Acinetobacter baumannii is recognized as an urgent public health threat by the Centers for Disease Control and Prevention (CDC). Current treatment options are scarce, particularly against carbapenem-resistant Acinetobacter baumannii (CRAB).
KEYWORDS: minocycline, polymyxin B, beta-lactams, continuous infusion, Acinetobacter baumannii
ABSTRACT
Acinetobacter baumannii is recognized as an urgent public health threat by the Centers for Disease Control and Prevention (CDC). Current treatment options are scarce, particularly against carbapenem-resistant Acinetobacter baumannii (CRAB). We simulated the impact of minocycline standard (200 mg load + 100 mg Q12h) and high (700 mg load + 350 mg Q12h) doses, polymyxin B (2.5 mg/kg Q12h), sulbactam (1 g Q6h and 9 g/24 h as continuous infusion), and meropenem (intermittent 1 or 2 g Q8h and 6 g/24 h as continuous infusion) alone or in combination against CRAB and non-CRAB isolates by simulating human therapeutic dosing regimens in a 72-h, in vitro pharmacodynamic (IVPD) model. There were no monotherapy regimens that demonstrated bactericidal activity against the tested non-CRAB and CRAB strains. Resistance development was common in monotherapy regimens. Against the CRAB isolate, the triple combination of high-dose minocycline (fAUC/MIC 21.2), polymyxin B (fAUC/MIC 15.6), and continuous-infusion sulbactam (67% T>MIC) was the most consistently active regimen. Against non-CRAB, the triple therapy regimen of high-dose minocycline (fAUC/MIC 84.8) with continuous-infusion meropenem (100% T>MIC) and continuous-infusion sulbactam (83% T>MIC), as well as the double therapy of high-dose minocycline (fAUC/MIC 84.8) with continuous-infusion meropenem (100% T>MIC), resulted in persistently bactericidal activity. In conclusion, triple therapy with high-dose minocycline, continuous-infusion sulbactam, and polymyxin B produced the most significant kill against the carbapenem-resistant Acinetobacter baumannii, with no regrowth and minimal resistance development.
TEXT
Carbapenem-resistant Acinetobacter baumannii (CRAB) was recently escalated to an urgent-level threat by the Centers for Disease Control and Prevention (CDC) due to both its propensity for being intrinsically drug resistant as well as its remarkable ability to acquire resistance against most classes of antimicrobials (1). A. baumannii causes a variety of health care-associated infections with notable clinical syndromes, including bloodstream infections (BSIs) and pneumonia, as well as surgical site infections, skin and soft tissue infections, urinary tract infections, and, less commonly, meningitis or peritonitis among patients receiving peritoneal dialysis (2). Infections are most frequently seen among the critically ill and immunocompromised patients, and are associated with high mortality rates (3). Specifically, nosocomial BSIs caused by A. baumannii have reported mortality rates as high as 34% (3), and as high as 43% among intensive care unit (ICU) patients (4). The CDC reported that in addition to an attributable cost of $281 million, there was an estimated 8,500 CRAB cases with 700 deaths among hospitalized patients in 2017 (1). This is particularly concerning, as mortality rates have been shown to double when infection is caused by CRAB compared to non-CRAB (5). Inappropriate empirical antimicrobial selection resulting in delayed therapy has been described as the driver of excess mortality rather than the resistance patterns (6, 7), highlighting the importance of early targeted treatment. To date, there is no “gold standard” treatment for Acinetobacter infections, and empirical therapy is driven by local susceptibility patterns. In an era of a sparse antimicrobial pipeline and increasingly limited treatment options against A. baumannii, one strategy to elucidate optimal antimicrobial therapy is through reevaluation of existing antimicrobial agents.
Minocycline is an example of such an existing drug that is of particular interest due to its safety profile (2, 8, 9). It is available as both an intravenous and oral formulation. It has been successfully used alone, but mostly in combination with other active agents against multidrug-resistant (MDR) A. baumannii (8). However, the optimal dose and role in combination therapy are unknown. Therefore, it is the primary goal of this study to evaluate minocycline alone and in combination with other commonly utilized antimicrobials, including meropenem, sulbactam, and polymyxin B, against CRAB and non-CRAB isolates using standard and pharmacodynamically optimized doses of the antimicrobial agents. Furthermore, we evaluated the combination of meropenem and sulbactam, taking into consideration the favorable safety profile associated with beta-lactams, and we hypothesized that combination therapy would yield greater activity than monotherapy.
(This work was presented in part at the 28th European Congress of Clinical Microbiology and Infectious Diseases, 22 April 2018, in Madrid, Spain.)
RESULTS
Susceptibility testing.
Susceptibility results for both the CRAB and non-CRAB isolates are reported in Table 1. In addition to carbapenem resistance, the CRAB isolate was a multidrug-resistant organism (MDRO), as shown by nonsusceptibility to ≥1 agent in ≥3 antimicrobial classes, including aminoglycosides (e.g., nonsusceptible to tobramycin and amikacin), antipseudomonal carbapenems (e.g., nonsusceptible to meropenem and imipenem), and antipseudomonal fluoroquinolones (e.g., nonsusceptible to levofloxacin) (Table 1) (10).
TABLE 1.
Isolate characteristics and baseline isolate minimum inhibitory concentrations (MIC)
| Isolatea | Source | MDR statusa | MIC μg/ml |
|||||
|---|---|---|---|---|---|---|---|---|
| Minocycline | Polymyxin B | Meropenem | Sulbactamb | Levofloxacina | Amikacin | |||
| CRAB | Human blood | MDRO | 2 | 2 | 128 | 4 | 6 | 128 |
| Non-CRAB | Human blood | Non-MDRO | 0.5 | 0.5 | 0.5 | 2 | NA | NA |
CRAB, carbapenem-resistant Acinetobacter baumannii; Non-CRAB, non-carbapenem-resistant Acinetobacter baumannii; MDR, multidrug resistant; MDRO, multidrug resistant organism; NA, not applicable.
Tested as ampicillin-sulbactam.
Pharmacodynamic models.
Results for monotherapy and combination therapy for non-CRAB and CRAB strains in 72-h in vitro pharmacodynamic models are shown in Fig. 1 and 2, and Fig. 3 and 4, respectively. Quantitative changes in the bacterial population, expressed as change in log CFU/ml, for each antimicrobial regimen are described in Table 2 for non-CRAB isolates and Table 3 for CRAB isolates.
