Trichosporon asahii is an opportunistic fungal pathogen that can cause severe infections with high mortality rates. Azole derivatives are the best-targeted therapy for T. asahii invasive infections, but azole-resistant isolates have been reported.
KEYWORDS: Trichosporon asahii, genotypes, epidemiology, antifungal resistance, opportunistic infections
ABSTRACT
Trichosporon asahii is an opportunistic fungal pathogen that can cause severe infections with high mortality rates. Azole derivatives are the best-targeted therapy for T. asahii invasive infections, but azole-resistant isolates have been reported. To investigate peculiarities in the antifungal susceptibility profile (ASP) of T. asahii clinical isolates, we analyzed the genotype distribution, isolation sources, and ASP of 284 strains collected from 1997 to 2019 in different Brazilian medical centers. Species identification and genotype characterization were performed by analysis of the intergenic spacer (IGS1) region of the ribosomal DNA (rDNA). Antifungal susceptibility testing (AST) for amphotericin B and azoles was with the CLSI M27, 4th edition, microdilution broth method. Trends in the ASP of Brazilian T. asahii isolates were investigated using epidemiological cutoff values. Five different genotypes were found among the 284 isolates tested (G1, 76%; G3, 10%; G4, 3%; G5, 7%; and G7, 4%). The isolates were collected mainly from urine (55%) and blood/catheter tip samples (25%) where G1 was the most frequent genotype found (P < 0.05). The G7 isolates exhibited the highest MIC90 values for azoles compared to those for the other genotypes (P < 0.05). Genotype 7 isolates also contributed to the increasing rates of voriconazole non-wild-type isolates found in recent years (P = 0.02). No significant differences were found among the AST results generated by isolates cultured from different anatomical sites. Monitoring T. asahii genotype distributions and antifungal susceptibility profiles is warranted to prevent the spread of azole-resistant isolates.
INTRODUCTION
First described by Akagi in 1929 (1), Trichosporon asahii is a basidiomycete yeast-like fungi that is considered an emergent opportunistic fungal pathogen capable of causing life-threatening invasive infections, mostly in neutropenic cancer patients, patients submitted for organ transplantation, or critically ill patients exposed to antibiotics, corticosteroids, or invasive medical procedures (2). The prognosis of patients with invasive trichosporonosis is a concern given that crude mortality rates as high as 42% to 87% have been reported by different series (2, 3).
Due to the lack of clinical trials, recommendations for the clinical management of invasive trichosporonosis rely mostly on data generated from animal models, in vitro susceptibility tests, and reports of small clinical series (3–6). In this scenario, most authors suggest that triazoles are the best therapy for invasive infections caused by Trichosporon spp., despite some reports illustrating that some species and strain variability may occur in their susceptibilities to fluconazole and voriconazole (3, 7, 8).
Nowadays, a total of 15 T. asahii genotypes has been characterized by different research groups based on the genetic diversity described in their intergenic spacer 1 (IGS1) ribosomal DNA (rDNA) sequences (9). Notably, there is a lack of robust data illustrating their distribution in different anatomical sites of patients colonized or infected by T. asahii. In addition, it remains controversial whether the genetic diversity described for T. asahii isolates has any impact in terms of virulence or antifungal susceptibility to different antifungal drugs (5, 6, 9).
The present study describes the prevalence rates of T. asahii genotypes found among 284 clinical isolates cultured from different patients as well as their in vitro susceptibility to four antifungal drugs.
RESULTS
Prevalence rates of T. asahii genotypes cultured from different anatomical sites.
Only five clinical centers were able to provide data on the annual incidence of T. asahii-positive cultures collected between 2010 and 2019. The pooled data showed that the incidence of cultures positive for T. asahii remained stable over the years, ranging from 0.5 to 2 isolates per 10,000 admissions each year. Clinical isolates were cultured from urine (n = 157 [55%]), blood samples/central venous catheter tips (n = 71 [25%]), respiratory tract samples (n = 16 [6%]), integumentary samples, including hair, nail, and skin samples (n = 11 [4%]), other sterile anatomical sites (n = 11 [4%]), and other sources (n = 18 [6%]).
