Abstract
Background
Many women experience perineal pain after childbirth, especially after having sustained perineal trauma. Perineal pain‐management strategies are an important part of postnatal care. Non‐steroidal anti‐inflammatory drugs (NSAIDs) are a commonly‐used type of medication in the management of postpartum pain, and their effectiveness and safety should be assessed. This is an update of a review first published in 2016.
Objectives
To determine the effectiveness of a single dose of an oral NSAID for relief of acute perineal pain in the early postpartum period.
Search methods
For this update, we searched the Cochrane Pregnancy and Childbirth Group's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (9 December 2019), OpenSIGLE and ProQuest Dissertations and Theses (28 February 2020), and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs) assessing a single dose of a NSAID versus a single dose of placebo, paracetamol or another NSAID for women with perineal pain in the early postpartum period. We excluded quasi‐RCTs and cross‐over trials. We included papers in abstract format only if they had sufficient information to determine that they met the review’s prespecified inclusion criteria.
Data collection and analysis
Two review authors (FW and VS) independently assessed all identified papers for inclusion and risks of bias, resolving any discrepancies through discussion. Two review authors independently conducted data extraction, including calculations of pain relief scores, and checked it for accuracy. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included 35 studies examining 16 different NSAIDs and involving 5136 women (none were breastfeeding). Studies were published between 1967 and 2013. Risk of bias due to random sequence generation, allocation concealment and blinding of outcome assessors was generally unclearly to poorly reported, but participants and caregivers were blinded, and outcome data were generally complete. We downgraded the certainty of evidence due to risk of bias, suspected publication bias, and imprecision for small numbers of participants.
NSAID versus placebo
Compared to women who receive a placebo, more women who receive a single‐dose NSAID may achieve adequate pain relief at four hours (risk ratio (RR) 1.91, 95% confidence interval (CI) 1.64 to 2.23; 10 studies, 1573 women; low‐certainty evidence) and at six hours (RR 1.92, 95% CI 1.69 to 2.17; 17 studies, 2079 women; very low‐certainty evidence), although we are less certain about the effects at six hours. At four hours after administration, women who receive a NSAID are probably less likely to needadditional analgesia compared to women who receive placebo (RR 0.39, 95% CI 0.26 to 0.58; 4 studies, 486 women; moderate‐certainty evidence) and may be less likely to need additional analgesia at six hours after initial administration, although the evidence was less certain at six hours (RR 0.32, 95% CI 0.26 to 0.40; 10 studies, 1012 women; very low‐certainty evidence).
One study reported that no adverse events were observed at four hours post‐administration (90 women). There may be little or no difference in maternal adverse effects between NSAIDs and placebo at six hours post‐administration (RR 1.38, 95% CI 0.71 to 2.70; 13 studies, 1388 women; low‐certainty evidence). Fourteen maternal adverse effects were reported in the NSAID group (drowsiness (5), abdominal discomfort (2), weakness (1), dizziness (2), headache (2), moderate epigastralgia (1), not specified (1)) and eight in the placebo group (drowsiness (2), light‐headedness (1), nausea (1), backache (1), dizziness (1), epigastric pain (1), not specified (1)), although not all studies assessed adverse effects. Neonatal adverse effects were not assessed in any of the studies.
NSAID versus paracetamol
NSAIDs may lead to more women achieving adequate pain relief at four hours, compared with paracetamol (RR 1.54, 95% CI 1.07 to 2.22; 3 studies, 342 women; low‐certainty evidence). We are uncertain if there is any difference in adequate pain relief between NSAIDs and paracetamol at six hours post‐administration (RR 1.82, 95% CI 0.61 to 5.47; 2 studies, 99 women; very low‐certainty evidence) or in the need foradditional analgesia at four hours (RR 0.55, 95% CI 0.27 to 1.13; 1 study, 73 women; very low‐certainty evidence). NSAIDs may reduce the risk of requiring additional analgesia at six hours compared with paracetamol (RR 0.28, 95% CI 0.12 to 0.67; 1 study, 59 women; low‐certainty evidence).
One study reported that no maternal adverse effects were observed at four hours post‐administration (210 women). Six hours post‐administration, we are uncertain if there is any difference between groups in the number of maternal adverse effects (RR 0.74, 95% CI 0.27 to 2.08; 3 studies, 300 women; very low‐certainty evidence), with one case of pruritis in the NSAID group and one case of sleepiness in the paracetamol group. Neonatal adverse effects were not assessed in any of the included studies.
Comparisons of different NSAIDs or doses did not demonstrate any differences in effectiveness for any primary outcome measures; however, few data were available on some NSAIDs.
None of the included studies reported on any of this review's secondary outcomes.
Authors' conclusions
In women who are not breastfeeding and who sustained perineal trauma, NSAIDs (compared to placebo or paracetamol) may provide greater pain relief for acute postpartum perineal pain and fewer women need additional analgesia, but uncertainty remains, as the evidence is rated as low‐ or very low‐certainty. The risk of bias was unclear for many studies, adverse effects were often not assessed and breastfeeding women were not included. While this review provides some indication of the likely effect, there is uncertainty in our conclusions. The main reasons for downgrading were the inclusion of studies at high risk of bias and inconsistency in the findings of individual studies.
Future studies could examine NSAIDs' adverse effects, including neonatal effects and the compatibility of NSAIDs with breastfeeding, and could assess other secondary outcomes. Future research could consider women with and without perineal trauma, including perineal tears. High‐quality studies could be conducted to further assess the efficacy of NSAIDs versus paracetamol and the efficacy of multimodal treatments.
Plain language summary
Anti‐inflammatory drugs for relief of perineal pain after childbirth
What is the issue?
Following childbirth, many women experience pain in the perineum, an area between the anus and vagina. This Cochrane Review asked if this pain can be reduced by one dose of a non‐steroidal anti‐inflammatory drug (NSAID), such as aspirin or ibuprofen.
Why is this important?
The pain some women experience in the perineum after childbirth can be particularly acute if the perineum tears during the birth, or needs to be cut (known as an episiotomy). Even a woman without tearing or surgery often experiences discomfort in her perineum, which can affect her mobility as well as her ability to care for her baby. This review is part of a series of reviews on the effectiveness of different drugs for pain relief for perineal pain immediately after birth. We are looking specifically at NSAIDs, such as aspirin and ibuprofen.
What evidence did we find?
We found 35 studies with 5136 women that examined 16 different NSAIDs (aspirin, ibuprofen, etc.). We included studies up to 9 December 2019. The studies we found only included women who had trauma of the perineum and who were not breastfeeding. Studies were conducted between 1967 and 2013 and had few women in them.
The studies showed that a single dose of a NSAID may provide greater pain relief at four hours (low‐certainty evidence) after taking the drug when compared to a placebo (dummy pill) or no treatment in non‐breastfeeding women who had sustained perineal trauma during childbirth. We are uncertain if there is any difference between NSAID and placebo in achieving adequate pain relief at six hours.
Women who received a single dose of NSAID are probably less likely to need additional pain relief at four hours (moderate‐certainty evidence) after taking the drug compared to women who received placebo or no treatment. We are uncertain if there is any difference between NSAIDs and placebo for women needing additional pain relief at six hours (very low‐certainty evidence). Not all of the studies assessed adverse effects of the drugs, but some studies reported maternal adverse effects such as drowsiness, headache, weakness, nausea, gastric discomfort. The evidence is very uncertain about the difference in maternal adverse effects between NSAIDs and placebo at six hours after administration (very low‐certainty evidence). One small study (90 women) reported that there were no maternal adverse effects at four hours after administration. None of the studies measured possible adverse effects on the baby.
A NSAID may also be better than paracetamol in providing pain relief at four hours after administration. We are uncertain if there is any difference between NSAID and paracetamol in achieving adequate pain relief at six hours or in the number of women who need additional pain relief at four hours after administration. Women who receive NSAID may be less likely to need additional pain relief at six hours compared to women who received paracetamol. One study reported that no maternal adverse effects were observed at four hours (210 women). Three small studies reported maternal adverse effects at six hours after administration but we are uncertain if there is any difference between the groups. Adverse effects on the baby were not reported in any of the included studies and all studies excluded women who were breastfeeding.
Comparisons of different NSAIDs and different doses of the same NSAID did not demonstrate any clear differences in their effectiveness on any of the main outcomes measured in this review. However, little information was available for some NSAIDs.
None of the included studies reported on any of this review's secondary outcomes, including: extended hospital stay or readmission to hospital for perineal pain; breastfeeding; perineal pain at six weeks after having the baby; women's views, postpartum depression or measures of disability due to perineal pain.
What does this mean?
For women who are not breastfeeding, a single dose of a NSAID may be better than placebo or paracetamol for perineal pain at four hours. No serious side effects were reported, but not all studies examined these. For women who breastfeed, there are no data and these women should seek help, as some NSAIDs are not recommended for women who breastfeed.
Summary of findings
Background
Description of the condition
The perineum in women is a diamond‐shaped area between the vagina and the anus (Chou 2009). Pain in this area is particularly common following childbirth. A study conducted in the UK found that 92% of all women, with or without perineal trauma, reported perineal pain in the first day after birth, although this resolved for 88% of women at two months postpartum (Andrews 2008). Macarthur 2004, in a prospective cohort study involving 447 women in Canada, reported an incidence of perineal pain in the first day after birth of 75% in women with an intact perineum. This shows that perineal pain is not limited to women who sustain perineal trauma. However, women who have perineal trauma, which is approximately 70% of women giving birth, more commonly experience perineal pain (Laws 2009), report more severe pain and are more likely to use analgesic medicines (Leeman 2009). Spontaneous trauma to the perineum during childbirth has a four‐degree classification system depending on the tissues affected, varying from tearing of only the skin, subcutaneous tissue and/or vaginal mucosa in a first‐degree tear, to tearing of the deep and superficial perineal muscles and anal sphincter in a third‐degree tear. In a fourth‐degree tear, the ano‐rectal epithelium is also disrupted (Kettle 2004). Episiotomy is another type of trauma to the perineum and involves a surgical incision of the perineum to increase the diameter of the vulval outlet (Kettle 2004). In Macarthur 2004, perineal pain was experienced by 95% (210/220) of women with first‐/second‐degree tears, 97% (94/96) of those who had undergone episiotomies, and 100% (46/46) of women with third‐/fourth‐degree tears, but by six weeks postpartum, the frequency of perineal pain was not different between trauma groups. Preventing perineal trauma, as far as is possible, is thus vital for minimising the experience of perineal pain. A Cochrane Review evaluating perineal techniques to avoid perineal trauma during childbirth (Aasheim 2017) found that the use of warm compresses reduces third‐ and fourth‐degree tears. However, perineal trauma is not fully preventable and women without perineal trauma also frequently experience perineal pain (Andrews 2008; Macarthur 2004). Consequently, pain‐management strategies for perineal pain are an important part of postpartum care, particularly as perineal pain can interfere with a woman’s mobility, affect her ability to care for her baby (East 2012a), and, if the pain persists, may be associated with urinary/faecal incontinence and dyspareunia (Andrews 2008; Thompson 2002).
Most women experience short‐term perineal pain following childbirth, but between 6% to 30% of women continue to report perineal pain at one year postpartum (Schytt 2007; Williams 2007). Definitions of acute and chronic pain vary in the literature, but chronic pain is often described as pain present for more than 12 weeks (Airaksinen 2006). Pain of up to 12 weeks duration is generally considered acute pain, although pain lasting between six and 12 weeks has been further classified as sub‐acute (Van Tulder 2006). More recently, rather than defining pain according to set time‐frames of duration, chronic pain has been defined as pain that persists longer than the usual course (Loeser 2011). Acute pain presenting in the early postpartum period should be differentiated from chronic perineal pain in this context (Chou 2009), because of different pathophysiological processes that occur when acute pain becomes chronic (Voscopoulus 2010). The term 'early postpartum period' is equally challenging to define and varies in time‐frame durations in the literature. Early postpartum period has previously been defined as a time period of between three and 12 weeks after a baby's birth (Moodley 2003; Nicklas 2013; O'Brien 2003), a time period of one week's duration (Abou Saleh 1997), a time period of up to six months postpartum (Goodman 2003; Teich 2014), or without any specified time limit. In this review, for consistency with previous Cochrane Reviews examining interventions for early postpartum perineal pain (Chou 2009; Chou 2013), we consider the first four weeks after the birth to be the 'early postpartum period'. When women experience postpartum perineal pain in this period, it can thus be considered acute pain.
Several methods of pharmacological and non‐pharmacological pain relief are currently being used in managing acute postpartum perineal pain. These include cooling treatments, topical anaesthetics, analgesics and non‐steroidal anti‐inflammatory drugs (NSAIDs). Previous Cochrane Reviews evaluating the effectiveness of several treatment strategies for acute perineal pain postpartum concluded that rectal NSAID suppositories are associated with less pain up to 24 hours postpartum compared to placebo (Hedayati 2003) and paracetamol provides more pain relief to women compared to placebo (Chou 2013). In addition, there is non‐compelling evidence for the use of topical anaesthesia (Hedayati 2005), limited evidence for the use of local cooling treatments (East 2012b), and a lack of evidence to support the use of therapeutic ultrasound (Hay‐Smith 1998).
In this review we focus on oral NSAIDs (single‐dose) for alleviating perineal pain in the early postpartum period, that is, during the first four weeks after birth.
Description of the intervention
NSAIDs are a group of medicines that have been used for centuries for their analgesic, anti‐pyretic and anti‐inflammatory properties. Salicin was first extracted from willow bark in 1829 by Leroux (Brunton 2011), and the derivative aspirin was produced in 1899 (Rao 2008). In the 20th century, many NSAIDs were developed, but it was not until the 1970s that a mechanism of action was identified (Rainsford 2007; Vane 1971), and our understanding of their effects as well as their use in the treatment and management of various conditions continue to evolve. NSAIDs are mainly categorised according to their inhibitory effects on two isoforms of cyclo‐oxygenase (COX1 and COX2), as described below.
Various routes for NSAID administration are available, including intra‐muscular injection, intravenously, per rectum, topically and orally (Tramèr 1998). This review examines the effectiveness of NSAIDs taken orally. More specifically, this review evaluates the effectiveness of a single oral dose of a NSAID, defining a single dose as a dose taken at one time rather than dosage regimens that would involve more than one dose of a given NSAID over time (Howard 2013). The speed at which an oral NSAID is absorbed into the bloodstream varies for different NSAIDs. For example, for ibuprofen, peak plasma concentrations are observed 15 to 30 minutes after ingesting the drug, with a half‐life in the plasma of approximately two hours (Davies 1998). This is an example of a fast‐acting NSAID, whereas slow‐acting NSAIDs such as naproxen show later peak plasma concentrations and have a longer half‐life (Vree 1993). The recommended dosage at which NSAIDs are administered also depends on the individual NSAID, as well as the route of administration and the reason for taking the drug. For acute pain, for instance, a single oral dose of 400 mg of ibuprofen is generally taken, which can be repeated every four to six hours up to a maximum daily dose of 2400 mg. Naproxen, as another example, has a maximum daily dose of 1250 mg and is given orally for acute pain in an initial dose of 500 mg followed by 250 mg doses every six to eight hours afterwards, as required (BNF 2014).
The most common adverse effects of NSAIDs include abdominal pain, nausea, dyspepsia, headache, pruritis, urticaria and other skin rashes. Rarely, NSAIDs can lead to perforation of gastric ulcers and gastrointestinal bleeding, hypersensitivity reactions, bronchospasm, haematopoietic disorders, hypertension, cardiac failure and renal failure. Adverse effects are more likely in elderly people and may be minimised by using the lowest effective dose for the shortest duration necessary (Irish Pharmaceutical Healthcare Association 2014).
How the intervention might work
During childbirth, due to pressure on or trauma of the perineum, a local inflammatory response occurs causing perineal pain. NSAIDs may improve perineal pain through their anti‐inflammatory action. Moreover, they have a known analgesic effect, particularly for pain that is associated with tissue trauma/injury and inflammation (Rao 2008). This review focuses on the effectiveness of a single dose of a NSAID in relieving perineal pain, which, if effective, will mainly be due to its early analgesic properties as its anti‐inflammatory effect will be minimal at this dosage. NSAIDs are believed to act peripherally by inhibiting COX enzymes that catalyse the conversion of arachidonic acid into prostaglandin (PG) (Rao 2008). There are two main isoforms of COX: COX1 and COX2. COX1 is normally present in most tissues and cells and is not related to inflammation, whereas COX2 is induced by inflammatory mediators and is only found in tissues in the presence of inflammation. In addition, COX2 catalyses the production of pro‐inflammatory prostaglandin G2 (Seibert 1994; Smith 1998). Selective COX2 inhibitors were developed to diminish the side effects of non‐selective NSAIDs that result from COX1 inhibition, particularly the inhibition of gastro‐protective prostaglandin synthesis. However, selective COX2 NSAIDs exhibit cardiovascular adverse effects (Solomon 2004).
Pain experienced in the perineal area is transmitted through the pudendal nerve to the spinal segments S2 to S4. NSAIDs thus act peripherally by inhibiting pro‐inflammatory prostaglandin production and by subsequently reducing inflammation in the perineal area and decreasing pudendal pain nerve fibres excitation.
This review examines the effectiveness of a single dose of any NSAID for the management of perineal pain in the early postpartum period.
Why it is important to do this review
Postpartum perineal pain is a very common post‐childbirth complaint. It can have negative consequences for mother and child, including disability in daily functioning for the mother; for example, it can interfere in her taking care of her infant and in breastfeeding. Early pain management is thus relevant to provide relief and prevent chronicity.
NSAIDs are commonly used in the management of postpartum pain (Leeman 2009). It is therefore important to consider their effectiveness and safety, including their safety for the neonate in breastfeeding mothers. The use of NSAID rectal suppositories has been examined in a previous Cochrane Review (Hedayati 2003). Adding to the evidence from previous Cochrane Reviews evaluating alternative management strategies for postpartum perineal pain, this systematic review evaluates and synthesises studies examining the effectiveness of NSAIDs that are administered orally and in a single dose.
Objectives
To determine the effectiveness of a single dose of an oral non‐steroidal anti‐inflammatory drug (NSAID) for relief of acute perineal pain in the early postpartum period.
Methods
Criteria for considering studies for this review
Types of studies
We include only randomised controlled trials (RCTs), comparing a non‐steroidal anti‐inflammatory drug (NSAID) with another NSAID, aspirin, paracetamol or placebo/no‐drug treatment. We exclude quasi‐RCTs and cross‐over trials. We included papers in abstract format only if they had sufficient information to determine that they met the review’s prespecified inclusion criteria. If they did not include this information, we excluded them, but first contacted authors to request the full‐text version.
Types of participants
All women who had acute perineal pain or who had been treated for acute perineal pain in the early postpartum period, i.e. the first four weeks after giving birth or as defined by the authors of the studies.
Types of interventions
Single dose of a NSAID compared with placebo/no drug treatment
Single dose of a NSAID compared with a single dose of paracetamol
Single dose of a NSAID compared with a single dose of another NSAID/aspirin
We excluded studies examining NSAIDs administered as suppositories as these have been examined in another Cochrane Review (Hedayati 2003). Our review only includes studies examining the effectiveness of NSAIDs administered orally (single‐dose). Studies that evaluated more than one dose of NSAIDs were included in the review if data on the effectiveness of a single dose were collected and reported separately.
Types of outcome measures
Primary outcomes
Adequate pain relief as reported by the woman, or by determination of greater than 50% relief of pain (either as stated by the woman or as calculated using a formula)*
Need for additional analgesia for relief of perineal pain
Maternal drug adverse effects, e.g. nausea, vomiting, sedation, constipation, diarrhoea, drowsiness, sleepiness, psychological impact
Neonatal drug adverse effects, e.g. nausea, vomiting, sedation, constipation, diarrhoea, drowsiness, sleepiness, psychological impact
* Assessment of 50% pain relief via Total Pain Relief (TOTPAR) and Summed Pain Intensity Difference (SPID) scores (see 'Assessment of pain' section)
Secondary outcomes
Prolonged hospitalisation due to perineal pain (days)
Rehospitalisation due to perineal pain
Fully breastfeeding at discharge
Mixed feeding at discharge
Fully breastfeeding at six weeks
Mixed feeding at six weeks
Perineal pain at six weeks
Maternal views (using a validated questionnaire), for example, women’s satisfaction with the intervention
Maternal postpartum depression, measured using a validated depression scale, for example the Edinburgh Postnatal Depression (EPD) scale
Instrumental measures of disability due to perineal pain/activities of daily living (ADLs)/quality of life (QoL), for example, 15D Health‐Related Quality of Life (HRQoL) instrument
Search methods for identification of studies
The following Methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (9 December 2019).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (9 December 2019) using the search methods detailed in Appendix 1.
We also searched the OpenSIGLE database to identify grey literature and the ProQuest Dissertations and Theses to retrieve dissertation theses related to our topic of interest (28 February 2020) (see: Appendix 1 for search methods used).
Searching other resources
We reviewed the reference lists of all selected papers to identify any additional potentially eligible studies not captured by the electronic searches. We also contacted experts in the field of pain relief and maternity care, and, where appropriate, authors of studies published in abstract format only, to identify any unpublished studies.
We did not apply any language or date restrictions.
Data collection and analysis
Assessment of pain
The number of women achieving adequate pain relief was defined as one of the following.
The number of women reporting 'good' or 'excellent' pain relief when asked about their level of pain relief four to six hours after receiving their allocated treatment (the data were extracted as dichotomous data).
The number of women who reported 50% pain relief or greater.
The number of women who achieved 50% pain relief or greater, as calculated by using derived pain relief scores (TOTPAR (total pain relief) or SPID (summed pain intensity differences)) over four to six hours.
TOTPAR or SPID (or both) were calculated provided sufficient data were present. Examples of possible pain measures included the five‐point pain relief (PR) scale with standard or comparable wording (none, slight, moderate, good, complete), the four‐point pain intensity (PI) scale (none, mild, moderate, severe), or the visual analogue scale (VAS) or both for pain relief or pain intensity. From these categorical scales, it was possible to convert results into dichotomous data (the proportion of women achieving at least 50% or greater, max TOTPAR) using standard formulae (Moore 1996; Moore 1997b). Conversion of data in this way allowed the use of these data in a meta‐analysis (Moore 1997a; Moore 1997b). We used the following equations to estimate the proportions of women achieving at least 50% of maximum TOTPAR:
Proportion with greater than 50% maxTOTPAR = (1.33 x mean %maxTOTPAR – 11.5)
With %maxTOTPAR = mean TOTPAR x 100/(maximum score x number of hours)
Proportion with greater than 50% maxTOTPAR = (1.36 x mean %maxSPID – 2.3)
With %maxSPID = mean SPID x 100/(maximum score x number of hours)
We then calculated the number of women achieving at least 50% maxTOTPAR by multiplying the proportions of women with at least 50% maxTOTPAR by the total number of women in the treatment groups. We then used the number of women with at least 50% maxTOTPAR to calculate the relative benefit and the number needed to treat for an additional beneficial outcome. Where studies used more than one method of calculating adequate pain relief, we preferred for analyses and reporting purposes (in order of decreasing preference) as follows: i) the proportion with at least 50% maxTOTPAR calculated using SPID; ii) the proportion with at least 50% maxTOTPAR calculated using TOTPAR; and iii) the number of women reporting 'good' or 'excellent' pain relief/number of women reporting at least 50% pain relief. We also assessed the number of women who remedicated in the period of four to eight hours, as well as the median time to remedication, if the information was available.
Selection of studies
Two review authors (FW and VS) independently assessed for inclusion all of the potential studies identified by the search strategy. We resolved any disagreement through discussion or, if necessary, we would have consulted a third person, but this was not required. We included studies presented only as abstracts if they had sufficient information to confirm that they met the review's prespecified inclusion criteria.
We created a study flow diagram to map out the number of records identified, included and excluded (Figure 1).
Data extraction and management
We designed a form to extract data based on the Cochrane Pregnancy and Childbirth Group's data extraction template form. For eligible studies, two review authors (FW and VS) extracted the data independently using the agreed form. We resolved discrepancies through discussion or, if necessary, we would have consulted a third person, but this was not required. We entered data into Review Manager 5 software (RevMan 2020), and FW and VS independently checked these data for accuracy.
Where information about any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (FW and VS) independently assessed risks of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or, if necessary, we would have involved a third assessor, but this was not required.
(1) Random sequence generation (checking for possible selection bias)
We describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random‐number table, computer random‐number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We describe for each included study the methods used to conceal allocation to interventions prior to assignment, and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation, consecutively‐numbered sealed opaque envelopes);
high risk of bias (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that a lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We describe for each included study, and for each outcome, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the relevant analyses.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data, missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups, ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We describe for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria described in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We intended to explore the impact of the level of bias in sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
We carried out statistical analyses using the Review Manager 5 software (RevMan 2020).
Dichotomous data
For dichotomous data, we presented the results as the summary risk ratio (RR) with a 95% confidence interval (CI).
Continuous data
For continuous data, we planned to use the mean difference (MD) if outcomes were measured in the same way between trials. We also planned to use the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods; however, these were not required as there were no continuous data in the included studies.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials on the topic. However, if we had found any, we would have included them using the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020).
Multi‐arm trials
For multi‐armed trials, only the comparisons of intervention arms that were relevant to this review were included. For example, if a study compared ibuprofen, codeine and placebo, we included only the ibuprofen versus placebo comparison.
If comparisons shared intervention or control groups then we divided the number of participants approximately evenly among the comparisons (Deeks 2020). For example, in the studies with two (or more) intervention groups and one control group we divided the number of participants and the number of events in the control group by half (or more where there were more intervention groups).
Dealing with missing data
For the included studies, we noted levels of attrition. We attempted to contact study authors to ask them to provide missing outcome data. Where this was not possible, we planned to explore the impact of including such studies in the overall assessment of results using a sensitivity analysis, but this was not required.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial is the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if an I2 was greater than 30% and either a Tau2 was greater than zero, or there was a low P value (< 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
Where there were 10 or more studies in a meta‐analysis we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually.
Where we suspected reporting bias, (see ‘Selective reporting bias’ above), we attempted to contact study authors to ask them to provide missing outcome data. Where this was not possible, we planned to explore the impact of including such studies in the overall assessment of results using a sensitivity analysis, but this was not required.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2020). We used a fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials examined the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we used a random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects, and we discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
If we used random‐effects analyses, the results are presented as the average treatment effect with a 95% confidence interval, and the estimates of Tau2 and I2.
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses. We considered whether an overall summary was meaningful in the presence of heterogeneity, and if it was, we used a random‐effects analysis to produce it.
We had planned to carry out the following subgroup analyses.
Drugs compatible with breastfeeding versus those that are not compatible with breastfeeding because they have adverse effects on the infant.
Primiparous versus multiparous women.
Women with perineal trauma versus women who gave birth over an intact perineum.
Women who used prior pain relief versus women who did not use prior pain relief.
Different time‐frames of when the dose was taken after the birth.
We were unable to carry out the planned subgroup analyses due to the absence of relevant data in the included studies.
We planned to use the following outcomes in subgroup analyses.
Adequate pain relief as reported by the woman, or by determination of 50% or greater relief of pain (either as stated by the woman or as calculated using a formula).*
Need for additional analgesia for relief of perineal pain.
Maternal drug adverse effects, e.g. nausea, vomiting, sedation, constipation, diarrhoea, drowsiness, sleepiness, psychological impact.
* Assessment of 50% pain relief via TOTPAR and SPID scores (see 'Assessment of pain' section).
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2020). We report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I2 value.
We had also planned to use the outcome 'Neonatal drug adverse effects' in subgroup analyses, but this outcome was not measured in any of the included studies.
Sensitivity analysis
Where appropriate, we carried out planned sensitivity analysis to explore the effect of risks of bias for important outcomes in the review. We carried out sensitivity analysis for the primary outcomes, where appropriate, by excluding those studies judged to be at a high risk of bias for any of the following 'Risk of bias' domains: random sequence generation, allocation concealment, blinding of participants and personnel, and incomplete outcome data, reporting bias or other bias.
We also planned to conduct sensitivity analysis to explore the impact of including studies with high levels of missing data, but we did not have sufficient numbers of studies.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach, as outlined in the GRADE handbook in order to assess the certainty of the body of evidence relating to the following outcomes for the main comparison (any NSAID versus placebo).
Adequate pain relief as reported by the woman, or by determination of 50% or greater relief of pain.
Need for additional analgesia for relief of perineal pain.
Maternal drug adverse effects.
Neonatal drug adverse effects.
We used GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2020) in order to create a 'Summary of findings’ table. We produced a summary of the intervention effect and a measure of certainty for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
See: Figure 1.
1.
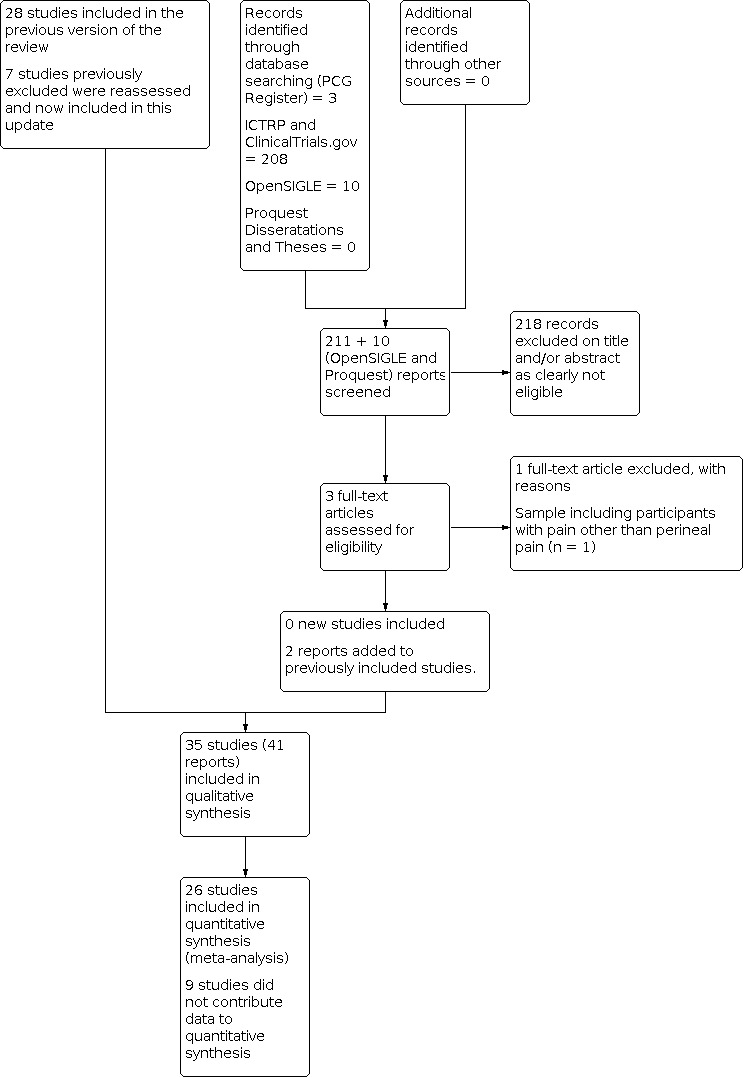
Study flow diagram.
We retrieved 221 records (3 CPC Register, 208 from ICTRP and ClnicalTrials.gov (none of these 208 were eligible), 10 from OpenSIGLE (none of these were eligible) and zero from Proquest). We added one report each to two previously included studies (De Vroey 1978; London 1983), and excluded one new trial report (Lataste 1981). Seven studies previously excluded have been included in this update.
Included studies
Design and setting
We include 35 studies (41 reports), of which two studies were reported in one publication (Laska 1981a; Laska 1981b). Twenty‐nine studies were multi‐arm studies; in such cases we only extracted the data for any non‐steroidal anti‐inflammatory drug (NSAIDs) compared with placebo, paracetamol or another NSAID. These studies are described in the Characteristics of included studies section. The effectiveness of paracetamol versus placebo has been examined in a previous Cochrane Review (Chou 2013) and the effectiveness of NSAIDs compared with other non‐NSAID drugs will be assessed in future reviews based on the generic protocol (Chou 2009).
Included studies were published between 1967 and 2013; one study was published in the 1960s, five in the 1970s, 22 in the 1980s and four in the 1990s. Only three studies were published since 2000; two in 2008 and one in 2013. Of the 35 included studies, 16 were conducted in the USA and eight in other high‐income countries (Canada, UK, Belgium, Spain, France, Italy). Six studies were conducted in Venezuela. The remaining five studies were conducted in other low‐ or middle‐income countries (India, Malaysia, Thailand, Iran).
Four studies reported the exact dates when the study had taken place (1965 ‐ 1966 Bloomfield 1967; 2006 Kamondetdecha 2008; 2005 Lim 2008; 2009 ‐ 2010 Suhrabi 2013). Similarly, reporting of declarations of interest was rare; only one study reported on this (Suhrabi 2013). Seven of the 35 studies had declared funding sources (public funding: Bloomfield 1967; Bloomfield 1974; funding from pharmaceutical industry: Hebertson 1986; Jain 1985; Melzack 1983; Olson 1997; Schachtel 1989). One study specifically stated that no funding or support was received (Suhrabi 2013).
Participants and sample sizes
A total of 5845 women were included in this review, of which 709 women received other drugs not included in this review and were subsequently excluded from the analysis. Of the 4837 women included in the analyses, 3145 received a NSAID and 1692 received placebo or paracetamol. Thirty‐four of the 35 studies examined the effectiveness of NSAIDs for relief of post‐episiotomy pain. One study (Lim 2008) only included women with any perineal trauma requiring repair but excluded third‐ or higher‐degree tears. All trials excluded women who were breastfeeding and none of the included trials reported neonatal adverse outcomes.
Interventions and comparisons
Sixteen different NSAIDs were examined in the studies included in this review. These were aspirin, ibuprofen, diclofenac, diflunisal, dipyrone, fenoprofen, fluproquazone, zomepirac, meclofenamate sodium, aceclofenac, ketoprofen, flurbiprofen, fendosal, piroxicam, tiaprofenic and celecoxib. Studies, or data from studies, reporting on indoprofen, zomepirac and fluproquazone were subsequently removed from the analyses, as these NSAIDs are currently withdrawn from the market due to causing the following adverse effects: fluproquazone for adverse effects on the liver (Kaplowitz 2013), indoprofen for reports of adverse reactions including reports of carcinogenicity in animal studies (Brayfield 2014), and zomepirac for being associated with fatal and near‐fatal anaphylactoid reactions (Brayfield 2014).
Doses of the intervention drugs varied across studies, with the different doses of individual drugs compared in subgroup analyses. Drugs deemed to have equivalent doses, i.e. aspirin 500 mg to 650 mg and ibuprofen 300 mg to 400 mg, were combined for purposes of analyses.
Outcomes
For 26 of the 35 included studies, some measure of adequate pain relief could be extracted four to six hours after drug administration. Seven studies provided data on adequate pain relief four hours after taking the medication, nine studies reported this outcome measure at six hours, and seven studies reported adequate pain relief at both four hours and six hours. In addition, three studies (two publications) reported adequate pain relief outcomes at five hours after drug administration (Jain 1985; Laska 1981a; Laska 1981b). We included these data in the six‐hours post‐administration outcomes for analysis purposes. Twenty studies reported summed pain intensity differences (SPIDs). Eleven of these studies also reported total pain relief (TOTPAR), and five studies also reported adequate pain relief as a good/excellent rating or the number of women reporting at least 50% pain relief. The remaining six studies only reported adequate pain relief as good/excellent or the number of women with at least 50% pain relief. In 20 of the 26 studies that reported SPID, we calculated the number of women with adequate pain relief using the SPID measure as per protocol. In four of the 11 studies that provided both SPID and TOTPAR (Gleason 1987; Hebertson 1986; Jain 1988; Schachtel 1989), the SPID and TOTPAR calculations of the number of women with adequate pain relief did not match and the raw data for pain intensity or pain relief were not available. In these cases, we used the SPID data to calculate the number of women with adequate pain relief. The reasons for the discrepancy in the number of women with adequate pain relief when calculated using SPID versus TOTPAR are not entirely clear, but they may be due to calculation errors in the reports or inaccurate time‐weighting. The formula to calculate %max TOTPAR contains the number of hours over which pain relief was measured. Some studies for example, measured pain relief at half‐hour and one‐hour post‐administration initially and then hourly thereafter up to six hours, providing a total of seven measurements of pain relief. To accurately apply these data to the formula, adjustments need to be made to account for the half‐hour periods, or the %maxTOTPAR would otherwise be overestimated. We noted this absence of adjustment in a study that additionally provided the raw data and we were able to check the calculations. Also, in one of the five studies that reported adequate pain relief as good/excellent, or as the number of women with at least 50% pain relief in addition to SPID, there was a significant unexplained discrepancy between these two measures of adequate pain relief (Hopkinson 1980).
Fifteen studies reported on the need for additional analgesia, and 18 studies reported on any maternal drug adverse effects. None of the secondary outcomes prespecified in the review were reported in any of the included studies. Furthermore, for seven studies that met the review's inclusion criteria, data were not available for analyses because the outcomes were reported beyond the review's maximum six‐hour time‐frame (e.g. at eight hours and 12 hours) (Bloomfield 1970; Melzack 1983), data for the unique medication groups were not provided (Gruber 1979), outcome data were presented in graphs or in a format that could not be accurately extracted (Jain 1978; Okun 1982), and the numbers randomised to the intervention and placebo groups were not reported (Sunshine 1987b; Trop 1983).
Excluded studies
Fifty‐eight of the 96 identified study reports did not meet the review's inclusion criteria and were excluded as follows: the intervention drug was not a NSAID or was administered by a route other than orally in 21 studies; two studies examined the effect of a NSAID in combination with another medication; the comparator was neither a placebo, paracetamol or an other NSAID in five studies; in 12 studies perineal pain was not reported separately for included women, but was reported collectively with other sources of pain or pain in other areas; 12 studies did not report on a single dose; and 4 studies were not RCTs. The remaining studies were excluded for the following reasons: one study (Cater 1985) was excluded because it only examined the NSAID zomepirac which was withdrawn voluntarily from the market by the manufacturer in 1983 because it was associated with fatal and near‐fatal anaphylactoid reactions; and one study (Pedronetto 1975) was excluded because it only examined the NSAID indoprofen, which was withdrawn from markets in the 1980s due to reports of adverse reactions including reports of carcinogenicity in animal studies.
Risk of bias in included studies
There was generally poor reporting in the studies included in this review, particularly around methods of randomisation sequence generation, allocation concealment, and blinding of the outcome assessor, with 21 studies receiving unclear judgements for all three of these 'Risk of bias' criteria (see Figure 2 and Figure 3).
2.

