Here, we report on the anti-influenza virus activity of the mannose-binding agents Hippeastrum hybrid agglutinin (HHA) and Galanthus nivalis agglutinin (GNA) and the (N-acetylglucosamine)n-specific Urtica dioica agglutinin (UDA). These carbohydrate-binding agents (CBA) strongly inhibited various influenza A(H1N1), A(H3N2), and B viruses in vitro, with 50% effective concentration values ranging from 0.016 to 83 nM, generating selectivity indexes up to 125,000.
KEYWORDS: antiviral, CBA, glycans, influenza, hemagglutinin, fusion
ABSTRACT
Here, we report on the anti-influenza virus activity of the mannose-binding agents Hippeastrum hybrid agglutinin (HHA) and Galanthus nivalis agglutinin (GNA) and the (N-acetylglucosamine)n-specific Urtica dioica agglutinin (UDA). These carbohydrate-binding agents (CBA) strongly inhibited various influenza A(H1N1), A(H3N2), and B viruses in vitro, with 50% effective concentration values ranging from 0.016 to 83 nM, generating selectivity indexes up to 125,000. Somewhat less activity was observed against A/Puerto Rico/8/34 and an A(H1N1)pdm09 strain. In time-of-addition experiments, these CBA lost their inhibitory activity when added 30 min postinfection (p.i.). Interference with virus entry processes was also evident from strong inhibition of virus-induced hemolysis at low pH. However, a direct effect on acid-induced refolding of the viral hemagglutinin (HA) was excluded by the tryptic digestion assay. Instead, HHA treatment of HA-expressing cells led to a significant reduction of plasma membrane mobility. Crosslinking of membrane glycoproteins, through interaction with HA, could also explain the inhibitory effect on the release of newly formed virions when HHA was added at 6 h p.i. These CBA presumably interact with one or more N-glycans on the globular head of HA, since their absence led to reduced activity against mutant influenza B viruses and HHA-resistant A(H1N1) viruses. The latter condition emerged only after 33 cell culture passages in the continuous presence of HHA, and the A(H3N2) virus retained full sensitivity even after 50 passages. Thus, these CBA qualify as potent inhibitors of influenza A and B viruses in vitro with a pleiotropic mechanism of action and a high barrier for viral resistance.
INTRODUCTION
Influenza A and B viruses are highly contagious respiratory pathogens causing seasonal influenza, which is responsible for ∼1 billion infections, 3 million to 5 million cases of severe illness, and 300,000 to 500,000 deaths each year. In addition, pandemic outbreaks sporadically occur when reassortant influenza A viruses of zoonotic origin enter the human population (1). Current influenza vaccines protect only against seasonal influenza, need annual updating, which occasionally results in mismatches between the circulating strains and those included in the vaccine, and are only partially efficient in some target populations due to low immunogenicity (2). Hence, antiviral therapy to suppress severe influenza virus infections is still indispensable. Two classes of antivirals have been used for many years, i.e., the obsolete M2 ion channel blockers (amantadine and rimantadine) and the neuraminidase (NA) inhibitors (oseltamivir, zanamivir, peramivir, and laninamivir) (3). However, the worldwide spread of amantadine- and oseltamivir-resistant viruses, even among untreated patients, is currently causing a shift in favor of inhibitors targeting the viral polymerase complex. Baloxavir, the recently approved endonuclease inhibitor, has a unique pharmacological profile allowing for a single oral dose to treat influenza A and B; however, caution is warranted since the resistance mutation PA-I38T/M/F occurred in 10% of the baloxavir recipients in a phase 3 trial (4, 5). Furthermore, the nucleobase analogue favipiravir, which is licensed only in Japan and China, requires high doses and showed inconsistent therapeutic efficacy (6). Hence, fundamentally different antiviral strategies must be developed for the prevention and treatment of influenza.
The acute onset of influenza virus infection and accompanying inflammation points to viral entry as an attractive process to interfere with (7). During influenza virus entry, the viral hemagglutinin (HA) plays a crucial and dual role (8). Its membrane-distal globular head region is responsible for attachment of the virus to sialylated receptors on the cell surface, which is followed by endocytosis of the virus. Upon acidification of the endosome, the HA undergoes drastic conformational rearrangements, resulting in fusion of the viral and endosomal membranes and eventually release of the viral ribonucleoproteins (vRNPs) in the cytoplasm (9, 10). The HA is a homotrimeric glycoprotein embedded in the viral envelope. Both the globular head domain and the stem region, comprising the fusion peptide, are posttranslationally modified with glycans at several asparagine residues. The stem glycans are most conserved among various influenza HAs and function in their correct folding, controlling cleavage in HA1 and HA2, and maintaining HA in its metastable conformation required for fusion activity. In contrast, there is extensive variation in the glycosylation pattern of the HA head region, which alters its affinity for the cellular receptor and plays an important role in immune evasion by masking antigenic epitopes (11, 12). Besides the HA, the viral NA, which facilitates release of newly formed virions, carries N-glycans, rendering the influenza virus a potential target for carbohydrate-binding agents (CBA) (13). CBA encompass both proteins and small agents and can be synthetic or isolated from a variety of species, including prokaryotes, sea corals, algae, plants, invertebrates and vertebrates (14, 15). Anti-influenza virus activity for the collectins surfactant protein D (SP-D) and mannose-binding lectin (MBL) and the prokaryotic cyanovirin-N (CV-N) has been described (16–18). Recently, the H84T banana lectin and the hyacinth bean lectin FRIL were reported to potently inhibit influenza A and B viruses (19, 20).
In this study, we focused on the plant lectins Hippeastrum hybrid agglutinin (HHA), Galanthus nivalis agglutinin (GNA), and Urtica dioica agglutinin (UDA). The (N-acetyl--d-glucosamine)n-specific UDA was isolated from the rhizomes of the stinging nettle (Urtica dioica). It exists as a monomeric protein of 8.5 kDa comprising two hevein-like domains, each containing an independent carbohydrate-binding domain (CBD). Chitotriose (GlcNAc3) appears to be the best substrate for both CBDs, although with significantly different binding affinities (21). In a cocrystal structure, one GlcNAc3 molecule is sandwiched between the high-affinity CBD of one UDA molecule and the low-affinity CBD of a second UDA molecule (22). Recently, UDA was also shown to have the ability to bind to high-mannose-type N-glycans (23).
The monocot lectins HHA and GNA were isolated from the bulbs of the amaryllis (Hippeastrum hybrid) and snowdrop (Galanthus nivalis), respectively (24). Sequence analysis and crystallization studies revealed a striking resemblance among HHA and GNA. Both lectins have a monomeric molecular size of ∼12.5 kDa and adopt a tetrameric form in solution. Each monomer contains three CBDs specific for α-d-mannose, yielding the potential of binding 12 monosaccharides (25, 26). Whereas HHA binds both internal and terminal α-(1,3)- or α-(1,6)-linked mannose residues, GNA exclusively targets terminal α-(1,3)-mannoses (27, 28).
Both the mannose-specific HHA and GNA and the (GlcNAc)n-specific UDA possess strong activity against several viruses such as HIV, hepatitis C virus (HCV), dengue virus (DENV), cytomegalovirus, and coronaviruses, including severe acute respiratory syndrome coronavirus (SARS-CoV) (29–35). For influenza virus, UDA has been reported to inhibit an A(H3N2) virus (33), and HHA can inhibit influenza A and B viruses (36).
Since no detailed anti-influenza virus study has been performed for neither of the three lectins, we first evaluated HHA, GNA, and UDA for their antiviral activity against a broad panel of influenza A(H1N1), A(H3N2), and B viruses in vitro. In a detailed mechanistic study, we found that these CBA interfere with both virus entry and release via interaction with the viral HA. A resistance selection study with A(H1N1) and A(H3N2) viruses highlighted that HHA has a high barrier for resistance and confirmed the involvement of HA in the antiviral effect of these CBA. In addition, mutant influenza B viruses lacking HA glycans were generated to evaluate the importance of specific glycans for the antiviral activity of HHA, GNA, and UDA.
RESULTS
CBA inhibit human influenza A and B viruses.
The CBA HHA, GNA, and UDA were evaluated for their antiviral activity against a broad panel of human influenza viruses belonging to the A(H1N1), A(H3N2), and B (sub)types, using a cell-based assay in Madin-Darby canine kidney (MDCK) cells. The antiviral data obtained by microscopic evaluation of the virus-induced cytopathic effect (CPE) were confirmed by the colorimetric 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell viability assay and expressed as the 50% effective concentration (EC50). The cytotoxicity of the compounds was expressed as the minimal cytotoxic concentration (MCC) or the 50% cytotoxic concentration (CC50).
All influenza B viruses, including strains belonging to the Yamagata and Victoria lineages, were consistently inhibited by HHA, GNA, and UDA, with EC50 values in the range of 0.15 to 1.8 nM, 0.016 to 0.89 nM, and 0.64 to 14 nM, respectively (Table 1). The strongest activity was observed for GNA displaying an EC50 of 16 pM against B/Malaysia/2506/2004. GNA was >460- and >40-fold more potent against this strain, compared to the reference compounds ribavirin and zanamivir, respectively.
TABLE 1.
Anti-influenza virus activity in MDCK cellsa
| Strain | No. of HA glycansb | HHA (nM) |
GNA (nM) |
UDA (nM) |
Ribavirin (µM) |
Zanamivir (µM) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Microscopy | MTS assay | Microscopy | MTS assay | Microscopy | MTS assay | Microscopy | MTS assay | Microscopy | MTS assay | ||
| Antiviral EC50c | |||||||||||
| A(H1N1) | |||||||||||
| A/Puerto Rico/8/34 | 6 | 121 ± 106 | 346 ± 331 | 268 ± 426 | 285 ± 464 | 435 ± 407 | 429 ± 488 | 7.1 ± 3.4 | 7.4 ± 3.3 | 0.51 ± 0.65 | 0.67 ± 0.82 |
| A/Fort Monmouth/1/47 | 7 | 0.052 ± 0.038 | 0.092 ± 0.109 | 0.14 ± 0.17 | 0.29 ± 0.50 | 24 ± 27 | 23 ± 17 | 15 ± 14 | 12 ± 13 | 1.3 ± 2.3 | 2.4 ± 5.6 |
| A/Netherlands/378/2005 | 9 | 2.3 ± 3.3 | 2.4 ± 3.3 | 0.62 ± 0.72 | 0.47 ± 0.56 | 5.0 ± 4.3 | 7.9 ± 3.8 | 12 ± 6 | 8.4 ± 2.5 | 2.8 ± 3.6 | 1.1 ± 0.9 |
| A(H1N1)pdm09 | |||||||||||
| A/Virginia/3/2009 | 6 | >2,000 | >2,000 | 170 ± 355 | 67 ± 72 | 74 ± 74 | 48 ± 59 | 17 ± 14 | 11 ± 5 | 37 ± 42 | 17 ± 13 |
| A(H3N2) | |||||||||||
| A/X-31 | 7 | 2.7 ± 2.4 | 1.3 ± 1.2 | 2.2 ± 2.3 | 3.0 ± 3.4 | 73 ± 79 | 47 ± 76 | 6.2 ± 3.7 | 7.6 ± 1.2 | 0.033 ± 0.040 | 0.040 ± 0.043 |
| A/Hong Kong/2/68 | 7 | 2.2 ± 3.4 | 2.4 ± 3.0 | 1.9 ± 3.5 | 2.8 ± 3.7 | 65 ± 97 | 59 ± 83 | 7.4 ± 2.5 | 8.2 ± 1.6 | 1.8 ± 3.9 | 1.7 ± 3.3 |
| A/Victoria/3/75 | 7 | 3.0 ± 3.4 | 2.4 ± 2.5 | 5.3 ± 10.3 | 6.4 ± 10.5 | 12 ± 20 | 18 ± 16 | 9.2 ± 0.9 | 5.6 ± 3.4 | 0.26 ± 0.36 | 0.86 ± 0.92 |
| A/Hong Kong/7/87 | 8 | 0.13 ± 0.09 | 0.21 ± 0.32 | 0.40 ± 0.47 | 0.57 ± 0.46 | 5.8 ± 8.0 | 9.3 ± 8.8 | 9.4 ± 4.1 | 8.4 ± 2.2 | 14 ± 20 | 19 ± 15 |
| A/Victoria/361/2011 | 10 | 0.41 ± 0.43 | 0.10 ± 0.10 | 0.72 ± 0.96 | 0.94 ± 0.97 | 81 ± 86 | 83 ± 81 | 8.6 ± 2.5 | 10 ± 6 | 0.29 ± 0.42 | 0.074 ± 0.081 |
| A/California/02/2014 | 10 | 0.91 ± 1.13 | 0.62 ± 0.65 | 0.76 ± 0.59 | 1.6 ± 1.4 | 17 ± 16 | 32 ± 25 | 6.1 ± 3.2 | 6.2 ± 4.9 | 1.1 ± 1.8 | 5.4 ± 11.5 |
| B | |||||||||||
| B/Hong Kong/5/72 | 10 | 0.49 ± 0.55 | 0.15 ± 0.19 | 0.75 ± 0.62 | 0.30 ± 0.36 | 8.8 ± 15.4 | 4.3 ± 8.1 | 27 ± 9 | 39 ± 12 | >100 | >100 |
| B(Yamagata) | |||||||||||
| B/Netherlands/537/2005 | 10 | 0.16 ± 0.28 | 0.31 ± 0.53 | 0.068 ± 0.104 | 0.19 ± 0.30 | 8.1 ± 15.1 | 10 ± 14 | 5.3 ± 2.4 | 13 ± 17 | 0.29 ± 0.36 | 1.2 ± 1.2 |
| B/Florida/4/2006 | 10 | 0.31 ± 0.55 | 0.25 ± 0.50 | 0.085 ± 0.127 | 0.053 ± 0.066 | 11 ± 18 | 14 ± 17 | 5.8 ± 2.3 | 7.2 ± 2.2 | 0.17 ± 0.19 | 0.34 ± 0.35 |
| B(Victoria) | |||||||||||
| B/Malaysia/2506/2004 | 11 | 0.25 ± 0.53 | 0.64 ± 0.55 | 0.016 ± 0.015 | 0.020 ± 0.008 | 0.64 ± 0.79 | 1.1 ± 0.7 | 8.7 ± 9.3 | 6.2 ± 7.0 | 0.53 ± 1.23 | 0.99 ± 2.03 |
| B/Brisbane/60/2008 | 12 | 1.8 ± 2.9 | 1.5 ± 2.1 | 0.75 ± 0.68 | 0.89 ± 0.73 | 0.77 ± 0.73 | 1.2 ± 1.1 | 9.7 ± 4.3 | 6.9 ± 3.0 | 2.2 ± 1.9 | 14 ± 24 |
| Cellular toxicityd | |||||||||||
| MCC | >2,000 | >2,000 | ≥12,000 | 73 ± 41 | >100 | ||||||
| CC50 | >2,000 | >2,000 | >12,000 | >100 | >100 | ||||||
Data shown are the mean ± SD of three to seven independent tests.
