Significance
Pulmonary hypertension (PH) is a serious disorder with a mortality rate of 40% over 5 y after diagnosis. Endothelial dysfunction is a major contributor to increased pulmonary arterial pressure (PAP) in PH. Here, we provide evidence that endothelial cell caveolin-1–TRPV4 channel signaling lowers resting PAP. Moreover, an impairment of endothelial caveolin-1–TRPV4 channel signaling elevates PAP in PH. In PH, there is an up-regulation of NOX1 and iNOS enzymes in endothelial cells, which increases peroxynitrite levels. Peroxynitrite, in turn, targets endothelial caveolin-1 and lowers endothelial caveolin-1–TRPV4 channel signaling. Finally, decreasing peroxynitrite levels rescues endothelial caveolin-1–TRPV4 channel signaling and lowers PAP in PH.
Keywords: endothelium, pulmonary hypertension, TRP channel, caveolin, peroxynitrite
Abstract
Recent studies have focused on the contribution of capillary endothelial TRPV4 channels to pulmonary pathologies, including lung edema and lung injury. However, in pulmonary hypertension (PH), small pulmonary arteries are the focus of the pathology, and endothelial TRPV4 channels in this crucial anatomy remain unexplored in PH. Here, we provide evidence that TRPV4 channels in endothelial cell caveolae maintain a low pulmonary arterial pressure under normal conditions. Moreover, the activity of caveolar TRPV4 channels is impaired in pulmonary arteries from mouse models of PH and PH patients. In PH, up-regulation of iNOS and NOX1 enzymes at endothelial cell caveolae results in the formation of the oxidant molecule peroxynitrite. Peroxynitrite, in turn, targets the structural protein caveolin-1 to reduce the activity of TRPV4 channels. These results suggest that endothelial caveolin-1–TRPV4 channel signaling lowers pulmonary arterial pressure, and impairment of endothelial caveolin-1–TRPV4 channel signaling contributes to elevated pulmonary arterial pressure in PH. Thus, inhibiting NOX1 or iNOS activity, or lowering endothelial peroxynitrite levels, may represent strategies for restoring vasodilation and pulmonary arterial pressure in PH.
Pulmonary hypertension (PH) is a degenerative disease characterized by increased pulmonary arterial pressure (PAP) (1). Current therapeutic options for PH patients are aimed at dilating pulmonary arteries (PAs) to lower PAP. However, treatments that target the mechanisms for impaired vasodilation are not available. Endothelial cells (ECs) promote vasodilation of PAs under normal conditions. Loss of endothelium-dependent vasodilation of resistance-sized PAs is a significant contributor to elevated PAP in PH (2). Therefore, a detailed understanding of the pathological signatures of arterial ECs is necessary for designing therapeutic options capable of rescuing endothelium-dependent vasodilation in PH.
Intracellular Ca2+ is an essential regulator of EC function in the pulmonary circulation. Recent studies show that Ca2+ influx through EC transient receptor potential vanilloid 4 (TRPV4EC) channels dilates resistance PAs via activation of endothelial nitric oxide synthase (eNOS) (3, 4). However, the physiological role of TRPV4EC channels in regulating PAP remains unknown, largely due to the lack of studies in cell-specific knockout mice. In this regard, activation of smooth muscle cell TRPV4 (TRPV4SMC) channels can induce PA constriction (5), whereas activation of TRPV4EC channels induces PA dilation (4). We hypothesized that TRPV4EC channel dysfunction contributes to the loss of vasodilation and elevation of PAP in PH.
Caveolin-1 (Cav-1) is an important scaffolding protein that interacts with and stabilizes several other proteins in the pulmonary circulation, including eNOS (6). Global Cav-1−/− mice show elevated PAP, and endothelial Cav-1 (Cav-1EC)–dependent signaling is altered in PH (7–11). Studies on pulmonary EC culture showed that the TRPV4EC channel coimmunoprecipitates with Cav-1 (12); however, direct functional evidence for the effect of Cav-1 on TRPV4EC channel activity is lacking. We postulated that Cav-1EC enhances TRPV4EC channel activity in PAs and that impaired Cav-1EC–TRPV4EC signaling contributes to the loss of vasodilation and elevated PAP in PH.
The generation of reactive oxygen species (ROS) appears to be central to vascular dysfunction in PH (13, 14). In this regard, NADPH oxidase 1 (NOX1) has emerged as a crucial source of endothelial superoxide radicals in PH (15–17). Interestingly, superoxide radicals were shown to augment TRPV4 channel-induced increases in global Ca2+ in pulmonary EC culture (18). Additionally, superoxide radicals can quickly react with NO to form the oxidant molecule peroxynitrite (PN) (19). PN is elevated in the lungs of PH patients (20) and is known to have deleterious effects on EC function (21). Importantly, inducible NOS (iNOS), which generates NO, is also up-regulated in mouse models of PH and in PH patients (22, 23). Therefore, we hypothesized that NOX1 and iNOS contribute to ROS-dependent inhibition of Cav-1EC–TRPV4EC channel signaling in PH.
In the current study, we used inducible, EC-specific TRPV4 channel knockout (TRPV4EC−/−) and endothelium-specific Cav-1–knockout (Cav-1EC−/−) mice to confirm a vital role for Cav-1EC–TRPV4EC signaling in lowering resting PAP. Cav-1EC–TRPV4EC signaling was impaired in resistance PAs from mouse models of PH and those from pulmonary arterial hypertension (PAH) patients. NADPH oxidase enzyme 1 (NOX1−/−) and inducible nitric oxide synthase (iNOS−/−) mice were protected against the impairment of Cav-1EC–TRPV4EC signaling in PH, and NOX1/iNOS/PN inhibitors rescued Cav-1EC–TRPV4EC signaling in PH. Collectively, our findings provide evidence that pathological signaling made possible by the colocalization of NOX1 and iNOS with Cav-1 underlies the loss of TRPV4EC channel activity and vasodilation in PH. Therefore, inhibiting NOX1/iNOS or suppressing PN formation may be a viable strategy for rescuing endothelial function and PAP in PH.
Results
Endothelial, but Not Smooth Muscle, TRPV4 Channels Control Resting PAP.
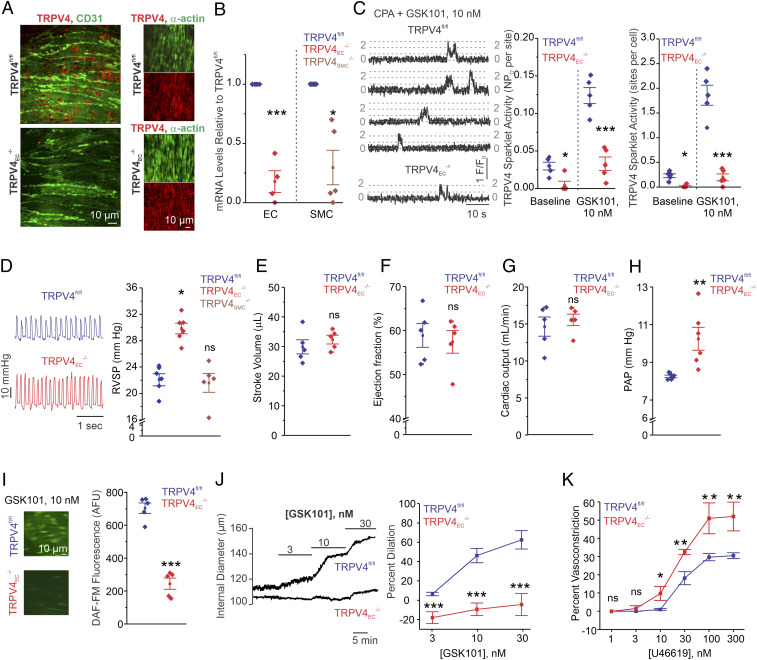
To determine the physiological role of TRPV4EC channels, we utilized inducible TRPV4EC−/− mice. Endothelial knockout of TRPV4 channels was confirmed at the protein level by immunofluorescence analysis of en face PA preparations (Fig. 1A) and at the mRNA level by RT-qPCR analysis of ECs from resistance-sized, fourth-order PAs (∼50 μm, Fig. 1B). Localized, unitary Ca2+ influx events through TRPV4EC channels, termed TRPV4EC sparklets (24), were recorded in en face PAs loaded with Fluo-4. Baseline TRPV4EC sparklet activity and activity induced by the specific TRPV4 channel agonist, GSK1016790A (10 nmol/L; hereafter, GSK101), were significantly reduced in PAs from TRPV4EC−/− mice compared with those in PAs from TRPV4fl/fl control mice (tamoxifen-injected TRPV4fl/fl Cre−; Fig. 1C). Thus, we can significantly reduce TRPV4EC channel expression and function specifically in the endothelium.
Fig. 1.
Inducible TRPV4EC−/− mice show elevated resting PAP. (A) TRPV4EC immunofluorescence images in en face fourth-order PAs from TRPV4fl/fl and TRPV4EC−/− mice. (B) Endothelial TRPV4 mRNA levels (left of the dotted line) relative to those in TRPV4fl/fl mice and SMC TRPV4 mRNA levels (right of the dotted line) relative to those in TRPV4fl/fl mice (n = 4 to 5; *P < 0.05, ***P < 0.001 versus TRPV4fl/fl; one-way ANOVA). (C, Left) TRPV4EC sparklet traces in one EC from a fluo-4–loaded en face PA in response to TRPV4 channel activator GSK101 (10 nmol/L). (Center) TRPV4EC sparklet activity in PAs, expressed as NPO per site (n = 5; *P < 0.05; ***P < 0.001 versus TRPV4fl/fl; two-way ANOVA). N is the number of channels per site, and PO is the open state probability of the channel. Experiments were performed in Fluo-4–loaded fourth-order PAs in the presence of cyclopiazonic acid (CPA; 20 μmol/L) to eliminate Ca2+ release from intracellular stores. (Right) TRPV4EC sparklet activity sites per cell in PAs (n = 5; *P < 0.05; ***P < 0.001 versus TRPV4fl/fl; two-way ANOVA). (D) Representative RVSP (millimeters of mercury) traces (Left) and averaged RVSP values (Right; n = 6; *P < 0.05 versus TRPV4fl/fl; ns indicates no significance; one-way ANOVA). (E) Averaged stroke volume (microliters) in TRPV4fl/fl and TRPV4EC−/− mice. Data represented as mean ± SEM (n = 5). (F) Averaged ejection fraction (%) in TRPV4fl/fl and TRPV4EC−/− mice (n = 5 mice; ns indicates no significance). (G) Cardiac output (milliliters per minute) in TRPV4fl/fl and TRPV4EC−/− mice (n = 5 mice). (H) Averaged PAP (mm Hg) in isolated perfused lungs from TRPV4fl/fl and TRPV4EC−/− mice (n = 6; **P < 0.01; t test). (I) DAF-FM fluorescence analysis of NO production in response to GSK101 (10 nmol/L) in TRPV4fl/fl and TRPV4EC−/− mice, respectively (n = 5; ***P < 0.001; t test). (J) Pressure myography traces (Left) and averaged percent vasodilation (Right) of PAs to GSK101 (3 to 30 nmol/L). Fourth-order PAs were pressurized to 15 mm Hg (n = 5 to 6; ***P < 0.001 versus TRPV4fl/fl; two-way ANOVA). (K) Percent vasoconstriction of PAs in response to the thromboxane A2 receptor agonist U46619 (1 to 300 nmol/L; n = 5; *P < 0.05 [10 nmol/L] and **P < 0.01 [30 to 300 nmol/L] versus TRPV4fl/fl, two-way ANOVA).
