Vancomycin induces exposure-related acute kidney injury. However, the pharmacokinetic-toxicodynamic (PK-TD) relationship remains unclear.
KEYWORDS: pharmacodynamics, pharmacokinetics, pharmacology, rat, toxicodynamics, vancomycin
ABSTRACT
Vancomycin induces exposure-related acute kidney injury. However, the pharmacokinetic-toxicodynamic (PK-TD) relationship remains unclear. Sprague-Dawley rats received intravenous (i.v.) vancomycin doses of 300 mg/kg/day and 400 mg/kg/day, divided into once-, twice-, three-times-, or four-times-daily doses (i.e., QD, BID, TID, or QID) over 24 h. Up to 8 samples plus a terminal sample were drawn during the 24-h dosing period. Twenty-four-hour urine was collected and assayed for kidney injury molecule-1 (KIM-1). Vancomycin was quantified via liquid chromatography-tandem mass spectrometry (LC-MS/MS). Following terminal sampling, nephrectomy and histopathologic analyses were conducted. PK analyses were conducted using Pmetrics. PK exposures (i.e., area under the concentration-time curve from 0 to 24 h [AUC0–24] and maximum concentration from 0 to 24 h [Cmax0–24]) were calculated for each rat, and PK-TD relationships were discerned. A total of 53-rats generated PK-TD data. A 2-compartment model fit the data well (Bayesian observed versus predicted concentrations; R2 = 0.96). KIM-1 values were greater in QD and BID groups (P for QD versus TID, <0.002; P for QD versus QID, <0.004; P for BID versus TID, <0.002; and P for BID versus QID, <0.004). Exposure–response relationships were observed between KIM-1 versus Cmax0–24 and AUC0–24 (R2 = 0.7 and 0.68). Corrected Akaike’s information criterion showed Cmax0–24 as the most predictive PK-TD driver for vancomycin-induced kidney injury (VIKI) (−5.28 versus −1.95). While PK-TD indices are often intercorrelated, maximal concentrations and fewer doses (for the same total daily amount) resulted in increased VIKI in our rat model.
INTRODUCTION
Vancomycin was approved for clinical use in 1958 and is still one of the most commonly used antibiotics in the hospital setting because of its activity against methicillin-resistant Staphylococcus aureus (MRSA) (1). The initial pharmacokinetic/pharmacodynamic (PK/PD) efficacy studies performed in neutropenic mouse models demonstrated that the exposures, calculated as area under the concentration-time curve (AUC) divided by organism MIC, explained efficacy (2, 3). Indeed, the 2020 vancomycin guidelines now recommend AUC monitoring to maximize efficacy for S. aureus infections (4). The guidelines also noted that AUC monitoring and tighter control of vancomycin exposures may result in less kidney injury.
The AUC therapeutic window for vancomycin has been described in human and animal studies. A prospective clinical trial demonstrated that acute kidney injury (AKI) increased above a 24-h AUC (AUC0–24) of 515 mg·h/liter and that efficacy did not increase for MRSA bloodstream treatments above these exposures (5). This ceiling threshold is consistent with other clinical data; a meta-analysis that included that study suggested a threshold AUC of 650 mg·h/liter (6). Preclinical rat studies have also found that a consistent target, an AUC threshold of 482.2, predicted 90% of maximal kidney injury biomarker response (7). Thus, there is considerable evidence to support AUC as a useful predictor of kidney injury, and thresholds are similar between humans and rats. It is still unknown if AUCs or maximal concentrations (Cmax) drive the toxicodynamic (TD) relationship for kidney injury. Herein, we present the results of dose fractionation experiments to better understand the PK-TD driver of vancomycin-induced kidney injury (VIKI).
RESULTS
Characteristics of animal cohort.
All 48 animals from the 300-mg and 400-mg total daily dose cohorts contributed pharmacokinetic model data (5 controls were not included in model since, by design, they did not have quantifiable vancomycin levels). Mean baseline weights were not significantly different between controls and animals from the vancomycin dosing protocol (307.4 g versus 313.6 g; P = 0.49). Overall 24-h urine output was significantly different between controls and the vancomycin-treated animals (4.2 versus 16.11 ml; P < 0001). Median histopathology scores differed numerically though not statistically significantly between controls and the entire vancomycin-treated cohort (1 versus 2; P = 0.088). However, median kidney injury molecule-1 (KIM-1) values were significantly different between controls and vancomycin dosing protocol animals (0.63 ng/ml versus 6.169 ng/ml; P < 0.001).
