Echinocandins are a first-line therapy for Candida infections through their ability to inhibit the synthesis of polymer β-(1,3)-d-glucan. However, there has been an emergence of multidrug-resistant fungal species necessitating the development of novel antifungal agents to combat invasive fungal infections.
KEYWORDS: ibrexafungerp; candidiasis; β-(1,3)-d-glucan; triterpenoid class; fungerp; SCY-247
ABSTRACT
Echinocandins are a first-line therapy for Candida infections through their ability to inhibit the synthesis of polymer β-(1,3)-d-glucan. However, there has been an emergence of multidrug-resistant fungal species necessitating the development of novel antifungal agents to combat invasive fungal infections. SCY-247, a second-generation glucan synthase inhibitor of the triterpenoid class (fungerps), is currently being developed as a potential therapy option. We determined the pharmacokinetics (PKs) of SCY-247 following oral (gavage) administration in mice and evaluated the efficacy of SCY-247 in a murine model of hematogenously disseminated candidiasis caused by Candida albicans. Plasma concentrations of SCY-247 were measurable through the last collected time point in all dose groups. Mean concentrations of SCY-247 increased with dose levels, with concentrations of SCY-247 higher after multiple doses than after a single dose. Treatment with SCY-247 resulted in decreased fungal burden and improvement in survival rates against C. albicans disseminated infection. Treatment with 10 mg/kg of body weight of SCY-247 showed a significant reduction in CFU compared with the untreated control (3-log decrease on average) (P = 0.008). Similarly, 40 mg/kg SCY-247 demonstrated a statistically significant reduction in kidney CFU compared with untreated mice (average log CFU ± SD of 2.38 ± 2.58 versus 6.26 ± 0.51; P = 0.001). Mice treated with SCY-247 at 40 mg/kg exhibited a 100% survival rate at the end of the study, contrasted with 62.5% (5 of 8) survival rate in untreated mice. The results of this investigation indicate that SCY-247 is a promising novel anti-fungal agent with activity against Candida infections.
INTRODUCTION
Candidiasis encompasses a wide range of diseases from superficial infections, such as oropharyngeal or esophageal candidiasis, to more serious conditions, including bloodstream infections or invasive candidiasis (1). Candida species are ranked as the fourth most common cause of nosocomial blood infections in the United States and are one of the most common causes of invasive fungal infections (IFIs) (2, 3). Candida albicans is the most prevalent and pathogenic among the Candida species (4). C. albicans is responsible for the majority of mucosal and systemic Candida infections and is a public health challenge, as systemic Candida infections may have mortality rates of up to 40% (5–8).
Therapy for candidiasis includes the antifungal classes of polyenes, azoles, and echinocandins (9). Echinocandins are glucan synthase inhibitors, generally recognized as standard-of-care for invasive Candida infections, which cover a broad range of Candida species and have a low incidence of adverse effects, but they are available only as intravenous formulations (10, 11). Furthermore, multidrug-resistant strains of fungi have emerged over recent years, and Candida resistance to echinocandins is concerning (12, 13). Given the increasing fungal resistance to echinocandins and the limited methods available for their administration (intravenous [i.v.] only), novel antifungals with oral bioavailability and broad-spectrum activity are needed to target these fungi.
Ibrexafungerp (IBX; formerly known as SCY-078), is a first-generation glucan synthase inhibitor of the triterpenoid antifungal class, a family commonly designated “fungerps” (Fig. 1). Fungerps and derivative compounds interfere with the synthesis of the cell wall polymer β-(1,3)-d-glucan and are being developed to address the dire need for novel classes of antifungal therapies. IBX is effective against Candida infections, both in vitro and in vivo, and phase III testing has been completed for IBX use in the treatment of vulvovaginal candidiasis (VVC). Currently, IBX is in phase II and III clinical testing involving patients with recurrent VVC (CANDLE; ClinicalTrials.gov identifier NCT04029116), refractory IFIs (FURI; NCT02244606), infections caused by Candida auris (CARES; NCT03363841), and invasive aspergillosis (SCYNERGIA; NCT03672292) (14–17). IBX is structurally distinct from echinocandins, possibly allowing for both oral and intravenous formulations. A second generation fungerp, SCY-247, is currently being screened for its differentiated profile of activity and pharmacokinetic properties. Preliminary evaluations show that SCY-247 demonstrates activity against C. albicans, non-C. albicans, and multidrug-resistant Candida (including C. auris), as well as Aspergillus spp. and dimorphic fungi. SCY-247 also has an excellent safety profile. Importantly, SCY-247 may be suitable for both intravenous and oral formulations (data not shown).
