2004; Mansueto 1989; Shah 1998; van der Linden 1993); Rudge 2006 administered a single dose of antibiotics intravenously (C1) versus multiple IM (P1); Alekwe 2008; Gidiri 2014; Kayihura 2003; Parulekar 2001 compared a single dose intravenous versus initial intravenous and then oral and Iintramuscular for remains of the course. In the remaining study, route was not explicitly described (Saltzman 1986). The final trial contributing data administered antibiotics via intraoperative irrigation in both groups rather than systemically (Lewis 1990). See Characteristics of included studies for detailed information on dose and regimen for each study.
Main comparisons (systemic administration)
See Table 3 for an overview of all the comparisons reported in the included studies.
2. Comparison matrix.
| Intervention/comparison class or sub‐class of antibiotic | Single class administered | Multiple classes administered | ||||||
| Antistaphylococcal cephalosporins (C1 and C2; 1st and 2nd generation) | Minimally antistaphylococcal cephalosporins (C3; 3rd generation) | Broad spectrum penicillins plus betalactamase inhibitors (P2+) | Lincosamide (L) plus aminoglycoside (A) | Antistaphylococcal cephalosporins (C1 and C2; 1st and 2nd generation) plus nitroimadazole (N) | Aminoglycaside (A) plus nitroimidazole (N) | Minimally antistaphylococcal cephalosporins (C3; 3rd generation) plus nitroimidazole (N) | ||
| Single class administered | Broad spectrum penicillins plus betalactamase inhibitors (P2+) | 8 trials (1540 women) | 2 trials (865 women) | Comparison not within scope of review | No trials | 1 trial (83 women) |
No trials | No trials |
| Non‐antistaphylococcal penicillins (P1 and P2; natural and broad spectrum) |
Systemic administration:
9 trials
(3093 women) Lavage: 1 trial (383 women) |
4 trials (854 women) | Comparison not within scope of review | 1 trial (88 women) |
1 trial (139 women) |
No trials | No trials | |
| Broad spectrum penicillins (P2) and antistaphylococcal penicillins (P3) | No trials | 1 trial (200 women) | Comparison not within scope of review | No trials | No trials | No trials | No trials | |
| Fluoroquinolones (F) | 1 trial (81 women) |
No trials | 1 trial (72 women) |
No trials | No trials | No trials | No trials | |
| Carbapenems (Ca) | No trials | 1 trial (48 women) |
No trials | No trials | No trials | No trials | No trials | |
| Macrolides (M) | 1 trial (70 women) |
No trials | No trials | No trials | No trials | No trials | No trials | |
| Multiple classes administered | Broad spectrum penicillin (P2) plus antistaphylococcal penicillin (P3) plus aminoglycoside (A) plus nitroimadazole (N) | No trials | 1 trial (200 women) |
Comparison not within scope of review | No trials | No trials | No trials | No trials |
| Antistaphylococcal penicillin (P3) plus aminoglycoside (A) | No trials | 1 trial (200 women) |
Comparison not within scope of review | No trials | No trials | No trials | No trials | |
| Natural penicillin (P1) plus nitroimidazole (N) plus macrolide (M) | No trials | No trials | Comparison not within scope of review | No trials | No trials | 1 trial (241 women) | No trials | |
| Non‐antistaphylococcal penicillins (P1 and P2; natural and broad spectrum) plus nitroimadazole (N) | No trials | No trials | Comparison not within scope of review | No trials | 2 trials (256 women) | No trials | No trials | |
| Non‐antistaphylococcal penicillins (P1 and P2; natural and broad spectrum) plus nitroimadazole (N) plus amphenicol (Am) | No trials | No trials | Comparison not within scope of review | No trials | No trials | No trials | 1 trial (232 women) |
|
We included three main comparisons.
Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) versus lincosamides
No included studies reported.
Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) versus lincosamides plus aminoglycosides
No included studies reported.
Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) versus broad spectrum penicillins plus betalactamase inhibitors P2+
Eight trials, which provided data on 1540 women, reported on our third main comparison (Bracero 1997; Busowski 2000; Jyothi 2010; Lumbiganon 1994; Noyes 1998; Saltzman 1985; Spinnato 2000; Ziogos 2010).
Additional comparisons (systemic administration)
Cephalosporins versus penicillins (pre‐specified comparisons)
For this update, we did not pool data on all generations of cephalosporins, or all subtypes of penicillins. We combined data on cephalosporins potentially active against staphylococcus aureus (1st and 2nd generation cephalosporins, C1 and C2), and we also combined data on non‐antistaphylocccal penicillins (natural and broad spectrum penicillins) (further details of drugs, their spectra of action, and key to abbreviations (C1, P1 etc) are described in Table 2). We did not pool data on other subtypes of these two classes of drug. According to this pre‐specified comparison structure, which is described in our Methods, the included studies reported on the following comparisons.
Cephalosporins potentially active against S. aureus C1 and C2 (1st and 2nd generation) versus non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum)
Twelve trials included this comparison for systemic administration (Benigno 1986; Chantharojwong 1993; De‐Lalla 1988; Faro 1990; Ford 1986; Graham 1993; Louie 1982; Mivumbi 2014; Rudge 2006; Saltzman 1986; Spinnato 2000; Voto 1986).
Three out of the 12 trials (De‐Lalla 1988; Graham 1993; Voto 1986) did not contribute data to the analyses.
Cephalosporins with minimal action against S. aureus C3 (3rd generation) versus non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum)
Five trials included this comparison (Faro 1990; Lehapa 1999; Louie 1982; Ng 1992; Rosaschino 1988), however Ng 1992 did not contribute data to the analyses.
Cephalosporins with minimal action against S. aureus C3 (3rd generation) versus broad spectrum penicillins plus betalactamase inhibitors P2+
Two trials included this comparison (Kamilya 2012; Koppel 1992).
No included studies gave women either cephalosporins potentially active against S. aureus, P. aeruginosa and A. baumannii C4 (4th generation), or cephalosporins plus betalactamase inhibitors C+.
Other cephalosporin (only) regimens versus other penicillin (only) regimens
Cephalosporins C3 (3rd generation) versus penicillins P2 and P3 (broad spectrum and antistaphylococcal)
One study (Ahmed 2004) included this comparison.
All other comparisons of a single class versus a single class of antibiotic
Fluoroquinolones F versus broad spectrum penicillins plus betalactamase inhibitors P2+
One study (Busowski 2000) included this comparison.
Fluoroquinolones F versus cephalosporins C2 (2nd generation)
One study (Busowski 2000) included this comparison.
Carbapenems Ca versus cephalosporins C2 (2nd generation)
One study (Mansueto 1989) included this comparison.
Macrolides M versus cephalosporins C1 (1st generation)
One study (Mothilal 2013) included this comparison.
Other antibiotic regimens (multiple classes) versus cephalosporin (only) regimens
Two studies included comparisons of other antibiotic regimens (multiple classes) versus cephalosporin (only) regimens. The findings were not pooled because the regimens differed substantially between trials.
Broad spectrum penicillin P2 plus antistaphylococcal penicillin P3 plus aminoglycoside A plus nitroimidazole N versus cephalosporin C3 (3rd generation)
One study (Alekwe 2008) included this comparison.
Antistaphylococcal penicillin P3 plus aminoglycoside A versus cephalosporin C3 (3rd generation)
One study (Parulekar 2001) included this comparison.
Other antibiotic regimens (multiple classes) versus penicillin (only) regimens
Three studies included comparisons of other antibiotic regimens (multiple classes) versus penicillin (only) regimens. The findings were not pooled because the regimens differed substantially between trials.
Lincosamide L plus aminoglycoside A versus natural penicillin P1
One study (Rehu 1980) included this comparison.
Cephalosporin C1 (1st generation) plus nitroimadazole versus broad spectrum penicillin P2
One study (Shah 1998) included this comparison.
Cephalosporin C2 (2nd generation) plus nitroimidazole N versus broad spectrum penicillin plus betalactamase inhibitors P2+
One study (van der Linden 1993) included this comparison.
Other antibiotic regimens (multiple classes) versus different antibiotic regimens (multiple classes)
Four studies included comparisons of other antibiotic regimens (multiple classes) versus different antibiotic regimens (multiple classes). The findings were not pooled because the regimens differed substantially between trials.
Aminoglycoside A plus nitroimidazole N versus natural penicillin P1 plus nitroimidazole N plus macrolide M
One study (Kayihura 2003) included this comparison.
Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) plus nitroimadazole N versus non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) nitroimadazole N
Two studies (Deng 2007; Rohan 2014) included this comparison.
Cephalosporin C3 (3rd generation) plus nitroimidazole N versus natural penicillin P1 plus broad spectrum penicillin P2 plus nitroimidazole N plus amphenicol Am
One study (Gidiri 2014) included this comparison.
Additional comparisons (irrigation/lavage administration)
Two trials (Dashow 1986; Lewis 1990) administered antibiotics via lavage, rather than systemically, and for this update they were considered separately from the trials using systemic administration.
Cephalosporins potentially active against S. aureus C1 and C2 (1st and 2nd generation) versus non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum)
Both trials (Dashow 1986; Lewis 1990) reported on this comparison, however Dashow 1986 did not contribute data to the analyses.
Excluded studies
We excluded 109 studies for the following reasons.
10 studies were quasi‐RCTs or not properly randomised (Beksac 1989; Bilgin 1998; De Palma 1982; Digumarthi 2008; Grujic 2009; Itskovitz 1979; Leveno 1984; Ovalle 1996; Prasuna 2011; Puri 1991)
26 studies compared different cephalosporins (Andrews 2003; Bernstein 1994; Carlson 1990; Crombleholme 1989; Ding 2000; Duff 1987; Fejgin 1993; Fugere 1983; Galask 1988; Galask 1989; Gonik 1994; Gordon 1982; Hager 1991; Hartert 1987; Kreutner 1979; Levin 1983; Major 1999; McGregor 1986; McGregor 1988; Meyer 2003; Parsons 1985; Periti 1988; Rayburn 1985; Stiver 1984; von Mandach 1993; Wagner 2006).
Three studies compared different penicillins (Chamberlain 1993; Chittacharoen 1998; O'Leary 1986)
10 studies compared different routes of administration (Berkeley 1990; Boothby 1984; Conover 1984; Donnenfeld 1986; Elliot 1986; Flaherty 1983; Gonen 1986; Lavery 1986; Mathelier 1992; Saravolatz 1985).
11 studies compared different doses (Crombleholme 1987; Elliot 1982; Gall 1987; Leonetti 1989; Luttkus 1997; Lyimo 2013; Neuman 1990; Patacchiola 2000; Stiver 1983; Teansutikul 1993; Zutshi 2008).
14 studies compared single versus multiple doses (Baheraie 1997; Gideon 2016; Gonik 1985; Hawrylyshyn 1983; Jakobi 1988; Masse 1988; Roex 1987; Tassi 1987; van Beekhuizen 2008; van Velzen 2009; Varner 1986; Vathana 2018Westen 2015; Wu 1991)
12 studies compared different timings of administration (Cunningham 1983; Gul 1999; Ijarotimi 2013; Macones 2008; Pevzner 2009; Rodriguez 1990; Seton 1996; Sullivan 2006; Sullivan 2007; Thigpen 2005; Wax 1997; Yildirim 2009).
Three studies compared single versus combinations of drugs (Meyer 2000; Rijhsinghani 1995; Xu 1997)
Four studies included different populations of women and not those having a caesarean (Mansani 1984; Mihailovic 1989; Roy 2003; Watts 1991).
Seven studies compared a cephalosporin versus the same cephalosporin plus another antibiotic (Azizi 2014; El Aish 2018; Jalai 2019; Jayawardena 2019; Mokhtar 2019; Sivasankari 2015; Wajsfeld 2019).
Nine studies were excluded for other reasons (D'Angelo 1980; De Palma 1980; Maggioni 1998; Opoku 2007; Peterson 1990; Scarpignato 1982; Shakya 2010; Tita 2016; Warnecke 1982).
Risk of bias in included studies
See Figure 2 for a summary of 'Risk of bias' assessments. We followed the methods described in the Cochrane Handbook (Higgins 2011) when assessing included studies for risk of bias.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
We considered six studies to have adequate sequence generation and allocation concealment (Alekwe 2008; Benigno 1986; Bracero 1997; Dashow 1986; Mivumbi 2014; Ziogos 2010). We assessed five further studies as low risk of bias for sequence generation but for allocation concealment they were unclear (Faro 1990; Graham 1993; Kamilya 2012; Rudge 2006) or high risk (Deng 2007). The reports for the remaining 27 studies were unclear about how adequately investigators had addressed these aspects to minimise bias with one study being unclear on sequence generation and high risk of bias for allocation concealment (Gidiri 2014).
Blinding
We assessed 13 studies as low risk for performance bias (Benigno 1986; Bracero 1997; Busowski 2000; Dashow 1986; Kamilya 2012; Koppel 1992; Lehapa 1999; Lewis 1990; Louie 1982; Rehu 1980; Saltzman 1985; Saltzman 1986; Spinnato 2000), 11 as high risk (Ahmed 2004; Alekwe 2008; Deng 2007; Faro 1990; Gidiri 2014; Graham 1993; Kayihura 2003; Mivumbi 2014; Rudge 2006; Voto 1986; Ziogos 2010), and we found the remaining 15 to be at unclear risk of performance bias.
For detection bias, we assessed eight studies as low risk (Benigno 1986; Busowski 2000; Dashow 1986; Kamilya 2012; Koppel 1992; Louie 1982; Rehu 1980; Rudge 2006), two as high risk (Kayihura 2003; Ziogos 2010) and the remaining 29 studies as unclear risk.
Incomplete outcome data
We found 29 studies to be at low risk of attrition bias (Ahmed 2004; Alekwe 2008; Bracero 1997; Busowski 2000; Chantharojwong 1993; Deng 2007; Ford 1986; Gidiri 2014; Graham 1993; Jyothi 2010; Kamilya 2012; Kayihura 2003; Koppel 1992; Louie 1982; Lumbiganon 1994; Mansueto 1989; Mivumbi 2014; Mothilal 2013; Ng 1992; Noyes 1998; Parulekar 2001; Rehu 1980; Rohan 2014; Rosaschino 1988; Rudge 2006; Saltzman 1986; Spinnato 2000; van der Linden 1993; Ziogos 2010). We found two studies to be at high risk for attrition bias where there was greater than 20% attrition post‐randomisation and attrition was imbalanced between groups (Benigno 1986; Voto 1986); for Voto 1986 attrition was so imbalanced that we considered the groups to be improperly randomised and the data from this study was not included in the meta‐analysis. For eight studies we found the risk of attrition bias to be unclear (Dashow 1986; De‐Lalla 1988; Faro 1990; Lehapa 1999; Lewis 1990; Saltzman 1985; Shah 1998; Wells 1994).
Selective reporting
We assessed that none of the included studies were low risk for selective reporting bias but we found seven studies to be high risk (Ahmed 2004; De‐Lalla 1988; Deng 2007; Lewis 1990; Noyes 1998; Saltzman 1985; Wells 1994), with the remaining 32 studies unclear.
Other potential sources of bias
All the included studies were at unclear risk of other sources of bias; many of the studies were quite old and it was difficult to assess if there were other possible biases.
Effects of interventions
See: Table 1
Main comparisons (systemic administration)
Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) versus lincosamides
No included trials reported this comparison.
Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) versus lincosamides plus aminoglycosides
No included trials reported this comparison.
Comparisons 1 to 3: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) versus broad spectrum penicillins plus betalactamase inhibitors P2+
Eight trials, which provided data on 1540 women reported on our third main comparison, 1st and 2nd generation cephalosporins C1 and C2 versus broad spectrum penicillins plus betalactamase inhibitors P2+ (Bracero 1997; Busowski 2000; Jyothi 2010; Lumbiganon 1994; Noyes 1998; Saltzman 1985; Spinnato 2000; Ziogos 2010).
The subtype of drugs that women received varied between trials (for ease of reference, see Table 4 for a detailed summary of drugs, doses and single/multiple dosing used in comparisons including more than two trials).
3. Interventions: drugs and doses.
|
Antistaphylococcal cephalosporins (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors (8 trials, 1 540 women) | ||||||
| Antistaphylococcal cephalosporins (1st and 2nd generation) | vs | Broad spectrum penicillins plus betalactamase inhibitors | ||||
| Drug | Dose | Number of women | Drug | Dose | Number of women | |
| Cefazolin | 1 g single dose | 289 | Ampicillin plus sulbactam | 1 g single dose | 87 | |
| 2 g single dose | 67 | 1.5 g single dose | 128 | |||
| Cefotetan | 1 g single dose | 224 | 3 g single dose | 192 | ||
| 2 g single dose | 96 | Co‐amoxyclav (amoxicillin plus clavulanic acid) | 1.2 g single dose | 188 | ||
| Cefoxitin | 2 g x 3 doses | 68 | 2.4 g single dose | 55 | ||
| Cefuroxime | 1.5 g single dose | 85 | Ticarcillin plus clavulanic acid | (3 g + 100 mg) x 3 doses | 61 | |
|
Antistaphylococcal cephalosporins (1st and 2nd generation) vs non‐antistaphylococcal penicillins (natural and broad spectrum) (9 trials, 3 093 women) | ||||||
| Antistaphylococcal cephalosporins (1st and 2nd generation) | vs | Non‐antistaphylococcal penicillins (natural and broad spectrum) | ||||
| Drug | Dose | Number of women | Drug | Dose | Number of women | |
| Cefazolin | 1 g single dose | 283 | Ampicillin | 2 g single dose | 315 | |
| 2 g single dose | 161 | 1 g x 3 doses | 113 | |||
| 1 g x 3 doses | 261 | Benzathine penicillin; and Procaine penicillin |
(1 200 000 IU and 400 000 IU) x 5 doses |
200 | ||
| Cefonicid | 1 g | 147 | Mezlocillin | 4 g single dose | 51 | |
| Cefotetan | 2 g, single dose | 244 | 2 g x 3 doses | 51 | ||
| Cefoxitin | 1 g single dose | 155 | Piperacillin | 4 g single dose | 155 | |
| 2 g single dose | 162 | 2 g x 3 doses | 268 | |||
| 2 g x 3 doses | 278 | |||||
| 4 g x 3 doses | 49 | |||||
| Cephalothin | 2 g single dose | 200 | ||||
|
Minimally antistaphylococcal cephalosporins (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors (2 trials, 865 women) | ||||||
| Minimally antistaphylococcal cephalosporins (3rd generation) | vs | Broad spectrum penicillins plus betalactamase inhibitors | ||||
| Drug | Dose | Number of women | Drug | Dose | Number of women | |
| Cefotaxime | 1 g single dose | 431 | Co‐amoxyclav (amoxicillin plus clavulanic acid) | 1.2 g single dose | 434 | |
|
Minimally antistaphylococcal cephalosporins (3rd generation) vs non‐antistaphylococcal penicillins (natural and broad spectrum) (4 trials, 854 women) | ||||||
| Minimally antistaphylococcal cephalosporins (3rd generation) | vs | Non‐antistaphylococcal penicillins (natural and broad spectrum) | ||||
| Drug | Dose | Number of women | Drug | Dose | Number of women | |
| Cefotaxime | 1 g x 3 | 55 | Ampicillin | 2 g | 148 | |
| Ceftizoxime | 1 g | 135 | 1 g x 3 | 59 | ||
| Ceftriaxone | 1 g | 145 | 1 g x 1; then 500mg x 4 | 125 | ||
| Mezlocillin | 2 g | 32 | ||||
| Piperacillin | 4 g | 155 | ||||
Two trials compared 1st generation cephalosporin (cefazolin) versus co‐amoxyclav (amoxicillin plus clavulanic acid) (Jyothi 2010; Lumbiganon 1994).
One further trial also included a cefazolin group, with the control receiving ampicillin plus sulbactam; this trial also included an additional intervention group who received 2nd generation cephalosporin (cefotetan); we pooled th the data from the two cephalosporin groups for the main analysis (Noyes 1998).
Three trials compared 2nd generation cephalosporin (cefotetan) versus ampicillin plus sulbactam (Bracero 1997; Busowski 2000; Spinnato 2000).
One trial compared 2nd generation cephalosporin (cefuroxime) versus ampicillin plus sulbactam (Ziogos 2010).
One trial compared 2nd generation cephalosporin (cefoxitin) versus ticarcillin plus clavulanic acid (Saltzman 1985).
Five of the trials were two‐arm (Bracero 1997; Jyothi 2010; Lumbiganon 1994; Saltzman 1985; Ziogos 2010) and the other three were three‐arm (as described above, the data for the two cephalosporin arms in Noyes 1998 were pooled in the main analysis; Busowski 2000 included a third group of women who received fluoroquinolone (ciprofloxacin) (see comparisons 14 and 15); Spinnato 2000 included a third group who received only broad spectrum penicillin (ampicillin) (see comparisons 4 to 7).
Seven of the trials administered a single dose of antibiotic in both groups (Bracero 1997; Busowski 2000; Jyothi 2010; Lumbiganon 1994; Noyes 1998; Spinnato 2000; Ziogos 2010), and the other trial (Saltzman 1985) administered multiple doses in both groups.
All studies had some limitations in their design. For selection bias, only two studies were assessed as low risk (for both sequence generation and allocation concealment) (Bracero 1997; Ziogos 2010), and the remainder of studies were at unclear risk. For blinding, only one study was assessed as low risk (both performance and detection) (Busowski 2000), and one study was assessed as high risk of both aspects of blinding (Ziogos 2010). Also for blinding, three further studies were assessed as low risk for performance bias but unclear risk for detection bias (Bracero 1997; Saltzman 1985; Spinnato 2000), and the remainder were assessed as unclear. For incomplete outcome data, all studies were assessed as low risk of bias except one study which was unclear (Saltzman 1985). All studies were assessed as unclear for selective reporting bias and other biases.
Primary outcomes
Maternal sepsis: only one small study reported 'bacteraemia', which we have reported under this outcome. One woman, in the intervention group had bacteraemia. When compared with broad spectrum penicillins plus betalactamase inhibitors, the effect of antistaphylococcal cephalosporins (1st and 2nd generation) on maternal sepsis is uncertain (risk ratio (RR) 2.37, 95% confidence interval (CI) 0.10 to 56.41, 1 study, 75 women; very low‐certainty evidence) (Analysis 1.1; Table 1).
1.1. Analysis.

Comparison 1: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 1: Maternal sepsis
Maternal endometritis: there may be little or no difference between antistaphylococcal cephalosporins and broad spectrum penicillin plus betalactamase inhibitors in preventing endometritis, however the 95% confidence interval is also compatible with both an increase or decrease in risk with antistaphylococcal cephalosporins (RR 1.10; 95% CI 0.76 to 1.60, 7 studies, 1161 women; low‐certainty evidence) (Analysis 1.2; Table 1).
1.2. Analysis.

Comparison 1: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 2: Maternal endometritis
No included studies reported on infant sepsis or infant oral thrush.
Subgroup analysis by type of caesarean section
For our primary outcomes, there were too few studies that defined the type of caesarean section for subgroup analyses to be meaningful (Analysis 2.1; Analysis 2.2).
2.1. Analysis.

Comparison 2: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ subgrouped by type of CS, Outcome 1: Maternal sepsis
2.2. Analysis.

Comparison 2: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ subgrouped by type of CS, Outcome 2: Maternal endometritis
Subgroup analysis by type of cephalosporin
Similarly by type of cephalosporin, only two trials (268 women) administered 1st generation cephalosporins while six (893 women) gave 2nd generation cephalosporins. There appeared to be no indication of a difference in effect by generation of cephalosporin, consistent with the hypothesis underpinning our Methods. However, due to the relatively small number of trials and this imbalanced distribution, these exploratory subgroup analyses could not be expected to detect real differences in effect (Analysis 3.1; Analysis 3.2).
3.1. Analysis.

Comparison 3: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ subgrouped by generation of cephalosporin, Outcome 1: Maternal sepsis
3.2. Analysis.

Comparison 3: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ subgrouped by generation of cephalosporin, Outcome 2: Maternal endometritis
Secondary outcomes
Maternal fever: RR 1.07, 95% CI 0.65 to 1.75, 3 studies, 678 women; low‐certainty evidence (Analysis 1.3).
1.3. Analysis.

Comparison 1: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 3: Maternal fever (febrile morbidity)
When compared with and broad spectrum penicillin plus betalactamase inhibitors, the effects of antistaphylococcal cephalosporins on all other reported secondary outcomes were uncertain.
Maternal wound infection: RR 0.78, 95% CI 0.32 to 1.90, 4 studies, 543 women; very low‐certainty evidence (Analysis 1.4; Table 1).
1.4. Analysis.

Comparison 1: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 4: Maternal wound infection
Maternal urinary tract infection: average RR 0.64, 95% CI 0.11 to 3.73, 4 studies, 496 women, random effects; very low‐certainty evidence (Analysis 1.5; Table 1). There was substantial statistical heterogeneity in these results (Tau² = 1.56; Chi² = 5.96, df = 2 (P = 0.05); I² = 66%). We could find no explanation for this heterogeneity. There were too few studies and women for planned subgroup analyses to suggest a plausible explanation.
1.5. Analysis.

Comparison 1: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 5: Maternal urinary tract infection
Maternal composite adverse effects: RR 0.96, 95% CI 0.09 to 10.50, 2 studies, 468 women; very low‐certainty evidence (Analysis 1.6; Table 1)
1.6. Analysis.

Comparison 1: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 6: Maternal composite adverse effects
Maternal allergic reactions: no events, not estimable, 2 studies, 373 women (Analysis 1.7)
1.7. Analysis.

Comparison 1: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 7: Maternal allergic reactions
Maternal skin rash: RR 1.08, 95% CI 0.28 to 4.11, 3 studies, 591 women; very low‐certainty evidence (Analysis 1.8)
1.8. Analysis.

Comparison 1: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 8: Maternal skin rash
Costs: two trials provided some information relating to cost of treatment. However, possibly due to differences in the specific type of cephalosporin administered, context, and time, their findings do not converge on the same overarching conclusion for this comparison:
Busowski 2000 described the cost of each drug to the patient at the hospital where the trial took place. Considering drug cost alone, the cephalosporin was more expensive: USD 72.00 for cefotetan 1 g versus USD 51.00 for ampicillin‐sulbactam 1.5 g.
Ziogos 2010 described the cost of antibiotics per women, cefuroxime EUR 2.38 versus ampicillin‐sulbactam EUR 5.07, suggesting that in Greece this cephalosporin may be cheaper.
The included studies did not report on any of our other secondary outcomes: maternal: thrush, composite serious infectious complication,other adverse effects (nausea, vomiting, diarrhoea); length of hospital stay; infections (post‐hospital discharge to 30 days postoperatively), readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development.
Other comparisons (systemic administration)
Comparisons 4 to 13: Cephalosporins versus penicillins (all remaining comparisons within these classes)
Comparisons 4 to 7: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) versus non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum)
Twelve trials included this comparison for systemic administration (Benigno 1986; Chantharojwong 1993; De‐Lalla 1988; Faro 1990; Ford 1986; Graham 1993; Louie 1982; Mivumbi 2014; Rudge 2006; Saltzman 1986; Spinnato 2000; Voto 1986). Three out of the 12 trials (De‐Lalla 1988; Graham 1993; Voto 1986) did not contribute data to the analyses. A total of nine trials contributed data on 3093 women to this comparison, however many trials did not report our priority outcomes (Benigno 1986; Chantharojwong 1993; Faro 1990; Ford 1986; Louie 1982; Mivumbi 2014; Rudge 2006; Saltzman 1986; Spinnato 2000).
For trials that contributed data to the analyses, the antibiotics that women received varied between trials, for both generation of cephalosporin and type of penicillin (for ease of reference, see Table 4 for a detailed summary of drugs, doses and single/multiple dosing used in comparisons including more than two trials).
One trial compared 1st generation cephalosporin (cephalothin) versus natural penicillins (benzathine penicillin and procaine penicillin) (Rudge 2006).
Three trials compared 1st generation cephalosporin (cefazolin) versus broad spectrum penicillin (ampicillin) (Chantharojwong 1993; Louie 1982; Mivumbi 2014).
One 10‐arm trial compared 1st generation cephalosporin (cefazolin, various doses/regimens) or 2nd generation cephalosporins (cefonicid or cefotetan, or cefoxitin various doses/regimens) versus broad spectrum penicillins (ampicillin or piperacillin). The results for each group given any dose or regimen of 1st generation cephalosporin, any dose or regimen of 2nd generation cephalosporins, or any broad spectrum penicillin, were pooled in our analyses (Faro 1990).
Two trials compared 2nd generation cephalosporin (cefoxitin) versus broad spectrum penicillin (piperacillin) (Benigno 1986; Ford 1986).
One trial compared 2nd generation cephalosporin (cefoxitin) versus broad spectrum penicillin (mezlocillin) (Saltzman 1986).
One trial compared 2nd generation cephalosporin (cefotetan) versus broad spectrum penicillin (ampicillin) (Spinnato 2000).
Six of the trials contributing data were two‐arm (Benigno 1986; Chantharojwong 1993; Ford 1986; Mivumbi 2014; Rudge 2006; Saltzman 1986), and the other three had multiple arms (Faro 1990; Louie 1982; Spinnato 2000). The results for different groups in Faro 1990 were combined as described above, and there was also another group who received 3rd generation cephalosporin ceftizoxime (see comparisons 8 to 10); Louie 1982 included a third group who received 3rd generation cephalosporin cefotaxime (see comparisons 8 to 10); Spinnato 2000 also a included a group who received penicillin plus betalactamase inhibitor (ampicillin plus sulbactam) (see comparisons 1 to 3).
Two trials administered women with a single dose of antibiotic in both groups (Mivumbi 2014; Spinnato 2000); one trial compared a single or multiple dose of antistaphylococcal cephalosporin versus a single dose of broad spectrum penicillin (Faro 1990); one trial compared multiple doses of antistaphylococcal cephalosporin versus a single or a multiple dose of broad spectrum penicillin (Saltzman 1986); one trial compared a single dose of cephalosporin versus multiple doses of penicillin (Rudge 2006); and the remaining four trials gave multiple doses of antibiotics to women in both groups (Benigno 1986; Chantharojwong 1993; Ford 1986; Louie 1982).
Of the nine studies contributing data to the analyses, all of them had some limitations in study design that could have biased the results. Only two studies were at low risk of selection bias for both sequence generation and allocation concealment (Benigno 1986; Mivumbi 2014), and all the others were at unclear risk for at least one or both sequence generation domains. Two studies reported blinding of participants, personnel and outcome assessors (Benigno 1986; Louie 1982), while three studies were at high risk of bias due to lack of blinding of participants and/or personnel (Faro 1990; Mivumbi 2014; Rudge 2006); and although the others were not at high risk of bias, they were at unclear risk for at least one blinding domain. One study was at high risk of bias due to incomplete outcome data (Benigno 1986), and all the other were at low risk. All studies were at unclear risk of bias due to selective reporting and other possible sources of bias.
Primary outcomes
Maternal endometritis: average RR 0.91, 95% CI 0.49 to 1.66, 6 studies, 2147 women, random effects (Analysis 4.1).
4.1. Analysis.

