Abstract
Background
People with neuromuscular disorders may have a weak, ineffective cough predisposing them to respiratory complications. Cough augmentation techniques aim to improve cough effectiveness and mucous clearance, reduce the frequency and duration of respiratory infections requiring hospital admission, and improve quality of life.
Objectives
To determine the efficacy and safety of cough augmentation techniques in adults and children with chronic neuromuscular disorders.
Search methods
On 13 April 2020, we searched the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL, and ClinicalTrials.gov for randomised controlled trials (RCTs), quasi‐RCTs, and randomised cross‐over trials.
Selection criteria
We included trials of cough augmentation techniques compared to no treatment, alternative techniques, or combinations thereof, in adults and children with chronic neuromuscular disorders.
Data collection and analysis
Two review authors independently assessed trial eligibility, extracted data, and assessed risk of bias. The primary outcomes were the number and duration of unscheduled hospitalisations for acute respiratory exacerbations. We assessed the certainty of evidence using GRADE.
Main results
The review included 11 studies involving 287 adults and children, aged three to 73 years. Inadequately reported cross‐over studies and the limited additional information provided by authors severely restricted the number of analyses that could be performed.
Studies compared manually assisted cough, mechanical insufflation, manual and mechanical breathstacking, mechanical insufflation‐exsufflation, glossopharyngeal breathing, and combination techniques to unassisted cough and alternative or sham interventions. None of the included studies reported on the primary outcomes of this review (number and duration of unscheduled hospital admissions) or listed 'adverse events' as primary or secondary outcome measures.
The evidence suggests that a range of cough augmentation techniques may increase peak cough flow compared to unassisted cough (199 participants, 8 RCTs), but the evidence is very uncertain. There may be little to no difference in peak cough flow outcomes between alternative cough augmentation techniques (216 participants, 9 RCTs).
There was insufficient evidence to determine the effect of interventions on measures of gaseous exchange, pulmonary function, quality of life, general function, or participant preference and satisfaction.
Authors' conclusions
We are very uncertain about the safety and efficacy of cough augmentation techniques in adults and children with chronic neuromuscular disorders and further studies are needed.
Keywords: Adolescent; Adult; Aged; Child; Child, Preschool; Humans; Middle Aged; Young Adult; Bias; Chronic Disease; Cough; Cough/physiopathology; Disease Progression; Hospitalization; Hospitalization/statistics & numerical data; Insufflation; Insufflation/methods; Mucociliary Clearance; Mucociliary Clearance/physiology; Neuromuscular Diseases; Neuromuscular Diseases/complications; Patient Satisfaction; Quality of Life; Respiration; Respiration Disorders; Respiration Disorders/etiology
Plain language summary
The safety and effectiveness of techniques to assist coughing in people with chronic neuromuscular disorders
Review question
We reviewed the evidence on the effectiveness and safety of techniques used to assist coughing in people with chronic neuromuscular disorders (cough augmentation techniques).
Background
People with neuromuscular disorders (nerve‐related conditions that affect the muscles) may have difficulty coughing and clearing mucous from the airways, placing them at risk of choking, recurrent chest infections, and ongoing lung disease. Cough augmentation techniques, such as manually assisted cough, bagging (using a self‐inflating bag commonly used for resuscitation), mechanical Cough Assist (a device that clears secretions by applying a positive pressure to the airway, then rapidly shifting to a negative pressure), 'frog' breathing (a method of breathing to help a person take in a bigger volume of air), and breathstacking (the person takes a number of sequential breaths in, stacking one breath on top of the other without breathing out in between breaths) aim to improve cough effectiveness, with the eventual aim of reducing the number or severity (or both) of chest infections, and improving the ability of people to perform daily activities (functional ability) and quality of life.
Methods
We carried out a wide database search for studies of cough augmentation techniques in adults and children with chronic neuromuscular disorders. We selected studies that assigned people to the treatment(s) or treatment order by chance, as this study type provides the best evidence.
Results and quality of the evidence
We found 11 studies with 287 people and several cough augmentation techniques. One study measured the long‐term effects of treatment, but was only published as an abstract without enough information to accurately analyse the study findings. Many included studies had problems with how they were performed, how their findings were reported, or both, which made it difficult to fully interpret their results. None of the studies reported on the outcomes we thought were the most important for making decisions about the effectiveness and safety of cough augmentation techniques. For example, the studies did not report on the number or duration of unscheduled hospital admissions for chest infections, survival, functional ability, or quality of life. The safety of cough augmentation techniques could not be determined. Some studies suggested that cough augmentation techniques may be better than an unassisted cough, but the results are very uncertain. There was not enough evidence to show that any one technique was better than another in improving cough effort.
Conclusions and recommendation
The findings of this review provided insufficient information to make decisions about when and how to use cough augmentation techniques in people with chronic neuromuscular disorders. There is currently very low certainty evidence for or against the safety and effectiveness of cough augmentation techniques in people with chronic neuromuscular diseases and more studies are needed.
The evidence is up‐to‐date to 13 April 2020.
Summary of findings
Summary of findings 1. Cough augmentation therapy compared with an alternative cough augmentation technique or combination of techniques for people with neuromuscular diseases.
| Cough augmentation compared with an alternative cough augmentation technique or combination technique | ||||||
|
Patient or population: participants with chronic neuromuscular diseases Settings: – Intervention: cough augmentation Comparison: alternative cough augmentation technique | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Alternative cough augmentation technique | Cough augmentation | |||||
| Number of unscheduled hospital admissions for 'maintenance therapy' | Not reported | |||||
| Duration of hospital stay (days) for 'rescue' therapy | Not reported | |||||
|
PCF Follow‐up: < 1 day ('rescue' and 'maintenance' therapy) |
8 RCTs (198 participants) studied various cough augmentation techniques or combinations of techniques.
Repeated measures data were reported and could not be meta‐analysed. |
— | 198 (8 RCTs (7 cross‐over, 1 parallel group) | ⊕⊝⊝⊝ Verylowa | Cough augmentation may improve PCF compared to unassisted cough, but the certainty of evidence was very low. See Table 2 for details. |
|
|
Any adverse events Follow‐up: < 1 day or 1–2 days ('rescue and maintenance therapy) |
4 cross‐over RCTs (64 participants) compared various cough augmentation techniques or combinations of techniques (including mechanical insufflation, mechanical exsufflation, MI‐E, MAC, MAC + manual breathstacking, MI‐E + MAC, MAC + manual breathstacking, MAC + mechanical insufflation).
|
— | 64 (4 cross‐over RCTs) | ⊕⊝⊝⊝ Verylowb | We are unable to draw a conclusion as the certainty of evidence is very low. See Table 3; Table 4 for details. | |
| Quality of life for 'maintenance' therapy | No study measured or reported quality of life. | |||||
| Participant preference or satisfaction for 'rescue' and 'maintenance' therapy | No study measured or reported participant preference or satisfaction. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MAC: manually assisted cough; MI‐E: mechanical insufflation‐exsufflation; PCF: peak cough flow; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded three levels – twice for study limitations – all studies were at high risk of bias in at least one domain and unclear in several. Data were based on repeated (dependent) measurements from seven cross‐over and one parallel‐group RCTs. We also downgraded the evidence for imprecision – all studies had a small sample size, wide CI, or both. The outcome was measured less than one day after the intervention, rather than in the medium and long term as specified. bDowngraded three levels – twice for study limitations – all studies were at high risk of bias in at least one domain and unclear in several. Data were based on repeated (dependent) measurements from seven cross‐over and one parallel‐group RCTs. We also downgraded the evidence for imprecision – all studies had a small sample size.
1. Summary of findings: cough augmentation therapy, short‐term outcomes – details of PCF by comparison.
| Mean difference in PCF post intervention‐baseline (L/min) | |||||||
| Comparison (experimental vs control/alternative therapy/sham therapy) | Summary of results | Illustrative comparative risks | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Control/comparator | Experimental | ||||||
|
Manual breathstacking vs mechanical breathstacking Follow‐up: < 1 day |
No evidence of a difference between manual and mechanical breathstacking in the change of PCF. | The mean PCF difference in the comparison group was 67 (SD 73) L/min | The mean PCF difference in the experimental group was 61 (SD 72) L/min | MD 6.00 (–33.43 to 45.43) | 52 (1) | ⊕⊕⊝⊝ Lowa | Based on 1 short‐term RCT with high risk of performance and detection bias and unclear allocation concealment (Toussaint 2016). |
|
Glossopharyngeal breathing vs manual breathstacking Follow‐up: < 1 day |
No evidence of a difference between glossopharyngeal breathing and manual breathstacking in the change of PCF. | The mean PCF difference in the comparison group was 72.86 (SD 61.84) L/min | The mean PCF difference in the experimental group was 32.14 (SD 26.44) L/min | MD –40.72 (–90.54 to 9.10) | 14 (1) | ⊕⊝⊝⊝ Verylowb | Based on first‐period data from 1 cross‐over RCT with unclear allocation concealment, very small sample size, imprecision of results (wide CI), and substantial risk of performance and detection bias (Torres‐Castro 2016). |
|
Mechanical insufflation + MAC vs MI‐E Follow‐up: < 1 day |
Mechanical insufflation + MAC produced a greater change in PCF compared to MI‐E alone. | The mean PCF difference in the comparison group was 53.4 (SD 51) L/min | The mean PCF difference in the experimental group was 124.8 (SD 38.4) L/min | MD 71.40 (18.08 to 124.72) | 11 (1) | ⊕⊝⊝⊝ Verylowc | Based on first‐period data of 1 cross‐over RCT with very small sample size, imprecision of results (wide CIs), and substantial risk of performance and other biases (Lacombe 2014). |
|
MI‐E + MAC vs MI‐E Follow‐up: < 1 day |
No clear evidence of a difference between MI‐E + MAC compared to MI‐E alone in the change in PCF. | The mean PCF difference in the comparison group was 53.4 (SD 51) L/min | The mean PCF difference in the experimental group was 106 (SD 50.4) L/min | MD 52.80 (–0.32 to 105.92) | 54 (2) | ⊕⊝⊝⊝ Verylowc | Analysis based on first‐period data of 1 randomised cross‐over study with very small sample size (n = 14), imprecision of results (wide CIs), and substantial risk of performance and other biases (Lacombe 2014). |
| Study reported significantly higher PCF with MI‐E + MAC compared to MI‐E alone | N/A | The second study was a cross‐over RCT with high risk of performance, detection and other bias (Kim 2016). Separate period data were not reported, precluding analysis and assessment of precision. |
|||||
|
MI‐E + MAC vs mechanical insufflation + MAC Follow‐up: < 1 day |
There was no evidence of a difference in PCF change between MI‐E + MAC and mechanical insufflation + MAC. | The mean PCF difference in the comparison group was 124.8 (SD 38.4) L/min | The mean PCF difference in the intervention groups was 106 (SD 50.4) L/min | MD 18.60 (–34.46 to 71.66) | 11 (1) | ⊕⊝⊝⊝ Verylowc | Based on the first‐period data of 1 randomised cross‐over study design with very small sample size, imprecision of results (wide CIs), and substantial risk of performance and other biases (Lacombe 2014). |
|
MAC vs mechanical insufflation Follow‐up: < 1 day |
We were unable to draw a conclusion. | Both studies reported no evidence of a difference in PCF between interventions. | N/A | 26 (2) | ⊕⊝⊝⊝ Verylowc | Based on 2 cross‐over RCTs with small sample sizes (Chatwin 2003: n = 4; Sivasothy 2001: n = 22). Separate period data were not reported or available, precluding analysis and assessment of precision. |
|
|
Mechanical insufflation + MAC vs MAC Follow‐up: < 1 day |
We were unable to draw a conclusion. | Reported no evidence of a difference in PCF between interventions. | N/A | 4 (1) | ⊕⊝⊝⊝ Verylowc | Based on 1 cross‐over RCT with 4 participants eligible for this review (Sivasothy 2001). Separate period data were not reported or available, precluding analysis and assessment of precision. |
|
|
MI‐E vs MAC Follow‐up: < 1 day |
We were unable to draw a conclusion. | MI‐E reported to produce a higher PCF than MAC. | N/A | 22 (1) | ⊕⊝⊝⊝ Verylowc | Based on 1 cross‐over RCT with 22 participants (Chatwin 2003). Separate period data were not reported or available, precluding analysis and assessment of precision. |
|
|
MI‐E vs mechanical exsufflation Follow‐up: < 1 day |
We were unable to draw a conclusion. | MI‐E reported to produce a higher PCF than mechanical exsufflation. | N/A | 22 (1) | ⊕⊝⊝⊝ Verylowc | Based on 1 cross‐over RCT with 22 participants (Chatwin 2003). Separate period data were not reported or available, precluding analysis and assessment of precision. |
|
|
MI‐E vs mechanical insufflation Follow‐up: < 1 day |
We were unable to draw a conclusion. | PCF reported to be higher with MI‐E than with mechanical insufflation. | N/A | 22 (1) | ⊕⊝⊝⊝ Verylowc | Based on 1 cross‐over RCT with 22 participants (Chatwin 2003). Separate period data were not reported or available, precluding analysis and assessment of precision. |
|
|
Manual breathstacking + MAC vs MI‐E Follow‐up: < 1 day |
We were unable to draw a conclusion. | PCF reported to be higher with MI‐E than with MAC + breathstacking. | N/A | 40 (1) | ⊕⊝⊝⊝ Verylowc | Based on 1 cross‐over RCT with 40 participants (Kim 2016). Separate period data were not reported or available, precluding analysis and assessment of precision. |
|
|
MI‐E + MAC vs manual breathstacking + MAC Follow‐up: < 1 day |
We were unable to draw a conclusion. | PCF reported to be higher with MI‐E + MAC than with MAC + breathstacking. | N/A | 40 (1) | ⊕⊝⊝⊝ Verylowc | Based on 1 cross‐over RCT with 40 participants (Kim 2016). Separate period data were not reported or available, precluding analysis and assessment of precision. |
|
|
MAC vs manual breathstacking + MAC Follow‐up: < 1 day |
We were unable to draw a conclusion. | PCF reported to be higher with manual breathstacking + MAC than with MAC alone. | N/A | 28 (1) | ⊕⊝⊝⊝ Verylowc | Based on 1 cross‐over RCT with 28 participants (Brito 2009). Separate period data were not reported or available, precluding analysis and assessment of precision. |
|
|
Manual breathstacking vs manual breathstacking + MAC Follow‐up: < 1 day |
We were unable to draw a conclusion | PCF reported to be higher with manual breathstacking + MAC than with manual breathstacking alone. | N/A | 28 (1) | ⊕⊝⊝⊝ Verylowc |
Based on 1 cross‐over RCT with 28 participants (Brito 2009). Separate period data were not reported or available, precluding analysis and assessment of precision. |
|
|
Mechanical breathstacking vs mechanical insufflation Follow‐up: < 1 day |
We were unable to draw a conclusion. | PCF reported to be higher with mechanical insufflation compared to mechanical breathstacking. Not quantitatively reported. | N/A | 20 (1) | ⊕⊝⊝⊝ Verylowc | Based on 1 cross‐over RCT with 20 participants (Del Amo Castrillo 2019). Data were presented graphically only and could not be precisely extracted from figures provided. Separate period data were not reported or available, precluding analysis and assessment of precision. |
|
| CI: confidence interval; MD: mean difference; MAC: manually assisted cough; MI‐E: mechanical insufflation‐exsufflation; min: minute; n: number of participants; N/A: not available; PCF: peak cough flow; RCT: randomised controlled trial; SD: standard deviation. | |||||||
aDowngraded twice because results come from a single short‐term RCT at high risk of bias. bDowngraded three times based on a single randomised cross‐over study design with very small sample size, imprecision of results (wide CIs), and high risk of performance and detection bias. cDowngraded three times based on a single randomised cross‐over study design with very small sample size, imprecision of results (wide CIs), and substantial risk of performance and other biases.
2. Study results grouped by outcome measures and interventions – cough augmentation therapy compared to alternative individual cough augmentation therapies.
| Outcome measure | Unassisted cough | MI | ME | MI‐E | MAC | Manual BS | Mechanical BS | Sham BS | GPB | Between‐group comparison |
| PCF (L/min) |
Chatwin 2003 (n = 22) Mean (95% CI) |
|||||||||
| 169 (129 to 209)a |
182 ( 147 to 217) |
235 (186 to 284) |
297 (246 to 350) |
188 (146 to 229) |
— | — | — | — | ME vs unassisted cough: P < 0.01 MI‐E vs unassisted cough: P < 0.001 MI‐E vs ME: P < 0.001 |
|
|
Toussaint 2016 (n = 52) Mean ± SD baseline to after intervention | ||||||||||
| — | — | — | — | — | 125 ± 52 to 186 ± 50; P < 0.001; n = 25 | 132 ± 55 to 199 ± 48; P = 0.001; n = 27 | — | — | P = 0.33 | |
|
Del Amo Castrillo 2019 (n = 20) Median/IQR | ||||||||||
| 176/68a | Data not reported | — | — | — | — | Data not reported | — | — | P < 0.001 comparing MI to baseline (favouring MI) P < 0.001 comparing MI to BS (favouring MI) P = 0.004 comparing BS to baseline (favouring BS) |
|
|
Torres‐Castro 2016 (n = 14) MD ± SD (95% CI) baseline to after interventionb | ||||||||||
| — | — | — | — | — | 72.86 ± 61.84 (15.67 to 130.05); P = 0.02 | — | — | 32.14 ± 26.44 (7.69 to 56.59); P = 0.018 | P = 0.14 | |
| Transcutaneous oxygen saturation (%) |
Jenkins 2014 (n = 23) Mean ± SD before to after intervention |
|||||||||
| — | — | — | — | — | 96 ± 3.2 to 96 ± 3 | — | 96 ± 3.6 to 96 ± 2.5 | — | NS | |
| Tidal volume (mL) |
Jenkins 2014 (n = 23) Mean ± SD before to after intervention |
|||||||||
| — | — | — | — | — | 277 ± 131 to 310 ± 148; P < 0.001 | — | 303 ± 141 to 289 ± 128; NS | — | Significance levels not reported | |
| Maximum inspiratory or insufflation capacity (L or mL) |
Toussaint 2016 (n = 52) mean ± SD, L |
|||||||||
| — | — | — | — | — | 1.344 ± 0.520; n = 25 | 1.481 ± 0.477; n = 27 | — | — | Mechanical vs manual BS: MD 0.14, 95% CI –0.13 to 0.41; P = 0.3 | |
|
Del Amo Castrillo 2019 (n = 20) median (IQR), L | ||||||||||
| — | 1.630 (1.247 to 1.870) |
— | — | — | — | 1.320 (1.085–1.755) |
— | — | P = 0.12 | |
|
Torres‐Castro 2016 (n = 14) MD between baseline vital capacity and postintervention maximum inspiratory capacityb mean ± SD (95% CI), mL | ||||||||||
| — | — | — | — | — | 435.0 ± 364.5 (98.61 to 772.82); P = 0.02 |
— | — | 454.29 ± 408.16 (76.80 to 831.77); P = 0.03 | MD 19.29, 95% CI –386.09 to 424.67; P = 0.93 | |
| Minute ventilation (L/min) |
Jenkins 2014 (n = 23) Mean ± SD before to after intervention |
|||||||||
| — | — | — | — | — | 6.8 ± 3.1 to 8.0 ± 3.5; P < 0.001 | — | 7.4 ± 4.9 to 6.9 ± 3.3; NS | — | Significance levels not reported | |
| Maximal expiratory pressure (cmH2O) |
Toussaint 2016 (n = 52) Mean ± SD |
|||||||||
| — | — | — | — | — | 26 ± 9 | 28 ± 10 | — | — | P = 0.45 | |
| Respiratory rate (breaths/minute) |
Jenkins 2014 (n = 23) Mean ± SD before to after intervention |
|||||||||
| — | — | — | — | — | 27 ± 9.2 to 28 ± 10.6; P < 0.05 | — | 26 ± 10.3 to 26 ± 10.4; NS | — | Significance levels not reported | |
| Ability to perform breath stacking (%) |
Toussaint 2016 (n = 52) | |||||||||
| — | — | — | — | — | 88 | 89 | — | — | P = 0.9 | |
| Number of insufflations to maximal insufflation capacity (n) |
Toussaint 2016 (n = 52) Mean ± SD |
|||||||||
| — | — | — | — | — | 1.8 ± 0.6 | 2.6 ± 0.6 | — | — | P < 0.001 | |
| Comfort, distress, and strength of cough (VAS 10‐point score) |
Chatwin 2003 (n = 22) Mean (95% CI) |
|||||||||
| 5.4 (4.5 to 6.3)a | 5.8 (4.8 to 6.8) (NS) |
6.9 (5.3 to 7.0) (NS) |
7.3 (6.6 to 8.0) (NS) |
5.9 (5.2 to 6.7) (NS) |
— | — | — | — | Separate VAS scores not presented Significance levels not reported |
|
| Comfort (VAS 10‐point score) |
Del Amo Castrillo 2019 (n = 20) Median (IQR) |
|||||||||
| — | 6.4 (5.2 to 7.6) | — | — | — | — | 6.5 (3.9–7.4) | — | — | P = 0.31 | |
| Subjective cough effectiveness (VAS 10‐point score) |
Del Amo Castrillo 2019 (n = 20) Median (IQR) |
|||||||||
| — | 6.0 (4.85 to 8.2) | — | — | — | — | 6.2 (5.1–7.1) | — | — | P = 0.17 | |
BS: breathstacking; CI: confidence interval; GPB: glossopharyngeal breathing; IQR: interquartile range; PCF: peak cough flow; MAC: manually assisted cough; MD: mean difference; ME: mechanical exsufflation; MI: mechanical insufflation; MI‐E: mechanical insufflation/exsufflation; min: minute; n: number of participants; NS: not significant; SD: standard deviation; VAS: visual analogue scale. aBaseline value – not a randomly assigned control. bUsing raw first‐period data provided by the author on request.
3. Study results grouped by outcome measures and interventions – comparison of individual and combination cough augmentation therapies with alternative individual and combination interventions.
| Outcome measure | Unassisted cough | MI | MI‐E | MAC | Manual BS | MAC + MI | MAC + manual BS | MAC + MI‐E | Between‐group differences |
| PCF (L/min) |
Sivasothy 2001 (n = 4) Median (range) |
||||||||
| 288 (175 to 367)a | 231 (148–597) | — | 193 (185–287) | — | 362 (218–440) | — | — | NS | |
|
Brito 2009 (n = 28) Mean ± SD | |||||||||
| 171 ± 67a | — | — | 231 ± 81 | 225 ± 80 | — | 292 ± 86 | — | Manual BS vs unassisted cough: P < 0.001 Manual BS vs MAC: NS MAC + BS vs unassisted cough: P < 0.001 MAC vs MAC + BS: P < 0.05 Manual BS vs MAC + BS: P < 0.05 |
|
|
Lacombe 2014 (n = 18) Mean ± SD | |||||||||
| — | — | Absolute valueb: 210.6 ± 52.8 MD from baselineb: 53.4 ± 51.0; n = 7 |
— | — | Absolute valueb: 225 ± 83.4 MD from baselineb: 124.8 ± 38.4; n = 4 |
— | Absolute valueb: 210.6 ± 50.4 MD from baselineb: 106.2 ± 50.4; n = 7 |
Comparison of MDs (intervention – baseline): MI + MAC vs MI‐E alone: MD 71.4, 95% CI 18.08 to 124.72); P = 0.009 MI‐E + MAC vs MI‐E alone: MD 52.8, 95% CI –0.32 to 105.92; P = 0.05 MI‐E + MAC vs MI + MAC: MD –18.6, 95% CI –71.61 to 34.41; P = 0.49 |
|
|
Kim 2016 (n = 40) Mean ± SD | |||||||||
| 95.7 ± 40.5 | — | 177.2 ± 33.9 | — | — | — | 155.9 ± 53.1 | 202.4 ± 46.6 | MAC + manual BS vs unassisted cough: P < 0.01 MI‐E vs unassisted cough: P < 0.01 MI‐E vs MAC + manual BS: P < 0.01 MI‐E + MAC vs unassisted cough: P < 0.01 MI‐E + MAC vs MAC + manual BS: P < 0.01 MI‐E + MAC vs MI‐E alone: P < 0.01 |
|
| Transcutaneous oxygen saturation (%) |
Chatwin 2009 (n = 8) Mean |
||||||||
| — | — | — | Data not reported | — | — | — | Data not reported | NS difference in group means | |
| Transcutaneous carbon dioxide tension (%) |
Chatwin 2009 (n = 8) Mean |
||||||||
| — | — | — | Data not reported | — | — | — | Data not reported | NS difference in group means | |
| Maximum inspiratory or insufflation capacity (L) |
Lacombe 2014 (n = 18) mean ± SD |
||||||||
| — | — | 1.55 ± 0.34b; n = 7 | — | — | 1.43 ± 0.34b; n = 4 | — | 1.39 ± 0.43b; n = 7 | Comparison of means: MI‐E vs MI + MAC: MD –0.12, 95% CI –33.44 to 33.20; P = 0.99 MI‐E vs MI‐E + MAC: MD –0.16, 95% CI –0.57 to 0.25; P = 0.44 MI+ MAC vs MI‐E + MAC: MD 0.04, 95% CI –0.42 to 0.50; P = 0.86 |
|
| Cough expiratory volume (L) |
Sivasothy 2001 (n = 4) Median (range) |
||||||||
| 0.9 (0.5–1.1)a | 0.7 (0.3–1.3) | — | 0.5 (0.41–1.01) | — | 0.6 (0.4–1.01) | — | — | NS | |
| Heart rate (beats per minute) |
Chatwin 2009 (n = 8) Not specified |
||||||||
| — | — | — | Data not reported | — | — | — | Data not reported | NS | |
| Effective cough time (ms) |
Lacombe 2014 (n = 18) Mean ± SD |
||||||||
| — | — | Absolute valueb: 70 ± 79 MD from baselineb: 54 ± 95; n = 7 |
— | — | Absolute valueb: 93 ± 111 MD from baselineb: 93 ± 111; n = 4 |
— | Absolute valueb: 22 ± 47 MD from baselineb: 20 ± 42; n = 7 |
MI‐E vs MI + MAC: MD 39.0, 95% CI –90.56 to 168.56; P = 0.56 MI‐E vs MI‐E + MAC: MD –34.00, 95% CI –110.95 to 42.95; P = 0.39 MI + MAC vs MI‐E + MAC: MD 73.00, 95% CI –40.14 to 186.14; P = 0.21 |
|
| Peak value time (ms) |
Sivasothy 2001 (n = 4) Median (range) |
||||||||
| 44 (40–50)a | 45 (30–60) | — | 50 (35–55) | — | 50 (45–120) | — | — | NS | |
| Treatment time after 30 minutes (min) |
Chatwin 2009 (n = 8) Median (range) |
||||||||
| — | — | — | 17 (0–35) | — | — | — | 0 (0–26) | P = 0.03 | |
| Auscultation score (VAS 10‐point score) |
Chatwin 2009 (n = 8) MD ± SD before to after intervention |
||||||||
| — | — | — | 3.4 ± 2.0 to 2.3 ± 2.2; P = 0.007 | — | — | — | 2.9 ± 1.9 to 1.8 ± 2.0; P = 0.02 | Significance level not reported | |
| Secretions (VAS 10‐point score) |
Chatwin 2009 (n = 8) MD ± SD before to after intervention |
||||||||
| — | — | — | 4.4 ± 2.5 to 3.0 ± 1.4; P = 0.03 | — | — | — | 4.0 ± 2.2 to 1.7 ± 0.4; P = 0.03 | Significance level not reported | |
| Comfort (VAS 10‐point score) |
Chatwin 2009 (n = 8) Baseline to after intervention |
||||||||
| — | — | — | Data not reported (NS) |
— | — | — | Data not reported (NS) | Data presented graphically only. Significance level not reported |
|
|
Lacombe 2014 (n = 18) Median (IQR) | |||||||||
| — | — | Original report: 6.4 (5.5 to –7.0) b5.7 (0.9) |
— | — | Original report: 7.0 (6.0–8.5) b5.9 (1.15) |
— | Original report: 6.6 (5.8–8.0) b6.8 (.7) |
NS | |
| Subjective cough effectiveness (VAS 10‐point score) |
Sivasothy 2001 (n = 4) Not reported |
||||||||
| Not reported* | Not reported | — | Not reported | — | Not reported | — | — | Participants did not report benefit of any intervention. | |
|
Lacombe 2014 (n = 18) Median (IQR) | |||||||||
| — | — | Original report: 6.4 (4.8–8.2) b7.2 (2.4) |
— | — | Original report: 8.3 (7.2–9.0) b7.1 (0.8) |
— | Original report: 8.5 (6.2–9.0) b8.0 (1.95) |
Original report: MI‐E + MAC vs MI‐E: P < 0.05 MAC + MI vs MI‐E: P < 0.05 |
|
| Breathlessness (VAS 10‐point score) |
Chatwin 2009 (n = 8) Baseline to after intervention score |
||||||||
| — | — | — | Data not reported (NS) |
— | — | — | Data not reported (NS) |
Data presented graphically only. Significance level not reported |
|
| Mood (VAS 10‐point score) |
Chatwin 2009 (n = 8) Baseline to after intervention score |
||||||||
| — | — | — | Data not reported (NS) |
— | — | — | Data not reported (NS) |
Data presented graphically only. Significance level not reported |
|
| Fatigue (VAS 10‐point score) |
Chatwin 2009 (n = 8) MD ± SD before to after intervention |
||||||||
| — | — | — | Data not reported (NS) | — | — | — | 3.2 ± 2.2 to 5.1 ± 2.6 (P = 0.005) |
Incomplete reporting. Significance level not reported |
|
BS: breathstacking; CI: confidence interval; GPB: glossopharyngeal breathing; IQR: interquartile range; PCF: peak cough flow; MAC: manually assisted cough; MD: mean difference; ME: mechanical exsufflation; MI: mechanical insufflation; MI‐E: mechanical insufflation/exsufflation; min: minute; n: number of participants; NS: not significant; SD: standard deviation; VAS: visual analogue scale.
aBaseline value – not a randomly assigned control.
bUsing raw first‐period data provided by the author on request.