The functional effect of TRPV4EC−/− was determined using right ventricular systolic pressure (RVSP) and PAP measurements, functional magnetic resonance imaging (MRI) analyses, and pressure myography studies in PAs. TRPV4EC−/− mice, but not TRPV4SMC−/− mice, exhibited elevated RVSP, an indirect indicator of PAP (Fig. 1D). To ensure that changes in RVSP were not influenced by altered cardiac function, we performed functional MRI analyses on TRPV4fl/fl and TRPV4EC−/− mice. The two genotypes showed no significant differences in heart rate, stroke volume, ejection fraction, and cardiac output (Fig. 1 E–G and SI Appendix, Table S1). Furthermore, isolated lung perfusion experiments showed that PAP was elevated in TRPV4EC−/− mice when compared with TRPV4fl/fl mice (Fig. 1H). Fulton index, a ratio of right ventricular (RV) weight to left ventricle and septal (LV+S) weight, was not altered in TRPV4EC−/− mice, suggesting a lack of RV hypertrophy in these mice (SI Appendix, Fig. S1).
Consistent with the previously reported TRPV4EC–eNOS signaling in PAs (4), GSK101 (10 nmol/L)-induced endothelial NO levels, measured using a fluorescent NO indicator (4-amino-5 methylamino-2′,7′difluorofluorescenin diacetate or DAF-FM, 5 μmol/L), were lower in TRPV4EC−/− mice (Fig. 1I and SI Appendix, Fig. S2). Reduced TRPV4EC sparklet activity resulted in impaired endothelium-dependent vasodilation to GSK101 (3 to 30 nmol/L) in isolated, pressurized PAs from TRPV4EC−/− mice compared with those from TRPV4fl/fl mice (Fig. 1J). Moreover, contractile responses to the thromboxane A2 receptor agonist U46619 were greater in pressurized PAs from TRPV4EC−/− mice (Fig. 1K). Together, these data provide evidence that TRPV4EC channel activity lowers PAP, an effect that is independent of changes in cardiac function.
Cav-1EC–Protein Kinase C Signaling Enhances TRPV4EC Channel Activity and Lowers PAP.
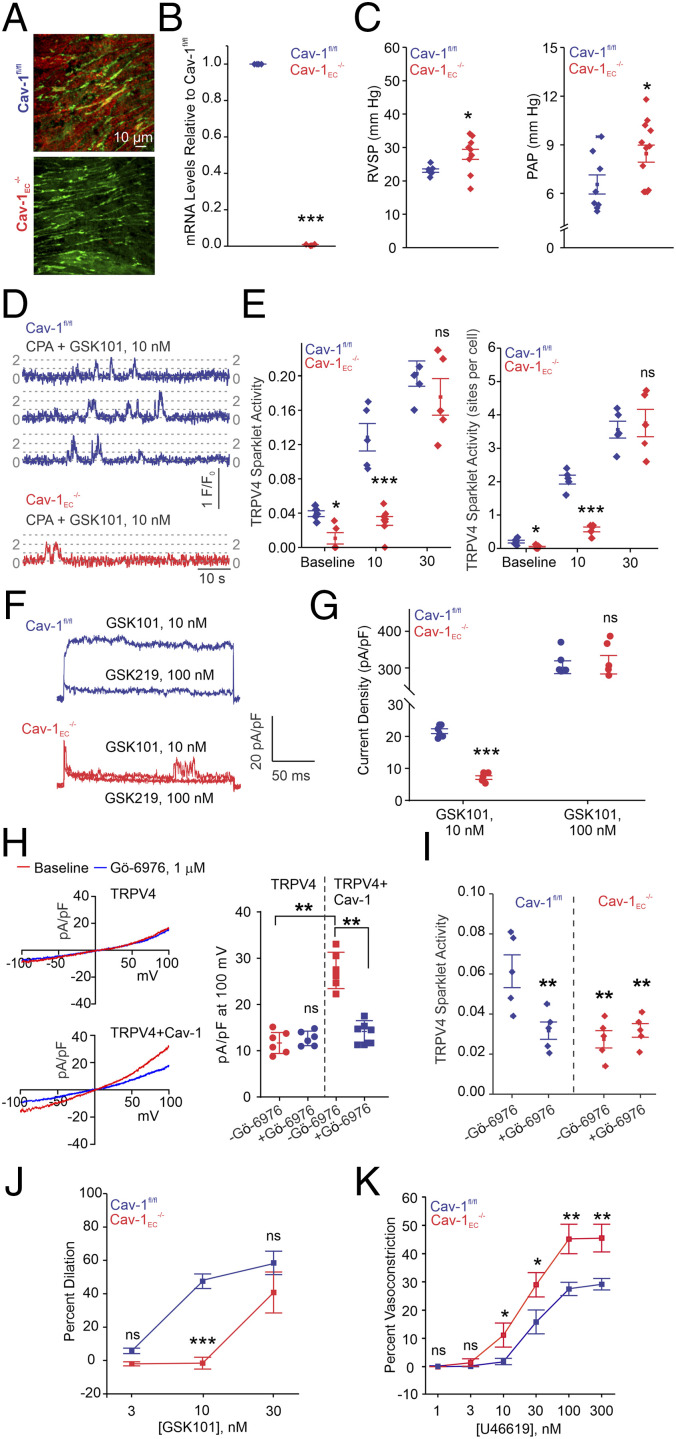
Cav-1 was shown to coimmunoprecipitate with TRPV4 channels in pulmonary EC culture (12). However, functional evidence for the effect of Cav-1 on TRPV4EC channel activity is lacking. We hypothesized that Cav-1EC enhances TRPV4EC channel activity in PAs. To determine whether Cav-1EC regulates TRPV4EC channel activity in PAs, we utilized an inducible Cav-1EC−/− mouse. Endothelial Cav-1EC knockout was confirmed by a reduction in Cav-1EC immunostaining (Fig. 2A) and Cav-1 messenger RNA (mRNA) levels (Fig. 2B). Loss of Cav-1EC resulted in elevated RVSP without RV hypertrophy (Fig. 2 C, Left and SI Appendix, Fig. S1). Isolated lung perfusion experiments showed that PAP was elevated in Cav-1EC−/− mice compared with floxed Cav-1 (Cav-1fl/fl) mice (Fig. 2 C, Right). In PAs from Cav-1EC−/− mice, baseline as well as GSK101-induced TRPV4EC sparklet activity was reduced when compared with the corresponding activities in PAs from Cav-1fl/fl mice, confirming Cav-1EC regulation of TRPV4EC channel activity (Fig. 2 D and E). At higher levels of activation (GSK101, 30 nmol/L), however, there was no difference in TRPV4EC sparklet activity between the two groups, indicating that Cav-1EC−/− does not reduce the total number of functional TRPV4EC channels per site. GSK101 (10 nmol/L)-induced currents through TRPV4EC channels were reduced in PAs from Cav-1EC−/− mice when compared to the Cav-1fl/fl mice (Fig. 2 F and G). Higher concentration of the agonist (100 nmol/L), however, elicited similar currents through the TRPV4EC channel (Fig. 2G), further supporting unaltered maximum functional TRPV4EC channels in PAs from Cav-1EC−/− and Cav-1fl/fl mice.
Fig. 2.
Cav-1EC–TRPV4EC signaling in PAs lowers resting PAP. (A) Cav-1EC immunofluorescence in en face preparations of fourth-order PAs. (B) Cav-1EC mRNA levels in PAs relative to those in Cav-1fl/fl mice (n = 4; ***P < 0.001; t test). (C) Averaged resting RVSP (Left; n = 5 to 9; *P < 0.05; t test) and PAP values (Right; n = 8 to 11; *P < 0.05; t test). (D) TRPV4EC sparklet traces in one EC from a fluo-4–loaded en face PAs in response to TRPV4 channel activator GSK101 (10 nmol/L) in Cav-1fl/fl and Cav-1EC−/− mice. (E, Left) Baseline or GSK101-induced (10 to 30 nmol/L) TRPV4EC sparklet activity, expressed as NPO per site (n = 5; *P < 0.05 versus Cav-1fl/fl [Baseline]; ***P < 0.001 versus Cav-1fl/fl [10 nmol/L]; ns indicates no significance; two-way ANOVA). Experiments were performed in fluo-4–loaded fourth-order PAs in the presence of cyclopiazonic acid (CPA; 20 μmol/L) to eliminate Ca2+ release from intracellular stores. (Right) TRPV4EC sparklet sites per cell in PAs from Cav-1fl/fl or Cav-1EC−/− mice (n = 5; *P < 0.05 versus Cav-1fl/fl [Baseline]; ***P < 0.001 versus Cav-1fl/fl [10 nmol/L]; two-way ANOVA). (F) Representative GSK101 (10 nmol/L)-induced outward TRPV4EC currents in freshly isolated ECs from Cav-1fl/fl or Cav-1EC−/− mice and effect of GSK2193874 (GSK219; 100 nmol/L) in the presence of GSK101 (10 nmol/L). (G) Scatterplot showing TRPV4EC currents in the presence of GSK101 (10 and 100 nmol/L; n = 5; ***P < 0.001 versus Cav-1fl/fl [10 nmol/L]; two-way ANOVA). (H, Left) Representative traces showing TRPV4 currents in the absence or presence of Gö-6976 (PKC-α/β inhibitor; 1 μmol/L) in HEK293 cells transfected with TRPV4 only or cotransfected with TRPV4 plus WT Cav-1, recorded in the whole-cell patch-clamp configuration. (Right) Current density plot of TRPV4 currents at +100 mV in the absence or presence of Gö-6976 (1 μmol/L) in HEK293 cells transfected with TRPV4 or TRPV4 + Cav-1 (n = 5; **P < 0.01 versus TRPV4 [−Gö-6976] and TRPV4 + Cav-1 [−Gö-6976]; two-way ANOVA). (I) Effect of Gö-6976 (1 μmol/L) on TRPV4EC sparklet activity in en face preparations of PAs from Cav-1fl/fl and Cav-1EC−/− mice, expressed as NPO per site (n = 5; **P < 0.01 versus Cav-1fl/fl [−Gö-6976]; two-way ANOVA). (J) Percent dilation of PAs in response to GSK101 (3 to 30 nmol/L; n = 6; *** P < 0.001 versus Cav-1fl/fl [10 nmol/L]; two-way ANOVA). (K) Percent constriction of PAs in response to the thromboxane A2 receptor agonist U46619 (1 to 300 nmol/L; n = 5; *P < 0.05 versus Cav-1fl/fl [10 and 30 nmol/L]; **P < 0.01 versus Cav-1fl/fl [100 and 300 nmol/L]; two-way ANOVA).