Comparison 4: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 1: Maternal endometritis
There was substantial statistical heterogeneity in the results for maternal endometritis (Tau² = 0.21; Chi² = 8.37, df = 5 (P = 0.14); I² = 40%). In line with our Methods, we, therefore, analysed the results using a random‐effects analysis. We note that the central effect estimate differs in direction when a fixed‐effect analysis is used (RR 1.15, 95% CI 0.86 to 1.56; Analysis 4.2), and the 95% CI shifts slightly although in both cases the CI is wide and does not clearly suggest a difference between interventions. Analysis using random effects gives more weight to small studies, and one smaller study Mivumbi 2014 reporting this outcome presented results that differed from other small studies, appearing to more clearly favour antistaphylococcal cephalosporins (cefazolin).
4.2. Analysis.

Comparison 4: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 2: Sensitivity analysis (Fixed effects) Maternal endometritis
The observed statistical heterogeneity was not explained by our pre‐specified subgroup analyses (see below). Further sensitivity analysis suggested that it was due to this outlying result from Mivumbi 2014, where there were a relatively large number of events in the penicillin group. The study authors did acknowledge some important limitations in this study themselves, including a lack of performance blinding, which may have contributed to the divergent results (as may the fact that all other studies had design limitations). It is also possible that timing of administration may have been a contributing factor. Mivumbi 2014 was the only study that gave antibiotics before skin incision (the authors describe timing of administration as 'no more than 60 minutes prior to skin incision'), whereas all other studies contributing data gave antibiotics at or after cord clamping. Although this hypothesis aligns with the findings of Mackeen 2014, and we did not identify other differences that would account for Mivumbi 2014's outlying results, further investigation would be required to confirm whether timing of administration is, in fact, an explanatory factor.
Maternal sepsis, infant sepsis, and infant oral thrush were not reported by any included trials.
Subgroup analysis by type of caesarean section
For the only primary outcome where we have data (maternal endometritis), no included studies reported data on only women giving birth by elective caesarean section, while four studies included only women having non‐elective caesarean section, and the remaining two included a mixture of women having elective and non‐elective caesarean section but did not provide data on separate subgroups (Analysis 5.1).
5.1. Analysis.

Comparison 5: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ subgrouped by type of CS, Outcome 1: Maternal endometritis
Subgroup analyses by generation of cephalosporin and type of penicillin
Subgroup analyses by generation of cephalosporin (C1 versus C2) did not indicate different effects by these subtypes of drug (Chi² = 0.22, df = 1 (P = 0.64), I² = 0%; Analysis 6.1). All included studies reporting on this primary outcome only administered broad spectrum penicillins (P2) (Analysis 7.1).
6.1. Analysis.

Comparison 6: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ subgrouped by generation of cephalosporin, Outcome 1: Maternal endometritis
7.1. Analysis.

Comparison 7: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ subgrouped by type of penicillin, Outcome 1: Maternal endometritis
Secondary outcomes
Maternal fever (febrile morbidity): average RR 0.74, 95% CI 0.39 to 1.41, 5 studies, 798 women, random effects (Analysis 4.3). There was substantial statistical heterogeneity in this result (Tau² = 0.28; Chi² = 8.66, df = 4 (P = 0.07); I² = 54%). Our planned subgroup analyses by type of caesarean section did not suggest an explanation (there were only five studies so any subgroup analysis will have limited validity; women underwent non‐elective caesarean section in two studies and mixed type of caesarean section in the other three), and nor did subgroup analysis by type of penicillin (all women received broad spectrum penicillins P2). It is possible that the administration of different generations of cephalosporin may have been a factor, with 1st generation cephalosporins potentially being more effective (Analysis 4.4; test for subgroup differences: Chi² = 4.65, df = 1 (P = 0.03), I² = 78.5%)). However, these results should be interpreted with caution given the small number of trials. Alternatively ‐ and similar to our results for endometritis ‐ sensitivity analysis suggested that this heterogeneity could be explained by outlying results in Mivumbi 2014: in which case, and as for endometritis above, it is possible that timing of administration may explain these differences (Mivumbi 2014 is the only trial that administered antibiotics pre‐operatively), however, this hypothesis would need to be confirmed by further investigation.
4.3. Analysis.

Comparison 4: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 3: Maternal fever (febrile morbidity)
4.4. Analysis.

Comparison 4: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 4: Subgroup analysis by type of cephalosporin Maternal fever (febrile morbidity)
Maternal wound infection: RR 1.15, 95% CI 0.59 to 2.26, 5 studies, 915 women (Analysis 4.5).
4.5. Analysis.

Comparison 4: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 5: Maternal wound infection
Maternal urinary tract infection: average RR 1.36, 95% CI 0.59 to 3.14, 4 studies, 515 women, random effects (Analysis 4.6). There was again evidence of statistical heterogeneity in this result (Tau² = 0.15; Chi² = 3.70, df = 3 (P = 0.30); I² = 19%), which appeared to be explained by outlying results in Mivumbi 2014. It was not possible to assess whether this heterogeneity could be explained by our planned subgroup analyses (there were only four studies; all women had either non‐elective or mixed caesarean section; only one study out of four gave 2nd generation cephalosporins; all studies gave broad spectrum penicillins P2). As for endometritis and maternal fever above, sensitivity analysis suggested that it is possible that timing of administration may explain these differences (Mivumbi 2014 is the only trial that administered antibiotics pre‐operatively), however this hypothesis would need to be confirmed by further investigation.
4.6. Analysis.

Comparison 4: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 6: Maternal urinary tract infection
Maternal composite adverse effects: RR 2.02, 95% CI 0.18 to 21.96, 2 studies, 1698 women (Analysis 4.7).
4.7. Analysis.

Comparison 4: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 7: Maternal composite adverse effects
Maternal allergic reactions: no events, not estimable, 2 studies, 329 women (Analysis 4.8).
4.8. Analysis.

Comparison 4: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 8: Maternal allergic reactions
Maternal length of hospital stay: mean difference (MD) 1.5 days fewer, 95% CI 2.46 days fewer to 0.54 days fewer, 1 study, 132 women; (Analysis 4.9).
4.9. Analysis.

Comparison 4: Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 9: Maternal length of hospital stay (days)
Costs: while most studies did not report this outcome, those that did provide information relating to cost of treatment calculated this in different ways (therefore, we have not meta‐analysed these data).
Ford 1986 reported the 'cost of prophylactic failure' in caesarean section, making an estimate of this cost based on many factors including hospital room, laboratory tests and fees, cost of drugs, pharmacy preparation and intravenous equipment; and possibly also psychological costs and clinician time although this was not entirely clear from the report (no breakdown of calculation given). The authors estimated the minimum cost of failure per 100 women to be cefoxitin USD 79, 074 versus piperacillin USD 26, 358, and argued that use of piperacillin for prophylaxis at caesarean section could realise significant cost savings. However, we note that these data relate to historic costs and may be out of date.
Louie 1982 reported a total figure per woman combining antibiotic costs and hospitalisation costs (which was calculated based on mean duration of hospitalisation of all successes and failures). Total costs per woman were: cefazolin CAD 1870.75 versus ampicillin CAD 2011.85, suggesting by contrast that the cephalosporin may be more cost‐effective. However, we note that these data relate to historic costs and may be out of date.
Rudge 2006 reported brief information on the unit cost of each drug in Brazil per woman, cephalothin USD 1.00 versus penicillins (benzathine penicillin and procaine penicillin) USD 1.17, suggesting the cephalosporin may be very slightly cheaper.
The included studies did not report on any of our other secondary outcomes: maternal: thrush, composite serious infectious complication, other adverse effects (nausea, vomiting, diarrhoea, skin rash), infections (post‐hospital discharge to 30 days postoperatively), readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development.
Comparisons 8 to 10: Minimally antistaphylococcal cephalosporins C3 (3rd generation) versus non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum)
Five trials included this comparison (Faro 1990; Lehapa 1999; Louie 1982; Ng 1992; Rosaschino 1988), however, Ng 1992 did not contribute data to the analyses. Four trials contributed data on 854 women to our analyses (Faro 1990; Lehapa 1999; Louie 1982; Rosaschino 1988).
Of the four trials that contributed data to our analyses, all trials compared 3rd generation cephalosporins C3 versus broad spectrum penicillins P2, however, the specific drugs used varied (for ease of reference, see Table 4 for a detailed summary of drugs, doses and single/multiple dosing used in comparisons including more than two trials).
One trial compared cefotaxime versus ampicillin (Louie 1982)
One trial compared ceftriaxone versus ampicillin (Lehapa 1999)
One trial compared ceftriaxone versus mezlocillin (Rosaschino 1988)
One trial compared ceftizoxime versus piperacillin or ampicillin (Faro 1990)
Lehapa 1999 and Rosaschino 1988 were two‐arm trials. Faro 1990 included 10 arms (see also comparisons 4 to 7), and in our analysis a single C3 arm was compared with combined results for two groups (one piperacillin, one ampicillin). Louie 1982 included three arms, but the third arm received C1 (see comparisons 4 to 7).
Two trials administered a single dose of the antibiotic to women in each group (Faro 1990; Rosaschino 1988), and the other two trials gave multiple dose to women in both groups (Lehapa 1999; Louie 1982).
All four of the studies contributing data to the analyses had limitations in study design that could have biased the results. Only one study reported reliable random sequence generation (Faro 1990), but for this study it was unclear whether the allocation was adequately concealed; and all three other studies were at unclear risk of bias for both selection bias domains. Blinding was patchy across the studies, with only one study reporting complete blinding (Louie 1982), and one study assessed at high risk of bias due to lack of blinding of participants and personnel (Faro 1990); the two other studies at unclear risk of bias due to lack of blinding for one or both domains. Two studies presented no concerns regarding incomplete outcome data (Louie 1982; Rosaschino 1988), while the other two were assessed to be at unclear risk for this domain. All studies were at unclear risk of selective reporting bias and other possible sources of bias.
Primary outcomes
Maternal sepsis: no events, not estimable, 1 study, 59 women (Analysis 8.1).
8.1. Analysis.

Comparison 8: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 1: Maternal sepsis
Maternal endometritis: RR 1.74, 95% CI 1.10 to 2.75, 2 studies, 562 women (Analysis 8.2).
8.2. Analysis.

Comparison 8: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 2: Maternal endometritis
Infant sepsis and infant oral thrush were not reported in any included trials.
Subgroup analyses by type of caesarean section and type of penicillin
For each of our primary outcomes where data were reported, all had the same type of caesarean section for any given outcome (Analysis 9.1; Analysis 9.2) and all women were administered broad spectrum penicillin P2 in all trials (Analysis 10.1; Analysis 10.2).
9.1. Analysis.

Comparison 9: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ subgrouped by type of CS, Outcome 1: Maternal sepsis
9.2. Analysis.

Comparison 9: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ subgrouped by type of CS, Outcome 2: Maternal endometritis
10.1. Analysis.

Comparison 10: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ subgrouped by type of penicillin, Outcome 1: Maternal sepsis
10.2. Analysis.

Comparison 10: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ subgrouped by type of penicillin, Outcome 2: Maternal endometritis
Secondary outcomes
Maternal fever (febrile morbidity): RR 0.89, 95% CI 0.29 to 2.76, 1 study, 114 women (Analysis 8.3).
8.3. Analysis.

Comparison 8: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 3: Maternal fever (febrile morbidity)
Maternal wound infection: RR 0.41, 95% CI 0.13 to 1.28, 3 studies, 406 women (Analysis 8.4).
8.4. Analysis.

Comparison 8: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 4: Maternal wound infection
Maternal urinary tract infection: RR 0.54, 95% CI 0.05 to 5.75, 2 studies, 173 women (Analysis 8.5).
8.5. Analysis.

Comparison 8: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 5: Maternal urinary tract infection
Maternal composite serious infectious complication: no events, not estimable, 1 study, 59 women (Analysis 8.6).
8.6. Analysis.

Comparison 8: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 6: Maternal composite serious infectious complication
Maternal composite adverse effects: no events, not estimable, 2 studies, 507 women (Analysis 8.7).
8.7. Analysis.

Comparison 8: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 7: Maternal composite adverse effects
Maternal allergic reactions: no events, not estimable, 1 study, 59 women (Analysis 8.8).
8.8. Analysis.

Comparison 8: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 8: Maternal allergic reactions
Maternal nausea: no events, not estimable, 1 study, 59 women (Analysis 8.9).
8.9. Analysis.

Comparison 8: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 9: Maternal nausea
Materanl vomiting: no events, not estimable, 1 study, 59 women (Analysis 8.10).
8.10. Analysis.

Comparison 8: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 10: Maternal vomiting
Maternal diarrhoea: no events, not estimable, 1 study, 59 women (Analysis 8.11).
8.11. Analysis.

Comparison 8: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 11: Maternal diarrhoea
Maternal skin rash: no events, not estimable, 1 study, 59 women (Analysis 8.12).
8.12. Analysis.

Comparison 8: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes, Outcome 12: Maternal skin rash
Costs:
Louie 1982 reported a total figure per woman combining antibiotic costs and hospitalisation costs (which was calculated based on mean duration of hospitalisation of all successes and failures). Total costs per woman were: cefotaxime CAD 1764.50 versus ampicillin CAD 2011.85, suggesting that the cephalosporin may be more cost‐effective. However, we note that these data are historic and may not reflect contemporary prices.
Lehapa 1999 made a brief comment on costs, stating that quote: "[c]eftriaxone was associated with lower management costs. This being attributable to its once daily dosage and less hospital stay for those who received this antibiotic. The net saving in cost by using ceftriaxone instead of ampicillin was [ZAR]883.54 per patient." Although the authors suggest that again that the cephalosporin may be more cost‐effective, the saving is hard to interpret in the absence on absolute costs for each group or details of how it was calculated.
The included studies did not report on any of our other secondary outcomes: maternal: thrush; infant: immediate adverse effects infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development; and costs).
Comparisons 11 and 12: Minimally antistaphylococcal cephalosporins C3 (3rd generation) versus broad spectrum penicillins plus betalactamase inhibitors P2+
Two trials reporting data on 865 women included this comparison (Kamilya 2012; Koppel 1992). Both studies compared cefotaxime versus co‐amoxyclav (amoxicillin plus clavulanic acid), and in both trials all women received a single dose.
Both studies appeared to have some limitations in study design that may have biased the results. While Kamilya 2012 reported adequate random sequence generation, this study was at unclear risk of bias for allocation concealment; and Koppel 1992 was at unclear risk of bias for both selection domains. Both studies reported complete blinding and no attrition, however, it was unclear whether there was a risk of bias due to selective reporting or any other possible sources of bias.
Primary outcomes
Maternal endometritis: RR 1.02, 95% CI 0.07 to 15.88, 2 studies, 865 women (Analysis 11.1).
11.1. Analysis.

Comparison 11: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 1: Maternal endometritis
The included studies did not report on our other primary outcomes, maternal sepsis, infant sepsis, and infant oral thrush.
Subgroup analysis by type of caesarean section
Data were only reported for one primary outcome (maternal endometritis), however the type of caesarean section that women underwent was either mixed (Kamilya 2012) or unclear (Koppel 1992) (Analysis 12.1).
12.1. Analysis.

Comparison 12: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ subgrouped by type of CS, Outcome 1: Maternal endometritis
Secondary outcomes
Maternal fever (febrile morbidity): RR 1.18, 95% CI 0.63 to 2.22, 1 study, 746 women (Analysis 11.2).
11.2. Analysis.

Comparison 11: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 2: Maternal fever (febrile morbidity)
Maternal wound infection: average RR 0.67, 95% CI 0.10 to 4.58, 2 studies, 865 women, random effects (Analysis 11.3). There was moderate statistical heterogeneity in this result (Tau² = 1.14; Chi² = 1.93, df = 1 (P = 0.16); I² = 48%). With only two studies included, it was not possible to assess whether type of caesarean section could have been a factor here (moreover, Kamilya 2012 included mixed caesarean section and in Koppel 1992 type of caesarean section was unclear). We did not identify any other likely explanation for the observed variation in effects.
11.3. Analysis.

Comparison 11: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 3: Maternal wound infection
Maternal urinary tract infection: RR 0.51, 95% CI 0.05 to 5.46, 2 studies, 865 women (Analysis 11.4).
11.4. Analysis.

Comparison 11: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 4: Maternal urinary tract infection
Maternal composite serious infectious complication: no events, not estimable, 1 study, 746 women (Analysis 11.5).
11.5. Analysis.

Comparison 11: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 5: Maternal composite serious infectious complication
Maternal composite adverse effects: no events, not estimable, 2 studies, 865 women (Analysis 11.6).
11.6. Analysis.

Comparison 11: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 6: Maternal composite adverse effects
Maternal allergic reactions: no events, not estimable, 2 studies, 865 women (Analysis 11.7).
11.7. Analysis.

Comparison 11: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 7: Maternal allergic reactions
Maternal nausea: no events, not estimable, 1 study, 119 women (Analysis 11.8).
11.8. Analysis.

Comparison 11: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 8: Maternal nausea
Maternal vomiting: no events, not estimable, 1 study, 119 women (Analysis 11.9).
11.9. Analysis.

Comparison 11: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 9: Maternal vomiting
Maternal diarrhoea: no events, not estimable, 1 study, 119 women (Analysis 11.10).
11.10. Analysis.

Comparison 11: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 10: Maternal diarrhoea
Maternal skin rash: no events, not estimable, 1 study, 119 women (Analysis 11.11).
11.11. Analysis.

Comparison 11: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 11: Maternal skin rash
Maternal length of hospital stay: MD 0.01 shorter; 95% CI 0.12 shorter to 0.10 longer, 1 study, 746 women; unit assumed to be days given reported quantities although this is not explicit in trial report (Analysis 11.12).
11.12. Analysis.

Comparison 11: Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes, Outcome 12: Maternal length of hospital stay
The included studies did not report on any of our other secondary outcomes: maternal: thrush, infections (post‐hospital discharge to 30 days postoperatively), readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development; costs.
Comparison 13: Other cephalosporin (only) regimens versus other penicillin (only) regimens
One study reporting on 200 women (Ahmed 2004) compared cephalosporins C3 (3rd generation) versus penicillins P2 and P3 (broad spectrum and antistaphylococcal) (comparison 13, subgroup 1). All women received a single dose of ceftriaxone C3 versus multiple doses of ampicillin P2 plus cloxacillin P3. All women had elective caesarean sections.
Primary outcomes
Maternal endometritis: RR 2.00, 95% CI 0.18, to 21.71, 1 study, 200 women (Analysis 13.1).
13.1. Analysis.

Comparison 13: Other cephalosporin (only) regimens vs other penicillin (only) regimens, Outcome 1: Maternal endometritis
This study did not report on our other primary outcomes (maternal endometritis, infant sepsis, and infant oral thrush).
Secondary outcomes
Maternal fever (febrile morbidity): RR 1.17, 95% CI 0.41 to 3.35, 1 study, 200 women (Analysis 13.2).
13.2. Analysis.

Comparison 13: Other cephalosporin (only) regimens vs other penicillin (only) regimens, Outcome 2: Maternal fever (febrile morbidity)
Maternal wound infection: RR 0.50, 95% CI 0.05 to 5.43, 1 study, 200 women (Analysis 13.3).
13.3. Analysis.

Comparison 13: Other cephalosporin (only) regimens vs other penicillin (only) regimens, Outcome 3: Maternal wound infection
Maternal vomiting: RR 7.00, 95% CI 0.37 to 133.78, 1 study, 200 women (Analysis 13.4).
13.4. Analysis.

Comparison 13: Other cephalosporin (only) regimens vs other penicillin (only) regimens, Outcome 4: Maternal vomiting
Maternal skin rash: RR 3.00, 95% CI 0.12 to 72.77, 1 study, 200 women (Analysis 13.5).
13.5. Analysis.

Comparison 13: Other cephalosporin (only) regimens vs other penicillin (only) regimens, Outcome 5: Maternal skin rash
This study did not report on any of our other secondary outcomes: maternal: urinary tract infection, thrush; composite serious infectious complication, composite adverse effects;allergic reactions;nausea;diarrhoea;length of hospital stay; infant: immediate adverse effects;unsettled;diarrhoea;skin rash;length of hospital stay;general health;frequency of hospital visits and costs.
Other comparisons of single class versus single class of antibiotic (comparisons 14 to 17)
Three studies (Busowski 2000; Mansueto 1989; Mothilal 2013) included four different comparisons of single classes of antibiotics with one another.
Comparison 14: Fluoroquinolones F versus penicillins plus betalactamase inhibitors P2+
A three‐arm trial, Busowski 2000, compared ciproflaxin F versus ampicillin plus sulbactam P2+, with a total of 72 women contributing data to this analysis. All women received a single dose of antibiotic, and the type of caesarean section was unclear. This study was of questionable quality as it provided no information on sequence generation or allocation concealment, although there were no concerns of risk of bias due to lack of blinding or attrition.
Primary outcomes
Maternal sepsis: RR 2.55, 95 CI 0.11 to 60.57, 1 study, 72 women (Analysis 14.1).
14.1. Analysis.

Comparison 14: Fluoroquinolones F vs broad spectrum penicillin plus betalactamase inhibitors P2+, Outcome 1: Maternal sepsis
Maternal endometritis: RR 1.17, 95% CI 0.68 to 2.01, 1 study, 72 women; (Analysis 14.2).
14.2. Analysis.

Comparison 14: Fluoroquinolones F vs broad spectrum penicillin plus betalactamase inhibitors P2+, Outcome 2: Maternal endometritis
Infant sepsis and infant oral thrush were not reported.
Secondary outcomes
Maternal wound infection: RR 4.25, 95% CI 0.21 to 85.51; 1 study, 72 women (Analysis 14.3).
14.3. Analysis.

Comparison 14: Fluoroquinolones F vs broad spectrum penicillin plus betalactamase inhibitors P2+, Outcome 3: Maternal wound infection
Maternal urinary tract infection: RR 0.09, 95% CI 0.01 to 1.69, 1 study, 72 women (Analysis 14.4).
14.4. Analysis.

Comparison 14: Fluoroquinolones F vs broad spectrum penicillin plus betalactamase inhibitors P2+, Outcome 4: Maternal urinary tract infection
Costs: this study described the cost of each drug to the patient at the hospital where the trial took place. Considering drug cost alone, the fluroquinolone was more expensive: USD 78.00 for ciproflaxin 200 mg versus USD 51.00 for ampicillin‐sulbactam 1.5 g.
This study did not report any of our other secondary outcomes: maternal: fever, serious infectious complication, adverse effects (allergic reactions, nausea, vomiting, diarrhoea, skin rash), length of hospital stay, infections, readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes, thrush), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development).
Comparison 15: Fluoroquinolones F versus cephalosporins C2 (2nd generation)
Busowski 2000 also compared ciproflaxin F versus cefotetan C2. All women received a single dose of antibiotic, and the type of caesarean section was unclear. This study was of questionable quality as it provided no information on the sequence generation or allocation concealment, although there were no concerns of risk of bias due to lack of blinding or attrition.
Primary outcomes
Maternal sepsis: RR 1.08, 95% CI 0.07 to 16.63, 1 study, 81 women (Analysis 15.1).
15.1. Analysis.

Comparison 15: Fluoroquinolones F vs cephalosporins C2 (2nd generation), Outcome 1: Maternal sepsis
Maternal endometritis: RR 1.29, 95% CI 0.76 to 2.19, 1 study, 81 women (Analysis 15.2).
15.2. Analysis.

Comparison 15: Fluoroquinolones F vs cephalosporins C2 (2nd generation), Outcome 2: Maternal endometritis
Infant sepsis and infant oral thrush were not reported.
Secondary outcomes
Maternal wound infection: RR 2.15, 95% CI 0.20 to 22.82, 1 study, 81 women (Analysis 15.3).
15.3. Analysis.

Comparison 15: Fluoroquinolones F vs cephalosporins C2 (2nd generation), Outcome 3: Maternal wound infection
Maternal urinary tract infection: no events, not estimable, 1 study, 81 women (Analysis 15.4).
15.4. Analysis.

Comparison 15: Fluoroquinolones F vs cephalosporins C2 (2nd generation), Outcome 4: Maternal urinary tract infection
Costs: this study described the cost of each drug to the patient at the hospital where the trial took place. Considering drug cost alone, the fluroquinolone was more expensive: USD 78.00 for ciproflaxin 200 mg versus USD 72.00 for cefotetan 1 g.
This study did not report any of our other secondary outcomes: maternal: fever, thrush, serious infectious complication, adverse effects (allergic reactions, nausea, vomiting, diarrhoea, skin rash), length of hospital stay, infections, readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development.
Comparison 16: Carbapenems versus cephalosporins C3 (3rd generation)
One trial reporting on 48 women (Mansueto 1989) compared a single dose of imipenem Ca versus multiple doses of cefotaxime C3. All women had non‐elective caesarean section. There was little information in the trial report and, therefore, this study was at unclear risk of bias for most domains, although there appeared to be no attrition.
Primary outcomes
Maternal endometritis: RR 1.18, 95% CI 0.08 to 17.82, 1 study, 48 women (Analysis 16.1).
16.1. Analysis.

Comparison 16: Carbapenems Ca vs cephalosporins C3 (3rd generation), Outcome 1: Maternal endometritis
Maternal sepsis, infant sepsis and infant oral thrush were not reported.
Secondary outcomes
Maternal fever (febrile morbidity): RR 0.59, 95% CI 0.06 to 6.09, 1 study, 48 women (Analysis 16.2).
16.2. Analysis.

Comparison 16: Carbapenems Ca vs cephalosporins C3 (3rd generation), Outcome 2: Maternal fever (febrile morbidity)
Maternal wound infection: RR 0.39, 95% CI 0.02 to 9.15, 1 study, 48 women (Analysis 16.3).
16.3. Analysis.

Comparison 16: Carbapenems Ca vs cephalosporins C3 (3rd generation), Outcome 3: Maternal wound infection
Maternal urinary tract infection: no events, not estimable, 1 study, 48 women (Analysis 16.4).
16.4. Analysis.

Comparison 16: Carbapenems Ca vs cephalosporins C3 (3rd generation), Outcome 4: Maternal urinary tract infection
This study did not report any of our other secondary outcomes: maternal: thrush, serious infectious complication, adverse effects (allergic reactions, nausea, vomiting, diarrhoea, skin rash), length of hospital stay, infections, readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development; costs.
Comparison 17: Macrolides versus cephalosporins C1 (1st generation)
One small study reporting on 70 women (Mothilal 2013) compared azithromycin M versus cefazolin C1. All women received a single dose of antibiotic. There was little information in the trial report and, therefore, this study was at unclear risk of bias for most domains, although there appeared to be no attrition.
Primary outcomes
This study did not report any of our primary outcomes: Maternal sepsis and endometritis; infant sepsis and oral thrush.
Secondary outcomes
Maternal fever (febrile morbidity): RR 7.00, 95% CI 0.37 to 130.69, 1 study, 70 women (Analysis 17.1).
17.1. Analysis.

Comparison 17: Macrolides M vs cephalosporins C1 (1st generation), Outcome 1: Maternal fever (febrile morbidity)
This study did not report any of our other secondary outcomes: maternal: wound infection, urinary tract infection, thrush, serious infectious complication, adverse effects (allergic reactions, nausea, vomiting, diarrhoea, skin rash), length of hospital stay, infections, readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development; costs.
Comparisons of other antibiotic regimens of multiple classes versus cephalosporins only (comparison 18)
For ease of reference, all trials making comparisons that fall within this category have been presented under a single comparison in our Data and analyses, however the data have not been totaled because the interventions and comparators varied substantially between trials.
Comparison 18 (subgroup 1): Broad spectrum penicillin P2 plus antistaphylococcal penicillin P3 plus aminoglycoside A plus nitroimidazole N versus cephalosporin C3 (3rd generation)
One study reporting data for 200 women (Alekwe 2008) compared multiple doses of ampicillin P2 plus cloxacillin P3 plus gentamicin A plus metronidazole N versus a single dose of ceftriaxone C3. All women had elective caesarean section. This study was at low risk of selection bias, but high risk for performance blinding, and unclear risk for detection bias. There appeared to be no attrition, while it was unclear whether there was risk of bias due to selective reporting or other possible sources of bias.
Primary outcomes
Maternal endometritis: RR 1.07, 95% CI 0.55 to 2.10, 1 study, 200 women (Analysis 18.1).
18.1. Analysis.

Comparison 18: Other antibiotic regimens (multiple classes) vs cephalosporin (only) regimens, Outcome 1: Maternal endometritis
This study did not report our other primary outcomes: maternal sepsis; infant sepsis and oral thrush.
Secondary outcomes
Maternal fever (febrile morbidity): RR 0.86, 95% CI 0.30 to 2.46, 1 study, 200 women (Analysis 18.2).
18.2. Analysis.

Comparison 18: Other antibiotic regimens (multiple classes) vs cephalosporin (only) regimens, Outcome 2: Maternal fever (febrile morbidity)
Maternal wound infection: RR 1.14, 95% CI 0.43 to 3.03, 1 study, 200 women (Analysis 18.3).
18.3. Analysis.

Comparison 18: Other antibiotic regimens (multiple classes) vs cephalosporin (only) regimens, Outcome 3: Maternal wound infection
Maternal urinary tract infection: RR 1.36, 95% CI 0.66 to 2.82, 1 study, 200 women (Analysis 18.4).
18.4. Analysis.

