The efficacy of fluconazole is related to the area under the plasma concentration-time curve (AUC) over the MIC of the microorganism. Physiological changes in critically ill patients may affect the exposure of fluconazole, and therefore dosing adjustments might be needed.
KEYWORDS: fluconazole, pharmacokinetics, invasive candidiasis, critically ill
ABSTRACT
The efficacy of fluconazole is related to the area under the plasma concentration-time curve (AUC) over the MIC of the microorganism. Physiological changes in critically ill patients may affect the exposure of fluconazole, and therefore dosing adjustments might be needed. The aim of this study was to evaluate variability in fluconazole drug concentration in intensive care unit (ICU) patients and to develop a pharmacokinetic model to support personalized fluconazole dosing. A prospective observational pharmacokinetic study was performed in critically ill patients receiving fluconazole either as prophylaxis or as treatment. The association between fluconazole exposure and patient variables was studied. Pharmacokinetic modeling was performed with a nonparametric adaptive grid (NPAG) algorithm using R package Pmetrics. Data from 33 patients were available for pharmacokinetic analysis. Patients on dialysis and solid organ transplant patients had a significantly lower exposure to fluconazole. The population was best described with a one-compartment model, where the mean volume of distribution was 51.52 liters (standard deviation [SD], 19.81) and the mean clearance was 0.767 liters/h (SD, 0.46). Creatinine clearance was tested as a potential covariate in the model, but was not included in the final population model. A significant positive correlation was found between the fluconazole exposure (AUC) and the trough concentration (Cmin). Substantial variability in fluconazole plasma concentrations in critically ill adults was observed, where the majority of patients were underexposed. Fluconazole Cmin therapeutic drug monitoring (TDM)-guided dosing can be used to optimize therapy in critically ill patients. (This study has been registered at ClinicalTrials.gov under identifier NCT02491151.)
INTRODUCTION
Invasive candidiasis remains a common nosocomial infection, with high mortality rates even among patients receiving antifungal treatment (1, 2). Although prevention of Candida bloodstream infection is important, with routine change or removal of indwelling catheters and appropriate antifungal prophylaxis in specific high-risk groups (3–6), timely initiation of treatment using adequate dosages of fluconazole is mandatory to improve the treatment outcome of invasive candidiasis (7, 8). Due to the increase of Candida species with reduced susceptibility or resistance to fluconazole, routine microbial surveillance of nonsterile and sterile sites in at-risk patients is suggested to permit clinicians to initiate effective treatment (7, 9, 10).
Echinocandins are recommended as primary antifungal agents for the treatment of invasive Candida infection (11). Treatment should be continued for at least 14 days following documented clearance of Candida species from the bloodstream and resolution of signs and symptoms attributable to infection (11). In addition to prophylaxis, fluconazole is used as a step-down treatment and targeted treatment against fluconazole-susceptible species, especially those with reduced susceptibility to echinocandins, such as Candida parapsilosis (11). Step-down treatment with fluconazole as early as 5 days after the start of intravenous treatment with an echinocandin appeared to be effective as antifungal treatment compared with an echinocandin administered for the full treatment course (12). Reduction of echinocandin use due to step-down treatment with fluconazole results in a decreased risk for the development of echinocandin-resistant microorganisms and significant cost savings (13).
Fluconazole has time- and concentration-dependent fungistatic activity and efficacy associated with the ratio of the area under the concentration-time curve (AUC) to the MIC (14). For treatment, an AUC for 24 h (AUC0–24 h) of 400 mg · h/liter and for prophylaxis an AUC0–24 h of 200 mg · h/liter are considered appropriate exposure for management of infections with fluconazole-susceptible species (15–18). Although therapeutic drug monitoring (TDM) is not routinely recommended for fluconazole (19), detection of underexposure of fluconazole in specific pediatric patient populations (20) and obese patients (21) has been demonstrated to be beneficial. Higher weight-based loading doses (12 mg/kg body weight) and maintenance doses (6 or 12 mg/kg/day, depending on renal function) are required to prevent underexposure (22). The heterogeneity of patients in the intensive care unit (ICU) results in differences in drug exposure, and therefore, standard dosing may not be appropriate for every patient (23). Furthermore, the dosage must be adapted to renal function, which may fluctuate significantly over time and is very difficult to estimate in ICU patients (24). Based on the highly variable drug exposure and the relationship between drug exposure and efficacy/toxicity, TDM could benefit ICU patients (25–28).
The aim of this study was to evaluate fluconazole drug concentration variability in ICU patients and to develop a pharmacokinetic (PK) model to support personalized fluconazole dosing to achieve adequate exposure in ≥95% of the patients after loading and during steady state.
RESULTS
In total, 49 patients were included in the study: 28 patients (57%) received fluconazole prophylaxis, and 21 patients (43%) received fluconazole treatment for invasive candidiasis (with 17 patients receiving fluconazole as initial therapy and 4 receiving fluconazole as step-down therapy) (Table 1). All prophylaxis recipients had at least two documented risk factors for invasive candidiasis, and 28 patients reported three or more risk factors. A total of 76.2% (16/21) of all patients receiving fluconazole as treatment underwent major surgery prior to the diagnosis of invasive candidiasis. The majority (93.8% [15/16]) underwent surgery for an indication other than solid organ transplantation, which consisted primarily of intestinal resection or bowel surgery.
TABLE 1.