FIG 1.
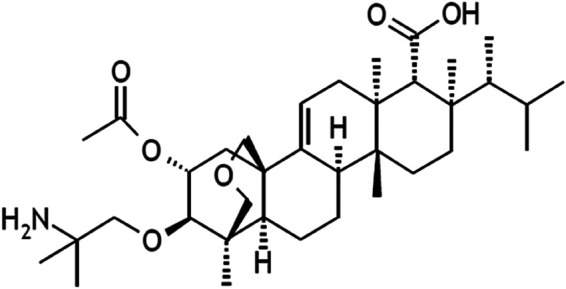
Chemical structure of SCY-247 (molecular weight [MW], 601).
This study was aimed at evaluating the efficacy of SCY-247 in a murine model of hematogenously disseminated candidiasis caused by C. albicans. We compared three dose groups of SCY-247 to IBX and evaluated efficacy as defined by changes in kidney fungal burden and animal survival relative to an untreated control.
RESULTS
Pharmacokinetic analysis.
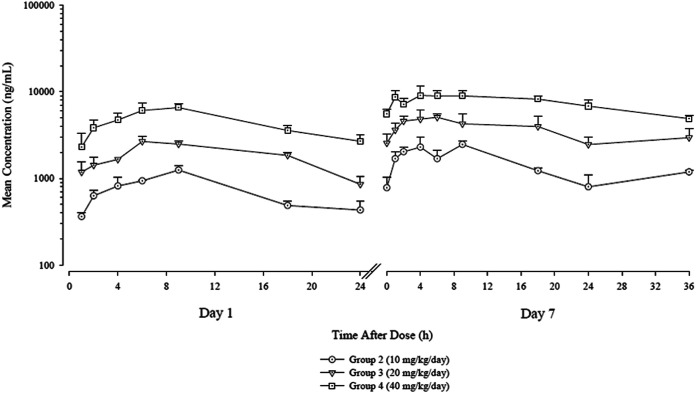
SCY-247 was evaluated at 40, 20, and 10 mg/kg of body weight per day and compared with the vehicle control-treated animals. All concentration values of SCY-247 in the vehicle control group were below the lower limit of quantitation (<5.00 ng/ml) on both evaluation days. The mean concentration-time profiles of SCY-247 in mouse plasma are shown in Fig. 2.
FIG 2.
Mean (+SD) concentrations (ng/ml) of SCY-247 in mouse plasma following daily oral gavage administration on days 1 and 7.
The pharmacokinetic parameters of SCY-247 in mouse plasma are presented in Table 1. After oral gavage administration, SCY-247 was absorbed with time to maximum concentration of drug in serum (Tmax) values ranging from 6.00 to 9.00 hours on day 1 and from 4.00 to 9.00 hours on day 7. Plasma concentrations of SCY-247 were measurable through the last collected time point in all dose groups on both evaluation days (24 hours and 36 hours for day 1 and day 7, respectively). The mean concentration-time profiles in mice demonstrated that mean concentrations of SCY-247 increased with the increase in dose level from 10 to 40 mg/kg/day. Results show that mean concentrations of SCY-247 were higher after multiple doses than after a single dose.
TABLE 1.