Comparison 18: Other antibiotic regimens (multiple classes) vs cephalosporin (only) regimens, Outcome 4: Maternal urinary tract infection
Maternal length of hospital stay: MD 0.11 days shorter, 95% CI 0.37 days shorter to 0.15 days longer, 1 study, 200 women (Analysis 18.5).
18.5. Analysis.

Comparison 18: Other antibiotic regimens (multiple classes) vs cephalosporin (only) regimens, Outcome 5: Maternal length of hospital stay (days)
Costs: MD 5.98 US dollars (USD) higher, 95% CI 4.28 USD higher to 7.68 USD higher, 1 study, 200 women (Analysis 18.6).
18.6. Analysis.

Comparison 18: Other antibiotic regimens (multiple classes) vs cephalosporin (only) regimens, Outcome 6: Costs
This study did not report any of our other secondary outcomes: maternal:thrush, serious infectious complication, adverse effects (allergic reactions, nausea, vomiting, diarrhoea, skin rash), infections, readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development.
Comparison 18 (subgroup 2): Antistaphylococcal penicillin P3 plus aminoglycaside A versus cephalosporin C3 (3rd generation)
One study including 200 women (Parulekar 2001) compared multiple doses of cloxacillin P3 plus gentamicin A versus a single dose of cefotaxime C3. The type of caesarean section was unclear. There was little information in the trial report and, therefore, this study was at unclear risk of bias for most domains, although there appeared to be no attrition.
Primary outcomes
Maternal endometritis: RR 17.00, 95% CI 0.99 to 290.62, 1 study, 200 women (Analysis 18.1).
This study did not report our other primary outcomes: maternal sepsis; infant sepsis and oral thrush.
Secondary outcomes
Maternal fever (febrile morbidity): RR 8.00, 95% CI 1.89 to 33.89, 1 study, 200 women (Analysis 18.2).
Costs: this trial reported the total cost of drugs and syringes per woman in each group as Indian rupees (INR) 320 versus INR 106, suggesting that a singe dose of C3 may be cheaper than the combined cost of multiple doses of the mixed regimen.
This study did not report any of our other secondary outcomes: maternal:wound infection, urinary tract infection, thrush, serious infectious complication, adverse effects (allergic reactions, nausea, vomiting, diarrhoea, skin rash), length of hospital stay, infections, readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development.
Comparisons of other antibiotic regimens of multiple classes versus penicillins only (comparison 19)
For ease of reference, all trials making comparisons that fall within this category have been presented under a single comparison, however the data have not been totaled because the interventions and comparators varied substantially between trials.
Comparison 19 (subgroup 1): Lincosamide plus aminoglycoside versus natural penicillin P1
One trial compared single doses of clindamycin L plus gentamicin A versus a single dose infusion of benzyl penicillin P1 (Rehu 1980). All women had non‐elective caesarean sections. This study was at unclear risk of selection bias, but low risk of bias for blinding and attrition. It was at unclear risk of selective reporting or other bias.
Primary outcomes
Maternal endometritis: RR 1.46, 95% CI 0.35 to 6.15, 1 study, 88 women (Analysis 19.1).
19.1. Analysis.

Comparison 19: Other antibiotic regimens (multiple classes) vs penicillin (only) regimens, Outcome 1: Maternal endometritis
This study did not report our other primary outcomes: maternal sepsis; infant sepsis and oral thrush.
Secondary outcomes
Maternal wound infection: RR 1.10, 95% CI 0.16 to 7.43, 1 study, 88 women (Analysis 19.3).
19.3. Analysis.

Comparison 19: Other antibiotic regimens (multiple classes) vs penicillin (only) regimens, Outcome 3: Maternal wound infection
This study did not report any of our other secondary outcomes: maternal: fever; urinary tract infection, thrush, serious infectious complication, adverse effects (allergic reactions, nausea, vomiting, diarrhoea, skin rash), length of hospital stay, infections, readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development; costs.
Comparison 19 (subgroup 2): Cephalosporin C1 (1st generation) plus nitroimadazole versus broad spectrum penicillin P2
One trial reporting on 139 women (Shah 1998) compared multiple doses of cephradine C1 plus metronidazole N versus a single dose of piperacillin P2. All women had elective caesarean sections. This study was at unclear risk of bias for all domains.
Primary outcomes
Maternal endometritis: RR 2.70, 95% CI 0.63 to 11.55, 1 study, 139 women (Analysis 19.1).
This study did not report our other primary outcomes: maternal sepsis; infant sepsis and oral thrush.
Secondary outcomes
Maternal fever (febrile morbidity): RR 2.36, 95% CI 0.84 to 6.62, 1 study, 139 women (Analysis 19.2).
19.2. Analysis.

Comparison 19: Other antibiotic regimens (multiple classes) vs penicillin (only) regimens, Outcome 2: Maternal fever (febrile morbidity)
Maternalwound infection: RR 2.02, 95% CI 0.42 to 9.63, 1 study, 139 women (Analysis 19.3).
This study did not report any of our other secondary outcomes: maternal:urinary tract infection, thrush, serious infectious complication, adverse effects (allergic reactions, nausea, vomiting, diarrhoea, skin rash), length of hospital stay, infections, readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development; costs.
Comparison 19 (subgroup 3): Cephalosporin C2 (2nd generation) plus nitroimidazole versus broad spectrum penicillin plus betalactamase inhibitors P2+
One study including 83 women (van der Linden 1993) compared multiple doses of cefuroxime C2 plus metronidazole N versus a single dose of co‐amoxyclav (amoxicillin plus clavulanic acid) P2+. Women had mixed elective and non‐elective caesarean sections. There was little information in the trial report and, therefore, this study was at unclear risk of bias for most domains, although there appeared to be no attrition.
Primary outcomes
Maternal endometritis: RR 0.33, 95% CI 0.01 to 7.77, 1 study, 83 women (Analysis 19.1).
This study did not report our other primary outcomes: maternal sepsis; infant sepsis and oral thrush.
Secondary outcomes
Maternal fever (febrile morbidity): RR 2.93, 95% CI 0.63 to 13.68, 1 study, 83 women (Analysis 19.2).
Maternalwound infection: RR 0.98, 95% CI 0.06 to 15.09, 1 study, 83 women (Analysis 19.3).
Maternal urinary tract infection: no events, not estimable, 1 study, 83 women (Analysis 19.4).
19.4. Analysis.

Comparison 19: Other antibiotic regimens (multiple classes) vs penicillin (only) regimens, Outcome 4: Maternal urinary tract infection
Costs: the authors commented that "[i]f drug costs only are calculated, the use of AMX/CL [P2+] saves dfl. 30.00/patient, a 60% difference. AMX/CL has the advantage of requiring fewer staff resources and materials associated with administration, and lower cost" (dfl = dutch guilder; now replaced by EUR). This comment reflected the fact that the P2+ group received a single dose, whereas the C2 plus N group received multiple doses. No other cost data were reported.
This study did not report any of our other secondary outcomes: maternal:thrush, serious infectious complication, adverse effects (allergic reactions, nausea, vomiting, diarrhoea, skin rash), length of hospital stay, infections, readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development.
Comparisons of other antibiotic regimens of multiple classes versus different antibiotic regimens of multiple classes (comparison 20)
For ease of reference, all trials making comparisons that fall within this category have been presented under a single comparison, however, the data have not been totaled because the interventions and comparators varied substantially between trials.
Comparison 20 (subgroup 1): Aminoglycoside A plus nitroimidazole N versus natural penicillin P1 plus nitroimidazole N plus macrolide M
One study reporting on 241 women (Kayihura 2003) compared single doses of gentamicin A plus metronidazole N versus multiple doses of crystalline penicillin P1 plus metronidazole N plus erythromycin M. All women had non‐elective caesarean sections. This study was at unclear risk of selection bias, and high risk of bias due to lack of blinding (both performance and detection bias). There was no evidence of attrition, but unclear risk of selective reporting or other bias.
Primary outcomes
Maternal sepsis: RR 0.81, 95% CI 0.29 to 2.26, 1 study, 241 women (Analysis 20.1).
20.1. Analysis.

Comparison 20: Other antibiotic regimens (multiple classes) versus different antibiotic regimens (multiple classes), Outcome 1: Maternal sepsis
This study did not report any of our other primary outcomes: maternal endometritis; infant sepsis and oral thrush.
Secondary outcomes
Maternalwound infection: RR 3.23, 95% CI 0.34 to 30.64, 1 study, 241 women (Analysis 20.4).
20.4. Analysis.

Comparison 20: Other antibiotic regimens (multiple classes) versus different antibiotic regimens (multiple classes), Outcome 4: Maternal wound infection
Maternal urinary tract infection: RR 1.08, 95% CI 0.07 to 17.03, 1 study, 241 women (Analysis 20.5).
20.5. Analysis.

Comparison 20: Other antibiotic regimens (multiple classes) versus different antibiotic regimens (multiple classes), Outcome 5: Maternal urinary tract infection
Maternal length of hospital stay: MD 0.30 shorter, 95% CI 0.78 shorter to 0.18 longer, 1 study, 241 women (Analysis 20.7).
20.7. Analysis.

Comparison 20: Other antibiotic regimens (multiple classes) versus different antibiotic regimens (multiple classes), Outcome 7: Maternal length of hospital stay
Costs: the trial report states that "a single dose of prophylactic antibiotics [A plus N] cost USD 0.78 whereas the standard postoperative scheme [P1 plus N plus M] followed at Maputo Central Hospital costs USD 8.37, the former regime thus being less than one‐tenth as expensive as the latter. The costs of the cesarean section, intravenous fluids, nursing and hospital stay are almost the same in both groups".
This study did not report any of our other secondary outcomes: maternal: fever, thrush, serious infectious complication, adverse effects (allergic reactions, nausea, vomiting, diarrhoea, skin rash), length of hospital stay, infections, readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development.
Comparison 20 (subgroup 2): Antistaphylococcal cephalosporins C1 and C2 (1stand 2nd generation) plus nitroimadazole N versus non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) plus nitroimadazole N
Two studies reporting on a total of 256 women included this comparison (Deng 2007; Rohan 2014). One study including 100 women (Deng 2007) compared multiple doses of cefazolin C1 plus metronidazole N versus multiple doses of benzylpenicillin P1 plus ampicillin P2 plus metronidazole N. The second study including 156 women (Rohan 2014) compared single doses of cefuroxime C2 plus metronidazole N versus single doses of ampicillin P2 plus metronidazole N. All women in both studies had elective caesarean sections.
Both studies had limitations in design. Deng 2007 was at low risk of bias for random sequence generation, but high risk of allocation concealment. This study was also at risk of performance bias, and unclear risk of detection bias. There were no concerns about attrition, however, it was at high risk of selective reporting bias, and unclear risk of selective reporting or other bias. There was little information in the trial report for Rohan 2014, and therefore this study was at unclear risk of bias for most domains, although there appeared to be no attrition.
Primary outcomes
Maternal endometritis: no events, not estimable, 1 study, 156 women (Analysis 20.2).
20.2. Analysis.

Comparison 20: Other antibiotic regimens (multiple classes) versus different antibiotic regimens (multiple classes), Outcome 2: Maternal endometritis
The studies did not report any of our other primary outcomes: maternal sepsis; infant sepsis and oral thrush.
Secondary outcomes
Maternal fever (febrile morbidity): RR 0.72, 95% CI 0.13 to 4.14, 1 study, 100 women (Analysis 20.3).
20.3. Analysis.

Comparison 20: Other antibiotic regimens (multiple classes) versus different antibiotic regimens (multiple classes), Outcome 3: Maternal fever (febrile morbidity)
Maternalwound infection: RR 2.00, 95% CI 0.19 to 21.61, 2 studies, 256 women (Analysis 20.4).
Maternal urinary tract infection: no events, not estimable, 1 study, 156 women (Analysis 20.5).
Maternal composite adverse events: no events, not estimable, 1 study, 100 women (Analysis 20.6).
20.6. Analysis.

Comparison 20: Other antibiotic regimens (multiple classes) versus different antibiotic regimens (multiple classes), Outcome 6: Maternal composite adverse effects
Maternal length of hospital stay: MD 0.53 shorter (unit assumed to be days from quantities reported), 95% CI 1.36 shorter to 0.30 longer, 1 study, 100 women (Analysis 20.7).
Costs: renminbi (RMB) 136.12 lower, 95% CI RMB 165.73 lower to RMB 106.51 lower, 1 study, 100 women (Analysis 20.8).
20.8. Analysis.

Comparison 20: Other antibiotic regimens (multiple classes) versus different antibiotic regimens (multiple classes), Outcome 8: Costs
This study did not report any of our other secondary outcomes: maternal: thrush, serious infectious complication, specific adverse effects (allergic reactions, nausea, vomiting, diarrhoea, skin rash), infections, readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development.
Comparison 20 (subgroup 3): Cephalosporin C3 (3rd generation) plus nitroimidazole N versus natural penicillin P1 plus broad spectrum penicillin P2 plus nitroimadazole N plus amphenicol Am
One study reporting on 232 women (Gidiri 2014) compared single doses of ceftriaxone C3 plus metronidazole N versus multiple doses of benzyl penicillin P1 plus amoxicillin P2 plus metronidazole N plus chloramphenicol Am. Women had mixed elective and non‐elective caesarean sections. This study was at unclear risk of bias for random sequence generation, and high risk due to concerns about concealment of allocation. There was high risk of performance bias, but risk of detection bias was unclear. There was no evidence of attrition, but unclear risk of selective reporting or other bias.
Primary outcomes
Maternal sepsis: RR 3.21, 95% CI 0.34 to 30.45, 1 study, 232 women (Analysis 20.1).
This study did not report any of our other primary outcomes: maternal endometritis; infant sepsis and oral thrush.
Secondary outcomes
Maternal fever (febrile morbidity): RR 1.22, 95% CI 0.46 to 3.27, 1 study, 232 women (Analysis 20.3).
Maternalwound infection: RR 1.29, 95% CI 0.40 to 4.10, 1 study, 232 women (Analysis 20.4).
Costs: this study provides some incomplete information relating to the costs of the drugs in each group, stating that the cost to the woman of the specified single doses of ceftriaxone plus metronidazole is USD 3, versus USD 10 for antibiotics in the P2 group (USD 10 appears to be the cost per dose; and the women would receive at least 13 doses according to the standard regimen given to the control group in this study).
This study did not report any of our other secondary outcomes: maternal: fever, urinary tract infection, thrush, serious infectious complication, adverse effects (allergic reactions, nausea, vomiting, diarrhoea, skin rash), length of hospital stay, infections, readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development; costs.
Other comparisons (lavage administration)
Two trials administered different classes of antibiotics by lavage. Both trials compared cephalosporins versus penicillins.
Data from Dashow 1986, which randomised 204 women to receive cephapirin C1 versus cefamandole C2 versus ampicillin P2, were not included in this update due to inconsistencies in the denominators in the trial report.
The other trial (Lewis 1990) reported data on 383 women. This trial compared lavage with cefoxitin C2 versus lavage with ticarcillin P2. Women had mixed elective and non‐elective caesarean sections. This study was at unclear risk of bias for all domains except performance bias (low risk) and selective reporting (high risk) due to lack of reporting of some outcomes for elective caesarean sections (unlike non‐elective caesarean sections).
Comparison 21 (subgroup 1): Cephalosporins C2 (2nd generation) versus broad spectrum penicillin P2
Primary outcomes
Maternal endometritis: RR 0.95, 95% CI 0.63 to 1.43, 1 study, 383 women (Analysis 21.1).
21.1. Analysis.

Comparison 21: (Irrigation/lavage) cephalosporins vs penicillins, Outcome 1: Maternal endometritis
This study also reported on maternal septicaemia. While these results are relevant to consideration of maternal sepsis, we have not included data in our analyses because the report only included information relating to women who had non‐elective caesarean sections (0/135 versus 0/152), and did not report results for women who had elective caesarean sections, or explain why they had not done so.
Secondary outcomes
Maternal fever (febrile morbidity): RR 0.95, 95% CI 0.63 to 1.43, 1 study, 383 women (Analysis 21.2).
21.2. Analysis.

Comparison 21: (Irrigation/lavage) cephalosporins vs penicillins, Outcome 2: Maternal fever (febrile morbidity)
Maternal wound infection: RR 1.06, 95% CI 0.27 to 4.17, 1 study, 383 women (Analysis 21.3).
21.3. Analysis.