The 284 clinical isolates were successfully identified as T. asahii by sequencing of the IGS1 rDNA region, with 100% of query coverage and percent identity and E value of 0.0. As illustrated in Fig. 1 and Fig. S1 in the supplemental material, the median-joining network analysis showed five different genotypes (G1, n = 216 [76%]; G3, n = 28 [10%]; G4, n = 8 [3%]; G5, n = 21 [7%]; G7, n = 11 [4%]), including representatives of G7 that were never previously reported among Brazilian isolates.
FIG 1.
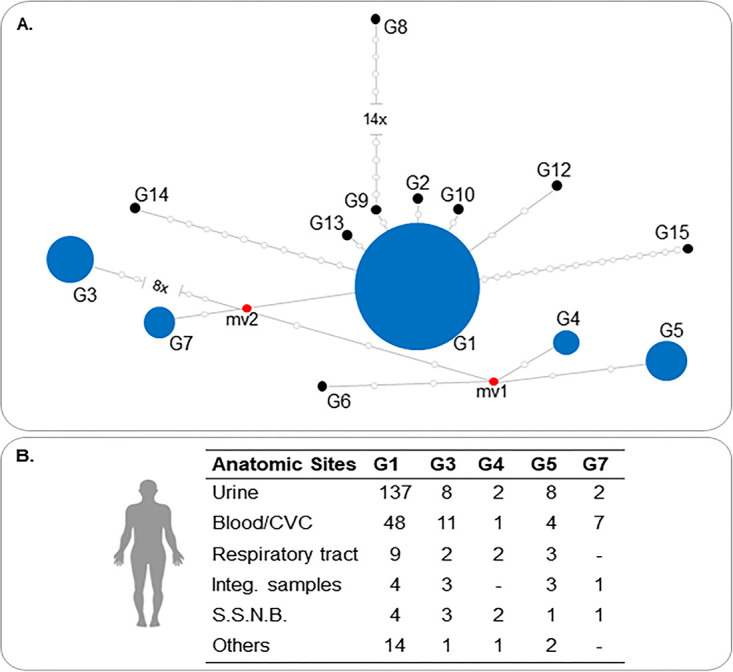
Median-joining genotypes network of T. asahii based on IGS1 rDNA sequences (A) and their distribution in anatomic sites (B). Circumference sizes are proportional to the frequencies of genotypes detected. Blue circles represent genotypes found in our study (G1, G3, G4, G5, and G7). Black circles represent genotypes that could not be grouped with any isolate from our collection. Red dots (median vectors [mv]) are hypothetical missing intermediates. CVC, catheter tips; Integ. samples, integumentary samples; S.S.N.B., sterile sites, nonblood.
Three to five distinct genotypes of T. asahii were found in each anatomic site. Genotype 1 was present in all evaluated sites and represented 76% of all typed isolates. In urine cultures, T. asahii G1 was responsible for 138 of 157 (88%) isolates studied (P < 0.001). Remarkably, all five genotypes were detected among the 71 isolates cultured from blood samples, including 48 isolates of G1 (68%) and 11 isolates of G3 (16%). The median-joining genotype network of T. asahii clinical isolates and the prevalence in each anatomical site are shown in Fig. 1.
We found a higher percentage of T. asahii G7 in blood/catheter tip samples than other anatomical sites (9.8% versus 1.8%, P = 0.005). Notably, the 11 T. asahii G7 isolates were cultured from patients admitted in 3 medical centers from different regions, instead of representing a cluster of infections documented in a single hospital.
Antifungal susceptibility tests.
Table 1 summarizes the MIC ranges (μg/ml), MIC50, MIC90, and geometric mean (GM) values obtained with the 284 T. asahii clinical isolates tested. At the 48-h reading, the MIC range values were 0.25 to ≥64 μg/ml for fluconazole (FLC), 0.03 to 2 μg/ml for both voriconazole (VRC) and posaconazole (POS), and 0.025 to ≥16 μg/ml for amphotericin B (AMB). The highest MIC90 values were found for FLC, followed by those for AMB, POS, and VRC.
TABLE 1.
