Molecular testing is rapidly becoming an integral component of global tuberculosis (TB) control. Uncommon mechanisms of resistance escape detection by these platforms and undermine our ability to contain outbreaks.
KEYWORDS: isoniazid, drug resistance evolution, drug resistance mechanisms, genotypic identification, phenotypic identification, tuberculosis
ABSTRACT
Molecular testing is rapidly becoming an integral component of global tuberculosis (TB) control. Uncommon mechanisms of resistance escape detection by these platforms and undermine our ability to contain outbreaks. This article is a systematic review of published articles that reported isoniazid (INH) resistance-conferring mutations between September 2013 and December 2019. The genes katG, inhA, and fabG1, and the intergenic region oxyR′-ahpC were considered in this review. Fifty-two articles were included that described 9,306 clinical isolates (5,804 INH resistant [INHr] and 3,502 INH susceptible [INHs]) from 31 countries. The three most frequently mutated loci continue to be locus 315 of katG (katG315; n = 4,271), locus −15 of inhA (inhA-15; n = 787), and locus −8 of inhA (inhA-8; 106). However, the diagnostic value of inhA-8 is far lower than previously thought, as it only appears in 25 (0.4%) of the INHr isolates lacking the first two mutations. I catalogued 45 new loci (29 katG, nine inhA, and seven ahpC) associated with INH resistance and identified 59 loci (common to this and previous reviews) as a reliable basis for molecular diagnostics. Including all observed mutations provides a cumulative sensitivity of 85.6%. In 14.4% of resistant isolates, no mechanism of resistance was detected, making them likely to escape molecular detection, and in the case of INH monoresistance, likely to convert to multidrug-resistant TB (MDR-TB). Integrating the information cataloged in this study into current diagnostic tools is essential for combating the emergence of MDR-TB, and its exclusion can lead to an unintended selection against common mechanisms and to diversifying evolution. Observation of many low-frequency resistance-conferring mutations points to an advantage of whole-genome sequencing (WGS) for diagnostics. Finally, I provide five recommendations for future diagnostic platforms.
INTRODUCTION
Tuberculosis (TB) is one of the most prevalent infectious diseases to date, with an estimated 10 million new cases in 2018 (1). It was the infectious disease with highest mortality in 2018, recently surpassing that of HIV/AIDS (2). One of the challenges in global TB control is the emergence of drug resistance. Globally, the World Health Organization (WHO) estimates a total of half a million rifampin (RIF)-resistant and multidrug-resistant (MDR) (resistant to RIF and isoniazid [INH]) TB cases in 2018, a number that has been on the rise in spite of declining total global TB cases (1, 3–6). Treatment success rate for multidrug-resistant TB (MDR-TB) is low (56% globally) (1), making its accurate diagnosis and prevention, when possible, critical pieces of global TB control. Traditionally, culture-based methods are used to determine resistance. Isolated bacteria (usually from the patient’s sputum) are cultured in the presence of a drug. If sufficient growth is observed in drug-containing culture compared to that in the drug-free control at a preset time, the case is considered resistant. Unfortunately, this process can take weeks, a period during which the patient is treated with ineffective drugs, allowing the resistant bacteria to further spread and potentially to develop resistance to additional drugs.
Drug resistance in Mycobacterium tuberculosis, the causative agent of TB, commonly emerges as a result of a point mutation in specific genes. This knowledge has been exploited in the development of molecular diagnostics as a rapid alternative to culture-based methods. Molecular testing, however, has an important disadvantage in that it can only detect resistant mutations that the platform was designed to detect. Bacteria that harbor uncommon mechanisms of resistance will therefore escape detection. As a result, the sensitivity in detecting resistance can suffer with increased prevalence of uncommon mechanisms of resistance. It is well documented that isoniazid-monoresistant (INHr) bacteria can harbor such mechanisms, escape detection, and develop into MDR-TB (7, 8). As a result, incorporation of all INH resistance-conferring mutations is critical for comprehensive molecular detection. For this reason, I set out to catalog and estimate the global prevalence of common (canonical) and uncommon mutations that confer INH resistance observed in the past 6 years. In doing so, I used the search criteria used in the previous systematic review by Seifert et al. (9), so the results of the two studies can be compared and temporal changes in prevalence can be estimated. While Seifert et al. surveyed articles published between year 2000 and August 2013, this study continued that survey from September 2013 until December 2019.
Previous studies have shown that mutations in katG, inhA, fabG1, and the oxyR′-ahpC intergenic region confer INH resistance (9, 10). katG codes for a catalase peroxidase that activates INH. Mutations in katG, in particular at codon 315 (katG315), are commonly observed to cause resistance to INH. The second most frequently observed mechanism of resistance is through mutations in the promoter of the gene inhA, particularly at position −15 (inhA-15). (9, 10) This leads to overexpression of the gene, which causes titration of the drug, leading to resistance. (8, 10) Mutations in the coding region of the inhA gene also have been associated with resistance (9, 10). Mutations in the oxyR′-ahpC intergenic region may help alleviate the fitness cost of the loss of KatG activity in many resistant isolates by increasing the expression of ahpC. (10) Finally, a synonymous mutation in fabG1, L203L (CTG203CTA), has been shown to cause INH resistance through the creation of an alternative promoter for inhA, hence causing its overexpression (11, 12).
In this review, I catalog the mutations reported as conferring INH resistance in the three genes and the intergenic region between September 2013 and December 2019, report their individual frequencies, and estimate the sensitivity of a molecular platform in detecting INH resistance, based solely on common mutations, as well as based on all mutations reported in this review. I then compare the results of this review with those of the previous systematic review to see how molecular epidemiology of INH resistance has changed in the last 5 years and offer a cumulative report for the 20-year combined timespan of both reviews (years 2000 through 2019).
RESULTS
Description of included studies.
The search through PubMed Medline using the terms indicated resulted in 601 articles published between 1 September 2013 and 31 December 2019. Out of the 601 potential studies, 52 studies met all inclusion criteria (7, 13–65) (see Table ST1 in reference 66) The PRISMA flow diagram shown in Fig. SF1 in reference 67 illustrates the breakdown of number of articles excluded because of the specified criteria.
In total, 5,804 INHr isolates were reported by the 52 included articles (see Table ST2 in reference 66). Table 1 shows the breakdown of these isolates per region of collection compared to those reported in Seifert et al.’s review (9). Overall, eight different molecular diagnostic methods were used in the 52 included studies for detection of mutations (see Table ST3 in reference 66). The most prevalent method was PCR, followed closely by whole-genome sequencing (WGS) and MTBDRplus. The three methods combined for sequencing over 82% of the included isolates. Although line probe assay (LPA) uses PCR, I distinguish between the two based on how each article preferred to refer to their method. Typically, articles that referred to their method as PCR used inhouse-designed primers (typically in academic settings). Among such studies, I only included those that had reported primer sequences for each of the canonical mutations Table ST3 in reference 66 presents the full list of nine methods and the numbers of included isolates they each sequenced. Compared to the previous review (9), perhaps the most notable change in sequencing methodology has been the increase in use of WGS.
TABLE 1.
Breakdown of INHr isolate counts by region of the world
| INHr isolate statistic | Isolate count or % by regionb: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Africa (n = 1,074; 18.5%) |
Asia (n = 4,122; 71.02%) |
Europe (n = 235; 4.05%) |
Americas (n = 373; 6.43%) |
|||||||||
| South | North | East | West | Central | South | East | West | South | East | South | North | |
| Isolate count | 415 | 39 | 620 | 141 | 348 | 1980 | 1653 | 51 | 8 | 176 | 344 | 29 |
| % of all isolatesa | 7.15 | 0.67 | 10.68 | 2.43 | 6.00 | 34.11 | 28.50 | 0.88 | 0.14 | 3.03 | 5.93 | 0.50 |
Percentages reflect the percentage of each count with respect to the total number (5,804) of INHr isolates included in this study.
For reference, INHr isolate counts (%) from the Seifert et al. review (9) per continent were 576 (5.0%), 4,424 (38.8%), 2,637 (23.1%), and 2,356 (20.6%) in Africa, Asia, Europe, and the Americas, respectively. Percentages from Seifert et al. do not add up to 100% because of 1,418 isolates (12.4%) with unknown origin in their report. Seifert et al. data reported in this table were curated from Table S6 in reference 9.
In total, a mutation was observed in 110 loci scattered across three genomic regions (katG, inhA promoter and open reading frame [ORF], and oxyR′-ahpC intergenic region and ahpC ORF). The breakdown of these loci per genomic region and of those reported in the previous systematic review by Seifert et al. (9), as well as the count of common loci, are listed in Table 2.
TABLE 2.
