Significance
The long-term effects of rising atmospheric carbon dioxide (CO2), high rates of nitrogen deposition, and declining plant biodiversity on ecosystem carbon pools are uncertain and rarely assessed in concert yet represent key feedbacks to global climate change. In a 19-y study in an open-air grassland experiment, increasing planted species richness substantially enhanced ecosystem carbon storage, while elevated CO2 and nitrogen addition treatments had only modest effects. Effects of the three global changes were largely additive, and stronger effects of species richness compared with CO2 or nitrogen resulted from large enhancements in plant productivity relative to ecosystem losses. Our results suggest that biodiversity losses may influence carbon storage as much as or more than rising CO2 or high nitrogen deposition rates.
Keywords: elevated CO2, biodiversity, nitrogen, carbon, grassland
Abstract
Whether the terrestrial biosphere will continue to act as a net carbon (C) sink in the face of multiple global changes is questionable. A key uncertainty is whether increases in plant C fixation under elevated carbon dioxide (CO2) will translate into decades-long C storage and whether this depends on other concurrently changing factors. We investigated how manipulations of CO2, soil nitrogen (N) supply, and plant species richness influenced total ecosystem (plant + soil to 60 cm) C storage over 19 y in a free-air CO2 enrichment grassland experiment (BioCON) in Minnesota. On average, after 19 y of treatments, increasing species richness from 1 to 4, 9, or 16 enhanced total ecosystem C storage by 22 to 32%, whereas N addition of 4 g N m−2 ⋅ y−1 and elevated CO2 of +180 ppm had only modest effects (increasing C stores by less than 5%). While all treatments increased net primary productivity, only increasing species richness enhanced net primary productivity sufficiently to more than offset enhanced C losses and substantially increase ecosystem C pools. Effects of the three global change treatments were generally additive, and we did not observe any interactions between CO2 and N. Overall, our results call into question whether elevated CO2 will increase the soil C sink in grassland ecosystems, helping to slow climate change, and suggest that losses of biodiversity may influence C storage as much as or more than increasing CO2 or high rates of N deposition in perennial grassland systems.
Only about half of anthropogenic carbon (C) emissions accumulate in the atmosphere, while the rest are stored by oceans and the terrestrial biosphere (1, 2). However, model projections of terrestrial C cycling in the face of climate change are highly variable, and whether the terrestrial biosphere will continue to act as a global net C sink is questionable (1, 3). A key uncertainty relates to whether the rise in atmospheric carbon dioxide (CO2) concentration is being slowed by enhanced photosynthesis and subsequent C storage in plants and soils (1). Moreover, other factors, such as soil nitrogen (N) availability, species composition, species diversity, climate warming and associated precipitation change, and altered disturbance rates may impact terrestrial C sinks and constrain how terrestrial ecosystems respond to elevated CO2 (eCO2) (4–11).
Elevated CO2 increases C fixed via photosynthesis and stimulates plant growth in a wide variety of species (4, 5, 12). Additional C acquisition under eCO2 can enhance plant and soil C pools, the latter by increasing plant-derived C inputs to soils, reducing litter quality and thereby slowing litter decomposition rates, and/or by stimulating physical protection of soil organic matter (SOM) from microbial decomposition (13–15). However, some studies have observed no C accumulation under eCO2 (16–21).
Conflicting results among studies have called into question whether short-term enhancement of plant C uptake under eCO2 (i.e., over days or years) will translate into long-term (i.e., decades or more) ecosystem C storage. The net impact of eCO2 on ecosystem C storage depends on the balance between eCO2 effects on net primary productivity (NPP) and on losses of C through microbial respiration and other pathways such as leaching, fire, and herbivory. To mitigate climate change over the long term (decades to centuries to millennia), some additional C captured under eCO2 must be transferred to biomass or SOM pools with slow turnover times (i.e., wood or physically or chemically protected SOM) (22, 23). However, eCO2 has been observed to increase the partitioning of C to rapidly cycling pools of labile C (e.g., roots, root exudates, and detritus), resulting in enhanced C loss via soil respiration (17–19, 22, 24, 25). Elevated CO2 can also increase soil moisture via enhanced plant water-use efficiency, thereby stimulating microbial decomposition and C loss (26). In ecosystems that are fire prone or that undergo managed fire regimes, like grasslands and savannas, enhanced biomass under eCO2 may also increase fuel load and thus fire intensity and spread, thereby enhancing fire-mediated C losses (10, 20, 27, 28).
Moreover, limited N availability often constrains positive responses of photosynthesis and plant growth to eCO2 (4, 5, 8, 29) as N is a key driver of photosynthesis and productivity (30, 31). Under ambient CO2, additional N inputs have been shown to promote C storage by increasing plant-derived C inputs to soils via litter and root production (32) and/or by suppressing organic matter decay (33). Under eCO2, N addition often alleviates down-regulation of photosynthetic and growth responses (4, 5, 8), potentially enhancing positive effects of eCO2 on plant productivity and soil C accumulation (13).
Plant species richness can also affect ecosystem C accumulation and interact with eCO2 and soil N supply (6, 34). Increased species richness has been shown to promote soil C storage in various grassland biodiversity experiments via enhanced productivity, belowground C allocation, and changes in microbial communities (35–42). For instance, at a companion experiment in Minnesota, increasing grassland species richness from 1 to 2, 4, 8, or 16 species following agricultural abandonment increased soil C storage by 60 to 178% due to greater aboveground and root biomass inputs (35). In a long-term grassland biodiversity experiment in Germany, higher plant diversity increased soil C storage by enhancing rhizosphere inputs of recently fixed C and increasing microbial biomass C (38, 40, 42). Plant growth responses to eCO2 and N addition may be stronger in more diverse plots (6, 34), potentially fueling interactive effects with diversity on ecosystem C accumulation. However, to our knowledge, no field experiment other than that described herein concurrently manipulates CO2, soil N supply, and species richness.
It remains unclear whether the eCO2-induced increases in plant C fixation commonly observed in short-term studies will translate into long-term C storage and whether this depends on soil N supply and/or species richness. We addressed this gap in understanding using the free-air CO2 enrichment experiment BioCON (6, 8), which concurrently manipulates CO2, N supply, and species richness in a frequently burned Minnesota grassland. Here, we used measurements of total soil C stocks to a depth of 60 cm coupled with long-term biomass, measures of aboveground and belowground NPP, and plant tissue chemistry data to assess how manipulations of CO2 (ambient, +180 ppm), soil N supply (ambient, +4 g N m−2 ⋅ y−1), and planted species richness (1, 4, 9, or 16 species) have influenced total ecosystem C accumulation over 19 y. In herbaceous systems that lack substantial woody biomass and where aboveground biomass C turns over annually, C storage is expected to occur primarily in soils (43). We expected this system to be in the aggrading stage of perennial vegetation growth following establishment of the experiment and to therefore accumulate C over time (35, 37).
Our global change treatments represent and span realistic scenarios for grassland ecosystems over the next seven or eight decades. We acknowledge it is challenging to project each factor with equal confidence and we make comparisons across experimentally selected treatments carefully. Our eCO2 treatment simulates atmospheric concentrations projected to be reached by the end of the century (44). Our N addition treatment simulates high rates of N deposition experienced in industrialized regions of the Northern Hemisphere and biomass burning regions in the tropics, and projections of N deposition rates over the next few decades are regionally variable and dependent on changes in emission regulations (45–48). The N treatment can also be used to understand variation in soil fertility; for example, the difference in N input rate between the control and N treatment is comparable to the range in net N mineralization rates between less fertile sites (frequently burned savannas) and more fertile forests within 1 km of our study site (49). Our species richness treatments span much of the range of diversity in native and anthropogenic grasslands in central Minnesota. As noted in Reich et al. (50), nearby native grasslands averaged 16.3 species per 1.0 m2 sampled, whereas BioCON plots planted with 16 species tend to have 9 to 11 species per 0.1 m2 sampled, due in part to local extinctions in individual plots and sampling only a small fraction of each 4 m2 plot. In contrast, postagricultural restored grasslands averaged 3.5 species (range 1 to 8) per 1.0 m2 sampled (45). Our treatments with 16 planted species are thus roughly representative of high-diversity native vegetation, while our 1 and 4 species planted treatments have diversity similar to low-diversity grasslands of anthropogenic origin. Grassland species richness is projected to decline substantially by the year 2100 because of climate change and other human disturbances (51), and the range of losses we simulate is relevant to both climate-driven, local-scale species losses experienced by natural grasslands (52) and more severe losses associated with land-use change (51). Our range of species richness levels is also relevant to grassland restoration, where managers must weigh the economic and ecological effects of planting monocultures versus diverse mixtures (e.g., ref. 53).
