Significance
In specific tissues, cell death does not represent the end of cell function. The death of the uppermost stratum granulosum keratinocytes (SG1 cells) facilitates their conversion into stratum corneum (SC) corneocytes. Such cells are not removed by efferocytosis as would occur after apoptosis or necrosis; instead, nonviable cell bodies contribute to the protective barrier function of the SC. The present study demonstrates that SG1 cell death is initiated via a single episode of prolonged intracellular Ca2+ elevation, followed by rapid acidification. Such intracellular ionic changes facilitate organellar degradation events specific to SC corneocyte formation. These findings further expand the current knowledge on cell death modes and highlight that nonviable cells contribute to physiological functions in specific contexts.
Keywords: corneoptosis, cornification, cell death, acidification, keratinocytes
Abstract
The stratum corneum (SC), the outermost epidermal layer, consists of nonviable anuclear keratinocytes, called corneocytes, which function as a protective barrier. The exact modes of cell death executed by keratinocytes of the upper stratum granulosum (SG1 cells) remain largely unknown. Here, using intravital imaging combined with intracellular Ca2+- and pH-responsive fluorescent probes, we aimed to dissect the SG1 death process in vivo. We found that SG1 cell death was preceded by prolonged (∼60 min) Ca2+ elevation and rapid induction of intracellular acidification. Once such intracellular ionic changes were initiated, they became sustained, irreversibly committing the SG1 cells to corneocyte conversion. Time-lapse imaging of isolated murine SG1 cells revealed that intracellular acidification was essential for the degradation of keratohyalin granules and nuclear DNA, phenomena specific to SC corneocyte formation. Furthermore, intravital imaging showed that the number of SG1 cells exhibiting Ca2+ elevation and the timing of intracellular acidification were both tightly regulated by the transient receptor potential cation channel V3. The functional activity of this protein was confirmed in isolated SG1 cells using whole-cell patch-clamp analysis. These findings provide a theoretical framework for improved understanding of the unique molecular mechanisms underlying keratinocyte-specific death mode, namely corneoptosis.
Cell death is an essential physiological phenomenon for the development and homeostasis of tissues and organs (1). To date, various types of cell death are classified according to their molecular and morphological features, including intrinsic/extrinsic apoptosis, mitochondrial permeability transition–driven necrosis, necroptosis, ferroptosis, pyroptosis, parthanatos, entotic cell death, NETotic cell death (NET: neutrophil extracellular traps), lysosome-dependent cell death, autophagy-dependent cell death, and immunogenic cell death (2). In these cell death types, the remnants of dead cells are removed by macrophages or adjacent cells through phagocytosis via efferocytosis. When dysregulated, the leakage of cellular contents, mitochondria, and DNA from dead cells can cause tissue inflammation (3). Notably, cell death generally implies the end of its functionality; dead cells and their products are generally unnecessary for organisms. However, in the case of epidermal keratinocytes, cell death does not indicate the end of their role; rather, dead epidermal keratinocytes converted into corneocytes constitute the stratum corneum (SC) that function as barriers (4–6). The SC enables terrestrial vertebrates to survive in the context of nonaquatic environments and even resist extreme environments by protecting the body against mechanical stresses, pathogen invasion, toxic substances, and dehydration (7).
Keratinocytes stem from the proliferative stratum basale (SB), differentiate, and move upward to form several stratum spinosum layers (8) (Fig. 1A). Subsequently, they form three layers of the stratum granulosum (SG) (SG1-3, the more superficial to the deepest layer) with a flattened Kelvin’s tetrakaidecahedron cell shape (9). Tight junctions (TJs), which enable individual cells to join as united topological barriers, are formed within the SG2 cell layer (10–12). SG1 cells above the TJs undergo cell death, forming a functional air–liquid interface barrier (i.e., the SC).
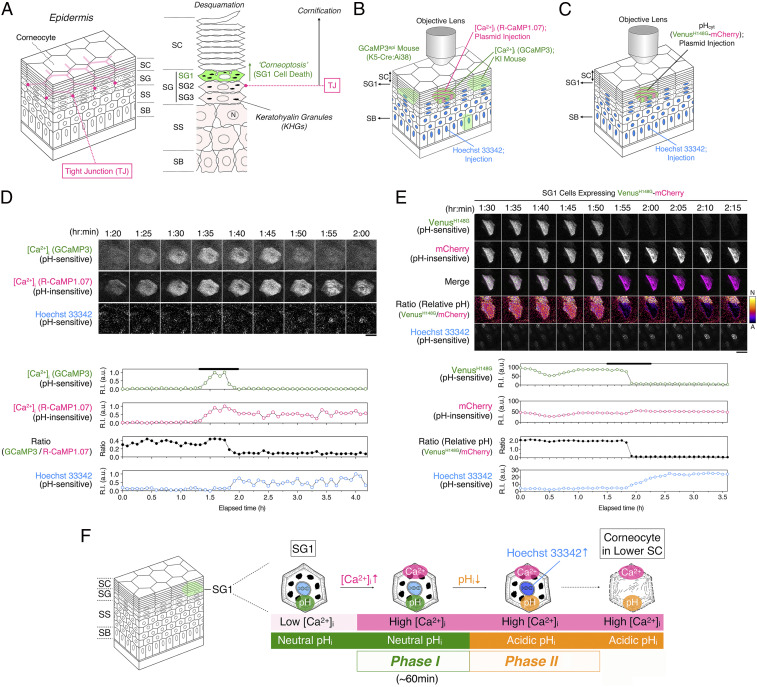
Fig. 1.
Intracellular acidification of SG1 cells in vivo. (A) Structure of the epidermis. The epidermis is composed of the SB, stratum spinosum (SS), SG, and SC. The SG is composed of SG1 (green), SG2, and SG3 cell layers. TJs are formed between adjacent SG2 cells (magenta line). Cornification involves multiple processes, including cell death of SG1 cells to form corneocytes and maturation of corneocytes that will constitute the SC. Corneocytes eventually shed from the surface of the SC (Desquamation). Here, we termed SG1 cell death as “corneoptosis.” (B) A schematic illustration of the intravital double [Ca2+]i imaging of SG1 cells coexpressing GCaMP3 (green) and R-CaMP1.07 (magenta) from the dorsal skin epidermis of an adult GCaMP3epi hairless mouse. Hoechst 33342 and pCMV-R-CaMP1.07 plasmid were intraepidermally and sequentially injected into the mouse’s dorsal skin. (C) A schematic illustration of intravital pHi imaging of SG1 cells expressing pHVenus–mCherry. Hoechst 33342 and the pCMV–pHVenus–mCherry plasmid were intraepidermally and sequentially injected into the dorsal skin of the adult hairless mice. (D) Representative time-lapse fluorescence images of an SG1 cell simultaneously expressing GCaMP3 and R-CaMP1.07a. Time-lapse images were obtained at 5 min intervals for 4 h 10 min. GCaMP3 fluorescence intensity gradually increased and peaked at around 1 h 45 min and then suddenly decreased to basal levels. R-CaMP1.07 fluorescence intensity peaked in a similar manner, but its levels remained high (scale bar, 20 µm). Changes in the fluorescent intensities of GCaMP3, R-CaMP1.07, and Hoechst 33342 signals detected in an SG1 cell plotted against the elapsed time. The fluorescence intensity ratios corresponding to the relative pH ratio (GCaMP3 to R-CaMP1.07) is shown. Notably, nuclear Hoechst 33342 intensity gradually increased after a decrease in the fluorescence intensity of GCaMP3. The black line indicates the duration time shown in images. See also SI Appendix, Fig. S2 A and B and Movie S2. (E) Representative time-lapse fluorescence images of an SG1 cell expressing the pHVenus–mCherry fusion protein and Hoechst 33342 are shown. Time-lapse images were obtained at a 5 min interval for 215 min. Immediately after the decrease in “cytoplasmic” pHVenus fluorescence intensity, Hoechst 33342 intensity gradually increased. In contrast, “cytoplasmic” mCherry intensity rarely changed (scale bar, 20 µm). Changes in the fluorescence intensities of pHVenus and mCherry detected in an SG1 cell are plotted against the elapsed time. Relative pH (ratio of pHVenus to mCherry signal) and Hoechst 33342 signal intensity are shown. Notably, just after the immediate cytoplasmic acidification of the SG1 cell, the Hoechst 33342 signal in the nucleus began to increase gradually. The black line indicates the duration time shown in images. See also Movie S3. (F) A schematic illustration of the changes observed in SG1 cell death via intravital imaging. Live SG1 cells exhibited a low [Ca2+]i and neutral pHi. Unidentified signal(s) induced an increase in [Ca2+]I under a neutral pHi for ∼60 min (phase I). Then, the pHi of SG1 cells decreased at high [Ca2+]i (phase II). Hoechst 33342 intensity began to increase in phase II. We assumed that these conditions were maintained in corneocytes in the lower SC where DNA, keratohyalin granules, and organelles are lost.