Number of mutated loci reported by Seifert et al. (9) and this study in INHr isolates
| Location of mutation(s) | No. of mutated loci reported |
|||
|---|---|---|---|---|
| Seifert et al. | This study | Common to both studiesc | Cumulative in both studies | |
| katG promoter | 4 | 0 | 0 (0) | 4 |
| katG ORF | 198 | 75 | 42 (18) | 231 |
| inhA promotera | 22 | 11 | 5 (22) | 28 |
| inhA ORF | 22 | 6 | 1 (5) | 27 |
| oxyR′-ahpC intergenic region | 22b | 16 | 11 (41) | 27 |
| ahpC ORF | 9 | 2 | 0 (0) | 11 |
inhA promoter mutations include mutations in fabG1 open reading frame (ORF) because they create alternative promoters for inhA and mutations upstream of fabG1 because they act as promoters of the entire operon, which includes inhA.
Seifert et al. reported a mutation in codon 13 of oxyR′. Because oxyR′ is an inactive pseudogene in M. tuberculosis (75), this position is not included in this analysis.
Numbers in parentheses are the percentages of mutated loci common to both studies.
(i) Mutations in KatG.
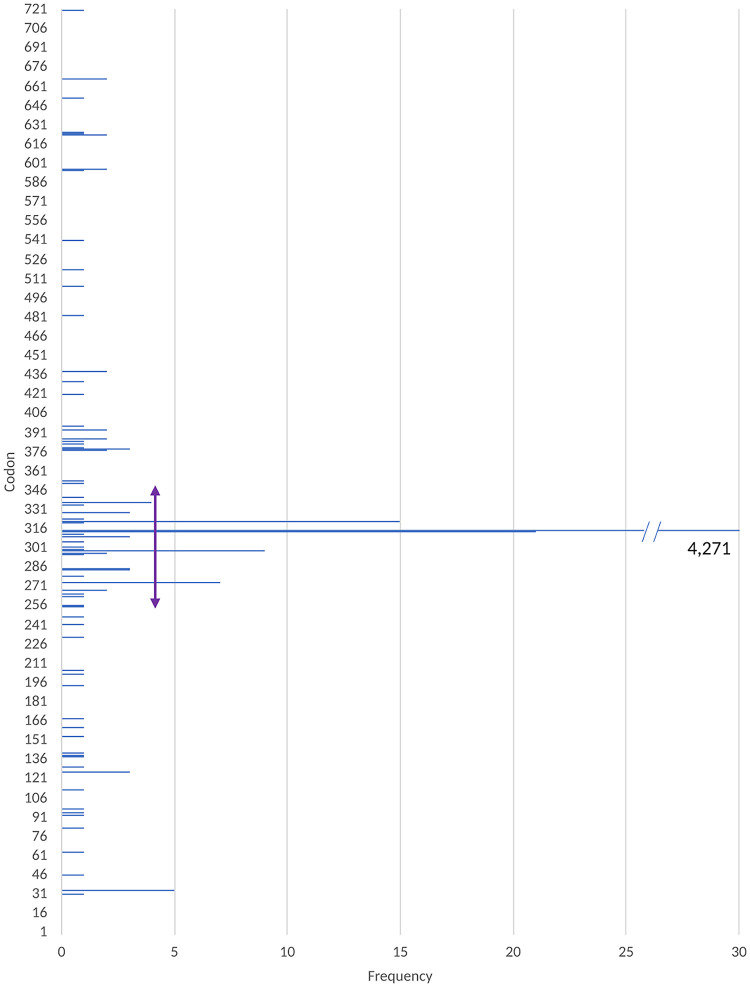
Figure 1 displays the mutated loci in katG and the number of isolates that harbored mutations at each locus. Table ST4 in reference 66 lists these loci, their counts, frequencies, and whether they were common to both systematic reviews. Two sets of mutations were excluded from consideration, namely, synonymous mutations in katG ORF that do not cause any change in the protein and mutations in codon 463, since they appear abundantly among INH-resistant (INHr) as well as INH-susceptible (INHs) isolates. Of the 740 KatG amino acids, 74 (9.8%) harbored a mutation in at least one INHr isolate. As shown in Fig. 1, these loci were scattered across the protein. Additionally, one study reported a resistant isolate with deleted katG (38), while a second reported the deletion of katG, as well as a substitution of C-52T in the oxyR′-ahpC intergenic region of a resistant strain (58). Deletion of katG has been shown to cause resistance to isoniazid (7, 8). In total, excluding codon 463, 4,391 (75.66%) resistant isolates harbored a nonsynonymous mutation in katG (Table 3). While mutations were observed across the gene, the range between codons 235 and 350 could be considered a relative hot spot, with 4,346 (74.88%) resistant isolates harboring a mutation in this region (Fig. 1 and Table ST2 in reference 66). Codon 315 in katG of 4,271 resistant isolates harbored a mutation, providing a diagnostic sensitivity of 73.59% (Table 3). As expected, this was the locus with the highest frequency of mutations. Among the varieties of mutations observed in this codon, katG S315T (AGC-ACC) was the most common variety and was harbored by 3,844 (or 66.23%) of resistant isolates (see Table ST4 in reference 66).
FIG 1.
Frequency of mutations in katG observed in INHr clinical isolates.
TABLE 3.
Twenty-year perspective on INHr mutants based on survey results
| Location of resistance mutation(s) | Results for survey period: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| September 2013 to December 2019 (current study) |
January 2000 to August 2013 (Seifert et al.) |
January 2000 to December 2019 (both studies) |
|||||||
| No. of INHr mutants | Total no. of INHr | % | No. of INHr mutants | Total no. of INHr | % | No. of INHr mutants | Total no. of INHr | % | |
| katG315 | 4,271 | 5,804 | 73.6 | 5,400 | 8,416 | 64.2 | 9,671 | 14,220 | 68.0 |
| inhA-15 | 787 | 5,804 | 13.6 | 1,189a | 6,192 | 19.2 | 1,976a | 11,996 | 16.5 |
| inhA-8 | 106 | 5,804 | 1.8 | 77a | 5,955 | 1.3e | 183a | 11,759 | 1.6 |
| katG | 4,391 | 5,804 | 75.7 | 4,068b | 6,136f | 66.3b | 8,459b | 11,940f | 70.8b |
| inhA | 978 | 5,804 | 16.9 | 926c | 4,495f | 20.6c | 1,904c | 10,299f | 18.5c |
| oxyR′-ahpC | 107 | 5,804 | 1.8 | 91d | 1,685f | 5.4d | 198d | 7,489f | 2.6d |
| katG and inhA | 4,894 | 5,804 | 84.3 | 1,271b,c | 1,582f | 80.3b,c | 6,165b,c | 7,386f | 83.5b,c |
| katG and inhA and oxyR′-ahpC | 4,967 | 5,804 | 85.6 | 1,328b,c,d | 1,583f | 83.9b,c,d | 6,295b,c,d | 7,387f | 85.2b,c,d |
| Unexplained INHr | 837 | 5,804 | 14.4 | 1,406f | 8,786 | 16 | 2,243f | 14,590 | 15.4 |
Calculated from the counts of total INHr isolates and frequency of mutation reported by Seifert et al. (9).
Only codons 309, 311, 315, and 316 were considered by Seifert et al. (9), although they report other mutations in the gene in their supplemental tables. This is thus a lower bound for the sensitivity of katG for their reported period.
Only positions −15, −8, −47, and −17 were considered by Seifert et al. (9), although they report other mutations in their supplemental tables. This is thus a lower bound for sensitivity of inhA for their reported period.
Only positions −10, −6, −39, −48, −15, −12, and −9 were considered by Seifert et al. (9), although they report other mutations in the region in their supplemental tables. This is thus a lower bound for the sensitivity of the oxyR′-ahpC intergenic region for their reported period.
This table reflects the value as reported by Seifert et al. However, I believe that this value was incorrectly calculated, and the correct frequency should be 2.25%. Please see Discussion.
Total count is calculated from mutant INHr count and the frequency of the mutation reported by Seifert et al. (9).
(ii) Mutations in inhA promoter and ORF.
As Table 3 depicts, the promoter region and ORF of inhA were mutated in 978 (16.8%) of the resistant isolates. Of these, 502 (7.62%) resistant isolates also had a mutation in KatG, while five INHr isolates had two mutations in the inhA promoter and/or its ORF. Mutations in 16 loci were reported in inhA or its promoter. Table ST5 in reference 66 lists the loci and the count and percentage of resistant isolates that harbored a mutation in each position. The most prevalent inhA promoter locus was −15, with 787 (13.56%) resistant isolates harboring a mutation at this locus. All mutations observed at this locus were inhA C-15T by all included studies.
Importantly, we have listed fabG1 ORF mutations as inhA mutations, since it has been shown that at least the synonymous mutation fabG1 L203L creates an alternative promoter for inhA (11, 12). Four INHr isolates harbored a fabG1 ORF mutation as well as a katG mutation, while one harbored only fabG1 L203L and no mutations in katG or inhA. These isolates have, therefore, contributed to inhA counts in Table 2 and 3.
(iii) Mutations in oxyR′-ahpC intergenic region or ahpC ORF.
Mutations in the 106-bp intergenic region between the pseudogene oxyR′ and the gene ahpC (H37Rv genome positions 2,726,088 to 2,726,192) are known to be associated with INH resistance (10).