In stratified dosing group analyses (i.e., 300 mg/kg/day and 400 mg/kg/day), there was a significant difference in median KIM-1 between fractionation schemes. In the 300-mg/kg/day group, median KIM-1 values were significantly different in the daily versus three-times-daily (TID) dose fractionation groups (10.7 ng/ml versus 2.3 ng/ml; P = 0.03) and daily versus four-times-daily (QID) dose fractionation groups (10.70 ng/ml versus 1.46 ng/ml; P < 0.01). In the 400-mg/kg/day group, median KIM-1 values differed only in the twice-daily (BID) versus QID (13.3 ng/ml versus 4 ng/ml) dose fractionation groups (P <0.001). Complete pairwise comparisons can be found in Table 1.
TABLE 1.
Biomarker KIM-1 summary for vancomycin-treated animals by dose fractionationa
| Vancomycin dosage (mg/kg/day) | Median KIM-1 concn (ng/ml) (IQR) [n] for dosing frequency |
|||
|---|---|---|---|---|
| QD | BID | TID | QID | |
| 300 | 10.70*,** (9.8–12.3) [5] | 9.5 (7.7–10.3) [5] | 2.3* (1.43–4.9) [6] | 1.46** (1.2–2.5) [6] |
| 400 | 10.5 (6.6–14.4) [5] | 13.3*** (13–16.5) [6] | 4*** (2.9–4.6) [9] | 6.4 (4.8–8.3) [6] |
Abbreviations: KIM-1, kidney injury molecule-1; IQR, interquartile range; QD, once daily; BID, twice daily; TID, three times daily; QID, four times daily. *, P = 0.03; **, P <0.01; ***, P < 0.001.
Vancomycin PK models, parameter estimates, and exposures.
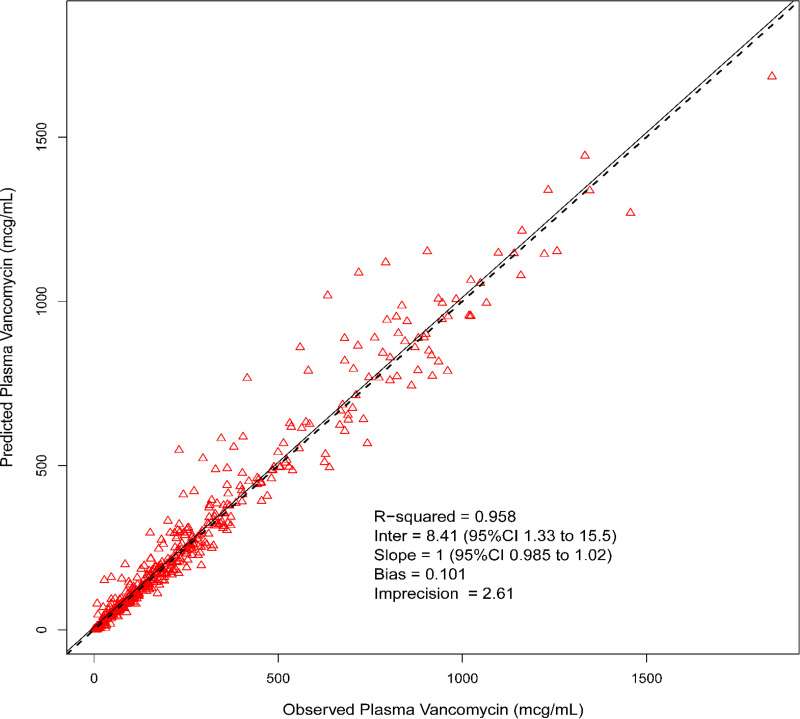
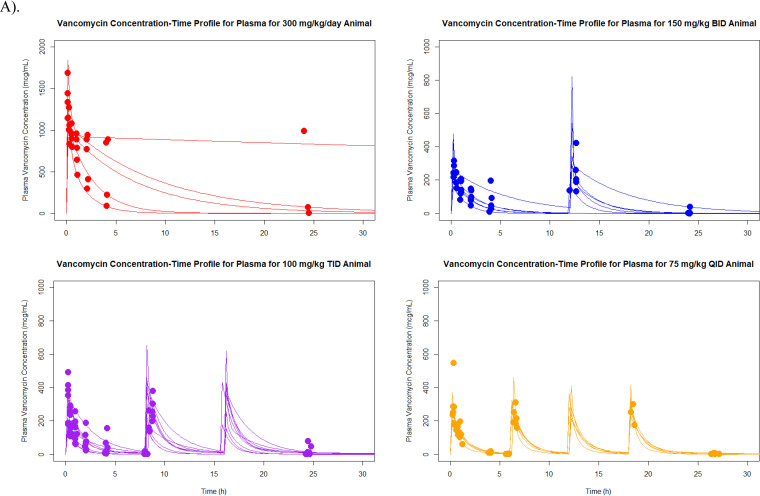
Median (coefficient of variation [CV]) parameter values for the pharmacokinetic model for elimination rate constant (kel), volume of distribution (V), rate of transfer from the central to the peripheral compartment (KCP), and rate of transfer from the peripheral to the central compartment (KPC) were 0.7 h−1 (60.81%), 0.07 liter (76.69%), 1.42 h−1 (136.87%), and 1.52 h−1 (160.86%), respectively. Model predictive performance demonstrated observed versus Bayesian predicted concentrations, bias, imprecision (i.e., bias-adjusted mean weighted squared prediction error), and coefficient of determination (R2) of 0.101 mg/liter, 2.61 (mg/liter)2, and 0.958, respectively (Fig. 1). A complete concentration-versus-time profile plot by dose fractionation for all animals in the 300-mg/kg/day and 400-mg/kg/day groups can be found in Fig. 2. There were no significant differences found in Cmax standardized by fractionation and milligrams in all animals.