Conceptually, on a timescale of two decades, eCO2, N addition, and increasing species richness could each increase ecosystem C accumulation because of enhanced NPP (i.e., enhanced plant-derived soil C inputs) (13, 15, 32, 35). However, pathways of C loss can also be stimulated, offsetting potential gains in ecosystem C (20, 22, 24, 25). Model projections of C storage by the end of the century show that grassland C storage may increase, decrease, or remain unchanged under future atmospheric composition and climate (3, 54, 55). Part of this uncertainty is due to a lack of empirical grounding for model assumptions regarding effects of global change factors on C cycling (56, 57). Here, the longevity of our experiment and factorial design allow us to empirically assess predictions of grassland C storage by the end of the century.
We constructed hypotheses about total ecosystem C responses to two decades of CO2, N, and species richness treatments in part based on a rich body of previous work performed in BioCON related to responses of biomass and C cycling to these treatments. After 4 y of treatments in a subexperiment of BioCON (including only 1 and 4 species plots), N addition, but not eCO2, modestly increased total soil %C (measured to a depth of 20 cm) (58). The lack of an eCO2 effect early in the experiment could reflect a balance between soil C inputs and losses in low-diversity plots; however, changes in soil C may take years to become apparent, may occur below a depth of 20 cm, and may be stronger with higher species richness. Past work in this experiment also indicated that these treatments stimulated both ecosystem C inputs and losses (based on measurements of photosynthesis, biomass, respiration, and fire-induced losses; 6, 8, 12, 20, 24, 34, 59, 60); for instance, early C gains in response to treatments were completely offset by greater fire-mediated C losses in years when biomass was burned, likely because enhanced litter production increased fuel loads (20). However, responses of soil C inputs and losses to global changes may be dynamic, driving periods of net positive, negative, and neutral C accumulation. Based on this prior work in the BioCON experiment, we hypothesized (H1) that neither eCO2 nor N addition would substantially enhance total ecosystem C pools because increased soil C inputs would be largely offset by enhanced C losses. In contrast, we hypothesized (H2) that total ecosystem C pools would increase with species richness, more so over time, as increased species richness enhanced biomass several-fold more than did enriched CO2 or N in this experiment (6). We also hypothesized (H3) that effects of eCO2, N addition, and species richness would be synergistic (i.e., more than additive) because of past work demonstrating resource colimitation and interactions with diversity for biomass production (6, 61). For instance, substantial positive effects of eCO2 on total ecosystem C might only be apparent at high levels of species richness or N.
Results
Total Ecosystem C.
As hypothesized, after 19 y, increasing species richness increased total ecosystem C more than did eCO2 or N addition (Fig. 1). All treatment effects on total ecosystem C were relatively modest (<10%) after 5 y of treatments (Fig. 2). The effect of species richness, however, grew substantially by 2007 (after 10 y of treatments) and then remained relatively stable (Fig. 2) despite a reversal in the direction of C accumulation over time in all treatments (Fig. 3). By contrast, effects of eCO2 and N addition remained modest throughout the experiment (Table 1, SI Appendix, Table S1, and Fig. 2). Thus, we focus on responses in the final measurement year (2016) here (Fig. 1). In terms of main effects, on average in 2016, increasing species richness from 1 to 4, 9, or 16 species enhanced total ecosystem C storage by 949 g C m−2 ⋅ y−1 (+22%), 1,094 g C m−2 ⋅ y−1 (+25%), and 1,360 g C m−2 ⋅ y−1 (+32%), respectively, supporting H2 (SR: P < 0.0001, Table 1, SI Appendix, Table S1, and Fig. 1A). The species richness effect was nonlinear (i.e., total ecosystem C increased more going from 1 to 4 species than from 4 to 9 or 9 to 16 species, Figs. 1 and 2 and SI Appendix, Fig. S1). Enriched N increased total ecosystem C by 190 g C m−2 ⋅ y−1 (+4%) on average in 2016 (N: P = 0.007, Table 1, SI Appendix, Table S1, and Fig. 1C). Given the modest main effect size, these results support our hypothesis that N addition would not substantially increase total ecosystem C (H1). There was no main effect of CO2 on total ecosystem C, as predicted (H1; Table 1, SI Appendix, Table S1, and Fig. 1B). On average in 2016, eCO2 increased total ecosystem C by 97.7 g C m−2 ⋅ y−1 (+2%).
Fig. 1.
Mean total ecosystem C in response to increasing species richness (A), eCO2 (B), and increased N supply (C) in 2016, after 19 y of treatment application. Total ecosystem pools are broken up into components: soils to 60 cm (brown, Bottom), root biomass to 20 cm (blue, Middle), and aboveground biomass (green, Top). Means were averaged across other treatments in all cases (e.g., mean for eCO2 is across all levels of species richness and N). The error bars represent ± 1 SE for total ecosystem C. n = 258.
Fig. 2.
Relative effect size (percent change) of increasing species richness (from 1 to 4, 9, or 16 species), elevated CO2 (eCO2), and increased N supply (eN) on total ecosystem C. Percent change values were calculated using means of the two treatment levels being compared (averaged across all other treatments) for each year that total ecosystem C was measured. Relative effect sizes of increases in species richness between other levels are shown in SI Appendix, Fig. S1.
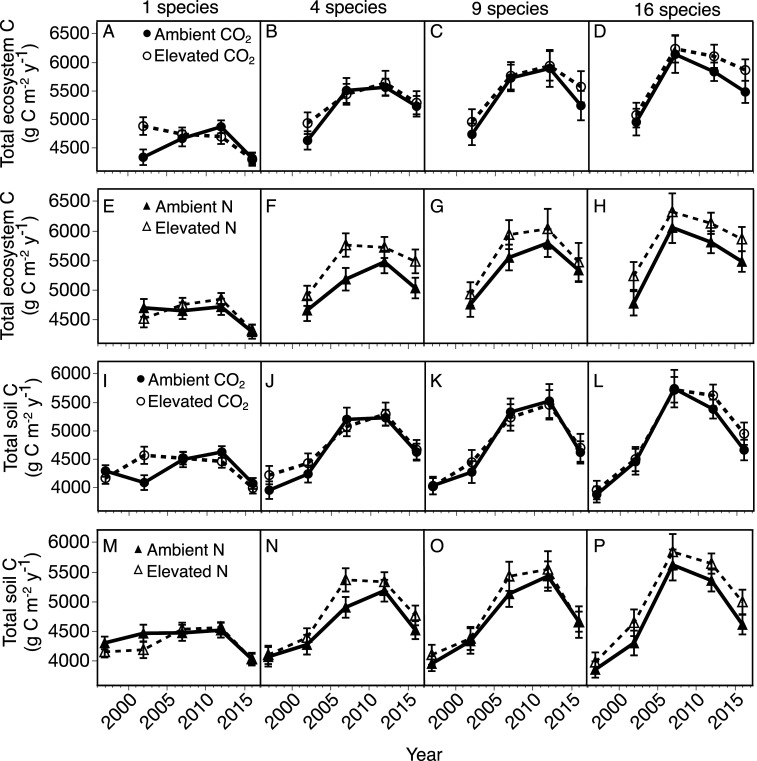
Fig. 3.
