Abstract
Background
This is a updated version of our Cochrane Review published in Issue 6, 2012. Sexually‐transmitted infections (STIs) continue to rise worldwide, imposing an enormous morbidity and mortality burden. Effective prevention strategies, including microbicides, are needed to achieve the goals of the World Heath Organization (WHO) global strategy for the prevention and control of these infections.
Objectives
To determine the effectiveness and safety of topical microbicides for preventing acquisition of STIs, including HIV.
Search methods
We undertook a comprehensive search of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, LILACS, CLIB, Web of Science, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, and reference lists of relevant articles up to August 2020. In addition, we contacted relevant organisations and experts.
Selection criteria
We included randomised controlled trials of vaginal microbicides compared to placebo (except for nonoxynol‐9 because it is covered in related Cochrane Reviews). Eligible participants were sexually‐active non‐pregnant, WSM and MSM, who had no laboratory confirmed STIs.
Data collection and analysis
Two review authors independently screened and selected studies, extracted data, and assessed risks of bias in duplicate, resolving differences by consensus. We conducted a fixed‐effect meta‐analysis, stratified by type of microbicide, and assessed the certainty of the evidence using the GRADE approach.
Main results
We included eight trials from the earlier version of the review and four new trials, i.e. a total of 12 trials with 32,464 participants (all WSM). We did not find any eligible study that enrolled MSM or reported fungal STI as an outcome. We have no study awaiting assessment.
All 12 trials were conducted in sub‐Saharan Africa, with one having a study site in the USA, and another having a site in India. Vaginal microbicides tested were BufferGel and PRO 2000 (1 trial, 3101 women), Carraguard (1 trial, 6202 women), cellulose sulphate (2 trials, 3069 women), dapivirine (2 trials, 4588 women), PRO 2000 (1 trial, 9385 women), C31G (SAVVY) (2 trials, 4295 women), and tenofovir (3 trials, 4958 women). All microbicides were compared to placebo and all trials had low risk of bias.
Dapivirine probably reduces the risk of acquiring HIV infection: risk ratio (RR) 0.71, (95% confidence interval (CI) 0.57 to 0.89, I2 = 0%, 2 trials, 4588 women; moderate‐certainty evidence). The other microbicides may result in little to no difference in the risk of acquiring HIV (low‐certainty evidence); including tenofovir (RR 0.83, 95% CI 0.68 to 1.02, cellulose sulphate (RR 1.20, 95% CI 0.74 to 1.95, BufferGel (RR 1.05, 95% CI 0.73 to 1.52), Carraguard (RR 0.89, 95% CI 0.71 to 1.11), PRO 2000 (RR 0.93, 95% CI 0.77 to 1.14), and SAVVY (RR 1.38, 95% CI 0.79 to 2.41).
Existing evidence suggests that cellulose sulphate (RR 0.99, 95% CI 0.37 to 2.62, 1 trial, 1425 women), and PRO 2000 (RR 0.95, 95% CI 0.73 to 1.23) may result in little to no difference in the risk of getting herpes simplex virus type 2 infection (low‐certainty evidence). Two studies reported data on tenofovir's effect on this virus. One suggested that tenofovir may reduce the risk (RR 0.55, 95% CI 0.36 to 0.82; 224 participants) while the other did not find evidence of an effect (RR 0.94, 95% CI 0.85 to 1.03; 1003 participants). We have not reported the pooled result because of substantial heterogeneity of effect between the two studies (l2 = 85%).
The evidence also suggests that dapivirine (RR 1.70, 95% CI 0.63 to 4.59), tenofovir (RR 1.27, 95% CI 0.58 to 2.78), cellulose sulphate (RR 0.69, 95% CI 0.26 to 1.81), and (Carraguard (RR 1.07, 95% CI 0.75 to 1.52) may have little or no effect on the risk of acquiring syphilis (low‐certainty evidence).
In addition, dapivirine (RR 0.97, 95% CI 0.89 to 1.07), tenofovir (RR 0.90, 95% CI 0.71 to 1.13), cellulose sulphate (RR 0.70, 95% CI 0.49 to 0.99), BufferGel (RR 0.97, 95% CI 0.65 to 1.45), Carraguard (RR 0.96, 95% CI 0.83 to 1.12), and PRO 2000 (RR 1.01, 95% CI 0.84 to 1.22) may result in little to no difference in the risk of acquiring chlamydia infection (low‐certainty evidence).
The evidence also suggests that current topical microbicides may not have an effect on the risk of acquiring gonorrhoea, condyloma acuminatum, trichomoniasis, or human papillomavirus infection (low‐certainty evidence). Microbicide use in the 12 trials, compared to placebo, did not lead to any difference in adverse event rates.
No study reported on acceptability of the intervention.
Authors' conclusions
Current evidence shows that vaginal dapivirine microbicide probably reduces HIV acquisition in women who have sex with men. Other types of vaginal microbicides have not shown evidence of an effect on acquisition of STIs, including HIV. Further research should continue on the development and testing of new microbicides.
Keywords: Female; Humans; Acrylic Resins; Acrylic Resins/administration & dosage; Adenine; Adenine/administration & dosage; Adenine/analogs & derivatives; Administration, Intravaginal; Agaricales; Agaricales/chemistry; Anti-HIV Agents; Anti-HIV Agents/administration & dosage; Anti-HIV Agents/adverse effects; Anti-Infective Agents, Local; Anti-Infective Agents, Local/administration & dosage; Bias; Cellulose; Cellulose/administration & dosage; Cellulose/adverse effects; Cellulose/analogs & derivatives; HIV Infections; HIV Infections/prevention & control; Naphthalenesulfonates; Naphthalenesulfonates/administration & dosage; Placebos; Placebos/administration & dosage; Polymers; Polymers/administration & dosage; Pyrimidines; Pyrimidines/administration & dosage; Pyrimidines/adverse effects; Seaweed; Seaweed/chemistry; Sexually Transmitted Diseases; Sexually Transmitted Diseases/prevention & control; Tenofovir; Tenofovir/administration & dosage; Tenofovir/adverse effects
Plain language summary
Vaginal inserts for prevention of sexually transmitted infections
Review question
In this Cochrane Review we assessed the effects of topical microbicides (chemical substances that can be applied inside the vagina or rectum), compared to placebo (inactive substance), to prevent women who have sex with men and men who have sex with men from getting sexually‐transmitted infections (STIs), including HIV.
Background
This is an updated version of our Cochrane Review published in 2012. Both curable and incurable STIs continue to rise, despite the prevention strategies implemented to date. Women often have the highest rates of STIs and account for a disproportionate number of new infections. STIs are often without symptoms. Despite their greater vulnerability, current options to reduce the spread of STI remain limited for women. There is thus a clear need for new and effective strategies to prevent people from getting STIs, including HIV.
Trial characteristics
Cochrane researchers searched the available literature up to August 2020 and included 12 trials with 32,464 women who have sex with men. The trials included seven types of inserts (six vaginal gels and one vaginal ring) that were compared with placebo, all conducted among women aged over 16 years. All trials were conducted in sub‐Saharan Africa, with one having a study site in India and another having a site in the USA. The Cochrane researchers did not find any studies that were conducted among men who have sex with men.
Key results
Compared with placebo, the rate of HIV infection was lower in the group that took vaginal inserts containing the antiretroviral drug known as dapivirine, but other STIs occurred at similar rates in dapivirine and placebo groups. Tenofovir gel may also reduce the rates of herpes simplex virus infection, but not other STIs. In addition, the cellulose sulphate gel resulted in lower rates of chlamydia infection, compared to placebo. When other microbicide gels were compared with placebo, could be little or no difference in the rates of STI. None of the trials reported fungal STI as outcome.
Compared with placebo, the rate of HIV infection was lower in the group that took vaginal inserts containing the antiretroviral drug known as dapivirine, but other STIs occurred at similar rates in dapivirine and placebo groups. Tenofovir gel probably reduces the rates of herpes simplex virus infection, but not other STIs. Cellulose sulphate gel probably results in lower rates of chlamydia infection, compared to placebo. When other microbicide gels were compared with placebo, could be, there is little or no difference in the rates of STI. None of the trials reported fungal STI as outcome.
Certainty of evidence
The certainty of evidence was low for most outcomes reported in this review, due to heterogeneity and small number of studies and participants for certain microbicides. This led to imprecision of the findings (ranging from large clinical benefits to substantial harm).
How up‐to‐date is this review?
The review authors searched for studies that were published up to August 2020.
Summary of findings
Summary of findings 1. Dapivirine vaginal microbicide for preventing sexually transmitted infections.
| Topical microbicides for preventing sexually transmitted infections | |||||
| Population: heterosexual women Settings: Local clinics and community based locations in Malawi, South Africa, Uganda, and Zimbabwe (1 study) and South Africa and Uganda (1 study) Intervention: dapivirine vaginal microbicide rings Comparison: identical placebo rings | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | |
| Placebo | Dapivirine | ||||
| HIV infection | 78 per 1000 |
55 per 1000 (44 to 69) |
RR 0.71 (0.57 to 0.89) |
4588 (2 studies) | ⊕⊕⊕⊝ Moderate1 |
| Herpes simplex virus | Data not reported | The dapivirine studies did not screen for herpes simplex virus | |||
| Gonorrhoea | 153 per 1000 |
153 per 1000 (133 to 175) |
RR 1.00 (0.87 to 1.15) | 4588 (2 studies) | ⊕⊕⊝⊝ low1,2 |
| Trichomoniasis | 144 per 1000 |
153 per 1000 (133 to 177) |
RR 1.06 (0.92 to 1.23) |
4588 (2 studies) | ⊕⊕⊝⊝ low3 |
| Chlamydia | 293 per 1000 |
285 per 1000 (261 to 314) |
RR 0.97 (0.89 to 1.07) |
4588 (2 studies) |
⊕⊕⊝⊝ moderate2 |
| Syphilis | 8 per 1000 |
13 per 1000 (5 to 35) |
RR 1.70 (0.63 to 4.59) |
1956 (1 study) |
⊕⊕⊝⊝ low3 |
| Serious adverse events | 110 per 1000 |
123 per 1000 (103 to 145) |
RR 1.12 (0.94 to 1.32) |
4588 (2 studies) | ⊕⊕⊝⊝ low1,2 |
| Acceptability | No study reported this outcome in a form that could be put in a forest plot | ||||
| Minor adverse events | No study reported this outcome in a form that could be put in a forest plot | ||||
| *The basis for the risk in the placebo group is the median risk across control groups of the included studies. The corresponding risk for dapivirine (and its 95% confidence interval) is based on the median risk in the dapivirine group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||
1 Downgraded by one level for imprecision, due to lack of optimal information size. 2 Downgraded by one level for inconsistency: I2 = 62% (gonorrhoea), I2 = 87% (serious adverse events) 3 Downgraded by two levels for substantial imprecision.
Summary of findings 2. Tenofovir vaginal microbicide for preventing sexually transmitted infections.
| Topical microbicides for preventing sexually transmitted infections | |||||
| Population: Heterosexual women Settings: Community clinics in South Africa (2 studies), Uganda and Zimbabwe (1 study) Intervention: Tenofovir vaginal microbicide gels Comparison: Identical placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | |
| Placebo | Tenofovir | ||||
| HIV infection | 78 per 1000 |
64 per 1000 (53 to 79) |
RR 0.83 (0.68 to 1.02) |
4958 (3 studies) | ⊕⊕⊝⊝ low1 |
| Herpes simplex virus | 426 per 1000 |
384 per 1000 (350 to 422) |
RR 0.90 (0.82 to 0.99) |
2439 (2 studies) | ⊕⊕⊝⊝ low2 |
| Gonorrhoea | 48 per 1000 |
24 per 1000 (14 to 39) |
RR 0.66 (0.40 to 1.10) |
2010 (1 study) | ⊕⊕⊝⊝ low1 |
| Trichomoniasis | 51 per 1000 |
62 per 1000 (43 to 64) |
RR 1.21 (0.84, 1.74) |
2010 (1 study) | ⊕⊕⊝⊝ low1 |
| Chlamydia | 129 per 1000 |
116 per 1000 (22 to 145) |
RR 0.90 (0.71 to 1.13) |
2010 (1 study) | ⊕⊕⊝⊝ low1 |
| Syphilis | 11 per 1000 |
14 per 1000 (6 to 30) |
RR 1.27 (0.58 to 2.78) |
2010 (1 study) | ⊕⊕⊝⊝ low1 |
| Serious adverse events | 63 per 1000 |
63 per 1000 (51 to 78) |
RR 1.00 (0.81 to 1.24) |
4958 (3 studies) | ⊕⊝⊝⊝ very low1,2 |
| Acceptability | No study reported this outcome in a form that could be put in a forest plot | ||||
| Minor adverse events | No study reported this outcome in a form that could be put in a forest plot | ||||
| *The basis for the risk in the placebo group is the median risk across control groups of the included studies. The corresponding risk for tenofovir (and its 95% confidence interval) is based on the median risk in the tenofovir group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||
1 Downgraded by two levels for substantial imprecision, due to lack of optimal information size. 2 Downgraded by two levels for substantial inconsistency: I2 = 85%.
Summary of findings 3. Cellulose sulphate vaginal microbicide for preventing sexually transmitted infections.
| Topical microbicide for preventing sexually transmitted infections | ||||||
|
Patient or population: Heterosexual women Settings: Community clinics Nigeria (1 study), South Africa, Uganda, Benin and India (1 study) Intervention: Cellulose sulphate vaginal gel Comparison: Identical placebo |
||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | ||
| Assumed risk | Corresponding risk | |||||
| Placebo | Cellulose sulphate | |||||
| HIV Infection | 19 per 1000 |
23 per 1000 (14 to 37) |
RR 1.2 (0.74 to 1.95) |
3069 (2 studies | ⊕⊕⊝⊝ low1 |
|
| Herpes simplex | 11 per 1000 | 11 per 1000 (4 to 30) |
RR 0.99 (0.37 to 2.62) |
1425 (1 study) | ⊕⊕⊝⊝ low1 |
|
| Gonorrhoea | 66 per 1000 | 59 per 1000 (44 to 77) | RR 0.89 (0.67 to 1.17) | 3069 (2 studies) | ⊕⊝⊝⊝ very low1,2 | |
| Trichomoniasis | 55 per 1000 | 53 per 1000 (34 to 82) | RR 0.96 (0.62 to 1.49) | 1425 (1 study) | ⊕⊕⊝⊝ low1 | |
| Chlamydia | 48 per 1000 | 34 per 1000 (24 to 48) | RR 0.70 (0.49 to 0.99) | 3069 (2 studies) | ⊕⊕⊝⊝ low1 |
|
| Syphilis | 14 per 1000 | 10 per 1000 (4 to 26) | RR 0.69 (0.26 to 1.81) | 1425 (1 study) | ⊕⊕⊝⊝ low1 | |
| Serious adverse events | 33 per 1000 |
42 per 1000 (29 to 60) |
RR 1.25 (0.87 to 1.79) |
3069 (2 studies) | ⊕⊕⊝⊝ low1 |
|
| Acceptability | No study reported this outcome in a form that could be put in a forest plot | |||||
| Minor adverse events | No study reported this outcome in a form that could be put in a forest plot | |||||
| *The basis for the risk in the placebo group is the median risk across control groups of the included studies. The corresponding risk for cellulose sulphate (and its 95% confidence interval) is based on the median risk in the cellulose sulphate group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by two levels for substantial imprecision, due to lack of optimal information size 2 Downgraded by one level for inconsistency: I2 = 50%
Background
This is an updated version of the original Cochrane review published in Issue 6, 2012 (Obiero 2012a)
Description of the condition
Sexually‐transmitted infections (STIs) refer to a variety of clinical syndromes and infections caused by pathogens that can be acquired and transmitted through sexual activity (CDC 2015). STIs are common global causes of illness, long‐term disability, and death with serious medical and psychological consequences for millions of people (Newman 2015). They have far‐reaching health, social, and economic consequences in countries and communities where socio‐economic conditions are poor (Tsai 2012; Vogenthaler 2013).There are over 30 bacterial, viral, and parasitic pathogens that can be transmitted sexually. According to 2008 World Health Organization (WHO) estimates, 499 million new curable cases of the four most common curable infections, chlamydia, gonorrhoea, syphilis and trichomoniasis, occur annually throughout the world (WHO 2012). In addition, over 500 million people are estimated to have a viral STI such as herpes simplex virus type 2 (HSV‐2) or human papillomavirus (HPV) at any point in time (Gotlieb 2014). In the developed world, viral infections have become increasingly common and important, whereas bacterial STIs are more common in low‐ and middle‐income countries, but even this is changing with the increasing recognition of viral infections (Adler 2004).
The three most common presenting symptoms of an STI are urethral discharge, genital ulceration and vaginal discharge with or without vulval irritation (Sonkar 2017), Assymptomatic STIs (C. trachomatis, N. gonorrhoea and syphilis are more frequent in women than in men and in women the risk of complications and sequels are higher and also there is the risk of maternal to child transmission (Oakeshott 2010; Wasserheit 1989: WHO 2007:WHO 2016; Walker 2007). STIs also frequently result in stigma due to their sexual nature of transmission, stereotyping and vulnerability, and have been associated with gender‐based violence (Amin 2013). Often, screening and treatment are not given priority within public health services, nor do STIs receive the political, socio‐economic and cultural attention they warrant (Ortayli 2014). Clinical symptoms of STIs can be non‐specific, and where possible the diagnosis needs to be confirmed by laboratory testing (Gaitán‐Duarte 2013). Current STI control is hampered by several behavioural, biological and implementation challenges including a large proportion of asymptomatic infections, lack of feasible diagnostic tests, antimicrobial resistance, repeat infections and barriers to intervention access, availability and scale‐up (Ortayli 2014). In low and middle income countries, laboratory testing is not always available and women and men reporting symptoms suggestive of an STI are often treated according to algorithms without confirmatory tests (Trollope‐Kumar 2006). This approach is effective for certain infections such as male urethritis and genital ulcers, but with vaginal discharge the risk of misdiagnosis is high (Sonkar 2016). Syndromic management of STI can therefore lead to over‐treatment and adverse social consequences such as stigma and intimate partner violence (White 2008). In addition, it misses asymptomatic infections, which are by far the greatest burden of disease (WHO 2013a).
The rising trend and complications of the infections suggest limited impact of prevention approaches to date (UNAIDS 2010; WHO 2013b). Both male and female condoms have been considered one of the most effective biological mechanisms for reducing the transmission of STIs (Matson 2011). However, the positive impact of their use may be affected by inconsistent and incorrect use. In addition, several factors are known to be associated with low uptake of this method, including perception of reduced pleasure (Sarkar 2008), discomfort (Crosby 2005), partner trust (Abdullah 2002), religious beliefs (Thomsen 2004), availability and accessibility (Kumar 2006), existing inequalities in gender‐based norms and values (Mayaud 2001), cost (Essien 2005), women's lack of empowerment (Sarkar 2008), and negative community attitudes towards homosexuality combined with legal restrictions among MSM (Adimora 2010).
Description of the intervention
Microbicides are compounds that can be applied inside the vagina or rectum to protect against STIs including HIV (Smriti 2012; WHO 2000). They can be formulated as gels, creams, films, rings, sponges or suppositories (Lopez 2016; Singh 2014). Microbicides would greatly empower women and men who have sex with men (MSM) to protect themselves, as they are a potential preventive option that they can easily control and do not require the co‐operation, consent or even knowledge of the partner. Microbicides may or may not have spermicidal activity (contraceptive effect). An ideal microbicide would be safe and effective against a range of STI‐causing organisms, available in both spermicidal and non‐spermicidal formulations, effective over relatively long periods, acceptable to potential users, bio‐diffusible, bio‐adhesive, can be effective immediately, are stable at high temperatures and economically affordable in middle‐ and low‐income countries (Han 2009). Microbicides that have been developed include vaginal gels and rings. The microbicides are applied intravaginally or rectally before or before and after each sexual episode (Abdool Karim 2010; Feldblum 2008), The rings are slow‐release devices that are effective for a longer period of time (Baeten 2016).
How the intervention might work
The principal target of microbicides is to reduce male‐to‐female and male‐to‐male STI transmission, although they could also potentially prevent female‐to‐male transmission. Topical microbicides are grouped based on their mode and site of action (Cutler 2008); They include:
Surfactants: These are detergent‐like products that disrupt cell membranes or change membrane structure, which make it more porous and liable to disruption. These products are non‐specific and impact on all cells (host, commensal.and pathogen). Hence these products have a wide spectrum of activity against several microbes, spermatozoa and cell membranes, e.g. SAVVY and nonoxynol‐9 (Peterson 2007; Wilkinson 2002a; Wilkinson 2002b).
Vaginal defence enhancers: These are acid‐buffering agents that assist the natural immune defences of the vagina to deactivate the pathogen. They augment vaginal mucosal defence by increasing microbicidal activity of genital secretions. Lactic acid bacteria, notably lactobacilli, occur naturally in the vagina and release a variety of antimicrobial compounds such as lactic acid, hydrogen peroxide, bacteriocins and bio‐surfactants. A disruption of the natural balance of the vaginal ecosystem raises the risk of STI infection. The vagina is usually maintained at a low pH of about 4 to 5, which is achieved through secretion of lactic acid bacteria. These microbicides maintain the colonisation or re‐colonisation of the vagina with lactobacilli when these commensal organisms have been adversely affected, for example by use of antibiotics or reproductive tract infections. They also assist the vagina to maintain low pH in the presence of alkaline semen, the effect of which on the vaginal pH results in the loss of barrier to pathogens, e. g. Buffergel (Abdool Karim 2011; Keller 2012).
Entry inhibitors: This class of microbicide agents blocks the attachment of the pathogen to the host cell, fusion with the host‐cell membrane or entry into the host cells. They are polymers that act against viruses, predominantly by interfering with attachment to host cells. The envelope of HIV, particularly the gp41 component, which enables fusion with the cell membrane, is a critical target for a potentially successful microbicide. Polymers act by blocking viral entry into susceptible cells by blocking CD4 attachment or receptor attachment, or both, e.g. Carragurard and Pro2000 (Abdool Karim 2011; Skoler‐Karpoff 2008).
Agents that prevent replication of the pathogen after it has entered the cell: These are antiretroviral drugs developed as microbicides because of their capacity to inhibit the replication process of the pathogen. They act locally in the reproductive tract mucosa at specific steps in the replication cycle of the pathogen and therefore have a narrow spectrum against viruses, notably HIV, e.g. tenofovir (Abdool Karim 2010; Delany‐Moretlwe 2018) and dapivirine (Baeten 2016).
Why it is important to do this review
Despite the greater vulnerability of women and of men who have sex with men, current options to reduce acquisition of STI remain limited for women and men who are receptive partners during anal sex (Nunes 2014). There is a clear need for new and effective prevention strategies that women and MSM can use and control to reduce their risk of sexual acquisition of STIs. In the absence of a definitive cure or preventative vaccine, microbicides could offer an alternative or a complement to condoms as the most feasible method for primary prevention of STIs.
A registered vaginal or rectal microbicide is not yet available, despite the fact that over 60 candidate agents have been identified which have in vitro activity against STIs (Singh 2014). Some of the microbicides have already undergone large‐scale phase lll trials (Obiero 2012a). There is thus a need for a comprehensive and up‐to‐date synthesis of this evidence. This Cochrane Review is an update of one published in 2012 (Obiero 2012a).
Objectives
To determine the effectiveness and safety of topical microbicides for preventing acquisition of STIs, including HIV.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), which followed up participants for at least 12 months. We exclude quasi‐randomised trials because these produce effect estimates that indicate more extreme benefits when they are compared with randomised trials (Higgins 2019). We exclude cross‐over and cluster trials, because of the nature of the condition and intervention (Higgins 2019). The unit of randomisation was the individual.
Types of participants
Eligible participants were sexually active non‐pregnant heterosexual women (i.e. WSM), and men who have sex with men (MSM), aged 16 years and above in any setting, who had no laboratory‐confirmed STIs at baseline.
Types of interventions
Eligible interventions were topical microbicides including detergent‐like products (surfactants) , vaginal defence enhancers, entry inhibitors, and agents that prevent replication of the pathogen after it has entered the host cell. We performed meta‐analyses separately for each STI, according to the assessed intervention based on its mechanism of action. We excluded nonoxynol‐9 because it is covered in other Cochrane Reviews (Wilkinson 2002a; Wilkinson 2002b). Eligible comparison interventions Included: placebo, no intervention, condom, diaphragm, vaginal sponge, and cervical cap. However, we did not find studies that compared microbicides with other active interventions.
Types of outcome measures
Primary outcomes
Laboratory confirmed incidence of STIs (viral, bacterial, fungal, protozoan). We reported the laboratory methods used for measurement of the outcomes included.
Serious adverse events, i.e. the proportion of participants who experience any adverse effect requiring hospitalisation or discontinuation of therapy, or both.
Secondary outcomes
Acceptability, i.e. proportion of participants that tolerated or allowed the intervention.
Minor adverse events, e.g. vaginal discharge, burning sensation, genital pain, rash, ulceration, erythema, vaginitis.
Search methods for identification of studies
We attempted to identify as many relevant randomised trials as possible of "topical microbicide", irrespective of language of publication or publication status (published or unpublished). We used both electronic searching in bibliographic databases and handsearching, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019)
Electronic searches
With the assistance of the Information Specialist of the Cochrane Sexually Transmitted Infections Group, we implemented a comprehensive search strategy to identify as many relevant trials as possible in electronic databases. We used a combination of controlled vocabulary (MeSH, Emtree, DeCS, including exploded terms) and free‐text terms (considering spelling variants, synonyms, acronyms and truncation) for topical microbicides, with field labels, proximity operators, and boolean operators.
For the initial version of the review published in 2012 (Obiero 2012a), we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (1980 to 22 July 2011), MEDLINE (1980 to July 2011), Embase (1980 to July 2011), Web of Science (1980 to May 2009), LILACS (1980 to May 2009), NML Gateway (1980 to December 2009), WHO International Clinical Trials Registry Platform (ICTRP: www.who.int/ictrp/search/en/) (22 July 2011), and ClinicalTrials.gov (22 July 2011).
For this review update, we conducted searches between June 2015 and August 2020 (Appendix 1; Appendix 2; Appendix 3; Appendix 4: Appendix 5; Appendix 6) in MEDLINE, Embase, LILACS, Web of Science, ICTRP, ClinicalTrials.gov and CENTRAL for trials that were done after 2011.
Searching other resources
We conducted searches of conference proceedings and reference lists of relevant journal articles, and contacted organisations involved in microbicide research.
Conference proceedings
We searched proceedings of the following conferences from August 2011 to 22 August 2020 for relevant studies:
International Conference on AIDS and STDs in Africa (ICASA)
Biennial meeting of the International Society for Sexually Transmitted Diseases Research
International Congress of Sexually Transmitted Infections
Biannual International Microbicide Conference and Modern Mucosal Vaccines, Adjuvants and Microbicides
Annual Conference on Retroviruses and Opportunistic Infections (CROI)
Researchers, organisations and pharmaceutical companies
We contacted organisations involved in microbicide research, including the Alliance for Microbicide Development, International Partnership for Microbicides (IPM), the Joint United Nations Programme on HIV/AIDS (UNAIDS), and WHO. We also contacted microbicide and HIV prevention experts.
Reference lists
We checked the reference lists of relevant previous reviews (Buckheit 2010; Cutler 2008; Garg 2009; Klasse 2008; Nuttall 2010; Singh 2014; Wilkinson 2002a; Wilkinson 2002b) and full‐text articles reviewed for inclusion in this review.
Data collection and analysis
Selection of studies
For the original review (Obiero 2012a), Jael Obiero (JO) and Charles Shey Wiysonge (CSW) independently screened all citations and abstracts identified by the search strategy for potentially eligible studies. For this review update, JO and Paul Ogongo (PO) independently performed screening and study selection. The two review authors independently assessed the full‐text articles of potentially relevant studies using the prespecified trial inclusion criteria. We resolved any disagreements by discussion and consensus. When a disagreement could not be resolved, a third review author (Peter Gichuhi Mwethera (PGM) for the original review and CSW for this update) arbitrated. We excluded potentially eligible studies that did not meet our inclusion criteria and documented the reasons for exclusion in the table of Characteristics of excluded studies.
Data extraction and management
For the current update, JO and PO independently extracted data from included trials using a data extraction form. Extracted information included trial methods, participant characteristics, interventions, and outcomes. For all trials, we extracted the number of participants randomised, the number of participants with each outcome, and the number analysed. The authors resolved differences by discussion and consensus, with arbitration by PGM and CSW.
Assessment of risk of bias in included studies
Three review authors (JO, PO, and CSW) independently assessed the risks of bias in each included trial addressing seven specific domains: generation of allocation sequence, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and 'other issues'. For each included trial, the three review authors independently described what the trial authors reported that they did for each domain and then made a decision relating to the risk of bias for that domain by assigning a judgement of 'low risk' of bias, 'high risk' of bias, or 'unclear risk' of bias. The review authors compared the results of their independent assessments of risks of bias and resolved any discrepancies by discussion and consensus (Higgins 2020). We assessed risk of bias using the Cochrane 'Risk of bias' tool. We provided justification for risk of bias (high, low, unclear) in the 'Risk of bias' table by direct reference to the relevant report.
Measures of treatment effect
Our outcomes were all dichotomous in nature, so we presented the trial results as risk ratios (RRs) with 95% confidence intervals (CIs). The RR is used as a relative effect measure that works well with a low or high rate of events, and is easy to interpret and use in clinical practice. We performed meta‐analyses separately for each STI, stratified by type of microbicide,compared to their respective placebos. This was because the microbicides in the trial belonged to different classes and their mechanisms of action differ.
Unit of analysis issues
The unit of analysis was the participant who received the preventive treatment.
Dealing with missing data
We identified the levels of attrition in the included trials and performed analyses for all outcomes as far as possible on an intention‐to‐treat basis, i.e. we analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau2, I2 statistic, and Chi2 test (Higgins 2019). We regarded heterogeneity as substantial if the I2 statistic value was greater than 40% and if either the Tau2 value was greater than zero or there was a P value less than 0.10 in the Chi2 test.
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity using the I2 statistic as follows: not relevant (l2 value below 40%), important (l2 value 40% to 75%), or substantial (l2 value above 75%) (Sutton 2008; Higgins 2020). We also assessed statistical heterogeneity in each meta‐analysis using the t2 and Chi2 statistics.
If we detected substantial heterogeneity, we explored possible explanations for it in subgroup analyses (see Subgroup analysis and investigation of heterogeneity). We took statistical heterogeneity into account if is not relevant we used fixed effects model, if heterogeneity is important we used a random‐effects analysis, and if heterogeneity is substantial and there is not explanation we did not pool data
Assessment of reporting biases
We planned to explore publication bias through assessment of funnel plot asymmetry and formal tests. For dichotomous outcomes we planned to use the test proposed by Harbord 2006. However, as we included fewer than 10 trials in the meta‐analyses we did not perform these analyses.
Data synthesis
We performed statistical analyses using Review Manager 5 (RevMan: RevMan 2014). For this version of the review, we used a fixed‐effect meta‐analysis, i.e. the trials examined the same intervention, and we judged the trials’ populations and methods to be sufficiently similar. We would have used a random‐effects model if there were clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials or if we detected substantial heterogeneity (I2 = 40% or greater) to produce an overall summary if we considered an average treatment effect across trials to be clinically meaningful. In such circumstances, we would have treated the random‐effects summary as the average range of possible treatment effects and discussed the clinical implications of treatment effects differing between trials.