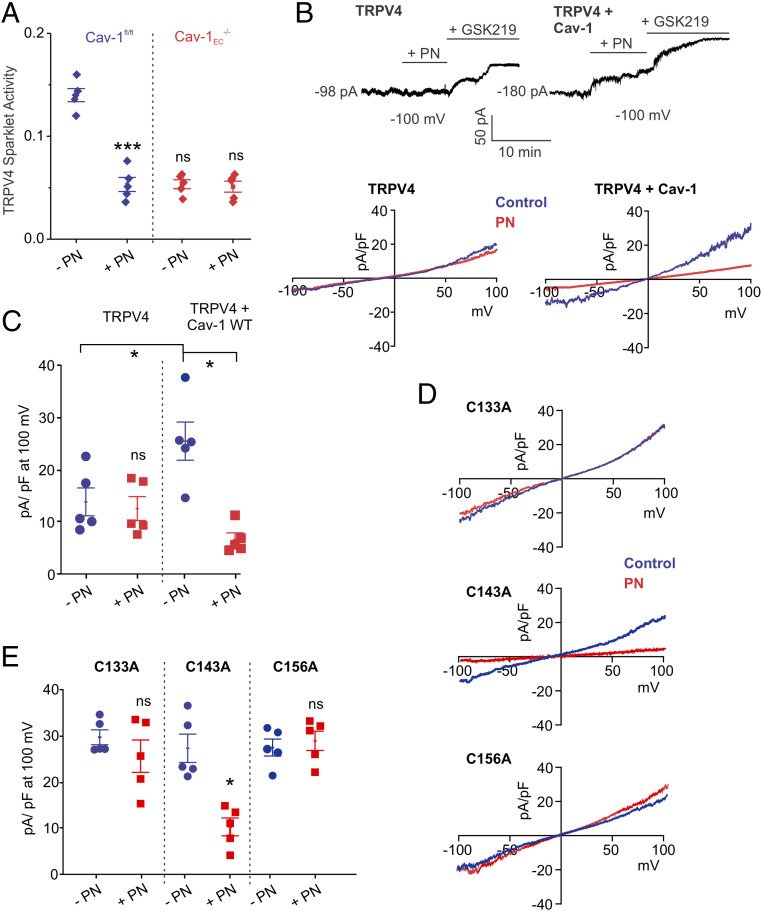
Protein kinase C (PKC) is a binding partner of Cav-1 (12) and a well-known activator of TRPV4EC channels (25). HEK293 cells expressing TRPV4 channels + Cav-1 showed higher TRPV4 currents compared to the cells expressing TRPV4 channels alone (Fig. 2H). The presence of selective PKC-α/β inhibitor Gö-6976 (1 μmol/L) abolished the difference in TRPV4 currents between TRPV4 and TRPV4 + Cav-1 expressing cells (Fig. 2H), supporting the PKC-α/β–dependent nature of Cav-1–TRPV4 channel interaction. Gö-6976 reduced TRPV4EC sparklet activity in Cav-1fl/fl mice but not Cav-1EC−/− mice (Fig. 2I), indicating that Cav-1EC–PKC signaling enhances TRPV4EC channel activity in PAs.
TRPV4EC channel-mediated dilation of PAs was substantially attenuated in Cav-1EC−/− mice when compared with that in Cav-1fl/fl mice (Fig. 2J). U46619-induced vasoconstriction was also greater in PAs from Cav-1EC−/− mice, confirming that PAs in these mice are more contractile (Fig. 2K). These results provide direct evidence that Cav-1EC enhances TRPV4EC channel activity in PAs and lowers PAP.
TRPV4EC Channel Activity Is Impaired in Resistance PAs from Mouse Models of PH and PH Patients.
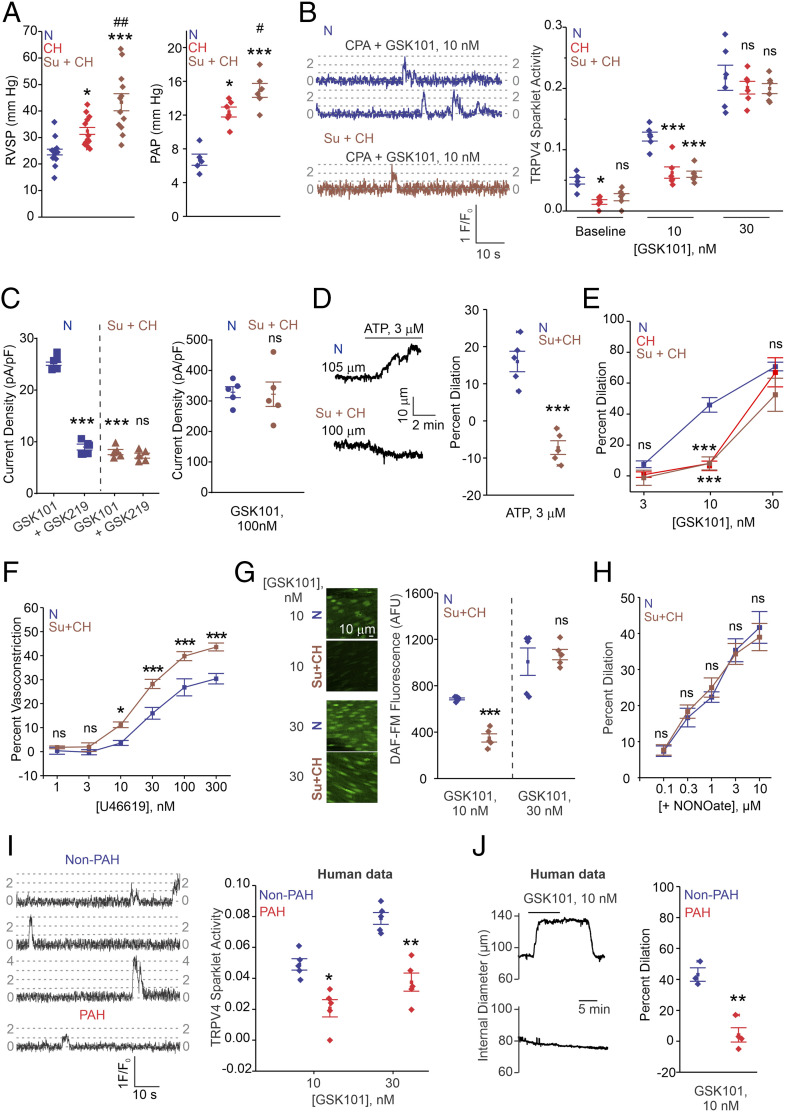
The mechanisms underlying the loss of endothelium-dependent dilation of PAs in PH are poorly understood. We hypothesized that impaired TRPV4EC channel activity contributes to the reduced vasodilation and elevated PAP in PH. TRPV4EC channel activity in resistance PAs was analyzed in two mouse models of PH: chronic hypoxia (CH) and SU5416 (VEGF receptor antagonist) + CH (Su+CH), which is known to cause a more profound PH phenotype than CH alone (26). CH mice showed elevated RVSP and PAP accompanied by RV hypertrophy, indicative of robust development of PH (Fig. 3A and SI Appendix, Fig. S1). In mice exposed to SU+CH, both RVSP and Fulton index increased to a greater extent than that observed in mice exposed to CH alone, consistent with earlier reports (26). Baseline and GSK101 (10 nmol/L)-induced TRPV4EC sparklet activity was lower in PAs from CH or Su+CH mice compared to PAs from normoxic (N) mice (Fig. 3B and SI Appendix, Table S2). There was, however, no difference in TRPV4EC sparklet activity among the groups at a higher level of activation (30 nmol/L GSK101; Fig. 3B), suggesting that the number of functional TRPV4EC channels is unaltered in PH. Ionic currents through TRPV4EC channels were also decreased in ECs from Su+CH mice when compared with N mice (Fig. 3C). At higher levels of TRPV4 channel activation (100 nmol/L GSK101), however, there was no difference in TRPV4EC currents between the groups, suggesting that the maximum number of functional TRPV4 channels is not altered in PH.
Fig. 3.