Mean total ecosystem C (A–H) and soil C (I–P) pools under ambient (closed symbols, solid lines) and elevated (open symbols, dashed lines) CO2 (circles) and N (triangles) at species richness of 1, 4, 9, or 16 (from left to right) over the 19 y experiment. Means are across levels of the other treatment. Note that the first time points for soil C are from pretreatment year 1997. The error bars represent ± 1 SE. n = 1,377.
Table 1.
Linear mixed-effects model probabilities (P > F) for NPP, total plant biomass (aboveground + roots to 20 cm) C, soil C to 60 cm, total ecosystem (soil + plant) C, and soil CO2 flux
| P > F | |||||
| Effect | NPP | Total plant C | Soil C | Total ecosystem C | Soil CO2 flux |
| Year | <0.0001 | 0.02 | 0.009 | 0.0001 | <0.0001 |
| CO2 | 0.004 | 0.01 | 0.92 | 0.85 | 0.004 |
| N | <0.0001 | <0.0001 | 0.07 | 0.007 | 0.02 |
| SR | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Year × CO2 | 0.67 | 0.67 | 0.01 | 0.005 | 0.006 |
| Year × N | 0.0005 | 0.06 | 0.27 | 0.46 | 0.13 |
| Year × SR | <0.0001 | <0.0001 | 0.0003 | <0.0001 | <0.0001 |
| CO2 × N | 0.31 | 0.71 | 0.59 | 0.80 | 0.85 |
| CO2 × SR | 0.62 | 0.56 | 0.78 | 0.78 | 0.10 |
| N × SR | 0.37 | 0.73 | 0.13 | 0.26 | 0.46 |
| Year × CO2 × N | 0.57 | 0.14 | 0.97 | 0.62 | 0.01 |
| Year × CO2 × SR | 0.99 | 0.81 | 0.009 | 0.005 | 0.03 |
| Year × N × SR | 0.17 | 0.0004 | 0.39 | 0.28 | 0.40 |
| CO2 × N × SR | 0.78 | 0.65 | 0.08 | 0.09 | 0.83 |
| Year × CO2 × N × SR | 0.39 | 0.96 | 0.14 | 0.27 | 0.11 |
F ratios are shown in SI Appendix, Fig. S1. P < 0.05 bolded to draw attention to the most important terms in the model. Degrees of freedom = 1 for all factors and interactions. N = nitrogen; SR = species richness.
Effects of treatments on total ecosystem C were generally additive (Table 1), with no interactions involving CO2 × N. We did observe small CO2 effects on total ecosystem C in monocultures early in the experiment and in 9- and especially 16-species plots later in the experiment, lending some modest support to H3 (year × CO2 × SR: P = 0.005, Table 1, SI Appendix, Table S1, and Fig. 3 A–D).
Time strongly influenced total ecosystem C, irrespective of treatments, particularly in mixtures (Fig. 3 A–H, year: P = 0.0001, Table 1 and SI Appendix, Table S1). Total ecosystem C increased with time initially but then declined (Fig. 3 A–H). Differences in total ecosystem C from one time interval to the next ranged from −436 to +511 g C m−2 on average across treatments, with an ultimate net mean difference of +129 g C m−2 from 2002 to 2016. These patterns were driven by soil C, which accumulated at a rate of 58 g C m−2 ⋅ y−1 on average over the first 15 y of the experiment before declining (Fig. 3 I–P). The highest rate of soil C accumulation occurred between years 5 to 10 at 120 g C m−2 ⋅ y−1 on average.
Soil and Plant C Pools.
Patterns in total ecosystem C were driven by soil C pools, which made up 90% of total ecosystem C; thus, effects on total ecosystem C described above reflect those for soil C (Fig. 3, Table 1, and SI Appendix, Table S1).
Total plant C pools (aboveground tissues + roots to 20 cm depth) accounted for only 10% of total ecosystem C on average (Fig. 1) and increased in response to all treatments (Table 1 and SI Appendix, Tables S1 and S2). As with total ecosystem and soil nutrient pools, effects of increasing species richness (+76 to 133% on average across years depending on species richness) outweighed those of eCO2 (+15% on average across years) and N addition (+21% on average across years); however, unlike for total ecosystem and soil C, a positive main effect of CO2 was detected on total plant C (Table 1 and SI Appendix, Tables S1 and S2). Positive effects occurred both above and belowground (SI Appendix, Table S2).
Net Primary Productivity.
Total annual NPP (aboveground biomass C + root ingrowth C to 20 cm) increased substantially in response to all treatments (Fig. 4). Enhancements in NPP with increasing species richness (+75 to +130% on average across years compared with monocultures, depending on SR level, P < 0.0001) were stronger than those with enriched N (+22%; P < 0.0001) and eCO2 (+17%; P = 0.004) (Fig. 4, Table 1, and SI Appendix, Table S1). Positive effects occurred both above and belowground (SI Appendix, Table S2). The divergence of NPP between monocultures versus mixtures grew stronger through time (year × species richness: P < 0.0001, Table 1 and SI Appendix, Table S1).
Fig. 4.
Relative effect size (percent change) of increasing species richness (A), eCO2 (B), and increased N supply (C) on mean total annual NPP (Left, blue) and mean rates of soil CO2 flux (Right, red). Soil CO2 flux is shown because it is the greatest route of C loss at this site (an order of magnitude greater than fire C loss). Percent change values here were first calculated for each of the 19 y using annual means of the two treatment levels being compared (averaged across all other treatments). We then averaged across annual percent change values and show these means and SEs here (i.e., N to calculate means shown here = 19). The error bars represent ± 1 SE (variance in percent change among years).
Responses of cumulative NPP across the entire experiment to treatments (i.e., calculated from NPP summed over 19 y) were positively correlated with, but were greater than, average gains in soil C in response to treatments by the final measurement year (SI Appendix, Fig. S2, R2 = 0.94, P = 0.007). Some portion of the additional NPP produced in response to treatments would be decomposed each year, but the large losses indicated here likely include other pathways (and are consistent with expectations given 10 prescribed fires over the course of the study). A total of 31, 26, and 23% of the additional cumulative NPP produced in response to increasing species richness from 1 to 4, 9, or 16, respectively, accumulated in soils by 2016, and we infer the remainder was lost (Fig. 5). A total of 15% of the additional cumulative NPP produced in response to N addition and only 5% produced in response to eCO2 accumulated in soils by 2016, suggesting that N addition and especially eCO2 disproportionately stimulated C losses relative to NPP compared with increasing species richness (Fig. 5).
Fig. 5.
Absolute effect size of eCO2 (A), increased N supply (B), and increased species richness from 1 to 4 (C), 1 to 9 (D), or 1 to 16 (E) on mean cumulative NPP across the entire 19 y (Left, blue) and inferred losses of this additional NPP (Right, red). Inferred losses were calculated as the absolute effect size for cumulative NPP minus the absolute effect size for soil C in 2016 (i.e., the amount of cumulative NPP produced in response to treatments minus the amount of C that accumulated in soils in response to treatments). The percentages above the bars indicate the inferred percent of cumulative NPP inputs that were retained.
Soil CO2 Flux.
Mean growing season (May through September) soil CO2 flux was increased by species richness, CO2, and N treatments (Fig. 4). On average across years, increasing species richness from 1 to 4, 9, or 16 species enhanced mean soil CO2 flux by 24, 28, and 30%, respectively (species richness: P < 0.0001, Table 1, SI Appendix, Table S1, and Fig. 4A). eCO2 increased mean soil CO2 flux by 21% on average (CO2: P = 0.004, Table 1, SI Appendix, Table S1, and Fig. 3B) and N addition increased mean soil CO2 flux by 5% (N: P = 0.02, Table 1, SI Appendix, Table S1, and Fig. 4C). Estimated cumulative growing season soil CO2 flux ranged from 639 to 822 g C m−2 ⋅ y−1 depending on treatment level (SI Appendix, Table S3), a magnitude that renders soil CO2 flux the most important C loss pathway in this system and thus an important regulator of total ecosystem C storage.