Subgroup analysis and investigation of heterogeneity
We planned to explore possible causes of any significant statistical heterogeneity of effects by using subgroup analyses, with subgroups defined by the sex of study participants (WSM or MSM), sexual route of transmission (vaginal or anal), population (high versus low risk), intervention type, and type of comparison group (placebo, condom, diaphragm, vaginal sponge, or cervical cap).
Sensitivity analysis
We conducted sensitivity analyses to investigate the effect of type of meta‐analysis (fixed‐effect versus random‐effects) on the robustness of the results.
Summary of findings and assessment of the certainty of the evidence
We graded our confidence in the evidence and summarised the findings in a 'Summary of findings' table using the approach recommended by the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Working Group (GRADEpro GDT 2015). We compared the effectiveness of topical microbicides (surfactants, vaginal defence enhancers, entry inhibitors and agents that prevent replication of the pathogen after it has entered the cell) with placebo, no intervention, condom, diaphragm, vaginal sponge, and cervical cap for prevention of STI for each of the following important outcomes: laboratory confirmed incidence of STIs (viral, bacterial, fungal, protozoan), severe adverse events, acceptability and minor adverse events. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and risk of bias) to assess the certainty of the evidence as it relates the outcomes (Balshem 2011; Guyatt 2011; Guyatt 2013) . We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020).
We plan to extract study data, format our comparisons in data tables and prepare a summary of findings table before writing the results and conclusions of our review.
Results
Description of studies
Results of the search
For the previous version of the review, we conducted searches up to July 2011 and included eight randomised trials (Obiero 2012a). For the current update, we conducted searches between June 2015 and August 2020 for trials conducted after 2011 and identified 1980 records (1834 without duplicates). From these records, we identified 16 potentially eligible studies (Figure 1). We included four of the studies, which met our inclusion criteria (Baeten 2016; Marrazzo 2015; Nel 2016; Delany‐Moretlwe 2018). Six of the 12 trials were stopped early due to safety concerns (Feldblum 2008: Halpern 2008; Marrazzo 2015; McCormack 2010; Peterson 2007; Van Damme 2008). We conducted sensitivity analyses and found that risk of bias (high versus moderate/low risk of bias) had no impact on the robustness of the findings.
1.
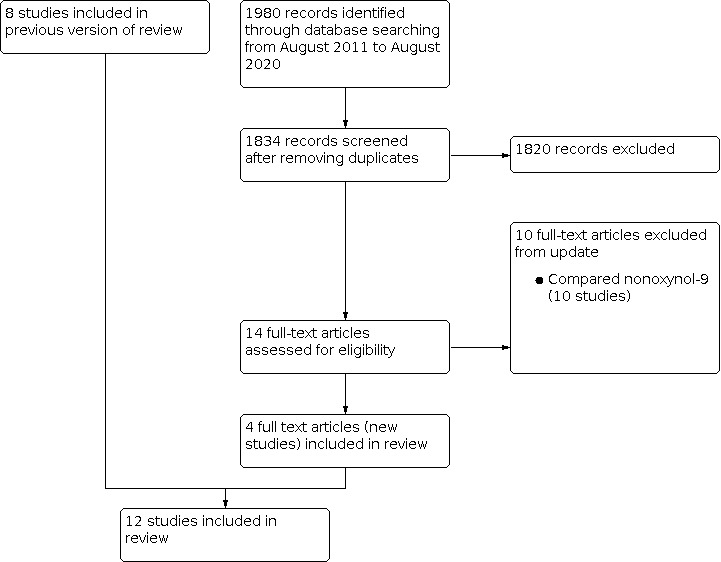
PRISMA diagram showing the search and selection of studies
Combining data from the previous version of the review and the recent searches, gives 14 excluded and 12 included studies (Figure 1; Table 4). In this update, we do not have any studies awaiting assessment.
1. Summary table with the description of included studies.
| Study | Participants | Intervention | Comparator | Outcome |
| Abdool Karim 2010 | 889 women | Tenofovir gel | Placebo gel | HIV, HSV‐2, HPV, Adverse events |
| Abdool Karim 2011 | 3101 women | Buffergel 0.5% PRO 2000 gel |
Placebo gel | HIV, Chlamydia, Gonorrhoea, Trichomoniasis, HSV‐2 Adverse events |
| Baeten 2016 | 2629 women | Dapivirine ring | Placebo ring | HIV‐1, Gonorrhoea, Trichomoniasis, Chlamydia, Adverse events |
| Feldblum 2008 | 2153 women | SAVVY gel | Placebo gel | HIV, Adverse events |
| Halpern 2008 | 1644 women | Cellulose sulphate gel | Placebo gel | HIV, Gonorrhoea, Chlamydia, Adverse events |
| Marrazzo 2015 | 2010 women | Tenofovir gel | Placebo gel | HIV, HSV, Gonorrhoea, Chlamydia, Syphilis, Trichomoniasis, Adverse events |
| Nel 2016 | 1959 women | Dapivirine ring | Placebo ring | HIV, Gonorrhoea, Chlamydia, Syphilis, Trichomoniasis, Adverse events |
| McCormack 2010 | 9385 women | 2% PRO 2000 gel 0.5% PRO 2000 gel |
Placebo gel | HIV, HSV, Gonorrhoea, Chlamydia |
| Peterson 2007 | 2142 women | SAVVY gel | Placebo gel | HIV, Adverse events |
| Delany‐Moretlwe 2018 | 2059 women | Tenofovir gel | Placebo gel | HIV, HSV, Adverse events |
| Skoler‐Karpoff 2008 | 6202 women | Carraguard gel | Placebo gel | HIV, Gonorrhoea, Chlamydia, Syphilis, Trichomoniasis, Adverse events |
| Van Damme 2008 | 1428 women | Cellulose sulphate gel | Placebo gel | HIV, HSV, Gonorrhoea, Chlamydia, Syphilis, Condyloma, Trichomoniasis |
Included studies
See: Characteristics of included studies.
Study design
All 12 included trials were parallel RCT. Seven trials were multicentre (Abdool Karim 2011; Baeten 2016; Delany‐Moretlwe 2018; Marrazzo 2015; McCormack 2010; Nel 2016; Van Damme 2008), with two having study sites outside Africa: one in the USA (Abdool Karim 2011) and one in India (Van Damme 2008). The rest of the trials were conducted solely in Africa (Abdool Karim 2010; Delany‐Moretlwe 2018; Feldblum 2008; Halpern 2008; Peterson 2007; Skoler‐Karpoff 2008). The trials lasted for at least two years, except for the trials that were stopped early for data‐dependent processes (Feldblum 2008; Halpern 2008; Marrazzo 2015; McCormack 2010: Peterson 2007; Van Damme 2008).
Population
The age of trial participants ranged from 16 to 72 years. These were non‐pregnant women who did not have any reproductive tract complications. The total number of participants in these trials was 32,464. The number of participants in each trial ranged from 889 (Abdool Karim 2010) to 9385 women (McCormack 2010).
We did not find any eligible trial that enrolled MSM.
Intervention
Two trials (Baeten 2016; Nel 2016) used davipirine using vaginal rings and the remaining trials used vaginal gels. In the trials that used vaginal rings, women randomised to the intervention arm used silicone elastomer vaginal matrix rings containing 25 mg of dapivirine. The women were instructed to wear the ring for the entire month and then replace it with a new ring at the monthly follow‐up visit.
In two trials that used 1% tenofovir (Abdool Karim 2010; Delany‐Moretlwe 2018), the women were instructed to insert one gel intravaginally in the 12 hours before vaginal sex and a second gel as soon as possible after the intercourse, with no more than two doses in a 24‐hour period. In the third tenofovir study (Marrazzo 2015), the gel was inserted up to one hour before each episode of vaginal intercourse. The gels were provided in prefilled applicators for single use.
For the other five types of vaginal microbicide gels that were used (cellulose sulphate (Halpern 2008; Van Damme 2008), SAVVY (Feldblum 2008; Peterson 2007), PRO 2000 (Abdool Karim 2011; McCormack 2010), BufferGel (Abdool Karim 2011), Carraguard (Skoler‐Karpoff 2008)), women were instructed to insert the contents of the applicator of their assigned gel into the vagina within the hour preceding each act of vaginal intercourse. All the gels were provided in prefilled applicators for single use. Follow‐up visits were on a monthly basis, and during the visits gels were re‐supplied to the women.
Comparators
All included trials used identical placebo. The two vaginal ring trials used identical placebo rings. The latter were flexible platinum‐catalysed‐cured silicone matrix rings, which contained no active drug. The microbicide gel studies used identical placebo gels. All the gels were similar to their respective microbicides in terms of appearance, packaging, and administration. All included studies provided condoms to both intervention and comparison arms. We did not find studies that had used condom, diaphragm, vaginal sponge, and cervical cap or not intervention.
Outcomes
All trials reported HIV incidence as the primary outcome. Except for four trials (Delany‐Moretlwe 2018; Feldblum 2008; Peterson 2007: Van Damme 2008), all studies reported other STIs as outcomes. However, they differed in the types of STIs reported. Other outcomes reported by individual trials included safety (Abdool Karim 2010; Abdool Karim 2011Baeten 2016: Delany‐Moretlwe 2018; Marrazzo 2015; ; Skoler‐Karpoff 2008), adherence (Baeten 2016; Delany‐Moretlwe 2018; Marrazzo 2015; Nel 2016), Other outcomes that were reported are: drug concentration (Baeten 2016; Marrazzo 2015; Nel 2016), drug resistance (Marrazzo 2015; Nel 2016), rates of pregnancy (Nel 2016), pharmacokinetics and delayed seroconversion (Delany‐Moretlwe 2018; Marrazzo 2015).
Length of follow up
In all trials, monthly follow‐up visits were scheduled for at least 12 months. During follow‐up visits, the participants were provided with comprehensive HIV prevention services (HIV pre‐ and post‐test counselling, HIV risk reduction counselling, condoms, and STI treatment) and reproductive health services, and assigned gels or rings as required.
Excluded studies
See: Characteristics of excluded studies.
We excluded 10 new studies for this update, making a total of 14 excluded studies. The most common reason for exclusion was that microbicide used in the study was nonoxynol‐9 which is not included this Cochrane review (12 studies), (Artz 2005; Barbone 1990; Cutler 1997; Ettiègne‐Traoré 1997; Kreiss 1992; Louv 1998; Niruthisard 1992; Roddy 1988; Rendon 1980; Rosenberg 1987a; Rosenberg 1987b; Sacks 1990). See Wilkinson 2002a and Wilkinson 2002b. One study used an in‐vitro or animal study (Zaneveld 2002) and one other study included HIV‐positive participants (Van der Straten 2007) (see Characteristics of excluded studies table).
Risk of bias in included studies
See below the risk of bias in included studies by domain.
Allocation
Random sequence generation
All trials adequately reported the random process of sequence generation, for example, random number table or computer random number generator, making selection bias unlikely.
Allocation concealment
For allocation concealment central randomisation was used in eight trials (Abdool Karim 2010; Abdool Karim 2011; Baeten 2016; Delany‐Moretlwe 2018; Marrazzo 2015; McCormack 2010; Nel 2016; Skoler‐Karpoff 2008) and sequentially‐numbered, sealed opaque envelopes in four (Feldblum 2008; Halpern 2008; Peterson 2007; Van Damme 2008). We judged the risk of bias to be low in all trials.
Blinding
Participants, trial personnel, and outcome assessors were blind to treatment allocation in all trials. In these trials, the intervention and placebo were identical in appearance and packaging. We therefore judged the risk of performance bias (blinding of participants and study personnel) and detection bias (blinding of outcome assessors) to be low in all trials.
Incomplete outcome data
All the included trials addressed completeness of outcome data adequately by providing information on participant dropout which was equally distributed between the intervention groups and also reported intention to treat data. The level of missing data in 10 trials (Abdool Karim 2010; Abdool Karim 2011; Baeten 2016; Delany‐Moretlwe 2018; Marrazzo 2015; McCormack 2010; Nel 2016; Peterson 2007; Skoler‐Karpoff 2008; Van Damme 2008) was less than 20% and the attrition probably was not related to the outcomes, making attrition bias unlikely. We thus considered these trials to be at low risk of attrition bias. In two trials (Halpern 2008; Feldblum 2008) loss to follow‐up was more than 20%, so we judged the risk of attrition bias as high in these two trials.
Selective reporting
Eleven trials reported outcomes as stated in the respective trial protocols, and we judged these to have low risk of reporting bias. However, one trial (Delany‐Moretlwe 2018) described the primary outcomes but stated that the other outcomes will be reported elsewhere, so we judged the risk of bias of selective reporting as unclear in this trial.
Other potential sources of bias
One trial has a potential source of bias as it was funded by a pharmaceutical company (McCormack 2010), but we do not think that necessarily introduced bias. The remaining 11 trials appeared to be free from other sources of bias.
Summary of risk of bias
Our judgements about the risk of bias in each included trial are summarised in Figure 2 and Figure 3. Overall, we judged nine trials to be at low risk of bias, one trial to be at moderate risk of bias (Delany‐Moretlwe 2018), and two to be at high risk of bias (Halpern 2008; Feldblum 2008).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We could not formally evaluate the risk of publication bias, because there were too few included trials in each comparison.
Effects of interventions
See: Table 1; Table 2; Table 3
Overall quality of the body of evidence: Summary of findings table
See: Table 1 Dapirine vaginal microbicide compared to placebo for preventing sexually transmitted infections; Table 2 Tenofovir vaginal microbicide for preventing sexually transmitted infections; Table 3 Cellulose sulphate vaginal microbicide for preventing sexually transmitted infections.
1. Primary outcomes
1.1. Laboratory confirmed incidence of viral sexually transmitted infections
1.1.1. HIV infection
Dapivirine vaginal ring (Baeten 2016; Nel 2016) probably decreases the risk of acquisition of HIV infection when compared to placebo (RR 0.71, 95% CI 0.57 to 0.89; I2 = 0%; 2 trials, 4588 women; moderate‐certainty evidence; Analysis 1.1). Our only concern with the data is that only two studies have so far assessed this microbicide (Table 1).
1.1. Analysis.

Comparison 1: Topical microbicide versus placebo ‐ dichotomous data, Outcome 1: HIV
Tenofovir vaginal gel (Abdool Karim 2010; Delany‐Moretlwe 2018; Marrazzo 2015; ) may not lead to any difference in the risk of HIV acquisition, compared to placebo (RR 0.83, 95% CI 0.68 to 1.02, I2 = 31%; 3 trials, 4958 women; low‐certainty evidence; Analysis 1.1). Our main concern with this evidence is imprecision, as the effect of tenofovir on HIV acquisition ranged from a large clinical benefit to a small increase in harm (Table 2).
None of the other microbicides tested showed evidence of an effect on the risk of acquiring HIV acquisition (Analysis 1.1):
BufferGel (Abdool Karim 2011): RR 1.05, 95% CI 0.73 to 1.52; 1 trial, 1546 women;
Carraguard (Skoler‐Karpoff 2008): RR 0.89, 95% CI 0.71 to 1.11; 1 trial, 6202 women;
Cellulose sulphate (Halpern 2008; Van Damme 2008): RR 1.20, 95% CI 0.74 to 1.95; I2 = 43%; 2 trials 3069 women;
PRO 2000 (Abdool Karim 2011; McCormack 2010): RR 0.93, 95% CI 0.77 to 1.14; I2 = 55%; 2 trials, 8191 women;
SAVVY (Feldblum 2008; Peterson 2007): RR 1.38, 95% CI 0.79 to 2.41; I2 = 22%; 2 trials, 4295 women.
1.1.2. Herpes simplex virus infection
Four studies reported herpes simplex virus (HSV) incidence (Abdool Karim 2010; Marrazzo 2015; McCormack 2010; Van Damme 2008). It is uncertain whether cellulose sulphate improved the incidence of HSV compared to placebo (RR 0.99, 95% CI 0.37 to 2.62; 1 trial, 1425 participants; very low‐certainty evidence; Analysis 1.2) or for 0.5% PRO 2000 (RR 0.95, 95% CI 0.73 to 1.23; 1 trial, 6651 participants; very low‐certainty evidence; Analysis 1.2). Two studies reported data on the effect of tenofovir. One study with 224 participants suggests that tenofovir may reduce the risk of getting HSV (RR 0.55, 95% CI 0.36 to 0.82; Abdool Karim 2010) but the other study found that it probably makes little or no difference in preventing this infection (RR 0.94, 95% CI 0.85 to 1.03; 1003 participants; Marrazzo 2015). We have not reported the pooled result because of substantial heterogeneity of effect (l2 = 85%; Analysis 1.2). Our concerns with the evidence were considerable unexplained heterogeneity for tenofovir (Table 2) and imprecision for the other microbicides (Table 3). The two dapivirine studies (Baeten 2016; Nel 2016 did not screen for HSV (Table 1).
1.2. Analysis.

Comparison 1: Topical microbicide versus placebo ‐ dichotomous data, Outcome 2: Herpes simplex
1.1.3 Condyloma acuminatum
We are uncertain whether the effect of cellulose sulphate on the risk of acquisition of condylomata acuminatum is different from that of placebo (RR 3.46, 95% CI 0.72 to 16.58; 1 trial (Van Damme 2008), 1425 women; very low‐certainty evidence; Analysis 1.3). Our main concern was substantial imprecision of the effect estimate.
1.3. Analysis.

Comparison 1: Topical microbicide versus placebo ‐ dichotomous data, Outcome 3: Condyloma acuminatum
1. 1.4 High‐risk Human papillomavirus
The risk of acquiring HPV was reported by a sub‐study of the Carraguard trial (Skoler‐Karpoff 2008) involving 1718 women. At the end of the study the incidence of high‐risk HPV infection was 23.5% in women on Carraguard and 23.0% in the placebo arm (RR 1.02, 95% CI 0.86 to 1.21; very low‐certainty evidence; Analysis 1.4). Our main concerns were imprecision and study limitations.
1.4. Analysis.

Comparison 1: Topical microbicide versus placebo ‐ dichotomous data, Outcome 4: High‐risk HPV
1.2. Laboratory confirmed incidence of bacterial sexually transmitted infections
1.2.1 Gonorrhoea infection
Seven trials reported gonorrhoea incidence, finding no evidence of a difference in effects between the microbicide and placebo groups (Analysis 1.5): Carraguard (RR 1.06, 95% CI 0.88 to 1.27; 1 trial, 6202 women; low‐certainty evidence); cellulose sulphate (RR 0.89, 95% CI 0.67 to 1.17; l2 = 50%; 2 trials, 3069 women; low‐certainty evidence); PRO 2000 (RR 1.15, 95% CI 0.87 to 1.52; I2 = 45%; 2 trials, 8191 women; low‐certainty evidence); BufferGel (RR 0.99, 95% CI 0.51 to 1.93; 1 trial, 1546 women; low‐certainty evidence); tenofovir (RR 0.66, 95% CI 0.40 to 1.10; 1 trial, 2010 women; low‐certainty evidence); dapivirine (RR 1.00, 95% CI 0.87 to 1.15; 2 trials, 4586 women; low‐certainty evidence). Our main concern was very serious imprecision of effects, as most of the findings range from large beneficial effects to large harmful effects (Table 1; Table 2: Table 3).
1.5. Analysis.

Comparison 1: Topical microbicide versus placebo ‐ dichotomous data, Outcome 5: Gonorrhoea
1.2.2. Chlamydia trachomatis
Eight trials reported Chlamydia trachomatis incidence, finding no evidence of a difference in effects between microbicide and placebo groups, except for cellulose sulphate. The results suggest that cellulose sulphate may reduce the risk of acquiring chlamydia infection (RR 0.70, 95% CI 0.49 to 0.99; I2 = 0%; 2 trials, 3069 women; low‐certainty evidence; Analysis 1.6). There was no evidence of an effect for other microbicides: BufferGel (RR 0.97, 95% CI 0.65 to 1.45; 1 trial, 1546 women; low‐certainty evidence; Analysis 1.6); Carraguard (RR 0.96, 95% CI 0.83 to 1.12; 1 trial, 6202 women; low‐certainty evidence; Analysis 1.6); dapivirine (RR 0.97, 95% CI 0.89 to 1.07; I2 = 0%; 2 trials, 4586 women; moderate‐certainty evidence; Analysis 1.6); PRO 2000 (RR 1.01, 95% CI 0.84 to 1.22; I2 = 0%; 2 trials, 8191 women; low‐certainty evidence; Analysis 1.6), and tenofovir (RR 0.90, 95% CI 0.71 to 1.13; 1 trial, 2010 women; low‐certainty evidence; Analysis 1.6). Our main concern was substantial imprecision in the effect estimates (Table 1,Table 2, and Table 3
1.6. Analysis.

Comparison 1: Topical microbicide versus placebo ‐ dichotomous data, Outcome 6: Chlamydia
1.2.3. Syphilis
From data reported by four trials (Marrazzo 2015; Nel 2016; Skoler‐Karpoff 2008; Van Damme 2008), the effects of the following microbicides on the risk of syphilis acquisition may not be different from those of placebo: Carraguard (RR 1.07, 95% CI 0.75 to 1.52; 1 trial, 6202 women; low‐certainty evidence; Analysis 1.7.1); cellulose sulphate (RR 0.69, 95% CI 0.26 to 1.81; 1 trial, 1425 women; low‐certainty evidence; Analysis 1.7); dapivirine (RR 1.70, 95% CI 0.63 to 4.59: 1 trial, 1956 women; low‐certainty evidence; Analysis 1.7); and tenofovir (RR 1.27, 95% CI 0.58 to 2.78; 1 trial, 2010 women; low‐certainty evidence; Analysis 1.7). We downgraded the evidence for each microbicide to low because of substantial imprecision with the effect estimates (Table 1; Table 2: Table 3).
1.7. Analysis.

Comparison 1: Topical microbicide versus placebo ‐ dichotomous data, Outcome 7: Syphilis
1.3. Laboratory confirmed incidence of protozoan sexually transmitted infections
1.3.1. Trichomoniasis
Six trials reported the effect of microbicides on the risk of acquiring trichomoniasis (Abdool Karim 2011; Baeten 2016; Marrazzo 2015; Nel 2016; Skoler‐Karpoff 2008; Van Damme 2008). Dapirivine may not lead to any difference in the risk of trichomoniasis acquisition, compared to placebo (RR 1.06, 95% CI 0.92 to 1.23; I2 = 0%: 2 trials, 4588 participants; low‐certainty evidence; Analysis 1.8). The effect of BufferGel (RR 0.96, 95% CI 0.80 to 1.15; 1 trial, 1546 women; low‐certainty evidence; Analysis 1.8); Carraguard (RR 0.85, 95% CI 0.72 to 1.01; 1 trial, 6202 women; low‐certainty evidence; Analysis 1.8); cellulose sulphate (RR 0.96, 95% CI 0.62 to 1.49; 1 trial, 1425 women; low‐certainty evidence; Analysis 1.8); PRO 2000 (RR 1.18, 95% CI 0.99 to 1.39; 1 trial, 1546 women; low‐certainty evidence; Analysis 1.8); and tenofovir (RR 1.21, 95% CI 0.84 to 1.74; 2010 women; low‐certainty evidence; Analysis 1.8) may not be different from that of placebo. We rated the evidence for each of these microbicides as low because of substantial imprecision in the effect estimates (Table 1; Table 2; Table 3).
1.8. Analysis.

Comparison 1: Topical microbicide versus placebo ‐ dichotomous data, Outcome 8: Trichomoniasis
1.4. Serious adverse events
All included microbicide trials reported serious adverse events which included death and hospitalisation. There was no clear evidence of a difference between microbicides used and placebo in the rate of serious adverse events (dapivirine vaginal ring, RR 1.12, 95% CI 0.94 to 1.32; I2 = 87%; 2 trials, 4588 women; tenofovir vaginal gel, RR 1.00, 95% CI 0.81 to1.24; I2 = 45%; 3 trials, 4958 women; BufferGel vaginal gel, RR 1.29, 95% CI 0.81 to 2.06;1 trial, 1546 women; Carraguard vaginal gel, RR 0.92, 95% CI 0.67to 1.27; 1 trial, 6202 women: cellulose sulphate vaginal gel, RR 1.25, 95% CI 0.87 to 1.79: I2 = 0; 2 trials, 3069 women: PRO 2000 vaginal gel, RR 1.18, 95% CI 0.96 to 1.46; I2 = 0; 2 trials, 8191 women; and SAVVY vaginal gel, RR 1.18, 95% CI 0.96 to 1.46; I2 = 67%; 2 trials, 4295 women, low‐certainty evidence (Analysis 1.9)
1.9. Analysis.

Comparison 1: Topical microbicide versus placebo ‐ dichotomous data, Outcome 9: Serious Adverse Events
2. Secondary outcomes
2.1. Acceptability
One study reported vaginal microbicide gel acceptability to be high, with 99% of women stating at study exit that they would use a microbicide gel if it were found to be effective (Abdool Karim 2011).
2.2. Minor adverse events
All trials reported minor adverse events with substantial variation in the number and type of events.Those reported by both dapivirine trials (Baeten 2016; Nel 2016) included urogenital symptoms (vulvovaginal candidiasis, menorrhagia, menometrorrhagia, bacterial vaginosis, cervical dysplasia,procedural, metrorrhagia, urinary tract infection, pelvic and procedural pain) upper respiratory tract infections and malaria, Additional common events reported by individual dapivirine trials were vaginal discharge gastroenteritis, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) increase, abdominal loss of weight, genitourinary chlamydia infection, vaginal pruritis, genitourinary tract gonococcal infection, haemoglobin decrease, neutrophil count increase (Baeten 2016) vulvovaginitis, viral rhinitis, nasopharyngitis, dysmenorrhoea, diarrhoea, myalgia, arthralgia.and headache (Nel 2016).
Events reported by all the three tenofovir trials (Abdool Karim 2010; Delany‐Moretlwe 2018; Marrazzo 2015) included vaginal discharge, vaginal candidiasis, vulvovaginitis, headache, urinary tract infections, disrupted epithelium e.g. genital ulceration, elevated AST) and ALT, gastrointestinal disorders (nausea, diarrhoea, abdominal pain, vomiting), anaemia and hypophosphataemia. Additional common events reported by at least two tenofovir trials were upper respiratory tract infections (Abdool Karim 2010; Delany‐Moretlwe 2018), neutropenia, and decreased bone density (Abdool Karim 2010; Marrazzo 2015). The other common events reported by individual trials included influenza, erythema, menorrhagia, raised creatinine, low potassium,raised sodium, fractures (Abdool Karim 2010), amenorrhoea,intermenstrual bleeding, vulvovaginal itching, reduced creatinine clearance (Delany‐Moretlwe 2018), genitourinary chlamydia infection, bacterial vaginitis, pelvic inflammatory disease, cervicitis, abnormal loss of weight, dizziness, migraine, haemorrhage in pregnancy, post abortion haemorrhage, proteinuria, dysuria, glycosuria, haematuria, pollakiuria cervical dysplasia, pelvic pain, menometrorrhagia, dysmenorrhoea, pelvic pain, and metrorrhagia (Marrazzo 2015).
For BufferGel trial (Abdool Karim 2011), events reported were vaginal discharge, vulvovaginal pruritis, metrorrhagia, cervical or uterine haemorrhage, menorrhagia, genital irritation events, abnormal genital bleeding, urinary tract infections, genital pain, intermenstrual bleeding, pregnancy events and coagulation abnormalities.
For Carraguard trial (Skoler‐Karpoff 2008), the following events were reported; vaginal discharge, dysmenorrhoea, genital pruritis, lower abdominal pain, injury, poisoning, and procedural complications, findings with disrupted epithelium, abnormal Papanicolaou smear and pregnancy outcomes (termination, live and still birth).
Events reported by both cellulose sulphate trials (Halpern 2008; Van Damme 2008) were bacterial vaginitis, genital pruritis, genital candidiasis, and vaginal discharge. Additional common events reported by individual trials included, malaria, abdominal pain, headache, pyrexia, respiratory tract infection, menstrual disorder (Halpern 2008); cervicitis, pelvic inflammatory disease, urinary tract infection, metrorrhagia pelvic pain genital ulceration and menorrhagia (Van Damme 2008).
Both PRO 2000 trials (Abdool Karim 2011; McCormack 2010) reported abnormal genital bleeding. However, additional events reported by individual trials included vaginal discharge, vulvovaginal pruritis, metrorrhagia, cervix haemorrhage uterine, menorrhagia, genital irritation events, urinary tract infections, genital pain, intermenstrual bleeding, pregnancy events and coagulation abnormalities (Abdool Karim 2011); ulcers, oedema and erythema (McCormack 2010).
Both SAVVY trials (Feldblum 2008; Peterson 2007) reported vaginal discharge, vaginal candidiasis, bacterial vaginitis, trichomonas vulvovaginitis, genital pruritis, irregular menstruation, gastrointestinal disorders, pregnancy, puerperium and perinatal conditions, renal and urinary disorders. Additional events reported by one study (Feldblum 2008) included dysmenorrhoea and pelvic inflammatory disease.
Other analyses
Subgroup analyses
There were insufficient studies to do planned subgroup analyses other than by the type of microbicide.
Sensitivity analyses
The method of meta‐analysis did not have an impact on the results.
Discussion
Summary of main results
We include in this updated review 12 randomised trials of seven different vaginal microbicides (BufferGel, Carraguard, cellulose sulphate, dapivirine, PRO 2000, SAVVY, and tenofovir), involving 32,849 sexually active women who have sex with men, conducted in 11 countries. All trials compared the use of microbicide versus placebo. No currently‐available trials have assessed the effect of topical microbicides during anal sex. There was moderate‐certainty evidence that dapivirine was superior to placebo in reducing the risk of HIV acquisition. There was clear evidence of an effect between any of the other microbicides and placebo in the risk of HIV acquisition. Two trials compared tenofovir to placebo, and provide low‐certainty evidence that tenofovir may slightly reduce the risk of acquiring herpes simplex virus type 2 infection. Two other trials provide low‐certainty evidence that cellulose sulphate may decrease the risk of acquisition of chlamydia infection. Our confidence in the evidence between any of the other microbicides and placebo in the risk of acquisition of STIs is very low, providing an indication of the unlikely effect.
Overall completeness and applicability of evidence
We conducted comprehensive searches to identify both published and unpublished RCs. In addition to two trial registers, we searched eight databases with no language restrictions, reducing the risk of publication bias (Egger 1997a, Egger 1997b, Moher 1996). We identified 12 trials published between 2007 and 2018 that examined effectiveness of four classes of microbicides with different mechanisms of action; surfactants (SAVVY), vaginal defence enhancers (Buffergel), entry inhibitors (carraguard, PRO 2000, cellulose suphate) and antiretroviral based microbidices (tenofovir and dapivirine). Being in gel form, the microbicides were packaged in single‐use microlax‐type applicators, filled to be dispensed vaginally before every act of vaginal intercourse.
Baseline factors appeared well balanced for all trials thus unlikely to cause any differences in outcome between the treatment groups. All trials used a defined range of age limits for inclusion criteria of the participants, thus providing a clear picture of the full range of ages. Ten trials reported including participants aged 18 years and over. One trial stated a lower age limit of 18 years old in their inclusion, but reported to have included participants of a lower age limit of 15 years old (McCormack 2010). One other trial included in the review had a lower age limit of 16 years old in their age criteria (Skoler‐Karpoff 2008). All trials except two (McCormack 2010; Skoler‐Karpoff 2008) presented the median age for the participants. This ranged between 23 and 26 years in 10 trials. One trial presented a higher median age (28 years one treatment group and 29 years in the other (Van Damme 2008).