In total, 107 (1.8%) resistant isolates harbored a mutation in 18 loci (Fig. 2) in this region. Of the 107, 32 (29.9%) isolates also harbored a KatG mutation, while 14 (13.08%) harbored an inhA (promoter or InhA) mutation. Seven (6.54%) of the 114 harbored a mutation both in KatG and inhA (promoter or InhA). Importantly, 68 (63.55%) of the 107 resistant isolates did not harbor a mutation in KatG or inhA (promoter or ORF). The 68 INHr isolates harbored a mutation in one of the 14 loci depicted in Fig. 2. Table ST6 in reference 66 lists the loci and the count and percentage of resistant isolates that harbored a mutation in each position.
FIG 2.
Mutations in intergenic region between the genes oxyR′ and ahpC (106 bp in H37Rv genome positions 2,726,088 to 2,726,192). Negative positions are nucleotide positions relative to the start of the gene ahpC on the positive strand, while positive positions are codon numbers in the gene’s open reading frame (ORF). Blue bars indicate isolates that also harbor KatG mutations, while orange bars indicate isolates that also harbor inhA (promoter or gene) mutations. Gray bars indicate isolates that harbor no mutations in inhA (promoter or gene) or in KatG. Seven isolates had a mutation in katG and inhA.
(iv) Regional stratification of frequencies.
Table 4 displays the regional prevalence of canonical mutations (katG315, inhA-15, and inhA-8), along with the prevalence of wild-type isolates and those with mutations in the oxyR′-ahpC region for comparison. Isolates that only harbor synonymous mutations in the three genomic regions considered in this article have been listed in the “Wild type” column.
TABLE 4.
Regional prevalence of canonical mutations (katG315, inhA-15, and inhA-8)
| Region | Prevalence of mutations (%) in locusc: |
Wild typeb | |||
|---|---|---|---|---|---|
| katG315 | inhA-15 | inhA-8 | oxyR′-ahpCa | ||
| Asia | 67.70% | 12.85% | 1.12% | 2.71% | 18.41% |
| Africa | 88.39% | 7.10% | 4.26% | 0.52% | 4.90% |
| Americas | 71.72% | 18.66% | 2.04% | – | 11.08% |
| Europe | 75.00% | 37.50% | – | – | – |
| Global | 73.59% | 13.56% | 1.83% | 1.84% | 14.42 |
The “oxyR′-ahpC” column reflects the prevalence of any mutation in this region.
The “Wild type” column displays the frequency of isoniazid-resistant isolates that harbored no mutations in the three considered regions. Isolates that only harbored synonymous mutations in coding regions have also been included in the “Wild type” column.
Because some isolates harbor multiple canonical mutations, percentages in each row add up to more than 100%. This table only includes 48 of the 52 included studies. The remaining four studies did not provide the regional information per isolate and are excluded from this table (–).
(v) Combined analysis.
To provide continuity in studying the prevalence of mutations over a 20-year period, I combined the results reported by Seifert et al. (9) and those reported in this review for a comprehensive list of mutations reported between January 2000 and December 2019. Table 3 reports the frequency of canonical mutations over the 20-year reporting span of both reviews.
Table 2 presents the number of loci reported in this article compared to those reported in Seifert et al. Importantly, the combination of the two reviews associates 230 (31%) of the katG gene’s 740 amino acids with resistance to INH. While there were many mutations that were unique in each review, the highest concordance between the two reviews (loci that I observed and those observed by Seifert et al.) was in the oxyR′-ahpC intergenic region, where 41% of all mutations were observed in both reviews.
Unfortunately, because of the way counts were reported, some raw counts could not be retrieved from Seifert et al. (9). This mostly stems from the fact that they allowed molecular evaluation of resistant isolates without evaluation of canonical mutations. (The total number of INHr isolates included in the study was reported as 8,786, but the total number of INHr isolates that were evaluated for katG315 was reported as 8,416 and the total number of INHr isolates evaluated for inhA-15 as 6,192.) Therefore, coexistence of these mutations with other mutations could not be assessed.
DISCUSSION
Molecular diagnostics are rapid, less expensive, often do not require a biosafety level 3 facility, and at times are more portable, promising to bring rapid testing to the bedside and even into the community at a low cost. They do, however, take testing one more step away from clinical outcomes. While phenotyping is intended to predict treatment outcome for a given regimen or drug, molecular platforms are often designed and tested as a proxy for the lengthier phenotypic testing. As a result, detailed knowledge of the performance profile of molecular testing and its assumptions is essential.
One of the dangers of molecular diagnostics is the selection that it could impose on to the molecular epidemiology of drug-resistant TB. A broad-scale and long-term application of molecular platforms that aim to detect only the canonical mutations increases the risk of lowering the incidence of isolates that harbor canonical mutations, since they are readily detected and appropriately treated, while those with uncommon mechanisms of resistance will escape detection and spread further. I will refer to this effect as the “artifactual” selection for uncommon mechanisms. This is of particular importance for isoniazid resistance, since its canonical mutations are well defined (e.g., katG315, inhA-15, and inhA-8), whereas quite a few other mutations are also known to cause resistance and frequently appear without the presence of canonical mutations. Such strains will benefit from artifactual selection and can cause future outbreaks.
This study was designed with three aims in mind, as follows: (i) to catalogue mutations associated with INH resistance; (ii) to assess the prevalence of canonical mutations; and (iii) estimate their global prevalence and co-occurrence and their utility in molecular diagnostics. For aim i, I include a summary of all the mutations reported in the 52 included studies. For aim ii, I report all mutation frequencies and compare these results to those of the previous survey by Seifert et al. (9), who reviewed articles published between January 2000 and August 2013. While this comparison is suboptimal, any notable differences provide additional evidence for my hypothesis that frequent global and regional reevaluation of sensitivity and specificity of molecular platforms are essential to prevention of emergence of MDR-TB.
Canonical mutations.
As defined for this study, I have considered mutations that appear in codon 315 of katG and promoter mutations at the −15 and −8 positions for inhA to be canonical. All molecular platforms reported in this survey have studied these positions for mutations. As such, the estimation of their global prevalence is the most accurate.
Campbell et al. (68) used 212 diverse INHr isolates from the WHO and the CDC to estimate the global frequency of katG315 mutations to be 85% in 2011. The data from this study estimates this frequency to be 73.6%, while Seifert et al. estimate this to be 64.2% (9) (68.8% from combining the two systematic reviews) (Table 3). Several important factors play a role in this variation. Among them, regional differences are perhaps the most important. Both reviews report a higher prevalence of katG315 in Africa than in all other regions. (Table 4) However, since only 5% of the previous review’s isolates originated from Africa, versus 18.5% in this review (Table 1), Africa’s higher katG315 frequency has a bigger impact on the global frequency of this mutation in my study compared to that in theirs, leading to a higher estimate for katG315 global prevalence than that in the previous review.
It is noteworthy that, among the isolates in this study, those from Asia had the lowest frequency of katG315 (67.7%), while Africa had the highest frequency for this mutation (88.4%) (Table 4). This highlights the need for frequent regional assessment of molecular epidemiology of resistance for isoniazid, but also quite possibly for all other drugs. Importantly, both reviews estimate the global prevalence of katG315 to be distinctly lower than the initial estimate by Campbell et al. (68), who estimated the global prevalence of inhA-15 mutants to be 17%, with a cumulative global prevalence of katG315 and inhA-15 of 91%. The current study estimates these frequencies to be 13.6% (Table 3) and 81.4%, respectively. Seifert and colleagues (9) estimated these frequencies to be 19.2% and 79.9%, respectively. This study’s individual inhA-15 frequency is lower than those in the other two studies, while the cumulative frequencies in this study and those of Seifert et al. are in line and are distinctly lower than initial estimates by Campbell et al. (68).
Finally, I estimate the global prevalence of inhA-8 to be 1.83%, whereas Seifert et al. estimated this value to be 1.3%. Combining isolates from the two studies results in a global frequency of 1.6% (Table 3) Importantly, Seifert et al. (9) reported separate mutations in mabA and fabG1. Since these two locus tags refer to the same gene, frequencies reported for this gene appear lower than they should be. Additionally, since fabG1 and inhA belong to the same operon, promoter loci are reported with respect to the beginning of the operon, which is the beginning of the fabG1 coding region. For example, fabG1-8 and inhA-8 tags point to the same locus, namely, eight positions upstream of fabG1. However, since the authors have reported these separately, the frequencies for these loci appear to be lower than they should have been. As such, combining the frequency reported for inhA-8 (1% in Table 4 of Seifert et al. [9]) and mabA-8 (1.25% in Supplemental Table 4 of Seifert et al. [9]), provides a corrected frequency of 2.25% for this locus.
Mutated loci in katG.