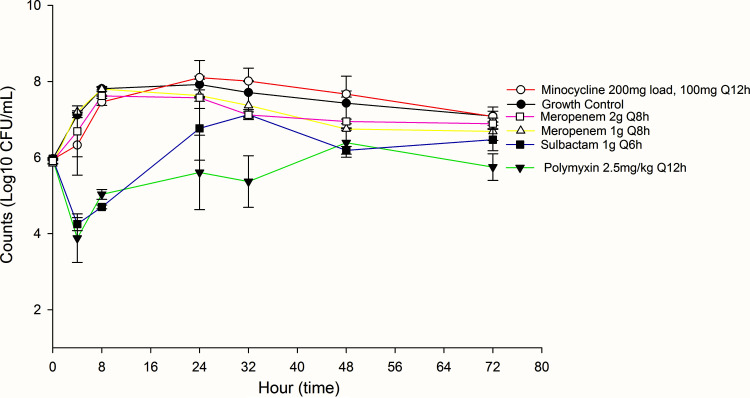
FIG 1.
Activities of tested monotherapies against non-carbapenem-resistant Acinetobacter baumannii (non-CRAB).
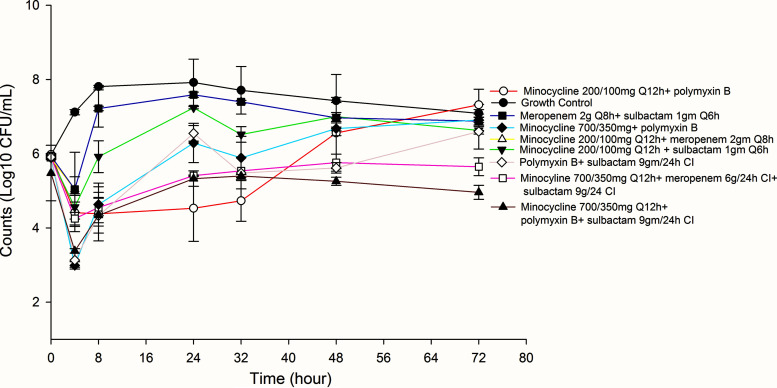
FIG 2.
Activities of tested combination therapies against non-carbapenem-resistant Acinetobacter baumannii (non-CRAB).
FIG 3.
Activities of tested monotherapies against carbapenem-resistant Acinetobacter baumannii (CRAB).
FIG 4.
Activities of tested combination therapies against carbapenem-resistant Acinetobacter baumannii (CRAB).
TABLE 2.
Inoculum change in non-CRAB compared to growth control
| Antimicrobial regimen | Change in bacterial density (log10 CFU/ml) over 24, 48, and 72 h |
||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Monotherapy | |||
| Minocycline 200 mg load, 100 mg Q12h (SD) | –0.59 | +0.64 | +0.23 |
| Minocycline 700 mg load, 350 mg Q12h (HD) | −2.01 | −1.11 | +0.17 |
| Meropenem 1 g Q8h | −2.24 | −0.79 | −0.30 |
| Polymyxin 2.5 mg/kg Q12h | −2.7d | −0.26 | −0.21 |
| Sulbactam 1 g Q6h | -0.59 | −0.5 | −0.39 |
| Dual Therapy | |||
| Minocycline SD + meropenem 1 g Q8h | −2.69d | −0.28 | −0.15 |
| Minocycline SD + sulbactam 1 g Q6h | −0.58 | −0.17 | −0.64 |
| Minocycline SD + polymyxin B 2.5 mg/kg Q12ha | −3.39d | −2.87d | −3.2d |
| Meropenem 1g Q8h + sulbactam 1 g Q6h | −2.59d | −0.66 | +0.07 |
| Minocycline HD + meropenem 6 g/24 h CIc | −4.63d | −4.52d | −3.96d |
| Minocycline HD + sulbactam 9 g/24 h CI | −2.82d | −1.51 | −1.51 |
| Triple Therapy | |||
| Minocycline HD + meropenem 6 g/24 h CI + sulbactam 9g/24h CIb | −3.97d | −3.92d | −4.32d |
Enhanced activity compared to minocycline SD alone at 24 h, 48 h.
Enhanced activity compared to minocycline HD alone at 48 h, 72 h.
Enhanced activity compared to minocycline HD alone at 24 h, 48 h, and 72 h; enhanced activity compared to polymyxin B alone at 72 h. CI, continuous infusion; HD, high dose; SD, standard dose.
P ≤ 0.05.
TABLE 3.
Inoculum change in CRAB compared to growth control
| Antimicrobial regimenc | Change in bacterial density (log10 CFU/ml) over 24, 48, and 72 h |
||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Monotherapy | |||
| Minocycline 200 mg load, 100 mg Q12h (SD) | +0.17 | +0.24 | −0.01 |
| Meropenem 1 g Q8h | −0.30 | −0.69 | −0.40 |
| Meropenem 2 g Q8h | −0.36 | −0.49 | −0.21 |
| Polymyxin B 2.5 mg/kg Q12h | −2.32d | −1.05 | −1.34d |
| Sulbactam 1 g Q6h | −1.15 | −1.25 | −0.62 |
| Dual Therapy | |||
| Minocycline SD + meropenem 2 g Q8h | −0.34 | −0.47 | −0.22 |
| Minocycline SD + sulbactam 1 g Q6h | −0.69 | −0.44 | −0.46 |
| Minocycline SD + polymyxin B 2.5 mg/kg Q12ha | −3.40d | −0.88 | +0.24 |
| Meropenem 2 g Q8hb + sulbactam 1 g Q6h | −0.93 | −0.62 | −0.29 |
| Minocycline HD + polymyxin B | −1.63 | −0.76 | −0.19 |
| Polymyxin B + sulbactam 9 g/24 h CI | −1.38 | −1.81d | −0.50 |
| Triple Therapy | |||
| Minocycline 700 mg load, 350 mg Q12h (HD) + meropenem 6 g/24 h CI + sulbactam 9 g/24 h CI | −2.51d | −1.68 | −1.45d |
| Minocycline HD + polymyxin B + sulbactam 9 g/24 h CI | −2.60d | −2.18d | −2.13d |
Enhanced activity compared to minocycline SD alone at 24 h
Meropenem exposure maximized for CRAB isolates due to resistance
CI, continuous infusion; HD, high dose; SD, standard dose.
P ≤ 0.05.
Non-carbapenem-resistant Acinetobacter baumannii (non-CRAB).