'Risk of bias' graph: review authors' judgements on each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements on each risk of bias item for each included study.
Allocation
Only six of the 35 studies described their sequence generation process. Five described using a computer‐generated random sequence (Hebertson 1986; Jain 1988; Olson 1997; Schachtel 1989; Sunshine 1983a), and one described using random numbers but not the method (Melzack 1983).
Adequate allocation concealment was described in six studies (Bloomfield 1967; Bloomfield 1974; Daftary 1980; Gruber 1979; Lim 2008; Suhrabi 2013), but was unclear for all other included studies.
Blinding
Thirty‐two of the 35 included studies were described as double‐blind, defined as blinding of the participants as well as the personnel providing the treatment to the participants, reducing performance bias. Two studies were single‐blind, with only the participants blinded to the treatment they received (Olson 1999; Suhrabi 2013). However, all but two studies (Kamondetdecha 2008; Trop 1983) did not clearly report whether or not the outcome assessor was blinded, making the extent of potential detection bias unclear.
Incomplete outcome data
We assessed three studies to have a high risk of attrition bias (Laska 1981a; Laska 1981b; Melzack 1983); in Laska 1981aand Laska 1981b information on withdrawals due to the need for rescue medication was not provided and in Melzack 1983 there was differential attrition.
We assessed four studies as being at unclear risk of attrition bias (Gruber 1979; Hopkinson 1980; Jain 1978; Sunshine 1987b). In Hopkinson 1980 there appeared to be missing data (possibly due to dropouts or withdrawals) at two‐, three‐ and four‐hour assessments without a clear statement of reasons for this in the study publication. In the other three unclear studies there was insufficient information in the trial report to assess the extent of incomplete outcome data.
We assessed all other studies to have a low risk of attrition bias because there was either low or non‐differential attrition, or both.
Selective reporting
We rated the potential for reporting bias as low for most of the studies, but it is important to note that in the absence of trial protocols it is not truly possible to assess for reporting bias. We judged four studies (Hopkinson 1980; Jain 1978; Laska 1981a; Laska 1981b) as being at high risk of bias because they did not report one of the outcomes they had prespecified in the Methods sections of their papers.
Other potential sources of bias
For two studies there was an imbalance in some baseline characteristics, so we judged them as unclear (Bloomfield 1970; Bloomfield 1974). Two studies (Honorato 1990; London 1983) did not provide a clear statement on whether baseline characteristics were balanced or not and we judged these at unclear risk of bias. We judged Sunshine 1987b as unclear on 'Other bias' as there was insufficient information to accurately assess this criterion, and we judged Bloomfield 1967 as unclear because there could have been potential carry‐over of effect of intrapartum analgesia.
One study that was stopped early due to administrative changes received a high risk of bias judgement (Olson 1999). Lastly, one study (Wisanto 1981) received a high risk of bias judgement for this criterion because the time‐lag between episiotomy and drug intake was significantly (P < 0.05) shorter in the placebo group (8.08 hours ± 0.81) compared to the intervention group (10.21 hours ± 0.70). We found no other potential sources of bias in any of the other included studies.
Effects of interventions
Summary of findings 1. NSAID compared with placebo for perineal pain in the early postpartum period.
| NSAID compared with placebo for perineal pain in the early postpartum period | ||||||
| Patient or population: women with perineal pain in the early postpartum period Settings: maternity hospitals in the USA, UK, Belgium, Spain, France, Italy, Venezuela, India, Malaysia, Thailand, and Iran Intervention: NSAID Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | NSAID | |||||
| Adequate pain relief (4 hours after administration) | 284 per 1000 | 543 per 1000 (466 to 634) | RR 1.91 (1.64 to 2.23) | 1573 (10 studies) | ⊕⊕⊝⊝ lowa,b | ‐ |
| Adequate pain relief (6 hours after administration) | 321 per 1000 | 615 per 1000 (542 to 696) | RR 1.92 (1.69 to 2.17) | 2079 (17 studies) | ⊕⊝⊝⊝ very lowb,c | ‐ |
| Need for additional analgesia (4 hours after administration) | 305 per 1000 | 119 per 1000 (79 to 177) | RR 0.39 (0.26 to 0.58) | 486 (4 studies) | ⊕⊕⊕⊝ moderated | ‐ |
| Need for additional analgesia (6 hours after administration) |
438 per 1000 | 140 per 1000 (114 to 175) | RR 0.32 (0.26 to 0.40) | 1012 (10 studies) | ⊕⊝⊝⊝ very lowb,c | ‐ |
| Maternal drug adverse effects (4 hours after administration) | See comment | Not estimable | 90 (1 RCT) |
One small study (90 women) reported no maternal drug adverse events in either the intervention or control group | ||
| Maternal drug adverse effects (6 hours after administration) | 22 per 1000 | 31 per 1000 (16 to 60) | RR 1.38 (0.71 to 2.70) | 1388 (13 studies) | ⊕⊕⊝⊝ lowa,e | ‐ |
| Neonatal drug adverse effects | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
aDowngraded one level for serious risk of bias: two studies included in this outcome had instances of high risk of bias. The remaining studies had a mix of low and unclear risk of bias. bDowngraded one level based on visual inspection of funnel plot which indicates likely publication bias. cDowngraded two levels for serious risk of bias: four studies included in this outcome had instances of high risk of bias. The remaining studies had a mix of low and unclear risk of bias. dDowngraded one level for serious risk of bias: one study included in this outcome had instances of high risk of bias. The remaining studies had a mix of low and unclear risk of bias.
eDowngraded one level for imprecision (few events); 95% CI around the pooled estimate includes no effect.
Summary of findings 2. NSAID (single administration, any dose) compared to paracetamol for perineal pain in the early postpartum period.
| NSAID (single administration, any dose) compared to paracetamol for perineal pain in the early postpartum period | ||||||
| Patient or population: women with perineal pain in the early postpartum period Setting: maternity hospitals in Italy, Spain, USA, France and Thailand Intervention: NSAID (single administration, any dose) Comparison: paracetamol | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with paracetamol | Risk with NSAID (single administration, any dose) | |||||
| Adequate pain relief (4 hours after administration) | Study population | RR 1.54 (1.07 to 2.22) | 342 (3 RCTs) | ⊕⊕⊝⊝ lowa,b | ‐ | |
| 205 per 1000 | 315 per 1000 (219 to 454) | |||||
| Adequate pain relief (6 hours after administration) | Study population | RR 1.82 (0.61 to 5.47) | 99 (2 RCTs) | ⊕⊝⊝⊝ very lowa,c | ‐ | |
| 200 per 1000 | 364 per 1000 (122 to 1000) | |||||
| Need for additional analgesia (4 hours after administration) | Study population | RR 0.55 (0.27 to 1.13) | 73 (1 RCT) | ⊕⊝⊝⊝ very lowa,c | ‐ | |
| 405 per 1000 | 223 per 1000 (109 to 458) | |||||
| Need for additional analgesia (6 hours after administration) | Study population | RR 0.28 (0.12 to 0.67) | 59 (1 RCT) | ⊕⊕⊝⊝ lowa,b | ‐ | |
| 571 per 1000 | 160 per 1000 (69 to 383) | |||||
| Maternal drug adverse effects (4 hours after administration) | See comment | not estimable | 210 (1 RCT) | 1 study (210 women) reported no maternal drug adverse events in either the intervention or control group | ||
| Maternal drug adverse effects (6 hours after administration) | Study population | RR 0.74 (0.27 to 2.08) | 300 (3 RCTs) | ⊕⊝⊝⊝ very lowa,c | ‐ | |
| 53 per 1000 | 39 per 1000 (14 to 111) | |||||
| Neonatal drug adverse effects ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for serious risk of bias: unclear risk of selection bias. bDowngraded one level for imprecision: few participants. cDowngraded two levels for imprecision: few participants and wide 95% confidence interval consistent with possible benefit and possible harm.
1. Any NSAID versus placebo
Primary outcomes
Adequate pain relief
At four hours after drug administration, more women who receive a NSAID may experience adequate pain relief compared to women who receive placebo (risk ratio (RR) 1.91, 95% confidence interval (CI) 1.64 to 2.23; 10 studies, 1573 women; Analysis 1.1; low‐certainty evidence; Table 1). Downgrading decisions were for risk of bias and possible publication bias (Figure 4).
1.1. Analysis.