In dying SG1 cells, intracellular changes enable the generation of functional barrier materials. Unique biochemical reactions occur in dying SG1 cells, including 1) the exclusion of lamellar lipids from specialized secretory vesicles formed in the epidermis called lamellar granules to the extracellular space; 2) the cell membrane transition to cornified envelopes (CEs), with highly cross-linked hydrophobic proteins produced by the activity of transglutaminases (TGases); 3) the formation of dense keratin networks; and 4) the degradation of organelles including the nucleus with terminal deoxynucleotidyl transferase–mediated dUTP Nick End Labeling–negative nuclear DNA, mitochondria, and Golgi complex, resulting in the formation of “corneocytes” (5, 6, 13–15). These processes contribute to the formation of the functional barrier component of the SC via further maturation of the corneocytes (16–19). The term “cornification” refers to the entire process of SC formation. Although the initial step in cornification is SG1 cell death, the molecular mechanisms are, to date, unclear owing to the lack of appropriate in vivo and in vitro experimental systems (20, 21).
Here, we combined various fluorescent probes and generated a system to dissect cell death in SG1 cells in vivo and in vitro. We analyzed major cytoplasmic events via intravital imaging of fluorescently labeled protein probes expressed in SG1 cells. To examine the impact of ionic changes in SG1 cells, we isolated and cultured them under different Ca2+ and pH conditions. Finally, we performed a whole-cell patch-clamp analysis of SG1 cells to identify the molecule(s) responsible for their unique physiological properties.
Results
A Single Long-Lasting Elevation in the Intracellular Ca2+ Levels of SG1 Cells Was Observed In Vivo.
To monitor time-dependent changes in intracellular Ca2+ ([Ca2+]i) in the epidermis during cornification in vivo, we generated a mouse expressing GCaMP3 in the epidermis (GCaMP3epi). We performed intravital imaging of GCaMP3 mice considering different ear epidermal depths (SB and SG planes) using two-photon microscopic analysis (SI Appendix, Fig. S1 and Movie S1). To detect SG1 cells based on the depth of the epidermis, nuclei of keratinocytes were stained using Hoechst 33342. Time-lapse imaging with 5 min intervals for 6 h revealed that numerous SG1 cells showed transient, long-lasting signals of GCaMP3 and a sudden drop after ∼60 min as reported (22). Some SB cells increasingly exhibited a transient, spike-like increase in GCaMP3 signal lasting less than 5 min. In addition, the nuclear fluorescence intensity of Hoechst 33342 in SG1 cells gradually intensified when the elevated GCaMP3 signal faded (SI Appendix, Fig. S1 C and E). This increase in the Hoechst 33342 signal was routinely used as the reference time point for the in vivo study of SG1 cells.
Intracellular Acidification Occurs in SG1 Cells In Vivo.
The fluorescence intensity of GCaMP3 is dependent on the surrounding pH (23). R-CaMP1.07 is another fluorescent [Ca2+]i indicator derived from red fluorescent protein; of note, its fluorescence is resistant to changes in the surrounding pH (24, 25). To test whether the observed reduced GCaMP3 signals were attributable to the intracellular acidification of SG1 cells in vivo, we devised a strategy that allowed the simultaneous expression of GCaMP3 and R-CaMP1.07 in single SG1 cells in vivo. We injected Hoechst 33342 intraepidermally, followed by a mammalian expression vector encoding R-CaMP1.07 into the dorsal skin of GCaMP3epi hairless mice and subsequently proceeded to confocal microscopy (Fig. 1 B and D, SI Appendix, Fig. S2 A and B, and Movie S2). GCaMP3 signal in single SG1 cells was sustained for ∼60 min and decreased subsequently. Importantly, the coexpressed R-CaMP1.07 signal intensity also gradually increased similarly to that of GCaMP3. However, the elevated signal of this pH-insensitive reporter was sustained (Fig. 1D and SI Appendix, Fig. S2B). The fluorescent ratio values of GCaMP3 to R-CaMP1.07 plotted against the elapsed time revealed that the simultaneous elevation of GCaMP3 and R-CaMP1.07 was followed by the reduction in GCaMP3 signals within 5 min. These results suggest that after a long-lasting [Ca2+]i elevation at a neutral pH in the SG1 cells, rapid intracellular acidification may occur while [Ca2+]i remains elevated. Importantly, we confirmed that Hoechst 33342 signals were gradually increased even after GCaMP3 signals decreased in all SG1 cells (Fig. 1D, SI Appendix, Fig. S2B, and Movie S2).
To confirm that the decrease in the GCaMP3 signal in SG1 cells correlated with a drop in the cytoplasmic pH in vivo, we attempted to visualize the pH change in SG1 cells. First, we developed a ratiometric pH probe. To achieve pH ratiometric imaging, we fused the pH-sensitive and -insensitive pHVenus and mCherry proteins, respectively [pHVenus–mCherry; SI Appendix, Fig. S2C (26)]. As this fusion protein expresses pHVenus and mCherry at same molar ratio, we observed relative pH changes via simultaneous ratiometric imaging. To validate the ratiometric changes in fluorescence levels of pHVenus–mCherry, we expressed it in HeLa cells by transient transfection. Formalin-fixed HeLa cells were stained with Hoechst 33342 and treated with phosphate buffers (pH 7.23, pH 6.37, and pH 5.60). pHVenus–mCherry was expressed in the cytoplasm and nucleus of HeLa cells (SI Appendix, Fig. S2D). The fluorescence of pHVenus was decreased at lower pH, whereas that of mCherry remained unaltered. Of note, Hoechst 33342 signals were increased at lower pH, suggesting that the fluorescence intensity of Hoechst 33342 in chemically fixed HeLa cells is affected by the pH. To observe the changes in intracellular pH (pHi) in SG1 cells in vivo, we intraepidermally injected Hoechst 33342 and the pCMV–pHVenus–mCherry plasmid into the dorsal skin of hairless mice (SI Appendix, Fig. S3 and Movie S3). Time-lapse confocal microscopic imaging revealed that SG1 cells expressing pHVenus–mCherry showed a time-dependent decrease in pHVenus fluorescence intensity, whereas that of mCherry rarely changed (Fig. 1 C and E and Movie S3). We speculate that living SG1 cells have a neutral pHi, and acidification occurs when pHVenus fluorescence decays. Of note, Hoechst 33342 signal was inversely related to that of pHVenus, indicating that this acidification of SG1 cells occurred after a long-lasting [Ca2+]i elevation.
Two High [Ca2+]i Phases Are Observed in Dying SG1 Cells In Vivo.
Intravital imaging of SG1 cells in vivo revealed that SG1 cells go through two phases during cell death (Fig. 1F). In phase I, SG1 cells exhibited high [Ca2+]i and neutral pHi for ∼60 min (phase I; high [Ca2+]i-neutral pHi). Then, in phase II, pHi dropped rapidly while high [Ca2+]i was sustained (phase II; high [Ca2+]i-acidic pHi). Having defined the characteristic phases of SG1 cell death in vivo, we next used primary cultured SG1 cells to study in vitro the mechanisms by which program(s) that coordinate these changes are maintained and controlled.