TRPV4EC channel activity and vasodilation are reduced in PAs from mouse models of PH and PAH patients. (A, Left) Averaged RVSP (mmHg) values in mice exposed to N, CH (3 wk; 10% O2), or Su+CH (n = 12; *P < 0.05 versus N; ***P < 0.001 versus N; ##P < 0.01 versus CH; one-way ANOVA). (Right) Averaged PAP (millimeters of mercury) values in mice exposed to N, CH, and Su+CH (n = 6; *P < 0.05 versus N; ***P < 0.001 versus N; #P < 0.05 versus CH; one-way ANOVA). (B, Left) TRPV4EC sparklet traces in one EC from a fluo-4–loaded en face PA from N and Su+CH mice in response to TRPV4 channel activator GSK101 (10 nmol/L). (Right) Baseline or GSK101 (10 and 30 nmol/L)-induced TRPV4EC sparklet activity in N, CH, or Su+CH mice, expressed as NPO per site (n = 5; *P < 0.05 versus N [baseline]; ***P < 0.001 versus N [10 nmol/L]; two-way ANOVA). Experiments were performed in fluo-4–loaded fourth-order PAs in the presence of cyclopiazonic acid (CPA; 20 μmol/L) to eliminate Ca2+ release from intracellular stores. (C, Left) GSK101 (10 nmol/L)-induced outward currents at +100 mV and inhibition by GSK2193874 (GSK219; 100 nmol/L; n = 5; ***P < 0.001 versus N [GSK101]; two-way ANOVA). (Right) GSK101 (100 nmol/L)-induced outward currents at +100 mV in PAs from N and Su+CH mice (n = 5). (D) Pressure myography trace (Left) and percent vasodilation (Right) of PAs in response to ATP (3 μmol/L) in N and Su+CH mice (n = 5; ***P < 0.001 versus N; t test). (E) Percent vasodilation of PAs from N, CH, and Su+CH mice in response to GSK101 (3 to 30 nmol/L; n = 5 to 10; ***P < 0.001 versus N [GSK101; 10 nmol/L]; two-way ANOVA). (F) Percent vasoconstriction to thromboxane A2 receptor agonist U46619 (1 to 300 nmol/L) in PAs from N or Su+CH mice (n = 5; *P < 0.05 [U46619; 10 nmol/L] and ***P < 0.001 [U466; 30 to 300 nmol/L] versus N; two-way ANOVA). (G) DAF-FM fluorescence analysis of NO production in response to GSK101 (10 and 30 nmol/L) in N and Su+CH mice, respectively (n = 5; ***P < 0.001 versus N [GSK101; 10 nmol/L]; two-way ANOVA). (H) Percent vasodilation of PAs from N and Su+CH mice in response to spermine NONOate (NO donor; 0.1 to 10 μmol/L; n = 5). (I) TRPV4EC sparklet traces (Left) and GSK101 (10 and 30 nmol/L)-induced sparklet activity (Right) in PAs from non-PAH and PAH patients (n = 5; *P < 0.05 [GSK101; 10 nmol/L] versus non-PAH; **P < 0.01 [GSK101; 30 nmol/L] versus non-PAH; two-way ANOVA). (J, Left) Representative diameter traces showing GSK101 (10 nmol/L)-induced dilation of PAs from non-PAH individuals and PAH patients, preconstricted with U46619 (50 nmol/L). (Right) Percent vasodilation of PAs from non-PAH and PAH patients in response to GSK101 (10 nmol/L; n = 3 to 4; **P < 0.01; t test).
Adenosine triphosphate (ATP), an endogenous purinergic receptor agonist, causes endothelium-dependent dilation of PAs through TRPV4EC channel activation (4). ATP-induced activation of TRPV4EC channels was impaired in PAs from mouse models of PH and PAH patients (SI Appendix, Fig. S3A). Furthermore, ATP-induced dilation of PAs was abolished in Su+CH mice (Fig. 3D). Consistent with lower TRPV4EC channel activity, PAs from CH and Su+CH mice showed reduced vasodilation and higher contractility (Fig. 3 E and F). TRPV4EC, Cav-1EC, and PKC mRNA levels were not different between N and Su+CH mice (SI Appendix, Fig. S4), further supporting impaired channel regulation instead of altered channel expression in PH.
TRPV4EC channels dilate PAs predominantly through eNOS activation (4). To test whether the reduced TRPV4EC channel-induced vasodilation in mouse models of PH is due to an impaired TRPV4EC–eNOS signaling linkage, we measured NO levels using DAF-FM. GSK101 (10 nmol/L)-induced endothelial NO levels were lower in PAs from Su+CH mice compared to the N mice (Fig. 3G). Notably, the GSK101 concentration that induced similar levels of TRPV4EC sparklet activation in PAs from N and Su+CH mice (30 nmol/L) produced comparable increases in DAF-FM fluorescence (Fig. 3G), indicating that activation of eNOS by TRPV4EC channels is not altered in PH. Furthermore, the NO donor, NONOate (0.1 to 10 μmol/L), was able to dilate PAs from N and CH mice to a similar extent (Fig. 3H), indicating that the signaling cascade downstream of NO is not altered in PH and that the impairment in endothelial signaling in PH occurs upstream of eNOS.
Support for the clinical relevance of our findings was provided by assessing TRPV4EC sparklet activity in resistance PAs (∼50 μm diameter) from patients of PAH (mean PAP > 25). Compared with the PAs from control individuals, PAs from PAH patients showed a significantly lower TRPV4EC sparklet activity (Fig. 3I and SI Appendix, Table S2). ATP activation of TRPV4EC sparklets was also impaired in PAs from PAH patients (SI Appendix, Fig. S3B). Moreover, TRPV4EC channel-induced dilation of PAs was significantly diminished in PAH patients compared with that in control individuals (Fig. 3J).
NOX1- and iNOS-Mediated PN Impairs TRPV4EC Channel Activity in PH.
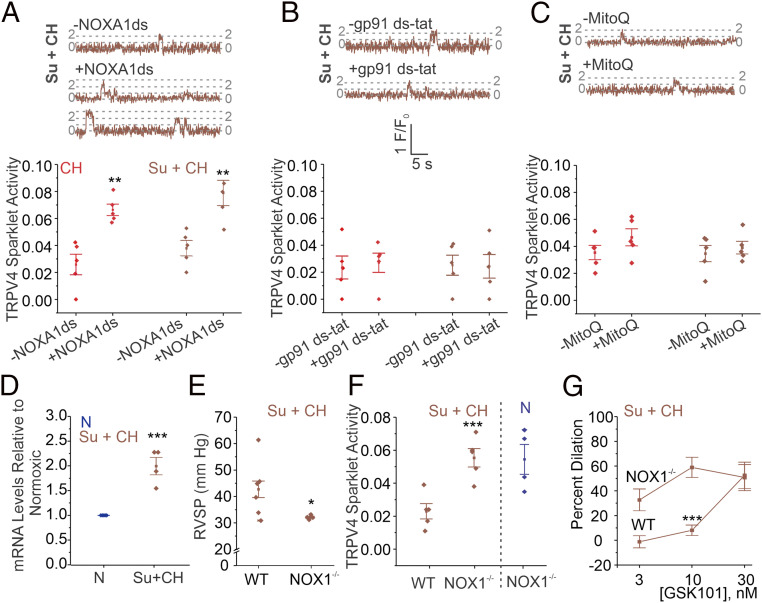
Elevated production of superoxide radicals (O2•−) has been implicated in the pathogenesis of PH (18, 27). In support of this concept, the O2•−-lowering compound, tempol, rescued TRPV4EC channel activity in Su+CH mice (SI Appendix, Fig. S5A). We then tested the effects of inhibiting three major sources of O2•− formation: NOX1 (NoxA1ds; 1 μmol/L), NOX2 (gp91ds-tat; 1 μmol/L), and mitochondria (mitoQ, 1 μmol/L; Fig. 4 A–C and SI Appendix, Table S3). Only NOX1 inhibition with NoxA1ds rescued TRPV4EC sparklet activity in the two mouse models of PH. qPCR studies showed that endothelial NOX1 mRNA levels were elevated in PAs from Su+CH mice (Fig. 4D). Importantly, the increase in RVSP observed in NOX1−/− mice exposed to Su+CH was diminished compared with that in wild-type (WT) Su+CH mice (Fig. 4E and SI Appendix, Fig. S6). Moreover, NOX1−/− mice exposed to Su+CH showed no impairment in TRPV4EC sparklet activity (Fig. 4F) or vasodilation (Fig. 4G), confirming an important role for endothelial NOX1-derived O2•− in reducing TRPV4EC channel activity in PH. However, excessive generation of O2•− using a hypoxanthine/xanthine oxidase system did not alter TRPV4EC channel activity in normal PAs, supporting the concept that O2•− by itself does not inhibit TRPV4EC channel activity in PAs (SI Appendix, Fig. S5B). Hypoxanthine/xanthine oxidase can also increase the production of hydrogen peroxide. In this regard, we previously demonstrated that hydrogen peroxide does not directly alter TRPV4EC sparklet activity (21). We therefore explored the possibility that O2•− reacts with NO, the predominant signaling molecule in the pulmonary circulation, to form the oxidant molecule PN.
Fig. 4.
Endothelial NOX1 impairs TRPV4EC channel activity in PH. (A–C) TRPV4EC sparklet traces in one EC from a fluo-4–loaded en face PA (Top) and TRPV4EC sparklet activity per site (Bottom; NPO per site) in the absence and presence of NOXA1ds (NOX 1 inhibitor; 1 μmol/L; A), gp91ds-tat (NOX2 inhibitor; 1 μmol/L; B), or mitoQ (mitochondrial antioxidant; 1 μmol/L; C) in PAs from C57BL6/J mice exposed to CH (3 wk; 10% O2) or Su+CH (n = 5; **P < 0.01 versus [−NOXA1ds]; two-way ANOVA), expressed as NPO per site. (D) NOX1 mRNA levels in ECs from N and Su+CH mice relative to N mice (n = 5; ***P < 0.001; t test). (E) Averaged resting RVSP (millimeters of mercury) values in WT and NOX1−/− mice exposed to Su+CH (n = 4 to 6; *P < 0.05; t test). (F) GSK101-induced (1 nmol/L) TRPV4EC sparklet activity in PAs from WT and NOX1−/− mice exposed to Su+CH and NOX1−/− mice exposed to N (n = 5; ***P < 0.001 versus WT [Su+CH]; two-way ANOVA). (G) Percent dilation of PAs from WT and NOX1−/− mice exposed to Su+CH in response to GSK101 (3 to 30 nmol/L; n = 5; ***P < 0.001 versus WT [3 and 10 nmol/L]; two-way ANOVA).
