Abstract
Background
Brachytherapy (permanent implantation of radioactive seeds) has emerged as an alternative to existing standard therapy with radical prostatectomy or external beam radiotherapy in the treatment of clinically localized (T1 and T2) prostate cancer. The Genitourinary Cancer Disease Site Group of the Cancer Care Ontario Practice Guidelines Initiative examined the role of brachytherapy in treating clinically localized prostate cancer.
Methods
A systematic review of articles published from 1988 to April 1999, retrieved through a search of MEDLINE and CANCERLIT databases, was combined with a consensus interpretation of the evidence in the context of conventional practice.
Results
Although there were no randomized trials comparing brachytherapy with standard treatment, evidence was available from 13 case series and 3 cohort studies. Rates of freedom from biochemical failure (biochemically no evidence of disease [bNED]) varied considerably from one series to another and were highly dependent on tumour stage, grade and pretreatment serum prostate-specific antigen (PSA) levels. Results in patients with favourable tumours (T1 or T2 tumour, Gleason score of 6 or lower, serum PSA level of 10 ng/mL [μg/L] or less) were comparable to those in patients undergoing radical prostatectomy. Acute urinary retention was reported in 1%–14% of patients. Long-term sequelae occurred in less than 5% of patients and included urinary incontinence, cystitis, urethral strictures and proctitis. Sexual potency was maintained after implantation in 86%–96% of patients.
Interpretation
At present, there is insufficient evidence to recommend the use of brachytherapy over current standard therapy for localized prostate cancer. Brachytherapy using transrectal ultrasound guidance for seed implantation is promising in terms of freedom from biochemical failure in selected patients with early-stage prostate cancer. Brachytherapy is currently available outside of clinical trials, but whenever possible patients should be asked to participate in randomized trials comparing brachytherapy and current standard therapy. Brachytherapy should be available to selected patients (those with T1c or T2a tumours, a Gleason score of 6 or lower and a serum PSA level of 10 μg/L or less), after discussion of the available data and potential adverse effects.
Otherwise healthy men with clinically localized prostate cancer have a choice of therapies. Although both radical prostatectomy and external beam radiotherapy have a risk of significant long-term morbidity, patients with low-risk prostate cancer have excellent 5-year biochemical progression-free rates with these standard therapies: 85%–96% after radical prostatectomy1,2,3 and 81%–94% after external beam radiotherapy.1,4,5 Given the long survival times of this population, biochemical freedom from relapse (bNED) is widely accepted as both appropriate and practical as a surrogate endpoint for ultimate cancer control.
Brachytherapy (permanent implantation of radioactive seeds in the prostate) (Fig. 1) is not new. In the 1970s and early 1980s, retropubic implantation of iodine 125 seeds was an attractive alternative to external beam radiotherapy for localized prostate cancer because of its ability to deliver a much higher dose of radiation. Unfortunately, 15-year follow-up data indicated that only 21% of patients were free of local failure.6 The free-hand technique used then to guide implantation is now recognized as being suboptimal (Table 1).
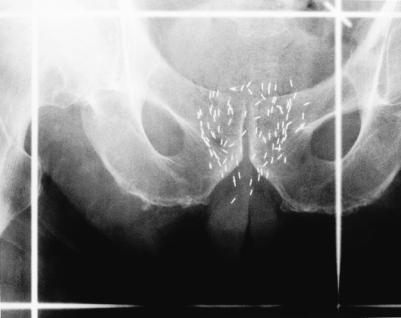
Fig. 1: Anteroposterior radiograph, showing radioactive seeds implanted into prostate gland.
Table 1
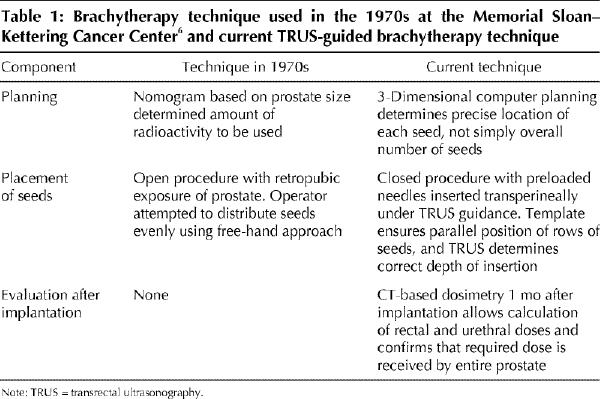
Modern brachytherapy, using either iodine 125 or palladium 103 seeds, is performed under the guidance of transrectal ultrasonography (TRUS) and is planned and evaluated using 3-dimensional computer software. Promising early results, a minimally invasive technique and rapidity of the outpatient procedure have made brachytherapy an attractive alternative to radical prostatectomy and external beam radiotherapy for localized prostate cancer. Although widely available and popular in the United States, brachytherapy has only recently become available in Canada.