Fire C Losses.
Estimated mean annual fire C losses ranged from 45 to 96 g C m−2 ⋅ y−1 (averaged across both burn and nonburn years (i.e., on an annual scale that facilitates comparison to the magnitude of other C loss routes), depending on treatment, with larger losses at elevated treatment levels (SI Appendix, Table S3). Because the vast majority of standing litter burns at this site, plots with higher aboveground biomass lost more C via fire in absolute terms. Fire C losses are of a magnitude to influence total ecosystem C storage over time but are an order of magnitude lower than C losses via soil CO2 flux.
Discussion
In this N-poor grassland, increasing species richness substantially increased total ecosystem (plant + soil to 60 cm) C pools over 19 y, whereas eCO2 and N addition had only modest effects. Specifically, after 19 y, increasing species richness from 1 to 4, 9, or 16 enhanced total ecosystem C storage by 22 to 32%, while average effects of N addition and eCO2 were <5%, supporting our hypotheses (H1 and H2). The species richness effect was initially modest but strengthened during the first decade and remained relatively stable over the following decade, whereas CO2 and N effects remained modest. We attribute the greater effect of species richness (compared with CO2 or N treatments) on C storage to proportionally larger enhancements in NPP (6) that grew over time (50) and thus on large organic matter inputs to soils relative to ecosystem losses. The effects of the global change treatments were generally additive, and thus we found little evidence for H3. We further discuss these results in relation to our hypotheses below.
Effects of Increasing Species Richness on C Pools.
The strong enhancement in total ecosystem C with increasing species richness agrees with our hypothesis (H2) and other studies showing greater C storage at higher species richness (35–42). However, the effect was nonlinear; that is, increasing species richness from 1 to 4 had a more positive effect on ecosystem C pools than going from 4 to 9 or 9 to 16 planted species treatments. Given that our highest diversity plots are most representative of natural grasslands, these results suggest that a 44% reduction in species richness in diverse grasslands might have modest negative effects on ecosystem C storage, similar to those of eCO2 and N addition, whereas a 75% or, in particular, a 94% reduction (or conversion to monoculture) will have stronger negative effects. This also suggests that planting mixtures, even those composed of only a few species, instead of monocultures is advantageous for grassland restoration efforts that prioritize soil C storage.
The positive effect of species richness on total ecosystem C was largely driven by greater soil C, though plant C pools were also enhanced. Species richness effects on soil C reflected responses of NPP and the importance of organic inputs to soils in driving treatment effects on soil C. Based upon prior work at BioCON, the approximately twofold enhancement in productivity in response to increasing species richness likely resulted from complementary interactions among species, such as niche differentiation or facilitation (34), which grew over time (50). As portions of those additional soil inputs accumulated, the species richness effect strengthened and appears to have stabilized. Large increases in root inputs may also have increased soil C storage by stabilizing SOM, including by promoting the formation of soil aggregates that physically protect SOM (62, 63).
However, responses of productivity did not fully explain soil C responses, as changes in soil C in response to increasing species richness (and to added CO2 and N) by the final measurement year were much smaller than responses of cumulative NPP summed over the 19 y duration. This suggests that increasing species richness (and addition of CO2 or N) also stimulated C loss pathways. Indeed, rates of soil CO2 flux, the greatest pathway for C loss at this site, were greater in mixtures than in monocultures, and our estimation of cumulative growing season soil CO2 flux was 183 g C m−2 ⋅ y−1 higher in 16-species plots than monocultures, though it is probable that a large portion of enhanced soil CO2 flux can be attributed to increases in root respiration and not solely to losses of soil C (64). Fire can also drive losses of soil C through the combustion of potential organic matter inputs and/or the stimulation of losses from deeper soil (10, 65), and mixtures at this site have exhibited greater fire-induced C losses than monocultures (20). While large amounts of belowground biomass produced in response to increasing species richness may have been resilient to fire, we estimated that fire C losses from aboveground biomass were over twice as high (+51.3 g C m−2 ⋅ y−1) in 16-species plots compared with monocultures because of higher fuel load. Regardless, any enhancement in soil respiration or other pathways of C loss with increasing species richness were outweighed by greater plant-derived C inputs to soils, as hypothesized (H2).
Effects of Increased N Supply on C Pools.
In contrast to species richness, increasing soil N supply only modestly enhanced total ecosystem and soil C in these nutrient-poor soils, despite enhancing NPP by 22% on average. This supports our hypothesis of modest effects of increased N supply on total ecosystem C (H1) and indicates that the influence of N supply on C storage may be limited in some systems, even in N-poor grasslands. It is likely that N-induced C losses mostly offset additional soil C inputs, similar to results of other grasslands in a meta-analysis of soil C storage in response to N addition (66). Repeated fire disturbances may have combusted additional organic matter inputs produced in response to N enrichment before they were stabilized in soils in prescribed burn years (10, 20). Indeed, we estimated that fire C losses were 13% (+14.6 g C m−2 ⋅ y−1) higher in enriched compared with ambient N plots (calculated including burn and nonburn years; +26% and +29.1 g C m−2 ⋅ y−1 on average if across burn years only). N addition may also have promoted soil respiration (24, 60). We did observe a modest enhancement in rates of soil CO2 flux with N addition and estimated that cumulative growing season soil CO2 flux was 38 g C m−2 ⋅ y−1 higher in enriched compared with ambient N plots. Leaching and herbivory are less likely to be substantial routes of enhanced C loss under N addition at this site. C losses via leaching are very low relative to other fluxes at BioCON (20, 67). Though C losses via herbivory have not been measured at BioCON, past work showed that neither N nor CO2 treatments affected plant damage of the legume Lespedeza capitata by herbivores or pathogens (68).
Our results are in contrast to a nearby 27 y grassland experiment following abandonment of agricultural fields in which N addition strongly increased total ecosystem and soil C storage, even when applied at rates lower than in our study (their N addition treatments ranged from +1 to 27 g N m−2 ⋅ y−1) (32). However, in that experiment, N addition also shifted the plant community from native C4 grasses to exotic C3 grasses, which may have increased C accrual through large increases in root biomass and thus root-derived soil C inputs through turnover and exudation (32). Fornara and Tilman (32) also suggest that plant species compositional shifts may have changed microbial communities such that they were less capable of decomposing recalcitrant soil C pools. Further work is needed to uncover the mechanisms controlling these varied responses of soil C storage to N addition in disturbed grasslands.
Effects of eCO2 on C Pools.
Though eCO2 stimulated total NPP by 17% on average, we did not detect a main effect of CO2 on total ecosystem C, in agreement with H1. However, effects of eCO2 on total ecosystem C were greater for some levels of species richness in some years. In particular, eCO2 increased total ecosystem C in monocultures in 2002 after 5 y of treatment (by 8%), but this modest effect disappeared within another 5 y. More recently, a trend of greater ecosystem C accumulation under eCO2 has emerged in 16-species plots, lending some modest support for H3. This trend suggests that in the species-rich (and most productive) community, which is most similar to natural grasslands, soil C may eventually increase under eCO2, becoming apparent only after many years in a grassland that is recovering from disturbance. Importantly, and in contrast to H3, we did not find evidence for the expectation proposed by others that N addition will enhance positive effects of eCO2 on soil C accumulation (13), despite N limitation of biomass responses to eCO2 at this site (8). This lack of dependency of the eCO2 response on N availability contrasts with results of a companion 8 y global change experiment, where in plots planted with nine species, total ecosystem C pool responses to eCO2 were strongly dependent on N, warming, and rainfall treatments (7), precluding any simple generalization about the likelihood of interactions among multiple global change drivers.