Patient demographicsa
| Demographic | Result for patient group: |
||
|---|---|---|---|
| All (n = 49) | Prophylaxis (n = 28) | Treatment (n = 21) | |
| Female, no. (%) | 13 (27.0) | 7 (24.1) | 6 (28.6) |
| Age, median yr (IQR) | 60 (52–66) | 56 (45–62) | 66 (59–73) |
| Wt, median kg (IQR) | 80.0 (71.0–88.0) | 81.0 (73.3–88.5) | 77.0 (71.0–93.5) |
| BMI, median (IQR) | 24.9 (23.6–27.6) | 25.3 (23.7–27.5) | 25.2 (23.6–30.1) |
| Underlying condition, no. (%) | |||
| Major surgery | 38 (77.5) | 22 (78.6) | 16 (76.2) |
| Solid organ transplant | 20 (40.1) | 19 (67.9) | 1 (4.8) |
| Severity of disease score, median (IQR) | |||
| APACHE II | 19 (15–24) | 19 (14–21) | 20 (16–24) |
| APACHE IV | 62 (50–88) | 61 (45–88) | 72 (51–89) |
| LODS | 6 (3–9) | 6 (3–8) | 7 (3–10) |
| SAPS | 40 (34–56) | 38 (33–54) | 45 (38–57) |
| Risk factor exposure, no. (%) | |||
| Central venous catheter | 45 (91.8) | 27 (96.4) | 18 (85.7) |
| Total parenteral nutrition | 8 (16.3) | 3 (10.7) | 5 (23.8) |
| Mechanical ventilation | 48 (98.0) | 27 (96.4) | 21 (100.0) |
| Immunosuppressive therapy | 20 (40.8) | 19 (67.9) | 1 (4.8) |
| Dialysis | 14 (28.6) | 9 (32.1) | 5 (23.8) |
| Corticosteroid therapy | 25 (51.0) | 21 (75.0) | 4 (19.0) |
| Broad-spectrum antibiotic use | 46 (93.9) | 25 (89.3) | 21 (100.0) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; APACHE IV, Acute Physiology and Chronic Health Evaluation IV; LODS, Logistic Organ Dysfunction System; SAPS, Simplified Acute Physiology Score.
Therapy and therapy outcome.
The median dose in mg per kg per day, the AUC0–24 h, and the trough concentration (Cmin) of fluconazole after a loading dose and during steady state (day 5 of fluconazole treatment) are displayed in Table 2. Despite a loading dose, the fluconazole exposure was significantly lower after 24 h compared with fluconazole exposure in steady state. Most patients (36/49 [73.4%]) ceased fluconazole after completion of successful therapy. Prophylaxis with fluconazole failed in 3 patients (10.7%) due to a breakthrough infection (n = 3 patients with Candida glabrata), and treatment with caspofungin or liposomal amphotericin B was initiated. Three patients (10.7%) discontinued prophylaxis with fluconazole due to lack of effectiveness (colonization of the gastrointestinal [GI] tract: two with C. glabrata and one with Candida albicans) and switched to amphotericin B orally. In the fluconazole treatment group, 6 patients (28.6%) died during fluconazole treatment. Death was related to the underlying condition in all patients, where 3 patients died from cardiac comorbidity, 2 patients from pancreatitis and 1 patient from advanced liver cirrhosis. A significant positive correlation was found between the fluconazole exposure (AUC) and the Cmin and fluconazole dose (Table 3). The correlation between the fluconazole exposure was significantly stronger (R2 = 0.9619 and 0.9854) than the exposure-dosage relationship (R2 = 0.3311 and 0.2512). Patients on dialysis, patients who underwent solid organ transplantation, and patients receiving immunosuppressive and corticosteroid therapy had significantly reduced fluconazole exposure. In the multiple linear regression analysis, variables obtained by the univariable analysis (P < 0.100) were included. Patients on dialysis and solid organ transplant patients had significantly reduced fluconazole exposure after multivariate analysis (Table 4).
TABLE 2.
Therapy and therapy outcomea
| Parameter | Result for recipients of: |
|
|---|---|---|
| Prophylaxis | Treatment | |
| Dose and exposure, median (IQR) | ||
| Loading dose, mg/kg/day | 4.7 (2.8–5.3) | 5.6 (3.8–8.8) |
| AUC0–24 h on day 1, mg · h/liter | 137.0 (11.5–169.7) | 174.0 (108.9–345.6) |
| Cmin on day 1, mg/liter | 4.5 (3.4–6.0) | 6.4 (3.9–12.7) |
| Dose on day 5, mg/kg/day | 1.4 (1.1–4.0) | 3.8 (2.7–6.7) |
| AUC0–24 h on day 5, mg · h/liter | 183.9 (70.3–469.4) | 405.0 (239.2–587.9) |
| Cmin on day 5, mg/liter | 6.5 (2.0–15.5) | 13.3 (7.7–20.7) |
| Therapy discontinuation, no. (%) | ||
| Successful therapy | 21 (75.0) | 15 (71.4) |
| Breakthrough infection | 3 (10.7) | NA |
| Adverse event | 0 (0.0) | 0 (0.0) |
| Lack of effectiveness | 3 (10.7) | 0 (0.0) |
| Death | 1 (3.6) | 6 (28.6) |
Abbreviations: AUC, area under the curve; Cmin, trough concentration; NA, not applicable.
TABLE 3.