The average bacterial density of the starting inoculum was 5.96 ± 0.06 standard deviation (SD) log10 CFU/ml across both A. baumannii isolates. Despite susceptible MICs, only polymyxin B at 24 h demonstrated a significant reduction in count (log10 CFU/ml), followed by regrowth (Table 2). Among combination therapies, standard-dose minocycline (i.e., 200 mg load with 100 mg every 12 hours [Q12h]) in combination with polymyxin B, as well as pharmacodynamically optimized combination therapies that included high-dose minocycline (i.e., 700 mg load followed by 350 mg Q12h) plus continuous-infusion meropenem and continuous-infusion sulbactam, and high-dose minocycline plus continuous-infusion (CI) meropenem, demonstrated significant reduction in bacterial counts at 24 h, 48 h, and 72 h. Although high-dose minocycline with sulbactam was associated with an initial drop in bacterial counts, regrowth occurred by hours 48 and 72. Regrowth was associated with an increase in MIC for all regimens, except high-dose minocycline with meropenem 6 g/24 h CI and sulbactam 9 g/24 h CI, and high-dose minocycline with meropenem 6 g/24 h CI.
Carbapenem-resistant Acinetobacter baumannii (CRAB).
Among monotherapy options, polymyxin B was associated with a significant reduction in bacterial counts at 24 h and 72 h (Table 3). Standard-dose minocycline with polymyxin B initially resulted in reduced bacterial counts, but regrowth was noted at 48 h and 72 h. While high-dose minocycline with meropenem 6 g/24 h CI and sulbactam 9 g/24 h CI yielded a significant drop in bacterial count at 24 h and 72 h, high-dose minocycline with polymyxin and continuous-infusion sulbactam demonstrated the highest and most consistent reduction in bacterial counts.
Detection of resistance.
Resistance was detected for all monotherapy regimens, with polymyxin B displaying a 128-fold increase in MIC by hour 72 for CRAB isolates (i.e., MIC increased from 2 to 256 μg/ml). All regimens that exhibited regrowth were associated with a rise in MIC with few exceptions. Regimens that did not result in resistance included: (i) high-dose minocycline plus continuous-infusion meropenem and continuous-infusion sulbactam, as well as (ii) high-dose minocycline plus meropenem against non-CRABs. Regimens that resulted in minimal resistance against CRABs included high-dose minocycline plus polymyxin B and continuous-infusion sulbactam. Detailed MICs at 24, 48, and 72 h are in Table 4 and 5.
TABLE 4.
MIC ranges at 24, 48, and 72 h for CRAB isolates
| Regimenc | MIC in µg/mla,b |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Minocycline (susceptible MIC = 2) |
Polymyxin B (susceptible MIC = 2) |
Sulbactam (susceptible MIC = 4) |
|||||||
| 24h | 48h | 72h | 24h | 48 h | 72 h | 24h | 48 h | 72 h | |
| Monotherapy | |||||||||
| Minocycline 200 mg load, 100 mg Q12h (SD) | 4–6 | 12–16 | 12–24 | – | – | – | – | – | – |
| Meropenem 1 g Q8h | – | – | – | – | – | – | – | – | – |
| Meropenem 2 g Q8h | – | – | – | – | – | – | – | – | – |
| Polymyxin B 2.5 mg/kg Q12h | – | – | – | 48–96 | 256–512 | 128–256 | – | – | – |
| Sulbactam 1 g Q6h | – | – | – | – | – | – | 64 | 64 | 24–96 |
| Dual therapy | |||||||||
| Minocycline SD + meropenem 2 g Q8h | 12 | 8–12 | 8 | – | – | – | – | – | – |
| Minocycline SD + sulbactam 1 g Q6h | 6 | 8–32 | 8–16 | – | – | – | 8–16 | 12–>256 | 12–>256 |
| Minocycline SD + polymyxin B 2.5 mg/kg Q12h | 4 | 16 | 24–64 | 3-4 | 16 | 16 | – | – | – |
| Meropenem 2 g Q8h + sulbactam 1 g Q6h | – | – | – | – | – | – | 3–16 | 32–96 | 16–32 |
| Minocycline HD + polymyxin B | 2–4 | 8 | 16 | 2 | 2 | 4 | – | – | – |
| Polymyxin B + sulbactam 9 g/24 h CI | – | – | – | 4–6 | 8–16 | 8–16 | 6–12 | >256 | >256 |
| Triple therapy | |||||||||
| Minocycline 700 mg load, 350 mg Q12h (HD) + meropenem 6 g/24 h CI + sulbactam 9 g/24 h CI | 6 | 8 | 8 | – | – | – | 4–6 | 12 | 12 |
| Minocycline HD + polymyxin B + sulbactam 9 g/24 h CI | 6–8 | 6–8 | 4–6 | 8–12 | 4 | 4 | 4 | 3–4 | 3–4 |
All MICs determined using E-tests and represent the range determined by duplicate runs. The meropenem MIC is 128 µg/ml.
CLSI breakpoints used for susceptibility: minocycline ≤4; polymyxin B ≤2, meropenem ≤2; and ampicillin/sulbactam ≤8/4; –, not applicable.
HD, high dose; SD, standard dose; CI, continuous infusion.
TABLE 5.