Comparison 1: NSAID (single administration, any dose) versus placebo, Outcome 1: Adequate pain relief (4 hours after administration)
4.

Funnel plot of comparison: 1 NSAID (single administration, any dose) versus placebo, outcome: 1.1 Adequate pain relief (4 hours after administration).
At six hours after drug administration, more women may also experience adequate pain relief in the NSAID compared to the placebo group, although the certainty of evidence is very low (RR 1.92, 95% CI 1.69 to 2.17; 17 studies, 2079 women; Analysis 1.2; Table 1). The number needed to treat for an additional outcome of have adequate pain relief is four (95% CI 3 to 4) at four hours after drug administration and four (95% CI 3 to 5) at six hours post‐administration. GRADE decisions for downgrading the certainty of the evidence for this outcome were based on risk of bias and possible publication bias (Figure 5).
1.2. Analysis.

Comparison 1: NSAID (single administration, any dose) versus placebo, Outcome 2: Adequate pain relief (6 hours after administration)
5.

Funnel plot of comparison: 1 NSAID (single administration, any dose) versus placebo, outcome: 1.2 Adequate pain relief (6 hours after administration).
Sensitivity analysis removing studies at high risk of bias did not substantially change the effect estimate at either four hours' follow‐up (RR 1.85, 95% CI 1.58 to 2.18) or at six hours (RR 1.74, 95% CI 1.53 to 1.97).
Additional analgesia
Women who received a NSAID are probably less likely to require additional analgesia at four hours (RR 0.39, 95% CI 0.26 to 0.58; 4 studies, 486 women; Analysis 1.3; moderate‐certainty evidence; Table 1).
1.3. Analysis.

Comparison 1: NSAID (single administration, any dose) versus placebo, Outcome 3: Need for additional analgesia (4 hours after administration)
At six hours after initial administration women who received a NSAID may be less likely to require additional analgesia, although the evidence for this is very uncertain (RR 0.32, 95% CI 0.26 to 0.40; 10 studies, 1012 women; Analysis 1.4; very low‐certainty evidence; Table 1). Downgrading was for risk of bias and possible publication bias (Figure 6).
1.4. Analysis.

Comparison 1: NSAID (single administration, any dose) versus placebo, Outcome 4: Need for additional analgesia (6 hours after administration)
6.

Funnel plot of comparison: 1 NSAID (single administration, any dose) versus placebo, outcome: 1.4 Need for additional analgesia (6 hours after administration).
Sensitivity analysis removing studies at high risk of bias did not substantially change the effect estimate at either four hours' follow‐up (RR 0.39, 95% CI 0.24 to 0.62) or at six hours (RR 0.32, 95% CI 0.26 to 0.41).
Maternal adverse effects
We could not estimate the RR for maternal drug adverse effects at four hours post‐administration, as no adverse effects were observed in either the NSAID (60 women) or the placebo groups (30 women) in the one study (with two treatment arms) reporting at this follow‐up time (Sunshine 1983b).
We are uncertain if there is any difference between NSAID and placebo in overall adverse effects at six hours post‐administration because the certainty of the evidence is low and the 95% CI is consistent with both possible benefit and possible harm (RR 1.38, 95% CI 0.71 to 2.70; 13 studies, 1388 women; Analysis 1.5; Table 1). A visual inspection of the funnel plot for this outcome (Figure 7) did not suggest evidence of publication bias.
1.5. Analysis.

Comparison 1: NSAID (single administration, any dose) versus placebo, Outcome 5: Maternal drug adverse effects (6 hours after administration)
7.

Funnel plot of comparison: 1 NSAID (single administration, any dose) versus placebo, outcome: 1.5 Maternal drug adverse effects (6 hours after administration).
Sensitivity analysis removing studies at high risk of bias did not change the effect estimate at six hours' follow‐up, because the studies at high risk of bias did not contribute to the effect estimate, as they had no events in either arm.
At six hours after drug administration, six of the 17 comparisons (a NSAID versus placebo) across 13 studies reported adverse effects (Analysis 1.5). These were drowsiness (n = 5), abdominal discomfort (n = 2), weakness (n = 1), dizziness (n = 2), headache (n = 2), moderate epigastralgia (n = 1) for the NSAID groups, and drowsiness (n = 2), light‐headedness (n = 1), nausea (n = 1), backache (n = 1), dizziness (n = 1) and epigastric pain (n = 1) for the placebo group. In two studies that reported adverse effects (Bloomfield 1967; Daftary 1980), the specific adverse effects were not stated.
Neonatal adverse effects
Neonatal drug adverse effects were not reported in any of the included studies.
Secondary outcomes
None of the studies assessed any of the review's prespecified secondary outcomes.
2. Any NSAID versus paracetamol
Primary outcomes
Adequate pain relief
At four hours after drug administration more women who receive any NSAID may experience adequate pain relief than women who received paracetamol (RR 1.54, 95% CI 1.07 to 2.22; 3 studies, 342 women; Analysis 2.1; low‐certainty evidence; Table 2). Only two studies (Movilia 1989; Yscla 1988) examined a NSAID (aceclofenac 100 mg) versus paracetamol six hours after administration. At six hours we are uncertain if there is a difference between NSAID and paracetamol in the number of women with adequate pain relief, because the certainty of evidence is very low (RR 1.82, 95% CI 0.61 to 5.47; 2 studies, 99 women; I2 = 59%; Analysis 2.2; Table 2).
2.1. Analysis.

Comparison 2: NSAID (single administration, any dose) versus paracetamol, Outcome 1: Adequate pain relief (4 hours after administration)
2.2. Analysis.

Comparison 2: NSAID (single administration, any dose) versus paracetamol, Outcome 2: Adequate pain relief (6 hours after administration)
We did not conduct a sensitivity analysis, since there were no studies at high risk of bias included in this comparison.
Additional analgesia
One study (Schachtel 1989) assessed the need for additional analgesia four hours after NSAID (ibuprofen) administration compared with paracetamol (1000 mg). We are uncertain if there is any difference between the two drugs, because the certainty of evidence is very low (RR 0.55, 95% CI 0.27 to 1.13; 73 women; Analysis 2.3; Table 2).
2.3. Analysis.

Comparison 2: NSAID (single administration, any dose) versus paracetamol, Outcome 3: Need for additional analgesia (4 hours after administration)
Another study (Behotas 1992) examined the need for additional analgesia six hours after NSAID (ibuprofen) administration compared with paracetamol (1000 mg). Women who receive NSAID may be less likely to need any additional analgesia compared with women who receive paracetamol (RR 0.28, 95% CI 0.12 to 0.67; 59 women; Analysis 2.4;, low‐certainty evidence; Table 2).
2.4. Analysis.

Comparison 2: NSAID (single administration, any dose) versus paracetamol, Outcome 4: Need for additional analgesia (6 hours after administration)
We did not conduct a sensitivity analysis, since there were no studies at high risk of bias included in this comparison.
Maternal adverse effects
No maternal adverse drugs adverse effects were reported in the one study (Kamondetdecha 2008; 210 women) that reported this outcome at four hours after drug administration. Six hours post‐administration, two of three studies reported the following maternal drug adverse effects; pruritis (n = 1) for the NSAID group and sleepiness (n = 1) for the paracetamol group. We are uncertain if there is any difference in overall adverse effects between the groups (RR 0.74, 95% CI 0.27 to 2.08; 3 studies, 300 women; Analysis 2.5; very low‐certainty evidence; Table 2).
2.5. Analysis.

Comparison 2: NSAID (single administration, any dose) versus paracetamol, Outcome 5: Maternal drug adverse effects (6 hours after administration)
We did not conduct a sensitivity analysis, since there were no studies at high risk of bias included in this comparison.
Neonatal adverse effects
Neonatal drug adverse effects were not reported in any of the included studies.
Secondary outcomes
None of the studies assessed any of the review's prespecified secondary outcomes.
3. NSAID versus another NSAID
Primary outcomes
Adequate pain relief
It is unclear if there is any difference in effectiveness between different NSAIDs in providing adequate pain relief at four hours after administration (Analysis 3.1) or six hours after administration (Analysis 3.2).
3.1. Analysis.

Comparison 3: NSAID versus a different NSAID, Outcome 1: Adequate pain relief (4 hours after administration)
3.2. Analysis.

Comparison 3: NSAID versus a different NSAID, Outcome 2: Adequate pain relief (6 hours after administration)
Even though comparisons between different NSAIDs did not show differences in benefit, the direction of effect was in favour of aspirin when compared to diflunisal, in favour of diclofenac when compared to aspirin, in favour of etodolac when compared to aspirin, in favour of dipyrone when compared to aspirin, in favour of ibuprofen when compared to aspirin at four hours but not at six hours, and in favour of flurbiprofen when compared to aspirin except at a lower dose (25 mg) (Analysis 3.1; Analysis 3.2).
Suhrabi 2013 was not included in the meta‐analysis, as it did not report any of the review's prespecified outcome measures, but only reported pain intensity scores four hours post‐administration on a 10‐cm visual analogue scale. Using this measure of pain, no difference between celecoxib 100 mg (mean 2.57, standard deviation (SD) 1.4) and ibuprofen 400 mg (mean 2.7, SD 1.4) was found.
We did not conduct a sensitivity analysis, since there were no studies at high risk of bias included in this comparison.
Additional analgesia
It is unclear if there is any difference in the need for additional analgesia between the different NSAID groups at four hours after administration (Analysis 3.3) or at six at hours after administration (Analysis 3.4).
3.3. Analysis.

Comparison 3: NSAID versus a different NSAID, Outcome 3: Need for additional analgesia (4 hours after administration)
3.4. Analysis.

Comparison 3: NSAID versus a different NSAID, Outcome 4: Need for additional analgesia (6 hours after administration)
We did not conduct a sensitivity analysis, since there were no studies at high risk of bias included in this comparison.
Maternal adverse effects
There were no reports of maternal adverse effects at four hours after administration in either of the NSAID groups (aspirin: 62 women; other NSAID:103 women) (De Vroey 1978; Sunshine 1983b).
It is unclear if there is any difference between the different NSAID groups in the risk of maternal adverse effects at six hours after administration (Analysis 3.5).
3.5. Analysis.

Comparison 3: NSAID versus a different NSAID, Outcome 5: Maternal drug adverse effects (6 hours after administration)
We did not conduct a sensitivity analysis, since there were no studies at high risk of bias included in this comparison.
Neonatal adverse effects
Neonatal drug adverse effects were not reported in any of the included studies.
Secondary outcomes
None of the studies assessed any of the review's prespecified secondary outcomes.
4. NSAID versus a different dose of the same NSAID
Primary outcomes
Adequate pain relief
It is unclear if there is any difference between different doses of the same NSAID for achieving adequate pain relief at four hours after administration (Analysis 4.1) or at six hours after administration (Analysis 4.2). The different doses of the same NSAID that were investigated were equally effective, with the exception of fenoprofen 50 mg providing adequate pain relief to more women than fenoprofen 100 mg six hours after administration (Laska 1981a). All but one comparison showed little or no difference, but the direction of effect in the included studies was in favour of a lower dose of diflunisal (125 mg) compared to a higher dose (250 mg or 500 mg), in favour of a higher dose of diclofenac (50 mg or 100 mg) versus a lower dose (25 mg), in favour of flurbiprofen 50 mg or 100 mg versus 25 mg, in favour of aceclofenac 150 mg versus 50 mg or 100 mg, in favour of etodolac 100 mg versus 25 mg, in favour of a higher dose of fenoprofen (25 mg, 50 mg, 100 mg, 200 mg, 300 mg) versus a lower dose of fenoprofen (12.5 mg, 25 mg, 50 mg). In contrast, there was no or minimal direction of effect between ibuprofen 300 mg to 400 mg and ibuprofen 800 mg, meclofenamate sodium 100 mg and 200 mg, aceclofenac 50 mg and 100 mg, ketoprofen 25 mg and 50 mg, flurbiprofen 50 mg and 100 mg, fenoprofen 25 mg and 50 mg, and between fenoprofen 100 mg, 200 mg and 300 mg (Analysis 4.1; Analysis 4.2).
4.1. Analysis.

Comparison 4: NSAID versus a different dose of the same NSAID, Outcome 1: Adequate pain relief (4 hours after administration)
4.2. Analysis.

Comparison 4: NSAID versus a different dose of the same NSAID, Outcome 2: Adequate pain relief (6 hours after administration)
We did not conduct a sensitivity analysis, since there were no studies at high risk of bias included in this comparison.
Additional analgesia
It is unclear if there is any difference in the need for additional analgesia between groups examining different doses of the same NSAID at fours after administration (Analysis 4.3) or at six hours after administration Analysis 4.4).
4.3. Analysis.

Comparison 4: NSAID versus a different dose of the same NSAID, Outcome 3: Need for additional analgesia (4 hours after administration)
4.4. Analysis.

Comparison 4: NSAID versus a different dose of the same NSAID, Outcome 4: Need for additional analgesia (6 hours after administration)
We did not conduct a sensitivity analysis, since there were no studies at high risk of bias included in this comparison.
Maternal adverse effects
There were no reports of maternal adverse effects at four hours after administration in either of the NSAID groups (Hopkinson 1980; De Vroey 1978). It is unclear if there is any difference between the different doses of NSAID in the risk of maternal adverse effects at six hours after administration (Analysis 4.5).
4.5. Analysis.