Comparison 21: (Irrigation/lavage) cephalosporins vs penicillins, Outcome 3: Maternal wound infection
This study also reported on urinary tract infection, however again data were only reported for women who had non‐elective caesarean sections (5/135 versus 3/152), and not for women who had elective caesarean section, therefore we have not included these data in our analyses.
This study did not report any of our other secondary outcomes: maternal:thrush, serious infectious complication, adverse effects (allergic reactions, nausea, vomiting, diarrhoea, skin rash), length of hospital stay, infections, readmissions; infant: immediate adverse effects (unsettled, diarrhoea, rashes), length of hospital stay, long‐term adverse effects (general health, frequency of hospital visits), immune system development; costs.
Publication bias
There were insufficient numbers of studies in all our comparisons to assess publication bias.
Sensitivity analyses
There were insufficient data from high‐quality studies for any meaningful sensitivity analyses.
Discussion
Antibiotic prophylaxis can be expected to produce a significant reduction in the incidence of maternal infectious morbidity (Smaill 2014). The type of antibiotic used prophylactically, as well as the optimal timing of administration, have been widely studied and discussed in the literature. Here we have addressed the comparisons between the different classes of antibiotics.
Summary of main results
Eight trials involving 1540 women contributed data to our main comparison of antistaphylococcal cephalosporins (1st and 2nd generation) versus broad spectrum penicillins plus betalactamase inhibitors (systemic administration). There may be little or no difference between these antibiotics in their ability to prevent endometritis and there was too little data to report on maternal sepsis. There was no conclusive evidence identified of any important difference between them for maternal fever, wound infection, urinary tract infection and maternal adverse effects.
For the other comparisons of specific types of cephalosporins versus specific types of penicillins, the findings were similarly inconclusive, although minimally antistaphylococcal cephalosporins (3rd generation) may be more effective than non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) in preventing maternal endometritis.
We did not identify any clear difference between other single classes of antibiotics, or for comparisons including multiple classes in one or both groups, for any clinical outcomes. However, we only identified small single studies for all but one of these comparisons, and more data are needed.
None of the studies assessed any infant outcomes. Most of the studies administered antibiotics at or after cord clamping. Best practice has now changed based on new evidence to support administration of antibiotics before skin incision (Mackeen 2014). This is a potential source of bias that could interfere with the results reported in this review. There is a need for further investigation of the efficacy and safety of different classes of antibiotics given prior to skin incision.
The absence of evidence on infant outcomes is a serious omission, as women will want to know if this intervention has any adverse effect on their babies. The absence of this information remains a concern even for studies where the antibiotic was given after the cord had been clamped and cut, as these drugs may pass to the baby through breastfeeding. In addition, none of the studies assessed readmissions and only three considered post‐discharge infections. This is a limitation of this analysis as late infections appear to constitute the majority of infections after caesarean section (Leth 2009). We have no information on whether prophylactic antibiotics impact on these infections and whether one class of antibiotic is better than another.
We also found very little difference on maternal side effects which can have different degrees of severity but can also be cumbersome for some women postpartum. The lack of information also warrants further investigation. We have found most of the outcomes to refer to in‐hospital infections, whereas surveillance should cover up to 30 days after the operation due to the fact that surgical site infections are frequently diagnosed post‐discharge (Sarah 2019).
Overall, 19 studies provided information or commented on costs of antibiotic prophylaxis, however in most cases this information was minimal. Given the limited and patchy cost data reported, it was not possible to provide a comprehensive picture of the relative costs or cost‐effectiveness of the wide array of antibiotics considered in the included studies. The relevance of cost information was also limited by substantial variation in the study dates and geographical locations.
Overall completeness and applicability of evidence
No trials addressed two of our three main comparisons. Due to the very large number of different comparisons made in different trials, of either different single classes or sub‐types of drugs, or diverse combinations of drugs, there were insufficient data to draw any firm conclusions about specific comparisons.
Other Cochrane Reviews have been undertaken that address specifically the timing (Mackeen 2014) and routes of administration (Nabhan 2016) of prophylactic antibiotics for preventing infectious morbidity in women undergoing caesarean section. In this review, most trials administered antibiotics at or after cord clamping, or post‐operatively, so results may have limited applicability to current practice which generally favours administration prior to skin incision (Mackeen 2014). We anticipate that the comparisons between the specific subclasses of penicillins and cephalosporins will also be more fully addressed in further (as yet unpublished) Cochrane Reviews (Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section and Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section); however, if these reviews remain unpublished, inclusion of intra‐class comparisons in a future update of the current review may be warranted.
Most trials in this review were undertaken some years ago, and patterns of antibiotic resistance may have changed in the intervening period; due to changing resistance patterns in different locations, choice of drug may need to be tailored to local circumstances.
Quality of the evidence
The risk of bias for most domains as assessed in the included studies was often unclear, possibly due to the inclusion of many older trials where the study design was not adequately reported, so as to rule out important possible risk of bias. For our main comparison of antistaphylococcal cephalosporins (1st and 2nd generation) and broad spectrum penicillins plus betalactamase inhibitors, the certainty of the evidence using GRADE was low for maternal endometritis, and very low for maternal sepsis, wound infection, urinary tract infection, and maternal composite adverse effects (Table 1). The outcomes were largely downgraded due to concerns about design limitations, wide confidence intervals crossing the line of no effect, and few events.
Potential biases in the review process
We attempted to minimise bias in a number of ways: two review authors assessed eligibility for inclusion and carried out data extraction, and at least two authors assessed risk of bias. Each worked independently. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements.
Agreements and disagreements with other studies or reviews
The previous version of this review concluded that there was no difference in efficacy between cephalosporins and penicillins when used to prevent infection in women undergoing caesarean section (Gyte 2014), however for this update we did not pool all cephalosporins or all penicillins. Nevertheless, the evidence available for this update was similar in that it did not support strong conclusions that one type of drug was beneficial when compared to another, nor that there was no difference between them, when all important outcomes signalling both safety and efficacy are considered. While we did not aim to investigate variations in regimen in this review, an earlier version of the review found no benefit from multiple doses as opposed to a single dose of antibiotics. This issue will hopefully be addressed in the remaining two reviews to be undertaken (Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section and Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section). The Cochrane Review on the timing of administration of prophylactic antibiotics at caesarean section suggests that preoperative administration is more effective (Mackeen 2014), however that review did not report on longer‐term adverse effects for the baby (while our review did not find evidence on long‐term outcomes for the baby). A further Cochrane Review on route of administration of antibiotic prophylaxis for women undergoing caesarean section did not yield any conclusions regarding the relative efficacy of different routes, due to a lack of good‐quality evidence (Nabhan 2016). In light of this uncertainty, for this update we did not pool data from systemic administration with lavage, due to known differences in the way that drugs are absorbed when given via irrigation rather than systemically.
While slightly different in scope, another systematic review relating to this topic also emphasised that the absence of evidence on neonatal outcomes ‐ especially in the context of administration pre‐incision ‐ needs to the addressed, and that microbial resistance should also be studied and factored into decision‐making (Tita 2009).
Authors' conclusions
Implications for practice.
Best current evidence suggests that there may be little or no difference in short‐term outcomes between antistaphylococcal cephalosporins (1st and 2nd generation) and 'broad spectrum penicillins plus betalactamase inhibitors' as prophylaxis for women undergoing caesarean section, although the impact on post‐discharge infections and outcomes for the infant are unknown, as is the impact on bacterial resistance. All are critical to decision‐making. The use of any antibiotic needs to be made on an individual basis, taking into account other medication the mother may be on, comorbidities and history of allergic reactions. The impact on the baby, for which there is no formal evidence, also needs to be considered, as does bacterial resistance. More costly extended‐spectrum penicillins, second or third‐generation cephalosporins, and combination regimens have not been demonstrated to be more effective, but there are few data upon which to make a clear judgement.
Considering that we did not identify differences between antibiotic regimens in terms of the measured outcomes indicative of effectiveness and safety, the decision of what antibiotic to use will depend on the woman's sensitivity to specific antibiotics, the physician's experience, the adverse events, the prevalence of pathogenic organisms according to previous epidemiological studies (if available), the availability and the costs in the different scenarios.
Implications for research.
There is a need for good‐quality trials to assess the most effective antibiotic to use at caesarean section and it is critical that short and long term outcomes for the baby, and post‐discharge outcomes for the mother, are more comprehensively assessed. In particular, trials need to do a better job of investigating possible harms; for instance relating to long‐term adverse effects of antibiotic exposure on the newborn's developing immune system and microbiome, especially in light of increasing rates of caesarean section and increasingly routine use of antibiotics pre‐incision. Trials could include the outcomes identified for this review, in particular outcomes on the baby and post‐discharge infections for the mother.
There is also a need for trials to differentiate between investigation of the most effective and safe of antibiotics at the most urgent caesarean deliveries (where infection control measures are likely to be compromised), and their use as part of the infection prevention package in surgeries that are scheduled. It would be helpful if triallists could employ a distinction between these different situations that is more precise than 'elective versus non‐elective', for instance employing the RCOG four‐fold definition of caesarean emergency, and clearly separating category 1 (the most urgent) surgeries from other scenarios (RCOG 2011).
The impact of routine antibiotics at caesarean section on antibiotic resistance needs to be investigated with some urgency, but can probably not be undertaken within a Cochrane Review due to the need for alternative methodology.
The absence of evidence for two of our main comparisons indicates that there is also a gap in knowledge regarding the comparability of 1st generation cephalosporins to clindamycin or clindamycin plus aminoglycoside, despite the fact that these are the recommended alternative regimens in several major guidelines (e.g. ACOG 2018; IDSA 2013; SOGC 2017).
Several studies (both complete and ongoing) identified by our 2019 search compared a combination of a 1st or 2nd generation cephalosporin plus azithromycin versus the same cephalosporin, with study authors citing an interest in investigating adjunctive azithromycin because it targets ureaplasma. These studies would at present fall within the scope of a different Cochrane Review (Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section) and were therefore excluded from our review, however that title remains vacant. For future updates of the present review, it may be important to revisit the scope to reflect shifts in research and clinical practice globally (e.g. to consider to including these studies).
What's new
| Date | Event | Description |
|---|---|---|
| 2 December 2019 | New search has been performed | Search updated. We assessed 33 new trial reports and two that were previously awaiting further classification. We included four new studies (Alekwe 2008; Deng 2007; Rohan 2014; Rudge 2006). For this update, we have not pooled the data for all penicillins or for all cephalosporins, because of important variations in spectra of action. We have meta‐analysed the results of trials of different sub‐classes separately. We have also revised our main comparisons. See Differences between protocol and review. We have deleted subgroup analyses by timing of administration and by route of administration, but we have analysed trial administering antibiotics systemically separately from those using lavage/irrigation. We have added two further outcomes 'Post‐discharge infections ‐ to 30 days' and 'Maternal readmissions to hospital'. We have removed the outcome 'Development of antibacterial resistance', because bacterial resistance is unlikely to be detected by randomised controlled trials (RCTs). Changes were made to the some data and analyses for Faro 1990; Kamilya 2012; Kayihura 2003; Noyes 1998 (see Characteristics of included studies). Some background references were updated. |
| 2 December 2019 | New citation required but conclusions have not changed | Updated search. |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 10, 2010
| Date | Event | Description |
|---|---|---|
| 30 September 2014 | New citation required but conclusions have not changed | Six new trials included. Conclusions remain the same. |
| 30 September 2014 | New search has been performed | Methods updated to include GRADE. Search updated, 17 new reports identified. |
| 13 July 2010 | New search has been performed | We have included further studies and re‐structured the review to address the comparisons between different classes of antibiotics. Further reviews will be undertaken to address comparisons within classes of antibiotics, including drug doses, and separate reviews will be undertaken on timings and routes of administration ‐ seeDifferences between protocol and review. |
Notes
The Hopkins 1999 published review on, Antibiotic prophylaxis regimens and drugs for caesarean section, has been subsequently ‘withdrawn’ from publication in the Cochrane Library because it has become out of date. The review has now been split into five separate reviews.
Different classes of antibiotics given to women routinely for preventing infection at caesarean section (this review)
Different regimens of penicillin antibiotics given to women routinely for preventing infection after caesarean section (vacant title)
Different regimens of cephalosporin antibiotic prophylaxis at caesarean section for reducing morbidity (vacant title)
Timing of prophylactic antibiotics for preventing infectious morbidity in women undergoing caesarean section (Mackeen 2014)
Routes of administration of antibiotic prophylaxis for preventing infection after caesarean section (Nabhan 2016)
Acknowledgements
We wish to thank Laura Hopkins and Fiona Smaill for all their work on the original version of this review, Lixia Dou, and Juan Vazquez for their work on previous updates, and Erika Ota for her contribution to earlier versions of the review. Also, we would like to thank Tim Neal for providing guidance on earlier updating of this review, and Neil Hotham and Wendy Jones who provided earlier guidance on the classification of the antibiotics. Also our thanks to Zarko Alfirevic for his contribution to previous versions of the review, particularly in helping to develop the structure and interpret the data, and to Joshua Vogel for suggesting the concept of Table 3 (comparison matrix).
Thanks to Austin Anderson Leirvik for translating Fugere 1983; Anne‐Marie Grant for translating Luttkus 1997; Danping He for translating Xu 1997; Alison Jenner for translating Warnecke 1982; Andrew MacDonald for translating Voto 1986; Cathryn Siegel‐Bergman for translating Koppel 1992; Tamara Stampalija for translating Mansani 1984 and Mansueto 1989; Caroline Summers for translating Wagner 2006; and Elizabeth Whiteley for translating Patacchiola 2000 and Rosaschino 1988.
This review is supported by funding from the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP) to Cochrane Pregnancy and Childbirth (University of Liverpool). HRP supports and coordinates research on a global scale, synthesizes research through systematic reviews of literature, builds research capacity in low‐ and middle‐income countries and develops dissemination tools to make efficient use of ever‐increasing research information. In addition to its cosponsors, the International Planned Parenthood Federation (IPPF) and UNAIDS are both members of HRP’s governing body.
As part of the pre‐publication editorial process, two peers (an editor and referee who is external to the editorial team) commented on this review, as well as a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the peer reviewer who wishes to remain anonymous.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Appendices
Appendix 1. Search methods ‐ ICTRP and ClinicalTrials.gov
ICTRP
cesarean AND antibiotic(s)
caesarean AND antibiotic(s)
ClinicalTrials.gov
Advanced search
Interventional studies | cesarean | antibiotics
Appendix 2. Data collection for studies identified before October 1998
All potential trials were selected for eligibility according to the criteria specified in the protocol and data were extracted from each publication by two reviewers. Any discrepancies were resolved by discussion. In addition to the main outcome measures listed above, information on the setting of the study (country, type of population, socioeconomic status), a detailed description of the antibiotic regimen used (drug, dose, frequency and timing), and definitions of the outcomes (if provided) were collected. An intention‐to‐treat analysis was performed where possible.
Trials were assessed for methodological quality using the standard Cochrane criteria of adequacy of allocation concealment: adequate (A), unclear (B), inadequate (C), or that allocation concealment was not used (D). Information on blinding of outcome assessment and loss to follow‐up were collected.
The main comparison of any treatment versus another treatment was to be stratified according to the indication for caesarean section.
Separate comparisons of different classes of antibiotics and regimens, grouped where appropriate by spectrum of activity, were made. If there were sufficient trials, separate comparisons were made between the timing of antibiotic administration, the number of doses given and the route of administration (whether systemic or lavage).
Summary relative risks were calculated using a fixed‐effect model (if there was no significant heterogeneity between trials).
Data and analyses
Comparison 1. Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Maternal sepsis | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.10, 56.41] |
| 1.2 Maternal endometritis | 7 | 1161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.76, 1.60] |
| 1.3 Maternal fever (febrile morbidity) | 3 | 678 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.65, 1.75] |
| 1.4 Maternal wound infection | 4 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.32, 1.90] |
| 1.5 Maternal urinary tract infection | 4 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.11, 3.73] |
| 1.6 Maternal composite adverse effects | 2 | 468 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.09, 10.50] |
| 1.7 Maternal allergic reactions | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.8 Maternal skin rash | 3 | 591 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.28, 4.11] |
Comparison 2. Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ subgrouped by type of CS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Maternal sepsis | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.10, 56.41] |
| 2.1.1 Elective CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.1.2 Non‐elective CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.1.3 Mixed type CS, or not defined | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.10, 56.41] |
| 2.2 Maternal endometritis | 7 | 1161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.76, 1.60] |
| 2.2.1 Elective CS | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.05, 12.83] |
| 2.2.2 Non‐elective CS | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.77, 3.84] |
| 2.2.3 Mixed type CS, or not defined | 5 | 747 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.61, 1.44] |
Comparison 3. Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ subgrouped by generation of cephalosporin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Maternal sepsis | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.10, 56.41] |
| 3.1.1 Cephalosporins C1 (1st generation) vs penicillins plus betalactamase inhibitors P2+ | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.1.2 Cephalosporins C2 (2nd generation) vs penicillins plus betalactamase inhibitors P2+ | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.10, 56.41] |
| 3.2 Maternal endometritis | 7 | 1161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.76, 1.60] |
| 3.2.1 Cephalosporins C1 (1st generation) vs penicillins plus betalactamase inhibitors P2+ | 2 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.59, 4.16] |
| 3.2.2 Cephalosporins C2 (2nd generation) vs penicillins plus betalactamase inhibitors P2+ | 6 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.68, 1.53] |
Comparison 4. Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Maternal endometritis | 6 | 2147 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.49, 1.66] |
| 4.2 Sensitivity analysis (Fixed effects) Maternal endometritis | 6 | 2147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.86, 1.56] |
| 4.3 Maternal fever (febrile morbidity) | 5 | 798 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.39, 1.41] |
| 4.4 Subgroup analysis by type of cephalosporin Maternal fever (febrile morbidity) | 5 | 798 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.39, 1.41] |
| 4.4.1 Cephalosporins C1 vs non‐antistaphylococcal penicillins P1 and P2 | 3 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.23, 0.97] |
| 4.4.2 Cephalosporins C2 vs non‐antistaphylococcal penicillins P1 and P2 | 2 | 434 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.75, 2.05] |
| 4.5 Maternal wound infection | 5 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.59, 2.26] |
| 4.6 Maternal urinary tract infection | 4 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.59, 3.14] |
| 4.7 Maternal composite adverse effects | 2 | 1698 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 21.96] |
| 4.8 Maternal allergic reactions | 2 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.9 Maternal length of hospital stay (days) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐2.46, ‐0.54] |
Comparison 5. Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ subgrouped by type of CS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Maternal endometritis | 6 | 2147 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.49, 1.66] |
| 5.1.1 Elective CS | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.1.2 Non‐elective CS | 4 | 1818 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.99, 1.89] |
| 5.1.3 Mixed type CS, or not defined | 2 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.04, 2.21] |
Comparison 6. Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ subgrouped by generation of cephalosporin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Maternal endometritis | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1.1 1st generation cephalosporins (C1) vs natural and broad spectrum penicillins (P1 and P2) | 4 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.33, 2.34] |
| 6.1.2 2nd generation cephalosporins (C2) vs natural and broad spectrum penicillins (P1 and P2) | 3 | 1111 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.72, 1.77] |
Comparison 7. Antistaphylococcal cephalosporins C1 and C2 (1st and 2nd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ subgrouped by type of penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Maternal endometritis | 6 | 2147 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.49, 1.66] |
| 7.1.1 1st and 2ndgeneration cephalosporins (C1 and C2) vs natural penicillins (P1) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 7.1.2 1st and 2ndgeneration cephalosporins (C1 and C2) vs broad spectrum penicillins (P2) | 6 | 2147 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.49, 1.66] |
Comparison 8. Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ all outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Maternal sepsis | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 8.2 Maternal endometritis | 2 | 562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.10, 2.75] |
| 8.3 Maternal fever (febrile morbidity) | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.29, 2.76] |
| 8.4 Maternal wound infection | 3 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.13, 1.28] |
| 8.5 Maternal urinary tract infection | 2 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.05, 5.75] |
| 8.6 Maternal composite serious infectious complication | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 8.7 Maternal composite adverse effects | 2 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 8.8 Maternal allergic reactions | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 8.9 Maternal nausea | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 8.10 Maternal vomiting | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 8.11 Maternal diarrhoea | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 8.12 Maternal skin rash | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 9. Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ subgrouped by type of CS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Maternal sepsis | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 9.1.1 Elective CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 9.1.2 Non‐elective CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 9.1.3 Mixed type CS, or not defined | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 9.2 Maternal endometritis | 2 | 562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.10, 2.75] |
| 9.2.1 Elective CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 9.2.2 Non‐elective CS | 2 | 562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.10, 2.75] |
| 9.2.3 Mixed type CS, or not defined | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 10. Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) ‐ subgrouped by type of penicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Maternal sepsis | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 10.1.1 Cephalosporins C3 (3rd generation) vs penicillins P1 (natural) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 10.1.2 Cephalosporins C3 (3rd generation) vs penicillins P2 (broad spectrum) | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 10.2 Maternal endometritis | 2 | 562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.10, 2.75] |
| 10.2.1 Cephalosporins C3 (3rd generation) vs penicillins P1 (natural) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 10.2.2 Cephalosporins C3 (3rd generation) vs penicillins P2 (broad spectrum) | 2 | 562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.10, 2.75] |
Comparison 11. Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ all outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Maternal endometritis | 2 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.07, 15.88] |
| 11.2 Maternal fever (febrile morbidity) | 1 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.63, 2.22] |
| 11.3 Maternal wound infection | 2 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.10, 4.58] |
| 11.4 Maternal urinary tract infection | 2 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.46] |
| 11.5 Maternal composite serious infectious complication | 1 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.6 Maternal composite adverse effects | 2 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.7 Maternal allergic reactions | 2 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.8 Maternal nausea | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.9 Maternal vomiting | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.10 Maternal diarrhoea | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.11 Maternal skin rash | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.12 Maternal length of hospital stay | 1 | 746 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.10] |
Comparison 12. Minimally antistaphylococcal cephalosporins C3 (3rd generation) vs broad spectrum penicillins plus betalactamase inhibitors P2+ ‐ subgrouped by type of CS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Maternal endometritis | 2 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.07, 15.88] |
| 12.1.1 Elective CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 12.1.2 Non‐elective CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 12.1.3 Mixed type CS, or not defined | 2 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.07, 15.88] |
Comparison 13. Other cephalosporin (only) regimens vs other penicillin (only) regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Maternal endometritis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1.1 Cephalosporins C3 (3rd generation) vs penicillins P2 and P3 (broad spectrum and antistaphyloccal) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.18, 21.71] |
| 13.2 Maternal fever (febrile morbidity) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.2.1 Cephalosporins C3 (3rd generation) vs penicillins P2 and P3 (broad spectrum and antistaphyloccal) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.41, 3.35] |
| 13.3 Maternal wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.3.1 Cephalosporins C3 (3rd generation) vs penicillins P2 and P3 (broad spectrum and antistaphyloccal) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.43] |
| 13.4 Maternal vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.4.1 Cephalosporins C3 (3rd generation) vs penicillins P2 and P3 (broad spectrum and antistaphyloccal) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.00 [0.37, 133.78] |
| 13.5 Maternal skin rash | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.5.1 Cephalosporins C3 (3rd generation) vs penicillins P2 and P3 (broad spectrum and antistaphyloccal) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.12, 72.77] |
Comparison 14. Fluoroquinolones F vs broad spectrum penicillin plus betalactamase inhibitors P2+.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Maternal sepsis | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.11, 60.57] |
| 14.2 Maternal endometritis | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.68, 2.01] |
| 14.3 Maternal wound infection | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.25 [0.21, 85.51] |
| 14.4 Maternal urinary tract infection | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.69] |
Comparison 15. Fluoroquinolones F vs cephalosporins C2 (2nd generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Maternal sepsis | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.07, 16.63] |
| 15.2 Maternal endometritis | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.76, 2.19] |
| 15.3 Maternal wound infection | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.20, 22.82] |
| 15.4 Maternal urinary tract infection | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 16. Carbapenems Ca vs cephalosporins C3 (3rd generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Maternal endometritis | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.08, 17.82] |
| 16.2 Maternal fever (febrile morbidity) | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.06, 6.09] |
| 16.3 Maternal wound infection | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.02, 9.15] |
| 16.4 Maternal urinary tract infection | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 17. Macrolides M vs cephalosporins C1 (1st generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Maternal fever (febrile morbidity) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.00 [0.37, 130.69] |
Comparison 18. Other antibiotic regimens (multiple classes) vs cephalosporin (only) regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Maternal endometritis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1.1 Broad spectrum penicillin P2 plus antistaphylococcal penicillin P3 plus aminoglycaside A plus nitroimadazole N vs cephalosporin C3 (3rd generation) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.55, 2.10] |
| 18.1.2 Antistaphylococcal penicillin P3 plus aminoglycaside A vs cephalosporin C3 (3rd generation) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.00 [0.99, 290.62] |
| 18.2 Maternal fever (febrile morbidity) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.2.1 Broad spectrum penicillin P2 plus antistaphylococcal penicillin P3 plus aminoglycaside A plus nitroimadazole N vs cephalosporin C3 (3rd generation) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.46] |
| 18.2.2 Antistaphylococcal penicillin P3 plus aminoglycaside A vs cephalosporin C3 (3rd generation) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.00 [1.89, 33.89] |
| 18.3 Maternal wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.3.1 Broad spectrum penicillin P2 plus antistaphylococcal penicillin P3 plus aminoglycaside A plus nitroimadazole N vs cephalosporin C3 (3rd generation) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.43, 3.03] |
| 18.3.2 Antistaphylococcal penicillin P3 plus aminoglycaside A vs cephalosporin C3 (3rd generation) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 18.4 Maternal urinary tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.4.1 Broad spectrum penicillin P2 plus antistaphylococcal penicillin P3 plus aminoglycaside A plus nitroimadazole N vs cephalosporin C3 (3rd generation) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.66, 2.82] |
| 18.4.2 Antistaphylococcal penicillin P3 plus aminoglycaside A vs cephalosporin C3 (3rd generation) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 18.5 Maternal length of hospital stay (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.5.1 Broad spectrum penicillin P2 plus antistaphylococcal penicillin P3 plus aminoglycaside A plus nitroimadazole N vs cephalosporin C3 (3rd generation) | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.37, 0.15] |
| 18.5.2 Antistaphylococcal penicillin P3 plus aminoglycaside A vs cephalosporin C3 (3rd generation) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18.6 Costs | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.6.1 Broad spectrum penicillin P2 plus antistaphylococcal penicillin P3 plus aminoglycaside A plus nitroimadazole N vs cephalosporin C3 (3rd generation) | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 5.98 [4.28, 7.68] |
| 18.6.2 Antistaphylococcal penicillin P3 plus aminoglycaside A vs cephalosporin C3 (3rd generation) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
Comparison 19. Other antibiotic regimens (multiple classes) vs penicillin (only) regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Maternal endometritis | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1.1 Lincosamide L plus aminoglycoside A vs natural penicillin P1 | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.35, 6.15] |
| 19.1.2 Cephalosporin C1 (1st generation) plus nitroimadazole vs broad spectrum penicillin P2 | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [0.63, 11.55] |
| 19.1.3 Cephalosporin C2 (2nd generation) plus nitroimadazole N vs broad spectrum penicillin plus betalactamase inhibitors P2+ | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.77] |
| 19.2 Maternal fever (febrile morbidity) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.2.1 Lincosamide L plus aminoglycoside A vs natural penicillin P1 | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 19.2.2 Cephalosporin C1 (1st generation) plus nitroimadazole N vs broad spectrum penicillin P2 | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.84, 6.62] |
