Candida endophthalmitis is a serious sight-threatening complication of candidemia that may occur before or during antifungal therapy. Hematogenous Candida meningoencephalitis (HCME) is also a serious manifestation of disseminated candidiasis in premature infants, immunosuppressed children, and immunocompromised adults.
KEYWORDS: fosmanogepix, APX001, manogepix, APX001A, Candida, endophthalmitis, hematogenous meningoencephalitis, rabbit, antifungal
ABSTRACT
Candida endophthalmitis is a serious sight-threatening complication of candidemia that may occur before or during antifungal therapy. Hematogenous Candida meningoencephalitis (HCME) is also a serious manifestation of disseminated candidiasis in premature infants, immunosuppressed children, and immunocompromised adults. We evaluated the antifungal efficacy and pharmacokinetics of the prodrug fosmanogepix (APX001) in a rabbit model of endophthalmitis/HCME. Manogepix (APX001A), the active moiety of prodrug fosmanogepix, inhibits the fungal enzyme Gwt1 and is highly active in vitro and in vivo against Candida spp., Aspergillus spp., and other fungal pathogens. Plasma pharmacokinetics of manogepix after oral administration of fosmanogepix on day 6 at 25, 50, and 100 mg/kg resulted in maximum concentration of drug in plasma (Cmax) of 3.96 ± 0.41, 4.14 ± 1.1, and 11.5 ± 1.1 μg/ml, respectively, and area under the concentration-time curve from 0 to 12 h (AUC0–12) of 15.8 ± 3.1, 30.8 ± 5.0, 95.9 ± 14 μg·h/ml, respectively. Manogepix penetrated the aqueous humor, vitreous, and choroid with liquid-to-plasma ratios ranging from 0.19 to 0.52, 0.09 to 0.12, and 0.02 to 0.04, respectively. These concentrations correlated with a significant decrease in Candida albicans burden in vitreous (>101 to 103 log CFU/g) and choroid (>101 to 103 log CFU/g) (P ≤ 0.05 and P ≤ 0.001, respectively). The aqueous humor had no detectable C. albicans in treatment and control groups. The tissue/plasma concentration ratios of manogepix in meninges, cerebrum, cerebellum, and spinal cord were approximately 1:1, which correlated with a >102 to 104 decline of C. albicans in tissue versus control (P ≤ 0.05). Serum and cerebrospinal fluid (CSF) (1→3)-β-d-glucan levels demonstrated significant declines in response to fosmanogepix treatment. These findings provide an experimental foundation for fosmanogepix in treatment of Candida endophthalmitis and HCME and derisk the clinical trials of candidemia and invasive candidiasis.
INTRODUCTION
Candida endophthalmitis is a serious and sight-threatening disease that is a well-known complication of candidemia (1–3), occurring in approximately 10 to 15% of cases. As a manifestation of disseminated candidiasis, Candida endophthalmitis is associated with high mortality (4–6). Antifungal triazoles, especially fluconazole and voriconazole, are effective when used intravenously for the treatment of Candida endophthalmitis (6–9). A key factor for this improved efficacy is the fact that fluconazole penetrates into choroid, retina, and vitreous in free drug concentrations that exceed the MIC of most Candida isolates.
Hematogenous Candida meningoencephalitis (HCME) is a common and serious manifestation of disseminated candidiasis in premature infants, immunosuppressed children, and immunocompromised adults (10–13). HCME has been associated with a high rate of mortality and severe neurodevelopmental abnormalities (10, 13). Currently, there are relatively few therapeutic options for HCME, and the outcome of current antifungal therapy is poor (10). Amphotericin B and fluconazole have historically been used for the treatment of this infection (14–16). Micafungin and anidulafungin also have been found to have demonstrable activity in the treatment of HCME (7, 17–20). However, echinocandins are not included in the current recommendations for treatment of Candida endophthalmitis. Echinocandins may be inadequate for the treatment of this infection, and Candida endophthalmitis may emerge due to insufficient free drug concentrations in the target tissues (21–23).
Fosmanogepix (APX001, formerly E1211 and FMGX) is an N-phosphonooxymethyl prodrug that is rapidly and completely metabolized by systemic phosphatases to the active moiety manogepix (APX001A) (24, 25). Manogepix is a first-in-class antifungal compound that has potent broad-spectrum antifungal activity. It inhibits the fungal enzyme Gwt1, which is required for acylation of inositol during glycosylphosphatidylinositol (GPI) anchor biosynthesis (24, 26, 27). Fosmanogepix is currently in clinical development for the treatment of invasive fungal infections. Several animal studies demonstrated encouraging activity of fosmanogepix in the treatment of disseminated candidiasis, aspergillosis, and fusariosis, as well as pulmonary scedosporiosis, aspergillosis, and mucormycosis (28–30). Recent studies demonstrated that treatment with fosmanogepix resulted in significant reductions in brain fungal burden for Candida auris infections (31, 32) as well as Fusarium and Scedosporium infections (30). Otherwise, there are limited data on the penetration of manogepix into the eye (33) as well as treatment of Candida encephalitis and HCME.
In this study, we evaluated the efficacy of prodrug fosmanogepix in the treatment of experimental Candida endophthalmitis and HCME in the nonneutropenic rabbit model and determined the distribution of manogepix in the eye compartments, brain, and spinal cord after fosmanogepix administration. These data are critical to the design of clinical trials and to broaden our understanding of the role of fosmanogepix in the treatment of candidemia and deeply invasive candidiasis, where Candida endophthalmitis and HCME are important complications.
RESULTS
Efficacy of fosmanogepix in a nonneutropenic rabbit model of Candida endophthalmitis and HCME.
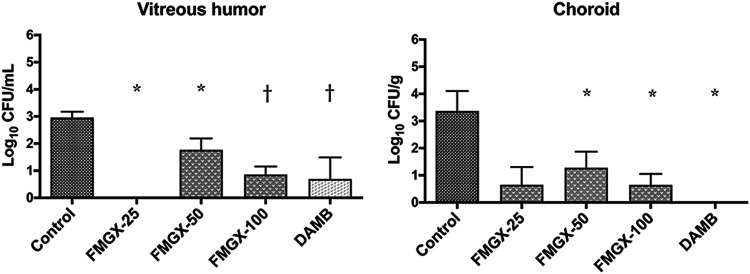
(i) Reduction in fungal burden by CFU count assessment. There was a significant reduction of 1 to >2 log10 in the residual fungal burden of C. albicans in the choroid and vitreous humor in rabbits treated with fosmanogepix or amphotericin B deoxycholate (DAMB), respectively (P ≤ 0.05) (Fig. 1). Notably, the aqueous humor, obtained from untreated control rabbits or treated with fosmanogepix or DAMB, had no detectable C. albicans. The reduction of residual fungal burden in vitreous humor and choroid by the highest dosage of fosmanogepix (100 mg/kg twice a day [BID]) was comparable to the efficacy of DAMB. The statistical significance of the fosmanogepix on the 25-mg/kg treatment group versus untreated control rabbit group was smaller due to the smaller size of the cohort (3 rabbits versus 6 rabbits).
FIG 1.
Comparison of the fungal burden in vitreous humor and choroid in rabbits treated with fosmanogepix or DAMB to that of untreated control rabbits (UC) (n = 6) in a model of Candida endophthalmitis. The aqueous humor, obtained from UC rabbits or treated with fosmanogepix or DAMB, had no detectable C. albicans. Values are given as means ± SEMs. *, P ≤ 0.05; †, P ≤ 0.01. FMGX-25, fosmanogepix at 25 mg/kg BID (n = 3); FMGX-50, fosmanogepix at 50 mg/kg BID (n = 6); FMGX-100, fosmanogepix at 100 mg/kg BID (n = 6); DAMB, deoxycholate amphotericin B at 1 mg/kg (n = 6).
There was a significant reduction (>2.0 log10) of the residual fungal burden in the cerebrum, cerebellum, spinal cord, meninges, and cerebrospinal fluid (CSF) at all dosages of fosmanogepix, as well as in DAMB-treated rabbits, in comparison to those of untreated controls (P ≤ 0.01) (Fig. 2). In particular, fosmanogepix at 50 mg/kg BID or 100 mg/kg BID resulted in the same reduction in CFU/g tissue as that of DAMB treatment.
FIG 2.
Comparison of the fungal burden in brain tissues and CSF in rabbits treated with prodrug fosmanogepix or DAMB to that of untreated control rabbits (UC) (n = 6) in a rabbit model of Candida hematogenous meningoencephalitis. Values are given as means ± SEMs. *, P ≤ 0.05; †, P ≤ 0.01. FMGX-25, fosmanogepix at 25 mg/kg BID (n = 3); FMGX-50, fosmanogepix at 50 mg/kg BID (n = 6); FMGX-100, fosmanogepix at 100 mg/kg BID (n = 6); DAMB, deoxycholate amphotericin B at 1 mg/kg (n = 6).