In vitro antifungal susceptibility of 284 Trichosporon asahii clinical isolates tested against triazoles and amphotericin B
| Anatomic site or genotypea | No. of isolates | MIC (μg/ml) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluconazole |
Voriconazole |
Posaconazole |
Amphotericin B |
||||||||||||||
| Range | 50% | 90% | GM | Range | 50% | 90% | GM | Range | 50% | 90% | GM | Range | 50% | 90% | GM | ||
| Anatomic sites | |||||||||||||||||
| Urine | 157 | 0.25 to ≥64 | 2 | 4 | 2.47 | 0.03–2 | 0.06 | 0.125 | 0.07 | 0.03–2 | 0.25 | 0.5 | 0.21 | 0.25 to ≥16 | 2 | 4 | 1.65 |
| Blood/CVC | 71 | 0.25 to 32 | 2 | 8 | 2.08 | 0.03–1 | 0.06 | 0.125 | 0.07 | 0.03–1 | 0.25 | 0.5 | 0.18 | 0.25 to ≥16 | 1 | 4 | 1.42 |
| Respiratory | 16 | 0.5 to 8 | 1 | 4 | 1.54 | 0.03–0.125 | 0.06 | 0.06 | 0.06 | 0.06–0.25 | 0.125 | 0.25 | 0.16 | 1 to 8 | 1 | 2 | 1.35 |
| Integumentary | 11 | 1 to 6 | 2 | 4 | 2.13 | 0.03–0.25 | 0.06 | 0.06 | 0.06 | 0.03–1 | 0.125 | 0.25 | 0.17 | 1 to 4 | 1 | 2 | 1.29 |
| S.S.N.B. | 11 | 1 to 16 | 2 | 4 | 2.57 | 0.03–1 | 0.125 | 0.125 | 0.12 | 0.06–1 | 0.25 | 0.25 | 0.21 | 0.5 to 4 | 1 | 4 | 1.55 |
| Others | 18 | 1 to 8 | 2 | 4 | 2.16 | 0.03–0.5 | 0.06 | 0.125 | 0.06 | 0.03–0.5 | 0.125 | 0.25 | 0.14 | 0.25 to ≥16 | 1 | 4 | 1.71 |
| Genotypes | |||||||||||||||||
| G1 | 216 | 0.25 to ≥64 | 2 | 8 | 2.30 | 0.03–2 | 0.06 | 0.25 | 0.06 | 0.03–2 | 0.25 | 0.5 | 0.18 | 0.25 to ≥16 | 2 | 4 | 1.57 |
| G3 | 28 | 0.5 to 16 | 2 | 2 | 1.95 | 0.03–1 | 0.06 | 0.125 | 0.08 | 0.06–1 | 0.25 | 0.25 | 0.22 | 0.5 to ≥16 | 1 | 1 | 1.19 |
| G4 | 8 | 1 to 4 | 1 | 2 | 1.54 | 0.03–0.125 | 0.06 | 0.06 | 0.05 | 0.125–0.25 | 0.125 | 0.125 | 0.13 | 0.5 to 2 | 1 | 2 | 1.09 |
| G5 | 21 | 0.5 to 4 | 2 | 2 | 1.61 | 0.03–0.125 | 0.06 | 0.125 | 0.04 | 0.03–0.5 | 0.25 | 0.5 | 0.16 | 0.25 to 4 | 2 | 4 | 1.14 |
| G7 | 11 | 1 to 32 | 2 | 16 | 4.83 | 0.06–1 | 0.125 | 0.5 | 0.19 | 0.06–1 | 0.5 | 1 | 0.36 | 1 to 4 | 2 | 4 | 2.13 |
CVC, catheter tips; SSNB, sterile sites, nonblood.
We found substantial variation in MIC90 values when comparing certain combinations of drugs and genotypes tested. In this scenario, the isolates representative of T. asahii G7, which emerged after 2013 in 3 medical centers only, exhibited higher MIC90 values for FLC (MIC90, 16 μg/ml; P = 0.03), VRC (MIC90, 0.5 μg/ml; P < 0.001), and POS (MIC90, 0.1 μg/ml; P < 0.02) than all other genotypes. No significant differences were found in AMB MIC values for the different T. asahii genotypes (P = 0.1). MIC values tended to be similar when checking isolates cultured from different anatomic sites (P > 0.05). Blood/catheter tips and sterile site isolates showed FLC, VRC, POS, and AMB MIC90 values of 8 μg/ml, 0.125 μg/ml, 0.5 μg/ml, and 4 μg/ml, respectively. Otherwise, isolates from all other anatomic sites showed FLC, VRC, POS, and AMB MIC90 values of 4 μg/ml (P = 0.7), 0.125 μg/ml (P = 0.2), 0.5 μg/ml (P = 0.7), and 4 μg/ml (P = 0.5), respectively.