Isolation of Intact SG1 Cells from Mouse Skin.
The primary culture of SG1 cells requires specific isolation of these cells with high purity. To achieve this, we generated knock-in mice expressing enhanced green fluorescent protein (EGFP) in the SG1 cell layer of the epidermis (EGFPSG1 mice; SI Appendix, Fig. S4 A–E). Intraepidermal injection of recombinant exfoliative toxin-A (ETA) protein into the dorsal skin of EGFPSG1 hairless mice resulted in the separation of the upper epidermal sheet containing the SG1, SG2, and SC layers (SI Appendix, Fig. S4 F and G) (20, 27). Subsequent trypsin treatment resulted in the isolation of SG1/2 cells (SI Appendix, Fig. S4H). Phase-contrast imaging revealed that SG1 (EGFP-positive) and SG2 (EGFP-negative) cells are morphologically indistinguishable from each other and contain numerous keratohyalin granules (KHGs) (Fig. 2A). Confocal microscopy analysis of isolated SG1 cells revealed that they maintained a polygonal saucer-like morphology (Fig. 2B and Movie S4). Scanning electron microscopic analysis of SG1 cells revealed that SG1 cells were shaped as Kelvin’s tetrakaidecahedron [Fig. 2 C and D (9)]. Moreover, their cell surface was covered with numerous fungiform protrusions and microvilli (Fig. 2C).
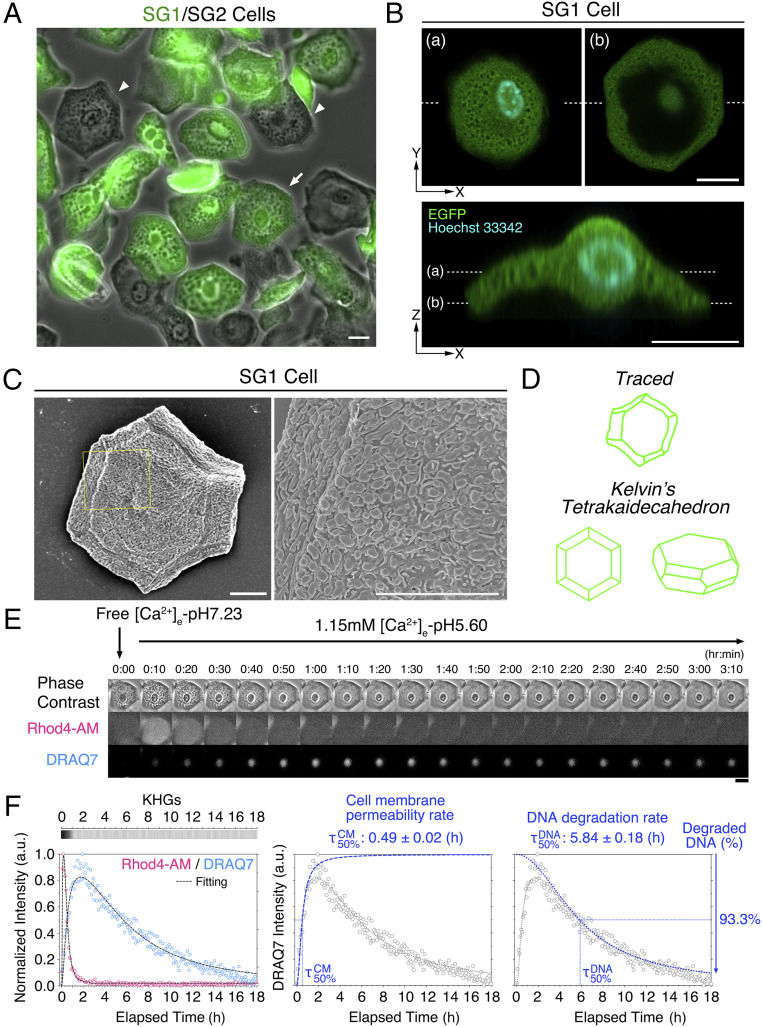
Fig. 2.
Isolation of mouse SG1 cells and identification of the cornification-triggering agent. (A) Isolated SG1 (EGFP-positive; green) and SG2 (EGFP-negative) cells obtained from ETA sheets of adult hairless EGFPSG1 mice. The arrow indicates the SG1 cell analyzed in E–F. The arrowheads indicate EGFP-negative SG2 cells (scale bars, 20 µm). (B) Confocal microscopic X-Y- and Z-sectioning image of an isolated SG1 cell (EGFP; green) stained with Hoechst 33342 (cyan). Plane (a) and (b) of the X-Y–scanning image were indicated by dashed lines (scale bars, 10 µm). See also Movie S4. (C) Scanning electron microscopic image of isolated SG1 cells. The yellow boxed areas are enlarged (Right). SG1 cells were covered with numerous fungiform protrusions and short microvilli (scale bars, 10 µm). (D) Traced images of E (Upper illustration) showed that isolated SG1 cell shape is similar to Kelvin’s tetrakaidecahedron (Lower illustration). (E) Representative time-lapse images of SG1 cells treated with high [Ca2+]e-pHe5.60 were captured every 5 min for 18 h. The morphology of KHGs was observed via phase-contrast microscopy. Rhod4-AM intensity was determined to measure relative [Ca2+]i. Cell membrane permeability was monitored via DRAQ7 nuclear staining. Rhod4-AM signals were highly and immediately increased at 10 min, followed by a gradual decrease within 1 h. Cell membrane permeability reached the maximum around 2 h after [Ca2+]i increase, followed by a gradual decrease up to 18 h. KHGs were rapidly eliminated within 1 h (scale bar, 20 µm). See also Movie S5. (F) Fluorescence intensity plots of Rhod4-AM and DRAQ7 against the elapsed time (18 h) (Left). The dashed lines indicate the fitting curves that reproduce experimental Rhod4-AM (magenta open circles) and DRAQ7 (blue open circles) signals. Time-dependent morphological changes in KHGs from 0 to 18 h are shown in the gray-scaled heatmap (whose depth of color depicts the presence of KHGs). Decomposed sigmoidal functions of 1) cell membrane permeability (Middle) and 2) DNA degradation (Right) compared with the experimental fluorescence intensity (open gray circles) and the best-fitted curve (blue dotted lines) for DRAQ7. The cell membrane permeability rate () and the DNA degradation rate () were defined as the time required to obtain half of the final values for each sigmoidal curve, respectively.
High Ca2+ and Low pH Levels Induce Morphological Changes in Isolated SG1 Cells In Vitro.
To reconstitute the intracellular changes in SG1 cells observed in vivo, we cultured SG1 cells in the context of different Ca2+ levels and pH levels. To assess both cell viability and the degradation of nuclear DNA, we used DRAQ7. An increase in the intensity of nuclear DRAQ7 corresponds to an increase in cell membrane permeability. To monitor [Ca2+]i, we used the fluorescent Ca2+ indicator, Rhod4-AM. The morphology of KHGs of SG1 cells was observed via phase-contrast microscopy.
High [Ca2+]i and low pHi, as phase II conditions, were observed in the intravital imaging of SG1 cells in vivo (Fig. 1). To examine conditions under which isolated SG1 cells undergo changes consistent with those observed in vivo, we cultured isolated SG1 cells under high [Ca2+]e (1 mM) levels at an acidic pHe (pH 5.60) (“high [Ca2+]e-pHe5.60”; Fig. 2 E and F and Movie S5). First, SG1 cells were suspended in “free [Ca2+]e-pHe7.26” conditions. After the medium was changed to “high [Ca2+]e-pHe5.60,” the Rhod4-AM intensity in most SG1 cells increased at 5 to 10 min, followed by a rapid decrease within 1 h, owing to the diffusion of Rhod4-AM along with increased cell membrane permeability. Additionally, the DRAQ7 intensity gradually increased for up to 2 h. Importantly, nuclei shrank, and nuclear DRAQ7 signals also decreased gradually for up to 18 h, suggesting that DNA degradation had occurred. KHGs gradually disappeared within 90 min resulting in a corneocyte-like appearance.