This review has several limitations in relation to the applicability of this evidence. HIV infection was the primary outcome in all the studies, but despite including 12 trials we could not pool the data due to the microbicides' different mechanisms of action, limiting data to perform meta‐analyses. Except for one trial that that had a study site in the USA (Abdool Karim 2011) the others were conducted in low and middle‐income countries, mainly in Africa, and the duration of follow up varied between trials. Because of the relatively low incidence of HIV infection among women in high‐income countries, effectiveness studies for this intervention may be limited predominantly in low‐income country sites,
Six of the 12 trials were stopped early due to safety concerns (Feldblum 2008: Halpern 2008; Marrazzo 2015; McCormack 2010; Peterson 2007; Van Damme 2008), indicating that surrogates for clinical safety of microbicides still need to be better defined.
The studies included in this review did not report all STIs. Only five trials assessed the effect of vaginal microbicides on other viral STIs Abdool Karim 2010; Marrazzo 2015; McCormack 2010; Skoler‐Karpoff 2008; Van Damme 2008). Eight trials assessed vaginal microbicide effects on gonorrhoea (Abdool Karim 2011; Baeten 2016; Halpern 2008; Marrazzo 2015; McCormack 2010; Nel 2016; Skoler‐Karpoff 2008; Van Damme 2008), eight on chlamydia (Abdool Karim 2011; Baeten 2016; Halpern 2008; Marrazzo 2015; McCormack 2010; Nel 2016; Skoler‐Karpoff 2008; Van Damme 2008), four on syphilis (Marrazzo 2015; Nel 2016; Skoler‐Karpoff 2008; Van Damme 2008) and six on trichomoniasis (Abdool Karim 2011; Baeten 2016; Marrazzo 2015; Nel 2016; Skoler‐Karpoff 2008; Van Damme 2008). Like in the case of HIV infections, data were insufficient to perform meta‐analyses for most STI outcomes due microbicide classification based on the mechanisms of action.
There was no trial that reported on the effect of microbicides on HIV or STI acquisition among men having sex with men.
The most promising intervention for preventing HIV and HSV infection were dapivirine and tenofovir respectively, with both trial sites being limited to Africa. In addition the relatively small sample size and few studies may restrict the broad generalisability of the finding that dapivirine vaginal ring reduces the risk of HIV acquisition (Baeten 2016; Nel 2016) and tenofovir reduces the risk of HSV acquisition (Abdool Karim 2010; Marrazzo 2015; Delany‐Moretlwe 2018) in sexually‐active women.
Quality of the evidence
We considered all 12 included trials to be at low risk of bias. These studies corresponded to the comparison of any microbicide versus placebo. For the comparison of the microbicide dapivirine versus placebo, our confidence in the effect estimate for HIV acquisition was reduced to moderate because only two studies have so far assessed this microbicide. Our confidence in the effect estimate of dapivirine on other STIs as well as the effect estimate of tenofovir and other microbicides on HIV and other STIs was low to very low, due to serious imprecision (i.e. few participants and few outcome events leading to effect estimates with very wide confidence intervals) and inconsistency (unexplained variability in some results) (Table 1; Table 2; Table 3).
Potential biases in the review process
This systematic review has many strengths regarding the review process: we adhered to the predefined objectives and study eligibility criteria; our literature search included an appropriate range of databases and sources, including relevant additional methods to identify relevant reports and retrieve as many eligible studies as possible; and we assessed all studies adequately for risk of bias. Two review authors independently screened the search results, selection of studies, and extraction of data. Intention‐to‐treat analysis was reported for all trials making incomplete data bias unlikely for this review. However, we had some concerns about publication bias. Publication bias is a possibility due to the limited number of trials for each comparison. Also, being an updated version, the review cannot be done blinded to the knowledge of the previous outcomes hence the potential to unconscious biasness.
Agreements and disagreements with other studies or reviews
Few systematic reviews have assessed the effectiveness of vaginal microbicides for for preventing STIs including HIV. There are four previous systematic reviews published on this topic (Obiero 2012b; Musekiwa 2020; Wilkinson 2002a; Wilkinson 2002b). . Evidence from two published systematic reviews (Wilkinson 2002a; Wilkinson 2002b) showed that one vaginal microbicide gel, nonoxynol‐9, may be harmful by increasing the risk of STI including HIV acquisition. In one systematic review, Musekiwa and colleagues searched two databases for studies published up to May 2019 on the effectiveness of microbicides to prevent HIV transmission (Musekiwa 2020), and reported lack of effect of early non‐HIV‐specific microbicides. Our current findings are consistent with previously‐published reviews, that topical microbicide research has had disappointing outcomes over a long period (Obiero 2012b). The authors of a recent systematic (Musekiwa 2020) reported moderate certainty of evidence supporting effectiveness of the intravaginal ring containing dapivirine. Our review update supports these results; a vaginal microbicide ring that contains the non‐nucleoside reverse‐transcriptase inhibitor dapivirine was found to be effective in reducing the risk of HIV acquisition. To the best of our knowledge, our review is the most comprehensive synthesis of existing evidence on topical microbicides for prevention of HIV infection and other STIs.
Authors' conclusions
Implications for practice.
Current evidence shows that vaginal dapivirine microbicide probably reduces HIV acquisition in heterosexual women. Due to the very low quality of the evidence, the effects of tenofovir in the management of HSV and cellulose sulphate in the management of chlamydia infection are uncertain. Our review does not consider the use of any vaginal microbicide for preventing STIs.
Implications for research.
Further high‐quality trials are needed to confirm the beneficial effects of microbicides containing dapivirine and tenofovir in vaginal sex. In addition, high‐quality research should continue on the development and testing of new topical microbicides. These trials should aim to explore the challenges experienced in the previous microbicide trials that led to the premature termination of some of the studies. Also, trials should aim to explore strategies for combining agents with different mechanisms of action to achieve synergistic or additive effects, such as maximized activity and broader spectrum of activity against STIs. As the evidence base grows, it would be helpful to conduct a network meta‐analysis to estimate the relative effectiveness and safety of the competing microbicide options.
What's new
| Date | Event | Description |
|---|---|---|
| 22 August 2020 | New citation required and conclusions have changed | We included four new studies (Baeten 2016; Delany‐Moretlwe 2018; Marrazzo 2015; Nel 2016) |
| 22 August 2020 | New search has been performed | Search updated in August 2020 |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 6, 2012
| Date | Event | Description |
|---|---|---|
| 19 July 2016 | New search has been performed | Contact author affiliations updated. |
Acknowledgements
For the update of this review, Jael Obiero was supported by a fellowship from the Effective Health Care Research Consortium (Grant 5242), administered by Cochrane South Africa at the South African Medical Research Council. Charles Shey Wiysonge's work is sponsored by the South African Medical Research Council and the National Research Foundation of South Africa (Grant Numbers: 106035 and 108571).
Appendices
Appendix 1. Search conducted in June 2015, STI Review Group
MEDLINE
| Search electronic report #1 | |
| Search type | Update |
| Databases | § MEDLINE § MEDLINE In‐Process & Other Non‐Indexed Citations § MEDLINE Daily Update |
| Platform | Ovid |
| Search date | 06/06/2015 |
| Update date | Undefined |
| Range of search date | 2011‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1 microbicid$.tw. (5151) 2 antimicrobial.tw. (95662) 3 1 or 2 (100041) 4 vagin$.tw. (89605) 5 rectal$.tw. (67721) 6 rectum.tw. (30777) 7 anus.tw. (6110) 8 anal$.tw. (4313850) 9 topical$.tw. (79474) 10 local$.tw. (984656) 11 4 or 5 or 6 or 7 or 8 or 9 or 10 (5206760) 12 3 and 11 (30984) 13 exp Anti‐Infective Agents, Local/ (184098) 14 ((anti adj1 infective$) and topical).tw. (112) 15 ((anti adj1 infective$) and local).tw. (131) 16 (antiinfective$ adj5 topical).tw. (15) 17 (antiinfective$ adj5 local).tw. (3) 18 (mercurial adj5 antiseptic).tw. (4) 19 (antifung$ adj5 topical).tw. (934) 20 (antivir$ adj5 topical).tw. (235) 21 (antibacteri$ adj5 topical).tw. (327) 22 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (185583) 23 12 or 22 (214580) 24 exp Sexually transmitted diseases/ or exp Sexually transmitted diseases, bacterial/ or exp Chancroid/ or exp Chlamydia infections/ or exp Lymphogranuloma venereum/ or exp Gonorrhea/ or exp Granuloma inguinale/ or exp Syphilis/ or exp Neurosyphilis/ or exp Tabes dorsalis/ or exp Syphilis, latent/ (296934) 25 exp Sexually transmitted diseases, viral/ or exp Condylomata acuminata/ or exp Herpes genitalis/ (236362) 26 (std or stds or sti or stis).tw. (17322) 27 ((sex$ adj5 transmitted) or (sex$ adj5 transmiss$)).tw. (29368) 28 ((venereal adj5 disease$) or (venereal adj5 infection$) or (venereal adj5 disorder$)).tw. (4219) 29 ((genital adj5 disease$) or (genital adj5 infection$) or (genital adj5 disorder$)).tw. (9327) 30 exp Chlamydia/ or exp Chlamydia trachomatis/ (12934) 31 chlamydia$.tw. (23287) 32 exp Treponema pallidum/ or treponema pallidum.tw. or condylom$.tw. or syphili$.tw. (28184) 33 (lymphogranuloma venereum or lymphogranuloma inguinale).tw. (980) 34 (granuloma inguinale or granuloma venereum or donovanosis or donovania).tw. (532) 35 exp Neisseria gonorrhoeae/ (8484) 36 (gonorrh$ or gonococc$).tw. (19345) 37 (nongonococcal urethritis or non‐gonococcal urethritis or ngu).tw. (1170) 38 exp Herpesvirus 1, human/ or exp Herpesvirus 2, human/ or (hsv1 or hsv‐1 or hsv2 or hsv‐2 or (herpes adj5 genital$)).tw. (19961) 39 (human cytomegalovirus or human herpesvirus‐5 or hhv‐5 or hcmv).tw. (8204) 40 exp Candidiasis/ or exp Candidiasis, vulvovaginal/ or exp Candidiasis, invasive/ or exp Candidemia/ or exp Candida albicans/ (41445) 41 (candidiasis or ((vagina$ adj5 candidosis) or ((vulvovagina$ adj5 candidosis) or (vagina$ adj5 mycoses)))).tw. (12498) 42 exp Vaginitis/ or exp Trichomonas vaginitis/ or exp Trichomonas vaginalis/ or exp Vulvovaginitis/ or (vaginitis or trichomoniasis or vaginalis).tw. (17816) 43 exp Vaginosis, bacterial/ or exp Gardnerella vaginalis/ or (vaginosis adj5 bacterial).tw. (4149) 44 exp Balanitis/ or balanitis.tw. (1040) 45 ((venereal adj5 ulcer$) or (venereal adj5 wart$)).tw. (113) 46 ((genital$ adj5 ulcer$) or (anogenital adj5 ulcer$) or (anorectal adj5 ulcer$) or (penile adj5 ulcer$) or (penis adj5 ulcer$)).tw. (2658) 47 ((genital$ adj5 wart$) or (anogenital adj5 wart$) or (anorectal adj5 wart$) or (penile adj5 wart$) or (penis adj5 wart$)).tw. (2510) 48 exp Alphapapillomavirus/ or exp Human papillomavirus 6/ or exp Human papillomavirus 11/ or exp Human papillomavirus 16/ or exp Human papillomavirus 18/ or exp Human papillomavirus 31/ or exp Betapapillomavirus/ or exp Gammapapillomavirus/ (5276) 49 (human papillomavirus$ or hpv or recurrent respiratory papillomatosis or condyloma$).tw. (36622) 50 exp Acquired Immunodeficiency Syndrome/ or exp HIV/ (148557) 51 ((human adj5 immun$ adj5 virus) or hiv or (acquired adj5 immun$ adj5 deficiency adj5 syndrome) or aids or (acquired adj5 immun$ adj5 syndrome)).tw. (329466) 52 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 (564437) 53 randomized controlled trial.pt. (397466) 54 controlled clinical trial.pt. (89683) 55 randomized.ab. (323220) 56 placebo.ab. (163406) 57 clinical trials as topic.sh. (173305) 58 randomly.ab. (232387) 59 trial.ti. (139792) 60 53 or 54 or 55 or 56 or 57 or 58 or 59 (966570) 61 exp animals/ not humans.sh. (4057817) 62 60 not 61 (891384) 63 23 and 52 and 62 (941) 64 limit 63 to yr="2011 ‐Current" (244) |
| # of records identified | 244 |
| # of records without duplicates | 213 |
EMBASE
| Search electronic report #2 | |
| Search type | Update |
| Database | EMBASE |
| Platform | EMBASE.com |
| Search date | 06/06/2015 |
| Update date | Undefined |
| Range of search date | 2011‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1 1. 'microbicide'/exp 1,517 2. microbicid*:ab,ti 5,691 3. antimicrobial:ab,ti 125,515 4. or/1‐3 130,714 5. vagin*:ab,ti 118,442 6. rectal*:ab,ti 94,192 7. rectum:ab,ti 42,697 8. anus:ab,ti 8,115 9. anal*:ab,ti 5,404,464 10. topical*:ab,ti 103,913 11. local*:ab,ti 1,177,195 12. or/5‐11 6,465,683 13. 4 AND 12 42,322 14. 'topical antiinfective agent'/exp 278,026 15. (anti NEXT/1 infective*):ab,ti AND topical:ab,ti 143 16. (anti NEXT/1 infective*):ab,ti AND local:ab,ti 164 17. (antiinfective* NEAR/5 topical):ab,ti 17 18. (antiinfective* NEAR/5 local):ab,ti 12 19. (mercurial NEAR/5 antiseptic):ab,ti 4 20. (antifung* NEAR/5 topical):ab,ti 1,294 21. (antivir* NEAR/5 topical):ab,ti 265 22. (antibacteri* NEAR/5 topical):ab,ti 437 23. or/14‐22 279,950 24. 13 or 23 319,139 25. 'sexually transmitted disease'/exp OR 99,944 'chlamydiasis'/exp OR 'condyloma'/exp OR 'condyloma acuminatum'/exp OR 'condyloma latum'/exp OR 'genital herpes'/exp OR 'gonorrhea'/exp OR 'granuloma inguinale'/exp OR 'lymphogranuloma venereum'/exp OR 'syphilis'/exp OR 'secondary syphilis'/exp OR 'tabes dorsalis'/exp OR 'ulcus molle'/exp 26. std:ab,ti OR stds:ab,ti OR sti:ab,ti OR 22,872 stis:ab,ti 27. (sex* NEAR/5 transmitted):ab,ti OR (sex* NEAR/5 33,248 transmiss*):ab,ti 28. (venereal NEAR/5 disease*):ab,ti OR (venereal 4,349 NEAR/5 infection*):ab,ti OR (venereal NEAR/5 disorder*):ab,ti 29. (genital NEAR/5 disease*):ab,ti OR (genital 11,299 NEAR/5 infection*):ab,ti OR (genital NEAR/5 disorder*):ab,ti 30. 'chlamydia'/exp OR 'chlamydia trachomatis'/exp 22,663 31. chlamydia*:ab,ti 27,615 32. syphili*:ab,ti OR chancre*:ab,ti OR 30,274 condylom*:ab,ti 33. 'lymphogranuloma venereum':ab,ti OR 1,102 'lymphogranuloma inguinale':ab,ti 34. 'granuloma inguinale':ab,ti OR 'granuloma 590 venereum':ab,ti OR donovanosis:ab,ti OR donovania:ab,ti 35. 'neisseria gonorrhoeae'/exp 13,702 36. gonorrh*:ab,ti OR gonococc*:ab,ti 22,251 37. 'nongonococcal urethritis'/exp OR 1,967 (nongonococcal:ab,ti AND urethritis:ab,ti OR 'non gonococcal':ab,ti AND urethritis:ab,ti) OR ngu:ab,ti 38. 'herpes simplex virus 1'/exp OR 'herpes simplex 28,336 virus 2'/exp OR hsv1:ab,ti OR 'hsv 1':ab,ti OR hsv2:ab,ti OR 'hsv 2':ab,ti OR (herpes NEAR/5 genital*):ab,ti 39. human:ab,ti AND cytomegalovirus:ab,ti OR 5,528 human:ab,ti AND 'herpesvirus 5':ab,ti OR 'hhv 5':ab,ti OR hcmv:ab,ti 40. 'candidiasis'/exp OR 'vagina candidiasis'/exp OR 72,274 'genital candidiasis'/exp OR 'invasive candidiasis'/exp OR 'candidemia'/exp OR 'candida albicans'/exp 41. candidiasis:ab,ti OR (vagina* NEAR/5 16,572 candid*):ab,ti OR (vulvovagina* NEAR/5 candid*):ab,ti OR (vagina* NEAR/5 mycoses):ab,ti 42. 'gardnerella infection'/exp OR 'gardnerella 4,894 vaginalis'/exp OR (vaginosis NEAR/5 bacterial):ab,ti 43. 'vaginitis'/exp OR 'vulvovaginitis'/exp OR 25,299 'trichomoniasis'/exp OR 'trichomonas vaginalis'/exp OR vaginitis:ab,ti OR trichomoniasis:ab,ti OR vaginalis:ab,ti 44. 'balanitis'/exp OR balanitis:ab,ti 1,546 45. (genital* NEAR/5 wart*):ab,ti OR (anogenital 3,185 NEAR/5 wart*):ab,ti OR (anorectal NEAR/5 wart*):ab,ti OR (penile NEAR/5 wart*):ab,ti OR (penis NEAR/5 wart*):ab,ti 46. (genital* NEAR/5 ulcer*):ab,ti OR (anogenital 3,440 NEAR/5 ulcer*):ab,ti OR (anorectal NEAR/5 ulcer*):ab,ti OR (penile NEAR/5 ulcer*):ab,ti OR (penis NEAR/5 ulcer*):ab,ti 47. (venereal NEAR/5 ulcer*):ab,ti OR (venereal 131 NEAR/5 wart*):ab,ti 48. 'human papillomavirus':ab,ti OR hpv:ab,ti 41,347 49. 'alphapapillomavirus'/exp OR 'human 9,441 papillomavirus type 11'/exp OR 'human papillomavirus type 16'/exp OR 'human papillomavirus type 18'/exp OR 'human papillomavirus type 31'/exp OR 'human papillomavirus type 6'/exp OR 'betapapillomavirus'/exp OR 'gammapapillomavirus'/exp 50. 'human immunodeficiency virus'/exp OR 'acquired 246,676 immune deficiency syndrome'/exp 51. (human NEAR/5 immun* NEAR/5 virus):ab,ti OR 381,316 hiv:ab,ti OR (acquired NEAR/5 immun* NEAR/5 deficiency NEAR/5 syndrome):ab,ti OR aids:ab,ti OR (acquired NEAR/5 immun* NEAR/5 syndrome):ab,ti 52. or/25‐51 685,883 53. 'randomized controlled trial'/exp OR 'single 1,163,675 blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR random*:ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti NOT ('animal'/de OR 'animal experiment'/de OR 'nonhuman'/de NOT ('animal'/de OR 'animal experiment'/de OR 'nonhuman'/de AND 'human'/de)) 54. 24 and 52 and 53 903 55. 54 and AND [2011‐2015]/py 329 |
| # of records identified | 329 |
| # of records without duplicates | 329 |
The Cochrane Central Register of Controlled Trials (CENTRAL)
| Search electronic report #3 | |
| Search type | Update |
| Database | The Cochrane Central Register of Controlled Trials (CENTRAL) |
| Platform | Ovid |
| Search date | 06/06/2015 |
| Update date | Undefined |
| Range of search date | 2011‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1 microbicid$.tw. (231) 2 antimicrobial.tw. (3042) 3 1 or 2 (3262) 4 vagin$.tw. (7640) 5 rectal$.tw. (4982) 6 rectum.tw. (1016) 7 anus.tw. (131) 8 anal$.tw. (191113) 9 topical$.tw. (13692) 10 local$.tw. (30193) 11 4 or 5 or 6 or 7 or 8 or 9 or 10 (227088) 12 3 and 11 (1218) 13 exp Anti‐Infective Agents, Local/ (5912) 14 ((anti adj1 infective$) and topical).tw. (12) 15 ((anti adj1 infective$) and local).tw. (7) 16 (antiinfective$ adj5 topical).tw. (3) 17 (antiinfective$ adj5 local).tw. (0) 18 (mercurial adj5 antiseptic).tw. (0) 19 (antifung$ adj5 topical).tw. (112) 20 (antivir$ adj5 topical).tw. (21) 21 (antibacteri$ adj5 topical).tw. (43) 22 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (6089) 23 12 or 22 (7102) 24 exp Sexually transmitted diseases/ or exp Sexually transmitted diseases, bacterial/ or exp Chancroid/ or exp Chlamydia infections/ or exp Lymphogranuloma venereum/ or exp Gonorrhea/ or exp Granuloma inguinale/ or exp Syphilis/ or exp Neurosyphilis/ or exp Tabes dorsalis/ or exp Syphilis, latent/ (8193) 25 exp Sexually transmitted diseases, viral/ or exp Condylomata acuminata/ or exp Herpes genitalis/ (7140) 26 (std or stds or sti or stis).tw. (1071) 27 ((sex$ adj5 transmitted) or (sex$ adj5 transmiss$)).tw. (987) 28 ((venereal adj5 disease$) or (venereal adj5 infection$) or (venereal adj5 disorder$)).tw. (29) 29 ((genital adj5 disease$) or (genital adj5 infection$) or (genital adj5 disorder$)).tw. (533) 30 exp Chlamydia/ or exp Chlamydia trachomatis/ (288) 31 chlamydia$.tw. (1000) 32 exp Treponema pallidum/ or treponema pallidum.tw. or condylom$.tw. or syphili$.tw. (561) 33 (lymphogranuloma venereum or lymphogranuloma inguinale).tw. (6) 34 (granuloma inguinale or granuloma venereum or donovanosis or donovania).tw. (6) 35 exp Neisseria gonorrhoeae/ (162) 36 (gonorrh$ or gonococc$).tw. (1062) 37 (nongonococcal urethritis or non‐gonococcal urethritis or ngu).tw. (145) 38 exp Herpesvirus 1, human/ or exp Herpesvirus 2, human/ or (hsv1 or hsv‐1 or hsv2 or hsv‐2 or (herpes adj5 genital$)).tw. (582) 39 (human cytomegalovirus or human herpesvirus‐5 or hhv‐5 or hcmv).tw. (49) 40 exp Candidiasis/ or exp Candidiasis, vulvovaginal/ or exp Candidiasis, invasive/ or exp Candidemia/ or exp Candida albicans/ (807) 41 (candidiasis or ((vagina$ adj5 candidosis) or ((vulvovagina$ adj5 candidosis) or (vagina$ adj5 mycoses)))).tw. (807) 42 exp Vaginitis/ or exp Trichomonas vaginitis/ or exp Trichomonas vaginalis/ or exp Vulvovaginitis/ or (vaginitis or trichomoniasis or vaginalis).tw. (1026) 43 exp Vaginosis, bacterial/ or exp Gardnerella vaginalis/ or (vaginosis adj5 bacterial).tw. (450) 44 exp Balanitis/ or balanitis.tw. (25) 45 ((venereal adj5 ulcer$) or (venereal adj5 wart$)).tw. (2) 46 ((genital$ adj5 ulcer$) or (anogenital adj5 ulcer$) or (anorectal adj5 ulcer$) or (penile adj5 ulcer$) or (penis adj5 ulcer$)).tw. (102) 47 ((genital$ adj5 wart$) or (anogenital adj5 wart$) or (anorectal adj5 wart$) or (penile adj5 wart$) or (penis adj5 wart$)).tw. (219) 48 exp Alphapapillomavirus/ or exp Human papillomavirus 6/ or exp Human papillomavirus 11/ or exp Human papillomavirus 16/ or exp Human papillomavirus 18/ or exp Human papillomavirus 31/ or exp Betapapillomavirus/ or exp Gammapapillomavirus/ (139) 49 (human papillomavirus$ or hpv or recurrent respiratory papillomatosis or condyloma$).tw. (1204) 50 exp Acquired Immunodeficiency Syndrome/ or exp HIV/ (3294) 51 ((human adj5 immun$ adj5 virus) or hiv or (acquired adj5 immun$ adj5 deficiency adj5 syndrome) or aids or (acquired adj5 immun$ adj5 syndrome)).tw. (11859) 52 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 (18532) 53 23 and 52 (533) 54 limit 53 to yr="2011 ‐Current" (122) |
| # of records identified | 122 |
| # of records without duplicates | 122 |
LILACS
| Search electronic report #4 | |
| Search type | Update |
| Database | LILACS http://lilacs.bvsalud.org/es/ |
| Platform | Biblioteca Virtual en Salud (BVS), interfaz iAHx |
| Search date | 06/06/2015 |
| Update date | Undefined |
| Range of search date | None |
| Language restrictions | None |
| Other limits | None |
| Search strategy | (ab:(microbicide*)) OR (ti:(microbicid*)) AND (ab:(topical*)) OR (ti:(topical*)) AND db:("LILACS") RCTs filter: ((PT:"ensayo clinico controlado aleatorio" OR PT:"ensayo clinico controlado" OR PT:"estudio multicéntrico" OR MH:"ensayos clinicos controlados aleatorios como asunto" OR MH:"ensayos clinicos controlados como asunto" OR MH:"estudio multicéntricos como asunto" OR MH:"distribución aleatoria" OR MH:"método doble ciego" OR MH:"metodo simple‐ciego") OR ((ensaio$ OR ensayo$ OR trial$) AND (azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego OR ((simple$ OR single OR duplo$ OR doble$ OR double$) AND (cego OR ciego OR blind OR mask))) AND clinic$)) AND NOT (MH:animales OR MH:conejos OR MH:ratones OR MH:ratas OR MH:primates OR MH:perros OR MH:gatos OR MH:porcinos OR PT:"in vitro") |
| # of records identified | 1 |
| # of records without duplicates | 1 |
Web of Science
| Search electronic report #5 | |
| Search type | Update |
| Database | Web of Science webofscience.com/ |
| Platform | Thomson Reuters |
| Search date | 06/06/2015 |
| Update date | Undefined |
| Range of search date | 2009‐current |
| Language restrictions | None |
| Other limits | None |
| Search strategy | TS=(topical*) AND TS=(microbicid*) AND TI=(trial) Refinado por: Años de publicación: ( 2010 OR 2009 OR 2014 OR 2011 OR 2012 ) |
| # of records identified | 22 |
| # of records without duplicates | 22 |
| Search electronic report #6 | |
| Search type | New |
| Database | International Clinical Trials Registry Platform www.who.int/ictrp/ |
| Platform | ICTRP Portal |
| Search date | 06/06/2015 |
| Update date | Undefined |
| Range of search date | None |
| Language restrictions | None |
| Other limits | None |
| Search strategy | topical microbicid* |
| # of records identified | 1 |
| # of records without duplicates | 1 |
Clinicaltrials.gov
| Search electronic report #7 | |
| Search type | New |
| Database | Clinicaltrials.gov |
| Platform | Clinicaltrials.gov |
| Search date | 06/06/2015 |
| Update date | Undefined |
| Range of search date | None |
| Language restrictions | None |
| Other limits | None |
| Search strategy | "topical microbicide" |
| # of records identified | 11 |
| # of records without duplicates | 11 |
Appendix 2. Search conducted in October 2016, STI Review Group
MEDLINE
| Search electronic report #1 | |
| Search type | Update |
| Databases | § MEDLINE § MEDLINE In‐Process & Other Non‐Indexed Citations § MEDLINE Daily Update |
| Platform | Ovid |
| Search date | 28/10/2016 |
| Update date | Undefined |