Pharmacokinetic parameters for SCY-247 in mouse plasma following daily oral gavage administration
| Dose (mg/kg/day) |
Cmax (ng/ml) |
DNa
Cmax [(ng/ml)/(mg/kg/day)] |
Tmax (h) |
AUC0–24 (h*ng/ml) |
DN AUC0–24 [(h*ng/ml)/(mg/kg/day)] |
Accumulation ratio |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cmax |
AUC0–24 |
|||||||||||
| Day 1 | Day 7 | Day 1 | Day 7 | Day 1 | Day 7 | Day 1 | Day 7 | Day 1 | Day 7 | Day 7 | Day 7 | |
| 10 | 1,250 | 2,500 | 125 | 250 | 9.00 | 9.00 | 17,700 | 408,00 | 1,770 | 4,080 | 2.00 | 2.31 |
| 20 | 2,670 | 5,130 | 134 | 257 | 6.00 | 6.00 | 44,700 | 98,000 | 2,230 | 4,900 | 1.92 | 2.19 |
| 40 | 6,550 | 9,170 | 164 | 229 | 9.00 | 4.00 | 107,000 | 201,000 | 2,670 | 5,020 | 1.40 | 1.88 |
DN, dose normalized.
SCY-247 maximum concentration of drug in serum (Cmax) and area under the concentration-time curve from 0 to 24 h (AUC0–24) dose proportionality ratios are also shown in Table 1. Exposure, as assessed by SCY-247 Cmax and AUC0–24 values, increased with the increase dose level from 10 to 40 mg/kg/day. The increases in SCY-247 Cmax and AUC0–24 values were generally dose proportional.
Tissue fungal burden.
The effect of treatment with SCY-247 on the kidney fungal burden of mice infected with C. albicans is summarized in Fig. 3. SCY-247 and IBX at 10 mg/kg showed similar activity in decreasing the average log CFU ± SD of mice infected with C. albicans (3.25 ± 2.60 and 3.26 ± 2.32, respectively). Both 10 mg/kg SCY-247- and IBX-treated mice showed a significant reduction in CFUs compared with the untreated control (3-log decrease in average) (P = 0.008 and P = 0.002, respectively). At 40 mg/kg, SCY-247 demonstrated a statistically significant reduction in kidney CFUs compared with untreated mice (average log CFU ± SD of 2.38 ± 2.58 versus 6.26 ± 0.51; P = 0.001). Additionally, mice treated with 20 mg/kg SCY-247 (5.54 ± 1.03 average log CFU ± SD) showed lower average log CFUs than those of the untreated control; however, this difference did not reach statistical significance (P = 0.266).
FIG 3.
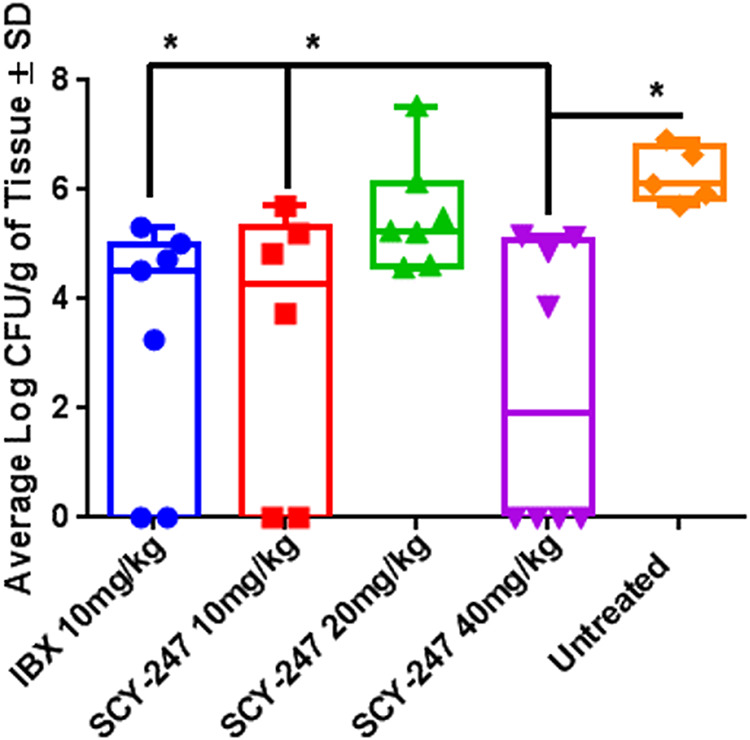
Average log CFU ± SD in kidneys of mice infected with C. albicans.
Survival.