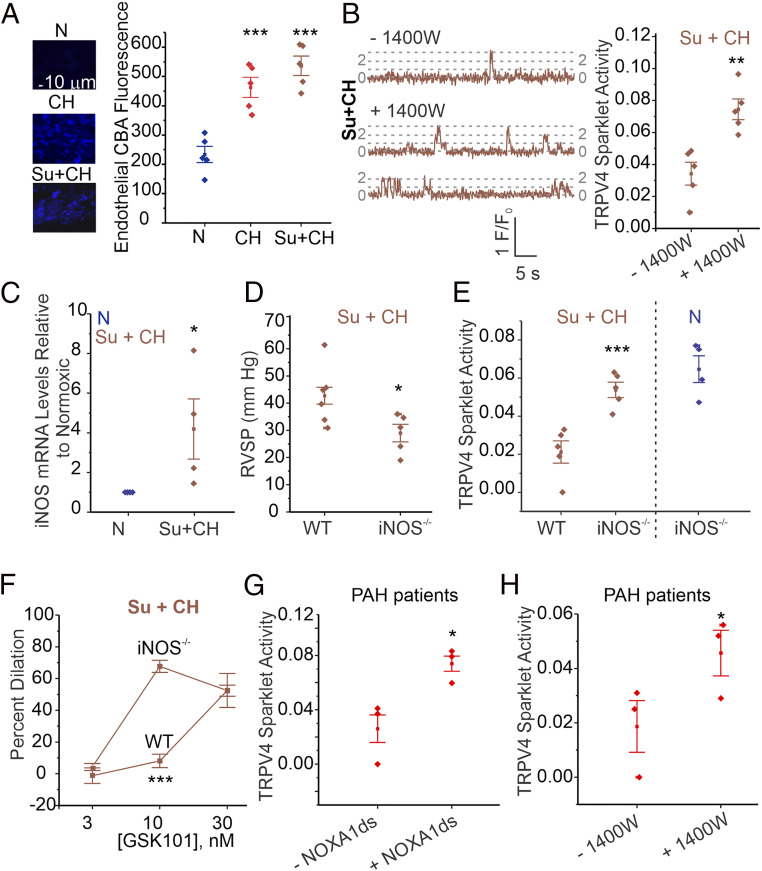
Pulmonary PN levels are elevated in PH (20, 28), and PN was shown to have detrimental effects on TRPV4EC channel activity and endothelial function in obesity (21). Measurement of PN levels using the fluorescent indicator, coumarin boronic acid, confirmed higher endothelial PN levels in PAs from mouse models of PH (Fig. 5A and SI Appendix, Fig. S2). While eNOS activity is impaired in PH (29), iNOS activity has been shown to be elevated (22, 23) and may provide NO for PN formation. The specific iNOS inhibitor 1400W rescued TRPV4EC sparklet activity in Su+CH mice (Fig. 5B and SI Appendix, Table S3), suggesting that iNOS contributes to PN formation in PH. Endothelial iNOS mRNA levels were elevated in Su+CH mice (Fig. 5C). RVSP in iNOS−/− mice exposed to Su+CH was lower than that in WT Su+CH mice (Fig. 5D and SI Appendix, Fig. S6), but TRPV4EC sparklet activity (Fig. 5E and SI Appendix, Table S3) and vasodilation (Fig. 5F) were not impaired in PAs from iNOS−/− mice exposed to Su+CH (SI Appendix, Fig. S6). Moreover, PN levels in both iNOS−/− and NOX1−/− mice exposed to Su+CH were lower than those in WT Su+CH mice (SI Appendix, Fig. S7A). Together, these results identify NOX1 and iNOS as the major contributors to endothelial PN formation in PH.
Fig. 5.
Endothelial iNOS/NOX1-generated PN impairs TRPV4EC channel activity in PH. (A, Left) Representative nonconfocal images for coumarin boronic acid (CBA, PN indicator) fluorescence in ECs from PAs of mice exposed to N, CH (3 wk; 10% O2), or Su+CH. (Right) Scatterplot of CBA fluorescence intensity in en face preparations of fourth-order PAs from mice exposed to N, CH, or Su+CH (n = 5; ***P < 0.001 versus N; one-way ANOVA). Experiments were performed in the presence of PEG-catalase (H2O2 metabolizing enzyme; 500 U/mL) and taurine (hypochlorous acid-lowering agent; 1 mmol/L). (B) TRPV4EC sparklet traces in one EC from fluo-4–loaded en face PAs (Left) and sparklet activity per site (Right; expressed as NPO per site) in the absence and presence of the 1400W (iNOS inhibitor; 1 μmol/L) in PAs from C57BL6/J mice exposed to Su+CH (n = 5; **P < 0.01; t test). (C) iNOS mRNA levels in ECs from PAs of N and Su+CH mice, expressed relative to N mice (n = 5; *P < 0.05; t test). (D) Averaged resting RVSP (millimeters of mercury) values in WT and iNOS−/− mice after exposure to Su+CH (n = 5 to 6; *P < 0.05; t test). (E) TRPV4EC sparklet activity in PAs from WT and iNOS−/− mice exposed to Su+CH and iNOS−/− mice exposed to N (n = 5; * P < 0.05; two-way ANOVA). (F) GSK101 (3–30 nmol/L)-induced vasodilation of PAs from WT and iNOS−/− mice exposed to Su+CH (n = 5; ***P < 0.001 versus WT [10 nmol/L]; two-way ANOVA). (G) Effect of the NOXA1ds (NOX1 inhibitor, 1 μmol/L) on GSK101 (10 nmol/L)-induced TRPV4EC sparklet activity in PAs from PAH patients (n = 3; *P < 0.05; t test). (H) Effect of the 1400W (1 μmol/L) on TRPV4EC sparklet activity in PAs from PAH patients (n = 3; *P < 0.05; t test).
Similar to the results from mouse models, studies in PAs from PAH patients and control individuals showed higher endothelial PN levels in PAH patients (SI Appendix, Fig. S7B). Moreover, TRPV4EC sparklet activity in PAs from PAH patients was rescued by inhibitors of iNOS or NOX1 (Fig. 5 G and H and SI Appendix, Table S3). These findings demonstrate that iNOS and NOX1 contribute to PN formation and impaired TRPV4EC channel activity in clinical PAH.
PN Targets Cav-1EC to Lower Cav-1EC–TRPV4EC Channel Signaling in PH.
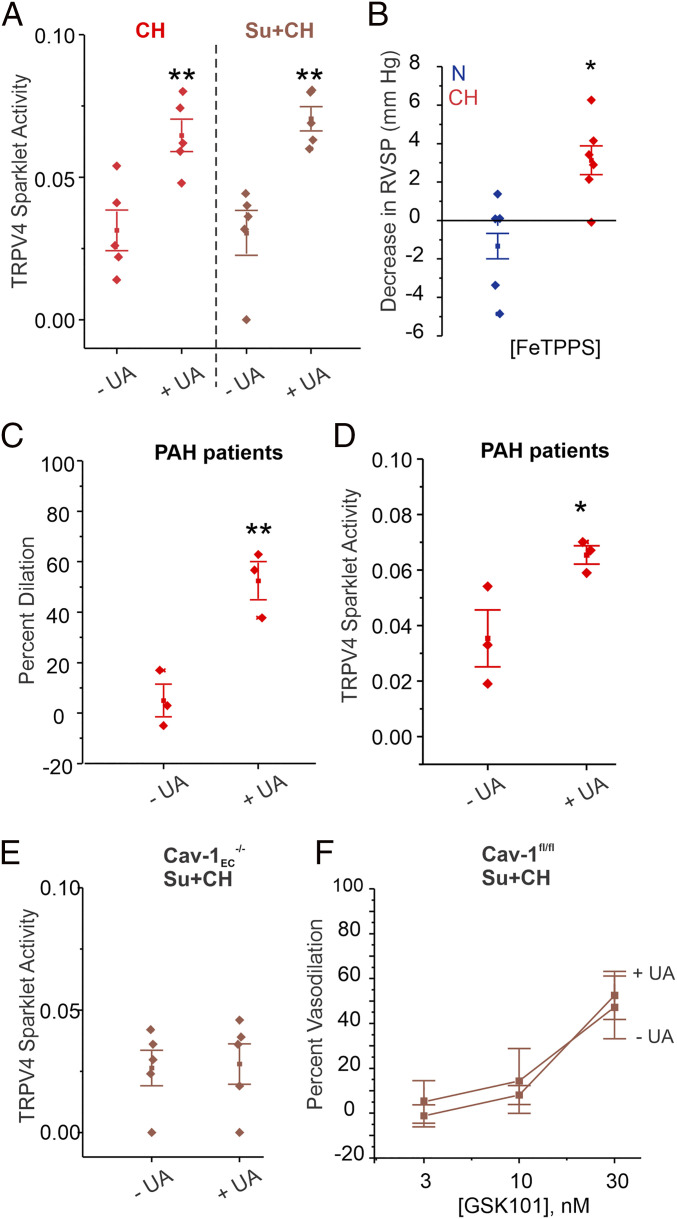
Specific PN inhibitors are not available; therefore, we used three different compounds that are known to lower PN levels: PN scavengers uric acid (UA; 200 μmol/L; Fig. 6A) and ebselen (1 μmol/L) and PN decomposer FeIII-tetra-(4-sulfonatophenyl)-porphyrin (FeTPPS; 1 μmol/L; SI Appendix, Fig. S8 A and B). All three compounds rescued TRPV4EC sparklet activity in PAs from mouse models of PH. PEG-catalase (500 U/mL), which breaks down H2O2, or taurine (1 mmol/L), which decreases hypochlorous acid levels, had no effect on TRPV4EC sparklet activity in PH (SI Appendix, Fig. S8 C and D), indicating that neither H2O2 nor hypochlorous acid are responsible for reducing TRPV4EC channel activity in PH. PN inhibition also rescued TRPV4EC channel-induced vasodilation in Su+CH mice (SI Appendix, Fig. S9 and Table S3). Strikingly, acute treatment with FeTPPS (30 mg/kg, intraperitoneally [i.p.]) reduced the RVSP of CH mice to normal levels but had no effect on RVSP in N mice (Fig. 6B). UA (200 μmol/L) also increased the activity of TRPV4EC channels and TRPV4EC channel-mediated vasodilation in PAs from PAH patients, indicating a clinically relevant role of PN formation in impairing TRPV4EC channel activity in PH (Fig. 6 C and D). Importantly, while UA was able to rescue TRPV4EC sparklet activity and vasodilation in PAs from WT mice exposed to Su+CH (Fig. 6A and SI Appendix, Fig. S9), it was unable to rescue TRPV4EC sparklet activity or vasodilation in Cav-1EC−/− mice exposed to Su+CH (Fig. 6 E and F), suggesting that PN may be targeting Cav-1EC to impair TRPV4EC channel activity.
Fig. 6.
PN inhibition rescues TRPV4EC channel activity in PH. (A) Effects of the UA (PN scavenger; 200 μmol/L) on TRPV4EC sparklet activity in PAs from mice treated with CH (3 wk; 10% O2) or Su+CH, expressed as NPO per site (n = 5; **P < 0.01 versus [−UA]; one-way ANOVA). (B) Scatter plot showing the FeTPPS (PN decomposer; 30 mg/kg i.p.)-induced decrease in RVSP (millimeters of mercury) in mice exposed to N or CH (n = 5 to 6; *P < 0.05; t test). (C) Effect of UA (200 μmol/L) on GSK101 (10 nmol/L)-induced vasodilation of PAs from PAH patients (n = 3; **P < 0.01; t test). (D) Effect of UA (200 μmol/L) on GSK101 (10 nmol/L)-induced TRPV4EC sparklet activity of PAs from PAH patients (n = 3; *P < 0.05; t test). (E) Effect of the UA (200 μmol/L) on TRPV4EC sparklet activity in PAs from Cav-1EC−/− mice exposed to Su+CH (n = 5). (F) Effect of UA (200 μmol/L) on GSK101 (3 to 30 nmol/L)-induced vasodilation of PAs from Cav-1EC−/− mice exposed to Su+CH (n = 5).