Comparison 4: NSAID versus a different dose of the same NSAID, Outcome 5: Maternal drug adverse effects (6 hours after administration)
Discussion
Summary of main results
This review involved 5136 women with perineal pain in the early postpartum period, mostly following episiotomy, of whom 3145 received non‐steroidal anti‐inflammatory drugs (NSAIDs) and 1692 were given paracetamol or placebo. Aspirin 500 mg to 650 mg was the most studied NSAID/dose in the included studies (nine studies), followed by ibuprofen (six studies), while many other NSAIDs were only examined in a single study.
For women who sustained perineal trauma during childbirth, any NSAID may be more effective at providing adequate pain relief than placebo at four hours (low‐certainty evidence) but we are less certain about the effects at six hours (very low‐certainty evidence; Table 1). Women who received a NSAID are probably less likely to need additional analgesia at four‐hour follow‐up compared with placebo (moderate‐certainty evidence), and may be less likely to need additional analgesia at six hours, although we are uncertain about this evidence (very low‐certainty evidence; Table 1).
NSAIDs may be more effective than paracetamol four hours after administration (low‐certainty evidence; Table 2), but we are uncertain about their relative effectiveness at six hours post‐administration (very low‐certainty evidence; Table 2). It may be that fewer women need additional analgesia at six hours after receiving a NSAID compared with paracetamol (low‐certainty evidence; Table 2).
Maternal adverse effects were rare in both the NSAID group and the placebo or paracetamol groups. We are uncertain if there is any difference between NSAID and placebo (Table 1) or between NSAID and paracetamol (Table 2) in overall adverse effects at six hours post‐administration.
In the studies that reported adverse effects, more than one adverse effect was reported in the aspirin, ibuprofen and dipyrone groups. Fewer adverse effects were reported for ibuprofen compared to aspirin. An equal number of adverse effects was reported at a lower dose of ibuprofen (300 mg to 400 mg) and a higher dose (800 mg), but their effectiveness in providing pain relief was also equivalent. Information on adverse effects of diclofenac, another commonly‐used NSAID, was not reported in the included studies, but more women in the diclofenac group reported adequate pain relief than women in the aspirin group.
It is unclear if there is any difference in effectiveness between the different NSAIDS or the different doses of the same NSAID examined in the included studies. For example, ibuprofen 300 mg to 400 mg was equally as effective in relieving perineal pain in the early postpartum period as ibuprofen 800 mg to 900 mg at four and at six hours after drug administration.
Overall completeness and applicability of evidence
Only three of the 14 prespecified outcomes were examined in the included studies. The studies in this review did not examine the compatibility of NSAIDs with breastfeeding and did not report on neonatal adverse effects. In general, NSAIDs should be used with caution when breastfeeding, with paracetamol as the preferred choice of analgesic for breastfeeding women (BNF 2014). If a NSAID is required, of the NSAIDs that were examined in the studies included in this review, ibuprofen, fenoprofen, diclofenac sodium would be preferred when breastfeeding, while diflunisal, aceclofenac, aspirin, celecoxib, etodolac, flurbiprofen, ketoprofen, dipyrone, meclofenamate sodium should be avoided (BNF 2014; LacMed 2015). Although NSAIDs seem to provide better pain relief than placebo at four and six hours postpartum, there is no evidence available to evaluate the effect of NSAIDs on neonatal outcomes, since the studies in this review only included non‐breastfeeding mothers. In addition, there are no data on outcomes such as disability and depression, that can be related to experiencing pain. Other prespecified outcomes that are important for women and service providers, including prolonged hospitalisation and rehospitalisation due to perineal pain, were also not examined in the included studies.
Quality of the evidence
Most of the included studies did not report details on how they generated the random sequence, whether allocation was concealed, and whether the outcome assessment was blinded. Only three studies were published after the year 2000, with most included studies conducted in the 1980s or earlier. Trial registries were only introduced in the past two decades; for example, ClinicalTrials.gov was launched in the year 2000. Moreover, the first CONSORT (Consolidated Standards of Reporting Trials) statement to improve reporting in clinical trials was only published in 1996 (Begg 1996). This may explain the lack of reporting of key methodological aspects in the studies included in this review.
We assessed the GRADE certainty of evidence for the main comparisons (NSAID versus placebo and NSAID versus paracetamol) for all primary outcomes at four‐ and six‐hour follow‐ups. The certainty of the evidence was downgraded for risks of bias, imprecision (due to low numbers of women and few events), and publication bias.
Asymmetry in the funnel plots (for the outcome of adequate pain relief) suggests that additional smaller studies comparing the use of a NSAID versus placebo may not have been published, which may lead to an overestimation of the intervention effect (Page 2020). In addition, one trial registration report identified in the search strategy included a letter from the authors confirming that their study would not be published (McCallum 1991). The funnel plots for additional analgesia and adverse effects at six hours post‐administration were more symmetrical, but still did not show an equal spread across the triangle.
Potential biases in the review process
We reduced bias in the review process as far as possible by conducting a comprehensive literature search with no language or publication‐status restrictions. We contacted study authors for missing data or to clarify information. Two review authors independently conducted study selection, data extraction and GRADE rating.
Agreements and disagreements with other studies or reviews
The results of this review are in agreement with a Cochrane Review assessing analgesia for relief of pain due to uterine cramping/involution after birth (Deussen 2020). This review found that NSAIDs were more effective than placebo in improving pain relief based on eleven studies including 946 women, but adverse effects reported were similar in the placebo and control groups.
Authors' conclusions
Implications for practice.
The findings of this review need to be interpreted in the context of the certainty of the evidence and risks of bias of the included studies, which was unclear for many because of a lack of reporting on the random sequence generation, allocation concealment and blinding of the outcome assessment.
NSAIDs may be effective analgesics for women with perineal pain postpartum. The different NSAIDs (examined in the included studies) are all effective compared to placebo, and no particular NSAIDs were more effective than others; the choice of NSAIDs may thus be guided by its compatibility with breastfeeding, which was not examined in this review, as all trials excluded women who were breastfeeding.
This review only examined the effectiveness of a single‐dose non‐steroidal anti‐inflammatory drug (NSAID), while in practice more than one dose is often given and women might receive a combination of paracetamol and a NSAID. The findings seem to support the practice of 'stepping up the pain ladder' to a NSAID if paracetamol does not provide sufficient pain relief, or providing multimodal pain relief (Berry 2001), combining paracetamol and a NSAID. The dosages that were compared in the included studies did not seem to impact on the effectiveness of a NSAID. Although NSAIDs may be more effective than paracetamol, the evidence is sparse and inconclusive. Other analgesia may also further be considered, but this is beyond the remit of this review and will be examined in further reviews in this series of reviews on pain relief for perineal pain in the early postpartum period.
Although there is limited evidence, maternal adverse effects seem rare.
None of the included studies reported on neonatal adverse effects or any data for the secondary outcomes of this review, including prolonged hospitalisation or rehospitalisation due to perineal pain, fully breastfeeding or mixed feeding at discharge and at six weeks, maternal views, postpartum depression, and disability
Implications for research.
Future studies may examine NSAIDs' adverse‐effects profile, including neonatal adverse effects and the compatibility of NSAIDs with breastfeeding, and may assess other important secondary outcomes of this review, including (re‐)hospitalisation, maternal disability and maternal views. Moreover, studies mostly included women who had episiotomies. Future research needs to be extended to women with and without perineal trauma, including perineal tears. Finally, the small size of the studies and poor reporting limit the strength of the results of this review. Methodologically high‐quality studies should be conducted to further assess the efficacy of NSAIDs versus paracetamol and the efficacy of multimodal treatments.
What's new
| Date | Event | Description |
|---|---|---|
| 9 December 2019 | New search has been performed | Search updated and 3 new studies assessed for inclusion. |
| 9 December 2019 | New citation required but conclusions have not changed | No new studies incorporated. Seven studies previously excluded have been included in this update. Conclusions remain unchanged. |
History
Protocol first published: Issue 10, 2014 Review first published: Issue 7, 2016
| Date | Event | Description |
|---|---|---|
| 22 October 2016 | Amended | Correction of a typographical error in the plain language summary. |
Acknowledgements
A Cochrane Fellowship (2013 ‐ 2015) was awarded by the Health Research Board Ireland to F Wuytack to support the conduct of this review (2016).
As part of the pre‐publication editorial process, the previous version of this review was commented on by five peers (an editor and four referees who are external to the editorial team), members of the Pregnancy and Childbirth Group's international panel of consumers, and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research (NIHR), via Evidence Synthesis Programme funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Appendices
Appendix 1. Search terms for ICTRP, ClinicalTrials.gov, OpenSIGLE and ProQuest Dissertations and Theses.
ICTRP
(searched with synonyms and each line was searched separately)
perine* AND pain AND postpartum
perine* AND pain AND postnatal
pain AND episiotomy
ClinicalTrials.gov
Advanced search
Interventional Studies | episiotomy pain
Interventional Studies | perineal pain
pain | Interventional Studies | perineum
OpenSIGLE and ProQuest Dissertations and Theses
episiotomy OR (perineal OR perineum) AND (tear OR tears OR pain)
Data and analyses
Comparison 1. NSAID (single administration, any dose) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Adequate pain relief (4 hours after administration) | 10 | 1573 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.64, 2.23] |
| 1.1.1 Aspirin 500 mg to 650 mg | 4 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.41, 5.10] |
| 1.1.2 Ibuprofen 300 mg to 400 mg | 3 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.62, 4.30] |
| 1.1.3 Ibuprofen 800 mg | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.87, 3.87] |
| 1.1.4 Diclofenac 25 mg | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.86, 4.65] |
| 1.1.5 Diclofenac 50 mg | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.96, 5.11] |
| 1.1.6 Diclofenac 100 mg | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.03, 5.42] |
| 1.1.7 Ketoprofen 25 mg | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [1.19, 9.34] |
| 1.1.8 Diflunisal 125 mg | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.44, 19.22] |
| 1.1.9 Meclofenamate sodium 100 mg | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.10, 1.82] |
| 1.1.10 Meclofenamate sodium 200 mg | 3 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.10, 1.83] |
| 1.1.11 Ketoprofen 50 mg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.23 [1.15, 9.10] |
| 1.1.12 Diflunisal 250 mg | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.35, 16.26] |
| 1.1.13 Diflunisal 500 mg | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.73 [0.57, 24.29] |
| 1.1.14 Flurbiprofen 25 mg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.41, 18.29] |
| 1.1.15 Flurbiprofen 50 mg | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [0.64, 26.76] |
| 1.1.16 Flurbiprofen 100 mg | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.87 [0.60, 25.09] |
| 1.2 Adequate pain relief (6 hours after administration) | 17 | 2079 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.69, 2.17] |
| 1.2.1 Aspirin 500 mg to 650 mg | 7 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.37, 2.24] |
| 1.2.2 Aspirin 900 mg | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.97, 10.26] |
| 1.2.3 Ibuprofen 300 mg to 400 mg | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.30, 3.32] |
| 1.2.4 Ibuprofen 900 mg | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [0.91, 9.74] |
| 1.2.5 Ketoprofen 25 mg | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [1.06, 8.49] |
| 1.2.6 Ketoprofen 50 mg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [1.08, 8.64] |
| 1.2.7 Meclofenamate sodium 100 mg | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.05, 1.76] |
| 1.2.8 Meclofenamate sodium 200 mg | 3 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.07, 1.83] |
| 1.2.9 Diflunisal 125 mg | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.48, 20.69] |
| 1.2.10 Diflunisal 250 mg | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.40, 17.86] |
| 1.2.11 Diflunisal 500 mg | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.27 [0.66, 27.51] |
| 1.2.12 Dipyrone 500 mg | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.44, 3.39] |
| 1.2.13 Aceclofenac 50 mg | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.57, 4.27] |
| 1.2.14 Aceclofenac 100 mg | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.62, 4.51] |
| 1.2.15 Aceclofenac 150 mg | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.67, 4.87] |
| 1.2.16 Etodolac 25 mg | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.34, 2.33] |
| 1.2.17 Etodolac 100 mg | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.49, 3.02] |
| 1.2.18 Antrafenine 300 mg | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.74, 16.36] |
| 1.2.19 Flurbiprofen 25 mg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [0.50, 21.31] |
| 1.2.20 Flurbiprofen 50 mg | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.97 [0.78, 31.75] |
| 1.2.21 Flurbiprofen 100 mg | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.90 [0.77, 31.33] |
| 1.2.22 Fenoprofen 12.5 mg | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.34, 12.80] |
| 1.2.23 Fenoprofen 25 mg | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [0.39, 14.53] |
| 1.2.24 Fenoprofen 50 mg | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.38 [0.93, 12.26] |
| 1.2.25 Fenoprofen 100 mg | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.95 [1.10, 14.19] |
| 1.2.26 Fenoprofen 200 mg | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.95 [1.10, 14.19] |
| 1.2.27 Fenoprofen 300 mg | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.93 [0.79, 30.74] |
| 1.3 Need for additional analgesia (4 hours after administration) | 4 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.26, 0.58] |
| 1.3.1 Aspirin 500 mg to 650 mg | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.23, 0.93] |
| 1.3.2 Ibuprofen 300 mg to 400 mg | 3 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.18, 0.56] |
| 1.3.3 Ibuprofen 800 mg | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.19, 1.36] |
| 1.4 Need for additional analgesia (6 hours after administration) | 10 | 1012 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.26, 0.40] |
| 1.4.1 Aspirin 500 mg to 650 mg | 3 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.22, 0.62] |
| 1.4.2 Aspirin 900 mg | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.81] |
| 1.4.3 Ibuprofen 300 mg to 400 mg | 3 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.20, 0.54] |
| 1.4.4 Ibuprofen 900 mg | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.81] |
| 1.4.5 Meclofenamate sodium 100 mg | 3 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.21, 0.53] |
| 1.4.6 Meclofenamate sodium 200 mg | 2 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.29, 0.70] |
| 1.4.7 Antrafenine 300 mg | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.13, 0.74] |
| 1.4.8 Flurbiprofen 25 mg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.49] |
| 1.4.9 Flurbiprofen 50 mg | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.56] |
| 1.4.10 Flurbiprofen 100 mg | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.53] |
| 1.5 Maternal drug adverse effects (6 hours after administration) | 13 | 1388 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.71, 2.70] |
| 1.5.1 Aspirin 500 mg to 650 mg | 6 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.38, 4.60] |
| 1.5.2 Aspirin 900 mg | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.24, 12.51] |
| 1.5.3 Ibuprofen 300 mg to 400 mg | 3 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.27, 3.85] |
| 1.5.4 Ibuprofen 900 mg | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.13, 8.52] |
| 1.5.5 Ketoprofen 25 mg | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5.6 Ketoprofen 50 mg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5.7 Aceclofenac 50 mg | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.04, 16.59] |
| 1.5.8 Aceclofenac 100 mg | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5.9 Aceclofenac 150 mg | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5.10 Diflunisal 125 mg | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5.11 Diflunisal 250 mg | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5.12 Diflunisal 500 mg | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5.13 Dipyrone 500 mg | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [0.49, 12.46] |
| 1.5.14 Antrafenine 300 mg | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5.15 Flurbiprofen 25 mg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5.16 Flurbiprofen 50 mg | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5.17 Flurbiprofen 100 mg | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 2. NSAID (single administration, any dose) versus paracetamol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Adequate pain relief (4 hours after administration) | 3 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.07, 2.22] |
| 2.1.1 Ibuprofen 300 mg to 400 mg versus paracetamol 1000 mg | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.93, 3.04] |
| 2.1.2 Ibuprofen 300 mg to 400 mg versus paracetamol 500 mg | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.86, 2.28] |
| 2.1.3 Aceclofenac 100 mg versus paracetamol 650 mg | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.57, 7.50] |
| 2.2 Adequate pain relief (6 hours after administration) | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.61, 5.47] |
| 2.2.1 Aceclofenac 100 mg versus paracetamol 650 mg | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.61, 5.47] |
| 2.3 Need for additional analgesia (4 hours after administration) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3.1 Ibuprofen 300 mg to 400 mg versus paracetamol 1000 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4 Need for additional analgesia (6 hours after administration) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4.1 Ibuprofen 300 mg to 400 mg versus paracetamol 1000 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.5 Maternal drug adverse effects (6 hours after administration) | 3 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.27, 2.08] |
| 2.5.1 Dipyrone 500 mg versus paracetamol 500 mg | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.15] |
| 2.5.2 Aceclofenac 100 mg versus paracetamol 650 mg | 2 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.07, 14.90] |
Comparison 3. NSAID versus a different NSAID.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Adequate pain relief (4 hours after administration) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 Aspirin 500 mg to 650 mg (A) versus Diflunisal 125 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.2 Aspirin 500 mg to 650 mg (A) versus Diflunisal 250 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.3 Aspirin 500 mg to 650 mg (A) versus Diflunisal 500 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.4 Aspirin 500 mg to 650 mg (A) versus Ibuprofen 300 mg to 400 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.5 Aspirin 500 mg to 650 mg (A) versus Diclofenac 25 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.6 Aspirin 500 mg to 650 mg (A) versus Diclofenac 50 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.7 Aspirin 500 mg to 650 mg (A) versus Diclofenac 100 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.8 Aspirin 500 mg to 650 mg (A) versus Flurbiprofen 25 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.9 Aspirin 500 mg to 650 mg (A) versus Flurbiprofen 50 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.10 Aspirin 500 mg to 650 mg (A) versus Flurbiprofen 100 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Adequate pain relief (6 hours after administration) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.1 Aspirin 900 mg (A) versus Ibuprofen 300 mg to 400 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.2 Aspirin 900 mg (A) versus Ibuprofen 900 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.3 Aspirin 500 mg to 650 mg (A) versus Diflunisal 125 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.4 Aspirin 500 mg to 650 mg (A) versus Diflunisal 250 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.5 Aspirin 500 mg to 650 mg (A) versus Diflunisal 500 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.6 Aspirin 500 mg to 650 mg (A) versus Etodolac 25 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.7 Aspirin 500 mg to 650 mg (A) versus Etodolac 100 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.8 Aspirin 500 mg to 650 mg (A) versus Flurbiprofen 25 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.9 Aspirin 500 mg to 650 mg (A) versus Flurbiprofen 50 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.10 Aspirin 500 mg to 650 mg (A) versus Flurbiprofen 100 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.11 Aspirin 500 mg to 650 mg (A) versus Dipyrone 500 mg (B) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Need for additional analgesia (4 hours after administration) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.3.1 Aspirin 500 mg to 650 mg (A) versus Ibuprofen 300 mg to 400 mg (B) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.00 [0.64, 190.53] |
| 3.4 Need for additional analgesia (6 hours after administration) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 Aspirin 900 mg (A) versus Ibuprofen 300 mg to 400 mg (B) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 69.52] |
| 3.4.2 Aspirin 900 mg (A) versus Ibuprofen 900 mg (B) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.4.3 Aspirin 500 mg to 650 mg (A) versus Flurbiprofen 25 mg (B) | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.07, 16.85] |
| 3.4.4 Aspirin 500 mg to 650 mg (A) versus Flurbiprofen 50 mg (B) | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 70.74] |
| 3.4.5 Aspirin 500 mg to 650 mg (A) versus Flurbiprofen 100 mg (B) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.20 [0.14, 75.55] |
| 3.5 Maternal drug adverse effects (6 hours after administration) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.5.1 Aspirin 900 mg (A) versus Ibuprofen 300 mg to 400 mg (B) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.46, 6.06] |
| 3.5.2 Aspirin 900 mg (A) versus Ibuprofen 900 mg (B) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.46, 6.06] |
| 3.5.3 Aspirin 500 mg to 650 mg (A) versus Dipyrone 500 mg (B) | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.5.4 Aspirin 500 mg to 650 mg (A) versus Flurbiprofen 25 mg (B) | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.5.5 Aspirin 500 mg to 650 mg (A) versus Flurbiprofen 50 mg (B) | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.5.6 Aspirin 500 mg to 650 mg (A) versus Flurbiprofen 100 mg (B) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.5.7 Aspirin 500 mg to 650 mg (A) versus Diflunisal 125 mg (B) | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.5.8 Aspirin 500 mg to 650 mg (A) versus Diflunisal 250 mg (B) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.5.9 Aspirin 500 mg to 650 mg (A) versus Diflunisal 500 mg (B) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 4. NSAID versus a different dose of the same NSAID.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Adequate pain relief (4 hours after administration) | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1.1 Ibuprofen 300 mg to 400 mg (A) versus Ibuprofen 800 mg (B) | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.63, 1.58] |
| 4.1.2 Diflunisal 125 mg (A) versus Diflunisal 250 mg (B) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.60, 2.46] |
| 4.1.3 Diflunisal 125 mg (A) versus Diflunisal 500 mg (B) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.41] |