Overall, enhanced C losses under eCO2 likely offset any increases in plant-derived soil C inputs. Indeed, eCO2 increased rates of soil CO2 flux by 21%, and we estimated that cumulative growing season soil CO2 flux was 132 g C m−2 ⋅ y−1 greater under eCO2. Past studies also showed that eCO2 strongly increased soil and microbial respiration at this site (24, 60). It is also probable that enhanced C losses via fire reduced the quantity of additional plant-derived C inputs to soils (20), and indeed we estimated that fire C losses were 8% greater (+9.6 g C m−2 ⋅ y−1) under eCO2 (calculated including burn and nonburn years; +15% and +18.3 g C m−2 ⋅ y−1 on average if across burn years only). Leaching and herbivory are less likely to be substantial routes of enhanced C losses under eCO2 at this site for the reasons described above.
Our finding that eCO2 did not promote soil C storage is consistent with some other, albeit shorter, studies in grasslands. For instance, in a calcareous grassland exposed to eCO2 for 6 y, soil C did not increase despite a 23% stimulation in the plant C pool (16). Similarly, another study revealed that eCO2 increased C uptake by vegetation in annual grasslands but also increased partitioning of C to rapidly cycling C pools with a net result of no change in soil C after three years of eCO2 (22). The coarse-textured, sandy soils at our site may possess a limited ability to stabilize C into pools with longer mean residence times in response to eCO2. While increased species richness may have promoted stabilization of SOM as a result of greater root C inputs, enhancements in root biomass and productivity under eCO2 (and N addition) were not as strong. Moreover, past studies at BioCON showed that eCO2 increased turnover of slow cycling C pools (21) and stimulated soil C losses via rhizosphere priming of SOM decomposition, suggesting that eCO2 may enhance the release of older, more recalcitrant soil C (69). Studies elsewhere, however, have observed positive effects of eCO2 on soil C, including in grasslands (13, 14). It is possible that changes in soil C content would occur very slowly given the modest stimulation in NPP we observed under eCO2 (70), and perhaps we are starting to detect this signal in 16-species plots. Regardless, our results indicate that any eCO2 effects on soil C in our study of 19 y of CO2 enrichment are modest. Based on our results, increases in plant C uptake (12, 71) and productivity under eCO2 may not translate into C storage even over multiple decades if NPP is only modestly stimulated and/or if the additional C released into soils is labile and decomposes quickly and/or stimulates losses of old soil C. Thus, our empirical results agree with models projecting little or no change in grassland soil C storage by the end of the century, but model predictions vary widely (3).
Changes in Total Ecosystem C through Time.
We observed strong temporal changes in total ecosystem C over 19 y, independent of treatments. These temporal trends were driven by C accumulation in soils, which initially reflected the aggrading stage of perennial vegetation growth following establishment of the experiment, as expected. As in other studies in aggrading grassland systems, soil C accumulated rapidly initially (35, 37). The average rate of soil C accumulation over the first 15 y of our experiment was greater than the average (but within the range) of reported rates during grassland establishment after land conversion from cultivated to perennial vegetation [58 g C m−2 ⋅ y−1 in our study versus 34 g C m−2 ⋅ y−1 on average for other converted agricultural fields at Cedar Creek (43) and 33 g C m−2 ⋅ y−1 on average in other establishing grasslands (72)]. However, in contrast to expectations, amounts of total soil C eventually decreased such that they have begun to approach pretreatment levels. The strongest reductions occurred between years 15 and 19. We explored whether changing weather and species composition over time could explain the temporal soil C pattern but did not find any notable patterns. Though we cannot say what drove the temporal reversal in soil C nor whether this trend will persist, we note that the timing of prescribed burns switched from early spring to late fall beginning in 2013 (year 16) and became more frequent after 2011. Perhaps fall burns were more intense and led to larger losses of nutrients and C (e.g., via nutrient volatilization, reduced inputs of organic matter, and enhanced leaching). It is also possible that by reducing the litter layer before winter, fall burns led to colder, more variable soil temperatures and more frequent freeze–thaw cycles (73) that may have affected biogeochemical cycles and stimulated losses of soil C through soil respiration and leaching.
Implications.
In this open-air grassland global change experiment, spanning nearly two decades, species richness treatments affected total ecosystem C more than eCO2 or N addition treatments; the effects of richness were larger even for changes that best match projections for this century. Though eCO2 and N addition influenced several aspects of C cycling, including NPP, this did not result in large or consistent changes in total ecosystem C pools. A portion of the additional organic matter produced in response to treatments was lost, likely preventing substantial soil C accumulation in response to CO2 and N treatments, whereas large enhancements in NPP with increasing species richness surpassed any concomitant stimulation in C losses. Moreover, effects of global change treatments were generally additive, with no interactions between CO2 and N, in contrast with other recent related studies from the same site (e.g., refs. 7, 56), indicating that our ability to forecast future interactions remains weak.
Grasslands, like many other biomes, are projected to experience large biodiversity losses by the end of the century in the face of global climate change and other human disturbances (51), and some have already undergone climate- and nutrient-driven species loss (52, 74). Our results suggest that the magnitude of effects of intermediate to high levels of species loss on grassland productivity and C storage rivals and can even surpass that of a ∼50% increase in atmospheric CO2 or high rates of N deposition experienced in industrialized regions (48). Thus, CO2 levels projected to be reached by the end of the century may have less of an impact on grassland soil C storage than concomitant reductions in species richness that surpass ∼40%. Additionally, the nonlinearity of the species richness effect has important implications to grassland restoration. Based on our results, planting even a relatively low-diversity mixture (i.e., 4 species) will notably increase soil C storage compared with planting a monoculture, but the addition of further species (i.e., species richness >4) may have only modest additional benefit from a C storage perspective. Overall, our results call into question whether ecosystem C storage will keep pace with CO2 rise and show that changes in species richness may have more profound effects on grassland C balances.
Materials and Methods
Site Description and Experimental Design.
This work was conducted at the BioCON (Biodiversity, CO2, and N) experiment within the Cedar Creek Ecosystem Science Reserve in East Bethel, Minnesota, part of the Cedar Creek Long Term Ecological Research program. BioCON (6) was established in 1997 on secondary successional grassland and is characterized by sandy, N-poor soils derived from a glacial outwash plain. Climate is continental, with mean January temperature of −11 °C, mean July temperature of 22 °C, and mean annual precipitation of 660 mm ⋅ y−1. All plots were burned roughly every other year (but we note a period of annual burns from 2011 to 2014) to mimic presettlement fire regimes in tall grass prairies.