MIC Ranges at 24, 48, and 72 h for non-CRAB isolates
| Regimenc | MIC in µg/mla,b |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minocycline (susceptible MIC = 0.5) |
Polymyxin B (susceptible MIC = 0.5) |
Meropenem (susceptible MIC = 0.5) |
Sulbactam (susceptible MIC = 2) |
|||||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24h | 48h | 72h | |
| Monotherapy | ||||||||||||
| Minocycline 200 mg load, 100 mg Q12h (SD) | 6–8 | 4–6 | 4–6 | – | – | – | – | – | – | – | – | – |
| Minocycline 700 mg load, 350 mg Q12h (HD) | 1 | 24 | 24–32 | – | – | – | – | – | – | – | – | – |
| Meropenem 1 g Q8h | – | – | – | – | – | – | 0.5–0.75 | 1.5 | 1–1.5 | – | – | – |
| Polymyxin B 2.5 mg/kg Q12h | – | – | – | 0.75–2 | 1.5–16 | 4–12 | – | – | – | – | – | – |
| Sulbactam 1 g Q6h | – | – | – | – | – | – | – | – | – | 3–4 | 6 | 6–12 |
| Dual therapy | ||||||||||||
| Minocycline SD + meropenem 1 g Q8h | 0.75–1.5 | 1.5–2 | 2 | – | – | – | 1 | 0.75–2 | 2 | – | – | – |
| Minocycline SD + sulbactam 1 g Q6h | 1.5–2 | 1.5 | 1.5–2 | – | – | – | – | – | – | 3 | 3 | 8 |
| Minocycline SD + polymyxin B 2.5 mg/kg Q12h | 0.38–0.75 | 1 | 0.75 | 16–24 | 12 | 12 | – | – | – | – | – | – |
| Meropenem 1g Q8h + sulbactam 1 g Q6h | – | – | – | – | – | – | 1.5 | 1.5 | 1 | 3–4 | 4 | 3 |
| Minocycline HD + meropenem 6 g/24 h CI | NG | NG | NG | – | – | – | NG | NG | NG | – | – | – |
| Minocycline HD + sulbactam 9 g/24 h CI | 1.5 | 1.5 | 1.5 | – | – | – | – | – | – | 2–3 | 3 | 3 |
| Triple therapy | ||||||||||||
| Minocycline HD + meropenem 6 g/24 h CI + sulbactam 9 g/24 h CI | 0.5 | 0.5 | 0.5 | – | – | – | 0.5 | 0.5 | 0.5 | 2 | 2 | 2 |
All MICs determined using E-tests and represent the range determined by duplicate runs.
CLSI breakpoints used for susceptibility: minocycline ≤4; polymyxin B ≤2, meropenem ≤2; and ampicillin/sulbactam ≤8/4; –, not applicable; NG, not enough growth to determine.
HD, high dose; SD, standard dose; CI, continuous infusion.
Pharmacodynamic/pharmacokinetic parameters.
The pharmacodynamic/pharmacokinetic (PK/PD) parameters for tested agents are displayed in Table 6. For the CRAB isolate, the achieved area under the concentration-time curve for the free fraction of the drug divided by the MIC (fAUC/MIC) was 7.04 and within 2% of the targeted fAUC/MIC of 7.18 for minocycline 200 mg load, 100 mg Q12h. High-dose (HD) minocycline (700 mg load, 350 mg Q12h) achieved an fAUC24h of 21.21, while targeted fAUC/MIC was 21.68. Published studies recommend an fAUC/MIC of 20 to 25. The achieved fAUC24h for polymyxin B was 31.26 μg · hour/ml, approximately 35% above the targeted fAUC24h of 21.84 μg · hour/ml, which is above the fAUC24h published parameters of 4 to 22 μg · hour/ml. The achieved fAUC/MIC associated with polymyxin B fAUC24h was 15.63, which was above the targeted fAUC/MIC of 10.92. Lastly, with a recommended target of the cumulative percentage of a 24-h period for which the concentration exceeds the MIC (fT>MIC) of 60%, meropenem achieved 0% fT>MIC, as expected with a MIC of 128 μg/ml, while the sulbactam fT>MIC was 67%.
TABLE 6.
Values of achieved and targeted pharmacokinetic parameters
| Regimena |
fCmax (μg/ml) |
Half-life (h) |
Ke |
% Protein binding |
fAUC (μg · h/ml) |
fAUC/MIC CRAB (non-CRAB) |
% T>MIC (CRAB [non-CRAB]) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Targeted | Achieved | Targeted | Achieved | Targeted | Achieved | Targeted | Targeted | Achieved | Targeted | Achieved | Targeted | Achieved | |
| Minocycline 200 mg load plus 100 mg Q12h | 0.84 (0.6–1.59) | 0.78b | 11 | 10.56 | 0.06 | 0.07 | 76 | 14.4 | 14.1 | 7.2 (28.7) | 7.0 (28.1) | — | — |
| Minocycline 700 mg load, 350 mg Q12h (HD) | 2.94 | 2.73b | 11 | 10.56 | 0.06 | 0.07 | 76 | 43.4 | 42.4 | 21.7 (86.7) | 21.2 (84.8) | — | — |
| Polymyxin B 2.5 mg/kg/day | 1.75 | 1.77b | 4.3 | 7.44 | 0.16 | 0.1 | 79 | 21.8 | 31.3 | 10.9 (43.7) | 15.6 (62.5) | — | — |
| Meropenem 1g Q8h | 48.02 | 38.36c | 1 | 1 | 0.69 | 0.69 | 2 | 335.8 | 287.5 | — | — | 0 (≥50) | 0d (100) |
| Sulbactame 1g Q6h | 26.66 | 35.14c | 1 | 1 | 0.69 | 0.69 | 38 | 253.7 | 338.8 | — | — | ≥60 (≥60) | 67 (83) |
Pharmacokinetic parameters extrapolated to other regimen based on achieved Ke.
Determined with in house bioassay.
Determined by HPLC analysis.
Unable to achieve T>MIC as this isolate was meropenem resistant.
Tested as ampicillin-sulbactam.
For non-CRAB isolates, the achieved fAUC/MIC of 28.14 was within 2% of the targeted fAUC/MIC of 28.74 for minocycline 200 mg load, 100 mg Q12h, and HD minocycline (700 mg load, 350 mg Q12h) achieved fAUC24h of 84.84 while targeted fAUC/MIC was 86.70. The achieved fAUC/MIC for polymyxin B was 62.52, and the targeted fAUC/MIC was 43.68. Free fT>MIC was 100% for meropenem and 83% for sulbactam for the non-CRAB isolate, and met recommendations of >60%. Pharmacokinetic parameters were extrapolated based on the achieved elimination rate constant (Ke) for all other dosing regimens used for pharmacodynamic models.
DISCUSSION
Acinetobacter baumannii is associated with a wide variety of intrinsic and acquired resistance mechanisms (2). Carbapenem resistance is associated with higher mortality rates than carbapenem-susceptible A. baumannii isolates, with an adjusted odds ratio of 2.49 (95% confidence interval [CI], 1.61 to 3.84) (5). A. baumannii can develop resistance to carbapenems through several mechanisms, including plasmid and chromosomally encoded carbapenemases (e.g., OXA-like class D beta-lactamases), metallo beta-lactamases, porin changes, penicillin-binding protein alterations, as well as efflux pumps. Due to this propensity for multidrug resistance, empirical therapy may miss coverage of CRAB isolates, creating a notable delay in therapy that may be responsible for the increased mortality, as previously demonstrated in Enterobacteriaceae (7). Furthermore, even upon organism identification and susceptibility reporting, optimal anti-CRAB therapy remains unclear. Therefore, our study aimed to evaluate the optimal monotherapy or combination therapy against CRAB and non-CRAB isolates by looking at standard doses as well as pharmacodynamically optimized doses (11–14).