We sought to derive numerical values to summarize (and quantify) the temporal changes we observed in individual SG1 cells. To this end, we fit Rhod4-AM and DRAQ7 fluorescence intensities into sigmoidal functions (SI Appendix, Eq. S1), which were consistently well matched to the experimental results (Fig. 2F). Therefore, we used the intensity I and the 50% transition time values given by these modeled functions to quantify the characteristic temporal patterns of fluorescence from SG1 cells in various conditions. With regards to DRAQ7, we particularly termed and as “cell membrane permeability rate” () and “DNA degradation rate” (), which represents half of the time required to incorporate DRAQ7 into nuclear DNA and DNA degradation, respectively. We also defined the intensity of the declining sigmoidal component, , as the ratio of “degraded DNA.” As shown in Fig. 2F, the best-fitted DRAQ7 intensity curve of a typical SG1 cell showed that was 0.49 ± 0.02 h and was 5.84 ± 0.18 h, with the “degraded DNA” ratio of 100% (fully degraded DNA) in the “high [Ca2+]e-pH5.60” condition. These indices enable us to quantitatively compare the intensity and rate of DRAQ7 signal changes.
Verification of the Effects of Phase I and II Conditions in Isolated SG1 Cells.
Our intravital imaging revealed that cell death in SG1 cells proceeded in two phases, phase I (high [Ca2+]i-neutral pHi) and phase II (high [Ca2+]i-acidic pHi) (Fig. 1F). To address the biological significance of these two conditions, we cultured isolated SG1 cells and analyzed them under various [Ca2+]e and pHe conditions (Fig. 3).
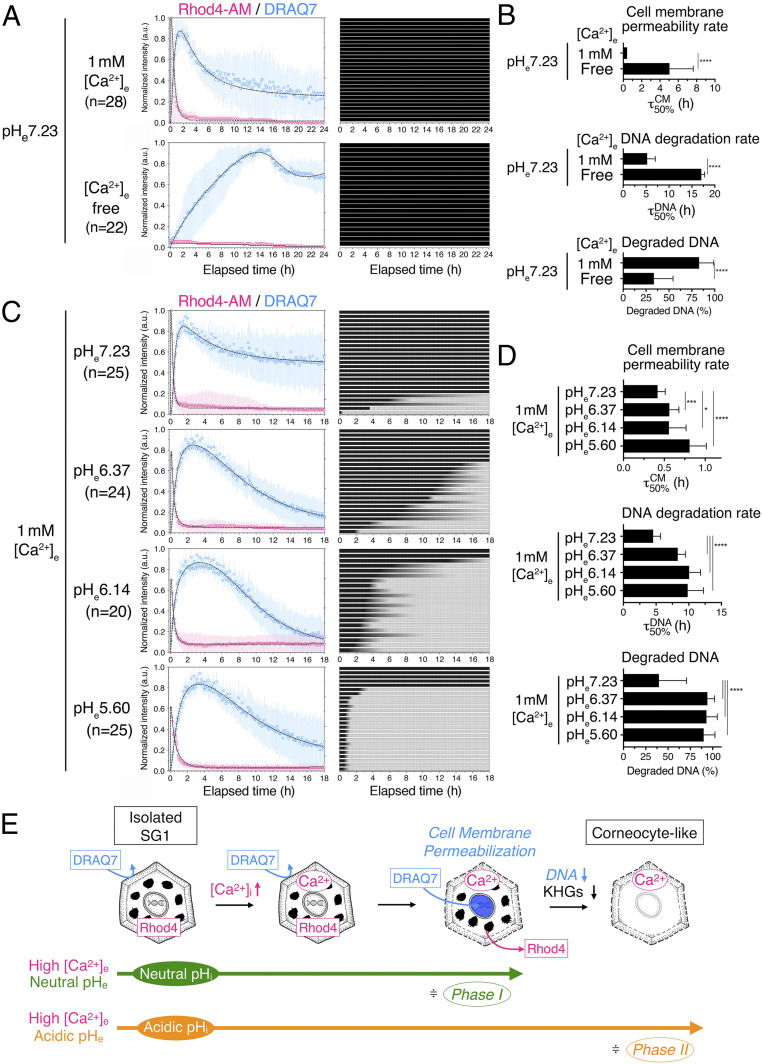
Fig. 3.
High [Ca2+]e and acidic pHe values differentially induce corneocyte-related morphological changes in isolated SG1 cells in vitro. (A) Time-dependent changes in the fluorescence intensities of Rhod4-AM and DRAQ7 in SG1 cells treated with 1 mM (n = 28) or free (n = 22) [Ca2+]e under neutral pHe conditions (Left). The best-fit curves with the experimental intensity plot are shown by dashed lines. High [Ca2+]e caused an increase in [Ca2+]i followed by cell membrane permeabilization within 2 h. The gradual degradation of nuclear DNA was not completed. In contrast, free [Ca2+]e hardly induced elevations in [Ca2+]i and cell membrane permeabilization. Each row of the heatmap depicts the time-dependent changes of the presence of KHGs (black color corresponds to an unchanged quantity of KHGs). (B) The cell membrane permeability rate () and DNA degradation rate () derived from the dual-sigmoidal curve fitting of DRAQ7 intensity in A. The uncertainty corresponds to the SD of the derived τ50%. ****P < 0.0001. See also SI Appendix, Table S1. (C) Time-dependent changes in the fluorescence intensities of Rhod4-AM and DRAQ7 in isolated SG1 cells treated with different pHe (7.23 (n = 25), 6.37 (n = 24), 6.14 (n = 20), and 5.60 (n = 25)) at 1 mM [Ca2+]e (Left). Irrespective of pHe, [Ca2+]i elevation was induced, followed by cell membrane permeabilization within 2 to 3 h. Only under the highest pHe (pH 7.23), an incomplete gradual decrease in DRAQ7 intensity was observed. The time-dependent changes in the presence of KHGs (darker color corresponds to a greater quantity of KHGs) showed that the dissipation rate of KHGs increased with decreasing pHe (Right heatmap). (D) The and with their SDs given in C are shown (Right). *P < 0.05, ***P < 0.0005, and ****P < 0.0001. See also SI Appendix, Table S1. (E) Schematic representation of the morphological changes observed in isolated SG1 cells in vitro. When SG1 cells preincorporated with Rhod4-AM (Rhod4) were treated with “high [Ca2+]e-neutral pHe” (phase I–mimicking conditions) in the presence of extracellular DRAQ7, an increase in [Ca2+]i was induced, as shown by the increase in cytoplasmic Rhod4 signals. Then, cell membrane permeabilization occurred, and extracellular DRAQ7 was incorporated into the nucleus and intercalated to nuclear DNA, while Rhod4 simultaneously leaked out. This “high [Ca2+]e-neutral pHe” is not sufficient to cause further intracellular changes in SG1 cells. In contrast, “high [Ca2+]i-acidic pHe” (phase II–mimicking conditions) induced additional DNA degradation and KHG elimination after the increase in [Ca2+]i and cell membrane permeabilization.
Comparison of Changes in SG1 Cells Subjected to High- and Free-[Ca2+]e Conditions at Neutral pHe (Verification of Phase I Conditions).