The BioCON experiment is a complete factorial design with a split-plot arrangement of treatments, including two atmospheric CO2 (ambient, +180 ppm), two soil N (ambient, +4 g N m−2 ⋅ y−1), and four species richness (1, 4, 9, and 16 species) treatments. Treatments began in 1998. CO2 is the whole-plot factor and was applied to three of six 20 m diameter free-air CO2 enrichment rings during daylight hours throughout the growing season. The N and species richness treatments are subplot factors and were randomly assigned to individual 2 × 2 m plots within each ring. N was applied as slow-release ammonium nitrate in three equal fractions over the growing season. Plots were initially planted with 1, 4, 9, or 16 perennial species belonging to four herbaceous plant functional groups: C3 grasses: Agropyron repens, Bromus inermis, Koeleria cristata, Poa pratensis; C4 grasses: Agropyron gerardii, Bouteloua gracilis, Schizachyrium scoparium, Sorghastrum nutans; nonleguminous forbs: Achillea millefolium, Anemone cylindrica, Asclepias tuberosa, Solidago rigida; and N-fixing legumes: Amorpha canescens, L. capitata, Lupinus perennis, Petalostemum villosum. Monocultures were replicated twice for each combination of species, CO2, and N, such that there were 32 total plots planted with 1 species. For 4 and 9 species plots, species were randomly selected from a pool of all 16 possible species. For each combination of CO2 and N under otherwise ambient conditions, there were 15 replicates for plots planted with 4 species, 15 replicates for plots planted with 9 species (until 2006; replicates were reduced to 9 beginning in 2007 and to 6 beginning in 2012), and 12 replicates for plots planted with 16 species. Thus, the main randomized experiment (used in this study) contained a total of 296 plots until 2006, 272 plots from 2007 to 2011, and 260 plots from 2012 onwards. The average realized species richness across 1998 to 2016 for both a permanent, undisturbed (aside from weeding) area (12.5% of plot) in the center of each plot used for percent cover measurements and from clipped aboveground biomass samples (from a separate area representing 2.5% of the plot) was 1, 3, 6, and 8 for 1, 4, 9, and 16 species plots, respectively. Note that average realized plot-level species richness was likely much higher in mixtures based on a one-time detailed mapping of the plant community in 80% of the area of 80 plots in 2019; 4 and 5 more species, respectively, were encountered on average in 9 and 16 species plots in the larger mapped area than the smaller areas sampled annually.
Soil C.
To determine total soil C, five soil cores were collected per plot in 1997 (pretreatment), 2002, 2007, 2012, and 2016, typically in early fall. One core was taken using a 2-cm diameter metal soil corer from each quadrant of the plot and one was taken in the middle of the north side of each plot. Cores were taken at four depth intervals (0 to 10, 10 to 20, 20 to 40, and 40 to 60 cm). All five cores for a given interval were combined for each plot and dried at 40 °C for at least 2 wks. Dried soil was sieved (2 mm) to remove roots and other particulate organic material. Soil from each plot and depth interval was then ground and analyzed for C concentration on an Elemental C-N Analyzer (ECS 4010 CHNSO Analyzer, Costech Analytical Technologies Inc. or NA 1500 CNS Analyzer, Carlo-Erba Instruments). Bulk density was measured in each plot in July of 2003. A 5 cm diameter schedule 40 PVC root corer was used for the 0 to 10 and 0 to 20 cm depths and a 5 cm diameter machined metal root corer was used for the 20 to 40 and 40 to 60 cm depths. Samples from each depth were dried at 40 °C and subsequently weighed.
To determine soil C mass, we multiplied soil C concentration by soil mass (g ⋅ m−2) for each depth interval. Soil mass was calculated from depth interval thickness and bulk density. We used mean bulk density for each species richness level from 0 to 10 cm because species richness affects bulk density in our experiment at this depth interval. For each depth interval below 10 cm, we used mean bulk density across all plots. Neither CO2 nor N treatments affected bulk density at this site. We summed soil C masses across depths to calculate total soil C pools to 60 cm.
Net Primary Productivity and Plant C Pools.
To determine aboveground biomass (which approximates aboveground NPP in this herbaceous system), a 10 × 100 cm strip was harvested to ∼1 cm above soil level in all plots each August, dried at 40 °C, and subsequently weighed. Three root cores per plot were taken from the same strip using a 5 cm diameter schedule 40 PVC root corer from 0 to 20 cm depth and combined. At this site, the majority of root biomass occurs above 20 cm. Root cores were then rinsed on a fine mesh screen with a gentle stream of water to remove sand, dried at 40 °C, weighed, and ground for C and N analyses. Aboveground and root biomass samples were analyzed for C concentration as above. Aboveground and root C masses were calculated as C concentration multiplied by aboveground or total (crown + coarse + fine) root biomass to a depth of 20 cm.
Total NPP (g ⋅ C ⋅ m−2) was calculated as a given year’s aboveground biomass C plus root in-growth biomass C. At this site, on average, aboveground NPP contributed 65% and belowground NPP 36% to total NPP during the first 19 y. Root ingrowth cores were taken to a depth of 20 cm. Roots were rinsed on a fine mesh screen with a gentle stream of water to remove sand, dried at 40 °C, and weighed. Because C content was not measured in root ingrowth samples, we multiplied plot root C concentration (described above) by root ingrowth biomass to estimate root ingrowth biomass C.
Soil CO2 Flux.
In every plot, we measured soil CO2 flux 12 to 15 times per year between early May and late September using a LI-COR 6400 gas exchange system with a LI-COR 6400-09 soil respiration chamber (LI-COR). We averaged these data to derive a single mean soil CO2 flux value for each plot each year. Vegetation is clipped within the flux measurement area, but measurements include root respiration.
We estimated cumulative growing season (May to September) soil CO2 flux by converting mean growing season values for each plot from units of μmol ∙ CO2 ⋅ m−2 ⋅ s−1 to g ⋅ C ⋅ m−2 ⋅ d−1 and multiplying by the number of days in May through September (153 d). Nongrowing season soil CO2 flux likely accounts for <10% of total annual soil CO2 flux in this system (20).
Fire C Loss Estimations.
We estimated annual C losses via fire to compare with other mechanisms of C losses by measuring % biomass loss through fire during a burn in fall of 2016 for 76 plots distributed across treatments. To measure pre- and postfire aboveground biomass, we measured biomass in adjacent 10 × 100 cm strips to ∼1 cm above soil level immediately before and after the burn (as above). As most plots experienced nearly complete biomass loss, regardless of treatment, we applied the median % biomass loss across all 76 plots (97%) to August biomass preceding burns to estimate losses of biomass C via fire in all burn years. We multiplied the resulting estimate of biomass loss (g ⋅ m−2 ⋅ y−1) by each plot’s respective August aboveground biomass C concentration. In years without prescribed burns, fire C losses were set to zero.
Statistical Analyses.
For all analyses, we used repeated measures linear mixed-effects models including main effects of year (as a continuous variable), species richness (as a continuous variable), CO2, N, and all interactions of the above. Ring was nested within CO2 as a random effect. Plot was nested within CO2 and N as a random effect. Data were checked for assumptions of normality and homogeneity of variances; soil and total ecosystem C pools were log10 transformed. Pretreatment soil C data from 1997 showed no differences among levels of CO2 and N but very modest decreases with species richness and were thus not included in analyses. All analyses were conducted with statistical analysis software (JMP Pro-14.2.0, 2018, SAS Institute Inc).
Supplementary Material
Acknowledgments
We greatly appreciate K. Worm and many undergraduate interns for assistance with experimental operation and data collection. This research was supported by NSF Long-Term Ecological Research Grants DEB-0620652, DEB-1234162, and DEB-1831944; Long-Term Research in Environmental Biology Grants DEB-1242531 and DEB-1753859; Biological Integration Institutes Grant NSF-DBI-2021898; Ecosystem Sciences Grant DEB-1120064; Biocomplexity Grant DEB-0322057; and US Department of Energy Programs for Ecosystem Research Grant DE-FG02-96ER62291; and by the University of Minnesota.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2016965118/-/DCSupplemental.
Data Availability
TEXT, HTML, and EML format data have been deposited in the Cedar Creek Ecosystem Science Reserve Data Catalog. Soil percent nitrogen and carbon data can be found at DOI: 10.6073/pasta/8fe02cded3d5d2979e4596d465b0e470 (EDI Package ID: knb-lter-cdr.339.8). Plant aboveground biomass carbon and nitrogen data can be found at DOI: 10.6073/pasta/4922002b6bc68b8947bf9f8f6905516f (EDI Package ID: knb-lter-cdr.305.9). Root carbon/nitrogen data can be found at DOI: 10.6073/pasta/cfc4c93aee56a6d8bb60b5fb206cca0d (EDI Package ID: knb-lter-cdr.298.9). Plant aboveground biomass data can be found at DOI: 10.6073/pasta/8524be9f00b40a9e71b73a8ba2dc9ed0 (EDI Package ID: knb-lter-cdr.302.9). Root biomass data can be found at DOI: 10.6073/pasta/c00662959002e588597bd77e0c7dbdbb (EDI Package ID: knb-lter-cdr.325.9). Root ingrowth biomass data can be found at DOI: 10.6073/pasta/d307637c4582713659a27cbd209a1d78 (EDI Package ID: knb-lter-cdr.299.10). Soil carbon flux data can be found at DOI: 10.6073/pasta/7378dc1bf52efbcd45dbf9741c925081 (EDI Package ID: knb-lter-cdr.300.8). Fire biomass loss data can be found at DOI: 10.6073/pasta/7cf202d217f7a959e9c08ad29d43f758.