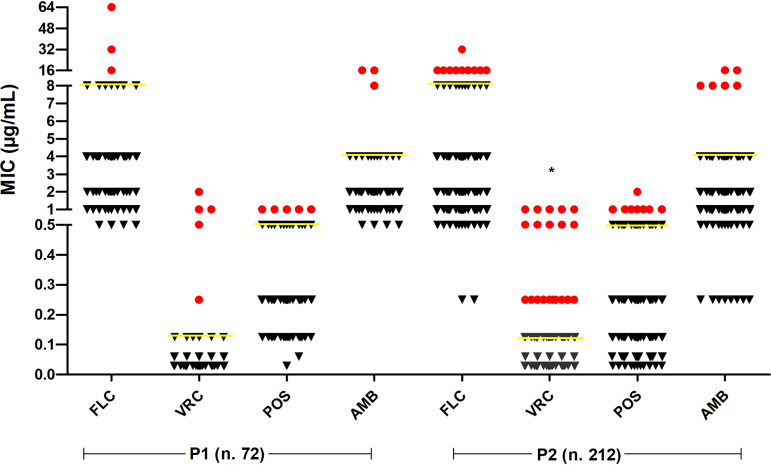
As shown in Fig. 2, by using the ECV values for T. asahii recently described by Francisco et al. (4), we found a trend for increasing rates of non-wild-type isolates of VRC in the second period of study (P1, 5/72 [7%] versus P2, 21/212 [10%]; P = 0.02). When isolates belonging to the G7 genotype were excluded from the analyses, the rates of non-wild-type isolates of VRC did not differ between the two periods (P1, 0/72 [0%] versus P2, 11/212 [5.2%]).
FIG 2.
Historical trends in the antifungal susceptibility of Brazilian clinical isolates of T. asahii collected over the last 20 years. The isolates that exhibited MICs higher than the epidemiologic cutoff values for each antifungal tested (yellow lines) were identified by red circles. FLC, fluconazole; VRC, voriconazole; POS, posaconazole; AMB, amphotericin B; P1, 1997 to 2008; P2, 2009 to 2019; *, P < 0.05.
DISCUSSION
Trichosporon asahii has recently attracted worldwide medical attention due to its capability to cause a broad spectrum of infections in humans, including life-threatening invasive infections in immunocompromised hosts and critically ill patients. Additionally, in contrast with Candida spp., Trichosporon is a poor target for echinocandins and amphotericin B (2–7).
Trichosporon asahii usually comprises the majority of all Trichosporon spp. found in urine and blood cultures (3–6). Data on the incidence of this pathogen in clinical samples are scarce, and our results show an approximate incidence of 0.5 to 2 cultures per 10,000 hospital admissions. While a higher incidence of positive cultures was reported in Taiwan, >12 per 100,000 admissions, much lower rates are reported in US or European centers (10, 11).
Despite the large number of T. asahii genotypes described so far, there is a lack of robust data on the real prevalence in different anatomical sites and potential peculiarities in terms of antifungal susceptibility.
Concerning the intraspecific diversity of T. asahii, our results showed the presence of five circulating genotypes in Brazilian patients: G1, G3, G4, G5, and G7. Consistent with previous studies, including Brazilian series, T. asahii G1 (76%) was the most common genotype, followed by G3 (9.8%), G5 (7.5%), G7 (3.9%), and G4 (2.8%) (5, 6, 12–22).
Interestingly, a high proportion of G7 isolates were found in blood/catheter tip samples. The G7 genotype was described in 2004 from blood samples of Japanese patients (21) and later found in Thailand, Taiwan, Greece, Argentina, Mexico, India, Tunisia, and China (5, 15–18, 23–25). These results suggest a more virulent trait of G7, but in vitro and in vivo studies comparing the virulence of all different described genotypes are warranted to confirm this issue.
We were not able to find any representative of genotypes 2, 6, or 8 to 15 in our T. asahii collection. These genotypes have been reported in different geographic regions, with variable prevalences and distributions. The first seven genotypes (G1 to G7) of T. asahii have been extensively recovered from a large diversity of clinical samples and patients living in different countries (5, 6, 12–22).
Otherwise, G8 was only mentioned by a single report published in 2010, and G9 has only been reported from Turkish studies (6, 19). Two different research groups, one from China and another from Greece, reported the isolation of genotypes 10 and 11 in their collections (23, 26). There are additional reports of isolation of G12 in patients from China, and T. asahii isolates representative of G13, G14, and G15 were reported in a single publication from Brazil (9, 26).