This work identifies several critical dosing strategies to consider. First, despite having low MICs, non-CRAB monotherapy generally led to regrowth that was associated with development of resistance within 24 to 72 h, depending on the regimen, but favoring pharmacodynamically optimized combination therapy. The pharmacodynamic index most associated with activity for polymyxin B is fAUC24h and fAUC/MIC for minocycline, although standard-dose minocycline (e.g., 200 mg) has been associated with development of resistance, similar to the observations within this study suggesting that an fAUC/MIC goal of 20 to 25 may be needed (11, 15). A previous dose-finding study found high-dose minocycline capped at 700 mg daily, after a loading dose up to 700 mg, was safe when used for its anti-inflammatory properties in ischemic stroke management for 6 total doses (14). Our study showed that, although regrowth occurs with monotherapy, combining high-dose minocycline with continuous-infusion meropenem with or without sulbactam led to the highest reduction in bacterial counts against non-CRAB strains. For CRAB isolates, triple therapy may be required as a 2-log kill was sustained at 24, 48, and 72 h only when treated with high-dose minocycline in combination with polymyxin B and continuous-infusion sulbactam. However, the safety profile for long-term therapy in human studies is needed.
Meropenem and sulbactam are time-dependent antimicrobial agents that exhibit activity when the free concentrations are maintained above the MIC (fT>MIC) at least 40 to 60% of the time (16, 17). Continuous or extended infusion meropenem was found to have superior cumulative fraction of response as determined by population pharmacokinetic modeling and Monte Carlo simulation (13). Therefore, this is a recommended dosing strategy, particularly for less-susceptible organisms, including A. baumannii, and Pseudomonas aeruginosa. Similarly, prolonging sulbactam infusion has been associated with favorable target attainment probability (12). While the current study did not use extended infusion (e.g., over 4 h) dosing strategies, it does highlight that including continuous-infusion beta-lactam therapy as double and/or triple therapy may yield greater reductions in bacterial count than intermittent dosing (e.g., Q8h 30 min infusion).
CRAB isolates remain difficult to treat, and optimal therapy that minimizes toxicity needs to be elucidated. While polymyxin B monotherapy demonstrated activity against the CRAB isolate, development of resistance was noted over the course of 72 h. Current literature comparing polymyxin monotherapy versus combination therapy against clinical outcomes is limited. However, as observed within this study, rapid emergence of resistance, particularly when used as monotherapy, is concerning and has resulted in clinical failure (18, 19). Furthermore, heteroresistance among clinical isolates (20) and a paradoxical amplification of resistance associated with increased polymyxin B exposure was previously observed, highlighting the pitfalls of monotherapy against A. baumannii (21). Our study demonstrated that triple therapy, particularly with high-dose minocycline, polymyxin B, and continuous-infusion sulbactam, resulted in the greatest reduction of bacterial counts without emergence of resistance among CRABs. This finding is supported by previous data of a 14-day hollow fiber model, where administration of high-dose sulbactam (tested as ampicillin/sulbactam) as a component of a combination regimen combated polymyxin B resistance, indicating that dose manipulation may aid in overcoming some resistance mechanisms (22). Of note, while tested as ampicillin/sulbactam, anti-Acinetobacter activity is attributed to the sulbactam component through the binding of penicillin binding protein 1 (PBP1) and PBP3 (23).
Our study has several limitations. This is an in vitro pharmacodynamic model with a limited duration of 72 h that does not consider patient’s own immune function, limiting extrapolation to clinical outcomes. Additionally, this study simulated only two strains of Acinetobacter baumannii at a fixed initial inoculum of 106 CFU/ml (i.e., final observations may not predict the results of a higher burden of infection). It evaluated only four antimicrobial agents, and assumed normal renal function. Therefore, it is unclear whether other antimicrobial agents provide more optimal reductions in bacterial counts, and how impaired renal function might alter pharmacodynamic effects against these isolates. Nonetheless, this study provides important insight on the difficulties associated with eradicating A. baumannii. Both CRAB and non-CRAB isolates demonstrate propensity for rapidly inducible resistance mechanisms, posing a serious threat to public health. Data indicate there is an unmet clinical need to identify optimal therapy for both CRAB and non-CRAB isolates in order to improve patient outcomes.
In conclusion, carbapenem-resistant Acinetobacter was recently escalated to an urgent public health threat by the CDC, representing a large burden among immunocompromised patients (1). This escalation in threat severity is attributed to the ease of resistance development, lack of current effective antibiotics, and lack of antibiotics in the development pipeline. Consequently, our study aimed to study minocycline alone and in various combinations with polymyxin B, meropenem, and sulbactam. Our data confirm that in vitro resistance can develop rapidly across both CRAB and non-CRAB isolates. This was associated with most regimens failing to sustain kill at 24, 48, and 72 h. Against CRAB isolates, the triple combination of high-dose minocycline, polymyxin B, and continuous-infusion sulbactam was the most consistently active regimen, while high-dose minocycline with continuous-infusion meropenem with and without continuous-infusion sulbactam was most persistently bactericidal against non-CRAB isolates. Our results should be applied to clinical practice cautiously, as confirmation from clinical outcome trials is necessary.
MATERIALS AND METHODS
Bacterial strains.
Two clinical isolates of Acinetobacter baumannii (NR-13382 and NR-17786) were tested. Both were isolated from human blood and obtained from the Biodefense and Emerging Infection Research Resources Repository, Manassas, VA (BEI). Strain NR-13382 was both multidrug resistant (MDR) in accordance with previous definitions (10) and a CRAB, while strain NR-17786 was a non-MDR, non-CRAB.
Media and antimicrobials.
Minocycline HCl powder (The Medicines Company; lot number M3401602), meropenem (Fresenius Kabi, LLC; lot number 0004D51), ampicillin/sulbactam (Pfizer Inc.; lot number 16/17000), and polymyxin B (X-Gen Pharmaceuticals; lot number AB7600) drug products were used in the experiments. Drug products were supplied by the Providence Veterans Affairs Medical Center Pharmacy Department. Antimicrobial stability was ensured in accordance with drug monographs and Trissel’s Stability of Compounded Formulations, accessed via Micromedex. Continuous infusion regimens were replaced with new drug approximately every 8 to 10 h. Cation-adjusted (calcium, 25 μg/ml and magnesium, 12.5 μg/ml) Mueller-Hinton broth (CAMHB; Difco Laboratories, Sparks, MD, USA) was used to determine susceptibility testing and for in vitro models.
