Ganciclovir is indicated for curative or preventive treatment of cytomegalovirus (CMV) infections. This study aimed to characterize ganciclovir pharmacokinetics, following intravenous ganciclovir and oral valganciclovir administration, to optimize dosing schemes.
KEYWORDS: antiviral drug, children, population pharmacokinetics
ABSTRACT
Ganciclovir is indicated for curative or preventive treatment of cytomegalovirus (CMV) infections. This study aimed to characterize ganciclovir pharmacokinetics, following intravenous ganciclovir and oral valganciclovir administration, to optimize dosing schemes. All children aged <18 years receiving ganciclovir or valganciclovir were included in this study. Pharmacokinetics were described using nonlinear mixed-effect modeling. Monte Carlo simulations were used to optimize the dosing regimen to maintain the area under the concentration-time curve (AUC) in the preventive or therapeutic target. Among the 105 children (374 concentration-time observations) included, 78 received intravenous (i.v.) ganciclovir, 19 received oral valganciclovir, and 6 received both drugs. A two-compartment model with first-order absorption for valganciclovir and first-order elimination best described the data. An allometric model was used to describe the bodyweight (BW) effect. Estimated glomerular filtration rate (eGFR) and medical status of critically ill children were significantly associated with ganciclovir elimination. Recommended doses were adapted for prophylactic treatment. To obtain a therapeutic exposure, doses should be increased to 40 mg/kg of body weight/day oral or 15 to 20 mg/kg/day i.v. in children with normal eGFR and to 56 mg/kg/day oral or 20 to 25 mg/kg/day i.v. in children with augmented eGFR. These doses should be prospectively confirmed, and therapeutic drug monitoring could be used to refine them individually. (This study has been registered at ClinicalTrials.gov under identifier NCT02539407.)
TEXT
Intravenous (i.v.) ganciclovir is indicated for curative or preventive treatment of cytomegalovirus (CMV) infections in immunocompromised patients (e.g., following an organ transplant or chemotherapy). The oral form valganciclovir is indicated for the prophylactic treatment of CMV infections after a solid-organ transplant and for the prevention of CMV reactivation in CMV+ patients (1, 2). The recommended ganciclovir doses are either 5 mg/kg of body weight every 12 h in curative treatment or 5 mg/kg/day in preventive treatment. Dose should be reduced by half for creatinine clearance between 50 and 69 ml/min, to a quarter for creatinine clearance between 25 and 49 ml/min, and divided by eight in patients with creatinine clearance between 10 and 24 ml/min. Oral doses of valganciclovir, the prodrug of ganciclovir, are recommended using the Pescovitz algorithm (3) based on body surface area (BSA) and creatinine clearance (CLCR).
Following oral administration, valganciclovir, ganciclovir’s prodrug, is rapidly and extensively metabolized to ganciclovir by intestinal and hepatic esterases. The absolute bioavailability is 60% after administration of food. Ganciclovir distributes with a steady-state volume of distribution from 0.54 to 0.87 liters/kg. Binding to plasma proteins is weak, between 1 and 2% for ganciclovir concentrations of 0.5 to 51 μg/ml. Ganciclovir is primarily eliminated by renal excretion after glomerular filtration and active tubular secretion of unchanged ganciclovir. In patients with normal renal function, more than 90% of the intravenous dose of ganciclovir is recovered as unchanged drug in the urine within 24 h of administration (2).
Relationships between concentrations and effects of ganciclovir have been shown in adults. They suggested an area under the concentration-time curve from 0 to 24 h (AUC0–24) or 0 to 12 h (AUC0–12) between 40 and 60 mg/liter·h to prevent or cure CMV infections, respectively (5).
However, few pharmacokinetics studies have been conducted in children, especially for ganciclovir as a curative treatment. These studies showed high variability and possible underdosing (6–8). Our study aimed to characterize ganciclovir pharmacokinetics (PK) following ganciclovir and valganciclovir administration with a population PK approach to identify relevant covariates that could explain interindividual variability (IIV). The final goal was to assess current dose recommendations for children and to suggest appropriate dosing for optimal exposure.
RESULTS
Plasma concentration data.
In total, 105 patients were included, with 374 ganciclovir plasma concentrations (1 to 18 samples per patient). Among the 105 children, 58 were from the pediatric immune-hematology unit (IHU), 29 were from pediatric intensive care unit (PICU), 2 were from cardiology, 8 were from pediatric hepatology, 1 was from pediatric pneumology, 1 was from pediatric nephrology, and 6 were from general pediatrics departments of Necker-Enfants Malades Hospital (Paris, France). Table 1 summarizes the patient characteristics. Construction of the model was made with the first 70 patients included, and validation was with the 35 following patients.
TABLE 1.