Seifert et al. (9) reported observing at least one mutation in 202 katG loci (198 codons in the ORF and four in the promoter) (Table 2). In this study, I report 75 such loci, all in the katG ORF. Of these, 42 were common to both reviews, whereas 33 loci are new observations compared to the Seifert report (see Table ST4 in reference 66). While the majority of the 42 common codons were in the first half of the genes, 23 of the 42 lie between codons 250 and 350, making this range a relative hot spot.
Mutated loci in inhA.
In total, Seifert et al. (9) report a mutation in 44 (22 promoter and 22 ORF) loci in inhA. I report only 16 (10 promoter and 6 ORF) such loci in the isolates in this review. Of these, five promoter (−34, −17, −15, −9, and −8) and one ORF (codon 94) loci were common to both reports, while five promoter and five ORF loci are new observations (see Table ST5 in reference 66).
Mutated loci in oxyR′-ahpC.
The role of mutations in this region in INH resistance remains a subject of discussion. Vilcheze et al. suggested that mutations in this region may not cause resistance but rather compensate (through overexpression of ahpC) for loss of peroxidase function caused by katG mutations (10). Under this hypothesis, those isolates that only harbor mutations in the oxyR′-ahpC region should be considered unexplained resistance cases. Regardless of the role, the strong association with resistance make these markers viable candidates for diagnostics.
Seifert et al. (9) reported observing mutations in 31 loci in this region (22 in the intergenic region and 9 in the ahpC ORF), whereas I report 18 mutated loci (16 in the intergenic region and two in the ahpC ORF) (see Table ST6 in reference 66). Of the 18 loci, 11 intergenic loci were common to both reports. No ahpC ORF loci were common to both reports. Five oxyR′-ahpC intergenic and two ahpC ORF loci are new observations compared to the previous systematic review. The 11 loci that appeared in both studies were −54, −52, −46, −39, −32, −30, −15, −12, −10, −9, and −6 upstream of ahpC. Interestingly, while some mutations in this region appeared only with canonical mutations, none of the 11 common loci belonged to this group. For each of the 11 common loci, there were several isolates that harbored a mutation at the locus without any mutations in katG or inhA (Fig. 2 and Table ST2 in reference 66).
Importantly, several studies did not report mutations in this region due to the limitations of the platform they used for detection of mutations (e.g., PCR without primers for this region). Hence, the frequencies reported for mutations in this region should be considered a lower bound for prevalence.
Basis for molecular diagnostics.
Seifert et al. (9) recommended the following 12 loci to be used for molecular diagnostics: katG codon 315, four inhA promoter loci (−15, −8, −47, and −17), and seven ahpC promoter loci (−10, −6, −39, −48, −15, −12, and −9). With this set of 12 loci, they reported a sensitivity of 84%. They selected these loci based on their higher frequencies. In this study, I have observed mutations at 11 of the 12 loci. ahpC-48 is the only one at which a mutation was never observed in this study. I recommend that all loci common to both studies should be added as a basis for future diagnostics. This would mean the addition of seven more positions, as follows: inhA-9, five ahpC promoter positions (−54, −52, −46, −32, and −30), and ahpC codon 94. This would bring the basis for molecular diagnostics to 18 positions (excluding ahpC-48). The significance of this position for INH resistance is unclear, and further study needs to be performed to determine its diagnostic value. Among the 5,804 INHr isolates in this review, this position was never mutated. Excluding ahpC-48, the remaining set of 18 loci in three regions provided a sensitivity of 83% in the set of 5,804 INHr isolates for molecular diagnostics of isoniazid resistance. Inclusion of all observed mutations (75 loci in katG, 16 loci in inhA, and 18 in ahpC) in the three regions would provide only marginally improved diagnostic sensitivity of 85.6%.
Alternative mechanisms of resistance.
Nearly 15% of the INHr isolates included in this study were reported by the respective studies as unexplained resistance. Several possibilities should be considered. The first is that not all molecular methods scan the full range of the three regions (katG, inhA ORF and promoter [including fabG1], and oxyR′-ahpC) considered to be associated with INH resistance. Even those that can (e.g., PCR and LPA) are used in a targeted fashion with primers that target only a very small portion of the three regions. As such, I believe that 15% should be considered a global upper bound. It is important to also understand that this value can differ regionally. For instance, the comparison of the data from this study and that of Seifert et al. (9) make it clear that the prevalence of katG mutations in Asia is notably lower than that in Africa. The most likely explanation is that there must be alternative mechanism(s) of isoniazid resistance that remain yet to be discovered. Further research and discovery of such mechanisms are critical for improvement of molecular diagnostics, reduction of misdiagnosis, and reduction of outbreaks that carry uncommon mechanisms of isoniazid resistance.
Phenotypic accuracy and heteroresistance.
In the discussions above, two important factors must be considered. The first is that, like all similar studies, I have used phenotypic drug susceptibility tests (DST) as the gold standard. It should be mentioned that incorrect phenotyping has been previously observed, especially when the inhibitory concentration of a strain lies near the critical cutoff for DST (69, 70). This would result in phenotypic-genotypic discordance and lower sensitivity or specificity of genetic markers.
Another cause of phenotypic-genotypic discordance and lower sensitivity is heteroresistance. Folkvardsen et al. demonstrated that the MGIT system can detect resistant subpopulations that are as small as 1% of the total bacterial population (71). In comparison, LPA cannot reliably detect heteroresistance below 5% (71). Such heteroresistance can cause genotypic-phenotypic discordance that could result in lower sensitivity of the molecular assay and overestimation of prevalence of alternative mechanism of resistance.
Evidence for continuing evolution.
Combining the data presented by this study with that of Seifert et al. (9) points to continuing evolution that required continuous adaptation of diagnostic platforms. First, it should be considered that the combination of the two studies have identified 327 loci (in three genomic regions) associated with INH resistance. Of these, 45 loci (29 katG, nine inhA, and seven ahpC) (Tables ST4 to ST6 in reference 66) have been identified just in the last 6 years (presented by this review), presenting an average reporting rate of 7.5 new loci per year for the past 6 years. Second, with 50 more loci in my study (in addition to the 45 above, four katG and one inhA loci were discovered during or before the period of last review, not listed in that review, but included in this review [see Tables ST4 and ST5 in reference 66]), overall sensitivity is still lower than that of Seifert et al. in 2013 (9). Using the 12 loci that they recommended for diagnostics, I was only able to reach 82% sensitivity (compared to their 84%). Considering all 59 loci recommended by this study would bring the sensitivity up to 83%, still shy of the 84% they were able to achieve with fewer markers. Considering all 327 loci from both studies would give us a sensitivity of 85.6%. Although Seifert et al. did not report the sensitivity reached with all their mutations, I believe that this would be in line with (or higher than) the total sensitivity in this study, albeit using 50 fewer loci. Although more data are needed, TB may be evolving faster in Asia (Table 4).
Effects of the choice of molecular platform on the conclusions.
To my surprise, the choice of molecular platform had little impact on the comparison between the two studies. Although WGS became more frequent during the period of this study, only nine out of 52 included studies used WGS (either completely or for some isolates). The majority of the mutations labeled as new with respect to the previous review were discovered by PCR or LPA—both platforms that were in use during the period of the last review. The fact that WGS still remains infeasible for many front-line TB clinics is an important factor here. Still, only a small fraction of TB cases are studied by WGS globally.
Effects of regional differences between two studies.
Table 4 displays regional differences in prevalence of mutations. Asia had the lowest frequency of katG315 mutations and the highest frequency of oxyR′-ahpC mutations, while Africa had the highest frequency of katG315 and lowest frequency of inhA-15 mutations (Table 4). For reviews such as this one and that of Seifert et al., because of these regional differences, estimation of global frequencies could differ with regional representation in the total isolate pool. A good example is the relative high prevalence of katG315 in Africa, as demonstrated by this and the previous review. While 25% of global TB cases are in Africa, only 5% of Seifert et al.’s isolates originated from this region (9). Combining these two facts leads me to believe that the estimate provided for this mutation’s prevalence by the previous review (64.3%) to be too low. In contrast, 18.5% of the isolates in this study originated from Africa, a number closer to the global prevalence of TB in the continent. As a result, my estimate (73.6%) for the global prevalence of katG315 mutations is higher than the previous review. I believe this to be closer to the true global prevalence of this mutation. Like Seifert et al., I struggled in finding sufficient studies from all regions that reported the required details for inclusion. Finally, perhaps due to geopolitical issues, some regions are poorly represented in both reports. The Middle East is one such example.
Specificity.
The 52 included articles also included 3,502 phenotypically INH-susceptible isolates. Among these, 23 isolates harbored mutations in the three genomic regions. Of these, one harbored a katG315 mutation, three harbored an inhA-8 mutation, and one harbored an inhA-15 mutation. The remaining 18 isolates harbored a mutation that was never observed in a resistant isolate and therefore were not considered for molecular diagnostics. As such, the specificity of the set of mutations presented this review is estimated to be 99.86%. The specificities of katG315, inhA-15, and inhA-8 are estimated to be 99.97%, 99.97, and 99.91%, respectively. The specificity of all other mutations reported in Table ST2 in reference 66 was 100%. The 18 isolates had mutations in the following loci: katG365, katG279, katG74, katG65, inhA253, inhA256, and ahpC10. It is premature to assume that mutations in these loci do not reduce susceptibility to INH. It is possible that the mutations in these loci cause low-level resistance, below critical concentration; therefore, isolates that solely harbor them as a mechanism of INH resistance are classified as susceptible.