First, to address the effect of phase I conditions (high [Ca2+]i-neutral pHi) on SG1 cells, we examined the effect of high (1 mM) or free (0.21 nM) [Ca2+]e on isolated SG1 cells under a neutral pHe (pH 7.23) (Fig. 3 A and B). Isolated SG1 cells were stimulated with either “high [Ca2+]e-pHe7.23” or “free [Ca2+]e-pHe7.23” media. In “high [Ca2+]e-pHe7.23,” SG1 cells showed an elevation in Rhod4-AM signals within 5 min, followed by a rapid decrease, possibly due to the permeabilized cell membrane. Consistently, DRAQ7 intensity was rapidly increased and maintained for up to 2 h, suggesting an increase in cell membrane permeability (; Fig. 3 A and B and SI Appendix, Table S1). In contrast, under “free-[Ca2+]e-pHe7.23” conditions, Rhod4-AM signals increased to a lesser degree. DRAQ7 intensity rose slightly over 14 h, suggesting that cell membrane permeabilization occurred slowly ( Fig. 3 A and B and SI Appendix, Table S1). Besides, the decrease of DRAQ7 intensity at the single-cell level indicated that partial DNA degradation was more frequent under “high-[Ca2+]e-pHe7.23” conditions than in the “free-[Ca2+]e-pHe7.23” ones ( Fig. 3 A and B nd SI Appendix, Table S1). Both conditions revealed that most cells did not eliminate their KHGs (Fig. 3A (heat map) and Fig. 3E). As a result, degraded DNA (%) in “free-[Ca2+]e-pHe7.23” conditions was lower than that in “high-[Ca2+]e-pHe7.23” conditions (Fig. 3 A, B, and E and SI Appendix, Table S1).
Comparison of Changes in SG1 Cells in High [Ca2+]e in the Context of Various pHe (Verification of Phase II Conditions).
To examine the effect of phase II conditions (high [Ca2+]i-acidic pHi) on isolated SG1 cells, we tested four different pHe conditions (pH 7.23, 6.37, 6.14, and 5.60) at high (1 mM) [Ca2+]e (Fig. 3 C and D and Movie S6). Under all pHe conditions, isolated SG1 cells showed a similar increase in Rhod4-AM intensity immediately after the culture medium replacement, followed by cell membrane permeabilization. More specifically, the cell membrane permeability rate ( revealed that lower-pH conditions slowed the permeabilization of the SG1 cells ( Fig. 3 C and D and SI Appendix, Table S1). The lower pHe also retarded the rate of nuclear DNA degradation (). However, the amount of degraded DNA increased under a lower pHe. The most striking difference was in the elimination of KHGs. The number of cells exhibiting KHG elimination increased at a lower pHe (Fig. 3 C and E). Furthermore, the isolated SG1 cells were stimulated with “free-[Ca2+]e-pHe6.14” or “high-[Ca2+]e-pHe6.14” media. DNA and KHG degradation in SG1 cells decreased upon stimulation with “free-[Ca2+]e-pHe6.14” media compared to that observed upon stimulation with “high-[Ca2+]e-pHe6.14” media (SI Appendix, Fig. S5 and Table S1), indicating that DNA degradation and KHG elimination require high [Ca2+]e.
Taken together, these results suggested that both high Ca2+ levels and acidic pH are required to degrade the DNA and KHGs.
Functional TRPV3 Is Highly Expressed in SG1 Cells.
To further address how [Ca2+]i and pHi are regulated in SG1 cells, we investigated the Ca2+ influx–regulated membrane properties of SG1 cells. Epidermal and ETA sheets containing SG1/SG2 cells were isolated from adult C57BL6/N mice ear skin (n = 3). Their total RNAs were subjected to RNA-seq analysis. We first examined the expression of transient receptor potential (TRP) channels in SG cells as attractive candidates for molecules regulating [Ca2+]i of SG1 cell. We analyzed the mRNA for all TRP channels, including TRPA, TRPC, TRPM, TRPP, and TRPV members. We found that Trpm7, Trpp1, and Trpv6 were slightly expressed in SG1/2 cells and highly expressed in the epidermis (SI Appendix, Fig. S6). In contrast, Trpm4, Trpml1, Trpv3, and Trpv4 were highly expressed in SG cells. To get a clear idea of the electrophysiological characteristics of SG1 cells, we used a whole-cell patch-clamp assay. We treated SG1 cells with TRP channel activators, including capsaicin (TRPV1), AITC (TRPA1), menthol (TRPM8), GSK1016790A (TRPV4), and carvacrol/2-APB (TRPV3) (Fig. 4A). The single SG1 cell recordings indicated that TRPV3 activators (carvacrol/2-APB) evoked large currents but capsaicin (TRPV1), AITC (TRPA1), menthol (TRPM8), and GSK1016790A (TRPV4) did not (n = 6). There was no obvious acid-sensing ion channel–like transient currents when an acidic solution (pH 5.4) was applied (Fig. 4B; n = 2). To determine whether the observed currents were derived from endogenous TRPV3, we modulated the bath temperature via the addition of preheated or cooled bath solutions after lifting the cell from the bottom of the recording chamber with the help of the patch pipette (Fig. 4C). Interestingly, we observed warm temperature–evoked currents, which is consistent with the previous reports that TRPV3 is activated at ambient temperature (32 to 39 °C) (28, 29) (Fig. 4 C and D).
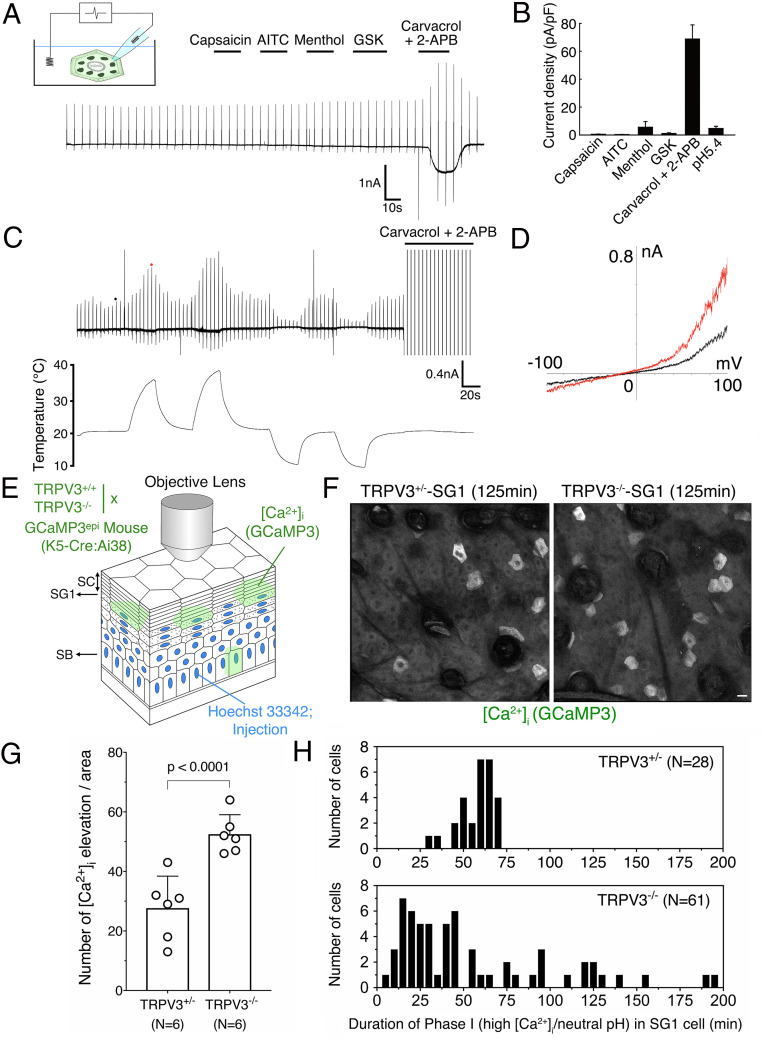
Fig. 4.