| Range of search date | 2015‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1 microbicid$.tw. (5440) 2 antimicrobial.tw. (108016) 3 1 or 2 (112605) 4 vagin$.tw. (96782) 5 rectal$.tw. (73380) 6 rectum.tw. (32867) 7 anus.tw. (6555) 8 anal$.tw. (4824067) 9 topical$.tw. (86022) 10 local$.tw. (1068952) 11 4 or 5 or 6 or 7 or 8 or 9 or 10 (5783746) 12 3 and 11 (35770) 13 exp Anti‐Infective Agents, Local/ (205199) 14 ((anti adj1 infective$) and topical).tw. (127) 15 ((anti adj1 infective$) and local).tw. (141) 16 (antiinfective$ adj5 topical).tw. (13) 17 (antiinfective$ adj5 local).tw. (4) 18 (mercurial adj5 antiseptic).tw. (5) 19 (antifung$ adj5 topical).tw. (1058) 20 (antivir$ adj5 topical).tw. (246) 21 (antibacteri$ adj5 topical).tw. (362) 22 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (206850) 23 12 or 22 (240434) 24 exp Sexually transmitted diseases/ or exp Sexually transmitted diseases, bacterial/ or exp Chancroid/ or exp Chlamydia infections/ or exp Lymphogranuloma venereum/ or exp Gonorrhea/ or exp Granuloma inguinale/ or exp Syphilis/ or exp Neurosyphilis/ or exp Tabes dorsalis/ or exp Syphilis, latent/ (317377) 25 exp Sexually transmitted diseases, viral/ or exp Condylomata acuminata/ or exp Herpes genitalis/ (251459) 26 (std or stds or sti or stis).tw. (18943) 27 ((sex$ adj5 transmitted) or (sex$ adj5 transmiss$)).tw. (31926) 28 ((venereal adj5 disease$) or (venereal adj5 infection$) or (venereal adj5 disorder$)).tw. (4450) 29 ((genital adj5 disease$) or (genital adj5 infection$) or (genital adj5 disorder$)).tw. (9901) 30 exp Chlamydia/ or exp Chlamydia trachomatis/ (13730) 31 chlamydia$.tw. (24536) 32 exp Treponema pallidum/ or treponema pallidum.tw. or condylom$.tw. or syphili$.tw. (30303) 33 (lymphogranuloma venereum or lymphogranuloma inguinale).tw. (1081) 34 (granuloma inguinale or granuloma venereum or donovanosis or donovania).tw. (564) 35 exp Neisseria gonorrhoeae/ (8952) 36 (gonorrh$ or gonococc$).tw. (20333) 37 (nongonococcal urethritis or non‐gonococcal urethritis or ngu).tw. (1216) 38 exp Herpesvirus 1, human/ or exp Herpesvirus 2, human/ or (hsv1 or hsv‐1 or hsv2 or hsv‐2 or (herpes adj5 genital$)).tw. (20819) 39 (human cytomegalovirus or human herpesvirus‐5 or hhv‐5 or hcmv).tw. (8724) 40 exp Candidiasis/ or exp Candidiasis, vulvovaginal/ or exp Candidiasis, invasive/ or exp Candidemia/ or exp Candida albicans/ (44240) 41 (candidiasis or ((vagina$ adj5 candidosis) or ((vulvovagina$ adj5 candidosis) or (vagina$ adj5 mycoses)))).tw. (13368) 42 exp Vaginitis/ or exp Trichomonas vaginitis/ or exp Trichomonas vaginalis/ or exp Vulvovaginitis/ or (vaginitis or trichomoniasis or vaginalis).tw. (18916) 43 exp Vaginosis, bacterial/ or exp Gardnerella vaginalis/ or (vaginosis adj5 bacterial).tw. (4436) 44 exp Balanitis/ or balanitis.tw. (1099) 45 ((venereal adj5 ulcer$) or (venereal adj5 wart$)).tw. (114) 46 ((genital$ adj5 ulcer$) or (anogenital adj5 ulcer$) or (anorectal adj5 ulcer$) or (penile adj5 ulcer$) or (penis adj5 ulcer$)).tw. (2855) 47 ((genital$ adj5 wart$) or (anogenital adj5 wart$) or (anorectal adj5 wart$) or (penile adj5 wart$) or (penis adj5 wart$)).tw. (2725) 48 exp Alphapapillomavirus/ or exp Human papillomavirus 6/ or exp Human papillomavirus 11/ or exp Human papillomavirus 16/ or exp Human papillomavirus 18/ or exp Human papillomavirus 31/ or exp Betapapillomavirus/ or exp Gammapapillomavirus/ (6227) 49 (human papillomavirus$ or hpv or recurrent respiratory papillomatosis or condyloma$).tw. (41498) 50 exp Acquired Immunodeficiency Syndrome/ or exp HIV/ (154327) 51 ((human adj5 immun$ adj5 virus) or hiv or (acquired adj5 immun$ adj5 deficiency adj5 syndrome) or aids or (acquired adj5 immun$ adj5 syndrome)).tw. (352730) 52 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 (605119) 53 randomized controlled trial.pt. (434179) 54 controlled clinical trial.pt. (91862) 55 randomized.ab. (366076) 56 placebo.ab. (177920) 57 randomly.ab. (260797) 58 trial.ti. (160235) 59 clinical trials as topic.sh. (180499) 60 53 or 54 or 55 or 56 or 57 or 58 or 59 (1061299) 61 exp animals/ not humans.sh. (4333932) 62 60 not 61 (977252) 63 23 and 52 and 62 (993) 64 limit 63 to yr="2015 ‐Current" (67) |
| # of records identified | 67 |
| # of records without duplicates | 55 |
EMBASE
| Search electronic report #2 | |
| Search type | Update |
| Database | EMBASE |
| Platform | EMBASE.com |
| Search date | 28/10/2016 |
| Update date | Undefined |
| Range of search date | 2015‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1. 'microbicide'/exp 1,679 2. microbicid*:ab,ti 6,167 3. antimicrobial:ab,ti 142,555 4. or/1‐3 148,176 5. vagin*:ab,ti 131,175 6. rectal*:ab,ti 104,352 7. rectum:ab,ti 46,719 8. anus:ab,ti 8,802 9. anal*:ab,ti 6,110,718 10. topical*:ab,ti 113,141 11. local*:ab,ti 1,291,279 12. or/5‐11 7,259,301 13. 4 AND 12 49,429 14. 'topical antiinfective agent'/exp 304,412 15. (anti NEXT/1 infective*):ab,ti AND topical:ab,ti 164 16. (anti NEXT/1 infective*):ab,ti AND local:ab,ti 183 17. (antiinfective* NEAR/5 topical):ab,ti 21 18. (antiinfective* NEAR/5 local):ab,ti 12 19. (mercurial NEAR/5 antiseptic):ab,ti 5 20. (antifung* NEAR/5 topical):ab,ti 1,430 21. (antivir* NEAR/5 topical):ab,ti 284 22. (antibacteri* NEAR/5 topical):ab,ti 481 23. or/14‐22 306,541 24. 13 or 23 352,376 25. 'sexually transmitted disease'/exp OR 107,884 'chlamydiasis'/exp OR 'condyloma'/exp OR 'condyloma acuminatum'/exp OR 'condyloma latum'/exp OR 'genital herpes'/exp OR 'gonorrhea'/exp OR 'granuloma inguinale'/exp OR 'lymphogranuloma venereum'/exp OR 'syphilis'/exp OR 'secondary syphilis'/exp OR 'tabes dorsalis'/exp OR 'ulcus molle'/exp 26. std:ab,ti OR stds:ab,ti OR sti:ab,ti OR 25,842 stis:ab,ti 27. (sex* NEAR/5 transmitted):ab,ti OR (sex* NEAR/5 36,665 transmiss*):ab,ti 28. (venereal NEAR/5 disease*):ab,ti OR (venereal 4,577 NEAR/5 infection*):ab,ti OR (venereal NEAR/5 disorder*):ab,ti 29. (genital NEAR/5 disease*):ab,ti OR (genital 12,201 NEAR/5 infection*):ab,ti OR (genital NEAR/5 disorder*):ab,ti 30. 'chlamydia'/exp OR 'chlamydia trachomatis'/exp 29,612 31. chlamydia*:ab,ti 29,507 32. syphili*:ab,ti OR chancre*:ab,ti OR 32,892 condylom*:ab,ti 33. 'lymphogranuloma venereum':ab,ti OR 1,246 'lymphogranuloma inguinale':ab,ti 34. 'granuloma inguinale':ab,ti OR 'granuloma 622 venereum':ab,ti OR donovanosis:ab,ti OR donovania:ab,ti 35. 'neisseria gonorrhoeae'/exp 14,433 36. gonorrh*:ab,ti OR gonococc*:ab,ti 23,729 37. 'nongonococcal urethritis'/exp OR 2,051 (nongonococcal:ab,ti AND urethritis:ab,ti OR 'non gonococcal':ab,ti AND urethritis:ab,ti) OR ngu:ab,ti 38. 'herpes simplex virus 1'/exp OR 'herpes simplex 30,087 virus 2'/exp OR hsv1:ab,ti OR 'hsv 1':ab,ti OR hsv2:ab,ti OR 'hsv 2':ab,ti OR (herpes NEAR/5 genital*):ab,ti 39. human:ab,ti AND cytomegalovirus:ab,ti OR 5,979 human:ab,ti AND 'herpesvirus 5':ab,ti OR 'hhv 5':ab,ti OR hcmv:ab,ti 40. 'candidiasis'/exp OR 'vagina candidiasis'/exp OR 78,630 'genital candidiasis'/exp OR 'invasive candidiasis'/exp OR 'candidemia'/exp OR 'candida albicans'/exp 41. candidiasis:ab,ti OR (vagina* NEAR/5 17,876 candid*):ab,ti OR (vulvovagina* NEAR/5 candid*):ab,ti OR (vagina* NEAR/5 mycoses):ab,ti 42. 'gardnerella infection'/exp OR 'gardnerella 5,348 vaginalis'/exp OR (vaginosis NEAR/5 bacterial):ab,ti 43. 'vaginitis'/exp OR 'vulvovaginitis'/exp OR 27,055 'trichomoniasis'/exp OR 'trichomonas vaginalis'/exp OR vaginitis:ab,ti OR trichomoniasis:ab,ti OR vaginalis:ab,ti 44. 'balanitis'/exp OR balanitis:ab,ti 1,650 45. (genital* NEAR/5 wart*):ab,ti OR (anogenital 3,489 NEAR/5 wart*):ab,ti OR (anorectal NEAR/5 wart*):ab,ti OR (penile NEAR/5 wart*):ab,ti OR (penis NEAR/5 wart*):ab,ti 46. (genital* NEAR/5 ulcer*):ab,ti OR (anogenital 3,785 NEAR/5 ulcer*):ab,ti OR (anorectal NEAR/5 ulcer*):ab,ti OR (penile NEAR/5 ulcer*):ab,ti OR (penis NEAR/5 ulcer*):ab,ti 47. (venereal NEAR/5 ulcer*):ab,ti OR (venereal 133 NEAR/5 wart*):ab,ti 48. 'human papillomavirus':ab,ti OR hpv:ab,ti 47,209 49. 'alphapapillomavirus'/exp OR 'human 11,129 papillomavirus type 11'/exp OR 'human papillomavirus type 16'/exp OR 'human papillomavirus type 18'/exp OR 'human papillomavirus type 31'/exp OR 'human papillomavirus type 6'/exp OR 'betapapillomavirus'/exp OR 'gammapapillomavirus'/exp 50. 'human immunodeficiency virus'/exp OR 'acquired 265,484 immune deficiency syndrome'/exp 51. (human NEAR/5 immun* NEAR/5 virus):ab,ti OR 415,741 hiv:ab,ti OR (acquired NEAR/5 immun* NEAR/5 deficiency NEAR/5 syndrome):ab,ti OR aids:ab,ti OR (acquired NEAR/5 immun* NEAR/5 syndrome):ab,ti 52. or/25‐51 745,783 53. 'randomized controlled trial'/exp OR 'single 1,311,714 blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR random*:ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti NOT ('animal'/de OR 'animal experiment'/de OR 'nonhuman'/de NOT ('animal'/de OR 'animal experiment'/de OR 'nonhuman'/de AND 'human'/de)) 54. 24 and 52 and 53 985 55. 54 and AND [2011‐2015]/py 80 |
| # of records identified | 80 |
| # of records without duplicates | 78 |
CENTRAL
| Search electronic report #3 | |
| Search type | Update |
| Database | The Cochrane Central Register of Controlled Trials (CENTRAL) |
| Platform | Ovid |
| Search date | 30/10/2016 |
| Update date | Undefined |
| Range of search date | 2015‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1 microbicid$.tw. (270) 2 antimicrobial.tw. (3403) 3 1 or 2 (3662) 4 vagin$.tw. (8645) 5 rectal$.tw. (5689) 6 rectum.tw. (1193) 7 anus.tw. (152) 8 anal$.tw. (230385) 9 topical$.tw. (14928) 10 local$.tw. (34806) 11 4 or 5 or 6 or 7 or 8 or 9 or 10 (270146) 12 3 and 11 (1425) 13 exp Anti‐Infective Agents, Local/ (6344) 14 ((anti adj1 infective$) and topical).tw. (13) 15 ((anti adj1 infective$) and local).tw. (10) 16 (antiinfective$ adj5 topical).tw. (3) 17 (antiinfective$ adj5 local).tw. (0) 18 (mercurial adj5 antiseptic).tw. (0) 19 (antifung$ adj5 topical).tw. (123) 20 (antivir$ adj5 topical).tw. (23) 21 (antibacteri$ adj5 topical).tw. (48) 22 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (6543) 23 12 or 22 (7746) 24 exp Sexually transmitted diseases/ or exp Sexually transmitted diseases, bacterial/ or exp Chancroid/ or exp Chlamydia infections/ or exp Lymphogranuloma venereum/ or exp Gonorrhea/ or exp Granuloma inguinale/ or exp Syphilis/ or exp Neurosyphilis/ or exp Tabes dorsalis/ or exp Syphilis, latent/ (9105) 25 exp Sexually transmitted diseases, viral/ or exp Condylomata acuminata/ or exp Herpes genitalis/ (7994) 26 (std or stds or sti or stis).tw. (1235) 27 ((sex$ adj5 transmitted) or (sex$ adj5 transmiss$)).tw. (1137) 28 ((venereal adj5 disease$) or (venereal adj5 infection$) or (venereal adj5 disorder$)).tw. (30) 29 ((genital adj5 disease$) or (genital adj5 infection$) or (genital adj5 disorder$)).tw. (613) 30 exp Chlamydia/ or exp Chlamydia trachomatis/ (303) 31 chlamydia$.tw. (1090) 32 exp Treponema pallidum/ or treponema pallidum.tw. or condylom$.tw. or syphili$.tw. (599) 33 (lymphogranuloma venereum or lymphogranuloma inguinale).tw. (8) 34 (granuloma inguinale or granuloma venereum or donovanosis or donovania).tw. (6) 35 exp Neisseria gonorrhoeae/ (164) 36 (gonorrh$ or gonococc$).tw. (1126) 37 (nongonococcal urethritis or non‐gonococcal urethritis or ngu).tw. (147) 38 exp Herpesvirus 1, human/ or exp Herpesvirus 2, human/ or (hsv1 or hsv‐1 or hsv2 or hsv‐2 or (herpes adj5 genital$)).tw. (641) 39 (human cytomegalovirus or human herpesvirus‐5 or hhv‐5 or hcmv).tw. (60) 40 exp Candidiasis/ or exp Candidiasis, vulvovaginal/ or exp Candidiasis, invasive/ or exp Candidemia/ or exp Candida albicans/ (850) 41 (candidiasis or ((vagina$ adj5 candidosis) or ((vulvovagina$ adj5 candidosis) or (vagina$ adj5 mycoses)))).tw. (864) 42 exp Vaginitis/ or exp Trichomonas vaginitis/ or exp Trichomonas vaginalis/ or exp Vulvovaginitis/ or (vaginitis or trichomoniasis or vaginalis).tw. (1100) 43 exp Vaginosis, bacterial/ or exp Gardnerella vaginalis/ or (vaginosis adj5 bacterial).tw. (500) 44 exp Balanitis/ or balanitis.tw. (25) 45 ((venereal adj5 ulcer$) or (venereal adj5 wart$)).tw. (2) 46 ((genital$ adj5 ulcer$) or (anogenital adj5 ulcer$) or (anorectal adj5 ulcer$) or (penile adj5 ulcer$) or (penis adj5 ulcer$)).tw. (113) 47 ((genital$ adj5 wart$) or (anogenital adj5 wart$) or (anorectal adj5 wart$) or (penile adj5 wart$) or (penis adj5 wart$)).tw. (236) 48 exp Alphapapillomavirus/ or exp Human papillomavirus 6/ or exp Human papillomavirus 11/ or exp Human papillomavirus 16/ or exp Human papillomavirus 18/ or exp Human papillomavirus 31/ or exp Betapapillomavirus/ or exp Gammapapillomavirus/ (175) 49 (human papillomavirus$ or hpv or recurrent respiratory papillomatosis or condyloma$).tw. (1473) 50 exp Acquired Immunodeficiency Syndrome/ or exp HIV/ (3546) 51 ((human adj5 immun$ adj5 virus) or hiv or (acquired adj5 immun$ adj5 deficiency adj5 syndrome) or aids or (acquired adj5 immun$ adj5 syndrome)).tw. (13808) 52 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 (21192) 53 23 and 52 (576) 54 limit 53 to yr="2015 ‐Current" (33) 55 limit 53 to yr="2015 ‐Current" (33) |
| # of records identified | 33 |
| # of records without duplicates | 29 |
LILACS
| Search electronic report #4 | |
| Search type | Update |
| Database | LILACS http://lilacs.bvsalud.org/es/ |
| Platform | Biblioteca Virtual en Salud (BVS), interfaz iAHx |
| Search date | 28/10/2016 |
| Update date | Undefined |
| Range of search date | None |
| Language restrictions | None |
| Other limits | None |
| Search strategy | (ab:(microbicide*)) OR (ti:(microbicid*)) AND (ab:(topical*)) OR (ti:(topical*)) AND db:("LILACS") RCTs filter: ((PT:"ensayo clinico controlado aleatorio" OR PT:"ensayo clinico controlado" OR PT:"estudio multicéntrico" OR MH:"ensayos clinicos controlados aleatorios como asunto" OR MH:"ensayos clinicos controlados como asunto" OR MH:"estudio multicéntricos como asunto" OR MH:"distribución aleatoria" OR MH:"método doble ciego" OR MH:"metodo simple‐ciego") OR ((ensaio$ OR ensayo$ OR trial$) AND (azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego OR ((simple$ OR single OR duplo$ OR doble$ OR double$) AND (cego OR ciego OR blind OR mask))) AND clinic$)) AND NOT (MH:animales OR MH:conejos OR MH:ratones OR MH:ratas OR MH:primates OR MH:perros OR MH:gatos OR MH:porcinos OR PT:"in vitro") Date filter: ( year_cluster:("2015" OR "2016")) |
| # of records identified | 0 |
| # of records without duplicates | 0 |
Web of Science
| Search electronic report #5 | |
| Search type | Update |
| Database | Web of Science webofscience.com/ |
| Platform | Thomson Reuters |
| Search date | 30/10/2016 |
| Update date | Undefined |
| Range of search date | 2015‐current |
| Language restrictions | None |
| Other limits | None |
| Search strategy | TS=(topical*) AND TS=(microbicid*) AND TI=(trial) Refinado por: Años de publicación: ( 2015 OR 2016 ) |
| # of records identified | 3 |
| # of records without duplicates | 3 |
| Search electronic report #6 | |
| Search type | New |
| Database | International Clinical Trials Registry Platform www.who.int/ictrp/ |
| Platform | ICTRP Portal |
| Search date | 30/10/2016 |
| Update date | Undefined |
| Range of search date | None |
| Language restrictions | None |
| Other limits | None |
| Search strategy | topical microbicid* |
| # of records identified | 0 (Published 2015‐2016) |
| # of records without duplicates | NA |
ClinicalTrials.gov
| Search electronic report #7 | |
| Search type | New |
| Database | Clinicaltrials.gov |
| Platform | Clinicaltrials.gov |
| Search date | 30/10/2016 |
| Update date | Undefined |
| Range of search date | None |
| Language restrictions | None |
| Other limits | None |
| Search strategy | "topical microbicide" |
| # of records identified | 1 (for 2015‐2016) |
| # of records without duplicates | 1 |
Appendix 3. Search conducted in February 2017, STI Review Group
MEDLINE
| Search electronic report #1 | |
| Search type | Update |
| Databases | § MEDLINE § MEDLINE In‐Process & Other Non‐Indexed Citations § MEDLINE Daily Update |
| Platform | Ovid |
| Search date | 02/2017 |
| Update date | Undefined |
| Range of search date | 2015‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1 microbicid$.tw. (5516) 2 antimicrobial.tw. (110812) 3 1 or 2 (115466) 4 vagin$.tw. (98504) 5 rectal$.tw. (74441) 6 rectum.tw. (33106) 7 anus.tw. (6592) 8 anal$.tw. (4892629) 9 topical$.tw. (87107) 10 local$.tw. (1079120) 11 4 or 5 or 6 or 7 or 8 or 9 or 10 (5860315) 12 3 and 11 (36910) 13 exp Anti‐Infective Agents, Local/ (205775) 14 ((anti adj1 infective$) and topical).tw. (131) 15 ((anti adj1 infective$) and local).tw. (143) 16 (antiinfective$ adj5 topical).tw. (13) 17 (antiinfective$ adj5 local).tw. (5) 18 (mercurial adj5 antiseptic).tw. (5) 19 (antifung$ adj5 topical).tw. (1069) 20 (antivir$ adj5 topical).tw. (246) 21 (antibacteri$ adj5 topical).tw. (366) 22 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (207451) 23 12 or 22 (242133) 24 exp Sexually transmitted diseases/ or exp Sexually transmitted diseases, bacterial/ or exp Chancroid/ or exp Chlamydia infections/ or exp Lymphogranuloma venereum/ or exp Gonorrhea/ or exp Granuloma inguinale/ or exp Syphilis/ or exp Neurosyphilis/ or exp Tabes dorsalis/ or exp Syphilis, latent/ (317542) 25 exp Sexually transmitted diseases, viral/ or exp Condylomata acuminata/ or exp Herpes genitalis/ (251572) 26 (std or stds or sti or stis).tw. (19080) 27 ((sex$ adj5 transmitted) or (sex$ adj5 transmiss$)).tw. (32083) 28 ((venereal adj5 disease$) or (venereal adj5 infection$) or (venereal adj5 disorder$)).tw. (4475) 29 ((genital adj5 disease$) or (genital adj5 infection$) or (genital adj5 disorder$)).tw. (10013) 30 exp Chlamydia/ or exp Chlamydia trachomatis/ (13779) 31 chlamydia$.tw. (24641) 32 exp Treponema pallidum/ or treponema pallidum.tw. or condylom$.tw. or syphili$.tw. (30355) 33 (lymphogranuloma venereum or lymphogranuloma inguinale).tw. (1079) 34 (granuloma inguinale or granuloma venereum or donovanosis or donovania).tw. (577) 35 exp Neisseria gonorrhoeae/ (8968) 36 (gonorrh$ or gonococc$).tw. (20409) 37 (nongonococcal urethritis or non‐gonococcal urethritis or ngu).tw. (1225) 38 exp Herpesvirus 1, human/ or exp Herpesvirus 2, human/ or (hsv1 or hsv‐1 or hsv2 or hsv‐2 or (herpes adj5 genital$)).tw. (20841) 39 (human cytomegalovirus or human herpesvirus‐5 or hhv‐5 or hcmv).tw. (8689) 40 exp Candidiasis/ or exp Candidiasis, vulvovaginal/ or exp Candidiasis, invasive/ or exp Candidemia/ or exp Candida albicans/ (44495) 41 (candidiasis or ((vagina$ adj5 candidosis) or ((vulvovagina$ adj5 candidosis) or (vagina$ adj5 mycoses)))).tw. (13518) 42 exp Vaginitis/ or exp Trichomonas vaginitis/ or exp Trichomonas vaginalis/ or exp Vulvovaginitis/ or (vaginitis or trichomoniasis or vaginalis).tw. (19097) 43 exp Vaginosis, bacterial/ or exp Gardnerella vaginalis/ or (vaginosis adj5 bacterial).tw. (4549) 44 exp Balanitis/ or balanitis.tw. (1105) 45 ((venereal adj5 ulcer$) or (venereal adj5 wart$)).tw. (115) 46 ((genital$ adj5 ulcer$) or (anogenital adj5 ulcer$) or (anorectal adj5 ulcer$) or (penile adj5 ulcer$) or (penis adj5 ulcer$)).tw. (2942) 47 ((genital$ adj5 wart$) or (anogenital adj5 wart$) or (anorectal adj5 wart$) or (penile adj5 wart$) or (penis adj5 wart$)).tw. (2742) 48 exp Alphapapillomavirus/ or exp Human papillomavirus 6/ or exp Human papillomavirus 11/ or exp Human papillomavirus 16/ or exp Human papillomavirus 18/ or exp Human papillomavirus 31/ or exp Betapapillomavirus/ or exp Gammapapillomavirus/ (6149) 49 (human papillomavirus$ or hpv or recurrent respiratory papillomatosis or condyloma$).tw. (41420) 50 exp Acquired Immunodeficiency Syndrome/ or exp HIV/ (154038) 51 ((human adj5 immun$ adj5 virus) or hiv or (acquired adj5 immun$ adj5 deficiency adj5 syndrome) or aids or (acquired adj5 immun$ adj5 syndrome)).tw. (354260) 52 24 or 25 or 26 or 27 or 28 or 29 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 (606865) 53 randomized controlled trial.pt. (448765) 54 controlled clinical trial.pt. (91958) 55 random*.ab. (871717) 56 placebo.ab. (181335) 57 clinical trials as topic.sh. (181463) 58 randomly.ab. (266643) 59 trial.ti. (170824) 60 53 or 54 or 55 or 56 or 57 or 58 or 59 (1287670) 61 exp animals/ not humans.sh. (4325121) 62 60 not 61 (1177804) 63 23 and 52 and 62 (1068) 64 limit 63 to yr="2015 ‐Current" (83) |
| # of records identified | 83 |
| # of records without duplicates | 66 |
EMBASE
| Search electronic report #2 | |
| Search type | Update |
| Database | EMBASE |
| Platform | EMBASE.com |
| Search date | 02/2017 |
| Update date | Undefined |
| Range of search date | 2015‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1. 'microbicide'/exp 2. microbicid*:ab,ti 3. antimicrobial:ab,ti 4. or/1‐3 5. vagin*:ab,ti 6. rectal*:ab,ti 7. rectum:ab,ti 8. anus:ab,ti 9. anal*:ab, 10. topical*:ab,ti 11. local*:ab,ti 12. or/5‐11 13. 4 AND 12 14. 'topical antiinfective agent'/exp 15. (anti NEXT/1 infective*):ab,ti AND topical:ab,ti 16. (anti NEXT/1 infective*):ab,ti AND local:ab,ti 17. (antiinfective* NEAR/5 topical):ab,ti 18. (antiinfective* NEAR/5 local):ab,ti 19. (mercurial NEAR/5 antiseptic):ab,ti 20. (antifung* NEAR/5 topical):ab,ti 21. (antivir* NEAR/5 topical):ab,ti 22. (antibacteri* NEAR/5 topical):ab,ti 23. or/14‐22 24. 13 or 23 25. 'sexually transmitted disease'/exp OR 'chlamydiasis'/exp OR 'condyloma'/exp OR 'condyloma acuminatum'/exp OR 'condyloma latum'/exp OR 'genital herpes'/exp OR 'gonorrhea'/exp OR 'granuloma inguinale'/exp OR 'lymphogranuloma venereum'/exp OR 'syphilis'/exp OR 'secondary syphilis'/exp OR 'tabes dorsalis'/exp OR 'ulcus molle'/exp 26. std:ab,ti OR stds:ab,ti OR sti:ab,ti OR stis:ab,ti 27. (sex* NEAR/5 transmitted):ab,ti OR (sex* NEAR/5 transmiss*):ab,ti 28. (venereal NEAR/5 disease*):ab,ti OR (venereal NEAR/5 infection*):ab,ti OR (venereal NEAR/5 disorder*):ab,ti 29. (genital NEAR/5 disease*):ab,ti OR (genital NEAR/5 infection*):ab,ti OR (genital NEAR/5 disorder*):ab,ti 30. 'chlamydia'/exp OR 'chlamydia trachomatis'/exp 31. chlamydia*:ab,ti 32. syphili*:ab,ti OR chancre*:ab,ti OR condylom*:ab,ti 33. 'lymphogranuloma venereum':ab,ti OR 'lymphogranuloma inguinale':ab,ti 34. 'granuloma inguinale':ab,ti OR 'granuloma venereum':ab,ti OR donovanosis:ab,ti OR donovania:ab,ti 35. 'neisseria gonorrhoeae'/exp 36. gonorrh*:ab,ti OR gonococc*:ab,ti 37. 'nongonococcal urethritis'/exp OR (nongonococcal:ab,ti AND urethritis:ab,ti OR 'non gonococcal':ab,ti AND urethritis:ab,ti) OR ngu:ab,ti 38. 'herpes simplex virus 1'/exp OR 'herpes simplex virus 2'/exp OR hsv1:ab,ti OR 'hsv 1':ab,ti OR hsv2:ab,ti OR 'hsv 2':ab,ti OR (herpes NEAR/5 genital*):ab,ti 39. human:ab,ti AND cytomegalovirus:ab,ti OR human:ab,ti AND 'herpesvirus 5':ab,ti OR 'hhv 5':ab,ti OR hcmv:ab,ti 40. 'candidiasis'/exp OR 'vagina candidiasis'/exp OR 'genital candidiasis'/exp OR 'invasive candidiasis'/exp OR 'candidemia'/exp OR 'candida albicans'/exp 41. candidiasis:ab,ti OR (vagina* NEAR/5 candid*):ab,ti OR (vulvovagina* NEAR/5 candid*):ab,ti OR (vagina* NEAR/5 mycoses):ab,ti 42. 'gardnerella infection'/exp OR 'gardnerella vaginalis'/exp OR (vaginosis NEAR/5 bacterial):ab,ti 43. 'vaginitis'/exp OR 'vulvovaginitis'/exp OR 'trichomoniasis'/exp OR 'trichomonas vaginalis'/exp OR vaginitis:ab,ti OR trichomoniasis:ab,ti OR vaginalis:ab,ti 44. 'balanitis'/exp OR balanitis:ab,ti 45. (genital* NEAR/5 wart*):ab,ti OR (anogenital NEAR/5 wart*):ab,ti OR (anorectal NEAR/5 wart*):ab,ti OR (penile NEAR/5 wart*):ab,ti OR (penis NEAR/5 wart*):ab,ti 46. (genital* NEAR/5 ulcer*):ab,ti OR (anogenital NEAR/5 ulcer*):ab,ti OR (anorectal NEAR/5 ulcer*):ab,ti OR (penile NEAR/5 ulcer*):ab,ti OR (penis NEAR/5 ulcer*):ab,ti 47. (venereal NEAR/5 ulcer*):ab,ti OR (venereal NEAR/5 wart*):ab,ti 48. 'human papillomavirus':ab,ti OR hpv:ab,ti 49. 'alphapapillomavirus'/exp OR 'human papillomavirus type 11'/exp OR 'human papillomavirus type 16'/exp OR 'human papillomavirus type 18'/exp OR 'human papillomavirus type 31'/exp OR 'human papillomavirus type 6'/exp OR 'betapapillomavirus'/exp OR 'gammapapillomavirus'/exp 50. 'human immunodeficiency virus'/exp OR 'acquired immune deficiency syndrome'/exp 51. (human NEAR/5 immun* NEAR/5 virus):ab,ti OR hiv:ab,ti OR (acquired NEAR/5 immun* NEAR/5 deficiency NEAR/5 syndrome):ab,ti OR aids:ab,ti OR (acquired NEAR/5 immun* NEAR/5 syndrome):ab,ti 52. or/25‐51 53. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR random*:ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti NOT ('animal'/de OR 'animal experiment'/de OR 'nonhuman'/de NOT ('animal'/de OR 'animal experiment'/de OR 'nonhuman'/de AND 'human'/de)) 54. 24 and 52 and 53 55. 54 and AND [2011‐2015]/py |
| # of records identified | 39 |
| # of records without duplicates | 38 |
CENTRAL
| Search electronic report #3 | |
| Search type | Update |
| Database | The Cochrane Central Register of Controlled Trials (CENTRAL) |
| Platform | Ovid |
| Search date | 02/2017 |
| Update date | Undefined |
| Range of search date | 2015‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1 microbicid$.tw. (279) 2 antimicrobial.tw. (3587) 3 1 or 2 (3855) 4 vagin$.tw. (8987) 5 rectal$.tw. (5901) 6 rectum.tw. (1243) 7 anus.tw. (165) 8 anal$.tw. (245360) 9 topical$.tw. (15395) 10 local$.tw. (36534) 11 4 or 5 or 6 or 7 or 8 or 9 or 10 (286479) 12 3 and 11 (1517) 13 exp Anti‐Infective Agents, Local/ (6445) 14 ((anti adj1 infective$) and topical).tw. (13) 15 ((anti adj1 infective$) and local).tw. (10) 16 (antiinfective$ adj5 topical).tw. (3) 17 (antiinfective$ adj5 local).tw. (0) 18 (mercurial adj5 antiseptic).tw. (0) 19 (antifung$ adj5 topical).tw. (130) 20 (antivir$ adj5 topical).tw. (24) 21 (antibacteri$ adj5 topical).tw. (50) 22 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (6653) 23 12 or 22 (7939) 24 exp Sexually transmitted diseases/ or exp Sexually transmitted diseases, bacterial/ or exp Chancroid/ or exp Chlamydia infections/ or exp Lymphogranuloma venereum/ or exp Gonorrhea/ or exp Granuloma inguinale/ or exp Syphilis/ or exp Neurosyphilis/ or exp Tabes dorsalis/ or exp Syphilis, latent/ (9292) 25 exp Sexually transmitted diseases, viral/ or exp Condylomata acuminata/ or exp Herpes genitalis/ (8167) 26 (std or stds or sti or stis).tw. (1297) 27 ((sex$ adj5 transmitted) or (sex$ adj5 transmiss$)).tw. (1187) 28 ((venereal adj5 disease$) or (venereal adj5 infection$) or (venereal adj5 disorder$)).tw. (31) 29 ((genital adj5 disease$) or (genital adj5 infection$) or (genital adj5 disorder$)).tw. (648) 30 exp Chlamydia/ or exp Chlamydia trachomatis/ (305) 31 chlamydia$.tw. (1116) 32 exp Treponema pallidum/ or treponema pallidum.tw. or condylom$.tw. or syphili$.tw. (613) 33 (lymphogranuloma venereum or lymphogranuloma inguinale).tw. (9) 34 (granuloma inguinale or granuloma venereum or donovanosis or donovania).tw. (6) 35 exp Neisseria gonorrhoeae/ (164) 36 (gonorrh$ or gonococc$).tw. (1140) 37 (nongonococcal urethritis or non‐gonococcal urethritis or ngu).tw. (148) 38 exp Herpesvirus 1, human/ or exp Herpesvirus 2, human/ or (hsv1 or hsv‐1 or hsv2 or hsv‐2 or (herpes adj5 genital$)).tw. (668) 39 (human cytomegalovirus or human herpesvirus‐5 or hhv‐5 or hcmv).tw. (62) 40 exp Candidiasis/ or exp Candidiasis, vulvovaginal/ or exp Candidiasis, invasive/ or exp Candidemia/ or exp Candida albicans/ (860) 41 (candidiasis or ((vagina$ adj5 candidosis) or ((vulvovagina$ adj5 candidosis) or (vagina$ adj5 mycoses)))).tw. (893) 42 exp Vaginitis/ or exp Trichomonas vaginitis/ or exp Trichomonas vaginalis/ or exp Vulvovaginitis/ or (vaginitis or trichomoniasis or vaginalis).tw. (1118) 43 exp Vaginosis, bacterial/ or exp Gardnerella vaginalis/ or (vaginosis adj5 bacterial).tw. (507) 44 exp Balanitis/ or balanitis.tw. (26) 45 ((venereal adj5 ulcer$) or (venereal adj5 wart$)).tw. (2) 46 ((genital$ adj5 ulcer$) or (anogenital adj5 ulcer$) or (anorectal adj5 ulcer$) or (penile adj5 ulcer$) or (penis adj5 ulcer$)).tw. (115) 47 ((genital$ adj5 wart$) or (anogenital adj5 wart$) or (anorectal adj5 wart$) or (penile adj5 wart$) or (penis adj5 wart$)).tw. (243) 48 exp Alphapapillomavirus/ or exp Human papillomavirus 6/ or exp Human papillomavirus 11/ or exp Human papillomavirus 16/ or exp Human papillomavirus 18/ or exp Human papillomavirus 31/ or exp Betapapillomavirus/ or exp Gammapapillomavirus/ (179) 49 (human papillomavirus$ or hpv or recurrent respiratory papillomatosis or condyloma$).tw. (1559) 50 exp Acquired Immunodeficiency Syndrome/ or exp HIV/ (3601) 51 ((human adj5 immun$ adj5 virus) or hiv or (acquired adj5 immun$ adj5 deficiency adj5 syndrome) or aids or (acquired adj5 immun$ adj5 syndrome)).tw. (14643) 52 24 or 25 or 26 or 27 or 28 or 29 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 (22260) 53 23 and 52 (591) 54 limit 53 to yr="2015 ‐Current" (46) |