The number of N-glycans attached to the viral HAs was predicted using NetNGlyc v1.0 software (www.cbs.dtu.dk/services/NetNGlyc); only sites with a potential score of >0.5 and a positive N-Glyc result are included. According to the PDB entry 4FQM crystal structure and the report by York et al. (43), one additional glycan with a potential score of 0.42 was included for all influenza B strains (i.e., glycan at position 492 in PDB entry 4FQM or position 506 in reference 43).
Antiviral activity was expressed as the EC50 value, defined as the compound concentration producing 50% inhibition of virus replication, estimated by light microscopic scoring of the CPE or by measuring cell viability in the formazan-based MTS assay.
Cellular toxicity was expressed as the MCC (compound concentration producing minimal changes in cell morphology, estimated by microscopy) or the CC50 (determined by the MTS cell viability assay).
Also, the replication of A(H3N2) viruses (encompassing viruses isolated in 1968 to 2014) was strongly inhibited, with EC50 values of 0.10 to 3.0 nM, 0.40 to 6.4 nM, and 5.8 to 83 nM for HHA, GNA, and UDA, respectively. Interestingly, as of the A/Hong Kong/7/87 virus, the sensitivity to HHA and GNA was increased by 14- and 8-fold, respectively, compared to older A(H3N2) viruses.
Regarding the A(H1N1) viruses, HHA, GNA, and UDA strongly blocked replication of the A/Fort Monmouth/1/47 and A/Netherlands/378/2005 viruses. However, decreased antiviral activity was observed against the A/Puerto Rico/8/34 and A/Virginia/3/2009 viruses.
In mock-infected cells, HHA and GNA produced no cellular toxicity at 2,000 nM, while minimal cytotoxic effects were noted for UDA around 12,000 nM. This leads to an exceptional selectivity index (i.e., ratio of the cytotoxic and antiviral concentrations) of up to 125,000 (e.g., for GNA against the B/Malaysia/2506/2004 virus).
CBA act at an early stage in the replication cycle.
In a one-cycle time-of-addition assay with the influenza A/Hong Kong/7/87 virus (H3N2), the compounds were added at different time points postinfection (p.i.), varying from −30 min to 8 h p.i. Besides GNA, HHA, and UDA, the following reference compounds were included: chloroquine (a weak base that raises the endosomal pH), ribavirin (inhibits viral RNA [vRNA] synthesis), nucleozin (aggregates viral nucleoprotein [NP]), and zanamivir (inhibits release of newly formed virions). At 10 h p.i., the cellular vRNA copies were quantified by two-step reverse transcription-quantitative PCR (RT-qPCR). The no-compound control showed a 153-fold increase in vRNA copies, relative to the virus added at 0 h (Fig. 1A). With HHA, GNA, or UDA, vRNA synthesis was potently inhibited when the compounds were added at −30 min or 0 h p.i., while the compounds lost their antiviral activity when added at 1 h p.i. or later. A similar curve was observed for chloroquine. The inhibition of ongoing vRNA synthesis was reflected in the gradual curve obtained with ribavirin. The NP-aggregating nucleozin still showed full inhibition when it was added at 5 h p.i., whereas zanamivir had no effect on first-round vRNA synthesis.
FIG 1.
HHA, GNA, and UDA inhibit influenza virus entry. (A) Inhibitory effects of HHA, GNA, and UDA on vRNA synthesis as a function of time of compound addition. MDCK cells were infected with A/Hong Kong/7/87 influenza virus, and compounds were added at −30 min, 0 h, 30 min, 1 h, 3 h, 5 h, or 8 h p.i. At 10 h p.i., the cells were subjected to RNA extraction for subsequent analysis by real-time RT-PCR. The vRNA synthesis is presented as the fold increase in vRNA copies at 10 h p.i., relative to the amount of added virus particles. Data shown are the mean ± standard error of the mean (SEM) of three independent experiments. (B) Localization of viral NP in influenza-infected MDCK cells treated with HHA, GNA, UDA, or 4c. After pretreatment with compound for 15 min, the cells were infected with A/Hong Kong/7/87 or B/Netherlands/537/2005 virus and further incubated in the presence of compound for 1 h at 35°C. After fixation and permeabilization, the cells were stained for NP (green), and nuclei were visualized with DAPI (blue). Bar, 25 µm.
The inhibition of influenza virus entry in the presence of CBA was visualized by confocal microscopy. MDCK cells were infected with A/Hong Kong/7/87 or B/Netherlands/537/2005 virus in the presence of compound, followed by NP staining at 1 h p.i. to localize the virus. In the absence of compound, the vRNPs were released from the endosomes and transported into the nucleus within 1 h p.i. (Fig. 1B, E1, E2, I1, and I2). In cells treated with HHA, GNA, or UDA, nuclear entry of the vRNPs was completely prevented (Fig. 1B, A1, A2, B1, B2, C1, C2, F1, F2, G1, G2, H1, and H2). In addition, neither cytoplasmic nor cell membrane-bound NP could be detected in the presence of the mannose-specific lectins HHA and GNA or in influenza B virus-infected cells treated with UDA. Only in the case of the A/Hong Kong/7/87 strain, the (GlcNAc)n-specific UDA seemed to induce the formation of large A(H3N2) virus aggregates at the cellular membrane and presumably in the endosomes. The fusion inhibitor 4c caused a punctate pattern in the cytoplasm, corresponding to the endosomal compartment (Fig. 1B, D1 and D2), as also reported (37). Both results indicate that HHA, GNA, and UDA inhibit the influenza virus entry process.
CBA interfere with HA-mediated membrane fusion.
Interference of both HHA and UDA with the first step in influenza virus entry, i.e., attachment of the viral HA to receptors on the cellular membrane, was excluded in a binding assay with the A/Hong Kong/7/87 virus. MDCK cells pretreated with compound were cooled on ice, followed by virus addition, further incubation on ice for 1 h, and quantification of the virus bound to the cell surface by RT-qPCR. While the sulfated sialyl lipid NMSO3 reduced the number of virions bound to the cells by 99%, HHA and UDA did not significantly affect virus binding (see Fig. S1 in the supplemental material).
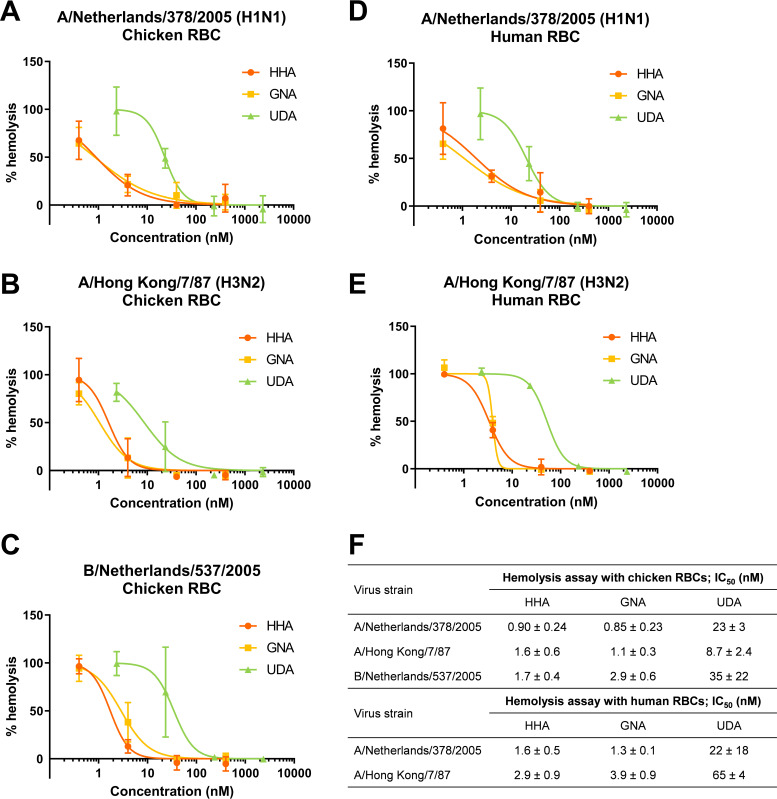
Next, a hemolysis inhibition assay was performed to evaluate the inhibitory effect of the CBA on the HA-mediated interaction with red blood cell (RBC) target membranes. After preincubation of the A/Netherlands/378/2005 (H1N1), A/Hong Kong/7/87 (H3N2), or B/Netherlands/537/2005 virus with chicken RBCs and removal of unbound virus, the virus-RBC pellet was resuspended in a low-pH buffer, leading to rupture of the RBC membranes. In the presence of HHA, GNA, and UDA, the virus-induced hemolysis was strongly inhibited, with 50% inhibitory concentration (IC50) values of 0.85 to 35 nM (Fig. 2A, B, C, and F). For the A/Netherlands/378/2005 and A/Hong Kong/7/87 viruses, the assay was repeated using human RBCs, yielding comparable IC50 values in the range of 1.3 to 65 nM for HHA, GNA, and UDA (Fig. 2D, E, and F). To verify whether the observed inhibition could be caused by inhibition of virus-RBC binding, we allowed the virus to interact with RBCs in the absence of compound. In fact, HHA and UDA still produced strong inhibition of A/Hong Kong/7/87-induced hemolysis when their addition was postponed to the acidic stage, thus after virus-RBC binding, with IC50 values that were increased by only 15- and 2.6-fold, respectively. However, HHA and UDA retained their full activity when the RBC-virus-compound pellet was washed with phosphate-buffered saline (PBS) after the adsorption stage and further incubated in an acidic buffer without compound. This observation indicates that the lectins tightly interact with their target.
FIG 2.
Inhibitory effects of HHA, GNA, and UDA on virus-induced hemolysis at low pH. (A to E) Virus-bound chicken (A, B, and C) or human RBCs (D and E) were resuspended in a low-pH buffer containing the compound and incubated for 25 min. After centrifugation, the optical density at 540 nm was measured. (F) IC50 values, i.e., the compound concentration producing 50% inhibition of virus-induced hemolysis. Data shown are the mean ± standard deviation (SD) of three independent experiments.
The inhibitory effect of the CBA on HA-mediated membrane fusion was confirmed using the syncytium formation assay with HA-expressing HeLa cells. When HeLa cells expressing the B/Netherlands/537/2005 HA were exposed to an acidic pH in the presence of HHA or UDA, cell-cell fusion leading to polykaryon formation was reduced in a dose-dependent manner, yielding IC50 values of 9.7 ± 6.2 nM and 25 ± 5 nM, respectively (Fig. 3A). To verify whether the plant lectins inhibit membrane fusion by preventing the acid-induced conformational change of HA, a trypsin susceptibility assay was performed, which is based on the exposure of trypsin cleavage sites upon the conformational change. As reference compounds, the A/H1-specific 9d and A/H3-specific 4c inhibitors were included in experiments with the A/Netherlands/378/2005 and A/Hong Kong/7/87 viruses, respectively (37, 38). However, in contrast to these small-molecule fusion inhibitors, HHA and UDA did not prevent the acid-induced conformational change at concentrations as high as 400 nM and 2,400 nM, respectively (Fig. 3B). Together, these findings show that these CBA interfere with HA-mediated membrane fusion without preventing the conformational change of HA.
FIG 3.