Excluded studies.
As in the previous systematic review, a great portion of relevant articles published during the period studied by this review lacked the details necessary for inclusion in this study. Undoubtedly, had the details for the excluded articles been available, the inclusion of their data would have shifted the frequencies reported here. In particular, since a great majority of excluded articles used WGS, it is likely that their inclusion would have introduced yet more rare mutations into this review’s analysis.
Recommendations.
Several recommendations emerge from comparing the data from this study to the previous study by Seifert et al. (9). (i) Due to the regionally variable molecular epidemiology of drug resistance, it is strongly recommended that similar systematic reviews to be performed for each region and globally. (ii) Because of the continuing evolution of this epidemiology, it is also recommended that such reviews be done globally and regionally, at least once every 2 years, in order to better understand the phenotypic evolvability and the changing efficacy of molecular testing globally as well as regionally. While this report offers a first perspective on the evolution of the epidemiology on a global scale, there is a significant lack of knowledge about the evolution of regional epidemiology. Because of regional differences, this lack of knowledge requires immediate attention. (iii) Because of the wide range of mutations that need to be considered for molecular diagnostics, approaches that consider the full range of the three regions associated with INH resistance should be favored over targeted approaches. Here, some innovation is needed for the development of an appropriate and feasible solution. Among current technologies, WGS offers the best potential but is still too costly to be relevant to low-income high-TB-burden settings. Some innovations introduced for inexpensive detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on a massive scale using extreme multiplexing (1,096-fold) (https://nanoporetech.com/about-us/news/oxford-nanopore-technologies-announces-advanced-development-lampore-rapid-highly) and molecular contact tracing (72) can be borrowed to make WGS-based TB diagnostics more relevant to low-income settings. The introduction of portable solutions in this domain such as Oxford Nanopore’s MinION platform (73, 74) also presents the potential for implementation in rural clinics. (iv) Because nearly 15% of resistant isolates (in both systematic reviews) harbor an undetected molecular mechanism of resistance, I recommend that clinicians keep in mind the possibility of uncommon mechanism of resistance in interpreting any molecular diagnostic result that indicates drug susceptibility but faces patient’s lack of response to treatment. The solution here depends on regional epidemiology of uncommon mechanisms of resistance. If such events are not uncommon (e.g., in East Asia) culture-based phenotypic testing could provide clarity. Here, factors such as laboratory capacity to perform additional phenotypic testing and time to response needs to be considered. (v) The ideal for molecular diagnostics of TB is a nontargeted and flexible approach that is cost effective and deployable in front-line clinics. Such a platform is ideally configurable onsite to regional epidemiological measures such as positive and negative predictive values. The closest solution to this ideal is WGS, which still suffers from high cost and lack of portability (most platforms). In the absence of such an ideal and because diagnostic platforms change infrequently, in designing a basis for targeted diagnostic platforms, I recommend the inclusion of mutations that stand the test of time rather than those that have a marginally higher frequency without temporal considerations. As such, I recommend including the 59 loci (Table 2) listed in Tables ST4, ST5, and ST6 in reference 66 (42 loci in katG, 6 in the inhA promoter, one in the inhA ORF, and 11 in the oxyR′-ahpC intergenic region). These loci are common to both studies (and hence have persisted through a 20-year time span) without appearing in any susceptible isolates (specificity of 100% in both studies). I recommend annual evaluation of this set both regionally and globally and development of region-specific sets using temporal prevalence data. The most important reasoning behind this recommendation is 2-fold; first, this approach is inclusive of all canonical mutations, and second, clonal expansions in a regional outbreak that artificially inflate local frequencies at a given time will carry less significance globally or over a longer span of time. A good example is the set of four katG codons recommended by Siefert et al. (9) for diagnostics: 309, 311, 315, and 316. These codons were the most frequently mutated codons in their study, where mutations were observed in 36, 27, 4,059, and 27 INHr isolates, respectively. Of this set of four, only one, codon 315, was observed in this review to harbor a mutation. The remaining three would not increase the sensitivity of molecular diagnostics in my set of 5,804 INHr isolates.
In summary, in addition to the 14.47% of isolates that harbor a yet to be detected mechanism of resistance, those that carry a known mechanism but one that is not included in current molecular diagnostics are a source of deep concern. With broad implementation of molecular diagnostics, the mechanisms of resistance that are included in molecular diagnostics will be identified and eradicated, while the remaining resistant strains will escape detection and continue infect others. This will result in an artifactual selection process that, over time, could result in lower regional detection rates and increased prevalence of uncommon mechanisms of resistance. To avoid this, systematic reviews such as this need to be annually repeated and the information discovered routinely included in molecular diagnostics.
MATERIALS AND METHODS
Literature search.
A search in PubMed was conducted on all peer-reviewed publications evaluating mutations in katG, inhA, fabG1, and oxyR′-ahpC intergenic region in INHr clinical isolates of M. tuberculosis. In order to continue the previous systematic review presented by Seifert et al. (9), I used the same search term used by the study with a slight modification. Seifert et al. used “(isoniazid OR inh) AND (resistance OR resistant) AND (mutation OR sequence) AND tuberculosis.” The search was limited to studies published between September 2013 and December 2019 in order to avoid the aggregation of strains that were reported by Seifert et al., who reported articles published through August 2013 (9).
Study selection criteria.
Study selection criteria were also the same as those listed by Seifert et al. (9) Studies were included if they (i) were written in English, (ii) presented original data, (iii) used clinical strains of M. tuberculosis, (iv) described the phenotypic DST method used as reference standard, and (v) included individual-level amino acid mutation data. Because some studies reported mutations observed but not their frequencies, I added the following criterion: (vi) reported frequency of mutations (or counts for each mutation and total counts so that frequencies could be calculated). Finally, because comutations (co-occurrence) of canonical mutations (katG315 and inhA-15 or inhA-8) are common and can skew frequencies, and some studies only reported individual frequencies, I required that the included studies (vii) provide detailed comutation (e.g., isolates harboring both katG315 and inhA-15 or inhA-8) counts.
Mutations in the putative regulatory regions or promoter region were included if available. Studies that performed DST on solid or liquid media were included as long as cutoff concentrations were clearly defined. A range of genotypic testing platforms were allowed: whole-genome sequencing (WGS) (PacBio RS), line probe assay (LPA) using GenoType MTBDRplus (Hain Lifescience, Nehren, Germany), PCR, and proprietary platforms (only if confirmatory data from a secondary platform was available).
The primary goal of this survey was to reassess the prevalence of canonical mutations.
Data quality assessment.
To ensure accurate data reported by each article, the following stepwise methodology was used.
(i) Accuracy of curation of mutations and their frequencies.
Each article was evaluated independently twice, once in February 2020 and a second time in March 2020. Each process resulted in an independent mutation frequency matrix stored in a tab-separated format.
(ii) Discordance identification and resolution.
A shell script in Linux compared the two files. The mismatching rows were investigated to identify the discrepant studies between the two rounds of evaluation. Eight studies were identified in this process as discrepant. The articles with discordant counts were assessed for a third time to resolve the discordance. The third round resolved seven of the eight discrepant records. One could not be resolved due to the vagueness of the language used in the article. Each round of evaluation interpreted the language differently. The third round could not resolve the issue, and therefore the study was included in the group of studies that did not report counts and as such was subsequently excluded from this review.
(iii) Study accuracy in reporting genomic change.
In this study, I report the locus of each mutation with respect to the M. tuberculosis H37Rv genome (NCBI accession number NC_000962.3). For each mutation reported by an included study, the reported reference amino acid was compared to the published H37Rv sequence (NCBI accession number NC_000962.3). Mutations reported with a reference amino acid discordant with that of H37Rv were excluded from my analysis.
(iv) Study accuracy in reporting frequencies.
Parity check was used to detect frequency reporting issues in each article. Two methods were used to detect frequency errors reported by each article:
(a) Per-locus parity.
If the sum of the mutations and wild-type observations reported for any locus did not add up to the total isolate count reported in the text, the study was excluded. Consequences of comutations were considered in this exercise.
(b) Isolate count parity.
If the number of mutant and WT isolates reported in the tables did not add up to the total isolate count reported, the article was excluded. Not reporting comutations was the common cause of this.
Data acquisition.