| 19.2.3 Cephalosporin C2 (2nd generation) plus nitroimadazole N vs broad spectrum penicillin plus betalactamase inhibitors P2+ | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.63, 13.68] |
| 19.3 Maternal wound infection | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.3.1 Lincosamide L plus aminoglycoside A vs natural penicillin P1 | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.16, 7.43] |
| 19.3.2 Cephalosporin C1 (1st generation) plus nitroimadazole N vs broad spectrum penicillin P2 | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.42, 9.63] |
| 19.3.3 Cephalosporin C2 (2nd generation) plus nitroimadazole N vs broad spectrum penicillin plus betalactamase inhibitors P2+ | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.09] |
| 19.4 Maternal urinary tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.4.1 Lincosamide L plus aminoglycoside A vs natural penicillin P1 | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 19.4.2 Cephalosporin C1 (1st generation) plus nitroimadazole N vs broad spectrum penicillin P2 | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 19.4.3 Cephalosporin C2 (2nd generation) plus nitroimadazole N vs broad spectrum penicillin plus betalactamase inhibitors P2+ | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 20. Other antibiotic regimens (multiple classes) versus different antibiotic regimens (multiple classes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 20.1 Maternal sepsis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1.1 Aminoglycoside A plus nitroimidazole N vs natural penicillin P1 plus nitroimidazole N plus macrolide M | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.29, 2.26] |
| 20.1.2 Antistaphylococcal cephalosporin C1 and C2 (1st and 2nd generation) plus nitroimadazole N vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) plus nitroimadazole N | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 20.1.3 Cephalosporin C3 (3rd generation) plus nitroimadazole N vs natural penicillin P1 plus broad spectrum penicillin P2 plus nitroimadazole N plus amphenicol Am | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.34, 30.45] |
| 20.2 Maternal endometritis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.2.1 Aminoglycoside A plus nitroimidazole N vs natural penicillin P1 plus nitroimidazole N plus macrolide M | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 20.2.2 Antistaphylococcal cephalosporin C1 and C2 (1st and 2nd generation) plus nitroimadazole N vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) plus nitroimadazole N | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 20.2.3 Cephalosporin C3 (3rd generation) plus nitroimadazole N vs natural penicillin P1 plus broad spectrum penicillin P2 plus nitroimadazole N plus amphenicol Am | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 20.3 Maternal fever (febrile morbidity) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.3.1 Aminoglycoside A plus nitroimidazole N vs natural penicillin P1 plus nitroimidazole N plus macrolide M | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 20.3.2 Antistaphylococcal cephalosporin C1 and C2 (1st and 2nd generation) plus nitroimadazole N vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) plus nitroimadazole N | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.13, 4.14] |
| 20.3.3 Cephalosporin C3 (3rd generation) plus nitroimadazole N vs natural penicillin P1 plus broad spectrum penicillin P2 plus nitroimadazole N plus amphenicol Am | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.46, 3.27] |
| 20.4 Maternal wound infection | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.4.1 Aminoglycoside A plus nitroimidazole N vs natural penicillin P1 plus nitroimidazole N plus macrolide M | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.34, 30.64] |
| 20.4.2 Antistaphylococcal cephalosporin C1 and C2 (1st and 2nd generation) plus nitroimadazole N vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) plus nitroimadazole N | 2 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.19, 21.61] |
| 20.4.3 Cephalosporin C3 (3rd generation) plus nitroimadazole N vs natural penicillin P1 plus broad spectrum penicillin P2 plus nitroimadazole N plus amphenicol Am | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.40, 4.10] |
| 20.5 Maternal urinary tract infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.5.1 Aminoglycoside A plus nitroimidazole N vs natural penicillin P1 plus nitroimidazole N plus macrolide M | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.07, 17.03] |
| 20.5.2 Antistaphylococcal cephalosporin C1 and C2 (1st and 2nd generation) plus nitroimadazole N vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) plus nitroimadazole N | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 20.5.3 Cephalosporin C3 (3rd generation) plus nitroimadazole N vs natural penicillin P1 plus broad spectrum penicillin P2 plus nitroimadazole N plus amphenicol Am | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 20.6 Maternal composite adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.6.1 Aminoglycoside A plus nitroimidazole N vs natural penicillin P1 plus nitroimidazole N plus macrolide M | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 20.6.2 Antistaphylococcal cephalosporin C1 and C2 (1st and 2nd generation) plus nitroimadazole N vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) plus nitroimadazole N | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 20.6.3 Cephalosporin C3 (3rd generation) plus nitroimadazole N vs natural penicillin P1 plus broad spectrum penicillin P2 plus nitroimadazole N plus amphenicol Am | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 20.7 Maternal length of hospital stay | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.7.1 Aminoglycoside A plus nitroimidazole N vs natural penicillin P1 plus nitroimidazole N plus macrolide M | 1 | 241 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.78, 0.18] |
| 20.7.2 Antistaphylococcal cephalosporin C1 and C2 (1st and 2nd generation) plus nitroimadazole N vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) plus nitroimadazole N | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.36, 0.30] |
| 20.7.3 Cephalosporin C3 (3rd generation) plus nitroimadazole N vs natural penicillin P1 plus broad spectrum penicillin P2 plus nitroimadazole N plus amphenicol Am | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.8 Costs | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.8.1 Aminoglycoside A plus nitroimidazole N vs natural penicillin P1 plus nitroimidazole N plus macrolide M | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.8.2 Antistaphylococcal cephalosporin C1 and C2 (1st and 2nd generation) plus nitroimadazole N vs non‐antistaphylococcal penicillins P1 and P2 (natural and broad spectrum) plus nitroimadazole N | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐136.12 [‐165.73, ‐106.51] |
| 20.8.3 Cephalosporin C3 (3rd generation) plus nitroimadazole N vs natural penicillin P1 plus broad spectrum penicillin P2 plus nitroimadazole N plus amphenicol Am | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
Comparison 21. (Irrigation/lavage) cephalosporins vs penicillins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 21.1 Maternal endometritis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1.1 Cephalosporins C2 (2nd generation) vs penicillins P2 (broad spectrum) | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.63, 1.43] |
| 21.2 Maternal fever (febrile morbidity) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.2.1 Cephalosporins C2 (2nd generation) vs penicillins P2 (broad spectrum) | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.63, 1.43] |
| 21.3 Maternal wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.3.1 Cephalosporins C2 (2nd generation) vs penicillins P2 (broad spectrum) | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.27, 4.17] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmed 2004.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 3rd generation cephalosporin (C3).
Comparator: broad spectrum penicillin (P2) plus antistaphylococcal penicillin (P3).
Subgroups
Comparison 13.1 |
|
| Outcomes | Postoperative febrile morbidity; postoperative infection; endometritis; wound infection; pelvic abscess; peritonitis; other febrile morbidity. | |
| Notes |
Dates: January to June 2001 Setting: Wad Medani Teaching Hospital, Central Sudan. Funding sources: the drugs were donated by Alhikma Company, Wad Medani, Sudan. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:“...were randomised...” |
| Allocation concealment (selection bias) | Unclear risk | Quote:“...were randomised...” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and the drug regimens were different, 1 a single dose, the other 3 doses. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | We did not assess trial protocol,but the publication reported more outcomes than their methods indicated e.g. low Apgar scores; death; maternal vomiting and maternal skin rash. |
| Other bias | Unclear risk | No statistical differences in admission variables between the 2 groups. Data and P values provided on temperature, weight, gestational age, pre‐operative Hb. However, other aspects of bias unclear. · Quote:"The drugs were donated by Alhikma Company, Wad Medani, Sudan." but it seems unclear whether this might give the company any influence or not. |
Alekwe 2008.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: other antibiotic regimen (multiple classes)
Comparator: 3rd generation cephalosporin (C3)
Subgroups
Comparison 18.1 |
|
| Outcomes | Endometritis, UTIs, febrile morbidities, wound infections, duration of hospital stay, cost of antibiotic therapy. | |
| Notes |
Dates: not reported. Setting: obstetric units of the Obafemi Awolowo University Teaching Hospital complex, Ile‐Ife, and the Seventh Day Adventist Hospital Ile‐Ife, Osun State, Nigeria. Funding sources: not reported. Declarations of interest: quote:“The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.” Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done using the method of block randomization with randomized numbers picked from a table of random numbers." |
| Allocation concealment (selection bias) | Low risk | Patients were allocated by quote: “...opening a sealed envelope; these were arranged serially”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different drug administration methods for the 2 groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Reported outcomes were as prespecified in the methods section, but we did not assess trial protocol. |
| Other bias | Unclear risk | No statistical differences in baseline variables between the 2 groups but it was unclear about other possible biases. |
Benigno 1986.
| Study characteristics | ||
| Methods | RCT. Individual women. Multi‐centre (6 centres). 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 2nd generation cephalosporin (C2).
Comparator: broad spectrum penicillin (P2).
Subgroups
Comparisons: 4 |
|
| Outcomes | Satisfactory prophylactic response; febrile morbidity (temperature > 38 ºC x 2 occasions, 6 hours apart, not included first 24 hours post operation; wound infection). | |
| Notes |
Dates of study: not reported. Setting: women from hospitals and universities of San Francisco, Atlanta, Memphis, Los Angeles, Phoenix, New York. Additional information
Funding sources: not reported. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “...a computer‐generated randomization schedule...” |
| Allocation concealment (selection bias) | Low risk | “...a computer‐generated randomization schedule maintained by each hospital pharmacy...” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The investigator and his (or her) staff were blinded as to antibiotic assignment. The code was not broken by the investigator until the last patient had been evaluated for prophylactic response.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The investigator and his (or her) staff were blinded as to antibiotic assignment. The code was not broken by the investigator until the last patient had been evaluated for prophylactic response.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Excluded after randomisation: cephalosporin group 30/177 (16.9%) and penicillin group 33/119 (19.5%). Also differential loss from 2 groups. |
| Selective reporting (reporting bias) | Unclear risk | Publication seemed to report on the outcomes listed in the methods section, but we did not assess trial protocol. |
| Other bias | Unclear risk | Study not stopped early; similar baseline characteristics for weight; height and race, but significant difference in mean age ‐ though not considered important. Other aspects of bias were unclear. No information on funding source of study. |
Bracero 1997.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 2nd generation cephalosporin (C2).
Comparator: broad spectrum penicillin plus betalactamase inhibitor (P2+)
Subgroups
Comparisons: 1, 2 (subgroup 3), 3 (subgroup 2) |
|
| Outcomes | Treatment success; incision site infection; endometritis; UTI; febrile morbidity; peak recorded temperature; days in hospital. | |
| Notes |
Dates of study: not reported. Setting: Westchester County Medical Center, USA. Funding sources: Quote: "This work was supported by a grant (89‐S‐0591, R‐0102) from The Reorig Division of Pfizer Inc." Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A computer was used to generate a list of random numbers for two groups.” |
| Allocation concealment (selection bias) | Low risk | “Treatment assignments were placed in numbered, sealed and opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Neither patient nor obstetrician was informed of the antibiotic assignment. The study drugs were administered by the anaesthesiologist in the operating room immediately after the umbilical cord was clamped” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “Neither patient nor obstetrician was informed of the antibiotic assignment", although it is unclear whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26/196 (13%) women were excluded because of protocol violations. |
| Selective reporting (reporting bias) | Unclear risk | Publication seemed to report on the outcomes listed in the methods section, but wee did not assess the trial protocol. |
| Other bias | Unclear risk | "This work was supported by a grant (89‐S‐0591, R‐0102) from The Reorig Division of Pfizer Inc." Not stopped early; no imbalance in baseline characteristics assessed on: on age; race; weight; height; BP; temperature and pulse but other aspects of bias were unclear. |
Busowski 2000.
| Study characteristics | ||
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 2nd generation cephalosporin (C2).
Comparator 1: broad spectrum penicillin plus betalactamase inhibitor (P2+)
Comparator 2: Fluroquinolone (F)
Subgroups
Comparisons: 1; 2 (subgroup 3); 3 (subgroup 2); 14; 15 |
|
| Outcomes | Endometritis; pneumonia; bacteraemia; UTI; would infection; postpartum stay > 6 days. | |
| Notes |
Dates of study: not reported Setting: Tampa General Hospital, USA. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “... prospectively randomised...” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “... prospectively randomised...” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Investigators were blinded to treatment.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Investigators were blinded to treatment.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Publication reported on the outcomes listed in the methods section, but we‐e did not assess trial protocol. |
| Other bias | Unclear risk | Baseline characteristics similar for: primaparous; weight; gestation; race; repeat CS; Hb but there was a statistically significant difference in age considered not to be clinically important. Other aspects of bias were unclear. No information about funding source of study. |
Chantharojwong 1993.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 1st generation cephalosporin (C1).
Comparator: broad spectrum penicillin (P2).
Subgroups
Comparisons: 4; 5 (subgroup 2); 6 (subgroup 1); 7 (subgroup 2) |
|
| Outcomes | Febrile morbidity; endometritis; parametritis; UTI; wound infection. | |
| Notes |
Dates: 1st January 1990 to 31 December 1992. Setting: Inburi Hospital, Thailand. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “... assigned randomly...” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “... assigned randomly...” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 women excluded. |
| Selective reporting (reporting bias) | Unclear risk | Publication reported on the outcomes listed in the methods section, but wee did not assess the trial protocol. |
| Other bias | Unclear risk | Baseline characteristics similar between groups on: age; height; weight; gravidity; gestation; preoperative haematocrit. However, other aspects of bias unclear. No information about funding source of study. |
Dashow 1986.
| Study characteristics | ||
| Methods | RCT. Individual women. 5‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention 1: 1st generation cephalosporin (C1).
Intervention 2: 2nd generation cephalosporin (C2).
Comparator: broad spectrum penicillin (P2).
4th group ‐ moxalactam disodium (1‐oxa‐beta‐lactam antibiotic: N = 64 in Table 4 (but 79 in Table 2). 5th group ‐ placebo (N = 77). Vitamin added to each solution for disguise. Subgroups
Comparison: this trial used lavage and therefore would have been analysed separately from IV trials, under comparison 21. However, we have not included data from this trial in the current update because there is inconsistency in the denominators detailed in the trial report. |
|
| Outcomes | Endometritis (temperature > 37.8 ºC, uterine tenderness, pelvic irritation without other localising signs); wound infection (breakdown, positive culture and/or cellulitis): febrile morbidity (temperature > 100.4 x 2. 6 hours apart, excluded first 24 hours); mean duration of hospital stay. | |
| Notes |
Dates: 1 December 1982 to 31 May 1984. Setting: Madigan Army Medical Center, USA. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A computer‐generated table of pseudo‐random numbers using the mixed congruential method was used by the pharmacy to assign each patient to one of five groups.” |
| Allocation concealment (selection bias) | Low risk | Quote: “A computer‐generated table of pseudo‐random numbers using the mixed congruential method was used by the pharmacy to assign each patient to one of five groups.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Patients and physicians were unaware of the group assignment until after completion of the study...” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Patients and physicians were unaware of the group assignment until after completion of the study...” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No explanation for differences between the numbers of the initially randomised groups and the groups included in the morbidity analysis (cephapirin 79 vs 70, cefamandole 70 vs 64, moxalactam 64 vs 79). The total of women included is the same (360); therefore we can assume that women originally assigned to 1 group received other treatment and they were not analysed by intention to treat. The uneven numbers may be due to lack of block‐randomisation. |
| Selective reporting (reporting bias) | Unclear risk | Publication reported on the outcomes listed in the methods section, but we did not assess trial protocol. |
| Other bias | Unclear risk | Baseline characteristics similar on: age; parity; gestation. Small difference on gravidity but not considered clinically important. However, other aspects of bias unclear. No information about funding source of study. |
De‐Lalla 1988.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 2nd generation cephalosporin (C2).
Comparator: broad spectrum penicillin (P2).
Subgroups
Comparisons: no usable data reported |
|
| Outcomes | Fever > 38 ºC; endometritis; wound infection; UTIs; asymptomatic bacteriuria. | |
| Notes |
Dates of study: not reported. Setting: Obstetric and Gynecologic department of UCSC, Como, Italy. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | High risk | We did not assess the trial protocol, but the conference abstract did not report pre‐specified outcomes to be assessed in the methods section. |
| Other bias | Unclear risk | No information provided. Also no information about funding source of study. |
Deng 2007.
| Study characteristics | ||
| Methods | RCT. 2 parallel‐arm study. Women randomised individually. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 1st generation cephalosporin (C1) + nitroimadazole (N)
Comparator: natural penicillin (P1) plus broad spectrum penicillin (P2) plus nitroimadazole (N)
Subgroups
Comparisons: 20.2 |
|
| Outcomes | Infection, duration of medication, cost of antibacterial agents and total drug cost during hospitalisation, adverse drug reactions, temperature at day after CS operation | |
| Notes |
Dates: Oct 2005 to Oct 2006 Setting: Central Hospital of Changnin District of Shanghai, China. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes were prepared. The surgeon “randomly” draw envelope to decide which group the patient will go. |
| Allocation concealment (selection bias) | High risk | Envelopes were prepared. The surgeon “randomly” draw envelope to decide which group the patient will go. This is akin to a revealed list, and it would have been easy to subvert the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo was used and the drug administration routes were different. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Publication reported on the outcomes listed in the methods section with the addition of adverse drug reactions, but we did not assess the trial protocol. |
| Other bias | Unclear risk | Baseline indicators (e.g. maternal age, Hb) were comparable between the 2 groups but it was unclear about other possible biases. |
Faro 1990.
| Study characteristics | ||
| Methods | RCT. Individual women. 10‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Interventions: 1st, 2nd and 3rd generation cephalosporins (C1, C2, C3).
Comparator: broad spectrum penicillins (P2).
Subgroups
Comparisons: 4; 5 (subgroup 2); 6 (subgroup 1); 7 (subgroup 2); 8; 10 (subgroup 2) |
|
| Outcomes | Endometritis (defined as temperature > 37.8 ºC x 2, 4 hours apart, excluding 24 hours after delivery plus tachycardia, white blood count > 14, uterine tenderness). | |
| Notes |
Dates: 7 December 1989 to 1 July 1989. Setting: Harris County Hospital, USA. Mixed ethnic population. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “...randomised...according to a computer‐generated schedule.” |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Prospective, open, randomised study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Prospective, open, randomised study but assessors may have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No loss to follow‐up as yet but study still on‐going at time of publication. |
| Selective reporting (reporting bias) | Unclear risk | Publication reported no maternal adverse reactions as well as the 1 pre‐specified outcome of endometritis, however, we did not assess trial protocol. |
| Other bias | Unclear risk | The difference in the numbers allocated to groups (ranging from 142 to 217) is reported as likely to be due to the study being on‐going and the randomisation schedule not complete or to some statistical issue, this is unclear. No information on funding source of study. |
Ford 1986.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (C2).
Comparator: penicillin (P2).
Subgroups
Comparison: 4 |
|
| Outcomes | Febrile morbidity; duration of hospitalisation; administration of systematic antibiotics postoperatively; wound healing; infection at operation site. | |
| Notes |
Dates of study: not reported. Setting: UCLA Medical Center, USA. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “...randomly assigned...”. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “...randomly assigned...”. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appeared to be no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Publication reported on the pre‐specified outcomes in the methods section, but we did not assess trial protocol. |
| Other bias | Unclear risk | No baseline imbalances on: age; weight; duration of surgery. However, other aspects of possible bias were unclear. No information on funding source of study. |
Gidiri 2014.
| Study characteristics | ||
| Methods | RCT. 2‐arm parallel study. Women randomised individually. | |
| Participants |
Inclusion criteria
Exclusion criteria Women who declined to participate in the study; severe immunosuppression of any cause; stage 3 and 4 HIV infection; prolonged rupture of membranes more than 12 hours; surgery longer than 3 hours; chorioamnionitis diagnosed pre‐operatively and obvious concurrent infection that requires therapeutic antibiotics. |
|
| Interventions |
Intervention: 3rd generation cephalosporin (C3) plus metronidazole (N).
Comparator: penicillin (P1) + amoxicillin (P2) + metronidazole(N) + chloramphenicol (Am)
Subgroups
Comparisons: 20.3 |
|
| Outcomes | Pyrexia; admission with puerperal sepsis; wound sepsis; death; duration of hospital stay; laparotomy for pelvic abscess. | |
| Notes |
Dates: 2 February 2012 to 30 May 2012. Setting: Parirenyatwa and Harare (tertiary) hospitals, Zimbabwe. Funding sources: not reported. Declarations of interest: authors report 'No conflict of interest' and they alone are responsible for the content and writing of the paper. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | High risk | Randomisation was by taking a ticket from a box. There was an A4 envelope of tickets at each of the 2 maternity units. Each envelope contained 75 tickets marked Arm 1 and 75 marked Arm 2. The ticket for Arm 1 was identical to that of Arm 2 in size, shape and material. This process could have been open to manipulation (e.g. returning a ticket and taking a different one). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not really possible to blind as 1 group had a single dose and the other had a weeks prescription. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information so most likely assessors were not blinded either. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Women lost to follow‐up were 24 in each group, 21% for intervention and 20% for comparator. |
| Selective reporting (reporting bias) | Unclear risk | Publication reported on all the outcomes pre‐specified in the methods section, but we did not assess the trial protocol. |
| Other bias | Unclear risk | Baseline data showed no imbalance on: age; marital status; education; occupation; booking status; HIV. Otherwise unclear. Authors report 'No conflict of interest' and they alone are responsible for the content and writing of the paper. So appears to have no drug company involvement. |
Graham 1993.
| Study characteristics | ||
| Methods | RCT. Individual women. Multi‐centre. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (C1).
Comparator: penicillin (P2).
Subgroups
Comparison: no data were analysed in this review |
|
| Outcomes | Genital tract cultures. | |
| Notes |
Dates of study: July 1st 1989 to December 31st 1990. Setting: University Medical Centre, Lubbock & LBJ General Hospital, Houston, Texas, USA. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer‐generated number..." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Antibiotic administration was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Antibiotic administration was not blinded but outcome assessor could have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions of women were reported. |
| Selective reporting (reporting bias) | Unclear risk | Publication reported on pre‐ and postoperative genital tract cultures as pre‐specified, but we did not asses trial protocol. |
| Other bias | Unclear risk | Baseline characteristics across groups not reported and other aspects of possible bias unclear. No information about funding source of study. |
Jyothi 2010.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 1st generation cephalosporin (C1).
Comparator: broad spectrum penicillin plus betalactamase inhibitor (P2+).
Subgroups
Comparisons: 1; 2 (subgroup 1); 3 (subgroup 1) |
|
| Outcomes |
Outcomes: fever and infection, endometritis. Reported outcomes: postoperative hospital stay, wound infection, asymptomatic bacteriuria, total infection, postoperative urinary infection. |
|
| Notes |
Dates of study: April 2004 to September 2005. Setting: Kasturba Hospital, Manipal, Karnataka, India. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up was reported. |
| Selective reporting (reporting bias) | Unclear risk | Publication seemed to report on the outcomes listed in the methods section, but we did not assess the trial protocol. |
| Other bias | Unclear risk | Baseline characteristics were similar for: age; BMI; associated disease; type of surgery (primary CS or not). Other possible biases were unclear. No information about funding source for study. |
Kamilya 2012.
| Study characteristics | ||
| Methods | RCT 2 parallel treatment groups, women randomised individually. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 3rd generation cephalosporin (C3).
Comparator: broad spectrum penicillin plus betalactamase inhibitor (P2+)
Subgroups
Comparisons: 11; 12 (subgroup 3) |
|
| Outcomes | Febrile morbidity, wound healing, endometritis, side effects of antibiotics. Reported outcomes: quote: “fever, mild or moderate wound infection, endometritis, UTI or any serious infection, fever in the 5th postoperative period, adverse reactions, duration of hospital stay.” |
|
| Notes |
Dates of study: not reported. Setting: tertiary care teaching hospital in Kolkata, India. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was done a priori by computer in blocks of 40.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization list remained in the custody of the principal investigator." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Being a double blind study, the nature or medication being received by individual trial subjects was not known to the subject or the project clinician”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Being a double blind study, the nature or medication being received by individual trial subjects was not known to the subject or the project clinician”. It seemed most likely considering the outcomes assessed that assessors were blinded as well. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “A total of 760 patients were recruited for the study. Eight patients in the cefotaxime group and six patients in the amoxicillin–clavulanic acid group had to be excluded from final analysis for various reasons”. |
| Selective reporting (reporting bias) | Unclear risk | Reported outcomes were as pre‐specified in the methods section, but we did not assess the trial protocol. |
| Other bias | Unclear risk | Baseline characteristics were similar for: age; parity; gestation. Other possible biases were unclear. No information about funding source of trial. |
Kayihura 2003.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: aminoglycoside (A) + nitroimidazole (N)
Comparator: natural penicillins (P1) + nitroimidazole (N) + macrolide (M)
Subgroups
Comparisons: 20.1 |
|
| Outcomes | Maternal endometritis; would infection; UTI; peritonitis; evisceration; postoperative infection; stillbirth, maternal length of hospital stay, costs | |
| Notes |
Dates: January to June 2000. Setting: Maternity Unit, Hospital Central de Maputo, Mozambique. Quaternary level care. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “...randomly constituted...”. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The anaesthetist administered the antibiotics according to the code written in the exercise book in the sequential order of admission to the theatre...the code was then written on the patient’s file so that the surgeon could prescribe...the doctor on duty was not aware of the group to which the patient was allocated. The principle investigator was not allowed either to select cases to be enrolled in the study or to follow the patients in the ward.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded comparative study. Although the doctor on duty was reported as not aware of the group to which the woman was allocated, the anaesthetist and surgeon were aware and overall the study was described as not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded comparative study. Quote: “Medical doctors allowing the patients to leave the maternity ward knew the regimen followed by the patients in order to not modify the antibiotics given…”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 288 women randomised and analysis on 241 so 47/288 (16.3%) loss. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol but the publication reported more outcomes that their methods indicated e.g. maternal and infant deaths, stillbirths, septicaemia with or without sepsis, however these outcomes are not reported in this Cochrane Review. |
| Other bias | Unclear risk | Baseline characteristics similar for:age; parity; gestation. However, other possible biases are unclear. No information about funding source of study. |
Koppel 1992.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 3rd generation cephalosporin (C3)
Comparator: broad spectrum penicillin plus betalactamase inhibitor (P2+)
Subgroups
Comparisons: 11; 12 (subgroup 3) |
|
| Outcomes | Endometritis (temperature > 37.5 ºC, uterine tenderness); UTI; wound infection. | |
| Notes |
Dates: 17 October 1987 to 24 February 1989. Setting: Kantonspital Hospital, Winterthur, Switzerland. Translation: from German. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The translation reports: "A midwife not involved in the study pulled the names from a randomised list and provided the medications in neutral syringes in the operating room." It is unclear if a 'randomised list', is the same as a 'random table' and until we are able to check this we are reporting this as unclear. |
| Allocation concealment (selection bias) | Unclear risk | A midwife who was not part of the study divided the women into 2 treatment groups, cefotaxime and amoxicillin/clavulanic acid, according to a randomised list. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol and there is no information in the translation which helps us assess if there is selective reporting. |
| Other bias | Unclear risk | Baseline characteristics similar for age. However, other possible biases unclear. No information about funding source of study. |
Lehapa 1999.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 3rd generation cephalosporin (C3).
Comparator: broad spectrum penicillin (P2).
Subgroups
Comparisons: 8 |
|
| Outcomes | Abdominal and/or wound sepsis; febrile morbidity; hospital stay; antibiotic and consumable costs. | |
| Notes |
Dates of study: not reported. Setting: Ga‐Rankuwa Hospital, South Africa. Conference abstract only. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind study although it is unclear whether assessors might have known allocation or not. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None reported, but there is no information on the denominators in either group for us to be sure there was no loss of participants. |
| Selective reporting (reporting bias) | Unclear risk | Reported outcomes in the conference abstract were as pre‐specified in the methods section, but we did not assess trial protocol. |
| Other bias | Unclear risk | 2 groups reported as similar in baseline characteristics on: age; gestation; type of incision; length of surgery, but no data provided. Other aspects of potential bias were unclear. No information about funding source of study. |
Lewis 1990.
| Study characteristics | ||
| Methods | RCT. Individual women. Study in 2 parts. Study 1: compared a penicillin vs placebo (so not included in this review). Study 2: compared a cephalosporin vs a penicillin. |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 2nd generation cephalosporin (C2).
Comparator: broad spectrum penicillin (P2).
Subgroups
Comparisons: antibiotics were administered by lavage, and therefore for the 2020 update this trial was analysed separately from IV trials, under comparison 21.1 |
|
| Outcomes | Endometritis; wound infection (criteria not specified): UTI (criteria not specified): sepsis. | |
| Notes |
Dates of study: June 1985 to April 1987. Quote: “The first part of the study encompassed seven months (Part 1) and the second part, 15 months, ending in April 1987 (Part 2).” Setting: Louisiana State University Hospital, USA. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “...random double‐blind fashion...”. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “...random double‐blind fashion...”. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although a double‐blind study, it is unclear whether the outcome assessors might have not know allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 396 women were reported to be included with 383 providing data, 186 in cephalosporin group and 197 in penicillin group (loss of 13/396 = 3.3%). Data were not reported for women with elective CS for UTI, but they were reported for non‐elective CS. |
| Selective reporting (reporting bias) | High risk | We did not assess trial protocol, but fewer outcomes were reported for elective CS (septicaemia and UTI missing) than for non‐elective CS. |
| Other bias | Unclear risk | Baseline characteristics were similar for age; gestation; gravidity; parity; length of labour. However, other potential biases were unclear. No information about funding source of study. |
Louie 1982.
| Study characteristics | ||
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention 1: 1st generation cephalosporin (C1).
Intervention 2: 3rd generation cephalosporin (C3).
Comparator: broad spectrum penicillin (P2).
Subgroups
Comparisons: 4; 5 (subgroup 2); 6 (subgroup 1); 7 (subgroup 2); 8; 9 (subgroup 2); 10 (subgroup 2) |
|