HHA and UDA inhibit HA-mediated fusion without affecting the low-pH-induced HA refolding. (A) HHA and UDA inhibit acid-induced syncytium formation in HA-expressing cells. HeLa cells expressing B/Netherlands/537/2005-HA were preincubated with compound for 15 min at 37°C, followed by a decrease of the pH to 5.4. After syncytium formation, the cells were fixed, stained, and imaged. Photographs are representative of four experiments (HHA) or two experiments (UDA). (B) HHA and UDA do not inhibit the acid-induced conformational change of HA. After preincubation of A/Netherlands/378/2005 or A/Hong Kong/7/87 virus with compound for 15 min at 37°C, the pH was lowered to 4.9. After neutralization and trypsin treatment, the viruses were lysed and subjected to Western blot analysis using an anti-HA1 antibody. Compound concentrations were 400 nM for HHA, 2,400 nM for UDA, and 100 µM for 9d and 4c.
CBA reduce release of newly formed virions.
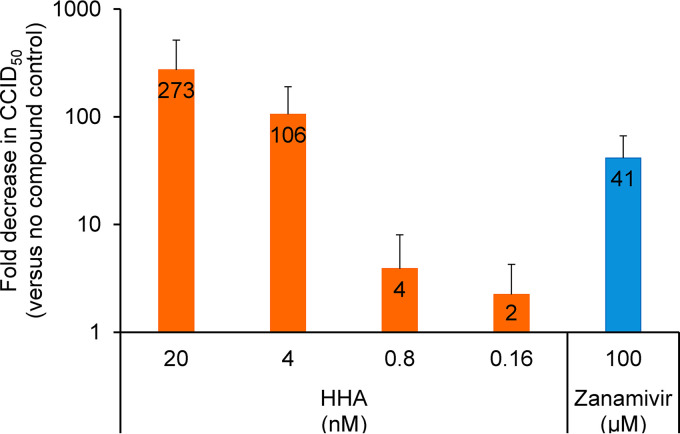
Besides its crucial role during influenza virus entry, the HA, together with the NA, plays an important role in virus budding (39). To assess whether HHA has an additional effect on the release of newly formed virions, we conducted a virus release assay. Therefore, A/Hong Kong/7/87-infected cells were extensively washed at 6 h p.i., followed by compound addition, and the infectious virus titer in the cell culture supernatant was determined at 12 h p.i. As shown in Fig. 4, HHA produced a clear and dose-dependent reduction of the infectious virus titer, compared to nontreated cells. Even at 4 nM, the effect was much more pronounced than for the NA inhibitor zanamivir at 100 µM (106-fold versus 41-fold).
FIG 4.
Inhibitory effect of HHA on virus release. MDCK cells were infected with A/Hong Kong/7/87 influenza virus. At 6 h p.i., the cells were washed and compounds were added. At 12 h p.i., the infectious virus titer of cell culture supernatants was determined by titration in MDCK cells. Data shown are the mean ± SD of four independent experiments.
To evaluate whether this effect is caused by a direct interaction of HHA with the viral NA, we performed a NA inhibition assay with the A/Hong Kong/7/87 virus. Incubation of influenza virus with 4-methylumbelliferyl N-acetyl-α-d-neuraminic acid (MUNANA) substrate leads to the release of the fluorescent product 4-methylumbelliferone. Whereas zanamivir showed an IC50 of 8.0 nM, HHA did not inhibit the viral NA at concentrations up to 400 nM (Fig. S2). Thus, the inhibitory effect of HHA on the release of progeny virus could not be attributed to inhibition of NA activity.
CBA reduce membrane mobility in HA-expressing cells.
Based on the combined effects of HHA on both membrane fusion and virus release, without direct interference with the HA conformational change or NA activity, we assumed that the lectins, which all possess multiple CBDs, could reduce membrane mobility by cross-linking membrane glycoproteins or glycolipids.
This hypothesis was verified by measuring the fluorescence recovery after photobleaching (FRAP), a technique that is used to study the dynamics of lipids and proteins in cellular membranes. Cell surface proteins of HA-transfected HeLa cells were fluorescently labeled with Alexa Fluor 488 dye. Then, a circular area of the cell surface with a diameter of 1 µm was photobleached, and the fluorescence recovery was monitored over time. The gradual mixing of photobleached and fluorescent proteins reflects the mobility of membrane proteins. In the absence of compound, the membrane fluorescence recovered to 82% of its intensity before bleaching (Fig. 5). Treatment of the cells with HHA led to a decrease of the fluorescence recovery, with 41% at 40 nM and 8.5% at 4 nM. Comparable results were obtained with UDA, displaying 42% and 17% reduction at 240 nM and 24 nM, respectively. Since HHA and UDA did not affect fluorescence recovery in untransfected HeLa cells, we concluded that these CBA act through interaction with the viral HA.
FIG 5.
HHA and UDA reduce membrane mobility in HA-expressing cells. HeLa cells expressing B/Netherlands/537/2005-HA were labeled with Alexa Fluor 488 and treated with HHA or UDA, followed by FRAP analysis. Data shown are the mean ± SD of three independent experiments. Statistical analysis showed a significant decrease for the curves for HHA at 40 nM (P < 0.001), UDA at 240 nM (P < 0.001), and UDA at 24 nM (P = 0.0051), compared to the no-compound control (Mann-Whitney test, two-tailed; confidence level, 99%).
Location of HA glycans crucial for antiviral activity of HHA, GNA, and UDA.
To assess the importance of HA glycans for the anti-influenza virus activity of the plant lectins and to localize the glycans interacting with these CBA, we created B/Brisbane/60/2008 mutants with glycan deletions at one or several glycosylation sites in HA. Based on the eight glycans attached to the B/Brisbane/60/2008 HA in a published crystal structure (PDB entry 4FQM [40]), we generated six mutant viruses missing the N25, N59, N145, N230, N301, or N492 glycan (Fig. 6) (HA numbering according to PDB entry 4FQM). Only the N330Q mutant could not be rescued as a virus causing full CPE in MDCK cells. Sequencing of our wild-type HA revealed an aspartic acid at position 194, instead of an asparagine carrying a glycan as in the PDB entry 4FQM structure. Hence, we also introduced this D194N substitution in our B/Brisbane/60/2008 virus. The mutant and wild-type viruses were evaluated for their sensitivity to the inhibitory effect of HHA, GNA, and UDA (Table 2), as well as their replication fitness (Fig. S3). Introduction of the N194 glycan caused 7- and 4-fold increased sensitivity to HHA and GNA, respectively, while the antiviral EC50 for UDA was unchanged, compared to the wild-type virus. Deletion of the N145 glycan had the most prominent effect on HHA, GNA, and UDA activity, with 53-, 1,840-, and 71-fold decreased sensitivity, respectively. Interestingly, the decreased sensitivity to HHA and GNA could be restored by introduction of the N194 glycan. Moreover, in the presence of this N194 glycan, removal of the N145 glycan resulted in no or only a moderate decrease of the antiviral EC50 for HHA, GNA, and UDA, by 3-, 1-, and 8-fold, respectively. The N59Q and N230Q substitutions produced 6- and 7-fold antiviral resistance to UDA, respectively, while they had only a slight effect on the antiviral activity of HHA. None of the mutants lacking glycans in the HA stem (i.e., N25Q, N301Q, and N492Q mutants) showed markedly decreased sensitivity to HHA, GNA, or UDA, indicating that these glycans are not involved in the interaction with these CBA. Compared to the wild-type virus, all mutants replicated to 10-fold lower levels of infectious virus, on average, indicating that their replication fitness is at least partially compromised (Fig. S3). Since deletion of glycans attached to the HA head, and not those on the HA stem, decreased the antiviral sensitivity to HHA, GNA, and UDA, our data indicate that these CBA interact with the globular head of HA.
FIG 6.
Positions of N-linked glycans attached to the HA trimer of influenza B and seasonal A(H1N1) viruses. (Left) Ribbon diagram showing the Asn residues that are glycosylated in a published crystal structure of B/Brisbane/60/2008 HA (N25, blue; N59, orange; N145, red; N194, pink; N230, yellow; N301, gray; N330, dark red; N492, purple); HA numbering is according to that of PDB entry 4FQM (40). (Right) Predicted N-glycosylation sites in seasonal H1 HA (summarized in Table S1 in the supplemental material). Residues N28 (blue), N40 (dark red), N71 (orange), N104 (red), N142 (yellow), N177 (pink), N 304 (gray), and N498 (purple) are indicated in a published crystal structure of A/Solomon Islands/3/2006 HA (PDB entry 5UGY [84]), with 97% sequence similarity to A/Netherlands/378/2005 HA. The monomers of the HA trimers are colored in olive drab (chains A and B), sea green (chains C and D), and dark green (chains E and F). The models were generated with UCSF Chimera (85).
TABLE 2.
Anti-influenza virus activity of mutant B/Brisbane/60/2008 viruses in MDCK cells
| Virus | HA head glycansa |
Antiviral EC50b |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 59 | 145 | 194 | 230 | HHA (nM) | GNA (nM) | UDA (nM) | Ribavirin (µM) | Zanamivir (µM) | |
| Wild-type | N | N | D | N | 1.7 ± 2.3 | 0.82 ± 0.66 | 0.96 ± 0.90 | 8.3 ± 3.8 | 8.9 ± 18 |
| N59Q | Q | N | D | N | 2.7 ± 2.4 | 3.5 ± 2.8 | 5.5 ± 2.9 | 10 ± 2.4 | >100 |
| N145Q | N | Q | D | N | 89 ± 146 | 1511 ± 906 | 69 ± 66 | 9.9 ± 2.3 | >100 |
| D194N | N | N | N | N | 0.25 ± 0.42 | 0.18 ± 0.10 | 0.94 ± 0.71 | 9.1 ± 2.1 | >100 |
| N230Q | N | N | D | Q | 3.0 ± 2.8 | 6.2 ± 3.6 | 6.8 ± 3.2 | 8.8 ± 1.6 | 38 ± 49 |
| N59Q-N145Q | Q | Q | D | N | 17 ± 15 | 13 ± 11 | 21 ± 14 | 8.0 ± 0.9 | 51 ± 40 |
| N145Q-D194N | N | Q | N | N | 0.63 ± 0.46 | 0.16 ± 0.10 | 7.8 ± 0.7 | 7.8 ± 2.8 | 51 ± 57 |
| N145Q-N230Q | N | Q | D | Q | 164 ± 15 | 1755 ± 384 | 40 ± 5 | 8.6 ± 2.8 | >100 |
| N25Q | N | N | D | N | 1.3 ± 0.2 | 0.30 ± 0.05 | 1.2 ± 0.8 | 9.5 ± 1.6 | >100 |
| N301Q | N | N | D | N | 0.93 ± 0.66 | 0.12 ± 0.11 | 0.89 ± 0.76 | 6.7 ± 3.5 | >100 |
| N492Q | N | N | D | N | 1.4 ± 0.2 | 0.21 ± 0.12 | 1.3 ± 0.5 | 9.6 ± 1.6 | >100 |
Amino acid substitutions were introduced in the HA head (N145, N194, and N230 on the HA top and N59 on the side of the HA head); N25, N301 and N492 are located in the HA stem. HA numbering is according to PDB entry 4FQM (40).
Antiviral activity was assessed in MDCK cells; values shown are the average EC50 values obtained by light microscopic scoring of the CPE and by measuring cell viability in the formazan-based MTS assay. Data shown are the mean ± SD based on three to six independent tests.
HHA displays a unique barrier to viral resistance.
To determine how readily influenza viruses acquire resistance to the three CBA upon prolonged exposure to the compound, we performed a resistance selection study with HHA using the viruses A/Netherlands/378/2005 and A/Hong Kong/7/87. A control condition was included to detect any potential changes associated with cell culture adaptation. After each virus passage, the supernatants from selected wells showing 5% to 100% CPE at day 3 p.i. were collected and used to infect fresh MDCK cells, in the presence of compound at gradually increasing concentrations. With the A/Netherlands/378/2005 virus, the emergence of HHA-resistant viruses was observed after 33 passages (about 3 months) (Fig. S4). In a CPE reduction assay, the isolated virus, designated HHA#33, showed >714-fold decreased sensitivity to HHA, compared to the virus that was passaged in the absence of compound (EC50 values of >2,000 nM versus 2.8 nM) (Table 3). While full cross-resistance was noted for GNA, HHA#33 retained moderate sensitivity to UDA, with an EC50 of 482 nM. The HHA#33 and control#33 clones displayed equal sensitivity to the reference compound ribavirin (Table 3) and similar replication fitness (data not shown). Continuation of the resistance selection study until passage 50 (about 5 months) and antiviral evaluation of the plaque-purified HHA#50 and control#50 clones did not demonstrate additional changes in the lectin resistance profile.
TABLE 3.