| 4.1.4 Diflunisal 250 mg (A) versus Diflunisal 500 mg (B) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.33, 1.25] |
| 4.1.5 Meclofenamate sodium 100 mg (A) versus Meclofenamate sodium 200 mg (B) | 3 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.17] |
| 4.1.6 Diclofenac 25 mg (A) versus Diclofenac 50 mg (B) | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.68, 1.21] |
| 4.1.7 Diclofenac 25 mg (A) versus Diclofenac 100 mg (B) | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.65, 1.11] |
| 4.1.8 Ketoprofen 25 mg (A) versus Ketoprofen 50 mg (B) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.73, 1.46] |
| 4.1.9 Aceclofenac 50 mg (A) versus Aceclofenac 100 mg (B) | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.65, 1.54] |
| 4.1.10 Aceclofenac 50 mg (A) versus Aceclofenac 150 mg (B) | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.56, 1.21] |
| 4.1.11 Aceclofenac 100 mg (A) versus Aceclofenac 150 mg (B) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.58, 1.17] |
| 4.1.12 Flurbiprofen 25 mg (A) versus Flurbiprofen 50 mg (B) | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.37, 1.20] |
| 4.1.13 Flurbiprofen 25 mg (A) versus Flurbiprofen 50 mg (B) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.39, 1.30] |
| 4.1.14 Flurbiprofen 50 mg (A) versus Flurbiprofen 100 mg (B) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.64, 1.77] |
| 4.2 Adequate pain relief (6 hours after administration) | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.2.1 Ibuprofen 300 mg to 400 mg (A) versus Ibuprofen 900 mg (B) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 4.2.2 Diflunisal 125 mg (A) versus Diflunisal 250 mg (B) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.61, 2.29] |
| 4.2.3 Diflunisal 125 mg (A) versus Diflunisal 500 mg (B) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.43, 1.27] |
| 4.2.4 Diflunisal 250 mg (A) versus Diflunisal 500 mg (B) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.34, 1.15] |
| 4.2.5 Meclofenamate sodium 100 mg (A) versus Meclofenamate sodium 200 mg (B) | 3 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.18] |
| 4.2.6 Ketoprofen 25 mg (A) versus Ketoprofen 50 mg (B) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.66, 1.46] |
| 4.2.7 Aceclofenac 50 mg (A) versus Aceclofenac 100 mg (B) | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.69, 1.27] |
| 4.2.8 Aceclofenac 50 mg (A) versus Aceclofenac 150 mg (B) | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.65, 1.14] |
| 4.2.9 Aceclofenac 100 mg (A) versus Aceclofenac 150 mg (B) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.73, 1.16] |
| 4.2.10 Etodolac 25 mg (A) versus Etodolac 100 mg (B) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.39, 1.39] |
| 4.2.11 Flurbiprofen 25 mg (A) versus Flurbiprofen 50 mg (B) | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.39, 1.09] |
| 4.2.12 Flurbiprofen 25 mg (A) versus Flurbiprofen 100 mg (B) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.40, 1.10] |
| 4.2.13 Flurbiprofen 50 mg (A) versus Flurbiprofen 100 mg (B) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.68, 1.51] |
| 4.2.14 Fenoprofen 50 mg (A) versus Fenoprofen 100 mg (B) | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.16] |
| 4.2.15 Fenoprofen 50 mg (A) versus Fenoprofen 200 mg (B) | 2 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.62, 1.17] |
| 4.2.16 Fenoprofen 50 mg (A) versus Fenoprofen 300 mg (B) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.31] |
| 4.2.17 Fenoprofen 100 mg (A) versus Fenoprofen 200 mg (B) | 2 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.34] |
| 4.2.18 Fenoprofen 100 mg (A) versus Fenoprofen 300 mg (B) | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.74, 1.46] |
| 4.2.19 Fenoprofen 200 mg (A) versus Fenoprofen 300 mg (B) | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.74, 1.46] |
| 4.2.20 Fenoprofen 12.5 mg (A) versus Fenoprofen 25 mg (B) | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.46, 1.65] |
| 4.2.21 Fenoprofen 12.5 mg (A) versus Fenoprofen 50 mg (B) | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.46, 1.65] |
| 4.2.22 Fenoprofen 12.5 mg (A) versus Fenoprofen 100 mg (B) | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.37, 1.12] |
| 4.2.23 Fenoprofen 12.5 mg (A) versus Fenoprofen 200 mg (B) | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.41, 1.33] |
| 4.2.24 Fenoprofen 25 mg (A) versus Fenoprofen 50 mg (B) | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.55, 1.83] |
| 4.2.25 Fenoprofen 25 mg (A) versus Fenoprofen 100 mg (B) | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.44, 1.23] |
| 4.2.26 Fenoprofen 25 mg (A) versus Fenoprofen 200 mg (B) | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.48, 1.48] |
| 4.3 Need for additional analgesia (4 hours after administration) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.3.1 Ibuprofen 300 mg to 400 mg (A) versus Ibuprofen 800 mg (B) | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.17, 1.88] |
| 4.4 Need for additional analgesia (6 hours after administration) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.4.1 Ibuprofen 300 mg to 400 mg (A) versus Ibuprofen 900 mg (B) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 69.52] |
| 4.4.2 Meclofenamate sodium 100 mg (A) versus Meclofenamate sodium 200 mg (B) | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.55, 1.50] |
| 4.4.3 Flurbiprofen 25 mg (A) versus Flurbiprofen 50 mg (B) | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.12, 64.42] |
| 4.4.4 Flurbiprofen 25 mg (A) versus Flurbiprofen 100 mg (B) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.12, 68.81] |
| 4.4.5 Flurbiprofen 50 mg (A) versus Flurbiprofen 100 mg (B) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.5 Maternal drug adverse effects (6 hours after administration) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.5.1 Ibuprofen 300 mg (A) versus Ibuprofen 900 mg (B) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.23, 4.37] |
| 4.5.2 Diflunisal 125 mg (A) versus Diflunisal 250 mg (B) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.5.3 Diflunisal 125 mg (A) versus Diflunisal 500 mg (B) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.5.4 Diflunisal 250 mg (A) versus Diflunisal 500 mg (B) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.5.5 Ketoprofen 25 mg (A) versus Ketoprofen 50 mg (B) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.5.6 Aceclofenac 50 mg (A) versus Aceclofenac 100 mg (B) | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.95 [0.17, 91.61] |
| 4.5.7 Aceclofenac 50 mg (A) versus Aceclofenac 150 mg (B) | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.47 [0.15, 80.35] |
| 4.5.8 Aceclofenac 100 mg (A) versus Aceclofenac 150 mg (B) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.5.9 Flurbiprofen 25 mg (A) versus Flurbiprofen 100 mg (B) | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.5.10 Flurbiprofen 25 mg (A) versus Flurbiprofen 100 mg (B) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.5.11 Flurbiprofen 50 mg (A) versus Flurbiprofen 100 mg (B) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Behotas 1992.
| Study characteristics | ||
| Methods | Multi‐arm RCT ‐ 3 groups | |
| Participants | Women whose post‐episiotomy pain warranted analgesia Hospital ‘Sainte‐Antonie’, Paris Participants were followed up on day‐1 postpartum Women with hepatic or renal malfunction, a previous duodenal ulcer and those whose condition contra‐indicated treatment with NSAIDs, were excluded |
|
| Interventions | Intervention: ibuprofen 400 mg (N = 31) Comparison: paracetamol 1 g (N = 28) and placebo (N = 31) |
|
| Outcomes |
4‐ and 6‐hourly pain data were only available for ibuprofen and not for the comparator treatments Need for additional analgesia data (6 hours) were the only outcome data available for inclusion in the review |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Identical white containers only – insufficient information as to whether these were sequentially‐numbered and sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all 90 participants (0% attrition rate in all groups) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | Participants were similar in terms of geographical background, socio‐professional status and their overall clinical picture |
Bloomfield 1967.
| Study characteristics | ||
| Methods | Multi‐arm RCT ‐ 5 groups | |
| Participants | All women having a painful mediolateral episiotomy, an uncomplicated labour and delivery, and consenting to take an investigational drug in the obstetric service of Cincinnati General Hospital, USA The study was conducted between December 7th 1965 and April 22nd 1966 Women who were breastfeeding, were under age 18 and were known to have ASA sensitivity were excluded |
|
| Interventions | Intervention: chlorphenesin 400 mg (N = 16); chlorphenesin 800 mg (N = 16); chlorphenesin 400 mg + ASA 300 mg (N = 18) and ASA 600 mg (N = 16) Comparison: placebo (N = 18) |
|
| Outcomes |
ASA 300 mg were the only data used in the review as the other drug treatment regimens were not NSAIDs Pain intensity was measured immediately before treatment and then hourly for 6 hours after administration |
|
| Notes | Dates of study: 7 Dec 1965 to 22 April 1966 Funding sources: USPHS grants HE 05622 and HE 07392 of the National Institutes of Health Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned under controlled conditions but does not state how sequence was generated |
| Allocation concealment (selection bias) | Low risk | Coded medication; identical black capsules but not clear if sequentially coded and sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; code could be broken without revealing the treatment received by other participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% attrition rate (1 of 16 women) in the ASA 600 mg group; 0% attrition in the placebo group (N = 18) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Unclear risk | Potential carry‐over of effect of intrapartum analgesia |
Bloomfield 1970.
| Study characteristics | ||
| Methods | Multi‐arm RCT ‐ 5 groups | |
| Participants | Women on the postnatal wards with moderate to very severe episiotomy pain within 48 hours of an uncomplicated birth at Cincinnati General Hospital, USA Women who were breastfeeding were excluded. |
|
| Interventions | Interventions: flufenisal 300 mg (N = 20); flufenisal 600 mg (N = 21); aspirin 600 mg (N = 20); aspirin 1200 mg (N = 20) Comparison: placebo (N = 16) |
|
| Outcomes |
Subjective evaluation by a trained nurse observer; each participant asked to estimate the severity of pain intensity by ‘How much do the stitches hurt you?’; each answer was transposed to an ordinal score of 0 = no pain (not at all); 1 = mild pain (a little); 2 = moderate pain (medium); 3 = severe pain (a lot); 4 = very severe pain (a whole lot). A pain intensity score was obtained immediately before the administration of medication and at hourly intervals for 8 hours after Pain relief was measured by 2 variables: % of participants with pain reduction > 50% and pain intensity difference (PID) scores |
|
| Notes | No data available for analyses as outcomes reported at 8 hours postpartum Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified block (blocks of 5) randomisation based on pain intensity (moderate, severe and very severe) – no clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | Pre‐packaged identical capsules, but not clear if sealed and sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all included participants reported |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in the study methods reported |
| Other bias | Unclear risk | Baseline imbalance in body weight between groups; the group of participants receiving 300 mg of flufenisal had a distinctly lower mean body weight than the other 4 groups, especially the placebo group; 114.1 versus 136.7 pounds, respectively |
Bloomfield 1974.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 4 groups. | |
| Participants | An homogenous population of postpartum women with moderate to very severe episiotomy pain (mediolateral or midline incision) within 48 hours of an uncomplicated delivery at the University of Cincinnati Medical Centre; homogenous population of postpartum women Women who were unmarried, < 18 years old (but included if married and < 18 years), with history of aspirin allergy, given analgesics or sedatives, or other psychotropic drugs in the previous 6 hours, breastfeeding and with known drug dependence were excluded |
|
| Interventions | Intervention: ibuprofen 300 mg (N = 20); ibuprofen 900 mg (N = 20) and aspirin 900 mg (N = 20) Comparison: lactose placebo (N = 20). |
|
| Outcomes |
Only 6‐hour data available in extractable format |
|
| Notes | Dates of study: NR Funding sources: United States Public Health Service Grant HL‐05622 and The Upjohn Company Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Low risk | All treatments were in the form of film‐coated tablets identical in appearance and taste and pre‐packaged in coded number individual dose‐vials |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all 80 participants (0% attrition rate in all groups) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Unclear risk | Some baseline differences between group characteristics (e.g. weight higher in group receiving ibuprofen 900 mg and more unmarried in ibuprofen 300 mg group). Chance occurrences, but with an uncertain influence on the results |
Daftary 1980.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 3 groups | |
| Participants | Postpartum women, reporting moderate and severe pain Noerosjee Wadia Hospital, Bombay, India Concurrent therapy with hypnotic‐sedative drugs was not permitted from 23.00 the previous night until the end of the evaluation |
|
| Interventions | Intervention: dipyrone 500 mg (N = 101) Comparison: placebo (N = 98) and paracetamol 500 mg (N = 100) |
|
| Outcomes |
Pain relief was evaluated at 30 minutes and hourly intervals thereafter up to 6 hours following therapy; but we were unable to accurately extract data on pain relief, as graphical data only were available; thus we extracted and reported side effects data only in the review |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Low risk | Tablets were identical in appearance and from numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all 299 participants (0% attrition rate in all groups) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | Participant baseline characteristics were comparable |
De Vroey 1978.
| Study characteristics | ||
| Methods | Multi‐arm RCT ‐ 5 groups | |
| Participants | Primiparous women aged between 16 ‐ 40 years who had a medio‐lateral episiotomy during the course of an otherwise uncomplicated delivery; delivery within previous 48 hours and complained of moderate to severe pain Department of Obstetrics and Gynaecology of the St. Christiana Clinic, Belgium |
|
| Interventions | Intervention: diflunisal 125 mg (N = 30); diflunisal 250 mg (N = 30); diflunisal 500 mg (N = 30) and aspirin 600 mg (N = 32) Comparison: placebo (N = 31) |
|
| Outcomes |
Pain severity was assessed before drug administration and then at hourly intervals up to 6 ‐ 8 hours; 4‐ and 6‐hour data available and extracted separately |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used. |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear information, including reasons, were provided on 5 exclusions after randomisation and on 3 withdrawals (9%) of the 32 women in the aspirin 600 mg group after treatment. (The attrition rate was 0% for all other groups) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | None obvious; all women were primiparas with equal distribution across groups, based on initial severity of pain |
Friedrich 1983.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 4 groups | |
| Participants | Women aged between 18 and 34 years with moderate or severe pain following episiotomy, at least 16 hours but no more than 48 hours following induction of anaesthesia Department of Obstetrics and Gynaecology, Barnes Hospital Plaza, Washington University, USA Women were excluded if they had current or recent history of gastrointestinal bleeding, peptic ulcer or other gastrointestinal disorders, alcohol or drug abuse, or disorders of the nervous system, kidney, heart or blood, known allergies to aspirin, or aspirin‐like analgesia, conditions likely to interfere with absorption, distribution, metabolism or excretion of drugs, other pain requiring narcotic analgesics, acute dermatitis or other skin lesions, past or present malignancies, taking corticosteroids or other NSAIDs, anticoagulants or other drugs that might interfere with the study medication, experiencing pain due to other conditions or were breastfeeding |
|
| Interventions | Intervention: aspirin 650 mg (N = 39); etodolac 25 mg (N = 40) and etodolac 100 mg (N = 40) Comparison: placebo (N = 40) |
|
| Outcomes |
Pain assessed at baseline, 30 minutes after and hourly thereafter up to 8 hours; data for 6 hours provided and used in the review as per protocol |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all 159 participants (0% attrition rate in all groups) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | Groups were balanced on demographics and clinical features |
Gleason 1987.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 4 groups | |
| Participants | Women aged 15 years and older with moderate to severe episiotomy pain within 24 hours of an uncomplicated vaginal birth and could read, comprehend and sign a consent form Hospital setting in Kansas, USA Nursing mothers, women with a history of reaction or hypersensitivity to NSAIDs or salicylates or women who had received topical perineal anaesthetic or analgesic, NSAID, sedative or psychotropic medication within 3½ hours of study entry, were excluded |
|
| Interventions | Intervention: meclofenamate 100 mg (N = 77); meclofenamate 200 mg (N = 80), and codeine 60 mg (N = 79) Comparison intervention: placebo (N = 79) |
|
| Outcomes |
Pain measured before 1st dose, 30 minutes after and thereafter hourly up to 6 hours |
|
| Notes | Codeine 60 mg was not considered in the review as not a NSAID Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | Only states each dose was packaged separately |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 327 women selected, 12 (4%) were excluded from the analysis due to protocol violations (taking other analgesic medication during the study). The attrition rates in the individual groups were: 1 of 81 (1%) in the meclofenamate 200 mg group, 3 of 80 (4%) in the meclofenamate 100 mg group, 3 of 83 (4%) in the placebo group (and 3 of 83 (4%) in the codeine group which was not included in this review) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | The 4 treatment groups did not differ significantly by demographic characteristics |
Gruber 1979.
| Study characteristics | ||
| Methods | Multi‐arm RCT ‐ 11 groups | |
| Participants | Hospitalised postpartum women without complicating disease reporting uterine cramp or post‐episiotomy wound pain (sub‐grouped separately) following vaginal birth | |
| Interventions | Intervention: propoxyphene napsylate 50 mg (N = 57), propoxyphene napsylate 100 mg (N = 54), propoxyphene napsylate 150 mg (N = 58), fenoprofen calcium 200 mg (N = 59), fenoprofen calcium 400 mg (N = 54), fenoprofen calcium 600 mg (N = 54), propoxyphene napsylate + fenoprofen calcium 50/200 mg (N = 55), propoxyphene napsylate+ fenoprofen calcium 100/400 mg (N = 50), propoxyphene napsylate + fenoprofen calcium 150/600 mg (N = 57), aspirin 650 mg (N = 59) Control: placebo (N = 56) |
|
| Outcomes |
|
|
| Notes | Propoxyphene napsylate groups and propoxyphene napsylate + fenoprofen calcium groups not considered in the review as not NSAID/pure NSAID. Unable also to extract data for NSAID medications only. Intervention and control group numbers reflect those treated for episiotomy pain only Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information; states only that 11 treatments were randomised in each successive series of 11 envelopes |
| Allocation concealment (selection bias) | Low risk | Identical capsules, numbered sequentially and dispensed in coded envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No clear statement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States that 1 observer at each institution gathered and recorded all of the data but not clear if blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to accurately assess due to how the data is presented in the Results section |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in the study methods are all reported |
| Other bias | Unclear risk | Groups were further sub‐grouped according to institution, type of pain (uterine or episiotomy) and intensity of pain at the start of study. If a sub‐group was too small it would be discarded; unclear if this occurred or possible effect on results |
Hebertson 1986.
| Study characteristics | ||
| Methods | Multi‐arm RCT– 4 groups | |
| Participants | Women aged 15 years and older, moderate to severe episiotomy pain and uncomplicated vaginal birth Latter Day Saints Hospital, Utah, USA Women with a history of reaction or hyper‐sensitivity to NSAIDs or salicylates, active gastric intestinal disease or other disease and received an analgesic, sedative or psychotropic medication or topical perineal anaesthetic within 3½ hours of entry into the study, were excluded |
|
| Interventions | Intervention: meclofenamate sodium 200 mg (N = 40); meclofenamate sodium 100 mg (N = 41) and codeine 60 mg (N = 39) Comparison: placebo (N = 41) |
|
| Outcomes |
Pain intensity was rated just prior to the treatment and together with pain relief was measured at 30 minutes after administration and hourly thereafter up to 6 hours |
|
| Notes | Codeine 60 mg was not considered in the review, as not a NSAID Dates of study: NR Funding sources: Warner‐Lambert/Parke‐Davis Pharmaceutical Research Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random table |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 168 women selected, 7 (4%) were excluded from the analysis due to protocol violations (i.e. had medication within 3½ hours before study entry). The attrition rates in the individual groups were: 2 of 42 (5%) in the meclofenamate 200 mg group, 1 of 42 (2%) in the meclofenamate 100 mg group, 3 of 42 (7%) in the placebo group (and 1 of 42 (2%) in the codeine group which was not included in this review) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | No differences in the distribution of participants across groups |
Honorato 1990.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 4 groups | |
| Participants | Women aged 18 ‐ 35 years with intense post‐episiotomy pain requiring analgesia 3 centres; Navarre Teaching Hospital, Pamplona, Spain; Legnano Civil Hospital, Legnano, Italy, and Montebelluna Civil Hospital, Montebelluna, Spain Women suffering little or no pain, who had peptic ulcer, serious liver or kidney failure, puerperal fever or any complication within the puerperium or any serious general disease that could interfere with the results and were already being treated with NSAID agents or systemic steroids, tranquillisers, sedatives, narcotics and/or local anaesthetics, were excluded Participants were not allowed to take any other drug that might interfere with the results |
|
| Interventions | Intervention: aceclofenac 50 mg (N = 18); aceclofenac 100 mg (N = 24) and aceclofenac 150 mg (N = 21) Comparison: placebo (N = 13) |
|
| Outcomes |
Degree of pain was measured at baseline, 30 minutes after and hourly thereafter up to 6 hours |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | Suggests only that treatments were prepared so that neither the participant nor the investigator could distinguish between the doses |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of blood sample data in 18 of 45 participants of the 4 groups (not specified which groups); reasons provided. However, this outcome (serum concentration of aceclofenac) was not included in this review. 0% attrition rate for outcomes included in this review |
| Selective reporting (reporting bias) | Low risk | Results for the outcome 'Overall evaluation' are not provided, but important pain outcome data were all reported |
| Other bias | Unclear risk | No clear statement on balanced between‐group characteristics; also fewer numbers in placebo group |
Hopkinson 1980.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 4 groups | |
| Participants | Women with moderate or severe post‐episiotomy pain Abington Memorial Hospital, Pennsylvania, USA Women with eclampsia and those who had received analgesic drugs within 12 hours prior to study, were excluded |
|
| Interventions | Intervention: ibuprofen 400 mg (N = 80); ibuprofen 800 mg (N = 80) and propoxyphene 65 mg (N = 81) Comparison: placebo (N = 81) |
|
| Outcomes |
Evaluations of pain were recorded at 30 minutes, and hourly thereafter up to 4 hours after treatment administration |
|
| Notes | Propoxyphene 65 mg was not considered in the review as not a NSAID Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on methods used |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appear to be missing data (dropouts or withdrawals) at 2‐, 3‐ and 4‐hour assessments; no clear statement as to reasons in study publication |
| Selective reporting (reporting bias) | High risk | Side effects not mentioned/reported; no reason provided |
| Other bias | Low risk | There were no statistically significant differences between any of the groups on baseline parameters or characteristics |