Characteristics of the population
| Characteristic, median (range) | Value by dataset |
||
|---|---|---|---|
| Building | Validation | Overall | |
| No. of patients (sampling) | 70 (275) | 35 (99) | 105 (374) |
| Administration route (p.o./i.v./i.v. + p.o.) | 13/51/6 | 6/27/0 | 19/78/6 |
| Oral dose (mg/kg/day) | 41 (14.6–83.8) | 32.4 (18.1–66.7) | 36 (14.6–83.8) |
| i.v. dose (mg/kg/day) | 10 (2.4–14.8) | 10 (1.2–15.4) | 10 (1.2–15.4) |
| No. of administrations/day (1/2/3/4) | 8/56/3/3 | 4/30/0/1 | 12/86/3/4 |
| Sex (no. male/female) | 40/30 | 19/16 | 59/46 |
| Age at inclusion (yr) | 2.3 (0.2–17.3) | 3.0 (0.01–16.0) | 2.5 (0.01–17.3) |
| No. aged 0–1 yr/1–12 yr/12–18 yr | 18/45/7 | 13/18/4 | 31/63/11 |
| Body weight (kg) | 11.3 (3.8–80) | 14.8 (2.6–49) | 11.7 (2.6–80) |
| Height (cm) | 85 (50–178) | 92 (47–165) | 85 (47–178) |
| Serum creatinine (µmol·liter-1) | 22 (10–159) | 26 (5–83) | 22 (5–159) |
| eGFR (ml/min/1.73m²) | 166 (43–392) | 172 (61–425) | 167 (43–425) |
Model building.
A data set with 70 patients (275 samples) allowed us to build the model. A two-compartment model with first-order absorption and first-order elimination best described oral and i.v. ganciclovir concentrations (see Fig. S1 in the supplemental material). Parameters of the model were bioavailability (F), absorption rate constant (Ka), volumes of distribution of central and peripheral compartments (V2 and V3), clearance (CL), and intercompartmental clearance (Q). Twenty-seven (10%) ganciclovir concentrations were lower than the lower limit of quantification (LOQ). These concentrations were kept in the data set and left-censored in the analysis. Data were not sufficient to estimate IIV for F, ka, Q, and V3, and fixing the variance of these random effects to zero had no influence on the objective function value (OFV). The residual variability was best described by a proportional error model. Adding the effect of body surface area (BSA) on CL decreased the OFV by 37 points, and the addition of the allometric model decreased the OFV by 71 U. The allometric model, using a median BW of 11.7 kg, was added on the structural model. The effect of estimated glomerular filtration rate (eGFR) on CL (median eGFR, 174 ml/min/1.73 m2) decreased the OFV by 102 U and the IIV from 0.43 to 0.24. The addition of critically ill medical status (children hospitalized in PICU) on CL decreased OFV by 8 U and IIV from 0.24 to 0.222. The effect of other covariates was not statistically significant. PK parameter estimates are summarized in Table 2.
TABLE 2.
Pharmacokinetic parameters for a BW of 11.7 kg in all children, with an eGFR of 167 ml/min/1.73 m2
| Fixed population effect | Estimate (% RSE) by dataset |
|
|---|---|---|
| Building | Building and validation | |
| Ka (h−1) | 0.55 (15) | 0.506 (12) |
| CL (liters· h−1) | 2.92 (6) | 2.55 (6) |
| V2 (liters) | 6.11 (11) | 5.96 (8) |
| Q (liters·h−1) | 0.484 (44) | 0.222 (38) |
| V3 (liters) | 1.75 (20) | 1.29 (19) |
| F | 0.444 (11) | 0.438 (11) |
| Effect of eGFR on CLa | 0.839 (8) | 0.763 (7) |
| Effect of critically ill on CLa | 0.763 (6) | 0.806 (6) |
| IIV | ||
| ωCL | 0.23 (13) | 0.236 (11) |
| ωV | 0.236 (46) | 0.22 (40) |
| Residual variability | ||
| Residual proportional error | 0.457 (5) | 0.477 (4) |
The equations for the final model were , with critically ill equal to 0 or 1, , , and .
Model assessment: validation on the remaining third of the data.
Graphically, the model showed good adequacy between predicted concentrations and observed concentrations (Fig. S2). Mean prediction error (MPE), root mean squared prediction error (RMSE), and mean absolute prediction error (MAE) were −0.41 mg/liter, 1.95 mg/liter, and 1.00 mg/liter, respectively.