Phenotypic and genotypic characterization of HHA-resistant viruses
| Clonea | Amino acid substitutionb |
Antiviral EC50c |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HA |
NA |
HHA (nM) | GNA (nM) | UDA (nM) | Ribavirin (µM) |
Zanamivir (µM) |
|||||||
| 106 | 177 | 179 | 233 | 428 | 519 | 177 | 309 | ||||||
| Control#33 | T | N | S | K | I | G | V | N | 2.8 ± 1.7 | 5.4 ± 3.1 | 28 ± 13 | 10 ± 0 | 6.5 ± 3.5 |
| HHA#33 | K | N | N | E | V | G | V | N | >2,000 | >2,000 | 482 ± 364 | 9.7 ± 0.6 | >100 |
| Control#50 | T | N | S | K | I | G | V | H | 1.4 ± 0.5 | 1.6 ± 1.1 | 4.1 ± 3.4 | 13 ± 5 | 26 ± 49 |
| HHA#50-cl1 | K | T | N | E | V | R | A | N | >2,000 | >2,000 | 968 ± 667 | 20 ± 12 | >100 |
| HHA#50-cl2 | K | N | N | E | V | R | A | N | >2,000 | >2,000 | 592 ± 537 | 19 ± 8 | 0.027 ± 0.031 |
| HHA#50-cl3 | K | T | N | E | V | R | A | N | >2,000 | >2,000 | 754 ± 470 | 15 ± 4 | >100 |
| HHA#50-cl4 | K | T | N | E | V | R | A | N | >2,000 | >2,000 | 961 ± 423 | 47 ± 46 | >100 |
| HHA#50-cl5 | K | N | N | E | V | R | A | N | >2,000 | >2,000 | 824 ± 490 | 21 ± 12 | >100 |
| A/Netherlands/378/2005 | T | N | S | K | V | G | V | N | 2.3 ± 3.3 | 0.62 ± 0.72 | 5.0 ± 4.3 | 12 ± 6 | 2.8 ± 3.6 |
HHA#33 and HHA#50 clones were selected in the continuous presence of increasing concentrations of HHA; control#33 and control#50 clones were selected in the absence of compound. A/Netherlands/378/2005 is the parent virus.
The HA sequence of A/Netherlands/378/2005 is 99.8% identical to that of A/Rheinland-Pfalz/58/2005 (NCBI accession number AJK00757.1 [86]). HA numbering is according to the full-length HA sequence of A/South Carolina/1/18 (NCBI accession number AF117241.1 [87]), starting from methionine. NA numbering is according to the NA sequence of A/Nordrhein-Westfalen/6/2005 (GenBank accession number ACI32775 [88]), which showed 100% similarity to the NA sequence obtained for A/Netherlands/378/2005.
Antiviral activity determined in MDCK cells was expressed as the EC50 value, defined as the compound concentration producing 50% inhibition of virus replication, as estimated by microscopic scoring of the CPE. Data shown are the mean ± SD of three to five independent tests.
Sequencing revealed that the HHA#33 virus contained three amino acid substitutions in HA, namely, T106K, S179N, and K233E, whereas the NA protein was unchanged (Table 3) (HA numbering according to the full-length HA sequence of A/South Carolina/1/18, starting from methionine). At passage 50, two additional mutations were identified in HA, i.e., G519R (in all HHA#50 clones) and N177T (in three of five HHA#50 clones). The HHA#50 clones also contained one mutation in NA, i.e., V177A. In the control clones obtained after 50 passages in the absence of compound, the following mutations were detected: V428I in HA, already present at passage 33, and N309H in NA, seen only at passage 50.
The impact of all observed mutations on the N-glycosylation of HA and NA was predicted using NetNGlyc v1.0 software (www.cbs.dtu.dk/services/NetNGlyc) (Table S1). Interestingly, two mutations identified in the HHA clones were predicted to affect the glycosylation of the HA head. The N-glycan at position 104 was deleted due to T106K, and S179N produced a shift of the N-glycan from position N177 to position N179. Hence, resistance against HHA appears to be associated with loss of glycans in the HA globular head.
In parallel, the A/Hong Kong/7/87 virus underwent 50 cell culture passages (about 5 months) in the presence of HHA at concentrations up to 80 nM (Fig. S4). Although no signs of resistance were observed, the resulting virus stock was subjected to plaque purification, followed by antiviral testing of three HHA#50 clones. Their antiviral EC50 values for HHA, GNA, UDA, ribavirin, and zanamivir were similar to the values obtained with the control#50 clone (data not shown). Sequencing showed that the viral HAs of all HHA#50 and control#50 clones were genotypically identical to that of the parent virus. Also, the NA of control#50 was unchanged, while two substitutions were detected in the HHA#50 clones, i.e., K328E (in all three clones) and E258G (in one clone). Thus, besides proving that CBA exhibit a high barrier for resistance, changes in the glycosylation pattern of the HA head in HHA-resistant viruses confirm the involvement of these glycans in interaction with HHA, GNA, and UDA.
DISCUSSION
Glycans on viral envelope proteins often play a vital role in efficient transmission of the pathogen and/or entry into its host cell and are also involved in escaping the immunological control of the host (41, 42). Therefore, CBA specifically interacting with these glycans might qualify as antivirals with activity against several unrelated viruses. The plant lectins HHA, GNA, and UDA have already been shown to be active against HIV, HCV, DENV, and coronaviruses, including SARS-CoV and recently SARS-CoV-2 (data on file) (30–35). Here, we demonstrate that HHA, GNA, and UDA are potent inhibitors of influenza A and B viruses with a high barrier for resistance. A detailed mechanistic study provided evidence that their inhibitory effect on virus entry and release is caused by reduced membrane mobility via interaction with glycans on the globular head of HA.
Despite the strong variation of HA head glycans among different virus strains, both the mannose-specific HHA and GNA and the (GlcNAc)n-specific UDA were shown to be potent inhibitors of influenza A and B viruses in vitro. The strongest activity was observed for GNA against the B/Malaysia/2506/2004 strain, with an EC50 of 16 pM. In combination with the absence of any cellular toxicity at 2,000 nM, an impressive selectivity index of 125,000 was noted. All influenza B viruses in our panel, belonging to either the Yamagata or the Victoria lineage, appeared highly sensitive to HHA, GNA, and UDA. This agrees with the fact that the number of N-glycans on the influenza B HAs has remained fairly constant at 10 to 12 (43). Also the A(H3N2) HAs are highly glycosylated, with a constant number of five N-linked glycans attached to the HA stem and an increasing number of glycans on the globular head, ranging from two on the 1968 pandemic virus up to seven on more recent isolates (44). Although all A(H3N2) viruses were nicely inhibited by HHA, GNA, and UDA, the antiviral potency of the mannose-specific HHA and GNA was increased against the more recent viruses. In fact, as of the A/Hong Kong/7/87 strain, position 246 of HA was occupied by an additional glycan, which appears to contain only high-mannose glycans (45). Deletion of this glycan was also reported to reduce the antiviral activity of the mannose-specific SP-D lectin (46). A similar trend of adding glycosylation sites to the HA globular head domain over time has been observed for A(H1N1) viruses (Table 1; also see Table S1 in the supplemental material). The 1918 pandemic virus and the A/Puerto Rico/8/34 and A/Virginia/3/2009 viruses included in our panel carry only a single glycan on their HA head, at position 104 (pandemic strains) or position 286 (A/Puerto Rico/8/34), explaining the moderate activity of the CBA against these strains (43). On the other hand, addition of glycans at position 144 (A/Fort Monmouth/1/47) or positions 71, 142, and 177 (A/Netherlands/378/2005) (Fig. 6) led to a substantial increase in sensitivity to the lectins. Accordingly, A(H1N1)pdm09 strains and A/Puerto Rico/8/34 were resistant to SP-D and MBL, while introduction of the N144 glycan in A/Puerto Rico/8/34 HA increased its sensitivity to both collectins (47).
The variations in their anti-influenza virus activities suggest that HHA, GNA, and UDA target glycans on the head of the viral HA. Interference with the influenza virus entry process was confirmed in time-of-addition experiments, in which HHA, GNA, and UDA lost their antiviral activity when added at 1 h p.i., and confocal microscopy studies showing that the nuclear entry of the vRNPs was virtually completely blocked in the presence of these CBA. Interestingly, in A(H3N2) virus-infected cells, UDA induced the formation of virus aggregates at the cell membrane and presumably in endosomal vesicles, whereas no viral NP could be visualized in similar experiments with influenza B virus. The aggregates might be formed by interaction of UDA with hybrid or complex glycans, which appear to have higher levels of occurrence on HAs of the A(H3N2) subtype, compared to influenza B viruses (45).
HHA, GNA, and UDA showed strong and dose-dependent inhibition of virus-induced hemolysis at low pH. The IC50 values were about 0.4- to 40-fold higher than those in the CPE reduction assay. This can be explained by the different experimental conditions, e.g., MDCK cells versus RBCs and multiplicity of infection (MOI) of 0.0004 PFU per cell versus undiluted allantoic virus. Even when the CBA were added at a postbinding step, UDA and HHA were still able to inhibit the virus-induced hemolysis, with EC50 values that were increased by only 2.6- and 15-fold, respectively. The finding that HHA preferably interacts with its target prior to virus-receptor binding was also observed in a study with a coronavirus (48). Plausibly, it is more convenient to interact with the HA after virus-receptor binding for the smaller UDA (8.5 kDa) than for HHA (50 kDa). The inhibitory effect of the lectins on the virus-induced fusion event was confirmed by dose-dependent inhibition of the acid-induced polykaryon formation in HA-expressing cells. Compared with the hemolysis inhibition assay, the IC50 values obtained were comparable for UDA and about 6-fold higher for HHA, which is in line with the hypothesis that HHA preferably interacts with its target before HA-receptor binding. Our findings with HHA, GNA, and UDA also agree with those of Covés-Datson et al., who recently reported that the mannose-specific H84T banana lectin inhibited intraendosomal membrane fusion, while attachment to the cell was only slightly decreased (19). Besides lectins, antibodies interacting with HA head glycans have been described (49, 50). The interactions of “Fab” and F005-126 with an A/H3 HA involve the glycans attached to residues 165 or 285, respectively. By interacting with two monomers in the HA trimer, these antibodies stabilize the HA in its neutral pH conformation, thereby preventing membrane fusion. However, in contrast to the small-molecule fusion inhibitors 9d and 4c, HHA and UDA did not inhibit the acid-induced conformational change, as we showed in tryptic digestion assays with A(H1N1) and A(H3N2) viruses.
Besides a crucial role for receptor binding, the functional balance of HA and NA activities is also considered to be important for efficient release of newly assembled virus particles (51). In a virus release assay with the A/Hong Kong/7/87 virus, HHA strongly decreased the infectious virus titer in the cell culture supernatant. In contrast to zanamivir, the inhibitory effect of HHA could not be attributed to inhibition of the NA activity. However, prior to virus release, the HA and NA are targeted to lipid rafts, leading to deformation of the host cell membrane and initiation of virus budding (39). Also, the θ-defensin retrocyclin 2 has been reported to cross-link membrane proteins due to interaction of this lectin with the viral HA (52). Similarly, treatment of HA-transfected cells with HHA at 40 nM and UDA at 240 nM caused a strong decrease of the membrane mobility by ∼40%, whereas no effect was observed in untransfected cells. Note that the lectins inhibited the low-pH-induced syncytium formation in the same HA-transfected cells at concentrations in a comparable range (IC50 values of 9.7 nM and 25 nM for HHA and UDA, respectively) and HHA blocked virus release at 4 nM and at 40 nM. Hence, we postulate that the combined effects on virus entry and release can be explained by limited lateral diffusion of membrane proteins in the presence of the CBA. Based on the HA-specific interaction of the lectins, we assume that virus entry is blocked at the level of intraendosomal fusion, rather than the process of endocytosis. We also think that this mechanism of action could cause the intraendosomal trapping of influenza virus in the presence of the FRIL lectin or the H84T banana lectin (19, 20). Of note, the latter proved to inhibit Ebola virus in cell culture and in mice (53).
As a matter of fact, the proposed mechanism of action for HHA, GNA, and UDA as anti-influenza virus inhibitors corresponds with studies performed with HIV, HCV, and coronavirus. For these viruses, these CBA do not interfere with attachment of the virus (via gp120, E1E2 or spike protein, respectively) to its cell receptor but rather intervene at a postbinding step (35, 48, 54). Moreover, in a study with SARS-CoV, HHA has been suggested to exert a dual inhibitory effect, namely, inhibition of virus entry and release (31).
To pinpoint which HA glycans interact with HHA, GNA, and UDA, we generated mutant influenza B viruses lacking one or more glycans on HA. In contrast to the glycans attached to the HA stem, several HA head glycans had an impact on the antiviral activity of HHA, GNA, and UDA. The glycosylation at N59, on the side of the globular head, mainly decreased the sensitivity to UDA. This observation suggests the presence of hybrid or complex glycans at this position, as recently reported for another influenza B strain (45). Nevertheless, the membrane-distal glycans at positions 145 and 194 proved to play the most crucial role in the antiviral activity of the CBA. Upon removal of the N145 glycan from our wild-type virus, which already lacked the N194 glycan, the antiviral activity of HHA, GNA, and UDA decreased by 53-, 1,840-, and 71-fold, respectively. The prominent effect on the GNA activity could be caused by the narrow binding spectrum of GNA, i.e., only terminal α-(1,3)-mannoses. The decreased antiviral activity caused by removal of the N145 glycan was partially (UDA) or completely (HHA and GNA) overcome by introduction of the N194 glycan. The presence of both glycans even increased the antiviral potency of HHA and GNA but had no added value for UDA. This finding could be attributed to the nature of the glycan at position 194, being a high-mannose glycan (45). The gain or loss of the N194 glycan was found to be responsible for antigenic variation in monoclonal antibody escape mutants or in clinical isolates (55–57). However, the glycosylation at position 145 first appeared in the B/Great Lakes/54 strain and has been maintained ever since. Wang et al. suggested that the presence of this glycan is advantageous for influenza B virus to evade human immunity, given its location on the 150 loop, a prominent neutralizing loop (58). This implies that escape mutants that might occur upon usage of the CBA could expose antigenic sites in the influenza B HA that render the mutant virus susceptible to immunological control. The mutants lacking even three HA glycans (N145 and N194 in combination with N59 or N230) retained some sensitivity to HHA and UDA. Together with the reduced replication fitness observed for all mutant viruses, these data suggest that CBA might have a high barrier for resistance.