FIG 1.
Best-fit plot for Bayesian observed versus predicted plasma vancomycin concentrations utilizing the final 2-compartment model.
FIG 2.
Concentration-versus-time plots for each dose fractionation group, all animals. (A) 300 mg/kg/day; (B) 400 mg/kg/day. Abbreviations: BID, twice daily; TID, three times daily; QID, four times daily.
Exposure-response relationships.
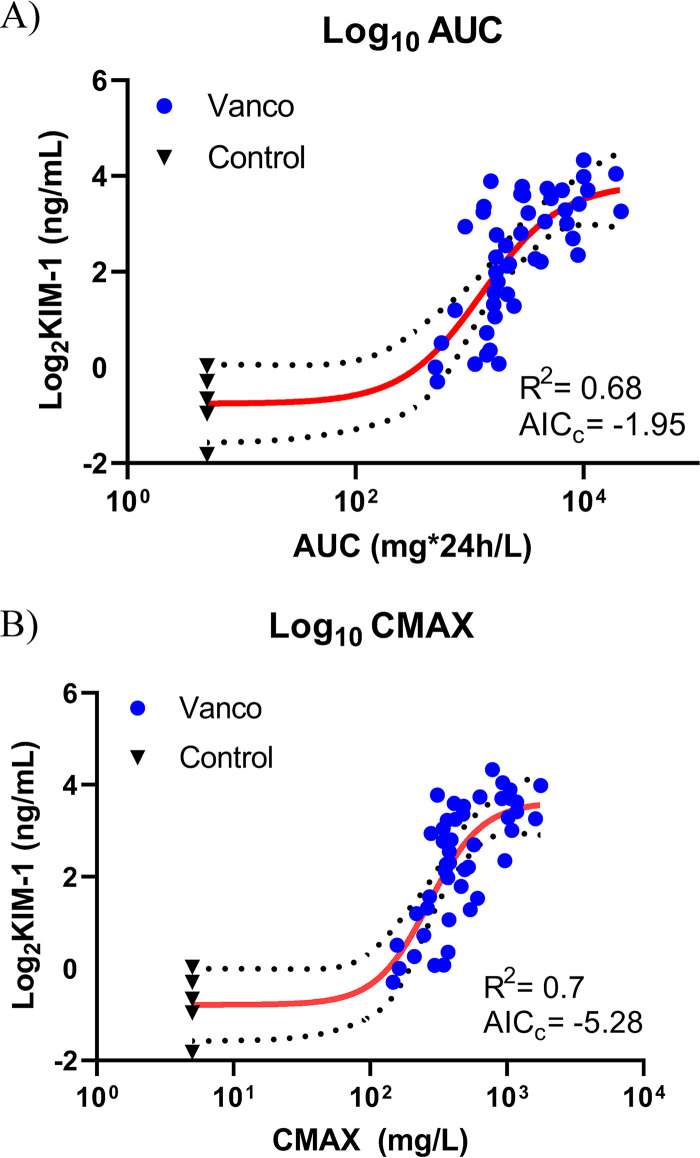
Four-parameter Hill models best described the exposure-biomarker relationships. Exposure-biomarker relationships were found between AUC0–24 versus KIM-1 (R2 = 0.68) and Cmax0–24 versus KIM-1 (R2 = 0.7). Overall, AUC0–24 versus KIM-1 was slightly less predictive than Cmax0–24 versus KIM-1, as the Cmax0–24 model performed better based on corrected Akaike information criterion (AICc) comparison (AICc = −5.28 versus AICc = −1.95). All exposure-biomarker relationships are shown in Fig. 3.
FIG 3.
(A) AUC0–24 and (B) Cmax0–24 versus the urinary biomarkers KIM-1, as calculated by the 4-parameter Hill model fit. The 4-parameter Hill model equation for TD is as follows: y = bottom + (top − bottom)/[1 + (EC50Hill slope)/(concnHill slope)]. Biomarker values were log2 transformed, and exposure values were log10 transformed. Abbreviations: AUC, area under the curve; Cmax, maximum concentration; KIM-1, kidney injury molecule-1.