| # of records identified | 46 |
| # of records without duplicates | 42 |
Appendix 4. Search conducted in March 2018, STI Review Group
| Search electronic report #1 | |
| Search type | Update |
| Databases | § MEDLINE § MEDLINE In‐Process & Other Non‐Indexed Citations § MEDLINE Daily Update |
| Platform | Ovid |
| Search date | 30/03/2018 |
| Update date | Undefined |
| Range of search date | 2016‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1 microbicid$.tw. (5730) 2 antimicrobial.tw. (123315) 3 1 or 2 (128127) 4 vagin$.tw. (103158) 5 rectal$.tw. (78475) 6 rectum.tw. (34357) 7 anus.tw. (6941) 8 anal$.tw. (5344032) 9 topical$.tw. (92856) 10 local$.tw. (1154675) 11 or/4‐10 (6369160) 12 3 and 11 (41707) 13 exp Anti‐Infective Agents, Local/ (211550) 14 ((anti adj1 infective$) and topical).tw. (137) 15 ((anti adj1 infective$) and local).tw. (170) 16 (antiinfective$ adj5 topical).tw. (13) 17 (antiinfective$ adj5 local).tw. (4) 18 (mercurial adj5 antiseptic).tw. (5) 19 (antifung$ adj5 topical).tw. (1149) 20 (antivir$ adj5 topical).tw. (273) 21 (antibacteri$ adj5 topical).tw. (382) 22 or/13‐21 (213363) 23 12 or 22 (252699) 24 exp Sexually transmitted diseases/ or exp Sexually transmitted diseases, bacterial/ or exp Chancroid/ or exp Chlamydia infections/ or exp Lymphogranuloma venereum/ or exp Gonorrhea/ or exp Granuloma inguinale/ or exp Syphilis/ or exp Neurosyphilis/ or exp Tabes dorsalis/ or exp Syphilis, latent/ (325375) 25 exp Sexually transmitted diseases, viral/ or exp Condylomata acuminata/ or exp Herpes genitalis/ (258572) 26 (std or stds or sti or stis).tw. (20520) 27 ((sex$ adj5 transmitted) or (sex$ adj5 transmiss$)).tw. (34152) 28 ((venereal adj5 disease$) or (venereal adj5 infection$) or (venereal adj5 disorder$)).tw. (4528) 29 ((genital adj5 disease$) or (genital adj5 infection$) or (genital adj5 disorder$)).tw. (10397) 30 exp Chlamydia/ or exp Chlamydia trachomatis/ (14119) 31 chlamydia$.tw. (25628) 32 exp Treponema pallidum/ or treponema pallidum.tw. or condylom$.tw. or syphili$.tw. (31480) 33 (lymphogranuloma venereum or lymphogranuloma inguinale).tw. (1117) 34 (granuloma inguinale or granuloma venereum or donovanosis or donovania).tw. (597) 35 exp Neisseria gonorrhoeae/ (9176) 36 (gonorrh$ or gonococc$).tw. (21138) 37 (nongonococcal urethritis or non‐gonococcal urethritis or ngu).tw. (1255) 38 exp Herpesvirus 1, human/ or exp Herpesvirus 2, human/ or (hsv1 or hsv‐1 or hsv2 or hsv‐2 or (herpes adj5 genital$)).tw. (21512) 39 (human cytomegalovirus or human herpesvirus‐5 or hhv‐5 or hcmv).tw. (9061) 40 exp Candidiasis/ or exp Candidiasis, vulvovaginal/ or exp Candidiasis, invasive/ or exp Candidemia/ or exp Candida albicans/ (45724) 41 (candidiasis or ((vagina$ adj5 candidosis) or ((vulvovagina$ adj5 candidosis) or (vagina$ adj5 mycoses)))).tw. (14174) 42 exp Vaginitis/ or exp Trichomonas vaginitis/ or exp Trichomonas vaginalis/ or exp Vulvovaginitis/ or (vaginitis or trichomoniasis or vaginalis).tw. (19593) 43 exp Vaginosis, bacterial/ or exp Gardnerella vaginalis/ or (vaginosis adj5 bacterial).tw. (4728) 44 exp Balanitis/ or balanitis.tw. (1147) 45 ((venereal adj5 ulcer$) or (venereal adj5 wart$)).tw. (126) 46 ((genital$ adj5 ulcer$) or (anogenital adj5 ulcer$) or (anorectal adj5 ulcer$) or (penile adj5 ulcer$) or (penis adj5 ulcer$)).tw. (3009) 47 ((genital$ adj5 wart$) or (anogenital adj5 wart$) or (anorectal adj5 wart$) or (penile adj5 wart$) or (penis adj5 wart$)).tw. (2931) 48 exp Alphapapillomavirus/ or exp Human papillomavirus 6/ or exp Human papillomavirus 11/ or exp Human papillomavirus 16/ or exp Human papillomavirus 18/ or exp Human papillomavirus 31/ or exp Betapapillomavirus/ or exp Gammapapillomavirus/ (6515) 49 (human papillomavirus$ or hpv or recurrent respiratory papillomatosis or condyloma$).tw. (44746) 50 exp Acquired Immunodeficiency Syndrome/ or exp HIV/ (155943) 51 ((human adj5 immun$ adj5 virus) or hiv or (acquired adj5 immun$ adj5 deficiency adj5 syndrome) or aids or (acquired adj5 immun$ adj5 syndrome)).tw. (371861) 52 or/24‐51 (634631) 53 randomized controlled trial.pt. (456550) 54 controlled clinical trial.pt. (92271) 55 randomized.ab. (406299) 56 placebo.ab. (187446) 57 randomly.ab. (287258) 58 trial.ti. (179586) 59 clinical trials as topic.sh. (183067) 60 or/53‐59 (1140383) 61 exp animals/ not humans.sh. (4437818) 62 60 not 61 (1050217) 63 23 and 52 and 62 (1006) 64 limit 63 to yr="2016 ‐Current" (69) |
| # of records identified | 69 |
| # of records without duplicates | 66 |
| Search electronic report #2 | |
| Search type | Update |
| Database | EMBASE |
| Platform | EMBASE.com |
| Search date | 30/03/2018 |
| Update date | Undefined |
| Range of search date | 2016‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1. 'microbicide'/exp (1770) 2. microbicid*:ab,ti (6563) 3. antimicrobial:ab,ti (162658) 4. #1 OR #2 OR #3 (168605) 5. vagin*:ab,ti (143413) 6. rectal*:ab,ti (114625) 7. rectum:ab,ti (50364) 8. anus:ab,ti (9389) 9. anal*:ab,ti (6931236) 10. topical*:ab,ti (124606) 11. local*:ab,ti (1419833) 12. #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 (8172688) 13. #4 AND #12 (57931) 14. 'topical antiinfective agent'/exp (328149) 15. ((anti NEXT/1 infective*):ab,ti) AND topical:ab,ti (182) 16. ((anti NEXT/1 infective*):ab,ti) AND local:ab,ti (219) 17. (antiinfective* NEAR/5 topical):ab,ti (24) 18. (antiinfective* NEAR/5 local):ab,ti (13) 19. (mercurial NEAR/5 antiseptic):ab,ti (5) 20. (antifung* NEAR/5 topical):ab,ti (1605) 21. (antivir* NEAR/5 topical):ab,ti (320) 22. (antibacteri* NEAR/5 topical):ab,ti (515) 23. #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 (330534) 24. #13 OR #23 (384300) 25. 'sexually transmitted disease'/exp OR 'chlamydiasis'/exp OR 'condyloma'/exp OR 'condyloma acuminatum'/exp OR 'condyloma latum'/exp OR 'genital herpes'/exp OR 'gonorrhea'/exp OR 'granuloma inguinale'/exp OR 'lymphogranuloma venereum'/exp OR 'syphilis'/exp OR 'secondary syphilis'/exp OR 'tabes dorsalis'/exp OR 'ulcus molle'/exp (113846) 26. std:ab,ti OR stds:ab,ti OR sti:ab,ti OR stis:ab,ti (28904) 27. ((sex* NEAR/5 transmitted):ab,ti) OR ((sex* NEAR/5 transmiss*):ab,ti) (40213) 28. ((venereal NEAR/5 disease*):ab,ti) OR ((venereal NEAR/5 infection*):ab,ti) OR ((venereal NEAR/5 disorder*):ab,ti) (4680) 29. ((genital NEAR/5 disease*):ab,ti) OR ((genital NEAR/5 infection*):ab,ti) OR ((genital NEAR/5 disorder*):ab,ti) (13081) 30. 'chlamydia'/exp OR 'chlamydia trachomatis'/exp (31162) 31. chlamydia*:ab,ti (31379) 32. syphili*:ab,ti OR chancre*:ab,ti OR condylom*:ab,ti (34107) 33. 'lymphogranuloma venereum':ab,ti OR 'lymphogranuloma inguinale':ab,ti (1290) 34. 'granuloma inguinale':ab,ti OR 'granuloma venereum':ab,ti OR donovanosis:ab,ti OR donovania:ab,ti (630) 35. 'neisseria gonorrhoeae'/exp (15074) 36. gonorrh*:ab,ti OR gonococc*:ab,ti (24800) 37. 'nongonococcal urethritis'/exp OR ((nongonococcal:ab,ti AND urethritis:ab,ti OR 'non gonococcal':ab,ti) AND urethritis:ab,ti) OR ngu:ab,ti (2124) 38. 'herpes simplex virus 1'/exp OR 'herpes simplex virus 2'/exp OR hsv1:ab,ti OR 'hsv 1':ab,ti OR hsv2:ab,ti OR 'hsv 2':ab,ti OR ((herpes NEAR/5 genital*):ab,ti) (31858) 39. (human:ab,ti AND cytomegalovirus:ab,ti OR human:ab,ti) AND 'herpesvirus 5':ab,ti OR 'hhv 5':ab,ti OR hcmv:ab,ti (6404) 40. 'candidiasis'/exp OR 'vagina candidiasis'/exp OR 'genital candidiasis'/exp OR 'invasive candidiasis'/exp OR 'candidemia'/exp OR 'candida albicans'/exp (85161) 41. candidiasis:ab,ti OR ((vagina* NEAR/5 candid*):ab,ti) OR ((vulvovagina* NEAR/5 candid*):ab,ti) OR ((vagina* NEAR/5 mycoses):ab,ti) (19254) 42. 'gardnerella infection'/exp OR 'gardnerella vaginalis'/exp OR ((vaginosis NEAR/5 bacterial):ab,ti) (5820) 43. 'vaginitis'/exp OR 'vulvovaginitis'/exp OR 'trichomoniasis'/exp OR 'trichomonas vaginalis'/exp OR vaginitis:ab,ti OR trichomoniasis:ab,ti OR vaginalis:ab,ti (28509) 44. 'balanitis'/exp OR balanitis:ab,ti (1786) 45. ((genital* NEAR/5 wart*):ab,ti) OR ((anogenital NEAR/5 wart*):ab,ti) OR ((anorectal NEAR/5 wart*):ab,ti) OR ((penile NEAR/5 wart*):ab,ti) OR ((penis NEAR/5 wart*):ab,ti) (3812) 46. ((genital* NEAR/5 ulcer*):ab,ti) OR ((anogenital NEAR/5 ulcer*):ab,ti) OR ((anorectal NEAR/5 ulcer*):ab,ti) OR ((penile NEAR/5 ulcer*):ab,ti) OR ((penis NEAR/5 ulcer*):ab,ti) (4101) 47. ((venereal NEAR/5 ulcer*):ab,ti) OR ((venereal NEAR/5 wart*):ab,ti) (143) 48. 'human papillomavirus':ab,ti OR hpv:ab,ti (53560) 49. 'alphapapillomavirus'/exp OR 'human papillomavirus type 11'/exp OR 'human papillomavirus type 16'/exp OR 'human papillomavirus type 18'/exp OR 'human papillomavirus type 31'/exp OR 'human papillomavirus type 6'/exp OR 'betapapillomavirus'/exp OR 'gammapapillomavirus'/exp (12888) 50. 'human immunodeficiency virus'/exp OR 'acquired immune deficiency syndrome'/exp (279681) 51. ((human NEAR/5 immun* NEAR/5 virus):ab,ti) OR hiv:ab,ti OR ((acquired NEAR/5 immun* NEAR/5 deficiency NEAR/5 syndrome):ab,ti) OR aids:ab,ti OR ((acquired NEAR/5 immun* NEAR/5 syndrome):ab,ti) (451328) 52. #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 (804275) 53. 'randomized controlled trial'/de (490928) 54. 'controlled clinical study'/de (423918) 55. random*:ti,ab (1272907) 56. 'randomization'/de (77078) 57. 'intermethod comparison'/de (232236) 58. placebo:ti,ab (266693) 59. compare:ti OR compared:ti OR comparison:ti (467839) 60. (evaluated:ab OR evaluate:ab OR evaluating:ab OR assessed:ab OR assess:ab) AND (compare:ab OR compared:ab OR comparing:ab OR comparison:ab) (1699926) 61. (open NEAR/1 label):ti,ab (62490) 62. ((double OR single OR doubly OR singly) NEAR/1 (blind OR blinded OR blindly)):ti,ab (205437) 63. 'double blind procedure'/de (147125) 64. (parallel NEXT/1 group*):ti,ab (21187) 65. crossover:ti,ab OR 'cross over':ti,ab (90967) 66. ((assign* OR match OR matched OR allocation) NEAR/5 (alternate OR group* OR intervention* OR patient* OR subject* OR participant*)):ti,ab (275657) 67. assigned:ti,ab OR allocated:ti,ab (323474) 68. (controlled NEAR/7 (study OR design OR trial)):ti,ab (287276) 69. volunteer:ti,ab OR volunteers:ti,ab (221053) 70. trial:ti (245818) 71. 'human experiment'/de (399875) 72. #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 (4208197) 73. #24 AND #52 AND #72 (2830) 74. #24 AND #52 AND #72 AND [embase]/lim AND [2016‐2018]/py (399) |
| # of records identified | 399 |
| # of records without duplicates | 372 |
| Search electronic report #3 | |
| Search type | Update |
| Database | The Cochrane Central Register of Controlled Trials (CENTRAL) |
| Platform | Ovid |
| Search date | 30/10/2016 |
| Update date | Undefined |
| Range of search date | 2015‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1 microbicid$.tw. (297) 2 antimicrobial.tw. (4118) 3 1 or 2 (4403) 4 vagin$.tw. (10071) 5 rectal$.tw. (6793) 6 rectum.tw. (1470) 7 anus.tw. (285) 8 anal$.tw. (300211) 9 topical$.tw. (16842) 10 local$.tw. (43291) 11 or/4‐10 (346155) 12 3 and 11 (1790) 13 exp Anti‐Infective Agents, Local/ (6725) 14 ((anti adj1 infective$) and topical).tw. (14) 15 ((anti adj1 infective$) and local).tw. (12) 16 (antiinfective$ adj5 topical).tw. (3) 17 (antiinfective$ adj5 local).tw. (0) 18 (mercurial adj5 antiseptic).tw. (0) 19 (antifung$ adj5 topical).tw. (143) 20 (antivir$ adj5 topical).tw. (25) 21 (antibacteri$ adj5 topical).tw. (54) 22 or/13‐21 (6952) 23 12 or 22 (8496) 24 exp Sexually transmitted diseases/ or exp Sexually transmitted diseases, bacterial/ or exp Chancroid/ or exp Chlamydia infections/ or exp Lymphogranuloma venereum/ or exp Gonorrhea/ or exp Granuloma inguinale/ or exp Syphilis/ or exp Neurosyphilis/ or exp Tabes dorsalis/ or exp Syphilis, latent/ (9866) 25 exp Sexually transmitted diseases, viral/ or exp Condylomata acuminata/ or exp Herpes genitalis/ (8705) 26 (std or stds or sti or stis).tw. (1457) 27 ((sex$ adj5 transmitted) or (sex$ adj5 transmiss$)).tw. (1357) 28 ((venereal adj5 disease$) or (venereal adj5 infection$) or (venereal adj5 disorder$)).tw. (34) 29 ((genital adj5 disease$) or (genital adj5 infection$) or (genital adj5 disorder$)).tw. (738) 30 exp Chlamydia/ or exp Chlamydia trachomatis/ (313) 31 chlamydia$.tw. (1201) 32 exp Treponema pallidum/ or treponema pallidum.tw. or condylom$.tw. or syphili$.tw. (672) 33 (lymphogranuloma venereum or lymphogranuloma inguinale).tw. (9) 34 (granuloma inguinale or granuloma venereum or donovanosis or donovania).tw. (6) 35 exp Neisseria gonorrhoeae/ (168) 36 (gonorrh$ or gonococc$).tw. (1191) 37 (nongonococcal urethritis or non‐gonococcal urethritis or ngu).tw. (148) 38 exp Herpesvirus 1, human/ or exp Herpesvirus 2, human/ or (hsv1 or hsv‐1 or hsv2 or hsv‐2 or (herpes adj5 genital$)).tw. (719) 39 (human cytomegalovirus or human herpesvirus‐5 or hhv‐5 or hcmv).tw. (75) 40 exp Candidiasis/ or exp Candidiasis, vulvovaginal/ or exp Candidiasis, invasive/ or exp Candidemia/ or exp Candida albicans/ (884) 41 (candidiasis or ((vagina$ adj5 candidosis) or ((vulvovagina$ adj5 candidosis) or (vagina$ adj5 mycoses)))).tw. (1001) 42 exp Vaginitis/ or exp Trichomonas vaginitis/ or exp Trichomonas vaginalis/ or exp Vulvovaginitis/ or (vaginitis or trichomoniasis or vaginalis).tw. (1185) 43 exp Vaginosis, bacterial/ or exp Gardnerella vaginalis/ or (vaginosis adj5 bacterial).tw. (558) 44 exp Balanitis/ or balanitis.tw. (30) 45 ((venereal adj5 ulcer$) or (venereal adj5 wart$)).tw. (3) 46 ((genital$ adj5 ulcer$) or (anogenital adj5 ulcer$) or (anorectal adj5 ulcer$) or (penile adj5 ulcer$) or (penis adj5 ulcer$)).tw. (119) 47 ((genital$ adj5 wart$) or (anogenital adj5 wart$) or (anorectal adj5 wart$) or (penile adj5 wart$) or (penis adj5 wart$)).tw. (256) 48 exp Alphapapillomavirus/ or exp Human papillomavirus 6/ or exp Human papillomavirus 11/ or exp Human papillomavirus 16/ or exp Human papillomavirus 18/ or exp Human papillomavirus 31/ or exp Betapapillomavirus/ or exp Gammapapillomavirus/ (195) 49 (human papillomavirus$ or hpv or recurrent respiratory papillomatosis or condyloma$).tw. (1901) 50 exp Acquired Immunodeficiency Syndrome/ or exp HIV/ (3723) 51 ((human adj5 immun$ adj5 virus) or hiv or (acquired adj5 immun$ adj5 deficiency adj5 syndrome) or aids or (acquired adj5 immun$ adj5 syndrome)).tw. (16796) 52 or/24‐51 (25215) 53 23 and 52 (636) 54 limit 53 to yr="2016 ‐Current" (59) |
| # of records identified | 59 |
| # of records without duplicates | 24 |
| Search electronic report #4 | |
| Search type | Update |
| Database | LILACS http://lilacs.bvsalud.org/es/ |
| Platform | Biblioteca Virtual en Salud (BVS), interfaz iAHx |
| Search date | 30/03/2018 |
| Update date | Undefined |
| Range of search date | 2016 ‐ Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy | (ab:(microbicide*)) OR (ti:(microbicid*)) AND (ab:(topical*)) OR (ti:(topical*)) AND db:("LILACS") |
| # of records identified | 0 |
| # of records without duplicates | 0 |
| Search electronic report #5 | |
| Search type | Update |
| Database | Web of Science webofscience.com/ |
| Platform | Thomson Reuters |
| Search date | 30/10/2016 |
| Update date | Undefined |
| Range of search date | 2016‐current |
| Language restrictions | None |
| Other limits | None |
| Search strategy | TS=(topical*) AND TS=(microbicid*) AND TI=(trial) Refinado por: Años de publicación: ( 2016 OR 2018 ) |
| # of records identified | 2 |
| # of records without duplicates | 2 |
| Search electronic report #6 | |
| Search type | New |
| Database | International Clinical Trials Registry Platform www.who.int/ictrp/ |
| Platform | ICTRP Portal |
| Search date | 30/03/2018 |
| Update date | Undefined |
| Range of search date | None |
| Language restrictions | None |
| Other limits | None |
| Search strategy | topical microbicid* |
| # of records identified | 0 (Published 2016‐2018) |
| # of records without duplicates | NA |
| Search electronic report #7 | |
| Search type | New |
| Database | Clinicaltrials.gov |
| Platform | Clinicaltrials.gov |
| Search date | 30/03/2018 |
| Update date | Undefined |
| Range of search date | None |
| Language restrictions | None |
| Other limits | None |
| Search strategy | "topical microbicide" |
| # of records identified | 0 (for 2016‐2018) |
| # of records without duplicates | N/A |
Appendix 5. Search conducted in May 2019, STI Review Group STI Review Group
| Search electronic report #1 | |
| Search type | Update |
| Databases | § MEDLINE § MEDLINE In‐Process & Other Non‐Indexed Citations § MEDLINE Daily Update |
| Platform | Ovid |
| Search date | 03/05/2019 |
| Update date | Undefined |
| Range of search date | 2018‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1 microbicid$.tw. (5546) 2 antimicrobial.tw. (118807) 3 1 or 2 (123476) 4 vagin$.tw. (99387) 5 rectal$.tw. (75698) 6 rectum.tw. (32568) 7 anus.tw. (6543) 8 anal$.tw. (5037640) 9 topical$.tw. (89091) 10 local$.tw. (1071218) 11 or/4‐10 (5991516) 12 3 and 11 (40526) 13 exp Anti‐Infective Agents, Local/ (223371) 14 ((anti adj1 infective$) and topical).tw. (132) 15 ((anti adj1 infective$) and local).tw. (153) 16 (antiinfective$ adj5 topical).tw. (13) 17 (antiinfective$ adj5 local).tw. (4) 18 (mercurial adj5 antiseptic).tw. (4) 19 (antifung$ adj5 topical).tw. (1083) 20 (antivir$ adj5 topical).tw. (267) 21 (antibacteri$ adj5 topical).tw. (370) 22 or/13‐21 (225070) 23 12 or 22 (263056) 24 exp Sexually transmitted diseases/ or exp Sexually transmitted diseases, bacterial/ or exp Chancroid/ or exp Chlamydia infections/ or exp Lymphogranuloma venereum/ or exp Gonorrhea/ or exp Granuloma inguinale/ or exp Syphilis/ or exp Neurosyphilis/ or exp Tabes dorsalis/ or exp Syphilis, latent/ (335973) 25 exp Sexually transmitted diseases, viral/ or exp Condylomata acuminata/ or exp Herpes genitalis/ (267689) 26 (std or stds or sti or stis).tw. (19834) 27 ((sex$ adj5 transmitted) or (sex$ adj5 transmiss$)).tw. (33057) 28 ((venereal adj5 disease$) or (venereal adj5 infection$) or (venereal adj5 disorder$)).tw. (4034) 29 ((genital adj5 disease$) or (genital adj5 infection$) or (genital adj5 disorder$)).tw. (10054) 30 exp Chlamydia/ or exp Chlamydia trachomatis/ (14578) 31 chlamydia$.tw. (25101) 32 exp Treponema pallidum/ or treponema pallidum.tw. or condylom$.tw. or syphili$.tw. (29080) 33 (lymphogranuloma venereum or lymphogranuloma inguinale).tw. (1043) 34 (granuloma inguinale or granuloma venereum or donovanosis or donovania).tw. (526) 35 exp Neisseria gonorrhoeae/ (9454) 36 (gonorrh$ or gonococc$).tw. (20286) 37 (nongonococcal urethritis or non‐gonococcal urethritis or ngu).tw. (1220) 38 exp Herpesvirus 1, human/ or exp Herpesvirus 2, human/ or (hsv1 or hsv‐1 or hsv2 or hsv‐2 or (herpes adj5 genital$)).tw. (21307) 39 (human cytomegalovirus or human herpesvirus‐5 or hhv‐5 or hcmv).tw. (8910) 40 exp Candidiasis/ or exp Candidiasis, vulvovaginal/ or exp Candidiasis, invasive/ or exp Candidemia/ or exp Candida albicans/ (47564) 41 (candidiasis or ((vagina$ adj5 candidosis) or ((vulvovagina$ adj5 candidosis) or (vagina$ adj5 mycoses)))).tw. (13637) 42 exp Vaginitis/ or exp Trichomonas vaginitis/ or exp Trichomonas vaginalis/ or exp Vulvovaginitis/ or (vaginitis or trichomoniasis or vaginalis).tw. (19350) 43 exp Vaginosis, bacterial/ or exp Gardnerella vaginalis/ or (vaginosis adj5 bacterial).tw. (4613) 44 exp Balanitis/ or balanitis.tw. (1109) 45 ((venereal adj5 ulcer$) or (venereal adj5 wart$)).tw. (112) 46 ((genital$ adj5 ulcer$) or (anogenital adj5 ulcer$) or (anorectal adj5 ulcer$) or (penile adj5 ulcer$) or (penis adj5 ulcer$)).tw. (2823) 47 ((genital$ adj5 wart$) or (anogenital adj5 wart$) or (anorectal adj5 wart$) or (penile adj5 wart$) or (penis adj5 wart$)).tw. (2810) 48 exp Alphapapillomavirus/ or exp Human papillomavirus 6/ or exp Human papillomavirus 11/ or exp Human papillomavirus 16/ or exp Human papillomavirus 18/ or exp Human papillomavirus 31/ or exp Betapapillomavirus/ or exp Gammapapillomavirus/ (6961) 49 (human papillomavirus$ or hpv or recurrent respiratory papillomatosis or condyloma$).tw. (43497) 50 exp Acquired Immunodeficiency Syndrome/ or exp HIV/ (159431) 51 ((human adj5 immun$ adj5 virus) or hiv or (acquired adj5 immun$ adj5 deficiency adj5 syndrome) or aids or (acquired adj5 immun$ adj5 syndrome)).tw. (358774) 52 or/24‐51 (617136) 53 randomized controlled trial.pt. (481012) 54 controlled clinical trial.pt. (93032) 55 randomized.ab. (392991) 56 placebo.ab. (182262) 57 randomly.ab. (270986) 58 trial.ti. (174701) 59 clinical trials as topic.sh. (186857) 60 or/53‐59 (1115080) 61 exp animals/ not humans.sh. (4576104) 62 60 not 61 (1017498) 63 23 and 52 and 62 (1013) 64 limit 63 to yr="2018 ‐Current" (23) |
| # of records identified | 23 |
| # of records without duplicates | 23 |
| Search electronic report #2 | |
| Search type | Update |
| Database | EMBASE |
| Platform | EMBASE.com |
| Search date | 07/05/2019 |
| Update date | Undefined |
| Range of search date | 2018‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1. 'microbicide'/exp 1952 2. microbicid*:ab,ti 7023 3. antimicrobial:ab,ti 180904 4. #1 OR #2 OR #3 187261 5. vagin*:ab,ti 153941 6. rectal*:ab,ti 123278 7. rectum:ab,ti 53529 8. anus:ab,ti 9961 9. anal*:ab,ti 7630027 10. topical*:ab,ti 133781 11. local*:ab,ti 1525262 12. #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 7948300 13. #3 AND #11 9321 14. 'topical antiinfective agent'/exp 585989 15. ((anti NEXT/1 infective*):ab,ti) AND topical:ab,ti 201 16. ((anti NEXT/1 infective*):ab,ti) AND local:ab,ti 238 17. (antiinfective* NEAR/5 topical):ab,ti 24 18. (antiinfective* NEAR/5 local):ab,ti 13 19. (mercurial NEAR/5 antiseptic):ab,ti 5 20. (antifung* NEAR/5 topical):ab,ti 1734 21. (antivir* NEAR/5 topical):ab,ti 347 22. (antibacteri* NEAR/5 topical):ab,ti 552 23. #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 596775 24. #13 OR #23 596775 25. 'sexually transmitted disease'/exp OR 'chlamydiasis'/exp OR 'condyloma'/exp OR 'condyloma acuminatum'/exp OR 'condyloma latum'/exp OR 'genital herpes'/exp OR 'gonorrhea'/exp OR 'granuloma inguinale'/exp OR 'lymphogranuloma venereum'/exp OR 'syphilis'/exp OR 'secondary syphilis'/exp OR 'tabes dorsalis'/exp OR 'ulcus molle'/exp 119122 26. std:ab,ti OR stds:ab,ti OR sti:ab,ti OR stis:ab,ti 31976 27. ((sex* NEAR/5 transmitted):ab,ti) OR ((sex* NEAR/5 transmiss*):ab,ti) 43565 28. ((venereal NEAR/5 disease*):ab,ti) OR ((venereal NEAR/5 infection*):ab,ti) OR ((venereal NEAR/5 disorder*):ab,ti) 4795 29. ((genital NEAR/5 disease*):ab,ti) OR ((genital NEAR/5 infection*):ab,ti) OR ((genital NEAR/5 disorder*):ab,ti) 13767 30. 'chlamydia'/exp OR 'chlamydia trachomatis'/exp 32791 31. chlamydia*:ab,ti 32947 32. syphili*:ab,ti OR chancre*:ab,ti OR condylom*:ab,ti 36001 33. 'lymphogranuloma venereum':ab,ti OR 'lymphogranuloma inguinale':ab,ti 1346 34. 'granuloma inguinale':ab,ti OR 'granuloma venereum':ab,ti OR donovanosis:ab,ti OR donovania:ab,ti 636 35. 'neisseria gonorrhoeae'/exp 15775 36. gonorrh*:ab,ti OR gonococc*:ab,ti 26100 37. 'nongonococcal urethritis'/exp OR ((nongonococcal:ab,ti AND urethritis:ab,ti OR 'non gonococcal':ab,ti) AND urethritis:ab,ti) OR ngu:ab,ti 2186 38. 'herpes simplex virus 1'/exp OR 'herpes simplex virus 2'/exp OR hsv1:ab,ti OR 'hsv 1':ab,ti OR hsv2:ab,ti OR 'hsv 2':ab,ti OR ((herpes NEAR/5 genital*):ab,ti) 33273 39. (human:ab,ti AND cytomegalovirus:ab,ti OR human:ab,ti) AND 'herpesvirus 5':ab,ti OR 'hhv 5':ab,ti OR hcmv:ab,ti 6752 40. 'candidiasis'/exp OR 'vagina candidiasis'/exp OR 'genital candidiasis'/exp OR 'invasive candidiasis'/exp OR 'candidemia'/exp OR 'candida albicans'/exp 90422 41. candidiasis:ab,ti OR ((vagina* NEAR/5 candid*):ab,ti) OR ((vulvovagina* NEAR/5 candid*):ab,ti) OR ((vagina* NEAR/5 mycoses):ab,ti) 20412 42. 'gardnerella infection'/exp OR 'gardnerella vaginalis'/exp OR ((vaginosis NEAR/5 bacterial):ab,ti) 6279 43. 'vaginitis'/exp OR 'vulvovaginitis'/exp OR 'trichomoniasis'/exp OR 'trichomonas vaginalis'/exp OR vaginitis:ab,ti OR trichomoniasis:ab,ti OR vaginalis:ab,ti 29916 44. 'balanitis'/exp OR balanitis:ab,ti 1867 45. ((genital* NEAR/5 wart*):ab,ti) OR ((anogenital NEAR/5 wart*):ab,ti) OR ((anorectal NEAR/5 wart*):ab,ti) OR ((penile NEAR/5 wart*):ab,ti) OR ((penis NEAR/5 wart*):ab,ti) 4066 46. ((genital* NEAR/5 ulcer*):ab,ti) OR ((anogenital NEAR/5 ulcer*):ab,ti) OR ((anorectal NEAR/5 ulcer*):ab,ti) OR ((penile NEAR/5 ulcer*):ab,ti) OR ((penis NEAR/5 ulcer*):ab,ti) 4412 47. ((venereal NEAR/5 ulcer*):ab,ti) OR ((venereal NEAR/5 wart*):ab,ti) 147 48. 'human papillomavirus':ab,ti OR hpv:ab,ti 58845 49. 'alphapapillomavirus'/exp OR 'human papillomavirus type 11'/exp OR 'human papillomavirus type 16'/exp OR 'human papillomavirus type 18'/exp OR 'human papillomavirus type 31'/exp OR 'human papillomavirus type 6'/exp OR 'betapapillomavirus'/exp OR 'gammapapillomavirus'/exp 14199 50. 'human immunodeficiency virus'/exp OR 'acquired immune deficiency syndrome'/exp 292514 51. ((human NEAR/5 immun* NEAR/5 virus):ab,ti) OR hiv:ab,ti OR ((acquired NEAR/5 immun* NEAR/5 deficiency NEAR/5 syndrome):ab,ti) OR aids:ab,ti OR ((acquired NEAR/5 immun* NEAR/5 syndrome):ab,ti) 481077 52. #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 853226 53. 'randomized controlled trial'/de 546921 54. 'controlled clinical study'/de 426684 55. random*:ti,ab 1399791 56. 'randomization'/de 81911 57. 'intermethod comparison'/de 247989 58. placebo:ti,ab 286445 59. compare:ti OR compared:ti OR comparison:ti 499591 60. (evaluated:ab OR evaluate:ab OR evaluating:ab OR assessed:ab OR assess:ab) AND (compare:ab OR compared:ab OR comparing:ab OR comparison:ab) 1900522 61. (open NEAR/1 label):ti,ab 70258 62. ((double OR single OR doubly OR singly) NEAR/1 (blind OR blinded OR blindly)):ti,ab 219419 63. 'double blind procedure'/de 160056 64. (parallel NEXT/1 group*):ti,ab 23227 65. crossover:ti,ab OR 'cross over':ti,ab 97922 66. ((assign* OR match OR matched OR allocation) NEAR/5 (alternate OR group* OR intervention* OR patient* OR subject* OR participant*)):ti,ab 302310 67. assigned:ti,ab OR allocated:ti,ab 355136 68. (controlled NEAR/7 (study OR design OR trial)):ti,ab 317871 69. volunteer:ti,ab OR volunteers:ti,ab 234759 70. trial:ti 274260 71. 'human experiment'/de 450646 72. #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 4614354 73. #24 AND #52 AND #72 2795 74. #24 AND #52 AND #72 AND [embase]/lim AND [2018‐2019]/py 226 |