Summary of findings 2. Cough augmentation therapy compared with standard care for people with neuromuscular diseases.
| Cough augmentation therapy compared with standard care for people with neuromuscular disease | ||||
|
Patient or population: participants with chronic neuromuscular diseases Settings: – Intervention: cough augmentation therapy Comparison: standard care | ||||
| Outcome | Summary of results | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Number of unscheduled hospital admissions for 'maintenance' therapy | No study reported the number of unscheduled admissions. | 1 parallel‐group RCT of manual breathstacking compared to standard care (67 participants) planned to measure these outcomes; however, only an abstract is available and data are not fully reported (Katz 2019). Lack of quantitative data precludes assessment of precision. |
||
| Duration of hospital stay (days) for 'rescue' therapy | No study reported the duration of hospital stay. | |||
| Quality of life for 'maintenance' therapy | No study reported quality of life | |||
| Peak cough flow for 'rescue' or 'maintenance' therapy | No study reported peak cough flow | |||
|
Any adverse events for 'rescue' and 'maintenance' therapy Follow‐up: 2 years |
1 parallel‐group RCT reported that no adverse events had occurred during the 2‐year study, but this outcome was not quantitatively reported and it was unclear how it was measured. | 67 (1 study) | ⊕⊝⊝⊝ Verylowa | We are unable to draw a conclusion. |
| Quality of life for 'maintenance' therapy | No study reported quality of life. | 1 parallel‐group RCT of manual breathstacking compared to standard care (67 participants) planned to measure quality of life; however, only an abstract is available and data are not fully reported (Katz 2019). | ||
| Participant preference or satisfaction for 'rescue' or 'maintenance' therapy | No study measured or reported participant preference. | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial. | ||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||
aDowngraded three times, twice for study limitations and once for imprecision. Data were from one parallel‐group RCT, with high risk of performance and reporting bias. This outcome was not quantitatively reported and unclear how it was measured. Lack of quantitative data precludes assessment of precision but the trial was small (67 participants).
Background
Description of the condition
A range of chronic neuromuscular disorders (NMDs) have been described in adults and children, including muscular dystrophies, congenital and metabolic myopathies, neuromuscular junction disorders, peripheral neuropathies, and anterior horn cell diseases (Gozal 2000). People affected by chronic NMDs are at risk of progressive respiratory insufficiency (breathing difficulties that worsen over time), primarily from a combination of respiratory muscle weakness and chest wall abnormalities (Boitano 2006; Finder 2010; Gozal 2000; Panitch 2009).
Many people with NMDs experience progressive respiratory insufficiency with advancing age. Infants with NMDs generally have normal lungs and normal mucociliary clearance mechanisms at birth, although pulmonary mechanics may be affected from baseline, depending on the underlying NMD (Panitch 2017). Chest deformities may develop from infancy, particularly with severe forms of spinal muscular atrophy (SMA), because of respiratory muscle weakness and chronic paradoxical chest wall or abdominal movement during breathing (or both), in conjunction with an initially very compliant chest wall (Panitch 2009; Panitch 2017; Papastamelos 1996). Respiratory muscle weakness causes chronic shallow breathing; the inability to take a sufficiently deep breath to sigh or yawn, which is required to maintain full lung expansion; an ineffective cough with secretion retention; and progressive loss of lung compliance (Fauroux 2008; Panitch 2009; Panitch 2017). Progressive thoracic deformities such as scoliosis, kyphosis, and spinal rigidity, together with fibrosis of the intercostal muscles, may further impact on lung function with a progressive decrease in chest wall compliance and ultimately a restrictive pattern of respiratory disease (Fauroux 2008; Gozal 2000; Panitch 2009; Wang 2007). Bulbar weakness and glottic dysfunction, as typically seen in children with SMA type 1 and other severe NMDs, also impact on the ability to cough effectively as well as increasing the risks of aspiration (Boitano 2006; Chatwin 2018; Toussaint 2018).
An effective cough is essential to clear pulmonary secretions from the airways (Panitch 2017). If the cough is ineffective, as is often the case in people with chronic NMD and respiratory muscle weakness, short‐term inability to clear secretions may lead to acute respiratory insufficiency and respiratory failure, while long‐term retention of secretions leads to a vicious cycle of obstruction, infection, inflammation, increased work of breathing, recurrent acute respiratory tract infections, and ultimately chronic lung disease and respiratory failure (Chatwin 2018; Homnick 2007; Panitch 2017). Respiratory tract infection with altered sputum viscosity and volume, difficult or ineffective swallowing (dysphagia), and gastro‐oesophageal reflux with chronic aspiration can all exacerbate secretion retention in people with NMD and respiratory muscle weakness (Farrero 2013; Finder 2010; Iannaccone 2007).
An effective cough requires: a sufficiently deep inspiration; brief closure of the glottis with simultaneous contraction of expiratory respiratory muscles to increase intrathoracic pressure; and finally the abrupt opening of the glottis at the start of the expiratory phase to produce a rapid, forceful flow of air from the lungs (Boitano 2006; Chatwin 2018; Farrero 2013; Panitch 2017; Toussaint 2018). Any or all these phases may be affected in people with NMD (Bach 2003; Boitano 2006; Finder 2010; Rokadia 2015).
Adults have a normal peak expiratory cough flow (PCF) range between 360 L/minutes and 1200 L/minutes (Anderson 2005; Leiner 1963; Mayer 2017; Tzeng 2000). Bach 1996 suggested that adults require a PCF greater than 160 L/minute for an effective cough. Furthermore, it has been suggested that adults with NMD require a PCF of more than 270 L/minute when well, to account for the expected decline in cough flows during intercurrent respiratory infections (Bach 1997). Cough augmentation may, therefore, be indicated if PCF falls below 270 L/minute in adults and adolescents with NMD (Toussaint 2018). In children with NMDs, an absolute PCF of less than 160 L/minute has been shown to be predictive of severe disease, but age or size‐adjusted reference values are not available (Dohna‐Schwake 2006). It must be noted that the normal range of PCF in young children is highly variable, with healthy children only able to achieve PCFs of 160 L/minute on the 5th percentile by six years of age (Bianchi 2008). Therefore, for children over the age of 12 years (when children attain adult PCF (Bianchi 2008), use of the adult values of 160 L/minute and 270 L/minute PCF cut‐offs may be appropriate (Hull 2012), but the corresponding levels in younger children are as yet unclear and this warrants further investigation.
Most episodes of respiratory failure in people with NMD are likely to be caused by ineffective coughing during intercurrent chest infections (Bach 2003; Boentert 2017; Chatwin 2018). The identification of the most effective, safe measures to optimise cough efficacy and promote secretion clearance is, therefore, vital to optimising pulmonary function, preventing morbidity, and improving the quality of life in people with chronic NMD (Toussaint 2018).
Description of the intervention
Many airway clearance techniques are used in clinical practice in people with chronic NMD. Some techniques aim to move secretions from the peripheral to the more central airways (secretion mobilisation techniques), while others aim to clear secretions from the central airways (cough augmentation techniques) (Chatwin 2018; Toussaint 2018). Secretion mobilisation and an effective cough are both needed for effective secretion clearance (Farrero 2013; Finder 2010).
Manual techniques to assist peripheral secretion mobilisation in adults and children with chronic NMD include positioning, chest wall shaking, percussion and vibrations (Chatwin 2018; Finder 2010; McCool 2006; Toussaint 2018; Wang 2007). Other secretion mobilisation techniques that have been suggested for people with NMD include the active cycle of breathing and forced expiratory techniques; autogenic drainage; positive expiratory pressure therapy; oscillatory positive pressure therapy; intermittent positive pressure breathing (IPPB); chest wall strapping; intrapulmonary percussive ventilation, and high‐frequency chest wall oscillation (Anderson 2005; Bott 2009; Chatwin 2018; Douglas 1981; Finder 2010; Hull 2012; Toussaint 2003; Toussaint 2018). Active breathing exercises, such as the active cycle of breathing and positive expiratory pressure therapy, are effort dependent and, therefore, may not be useful in people with severe respiratory muscle weakness (Finder 2010; Hull 2012), unless concomitant ventilatory support is given (Chatwin 2018; Toussaint 2018).
Cough augmentation for proximal secretion clearance can be performed using manual or mechanical methods, alone or in combination, to support different components of the cough (Chatwin 2018; Finder 2010; Panitch 2017; Toussaint 2018). These may also be done in different body positions to optimise secretion clearance (Marques 2020). Techniques such as breath‐ or airstacking, glossopharyngeal breathing (GPB), and mechanical or manual single‐breath insufflations (blowing air into the lungs), augment inspiration to achieve sufficient inspiratory lung volumes before a cough (Bott 2009; Chatwin 2018; Toussaint 2018). People can achieve lung insufflation using positive pressure devices including ventilators (invasively or non‐invasively) and IPPB devices, with set pressure or volume limits, or both. They may achieve breathstacking independently (with glottic closure) or through use of an external self‐inflating manual resuscitator bag with a one‐way valve, if needed, to prevent air leak (Chatwin 2018; Toussaint 2018). For breathstacking, a person takes or receives multiple inspiratory breaths, without exhalation between breaths, until they achieve maximal insufflation capacity (MIC) (Bach 2007; Chatwin 2018; Marques 2014; Toussaint 2018). Thereafter, the individual releases the breath in a spontaneous or assisted forced expiratory manoeuvre or cough (Chatwin 2018; Marques 2014). MIC refers to the maximum tolerable inspiratory lung volume (Bach 2007; Chatwin 2018; Kang 2000). GPB or 'frog breathing,' which does not use any external equipment, requires the person with NMD to actively 'gulp' air into the lungs by opening and closing the glottis until MIC is reached (Bach 2007; Chatwin 2018; Nygren‐Bonnier 2009; Toussaint 2018).
Mechanical exsufflation (forcible expulsion of air from the lungs by artificial means) and manually assisted cough (MAC), the latter achieved by manually compressing the thorax, abdomen, or both, aim to improve expiratory flow rates by rapidly increasing intrathoracic pressure (Anderson 2005; Chatwin 2018; Finder 2010; Panitch 2017; Toussaint 2018).
Mechanical insufflation‐exsufflation (MI‐E) supports both insufflation and exsufflation, using a device that delivers a preset positive pressure into the airways for a set duration during inspiration (insufflation), immediately followed by an abrupt change to a preset negative exsufflation pressure, thereby simulating a cough with high expiratory flow rates (Anderson 2005; Chatwin 2018; Fauroux 2008; Morrow 2013; Panitch 2017; Toussaint 2018).
How the intervention might work
Both inspiratory and expiratory cough augmentation techniques aim to optimise cough efficacy by improving PCF when respiratory muscles are too weak to independently achieve sufficient flow rates for secretion clearance. The mechanism by which PCF is affected varies among different cough augmentation techniques (Chatwin 2018; Toussaint 2018).
Inspiratory cough augmentation techniques aim to augment inspiratory lung volumes to those required for an effective cough (MIC). By increasing inspiratory volume, these techniques enhance expiratory flow bias (creating higher expiratory than inspiratory air flow) during a spontaneous or assisted cough, thereby effectively mobilising and clearing secretions (Chatwin 2018). Inhaling a large volume of air before the compressive and expiratory phases of the cough optimises the length–tension relationship of expiratory muscles and may generate higher intrathoracic pressures and PCF (Boitano 2006; Chatwin 2018).
Expiratory cough augmentation techniques, whether manual or mechanical, aim to assist the weak expiratory muscles in generating sufficient intrathoracic pressures thereby increasing the expiratory flow generated during the cough. The overall aim is to increase PCF enough to effectively clear secretions from the central airways (Boitano 2006; Chatwin 2018; Toussaint 2018).
Some investigators have suggested that combining inspiratory and expiratory cough augmentation techniques could optimise cough clearance in people with NMD (Boitano 2006; Chatwin 2018; Hull 2012; Toussaint 2018; Trebbia 2005).
Why it is important to do this review
Cough augmentation techniques are considered essential to prevent pulmonary morbidity and progression to respiratory failure in people with NMD (Bach 2003; Chatwin 2018). In addition, they prevent acute respiratory failure, improve work of breathing, and relieve distress caused by retained secretions in the short term. However, it is still unclear which technique(s) offer the greatest clinical benefit with the least risk of harm.
Any application of positive pressure to the airways carries a risk of complications including abdominal distention, discomfort, gastro‐oesophageal reflux, cardiovascular effects such as changes in blood pressure and cardiac arrhythmia, and pneumothorax (Chatwin 2018; Homnick 2007; Morrow 2013; Toussaint 2018). Pneumothorax has been described in adults following the use of MI‐E (Suri 2008), breathstacking (Westermann 2013), and long‐term non‐invasive positive pressure ventilation (Vianello 2004). There may be greater risk of barotrauma and volutrauma in infants and young children with NMD compared to older children or adults, considering their different respiratory anatomy and physiology. Application of positive pressure will affect the lungs differently according to, for example, lung volumes and respiratory system compliance and resistance, all of which vary with age and NMD condition (Gattinoni 2003; Gattinoni 2010). The effects of MAC may be altered by chest wall compliance, which is almost twice that of controls in infants with NMD (Papastamelos 1996), and may be substantially reduced in adults with NMD, particularly in the case of chest wall deformities (Gozal 2000; Panitch 2009). During MI‐E specifically, applied insufflation volume is not usually measured in clinical practice, and a rapid swing to negative pressure follows insufflation. The combination of high applied tidal volume and atelectrauma (lung injury caused by repeated expansion and collapse of lung units) has been associated with lung injury in the context of invasive mechanical ventilation (Albuali 2007; Saharan 2010). The safety of MI‐E and other insufflation techniques is unclear in this regard and warrants further research.
Some cough augmentation techniques recommended in international guidelines for the treatment of people with NMD require equipment or expertise that are not readily available in lower‐resourced environments (Bott 2009; Chatwin 2018; Finder 2004; McCool 2006; Rosière 2009; Toussaint 2018; Wang 2007; Wang 2010), while cheaper and more readily available techniques may be equally effective (Anderson 2005; Finder 2010). Currently, people living with NMD and their caregivers generally manage their airway clearance according to perceived need, and clinical management is responsive to changes in the patient's condition (Toussaint 2018). The management approach also depends on availability of equipment and local expertise, which may vary substantially at a global level (Toussaint 2018). It is not yet clear what people with NMD and their caregivers prefer when considering the choice of cough augmentation technique, and this warrants investigation.
To advocate for the best and most appropriate treatment in different sociogeographical contexts, it is necessary to first determine which cough augmentation technique(s), dosages and frequencies are effective and safe for use in people with chronic NMD, using clinically relevant outcome measures.
Objectives
To determine the efficacy and safety of cough augmentation techniques in adults and children with chronic neuromuscular diseases.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), quasi‐RCTs, and randomised cross‐over trials. We considered quasi‐RCTs (those in which participants were allocated using methods that were partly systematic, such as by case record number, date of birth, or alternation) were considered for inclusion, considering the likely paucity of high‐level RCTs in the field. We included studies reported as full text and those published as abstract only. There were no language restrictions.
Types of participants
We included adults, adolescents, and children with a diagnosis of chronic NMD that may affect the muscles of respiration.
Owing to age‐related changes in respiratory anatomy and physiology, we planned to stratify participants according to age. For the purposes of this review, 'infants' referred to children under the age of one year; 'children' from one to 13 years of age; and 'adolescents/adults' over the age of 13 years. We chose this cut‐off, as peak cough flow normally reaches adult levels above 12 years of age (Bianchi 2008). We also planned to stratify participants according to whether the intervention was 'rescue' therapy (i.e. intercurrent acute chest infection in a person with chronic NMD) or maintenance therapy, where possible.
We excluded people with the following comorbidities/characteristics.
Amyotrophic lateral sclerosis/motor neuron disease (ALS/MND), which is the focus of another review.
Acute NMD with likelihood of resolution (e.g. Guillain‐Barré syndrome).
Spinal cord injuries.
Neonates in the first month of life, as they are pathophysiologically and anatomically a unique patient group warranting a separate review.
We considered studies with mixed eligible and non‐eligible population groups for inclusion, but only extracted data for participants meeting eligibility criteria for synthesis and analysis. Where separate data were not available, we contacted trial authors to obtain subgroup data. Where we could not obtain additional data, we presented results for all participants narratively, noting the mixed nature of the population.
Types of interventions
We included trials comparing any cough augmentation technique or combination of techniques, whether provided as maintenance therapy or for treatment of intercurrent respiratory tract infection, with no treatment (unassisted cough), alternative cough augmentation techniques, or combinations thereof. We allowed co‐interventions if they were provided to each group equally.
Cough augmentation techniques included, but were not limited to, the following alone or in combination):
manual or mechanical insufflation;
air‐ or breathstacking;
GPB ('frog' breathing);
MI‐E;
mechanical exsufflation;
and MAC.
Types of outcome measures
In formulating primary and secondary outcome measures, we differentiated between cough augmentation techniques used for 'rescue' therapy (e.g. during intercurrent respiratory exacerbations) and maintenance therapy.
In addition to the formal outcome measures listed below, we planned to informally include any valid measure of economic comparison between cough augmentation techniques relative to health outcomes.
The outcomes listed here were not eligibility criteria for this review, but rather outcomes of interest within included studies.
Primary outcomes
Number of unscheduled hospital admissions for episodes of acute respiratory exacerbations over one year for 'maintenance' therapy.
Duration of hospital stay (days) for 'rescue' therapy.
Secondary outcomes
Peak cough flow (PCF) measured before and after intervention for 'rescue' therapy and measured over the medium term (between three months and one year) and long term (one year and longer) for 'maintenance' therapy.
Any adverse events, including, but not limited to: pneumothorax, rib fractures, lung injury, aerophagia/abdominal distension, and death for both 'maintenance' and 'rescue' therapy.
Measures of gaseous exchange (e.g. oxygen saturation in arterial blood (SaO2) and expired carbon dioxide (CO2; end tidal carbon dioxide; ETCO2)) measured before and after the intervention for 'rescue' therapy, and measured over the medium term (between three months and one year) and long term (one year and longer) for 'maintenance' therapy.
Pulmonary function measured by forced expiratory volume in one second (FEV1), forced vital capacity (FVC), vital capacity (VC), and peak expiratory flow rate (PEFR), over the short term (less than three months); medium term (between three months and one year); and long term (one year and longer), for 'maintenance' therapy. Where possible, values were presented as percentages predicted according to age, gender, and height; or as Global Lung Function Initiative multiethnic norm‐referenced Z score values (Quanjer 2012).
Quality of life measured by any validated measure over the medium term (between three months and one year) and long term (one year and longer) for 'maintenance' therapy.
Validated measures of function, including measures of perceived exertion, exercise tolerance, and motor function measured over the medium term (between three months and one year) and long term (one year and longer) for 'maintenance' therapy.
Participant preference for, or satisfaction with, specific cough augmentation techniques, expressed as a proportion or percentage of the sample (preference) or any validated measure (satisfaction) for both 'rescue' and 'maintenance' therapy.
Search methods for identification of studies
Electronic searches
On 10 January 2019 and 13 April 2020, the Information Specialist searched the following databases.
Cochrane Neuromuscular Specialised Register via the Cochrane Register of Studies (CRS‐Web; Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web; Appendix 2).
MEDLINE OvidSP (1946 to 10 April 2020; Appendix 3).
Embase OvidSP (1974 to 2020 Week 15; Appendix 4).
Cumulative Index of Nursing and Allied Health Literature (CINAHL) EBSCOhost (1937 to 13 April 2020; Appendix 1).
We also searched the following trials registries.
WHO International Clinical Trials Registry Platform (ICTRP; inaccessible on 13 April 2020; Appendix 2).
US National Institutes for Health Clinical Trials Registry (ClinicalTrials.gov; Appendix 3).
We searched all databases from their inception to the search date, and imposed no restriction based on language of publication, or by publication status (abstract only, 'in press,' 'grey' literature, full text, etc.).
Searching other resources
We searched reference lists of all primary studies and review articles for additional references. We also searched relevant manufacturers' websites for trial information and we searched for errata or retractions from included studies. We further performed handsearches for conference proceedings.
Data collection and analysis
Selection of studies
Using Covidence (Covidence), two review authors (BM and AH) independently screened titles and abstracts of all the studies identified from the search for inclusion criteria, and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve.' We retrieved the full‐text study reports/publications, and two review authors (AH and LC) independently screened the full text and identified studies for inclusion, as well as identifying and recording reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion and planned, if required, to consult a third review author as arbiter (BM). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009).
Data extraction and management
We used a data extraction form for study characteristics and outcome data, which we piloted on one study in the review. One review author (BM) extracted study characteristics from included studies. We extracted data on:
study design and setting;
characteristics of participants (e.g. disease severity and age);
eligibility criteria;
intervention details;
outcomes assessed;
source(s) of study funding;
conflicts of interest among investigators.
Two review authors (AH and LC) independently extracted outcome data from included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way, and planned to resolve disagreements by consensus or by involving a third review author if necessary (MT). One review author (BM) transferred data into Review Manager 5 (Review Manager 2020). A second author checked the outcome data entries (AH). Another review author (MZ) spot‐checked study characteristics for accuracy against trial reports.
If reports required translation, it was planned that the translator would extract data directly using a data extraction form, or authors would extract data from the translation provided. Where possible a review author planned to check numerical data in the translation against the study report.
Assessment of risk of bias in included studies
Two review authors (LC and AH) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020a). We made summary assessments of the risk of bias for each important outcome (across domains) within and across studies comparing the same interventions. We resolved any disagreements by discussion or by involving another review author (BM) where necessary. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear, and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We planned to summarise the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary. If information on risk of bias had been related to unpublished data or correspondence with an author, we planned to note this in the 'Risk of bias' table.
When considering treatment effects, we considered the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol, and reported any deviations in the 'Differences between protocol and review' section (Morrow 2018).
Measures of treatment effect
We planned to analyse all data for 'rescue' and maintenance therapy using cough augmentation techniques separately. We planned to analyse dichotomous data as risk ratios (RRs) and continuous data as mean difference (MD) when studies used the same scale, or standardised mean difference (SMD) for results across studies with outcomes that were conceptually the same but measured in different ways. We reported 95% confidence intervals (CI). Where studies reported standard errors of the means (SEMs), we planned to convert these to standard deviations (SDs) where possible. We entered data presented as a scale with a consistent direction of effect.
We planned to calculate a Peto odds ratio (Peto OR) and corresponding 95% CI for rare adverse events. In the case of statistically significant results, we planned to calculate the risk difference (RD) and 95% CI and the number needed to treat for an additional beneficial outcome or for an additional harmful outcome as appropriate.
We planned to undertake meta‐analyses only where this was meaningful (i.e. where treatments, participants, and the underlying clinical questions were similar enough for pooling to be meaningful). We reported the types of cough augmentation techniques and different underlying conditions which could not be pooled separately (if the number of trials permitted).
We planned to describe skewed data reported as medians and interquartile ranges (IQRs).
Unit of analysis issues
We only included first‐period data from cross‐over trials for purposes of analysis, when sufficient data were available (Elbourne 2002; Higgins 2020b). Long‐term studies with multiple repeated measures of outcome could be included, in which case we planned to define outcomes based on the specified time points (Higgins 2020b).
Where multiple trial arms were reported in a single trial, we planned to only include the treatment arms relevant to the review topic. If two comparisons (e.g. treatment A versus no treatment and treatment B versus no treatment) were combined in the same meta‐analysis, we planned to follow guidance in Section 23.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions to avoid double‐counting (Higgins 2020b). Our preferred approach was to halve the control group.