TRPV3 regulates the [Ca2+]i elevation and intracellular acidification timing occurred in SG1 cells. (A) A representative membrane current trace from whole-cell patch-clamp recordings in isolated SG1 cells is shown. Large currents were observed upon stimulation with TRPV3 activators (250 μM carvacrol and 100 μM 2-APB) but not with TRPV1 (1 μM capsaicin), TRPA1 (100 μM AITC), TRPM8 (100 μM menthol), or TRPV4 agonists (0.5 μM GSK1016790A) (n = 6). (B) The peak current density was increased by TRPV3 channel activators (n = 4 to 6) and not by a pH 5.4 (n = 2). (C) A representative membrane current trace of changes in temperature in isolated SG1 cells. Large currents were observed upon warm stimulation (n = 4). After two rounds of warm stimulation, an SG1 cell was stimulated with 250 μM carvacrol and 100 μM 2-APB (The peak current was out of range because the current was too large). (D) Current–voltage relationship of warm temperature–evoked currents is shown in the Right graph. The black and red dots in trace C indicate points where current–voltage curves were generated. (E) A schematic illustration of intravital GCaMP3 imaging in TRPV3−/− mice. Time-lapse images of GCaMP3 and Hoechst 33342 were obtained at 5 min intervals for 305 min. The scanned plane containing SG1 cells is indicated by an arrow. (F) Representative images of TRPV3+/− and TRPV3−/− SG1 cells obtained from GCaMP3epi mice at the 125 min time point of intravital imaging. Notably, the number of SG1 cells exhibiting increased GCaMP3 signals were increased in TRPV3−/− SG1 cells (scale bar, 20 µm). See also SI Appendix, Fig. S4 and Movie S7. (G) The number of cells exhibiting elevation in [Ca2+]i is shown in the bar graph. Time-lapse images of five different areas of TRPV3+/− (n = 6) or TRPV3−/− (n = 6) epidermis were obtained via confocal microscopy and analyzed. The number of SG1 cells exhibiting increased [Ca2+]i was counted. TRPV3−/− SG1 cells showed an increased elevation in [Ca2+]i. (H) The number of cells was plotted against the duration time of phase I (high [Ca2+]i-neutral pHi) for TRPV3+/+ (n = 28) or TRPV3−/− (n = 61) SG1 cells.
Alteration of the Frequency of [Ca2+]i Elevation and the Intracellular Acidification Timing in TRPV3-Deficient SG1 Cells In Vivo.
To test the physiological role of TRPV3 on SG1 cell death, we generated Trpv3 knockout (TRPV3−/−) hairless mice expressing GCaMP3 in the epidermis and subjected them to intravital [Ca2+]i imaging (Fig. 4E). Three-dimensional time-lapse images of GCaMP3 fluorescence signals were obtained in the mouse back skin epidermis via confocal microscopy every 5 min for 305 min (Fig. 4F, SI Appendix, Figs. S7 and S8, and Movie S7). Z projection images of the epidermis revealed that TRPV3−/−-SG1 had an increased frequency of [Ca2+]i elevation per epidermal area, compared to that in heterozygous (TRPV3+/−) mice (Fig. 4 F and G and SI Appendix, Figs. S7 and S8). Strikingly, the duration time of sustained elevation of GCaMP3 signals was dysregulated and became either prolonged or shortened in TRPV3−/−-SG1 cells (2∼195 min), compared to that in TRPV3+/−-SG1 cells (approximately ∼60 min; Fig. 4H and SI Appendix, Figs. S7B and S8). The increase in Hoechst 33342 signals was detected after the drop of the elevated GCaMP3 signal in both TRPV3+/−- and TRPV3−/−-SG1 cells (SI Appendix, Figs. S7B and S8 and Movie S7). Furthermore, we expressed pHVenus–mCherry in the dorsal skin of TRPV3+/− and TRPV3−/− hairless mice via plasmid injection and subjected them to intravital pHi imaging (SI Appendix, Fig. S9). This revealed that SG1 cells expressing pHVenus–mCherry displayed sustained mCherry signal but diminished pHVenus signal. These results suggested that the intracellular acidification of SG1 cells occurs normally in TRPV3−/− mice. Therefore, these data suggest that the interval between the [Ca2+]i elevation and intracellular acidification (i.e., the duration time of phase I) is dysregulated in TRPV3−/−-SG1 cells (Fig. 4H, SI Appendix, Figs. S7B and S8, and Movie S7).
Discussion
Possible Role of Phase I; [Ca2+]i Elevation and Cell Membrane Permeabilization Triggered by High [Ca2+]i.
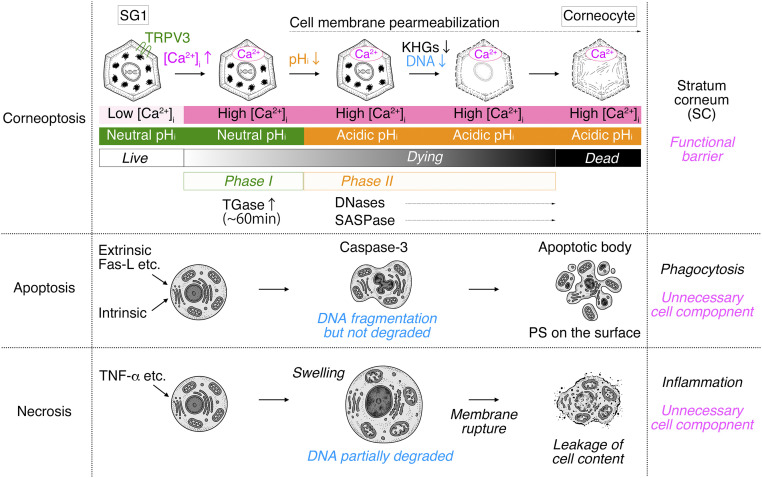
Combining in vivo imaging and cell biological studies, we clarified the sequential events occurring during SG1 cell death (i.e., phase I (high [Ca2+]i at neutral pHi) and phase II (high [Ca2+]i at acidic pHi) (summarized in Fig. 5). Previous electon microscopic observation also described the existence of infrequently observed cells between SG and SC as “transitional cells” which possibly corresponded to the dying SG1 cells at late stage of phase II (30, 31).
Fig. 5.
A schematic illustration of the hypothetical cell death process of corneoptosis. In “corneoptosis,” corneocytes are formed after SG1 cell death through two sequential, intracellular states. “phase I:” high [Ca2+]i-neutral pHi activates TGases and lasts for ∼60 min. “phase II:” high [Ca2+]i-acidic pHi induces the activation of DNases (DNase1L1 and DNase2), SASPase, and changes in the keratin network. As a result, KHGs are eliminated, the DNA is completely degraded, then the process of SG1 cell death is completed (black shaded bar), and corneocytes are formed. In apoptosis, extrinsic (Fas-L) or intrinsic factors activate the apoptotic pathway via the activation of effector caspases (such as caspase-3). Activated DNases fragment DNAs but do not degrade them completely. Organelles are degraded. Cytochrome C is released from the mitochondria. Chromatin is condensed. Cells finally form apoptotic bodies that present phosphatidylserine (PS) on their surface. PS is recognized by macrophages or adjacent cells that phagocytize dead cells and degrade all dead cell components. In necrosis, dead cells become swollen, and organelles/DNAs are partially degraded; subsequently, cell membranes are finally ruptured, and cell contents are leaked. This causes immune responses resulting in inflammation and the removal of cell remnants by immune cells via efferocytosis.
What is the biological meaning of a prolonged high [Ca2+]i (∼60 min) under a “neutral pHi” (phase I) versus short, spike-like [Ca2+]i elevations that are generally observed in cultured cells? Intracellular Ca2+ overload is observed in other cell death types, including apoptosis, necrosis, autophagic cell death, pyroptosis, and NETosis (32–36). In SG1 cells, the prolonged increase in free cytosolic Ca2+ levels is assumed to either activate or induce conformational changes that influence various enzymes, such as caspases, calpains, other proteases, etc. Among these, TGases are Ca2+-activated enzymes that catalyze protein covalent cross-linking events via the formation of isopeptide bonds (37). The CE is an insoluble protein structure of corneocytes replaced from plasma membranes. Elevated [Ca2+]i in SG1 cells causes the activation of TGase 1 and TGase 3, which aid CE assembly underneath the plasma membrane, by cross-linking hydrophobic proteins, such as keratin 1/10, involucrin, loricrin, etc (13). Thus, maintaining high [Ca2+]i in SG1 cells for a prolonged duration at a neutral pH, as seen in phase I, may be essential for achieving a complete cross-linking reaction at the site of CE formation.