As shown in Fig. 4, all SCY-247-treated groups showed improvements in survival, with mice treated with SCY-247 at 40 mg/kg exhibiting a 100% survival rate at the end of the study. In contrast, untreated mice had a 62.5% (5 of 8) survival rate. While the difference in survival rates indicates an advantage in mice treated with 40 mg/kg SCY-247, the differences did not reach statistical significance.
FIG 4.

Survival curve of mice in all treatment groups through 8 days.
DISCUSSION
We examined the in vivo activity of the second-generation fungerp SCY-247 using a clinical isolate of C. albicans. Our findings demonstrate that SCY-247 shows antifungal efficacy against C. albicans that was at least as effective as the first-generation fungerp (IBX) at 10 mg/kg. SCY-247 demonstrated excellent antifungal activity when administered at 40 mg/kg against a C. albicans disseminated infection.
Invasive fungal infections are an important cause of morbidity and mortality in certain high-risk groups of patients, especially immunocompromised patients. C. albicans remains the predominant cause of invasive candidiasis, making up over half of all reported cases (18, 19). Echinocandins are used as a first-line therapy against Candida infections in various scenarios due to their broad spectrum of activity, low toxicity, and high potency (20, 21). However, echinocandins are large lipopeptides (∼1,200 kDa) and have low oral bioavailability, and therefore, echinocandin-resistant fungal species have recently emerged.
IBX was developed to address the need for effective antifungal agents to treat both echinocandin-sensitive and echinocandin-resistant fungi. IBX has shown in vitro and in vivo effectiveness against Candida species, including azole-resistant and echinocandin-resistant strains (14, 22, 23). However, there is a further need to develop new antifungal agents, and new analogues of IBX, such as SCY-247, are currently being developed and tested against a broad spectrum of fungal species. Previous in vitro analysis has shown that SCY-247 demonstrated potent anti-Candida, anti-Aspergillus, and anti-Scedosporium activity and maintained its activity and structural stability under acidic environments (24).
SCY-247 has broad-spectrum activity against various yeasts, molds, and dimorphic fungi. Additionally, SCY-247 has a lower molecular weight than IBX, improving its oral bioavailability, allowing for it to be delivered intravenously using a simple formulation, and also demonstrating meaningful central nervous system penetration. Thus, SCY-247 may be a candidate for treatment of IFIs involving the central nervous system. Our data show that SCY-247 exposure, as assessed by Cmax and AUC0–24, increased with the increase in dosages from 10 to 40 mg/kg/day. The increases in Cmax and AUC0–24 values were generally dose proportional. Accumulation of SCY-247 was observed after multiple doses in female mice.
Given the demonstrated antifungal properties against C. albicans in vivo and the favorable pharmacokinetic profile, SCY-247 may be a promising option for Candida infections.
MATERIALS AND METHODS
Procurement and housing of animals.
All procedures were performed in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Office of Laboratory Animal Welfare. Mice were used upon review and approval of an addendum to an existing protocol by the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University (CWRU). Female BALB/c mice (Charles River Laboratories, Wilmington, MA), with a body weight of ∼20 g, were purchased through the CWRU Animal Resource Center (ARC). Animals were acclimatized for a minimum of 5 days prior to use. Environmental controls for the animal room were set to maintain a temperature of 16 to 22°C, a relative humidity of 30% to 70%, and a 12:12 light:dark cycle.
Pharmacokinetic analysis.
Doses of SCY-247 were administered daily via oral gavage for at least 7 days. Blood samples were collected from three animals/SCY-247 treated-group/time point, on days 1 and 7 predose and at 1, 2, 4, 6, 9, 18, 24, and 36 (day 7 only) hours postdose. Blood samples were also collected from three animals/time point in the control group at 1, 4, and 24 hours postdose. Blood samples for PK studies were collected into tubes containing K2EDTA anticoagulant and stored on wet ice until centrifuged and processed for plasma. SCY-247 concentrations were determined by liquid chromatography-tandem mass spectrometry. A noncompartmental model was used in the pharmacokinetic analysis using the Phoenix WinNonlin program.
Preparation of standard inoculum.