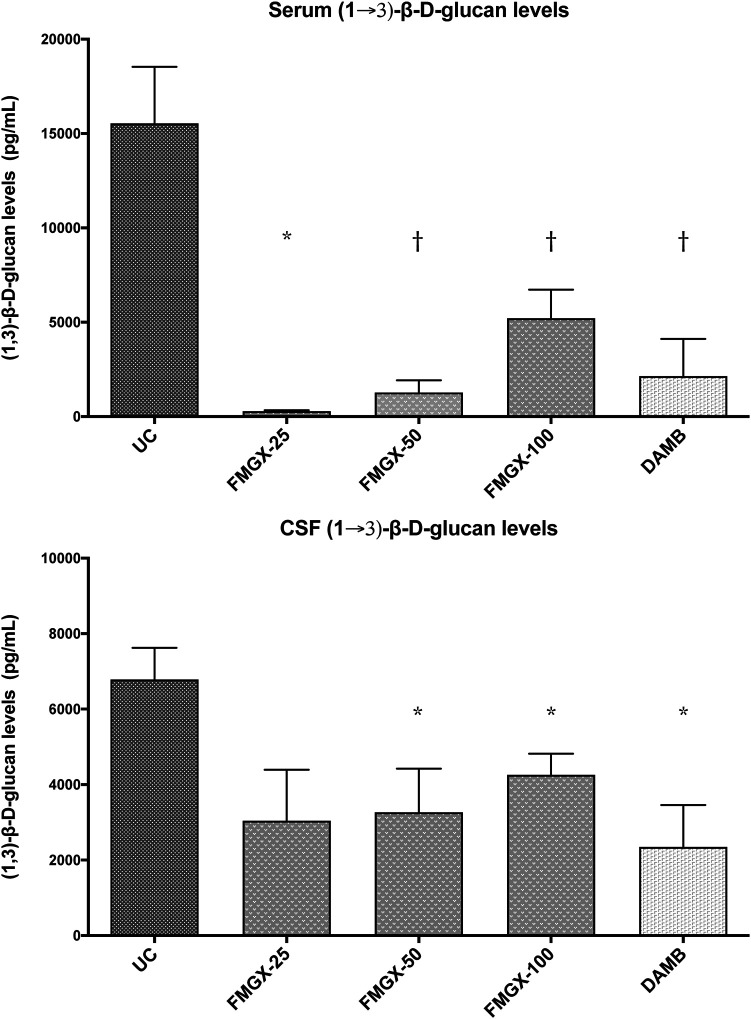
(ii) Assessment of (1→3)-β-d-glucan levels. Serum and CSF levels of (1→3)-β-d-glucan, cell wall polysaccharide, were monitored as a biomarker for the antifungal therapeutic response, as previously described (34–36). Consistent with the therapeutic response of fosmanogepix or DAMB in reducing the residual fungal burden of C. albicans in the CNS tissues and CSF, significant reductions of (1→3)-β-d-glucan levels were observed in CSF of rabbits treated with fosmanogepix at 50 mg/kg BID and 100 mg/kg BID, or DAMB at 1 mg/kg once daily (QD) (P ≤ 0.05) (Fig. 3). There was a parallel reduction of (1→3)-β-d-glucan levels in serum of rabbits treated with fosmanogepix at 50 mg/kg BID and 100 mg/kg BID and DAMB treated (P ≤ 0.01). A greater reduction of (1→3)-β-d-glucan levels was observed in serum than in CSF, reflecting the compartmentalization of (1→3)-β-d-glucan within the CSF.
FIG 3.
Serum and CSF (1→3)-β-d-glucan levels in a nonneutropenic rabbit model of Candida endophthalmitis and hematogenous meningoencephalitis in untreated control rabbits (UC) (n = 6) and rabbits treated with treated with prodrug fosmanogepix or DAMB. The last sample of serum was collected before euthanasia, and CSF was obtained postmortem for detection of (1→3)-β-d-glucan levels. Values are presented as means ± SEMs. *, P ≤ 0.05; †, P ≤ 0.01. FMGX-25, fosmanogepix at 25 mg/kg BID (n = 3); FMGX-50, fosmanogepix at 50 mg/kg BID (n = 6); FMGX-100, fosmanogepix at 100 mg/kg BID (n = 6); DAMB, deoxycholate amphotericin B at 1 mg/kg (n = 6).
Pharmacokinetics of manogepix and DAMB in rabbits.
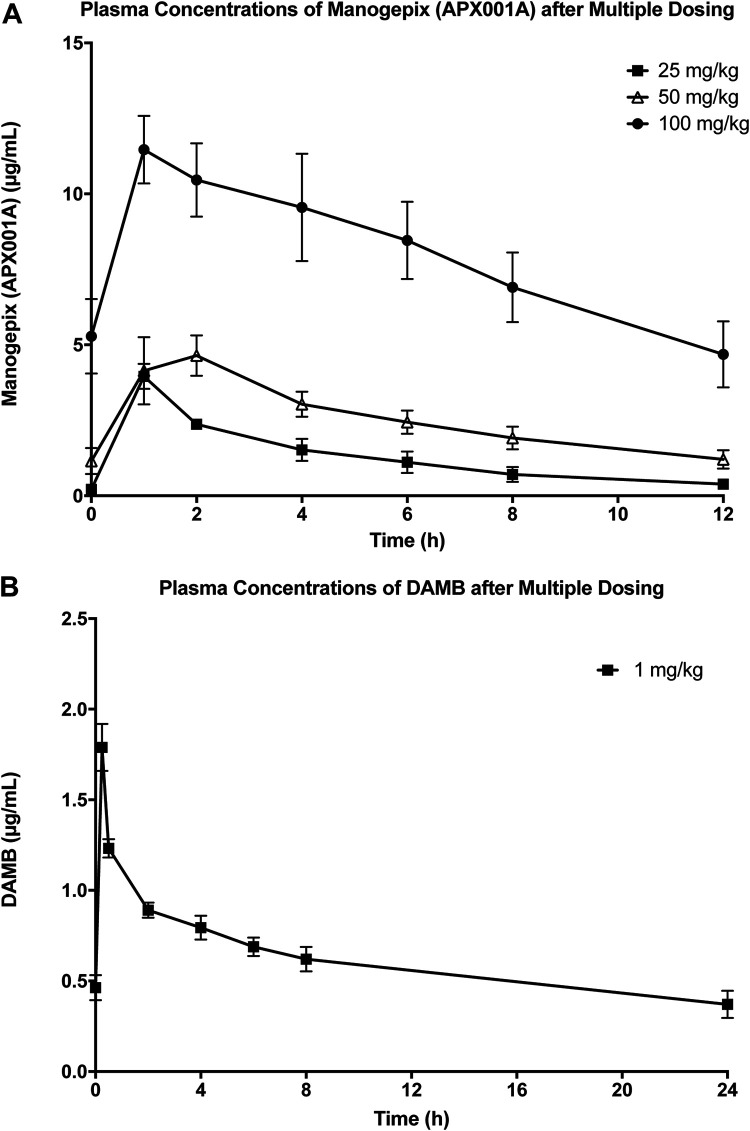
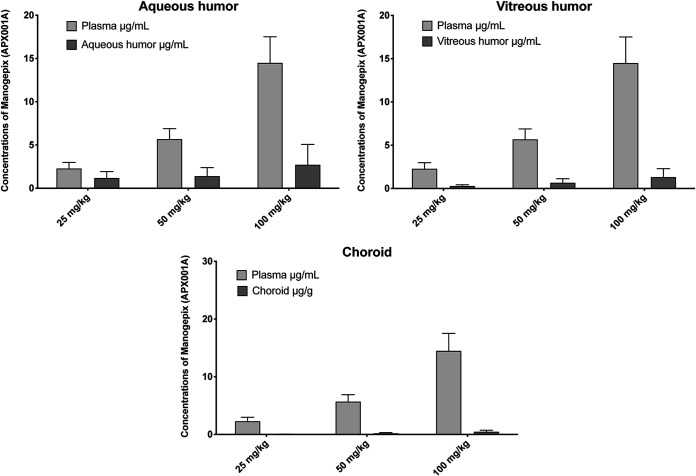
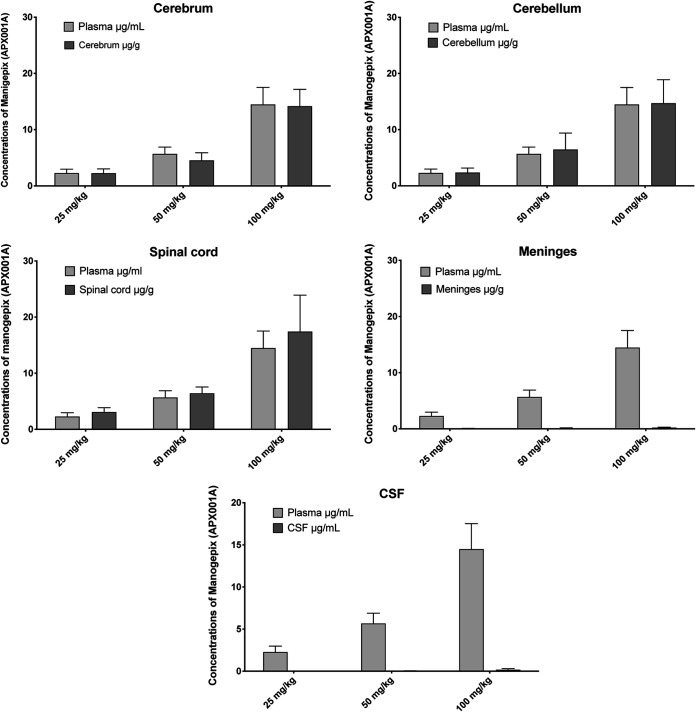
Plasma pharmacokinetics (PKs) of manogepix at dosages of oral fosmanogepix 25 mg/kg, 50 mg/kg, and 100 mg/kg resulted in maximum concentration of drug in plasma (Cmax) values of 3.96 ± 0.41, 4.14 ± 1.1, and 11.5 ± 1.1 μg/ml, respectively, and the area under the concentration-time curve from 0 to 12 h (AUC0–12) values of 15.8 ± 3.1, 30.8 ± 5.0, and 95.9 ± 15 μg·h/ml, respectively (Table 1). The Cmax and AUC0–12 were dose proportional between dosages of 25 mg/kg and 50 mg/kg, while the dose of 100 mg/kg was supraproportional (Table 1; Fig. 4). These three dosages of fosmanogepix conferred penetration into aqueous humor from 1.18 ± 0.43 μg/ml to 2.72 ± 0.24 μg/ml with liquid/plasma ratios from 0.52 to 0.19 (Table 2; Fig. 5). Similarly, the penetration into vitreous humor ranged from 0.28 ± 0.08 μg/ml to 1.32 ± 0.39 μg/ml with liquid/plasma ratios from 0.12 to 0.09. The penetration into choroid ranged from 0.05 ± 0.01 μg/ml to 0.48 ± 0.10 μg/ml with tissue/plasma ratios from 0.02 to 0.04 (Table 2, Fig. 5).
TABLE 1.
Plasma pharmacokinetics of manogepix after multiple oral dosing of prodrug fosmanogepix and DAMB after multiple intravenous dosinga
| Drug and dose (mg/kg) | Cmax (μg/ml) | AUC0–12 (μg·h/ml) | AUC(0–24) (μg·h/ml) | CL (ml/h/kg) |
|---|---|---|---|---|
| Fosmanogepix | ||||
| 25 | 3.96 ± 0.41 | 15.78 ± 3.12 | 1.54 ± 0.34 | |
| 50 | 4.14 ± 1.11 | 30.79 ± 5.01 | 1.48 ± 0.28 | |
| 100 | 11.46 ± 1.12 | 95.91 ± 14.56 | 0.79 ± 0.18 | |
| DAMB | ||||
| 1 | 1.79 ± 0.13 | 15.58 ± 1.64 | 42.9 ± 7.04 |
All values are expressed as the mean ± SEM. Cmax, maximum concentration of drug in plasma; AUC0–12, AUC from 0 to 12 h; AUC0–24, AUC from 0 to 24 h; CL, clearance.
FIG 4.
(A) Manogepix plasma concentration-time curves after multiple oral dosing of prodrug fosmanogepix. FMGX-25, fosmanogepix at 25 mg/kg BID (n = 3); FMGX-50, fosmanogepix at 50 mg/kg BID (n = 6); FMGX-100, fosmanogepix at 100 mg/kg BID (n = 6). (B) DAMB plasma concentration-time curve after multiple intravenous dosing of deoxycholate amphotericin B at 1 mg/kg (n = 5). Values are presented as the mean ± SEM.