Possible Roles of Phase II; Changes in Keratin and KHGs Triggered by Acidic pHi.
What is the biological meaning of rapid intracellular acidification under high [Ca2+]i in SG1 cells? KHGs are composed of keratin filament binding proteins with amorphous aggregated keratin filaments, such as profilaggrin (precursor of filaggrin), loricrin, and trichohyalin (18). Keratin intermediate filaments are supposedly affected by the intracellular acidification of SG1 cells (38–40). Furthermore, the disappearance of KHGs, which exist in a state of liquid–liquid phase separation, correlates with the drop in pHi (below pH 6.2) of SG1 cells in vivo (41). This finding suggests that coincident with the rapid drop of pHi in SG1 cells after ∼60 min of “high [Ca2+]i-neutral pHi” (phase I), KHGs, and bundled keratin filaments change properties to become a component of stiff corneocytes.
For the disassembly of KHGs, profilaggrin-to-filaggrin processing is another key factor. Profilaggrin is composed of an N terminus, two Ca2+-binding domains, tandemly connected filaggrin monomers (10 to 12 in human), and a C-terminus domain (18, 42, 43), and is localized at KHGs. During the SG1-to-SC transition, the linker sequences of profilaggrin are cleaved by specific proteases (44), such as SASPase, which is a retroviral-like aspartic (acid) protease with an optimum acidic pH for its activity (5.77 in mouse SASPase) (45, 46). One hypothesis raised by our results is that intracellular acidification may activate SASPase to accelerate profilaggrin processing. Cleaved filaggrin in corneocytes located in lower layers of the SC is involved in the bundling of keratin filaments (47).
Therefore, at the beginning of phase II (intracellular acidification of SG1 cells), the direct effect of an acidic pH as well as the bundling activity by the filaggrin monomer were suggested to enable the tight packing of keratin filaments well ahead of the formation of stiff corneocytes at the SG1-to-SC transition stage, at which point they begin to act as barriers in the SC (18).
Possible Roles of Phase II; Completion of DNA Degradation by Acidic pHi.
We demonstrated that isolated SG1 cells’ nuclear DNA was partially degraded in phase I and completely in phase II–mimicking conditions. The nuclei of SG1 cells are absent in the first layer of SC. Thus, nuclear degradation events must be completed before/when the cells enter this layer. The major DNases expressed in differentiated epidermal layers are DNase1-like 2 (DNase1L2) and DNase2 (48, 49) and both have acidic optimal pH (50, 51). Moreover, the double inactivation of DNase1L2 and DNase2 in mouse skin showed a defect in the degradation of nuclear DNA in SG1 cells (52). It is reasonable to assume that nuclear acidification of SG1 cells is required for the full activation of these acid-activated DNases by phase II conditions for the completion of nuclear DNA degradation.
Corneoptosis as a Unique Cell Death Type Observed in SG1 Keratinocyte Cells.
Taken together, our data suggest that SG1 cell death is complex and unique. We propose that several steps in the SG1 cell death process are essential to initiate cornification: 1) phase I—a long-lasting elevation in [Ca2+]i at neutral pHi for ∼60 min, 2) phase II—a rapid drop in pHi to achieve acidic conditions; and 3) elimination of KHGs and DNA and possibly other organelles, to form a mature corneocyte in the lower SC. Afterward, the corneocytes are retained in the SC, move upward, mature further, and eventually reach the SC surface and ultimately are shed (Fig. 5). In apoptosis or necrosis, dead cells are constantly removed by macrophages or nearby cells via efferocytosis. Otherwise, there is a leakage of cell content resulting in inflammation, as observed in necrosis (Fig. 5). The unique feature of SG1 cell death is that the dead cells (corneocytes) are not eliminated; rather, they function as an essential component of the SC barrier. Considering all the unique aspects of this cell death, we termed the SG1 cell death “corneoptosis,” the initial cornification process.
TRPV3 Acts as a Timekeeper for Phase I and Phase II during Corneoptosis in SG1 Cells.
The observation of high temperature–evoked TRPV3-like currents in SG1 cells implied that the regulation of SG1 cell death by TRPV3 is differentially regulated at various body sites with different skin temperatures. Intravital [Ca2+]i imaging of TRPV3-deficient mice revealed that increased [Ca2+]i elevation frequencies were accompanied by the dysregulation of the timing of acidification in SG1 cells. This suggested that TRPV3 acts as a timekeeper for phase I and II conditions and also indicated that besides TRPV3, there might be another unidentified molecule(s) responsible for SG1-specific [Ca2+]i elevation.
How does TRPV3 serve a timekeeper function? A unique feature of TRPV3 is its “sensitization mechanism;” the repeated stimulation of TRPV3 evokes, prolongs, and enhances multiple TRPV3 channel activities, possibly due to its structural characteristics (53–56). TRPV3−/− mice exhibited aberrant epidermal growth factor receptor signaling, resulting in abnormal hair morphogenesis and barrier formation–related defects (57). Moreover, a defect in the TGase activity was observed in the entire layer of the TRPV3-deficient epidermis. These mechanisms are attributable to the specific TRPV3-deficiency phenotype in SG1 cells, even though TRPV3 is expressed in all layers of the epidermis in humans and mice. A possible link between intracellular acidification and TRPV3 activation has been reported in case of nitric oxide production in cultured cells (58). Further studies are needed to understand the mechanism underlying the SG1-specific regulation of TRPV3.
Taken together, TRPV3 is suggested to play an important role in controlling the timing of acidification after [Ca2+]i elevation in SG1 cells and maintaining the spatial and temporal homeostasis of corneoptosis in the epidermis.
Future Perspectives on the Study of Corneoptosis.
Studying the corneoptosis process, which still requires the development of several new technologies, will allow us to understand the quality of the SC. The investigation of corneoptosis from the SG1 cell biological perspective will enable us to understand new aspects regarding cell death, which has a special biological meaning considering the mammalian skin.
Materials and Methods
Detailed experimental methods for intravital multiphoton excitation microscopy, confocal microscopy, isolation of SG1 cells, and whole-cell patch-clamp analysis of SG1 cells appear in SI Appendix.
Supplementary Material
Acknowledgments
We thank Keiko Mizuno, Ritsuko Ozawa, Rika Yokoo, Sachie Marushima, Yuki Yamagishi, and Hachiro Iseki for technical assistance. We also thank Takashi Kondoh and Yusuke Iizuka, and Chieko Tezuka for generating TRPV3-deficient mice. We thank Dr. Takashi Hiiragi (European Molecular Biology Laboratory) and the Institute for Integrated Cell-Material Sciences, Kyoto University, for supporting this project. This study was supported by a Japan Agency for Medical Research and Development (AMED) under the Grant Numbers 19gm1010001, 18gm1010001, 19ek0410058, and 18ek0410028 by Health and Labour Sciences Research Grants for Research on Allergic Diseases and Immunology from the Ministry of Health, Labour and Welfare and by a Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (C) under the Grant Number JP25461667, by the Takeda Science Foundation, by the Naito Foundation, and by a Cooperative Study Program by the National Institute for Physiological Sciences, Japan. Y.F. was supported by a RIKEN Junior Research Associate Program. N.K. was supported by JSPS Grant-in-Aid for JSPS Fellows under Grant Number 19J00968. We thank Dr. Nick Parris for his critical reading of the manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2020722118/-/DCSupplemental.
Data Availability
Bam fastq data have been deposited in Gene Expression Omnibus (GSE168011).