The clinical C. albicans strain SC5314 originating from the American Type and Culture Collection and obtained from the Center for Medical Mycology (CMM) Culture Collection was used as the infecting organism. Susceptibility testing was performed prior to study initiation in accordance with the CLSI M27-A4 standard, with both SCY-247 and IBX demonstrating MIC values of 0.125 µg/ml. C. albicans SC5314 was plated onto potato dextrose agar (PDA; Difco Laboratories) and incubated at 37°C for 24 hours. Yeast cells were harvested by centrifugation and phosphate-buffered saline (PBS; Corning) washes. An inoculum of 6 × 104 CFU/ml (based on an inoculum-finding preliminary experiment in which 50% mortality of an untreated control group was achieved) was resuspended in 100 μl PBS. To validate the inoculum count, 10-fold dilutions of the working suspension was plated onto PDA media. The plates were incubated at 37°C for 2 days, and the CFUs were determined.
Treatment efficacy evaluations.
Mice were randomized into the following groups: IBX, 10 mg/kg twice a day by oral gavage (n = 8); SCY-247, 10 mg/kg twice a day by oral gavage (n = 8); SCY-247, 20 mg/kg twice a day by oral gavage (n = 8); SCY-247, 40 mg/kg twice a day by oral gavage (n = 8); and untreated control (n = 11).
Schedule of treatment.
Interventional treatment began on day 1, 2 hours postinoculation, and continued for a period of 7 days for a total of 14 doses. On day 8, mice were euthanized and kidneys were harvested for determination of tissue fungal burden by CFUs. Animal survival was also recorded daily throughout the 8-day period.
Tissue sample collection for fungal enumeration.
To confirm successful inoculation, three mice from the untreated control group were euthanized 24 hours after inoculation, and kidneys were harvested for determination of tissue fungal burden. Surviving mice were sacrificed 12 hours after the final dose on the last day of treatment, and kidneys were removed aseptically and weighed. Tissues were homogenized and serially diluted in PBS. The homogenates were cultured for 48 hours on PDA plates to determine CFUs. Tissue fungal burden was expressed as average log CFU/gram of tissue ± SD. Survival was monitored for 8 days postinoculation.
Statistical analysis.
Differences in mean CFUs in treated kidneys were determined by comparison with control animals using an independent-sample Kruskal-Wallis test. Statistical analysis to assess the significance in survival between the different groups was performed using the Kaplan-Meier estimate, and a P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health (NIH; grant number R21 AI143305-01) and in part by Scynexis, Inc.
In compliance with the ICMJE uniform disclosure form, we declare the following financial relationships: K.B.-E. and S.B. are employees of Scynexis, Inc. who manufactures and commercializes ibrexafungerp, and M.A.G. declares a consultation role for Scynexis, Inc. All other authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
REFERENCES
- 1.Pappas PG. 2006. Invasive candidiasis. Infect Dis Clin North Am 20:485–506. doi: 10.1016/j.idc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rueping MJ, Vehreschild JJ, Cornely OA. 2009. Invasive candidiasis and candidemia: from current opinions to future perspectives. Expert Opin Invest Drugs 18:735–748. doi: 10.1517/13543780902911440. [DOI] [PubMed] [Google Scholar]
- 4.McManus BA, Coleman DC. 2014. Molecular epidemiology, phylogeny and evolution of Candida albicans. Infect Genet Evol 21:166–178. doi: 10.1016/j.meegid.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Ruping MJ, Vehreschild JJ, Cornely OA. 2008. Patients at high risk of invasive fungal infections: when and how to treat. Drugs 68:1941–1962. doi: 10.2165/00003495-200868140-00002. [DOI] [PubMed] [Google Scholar]
- 6.Thompson GR, III, Patel PK, Kirkpatrick WR, Westbrook SD, Berg D, Erlandsen J, Redding SW, Patterson TF. 2010. Oropharyngeal candidiasis in the era of antiretroviral therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:488–495. doi: 10.1016/j.tripleo.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zomorodian K, Haghighi NN, Rajaee N, Pakshir K, Tarazooie B, Vojdani M, Sedaghat F, Vosoghi M. 2011. Assessment of Candida species colonization and denture-related stomatitis in complete denture wearers. Med Mycol 49:208–211. doi: 10.3109/13693786.2010.507605. [DOI] [PubMed] [Google Scholar]