The Genitourinary Cancer Disease Site Group of the Cancer Care Ontario Practice Guidelines Initiative conducted a systematic review of the literature to clarify the role of brachytherapy in treating clinically localized (T1 and T2) prostate cancer. The group comprises urologists, radiation oncologists, medical oncologists, a pathologist and 2 community representatives.
Methods
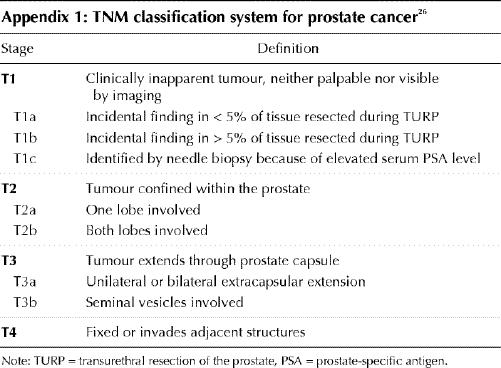
A systematic search of the MEDLINE and CANCERLIT databases was carried out for articles published from 1988 to April 1999 using the search terms “prostate cancer,” “prostate neoplasm,” “brachytherapy,” “seed implant,” “interstitial radiotherapy,” “practice guideline,” “meta-analysis,” “randomized clinical trial” and “clinical trial.” No randomized trials comparing brachytherapy with standard treatment were found. Relevant articles evaluating permanent seed implantation for clinically localized prostate cancer were reviewed. Articles had to meet the following criteria: series limited to T1 or T2 prostate cancer (Appendix 1); brachytherapy performed under ultrasound or CT guidance; outcome data reported in terms of freedom from biochemical failure (bNED [biochemically no evidence of disease]), biopsy results or toxicity; and report not published as an abstract.
Although survival is the ultimate and irrefutable measure of successful treatment of cancer, the long natural history of prostate cancer promotes the use of surrogate endpoints such as the serum prostate-specific antigen (PSA) level and post-treatment biopsy results. These endpoints antedate clinical progression and ultimate death from prostate cancer by years and thus permit more rapid evaluation of treatment efficacy. Their use, however, is not without controversy.
The 1997 publication of the American Society for Therapeutic Radiology and Oncology consensus guideline8 provided criteria for defining biochemical failure after radiotherapy. Before this guideline, many different definitions of PSA failure were used, based either on a threshold9,10 or on consecutive rises in PSA level.11,12,13
Biopsy after radiotherapy is not routinely used and is associated with problems of false-positive and false-negative results and of patient selection bias. Biopsy status cannot be considered a “gold standard” of treatment efficacy.
Evidence was selected and reviewed by a member of the Genitourinary Cancer Disease Site Group. The group reviewed and discussed a draft of the evidence summary. The final version was approved by the group and the Practice Guidelines Coordinating Committee of Cancer Care Ontario.7
Results
The following studies were reviewed: 10 case series and 1 cohort study of brachytherapy alone; 1 case series of external beam radiotherapy followed by brachytherapy as a boost, and 2 cohort studies comparing this combination with brachytherapy alone; and 2 case series of brachytherapy followed by external beam radiotherapy.
Brachytherapy alone
Results from 6 case series9,10,11,12,14,15 are summarized in Table 2. Rates of freedom from biochemical failure (bNED) varied considerably from one series to another, from 63% at 4 years (n = 92)15 to 93% at 5 years (n = 197).12 This variation was largely due to differences in patient selection criteria.