| # of records identified | 226 |
| # of records without duplicates | 223 |
| Search electronic report #3 | |
| Search type | Update |
| Database | The Cochrane Central Register of Controlled Trials (CENTRAL) |
| Platform | Ovid |
| Search date | 03/05/2019 |
| Update date | Undefined |
| Range of search date | 2018‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1. microbicid$.tw. (402) 2. antimicrobial.tw. (5762) 3. 1 or 2 (6146) 4. vagin$.tw. (15260) 5. rectal$.tw. (9853) 6. rectum.tw. (2534) 7. anus.tw. (524) 8. anal$.tw. (421185) 9. topical$.tw. (23828) 10. local$.tw. (68328) 11. or/4‐10 (491385) 12. 3 and 11 (2636) 13. exp Anti‐Infective Agents, Local/ (8394) 14. ((anti adj1 infective$) and topical).tw. (20) 15. ((anti adj1 infective$) and local).tw. (16) 16. (antiinfective$ adj5 topical).tw. (3) 17. (antiinfective$ adj5 local).tw. (0) 18. (mercurial adj5 antiseptic).tw. (0) 19. (antifung$ adj5 topical).tw. (191) 20. (antivir$ adj5 topical).tw. (40) 21. (antibacteri$ adj5 topical).tw. (86) 22. or/13‐21 (8722) 23. 12 or 22 (11056) 24. exp Sexually transmitted diseases/ or exp Sexually transmitted diseases, bacterial/ or exp Chancroid/ or exp Chlamydia infections/ or exp Lymphogranuloma venereum/ or exp Gonorrhea/ or exp Granuloma inguinale/ or exp Syphilis/ or exp Neurosyphilis/ or exp Tabes dorsalis/ or exp Syphilis, latent/ (12530) 25. exp Sexually transmitted diseases, viral/ or exp Condylomata acuminata/ or exp Herpes genitalis/ (11167) 26. (std or stds or sti or stis).tw. (2207) 27. ((sex$ adj5 transmitted) or (sex$ adj5 transmiss$)).tw. (2022) 28. ((venereal adj5 disease$) or (venereal adj5 infection$) or (venereal adj5 disorder$)).tw. (38) 29. ((genital adj5 disease$) or (genital adj5 infection$) or (genital adj5 disorder$)).tw. (1491) 30. exp Chlamydia/ or exp Chlamydia trachomatis/ (327) 31. chlamydia$.tw. (1525) 32. exp Treponema pallidum/ or treponema pallidum.tw. or condylom$.tw. or syphili$.tw. (936) 1. (lymphogranuloma venereum or lymphogranuloma inguinale).tw. (12) 2. (granuloma inguinale or granuloma venereum or donovanosis or donovania).tw. (6) 3. exp Neisseria gonorrhoeae/ (170) 4. (gonorrh$ or gonococc$).tw. (1392) 5. (nongonococcal urethritis or non‐gonococcal urethritis or ngu).tw. (160) 6. exp Herpesvirus 1, human/ or exp Herpesvirus 2, human/ or (hsv1 or hsv‐1 or hsv2 or hsv‐2 or (herpes adj5 genital$)).tw. (888) 7. (human cytomegalovirus or human herpesvirus‐5 or hhv‐5 or hcmv).tw. (110) 8. exp Candidiasis/ or exp Candidiasis, vulvovaginal/ or exp Candidiasis, invasive/ or exp Candidemia/ or exp Candida albicans/ (996) 9. (candidiasis or ((vagina$ adj5 candidosis) or ((vulvovagina$ adj5 candidosis) or (vagina$ adj5 mycoses)))).tw. (1392) 10. exp Vaginitis/ or exp Trichomonas vaginitis/ or exp Trichomonas vaginalis/ or exp Vulvovaginitis/ or (vaginitis or trichomoniasis or vaginalis).tw. (1606) 11. exp Vaginosis, bacterial/ or exp Gardnerella vaginalis/ or (vaginosis adj5 bacterial).tw. (847) 12. exp Balanitis/ or balanitis.tw. (37) 13. ((venereal adj5 ulcer$) or (venereal adj5 wart$)).tw. (17) 14. ((genital$ adj5 ulcer$) or (anogenital adj5 ulcer$) or (anorectal adj5 ulcer$) or (penile adj5 ulcer$) or (penis adj5 ulcer$)).tw. (153) 15. ((genital$ adj5 wart$) or (anogenital adj5 wart$) or (anorectal adj5 wart$) or (penile adj5 wart$) or (penis adj5 wart$)).tw. (405) 16. exp Alphapapillomavirus/ or exp Human papillomavirus 6/ or exp Human papillomavirus 11/ or exp Human papillomavirus 16/ or exp Human papillomavirus 18/ or exp Human papillomavirus 31/ or exp Betapapillomavirus/ or exp Gammapapillomavirus/ (212) 17. (human papillomavirus$ or hpv or recurrent respiratory papillomatosis or condyloma$).tw. (2975) 18. exp Acquired Immunodeficiency Syndrome/ or exp HIV/ (4377) 19. ((human adj5 immun$ adj5 virus) or hiv or (acquired adj5 immun$ adj5 deficiency adj5 syndrome) or aids or (acquired adj5 immun$ adj5 syndrome)).tw. (26487) 20. or/24‐51 (38235) 21. 23 and 52 (827) 22. 54 limit 53 to yr="2018 ‐Current" (34) |
| # of records identified | 34 |
| # of records without duplicates | 27 |
| Search electronic report #4 | |
| Search type | Update |
| Database | LILACS http://lilacs.bvsalud.org/es/ |
| Platform | Biblioteca Virtual en Salud (BVS), interfaz iAHx |
| Search date | 07/05/2019 |
| Update date | Undefined |
| Range of search date | 2018‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy | (ti:(microbicid*)) OR (ab:(microbicid*)) AND (ti:(topic*)) AND (ab:(topic*)) AND (instance:"regional") AND ( db:("LILACS")) |
| # of records identified | 0 |
| # of records without duplicates | 0 |
Appendix 6. Search conducted in August 2020, STI Review Group
| Search electronic report #1 | |
| Search type | Update |
| Databases |
|
| Platform | Ovid |
| Search date | 22/08/2020 |
| Update date | Undefined |
| Range of search date | 2019‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1 microbicid$.tw. (5791) 2 antimicrobial.tw. (131733) 3 1 or 2 (136588) 4 vagin$.tw. (104602) 5 rectal$.tw. (79852) 6 rectum.tw. (33761) 7 anus.tw. (6812) 8 anal$.tw. (5463612) 9 topical$.tw. (94489) 10 local$.tw. (1130675) 11 or/4‐10 (6461416) 12 3 and 11 (45846) 13 exp Anti‐Infective Agents, Local/ (232470) 14 ((anti adj1 infective$) and topical).tw. (145) 15 ((anti adj1 infective$) and local).tw. (176) 16 (antiinfective$ adj5 topical).tw. (13) 17 (antiinfective$ adj5 local).tw. (4) 18 (mercurial adj5 antiseptic).tw. (4) 19 (antifung$ adj5 topical).tw. (1154) 20 (antivir$ adj5 topical).tw. (285) 21 (antibacteri$ adj5 topical).tw. (388) 22 or/13‐21 (234300) 23 12 or 22 (277426) 24 exp Sexually transmitted diseases/ or exp Sexually transmitted diseases, bacterial/ or exp Chancroid/ or exp Chlamydia infections/ or exp Lymphogranuloma venereum/ or exp Gonorrhea/ or exp Granuloma inguinale/ or exp Syphilis/ or exp Neurosyphilis/ or exp Tabes dorsalis/ or exp Syphilis, latent/ (351393) 25 exp Sexually transmitted diseases, viral/ or exp Condylomata acuminata/ or exp Herpes genitalis/ (281560) 26 (std or stds or sti or stis).tw. (21251) 27 ((sex$ adj5 transmitted) or (sex$ adj5 transmiss$)).tw. (35298) 28 ((venereal adj5 disease$) or (venereal adj5 infection$) or (venereal adj5 disorder$)).tw. (4087) 29 ((genital adj5 disease$) or (genital adj5 infection$) or (genital adj5 disorder$)).tw. (10419) 30 exp Chlamydia/ or exp Chlamydia trachomatis/ (15115) 31 chlamydia$.tw. (26038) 32 exp Treponema pallidum/ or treponema pallidum.tw. or condylom$.tw. or syphili$.tw. (30218) 33 (lymphogranuloma venereum or lymphogranuloma inguinale).tw. (1079) 34 (granuloma inguinale or granuloma venereum or donovanosis or donovania).tw. (531) 35 exp Neisseria gonorrhoeae/ (9858) 36 (gonorrh$ or gonococc$).tw. (21092) 37 (nongonococcal urethritis or non‐gonococcal urethritis or ngu).tw. (1255) 38 exp Herpesvirus 1, human/ or exp Herpesvirus 2, human/ or (hsv1 or hsv‐1 or hsv2 or hsv‐2 or (herpes adj5 genital$)).tw. (22003) 39 (human cytomegalovirus or human herpesvirus‐5 or hhv‐5 or hcmv).tw. (9254) 40 exp Candidiasis/ or exp Candidiasis, vulvovaginal/ or exp Candidiasis, invasive/ or exp Candidemia/ or exp Candida albicans/ (49527) 41 (candidiasis or ((vagina$ adj5 candidosis) or ((vulvovagina$ adj5 candidosis) or (vagina$ adj5 mycoses)))).tw. (14283) 42 exp Vaginitis/ or exp Trichomonas vaginitis/ or exp Trichomonas vaginalis/ or exp Vulvovaginitis/ or (vaginitis or trichomoniasis or vaginalis).tw. (20020) 43 exp Vaginosis, bacterial/ or exp Gardnerella vaginalis/ or (vaginosis adj5 bacterial).tw. (4850) 44 exp Balanitis/ or balanitis.tw. (1140) 45 ((venereal adj5 ulcer$) or (venereal adj5 wart$)).tw. (114) 46 ((genital$ adj5 ulcer$) or (anogenital adj5 ulcer$) or (anorectal adj5 ulcer$) or (penile adj5 ulcer$) or (penis adj5 ulcer$)).tw. (2945) 47 ((genital$ adj5 wart$) or (anogenital adj5 wart$) or (anorectal adj5 wart$) or (penile adj5 wart$) or (penis adj5 wart$)).tw. (2965) 48 exp Alphapapillomavirus/ or exp Human papillomavirus 6/ or exp Human papillomavirus 11/ or exp Human papillomavirus 16/ or exp Human papillomavirus 18/ or exp Human papillomavirus 31/ or exp Betapapillomavirus/ or exp Gammapapillomavirus/ (7508) 49 (human papillomavirus$ or hpv or recurrent respiratory papillomatosis or condyloma$).tw. (47220) 50 exp Acquired Immunodeficiency Syndrome/ or exp HIV/ (164394) 51 ((human adj5 immun$ adj5 virus) or hiv or (acquired adj5 immun$ adj5 deficiency adj5 syndrome) or aids or (acquired adj5 immun$ adj5 syndrome)).tw. (377669) 52 or/24‐51 (646738) 53 randomized controlled trial.pt. (511315) 54 controlled clinical trial.pt. (93789) 55 randomized.ab. (432043) 56 placebo.ab. (192863) 57 randomly.ab. (292602) 58 trial.ti. (196263) 59 clinical trials as topic.sh. (192609) 60 or/53‐59 (1192581) 61 exp animals/ not humans.sh. (4727456) 62 60 not 61 (1088647) 63 23 and 52 and 62 (1040) 64 limit 63 to yr="2019 ‐Current" (31) |
| # of records identified | 31 |
| Search electronic report #2 | |
| Search type | Update |
| Database | EMBASE |
| Platform | EMBASE.com |
| Search date | 22/08/2020 |
| Update date | Undefined |
| Range of search date | 2019‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1. 'microbicide'/exp 2013 2. microbicid*:ab,ti 7355 3. antimicrobial:ab,ti 210527 4. #1 OR #2 OR #3 217126 5. vagin*:ab,ti 166224 6. rectal*:ab,ti 134007 7. rectum:ab,ti 57480 8. anus:ab,ti 10671 9. anal*:ab,ti 8490379 10. topical*:ab,ti 145038 11. local*:ab,ti 1655851 12. #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 9906124 13. #4 AND #12 78334 14. 'topical antiinfective agent'/exp 631590 15. ((anti NEXT/1 infective*):ab,ti) AND topical:ab,ti 226 16. ((anti NEXT/1 infective*):ab,ti) AND local:ab,ti 269 17. (antiinfective* NEAR/5 topical):ab,ti 115 18. (antiinfective* NEAR/5 local):ab,ti 52 19. (mercurial NEAR/5 antiseptic):ab,ti 5 20. (antifung* NEAR/5 topical):ab,ti 1966 21. (antivir* NEAR/5 topical):ab,ti 383 22. (antibacteri* NEAR/5 topical):ab,ti 599 23. #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 634475 24. #13 OR #23 706154 25. 'sexually transmitted disease'/exp OR 'chlamydiasis'/exp OR 'condyloma'/exp OR 'condyloma acuminatum'/exp OR 'condyloma latum'/exp OR 'genital herpes'/exp OR 'gonorrhea'/exp OR 'granuloma inguinale'/exp OR 'lymphogranuloma venereum'/exp OR 'syphilis'/exp OR 'secondary syphilis'/exp OR 'tabes dorsalis'/exp OR 'ulcus molle'/exp 124752 26. std:ab,ti OR stds:ab,ti OR sti:ab,ti OR stis:ab,ti 35074 27. ((sex* NEAR/5 transmitted):ab,ti) OR ((sex* NEAR/5 transmiss*):ab,ti) 47056 28. ((venereal NEAR/5 disease*):ab,ti) OR ((venereal NEAR/5 infection*):ab,ti) OR ((venereal NEAR/5 disorder*):ab,ti) 4895 29. ((genital NEAR/5 disease*):ab,ti) OR ((genital NEAR/5 infection*):ab,ti) OR ((genital NEAR/5 disorder*):ab,ti) 14518 30. 'chlamydia'/exp OR 'chlamydia trachomatis'/exp 34262 31. chlamydia*:ab,ti 34688 32. syphili*:ab,ti OR chancre*:ab,ti OR condylom*:ab,ti 38042 33. 'lymphogranuloma venereum':ab,ti OR 'lymphogranuloma inguinale':ab,ti 1400 34. 'granuloma inguinale':ab,ti OR 'granuloma venereum':ab,ti OR donovanosis:ab,ti OR donovania:ab,ti 644 35. 'neisseria gonorrhoeae'/exp 16701 36. gonorrh*:ab,ti OR gonococc*:ab,ti 27649 37. 'nongonococcal urethritis'/exp OR ((nongonococcal:ab,ti AND urethritis:ab,ti OR 'non gonococcal':ab,ti) AND urethritis:ab,ti) OR ngu:ab,ti 2268 38. 'herpes simplex virus 1'/exp OR 'herpes simplex virus 2'/exp OR hsv1:ab,ti OR 'hsv 1':ab,ti OR hsv2:ab,ti OR 'hsv 2':ab,ti OR ((herpes NEAR/5 genital*):ab,ti) 34907 39. (human:ab,ti AND cytomegalovirus:ab,ti OR human:ab,ti) AND 'herpesvirus 5':ab,ti OR 'hhv 5':ab,ti OR hcmv:ab,ti 7192 40. 'candidiasis'/exp OR 'vagina candidiasis'/exp OR 'genital candidiasis'/exp OR 'invasive candidiasis'/exp OR 'candidemia'/exp OR 'candida albicans'/exp 96898 41. candidiasis:ab,ti OR ((vagina* NEAR/5 candid*):ab,ti) OR ((vulvovagina* NEAR/5 candid*):ab,ti) OR ((vagina* NEAR/5 mycoses):ab,ti) 21868 42. 'gardnerella infection'/exp OR 'gardnerella vaginalis'/exp OR ((vaginosis NEAR/5 bacterial):ab,ti) 6837 43. 'vaginitis'/exp OR 'vulvovaginitis'/exp OR 'trichomoniasis'/exp OR 'trichomonas vaginalis'/exp OR vaginitis:ab,ti OR trichomoniasis:ab,ti OR vaginalis:ab,ti 31495 44. 'balanitis'/exp OR balanitis:ab,ti 1939 45. ((genital* NEAR/5 wart*):ab,ti) OR ((anogenital NEAR/5 wart*):ab,ti) OR ((anorectal NEAR/5 wart*):ab,ti) OR ((penile NEAR/5 wart*):ab,ti) OR ((penis NEAR/5 wart*):ab,ti) 4356 46. ((genital* NEAR/5 ulcer*):ab,ti) OR ((anogenital NEAR/5 ulcer*):ab,ti) OR ((anorectal NEAR/5 ulcer*):ab,ti) OR ((penile NEAR/5 ulcer*):ab,ti) OR ((penis NEAR/5 ulcer*):ab,ti) 4751 47. ((venereal NEAR/5 ulcer*):ab,ti) OR ((venereal NEAR/5 wart*):ab,ti) 150 48. 'human papillomavirus':ab,ti OR hpv:ab,ti 65259 49. 'alphapapillomavirus'/exp OR 'human papillomavirus type 11'/exp OR 'human papillomavirus type 16'/exp OR 'human papillomavirus type 18'/exp OR 'human papillomavirus type 31'/exp OR 'human papillomavirus type 6'/exp OR 'betapapillomavirus'/exp OR 'gammapapillomavirus'/exp 15803 50. 'human immunodeficiency virus'/exp OR 'acquired immune deficiency syndrome'/exp 305160 51. ((human NEAR/5 immun* NEAR/5 virus):ab,ti) OR hiv:ab,ti OR ((acquired NEAR/5 immun* NEAR/5 deficiency NEAR/5 syndrome):ab,ti) OR aids:ab,ti OR ((acquired NEAR/5 immun* NEAR/5 syndrome):ab,ti) 512758 52. #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 907580 53. 'randomized controlled trial'/de 615806 54. 'controlled clinical study'/de 430742 55. random*:ti,ab 1558879 56. 'randomization'/de 87241 57. 'intermethod comparison'/de 264352 58. placebo:ti,ab 309415 59. compare:ti OR compared:ti OR comparison:ti 536862 60. (evaluated:ab OR evaluate:ab OR evaluating:ab OR assessed:ab OR assess:ab) AND (compare:ab OR compared:ab OR comparing:ab OR comparison:ab) 2143515 61. (open NEAR/1 label):ti,ab 80276 62. ((double OR single OR doubly OR singly) NEAR/1 (blind OR blinded OR blindly)):ti,ab 236261 63. 'double blind procedure'/de 175158 64. (parallel NEXT/1 group*):ti,ab 25806 65. crossover:ti,ab OR 'cross over':ti,ab 106293 66. ((assign* OR match OR matched OR allocation) NEAR/5 (alternate OR group* OR intervention* OR patient* OR subject* OR participant*)):ti,ab 334917 67. assigned:ti,ab OR allocated:ti,ab 392870 68. (controlled NEAR/7 (study OR design OR trial)):ti,ab 355354 69. volunteer:ti,ab OR volunteers:ti,ab 249734 70. trial:ti 311663 71. 'human experiment'/de 509802 72. #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 5105783 73. #24 AND #52 AND #72 4852 74. #24 AND #52 AND #72 AND [embase]/lim AND [2019‐2020]/py 485 |
| # of records identified | 485 |
| Search electronic report #3 | |
| Search type | Update |
| Database | The Cochrane Central Register of Controlled Trials (CENTRAL) |
| Platform | Ovid |
| Search date | 22/08/2020 |
| Update date | Undefined |
| Range of search date | 2019‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy (results) | 1. microbicid$.tw. (456) 2. antimicrobial.tw. (6727) 3. 1 or 2 (7161) 4. vagin$.tw. (17399) 5. rectal$.tw. (11092) 6. rectum.tw. (2915) 7. anus.tw. (606) 8. anal$.tw. (484985) 9. topical$.tw. (26471) 10. local$.tw. (79189) 11. or/4‐10 (564220) 12. 3 and 11 (3145) 13. exp Anti‐Infective Agents, Local/ (9196) 14. ((anti adj1 infective$) and topical).tw. (23) 15. ((anti adj1 infective$) and local).tw. (19) 16. (antiinfective$ adj5 topical).tw. (3) 17. (antiinfective$ adj5 local).tw. (0) 18. (mercurial adj5 antiseptic).tw. (0) 19. (antifung$ adj5 topical).tw. (212) 20. (antivir$ adj5 topical).tw. (41) 21. (antibacteri$ adj5 topical).tw. (91) 22. or/13‐21 (9554) 23. 12 or 22 (12373) 24. exp Sexually transmitted diseases/ or exp Sexually transmitted diseases, bacterial/ or exp Chancroid/ or exp Chlamydia infections/ or exp Lymphogranuloma venereum/ or exp Gonorrhea/ or exp Granuloma inguinale/ or exp Syphilis/ or exp Neurosyphilis/ or exp Tabes dorsalis/ or exp Syphilis, latent/ (14158) 25. exp Sexually transmitted diseases, viral/ or exp Condylomata acuminata/ or exp Herpes genitalis/ (12670) 26. (std or stds or sti or stis).tw. (2594) 27. ((sex$ adj5 transmitted) or (sex$ adj5 transmiss$)).tw. (2367) 28. ((venereal adj5 disease$) or (venereal adj5 infection$) or (venereal adj5 disorder$)).tw. (40) 29. ((genital adj5 disease$) or (genital adj5 infection$) or (genital adj5 disorder$)).tw. (1626) 30. exp Chlamydia/ or exp Chlamydia trachomatis/ (335) 31. chlamydia$.tw. (1685) 32. exp Treponema pallidum/ or treponema pallidum.tw. or condylom$.tw. or syphili$.tw. (1041) 33. (lymphogranuloma venereum or lymphogranuloma inguinale).tw. (12) 34. (granuloma inguinale or granuloma venereum or donovanosis or donovania).tw. (6) 35. exp Neisseria gonorrhoeae/ (175) 36. (gonorrh$ or gonococc$).tw. (1520) 37. (nongonococcal urethritis or non‐gonococcal urethritis or ngu).tw. (165) 38. exp Herpesvirus 1, human/ or exp Herpesvirus 2, human/ or (hsv1 or hsv‐1 or hsv2 or hsv‐2 or (herpes adj5 genital$)).tw. (975) 39. (human cytomegalovirus or human herpesvirus‐5 or hhv‐5 or hcmv).tw. (126) 40. exp Candidiasis/ or exp Candidiasis, vulvovaginal/ or exp Candidiasis, invasive/ or exp Candidemia/ or exp Candida albicans/ (1053) 41. (candidiasis or ((vagina$ adj5 candidosis) or ((vulvovagina$ adj5 candidosis) or (vagina$ adj5 mycoses)))).tw. (1517) 42. exp Vaginitis/ or exp Trichomonas vaginitis/ or exp Trichomonas vaginalis/ or exp Vulvovaginitis/ or (vaginitis or trichomoniasis or vaginalis).tw. (1762) 43. exp Vaginosis, bacterial/ or exp Gardnerella vaginalis/ or (vaginosis adj5 bacterial).tw. (961) 44. exp Balanitis/ or balanitis.tw. (38) 45. ((venereal adj5 ulcer$) or (venereal adj5 wart$)).tw. (20) 46. ((genital$ adj5 ulcer$) or (anogenital adj5 ulcer$) or (anorectal adj5 ulcer$) or (penile adj5 ulcer$) or (penis adj5 ulcer$)).tw. (175) 47. ((genital$ adj5 wart$) or (anogenital adj5 wart$) or (anorectal adj5 wart$) or (penile adj5 wart$) or (penis adj5 wart$)).tw. (447) 48. exp Alphapapillomavirus/ or exp Human papillomavirus 6/ or exp Human papillomavirus 11/ or exp Human papillomavirus 16/ or exp Human papillomavirus 18/ or exp Human papillomavirus 31/ or exp Betapapillomavirus/ or exp Gammapapillomavirus/ (229) 49. (human papillomavirus$ or hpv or recurrent respiratory papillomatosis or condyloma$).tw. (3594) 50. exp Acquired Immunodeficiency Syndrome/ or exp HIV/ (4744) 51. ((human adj5 immun$ adj5 virus) or hiv or (acquired adj5 immun$ adj5 deficiency adj5 syndrome) or aids or (acquired adj5 immun$ adj5 syndrome)).tw. (30807) 52. or/24‐51 (43934) 53. 23 and 52 (949) 54. limit 53 to yr="2019 ‐Current" (70) |
| # of records identified | 70 |
| Search electronic report #4 | |
| Search type | Update |
| Database | LILACS http://lilacs.bvsalud.org/es/ |
| Platform | Biblioteca Virtual en Salud (BVS), interfaz iAHx |
| Search date | 22/08/2020 |
| Update date | Undefined |
| Range of search date | 2019‐Current |
| Language restrictions | None |
| Other limits | None |
| Search strategy | (ti:(microbicid*)) OR (ab:(microbicid*)) AND (ti:(topic*)) AND (ab:(topic*)) AND (instance:"regional") AND ( db:("LILACS")) |
| # of records identified | 0 |
Data and analyses
Comparison 1. Topical microbicide versus placebo ‐ dichotomous data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 HIV | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2 Herpes simplex | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Tenofovir | 2 | 2439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.82, 0.99] |
| 1.2.2 Cellulose sulphate | 1 | 1425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.37, 2.62] |
| 1.2.3 PRO 2000 | 1 | 6651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.73, 1.23] |
| 1.3 Condyloma acuminatum | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 Cellulose sulphate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 High‐risk HPV | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5 Gonorrhoea | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Carraguard | 1 | 6202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.88, 1.27] |
| 1.5.2 Cellulose sulphate | 2 | 3069 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.67, 1.17] |
| 1.5.3 PRO 2000 | 2 | 8191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.87, 1.52] |
| 1.5.4 BufferGel | 1 | 1546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.51, 1.93] |
| 1.5.5 Tenofovir | 1 | 2010 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.40, 1.10] |
| 1.5.6 Dapivirine | 2 | 4586 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 1.6 Chlamydia | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 Cellulose sulphate | 2 | 3069 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.49, 0.99] |
| 1.6.2 BufferGel | 1 | 1546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.45] |
| 1.6.3 Carraguard | 1 | 6202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.12] |
| 1.6.4 Dapirivine | 2 | 4586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.07] |
| 1.6.5 PRO 2000 | 2 | 8191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.84, 1.22] |
| 1.6.6 Tenofovir | 1 | 2010 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.13] |
| 1.7 Syphilis | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Carraguard | 1 | 6202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.75, 1.52] |
| 1.7.2 Cellulose sulphate | 1 | 1425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.26, 1.81] |
| 1.7.3 Dapivirine | 1 | 1956 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.63, 4.59] |
| 1.7.4 Tenofovir | 1 | 2010 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.58, 2.78] |
| 1.8 Trichomoniasis | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 Dapivirine | 2 | 4588 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.92, 1.23] |
| 1.8.2 BufferGel | 1 | 1546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.80, 1.15] |
| 1.8.3 Carraguard | 1 | 6202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.72, 1.01] |
| 1.8.4 Cellulose sulphate | 1 | 1425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.49] |
| 1.8.5 PRO 2000 | 1 | 1546 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.99, 1.39] |
| 1.8.6 Tenofovir | 1 | 2010 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.84, 1.74] |
| 1.9 Serious Adverse Events | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.9.1 Dapivirine | 2 | 4588 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.94, 1.32] |
| 1.9.2 Tenofovir | 3 | 4958 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.81, 1.24] |
| 1.9.3 BufferGel | 1 | 1546 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.81, 2.06] |
| 1.9.4 Carraguard | 1 | 6202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.67, 1.27] |
| 1.9.5 Cellulose sulphate | 2 | 3069 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.87, 1.79] |
| 1.9.6 PRO 2000 | 2 | 8191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.96, 1.46] |
| 1.9.7 SAVVY | 2 | 4295 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.79, 2.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdool Karim 2010.