- 8.Mavor AL, Thewes S, Hube B. 2005. Systemic fungal infections caused by Candida species: epidemiology, infection process and virulence attributes. Curr Drug Targets 6:863–874. doi: 10.2174/138945005774912735. [DOI] [PubMed] [Google Scholar]
- 9.Fluckiger U, Marchetti O, Bille J, Eggimann P, Zimmerli S, Imhof A, Garbino J, Ruef C, Pittet D, Tauber M, Glauser M, Calandra T. 2006. Treatment options of invasive fungal infections in adults. Swiss Med Wkly 136:447–463. doi: 10.2006/29/smw-11392. [DOI] [PubMed] [Google Scholar]
- 10.Denning DW. 2003. Echinocandin antifungal drugs. Lancet 362:1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee PK, Sheehan D, Puzniak L, Schlamm H, Ghannoum MA. 2011. Echinocandins: are they all the same? J Chemother 23:319–325. doi: 10.1179/joc.2011.23.6.319. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller MA. 2012. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 125:S3–S13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Arendrup MC, Perlin DS. 2014. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis 27:484–492. doi: 10.1097/QCO.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scorneaux B, Angulo D, Borroto-Esoda K, Ghannoum M, Peel M, Wring S. 2017. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother 61:e01961-16. doi: 10.1128/AAC.01961-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghannoum M, Long L, Isham N, Hager C, Wilson R, Borroto-Esoda K, Barat S, Angulo D. 2019. Activity of a novel 1,3-beta-d-glucan synthase inhibitor, ibrexafungerp (formerly SCY-078), against Candida glabrata. Antimicrob Agents Chemother 63:e01510-19. doi: 10.1128/AAC.01510-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepak AJ, Marchillo K, Andes DR. 2015. Pharmacodynamic target evaluation of a novel oral glucan synthase inhibitor, SCY-078 (MK-3118), using an in vivo murine invasive candidiasis model. Antimicrob Agents Chemother 59:1265–1272. doi: 10.1128/AAC.04445-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scynexis Inc. 2020. Phase 3 study of oral ibrexafungerp (SCY-078) vs. placebo in subjects with recurrent vulvovaginal candidiasis (VVC) (CANDLE). https://clinicaltrials.gov/ct2/show/NCT04029116?term=scynexis&draw=2.
- 18.Richardson M, Lass-Flörl C. 2008. Changing epidemiology of systemic fungal infections. Clin Microbiol Infect 14:5–24. doi: 10.1111/j.1469-0691.2008.01978.x. [DOI] [PubMed] [Google Scholar]
- 19.Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Ellis D, Tullio V, Rodloff A, Fu W, Ling TA, Global Antifungal Surveillance Group . 2010. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol 48:1366–1377. doi: 10.1128/JCM.02117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg B-J, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaller MA, Boyken L, Hollis RJ, Messer SA, Tendolkar S, Diekema DJ, Benjamin DK, Jr. 2006. In vitro susceptibilities of Candida spp. to caspofungin: four years of global surveillance. J Clin Microbiol 44:760–763. doi: 10.1128/JCM.44.3.760-763.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller MA, Messer SA, Rhomberg PR, Borroto-Esoda K, Castanheira M. 2017. Differential activity of the oral glucan synthase inhibitor SCY-078 against wild-type and echinocandin-resistant strains of Candida species. Antimicrob Agents Chemother 61:e00161-17. doi: 10.1128/AAC.00161-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larkin E, Hager C, Chandra J, Mukherjee PK, Retuerto M, Salem I, Long L, Isham N, Kovanda L, Borroto-Esoda K, Wring S, Angulo D, Ghannoum M. 2017. The emerging pathogen Candida auris: growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother 61:e02396-16. doi: 10.1128/AAC.02396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu S, Long L, Sherif R, McCormick TS, Borroto-Esoda K, Barat S, Ghannoum MA. 2020. A second generation fungerp analog SCY-247, shows potent in vitro activity against Candida auris and other clinically relevant fungal isolates. Antimicrob Agents Chemother doi: 10.1128/AAC.01988-20. [DOI] [PMC free article] [PubMed] [Google Scholar]