The following data were extracted from articles that met the inclusion criteria: PubMed identifier (ID), author names, title, journal name, publication year, country of isolate collection, Digital Object Identifier (DOI), total numbers of resistant and susceptible isolates (reported and calculated from provided tables), total number of isolates harboring each mutation/combination, method of determining DST, and the method for detecting mutations. Each paper was examined for individual mutations and combination of mutations in katG, inhA, fabG1, and the oxyR′-ahpC intergenic region. Mutations in the same locus but with a different change were reported independently. The frequency of combinations of mutations (mutations harbored by the same isolate, e.g., katG315 and inhA-15) was reported as a combination. Unless otherwise indicated, frequency of an individual mutation is the count of isolates in which the mutation appears as a standalone mutation. As such, the frequency of individual mutations does not include isolates that harbor the mutation along with others conferring INH resistance.
Statistical analysis.
(i) Per-mutation analysis. The total number of isolates harboring each distinct mutation (or combination thereof) was calculated across all included studies and divided by the total number of resistant isolates listed in all included studies to estimate the diagnostic sensitivity of the mutation.
(ii) Cumulative analysis per locus. The total number of resistant isolates that harbored a mutation in each distinct locus was calculated across included studies (regardless of the change or type). The total at each locus was then divided by the total number of resistant isolates included in all studies to estimate the diagnostic sensitivity of the locus. Since the purpose of this analysis is to indicate the importance of inclusion of the locus in diagnostics, combination of mutations that included the locus were also included in this analysis.
(iii) Cumulative analysis per region/gene. To estimate the diagnostic significance of a gene or a region (oxyR′-ahpC intergenic region), the total number of resistant isolates reported to harbor a mutation in each region was calculated across the included studies and divided by the total number of resistant isolates from included studies.
Additional data.
All supplemental tables are available on Zenodo (https://doi.org/10.5281/zenodo.4308969) (66). Supplemental Fig. SF1 is available at https://doi.org/10.5281/zenodo.4067201 (67).
ACKNOWLEDGMENTS
I acknowledge the mentorship of Michael Botte (Scripps Clinic) in all aspects of medical practice, including surgery. His mentorship played an important role in maintaining my interest in medicine and hence the perseverance that was needed for completion of this work. I also acknowledge Wael Elmaraachli (UCSD Division of Pulmonary Medicine) for his vast subject area expertise and his mentorship in pulmonary medicine, particularly in the treatment of tuberculosis complications relating to drug resistance.
REFERENCES
- 1.World Health Organization. 2019. Global tuberculosis report 2019. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.CDC. 2019. Global health report: tuberculosis. CDC, Atlanta, GA. [Google Scholar]
- 3.World Health Organization. 2018. Global tuberculosis report 2018. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.World Health Organization. 2017. Global tuberculosis report 2017. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 5.World Health Organization. 2016. Global tuberculosis report 2016. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 6.World Health Organization. 2015. Global tuberculosis report 2015. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 7.Torres JN, Paul LV, Rodwell TC, Victor TC, Amallraja AM, Elghraoui A, Goodmanson AP, Ramirez-Busby SM, Chawla A, Zadorozhny V, Streicher EM, Sirgel FA, Catanzaro D, Rodrigues C, Gler MT, Crudu V, Catanzaro A, Valafar F. 2015. Novel katG mutations causing isoniazid resistance in clinical M. tuberculosis isolates. Emerg Microbes Infect 4:1–9. doi: 10.1038/emi.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilchèze C, Jacobs WR. 2019. The isoniazid paradigm of killing, resistance, and persistence in Mycobacterium tuberculosis. J Mol Biol 431:3450–3461. doi: 10.1016/j.jmb.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seifert M, Catanzaro D, Catanzaro A, Rodwell TC. 2015. Genetic mutations associated with isoniazid resistance in Mycobacterium tuberculosis: a systematic review. PLoS One 10:e0119628. doi: 10.1371/journal.pone.0119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vilchèze C, Jacobs WR, Vilcheze C, Jacobs WRJ. 2014. Resistance to isoniazid and ethionamide in Mycobacterium tuberculosis: genes, mutations, and causalities. Microbiol Spectr 2:MGM2-0014-2013. doi: 10.1128/microbiolspec.MGM2-0014-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramaswamy SV, Reich R, Dou S-J, Jasperse L, Pan X, Wanger A, Quitugua T, Graviss EA. 2003. Single nucleotide polymorphisms in genes associated with isoniazid resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 47:1241–1250. doi: 10.1128/aac.47.4.1241-1250.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ando H, Miyoshi-Akiyama T, Watanabe S, Kirikae T. 2014. A silent mutation in mabA confers isoniazid resistance on Mycobacterium tuberculosis. Mol Microbiol 91:538–547. doi: 10.1111/mmi.12476. [DOI] [PubMed] [Google Scholar]
- 13.Kazemian H, Kardan-Yamchi J, Bahador A, Khonsari S, Nasehi M, Hamzehloo G, Vaziri F, Salehi MR, Feizabadi MM. 2019. Efficacy of line probe assay in detection of drug-resistant pulmonary tuberculosis in comparison with GeneXpert and phenotypic methods in Iran and genetic analysis of isolates by MIRU-VNTR. Infect Drug Resist 12:3585–3593. doi: 10.2147/IDR.S222905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negi SS, Singh P, Bhargava A, Chandrakar S, Gaikwad U, Das P, Behra A. 2018. Effective pragmatic approach of diagnosis of multidrug-resistant tuberculosis by high-resolution melt curve assay. Int J Mycobacteriol 7:228–235. doi: 10.4103/ijmy.ijmy_100_18. [DOI] [PubMed] [Google Scholar]
- 15.Purkan P, Ihsanawati I, Natalia D, Syah YM, Retnoningrum DS, Siswanto I. 2018. Molecular analysis of katG encoding catalase-peroxidase from clinical isolate of isoniazid-resistant Mycobacterium tuberculosis. J Med Life 11:160–167. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HY, Uh Y, Kim S, Cho E, Lee JS, Lee H. 2018. Detection of rifampicin- and isoniazid-resistant Mycobacterium tuberculosis using the QuantaMatrix multiplexed assay platform system. Ann Lab Med 38:569–577. doi: 10.3343/alm.2018.38.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karunaratne GHRE, Wijesundera SS, Vidanagama D, Adikaram CP, Perera J. 2018. Significance of coexisting mutations on determination of the degree of isoniazid resistance in Mycobacterium tuberculosis strains. Microb Drug Resist 24:844–851. doi: 10.1089/mdr.2017.0330. [DOI] [PubMed] [Google Scholar]
- 18.Park J, Shin SY, Kim K, Park K, Shin S, Ihm C. 2018. Determining genotypic drug resistance by ion semiconductor sequencing with the Ion AmpliSeq™ TB panel in multidrug-resistant Mycobacterium tuberculosis isolates. Ann Lab Med 38:316–323. doi: 10.3343/alm.2018.38.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kebede A, Demisse D, Assefa M, Getachew Z, Yenew B, Tedla Y, Ameni G. 2017. Performance of MTBDRplus assay in detecting multidrug resistant tuberculosis at hospital level. BMC Res Notes 10:661. doi: 10.1186/s13104-017-2989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unissa AN, Dusthackeer VNA, Kumar MP, Nagarajan P, Sukumar S, Kumari VI, Lakshmi AR, Hanna LE. 2017. Variants of katG, inhA and nat genes are not associated with mutations in efflux pump genes (mmpL3 and mmpL7) in isoniazid-resistant clinical isolates of Mycobacterium tuberculosis from India. Tuberculosis (Edinb) 107:144–148. doi: 10.1016/j.tube.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Karimi H, En-Nanai L, Oudghiri A, Chaoui I, Laglaoui A, Bourkadi JE, El Mzibri M, Abid M. 2018. Performance of GenoType MTBDRplus assay in the diagnosis of drug-resistant tuberculosis in Tangier, Morocco. J Glob Antimicrob Resist 12:63–67. doi: 10.1016/j.jgar.