Table 2
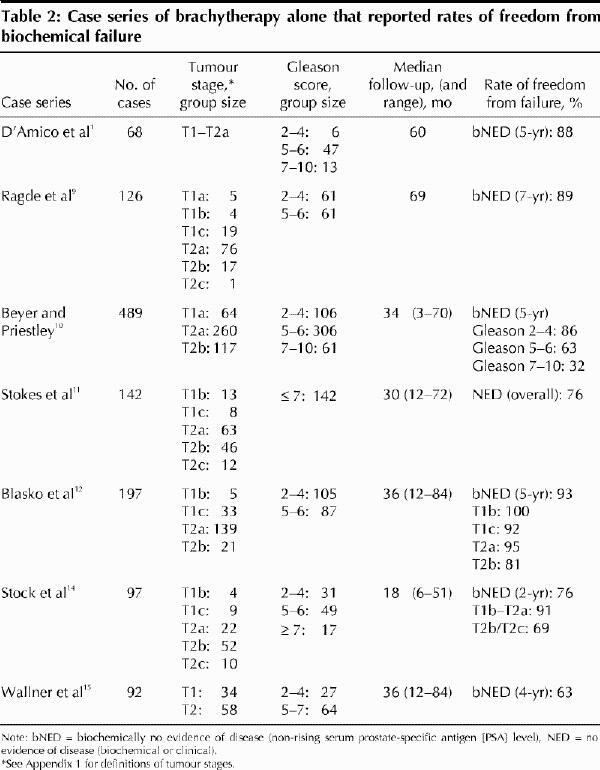
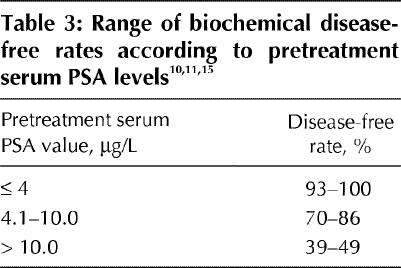
Beyer and Priestley10 reported the largest case series (n = 489) and elucidated the main prognostic factors for freedom from biochemical failure. Their series, and others, have documented decreasing 5-year actuarial bNED rates with increasing Gleason scores,16 higher pretreatment PSA levels (Table 3) and increasing tumour stage. BNED rates of 90%–94% have been reported for T1 tumours, 70%–75% for T2a tumours and 34% for T2b and T2c tumours.10,11
Table 3
D'Amico and associates1 described 68 patients who received 103Pd seed implants as part of a cohort study comparing brachytherapy, external beam radiotherapy and radical prostatectomy. The known prognostic variables (tumour stage, serum PSA level and Gleason score) were combined to create low-, intermediate- and high-risk groups. For low-risk patients (T1c or T2a tumour, Gleason score of 6 or lower, and serum PSA level of 10 ng/mL [μg/L] or less) the 5-year bNED rate was 88% (28/32). For those at intermediate risk (T2b tumour, Gleason score of 7 and serum PSA level greater than 10 μg/L) the bNED rate fell to 33% (5/15). For the high-risk patients (T2c tumour, Gleason score greater than 7 and serum PSA level greater than 20 μg/L) the rate was 0% (0/19) at 3 years.
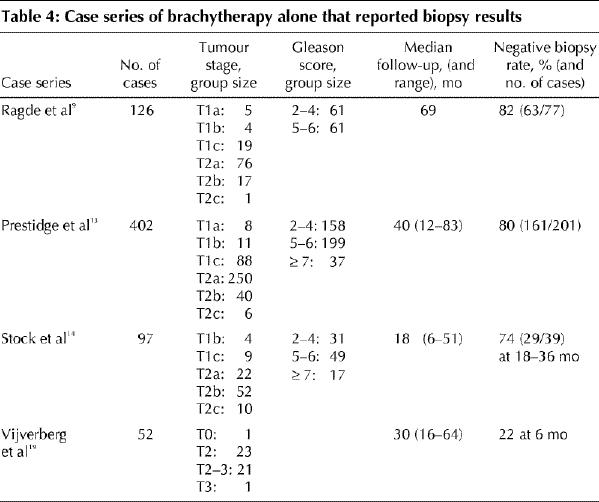
Seven case series reported biopsy results.9,11,13,14,17,18,19 Table 4 provides negative biopsy rates for 4 of these series.9,13,14,19 Case selection and difficulties in obtaining biopsy specimens make comparison of the results difficult. Prestidge and associates13 and Ragde and associates,9 reporting from the same centre, found positive biopsy results in 3%–5% of cases and negative results in 80%–82% of cases; the remaining results were indeterminate. Others14,18 reported positive biopsy results in 16%–26% of cases at 18–36 months after brachytherapy.