Association between patient characteristics, clinical parameters, and fluconazole exposure (AUC0–24 h)a
| Parameter | Correlation coefficient | P value |
|---|---|---|
| Day 1 | ||
| Cmin (mg/liter) | 0.951 | <0.001 |
| Loading dose (mg/kg/day) | 0.678 | <0.001 |
| Body wt | 0.112 | 0.446 |
| BMI | 0.195 | 0.180 |
| Dialysis use (CVVH) | −0.319 | 0.025 |
| SOT | −0253 | 0.080 |
| Diabetes | −0.090 | 0.541 |
| Corticosteroid use | −0.248 | 0.085 |
| APACHE II | −0.004 | 0.979 |
| APACHE IV | −0.088 | 0.583 |
| LODS | 0.042 | 0.776 |
| MPM0 | 0.006 | 0.972 |
| MPMII | 0.134 | 0.405 |
| SAPS | 0.135 | 0.353 |
| Creatinine | −0.201 | 0.166 |
| ALP | 0.184 | 0.237 |
| ALAT | −0.196 | 0.208 |
| ASAT | −0.182 | 0.249 |
| γGT | 0.144 | 0.357 |
| Day 5 | ||
| Cmin (mg/liter) | 0.993 | <0.001 |
| Dose (mg/kg/day) | 0.736 | <0.001 |
| Body wt | 0.103 | 0.596 |
| BMI | 0.109 | 0.573 |
| Dialysis use (CVVH) | −0.526 | 0.003 |
| SOT | −0.481 | 0.008 |
| Diabetes | 0.012 | 0.951 |
| Corticosteroid use | −0.494 | 0.006 |
| APACHE II | 0.015 | 0.943 |
| APACHE IV | −0.181 | 0.386 |
| LODS | −0.065 | 0.737 |
| MPM0 | −0.148 | 0.479 |
| MPMII | −0.055 | 0.793 |
| SAPS | 0.112 | 0.564 |
| Creatinine | −0.135 | 0.486 |
| ALP | 0.098 | 0.642 |
| ALAT | −0.140 | 0.504 |
| ASAT | −0.122 | 0.563 |
| γGT | 0.072 | 0.731 |
The Spearman coefficient was used for analysis. Abbreviations: Cmin, trough concentration; BMI, body mass index; CVVH, continuous veno-venous hemofiltration; SOT, solid organ transplant; APACHE II, Acute Physiology and Chronic Health Evaluation II; APACHE IV, Acute Physiology and Chronic Health Evaluation IV; LODS, Logistic Organ Dysfunction System; SAPS, Simplified Acute Physiology Score; ALP, alkaline phosphatase; ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; γGT, γ-glutamyl transferase.
TABLE 4.
Multiple linear regression model of factors significantly correlated with fluconazole exposure (AUC0–24 h) on day 5a
| Factor | Effect | 95% CI | P value |
|---|---|---|---|
| Dialysis use | −0.442 | −410.859 to −68.279 | 0.008 |
| SOT | −0.382 | −344.177 to −35.525 | 0.020 |
R2 of model 1 = 0.421, and R2 of model 2 = 0.407, compared with the model with all variables included. Abbreviations: CI, confidence interval; SOT, solid organ transplant.
Pharmacokinetic modeling.
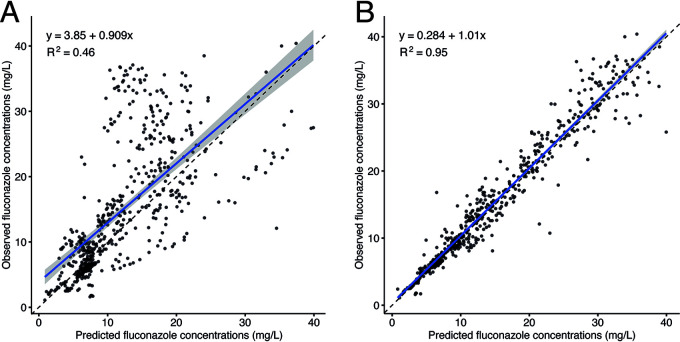
For the development of the population pharmacokinetic (PK) model, data from 33 patients with 561 fluconazole concentrations were used. Patients on dialysis (n = 13) and receiving fluconazole orally (n = 3) were excluded due to the possible different pharmacokinetic profile and missing detailed data on dialysis. This population was best described with a one-compartment pharmacokinetic model. Creatinine was tested as a possible covariate on clearance (CL); however, including it did not improve the goodness of fit or other parameters of the model. The final population pharmacokinetic model estimates are described in Table 5. The individual-predicted and population-predicted versus observed goodness-of-fit plots are presented in Fig. 1. Figure 1B shows that the fit could not be described for some subpopulations by covariates, even after excluding patients on dialysis. The developed model showed good precision when estimating Bayesian posterior predictions (R2 = 0.79). The external validation is presented in Fig. 2.
TABLE 5.
Final one-compartment model population pharmacokinetic estimatesa
| Parameter | Mean (SD) | CV (%) | Median |
|---|---|---|---|
| V | 51.52 (19.81) liters | 38.45 | 50.54 liters |
| CL | 0.767 (0.46) liter/h | 60.21 | 0.82 liter/h |
Abbreviations: SD, standard deviation; CV, coefficient of variation; V, volume of distribution; CL, mean clearance.
FIG 1.
(A) The population-predicted versus observed goodness-of-fit plots for the final one-compartment model. (B) The individual predicted versus observed goodness-of-fit plots for the final one-compartment model.
FIG 2.
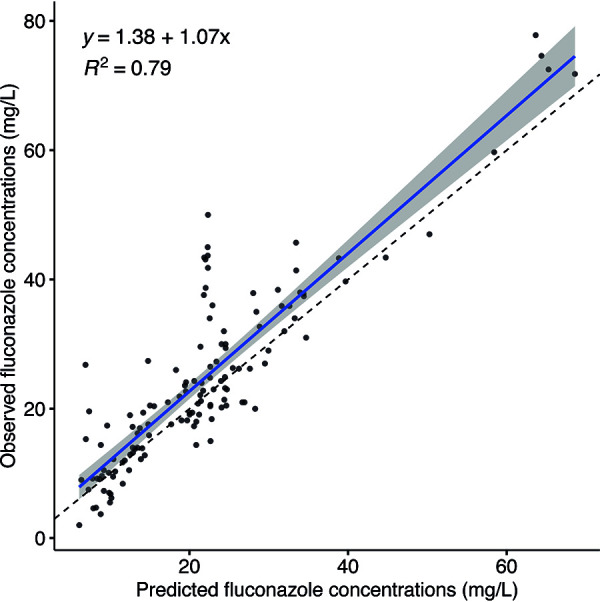
The Bayesian posterior predictions for the external data set.
Probability of target attainment.