TABLE 2.
Tissue/plasma or liquid/plasma concentration ratios of manogepix administered orally as prodrug fosmanogepixa
| Tissue or liquid | Liquid/plasma concentration ration at dose of: |
||
|---|---|---|---|
| 25 mg/kg | 50 mg/kg | 100 mg/kg | |
| Aqueous humor | 0.52 ± 0.12 | 0.25 ± 0.07 | 0.19 ± 0.05 |
| Vitreous humor | 0.12 ± 0.03 | 0.12 ± 0.03 | 0.09 ± 0.02 |
| Choroid | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.04 ± 0.00 |
| Cerebrum | 0.99 ± 0.02 | 0.80 ± 0.06 | 0.98 ± 0.03 |
| Cerebellum | 1.03 ± 0.05 | 1.13 ± 0.13 | 1.02 ± 0.08 |
| Spinal cord | 1.35 ± 0.13 | 1.13 ± 0.08 | 1.20 ± 0.15 |
| Meninges | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| CSF | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
Tissue or liquid/plasma ratio calculations performed from samples obtained 1 h after the last dose of fosmanogepix on day 7 of antifungal therapy at the end of the study. All values are expressed as the ratio means.
FIG 5.
Concentrations of manogepix in aqueous humor, vitreous humor, and choroid versus plasma levels after oral administration of prodrug fosmanogepix. FMGX-25, fosmanogepix at 25 mg/kg BID (n = 3); FMGX-50, fosmanogepix at 50 mg/kg BID (n = 6); FMGX-100, fosmanogepix at 100 mg/kg BID (n = 6). Plasma was collected before rabbits were euthanized 1 h after the last dose of fosmanogepix. Aqueous humor, vitreous humor, and choroid were obtained after rabbits were euthanized 1 h after the last dose of fosmanogepix.
Penetration of the drug into the cerebrum, cerebellum, and spinal cord achieved high concentrations with an approximate 1:1 tissue/plasma concentration ratio across all three dosages (Table 2). By comparison, manogepix was virtually nondetectable in CSF (Table 2; Fig. 6). The high concentrations of manogepix in brain tissues and spinal cord were consistent with the >2.0-log10 CFU/g reduction in the residual fungal burden of C. albicans at all dosages (P ≤ 0.01) (Fig. 6).
FIG 6.
Concentrations of manogepix in cerebrum, cerebellum, spinal cord, meninges, and CSF versus plasma levels after oral administration of prodrug fosmanogepix. FMGX-25, fosmanogepix at 25 mg/kg BID (n = 3); FMGX-50, fosmanogepix at 50 mg/kg BID (n = 6); FMGX-100, fosmanogepix at 100 mg/kg BID (n = 6). Plasma was collected before rabbits were euthanized 1 h after the last dose of fosmanogepix. CSF, meninges, cerebrum, cerebellum, and spinal cord were obtained after rabbits were euthanized 1 h after the last dose of fosmanogepix.
Blood samples for plasma concentrations of DAMB, administered intravenously at 1 mg/kg, were drawn predose and at time points of 0.5, 1, 2, 4, 6, 8, and 24 h. Plasma pharmacokinetics of DAMB demonstrated plasma Cmax of 1.79 ± 0.13 μg/ml and AUC0–12 of 15.6 ± 1.64 μg·h/ml (Table 1).
Plasma chemistry panel.
As demonstrated in Table 3, untreated infected control rabbits demonstrated significant increases in their serum creatinine levels compared to those treated with fosmanogepix at 25 mg/kg and 50 mg/kg (P < 0.01). There were no significant differences in serum creatinine levels among fosmanogepix groups. There also were significant decreases in serum urea nitrogen in rabbits treated with fosmanogepix at 25, 50, and 100 mg/kg in comparison to that of untreated rabbits (P ≤ 0.05). By comparison, DAMB-treated animals demonstrated significant elevations of serum creatinine and serum urea nitrogen, consistent with its known nephrotoxicity. Serum potassium levels were unchanged among all treatment groups and the untreated control rabbits (Table 3).
TABLE 3.