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Tam KKG, Leung KSS, To SWC, Siu GKH, Lau TCK, Shek VCM, Tse CWS, Wong SSY, Ho PL, Yam WC. 2017. Direct detection of Mycobacterium tuberculosis and drug resistance in respiratory specimen using Abbott RealTime MTB detection and RIF/INH resistance assay. Diagn Microbiol Infect Dis 89:118–124. doi: 10.1016/j.diagmicrobio.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Li GL, Chen C, Wang JM, Martinez L, Lu W, Zhu LM. 2017. Diagnostic performance of the genotype MTBDR plus and MTBDR sl assays to identify tuberculosis drug resistance in eastern China. Chin Med J (Engl) 130:1521–1528. doi: 10.4103/0366-6999.208248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen TLH, Hijikata M, Maeda S, Thuong PH, Ohashi J, Huan HV, Hoang NP, Miyabayashi A, Cuong VC, Seto S, Hung NV, Keicho N. 2019. Whole genome sequencing, analyses of drug resistance-conferring mutations, and correlation with transmission of Mycobacterium tuberculosis carrying katG-S315T in Hanoi. Sci Rep 9:15354. doi: 10.1038/s41598-019-51812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narang A, Giri A, Gupta S, Garima K, Bose M, Varma-Basil M. 2017. Contribution of putative efflux pump genes to isoniazid resistance in clinical isolates of Mycobacterium tuberculosis. Int J Mycobacteriol 6:177–183. doi: 10.4103/ijmy.ijmy_26_17. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Peng P, Du Y, Ren Y, Chen L, Rao Y, Wang W. 2017. Early detection of multidrug- and pre-extensively drug-resistant tuberculosis from smear-positive sputum by direct sequencing. BMC Infect Dis 17:300. doi: 10.1186/s12879-017-2409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brhane M, Kebede A, Petros Y. 2017. Molecular detection of multidrug-resistant tuberculosis among smear-positive pulmonary tuberculosis patients in Jigjiga town, Ethiopia. Infect Drug Resist 10:75–83. doi: 10.2147/IDR.S127903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jo KW, Yeo Y, Sung H, Kim MN, Shim TS. 2017. Analysis of discrepant results between the Genotype MTBDRplus assay and an antimicrobial drug susceptibility test for isoniazid-resistant tuberculosis. Respir Med 122:12–17. doi: 10.1016/j.rmed.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Bedewi Omer Z, Mekonnen Y, Worku A, Zewde A, Medhin G, Mohammed T, Pieper R, Ameni G. 2016. Evaluation of the GenoType MTBDRplus assay for detection of rifampicin- and isoniazid-resistant Mycobacterium tuberculosis isolates in central Ethiopia. Int J Mycobacteriol 5:475–481. doi: 10.1016/j.ijmyco.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Dookie N, Sturm AW, Moodley P. 2016. Mechanisms of first-line antimicrobial resistance in multi-drug and extensively drug resistant strains of Mycobacterium tuberculosis in KwaZulu-Natal, South Africa. BMC Infect Dis 16:609. doi: 10.1186/s12879-016-1906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamze M, Ismail MB, Rahmo AK, Dabboussi F. 2015. Pyrosequencing for rapid detection of tuberculosis resistance to rifampicin and isoniazid in Syrian and Lebanese clinical isolates. Int J Mycobacteriol 4:228–232. doi: 10.1016/j.ijmyco.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Kumari R, Tripathi R, Pandey AP, Banerjee T, Sinha P, Anupurba S. 2016. Rapid screening of MDR-TB in cases of extra pulmonary tuberculosis using Geno Type MTBDRplus. PLoS One 11:e0159651. doi: 10.1371/journal.pone.0159651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bollela VR, Namburete EI, Feliciano CS, Macheque D, Harrison LH, Caminero JA. 2016. Detection of katG and inhA mutations to guide isoniazid and ethionamide use for drug-resistant tuberculosis. Int J Tuber Lung Dis 20:1099–1104. doi: 10.5588/ijtld.15.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tadesse M, Aragaw D, Dimah B, Efa F, Abdella K, Kebede W, Abdissa K, Abebe G. 2016. Drug resistance-conferring mutations in Mycobacterium tuberculosis from pulmonary tuberculosis patients in Southwest Ethiopia. Int J Mycobacteriol 5:185–191. doi: 10.1016/j.ijmyco.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Imperiale BR, Di Giulio ÁB, Mancino MB, Zumárraga MJ, Morcillo NS. 2019. Surveillance and characterization of drug-resistant Mycobacterium tuberculosis isolated in a reference hospital from Argentina during 8 years’ period. Int J Mycobacteriol 8:223–228. doi: 10.4103/ijmy.ijmy_94_19. [DOI] [PubMed] [Google Scholar]
- 36.Georghiou SB, Seifert M, Catanzaro D, Garfein RS, Valafar F, Crudu V, Rodrigues C, Victor TC, Catanzaro A, Rodwell TC. 2016. Frequency and distribution of tuberculosis resistance-associated mutations between Mumbai, Moldova, and Eastern Cape. Antimicrob Agents Chemother 60:3994–4004. doi: 10.1128/AAC.00222-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aye KS, Nakajima C, Yamaguchi T, Win MM, Shwe MM, Win AA, Lwin T, Nyunt WW, Ti T, Suzuki Y. 2016. Genotypic characterization of multi-drug-resistant Mycobacterium tuberculosis isolates in Myanmar. J Infect Chemother 22:174–179. doi: 10.1016/j.jiac.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Igarashi Y, Chikamatsu K, Aono A, Yi L, Yamada H, Takaki A, Mitarai S. 2017. Laboratory evaluation of the Anyplex™ II MTB/MDR and MTB/XDR tests based on multiplex real-time PCR and melting-temperature analysis to identify Mycobacterium tuberculosis and drug resistance. Diagn Microbiol Infect Dis 89:276–281. doi: 10.1016/j.diagmicrobio.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Rueda J, Realpe T, Mejia GI, Zapata E, Rozo JC, Ferro BE, Robledo J. 2015. Genotypic analysis of genes associated with independent resistance and cross-resistance to isoniazid and ethionamide in Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother 59:7805–7810. doi: 10.1128/AAC.01028-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alame-Emane AK, Xu P, Pierre-Audigier C, Cadet-Daniel V, Shen X, Sraouia M, Siawaya JFD, Takiff H, Gao Q, Gicquel B. 2015. Pyrazinamide resistance in Mycobacterium tuberculosis arises after rifampicin and fluoroquinolone resistance. Int j Tuber Lung Dis 19:679–684. doi: 10.5588/ijtld.14.0768. [DOI] [PubMed] [Google Scholar]
- 41.Kambli P, Ajbani K, Sadani M, Nikam C, Shetty A, Udwadia Z, Georghiou SB, Rodwell TC, Catanzaro A, Rodrigues C. 2015. Defining multidrug-resistant tuberculosis: correlating GenoType MTBDRplus assay results with minimum inhibitory concentrations. Diagn Microbiol Infect Dis 82:49–53. doi: 10.1016/j.diagmicrobio.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramasubban G, Therese KL, Lakshmipathy D, Sridhar R, Meenakshi N, Madhavan HN. 2015. Detection of novel and reported mutations in the rpoB, katG and inhA genes in multidrug-resistant tuberculosis isolates: a hospital-based study. J Glob Antimicrob Resist 3:1–4. doi: 10.1016/j.jgar.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Singhal R, Myneedu VP, Arora J, Singh N, Sah GC, Sarin R. 2014. Detection of multi-drug resistance and characterization of mutations in Mycobacterium tuberculosis isolates from North- Eastern States of India using GenoType MTBDRplus assay. Indian J Med Res 140:501–506. [PMC free article] [PubMed] [Google Scholar]
- 44.Haeili M, Fooladi AI, Bostanabad SZ, Sarokhalil DD, Siavoshi F, Feizabadi MM. 2014. Rapid screening of rpoB and katG mutations in Mycobacterium tuberculosis isolates by high-resolution melting curve analysis. Indian J Med Microbiol 32:398–403. doi: 10.4103/0255-0857.142245. [DOI] [PubMed] [Google Scholar]
- 45.Abate D, Tedla Y, Meressa D, Ameni G. 2014. Isoniazid and rifampicin resistance mutations and their effect on second-line anti-tuberculosis treatment. Int J Tuber Lung Dis 18:946–951. doi: 10.5588/ijtld.13.0926. [DOI] [PubMed] [Google Scholar]
- 46.Hofmann-Thiel S, Molodtsov N, Duffner C, Kadyrov A, Kalmambetova G, Kabirov O, Rajabov A, Parpieva N, Sayfutdinov Z, Vogel M, Vogel H, Antonenka U, Hoffmann H. 2019. Capacity of Abbott RealTime MTB RIF/INH to detect rifampicinand isoniazid-resistant tuberculosis. Int j Tuber Lung Dis 23:458–464. doi: 10.5588/ijtld.18.0615. [DOI] [PubMed] [Google Scholar]
- 47.Abbas Ali IF, Babak F, Fazlollah MS, Nematollah JJ. 2014. Rapid detection of MDR-Mycobacterium tuberculosis using modified PCR-SSCP from clinical specimens. Asian Pac J Trop Biomed 4:S165–S170. doi: 10.12980/APJTB.4.2014C1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta A, Kulkarni S, Rastogi N, Anupurba S. 2014. A study of Mycobacterium tuberculosis genotypic diversity and drug resistance mutations in Varanasi, North India. Indian J Med Res 139:892–902. [PMC free article] [PubMed] [Google Scholar]