Jain 1978.
| Study characteristics | ||
| Methods | Multi‐arm RCT ‐ 4 groups | |
| Participants | Consenting postpartum women within 48 hrs of birth, aged between 18 and 39 years with moderate to severe episiotomy pain Tulane University School of Medicine, New Orleans, USA Nursing mothers, those with systemic diseases and those allergic to aspirin were excluded |
|
| Interventions | Intervention: piroxicam 20 mg (N = 31), piroxicam 40 mg (N = 29) and aspirin 648 mg (N = 30) Control: placebo (N = 30) |
|
| Outcomes |
Measurement was at baseline and hourly up to 4 hours thereafter. Observer rating of intensity on a 4‐point scale of 0 = none, 1 = slight, 2 = moderate and 3 = severe. Observer rating of relief on a 5‐point scale of 0 = none, 1 = slight, 2 = moderate, 3 = marked and 4 = complete |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to accurately assess from the data presented in paper |
| Selective reporting (reporting bias) | High risk | Prespecified outcomes (in study methods) of pain intensity and pain relief by observer rating are not reported (only states that it is comparable to self‐rating); additional analgesia is also not reported |
| Other bias | Low risk | Groups were comparable in age, weight, height and initial degree of pain |
Jain 1985.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 4 groups | |
| Participants | Postpartum women at least 18 years old who had undergone episiotomy and had at least moderate intensity pain Tulane University School of Medicine and Clinical Research Centre, New Orleans, USA Women who received analgesics or tranquillisers within at least 4 hours of study entry, intended to breastfeed, had a history of convulsive disorders, known peptic ulcer, renal, hepatic or haematological disease or known allergic reactions to NSAIDs, were excluded |
|
| Interventions | Intervention: aspirin 600 mg (N = 30); indoprofen 50 mg (N = 30); indoprofen 100 mg (N = 30) Comparison: placebo (N = 30) |
|
| Outcomes |
Pain assessed at baseline, 30 minutes after and thereafter through to 5 hours. 5‐hour data were used in the review (considered within 6‐hour time‐frame data) |
|
| Notes | Only aspirin included in the review, since indoprofen was withdrawn from the market Dates of study: NR Funding sources: Adria Laboratories Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all 120 participants (0% attrition rate in all groups) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | Demographic data indicated groups were similar in age, race, height, weight and initial pain intensity |
Jain 1988.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 3 groups | |
| Participants | Women with moderate to severe pain following episiotomy Clinical research centre, New Orleans, USA Women with known hypersensitivity to NSAID agents, history of allergy to aspirin, ibuprofen or caffeine, history of asthma, clinically significant renal, hepatic, endocrine, pulmonary, cardiac, neurologic, or cerebral dysfunction or with a history of peptic ulcer disease or gastrointestinal blood loss, uncontrolled diabetes, drug abuse or alcoholism, were excluded Use of caffeine, anti‐inflammatory agent, tranquilliser or sedative was prohibited during the 4 hours prior to administration of test medication as well as during the study period |
|
| Interventions | Intervention: caffeine 100 mg + ibuprofen 200 mg (N = 50) and ibuprofen 400 mg (N = 49) Comparison: placebo (N = 48) |
|
| Outcomes |
Pain assessed at baseline and 30 minutes after and thereafter hourly up to 6 hours. Only 6‐hour data used in this review (4‐hour data could not be extracted) |
|
| Notes | Caffeine 100 mg + ibuprofen 200 mg combination therapy not include in the review, as not pure NSAID Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Supplied in unit dose bottles containing identical tablets; no information if sequentially‐numbered and sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 161 women selected, 14 (9%) were excluded from the analysis; 11 due to rescue medication before the 2‐hour follow‐up (4 in the ibuprofen 400 mg group, 4 in the placebo group (and 3 in the ibuprofen 200 mg + caffeine group which was not included in this review)), 2 due to use of confounding agents and 1 because she was under 18 years old (not specified from which group). Attrition rates could not be calculated due to non‐report of the exact number of participants in each group at the start of the study |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | No statistical differences across groups by demographic variables and baseline pain intensity measures |
Kamondetdecha 2008.
| Study characteristics | ||
| Methods | Parallel RCT ‐ 2 groups. | |
| Participants | Women with mediolateral episiotomy without a 3rd‐ or 4th‐degree tear after a normal uncomplicated delivery, at term, who had not used any analgesic drugs within 4 hours preceding the study King Chulalongkorn Memorial Hospital, Bangkok, Thailand Women with an allergy to either study drug, a history of drug dependence, regular use of analgesic drugs before or during pregnancy, and any medical condition known to be potentially exacerbated by acetaminophen or NSAIDS, including a history of gastrointestinal ulcer or bleeding, significant renal or liver impairment and asthma, postpartum haemorrhage or any other major postpartum complications, were excluded |
|
| Interventions | Intervention: ibuprofen 400 mg (N = 106) Comparison: acetaminophen 500 mg (N = 104) |
|
| Outcomes |
An initial pain rating was recorded before participants took the first dose of analgesia and at 1, 2, 3 and 4 hours after |
|
| Notes | Dates of study: June to November 2006 Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used; states only "stratified random sampling technique". |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blind to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all 210 participants (0% attrition rate in all groups) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | Treatment groups were similar in demographic data and clinical features; the severity of perineal pain did not differ between the groups before the treatment |
Laska 1981a.
| Study characteristics | ||
| Methods | Multi‐arm RCT (2 studies reported in 1 publication; study E1). | |
| Participants | Women with severe pain only, who gave consent, no complicating illness, were not breastfeeding and were expected to tolerate medication well Maternity hospital, Caracus, Venezuela |
|
| Interventions | Intervention: E1 study: fenoprofen 50 mg (N = 27); fenoprofen 100 mg (N = 27); fenoprofen 200 mg (N = 26) and fenoprofen 300 mg (N = 27) | |
| Outcomes |
Data were obtained at baseline, and 1, 2, 3, 4 and 5 hours after taking the medication. 5‐hour data used in this review (included at 6‐hour time‐frame) |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on withdrawals due to need for rescue medication at < 2 hours not provided (or referred to in Results section) |
| Selective reporting (reporting bias) | High risk | Need for rescue medication prespecified in the study methods but not reported |
| Other bias | Low risk | None apparent; "no significant differences between treatment groups". |
Laska 1981b.
| Study characteristics | ||
| Methods | Multi‐arm RCT (2 studies reported in 1 publication; study E2) | |
| Participants | Women with severe pain only, who gave consent, no complicating illness, were not breastfeeding and were expected to tolerate medication well Maternity hospital, Caracus, Venezuela |
|
| Interventions |
E2 study: fenoprofen 12.5 mg (N = 24); fenoprofen 25 mg (N = 23); fenoprofen 50 mg (N = 23); fenoprofen 100 mg (N = 23) and fenoprofen 200 mg (N = 23) Comparison: placebo in both E1 study (N = 27) and E2 study (N = 23) |
|
| Outcomes |
Data were obtained at baseline, and 1, 2, 3, 4 and 5 hours after taking the medication. 5‐hour data used in this review (included at 6‐hour time‐frame) |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on withdrawals due to need for rescue medication at < 2 hours not provided (or referred to in results section) |
| Selective reporting (reporting bias) | High risk | Need for rescue medication prespecified in the study methods but not reported. |
| Other bias | Low risk | None apparent; "no significant differences between treatment groups". |
Lim 2008.
| Study characteristics | ||
| Methods | Parallel RCT – 2 groups. | |
| Participants | Women who spontaneously delivered a singleton fetus and sustained perineal damage requiring repair; women were randomised as soon as possible after completion of perineal suturing A state‐funded public maternity hospital which also serves as an affiliated teaching hospital in Penang, Malaysia Women with known allergy to NSAIDs, epidural during labour, 3rd‐ or higher‐degree tear, instrumental vaginal birth, a history of peptic ulcer, asthma, thrombocytopenia, renal impairment or severe postpartum haemorrhage > 1500 mL, were excluded |
|
| Interventions | Intervention: celecoxib 200 mg (N = 163) Comparison: diclofenac 100 mg (N = 165) |
|
| Outcomes |
Pain score (VAS) were completed by women at baseline and 1, 2, 4, 8, 12 and 24 hours. 4‐hour data used in the review |
|
| Notes | Dates of study: January to June 2006 Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data balanced across groups (attrition rates: 15 of 164 (9%) in the celecoxib groups and 15 of 165 (9%) in the diclofenac group). Analysis by intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | No significant differences between the groups in any characteristics |
London 1983.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 4 groups | |
| Participants | Women between the ages of 18 and 40, no systemic medical illness and experienced moderate to severe episiotomy pain within 48 hours following an otherwise uncomplicated vaginal delivery Department of Obstetrics and Gynaecology, Sinai Hospital of Baltimore, USA Exclusion criteria were those used by Hermann et al 1980 and included; women with pain not due directly to the episiotomy (e.g. uterine cramps), except for cleansing, all wound care was suspended for the whole study period; patients who appeared unlikely to communicate meaningful information about their pain; women with only mild pain; those under 16 years of age; history of drug allergy; any relevant psychiatric, neurologic, cardiovascular, pulmonary, hepatic, gastrointestinal, or renal disorders and women given analgesics, sedatives, or other psychotropic drugs within 3 hours were excluded |
|
| Interventions | Intervention: fluproquazone 200 mg (N = 39); fluproquazone 100 mg (N = 41), and aspirin 650 mg (N = 40) Comparison: placebo (N = 40) |
|
| Outcomes |
Rating scale information for pain intensity and pain relief measures are not provided; unable to use formula to calculate adequate pain relief, but adequate pain relief could be derived from good to excellent overall impression rating |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement |
| Allocation concealment (selection bias) | Unclear risk | States sealed envelopes, but unclear if consecutively‐numbered or opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The attrition rate was 0%, but of the 166 participants, 160 were included in the analysis (4% excluded). Provides reasons for exclusions after entering study; the data of 6 patients were not included since they did not follow the assigned protocol. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported. |
| Other bias | Unclear risk | No clear statement on baseline characteristics or balance across groups |
Melzack 1983.
| Study characteristics | ||
| Methods | Multi‐arm RCT ‐ 3 groups | |
| Participants | Women in Quebec, Canada who had uncomplicated vaginal births, were judged to be in excellent health, did not receive any analgesics within 6 hrs of the study, and had moderate to severe post‐episiotomy pain | |
| Interventions | Intervention: Diflunisal 1000 mg (N = 30) and acetominophen 650 mg (N = 30) Control: Placebo (N = 30) |
|
| Outcomes |
Outcomes measured at 30, 60, 90 minutes, and hourly from 2 to 12 hours thereafter |
|
| Notes | Acetominophen 650 mg not considered for the review as not an NSAID. Data only extractable for outcomes at 12 hours post‐treatment only Dates of study: NR Funding sources: This was supported by a grant from Merck Frosst Canada Inc Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number allocator |
| Allocation concealment (selection bias) | Unclear risk | States only that all capsules were identical in appearance, odourless, peach‐coloured and film‐coated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% (6/30) were excluded from the intervention group after randomisation as 5 requested additional analgesia and 1 had loss of data; 40% (12/30) were excluded from the intervention group as 11 requested additional analgesia and 1 had loss of data. Although the reasons for attrition are provided, attrition is high and imbalanced across groups. The analysis is not by intention‐to‐treat as those who requested additional medication during the study were excluded; twice the number in the placebo group compared to the NSAID group were excluded (5 versus 11) which has the potential to influence results |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in the study Methods are all reported |
| Other bias | Low risk | Nothing to indicate any other source of bias |
Movilia 1989.
| Study characteristics | ||
| Methods | Parallel RCT– 2 groups | |
| Participants | Women aged 18 ‐ 38 years with intense post‐episiotomy pain requiring analgesia Legnano Civil Hospital, Italy Women with liver or kidney failure, peptic ulcer and hypersensitivity to paracetamol or NSAID, were excluded |
|
| Interventions | Intervention: aceclofenac single tablet 100 mg (N = 30; pain intensity could not be assessed in 1 due to vomiting; N = 29) Comparison: paracetamol 650 mg (N = 30) |
|
| Outcomes |
Pain assessed before treatment administration, at 30 minutes after and hourly thereafter through to 6 hours |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 (3%) of 30 lost to analysis for pain intensity in aceclofenac 100 mg group due to vomiting. 0% attrition rate for the paracetamol 650 mg group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | Randomisation produced 2 homogenous groups |
Mukherjee 1980.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 3 groups | |
| Participants | Women were selected from an otherwise healthy population of parturient women whose chief complaint was moderate to severe pain following episiotomy New Delhi, India Women with known sensitivity to dipyrone and aspirin or who had received analgesics 8 hours before entry to the study, were excluded |
|
| Interventions | Intervention: dipyrone 500 mg (N = 89) and aspirin 500 mg (N = 90) Comparison: placebo (N = 88) |
|
| Outcomes |
Pain relief was measured at baseline, at 30 minutes post‐treatment and hourly thereafter to 6 hours. We were unable to accurately extract mean pain relief scores from the graphs; numbers converted from percentages of reported pain as provided in the paper for 6‐hour time‐frame |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all 267 participants (0% attrition rate in all groups) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | Baseline characteristics comparable |
Okun 1982.
| Study characteristics | ||
| Methods | Multi‐arm RCT ‐ 5 groups | |
| Participants | Hospitalised women within 48hrs of birth and with moderate, severe or very severe uterine or episiotomy pain Department of Clinical Pharmacology, Cedars‐Sinai Medical Center, Los Angeles, USA Women who were breast feeding, and/or had received any analgesic, sedative or psychotropic medication within 6 hrs of the study were excluded |
|
| Interventions | Interventions: fendosal 100 mg (N = 19), fendosal 200 mg (N = 19), fendosal 400 mg (N = 18) and aspirin 650 mg (N = 20) Control: placebo (N = 18) |
|
| Outcomes |
Pain intensity was measured on a 5‐point scale of 1 = no pain, 2 = mild pain, 3 = moderate pain, 4 = severe pain and 5 = very severe pain, just before and then hourly from drug administration up to 8 hours, which was the study period. Participants were questioned at 1 and 2 hours whether their pain relief was 50% or greater |
|
| Notes | Results are extractable for 8 hours after the intervention only Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement |
| Allocation concealment (selection bias) | Unclear risk | States only that the capsules were identical looking; unclear if numbered sequentially or coded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | 1 nurse observer measured the outcomes, but it is not clear if the observer was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing or incomplete data |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in the study methods are all reported |
| Other bias | Low risk | Mean ages, body weights, and pain intensity and type of pain at baseline were comparable across the 5 groups |
Olson 1997.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 5 groups | |
| Participants | Women 18 years or older and able to communicate meaningfully with the nurse‐observer, severe episiotomy pain after uncomplicated delivery, could tolerate oral medication and had no medications that might confound the results were permitted during the study or for the 4 hours before entry to the study Hospital Maternidad Concepcion Palacios, Caracas, Venezuela Women planning to breastfeed within 24 hours after administration of study medication, with serious complicating illness, abnormal postpartum bleeding, active peptic ulcer disease or other gastrointestinal disease associated with blood loss, who received any other investigational drug within 1 month before enrolment in study or with a history of drug or alcohol abuse or known allergic sensitivities to the study medications, were excluded |
|
| Interventions | Intervention: diclofenac 25 mg (N = 52); diclofenac 50 mg (N = 50); diclofenac 100 mg (N = 51) and aspirin 650 mg (N = 50) Comparison: placebo (N = 52) |
|
| Outcomes |
Pain intensity was measured prior to, 30 minutes after, and hourly thereafter up to 8 hours following drug administration. 4‐hour data used in the review (6‐hour data could not be extracted) |
|
| Notes | Dates of study: NR Funding sources: Ciba‐Geigy Corporation Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permutation |
| Allocation concealment (selection bias) | Unclear risk | Doses were identical in appearance and packaging but no indication if sequentially numbered and sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all 255 participants (0% attrition rate in all groups) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | No significant differences between groups in terms of characteristics and clinical features; all had severe episiotomy pain on entry to the study |
Olson 1999.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 4 groups | |
| Participants | Women 18 years or older and able to communicate meaningfully with nurse‐observer, hospitalised and were in good health, could tolerate oral medication and had severe post‐episiotomy pain after term delivery with no medical complications Hospital Maternidad Concepcion Palacios, Caracas, Venezuela Women who were breastfeeding or who planned to breastfeed within 48 hours after drug administration, with none or suspected hypersensitivity to dipyrone, ketoprofen or other NSAID agents or who received any other investigational drug within 1 month prior to enrolment in the study, were excluded |
|
| Interventions | Intervention: ketoprofen oral solution 5%, 25 mg prepared in 0.45 mL (N = 28); ketoprofen oral solution 5%, 50 mg prepared in 0.90 mL (N = 26) and dipyrone oral solution 500 mg prepared in 1 mL or 30 drops (N = 27) Comparison: placebo oral solution (N = 27) |
|
| Outcomes |
Pain intensity and relief collected at baseline prior to treatment, at; 15, 30, 60, 90 and 120 minutes after treatment and hourly thereafter for a total of 6 hours |
|
| Notes | Dipyrone oral solution not considered for the review as not NSAID Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | States individual randomisation envelope was prepared for each participant, and sealed (and opened later by nurse A), but does not state if opaque and consecutively labelled |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Nurse A prepared and gave the medication to the participants, so was aware of their allocation; although Nurse B was the observer and did not know the allocations, there is a risk of bias here as nurse A (although instructed not to disclose drug) could have |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all 108 participants (0% attrition rate in all groups) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | High risk | No significant differences among the treatment groups for characteristics and clinical features; The study was prematurely terminated due to administrative changes, so fewer than half of the sample size estimate was recruited |
Schachtel 1989.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 3 groups | |
| Participants | Hospitalised women with moderate or severe episiotomy pain after normal uncomplicated birth New York Women with eclampsia or any other medical complication, allergic hypersensitivity to treatment drugs or had any analgesia or NSAID in 4 hours prior to study entry, were excluded |
|
| Interventions | Intervention: ibuprofen 400 mg (N = 36) Comparison: placebo (N = 38) and acetaminophen 1000 mg (N = 37) |
|
| Outcomes |
Pain measured before medication, 30 minutes and 1 ‐ 4 hours after |
|
| Notes | Dates of study: NR Funding sources: Whitehall Laboratories Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random code |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 of 115 (3%) participants removed after randomisation due to re‐medicated but time not known and not specified from which group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | The groups were similar in demographics and clinical features |
Suhrabi 2013.
| Study characteristics | ||
| Methods | Parallel RCT – 2 groups | |
| Participants | Women 18 ‐ 35 years of age, vaginal birth with mediolateral episiotomy, absorbable and continuous sutures and singleton, live baby. Iran. Women were excluded if they had known allergy to NSAIDs or a history of alimentary canal disorders, underlying illness, instrumental delivery, perineal rupture (3rd or 4th degree), postpartum haemorrhage, pre‐eclampsia and eclampsia |
|
| Interventions | Intervention: celecoxib 100 mg every 12 hours (N = 85) Comparison: ibuprofen 400 mg every 6 hours (N = 85) |
|
| Outcomes |
Pain levels were measured before the intervention and at 1, 2, 4, 8 and 12 hours after suturing using a VAS; but no information provided on scale intervals 4‐hour continuous data only included in the review |
|
| Notes | Dates of study: March 2009 to November 2010 Funding sources: there was no funding or support Declarations of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind only (the person assessing pain was blinded, but no report of blinding of the participants) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all 170 participants (0% attrition rate in all groups) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | No significant differences between the groups in terms of demographics or clinical characteristics |
Sunshine 1983a.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 5 groups | |
| Participants | Women 18 years or older who were able to communicate meaningfully with the nurse observer and gave written consent and had moderate or severe post‐episiotomy pain after an uncomplicated birth and could tolerate oral medication Hospital Maternidad Concepcion Palacios, Caracas, Venezuela Women who were breastfeeding, had any complicating illness or abnormal postpartum bleeding, received any other investigational drug within 1 month prior to enrolment in the study or history of drug dependence or known allergic sensitivities to propionic acid derivatives and aspirin, were excluded |
|
| Interventions | Intervention: aspirin 600 mg (N = 29); flurbiprofen 25 mg (N = 32); flurbiprofen 50 mg (N = 29) and flurbiprofen 100 mg (N = 31) Comparison: placebo (N = 31) |
|
| Outcomes |
Pain assessed prior to and hourly up to 6 hours after drug administration |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | States identical in appearance and packaging but insufficient information about sequentially numbered and sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 of 168 (10%) were dropped from the analysis because they received oxytocic medication (4 of 33 (12%) in the aspirin 600 mg group, 3 of 35 (9%) in the flurbiprofen 25 mg group, 5 of 34 (15%) in the flurbiprofen 50 mg group, 3 of 34 (9%) in the flurbiprofen 100 mg group, and 1 of 32 (3%) in the placebo group) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | No significant differences between groups in characteristics and clinical features; all had moderate or severe episiotomy pain on entry to the study |
Sunshine 1983b.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 4 groups | |
| Participants | Women with severe post‐episiotomy pain after uncomplicated delivery, > 18 years old and could tolerate oral medication Hospital Maternidad Concepcion Palacios, Caracus, Venezuela Women with known allergic sensitivities to study medications, abnormal postpartum bleeding, or complicated illnesses, breastfeeding or with a history of drug dependence or had received other investigational drugs prior to entry into study, were excluded |
|