Vancomycin dose fractionation-versus-biomarker relationships.
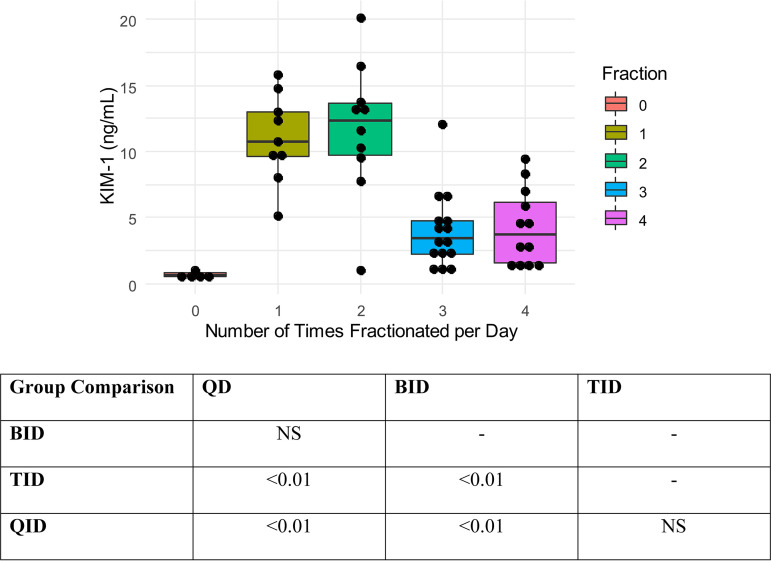
The plot in Fig. 4 visually displays decreasing urinary KIM-1 concentrations as doses were increasingly fractionated between once daily (QD) and four times daily for both daily-dose groups (i.e., 300 and 400 mg/kg/day). Pairwise comparison showed that there was a significant difference in KIM-1 in QD versus TID and QID groups and in BID versus TID and QID groups (all P values <0.01). A visual representation of these trends and differences is shown in Fig. 4.
FIG 4.
Dose fractionation versus KIM-1 relationship for all animals. Abbreviations: KIM-1, kidney injury molecule-1; IQR, interquartile range; QD, daily; BID, twice daily; TID, three times daily; QID, four times daily. Controls were excluded from statistical analysis, given that they did not receive vancomycin, and are shown graphically only to demonstrate KIM-1 values without therapy but with sham procedures.
DISCUSSION
This preclinical study provides continued evidence that VIKI is caused by high vancomycin concentrations (i.e., concentration-dependent toxicity) and elevated exposures. Importantly, in our model, Cmax was marginally better than AUC in explaining toxicodynamic relationships. These findings, along with studies that compared prolonged infusion vancomycin to standard intermittently infused vancomycin, suggest that giving the same total daily dose in a fractionated fashion or with continuous infusion may improve kidney outcomes (8–12). In our trial, where we were able to fractionate doses, we determined that Cmax exhibited a slightly improved relationship with KIM-1 compared to the AUC 4-parameter Hill model fit (for Cmax verus KIM-1, R2 = 0.7 and AICc = −5.28; for AUC versus KIM-1, R2 =0.68 and AICc = −1.95). Further, the fractionation scheme resulted in lower KIM-1. That is, when the daily dose was split into several doses, kidney injury was less. For the fractionation schemes, we demonstrated that Cmax remained constant between the groups (P = 0.34) and that urinary KIM-1 was lower in TID and QID groups than QD and BID groups (all P values < 0.01).
The rat is a highly relevant preclinical model for drug-induced acute kidney injury, as quantitative biomarkers that describe degree of injury are shared between humans and rats (13, 14). Adequate blood sample volumes are possible in the rat, thus allowing a richly sampled PK design and careful characterization of vancomycin exposures in order to assess pharmacokinetic-toxicodynamic (PK-TD) relationships at the individual-animal level. Further, PK-TD relationships are similar between this rat model and human VIKI. In both species, AUC toxicity thresholds are ∼500 to 600 mg/liter·24 h (5–7). KIM-1 was utilized as the surrogate of VIKI in this study, as it is causally linked to histopathologic damage in vancomycin-treated rats. KIM-1 is a specific marker of histopathologic proximal tubule injury in VIKI (7, 15). KIM-1 has predicted histopathologic rise (P < 0.001) and was the best predictor of a histopathologic damage score of ≥2 on each study day (i.e., days 1, 2, 3, and 6) as determined by ROC area. Quantitatively, every 1-ng/ml increase of KIM-1 increased the likelihood of a histopathologic score of ≥2 by 1.3-fold (P < 0.001) (5). The use of KIM-1 as a marker for VIKI is clinically relevant, given that it is one of the biomarkers specifically qualified by the FDA for drug-induced acute kidney injury in both human and rat drug trials (16).