References
- 1.Ciais P., et al., “Carbon and other biogeochemical cycles” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Stocker T. F., Ed. (Cambridge University Press, Cambridge, United Kingdom, and New York, 2013), pp. 465–570. [Google Scholar]
- 2.Houghton R., Balancing the global carbon budget. Annu. Rev. Earth Planet. Sci. 35, 313–347 (2007). [Google Scholar]
- 3.Todd-Brown K., et al., Causes of variation in soil carbon simulations from CMIP5 Earth system models and comparison with observations. Biogeosciences 10, 1717–1736 (2013). [Google Scholar]
- 4.Ainsworth E. A., Rogers A., The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 30, 258–270 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Nowak R. S., Ellsworth D. S., Smith S. D., Functional responses of plants to elevated atmospheric CO2–do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol. 162, 253–280 (2004). [Google Scholar]
- 6.Reich P. B., et al., Plant diversity enhances ecosystem responses to elevated CO2 and nitrogen deposition. Nature 410, 809–812 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Reich P. B., et al., Synergistic effects of four climate change drivers on terrestrial carbon cycling. Nat. Geosci. 13, 787–793 (2020). [Google Scholar]
- 8.Reich P. B., Hobbie S. E., Decade-long soil nitrogen constraint on the CO2 fertilization of plant biomass. Nat. Clim. Chang. 3, 278–282 (2013). [Google Scholar]
- 9.Reich P. B., Hobbie S. E., Lee T. D., Pastore M. A., Unexpected reversal of C3 versus C4 grass response to elevated CO2 during a 20-year field experiment. Science 360, 317–320 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Pellegrini A. F. A., et al., Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity. Nature 553, 194–198 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Niklaus P., Leadley P., Schmid B., Körner C., A long‐term field study on biodiversity × elevated CO2 interactions in grassland. Ecol. Monogr. 71, 341–356 (2001). [Google Scholar]
- 12.Pastore M. A., Lee T. D., Hobbie S. E., Reich P. B., Strong photosynthetic acclimation and enhanced water-use efficiency in grassland functional groups persist over 21 years of CO2 enrichment, independent of nitrogen supply. Glob. Change Biol. 25, 3031–3044 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Luo Y., Hui D., Zhang D., Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: A meta-analysis. Ecology 87, 53–63 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Jones M. B., Donnelly A., Carbon sequestration in temperate grassland ecosystems and the influence of management, climate and elevated CO2. New Phytol. 164, 423–439 (2004). [Google Scholar]
- 15.Jastrow J. D., et al., Elevated atmospheric carbon dioxide increases soil carbon. Glob. Change Biol. 11, 2057–2064 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Niklaus P. A., Wohlfender M., Siegwolf R., Körner C., Effects of six years atmospheric CO2 enrichment on plant, soil, and soil microbial C of a calcareous grassland. Plant Soil 233, 189–202 (2001). [Google Scholar]
- 17.Hungate B. A., et al., Cumulative response of ecosystem carbon and nitrogen stocks to chronic CO2 exposure in a subtropical oak woodland. New Phytol. 200, 753–766 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang M., et al., The fate of carbon in a mature forest under carbon dioxide enrichment. Nature 580, 227–231 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Song J., et al., Elevated CO2 does not stimulate carbon sink in a semi-arid grassland. Ecol. Lett. 22, 458–468 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Adair E. C., Reich P. B., Hobbie S. E., Knops J. M., Interactive effects of time, CO2, N, and diversity on total belowground carbon allocation and ecosystem carbon storage in a grassland community. Ecosystems (N. Y.) 12, 1037–1052 (2009). [Google Scholar]
- 21.Reid J. P., Adair E. C., Hobbie S. E., Reich P. B., Biodiversity, nitrogen deposition, and CO2 affect grassland soil carbon cycling but not storage. Ecosystems (N. Y.) 15, 580–590 (2012). [Google Scholar]
- 22.Hungate B. A., et al., The fate of carbon in grasslands under carbon dioxide enrichment. Nature 388, 576–579 (1997). [Google Scholar]
- 23.Harrison K., Broecker W., Bonani G., A strategy for estimating the impact of CO2 fertilization on soil carbon storage. Global Biogeochem. Cycles 7, 69–80 (1993). [Google Scholar]
- 24.Adair C. E., Reich P. B., Trost J. J., Hobbie S. E., Elevated CO2 stimulates grassland soil respiration by increasing carbon inputs rather than by enhancing soil moisture. Glob. Change Biol. 17, 3546–3563 (2011). [Google Scholar]
- 25.Luo Y., Jackson R. B., Field C. B., Mooney H. A., Elevated CO2 increases belowground respiration in California grasslands. Oecologia 108, 130–137 (1996). [DOI] [PubMed] [Google Scholar]
- 26.Marhan S., Kandeler E., Rein S., Fangmeier A., Niklaus P. A., Indirect effects of soil moisture reverse soil C sequestration responses of a spring wheat agroecosystem to elevated CO2. Glob. Change Biol. 16, 469–483 (2010). [Google Scholar]
- 27.Byram G. M., “Combustion of forest fuels” in Forest Fire, Control and Use, Davis K. P., Ed. (McGraw-Hill, New York, 1959), chap. 3, pp. 61–89. [Google Scholar]
- 28.Rao L. E., Allen E. B., Meixner T., Risk-based determination of critical nitrogen deposition loads for fire spread in southern California deserts. Ecol. Appl. 20, 1320–1335 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Luo Y., et al., Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54, 731–739 (2004). [Google Scholar]
- 30.Reich P. B., Walters M. B., Ellsworth D. S., From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. U.S.A. 94, 13730–13734 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeBauer D. S., Treseder K. K., Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89, 371–379 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Fornara D. A., Tilman D., Soil carbon sequestration in prairie grasslands increased by chronic nitrogen addition. Ecology 93, 2030–2036 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Frey S. D., et al., Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 121, 305–316 (2014). [Google Scholar]
- 34.Reich P. B., et al., Species and functional group diversity independently influence biomass accumulation and its response to CO2 and N. Proc. Natl. Acad. Sci. U.S.A. 101, 10101–10106 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y., Tilman D., Furey G., Lehman C., Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat. Commun. 10, 718 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tilman D., et al., Diversity and productivity in a long-term grassland experiment. Science 294, 843–845 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Fornara D., Tilman D., Plant functional composition influences rates of soil carbon and nitrogen accumulation. J. Ecol. 96, 314–322 (2008). [Google Scholar]
- 38.Lange M., et al., Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 6, 6707 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Cong W. F., et al., Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J. Ecol. 102, 1163–1170 (2014). [Google Scholar]
- 40.Steinbeiss S., et al., Plant diversity positively affects short‐term soil carbon storage in experimental grasslands. Glob. Change Biol. 14, 2937–2949 (2008). [Google Scholar]
- 41.De Deyn G. B., et al., Additional carbon sequestration benefits of grassland diversity restoration. J. Appl. Ecol. 48, 600–608 (2011). [Google Scholar]