| Interventions | Intervention: ibuprofen 400 mg (N = 30); zomepirac 100 mg (N = 30) and aspirin 600 mg (N = 30) Comparison: placebo (N = 30) |
|
| Outcomes |
Measurements were taken at the time of medication, 30 minutes later, and then hourly up to 4 hours. No medications were given within 4 hours of the study medication being given; if women required additional analgesia within 1 hour after administration of study medication, they were subsequently excluded from the study |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all 120 participants (0% attrition rate in all groups) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | Similar group baseline characteristics |
Sunshine 1987b.
| Study characteristics | ||
| Methods | Multi‐arm RCT ‐ 5 groups | |
| Participants | Patients with post‐episiotomy pain (no other information provided). | |
| Interventions | Intervention: flurbiprofen 25 mg, flurbiprofen 50 mg, BTS 24332 12.5 mg and BTS 24332 25 mg Control: placebo Numbers per group are not provided, only the total number included in the study of N = 149 |
|
| Outcomes | Not described explicitly; assume measures of pain | |
| Notes | Abstract publication; unable to obtain numbers per groups Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to accurately assess |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to accurately assess |
| Other bias | Unclear risk | Insufficient information accurately assess |
Trop 1983.
| Study characteristics | ||
| Methods | Multi‐arm RCT ‐ 5 groups | |
| Participants | Parturient women aged 16 ‐ 38 who had an episiotomy in 1 obstetric and gynaecology unit in Quebec, Canada. Women who received tranquillizers, sedatives, hypnotics or other analgesics during the 4 hours preceding the study were excluded, as well as those who were breastfeeding their babies |
|
| Interventions | Interventions: tiaprofenic acid 200 mg + placebo, tiaprofenic acid 400 mg alone Controls: ASA 600 mg + placebo and ASA 1200 mg alone The medications were administered 10.5 to 14.4 hours after episiotomy. The total number randomised to each group is not provided; states only that 150 women were included in the study |
|
| Outcomes |
Pain intensity was measured using a 20‐cms VAS divided into equal sections labelled no pain, slight, moderate, severe, unbearable; measured by putting a stroke on the appropriate place on the scale before drug administration and hourly thereafter for 4 hours. The research nurse also independently recorded her evaluation of the analgesic effect of the medication on a scale of 0 ‐ 4 corresponding to no pain to pain worse than before |
|
| Notes | Unable to obtain numbers randomised to each group. Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement |
| Allocation concealment (selection bias) | Unclear risk | States only that tablets were identical; no other information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The nurse evaluated the participants’ 5 VAS scales only at the end of the testing period by superimposing them over a transparent ruler that recorded the level of pain on a scale of 0 ‐ 20. By proceeding this way, the nurse was kept unaware of the participant's evaluation scores |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 150 participants completed the study |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in the study methods were all reported |
| Other bias | Low risk | Baseline characteristics equivalent across the groups |
Wisanto 1981.
| Study characteristics | ||
| Methods | Parallel RCT – 2 groups | |
| Participants | Primiparas over 18 years old, had deliveries and episiotomies without complications and moderate to severe pain within 48 hours of procedure with an intensity of at least 60% on a VAS. All participants had undergone medio‐lateral episiotomies with 3 ‐ 4 non‐absorbable surgical sutures using a local anaesthetic Belgium. Women with mild pain (< 60% on VAS), breastfeeding and excessive anxiety or emotional instability, were excluded |
|
| Interventions | Intervention: antrafenine 300 mg (N = 30) Comparison: placebo (N = 30) |
|
| Outcomes |
Pain measured at baseline, before drug administration, and hourly thereafter up to 6 hours. Only 6‐hour data included in the review (4‐hour data could not be extracted) 1 dose of rescue analgesia was permitted in cases of excessive pain 2 hours after study drugs administered ‐ all other treatments were excluded |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | States identical‐appearing tablets but no other details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 of 60 (3%) of participants were excluded from the study after taking another analgesic (1 (3%) in each group) |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes were all reported. |
| Other bias | High risk | Delay between episiotomy and drug intake was significantly shorter in the placebo group; also 16 placebo‐treated participants withdrew from the study due to lack of efficacy compared to 5 in the antrafenine group. (Participants who withdrew from the study due to lack of efficacy were still included in the analysis: their last self‐rating on VAS was repeated) |
Yonkeura 1987.
| Study characteristics | ||
| Methods | Multi‐arm RCT – 4 group | |
| Participants | Women > 15 years with moderate to severe episiotomy pain after normal vaginal birth Women’s Hospital, Los Angeles County, USA Women with a history of reaction or hypersensitivity to NSAID or salicylates, receiving perineal < 1 hour before entry to study, received analgesics, sedatives, psychotropic medications, or topical perineal anaesthetics within 4 hours of entering study or had an active disease that might interfere with the evaluation of study medications, were excluded |
|
| Interventions | Intervention: meclofenamate 200 mg (N = 55); meclofenamate 100 mg (N = 55) and codeine 60 mg (N = 53) Comparison: placebo (N = 52) |
|
| Outcomes |
Pain measures were assessed at baseline prior to treatment, at 30 minutes after and hourly thereafter to 6 hours |
|
| Notes | Codeine 60 mg not considered for the review as not an NSAID Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | No clear statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The attrition rate was 0%, but 5 of 220 (2%) participants were excluded from efficacy analysis because of protocol violations |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in the study methods were all reported |
| Other bias | Low risk | No differences in baseline characteristics |
Yscla 1988.
| Study characteristics | ||
| Methods | Parallel RCT – 2 groups | |
| Participants | Only women with severe episiotomy pain, aged 18 ‐ 39 years in the 48 hours following an uncomplicated delivery Mollet General Hospital, Barcelona, Spain Women with slight or moderate pain, gastroduodenal disorders, and liver or kidney failure, a known history of hypersensitivity to phynylacetic acid derivatives or to acetylsalicylic acid and similar substances, treated with NSAIDs or systemic steroids or exceeding the age limits and who did not give consent, were excluded |
|
| Interventions | Intervention: aceclofenac 100 mg (N = 20) Comparison: placebo (N = 20) |
|
| Outcomes |
Pain intensity was measured before treatment, 30 minutes and 1 hourly to 6 hours thereafter, but these data could not be extracted |
|
| Notes | Dates of study: NR Funding sources: NR Declarations of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement on method used |
| Allocation concealment (selection bias) | Unclear risk | Packaged in identical packs identifiable only by the letters A and B; but does not state if sequentially numbered and sealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all 40 participants (0% attrition rate in all groups) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were all reported |
| Other bias | Low risk | Groups similar at baseline |
ASA: acetylsalicylic acid; NR: not reported; NSAID: non‐steroidal anti‐inflammatory drug; PID: pain intensity difference; RCT: randomised controlled trial; SPID: Summed Pain Intensity Difference TOTPAR: Total Pain Relief; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abedzadeh 2009 | Treatment was not oral (gel versus suppository) |
| Akil 2014 | Treatment was not oral (intravenous) |
| Altungul 2012 | Treatment was not oral (suppository). |
| Bettigole 1981 | Not a single dose |
| Bhounsule 1990 | Not a RCT; quasi‐RCT with concurrent allocation – 2‐group comparison without randomisation |
| Bloomfield 1979 | Treatment not a NSAID |
| Bloomfield 1980 | Treatment not a NSAID |
| Bloomfield 1991 | Not a RCT; personal communication related to non‐completion of a registered trial |
| Bruni 1965 | Not specific to perineal pain; rather postpartum pain in general |
| Bucheli 1994 | Not a single dose |
| Buck 1978 | Not a single dose |
| Cater 1985 | Ibuprofen and codeine phosphate combination treatment was excluded from the review as not a pure NSAID. Zomepirac is an NSAID but was withdrawn voluntarily from the market by the manufacturer in 1983 because it was associated with fatal and near‐fatal anaphylactoid reactions |
| Choi 2000 | Treatment not oral (suppository) |
| Coburn 1966 | Not a single dose |
| Cunha 2011 | Not a single dose |
| De los Santos 1998 | Comparator drug is a combination of paracetamol and codeine |
| Delaram 2012 | Treatment not a NSAID |
| Delaram 2014 | Comparator drug is Lidocaine topical cream |
| Facchinetti 2005 | Examine the effectiveness of multiple doses (not a single dose) |
| Finch 1971 | Not a RCT; cross‐over trial and not exclusive to perineal pain |
| Fragen 1982 | Comparator drug is a combination of NSAID and opioid |
| Gindhart 1971 | Treatment not a NSAID |
| Gruber 1962 | Examines postpartum pain (not specifically perineal pain) |
| Gruber 1976 | Comparator drug is not an NSAID |
| Harrison 1987 | Not exclusively oral treatment |
| Harrison 1992 | Not a RCT; cross‐over trial |
| Kantor 1984 | Data not exclusive to perineal pain; treatment was administered for postpartum pain which may or may not have included post‐episiotomy pain |
| Lataste 1981 | Includes participants with pain other than perineal pain |
| Levin 1978 | Not clear that the intervention is a single‐dose of a NSAID. Author's contact details not found |
| Mazzarella 1989 | Treatment not a NSAID |
| McCallum 1991 | Registration form only – does not appear to focus on management of perineal pain |
| Norman 1985 | Intervention drug is a combination of NSAID and opioid |
| Odigie 1988 | Treatment was not oral (suppository) |
| Offen 1985 | Examines postpartum pain and pain related to general surgery (not specifically perineal pain) |
| Ogunbode 1987 | Not a single dose |
| Olson 1984 | Not exclusive to perineal pain as includes other types of postpartum pain |
| Pedronetto 1975 | Indoprofen was withdrawn from the market worldwide following reports of adverse reactions including reports of carcinogenicity in animal studies |
| Peter 2001 | Comparator drug is not a NSAID |
| Pitton 1982 | Treatment was not oral (intra‐muscular injection) |
| Radman 1961 | Not a single dose |
| Ray 1993 | Treatment was not oral (suppository) |
| Rezaei 2014 | Treatment was not oral (suppository) |
| Searles 1995 | Treatment was not oral (suppository) |
| Searles 1998 | Treatment was not oral (suppository) |
| Sunshine 1982 | Treatment not a NSAID |
| Sunshine 1983c | Not exclusive to perineal pain as includes other types of postpartum pain |
| Sunshine 1983d | Treatment not a NSAID |
| Sunshine 1985 | Abstract only; insufficient information to include; unclear also if pain is reported separately by pain type. Author's contact details could not be identified |
| Sunshine 1986 | Examines postpartum pain (not specifically perineal pain) |
| Sunshine 1987a | Examines postpartum pain and incisional pain following surgery.(not specifically perineal pain) |
| Sunshine 1989 | Measures the adjuvant effect of caffeine on NSAID not NSAID alone versus another NSAID or placebo |
| Szabados 1986 | Not a single dose |
| Taina 1981 | Not a single dose |
| Van Wering 1972 | Examines postpartum pain (not specifically perineal pain) |
| Von Pein 1974 | Not a single dose |
| Walters 1984 | Comparator treatment does not meet inclusion criteria |
| Walters 1985 | Comparator is an opioid; does not meet comparator inclusion criteria |
| Yoong 1997 | Treatment was not oral (suppository) |
NSAID: non‐steroidal anti‐inflammatory drug; RCT: randomised controlled trial
Differences between protocol and review
We have used GRADE to assess the certainty of the body of the evidence and produced Table 1 and Table 2. This was not prespecified in our protocol (Wuytack 2014).
Contributions of authors
F Wuytack drafted the protocol and is guarantor for the review. F Wuytack and V Smith conducted the study selection, data extraction and 'Risk of bias' assessment. F Wuytack, V Smith and B Cleary conceptualised the data analysis. F Wuytack entered data into RevMan, performed the analysis and drafted the manuscript. F Wuytack, V Smith and B Cleary reviewed the review for intellectual content and interpretation of the data. All authors read and commented on the final draft of the manuscript prior to submission.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Health Research Board, Ireland
A Health Research Board, Ireland, Cochrane Fellowship was awarded to F Wuytack to support the conduct of this review (2016 update).
Declarations of interest
Francesca Wuytack: has received a Cochrane Fellowship (2013 ‐ 2015), awarded by the Health Research Board Ireland, to support the conduct of this review.
Valerie Smith has acted in the capacity of Ms Wuytack's supervisor and principal investigator (PI) on the Cochrane HRB Fellowship grant application. The awarded grant is not salary or such support, but rather support for Dr Wuytack to attend Cochrane training workshops and other necessary consumables.
Brian J Cleary's institution has received EUR 500 from the State Claims Agency for a lecture that he gave about the historical context of the development and marketing of thalidomide in Ireland to a mediation process between the Irish state and survivors of the Thalidomide disaster. A sum of EUR 1500 was also paid to his institution (by Ferring Pharmaceuticals) for training provided to private‐sector pharmacies and an infertility clinic on the use of a specialised drug delivery system used to delivery an infertility treatment from Ferring (Lutrelef). Brian's brother works for a drug company that sells analgesic products (Grunenthal). However Brian's institution does not currently use their products for the management of peripartum pain and their products are irrelevant to the content of this review. Brian has been publicly critical of Grunenthal and their historical actions in the context of the thalidomide disaster.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Behotas 1992 {published data only}
- Behotas S, Chauvin A, Castiel J, Martin A, Boureau F, Barrat J, et al. Analgesic effect of ibuprofen in pain after episiotomy [Effets antalgiques de l'ibuprofene dans les douleurs apres episiotomie]. Annales Francaises d'Anesthesie et de Reanimation 1992;11(1):22-6. [DOI] [PubMed] [Google Scholar]
Bloomfield 1967 {published data only}
- Bloomfield SS, Gaffney TE, Howett M. Comparative analgesic efficacy of chlorphenesin carbamate and acetylsalicylic acid after episiotomy. Anesthesia and Analgesia 1967;46(5):515-20. [PubMed] [Google Scholar]
Bloomfield 1970 {published data only}
- Bloomfield SS, Barden TP, Hille R. Clinical evaluation of flufenisal, a long-acting analgesic. Clinical Pharmacology and Therapeutics 1970;11(5):747-54. [DOI] [PubMed] [Google Scholar]
Bloomfield 1974 {published data only}
- Bloomfield SS, Barden TP, Mitchell J. Comparative efficacy of ibuprofen and aspirin in episiotomy pain. Clinical Pharmacology and Therapeutics 1974;15(6):565-70. [DOI] [PubMed] [Google Scholar]
Daftary 1980 {published data only}
- Daftary SN, Mehta AC, Nanavati M. A controlled comparison of dipyrone and paracetamol in post-episiotomy pain. Current Medical Research and Opinion 1980;6(9):614-8. [DOI] [PubMed] [Google Scholar]
De Vroey 1978 {published data only}
- De Vroey P. A double-blind comparison of diflunisal and aspirin in the treatment of post-operative pain after episiotomy. Current Medical Research and Opinion 1978;5(7):544-7. [DOI] [PubMed] [Google Scholar]
- De Vroey P. The treatment of postoperative pain with a single dose of diflunisal. Clinical Therapeutics 1977;1(Suppl A):30-3. [Google Scholar]
- Devroey P, Steelman SL, Caudron J, . A double blind, placebo controlled, single dose study comparing three dose levels of diflunisal with aspirin and placebo in patients with pain due to episiotomy. Acta Therapeutica 1977;3(3):205-16. [CENTRAL: CN-00186600] [EMBASE: 1978210653] [Google Scholar]
Friedrich 1983 {published data only}
- Friedrich E. A comparison of etodolac (Ultradol) with aspirin and placebo in patients with episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1983;33(1):100-7. [Google Scholar]
Gleason 1987 {published data only}
- Gleason JA, Winer W, Turner JL. Comparison of meclofenamate sodium with codeine and placebo for the treatment of episiotomy pain. Clinical Therapeutics 1987;9(6):585-93. [PubMed] [Google Scholar]
Gruber 1979 {published data only}
- Gruber CM, Bauer RO, Bettigole JB, Lash AF, McDonald JS. A multicenter study for analgesia involving fenoprofen, propoxyphene [alone or in combination] with placebo and aspirin controls in postpartum pain. Journal of Medicine 1979;10(1-2):65-98. [PubMed] [Google Scholar]
Hebertson 1986 {published data only}
- Hebertson RM, Storey N, Turner JL. Analgesic efficacy of meclofenamate sodium in episiotomy pain. Pharmacotherapy 1986;6(5):205-10. [DOI] [PubMed] [Google Scholar]
Honorato 1990 {published data only}
- Honorato J, Caballero R, Giorgiani G, Movilia PG, Tapounet R. Dose-analgesic response study and aceclofenac plasma levels in humans. Current Therapeutic Research, Clinical and Experimental 1990;47(4):605-11. [Google Scholar]
Hopkinson 1980 {published data only}
- Hopkinson JH. Ibuprofen vs propoxyphene hydrochloride and placebo in the relief of postepisiotomy pain. Current Therapeutic Research 1980;27:55-63. [Google Scholar]
Jain 1978 {published data only}
- Jain AK, McMahon FG, Ryan JR, Raphan H, Richard W. Piroxicam, a novel analgesic in postpartum pain. European Journal of Rheumatology and Inflammation 1978;1(3):356-9. [Google Scholar]
Jain 1985 {published data only}
- Jain AK, McMahon FG, Ryan JR, Smith GB. Analgesic efficacy of indoprofen in postpartum episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1985;38(5):677-81. [Google Scholar]
Jain 1988 {published data only}
- Jain AK, McMahon FG, Ryan JR, Narcisse C. A double-blind study of ibuprofen 200 mg in combination with caffeine 100 mg, ibuprofen 400 mg, and placebo in episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1988;43(4):762-9. [Google Scholar]
Kamondetdecha 2008 {published data only}
- Kamondetdecha R, Tannirandorn Y. Ibuprofen versus acetaminophen for the relief of perineal pain after childbirth: a randomized controlled trial. Journal of the Medical Association of Thailand 2008;91(3):282-6. [PubMed] [Google Scholar]
Laska 1981a {published data only}
- Laska EM, Sunshine A. Fenoprofen and codeine analgesia [E1 study]. Clinical Pharmacology and Therapeutics 1981;29(5):606-16. [DOI] [PubMed] [Google Scholar]
- Sunshine A, Laska E, Zighelboim I, Desenne J. A comparison of the analgesic responses of fenoprofen, codeine, and placebo in postpartum and postoperative pain. Current Therapeutic Research, Clinical and Experimental 1981;29(5):771-7. [Google Scholar]
Laska 1981b {published data only}
- Laska EM, Sunshine A. Fenoprofen and codeine analgesia [E2 study]. Clinical Pharmacology and Therapeutics 1981;29(5):606-16. [DOI] [PubMed] [Google Scholar]
Lim 2008 {published data only}
- Lim SS, Tan PC, Sockalingam JK, Omar SZ. Oral celecoxib versus oral diclofenac for post-perineal repair analgesia after spontaneous vaginal birth: a randomised trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2008;48(1):71-7. [DOI] [PubMed] [Google Scholar]
London 1983 {published data only}
- London R, Sundaram GS, Feldman S, Goldstein P. Evaluation of analgesic efficacy and safety of fluproquazone compared to aspirin in the treatment of episiotomy pain. Federation Proceedings 1982;41(4):NO. 5573. [CENTRAL: CN-00191231] [EMBASE: 1982157809] [Google Scholar]
- London R, Sundaram GS, Feldman S, Goldstein PJ. Episiotomy pain: efficacy and safety of fluproquazone compared to aspirin and placebo. International Journal of Gynecology & Obstetrics 1983;21(3):251-5. [DOI] [PubMed] [Google Scholar]
Melzack 1983 {published data only}
- Melzack R, Jeans ME, Kinch RA, Katz J. Diflunisal (1000 mg single dose) vs acetaminophen (650 mg) and placebo for the relief of post-episiotomy pain. Current Therapeutic Research 1983;34:929-39. [Google Scholar]
Movilia 1989 {published data only}
- Movilia PG. Evaluation of the analgesic activity and tolerability of aceclofenac in the treatment of post-episiotomy pain. Drugs under Experimental and Clinical Research 1989;15(1):47-51. [PubMed] [Google Scholar]
Mukherjee 1980 {published data only}
- Mukherjee S, Sood S. A controlled evaluation of orally administered aspirin dipyrone and placebo in patients with post-operative pain. Current Medical Research and Opinion 1980;6(9):619-23. [DOI] [PubMed] [Google Scholar]
Okun 1982 {published data only}
- Okun R. Evaluation of the analgesic effect of fendosal in patients with postpartum uterine cramp or episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1982;32(1):65-73. [Google Scholar]
Olson 1997 {published data only}
- Olson NZ, Sunshine A, Zighelboim I, DeCastro A. Onset and duration of analgesia of diclofenac potassium in the treatment of postepisiotomy pain. American Journal of Therapeutics 1997;4(7-8):239-46. [DOI] [PubMed] [Google Scholar]
Olson 1999 {published data only}
- Olson NZ, Sunshine A, Zighelboim I, Lange R. Analgesic efficacy of liquid ketoprofen compared to liquid dipyrone and placebo administered orally as drops in postepisiotomy pain. International Journal of Clinical Pharmacology & Therapeutics 1999;37(4):168-74. [PubMed] [Google Scholar]
Schachtel 1989 {published data only}
- Schachtel BP, Thoden WR, Baybutt RI. Ibuprofen and acetaminophen in the relief of postpartum episiotomy pain. Journal of Clinical Pharmacology 1989;29(6):550-3. [DOI] [PubMed] [Google Scholar]
- Schachtel BP, Thoden WR. Efficacy of ibuprofen 400mg, acetaminophen 1000mg and placebo in post-episiotomy pain. Clinical Pharmacology and Therapeutics 1989;45:175. [Google Scholar]
Suhrabi 2013 {published data only}
- Suhrabi Z, Taghinejad H. A comparative study on the efficacy of Ibuprofen and celecoxib on the intensity of perineal pain following episiotomy: a randomized clinical trial. Iranian Red Crescent Medical Journal 2013;15(12):e9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sunshine 1983a {published data only}
- Sunshine A, Olson NZ, Laska EM, Zighelboim I, De Castro A, De Sarrazin C. Analgesic effect of graded doses of flurbiprofen in post-episiotomy pain. Pharmacotherapy 1983;3(3):177-81. [DOI] [PubMed] [Google Scholar]
Sunshine 1983b {published data only}
- Sunshine A, Olson NZ, Laska EM, Zighelboim I, De Castro A, De Sarrazin C. Ibuprofen, zomepirac, aspirin, and placebo in the relief of postepisiotomy pain. Clinical Pharmacology and Therapeutics 1983;34(2):254-8. [DOI] [PubMed] [Google Scholar]
Sunshine 1987b {published data only}
- Sunshine A, Zighelboim I, Olson N, Laska E. Flurbiprofen, flurbiprofen dextrorotatory component (BTS 24332) and placebo in post-episiotomy pain. Clinical Pharmacology and Therapeutics 1987;41:162. [Google Scholar]
Trop 1983 {published data only}
- Trop D, Nucci C, Elie R, Gareau J. Double-blind comparative evaluation of tiaprofenic acid (Surgam) versus acetylsalicylic acid (ASA) in relieving pain following episiotomy. Current Therapeutic Research, Clinical and Experimental 1983;34(2I):274-9. [Google Scholar]
Wisanto 1981 {published data only}
- Visanto J, Dubois D, Coquelin JP. Comparative study between antrafenine and placebo in patients with pain due to episiotomy [abstract]. European Journal of Clinical Investigation 1980;10:39; Abstract no: 229. [Google Scholar]
- Wisanto A, Caudron J, Dubois D, Narbonne G, Coquelin JPH. A double-blind placebo-controlled single-dose study comparing antrafenine and placebo in patients with post-episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1981;29:171-82. [Google Scholar]
Yonkeura 1987 {published data only}
- Yonkeura ML, Petrone S, Turner JL, Di Zerega GS. Double-blind comparison of meclofenamate sodium with codeine and placebo for the pain of episiotomy. Clinical Therapeutics 1987;9(6):578-84. [PubMed] [Google Scholar]
Yscla 1988 {published data only}
- Yscla A. Aceclofenac and paracetamol in episiotomal pain. Drugs Under Experimental and Clinical Research 1988;14(7):491-4. [PubMed] [Google Scholar]
References to studies excluded from this review
Abedzadeh 2009 {published data only}
- Abedzadeh M, Sadat Z, Saberi F. The efficacy of 2% lidocaine gel versus diclofenac suppository in pain relieving after episiotomy. Koomesh 2009;10(4):301-5. [Google Scholar]
Akil 2014 {published data only}
- Akil A, Api O, Bektas Y, Yilmaz AO, Yalti S, Unal O. Paracetamol vs dexketoprofen for perineal pain relief after episiotomy or perineal tear. Journal of Obstetrics and Gynaecology 2014;34(1):25-8. [DOI] [PubMed] [Google Scholar]
Altungul 2012 {published data only}
- Altungul AC, Sapmaz E, Kale A. Comparison of diclofenac sodium with indomethacin suppositories for mediolateral episiotomies. Clinical and Experimental Obstetrics and Gynecology 2012;39(1):112-4. [PubMed] [Google Scholar]