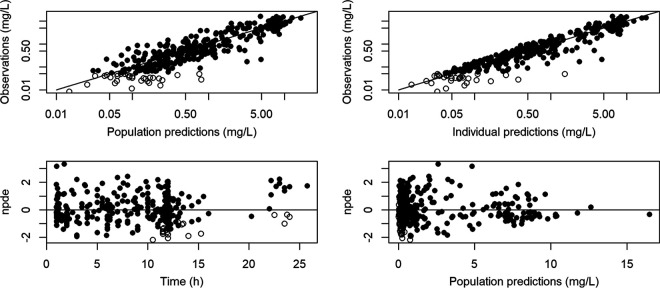
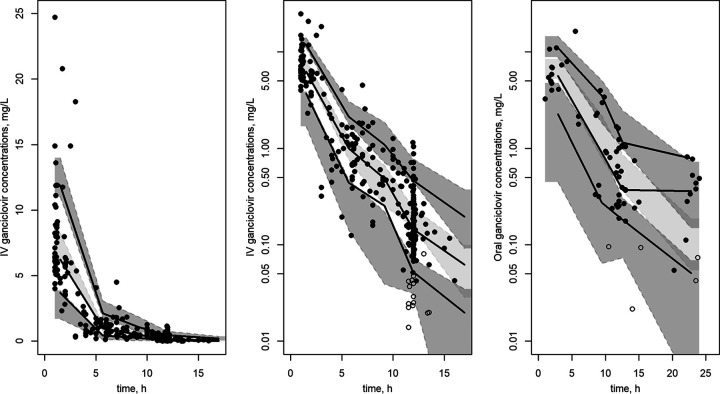
Thus, building and validation data sets could be modeled together (Fig. S3). Final PK parameters are summarized in Table 2, and measured concentrations for the full data set as a function of time are represented in Fig. S3. Diagnostic plots from the final model are shown in Fig. 1. Prediction-corrected visual predictive check (pc-VPC) of the final population PK model showed the comparison between the 5th, 50th, and 95th predicted percentiles for the 1,000 simulations and the observed concentrations of ganciclovir for oral and i.v. administration (Fig. 2).
FIG 1.
Observation data versus population predictions (upper left) and versus individual predictions (upper right) in the final model, noncensored data (full circle), and censored data (empty circle), represented with a log-log scale. The black line is the identity line. Normalized prediction distribution error versus time (bottom left) and predictions (bottom right). The horizontal line is the theoretical mean (0).
FIG 2.
Prediction-corrected visual predictive check for i.v. (linear scale on the left and log-linear scale in the middle) and oral (right) administration. Colored areas represent 95% CIs of 5th, 50th, and 95th simulated percentiles. Lines are empirical (observed) 5th, 50th, and 95th percentiles. Dots are observed data. Full and empty circles are the noncensored and censored data, respectively.
Subpopulation definition.
The final model was used to define different subpopulations according to the covariates included in the model (BW and eGFR). Three groups were distinguished: children with low eGFR (eGFR < 70 ml/min/1.73 m2), children with medium eGFR (70 ≤ eGFR < 200 ml/min/1.73 m2), and children with high eGFR (eGFR ≥ 200 ml/min/1.73 m2).
Plasma concentration simulation.
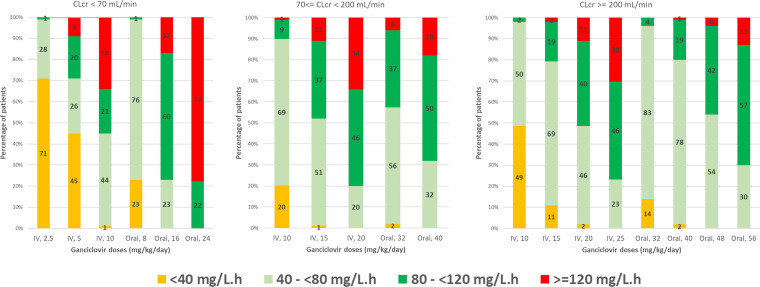
Several i.v. and oral dosing regimens were simulated, and the percentages of patients with ganciclovir AUC0–24 (i) with insufficient exposure (below 40 mg/liter·h), (ii) preventive exposure (between 40 and 80 mg/liter·h), (iii) curative exposure (between 80 and 120 mg/liter·h), and (iv) exposure at risk of toxicity (above 120 mg/liter·h) were reported in Fig. 3. These percentages were also estimated after a simulation of an oral administration of doses calculated with Pescovitz et al. (3), Åsberg et al. (8), and Villeneuve et al. (9) algorithms (Table 3).
FIG 3.
Percentage of patients with an AUC0–24 below 40 mg/liter·h, between 40 and 80 mg/liter·h, between 80 and 120 mg/liter·h, and above 120 mg/liter·h after 2.5- to 25-mg/kg/day i.v. dose and 8- to 56-mg/kg/day oral dose according to eGFR.
TABLE 3.