References
- 1.Kerr J. F., Wyllie A. H., Currie A. R., Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galluzzi L., et al., Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25, 486–541 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majno G., Joris I., Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 146, 3–15 (1995). [PMC free article] [PubMed] [Google Scholar]
- 4.Lippens S., Denecker G., Ovaere P., Vandenabeele P., Declercq W., Death penalty for keratinocytes: Apoptosis versus cornification. Cell Death Differ. 12, 1497–1508 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Eckhart L., Lippens S., Tschachler E., Declercq W., Cell death by cornification. Biochim. Biophys. Acta 1833, 3471–3480 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Matsui T., Amagai M., Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 27, 269–280 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Alibardi L., Adaptation to the land: The skin of reptiles in comparison to that of amphibians and endotherm amniotes. J. Exp. Zoolog. B Mol. Dev. Evol. 298, 12–41 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Watt F. M., Terminal differentiation of epidermal keratinocytes. Curr. Opin. Cell Biol. 1, 1107–1115 (1989). [DOI] [PubMed] [Google Scholar]
- 9.Yokouchi M., et al., Epidermal cell turnover across tight junctions based on Kelvin’s tetrakaidecahedron cell shape. eLife 5, e19593 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto K., Intercellular spaces of the human epidermis as demonstrated with lanthanum. J. Invest. Dermatol. 57, 17–31 (1971). [DOI] [PubMed] [Google Scholar]
- 11.Furuse M., et al., Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 156, 1099–1111 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuruta D., Green K. J., Getsios S., Jones J. C., The barrier function of skin: How to keep a tight lid on water loss. Trends Cell Biol. 12, 355–357 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Candi E., Schmidt R., Melino G., The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6, 328–340 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Ipponjima S., Umino Y., Nagayama M., Denda M., Live imaging of alterations in cellular morphology and organelles during cornification using an epidermal equivalent model. Sci. Rep. 10, 5515 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson C. L., et al., NIX initiates mitochondrial fragmentation via DRP1 to drive epidermal differentiation. Cell Rep. 34, 108689 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding C. R., et al., The cornified cell envelope: An important marker of stratum corneum maturation in healthy and dry skin. Int. J. Cosmet. Sci. 25, 157–167 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Richter T., et al., Dead but highly dynamic–The stratum corneum is divided into three hydration zones. Skin Pharmacol. Physiol. 17, 246–257 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Sandilands A., Sutherland C., Irvine A. D., McLean W. H., Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 122, 1285–1294 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubo A., et al., The stratum corneum comprises three layers with distinct metal-ion barrier properties. Sci. Rep. 3, 1731 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elias P. M., et al., The secretory granular cell: The outermost granular cell as a specialized secretory cell. J. Investig. Dermatol. Symp. Proc. 3, 87–100 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Holbrook K. A., Biologic structure and function: Perspectives on morphologic approaches to the study of the granular layer keratinocyte. J. Invest. Dermatol. 92, 84S–104S (1989). [DOI] [PubMed] [Google Scholar]
- 22.Murata T., et al., Transient elevation of cytoplasmic calcium ion concentration at a single cell level precedes morphological changes of epidermal keratinocytes during cornification. Sci. Rep. 8, 6610 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bokman S. H., Ward W. W., Renaturation of Aequorea gree-fluorescent protein. Biochem. Biophys. Res. Commun. 101, 1372–1380 (1981). [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y., et al., An expanded palette of genetically encoded Ca2+ indicators. Science 333, 1888–1891 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohkura M., Sasaki T., Kobayashi C., Ikegaya Y., Nakai J., An improved genetically encoded red fluorescent Ca2+ indicator for detecting optically evoked action potentials. PLoS One 7, e39933 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tojima T., et al., Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat. Neurosci. 10, 58–66 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Amagai M., Matsuyoshi N., Wang Z. H., Andl C., Stanley J. R., Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat. Med. 6, 1275–1277 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Peier A. M., et al., A heat-sensitive TRP channel expressed in keratinocytes. Science 296, 2046–2049 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Xu H., et al., TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418, 181–186 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Brody I., An ultrastructural study on the role of the keratohyalin granules in the keratinization process. J. Ultrastruct. Res. 3, 84–104 (1959). [DOI] [PubMed] [Google Scholar]
- 31.Brody I., The ultrastructure of the tonofibrils in the keratinization process of normal human epidermis. J. Ultrastruct. Res. 4, 264–297 (1960). [Google Scholar]
- 32.Orrenius S., Zhivotovsky B., Nicotera P., Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 4, 552–565 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Harr M. W., Distelhorst C. W., Apoptosis and autophagy: Decoding calcium signals that mediate life or death. Cold Spring Harb. Perspect. Biol. 2, a005579 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhivotovsky B., Orrenius S., Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium 50, 211–221 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Gupta A. K., Giaglis S., Hasler P., Hahn S., Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS One 9, e97088 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Vasconcelos N. M., Van Opdenbosch N., Van Gorp H., Parthoens E., Lamkanfi M., Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ. 26, 146–161 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorand L., Graham R. M., Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 4, 140–156 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Lee C. H., Coulombe P. A., Self-organization of keratin intermediate filaments into cross-linked networks. J. Cell Biol. 186, 409–421 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma L., Yamada S., Wirtz D., Coulombe P. A., A ‘hot-spot’ mutation alters the mechanical properties of keratin filament networks. Nat. Cell Biol. 3, 503–506 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Yamada S., Wirtz D., Coulombe P. A., Pairwise assembly determines the intrinsic potential for self-organization and mechanical properties of keratin filaments. Mol. Biol. Cell 13, 382–391 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quiroz F. G., et al., Liquid-liquid phase separation drives skin barrier formation. Science 367, eaax9554 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Presland R. B., Bassuk J. A., Kimball J. R., Dale B. A., Characterization of two distinct calcium-binding sites in the amino-terminus of human profilaggrin. J. Invest. Dermatol. 104, 218–223 (1995). [DOI] [PubMed] [Google Scholar]
- 43.Brown S. J., McLean W. H., One remarkable molecule: Filaggrin. J. Invest. Dermatol. 132, 751–762 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Veer S. J., Furio L., Harris J. M., Hovnanian A., Proteases: Common culprits in human skin disorders. Trends Mol. Med. 20, 166–178 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Matsui T., et al., Mouse homologue of skin-specific retroviral-like aspartic protease involved in wrinkle formation. J. Biol. Chem. 281, 27512–27525 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Matsui T., et al., SASPase regulates stratum corneum hydration through profilaggrin-to-filaggrin processing. EMBO Mol. Med. 3, 320–333 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dale B. A., Holbrook K. A., Steinert P. M., Assembly of stratum corneum basic protein and keratin filaments in macrofibrils. Nature 276, 729–731 (1978). [DOI] [PubMed] [Google Scholar]
- 48.Fischer H., et al., DNase1L2 degrades nuclear DNA during corneocyte formation. J. Invest. Dermatol. 127, 24–30 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Fischer H., et al., DNase 2 is the main DNA-degrading enzyme of the stratum corneum. PLoS One 6, e17581 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiokawa D., Tanuma S., Characterization of human DNase I family endonucleases and activation of DNase gamma during apoptosis. Biochemistry 40, 143–152 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Eckhart L., Fischer H., Barken K. B., Tolker-Nielsen T., Tschachler E., DNase1L2 suppresses biofilm formation by Pseudomonas aeruginosa and Staphylococcus aureus. Br. J. Dermatol. 156, 1342–1345 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Fischer H., Buchberger M., Napirei M., Tschachler E., Eckhart L., Inactivation of DNase1L2 and DNase2 in keratinocytes suppresses DNA degradation during epidermal cornification and results in constitutive parakeratosis. Sci. Rep. 7, 6433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moqrich A., et al., Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307, 1468–1472 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Singh A. K., et al., Structural basis of temperature sensation by the TRP channel TRPV3. Nat. Struct. Mol. Biol. 26, 994–998 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng Z., et al., Gating of human TRPV3 in a lipid bilayer. Nat. Struct. Mol. Biol. 27, 635–644 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimada H., et al., The structure of lipid nanodisc-reconstituted TRPV3 reveals the gating mechanism. Nat. Struct. Mol. Biol. 27, 645–652 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Cheng X., et al., TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 141, 331–343 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyamoto T., Petrus M. J., Dubin A. E., Patapoutian A., TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat. Commun. 2, 369 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Bam fastq data have been deposited in Gene Expression Omnibus (GSE168011).