Effects of oral prodrug fosmanogepix and DAMB on serum creatinine, urea nitrogen, ALT, and AST in a rabbit model of Candida endophthalmitis and hematogenous meningoencephalitisa
| Exptl group (no.) | Serum creatinine (mg/dl) | Serum urea nitrogen (mg/dl) | Serum ALT (U/liter) | Serum AST (U/liter) | Serum potassium (mEq/liter) |
|---|---|---|---|---|---|
| Healthy rabbits (baseline) (26) | 0.93 ± 0.04 | 21.81 ± 0.82 | 28.38 ± 2.26 | 12.31 ± 0.72 | 4.07 ± 0.12 |
| Untreated controls (6) | 3.58 ± 0.99 | 68.50 ± 13.73 | 78.60 ± 16.86 | 91.60 ± 27.61 | 3.63 ± 0.48 |
| Treatment group | |||||
| FMGX-25 (3) | 0.92 ± 0.10* | 16.00 ± 0.58b | 31.00 ± 3.22 | 11.33 ± 0.67b | 4.00 ± 0.21 |
| FMGX-50 (6) | 1.02 ± 0.08* | 15.33 ± 1.17b | 24.67 ± 6.19c | 14.33 ± 4.41c | 3.98 ± 0.10 |
| FMGX-100 (6) | 1.65 ± 0.29 | 28.00 ± 7.30b | 19.67 ± 1.78c | 9.17 ± 0.17c | 3.58 ± 0.24 |
| DAMB (6) | 2.53 ± 1.16 | 49.13 ± 21.44 | 25.30 ± 2.00c | 11.83 ± 1.33c | 4.26 ± 0.17 |
All values are expressed as the mean ± SEM. Comparisons to untreated control rabbit group were performed using Mann-Whitney U test.
P ≤ 0.05.
P ≤ 0.01.
Neither fosmanogepix nor DAMB-treated rabbits demonstrated elevated serum hepatic transaminase levels. On the other hand, there was a significant increase in alanine aminotransaminase (ALT) and aspartyl aminotransaminase (AST) in untreated infected control rabbits versus all treatment groups (P < 0.01) (Table 3).
DISCUSSION
The purpose of this study was to determine the efficacy, plasma pharmacokinetics, tissue distribution, and safety of manogepix after oral administration of the prodrug fosmanogepix in the treatment of Candida endophthalmitis and HCME in an experimental rabbit model. The study elucidated the compartmental CNS distribution of manogepix and demonstrated drug concentrations sufficient for a reduction of >2-log10 CFU/g of fungal burden of C. albicans in CNS tissues. In addition, penetration into different compartments of ocular tissue was demonstrated with a corresponding reduction in log10 CFU/ml in ocular fluids. These findings are consistent with 14C-manogepix distribution studies that demonstrated the significant radioactivity in tissues associated with invasive fungal infections, including brain and ocular tissue (33). Such findings provide a scientific foundation for the application of fosmanogepix in the treatment of hematogenous Candida endophthalmitis and HCME, particularly in premature infants, immunosuppressed children, and immunocompromised patients. In addition, these findings are consistent with the achievement of sufficient ocular penetration for the treatment of Candida endophthalmitis. Investigation of clinically applicable animal systems is essential to providing confidence in the antifungal activity of this first-in-class agent in both CNS and eye infections. These detailed studies of the efficacy of fosmanogepix in brain, spinal cord, and eye complement previous mouse disseminated infection models, demonstrating the efficacy of fosmanogepix administration reduces brain, lung, kidney, and spleen burdens (28, 31, 37–39).
Fosmanogepix CNS tissue distribution and efficacy (reduction of CFU counts) were similar to that of DAMB in experimental HCME. The plasma and CNS pharmacokinetics of DAMB and the lipid formulations of amphotericin B were studied extensively in rabbits by Groll et al. (17). Following intravenous infusion of DAMB at 1 mg/kg, mean DAMB Cmax values of 1.82 μg/ml corresponded to a mean CSF concentration of 0.023 μg/ml and a CNS tissue (combined cerebrum and cerebellum) concentration of 0.33 μg/g with a tissue/plasma ratio of 0.18. By comparison, fosmanogepix at 25 mg/kg achieved a Cmax of 3.96 μg/ml, a CSF concentration of 0.01 μg/ml, and tissue/plasma ratios in cerebrum and cerebellum of 0.99 and 1.03, respectively. Although the 100-mg/kg dose demonstrated supraproportionality in plasma, both the 50-mg/kg and 100-mg/kg doses preserved the tissue/plasma ratio of approximately 1.0. A similar ratio of 1.0 was also observed in the spinal cord tissue. Of note is that the total daily exposures (AUC) in rabbits resulting from oral administration of fosmanogepix at 100 mg/kg BID (192 μg·h/ml) were similar to total daily exposures observed in phase 1 studies of healthy human volunteers at an oral dose of 500 mg (205 μg·h/ml) (40).
The AUC0–12 is linear and dose proportional between oral fosmanogepix at 25 mg/kg and 50 mg/kg with values of 15.78 ± 3.12 μg·h/ml and 30.79 ± 5.01 μg·h/ml, respectively, with clearance remaining relatively unchanged. However, when the AUC0–12 of 11.46 ± 1.12 μg·h/ml is considered, the AUC0–12 is disproportionally higher at 95.91 μg·h/ml. This higher AUC0–12 may be caused by the wide interanimal oral bioavailability with AUC0–12, ranging from 47.99 μg·h/ml to 144.1 μg·h/ml. Since clearance equals dose/AUC, as AUC is disproportionally increased, due to variation in bioavailability, clearance will proportionally decrease. Thus, we believe that higher AUC and lower clearance may reflect variable bioavailability of manogepix.
Thus, these patterns of plasma and tissue distribution would predict that fosmanogepix would be comparable in efficacy against HCME to that of DAMB. Supporting this hypothesis, there was a dose-response relationship of fosmanogepix in the cerebral and cerebellar tissue that achieved significant reductions in residual fungal burden comparable to those of DAMB. Fosmanogepix also exerted antifungal efficacy in the CSF despite very low concentrations. This activity may be explained by the presence of manogepix in the meninges at a level comparable to that of other CNS sites. Organisms adhering to the meninges would be damaged by manogepix. The paucity of protein in the CSF may also permit the diffusion of low levels of unbound active drug from the meninges. These findings of efficacy in HCME that correlate with manogepix tissue concentrations in cerebral and cerebellar tissues support the use of fosmanogepix in the treatment of patients with Candida endophthalmitis and hematogenous meningoencephalitis.
The data of CSF (1→3)-β-d-glucan and residual fungal burden (log CFU per gram) are internally consistent and replicate previous work in HCME. The study demonstrates a significant reduction of residual fungal burden in cerebrum, as well as other CNS tissues. These reductions correlate with a significant reduction of CNS (1→3)-β-d-glucan. These data also are consistent with the earlier report establishing a correlation between residual fungal burden cerebral tissue and CSF (1→3)-β-d-glucan (r = 0.842; P ≤ 0.001) (35). Due to the compartmentalization of the large (1→3)-β-d-glucan molecules, there still remains, at the end of therapy, some persistent cell wall carbohydrate in this and earlier studies. However, when measured over time, CSF (1→3)-β-d-glucan continues to resolve as a reliable biomarker in patients with HCME who are successfully treated. For example, Salvatore et al. demonstrated time-dependent resolution of CSF (1→3)-β-d-glucan in pediatric patients who were successfully treated for HCME (36). Time-dependent resolution of CSF (1→3)-β-d-glucan also occurs in successfully treated patients with cryptococcal meningoencephalitis (41), CNS aspergillosis (42), and Exserohilum rostratum meningitis (43, 44).
Fosmanogepix also is similar in its properties of ocular tissue distribution and antifungal efficacy to those of DAMB in experimental Candida endophthalmitis. Consistent with the relatively lower tissue/plasma ratios in the vitreous humor and choroid, the antifungal response was somewhat less than that achieved in the cerebral, cerebellar, and spinal cord tissue. Nonetheless, the antifungal activity of fosmanogepix in vitreous and choroid was comparable to that of DAMB, which also has relatively low tissue/plasma ratios.
We recently characterized an important mechanism of penetration of amphotericin B through the blood-brain barrier and its distribution in brain tissue to account for its efficacy in the experimental HCME rabbit model (18, 19). Petraitis et al. (19) compared the exposures of two amphotericin B formulations (DAMB and liposomal amphotericin B) in the brain and found significantly greater concentrations of the polyenes within the abscesses in cerebrum in comparison to that of normal cerebrum tissue. Petraitis et al. postulated that localized blood-brain barrier disruption and the focally destructive nature of the abscesses allowed high concentrations of amphotericin B within infected tissues, despite the presence of low CSF concentrations (19). Fosmanogepix has also demonstrated a reduction in CNS fungal burden in several animal models of disseminated infection and also demonstrated extensive manogepix CNS exposure in animal absorption, distribution, metabolism, and excretion (ADME) studies (32, 33). The manogepix brain tissue/plasma concentration ratio was also high (approximately 1.0). Due to the smaller molecular weight (manogepix, 358.401, versus DAMB, 924.079), the CNS penetration of manogepix may reflect diffusion across the blood-brain barrier.