| Study characteristics | ||
| Methods |
Setting: urban and rural clinics in South Africa Trial design: 2‐arm double‐blind, randomised placebo‐controlled trial; allocation sequential identification number corresponded to a unique envelope (accessible to each site pharmacist) and randomly allocated using permuted block randomisation of various sizes Duration of enrolment: May 2007 to January 2009 Loss to follow‐up: 5% both groups Follow‐up duration: 2.5 years Analyses were performed on an ITT basis |
|
| Participants |
Number enrolled: 1085 (445 allocated to tenofovir and 444 to placebo) Mean age: tenofovir 23.6 years; placebo 24.2 years Inclusion criteria: 18 to 40 years, sexually active, not pregnant and using a non‐barrier form of contraceptive Exclusion criteria: history of adverse reactions to latex, planning to either travel away from the study site for more than 30 consecutive days, relocate away from the study site, becoming pregnant, or enrol in any other behavioural or investigational product study,creatinine clearance < 50 mL/minute,evidence of genital deep epithelial disruption, participation in any research related to any vaginally‐applied product(s) or had an untreated STI or reproductive tract infection in the past year |
|
| Interventions |
Intervention arm: 1% tenofovir gel and condom Control arm: placebo gel and condom 1 dose of each gel inserted within 12 hours before sex and a second as soon as possible after sex and no more than 2 doses in a 24‐hour period. Both gels appeared identical and were dispensed in the same pre‐filled vaginal applicators with identical packaging. |
|
| Outcomes |
Primary: HIV incidence, safety Secondary: incidence of deep epithelial irritation, viral load, resistance, pregnancy rates and outcomes, product hold at study exit on HIV infection and tenofovir resistance Tertiary: impact of tenofovir gel in preventing STIs, including HSV‐2 and HPV infections |
|
| Notes | The study was approved by the University of KwaZulu‐Natal's Biomedical Research Ethics Committee (E111/06), Family Health International's Protection of Human Subjects Committee (#9946) and the South African Medicines Control Council (#20060835). Participants signed informed written consent. Funding sources: The trial was supported by National Institutes of Health ClinicalTrials.gov number NCT00441298 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in blocks of 12 and 18 assumed to have been done by computer |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study gels Identical in appearance and packaging |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not aware of the assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was 5% in both arms |
| Selective reporting (reporting bias) | Low risk | Reported all primary and secondary outcomes as stated in protocol |
| Other bias | Low risk | No other potential sources of bias identified |
Abdool Karim 2011.
| Study characteristics | ||
| Methods |
Setting: community clinics in Malawi, South Africa, Zambia, Zimbabwe, and USA Trial design: multicentre randomised, placebo‐controlled trial with 3 double‐blinded gel arms and 1 open‐label no‐gel arm. Randomisation was stratified by site in blocks of size 12 or 24, distributed randomly. Each random sequence was determined through generation of uniform random variates in a computer programme Trial duration: 2005 to 2009 Loss to follow‐up: about 5% in each group Mean duration of follow‐up: 20.4 months Analyses performed on the ITT basis |
|
| Participants |
Number enrolled: 3087 (775 allocated to BufferGel, 769 to 0.5% PRO 2000, 771 to placebo gel, 722 to no gel). Mean age: Buffergel 26.2 years, PRO 2000 26.3 years, placebo gel 26.5 years, no gel 26.3 years Inclusion criteria: HIV‐negative non‐pregnant women, 18 years and older, sexually active, able to provide adequate contact information to study officials for purposes of follow‐up Exclusion criteria: history of adverse reactions to latex, use of non‐therapeutic injection drugs in the past 12 months and a history of vaginal intercourse more than an average of twice per day in the past 2 weeks, planned to become pregnant in the 30 months after study entry, to travel away from the study site for more than 3 consecutive months in the 30 months after study entry, to relocate away from the study site in the 30 months after study entry, participation in another clinical trial of a vaginal product, pregnant within 42 days of study entry, had an STI or other reproductive tract infection abnormal pelvic examination, condition that in the opinion of the investigator may interfere with the study, liver or kidney function abnormality of grade 3 or higher, blood or blood‐clotting abnormality of grade 4 or higher |
|
| Interventions |
Intervention arm: 1. BufferGel 2. 5% PRO 2000 Control arm: 1. Placebo gel 2. No gel One single‐use, prefilled applicator of both gels inserted intravaginally up to 1 hour before each episode of vaginal intercourse. Study gels were similar in appearance and packaging |
|
| Outcomes |
Primary: 1. Safety: deep epithelial disruption, other genital symptoms or other systemic symptoms, adverse genital signs and symptoms, as well as haematological hepatic and renal abnormalities of grade 3 or higher severity based on the Division of AIDS Table for Grading Adult and Paediatric Adverse Events, 2004 2. HIV infection (laboratory tests) Secondary: Incidence of STIs: Chlamydia, genital ulcer disease, gonorrhoea, syphilis, trichomoniasis, HSV‐2; bacterial vaginosis; pregnancy; acceptability of study product; number of behavioural risk assessment questions not answered in self‐reported interviews, rates of condom use versus gel use, establishment of repository of vaginal swab specimens for long‐term storage and future research testing on biomarkers |
|
| Notes | The study was approved by 11 institutional review boards that oversee research conducted at 8 study sites, as well as regulatory authorities in the USA, South Africa and Zimbabwe. All participants provided written informed consent Funding sources: National Institutes of Health ClinicalTrials.gov number NCT00074425 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All the study gels were similar in appearance and packaging. Blinding was not possible in the open‐label arm, but the outcomes were unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not aware of the assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was about 5% in both treatment arms |
| Selective reporting (reporting bias) | Low risk | All prespecified secondary outcomes have been reported |
| Other bias | Low risk | No other potential sources of bias suspected |
Baeten 2016.
| Study characteristics | ||
| Methods |
Setting: sexually‐transmitted disease clinics, family planning clinics, and community‐based locations in Malawi, South Africa, Uganda, and Zimbabwe Trial design: randomised, double‐blind placebo‐controlled trial with the use of fixed‐block size block randomisation, stratified according to site either to the treatment or placebo vaginal ring. Trial duration: August 2012 to June 2015 Median follow‐up 1.6 years; maximum follow‐up 2.6 years Loss to follow‐up: less than 1% in both groups Follow‐up: monthly The median follow‐up was 1.6 years and the maximum follow‐up was 2.6 years |
|
| Participants |
Number enrolled: 2629 (1313 allocated to dapivirine, 1316 allocated to placebo) Mean age (SD): dapivirine 27.2 (6.1) years, placebo 27.3 (6.3) years Inclusion criteria: healthy women aged 18 through 45 years, sexually active, able and willing to provide adequate locator information and written informed consent, HIV‐1‐seronegative, using an effective method of contraception except contraceptive rings, intrauterine device and sterilisation, agree not participate in other research studies involving drugs, medical devices, vaginal products or vaccines during study participation Exclusion criteria: pregnant or intending to become pregnant, planning to relocate or planning to travel away from the study site for more than 8 consecutive weeks during study participation, breast‐feeding, infection with urinary tract or pelvic inflammatory disease, an STI or reproductive tract infection, having clinical apparent Grade 2 or higher pelvic finding, clinical evidence of adverse study products or condom, or participation in any HIV prevention study using systemic or topical antiretroviral medications within 12 months of enrolment |
|
| Interventions |
Intervention arm: silicone elastomer vaginal matrix ring containing 25 mg of dapivirine Control arm: placebo vaginal ring, a flexible platinum‐catalysed‐cured silicone matrix ring which contains no active drug. The rings were indistinguishable and were used for 4 weeks and then replaced with another. Women were taught how to insert and remove the vaginal ring and counselled to wear it for the entire month |
|
| Outcomes |
Primary: 1. Incidence of HIV‐1 infection; indicated by presence of HIV‐1 RNA PCR assay 2. Safety: indicated by a composite of any serious adverse event, any grade 3 or 4 adverse event, and any grade 2 adverse event that was assessed by the trial clinicians as being related to dapivirine Secondary: 1. Adherence: detected by use of validated ultra‐performance liquid chromatography assay of collected plasma samples. Women were defined as being adherent if the returned ring contained less the 23.5 mg of dapivirine 2. Drug concentration: determined by measuring drug concentration in blood plasma |
|
| Notes | The trial protocol was approved by the ethics review committee at each site. All participants signed written informed consent. The ring was manufactured by QPharma, which had no role in design or implementation of the trial Funding: National Institutes of Health ClinicalTrials.gov number NCT01617096 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation scheme was computer‐generated |
| Allocation concealment (selection bias) | Low risk | There was central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The rings were indistinguishable With the exception of staff members at the CDSC, investigators and participants were unaware of randomisation assignments until completion of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The intervention and control rings were indistinguishable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was less than 1% in each treatment arm |
| Selective reporting (reporting bias) | Low risk | All outcomes in the protocol have now been reported |
| Other bias | Low risk | None identified |
Delany‐Moretlwe 2018.
| Study characteristics | ||
| Methods |
Setting: urban, peri‐urban, and rural clinics in South Africa Trial design: multicentre double‐blind, 2‐arm, randomised, placebo‐controlled Trial duration: October 2011 to August 2014 |
|
| Participants |
Number enrolled: 2059 women (1032 allocated to tenofovir gel, 1027 to placebo gel) Mean age: tenofovir 23 years, placebo 23 years Inclusion criteria:age 18 to 30 years,HIV‐negative, willing to provide written informed consent and adequate locater information, sexually active, no evidence of glycosuria and proteinuria greater than trace, no history of pathological bone fractures, non‐pregnant, not breast‐feeding, agreed to use a study‐approved effective non‐barrier form of contraception, adherent to study visits and procedures, willing to use study gel as advised and not using or taking any of the following groups of medication; nephrotic agents, drugs that slow renal excretion, immune system modulators or other antiretrovirals Exclusion criteria: history of adverse reaction to latex, planning to travel away from the study site for more than 30 consecutive days, relocate away from the study site, becoming pregnant, enrolment in another study of an investigational product or behaviour modification related to HIV prevention, those with inadequate renal function, Grade 3 ALT or AST at screening or any of liver disease, abnormal serum phosphate levels (Grade 3 and above, clinically‐apparent finding on speculum pelvic examination, previously or receiving an experimental HIV vaccine, participating in another HIV prevention study or in other clinical trial with a biomedical intervention in the last 6 months, current STI symptoms or other reproductive tract infection requiring treatment and those with evidence of untreated cervical abnormalities |
|
| Interventions |
Intervention arm: tenofovir 1% gel, pH 4 ‐ 5, supplied in a single‐use applicator containing approximately 4 grams of gel equivalent to approximately 40 mg of tenofovir Control arm: universal placebo gel, an inert gel containing HEC as the gelling agent with each applicator containing approximately 4 ml placebo gel Participants were requested to insert a single dose of assigned gel intravaginally up to 12 hours before coitus and the second dose within 12 hours after coitus. but no more than 2 applicators within a 24‐hour period |
|
| Outcomes |
Primary: 1. Incidence of HIV‐1 infection: determined by detection of HIV antibodies using 2 HIV rapid tests, 1 detecting both HIV‐1 and HIV‐2 and the other specific for HIV‐1 2. Safety: Grade 2, 3 and 4 clinical and laboratory adverse events as defined by the DAIDS toxicity table Secondary: Incidence of HSV‐2 infection: determined by HSV western blot |
|
| Notes |
Funding source: USAID, the Bill & Melinda Gates Foundation and the South African Department of Science and Technology and Department of Health ClinicalTrials.gov number NCT01386294 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in block sizes of 8 and 16 within each study site assumed to have been done by computer |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All staff and participants were blinded to study group allocation. sequentially numbered sealed tamper‐proof envelopes were used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not aware of the assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was less than 8% in both treatment arms |
| Selective reporting (reporting bias) | Unclear risk | All the potential outcomes were not reported |
| Other bias | Low risk | No other potential source of bias suspected |
Feldblum 2008.
| Study characteristics | ||
| Methods |
Setting: community clinics and colleges in Nigeria Trial design: double‐blind, randomised, placebo‐controlled. Allocation sequence developed using a computer random‐number generator and randomly varied blocks of different sizes stratified by site. Sequentially‐numbered, sealed opaque envelopes used to assign participants to a group Trial duration: September 2004 to December 2006 Loss to follow‐up: 23% in both groups Follow‐up: Monthly for 12 months ITT analyses |
|
| Participants |
Number enrolled: 2153 (1076 allocated to SAVVY, 1077 to placebo) Mean age (SD): SAVVY 23.5 (3.7) years, placebo 23.6 (3.8) years Inclusion criteria: women aged 18 to 35 years, non‐pregnant, HIV‐negative, sexually active, willing to use vaginal gel and condom for 12 months Exclusion criteria: HIV‐positive, pregnant |
|
| Interventions |
Inervention arm: 1.0% C31G (SAVVY) gel and condom Control arm: HEC (placebo) as the gelling agent and sorbic acid as preservative Both gels were similar in appearance (clear viscous) dispensed in 3.5 mL doses with applicator and had pH 4.4. The gels were Inserted before intercourse (and a second time if more than 1 hour had elapsed between the first application and sexual intercourse), and condoms used at every at every coital act |
|
| Outcomes | Incidence of HIV infection | |
| Notes | The study was approved by the University of Ibadan, the Nigerian Institute of Medical Research, and the Protection of Human Subjects Committee, FHI (US). Participants signed written informed consent. Investigational products donated by Cellegy Pharmaceuticals. Funding source: USAID Study was prematurely terminated in August 2006. The DSMB recommended that the study be stopped because the HIV incidence was less than half the expected rate. It was estimated that the study needed to enrol approximately 1980 additional participants to identify the number of HIV infections that would offer the desired study power ClinicalTrials.gov number NCT00130078 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, sealed opaque envelopes were used to assign participants to a group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both study gels were similar in appearance and packaging and trial staff (participants, field staff, monitors, statisticians, other staff) not aware of colours used to differentiate identical packed gels |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not aware of the assignments |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up data were not available for more than 20% of women in both groups, because the trial was stopped early. Although the loss to follow‐up was not linked to treatment allocation, we judged the risk of attrition bias as high. |
| Selective reporting (reporting bias) | Low risk | HIV incidence was the outcome stated in the protocol and it has been reported |
| Other bias | Low risk | Trial stopped early for data‐dependent processes, but we do not think that this could have introduced bias to the study |
Halpern 2008.
| Study characteristics | ||
| Methods |
Setting: community clinics in Nigeria Trial design: double‐blind, randomised, placebo‐controlled trial. Sequentially‐numbered sealed opaque envelopes were used to assign participants to 1 of the 6 colour groups Trial duration: November 2004 to March 2007 Loss to follow‐up: 30% in both treatment groups Analysis performed based on ITT. |
|
| Participants |
Number enrolled: 1644 women (820 allocated to CS, 824 to placebo) Mean age (SD): cellulose sulphate 23.4 (3.7) years, placebo 23.3 (3.5) years Inclusion criteria: Age 18 to 35 years HIV‐antibody negative, sexually active, no STI, not pregnant Exclusion criteria: Pregnant, HIV‐positive, participation in another microbicide trial, less than 3 months since their last pregnancy or desire to become pregnant in the next 12 months, and drug injection use |
|
| Interventions |
Intervention arm: cellulose sulphate and condom. Each 3.5 mL application of 6% cellulose sulphate gel contained 231 mg of the active ingredient, sodium, and had a pH of 7.5 Control arm: placebo gel and condom. The gel contained HEC as gelling agent, no cell toxicity or anti‐HIV properties, and had a pH of 4.4 Both cellulose sulphate and placebo were administered in a 3.5 mL dose via a plastic single‐use applicator. The contents of the assigned gels of 1 full applicator were inserted into the vagina immediately prior to each act of sexual intercourse and re‐applied if intercourse did not take place throughout the 12 months Participants were instructed to use condoms for all acts of sexual intercourse regardless of gel use, not to douche after sex, not to use any other vaginal products and not to use the study gel in anal intercourse. Follow‐up visits were held every month for 12 months |
|
| Outcomes |
Primary: incidence of HIV‐1 and HIV‐2 infections Secondary: incidence of STI (gonorrhoea or chlamydial infection) |
|
| Notes | The trial received ethical approval from the College of Medicine, University of Lagos; the University of Port Harcourt Teaching Hospital and FHI. Written informed consent was required and measures were put in place to ensure the process was adequate for illiterate participants. Informed consent was obtained Funding source: USAID Premature stoppage: an unplanned interim safety analysis was conducted in January 2007 after an apparent increased risk of HIV in the cellulose sulphate arm was found in a parallel trial (Van Damme 2008). The DSMB found no increased HIV risk in the CS arm of this study but recommended that the trial be stopped due to safety concerns arising from the results of the parallel trial (Van Damme 2008). Last follow‐up visit was in March 2007 ClinicalTrials.gov number NCT00120770 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assumed to be computer‐generated (block randomisation with varying block sizes) |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered sealed opaque envelopes were used to assign participants to 1 of the 6 colour groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both gels were identical in packaging and labelling. 6 product label colours (3 for CS and 3 for placebo) were used to improve blinding. There was no indication that any unblinding occurred during the study. Both CS and placebo gels were identical in packaging and labelling |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not aware of the assignments. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up was 30% due to early stopping of the trial. Although the loss to follow‐up was not linked to treatment allocation, we judged the risk of attrition bias as high. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as stated in the protocol |
| Other bias | Low risk | Trial stopped early for data‐dependent processes, but we do not think that this could have introduced bias to the study other than attrition bias as indicated above |
Marrazzo 2015.
| Study characteristics | ||
| Methods |
Setting: urban and rural clinics South Africa, Uganda, Zimbabwe Trial design: a 5‐arm randomised placebo‐controlled trial Trial duration: September 200 to June 2011 Loss to follow‐up: intervention arm 7.8%, control arm 6.8% |
|
| Participants |
Number enrolled: 5029 women (3 arms composed of 3019 allocated to oral interventions, 2010 allocated to gel use (1007 to 1% tenofovir, 1003 to placebo gel)) Mean age (SD): tenofovir gel 25.3 (5.2) years, placebo gel 25.3 (5.1) years Inclusion criteria: women aged 18 to 45 years, sexually active, HIV‐negative, not pregnant or breast‐feeding, using effective contraception and willing to use effective method for the next 24 months, able and willing to provide written informed consent and locator information, having normal haematologic and hepatic function,agreed not to join studies of drugs, medical devices or vaginal products for the next 24 months Exclusion criteria: women who reported any of the following: adverse reaction to any of the study product or latex, pathologic bone fracture not related to trauma, non‐therapeutic infection drug use in past 12 months, post‐exposure prophylaxis for HIV exposure in the past 6 months, being pregnant or had gynaecologic or genital procedures in past 42 days, participation in any other study of drugs, medical devices or vaginal products in past 30 days, breast‐feeding, using spermicide, interferon or interleukin therapy, medication with nephrotoxic potential,, significant uncontrolled active or chronic cardiovascular, renal, liver, haematologic, neurologic, immunologic disorders or infectious disease, laboratory abnormalities, intending to become pregnant in the next 24 months, planned to relocate away from site for more than 8 consecutive weeks, urinary tract infections, pelvic inflammatory diseases, STIs, grade 2 or higher exam pelvic finding |
|
| Interventions |
Intervention arm: 1% tenofovir gel Control arm: placebo gel Participants were counselled to use products daily. 1 single‐use, prefilled applicator of both gels inserted intravaginally up to 1 hour before each episode of vaginal intercourse. Study gels were similar in appearance and were packaged in identical vaginal applicators |
|
| Outcomes |
Primary: 1. Incidence of HIV‐1 infection, determined by any rapid HIV assay and confirmed by means on an enzyme‐linked immunosorbent assay and subsequent Western blotting 2. Safety (Grades 2, 3, and 4 clinical and laboratory adverse events) Secondary: 1. Adherence/behavioural 2. HIV‐1 drug resistance 3. Pharmacokinetic 4. Delayed seroconversion |
|
| Notes | This was a 5‐arm study that enrolled 5020 women and 2 study arms were assigned to receive vaginal 1% tenofovir gel and placebo gel. In November 2011, the DSMB recommended that the treatment with 1% tenofovir gel be discontinued for futility Funding source: National Institutes of Health The study was approved annually by the institutional review boards and ethics committees at all participating institutions. ClinicalTrials.gov number: NCT00705679 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All end points were reviewed by an HIV end point adjudication committee, the members of which were unaware of the study group assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow ‐up was less than 10% with similar reasons for missing outcome data in both treatment groups |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in protocol reported |
| Other bias | Low risk | None |
McCormack 2010.
| Study characteristics | ||
| Methods |
Setting: clinics in South Africa, Uganda, Zambia, and Tanzania Trial design: randomised, double‐blind parallel‐group trial, 3‐arm. Trial duration: September 2005 to August 2008 Loss to follow‐up: 2% PRO2000 1.7%, 0.5% PRO 2000 8.7%, placebo 10.6% ITT analyses |
|
| Participants |
Number enrolled: 9385 women (2734 assigned to 2% PRO2000, 3326 to 0.5% PRO2000, 3325 to placebo) Age: ‐2% PRO 2000: 15 to 24 years (37%), 25 to 34 years (35%), 34 t0 44 years (19%), ≥ 45 years (8%) ‐0.5% PRO 2000: 15 to 24 years (38%), 25 to 34 years (32%), 35 to 44 years (19%), ≥ 45 years 8% ‐Placebo: 15 to 24 years (38%), 25 t0 34 years (33%) 35 to 44 years (21%), ≥ 45 years (8%) Inclusion criteria: 18 years or older, (> 16 years in Tanzania and Uganda), HIV‐1‐negative, willing to have regular speculum examinations and urinary pregnancy tests, willing to use gel as instructed, ,sexually active, willing to receive health education about condoms, able to give informed consent Exclusion criteria: unable or unwilling to provide a reliable method of contact, were likely to move out of the area within 12 months, were likely to have sex more than 14 times a week on a regular basis ( a regulatory requirement was that no more than 60 applicators were to be dispensed at every 4‐weekly visit), used spermicides regularly, were pregnant or within 6 weeks' post partum, had a severe clinical or laboratory abnormality, needed referral for assessment of a suspicious cervical lesion, had received treatment to the cervix or other gynaecological procedure within 30 days of enrolment, were allergic to latex, or were participating or had participated in another clinical trial that was likely to affect the primary efficacy end point within 30 days before enrolment |
|
| Interventions |
Intervention arm: 1. 2% PRO 2000 and condom 2. 0.5% PRO 2000 gels and condom Control arm: HEC placebo gel and condom Gel was dispensed in identical applicators, in packs of 10 prefilled single‐dose applicators (maximum of 60 applicators in 10 packs per visit). Women were instructed to apply gel within 1 hour before sexual intercourse. They were counselled to use condoms during all sex acts and received unrestricted supplies of free condoms at the research clinics. Follow‐up at every 4‐week visit |
|
| Outcomes |
Primary: 1. HIV‐1 infection 2. Safety Seconday: STIs (HSV N. gonorrhoea, C trachomatis) |
|
| Notes | The protocol was approved by local and national ethics committees, in all participating countries and in the UK. Authorisation was obtained from the national regulatory authority in all participating countries and the US Food and Drug Administration. Participants provided written informed consent or witnessed thumbprint. Enrolment on protocol; 9404 Funding source: UK Department for International Development, UK Medical Research Council, European and Developing Countries Clinical Trials Partnership, IPM and Endo Pharmaceuticals Solutions The 2% PRO 2000 gel was discontinued early (14 February 2008) after a review by the DSMB, which advised there was little chance of the 2% gel showing benefit, given the planned sample size and postulated effect size. However, the conditional power for significant benefit from the 0.5% PRO 2000 dose (based on the original sample size assumptions) was sufficiently high to warrant trial continuation ClinicalTrials.gov number, NCT00153777 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated with a computerised random‐number generator |
| Allocation concealment (selection bias) | Low risk | Central allocation (pharmacy controlled) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators and participants were masked to group assignment. Site pharmacists dispensed gel in identical applicators on the basis of the trial number and the assigned study product codes on the clinic randomisation list. Only statisticians responsible for the preparation of the DSMB reports and essential manufacturing and distribution staff had access to the list matching study product codes to gel. No other site personnel had access to the list |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not aware of the assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less that 20% with similar reasons for missing date in each treatment group |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the protocol are reported |
| Other bias | Low risk | Funding by a pharmaceutical company (Endo Pharmaceuticals Solutions). Trial stopped early for data‐dependent processes, but we do not think that this could have introduced bias to the study |
Nel 2016.
| Study characteristics | ||
| Methods |
Setting: urban and rural clinics in South Africa, Uganda Trial design: Randomised, double‐blind, placebo‐controlled Trial duration: March 2012 to December 2016 Loss to follow‐up: 3.1% in both study arms Follow‐up: Every 6 months for 24 months. Additional 6 weeks of follow‐up after ring discontinuation ITT analyses performed |
|
| Participants |
Number enrolled: 1959 women (1307 allocated to dapivirine, 652 to placebo) Mean age (SD): dapivirine 25.9 (5.8), placebo (26.1 (5.9) Inclusion criteria: age 18 to 45 years, HIV‐negative, availability for all visits and consent to follow all trial procedures, self‐reported sexually active (defined as an average of at least 1 penetrative penile vaginal coital act per month for the last 3 months prior to screening) being on stable contraception and willing to continue with it for the trial duration unless postmenopausal or surgically sterilised, asymptomatic for genital infections, willing to: answer questions about adherence, sexual behavior, and ring acceptability; provide adequate locator information and be reachable by local standard procedures; refrain from participating in another research trial using drugs, vaccines, medical devices, microbicides or oral pre‐exposure prophylaxis investigational drugs; willing to refrain from use of vaginal products or objects (except tampons) within 14 days from enrolment and for the trial duration Exclusion criteria: pregnant or last pregnancy within 3 months prior to screening, breast‐feeding, having had a hysterectomy, participated in another research trial using drugs, medical devices, microbicides or oral pre‐exposure prophylaxis within 90 days prior to screening, previously participated or participating in any HIV vaccine trial, untreated or clinically significant urogenital infections or other STIs or other gynaecological symptoms within 1 week prior to enrolment, Grade 2 or higher pelvic examination finding, history of a significant urogenital or uterine prolapse, undiagnosed vaginal bleeding, diagnosed chronic and /or recurrent vulvo‐vaginal candidiasis urethral obstruction, incontinence or urge incontinence, any gynaecological surgery within 90 days prior to screening, any Grade 2, 3 or 4 haematology, chemistry or urinalysis laboratory value according to the division of AIDS Table for Grading severity of Adult and Pediatric Adverse Events, any history of anaphylaxis or severe allergy resulting in angioedema or a history of sensitivity/allergy to latex or a silicone elastomer, any history of diabetes mellitus and chronic use of oral corticosteroid therapy and any uncontrolled serious chronic or progressive disease, cervical cytology at screening that requires cryotherapy, biopsy or treatment (other than infection), any condition(s) that, in the opinion of the investigator, might put the participant at risk, or interfere with the trial objectives, or adherence to trial requirements |
|
| Interventions |
Intervention arm: dapivirine vaginal ring which contains 25 mg of dapivirine dispersed in a platinum‐catalysed silicone matrix Control arm: a matched placebo Participants inserted the rings themselves every 4 weeks for up to 24 months |
|
| Outcomes |
Primary: 1. Rate of HIV‐1 seroconversion (assessed on the basis of PCR assay) 2. Safety: Grade 2 adverse events (judged to be related to the investigational product; Grade 3 and 4 adverse events. Determination of all adverse events as measured by self‐reports, physical examination, gynaecologic assessments (including pelvic examination with the use of a speculum), laboratory tests, and other indicated investigations Secondary: 1. Incidence rate of curable STIs 2. Adherance to ring use 3. Incidence of pregnancy 4. HIV‐1 drug mutations in women infected during the trial |
|
| Notes | The trial protocol was approved by the ethics committees for each site. All participants provided informed consent Funding: International Partnership for Microbicides (IPM) IPM donated the vaginal rings which were manufactured by QPharma. QPharma had no other role in the trial CinicalTrials.gov number NCT01539226 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | A unique participant identification number was assigned to a participant using an automated response |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The microbicide and placebo rings were identical in every respect |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The microbicide and placebo rings were identical in every respect |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3.1% were lost to follow‐up in both the treatment groups |
| Selective reporting (reporting bias) | Low risk | 2 outcomes (acceptability and HIV‐2) mentioned in the protocol were not reported in the single publication but, since this is a recent trial, we expect that these will be reported in subsequent publications |
| Other bias | Low risk | We did not identify any other sources of bias |
Peterson 2007.