Table 4
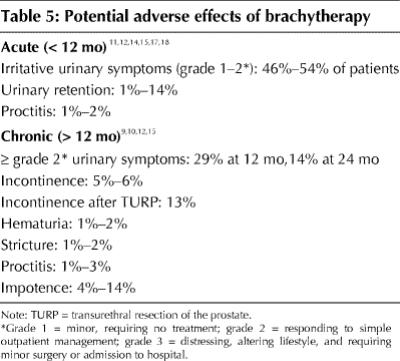
Potential adverse effects of brachytherapy are summarized in Table 5. About 50% of patients experienced acute irritative or obstructive urinary symptoms requiring drug treatment,15,17 which persisted in 29% of patients at 12 months and in 14% at 24 months.15 Acute urinary retention was reported to occur in 1%–14% of patients11,12,15,17 and proctitis in 1%–2%.11,12,14,18 Urinary incontinence was present in 5%–6% of patients9,10,12 but was much more common (13%) after transurethral resection of the prostate (TURP).9 Potency14,15 was maintained in 86%–96% of patients at 2–3 years after implantation.
Table 5
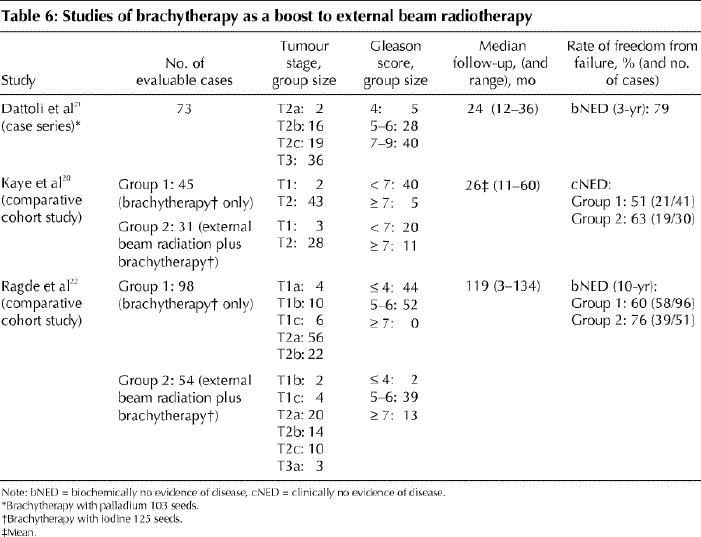
Brachytherapy following external beam radiotherapy
Despite selection of less favourable tumours for combined treatment (45 Gy in 25 fractions plus brachytherapy with 125I seeds), there was a trend toward improved bNED rates with combined treatment compared with brachytherapy alone (Table 6). Ragde and colleagues22 reported positive biopsy results in 27% of 108 patients in the combined treatment and the brachytherapy groups (at median 55 months), whereas Kaye and colleagues20 found no difference in positive biopsy rates between the combined treatment group and the brachytherapy group (20% v. 17% respectively).
Table 6
The most commonly reported adverse effects were grade 1 and 2 diarrhea and dysuria (in 88% of cases in the 3 studies). Grade 3 dysuria and diarrhea occurred in 3% of 33 patients. Rectal pain or tenesmus occurred in 55% of patients and persistent urinary retention in 21%. Other symptoms included urinary incontinence (in 9%), urinary tract infection (in 6%) and persistent perineal pain (in 12%). Chronic toxicity was not well described. In the one series reporting potency,21 82% of the patients maintained potency at 1 year and 77% at 2 years.
Brachytherapy preceding external beam radiotherapy
Critz and associates23 reported experience with brachytherapy using 125I seeds followed by external beam radiotherapy in 1020 men treated between 1984 and 1996. A retropubic freehand technique was used to guide implantation in the early years. Three weeks after implantation external beam radiotherapy (45 Gy in 30 fractions) was delivered to the prostate bed. Median follow-up was 2 years for patients whose implantation was guided by transrectal ultrasound. At 5 years, 92% were biochemically free of recurrence.
Although Critz and associates did not report biopsy data, Iversen and associates24 did. They obtained annual biopsy specimens from 32 patients given a similar combination of brachytherapy plus external beam radiotherapy; the median follow-up was 35 months. Twelve (48%) of 25 patients had positive biopsy results.
Adverse effects were reported in one series.24 Of the 32 patients 75% experienced mild and transient cystitis or diarrhea. Rectovesical fistula occurred in 6%, anal ulcer in 3%, hemorrhagic proctitis in 16% and severe persistent cystitis in 25%.
Interpretation
The Memorial Sloan–Kettering Cancer Center experience of the 1970s6 did not meet the technical criteria for inclusion in this review. However, the local relapse-free rate of 21% at 15 years is sobering evidence of the continued risk of late local recurrence and the necessity of adequate follow-up. With the modern brachytherapy technique, transrectal ultrasound guidance has resulted in improved seed alignment and spacing, and improved implant homogeneity, whereas treatment-planning software has provided precision in preplanning the implant and evaluating the final result.