To our knowledge, the only lectin that has been the subject of a resistance study with influenza virus is CV-N, a mannose-specific lectin (59). Within two cell culture passages of an A(H1N1) virus in the presence of CV-N, loss of a single glycan at position N104 in HA resulted in resistance to this lectin (corresponding to N94 in reference 59). In comparison, HIV-1 attained resistance to CV-N after 19 cell culture passages and deletion of three glycosylation sites on gp120, while 65 to 90 passages accompanied by loss of seven or eight glycans on gp120 were required to obtain resistance to HHA and GNA (60, 61). For UDA, even loss of nine glycosylation sites on gp120 reduced its anti-HIV activity to only a limited extent (54).
To address the question of how fast influenza viruses acquire resistance to the plant lectins, we performed a resistance study with HHA using two virus strains, A/Netherlands/378/2005 (H1N1) and A/Hong Kong/7/87 (H3N2). For the A/Netherlands/378/2005 virus, HHA-resistant viruses could be isolated after 33 passages (about 3 months), much more than the 5 and 7 passages needed to obtain resistance to amantadine and oseltamivir, respectively, as we found in a previous study using the same virus strain and methodology (37). Genotypic analysis of the HHA-resistant viruses revealed not only deletion of the N104 glycan on HA, which already proved to be important for SP-D, conglutinin, and CV-N activity (59, 62, 63), but also migration of the N-glycan at position 177 to 179 near the receptor-binding site (RBS). The third mutation, K233E, is located very close to N179 and therefore could be a compensatory mutation or could cause allosteric effects resulting in reduced binding to HHA. The same speculations apply to the N177T substitution, which was found after 50 passages in the presence of HHA. A modeling study by Sun et al. (64) suggested that relocation of the N177 glycan to N179 increases the obstruction of the HA RBS because the N179 glycan shields the RBS not only on the same subunit but also on the adjacent HA subunit. This effect could be countered by loss of the N104 glycan, which would reduce obstruction of the RBS (64). In addition, the N177 and N179 glycans would both shield antigenic sites (65). Interestingly, a mutant lacking the N177 glycan could not be rescued in a study using a closely related A(H1N1) HA (corresponding to N160 in the report by Hillaire et al. 66). Finally, the NA substitution identified in the HHA#50 clones, V177A, could compensate for the HA mutations by restoring the functional balance of HA, NA, and the receptor (51).
The HHA-resistant clones showed cross-resistance to the mannose-specific GNA and, to a lesser extent, the (GlcNAc)n-binding UDA, as well as zanamivir. The latter observation could be caused by loss of the N104 glycan (corresponding to N87 in the report by Mishin et al. [67]), which has been reported to increase the antiviral activity of NA inhibitors.
The resistance profile of HHA appeared to be even more favorable for the A(H3N2) virus, since no sign of resistance to HHA, GNA, or UDA was observed after 50 passages, or 5 months, in the presence of HHA. We hypothesize that the high barrier for resistance is related to the receptor usage of influenza A viruses. Binding of HA to sialic acid residues on the cell surface is considered to be the first step in influenza virus infection. However, little is known about the identity of specific (co)receptors that mediate virus internalization, and sialic acid-independent entry and infection have been reported (68, 69). Virus strains that are expected to be highly sensitive to HHA, GNA, and UDA [seasonal A(H1N1) and A(H3N2)] were able to infect DC-SIGN-, L-SIGN-, or langerin-expressing Lec2 cells, a CHO cell line deficient in sialic acid (68, 70, 71). In contrast, the DC-SIGN-, L-SIGN-, or langerin-expressing cells were largely resistant to A/Puerto Rico/8/34, a virus that is only moderately sensitive to the plant lectins. These receptors, supporting clathrin-mediated endocytosis of the virus, are probably blocked in the presence of CBA (70). Plausibly, the virus would bind to other cell surface receptors, leading to less efficient entry of the virus. Since DC-SIGN, L-SIGN, and langerin recognize HA glycans, removal of glycans, resulting in resistance to CBA, would also lead to inefficient virus entry. In addition, these viruses would probably be prone to immunological control.
Interestingly, the absence of any cellular toxicity with 2,000 nM HHA or GNA and the limited toxicity observed for UDA at 12,000 nM in the highly proliferating MDCK cells highlight the favorable cell viability profiles of these CBA. Also, it was reported previously that intravenous treatment of mice with HHA or GNA at 1,000 nM or UDA at 3,000 nM as a bolus injection did not provoke visible toxic side effects (54, 72). In the case of influenza virus infections, toxic effects caused by interaction with host cell glycans are expected to be minimized by the possibility of intranasal application and short-term treatment. The high-mannose-binding lectins CV-N and H84T banana lectin have already been shown to be protective against influenza virus challenge in mice by intranasal administration, and in the case of H84T banana lectin even intraperitoneal administration (19, 73, 74). CBA could also be useful as coatings on masks or as aerosols in closed spaces, such as airplane cabins, to reduce virus transmission. However, clinical use of CBA will require vigilant clinical trials to evaluate their safety and efficacy.
In summary, we demonstrated that the CBA HHA, GNA, and UDA are potent inhibitors of influenza A and B viruses in vitro. Through interaction with glycans on the HA head, they decrease the membrane mobility, which results in profound inhibition of influenza virus entry and release. Finally, their excellent resistance and cellular toxicity profiles underline the therapeutic potential of these CBA.
MATERIALS AND METHODS
Cells and viruses.
MDCK cells (a kind gift from M. Matrosovich, Marburg, Germany) and HEK293T cells (product number HCL4517; Thermo Fisher Scientific) were cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum (FCS), 1 mM sodium pyruvate, and 0.075% sodium bicarbonate. The HeLa cell (ATCC CCL-2) growth medium consisted of minimal essential medium (MEM) supplemented with 10% FCS, 0.1 mM nonessential amino acids, 2 mM l-glutamine, and 10 mM HEPES. During virus experiments, the MDCK cells were maintained in UltraMDCK medium (Lonza) supplemented with 2 mM l-glutamine, 0.0225% sodium bicarbonate, and 2 µg/ml tosylphenylalanylchloromethylketone (TPCK)-treated trypsin (Sigma).
The influenza A/Virginia/3/2009 [(H1N1)pdm09], B/Hong Kong/5/72, and B/Florida/4/2006 (Yamagata) virus strains were purchased from ATCC, whereas the B/Malaysia/2506/2004 (Victoria) strain was obtained from BEI Resources (Biodefense and Emerging Infections Research Resources Repository). The A/X-31 (chimeric virus consisting of the A/Puerto Rico/8/34 backbone and the HA and NA of A/Aichi/1/68) and A/Hong Kong/7/87 (H3N2) strains were obtained from J. Neyts, while the following strains were kindly provided by R. Fouchier (Rotterdam, Netherlands): A/Fort Monmouth/1/47 (H1N1), A/Netherlands/378/2005 (H1N1), A/Hong Kong/2/68 (H3N2), A/Victoria/3/75 (H3N2), and B/Netherlands/537/2005 (Yamagata). Virus stocks of these strains were prepared by propagation in 10-day-old embryonated chicken eggs. The recent A(H3N2) viruses A/Victoria/361/2011 (kind gift from G. Rimmelzwaan, Rotterdam, Netherlands) and A/California/02/2014 (purchased from Influenza Reagent Resource) were expanded on MDCK cells. The A/Puerto Rico/8/34 virus was generated by reverse genetics with plasmids kindly provided by M. Kim (Daejeon, Republic of Korea) following the protocol described previously (75).
Test compounds.
The mannose-binding lectins GNA and HHA were purified from the bulbs of the Galanthus nivalis and Hippeastrum hybrid, respectively. The (GlcNAc)n-specific UDA was isolated from Urtica dioica rhizomes. All lectins were purified by multiple rounds of affinity and/or ion-exchange chromatography, using established protocols previously described by Van Damme et al. (76). The purity of all lectins was confirmed by SDS-PAGE, indicating that the proteins were essentially pure. GNA was also purchased from Medicago (Uppsala, Sweden).
The following reference compounds were included: ribavirin (Virazole from ICN Pharmaceuticals), zanamivir (Relenza from GlaxoSmithKline), NMSO3 (77) (a generous gift from G. Wright, Microbiotix, Worcester, MA), chloroquine (Sigma), 4c [6-methyl-N-(2,8-dimethyl-3-oxo-1-thia-4-azaspiro[4.5]dec-4-yl)imidazo[2,1-b][1,3]thiazole-5-carboxamide] (synthesized by N. Cesur, Istanbul, Turkey) (78), 9d (2-isopropyl-N-[2-(piperidin-1-yl)ethyl]aniline hydrochloride) (synthesized by S. Vazquez, Barcelona, Spain) (38), and nucleozin (Sigma).
In several mechanistic assays (time-of-addition assay, confocal microscopy, binding assay, and trypsin digestion assay), compound concentrations that ensured complete virus blockage even at high virus titers, but without causing cellular toxicity, were chosen.
Antiviral assay.
The antiviral activity of the compounds against a broad panel of influenza viruses was evaluated as described before (78). Briefly, MDCK cells were seeded into 96-well dishes at 7,500 cells per well. After 16 h at 35°C, serial dilutions of the compounds were added to the cells prior to infection with influenza virus at a MOI of 0.0004 PFU per cell (corresponding to 50 times the CCID50 per well). At 3 days p.i., the virus-induced cytopathogenic effect was evaluated by microscopy and the antiviral activity was expressed as the EC50. In parallel, the compound concentration causing minimal morphological changes to the cells (MCC) was derived from mock-infected cells. Then, the formazan-based MTS cell viability assay (CellTiter 96 AQueous One Solution cell proliferation assay from Promega, Madison, WI) was performed, and the spectrophotometric data were used to calculate the antiviral EC50 and CC50.
Time-of-addition assay.
MDCK cells were seeded into 24-well dishes at 125,000 cells per well. After 16 h of incubation at 35°C, the cells were cooled on ice for 1 h, followed by the addition of the influenza A/Hong Kong/7/87 virus at an MOI of 0.0004 PFU per cell and further incubation at 35°C. The compounds were added at −30 min, 0 h, 30 min, 1 h, 3 h, 5 h, or 8 h p.i. At 10 h p.i., total cellular RNA extracts were prepared for all conditions, using the ReliaPrep RNA cell miniprep system (Promega). To quantify the vRNA, two-step real-time RT-PCR was performed, using the M1-FOR primer (5′-CCTGGTATGTGCAACATGTG-3′), the M1-REV primer (5′-GGCCTGACTAGCAATCTCCA-3′), and the M1 probe (6-carboxyfluorescein-5′-CTGACTCCC/ZEN/AGCACAGGTCTCATAGGC-3′-Iowa Black FQ; IDT, Leuven, Belgium), derived from the influenza A virus M1 gene sequence. First, 1 µg of total RNA was subjected to cRNA synthesis with the high-capacity cDNA reverse transcription kit (Life Technologies) and 100 nM M1-FOR primer. Then, real-time PCR was performed with the qPCR MasterMix (Eurogentec). The thermal profile consisted of a 10-min initial activation at 95°C, followed by 40 thermal cycles of 15 s at 95°C, 30 s at 60°C, and 90 s at 72°C. In each experiment, a standard curve (R2 of ≥0.99 within the range of 103 to 108 copies per reaction) was drawn to convert the respective cycle threshold (CT) values into the number of viral genome copies. The standard consisted of a pCR2.1-TOPO plasmid construct in which a 473-bp sequence of influenza virus A/Hong Kong/7/87 segment 7 was cloned. All samples were analyzed in duplicate.
Binding inhibition assay.
MDCK cells were seeded into 96-well plates at 50,000 cells per well and incubated for 16 h at 35°C. After 2 h of preincubation with compound at 35°C, the cells were cooled on ice for 15 min, followed by infection with precooled A/Hong Kong/7/87 virus at an MOI of 0.04 PFU per cell and incubation on ice for 1 h. After two washes with ice-cold UltraMDCK infection medium and one wash with ice-cold PBS, the cells were lysed with 5 µl lysis enhancer and 50 µl resuspension buffer (both from the CellsDirect One-Step qRT-PCR kit; Invitrogen). The cell lysates were then incubated at 75°C for 10 min, and the vRNA was quantified using the CellsDirect One-Step qRT-PCR kit, the M1-FOR and M1-REV primers, and the M1 probe (described above). Amplification was performed on an ABI Prism 7500 system (Applied Biosystems, Foster City, CA) and consisted of 15 min of cDNA synthesis at 50°C and a 2-min initial activation at 95°C, followed by 40 thermal cycles of 15 s at 95°C and 90 s at 60°C. In each experiment, a standard curve (R2 of >0.99 within the range of 103 to 108 copies per reaction) was drawn to convert the respective CT values into the number of viral genome copies. All samples were run in duplicate.