Dealing with missing data
We attempted to contact investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was available as an abstract only; where only pooled data or estimates of results were presented; and where separate period data were not presented for cross‐over studies). Where this was not possible, we considered the studies adequate if more than 85% of the participants were included in the outcome analysis or if fewer participants were analysed, but sufficient measures were taken to ensure or demonstrate that this did not bias the results. Where this was unclear, we planned to conduct an intention‐to‐treat analysis from extrapolated data. If we suspected that missing data may have introduced serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Assessment of heterogeneity
We planned to use the I2 statistic to measure heterogeneity among the trials in each analysis. We planned to avoid the use of absolute cut‐off values, but to interpret the I2 statistic in relation to the size and direction of effects and strength of evidence for heterogeneity (e.g. P value from the Chi2 test, or CI for the I2 statistic).
We planned to use the rough guide to interpretation as outlined in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017), as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
If we had identified substantial unexplained heterogeneity, we planned to report it and explore possible causes with prespecified subgroup analysis.
Assessment of reporting biases
If we had been able to pool more than 10 trials, we planned to create and examine a funnel plot to explore possible small‐study biases.
Data synthesis
We were unable to pool more than one study in any meta‐analysis due to inadequate presentation of results, as well as clinical and methodological heterogeneity. Where we could not source additional information, and there was insufficient information supplied, we reported the individual results as described in the original trials in qualitative, tabular, and narrative form. If the included trials had been similar enough to combine them, we would have performed a statistical pooling of effect measures using a random‐effects model, as this is more conservative, and explore possible causes of heterogeneity by subgroup analyses if there were sufficient studies to do so. We reported the results for each review outcome measure and comparison separately, where possible. We compiled the review using Review Manager 5 (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Infants versus children.
Children versus adolescents or adults, or both.
We planned to use the following outcomes in subgroup analyses.
Number of hospital admissions over one year (for maintenance use).
Duration of hospital stay (days) for 'rescue' use.
We planned to use the formal test for subgroup interactions in Review Manager 5 (Review Manager 2020). Owing to inadequate data, we were unable to conduct subgroup analyses, and this is recommended for future versions of the review.
Sensitivity analysis
We planned the following sensitivity analyses, but could not conducted them owing to insufficient data. This should be considered for future versions of this review.
Repeat the analysis excluding unpublished studies (if there were any).
Repeat the analysis excluding studies with high risk of bias (e.g. randomised versus quasi‐randomised). We planned to rate studies at overall high risk of bias if there was a high risk of bias for one or more key domains (Higgins 2020a).
In the case of including one or more very large study, repeat the analysis excluding these to determine to what extent they dominated the results.
Repeat the analysis using different statistical models (fixed‐effect versus random‐effects).
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice and our implications for research suggest priorities for future research and outline what the remaining uncertainties are in the area.
Summary of findings and assessment of the certainty of the evidence
We planned to create separate 'Summary of findings' tables for 'rescue' and 'maintenance therapy' using cough augmentation techniques, using GRADEpro GDT software, presenting the following outcomes.
Number of unscheduled hospital admissions for episodes of respiratory exacerbations over the medium term (between three months and one year) and long term (one year and longer) for 'maintenance' therapy.
Duration of hospital stay (days) for 'rescue' therapy.
PCF measured before and after intervention(s) for 'rescue' and maintenance therapy and measured over the medium term (between three months and one year) and long term (one year and longer) for maintenance therapy.
Any adverse events measured over the short term, medium term (three months to one year), and long term (one year or longer) ('rescue' and maintenance therapy).
Quality of life measured by any validated measure over the medium term (between three months and one year) and long term (one year and longer) (maintenance therapy).
Participant preference for, or satisfaction with specific cough augmentation techniques ('rescue' and maintenance therapy), measured over the short term, medium term (three months to one year) and long term (one year or longer).
However, based on the included studies, we chose to rather present separate 'Summary of findings' tables for the comparison between cough augmentation technique(s) and alternative cough augmentation technique(s) and for the comparison between cough augmentation technique(s) and standard of care, for the above outcome measures.
Two review authors (BM and AH) independently assessed the certainty of the body of evidence (studies that contributed data for the prespecified outcomes) using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias). We used methods and recommendations described in Chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017a; Schünemann 2017b). We resolved any disagreements by discussion or by involving another review author (LC) where necessary. We considered RCTs as high‐certainty evidence if the five factors above were not present to any serious degree, but could downgrade the certainty to moderate, low, or very low. We downgraded evidence once if a GRADE consideration was present to a serious degree, twice if very serious, and three times based on several GRADE concerns. We justified all decisions to downgrade or upgrade the certainty of evidence using footnotes, and made comments to aid readers' understanding of the review where necessary.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
The literature search identified 390 papers (see Figure 1 for study flow diagram): 20 from the Cochrane Neuromuscular Specialized Register, 142 from CENTRAL, 81 from MEDLINE, 55 from CINAHL, and 92 from EMBASE.
1.
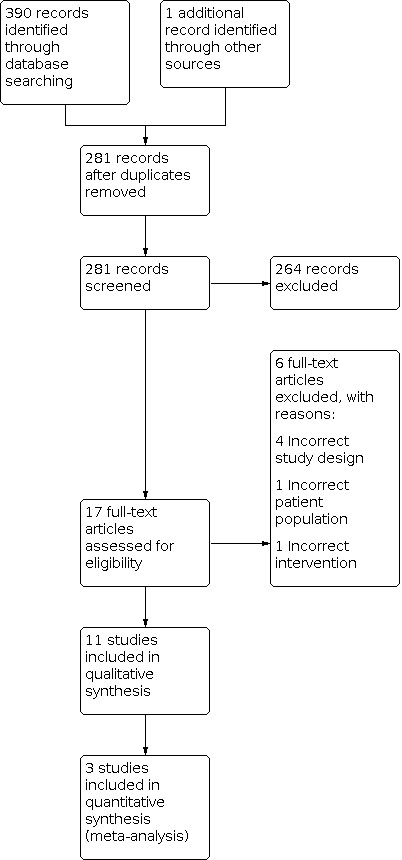
Study flow diagram.
From ClinicalTrials.gov, we identified 76 potentially relevant ongoing clinical trials and 64 ongoing trials from ICTRP, from which we identified five studies for possible inclusion in future reviews (NCT01518439; NCT02651805; NCT03355105; NCT04081116; PACTR201506001171421) (see Characteristics of ongoing studies table).
We identified one study through other methods, after reviewing the published study protocol (Katz 2019). After removing duplicates, we reviewed the titles and abstracts of 281 papers, and identified a further two duplicates in this process. We selected 17 studies for full‐text review and excluded six of these studies, with reasons (Bianchi 2014; Kang 2000; Silva 2012; Toussaint 2003; Toussaint 2009; Winck 2004; see Characteristics of excluded studies table). Eleven studies met the inclusion criteria for the review (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Katz 2019; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016; Toussaint 2016).
Included studies
Ten included studies were full published articles (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016; Toussaint 2016), while one study was a congress abstract, with detailed methodology published on ClinicalTrials.gov (Katz 2019). Full details of the Katz 2019 study results were not available, and attempts to contact the author were unsuccessful.
Region and setting
Three included studies were from the UK (Chatwin 2003; Chatwin 2009; Sivasothy 2001); three from Europe (two from France (Del Amo Castrillo 2019; Lacombe 2014) and one from Belgium (Toussaint 2016)); two from Canada (Jenkins 2014; Katz 2019); one from Brazil (Brito 2009); one from Korea (Kim 2016); and one was from Chile (Torres‐Castro 2016). Ten were short term (i.e. two days or less in duration) studies of the immediate effects of cough augmentation techniques in a hospital or clinic setting (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016; Toussaint 2016). One two‐year study investigated the long‐term effects of maintenance interventions performed outside the hospital setting (Katz 2019).
Study design
Two studies were prospective parallel‐group RCTs (Katz 2019; Toussaint 2016). Toussaint 2016 was a single‐centre, short‐term trial of a single intervention (52 participants); while the study by Katz 2019 was a long‐term multicentre study conducted over two years (67 participants). Katz 2019 further used a minimisation technique to allocate participants to intervention arms to ensure between‐group matching. With minimisation, allocation of the next participant depends wholly or partly on the characteristics of participants already enrolled in the trial, with only the first participant being truly randomised (Altman 2005). Minimisation is considered a valid alternative to ordinary randomisation, and has the advantage of better balancing intervention groups, especially in smaller trials (Altman 2005). Sufficient data for analysis were available for Toussaint 2016; however, the abstract of Katz 2019 did not provide sufficient data for analysis.
Most studies were cross‐over trials in which all participants received every intervention in random order, in a single session, with variable washout periods between interventions (Brito 2009; Chatwin 2003; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016). One study was a randomised cross‐over trial conducted over two days, in which eight participants were randomly assigned to receive MI‐E for one treatment session and no MI‐E for a second treatment session, with a reverse cross‐over the following day (Chatwin 2009). Only the first part of Jenkins 2014 was randomised, a second substudy involved systematically assigned interventions and, therefore, we did not include it in this review. Torres‐Castro 2016 and Lacombe 2014 provided additional first‐period data on request, which could be analysed. The remaining cross‐over trials did not present separate first‐period data, or make these data available, precluding meta‐analysis (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Sivasothy 2001).
Participants
Participants were adults and children (total 287) with a variety of NMDs ranging in age from three to 73 years. Four studies included adults only: Lacombe 2014 included 18 adults aged 21 to 68 years; Toussaint 2016 included 52 adults with a mean age of 25.3 (SD 5.1) years (27 participants) in the mechanical breathstacking group and 24.7 (SD 5.7) years (25 participants) in the manual stacking group; Del Amo Castrillo 2019 included 20 adults aged 21 to 71 years; and Sivasothy 2001 included four adults with respiratory muscle weakness and scoliosis secondary to NMD, aged 44 to 66 years. Katz 2019 included 67 children and adolescents aged six to 16 years (median 11.4 years) and Torres‐Castro 2016 included 14 children and adolescents aged from nine to 18 years. The remaining studies had mixed child, adolescent, and adult populations: Chatwin 2003 included eight children and adolescents aged 10 to 17 years, and 14 adults aged 18 to 56 years. Chatwin 2009 included two children aged four and 12 years, and six adults aged 21 to 44 years. Jenkins 2014 included 13 children and adolescents with NMDs aged four to 18 years, and one adult aged 19 years. Kim 2016 did not report separate paediatric and adult data, but enrolled 40 participants with a mean age of 20.9 (SD 7.2) years. Similarly, Brito 2009 included 28 participants over 10 years old (mean 20, SD 4 years), and did not report separate data for children, adolescents, and adults. Reports provided insufficient information to enable subgroup analysis for different age groups or comorbid conditions.
Conditions
Duchenne muscular dystrophy (DMD) was the most commonly reported condition (207 participants), with three studies only including participants with DMD (Brito 2009; Katz 2019; Toussaint 2016). The other studies included a range of NMDs including DMD (Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016); SMA (39 participants) (Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016); poliomyelitis or postpolio syndrome (six participants) (Chatwin 2003; Del Amo Castrillo 2019; Sivasothy 2001); congenital muscular dystrophy (CMD) (four participants) (Chatwin 2003; Lacombe 2014); congenital myopathy (five participants) (Chatwin 2009; Kim 2016; Torres‐Castro 2016); Becker muscular dystrophy (BMD) (three participants) (Del Amo Castrillo 2019; Jenkins 2014; Lacombe 2014); gamma‐sarcoglycanopathy (four participants) (Del Amo Castrillo 2019; Lacombe 2014); acid maltase deficiency (three participants) (Del Amo Castrillo 2019; Lacombe 2014), and other NMDs, including Ulrich Syndrome (two participants) and facio‐scapulo‐humeral muscular dystrophy, vacuolar myopathy, congenital fibre type disproportion (myopathy), limb girdle muscular dystrophy, Charcot‐Marie‐Tooth Type 1 disease, progressive muscular dystrophy, and myasthenia gravis (one participant each) (Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014).
Two studies included comparative participant groups without NMD (Chatwin 2003; Sivasothy 2001), healthy controls (Chatwin 2003; Sivasothy 2001), or controls with chronic obstructive pulmonary disease (COPD) (Sivasothy 2001), which were not eligible for inclusion in this review. Therefore, we only included data for the groups of participants with NMD. Jenkins 2014 also included participants with other central nervous system (CNS) disorders (including cerebral palsy (two participants), and seizure disorder, spinal cord injury, Rett syndrome, encephalomalacia, hypoxic brain injury, Batten disease, and Cri‐du‐Chat syndrome (one participant each)), but did not provide separate data for participants with NMDs versus CNS disorders. Similarly, Torres‐Castro 2016 included one participant with spinal cord injury, but it was not possible to analyse participants with NMD separately. Sivasothy 2001 included seven of eight participants in a non‐scoliotic participant group with ALS; therefore, we did not include this group's data in the review. We only included and described data from the participant group with eligible NMD and scoliosis (four participants) in this review (Sivasothy 2001).
One study investigated participants admitted to hospital with acute respiratory tract infections, thereby receiving 'rescue' therapy (Chatwin 2009); while one other study investigated the effects of a two‐year course of cough augmentation therapy as maintenance therapy (Katz 2019). Jenkins 2014 included both inpatients and outpatients but did not distinguish between results obtained with rescue and maintenance therapy. This study did not report participants' respiratory infection status, although participants requiring oxygen therapy were excluded (Jenkins 2014). Eight studies specifically investigated stable participants without intercurrent infection (Brito 2009; Chatwin 2003; Del Amo Castrillo 2019; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016; Toussaint 2016).
There were insufficient data to allow for subgroup analysis among different conditions, participant ages, and therapy circumstances ('rescue' or maintenance therapy).
Interventions
Studies compared cough augmentation techniques to alternative and combination techniques (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016; Toussaint 2016); standard or conventional management (Katz 2019); spontaneous unassisted cough (Kim 2016); or sham interventions (Jenkins 2014). Ten studies reported a change in outcome measurements from baseline or preintervention unassisted cough to intervention‐assisted cough, but unassisted cough in these studies was not a randomly assigned intervention (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Katz 2019; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016; Toussaint 2016). A summary of interventions and main results is presented in Table 3; Table 4; and Table 6, and descriptions of the interventions are fully described in the Characteristics of included studies table.
4. Study results grouped by outcome measures and interventions – cough augmentation therapy compared to standard care.
| Outcome measure |
Study identifier Sample size Data presentation |
Unassisted cough | Manual BS | Standard care | Between‐group differences |
| Number and duration of unscheduled hospital and ICU admissions |
Katz 2019 n = 67 Units not specified |
— | Not reported | Not reported | No results reported |
| Unassisted PCF |
Katz 2019 n = 67 Units not specified |
— | Not reported | Not reported | No results reported |
| Health‐related quality of life |
Katz 2019 n = 67 Pediatric Quality of Life Inventory score |
— | Not reported | Not reported | No results reported |
| FVC |
Katz 2019 n = 67 Median % predicted |
85.5 (entire cohort)a | 4.1% change | 6.4% change | Adjusted MD 2.0, 95% CI –8.2 to 12.3 |
| Time to 10% decline in FVC |
Katz 2019 n = 67 Not reported |
— | Data not reported | Data not reported | Manual BS vs standard care: P = 0.5 |
| Maximal inspiratory or insufflation capacity |
Katz 2019 n = 67 Units not specified |
— | Not reported | Not reported | No results reported |
| MEP |
Katz 2019 n = 67 Units not specified |
— | Not reported | Not reported | No results reported |
| MIP |
Katz 2019 n = 67 Units not specified |
— | Not reported | Not reported | No results reported |
| Number and duration of outpatient oral antibiotic courses |
Katz 2019 n = 67 Units not specified |
— | Not reported | Not reported | No results reported |
BS: breathstacking; CI: confidence interval; FVC: forced vital capacity; ICU: intensive care unit; MD: mean difference; MEP: maximal expiratory pressure; MIP: maximal inspiratory pressure; n: number of participants; PCF: peak cough flow.
aBaseline value – not a randomly assigned control
Cough augmentation techniques included mechanical insufflation (Chatwin 2003; Del Amo Castrillo 2019; Sivasothy 2001); mechanical exsufflation (Chatwin 2003); MI‐E (Chatwin 2003; Kim 2016; Lacombe 2014); MAC (Brito 2009; Chatwin 2003; Chatwin 2009; Sivasothy 2001); manual or ventilator‐assisted breathstacking, or both (Brito 2009; Del Amo Castrillo 2019; Jenkins 2014; Katz 2019; Torres‐Castro 2016; Toussaint 2016); GPB (Torres‐Castro 2016); and breathstacking plus MAC (Brito 2009; Kim 2016); MAC plus MI‐E (Chatwin 2009; Kim 2016; Lacombe 2014); and mechanical insufflation plus MAC (Del Amo Castrillo 2019; Lacombe 2014; Sivasothy 2001).
One RCT conducted over two years compared conventional treatment (which could have included chest physiotherapy or peripheral airway clearance techniques, or both; nutritional support; antibiotics; non‐invasive ventilation (NIV) and systemic steroids) to conventional treatment plus twice daily lung volume recruitment/breathstacking, using a self‐inflating resuscitation bag with a one‐way valve (Katz 2019). Measures of adherence to the intervention were not reported (Katz 2019). One randomised cross‐over trial conducted over two days compared standardised airway clearance therapy (with MAC and ventilator‐assisted active cycle of breathing technique) with and without MI‐E (Chatwin 2009). Ventilator tidal volumes and pressures applied for the thoracic expansion component of the active cycle of breathing technique and preinsufflation part of the cough were not reported (Chatwin 2009). Toussaint 2016 compared mechanical breathstacking using a home mechanical ventilator to manual breathstacking with a resuscitation bag. Del Amo Castrillo 2019 compared standard, mechanical breathstacking using a home ventilator to augmented mechanical insufflation using the ventilator's volumetric cough mode, which provides a programmable intermittent deep breath set at a percentage of the baseline tidal volume. Torres‐Castro 2016 compared manual breathstacking (using a resuscitation bag and one‐way valve) to GPB. Brito 2009 compared MAC to manual breathstacking (using a resuscitation bag) and manual breathstacking plus MAC; Chatwin 2003 compared baseline maximal unassisted cough to 1. standard "physiotherapy assisted cough;" 2. cough after supported inspiration by a non‐invasive positive pressure ventilator (mechanical insufflation); 3. exsufflation‐assisted cough with negative pressure initiated manually at end‐inspiration; 4. insufflation‐assisted cough using a mechanical in‐exsufflator; and 5. mechanical exsufflation‐assisted cough with negative pressure delivered immediately preceding the cough effort. Chatwin 2003 did not clearly describe the method of performing "standard physiotherapy‐assisted cough," but we presumed it to include or be equivalent to MAC. Jenkins 2014 compared involuntary manual breathstacking using a self‐inflating resuscitator bag and one‐way valve, to sham breathstacking; Kim 2016 compared unassisted cough, MAC performed after manual breathstacking to maximal inspiratory capacity, MI‐E and MI‐E plus MAC; Lacombe 2014 compared mechanical insufflation (using a positive pressure ventilator) plus MAC, MI‐E plus MAC and MI‐E alone; and Sivasothy 2001 compared MAC alone to mechanical insufflation (delivered using an MI‐E device) and to mechanical insufflation plus MAC.
Studies applied MAC using pressure to the abdomen (Chatwin 2009; Jenkins 2014; Kim 2016), chest (Brito 2009), or both abdomen and chest (Sivasothy 2001); while Lacombe 2014 used abdominal, thoracic, or thoraco‐abdominal compression according to participant comfort. Chatwin 2003 did not describe the therapist's hand position for "physiotherapy assisted cough," but, in the study's literature review, MAC is mentioned as forming part of standard physiotherapy treatment and we assumed that the techniques were equivalent (Chatwin 2003).
Studies applied insufflation‐assisted cough mechanically using a non‐invasive positive pressure ventilator (Chatwin 2003; Del Amo Castrillo 2019; Lacombe 2014) or MI‐E devices (Sivasothy 2001). Ventilators used for insufflation were a bilevel positive airway pressure ventilator (BiPAP: Respironics Inc. Murraysville, North Carolina, USA or Breas MedicalSweden), with insufflation pressures titrated to participant comfort (Chatwin 2003); a ventilator equipped with volumetric cough mode (Astral 150, Resmed, Saint‐Priest, France) (Del Amo Castrillo 2019); and an Alpha 200C ventilator (Air Liquide, France), set to provide IPPB with a low inspiratory trigger and gradually increased inspiratory pressure to the highest tolerated value, to a maximum of 40 cmH2O, with inspiratory flow set according to patient comfort (Lacombe 2014). Sivasothy 2001 used a "CoughAssist" MI‐E device (JH Emersen, Cambridge, Massachusetts, USA) to provide insufflation, in which two cycles of both insufflation and exsufflation (set at +20 cmH2O/–20 cmH2O) were followed by a third insufflation and maximal spontaneous cough, which was measured without assistance or exsufflation support (Sivasothy 2001).
Exsufflation‐assisted cough was applied using a "CoughAssist" MI‐E device (JH Emersen, Cambridge, Massachusetts, USA) with the negative pressure applied manually at the end of inspiration (Chatwin 2003). Exsufflation pressures were titrated for participant comfort and reported to have a mean of –15 (SD 9) cmH2O (Chatwin 2003).
Five studies applied breathstacking using a manual resuscitation bag and face mask interface (Brito 2009; Jenkins 2014; Katz 2019; Torres‐Castro 2016; Toussaint 2016). Brito 2009, Jenkins 2014, Katz 2019, and Torres‐Castro 2016 specified use of a unidirectional valve during manual breathstacking. Brito 2009 and Jenkins 2014 applied three consecutive stacking breaths without exhalation before the maximum exhalation or cough, while Katz 2019 and Torres‐Castro 2016 did not specify the required number of stacked breaths to reach maximal insufflation. Toussaint 2016 individualised the number of successive inspirations for each participant, but participants were typically instructed to take "two to three successive insufflations" without breathing out in‐between. Jenkins 2014 applied sham breathstacking using the same technique as involuntary resuscitation bag breathstacking, but in the absence of a directional valve. Toussaint 2016 specified using a 2 L resuscitator bag (Resutator 2000, Dräger, Germany), while other studies did not specify the size of the resuscitator bag used.
Toussaint 2016 and Del Amo Castrillo 2019 applied mechanical breathstacking using volume‐cycled home mechanical ventilators and nasal mask (Toussaint 2016) or face mask (Del Amo Castrillo 2019) interfaces. Del Amo Castrillo 2019 specified that consecutive inspiratory‐hold insufflations were performed until participants felt their lungs were fully expanded or until the insufflation pressure plateau was 50 cmH2O.
Two studies delivered mechanical insufflation/exsufflation‐assisted cough using the CoughAssist device manufactured by JH Emerson Co (Cambridge, Massachusetts, USA) (Chatwin 2003; Lacombe 2014), and two studies used the Philips Respironics (Murraysville, Pennsylvania, USA) (Chatwin 2009; Kim 2016). All four studies reporting MI‐E used a full‐face mask interface (Chatwin 2003; Chatwin 2009; Kim 2016; Lacombe 2014). Insufflation pressures ranged from +15 (SD 3) cmH2O (Chatwin 2003, titrated for patient comfort); through +20 cmH2O (range 15 cmH2O to 35 cmH2O) (Chatwin 2009, titrated for patient comfort); up to +40 cm H2O (Kim 2016; Lacombe 2014). Exsufflation pressures ranged from –40 cmH2O (Kim 2016; Lacombe 2014); –20 cmH2O (range –20 cmH2O to –40 cmH2O) (Chatwin 2009); to –15 (SD 9) cmH2O (Chatwin 2003). Kim 2016 set the MI‐E device to deliver ± 40 cmH2O pressures as standard, while Lacombe 2014 reported gradually increasing or decreasing insufflation/exsufflation pressures to the highest or lowest tolerated values. All studies used the MI‐E device in manual mode (Chatwin 2003; Chatwin 2009; Kim 2016; Lacombe 2014). Chatwin 2003 and Lacombe 2014 did not describe insufflation/exsufflation and pause times, while Chatwin 2009 used an insufflation time of two seconds to four seconds and exsufflation time of four seconds to five seconds and Kim 2016 used an insufflation time of three seconds and exsufflation time of two seconds. Only Kim 2016 reported a three‐second pause between cycles. Lacombe 2014 set insufflation flow (and therefore insufflation time) according to participant comfort. Chatwin 2003 and Lacombe 2014 did not describe the number of MI‐E cycles delivered, while Kim 2016 applied five cycles of insufflation and exsufflation.
Torres‐Castro 2016 included GPB, in which participants were instructed to perform successive air "swallowing" manoeuvres, until they achieved maximum volume.
Outcomes
Ten studies reported only short‐term outcome measures of interventions, mostly in the context of single treatment sessions (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016; Toussaint 2016). Only one studies planned to report on this review's primary outcome measures (number of unscheduled hospital admissions and duration of hospital stay) in 67 participants; however, the results of this outcome measure were not reported in the published abstract and, therefore, could not be included in qualitative or quantitative analysis (Katz 2019). Although some studies reported our secondary short‐term outcome measures of PCF and gaseous exchange, measured before and after intervention, we note that only one study measured them in the context of 'rescue' therapy, in eight participants (Chatwin 2009).
Objective outcomes measured in the included studies were: PCF (265 participants) (Brito 2009; Chatwin 2003; Del Amo Castrillo 2019; Katz 2019; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016; Toussaint 2016); FVC and time to reach a 10% decline in FVC (67 participants) (Katz 2019); physiological variables of heart rate (eight participants) (Chatwin 2009); transcutaneous oxygen saturation (31 participants) (Chatwin 2009; Jenkins 2014); transcutaneous carbon dioxide tension (PtcCO2) (eight participants) (Chatwin 2009); respiratory rate (23 participants) (Jenkins 2014); cough expiratory volume and peak value time (four participants) (Sivasothy 2001); treatment time (eight participants) (Chatwin 2009); oesophageal or gastric pressures (four participants) (Sivasothy 2001); tidal volume (23 participants) (Jenkins 2014); inspiratory or insufflation capacity (171 participants) (Del Amo Castrillo 2019; Katz 2019; Lacombe 2014; Torres‐Castro 2016; Toussaint 2016); effective cough time (time with PCF of more than 3 L/second) (18 participants) (Lacombe 2014); maximal expiratory pressure (MEP) (119 participants) (Katz 2019; Toussaint 2016); maximal inspiratory pressure (MIP) (67 participants) (Katz 2019); and the number of insufflations to reach MIC (52 participants) (Toussaint 2016).