Our findings by fractionation group are similar to those reported by Konishi et al. (17) from a study in Wistar rats. They found that creatinine clearance and superoxide dismutase were decreased in rats treated once daily versus twice daily (17). Our study is different in that we administered vancomycin intravenously and were additionally able to precisely estimate exposures for each rat in order to define the PK-TD relationships. Konishi et al. studied animals over 7 days, while we studied a single day (as this is the PK-TD model that has best linked rat outcomes to human outcomes) (5, 7, 17). Their findings that vancomycin saturated similarly in the kidney between the two groups may suggest that outcomes will eventually converge toward equivocal if treatment is prolonged. That is, fractionating doses may provide a benefit early; however, it will remain prudent to discontinue nephrotoxic drugs when they are not needed, as human trials and animal models consistently show the kidney toxicity of vancomycin (18).
Randomized studies will be necessary to discern if fractionating the daily dose of vancomycin ultimately improves outcomes for humans. Indeed, small clinical studies and meta-analyses have demonstrated that continuous infusions of vancomycin might result in less kidney injury than traditional intermittent infusions (8, 10, 11). In theory, limiting the Cmax even for equivalent AUC exposures could improve the renal safety of vancomycin. The single prospective human trial that randomized patients to continuous infusion vancomycin (n = 61) or traditional intermittent infusions (n = 58) did not find a difference in renal outcomes between the groups. However, the study was relatively underpowered to assess this outcome in the setting of a heterogenous study population receiving varied concomitant therapies (19). Prolonged vancomycin administration has also been considered in recent national guidelines which concluded with moderate evidence that the risk of developing nephrotoxicity with continuous infusion appears to be similar to or lower than that with intermittent dosing (4).
There are several limitations to this study. First, this study was limited to 24-h dosing for our dose fractionation protocol. However, as previously noted, elevations in biomarkers have already been linked to histopathologic damage within this time period (20). Second, we did not utilize KIM-1 as a binary variable, like some preclinical toxicology screenings. Currently there is no consensus on the threshold of significant elevation, and appropriate exposure response-versus-biomarker kinetic studies are needed to establish accurate and reliable KIM-1 thresholds. We believe our preliminary work does this. Third, this employed allometric scaled doses that are known to result in toxicity, i.e., Cmax, were not humanized. Additional studies will be needed to understand if the TD relationship found in this study is reproducible if Cmax is scaled to humanized values. It is notable that it is not possible to utilize standard practices of administering nephrotoxic agents to animals to match human clearance when the outcome being assessed is AKI. Thus, a complex continuous infusion animal model may be the best way to parameter scale Cmax, and it is not clear if those studies will be more translational than the current approaches.
In summary, these data demonstrate that VIKI may be driven by Cmax0–24 (i.e., concentration-dependent toxicity). These findings have clinical implications, as dosing strategies can be designed to dose fractionate a total daily vancomycin dose in efforts to maintain efficacy by maintaining AUC while decreasing toxicity. Further studies employing continuous infusion dosing strategies are warranted to further assess if administration scheme can mitigate toxicity.
MATERIALS AND METHODS
This PK-TD study was conducted at Midwestern University in Downers Grove, IL. All study methods were approved by the Institutional Animal Care and Use Committee (IACUC; protocol number 2295) and conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (21).
Experimental design and animals.
Experimental methods and design were similar to those described previously (7). Male Sprague-Dawley rats (n = 53; approximately 8 to 10 weeks old; mean purchase weight, 310 g) were housed individually in a light- and temperature-controlled room for the duration of the study and allowed free access to water and food. Rats (n = 5 to 9 per dosing protocol) were administered intravenous (i.v.) injections of clinical-grade vancomycin (n = 48) in normal saline (NS) or NS only (n = 5; control) as previously described (7). In brief, rats were placed into a treatment or control group (treatment receiving vancomycin or normal saline, respectively). Vancomycin-treated rats received total daily doses of 300 or 400 mg/kg as either a three- or four-times-daily divided dose over 24 h (e.g., 300 mg/kg was given as three 100-mg/kg injections daily or as four 75-mg/kg injections daily for a total of 24 h). Previous animal data from our lab constituted the 300- and 400-mg/kg/day daily and twice-daily cohorts (i.e., QD and BID) (7). A complete animal dosing flow chart can be found in Fig. S1 in the supplemental material. The 300- to 400-mg/kg/day dosing range was chosen based on the known nephrotoxic effect observed in our previous study of i.v. dosing (7) and to span the higher end of the clinical allometric range. For example, the clinical kidney injury threshold of ≥4 g/day in a 70-kg patient (i.e., 57 mg/kg/day in humans) scales allometrically to 350 mg/kg in rats (22, 23). Data were analyzed for all animals that were included in the protocol.