To confirm the inhibitory effect of exogenous PN on TRPV4EC channel activity, we recorded TRPV4EC currents from freshly isolated pulmonary ECs and TRPV4EC sparklet activity in en face PAs. The addition of exogenous PN (5 μmol/L) attenuated TRPV4EC channel currents (SI Appendix, Fig. S10A) and decreased TRPV4EC sparklet activity (Fig. 7A). PN also decreased TRPV4EC channel-induced vasodilation (SI Appendix, Fig. S10B) but did not affect vasodilation induced by the NO donor, spermine NONOate (SI Appendix, Fig. S10C), suggesting that PN does not alter signaling elements downstream of NO. Importantly, PN inhibition of TRPV4EC channel activity was absent in PAs from Cav1EC−/− mice (Fig. 7A), further supporting the idea that PN may be acting on Cav-1EC, rather than TRPV4EC channels, to lower Cav-1EC–TRPV4EC signaling. The Cav-1EC–dependent nature of PN inhibition of TRPV4EC channels was further confirmed by studies in HEK293 cells expressing TRPV4 channels alone or coexpressing TRPV4 channels and Cav-1 (Fig. 7B). PN was only able to inhibit TRPV4 channel currents in the presence of Cav-1, supporting the specificity of PN effects on Cav-1 (Fig. 7C). Collectively, these results show that the PN-dependent impairment of Cav1EC–TRPV4EC channel signaling contributes to reduced vasodilation and elevated RVSP in PH.
Fig. 7.
PN inhibits TRPV4 channel activity by targeting cysteine residues on Cav-1. (A) GSK101 (10 nmol/L)-induced TRPV4EC sparklet activity in PAs from Cav-1fl/fl and Cav-1EC−/− mice in the presence or absence of PN (5 μmol/L), expressed as NPO per site (n = 5; ***P < 0.001 versus Cav-1fl/fl [−PN]; two-way ANOVA). (B, Top) Patch-clamp traces showing continuous recordings of TRPV4 currents at −100 mV under basal conditions, followed by PN (5 μmol/L) and GSK2193874 (GSK219; 100 nmol/L) in HEK293 cells transfected with TRPV4 alone or TRPV4 + Cav-1. Cells were held at −100 mV in the whole-cell patch-clamp configuration. (Bottom) Representative traces showing TRPV4 currents in the absence or presence of PN (5 μmol/L) in HEK293 cells transfected with TRPV4 alone or TRPV4 + Cav-1, recorded in the whole-cell patch-clamp configuration. (C) Current density plot of TRPV4 currents at +100 mV in the absence or presence of PN in HEK293 cells transfected with TRPV4 or TRPV4 + WT Cav-1 (n = 5; *P < 0.05 versus TRPV4 + Cav-1 [−PN]; two-way ANOVA). (D) Representative traces showing TRPV4 currents in the absence or presence of PN in HEK293 cells transfected with TRPV4 plus Cav-1 mutants, recorded in the whole-cell patch-clamp configuration. (E) Current density plot of TRPV4 currents at +100 mV in the absence or presence of PN (5 μmol/L) in HEK293 cells transfected with TRPV4 + Cav-1C133A, TRPV4 + Cav-1C143A, or TRPV4 + Cav-1C156A (n = 5; * P < 0.05 versus [−PN; TRPV4 + Cav-1C143A]; two-way ANOVA).
PN Induces Cysteine Modifications on Cav-1 to Lower TRPV4 Channel Activity.
PN is known to induce tyrosine nitration and cysteine modification of proteins. Because the observed PN effects were reversible, we postulated that PN causes reversible cysteine modification(s) on Cav-1. To test this, we generated Cav-1 mutants containing Cys-to-Ala substitutions of each of the three cysteine residues on Cav-1 and exogenously expressed them in HEK293 cells (Fig. 7D). The Cys133Ala and Cys156Ala mutations inhibited the effect of PN on TRPV4 channel activity, whereas the Cys-to-Ala modification at Cys143 did not (Fig. 7E). Taken together, these results suggest that PN targets Cys133 and Cys156 on Cav-1, thereby reducing TRPV4 channel activity and increasing PAP in PH.
NOX1 and iNOS Colocalize with Cav-1EC in PH.
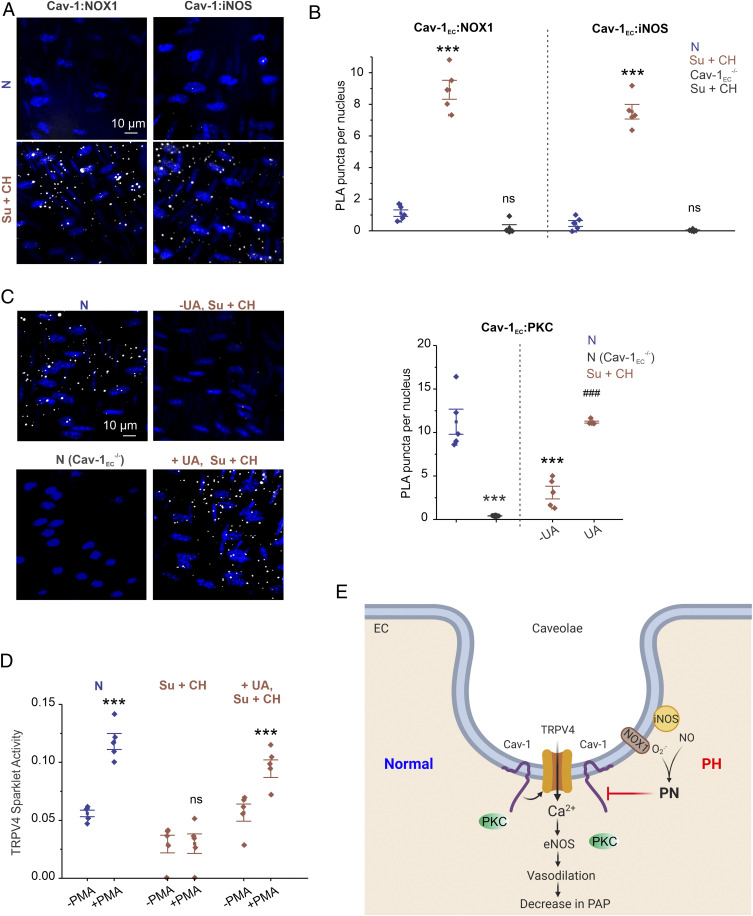
Physiological rescue of TRPV4EC channel activity by PN inhibitors in PH-supported impaired channel regulation, rather than channel expression, as a cause of reduced TRPV4EC channel activity. To that end, the nanometer proximity between Cav-1EC and TRPV4EC channels, assessed with in situ proximity ligation assays (PLAs), was not affected in PH (SI Appendix, Fig. S11). However, the nanometer proximity of Cav-1EC with NOX1 and iNOS was increased in Su+CH mice compared with N mice (Fig. 8 A and B). These results demonstrate higher colocalization of iNOS and NOX1 with Cav-1EC in PH and suggest that PN generation at caveolae may impair Cav-1EC–TRPV4EC signaling. In HEK293 cells overexpressing Cav-1 with NOX1 or iNOS, Cav-1 coimmunoprecipitated with NOX1 as well as iNOS (SI Appendix, Fig. S12), supporting the ability of Cav-1 to associate with iNOS and NOX1.
Fig. 8.
Colocalization of endothelial NOX1 and iNOS with Cav-1EC impairs Cav-1EC:PKC interaction in PH. (A) Representative merged images of PLA showing EC nuclei and Cav-1:NOX1 (Left) or Cav-1:iNOS (Right) colocalization in fourth-order PAs from mice treated with N or CH (3 wk; 10% O2) + SU5416. (B) Quantification of Cav-1:NOX1 and Cav-1:iNOS colocalization in PAs of N and Su+CH mice and Cav-1EC−/− mice treated with Su+CH (n = 5; ***P < 0.001 versus N; two-way ANOVA). (C, Left) Representative merged images of PLA showing EC nuclei and Cav-1EC:PKC colocalization in PAs from N mice (Top Left), N Cav-1EC−/− mice (Bottom Left), and Su+CH mice in the absence (Top Right) or presence (Bottom Right) of UA (PN scavenger; 200 μmol/L). (Right) Quantification of Cav-1EC:PKC colocalization in PAs from N mice, N Cav-1EC−/− mice, and Su+CH mice in the absence or presence of UA (200 μmol/L; n = 5; ***P < 0.001 versus N; ###P < 0.001 versus Su+CH [−UA]; two-way ANOVA). (D) TRPV4EC sparklet activity in PAs from mice treated with N, Su+CH, or Su+CH + UA (200 μmol/L) in the absence or presence of Phorbol 12-myristate 13-acetate (PMA, PKC activator; 10 nmol/L), expressed as NPO per site (n = 5; ***P < 0.001 versus N [−PMA]; ***P < 0.001 versus Su+CH + UA [−PMA]). (E) Schematic depiction of the PN-dependent impairment of endothelial function in PH. TRPV4EC channel-dependent vasodilation reduces PAP. Cav-1EC enhances TRPV4EC channel activity via its interaction with PKC. Up-regulation of iNOS and NOX1 enzymes in caveolae results in increased PN formation in PH. PN, in turn, disrupts Cav-1EC:PKC localization, impairs TRPV4EC channel signaling and vasodilation, and increases PAP in PH.
Cav-1EC showed nanometer proximity with PKC in PAs from normal mice (Fig. 8C). However, in Su+CH mice, the localization of PKC with Cav-1EC was highly reduced (Fig. 8C). PN scavenger UA restored the Cav-1:PKC localization (Fig. 8C) and PKC activation of TRPV4EC sparklets in PAs from Su+CH mice (Fig. 8D). These results supported the concept that PN-induced cysteine modification on Cav-1 impairs Cav-1EC:PKC localization and TRPV4EC channel activity in PH. The summary of our results is illustrated in Fig. 8E.
Discussion
Despite an emerging interest in the contribution of endothelial TRPV4 channels to pulmonary pathologies, including lung edema and lung injury (30–32), the physiological roles of pulmonary TRPV4EC channels remain unknown. Using cell-specific knockout mice, we show that arterial TRPV4EC channels lower resting PAP and that impaired TRPV4EC channel activity contributes to higher PAP in PH. Importantly, data from PAH patients provide evidence that clinical PAH is also associated with the lowering of TRPV4EC channel activity in small PAs. Detailed mechanistic studies show increased association between Cav-1EC and NOX1/iNOS in PH, resulting in localized PN formation and impaired Cav-1EC–TRPV4EC channel signaling. Overall, our studies identify specific mechanisms that impair the activity of TRPV4EC channels in resistance PAs in PH and lay the foundation for targeting these pathological mechanisms to rescue endothelial function. Recent studies have focused on the detrimental effects of capillary TRPV4EC channels in pulmonary vascular disorders. Indeed, inhibition of TRPV4 channels has been proposed as a treatment option for pulmonary edema (30, 31). Our results indicate that direct TRPV4 channel inhibitor therapy may have undesirable effects on PAP through inhibition of TRPV4EC channels in resistance PAs.