Amphotericin B, fluconazole, micafungin, and anidulafungin have demonstrable activity in the treatment of Candida HCME (17). Due to its extensive CNS penetration (14, 15), fluconazole is another potential option for the treatment of HCME. However, refractory cases of HCME treated with fluconazole had been reported, possibly because of its lack of fungicidal activity. Consequently, fluconazole is recognized as second-line therapy for the treatment of HCME (7). The echinocandins represent a class of antifungal agents which exhibit broad candidacidal activity (20). The echinocandins in currently approved doses in adults do not achieve sufficient therapeutic concentrations in the eye and brain. Studies of higher dosages (45), which achieve therapeutic concentrations of micafungin in CNS tissue, have been recently completed in pediatric patients (46, 47).
As depicted in the serum chemistry of elevated serum creatinine, urea, and transaminases AST and ALT, the untreated control rabbits reflect a pattern of organism-mediated tissue injury caused by C. albicans in kidney and liver in a process of disseminated candidiasis (48–51). Deoxycholate amphotericin B effectively eradicated C. albicans while also reflecting its nephrotoxicity by elevated serum creatinine and urea. Fosmanogepix exerted extensive antifungal activity, significantly reducing Candida burden in multiple CNS and eye tissues. Although serum creatinine levels in the FMGX-100 group differed from levels in the FMGX-25 and FMGX-50 groups, the changes were not statistically different from other study groups and may be the result of variable contracted volume during the disseminated infection.
These preclinical data are critical to understanding the role of fosmanogepix in clinical treatment of candidemia and invasive candidiasis. Overall, the study demonstrated exposure of manogepix in several key CNS and eye compartments. Likewise, amphotericin B, the gold standard for treatment of endophthalmitis and CNS Candida infection, also demonstrated similar reductions in CNS tissue fungal burden. However, amphotericin B was associated with elevations in parameters of renal impairment, whereas fosmanogepix was not. These results, while preliminary, provide evidence for the potential of fosmanogepix to be used in the treatment of endophthalmitis and CNS Candida infections, where the current treatment options remain suboptimal.
MATERIALS AND METHODS
Animals.
Female immunocompetent New Zealand White rabbits (Oryctolagus cuniculus) (n = 27) (Covance Research Products, Inc., Denver, PA) weighing 2.8 to 3.7 kg were housed individually and maintained with water and standard autoclaved rabbit feed ad libitum. Rabbits were monitored under humane care in the facilities, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) and according to the guidelines of the National Research Council (52) for the care and use of laboratory animals. The Institutional Animal Care and Use Committee of Weill Cornell Medicine of Cornell University approved the study. Each rabbit had established vascular access under general anesthesia by the surgical placement of a subcutaneous Silastic central venous catheter at least 72 h before studies (53). The Silastic catheter permitted nontraumatic venous access for the administration of Candida inoculum, parental agents, and repeated blood sampling for plasma pharmacokinetics, serum (1→3)-β-d-glucan levels, and biochemical and hematological parameters.
A nonneutropenic, well-established rabbit model of Candida endophthalmitis and HCME, described elsewhere (17, 35, 45), was used for all studies. Rabbits were euthanized according to the Animal Care and Use Committee-approved prespecified humane endpoints by intravenous administration of pentobarbital (65 mg of pentobarbital sodium/kg of body weight; SomnaSol Euthanasia-III Solution C3N; Henry Schein Animal Health, Dublin, OH) at the end of the study.
Organism and inoculum.
A well-characterized clinical isolate of C. albicans, NIH-8621 (ATCC MYA-1237), was used, and inoculum was prepared as previously described in a rabbit model of Candida endophthalmitis and HCME (17, 35, 45). Briefly, the C. albicans isolate was subcultured from a frozen stock culture stored at −80°C on potato dextrose agar slants onto Sabouraud dextrose agar (SGA) plates and incubated at 37°C for 24 h. A few colonies were sampled from freshly grown culture plates, suspended into 50 ml of Emmon’s modified Sabouraud glucose broth in a 250-ml Erlenmeyer flask, incubated in a shaking water bath at 80 oscillations per min at 37°C for 18 h, then centrifuged at 1,600 × g for 10 min, and washed three times with sterile 0.9% normal saline. The concentration then was adjusted by use of a hemacytometer and confirmed by quantitative culture of a 10-fold serial dilution. An inoculum of 1 × 106 blastoconidia, suspended in 5 ml of sterile normal saline, was administered to each rabbit over 1 min via an indwelling Silastic central venous catheter to establish a nonlethal model of Candida endophthalmitis and HCME in nonneutropenic rabbits.
Study drugs.
Fosmanogepix (provided by Amplyx Pharmaceuticals, Inc., San Diego, CA) was prepared according to recommendations as follows: 100 mg of fosmanogepix powder was added to 1,000 μl of 0.21 M NaOH and vortexed for approximately 1 min with gentle heat until the liquid became a pale yellow solution at pH 7.5. The solution at a concentration of 100 mg/ml was then diluted with sterile water to prepare the appropriate dosing solutions.
Amphotericin B deoxycholate (DAMB; XGen Pharmaceuticals Inc., Big Flats, NY) was investigated for antifungal efficacy as a positive control. Lyophilized powder of DAMB (50 mg of amphotericin B) was reconstituted with 10 ml of sterile water to produce a solution of 5 mg/ml and further diluted in 5% dextrose in water (D5W) to achieve a final concentration of 1 mg/ml.
Antifungal therapy.
The study was conducted in two replicate experiments with all study cohorts. The treatment groups consisted of oral fosmanogepix at 25 mg/kg (FMGX-25) BID (n = 3), 50 mg/kg BID (FMGX-50) (n = 6), and 100 mg/kg BID (FMGX-100) (n = 6) and intravenous (i.v.) DAMB at 1 mg/kg QD (a standard dosage of DAMB used in the treatment of HCME; n = 6). The antifungal therapy with fosmanogepix or DAMB was initiated 48 h after inoculation of C. albicans and administered for 7 days. Antifungal efficacy in rabbits was assessed in comparison to the untreated control group. Surviving rabbits were euthanized (on day 8 of treatment) 1 h after the last 15th dose of fosmanogepix or 30 min after the last 8th dose of DAMB.
Therapeutic endpoints of Candida endophthalmitis and HCME.
The therapeutic endpoints consist of the reduction in the residual fungal burden (log CFU per gram) and declining of CSF and serum (1→3)-β-d-glucan. The changes in the residual fungal burden were measured in the following fluids and tissues: aqueous humor, vitreous humor, choroid, CSF, meninges, cerebrum, cerebellum, and spinal cord.
Postmortem analysis.
The duration of survival in days postinoculation was recorded for each rabbit. Surviving rabbits were euthanized on day 8 of treatment 1 h after the last dose of fosmanogepix or 30 min after the last dose of DAMB. All harvested tissues and fluids were quantitatively cultured and submitted for analytical determination of manogepix concentrations.
Quantitation of C. albicans in body fluids and tissues.
The antifungal efficacy in the model of Candida endophthalmitis and HCME was determined by the quantitative clearance of C. albicans from the blood, aqueous humor, vitreous humor, CSF, choroid, meninges, cerebrum, cerebellum, and spinal cord. Methods for obtaining individual samples for analysis were as previously described (17, 35). Briefly, just after euthanasia of each rabbit, CSF samples were collected, aqueous humor was obtained using a 3-ml syringe with a 25-gauge needle before dissecting the eyes, and then vitreous humor and choroid were collected for analysis. Representative sections of tissues (meninges, cerebrum, cerebellum, and spinal cord) were weighed, and each tissue sample was then homogenized.
The CSF, aqueous humor, or vitreous humor specimen or each tissue homogenate was serially diluted to 10−1,10−2, or 10−4 in sterile 0.9% normal saline. Aliquots (100 μl) of CSF, aqueous humor, vitreous, or undiluted tissue homogenates and serial dilutions of 10−1,10−2, or 10−4 in sterile 0.9% normal saline were separately plated onto SGA plates. Culture plates were incubated at 37°C for 24 h, CFU were counted, and the numbers of CFU per milliliter (for CSF, aqueous humor, and vitreous humor) or CFU per gram (for chorioretinal and brain tissues) were calculated. Performing serial dilutions minimized potential carryover of drug. The lower limit of detection was 10 CFU/ml or 10 CFU/g. The culture-negative plates were counted as 0 CFU/ml or 0 CFU/g. Data were graphed as the means of log10 (CFU/ml or CFU/g) ± standard errors of the means.