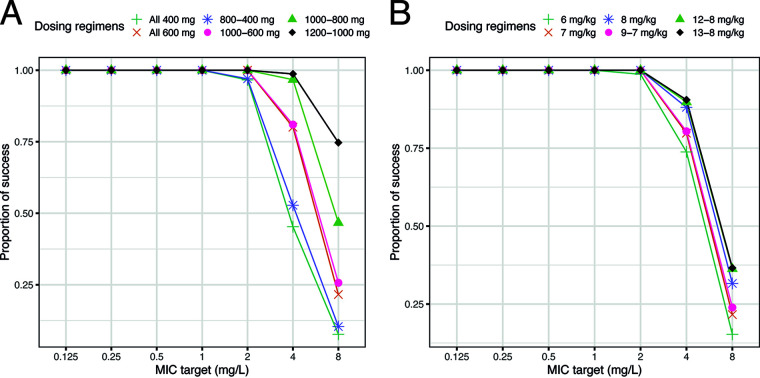
The probability of target attainment is presented in Table 6 and Fig. 3. The lowest dose that achieved a target attainment of more than 95% was for a fixed dosing regimen of 1,200 mg loading + 1,000 mg daily (98% on the first day and 100% on the third and fifth days); for a weight-based dosing regimen, the lowest dose for target attainment of more than 95% was 13 mg/kg loading + 8 mg/kg daily (96% on the first day and 100% on the third and fifth days). None of the simulated patients receiving a fixed dosing regimen (1,200 mg loading + 1,000 mg daily) reached a fluconazole blood concentration of 80 mg/liter after the loading dose, 4% of the patients reached 80 mg/liter after day 3, and 7% of the simulated patients reached 80 mg/liter after day 5 of fluconazole dosing. A lower percentage of patients dosed with a weight-based dosing regimen of 13 mg/kg loading + 8 mg/kg daily reached the 80-mg/liter cutoff value (0% after the loading dose, 1% after day 3, and 5% after day 5 of dosing). Using the linear model AUC∼Cmin, the Cmin to be targeted on day 3 of therapy to achieve adequate AUC values was 14 mg/liter (90% confidence interval [CI] of 13.7 to 14.3 mg/liter).
TABLE 6.
Probability of target attainment for seven dosing regimens with fAUC/MIC of >100 and MIC of ≥2 mg/liter for the first and third days of therapya
| Fixed dosing regimen | PTA for fixed dosing (%) |
Wt-based dosing regimen | PTA for wt-based dosing (%) |
||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 1 | Day 3 | Day 5 | ||
| 400 mg daily | 16 | 87 | 97 | 6 mg/kg daily | 36 | 97 | 99 |
| 600 mg daily | 60 | 98 | 100 | 7 mg/kg daily | 58 | 98 | 100 |
| 800 mg loading + 400 mg daily | 87 | 96 | 97 | 8 mg/kg daily | 73 | 100 | 100 |
| 1,000 mg loading + 600 mg daily | 94 | 100 | 100 | 9 mg/kg loading + 7 mg/kg daily | 83 | 99 | 100 |
| 1,000 mg loading + 800 mg daily | 94 | 100 | 100 | 12 mg/kg loading + 8 mg/kg daily | 94 | 100 | 100 |
| 1,200 mg loading + 1,000 mg daily | 98 | 100 | 100 | 13 mg/kg loading + 8 mg/kg daily | 96 | 100 | 100 |
Abbreviation: PTA, probability of target attainment.
FIG 3.
(A) The PTAs for an fAUC/MIC target of 100 for the third day of therapy for fixed dosing. The x axis presents the MIC targets, and the y axis presents the proportion of success. (B) The PTAs for an fAUC/MIC target of 100 for the third day of therapy for weight-based dosing. The x axis presents the MIC targets, and the y axis presents the proportion of success.
DISCUSSION
This study demonstrated large variability in the fluconazole exposure in critically ill adults. Almost 50% of the patients did not reach the target for treatment (400 mg · h/liter) or prophylaxis (200 mg · h/liter) when Pfizer Summary of Product Characteristics (SPC) recommended fluconazole dosages were applied (24).
Leftover clinical samples from critically ill adults were used to construct concentration-time curves and to develop a pharmacokinetic model (29, 30). This approach results in no additional burden for the patient. Before applying this strategy for other drugs, stability during processing and storage needs to be confirmed.
As clinical samples are frequently collected in ICU patients, we had a large number of plasma samples, and the resulting pharmacokinetic model was able to predict fluconazole concentrations in an external data set with good precision of R2 = 0.79, supporting the strategy. Although our model was able to predict the drug exposure in most of the patients, a number of patients did not fit the model; this could not be explained by covariate analysis. Due the heterogeneity of the ICU population, the sample size of our study was possibly too small, and this could be considered a limitation of the study (31).
Our study population was diverse, reflecting a “real-life” population. Analyzing subgroups, we noted that solid organ transplant recipients and patients on renal replacement therapy (RRT; usually, continuous veno-venous hemofiltration [CVVH]) were associated with a lower fluconazole exposure, which is consistent with earlier findings in patients on RRT and patients with various rates of creatinine clearance (CLCR) (21, 22, 32, 33). Patel et al. found a significantly higher clearance of fluconazole in patients receiving continuous veno-venous hemodiafiltration (CVVHDF) compared with patients not undergoing continuous dialysis (32). Furthermore, significant underexposure due to increased fluconazole clearance in obese patients with CLCR higher than 150 ml/min has been described by Alobaid et al. (21) These observations suggest that individualization of therapy for patients undergoing dialysis could be warranted.