| Outcomes | Endometritis (temperature > 38 ºC, foul lochia, uterine tenderness); UTI; wound infection; febrile morbidity (temp > 38 ºC x 2, 6 hours apart, excluding first 24 hours), antibiotic and hospitalisation costs | |
| Notes |
Dates: December 1979 to December 1981. Setting: Women's Centre at the Health Sciences Winnipeg, Canada. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “...randomised...”. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “..unlabelled but number‐coded, previously randomised vials...”. Also, unclear because sequence generation is unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Only pharmacist was aware of the drug code.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/195 (3.6%) lost to follow‐up. Similar across groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol, but the study did not report the Apgar scores, meconium staining and sepsis in the infant as pre‐specified in the methods section, however these are not priority outcomes in this Cochrane Review. |
| Other bias | Unclear risk | Baseline characteristics similar for age and race. However, other possible biases were unclear. No information about funding source of study. |
Lumbiganon 1994.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 1st generation cephalosporin (C1).
Comparator: broad spectrum penicillin plus betalactamase inhibitors (P2+)
Subgroups
Comparisons: 1 |
|
| Outcomes | Febrile and infectious morbidity. | |
| Notes |
Dates of study: not reported. Setting: Department of Obstetric and Gynecology, Khon Kaen University, Thailand. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | Although the published abstract provided no information, the registration of the study with the Oxford Database of Perinatal Trials reported allocation was "by sealed, numbered envelopes" but it is unclear if these had to be used in sequential order. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | ‘Partially blinded’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | ‘Partially blinded’. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration form only asks for principle outcomes (febrile morbidity & infectious morbidity) both of which are reported in the Conference abstract, but we did not assess the trial protocol. |
| Other bias | Unclear risk | No baseline data provided and other possible biases unclear. No information about funding source of study. |
Mansueto 1989.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 0ther betalactam: carbapenem (Ca).
Comparator: 3rd generation cephalosporin (C3).
Subgroups
Comparisons: 16 |
|
| Outcomes | Infective complications after the CS (endometritis, infection of the wound, peritonitis, urinary infections, other causes, fever morbidity). | |
| Notes |
Dates: 1 January 1988 to 30 September 1988. Setting: Umberto I Hospital, Frosinone, Italy. Funding sources: not reported. Declarations of interest: unclear. The original paper was not in English. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women reported to be divided randomly into 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol and were unable to assess if there was selective reporting from the translation. |
| Other bias | Unclear risk | No differences in baseline characteristics for: age, parity, length of PROM and number of vaginal examination between 2 groups. Other possible biases were unclear. No information about funding source of study. |
Mivumbi 2014.
| Study characteristics | ||
| Methods | A prospective, randomised, open‐label, single‐site study,with 2 parallel arms. Women randomised individually. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 1st generation cephalosporin (C1).
Comparator: (usual care): broad spectrum penicillin (P2)
Subgroups
Comparisons: 1; 2 (subgroup 3); 3 (subgroup 1); 4 (subgroup 2) |
|
| Outcomes |
Outcomes: the primary outcome variable was postoperative febrile morbidity. Secondary outcomes were infection‐related complications defined as endometritis, wound infection, UTI, fever with unexplained source, need for therapeutic antibiotics, and length of postoperative days in hospital (starting the day after surgery and including the day of discharge). Reported outcomes: febrile morbidity, endometritis, wound infection, UTI, unexplained fever(febrile morbidity), required therapeutic antibiotics, length of postoperative stay, allergic reactions. |
|
| Notes |
Dates: March 1 to May 31, 2012. Setting: The Centre Hospitalier Universitaire de Kigali/University Teaching Hospital of Kigali (CHUK), which is located in Kigali, the capital of Rwanda, is 1 of 3 tertiary care referral hospitals in the Rwandan healthcare system and, compared with district hospitals, receives a disproportionate number of women needing CS. Funding sources: not reported. Declarations of interest: reported that authors have no conflict of interest. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The cards inside the envelopes were randomized by the principle investigator using a random integer generator." |
| Allocation concealment (selection bias) | Low risk | Quote: “The women were preoperatively randomized to one of two study groups via numerically ordered cards in sealed envelopes...The allocated envelopes were opened by clinicians only after the decision for cesarean delivery was made." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “...open‐label...”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As an open‐label RCT the investigators were not blinded but the assessors could have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No women were lost to follow‐up”. |
| Selective reporting (reporting bias) | Unclear risk | Reported outcomes were as pre‐specified in the methods section, but we did not assess the trial protocol. |
| Other bias | Unclear risk | Not known. Reported that authors have no conflict of interest. Funding source of study not reported. |
Mothilal 2013.
| Study characteristics | ||
| Methods | A randomised prospective study with 2 parallel arms. Women randomised individually. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: macrolide (M).
Comparator: 1st generation cephalosporin (C1).
Subgroups
Comparisons: 17 |
|
| Outcomes | Reported outcomes: postoperative fever, wound healing duration (healing within 10 days, healing within 20 days), pain for 6 days, pain for 7‐9 days, infection, PV discharge. | |
| Notes |
Study dates: September 2011 to February 2012. Follow‐up of the cases were finished in March 2012. Setting: Department of Obstetrics & Gynaecology, SRM Medical Research Centre and Hospital in Kattankulathur, Kancheepuram District, India. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The pregnant women were randomly given either Azithromycin or cefazolinas prophylactic antibiotics” and "(Antibiotics) were given ... in a random order ...”. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The pregnant women were randomly given either Azithromycin or Cefazolinas prophylactic antibiotics". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on this. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on this. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses of follow‐up were reported. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes were fever or infection without specifying the type of infections which were reported on, and we did not assess the trial protocol |
| Other bias | Unclear risk | Not known. No information about funding source of study. |
Ng 1992.
| Study characteristics | ||
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention 1: cephalosporin (C3)
Intervention 2: penicillin (P2)
Comparator: ‐ data not used in this review
Subgroups
Comparisons: this trial would have been reported under comparison 8, however due to inconsistencies in the published report, data were not included in the analysis. |
|
| Outcomes | Febrile morbidity; wound infection. | |
| Notes |
Dates: March to August 1991. Setting: Ipoh General Hospital, Ipoh, Perak Darul Ridzuan, Malasyia. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A randomised trial. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women excluded ‐ 1 from each group. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes were 'wound infection' without being specific on the measures to be reported and wee did not assess the trial protocol. |
| Other bias | Unclear risk | Baseline characteristics similar for: age; race; parity; gestation. Other possible biases were unclear. No information about funding source of study. |
Noyes 1998.
| Study characteristics | ||
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention 1: 1st generation cephalosporin (C1).
Intervention 2: 2nd generation cephalosporin (C2).
Comparator: broad spectrum penicillin plus betalactamase inhibitor (P2+)
Subgroups
Comparisons: 1; 2 (subgroup 2); 3 (subgroups 1 and 2) |
|
| Outcomes | Endometritis. | |
| Notes |
Dates: July 1988 to November 1990. Setting: New York Hospital, University‐based, USA. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Prospective randomized trial”. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 out of 300 women (2.7%) were excluded from the analysis. |
| Selective reporting (reporting bias) | High risk | We did not assess the trial protocol, but additional outcomes were reported which were not pre‐specified, e.g. complications, hospital stay, side effects. |
| Other bias | Unclear risk | There was insufficient information to assess other possible biases. No information about funding source of study. |
Parulekar 2001.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion
Exclusion
|
|
| Interventions |
Intervention: antistaphylococcal penicillin (P3) plus aminoglycoside (A)
Comparator: 3rd generation cephalosporin (C3).
Subgroups
Comparisons: 18.2 |
|
| Outcomes | Postpartum infection; wound infection; fever; duration in hospital. | |
| Notes |
Dates of study: July 1995 to July 1997. Setting: Naval Hospital INHS Asvini Colaba, Mumbai, India. Funding sources: not reported. Declarations of interest: not reported. Further information:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly assigned...". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up was reported. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes only were reported but we did not assess the trial protocol. |
| Other bias | Unclear risk | Insufficient information to assess other possible biases. No information about funding source of study. |
Rehu 1980.
| Study characteristics | ||
| Methods | RCT. Individual women. 4 groups. | |
| Participants |
Inclusion
Exclusion
|
|
| Interventions |
Intervention 1 (Group 2): lincosamide antibiotic (L) + aminoglycoside (A).
Intervention 2 (Group 1): natural penicillin (P1).
Comparator 1 (Group 3): placebo 1 (not included in this review).
Comparator 2 (Group 4): placebo 2 (not included in this review).
Subgroups
Comparisons: 19.1 |
|
| Outcomes | Endometritis; wound infection; duration of hospital stay; number of women receiving postoperative treatment. | |
| Notes |
Dates: September 1977 and January 1978. Setting: State/maternity hospital, Helsinki, Finland. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...assigned at random...". |
| Allocation concealment (selection bias) | Unclear risk | Antibiotic preparations for IV use were supplied in solution in bottles carrying code numbers. Still unclear if there was allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The code was kept secret for persons performing the operations and observing the patients in the postoperative period." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The code was kept secret for persons performing the operations and observing the patients in the postoperative period". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 out of the 147 women receiving antibiotics of other reasons during the preoperative period were later excluded from the series. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes only were reported but we did not assess the trial protocol. |
| Other bias | Unclear risk | There was no information on baseline characteristics of the women in the groups, and other possible biases were unclear. No information about funding source of study. |
Rohan 2014.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion
Exclusion
|
|
| Interventions |
Intervention: 2nd generation cefuroxime (C2) and nitroimadazole (N)
Comparator: broad spectrum penicillin (P2) and nitroimadazole (N)
Subgroups
Comparisons: 20.2 |
|
| Outcomes | Puerperal pyrexia, postpartum endometritis, wound infection and UTI | |
| Notes |
Dates: from October 2012 to April 2013. Setting: in a tertiary care hospital in Sri Lanka. Trial funding sources: not reported. Declarations of interest: not reported Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “by pre‐determined randomized allocation sequence.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “by pre‐determined randomized allocation sequence.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes only were reported in the conference abstract but we did not assess the trial protocol. |
| Other bias | Unclear risk | Quote: “There were no demographic differences between two groups” However, there was no information on the methodology reported in this Conference Abstract. |
Rosaschino 1988.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 3rd generation cephalosporin (C3)
Comparator: broad spectrum penicillin (P2)
Subgroups
Comparisons: 8; 9 (subgroup 3); 10 (subgroup 2) |
|
| Outcomes | Tolerability, wound infections, urinary or respiratory infections, complications, side effects. | |
| Notes |
Dates of study: not reported. Setting: Bolognini di Seriate (BG) hospital, obstetric and gynaecological clinic, Italy. Translation: paper in Italian with summary in English. We had information extracted for us in English. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information in the translation for us to look at possible reporting bias and we did not assess the trial protocol. |
| Other bias | Unclear risk | Unable to assess other possible biases. No information about funding source of study. |
Rudge 2006.
| Study characteristics | ||
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention 1: 1st generation cephalosporin (C1)
Intervention 2: natural penicillins(standard at hospital).
Comparator: no antibiotics ‐ data not used in this review
Subgroups
Comparisons: 4 |
|
| Outcomes | Puerperal infection; wound infection; post CS infection; costs of antibiotics. | |
| Notes |
Dates of study: March 1994 to July 1996. Setting: Unversity Teaching Hospital, Botucata School of Medicine, Sao Paulo State University, UNESP, Brazil. 1500 births annually. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided and routes of administration are different so likely the clinicians knew. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the outcome observers were not informed about which group the patients had been allocated to” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes only were reported but we did not assess trial protocol. |
| Other bias | Unclear risk | Baseline characteristics similar. ITT analysis. Too little methodological information to assess if other biases. |
Saltzman 1985.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 2nd generation cephalosporin (C2)
Comparator: broad spectrum penicillin plus betalactamase inhibitor (P2+).
Subgroups
Comparisons: 1; 2 (subgroup 3); 3 (subgroup 2) |
|
| Outcomes | Febrile morbidity; endometritis; UTI. | |
| Notes |
Dates of study: 1983. Setting: Virginia, USA. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...randomly assigned...”. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind study but no mention about whether assessors were blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 18 out of 147 women (12.2%) were excluded from the analysis. However, the report does not describe the group to which they were originally allocated. The only reason given for their exclusion is that they did not complete the study. |
| Selective reporting (reporting bias) | High risk | We did not assess the trial protocol, but the published brief report, In addition to the pre‐specified outcomes of febrile morbidity, endometritis and urinary tract infections, reported on chorioamnionitis (but this seems a subgroup of endometritis ‐ so maybe doesn't count as additional outcome) and maternal skin rash. |
| Other bias | Unclear risk | No information on baseline characteristics. Other possible biases were unclear. No information about funding source of study. |
Saltzman 1986.
| Study characteristics | ||
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 2nd generation cephalosporin (C2)
Comparator 1: broad spectrum penicillin (P2)
Comparator 2: broad spectrum penicillin (P2)
Subgroups
Comparisons: 4; 5 (subgroup 2); 6 (subgroup 2); 7 (subgroup 2) |
|
| Outcomes | Febrile morbidity (temperature > 38 x 2, 8 hours apart, excluding first 24 hours postoperatively; endometritis (temperature > 38 plus foul lochia or uterine tenderness); wound infection (wound surrounded by cellulitis and/or draining purulent material); UTI. | |
| Notes |
Dates: October 1982 to April 1983. Setting: Virginia, USA. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “...randomly assigned...”. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “...randomly assigned...”. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “...double‐blind study...”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind study but no mention of whether assessors were blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 out of 158 women (4.4%) were removed for failure to fulfil the study criteria. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes only were reported but we did not assess trial protocol. |
| Other bias | Unclear risk | Baseline characteristics similar on: age; parity; gestation. However other aspects of potential bias were unclear. No information about funding source of study. |
Shah 1998.
| Study characteristics | ||
| Methods | RCT. Individual women. 4‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 1st generation cephalosporin (C1) + Nitroimidazole (N)
Comparator 1: broad spectrum penicillin (P2).
Comparator 2: broad spectrum penicillin (P2)
Comparator 3: no antibiotics ‐ data not included in this review.
Subgroups
Comparisons: 19.2 |
|
| Outcomes | Postoperative febrile morbidity; metritis with pelvic cellulitis; wound infection. | |
| Notes |
Dates: January 1995 to mid‐1996. Setting: Abu‐Dhabi, United Arab Emirates. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reports women were randomised and no further details. |
| Allocation concealment (selection bias) | Unclear risk | ‘...consecutively numbered sealed envelopes...'. However, the envelopes were not describes as opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8 out of 147 of the women included in our analyses (5.4%) were excluded during the course of the study, with attrition being somewhat imbalanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes only were reported but we did not assess trial protocol. |
| Other bias | Unclear risk | No information on the baseline characteristics of women in each group. Also other aspects of possible bias were unclear. No information about funding source of study. |
Spinnato 2000.
| Study characteristics | ||
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention (publication Group 2): 2nd generation cephalosporin (C2)
Comparator: 1 (publication Group 3): broad spectrum penicillin (P2)
Comparator: 2 (publication Group 1): broad spectrum penicillin plus betalactamase inhibitor (P2+)
Subgroups
Comparisons: 1; 2 (subgroup 3); 3 (subgroup 2); 4; 5 (subgroup 3); 6 (subgroup 2); 7 (subgroup 2) |
|
| Outcomes | Endomyometritis; wound complications. | |
| Notes |
Dates: 24 January 1994 to 12 December 1996. Setting: University of Louisville Hospital, USA. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “...patients were randomized...”. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “...double‐blinded...”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blinded but unclear if the outcome assessors were blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 out of 301 women (1.0%) were not evaluated due to incomplete information data that restricted chart retrieval. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes of endomyometritis and wound infection only were reported, however, for wound infection only the overall incidence was reported so could not be included in this review. We did not assess trial protocol. |
| Other bias | Unclear risk | Baseline characteristics were similar on: age; gestational age; height and weight. Other possible biases were unclear. No information about funding source of study. |
van der Linden 1993.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study, stratified by type of operation, CS or hysterectomy. We only report on women having CS here. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 2nd generation cephalosporin (C2) + nitroimidazole (N)
Comparator: broad spectrum penicillin plus betalactamase inhibitor (P2+)
Subgroups
Comparisons: 19.3 |
|
| Outcomes | UTI; febrile temperature; abdominal wound infection; endometritis and infiltrates at the top of the vaginal vault. | |
| Notes |
Dates: 1 August 1988 to 15 December 1989. Setting: Leyenburg Hospital, Netherlands. Funding sources: quote: "This study was sponsored by Smith Kline and Beecham Pharmaceuticals." Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were “randomly allocated to one of two treatment regimes”. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 out of 215 (7.4%) women were excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes only were reported but we did not assess trial protocol. |
| Other bias | Unclear risk | Baseline characteristics were similar on: age and weight. Other possible biases were unclear. The study was sponsored by Smith Kline and Beecham Pharmaceuticals. |
Voto 1986.
| Study characteristics | ||
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 2nd generation cephalosporin (C2)
Comparator: broad spectrum penicillin (P2)
Subgroups
Comparisons: Although this study would have been included in comparison 4, no data are included in this review due to high rate of attrition from the penicillin group. |
|
| Outcomes | Analyses of cultures at endocervix, skin and tissue and urine. | |
| Notes |
Dates of study: not reported. Setting: maternity ward, the Hospital Juan A. Fernandez, Buenos Aires, Argentina. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Women were divided, at random, into two groups of which one was administered cefoxitin and the other ampicillin.” |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 80 women included (GpA = 39 and GpB = 40) authors only report data on GpA = 37 and GpB = 17. Suggest loss too high and very uneven, so groups are not randomised groups. We have not used data in the meta‐analyses. |
| Selective reporting (reporting bias) | Unclear risk | It is not clear from the translation if all the outcomes listed in the methods are reported on and we did not assess the trial protocol. |
| Other bias | Unclear risk | No information on baseline characteristics and other possible biases unclear. No information about funding source of study. |
Wells 1994.
| Study characteristics | ||
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention 1: 2nd generation cephalosporin (C2) + nitroimidazole (N)
Intervention 2: nitroimidazole (N)
Comparator: placebo.
Subgroups
Comparisons: No data for this review |
|
| Outcomes | Temperature; wound infection; offensive lochia; UTI. | |
| Notes |
Dates of study: not reported. Setting: authors from Grey's Hospital, London, UK. Funding sources: not reported. Declarations of interest: not reported. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...were randomised...". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | High risk | The conference abstract indicates pre‐specified outcomes of: temperature; wound infection; offensive lochia and urinary tract infection, but the abstract only reports on infectious morbidity. We did not assess trial protocol. |
| Other bias | Unclear risk | No baseline data and other aspects of possible bias were unclear. No information about funding source of study. |
Ziogos 2010.
| Study characteristics | ||
| Methods | Prospective RCT ‐ 2 parallel arms ‐ women randomised individually. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 2nd generation cephalosporin (C2)
Comparator: broad spectrum penicillin plus betalactamase inhibitor (P2+)
Subgroups
Comparisons: 1; 2 (subgroup 3); 3 (subgroup 2) |
|
| Outcomes |
Outcomes: the primary outcome was development of an infection either at the surgical site or elsewhere, e.g. UTI, endometritis. Reported outcomes: postoperative infections, surgical site infection (SSI), endometritis, duration of hospitalisation in days median (IQR), duration of hospitalisation postoperatively in days median (IQR), adverse drug reactions. |
|
| Notes |
Study dates July 2004 to December 2008 Setting Major tertiary care hospital, Nikaia’s Regional General Hospital “Agios Panteleimon”, Athens, Greece. Funding sources: Attikon Hospital was the research sponsor (Clinicaltrials.gov identifier: NCT01138852). Declarations of interest: authors reported that had no competing interests. Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Using a random‐number generator”. |
| Allocation concealment (selection bias) | Low risk | Quote: “The sequence was obtained using a central telephone number and it was concealed until interventions were assigned.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Participants ... were blinded to the intervention, however the physician administering the intervention and assessing the outcomes was not.” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “Participants ... were blinded to the intervention, however the physician administering the intervention and assessing the outcomes was not.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were reported |
| Selective reporting (reporting bias) | Unclear risk | Methods section indicates the primary outcomes will be postoperative infections at surgical site and elsewhere, so it is not clear about specific outcomes to be measured. We did not assess the trial protocol and the trial registration form does not report outcomes to be collected. |
| Other bias | Unclear risk | Not known. Authors reported that had no competing interests. |
BMI: body mass index BP: blood pressure CI: confidence interval CS: caesarean section Hb: haemoglobin IM: intramuscular IQR: interquartile range ITT: inetntion‐to‐treat IV: intravenous NaCl: sodium chloride PCV: packed cell volume PROM: premature rupture of membranes RCT: randomised controlled trial RR: risk ratio sg: subgroup UTI: urinary tract infection vs: versus
P. Penicillins
P1. Natural penicillins P2. Broad spectrum penicillins
P2+. Broad spectrum penicillins plus betalactamase inhibitors. P3. Antistaphylococcal penicillins
C. Cephalosporins
C1. First‐generation cephalosporins C2. Second‐generation cephalosporins C3. Third‐generation cephalosporins C4. Fourth‐generation cephalosporins
F. Fluoroquinolones
T. Tetracyclines
M. Macrolides
Ca. Beta‐lactams/carbapenems
A. Aminoglycosides
L. Lincosamides
N. Nitroimidazoles
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrews 2003 | Study compared 2 different 2nd generation cephalosporins. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Andrews 2003). |
| Azizi 2014 | Study compared a single cephalosporin (cephalothin C1) versus a cephalosporin combination (cephalothin C1 plus cephalexin C1 plus azithromycin). Study should be considered for inclusion in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Azizi 2014). |
| Baheraie 1997 | Compares single vs multiple doses of same class of antibiotic (cephalosporin). Study will be considered in the review ‘Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section’. (Baheraie 1997). |
| Beksac 1989 | Quasi‐RCT ‐ "...randomly assigned according to the last number of her hospital notes..." (Beksac 1989). |
| Berkeley 1990 | Study compared a cephalosporin (cefotaxime) by 2 different routes of administration, IV and lavage. (Berkeley 1990). |
| Bernstein 1994 | Study compared 2 different cephalosporins, cefotetan versus cefoxitin. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Bernstein 1994). |
| Bilgin 1998 | Quasi‐RCT, allocated women to groups according to last digit of hospital number. (Bilgin 1998). |
| Boothby 1984 | Study compared a cephalosporin (cefoxitin) by 2 different routes of administration, IV and lavage. (Boothby 1984). |
| Carlson 1990 | Study compared 2 different cephalosporins, cefazolin versus cefotetan. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Carlson 1990). |
| Chamberlain 1993 | Study compared ampicillins plus sulbactam versus ampicillin alone. Study will be considered in the review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. (Chamberlain 1993). |
| Chittacharoen 1998 | Study compared 2 different ampicillins (augmentin vs ampicillin). Study will be considered in the review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. (Chittacharoen 1998). |
| Conover 1984 | Study compared a cephalosporin (cefoxitin) by 2 different routes of administration, IV and irrigation. (Conover 1984). |
| Crombleholme 1987 | Study compared 2 versus 3 doses of a penicillin (mezlocillin). Study will be considered in review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. (Crombleholme 1987). |
| Crombleholme 1989 | Study compared 2 different 2nd generation cephalosporins. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Crombleholme 1989). |
| Cunningham 1983 | Study compared 2 differing timings of giving the prophylactic antibiotics, before and after cord clamping. (Cunningham 1983). |
| D'Angelo 1980 | Comparison of short‐ versus long‐course prophylactic antibiotic treatment. Authors do not list dose of drug at time of first administration, nor do they indicate the time of administration (preoperative, cord clamp). The authors are not even clear about the identity of the drug which begins the prophylactic regimen. They state that it is a random study but provide no details of mechanism. (D'Angelo 1980). |
| De Palma 1980 | At the start of the study 2 arms: 1 a no treatment arm, the other composed of women given either cefamandole or penicillin plus gentamicin. It would have been possible to try and dissect important information from the study except that they changed the antibiotic regimen after treating 57/105 women in the cefamandole subgroup. A co‐intervention (addition of chloramphenicol) was also applied to 3/105 women in the cefamandole subgroup and 4/104 women in the penicillin/gentamicin arm. (De Palma 1980). |
| De Palma 1982 | Timing of delivery of antibiotics for prophylaxis not specified. Authors state antibiotics given within 90 minutes of delivery with no indication as to whether these might have been given pre‐, post‐ or intra‐operatively. Mechanism of randomisation clearly inadequate. (De Palma 1982). |
| Digumarthi 2008 | Quasi‐RCT, allocated women to groups alternatively. (Digumarthi 2008). |
| Ding 2000 | Study compared 2 different cephalosporins from 1st and 2nd generations. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Ding 2000). |
| Donnenfeld 1986 | Study compared a cephalosporin (cefazolin) by 2 different routes of administration, IV and lavage. (Donnenfeld 1986). |
| Duff 1987 | Study compared 2 different cephalosporins, cefazolin versus cefonicid; 1 g after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Duff 1987). |
| El Aish 2018 | Study compared a single cephalosporin (cefazolin C1) versus a cephalosporin combination (cefazolin C1 plus azithromycin plus nitroimadazole). Study should be considered for inclusion in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (El Aish 2018). |
| Elliot 1982 | Study compared a penicillin, ampicillin, given in differing multiple doses. Study will be considered in review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. (Elliot 1982). |
| Elliot 1986 | Study compared a cephalosporin, cefoxitin, given in different ways, IV or lavage, or a combination of IV plus lavage. (Elliot 1986). |
| Fejgin 1993 | This study compared 2 different cephalosporins from 2nd and 3rd generations. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Fejgin 1993). |
| Flaherty 1983 | Comparison of pharmacokinetics of cefoxitin when administered by intravenous versus intraperitoneal lavage. Outcome variable of interest: concentration of drug in decidua. No outcomes of interest in our review are listed or were collected (i.e. febrile morbidity, endometritis, etc). (Flaherty 1983). |
| Fugere 1983 | Study compared 2 different cephalosporins, cefoxitin (2 g) versus cefazolin (1 g). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Fugere 1983). |
| Galask 1988 | Study compared 2 different cephalosporins, cefotetan (2 g, single dose, IV dose) versus cefoxitin (6 g, multiple doses (2 g each dose), IV); after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Galask 1988). |
| Galask 1989 | Study compared 2 different 2nd generation cephalosporins. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Galask 1989). |
| Gall 1987 | Study compared a penicillin, piperacillin (4 g, IV, after cord clamped) single dose versus multiple doses. Study will be considered in review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. (Gall 1987). |
| Gideon 2016 | Comparing the same antibiotics but single vs multiple doses. (Gideon 2016). |
| Gonen 1986 | Study compared a cephalosporin, cefamandole, by 2 different routes of administration, lavage and multiple doses IV. (Gonen 1986). |
| Gonik 1985 | Study compared a cephalosporin, cefotaxime (IV, after cord clamped) single dose (1 g) versus multiple doses (3 x 1 g). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Gonik 1985). |
| Gonik 1994 | Study compared 2 different 2nd generation cephalosporins. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Gonik 1994). |
| Gordon 1982 | Study compared 2 different cephalosporins from 2nd and 3rd generations. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Gordon 1982). |
| Grujic 2009 | Study not randomised, authors report that women were allocated to groups according to the type of antibiotics prophylaxis administered as a single dose. (Grujic 2009). |
| Gul 1999 | Study compared different timings of the antibiotic prophylaxis (before versus after cord clamping). (Gul 1999). |
| Hager 1991 | Study compared 3 cephalosporins, cefazolin (1 g) versus cefoxitin (2 g) versus cefotaxime (1 g); IV; after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Hager 1991). |
| Hartert 1987 | Study compared 2 cephalosporins, single dose cefonicid (1 g) versus multiple doses cefoxitin (2 g each); IV; after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Hartert 1987). |
| Hawrylyshyn 1983 | Study compared a cephalosporin, cefoxitin, single dose (2 g) versus multiple dosed (2 g each); IV; after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Hawrylyshyn 1983). |
| Ijarotimi 2013 | Comparing the same antibiotics but using different time scales (24 hours vs 48 hours + oral for 5 days). Study may be considered for possible inclusion in another review. (Ijarotimi 2013). |
| Itskovitz 1979 | Quasi‐RCT. Women were assigned to each of the 2 wings of the department according to the day of their admission. (Itskovitz 1979). |
| Jakobi 1988 | Study compared a cephalosporin, cefazolin, single dose (1 g) versus multiple doses (2 g each); IV; after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Jakobi 1988). |
| Jalai 2019 | This study compared Cefazolin (C1) + azithromycin (M) versus the same Cefazolin (C1). It will be considered in the review 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. Principle investigator, Dr Zeinab Jalai, Shahid Sayyad Shirazi Hospital, Gorgan, Golestan, Iran. IRCT20190609043847N1 2019. |
| Jayawardena 2019 | This study compared Cefazolin (C1) + azithromycin (M) versus the same Cefazolin (C1). It should be considered in the review 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. Principle investigator: Dr Ishani Jayawardena, Australia. ACTRN12619000018112 2019. |
| Kreutner 1979 | Study compared 2 cephalosporins, cephalothin versus cefamandole; 1 g; IV at differing times of administration. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Kreutner 1979). |
| Lavery 1986 | Study compared penicillin (mezlocillin) by differing routes of administration and single versus multiple doses. Study will be considered in review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. (Lavery 1986). |
| Leonetti 1989 | Study compared penicillin (piperacillin, IV after cord clamping) single (4 g) versus multiple doses (4 g each). Study will be considered in review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. (Leonetti 1989). |
| Leveno 1984 | Study compared a cephalosporin (cefamandole 2 g) by 2 routes of administration, lavage versus IV. (Leveno 1984). |
| Levin 1983 | Study compared 2 different cephalosporins, cefoxitin versus cephapirin (2 g/L by irrigation). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Levin 1983). |
| Luttkus 1997 | Compares different doses of the same cephalosporin. Study will be considered in the review on ‘Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section (0732)’. (Luttkus 1997). |
| Lyimo 2013 | Compares different doses of the same combination of antibiotics. Study may be considered for possible inclusion in another review. (Lyimo 2013). |
| Macones 2008 | Study compared different timings of giving the prophylactic antibiotic. (Macones 2008). |
| Maggioni 1998 | Study compared 2 different B‐lactams (F). (Maggioni 1998). |
| Major 1999 | Study compared 2 different cephalosporins from 1st and 2nd generations. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Major 1999). |
| Mansani 1984 | Study on antibiotics for women undergoing hysterectomy. (Mansani 1984). |
| Masse 1988 | Study compared a cephalosporin, cefoxitin (2 g IV after cord clamped) single dose versus multiple doses. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Masse 1988). |