Bettigole 1981 {published data only}
- Bettigole JB. A double-blind comparison of placebo, codeine, and fenoprofen in patients with postpartum pain. Current Therapeutic Research 1981;29:778-84. [Google Scholar]
Bhounsule 1990 {published data only}
- Bhounsule SA, Nevreker PR, Agshikar NV, Pal MN, Dhume VG. A comparison of four analgesics in post-episiotomy pain. Indian Journal of Physiology and Pharmacology 1990;34(1):34-8. [PubMed] [Google Scholar]
Bloomfield 1979 {published data only}
- Bloomfield SS, Barden TP, Mitchell J. Nefopam and propoxyphene in episiotomy pain [abstract]. Clinical Pharmacology & Therapeutics 1979;25(2):214-5. [DOI] [PubMed] [Google Scholar]
Bloomfield 1980 {published data only}
- Bloomfield SS, Barden TP, Mitchell J. Nefopam and propoxyphene in episiotomy pain. Clinical Pharmacology and Therapeutics 1980;27(4):502-7. [DOI] [PubMed] [Google Scholar]
Bloomfield 1991 {published data only}
- Bloomfield SS [pers comm]. The comparative efficacy of Voltaren(R) (diclofenac), naproxen sodium and placebo in the treatment of postepisiotomy pain. Email to: Francesca Wuytack (2016) 1991.
Bruni 1965 {published data only}
- Bruni JR, Holt RE. Controlled double-blind evaluation of three analgesic medications for postpartum discomfort. Obstetrics & Gynecology 1965;25:76-81. [PubMed] [Google Scholar]
Bucheli 1994 {published data only}
- Bucheli R, Davalos V, Neto N, Naranjo I, Calderon D, Alamo C, et al. A randomized, double-blind study of the efficacy and safety of microcapsulated butibufen and naproxen in the treatment of post-episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1994;55(12):1527-37. [Google Scholar]
Buck 1978 {published data only}
- Buck ME, Paintin DB. Diflunisal in post-episiotomy pain: a preliminary report of a double-blind comparative study. Current Medical Research and Opinion 1978;5(7):548-9. [DOI] [PubMed] [Google Scholar]
Cater 1985 {published data only}
- Cater M, O'Brien PM, Pickvance NJ. A double-blind comparison of the new ibuprofen-codeine phosphate combination, zomepirac, and placebo in the relief of postepisiotomy pain. Clinical Therapeutics 1985;7(4):442-7. [PubMed] [Google Scholar]
Choi 2000 {published data only}
- Choi DM, Peter EA, Douglas MJ, Janssen P. Naproxen and epidural morphine for perineal pain after forceps delivery [abstract]. Anesthesiology 2000;92 Suppl:Abstract no: A79. [Google Scholar]
Coburn 1966 {published data only}
- Coburn WA, Rutherford RN, Banks AL. Short-term use of oxyphenbutazone in the postpartum period. Obstetrics & Gynecology 1966;28(4):484-90. [PubMed] [Google Scholar]
Cunha 2011 {published data only}
- Cunha A, Almeida A, Almeida L, Cruz A, Vilhena I, Costa C, et al. Pospartum analgesia - What can we do? Regional Anesthesia and Pain Medicine 2011;36(5 Suppl 2):E239. [Google Scholar]
Delaram 2012 {published data only}
- Delaram M. Comparison of the effects of lidocaine pomade, indomethacin suppository and mefenamic acid on the relief of post episiotomy pain in primiparous women. IRCT Iranian Registry of Clinical Trials 2012 (accessed 15 March 2014_. [IRCT201104253078N7]
Delaram 2014 {published data only}
- Delaram M, Dadkhah N. Comparing the effects of lidocaine cream and mefenamic acid on post episiotomy pain. Iranian Journal of Obstetrics, Gynecology and Infertility 2014;17(96):6-11. [Google Scholar]
De los Santos 1998 {published data only}
- De los Santos AR, Marti MI, Espinosa D, Di Girolamo G, Vinacur JC, Casadei-A. Lysine clonixinate vs paracetamol/codeine in postepisiotomy pain. Acta Physiologica, Pharmacologica et Therapeutica Latinoamericana 1998;48:52-8. [PubMed] [Google Scholar]
Facchinetti 2005 {published data only}
- Facchinetti F, Casini ML, Costabile L, Malavasi B, Unfer V. Diclofenac pyrrolidine versus ketoprofen for the relief of pain from episiotomy: a randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2005;84(10):951-5. [DOI] [PubMed] [Google Scholar]
Finch 1971 {published data only}
- Finch JS, DeKornfeld TJ. Clonixin: a clinical evaluation of a new oral analgesic. Journal of Clinical Pharmacology & New Drugs 1971;11(5):371-7. [PubMed] [Google Scholar]
Fragen 1982 {published data only}
- Fragen RJ, Vlasuk L. Analgesic efficacy of a combination of fenoprofen and codeine administered orally. Current Therapeutic Research, Clinical and Experimental 1982;31(2):129-37. [Google Scholar]
Gindhart 1971 {published data only}
- Gindhart JD. A rationale for studying analgesia. A double blind study in postpartum patients. Current Therapeutic Research 1971;13(4):240-50. [PubMed] [Google Scholar]
Gruber 1962 {published data only}
- Gruber CM Jr, Baptisti A, Chernish SM. Comparative evaluation of analgesic agents in postpartum patients: oral dextropropoxyphene, codeine and meperidine. Anesthesia and Analgesia 1962;41:538-44. [PubMed] [Google Scholar]
Gruber 1976 {published data only}
- Gruber CM Jr. Evaluating interactions between fenoprofen and propoxyphene: analgesia and adverse reports by postepisiotomy patients. Journal of Clinical Pharmacology 1976;16(8-9):407-17. [DOI] [PubMed] [Google Scholar]
Harrison 1987 {published data only}
- Harrison RF, Brennan M. Comparison of two formulations of lignocaine spray with mefenamic acid in the relief of post-episiotomy pain: a placebo-controlled study. Current Medical Research and Opinion 1987;10(6):375-9. [DOI] [PubMed] [Google Scholar]
Harrison 1992 {published data only}
- Harrison RF, Devitt M. Indomethacin and ethamsylate alone and in combination for the relief of post episiotomy pain. Irish Journal of Medical Science 1992;161(8):493-7. [DOI] [PubMed] [Google Scholar]
Kantor 1984 {published data only}
- Kantor T, Cavaliere MB, Hopper M, Roepke S. A double-blind parallel comparison of ketoprofen, codeine, and placebo in patients with moderate to severe postpartum pain. Journal of Clinical Pharmacology 1984;24(5-6):228-34. [DOI] [PubMed] [Google Scholar]
Lataste 1981 {published data only}
- Lataste X, Berchier P. Clinical evaluation of fluproquazone in post-operative pain. A report of double-blind comparative trials in patients after surgical interventions. Arzneimittel-Forschung 1981;31(5a):920-4. [CENTRAL: CN-00025847] [EMBASE: 1981168615] [PMID: ] [PubMed] [Google Scholar]
Levin 1978 {published data only}
- Levin HM. Relative potency assay comparing zomepirac sodium, propoxyphene napsylate, propoxyphene napsylate with acetaminophen, codeine, and placebo in patients with episiotomy pain. International Journal of Clinical Pharmacology and Therapeutics 1978;23(1):118. [Google Scholar]
Mazzarella 1989 {published data only}
- Mazzarella B, Mastronardi P, Rossi AE, Cafiero T, Chiefari M, Ceccarelli G. Controlled clinical evaluation of the analgesic efficacy of flupirtine and ketoprofen in postepisiotomy pain. In: 4th European Congress of Allied Specialists in Maternal and Neonatal Care; 1989 Sept; Bruges, Belgium. 1989.
McCallum 1991 {published data only}
- McCallum KA [pers comm]. Trial to assess Ponstan vs placebo, given within an hour of birth, to women who had had epidural and forceps deliveries, as a means of prophylactic analgesia, by measuring time to first postnatal analgesic consumption. Email to: Francesa Wuytack (2016) 1991.
Norman 1985 {published data only}
- Norman SL, Jeavons BI, O'Brien PM, Johnson IR, Hitchcock A, Noyelle RM, et al. A double-blind comparison of a new ibuprofen-codeine phosphate combination, codeine phosphate, and placebo in the relief of postepisiotomy pain. Clinical Therapeutics 1985;7(5):549-54. [PubMed] [Google Scholar]
Odigie 1988 {published data only}
- Odigie EA. Effectiveness of indomethacin (indocid) suppositories as post-episiotomy analgesia. International Journal of Gynecology & Obstetrics 1988;26(1):57-60. [DOI] [PubMed] [Google Scholar]
Offen 1985 {published data only}
- Offen WW, Gruber CM Jr. Dose response to fenoprofen calcium using placebo and codeine as controls. Journal of Medicine 1985;16(4):439-52. [PubMed] [Google Scholar]
Ogunbode 1987 {published data only}
- Ogunbode O. A comparative trial of piroxicam and paracetamol after episiotomy wound repair. Current Therapeutic Research, Clinical & Experimental 1987;41:89-94. [Google Scholar]
Olson 1984 {published data only}
- Olson N, Sunshine A, Roure C, Colon A, Laska EM, Santiago H, et al. Analgesic efficacy of suprofen, codeine and placebo. Pain 1984;2 Suppl:238. [Google Scholar]
Pedronetto 1975 {published data only}
- Pedronetto S, Gorini F, Mandelli V, Fuccella LM. Double blind trial of the new analgesic and anti inflammatory drug indoprofen in post episiotomic pain. Journal of International Medical Research 1975;3:16-20. [Google Scholar]
Peter 2001 {published data only}
- Peter EA, Janssen PA, Grange CS, Douglas MJ. Ibuprofen versus acetaminophen with codeine for the relief of perineal pain after childbirth: a randomized controlled trial. CMAJ: Canadian Medical Association Journal 2001;165(9):1203-9. [PMC free article] [PubMed] [Google Scholar]
Pitton 1982 {published data only}
- Pitton MA, Vincenti E, Polato D, Tambuscio B, Salvia D. Double-blind study on diclofenac in pain relief after episiotomy [Studio clinico a doppio cieco con diclofenac sodico nel controllo del dolore post-operatorio in ostetricia. Ii. Episiotomia]. Acta Anaesthesiologica Italica 1982;33(4):717-20. [Google Scholar]
Radman 1961 {published data only}
- Radman HM, Campbell C, Coplan RS. Anti-inflammatory drugs in obstetrics and gynaecology. American Journal of Obstetrics and Gynecology 1961;81:344-9. [DOI] [PubMed] [Google Scholar]
Ray 1993 {published data only}
- Ray S, Swami A, Kadim M, Morgan B. Efficacy of diclofenac in a single prophylactic dose in post partum pain. International Journal of Obstetric Anesthesia 1993;2:58. [Google Scholar]
Rezaei 2014 {published data only}
- Rezaei Z, Haghighi Z, Haeri G, Hekmatdoust A. A comparative study on relieving post-episiotomy pain with diclofenac and indomethacin suppositories or placebo. Journal of Obstetrics and Gynaecology 2014;34(4):293-6. [DOI] [PubMed] [Google Scholar]
Searles 1995 {published data only}
- Searles JA, Pring DW. Improving perineal pain relief. In: 27th British Congress of Obstetrics and Gynaecology; 1995 July 4-7; Dublin, Ireland. 1995:Abstract no: 499.
Searles 1998 {published data only}
- Searles JA, Pring DW. Effective analgesia following perineal injury during childbirth: a placebo controlled trial of prophylactic rectal diclofenac. British Journal of Obstetrics and Gynaecology 1998;105(6):627-31. [DOI] [PubMed] [Google Scholar]
Sunshine 1982 {published data only}
- Sunshine A, Zighelboim I, Laska E. Oral analgesic study of nalbuphine hydrochloride, codeine, and placebo in postpartum pain. Clinical Pharmacology & Therapeutics 1982;31(2):274. [Google Scholar]
Sunshine 1983c {published data only}
- Sunshine A, Olson JZ, Siegel C, Laska EM. Oral analgesic study of ketoprofen, aspirin and placebo in post-partum pain. Clinical Pharmacology and Therapeutics 1983;33:154. [Google Scholar]
Sunshine 1983d {published data only}
- Sunshine A, Zighelboim I, De Sarrazin C. A study of the analgesic efficacy of nalbuphine hydrochloride in patients with postpartum pain. Current Therapeutic Research, Clinical and Experimental 1983;33(1):108-14. [Google Scholar]
Sunshine 1985 {published data only}
- Sunshine A, Roure C, Zorrilla C, Laska EM, Olson N, Rivera J. The analgesic efficacy of ibuprofen, codeine and placebo. Clinical Pharmacology and Therapeutics 1985;37:232. [DOI] [PubMed] [Google Scholar]
Sunshine 1986 {published data only}
- Sunshine A, Zighelboim I, Laska E, Siegel C, Olson NZ, De Castro A. A double-blind, parallel comparison of ketoprofen, aspirin, and placebo in patients with postpartum pain. Journal of Clinical Pharmacology 1986;26(8):706-11. [DOI] [PubMed] [Google Scholar]
Sunshine 1987a {published data only}
- Sunshine A, Roure C, Olson N, Laska EM, Zorrilla C, Rivera J. Analgesic efficacy of two ibuprofen-codeine combinations for the treatment of postepisiotomy and postoperative pain. Clinical Pharmacology and Therapeutics 1987;42(4):374-80. [DOI] [PubMed] [Google Scholar]
Sunshine 1989 {published data only}
- Sunshine A, Laska E, Siegel C, Zighelboim I, De Castro A, Sorrentino J, et al. Analgesic adjuvancy of caffeine with ibuprofen in three different postpartum pain populations. Clinical Pharmacology and Therapeutics 1989;45:174. [Google Scholar]
Szabados 1986 {published data only}
- Szabados T. Acemetacine and phenylbutazone: Double-blind study in pain and inflammations following episiotomy [Doppelblindprüfung von Acemetacin und Phenylbutazon bei Schmerzen und Entzündungen nach Episiotomie]. Medizinische Welt 1986;37:703-6. [Google Scholar]
Taina 1981 {published data only}
- Taina E. Ibuprofen vs placebo in the relief of post-episiotomy pain. Current Medical Research and Opinion 1981;7(7):423-8. [DOI] [PubMed] [Google Scholar]
Van Wering 1972 {published data only}
- Van Wering RF, Bleker OP. Oral analgesia in post-partum pain: a comparison of ibuprofen ('Brufen') and dextropropoxyphene. Current Medical Research and Opinion 1972;1(1):49-52. [DOI] [PubMed] [Google Scholar]
Von Pein 1974 {published data only}
- Von Pein W. Double blind study using benzydamine in the puerperium. Gynakologische Rundschau 1974;14(4):327-8. [PubMed] [Google Scholar]
Walters 1984 {published data only}
- Walters BN, Smith VA, De Swiet M. Comparative trial of suprofen and dihydrocodeine in post-episiotomy pain. Pain 1984;2:236. [DOI] [PubMed] [Google Scholar]
Walters 1985 {published data only}
- Walters BN, Smith VA, De Swiet M, Mustill TA. Pain relief after episiotomy - a comparative study of suprofen and dihydrocodeine. British Journal of Obstetrics and Gynaecology 1985;92(11):1160-3. [DOI] [PubMed] [Google Scholar]
Yoong 1997 {published data only}
- Yoong WC, Biervliet F, Nagrani R. The prophylactic use of diclofenac (voltarol) suppositories in perineal pain after episiotomy. A random allocation double-blind study. Journal of Obstetrics & Gynaecology 1997;17(1):39-41. [DOI] [PubMed] [Google Scholar]
Additional references
Aasheim 2017
- Aasheim V, Nilsen AB, Reinar L, MLukasse M. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database of Systematic Reviews 2017, Issue 6. Art. No: CD006672. [DOI: 10.1002/14651858.CD006672.pub3] [DOI] [PubMed] [Google Scholar]
Abou Saleh 1997
- Abou-Saleh MT, Ghubash R. The prevalence of early postpartum psychiatric morbidity in Dubai: transcultural perspective. Acta Psychiatrica Scandinavica 1997;95(5):428-32. [DOI] [PubMed] [Google Scholar]
Airaksinen 2006
- Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. European Spine Journal 2006;15(Suppl 2):S192-S300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrews 2008
- Andrews V, Thakar R, Sultan AH, Jones PW. Evaluation of postpartum perineal pain and dyspareunia--a prospective study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2008;137(2):152-6. [DOI] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637-9. [DOI] [PubMed] [Google Scholar]
Berry 2001
- Berry PH, Chapman CR, Covington EC, Dahl JL, Katz JA, Miaskowski C, et al. Pain: Current Understanding of Assessment, Management, and Treatments. Reston: National Pharmaceutical Council, Inc, 2001. [Google Scholar]
BNF 2014
- BNF: Joint Formulary Committee. British National Formulary. 67 edition. London: BMJ Publishing Group and Pharmaceutical Press, 2014. [Google Scholar]
Brayfield 2014
- Brayfield A. Martindale: The Complete Drug Reference. Pharmaceutical Press, 2014. [Google Scholar]
Brunton 2011
- Brunton L. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 12th edition. McGraw-Hill Companies, Inc, 2011. [Google Scholar]
Chou 2009
- Chou D, Abalos E, Gyte GM, Gülmezoglu AM. Drugs for perineal pain in the early postpartum period: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007734. [DOI: 10.1002/14651858.CD007734] [DOI] [PubMed] [Google Scholar]
Chou 2013
- Chou D, Abalos E, Gyte GM, Gülmezoglu AM. Paracetamol/acetaminophen (single administration) for perineal pain in the early postpartum period. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD008407. [DOI: 10.1002/14651858.CD008407.pub2] [DOI] [PubMed] [Google Scholar]
Cooper 1991
- Cooper SA. Commentary: Single-dose analgesic studies: the upside and downside of assay sensitivity. In: Max M, Portenov R, Laska E, editors(s). Advances in Pain Research and Therapy. Vol. 18. New York: Raven Press, 1991:117–24. [Google Scholar]
Davies 1998
- Davies NM. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clinical Pharmacokinetics 1998;34(2):101-54. [DOI] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Deussen 2020
- Deussen AR, Ashwood P, Martis R, Stewart F, Grzeskowiak LE. Relief of pain due to uterine cramping/involution after birth. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD004908. [DOI: 10.1002/14651858.CD004908.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
East 2012a
- East CE, Sherburn M, Nagle C, Said J, Forster D. Perineal pain following childbirth: prevalence, effects on postnatal recovery and analgesia usage. Midwifery 2012;28(1):93-7. [DOI] [PubMed] [Google Scholar]
East 2012b
- East CE, Dorward ED, Whale EW, Liu J. Local cooling for relieving pain from perineal trauma sustained during childbirth. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD006304. [DOI: 10.1002/14651858.CD006304.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodman 2003
- Goodman JH. Postpartum depression beyond the early postpartum period. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2003;33(4):410-20. [DOI: 10.1177/0884217504266915] [DOI] [PubMed] [Google Scholar]
Hay‐Smith 1998
- Hay-Smith EJ. Therapeutic ultrasound for postpartum perineal pain and dyspareunia. Cochrane Database of Systematic Reviews 1998, Issue 3. Art. No: CD000495. [DOI: 10.1002/14651858.CD000495] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedayati 2003
- Hedayati H, Parsons J, Crowther CA. Rectal analgesia for pain from perineal trauma following childbirth. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD003931. [DOI: 10.1002/14651858.CD003931] [DOI] [PubMed] [Google Scholar]
Hedayati 2005
- Hedayati H, Parsons J, Crowther CA. Topically applied anaesthetics for treating perineal pain after childbirth. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD004223. [DOI: 10.1002/14651858.CD004223.pub2] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2020
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Howard 2013
- Howard CA. Pharmaceutical Calculations. 14th edition. Lippincott Williams & Wilkins, 2013. [Google Scholar]
Irish Pharmaceutical Healthcare Association 2014
- Irish Pharmaceutical Healthcare Association. Summary of product characteristics Nurofen 200 mg coated tablets. www.medicines.ie 2014.
Kaplowitz 2013
- Kaplowitz N, DeLeve LD. Drug-induced Liver Disease. 3 edition. London: Elsevier Inc, 2013. [Google Scholar]
Kettle 2004
- Kettle C. The pelvic floor. In: Henderson C, Macdonald S, editors(s). Mayes’ Midwifery: A Textbook for Midwives. Vol. 13. Edinburgh: Bailliere Tindall, 2004. [Google Scholar]
LacMed 2015
- LacMed, a ToxNet database. U.S. National Library of Medicine, National Institutes of Health, Health & Human Services 2015;https://www.ncbi.nlm.nih.gov/books/NBK501922/?report=classic.
Laws 2009
- Laws P, Sullivan EA. Australia’s Mothers and Babies 2007. AIHW National Perinatal Statistics Unit, Sydney 2009.
Leeman 2009
- Leeman L, Fullilove AM, Borders N, Manocchio R, Albers LL, Rogers RG. Postpartum perineal pain in a low episiotomy setting: association with severity of genital trauma, labor care, and birth variables. Birth 2009;36(4):283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loeser 2011
- Loeser JD, Arendt-Nielsen L, Baron R, Basbaum A, Bond M, Breivik H, et al. Part III: Pain terms: A current list with definitions and notes on usage. In: Merskey H, Bogduk N, editors(s). Classification of Chronic Pain. 2nd edition. Seattle: International Society of the Study of Pain Press, 2011. [Google Scholar]
Macarthur 2004
- Macarthur AJ, Macarthur C. Incidence, severity, and determinants of perineal pain after vaginal delivery: a prospective cohort study. American Journal of Obstetrics and Gynecology 2004;191(4):1199-204. [DOI] [PubMed] [Google Scholar]
Moodley 2003
- Moodley D, Moodley J, Coovadia H, Gray G, McIntyre J, Hofmyer J, et al. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother-to-child transmission of human immunodeficiency virus type 1. Journal of Infectious Diseases 2003;187(5):725–35. [DOI: ] [DOI] [PubMed] [Google Scholar]
Moore 1996
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66(2-3):229–37. [DOI] [PubMed] [Google Scholar]
Moore 1997a
- Moore A, Moore O, McQuary H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesia: use of pain intensity and visual analogue scales. Pain 1997;69(3):311–5. [DOI] [PubMed] [Google Scholar]
Moore 1997b
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesia: verification from independent data. Pain 1997;69(1-2):127–30. [DOI] [PubMed] [Google Scholar]
Nicklas 2013
- Nicklas JM, Miller LJ, Zera CA, Davis RB, Levkoff SE, Seely EW. Factors associated with depressive symptoms in the early postpartum period among women with recent gestational diabetes mellitus. Maternal and Child Health Journal 2013;17(9):1665-72. [DOI: 10.1007/s10995-012-1180-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 2003
- O’Brien KO, Nathanson MS, Mancini J, Witter FR. Calcium absorption is significantly higher in adolescents during pregnancy than in the early postpartum period. American Journal of Clinical Nutrition 2003;78(6):1188–93. [DOI] [PubMed] [Google Scholar]
Page 2020
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Rainsford 2007
- Rainsford KD. Anti-inflammatory drugs in the 21st century. Sub-cellular Biochemistry 2007;42:3-27. [DOI] [PubMed] [Google Scholar]
Rao 2008
- Rao PN, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. Journal of Pharmacy and Pharmaceutical Sciences 2008;11(2):81s-110s. [DOI] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Schytt 2007
- Schytt E, Waldenstrom U. Risk factors for poor self-rated health in women at 2 months and 1 year after childbirth. Journal of Women's Health 2007;16(3):390-405. [DOI: 10.1089/jwh.2006.0030] [DOI] [PubMed] [Google Scholar]
Seibert 1994
- Seibert K, Hang Y, Leahy K, Hauser S, Masferrer J, Perkins W, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase-2 in inflammation and pain. Proceedings of the National Academy of Sciences of the USA 1994;91(25):12013-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1998
- Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, et al. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proceedings of the National Academy of Sciences of the USA 1998;95(22):13313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2004
- Solomon DH, Schneeweiss S, Glynn RJ, Kiyota Y, Levin R, Mogun H, et al. Relationship between selective cyclooxygenase- 2 inhibitors and acute myocardial infarction in older adults. Circulation 2004;109(17):2068-73. [DOI] [PubMed] [Google Scholar]
Teich 2014
- Teich AS, Barnett J, Bonuck K. Women's perceptions of breastfeeding barriers in early postpartum period: a qualitative analysis nested in two randomized controlled trials. Breastfeeding Medicine 2014;9(1):9-15. [DOI: 10.1089/bfm.2013.0063] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2002
- Thompson JF, Roberts CL, Currie M, Ellwood DA. Prevalence and persistence of health problems after childbirth: associations with parity and method of birth. Birth 2002;29(2):83-93. [DOI] [PubMed] [Google Scholar]
Tramèr 1998
- Tramèr MR, Williams JE, Carroll D, Wiffen PJ, Moore RA, McQuay HJ. Comparing analgesic efficacy of non-steroidal anti-inflammatory drugs given by different routes in acute and chronic pain: a qualitative systematic review. Acta Anaesthesiologica Scandinavica 1998;42(1):71-9. [DOI] [PubMed] [Google Scholar]
Van Tulder 2006
- Van Tulder M, Becker A, Bekkering T, Breen A, Real MT, Hutchinson A, et al. Chapter 3. European guidelines for the management of acute nonspecific low back pain in primary care. European Spine Journal 2006;15 Suppl 2(Suppl 2):S169-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vane 1971
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature: New Biology 1971;43:232-5. [DOI] [PubMed] [Google Scholar]
Voscopoulus 2010
- Voscopoulos C, Lema M. When does acute pain become chronic? British Journal of Anaesthesia 2010;105 Suppl 1:69-85. [DOI: 10.1093/bja/aeq323] [DOI] [PubMed] [Google Scholar]
Vree 1993
- Vree TB, Van den Biggelaar-Martea M, Verwey-van Wissen CP, Vree JB, Guelen PJ. Pharmacokinetics of naproxen, its metabolite O-desmethylnaproxen, and their acyl glucuronides in humans. Biopharmaceutics & Drug Diposition 1993;14(6):491-502. [DOI] [PubMed] [Google Scholar]
Williams 2007
- Williams A, Herron-Marx S, Knibb R. The prevalence of enduring postnatal perineal morbidity and its relationship to type of birth and birth risk factors. Journal of Clinical Nursing 2007;16(3):549-61. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wuytack 2014
- Wuytack F, Smith V. Oral non-steroidal anti-inflammatory drugs (single dose) for perineal pain in the early postpartum period. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD011352. [DOI: 10.1002/14651858.CD011352] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wuytack 2016
- Wuytack F, Smith V, Cleary BJ. Oral non-steroidal anti-inflammatory drugs (single dose) for perineal pain in the early postpartum period. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No: CD011352. [DOI: 10.1002/14651858.CD011352.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