Blood and urine sampling.
Surgical catheters were implanted 24 h prior to protocol initiation. Blood samples were drawn from a single right-side internal jugular vein catheter, and dosing occurred via the left-side internal jugular vein catheter. A maximum of 8 samples plus terminal draw per animal were obtained and scheduled at 0, 5, 15, 30, 60, 120, 240, and 1,440 min after the first dose for the once-daily dosing. The twice-daily dosing protocol animals were sampled at 0, 15, 30, 60, 120, 240, 750, and 1,440 min. The animals in the TID dosing treatment protocol were sampled at 0, 15, 30, 60, 120, 240, 480, 504, and 1,440 min. The animals in the QID dosing treatment protocol were sampled at 0, 15, 30, 60, 240, 360, 384, 1,094, and 1,440 min. Each sample (0.25-ml aliquot) was replaced with an equivalent volume of NS to maintain euvolemia. Blood samples from vancomycin-treated animals were immediately transferred to a disodium EDTA (Sigma-Aldrich Chemical Company, Milwaukee, WI)-treated microcentrifuge tube and centrifuged at 3,000 rpm for 10 min. Plasma supernatant was collected and stored at −80°C for batch sample analysis.
Following the 2-h blood sample, animals were placed in metabolic cages for urine collection (catalog number 650-0350; Nalgene, Rochester, NY) for the remainder of the 24-h study (with the exception that they were briefly removed for scheduled blood samples and vancomycin doses). Urine volume was measured at 12 and 24 h. Urine was centrifuged at 400 × g for 5 min, and the supernatant was stored at −80°C until batch analysis.
Chemicals and reagents.
Animals were administered clinical-grade vancomycin hydrochloride for injection (lot number 591655DD) obtained commercially (Hospira, Lake Forrest, IL). All solvents were of liquid chromatography-tandem mass spectrometry (LC-MS/MS) grade. For LC-MS/MS assay purposes, vancomycin hydrochloride (United States Pharmacopeia) was used (Enzo Life Science, Farmingdale, NY) with a purity of 99.3%. Polymyxin B (Sigma-Aldrich, St. Louis, MO), acetonitrile, and methanol were purchased from VWR International (Radnor, PA). Formic acid was obtained from Fischer Scientific (Waltham, MA). Frozen, nonmedicated, nonimmunized, pooled Sprague-Dawley rat plasma (anticoagulated with disodium EDTA) was used for calibration of standard curves (BioreclamationIVT, Westbury, NY).
Determination of vancomycin concentrations in plasma.
Plasma concentrations of vancomycin were quantified with LC and column conditions similar to those used in our previous study (7). The lower limit of quantification was 0.25 mg/liter. Precision was <8.6% for all measurements, including intra- and interday assay measurements. More than 92% of the analyte was recovered in all samples tested with an overall mean assay accuracy of 100%. Any samples measuring above the upper limit of quantification were diluted per standard protocol and requantified.
Determination of urinary biomarkers of AKI.
Urine samples were analyzed in batch to determine concentrations of KIM-1. Microsphere-based Luminex X-MAP technology was used for the determination of all biomarker concentrations, as previously described (24, 25). Urine samples were aliquoted into 96-well plates supplied with Milliplex MAP rat kidney toxicity magnetic bead panels 1 and 2 (EMD Millipore Corporation, Charles, MO), prepared and analyzed according to the manufacturer’s recommendations.
Histopathology kidney scoring.
Following terminal blood sampling, bilateral nephrectomy was performed under anesthesia as previously described (15). Briefly, kidneys were washed in cold isotonic saline and preserved in 10% formalin solution for histologic examination. Histopathologic analyses were conducted by IDEXX BioAnalytics (Westbrook, ME.). Pathologists received access only to nominal dosing group assignment. Scoring was conducted according to the Critical Path Institute’s Predictive Safety Testing Consortium Nephrotoxicity Working Group’s histologic injury lexicon, which utilizes a 0- to 5-point ordinal scale (14). This scoring system assigns higher scores to increasing levels of damage (0, no evidence of damage; 1, minimal; 2, mild; 3, moderate; 4, marked; 5, severe/massive) and was validated previously (14, 15). The composite score for each animal was calculated as the highest ordinal score for histopathologic changes at any kidney site (14).
Vancomycin pharmacokinetic model and exposure determination.
We employed the Bayesian priors from our previously published pharmacokinetic model (7) to generated Bayesian posteriors for all 48 animals reported here. Pharmacokinetic analyses were completed using the Pmetrics package version 1.5.0 (Pmetrics, Los Angeles, CA) for R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria) (26, 27) with model assessment as previously described (7).
Estimation of PK exposure profiles and statistical analysis.