| Mathelier 1992 | Study compared a cephalosporin, cefazolin, by different routes of administration, '2 g IV after cord clamped and saline irrigation of abdomen' versus '1 g IV after cord clamped and 1 g in 500 mL normal saline by irrigation'. (Mathelier 1992). |
| McGregor 1986 | Study compared 2 cephalosporins, cefotetan (2 g IV after cord clamped) versus cefoxitin (2 g IV and 2 further doses at 4 and 8 hours postoperatively). (McGregor 1986). |
| McGregor 1988 | Study compared 2 cephalosporins, cefotetan (2 g IV after cord clamped) versus cefoxitin (2 g IV and 2 further doses at 4 and 8 hours postoperatively). (McGregor 1988). |
| Meyer 2000 | This study compared a cephalosporin versus the same cephalosporin plus another antibiotic. More specifically, cefazolin versus cefalozin‐metronidazole. So this study compared C1 versus C1 + N and will be considered in the review 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Meyer 2000). |
| Meyer 2003 | Study compared 2 different 1st generation cephalosporins 1 in combination with metronidazole versus the antibiotic alone. (Meyer 2003). |
| Mihailovic 1989 | This study includes women having a hysterectomy or a caesarean. Randomisation was for the whole group with no suggestion of stratification. Hence, women having a caesarean are not randomised to intervention and comparator groups. (Mihailovic 1989). |
| Mokhtar 2019 | This study compared Cefazolin (C1) + azithromycin (M) versus the same Cefazolin (C1). It will be considered in the review 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. Principle investigator: Reda Mokhtar, Egypt. NCT04062175 2019. |
| Neuman 1990 | Study comparing penicillin G (10 million units IV after cord clamped) plus tetracycline (250 mg IM after cord clamped) versus ampicillin (2 g) plus tetracycline (1.5 g per day, to complete 3 days). Study will be considered in review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. (Neuman 1990). |
| O'Leary 1986 | Study compared a penicillin (ampicillin 2 g IV intraoperatively and 7 further doses) versus the same penicillin regime plus another antibiotic (gentamicin 2 g IV after cord clamping and 6 further doses). So the study compared P2 versus P2 + A and will be considered in the review 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. (O'Leary 1986). |
| Opoku 2007 | Study compared a regimen of multiple classes of antibiotic including a penicillin (ampicillin P2+ 1.0 g plus gentamycin A 80 mg plus metronidazole N 500 mg; multiple doses) versus a different regimen of multiple classes including a penicillin (co‐amoxyclav, amoxicillin plus clavulanic acid P2+ 1.2 g; multiple doses). This study will be considered in the review 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. (Opoku 2007). |
| Ovalle 1996 | Quasi‐RCT as quote: “Patients were distributed strictly by order of admission in five groups”. (Ovalle 1996). |
| Parsons 1985 | Study compared 2 cephalosporins, cefonicid (1 g IV after cord clamped) versus cefoxitin (2 g IV after cord clamped and 4 additional doses). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Parsons 1985). |
| Patacchiola 2000 | Study compared 2 3rd generation penicillins at differing doses. Study will be considered in the review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. (Patacchiola 2000). |
| Periti 1988 | Study compared 2 different cephalosporins from 1st and 2nd generations. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Periti 1988). |
| Peterson 1990 | Study compared 2 different cephalosporins by 2 different routes of administration. Cefazolin (2 g IV after cord clamped) versus cefamandole (2 g IV after cord clamped) versus cefazolin (2 g in 1 L in normal saline by lavage) versus cefamandole (2 g in 1 L normal saline by lavage). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Peterson 1990). |
| Pevzner 2009 | Compares giving prophylactic antibiotics before or after cord clamping. Study will be considered for review on 'Timing of prophylactic antibiotics for preventing infectious morbidity in women undergoing caesarean section'. (Pevzner 2009). |
| Prasuna 2011 | Quasi‐RCT, allocated women to groups alternately. (Prasuna 2011). |
| Puri 1991 | Quasi‐RCT, allocated women to groups alternately. (Puri 1991). |
| Rayburn 1985 | Study compared a 1st and 3rd generation cephalosporins. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Rayburn 1985). |
| Rijhsinghani 1995 | This study compares a single penicillin with a penicillin combination so should be considered for review ‘Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section’. (Rijhsinghani 1995). |
| Rodriguez 1990 | Study compared different timings of giving prophylactic antibiotics. (Rodriguez 1990). |
| Roex 1987 | Study compared a cephalosporin, cefoxitin (2 g IV after cord clamped) single dose versus multiple doses. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Roex 1987). |
| Roy 2003 | Study looked at women with acute pelvic infections. (Roy 2003). |
| Saravolatz 1985 | Study compared cephalosporin (ceforanide) by 2 routes of administration, 2 g IV after cord clamped versus 2 g in 1 L normal saline by irrigation. (Saravolatz 1985). |
| Scarpignato 1982 | Study compared a cephalosporin (cefuroxime) by different length of administration, 750 mg IM 30 to 60 minutes preoperatively and again postoperatively at 8 and 16 hours versus 750 mg IM to complete 5 days of therapy, first dose postoperatively after return of woman to the ward. (Scarpignato 1982). |
| Seton 1996 | Study looked at different timings of giving 3rd generation cephalosporins. (Seton 1996). |
| Shakya 2010 | Study is unclear how women were allocated to groups and also compared single dose (Cefazolin + Metronidazole) vs multiple doses antibiotics. (Shakya 2010). |
| Sivasankari 2015 | This study compared Cefazolin (C1) + azithromycin (M) versus the same Cefazolin (C1). It should be considered in the review 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. Principle investigator, Dr Sivasankari and Dr Ruby Jose, Tamil Nadu, India. CTRI/2015/10/006329 2015 |
| Stiver 1983 | Study compared a cephalosporin (cefoxitin) at different doses, 1 g IV after cord clamped versus 2 g IV after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Stiver 1983). |
| Stiver 1984 | Study compared 2 different cephalosporins from 1st and 2nd generations. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Stiver 1984). |
| Sullivan 2006 | Study looked at different timings of giving 1st generation cephalosporins for prophylaxis. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Sullivan 2006). |
| Sullivan 2007 | Study looked at different timings of giving 1st generation cephalosporins for prophylaxis. (Sullivan 2007). |
| Tassi 1987 | Study compared a cephalosporin (ceftazidime) by single (2 g IM 1 hour preoperative) versus multiple doses (2 g IM 1 hour preoperative plus 2 additional doses). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Tassi 1987). |
| Teansutikul 1993 | Study compared different doses of ampicillins. Study will be considered in the review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. (Teansutikul 1993). |
| Thigpen 2005 | Study looked at different timings of giving 1st generation cephalosporins for prophylaxis. (Thigpen 2005). |
| Tita 2016 | Trial compared azithromycin vs placebo. All women in both groups also received local standard care of either cefazolin, clindamycin, or clindamycin plus gentamicin, but the specific drug that women received as standard care was not reported. |
| van Beekhuizen 2008 | Study looked at single dose of ampicillin plus metronidazole versus multiple doses in low‐income setting. (van Beekhuizen 2008). |
| van Velzen 2009 | Study compared a single dose of ampicillin + metronidazole versus multiple doses. (van Velzen 2009). |
| Varner 1986 | Study compared a cephalosporin (cefotetan) by single (2 g IV after cord clamping) versus multiple doses (2 g IV after cord clamping plus 2 additional doses). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Varner 1986). |
| Vathana 2018 | Study compares single dose of cefuroxime (C2) and metronidazole versus multiple doses of cefuroxime (C2) and metronidazole. It should be considered for the review of ‘Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section’. Principle Investigators: Dr M Vathana and Dr K Muhumthan, Sri Lanka. (Vathana 2018). |
| von Mandach 1993 | Study compared 2 cephalosporins, ceftriaxone 1 g IV after cord clamped versus cefoxitin 1 g IV after cord clamped and 2 additional doses at 8 and 16 hours after first dose. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (von Mandach 1993). |
| Wagner 2006 | Study compared 2 different 3rd generation cephalosporins. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Wagner 2006). |
| Wajsfeld 2019 | This study compares cefoxitin (C2) + azithromycin (M) versus the same cefoxitin (C2). It should be considered in the review 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. Principle Investigator: Dr Tali Wajsfeld, New Jersey, USA. (Wajsfeld 2019). |
| Warnecke 1982 | Study compares prophylactic antibiotics with what we presume is 'no treatment' as the English summary only refers to the ‘control group’. Study will be considered for review on 'Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section'. (Warnecke 1982). |
| Watts 1991 | Study looked at upper genital isolates at birth as predictors of infection. (Watts 1991). |
| Wax 1997 | Study looked at different timings of cephalosporin administration (before and after cord clamping). (Wax 1997). |
| Westen 2015 | This study compares the same drugs but single vs multiple doses. It belongs in another review. Our review compares different drug classes. (Westen 2015). |
| Wu 1991 | Study compared a penicillin versus the same penicillin plus another antibiotic. More specifically, ampicillin versus ampicillin‐gentamicin. So the study compared A4 versus A4 + G and will be considered in the review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. (Wu 1991). |
| Xu 1997 | Study looked at cephalosporins versus penicillins but the penicillins were sometimes given with other classes of antibiotics and the data could not be separated. (Xu 1997). |
| Yildirim 2009 | Study compares administration of antibiotics before and after cord clamping. Study will be considered for review on ‘Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. (Yildirim 2009). |
| Zutshi 2008 | Compared different dosage regimens of the same antibiotic, but the specific antibiotic is not given in this conference abstract. (Zutshi 2008). |
IM: intramuscular IV: intravenous RCT: randomised controlled trial vs: versus.
Characteristics of ongoing studies [ordered by study ID]
Abdalmageed 2019.
| Study name | Single dose cefepime versus cefuroxime plus metronidazole as a prophylactic antibiotic during emergency intrapartum cesarean section |
| Methods | RCT |
| Participants | Women having intrapartum (emergency) caesarean section |
| Interventions | Cefepime (C4) versus cefuroxime (C2) + metronidazole (N) |
| Outcomes | Surgical wound infection, etc. |
| Starting date | 1 July 2019 |
| Contact information | Contact: Abdalmageed Abdalmageed email: drosamast@yahoo.com.au and Osama Abdalmageed email: drosamast1981@gmail.com. |
| Notes | Setting: Assiut University, Assiut, Egypt, 71515. NCT04009772 |
Karamali 2013.
| Study name | Comparison of prophylactic administration of cefazolin, ampicillin and azithromycin in prevention of postpartum infections after cesarean |
| Methods | RCT |
| Participants | Women ≥ 28 weeks' gestation having non‐elective caesarean section |
| Interventions | Intervention 1: cefazolin (C1) Intervention 2: cefazolin (C1) + azithromycin (M) Intervention 3: ampicillin (P2) + azithromycin (M) |
| Outcomes | Postpartum infection etc |
| Starting date | October 2012 |
| Contact information | Dr. Maryam Karamali, email: dr.karamali@arakmu.ac.ir; drkaramali88@yahoo.com. |
| Notes | Setting: Arak, Emam Khomaini Street, Taleghani Hospital, Arak, Iran. IRCT2013080710511N2 2013 |
RCT: randomised controlled trial
Differences between protocol and review
From the original protocol, this review has been separated into three reviews as described in the updated protocol sections of this review and a further two reviews will provide information on this topic.
Different classes of antibiotics given to women routinely for preventing infection after caesarean section (this review)
Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section (vacant title)
Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section (vacant title)
Timing of intravenous prophylactic antibiotics for preventing postpartum infectious morbidity in women undergoing caesarean delivery (Mackeen 2014)
Routes of administration for antibiotic given to women routinely for preventing infection after caesarean section (Nabhan 2016)
Change in definition of caesarean subgroups in 2020 update
We intended to revise the definition of type of caesarean surgery by urgency in line with RCOG definitions, due to the fact that other infection control measures are compromised in the most urgent surgeries. However, there was inadequate information in the available trial reports to support this planned revision.
Removal of subgroup analyses in 2020 update We have deleted subgroup analyses by timing of administration because these issues are investigated in Mackeen 2014. We have also removed subgroup analyses by route of administration because this is investigated in Nabhan 2016. However, we have analysed trial administering antibiotics systemically separately from those using lavage/irrigation (see note below).
Separation of results from trials using lavage from those administering different classes of antibiotics systemically
For this update, we did not pool results from two trials that gave women antibiotics via lavage with results from trials administering them systemically (usually IV), because antibiotics penetrate tissues very differently in each case. The intravenous route allows the antibiotic to penetrate not only the endometrium, but also the skin, the fat tissues beneath it the muscles and the uterus as a whole, and also the urinary tract.
Revised analyses of cephalosporins and penicillins in 2020 update The main causative agents of caesarean section infection are skin colonizers, primarily gram‐positive cocci (particularly including Staphylococcus aureus and Streptococci); and vaginal colonizers, including anaerobes and, to a lesser extent, gram‐negative bacilli. In the previous update of this review, the main comparison was between cephalosporins and penicillins. For this update, we have not pooled the data for all penicillins or for all cephalosporins, because of important variations in spectra of action between different sub‐classes of both of these classes of drugs (including different generations of cephalosporins, types of penicillins, and co‐formulations of both classes). Where sub‐classes of these drugs are known to differ in their potential to act against agents that are the principle causes of infection at caesarean section, we have meta‐analysed the results of trials of different sub‐classes separately. Where sub‐classes of drugs are known to have similar potential action against these agents, we have pooled the results.
Revised main comparisons
In the previous update of this review, the main comparison was between cephalosporins and penicillins. We have revised our main comparisons to reflect trends in global practice, to include the following.
Cephalosporins C1 and C2 (1st and 2nd generation) versus lincosamides (especially clindamycin)
Cephalosporins C1 and C2 (1st and 2nd generation) versus lincosamides (especially clindamycin) plus aminoglycosides (especially gentamicin)
Cephalosporins C1 and C2 (1st and 2nd generation) versus penicillins P2+ (broad spectrum penicillins plus betalactamase inhibitors)
Revised outcomes We have added two further outcomes 'Post‐discharge infections ‐ to 30 days' and 'Maternal readmissions to hospital'. We have removed the outcome 'Development of antibacterial resistance', because bacterial resistance is unlikely to be detected by randomised controlled trials (RCTs). Other types of trial are more appropriate for investigating this outcome.
Search For the 2020 update, we added in an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
Contributions of authors
For the 2020 update, Gill Gyte (GG) and Myfanwy Williams (MW) undertook the data extraction, GG entered the data, and MW checked this. MW drafted the 'Summary of findings' table, and all assessments were checked and agreed with GG. MW and GG drafted all other text. Carolina Carvalho Ribeiro do Valle (CV) provided expert guidance on the structural revisions for this update, and helped to draft text explaining these revisions. CV also advised on the content of the background section and updated information about different types of antibiotics. GG, MW and CV reviewed and agreed all discussion sections and review conclusions.
Sources of support
Internal sources
The University of Liverpool, UK
External sources
-
World Health Organization (WHO) and the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Switzerland, Switzerland
This review is supported by funding to Cochrane Pregnancy and Childbirth (University of Liverpool)
Declarations of interest
Gillian ML Gyte: GG received royalties from John Wiley & Sons in respect of ‘A Cochrane Pocketbook – Pregnancy and Childbirth’ Hofmeyr GJ et al. 2008.
Myfanwy J Williams: is employed by the University of Liverpool as a Research Associate at Cochrane Pregnancy and Childbirth. Her role is supported by the World Health Organization.
Carolina Carvalho Ribeiro do Valle: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ahmed 2004 {published data only}
- Ahmed ET, Mirghani OA, Gerais AS, Adam I. Ceftriaxone versus ampicillin/cloxacillin as antibiotic prophylaxis in elective caesarean section. East Mediterranean Health Journal 2004;10(3):277-88. [PubMed] [Google Scholar]
Alekwe 2008 {published data only}
- Alekwe LO, Kuti O, Orji EO, Ogunniyi SO. Comparison of ceftriaxone versus triple drug regimen in the prevention of cesarean section infectious morbidities. Journal of Maternal-Fetal & Neonatal Medicine 2008;21(9):638-42. [DOI] [PubMed] [Google Scholar]
Benigno 1986 {published data only}
- Benigno B, Ford L, Lawrence W, Ledger W, Ling F, McNeeley S. A double-blind, controlled comparison of piperacillin and cefoxitin in the prevention of postoperative infection in patients undergoing cesarean section. Surgery, Gynecology and Obstetrics 1986;162(1):1-7. [PubMed] [Google Scholar]
Bracero 1997 {published data only}
- Bracero LA. Ampicillin/sulbactam versus cefotetan for the prevention of infection following cesarean delivery in high-risk patients: a randomized double-blind trial. Gynecologic and Obstetric Investigation 1997;44:21-5. [DOI] [PubMed] [Google Scholar]
Busowski 2000 {published data only}
- Busowski JD, Porter KB, Pendergraft S, O'Brien F, Vodra J. Antibiotic prophylaxis for cesarean delivery: a randomized trial of cefotetan, ampicillin-sulbactam and ciprofloxacin. Prenatal and Neonatal Medicine 2000;5:357-62. [Google Scholar]
Chantharojwong 1993 {published data only}
- Chantharojwong P. An efficacy study of ampicillin vs cefazolin prophylaxis in patients undergoing cesarean section. Journal of the Medical Association of Thailand 1993;76:165-70. [PubMed] [Google Scholar]
Dashow 1986 {published data only}
- Dashow E, Read J, Coleman F. Randomized comparison of five irrigation solutions at cesarean section. Obstetrics & Gynecology 1986;68(4):473-8. [PubMed] [Google Scholar]
De‐Lalla 1988 {published data only}
- De-Lalla F, Oliva GC, Fratoni A, Papadia LS, Mancuso S. Single-dose mezlocillin versus single-dose cefotetan for prophylaxis in cesarean section. In: 12th World Congress of Gynecology and Obstetrics; 1988 Oct 23-28; Rio de Janeiro, Brazil. 1988.
Deng 2007 {published data only}
- Deng DM, Huang Z, Yuan WJ, Ding LZ, Chen XY. Clinical study on coadministration of cefazolin sodium and metronidazole for infection prophylaxis in perioperative period of cesarean section. Pharmaceutical Care and Research 2007;7(4):278-80. [Google Scholar]
Faro 1990 {published data only}
- Faro S, Martens M, Hammill H, Riddle G, Tortolero G. Antibiotic prophylaxis: is there a difference? American Journal of Obstetrics and Gynecology 1990;162:900-9. [DOI] [PubMed] [Google Scholar]
Ford 1986 {published data only}
- Ford L. Cost of antibiotic prophylaxis in cesarean section. Drug Intelligence and Clinical Pharmacy 1986;20:592-3. [DOI] [PubMed] [Google Scholar]
Gidiri 2014 {published data only}
- Gidiri MF, Ziruma A. A randomised clinical trial evaluating prophylactic single-dose versus a prolonged week-long course of antibiotics for caesarean section at two teaching hospitals in Zimbabwe. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):12. [DOI] [PubMed] [Google Scholar]
- Gidiri MF, Ziruma A. A randomized clinical trial evaluating prophylactic single-dose vs prolonged course of antibiotics for caesarean section in a high HIV-prevalence setting. Journal of Obstetrics and Gynaecology 2014;34(2):160-4. [DOI] [PubMed] [Google Scholar]
Graham 1993 {published data only}
- Graham JM, Blanco JD, Oshiro BT, Magee KP, Monga M, Eriksen N. Single-dose ampicillin prophylaxis does not eradicate enterococcus from the lower genital tract. Obstetrics & Gynecology 1993;81:115-7. [PubMed] [Google Scholar]
Jyothi 2010 {published data only}
- Jyothi S, Vyas M, Pratap K, Asha K. Antibiotic prophylaxis for hysterectomy and cesarean section: Amoxicillin-clavulanic acid versus cefazolin. Journal of Obstetrics and Gynecology of India 2010;60(5):419-23. [Google Scholar]
Kamilya 2012 {published data only}
- Kamilya G, Seal SL, Mukherji J, Roy H, Bhattacharyya SK, Hazra A. A randomized controlled trial comparing two different antibiotic regimens for prophylaxis at cesarean section. Journal of Obstetrics and Gynecology of India 2012;62(1):35-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kayihura 2003 {published data only}
- Kayihura V, Osman NB, Bugalho A, Bergstrom S. Choice of antibiotics for infection prophylaxis in emergency cesarean sections in low-income countries: a cost-benefit study in Mozambique. Acta Obstetricia et Gynecologica Scandinavica 2003;82(7):636-41. [DOI] [PubMed] [Google Scholar]
Koppel 1992 {published data only}
- Koppel R, Kaehler D, Benz J. Effectiveness of single dose antibiotic prophylaxis in caesarean section: comparison between cefotaxim and amoxicillin plus clavulanic acid. Geburtshilfe und Frauenheilkunde 1992;52(2):113-6. [DOI] [PubMed] [Google Scholar]
Lehapa 1999 {published data only}
- Lehapa AM, Towobola OA, Mahapa DH. The comparative efficacy and cost benefit of ceftriaxone versus ampicillin as prophylaxis in patients undergoing caesarean section. In: Women's Health - into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3-6; Cape Town South Africa. RCOG, 1999:83.
Lewis 1990 {published data only}
- Lewis D, Otterson W, Dunnihoo D. Antibiotic prophylactic uterine lavage in cesarean section: a double-blind comparison of saline, ticarcillin, and cefoxitin irrigation in indigent patients. Southern Medical Journal 1990;83(3):274-6. [DOI] [PubMed] [Google Scholar]
Louie 1982 {published data only}
- Louie TJ, Binns B, Baskett T, Ross J, Koss J. Cefotaxime, cefazolin or ampicillin prophylaxis of febrile morbidity in emergency cesarean sections. Clinical Therapeutics 1982;5:83-96. [PubMed] [Google Scholar]
Lumbiganon 1994 {published data only}
- Lumbiganon P, Sirivatanapa P, Prasertchareonsook W, Kumlertkit S, Anansuwanchai J. A randomized controlled trial of augmentin vs cefazolin in emergency cesarean section. International Journal of Gynecology & Obstetrics 1994;46:77. [Google Scholar]
Mansueto 1989 {published data only}
- Mansueto G, Tomaselli F. Antibiotic prophylaxis in non-elective caesarean section with single dose imipenem versus multiple-dose cefotaxime [Profilassi antibiotica in pazieti sottoposte a taglio cesareo non di elezione con Imipenem in "single dose" versus cefotaxime in "multiple doses"]. European Review for Medical and Pharmacological Sciences 1989;11:65-8. [PubMed] [Google Scholar]
Mivumbi 2014 {published data only}
- Mivumbi V, Greenberg J, Rulisa S. Randomized trial of prophylactic ampicillin vs cefazolin for prevention of post cesarean delivery infectious morbidity in Rwanda. In: 1st FIGO African Regional Conference of Gynecology and Obstetrics; 2013 Oct 2-5; Addis Ababa, Ethiopia. 2013.
- Mivumbi VN, Little SE, Rulisa S, Greenberg JA. Prophylactic ampicillin versus cefazolin for the prevention of post-cesarean infectious morbidity in Rwanda. International Journal of Gynecology and Obstetrics 2014;124:244-7. [DOI] [PubMed] [Google Scholar]
- Mivumbi VN, Little SE, Rulisa S, Greenberg JA. Prophylactic ampicillin versus cefazolin for the prevention of post-cesarean infectious morbidity in Rwanda. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E284. [DOI] [PubMed] [Google Scholar]
Mothilal 2013 {published data only}
- Mothilal M, Thivya R, Anjalakshi C, Ramesh A, Damodharan N. Comparison of effectiveness of azithromycin and cefazolin in post caesarean section infection. International Journal of Pharmacy and Pharmaceutical Sciences 2013;5(Suppl 3):92-4. [Google Scholar]
Ng 1992 {published data only}
- Ng NK, Sivalingam N. The role of prophylactic antibiotics in caesarean section - a randomized trial. Medical Journal of Malaysia 1992;47(4):273-9. [PubMed] [Google Scholar]
Noyes 1998 {published data only}
- Noyes N, Berkeley AS, Freedman K, Ledger W. Incidence of postpartum endomyometritis following single-dose antibiotic prophylaxis with either ampicillin/sulbactam, cefazolin, or cefotetan in high-risk cesarean section patients. Infectious Diseases in Obstetrics and Gynecology 1998;6(5):220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parulekar 2001 {published data only}
- Parulekar P, Kumar S, Awasthi RT, Tarneja P. A single dose of cefotoxime - as a prophylaxis during caesarean section. Journal of Obstetrics and Gynecology of India 2001;51(5):118-21. [Google Scholar]
Rehu 1980 {published data only}
- Rehu M, Jahkola M. Prophylactic antibiotics in caesarean section: effect of a short preoperative course of benzyl penicillin or clindamycin plus gentamicin on postoperative infectious morbidity. Annals of Clinical Research 1980;12:45-8. [PubMed] [Google Scholar]
Rohan 2014 {published data only}
- Rohan LC, Hemapriya S, Kodithuwakku KA. Ampicillin and metronidazole versus cefuroxime and metronidazole as antimicrobial prophylaxis for antepartum caesarean section. Sri Lanka Journal of Obstetrics and Gynaecology 2014;36(Suppl 1):67-8, Abstract no: P 42. [Google Scholar]
Rosaschino 1988 {published data only}
- Rosaschino P, Palmerio G, Ziliani A, Gambarini A, Zambetti E, Bagolan M. Antibiotic prophylaxis in cesarean section: a controlled randomized study of mezlocillin vs. ceftriaxone [La profilassi antibiotica nel taglio cesareo: mezlocillina vs. cetriaxone. Studio controllato randomizzato]. Giornale Italiano di Ostetricia e Ginecologia 1988;10(8):587-8. [Google Scholar]
Rudge 2006 {published data only}
- Rudge M, Atallah AN, Peracoli JC, Tristao AD, Neto MM. Randomized controlled trial on prevention of postcesarean infection using penicillin and cephalothin in Brazil. Acta Obstetricia et Gynecologica Scandinavica 2006;85(8):945-8. [DOI] [PubMed] [Google Scholar]
Saltzman 1985 {published data only}
- Saltzman DH, Eron LJ, Toy C, Protomastro LJ, Sites JG. Ticarcillin plus clavulanic acid versus cefoxitin in the prophylaxis of infection after cesarean section. American Journal of Medicine 1985;79(Suppl 5B):172-3. [DOI] [PubMed] [Google Scholar]
Saltzman 1986 {published data only}
- Saltzman D, Eron L, Tuomala R, Protomastro L, Sites J. Single-dose antibiotic prophylaxis in high-risk patients undergoing cesarean section. A comparative trial. Journal of Reproductive Medicine 1986;31:709-12. [PubMed] [Google Scholar]
Shah 1998 {published data only}
- Shah S, Mazher Y, John IS. Single or triple dose piperacillin prophylaxis in elective cesarean section. International Journal of Gynecology & Obstetrics 1998;62(1):23-9. [DOI] [PubMed] [Google Scholar]
Spinnato 2000 {published data only}
- Spinnato JA, Youkilis B, Cook VD, Pietrantoni M, Clark AL, Gall SA. Antibiotic prophylaxis at cesarean delivery. Journal of Maternal-Fetal Medicine 2000;9:348-50. [DOI] [PubMed] [Google Scholar]
van der Linden 1993 {published data only}
- Der Linden MC, Van Erp EJ, Ruijs GJ, Holm JP. A prospective randomized study comparing amoxycillin/clavulanate with cefuroxime plus metronidazole for perioperative prophylaxis in gynaecological surgery. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;50:141-5. [DOI] [PubMed] [Google Scholar]
Voto 1986 {published data only}
- Voto LS, Benoliel LA, Amadora Muñiz A, Trepat A, Balsechi EE, Margulies M. Prophylaxis of post cesarean section puerperal infection with the using of cefoxitin antibiotics [Profilaxis de la infección puerperal post-cesarea mediante el uso del antibiótico cefoxitina: I - Resultados de su empleo valorados a través del cultivo sistemático pre, intra y postquirúrgico]. Obstetricia y Ginecologia Latino-Americanas 1986;44:419-24. [Google Scholar]
Wells 1994 {published data only}
- Wells M, McCullough W, Rymer J. Antibiotic prophylaxis in emergency cesarean section. International Journal of Gynecology and Obstetrics 1994;46:77. [Google Scholar]
Ziogos 2010 {published data only}
- Ziogos E, NCT01138852. Ampicillin/sulbactam versus cefuroxime as antimicrobial prophylaxis for cesarean section: a randomized study. clinicaltrials.gov/ct2/show/record/NCT01138852 (first received 7 June 2010).
- Ziogos E, Tsiodras S, Matalliotakis I, Giamarellou H, Kanellakopoulou K. Ampicillin/sulbactam versus cefuroxime as antimicrobial prophylaxis for cesarean delivery: a randomized study. BMC Infectious Diseases 2010;10:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Andrews 2003 {published data only}
- Andrews WW, Hauth JC, Cliver SP, Savage K, Goldenberg RL. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstetrics & Gynecology 2003;101:1183-9. [DOI] [PubMed] [Google Scholar]
Azizi 2014 {published data only}
- Azizi M, IRCT2014041017212N1. Comparison between azithromycin and cephalexin for preventing infection after cesarean section in obese patients. en.irct.ir/trial/15882 (first received 16 June 2016).
- Azizi M, Rajaei M, Abbasian M, Ghanbarnejad A Najafian A, Iranfar M. Comparison between azithromycin and cephalexin for preventing Infection after cesarean section in obese patient. International Journal of Clinical Medicine 2014;5:1214-20. [Google Scholar]
- IRCT2014041017212N1. Reduction of infection after caesarean section in obese women with antibiotic prophylaxis [Comparison between azithromycin and cephalexin for preventing infection after cesarean section in obese patients]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014041017212N1 2014. [CENTRAL: CN-01835673]
Baheraie 1997 {published data only}
- Baheraie A, Modares M, Azimi KH, Mahmodi M. Single-dose cefazolin prophylaxis for cesarean section. Acta Obstetricia et Gynecologica Scandinavica 1997;76:28. [Google Scholar]
Beksac 1989 {published data only}
- Beksac MS, Urman CB, Akalin E, Baykal M. A randomized comparative study of a single-dose 3rd generation cephalosporin (ceftriaxone) and a broad spectrum ureidopenicillin (mezlocillin) in a controlled group trial for cesarean section prophylaxis. International Journal of Experimental and Clinical Chemotherapy 1989;2(1):55-7. [Google Scholar]
Berkeley 1990 {published data only}
- Berkeley A, Hirsch J, Freedman K, Ledger W. Cefotaxime for cesarean section prophylaxis in labor; intravenous administration vs lavage. Journal of Reproductive Medicine 1990;35(3):214-8. [PubMed] [Google Scholar]
Bernstein 1994 {published data only}
- Bernstein P, Novosel S, Collins JA, Jarrell JF, Joly J, Moutquin JM. Cefotetan versus cefoxitin in cesarean section prophylaxis. Annals of the Royal College of Physicians and Surgeons of Canada 1994;27(7):401-4. [Google Scholar]
Bilgin 1998 {published data only}
- Bilgin T, Ozan H, Dirgen A, Esmer A. Comparison of four different antibiotics as prophylaxis in caesarean section. Journal of Obstetrics and Gynaecology 1998;18(6):546-7. [DOI] [PubMed] [Google Scholar]
Boothby 1984 {published data only}
- Boothby R, Benrubi G, Ferrell E. Comparison of intravenous cefoxitin prophylaxis with intraoperative cefoxitin irrigation for the prevention of post-cesarean-section endometritis. Journal of Reproductive Medicine 1984;29(11):830-2. [PubMed] [Google Scholar]
Carlson 1990 {published data only}
- Carlson C, Duff P. Antibiotic prophylaxis for cesarean delivery: is an extended-spectrum agent necessary? Obstetrics & Gynecology 1990;76(3):343-6. [PubMed] [Google Scholar]
Chamberlain 1993 {published data only}
- Chamberlain A, White S, Bawdon R, Thomas S, Larsen B. Pharmacokinetics of ampicillin and sulbactam in pregnancy. American Journal of Obstetrics and Gynecology 1993;168:667-73. [DOI] [PubMed] [Google Scholar]
Chittacharoen 1998 {published data only}
- Chittacharoen A, Manonai J, Suthutvoravut S, Phaupradit W. Single-dose amoxycillin-clavulanic acid vs ampicillin prophylaxis in emergency cesarean section. International Journal of Gynecology & Obstetrics 1998;62:249-54. [Google Scholar]
Conover 1984 {published data only}
- Conover W, Moore T. Comparison of irrigation and intravenous antibiotic prophylaxis at cesarean section. Obstetrics & Gynecology 1984;63(6):787-91. [PubMed] [Google Scholar]
- Conover WB, Moore TR. Comparison of irrigation and intravenous antibiotic prophylaxis at cesarean section. Obstetrical and Gynecological Survey 1984;39:692-3. [PubMed] [Google Scholar]
Crombleholme 1987 {published data only}
- Crombleholme W, Green J, Ohm-Smith M, Dahrouge D, DeKay V, Rideout A, et al. Cesarean section prophylaxis: comparison of two doses with three doses of mezlocillin. American Journal of Reproductive Immunology 1987;13:71-5. [DOI] [PubMed] [Google Scholar]
Crombleholme 1989 {published data only}
- Crombleholme WR, Green JR, Ohm-Smith M, DeKay V, Klaisle C, Luft J, et al. Prophylaxis in caesarean section with cefmetazole and cefoxitin. Journal of Antimicrobial Chemotherapy 1989;23:97-104. [DOI] [PubMed] [Google Scholar]
Cunningham 1983 {published data only}
- Cunningham FG, Leveno KJ, De Palma RT, Roark ML, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping? Obstetrics & Gynecology 1983;62:151-4. [PubMed] [Google Scholar]
D'Angelo 1980 {published data only}
- D'Angelo L, Sokol R. Short- versus long-course prophylactic antibiotic treatment in cesarean section patients. Obstetrics & Gynecology 1980;55(5):583-6. [PubMed] [Google Scholar]
De Palma 1980 {published data only}
- De Palma R, Leveno K, Cunningham FG, Pope T, Kappus S, Roark M, et al. Identification and management of women at high risk for pelvic infection following cesarean section. Obstetrics & Gynecology 1980;55(5):185S-191S. [DOI] [PubMed] [Google Scholar]
De Palma 1982 {published data only}
- De Palma R, Cunningham G, Leveno K, Roark M. Continuing investigation of women at high risk for infection following cesarean delivery. Obstetrics & Gynecology 1982;60(1):53-9. [PubMed] [Google Scholar]
Digumarthi 2008 {published data only}
- Digumarthi L, Gupta S. A single dose of co-amoxiclav vs. five-day antibiotics in elective and emergency caesarean section. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):159. [Google Scholar]
Ding 2000 {published data only}
- Ding H, Wang Y, Liu X. Effects of elective cesarean section and antibiotics to the bacterial flora in female genital tract. Chung-Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2000;35(6):342-4. [PubMed] [Google Scholar]
Donnenfeld 1986 {published data only}
- Donnenfeld A, Otis C, Weiner S. Antibiotic prophylaxis in cesarean section. Comparison of intrauterine lavage and intravenous administration. Journal of Reproductive Medicine 1986;31(1):15-8. [PubMed] [Google Scholar]
Duff 1987 {published data only}
- Duff P, Robertson A, Read J. Single-dose cefazolin versus cefonicid for antibiotic prophylaxis in cesarean delivery. Obstetrics & Gynecology 1987;70(5):718-21. [PubMed] [Google Scholar]
El Aish 2018 {published data only}
- El Aish KA, Zourob H, Madi W, El Hams S. Cefazolin alone versus cefazolin, gentamicin, and metronidazole for prophylaxis in women undergoing caesarean section: a randomised controlled trial. Lancet 2018;391:15. [Google Scholar]
Elliot 1982 {published data only}
- Elliott J, Freeman R, Dorchester W. Short versus long course of prophylactic antibiotics in cesarean section. American Journal of Obstetrics and Gynecology 1982;143:740-4. [DOI] [PubMed] [Google Scholar]
Elliot 1986 {published data only}
- Elliott J, Flaherty J. Comparison of lavage or intravenous antibiotics at cesarean section. Obstetrics & Gynecology 1986;67(1):29-32. [PubMed] [Google Scholar]
Fejgin 1993 {published data only}
- Fejgin MD, Markov S, Goshen S, Segal J, Arbel Y, Lang R. Antibiotic for cesarean section: the case for 'true' prophylaxis. International Journal of Gynecology & Obstetrics 1993;43:257-61. [DOI] [PubMed] [Google Scholar]
Flaherty 1983 {published data only}
- Flaherty J, Boswell G, Winkel C, Elliott J. Pharmacokinetics of cefoxitin in patients at term gestation; lavage versus intravenous administration. American Journal of Obstetrics and Gynecology 1983;146:760-6. [DOI] [PubMed] [Google Scholar]
Fugere 1983 {published data only}
- Fugere P, Turgeon P, Boucher M, Verscheiden G, Lemay M. Utilisation des cephalosporines comme antibioprophylaxie lors de cesariennes. Canadian Medical Association Journal 1983;129:132-5. [PMC free article] [PubMed] [Google Scholar]
Galask 1988 {published data only}
- Galask R, Benigno B, Cunningham G, Elliott J, Makowski E, McGregor J, et al. Results of a multicenter comparative study of single-dose cefotetan and multiple-dose cefoxitin as prophylaxis in patients undergoing cesarean section. American Journal of Surgery 1988;155:86-90. [DOI] [PubMed] [Google Scholar]