Although 15 different genotypes for T. asahii have been described around the world, some of them have only been found in single reports (9, 19) and require confirmation to determine if they represent the genetic diversity of this pathogen or if their description was related to some artifacts faced during their sequence analysis.
Despite the lack of statistical differences in the AST results obtained with isolates cultured from several anatomical sites, attention is drawn to the high MIC90 values for FLC (8 µg/ml) in T. asahii isolates obtained from blood samples/central venous catheters. These data suggest that MIC determination may have a role in defining the best strategy to treat patients with T. asahii fungemia.
Voriconazole exhibits the best in vitro activity against T. asahii clinical isolates and is considered first-line therapy, especially in hematological patients (3). High MIC values in T. asahii isolates are not commonly reported for this compound. In our study, the increase of rates of non-wild-type (NWT) T. asahii clinical isolates for VRC over the last 10 years was certainly influenced by the emergence of G7 isolates. Similar results were found in G7 clinical isolates tested against triazoles in a center from Greece (23). As reported in other yeast genera (Candida and Cryptococcus), we believe that the low susceptibility of G7 for azoles can be influenced by the selective pressure of their widespread use prophylactically or empirically (8). Otherwise, until now, these organisms have represented only a small proportion of the T. asahii clinical isolates found in Brazilian medical centers, and less than 15% in series published in other countries (5, 15–18, 23–25). A previous systematic review on Trichosporon fungemia shows a notable increase of azole-based treatments in the late 2000s, and the emergence of resistant isolates in recent years is a concern (27).
In conclusion, the present paper represents the largest series of T. asahii isolates characterized by sequencing IGS1 of rDNA region and antifungal susceptibility testing. We were able to demonstrate four main aspects: (i) despite the fact that 15 different T. asahii genotypes have been described by different authors, only 5 of them appeared in this large Brazilian isolate series; (ii) the five T. asahii genotypes found were cultivated from different clinical samples, illustrating their ability to colonize and infect several anatomic sites; (iii) isolates recovered from different anatomical sites exhibited similar MIC values to all tested drugs; (iv) isolates of G7 emerged throughout the last 10 years and exhibit limited susceptibility to all triazoles investigated as well as to amphotericin B.
Based on our findings, we may suggest that monitoring of T. asahii genotype distributions and antifungal susceptibility profiles is warranted to prevent the spread of azole-resistant isolates.
MATERIALS AND METHODS
Selection of T. asahii clinical isolates.
We selected a total of 284 T. asahii clinical isolates obtained from different patients that were admitted in 24 Brazilian medical centers at different times in the period between January 1997 and May 2019. The isolates were obtained from different anatomical sites and sent to Laboratório Especial de Micologia, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil, for further identification and antifungal susceptibility testing.
Only one isolate per patient was selected for further molecular identification, genotyping investigation, and antifungal susceptibility testing. The medical centers were encouraged to provide the annual incidence of T. asahii cultures per 10,000 admissions/year. The study protocol was approved by UNIFESP’s ethics committee (CEP-UNIFESP number 401.893/2013).
Molecular species identification of T. asahii isolates.
Molecular identification was performed by sequencing the IGS1 region of rDNA using the 26SF and 5SR primer pair as previously described (12). Contigs were assembled based on two reads per isolates and edited using Phred-Phrap-Consed, targeting a Phred score of >30 (28, 29). The consensus was compared with the sequences deposited in the NCBI database (http://ncbi.nlm.nih.gov) using the BLASTn tool, looking for the percentage of identity and sequence coverage of ≥98% and an E value of <10−5 (4). Additionally, the nucleotide sequences were aligned with sequences of T. asahii type strains deposited in the GenBank (https://www.ncbi.nlm.nih.gov/genbank/). The phylogenetic tree was created by using the neighbor-joining method based on the Kimura two-parameter model with 1,000 bootstrap pseudoreplicates in MEGA7 (30–33).
Genotyping analysis of T. asahii.