Percentage of patients with an AUC0–24 of <40, from 40 to 80, from 80 to 120, and >120 mg/liter·h following oral administrations of doses calculated with published algorithms
| Dose according to algorithm | % of patients with an AUC0–24 (mg/liter·h) of: |
|||
|---|---|---|---|---|
| <40 | 40–80 | 80–120 | ≥120 | |
| Pescovitz et al. dose algorithm (3) | 0 | 12 | 55 | 33 |
| Åsberg et al. dose algorithm (8) (applying the proposed 20% increase) | 13 | 60 | 13 | 14 |
| Villeneuve et al. dose algorithm (9) (interval for 28–32 mg/kg/day) | <1 | 53–61 | 20–30 | 11–14 |
| Our oral proposed doses: 16, 40, and 56 mg/kg/day for low, medium, and high eGFR (maximum, 1,800 mg daily) | 0 | 23–32 | 50–60 | 13–18 |
DISCUSSION
The pharmacokinetics of ganciclovir have been described with a 2-compartment model with first-order absorption for the oral route and first-order elimination. This structural model was consistent with previous analysis in children and adults (10–12). In our study, PK parameters were comparable to those previously reported. Clearance and volume of distribution were 0.22 liters/h/kg and 0.51 liters/kg, respectively, compared to 0.19 liters/h/kg and 0.38 liters/kg in Åsberg et al. (8), 0.21 liters/h/kg and 1.25 liters/kg in Vezina et al. (12), and 0.24 liters/h/kg and 0.23 liters/kg in Pescovitz et al. (3).
An allometric model was used to represent the physiological process of child development. The effects of both BW and BSA were tested, as evidenced previously (3, 10, 12), but the effect of BW was much more significant than that of BSA in our population. No additional effect of age was evidenced, although 31 children were younger than 1 year at inclusion. However, an age effect (<2 years versus 2 to 13 years) has indirectly been included on ganciclovir clearance through the inclusion of eGFR by the Schwartz formula.
The effect of eGFR on ganciclovir CL is easily explained, since ganciclovir is eliminated via the kidneys. This effect has already been reported in previous studies in children (3, 8, 10). Renal clearance was described by eGFR using the Schwartz formula (13). However, this formula does not take into account gender, muscle mass, nutrition/hydration status, or liver dysfunction and could under- or overestimate GFR. An effect of critically ill children was found to decrease elimination clearance by 20% in our model. This effect was found whatever the level of eGFR. We attributed it to an overestimation of eGFR with the Schwartz formula for critically ill children, leading to an overestimation of ganciclovir clearance. The critically ill effect, which decreases ganciclovir clearance, allows us to correct this overestimation. A more precise approach would be to use creatinine clearance (CLCR), calculated with urine (Ucr) and plasmatic (Scr) creatinine concentrations. Unfortunately, data on Ucr and urine output were not available for most of our patients (14).
On the one hand, it has been reported that in prophylactic treatment, an AUC0–24 of 50 mg/liter·h led to an incidence of viremia of 1.3%, whereas an AUC0–24 of 25 mg/liter·h was associated with an 8-fold higher risk of viremia. On the other hand, it has been shown that an AUC0–24 of >60 mg/liter·h corresponded to an incidence of leukopenia and neutropenia of 20% and 50%, respectively. Thus, for therapeutic drug monitoring, the target exposure is between 40 and 60 mg/liter·h, being AUC0–24 for prophylaxis and AUC0–12 for treatment of active disease (5). In our simulations, four windows of ganciclovir AUC0–24 were determined: below 40 mg/liter·h (underexposure), between 40 and 80 mg/liter·h (preventive range), between 80 and 120 mg/liter·h (curative range), and above 120 mg/liter·h (at risk of toxicity).
Three algorithms were developed to predict appropriate valganciclovir pediatric-specific doses, two of which implemented the eGFR. Pescovitz et al. (3) proposed the equation dose (mg/day) = 7 × BSA (m2) × CLCR (ml/min/1.73 m2), with CLCR calculated with the Schwartz formula and BSA as the square root of height (HT) (cm) × BW (kg)/3,600. This algorithm is implemented in various recommendations (1). This algorithm has been developed in 26 children with renal transplantation, with a mean ± standard deviation (SD) CLCR of 109.9 ± 43.6 ml/min and 20 children with liver transplantation with a mean ± SD CLCR of 153.4 ± 75.3 ml/min. Applying this formula for dose calculation to our population, our PK model predicted that 0%, 12%, 55%, and 33% of children had an AUC0–24 of <40 mg/liter·h, 40 to 80 mg/liter·h, 80 to 120 mg/liter·h, and >120 mg/liter·h, respectively. With a mean CLCR in our population of 175.5 ± 66.9 ml/min/1.73 m2, the impact of a linear relationship of CLCR on predicted dose seemed too important for our population, leading to a third of the patients having very high exposure. More recently, Åsberg et al. (8) evaluated valganciclovir PK among 104 pediatric solid-organ transplant recipients with median (range) GFR of 112 (46 to 212) ml/min and BW of 25.9 (5.8 to 89.4) kg, using BW instead of BSA and reducing the impact of GFR in this new dosing algorithm: dose (mg) = WT (kg) × [0.07 × GFR (ml/min) + k], where k = 5 for GFR ≤ 30 ml/min, k = 10 for GFR > 30 ml/min, and WT > 30 kg and k = 15 for GFR > 30 ml/min and WT ≤ 30 kg. GFR was estimated with the Cockroft-Gault formula. However, the percentage of children achieving the target AUC0–24 of 80 to 120 mg/liter·h was low; thus, the authors suggested that this equation should be multiplied by 1.2 for all age and GFR ranges. This last equation (with addition of coefficient 1.2) was tested in our population, and 13%, 60%, 13%, and 14% had an AUC0–24 of <40, 40 to 80, 80 to 120, and >120 mg/liter·h, respectively. Those levels would be sufficient for prophylaxis but too low for therapy. Villeneuve et al. (9) used a simple weight-based regimen of 14 to 16 mg/kg twice a day. In our population, <1%, 53 to 61%, 20 to 30%, and 11 to 14% of the children had an AUC0–24 of <40, 40 to 80, 80 to 120, and >120 mg/liter·h, respectively. Again, this regimen could be used for prophylaxis but would be insufficient for curative treatment.