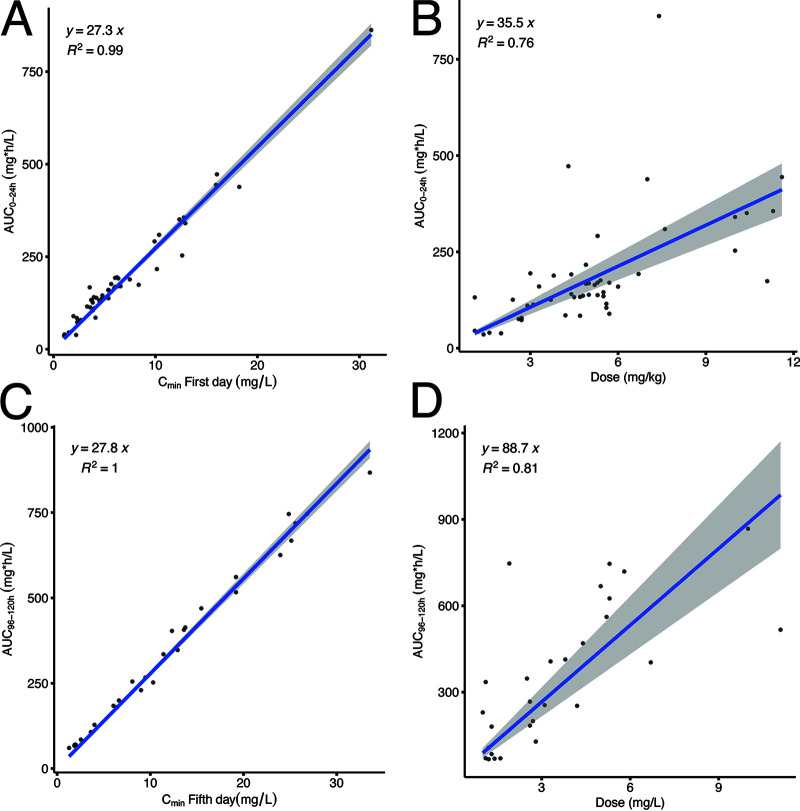
Probability of target attainment (PTA) values for multiple dosing regimens were calculated using the developed population PK model. Higher dosing (1,200 mg loading + 1,000 mg daily or 13 mg/kg loading + 8 mg/kg daily) than currently recommended appeared necessary to achieve a PTA of ≥95% throughout the treatment period. Although almost 50% of the patients were underexposed, a substantial portion of the patients (20%) could be considered overexposed, with AUCs ranging from 600 mg · h/liter to 800 mg · h/liter. Anaissie et al. (34) concluded that fluconazole dosing up to 1,600 mg daily was well tolerated, but toxic effects possibly related to higher fluconazole dosing in 38.5% of the patients were observed. Despite a good safety profile, hepatotoxicity has been associated with high fluconazole exposure, and fluconazole is recognized to have clinically relevant cytochrome P450 (CYP)-mediated drug-drug interactions. Routine administration of higher fluconazole dosages in ICU patients may be beneficial for patients at risk for underexposure, but result in an increased risk for adverse events in some of the critically ill adults. In our opinion, fluconazole TDM‐guided dosing adjustments represent a more appropriate solution (33). Although data from randomized studies are lacking, TDM‐guided fluconazole dosing in patients with deep intra-abdominal Candida infection, targeting a plasma concentration of 15 mg/liter for 14 days of therapy, was considered to have contributed to successful therapy outcome (35). In our study, fluconazole dose and fluconazole trough concentration (Cmin) were associated with the fluconazole exposure (AUC), as shown in Fig. 4; however, only the Cmin proved to be a good predictor for the AUC, making TDM very feasible. We believe that fluconazole could be an interesting and useful addition to the panel of drugs for which a pharmacy-based active TDM service is an effective tool to optimize drug dosing (as demonstrated for aminoglycoside antibiotics), resulting in higher antimicrobial efficacy, shorter hospitalization, and a reduced incidence of adverse events (36).
FIG 4.
(A) The fluconazole exposure after a loading dose correlated with the trough concentration. (B) The fluconazole exposure after a loading dose correlated with the dose. (C) The fluconazole exposure after 5 days of dosing correlated with the trough concentration. (D) The fluconazole exposure after 5 days of dosing correlated with the dose.
Over the last 2 decades, multiple studies have demonstrated significant variability in fluconazole exposure in different patient populations. Increased fluconazole dosing is proposed to avoid underexposure, but we demonstrated the potential risk for unnecessary overexposure in a heterogeneous patient population. A randomized clinical trial with critically ill patients receiving fluconazole to compare different dosing strategies (e.g., SPC dosing [800 mg loading + 400 mg maintenance]), TDM-guided dosing, higher fixed dosing (e.g., 1,200 mg loading + 600 mg maintenance), and weight-based dosing (e.g., 13 mg/kg loading + 8 mg/kg maintenance) will provide stronger evidence for optimal dosing with fluconazole. However, it is unlikely that such a trial will be funded for a generic drug. The use of TDM for fluconazole remains, therefore, at the discretion of clinicians to be used in selected cases. In this study, leftover blood samples were collected to obtain plasma samples to determine the fluconazole plasma concentrations in each patient. The use of leftover samples for a stable compound such as fluconazole has been reported in other studies, and comparable results to scheduled PK sampling were demonstrated (29, 30). This noninvasive approach to obtain plasma concentrations for other compounds could be very useful in patient populations that are difficult to study. Before using a scavenge sample approach for other drugs, the stability of the proposed drug must first be established for the conditions under which the drug will be stored and processed.
In conclusion, we observed substantial variability in fluconazole plasma concentrations in critically ill adults. Fluconazole exposure was strongly correlated with fluconazole trough concentration. Considering the large variability in exposure and in particular the observed underexposure in ICU patients, fluconazole Cmin TDM-guided dosing could be a valuable tool to optimize antifungal therapy with fluconazole in critically ill patients, solid organ transplant recipients, and patients on dialysis.