(1→3)-β-d-glucan levels in serum and CSF.
The collection of blood from each rabbit was performed every other day for determination of serum (1→3)-β-d-glucan levels. CSF was collected postmortem. The assay was performed according to the instructions of the manufacturer using the Fungitell assay kit, and the levels of (1→3)-β-d-glucan were determined by comparison to a standard curve. When absorbance was outside the range of the standard curve, the sera were serially diluted in β-glucan-free reagent-grade water and tested again. Interpretation of (1→3)-β-d-glucan values, according to the manufacturer’s instructions for use, was as follows: 60 pg/ml, negative; 60 to 79 pg/ml, indeterminate; and 80 pg/ml, positive. The mean (1→3)-β-d-glucan level in the serum from 60 noninfected rabbits was 13 ± 1.21 pg/ml (range, 0 to 35.2 pg/ml).
Pharmacokinetics of manogepix and DAMB in rabbits.
The PK study was conducted in two replicate experiments with all study cohorts.
Pharmacokinetics of manogepix.
The pharmacokinetics of manogepix was performed on day 6 of antifungal therapy. Blood samples for manogepix concentrations were collected from three rabbits in the FMGX-25 treatment group and six rabbits in each cohort of FMGX-50 and FMGX-100 into BD vacutainer K2 EDTA tubes before oral administration of the 11th dose of fosmanogepix (time point 0) and further at time points of 1, 2, 4, 6, 8, and 12 h after administration. Plasma samples were stored at −80°C until they were analyzed.
For correlation of manogepix tissue concentrations and manogepix plasma levels, plasma samples were obtained 1 h after the last 15th dose of fosmanogepix on day 8 of antifungal therapy at the end of the study, prior to euthanasia. Aqueous humor, vitreous humor, choroid, CSF, cerebrum, cerebellum, and spinal cord were collected after euthanasia and stored at −80°C until they were analyzed. Ratios were calculated as tissue or liquid concentrations over plasma concentrations (CT/CP or CL/CP, respectively).
Plasma and tissue samples were analyzed for manogepix concentrations using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method at QPS LLC (Newark, DE) with a triple quadrupole mass spectrometer (API 4000 LC-MS/MS system; Applied Biosystems, Inc., Waltham, MA) and utilized an internal standard (IS) ER-509542-00.
Calibration standards were prepared by adding 25 μl of working standard solutions at each concentration into a preparation plate; 25 μl of rabbit plasma was added into the blank, blank plus IS, and carryover blank wells. To quality control (QC) wells, 25 μl of QC samples was added at the appropriate concentrations and number of replicates. We added 25 μl of each study sample to the appropriate wells. We added 25 μl of IS into the blank plus IS calibration wells. We added 25 μl of acetonitrile/water at 50:50 (vol/vol) to the blank and carryover blank wells. We added 50 μl of type I water into all wells. The plate was capped and centrifuged for about 1 min at 1,000 rpm. The plate was vortex mixed for approximately 1 min at high speed.
Protein precipitation extraction was performed using a Tomtec Quadra 4 (Tomtec, Inc., Hamden, CT). An aliquot of 400 μl of acetonitrile was added to all the wells with a repeater pipette. The plate was capped tightly and vortex mixed for approximately 5 min at high speed and then centrifuged for approximately 5 min at 3,000 rpm. On the Tomtec, 25 μl of supernatant was transferred from the preparation plate into the collection plate I for manogepix analysis. An aliquot of 300 μl of acetonitrile/water at 20:80 (vol/vol) was added into all samples in the collection plate I for manogepix. The collection plate I was vortex mixed for approximately 1 min at medium speed and then centrifuged at approximately 3,000 rpm for 1 min. At this time, extraction was completed for manogepix.
A 2.1- by 50-mm, 3.5-μm-particle-size XBridge phenyl column (Waters Corp., Milford, MA) was used for chromatography. Elution was carried out in isocratic flow with the following mobile phase composition: A-acetronitrile/water/1 M ammonium acetate at 20:80:0.5 (vol/vol/vol) and B-acetronitrile/water/1 M ammonium acetate at 95:5:0.5 (vol/vol/vol). An autoinjector temperature was set to 4°C. Wash solvents (R0)-acetonitrile/water at 70:30 (vol/vol) and (R3)-acetonitrile/isopropanol/dimethyl sulfoxide at 30:30:30 (vol/vol/vol) were used. The flow rate in the column was 0.5 ml/min with analysis time ∼2.7 min and injection volume of 2 to 5 μl. The retention times for manogepix and the IS (ER-509542-00) were ∼0.77 min and ∼0.76 min, respectively.
For each group, mean plasma concentrations at each nominal sample time were used for PK analysis. Values below the quantification limit (BQL; <0.050 μg/ml) were set to zero for determining the mean. PK data analysis was performed for plasma concentration versus time data using noncompartmental analysis in Phoenix WinNonlinTM, version 6.3 (Pharsight Corporation, a Certara Company, Princeton, NJ).
Plasma pharmacokinetics of DAMB.
Blood samples for DAMB concentrations were collected in heparinized syringes from six rabbits on day 6 of antifungal therapy at baseline and time points of 0.25, 0.5, 2, 4, 6, 8, and 24 h after administration of DAMB.
Concentrations of amphotericin B in plasma were determined by an internally validated, reversed-phase high-performance liquid chromatographic (HPLC) method (17, 54), modified at Keystone Bioanalytical, Inc. Briefly, extraction from 250-μl samples was performed by precipitation of proteins using 1:2 (vol/vol) methanol-to-plasma ratio. Then, samples were incubated for 30 min at 4°C and centrifuged at 2,000 × g for 10 min. The methanolic supernatant was transferred into a 0.22-μm Durapore filter tube and centrifuged at 4,000 × g for 4 min for injection. Standards and quality controls were similarly prepared from pooled normal rabbit plasma. A commercial amphotericin B was used as reference standard (MilliporeSigma).
HPLC was performed with a modular liquid chromatograph equipped with a Shimadzu LC-20AD pump, a Shimadzu Sil-30ACMP autosampler fitted to a 100-μl sampling loop, and a Shimadzu SPD-20A UV detector. Amphotericin B was separated on a 100- by 3-mm, 3-μm-particle-size Unison UK-C18 reverse-phase column. Elution was carried out isocratically with a mobile phase consisting of an acetonitrile-acetic acid-water (60% of mobile phase A, 0.75% acetic acid in water; 40% of mobile phase B, 0.75% acetic acid in acetonitrile) mixture. It flowed at a constant flow rate of 0.8 ml/min, and the effluent was monitored at 408 nm. The chromatographic run time was 4.5 min per sample with injection volume of 20 μl. A linear response over the concentration (calibration) range was 0.1 to 50 μg/ml, and the lower limit of quantitation was 0.1 μg/ml in plasma.
Plasma chemistry panel.
Creatinine, urea nitrogen, ALT, AST, and potassium concentrations were determined in plasma by the Central Laboratory and Clinical Chemistry Services of Clinical Pathology and Laboratory Medicine of Weill Cornell Medicine of Cornell University on the last sample collected from each rabbit (day 8 of treatment).
Statistical analysis.
The following hypotheses were studied: manogepix achieves effective free drug concentrations throughout all compartments of the CNS, including specific tissues of the eye; fosmanogepix is effective in the treatment of Candida endophthalmitis and HCME. The quantitative cultures of C. albicans and levels of (1→3)-β-d-glucan in serum and CSF were analyzed as dependent variables of tissue and serum concentrations of manogepix. Comparisons between the experimental groups were performed by Kruskal-Wallis test (nonparametric analysis of variance [ANOVA]) or the Mann-Whitney U-test, as appropriate. A two-tailed P value of ≤0.05 was considered to be statistically significant. Values were expressed as means ± standard error of the means (SEMs).
ACKNOWLEDGMENTS
We thank QPS (Newark, DE) for their assistance in pharmacokinetic studies.
This study was supported by Amplyx Pharmaceuticals.
T.J.W. has received research support from and served as a consultant to Astellas, ContraFect, Drais, iCo, Novartis, Pfizer, Methylgene, SigmaTau, and Trius. K.J.S. was previously an employee of Amplyx Pharmaceuticals and is now an independent consultant at Hearts Consulting Group, LLC. M.R.H. is an employee of Amplyx. R.S.M. is a consultant for Amplyx. M.A.F. is an employee of Associates of Cape Cod, Inc. All other authors declare no conflicts of interest.