For non-CRAB isolates, monotherapy regimens included: (i) minocycline 200 mg load, followed by 100 mg Q12h (i.e., standard dose, SD); (ii) minocycline 700 mg load, followed by 350 mg Q12h (i.e., high-dose, HD); (iii) meropenem 1 g Q8h; (iv) polymyxin 2.5 mg/kg Q12h; or (v) sulbactam 1g Q6h. Dual therapy regimens included: (i) minocycline SD + meropenem 1g Q8h; (ii) minocycline SD + sulbactam 1g Q6h; (iii) minocycline SD + polymyxin 2.5 mg/kg Q12h; (iv) meropenem 1g Q8h + sulbactam 1g Q6h; (v) minocycline HD + meropenem 6 g/24 h continuous infusion (CI); or (vi) minocycline HD + sulbactam 9 g/24 h CI. Finally, triple therapy maximizing drug exposure included minocycline HD + meropenem 6 g/24 h CI + sulbactam 9 g/24 h CI. Initial doses were selected based on MIC. For example, lower drug exposures were utilized for non-CRAB isolates because eradication was anticipated. These doses were evaluated against pharmacodynamically optimized doses (i.e., continuous infusion and higher doses) to assess differences in isolate eradication.
For CRAB isolates, monotherapy regimens included: (i) minocycline 200 mg load, followed by 100 mg Q12h (i.e., standard dose, SD); (ii) meropenem 1 g Q8h; (iii) meropenem 2 g Q8h; (iv) polymyxin 2.5 mg/kg Q12h; or (v) sulbactam 1g Q6h. Dual therapy regimens included: (i) minocycline SD + meropenem 2 g Q8h; (ii) minocycline SD + sulbactam 1g Q6h; (iii) minocycline SD + polymyxin B 2.5 mg/kg Q12h; (iv) meropenem 2 g Q8h + sulbactam 1g Q6h; (v) minocycline HD + polymyxin B 2.5 mg/kg Q12h; or (vi) polymyxin B 2.5 mg/kg Q12h + sulbactam 9 g/24 h CI. Finally, triple therapy maximizing drug exposure included (i) minocycline HD + meropenem 6 g/24 h CI + sulbactam 9 g/24 h CI and (ii) minocycline HD + polymyxin B 2.5 mg/kg Q12h + sulbactam 9 g/24 h CI. A higher dose of meropenem was selected for dual therapy due to carbapenem resistance and the necessity for optimized exposures. Additionally, more polymyxin B-containing regimens were included because of inherent challenges of eradicating CRAB isolates. Pharmacodynamically optimized doses were chosen to maximize exposures while maintaining clinically achievable concentrations previously utilized in practice.
Susceptibility testing.
MIC was determined via broth microdilution in accordance with Clinical and Laboratory Standards Institute (CLSI) standards using cation-adjusted Mueller-Hinton broth (24, 25). Combination MICs were not conducted. To assess the activity of ampicillin, isolates were tested against sulbactam alone prior to use of ampicillin/sulbactam and no difference in MIC was noted. Baseline isolate characteristics are further depicted in Table 1.
In vitro pharmacodynamic model.
An in vitro 72-h pharmacodynamic (IVPD) model using a 250-ml one-compartment chamber with ports for removal of medium, administration of antimicrobials, and collection of bacterial samples was employed using previously established methodology for monotherapy and combination therapy (26–28). The chamber was prefilled with medium, and isolate inoculation as well as intermittent antimicrobial boluses were slowly (over ∼1 min) administered via injection port. Continuous-infusion antimicrobials were combined with broth. All models were simulated in duplicate to ensure reproducibility. Prior to each experiment, A. baumannii colonies from an overnight growth on tryptic soy agar (TSA) (Difco, Becton, Dickinson Co., Sparks, MD, USA) were suspended in 0.9% sodium chloride to obtain a 0.5-McFarland standard suspension for an initial starting inoculum of 106 CFU/ml. Models were placed in a 37°C water bath with a magnetic stir bar for continuous mixing of medium for the duration of the experiment. Antibiotic-containing broth was continuously replaced using a peristaltic pump (Masterflex; Cole-Parmer Instrument, Chicago, IL) at a rate simulating the half-life elimination for the respective antibiotic. Combination regimen experiments were set for the rate of the drug with the shortest half-life, and the drug with the longer half-life was supplemented in accordance with previously established methods (27, 28). Of note, because three different half-lives could not be simulated, for the combination of high-dose minocycline with polymyxin B and continuous-infusion sulbactam, polymyxin B was utilized as the fastest half-life drug and sulbactam concentration was maintained as a constant (i.e., the broth contained sulbactam throughout the experiment). Consequently, the aforementioned regimen did not receive a loading dose of sulbactam, while all other continuous infusion regimens did. All antimicrobials were administered to simulate humanized doses corresponding to exposures reflecting approximate free drug concentrations (i.e., fCmax), based on protein binding affinity at standard doses and pharmacodynamically optimized doses (Table 6).
Pharmacodynamic analysis.
Approximately 1-ml samples were collected from each model at 0, 4, 8, 24, 32, 48, and 72 h and serially diluted with 0.9% sodium chloride. Bacterial counts were determined by inoculating three 20-μl drops of each dilution onto TSA plates. Plated samples were incubated at 37°C for 24 h and colonies were subsequently counted (CFU per milliliter) with a limit of detection of 2.0 log10 CFU/ml (29–31). Growth curves were conducted for each pathogen at the fastest and slowest half-life of each model. Antimicrobial carryover was accounted for by serial dilution of all plated samples. When the anticipated dilution was near the MIC, vacuum filtration was utilized to remove antimicrobial agents through the use of a 0.45-μm filter and sterile water. Filters were plated on TSA plates and incubated at 37°C for 24 h. Time-kill curves of colony counts (log 10 CFU/ml) versus time were plotted for each model (SigmaPlot V13.0, Systat Software, Inc.). Bactericidal activity (i.e., 99.9% kill) was defined as a ≥3 log10 CFU/ml reduction in colony counts compared to the initial inoculum, while bacteriostatic activity was defined as a <3 log10 CFU/ml reduction in colony count (26). Models without change in colony count were deemed inactive (26). When comparing combination regimens, enhanced activity was defined as a ≥2 log10 CFU/ml increase in kill compared to the most active single agent within that combination, while improvement was defined as a one to two log10 CFU/ml increase in kill compared to the most active single agent. Colony count reductions were measured over a 72-h period at 24, 48, and 72 h.