Subjective outcome measures were: scores on a visual analogue scale (VAS) for participant comfort (46 participants) (Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Lacombe 2014); distress (22 participants) (Chatwin 2003); breathlessness (eight participants) (Chatwin 2009); fatigue (eight participants) (Chatwin 2009); mood (eight participants) (Chatwin 2009); secretion production (eight participants) (Chatwin 2009); and cough effectiveness or strength (42 participants) (Chatwin 2003; Del Amo Castrillo 2019; Lacombe 2014). Chatwin 2009 (eight participants) measured auscultation score. Sivasothy 2001 asked four participants to report whether the intervention had aided, impaired, or had no effect on their cough. None of the included studies included standardised, valid measures of function, or participant preference.
Katz 2019 planned to measure health‐related quality of life, using the Pediatric Quality of Life Inventory (PedQL) Score in 67 participants; however, this outcome was not reported in the published abstract.
None of the studies listed adverse events as a primary or secondary outcome. Chatwin 2003 (22 participants) and Sivasothy 2001 (four participants) reported there had been no adverse events. Kim 2016 (40 participants) reported that the interventions were "well tolerated." Chatwin 2009 (eight participants) reported fatigue as an adverse effect of MI‐E.
It was unclear whether serious adverse events such as pneumothorax were systematically investigated in any of the studies.
Potential conflicts of interest
Chatwin 2009 disclosed a relationship with a healthcare company that manufactured ventilation equipment, although the nature of the relationship and the relevance to this study was unclear. Del Amo Castrillo 2019 disclosed a relationship with ResMed France, the company who manufacture the ventilator device with volumetric cough mode used in their study. The exact nature of the relationship was unclear. Katz 2019 declared relationships with a pharmaceutical company, but it was unclear whether these relationships would have constituted a source of bias. Jenkins 2014 and Brito 2009 declared their funding source, which, in both studies, was unlikely to constitute a conflict of interest. Chatwin 2003, Lacombe 2014, Sivasothy 2001, Torres‐Castro 2016, and Toussaint 2016 did not declare funding sources or other potential conflicts of interest. Kim 2016 declared no financial conflicts of interests, but other interests were not declared.
Excluded studies
We excluded six studies (see Characteristics of excluded studies table): four due to incorrect study design (Bianchi 2014; Kang 2000; Toussaint 2009; Winck 2004); one because of the incorrect population (Silva 2012), and one did not describe a cough augmentation technique and we excluded it based on studying the incorrect intervention (Toussaint 2003).
Kang 2000 investigated the relationships between VC, MIC, and both unassisted and assisted PCF (using manual insufflation versus unassisted or spontaneous PCF in two groups of participants with MIC greater than or equal to their VC). The study was not designed to determine effectiveness of the cough augmentation interventions, and neither the allocation nor order of intervention was randomised.
Toussaint 2009 conducted a prospective cross‐sectional observational study investigating three cough augmentation techniques (MAC, breathstacking, and breathstacking with MAC) in 179 clinically stable participants with a range of NMDs. Breathstacking as well as breathstacking plus MAC was only conducted in a subgroup of 60 participants receiving NIV.
Winck 2004 conducted a prospective observational study to evaluate the tolerance of three different MI‐E pressures (+15 cmH2O to –15 cmH2O, +30 cmH2O to –30 cm H2O, and +40 cmH2O to –40 cm H2O) in a heterogeneous sample of people with NMD (seven participants), ALS (13 participants), and COPD (nine participants). Data for each participant group were provided separately. The MI‐E pressures were increased systematically for each participant, without randomisation of order.
Silva 2012 studied the effect of MAC alone or in association with increased positive end‐expiratory pressure (PEEP) and inspiratory time on peak expiratory flow and respiratory mechanics in mechanically ventilated participants diagnosed with head trauma, stroke, congestive heart failure, and ventilator‐associated pneumonia. The study did not include participants with NMD.
Toussaint 2003 conducted a randomised cross‐over study comparing mucous clearance techniques with and without intrapulmonary percussive ventilation (IPV), in eight participants with DMD (five with mucous hypersecretion). IPV is considered a peripheral airway clearance technique, not a proximal clearance (cough augmentation) technique, and we determined the intervention ineligible for this review.
Bianchi 2014 conducted a prospective observational study on 18 participants (aged 21.1 (SD 5.4) years) with muscular dystrophy, comparing unassisted PCF to augmented PCF using various interventions, including GPB; a self‐induced thoracic or abdominal thrust (by independently manoeuvring a wheelchair into a table); assistant‐delivered MAC; breathstacking; and combination techniques. There was no randomisation or allocation to different interventions or order of interventions, and this study was ineligible for inclusion in this review.
Risk of bias in included studies
See Figure 2, Figure 3, and the Characteristics of included studies table.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Eight studies provided no details about the method of randomisation (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Sivasothy 2001). Katz 2019 used a minimisation technique, but provided no details of the minimisation methodology. Therefore, we judged these studies at unclear risk of bias for the generation of randomisation sequence. Toussaint 2016 conducted an RCT that randomised participants to receive one of the two interventions by means of a coin toss. Torres‐Castro 2016 described using freely available software to generate random number lists. We judged these two studies at low risk of selection bias. None of the included studies described allocation concealment, leading to a judgement of unclear risk of selection bias for all included studies.
Blinding
Considering the nature of cough augmentation interventions, it is highly unlikely that participant or clinician blinding would have been possible, leading to a high risk of performance bias in 10 studies (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Katz 2019; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016; Toussaint 2016).
Jenkins 2014 used a sham intervention as a control, but it was unclear if blinding was successfully achieved. Both involuntary breathstacking and sham interventions were performed in the same way except for the presence or absence of a valve. It was unclear if the masks looked identical, with a sham valve, or whether the valve was simply not added to the mask circuit for the sham intervention. Therefore, the risk of performance bias for participants was unclear. Considering therapists applying the intervention would likely have known whether a one‐way valve was applied or not, we judged the risk of personnel (therapist) performance bias as high. Overall, therefore, we judged the risk of performance bias in Jenkins 2014 as unclear.
Owing to insufficient methodological information, it was unclear whether six studies blinded outcome assessors, which we judged at unclear risk of detection bias (Chatwin 2003; Chatwin 2009; Jenkins 2014; Katz 2019; Lacombe 2014; Sivasothy 2001). Five studies measured outcomes while investigators were performing the study interventions, and, therefore, outcome assessment could not have been blinded, leading to a high risk of detection bias (Brito 2009; Del Amo Castrillo 2019; Kim 2016; Torres‐Castro 2016; Toussaint 2016).
Incomplete outcome data
All participants completed the interventions, or were appropriately accounted for, in eight studies, leading to a judgement of low risk of attrition bias (Brito 2009; Chatwin 2003; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Sivasothy 2001; Toussaint 2016). In two studies, it was unclear whether all included participants completed all outcome measurements, as this was not explicitly stated in the text (Chatwin 2009; Katz 2019). In one study, three participants were excluded after screening, but the trial authors did not provide clear reasons for exclusion (Torres‐Castro 2016). We judged the risk of attrition bias for these three studies as unclear (Chatwin 2009; Katz 2019; Torres‐Castro 2016).
Selective reporting
We judged three studies at high risk of reporting bias (Brito 2009; Chatwin 2009; Katz 2019). Brito 2009 did not present all stated baseline measurements (SpO2; expired CO2) but fully reported the primary outcome of PCF. Chatwin 2009 presented no data for the primary physiological outcome measures of peripheral capillary oxygen saturation (SpO2), heart rate, and PtcCO2. In addition, the study only presented VAS scores for comfort, breathlessness, and mood as graphs, and we could not extract the data precisely. Katz 2019 presented selected outcome measures in the published abstract, while the published protocol presented several primary and secondary outcome measures that were not reported in the abstract. Efforts to obtain missing data for these studies were unsuccessful.
We judged three studies at unclear risk of reporting bias (Chatwin 2003; Lacombe 2014; Sivasothy 2001). Chatwin 2003 did not report separate VAS scores of patient comfort, distress, and strength of cough. Lacombe 2014 presented several outcome measures graphically, with specific values not reported for PCF, inspiratory capacity, and effective cough time. Sivasothy 2001 did not clearly describe the study's primary and secondary outcome measures and did not mention a trial registration number, so the review authors could not confirm the outcome measures by checking the predescribed protocol. The report did not present gastric and oesophageal pressures; however, the trial authors acknowledged this and ascribed it to a measurement problem owing to collapse of balloons in the control groups. The trial authors did not fully report the subjective outcome measure of cough effectiveness, but simply stated that participants did not report any benefit of any assisted cough interventions. Sivasothy 2001 fully reported other measured outcomes.
The other five studies reported all the prespecified primary and secondary outcome measures and we judged these studies at low risk of reporting bias (Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Torres‐Castro 2016; Toussaint 2016).
Other potential sources of bias
We judged one study at low risk of other biases (Toussaint 2016), and two studies at unclear risk (Katz 2019; Torres‐Castro 2016). Torres‐Castro 2016 did not explicitly identify primary and secondary outcomes and Katz 2019 provided insufficient information to judge the risk of other biases. We judged eight studies at high risk of other bias, considering they were all short‐term cross‐over trials with no analysis of carry‐over effect, and no separate period reporting in the primary publication (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Sivasothy 2001). A short‐term cross‐over study design may not be the most appropriate for studies on conditions such as NMD, which require long‐term follow‐up. Also, none of these studies considered the potential confounder of learning effect on outcome measurement, and this may have influenced the results. 'Learning effect' refers to participants improving their ability to perform or co‐ordinate the outcome assessment (e.g. PCF technique) through practice and learning, rather than showing an objective improvement in the actual outcome being measured. Lacombe 2014 and Torres‐Castro 2016 provided separate baseline data for group allocation; for the other cross‐over studies it was unclear whether groups were well balanced at baseline, or whether the groups were treated the same except for the intervention (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Sivasothy 2001). Other potential confounders in studies included: presence of comorbid conditions (Brito 2009; Del Amo Castrillo 2019; Katz 2019); oral/bulbar control (Chatwin 2003; Jenkins 2014; Sivasothy 2001; Torres‐Castro 2016); heterogeneity of included conditions or ages, or both (Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Torres‐Castro 2016); and concomitant use of NIV (Katz 2019; Kim 2016).
Effects of interventions
One study was a short‐term RCT (Toussaint 2016), the main results of which are presented in Analysis 1.1 and Analysis 1.2. Katz 2019 conducted a long‐term RCT; however, the published abstract provided insufficient data. Attempts to contact the author were unsuccessful, and we could not perform any additional analysis.
1.1. Analysis.

Comparison 1: Manual versus mechanical breathstacking (BS), Outcome 1: Peak cough flow
1.2. Analysis.

Comparison 1: Manual versus mechanical breathstacking (BS), Outcome 2: Maximal insufflation capacity
Eight studies applied every intervention to every included participant in a single session, in random order (Brito 2009; Chatwin 2003; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016). Therefore, each participant received several interventions. None of these published reports presented individual responses to each intervention, which could have allowed secondary analysis. However, Torres‐Castro 2016 and Lacombe 2014 provided additional individual data on request, allowing separate first‐period analysis of one our secondary outcome measures, PCF (Analysis 2.1; Analysis 3.1; Analysis 4.1; Analysis 5.1). Meta‐analysis and pooling of the results of the remaining six studies was not possible, owing to the repeat counting that occurred, which would cause unit‐of‐analysis errors from the unaddressed correlation between the estimated intervention effects of multiple comparisons (Higgins 2020b). The two‐day cross‐over study by Chatwin 2009 also did not report data separately for the two periods of the study, precluding inclusion in a meta‐analysis. All the reported quantitative results of the included studies are presented in Table 3; Table 4; and Table 6. The main results for studies comparing cough augmentation technique(s) with alternate cough augmentation technique(s) are summarised in Table 1 and results for studies comparing cough augmentation technique(s) with standard of care are presented in Table 5.
2.1. Analysis.

Comparison 2: Glossopharyngeal breathing (GPB) versus manual breathstacking (BS), Outcome 1: Peak cough flow
3.1. Analysis.

Comparison 3: Mechanical insufflation‐exsufflation (MI‐E) versus mechanical insufflation (MI) plus manually assisted cough (MAC), Outcome 1: Peak cough flow
4.1. Analysis.

Comparison 4: Mechanical insufflation‐exsufflation (MI‐E) versus mechanical insufflation‐exsufflation (MI‐E) plus manually assisted cough (MAC), Outcome 1: Peak cough flow
5.1. Analysis.

Comparison 5: Mechanical insufflation (MI) plus manually assisted cough (MAC) versus mechanical insufflation‐exsufflation (MI‐E) plus MAC, Outcome 1: Peak cough flow
Cough augmentation therapy compared with alternative cough augmentation therapy
Ten studies compared cough augmentation therapies to alternative individual or combination cough augmentation therapies (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016; Toussaint 2016). See Table 1.
Primary outcomes
1. Number of unscheduled hospital admissions for episodes of acute respiratory exacerbations over one year, for 'maintenance' therapy
No studies reported number of hospital admissions for episodes of acute respiratory exacerbations over one year, for 'maintenance use.'
2. Duration of hospital stay (days) for 'rescue' therapy
No studies reported duration of hospital stay (days) for 'rescue' use.
Secondary outcomes
1. PCF measured before and after intervention for 'rescue' therapy and measured over the medium term (between three months and one year) and long term (one year and longer) for 'maintenance' therapy
PCF was the most common outcome, measured in eight studies with 198 participants (Brito 2009; Chatwin 2003; Del Amo Castrillo 2019; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016; Toussaint 2016). These studies reported the immediate effect on PCF of the cough augmentation techniques, whether for acute or maintenance use (Table 1; Table 2). We considered the certainty of evidence for this outcome very low, downgrading twice for very serious study limitations (risk of bias) and once for imprecision (all studies had a small sample size, wide CIs, or both).
Manual versus mechanical breathstacking
Toussaint 2016 compared PCF (the primary outcome) with mechanical breathstacking compared to manual breathstacking. The mean (± SD) PCF increased in the mechanical breathstacking group from 132 (SD 55) L/minute to 199 (SD 48 L/min) (within‐group P = 0.001) compared to the manual breathstacking group, in which PCF increased from 125 (SD 52) L/min to 186 (SD 50 L/min) (within‐group P < 0.001). This study reported no evidence of a difference between the two intervention groups in the PCF change (between‐group MD 6.00 L/minute, 95% CI –33.43 to 45.43; P = 0.3; 52 participants; Analysis 1.1). The conclusion of lack of difference between resuscitator bag and ventilator breathstacking on PCF was based on a low‐certainty evidence, double downgraded as results were from a single study (Toussaint 2016), with substantial risk of bias due to lack of blinding of personnel, participants, or assessors; and unclear allocation concealment.
Glossopharyngeal breathing versus manual breathstacking
Torres‐Castro 2016 provided data for the first cross‐over period, which could be analysed. This study compared PCF at baseline and after either GPB or breathstacking using a self‐inflating resuscitator bag, in adults with DMD. In the first period of cross‐over, mean PCF in the manual breathstacking group increased from 162.86 (SD 77.4) L/min to 235.71 (SD 125.01) L/min (MD 72.86 (SD 61.84) L/minute, 95% CI 15.67 to 130.05; within‐group P = 0.02); while PCF in the GPB group increased from 167.14 (SD 42.71) L/min to 199.29 (SD 52.95) L/min (MD 32.14 (SD 26.44) L/min, 95% CI 7.69 to 56.59; within‐group P = 0.02). There was no evidence of a difference in the change of PCF between groups (between‐group MD –40.72, 95% CI –90.54 to 9.10; P = 0.14; 14 participants; Analysis 2.1). The conclusion that GPB and manual breathstacking have a similar effect on PCF was based very low‐certainty evidence, triple downgraded based on data extracted from a single randomised cross‐over study design (Torres‐Castro 2016), with unclear allocation concealment, very small sample size, imprecision of results (wide CIs), and substantial risk of performance and detection bias.
MI‐E versus mechanical insufflation plus MAC
Lacombe 2014, in adults with a range of NMD, provided separate allocation data for the first period of cross‐over, which could be analysed. The first period of cross‐over reported increases from baseline with both MI‐E and mechanical insufflation plus MAC. Mean PCF increased from 157.2 (SD 64.2) L/min (unassisted cough) to 210.6 (SD 52.8) L/min with MI‐E alone (MD 53.4 (SD 51.0) L/min) and from 100.8 (SD 69) L/min to 225 (SD 83.4) L/min with mechanical insufflation plus MAC (MD 124.8 (SD 38.4) L/min). Mechanical insufflation plus MAC produced a greater change in PCF compared to MI‐E alone (between‐group MD 71.40 L/minute, 95% CI 18.08 to 124.72; P = 0.009; 11 participants; Analysis 3.1).
MI‐E versus MI‐E plus MAC
Lacombe 2014 compared baseline unassisted PCF to PCF produced with MI‐E and MI‐E plus MAC. The study reported increases from baseline with both interventions. In the first period of cross‐over, mean PCF increased from 157.2 (SD 64.2) L/min (unassisted cough) to 210.6 (SD 52.8) L/min with MI‐E alone (MD 53.4 (SD 51.0) L/min) and from 104.4 (SD 41.4) L/min to 210.6 (SD 50.4) L/min with MI‐E plus MAC (MD 106.2 (SD 50.4) L/min). There was a slightly greater increase in PCF with MI‐E plus MAC compared to MI‐E alone (between‐group MD 52.80, 95% CI –0.32 to 105.92; P = 0.05; 14 participants; Analysis 4.1).
Kim 2016 reported increased PCF with both MI‐E and MI‐E plus MAC compared to unassisted cough, in children and adolescents with DMD. The PCF generated with MI‐E plus MAC was greater than with MI‐E alone. Separate data for the two periods of cross‐over were not available for analysis.
MI‐E plus MAC versus mechanical insufflation plus MAC
In the first period of cross‐over RCT, Lacombe 2014 reported that mean PCF increased from 101 (SD 69) L/min (baseline unassisted cough) to 225 (SD 83) L/min with mechanical insufflation plus MAC (MD 124 (SD 38.4) L/min); and from 104 (SD 41) L/min to 211 (SD 50) L/min with MI‐E plus MAC (MD 106 (SD 50.4) L/min). There was no evidence of a difference in the change in PCF from baseline between the MI‐E plus MAC and mechanical insufflation plus MAC (between‐group MD –18.60, 95% CI –34.46 to 71.66; P = 0.49; 11 participants; Analysis 5.1).
MAC versus mechanical insufflation
Sivasothy 2001 reported no evidence of a change from baseline PCF measurement with MAC or mechanical insufflation in four adults with NMD and scoliosis, and no evidence of between‐group differences. The very small sample size eligible for inclusion in this review limited the interpretation of these results. Separate data for the two periods of cross‐over were not available for analytical purposes.
Chatwin 2003 reported no evidence of a difference in PCF between "physiotherapy‐assisted cough" (MAC) and mechanical insufflation‐assisted cough using a non‐invasive ventilator device in 22 participants. Moreover, there was no evidence of a difference between PCF with unassisted cough and either MAC or mechanical insufflation alone. Separate data for the two periods of cross‐over were not available for analytical purposes.
MAC versus mechanical insufflation plus MAC
Sivasothy 2001 reported no evidence of a change from baseline PCF measurement with MAC or MAC plus mechanical insufflation, in four adults with NMD and scoliosis. There was no evidence of a difference in PCF change between interventions. The very small sample size eligible for inclusion in this review limited the interpretation of these results. Separate data for the two periods of cross‐over were not available for analytical purposes.
MI‐E versus MAC
In 22 adults and children with NMD presenting with severe respiratory muscle weakness (MIP 25 (SD 16) cmH2O; MEP 26 (SD 22) cmH2O), Chatwin 2003 reported that MI‐E assisted cough produced a higher PCF than MAC, while only MI‐E increased PCF significantly above baseline unassisted cough. Separate data for the two periods of cross‐over were not available for analytical purposes.
MI‐E versus mechanical exsufflation‐assisted cough
In 22 adults and children with NMD and severe respiratory muscle weakness, Chatwin 2003 reported that MI‐E‐assisted cough produced a higher PCF than exsufflation‐assisted cough, while both interventions produced a higher PCF than unassisted cough. Separate data for the two periods of cross‐over were not available for analytical purposes.
MI‐E versus mechanical insufflation
Chatwin 2003, in 22 participants, reported that MI‐E‐assisted cough produced a higher PCF than mechanical insufflation‐assisted cough, while only MI‐E increased PCF significantly above baseline unassisted cough. Separate data for the two periods of cross‐over were not available for analytical purposes.
MI‐E versus manual breathstacking plus MAC
Kim 2016 reported increased PCF with MAC plus breathstacking and MI‐E, compared to unassisted cough, in 40 children and adolescents with DMD. The PCF generated with MI‐E was significantly higher than with MAC plus breathstacking. Separate data for the two periods of cross‐over were not available for analytical purposes.
MI‐E plus MAC versus manual breathstacking plus MAC
Kim 2016 reported significantly increased PCF with both MAC plus breathstacking and MI‐E plus MAC compared to unassisted cough in 40 participants. The PCF generated with the MI‐E plus MAC produced greater PCF than manual breathstacking plus MAC. Separate data for the two periods of cross‐over were not available for analytical purposes.
MAC versus manual breathstacking
Brito 2009 reported that, in 28 adults with DMD, PCF increased with MAC and manual breathstacking compared to unassisted cough. There was no difference between PCF generated with MAC compared to manual breathstacking. Separate data for the two periods of cross‐over were not available for analytical purposes.
MAC versus manual breathstacking plus MAC
Brito 2009 reported that PCF increased with both MAC and MAC plus breathstacking, compared to unassisted cough, in 28 adults with DMD. PCF was higher when using manual breathstacking plus MAC compared to MAC alone. Separate data for the two periods of cross‐over were not available for analytical purposes.
Manual breathstacking versus manual breathstacking plus MAC
In 28 adults with DMD, PCF increased significantly with both manual breathstacking and manual breathstacking plus MAC, compared to unassisted cough. PCF was higher when using manual breathstacking plus MAC compared to manual breathstacking alone (Brito 2009). Separate data for the two periods of cross‐over were not available for analytical purposes.
Mechanical breathstacking versus mechanical insufflation
Del Amo Castrillo 2019 reported that, in 20 adults with NMD, both mechanical breathstacking (using a ventilator) and mechanical insufflation using a ventilator's volumetric cough mode were associated with an increase in PCF, but that mean PCF was higher with mechanical insufflation (using volumetric cough mode) than with mechanical breathstacking (P < 0.01). Data were presented graphically, and we could not extract data precisely from the figures provided. Attempts to contact the author for additional data were unsuccessful.
2. Any adverse events, including, but not limited to: pneumothorax, rib fractures, lung injury, aerophagia/abdominal distension, and death for both 'maintenance' and 'rescue' therapy
Chatwin 2009 recorded fatigue using a VAS, in eight adults and children with a range of NMD conditions, but did not report other adverse events. Reporting for the outcome measure of fatigue was, however, incomplete, with fatigue VAS values only reported for the intervention MAC plus MI‐E, and there were no data for MAC alone (Chatwin 2009). In the latter group, fatigue was only reported as being not significantly different before to after intervention, while with MAC plus MI‐E, mean fatigue VAS increased from 3.2 (SD 2.2) before the intervention to 5.1 (SD 2.6) after the intervention (P = 0.005; see Table 4). Separate data for the two periods of cross‐over were not available for analytical purposes. The lack of comparable data makes meaningful conclusions difficult. The evidence was very‐low certainty due to very serious study limitations and imprecision due to the small study size (eight participants).
None of the included studies specified adverse events as primary or secondary outcome measures, and six studies with 155 participants did not report on this outcome measure (Brito 2009; Del Amo Castrillo 2019; Jenkins 2014; Lacombe 2014; Torres‐Castro 2016; Toussaint 2016). Chatwin 2003 (22 participants) reported that no adverse events occurred during the study, and that participants tolerated the interventions well. Kim 2016 (40 participants) also reported that all three interventions were "well tolerated" and Sivasothy 2001 (four participants) reported that no adverse events had occurred. Although the studies reported no serious adverse events, it was unclear whether these were systematically investigated. We downgraded the body of evidence for adverse effects three times to very‐low certainty – twice for very serious study limitations and once for imprecision (see Table 1).
3. Measures of gaseous exchange measured before and after the intervention for 'rescue' therapy, and measured over the medium term (between three months and one year) and long term (one year and longer) for 'maintenance' therapy
None of the studies investigated the medium‐ or long‐term effects of cough augmentation techniques on measures of gaseous exchange. Chatwin 2009 (eight participants) measured the short‐term effects of interventions on physiological variables of heart rate, transcutaneous oxygen saturation, and PtcCO2 in adults and children with NMD; however, this study did not provide separate data for the interventions. Instead it simply reported that there was no difference between intervention groups for these physiological parameters. Jenkins 2014 (23 participants) reported that there was no difference in the change of transcutaneous oxygen saturation from before to after manual breathstacking using a resuscitator bag compared to sham breathstacking (see Table 3).
4. Pulmonary function measured by FEV1, FVC, VC, and PEFR, over the short term (less than three months); medium term (between three months and one year), and long term (one year and longer) for 'maintenance' therapy
None of the studies reported pulmonary function.
5. Quality of life measured by any validated measure over the medium term (between three months and one year) and long term (one year and longer) for 'maintenance' use
None of the studies reported quality of life.
6. Validated measures of function, including measures of perceived exertion, exercise tolerance, and motor function measured over the medium term (between three months and one year) and long term (one year and longer) for 'maintenance' therapy
Chatwin 2009 measured the level of perceived breathlessness with MI‐E plus MAC and MAC alone, using a 10‐point VAS, in eight adults and children. The validity of the scale was not determined, and data were not presented for separate interventions. It was simply reported that there were no significant changes from baseline to after intervention (see Table 4).
7. Participant preference for, or satisfaction with, specific cough augmentation techniques, expressed as a proportion or percentage of the sample for both 'rescue' and 'maintenance' therapy
None of the studies reported participants preference or satisfaction.
8. Other outcome measures
We presented data for other outcome measures in Table 3 and Table 4.
8.1. Tidal volume
One study, with 23 participants, reported an increase in tidal volume from before to after intervention with manual breathstacking using a resuscitation bag (P < 0.0001) compared to a no change with sham breathstacking (Jenkins 2014). Authors did not report between‐group significance levels, and as separate cross‐over period data were not available, these could not be calculated.
8.2. Maximum inspiratory or insufflation capacity
Four studies measured maximal inspiratory or insufflation capacity in 104 participants (Del Amo Castrillo 2019; Lacombe 2014; Torres‐Castro 2016; Toussaint 2016).
8.2.1. Manual breathstacking versus mechanical breathstacking
Toussaint 2016 reported that mean MIC achieved by participants performing manual breathstacking was 1.344 (SD 0.520) L compared to 1.481 (SD 0.477) L for those performing breathstacking using a ventilator (between‐group MD 0.14 L, 95% CI –0.13 to 0.41; P = 0.3; 52 participants; Analysis 1.2). Therefore, there was no evidence of a difference between manual and mechanical breathstacking in achieved MIC.