- 42.Prommer J., et al., Increased microbial growth, biomass, and turnover drive soil organic carbon accumulation at higher plant diversity. Glob. Change Biol. 26, 669–681 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knops J. M., Bradley K. L., Soil carbon and nitrogen accumulation and vertical distribution across a 74-year chronosequence. Soil Sci. Soc. Am. J. 73, 2096–2104 (2009). [Google Scholar]
- 44.Collins M., et al., “Long-term climate change: projections, commitments and irreversibility” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Stocker T. F., Ed. (Cambridge University Press, Cambridge, United Kingdom, and New York, 2013), pp. 1029–1136. [Google Scholar]
- 45.Ackerman D., Millet D. B., Chen X., Global estimates of inorganic nitrogen deposition across four decades. Global Biogeochem. Cycles 33, 100–107 (2019). [Google Scholar]
- 46.Kanakidou M., et al., Past, present, and future atmospheric nitrogen deposition. J. Atmos. Sci. 73, 2039–2047 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galloway J. N., et al., Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 320, 889–892 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Dentener F., et al., Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation. Global Biogeochem. Cycles 20, GB4003 (2006). [Google Scholar]
- 49.Reich P. B., Peterson D. W., Wedin D. A., Wrage K., Fire and vegetation effects on productivity and nitrogen cycling across a forest–grassland continuum. Ecology 82, 1703–1719 (2001). [Google Scholar]
- 50.Reich P. B., et al., Impacts of biodiversity loss escalate through time as redundancy fades. Science 336, 589–592 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Sala O. E., et al., Global biodiversity scenarios for the year 2100. Science 287, 1770–1774 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Harrison S. P., Gornish E. S., Copeland S., Climate-driven diversity loss in a grassland community. Proc. Natl. Acad. Sci. U.S.A. 112, 8672–8677 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zilverberg C. J., et al., Biomass yield from planted mixtures and monocultures of native prairie vegetation across a heterogeneous farm landscape. Agric. Ecosyst. Environ. 186, 148–159 (2014). [Google Scholar]
- 54.Smith J., et al., Projected changes in mineral soil carbon of European croplands and grasslands, 1990–2080. Glob. Change Biol. 11, 2141–2152 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Zhang J., Felzer B. S., Troy T. J., Projected changes of carbon balance in mesic grassland ecosystems in response to warming and elevated CO2 using CMIP5 GCM results in the Central Great Plains, USA. Ecol. Modell. 434, 109247 (2020). [Google Scholar]
- 56.Gao Q., et al., Stimulation of soil respiration by elevated CO2 is enhanced under nitrogen limitation in a decade-long grassland study. Proc. Natl. Acad. Sci. U.S.A. 117, 33317–33324 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo Y., et al., Toward more realistic projections of soil carbon dynamics by Earth system models. Global Biogeochem. Cycles 30, 40–56 (2016). [Google Scholar]
- 58.Dijkstra F. A., Hobbie S. E., Reich P. B., Knops J. M., Divergent effects of elevated CO2, N fertilization, and plant diversity on soil C and N dynamics in a grassland field experiment. Plant Soil 272, 41–52 (2005). [Google Scholar]
- 59.Craine J. M., et al., The role of plant species in biomass production and response to elevated CO2 and N. Ecol. Lett. 6, 623–625 (2003). [Google Scholar]
- 60.West J. B., Hobbie S. E., Reich P. B., Effects of plant species diversity, atmospheric [CO2], and N addition on gross rates of inorganic N release from soil organic matter. Glob. Change Biol. 12, 1400–1408 (2006). [Google Scholar]
- 61.Reich P. B., Hobbie S. E., Lee T. D., Plant growth enhancement by elevated CO2 eliminated by joint water and nitrogen limitation. Nature Geoscience 7, 920–924 (2014). [Google Scholar]
- 62.Poirier V., Roumet C., Munson A. D., The root of the matter: Linking root traits and soil organic matter stabilization processes. Soil Biol. Biochem. 120, 246–259 (2018). [Google Scholar]
- 63.Lützow M. v., et al., Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions–a review. Eur. J. Soil Sci. 57, 426–445 (2006). [Google Scholar]
- 64.Hanson P., Edwards N., Garten C. T., Andrews J., Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 48, 115–146 (2000). [Google Scholar]
- 65.Pellegrini A. F., et al., Frequent burning causes large losses of carbon from deep soil layers in a temperate savanna. J. Ecol. 108, 1426–1441 (2020). [Google Scholar]
- 66.Lu M., et al., Minor stimulation of soil carbon storage by nitrogen addition: A meta-analysis. Agric. Ecosyst. Environ. 140, 234–244 (2011). [Google Scholar]
- 67.Dijkstra F. A., West J. B., Hobbie S. E., Reich P. B., Trost J., Plant diversity, CO2, and N influence inorganic and organic N leaching in grasslands. Ecology 88, 490–500 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Lau J. A., Strengbom J., Stone L. R., Reich P. B., Tiffin P., Direct and indirect effects of CO2, nitrogen, and community diversity on plant-enemy interactions. Ecology 89, 226–236 (2008). [DOI] [PubMed] [Google Scholar]
- 69.Kazanski C., “Soil carbon cycling responses to elevated CO2 and nitrogen addition.” Ph.D. dissertation, Retrieved from the University of Minnesota Digital Conservancy, (Minneapolis, MN, 2017).
- 70.Hungate B. A., Jackson R. B., Field C. B., Chapin F. S., Detecting changes in soil carbon in CO2 enrichment experiments. Plant Soil 187, 135–145 (1996). [Google Scholar]
- 71.Lee T. D., Barrott S. H., Reich P. B., Photosynthetic responses of 13 grassland species across 11 years of free‐air CO2 enrichment is modest, consistent and independent of N supply. Glob. Change Biol. 17, 2893–2904 (2011). [Google Scholar]
- 72.Post W. M., Kwon K. C., Soil carbon sequestration and land‐use change: Processes and potential. Glob. Change Biol. 6, 317–327 (2000). [Google Scholar]
- 73.Henn J. J., Damschen E. I., “The interactive effects of prescribed fire timing and climate change on Midwestern tallgrass prairie communities” (2020). https://www.firescience.gov/projects/16-2-01-26/project/16-2-01-26_final_report.pdf. Accessed 1 April 2021.
- 74.Wesche K., Krause B., Culmsee H., Leuschner C., Fifty years of change in Central European grassland vegetation: Large losses in species richness and animal-pollinated plants. Biol. Conserv. 150, 76–85 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
TEXT, HTML, and EML format data have been deposited in the Cedar Creek Ecosystem Science Reserve Data Catalog. Soil percent nitrogen and carbon data can be found at DOI: 10.6073/pasta/8fe02cded3d5d2979e4596d465b0e470 (EDI Package ID: knb-lter-cdr.339.8). Plant aboveground biomass carbon and nitrogen data can be found at DOI: 10.6073/pasta/4922002b6bc68b8947bf9f8f6905516f (EDI Package ID: knb-lter-cdr.305.9). Root carbon/nitrogen data can be found at DOI: 10.6073/pasta/cfc4c93aee56a6d8bb60b5fb206cca0d (EDI Package ID: knb-lter-cdr.298.9). Plant aboveground biomass data can be found at DOI: 10.6073/pasta/8524be9f00b40a9e71b73a8ba2dc9ed0 (EDI Package ID: knb-lter-cdr.302.9). Root biomass data can be found at DOI: 10.6073/pasta/c00662959002e588597bd77e0c7dbdbb (EDI Package ID: knb-lter-cdr.325.9). Root ingrowth biomass data can be found at DOI: 10.6073/pasta/d307637c4582713659a27cbd209a1d78 (EDI Package ID: knb-lter-cdr.299.10). Soil carbon flux data can be found at DOI: 10.6073/pasta/7378dc1bf52efbcd45dbf9741c925081 (EDI Package ID: knb-lter-cdr.300.8). Fire biomass loss data can be found at DOI: 10.6073/pasta/7cf202d217f7a959e9c08ad29d43f758.