REFERENCES
- 1.Edwards JE, Jr, Foos RY, Montgomerie JZ, Guze LB. 1974. Ocular manifestations of Candida septicemia: review of seventy-six cases of hematogenous Candida endophthalmitis. Medicine (Baltimore, MD) 53:47–75. doi: 10.1097/00005792-197401000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein AJ, Johnson EH, Moellering RC, Jr. 1973. Candida endophthalmitis. A complication of candidemia. Arch Intern Med 132:749–752. doi: 10.1001/archinte.1973.03650110083018. [DOI] [PubMed] [Google Scholar]
- 3.Sallam A, Lynn W, McCluskey P, Lightman S. 2006. Endogenous Candida endophthalmitis. Expert Rev Anti Infect Ther 4:675–685. doi: 10.1586/14787210.4.4.675. [DOI] [PubMed] [Google Scholar]
- 4.Brooks RG. 1989. Prospective study of Candida endophthalmitis in hospitalized patients with candidemia. Arch Intern Med 149:2226–2228. doi: 10.1001/archinte.1989.00390100056014. [DOI] [PubMed] [Google Scholar]
- 5.Menezes AV, Sigesmund DA, Demajo WA, Devenyi RG. 1994. Mortality of hospitalized patients with Candida endophthalmitis. Arch Intern Med 154:2093–2097. doi: 10.1001/archinte.1994.00420180103012. [DOI] [PubMed] [Google Scholar]
- 6.Riddell J, Comer GM, Kauffman CA. 2011. Treatment of endogenous fungal endophthalmitis: focus on new antifungal agents. Clin Infect Dis 52:648–653. doi: 10.1093/cid/ciq204. [DOI] [PubMed] [Google Scholar]
- 7.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:409–417. doi: 10.1093/cid/civ1194. [DOI] [PubMed] [Google Scholar]
- 8.Ruhnke M, Rickerts V, Cornely OA, Buchheidt D, Glockner A, Heinz W, Hohl R, Horre R, Karthaus M, Kujath P, Willinger B, Presterl E, Rath P, Ritter J, Glasmacher A, Lass-Florl C, Groll AH, German Speaking Mycological Society, Paul-Ehrlich-Society for Chemotherapy. 2011. Diagnosis and therapy of Candida infections: joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses 54:279–310. doi: 10.1111/j.1439-0507.2011.02040.x. [DOI] [PubMed] [Google Scholar]
- 9.Feman SS, Nichols JC, Chung SM, Theobald TA. 2002. Endophthalmitis in patients with disseminated fungal disease. Trans Am Ophthalmol Soc 100:67–70. [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, Duara S, Poole K, Laptook A, Goldberg R. 2006. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 11.McCullers JA, Vargas SL, Flynn PM, Razzouk BI, Shenep JL. 2000. Candidal meningitis in children with cancer. Clin Infect Dis 31:451–457. doi: 10.1086/313987. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez M, Moylett EH, Noyola DE, Baker CJ. 2000. Candidal meningitis in neonates: a 10-year review. Clin Infect Dis 31:458–463. doi: 10.1086/313973. [DOI] [PubMed] [Google Scholar]
- 13.Friedman S, Richardson SE, Jacobs SE, O'Brien K. 2000. Systemic Candida infection in extremely low birth weight infants: short term morbidity and long term neurodevelopmental outcome. Pediatr Infect Dis J 19:499–504. doi: 10.1097/00006454-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Arndt CA, Walsh TJ, McCully CL, Balis FM, Pizzo PA, Poplack DG. 1988. Fluconazole penetration into cerebrospinal fluid: implications for treating fungal infections of the central nervous system. J Infect Dis 157:178–180. doi: 10.1093/infdis/157.1.178. [DOI] [PubMed] [Google Scholar]
- 15.Walsh TJ, Foulds G, Pizzo PA. 1989. Pharmacokinetics and tissue penetration of fluconazole in rabbits. Antimicrob Agents Chemother 33:467–469. doi: 10.1128/aac.33.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh TJ, Lee J, Aoki S, Mechinaud F, Bacher J, Lecciones J, Thomas V, Rubin M, Pizzo PA. 1990. Experimental basis for use of fluconazole for preventive or early treatment of disseminated candidiasis in granulocytopenic hosts. Rev Infect Dis 12(Suppl 3):S307–S317. doi: 10.1093/clinids/12.supplement_3.s307. [DOI] [PubMed] [Google Scholar]
- 17.Groll AH, Giri N, Petraitis V, Petraitiene R, Candelario M, Bacher JS, Piscitelli SC, Walsh TJ. 2000. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J Infect Dis 182:274–282. doi: 10.1086/315643. [DOI] [PubMed] [Google Scholar]
- 18.Pyrgos V, Mickiene D, Sein T, Cotton M, Fransesconi A, Mizrahi I, Donoghue M, Bundrant N, Kim SY, Hardwick M, Shoham S, Walsh TJ. 2010. Effects of immunomodulatory and organism-associated molecules on the permeability of an in vitro blood-brain barrier model to amphotericin B and fluconazole. Antimicrob Agents Chemother 54:1305–1310. doi: 10.1128/AAC.01263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petraitis V, Petraitiene R, Valdez JM, Pyrgos V, Lizak MJ, Klaunberg BA, Kalasauskas D, Basevicius A, Bacher JD, Benjamin DK, Jr, Hope WW, Walsh TJ. 2019. Amphotericin B penetrates into the central nervous system through focal disruption of the blood-brain barrier in experimental hematogenous Candida meningoencephalitis. Antimicrob Agents Chemother 63:e01626-19. doi: 10.1128/AAC.01626-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denning DW. 2003. Echinocandin antifungal drugs. Lancet 362:1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 21.Gauthier GM, Nork TM, Prince R, Andes D. 2005. Subtherapeutic ocular penetration of caspofungin and associated treatment failure in Candida albicans endophthalmitis. Clin Infect Dis 41:e27–e28. doi: 10.1086/431761. [DOI] [PubMed] [Google Scholar]
- 22.Mochizuki K, Murase H, Yasuda Y, Suematsu H, Yamagishi Y, Mikamo H. 2012. Discrepancy of in-vitro data and clinical efficacy of micafungin against Candida tropicalis endophthalmitis. J Infect Chemother 18:786–789. doi: 10.1007/s10156-012-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mochizuki K, Sawada A, Suemori S, Kawakami H, Niwa Y, Kondo Y, Ohkusu K, Yamada N, Ogura S, Yaguchi T, Nishimura K, Kishino S. 2013. Intraocular penetration of intravenous micafungin in inflamed human eyes. Antimicrob Agents Chemother 57:4027–4030. doi: 10.1128/AAC.02300-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe NA, Miyazaki M, Horii T, Sagane K, Tsukahara K, Hata K. 2012. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob Agents Chemother 56:960–971. doi: 10.1128/AAC.00731-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osherov N, Kontoyiannis DP. 2017. The anti-Aspergillus drug pipeline: is the glass half full or empty? Med Mycol 55:118–124. doi: 10.1093/mmy/myw060. [DOI] [PubMed] [Google Scholar]
- 26.Hata K, Horii T, Miyazaki M, Watanabe NA, Okubo M, Sonoda J, Nakamoto K, Tanaka K, Shirotori S, Murai N, Inoue S, Matsukura M, Abe S, Yoshimatsu K, Asada M. 2011. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob Agents Chemother 55:4543–4551. doi: 10.1128/AAC.00366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazaki M, Horii T, Hata K, Watanabe NA, Nakamoto K, Tanaka K, Shirotori S, Murai N, Inoue S, Matsukura M, Abe S, Yoshimatsu K, Asada M. 2011. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob Agents Chemother 55:4652–4658. doi: 10.1128/AAC.00291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gebremariam T, Alkhazraji S, Alqarihi A, Jeon HH, Gu Y, Kapoor M, Shaw KJ, Ibrahim AS. 2018. APX001 is effective in the treatment of murine invasive pulmonary aspergillosis. Antimicrob Agents Chemother 63:e01713-18. doi: 10.1128/AAC.01713-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gebremariam T, Alkhazraji S, Alqarihi A, Wiederhold NP, Shaw KJ, Patterson TF, Filler SG, Ibrahim AS. 2020. Fosmanogepix (APX001) is effective in the treatment of pulmonary murine mucormycosis due to Rhizopus arrhizus. Antimicrob Agents Chemother 64:e00178-20. doi: 10.1128/AAC.00178-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alkhazraji S, Gebremariam T, Alqarihi A, Gu Y, Mamouei Z, Singh S, Wiederhold NP, Shaw KJ, Ibrahim AS. 2019. Fosmanogepix (APX001) is effective in the treatment of immunocompromised mice infected with invasive pulmonary scedosporiosis or disseminated fusariosis. Antimicrob Agents Chemother 64:e01735-19. doi: 10.1128/AAC.01735-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiederhold NP, Najvar LK, Shaw KJ, Jaramillo R, Patterson H, Olivo M, Catano G, Patterson TF. 2019. Efficacy of delayed therapy with fosmanogepix (APX001) in a murine model of Candida auris invasive candidiasis. Antimicrob Agents Chemother 63:e01120-19. doi: 10.1128/AAC.01120-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hager CL, Larkin EL, Long L, Zohra Abidi F, Shaw KJ, Ghannoum MA. 2018. In vitro and in vivo evaluation of the antifungal activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother 62:e02319-17. doi: 10.1128/AAC.02319-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mansbach RSSK, Hodges MR, Coleman S, Fitzsimmons ME. 2017. Absorption, distribution, and excretion of [14C]-APX001 after single-dose administration to rats and monkeys, abstr 1513. IDWeek 2017, San Diego, CA. [Google Scholar]