MATERIALS AND METHODS
This prospective study was conducted at the University Medical Center Groningen. Patients were eligible for inclusion if they were at least 18 years of age, were admitted to the ICU, and received antifungal therapy or prophylaxis with fluconazole. This study was evaluated by the local ethics committee (Institutional Review Board 2014, METc 2014.363), and according to Dutch law, a waiver was obtained for this study according to the Medical Research Involving Human Subjects Act due to its noninvasive nature: i.e., fluconazole plasma concentrations were determined in discarded samples. Patients were included between October 2014 and February 2017 (ClinicalTrials.gov identifier NCT02491151).
Data collection.
Data were collected (J.B.) using a standardized case report form and verified by a second investigator (A.-G.M.). Data included demographics, clinical data, and therapy related data: i.e., age, gender, weight, length, underlying condition, length of stay in the ICU, leukocyte count, Candida species, and MIC. The presence of risk factors for invasive candidiasis was reviewed, including the presence of a central venous catheter, total parenteral nutrition, mechanical ventilation, dialysis, corticosteroid use, previous use of broad-spectrum antibiotics, and immunosuppressive therapy (37). Information was collected about the indication for antifungal therapy (prophylaxis or treatment), fluconazole dose (mg/day), route of administration, fluconazole concentrations, and dose adjustments. Leftover samples from routinely collected blood specimens were used for analysis. Whole-blood samples were collected on the ICU and stored directly at 2 to 8°C. Blood samples were processed within 24 h of collection, and plasma was stored at −20°C until analysis to determine the fluconazole concentration. This strategy has been used before for fluconazole and showed comparable results to scheduled PK sampling (29, 30). Total (bound and unbound) fluconazole concentrations were determined using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay (38).
Therapy and therapy outcome.
Prophylaxis with fluconazole for 5 days was administered only to patients who underwent major abdominal surgery according to the hospital protocol. These patients received doses of 400 mg on day 1, 200 mg on day 2, and 100 mg on days 3, 4, and 5, starting on the day of surgery. Based on the clinical status of the patient and microbiological surveillance data from nonsterile sites, prophylaxis was continued after day 5 with 100 mg fluconazole daily until clearance of Candida from the nonsterile sites or the absence of complications due to the surgical intervention. The response to fluconazole prophylaxis was determined 14 days after initiation; it was considered successful if there was no breakthrough infection or if therapy was not switched to another antifungal agent due to lack of clinical effectiveness. Patients receiving fluconazole for the treatment of invasive candidiasis were dosed with 800 mg on day 1, followed by 400 mg daily as the maintenance dose, according to the Pfizer Summary of Product Characteristics (SPC) (24). Initiation of treatment with fluconazole was based on growth of Candida species susceptible to fluconazole at sterile sites. For therapy outcome, possible reasons for therapy discontinuation were determined, including death, switching to another antifungal agent due to lack of efficacy, successful treatment, or the onset of an adverse event.
Pharmacokinetic modeling.
Pharmacokinetic modeling was performed with a nonparametric adaptive grid (NPAG) algorithm using R package Pmetrics, R (Los Angeles, CA, USA) (39). One- and two-compartment pharmacokinetic models were parameterized with central volume of distribution (V; liters), clearance (CL; liters/h), rate constant for fluconazole distribution from the peripheral to the central compartment (kpc; h−1), and rate constant for fluconazole distribution from the central to the peripheral compartment (kcp; h−1). The goodness of fit of individual pharmacokinetic models was analyzed using the individual-predicted and population-predicted versus observed goodness-of-fit plots, the Akaike information criterion (AIC), the Bayesian information criterion (BIC), the log-likelihood value, and the bias and imprecision of the observed-predicted plots. Age, gender, weight and creatinine (μmol/liter) were tested as potential covariates to be included in the model. Covariates were included in the final model if the stepwise linear regression resulted in P < 0.05 or if other population pharmacokinetic parameters improved. The model was validated using an independent external data set with 132 samples from 30 patients (40). For the validation, the final model was used as a nonuniform prior, the validation data set was used as data, and cycles were set to 0. This created only posterior Bayesian predictions.
Probability of target attainment.
Using the final model, probability of target attainment (PTA) was calculated using Monte Carlo simulations (n = 1,000). The pharmacokinetic/pharmacodynamic (PK/PD) target of unbound fluconazole AUC to the MIC (fAUC/MIC) was set to 100, as defined by the European Committee for Antimicrobial Susceptibility and Testing (EUCAST) (41). Fluconazole plasma protein binding was estimated to be 12% in all simulations (24). Different dosages were simulated to explore the probability of reaching the highest target: thus, higher-than-routine dosing regimens were tested. A wide dosing range was examined where six fixed dosing regimens were simulated: 400 mg daily, 600 mg daily, 800 mg loading + 400 mg daily, 1,000 mg loading + 600 mg daily, 1,000 mg loading + 800 mg daily, and 1,200 mg loading + 1,000 mg daily. Six weight-based dosing regimens were simulated: 6 mg/kg daily, 7 mg/kg daily, 8 mg/kg daily, 9 mg/kg loading + 7 mg/kg daily, 12 mg/kg loading + 8 mg/kg daily, and 13 mg/kg loading + 8 mg/kg daily. The regimen was designed to cover C. albicans and Candida tropicalis, and a MIC of 2 mg/liter was used for PTA calculations, as the epidemiological cutoff (ECOFF) value for each of these pathogens is 2 mg/liter, respectively (41). The PTA target was to achieve adequate exposure in ≥95% of the patients after day 1 of fluconazole therapy. The number of patients reaching a toxic exposure (80 mg/liter) was simulated for the proposed dosing regimens (34). A linear model was used to determine the proposed Cmin range for day 3 in our data set: 400 mg · h/liter for AUC∼Cmin (41).
Statistical analysis.
For the univariable analysis, a Spearman correlation coefficient was calculated to determine correlations between 2 continuous variables. For comparison of 2 groups, the Mann-Whitney U test was used. Variables obtained from univariable analysis (P < 0.1) were included in multiple linear regression analysis using a backward procedure, thereby removing nonsignificant variables, starting with the one with the highest P value.