- 49.Siu GKH, Yam WC, Zhang Y, Kao RYT. 2014. An upstream truncation of the furA-katG operon confers high-level isoniazid resistance in a Mycobacterium tuberculosis clinical isolate with no known resistance-associated mutations. Antimicrob Agents Chemother 58:6093–6100. doi: 10.1128/AAC.03277-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martínez-Lirola MJ, Munoz-Dávila MJ, García-De Viedma D, Cabezas Fernández T, Luzón García P, Indal-Tb Group . 2014. Usefulness of GenoType MTBDRplus assay in acid-fast bacilli positive smear specimens in Almeria, Spain. Enferm Infecc Microbiol Clin 32:511–514. doi: 10.1016/j.eimc.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 51.Zhao LL, Chen Y, Chen ZN, Liu HC, Hu PL, Sun Q, Zhao XQ, Jiang Y, Li GL, Tan YH, Wan KL. 2014. Prevalence and molecular characteristics of drug-resistant mycobacterium tuberculosis in Hunan, China. Antimicrob Agents Chemother 58:3475–3480. doi: 10.1128/AAC.02426-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang ZK, Luo Q, Xiong GL, Li WT, Xu XM, Li J. 2014. Evaluation of indirect drug susceptibility testing using the MODS assay for the detection of XDR-TB in China. Int J Tuber Lung Dis 18:461–465. doi: 10.5588/ijtld.13.0736. [DOI] [PubMed] [Google Scholar]
- 53.Yakrus MA, Driscoll J, Lentz AJ, Sikes D, Hartline D, Metchock B, Starks AM. 2014. Concordance between molecular and phenotypic testing of Mycobacterium tuberculosis complex isolates for resistance to rifampin and isoniazid in the United States. J Clin Microbiol 52:1932–1937. doi: 10.1128/JCM.00417-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu J, Jiang S, Liu QY, Ma S, Li Y, Li CP. 2014. Analysis of mutational characteristics of the drug-resistant gene katG in multi-drug resistant Mycobacterium tuberculosis L-form among patients with pneumoconiosis complicated with tuberculosis. Mol Med Rep 9:2031–2035. doi: 10.3892/mmr.2014.2045. [DOI] [PubMed] [Google Scholar]
- 55.Hu S, Li G, Li H, Liu X, Niu J, Quan S, Wang F, Wen H, Xu Y, Li Q. 2014. Rapid detection of isoniazid resistance in Mycobacterium tuberculosis isolates by use of real-time-PCR-based melting curve analysis. J Clin Microbiol 52:1644–1652. doi: 10.1128/JCM.03395-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma BK, Bhandari S, Maharjan B, Shrestha B, Banjara MR. 2014. Rapid detection of rifampicin and isoniazid resistant Mycobacterium tuberculosis using genotype MTBDR plus assay in Nepal. Int Sch Res Not 2014:1–6. doi: 10.1155/2014/648294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Q, Wang Y, Li Y, Gao H, Zhang Z, Feng F, Dai E. 2019. Characterisation of drug resistance-associated mutations among clinical multidrug-resistant Mycobacterium tuberculosis isolates from Hebei Province, China. J Glob Antimicrob Resist 18:168–176. doi: 10.1016/j.jgar.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Yu XL, Wen ZL, Chen GZ, Li R, Ding BB, Yao YF, Li Y, Wu H, Guo XK, Wang HH, Zhang SL. 2014. Molecular characterization of multidrug-resistant Mycobacterium tuberculosis isolated from south-central in China. J Antibiot (Tokyo) 67:291–297. doi: 10.1038/ja.2013.133. [DOI] [PubMed] [Google Scholar]
- 59.Kumar P, Balooni V, Sharma BK, Kapil V, Sachdeva KS, Singh S. 2014. High degree of multi-drug resistance and hetero-resistance in pulmonary TB patients from Punjab state of India. Tuberculosis (Edinb) 94:73–80. doi: 10.1016/j.tube.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 60.Tseng ST, Tai CH, Li CR, Lin CF, Shi ZY. 2015. The mutations of katG and inhA genes of isoniazid-resistant Mycobacterium tuberculosis isolates in Taiwan. J Microbiol Immunol Infect 48:249–255. doi: 10.1016/j.jmii.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 61.Isakova J, Sovkhozova N, Vinnikov D, Goncharova Z, Talaibekova E, Aldasheva N, Aldashev A. 2018. Mutations of rpoB, katG, inhA and ahp genes in rifampicin and isoniazid-resistant Mycobacterium tuberculosis in Kyrgyz Republic. BMC Microbiol 18:22. doi: 10.1186/s12866-018-1168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogari CO, Nyamache AK, Nonoh J, Amukoye E. 2019. Prevalence and detection of drug resistant mutations in Mycobacterium tuberculosis among drug naïve patients in Nairobi, Kenya. BMC Infect Dis 19:279. doi: 10.1186/s12879-019-3911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stephen S, Muzhizhizhi D, Dhibi N, Chidemo T, Samaneka W, Matubu TA, Hakim JG, Chirenje ZM. 2019. Validation of the GenoType MTBDRplus ver 2.0 assay for detection of rifampicin and isoniazid resistance in Mycobacterium tuberculosis complex isolates at UZCHS-CTRC TB research laboratory. Int J Mycobacteriol 8:83–88. doi: 10.4103/ijmy.ijmy_170_18. [DOI] [PubMed] [Google Scholar]
- 64.Zurita J, Espinel N, Barba P, Ortega-Paredes D, Zurita-Salinas C, Rojas Y, Alcocer I. 2019. Genetic diversity and drug resistance of Mycobacterium tuberculosis in Ecuador. Int J Tuber Lung Dis 23:166–173. doi: 10.5588/ijtld.18.0095. [DOI] [PubMed] [Google Scholar]
- 65.Sirous M, Khosravi AD, Tabandeh MR, Salmanzadeh S, Ahmadkhosravi N, Amini S. 2018. Molecular detection of rifampin, isoniazid, and ofloxacin resistance in Iranian isolates of Mycobacterium tuberculosis by high-resolution melting analysis. Infect Drug Resist 11:1819–1829. doi: 10.2147/IDR.S178831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valafar S. 2020. Supplementary tables ST1–6 for isoniazid systematic review. Zenodo 10.5281/zenodo.4308969. [DOI]
- 67.Valafar S. 2020. Supplementary figure. Zenodo 10.5281/ZENODO.4067201. [DOI]
- 68.Campbell PJ, Morlock GP, Sikes RD, Dalton TL, Metchock B, Starks AM, Hooks DP, Cowan LS, Plikaytis BB, Posey JE. 2011. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob Agents Chemother 55:2032–2041. doi: 10.1128/AAC.01550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Horne DJ, Pinto LM, Arentz M, Lin S-YG, Desmond E, Flores LL, Steingart KR, Minion J. 2013. Diagnostic accuracy and reproducibility of WHO-endorsed phenotypic drug susceptibility testing methods for first-line and second-line antituberculosis drugs. J Clin Microbiol 51:393–401. doi: 10.1128/JCM.02724-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nikolayevskyy V, Hillemann D, Richter E, Ahmed N, van der Werf MJ, Kodmon C, Drobniewski F, Ruesch-Gerdes S, ERLTB-Net Network . 2016. External quality assessment for tuberculosis diagnosis and drug resistance in the European Union: a five year multicentre implementation study. PLoS One 11:e0152926. doi: 10.1371/journal.pone.0152926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Folkvardsen DB, Svensson E, Thomsen V, Rasmussen EM, Bang D, Werngren J, Hoffner S, Hillemann D, Rigouts L. 2013. Can molecular methods detect 1% isoniazid resistance in Mycobacterium tuberculosis? J Clin Microbiol 51:1596–1599. doi: 10.1128/JCM.00472-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fauver JR, Petrone ME, Hodcroft EB, Shioda K, Ehrlich HY, Watts AG, Vogels CBF, Brito AF, Alpert T, Muyombwe A, Razeq J, Downing R, Cheemarla NR, Wyllie AL, Kalinich CC, Ott IM, Quick J, Loman NJ, Neugebauer KM, Greninger AL, Jerome KR, Roychoudhury P, Xie H, Shrestha L, Huang ML, Pitzer VE, Iwasaki A, Omer SB, Khan K, Bogoch II, Martinello RA, Foxman EF, Landry ML, Neher RA, Ko AI, Grubaugh ND. 2020. Coast-to-coast spread of SARS-CoV-2 during the early epidemic in the United States. Cell 181:990–996.e5. doi: 10.1016/j.cell.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bainomugisa A, Duarte T, Lavu E, Pandey S, Coulter C, Marais BJ, Coin LM. 2018. A complete high-quality MinION nanopore assembly of an extensively drug-resistant Mycobacterium tuberculosis Beijing lineage strain identifies novel variation in repetitive PE/PPE gene regions. Microb Genom 4:e000188. doi: 10.1099/mgen.0.000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castro-Wallace SL, Chiu CY, John KK, Stahl SE, Rubins KH, McIntyre ABR, Dworkin JP, Lupisella ML, Smith DJ, Botkin DJ, Stephenson TA, Juul S, Turner DJ, Izquierdo F, Federman S, Stryke D, Somasekar S, Alexander N, Yu G, Mason CE, Burton AS. 2017. Nanopore DNA sequencing and genome assembly on the International Space Station. Sci Rep 7:18022. doi: 10.1038/s41598-017-18364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pagán-Ramos E, Master SS, Pritchett CL, Reimschuessel R, Trucksis M, Timmins GS, Deretic V. 2006. Molecular and physiological effects of mycobacterial oxyR inactivation. J Bacteriol 188:2674–2680. doi: 10.1128/JB.188.7.2674-2680.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]