The pharmacokinetic model was utilized to obtain median maximum a posteriori probability (MAP) Bayesian vancomycin plasma concentration estimates at 12-min intervals over the 24-h study period, generated from each animal’s measured vancomycin concentrations, exact dose, and dosing schedule. Bayesian posteriors for each animal were used to determine exposures over the 24-h time period (i.e., AUC0–24 and Cmax0–24). The pharmacokinetic value Cmax0–24 was calculated using makeNCA within Pmetrics (Los Angeles, CA, USA) (26, 28). The highest Bayesian posterior concentration was determined to be each individual animal’s Cmax0–24. Twenty-four hour exposure, as measured by AUC0–24, was calculated using the trapezoidal rule within the Pmetrics command makeAUC (26, 28). Cumulative Cmax0–24 was also calculated for the dose fractionation groups (i.e., BID Cmax multiplied by 2, TID Cmax multiplied by 3, and QID Cmax multiplied by 4) and standardized to milligrams to allow comparison and assess successfulness of varying Cmax0–24 while holding AUC0–24 constant.
Association of PK measures with the urinary AKI biomarker KIM-1.
Pharmacokinetic exposure estimates were assessed for relationships with KIM-1 using GraphPad Prism version 7.02 (GraphPad Software Inc., La Jolla, CA). PK-TD exposure-response relationships with KIM-1 were evaluated using Spearman’s rank correlation coefficient. Hill-type functions and log transformations of variables were employed to explore the relationship between PK exposures and KIM-1. Correlation coefficients (R2) and corrected Akaike information criteria (AICc) were calculated and compared between exposures metrics (Cmax versus AUC) to evaluate overall fit. KIM-1 was the primary biomarker of interest given specificity for proximal tubule damage and identification of VIKI in the rat model (7, 15, 29).
Statistical analysis for between-treatment-group comparisons.
Statistical analysis for between-treatment-group comparisons was performed using Intercooled Stata, version 14.2 (StataCorp LP, College Station, TX). PK exposure measurements, i.e., AUC0–24 and Cmax0–24, were compared across vancomycin total daily dose and dosing frequency groups for 300 mg/kg/day and 400 mg/kg/day. Log transformations were employed as needed. Differences between treatment groups for KIM-1 and standardized Cmax were visualized with locally weighted scatterplot smoothing (LOWESS) regression and compared with the Kruskal-Wallis Dunn pairwise comparison with the Bonferroni adjustment. All tests were two tailed, with an a priori level of statistical significance set at an alpha of 0.05.
Supplementary Material
ACKNOWLEDGMENTS
We kindly acknowledge the Core Facility at Midwestern University for access to the LC-MS/MS.
M. H. Scheetz has ongoing research contracts and serves as a consultant for Nevakar and SuperTrans Medical and filed patent US10688195B2. All other authors have no other related conflicts of interest to declare.
Research reported in this publication was supported in part by National Institute of Allergy and Infectious Diseases under award numbers R15-AI105742 and R21-AI149026. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Scheetz MH. 2020. Vancomycin: the pendulum swings. Am J Health Syst Pharm 77:810–811. doi: 10.1093/ajhp/zxaa076. [DOI] [PubMed] [Google Scholar]
- 2.Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 17:479–501. doi: 10.1016/s0891-5520(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 3.Rybak MJ. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis 42(Suppl 1):S35–S39. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 4.Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, Rodvold KA, Maples HD, Lomaestro BM. 2020. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 77:835–864. doi: 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 5.Pais GM, Avedissian SN, O’Donnell JN, Rhodes NJ, Lodise TP, Prozialeck WC, Lamar PC, Cluff C, Gulati A, Fitzgerald JC, Downes KJ, Zuppa AF, Scheetz MH. 2019. Comparative performance of urinary biomarkers for vancomycin-induced kidney injury according to timeline of injury. Antimicrob Agents Chemother 63:e00079-19. doi: 10.1128/AAC.00079-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aljefri DM, Avedissian SN, Rhodes NJ, Postelnick MJ, Nguyen K, Scheetz MH. 2019. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis 69:1881–1887. doi: 10.1093/cid/ciz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avedissian SN, Pais GM, O'Donnell JN, Lodise TP, Liu J, Prozialeck WC, Joshi MD, Lamar PC, Becher L, Gulati A, Hope W, Scheetz MH. 2019. Twenty-four hour pharmacokinetic relationships for intravenous vancomycin and novel urinary biomarkers of acute kidney injury in a rat model. J Antimicrob Chemother 74:2326–2334. doi: 10.1093/jac/dkz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flannery AH, Bissell BD, Bastin MT, Morris PE, Neyra JA. 2020. Continuous versus intermittent infusion of vancomycin and the risk of acute kidney injury in critically ill adults: a systematic review and meta-analysis. Crit Care Med 48:912–918. doi: 10.1097/CCM.0000000000004326. [DOI] [PubMed] [Google Scholar]
- 9.Hanrahan T, Whitehouse T, Lipman J, Roberts JA. 2015. Vancomycin-associated nephrotoxicity: a meta-analysis of administration by continuous versus intermittent infusion. Int J Antimicrob Agents 46:249–253. doi: 10.1016/j.ijantimicag.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Hao JJ, Chen H, Zhou JX. 2016. Continuous versus intermittent infusion of vancomycin in adult patients: a systematic review and meta-analysis. Int J Antimicrob Agents 47:28–35. doi: 10.1016/j.ijantimicag.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Cataldo MA, Tacconelli E, Grilli E, Pea F, Petrosillo N. 2012. Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother 67:17–24. doi: 10.1093/jac/dkr442. [DOI] [PubMed] [Google Scholar]
- 12.Ingram PR, Lye DC, Tambyah PA, Goh WP, Tam VH, Fisher DA. 2008. Risk factors for nephrotoxicity associated with continuous vancomycin infusion in outpatient parenteral antibiotic therapy. J Antimicrob Chemother 62:168–171. doi: 10.1093/jac/dkn080. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration. 2009. Review of qualification data for biomarkers of nephrotoxicity submitted by the Predictive Safety Testing Consortium. Accessed 11 May 2020. https://www.fda.gov/media/87781/download.