All statistical analyses were performed using SPSS for Windows, version 20.0 (IBM SPSS, Chicago, IL), and R version 3.6.1. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
We are grateful to and thank all patients who participated in this study. We also thank the medical and nursing staff of the ICU and the analytical staff of the pharmacy.
A.G.M. was funded by Marie Skłodowska-Curie Actions (grant agreement no. 713660—PRONKJEWAIL—H2020-MSCA-COFUND-2015). None of the financial support is related to this article.
REFERENCES
- 1.Kullberg BJ, Arendrup MC. 2015. Invasive candidiasis. N Engl J Med 373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 2.Koehler P, Stecher M, Cornely OA, Koehler D, Vehreschild MJGT, Bohlius J, Wisplinghoff H, Vehreschild JJ. 2019. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect 25:1200–1212. doi: 10.1016/j.cmi.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Ostrosky-Zeichner L. 2004. Prophylaxis or preemptive therapy of invasive candidiasis in the intensive care unit? Crit Care Med 32:2552–2553. doi: 10.1097/01.ccm.0000148226.95597.7e. [DOI] [PubMed] [Google Scholar]
- 4.Knitsch W, Vincent J-L, Utzolino S, François B, Dinya T, Dimopoulos G, Özgüneş İ, Valía JC, Eggimann P, León C, Montravers P, Phillips S, Tweddle L, Karas A, Brown M, Cornely OA. 2015. A randomized, placebo-controlled trial of preemptive antifungal therapy for the prevention of invasive candidiasis following gastrointestinal surgery for intra-abdominal infections. Clin Infect Dis 61:1671–1678. doi: 10.1093/cid/civ707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garbino J, Lew DP, Romand JA, Hugonnet S, Auckenthaler R, Pittet D. 2002. Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med 28:1708–1717. doi: 10.1007/s00134-002-1540-y. [DOI] [PubMed] [Google Scholar]
- 6.Pelz RK, Hendrix CW, Swoboda SM, Diener-West M, Merz WG, Hammond J, Lipsett PA. 2001. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann Surg 233:542–548. doi: 10.1097/00000658-200104000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 8.Garey KW, Pai MP, Suda KJ, Turpin RS, Rege MD, Mingo DE, Bearden DT. 2007. Inadequacy of fluconazole dosing in patients with candidemia based on Infectious Diseases Society of America (IDSA) guidelines. Pharmacoepidemiol Drug Safe 16:919–927. doi: 10.1002/pds.1365. [DOI] [PubMed] [Google Scholar]
- 9.Cleveland AA, Farley MM, Harrison LH, Stein B, Hollick R, Lockhart SR, Magill SS, Derado G, Park BJ, Chiller TM. 2012. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin Infect Dis 55:1352–1361. doi: 10.1093/cid/cis697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zilberberg MD, Kollef MH, Arnold H, Labelle A, Micek ST, Kothari S, Shorr AF. 2010. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study. BMC Infect Dis 10:150. doi: 10.1186/1471-2334-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vazquez J, Reboli AC, Pappas PG, Patterson TF, Reinhardt J, Chin-Hong P, Tobin E, Kett DH, Biswas P, Swanson R. 2014. Evaluation of an early step-down strategy from intravenous anidulafungin to oral azole therapy for the treatment of candidemia and other forms of invasive candidiasis: results from an open-label trial. BMC Infect Dis 14:97. doi: 10.1186/1471-2334-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grau S, Pozo JC, Roma E, Salavert M, Barrueta JA, Peral C, Rodriguez I, Rubio-Rodriguez D, Rubio-Terres C. 2015. Cost-effectiveness of three echinocandins and fluconazole in the treatment of candidemia and/or invasive candidiasis in nonneutropenic adult patients. Clinicoecon Outcomes Res 7:527–535. doi: 10.2147/CEOR.S91587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andes D, van Ogtrop M. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob Agents Chemother 43:2116–2120. doi: 10.1128/AAC.43.9.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watt KM, Gonzalez D, Benjamin DK, Jr, Brouwer KL, Wade KC, Capparelli E, Barrett J, Cohen-Wolkowiez M. 2015. Fluconazole population pharmacokinetics and dosing for prevention and treatment of invasive candidiasis in children supported with extracorporeal membrane oxygenation. Antimicrob Agents Chemother 59:3935–3943. doi: 10.1128/AAC.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clancy CJ, Yu VL, Morris AJ, Snydman DR, Nguyen MH. 2005. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob Agents Chemother 49:3171–3177. doi: 10.1128/AAC.49.8.3171-3177.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai MP, Turpin RS, Garey KW. 2007. Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob Agents Chemother 51:35–39. doi: 10.1128/AAC.00474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiemenz J, Cagnoni P, Simpson D, Devine S, Chao N, Keirns J, Lau W, Facklam D, Buell D. 2005. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob Agents Chemother 49:1331–1336. doi: 10.1128/AAC.49.4.1331-1336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. 2014. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother 69:1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Elst KC, Pereboom M, van den Heuvel ER, Kosterink JG, Scholvinck EH, Alffenaar JW. 2014. Insufficient fluconazole exposure in pediatric cancer patients and the need for therapeutic drug monitoring in critically ill children. Clin Infect Dis 59:1527–1533. doi: 10.1093/cid/ciu657. [DOI] [PubMed] [Google Scholar]
- 21.Alobaid AS, Wallis SC, Jarrett P, Starr T, Stuart J, Lassig-Smith M, Mejia JL, Roberts MS, Sinnollareddy MG, Roger C, Lipman J, Roberts JA. 2016. Effect of obesity on the population pharmacokinetics of fluconazole in critically ill patients. Antimicrob Agents Chemother 60:6550–6557. doi: 10.1128/AAC.01088-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yagasaki K, Gando S, Matsuda N, Kameue T, Ishitani T, Hirano T, Iseki K. 2003. Pharmacokinetics and the most suitable dosing regimen of fluconazole in critically ill patients receiving continuous hemodiafiltration. Intensive Care Med 29:1844–1848. doi: 10.1007/s00134-003-1980-z. [DOI] [PubMed] [Google Scholar]
- 23.Pea F. 2020. From bench to bedside: perspectives on the utility of pharmacokinetics/pharmacodynamics in predicting the efficacy of antifungals in invasive candidiasis. Mycoses 63:854–858. doi: 10.1111/myc.13121. [DOI] [PubMed] [Google Scholar]
- 24.Pfizer. 2019. Diflucan: summary of product characteristics revision February 2019. http://www.pfizer.com/products/product-detail/diflucan. Accessed 14 July 2019.