Sequences of the IGS1 of rDNA region from 284 clinical isolates identified at the species level were aligned with sequences deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/) representative of the 15 identified genotypes of T. asahii. Alignments were performed using the muscle algorithm implemented by SeaView 4.2.12 and adjusted by eye before performing haplotype analyses (33, 34). Genotype analysis was implemented in DNAsp 5.0, excluding gap positions, and the median-joining network was constructed and visualized using the software Network 5.0.1.1 (35, 36). To check the consistency of the network results, the T. asahii genotypes were also investigated by the neighbor-joining analysis performed by SeaView 4.2.12 (34), according to previous studies (5, 6, 13, 19, 21, 23–25).
Antifungal susceptibility testing of T. asahii.
Antifungal susceptibility testing (AST) for fluconazole (FLC), voriconazole (VRC), posaconazole (POS), and amphotericin B (AMB) (Sigma, St. Louis, MO, USA) was performed by using the CLSI broth microdilution method according to the document M27, 4th edition (37). The MIC values were determined after 48 h of incubation (4, 37). The following drug concentration ranges were tested: 0.125 to 64 μg/ml for FLC and 0.03 to 16 μg/ml for VRC, POS, and AMB. Quality control organisms were included in all experiments and were represented by the ATCC 6258 Candida krusei and ATCC 22019 Candida parapsilosis strains. Antifungal MIC distributions obtained with 284 T. asahii isolates cultured from different anatomical sites and representatives of different genotypes were compared using analysis of variance (ANOVA) performed with the aid of SPSS program, and P values of <0.05 were considered significant.
To check for historical trends in the prevalence rates of non-wild-type (NWT) clinical isolates regarding their susceptibility to antifungals, the total collection of isolates was divided into two different periods: (i) P1, including 72 clinical isolates collected between 1997 and 2008, and (ii) P2, including 212 clinical isolates collected between 2009 and 2019. The definition of wild-type and non-wild-type T. asahii isolates was based on the epidemiological cutoff values (ECVs) recently proposed by Francisco et al. (4): 8 μg/ml for FLC, 0.125 μg/ml for VRC, 0.5 μg/ml for POS, and 4 μg/m for AMB. The distributions of MICs obtained with all isolates collected during both periods (P1 versus P2) were compared by Student's t test (P < 0.05).
Data availability.
The rDNA IGS sequences of the 284 Trichosporon asahii clinical isolates were deposited in the GenBank database under accession numbers MT460677 to MT460907, FJ169359 to FJ169365, FJ172160 to FJ172173, FJ187679 to FJ187697, KM488282 to KM488284, EU934808 to EU938058, and EU980326. For complete information, see Table S1 in the supplemental material.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ângela Tavares Paes from Pró-Reitoria de Pós-Graduação e Pesquisa da UNIFESP/EPM for helping with the statistical analysis.
The study was partially supported by a grant received from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 484020-2013-7 and CNPq 307510/2015-8). E.C.F. received a scholarship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 138258/2013-9 and 165230/2015-0).
A.L.C. received research grants from Astellas and Pfizer. A.L.C. has also received educational grants from Astellas, Biotoscana, United Medical, Gilead, MSD, and Pfizer. The other authors report no conflicts of interest that may be relevant to this article.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Akagi S. 1929. Uber eine neue mykotische Hautkrankheit. Jpn J Dermatol Urol 29:53–55. [Google Scholar]