In our simulations, 3 groups of patients were constituted, according to their eGFR: <70, 70 to <200, and ≥200 ml/min/1.73 m2. Increased creatinine clearance is frequently observed in critically ill patients (16% to 80% of patients with creatinine clearance of >130 ml/min/1.73 m2) (15, 16) and could lead to underexposure for many drugs, such as antibiotics (17). With oral administration, only 2 children had an eGFR of <70 ml/min/1.73 m2, and 16 mg/kg daily would be suitable for them. A dose of 32 mg/kg/day would be adapted for prophylaxis in children with an eGFR of ≥70 ml/min/1.73 m2. For curative treatment, 40 mg/kg/day in children with eGFR from 70 to <200 ml/min/1.73 m2 and 56 mg/kg/day with eGFR of ≥200 ml/min/1.73 m2 should be prescribed. All of these doses were limited to a daily amount of 1,800 mg. Using our simulations instead of the Pescovitz formula, 13 to 18% of the children versus 33% would be at risk of toxicity, and 23 to 32% of the children versus 12% would attain the preventive target.
Pharmacokinetic data of i.v. ganciclovir in children are sparse. Pescovitz et al. (3) concluded that patients <5 years of age had AUC values approximately 50% lower than those of older age ranges (around 22 versus 39 mg/liter·h). Launay et al. (6) reported data from 10 children with a median age of 5.2 years, receiving 10 mg/kg/day; median daily exposure was 22.9 mg/liter·h. In our cohort (median age of 2.5 years), we also found that exposures were low.
According to our simulations, for the 3 patients with i.v. administration with an eGFR of <70 ml/min/1.73 m2 (one at 43 ml/min/1.73 m2), a half dose of 5 mg/kg/day would lead to low exposure, and an increase could be needed to reach prophylactic and therapeutic targets. For patients with 70 ≤ eGFR <200 ml/min/1.73 m2, the ganciclovir i.v. recommended dose of 10 mg/kg/day produced prophylactic exposure in 67% of children and curative exposure in 9% of them. An increased dose to 15 or 20 mg/kg would allow 37 and 46% of children to be in the therapeutic range. For children with eGFR of ≥200 ml/min/1.73 m2, a 10-mg/kg dose is insufficient, an i.v. 15-mg/kg/day dose seems sufficient for prevention, and an i.v. dose of 20 to 25 mg/kg/day should be more adequate for curative purposes.
Jorga et al. (18, 19) proposed an adaptation of the Pescovitz algorithm for i.v. administration: dose (mg/day) = 3 × BSA (m2) × CLCR (ml/min/m2). In our population, 0%, 12%, 50%, and 38% of children had an AUC0–24 of <40, 40 to 80, 80 to 120, and >120 mg/liter·h. As for oral administration, the impact of a linear relationship of CLCR on the dose seemed too important for our population, and this algorithm would lead to an overdosage in 38% of the children.
Our study has limitations. No information was reported on CMV viral load or related toxicity effects. The active phosphorylated form was not measured; however, relationships were reported between effect (efficacy and toxicity) and exposure in plasma. Only 6 children received both i.v. and oral doses. The estimated bioavailability, which is lower than that in previous studies (44% versus 55% [3] and 59% [8]), might have been slightly underestimated, although both i.v. and oral concentrations seemed correctly described. Only 2 children had an eGFR of <70 ml/min/1.73 m2; thus, we were not able to propose an oral dose for these patients, and the proposed oral dose for 70 < eGFR ≤ 200 ml/min/1.73 m2 may need to be refined for children with an eGFR of around 70 to 80 ml/min/1.73 m2.
Our dosing regimen propositions need to be confirmed prospectively. Estimated GFR is an important covariate and is susceptible to increase or decrease quickly in critically ill patients; thus, therapeutic drug monitoring, combining drug measurement with Bayesian estimation of the area under the curve from a population model, could become an everyday tool to adapt the dose individually.