Pretreatment prognostic factors such as tumour stage, Gleason score and serum PSA level influence the outcome of any definitive treatment of localized prostate cancer. Clinical experience indicates that permanent implantation of 125I or 103Pd seeds under transrectal ultrasound guidance yields promising short- and intermediate-term rates of freedom from biochemical failure among selected patients with early-stage prostate cancer. Results appear to be comparable among selected patients with T1c or T2a tumours, a Gleason score of 6 or lower and a serum PSA level of 10 μg/L or less. For less favourable tumours, the results of brachytherapy as monotherapy are inferior to other modalities. The addition of external beam radiotherapy may improve results by increasing the margin of coverage in the periprostatic tissue, but alternatives such as dose-escalated 3-dimensional conformal radiotherapy should be considered.
Patient selection is also important for technical reasons. Prior transurethral resection of the prostate is a relative contraindication because the surgical defect can interfere with optimal seed placement. Pubic arch interference can pose problems in implanting seeds in the anterolateral aspects of larger prostates. Therefore, prostate glands should ideally be less than 45–50 mL in size.
The spectrum of adverse effects associated with brachytherapy differs from that associated with external beam radiotherapy. Acute urinary symptoms tend to be more prolonged and more severe with brachytherapy. When brachytherapy is combined with external beam radiotherapy, the potential toxicity is additive. The spectrum of possible side effects after brachytherapy may be more acceptable to patients than that associated with external beam radiotherapy or radical prostatectomy. Issues such as quality of life, patient preference and cost are important considerations.
Results from randomized trials with adequate follow-up are key in the evaluation of new and emerging therapies such as brachytherapy. At present, there is insufficient evidence to unconditionally recommend the use of brachytherapy over current standard therapies. Whenever possible, patients should be asked to participate in randomized trials comparing brachytherapy and standard treatment with radical prostatectomy or external beam radiotherapy.
To minimize the risk of recurrence from subclinical extraprostatic disease,25 brachytherapy should be offered only to selected patients with favourable disease (T1c or T2a tumour, Gleason score of 6 or lower and serum PSA level of 10 μg/L or less). Patients should be well informed about alternative therapies and potential adverse effects.
Appendix 1.
Footnotes
Members of the Genitourinary Cancer Disease Site Group appear at the end of the article.
This article has been peer reviewed.
Acknowledgement: This study was sponsored by Cancer Care Ontario and the Ontario Ministry of Health.
Competing interests: None declared.
Reprint requests to: Dr. Himu Lukka, Hamilton Regional Cancer Centre, 699 Concession St., Hamilton ON L8V 5C2; fax 905 575-6326; himu.lukka@hrcc.on.ca
References
- 1.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969-74. [DOI] [PubMed]
- 2.Epstein JI, Partin AW, Sauvageot J, Walsh PC. Prediction of progression following radical prostatectomy. A multivariate analysis of 721 men with long-term follow-up. Am J Surg Pathol 1996;20:286-92. [DOI] [PubMed]
- 3.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst 1998;90:766-71. [DOI] [PubMed]
- 4.Shipley WU, Thames HD, Sandler HM, Hanks GE, Zietman AL, Perez CA, et al. Radiation therapy for clinically localized prostate cancer: a multi- institutional pooled analysis. JAMA 1999;281:1598-604. [DOI] [PubMed]
- 5.Pisansky TM, Kahn MJ, Rasp GM, Cha SS, Haddock MG, Bostwick DG. A multiple prognostic index predictive of disease outcome after irradiation for clinically localized prostate cancer. Cancer 1997;79:337-44. [DOI] [PubMed]
- 6.Fuks Z, Leibel SA, Wallner KE, Begg CB, Fair WR, Anderson LL, et al. The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with 125I implantation. Int J Radiat Oncol Biol Phys 1991;21:537-47. [DOI] [PubMed]
- 7.Browman GP. The evidence summary: a tool for informing clinical cancer policy recommendations based on weak evidence from health-care research [editorial]. Current Oncology 1999–2000;6:203-4.