Pharmacokinetic analysis.
Samples for pharmacokinetic analyses were obtained through the injection port at 0, 0.5, and 4 h for verification of target antibiotic concentrations. All samples were stored at –80°C until analysis. Meropenem and ampicillin-sulbactam concentrations were determined by a previously described and validated high-pressure liquid chromatography (HPLC) method (Center for Anti-Infective Research and Development, Hartford, CT) (32). Polymyxin B concentrations were determined by a modified microbioassay utilizing Micrococcus luteus ATCC 49732 as the reference organism (33, 34). Bioassay models were run at a higher concentration of 30 μg/ml in order to achieve zones of inhibition in the agar. As previously described, the bacteria were incorporated into molten cation-adjusted MHA to achieve a final concentration of approximately 5 log 10 CFU/ml (34). Three standard solutions (60, 30, and 15 μg/ml) and duplicates of three test samples from the model were pipetted into a 6-mm diameter hole in the agar. Plates were incubated at 35°C for 24 h and the zones of inhibition were measured using calipers. Minocycline concentrations were determined by standard agar diffusion assay using a lawn of 0.5 McFarland of Micrococcus luteus ATCC 49732 on cation-adjusted MHA (33). Bioassay models were run at a higher concentration of 30 μg/ml in order to achieve zones of inhibition on the agar. Three standard solutions (30, 15, and 7.5 μg/ml) and duplicates of three test samples from the model were pipetted onto a 6-mm blank disk on the agar. Plates were incubated at 35°C for 24 h, and the zones of inhibition were measured using calipers. Standard curves for the assays were made by plotting the inhibition zone diameter versus the standard drug concentrations. Both assays were linear over their concentration ranges; minocycline (R2 = 0.9643) and polymyxin B (R2 = 0.9944).
Based on previously described pharmacodynamic parameters, we targeted a higher fAUC/MIC of 20 to 25 for minocycline monotherapy (11). However, in order to test for enhanced activity of minocycline in combination with beta-lactams, we targeted a lower fAUC/MIC. Optimal polymyxin B fAUC/MIC for Acinetobacter is recommended to be around 12 to 48 (15, 35, 36). For sulbactam (12, 16) and meropenem (13, 17), we targeted a fT>MIC of at least 40 to 60%. The AUC was calculated based on the trapezoidal rule in Excel.
Resistance.
Samples from 0, 24, 48, and 72 h were taken and approximately 100 μl was plated on Mueller-Hinton agar (MHA) for MIC determination via Etest for each antimicrobial agent.
Statistical analysis.
Bacterial colony counts (i.e., log10 CFU/ml) were compared at 24, 48, and 72 h by two-way analysis of variance using Tukey’s post hoc test. A P value of ≤0.05 was considered significant. Data were plotted and graphed using SigmaPlot V13.0 software (Systat Software, Inc.). All data were analyzed using SPSS statistical software (SPSS version 24 Inc. Chicago, IL).
ACKNOWLEDGMENTS
This work was not supported by funding. However, the minocycline product was received from The Medicines Company.
We thank BEI Resources, NIAID, NIH for the Acinetobacter baumannii isolate 9 (NR-13382).
The views expressed are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs.
This work has been supported in part by the Office of Academic Affiliations, Department of Veterans Affairs, and with resources and the use of facilities at Providence VA Medical Center.
Kerry L. LaPlante has received research funding or acted as an advisor or consultant for Merck, Davol/BARD, Actavis, Melinta Therapeutics, and Pfizer Inc. Maya Beganovic, Megan K. Luther, and Kathryn E. Daffinee have no actual or potential conflicts of interest to disclose.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2019. Antibiotic resistance threats in the United States. U.S. Department of Health and Human Services, Atlanta, GA. [Google Scholar]
- 2.Karageorgopoulos DE, Falagas ME. 2008. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis 8:751–762. doi: 10.1016/S1473-3099(08)70279-2. [DOI] [PubMed] [Google Scholar]
- 3.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 4.Falagas ME, Bliziotis IA, Siempos II. 2006. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care 10:R48. doi: 10.1186/cc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemos EV, de la Hoz FP, Einarson TR, McGhan WF, Quevedo E, Castaneda C, Kawai K. 2014. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect 20:416–423. doi: 10.1111/1469-0691.12363. [DOI] [PubMed] [Google Scholar]
- 6.Huang ST, Chiang MC, Kuo SC, Lee YT, Chiang TH, Yang SP, Ti Y, Chen TL, Fung CP. 2012. Risk factors and clinical outcomes of patients with carbapenem-resistant Acinetobacter baumannii bacteremia. J Microbiol Immunol Infect 45:356–362. doi: 10.1016/j.jmii.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Lodise TP, Berger A, Altincatal A, Wang R, Bhagnani T, Gillard P, Bonine NG. 2019. Antimicrobial resistance or delayed appropriate therapy—does one influence outcomes more than the other among patients with serious infections due to carbapenem-resistant versus carbapenem-susceptible Enterobacteriaceae? Open Forum Infect Dis 6:ofz194. doi: 10.1093/ofid/ofz194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff DA, Bauer KA, Mangino JE. 2014. Bad bugs need old drugs: a stewardship program's evaluation of minocycline for multidrug-resistant Acinetobacter baumannii infections. Clin Infect Dis 59 (Suppl 6):S381–7. doi: 10.1093/cid/ciu593. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie DJ, Garavaglia-Wilson A. 2014. A review of intravenous minocycline for treatment of multidrug-resistant Acinetobacter infections. Clin Infect Dis 59 (Suppl 6):S374–80. doi: 10.1093/cid/ciu613. [DOI] [PubMed] [Google Scholar]
- 10.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 11.Alfouzan WA, Noel AR, Bowker KE, Attwood MLG, Tomaselli SG, MacGowan AP. 2017. Pharmacodynamics of minocycline against Acinetobacter baumannii studied in a pharmacokinetic model of infection. Int J Antimicrob Agents 50:715–717. doi: 10.1016/j.ijantimicag.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Jaruratanasirikul S, Wongpoowarak W, Aeinlang N, Jullangkoon M. 2013. Pharmacodynamics modeling to optimize dosage regimens of sulbactam. Antimicrob Agents Chemother 57:3441–3444. doi: 10.1128/AAC.00342-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J. 2009. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother 64:142–150. doi: 10.1093/jac/dkp139. [DOI] [PubMed] [Google Scholar]