Conclusions.
This study described ganciclovir and valganciclovir PK in children and showed the impact of bodyweight and eGFR on drug clearance. Simulations from modeling suggested that the recommended dose is adequate for prophylaxis, but doses should be increased for curative treatment in children with normal and high eGFR.
MATERIALS AND METHODS
Patients.
This prospective study was conducted in several pediatric units: the intensive care unit, immune-hematology, cardiology, pneumology nephrology, and general pediatric departments of Necker-Enfants Malades Hospital (Paris, France). All children, aged less than 18 years old and weighing more than 2.5 kg, receiving i.v. ganciclovir or oral valganciclovir were included. These inclusions took part in the Optimome study, which was approved by the Ethics Committee of the Necker Enfants-Malades Hospital and registered at www.clinicaltrials.gov (NCT02539407). Prior to inclusion, oral consent was obtained from the patient’s legal representative after oral and written information. Patients with hemofiltration or extracorporeal membrane oxygenation were excluded.
Baseline patient characteristics were recorded, including sex, age, bodyweight (BW), height (HT), body surface area (BSA), creatinine level, and indication for drug administration. Estimated glomerular filtration rate (eGFR; ml/min/1.73 m2) was calculated with the Schwartz formula (13) as , where KCR is a coefficient equal to 0.45 for ages of <2 years and BW of ≥2.5 kg, 0.55 for ages of ≥2 and <13 years and for females aged ≥13 years, and 0.7 for males aged ≥13 years. Critically ill medical status was defined as a binary covariate coded 1 for children admitted to the critical care unit and 0 for other children.
Drug administration and blood sampling.
The prescribed doses referred to medical child history (preventive or curative treatment), the disease, local protocols, and the route of administration. In most cases, ganciclovir and valganciclovir were administered twice daily but were sometimes given every 6, 8, or 24 h. Blood samples were collected during routine laboratory testing regardless of the delay since the administration. Median (range) daily doses were 10 (1.2 to 15.4) mg/kg/day for i.v. ganciclovir and 36 (14.6 to 83.8) mg/kg/day for oral valganciclovir. The median delay between drug intake and sample collection was 11 h, and the interquartile range was 5 to 12 h.
Assay.
Ganciclovir concentrations in plasma samples were measured using a high-performance liquid chromatography tandem mass spectrometry method. Samples were centrifuged (4,000 rpm, 5 min) to yield plasma and then stored at –20°C before analysis. The analysis was performed using a TSQ quantum discovery max chromatographic system (Fisher Scientific, Les Ulis, France). A volume of 100 μl of plasma samples was precipitated with 500 μl of acetonitrile. The supernatants were evaporated to dryness under a 40°C nitrogen flow. The residues were then reconstituted in 500 μl of water, and a volume of 10 μl was injected into the chromatographic system. The chromatographic separation was carried out on an Atlantis T3 C18 column (Waters, Saint-Quentin, France) using a mobile phase composed of water (0.05%, vol/vol, formic acid) and methanol (0.05%, vol/vol, formic acid). The method was fully validated according to Food and Drug Administration guidelines for validation of bioanalytical assays (20). The calibration of ganciclovir was linear over the range of 0.05 to 16 µg/ml. The lower limit of quantification (LOQ) was 0.05 µg/ml, determined based on the signal-to-noise ratio, which was at least 10:1, and checking that the bias and coefficient of variation of LOQ did not exceed 20% during the analysis of six different samples.
Population pharmacokinetics.
Data were analyzed using the nonlinear mixed-effect modeling software Monolix 2018R1 (version 2018R1; https://lixoft.com). Parameters were estimated by computing the maximum likelihood estimator of the parameters using the stochastic approximation expectation maximization (SAEM) algorithm combined with a Markov chain Monte Carlo (MCMC) procedure. The number of MCMC chains was fixed to 5 for all estimations. The data set was divided into two parts: two-thirds of the children were used to build the model, and one-third was used for the validation of the model. Finally, all data were analyzed simultaneously to perform dose simulations. To model simultaneously ganciclovir and valganciclovir PK, doses and concentrations were converted to molarity, e.g., dividing mass concentrations by the molar mass of each compound. In Monolix, the extension of the SAEM algorithm performed a simulation of the left-censored data in a right-truncated Gaussian distribution with an integration below the limit of quantification (BLOQ) to obtain the probability of BLOQ (21). Analytical solutions were used to code i.v. and oral routes simultaneously. Numerical and graphical outputs were obtained with R software (version 3.6.1). Simulations were performed with NONMEM 7.4. The area under the concentration-time curve and the percentages of patients in each interval were calculated using R and represented with Excel.
(i) Structural model building.
One- and two-compartment models were tested, with first-order absorption, with or without lag time and first-order elimination. Ganciclovir concentrations below the LOQ were left-censored. Proportional, additive, and combined models were investigated to describe residual variability. IIV was defined by an exponential model.