- 8.American Society for Therapeutic Radiology and Oncology Consensus Panel. Consensus statement: guidelines for PSA following radiotherapy. Int J Radiat Oncol Biol Phys 1997;37:1035-41. [PubMed]
- 9.Ragde H, Blasko JC, Grimm PD, Kenny GM, Sylvester JE, Hoak DC, et al. Interstitial iodine-125 radiation without adjuvant therapy for clinically localized prostate cancer. Cancer 1997;80:442-53. [DOI] [PubMed]
- 10.Beyer DC, Priestley JB. Biochemical disease-free survival following 125I prostate implantation. Int J Radiat Oncol Biol Phys 1997;37:559-63. [DOI] [PubMed]
- 11.Stokes SH, Real JD, Adams PW, Clements JC, Wuertzer S, Kan W. Transperineal ultrasound-guided radioactive seed implantation for organ-confined carcinoma of the prostate. Int J Radiat Oncol Biol Phys 1997;37:337-41. [DOI] [PubMed]
- 12.Blasko JC, Wallner K, Grimm PD, Ragde H. Prostate specific antigen based disease control following ultrasound guided 125Iodine implantation for stage T1/T2 prostatic carcinoma. J Urol 1995;154:1096-9. [PubMed]
- 13.Prestidge BR, Hoak DC, Grimm PD, Ragde H, Cavanagh W, Blasko JC. Posttreatment biopsy results following interstitial brachytherapy in early-stage prostate cancer. Int J Radiat Oncol Biol Phys 1997;37:31-9. [DOI] [PubMed]
- 14.Stock RG, Stone NN, DeWyngaert JK, Lavagnini P, Unger PD. Prostate specific antigen findings and biopsy results following interactive ultrasound guided transperineal brachytherapy for early stage prostate carcinoma. Cancer 1996; 77:2386-92. [DOI] [PubMed]
- 15.Wallner K, Roy J, Harrison L. Tumor control and morbidity following transperineal iodine 125 implantation for stage T1/T2 prostatic carcinoma. J Clin Oncol 1996;14:449-53. [DOI] [PubMed]
- 16.Peterson RO. Urologic pathology. Philadelphia: JB Lippincott; 1986.
- 17.Brosman SA, Tokita K. Transrectal ultrasound-guided interstitial radiation therapy for localized prostate cancer. Urology 1991;38:372-6. [DOI] [PubMed]
- 18.Stone NN, Ramin SA, Wesson MF, Stock R, Unger O, Klein G. Laparoscopic pelvic lymph node dissection combined with real-time interactive transrectal ultrasound guided transperineal radioactive seed implantation of the prostate. J Urol 1995;153:1555-60. [PubMed]
- 19.Vijverberg PLM, Blank LEC, Dabhoiwala NF, de Reijke THM, Koedooder C, Hart AAM, et al. Analysis of biopsy findings and implant quality following ultrasonically guided 125I implantation for localised prostatic carcinoma. Br J Urol 1993;72:470-7. [DOI] [PubMed]
- 20.Kaye KW, Olson DJ, Payne JT. Detailed preliminary analysis of 125iodine implantation for localized prostate cancer using percutaneous approach. J Urol 1995;153:1020-5. [PubMed]
- 21.Dattoli M, Wallner K, Sorace R, Koval J, Cash J, Acosta R, et al. 103Pd brachytherapy and external beam irradiation for clinically localized, high-risk prostatic carcinoma. Int J Radiat Oncol Biol Phys 1996;35:875-9. [DOI] [PubMed]
- 22.Ragde H, Elgamal AA, Snow PB, Brandt J, Bartolucci AA, Nadir BS, et al. Ten-year disease free survival after transperineal sonography-guided iodine-125 brachytherapy with or without 45-gray external beam irradiation in the treatment of patients with clinically localized, low to high gleason grade prostate carcinoma. Cancer 1998;83:989-1000. [DOI] [PubMed]
- 23.Critz FA, Levinson AK, Williams WH, Holladay CT, Griffin VD, Holladay DA. Simultaneous radiotherapy for prostate cancer: 125I prostate implant followed by external-beam radiation. Cancer J Sci Am 1998;4:359-63. [PubMed]
- 24.Iversen P, Bak M, Juul N, Laursen F, von der Maase H, Nielsen L, et al. Ultrasonically guided 125iodine seed implantation with external radiation in management of localized prostatic carcinoma. Urology 1989;34:181-6. [DOI] [PubMed]
- 25.Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA 1997;277:1445-51. [PubMed]
- 26.Sobin LH, Wittekind C, editors. TNM classification of malignant tumors. 5th ed. Toronto: John Wiley & Sons; 1997.