- 34.Pickering JW, Sant HW, Bowles CA, Roberts WL, Woods GL. 2005. Evaluation of a (1->3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol 43:5957–5962. doi: 10.1128/JCM.43.12.5957-5962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petraitiene R, Petraitis V, Hope WW, Mickiene D, Kelaher AM, Murray HA, Mya-San C, Hughes JE, Cotton MP, Bacher J, Walsh TJ. 2008. Cerebrospinal fluid and plasma (1–>3)-beta-D-glucan as surrogate markers for detection and monitoring of therapeutic response in experimental hematogenous Candida meningoencephalitis. Antimicrob Agents Chemother 52:4121–4129. doi: 10.1128/AAC.00674-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvatore CM, Chen TK, Toussi SS, DeLaMora P, Petraitiene R, Finkelman MA, Walsh TJ. 2016. (1–>3)-beta-d-glucan in cerebrospinal fluid as a biomarker for Candida and Aspergillus infections of the central nervous system in pediatric patients. J Ped Infect Dis 5:277–286. doi: 10.1093/jpids/piv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao M, Lepak AJ, VanScoy B, Bader JC, Marchillo K, Vanhecker J, Ambrose PG, Andes DR. 2018. In vivo pharmacokinetics and pharmacodynamics of APX001 against Candida spp. in a neutropenic disseminated candidiasis mouse model. Antimicrob Agents Chemother 62:e02542-17. doi: 10.1128/AAC.02542-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Lee MH, Paderu P, Lee A, Jimenez-Ortigosa C, Park S, Mansbach RS, Shaw KJ, Perlin DS. 2018. Significantly improved pharmacokinetics enhances in vivo efficacy of APX001 against echinocandin- and multidrug-resistant Candida isolates in a mouse model of invasive candidiasis. Antimicrob Agents Chemother 62:e00425-18. doi: 10.1128/AAC.00425-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viriyakosol S, Kapoor M, Okamoto S, Covel J, Soltow QA, Trzoss M, Shaw KJ, Fierer J. 2018. APX001 and other Gwt1 inhibitor prodrugs are effective in experimental Coccidioides immitis pneumonia. Antimicrob Agents Chemother 63:e01715-18. doi: 10.1128/AAC.01715-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodges MROE, Shaw KJ, Mansbach RS, van Marle S, van Hoogdalem E, Kramer W, Wedel P. 2017. Phase 1 study to assess safety, tolerability and pharmacokinetics of single and multiple oral doses of APX001 and to investigate the effect of food on APX001 bioavailability. Poster 1860. Abstr IDWeek 2017, San Diego, CA. [Google Scholar]
- 41.Rhein J, Bahr NC, Morawski BM, Schutz C, Zhang Y, Finkelman M, Meya DB, Meintjes G, Boulware DR. 2014. Detection of high cerebrospinal fluid levels of (1–>3)-beta-d-glucan in cryptococcal meningitis. Open Forum Infect Dis 1:ofu105. doi: 10.1093/ofid/ofu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen TK, Groncy PK, Javahery R, Chai RY, Nagpala P, Finkelman M, Petraitiene R, Walsh TJ. 2017. Successful treatment of Aspergillus ventriculitis through voriconazole adaptive pharmacotherapy, immunomodulation, and therapeutic monitoring of cerebrospinal fluid (1–>3)-beta-D-glucan. Med Mycol 55:109–117. doi: 10.1093/mmy/myw118. [DOI] [PubMed] [Google Scholar]
- 43.Litvintseva AP, Lindsley MD, Gade L, Smith R, Chiller T, Lyons JL, Thakur KT, Zhang SX, Grgurich DE, Kerkering TM, Brandt ME, Park BJ. 2014. Utility of (1–3)-beta-D-glucan testing for diagnostics and monitoring response to treatment during the multistate outbreak of fungal meningitis and other infections. Clin Infect Dis 58:622–630. doi: 10.1093/cid/cit808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petraitiene R, Petraitis V, Maung BW, Naing E, Kavaliauskas P, Walsh TJ. 2020. Posaconazole alone and in combination with caspofungin for treatment of experimental Exserohilum rostratum meningoencephalitis: developing new strategies for treatment of phaeohyphomycosis of the central nervous system. J Fungi (Basel) 6:33. doi: 10.3390/jof6010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hope WW, Mickiene D, Petraitis V, Petraitiene R, Kelaher AM, Hughes JE, Cotton MP, Bacher J, Keirns JJ, Buell D, Heresi G, Benjamin DK, Jr, Groll AH, Drusano GL, Walsh TJ. 2008. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J Infect Dis 197:163–171. doi: 10.1086/524063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benjamin DK, Jr, Kaufman DA, Hope WW, Smith PB, Arrieta A, Manzoni P, Kovanda LL, Lademacher C, Isaacson B, Jednachowski D, Wu C, Kaibara A, Walsh TJ. 2018. A phase 3 study of micafungin versus amphotericin B deoxycholate in infants with invasive candidiasis. Pediatr Infect Dis J 37:992–998. doi: 10.1097/INF.0000000000001996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovanda LL, Walsh TJ, Benjamin DK, Jr, Arrieta A, Kaufman DA, Smith PB, Manzoni P, Desai AV, Kaibara A, Bonate PL, Hope WW. 2018. Exposure-response analysis of micafungin in neonatal candidiasis: pooled analysis of two clinical trials. Pediatr Infect Dis J 37:580–585. doi: 10.1097/INF.0000000000001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spellberg B, Ibrahim AS, Edwards JE, Jr, Filler SG. 2005. Mice with disseminated candidiasis die of progressive sepsis. J Infect Dis 192:336–343. doi: 10.1086/430952. [DOI] [PubMed] [Google Scholar]
- 49.Leelahavanichkul A, Somparn P, Bootprapan T, Tu H, Tangtanatakul P, Nuengjumnong R, Worasilchai N, Tiranathanagul K, Eiam-Ong S, Levine M, Chinampon A, Srisawat N. 2015. High-dose ascorbate with low-dose amphotericin B attenuates severity of disease in a model of the reappearance of candidemia during sepsis in the mouse. Am J Physiol Regul Integr Comp Physiol 309:R223–R234. doi: 10.1152/ajpregu.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petraitiene R, Petraitis V, Groll AH, Candelario M, Sein T, Bell A, Lyman CA, McMillian CL, Bacher J, Walsh TJ. 1999. Antifungal activity of LY303366, a novel echinocandin B, in experimental disseminated candidiasis in rabbits. Antimicrob Agents Chemother 43:2148–2155. doi: 10.1128/AAC.43.9.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Groll AH, Petraitis V, Petraitiene R, Field-Ridley A, Calendario M, Bacher J, Piscitelli SC, Walsh TJ. 1999. Safety and efficacy of multilamellar liposomal nystatin against disseminated candidiasis in persistently neutropenic rabbits. Antimicrob Agents Chemother 43:2463–2467. doi: 10.1128/AAC.43.10.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington (DC). doi: 10.17226/12910. [DOI] [Google Scholar]
- 53.Walsh TJ, Bacher J, Pizzo PA. 1988. Chronic silastic central venous catheterization for induction, maintenance and support of persistent granulocytopenia in rabbits. Lab Anim Sci 38:467–471. [PubMed] [Google Scholar]
- 54.Wasan KM, Vadiei K, Lopez-Berestein G, Luke DR. 1990. Pharmacokinetics, tissue distribution, and toxicity of free and liposomal amphotericin B in diabetic rats. J Infect Dis 161:562–566. doi: 10.1093/infdis/161.3.562. [DOI] [PubMed] [Google Scholar]