- 25.Bronstein JA, Gros P, Hernandez E, Larroque P, Molinie C. 1997. Fatal acute hepatic necrosis due to dose-dependent fluconazole hepatotoxicity. Clin Infect Dis 25:1266–1267. doi: 10.1086/516975. [DOI] [PubMed] [Google Scholar]
- 26.Wells C, Lever AM. 1992. Dose-dependent fluconazole hepatotoxicity proven on biopsy and rechallenge. J Infect 24:111–112. doi: 10.1016/0163-4453(92)91346-D. [DOI] [PubMed] [Google Scholar]
- 27.Sinnollareddy MG, Roberts JA, Lipman J, Akova M, Bassetti M, De Waele JJ, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Dimopoulos G, DALI Study authors. 2015. Pharmacokinetic variability and exposures of fluconazole, anidulafungin, and caspofungin in intensive care unit patients: data from multinational Defining Antibiotic Levels in Intensive care unit (DALI) patients study. Crit Care 19:33. doi: 10.1186/s13054-015-0758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gómez-López A. 2020. Antifungal therapeutic drug monitoring: focus on drugs without a clear recommendation. Clin Microbiol Infect 26:1481–1487. doi: 10.1016/j.cmi.2020.05.037. [DOI] [PubMed] [Google Scholar]
- 29.Momper JD, Capparelli EV, Wade KC, Kantak A, Dhanireddy R, Cummings JJ, Nedrelow JH, Hudak ML, Mundakel GT, Natarajan G, Gao J, Laughon M, Smith PB, Benjamin DK, Jr. 2016. Population pharmacokinetics of fluconazole in premature infants with birth weights less than 750 grams. Antimicrob Agents Chemother 60:5539–5545. doi: 10.1128/AAC.00963-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wade KC, Wu D, Kaufman DA, Ward RM, Benjamin DK, Jr, Sullivan JE, Ramey N, Jayaraman B, Hoppu K, Adamson PC, Gastonguay MR, Barrett JS, National Institute of Child Health and Development Pediatric Pharmacology Research Unit Network. 2008. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother 52:4043–4049. doi: 10.1128/AAC.00569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL, International Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases. 2014. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel K, Roberts JA, Lipman J, Tett SE, Deldot ME, Kirkpatrick CM. 2011. Population pharmacokinetics of fluconazole in critically ill patients receiving continuous venovenous hemodiafiltration: using Monte Carlo simulations to predict doses for specified pharmacodynamic targets. Antimicrob Agents Chemother 55:5868–5873. doi: 10.1128/AAC.00424-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Righi E, Carnelutti A, Baccarani U, Sartor A, Cojutti P, Bassetti M, Pea F. 2019. Treatment of Candida infections with fluconazole in adult liver transplant recipients: is TDM-guided dosing adaptation helpful? Transpl Infect Dis 21:e13113. doi: 10.1111/tid.13113. [DOI] [PubMed] [Google Scholar]
- 34.Anaissie EJ, Kontoyiannis DP, Huls C, Vartivarian SE, Karl C, Prince RA, Bosso J, Bodey GP. 1995. Safety, plasma concentrations, and efficacy of high-dose fluconazole in invasive mold infections. J Infect Dis 172:599–602. doi: 10.1093/infdis/172.2.599. [DOI] [PubMed] [Google Scholar]
- 35.Pea F, Righi E, Cojutti P, Carnelutti A, Baccarani U, Soardo G, Bassetti M. 2014. Intra-abdominal penetration and pharmacodynamic exposure to fluconazole in three liver transplant patients with deep-seated candidiasis. J Antimicrob Chemother 69:2585–2586. doi: 10.1093/jac/dku169. [DOI] [PubMed] [Google Scholar]
- 36.van Lent-Evers NA, Mathot RA, Geus WP, van Hout BA, Vinks AA. 1999. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: a cost-effectiveness analysis. Ther Drug Monit 21:63–73. doi: 10.1097/00007691-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Muskett H, Shahin J, Eyres G, Harvey S, Rowan K, Harrison D. 2011. Risk factors for invasive fungal disease in critically ill adult patients: a systematic review. Crit Care 15:R287. doi: 10.1186/cc10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alffenaar JW, Wessels AM, van Hateren K, Greijdanus B, Kosterink JG, Uges DR. 2010. Method for therapeutic drug monitoring of azole antifungal drugs in human serum using LC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 878:39–44. doi: 10.1016/j.jchromb.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandaradura I, Norris R, Marriott D, Day R, Reuter S. 2016. Modelling and simulation of fluconazole in critically ill adult patients, poster 217. World Conf Pharmacokinet 2016, Brisbane, Australia. Poster 217. https://go-wcop.org/wp-content/uploads/2016/08/WCoP-2016-Book-of-abstracts-Poster-presentations.pdf.
- 41.European Committee on Antimicrobial Susceptibility Testing-Subcommittee on Antifungal Susceptibility Testing (EUCAST-AFST). 2008. EUCAST technical note on fluconazole. Clin Microbiol Infect 14:193–195. doi: 10.1111/j.1469-0691.2007.01899.x. [DOI] [PubMed] [Google Scholar]