- 2.Colombo AL, Padovan ACB, Chaves GM. 2011. Current knowledge of Trichosporon spp. and trichosporonosis. Clin Microbiol Rev 24:682–700. doi: 10.1128/CMR.00003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Almeida Júnior JN, Hennequin C. 2016. Invasive Trichosporon infection: a systematic review on a re-emerging fungal pathogen. Front Microbiol 7:1629. doi: 10.3389/fmicb.2016.01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francisco EC, de Almeida Junior JN, de Queiroz Telles F, Aquino VR, Mendes AVA, de Andrade Barberino MGM, de Tarso O Castro P, Guimarães T, Hahn RC, Padovan ACB, Chaves GM, Colombo AL. 2019. Species distribution and antifungal susceptibility of 358 Trichosporon clinical isolates collected in 24 medical centres. Clin Microbiol Infect 25:909.e1–909.e5. doi: 10.1016/j.cmi.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Guo L-N, Yu S-Y, Hsueh P-R, Al-Hatmi AMS, Meis JF, Hagen F, Xiao M, Wang H, Barresi C, Zhou M-L, de Hoog GS, Xu Y-C. 2019. Invasive infections due to Trichosporon: species distribution, genotyping, and antifungal susceptibilities from a multicenter study in China. J Clin Microbiol 57:e01505-18. doi: 10.1128/JCM.01505-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hazirolan G, Koçak N, Karagöz A. 2018. Sequence-based identification, genotyping and virulence factors of Trichosporon asahii strains isolated from urine samples of hospitalized patients (2011–2016). J Mycol Med 28:452–456. doi: 10.1016/j.mycmed.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O, European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, European Confederation of Medical Mycology. 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect 20 (Suppl 3):76–98. doi: 10.1111/1469-0691.12569. [DOI] [PubMed] [Google Scholar]
- 8.Padovan ACB, Rocha W. P d S, Toti A. C d M, Freitas de Jesus DF, Chaves GM, Colombo AL. 2019. Exploring the resistance mechanisms in Trichosporon asahii: triazoles as the last defense for invasive trichosporonosis. Fungal Genet Biol 133:103267. doi: 10.1016/j.fgb.2019.103267. [DOI] [PubMed] [Google Scholar]
- 9.de Almeida AA, Crispim B.dA, Grisolia AB, Svidzinski TIE, Ortolani LG, Pires de Oliveira KM. 2016. Genotype, antifungal susceptibility, and biofilm formation of Trichosporon asahii isolated from the urine of hospitalized patients. Rev Argent Microbiol 48:62–66. doi: 10.1016/j.ram.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Ruan S-Y, Chien J-Y, Hsueh P-R. 2009. Invasive trichosporonosis caused by Trichosporon asahii and other unusual Trichosporon species at a medical center in Taiwan. Clin Infect Dis 49:e11–e17. doi: 10.1086/599614. [DOI] [PubMed] [Google Scholar]
- 11.Kontoyiannis DP, Torres HA, Chagua M, Hachem R, Tarrand JJ, Bodey GP, Raad II. 2004. Trichosporonosis in a tertiary care cancer center: risk factors, changing spectrum and determinants of outcome. Scand J Infect Dis 36:564–569. doi: 10.1080/00365540410017563. [DOI] [PubMed] [Google Scholar]
- 12.Sugita T, Nakajima M, Ikeda R, Matsushima T, Shinoda T. 2002. Sequence analysis of the ribosomal DNA intergenic spacer 1 regions of Trichosporon species. J Clin Microbiol 40:1826–1830. doi: 10.1128/jcm.40.5.1826-1830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.do Espírito Santo EPT, Monteiro RC, da Costa ARF, Marques-da-Silva SH. 2020. Molecular identification, genotyping, phenotyping, and antifungal susceptibilities of medically important Trichosporon, Apiotrichum, and Cutaneotrichosporon species. Mycopathologia 185:307–317. doi: 10.1007/s11046-019-00407-x. [DOI] [PubMed] [Google Scholar]
- 14.Cho O, Matsukura M, Sugita T. 2015. Molecular evidence that the opportunistic fungal pathogen Trichosporon asahii is part of the normal fungal microbiota of the human gut based on rRNA genotyping. Int J Infect Dis 39:87–88. doi: 10.1016/j.ijid.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Sellami H, Trabelsi H, Neji S, Amouri I, Cheikhrouhou F, Makni F, Ayadi A. 2017. First genotype identification of Trichosporon asahii in Sfax, Tunisia. J Med Microbiol 66:397–401. doi: 10.1099/jmm.0.000442. [DOI] [PubMed] [Google Scholar]
- 16.Montoya AM, Sánchez González A, Palma-Nicolás JP, Gómez-Treviño A, González JG, González GM. 2015. Genotyping, extracellular compounds, and antifungal susceptibility testing of Trichosporon asahii isolated from Mexican patients. Med Mycol 53:505–511. doi: 10.1093/mmy/myv009. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y-L, Liu Y-W, Chen H-T, Tsai M-S, Chu W-L, Lo H-J. 2013. Genotype analysis based on intergenic spacer 1 sequences of Trichosporon asahii collected in Taiwan. Med Mycol 51:880–883. doi: 10.3109/13693786.2013.800240. [DOI] [PubMed] [Google Scholar]