Confocal microscopy.
To monitor the virus entry process by confocal microscopy, the method described by Vanderlinden et al. (37) was slightly adapted. MDCK cells were plated in 8-well Ibidi µ-slides at 70,000 cells per well and incubated for 16 h at 35°C. Then, the cells were preincubated with compound for 15 min at 35°C and cooled on ice for 10 min, followed by addition of ultracentrifuged A/Hong Kong/7/87 or B/Netherlands/537/2005 virus at an MOI of 4 PFU per cell. After 1 h at 35°C, immunostaining for the viral NP was performed at room temperature with the following steps: three washes with PBS, fixation for 15 min with 4% formaldehyde, two washes, permeabilization for 10 min with 0.1% Triton X-100, two washes, blocking for 30 min with 10% normal goat serum diluted in PBS, incubation with anti-NP antibody (Hytest 3IN5-InA108 for A/Hong Kong/7/87 or RIF17-R2/3 for B/Netherlands/537/2005, both diluted 1:250 in PBS with 10% normal goat serum), four washes, incubation with an Alexa Fluor 488-labeled goat anti-mouse IgG antibody (Invitrogen A11001, diluted 1:500 in PBS with 10% normal goat serum), and four washes. Finally, the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) diluted in PBS, and the cells were analyzed with a Leica TCS SP5 confocal microscope (Leica Microsystems) equipped with an acousto-optical beam splitter (AOBS), using an HCX PL APO 63× water immersion lens (numerical aperture, 1.2). Different fluorochromes were detected sequentially using excitation lines of 405 nm (DAPI) and 488 nm (Alexa Fluor 488), and emission was detected between 410 and 480 nm and between 493 and 565 nm, respectively.
Hemolysis inhibition assay.
The hemolysis inhibition assay was performed as described previously (78). Briefly, allantoic virus was preincubated with compound for 1 h at 37°C. After addition of an equal volume of RBCs (chicken or human, 2% in PBS) and an additional incubation for 10 min at 37°C, the RBCs were pelleted by centrifugation. The pellet was resuspended in a low-pH buffer (pH 5.0 for A/Netherlands/378/2005 and A/Hong Kong/7/87 and pH 5.25 for B/Netherlands/537/2005) containing the compound. After incubation for 25 min at 37°C, the mixture was neutralized, followed by centrifugation and measurement of the optical density of the supernatant at 540 nm. Data were expressed as the IC50, representing the concentration causing 50% reduction of hemolysis, compared to the no-compound control.
To evaluate the reversibility of its interaction with the virus, the compound was washed away by resuspending the RBC pellet in PBS without compound, followed by an additional centrifugation step and resuspension of the RBC pellet in low-pH buffer without compound.
To rule out an effect on virus binding, the virus was incubated with RBCs in the absence of compound. The compound was added only when the RBC pellet was resuspended in low-pH buffer (containing compound).
Trypsin digestion assay.
To determine the inhibitory effect of the compounds on the acid-induced conformational change of HA, the protocol for the trypsin digestion assay was slightly adapted from that described by Vanderlinden et al. (78). Briefly, the A/Netherlands/378/2005 or A/Hong Kong/7/87 virus was preincubated with compound for 15 min at 37°C. After lowering the pH to 4.9 with acetic acid, the mixture was incubated for 10 min at 37°C, followed by neutralization to pH 7.2 by the addition of 0.5 M Tris-HCl. Then, the samples were incubated with 10 mg per ml trypsin for 1 h at 37°C. The reaction was terminated by mixing the samples with protein extraction buffer (RIPA buffer supplemented with a protease inhibitor cocktail mixture; Thermo Fisher Scientific), followed by electrophoresis on 4 to 12% Criterion Bis-Tris XT precast gels (Bio-Rad) and electroblotting onto polyvinylidene difluoride membranes (Bio-Rad). Western blot analysis was performed using the primary antibodies anti-HA1 mouse monoclonal antibody clone InA97 (for A/Netherlands/378/2005) or InA246 (for A/Hong Kong/7/87) (both from Hytest) and the secondary antibody horseradish peroxidase-linked goat anti-mouse immunoglobulin polyclonal antibody (Dako). The protein bands were visualized using the SuperSignal West Femto maximum sensitivity substrate (Thermo Fisher Scientific) and a ChemiDoc XRS+ system (Bio-Rad).
Polykaryon assay.
The procedure for the polykaryon assay was based on the methods described by Vanderlinden et al. (78) and Laporte et al. (79). HeLa cells were reverse transfected with the pCAGEN plasmid containing the HA coding sequence of the B/Netherlands/537/2005 virus (pCAGEN-BNed05, derived from the pCAGEN expression vector kindly provided by C. Cepko [Boston, MA] via Addgene [plasmid number 11160]). Therefore, a mixture of 50 ng plasmid DNA and 0.2 µl FuGENE 6 reagent (Promega) in serum-free medium was transferred into black 96-well plates with a transparent bottom, followed by the addition of HeLa cells in growth medium at a density of 17,500 cells per well. At 1 day posttransfection, the medium was replaced by MEM containing 0.3% bovine serum albumin (BSA) and 0.2% FCS, and the cells were further incubated at 37°C. One day later, the cells were washed with MEM, and the expressed HA was cleaved by incubation with TPCK-treated trypsin at 5 µg/ml for 15 min at 37°C. After two washes with PBS containing Ca2+ and Mg2+ (PBS-CM), the cells were preincubated for 15 min at 37°C with 100 µl of compound diluted in PBS-CM. Then, the compound was removed, and the cells were incubated with 100 µl of acidic buffer (PBS-CM adjusted to pH 5.4 with 0.1 M citric acid) containing the corresponding concentration of test compound. After 15 min of incubation at 37°C, the cells were rinsed with PBS-CM, and 100 µl growth medium was added, followed by 3 h of incubation at 37°C. Finally, the cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 for 15 min, and stained with HCS CellMask staining solution for 30 min (Thermo Fisher Scientific). The plates were imaged using a CellInsight CX5 high-content imaging platform (Thermo Fisher Scientific). Nine images were taken per well, and the number of polykaryons (containing five or more nuclei) was counted. Data were expressed as the IC50, representing the concentration causing 50% reduction of the number of polykaryons, compared to the no-compound control.
Virus release assay.
MDCK cells were seeded in 24-well plates at 125,000 cells per well 16 h prior to infection with the A/Hong Kong/7/87 virus at 0.4 PFU/cell. After 6 h of incubation at 35°C, cells were washed five times with prewarmed medium, followed by compound addition and further incubation at 35°C. At 12 h p.i., cell culture supernatants were harvested and subjected to virus titration on MDCK cells. Serial (4-fold) dilutions of these samples were added to MDCK cells seeded in 96-well plates. After 72 h of incubation at 35°C, the viral CPE was evaluated microscopically, and the CCID50 was calculated following the method of Reed and Muench (80).
NA assay.
The procedure to determine the inhibitory effect of the compounds on NA activity was slightly adapted from that described by Woods et al. (81). Briefly, allantoic A/Hong Kong/7/87 virus (final dilution, 1:7.5) and the test compounds were diluted in 2-(N-morpholino)ethanesulfonic acid (MES) buffer (32.5 mM MES, 4 mM calcium dichloride [pH 6.0]) and transferred into black 96-well plates with a transparent bottom (total volume, 50 µl). After 10 min of preincubation at 37°C, 50 µl of prewarmed MUNANA substrate (diluted in MES buffer; final concentration, 100 µM) was added to the wells, followed by incubation at 37°C in a Tecan Spark 10M plate reader. Fluorescence (excitation at 355 nm and emission at 460 nm) was measured at 0, 30, 60, 90, and 120 min. A sample containing only MUNANA substrate was included to perform background correction. The IC50 was calculated as the concentration causing 50% reduction of the fluorescent signal at 120 min, relative to the no-compound control.
Fluorescence recovery after photobleaching.
The protocol was adapted from that described by Leikina et al. (52). HeLa cells were seeded in 35-mm glass-bottom culture dishes at 250,000 cells/well using growth medium, and the HA expression plasmid (pCAGEN-BNed05 at 725 ng per well) was reverse transfected using FuGENE 6 reagent and Opti-MEM I. After 24 h at 37°C, the cell culture medium was replaced by MEM with 0.3% BSA and 0.2% FCS, and the cells were further incubated at 37°C for 24 h. To label membrane proteins, the cells were washed with bicarbonate-PBS buffer (PBS supplemented with 0.1 M sodium bicarbonate) and incubated for 1 h at 37°C with Alexa Fluor 488 succinimidyl ester (10 µg per well; Life Technologies). The cells were then washed three times with PBS, and compounds were added. FRAP experiments were performed at 22°C with a Leica TCS SP5 confocal microscope (Leica Microsystems) equipped with an AOBS, using an HCX PL APO CS 63× water immersion lens (numerical aperture, 1.2). To visualize Alexa Fluor 488, the excitation line of 488 nm was used, and emission was detected between 493 and 565 nm. A circular area of the membrane with a diameter of 1 µm was bleached, and fluorescence recovery was monitored (prebleach, 20 cycles of 0.66 s; postbleach, 20 cycles of 0.66 s, 20 cycles of 1 s, and 20 cycles of 5 s). The mean intensity of fluorescence of the bleached area was corrected for natural photobleaching with a control zone on the same membrane. For the time course of fluorescence recovery, the average fluorescence of the bleached area before bleaching and at the first measurement after bleaching were considered 100% and 0%, respectively. For each sample, 10 curves were obtained and the average value was calculated. Three independent experiments were performed.
Generation of wild-type and mutant B/Brisbane/60/2008 viruses.
The B/Brisbane/60/2008 virus was generated using the pDP2002-PB1, pDP2002-PB2, pDP2002-PA, pDP2002-NP, pDP2002-HA, pDP2002-NA, pDP2002-M, and pDP2002-NS plasmids, kindly provided by D. Perez (University of Georgia, USA) (82). To create HA mutants with disrupted N-glycosylation sites at position N25, N59, N145, N230, N301, N330, or N492, the corresponding Asn in the pDP2002-HA plasmid was mutated to the structurally related Gln using the QuikChange Lightning site-directed mutagenesis kit (Agilent). In addition, the N-glycosylation site at position 194 was introduced by the D194N substitution (amino acid numbering according to that of PDB entry 4FQM [40]). The entire HA sequence was verified by Sanger sequencing to check for the absence of unwanted mutations. Plasmid DNA was purified using the PureYield plasmid midiprep system (Promega), followed by ethanol precipitation.
To generate the wild-type or mutant viruses, the procedure described by Hoffmann et al. (83) was slightly adapted. A coculture of MDCK and HEK293T cells was seeded in 6-well plates at 200,000 cells per well each in Opti-MEM I and incubated at 37°C. After 16 h, the cells were transfected with a mixture of 8 plasmids (1 µg per well for each plasmid) using Lipofectamine 2000 (8 µl per well; Thermo Fisher Scientific) and further incubated at 33°C. One day later, the medium was replaced by Opti-MEM I containing 1 µg/ml TPCK-treated trypsin. At 3 days posttransfection, the cell culture supernatant was harvested and expanded twice on freshly seeded MDCK cells. For the HA-N301Q and HA-N492Q viruses, a third passage on MDCK cells was required to obtain a virus stock. The HA and NA sequences of wild-type and mutant viruses were verified by Sanger sequencing. To assess the viral fitness of wild-type and mutant B/Brisbane/60/2008 viruses, MDCK cells were infected at the same MOI as for antiviral assays, followed by titration of the virus in the cell culture supernatant at 24, 48, and 72 h p.i.
Selection and characterization of HHA-resistant viruses.
Plaque-purified A/Netherlands/378/2005 and A/Hong Kong/7/87 influenza viruses underwent 50 passages on MDCK cells in the presence of 3.2 to 2,000 nM and 3.2 to 80 nM HHA, respectively. The final supernatants were plaque purified on 1% agarose, followed by expansion of individual clones on MDCK cells in the presence of HHA (20 nM for A/Netherlands/378/2005 and 3.2 nM for A/Hong Kong/7/87). The supernatant fractions were subjected to RNA extraction with the QIAamp viral RNA minikit (Qiagen), followed by amplification of the HA and NA genes using the SuperScript IV One-Step RT-PCR system (Life Technologies) with segment-specific primers and Sanger sequencing. In parallel, these supernatant fractions were used as virus stocks in an antiviral assay as described above. To assess the viral fitness of HHA-resistant A/Netherlands/378/2005 viruses, MDCK cells were infected at the same MOI as for antiviral assays, followed by titration of the virus in the cell culture supernatant at 24, 48, and 72 h p.i.
Statistical analysis.
To compare the data in Fig. 5, the Mann-Whitney test (two-tailed; confidence level of 99%) was performed using GraphPad Prism 8. Dose-response curves and IC50 values in Fig. 2 were obtained using GraphPad Prism 8 (nonlinear regression).