- 14.Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, Hall CE, Switzer JA, Ergul A, Hess DC. 2010. Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke 41:2283–2287. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, Kaye D, Mouton JW, Tam VH, Thamlikitkul V, Wunderink RG, Li J, Nation RL, Kaye KS. 2019. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 39:10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama Y, Matsumoto K, Ikawa K, Watanabe E, Shigemi A, Umezaki Y, Nakamura K, Ueno K, Morikawa N, Takeda Y. 2014. Pharmacokinetic/pharmacodynamic evaluation of sulbactam against Acinetobacter baumannii in in vitro and murine thigh and lung infection models. Int J Antimicrob Agents 43:547–552. doi: 10.1016/j.ijantimicag.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Roberts JA, Lipman J, Blot S, Rello J. 2008. Better outcomes through continuous infusion of time-dependent antibiotics to critically ill patients? Curr Opin Crit Care 14:390–396. doi: 10.1097/MCC.0b013e3283021b3a. [DOI] [PubMed] [Google Scholar]
- 18.Marchaim D, Chopra T, Pogue JM, Perez F, Hujer AM, Rudin S, Endimiani A, Navon-Venezia S, Hothi J, Slim J, Blunden C, Shango M, Lephart PR, Salimnia H, Reid D, Moshos J, Hafeez W, Bheemreddy S, Chen TY, Dhar S, Bonomo RA, Kaye KS. 2011. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 55:593–599. doi: 10.1128/AAC.01020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rojas LJ, Salim M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Marshall S, Rudin SD, Domitrovic TN, Hujer AM, Hujer KM, Doi Y, Kaye KS, Evans S, Fowler VG, Jr, Bonomo RA, van Duin D, Antibacterial Resistance Leadership Group. 2017. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae: laboratory detection and impact on mortality. Clin Infect Dis 64:711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, Liolios L. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 50:2946–2950. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuji BT, Landersdorfer CB, Lenhard JR, Cheah SE, Thamlikitkul V, Rao GG, Holden PN, Forrest A, Bulitta JB, Nation RL, Li J. 2016. Paradoxical effect of polymyxin B: high drug exposure amplifies resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 60:3913–3920. doi: 10.1128/AAC.02831-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenhard JR, Smith NM, Bulman ZP, Tao X, Thamlikitkul V, Shin BS, Nation RL, Li J, Bulitta JB, Tsuji BT. 2017. High-dose ampicillin-sulbactam combinations combat polymyxin-resistant Acinetobacter baumannii in a hollow-fiber infection model. Antimicrob Agents Chemother 61:e01268-16. doi: 10.1128/AAC.01268-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penwell WF, Shapiro AB, Giacobbe RA, Gu RF, Gao N, Thresher J, McLaughlin RE, Huband MD, DeJonge BL, Ehmann DE, Miller AA. 2015. Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii. Antimicrob Agents Chemother 59:1680–1689. doi: 10.1128/AAC.04808-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2017. M100-S27. Performance standards for antimicrobial susceptibility testing: 27th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2015. M07-A10. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard- tenth edition. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.LaPlante KL, Rybak MJ, Leuthner KD, Chin JN. 2006. Impact of Enterococcus faecalis on the bactericidal activities of arbekacin, daptomycin, linezolid, and tigecycline against methicillin-resistant Staphylococcus aureus in a mixed-pathogen pharmacodynamic model. Antimicrob Agents Chemother 50:1298–1303. doi: 10.1128/AAC.50.4.1298-1303.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaser J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J Antimicrob Chemother 15 (Suppl A):125–130. doi: 10.1093/jac/15.suppl_a.125. [DOI] [PubMed] [Google Scholar]
- 28.McConeghy KW, LaPlante KL. 2010. In vitro activity of tigecycline in combination with gentamicin against biofilm-forming Staphylococcus aureus. Diagn Microbiol Infect Dis 68:1–6. doi: 10.1016/j.diagmicrobio.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 29.LaPlante KL, Rybak MJ. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 48:4665–4672. doi: 10.1128/AAC.48.12.4665-4672.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luther MK, Arvanitis M, Mylonakis E, LaPlante KL. 2014. Activity of daptomycin or linezolid in combination with rifampin or gentamicin against biofilm-forming Enterococcus faecalis or E. faecium in an in vitro pharmacodynamic model using simulated endocardial vegetations and an in vivo survival assay using Galleria mellonella larvae. Antimicrob Agents Chemother 58:4612–4620. doi: 10.1128/AAC.02790-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luther MK, Mermel LA, LaPlante KL. 2014. Comparison of ML8-X10 (a prototype oil-in-water micro-emulsion based on a novel free fatty acid), taurolidine/citrate/heparin and vancomycin/heparin antimicrobial lock solutions in the eradication of biofilm-producing staphylococci from central venous catheters. J Antimicrob Chemother 69:3263–3267. doi: 10.1093/jac/dku281. [DOI] [PubMed] [Google Scholar]
- 32.Steed M, Vidaillac C, Rybak MJ. 2011. Evaluation of ceftaroline activity versus daptomycin (DAP) against DAP-nonsusceptible methicillin-resistant Staphylococcus aureus strains in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 55:3522–3526. doi: 10.1128/AAC.00347-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowker KE, Noel AR, Macgowan AP. 2008. Pharmacodynamics of minocycline against Staphylococcus aureus in an in vitro pharmacokinetic model. Antimicrob Agents Chemother 52:4370–4373. doi: 10.1128/AAC.00922-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tam VH, Schilling AN, Vo G, Kabbara S, Kwa AL, Wiederhold NP, Lewis RE. 2005. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob Agents Chemother 49:3624–3630. doi: 10.1128/AAC.49.9.3624-3630.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zavascki AP, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL, Li J. 2008. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis 47:1298–1304. doi: 10.1086/592577. [DOI] [PubMed] [Google Scholar]
- 36.Kuti JL. 2016. Optimizing antimicrobial pharmacodynamics: a guide for your stewardship program. Revista Médica Clínica Las Condes 27:615–624. doi: 10.1016/j.rmclc.2016.08.001. [DOI] [Google Scholar]