8.2.2. Glossopharyngeal breathing versus manual breathstacking
Torres‐Castro 2016 reported the change from baseline VC to postintervention MIC. The published article reported that the median MIC achieved with manual breathstacking was 290 mL (IQR 168 to 567) greater than was achieved using GPB (P = 0.002); however, on analysing the provided separate first‐period data, the MD in postintervention MIC between groups was calculated as 90 mL (P = 0.76). There was no evidence of between‐groups difference in the MIC change from baseline to after intervention, with an MD from baseline to postintervention MIC in the breathstacking group of 435.0 (SD 364.5) mL compared to 454.29 (408.16) mL in the group receiving GPB (between‐group MD 19.29 mL, 95% CI –386.09 to 424.67; P = 0.9; 14 participants; Analysis 2.2).
2.2. Analysis.

Comparison 2: Glossopharyngeal breathing (GPB) versus manual breathstacking (BS), Outcome 2: Inspiratory capacity
8.2.3. MI‐E versus mechanical insufflation plus MAC
First‐period data provided on request by Lacombe 2014 compared mean inspiratory capacity between MI‐E alone (1.55 (SD 0.34) L) and mechanical insufflation plus MAC (1.43 (SD 0.34) L). There was no evidence of a difference in inspiratory capacity achieved between interventions (MD –0.12 L, 95% CI –33.44 to 33.20; P = 0.99; 11 participants; Analysis 3.2).
3.2. Analysis.

Comparison 3: Mechanical insufflation‐exsufflation (MI‐E) versus mechanical insufflation (MI) plus manually assisted cough (MAC), Outcome 2: Inspiratory capacity
8.2.4. MI‐E versus MI‐E plus MAC
Lacombe 2014 provided first‐period data for mean inspiratory capacity with MI‐E alone (1.55 (SD 0.34) L) and MI‐E plus MAC (1.39 (SD 0.43) L). There was no evidence of a difference in inspiratory capacity achieved between the interventions (between‐group MD –0.16, 95% CI –0.57 to 0.25; P = 0.44; 14 participants; Analysis 4.2).
4.2. Analysis.

Comparison 4: Mechanical insufflation‐exsufflation (MI‐E) versus mechanical insufflation‐exsufflation (MI‐E) plus manually assisted cough (MAC), Outcome 2: Inspiratory capacity
8.2.5. Mechanical insufflation plus MAC versus MI‐E plus MAC
First‐period data for mean inspiratory capacity for mechanical insufflation plus MAC (1.43 (SD 0.34) L) and MI‐E plus MAC (1.39 (SD 0.43) L) showed no evidence of difference between interventions (between‐group MD 0.04, 95% CI –0.42 to 0.50; P = 0.86; 11 participants; Analysis 5.2) (Lacombe 2014).
5.2. Analysis.

Comparison 5: Mechanical insufflation (MI) plus manually assisted cough (MAC) versus mechanical insufflation‐exsufflation (MI‐E) plus MAC, Outcome 2: Inspiratory capacity
8.2.6. Mechanical breathstacking versus mechanical insufflation
Del Amo Castrillo 2019 reported no difference in inspiratory capacity between breathstacking using a ventilator compared to mechanical insufflation using the ventilator's volumetric cough mode in 20 participants (P = 0.12). Separate cross‐over period data were not available, precluding analysis.
8.3. Minute ventilation
One study reported minute ventilation in 23 participants (Jenkins 2014). Minute ventilation increased from baseline with breathstacking using a manual resuscitation bag (P < 0.001) compared to a non‐significant change with sham breathstacking. Authors did not report between‐group significance levels, and, as separate period data were not available for the cross‐over RCT, these could not be calculated.
8.4. Maximal expiratory pressure
Toussaint 2016 reported that mean maximal expiratory pressure was 26 (SD 9) cmH2O in the group receiving resuscitator bag breathstacking compared to 28 (SD 10) cmH2O in those receiving ventilator breathstacking (MD 2.00 cmH2O, 95% CI –3.16 to 7.16; 52 participants).
8.5. Cough expiratory volume
One study reported cough expiratory volume in four participants (Sivasothy 2001). Median cough expiratory volumes were not different between mechanical insufflation, MAC, and MAC plus mechanical insufflation. The small sample size and lack of separate period data limit interpretation of these results.
8.6. Respiratory rate
Jenkins 2014 (23 participants) reported respiratory rate increased from 27 (SD 9.2) breaths/min to 28 (SD 10.6) breaths/min (P < 0.05) with manual breathstacking using a resuscitator bag compared to a non‐significant change from 26 (SD 10.3) breaths/min to 26 (SD 10.4) breaths/min with sham breathstacking. Between‐groups significance levels were not provided, and separate period data were not available, precluding analysis.
8.7. Heart rate
Chatwin 2009 (eight participants) reported heart rate; however, although the trial authors reported that there were no differences in heart rate between standard airway clearance therapy with and without MI‐E, data and significance levels were not reported. Attempts to obtain additional data were unsuccessful.
8.8. Effective cough time (time with PCF greater than 3 L/sec or greater than 180 L/min)
One study reported effective cough time in 18 participants (Lacombe 2014). Based on first‐period data received from the author on request, the MD in effective cough time from baseline with MI‐E alone was 54 (SD 95) ms; 93 (SD 111) ms with mechanical insufflation plus MAC; and 20 (SD 42) ms with MI‐E plus MAC. Although the trial authors reported, based on the combined cross‐over data, that the increase in effective cough time was smaller with MI‐E alone than with both the combined techniques using MAC, on analysis of separate first‐period data, there was no evidence of differences between any intervention: MI‐E versus mechanical insufflation plus MAC (MD 39.0 ms, 95% CI –90.56 to 168.56; P = 0.56; 11 participants); MI‐E versus MI‐E plus MAC (MD –34.00 ms, 95% CI –110.95 to 42.95; P = 0.39; 11 participants); and mechanical insufflation plus MAC versus MI‐E plus MAC (MD 73.00 ms, 95% CI –40.14 to 186.14; P = 0.21; 14 participants).
8.9. Peak value time (time from onset of expiratory flow to peak expiratory cough flow)
One study reported peak value time (Sivasothy 2001). In four participants, median peak value time was reported not to be significantly different between mechanical insufflation, MAC, and MAC plus mechanical insufflation. Separate first‐period data were not available, precluding analysis.
8.10. Ability to perform breathstacking
One study compared the ability to breathstack using a resuscitator bag (manual breathstacking) compared with ventilator (mechanical) breathstacking (Toussaint 2016). There was no evidence of a difference in the ability to breathstack between groups, with 88% in the resuscitator bag group versus 89% in the ventilator group being able to perform the technique (RR 0.93, 95% CI 0.21 to 4.17; P = 0.33; 52 participants).
8.11. Number of insufflations to achieve MIC
Toussaint 2016 reported that a mean of 1.8 (SD 0.6) insufflations were required to reach MIC with manual breathstacking using a resuscitator bag compared to a mean of and 2.6 (SD 0.6) insufflations with mechanical breathstacking using a ventilator (between‐group MD 0.80 insufflations, 95% CI 0.47 to 1.13; P < 0.001; 52 participants).
8.12. Subjective outcome measures
One study reported auscultation score, measured using a 10‐point VAS (eight participants) (Chatwin 2009). Auscultation VAS decreased significantly with both MAC (P = 0.007) and MAC plus MI‐E (P = 0.02). Between‐groups significance levels were not reported, and we could not obtain separate period data, precluding analysis.
Chatwin 2003 (22 participants) reported a combined outcome of comfort, distress, and cough strength. The trial authors reported that there were no changes from baseline in VAS results on a 10‐point scale for any intervention (MAC, mechanical insufflation, mechanical exsufflation, or MI‐E). Between‐group significance levels were not reported, and we could not obtain separate period data, precluding analysis.
Chatwin 2009 (eight participants) reported participants' perceived presence of secretions, using a 10‐point VAS, improved from before to after intervention with standard therapy including MAC (P = 0.03) and with standard therapy with MAC plus MI‐E (P = 0.03). Between‐group significance levels were not reported, and we could not obtain separate period data, precluding analysis.
Three studies (46 participants) reported participant comfort using a 10‐point VAS (Chatwin 2009; Del Amo Castrillo 2019; Lacombe 2014). Chatwin 2009 (eight participants) presented results graphically only, and data could not be extracted from the figures. Lacombe 2014 (18 participants) reported no significant differences in subjective comfort between MI‐E, mechanical insufflation plus MAC, and MI‐E plus MAC, but did not present significance levels. Del Amo Castrillo 2019 (20 participants) reported no significant difference in comfort VAS between ventilator breathstacking and mechanical insufflation using the ventilator's volumetric cough mode.
Two studies (38 participants) reported subjective cough effectiveness (Del Amo Castrillo 2019; Lacombe 2014). Aggregate results from the cross‐over study by Lacombe 2014 (18 participants) suggested a significant difference in perceived cough effectiveness between MI‐E alone and MI‐E plus MAC (P < 0.05, favouring MI‐E plus MAC) and between mechanical insufflation plus MAC and MI‐E alone (P < 0.05, favouring mechanical insufflation plus MAC). Median values provided for the first period of cross‐over study by Lacombe 2014 (see Table 4), however, suggested a possible difference between MI‐E and MI‐E plus MAC, and no evidence of a difference between MI‐E and mechanical insufflation plus MAC in perceived cough effectiveness (measured using a 10‐point VAS). There were insufficient data to confirm the size or precision of the effect. Del Amo Castrillo 2019 (20 participants) reported no difference in perceived cough effectiveness with mechanical breathstacking compared to mechanical insufflation using volumetric cough mode (P = 0.17). Separate period data could not be obtained for this study, precluding analysis.
One study measured participant mood using a 10‐point VAS (eight participants) (Chatwin 2009). The report only presented data graphically and results could not be extracted accurately from the figures. The study reported that no within‐groups changes from baseline to after either intervention: standard treatment with MAC, or standard treatment with MAC plus MI‐E. No between‐group values or significance levels were reported, and attempts to obtain additional information were unsuccessful.
None of the studies provided cost‐effectiveness analyses, and we could not evaluate it as part of this review.
Cough augmentation therapy compared to standard of care
One study, in 67 children and adolescents with DMD, compared twice daily manual breathstacking compared to standard care, over two years (Katz 2019). Reported outcomes are presented in Table 6 and Table 5.
Primary outcomes
1. Number of unscheduled hospital admissions for episodes of acute respiratory exacerbations over one year for 'maintenance' therapy
The study did not report number of hospital admissions for episodes of acute respiratory exacerbations over one year, for 'maintenance use.'
2. Duration of hospital stay (days) for 'rescue' therapy
The study protocol by Katz 2019 included, as a secondary outcome measure, the number and duration of hospital admissions over two years in 67 participants. However, the published abstract did not present these outcome data. Attempts to contact the author were unsuccessful.
Secondary outcomes
1. PCF measured before and after intervention for 'rescue' therapy and measured over the medium term (between three months and one year) and long term (one year and longer) for 'maintenance' therapy
The study did not report PCF.
2. Any adverse events, including, but not limited to: pneumothorax, rib fractures, lung injury, aerophagia/abdominal distension, and death for both 'maintenance' and 'rescue' therapy
Katz 2019 did not include adverse events as a primary or secondary outcome measure but reported that no adverse events had occurred (67 participants; very low‐certainty evidence).
3. Measures of gaseous exchange measured before and after the intervention for 'rescue' therapy, and measured over the medium term (between three months and one year) and long term (one year and longer) for 'maintenance' therapy
The study did not report measures of gaseous exchange.
4. Pulmonary function measured by FEV1, FVC, VC, and PEFR, over the short term (less than three months); medium term (between three months and one year); and long term (one year and longer) for 'maintenance' therapy
Katz 2019 measured change in FVC (as percentage predicted) over two years from baseline. Change in FVC among participants in the breathstacking group was 4.1% compared to 6.4% in the conventional treatment group (adjusted MD 2.0%, 95% CI –8.2 to 12.3; 67 participants). Sufficient data, including number of participants per group, separate allocation baseline data, and SD of the mean, were not available for analysis. This study also reported that the time to 10% decline in FVC% predicted was not significantly different between groups (P = 0.5) but did not provide data, precluding analysis. We may be able to include complete results from this study in analysis in updates of this review, if data become available.
5. Quality of life measured by any validated measure over the medium term (between three months and one year) and long term (one year and longer) for 'maintenance' therapy
Katz 2019 planned to report health‐related quality of life using the PedsQL 4.0. However, the published abstract did not report this outcome measure, and attempts to contact the author for additional data were unsuccessful. If data become available, we may be able to include health‐related quality of life data in updates of this review.
6. Validated measures of function, including measures of perceived exertion, exercise tolerance, and motor function measured over the medium term (between three months and one year) and long term (one year and longer) for 'maintenance' therapy
The study did not report validated measures of function.
7. Participant preference for, or satisfaction with, specific cough augmentation techniques, expressed as a proportion or percentage of the sample for both 'rescue' and 'maintenance' therapy
The study did not report participant preference or satisfaction.
8. Other outcome measures
We presented data for other outcome measures in Table 6.
Katz 2019 included MIC, MEP, MIP, and the number and duration of outpatient oral antibiotic courses as additional outcome measures in the published protocol (67 participants). However, these data were not reported in the published abstract and attempts to contact the author were unsuccessful. Results from this study may be able to be included in updates of this review, if data become available.
Discussion
Summary of main results
Eleven studies involving 287 children, adolescents, and adults, with a variety of NMDs, met this review's inclusion criteria. Sample sizes in individual studies ranged from four to 67 eligible participants; 10 studies were full‐text articles and one was in abstract form.
Included studies compared a range of cough augmentation technique(s) to alternative interventions (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016); standard care (control) (Katz 2019); unassisted cough (Kim 2016), or sham intervention (Jenkins 2014), for several outcome measures. Most studies compared intervention‐assisted cough outcomes with preintervention or baseline measurements of unassisted cough (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Katz 2019; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016; Toussaint 2016). Only one study was of long‐term duration, lasting two years (Katz 2019), but there were limited data presented in abstract format only. One study was a two‐day cross‐over trial (Chatwin 2009), while the remainder measured the immediate effects of single intervention sessions. Only two studies were prospective RCTs (Katz 2019; Toussaint 2016), the remaining nine were short‐term randomised cross‐over trials (Brito 2009; Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019; Jenkins 2014; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016). Two cross‐over studies provided first‐period data (Lacombe 2014; Torres‐Castro 2016), which constitutes a major limitation of this review. The large number of inadequately reported results from cross‐over studies, and the limited information provided by authors on request, severely restricted the number of analyses that could be performed.
Cough augmentation techniques aim to improve cough efficiency, with potential for both short‐ and long‐term effects on pulmonary morbidity. During acute respiratory exacerbations, cough augmentation techniques aim to clear obstructed secretions to prevent the progression to respiratory failure, improve work of breathing and gaseous exchange, and potentially reduce the need for hospital admission and, if admitted, reduce the length of stay. In the longer term, regular use of cough augmentation is hoped to reduce the incidence or severity (or both) of respiratory tract infections requiring unscheduled hospitalisation. Although one long‐term RCT planned to measure this review's primary outcome measures of number and duration of hospital admissions (Katz 2019), the published abstract of the study did not report these outcomes and attempts to contact the author for additional data were unsuccessful, therefore, these data could not be included in this review. None of the other included studies measured or reported on this review's primary outcomes. Therefore, the evidence is very uncertain about the efficacy of any cough augmentation technique for reducing the number or duration (or both) of hospital admissions for respiratory exacerbations in people with NMD (see Table 1; Table 5).
Clinically important secondary outcomes of this review were selected for their utility in measuring the safety of cough augmentation techniques and their effect on cough efficiency (PCF), gas exchange (oxygenation and carbon dioxide clearance), as well as objective and subjective measures of pulmonary and general function, quality of life, and participant preference or satisfaction. Only three studies provided sufficient data for analysis of one of this review's secondary outcome measures of PCF (Lacombe 2014; Torres‐Castro 2016; Toussaint 2016). None of the included studies provided sufficient data for analysis of any of this review's other secondary outcome measures. Therefore, the evidence is very uncertain about the effect of cough augmentation techniques on measures of safety, gaseous exchange, pulmonary function, quality of life, general function, or participant preference or satisfaction.
Although four studies reported that no adverse events had occurred (Chatwin 2003; Katz 2019; Kim 2016; Sivasothy 2001), none of the included studies listed "adverse events" as primary or secondary outcome measures. Chatwin 2009 reported that fatigue increased in participants receiving MAC plus MI‐E, with no change in fatigue in those receiving MAC alone; however, there were insufficient data for analysis (Table 1). The evidence is therefore very uncertain about the safety of any of the included cough augmentation interventions.
One RCT with 67 participants planned to measure the long‐term effect of manual breathstacking on PCF (Katz 2019); however, this outcome measure was not reported in the published abstract and data could not be included in this review (Table 5).
Eight studies with 198 participants compared the PCF generated with various cough augmentation techniques to baseline unassisted cough, as a repeated measure for each participant (Brito 2009; Chatwin 2003; Del Amo Castrillo 2019; Kim 2016; Lacombe 2014; Sivasothy 2001; Torres‐Castro 2016; Toussaint 2016). All but two cross‐over RCTs with small sample sizes (Chatwin 2003; Sivasothy 2001), showed significant increases in PCF with cough augmentation therapy from baseline. However, "unassisted cough" in all studies, except for Kim 2016, was measured at baseline or before intervention, and was not a randomly assigned control intervention. Kim 2016 did not provide separate period data. Therefore, there is only very low‐certainty evidence that manual and mechanical breathstacking; GPB; MI‐E; mechanical exsufflation; MAC; MAC plus MI‐E; MAC plus breathstacking; and mechanical insufflation may all increase PCF above unassisted cough (Table 3; Table 4; Table 2).
Based on one single‐centre, short‐term RCT (52 participants), with high risk of performance and assessor bias, and unclear allocation concealment (Toussaint 2016), there was low‐certainty evidence that manual breathstacking using a resuscitation bag may result in little to no difference in PCF in the short‐term, compared to mechanical breathstacking (using a ventilator). Further results from RCTs are very likely to have an important impact on our confidence in the estimate of effect and are likely to change this estimate (Table 2).
Based on the results of the first‐period data of one short‐term, randomised cross‐over study (14 participants) (Torres‐Castro 2016), there may be little to no difference in the short‐term outcome of PCF between GPB compared with manual breathstacking (Table 2). Considering the very small sample size of this single study, and high risk of performance and detection bias, we are very uncertain about this estimate, and sufficiently powered RCTs to compare the effects of these interventions on PCF are warranted.
Based on the first period results of one short‐term, randomised cross‐over study with small sample size (18 participants), very wide CIs, and substantial risk of performance and other biases (Lacombe 2014), there is very low‐certainty evidence that, in adults with chronic NMD, mechanical insufflation plus MAC may improve PCF more than MI‐E alone in the short term. The evidence suggests little to no difference in the change in PCF between MI‐E plus MAC and mechanical insufflation plus MAC. We are very uncertain about these estimates, and further adequately powered RCTs are required to confirm or refute these results (Table 2).
Aggregate results of short‐term cross‐over trials, without provision of separate period data, reported little to no difference in PCF between MAC and manual breathstacking (Brito 2009), or among MAC, mechanical insufflation, and MAC plus mechanical insufflation (Sivasothy 2001). Higher PCF was reported with MI‐E compared to mechanical exsufflation (Chatwin 2003) and MAC plus manual breathstacking (Kim 2016); with MAC plus manual breathstacking compared to either MAC or manual breathstacking individually (Brito 2009); with MAC plus MI‐E compared to MI‐E alone (Kim 2016) and MAC plus manual breathstacking (Kim 2016); and mechanical insufflation compared to mechanical breathstacking (Del Amo Castrillo 2019). Overall, the evidence suggests there may be little to no difference between alternate cough augmentation interventions in improving PCF (Table 2). The evidence for this is, however, very uncertain.
Two cross‐over studies measured the short‐term effect of interventions on gaseous exchange. Chatwin 2009 reported there was no difference in transcutaneous oxygen saturation and PtcCO2 between MAC and MAC plus MI‐E; Jenkins 2014 reported no difference in transcutaneous oxygen saturation between manual and sham breathstacking. One long‐term RCT measured pulmonary function (FVC) (Katz 2019); however, there were insufficient data, precluding analysis. This study reported no change in FVC or change in the time to 10% decline in FVC between participants receiving manual breathstacking compared to standard care. One study planned to report the long‐term effects of interventions on health‐related quality of life (Katz 2019); however, no data were available. The evidence is therefore very uncertain about the effect of any cough augmentation technique on gaseous exchange or health‐related quality of life.
Other outcome measures were variably reported in included studies. Based on aggregated reported results of three cross‐over studies (Chatwin 2003; Chatwin 2009; Del Amo Castrillo 2019), and first‐period data from one cross‐over study (total 68 participants) (Lacombe 2014), there was no evidence of superior participant comfort with any cough augmentation technique. Although no study reported participant preference, short‐term perceived cough effectiveness was reported in the aggregate results of three cross‐over studies with 42 participants (Del Amo Castrillo 2019; Lacombe 2014; Sivasothy 2001). Lacombe 2014 reported that perceived effectiveness was higher with MAC plus MI‐E compared with MI‐E alone. The potential greater efficacy of combined cough augmentation techniques compared with single techniques requires attention in future RCTs.
Four studies with 104 participants reported maximal insufflation or inflation capacity (MIC) (Del Amo Castrillo 2019; Lacombe 2014; Torres‐Castro 2016; Toussaint 2016), with three studies (84 participants) providing sufficient data for analysis (Lacombe 2014; Torres‐Castro 2016; Toussaint 2016). Based on these studies, there may be little to no difference in MIC with MAC, MI‐E, and MAC plus MI‐E; manual breathstacking versus GPB; mechanical versus manual breathstacking; or mechanical breathstacking versus mechanical insufflation (using volumetric cough mode).
Overall completeness and applicability of evidence
We identified 11 studies for inclusion in this review, nine of which were short‐term cross‐over studies of which only two provided separate first‐period data for analysis. These methodological limitations substantially impact on the internal and external validity of this review. We found only one long‐term trial for maintenance therapy; however, complete data were not available for inclusion in this review. None of the included studies reported clearly on the short‐ or long‐term effects of cough augmentation interventions on clinically relevant outcomes of morbidity and safety, and this study could not, therefore, address the objective of this review in determining the efficacy and safety of cough augmentation techniques for adults and children with chronic NMD and respiratory muscle weakness. Furthermore, none of the studies compared different dosages or frequencies of application of any cough augmentation technique and the evidence is therefore very uncertain regarding optimal safe and effective prescription of cough augmentation techniques in people with NMD.
Participant numbers were generally small, with no possibility of subgroup analyses for different age groups or conditions. As seen in Table 3; Table 4; and Table 6, studies compared a variety of interventions, with variable techniques, and a wide range of outcome measures. Studies were conducted in Europe (three), the UK (three), Canada (two), Korea (one) and South America (two). External generalisability to other geographical regions and socioeconomic contexts cannot be determined. None of the studies provided any estimate of cost‐effectiveness.
Quality of the evidence
Key limitations of included studies were: study design; small sample sizes; unreliable or clinically irrelevant outcome measures; and unclear to high risk of bias, specifically related to poorly reported methods of allocation concealment, randomisation sequence generation, insufficient blinding of participants and personnel, and insufficient reporting of data. The overall certainty of the evidence of included studies was low or very low, with most being short‐term randomised cross‐over trials, in which participants received two or more interventions in randomly assigned order, with undetermined and untested carry‐over effects (Mills 2009) and, in all but three studies, insufficient information to allow data analysis. In several studies, the investigators compared cough augmentation techniques and unassisted cough; however, unassisted coughing was not a randomly assigned controlled intervention except in one study (Kim 2016), and definitive conclusions regarding efficacy of interventions cannot therefore be made.
Cross‐over study designs are considered suitable for evaluating interventions with a temporary effect in participants with stable or chronic conditions (Nolan 2016), and may, therefore, be appropriate for measuring outcome measures such as PCF, a secondary outcome of interest for this review. However, short‐term cross‐over designs are generally not the most appropriate for measuring longer‐term health‐related outcomes of chronic life‐limiting and progressive conditions such as NMDs. The immediate effects of an intervention may not translate into longer‐term benefit, and results of such studies must, therefore, be interpreted with caution. The decision to include cross‐over trials in this review was based on the knowledge that this is the most common study design used among this population group, likely owing to various factors including the fact that NMDs are rare conditions, and generally a smaller overall sample size is needed for cross‐over compared to parallel‐group RCTs (Nolan 2016). In addition, well‐conducted cross‐over trials may yield more precise results than parallel‐group designs, owing to lower variability with individual compared to between‐participant responses (Elbourne 2002). The inability to pool or individually analyse data from most cross‐over trials limits the validity of this review. It is not considered methodologically acceptable to simply treat cross‐over trials as parallel‐group RCTs for the purposes of systematic reviews and meta‐analysis (Elbourne 2002).
Blinding of participants and research personnel is generally not possible for interventions such as cough augmentation techniques, increasing the risk of bias of studies. Limited available information regarding methodology (e.g. allocation concealment, washout periods, and randomisation sequence generation) further increased the risk of bias in included studies. The publications of all included cross‐over trials presented results as though from parallel‐group RCTs, and we judged the data unsuitable for meta‐analysis (Elbourne 2002).
The body of evidence included in this review did not allow any clear conclusions to be reached regarding the efficacy or safety of cough augmentation techniques in people with chronic NMD.
Potential biases in the review process
There were no major deviations from the published protocol in conducting this review. Our literature search was comprehensive, and included searches for unpublished material through trial registration platforms and congress abstract reports. There were no geographical, time, or language constraints to this review. However, it is possible that some studies may have been overlooked, particularly if published in non‐peer reviewed journals or presented at small or regional congresses. Further, we cannot control for inherent publication bias.
We contacted the corresponding authors of included studies, where appropriate, to obtain missing results or additional information but most did not respond and only one author was able to provide all the necessary information. We could not obtain missing data on the primary outcomes of this review, as measured by Katz 2019, and this may have substantially impacted on this review, which is an unavoidable source of bias. We hope that these data will be available for future versions.
Agreements and disagreements with other studies or reviews
A previous Cochrane systematic review concluded that there was insufficient evidence for or against the use of MI‐E as a cough augmentation technique in people with NMD, for efficacy and safety outcome measures (Morrow 2013). This review was also unable to present moderate or high certainty evidence for or against the safety or efficacy of either MI‐E or any other cough augmentation technique in people with NMD. Further, the evidence from this review suggests there may be little to no difference between any alternate cough augmentation technique, for a range of short‐ or long‐term outcome measures.