EC and smooth muscle cell (SMC) components in the TRPV4 channel control of PAP have been difficult to define. Global TRPV4−/− mice show no changes in resting RVSP (5). Administration of the TRPV4 channel activator GSK101 was shown to reduce RVSP in rats (33), although the exact cell types involved in this effect were not clear. TRPV4 channels are also detected at mRNA and protein levels in pulmonary vascular SMCs (34). SMC TRPV4 channels promote PA contractility (35) and are up-regulated following CH (5, 36). Thus, EC and SMC TRPV4 channels appear to have contrasting effects on contractility, similar to intracellular Ca2+ in ECs and SMCs (37). In other words, it appears that EC TRPV4 channels are dilatory, whereas SMC TRPV4 channels are contractile in PAs. These findings further exemplify the unique cellular activation/function of TRPV4 channels in ECs or SMCs, which would be difficult to decipher using global knockout mice. We provide evidence that resting RVSP is not altered in TRPV4SMC−/− mice (Fig. 1D), suggesting that SMC TRPV4 channels may not play a role in regulating the resting PAP. The lack of a resting PAP phenotype in global TRPV4−/− mice (5) but elevated PAP in endothelial TRPV4−/− mice are consistent with previously published findings of unaltered resting systemic blood pressure in TRPV4−/− mice (38) and elevated systemic blood pressure in endothelial TRPV4−/− mice (21). Indeed, many global knockout mice are known to have developmental compensations to ensure physiological homeostasis, masking individual cellular function (reviewed in ref. 39). TRPV4 channels have also been reported in alveolar epithelial cells (40), macrophages (41), airway SMCs (42), and epithelial cells (43). Therefore, using direct TRPV4 channel activators to improve vasodilation in PH would likely be associated with multiple side effects. Targeting pathological elements upstream of TRPV4EC channels, namely PN, iNOS, or NOX1, may result in a more specific rescue of TRPV4EC channel activity in resistance PAs in PH.
Endothelial Ca2+ has distinct effects on capillary and arterial function. TRPV4EC channel activity in capillary ECs has mostly been associated with detrimental effects in pulmonary pathologies. Excessive capillary TRPV4EC channel activity contributes to increased endothelial permeability (44, 45), lung injury (40), and pulmonary edema (44, 45). A study by Thorneloe et al. (44) showed that chronic treatment with TRPV4 channel inhibitor restored the integrity of the capillary endothelial barrier in heart failure–induced pulmonary edema. However, arterial TRPV4EC channel activity remains an unexplored topic in pulmonary vascular disorders. Our data show that acute treatment with TRPV4 channel inhibitor elevates RVSP, and this effect is lost in TRPV4EC−/− mice (SI Appendix, Fig. S13). Capillary and arterial ECs are structurally and functionally heterogeneous (24, 46, 47). Moreover, TRPV4EC channels and Ca2+ signaling mechanisms have distinct effects on capillary and arterial function (3, 4, 40, 44). Our data, in conjunction with current evidence in the literature, suggest that pathological conditions may affect capillary and arterial TRPV4EC channels differently.
Pulmonary circulation is a high-flow circulation. Therefore, flow-induced shear stress is a potential activator of TRPV4EC channels in PAs (48–50). Shear stress has also been shown to increase the release of ATP from the pulmonary endothelium (51). Extracellular ATP is a physiological activator of TRPV4EC channels in PAs (4). We show that basal and ATP-induced TRPV4EC channel activity is reduced in PH (Fig. 3D and SI Appendix, Fig. S3A). Therefore, it is possible that flow-induced TRPV4EC channel activation is impaired in PH, which would result in reduced endothelium-dependent vasodilation.
Cav-1EC is a key structural protein in the pulmonary circulation, as demonstrated by the observed increase in RVSP in global Cav-1−/− mice (11, 52). Moreover, Cav-1EC–dependent signaling is impaired in PH (7–9). We found that abnormal Cav-1EC–TRPV4EC signaling, rather than reduced Cav-1EC or TRPV4EC expression, contributes to endothelial dysfunction in PH. Although qPCR analysis showed a minor decrease in Cav-1EC mRNA levels in PH (SI Appendix, Fig. S4), Cav-1EC:TRPV4EC localization was not altered in PH. The instantaneous rescue of TRPV4EC channel activity by PN inhibitors also supports the concept that the signaling impairment in PH is not attributable to altered protein expression. Cav-1 is also a well-known anchor protein for eNOS (6), and global Cav-1−/− mice show higher NO and PN levels (52). TRPV4EC channel activity remained low in Cav-1EC−/− mice in the presence of the NOS inhibitor l-NNA (100 μmol/L; SI Appendix, Fig. S14), supporting the idea that the decrease in TRPV4EC channel activity in Cav-1EC−/− mice is not due to elevated NO levels.
The spatial proximity of iNOS and NOX1 with Cav-1EC facilitates the formation of endothelial PN in PH. PN is known to have harmful effects on the vascular function in multiple disease conditions. Our previous studies showed that PN targets A-kinase anchoring protein 150 (AKAP150)–TRPV4EC channel signaling in systemic resistance arteries, contributing to systemic hypertension. Notably, AKAP150 is not expressed in PA endothelium. Instead, PN-dependent inhibition of Cav-1EC–TRPV4EC channel signaling elevates PAP in PH. Importantly, superoxide radicals do not directly inhibit TRPV4EC channel activity (SI Appendix, Fig. S5B). In this regard, a previous study on capillary EC culture suggested that superoxide radicals activate TRPV4EC channel-dependent increases in Ca2+ (18). TRPV4EC channels promote inositol 1,4,5-trisphosphate (IP3) receptor (IP3R)-mediated Ca2+ release from the endoplasmic reticulum (ER) (53), and superoxide radicals have been shown to potentiate IP3Rs (54). Therefore, it is possible that superoxide radicals act on IP3Rs to promote TRPV4–IP3R-mediated increases in intracellular Ca2+.
iNOS activity has mainly been associated with immune cells (55). The increase in endothelial iNOS mRNA levels (Fig. 5C) confirms the up-regulation of endothelial iNOS in PH. However, it should be noted that this increase in iNOS activity did not result in pulmonary vasodilation. We postulate that the reduced bioavailability of NO despite higher iNOS activity is due to the near-instantaneous reaction of NO with NOX1-generated superoxide radicals to form PN. Uncoupling of eNOS has also been proposed as a cause of reduced NO bioavailability in PH (29). However, our results indicate that the TRPV4EC–eNOS signaling linkage is not altered in PH (Fig. 3G); instead, the signaling abnormalities occur upstream of TRPV4EC channels. PN modification of cysteine residues on Cav-1EC appears to be the mechanism underlying PN-induced impairment of TRPV4EC channel activity. The reversibility of the PN effect on TRPV4EC channel activity implies that PN-induced tyrosine nitration, which is thought to be a permanent modification (56), is unlikely to be the underlying mechanism. The main PN-induced cysteine modifications include disulfide bond formation and S-nitrosylation (56). Previous studies have suggested that Cys156 S-nitrosylation can impair Cav-1–dependent signaling in PAH (7), although identifying which specific type of PN-induced cysteine modification underlies impaired Cav-1EC–TRPV4EC signaling will require further investigation.
The current manuscript presents in vivo RVSP and ex vivo PAP measurements. A significant increase in resting RVSP and PAP within 1 mo of the induction of endothelial knockout is striking. One limitation of the study is that the direct in vivo PAP measurements were not performed. Besides PA contractility, other crucial factors such as blood viscosity may also play a role in regulating PAP and will need to be investigated separately.
In conclusion, arterial Cav-1EC–TRPV4EC channel signaling promotes pulmonary vasodilation, and its impairment contributes to elevated PAP in PH. In PH, up-regulation of NOX1 and iNOS in endothelial caveolae increases PN formation and inhibits the Cav-1EC–TRPV4EC channel signaling linkage. Decreasing PN levels or NOX1/iNOS activity rescues Cav-1EC–TRPV4EC signaling, improves vasodilation, and lowers PAP in PH. These results identify a pathological mechanism that could be targeted for rescuing endothelial Ca2+ signaling and vasodilation in PH.
Materials and Methods
Animal Protocols.
All animal protocols were approved by the University of Virginia Animal Care and Use Committee (protocols 4,100 and 4,120). Both male and female mice were used in this study. Comparison groups were age and sex matched. A total of 247 mice were used in the study. C57BL6/J were obtained from the Jackson Laboratory. TRPV4EC−/−, SMC-specific TRPV4 channel knockout (TRPV4SMC−/−), Cav-1EC−/−, iNOS−/−, and NOX1−/− knockout mice (10 to 14 wk old) were used. Mice were euthanized with pentobarbital (90 mg/kg−1 i.p.; Diamondback Drugs) followed by cranial dislocation for harvesting lung tissue. Fourth‐order PAs (∼50 μm diameter) were isolated in cold Hepes‐buffered physiological salt solution (Hepes‐PSS, in millimoles per liter, 10 Hepes, 134 NaCl, 6 KCl, 1 MgCl2 hexahydrate, 2 CaCl2 dihydrate, and 7 dextrose, pH adjusted to 7.4 using 1 mol/L NaOH).
Study Approval for Human PAs.
Deidentified lung tissue samples from patients of scleroderma PAH (3 samples) and non-PAH individuals (3 samples) were obtained in accordance with the University of Virginia Institutional Review Board (IRB #19044) and the University of Pittsburgh Committee for Oversight of Research and Clinical Training Involving Descents. Small PAs (∼50 μm) were used for pressure myography and Ca2+ imaging experiments.
Mouse Models of PH.
Two different mouse models of PH were used in this study: CH (10% O2) for 3 wk or CH with SU5416 [20 mg/kg; subcutaneously; once a week (26) for 3 wk]. SU5416 was dissolved in DMSO. Mice were exposed to CH conditions in a vinyl hypoxic chamber (Coy Laboratory Products, Inc.) connected to an autopurge airlock inlet. The oxygen concentration in the glove box was regulated by an oxygen controller and oxygen sensor (Coy Laboratory Products, Inc.). Control mice were maintained in room air for 3 wk.
Generation of TRPV4EC−/−, Cav-1EC−/−, and TRPV4SMC−/− Mice.