Galask 1989 {published data only}
- Galask RP, Weiner C, Petzold CR. Comparison of single-dose cefmetazole and cefotetan prophylaxis in women undergoing primary caesarean section. Journal of Antimicrobial Chemotherapy 1989;23:105-8. [DOI] [PubMed] [Google Scholar]
Gall 1987 {published data only}
- Gall S, Hill G. Single-dose versus multiple-dose piperacillin prophylaxis in primary cesarean operation. American Journal of Obstetrics and Gynecology 1987;157:502-6. [DOI] [PubMed] [Google Scholar]
Gideon 2016 {published data only}
- Gideon MA, NCT02736682. Single dose ceftriaxone and metronidazole versus multiple doses for antibiotic prophylaxis at elective cesarean section in mulago hospital. a randomized clinical trial. clinicaltrials.gov/show/NCT02736682 (first received 13 April 2016).
Gonen 1986 {published data only}
- Gonen R, Samberg I, Levinski R, Levitan Z, Sharf M. Effect of irrigation or intravenous antibiotic prophylaxis on infectious morbidity at cesarean section. Obstetrics & Gynecology 1986;67:545-8. [PubMed] [Google Scholar]
Gonik 1985 {published data only}
- Gonik B. Single- versus three-dose cefotaxime prophylaxis for cesarean section. Obstetrics & Gynecology 1985;65:189-93. [PubMed] [Google Scholar]
Gonik 1994 {published data only}
- Gonik B, Mcgregor J. Comparison of short vs. long half-life single-dose prophylactic antibiotics for cesarean section. Infectious Diseases in Obstetrics & Gynecology 1994;2:120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 1982 {published data only}
- Gordon SF, Russell J. A randomized controlled study comparing ceftizoxime, cefamandole, and cefoxitin in obstetric and gynaecological surgery: a preliminary report. Journal of Antimicrobial Chemotherapy 1982;10 Suppl C:289-92. [DOI] [PubMed] [Google Scholar]
Grujic 2009 {published data only}
- Grujic Z, Bogavac M, Ilija G, Nikolic A. Is this necessary to give antibiotic prophylaxis in elective caesarean sections? Journal of Maternal-Fetal and Neonatal Medicine 2010;23(S1):230. [Google Scholar]
- Grujic Z, Sabo A, Grujic I, Kopitovic V, Papovic M. Single dose of antibiotic prophylaxis in elective cesarean sections. Medicinski Pregled 2009;62(3-4):101-6. [DOI] [PubMed] [Google Scholar]
Gul 1999 {published data only}
- Gul A, Zeteroilu I, Surucu R. The comparison of piperacillin use for prophylaxis of post cesarean section infection before and after clamping umbilical cord [abstract]. Infectious Diseases in Obstetrics & Gynecology 1999;7(6):306. [Google Scholar]
Hager 1991 {published data only}
- Hager W, Rapp R, Billeter M, Bradley B. Choice of antibiotic in nonelective cesarean section. Antimicrobial Agents and Chemotherapy 1991;35(9):1782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartert 1987 {published data only}
- Hartert R, Benrubi G, Thompson R, Nuss R. Cefonicid vs. cefoxitin for cesarean section prophylaxis. Journal of Reproductive Medicine 1987;32(12):907-10. [PubMed] [Google Scholar]
Hawrylyshyn 1983 {published data only}
- Hawrylyshyn P, Bernstein P, Papsin F. Short-term antibiotic prophylaxis in high-risk patients following cesarean section. American Journal of Obstetrics and Gynecology 1983;145:285-9. [DOI] [PubMed] [Google Scholar]
Ijarotimi 2013 {published data only}
- Ijarotimi AO, Badejoko OO, Ijarotimi O, Loto OM, Orji EO, Fasubaa OB. Comparison of short versus long term antibiotic prophylaxis in elective caesarean section at the Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria. Nigerian Postgraduate Medical Journal 2013;20(4):325-30. [PubMed] [Google Scholar]
- Ijarotimi OA, Badejoko OO, Ijarotimi OO, Orji EO, Loto OM, Fasubaa OB. Comparison of short versus long term antibiotic prophylaxis in elective caesarean section-the ile-ife experience. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S375. [Google Scholar]
Itskovitz 1979 {published data only}
- Itskovitz J, Paldi E, Katz M. The effect of prophylactic antibiotics on febrile morbidity following cesarean section. Obstetrics & Gynecology 1979;53(2):162-5. [PubMed] [Google Scholar]
Jakobi 1988 {published data only}
- Jakobi P, Weissman A, Zimmer E, Paldi E. Single-dose cefazolin prophylaxis for cesarean section. American Journal of Obstetrics and Gynecology 1988;158:1049-52. [DOI] [PubMed] [Google Scholar]
Jalai 2019 {published data only}
- IRCT20190609043847N1. Prevention of post-cesarean infection [Comparison of cefazolin and cefazolin plus azithromycin regimens in prevention of infection in caesarean section in Shahid Sayyad Shirazi hospital, 2017-2019]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20190609043847N1 2019. [CENTRAL: CN-01973986]
Jayawardena 2019 {published data only}
- ACTRN12619000018112. The Impact of Azithromycin on Surgical Site Infection Rates [The Impact of Azithromycin on Surgical Site Infection Rates (TIASSIR) in pregnant women undergoing an emergency caesarean section at Redland Hospital]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12619000018112 2019. [CENTRAL: CN-01948712]
Kreutner 1979 {published data only}
- Kreutner K, Del Bene V, DeLamar D, Bodden J, Loadholt C. Perioperative cephalosporin prophylaxis in cesarean section: effect on endometritis in the high-risk patient. American Journal of Obstetrics and Gynecology 1979;134:925-33. [DOI] [PubMed] [Google Scholar]
Lavery 1986 {published data only}
- Lavery J, Huang K, Koontz W, Reinstine J, Marcell C, Rosenberg N. Mezlocillin prophylaxis against infection after cesarean section: a comparison of techniques. Southern Medical Journal 1986;79(10):1248-351. [DOI] [PubMed] [Google Scholar]
Leonetti 1989 {published data only}
- Leonetti H, Yun H, O'Leary J, Greenberg A. Single versus multiple dose piperacillin in high risk primary cesarean section. American Journal of Gynecologic Health 1989;3(6):29-32. [Google Scholar]
Leveno 1984 {published data only}
- Leveno K, Quirk J, Cunningham F, Nelson S, Bawdon R. Perioperative antimicrobials at cesarean section: lavage versus three intravenous doses. American Journal of Obstetrics and Gynecology 1984;149:463-4. [DOI] [PubMed] [Google Scholar]
Levin 1983 {published data only}
- Levin DK, Gorchels C, Andersen R. Reduction of post-cesarean section infectious morbidity by means of antibiotic irrigation. American Journal of Obstetrics and Gynecology 1983;147(3):273-6. [DOI] [PubMed] [Google Scholar]
Luttkus 1997 {published data only}
- Luttkus A, Fiebelkorn J, Dudenhausen W. Antibiotic prophylaxis in cases of emergency caesarean section. Geburtshilfe und Frauenheilkunde 1997;57(9):510-4. [Google Scholar]
Lyimo 2013 {published data only}ISRCTN44462542
- Lyimo FM, Massinde AN, Kidenya BR, Konje E, Mshana SE. Efficacy of single dose of gentamicin in combination with metronidazole versus multiple doses for prevention of post-caesarean infection: study protocol for a randomized controlled trial. Trials 2012;13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyimo FM, Massinde AN, Kidenya BR, Konje ET, Mshana SE. Single dose of gentamicin in combination with metronidazole versus multiple doses for prevention of post-caesarean infection at Bugando Medical Centre in Mwanza, Tanzania: a randomized, equivalence, controlled trial. BMC Pregnancy and Childbirth 2013;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massinde A, ISRCTN44462542. Efficacy of single dose of gentamicin in combination with metronidazole versus multiple doses for prevention of post caesarean infection: a randomised controlled trial. isrctn.com/ISRCTN44462542 (first received 5 September 2011).
Macones 2008 {published data only}
- Macones G, Cleary K, Odibo A, Gross G, Parry S, Rampersad R, et al. Timing of antibiotic prophylaxis at cesarean - results of an RCT. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S33. [Google Scholar]
Maggioni 1998 {published data only}
- Maggioni P, Di Stefano F, Facchini V, Irato S, Mancuso S, Colombo M, et al. Treatment of obstetric and gynecologic infections with meropenem: comparison with imipenem/cilastatin. Journal of Chemotherapy 1998;10:114-21. [DOI] [PubMed] [Google Scholar]
Major 1999 {published data only}
- Major CA, Reimbold T, Morgan MA. Cephararin versus cefoxitin prophylaxis for cesarean sections. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S75. [Google Scholar]
Mansani 1984 {published data only}
- Mansani FE, Caltabiano M, Condemi V, Scarpignato C. Short- or long-term antibiotic prophylaxis in obstetrical and gynecological surgery? Acta Bio-Medica de l Ateneo Parmense 1984;55:147-51. [PubMed] [Google Scholar]
Masse 1988 {published data only}
- Masse A, Turgeon P, Gay N, Verschelden G. To compare the efficacy of antibiotic prophylaxis with cefoxitine using one or three doses at caesarean [Efficacite comparee de l'antibioprophylaxie par la cefoxitine en une ou trois doses dans la cesarienne]. Canadian Medical Association Journal 1988;138:921-4. [PMC free article] [PubMed] [Google Scholar]
Mathelier 1992 {published data only}
- Mathelier A. A comparison of postoperative morbidity following prophylactic antibiotic administration by combined irrigation and intravenous route or by intravenous route alone during cesarean section. Journal of Perinatal Medicine 1992;20:177-82. [DOI] [PubMed] [Google Scholar]
McGregor 1986 {published data only}
- McGregor J, French J, Makowski E. Single-dose cefotetan versus multidose cefoxitin for prophylaxis in cesarean section in high-risk patients. American Journal of Obstetrics and Gynecology 1986;154:955-60. [DOI] [PubMed] [Google Scholar]
McGregor 1988 {published data only}
- McGregor J, Gordon S, Krotec J, Poindexter A. Results of a randomized, multicenter, comparative trial of a single dose of cefotetan versus multiple doses of cefoxitin as prophylaxis in cesarean section. American Journal of Obstetrics and Gynecology 1988;158:701-6. [DOI] [PubMed] [Google Scholar]
Meyer 2000 {published data only}
- Meyer NL, Scott K, Hosier K, Sibai B. Cefazolin versus cefazolin/metronidazole for antibiotic prophylaxis at cesarean section [abstract]. Obstetrics & Gynecology 2000;95(4 Suppl):74S. [Google Scholar]
Meyer 2003 {published data only}
- Meyer NL, Hosier KV, Scott K, Lipscomb GH. Cefazolin versus cefazolin plus metronidazole for antibiotic prophylaxis at cesarean section. Southern Medical Journal 2003;96(10):992-5. [DOI] [PubMed] [Google Scholar]
Mihailovic 1989 {published data only}
- Mihailovic M, Hani A, Klainguti A, Soldini G. Antimicrobial prophylaxis in non-infected patients undergoing abdominal or vaginal hysterectomy or cesarean section. Comparative efficacy of a single preoperative dose of ceftriaxone and of multiple doses of combined amoxicillin plus metronidazole and of amoxicillin alone. Journal of Chemotherapy 1989;1(4 Suppl):1029-30. [PubMed] [Google Scholar]
Mokhtar 2019 {published data only}
- NCT04062175. Efficacy of adding azithromycin to cephalosporin before cesarean delivery [Efficacy of adding azithromycin to cephalosporin for the prophylaxis against infectious morbidity following cesarean delivery in high risk women]. Https://clinicaltrials.gov/show/nct04062175 (first received 20 August 2020). [CENTRAL: CN-01983582]
Neuman 1990 {published data only}
- Neuman M, Langer R, Bachar R, Golan A, Bukovsky I, Caspi E. Penicillin-tetracycline prophylaxis in cesarean delivery: prospective and randomized comparison of short and long term therapy. Journal of Perinatal Medicine 1990;18:145-8. [DOI] [PubMed] [Google Scholar]
O'Leary 1986 {published data only}
- O'Leary J, Mullins J, Andrinopoulos G. Ampicillin vs. ampicillin-gentamicin prophylaxis in high-risk primary cesarean section. Journal of Reproductive Medicine 1986;31(1):27-30. [PubMed] [Google Scholar]
Opoku 2007 {published data only}
- Opoku B. Prophylactic antibiotic during caesarean sections at Komfo Anokye Teaching Hospital, Kumasi. Ghana Medical Journal 2007;41(2):48-51. [PMC free article] [PubMed] [Google Scholar]
Ovalle 1996 {published data only}
- Ovalle SA, Robinovich Tannenbaum J, Leyton H, Nilo C, Nasra J, Aliaga J, et al. Antibiotic prophylaxis in the cesarean surgery [Profilaxis antibiótica en la operación cesárea]. Revista Chilena De Obstetricia y Ginecología 1996;61(4):243-9. [Google Scholar]
Parsons 1985 {published data only}
- Parsons M, Gibson R, Rimando-Ramos J, Spellacy W. Comparison of single-dose cefonicid and multiple-dose cefoxitin for surgical prophylaxis in women undergoing cesarean section. Advances in Therapy 1985;2(5):233-9. [Google Scholar]
Patacchiola 2000 {published data only}
- Patacchiola F, Di Paolantonio L, Palermo P, Di Stefano L, Mascaretti G, Moscarini M. Antibiotic prophylaxis of infective complications after cesarean section. Our experience [Profilassi antibiotica delle complicanze infettive dopo taglio cesareo. Nostra esperienza]. Minerva Ginecologica 2000;52(10):385-9. [PubMed] [Google Scholar]
Periti 1988 {published data only}
- Periti P, Mazzei T, Periti E. Prophylaxis in gynaecological and obstetric surgery: a comparative randomised multicentre study of single-dose cefotetan versus two doses of cefazolin. Chemioterapia 1988;7(4):245-52. [PubMed] [Google Scholar]
Peterson 1990 {published data only}
- Peterson CM, Medchill M, Gordon D, Chard H. Cesarean prophylaxis: a comparison of cefamandole and cefazolin by both intravenous and lavage routes, and risk factors associated with endometritis. Obstetrics & Gynecology 1990;75:179-82. [PubMed] [Google Scholar]
Pevzner 2009 {published data only}
- Pevzner L, Chan K. Optimal timing for antibiotic prophylaxis during elective cesarean: before or after cord clamping. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S225-S226. [Google Scholar]
Prasuna 2011 {published data only}
- Prasuna NJ. Comparative study between prophylactic and routine antibiotic in elective and emergency caesarean section, using coamoxyclav vs ampicillin and gentamicin respectively. In: 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5-9; Hyderabad, Andhra Pradesh, India. 2011:124.
Puri 1991 {published data only}
- Puri M, Chadda P, Nagrani R, Tandon G. Single dose cephexin prophylaxis for caesarean section. Journal of Obstetrics and Gynaecology of India 1991;41(5):643-6. [Google Scholar]
Rayburn 1985 {published data only}
- Rayburn W, Varner M, Galask R, Petzold CR, Piehl E. Comparison of moxalactam and cefazolin as prophylactic antibiotics during cesarean section. Antimicrobial Agents and Chemotherapy 1985;27:337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rijhsinghani 1995 {published data only}
- Rijhsinghani A, Savapoulos SE, Walters JK, Huggins G, Hibbs JR. Ampicillin/sulbactam vs ampicillin alone for cesarean section prophylaxis: a randomized double-blind trial. American Journal of Perinatology 1995;12:322-4. [DOI] [PubMed] [Google Scholar]
Rodriguez 1990 {published data only}
- Rodriguez GC, Gall SA, Parsons MT. Timing of prophylactic antibiotic administration at cesarean section. In: Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23-27; Houston, Texas, USA. 1990:311.
Roex 1987 {published data only}
- Roex AJ, Puyenbroek JI, Van Loenen AC, Arts NF. Single- versus three-dose cefoxitin prophylaxis in caesarean section: a randomized clinical trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 1987;25:293-8. [DOI] [PubMed] [Google Scholar]
Roy 2003 {published data only}
- Roy S, Higareda I, Angel-Muller E, Ismail M, Hague C, Adeyi B, et al. Ertapenem once a day versus piperacillin-tazobactam every 6 hours for treatment of acute pelvic infections: a prospective, multicenter, randomized, double-blind study. Infectious Diseases in Obstetrics and Gynecology 2003;11(1):27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saravolatz 1985 {published data only}
- Saravolatz L, Lee C, Drukker B. Comparison of intravenous administration with intrauterine irrigation with ceforanide for nonelective cesarean section. Obstetrics & Gynecology 1985;66(4):513-6. [PubMed] [Google Scholar]
Scarpignato 1982 {published data only}
- Scarpignato C, Caltabiano M, Condemi V, Mansani F. Short-term versus long-term cefuroxime prophylaxis in patients undergoing emergency cesarean section. Clinical Therapeutics 1982;5(2):186-2. [PubMed] [Google Scholar]
Seton 1996 {published data only}
- Seton DN, Vellema DM, Schoon MG, Grobler JM. Prophylactic antibiotics and caesarean section: comparing ceftriaxone administration pre- and post- umbilical cord clamping in terms of maternal and neonatal morbidity. In: 15th Conference on Priorities in Perinatal Care in Southern Africa; 1996 March 5-8; Goudini Spa, South Africa. 1996.
Shakya 2010 {published data only}
- Shakya A, Sharma J. Comparison of single versus multiple doses of antibiotic prophylaxis in reducing post-elective caesarean section infectious morbidity. Kathmandu University Medical Journal 2010;8(30):179-84. [DOI] [PubMed] [Google Scholar]
Sivasankari 2015 {published data only}
- CTRI/2015/10/006329. Additional injection to prevent infection after cesarean delivery [Randomized double blinded controlled trial to compare the efficacy of extended spectrum antibiotics with narrow spectrum antibiotic as prophylaxis in cesarean delivery for the prevention of post cesarean endometritis. - PACS]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2015/10/006329 (first received 2015). [CENTRAL: CN-01813607]
- Sivasankari, CTRI/2015/10/006329. Randomized double blinded controlled trial to compare the efficacy of extended spectrum antibiotics with narrow spectrum antibiotic as prophylaxis in cesarean delivery for the prevention of post cesarean endometritis. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=10952 (first received 29 October 2015).
Stiver 1983 {published data only}
- Stiver HG, Forward K, Livingstone R, Fugere P, LeMay M, Verschelden G, et al. Muliticenter comparison of cefoxitin versus cefazolin for prevention of infectious morbidity after nonelective cesarean section. Obstetrics & Gynecology 1983;143:158-63. [DOI] [PubMed] [Google Scholar]
Stiver 1984 {published data only}
- Stiver HG, Forward KR, Tyrrell DL, Livingstone RA, Fugere P, Lemay M, et al. Comparative cervical microflora shifts after cefoxitin or cefazolin prophylaxis against infection following cesarean section. American Journal of Obstetrics and Gynecology 1984;149:718-21. [DOI] [PubMed] [Google Scholar]
Sullivan 2006 {published data only}
- Sullivan S, Smith T, Chang E, Hulsey T, Van Dorsten J, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S17. [DOI] [PubMed] [Google Scholar]
Sullivan 2007 {published data only}
- Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized, controlled trial. American Journal of Obstetrics and Gynecology 2007;196(5):455.e1-455.e5. [DOI] [PubMed] [Google Scholar]
Tassi 1987 {published data only}
- Tassi P, Tarantini M, Cadenelli G, Gastaldi A, Benedetti M. Coftazidime in antibiotic prophylaxis for emergency cesarean section: a randomized prospective study. International Journal of Clinical Pharmacology, Therapy and Toxicology 1987;25(10):582-8. [PubMed] [Google Scholar]
Teansutikul 1993 {published data only}
- Teansutikul C. Comparative study of 2 gram single dose versus 1 gram three doses of ampicillin prophylaxis in high risk emergency cesarean section patients. Chon Buri Hospital Journal 1993;18(1):1-18. [Google Scholar]
Thigpen 2005 {published data only}
- Thigpen BD, Hood WA, Chauhan S, Bufkin L, Bofill J, Magann E, et al. Timing of prophylactic antibiotic administration in the uninfected laboring gravida: a randomized clinical trial. American Journal of Obstetrics and Gynecology 2005;192:1864-71. [DOI] [PubMed] [Google Scholar]
Tita 2016 {published data only}
- Ausbeck EB, Jauk VC, Boggess KA, Saade GR, Longo S, Clark EA, et al. Impact of azithromycin-based extended-spectrum antibiotic prophylaxis on noninfectious cesarean wound complications. American Journal of Perinatology 2019;36(9):886-90. [CENTRAL: CN-01976509] [EMBASE: 628740946] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Boggess K, Tita A, Jauk V, Saade G, Longo S, Clark E, et al. Clinical risk factors for post-cesarean surgical site infection despite pre-incision azithromycin-based extended spectrum antibiotic prophylaxis. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S118-S119. [CENTRAL: CN-01199679] [EMBASE: 72164387] [Google Scholar]
- Pasko DN, Tita AT. Does the effect of adjunctive azithromycin on cesarean infection vary with cefazolin dose? American Journal of Obstetrics and Gynecology 2018;218(1 Suppl):S522. [CENTRAL: CN-02004401] [EMBASE: 620310418] [Google Scholar]
- Subramaniam A, Waites KB, Jauk VC, Biggio JR, Sutton AL, Szychowski JM, et al. Azithromycin-based extended-spectrum antibiotic prophylaxis for cesarean: role of placental colonization with genital ureaplasmas and mycoplasmas. American Journal of Perinatology 2019;36(10):1002-8. [CENTRAL: CN-01976600] [EMBASE: 628888856] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Tita A, Szychowski J, Boggess K, Saade G, Longo S, Clark E, et al. Azithromycin-based extended spectrum antibiotic prophylaxis for non-elective cesarean delivery: a pragmatic multicenter placebo-controlled double-blind RCT. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S3. [CENTRAL: CN-01199691] [EMBASE: 72164200] [Google Scholar]
- Tita A, Waites K, Jauk V, Biggio J, Sutton A, Szychowski J, et al. RCT of azithromycin-based extended-spectrum antibiotic prophylaxis for cesarean delivery: role of placental colonization with ureaplasma or mycoplasma. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S70-1. [CENTRAL: CN-01199681] [EMBASE: 72164299] [DOI] [PubMed] [Google Scholar]
- Tita A. Impact of azithromycin-based extended spectrum antibiotic prophylaxis on non-infectious cesarean wound complications. American Journal of Obstetrics and Gynecology 2017;216(1 Suppl 1):S116. [CENTRAL: CN-01304138] [EMBASE: 614090253] [Google Scholar]
- Tita AT, Szychowski JM, Boggess K, Saade G, Longo S, Clark E, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. New England Journal of Medicine 2016;375(13):1231-41. [CENTRAL: CN-01260880] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Beekhuizen 2008 {published data only}
- Beekhuizen H, ISRCTN06127083. Is the administration of a single prophylactic dose of ampicillin and metronidazole before caesarean section as effective as a multiple day regimen of these antibiotics to prevent postpartum maternal infection in a low resource setting? A randomised controlled trial. isrctn.com/ISRCTN06127083 (first received 5 February 2008).
van Velzen 2009 {published data only}
- Van Velzen C, Kolk P, Westen E, Mmuni N, Hamisi A, Nakua R, et al. Comparison of a single prophylactic dose of ampicillin and metronidazole before caesarean section with a multiple day regimen of these antibiotics in prevention of postpartum maternal infection in a low resource setting: a randomized controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S369. [Google Scholar]
Varner 1986 {published data only}
- Varner M, Weiner C, Petzold R, Galask R. Comparison of cefotetan and cefoxitin as prophylaxis in cesarean section. American Journal of Obstetrics and Gynecology 1986;154:951-4. [DOI] [PubMed] [Google Scholar]
Vathana 2018 {published data only}
- Vathana M, Muhumthan K. A randomized control trial of single dose versus multiple doses of IV antibiotic prophylaxis in caesarean delivery. Sri Lanka Journal of Obstetrics and Gynaecology 2018;40(4):92-100. [Google Scholar]
- Vathana M, Muhunthan K. A randomized control trial of single dose versus 24 hours IV antibiotic prophylaxis in caesarean delivery - an attempt to reduce antibiotic use. Sri Lanka Journal of Obstetrics and Gynaecology 2014;36(Suppl 1):13, Abstract no: FC 4.4. [Google Scholar]
von Mandach 1993 {published data only}
- Mandach U, Huch R, Malinverni R, Huch A. Ceftriaxone (single dose) versus cefoxitin (multiple doses): success and failure of antibiotic prophylaxis in 1052 cesarean sections. Journal of Perinatal Medicine 1993;21:385-97. [DOI] [PubMed] [Google Scholar]
Wagner 2006 {published data only}
- Wagner KJ, Bier U, Callies R, Regidor PA, Schindler AE. Antibiotic prophylaxis in cesarean section - piperacillin 4 g versus piperacillin/tazobactam 4.5 g in 300 cesarean sections [Antibiotikaprophylaxe bei Sectio Caesarea - Piperacillin 4 g versus Piperacillin/Tazobactam 4.5 g]. Zentralblatt fur Gynakologie 2006;128(3):149-52. [DOI] [PubMed] [Google Scholar]
Wajsfeld 2019 {published data only}
- NCT03960970. Two-drug antibiotic prophylaxis in scheduled cesarean deliveries [Azithromycin-based extended-spectrum prophylaxis in scheduled cesarean deliveries]. Https://clinicaltrials.gov/show/NCT03960970 (first received 23 May 2019). [CENTRAL: CN-01945017]
Warnecke 1982 {published data only}
- Warnecke HH, Graeff H, Selbmann HK, Preac-Mursic V, Adam D, Gloning K, et al. Perioperative short-term prophylaxis with antibiotics in caesarean section. Geburtshilfe und Frauenheilkunde 1982;42(9):654-61. [DOI] [PubMed] [Google Scholar]
Watts 1991 {published data only}
- Watts DH, Hillier SL, Eschenbach DA. Upper genital tract isolates at delivery as predictors of post-Cesarean infections among women receiving antibiotic prophylaxis. Obstetrics & Gynecology 1991;77:287-92. [DOI] [PubMed] [Google Scholar]
Wax 1997 {published data only}
- Wax JR, Hersey K, Philput C, Wright MS, Nichols KV, Eggleston MK, et al. Single dose cefazolin prophylaxis for postcesarean infections: before vs after cord clamping. Journal of Maternal Fetal Medicine 1997;6(1):61-5. [DOI] [PubMed] [Google Scholar]
Westen 2015 {published data only}
- Westen EH, Kolk PR, Velzen CL, Unkels R, Mmuni NS, Hamisi AD, et al. Single-dose compared to multiple day antibiotic prophylaxis for cesarean section in low-resource settings, a randomized controlled, non-inferiority trial. Acta Obstetricia et Gynecologica Scandinavica 2015;94:43-9. [DOI] [PubMed] [Google Scholar]
Wu 1991 {published data only}
- Wu Y, Zhan L, Jing Y. Prevention of post-operative infection by uterine and intraperitoneal irrigation with ampicillin during cesarean section. International Journal of Experimental and Clinical Chemotherapy 1991;4(3):132-6. [Google Scholar]
Xu 1997 {published data only}
- Xu X, Zhang W, He X. Use of cefotaxime to control post-operative infection in patients who underwent C-section. Acta Academiae Medicinae Hubei 1997;18:74-6. [Google Scholar]
Yildirim 2009 {published data only}
- Yildirim G, Gungorduk K, Guven HZ, Aslan H, Celikkol O, Sudolmus S, et al. When should we perform prophylactic antibiotics in elective cesarean cases? Archives of Gynecology and Obstetrics 2009;280(1):13-8. [DOI] [PubMed] [Google Scholar]
Zutshi 2008 {published data only}
- Zutshi V. Prophylactic antibiotics - a concept forgotten by gynaecologists? BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):104. [Google Scholar]
References to ongoing studies
Abdalmageed 2019 {published data only}
- NCT04009772. Single dose cefepime versus cefuroxime plus metronidazole as a prophylactic antibiotic during emergency intrapartum cesarean section [Single dose cefepime versus cefuroxime plus metronidazole as a prophylactic antibiotic: a randomized controlled study]. Https://clinicaltrials.gov/show/nct04009772 (first received 4 July 2019). [CENTRAL: CN-01951287]
Karamali 2013 {published data only}
- IRCT2013080710511N2. Comparison of prophylactic administration of cefazolin, ampicillin and azithromycin in prevention of postpartum infections after cesarean. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2013080710511N2 (first received 2013. [CENTRAL: CN-01816174]
Additional references
ACOG 2018
- Use of prophylactic antibiotics in labor and delivery. ACOG Practice Bulletin (summary at https://www.obgproject.com/2018/08/29/acog-guidance-antibiotic-prophylaxis-during-labor-and-delivery/) 2018;199.
Bayarski 2006
- Bayarski Y. Antibiotics classification and side effects. http://ezinearticles.com/?Antibiotics-Classification-And-Side-Effects&id=288137 (accessed 14 April 2010).
Bedford Russell 2006
- Bedford Russell A, Murch S. Could peripartum antibiotics have delayed health consequences for the infant? BJOG: an international journal of obstetrics and gynaecology 2006;113:758-65. [DOI] [PubMed] [Google Scholar]
BNF 2020
- Britich National Formulary. Tetracycline. https://bnf.nice.org.uk/drug/tetracycline.html#indicationsAndDoses (accessed 3 September 2020).
Boerma 2018
- Boerma T, Ronsmans C, Melesse DY, Barros AJ, Barros FC, Juan L, et al. Global epidemiology of use of and disparities in caesarean sections. Lancet 2018;392(10155):1341-8. [DOI: 10.1016/S0140-6736(18)31928-7] [DOI] [PubMed] [Google Scholar]
Csaba 2007
- Csaba G. Hormonal imprinting: phylogeny, ontogeny, diseases and possible role in present-day human evolution. Cell Biochemistry and Function 2007;26(1):1-10. [DOI] [PubMed] [Google Scholar]
Dancer 2004
- Dancer SJ. How antibiotics can make us sick: the less obvious adverse effects of antimicrobial chemotherapy. Lancet Infectious Diseases 2004;4:611-9. [DOI] [PubMed] [Google Scholar]
Declercq 2007
- Declercq E, Barger M, Cabral HJ, Evans SR, Kotelchuck M, Simon C, et al. Maternal outcomes associated with planned primary cesarean births compared with planned vaginal births. Obstetrics and Gynecology 2007;109(3):669-77. [DOI: 10.1097/01.AOG.0000255668.20639.40] [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-analysis in Context. 2nd edition. London (UK): BMJ Publications Group, 2001. [Google Scholar]
Drew 2020
- Drew, R. Aminoglycasides. UpToDate (accessed 3 September 2020).
eMedExpert 2009
- eMedExpert. Antibiotics: types and side effects. http://www.emedexpert.com/classes/antibiotics.shtml (accessed 14 April 2010).
Glasgow 2005
- Glasgow TS, Young PC, Wallin J, Kwok C, Stoddard G, Firth S, et al. Association of intrapartum antibiotic exposure and late-onset serious bacterial infections in infants. Pediatrics 2005;116(3):696-702. [DOI] [PubMed] [Google Scholar]
Goodman 2008
- Goodman L, Gilman A. Section V111: Chemotherapy of microbial diseases. In: Brunton L, Parker K, Blumenthal D, Buxton I, editors(s). Goodman & Gillman's Manual of Pharmacology and Therapeutics. 11th edition. New York: McGraw-Hill, 2008:707-852. [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hofmeyr 2010
- Hofmeyr GJ, Smaill FM. Antibiotic prophylaxis for cesarean section. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD000933. [DOI: 10.1002/14651858.CD000933.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
IDSA 2013
- Bratzler D, Patchen Delinger E, Olsen K, Perl, T, Auwaerter P, Bolon M, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. American Journal of Health-System Pharmacy 2013;70(3):195-283. [DOI] [PubMed] [Google Scholar]
Kapoor 2017
- Kapoor G, Saigal S, Elongavan A. Action and resistance mechanisms of antibiotics: A guide for clinicians. Journal of Anaesthesiology, Clinical Pharmacology 2017;33(3):300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Korpela 2018
- Korpela K, Salonen A, Vepsäläinen O, Suomalainen M, Kolmeder C, Varjosalo M, et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome 2018;16(6[1]):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamont 2011
- Lamont RF, Sobel JD, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Kim SK, et al. Current debate on the use of antibiotic prophylaxis for cesarean section. British Journal of Obstetrics and Gynaecology 2011;118(2):193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leth 2009
- Leth RA, Møller JK, Thomsen RW, Uldbjerg N, Nørgaard M. Risk of selected postpartum infections after cesarean section compared with vaginal birth: a five-year cohort study of 32,468 women. Acta Obstetricia et Gynecologica Scandinavica 2009;88(9):976-83. [DOI] [PubMed] [Google Scholar]
Letourneau 2020a
- Letourneau A. Beta-lactam antibiotics: mechanisms of action and resistance and adverse effects. UpToDate (accessed 3 September 2020).
Letourneau 2020b
- Letourneau A. Cephalosporins. UpToDate (accessed 3 September 2020).
Letourneau 2020c
- Letourneau A. Combination beta-lactamase inhibitors, carbapenems, and monobactams. UpToDate (accessed 3 September 2020).
Letourneau 2020d
- Letourneau A. Penicillin, antistaphylococcal penicillins, and broad-spectrum penicillins. UptoDate (accessed 3 September 2020).
Mackeen 2014
- Mackeen AD, Packard RE, Ota E, Berghella V, Baxter JK. Timing of intravenous prophylactic antibiotics for preventing postpartum infectious morbidity in women undergoing cesarean delivery. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD009516. [DOI: 10.1002/14651858.CD009516.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Madhok 2015
- Madhok V, Futamura M, Thomas KS, Barbarot S. What's new in atopic eczema? An analysis of systematic reviews published in 2012 and 2013. Part 1. Epidemiology, mechanisms of disease and methodological issues. Clinical and Experimental Dermatology 2015;40(3):238-42. [DOI] [PubMed] [Google Scholar]
Martin 2014
- Martin E. Preventing infections following caesarean section. Australian Nursing & Midwifery Journal 2014;22(3):37. [PubMed] [Google Scholar]
Martin 2018
- Martin EK, Beckmann MM, Barnsbee LN, Halton KA, Merollini K, Graves N. Best practice perioperative strategies and surgical techniques for preventing caesarean section surgical site infections: a systematic review of reviews and meta-analyses. British Journal of Obstetrics and Gynaecology 2018;125(8):956-64. [DOI] [PubMed] [Google Scholar]
Martinez de Tejada 2014
- Martinez de Tejada B. Antibiotic use and misuse during pregnancy and delivery: benefits and risks. International Journal of Environmental Research and Public Health 2014;11(8):7993-8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mascarello 2017
- Mascarello KC, Horta BL, Silveira MF. Maternal complications and cesarean section without indication: systematic review and meta-analysis. Revista de Saude Publica 2017;51:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mueller 2015
- Mueller NT, Whyatt R, Hoepner L, Oberfield S, Dominguez-Bello MG, Widen EM, et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. International Journal of Obesity 2015;39(4):665-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabhan 2016
- Nabhan AF, Allam NE, Hamed Abdel-Aziz Salama M. Routes of administration of antibiotic prophylaxis for preventing infection after caesarean section. Cochrane Database of Systematic Reviews 2016, Issue 6. Art. No: CD011876. [DOI: 10.1002/14651858.CD011876.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Penders 2006
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006;118(2):511-21. [DOI] [PubMed] [Google Scholar]
Pierson 2018
- Pierson RC, Scott NP, Briscoe KE, Haas DM. A review of post-caesarean infectious morbidity: how to prevent and treat. Journal of Obstetrics and Gynaecology 2018;38(5):591-7. [DOI] [PubMed] [Google Scholar]
RCOG 2011
- National Collaborating Centre for Women's and Children's Health UK . Caesarean Section. London, UK: RCOG Press, 2011. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sarah 2019
- Sarah HY, Perkins KM, Kazokova SV, Hatfield KM, Kleinbaum DG, Baggs J, et al. Surgical site infection risk following cesarean deliveries covered by Medicaid or private insurance. Infection Control and Hospital Epidemiology 2019;40(6):639-48. [DOI: 10.1017/ice.2019.66] [DOI] [PubMed] [Google Scholar]
Schultz 1995
- Schultz KF. Subverting randomization in controlled trials. JAMA 1995;274:1456-8. [PubMed] [Google Scholar]
Seedat 2017
- Seedat F, Stinton C, Patterson J, Geppert J, Tan B, Robinson ER, et al. Adverse events in women and children who have received intrapartum antibiotic prophylaxis treatment: a systematic review. BMC Pregnancy and Childbirth 2017;26(17[1]):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skeith 2017
- Skeith AE, Niu B, Valent AM, Tuuli MG, Caughey AB. Adding azithromycin to cephalosporin for cesarean delivery infection prophylaxis: a cost-effectiveness analysis. Obstetrics and Gynecology 2017;130(6):1279-84. [DOI] [PubMed] [Google Scholar]
Smaill 2014
- Smaill FM, Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD007482. [DOI: 10.1002/14651858.CD007482.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
SOGC 2017
- Schalwyk J, Van Eyk N. SOGC reaffirmed guidelines no. 247- Antibiotic prophylaxis in obstetric procedures. Journal of Obstetrics and Gynaecology Canada 2017;39(9):e293-e299. [DOI] [PubMed] [Google Scholar]
Tita 2009
- Tita AT, Rouse DJ, Blackwell S, Saade GR, Spong CY, Andrews WW. Emerging concepts in antibiotic prophylaxis for cesarean delivery: a systematic review. Obstetrics and Gynecology 2009;113(3):675-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Dillen 2010
- Dillen J, Zwart J, Schutte J, Roosmalen J. Maternal sepsis: epidemiology, etiology and outcome. Current Opinion in Infectious Diseases 2010;23(3):249-54. [DOI] [PubMed] [Google Scholar]
Walsh 2010
- Walsh CA. Evidence-based cesarean technique. Current Opinion in Obstetrics & Gynecology 2010;22(2):110-5. [DOI] [PubMed] [Google Scholar]
WHO 2020
- World Health Organization Centre for Drug Statistics Methodology. Antiinfectives for systemic use. https://www.whocc.no/atc_ddd_index/?code=J01BA&showdescription=yes (accessed 3 September 2020).
Wilson 2018
- Wilson RD, Caughey AB, Wood SL, Macones GA, Wrench IJ, Huang J, et al. Guidelines for antenatal andpPreoperative care in cesarean delivery: enhanced recovery after Surgery Society recommendations (Part 1). American Journal of Obstetrics and Gynecology 2018;219(6):523.e1-523.e15. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Alfirevic 2010
- Alfirevic Z, Gyte GM, Dou L. Different classes of antibiotics given to women routinely for preventing infection at caesarean section. Cochrane Database of Systematic Reviews 2010, Issue 10. Art. No: CD008726. [DOI: 10.1002/14651858.CD008726] [DOI] [PubMed] [Google Scholar]
Gyte 2014
- Gyte GM, Dou L, Vazquez JC. Different classes of antibiotics given to women routinely for preventing infection at caesarean section. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD008726. [DOI: 10.1002/14651858.CD008726.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopkins 1999
- Hopkins L, Smaill F. Antibiotic prophylaxis regimens and drugs for cesarean section. Cochrane Database of Systematic Reviews 1999, Issue 2. Art. No: CD001136. [DOI: 10.1002/14651858.CD001136] [DOI] [PubMed] [Google Scholar]