A previous 'state of the art' narrative review systematically reviewed the evidence‐base for airway clearance techniques (including both peripheral and proximal techniques) in adults and children with NMD, including participants with ALS (Chatwin 2018). The review reported on all study designs, including case studies and retrospective audits. Chatwin 2018 suggested that all cough augmentation techniques, including MAC, single‐breath assisted inspiration (manual insufflation), breathstacking, GPB, and MI‐E effectively increase PCF, as is also suggested in this review, based on very low‐certainty evidence. Chatwin 2018 also suggested that combining a technique augmenting inspiration with one that enhances expiration may further increase cough efficacy; however, this review demonstrated that the evidence for better cough efficiency with combination compared to single techniques is very uncertain and further research is warranted in this regard. Based largely on observational studies, Chatwin 2018 recommended using MI‐E preferentially for weaker patients with NMD. This recommendation is not supported or refuted by the results of this review. The review by Chatwin 2018 was limited by the lack of defined review objectives, the inclusion of all study types, and the lack of a risk of bias or GRADE assessments for the included studies. Owing to the differing purpose, methodologies, and reporting, direct agreements or disagreements between reviews cannot be made.
Authors' conclusions
Implications for practice.
The results of this review do not provide sufficient certainty of evidence to guide clinical practice, as we were unable to address important short‐ and long‐term clinically relevant outcomes, including measures of safety. There is very low‐certainty evidence that a range of cough augmentation techniques may increase peak cough flow (PCF) above that of unassisted cough; however, there is insufficient certainty of evidence to determine whether any one technique is superior to another technique or combination of techniques in this regard. The evidence is currently very uncertain about the safety and effectiveness of cough augmentation techniques in adults and children with chronic neuromuscular disease (NMD). Considering that respiratory decompensation in people with NMD may occur as a consequence of the inability to clear secretions during cough (Toussaint 2018), and given the very low‐certainty evidence supporting the effect of cough augmentation techniques on PCF, practitioners may continue to implement this therapy in people with chronic NMD and respiratory muscle weakness, as recommended previously (Chatwin 2018; Toussaint 2018). However, as there is no moderate or high certainty evidence for the superiority of any cough augmentation technique/s, the choice of techniques may take other factors into account, including cost, patient preference and ability, therapist knowledge and proficiency, and equipment availability. Further, there is insufficient evidence to inform safe and effective frequency or dosage of cough augmentation techniques in the management of people with respiratory muscle weakness caused by chronic NMD.
Implications for research.
Further research is required to establish the safety and efficacy of cough augmentation techniques in people with NMDs, for both long‐term maintenance use, and during respiratory exacerbations or acute obstructive episodes, for 'rescue' use. We need future studies to measure longer‐term, clinically relevant outcomes that will inform the effects of interventions on morbidity, mortality, and health‐related quality of life. We also need systematic reporting of adverse events to obtain safety data, and reporting on participant choice of techniques. Studies comparing dosage and frequency regimens would be useful in this regard. Choice of comparators would depend on local standard of care. Sham treatment or non‐intervention controls may be difficult to support ethically over the long term, but comparative studies of different interventions could be ethically justifiable, as there is clearly equipoise between intervention types. Given the diversity of NMD and of age groups affected, we need studies that either focus on a specific age group or condition, or provide sufficient data for subgroup analyses in systematic reviews.
In terms of study design, long‐term parallel‐group RCTs provide the best evidence, but NMDs are rare and attaining sufficient sample size is often difficult. Researchers should therefore be encouraged to consider multisite collaborative studies to reach sufficient sample sizes for adequate power, and to allow meaningful subgroup analyses. It is particularly important to consider paediatric and adult data separately, owing to the anatomical and physiological differences between these participant groups, which likely translate into different safety and efficacy profiles.
For short‐ and medium‐term outcomes such as immediate change in PCF, cross‐over trials may be useful, as smaller samples may yield equivalent power to a parallel‐group RCT. Importantly, comprehensive reporting of data from cross‐over trials will allow for systematic review synthesis and analysis (Elbourne 2002; Nolan 2016). This includes providing full details on methods (including allocation concealment, sequence generation, washout periods, and carry‐over effects) and either individual level data (including allocation information) or appropriately summarised, separate data for both periods (Elbourne 2002). Our findings support the recommendation that minimum standards for the transparent reporting of cross‐over trials are urgently needed (Mills 2009), as currently the results of many of these trials are essentially lost, because they cannot be included in important meta‐analyses to inform clinical practice.
Involvement of people living with or affected by NMDs when designing clinical trials can ensure that outcome measures and interventions are appropriate and responsive to their needs and experiences. Cost‐effective analyses are also warranted to relate the potential benefits of interventions with financial, physical, and social costs or harms.
History
Protocol first published: Issue 11, 2018 Review first published: Issue 4, 2021
Notes
This review will partially supersede 'Mechanical insufflation‐exsufflation for people with neuromuscular disorders' (Morrow 2013).
Acknowledgements
The Information Specialist of Cochrane Neuromuscular, Angela Gunn, developed the search strategy in consultation with the review authors.
The Methods section of this protocol was based on a template developed by the Cochrane Neuromuscular Disease Group from an original created by the Cochrane Airways Group.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Disease.
Appendices
Appendix 1. CINAHL (EBSCOhost) search strategy
Monday, April 13, 2020 8:49:22 AM
CINAHL Plus with Full Text
S30 S29 Limiters – Exclude MEDLINE records 55
S29 S18 AND S28 102
S28 S19 OR S20 OR S21 OR S22 OR S27 284
S27 S25 AND S26 103
S26 breath* or resp* 1,091,462
S25 S23 OR S24 90 117
S24 "manual insufflation" 4
S23 mechanical N4 (insufflation or exsufflation) 117
S22 "frog breath*" 1
S21 "glossopharyngeal breath*" 25
S20 "breath stack*" or "air stack*" 48
S19 assist* N2 cough* 167
S18 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 1,529,197
S17 ABAB design* 162
S16 TI random* or AB random* 364,861
S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 734,319
S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 280,652
S13 ( TI (meta?analys* or "systematic review*") ) or ( AB (meta?analys* or "systematic review*") ) 98,430
S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 55,477
S11 PT ("clinical trial" or "systematic review") 224,844
S10 (MH "Factorial Design") 1,352
S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 464,917
S8 (MH "Meta Analysis") 49,933
S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 120
S6 (MH "Quasi‐Experimental Studies") 14,347
S5 (MH "Placebos") 13,662
S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 49,848
S3 (MH "Clinical Trials+") 317,588
S2 (MH "Crossover Design") 21,101
S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 114,917
Appendix 2. ICTRP Platform search strategy
ICTRP was not accessible at the time of update search on 13 April 2020.
Advanced search
Intervention: (cough AND assist) OR (assisted coughing) OR (breath stacking) OR (air stacking) OR (mechanical exsufflation)
Recruitment status: ALL
Appendix 3. ClinicalTrials.gov search strategy
Advanced Search
Study type: Interventional (Clinical Trials)
Intervention/treatment: (Cough AND Assist) OR Assisted Coughing OR Breath Stacking OR Air Stacking OR Mechanical Exsufflation
76 Studies Found
Appendix 4. Embase (OvidSP) search strategy
Database: Embase <1974 to 2020 Week 15>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 crossover‐procedure.sh. (62746)
2 double‐blind procedure.sh. (171274)
3 single‐blind procedure.sh. (38496)
4 randomized controlled trial.sh. (598106)
5 (random* or crossover* or cross over* or placebo* or (doubl* adj blind*) or allocat*).tw,ot. (1760750)
6 trial.ti. (296091)
7 controlled clinical trial/ (463970)
8 or/1‐7 (2085723)
9 exp animal/ or exp invertebrate/ or animal.hw. or non human/ or nonhuman/ (27204367)
10 human/ or human cell/ or human tissue/ or normal human/ (20829599)
11 9 not 10 (6444888)
12 8 not 11 (1854230)
13 limit 12 to (conference abstracts or embase) (1561015)
14 (assist* adj2 cough*).mp. (503)
15 (breath stack* or air stack*).mp. (147)
16 glossopharyngeal breath*.mp. (77)
17 frog breath*.mp. (2)
18 (mechanical adj4 (insufflation or exsufflation)).mp. (296)
19 manual insufflation.mp. (11)
20 18 or 19 (307)
21 (breath* or resp*).mp. (8827319)
22 20 and 21 (258)
23 or/14‐17,22 (810)
24 13 and 23 (92)
25 remove duplicates from 24 (92)
Appendix 5. Cochrane Neuromuscular Specialised Register via the Cochrane Register of Studies (CRS‐Web) search strategy
1 assist* NEAR2 cough* AND INREGISTER 13
2 "breath stack*" or "air stack*" AND INREGISTER 7
3 "glossopharyngeal breath*" AND INREGISTER 0
4 "frog breath*" AND INREGISTER 0
5 mechanical NEAR4 (insufflation or exsufflation) AND INREGISTER 10
6 "manual insufflation" AND INREGISTER 0
7 #5 OR #6 10
8 breath* OR resp* AND INREGISTER 2723
9 #7 AND #8 10
10 #1 OR #2 OR #3 OR #4 OR #9 20
Appendix 6. CENTRAL via the Cochrane Register of Studies (CRS‐Web) search strategy
1 assist* NEAR2 cough* AND CENTRAL:TARGET7 9
2 "breath stack*" or "air stack*" AND CENTRAL:TARGET 46
3 "glossopharyngeal breath*" AND CENTRAL:TARGET 1
4 "frog breath*" AND CENTRAL:TARGET 0
5 mechanical NEAR4 (insufflation or exsufflation) AND CENTRAL:TARGET 51
6 "manual insufflation" AND CENTRAL:TARGET 0
7 #5 OR #6 51
8 breath* or resp* AND CENTRAL:TARGET 538201
9 #7 AND #8 46
10 #1 OR #2 OR #3 OR #4 OR #9 142
Appendix 7. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) ALL <1946 to April 10, 2020>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 randomized controlled trial.pt. (503706)
2 controlled clinical trial.pt. (93614)
3 randomi#ed.ti,ab. (613469)
4 placebo.ab. (206754)
5 drug therapy.fs. (2194104)
6 randomly.ab. (330921)
7 trial.ab. (501216)
8 groups.ab. (2032325)
9 or/1‐8 (4703106)
10 exp animals/ not humans.sh. (4689538)
11 9 not 10 (4080041)
12 (assist* adj2 cough*).mp. (273)
13 (breath stack* or air stack*).mp. (77)
14 glossopharyngeal breath*.mp. (64)
15 frog breath*.mp. (5)
16 (mechanical adj4 (insufflation or exsufflation)).mp. (180)
17 manual insufflation.mp. (8)
18 16 or 17 (188)
19 (breath* or resp*).mp. (6507692)
20 18 and 19 (161)
21 or/12‐15,20 (482)
22 11 and 21 (81)
23 remove duplicates from 22 (81)
Data and analyses
Comparison 1. Manual versus mechanical breathstacking (BS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Peak cough flow | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 6.00 [‐33.43, 45.43] |
| 1.2 Maximal insufflation capacity | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.13, 0.41] |
Comparison 2. Glossopharyngeal breathing (GPB) versus manual breathstacking (BS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Peak cough flow | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐40.72 [‐90.54, 9.10] |
| 2.2 Inspiratory capacity | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 19.29 [‐386.09, 424.67] |
Comparison 3. Mechanical insufflation‐exsufflation (MI‐E) versus mechanical insufflation (MI) plus manually assisted cough (MAC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Peak cough flow | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 71.40 [18.08, 124.72] |
| 3.2 Inspiratory capacity | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐33.44, 33.20] |
Comparison 4. Mechanical insufflation‐exsufflation (MI‐E) versus mechanical insufflation‐exsufflation (MI‐E) plus manually assisted cough (MAC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Peak cough flow | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 52.80 [‐0.32, 105.92] |
| 4.2 Inspiratory capacity | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.57, 0.25] |
Comparison 5. Mechanical insufflation (MI) plus manually assisted cough (MAC) versus mechanical insufflation‐exsufflation (MI‐E) plus MAC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Peak cough flow | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | 18.60 [‐34.46, 71.66] |
| 5.2 Inspiratory capacity | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.42, 0.50] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brito 2009.
| Study characteristics | ||
| Methods |
Study design: prospective randomised cross‐over trial comparing MAC, breathstacking, and MAC + breathstacking Study grouping: cross‐over 'Rescue' vs maintenance therapy: maintenance Ethics: ethical clearance provided by Federal University of São Paulo (UNIFESP) Research Ethics Committee (CEP 0775/06) |
|
| Participants |
Baseline characteristics Separate data were not documented for group allocations in the first period of cross‐over. Data were presented for all participants, who received all interventions.
Inclusion criteria
Exclusion criteria
Pretreatment
|
|
| Interventions |
Intervention characteristics Baseline spontaneous MEE
Manual chest compression (MAC)
Breathstacking using a manual resuscitation bag
Chest compressions (MAC) + breathstacking
|
|
| Outcomes | Separate first‐period data were not presented, precluding analysis. PCF
Adverse events: not reported |
|
| Identification |
Funding source: financial support was provided by the Associação Fundo de Incentivo à Psicofarmacologia (AFIP, Association for the Incentive Funding of Psychopharmacology). Conflict of interest statement: funding source declared and unlikely to constitute a conflict of interest. Country: Brazil Setting: outpatient clinic: Pediatric Sector of the Noninvasive Mechanical Ventilation Outpatient Clinic of the Psychobiology Department of the Sleep Institute at UNIFESP, Federal University of São Paulo Author name: Magneide Fernandes Brito (corresponding author) Institution: Sleep Medicine and Biology Division of the Psychobiology Department; Federal University of São Paulo Email: magneide@gmail.com Address: Rua Marselhesa, 500, 14° andar, Vila Clementino, CEP 04020‐060, São Paulo, SP, Brazil |
|
| Notes | Attempts to contact the corresponding author for additional data were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "To avoid the influence of the order of the maneuvers and minimize patient fatigue, the sequence of the time points (other than, obviously, baseline) was random." Comment: there was no intervention and control group, each participant acted as their own control in a cross‐over design. All participants had baseline measurements, which were compared to various cough augmentation interventions. Although there was random allocation of intervention order, there was no indication of how randomisation was done. Separate group/period data were not provided so baseline imbalances could not be determined. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. Unclear whether or how allocation concealment was maintained. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: although not stated in the study, this was likely not achieved, as both participants and personnel performing the interventions would have been aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the PCF measurements were taken during a spontaneous MEE accompanied by chest compression…" Quote: "For the air stacking‐only time point, the PCF measurements were made after air stacking…" Quote: "…after the third insufflation, the patient made a forced exhalation, and the PCF with MEE was measured." Quote: "For the combined technique time point, the PCF was measured after the use of air stacking with a manual resuscitation bag followed by chest compression with MEE." Comment: PCF values were measured while performing the cough assist techniques and, therefore, assessment could not have been performed blinded. This leads to potential detection bias. The same examiner performed all measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "We excluded 2 patients for not having the intellectual capacity to understand and perform the maneuvers involved in the spirometry and PCF measurements." Comment: 2 participants were excluded with reasons provided; there were no other missing data or dropouts reported. All 28 participants' data were presented in Figure 2 of the publication. |
| Selective reporting (reporting bias) | High risk | Comment: not all the prespecified outcome measures were reported in the results, e.g. SpO2, expired CO2, and all spirometric measures. Of the last‐mentioned, only FVC was reported (mean FVC% predicted 29% (SD 12%)); no other spirometry or bio‐demographic values were provided. Data for the primary outcome measure, PCF, were presented; however, separate period data were not provided, precluding the possibility of meta‐analysis. |
| Other bias | High risk | Comment: the following factors placed the study at high risk of other bias.
|
Chatwin 2003.
| Study characteristics | ||
| Methods |
Study design: prospective randomised cross‐over trial, comparing MI‐E to other cough augmentation techniques* Study grouping: cross‐over 'Rescue' vs maintenance therapy: maintenance Ethics: local ethics committee approval. All participants, parents, or both, provided informed consent |
|
| Participants |
Baseline characteristics Separate data were not available for group allocations in the first period of cross‐over. Data were presented for all participants, who received all interventions. Neuromuscular disease group
Inclusion criteria (NMD group)
Exclusion criteria (NMD group)
Age‐matched controls
Inclusion criteria (controls)
Exclusion criteria (controls)
Pretreatment: no separate data were provided for the first period of cross‐over, or for separate intervention order groups. |
|
| Interventions | For all participants, baseline unassisted cough was compared, in random order: to standard physiotherapy and assisted cough; cough after inspiration supported by NIPPV (BiPAP); exsufflation‐assisted cough, with negative pressure initiated manually at end of inspiration; insufflation‐assisted cough; and exsufflation‐assisted cough with negative pressure delivered immediately preceding the cough effort. Baseline unassisted cough
Standard "physiotherapy assisted cough"
Cough after inspiration supported by a non‐invasive positive pressure ventilator
Exsufflation‐assisted cough, with negative pressure initiated manually at end inspiration
Insufflation‐exsufflation‐assisted cough
Exsufflation‐assisted cough with negative pressure delivered immediately preceding the cough effort
|
|
| Outcomes | Separate first‐period data were not presented, precluding analysis. PCF
VAS comfort, distress, and perceived cough strength
Adverse events: no adverse events reported and participants were reported to tolerate all interventions well. |
|
| Identification |
Funding source: Jennifer Trust for Spinal Muscular Atrophy,UK (M Chatwin); Brompton Breathers Trust Fund, UK (patient expenses support); Cystic Fibrosis Trust, UK (E Ross); British Lung Foundation, UK (AH Nickol); Association Française Contre Les Myopathies, France (N Hart) Conflict of interest: funding sources identified and unlikely to constitute a conflict of interests. Country: UK Setting: Sleep and Ventilation Unit, Royal Brompton Hospital Comments: no conflict of interest mentioned First author name: M Chatwin Institution: Sleep and Ventilation Unit, Royal Brompton Hospital, London, UK Email: M.Chatwin@rbh.nthames.nhs.uk Address: Sleep and Ventilation Unit, Royal Brompton Hospital, Sydney Street, London, UK |
|
| Notes | *The study was designed as a parallel non‐randomised clinical trial comparing participants with NMD to 19 healthy, age‐matched controls (parallel grouping). Only data from the prospective randomised cross‐over trial within the NMD participant group was eligible for inclusion in this review. We contacted the author who was unable to provide additional data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…random order by coughs assisted by physiotherapy, noninvasive ventilation, insufflation and exsufflation, and exsufflation alone." Quote: "Initial assessment consisted of at least six maximal unaided coughs followed in random order by the cough intervention techniques;" Comment: the interventions in the NMD arm of the study were performed in random order to reduce bias. However, it is unclear how randomisation was performed and whether there were baseline differences between groups. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information was provided. Unclear whether or how allocation concealment was maintained. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel would have been aware of group allocation, given the nature of the interventions. Those performing the interventions could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: PCF was assessed during the cough assist and, therefore, was likely done by a non‐blinded outcome assessor; however, this is not clear from the article. The outcome "comfort" was rated on a VAS scale by the participants, who were aware of the intervention they were given. There was no mention of whether the same assessor performed all measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no missing data. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Following each intervention, the subjects rated comfort of intervention, distress and strength of cough produced (0: least; 10: most)." Comment: separate VAS scores for patient comfort, distress, and strength of cough were not reported; only 1 VAS result was presented, and it is unclear how this was calculated. It was stated that there was no significant change from baseline in results for comfort or distress of intervention on the VAS. |
| Other bias | High risk | Comment: the following factors placed the study at high risk of other bias.
|
Chatwin 2009.
| Study characteristics | ||
| Methods |
Study design: 2‐day randomised controlled cross‐over trial comparing standard chest physiotherapy with and without MI‐E Study grouping: cross‐over 'Rescue' vs maintenance therapy: rescue Ethics: ethical clearance obtained. All participants, caregivers, or both, provided informed consent |
|
| Participants |
Baseline characteristics Separate period data for the cross‐over trial were not available, therefore, overall baseline data were presented for the entire sample only. Overall
Inclusion criteria
Exclusion criteria
Pretreatment: cross‐over study. Separate data were not provided for allocation groups at baseline or for the first period of cross‐over. Sputum growth/culture: 3 sputum cultures were positive Chest x‐ray: at baseline 5 participants presented with changes on chest x‐ray |
|
| Interventions |
Intervention characteristics Participants received MI‐E for 1 treatment session and no MI‐E for the second treatment session, in a randomly assigned order, with reverse cross‐over the following day. Standard airway clearance therapy without in‐exsufflation
Standard airway clearance therapy with in‐exsufflation
|
|
| Outcomes | Separate first‐period data were not presented, precluding analysis. Transcutaneous oxygen saturation (SpO2)
PtcCO2
Treatment time after 30 min
Auscultation score
VAS for comfort, mood, breathlessness, fatigue, and presence of sputum
Adverse events: fatigue based on VAS; no other adverse events reported on. |
|
| Identification |
Sponsorship source: partly sponsored by the Jennifer Trust for Spinal Muscular Atrophy, UK Conflict of interest statement: disclosed a relationship with a healthcare company (Breas Medical) that manufactures ventilation equipment, although the nature of the relationship and the relevance to this study was unclear. Country: UK Setting: Royal Brompton Hospital (adult and paediatric wards), London Comments: M Chatwin disclosed a relationship with Breas Medical, Molnlycke, Sweden; A Simonds had no conflicts of interest. First author name: Michelle Chatwin Institution: Royal Brompton Hospital Email: m.chatwin@rbht.nhs.uk Address: Sleep and Ventilation Unit; Royal Brompton Hospital; Sydney street, London, UK |
|
| Notes | Author was contacted for first‐period baseline and outcome data; however, these data were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "2‐day randomized crossover treatment program." Quote: "Patients were randomized to group 1 or group 2." Comment: 2‐day randomised cross‐over trial; however, unclear how randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. Unclear whether or how allocation concealment was maintained. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and therapists performing the interventions could not feasibly have been blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the assessor performing the auscultation and determining the auscultation score was blinded to allocation. No information provided regarding blinding of other outcome assessors. Potential high level of assessor bias for the outcome measure "treatment time." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unclear whether outcome data on all 8 included participants were presented. This was not explicitly mentioned in the text. |
| Selective reporting (reporting bias) | High risk | Comment: data were not presented for the primary physiological outcome measures of SpO2, heart rate, and PtcCO2. Other prespecified outcome measures were reported. VAS scores for comfort, breathlessness, and mood were only presented as graphs and data could not be extracted precisely. |
| Other bias | High risk | Quote: "Airway clearance sessions were standardized to prevent treatment bias. Individuals had their randomized treatment at standardized times, as in other airway clearance studies." Quote: "Patients then continued to have treatment until they were fatigued or there were no longer any secretions produced." Comment: the following factors placed the study at high risk for other bias:
|
Del Amo Castrillo 2019.
| Study characteristics | ||
| Methods |
Study design: randomised, open, single‐centre, cross‐over study comparing breathstacking and mechanical insufflation using VCM, both using a home ventilator Study grouping: cross‐over 'Rescue' vs maintenance therapy: maintenance Ethics: ethical clearance obtained. All participants or caregivers (or both) provided informed consent |
|
| Participants |
Baseline characteristics (n = 20) Separate data were not available for group allocations in the first period of cross‐over. Data were presented for all participants who received all interventions.
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention characteristics Breathstacking
Mechanical insufflation using ventilator VCM
|
|
| Outcomes | Separate first‐period data were not presented, precluding analysis. Primary outcome measures PCF
All measurements were taken with the participant in a sitting position. The PCF measurements were made using a pneumotachograph (Fleisch No. 4, Lausanne, Switzerland). Flow increases were linear at 600 L/min, therefore the volume measured during calibration with a syringe was not influenced by flows of 30–600 L/min. The flow signal was sampled at 1000 Hz and recorded using an analogue‐numeric system (MP100, Biopac System, Goleta, California, USA) and its software (AcqKnowledge).
Inspiratory capacity
Secondary outcome measures Subjective ratings of breathing comfort
Subjective ratings of cough effectiveness
Oxygen saturation and heart rate were recorded but not listed as primary or secondary outcome measures. Adverse events: not reported |
|
| Identification |
Sponsorship source: not stated Conflict of interest: the authors disclosed a relationship with ResMed France, the company who manufacture the VCM ventilator device. The exact nature of the relationship was unclear. Country: France Setting: home ventilation unit of the medical ICU of the Raymond Poincaré Teaching Hospital, Garxhes, France Comments: authors disclosed a relationship with ResMed, France First author name: Del Amo Castrillo Institution: Ms Del Amo Castrillo, Mr Lacombe, and Mr Bore were affiliated with the ICU at Hôpital Raymond Poincaré, AP‐HP, Garches, France. Ms Vaugier and Dr Orlikowski were affiliated with Hôpital Raymond Poincaré, INSERM CIC 1429, Garches, France. Ms Falaize and Dr Prigent, and Dr Lofaso were affiliated with Service de Physiologie‐Explorations Fonctionnelles, Hôpital Raymond Poincaré, AP‐HP, Garches, France Email: f.lofaso@rpc.aphp.fr Address: Frédéric Lofaso MD PhD, Services de Physiologie et Explorations Fonctionnelles, Hôpital Raymond Poincaré, AP‐HP, 92380, Garches, France |
|
| Notes | Study was approved by the French Ethics Committee (Comité de Protection des Personnes) of Saint‐Germain‐en‐Laye, France, on 3 September 2015 (NCPP15031) and registered on ClinicalTrials.gov as NCT02847299. Attempts to contact the trial author for additional data were unsuccessful. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We used a randomized, open, single‐center, crossover design…" Comment: method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not mentioned in the article. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "One physiotherapist and one technician carried out the tests, prevented leakage and performing measurements." Comment: the same personnel conducted all interventions and measurements, and, therefore, it was highly unlikely that they would have been blinded. The nature of the interventions suggested that participants could not have been blinded to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "One physiotherapist and one technician carried out the tests, prevented leakage and performing measurements." Comment: all outcome assessments were measured by the same technician, who could not feasibly have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome measures were reported for all participants. |
| Selective reporting (reporting bias) | Low risk | Comment: all primary and secondary outcome measures were fully reported. |
| Other bias | High risk | Comment: the following factors placed this study at high risk of other sources of bias.
|
Jenkins 2014.
| Study characteristics | ||
| Methods |
Study design: RCT comparing IBS to sham intervention* Study grouping: cross‐over 'Rescue' vs maintenance therapy: both inpatients and outpatients were included, no differentiation made between rescue and maintenance therapy. Ethics: no information available regarding ethical review provided for the RCT. For the second, non‐randomised study, approval by the University of Manitoba's Research Ethics Board was reported. Written informed consent was obtained from all caregivers, and assent was obtained from participants where applicable. |
|
| Participants |
Baseline characteristics No separate data were provided for the first period of cross‐over; therefore, aggregated data are provided for all participants, who underwent all interventions.