TRPV4fl/fl (57) and Cav-1fl/fl (10, 58, 59) mice were crossed with VE-Cadherin (Cdh5, endothelial) Cre mice (60) or SMMHC (smooth muscle) Cre mice (61). EC- or SMC-specific knockout of TRPV4 or Cav-1 was induced by injecting 6 wk old TRPV4fl/fl Cre+ or Cav-1fl/fl Cre+ mice with tamoxifen (40 mg/kg per day for 10 d; i.p.). Tamoxifen-injected TRPV4fl/fl Cre− and Cav-1fl/fl Cre− mice were used as controls. Mice were used for experiments after a 2 wk tamoxifen washout period.
Measurement of PAP and RVSP.
PAP was evaluated using an IPL-1 ex vivo murine lung perfusion system (Harvard Apparatus), as previously described (62). RVSP was measured in anesthetized mice using a Mikro Tip pressure catheter (Millar Instruments).
Statistics.
Results are presented as mean ± SEM. Data were analyzed using two-tailed paired or independent t test (for comparison of data collected from two different treatments) and one-way ANOVA or two-way ANOVA (to investigate statistical differences among more than two different treatments). Statistical significance was determined as a P value less than 0.05.
Supplementary Material
Acknowledgments
This work was supported by a predoctoral fellowship from the American Heart Association to M.O. (20PRE35210176) and grants from the NIH to E.A.G. (HL113178 and HL130261) and S.K.S. (HL146914, HL142808, and HL147555). We thank Dr. Wolfgang Liedtke (Duke University) for the TRPV4fl/fl mice.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2023130118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Rich S., Haworth S. G., Hassoun P. M., Yacoub M. H., Pulmonary hypertension: The unaddressed global health burden. Lancet Respir. Med. 6, 577–579 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Budhiraja R., Tuder R. M., Hassoun P. M., Endothelial dysfunction in pulmonary hypertension. Circulation 109, 159–165 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Ottolini M., et al., Mechanisms underlying selective coupling of endothelial Ca2+ signals with eNOS versus IK/SK channels in systemic and pulmonary arteries. J. Physiol. 598, 3577–3596 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marziano C., et al., Nitric oxide-dependent feedback loop regulates transient receptor potential vanilloid 4 (TRPV4) channel cooperativity and endothelial function in small pulmonary arteries. J. Am. Heart Assoc. 6, e007157 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia Y., et al., TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am. J. Physiol. Cell Physiol. 305, C704–C715 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernatchez P. N., et al., Dissecting the molecular control of endothelial NO synthase by caveolin-1 using cell-permeable peptides. Proc. Natl. Acad. Sci. U.S.A. 102, 761–766 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakhshi F. R., et al., Nitrosation-dependent caveolin 1 phosphorylation, ubiquitination, and degradation and its association with idiopathic pulmonary arterial hypertension. Pulm. Circ. 3, 816–830 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maniatis N. A., et al., Increased pulmonary vascular resistance and defective pulmonary artery filling in caveolin-1-/- mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L865–L873 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nickel N. P., et al., Elafin reverses pulmonary hypertension via caveolin-1-dependent bone morphogenetic protein signaling. Am. J. Respir. Crit. Care Med. 191, 1273–1286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira S. D. S., et al., Injury-induced shedding of extracellular vesicles depletes endothelial cells of Cav-1 (caveolin-1) and enables TGF-β (transforming growth factor-β)-dependent pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 39, 1191–1202 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y.-Y., et al., Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc. Natl. Acad. Sci. U.S.A. 99, 11375–11380 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saliez J., et al., Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117, 1065–1074 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Daneva Z., Laubach V. E., Sonkusare S. K., Novel regulators and targets of redox signaling in pulmonary vasculature. Curr. Opin. Physiol. 9, 87–93 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulton D. J. R., et al., Reactive oxygen and nitrogen species in the development of pulmonary hypertension. Antioxidants (Basel) 6, 54 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carnesecchi S., et al., NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am. J. Respir. Crit. Care Med. 180, 972–981 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jesus D. S., et al., Nox1/Ref-1-mediated activation of CREB promotes Gremlin1-driven endothelial cell proliferation and migration. Redox Biol. 22, 101138 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghouleh I. A., et al., Endothelial Nox1 oxidase assembly in human pulmonary arterial hypertension; Driver of Gremlin1-mediated proliferation. Clin. Sci. (Lond.) 131, 2019–2035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suresh K., et al., Reactive oxygen species induced Ca2+ influx via TRPV4 and microvascular endothelial dysfunction in the SU5416/hypoxia model of pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 314, L893–L907 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radi R., Beckman J. S., Bush K. M., Freeman B. A., Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 266, 4244–4250 (1991). [PubMed] [Google Scholar]
- 20.Bowers R., et al., Oxidative stress in severe pulmonary hypertension. Am. J. Respir. Crit. Care Med. 169, 764–769 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Ottolini M., et al., Local peroxynitrite impairs endothelial transient receptor potential vanilloid 4 channels and elevates blood pressure in obesity. Circulation 141, 1318–1333 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hampl V., et al., Pulmonary vascular iNOS induction participates in the onset of chronic hypoxic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L11–L20 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Hoehn T., Stiller B., McPhaden A. R., Wadsworth R. M., Nitric oxide synthases in infants and children with pulmonary hypertension and congenital heart disease. Respir. Res. 10, 110 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonkusare S. K., et al., Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336, 597–601 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan H.-C., Zhang X., McNaughton P. A., Activation of the TRPV4 ion channel is enhanced by phosphorylation. J. Biol. Chem. 284, 27884–27891 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitali S. H., et al., The Sugen 5416/hypoxia mouse model of pulmonary hypertension revisited: Long-term follow-up. Pulm. Circ. 4, 619–629 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jernigan N. L., et al., Contribution of reactive oxygen species to the pathogenesis of pulmonary arterial hypertension. PLoS One 12, e0180455 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravi Y., et al., Pulmonary hypertension secondary to left-heart failure involves peroxynitrite-induced downregulation of PTEN in the lung. Hypertension 61, 593–601 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klinger J. R., Abman S. H., Gladwin M. T., Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 188, 639–646 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Cheung M., et al., Discovery of GSK2193874: An orally active, potent, and selective blocker of transient receptor potential vanilloid 4. ACS Med. Chem. Lett. 8, 549–554 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuebler W. M., Jordt S.-E., Liedtke W. B., Urgent reconsideration of lung edema as a preventable outcome in COVID-19: Inhibition of TRPV4 represents a promising and feasible approach. Am. J. Physiol. Lung Cell. Mol. Physiol. 318, L1239–L1243 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin M. T., et al., Functional coupling of TRPV4, IK, and SK channels contributes to Ca(2+)-dependent endothelial injury in rodent lung. Pulm. Circ. 5, 279–290 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pankey E. A., Zsombok A., Lasker G. F., Kadowitz P. J., Analysis of responses to the TRPV4 agonist GSK1016790A in the pulmonary vascular bed of the intact-chest rat. Am. J. Physiol. Heart Circ. Physiol. 306, H33–H40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin E., et al., Involvement of TRPV1 and TRPV4 channels in migration of rat pulmonary arterial smooth muscle cells. Pflugers Arch. 464, 261–272 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Song S., et al., Flow shear stress enhances intracellular Ca2+ signaling in pulmonary artery smooth muscle cells from patients with pulmonary arterial hypertension. Am. J. Physiol. Cell Physiol. 307, C373–C383 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X.-R., et al., Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L555–L568 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ottolini M., Hong K., Sonkusare S. K., Calcium signals that determine vascular resistance. Wiley Interdiscip. Rev. Syst. Biol. Med. 11, e1448 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong K., et al., TRPV4 (transient receptor potential vanilloid 4) channel-dependent negative feedback mechanism regulates Gq protein-coupled receptor-induced vasoconstriction. Arterioscler. Thromb. Vasc. Biol. 38, 542–554 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Brolosy M. A., Stainier D. Y. R., Genetic compensation: A phenomenon in search of mechanisms. PLoS Genet. 13, e1006780 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez D. F., et al., Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: A novel mechanism of acute lung injury. Circ. Res. 99, 988–995 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamanaka K., et al., TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 299, L353–L362 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia Y., et al., Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L272–L278 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Lorenzo I. M., Liedtke W., Sanderson M. J., Valverde M. A., TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 105, 12611–12616 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorneloe K. S., et al., An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci. Transl. Med. 4, 159ra148 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Yin J., et al., Negative-feedback loop attenuates hydrostatic lung edema via a cGMP-dependent regulation of transient receptor potential vanilloid 4. Circ. Res. 102, 966–974 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Stevens T., Functional and molecular heterogeneity of pulmonary endothelial cells. Proc. Am. Thorac. Soc. 8, 453–457 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Longden T. A., et al., Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci. 20, 717–726 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendoza S. A., et al., TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am. J. Physiol. Heart Circ. Physiol. 298, H466–H476 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Köhler R., et al., Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler. Thromb. Vasc. Biol. 26, 1495–1502 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Hartmannsgruber V., et al., Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One 2, e827 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gödecke S., et al., Thrombin-induced ATP release from human umbilical vein endothelial cells. Am. J. Physiol. Cell Physiol. 302, C915–C923 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y.-Y., et al., Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J. Clin. Invest. 119, 2009–2018 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heathcote H. R., et al., Endothelial TRPV4 channels modulate vascular tone by Ca2+ -induced Ca2+ release at inositol 1,4,5-trisphosphate receptors. Br. J. Pharmacol. 176, 3297–3317 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madesh M., et al., Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J. Cell Biol. 170, 1079–1090 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bogdan C., Nitric oxide synthase in innate and adaptive immunity: An update. Trends Immunol. 36, 161–178 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Radi R., Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 288, 26464–26472 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore C., et al., UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc. Natl. Acad. Sci. U.S.A. 110, E3225–E3234 (2013).Corrected in: Proc. Natl. Acad. Sci. U.S.A.110, 15502 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Z., et al., Reciprocal regulation of eNOS and caveolin-1 functions in endothelial cells. Mol. Biol. Cell 29, 1190–1202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oliveira S. D. S., et al., Inflammation-induced caveolin-1 and BMPRII depletion promotes endothelial dysfunction and TGF-β-driven pulmonary vascular remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol. 312, L760–L771 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sörensen I., Adams R. H., Gossler A., DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood 113, 5680–5688 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Wirth A., et al., G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat. Med. 14, 64–68 (2008). Correction in: Nat. Med.14, 222 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Anvari F., et al., Tissue-derived proinflammatory effect of adenosine A2B receptor in lung ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 140, 871–877 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sonkusare S. K., et al., AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci. Signal. 7, ra66 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.