- 18.Mekha N, Sugita T, Ikeda R, Nishikawa A, Autthateinchai R, Poonwan N, Sawanpanyalert P. 2010. Genotyping and antifungal drug susceptibility of the pathogenic yeast Trichosporon asahii isolated from Thai patients. Mycopathologia 169:67–70. doi: 10.1007/s11046-009-9225-5. [DOI] [PubMed] [Google Scholar]
- 19.Kalkanci A, Sugita T, Arikan S, Yucesoy M, Ener B, Otag F, Kiraz N, Kustimur S, Sancak B, Evci C, Emektas G. 2010. Molecular identification, genotyping, and drug susceptibility of the basidiomycetous yeast pathogen Trichosporon isolated from Turkish patients. Med Mycol 48:141–146. doi: 10.3109/13693780902977984. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Tudela JL, Diaz-Guerra TM, Mellado E, Cano V, Tapia C, Perkins A, Gomez-Lopez A, Rodero L, Cuenca-Estrella M. 2005. Susceptibility patterns and molecular identification of Trichosporon species. Antimicrob Agents Chemother 49:4026–4034. doi: 10.1128/AAC.49.10.4026-4034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugita T, Ikeda R, Nishikawa A. 2004. Analysis of Trichosporon isolates obtained from the houses of patients with summer-type hypersensitivity pneumonitis. J Clin Microbiol 42:5467–5471. doi: 10.1128/JCM.42.12.5467-5471.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iturrieta-González IA, Padovan ACB, Bizerra FC, Hahn RC, Colombo AL. 2014. Multiple species of Trichosporon produce biofilms highly resistant to triazoles and amphotericin B. PLoS One 9:e109553. doi: 10.1371/journal.pone.0109553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arabatzis M, Abel P, Kanellopoulou M, Adamou D, Alexandrou-Athanasoulis H, Stathi A, Platsouka E, Milioni A, Pangalis A, Velegraki A. 2014. Sequence-based identification, genotyping and EUCAST antifungal susceptibilities of Trichosporon clinical isolates from Greece. Clin Microbiol Infect 20:777–783. doi: 10.1111/1469-0691.12501. [DOI] [PubMed] [Google Scholar]
- 24.Taverna CG, Córdoba S, Murisengo OA, Vivot W, Davel G, Bosco-Borgeat ME. 2014. Molecular identification, genotyping, and antifungal susceptibility testing of clinically relevant Trichosporon species from Argentina. Med Mycol 52:356–366. doi: 10.1093/mmy/myt029. [DOI] [PubMed] [Google Scholar]
- 25.Rastogi V, Honnavar P, Rudramurthy SM, Pamidi U, Ghosh A, Chakrabarti A. 2016. Molecular characterisation and antifungal susceptibility of clinical Trichosporon isolates in India. Mycoses 59:528–534. doi: 10.1111/myc.12511. [DOI] [PubMed] [Google Scholar]
- 26.Xia Z, Yang R, Wang W, Cong L. 2012. Genotyping and antifungal drug susceptibility of Trichosporon asahii isolated from Chinese patients. Mycopathologia 173:127–133. doi: 10.1007/s11046-011-9478-7. [DOI] [PubMed] [Google Scholar]
- 27.Liao Y, Lu X, Yang S, Luo Y, Chen Q, Yang R. 2015. Epidemiology and outcome of Trichosporon fungemia: a review of 185 reported cases from 1975 to 2014. Open Forum Infect Dis 2:ofv141. doi: 10.1093/ofid/ofv141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ewing B, Green P. 1998. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res 8:186–194. doi: 10.1101/gr.8.3.186. [DOI] [PubMed] [Google Scholar]
- 29.Gordon D. 2003. Viewing and editing assembled sequences using Consed. Curr Protoc Bioinformatics Chapter 11:Unit11.2. doi: 10.1002/0471250953.bi1102s02. [DOI] [PubMed] [Google Scholar]
- 30.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 31.Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gouy M, Guindon S, Gascuel O. 2010. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 35.Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 36.Bandelt HJ, Forster P, Röhl A. 1999. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 37.Clinical and Laboratory Standards Institute. 2017. Reference method for broth dilution antifungal susceptibility testing of yeasts. CLSI document M27-4th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The rDNA IGS sequences of the 284 Trichosporon asahii clinical isolates were deposited in the GenBank database under accession numbers MT460677 to MT460907, FJ169359 to FJ169365, FJ172160 to FJ172173, FJ187679 to FJ187697, KM488282 to KM488284, EU934808 to EU938058, and EU980326. For complete information, see Table S1 in the supplemental material.