Inclusion criteria
Exclusion criteria None indicated Pretreatment No separate group data reported |
|
| Interventions |
Intervention characteristics IBS
Sham
|
|
| Outcomes | Separate first‐period data were not presented, precluding analysis. Tidal volume
Respiratory rate
SaO2
Adverse events: not reported |
|
| Identification |
Funding source: Children's Hospital Foundation of Manitoba and Health Sciences Centre Foundation, Winnipeg, Mannitoba, Canada Conflict of interest: funding source declared and unlikely to constitute a conflict of interest. Country: Canada Setting: Winnipeg Children's Hospital for inpatients; Muscular Dystrophy Clinic at The Rehabilitation Centre for Children or Children's Hospital Physiotherapy for outpatient follow‐up Comments: some participants were unable to communicate verbally or follow instructions (due to age and cognition level). This study formed the basis of the Masters thesis by HML Jenkins. First author name: Heather ML Jenkins Institution: Department of Physiotherapy Services, Winnipeg Children's Hospital Email: hjenkins@hsc.mb.ca Address: Department of Physiotherapy Services, Winnipeg Children's hospital, CH246‐840 Sherbrook Street, Winnipeg, Manitoba, Canada R2A 1S1 |
|
| Notes | Attempts to contact corresponding author for first‐period data were unsuccessful. *This paper also reported on a second study, which was a "comparative study" of voluntary and supported, compared to IBS in 6 children and adolescents with DMD. Although the order of voluntary and IBS was randomised, all received supported breathstacking as the final intervention. Therefore, this study was an observational, non‐randomised study, which did not qualify for inclusion in this review. Only data for the randomised cross‐over trial are therefore presented. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This initial study of IBS followed a randomized cross over design. Inpatient subjects were assigned by blocked order randomization to two streams, either receiving the intervention or the sham in the morning and the reverse in the afternoon of the same day." Quote: "randomized cross over design." Quote: "assigned by blocked order randomization to two streams," Quote: "For each participant the sequence of interventions was randomized." Comment: randomised cross‐over trial; however, no indication was given as to how randomisation was achieved. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear how allocation concealment was maintained. No information was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The sham trial was done to rule out the possible effects of the placement of a mask on the child’s face and of the effect of dead space ventilation during the 1 min of recording time." Comment: unclear if blinding was successfully achieved. Both IBS and sham were performed in the same way except for the presence or absence of a valve. Unclear if the masks looked identical, with a sham valve, or whether the valve was simply not added to the mask circuit for the sham intervention. It is likely that the intervention would have "felt" different, and in that way could have unblinded participants. Some participants were not cognitively aware/could not communicate. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to allow judgement. Not stated if the assessments were performed by the physiotherapists performing the interventions/sham or if they were performed by an independent assessor. If the assessor was present during the stacking manoeuvres to take the measurements, they would have been aware of the intervention (allocation). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Twenty‐four children and adolescents participated in the study of IBS. Data from one patient were excluded due to a face mask leak. Twenty‐three children, 15 inpatients and 8 outpatients, were included in the final analysis." Comment: it was mentioned that 1 participant's results were excluded due to a facial mask leak, and this was considered unlikely to have introduced bias. Data of 4 participants who could not breathstack were described in the text (e.g. all had a tidal volume lower than the dead space of the mask). |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures described in the methods section were reported. |
| Other bias | High risk | Comment: the following factors placed the study at high risk for other bias.
|
Katz 2019.
| Study characteristics | ||
| Methods |
Study design: multicentre 2‐year RCT comparing LVR (breathstacking exercises) as an add‐on to conventional treatment and conventional treatment alone* Study grouping: parallel‐group assignment 'Rescue' vs maintenance therapy: maintenance Ethics: no information regarding ethical review or informed consent was provided. |
|
| Participants |
Baseline characteristics (entire sample, n = 67)
Conventional treatment + LVR No separate group data were presented Conventional treatment No separate group data were presented Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Conventional treatment This could have included:
LVR (breathstacking) Participants were instructed to use LVR (breathstacking) twice per day, using an inexpensive, portable self‐inflating resuscitation bag containing a 1‐way valve and mouthpiece. Details regarding the number of repetitions/sets per session were not provided. Duration: 2 years |
|
| Outcomes |
Primary outcome measures
Secondary outcome measures
Other outcome measures
Adverse events: not listed as a primary or secondary outcome in the published protocol, but were reported in the abstract. It is unclear what adverse events were monitored or recorded. |
|
| Identification |
Funding source: Children's Hospital of Eastern Ontario Conflict of interest: Craig Campbell declared a "Scientific Medical Advisor relationship with Biogen, Genzyme, PTC Therapeutics" and Sherri Katz disclosed a financial speaker relationship with Biogen. Unclear how declared interests may have influenced the study. Country: Canada Setting: participants were recruited from 9 tertiary care paediatric hospitals across Canada. Interventions were conducted at the participants' homes. Comments: none First author name: Sherri Katz Institutions: Canadian study sites listed on the protocol: Alberta Children's Hospital; Stollery Children's Hospital; BC Children's Hospital; McMaster University; London Health Sciences; Children's Hospital of Eastern Ontario; Holland Bloorview Kids Rehabilitation Hospital; SickKids Hospital; Hôpital Ste. Justine Email: not provided Address: not provided |
|
| Notes | *Information was sourced from a published abstract of findings as well as from ClinicalTrials.org. Adherence to all aspects of the published protocol could not be assessed based on the published abstract. No contact information was available on either the abstract or protocol; however, a current email address of the corresponding author was identified using "Google" search and she was contacted (unsuccessfully) for additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "multi‐centre randomized controlled trial." Quote: "Participants were allocated with a minimisation procedure to receive conventional treatment or conventional treatment plus twice daily lung volume recruitment exercises." Comment: unclear whether the minimisation procedure biased the randomisation procedure, as there was insufficient methodological information. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Single (Investigator) blinding." Comment: no information provided regarding participant blinding; however, considering they were randomised to receiving breathstacking with standard care or standard care alone, blinding of participants seems unlikely to have been achievable. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: study was described as single blinded, but there was no description provided of how blinding was maintained. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Primary analysis was by intention to treat." Quote: "Multiple imputation was used to account for longitudinal missing data." Comment: there was no indication of dropout numbers or reasons for loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | Comment: published protocol listed numerous outcome measures. Only FVC, time to 10% decline in FVC, and adverse events (adverse events was not an a priori listed outcome measure) were reported in the published abstract. |
| Other bias | Unclear risk | Comment: insufficient detail provided in the description of interventions. |
Kim 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled cross‐over trial comparing unassisted cough and cough augmentation using MAC, MIE, and MIE + MAC. Study grouping: cross‐over 'Rescue' vs maintenance therapy: maintenance Ethics: all 40 participants provided written informed consent. Ethical approval was obtained from the local ethics committee (no reference number provided). |
|
| Participants |
Baseline characteristics Separate period data were not provided for the cross‐over trial; therefore, only overall sample data were reported.
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention characteristics Unassisted cough
Manual thrust (MAC) following manual breathstacking
MI‐E
MI‐E + manual thrust
|
|
| Outcomes | Separate first‐period data were not presented, precluding analysis. PCF
Adverse events: not reported. All 3 cough augmentation techniques were reported to be "well tolerated." |
|
| Identification |
Sponsorship source: none mentioned Conflict of interest: declared no financial conflicts of interests, but other interests were not declared. Country: Korea Setting: not specifically stated Comments: seemed to have been in an outpatient setting; however, the exact setting was unclear. First author name: Sun Mi Kim Corresponding author: Dr Seong‐Woong Kang Institution: Yonsei University College of Medicine Email: kswoong@yuhs.ac Address: Department of Rehabilitation Medicine, Gangnam Severance Hospital, Rehabilitation Institute of Neuromuscular Disease, Yonsei University College of Medicine, 211 Eonju‐ro, Gangnam‐gu, Seoul 06273, Korea |
|
| Notes | Attempts to contact authors for first‐period data were unsuccessful. A new device was used for measurement of PCF (CoughAid), and authors indicated that it accurately measured PCF in people with ALS. However, the article referred to the effectiveness of the CoughAid as a cough augmentation device, not as a measurement device for PCF. Therefore, the validity of this device was unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…following an MIC maneuver, MI‐E, and MI‐E in combination with manual thrust, with a 10‐minute washout period between conditions. The order of the PCF measurements was randomized." Quote: "randomized crossover single‐center controlled trial," Comment: randomised cross‐over trial with randomisation of order of cough augmentation techniques. However, the method of randomisation was unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to allow judgement (method of concealment not described). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: neither participants nor personnel could feasibly be blinded to cough augmentation intervention allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: same staff performing the cough intervention also performed the assessment; there was no attempt to blind outcome measurement. However, it noted that inter‐rater (0.98) and intra‐rater (0.99) reliability for measuring the primary outcome of PCF was high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no attritions, and all participants performed all interventions. There were no dropouts or loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: the primary outcome measure of PCF was fully reported. No secondary outcome measures were defined. |
| Other bias | High risk | Comment: the following factors placed the study at high risk for other bias.
|
Lacombe 2014.
| Study characteristics | ||
| Methods |
Design: randomised controlled cross‐over trial comparing mechanical insufflation + MAC, MI‐E, and MI‐E + MAC Group: cross‐over 'Rescue' vs maintenance therapy: maintenance Ethics: approved by hospital's ethics committee. Patients provided written informed consent before participating. Registered on ClinicalTrials.gov (NCT01518439). Time frame: March 2012 to June 2013 Additional comments on methodology: open, single‐centre, randomised (block size of 6) cross‐over study |
|
| Participants | The author provided baseline separate group allocation data for the first period of cross‐over on request. Baseline characteristics Entire sample
Mechanical insufflation + MAC
MI‐E
MI‐E + MAC
Inclusion criteria
Exclusion criteria None specified Pretreatment Baseline VC was considerably different among participant groups, with the lowest value recorded in the MI‐E + MAC group. This suggests there may have been baseline differences in participant groups, which may have influenced the outcomes. |
|
| Interventions |
Mechanical insufflation + MAC Position: seated in wheelchair Technique description: insufflation was provided by an Alpha 200 C ventilator (Air Liquide, Antony, France). Participants started IPPB insufflation with an inspiratory effort, then allowed the insufflation to continue passively until the selected inspiratory pressure was reached in about 5 s. The lowest inspiratory trigger was chosen to facilitate the start of insufflation. Inspiratory pressure (insufflation) with IPPB was increased gradually to the highest tolerated value, or 40 cmH2O. Inspiratory flow was set to maximise participant comfort. Once target inspiratory pressure was reached, 1 physiotherapist removed the IPPB circuit to avoid resistance while coughing. At the same time, MAC (compression to the abdomen, thorax, or both, participant and on cough efficiency as perceived by the participant and physiotherapist. Interface: IPPB was applied using a face mask, chosen to fit each participant individually. MI‐E Participant position: seated in wheelchair Technique description: MI‐E was performed using the CoughAssist device (JH Emerson Co., Cambridge, Massachusetts, USA) in manual mode. After each insufflation, a physiotherapist delivered the exsufflation while simultaneously asking the participant to cough. Inspiratory and expiratory pressures were increased/decreased gradually to the highest/lowest tolerated values, up to +40 cmH2O for inspiratory pressure and down to –40 cmH2O for expiratory pressure. Insufflation flow adjustment (high or low insufflation flow) was set according to participant comfort. Interface: MI‐E was applied using a face mask, chosen to fit each participant individually. MI‐E + MAC MI‐E and MAC interventions as described above. |
|
| Outcomes | Separate first‐period data were not presented, precluding analysis. Primary objective outcome PCF
Secondary objective outcomes Effective cough time: time with PCF > 3 L/s or 180 L/min
Inspiratory capacity
Secondary subjective outcomes Comfort ratings
Subjective cough effectiveness
Adverse events: not reported |
|
| Identification |
Sponsorship source: no declaration of funding source Conflict of interest: not declared Country: France Setting: home ventilation unit of the medical ICU, Raymond Poincare Teaching Hospital, Garches Comments: registered on ClinicalTrials.gov (NCT01518439) Author name: Matthieu Lacombe was the primary author; Prof F Lofaso is the contact author Institution: Hôpital Raymond Poincaré Email: f.lofaso@rpc.aphp.fr Address: Réanimation Médicale, Physiologie – Explorations Fonctionnelles, Centre d’Innovations Technologiques UMR 805, Hôpital Raymond Poincaré, AP‐HP, Garches; EA 4497, Université de Versailles Saint‐Quentin‐en‐Yvelines, Versailles, France" |
|
| Notes | Separate baseline data and postintervention data for first‐period group allocation were provided by author on request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: cross‐over trial during which cough assist techniques were applied in random order; however, it was unclear how randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information was provided to determine if participants/physiotherapists involved in the study could have foreseen the cough augmentation technique allocated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it would not be possible for participants and those implementing the cough augmentation techniques to be blinded to the cough augmentation used. 3 physiotherapists (MLa, LDAC, and AB) were involved in interventions and assessments. For each participant, 2 physiotherapists were needed for the intervention: 1 for using the device and 1 for MAC manoeuvres. The same physiotherapist performed the different cough techniques for a single participant. In addition, the same technician (MLe) performed the measurements. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: unclear whether the outcome assessor was blinded to group allocation, but seems likely that blinding would not have been possible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no incomplete data in the study report. |
| Selective reporting (reporting bias) | Unclear risk | Comment: primary outcome measure of PCF and secondary outcomes of effective cough time and inspiratory capacity were only presented graphically, with no specific values provided. Subjective secondary outcome measures of comfort and cough effectiveness (VAS) were fully reported. |
| Other bias | High risk | Comment: the following factors placed the study at high risk of other bias.
|
Sivasothy 2001.
| Study characteristics | ||
| Methods |
Study design: randomised controlled cross‐over trial comparing 3 cough augmentation techniques: MAC; mechanical insufflation, and mechanical insufflation with MAC* Study grouping: cross‐over 'Rescue' vs maintenance therapy: maintenance Ethics: no mention of ethical clearance/number. All participants provided informed consent prior to participation. |
|
| Participants |
Baseline characteristics* Respiratory muscle weakness participant group – with scoliosis (separate data not presented for 2 periods of cross‐over trial)
Inclusion criteria
Exclusion criteria
Respiratory muscle weakness participant group ‐ without scoliosis
Inclusion criteria
Exclusion criteria
Chronic obstructive pulmonary disease group (COPD)
Inclusion criteria
Exclusion criteria
"Normal" volunteers
Inclusion criteria
Exclusion criteria
Pretreatment No data were presented for different allocation groups at baseline; neither were separate data presented for the 2 periods of cross‐over. |
|
| Interventions |
Intervention characteristics MAC Participant position: semi‐recumbent Technique description: performed by an experienced physiotherapist. Manual thoracoabdominal compression during the expulsive phase of the maximal voluntary cough. Hand position was optimised for participants with scoliosis by placing the hand used for thoracic compression on the hyperinflated hemithorax. Washout time: ≥ 5 min was allowed between each cough manoeuvre. Mechanical insufflation Participant position: semi‐recumbent Technique description: performed with an in‐exsufflator (JH Emerson Co, Cambridge, Massachusetts, USA) set to give 20 cmH2O inspiratory and –20 cmH2O expiratory pressure. 2 in‐exsufflation cycles were delivered and after the third insufflation, the participant was asked to make a maximal voluntary cough without the assistance of negative pressure. Washout time: ≥ 5 min was allowed between each cough manoeuvre. Mechanical insufflation with MAC Participant position: semi‐recumbent Technique description: the 2 techniques above were combined but the technique was not described separately. Washout time: ≥ 5 min was allowed each cough manoeuvre. |
|
| Outcomes | Separate first‐period data were not presented, precluding analysis. Maximal peak cough expiratory flow (PCF)
CEV
Peak value time
Oesophageal and gastric pressures (as proxies to pleural and abdominal pressure)
Subjective cough effectiveness
Adverse events: reported as none having occurred. |
|
| Identification |
Sponsorship source: no sponsorship source declared. Conflict of interest: not declared Country: UK Setting: setting of data collected not well described, assumed to be the Respiratory Support and Sleep Centre/Papworth Hospital and 9 healthy volunteers that participated. Comments: none Author name: Dr P Sivasothy Institution: Respiratory Support and Sleep Centre; Papworth Hospital Email: ps247@cus.cam.ac.uk Address: Respiratory Support and Sleep Centre, Papworth Hospital, Papworth Everard, Cambridge, UK |
|
| Notes | Attempts to contact the author for additional data were not successful. *This study was reported as a non‐randomised clinical trial of parallel groups (healthy controls; 1 group with chronic obstructive pulmonary disease and 2 groups with NMD and respiratory muscle weakness – 1 with and 1 without scoliosis). Only data from the cross‐over component of the trial within the NMD group was eligible for inclusion in this review. The group with NMD and without scoliosis was further excluded, because 7/8 participants had ALS, an excluded condition in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "To exclude bias the order of the treatments was randomised for each subject." Comment: unclear how randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: neither participants nor personnel were blinded to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: unclear whether the outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were accounted for, no dropouts or missing data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: from the methods section, it was unclear which were the study's primary and secondary outcome measures. There was no trial registration number mentioned so we could not check the predescribed protocol. Gastric and oesophageal pressures were not presented due to the collapse of the balloons in the control groups. The subjective outcome measure of cough effectiveness was not fully reported, it was simply stated that participants did not report any benefit of any assisted cough interventions. |
| Other bias | High risk | Quote: "All subjects practised with both manually assisted cough and mechanical." Quote: "All subjects practised coughing while the face mask was adjusted to minimise air leaks. An investigator held the subject’s cheeks…" Comment: the following factors placed this study at high risk of other bias.
|
Torres‐Castro 2016.
| Study characteristics | ||
| Methods |
Study design: prospective randomised cross‐over study comparing air (breath) stacking and glossopharyngeal breathing Study grouping: cross‐over 'Rescue' vs maintenance therapy: maintenance Ethics: informed consent was obtained from participants. Ethical approval was obtained from the Ethics Committee for Research Involving Human Beings, Faculty of Medicine, University of Chile. Additional information: the author provided selected separate period data on email request. |
|
| Participants |
Baseline characteristics Overall sample data
First‐period baseline data, as provided by author on request Breathstacking
Glossopharyngeal breathing
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention characteristics Air/ breathstacking
Glossopharyngeal breathing
|
|
| Outcomes | Primary and secondary outcomes were not explicitly identified. Baseline VC and postintervention MIC
PCF
Adverse events: not reported |
|
| Identification |
Sponsorship source: no sponsorship source declared. Conflict of interest: no conflict‐of‐interest statement presented. Country: Santiago, Chile Setting: domiciliary NIV programme recipients, in the participants' own homes Comments: authors declared no competing interests exist. Author names: Rodrigo Torres‐Castro, Jordi Vilaró, Roberto Vera‐Uribe, Luis Vasconcello, Homero Puppo Institution: Rodrigo Torres‐Castro and Homero Puppo: Department of Kinesiology, University of Chile, Chile Jordi Vilaró: Faculty of Health Sciences Blanquerna, Research Group of Physiotherapy (GReFis) Roberto Vera‐Uribe and Luis Vasconcello: National Program of Non‐Invasive Ventilation, Ministry of Health, Chile Email: klgorodrigotorres@gmail.com Address: not provided |
|
| Notes | Additional separate‐period baseline and outcome data were provided by author on request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To minimize the risk of bias, the order of execution of each cough assistance technique was performed randomly for each participant." Quote: "Randomization was performed by a free software (for www.randomization.com) specifically designed for generating random number lists." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "To minimize the risk of bias, the order of execution of each cough assistance technique was performed randomly for each participant. Comment: the article did not describe whether or how allocation concealment was achieved. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A chest physiotherapist insufflated the patient during the inspiratory phase." Quote: "The protocol was implemented in the patients' homes by a trained chest physiotherapist." Comment: the chest physiotherapist was physically performing the insufflation interventions; therefore, it would be impossible to blind them. Similarly, the participants could not feasibly be blinded to allocation considering the nature of the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "A chest physiotherapist insufflated the patient during the inspiratory phase." Quote: "The protocol was implemented in the patients' homes by a trained chest physiotherapist." Quote: "Baseline PCF assessment was performed with the patient seated and with a respiratory physiotherapist monitoring the seal between the entire surface of the mask and the flowmeter to avoid any leakage." Comment: the baseline, intervention, and outcome assessments seem to have been performed by the same physiotherapist, who could not have been blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 3 participants were excluded after screening, but clear reasons for exclusion were not provided. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures listed in the protocol were reported. |
| Other bias | Unclear risk | Comment: the following factors placed this study at unclear risk of other bias.
|
Toussaint 2016.
| Study characteristics | ||
| Methods |
Study design: prospective RCT comparing a single session of air (breath) stacking using a resuscitator bag (manual breathstacking) compared to a home ventilator (mechanical breathstacking). Study grouping: parallel‐group 'Rescue' vs maintenance therapy: maintenance Ethics: informed consent was obtained from patients before recruitment. Approval obtained from the Ethics committee at the institution (Inkendaal Rehabilitation Hospital). Additional information: participants were randomly allocated to 1 of 2 interventions; there was no control group where participants received no intervention. This study was conducted over 2 years (January 2012 to December 2013). |
|
| Participants |
Baseline characteristics Overall
Mechanical breathstacking
Manual breathstacking
Inclusion criteria
Exclusion criteria
Pretreatment: demographics, lung function values, and ventilation parameters were similar between the 2 groups (no statistically significant difference between groups, Table 1 of publication). |
|
| Interventions |
Intervention characteristics Mechanical breathstacking
Manual breathstacking
|
|
| Outcomes |
Able to perform breath/airstacking
Number of insufflations to maximal insufflation capacity
Breathstacking‐assisted PCF (primary outcome measure)
Maximal insufflation capacity
MEP following breathstacking
Adverse events: not reported on |
|
| Identification |
Sponsorship source: not mentioned Conflict of interest: no conflict‐of‐interest statement presented Country: Belgium Setting: Neuromuscular Excellency Centre and Centre for Home Mechanical Ventilation Inkendaal Rehabilitation Hospital Comments: – Author name: Michel Toussaint Institution: Rehabilitation Hospital (Ziekenhuis), Inkendaal Email: michel.toussaint@inkendaal.be Address: Rehabilitation Hospital, InkendaalInkendaalstraat 1B‐1602 Vlezenbeek (Brussels), Belgium |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were allocated by coin toss to a single session of either air stacking via home ventilator or air stacking via a resuscitator bag at a routine clinical visit." Comment: randomisation to intervention was determined by coin toss (air stacking with ventilator or resuscitator bag), therefore, selection bias was low. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided regarding allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and therapists were not blinded to treatment. All participants were trained in both air stacking techniques, before randomisation was implemented. However, it was not possible to blind participants to the techniques that were used. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: a blinded outcome assessor gathered baseline data prior to group allocation. However, the postintervention outcome assessment seemed to have been done by the attending therapist, who was not blinded to group allocation. The best of 3 values was recorded and standardised assessment guidelines were followed, but insufficient information was provided about who performed the assessments. As with blinding of participants and staff, it would have been difficult to blind the person assessing PCF, as they would have been aware of intervention allocation and air stacking technique. Other measures such as FVC and MEP were routinely assessed at the centre. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants allocated to a group were assessed in the group, with no loss to follow‐up/missing data. Eligibility criteria were clear. 6 participants could not perform breath stacking (as defined by the study) and the number was too small to make a comparison between the 46 that could perform the procedure and those that could not. There was full transparency of the research process and alignment with the protocol. |
| Selective reporting (reporting bias) | Low risk | Quote: "primary outcome measure was air stacking‐assisted cough peak flow." Quote: "Lung volume was recorded from a maximal effort unassisted breath (FVC) and following an air stacking‐assisted breath (maximum insufflation capacity). Maximal expiratory pressure (P Emax) was recorded from total lung capacity as per American Thoracic Society/European Respiratory Society guidelines." Quote: "no difference in air stacking‐assisted cough peak flow between groups." Quote: "P.001). Similarly, there were comparable expired volumes between techniques, with maximum insufflation capacity values greater than spontaneous FVC in both groups (mean within group change: 672)." Quote: "difference in maximum insufflation capacity between groups (Table 2)." Quote: "Table 2. Comparison of Air Stacking via Ventilator Versus via Resuscitator Bag." Comment: all outcome measures reported in the methods section were analysed and presented in the results section (Table 2 and in text). The study protocol was well described regarding study procedure, participants, and outcome measures (reproducible) and all the specified outcomes were reported in the prespecified way. |
| Other bias | Low risk | Quote: "Able to perform air stacking, n (%) 24/27 (89) 22/25 (88) NS [not significant]" Comment: the following factors placed the study at low risk of other bias.
|
ACBT: active cycle of breathing technique; ALS: amyotrophic lateral sclerosis; BiPAP: bilevel positive airway pressure; BMD: Becker muscular dystrophy; CEV: cough expiratory volume; CMD: congenital muscular dystrophy; CNS: central nervous system; CO2: carbon dioxide; DMD: Duchenne muscular dystrophy; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; IBS: involuntary breathstacking; ICU: intensive care unit; IQR: interquartile range; IPPB: intermittent positive pressure breathing; LVR: lung volume recruitment; MAC: manually assisted cough; MD: mean difference; MEE: maximal expiration effort; MEP: maximal expiratory pressure; MI‐E: mechanical insufflation‐exsufflation; MIC: maximal inspiratory or insufflation capacity; MIP: maximum inspiratory pressure; min: minute; n: number of participants; MVV: maximal voluntary ventilation; N/A: not available; NIPPV: non‐invasive positive pressure ventilation; NIV: non‐invasive ventilation; NMD: neuromuscular disease; PCF: peak cough flow; PECF: peak expiratory cough flow; PEFR: peak expiratory flow rate; PETCO2: end‐tidal carbon dioxide tension; Pmo,w: maximal pressure within the mouth; PtcCO2: transcutaneous carbon dioxide tension; PVT: peak value time; RCT: randomised controlled trial; RR: risk ratio; s: second; SaO2: oxygen saturation in arterial blood; SD: standard deviation; SMA: spinal muscular atrophy; SNIP: sniff nasal inspiratory pressure; SpO2: peripheral capillary oxygen saturation; VAS: visual analogue scale; VC: vital capacity; VCM: volumetric cough mode; COPD: chronic obstructive pulmonary disease
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bianchi 2014 | Incorrect study design: prospective observational study. |
| Kang 2000 | Incorrect study design: observational study design. |
| Silva 2012 | Incorrect patient population: mechanically ventilated people diagnosed with head trauma, stroke, congestive heart failure, and ventilator‐associated pneumonia. No participants with NMD. |
| Toussaint 2003 | Incorrect intervention: intrapulmonary percussive ventilation, which is a peripheral airway clearance technique and not a cough augmentation technique. |
| Toussaint 2009 | Incorrect study design: prospective cross‐sectional observational study. |
| Winck 2004 | Incorrect study design: prospective observational study. |
NMD: neuromuscular disease.