Supplementary Material
ACKNOWLEDGMENTS
This paper is in honor of the 80th birthday (in 2021) of Em. Prof. Erik De Clercq, our respected mentor and pioneer in antiviral therapies.
We thank Dirk Daelemans for providing the high-content imaging infrastructure, Els Vanstreels for support with the confocal microscope, and Jeroen Sajdak, Ria Van Berwaer, and Valerie Raeymaekers for their dedicated technical assistance.
Molecular graphics in Fig. 6 were generated with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH grant P41-GM103311.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, Palese P, Shaw ML, Treanor J, Webster RG, García-Sastre A. 2018. Influenza. Nat Rev Dis Primers 4:3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamayoshi S, Kawaoka Y. 2019. Current and future influenza vaccines. Nat Med 25:212–220. doi: 10.1038/s41591-018-0340-z. [DOI] [PubMed] [Google Scholar]
- 3.Shie JJ, Fang JM. 2019. Development of effective anti-influenza drugs: congeners and conjugates: a review. J Biomed Sci 26:84. doi: 10.1186/s12929-019-0567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayden FG, Sugaya N, Hirotsu N, Lee N, de Jong MD, Hurt AC, Ishida T, Sekino H, Yamada K, Portsmouth S, Kawaguchi K, Shishido T, Arai M, Tsuchiya K, Uehara T, Watanabe A. 2018. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 379:913–923. doi: 10.1056/NEJMoa1716197. [DOI] [PubMed] [Google Scholar]
- 5.O'Hanlon R, Shaw ML. 2019. Baloxavir marboxil: the new influenza drug on the market. Curr Opin Virol 35:14–18. doi: 10.1016/j.coviro.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Hayden FG, Shindo N. 2019. Influenza virus polymerase inhibitors in clinical development. Curr Opin Infect Dis 32:176–186. doi: 10.1097/QCO.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taubenberger JK, Morens DM. 2008. The pathology of influenza virus infections. Annu Rev Pathol 3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamblin SJ, Skehel JJ. 2010. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J Biol Chem 285:28403–28409. doi: 10.1074/jbc.R110.129809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertram S, Glowacka I, Steffen I, Kühl A, Pöhlmann S. 2010. Novel insights into proteolytic cleavage of influenza virus hemagglutinin. Rev Med Virol 20:298–310. doi: 10.1002/rmv.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell CJ, Hu M, Okda FA. 2018. Influenza hemagglutinin protein stability, activation, and pandemic risk. Trends Microbiol 26:841–853. doi: 10.1016/j.tim.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanderlinden E, Naesens L. 2014. Emerging antiviral strategies to interfere with influenza virus entry. Med Res Rev 34:301–339. doi: 10.1002/med.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkpatrick E, Qiu X, Wilson PC, Bahl J, Krammer F. 2018. The influenza virus hemagglutinin head evolves faster than the stalk domain. Sci Rep 8:10432. doi: 10.1038/s41598-018-28706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim P, Jang YH, Kwon SB, Lee CM, Han G, Seong BL. 2018. Glycosylation of hemagglutinin and neuraminidase of influenza A virus as signature for ecological spillover and adaptation among influenza reservoirs. Viruses 10:183. doi: 10.3390/v10040183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahalingam A, Geonnotti AR, Balzarini J, Kiser PF. 2011. Activity and safety of synthetic lectins based on benzoboroxole-functionalized polymers for inhibition of HIV entry. Mol Pharm 8:2465–2475. doi: 10.1021/mp2002957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balzarini J. 2007. Carbohydrate-binding agents: a potential future cornerstone for the chemotherapy of enveloped viruses? Antivir Chem Chemother 18:1–11. doi: 10.1177/095632020701800101. [DOI] [PubMed] [Google Scholar]
- 16.O'Keefe BR, Smee DF, Turpin JA, Saucedo CJ, Gustafson KR, Mori T, Blakeslee D, Buckheit R, Boyd MR. 2003. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob Agents Chemother 47:2518–2525. doi: 10.1128/aac.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillaire ML, van Eijk M, Vogelzang-van Trierum SE, Fouchier RA, Osterhaus AD, Haagsman HP, Rimmelzwaan GF. 2014. Recombinant porcine surfactant protein D inhibits influenza A virus replication ex vivo. Virus Res 181:22–26. doi: 10.1016/j.virusres.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 18.Hillaire ML, van Eijk M, Vogelzang-van Trierum SE, Nieuwkoop NJ, van Riel D, Fouchier RA, Kuiken T, Osterhaus AD, Haagsman HP, Rimmelzwaan GF. 2015. Assessment of the antiviral properties of recombinant surfactant protein D against influenza B virus in vitro. Virus Res 195:43–46. doi: 10.1016/j.virusres.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Covés-Datson EM, King SR, Legendre M, Gupta A, Chan SM, Gitlin E, Kulkarni VV, Pantaleón García J, Smee DF, Lipka E, Evans SE, Tarbet EB, Ono A, Markovitz DM. 2020. A molecularly engineered antiviral banana lectin inhibits fusion and is efficacious against influenza virus infection in vivo. Proc Natl Acad Sci U S A 117:2122–2132. doi: 10.1073/pnas.1915152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YM, Shahed-Al-Mahmud M, Chen X, Chen TH, Liao KS, Lo JM, Wu YM, Ho MC, Wu CY, Wong CH, Jan JT, Ma C. 2020. A carbohydrate-binding protein from the edible Lablab beans effectively blocks the infections of influenza viruses and SARS-CoV-2. Cell Rep doi: 10.1016/j.celrep.2020.108016:108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibuya N, Goldstein IJ, Shafer JA, Peumans WJ, Broekaert WF. 1986. Carbohydrate binding properties of the stinging nettle (Urtica dioica) rhizome lectin. Arch Biochem Biophys 249:215–224. doi: 10.1016/0003-9861(86)90577-1. [DOI] [PubMed] [Google Scholar]
- 22.Harata K, Muraki M. 2000. Crystal structures of Urtica dioica agglutinin and its complex with tri-N-acetylchitotriose. J Mol Biol 297:673–681. doi: 10.1006/jmbi.2000.3594. [DOI] [PubMed] [Google Scholar]
- 23.Itakura Y, Nakamura-Tsuruta S, Kominami J, Tateno H, Hirabayashi J. 2017. Sugar-binding profiles of chitin-binding lectins from the hevein family: a comprehensive study. Int J Mol Sci 18:1160. doi: 10.3390/ijms18061160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Damme EJM, Goldstein IJ, Peumans WJ. 1991. A comparative study of mannose-binding lectins from the Amaryllidaceae and Alliaceae. Phytochemistry 30:509–514. doi: 10.1016/0031-9422(91)83716-X. [DOI] [Google Scholar]
- 25.Chantalat L, Wood SD, Rizkallah P, Reynolds CD. 1996. X-ray structure solution of amaryllis lectin by molecular replacement with only 4% of the total diffracting matter. Acta Crystallogr D Biol Crystallogr 52:1146–1152. doi: 10.1107/S090744499600546X. [DOI] [PubMed] [Google Scholar]
- 26.Hester G, Wright CS. 1996. The mannose-specific bulb lectin from Galanthus nivalis (snowdrop) binds mono- and dimannosides at distinct sites: structure analysis of refined complexes at 2.3 Å and 3.0 Å resolution. J Mol Biol 262:516–531. doi: 10.1006/jmbi.1996.0532. [DOI] [PubMed] [Google Scholar]
- 27.Kaku H, Van Damme EJM, Peumans WJ, Goldstein IJ. 1990. Carbohydrate-binding specificity of the daffodil (Narcissus pseudonarcissus) and amaryllis (Hippeastrum hybr.) bulb lectins. Arch Biochem Biophys 279:298–304. doi: 10.1016/0003-9861(90)90495-K. [DOI] [PubMed] [Google Scholar]
- 28.Shibuya N, Goldstein IJ, Van Damme EJM, Peumans WJ. 1988. Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J Biol Chem 263:728–734. [PubMed] [Google Scholar]
- 29.Gordts SC, Renders M, Ferir G, Huskens D, Van Damme EJM, Peumans WJ, Balzarini J, Schols D. 2015. NICTABA and UDA, two GlcNAc-binding lectins with unique antiviral activity profiles. J Antimicrob Chemother 70:1674–1685. doi: 10.1093/jac/dkv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alen MM, De Burghgraeve T, Kaptein SJ, Balzarini J, Neyts J, Schols D. 2011. Broad antiviral activity of carbohydrate-binding agents against the four serotypes of dengue virus in monocyte-derived dendritic cells. PLoS One 6:e21658. doi: 10.1371/journal.pone.0021658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keyaerts E, Vijgen L, Pannecouque C, Van Damme EJM, Peumans WJ, Egberink H, Balzarini J, Van Ranst M. 2007. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res 75:179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Meer FJ, de Haan CA, Schuurman NM, Haijema BJ, Peumans WJ, Van Damme EJM, Delputte PL, Balzarini J, Egberink HF. 2007. Antiviral activity of carbohydrate-binding agents against Nidovirales in cell culture. Antiviral Res 76:21–29. doi: 10.1016/j.antiviral.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balzarini J, Neyts J, Schols D, Hosoya M, Van Damme EJM, Peumans WJ, De Clercq E. 1992. The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Res 18:191–207. doi: 10.1016/0166-3542(92)90038-7. [DOI] [PubMed] [Google Scholar]
- 34.Balzarini J, Schols D, Neyts J, Van Damme EJM, Peumans WJ, De Clercq E. 1991. α-(1–3)- and α-(1–6)-d-mannose-specific plant lectins are markedly inhibitory to human immunodeficiency virus and cytomegalovirus infections in vitro. Antimicrob Agents Chemother 35:410–416. doi: 10.1128/aac.35.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kachko A, Loesgen S, Shahzad-Ul-Hussan S, Tan W, Zubkova I, Takeda K, Wells F, Rubin S, Bewley CA, Major ME. 2013. Inhibition of hepatitis C virus by the cyanobacterial protein Microcystis viridis lectin: mechanistic differences between the high-mannose specific lectins MVL, CV-N, and GNA. Mol Pharm 10:4590–4602. doi: 10.1021/mp400399b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gondim ACS, Roberta da Silva S, Mathys L, Noppen S, Liekens S, Holanda Sampaio A, Nagano CS, Renata Costa Rocha C, Nascimento KS, Cavada BS, Sadler PJ, Balzarini J. 2019. Potent antiviral activity of carbohydrate-specific algal and leguminous lectins from the Brazilian biodiversity. Medchemcomm 10:390–398. doi: 10.1039/c8md00508g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanderlinden E, Vanstreels E, Boons E, ter Veer W, Huckriede A, Daelemans D, Van Lommel A, Rőth E, Sztaricskai F, Herczegh P, Naesens L. 2012. Intracytoplasmic trapping of influenza virus by a lipophilic derivative of aglycoristocetin. J Virol 86:9416–9431. doi: 10.1128/JVI.07032-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leiva R, Barniol-Xicota M, Codony S, Ginex T, Vanderlinden E, Montes M, Caffrey M, Luque FJ, Naesens L, Vázquez S. 2018. Aniline-based inhibitors of influenza H1N1 virus acting on hemagglutinin-mediated fusion. J Med Chem 61:98–118. doi: 10.1021/acs.jmedchem.7b00908. [DOI] [PubMed] [Google Scholar]
- 39.Rossman JS, Lamb RA. 2011. Influenza virus assembly and budding. Virology 411:229–236. doi: 10.1016/j.virol.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, van der Vlugt R, Lamrani M, Korse HJ, Geelen E, Sahin Ö, Sieuwerts M, Brakenhoff JP, Vogels R, Li OT, Poon LL, Peiris M, Koudstaal W, Ward AB, Wilson IA, Goudsmit J, Friesen RH. 2012. Highly conserved protective epitopes on influenza B viruses. Science 337:1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balzarini J. 2007. Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy. Nat Rev Microbiol 5:583–597. doi: 10.1038/nrmicro1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Breedam W, Pohlmann S, Favoreel HW, de Groot RJ, Nauwynck HJ. 2014. Bitter-sweet symphony: glycan-lectin interactions in virus biology. FEMS Microbiol Rev 38:598–632. doi: 10.1111/1574-6976.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.York IA, Stevens J, Alymova IV. 2019. Influenza virus N-linked glycosylation and innate immunity. Biosci Rep 39:BSR20171505. doi: 10.1042/BSR20171505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alymova IV, York IA, Air GM, Cipollo JF, Gulati S, Baranovich T, Kumar A, Zeng H, Gansebom S, McCullers JA. 2016. Glycosylation changes in the globular head of H3N2 influenza hemagglutinin modulate receptor binding without affecting virus virulence. Sci Rep 6:36216. doi: 10.1038/srep36216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.An Y, Parsons LM, Jankowska E, Melnyk D, Joshi M, Cipollo JF. 2019. N-Glycosylation of seasonal influenza vaccine hemagglutinins: implication for potency testing and immune processing. J Virol 93:e01693-18. doi: 10.1128/JVI.01693-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reading PC, Pickett DL, Tate MD, Whitney PG, Job ER, Brooks AG. 2009. Loss of a single N-linked glycan from the hemagglutinin of influenza virus is associated with resistance to collectins and increased virulence in mice. Respir Res 10:117. doi: 10.1186/1465-9921-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Job ER, Deng YM, Tate MD, Bottazzi B, Crouch EC, Dean MM, Mantovani A, Brooks AG, Reading PC. 2010. Pandemic H1N1 influenza A viruses are resistant to the antiviral activities of innate immune proteins of the collectin and pentraxin superfamilies. J Immunol 185:4284–4291. doi: 10.4049/jimmunol.1001613. [DOI] [PubMed] [Google Scholar]