Data for ganciclovir and valganciclovir then were fitted jointly. Only significant interindividual variabilities on the PK parameters were kept, i.e., a decrease in the objective function value (OFV) of ≥3.84 U.
The continuous covariates considered were BW, age, HT, BSA, and eGFR using the Schwarz formula (13). Continuous covariates were integrated as , where is the typical value of clearance or volume of distribution for a patient with the median covariate value and β is the estimated influential factor for the continuous covariate estimated by the modeling software. For BW (median, 11.7 kg), according to the allometric rule, β was fixed at 0.75 for the clearance and 1 for the volume of distribution (22). Categorical covariates, such as sex, were tested as , where COVi is equal to 0 or 1 and β is the estimated factor for the covariate.
Covariates were retained in the model if their effects were biologically plausible, if they produced a reduction in the PK parameter IIV, and if the OFV was decreased by at least 3.84 (equal to chi-squared with one degree of freedom, equivalent to a P value of 0.05) in the forward inclusion phase and was increased by more than 6.63 (equal to chi-squared with one degree of freedom, equivalent to a P value of 0.01) in the backward phase.
(ii) Model evaluation. (a) Validation on the remaining third of the data.
To assess predictive performance of the model, we first compared goodness-of-fit plots of the population-predicted concentrations and the observed concentration. We calculated mean prediction error (MPE) (equation 1) and mean absolute prediction error (MAE) (equation 2) to assess bias and root mean squared prediction error (RMSE) (equation 3) to assess precision between population predicted concentrations of the model and observed concentrations of the validation data set (23).
| (1) |
| (2) |
| (3) |
(b) Evaluation of the final model with building and validation data set.
The following goodness-of-fit plots were generated: observed concentrations versus population predictions and versus individual predictions, normalized prediction distribution error metrics (npde) versus time and versus predictions.
From the final model, Monte Carlo simulations were performed to compute the prediction-corrected visual predictive check (pc-VPC). The observed concentration data were overlaid on the 5th, 50th, and 95th percentiles of the simulated concentrations at each time, and a visual inspection was performed.
(iii) Simulations.
One thousand Monte Carlo simulations were performed from the final population PK model to simulate ganciclovir area under the curve. According to previously published reports, prophylactic and curative antiviral efficiency is reached for ganciclovir AUC0–24 at 40 to 60 mg/liter·h and AUC0–12 at 40 to 60 mg/liter·h, respectively (5, 24). For an easier presentation of the results, the therapeutic targets were converted to an AUC0–24 between 80 and 120 mg/liter·h, and the preventive range has been expanded to an AUC0–24 between 40 and 80 mg/liter·h. Several dosing regimens were tested, from 2.5 mg/kg/day to 25 mg/kg/day, for i.v. administration. For oral administration, doses from 8 to 56 mg/kg/day were simulated, corresponding to an increase of 75% of an oral dose of 32 mg/kg/day. The percentages of patients with an AUC0–24 below 40 mg/liter·h, between 40 and 80 mg/liter·h, between 80 and 120 mg/liter·h, and above 120 mg/liter·h were reported.
Supplementary Material
ACKNOWLEDGMENT
We have no conflicts of interests to declare.
REFERENCES
- 1.Agence nationale de sécurité du médicament et des producits de santé. 2019. Ganciclovir - résumé des caractéristiques du produit. ANSM, Saint Denis, France. [Google Scholar]
- 2.Agence nationale de sécurité du médicament et des producits de santé. 2019. Valganciclovir - résumé des caractéristiques du produit. ANSM, Saint Denis, France. [Google Scholar]
- 3.Pescovitz MD, Ettenger RB, Strife CF, Sherbotie JR, Thomas SE, McDiarmid S, Bartosh S, Ives J, Bouw MR, Bucuvalas J. 2009. Pharmacokinetics of oral valganciclovir solution and intravenous ganciclovir in pediatric renal and liver transplant recipients. Transpl Infect Dis off J Transplant Soc 12:195–203. doi: 10.1111/j.1399-3062.2009.00478.x. [DOI] [PubMed] [Google Scholar]
- 4.Reference deleted.