Characteristics of ongoing studies [ordered by study ID]
NCT01518439.
| Study name | Instrumental and manual Increase of couch[sic] in neuromuscular patients (OPTICOUGH) |
| Methods | Open monocentric randomised cross‐over study |
| Participants | People with NMD with cough inefficiency in a stable respiratory state on recruitment |
| Interventions |
Mechanical insufflation: Alpha 200 inspiratory capacity increased with a constant pressure device: Alpha 200 (presume mechanical insufflation) Mechanical insufflation + physiotherapy: Alpha 200 combined with physiotherapy – inspiratory capacity is increased with the use of constant pressure device (Alpha 200) combined with the manual pressures techniques to increase cough by the physiotherapist (presume mechanical insufflation + MAC) MI‐E: CoughAssist – increased inspiratory capacity and mechanical exsufflation with the use of insufflation‐exsufflation device (Cough Assist) MI‐E + MAC: CoughAssist + physiotherapy – increased inspiratory capacity and mechanical exsufflation with the use of insufflation‐exsufflation device (Cough Assist) + the "manual pressures techniques" (presume MAC) to increase cough by the physiotherapist (MI‐E + MAC) MAC: physiotherapist manual pressures techniques to increase cough applied by the physiotherapist (presumed MAC alone) |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | January 2012 |
| Contact information | Contact: Helene Prigent, helene.prigent@rpc.aphp.fr Contact: Sandra Pottier, sandra.pottier@rpc.aphp.fr Principal investigator: Helene Prigent, MD, PhD Subinvestigator: Frederic Lofaso, MD, PhD Subinvestigator: David Orlikowski, MD, PhD |
| Notes | Attempts to contact the corresponding investigators were unsuccessful. |
NCT02651805.
| Study name | Mechanical insufflator‐exsufflator to control mucus hypersecretion in patients in palliative care – a feasibility study |
| Methods | RCT |
| Participants | Hospitalised palliative care patients with mucous hypersecretion |
| Interventions | MI‐E vs usual care |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | February 2016 |
| Contact information | Juliano Ferreira Arcuri, Universidade Federal de Sao Carlos, Brazil |
| Notes | Attempts to contact the corresponding investigator were unsuccessful. |
NCT03355105.
| Study name | Comparison of two methods of adjusting mechanical in‐exsufflation in neuromuscular adult patients (EXSUFLOW) |
| Methods | Bicentric, prospective cross‐over, randomised, open‐label trial |
| Participants | 50 adults with stable NMDs |
| Interventions |
Objective adjustment of the MI‐E Pe and evaluation of cough effort: the Pes will be progressively increased starting from –20 cmH2O with 10 cmH2O increments. 3 cough efforts will be performed for each level, until obtaining a 10% of area under the curve decrease or a PCF decline of ≥ 10% over last 2 levels, and without exceeding –70 cmH2O. The selected setting will be the one allowing the largest PCF without presence of collapse. An intermediate bearing between 2 bearings may be tested to approach an optimum adjustment threshold < 5 cmH2O. Subjective adjustment of the MI‐E Pe and evaluation of cough effort: the Pes will be gradually increased from –20 cmH2O, with 10 cmH2O increments up to a maximum of –70 cmH2O. The selected level will be the one selected both by the patient and the "clinician" therapist and considered as making it possible to obtain the most effective cough according to the return of the patient. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 28 November 2017 |
| Contact information | Contact: Frédéric LOFASO, MD, PhD: f.lofaso@aphp.fr Contact: Aurélien BORÉ: kines.widal3@rpc.aphp.fr |
| Notes | Attempts to contact corresponding authors were unsuccessful. |
NCT04081116.
| Study name | Mechanical insufflation‐exsufflation in children with NMD and weak cough |
| Methods | Randomised controlled, single‐group assignment; quadruple‐blinded cross‐over trial |
| Participants | 100 children aged 6 months to 18 years Inclusion criteria
Exclusion criteria
|
| Interventions |
3 MI‐E settings will be tested on the same day, in random order:
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 1 January 2019 |
| Contact information | Brit Hov (MSc); +4723015667; uxbrov@ous‐hf.no Vegard Hovland (PhD); +4722118765 |
| Notes | Study still recruiting until end 2023. |
PACTR201506001171421.
| Study name | The effect of mechanical insufflation‐exsufflation (MI‐E) (and inspiratory muscle training) on clinical outcomes and health‐related quality of life in paediatric and adolescent patients with neuromuscular diseases presenting with respiratory muscle weakness: a multi‐centre trial |
| Methods | RCT with parallel‐group assignment |
| Participants | Children with NMD, aged 5–18 years Inclusion criteria
Exclusion criteria
|
| Interventions |
Standard management with bi‐daily MI‐E (using Nippy Clearway CoughAssist device) during hospital admission (Pi/Pe 10–30 cmH2O; 4 sets consisting of 5 breaths, 1–2 min of rest between sets Standard management with bi‐daily MAC (thoracic compressions) during hospital admission |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | July 2016 |
| Contact information | Anri Human; anrihuman@gmail.com Brenda Morrow (PhD); brenda.morrow@uct.ac.za |
| Notes | Only the MI‐E arm (and not the IMT intervention) of this study would potentially be eligible for inclusion in updates of this review. The PACT site states that the study has not started recruiting; however the study has enrolled participants but is temporarily halted owing to feasibility issues. Data are not yet available for analysis. |
AUC: area under the curve; bpm: beats per minute; FiO2: fraction of inspired oxygen; IMT: inspiratory muscle training; MAC: manually assisted cough; MI‐E: mechanical insufflation‐exsufflation; min: minute; NMD: neuromuscular disease; PaCO2: partial pressure of carbon dioxide; PACT: Pan African Clinical Trials; PCF: peak cough flow; Pe: exsufflation pressure; PECF: peak expiratory cough flow; PETCO2: end‐tidal carbon dioxide tension; Pi: insufflation pressure; RCT: randomised controlled trial; SpO2: peripheral capillary oxygen saturation; Te: time for exsufflation; Ti: time for insufflation; VAS: visual analogue scale.
Differences between protocol and review
There were no other major deviations from the published protocol of this review (Morrow 2018).
We had planned to create separate 'Summary of findings' tables for 'rescue' and 'maintenance therapy' using cough augmentation techniques; however, considering the lack of data, we instead presented two separate 'Summary of Findings' tables for the comparison between cough augmentation technique(s) and alternative cough augmentation technique(s) and for the comparison between cough augmentation technique(s) and standard of care. All predetermined outcome measures were presented in the 'Summary of findings' tables, for 'rescue' and 'maintenance' therapy.
Contributions of authors
Conception of the review: BM, MZ, AH, LC, AA, MT.
Design of the review: BM, MZ, AH, LC, AA, MT.
Co‐ordination of the review: BM.
Search and selection of studies: BM, AH, LC.
Extraction of data: BM, AH, LC.
Assessment of risk of bias: BM, AH, LC.
Data analysis: BM.
Interpretation of results: BM, MZ, AH, LC, AA, MT.
Assessment of certainty: BM, AH.
Writing of the review: BM wrote the review and MZ, AH, LC, AA, and MT reviewed and contributed to the manuscript.
Sources of support
Internal sources
-
University of Cape Town, South Africa
Salary
External sources
None received, UK
Declarations of interest
BM: has no particular conflict of interest to declare in respect of this review. She received an equipment grant from the University of Cape Town, which supported the purchase of a "Nippy" mechanical In‐exsufflation device as well as an unconditional donation of consumables for this device from Bakoni Medical company for an ongoing clinical trial of MI‐E (PACTR201506001171421). BM is principal investigator of this trial, which may be eligible for inclusion in later versions of this review, in which case, she will recuse herself from that data extraction and analysis.
AA: is a specialist (paediatric critical care) physician and manages patients with a variety of conditions. He has no particular conflicts of interest to declare in respect of this review.
MZ: is a specialist (paediatric pulmonology) and manages patients with a variety of conditions, including NMD. He has no particular conflicts of interest to declare in respect of this review.
AH: as student investigator of the ongoing PACTR201506001171421trial, AH was also a recipient of an equipment grant from the University of Cape Town, which supported the purchase of a Nippy mechanical In‐exsufflation device as well as an unconditional donation of consumables for this device from Bakoni Medical company. This trial may be eligible for inclusion in later versions of this review, in which case, she will recuse herself from that data extraction and analysis.
LC: is a physiotherapist and lecturer. She has no conflicts of interest to declare in respect of this review.
MT: is the first author of a study included in this review (Toussaint 2016), as well as two excluded studies (Toussaint 2003; Toussaint 2009); however, he did not participate in any methodological or analytical processes related to these studies. He has no other conflicts of interest to declare in respect of this review.
New
References
References to studies included in this review
Brito 2009 {published data only}
- Brito MF, Moreira GA, Pradella-Hallinan M, Tufik S. Air stacking and chest compression increase peak cough flow in patients with Duchenne muscular dystrophy. Jornal Brasileiro de Pneumologia 2009;35(10):973-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chatwin 2003 {published data only}
- Chatwin M, Ross E, Hart N, Nickol AH, Polkey MI, Simonds AK. Cough augmentation with mechanical insufflation/exsufflation in patients with neuromuscular weakness. European Respiratory Journal 2003;21(3):502-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chatwin 2009 {published data only}
- Chatwin M, Simonds AK. The addition of mechanical insufflation/exsufflation shortens airway-clearance sessions in neuromuscular patients with chest infection. Respiratory Care 2009;54(11):1473-9. [PMID: ] [PubMed] [Google Scholar]
Del Amo Castrillo 2019 {published data only}
- Del Amo Castrillo L, Lacombe M, Boré A, Vaugier I, Falaize L, Orlikowski D, et al. Comparison of two cough-augmentation techniques delivered by a home ventilator in subjects with neuromuscular disease. Respiratory Care 2019;64(3):255-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jenkins 2014 {published data only}
- Jenkins HM, Stocki A, Kriellaars D, Pasterkamp H. Breath stacking in children with neuromuscular disorders. Pediatric Pulmonology 2014;49(6):544-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Katz 2019 {published data only}
- Katz S, Momoli F, Barrowman N, Bijelic V, Mah J, McMillan H, et al. Stacking exercises attenuate the decline in FVC and sick time (STEADFAST). Chest 2019;156(4 Suppl):A245-6. [Google Scholar]
Kim 2016 {published data only}
- Kim SM, Choi WA, Won YH, Kang SW. A comparison of cough assistance techniques in patients with respiratory muscle weakness. Yonsei Medical Journal 2016;57(6):1488-93. [DOI: 10.3349/ymj.2016.57.6.1488] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lacombe 2014 {published data only}
- Lacombe M, Castrillo LD, Boré A, Chapeua D, Horvat E, Vaugier I, et al. Comparison of three cough-augmentation techniques in neuromuscular patients: mechanical insufflation combined with manually assisted cough, insufflation-exsufflation alone and insufflation-exsufflation combined with manually assisted cough. Respiration 2014;88(3):215-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sivasothy 2001 {published data only}
- Sivasothy P, Brown L, Smith IE, Shneerson JM. Effect of manually assisted cough and mechanical insufflation on cough flow of normal subjects, patients with chronic obstructive pulmonary disease (COPD), and patients with respiratory muscle weakness. Thorax 2001;56(6):438-44. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torres‐Castro 2016 {published data only}
- Torres-Castro R, Vilaró J, Vera-Uribe R, Vasconcello L, Puppo H. Acute effects of air stacking versus glossopharyngeal breathing in patients with neuromuscular disease. British Journal of Medicine and Medical Research 2016;14(3):1-8. [Google Scholar]
Toussaint 2016 {published data only}
- Toussaint M, Pernet K, Steens M, Haan J, Sheers N. Cough augmentation in subjects with Duchenne muscular dystrophy: comparison of air stacking via a resuscitator bag versus mechanical ventilation. Respiratory Care 2016;61(1):61-7. [DOI: 10.4187/respcare.04033] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bianchi 2014 {published data only}
- Bianchi C, Carrara R, Khirani S, Tuccio MC. Independent cough flow augmentation by glossopharyngeal breathing plus table thrust in muscular dystrophy. American Journal of Physical Medicine and Rehabilitation 2014;93(1):43-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kang 2000 {published data only}
- Kang SW, Bach JR. Maximum insufflation capacity: vital capacity and cough flows in neuromuscular disease. American Journal of Physical Medicine & Rehabilitation 2000;79(3):222-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Silva 2012 {published data only}
- Silva AR, Fluhr SA, Bezerra AL, Correia MA Jr, Franca EE, Andrade FM. Expiratory peak flow and respiratory system resistance in mechanically ventilated patients undergoing two different forms of manually assisted cough. Revista Brasileira de Terapia Intensiva 2012;24(1):58-63. [PMID: ] [PubMed] [Google Scholar]
Toussaint 2003 {published data only}
- Toussaint M, De Win H, Steens M, Soudon P. Effect of intrapulmonary percussive ventilation on mucus clearance in Duchenne muscular dystrophy patients: a preliminary report. Respiratory Care 2003;48(10):940-7. [PMID: ] [PubMed] [Google Scholar]
Toussaint 2009 {published data only}
- Toussaint M, Boitano LJ, Gathot V, Steens M, Soudon P. Limits of effective cough-augmentation techniques in patients with neuromuscular disease. Respiratory Care 2009;54(3):359-66. [PMID: 19245730 ] [PubMed] [Google Scholar]
Winck 2004 {published data only}
- Winck JC, Goncalves MR, Lourenco C, Viana P, Almeida J, Bach JR. Effects of mechanical insufflation-exsufflation on respiratory parameters for patients with chronic airway secretion encumbrance. Chest 2004;126(3):774-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01518439 {published data only}
- NCT01518439. Instrumental and manual increase of couch in neuromuscular patients (OPTICOUGH). clinicaltrials.gov/ct2/show/NCT01518439 (first received 26 January 2012).
NCT02651805 {published data only (unpublished sought but not used)}
- NCT02651805. Mechanical insufflator-exsufflator to control mucus hypersecretion in patients in palliative care – a feasibility study. clinicaltrials.gov/ct2/show/NCT02651805 (first received 11 January 2016).
NCT03355105 {published data only (unpublished sought but not used)}
- NCT03355105. Comparison of two methods of adjusting the mechanical in-exsuflation in neuromuscular adult patients (EXSUFLOW). clinicaltrials.gov/ct2/show/NCT03355105 (first received 28 November 2017).
NCT04081116 {published data only}
- NCT04081116. Mechanical insufflation-exsufflation in children with NMD and weak cough. clinicaltrials.gov/ct2/show/NCT04081116 (first received 9 September 2019).
PACTR201506001171421 {published and unpublished data}
- PACTR201506001171421. The effect of cough assist and inspiratory muscle training in patients with NMD. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=1171 (first received 10 June 2015).
Additional references
Albuali 2007
- Albuali WH, Singh RN, Fraser DD, Seabrook JA, Kavanagh BP, Parshuram CS, et al. Have changes in ventilation practice improved outcome in children with acute lung injury? Pediatric Critical Care Medicine 2007;8(4):324-30. [DOI] [PubMed] [Google Scholar]
Altman 2005
- Altman DG, Bland MJ. Statistic notes: treatment allocation by minimisation. BMJ 2005;330:843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2005
- Anderson JL, Hasney KM, Beaumont NE. Systematic review of techniques to enhance peak cough flow and maintain vital capacity in neuromuscular disease: the case for mechanical insufflation-exsufflation. Physical Therapy Reviews 2005;10(1):25-33. [Google Scholar]
Bach 1996
- Bach JR, Saporito LR. Criteria for extubation and tracheostomy tube removal for patients with ventilatory failure. A different approach to weaning. Chest 1996;110(6):1566-71. [DOI] [PubMed] [Google Scholar]
Bach 1997
- Bach JR, Ishikawa Y, Kim H. Prevention of pulmonary morbidity for patients with Duchenne muscular dystrophy. Chest 1997;112(4):1024-8. [DOI] [PubMed] [Google Scholar]
Bach 2003
Bach 2007
- Bach JR, Bianchi C, Vidigal-Lopes M, Turi S, Felisari G. Lung inflation by glossopharyngeal breathing and "air stacking" in Duchenne muscular dystrophy. American Journal of Physical Medicine & Rehabilitation 2007;86(4):295-300. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bianchi 2008
- Bianchi C, Baiardi P. Cough peak flows: standard values for children and adolescents. American Journal of Physical Medicine & Rehabilitation 2008;87(6):461-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Boentert 2017
- Boentert M, Wenninger S, Sansone VA. Respiratory involvement in neuromuscular disorders. Current Opinion in Neurology 2017;30(5):529-37. [DOI] [PubMed] [Google Scholar]
Boitano 2006
- Boitano LJ. Management of airway clearance in neuromuscular disease. Respiratory Care 2006;51(8):913-22; discussion 922-4. [PMID: ] [PubMed] [Google Scholar]
Bott 2009
- Bott J, Blumenthal S, Buxton M, Ellum S, Falconer C, Garrod R, et al. Guidelines for the physiotherapy management of the adult, medical, spontaneously breathing patient. Thorax 2009;64(Suppl 1):i1-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chatwin 2018
- Chatwin M, Toussaint M, Goncalvez MR, Sheers N, Mellies U, Gonzales-Bermejo J, et al. Airway clearance techniques in neuromuscular disorders: a state of the art review. Respiratory Medicine 2018;136:98-110. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed March 2020. Available at covidence.org.
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Dohna‐Schwake 2006
- Dohna-Schwake C, Ragette R, Teschler H, Voit T, Mellies U. IPPB-assisted coughing in neuromuscular disorders. Pediatric Pulmonology 2006;41(6):551-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Douglas 1981
- Douglas NJ, Drummond GB, Sudlow MF. Breathing at low lung volumes and chest strapping: a comparison of lung mechanics. Journal of Applied Physiology 1981;50:650-7. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Farrero 2013
- Farrero E, Antonio A, Egeac CJ, Almaraz MJ, Masae JF, Utraboe I, et al. Guidelines for the management of respiratory complications in patients with neuromuscular disease. Archivos de Bronconeumologia 2013;49(7):306-13. [DOI] [PubMed] [Google Scholar]
Fauroux 2008
- Fauroux B, Guillemot N, Aubertin G, Nathan N, Labit A, Clement A, et al. Physiologic benefits of mechanical insufflation-exsufflation in children with neuromuscular diseases. Chest 2008;133(1):161-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Finder 2004
- Finder JD, Birnkrant D, Carl J, Farber HJ, Gozal D, Iannaccone ST, et al. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. American Journal of Respiratory and Critical Care Medicine 2004;170(4):456-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Finder 2010
- Finder JD. Airway clearance modalities in neuromuscular disease. Paediatric Respiratory Reviews 2010;11(1):31-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gattinoni 2003
- Gattinoni L, Carlesso E, Cadringher P, Valenza F, Vagginelli F, Chiumello D. Physical and biological triggers of ventilator-induced lung injury and its prevention. European Respiratory Journal 2003;22(47 Suppl):15s-25s. [DOI] [PubMed] [Google Scholar]
Gattinoni 2010
- Gattinoni L, Protti A, Caironi P, Carlesso E. Ventilator-induced lung injury: the anatomical and physiological framework. Critical Care Medicine 2010;38(10 Suppl):S539-48. [DOI] [PubMed] [Google Scholar]
Gozal 2000
- Gozal D. Pulmonary manifestations of neuromuscular disease with special reference to Duchenne muscular dystrophy and spinal muscular atrophy. Pediatric Pulmonology 2000;29(2):141-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2020a
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Higgins 2020b
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Homnick 2007
- Homnick DN. Mechanical insufflation-exsufflation for airway mucus clearance. Respiratory Care 2007;52(10):1296-305; discussion 1306-7. [PMID: ] [PubMed] [Google Scholar]
Hull 2012
- Hull J, Aniapravan R, Chan E, Chatwin M, Forton J, Gallagher J, et al. British Thoracic Society guideline for respiratory management of children with neuromuscular weakness. Thorax 2012;67(1):1-40. [DOI] [PubMed] [Google Scholar]
Iannaccone 2007
- Iannaccone ST. Modern management of spinal muscular atrophy. Journal of Child Neurology 2007;22(8):974-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leiner 1963
- Leiner GC, Brramowitz S, Small MJ, Stenby VB, Lewis WA. Expiratory peak flow rate. Standard values for normal subjects. Use as a clinical test of ventilatory function. American Review of Respiratory Disease 1963;88:644-51. [DOI] [PubMed] [Google Scholar]
Marques 2014
- Marques TB, Neves Jde C, Portes LA, Salge JM, Zanoteli E, Reed UC. Air stacking: effects on pulmonary function in patients with spinal muscular atrophy and in patients with congenital muscular dystrophy. Jornal Brasileiro de Pneumologia 2014;40(5):528-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marques 2020
- Marques L, Freitas Fregonezi GA, Santos IP, Marcelino AA, Medeiros da Fonsêca JD, Dourado-Júnior ME, et al. Effects of positioning on cough peak flow and muscular electromyographic activation in Duchenne muscular dystrophy. Respiratory Care 2020;65(11):1668-77. [DOI] [PubMed] [Google Scholar]
Mayer 2017
- Mayer OH, Henricson EK, McDonald CM, Buyse GM. Advances in pulmonary care in Duchenne muscular dystrophy. US Neurology 2017;13(1):35-41. [Google Scholar]
McCool 2006
- McCool FD, Rosen MJ. Nonpharmacologic airway clearance therapies: ACCP evidence-based clinical practice guidelines. Chest 2006;129(1 Suppl):250S-9S. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mills 2009
- Mills EJ, Chan AW, Wu P, Vail A, Guyatt GH, Altman DG. Design, analysis, and presentation of crossover trials. Trials 2009;10(27):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrow 2013
- Morrow B, Zampoli M, Van Aswegen H, Argent A. Mechanical insufflation-exsufflation for people with neuromuscular disorders. Cochrane Database of Systematic Reviews 2013, Issue 12. Art. No: CD010044. [DOI: 10.1002/14651858.CD010044.pub2] [DOI] [PubMed] [Google Scholar]
Nolan 2016
- Nolan SJ, Hambleton I, Dwan K. The use and reporting of the cross-over study design in clinical trials and systematic reviews: a systematic assessment. PloS One 2016;11(7):e0159014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nygren‐Bonnier 2009
- Nygren-Bonnier M, Markström A, Lindholm P, Mattsson E, Klefbeck B. Glossopharyngeal pistoning for lung insufflation in children with spinal muscular atrophy type II. Acta Paediatrica 2009;98:1324–8. [DOI] [PubMed] [Google Scholar]
Panitch 2009
- Panitch HB. The pathophysiology of respiratory impairment in pediatric neuromuscular diseases. Pediatrics 2009;123(Suppl 4):S215-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Panitch 2017
- Panitch HB. Respiratory implications of pediatric neuromuscular disease. Respiratory Care 2017;62(6):826-48. [DOI] [PubMed] [Google Scholar]
Papastamelos 1996
- Papastamelos C, Panitch HB, Allen JL. Chest wall compliance in infants and children with neuromuscular disease. American Journal of Respiratory and Critical Care Medicine 1996;154(4 Pt 1):1045-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Quanjer 2012
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al, ERS Globalet al Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. European Respiratory Journal 2012;40(6):1324-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rokadia 2015
- Rokadia HK, Adams JR, McCarthy K, Aboussouan LS, Mireles-Cabodevila E. Cough augmentation in a patient with neuromuscular disease. Annals of the American Thoracic Society 2015;12(12):1888-91. [DOI] [PubMed] [Google Scholar]
Rosière 2009
- Rosière J, Vader JP, Cavin MS, Grant K, Larcinese A, Voellinger R, et al. Appropriateness of respiratory care: evidence-based guidelines. Swiss Medical Weekly 2009;139(27-28):387-92. [DOI] [PubMed] [Google Scholar]
Saharan 2010
- Saharan S, Lodha R, Kabra SK. Management of acute lung injury/ARDS. Indian Journal of Pediatrics 2010;77(11):1296-302. [DOI] [PubMed] [Google Scholar]
Schünemann 2017a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. Chapter 11: Completing 'Summary of findings' tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Schünemann 2017b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Suri 2008
- Suri P, Burns SP, Bach JR. Pneumothorax associated with mechanical insufflation-exsufflation and related factors. American Journal of Physical Medicine & Rehabilitation 2008;87(11):951-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Toussaint 2018
- Toussaint M, Chatwin M, Gonzalez J, Berlowitz DJ, the ENMC Respiratory Therapy Consortium. Airway clearance techniques in neuromuscular disorders. Neuromuscular Disorders 2018;28:289-98. [DOI] [PubMed] [Google Scholar]
Trebbia 2005
- Trebbia G, Lacombe M, Fermanian C, Falaize L, Lejaille M, Louis A, et al. Cough determinants in patients with neuromuscular disease. Respiratory Physiology and Neurobiology 2005;146(2-3):291-300. [DOI] [PubMed] [Google Scholar]
Tzeng 2000
- Tzeng AC, Bach JR. Prevention of pulmonary morbidity for patients with neuromuscular disease. Chest 2000;118(5):1390-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vianello 2004
- Vianello A, Arcaro G, Gallan F, Ori C, Bevilacqua M. Pneumothorax associated with long-term non-invasive positive pressure ventilation in Duchenne muscular dystrophy. Neuromuscular Disorders 2004;14(6):353-5. [DOI] [PubMed] [Google Scholar]
Wang 2007
- Wang CH, Finkel RS, Bertini ES, Schroth M, Simonds A, Wong B, et al, Participants of the International Conference on SMA Standard of Care. Consensus statement for standard of care in spinal muscular atrophy. Journal of Child Neurology 2007;22(8):1027-49. [DOI] [PubMed] [Google Scholar]
Wang 2010
- Wang CH, Bonnemann CG, Rutkowski A, Sejersen T, Bellini J, Battista V, et al. Consensus statement on standard of care for congenital muscular dystrophies. Journal of Child Neurology 2010;25(12):1559-81. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Westermann 2013
- Westermann EJ, Jans M, Gaytant MA, Bach JR, Kampelmacher MJ. Pneumothorax as a complication of lung volume recruitment. Jornal Brasileiro Pneumologia 2013;39(3):382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Morrow 2018
- Morrow B, Argent A, Zampoli M, Human A, Corten L, Toussaint M. Cough augmentation techniques for people with chronic neuromuscular disorders. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD013170. [DOI: 10.1002/14651858.CD013170] [DOI] [PMC free article] [PubMed] [Google Scholar]