| Study characteristics | ||
| Methods |
Setting: Community clinics Ghana Trial design: double‐blind, randomised, placebo‐controlled trial. Trial duration: March 2004 to February 2006 Loss to follow‐up: SAVVY arm 13.3%, placebo arm 15.6% ITT analyses performed |
|
| Participants |
Number enrolled: 2142 women (1073 allocated to SAVVY, 1069 to placebo) Mean age (SD): SAVVY 22.7 (3.6), placebo 22.7 (3.6) Inclusion criteria: Aged 18 to 35 years, HIV‐antibody‐negative, non‐pregnant, agreed to use the study gel as directed and follow study participation and report self‐medication with antibiotics during study procedures, avoided use of spermicides or other vaginal contraceptives or lubricants during the study Exclusion criteria: Intended to become pregnant, had a history of latex allergy, were injecting drug users or had gynaecological conditions that could affect the safety or effectiveness of the study gel |
|
| Interventions |
Intervention arm: 1% C31G (SAVVY) and condom Control arm: HEC (placebo) as the gelling agent and sorbic acid as preservative and condom Both gels were similar in appearance (clear, viscous), dispensed in 3.5 mL doses with applicator and had a pH of 4.4. The gels were inserted vaginally before each act of sexual intercourse (a second dose was inserted if more than 1 hour had elapsed between the first application and sexual intercourse) and condom use was advised for all sexual contacts with all partners Follow‐up visits took place monthly for 12 months |
|
| Outcomes | Incidence of HIV‐1 and HIV‐2, measured by detecting antibodies in ORT (Rapid HIV‐1/2 test) and confirmed by ELISA, Western blot or both | |
| Notes | The study protocol was approved by the Committee on Human Research, Publications and Ethics, School of Medical Sciences, University of Science and Technology, Kumasi, Ghana; Noguchi Memorial Institute for Medical Research IRB, University of Ghana, Legon, Ghana; and the Protection of Human Subjects Committee FHI, US. Written informed consent was required. Investigational products donated by Cellegy Pharmaceuticals Funding source: USAID The study was stopped prematurely (November 2005) following recommendations of DSMB because the HIV incidence among enrolled participants was substantially lower than expected. The study statistician estimated that approximately 3500 additional participants (beyond the 2124 planned sample size) would be needed to achieve the required number of HIV infections ClinicalTrials.gov number NCT00129532 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated using a computer random‐number generator |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered sealed opaque envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The gels were similar in appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not aware of the assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was less than 20%, but was not linked to treatment allocation |
| Selective reporting (reporting bias) | Low risk | Incidence of HIV‐1 or HIV‐2 infection were the main outcomes stated in the protocol. The reported outcome is HIV without specifying the type, but we do not regard this as selective reporting |
| Other bias | Low risk | Trial stopped early for data‐dependent processes, but we do not think that this could have introduced bias to the study other than attrition bias mentioned above |
Skoler‐Karpoff 2008.
| Study characteristics | ||
| Methods |
Setting: local health clinics, malls, churches, taxi ranks, and other community venues in South Africa Trial design: randomised, 2‐arm, double‐blind, placebo‐controlled trial Trial duration: March 2004 to March 2007 Loss to follow‐up: Carraguard 7.1%, placebo 6.7% Follow‐up duration: every 3 months for up to 2 years (a minimum of 9 months and maximum of 24 months) A sub‐study assessed the association of HR‐HPV in women at study end and Carraguard use. Participants entered the HR‐HPV study in October 2006, 6 months before the study end |
|
| Participants |
Number enrolled: 6202 women (3103 allocated to Carraguard gel, 3099 to placebo) Inclusion criteria: aged 16 years and older, sexually active, HIV‐negative, sexually active, willing to give informed consent and provide locator information to study staff, comply with all aspects of the study protocol, citizen or permanent resident of South Africa, no use of any vaginal products except those prescribed or approved by the study clinician Exclusion criteria: pregnant or a desire to become pregnant in the next 2 years at the time of screening, within 4 weeks of last pregnancy outcome at time of enrolment, Pap smear at screening grades as carcinoma, injected illicit drugs in the 12 months before screening or were participating in any other clinical trial or HIV prevention study |
|
| Interventions |
Intervention arm: Carraguard gel and condom Control arm: methylcellulose (placebo) gel and condom Both gels were packaged in single‐use microlax‐type applicators, each filled with 7 mL of gel to dispense approximately 4 mL. Appearance and frequency of administration of the gels were similar. Participants were instructed to vaginally insert the study gel up to 1 hour before every act of vaginal intercourse, use condoms together with the gel and not to insert any other vaginal products (apart from the medication prescribed by study clinicians) |
|
| Outcomes |
Primary: HIV infection Secondary: 1. Safety 2. Incidence of STIs |
|
| Notes | The study was reviewed and approved by the Population Council Institutional Review Board, NY, USA, the University of KwaZulu‐Natal Biomedical Research Ethics Committee for the Medical Research Council; the University of Limpopo, Medunsa Campus, Research, Ethics and Publications Committee; the University of Cape Town Research Ethics Committee; and the South African Medicines Control Council, and was undertaken in accordance with the declaration of Helsinki. Participants gave written informed consent Funding source: US Agency for International Development, Bill and Melinda Gates Foundation ClinicalTrials.gov number NCT00213083 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer pseudo‐random‐number generator was used |
| Allocation concealment (selection bias) | Low risk | There was central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The gels were similar in appearance and packaging. To maintain blinding, only 2 non‐clinical staff members at the Population Council, who were not involved in daily procedures, data cleaning or analysis, had access to records showing which barcodes corresponded to Carraguard or placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not aware of the assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was 7.1% in the intervention group and 6.7% in the control group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as per protocol |
| Other bias | Low risk | No other potential sources of bias |
Van Damme 2008.
| Study characteristics | ||
| Methods |
Setting: community clinics in South Africa, Uganda, Benin, and India Trial design: randomised, 2‐arm, double‐blind, placebo‐controlled trial Trial duration: July 2005 to March 2007 Loss to follow‐up: cellulose sulphate 10.7%, placebo 9.0% Follow‐up: 12 monthly follow‐up visits |
|
| Participants |
Number enrolled: 1428 women (717 allocated to cellulose sulphate, 708 to placebo) Median age: cellulose sulphate 28 years, placebo 29 years Inclusion criteria: aged at least 18 years, negative HIV‐antibody test, sexually active, agreed to come to the clinic for 12 monthly follow‐up visits Exclusion criteria: allergy to latex or spermicides, pregnancy or wanted to become pregnant in the next year, intravenous drug users, participation in another trial, had already been screened for this trial, or had any condition that made participation unsafe or that the investigator believed could complicate interpretation of the data |
|
| Interventions |
Intervention arm: 6% cellulose sulphate gel and condom. CS had a pH of 7.5 Control arm: placebo gel and condom. The placebo gel had a pH of 4.4 The gels were identical in appearance, delivered in 3.5 mL single‐use opaque applicators and frequency of administration was similar. The gels were to be inserted vaginally within 1 hour before each act of vaginal intercourse, during a period of 1 year |
|
| Outcomes | incidence of HIV‐1 and HIV‐2 | |
| Notes | The study was approved by the Institutional Review Board of the Eastern Virginia Medical School and by local ethical committees at the sites where women were recruited. Written informed consent was required Funding source: USAID and the Bill & Melinda Gates Foundation The trial was stopped prematurely after the Independent Data Monitoring Committee determined that CS gel may have increased the risk of HIV infection as compared with placebo. In January 2007, sites were instructed to withdraw the product as soon as possible. Last follow‐up visit was in March 2007 ClinicalTrials.gov number NCT00153777 3 participants had positive PCR test for RNA at enrolment and were excluded (1 in South Africa 1 in Uganda1 in Benin) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random‐number generator was used |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, sealed opaque envelopes were used, that were kept in a secure office at each site |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind outcome assessment. Outcome assessors were not aware of the assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up comprised 10.7% in the intervention group and 9.0% in the control group |
| Selective reporting (reporting bias) | Low risk | HIV‐1 and HIV‐2 are listed as primary outcomes and, although the results reported do not specify the type of HIV, we do not think there is selective reporting |
| Other bias | Low risk | Trial stopped early for data‐dependent processes, but we do not think that this could have introduced bias to the study |
AIDS: acquired immunodeficiency syndrome; ARV: antiretroviral; CAPRISA: Centre for the AIDS Programme of Research in South Africa; CS: cellulose sulphate; DAIDS: Division of AIDS; DNA: deoxyribonucleic acid; DSMB: Data Safety Monitoring Board; ELISA: enzyme‐linked immunosorbent assay; FHI: Family Health International; HEC: hydroxy ethylcellulose; HIV: human immunodeficiency virus; HR‐HPV: high‐risk human papillomavirus; HSV: herpes simplex virus; IPM: International Partnership for Microbicides; ITT: intention‐to‐treat; NIAID: National Institute of Allergy and Infectious Diseases; ORT: oral mucosal transudate; PCR: polymerase chain reaction; PEPFAR: President's Emergency Plan for AIDS Relief; PMPA: 9‐[(R)‐2‐phosphonomethoxy)propyl]adenine monohydrate; RNA: ribonucleic acid; RPR: rapid plasma reagin; SDA: strand displacement amplification; SDMC: Statistical and Data Management Centre; STI: sexually transmitted infections; TPHA: treponema pallidum haemagglutination assay; USAID: United States Agency for International Development.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Artz 2005 | The microbicide used in the study was nonoxynol‐9, which is not included in this review. This microbicide has been covered in other Cochrane Reviews. See Wilkinson 2002a and Wilkinson 2002b |
| Barbone 1990 | The microbicide used in the study was nonoxynol‐9, which is not included in this review. See Wilkinson 2002a and Wilkinson 2002b |
| Cutler 1997 | The microbicide used in the study was nonoxynol‐9, which is not included in this review. See Wilkinson 2002a and Wilkinson 2002b |
| Ettiègne‐Traoré 1997 | The microbicide used in the study was nonoxynol‐9, which is not included in this review. See Wilkinson 2002a and Wilkinson 2002b |
| Kreiss 1992 | The microbicide used in the study was nonoxynol‐9, which is not included in this review. See Wilkinson 2002a and Wilkinson 2002b |
| Louv 1998 | The microbicide used in the study was nonoxynol‐9, which is not included in this review. See Wilkinson 2002a and Wilkinson 2002b |
| Niruthisard 1992 | The microbicide used in the study was nonoxynol‐9, which is not included in this review. See Wilkinson 2002a and Wilkinson 2002b |
| Rendon 1980 | The microbicide used in the study was nonoxynol‐9, which is not included in this review. See Wilkinson 2002a and Wilkinson 2002b |
| Roddy 1988 | The microbicide used in the study was Nonoxynol‐9, which is not included in this review. See Wilkinson 2002a and Wilkinson 2002b |
| Rosenberg 1987a | The microbicide used in the study was nonoxynol‐9, which is not included in this review. See Wilkinson 2002a and Wilkinson 2002b |
| Rosenberg 1987b | The microbicide used in the study was nonoxynol‐9, which is not included in this review. See Wilkinson 2002a and Wilkinson 2002b |
| Sacks 1990 | The microbicide used in the study was nonoxynol‐9, which is not included in this review. See Wilkinson 2002a and Wilkinson 2002b |
| Van der Straten 2007 | The study included HIV‐positive women. |
| Zaneveld 2002 | This is an in vitro and animal study |
Differences between protocol and review
In the previous version of this review, we pooled outcome data from all microbicides. Since the different types of microbicides have different mechanisms of action, we have now decided not to combine the data.
Contributions of authors
For this update JO and PO carried out searches, assessed studies for inclusion, and extracted data. JO, PO and CSW assessed risk of bias. All review authors participated in writing the review.
Sources of support
Internal sources
-
South African Medical Research Council (CSW), South Africa
Grant Number 106035
-
National Research Foundation of South Africa (CSW), South Africa
Grant Number 108571
University of Cape Town (CSW), South Africa
-
Effective Health Care Research Consortium (JAO), South Africa
Grant 5242 administered by Cochrane South Africa at the South African Medical Research Council
Institute of Primate Research, Nairobi (JAO, POO, PGM), Kenya
External sources
Reviews for Africa Program, South African Medical Research Council (JAO), South Africa
Declarations of interest
JO: none known
PO: none known
PGM: none known
CSW: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abdool Karim 2010 {published data only}
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor EL, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010;329(5996):1168-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdool Karim SS, Abdool Karim Q, Kharsany AB, Baxter C, Grobler AC, Werner L. Tenofovir gel for the prevention of herpes simplex virus type 2 infection. New England Journal of Medicine 2015 2015;373(6):530-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim QA, Kharsany AB, Frohlich JA, Baxter C, Yende N, Mansoor LE, et al. Recruitment of high risk women for HIV prevention trials: baseline HIV prevalence and sexual behavior in the CAPRISA 004 tenofovir gel trial. Trials 2011;12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM. AIDS 2010, XVIII International AIDS Conference: Tenofovir vaginal gel first microbicide to prevent HIV, HSV infections. Medscape Medical News July 2010.
Abdool Karim 2011 {published data only}
- Abdool Karim SS, Richardson BA, Ramjee G, Hoffman IF, Chirenje ZM, Taha T, et al. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS 2011;25(7):957-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffey MB, Richardson B, Husnik M, Makanani B, Chilongozi D, Yu E, et al. HPTN 035 Phase II/IIb Randomized safety and effectiveness study of the vaginal microbicides BufferGel and 0.5% PRO 2000 for the prevention of sexually transmitted infections in women. Sexually Transmitted Infections 2014;90(5):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baeten 2016 {published data only}
- Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. New England Journal of Medicine 2016;375(22):2121-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MTN 2016. Dapivirine vaginal ring helped protect women against HIV in ASPIRE Phase lll trial. Media release.
Delany‐Moretlwe 2018 {published data only}
- Delany-Moretlwe S, Lombard C, Baron D, Bekker LG, Nkala B, Ahmed K et al. Tenofovir 1% vaginal gel for prevention of HIV-1 infection in women in South Africa (FACTS-001): a phase 3, randomised,double-blind, placebo-controlled trial. The Lancet Infectious Diseases 2018;18(11):1241-50. [DOI] [PubMed] [Google Scholar]
- Rees HS, Sinead A, Delany- Moretiwa S, Baron D, Pachia R, et al. In: FACTS 001 Phase III Trial of Pericoital Tenofovir 1% Gel for HIV Prevention in Women.The 22nd Conference on Retroviruses and Opportunistic Infections;2015 Feb 23-26; Seattle Washington. 2015.
Feldblum 2008 {published data only}
- Feldblum PJ, Adeiga A, Bakare R, Wevill S, Lendvay A, Obadaki F, et al. SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomised controlled trial in Nigeria. PLoS One 2008;3(1):e1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halpern 2008 {published data only}
- Halpern V, Ogunsola F, Obunge O, Wang CH, Onyejepu N, Oduyebo O, et al. Effectiveness of cellulose sulphate vaginal gel for the prevention of HIV infection: results of a phase III trial in Nigeria. PLoS One 2008;3(11):e3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marrazzo 2015 {published data only}
- Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. New England Journal of Medicine 2015;372(6):509-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCormack 2010 {published data only}
- McCormack S, Ramjee G, Kamali A, Rees H, Crook AM, Gafos M, et al. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet 2010;376(9749):1329-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nel 2016 {published data only}
- Nel A, Van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. New England Journal of Medicine 2016;375(22):2133-45. [DOI] [PubMed] [Google Scholar]
Peterson 2007 {published data only}
- Peterson L, Nanda K, Opoku BK, Ampofo WK, Owusu-Amoako M, Boakye AY, et al. SAVVY (C31G) gel for prevention of HIV infection in women: a phase 3, double-blind, randomised, placebo-controlled trial in Ghana. PLoS One 2007;2(12):e1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skoler‐Karpoff 2008 {published data only}
- Marais D, Gawarecki D, Allan B, Ahmed K, Altini L, Cassim N, et al. The effectiveness of Carraguard, a vaginal microbicide, in protecting women against high-risk human papillomavirus infection. Antiviral Therapy 2011;16(8):1219-26. [DOI] [PubMed] [Google Scholar]
- Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2008;372(9654):1977-87. [DOI] [PubMed] [Google Scholar]
Van Damme 2008 {published data only}
- Van Damme L, Govinden R, Mirembe FM, Guédou F, Solomon S, Becker ML, et al. Lack of effectiveness of cellulose sulphate gel for the prevention of vaginal HIV transmission. New England Journal of Medicine 2008;359(5):463-73. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Artz 2005 {published data only}
- Artz L, Macaluso M, Meinzen-Derr J, Kelaghan J, Austin H, Fleenor M, et al. A randomised trial of clinician-delivered interventions promoting barrier contraception for sexually transmitted disease prevention. Sexually Transmitted Diseases 2005;32:672-9. [DOI] [PubMed] [Google Scholar]
Barbone 1990 {published data only}
- Barbone F, Austin H, Louv WC, Alexander WJ. A follow-up study of methods of contraception, sexual activity, and rates of trichomoniasis, candidiasis, and bacterial vaginosis. American Journal of Obstetrics and Gynaecology 1990;163(2):510-4. [DOI] [PubMed] [Google Scholar]
Cutler 1997 {published data only}
- Cutler JC, Singh B, Carpenter U, Nickens O, Scarola A, Sussman N, et al. Vaginal contraceptives as prophylaxis against gonorrhoeaand other sexually transmissible diseases. Advances in Planned Parenthood 1977;12:45-56. [Google Scholar]
Ettiègne‐Traoré 1997 {published data only}
- Ettiègne-Traoré V, Ghys PD, Van Damme L, N'Krumah M, Maïga A, Tiémelé A, et al. Acceptability and feasibility of a clinical trial to assess the efficacy of a microbicide-containing vaginal gel to prevent HIV infection among female sex workers in Abidjan, Cote d'Ivoire. AIDS 1997;11(13):1660-2. [PubMed] [Google Scholar]
Kreiss 1992 {published data only}
- Kreiss J, Ngugi E, Holmes KK, Ndinya-Achola J, Waiyaki P, Roberts PL, et al. Efficacy of nonoxynol-9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA 1992;268(4):477-82. [PubMed] [Google Scholar]
Louv 1998 {published data only}
- Louv WC, Austin H, Alexander WJ, Stagno S, Cheeks J. A clinical trial of nonoxynol-9 for preventing gonococcal and chlamydial infection. Journal of Infectious Diseases 1988;158(3):518-23. [DOI] [PubMed] [Google Scholar]
Niruthisard 1992 {published data only}
- Niruthisard S, Roddy RE, Chutivongse S. Use of nonoxynol-9 and reduction in rate of gonococcal and chlamydial cervical infections. Lancet 1992;339(8806):1371-5. [DOI] [PubMed] [Google Scholar]
Rendon 1980 {published data only}
- Rendon AL, Covarrubias J, McCarney KE, Marion-Landais G, Luna del Villar J. A controlled comparative study of phenyl mercuric acetate, nonoxynol-9 and placebo vaginal suppositories as prophylactic agents against gonorrhoea. Current Therapeutic Research 1980;27:780-3. [Google Scholar]
Roddy 1988 {published data only}
- Roddy RE, Zekeng L, Ryan KA, Tamoufé U, Weir SS, Wong EL. A controlled trial of nonoxynol-9 film to reduce male to female transmission of sexually transmitted diseases. New England Journal of Medicine 1998;339(8):504–10. [DOI] [PubMed] [Google Scholar]
Rosenberg 1987a {published data only}
- Rosenberg MJ, Feldblum PJ, Rojanapithayakorn W, Sawasdivorn W. The contraceptive sponge's protection against Chlamydia trachomatis and Neisseria gonorrhoeae. Sexually Transmitted Diseases 1987;14(3):147-52. [DOI] [PubMed] [Google Scholar]
Rosenberg 1987b {published data only}
- Rosenberg MJ, Rojanapithayakorn W, Feldblum PJ, Higgins JE. Effect of the contraceptive sponge on chlamydial infection, gonorrhoea, and candidiasis - a comparative clinical trial. JAMA 1987;257(17):780–3. [PubMed] [Google Scholar]
Sacks 1990 {published data only}
- Sacks SL, Varner TL, Davies KS, Rekart ML, Stiver HG, DeLong ER, et al. Randomised, double-blind,placebo-controlled, patient initiated study of topical high and low-dose interferon-alpha with nonoxynol-9 in the treatment of recurrent genital herpes. Journal of Infectious Diseases 1990;161:692–8. [DOI] [PubMed] [Google Scholar]
Van der Straten 2007 {published data only}
- Van der Straten A, Napierala S, Cheng H, Mauck C, Depineres T, Dhlakama P, et al. A randomised controlled safety trial of the diaphragm and cellulose sulphate microbicide gel in sexually active women in Zimbabwe. Contraception 2007;76:389-99. [DOI] [PubMed] [Google Scholar]
Zaneveld 2002 {published data only}
- Zaneveld LJ, Anderson RA, Diao XH, Waller DP, Chany C, Feathergill K, et al. Use of mandelic acid condensation polymer (SAMMA), a new antimicrobial contraceptive agent, for vaginal prophylaxis. Fertility and Sterility 2002;78(5):1107-15. [DOI] [PubMed] [Google Scholar]
Additional references
Abdullah 2002
- Abdullah AS, Fielding R, Hedley AJ, Ebrahim SH, Luk YK. Reasons for not using condoms among the HongKong Chinese population: implications for HIV and STD prevention. Sexually Transmitted Infections 2002;78(3):180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adimora 2010
- Adimora AA, Auerbach JD. Structural interventions for HIV prevention in the United States. Journal of AcquiredImmune Deficiency Syndromes 2010;55(S132–5):1525–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adler 2004
- Adler M, Cowan, F, French P, Mitchell L, Richens J. ABC of Sexually Transmitted Infections, Fifth Edition. BMJ Publishig Group, 2004. [Google Scholar]
Amin 2013
- Amin A, Garcia Moreno C. Addressing gender-based violence to reduce risk of STI and HIV. Sexually Transmitted Infections. 2013;89:A8. [Google Scholar]
Balshem 2011
- Balshem B, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Broze J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [DOI] [PubMed] [Google Scholar]
Buckheit 2010
- Buckheit RW Jr, Watson KM, Morrow KM, Ham AS. Development of topical microbicides to prevent the sexual transmission of HIV. Antiviral Research 2010;85(1):142-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2015
- CDC 2015. Sexually transmitted diseases treatment guidelines, 2015. www.cdc.gov/mmwr/preview/mmwrhtml/rr6403a1.htm.
Crosby 2005
- Crosby R, Yarber WL, Sanders SA, Graham CA. Condom discomfort and associated problems with their use among university students. Journal of American College Health 2005;54(3):143-7. [DOI] [PubMed] [Google Scholar]
Cutler 2008
- Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infectious Diseases 2008;8(11):685-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997a
- Egger M, Zellweger-Zahner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet 1997;350:326-9. [DOI] [PubMed] [Google Scholar]
Egger 1997b
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Essien 2005
- Essien E, Meshack A, Peters R, Ogungbade G, Osemene N. Strategies to prevent HIV transmission among heterosexual African-American men. BMC Public Health 2005;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaitán‐Duarte 2013
- Gaitán-Duarte HG, Rodríguez-Hernández AE, Arévalo-Rodríguez I, Angel-Müller E, López-Ramos HE, Estrada-Mesa, JS, et al. Clinical practice guideline for syndromic management of patients with sexually transmitted infections and other genital tract infections. Revista Colombia Obstetrics and Gynecology 2013;64:126-77. [Google Scholar]
Garg 2009
- Garg AB, Nuttall J, Romano J. The future of HIV microbicides: challenges and opportunities. Antiviral Chemistry & Chemotherapy 2009;19(4):143-50. [DOI] [PubMed] [Google Scholar]
Gotlieb 2014
- Gottlieb SL, Low N, Newman LM, Bolan G, Kamb M, Broute N. Toward global prevention of sexually transmitted infections (STIs): The need for STI vaccines. Vaccine 2014;34:1527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Guyatt 2013
- Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 2013;66(2):151-157. [DOI] [PubMed] [Google Scholar]
Han 2009
- Han L, Lv F, Xu P, Zhang G, Juniper NS, Wu Z. Microbicide acceptability among female sex workers in Beijing, China:results from a pilot study.. Journal of Womens Health (Larchmt) 2009;18(9):1377-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25.(20):3443-57. [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J, (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated August 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2020
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Keller 2012
- Keller MJ, Carpenter CA, Lo Y, Einstein MH, Liu C, Fredricks DN, et al. Phase I randomized safety study of twice daily dosing of acid form vaginal gel: candidate antimicrobial contraceptive. PLOS One 2012;7:10:e46901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klasse 2008
- Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annual Review of Medicine 2008;59:455-71. [DOI] [PubMed] [Google Scholar]
Kumar 2006
- Kumar GA, Dandona R, Gutierrez JP, McPherson S, Bertozzi SM, Dandona L. Access to condoms for female sex workers in Andhra Pradesh. National Medical Journal of India 2006;19(6):306-12. [PubMed] [Google Scholar]
Lopez 2016
- Lopez LM, Grey TW, Chen M, Denison J, Stuart G. Behavioral interventions for improving contraceptive use among women living with HIV. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD010243. [DOI: 10.1002/14651858.CD010243.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matson 2011
- Matson PA, Adler NE, Millstein SG, Tschann JM, Ellen JM. Developmental changes in condom use among urban adolescent females: influence of partner context. Journal of Adolescent Health 2011;48(4):386–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayaud 2001
- Mayaud P, McComick D. The changing face of HIV and AIDS. British Medical Bulletin 2001;58:129-53. [DOI] [PubMed] [Google Scholar]
Moher 1996
- Moher D, Fortin P, Jada AR, Juni P, Klassen T, Le Lorier J, et al. Completeness of reporting of trials published in languages other than English: implications for conduct and reporting of systematic reviews. Lancet 1996;347:363-6. [DOI] [PubMed] [Google Scholar]
Musekiwa 2020
- Musekiwa A, Fernando MB, Abariga SA. Effectiveness of vaginal microbicides in preventing HIVtransmission. Tropical medicine and international health 2020;25(7):790-802. [DOI] [PubMed] [Google Scholar]
Newman 2015
- Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global estimates of the prevalence and Incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PloS One 2015;10(12):e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nunes 2014
- Nunes R, Sarmento B, Das Neves J. Formulation and delivery of anti-HIV rectal microbicides: advances and challenges. Journal of Control Release 2014;194:278-94. [DOI] [PubMed] [Google Scholar]
Nuttall 2010
- Nuttall J. Microbicides in the prevention of HIV infection: current status and future directions. Drugs 2010;70(10):1231-43. [DOI] [PubMed] [Google Scholar]
Oakeshott 2010
- Oakeshott P, Kerry S, Aghaizu A, Atherton H, Hay S, Taylor-Robinson D, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. BMJ 2010;340:c1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ortayli 2014
- Ortayli N, Ringheim K, Collins L, Sladden T. Sexually transmitted infections: progress and challenges since the 1994 International Conference on Population and Development (ICPD). Contraception 2014;90:(6 Suppl):S22-31. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sarkar 2008
- Sarkar NN. Barriers to condom use. European Journal of Contraception and Reprodive Health Care 2008;13(2):114-22. [DOI] [PubMed] [Google Scholar]
Singh 2014
- Singh O, Garg T, Rath G, Goyal AK. Microbicides for the treatment of sexually transmitted HIV Infections. Journal of Pharmceutics 2014;2014:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smriti 2012
- Smriti N, Marfatia YS, Prasad TL. Microbicides and HIV: A Review and an update. Indian Journal of Sexually Transmitted Diseases 2012;33(2):81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sonkar 2016
- Sonkar SC, Wasnik K, Kumar A, Mittal P, Saluja D. Comparative analysis of syndromic and PCR-based diagnostic assay reveals misdiagnosis/ overtreatment for trichomoniasis based on subjective judgment in symptomatic patients. Infectious Diseases of Porvety 2016;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sonkar 2017
- Sonkar SC, Wasnik K, Kumar A, Sharma V, Mittal P, Mishra PK, et al. Evaluating the utility of syndromic case management for three sexually transmitted infections in women visiting hospitals in Delhi, India. Scientific Reports 2017;7(1):1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomsen 2004
- Thomsen S, Stalker M, Toroitich-Ruto C. Fifty ways to leave your rubber: how men in Mombasa rationalise unsafe sex. Sexually Transmitted Infections 2004;80(6):430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trollope‐Kumar 2006
- Trollope-Kumar K, Guyatt G. Syndromic approach for treatment of STIs: time for a change. Lancet 2006;367(9520):1380-1. [DOI] [PubMed] [Google Scholar]
Tsai 2012
- Tsai CA, Hung JK, Weiser DS. Is food insecurity associated with HIV risk? cross-sectional evidence from sexually active women in Brazil. PLoS Medicine. 2012;9(4):e1001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
UNAIDS 2010
- UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. UNAIDS/10.11E/JC1958E. www.unaids.org/globalreport/documents/20101123_GlobalReport_Foreword_em.pdf (accessed 02 June 2018).
Vogenthaler 2013
- Vogenthaler SN, Kushel BM, Craig H, Frongillo Jr AE, Riley DE Bangsberg RD, et al. Food insecurity and risky sexual behaviours among homeless and marginally housed HIV-Infected individuals in San Francisco. AIDS Behaviour 2013;17(5):1688-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2007
- Walker GJA, Walker DG. Congenital syphilis: A continuing but neglected problem. Seminars in Fetal & Neonatal Medicine 2007;12:198-206. [DOI] [PubMed] [Google Scholar]
Wasserheit 1989
- Wasserheit JN. The significance and scope of reproductive tract infections among third world women. International Journal of Gynecology and Obstetrics 1989;Suppl 3:145-68. [DOI] [PubMed] [Google Scholar]
White 2008
- White RG, Moodley P, McGrath N, Hosegood V, Zaba BK, Herbst K, et al. Low effectiveness of syndromic treatment services for curable sexually transmitted infections in rural South Africa. Sexually Transmitted Infections 2008;84(7):528-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. Microbicides. www.who.int/hiv/topics/microbicides/microbicides/en/ (accessed 02 June 2018).
WHO 2007
- World Health Organization. Global strategy for the prevention and control of sexually transmitted infections: 2006–2015: Breaking the chain of transmission. apps.who.int/iris/bitstream/handle/10665/69361/WHO_RHR_06.10_eng.pdf;jsessionid=76C8F0A1DD8BEEDE7B3B675018C3E3C1?sequence=1 2007 (accessed 02 June 2018).
WHO 2012
- World Health Organization. Global incidence and prevalence of selected sexually transmitted infections–2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/ (accessed 02 June 2018).
WHO 2013a
- World Health Organization. Sexually transmitted infections (STIs). apps.who.int/iris/bitstream/handle/10665/82207/WHO_RHR_13.02_eng.pdf?sequence=1 (accessed 02 June 2018).
WHO 2013b
- World Health Organization. The importance of a renewed commitment to STI prevention and control in achieving global sexual and reproductive health. apps.who.int/iris/handle/10665/75838?mode=full (accessed 02 June 2018).
WHO 2016
- World Health Organization. Treatment of Trepanoma pallidum. apps.who.int/iris/bitstream/10665/249572/1/9789241549806-eng.pdf (accessed 08 June 2018).
Wilkinson 2002a
- Wilkinson D, Ramjee G, Tholandi M, Rutherford G. Nonoxynol-9 for preventing vaginal acquisition of sexually transmitted infections by women from men. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No: CD003939. [DOI: 10.1002/14651858.CD003939] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkinson 2002b
- Wilkinson D, Ramjee G, Tholandi M, Rutherford G. Nonoxynol-9 for preventing vaginal acquisition of HIV infection by women from men. Cochrane Database of Systematic Reviews 2002, Issue 3. Art. No: CD003936. [DOI: 10.1002/14651858.CD003936] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
GRADEpro GDT 2015
- GRADEpro GDT [GRADEpro GDT]. GRADE Working Group, Version 2015. Canada: McMaster University, 2015. gradepro.org..
Obiero 2009
- Obiero J, Wiysonge CS, Mwethera PG. Topical microbicides for prevention of sexually transmitted infections. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007961. [DOI: 10.1002/14651858.CD007961] [DOI] [PubMed] [Google Scholar]
Obiero 2012a
- Obiero J, Mwethera PG, Wiysonge CS. Topical microbicides for prevention of sexually transmitted infections. Cochrane Database of Systematic Reviews 2012, Issue 6. Art. No: CD007961. [DOI: 10.1002/14651858.CD007961.pub2] [DOI] [PubMed] [Google Scholar]
Obiero 2012b
- Obiero J, Mwethera PG, Hussey GD, Wiysonge CS. Vaginal microbicides for reducing the risk of sexual acquisition of HIV infection in women: systematic review and meta-analysis. BMC Infectious Diseases 2012;12:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