- 48.van der Meer F, de Haan C, Schuurman N, Haijema B, Verheije M, Bosch B, Balzarini J, Egberink H. 2007. The carbohydrate-binding plant lectins and the non-peptidic antibiotic pradimicin A target the glycans of the coronavirus envelope glycoproteins. J Antimicrob Chemother 60:741–749. doi: 10.1093/jac/dkm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iba Y, Fujii Y, Ohshima N, Sumida T, Kubota-Koketsu R, Ikeda M, Wakiyama M, Shirouzu M, Okada J, Okuno Y, Kurosawa Y, Yokoyama S. 2014. Conserved neutralizing epitope at globular head of hemagglutinin in H3N2 influenza viruses. J Virol 88:7130–7144. doi: 10.1128/JVI.00420-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barbey-Martin C, Gigant B, Bizebard T, Calder LJ, Wharton SA, Skehel JJ, Knossow M. 2002. An antibody that prevents the hemagglutinin low pH fusogenic transition. Virology 294:70–74. doi: 10.1006/viro.2001.1320. [DOI] [PubMed] [Google Scholar]
- 51.de Vries E, Du W, Guo H, de Haan CAM. 2020. Influenza A virus hemagglutinin-neuraminidase-receptor balance: preserving virus motility. Trends Microbiol 28:57–67. doi: 10.1016/j.tim.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leikina E, Delanoe-Ayari H, Melikov K, Cho MS, Chen A, Waring AJ, Wang W, Xie Y, Loo JA, Lehrer RI, Chernomordik LV. 2005. Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat Immunol 6:995–1001. doi: 10.1038/ni1248. [DOI] [PubMed] [Google Scholar]
- 53.Coves-Datson EM, Dyall J, DeWald LE, King SR, Dube D, Legendre M, Nelson E, Drews KC, Gross R, Gerhardt DM, Torzewski L, Postnikova E, Liang JY, Ban B, Shetty J, Hensley LE, Jahrling PB, Olinger GG, Jr, White JM, Markovitz DM. 2019. Inhibition of Ebola virus by a molecularly engineered banana lectin. PLoS Negl Trop Dis 13:e0007595. doi: 10.1371/journal.pntd.0007595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balzarini J, Van Laethem K, Hatse S, Froeyen M, Peumans WJ, Van Damme EJM, Schols D. 2005. Carbohydrate-binding agents cause deletions of highly conserved glycosylation sites in HIV GP120: a new therapeutic concept to hit the Achilles heel of HIV. J Biol Chem 280:41005–41014. doi: 10.1074/jbc.M508801200. [DOI] [PubMed] [Google Scholar]
- 55.Berton MT, Naeve CW, Webster RG. 1984. Antigenic structure of the influenza B virus hemagglutinin: nucleotide sequence analysis of antigenic variants selected with monoclonal antibodies. J Virol 52:919–927. doi: 10.1128/JVI.52.3.919-927.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ikonen N, Pyhälä R, Axelin T, Kleemola M, Korpela H. 2005. Reappearance of influenza B/Victoria/2/87-lineage viruses: epidemic activity, genetic diversity and vaccination efficacy in the Finnish Defence Forces. Epidemiol Infect 133:263–271. doi: 10.1017/s0950268804003462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakagawa N, Kubota R, Maeda A, Okuno Y. 2004. Influenza B virus Victoria group with a new glycosylation site was epidemic in Japan in the 2002–2003 season. J Clin Microbiol 42:3295–3297. doi: 10.1128/JCM.42.7.3295-3297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Q, Cheng F, Lu M, Tian X, Ma J. 2008. Crystal structure of unliganded influenza B virus hemagglutinin. J Virol 82:3011–3020. doi: 10.1128/JVI.02477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smee DF, Wandersee MK, Checketts MB, O'Keefe BR, Saucedo C, Boyd MR, Mishin VP, Gubareva LV. 2007. Influenza A (H1N1) virus resistance to cyanovirin-N arises naturally during adaptation to mice and by passage in cell culture in the presence of the inhibitor. Antivir Chem Chemother 18:317–327. doi: 10.1177/095632020701800604. [DOI] [PubMed] [Google Scholar]
- 60.Witvrouw M, Fikkert V, Hantson A, Pannecouque C, O'Keefe BR, McMahon J, Stamatatos L, de Clercq E, Bolmstedt A. 2005. Resistance of human immunodeficiency virus type 1 to the high-mannose binding agents cyanovirin N and concanavalin A. J Virol 79:7777–7784. doi: 10.1128/JVI.79.12.7777-7784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balzarini J, Van Laethem K, Hatse S, Froeyen M, Van Damme EJM, Bolmstedt A, Peumans WJ, De Clercq E, Schols D. 2005. Marked depletion of glycosylation sites in HIV-1 gp120 under selection pressure by the mannose-specific plant lectins of Hippeastrum hybrid and Galanthus nivalis. Mol Pharmacol 67:1556–1565. doi: 10.1124/mol.104.005082. [DOI] [PubMed] [Google Scholar]
- 62.Hartley CA, Jackson DC, Anders EM. 1992. Two distinct serum mannose-binding lectins function as β inhibitors of influenza virus: identification of bovine serum β inhibitor as conglutinin. J Virol 66:4358–4363. doi: 10.1128/JVI.66.7.4358-4363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hartshorn KL, Webby R, White MR, Tecle T, Pan C, Boucher S, Moreland RJ, Crouch EC, Scheule RK. 2008. Role of viral hemagglutinin glycosylation in anti-influenza activities of recombinant surfactant protein D. Respir Res 9:65. doi: 10.1186/1465-9921-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun S, Wang Q, Zhao F, Chen W, Li Z. 2012. Prediction of biological functions on glycosylation site migrations in human influenza H1N1 viruses. PLoS One 7:e32119. doi: 10.1371/journal.pone.0032119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al Khatib HA, Al Thani AA, Yassine HM. 2018. Evolution and dynamics of the pandemic H1N1 influenza hemagglutinin protein from 2009 to 2017. Arch Virol 163:3035–3049. doi: 10.1007/s00705-018-3962-z. [DOI] [PubMed] [Google Scholar]
- 66.Hillaire ML, van Eijk M, Nieuwkoop NJ, Vogelzang-van Trierum SE, Fouchier RA, Osterhaus AD, Haagsman HP, Rimmelzwaan GF. 2012. The number and position of N-linked glycosylation sites in the hemagglutinin determine differential recognition of seasonal and 2009 pandemic H1N1 influenza virus by porcine surfactant protein D. Virus Res 169:301–305. doi: 10.1016/j.virusres.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Mishin VP, Novikov D, Hayden FG, Gubareva LV. 2005. Effect of hemagglutinin glycosylation on influenza virus susceptibility to neuraminidase inhibitors. J Virol 79:12416–12424. doi: 10.1128/JVI.79.19.12416-12424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Londrigan SL, Turville SG, Tate MD, Deng YM, Brooks AG, Reading PC. 2011. N-linked glycosylation facilitates sialic acid-independent attachment and entry of influenza A viruses into cells expressing DC-SIGN or L-SIGN. J Virol 85:2990–3000. doi: 10.1128/JVI.01705-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stray SJ, Cummings RD, Air GM. 2000. Influenza virus infection of desialylated cells. Glycobiology 10:649–658. doi: 10.1093/glycob/10.7.649. [DOI] [PubMed] [Google Scholar]
- 70.Gillespie L, Roosendahl P, Ng WC, Brooks AG, Reading PC, Londrigan SL. 2016. Endocytic function is critical for influenza A virus infection via DC-SIGN and L-SIGN. Sci Rep 6:19428. doi: 10.1038/srep19428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ng WC, Londrigan SL, Nasr N, Cunningham AL, Turville S, Brooks AG, Reading PC. 2016. The C-type lectin langerin functions as a receptor for attachment and infectious entry of influenza A virus. J Virol 90:206–221. doi: 10.1128/JVI.01447-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balzarini J, Hatse S, Vermeire K, Princen K, Aquaro S, Perno CF, De Clercq E, Egberink H, Vanden Mooter G, Peumans WJ, Van Damme EJM, Schols D. 2004. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob Agents Chemother 48:3858–3870. doi: 10.1128/AAC.48.10.3858-3870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swanson MD, Boudreaux DM, Salmon L, Chugh J, Winter HC, Meagher JL, André S, Murphy PV, Oscarson S, Roy R, King S, Kaplan MH, Goldstein IJ, Tarbet EB, Hurst BL, Smee DF, de la Fuente C, Hoffmann HH, Xue Y, Rice CM, Schols D, Garcia JV, Stuckey JA, Gabius HJ, Al-Hashimi HM, Markovitz DM. 2015. Engineering a therapeutic lectin by uncoupling mitogenicity from antiviral activity. Cell 163:746–758. doi: 10.1016/j.cell.2015.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smee DF, Bailey KW, Wong MH, O'Keefe BR, Gustafson KR, Mishin VP, Gubareva LV. 2008. Treatment of influenza A (H1N1) virus infections in mice and ferrets with cyanovirin-N. Antiviral Res 80:266–271. doi: 10.1016/j.antiviral.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stevaert A, Dallocchio R, Dessì A, Pala N, Rogolino D, Sechi M, Naesens L. 2013. Mutational analysis of the binding pockets of the diketo acid inhibitor L-742,001 in the influenza virus PA endonuclease. J Virol 87:10524–10538. doi: 10.1128/JVI.00832-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Damme EJM, Peumans WJ, Pusztai A, Bardocz S. 1998. Handbook of plant lectins: properties and biomedical applications. John Wiley & Sons, Chichester, West Sussex, England. [Google Scholar]
- 77.Kimura K, Mori S, Tomita K, Ohno K, Takahashi K, Shigeta S, Terada M. 2000. Antiviral activity of NMSO3 against respiratory syncytial virus infection in vitro and in vivo. Antiviral Res 47:41–51. doi: 10.1016/s0166-3542(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 78.Vanderlinden E, Göktas F, Cesur Z, Froeyen M, Reed ML, Russell CJ, Cesur N, Naesens L. 2010. Novel inhibitors of influenza virus fusion: structure-activity relationship and interaction with the viral hemagglutinin. J Virol 84:4277–4288. doi: 10.1128/JVI.02325-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laporte M, Stevaert A, Raeymaekers V, Boogaerts T, Nehlmeier I, Chiu W, Benkheil M, Vanaudenaerde B, Pöhlmann S, Naesens L. 2019. Hemagglutinin cleavability, acid stability, and temperature dependence optimize influenza B virus for replication in human airways. J Virol 94:e01430-19. doi: 10.1128/JVI.01430-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am J Epidemiol 27:493–497. doi: 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 81.Woods JM, Bethell RC, Coates JA, Healy N, Hiscox SA, Pearson BA, Ryan DM, Ticehurst J, Tilling J, Walcott SM. 1993. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob Agents Chemother 37:1473–1479. doi: 10.1128/aac.37.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nogales A, Perez DR, Santos J, Finch C, Martínez-Sobrido L. 2017. Reverse genetics of influenza B viruses. Methods Mol Biol 1602:205–238. doi: 10.1007/978-1-4939-6964-7_14. [DOI] [PubMed] [Google Scholar]
- 83.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A 97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whittle JR, Zhang R, Khurana S, King LR, Manischewitz J, Golding H, Dormitzer PR, Haynes BF, Walter EB, Moody MA, Kepler TB, Liao HX, Harrison SC. 2011. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc Natl Acad Sci U S A 108:14216–14221. doi: 10.1073/pnas.1111497108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera: a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 86.Bedford T, Riley S, Barr IG, Broor S, Chadha M, Cox NJ, Daniels RS, Gunasekaran CP, Hurt AC, Kelso A, Klimov A, Lewis NS, Li X, McCauley JW, Odagiri T, Potdar V, Rambaut A, Shu Y, Skepner E, Smith DJ, Suchard MA, Tashiro M, Wang D, Xu X, Lemey P, Russell CA. 2015. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature 523:217–220. doi: 10.1038/nature14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reid AH, Fanning TG, Hultin JV, Taubenberger JK. 1999. Origin and evolution of the 1918 “Spanish” influenza virus hemagglutinin gene. Proc Natl Acad Sci U S A 96:1651–1656. doi: 10.1073/pnas.96.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bauer K, Richter M, Wutzler P, Schmidtke M. 2009. Different neuraminidase inhibitor susceptibilities of human H1N1, H1N2, and H3N2 influenza A viruses isolated in Germany from 2001 to 2005/2006. Antiviral Res 82:34–41. doi: 10.1016/j.antiviral.2009.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.