- 14.Food and Drug Administration. 2015. Biomarker Qualification Program Office of Clinical Pharmacology full qualification package review. Accessed 11 May 2020. https://www.fda.gov/media/93150/download.
- 15.O'Donnell JN, Rhodes NJ, Lodise TP, Prozialeck WC, Miglis CM, Joshi MD, Venkatesan N, Pais G, Cluff C, Lamar PC, Briyal S, Day JZ, Gulati A, Scheetz MH. 2017. 24-hour pharmacokinetic relationships for vancomycin and novel urinary biomarkers of acute kidney injury. Antimicrob Agents Chemother 61:e00416-17. doi: 10.1128/AAC.00416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. 2015. List of qualified biomarkers. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/ucm535383.htm.
- 17.Konishi H, Morita Y, Mizumura M, Iga I, Nagai K. 2013. Difference in nephrotoxicity of vancomycin administered once daily and twice daily in rats. J Chemother 25:273–278. doi: 10.1179/1973947812Y.0000000067. [DOI] [PubMed] [Google Scholar]
- 18.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. 2012. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54:621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 19.Wysocki M, Delatour F, Faurisson F, Rauss A, Pean Y, Misset B, Thomas F, Timsit JF, Similowski T, Mentec H, Mier L, Dreyfuss D. 2001. Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 45:2460–2467. doi: 10.1128/AAC.45.9.2460-2467.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. 2010. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 22.Lodise TP, Lomaestro B, Graves J, Drusano GL. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 52:1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckley MS, Hartsock NC, Berry AJ, Bikin DS, Richards EC, Yerondopoulos MJ, Kobic E, Wicks LM, Hammond DA. 2018. Comparison of acute kidney injury risk associated with vancomycin and concomitant piperacillin/tazobactam or cefepime in the intensive care unit. J Crit Care 48:32–38. doi: 10.1016/j.jcrc.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Prozialeck WC, Edwards JR, Lamar PC, Liu J, Vaidya VS, Bonventre JV. 2009. Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicol Appl Pharmacol 238:306–314. doi: 10.1016/j.taap.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prozialeck WC, Edwards JR, Vaidya VS, Bonventre JV. 2009. Preclinical evaluation of novel urinary biomarkers of cadmium nephrotoxicity. Toxicol Appl Pharmacol 238:301–305. doi: 10.1016/j.taap.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatarinova T, Neely M, Bartroff J, van Guilder M, Yamada W, Bayard D, Jelliffe R, Leary R, Chubatiuk A, Schumitzky A. 2013. Two general methods for population pharmacokinetic modeling: non-parametric adaptive grid and non-parametric Bayesian. J Pharmacokinet Pharmacodyn 40:189–199. doi: 10.1007/s10928-013-9302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Donnell JN, O'Donnell EP, Kumar EJ, Lavhale MS, Andurkar SV, Gulati A, Scheetz MH. 2016. Pharmacokinetics of centhaquin citrate in a dog model. J Pharm Pharmacol 68:803–809. doi: 10.1111/jphp.12554. [DOI] [PubMed] [Google Scholar]
- 29.Wong-Beringer A, Joo J, Tse E, Beringer P. 2011. Vancomycin-associated nephrotoxicity: a critical appraisal of risk with high-dose therapy. Int J Antimicrob Agents 37:95–101. doi: 10.1016/j.ijantimicag.2010.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.