- 5.Wiltshire H, Paya CV, Pescovitz MD, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Zuideveld KP, Valganciclovir Solid Organ Transplant Study Group. 2005. Pharmacodynamics of oral ganciclovir and valganciclovir in solid organ transplant recipients. Transplantation 79:1477–1483. doi: 10.1097/01.tp.0000164512.99703.ad. [DOI] [PubMed] [Google Scholar]
- 6.Launay E, Théôret Y, Litalien C, Duval M, Alvarez F, Lapeyraque A-L, Phan V, Larocque D, Poirier N, Lamarre V, Ovetchkine P. 2012. Pharmacokinetic profile of valganciclovir in pediatric transplant recipients. Pediatr Infect Dis J 31:405–407. doi: 10.1097/INF.0b013e3182463a19. [DOI] [PubMed] [Google Scholar]
- 7.Vethamuthu J, Feber J, Chretien A, Lampe D, Filler G. 2007. Unexpectedly high inter- and intrapatient variability of ganciclovir levels in children. Pediatr Transplant 11:301–305. doi: 10.1111/j.1399-3046.2006.00669.x. [DOI] [PubMed] [Google Scholar]
- 8.Åsberg A, Bjerre A, Neely M. 2014. New algorithm for valganciclovir dosing in pediatric solid organ transplant recipients. Pediatr Transplant 18:103–111. doi: 10.1111/petr.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villeneuve D, Brothers A, Harvey E, Kemna M, Law Y, Nemeth T, Gantt S. 2013. Valganciclovir dosing using area under the curve calculations in pediatric solid organ transplant recipients. Pediatr Transplantation 17:80–85. doi: 10.1111/petr.12030. [DOI] [PubMed] [Google Scholar]
- 10.Facchin A, Elie V, Benyoub N, Magreault S, Maisin A, Storme T, Zhao W, Deschenes G, Jacqz-Aigrain E. 2019. Population pharmacokinetics of ganciclovir after valganciclovir in renal transplant children. Antimicrob Agents Chemother 63:e01192-19. doi: 10.1128/AAC.01192-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldés A, Colom H, Armendariz Y, Garrido MJ, Troconiz IF, Gil-Vernet S, Lloberas N, Pou L, Peraire C, Grinyó JM. 2009. Population pharmacokinetics of ganciclovir after intravenous ganciclovir and oral valganciclovir administration in solid organ transplant patients infected with cytomegalovirus. Antimicrob Agents Chemother 53:4816–4824. doi: 10.1128/AAC.00085-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vezina HE, Brundage RC, Balfour HH. 2014. Population pharmacokinetics of valganciclovir prophylaxis in paediatric and adult solid organ transplant recipients. Br J Clin Pharmacol 78:343–352. doi: 10.1111/bcp.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A. 1976. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263. [PubMed] [Google Scholar]
- 14.Lubowitz H, Slatopolsky E, Shankel S, Rieselbach RE, Bricker NS. 1967. Glomerular filtration rate. Determination in patients with chronic renal disease. JAMA 199:252–256. doi: 10.1001/jama.199.4.252. [DOI] [PubMed] [Google Scholar]
- 15.Dhont E, Van Der Heggen T, De Jaeger A, Vande Walle J, De Paepe P, De Cock PA. 2020. Augmented renal clearance in pediatric intensive care: are we undertreating our sickest patients? Pediatr Nephrol 35:25–39. doi: 10.1007/s00467-018-4120-2. [DOI] [PubMed] [Google Scholar]
- 16.Baptista JP, Roberts JA, Udy AA. 2019. Augmented renal clearance: a real phenomenon with an uncertain cause. Anaesth Crit Care Pain Med 38:335–336. doi: 10.1016/j.accpm.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Cook AM, Hatton-Kolpek J. 2019. Augmented renal clearance. Pharmacotherapy 39:346–354. doi: 10.1002/phar.2231. [DOI] [PubMed] [Google Scholar]
- 18.Jorga K, Reigner B, Chavanne C, Alvaro G, Frey N. 2019. Pediatric dosing of ganciclovir and valganciclovir: how model-based simulations can prevent underexposure and potential treatment failure. CPT Pharmacometrics Syst Pharmacol 8:167–176. doi: 10.1002/psp4.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorga K, Chavanne C, Frey N, Lave T, Lukacova V, Parrott N, Peck R, Reigner B. 2016. Bottom-up meets top-down: complementary physiologically based pharmacokinetic and population pharmacokinetic modeling for regulatory approval of a dosing algorithm of valganciclovir in very young children. Clin Pharmacol Ther 100:761–769. doi: 10.1002/cpt.449. [DOI] [PubMed] [Google Scholar]
- 20.U.S. FDA. 2018. Bioanalytical method validation guidance for industry. U.S. FDA, Silver Spring, MD. [Google Scholar]
- 21.Samson A, Lavielle M, Mentré F. 2006. Extension of the SAEM algorithm to left-censored data in nonlinear mixed-effects model: application to HIV dynamics model. Comput Stat Data Anal 51:1562–1574. doi: 10.1016/j.csda.2006.05.007. [DOI] [Google Scholar]
- 22.Anderson BJ, Holford NHG. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 23.Sheiner LB, Beal SL. 1981. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503–512. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 24.Stockmann C, Roberts JK, Knackstedt ED, Spigarelli MG, Sherwin CM. 2015. Clinical pharmacokinetics and pharmacodynamics of ganciclovir and valganciclovir in children with cytomegalovirus infection. Expert Opin Drug Metab Toxicol 11:205–219. doi: 10.1517/17425255.2015.988139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.